User login
Group calls for more investment in radiotherapy

woman for radiotherapy
Photo by Rhoda Baer
VIENNA—Millions of people throughout the world are dying from potentially treatable cancers because of a chronic underinvestment in radiotherapy resources, according to a new report.
The report suggests that expanding access to radiotherapy services will require a sizeable investment upfront, but that investment could bring economic benefits of up to $365 billion in developing countries over the next 20 years.
The report was published in The Lancet Oncology and presented at the 2015 European Cancer Congress.
The report estimates that 204 million fractions of radiotherapy will be needed to treat the 12 million cancer patients worldwide who could benefit from treatment in 2035.
But the cost per fraction is highly cost-effective and low compared to the price of many new cancer drugs, according to the report’s authors.
They estimate that full access to radiotherapy could be achieved for all patients in need in low-and middle income countries (LMIC) by 2035 for $97 billion, with potential health benefits of 27 million life-years saved and economic benefits ranging from $278 billion to $365 billion over the next 20 years.
“There is a widespread misconception that the costs of providing radiotherapy put it beyond the reach of all but the richest countries, [but] nothing could be further from the truth,” said Rifat Atun, MBBS, of Harvard University in Boston, Massachusetts.
“Our work . . . clearly shows that not only can this essential service be deployed safely and high quality treatment delivered in low- and middle-income countries, but that scale-up of radiotherapy capacity is a feasible and highly cost-effective investment.”
The report provides details on access to radiotherapy services across the world, on a country-by-country basis. The authors calculated the costs and benefits of meeting the worldwide shortfall in resources and bridging the gap in access to effective treatment.
Estimates suggest that, at present, about 40% to 60% of cancer patients worldwide have access to radiotherapy. Even in high-income countries like Canada, Australia, and the UK, numbers of radiotherapy facilities, equipment, and trained staff are inadequate.
Access is worst in low-income countries, where as many as 9 out of 10 people cannot access radiotherapy. The problem of access is especially acute in Africa. In most African countries, radiotherapy is virtually non-existent. Forty countries have no radiotherapy facilities at all.
“The time has come to agree and implement immediate actions to tackle the global shortfall in radiotherapy services and the crisis of access to this highly effective treatment,” Dr Atun said.
With that in mind, he and his colleagues called for the following 6 targets to be met.
By 2020:
- 80% of countries to have comprehensive cancer plans that include radiotherapy.
- Each LMIC to create 1 new center for treatment and training.
- 80% of LMICs to include radiotherapy services in their universal health coverage plans.
By 2025:
- A 25% increase in radiotherapy treatment capacity.
- LMICs to train 7500 radiation oncologists, 20,000 radiotherapy radiographers, and 6000 medical physicists.
- $46 billion of upfront investment to be raised to establish radiotherapy infrastructure and training in LMICs (with help from international banks and the private sector).
“The evidence outlined in the [report] reinforces the case for investing in radiotherapy as an essential component of cancer control,” said Mary Gospodarowicz, MD, co-chair of the UICC Global Task Force on Radiotherapy for Cancer Control.
“The building of radiotherapy capacity will require large initial investment. However, the treatment is more cost-effective than chemotherapy and surgery for treating cancer, and the health and economic benefits will be realized in just 10 to 15 years.” ![]()

woman for radiotherapy
Photo by Rhoda Baer
VIENNA—Millions of people throughout the world are dying from potentially treatable cancers because of a chronic underinvestment in radiotherapy resources, according to a new report.
The report suggests that expanding access to radiotherapy services will require a sizeable investment upfront, but that investment could bring economic benefits of up to $365 billion in developing countries over the next 20 years.
The report was published in The Lancet Oncology and presented at the 2015 European Cancer Congress.
The report estimates that 204 million fractions of radiotherapy will be needed to treat the 12 million cancer patients worldwide who could benefit from treatment in 2035.
But the cost per fraction is highly cost-effective and low compared to the price of many new cancer drugs, according to the report’s authors.
They estimate that full access to radiotherapy could be achieved for all patients in need in low-and middle income countries (LMIC) by 2035 for $97 billion, with potential health benefits of 27 million life-years saved and economic benefits ranging from $278 billion to $365 billion over the next 20 years.
“There is a widespread misconception that the costs of providing radiotherapy put it beyond the reach of all but the richest countries, [but] nothing could be further from the truth,” said Rifat Atun, MBBS, of Harvard University in Boston, Massachusetts.
“Our work . . . clearly shows that not only can this essential service be deployed safely and high quality treatment delivered in low- and middle-income countries, but that scale-up of radiotherapy capacity is a feasible and highly cost-effective investment.”
The report provides details on access to radiotherapy services across the world, on a country-by-country basis. The authors calculated the costs and benefits of meeting the worldwide shortfall in resources and bridging the gap in access to effective treatment.
Estimates suggest that, at present, about 40% to 60% of cancer patients worldwide have access to radiotherapy. Even in high-income countries like Canada, Australia, and the UK, numbers of radiotherapy facilities, equipment, and trained staff are inadequate.
Access is worst in low-income countries, where as many as 9 out of 10 people cannot access radiotherapy. The problem of access is especially acute in Africa. In most African countries, radiotherapy is virtually non-existent. Forty countries have no radiotherapy facilities at all.
“The time has come to agree and implement immediate actions to tackle the global shortfall in radiotherapy services and the crisis of access to this highly effective treatment,” Dr Atun said.
With that in mind, he and his colleagues called for the following 6 targets to be met.
By 2020:
- 80% of countries to have comprehensive cancer plans that include radiotherapy.
- Each LMIC to create 1 new center for treatment and training.
- 80% of LMICs to include radiotherapy services in their universal health coverage plans.
By 2025:
- A 25% increase in radiotherapy treatment capacity.
- LMICs to train 7500 radiation oncologists, 20,000 radiotherapy radiographers, and 6000 medical physicists.
- $46 billion of upfront investment to be raised to establish radiotherapy infrastructure and training in LMICs (with help from international banks and the private sector).
“The evidence outlined in the [report] reinforces the case for investing in radiotherapy as an essential component of cancer control,” said Mary Gospodarowicz, MD, co-chair of the UICC Global Task Force on Radiotherapy for Cancer Control.
“The building of radiotherapy capacity will require large initial investment. However, the treatment is more cost-effective than chemotherapy and surgery for treating cancer, and the health and economic benefits will be realized in just 10 to 15 years.” ![]()

woman for radiotherapy
Photo by Rhoda Baer
VIENNA—Millions of people throughout the world are dying from potentially treatable cancers because of a chronic underinvestment in radiotherapy resources, according to a new report.
The report suggests that expanding access to radiotherapy services will require a sizeable investment upfront, but that investment could bring economic benefits of up to $365 billion in developing countries over the next 20 years.
The report was published in The Lancet Oncology and presented at the 2015 European Cancer Congress.
The report estimates that 204 million fractions of radiotherapy will be needed to treat the 12 million cancer patients worldwide who could benefit from treatment in 2035.
But the cost per fraction is highly cost-effective and low compared to the price of many new cancer drugs, according to the report’s authors.
They estimate that full access to radiotherapy could be achieved for all patients in need in low-and middle income countries (LMIC) by 2035 for $97 billion, with potential health benefits of 27 million life-years saved and economic benefits ranging from $278 billion to $365 billion over the next 20 years.
“There is a widespread misconception that the costs of providing radiotherapy put it beyond the reach of all but the richest countries, [but] nothing could be further from the truth,” said Rifat Atun, MBBS, of Harvard University in Boston, Massachusetts.
“Our work . . . clearly shows that not only can this essential service be deployed safely and high quality treatment delivered in low- and middle-income countries, but that scale-up of radiotherapy capacity is a feasible and highly cost-effective investment.”
The report provides details on access to radiotherapy services across the world, on a country-by-country basis. The authors calculated the costs and benefits of meeting the worldwide shortfall in resources and bridging the gap in access to effective treatment.
Estimates suggest that, at present, about 40% to 60% of cancer patients worldwide have access to radiotherapy. Even in high-income countries like Canada, Australia, and the UK, numbers of radiotherapy facilities, equipment, and trained staff are inadequate.
Access is worst in low-income countries, where as many as 9 out of 10 people cannot access radiotherapy. The problem of access is especially acute in Africa. In most African countries, radiotherapy is virtually non-existent. Forty countries have no radiotherapy facilities at all.
“The time has come to agree and implement immediate actions to tackle the global shortfall in radiotherapy services and the crisis of access to this highly effective treatment,” Dr Atun said.
With that in mind, he and his colleagues called for the following 6 targets to be met.
By 2020:
- 80% of countries to have comprehensive cancer plans that include radiotherapy.
- Each LMIC to create 1 new center for treatment and training.
- 80% of LMICs to include radiotherapy services in their universal health coverage plans.
By 2025:
- A 25% increase in radiotherapy treatment capacity.
- LMICs to train 7500 radiation oncologists, 20,000 radiotherapy radiographers, and 6000 medical physicists.
- $46 billion of upfront investment to be raised to establish radiotherapy infrastructure and training in LMICs (with help from international banks and the private sector).
“The evidence outlined in the [report] reinforces the case for investing in radiotherapy as an essential component of cancer control,” said Mary Gospodarowicz, MD, co-chair of the UICC Global Task Force on Radiotherapy for Cancer Control.
“The building of radiotherapy capacity will require large initial investment. However, the treatment is more cost-effective than chemotherapy and surgery for treating cancer, and the health and economic benefits will be realized in just 10 to 15 years.” ![]()
Studies raise concerns about drug approval process

