User login
Mild Gestational Diabetes Treatment Is Beneficial : Treatment reduced macrosomia, lowered neonatal fat mass, rates of shoulder dystocia and C-section.
SAN DIEGO — Treatment of mild gestational diabetes did not reduce the frequency of several commonly reported morbidities associated with diabetic pregnancy, results from a large multicenter randomized trial demonstrated.
However, treatment did lower birth weight and resulted in a 50% reduction in macrosomia, as well as lower neonatal fat mass, rates of shoulder dystocia, cesarean delivery, preeclampsia, and gestational hypertension.
“Identification and treatment of mild gestational diabetes is clearly associated with significant clinical benefits,” principal investigator Dr. Mark B. Landon said at the annual meeting of the Society for Maternal-Fetal Medicine.
The incidence of gestational diabetes, defined as glucose intolerance with onset or first recognition during pregnancy, is rising in the United States, said Dr. Landon, professor of obstetrics and gynecology at the Ohio State University, Columbus.
More than 45 years ago researchers “first proposed criteria for the diagnosis, which were based on the subsequent development of adult onset diabetes and not on any association between carbohydrate intolerance and adverse pregnancy outcomes,” he said. “Thus, the clinical significance of gestational diabetes and, in particular, mild gestational diabetes as it relates to perinatal morbidity is unclear and has been challenged for decades.”
He went on to note that, based largely on results of retrospective single-center studies to date, there has been “widespread acceptance of screening and treatment of gestational diabetes by professional organizations with little evidence of demonstrable benefit.”
However, in 2003 and in 2008 the U.S. Preventive Services Task Force issued statements concluding that there is insufficient evidence to determine if a health benefit to the treatment of mild gestational diabetes exists.
The controversy prompted the maternal-fetal medicine units network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development to conduct a randomized trial to determine if treatment of mild gestational diabetes reduced perinatal morbidity.
For the study, 958 women with a singleton gestation and who met criteria for mild gestational diabetes (defined as a fasting value of less than 95 mg/dL on a blinded 3-hour oral glucose tolerance test) were allocated to one of two groups.
The 485 women in the treatment group received formal nutrition counseling, instruction on self-monitoring of blood glucose, and insulin administration, if necessary.
The 473 women in the control group received standard routine obstetric care, and clinicians and study participants were unaware of their glucose tolerance test results.
The primary end point was a composite outcome that consisted of perinatal mortality; neonatal hypoglycemia defined as a value less than 35 mg/dL during the first 2 hours of life without feeding; a serum bilirubin greater than 8 mg/dL between 16 and 36 hours of life, hyperinsulinemia as reflected by a cord blood C-peptide greater than the 95th percentile, or birth trauma.
Dr. Landon reported that the average age of the study participants was 29 years.
There were no differences between the groups in the frequency of composite primary neonatal outcome (32% in the treatment group vs. 37% in the control group).
Among secondary outcomes, the researchers observed a significant difference between the treatment and control groups in terms of mean birth weight (3,302 g vs. 3,408 g, respectively), fetal fat mass (427 g vs. 464 g), and the frequency of infants weighing greater than 4,000 g at birth (6% vs. 14%).
There were no differences between the two groups in terms of NICU admission, preterm delivery, respiratory distress syndrome, or need for intravenous glucose treatment.
As for maternal outcomes, induction of labor rates were similar between the two groups (about 27%), but women in the treatment group had significantly lower overall rates of cesarean delivery (27% vs. 34%) and rates of cesarean corrected for abnormal presentation and prior cesarean (13% vs. 20%).
The rate of shoulder dystocia also was reduced with treatment (2% vs. 4%) as was the rate of preeclampsia and gestational hypertension as a composite (9% vs. 14%).
Dr. Landon concluded that the study findings “should complement the ongoing analysis of the recent Hyperglycemia and Adverse Pregnancy Outcome study as experts develop a consensus for the diagnosis and treatment of carbohydrate intolerance during pregnancy.”
Dr. Landon had no conflicts to disclose.
The rate of preeclampsia and gestational hypertension as a composite was reduced with treatment. DR. LANDON
SAN DIEGO — Treatment of mild gestational diabetes did not reduce the frequency of several commonly reported morbidities associated with diabetic pregnancy, results from a large multicenter randomized trial demonstrated.
However, treatment did lower birth weight and resulted in a 50% reduction in macrosomia, as well as lower neonatal fat mass, rates of shoulder dystocia, cesarean delivery, preeclampsia, and gestational hypertension.
“Identification and treatment of mild gestational diabetes is clearly associated with significant clinical benefits,” principal investigator Dr. Mark B. Landon said at the annual meeting of the Society for Maternal-Fetal Medicine.
The incidence of gestational diabetes, defined as glucose intolerance with onset or first recognition during pregnancy, is rising in the United States, said Dr. Landon, professor of obstetrics and gynecology at the Ohio State University, Columbus.
More than 45 years ago researchers “first proposed criteria for the diagnosis, which were based on the subsequent development of adult onset diabetes and not on any association between carbohydrate intolerance and adverse pregnancy outcomes,” he said. “Thus, the clinical significance of gestational diabetes and, in particular, mild gestational diabetes as it relates to perinatal morbidity is unclear and has been challenged for decades.”
He went on to note that, based largely on results of retrospective single-center studies to date, there has been “widespread acceptance of screening and treatment of gestational diabetes by professional organizations with little evidence of demonstrable benefit.”
However, in 2003 and in 2008 the U.S. Preventive Services Task Force issued statements concluding that there is insufficient evidence to determine if a health benefit to the treatment of mild gestational diabetes exists.
The controversy prompted the maternal-fetal medicine units network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development to conduct a randomized trial to determine if treatment of mild gestational diabetes reduced perinatal morbidity.
For the study, 958 women with a singleton gestation and who met criteria for mild gestational diabetes (defined as a fasting value of less than 95 mg/dL on a blinded 3-hour oral glucose tolerance test) were allocated to one of two groups.
The 485 women in the treatment group received formal nutrition counseling, instruction on self-monitoring of blood glucose, and insulin administration, if necessary.
The 473 women in the control group received standard routine obstetric care, and clinicians and study participants were unaware of their glucose tolerance test results.
The primary end point was a composite outcome that consisted of perinatal mortality; neonatal hypoglycemia defined as a value less than 35 mg/dL during the first 2 hours of life without feeding; a serum bilirubin greater than 8 mg/dL between 16 and 36 hours of life, hyperinsulinemia as reflected by a cord blood C-peptide greater than the 95th percentile, or birth trauma.
Dr. Landon reported that the average age of the study participants was 29 years.
There were no differences between the groups in the frequency of composite primary neonatal outcome (32% in the treatment group vs. 37% in the control group).
Among secondary outcomes, the researchers observed a significant difference between the treatment and control groups in terms of mean birth weight (3,302 g vs. 3,408 g, respectively), fetal fat mass (427 g vs. 464 g), and the frequency of infants weighing greater than 4,000 g at birth (6% vs. 14%).
There were no differences between the two groups in terms of NICU admission, preterm delivery, respiratory distress syndrome, or need for intravenous glucose treatment.
As for maternal outcomes, induction of labor rates were similar between the two groups (about 27%), but women in the treatment group had significantly lower overall rates of cesarean delivery (27% vs. 34%) and rates of cesarean corrected for abnormal presentation and prior cesarean (13% vs. 20%).
The rate of shoulder dystocia also was reduced with treatment (2% vs. 4%) as was the rate of preeclampsia and gestational hypertension as a composite (9% vs. 14%).
Dr. Landon concluded that the study findings “should complement the ongoing analysis of the recent Hyperglycemia and Adverse Pregnancy Outcome study as experts develop a consensus for the diagnosis and treatment of carbohydrate intolerance during pregnancy.”
Dr. Landon had no conflicts to disclose.
The rate of preeclampsia and gestational hypertension as a composite was reduced with treatment. DR. LANDON
SAN DIEGO — Treatment of mild gestational diabetes did not reduce the frequency of several commonly reported morbidities associated with diabetic pregnancy, results from a large multicenter randomized trial demonstrated.
However, treatment did lower birth weight and resulted in a 50% reduction in macrosomia, as well as lower neonatal fat mass, rates of shoulder dystocia, cesarean delivery, preeclampsia, and gestational hypertension.
“Identification and treatment of mild gestational diabetes is clearly associated with significant clinical benefits,” principal investigator Dr. Mark B. Landon said at the annual meeting of the Society for Maternal-Fetal Medicine.
The incidence of gestational diabetes, defined as glucose intolerance with onset or first recognition during pregnancy, is rising in the United States, said Dr. Landon, professor of obstetrics and gynecology at the Ohio State University, Columbus.
More than 45 years ago researchers “first proposed criteria for the diagnosis, which were based on the subsequent development of adult onset diabetes and not on any association between carbohydrate intolerance and adverse pregnancy outcomes,” he said. “Thus, the clinical significance of gestational diabetes and, in particular, mild gestational diabetes as it relates to perinatal morbidity is unclear and has been challenged for decades.”
He went on to note that, based largely on results of retrospective single-center studies to date, there has been “widespread acceptance of screening and treatment of gestational diabetes by professional organizations with little evidence of demonstrable benefit.”
However, in 2003 and in 2008 the U.S. Preventive Services Task Force issued statements concluding that there is insufficient evidence to determine if a health benefit to the treatment of mild gestational diabetes exists.
The controversy prompted the maternal-fetal medicine units network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development to conduct a randomized trial to determine if treatment of mild gestational diabetes reduced perinatal morbidity.
For the study, 958 women with a singleton gestation and who met criteria for mild gestational diabetes (defined as a fasting value of less than 95 mg/dL on a blinded 3-hour oral glucose tolerance test) were allocated to one of two groups.
The 485 women in the treatment group received formal nutrition counseling, instruction on self-monitoring of blood glucose, and insulin administration, if necessary.
The 473 women in the control group received standard routine obstetric care, and clinicians and study participants were unaware of their glucose tolerance test results.
The primary end point was a composite outcome that consisted of perinatal mortality; neonatal hypoglycemia defined as a value less than 35 mg/dL during the first 2 hours of life without feeding; a serum bilirubin greater than 8 mg/dL between 16 and 36 hours of life, hyperinsulinemia as reflected by a cord blood C-peptide greater than the 95th percentile, or birth trauma.
Dr. Landon reported that the average age of the study participants was 29 years.
There were no differences between the groups in the frequency of composite primary neonatal outcome (32% in the treatment group vs. 37% in the control group).
Among secondary outcomes, the researchers observed a significant difference between the treatment and control groups in terms of mean birth weight (3,302 g vs. 3,408 g, respectively), fetal fat mass (427 g vs. 464 g), and the frequency of infants weighing greater than 4,000 g at birth (6% vs. 14%).
There were no differences between the two groups in terms of NICU admission, preterm delivery, respiratory distress syndrome, or need for intravenous glucose treatment.
As for maternal outcomes, induction of labor rates were similar between the two groups (about 27%), but women in the treatment group had significantly lower overall rates of cesarean delivery (27% vs. 34%) and rates of cesarean corrected for abnormal presentation and prior cesarean (13% vs. 20%).
The rate of shoulder dystocia also was reduced with treatment (2% vs. 4%) as was the rate of preeclampsia and gestational hypertension as a composite (9% vs. 14%).
Dr. Landon concluded that the study findings “should complement the ongoing analysis of the recent Hyperglycemia and Adverse Pregnancy Outcome study as experts develop a consensus for the diagnosis and treatment of carbohydrate intolerance during pregnancy.”
Dr. Landon had no conflicts to disclose.
The rate of preeclampsia and gestational hypertension as a composite was reduced with treatment. DR. LANDON
Contingency Mx Helps Pregnant Women Stop Smoking
BOCA RATON, FLA. — In a pilot study of pregnant women who continued to smoke cigarettes despite knowing they were pregnant, 11 (37%) of 30 women who received contingency management achieved abstinence, compared with just 2 (10%)) of 23 women who did not.
This result highlights the effectiveness of contingency management as a strategy to help pregnant women stop smoking, Dr. Sarah Heil said at the annual meeting of the American Academy of Addiction Psychiatry.
The women in both the contingent group and noncontingent group were seen every day for the first 5 days of the study.
During this time, abstinence was based on a breath carbon monoxide level of 6 parts per million or less, said Dr. Heil of the University of Vermont, Burlington.
After the first 5 days, the women were seen according to the following schedule:
▸ Twice a week for 7 weeks.
▸ Once a week for the next 11 weeks.
▸ Once every other week until delivery.
▸ Once a week for the first 4 weeks post partum.
▸ Every other week for the next 8 weeks.
Abstinence in this phase of the study was assessed by measuring urine cotinine levels; levels of 80 ng/mL or less were indicative of abstinence.
The women were rewarded with vouchers, which were earned contingent on biochemically verified abstinence.
The voucher value began at $6.25 and escalated at a rate of $1.25 per consecutive negative sample up to a maximum of $45.
“These vouchers are like having a bank account with us. We put their money into an account, and they are allowed to spend it on things we believe are appropriate. So there were a lot of gift certificates, paying of credit card bills, and shopping at Wal-Mart and grocery stores,” Dr. Heil said.
