User login
Paper-based diagnostic device is like ‘portable lab’
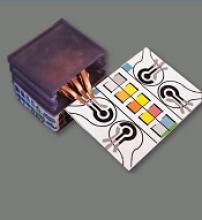
Researchers say they have developed self-powered, paper-based electrochemical devices (SPEDs) that can provide sensitive diagnostics in low-resource settings and at the point of care.
The SPEDs can detect biomarkers in the blood and identify conditions such as anemia by performing electrochemical analyses that are powered by the user’s touch.
The devices produce color-coded test results that are easy for non-experts to understand.
“You could consider this a portable laboratory that is just completely made out of paper, is inexpensive, and can be disposed of through incineration,” said Ramses V. Martinez, PhD, of Purdue University in West Lafayette, Indiana.
“We hope these devices will serve untrained people located in remote villages or military bases to test for a variety of diseases without requiring any source of electricity, clean water, or additional equipment.”
Dr Martinez and his colleagues developed the SPEDs and described them in a paper published in Advanced Materials Technologies.
SPED testing is initiated by placing a pinprick of blood in a circular feature on the device, which is less than 2-inches square. The SPEDs also contain “self-pipetting test zones” that can be dipped into a sample instead of using a finger-prick test.
The top layer of each SPED is made of untreated cellulose paper with patterned hydrophobic domains that define channels that wick up blood samples for testing. These microfluidic channels allow for assays that change color to indicate specific test results.
The researchers also created a machine-vision diagnostic application to identify and quantify each of these colorimetric tests from a digital image of the SPED, perhaps taken with a cell phone. This provides rapid results for the user and allows for consultation with a remote expert if necessary.
The bottom layer of the SPED is a triboelectric generator (TEG), which generates the electric current necessary to run the diagnostic test by rubbing or pressing it.
An inexpensive, hand-held device called a potentiostat can be plugged into the SPED to automate the diagnostic tests so they can be performed by untrained users. The battery powering the potentiostat can be recharged using the TEG built into the SPEDs.
“To our knowledge, this work reports the first self-powered, paper-based devices capable of performing rapid, accurate, and sensitive electrochemical assays in combination with a low-cost, portable potentiostat that can be recharged using a paper-based TEG,” Dr Martinez said.
SPEDs can perform multiplexed analyses, enabling the detection of various targets for a range of point-of-care testing applications. In addition, the devices are compatible with mass-printing technologies, such as roll-to-roll printing or spray deposition. And the SPEDs can be used to power other electronic devices to facilitate telemedicine applications in resource-limited settings.
Dr Martinez and his colleagues used the SPEDs to detect biomarkers such as glucose, uric acid and L-lactate, ketones, and white blood cells, which indicate factors related to liver and kidney function, malnutrition, and anemia.
The researchers said future versions of the technology will contain several additional layers for more complex assays to detect diseases such as malaria, dengue fever, yellow fever, hepatitis, and HIV. ![]()
Researchers say they have developed self-powered, paper-based electrochemical devices (SPEDs) that can provide sensitive diagnostics in low-resource settings and at the point of care.
The SPEDs can detect biomarkers in the blood and identify conditions such as anemia by performing electrochemical analyses that are powered by the user’s touch.
The devices produce color-coded test results that are easy for non-experts to understand.
“You could consider this a portable laboratory that is just completely made out of paper, is inexpensive, and can be disposed of through incineration,” said Ramses V. Martinez, PhD, of Purdue University in West Lafayette, Indiana.
“We hope these devices will serve untrained people located in remote villages or military bases to test for a variety of diseases without requiring any source of electricity, clean water, or additional equipment.”
Dr Martinez and his colleagues developed the SPEDs and described them in a paper published in Advanced Materials Technologies.
SPED testing is initiated by placing a pinprick of blood in a circular feature on the device, which is less than 2-inches square. The SPEDs also contain “self-pipetting test zones” that can be dipped into a sample instead of using a finger-prick test.
The top layer of each SPED is made of untreated cellulose paper with patterned hydrophobic domains that define channels that wick up blood samples for testing. These microfluidic channels allow for assays that change color to indicate specific test results.
The researchers also created a machine-vision diagnostic application to identify and quantify each of these colorimetric tests from a digital image of the SPED, perhaps taken with a cell phone. This provides rapid results for the user and allows for consultation with a remote expert if necessary.
The bottom layer of the SPED is a triboelectric generator (TEG), which generates the electric current necessary to run the diagnostic test by rubbing or pressing it.
An inexpensive, hand-held device called a potentiostat can be plugged into the SPED to automate the diagnostic tests so they can be performed by untrained users. The battery powering the potentiostat can be recharged using the TEG built into the SPEDs.
“To our knowledge, this work reports the first self-powered, paper-based devices capable of performing rapid, accurate, and sensitive electrochemical assays in combination with a low-cost, portable potentiostat that can be recharged using a paper-based TEG,” Dr Martinez said.
SPEDs can perform multiplexed analyses, enabling the detection of various targets for a range of point-of-care testing applications. In addition, the devices are compatible with mass-printing technologies, such as roll-to-roll printing or spray deposition. And the SPEDs can be used to power other electronic devices to facilitate telemedicine applications in resource-limited settings.
Dr Martinez and his colleagues used the SPEDs to detect biomarkers such as glucose, uric acid and L-lactate, ketones, and white blood cells, which indicate factors related to liver and kidney function, malnutrition, and anemia.
The researchers said future versions of the technology will contain several additional layers for more complex assays to detect diseases such as malaria, dengue fever, yellow fever, hepatitis, and HIV. ![]()
Researchers say they have developed self-powered, paper-based electrochemical devices (SPEDs) that can provide sensitive diagnostics in low-resource settings and at the point of care.
The SPEDs can detect biomarkers in the blood and identify conditions such as anemia by performing electrochemical analyses that are powered by the user’s touch.
The devices produce color-coded test results that are easy for non-experts to understand.
“You could consider this a portable laboratory that is just completely made out of paper, is inexpensive, and can be disposed of through incineration,” said Ramses V. Martinez, PhD, of Purdue University in West Lafayette, Indiana.
“We hope these devices will serve untrained people located in remote villages or military bases to test for a variety of diseases without requiring any source of electricity, clean water, or additional equipment.”
Dr Martinez and his colleagues developed the SPEDs and described them in a paper published in Advanced Materials Technologies.
SPED testing is initiated by placing a pinprick of blood in a circular feature on the device, which is less than 2-inches square. The SPEDs also contain “self-pipetting test zones” that can be dipped into a sample instead of using a finger-prick test.
The top layer of each SPED is made of untreated cellulose paper with patterned hydrophobic domains that define channels that wick up blood samples for testing. These microfluidic channels allow for assays that change color to indicate specific test results.
The researchers also created a machine-vision diagnostic application to identify and quantify each of these colorimetric tests from a digital image of the SPED, perhaps taken with a cell phone. This provides rapid results for the user and allows for consultation with a remote expert if necessary.
The bottom layer of the SPED is a triboelectric generator (TEG), which generates the electric current necessary to run the diagnostic test by rubbing or pressing it.
An inexpensive, hand-held device called a potentiostat can be plugged into the SPED to automate the diagnostic tests so they can be performed by untrained users. The battery powering the potentiostat can be recharged using the TEG built into the SPEDs.
“To our knowledge, this work reports the first self-powered, paper-based devices capable of performing rapid, accurate, and sensitive electrochemical assays in combination with a low-cost, portable potentiostat that can be recharged using a paper-based TEG,” Dr Martinez said.
SPEDs can perform multiplexed analyses, enabling the detection of various targets for a range of point-of-care testing applications. In addition, the devices are compatible with mass-printing technologies, such as roll-to-roll printing or spray deposition. And the SPEDs can be used to power other electronic devices to facilitate telemedicine applications in resource-limited settings.
Dr Martinez and his colleagues used the SPEDs to detect biomarkers such as glucose, uric acid and L-lactate, ketones, and white blood cells, which indicate factors related to liver and kidney function, malnutrition, and anemia.
The researchers said future versions of the technology will contain several additional layers for more complex assays to detect diseases such as malaria, dengue fever, yellow fever, hepatitis, and HIV. ![]()
Study: Reference pricing does reduce prescription costs
Reference pricing effectively encourages patients to spend significantly less on prescription drugs, according to research published in NEJM.