Photo courtesy of the FDA
Two newly published studies have raised concerns about the drug approval process in the US.
One study showed that, over the past two decades, the US Food and Drug Administration (FDA) has significantly increased its use of expedited development or review programs when approving new drugs.
Investigators said this increase cannot be attributed to an increase in the number of innovative new drug classes.
The other study revealed “wide variations” in evidence supporting the approval of supplemental drug applications.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted these studies and reported the results in The BMJ.
Authors of a related editorial wrote that these studies “give cause for concern about whether most new drugs are any more effective than existing products or whether their safety has been adequately assessed.”
Expedited approval
For the first study, the investigators looked at the FDA’s use of expedited development and review programs for drugs newly approved between 1987 and 2014. This included the orphan designation, fast track designation, priority review, and accelerated approval programs.
The FDA approved 774 drugs during the study period, and 33% of these were first-in-class agents. Priority review (43%) was the most-used program, followed by orphan designation (25%), fast track designation (19%), and accelerated approval (9%).
The investigators observed an increase of 2.6% per year in the number of expedited review and approval programs granted to each newly approved drug (P<0.001) and a 2.4% increase in the proportion of drugs associated with at least one of the programs (P=0.009).
The team noted that “this trend is being driven by drugs that are not first in class and thus potentially less innovative.”
They also said that, by the end of the study period, most newly approved drugs were associated with at least one of the programs. The peak was in 2005, when 75% (15/20) of newly approved drugs were associated with at least one program.
Supplemental approval
For the second study, Dr Kesselheim and his colleagues evaluated the quality of evidence underpinning FDA approval of supplemental drug applications (uses beyond a drug’s original indication) between 2005 and 2014.
The team assessed 295 supplemental drug approvals. They found a lack of trials using clinical outcome endpoints, a lack of trials including active comparators, and differences in the evidence according to types of approval.
Thirty percent of drug approvals for new indications were supported by trials with active comparators, as were 51% of modified-use approvals and 11% of approvals expanding the patient population (P<0.001).
Thirty-two percent of drug approvals for new indications were supported by trials using clinical outcome endpoints, as were 30% of modified-use approvals and 22% of expanded-population approvals (P=0.29).
The investigators said these findings “underscore the need for a robust system of post-approval drug monitoring for efficacy and safety, timely confirmatory studies, and re-examination of existing legislative incentives to promote the optimal delivery of evidence-based medicine.” ![]()

Photo courtesy of the FDA
Two newly published studies have raised concerns about the drug approval process in the US.
One study showed that, over the past two decades, the US Food and Drug Administration (FDA) has significantly increased its use of expedited development or review programs when approving new drugs.
Investigators said this increase cannot be attributed to an increase in the number of innovative new drug classes.
The other study revealed “wide variations” in evidence supporting the approval of supplemental drug applications.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted these studies and reported the results in The BMJ.
Authors of a related editorial wrote that these studies “give cause for concern about whether most new drugs are any more effective than existing products or whether their safety has been adequately assessed.”
Expedited approval
For the first study, the investigators looked at the FDA’s use of expedited development and review programs for drugs newly approved between 1987 and 2014. This included the orphan designation, fast track designation, priority review, and accelerated approval programs.
The FDA approved 774 drugs during the study period, and 33% of these were first-in-class agents. Priority review (43%) was the most-used program, followed by orphan designation (25%), fast track designation (19%), and accelerated approval (9%).
The investigators observed an increase of 2.6% per year in the number of expedited review and approval programs granted to each newly approved drug (P<0.001) and a 2.4% increase in the proportion of drugs associated with at least one of the programs (P=0.009).
The team noted that “this trend is being driven by drugs that are not first in class and thus potentially less innovative.”
They also said that, by the end of the study period, most newly approved drugs were associated with at least one of the programs. The peak was in 2005, when 75% (15/20) of newly approved drugs were associated with at least one program.
Supplemental approval
For the second study, Dr Kesselheim and his colleagues evaluated the quality of evidence underpinning FDA approval of supplemental drug applications (uses beyond a drug’s original indication) between 2005 and 2014.
The team assessed 295 supplemental drug approvals. They found a lack of trials using clinical outcome endpoints, a lack of trials including active comparators, and differences in the evidence according to types of approval.
Thirty percent of drug approvals for new indications were supported by trials with active comparators, as were 51% of modified-use approvals and 11% of approvals expanding the patient population (P<0.001).
Thirty-two percent of drug approvals for new indications were supported by trials using clinical outcome endpoints, as were 30% of modified-use approvals and 22% of expanded-population approvals (P=0.29).
The investigators said these findings “underscore the need for a robust system of post-approval drug monitoring for efficacy and safety, timely confirmatory studies, and re-examination of existing legislative incentives to promote the optimal delivery of evidence-based medicine.” ![]()

Photo courtesy of the FDA
Two newly published studies have raised concerns about the drug approval process in the US.
One study showed that, over the past two decades, the US Food and Drug Administration (FDA) has significantly increased its use of expedited development or review programs when approving new drugs.
Investigators said this increase cannot be attributed to an increase in the number of innovative new drug classes.
The other study revealed “wide variations” in evidence supporting the approval of supplemental drug applications.
Aaron S. Kesselheim, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues conducted these studies and reported the results in The BMJ.
Authors of a related editorial wrote that these studies “give cause for concern about whether most new drugs are any more effective than existing products or whether their safety has been adequately assessed.”
Expedited approval
For the first study, the investigators looked at the FDA’s use of expedited development and review programs for drugs newly approved between 1987 and 2014. This included the orphan designation, fast track designation, priority review, and accelerated approval programs.
The FDA approved 774 drugs during the study period, and 33% of these were first-in-class agents. Priority review (43%) was the most-used program, followed by orphan designation (25%), fast track designation (19%), and accelerated approval (9%).
The investigators observed an increase of 2.6% per year in the number of expedited review and approval programs granted to each newly approved drug (P<0.001) and a 2.4% increase in the proportion of drugs associated with at least one of the programs (P=0.009).
The team noted that “this trend is being driven by drugs that are not first in class and thus potentially less innovative.”
They also said that, by the end of the study period, most newly approved drugs were associated with at least one of the programs. The peak was in 2005, when 75% (15/20) of newly approved drugs were associated with at least one program.
Supplemental approval
For the second study, Dr Kesselheim and his colleagues evaluated the quality of evidence underpinning FDA approval of supplemental drug applications (uses beyond a drug’s original indication) between 2005 and 2014.
The team assessed 295 supplemental drug approvals. They found a lack of trials using clinical outcome endpoints, a lack of trials including active comparators, and differences in the evidence according to types of approval.
Thirty percent of drug approvals for new indications were supported by trials with active comparators, as were 51% of modified-use approvals and 11% of approvals expanding the patient population (P<0.001).
Thirty-two percent of drug approvals for new indications were supported by trials using clinical outcome endpoints, as were 30% of modified-use approvals and 22% of expanded-population approvals (P=0.29).
The investigators said these findings “underscore the need for a robust system of post-approval drug monitoring for efficacy and safety, timely confirmatory studies, and re-examination of existing legislative incentives to promote the optimal delivery of evidence-based medicine.” ![]()
Inflammation, coagulation may contribute to CM pathogenesis
Image by Bruce Blaus
Cells associated with inflammation and coagulation accumulate in the brains of children with cerebral malaria (CM), according to research published in mBio.
The researchers studied autopsied brain tissue from more than 100 African children and found that children with CM had a more than 9-fold greater number of intravascular monocytes and platelets than children who did not have malaria.
In addition, HIV-positive children had more than twice the amount of intravascular monocytes and platelets than was observed in children who were not HIV-positive.
“Our study clearly shows that HIV exacerbates the disease process in cerebral malaria and also leads to some really interesting insights into what may be going on with children who are dying of cerebral malaria, which has been very controversial,” said study author Kami Kim, MD, of the Albert Einstein College of Medicine in the Bronx, New York.
“Children who are HIV-positive and at risk for malaria may benefit from targeted antimalaria drugs, and adjunctive therapies that target inflammation or blood clotting may improve outcomes from CM.”
CM is one of the most severe complications of malaria and can lead to behavioral problems, seizures, coma, or death. It is mainly seen in children younger than 5 living in sub-Saharan Africa.
CM is fairly rare, affecting about 2% of children with malaria, but CM is also thought to be responsible for half of malaria deaths.
“So it’s a big deal,” Dr Kim said. “The more we know about CM, the more that we can theoretically do something either to better treat or prevent it.”
With this goal in mind, Dr Kim and her colleagues analyzed children in an ongoing study of pediatric CM in Blantyre, Malawi. The researchers enrolled more than 3000 participants and completed 103 autopsies in those who died from either CM or other causes of coma.
HIV prevalence was higher than expected and led to higher mortality in CM patients. The prevalence of HIV was 14.5% in children enrolled in the study, compared to 2% in the general Malawi pediatric population.
Twenty-three percent of HIV-positive children died, while 17% of those without HIV died. Twenty percent of autopsy cases were HIV-positive.
The researchers noted that HIV-infected children with CM were older than children without HIV (an average of 99 months vs 32 months) and were not severely immunocompromised.
In addition, monocytes and platelets were significantly more prevalent in HIV-positive children with CM than neutrophils.
“We identified a unique and pervasive pathology pattern in pediatric CM, marked by monocytes and platelets, which is more severe in HIV-positive children,” Dr Kim said.
“It doesn’t prove that these cells cause clinical disease, but the fact that they’re there in huge abundance when there’s a lot of parasites is pretty strongly suggestive evidence that they’re doing something. We never see that in healthy brain tissue.”
Additional studies of children with varying severities of malaria are necessary in order to design better treatment algorithms, Dr Kim added. ![]()