Women who were randomized to noncontingency management got vouchers independent of their smoking status.
The vouchers were a flat $11.50 per antepartum visit, and $20 per each postpartum visit.
The women in the study had been smoking for about 8 years; most of them lived with other smokers.
They smoked approximately one pack of cigarettes a day before pregnancy, but had reduced this amount by roughly 50% by the time they entered the study.
“They had very high intentions to quit while they are pregnant,” Dr. Heil noted.
Most of the women had less than a high school education, and few of the women were married.
To be considered abstinent at each time point, the women had to self-report that they had not had a cigarette—“not even a puff”—in the last 7 days, as well as the appropriate urine cotinine level.
The effects obtained in the study persisted 3 months after delivery, and for a further 3 months, even though the voucher program was discontinued at 3 months post partum.
This was true for women in the contingent and noncontingent groups, Dr. Heil said.
Importantly, fetuses in the contingent group gained weight faster than those in the noncontingent group. Fetal weight was estimated by measuring fetal length and abdominal circumference by ultrasound.
“We are really excited by these results,” she said.
Cigarette smoking is the leading preventable cause of poor pregnancy outcomes in the United States.
Placental abruptions, small gestational age, preterm and still birth, low birth weight, and increased risk for sudden infant death syndrome are all associated with cigarette smoking by the mother.
The adverse effects of smoking on the neonate cost $1,630/birth per year in 2008 dollars.
Dr. Heil said she hopes to extend her research on contingency management to include pregnant smokers who are also opioid dependent.
The women were rewarded with vouchers—earned contingent on biochemically verified abstinence. DR. HEIL
BOCA RATON, FLA. — In a pilot study of pregnant women who continued to smoke cigarettes despite knowing they were pregnant, 11 (37%) of 30 women who received contingency management achieved abstinence, compared with just 2 (10%)) of 23 women who did not.
This result highlights the effectiveness of contingency management as a strategy to help pregnant women stop smoking, Dr. Sarah Heil said at the annual meeting of the American Academy of Addiction Psychiatry.
The women in both the contingent group and noncontingent group were seen every day for the first 5 days of the study.
During this time, abstinence was based on a breath carbon monoxide level of 6 parts per million or less, said Dr. Heil of the University of Vermont, Burlington.
After the first 5 days, the women were seen according to the following schedule:
▸ Twice a week for 7 weeks.
▸ Once a week for the next 11 weeks.
▸ Once every other week until delivery.
▸ Once a week for the first 4 weeks post partum.
▸ Every other week for the next 8 weeks.
Abstinence in this phase of the study was assessed by measuring urine cotinine levels; levels of 80 ng/mL or less were indicative of abstinence.
The women were rewarded with vouchers, which were earned contingent on biochemically verified abstinence.
The voucher value began at $6.25 and escalated at a rate of $1.25 per consecutive negative sample up to a maximum of $45.
“These vouchers are like having a bank account with us. We put their money into an account, and they are allowed to spend it on things we believe are appropriate. So there were a lot of gift certificates, paying of credit card bills, and shopping at Wal-Mart and grocery stores,” Dr. Heil said.
Women who were randomized to noncontingency management got vouchers independent of their smoking status.
The vouchers were a flat $11.50 per antepartum visit, and $20 per each postpartum visit.
The women in the study had been smoking for about 8 years; most of them lived with other smokers.
They smoked approximately one pack of cigarettes a day before pregnancy, but had reduced this amount by roughly 50% by the time they entered the study.
“They had very high intentions to quit while they are pregnant,” Dr. Heil noted.
Most of the women had less than a high school education, and few of the women were married.
To be considered abstinent at each time point, the women had to self-report that they had not had a cigarette—“not even a puff”—in the last 7 days, as well as the appropriate urine cotinine level.
The effects obtained in the study persisted 3 months after delivery, and for a further 3 months, even though the voucher program was discontinued at 3 months post partum.
This was true for women in the contingent and noncontingent groups, Dr. Heil said.
Importantly, fetuses in the contingent group gained weight faster than those in the noncontingent group. Fetal weight was estimated by measuring fetal length and abdominal circumference by ultrasound.
“We are really excited by these results,” she said.
Cigarette smoking is the leading preventable cause of poor pregnancy outcomes in the United States.
Placental abruptions, small gestational age, preterm and still birth, low birth weight, and increased risk for sudden infant death syndrome are all associated with cigarette smoking by the mother.
The adverse effects of smoking on the neonate cost $1,630/birth per year in 2008 dollars.
Dr. Heil said she hopes to extend her research on contingency management to include pregnant smokers who are also opioid dependent.
The women were rewarded with vouchers—earned contingent on biochemically verified abstinence. DR. HEIL
BOCA RATON, FLA. — In a pilot study of pregnant women who continued to smoke cigarettes despite knowing they were pregnant, 11 (37%) of 30 women who received contingency management achieved abstinence, compared with just 2 (10%)) of 23 women who did not.
This result highlights the effectiveness of contingency management as a strategy to help pregnant women stop smoking, Dr. Sarah Heil said at the annual meeting of the American Academy of Addiction Psychiatry.
The women in both the contingent group and noncontingent group were seen every day for the first 5 days of the study.
During this time, abstinence was based on a breath carbon monoxide level of 6 parts per million or less, said Dr. Heil of the University of Vermont, Burlington.
After the first 5 days, the women were seen according to the following schedule:
▸ Twice a week for 7 weeks.
▸ Once a week for the next 11 weeks.
▸ Once every other week until delivery.
▸ Once a week for the first 4 weeks post partum.
▸ Every other week for the next 8 weeks.
Abstinence in this phase of the study was assessed by measuring urine cotinine levels; levels of 80 ng/mL or less were indicative of abstinence.
The women were rewarded with vouchers, which were earned contingent on biochemically verified abstinence.
The voucher value began at $6.25 and escalated at a rate of $1.25 per consecutive negative sample up to a maximum of $45.
“These vouchers are like having a bank account with us. We put their money into an account, and they are allowed to spend it on things we believe are appropriate. So there were a lot of gift certificates, paying of credit card bills, and shopping at Wal-Mart and grocery stores,” Dr. Heil said.
Women who were randomized to noncontingency management got vouchers independent of their smoking status.
The vouchers were a flat $11.50 per antepartum visit, and $20 per each postpartum visit.
The women in the study had been smoking for about 8 years; most of them lived with other smokers.
They smoked approximately one pack of cigarettes a day before pregnancy, but had reduced this amount by roughly 50% by the time they entered the study.
“They had very high intentions to quit while they are pregnant,” Dr. Heil noted.
Most of the women had less than a high school education, and few of the women were married.
To be considered abstinent at each time point, the women had to self-report that they had not had a cigarette—“not even a puff”—in the last 7 days, as well as the appropriate urine cotinine level.
The effects obtained in the study persisted 3 months after delivery, and for a further 3 months, even though the voucher program was discontinued at 3 months post partum.
This was true for women in the contingent and noncontingent groups, Dr. Heil said.
Importantly, fetuses in the contingent group gained weight faster than those in the noncontingent group. Fetal weight was estimated by measuring fetal length and abdominal circumference by ultrasound.
“We are really excited by these results,” she said.
Cigarette smoking is the leading preventable cause of poor pregnancy outcomes in the United States.
Placental abruptions, small gestational age, preterm and still birth, low birth weight, and increased risk for sudden infant death syndrome are all associated with cigarette smoking by the mother.
The adverse effects of smoking on the neonate cost $1,630/birth per year in 2008 dollars.
Dr. Heil said she hopes to extend her research on contingency management to include pregnant smokers who are also opioid dependent.
The women were rewarded with vouchers—earned contingent on biochemically verified abstinence. DR. HEIL
Pulmonary HT in Pregnancy With 100% Survival
PHILADELPHIA — A Milwaukee cardiologist seems to have defied the textbook on what happens to women who develop pulmonary hypertension while pregnant.
The medical literature generally says that about half of these women will die during or soon after delivery if they carry the pregnancy close to term.
But a series of 40 pregnant women with pulmonary hypertension who were seen or consulted on by Dr. Dianne L. Zwicke during 2000–2008 have all survived, and 39 of them delivered healthy infants, she reported at the annual meeting of the American College of Chest Physicians.
The pregnancy of the 40th woman was electively terminated at 22 weeks.
Despite this perfect survival record so far, Dr. Zwicke is very cautious about the immediate implications of her series.
“I have not encouraged any women with pulmonary hypertension to get pregnant. We don't have enough data to say that yet.” In all except one instance the 27 women directly treated by Dr. Zwicke and the 13 cases for which she provided ongoing consultation involved women who were diagnosed with pulmonary hypertension after they became pregnant.
She also warns the women who choose to continue their pregnancy that they must be willing to do everything she instructs them to do, they must come in for every appointment, and they must understand what the medical literature says: that in the past many of these pregnancies have been fatal for the mothers.
The cases are generally first identified at 15–20 weeks gestation. The first clue that something is wrong is when exercise-induced dyspnea or the woman's weight gain is disproportionate to the pregnancy, said Dr. Zwicke, a cardiologist in a group practice in Milwaukee.
The patients that she has managed had an average age of 27 years (range 19–36 years). The most common cause of their pulmonary hypertension was idiopathic, in 50%, followed by congenital heart disease in 18%, mitral valve disease in 10%, and lupus in 10%.
Their average pulmonary artery pressure was 59 mm Hg (range of 37–86 mm Hg), their average right-ventricular ejection fraction was 30%–35% (range of 20%–45%), and their average right atrial pressure was 10 mm Hg (range 7–20 mm Hg).
Thirty-nine of the mothers were treated with intravenous prostaglandin infusions; 1 patient was primarily treated with a calcium channel blocker. Several other drugs were also used throughout pregnancy, but prostaglandin infusion is key. “There is never too much prostaglandin,” she said. “The dosage must rise as soon as possible.”
Thirty-four of the babies were delivered vaginally following induction. The remaining five live births were by cesarean section. No pregnant mother with pulmonary hypertension should expect a natural delivery, Dr. Zwicke said.
One newborn required 2 days on a ventilator after delivery. All of the other 38 were ready to go home by the third day after delivery.
Following diagnosis, the most critical time during pregnancy is weeks 30–36, when hormonal and fluid shifts start to become dramatic.
Prior to 30 weeks' gestation, women can be monitored every 4 weeks by ultrasound.
Starting at week 30, ultrasound examination of the right heart must be done weekly. These exams should be done by the same echocardiographic technician to help insure consistent images.
The key member of the delivery team is a cardiologist or pulmonologist, who is the person reading the weekly echocardiograms and deciding whether the patient's clinical state and right-heart function has deteriorated so severely during the prior week that delivery must occur immediately. This happens when the patient can no longer do the exertional tasks that they could do the prior week, and when their right-side ejection fraction, right ventricular size, and right atrial size have all become very compromised.
During this key period of weeks 30–36, if “I can identify a reason why the patient is deteriorating and I can fix it, then we'll let the pregnancy continue.” But if there is no obvious explanation for the deterioration and their status is worrisome or worsening, then delivery is immediately begun.
Right-heart status is more important than their pulmonary artery pressure, Dr. Zwicke said. If no crisis occurs, delivery is induced after 36 weeks' gestation.
Once delivery starts, the key to success is careful fluid control. Every milliliter that enters the women must eventually get taken out because of the high risk from excess fluid in these patients. “It's all about the right ventricle being able to handle the fluid,” she said.
After delivery, the mother is taken to the ICU and closely monitored and treated so that she loses an average of three liters of fluid a day for 3 days.
This is another critical time for the mother, especially the last several hours leading up to a full 72 hours elapsed following delivery. The normal redistribution of fluid within the mother that occurs at this time must be very tightly monitored.
The mother can usually be discharged on a low-dose diuretic 7 days after delivery and should be completely stable within 6–8 weeks.
Dr. Zwicke has follow-up data on her 40 patients for a minimum of 2–3 months following delivery and during that period there were no complications.
Right-heart status is more important than their pulmonary artery pressure. DR. ZWICKE
PHILADELPHIA — A Milwaukee cardiologist seems to have defied the textbook on what happens to women who develop pulmonary hypertension while pregnant.
The medical literature generally says that about half of these women will die during or soon after delivery if they carry the pregnancy close to term.
But a series of 40 pregnant women with pulmonary hypertension who were seen or consulted on by Dr. Dianne L. Zwicke during 2000–2008 have all survived, and 39 of them delivered healthy infants, she reported at the annual meeting of the American College of Chest Physicians.
The pregnancy of the 40th woman was electively terminated at 22 weeks.
Despite this perfect survival record so far, Dr. Zwicke is very cautious about the immediate implications of her series.
“I have not encouraged any women with pulmonary hypertension to get pregnant. We don't have enough data to say that yet.” In all except one instance the 27 women directly treated by Dr. Zwicke and the 13 cases for which she provided ongoing consultation involved women who were diagnosed with pulmonary hypertension after they became pregnant.
She also warns the women who choose to continue their pregnancy that they must be willing to do everything she instructs them to do, they must come in for every appointment, and they must understand what the medical literature says: that in the past many of these pregnancies have been fatal for the mothers.
