User login
Drug approved as part of frontline therapy for HL
The Japanese Ministry of Health, Labour and Welfare has approved brentuximab vedotin (Adcetris) in combination with doxorubicin, vinblastine, and dacarbazine as a frontline treatment option for CD30-positive Hodgkin lymphoma (HL).
The approval was based on the phase 3 ECHELON-1 trial.
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
In this trial, researchers compared brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review committee, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
The Japanese Ministry of Health, Labour and Welfare has approved brentuximab vedotin (Adcetris) in combination with doxorubicin, vinblastine, and dacarbazine as a frontline treatment option for CD30-positive Hodgkin lymphoma (HL).
The approval was based on the phase 3 ECHELON-1 trial.
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
In this trial, researchers compared brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review committee, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
The Japanese Ministry of Health, Labour and Welfare has approved brentuximab vedotin (Adcetris) in combination with doxorubicin, vinblastine, and dacarbazine as a frontline treatment option for CD30-positive Hodgkin lymphoma (HL).
The approval was based on the phase 3 ECHELON-1 trial.
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in The New England Journal of Medicine.
In this trial, researchers compared brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (A+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review committee, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
Product approved to treat hemophilia A in Japan
The Japanese Ministry of Health, Labour and Welfare has approved Jivi® (also known as damoctocog alfa pegol or antihemophilic factor [recombinant] PEGylated-aucl) for the treatment of hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate approved for use in hemophilia A patients age 12 and older.
Jivi is approved for on-demand treatment and control of bleeding episodes, for perioperative management of bleeding, and as routine prophylaxis to reduce the frequency of bleeding episodes.
As prophylaxis, Jivi is typically given twice weekly, but it can also be given every 5 days or once a week, depending on patient needs.
The approval of Jivi in Japan is supported by data from the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated Jivi for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
The Japanese Ministry of Health, Labour and Welfare has approved Jivi® (also known as damoctocog alfa pegol or antihemophilic factor [recombinant] PEGylated-aucl) for the treatment of hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate approved for use in hemophilia A patients age 12 and older.
Jivi is approved for on-demand treatment and control of bleeding episodes, for perioperative management of bleeding, and as routine prophylaxis to reduce the frequency of bleeding episodes.
As prophylaxis, Jivi is typically given twice weekly, but it can also be given every 5 days or once a week, depending on patient needs.
The approval of Jivi in Japan is supported by data from the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated Jivi for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
The Japanese Ministry of Health, Labour and Welfare has approved Jivi® (also known as damoctocog alfa pegol or antihemophilic factor [recombinant] PEGylated-aucl) for the treatment of hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate approved for use in hemophilia A patients age 12 and older.
Jivi is approved for on-demand treatment and control of bleeding episodes, for perioperative management of bleeding, and as routine prophylaxis to reduce the frequency of bleeding episodes.
As prophylaxis, Jivi is typically given twice weekly, but it can also be given every 5 days or once a week, depending on patient needs.
The approval of Jivi in Japan is supported by data from the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated Jivi for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
CHMP reconsiders new indication for blinatumomab
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) said it will re-examine a recent opinion on blinatumomab (Blincyto).
In July, the CHMP recommended against approving blinatumomab to treat patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who have minimal residual disease (MRD).
However, the CHMP has agreed to re-examine its position and issue a final recommendation.
Blinatumomab is currently approved by the European Commission (EC) as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
Blinatumomab is also approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
Amgen is seeking an extension of the marketing authorization for blinatumomab to include BCP-ALL patients with MRD.
The CHMP previously recommended against approving blinatumomab for these patients based on data from the BLAST study. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab helped clear away residual cells in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP has complied.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) said it will re-examine a recent opinion on blinatumomab (Blincyto).
In July, the CHMP recommended against approving blinatumomab to treat patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who have minimal residual disease (MRD).
However, the CHMP has agreed to re-examine its position and issue a final recommendation.
Blinatumomab is currently approved by the European Commission (EC) as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
Blinatumomab is also approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
Amgen is seeking an extension of the marketing authorization for blinatumomab to include BCP-ALL patients with MRD.
The CHMP previously recommended against approving blinatumomab for these patients based on data from the BLAST study. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab helped clear away residual cells in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP has complied.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) said it will re-examine a recent opinion on blinatumomab (Blincyto).
In July, the CHMP recommended against approving blinatumomab to treat patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who have minimal residual disease (MRD).