Image by Bruce Blaus
Cells associated with inflammation and coagulation accumulate in the brains of children with cerebral malaria (CM), according to research published in mBio.
The researchers studied autopsied brain tissue from more than 100 African children and found that children with CM had a more than 9-fold greater number of intravascular monocytes and platelets than children who did not have malaria.
In addition, HIV-positive children had more than twice the amount of intravascular monocytes and platelets than was observed in children who were not HIV-positive.
“Our study clearly shows that HIV exacerbates the disease process in cerebral malaria and also leads to some really interesting insights into what may be going on with children who are dying of cerebral malaria, which has been very controversial,” said study author Kami Kim, MD, of the Albert Einstein College of Medicine in the Bronx, New York.
“Children who are HIV-positive and at risk for malaria may benefit from targeted antimalaria drugs, and adjunctive therapies that target inflammation or blood clotting may improve outcomes from CM.”
CM is one of the most severe complications of malaria and can lead to behavioral problems, seizures, coma, or death. It is mainly seen in children younger than 5 living in sub-Saharan Africa.
CM is fairly rare, affecting about 2% of children with malaria, but CM is also thought to be responsible for half of malaria deaths.
“So it’s a big deal,” Dr Kim said. “The more we know about CM, the more that we can theoretically do something either to better treat or prevent it.”
With this goal in mind, Dr Kim and her colleagues analyzed children in an ongoing study of pediatric CM in Blantyre, Malawi. The researchers enrolled more than 3000 participants and completed 103 autopsies in those who died from either CM or other causes of coma.
HIV prevalence was higher than expected and led to higher mortality in CM patients. The prevalence of HIV was 14.5% in children enrolled in the study, compared to 2% in the general Malawi pediatric population.
Twenty-three percent of HIV-positive children died, while 17% of those without HIV died. Twenty percent of autopsy cases were HIV-positive.
The researchers noted that HIV-infected children with CM were older than children without HIV (an average of 99 months vs 32 months) and were not severely immunocompromised.
In addition, monocytes and platelets were significantly more prevalent in HIV-positive children with CM than neutrophils.
“We identified a unique and pervasive pathology pattern in pediatric CM, marked by monocytes and platelets, which is more severe in HIV-positive children,” Dr Kim said.
“It doesn’t prove that these cells cause clinical disease, but the fact that they’re there in huge abundance when there’s a lot of parasites is pretty strongly suggestive evidence that they’re doing something. We never see that in healthy brain tissue.”
Additional studies of children with varying severities of malaria are necessary in order to design better treatment algorithms, Dr Kim added. ![]()

Image by Bruce Blaus
Cells associated with inflammation and coagulation accumulate in the brains of children with cerebral malaria (CM), according to research published in mBio.
The researchers studied autopsied brain tissue from more than 100 African children and found that children with CM had a more than 9-fold greater number of intravascular monocytes and platelets than children who did not have malaria.
In addition, HIV-positive children had more than twice the amount of intravascular monocytes and platelets than was observed in children who were not HIV-positive.
“Our study clearly shows that HIV exacerbates the disease process in cerebral malaria and also leads to some really interesting insights into what may be going on with children who are dying of cerebral malaria, which has been very controversial,” said study author Kami Kim, MD, of the Albert Einstein College of Medicine in the Bronx, New York.
“Children who are HIV-positive and at risk for malaria may benefit from targeted antimalaria drugs, and adjunctive therapies that target inflammation or blood clotting may improve outcomes from CM.”
CM is one of the most severe complications of malaria and can lead to behavioral problems, seizures, coma, or death. It is mainly seen in children younger than 5 living in sub-Saharan Africa.
CM is fairly rare, affecting about 2% of children with malaria, but CM is also thought to be responsible for half of malaria deaths.
“So it’s a big deal,” Dr Kim said. “The more we know about CM, the more that we can theoretically do something either to better treat or prevent it.”
With this goal in mind, Dr Kim and her colleagues analyzed children in an ongoing study of pediatric CM in Blantyre, Malawi. The researchers enrolled more than 3000 participants and completed 103 autopsies in those who died from either CM or other causes of coma.
HIV prevalence was higher than expected and led to higher mortality in CM patients. The prevalence of HIV was 14.5% in children enrolled in the study, compared to 2% in the general Malawi pediatric population.
Twenty-three percent of HIV-positive children died, while 17% of those without HIV died. Twenty percent of autopsy cases were HIV-positive.
The researchers noted that HIV-infected children with CM were older than children without HIV (an average of 99 months vs 32 months) and were not severely immunocompromised.
In addition, monocytes and platelets were significantly more prevalent in HIV-positive children with CM than neutrophils.
“We identified a unique and pervasive pathology pattern in pediatric CM, marked by monocytes and platelets, which is more severe in HIV-positive children,” Dr Kim said.
“It doesn’t prove that these cells cause clinical disease, but the fact that they’re there in huge abundance when there’s a lot of parasites is pretty strongly suggestive evidence that they’re doing something. We never see that in healthy brain tissue.”
Additional studies of children with varying severities of malaria are necessary in order to design better treatment algorithms, Dr Kim added. ![]()
Study reveals mechanism of multidrug resistance in malaria

Image by Peter H. Seeberger
Researchers say they have identified a mechanism of multidrug resistance in malaria that represents a major threat to antimalarial drug policy.
The team exposed a strain of malaria parasites to artemisinin (the base compound for standard therapy) long-term and found the parasites developed widespread resistance to most other antimalarial drugs.
The group also discovered that this type of resistance cannot be detected by current assays.
Françoise Benoit-Vical, PhD, of Université de Toulouse in France, and his colleagues described this work in Emerging Infectious Diseases.
The researchers set out to study resistance mechanisms in Plasmodium falciparum parasites. So they exposed the parasites to artemisinin in vitro for 5 years.
Parasites that survived in the presence of artemisinin developed resistance to most other artemisinin-based or non-artemisinin-based antimalarial therapies, including partner molecules present in combination therapies used in endemic areas.
These parasites did not exhibit any known mutation in resistance genes. However, the researchers found the parasites could circumvent the toxic effect of the drugs by quiescence.
The parasites were able to suspend their development during exposure to antimalarial agents. As soon as they were no longer subjected to antimalarial therapy, they “woke up” and began to proliferate again.
The researchers also found that multidrug resistance based on this quiescence phenomenon cannot be detected by tests currently used to analyze parasitic resistance.
“In vitro tests carried out using the patient’s blood predict high sensitivity and, therefore, the treatment’s effectiveness, while parasites are resistant because they are quiescent,” Dr Benoit-Vical said.
“As such, it is essential to conduct research with relevant and appropriate tests in the field if the multi[drug]-resistant phenomenon that we identified in vitro is also present, in order to design therapeutic strategies accordingly.” ![]()

Image by Peter H. Seeberger
Researchers say they have identified a mechanism of multidrug resistance in malaria that represents a major threat to antimalarial drug policy.
The team exposed a strain of malaria parasites to artemisinin (the base compound for standard therapy) long-term and found the parasites developed widespread resistance to most other antimalarial drugs.
The group also discovered that this type of resistance cannot be detected by current assays.
Françoise Benoit-Vical, PhD, of Université de Toulouse in France, and his colleagues described this work in Emerging Infectious Diseases.
The researchers set out to study resistance mechanisms in Plasmodium falciparum parasites. So they exposed the parasites to artemisinin in vitro for 5 years.
Parasites that survived in the presence of artemisinin developed resistance to most other artemisinin-based or non-artemisinin-based antimalarial therapies, including partner molecules present in combination therapies used in endemic areas.
These parasites did not exhibit any known mutation in resistance genes. However, the researchers found the parasites could circumvent the toxic effect of the drugs by quiescence.
The parasites were able to suspend their development during exposure to antimalarial agents. As soon as they were no longer subjected to antimalarial therapy, they “woke up” and began to proliferate again.
The researchers also found that multidrug resistance based on this quiescence phenomenon cannot be detected by tests currently used to analyze parasitic resistance.
“In vitro tests carried out using the patient’s blood predict high sensitivity and, therefore, the treatment’s effectiveness, while parasites are resistant because they are quiescent,” Dr Benoit-Vical said.
“As such, it is essential to conduct research with relevant and appropriate tests in the field if the multi[drug]-resistant phenomenon that we identified in vitro is also present, in order to design therapeutic strategies accordingly.” ![]()

Image by Peter H. Seeberger
Researchers say they have identified a mechanism of multidrug resistance in malaria that represents a major threat to antimalarial drug policy.
The team exposed a strain of malaria parasites to artemisinin (the base compound for standard therapy) long-term and found the parasites developed widespread resistance to most other antimalarial drugs.
The group also discovered that this type of resistance cannot be detected by current assays.
Françoise Benoit-Vical, PhD, of Université de Toulouse in France, and his colleagues described this work in Emerging Infectious Diseases.
The researchers set out to study resistance mechanisms in Plasmodium falciparum parasites. So they exposed the parasites to artemisinin in vitro for 5 years.
Parasites that survived in the presence of artemisinin developed resistance to most other artemisinin-based or non-artemisinin-based antimalarial therapies, including partner molecules present in combination therapies used in endemic areas.
These parasites did not exhibit any known mutation in resistance genes. However, the researchers found the parasites could circumvent the toxic effect of the drugs by quiescence.
The parasites were able to suspend their development during exposure to antimalarial agents. As soon as they were no longer subjected to antimalarial therapy, they “woke up” and began to proliferate again.
The researchers also found that multidrug resistance based on this quiescence phenomenon cannot be detected by tests currently used to analyze parasitic resistance.
“In vitro tests carried out using the patient’s blood predict high sensitivity and, therefore, the treatment’s effectiveness, while parasites are resistant because they are quiescent,” Dr Benoit-Vical said.
“As such, it is essential to conduct research with relevant and appropriate tests in the field if the multi[drug]-resistant phenomenon that we identified in vitro is also present, in order to design therapeutic strategies accordingly.” ![]()
Optimizing investment in US research