The cases are generally first identified at 15–20 weeks gestation. The first clue that something is wrong is when exercise-induced dyspnea or the woman's weight gain is disproportionate to the pregnancy, said Dr. Zwicke, a cardiologist in a group practice in Milwaukee.
The patients that she has managed had an average age of 27 years (range 19–36 years). The most common cause of their pulmonary hypertension was idiopathic, in 50%, followed by congenital heart disease in 18%, mitral valve disease in 10%, and lupus in 10%.
Their average pulmonary artery pressure was 59 mm Hg (range of 37–86 mm Hg), their average right-ventricular ejection fraction was 30%–35% (range of 20%–45%), and their average right atrial pressure was 10 mm Hg (range 7–20 mm Hg).
Thirty-nine of the mothers were treated with intravenous prostaglandin infusions; 1 patient was primarily treated with a calcium channel blocker. Several other drugs were also used throughout pregnancy, but prostaglandin infusion is key. “There is never too much prostaglandin,” she said. “The dosage must rise as soon as possible.”
Thirty-four of the babies were delivered vaginally following induction. The remaining five live births were by cesarean section. No pregnant mother with pulmonary hypertension should expect a natural delivery, Dr. Zwicke said.
One newborn required 2 days on a ventilator after delivery. All of the other 38 were ready to go home by the third day after delivery.
Following diagnosis, the most critical time during pregnancy is weeks 30–36, when hormonal and fluid shifts start to become dramatic.
Prior to 30 weeks' gestation, women can be monitored every 4 weeks by ultrasound.
Starting at week 30, ultrasound examination of the right heart must be done weekly. These exams should be done by the same echocardiographic technician to help insure consistent images.
The key member of the delivery team is a cardiologist or pulmonologist, who is the person reading the weekly echocardiograms and deciding whether the patient's clinical state and right-heart function has deteriorated so severely during the prior week that delivery must occur immediately. This happens when the patient can no longer do the exertional tasks that they could do the prior week, and when their right-side ejection fraction, right ventricular size, and right atrial size have all become very compromised.
During this key period of weeks 30–36, if “I can identify a reason why the patient is deteriorating and I can fix it, then we'll let the pregnancy continue.” But if there is no obvious explanation for the deterioration and their status is worrisome or worsening, then delivery is immediately begun.
Right-heart status is more important than their pulmonary artery pressure, Dr. Zwicke said. If no crisis occurs, delivery is induced after 36 weeks' gestation.
Once delivery starts, the key to success is careful fluid control. Every milliliter that enters the women must eventually get taken out because of the high risk from excess fluid in these patients. “It's all about the right ventricle being able to handle the fluid,” she said.
After delivery, the mother is taken to the ICU and closely monitored and treated so that she loses an average of three liters of fluid a day for 3 days.
This is another critical time for the mother, especially the last several hours leading up to a full 72 hours elapsed following delivery. The normal redistribution of fluid within the mother that occurs at this time must be very tightly monitored.
The mother can usually be discharged on a low-dose diuretic 7 days after delivery and should be completely stable within 6–8 weeks.
Dr. Zwicke has follow-up data on her 40 patients for a minimum of 2–3 months following delivery and during that period there were no complications.
Right-heart status is more important than their pulmonary artery pressure. DR. ZWICKE
PHILADELPHIA — A Milwaukee cardiologist seems to have defied the textbook on what happens to women who develop pulmonary hypertension while pregnant.
The medical literature generally says that about half of these women will die during or soon after delivery if they carry the pregnancy close to term.
But a series of 40 pregnant women with pulmonary hypertension who were seen or consulted on by Dr. Dianne L. Zwicke during 2000–2008 have all survived, and 39 of them delivered healthy infants, she reported at the annual meeting of the American College of Chest Physicians.
The pregnancy of the 40th woman was electively terminated at 22 weeks.
Despite this perfect survival record so far, Dr. Zwicke is very cautious about the immediate implications of her series.
“I have not encouraged any women with pulmonary hypertension to get pregnant. We don't have enough data to say that yet.” In all except one instance the 27 women directly treated by Dr. Zwicke and the 13 cases for which she provided ongoing consultation involved women who were diagnosed with pulmonary hypertension after they became pregnant.
She also warns the women who choose to continue their pregnancy that they must be willing to do everything she instructs them to do, they must come in for every appointment, and they must understand what the medical literature says: that in the past many of these pregnancies have been fatal for the mothers.
The cases are generally first identified at 15–20 weeks gestation. The first clue that something is wrong is when exercise-induced dyspnea or the woman's weight gain is disproportionate to the pregnancy, said Dr. Zwicke, a cardiologist in a group practice in Milwaukee.
The patients that she has managed had an average age of 27 years (range 19–36 years). The most common cause of their pulmonary hypertension was idiopathic, in 50%, followed by congenital heart disease in 18%, mitral valve disease in 10%, and lupus in 10%.
Their average pulmonary artery pressure was 59 mm Hg (range of 37–86 mm Hg), their average right-ventricular ejection fraction was 30%–35% (range of 20%–45%), and their average right atrial pressure was 10 mm Hg (range 7–20 mm Hg).
Thirty-nine of the mothers were treated with intravenous prostaglandin infusions; 1 patient was primarily treated with a calcium channel blocker. Several other drugs were also used throughout pregnancy, but prostaglandin infusion is key. “There is never too much prostaglandin,” she said. “The dosage must rise as soon as possible.”
Thirty-four of the babies were delivered vaginally following induction. The remaining five live births were by cesarean section. No pregnant mother with pulmonary hypertension should expect a natural delivery, Dr. Zwicke said.
One newborn required 2 days on a ventilator after delivery. All of the other 38 were ready to go home by the third day after delivery.
Following diagnosis, the most critical time during pregnancy is weeks 30–36, when hormonal and fluid shifts start to become dramatic.
Prior to 30 weeks' gestation, women can be monitored every 4 weeks by ultrasound.
Starting at week 30, ultrasound examination of the right heart must be done weekly. These exams should be done by the same echocardiographic technician to help insure consistent images.
The key member of the delivery team is a cardiologist or pulmonologist, who is the person reading the weekly echocardiograms and deciding whether the patient's clinical state and right-heart function has deteriorated so severely during the prior week that delivery must occur immediately. This happens when the patient can no longer do the exertional tasks that they could do the prior week, and when their right-side ejection fraction, right ventricular size, and right atrial size have all become very compromised.
During this key period of weeks 30–36, if “I can identify a reason why the patient is deteriorating and I can fix it, then we'll let the pregnancy continue.” But if there is no obvious explanation for the deterioration and their status is worrisome or worsening, then delivery is immediately begun.
Right-heart status is more important than their pulmonary artery pressure, Dr. Zwicke said. If no crisis occurs, delivery is induced after 36 weeks' gestation.
Once delivery starts, the key to success is careful fluid control. Every milliliter that enters the women must eventually get taken out because of the high risk from excess fluid in these patients. “It's all about the right ventricle being able to handle the fluid,” she said.
After delivery, the mother is taken to the ICU and closely monitored and treated so that she loses an average of three liters of fluid a day for 3 days.
This is another critical time for the mother, especially the last several hours leading up to a full 72 hours elapsed following delivery. The normal redistribution of fluid within the mother that occurs at this time must be very tightly monitored.
The mother can usually be discharged on a low-dose diuretic 7 days after delivery and should be completely stable within 6–8 weeks.
Dr. Zwicke has follow-up data on her 40 patients for a minimum of 2–3 months following delivery and during that period there were no complications.
Right-heart status is more important than their pulmonary artery pressure. DR. ZWICKE
You should add the Bakri balloon to your treatments for OB bleeds
Every obstetrician fears the day when one of her (or his) patients has a massive postpartum hemorrhage. For good reason, we endeavor continuously to improve our ability to respond to this obstetric emergency (TABLE): Postpartum hemorrhage is a major cause of pregnancy-related death in both developed and developing nations.
One of those improvements in care is the Bakri balloon (Bakri Postpartum Balloon, Cook Medical) (FIGURE), which, I believe, is one of the most important recent advances for treating serious postpartum hemorrhage. I’ll explain here why this device should be utilized more often when treatment with a uterotonic hasn’t adequately resolved bleeding.
TABLE
At your disposal, a range of interventions for postpartum hemorrhage
| Pharmacotherapy | ||
| Carboprost Methergine | Misoprostol Oxytocin | Vasopressin |
| Blood banking | ||
| Cryoprecipitate Fresh-frozen plasma | Platelets | Red blood cells |
| Surgery | ||
| Repair of lacerations B-Lynch suture Hysterectomy, supracervical Hysterectomy, total | Ligation of the hypogastric artery Other uterine compression sutures | Pelvic packing Pelvic tourniquet |
| Nonsurgical procedures | ||
| Bakri Postpartum Balloon | Uterine balloon tamponade | Uterine packing |
| Interventional radiology | ||
| Uterine artery balloons | Uterine embolization | |
| Consultation | ||
| Anesthesiologist–intensivist Gynecologic oncologist | Interventional radiologist Trauma surgeon | Urologist |
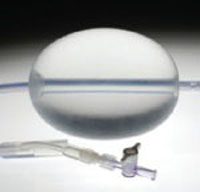
FIGURE Bakri Postpartum Balloon
SOURCE: COOK MEDICAL, INC. USED WITH PERMISSION.
How, and how well, the Bakri balloon works
The Bakri balloon is a 24-French, 54-cm long, silicone catheter that contains a large central lumen and a 500-mL balloon that can be inflated once the device is inserted in the uterus. Downward traction on the balloon helps to ensure that the surface of the balloon is tightly opposed to the lining of the lower uterine segment. The catheter provides temporary reduction of postpartum uterine bleeding if management with uterotonics, repair of genital lacerations, and removal of retained placental tissue has been unsuccessful.
The literature on the use of the Bakri balloon comprises case reports and case series.
Bakri’s first report described how he placed—simultaneously—10 (or more) standard Foley balloons in the lower uterine cavity to control postpartum hemorrhage.1 Building on this success, he and his team later developed a single, large balloon with a large central lumen and used it successfully in women experiencing postpartum hemorrhage.
In an initial case-series report, the single balloon was used to treat postpartum hemorrhage in women with placenta previa or low-lying placenta and a suspicion of placental accreta.2 Two subjects had placenta previa and a history of, in one case, three and, in the other, five prior cesarean deliveries—putting them both at greatly increased risk of placenta accreta. The Bakri balloon was placed at the time of cesarean delivery for those two women.
“How” in cesarean delivery. When the balloon is placed at the time of cesarean delivery, an assistant working from below helps pull the distal end of the balloon shaft through the cervix into the vagina. Next:
- The hysterotomy incision is closed
- A vaginal pack is placed to keep the balloon seated in the uterus
- The balloon is filled with 250 to 500 mL of sterile isotonic fluid
- The distal end of the balloon is attached to a weight, such as a liter intravenous fluid bag, to ensure that a tamponade effect is maintained.
- A Foley catheter is inserted in the bladder to monitor urine output and reduce bladder volume
- The uterus is examined to ensure that there are no retained placental fragments
- The balloon is inserted into the uterus so that the entire balloon is past the internal cervical os; ultrasonographic guidance can help determine proper placement
- Using a syringe, the balloon is filled with sterile saline to the desired volume—again, typically, 250 to 500 mL
- Gentle downward traction is placed on the balloon stem to ensure that a tamponade effect is maintained
- A balloon that protrudes through the cervix can be deflated, repositioned, and reinflated
- Vaginal packing may be useful to help keep the balloon within the lower uterine segment
- The drainage port of the balloon is connected to a fluid collection bag to monitor uterine bleeding; the drainage port and tubing can, if necessary, be flushed with sterile saline to ensure patency and prevent clots from obstructing the drainage port
- The balloon is kept inflated for 12 to 24 hours—the maximum indwell.
Most clinicians administer an antibiotic until the balloon is removed. When the decision is made to remove the balloon, it can be deflated 1) all at once or 2) in two or three stages, with attention to the onset of new bleeding. An alternative approach, such as exploratory laparotomy or interventional radiology (IR), is indicated if a patient continues to bleed after the balloon is placed.
Advantages. The Bakri balloon is easily placed and removed. Its silicone composition reduces the chances that the balloon will “stick” to the uterine lining. The large central port provides real-time assessment of the effectiveness of balloon tamponade. This property of the device allows it to be used in a diagnostic manner—the so-called tamponade test.
Tamponade test
A common challenge for the OB when a woman has a massive postpartum hemorrhage is to determine—quickly—if she requires exploratory laparotomy or can be treated with conservative measures. In one case series, investigators concluded that, in women who have severe postpartum hemorrhage that is unresponsive to uterotonics, immediate placement of a uterine tamponade balloon is an effective test for rapidly distinguishing patients who need exploratory laparotomy from those whose condition can be managed conservatively.3
In that series, women who developed postpartum hemorrhage were treated first with uterotonics, including oxytocin, ergometrine, and carboprost. Next, they underwent initial exploration to look for lacerations of the uterus, cervix, and vagina and to ensure that no placental tissue had been retained.