However, the CHMP has agreed to re-examine its position and issue a final recommendation.
Blinatumomab is currently approved by the European Commission (EC) as monotherapy for adults with Philadelphia chromosome-negative, CD19-positive, relapsed or refractory BCP-ALL.
Blinatumomab is also approved as monotherapy for pediatric patients age 1 year or older who have relapsed/refractory, Philadelphia chromosome-negative, CD19-positive BCP-ALL and have received at least two prior therapies or relapsed after allogeneic hematopoietic stem cell transplant.
Amgen is seeking an extension of the marketing authorization for blinatumomab to include BCP-ALL patients with MRD.
The CHMP previously recommended against approving blinatumomab for these patients based on data from the BLAST study. Results from this phase 2 trial were published in Blood in April.
The CHMP noted that, although blinatumomab helped clear away residual cells in many patients in the BLAST trial, there is no strong evidence that this leads to improved survival.
Given the uncertainty, the CHMP was of the opinion that the benefits of blinatumomab do not outweigh its risks in MRD-positive BCP-ALL patients.
However, Amgen request a re-examination of the CHMP’s opinion, and the CHMP has complied.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
CHMP backs proposed biosimilars of pegfilgrastim
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for three proposed biosimilars of pegfilgrastim—Ziextenzo, Pelmeg, and Fulphila.
If approved by the European Commission (EC), these products would be used for the same indication as the reference medicine, Neulasta (pegfilgrastim).
Neulasta has been EC-approved since 2002 to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy to treat malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
According to the CHMP, data suggest that Ziextenzo, Pelmeg, and Fulphila all have quality, efficacy, and safety profiles comparable to Neulasta.
The EC is expected to make a decision on the approval of Ziextenzo, Pelmeg, and Fulphila within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions based on the EC’s judgement.
Ziextenzo is being developed by Sandoz GmbH, Pelmeg is being developed by Cinfa Biotech S.L., and Fulphila is being developed by MYLAN S.A.S.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for three proposed biosimilars of pegfilgrastim—Ziextenzo, Pelmeg, and Fulphila.
If approved by the European Commission (EC), these products would be used for the same indication as the reference medicine, Neulasta (pegfilgrastim).
Neulasta has been EC-approved since 2002 to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy to treat malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
According to the CHMP, data suggest that Ziextenzo, Pelmeg, and Fulphila all have quality, efficacy, and safety profiles comparable to Neulasta.
The EC is expected to make a decision on the approval of Ziextenzo, Pelmeg, and Fulphila within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions based on the EC’s judgement.
Ziextenzo is being developed by Sandoz GmbH, Pelmeg is being developed by Cinfa Biotech S.L., and Fulphila is being developed by MYLAN S.A.S.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for three proposed biosimilars of pegfilgrastim—Ziextenzo, Pelmeg, and Fulphila.
If approved by the European Commission (EC), these products would be used for the same indication as the reference medicine, Neulasta (pegfilgrastim).
Neulasta has been EC-approved since 2002 to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults who receive cytotoxic chemotherapy to treat malignancies except chronic myeloid leukemia and myelodysplastic syndromes.
According to the CHMP, data suggest that Ziextenzo, Pelmeg, and Fulphila all have quality, efficacy, and safety profiles comparable to Neulasta.
The EC is expected to make a decision on the approval of Ziextenzo, Pelmeg, and Fulphila within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions based on the EC’s judgement.
Ziextenzo is being developed by Sandoz GmbH, Pelmeg is being developed by Cinfa Biotech S.L., and Fulphila is being developed by MYLAN S.A.S.
CHMP recommends factor VIII therapy for hemophilia A
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for damoctocog alfa pegol, a recombinant human factor VIII therapy.
Bayer is seeking European marketing authorization for damoctocog alfa pegol (formerly BAY94-9027) for the treatment and prophylaxis of bleeding in previously treated patients age 12 and older with hemophilia A.
The CHMP’s recommendation for damoctocog alfa pegol, which is approved in the U.S. under the name Jivi, will be reviewed by the European Commission (EC).
The EC typically makes a decision about marketing authorization within 67 days of the CHMP’s opinion. The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for damoctocog alfa pegol is supported by the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for damoctocog alfa pegol used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated damoctocog alfa pegol for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received damoctocog alfa pegol for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Damoctocog alfa pegol provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for damoctocog alfa pegol, a recombinant human factor VIII therapy.