Under the reference pricing strategy, the insurer or employer establishes its maximum contribution toward the price of therapeutically similar drugs, and the patient must pay the remainder out of pocket.
The insurer/employer contribution is based on the price of the lowest-priced drug in the therapeutic class, called the reference drug.
“If the patient chooses a cheap or moderately priced option, the employer’s contribution will cover most of the cost,” said study author James C. Robinson, PhD, of the University of California at Berkeley.
“However, if the patient insists on a particularly high-priced option, he or she will need to make a meaningful payment from personal resources.”
It has been theorized that this policy would encourage patients to save money by selecting cheaper drugs. However, little is known about how the policy has actually influenced patient spending.
The new study showed that reference pricing was associated with significant changes in drug selection and spending for patients covered by employment-based insurance in the US.
Researchers analyzed changes in prescriptions and pricing for 1302 drugs in 78 therapeutic classes in the US, before and after an alliance of private employers began using reference pricing.
The trends were compared to a cohort without reference pricing. The study’s dataset included 1.1 million prescriptions reimbursed from 2010 to 2014.
Implementation of reference pricing was associated with a 7% increase in prescriptions filled for the low-price reference drug within its therapeutic class, a 14% decrease in average price paid, and a 5% increase in consumer cost-sharing.
In the first 18 months after implementation, employers’ spending dropped $1.34 million, and employees’ cost-sharing increased $120,000.
Based on these findings, Dr Robinson and his colleagues concluded that reference pricing may be one instrument for influencing patients’ drug choices and drug prices paid by employers and insurers. The team believes that, in the future, pharmaceutical companies charging “premium prices” may need to demonstrate that their drugs provide “premium performance.”
“There is huge and unjustified variation within and across geographic areas in the prices charged for almost every test and treatment, drug and device, office visit and hospitalization,” Dr Robinson said.
“It’s not a surprise when one considers that most patients are covered by health insurance and, hence, do not shop among competing providers on the basis of price. Some providers look at price-unconscious consumer demand and ask themselves, ‘Why don’t we raise our prices?’”
This research was funded by the US Agency for Healthcare Research and Quality and the Genentech Foundation. ![]()
Reference pricing effectively encourages patients to spend significantly less on prescription drugs, according to research published in NEJM.
Under the reference pricing strategy, the insurer or employer establishes its maximum contribution toward the price of therapeutically similar drugs, and the patient must pay the remainder out of pocket.
The insurer/employer contribution is based on the price of the lowest-priced drug in the therapeutic class, called the reference drug.
“If the patient chooses a cheap or moderately priced option, the employer’s contribution will cover most of the cost,” said study author James C. Robinson, PhD, of the University of California at Berkeley.
“However, if the patient insists on a particularly high-priced option, he or she will need to make a meaningful payment from personal resources.”
It has been theorized that this policy would encourage patients to save money by selecting cheaper drugs. However, little is known about how the policy has actually influenced patient spending.
The new study showed that reference pricing was associated with significant changes in drug selection and spending for patients covered by employment-based insurance in the US.
Researchers analyzed changes in prescriptions and pricing for 1302 drugs in 78 therapeutic classes in the US, before and after an alliance of private employers began using reference pricing.
The trends were compared to a cohort without reference pricing. The study’s dataset included 1.1 million prescriptions reimbursed from 2010 to 2014.
Implementation of reference pricing was associated with a 7% increase in prescriptions filled for the low-price reference drug within its therapeutic class, a 14% decrease in average price paid, and a 5% increase in consumer cost-sharing.
In the first 18 months after implementation, employers’ spending dropped $1.34 million, and employees’ cost-sharing increased $120,000.
Based on these findings, Dr Robinson and his colleagues concluded that reference pricing may be one instrument for influencing patients’ drug choices and drug prices paid by employers and insurers. The team believes that, in the future, pharmaceutical companies charging “premium prices” may need to demonstrate that their drugs provide “premium performance.”
“There is huge and unjustified variation within and across geographic areas in the prices charged for almost every test and treatment, drug and device, office visit and hospitalization,” Dr Robinson said.
“It’s not a surprise when one considers that most patients are covered by health insurance and, hence, do not shop among competing providers on the basis of price. Some providers look at price-unconscious consumer demand and ask themselves, ‘Why don’t we raise our prices?’”
This research was funded by the US Agency for Healthcare Research and Quality and the Genentech Foundation. ![]()
Reference pricing effectively encourages patients to spend significantly less on prescription drugs, according to research published in NEJM.
Under the reference pricing strategy, the insurer or employer establishes its maximum contribution toward the price of therapeutically similar drugs, and the patient must pay the remainder out of pocket.
The insurer/employer contribution is based on the price of the lowest-priced drug in the therapeutic class, called the reference drug.
“If the patient chooses a cheap or moderately priced option, the employer’s contribution will cover most of the cost,” said study author James C. Robinson, PhD, of the University of California at Berkeley.
“However, if the patient insists on a particularly high-priced option, he or she will need to make a meaningful payment from personal resources.”
It has been theorized that this policy would encourage patients to save money by selecting cheaper drugs. However, little is known about how the policy has actually influenced patient spending.
The new study showed that reference pricing was associated with significant changes in drug selection and spending for patients covered by employment-based insurance in the US.
Researchers analyzed changes in prescriptions and pricing for 1302 drugs in 78 therapeutic classes in the US, before and after an alliance of private employers began using reference pricing.
The trends were compared to a cohort without reference pricing. The study’s dataset included 1.1 million prescriptions reimbursed from 2010 to 2014.
Implementation of reference pricing was associated with a 7% increase in prescriptions filled for the low-price reference drug within its therapeutic class, a 14% decrease in average price paid, and a 5% increase in consumer cost-sharing.
In the first 18 months after implementation, employers’ spending dropped $1.34 million, and employees’ cost-sharing increased $120,000.
Based on these findings, Dr Robinson and his colleagues concluded that reference pricing may be one instrument for influencing patients’ drug choices and drug prices paid by employers and insurers. The team believes that, in the future, pharmaceutical companies charging “premium prices” may need to demonstrate that their drugs provide “premium performance.”
“There is huge and unjustified variation within and across geographic areas in the prices charged for almost every test and treatment, drug and device, office visit and hospitalization,” Dr Robinson said.
“It’s not a surprise when one considers that most patients are covered by health insurance and, hence, do not shop among competing providers on the basis of price. Some providers look at price-unconscious consumer demand and ask themselves, ‘Why don’t we raise our prices?’”
This research was funded by the US Agency for Healthcare Research and Quality and the Genentech Foundation. ![]()
Team uses genetic barcodes to track hematopoiesis
New research suggests the classical yet contested “tree” model of hematopoiesis is accurate.
In this model, the tree trunk is composed of hematopoietic stem cells (HSCs), and the branches consist of various progenitor cells that give rise to a number of distinct cell types.
Because previous research raised doubts about this model, investigators set out to track hematopoiesis more effectively than ever before.
The team used a “random generator” to label HSCs with genetic barcodes, and this allowed them to trace which cell types arise from an HSC.
Hans-Reimer Rodewald, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg, and his colleagues described this research in Nature.
“Genetic barcodes have been developed and applied before, but they were based on methods that can also change cellular properties,” Dr Rodewald said. “Our barcodes are different. They can be induced tissue-specifically and directly in the genome of mice–without influencing the animals’ physiological development.”
The basis of the new technology is the Cre/loxP system that is used to rearrange or remove specially labeled DNA segments.
The investigators bred mice whose genomes exhibit the basic elements of the barcode. At a selected site, where no genes are encoded, it contains 9 DNA fragments from a plant called Arabidopsis thaliana. These elements are flanked by 10 genetic cutting sites called IoxP sites.
By administering a pharmacological agent, the matching molecular scissors, called “Cre,” can be activated in the animals’ HSCs. Then, code elements are randomly rearranged or cut out.
“This genetic, random DNA barcode generator can generate up to 1.8 million genetic barcodes, and we can identify the codes that arise only once in an experiment,” said study author Thomas Höfer, PhD, also of DKFZ.
When these specially labeled HSCs divide and mature, the barcodes are preserved.