Sixteen subjects (most of whom had uterine atony as a provisional diagnosis) continued to have massive bleeding after those first two steps were employed. They next had a Sengstaken-Blakemore esophageal catheter placed in the uterine cavity. The catheter was then filled with 70 to 300 mL of warm saline. Gentle downward traction was applied to the catheter to ensure contact of the catheter with the lining of the uterus.
Uterine bleeding stopped quickly in 14 women after the intrauterine balloon was filled to produce the tamponade effect. Bleeding continued in two women; they underwent exploratory laparotomy. Route of delivery for these 16 women included 10 vaginal and six cesarean deliveries; two of the 10 vaginal deliveries involved prostaglandin-induced second trimester abortions. This indicates that the tamponade test can be used after either vaginal or cesarean delivery and in either the second or third trimester.
The authors of a more recent case series also reported that balloon tamponade is successful for postpartum hemorrhage in most cases.4
The Bakri balloon can also be used in conjunction with additional interventions, including uterine compression sutures and uterine artery embolization.
The uterine sandwich
Combined use of a uterine compression stitch (B-Lynch compression suture using a 1-Vicryl suture) along with the placement of the Bakri balloon—the so-called uterine sandwich—may be indicated when postpartum hemorrhage occurs after C-section in select patients. In a report of a case series of five women in whom this combined technique was first described, uterotonics had failed to control the hemorrhage and no retained placental products were detected.5 Management proceeded as follows:
- A B-Lynch compression suture was placed with a 1-Vicryl suture
- A Bakri balloon was also placed, if determined to be necessary, with the stem brought out through the vagina
- The Bakri balloon was filled with, on average, 100 mL of fluid (range, 60 to 250 mL).
This approach controlled bleeding in all five cases.
Balloon combined with uterine artery embolization
Treatment of severe postpartum hemorrhage can also be accomplished by combining the Bakri balloon and uterine artery embolization. Initial management steps include, as mentioned, administration of uterotonics; uterine massage; fluid resuscitation; examination to detect and repair cervical and vaginal lacerations; and evaluation for retained placental tissue.
If those steps do not stop hemorrhage, a Bakri balloon can be placed and IR consultation requested to perform uterine artery embolization. Gathering a team to perform uterine artery embolization often takes 1 to 4 hours; in this setting, a Bakri balloon is a useful temporizing step that reduces bleeding while the IR team is assembled.
Potentially, a lifesaver
Postpartum hemorrhage occurs after approximately 5% of deliveries. A clinician who performs, say, 160 deliveries a year will confront this complication about eight times in that span. For an event that occurs only every 6 weeks, you need to develop, and rehearse, a systematic plan of response6 (TABLE). One component of a good plan, I believe, should be the Bakri balloon, which can be a life-saving device for women whose severe postpartum hemorrhage hasn’t responded to first-line measures.
1. Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
2. Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
3. Condous GS, Arulkumaran S, Symonds I, Chapman R, Sinha A, Razvi K. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
4. Dabelea V, Schultze PM, McDuffie RS, Jr. Intrauterine balloon tamponade in the management of postpartum hemorrhage. Am J Perinatol. 2007;24:359-364.
5. Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B- Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
6. American College of Obstetricians and Gynecologists ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039-1047.
Every obstetrician fears the day when one of her (or his) patients has a massive postpartum hemorrhage. For good reason, we endeavor continuously to improve our ability to respond to this obstetric emergency (TABLE): Postpartum hemorrhage is a major cause of pregnancy-related death in both developed and developing nations.
One of those improvements in care is the Bakri balloon (Bakri Postpartum Balloon, Cook Medical) (FIGURE), which, I believe, is one of the most important recent advances for treating serious postpartum hemorrhage. I’ll explain here why this device should be utilized more often when treatment with a uterotonic hasn’t adequately resolved bleeding.
TABLE
At your disposal, a range of interventions for postpartum hemorrhage
| Pharmacotherapy | ||
| Carboprost Methergine | Misoprostol Oxytocin | Vasopressin |
| Blood banking | ||
| Cryoprecipitate Fresh-frozen plasma | Platelets | Red blood cells |
| Surgery | ||
| Repair of lacerations B-Lynch suture Hysterectomy, supracervical Hysterectomy, total | Ligation of the hypogastric artery Other uterine compression sutures | Pelvic packing Pelvic tourniquet |
| Nonsurgical procedures | ||
| Bakri Postpartum Balloon | Uterine balloon tamponade | Uterine packing |
| Interventional radiology | ||
| Uterine artery balloons | Uterine embolization | |
| Consultation | ||
| Anesthesiologist–intensivist Gynecologic oncologist | Interventional radiologist Trauma surgeon | Urologist |

FIGURE Bakri Postpartum Balloon
SOURCE: COOK MEDICAL, INC. USED WITH PERMISSION.
How, and how well, the Bakri balloon works
The Bakri balloon is a 24-French, 54-cm long, silicone catheter that contains a large central lumen and a 500-mL balloon that can be inflated once the device is inserted in the uterus. Downward traction on the balloon helps to ensure that the surface of the balloon is tightly opposed to the lining of the lower uterine segment. The catheter provides temporary reduction of postpartum uterine bleeding if management with uterotonics, repair of genital lacerations, and removal of retained placental tissue has been unsuccessful.
The literature on the use of the Bakri balloon comprises case reports and case series.
Bakri’s first report described how he placed—simultaneously—10 (or more) standard Foley balloons in the lower uterine cavity to control postpartum hemorrhage.1 Building on this success, he and his team later developed a single, large balloon with a large central lumen and used it successfully in women experiencing postpartum hemorrhage.
In an initial case-series report, the single balloon was used to treat postpartum hemorrhage in women with placenta previa or low-lying placenta and a suspicion of placental accreta.2 Two subjects had placenta previa and a history of, in one case, three and, in the other, five prior cesarean deliveries—putting them both at greatly increased risk of placenta accreta. The Bakri balloon was placed at the time of cesarean delivery for those two women.
“How” in cesarean delivery. When the balloon is placed at the time of cesarean delivery, an assistant working from below helps pull the distal end of the balloon shaft through the cervix into the vagina. Next:
- The hysterotomy incision is closed
- A vaginal pack is placed to keep the balloon seated in the uterus
- The balloon is filled with 250 to 500 mL of sterile isotonic fluid
- The distal end of the balloon is attached to a weight, such as a liter intravenous fluid bag, to ensure that a tamponade effect is maintained.
- A Foley catheter is inserted in the bladder to monitor urine output and reduce bladder volume
- The uterus is examined to ensure that there are no retained placental fragments
- The balloon is inserted into the uterus so that the entire balloon is past the internal cervical os; ultrasonographic guidance can help determine proper placement
- Using a syringe, the balloon is filled with sterile saline to the desired volume—again, typically, 250 to 500 mL
- Gentle downward traction is placed on the balloon stem to ensure that a tamponade effect is maintained
- A balloon that protrudes through the cervix can be deflated, repositioned, and reinflated
- Vaginal packing may be useful to help keep the balloon within the lower uterine segment
- The drainage port of the balloon is connected to a fluid collection bag to monitor uterine bleeding; the drainage port and tubing can, if necessary, be flushed with sterile saline to ensure patency and prevent clots from obstructing the drainage port
- The balloon is kept inflated for 12 to 24 hours—the maximum indwell.
Most clinicians administer an antibiotic until the balloon is removed. When the decision is made to remove the balloon, it can be deflated 1) all at once or 2) in two or three stages, with attention to the onset of new bleeding. An alternative approach, such as exploratory laparotomy or interventional radiology (IR), is indicated if a patient continues to bleed after the balloon is placed.
Advantages. The Bakri balloon is easily placed and removed. Its silicone composition reduces the chances that the balloon will “stick” to the uterine lining. The large central port provides real-time assessment of the effectiveness of balloon tamponade. This property of the device allows it to be used in a diagnostic manner—the so-called tamponade test.
Tamponade test
A common challenge for the OB when a woman has a massive postpartum hemorrhage is to determine—quickly—if she requires exploratory laparotomy or can be treated with conservative measures. In one case series, investigators concluded that, in women who have severe postpartum hemorrhage that is unresponsive to uterotonics, immediate placement of a uterine tamponade balloon is an effective test for rapidly distinguishing patients who need exploratory laparotomy from those whose condition can be managed conservatively.3
In that series, women who developed postpartum hemorrhage were treated first with uterotonics, including oxytocin, ergometrine, and carboprost. Next, they underwent initial exploration to look for lacerations of the uterus, cervix, and vagina and to ensure that no placental tissue had been retained.
Sixteen subjects (most of whom had uterine atony as a provisional diagnosis) continued to have massive bleeding after those first two steps were employed. They next had a Sengstaken-Blakemore esophageal catheter placed in the uterine cavity. The catheter was then filled with 70 to 300 mL of warm saline. Gentle downward traction was applied to the catheter to ensure contact of the catheter with the lining of the uterus.
Uterine bleeding stopped quickly in 14 women after the intrauterine balloon was filled to produce the tamponade effect. Bleeding continued in two women; they underwent exploratory laparotomy. Route of delivery for these 16 women included 10 vaginal and six cesarean deliveries; two of the 10 vaginal deliveries involved prostaglandin-induced second trimester abortions. This indicates that the tamponade test can be used after either vaginal or cesarean delivery and in either the second or third trimester.
The authors of a more recent case series also reported that balloon tamponade is successful for postpartum hemorrhage in most cases.4
The Bakri balloon can also be used in conjunction with additional interventions, including uterine compression sutures and uterine artery embolization.
The uterine sandwich
Combined use of a uterine compression stitch (B-Lynch compression suture using a 1-Vicryl suture) along with the placement of the Bakri balloon—the so-called uterine sandwich—may be indicated when postpartum hemorrhage occurs after C-section in select patients. In a report of a case series of five women in whom this combined technique was first described, uterotonics had failed to control the hemorrhage and no retained placental products were detected.5 Management proceeded as follows:
- A B-Lynch compression suture was placed with a 1-Vicryl suture
- A Bakri balloon was also placed, if determined to be necessary, with the stem brought out through the vagina
- The Bakri balloon was filled with, on average, 100 mL of fluid (range, 60 to 250 mL).
This approach controlled bleeding in all five cases.
Balloon combined with uterine artery embolization
Treatment of severe postpartum hemorrhage can also be accomplished by combining the Bakri balloon and uterine artery embolization. Initial management steps include, as mentioned, administration of uterotonics; uterine massage; fluid resuscitation; examination to detect and repair cervical and vaginal lacerations; and evaluation for retained placental tissue.
If those steps do not stop hemorrhage, a Bakri balloon can be placed and IR consultation requested to perform uterine artery embolization. Gathering a team to perform uterine artery embolization often takes 1 to 4 hours; in this setting, a Bakri balloon is a useful temporizing step that reduces bleeding while the IR team is assembled.
Potentially, a lifesaver
Postpartum hemorrhage occurs after approximately 5% of deliveries. A clinician who performs, say, 160 deliveries a year will confront this complication about eight times in that span. For an event that occurs only every 6 weeks, you need to develop, and rehearse, a systematic plan of response6 (TABLE). One component of a good plan, I believe, should be the Bakri balloon, which can be a life-saving device for women whose severe postpartum hemorrhage hasn’t responded to first-line measures.
Every obstetrician fears the day when one of her (or his) patients has a massive postpartum hemorrhage. For good reason, we endeavor continuously to improve our ability to respond to this obstetric emergency (TABLE): Postpartum hemorrhage is a major cause of pregnancy-related death in both developed and developing nations.
One of those improvements in care is the Bakri balloon (Bakri Postpartum Balloon, Cook Medical) (FIGURE), which, I believe, is one of the most important recent advances for treating serious postpartum hemorrhage. I’ll explain here why this device should be utilized more often when treatment with a uterotonic hasn’t adequately resolved bleeding.
TABLE
At your disposal, a range of interventions for postpartum hemorrhage
| Pharmacotherapy | ||
| Carboprost Methergine | Misoprostol Oxytocin | Vasopressin |
| Blood banking | ||
| Cryoprecipitate Fresh-frozen plasma | Platelets | Red blood cells |
| Surgery | ||
| Repair of lacerations B-Lynch suture Hysterectomy, supracervical Hysterectomy, total | Ligation of the hypogastric artery Other uterine compression sutures | Pelvic packing Pelvic tourniquet |
| Nonsurgical procedures | ||
| Bakri Postpartum Balloon | Uterine balloon tamponade | Uterine packing |
| Interventional radiology | ||
| Uterine artery balloons | Uterine embolization | |
| Consultation | ||
| Anesthesiologist–intensivist Gynecologic oncologist | Interventional radiologist Trauma surgeon | Urologist |

FIGURE Bakri Postpartum Balloon
SOURCE: COOK MEDICAL, INC. USED WITH PERMISSION.
How, and how well, the Bakri balloon works
The Bakri balloon is a 24-French, 54-cm long, silicone catheter that contains a large central lumen and a 500-mL balloon that can be inflated once the device is inserted in the uterus. Downward traction on the balloon helps to ensure that the surface of the balloon is tightly opposed to the lining of the lower uterine segment. The catheter provides temporary reduction of postpartum uterine bleeding if management with uterotonics, repair of genital lacerations, and removal of retained placental tissue has been unsuccessful.