Bayer is seeking European marketing authorization for damoctocog alfa pegol (formerly BAY94-9027) for the treatment and prophylaxis of bleeding in previously treated patients age 12 and older with hemophilia A.
The CHMP’s recommendation for damoctocog alfa pegol, which is approved in the U.S. under the name Jivi, will be reviewed by the European Commission (EC).
The EC typically makes a decision about marketing authorization within 67 days of the CHMP’s opinion. The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for damoctocog alfa pegol is supported by the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for damoctocog alfa pegol used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated damoctocog alfa pegol for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received damoctocog alfa pegol for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Damoctocog alfa pegol provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for damoctocog alfa pegol, a recombinant human factor VIII therapy.
Bayer is seeking European marketing authorization for damoctocog alfa pegol (formerly BAY94-9027) for the treatment and prophylaxis of bleeding in previously treated patients age 12 and older with hemophilia A.
The CHMP’s recommendation for damoctocog alfa pegol, which is approved in the U.S. under the name Jivi, will be reviewed by the European Commission (EC).
The EC typically makes a decision about marketing authorization within 67 days of the CHMP’s opinion. The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for damoctocog alfa pegol is supported by the phase 2/3 PROTECT VIII trial. Some results from this trial were published in the Journal of Thrombosis and Haemostasis in 2016. Additional results are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for damoctocog alfa pegol used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A.
In part B, researchers evaluated damoctocog alfa pegol for perioperative management.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received damoctocog alfa pegol for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Damoctocog alfa pegol provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
CHMP supports new indication for venetoclax
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that the European Commission (EC) approve a new indication for venetoclax (Venclyxto®).
AbbVie is seeking EC approval for venetoclax in combination with rituximab for the treatment of patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who have received at least one prior therapy.
The EC typically makes an approval decision within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
Venetoclax is already EC-approved as monotherapy for:
- Adults with CLL who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with a B-cell receptor pathway inhibitor
- Adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.
The CHMP’s recommendation to approve venetoclax in combination with rituximab is supported by the phase 3 MURANO trial. Results from MURANO were published in The New England Journal of Medicine in March.
The trial included 389 CLL patients who were randomized to receive venetoclax plus rituximab (VEN+R) or bendamustine plus rituximab (B+R). The median follow-up was 23.8 months.
According to investigators, the median progression-free survival was not reached in the VEN+R arm and was 17.0 months in the B+R arm (hazard ratio, 0.17; P<0.0001).
According to an independent review committee, the median progression-free survival was not reached in the VEN+R arm and was 18.1 months in the B+R arm (hazard ratio, 0.20; P<0.0001).
Grade 3/4 adverse events (AEs) with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) included:
- Neutropenia (57.7% and 38.8%)
- Infections and infestations (17.5% and 21.8%)
- Anemia (10.8% and 13.8%)
- Thrombocytopenia (5.7% and 10.1%)
- Febrile neutropenia (3.6% and 9.6%)
- Pneumonia (5.2% and 8.0%)
- Infusion-related reaction (1.5% and 5.3%)
- Tumor lysis syndrome (3.1% and 1.1%)
- Hypotension (0% and 2.7%)
- Hyperglycemia (2.1% and 0%)
- Hypogammaglobulinemia (2.1% and 0%).
Serious AEs with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) were:
- Pneumonia (8.2% and 8.0%)
- Febrile neutropenia (3.6% and 8.5%)
- Pyrexia (2.6% and 6.9%)
- Anemia (1.5% and 2.7%)
- Infusion-related reaction (0.5% and 3.2%)
- Sepsis (0.5% and 2.1%)
- Tumor lysis syndrome (2.1% and 0.5%)
- Hypotension (0% and 2.7%).
Fatal AEs occurred in 5.2% of patients in the VEN+R arm and 5.9% in the B+R arm.
Fatal AEs in the VEN+R arm included pneumonia (n=3), sepsis (n=1), thrombocytopenia (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1). Two cases of pneumonia occurred in the setting of progression/Richter transformation.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that the European Commission (EC) approve a new indication for venetoclax (Venclyxto®).
AbbVie is seeking EC approval for venetoclax in combination with rituximab for the treatment of patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who have received at least one prior therapy.
The EC typically makes an approval decision within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
Venetoclax is already EC-approved as monotherapy for:
- Adults with CLL who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with a B-cell receptor pathway inhibitor
- Adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.
The CHMP’s recommendation to approve venetoclax in combination with rituximab is supported by the phase 3 MURANO trial. Results from MURANO were published in The New England Journal of Medicine in March.