The investigators performed comprehensive barcode analyses in order to trace an individual blood cell back to the HSC from which it originated.
These analyses revealed 2 large developmental branches “growing” from the HSC “tree trunk.” In one branch, T cells and B cells develop. In the other, red blood cells and other white blood cells, such as granulocytes and monocytes, form.
“Our findings show that the classical model of a hierarchical developmental tree that starts from multipotent stem cells holds true for hematopoiesis,” Dr Rodewald said.
He and his colleagues believe their genetic barcode system can be used for purposes other than studying blood cell development. In the future, it might be used for experimentally tracing the origin of leukemias and other cancers. ![]()
New research suggests the classical yet contested “tree” model of hematopoiesis is accurate.
In this model, the tree trunk is composed of hematopoietic stem cells (HSCs), and the branches consist of various progenitor cells that give rise to a number of distinct cell types.
Because previous research raised doubts about this model, investigators set out to track hematopoiesis more effectively than ever before.
The team used a “random generator” to label HSCs with genetic barcodes, and this allowed them to trace which cell types arise from an HSC.
Hans-Reimer Rodewald, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg, and his colleagues described this research in Nature.
“Genetic barcodes have been developed and applied before, but they were based on methods that can also change cellular properties,” Dr Rodewald said. “Our barcodes are different. They can be induced tissue-specifically and directly in the genome of mice–without influencing the animals’ physiological development.”
The basis of the new technology is the Cre/loxP system that is used to rearrange or remove specially labeled DNA segments.
The investigators bred mice whose genomes exhibit the basic elements of the barcode. At a selected site, where no genes are encoded, it contains 9 DNA fragments from a plant called Arabidopsis thaliana. These elements are flanked by 10 genetic cutting sites called IoxP sites.
By administering a pharmacological agent, the matching molecular scissors, called “Cre,” can be activated in the animals’ HSCs. Then, code elements are randomly rearranged or cut out.
“This genetic, random DNA barcode generator can generate up to 1.8 million genetic barcodes, and we can identify the codes that arise only once in an experiment,” said study author Thomas Höfer, PhD, also of DKFZ.
When these specially labeled HSCs divide and mature, the barcodes are preserved.
The investigators performed comprehensive barcode analyses in order to trace an individual blood cell back to the HSC from which it originated.
These analyses revealed 2 large developmental branches “growing” from the HSC “tree trunk.” In one branch, T cells and B cells develop. In the other, red blood cells and other white blood cells, such as granulocytes and monocytes, form.
“Our findings show that the classical model of a hierarchical developmental tree that starts from multipotent stem cells holds true for hematopoiesis,” Dr Rodewald said.
He and his colleagues believe their genetic barcode system can be used for purposes other than studying blood cell development. In the future, it might be used for experimentally tracing the origin of leukemias and other cancers. ![]()
New research suggests the classical yet contested “tree” model of hematopoiesis is accurate.
In this model, the tree trunk is composed of hematopoietic stem cells (HSCs), and the branches consist of various progenitor cells that give rise to a number of distinct cell types.
Because previous research raised doubts about this model, investigators set out to track hematopoiesis more effectively than ever before.
The team used a “random generator” to label HSCs with genetic barcodes, and this allowed them to trace which cell types arise from an HSC.
Hans-Reimer Rodewald, PhD, of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg, and his colleagues described this research in Nature.
“Genetic barcodes have been developed and applied before, but they were based on methods that can also change cellular properties,” Dr Rodewald said. “Our barcodes are different. They can be induced tissue-specifically and directly in the genome of mice–without influencing the animals’ physiological development.”
The basis of the new technology is the Cre/loxP system that is used to rearrange or remove specially labeled DNA segments.
The investigators bred mice whose genomes exhibit the basic elements of the barcode. At a selected site, where no genes are encoded, it contains 9 DNA fragments from a plant called Arabidopsis thaliana. These elements are flanked by 10 genetic cutting sites called IoxP sites.
By administering a pharmacological agent, the matching molecular scissors, called “Cre,” can be activated in the animals’ HSCs. Then, code elements are randomly rearranged or cut out.
“This genetic, random DNA barcode generator can generate up to 1.8 million genetic barcodes, and we can identify the codes that arise only once in an experiment,” said study author Thomas Höfer, PhD, also of DKFZ.
When these specially labeled HSCs divide and mature, the barcodes are preserved.
The investigators performed comprehensive barcode analyses in order to trace an individual blood cell back to the HSC from which it originated.
These analyses revealed 2 large developmental branches “growing” from the HSC “tree trunk.” In one branch, T cells and B cells develop. In the other, red blood cells and other white blood cells, such as granulocytes and monocytes, form.
“Our findings show that the classical model of a hierarchical developmental tree that starts from multipotent stem cells holds true for hematopoiesis,” Dr Rodewald said.
He and his colleagues believe their genetic barcode system can be used for purposes other than studying blood cell development. In the future, it might be used for experimentally tracing the origin of leukemias and other cancers. ![]()
Researchers compare world health authorities
A new study has revealed substantial differences between health authorities in different regions of the world.
A pair of researchers compared 12 different regulatory authorities responsible for approving drugs and medical products.
The researchers collected data* on annual budgets, new drug approvals per year, numbers of reviewers, standard and median review times, fees for new drug applications (NDAs), and other measurements.
The results were published in Nature Reviews Drug Discovery.
For the 2015 fiscal year, the US Food and Drug Administration (FDA) had the highest budget—$1.19 billion—and India’s Central Drugs Standard Control Organization (CDSCO) had the lowest—$26 million.
In 2016, the FDA again had the highest budget—$1.23 billion—while Health Canada and Switzerland’s SwissMedic had the lowest—$108 million.
In 2016, the European Medicines Agency (EMA) had the highest number of reviewers—4500—and SwissMedic had the lowest—60. (Data from 2015 were not included.)
In 2015, Japan’s Pharmaceuticals and Medical Devices Agency had the highest number of NDA submissions—127—and Health Canada had the lowest—27. Meanwhile, the Chinese FDA had the highest number of new drug approvals—72—and India’s CDSCO had the lowest—17.
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) technically had the most new drug approvals in 2015, at 146, but not all of these were unique, as the number included all decentralized applications, both with the UK as the reference member state and approvals from concerned member states.
In 2016, the EMA had the highest number of NDA submissions—68—and Health Canada had the lowest—25. Singapore’s Health Sciences Authority had the highest number of new drug approvals—72—while the US FDA and India’s CDSCO had the lowest—22.
The shortest standard review period was 210 days. This is the standard for the EMA, the UK’s MHRA, and Russia’s Roszdravnadzor. The regulatory agency with the longest standard review time—900 days—is the Chinese FDA.
The shortest median time to new drug approval in 2015 was 230 days, for the UK’s MHRA. The longest was 834 days, for the Brazilian Health Surveillance Agency.
The highest NDA review fees were those charged by the US FDA—$2.3 million. The lowest were those charged by India’s CDSCO—50,000 Indian rupees or about USD$1000.
The researchers noted that these data suggest products are being evaluated via different processes and according to different standards, which makes it challenging for pharmaceutical companies to develop drugs for simultaneous submission to all regulatory authorities.
Therefore, a harmonization of approval requirements and processes could significantly improve efficiency.
“Patients would profit especially since new drugs would be available faster and at lower prices,” said study author Thomas D. Szucs, MD, PhD, of the University of Basel in Switzerland.
“This suggests that companies and authorities should strengthen their international collaboration and communicate better with each other.” ![]()
*Some data were missing for most of the 12 agencies studied.
A new study has revealed substantial differences between health authorities in different regions of the world.
A pair of researchers compared 12 different regulatory authorities responsible for approving drugs and medical products.
The researchers collected data* on annual budgets, new drug approvals per year, numbers of reviewers, standard and median review times, fees for new drug applications (NDAs), and other measurements.
The results were published in Nature Reviews Drug Discovery.
For the 2015 fiscal year, the US Food and Drug Administration (FDA) had the highest budget—$1.19 billion—and India’s Central Drugs Standard Control Organization (CDSCO) had the lowest—$26 million.
In 2016, the FDA again had the highest budget—$1.23 billion—while Health Canada and Switzerland’s SwissMedic had the lowest—$108 million.