The literature on the use of the Bakri balloon comprises case reports and case series.
Bakri’s first report described how he placed—simultaneously—10 (or more) standard Foley balloons in the lower uterine cavity to control postpartum hemorrhage.1 Building on this success, he and his team later developed a single, large balloon with a large central lumen and used it successfully in women experiencing postpartum hemorrhage.
In an initial case-series report, the single balloon was used to treat postpartum hemorrhage in women with placenta previa or low-lying placenta and a suspicion of placental accreta.2 Two subjects had placenta previa and a history of, in one case, three and, in the other, five prior cesarean deliveries—putting them both at greatly increased risk of placenta accreta. The Bakri balloon was placed at the time of cesarean delivery for those two women.
“How” in cesarean delivery. When the balloon is placed at the time of cesarean delivery, an assistant working from below helps pull the distal end of the balloon shaft through the cervix into the vagina. Next:
- The hysterotomy incision is closed
- A vaginal pack is placed to keep the balloon seated in the uterus
- The balloon is filled with 250 to 500 mL of sterile isotonic fluid
- The distal end of the balloon is attached to a weight, such as a liter intravenous fluid bag, to ensure that a tamponade effect is maintained.
- A Foley catheter is inserted in the bladder to monitor urine output and reduce bladder volume
- The uterus is examined to ensure that there are no retained placental fragments
- The balloon is inserted into the uterus so that the entire balloon is past the internal cervical os; ultrasonographic guidance can help determine proper placement
- Using a syringe, the balloon is filled with sterile saline to the desired volume—again, typically, 250 to 500 mL
- Gentle downward traction is placed on the balloon stem to ensure that a tamponade effect is maintained
- A balloon that protrudes through the cervix can be deflated, repositioned, and reinflated
- Vaginal packing may be useful to help keep the balloon within the lower uterine segment
- The drainage port of the balloon is connected to a fluid collection bag to monitor uterine bleeding; the drainage port and tubing can, if necessary, be flushed with sterile saline to ensure patency and prevent clots from obstructing the drainage port
- The balloon is kept inflated for 12 to 24 hours—the maximum indwell.
Most clinicians administer an antibiotic until the balloon is removed. When the decision is made to remove the balloon, it can be deflated 1) all at once or 2) in two or three stages, with attention to the onset of new bleeding. An alternative approach, such as exploratory laparotomy or interventional radiology (IR), is indicated if a patient continues to bleed after the balloon is placed.
Advantages. The Bakri balloon is easily placed and removed. Its silicone composition reduces the chances that the balloon will “stick” to the uterine lining. The large central port provides real-time assessment of the effectiveness of balloon tamponade. This property of the device allows it to be used in a diagnostic manner—the so-called tamponade test.
Tamponade test
A common challenge for the OB when a woman has a massive postpartum hemorrhage is to determine—quickly—if she requires exploratory laparotomy or can be treated with conservative measures. In one case series, investigators concluded that, in women who have severe postpartum hemorrhage that is unresponsive to uterotonics, immediate placement of a uterine tamponade balloon is an effective test for rapidly distinguishing patients who need exploratory laparotomy from those whose condition can be managed conservatively.3
In that series, women who developed postpartum hemorrhage were treated first with uterotonics, including oxytocin, ergometrine, and carboprost. Next, they underwent initial exploration to look for lacerations of the uterus, cervix, and vagina and to ensure that no placental tissue had been retained.
Sixteen subjects (most of whom had uterine atony as a provisional diagnosis) continued to have massive bleeding after those first two steps were employed. They next had a Sengstaken-Blakemore esophageal catheter placed in the uterine cavity. The catheter was then filled with 70 to 300 mL of warm saline. Gentle downward traction was applied to the catheter to ensure contact of the catheter with the lining of the uterus.
Uterine bleeding stopped quickly in 14 women after the intrauterine balloon was filled to produce the tamponade effect. Bleeding continued in two women; they underwent exploratory laparotomy. Route of delivery for these 16 women included 10 vaginal and six cesarean deliveries; two of the 10 vaginal deliveries involved prostaglandin-induced second trimester abortions. This indicates that the tamponade test can be used after either vaginal or cesarean delivery and in either the second or third trimester.
The authors of a more recent case series also reported that balloon tamponade is successful for postpartum hemorrhage in most cases.4
The Bakri balloon can also be used in conjunction with additional interventions, including uterine compression sutures and uterine artery embolization.
The uterine sandwich
Combined use of a uterine compression stitch (B-Lynch compression suture using a 1-Vicryl suture) along with the placement of the Bakri balloon—the so-called uterine sandwich—may be indicated when postpartum hemorrhage occurs after C-section in select patients. In a report of a case series of five women in whom this combined technique was first described, uterotonics had failed to control the hemorrhage and no retained placental products were detected.5 Management proceeded as follows:
- A B-Lynch compression suture was placed with a 1-Vicryl suture
- A Bakri balloon was also placed, if determined to be necessary, with the stem brought out through the vagina
- The Bakri balloon was filled with, on average, 100 mL of fluid (range, 60 to 250 mL).
This approach controlled bleeding in all five cases.
Balloon combined with uterine artery embolization
Treatment of severe postpartum hemorrhage can also be accomplished by combining the Bakri balloon and uterine artery embolization. Initial management steps include, as mentioned, administration of uterotonics; uterine massage; fluid resuscitation; examination to detect and repair cervical and vaginal lacerations; and evaluation for retained placental tissue.
If those steps do not stop hemorrhage, a Bakri balloon can be placed and IR consultation requested to perform uterine artery embolization. Gathering a team to perform uterine artery embolization often takes 1 to 4 hours; in this setting, a Bakri balloon is a useful temporizing step that reduces bleeding while the IR team is assembled.
Potentially, a lifesaver
Postpartum hemorrhage occurs after approximately 5% of deliveries. A clinician who performs, say, 160 deliveries a year will confront this complication about eight times in that span. For an event that occurs only every 6 weeks, you need to develop, and rehearse, a systematic plan of response6 (TABLE). One component of a good plan, I believe, should be the Bakri balloon, which can be a life-saving device for women whose severe postpartum hemorrhage hasn’t responded to first-line measures.
1. Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
2. Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
3. Condous GS, Arulkumaran S, Symonds I, Chapman R, Sinha A, Razvi K. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
4. Dabelea V, Schultze PM, McDuffie RS, Jr. Intrauterine balloon tamponade in the management of postpartum hemorrhage. Am J Perinatol. 2007;24:359-364.
5. Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B- Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
6. American College of Obstetricians and Gynecologists ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039-1047.
1. Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
2. Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
3. Condous GS, Arulkumaran S, Symonds I, Chapman R, Sinha A, Razvi K. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
4. Dabelea V, Schultze PM, McDuffie RS, Jr. Intrauterine balloon tamponade in the management of postpartum hemorrhage. Am J Perinatol. 2007;24:359-364.
5. Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B- Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
6. American College of Obstetricians and Gynecologists ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039-1047.
Study on Genes Vs. Environment Recruiting Now
Researchers in North Carolina and New York are beginning to recruit the first volunteers as part of a huge federal study that will examine the relative effects of genes and environment on development.
The researchers plan to track 100,000 women through pregnancy and then follow their children through age 21 years as part of the National Children's Study. In addition to tracking their development, the researchers will collect biological and environmental samples from study participants. Researchers aim to use this information to help identify the environmental and genetic factors that contribute to conditions such as autism, cerebral palsy, learning disabilities, birth defects, diabetes, asthma, and obesity.
Women are being recruited and enrolled from areas of the country that are representative of the diversity of U.S. children in terms of race, ethnicity, socioeconomic status, and community size. The initial recruitment began in mid-January in the borough of Queens, N.Y., and in Duplin County, N.C. In April, five other centers will begin recruiting. Ultimately, the study is expected to recruit participants from 105 locations across the country.
The National Children's Study was authorized by Congress in the Children's Health Act of 2000 and has been developed by the National Institutes of Health, the Centers for Disease Control and Prevention, and the Environmental Protection Agency.
Researchers estimate that the first children to be part of the study will be born this summer. As a result, the study could yield data in the next few years on conditions such as prematurity and birth defects, according to the NIH.
“Findings from the study will ultimately benefit all Americans by providing researchers, health care providers, and public health officials with information from which to develop prevention strategies, health and safety guidelines, and possibly new treatments for disease,” Dr. Peter Scheidt, director of the National Children's Study, said during a press briefing last month to announce the recruitment phase of the study.
Researchers in North Carolina and New York are beginning to recruit the first volunteers as part of a huge federal study that will examine the relative effects of genes and environment on development.
The researchers plan to track 100,000 women through pregnancy and then follow their children through age 21 years as part of the National Children's Study. In addition to tracking their development, the researchers will collect biological and environmental samples from study participants. Researchers aim to use this information to help identify the environmental and genetic factors that contribute to conditions such as autism, cerebral palsy, learning disabilities, birth defects, diabetes, asthma, and obesity.
Women are being recruited and enrolled from areas of the country that are representative of the diversity of U.S. children in terms of race, ethnicity, socioeconomic status, and community size. The initial recruitment began in mid-January in the borough of Queens, N.Y., and in Duplin County, N.C. In April, five other centers will begin recruiting. Ultimately, the study is expected to recruit participants from 105 locations across the country.
The National Children's Study was authorized by Congress in the Children's Health Act of 2000 and has been developed by the National Institutes of Health, the Centers for Disease Control and Prevention, and the Environmental Protection Agency.
Researchers estimate that the first children to be part of the study will be born this summer. As a result, the study could yield data in the next few years on conditions such as prematurity and birth defects, according to the NIH.
“Findings from the study will ultimately benefit all Americans by providing researchers, health care providers, and public health officials with information from which to develop prevention strategies, health and safety guidelines, and possibly new treatments for disease,” Dr. Peter Scheidt, director of the National Children's Study, said during a press briefing last month to announce the recruitment phase of the study.
Researchers in North Carolina and New York are beginning to recruit the first volunteers as part of a huge federal study that will examine the relative effects of genes and environment on development.
The researchers plan to track 100,000 women through pregnancy and then follow their children through age 21 years as part of the National Children's Study. In addition to tracking their development, the researchers will collect biological and environmental samples from study participants. Researchers aim to use this information to help identify the environmental and genetic factors that contribute to conditions such as autism, cerebral palsy, learning disabilities, birth defects, diabetes, asthma, and obesity.
Women are being recruited and enrolled from areas of the country that are representative of the diversity of U.S. children in terms of race, ethnicity, socioeconomic status, and community size. The initial recruitment began in mid-January in the borough of Queens, N.Y., and in Duplin County, N.C. In April, five other centers will begin recruiting. Ultimately, the study is expected to recruit participants from 105 locations across the country.
The National Children's Study was authorized by Congress in the Children's Health Act of 2000 and has been developed by the National Institutes of Health, the Centers for Disease Control and Prevention, and the Environmental Protection Agency.
Researchers estimate that the first children to be part of the study will be born this summer. As a result, the study could yield data in the next few years on conditions such as prematurity and birth defects, according to the NIH.
“Findings from the study will ultimately benefit all Americans by providing researchers, health care providers, and public health officials with information from which to develop prevention strategies, health and safety guidelines, and possibly new treatments for disease,” Dr. Peter Scheidt, director of the National Children's Study, said during a press briefing last month to announce the recruitment phase of the study.
Birth Weight's Tie to Diabetes Risk May Change
In most present-day middle-aged populations, the risk of developing type 2 diabetes is inversely related to birth weight, according to a report in JAMA.
In other words, adults at highest risk for type 2 diabetes had the lowest birth weights, independent of potentially confounding factors such as body size and socioeconomic status in adulthood, wrote Peter H. Whincup, Ph.D., of the University of London, and his associates.
However, this association may not hold true for long. It likely reflects fetal undernutrition during the mid-20th century. The current epidemic of overweight and obesity may well reverse this association, with people who had very high birth weights eventually showing a greater propensity to develop diabetes later in life, the researchers said.
The association between low birth weight and later development of diabetes has been known since the early 1990s, but the strength and consistency of the link and its independence from confounding factors has been questioned.
Dr. Whincup and associates performed a meta-analysis of 30 studies published between 1950 and early 2008 involving 6,090 cases of diabetes among 152,084 subjects (JAMA 2008;300:2886-97). Twenty-three of the 30 showed an inverse association between birth weight and later diabetes risk, and the association was statistically significant in 9 of them. Two involved Native American populations with exceptionally high prevalences of obesity, gestational diabetes, and type 2 diabetes. In these, birth weight showed a positive, not an inverse, correlation with later diabetes risk.
In most present-day middle-aged populations, the risk of developing type 2 diabetes is inversely related to birth weight, according to a report in JAMA.
In other words, adults at highest risk for type 2 diabetes had the lowest birth weights, independent of potentially confounding factors such as body size and socioeconomic status in adulthood, wrote Peter H. Whincup, Ph.D., of the University of London, and his associates.