Photo by Daniel Sone
A new report suggests a need to change regulations for federally funded research in the US.
According to the report, the current regulatory burden is diminishing the effectiveness of the US scientific enterprise and lowering the return on
the federal investment in research by directing investigators’ time away from research and toward administrative matters.
The report, Optimizing the Nation’s Investment in Academic Research: A New Regulatory Framework for the 21st Century: Part 1 (2015), was
compiled by the National Academies of Sciences, Engineering, and Medicine.
“Federal regulations and reporting requirements, which began as a way to exercise responsible oversight, have increased dramatically in recent
decades and are now unduly encumbering the very research enterprise they were intended to facilitate,” said Larry Faulkner, chair of the committee that conducted the study and wrote the report, and president emeritus of the University of Texas, Austin.
“A significant amount of investigators’ time is now spent complying with regulations, taking valuable time from research, teaching, and scholarship.”
The report lists specific actions the government and research institutions should take to reduce the regulatory burden.
New framework needed
The report says a new framework is needed to approach regulation in a holistic, rather than piecemeal, way. Regulatory requirements should be harmonized across funding agencies, and we need a more effective and efficient partnership between funding agencies and research institutions.
Congress should create a Research Policy Board to serve as a public-private forum for discussions related to regulation of federally funded research programs. The board should be a government-enabled, private-sector entity that will foster more effective conception, development, and synchronization of research policies.
The board should be formally connected to government through a new associate director position at the White House Office of Science and Technology Policy (OSTP) and through the Office of Information and Regulatory Affairs at the White House Office of Management and Budget (OMB).
Strengthening the research partnership also requires that universities demand the highest standards in institutional and individual behavior, foster a culture of integrity, and mete out appropriate sanctions when behavior deviates from ethical and professional norms, the report says.
The Research Policy Board should collaborate with research institutions to develop a policy that holds universities accountable, sanctioning institutions that fail to enforce standards.
The report also recommends a number of specific actions—a sample of which are listed below—that are intended to improve the efficiency of federal regulation and reduce duplication.
Congress should:
- Work with OMB to conduct a review of agency research grant proposal documents for the purpose of developing a uniform format to be used by all funding agencies
- Work with OSTP and research institutions to develop a single financial conflict-of-interest policy to be used by all research funding agencies
- Task a single agency with overseeing and unifying efforts to develop a central database of investigators and their professional output
- Direct agencies to align and harmonize their regulations and definitions concerning the protection of human subjects
- Instruct OSTP to convene representatives from federal agencies that fund animal research and from the research community to assess and report back to Congress on the feasibility and usefulness of a unified federal approach to policies and regulations pertaining to the care and use of research animals.
The White House OMB should:
- Require that research funding agencies use a uniform format for research progress reporting
- Amend the new Uniform Guidance to improve the efficiency and consistency of procurement standards, financial reporting, and cost accounting.
Federal agencies should:
- Limit research proposals to the minimum information necessary to permit peer evaluation of the merit of the scientific questions being asked, the feasibility of answering those questions, and the ability of the investigator to carry out that research. Any supplementary information—internal review board approval, conflict-of-interest disclosures, detailed budgets, etc.—should be provided “just in time,” after the research proposal is deemed likely to be funded
- Reduce and streamline reporting, assurances, and verifications. Agencies should also develop a central repository to house assurances.
Universities should:
- Conduct a review of institutional policies developed to comply with federal regulations of research to determine whether the institution itself has created excessive or unnecessary self-imposed burden
- Revise self-imposed burdensome institutional policies that go beyond those necessary and sufficient to comply with federal, state, and local requirements.
The release of the report completes the first phase of the committee’s study, which was expedited at the request of Congress.
The committee will now continue its assessment and issue a spring 2016 addendum report addressing issues it has been unable to address in the first phase. ![]()

Photo by Daniel Sone
A new report suggests a need to change regulations for federally funded research in the US.
According to the report, the current regulatory burden is diminishing the effectiveness of the US scientific enterprise and lowering the return on
the federal investment in research by directing investigators’ time away from research and toward administrative matters.
The report, Optimizing the Nation’s Investment in Academic Research: A New Regulatory Framework for the 21st Century: Part 1 (2015), was
compiled by the National Academies of Sciences, Engineering, and Medicine.
“Federal regulations and reporting requirements, which began as a way to exercise responsible oversight, have increased dramatically in recent
decades and are now unduly encumbering the very research enterprise they were intended to facilitate,” said Larry Faulkner, chair of the committee that conducted the study and wrote the report, and president emeritus of the University of Texas, Austin.
“A significant amount of investigators’ time is now spent complying with regulations, taking valuable time from research, teaching, and scholarship.”
The report lists specific actions the government and research institutions should take to reduce the regulatory burden.
New framework needed
The report says a new framework is needed to approach regulation in a holistic, rather than piecemeal, way. Regulatory requirements should be harmonized across funding agencies, and we need a more effective and efficient partnership between funding agencies and research institutions.
Congress should create a Research Policy Board to serve as a public-private forum for discussions related to regulation of federally funded research programs. The board should be a government-enabled, private-sector entity that will foster more effective conception, development, and synchronization of research policies.
The board should be formally connected to government through a new associate director position at the White House Office of Science and Technology Policy (OSTP) and through the Office of Information and Regulatory Affairs at the White House Office of Management and Budget (OMB).
Strengthening the research partnership also requires that universities demand the highest standards in institutional and individual behavior, foster a culture of integrity, and mete out appropriate sanctions when behavior deviates from ethical and professional norms, the report says.
The Research Policy Board should collaborate with research institutions to develop a policy that holds universities accountable, sanctioning institutions that fail to enforce standards.
The report also recommends a number of specific actions—a sample of which are listed below—that are intended to improve the efficiency of federal regulation and reduce duplication.
Congress should:
- Work with OMB to conduct a review of agency research grant proposal documents for the purpose of developing a uniform format to be used by all funding agencies
- Work with OSTP and research institutions to develop a single financial conflict-of-interest policy to be used by all research funding agencies
- Task a single agency with overseeing and unifying efforts to develop a central database of investigators and their professional output
- Direct agencies to align and harmonize their regulations and definitions concerning the protection of human subjects
- Instruct OSTP to convene representatives from federal agencies that fund animal research and from the research community to assess and report back to Congress on the feasibility and usefulness of a unified federal approach to policies and regulations pertaining to the care and use of research animals.
The White House OMB should:
- Require that research funding agencies use a uniform format for research progress reporting
- Amend the new Uniform Guidance to improve the efficiency and consistency of procurement standards, financial reporting, and cost accounting.
Federal agencies should:
- Limit research proposals to the minimum information necessary to permit peer evaluation of the merit of the scientific questions being asked, the feasibility of answering those questions, and the ability of the investigator to carry out that research. Any supplementary information—internal review board approval, conflict-of-interest disclosures, detailed budgets, etc.—should be provided “just in time,” after the research proposal is deemed likely to be funded
- Reduce and streamline reporting, assurances, and verifications. Agencies should also develop a central repository to house assurances.
Universities should:
- Conduct a review of institutional policies developed to comply with federal regulations of research to determine whether the institution itself has created excessive or unnecessary self-imposed burden
- Revise self-imposed burdensome institutional policies that go beyond those necessary and sufficient to comply with federal, state, and local requirements.
The release of the report completes the first phase of the committee’s study, which was expedited at the request of Congress.
The committee will now continue its assessment and issue a spring 2016 addendum report addressing issues it has been unable to address in the first phase. ![]()