In 2016, the European Medicines Agency (EMA) had the highest number of reviewers—4500—and SwissMedic had the lowest—60. (Data from 2015 were not included.)
In 2015, Japan’s Pharmaceuticals and Medical Devices Agency had the highest number of NDA submissions—127—and Health Canada had the lowest—27. Meanwhile, the Chinese FDA had the highest number of new drug approvals—72—and India’s CDSCO had the lowest—17.
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) technically had the most new drug approvals in 2015, at 146, but not all of these were unique, as the number included all decentralized applications, both with the UK as the reference member state and approvals from concerned member states.
In 2016, the EMA had the highest number of NDA submissions—68—and Health Canada had the lowest—25. Singapore’s Health Sciences Authority had the highest number of new drug approvals—72—while the US FDA and India’s CDSCO had the lowest—22.
The shortest standard review period was 210 days. This is the standard for the EMA, the UK’s MHRA, and Russia’s Roszdravnadzor. The regulatory agency with the longest standard review time—900 days—is the Chinese FDA.
The shortest median time to new drug approval in 2015 was 230 days, for the UK’s MHRA. The longest was 834 days, for the Brazilian Health Surveillance Agency.
The highest NDA review fees were those charged by the US FDA—$2.3 million. The lowest were those charged by India’s CDSCO—50,000 Indian rupees or about USD$1000.
The researchers noted that these data suggest products are being evaluated via different processes and according to different standards, which makes it challenging for pharmaceutical companies to develop drugs for simultaneous submission to all regulatory authorities.
Therefore, a harmonization of approval requirements and processes could significantly improve efficiency.
“Patients would profit especially since new drugs would be available faster and at lower prices,” said study author Thomas D. Szucs, MD, PhD, of the University of Basel in Switzerland.
“This suggests that companies and authorities should strengthen their international collaboration and communicate better with each other.” ![]()
*Some data were missing for most of the 12 agencies studied.
A new study has revealed substantial differences between health authorities in different regions of the world.
A pair of researchers compared 12 different regulatory authorities responsible for approving drugs and medical products.
The researchers collected data* on annual budgets, new drug approvals per year, numbers of reviewers, standard and median review times, fees for new drug applications (NDAs), and other measurements.
The results were published in Nature Reviews Drug Discovery.
For the 2015 fiscal year, the US Food and Drug Administration (FDA) had the highest budget—$1.19 billion—and India’s Central Drugs Standard Control Organization (CDSCO) had the lowest—$26 million.
In 2016, the FDA again had the highest budget—$1.23 billion—while Health Canada and Switzerland’s SwissMedic had the lowest—$108 million.
In 2016, the European Medicines Agency (EMA) had the highest number of reviewers—4500—and SwissMedic had the lowest—60. (Data from 2015 were not included.)
In 2015, Japan’s Pharmaceuticals and Medical Devices Agency had the highest number of NDA submissions—127—and Health Canada had the lowest—27. Meanwhile, the Chinese FDA had the highest number of new drug approvals—72—and India’s CDSCO had the lowest—17.
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) technically had the most new drug approvals in 2015, at 146, but not all of these were unique, as the number included all decentralized applications, both with the UK as the reference member state and approvals from concerned member states.
In 2016, the EMA had the highest number of NDA submissions—68—and Health Canada had the lowest—25. Singapore’s Health Sciences Authority had the highest number of new drug approvals—72—while the US FDA and India’s CDSCO had the lowest—22.
The shortest standard review period was 210 days. This is the standard for the EMA, the UK’s MHRA, and Russia’s Roszdravnadzor. The regulatory agency with the longest standard review time—900 days—is the Chinese FDA.
The shortest median time to new drug approval in 2015 was 230 days, for the UK’s MHRA. The longest was 834 days, for the Brazilian Health Surveillance Agency.
The highest NDA review fees were those charged by the US FDA—$2.3 million. The lowest were those charged by India’s CDSCO—50,000 Indian rupees or about USD$1000.
The researchers noted that these data suggest products are being evaluated via different processes and according to different standards, which makes it challenging for pharmaceutical companies to develop drugs for simultaneous submission to all regulatory authorities.
Therefore, a harmonization of approval requirements and processes could significantly improve efficiency.
“Patients would profit especially since new drugs would be available faster and at lower prices,” said study author Thomas D. Szucs, MD, PhD, of the University of Basel in Switzerland.
“This suggests that companies and authorities should strengthen their international collaboration and communicate better with each other.” ![]()
*Some data were missing for most of the 12 agencies studied.
Test uses nanotechnology to diagnose Zika virus
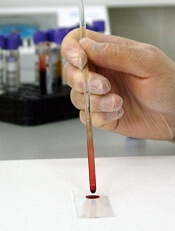
Researchers say they have developed a point-of-care, paper-based test that quickly detects the presence of Zika virus in blood.
Currently, testing for Zika requires that a blood sample be refrigerated and shipped to a medical center or laboratory, delaying diagnosis and possible treatment.
The new test, on the other hand, does not require refrigeration and can produce results in minutes.
“If an assay requires electricity and refrigeration, it defeats the purpose of developing something to use in a resource-limited setting, especially in tropical areas of the world,” said Srikanth Singamaneni, PhD, of Washington University in St. Louis, Missouri.
“We wanted to make the test immune from variations in temperature and humidity.”
Dr Singamaneni and his colleagues described this test in Advanced Biosystems.
The researchers used the test on blood samples from 4 subjects known to be infected with Zika virus and samples from 5 subjects who did not have the virus.
The test showed positive results for the Zika-infected patients and negative results for controls. There were no false-positives.
How the test works
The test uses gold nanorods mounted on paper to detect Zika infection in the blood.
The test relies on a protein made by Zika virus that causes an immune response in infected individuals— ZIKV-nonstructural protein 1 (NS1). The ZIKV-NS1 protein is attached to gold nanorods mounted on a piece of paper.
The paper is then covered with protective nanocrystals. The nanocrystals allow the diagnostic nanorods to be shipped and stored without refrigeration prior to use.
To use the test, a technician rinses the paper with slightly acidic water, removing the protective crystals and exposing the protein mounted on the nanorods.
Then, a drop of the patient’s blood is applied. If the patient has come into contact with the virus, the blood will contain immunoglobulins that react with the ZIKV-NS1 protein.
“We’re taking advantage of the fact that patients mount an immune attack against this viral protein,” said study author Jeremiah J. Morrissey, PhD, of Washington University.
“The immunoglobulins persist in the blood for a few months, and when they come into contact with the gold nanorods, the nanorods undergo a slight color change that can be detected with a hand-held spectrophotometer. With this test, results will be clear before the patient leaves the clinic, allowing immediate counseling and access to treatment.”
The color change cannot be seen with the naked eye, but the researchers are working to change that. They’re also working on developing ways to use saliva rather than blood.
Although the test uses gold, the nanorods are very small. The researchers estimate the cost of the gold used in one of the assays would be 10 to 15 cents. ![]()
Researchers say they have developed a point-of-care, paper-based test that quickly detects the presence of Zika virus in blood.
Currently, testing for Zika requires that a blood sample be refrigerated and shipped to a medical center or laboratory, delaying diagnosis and possible treatment.
The new test, on the other hand, does not require refrigeration and can produce results in minutes.
“If an assay requires electricity and refrigeration, it defeats the purpose of developing something to use in a resource-limited setting, especially in tropical areas of the world,” said Srikanth Singamaneni, PhD, of Washington University in St. Louis, Missouri.
“We wanted to make the test immune from variations in temperature and humidity.”
Dr Singamaneni and his colleagues described this test in Advanced Biosystems.
The researchers used the test on blood samples from 4 subjects known to be infected with Zika virus and samples from 5 subjects who did not have the virus.
The test showed positive results for the Zika-infected patients and negative results for controls. There were no false-positives.
How the test works
The test uses gold nanorods mounted on paper to detect Zika infection in the blood.
The test relies on a protein made by Zika virus that causes an immune response in infected individuals— ZIKV-nonstructural protein 1 (NS1). The ZIKV-NS1 protein is attached to gold nanorods mounted on a piece of paper.
The paper is then covered with protective nanocrystals. The nanocrystals allow the diagnostic nanorods to be shipped and stored without refrigeration prior to use.