However, this association may not hold true for long. It likely reflects fetal undernutrition during the mid-20th century. The current epidemic of overweight and obesity may well reverse this association, with people who had very high birth weights eventually showing a greater propensity to develop diabetes later in life, the researchers said.
The association between low birth weight and later development of diabetes has been known since the early 1990s, but the strength and consistency of the link and its independence from confounding factors has been questioned.
Dr. Whincup and associates performed a meta-analysis of 30 studies published between 1950 and early 2008 involving 6,090 cases of diabetes among 152,084 subjects (JAMA 2008;300:2886-97). Twenty-three of the 30 showed an inverse association between birth weight and later diabetes risk, and the association was statistically significant in 9 of them. Two involved Native American populations with exceptionally high prevalences of obesity, gestational diabetes, and type 2 diabetes. In these, birth weight showed a positive, not an inverse, correlation with later diabetes risk.
In most present-day middle-aged populations, the risk of developing type 2 diabetes is inversely related to birth weight, according to a report in JAMA.
In other words, adults at highest risk for type 2 diabetes had the lowest birth weights, independent of potentially confounding factors such as body size and socioeconomic status in adulthood, wrote Peter H. Whincup, Ph.D., of the University of London, and his associates.
However, this association may not hold true for long. It likely reflects fetal undernutrition during the mid-20th century. The current epidemic of overweight and obesity may well reverse this association, with people who had very high birth weights eventually showing a greater propensity to develop diabetes later in life, the researchers said.
The association between low birth weight and later development of diabetes has been known since the early 1990s, but the strength and consistency of the link and its independence from confounding factors has been questioned.
Dr. Whincup and associates performed a meta-analysis of 30 studies published between 1950 and early 2008 involving 6,090 cases of diabetes among 152,084 subjects (JAMA 2008;300:2886-97). Twenty-three of the 30 showed an inverse association between birth weight and later diabetes risk, and the association was statistically significant in 9 of them. Two involved Native American populations with exceptionally high prevalences of obesity, gestational diabetes, and type 2 diabetes. In these, birth weight showed a positive, not an inverse, correlation with later diabetes risk.
50,000 Women Aged 18–45 Have Bariatric Surgery Each Year
An estimated 50,000 women of childbearing age undergo inpatient bariatric surgery each year, and an unknown number have outpatient bariatric procedures, according to a report in the Journal of the American Medical Association.
Unfortunately, data about the surgery's effects on pregnancy, fertility, and contraception are still so limited that researchers are precluded from drawing firm conclusions and clinicians cannot make informed decisions regarding these patients, said Dr. Melinda A. Maggard of the Rand Corp., Santa Monica, Calif., and her associates.
The investigators used data from a national health care sample to assess trends in inpatient bariatric surgeries between 1998 and 2005, the most recent year for which information was available. This sample included data on up to 8 million hospitalizations at approximately 1,000 medical centers.
Outpatient bariatric surgeries were not assessed in this study.
The rate of inpatient bariatric procedures—laparoscopic adjustable gastric banding, vertical-banded gastroplasty, Roux-en-Y gastric bypass, and biliopancreatic diversion/duodenal switch—increased by 800% during this interval, from just more than 12,000 to more than 113,500 cases annually.
From 2003 to 2005, women of childbearing age (ages 18–45) accounted for around half of these surgeries (50,000) annually.
Dr. Maggard and her colleagues also reviewed 75 studies in the literature that compared ob.gyn. data between women who underwent bariatric surgery and those who did not.
“The available evidence suggests that risks for maternal complications, such as gestational diabetes and preeclampsia, may be lower following surgically induced weight loss than the risks in obese women, and may approach community rates,” they said (JAMA 2008;300:2286–96).
However, there have been 20 reports of complications during pregnancy that were directly related to the bariatric surgery, including bowel obstructions, band malfunctions, ulcers, and staple-line stricture. Three mothers and five neonates in these cases died.
Bariatric surgery doesn't appear to strongly influence the rate of cesarean deliveries or that of delivery complications such as blood loss or operative injury.
However, few studies have assessed such outcomes in this patient population, the researchers wrote.
Neonatal complications such as preterm delivery and low birth weight may be less common in pregnancies following bariatric surgery.
Similarly, neonatal outcomes such as macrosomia appear to be less frequent. However, the rate of miscarriage appears to be higher in women who have undergone bariatric surgery than in those who have not.
Moreover, two studies reported higher than expected rates of neural tube defects in neonates of women who had undergone bariatric surgery, possibly related to the mothers' noncompliance with vitamin supplementation.
Nutritional problems during these pregnancies seem to be uncommon and, like neural tube defects, are often attributed to the women's noncompliance with taking the recommended nutritional supplements.
Most studies of fertility after bariatric surgery were small and incomplete, the researchers reported. However, their results suggested that the procedures normalized hormone levels and menstrual irregularities, thus improving fertility.
Dr. Maggard and her associates also reported finding that no randomized trials have yet explored the theoretical possibility that bariatric surgery impairs absorption of oral contraceptives, rendering them less effective.
Risks for maternal complications may be lower following surgically induced weight loss. DR. MAGGARD
An estimated 50,000 women of childbearing age undergo inpatient bariatric surgery each year, and an unknown number have outpatient bariatric procedures, according to a report in the Journal of the American Medical Association.
Unfortunately, data about the surgery's effects on pregnancy, fertility, and contraception are still so limited that researchers are precluded from drawing firm conclusions and clinicians cannot make informed decisions regarding these patients, said Dr. Melinda A. Maggard of the Rand Corp., Santa Monica, Calif., and her associates.
The investigators used data from a national health care sample to assess trends in inpatient bariatric surgeries between 1998 and 2005, the most recent year for which information was available. This sample included data on up to 8 million hospitalizations at approximately 1,000 medical centers.
Outpatient bariatric surgeries were not assessed in this study.
The rate of inpatient bariatric procedures—laparoscopic adjustable gastric banding, vertical-banded gastroplasty, Roux-en-Y gastric bypass, and biliopancreatic diversion/duodenal switch—increased by 800% during this interval, from just more than 12,000 to more than 113,500 cases annually.
From 2003 to 2005, women of childbearing age (ages 18–45) accounted for around half of these surgeries (50,000) annually.
Dr. Maggard and her colleagues also reviewed 75 studies in the literature that compared ob.gyn. data between women who underwent bariatric surgery and those who did not.
“The available evidence suggests that risks for maternal complications, such as gestational diabetes and preeclampsia, may be lower following surgically induced weight loss than the risks in obese women, and may approach community rates,” they said (JAMA 2008;300:2286–96).
However, there have been 20 reports of complications during pregnancy that were directly related to the bariatric surgery, including bowel obstructions, band malfunctions, ulcers, and staple-line stricture. Three mothers and five neonates in these cases died.
Bariatric surgery doesn't appear to strongly influence the rate of cesarean deliveries or that of delivery complications such as blood loss or operative injury.
However, few studies have assessed such outcomes in this patient population, the researchers wrote.
Neonatal complications such as preterm delivery and low birth weight may be less common in pregnancies following bariatric surgery.
Similarly, neonatal outcomes such as macrosomia appear to be less frequent. However, the rate of miscarriage appears to be higher in women who have undergone bariatric surgery than in those who have not.
Moreover, two studies reported higher than expected rates of neural tube defects in neonates of women who had undergone bariatric surgery, possibly related to the mothers' noncompliance with vitamin supplementation.
Nutritional problems during these pregnancies seem to be uncommon and, like neural tube defects, are often attributed to the women's noncompliance with taking the recommended nutritional supplements.
Most studies of fertility after bariatric surgery were small and incomplete, the researchers reported. However, their results suggested that the procedures normalized hormone levels and menstrual irregularities, thus improving fertility.
Dr. Maggard and her associates also reported finding that no randomized trials have yet explored the theoretical possibility that bariatric surgery impairs absorption of oral contraceptives, rendering them less effective.
Risks for maternal complications may be lower following surgically induced weight loss. DR. MAGGARD
An estimated 50,000 women of childbearing age undergo inpatient bariatric surgery each year, and an unknown number have outpatient bariatric procedures, according to a report in the Journal of the American Medical Association.
Unfortunately, data about the surgery's effects on pregnancy, fertility, and contraception are still so limited that researchers are precluded from drawing firm conclusions and clinicians cannot make informed decisions regarding these patients, said Dr. Melinda A. Maggard of the Rand Corp., Santa Monica, Calif., and her associates.
The investigators used data from a national health care sample to assess trends in inpatient bariatric surgeries between 1998 and 2005, the most recent year for which information was available. This sample included data on up to 8 million hospitalizations at approximately 1,000 medical centers.
Outpatient bariatric surgeries were not assessed in this study.
The rate of inpatient bariatric procedures—laparoscopic adjustable gastric banding, vertical-banded gastroplasty, Roux-en-Y gastric bypass, and biliopancreatic diversion/duodenal switch—increased by 800% during this interval, from just more than 12,000 to more than 113,500 cases annually.
From 2003 to 2005, women of childbearing age (ages 18–45) accounted for around half of these surgeries (50,000) annually.
Dr. Maggard and her colleagues also reviewed 75 studies in the literature that compared ob.gyn. data between women who underwent bariatric surgery and those who did not.
“The available evidence suggests that risks for maternal complications, such as gestational diabetes and preeclampsia, may be lower following surgically induced weight loss than the risks in obese women, and may approach community rates,” they said (JAMA 2008;300:2286–96).
However, there have been 20 reports of complications during pregnancy that were directly related to the bariatric surgery, including bowel obstructions, band malfunctions, ulcers, and staple-line stricture. Three mothers and five neonates in these cases died.
Bariatric surgery doesn't appear to strongly influence the rate of cesarean deliveries or that of delivery complications such as blood loss or operative injury.
However, few studies have assessed such outcomes in this patient population, the researchers wrote.
Neonatal complications such as preterm delivery and low birth weight may be less common in pregnancies following bariatric surgery.
Similarly, neonatal outcomes such as macrosomia appear to be less frequent. However, the rate of miscarriage appears to be higher in women who have undergone bariatric surgery than in those who have not.
Moreover, two studies reported higher than expected rates of neural tube defects in neonates of women who had undergone bariatric surgery, possibly related to the mothers' noncompliance with vitamin supplementation.
Nutritional problems during these pregnancies seem to be uncommon and, like neural tube defects, are often attributed to the women's noncompliance with taking the recommended nutritional supplements.
Most studies of fertility after bariatric surgery were small and incomplete, the researchers reported. However, their results suggested that the procedures normalized hormone levels and menstrual irregularities, thus improving fertility.
Dr. Maggard and her associates also reported finding that no randomized trials have yet explored the theoretical possibility that bariatric surgery impairs absorption of oral contraceptives, rendering them less effective.
Risks for maternal complications may be lower following surgically induced weight loss. DR. MAGGARD
Miscarriages, Terminations Rise After RA Onset
SAN FRANCISCO — Women who got pregnant after the onset of rheumatoid arthritis had a slightly higher risk of miscarriage, compared with pregnant women as a whole, according to a study of 1,461 pregnancies in 636 women with rheumatoid arthritis.
Many more pregnancies in the retrospective study occurred before rheumatoid arthritis symptoms appeared than occurred after disease onset.
The 2% rate of pregnancy termination in the 86% of women who got pregnant before the onset of rheumatoid arthritis symptoms was significantly lower than a 6% termination rate among the 14% of women who got pregnant after developing arthritis, Dr. Cecilia Friden and her associates reported at the annual meeting of the American College of Rheumatology.
The investigators compared data on pregnancy histories in women who were enrolled in a hospital-based rheumatoid arthritis registry in Boston with U.S. data on pregnant women of comparable ages (20–44 years), and compared outcomes between women who became pregnant before or after developing arthritis.
The cohort's history of 1,461 pregnancies included 1,146 live births, with birth defects in 2% of neonates.
There was no association between the risk of birth defects and the timing of the onset of rheumatoid arthritis, said Dr. Friden of the Karolinska Institute, Stockholm.
The study did not collect information on the use of disease-modifying anti-rheumatic drugs.
Although the effects of pregnancy on rheumatoid arthritis (disease activity decreases during pregnancy and increases during the postpartum period and with breast-feeding) are well known, little has been known about the effects of rheumatoid arthritis on pregnancy outcomes, she noted.
More research will be needed to be able to correctly inform women with rheumatoid arthritis about the potential risks of pregnancy with the disease, Dr. Friden added.
Compared with the nationwide data on pregnant women, the investigators found that the women who had rheumatoid arthritis had slightly higher rates of miscarriage but slightly lower rates of stillbirths.
Pregnancy outcomes did not differ significantly between the pre- and post-arthritis pregnancy groups except for the termination rate.
The rate of miscarriages, stillbirths, multiple births, low-birth-weight babies, and infants born before or after 32 weeks did not differ significantly between the two arthritis groups.
Dr. Friden stated that she had no relevant conflicts of interest to report.
Her associates in the study have received research funds from pharmaceutical companies (some of which make arthritis medications) including Amgen Inc., Biogen Idec Inc., Bristol-Myers Squibb Co., and Millennium Pharmaceuticals Inc.