Photo by Daniel Sone
A new report suggests a need to change regulations for federally funded research in the US.
According to the report, the current regulatory burden is diminishing the effectiveness of the US scientific enterprise and lowering the return on
the federal investment in research by directing investigators’ time away from research and toward administrative matters.
The report, Optimizing the Nation’s Investment in Academic Research: A New Regulatory Framework for the 21st Century: Part 1 (2015), was
compiled by the National Academies of Sciences, Engineering, and Medicine.
“Federal regulations and reporting requirements, which began as a way to exercise responsible oversight, have increased dramatically in recent
decades and are now unduly encumbering the very research enterprise they were intended to facilitate,” said Larry Faulkner, chair of the committee that conducted the study and wrote the report, and president emeritus of the University of Texas, Austin.
“A significant amount of investigators’ time is now spent complying with regulations, taking valuable time from research, teaching, and scholarship.”
The report lists specific actions the government and research institutions should take to reduce the regulatory burden.
New framework needed
The report says a new framework is needed to approach regulation in a holistic, rather than piecemeal, way. Regulatory requirements should be harmonized across funding agencies, and we need a more effective and efficient partnership between funding agencies and research institutions.
Congress should create a Research Policy Board to serve as a public-private forum for discussions related to regulation of federally funded research programs. The board should be a government-enabled, private-sector entity that will foster more effective conception, development, and synchronization of research policies.
The board should be formally connected to government through a new associate director position at the White House Office of Science and Technology Policy (OSTP) and through the Office of Information and Regulatory Affairs at the White House Office of Management and Budget (OMB).
Strengthening the research partnership also requires that universities demand the highest standards in institutional and individual behavior, foster a culture of integrity, and mete out appropriate sanctions when behavior deviates from ethical and professional norms, the report says.
The Research Policy Board should collaborate with research institutions to develop a policy that holds universities accountable, sanctioning institutions that fail to enforce standards.
The report also recommends a number of specific actions—a sample of which are listed below—that are intended to improve the efficiency of federal regulation and reduce duplication.
Congress should:
- Work with OMB to conduct a review of agency research grant proposal documents for the purpose of developing a uniform format to be used by all funding agencies
- Work with OSTP and research institutions to develop a single financial conflict-of-interest policy to be used by all research funding agencies
- Task a single agency with overseeing and unifying efforts to develop a central database of investigators and their professional output
- Direct agencies to align and harmonize their regulations and definitions concerning the protection of human subjects
- Instruct OSTP to convene representatives from federal agencies that fund animal research and from the research community to assess and report back to Congress on the feasibility and usefulness of a unified federal approach to policies and regulations pertaining to the care and use of research animals.
The White House OMB should:
- Require that research funding agencies use a uniform format for research progress reporting
- Amend the new Uniform Guidance to improve the efficiency and consistency of procurement standards, financial reporting, and cost accounting.
Federal agencies should:
- Limit research proposals to the minimum information necessary to permit peer evaluation of the merit of the scientific questions being asked, the feasibility of answering those questions, and the ability of the investigator to carry out that research. Any supplementary information—internal review board approval, conflict-of-interest disclosures, detailed budgets, etc.—should be provided “just in time,” after the research proposal is deemed likely to be funded
- Reduce and streamline reporting, assurances, and verifications. Agencies should also develop a central repository to house assurances.
Universities should:
- Conduct a review of institutional policies developed to comply with federal regulations of research to determine whether the institution itself has created excessive or unnecessary self-imposed burden
- Revise self-imposed burdensome institutional policies that go beyond those necessary and sufficient to comply with federal, state, and local requirements.
The release of the report completes the first phase of the committee’s study, which was expedited at the request of Congress.
The committee will now continue its assessment and issue a spring 2016 addendum report addressing issues it has been unable to address in the first phase. ![]()
Cancer report highlights progress, makes predictions

Photo courtesy of the FDA
Despite recent progress in the fight against cancers, these diseases continue to exert “an immense toll” in the US, according to the AACR Cancer Progress Report 2015.
The report highlights the recent approval by the US Food and Drug Administration (FDA) of several anticancer therapies, a vaccine, and 2 diagnostic aids.
But the report also includes data suggesting that cancer cases, and costs related to cancer care, are on the rise.
The report states that, between Aug. 1, 2014, and July 31, 2015, the FDA approved 9 anticancer therapies, either for the first time or for new indications.
During the same period, the FDA approved a new cancer vaccine, a new cancer screening test, and a new use for a previously approved imaging agent.
| Cancer-related products approved from Aug. 1, 2014 to July 31, 2015 | |
| Drug | Approved indication |
| bevacizumab (Avastin) | cervical, ovarian, fallopian
tube, and peritoneal cancers |
| blinatumomab (Blincyto) | acute lymphoblastic leukemia |
| denosumab (Xgeva) | potentially lethal complication
of advanced cancers |
| dinutuximab (Unituxin) | neuroblastoma |
| gefitinib (Iressa) | lung cancer |
| ibrutinib (Imbruvica) | Waldenstrom macroglobulinemia |
| lenvatinib (Lenvima) | thyroid cancer |
| nivolumab (Opdivo) | melanoma, lung cancer |
| olaparib (Lynparza) | ovarian cancer |
| palbociclib (Ibrance) | breast cancer |
| panobinostat (Farydak) | multiple myeloma |
| pembrolizumab (Keytruda) | melanoma |
| ramucirumab (Cyramza) | colorectal and lung cancers |
| sonidegib (Odomzo) | skin cancer |
| Imaging agent | Approved indication |
| technetium 99m tilmanocept
(Lymphoseek) |
lymphatic mapping in solid tumors |
| Vaccine | Approved indication |
| human papillomavirus
9-valent vaccine (Gardasil 9) |
cervical, vulvar,
vaginal, and anal cancers |
| Screening test | Approved indication |
| Cologuard (no generic name) | colorectal cancer |
Despite these advances, cancers continue to exert personal and economic tolls, according to the report.
It states that cancer is the number 1 cause of disease-related death among US children. And more than 589,000 people in the US are projected to die from cancer in 2015.
The number of new cancer cases in the US is predicted to rise from 1.7 million in 2015 to 2.4 million in 2035.
In addition, estimates suggest the direct medical costs of cancer care in the US in 2010 were nearly $125 billion, and these costs are predicted to rise to $156 billion in 2020.
These data underscore the need for more research to develop new approaches to cancer prevention and treatment, according to the report.
Its authors call for Congress and the administration to provide the National Institutes of Health, National Cancer Institute, and FDA with annual funding increases.
“We have made spectacular progress against cancer, which has saved the lives of millions of individuals in the United States and around the world,” said Margaret Foti, PhD, MD, chief executive officer of the AACR.
“However, without increased federal funding for cancer research, we will not be able to realize the promise of recent discoveries and technological advances.” ![]()

Photo courtesy of the FDA
Despite recent progress in the fight against cancers, these diseases continue to exert “an immense toll” in the US, according to the AACR Cancer Progress Report 2015.
The report highlights the recent approval by the US Food and Drug Administration (FDA) of several anticancer therapies, a vaccine, and 2 diagnostic aids.
But the report also includes data suggesting that cancer cases, and costs related to cancer care, are on the rise.
The report states that, between Aug. 1, 2014, and July 31, 2015, the FDA approved 9 anticancer therapies, either for the first time or for new indications.
During the same period, the FDA approved a new cancer vaccine, a new cancer screening test, and a new use for a previously approved imaging agent.
| Cancer-related products approved from Aug. 1, 2014 to July 31, 2015 | |
| Drug | Approved indication |
| bevacizumab (Avastin) | cervical, ovarian, fallopian
tube, and peritoneal cancers |
| blinatumomab (Blincyto) | acute lymphoblastic leukemia |
| denosumab (Xgeva) | potentially lethal complication
of advanced cancers |
| dinutuximab (Unituxin) | neuroblastoma |
| gefitinib (Iressa) | lung cancer |
| ibrutinib (Imbruvica) | Waldenstrom macroglobulinemia |
| lenvatinib (Lenvima) | thyroid cancer |
| nivolumab (Opdivo) | melanoma, lung cancer |
| olaparib (Lynparza) | ovarian cancer |
| palbociclib (Ibrance) | breast cancer |
| panobinostat (Farydak) | multiple myeloma |
| pembrolizumab (Keytruda) | melanoma |
| ramucirumab (Cyramza) | colorectal and lung cancers |
| sonidegib (Odomzo) | skin cancer |
| Imaging agent | Approved indication |
| technetium 99m tilmanocept
(Lymphoseek) |
lymphatic mapping in solid tumors |
| Vaccine | Approved indication |
| human papillomavirus
9-valent vaccine (Gardasil 9) |
cervical, vulvar,
vaginal, and anal cancers |
| Screening test | Approved indication |
| Cologuard (no generic name) | colorectal cancer |
Despite these advances, cancers continue to exert personal and economic tolls, according to the report.
It states that cancer is the number 1 cause of disease-related death among US children. And more than 589,000 people in the US are projected to die from cancer in 2015.
The number of new cancer cases in the US is predicted to rise from 1.7 million in 2015 to 2.4 million in 2035.
In addition, estimates suggest the direct medical costs of cancer care in the US in 2010 were nearly $125 billion, and these costs are predicted to rise to $156 billion in 2020.
These data underscore the need for more research to develop new approaches to cancer prevention and treatment, according to the report.
Its authors call for Congress and the administration to provide the National Institutes of Health, National Cancer Institute, and FDA with annual funding increases.
“We have made spectacular progress against cancer, which has saved the lives of millions of individuals in the United States and around the world,” said Margaret Foti, PhD, MD, chief executive officer of the AACR.
“However, without increased federal funding for cancer research, we will not be able to realize the promise of recent discoveries and technological advances.” ![]()