To use the test, a technician rinses the paper with slightly acidic water, removing the protective crystals and exposing the protein mounted on the nanorods.
Then, a drop of the patient’s blood is applied. If the patient has come into contact with the virus, the blood will contain immunoglobulins that react with the ZIKV-NS1 protein.
“We’re taking advantage of the fact that patients mount an immune attack against this viral protein,” said study author Jeremiah J. Morrissey, PhD, of Washington University.
“The immunoglobulins persist in the blood for a few months, and when they come into contact with the gold nanorods, the nanorods undergo a slight color change that can be detected with a hand-held spectrophotometer. With this test, results will be clear before the patient leaves the clinic, allowing immediate counseling and access to treatment.”
The color change cannot be seen with the naked eye, but the researchers are working to change that. They’re also working on developing ways to use saliva rather than blood.
Although the test uses gold, the nanorods are very small. The researchers estimate the cost of the gold used in one of the assays would be 10 to 15 cents. ![]()
Researchers say they have developed a point-of-care, paper-based test that quickly detects the presence of Zika virus in blood.
Currently, testing for Zika requires that a blood sample be refrigerated and shipped to a medical center or laboratory, delaying diagnosis and possible treatment.
The new test, on the other hand, does not require refrigeration and can produce results in minutes.
“If an assay requires electricity and refrigeration, it defeats the purpose of developing something to use in a resource-limited setting, especially in tropical areas of the world,” said Srikanth Singamaneni, PhD, of Washington University in St. Louis, Missouri.
“We wanted to make the test immune from variations in temperature and humidity.”
Dr Singamaneni and his colleagues described this test in Advanced Biosystems.
The researchers used the test on blood samples from 4 subjects known to be infected with Zika virus and samples from 5 subjects who did not have the virus.
The test showed positive results for the Zika-infected patients and negative results for controls. There were no false-positives.
How the test works
The test uses gold nanorods mounted on paper to detect Zika infection in the blood.
The test relies on a protein made by Zika virus that causes an immune response in infected individuals— ZIKV-nonstructural protein 1 (NS1). The ZIKV-NS1 protein is attached to gold nanorods mounted on a piece of paper.
The paper is then covered with protective nanocrystals. The nanocrystals allow the diagnostic nanorods to be shipped and stored without refrigeration prior to use.
To use the test, a technician rinses the paper with slightly acidic water, removing the protective crystals and exposing the protein mounted on the nanorods.
Then, a drop of the patient’s blood is applied. If the patient has come into contact with the virus, the blood will contain immunoglobulins that react with the ZIKV-NS1 protein.
“We’re taking advantage of the fact that patients mount an immune attack against this viral protein,” said study author Jeremiah J. Morrissey, PhD, of Washington University.
“The immunoglobulins persist in the blood for a few months, and when they come into contact with the gold nanorods, the nanorods undergo a slight color change that can be detected with a hand-held spectrophotometer. With this test, results will be clear before the patient leaves the clinic, allowing immediate counseling and access to treatment.”
The color change cannot be seen with the naked eye, but the researchers are working to change that. They’re also working on developing ways to use saliva rather than blood.
Although the test uses gold, the nanorods are very small. The researchers estimate the cost of the gold used in one of the assays would be 10 to 15 cents. ![]()
Study reveals elevated cancer risk in Holocaust survivors
A new study indicates that survivors of the Holocaust have experienced a small but consistent increase in the risk of developing cancer.
The findings, published in the journal Cancer, offer an example of how extreme population-level tragedies can have an impact on health.
Holocaust survivors were exposed to a variety of factors that have been linked with cancer.
So researchers set out to investigate whether the starvation, overcrowding, infectious diseases, and psychological stress that survivors endured might have contributed to the development of cancer in some individuals.
The team studied 152,622 Holocaust survivors who were followed for more than 45 years.
The researchers used 2 definitions of exposure to classify the survivors.
One definition was based on an individual’s entitlement for compensation for suffering persecution during the war. The other was based on the country of origin, dividing countries into those that were directly governed by Nazi Germany and those that were not occupied by Nazis.
The cancer incidence was significantly higher in survivors who were granted compensation than in those who were not—21.9% and 16.1%, respectively (P<0.0001).
However, the difference between survivors from occupied and non-occupied countries was not significant—22.7% and 21.4%, respectively.
On the other hand, when the researchers adjusted for confounding factors, survivors who had been exposed by either definition had a significantly increased risk of cancer.
For survivors who were granted compensation, the hazard ratio (HR) was 1.06 (P<0.001). For survivors born in occupied countries, the HR was 1.08 (P<0.001).
There was no increased risk of acute or chronic leukemia among patients who received compensation. And there was no increased risk of acute leukemia for survivors born in occupied countries.
However, there was a significantly increased risk of chronic leukemia among survivors born in occupied countries (HR=1.33, P=0.001)
“The data emphasize the importance of learning about the combined effect of several exposures occurring intensely and contemporaneously on cancer risk, such as those that unfortunately occurred during World War II,” said study author Siegal Sadetzki, MD, of the Chaim Sheba Medical Center in Tel HaShomer, Israel.
An editorial related to this study noted that the association between cancer and the extreme deprivation experienced by Holocaust survivors may also have parallels with other extreme population-level events, including in racial/ethnic minority groups experiencing severe social deprivation over time. ![]()
A new study indicates that survivors of the Holocaust have experienced a small but consistent increase in the risk of developing cancer.
The findings, published in the journal Cancer, offer an example of how extreme population-level tragedies can have an impact on health.
Holocaust survivors were exposed to a variety of factors that have been linked with cancer.
So researchers set out to investigate whether the starvation, overcrowding, infectious diseases, and psychological stress that survivors endured might have contributed to the development of cancer in some individuals.
The team studied 152,622 Holocaust survivors who were followed for more than 45 years.
The researchers used 2 definitions of exposure to classify the survivors.
One definition was based on an individual’s entitlement for compensation for suffering persecution during the war. The other was based on the country of origin, dividing countries into those that were directly governed by Nazi Germany and those that were not occupied by Nazis.
The cancer incidence was significantly higher in survivors who were granted compensation than in those who were not—21.9% and 16.1%, respectively (P<0.0001).
However, the difference between survivors from occupied and non-occupied countries was not significant—22.7% and 21.4%, respectively.
On the other hand, when the researchers adjusted for confounding factors, survivors who had been exposed by either definition had a significantly increased risk of cancer.
For survivors who were granted compensation, the hazard ratio (HR) was 1.06 (P<0.001). For survivors born in occupied countries, the HR was 1.08 (P<0.001).
There was no increased risk of acute or chronic leukemia among patients who received compensation. And there was no increased risk of acute leukemia for survivors born in occupied countries.
However, there was a significantly increased risk of chronic leukemia among survivors born in occupied countries (HR=1.33, P=0.001)
“The data emphasize the importance of learning about the combined effect of several exposures occurring intensely and contemporaneously on cancer risk, such as those that unfortunately occurred during World War II,” said study author Siegal Sadetzki, MD, of the Chaim Sheba Medical Center in Tel HaShomer, Israel.
An editorial related to this study noted that the association between cancer and the extreme deprivation experienced by Holocaust survivors may also have parallels with other extreme population-level events, including in racial/ethnic minority groups experiencing severe social deprivation over time. ![]()
A new study indicates that survivors of the Holocaust have experienced a small but consistent increase in the risk of developing cancer.
The findings, published in the journal Cancer, offer an example of how extreme population-level tragedies can have an impact on health.
Holocaust survivors were exposed to a variety of factors that have been linked with cancer.
So researchers set out to investigate whether the starvation, overcrowding, infectious diseases, and psychological stress that survivors endured might have contributed to the development of cancer in some individuals.
The team studied 152,622 Holocaust survivors who were followed for more than 45 years.
The researchers used 2 definitions of exposure to classify the survivors.
One definition was based on an individual’s entitlement for compensation for suffering persecution during the war. The other was based on the country of origin, dividing countries into those that were directly governed by Nazi Germany and those that were not occupied by Nazis.
The cancer incidence was significantly higher in survivors who were granted compensation than in those who were not—21.9% and 16.1%, respectively (P<0.0001).
However, the difference between survivors from occupied and non-occupied countries was not significant—22.7% and 21.4%, respectively.