One associate has been a consultant for GlaxoSmithKline.
ELSEVIER GLOBAL MEDICAL NEWS
Reports on Birth Outcomes in Autoimmune Diseases From the ACR Meeting
Several posters at the annual meeting of the American College of Rheumatology reported on the following pregnancy outcomes for women who had autoimmune diseases or were taking drugs for autoimmune diseases:
▸ Lupus. A study of 198 women with clinically stable or mildly active systemic lupus erythematosus (SLE) at the time of conception found that they rarely developed severe SLE flares and generally had good pregnancy outcomes, Dr. Jane E. Salmon of the Hospital for Special Surgery, New York, and her associates reported.
Mild or moderate disease flares occurred in 6% of women within 20 weeks of gestation, in 5% at 32 weeks, and in 8% during the postpartum period, according to data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study.
No severe flares occurred by 20 weeks. Severe flares were seen in fewer than 1% of women by 32 weeks and in 1.5% during the postpartum period in the ongoing prospective, multicenter, observational study.
Of the four severe SLE flares, two involved the central nervous system, one involved kidney disease, and the other was an arthritis flare.
Pregnancy complications occurred in 15% of women, and included fetal death (in 8%), neonatal death (3%), preeclampsia (15%), and fetal growth restriction (10%), with some women having more than one complication.
Although 28% of women had a past history of renal disease and 39% had blood abnormalities associated with active lupus, these were not associated with flares or poor pregnancy outcomes, Dr. Salmon reported. The investigators reported no relevant conflicts of interest.
▸ Etanercept. Babies born to 139 women who took etanercept for autoimmune diseases during the first trimester of pregnancy had more than twice the rate of congenital anomalies, compared with the newborns of 67 women with the same diseases who were not on etanercept. The data came from the ongoing Autoimmune Diseases in Pregnancy Project run by OTIS (Organization of Teratology Information Specialists), a network of telephone-based teratogen counseling services in North American universities and hospitals. The project recruits women in a prospective cohort; the women are interviewed three times during pregnancy and their medical records studied. Pediatric specialists examine all live-born infants and follow them for up to a year.
In the etanercept group, major defects were seen in 9.4% of all pregnancies in the etanercept group and in 4.5% of the control group. Pregnancies resulted in live births in 94% of the etanercept group and 88% of the control group (due to a higher rate of spontaneous abortion in the control group).
Among live births, 8.5% in the etanercept group had major defects, compared with 1.7% of the control group, reported Dr. Diana L. Johnson of the University of California, San Diego, and her associates.
The two groups did not differ significantly in mean birth weight of full-term infants, mean gestational age at delivery of live births, or pregnancy terminations.
The women took etanercept to treat rheumatoid arthritis; psoriasis or psoriatic arthritis; ankylosing spondylitis; or multiple diseases.
The project is sponsored in part by grants from 10 pharmaceutical companies including Amgen Inc., which markets etanercept with Wyeth Pharmaceuticals.
▸ Adalimumab. The OTIS Autoimmune Diseases in Pregnancy Project also followed 33 women who took adalimumab for rheumatoid arthritis during the first trimester of pregnancy and followed them in the same manner as the etanercept cohort. Their birth outcomes were compared with birth outcomes of 52 pregnant women with the same disease but no adalimumab treatment and 45 pregnant women without rheumatoid arthritis.
Preliminary data from the ongoing study suggest that rates of spontaneous abortion, stillbirth, congenital defects, and preterm deliveries are similar between groups and within the expected range in the general population, Dr. Johnson and her associates reported in a separate poster. A larger sample size is needed, however, to draw firm conclusions, she added.
The companies that fund the project include Abbott Laboratories, which markets adalimumab.
▸ Systemic sclerosis. Worsening of systemic sclerosis and progression to organ problems in three out of five Hungarian women after pregnancy surprised investigators in a separate study. Previous reports identified a higher risk for maternal scleroderma renal crisis in the third trimester, but other than that have suggested that disease symptoms generally do not change or may improve during pregnancy.
The five pregnancies among 400 women with systemic sclerosis who were seen at two medical centers from 1995 to 2007 resulted in five infants with no severe organ complications, reported Dr. G. Szucs of the University of Debrecen (Hungary), and associates.
One mother with limited cutaneous disease had a normal, full-term delivery. One with diffuse cutaneous disease had a spontaneous preterm birth. Three women (one with limited cutaneous disease and two with diffuse systemic sclerosis) were delivered by C-section because of maternal hypertension and proteinuria with a high risk for renal crisis.
The hypertension and proteinuria disappeared after C-section delivery in one woman with diffuse disease, who was treated with an ACE inhibitor to avert renal crisis.
Three other women (one with limited disease and two with diffuse disease) developed severe manifestations of systemic sclerosis after delivery—fibrosing alveolitis; cardiomyopathy with arrhythmias or cardiac failure; renal failure; and/or rapidly progressing skin symptoms.
Pregnant women with systemic sclerosis “should be monitored often and carefully after delivery for not only renal but other life-threatening complications,” Dr. Szucs suggested.
SAN FRANCISCO — Women who got pregnant after the onset of rheumatoid arthritis had a slightly higher risk of miscarriage, compared with pregnant women as a whole, according to a study of 1,461 pregnancies in 636 women with rheumatoid arthritis.
Many more pregnancies in the retrospective study occurred before rheumatoid arthritis symptoms appeared than occurred after disease onset.
The 2% rate of pregnancy termination in the 86% of women who got pregnant before the onset of rheumatoid arthritis symptoms was significantly lower than a 6% termination rate among the 14% of women who got pregnant after developing arthritis, Dr. Cecilia Friden and her associates reported at the annual meeting of the American College of Rheumatology.
The investigators compared data on pregnancy histories in women who were enrolled in a hospital-based rheumatoid arthritis registry in Boston with U.S. data on pregnant women of comparable ages (20–44 years), and compared outcomes between women who became pregnant before or after developing arthritis.
The cohort's history of 1,461 pregnancies included 1,146 live births, with birth defects in 2% of neonates.
There was no association between the risk of birth defects and the timing of the onset of rheumatoid arthritis, said Dr. Friden of the Karolinska Institute, Stockholm.
The study did not collect information on the use of disease-modifying anti-rheumatic drugs.
Although the effects of pregnancy on rheumatoid arthritis (disease activity decreases during pregnancy and increases during the postpartum period and with breast-feeding) are well known, little has been known about the effects of rheumatoid arthritis on pregnancy outcomes, she noted.
More research will be needed to be able to correctly inform women with rheumatoid arthritis about the potential risks of pregnancy with the disease, Dr. Friden added.
Compared with the nationwide data on pregnant women, the investigators found that the women who had rheumatoid arthritis had slightly higher rates of miscarriage but slightly lower rates of stillbirths.
Pregnancy outcomes did not differ significantly between the pre- and post-arthritis pregnancy groups except for the termination rate.
The rate of miscarriages, stillbirths, multiple births, low-birth-weight babies, and infants born before or after 32 weeks did not differ significantly between the two arthritis groups.
Dr. Friden stated that she had no relevant conflicts of interest to report.
Her associates in the study have received research funds from pharmaceutical companies (some of which make arthritis medications) including Amgen Inc., Biogen Idec Inc., Bristol-Myers Squibb Co., and Millennium Pharmaceuticals Inc.
One associate has been a consultant for GlaxoSmithKline.
ELSEVIER GLOBAL MEDICAL NEWS
Reports on Birth Outcomes in Autoimmune Diseases From the ACR Meeting
Several posters at the annual meeting of the American College of Rheumatology reported on the following pregnancy outcomes for women who had autoimmune diseases or were taking drugs for autoimmune diseases:
▸ Lupus. A study of 198 women with clinically stable or mildly active systemic lupus erythematosus (SLE) at the time of conception found that they rarely developed severe SLE flares and generally had good pregnancy outcomes, Dr. Jane E. Salmon of the Hospital for Special Surgery, New York, and her associates reported.
Mild or moderate disease flares occurred in 6% of women within 20 weeks of gestation, in 5% at 32 weeks, and in 8% during the postpartum period, according to data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study.
No severe flares occurred by 20 weeks. Severe flares were seen in fewer than 1% of women by 32 weeks and in 1.5% during the postpartum period in the ongoing prospective, multicenter, observational study.
Of the four severe SLE flares, two involved the central nervous system, one involved kidney disease, and the other was an arthritis flare.
Pregnancy complications occurred in 15% of women, and included fetal death (in 8%), neonatal death (3%), preeclampsia (15%), and fetal growth restriction (10%), with some women having more than one complication.
Although 28% of women had a past history of renal disease and 39% had blood abnormalities associated with active lupus, these were not associated with flares or poor pregnancy outcomes, Dr. Salmon reported. The investigators reported no relevant conflicts of interest.
▸ Etanercept. Babies born to 139 women who took etanercept for autoimmune diseases during the first trimester of pregnancy had more than twice the rate of congenital anomalies, compared with the newborns of 67 women with the same diseases who were not on etanercept. The data came from the ongoing Autoimmune Diseases in Pregnancy Project run by OTIS (Organization of Teratology Information Specialists), a network of telephone-based teratogen counseling services in North American universities and hospitals. The project recruits women in a prospective cohort; the women are interviewed three times during pregnancy and their medical records studied. Pediatric specialists examine all live-born infants and follow them for up to a year.
In the etanercept group, major defects were seen in 9.4% of all pregnancies in the etanercept group and in 4.5% of the control group. Pregnancies resulted in live births in 94% of the etanercept group and 88% of the control group (due to a higher rate of spontaneous abortion in the control group).
Among live births, 8.5% in the etanercept group had major defects, compared with 1.7% of the control group, reported Dr. Diana L. Johnson of the University of California, San Diego, and her associates.
The two groups did not differ significantly in mean birth weight of full-term infants, mean gestational age at delivery of live births, or pregnancy terminations.
The women took etanercept to treat rheumatoid arthritis; psoriasis or psoriatic arthritis; ankylosing spondylitis; or multiple diseases.
The project is sponsored in part by grants from 10 pharmaceutical companies including Amgen Inc., which markets etanercept with Wyeth Pharmaceuticals.
▸ Adalimumab. The OTIS Autoimmune Diseases in Pregnancy Project also followed 33 women who took adalimumab for rheumatoid arthritis during the first trimester of pregnancy and followed them in the same manner as the etanercept cohort. Their birth outcomes were compared with birth outcomes of 52 pregnant women with the same disease but no adalimumab treatment and 45 pregnant women without rheumatoid arthritis.
Preliminary data from the ongoing study suggest that rates of spontaneous abortion, stillbirth, congenital defects, and preterm deliveries are similar between groups and within the expected range in the general population, Dr. Johnson and her associates reported in a separate poster. A larger sample size is needed, however, to draw firm conclusions, she added.
The companies that fund the project include Abbott Laboratories, which markets adalimumab.
▸ Systemic sclerosis. Worsening of systemic sclerosis and progression to organ problems in three out of five Hungarian women after pregnancy surprised investigators in a separate study. Previous reports identified a higher risk for maternal scleroderma renal crisis in the third trimester, but other than that have suggested that disease symptoms generally do not change or may improve during pregnancy.
The five pregnancies among 400 women with systemic sclerosis who were seen at two medical centers from 1995 to 2007 resulted in five infants with no severe organ complications, reported Dr. G. Szucs of the University of Debrecen (Hungary), and associates.
One mother with limited cutaneous disease had a normal, full-term delivery. One with diffuse cutaneous disease had a spontaneous preterm birth. Three women (one with limited cutaneous disease and two with diffuse systemic sclerosis) were delivered by C-section because of maternal hypertension and proteinuria with a high risk for renal crisis.
The hypertension and proteinuria disappeared after C-section delivery in one woman with diffuse disease, who was treated with an ACE inhibitor to avert renal crisis.
Three other women (one with limited disease and two with diffuse disease) developed severe manifestations of systemic sclerosis after delivery—fibrosing alveolitis; cardiomyopathy with arrhythmias or cardiac failure; renal failure; and/or rapidly progressing skin symptoms.
Pregnant women with systemic sclerosis “should be monitored often and carefully after delivery for not only renal but other life-threatening complications,” Dr. Szucs suggested.
SAN FRANCISCO — Women who got pregnant after the onset of rheumatoid arthritis had a slightly higher risk of miscarriage, compared with pregnant women as a whole, according to a study of 1,461 pregnancies in 636 women with rheumatoid arthritis.
Many more pregnancies in the retrospective study occurred before rheumatoid arthritis symptoms appeared than occurred after disease onset.
The 2% rate of pregnancy termination in the 86% of women who got pregnant before the onset of rheumatoid arthritis symptoms was significantly lower than a 6% termination rate among the 14% of women who got pregnant after developing arthritis, Dr. Cecilia Friden and her associates reported at the annual meeting of the American College of Rheumatology.
The investigators compared data on pregnancy histories in women who were enrolled in a hospital-based rheumatoid arthritis registry in Boston with U.S. data on pregnant women of comparable ages (20–44 years), and compared outcomes between women who became pregnant before or after developing arthritis.