Photo courtesy of the FDA
Despite recent progress in the fight against cancers, these diseases continue to exert “an immense toll” in the US, according to the AACR Cancer Progress Report 2015.
The report highlights the recent approval by the US Food and Drug Administration (FDA) of several anticancer therapies, a vaccine, and 2 diagnostic aids.
But the report also includes data suggesting that cancer cases, and costs related to cancer care, are on the rise.
The report states that, between Aug. 1, 2014, and July 31, 2015, the FDA approved 9 anticancer therapies, either for the first time or for new indications.
During the same period, the FDA approved a new cancer vaccine, a new cancer screening test, and a new use for a previously approved imaging agent.
| Cancer-related products approved from Aug. 1, 2014 to July 31, 2015 | |
| Drug | Approved indication |
| bevacizumab (Avastin) | cervical, ovarian, fallopian
tube, and peritoneal cancers |
| blinatumomab (Blincyto) | acute lymphoblastic leukemia |
| denosumab (Xgeva) | potentially lethal complication
of advanced cancers |
| dinutuximab (Unituxin) | neuroblastoma |
| gefitinib (Iressa) | lung cancer |
| ibrutinib (Imbruvica) | Waldenstrom macroglobulinemia |
| lenvatinib (Lenvima) | thyroid cancer |
| nivolumab (Opdivo) | melanoma, lung cancer |
| olaparib (Lynparza) | ovarian cancer |
| palbociclib (Ibrance) | breast cancer |
| panobinostat (Farydak) | multiple myeloma |
| pembrolizumab (Keytruda) | melanoma |
| ramucirumab (Cyramza) | colorectal and lung cancers |
| sonidegib (Odomzo) | skin cancer |
| Imaging agent | Approved indication |
| technetium 99m tilmanocept
(Lymphoseek) |
lymphatic mapping in solid tumors |
| Vaccine | Approved indication |
| human papillomavirus
9-valent vaccine (Gardasil 9) |
cervical, vulvar,
vaginal, and anal cancers |
| Screening test | Approved indication |
| Cologuard (no generic name) | colorectal cancer |
Despite these advances, cancers continue to exert personal and economic tolls, according to the report.
It states that cancer is the number 1 cause of disease-related death among US children. And more than 589,000 people in the US are projected to die from cancer in 2015.
The number of new cancer cases in the US is predicted to rise from 1.7 million in 2015 to 2.4 million in 2035.
In addition, estimates suggest the direct medical costs of cancer care in the US in 2010 were nearly $125 billion, and these costs are predicted to rise to $156 billion in 2020.
These data underscore the need for more research to develop new approaches to cancer prevention and treatment, according to the report.
Its authors call for Congress and the administration to provide the National Institutes of Health, National Cancer Institute, and FDA with annual funding increases.
“We have made spectacular progress against cancer, which has saved the lives of millions of individuals in the United States and around the world,” said Margaret Foti, PhD, MD, chief executive officer of the AACR.
“However, without increased federal funding for cancer research, we will not be able to realize the promise of recent discoveries and technological advances.” ![]()
Bringing the B-cell surface into focus

clusters on the cell surface
Image courtesy of V.
Altounian/Science Signaling
New research has provided a clearer picture of the B-cell surface, unearthing new insights regarding antigen receptors.
Mature B cells have 2 classes of antigen receptors on their surface, immunoglobulin M (IgM) and immunoglobulin D (IgD).
Using multiple imaging techniques, researchers studied the spatial relationship of these receptor types in B cells from cell lines, mice, and human blood.
The team described this work in Science Signaling.
Receptors on the surface of resting T cells are thought to reside in preformed clusters known as protein islands, but whether these islands exist on B cells has been unclear.
Palash Maity, PhD, of the University of Freiburg in Germany, and colleagues found these islands do exist on B cells, but their structure changes upon B-cell activation.
Using 2-color direct stochastical optical reconstruction microscopy (dSTORM), the researchers found that IgM and IgD reside on the plasma membrane of resting B cells in separate protein islands of approximately 150 and 240 nanometers, respectively.
The team also observed this class-specific compartmentalization of the antigen receptors using transmission electron microscopy (TEM) and Fab-based proximity-ligation assay (Fab-PLA).
However, the researchers noted a change during B-cell activation. Upon activation, the IgM and IgD protein islands broke up into smaller islands, allowing the 2 types to intermingle, although they did not make direct contact.
The researchers said it is not clear whether this increased affinity between the receptor types is a result of a direct interaction or is mediated by an adaptor protein. The function of the association between the receptors is not clear, either.
But the team believes that one possibility is that, upon B-cell activation, the IgM and IgD protein islands form nanosynapses that allow the islands to exchange proteins and lipids with one another.
The researchers hope these new insights into the nanoscale organization of antigen receptors will support the design of more efficient vaccines or better treatments for B-cell tumors, in which membrane organization is often altered. ![]()

clusters on the cell surface
Image courtesy of V.
Altounian/Science Signaling
New research has provided a clearer picture of the B-cell surface, unearthing new insights regarding antigen receptors.
Mature B cells have 2 classes of antigen receptors on their surface, immunoglobulin M (IgM) and immunoglobulin D (IgD).
Using multiple imaging techniques, researchers studied the spatial relationship of these receptor types in B cells from cell lines, mice, and human blood.
The team described this work in Science Signaling.
Receptors on the surface of resting T cells are thought to reside in preformed clusters known as protein islands, but whether these islands exist on B cells has been unclear.
Palash Maity, PhD, of the University of Freiburg in Germany, and colleagues found these islands do exist on B cells, but their structure changes upon B-cell activation.
Using 2-color direct stochastical optical reconstruction microscopy (dSTORM), the researchers found that IgM and IgD reside on the plasma membrane of resting B cells in separate protein islands of approximately 150 and 240 nanometers, respectively.
The team also observed this class-specific compartmentalization of the antigen receptors using transmission electron microscopy (TEM) and Fab-based proximity-ligation assay (Fab-PLA).
However, the researchers noted a change during B-cell activation. Upon activation, the IgM and IgD protein islands broke up into smaller islands, allowing the 2 types to intermingle, although they did not make direct contact.
The researchers said it is not clear whether this increased affinity between the receptor types is a result of a direct interaction or is mediated by an adaptor protein. The function of the association between the receptors is not clear, either.
But the team believes that one possibility is that, upon B-cell activation, the IgM and IgD protein islands form nanosynapses that allow the islands to exchange proteins and lipids with one another.
The researchers hope these new insights into the nanoscale organization of antigen receptors will support the design of more efficient vaccines or better treatments for B-cell tumors, in which membrane organization is often altered. ![]()

clusters on the cell surface
Image courtesy of V.
Altounian/Science Signaling
New research has provided a clearer picture of the B-cell surface, unearthing new insights regarding antigen receptors.
Mature B cells have 2 classes of antigen receptors on their surface, immunoglobulin M (IgM) and immunoglobulin D (IgD).
Using multiple imaging techniques, researchers studied the spatial relationship of these receptor types in B cells from cell lines, mice, and human blood.
The team described this work in Science Signaling.
Receptors on the surface of resting T cells are thought to reside in preformed clusters known as protein islands, but whether these islands exist on B cells has been unclear.
Palash Maity, PhD, of the University of Freiburg in Germany, and colleagues found these islands do exist on B cells, but their structure changes upon B-cell activation.
Using 2-color direct stochastical optical reconstruction microscopy (dSTORM), the researchers found that IgM and IgD reside on the plasma membrane of resting B cells in separate protein islands of approximately 150 and 240 nanometers, respectively.
The team also observed this class-specific compartmentalization of the antigen receptors using transmission electron microscopy (TEM) and Fab-based proximity-ligation assay (Fab-PLA).
However, the researchers noted a change during B-cell activation. Upon activation, the IgM and IgD protein islands broke up into smaller islands, allowing the 2 types to intermingle, although they did not make direct contact.
The researchers said it is not clear whether this increased affinity between the receptor types is a result of a direct interaction or is mediated by an adaptor protein. The function of the association between the receptors is not clear, either.
But the team believes that one possibility is that, upon B-cell activation, the IgM and IgD protein islands form nanosynapses that allow the islands to exchange proteins and lipids with one another.
The researchers hope these new insights into the nanoscale organization of antigen receptors will support the design of more efficient vaccines or better treatments for B-cell tumors, in which membrane organization is often altered.
Platelet mimics provide targeted drug delivery
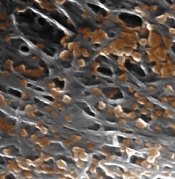
nanoparticles binding to the
lining of a damaged artery
Image courtesy of the
Zhang Research Group
Nanoparticles disguised as human platelets can provide targeted drug delivery, according to research published in Nature.
The nanoparticles are made of a biodegradable polymer coated with human platelet membranes.
This coating allows the nanoparticles to circulate in the bloodstream without being attacked by the immune system and to preferentially bind to damaged blood vessels and certain pathogens.
Murine experiments showed that these platelet-mimicking nanoparticles can deliver drugs to targeted sites, thereby increasing the therapeutic effect.
“Because of their targeting ability, platelet-mimicking nanoparticles can directly provide a much higher dose of medication specifically to diseased areas without saturating the entire body with drugs,” said study author Liangfang Zhang, PhD, of the University of California San Diego.
Creating the platelet mimics
To make the nanoparticles, Dr Zhang and his colleagues first separated platelets from whole blood samples using a centrifuge. The platelets were then processed to isolate the membranes from the platelets.
Next, the platelet membranes were broken up into much smaller pieces and fused to the surface of the nanoparticle cores. The resulting platelet-membrane-coated nanoparticles were approximately 100 nanometers in diameter.
This cloaking technology is based on a strategy Dr Zhang’s group had developed to cloak nanoparticles in red blood cell membranes. The researchers previously demonstrated that nanoparticles disguised as red blood cells are capable of removing toxins from the bloodstream.
With the current work, the researchers were able to produce platelet mimics that contain the complete set of surface receptors, antigens, and proteins naturally present on platelet membranes.
“Our technique takes advantage of the unique natural properties of human platelet membranes, which have a natural preference to bind to certain tissues and organisms in the body,” Dr Zhang said.
This targeting ability, which red blood cell membranes do not have, makes platelet membranes extremely useful for targeted drug delivery, according to the researchers.
Platelet mimics at work
The researchers packed the platelet-mimicking nanoparticles with docetaxel, a drug used to prevent scar tissue formation in the lining of damaged blood vessels, and administered them to rats afflicted with injured arteries.
The docetaxel-containing nanoparticles collected at the damaged sites of arteries and healed them.
The researchers then injected nanoparticles containing one-sixth the clinical dose of the antibiotic vancomycin into a group of mice systemically infected with methicillin-resistant Staphylococcus aureus bacteria.
Bacterial counts in the organs of these mice were up to 1000 times lower than in mice treated with the clinical dose of vancomycin alone.
“Our platelet-mimicking nanoparticles can increase the therapeutic efficacy of antibiotics because they can focus treatment on the bacteria locally without spreading drugs to healthy tissues and organs throughout the rest of the body,” Dr Zhang said. “We hope to develop platelet-mimicking nanoparticles into new treatments for systemic bacterial infections and cardiovascular disease.”
Dr Zhang noted that this drug delivery technique could potentially be used in other diseases as well, including cancers.