On the other hand, when the researchers adjusted for confounding factors, survivors who had been exposed by either definition had a significantly increased risk of cancer.
For survivors who were granted compensation, the hazard ratio (HR) was 1.06 (P<0.001). For survivors born in occupied countries, the HR was 1.08 (P<0.001).
There was no increased risk of acute or chronic leukemia among patients who received compensation. And there was no increased risk of acute leukemia for survivors born in occupied countries.
However, there was a significantly increased risk of chronic leukemia among survivors born in occupied countries (HR=1.33, P=0.001)
“The data emphasize the importance of learning about the combined effect of several exposures occurring intensely and contemporaneously on cancer risk, such as those that unfortunately occurred during World War II,” said study author Siegal Sadetzki, MD, of the Chaim Sheba Medical Center in Tel HaShomer, Israel.
An editorial related to this study noted that the association between cancer and the extreme deprivation experienced by Holocaust survivors may also have parallels with other extreme population-level events, including in racial/ethnic minority groups experiencing severe social deprivation over time. ![]()
FDA provides tool for evaluating Zika tests
The US Food and Drug Administration (FDA) has made available a panel of human plasma samples that can be used to evaluate serological tests for detecting Zika virus infection.
The panel consists of plasma samples from anonymous individuals infected with Zika, West Nile, or dengue viruses.
Diagnostic developers can use the panel to assess whether their tests can help distinguish recent Zika virus infection from infection with West Nile or dengue.
The panel is available to developers who have interacted with the FDA and are seeking Emergency Use Authorization (EUA) for devices that are in the final stages of validation.
Other developers interested in requesting a panel may contact the FDA at [email protected].
The agency said the panel can also be used to compare the performance of Zika virus tests that are already available under an EUA.
To date, the FDA has granted EUAs to 3 serological tests for detection of recent Zika virus infection: Zika MAC-ELISA, ZIKV Detect IgM Capture ELISA, and LIAISON XL Zika Capture IgM Assay.
“At the onset of the Zika virus outbreak, when little was known about the disease or how to diagnose it, the FDA worked quickly with manufacturers to encourage the development of diagnostic tests and ensure they were available using its Emergency Use Authorization authorities,” said FDA Commissioner Scott Gottlieb, MD.
“By providing manufacturers of these tests with standardized patient samples to use in properly validating these diagnostics, we will be able to better assess how well their tests perform. This is part of our effort to ultimately bring these tests through the FDA’s formal review process to better ensure their reliability, and to enable broader access to Zika diagnostic testing.”
The FDA panel was prepared using samples from Zika virus-infected individuals provided by Blood Systems Research Institute. The samples from individuals infected with dengue and West Nile virus were obtained separately. ![]()
The US Food and Drug Administration (FDA) has made available a panel of human plasma samples that can be used to evaluate serological tests for detecting Zika virus infection.
The panel consists of plasma samples from anonymous individuals infected with Zika, West Nile, or dengue viruses.
Diagnostic developers can use the panel to assess whether their tests can help distinguish recent Zika virus infection from infection with West Nile or dengue.
The panel is available to developers who have interacted with the FDA and are seeking Emergency Use Authorization (EUA) for devices that are in the final stages of validation.
Other developers interested in requesting a panel may contact the FDA at [email protected].
The agency said the panel can also be used to compare the performance of Zika virus tests that are already available under an EUA.
To date, the FDA has granted EUAs to 3 serological tests for detection of recent Zika virus infection: Zika MAC-ELISA, ZIKV Detect IgM Capture ELISA, and LIAISON XL Zika Capture IgM Assay.
“At the onset of the Zika virus outbreak, when little was known about the disease or how to diagnose it, the FDA worked quickly with manufacturers to encourage the development of diagnostic tests and ensure they were available using its Emergency Use Authorization authorities,” said FDA Commissioner Scott Gottlieb, MD.
“By providing manufacturers of these tests with standardized patient samples to use in properly validating these diagnostics, we will be able to better assess how well their tests perform. This is part of our effort to ultimately bring these tests through the FDA’s formal review process to better ensure their reliability, and to enable broader access to Zika diagnostic testing.”
The FDA panel was prepared using samples from Zika virus-infected individuals provided by Blood Systems Research Institute. The samples from individuals infected with dengue and West Nile virus were obtained separately. ![]()
The US Food and Drug Administration (FDA) has made available a panel of human plasma samples that can be used to evaluate serological tests for detecting Zika virus infection.
The panel consists of plasma samples from anonymous individuals infected with Zika, West Nile, or dengue viruses.
Diagnostic developers can use the panel to assess whether their tests can help distinguish recent Zika virus infection from infection with West Nile or dengue.
The panel is available to developers who have interacted with the FDA and are seeking Emergency Use Authorization (EUA) for devices that are in the final stages of validation.
Other developers interested in requesting a panel may contact the FDA at [email protected].
The agency said the panel can also be used to compare the performance of Zika virus tests that are already available under an EUA.
To date, the FDA has granted EUAs to 3 serological tests for detection of recent Zika virus infection: Zika MAC-ELISA, ZIKV Detect IgM Capture ELISA, and LIAISON XL Zika Capture IgM Assay.
“At the onset of the Zika virus outbreak, when little was known about the disease or how to diagnose it, the FDA worked quickly with manufacturers to encourage the development of diagnostic tests and ensure they were available using its Emergency Use Authorization authorities,” said FDA Commissioner Scott Gottlieb, MD.
“By providing manufacturers of these tests with standardized patient samples to use in properly validating these diagnostics, we will be able to better assess how well their tests perform. This is part of our effort to ultimately bring these tests through the FDA’s formal review process to better ensure their reliability, and to enable broader access to Zika diagnostic testing.”
The FDA panel was prepared using samples from Zika virus-infected individuals provided by Blood Systems Research Institute. The samples from individuals infected with dengue and West Nile virus were obtained separately.
FDA allows emergency use of multiplex Zika test
The US Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the CII-ArboViroPlex rRT-PCR Test.
It is the first multiplex assay that simultaneously tests for the presence of Zika virus, all serotypes of dengue virus, chikungunya virus, and West Nile virus, as well as a host gene that ensures the accuracy of results.
The EUA does not mean the CII-ArboViroPlex rRT-PCR Test is FDA cleared or approved.
An EUA allows for the use of unapproved medical products in an emergency. The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
The EUA for the CII-ArboViroPlex rRT-PCR Test means the test is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
About the test
The CII-ArboViroPlex rRT-PCR Test is an assay that detects viral RNA matching Zika virus (ZIKV), dengue virus types 1-4 (DENV), chikungunya virus (CHIKV), and West Nile virus (WNV) with a human housekeeping gene, viral RNA controls, and extraction controls that ensure the integrity of the test from nucleic extraction to the final result.
Named for the 4 arboviruses it targets and the real-time reverse transcription polymerase chain reaction (rRT-PCR) technique it employs, the test can simultaneously detect ZIKV, DENV, CHIKV, and WNV in up to 88 samples of blood in less than 2 hours and ZIKV in urine (collected alongside a patient-matched serum specimen).
The CII-ArboViroPlex rRT-PCR Test was developed by scientists at the Center for Infection and Immunity (CII) at Columbia University’s Mailman School of Public Health in New York, New York. The manufacture of the test will be overseen by CII.
The CII-ArboViroPlex rRT-PCR Test is authorized to be used by laboratories in the US that are certified under the Clinical Laboratory Improvement Amendments of 1988, 42 U.S.C. § 263a, to perform high complexity tests, or by similarly qualified non-US laboratories.
The CII-ArboViroPlex rRT-PCR Test is authorized to be performed with the NucliSENS® easyMag® automated extraction platform (bioMérieux), the RNA UltraSense™ One-Step Quantitative RT-PCR System (Thermo Fisher), and CFX96 Real-Time PCR Detection System (Bio-Rad).
“The ArboViroPlex Test provides an easy and efficient means to simultaneously detect Zika and 3 other mosquito-borne viral infections that may present with similar clinical features,” said Nischay Mishra, of CII.
“The FDA decision to issue the EUA gives clinicians and researchers a powerful tool to diagnose and prevent the spread of Zika,” added W. Ian Lipkin, director of CII.
More information on the CII-ArboViroPlex rRT-PCR Test Virus Kit and other Zika tests granted EUAs can be found on the FDA’s EUA page.