The trial included 389 CLL patients who were randomized to receive venetoclax plus rituximab (VEN+R) or bendamustine plus rituximab (B+R). The median follow-up was 23.8 months.
According to investigators, the median progression-free survival was not reached in the VEN+R arm and was 17.0 months in the B+R arm (hazard ratio, 0.17; P<0.0001).
According to an independent review committee, the median progression-free survival was not reached in the VEN+R arm and was 18.1 months in the B+R arm (hazard ratio, 0.20; P<0.0001).
Grade 3/4 adverse events (AEs) with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) included:
- Neutropenia (57.7% and 38.8%)
- Infections and infestations (17.5% and 21.8%)
- Anemia (10.8% and 13.8%)
- Thrombocytopenia (5.7% and 10.1%)
- Febrile neutropenia (3.6% and 9.6%)
- Pneumonia (5.2% and 8.0%)
- Infusion-related reaction (1.5% and 5.3%)
- Tumor lysis syndrome (3.1% and 1.1%)
- Hypotension (0% and 2.7%)
- Hyperglycemia (2.1% and 0%)
- Hypogammaglobulinemia (2.1% and 0%).
Serious AEs with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) were:
- Pneumonia (8.2% and 8.0%)
- Febrile neutropenia (3.6% and 8.5%)
- Pyrexia (2.6% and 6.9%)
- Anemia (1.5% and 2.7%)
- Infusion-related reaction (0.5% and 3.2%)
- Sepsis (0.5% and 2.1%)
- Tumor lysis syndrome (2.1% and 0.5%)
- Hypotension (0% and 2.7%).
Fatal AEs occurred in 5.2% of patients in the VEN+R arm and 5.9% in the B+R arm.
Fatal AEs in the VEN+R arm included pneumonia (n=3), sepsis (n=1), thrombocytopenia (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1). Two cases of pneumonia occurred in the setting of progression/Richter transformation.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that the European Commission (EC) approve a new indication for venetoclax (Venclyxto®).
AbbVie is seeking EC approval for venetoclax in combination with rituximab for the treatment of patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who have received at least one prior therapy.
The EC typically makes an approval decision within 67 days of the CHMP’s opinion.
The EC’s decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
Venetoclax is already EC-approved as monotherapy for:
- Adults with CLL who have 17p deletion or TP53 mutation and are unsuitable for or have failed treatment with a B-cell receptor pathway inhibitor
- Adults with CLL who do not have 17p deletion or TP53 mutation but have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.
The CHMP’s recommendation to approve venetoclax in combination with rituximab is supported by the phase 3 MURANO trial. Results from MURANO were published in The New England Journal of Medicine in March.
The trial included 389 CLL patients who were randomized to receive venetoclax plus rituximab (VEN+R) or bendamustine plus rituximab (B+R). The median follow-up was 23.8 months.
According to investigators, the median progression-free survival was not reached in the VEN+R arm and was 17.0 months in the B+R arm (hazard ratio, 0.17; P<0.0001).
According to an independent review committee, the median progression-free survival was not reached in the VEN+R arm and was 18.1 months in the B+R arm (hazard ratio, 0.20; P<0.0001).
Grade 3/4 adverse events (AEs) with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) included:
- Neutropenia (57.7% and 38.8%)
- Infections and infestations (17.5% and 21.8%)
- Anemia (10.8% and 13.8%)
- Thrombocytopenia (5.7% and 10.1%)
- Febrile neutropenia (3.6% and 9.6%)
- Pneumonia (5.2% and 8.0%)
- Infusion-related reaction (1.5% and 5.3%)
- Tumor lysis syndrome (3.1% and 1.1%)
- Hypotension (0% and 2.7%)
- Hyperglycemia (2.1% and 0%)
- Hypogammaglobulinemia (2.1% and 0%).
Serious AEs with at least a 2% difference in incidence between the treatment groups (in the VEN+R and B+R arms, respectively) were:
- Pneumonia (8.2% and 8.0%)
- Febrile neutropenia (3.6% and 8.5%)
- Pyrexia (2.6% and 6.9%)
- Anemia (1.5% and 2.7%)
- Infusion-related reaction (0.5% and 3.2%)
- Sepsis (0.5% and 2.1%)
- Tumor lysis syndrome (2.1% and 0.5%)
- Hypotension (0% and 2.7%).
Fatal AEs occurred in 5.2% of patients in the VEN+R arm and 5.9% in the B+R arm.