nanoparticles binding to the
lining of a damaged artery
Image courtesy of the
Zhang Research Group
Nanoparticles disguised as human platelets can provide targeted drug delivery, according to research published in Nature.
The nanoparticles are made of a biodegradable polymer coated with human platelet membranes.
This coating allows the nanoparticles to circulate in the bloodstream without being attacked by the immune system and to preferentially bind to damaged blood vessels and certain pathogens.
Murine experiments showed that these platelet-mimicking nanoparticles can deliver drugs to targeted sites, thereby increasing the therapeutic effect.
“Because of their targeting ability, platelet-mimicking nanoparticles can directly provide a much higher dose of medication specifically to diseased areas without saturating the entire body with drugs,” said study author Liangfang Zhang, PhD, of the University of California San Diego.
Creating the platelet mimics
To make the nanoparticles, Dr Zhang and his colleagues first separated platelets from whole blood samples using a centrifuge. The platelets were then processed to isolate the membranes from the platelets.
Next, the platelet membranes were broken up into much smaller pieces and fused to the surface of the nanoparticle cores. The resulting platelet-membrane-coated nanoparticles were approximately 100 nanometers in diameter.
This cloaking technology is based on a strategy Dr Zhang’s group had developed to cloak nanoparticles in red blood cell membranes. The researchers previously demonstrated that nanoparticles disguised as red blood cells are capable of removing toxins from the bloodstream.
With the current work, the researchers were able to produce platelet mimics that contain the complete set of surface receptors, antigens, and proteins naturally present on platelet membranes.
“Our technique takes advantage of the unique natural properties of human platelet membranes, which have a natural preference to bind to certain tissues and organisms in the body,” Dr Zhang said.
This targeting ability, which red blood cell membranes do not have, makes platelet membranes extremely useful for targeted drug delivery, according to the researchers.
Platelet mimics at work
The researchers packed the platelet-mimicking nanoparticles with docetaxel, a drug used to prevent scar tissue formation in the lining of damaged blood vessels, and administered them to rats afflicted with injured arteries.
The docetaxel-containing nanoparticles collected at the damaged sites of arteries and healed them.
The researchers then injected nanoparticles containing one-sixth the clinical dose of the antibiotic vancomycin into a group of mice systemically infected with methicillin-resistant Staphylococcus aureus bacteria.
Bacterial counts in the organs of these mice were up to 1000 times lower than in mice treated with the clinical dose of vancomycin alone.
“Our platelet-mimicking nanoparticles can increase the therapeutic efficacy of antibiotics because they can focus treatment on the bacteria locally without spreading drugs to healthy tissues and organs throughout the rest of the body,” Dr Zhang said. “We hope to develop platelet-mimicking nanoparticles into new treatments for systemic bacterial infections and cardiovascular disease.”
Dr Zhang noted that this drug delivery technique could potentially be used in other diseases as well, including cancers.

nanoparticles binding to the
lining of a damaged artery
Image courtesy of the
Zhang Research Group
Nanoparticles disguised as human platelets can provide targeted drug delivery, according to research published in Nature.
The nanoparticles are made of a biodegradable polymer coated with human platelet membranes.
This coating allows the nanoparticles to circulate in the bloodstream without being attacked by the immune system and to preferentially bind to damaged blood vessels and certain pathogens.
Murine experiments showed that these platelet-mimicking nanoparticles can deliver drugs to targeted sites, thereby increasing the therapeutic effect.
“Because of their targeting ability, platelet-mimicking nanoparticles can directly provide a much higher dose of medication specifically to diseased areas without saturating the entire body with drugs,” said study author Liangfang Zhang, PhD, of the University of California San Diego.
Creating the platelet mimics
To make the nanoparticles, Dr Zhang and his colleagues first separated platelets from whole blood samples using a centrifuge. The platelets were then processed to isolate the membranes from the platelets.
Next, the platelet membranes were broken up into much smaller pieces and fused to the surface of the nanoparticle cores. The resulting platelet-membrane-coated nanoparticles were approximately 100 nanometers in diameter.
This cloaking technology is based on a strategy Dr Zhang’s group had developed to cloak nanoparticles in red blood cell membranes. The researchers previously demonstrated that nanoparticles disguised as red blood cells are capable of removing toxins from the bloodstream.
With the current work, the researchers were able to produce platelet mimics that contain the complete set of surface receptors, antigens, and proteins naturally present on platelet membranes.
“Our technique takes advantage of the unique natural properties of human platelet membranes, which have a natural preference to bind to certain tissues and organisms in the body,” Dr Zhang said.
This targeting ability, which red blood cell membranes do not have, makes platelet membranes extremely useful for targeted drug delivery, according to the researchers.
Platelet mimics at work
The researchers packed the platelet-mimicking nanoparticles with docetaxel, a drug used to prevent scar tissue formation in the lining of damaged blood vessels, and administered them to rats afflicted with injured arteries.
The docetaxel-containing nanoparticles collected at the damaged sites of arteries and healed them.
The researchers then injected nanoparticles containing one-sixth the clinical dose of the antibiotic vancomycin into a group of mice systemically infected with methicillin-resistant Staphylococcus aureus bacteria.
Bacterial counts in the organs of these mice were up to 1000 times lower than in mice treated with the clinical dose of vancomycin alone.
“Our platelet-mimicking nanoparticles can increase the therapeutic efficacy of antibiotics because they can focus treatment on the bacteria locally without spreading drugs to healthy tissues and organs throughout the rest of the body,” Dr Zhang said. “We hope to develop platelet-mimicking nanoparticles into new treatments for systemic bacterial infections and cardiovascular disease.”
Dr Zhang noted that this drug delivery technique could potentially be used in other diseases as well, including cancers.
Tool can guide allocation of research resources

Photo by Bill Branson
Scientists say they have developed a tool that can guide the allocation of resources for biomedical research in an unbiased way.
The tool, called the Research Opportunity Index (ROI), measures disparities between resources dedicated to a disease and its relative burden on society.
This reveals diseases that receive a disproportionate share of resources and opportunities for high-impact investment or the realignment of existing resources.
The ROI was designed to provide an unbiased, data-driven framework to help scientific and political communities assess resource investment and identify unmet medical needs.
The tool is described in a letter to Nature Biotechnology.
“The misalignment of resources in biomedical research could be likened to poor budgeting of household finances,” said Andrey Rzhetsky, PhD, of the University of Chicago in Illinois.
“It would be bad to spend all your money on food, for example, and have nothing for rent. Resources are finite, and attention to each problem, ideally, should be proportional to the need.”
With this in mind, Dr Rzhetsky and his colleagues created the ROI. They used it to estimate the societal burden of 1400 medical conditions in the US over a 12-year timespan, based on frequency of diagnosis and healthcare insurance costs, as well as research publications, awarded grants, and clinical trials for each condition.
The tool revealed misalignments, which allowed the scientists to create an “investment portfolio” of the resources dedicated to each disease, relative to its burden on the US healthcare system.
The team found that breast cancer, for example, stands out as a disease with higher dedicated resources than its relative societal burden. On the opposite end of the spectrum, Hashimoto’s thyroiditis is among the conditions with the most investment potential.
While Hashimoto’s disease has nearly the same incidence among women as breast cancer, there were only 16 open clinical trials for Hashimoto’s disease as of August 2015, according to a list on clinicaltrials.gov. Breast cancer had 2205 open trials.
Dr Rzhetsky and his colleagues acknowledge that the question of what makes a condition more deserving of funding than any other ailment is complex and multifaceted. But the team hopes this new tool can aid decisions on how best to allocate funds and other resources.
By providing a framework based on unbiased quantitative analytics and big data, they hope to identify diseases that are high-impact and rewarding targets for additional investment.
“Some diseases stick in the public’s attention,” Dr Rzhetsky said. “We have a distorted map of the frequency and importance of events from media and articles, and, without special efforts to reconcile the reality, we are inherently biased.”
The team is now working to incorporate other models of funding distribution into their tool to account for additional variables.
For example, the “trendy model,” which supports investment for diseases with large emotional impact, suggests that even though this reduces funding for other diseases, there may still be benefits as basic science discoveries are added to the scientific and medical community.