The US Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the CII-ArboViroPlex rRT-PCR Test.
It is the first multiplex assay that simultaneously tests for the presence of Zika virus, all serotypes of dengue virus, chikungunya virus, and West Nile virus, as well as a host gene that ensures the accuracy of results.
The EUA does not mean the CII-ArboViroPlex rRT-PCR Test is FDA cleared or approved.
An EUA allows for the use of unapproved medical products in an emergency. The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
The EUA for the CII-ArboViroPlex rRT-PCR Test means the test is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
About the test
The CII-ArboViroPlex rRT-PCR Test is an assay that detects viral RNA matching Zika virus (ZIKV), dengue virus types 1-4 (DENV), chikungunya virus (CHIKV), and West Nile virus (WNV) with a human housekeeping gene, viral RNA controls, and extraction controls that ensure the integrity of the test from nucleic extraction to the final result.
Named for the 4 arboviruses it targets and the real-time reverse transcription polymerase chain reaction (rRT-PCR) technique it employs, the test can simultaneously detect ZIKV, DENV, CHIKV, and WNV in up to 88 samples of blood in less than 2 hours and ZIKV in urine (collected alongside a patient-matched serum specimen).
The CII-ArboViroPlex rRT-PCR Test was developed by scientists at the Center for Infection and Immunity (CII) at Columbia University’s Mailman School of Public Health in New York, New York. The manufacture of the test will be overseen by CII.
The CII-ArboViroPlex rRT-PCR Test is authorized to be used by laboratories in the US that are certified under the Clinical Laboratory Improvement Amendments of 1988, 42 U.S.C. § 263a, to perform high complexity tests, or by similarly qualified non-US laboratories.
The CII-ArboViroPlex rRT-PCR Test is authorized to be performed with the NucliSENS® easyMag® automated extraction platform (bioMérieux), the RNA UltraSense™ One-Step Quantitative RT-PCR System (Thermo Fisher), and CFX96 Real-Time PCR Detection System (Bio-Rad).
“The ArboViroPlex Test provides an easy and efficient means to simultaneously detect Zika and 3 other mosquito-borne viral infections that may present with similar clinical features,” said Nischay Mishra, of CII.
“The FDA decision to issue the EUA gives clinicians and researchers a powerful tool to diagnose and prevent the spread of Zika,” added W. Ian Lipkin, director of CII.
More information on the CII-ArboViroPlex rRT-PCR Test Virus Kit and other Zika tests granted EUAs can be found on the FDA’s EUA page.
The US Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the CII-ArboViroPlex rRT-PCR Test.
It is the first multiplex assay that simultaneously tests for the presence of Zika virus, all serotypes of dengue virus, chikungunya virus, and West Nile virus, as well as a host gene that ensures the accuracy of results.
The EUA does not mean the CII-ArboViroPlex rRT-PCR Test is FDA cleared or approved.
An EUA allows for the use of unapproved medical products in an emergency. The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
The EUA for the CII-ArboViroPlex rRT-PCR Test means the test is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
About the test
The CII-ArboViroPlex rRT-PCR Test is an assay that detects viral RNA matching Zika virus (ZIKV), dengue virus types 1-4 (DENV), chikungunya virus (CHIKV), and West Nile virus (WNV) with a human housekeeping gene, viral RNA controls, and extraction controls that ensure the integrity of the test from nucleic extraction to the final result.
Named for the 4 arboviruses it targets and the real-time reverse transcription polymerase chain reaction (rRT-PCR) technique it employs, the test can simultaneously detect ZIKV, DENV, CHIKV, and WNV in up to 88 samples of blood in less than 2 hours and ZIKV in urine (collected alongside a patient-matched serum specimen).
The CII-ArboViroPlex rRT-PCR Test was developed by scientists at the Center for Infection and Immunity (CII) at Columbia University’s Mailman School of Public Health in New York, New York. The manufacture of the test will be overseen by CII.
The CII-ArboViroPlex rRT-PCR Test is authorized to be used by laboratories in the US that are certified under the Clinical Laboratory Improvement Amendments of 1988, 42 U.S.C. § 263a, to perform high complexity tests, or by similarly qualified non-US laboratories.
The CII-ArboViroPlex rRT-PCR Test is authorized to be performed with the NucliSENS® easyMag® automated extraction platform (bioMérieux), the RNA UltraSense™ One-Step Quantitative RT-PCR System (Thermo Fisher), and CFX96 Real-Time PCR Detection System (Bio-Rad).
“The ArboViroPlex Test provides an easy and efficient means to simultaneously detect Zika and 3 other mosquito-borne viral infections that may present with similar clinical features,” said Nischay Mishra, of CII.
“The FDA decision to issue the EUA gives clinicians and researchers a powerful tool to diagnose and prevent the spread of Zika,” added W. Ian Lipkin, director of CII.
More information on the CII-ArboViroPlex rRT-PCR Test Virus Kit and other Zika tests granted EUAs can be found on the FDA’s EUA page.
Researchers find higher opioid use among cancer survivors
A study of residents in Ontario, Canada, showed that opioid prescription use was more common in cancer survivors than in individuals without a history of cancer.
This was true even among survivors who were 10 or more years past their cancer diagnosis.
Rinku Sutradhar, PhD, of the University of Toronto in Ontario, Canada, and her colleagues reported these findings in Cancer.
The researchers said little is known about prescribing opioids to relieve pain in individuals who have survived cancer.
To investigate, the team looked at opioid prescribing among residents of Ontario, Canada, with and without a history of cancer.
The study included 8601 adults who were at least 5 years past a cancer diagnosis. These subjects were were matched with 8601 individuals without a prior cancer diagnosis. The subjects were matched based on sex and calendar year of birth.
The researchers looked for opioid prescriptions filled at a pharmacy during the observation period. Follow-up was stopped at any indication of cancer recurrence, second malignancy, or new cancer diagnosis.
The rate of opioid prescribing was 1.22 times higher among cancer survivors than corresponding matched controls.
Over a 36-month period, the average number of opioid prescriptions filled by cancer survivors was 7.7, compared with 6.3 for controls.
This increased rate of opioid prescribing was also seen among survivors who were 10 or more years past their cancer diagnosis.
Individuals with lower income and those who were younger, from rural neighborhoods, and with more comorbidities had significantly higher prescribing rates. Sex was not associated with prescribing rates.
“Our research findings raise concerns about the diagnosis and management of chronic pain problems among survivors stemming from their cancer diagnosis or treatment,” Dr Sutradhar said. “Physicians providing primary care to cancer survivors should consider close examination of reasons for continued opioid use to differentiate chronic pain from dependency.”
A study of residents in Ontario, Canada, showed that opioid prescription use was more common in cancer survivors than in individuals without a history of cancer.
This was true even among survivors who were 10 or more years past their cancer diagnosis.
Rinku Sutradhar, PhD, of the University of Toronto in Ontario, Canada, and her colleagues reported these findings in Cancer.
The researchers said little is known about prescribing opioids to relieve pain in individuals who have survived cancer.
To investigate, the team looked at opioid prescribing among residents of Ontario, Canada, with and without a history of cancer.
The study included 8601 adults who were at least 5 years past a cancer diagnosis. These subjects were were matched with 8601 individuals without a prior cancer diagnosis. The subjects were matched based on sex and calendar year of birth.
The researchers looked for opioid prescriptions filled at a pharmacy during the observation period. Follow-up was stopped at any indication of cancer recurrence, second malignancy, or new cancer diagnosis.
The rate of opioid prescribing was 1.22 times higher among cancer survivors than corresponding matched controls.
Over a 36-month period, the average number of opioid prescriptions filled by cancer survivors was 7.7, compared with 6.3 for controls.
This increased rate of opioid prescribing was also seen among survivors who were 10 or more years past their cancer diagnosis.
Individuals with lower income and those who were younger, from rural neighborhoods, and with more comorbidities had significantly higher prescribing rates. Sex was not associated with prescribing rates.
“Our research findings raise concerns about the diagnosis and management of chronic pain problems among survivors stemming from their cancer diagnosis or treatment,” Dr Sutradhar said. “Physicians providing primary care to cancer survivors should consider close examination of reasons for continued opioid use to differentiate chronic pain from dependency.”