The cohort's history of 1,461 pregnancies included 1,146 live births, with birth defects in 2% of neonates.
There was no association between the risk of birth defects and the timing of the onset of rheumatoid arthritis, said Dr. Friden of the Karolinska Institute, Stockholm.
The study did not collect information on the use of disease-modifying anti-rheumatic drugs.
Although the effects of pregnancy on rheumatoid arthritis (disease activity decreases during pregnancy and increases during the postpartum period and with breast-feeding) are well known, little has been known about the effects of rheumatoid arthritis on pregnancy outcomes, she noted.
More research will be needed to be able to correctly inform women with rheumatoid arthritis about the potential risks of pregnancy with the disease, Dr. Friden added.
Compared with the nationwide data on pregnant women, the investigators found that the women who had rheumatoid arthritis had slightly higher rates of miscarriage but slightly lower rates of stillbirths.
Pregnancy outcomes did not differ significantly between the pre- and post-arthritis pregnancy groups except for the termination rate.
The rate of miscarriages, stillbirths, multiple births, low-birth-weight babies, and infants born before or after 32 weeks did not differ significantly between the two arthritis groups.
Dr. Friden stated that she had no relevant conflicts of interest to report.
Her associates in the study have received research funds from pharmaceutical companies (some of which make arthritis medications) including Amgen Inc., Biogen Idec Inc., Bristol-Myers Squibb Co., and Millennium Pharmaceuticals Inc.
One associate has been a consultant for GlaxoSmithKline.
ELSEVIER GLOBAL MEDICAL NEWS
Reports on Birth Outcomes in Autoimmune Diseases From the ACR Meeting
Several posters at the annual meeting of the American College of Rheumatology reported on the following pregnancy outcomes for women who had autoimmune diseases or were taking drugs for autoimmune diseases:
▸ Lupus. A study of 198 women with clinically stable or mildly active systemic lupus erythematosus (SLE) at the time of conception found that they rarely developed severe SLE flares and generally had good pregnancy outcomes, Dr. Jane E. Salmon of the Hospital for Special Surgery, New York, and her associates reported.
Mild or moderate disease flares occurred in 6% of women within 20 weeks of gestation, in 5% at 32 weeks, and in 8% during the postpartum period, according to data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study.
No severe flares occurred by 20 weeks. Severe flares were seen in fewer than 1% of women by 32 weeks and in 1.5% during the postpartum period in the ongoing prospective, multicenter, observational study.
Of the four severe SLE flares, two involved the central nervous system, one involved kidney disease, and the other was an arthritis flare.
Pregnancy complications occurred in 15% of women, and included fetal death (in 8%), neonatal death (3%), preeclampsia (15%), and fetal growth restriction (10%), with some women having more than one complication.
Although 28% of women had a past history of renal disease and 39% had blood abnormalities associated with active lupus, these were not associated with flares or poor pregnancy outcomes, Dr. Salmon reported. The investigators reported no relevant conflicts of interest.
▸ Etanercept. Babies born to 139 women who took etanercept for autoimmune diseases during the first trimester of pregnancy had more than twice the rate of congenital anomalies, compared with the newborns of 67 women with the same diseases who were not on etanercept. The data came from the ongoing Autoimmune Diseases in Pregnancy Project run by OTIS (Organization of Teratology Information Specialists), a network of telephone-based teratogen counseling services in North American universities and hospitals. The project recruits women in a prospective cohort; the women are interviewed three times during pregnancy and their medical records studied. Pediatric specialists examine all live-born infants and follow them for up to a year.
In the etanercept group, major defects were seen in 9.4% of all pregnancies in the etanercept group and in 4.5% of the control group. Pregnancies resulted in live births in 94% of the etanercept group and 88% of the control group (due to a higher rate of spontaneous abortion in the control group).
Among live births, 8.5% in the etanercept group had major defects, compared with 1.7% of the control group, reported Dr. Diana L. Johnson of the University of California, San Diego, and her associates.
The two groups did not differ significantly in mean birth weight of full-term infants, mean gestational age at delivery of live births, or pregnancy terminations.
The women took etanercept to treat rheumatoid arthritis; psoriasis or psoriatic arthritis; ankylosing spondylitis; or multiple diseases.
The project is sponsored in part by grants from 10 pharmaceutical companies including Amgen Inc., which markets etanercept with Wyeth Pharmaceuticals.
▸ Adalimumab. The OTIS Autoimmune Diseases in Pregnancy Project also followed 33 women who took adalimumab for rheumatoid arthritis during the first trimester of pregnancy and followed them in the same manner as the etanercept cohort. Their birth outcomes were compared with birth outcomes of 52 pregnant women with the same disease but no adalimumab treatment and 45 pregnant women without rheumatoid arthritis.
Preliminary data from the ongoing study suggest that rates of spontaneous abortion, stillbirth, congenital defects, and preterm deliveries are similar between groups and within the expected range in the general population, Dr. Johnson and her associates reported in a separate poster. A larger sample size is needed, however, to draw firm conclusions, she added.
The companies that fund the project include Abbott Laboratories, which markets adalimumab.
▸ Systemic sclerosis. Worsening of systemic sclerosis and progression to organ problems in three out of five Hungarian women after pregnancy surprised investigators in a separate study. Previous reports identified a higher risk for maternal scleroderma renal crisis in the third trimester, but other than that have suggested that disease symptoms generally do not change or may improve during pregnancy.
The five pregnancies among 400 women with systemic sclerosis who were seen at two medical centers from 1995 to 2007 resulted in five infants with no severe organ complications, reported Dr. G. Szucs of the University of Debrecen (Hungary), and associates.
One mother with limited cutaneous disease had a normal, full-term delivery. One with diffuse cutaneous disease had a spontaneous preterm birth. Three women (one with limited cutaneous disease and two with diffuse systemic sclerosis) were delivered by C-section because of maternal hypertension and proteinuria with a high risk for renal crisis.
The hypertension and proteinuria disappeared after C-section delivery in one woman with diffuse disease, who was treated with an ACE inhibitor to avert renal crisis.
Three other women (one with limited disease and two with diffuse disease) developed severe manifestations of systemic sclerosis after delivery—fibrosing alveolitis; cardiomyopathy with arrhythmias or cardiac failure; renal failure; and/or rapidly progressing skin symptoms.
Pregnant women with systemic sclerosis “should be monitored often and carefully after delivery for not only renal but other life-threatening complications,” Dr. Szucs suggested.
Diagnostic Keys to Ectopic Pregnancy
Early, Late Preeclampsia May Be Hemodynamically Distinct
CHICAGO — A retrospective study of 1,300 women at 24 weeks' gestation suggests that early preeclampsia and late preeclampsia may be two different hemodynamic forms of disease.
Early preeclampsia was associated with normal prepregnancy BMI, high total vascular resistance (TVR), and bilateral notching of the uterine artery on Doppler evaluation, and late preeclampsia was associated with high prepregnancy BMI and low TVR, Dr. Barbara Vasapollo of University of Rome Tor Vergata reported in a presentation at the World Congress on Ultrasound in Obstetrics and Gynecology.
“This is not the first study to suggest that early and late preeclampsia are two different entities, but it is the first to demonstrate that they are two different hemodynamic entities in the latent phase,” Dr. Vasapollo said in an interview.
Researchers reviewed data on 1,345 nulliparous normotensive women who had undergone uterine artery Doppler and maternal echocardiography to determine TVR at 24 weeks' gestation between 1999 and 2007.
Of these patients, 155 had bilateral notching of the uterine artery, and 107 of this group developed preeclampsia (defined as blood pressure greater than 140/90 mm Hg and proteinuria greater than 300 mg/dL).
Thirty-two patients developed late preeclampsia (more than 34 weeks' gestation), and 75 developed early preeclampsia (less than 34 weeks' gestation).
Significantly more early preeclampsia patients (60%) showed bilateral notching of the uterine artery than late preeclampsia patients (15.6%) at the 24 weeks' examination.
TVR was significantly lower in the group who subsequently developed late preeclampsia than in the group who developed early preeclampsia (741 dyn-s/cm
Dr. Vasapollo said the findings are consistent with other research that links late preeclampsia with maternal constitutional factors such as BMI and early preeclampsia with defective trophoblast invasion (Hypertension 2008; 51:970–5, 989–90).
TVR appears to be one of the most reliable predictors of early or late preeclampsia, she said.
“The ROC curves built to predict early and late preeclampsia show a very good sensitivity and specificity,” according to a study by Dr. Vasapollo and her colleagues. “When considering early severe complications, almost all preeclamptic women show a TVR of greater than 1,400,” she noted (Hypertension 2008;51:1020–6).
Dr. Vasapollo and her colleagues plan to investigate a preventive pharmacologic approach to treatment that is guided by maternal hemodynamics.
CHICAGO — A retrospective study of 1,300 women at 24 weeks' gestation suggests that early preeclampsia and late preeclampsia may be two different hemodynamic forms of disease.
Early preeclampsia was associated with normal prepregnancy BMI, high total vascular resistance (TVR), and bilateral notching of the uterine artery on Doppler evaluation, and late preeclampsia was associated with high prepregnancy BMI and low TVR, Dr. Barbara Vasapollo of University of Rome Tor Vergata reported in a presentation at the World Congress on Ultrasound in Obstetrics and Gynecology.
“This is not the first study to suggest that early and late preeclampsia are two different entities, but it is the first to demonstrate that they are two different hemodynamic entities in the latent phase,” Dr. Vasapollo said in an interview.
Researchers reviewed data on 1,345 nulliparous normotensive women who had undergone uterine artery Doppler and maternal echocardiography to determine TVR at 24 weeks' gestation between 1999 and 2007.
Of these patients, 155 had bilateral notching of the uterine artery, and 107 of this group developed preeclampsia (defined as blood pressure greater than 140/90 mm Hg and proteinuria greater than 300 mg/dL).
Thirty-two patients developed late preeclampsia (more than 34 weeks' gestation), and 75 developed early preeclampsia (less than 34 weeks' gestation).
Significantly more early preeclampsia patients (60%) showed bilateral notching of the uterine artery than late preeclampsia patients (15.6%) at the 24 weeks' examination.
TVR was significantly lower in the group who subsequently developed late preeclampsia than in the group who developed early preeclampsia (741 dyn-s/cm
Dr. Vasapollo said the findings are consistent with other research that links late preeclampsia with maternal constitutional factors such as BMI and early preeclampsia with defective trophoblast invasion (Hypertension 2008; 51:970–5, 989–90).
TVR appears to be one of the most reliable predictors of early or late preeclampsia, she said.
“The ROC curves built to predict early and late preeclampsia show a very good sensitivity and specificity,” according to a study by Dr. Vasapollo and her colleagues. “When considering early severe complications, almost all preeclamptic women show a TVR of greater than 1,400,” she noted (Hypertension 2008;51:1020–6).
Dr. Vasapollo and her colleagues plan to investigate a preventive pharmacologic approach to treatment that is guided by maternal hemodynamics.
CHICAGO — A retrospective study of 1,300 women at 24 weeks' gestation suggests that early preeclampsia and late preeclampsia may be two different hemodynamic forms of disease.
Early preeclampsia was associated with normal prepregnancy BMI, high total vascular resistance (TVR), and bilateral notching of the uterine artery on Doppler evaluation, and late preeclampsia was associated with high prepregnancy BMI and low TVR, Dr. Barbara Vasapollo of University of Rome Tor Vergata reported in a presentation at the World Congress on Ultrasound in Obstetrics and Gynecology.
“This is not the first study to suggest that early and late preeclampsia are two different entities, but it is the first to demonstrate that they are two different hemodynamic entities in the latent phase,” Dr. Vasapollo said in an interview.
Researchers reviewed data on 1,345 nulliparous normotensive women who had undergone uterine artery Doppler and maternal echocardiography to determine TVR at 24 weeks' gestation between 1999 and 2007.
Of these patients, 155 had bilateral notching of the uterine artery, and 107 of this group developed preeclampsia (defined as blood pressure greater than 140/90 mm Hg and proteinuria greater than 300 mg/dL).
Thirty-two patients developed late preeclampsia (more than 34 weeks' gestation), and 75 developed early preeclampsia (less than 34 weeks' gestation).
Significantly more early preeclampsia patients (60%) showed bilateral notching of the uterine artery than late preeclampsia patients (15.6%) at the 24 weeks' examination.
TVR was significantly lower in the group who subsequently developed late preeclampsia than in the group who developed early preeclampsia (741 dyn-s/cm
Dr. Vasapollo said the findings are consistent with other research that links late preeclampsia with maternal constitutional factors such as BMI and early preeclampsia with defective trophoblast invasion (Hypertension 2008; 51:970–5, 989–90).
TVR appears to be one of the most reliable predictors of early or late preeclampsia, she said.
“The ROC curves built to predict early and late preeclampsia show a very good sensitivity and specificity,” according to a study by Dr. Vasapollo and her colleagues. “When considering early severe complications, almost all preeclamptic women show a TVR of greater than 1,400,” she noted (Hypertension 2008;51:1020–6).
Dr. Vasapollo and her colleagues plan to investigate a preventive pharmacologic approach to treatment that is guided by maternal hemodynamics.