Fatal AEs in the VEN+R arm included pneumonia (n=3), sepsis (n=1), thrombocytopenia (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1). Two cases of pneumonia occurred in the setting of progression/Richter transformation.
CHMP recommends mogamulizumab for MF, SS
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for mogamulizumab (Poteligeo).
Kyowa Kirin Limited is seeking European Commission (EC) approval for mogamulizumab as a treatment for adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
The CHMP’s recommendation to approve mogamulizumab will be reviewed by the EC, and the EC is expected to make its decision about the drug by the end of this year.
The decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for mogamulizumab is supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to independent review, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for mogamulizumab (Poteligeo).
Kyowa Kirin Limited is seeking European Commission (EC) approval for mogamulizumab as a treatment for adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
The CHMP’s recommendation to approve mogamulizumab will be reviewed by the EC, and the EC is expected to make its decision about the drug by the end of this year.
The decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for mogamulizumab is supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to independent review, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for mogamulizumab (Poteligeo).
Kyowa Kirin Limited is seeking European Commission (EC) approval for mogamulizumab as a treatment for adults with mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
The CHMP’s recommendation to approve mogamulizumab will be reviewed by the EC, and the EC is expected to make its decision about the drug by the end of this year.
The decision will apply to the European Union, Norway, Iceland, and Liechtenstein.
The CHMP’s recommendation for mogamulizumab is supported by the phase 3 MAVORIC trial. Results from this trial were published in The Lancet Oncology in August.
MAVORIC was a comparison of mogamulizumab and vorinostat in 372 adults with MF or SS who had received at least one prior systemic therapy.
Mogamulizumab provided a significant improvement in progression-free survival (PFS), the study’s primary endpoint.
According to investigators, the median PFS was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio=0.53, P<0.0001).
According to independent review, the median PFS was 6.7 months and 3.8 months, respectively (hazard ratio=0.64, P<0.0007).
There was a significant improvement in overall response rate (ORR) with mogamulizumab.
According to independent review, the global ORR was 23% (43/186) in the mogamulizumab arm and 4% (7/186) in the vorinostat arm (risk ratio=19.4, P<0.0001).
According to investigators, the global ORR was 28% (52/186) and 5% (9/186), respectively (risk ratio=23.1, P<0.0001).
For patients with MF, the investigator-assessed ORR was 21% (22/105) with mogamulizumab and 7% (7/99) with vorinostat.
For SS patients, the investigator-assessed ORR was 37% (30/81) and 2% (2/87), respectively.
Grade 3 adverse events (AEs) in the mogamulizumab arm included drug eruptions (n=8), hypertension (n=8), pneumonia (n=6), fatigue (n=3), cellulitis (n=3), infusion-related reactions (n=3), sepsis (n=2), decreased appetite (n=2), AST increase (n=2), weight decrease (n=1), pyrexia (n=1), constipation (n=1), nausea (n=1), and diarrhea (n=1).
Grade 4 AEs with mogamulizumab were cellulitis (n=1) and pneumonia (n=1). Grade 5 AEs included pneumonia (n=1) and sepsis (n=1).
FDA approves drug for hairy cell leukemia
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
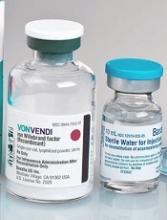
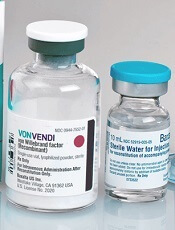
EC approves product for von Willebrand disease
The European Commission (EC) has granted marketing authorization for vonicog alfa (Veyvondi), a recombinant von Willebrand factor (rVWF) product.
The EC approved vonicog alfa for the treatment of bleeding events and treatment/prevention of surgical bleeding in adults (age 18 and older) with von Willebrand disease (VWD) when desmopressin treatment alone is ineffective or not indicated.
The approval means Shire is authorized to market vonicog alfa in the European Union as well as in Iceland, Lichtenstein, and Norway.
The EC’s approval of vonicog alfa was based on outcomes from three clinical trials. This includes a phase 1 study and a pair of phase 3 trials—one in a surgical setting and one in a non-surgical setting.
Data from these studies are available in the Summary of Product Characteristics for vonicog alfa.
Phase 1 trial
This trial (NCT00816660, 070701) enrolled patients with type 3 or severe type 1 VWD.