A study of residents in Ontario, Canada, showed that opioid prescription use was more common in cancer survivors than in individuals without a history of cancer.
This was true even among survivors who were 10 or more years past their cancer diagnosis.
Rinku Sutradhar, PhD, of the University of Toronto in Ontario, Canada, and her colleagues reported these findings in Cancer.
The researchers said little is known about prescribing opioids to relieve pain in individuals who have survived cancer.
To investigate, the team looked at opioid prescribing among residents of Ontario, Canada, with and without a history of cancer.
The study included 8601 adults who were at least 5 years past a cancer diagnosis. These subjects were were matched with 8601 individuals without a prior cancer diagnosis. The subjects were matched based on sex and calendar year of birth.
The researchers looked for opioid prescriptions filled at a pharmacy during the observation period. Follow-up was stopped at any indication of cancer recurrence, second malignancy, or new cancer diagnosis.
The rate of opioid prescribing was 1.22 times higher among cancer survivors than corresponding matched controls.
Over a 36-month period, the average number of opioid prescriptions filled by cancer survivors was 7.7, compared with 6.3 for controls.
This increased rate of opioid prescribing was also seen among survivors who were 10 or more years past their cancer diagnosis.
Individuals with lower income and those who were younger, from rural neighborhoods, and with more comorbidities had significantly higher prescribing rates. Sex was not associated with prescribing rates.
“Our research findings raise concerns about the diagnosis and management of chronic pain problems among survivors stemming from their cancer diagnosis or treatment,” Dr Sutradhar said. “Physicians providing primary care to cancer survivors should consider close examination of reasons for continued opioid use to differentiate chronic pain from dependency.”
Insured cancer patients report ‘overwhelming’ financial distress
A study of 300 US cancer patients showed that paying for care can cause “overwhelming” financial distress, even when patients have health insurance.
Sixteen percent of the patients studied reported “high or overwhelming” financial distress, spending a median of 31% of their monthly household income on healthcare, not including insurance premiums.
They had a median monthly out-of-pocket cost of $728 (range, $6 to $47,250).
Fumiko Chino, MD, of Duke University Medical Center in Durham, North Carolina, and her colleagues reported these findings in a letter to JAMA Oncology.
The researchers interviewed 300 insured cancer patients for this study. They had a median age of 59.6, and 68.3% were married.
Fifty-six percent of patients had private insurance, 35.7% had Medicare, and 7.3% had Medicaid.
Annual household incomes were as follows:
- 45.7%, $60,000 or greater
- 15.7%, $40,000 to $59,999
- 17.7%, $20,000 to 39,999
- 13.7%, lower than $20,000
- 7.3%, unknown.
The median monthly out-of-pocket cost for care was $592 (range, $3-$47,250), not including insurance premiums. The median relative cost of care was 11% of a patient’s monthly household income.
“Those who spend more than 10% of their income on healthcare costs are considered underinsured,” Dr Chino said. “Learning about the cost-sharing burden on some insured patients is important right now, given the uncertainty in health insurance.”
Most of the patients studied (83.7%, n=251) reported no, low, or average financial distress. Their median relative cost of care was 10% of their monthly household income, and their median monthly out-of-pocket cost was $565 (range, $3 to $26,756). Six percent of these patients had Medicaid, 39% had Medicare, and 53.8% had private insurance.
For the 16.3% of patients (n=49) who reported high or overwhelming financial distress, 67.3% had private insurance, 18.4% had Medicare, and 14.3% had Medicaid. As stated above, their median relative cost of care was 31% of their monthly household income, and their median monthly out-of-pocket cost was $728 (range, $6 to $47,250).
“This study adds to the growing evidence that we need to intervene,” said study author Yousuf Zafar, MD, of Duke Cancer Institute.
“We know there are a lot of barriers that prevent patients from talking about cost with their providers. We need to create tools for patients at risk of financial toxicity and connect them with resources in a timely fashion so they can afford their care.”
A study of 300 US cancer patients showed that paying for care can cause “overwhelming” financial distress, even when patients have health insurance.
Sixteen percent of the patients studied reported “high or overwhelming” financial distress, spending a median of 31% of their monthly household income on healthcare, not including insurance premiums.
They had a median monthly out-of-pocket cost of $728 (range, $6 to $47,250).
Fumiko Chino, MD, of Duke University Medical Center in Durham, North Carolina, and her colleagues reported these findings in a letter to JAMA Oncology.
The researchers interviewed 300 insured cancer patients for this study. They had a median age of 59.6, and 68.3% were married.
Fifty-six percent of patients had private insurance, 35.7% had Medicare, and 7.3% had Medicaid.
Annual household incomes were as follows:
- 45.7%, $60,000 or greater
- 15.7%, $40,000 to $59,999
- 17.7%, $20,000 to 39,999
- 13.7%, lower than $20,000
- 7.3%, unknown.
The median monthly out-of-pocket cost for care was $592 (range, $3-$47,250), not including insurance premiums. The median relative cost of care was 11% of a patient’s monthly household income.
“Those who spend more than 10% of their income on healthcare costs are considered underinsured,” Dr Chino said. “Learning about the cost-sharing burden on some insured patients is important right now, given the uncertainty in health insurance.”
Most of the patients studied (83.7%, n=251) reported no, low, or average financial distress. Their median relative cost of care was 10% of their monthly household income, and their median monthly out-of-pocket cost was $565 (range, $3 to $26,756). Six percent of these patients had Medicaid, 39% had Medicare, and 53.8% had private insurance.
For the 16.3% of patients (n=49) who reported high or overwhelming financial distress, 67.3% had private insurance, 18.4% had Medicare, and 14.3% had Medicaid. As stated above, their median relative cost of care was 31% of their monthly household income, and their median monthly out-of-pocket cost was $728 (range, $6 to $47,250).
“This study adds to the growing evidence that we need to intervene,” said study author Yousuf Zafar, MD, of Duke Cancer Institute.
“We know there are a lot of barriers that prevent patients from talking about cost with their providers. We need to create tools for patients at risk of financial toxicity and connect them with resources in a timely fashion so they can afford their care.”
A study of 300 US cancer patients showed that paying for care can cause “overwhelming” financial distress, even when patients have health insurance.
Sixteen percent of the patients studied reported “high or overwhelming” financial distress, spending a median of 31% of their monthly household income on healthcare, not including insurance premiums.
They had a median monthly out-of-pocket cost of $728 (range, $6 to $47,250).
Fumiko Chino, MD, of Duke University Medical Center in Durham, North Carolina, and her colleagues reported these findings in a letter to JAMA Oncology.
The researchers interviewed 300 insured cancer patients for this study. They had a median age of 59.6, and 68.3% were married.
Fifty-six percent of patients had private insurance, 35.7% had Medicare, and 7.3% had Medicaid.
Annual household incomes were as follows:
- 45.7%, $60,000 or greater
- 15.7%, $40,000 to $59,999
- 17.7%, $20,000 to 39,999
- 13.7%, lower than $20,000
- 7.3%, unknown.
The median monthly out-of-pocket cost for care was $592 (range, $3-$47,250), not including insurance premiums. The median relative cost of care was 11% of a patient’s monthly household income.
“Those who spend more than 10% of their income on healthcare costs are considered underinsured,” Dr Chino said. “Learning about the cost-sharing burden on some insured patients is important right now, given the uncertainty in health insurance.”
Most of the patients studied (83.7%, n=251) reported no, low, or average financial distress. Their median relative cost of care was 10% of their monthly household income, and their median monthly out-of-pocket cost was $565 (range, $3 to $26,756). Six percent of these patients had Medicaid, 39% had Medicare, and 53.8% had private insurance.
For the 16.3% of patients (n=49) who reported high or overwhelming financial distress, 67.3% had private insurance, 18.4% had Medicare, and 14.3% had Medicaid. As stated above, their median relative cost of care was 31% of their monthly household income, and their median monthly out-of-pocket cost was $728 (range, $6 to $47,250).
“This study adds to the growing evidence that we need to intervene,” said study author Yousuf Zafar, MD, of Duke Cancer Institute.
“We know there are a lot of barriers that prevent patients from talking about cost with their providers. We need to create tools for patients at risk of financial toxicity and connect them with resources in a timely fashion so they can afford their care.”