The goal was to assess the safety and pharmacokinetics of vonicog alfa (rVWF) combined at a fixed ratio with recombinant factor VIII (rFVIII)—referred to as “rVWF-rFVIII.” The researchers compared rVWF-rFVIII to plasma-derived (pd) VWF combined with pdFVIII (pdVWF-pdFVIII).
The safety analysis included 32 patients who received rVWF-rFVIII. There were no thrombotic events, serious adverse events, or new cases of inhibitors to VWF or FVIII in these patients.
The pharmacokinetic analysis included 19 patients. The researchers said the pharmacokinetics of rVWF ristocetin cofactor activity, VWF antigen, and collagen-binding activity were similar with rVWF-rFVIII and pdVWF-pdFVIII.
FVIII levels were higher after infusion with rVWF-rFVIII than with pdVWF-pdFVIII, even after 72 hours. The researchers said this suggests that rVWF alone “could maintain sufficient FVIII activity to treat a bleeding episode once the initial FVIII level has reached a therapeutic threshold.”
These results were published in Blood in 2013.
Phase 3: Non-surgical
This study (NCT01410227, 071001) included 49 patients with VWD who received vonicog alfa with or without rFVIII.
All participants had successful treatment of bleeding episodes. Most (96.9%) treated bleeds (192 bleeds in 22 patients) were given an “excellent” efficacy rating (as good as or better than expected).
Most bleeds (81.8%) were resolved with a single infusion of vonicog alfa, and the treatment had a mean half-life of 21.9 hours.
There were 8 adverse events considered related to vonicog alfa, and 2 were serious. One patient experienced 2 simultaneous serious events—chest discomfort and increased heart rate—but these were resolved.
There were no thrombotic events in this trial, no treatment-related binding or neutralizing antibodies against VWF, and no neutralizing antibodies against FVIII.
These results were published in Blood in 2015.
Phase 3: Surgical setting
This trial (NCT02283268, 071101) enrolled 15 adults with severe VWD who were undergoing elective surgical procedures (10 of them major procedures).
Patients received vonicog alfa at 40 to 60 IU per kg of body weight 12 to 24 hours before surgery. Within 3 hours of surgery, each patient’s FVIII level (FVIII:C) was assessed, with a target of 30 IU/dL for minor surgeries and 60 IU/dL for major surgeries.
Within an hour of surgery, patients received a dose of vonicog alfa, with or without rFVIII, depending on the target FVIII:C levels at the 3-hour assessment.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
Vonicog alfa demonstrated overall hemostatic efficacy, as assessed 24 hours after the last perioperative infusion or the completion of the study visit, whichever occurred earlier.
Intra- and post-operative hemostasis was rated as “excellent” (as good as or better than expected) in 60% of patients and “good” (probably as good as expected) in 40% of patients.
One patient developed deep vein thrombosis 3 days after undergoing hip replacement surgery.
One patient tested positive for binding antibodies to VWF. None of the patients developed binding antibodies against potential impurities such as rFurin, CHO-protein, or mouse IgG.
These results were presented at the WFH 2018 World Congress.
The European Commission (EC) has granted marketing authorization for vonicog alfa (Veyvondi), a recombinant von Willebrand factor (rVWF) product.
The EC approved vonicog alfa for the treatment of bleeding events and treatment/prevention of surgical bleeding in adults (age 18 and older) with von Willebrand disease (VWD) when desmopressin treatment alone is ineffective or not indicated.
The approval means Shire is authorized to market vonicog alfa in the European Union as well as in Iceland, Lichtenstein, and Norway.
The EC’s approval of vonicog alfa was based on outcomes from three clinical trials. This includes a phase 1 study and a pair of phase 3 trials—one in a surgical setting and one in a non-surgical setting.
Data from these studies are available in the Summary of Product Characteristics for vonicog alfa.
Phase 1 trial
This trial (NCT00816660, 070701) enrolled patients with type 3 or severe type 1 VWD.
The goal was to assess the safety and pharmacokinetics of vonicog alfa (rVWF) combined at a fixed ratio with recombinant factor VIII (rFVIII)—referred to as “rVWF-rFVIII.” The researchers compared rVWF-rFVIII to plasma-derived (pd) VWF combined with pdFVIII (pdVWF-pdFVIII).
The safety analysis included 32 patients who received rVWF-rFVIII. There were no thrombotic events, serious adverse events, or new cases of inhibitors to VWF or FVIII in these patients.
The pharmacokinetic analysis included 19 patients. The researchers said the pharmacokinetics of rVWF ristocetin cofactor activity, VWF antigen, and collagen-binding activity were similar with rVWF-rFVIII and pdVWF-pdFVIII.
FVIII levels were higher after infusion with rVWF-rFVIII than with pdVWF-pdFVIII, even after 72 hours. The researchers said this suggests that rVWF alone “could maintain sufficient FVIII activity to treat a bleeding episode once the initial FVIII level has reached a therapeutic threshold.”
These results were published in Blood in 2013.
Phase 3: Non-surgical
This study (NCT01410227, 071001) included 49 patients with VWD who received vonicog alfa with or without rFVIII.
All participants had successful treatment of bleeding episodes. Most (96.9%) treated bleeds (192 bleeds in 22 patients) were given an “excellent” efficacy rating (as good as or better than expected).
Most bleeds (81.8%) were resolved with a single infusion of vonicog alfa, and the treatment had a mean half-life of 21.9 hours.
There were 8 adverse events considered related to vonicog alfa, and 2 were serious. One patient experienced 2 simultaneous serious events—chest discomfort and increased heart rate—but these were resolved.
There were no thrombotic events in this trial, no treatment-related binding or neutralizing antibodies against VWF, and no neutralizing antibodies against FVIII.
These results were published in Blood in 2015.
Phase 3: Surgical setting
This trial (NCT02283268, 071101) enrolled 15 adults with severe VWD who were undergoing elective surgical procedures (10 of them major procedures).
Patients received vonicog alfa at 40 to 60 IU per kg of body weight 12 to 24 hours before surgery. Within 3 hours of surgery, each patient’s FVIII level (FVIII:C) was assessed, with a target of 30 IU/dL for minor surgeries and 60 IU/dL for major surgeries.
Within an hour of surgery, patients received a dose of vonicog alfa, with or without rFVIII, depending on the target FVIII:C levels at the 3-hour assessment.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
Vonicog alfa demonstrated overall hemostatic efficacy, as assessed 24 hours after the last perioperative infusion or the completion of the study visit, whichever occurred earlier.
Intra- and post-operative hemostasis was rated as “excellent” (as good as or better than expected) in 60% of patients and “good” (probably as good as expected) in 40% of patients.
One patient developed deep vein thrombosis 3 days after undergoing hip replacement surgery.
One patient tested positive for binding antibodies to VWF. None of the patients developed binding antibodies against potential impurities such as rFurin, CHO-protein, or mouse IgG.
These results were presented at the WFH 2018 World Congress.
The European Commission (EC) has granted marketing authorization for vonicog alfa (Veyvondi), a recombinant von Willebrand factor (rVWF) product.
The EC approved vonicog alfa for the treatment of bleeding events and treatment/prevention of surgical bleeding in adults (age 18 and older) with von Willebrand disease (VWD) when desmopressin treatment alone is ineffective or not indicated.
The approval means Shire is authorized to market vonicog alfa in the European Union as well as in Iceland, Lichtenstein, and Norway.
The EC’s approval of vonicog alfa was based on outcomes from three clinical trials. This includes a phase 1 study and a pair of phase 3 trials—one in a surgical setting and one in a non-surgical setting.
Data from these studies are available in the Summary of Product Characteristics for vonicog alfa.
Phase 1 trial
This trial (NCT00816660, 070701) enrolled patients with type 3 or severe type 1 VWD.
The goal was to assess the safety and pharmacokinetics of vonicog alfa (rVWF) combined at a fixed ratio with recombinant factor VIII (rFVIII)—referred to as “rVWF-rFVIII.” The researchers compared rVWF-rFVIII to plasma-derived (pd) VWF combined with pdFVIII (pdVWF-pdFVIII).
The safety analysis included 32 patients who received rVWF-rFVIII. There were no thrombotic events, serious adverse events, or new cases of inhibitors to VWF or FVIII in these patients.
The pharmacokinetic analysis included 19 patients. The researchers said the pharmacokinetics of rVWF ristocetin cofactor activity, VWF antigen, and collagen-binding activity were similar with rVWF-rFVIII and pdVWF-pdFVIII.
FVIII levels were higher after infusion with rVWF-rFVIII than with pdVWF-pdFVIII, even after 72 hours. The researchers said this suggests that rVWF alone “could maintain sufficient FVIII activity to treat a bleeding episode once the initial FVIII level has reached a therapeutic threshold.”
These results were published in Blood in 2013.
Phase 3: Non-surgical
This study (NCT01410227, 071001) included 49 patients with VWD who received vonicog alfa with or without rFVIII.
All participants had successful treatment of bleeding episodes. Most (96.9%) treated bleeds (192 bleeds in 22 patients) were given an “excellent” efficacy rating (as good as or better than expected).
Most bleeds (81.8%) were resolved with a single infusion of vonicog alfa, and the treatment had a mean half-life of 21.9 hours.
There were 8 adverse events considered related to vonicog alfa, and 2 were serious. One patient experienced 2 simultaneous serious events—chest discomfort and increased heart rate—but these were resolved.
There were no thrombotic events in this trial, no treatment-related binding or neutralizing antibodies against VWF, and no neutralizing antibodies against FVIII.
These results were published in Blood in 2015.
Phase 3: Surgical setting
This trial (NCT02283268, 071101) enrolled 15 adults with severe VWD who were undergoing elective surgical procedures (10 of them major procedures).
Patients received vonicog alfa at 40 to 60 IU per kg of body weight 12 to 24 hours before surgery. Within 3 hours of surgery, each patient’s FVIII level (FVIII:C) was assessed, with a target of 30 IU/dL for minor surgeries and 60 IU/dL for major surgeries.
Within an hour of surgery, patients received a dose of vonicog alfa, with or without rFVIII, depending on the target FVIII:C levels at the 3-hour assessment.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
Vonicog alfa demonstrated overall hemostatic efficacy, as assessed 24 hours after the last perioperative infusion or the completion of the study visit, whichever occurred earlier.
Intra- and post-operative hemostasis was rated as “excellent” (as good as or better than expected) in 60% of patients and “good” (probably as good as expected) in 40% of patients.
One patient developed deep vein thrombosis 3 days after undergoing hip replacement surgery.
One patient tested positive for binding antibodies to VWF. None of the patients developed binding antibodies against potential impurities such as rFurin, CHO-protein, or mouse IgG.
These results were presented at the WFH 2018 World Congress.
Hemophilia B drug available in larger vial
CSL Behring has announced that Idelvion (Coagulation Factor IX [Recombinant], Albumin Fusion Protein) is now available in a 3500 IU vial size.
Idelvion is also available in 250 IU, 500 IU, 1000 IU, and 2000 IU vial sizes.
For some patients requiring high doses of Idelvion, the new 3500 IU vial size will reduce the reconstitution time needed to prepare multiple vials for a similar dose.
Idelvion is a fusion protein linking recombinant coagulation factor IX with recombinant albumin, and it is approved by the U.S. Food and Drug Administration to treat children and adults with hemophilia B.
Idelvion can be used as routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for on-demand control and prevention of bleeding episodes, and for the perioperative management of bleeding.
Idelvion is approved for up to 14-day dosing in appropriate patients.
For more details on Idelvion, see the prescribing information.
CSL Behring has announced that Idelvion (Coagulation Factor IX [Recombinant], Albumin Fusion Protein) is now available in a 3500 IU vial size.
Idelvion is also available in 250 IU, 500 IU, 1000 IU, and 2000 IU vial sizes.
For some patients requiring high doses of Idelvion, the new 3500 IU vial size will reduce the reconstitution time needed to prepare multiple vials for a similar dose.
Idelvion is a fusion protein linking recombinant coagulation factor IX with recombinant albumin, and it is approved by the U.S. Food and Drug Administration to treat children and adults with hemophilia B.
Idelvion can be used as routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for on-demand control and prevention of bleeding episodes, and for the perioperative management of bleeding.
Idelvion is approved for up to 14-day dosing in appropriate patients.
For more details on Idelvion, see the prescribing information.
CSL Behring has announced that Idelvion (Coagulation Factor IX [Recombinant], Albumin Fusion Protein) is now available in a 3500 IU vial size.
Idelvion is also available in 250 IU, 500 IU, 1000 IU, and 2000 IU vial sizes.
For some patients requiring high doses of Idelvion, the new 3500 IU vial size will reduce the reconstitution time needed to prepare multiple vials for a similar dose.
Idelvion is a fusion protein linking recombinant coagulation factor IX with recombinant albumin, and it is approved by the U.S. Food and Drug Administration to treat children and adults with hemophilia B.
Idelvion can be used as routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for on-demand control and prevention of bleeding episodes, and for the perioperative management of bleeding.
Idelvion is approved for up to 14-day dosing in appropriate patients.
For more details on Idelvion, see the prescribing information.