Photo by Bill Branson
Scientists say they have developed a tool that can guide the allocation of resources for biomedical research in an unbiased way.
The tool, called the Research Opportunity Index (ROI), measures disparities between resources dedicated to a disease and its relative burden on society.
This reveals diseases that receive a disproportionate share of resources and opportunities for high-impact investment or the realignment of existing resources.
The ROI was designed to provide an unbiased, data-driven framework to help scientific and political communities assess resource investment and identify unmet medical needs.
The tool is described in a letter to Nature Biotechnology.
“The misalignment of resources in biomedical research could be likened to poor budgeting of household finances,” said Andrey Rzhetsky, PhD, of the University of Chicago in Illinois.
“It would be bad to spend all your money on food, for example, and have nothing for rent. Resources are finite, and attention to each problem, ideally, should be proportional to the need.”
With this in mind, Dr Rzhetsky and his colleagues created the ROI. They used it to estimate the societal burden of 1400 medical conditions in the US over a 12-year timespan, based on frequency of diagnosis and healthcare insurance costs, as well as research publications, awarded grants, and clinical trials for each condition.
The tool revealed misalignments, which allowed the scientists to create an “investment portfolio” of the resources dedicated to each disease, relative to its burden on the US healthcare system.
The team found that breast cancer, for example, stands out as a disease with higher dedicated resources than its relative societal burden. On the opposite end of the spectrum, Hashimoto’s thyroiditis is among the conditions with the most investment potential.
While Hashimoto’s disease has nearly the same incidence among women as breast cancer, there were only 16 open clinical trials for Hashimoto’s disease as of August 2015, according to a list on clinicaltrials.gov. Breast cancer had 2205 open trials.
Dr Rzhetsky and his colleagues acknowledge that the question of what makes a condition more deserving of funding than any other ailment is complex and multifaceted. But the team hopes this new tool can aid decisions on how best to allocate funds and other resources.
By providing a framework based on unbiased quantitative analytics and big data, they hope to identify diseases that are high-impact and rewarding targets for additional investment.
“Some diseases stick in the public’s attention,” Dr Rzhetsky said. “We have a distorted map of the frequency and importance of events from media and articles, and, without special efforts to reconcile the reality, we are inherently biased.”
The team is now working to incorporate other models of funding distribution into their tool to account for additional variables.
For example, the “trendy model,” which supports investment for diseases with large emotional impact, suggests that even though this reduces funding for other diseases, there may still be benefits as basic science discoveries are added to the scientific and medical community.

Photo by Bill Branson
Scientists say they have developed a tool that can guide the allocation of resources for biomedical research in an unbiased way.
The tool, called the Research Opportunity Index (ROI), measures disparities between resources dedicated to a disease and its relative burden on society.
This reveals diseases that receive a disproportionate share of resources and opportunities for high-impact investment or the realignment of existing resources.
The ROI was designed to provide an unbiased, data-driven framework to help scientific and political communities assess resource investment and identify unmet medical needs.
The tool is described in a letter to Nature Biotechnology.
“The misalignment of resources in biomedical research could be likened to poor budgeting of household finances,” said Andrey Rzhetsky, PhD, of the University of Chicago in Illinois.
“It would be bad to spend all your money on food, for example, and have nothing for rent. Resources are finite, and attention to each problem, ideally, should be proportional to the need.”
With this in mind, Dr Rzhetsky and his colleagues created the ROI. They used it to estimate the societal burden of 1400 medical conditions in the US over a 12-year timespan, based on frequency of diagnosis and healthcare insurance costs, as well as research publications, awarded grants, and clinical trials for each condition.
The tool revealed misalignments, which allowed the scientists to create an “investment portfolio” of the resources dedicated to each disease, relative to its burden on the US healthcare system.
The team found that breast cancer, for example, stands out as a disease with higher dedicated resources than its relative societal burden. On the opposite end of the spectrum, Hashimoto’s thyroiditis is among the conditions with the most investment potential.
While Hashimoto’s disease has nearly the same incidence among women as breast cancer, there were only 16 open clinical trials for Hashimoto’s disease as of August 2015, according to a list on clinicaltrials.gov. Breast cancer had 2205 open trials.
Dr Rzhetsky and his colleagues acknowledge that the question of what makes a condition more deserving of funding than any other ailment is complex and multifaceted. But the team hopes this new tool can aid decisions on how best to allocate funds and other resources.
By providing a framework based on unbiased quantitative analytics and big data, they hope to identify diseases that are high-impact and rewarding targets for additional investment.
“Some diseases stick in the public’s attention,” Dr Rzhetsky said. “We have a distorted map of the frequency and importance of events from media and articles, and, without special efforts to reconcile the reality, we are inherently biased.”
The team is now working to incorporate other models of funding distribution into their tool to account for additional variables.
For example, the “trendy model,” which supports investment for diseases with large emotional impact, suggests that even though this reduces funding for other diseases, there may still be benefits as basic science discoveries are added to the scientific and medical community.
Living near dams increases malaria risk, study shows

Photo courtesy of the
International Water
Management Institute
More than 1 million people in sub-Saharan Africa will contract malaria this year because they live near a large dam, according to a study published in Malaria Journal.
For the first time, researchers correlated the location of large dams in sub-Saharan Africa with the incidence of malaria.
And they found evidence to suggest that construction of an expected 78 major new dams over the next few years will lead to an additional 56,000 malaria cases annually.
The researchers said these findings have major implications for new dam projects and how health impacts should be assessed prior to construction.
“Dams are at the center of much development planning in Africa,” said study author Solomon Kibret, a graduate student at the University of New England in Armidale, New South Wales, Australia.
“While dams clearly bring many benefits—contributing to economic growth, poverty alleviation, and food security—adverse malaria impacts need to be addressed or they will undermine the sustainability of Africa’s drive for development.”
As part of the CGIAR Research Program on Water, Land, and Ecosystems, Kibret and colleagues looked at 1268 dams in sub-Saharan Africa. Of these, just under two-thirds (n=723) are in malarious areas.
The researchers compared detailed maps of malaria incidence with the dam sites. The number of annual malaria cases associated with the dams was estimated by comparing the number of cases for communities less than 5 kilometers from the dam reservoir with the number of cases for communities further away.
The team found that 15 million people live within 5 kilometers of dam reservoirs and are therefore at risk of contracting malaria. And at least 1.1 million malaria cases annually are linked to the presence of the dams.
“Our study showed that the population at risk of malaria around dams is at least 4 times greater than previously estimated,” Kibret said, noting that the authors were conservative in all their analyses.
The risk is particularly high in areas of sub-Saharan Africa with “unstable” malaria transmission, where malaria is seasonal. The study indicated that the impact of dams on malaria in unstable areas could either lead to intensified malaria transmission or change the nature of transmission from seasonal to perennial.
Explaining the risk
Previous research revealed increases in malaria incidence near major sub-Saharan dams such as the Akosombo Dam in Ghana, the Koka Dam in Ethiopia, and the Kamburu Dam in Kenya. But until now, no attempt has been made to assess the cumulative effect of large dam-building on malaria.
Malaria is transmitted by the Anopheles mosquito, which needs slow-moving or stagnant water in which to breed. Dam reservoirs, particularly shallow puddles that often form along shorelines, provide a perfect environment for the insects to multiply. Thus, dam construction can intensify transmission and shift patterns of malaria infection.
Many African countries are planning new dams to help drive economic growth and increase water security. Improved water storage for growing populations, irrigation, and hydropower generation are needed for a fast-developing continent, but the researchers warn that building new dams has potential costs as well as benefits.
“Dams are an important option for governments anxious to develop,” said study author Matthew McCartney, PhD, of the International Water Management Institute in Vientiane, Laos.
“But it is unethical that people living close to them pay the price of that development through increased suffering and, possibly in extreme cases, loss of life due to disease.”
Lowering the risk
The researchers noted that, despite growing evidence of the impact of dams on malaria, there is scant evidence of their negative impacts being fully offset.
The team therefore made recommendations for managing the increased malaria risk. They said dam reservoirs could be more effectively designed and managed to reduce mosquito breeding. For instance, one option is to adopt operating schedules that, at critical times, dry out shoreline areas where mosquitoes tend to breed.
The researchers said dam developers should also consider increasing investment in integrated malaria intervention programs that include measures such as bed net distribution. Other environmental controls, such as introducing fish that eat mosquito larva in dam reservoirs, could also help reduce malaria cases in some instances.
“The bottom line is that adverse malaria impacts of dams routinely receive recognition in Environmental Impact Assessments, and areas around dams are frequently earmarked for intensive control efforts,” said study author Jonathan Lautze, PhD, of the International Water Management Institute in Pretoria, South Africa.
“The findings of our work hammer home the reality that this recognition and effort—well-intentioned though it may be—is simply not sufficient. Given the need for water resources development in Africa, malaria control around dams requires interdisciplinary cooperation, particularly between water and health communities. Malaria must be addressed while planning, designing, and operating African dams.”

Photo courtesy of the
International Water
Management Institute
More than 1 million people in sub-Saharan Africa will contract malaria this year because they live near a large dam, according to a study published in Malaria Journal.
For the first time, researchers correlated the location of large dams in sub-Saharan Africa with the incidence of malaria.
And they found evidence to suggest that construction of an expected 78 major new dams over the next few years will lead to an additional 56,000 malaria cases annually.
The researchers said these findings have major implications for new dam projects and how health impacts should be assessed prior to construction.
“Dams are at the center of much development planning in Africa,” said study author Solomon Kibret, a graduate student at the University of New England in Armidale, New South Wales, Australia.
“While dams clearly bring many benefits—contributing to economic growth, poverty alleviation, and food security—adverse malaria impacts need to be addressed or they will undermine the sustainability of Africa’s drive for development.”
As part of the CGIAR Research Program on Water, Land, and Ecosystems, Kibret and colleagues looked at 1268 dams in sub-Saharan Africa. Of these, just under two-thirds (n=723) are in malarious areas.
The researchers compared detailed maps of malaria incidence with the dam sites. The number of annual malaria cases associated with the dams was estimated by comparing the number of cases for communities less than 5 kilometers from the dam reservoir with the number of cases for communities further away.
The team found that 15 million people live within 5 kilometers of dam reservoirs and are therefore at risk of contracting malaria. And at least 1.1 million malaria cases annually are linked to the presence of the dams.
“Our study showed that the population at risk of malaria around dams is at least 4 times greater than previously estimated,” Kibret said, noting that the authors were conservative in all their analyses.
The risk is particularly high in areas of sub-Saharan Africa with “unstable” malaria transmission, where malaria is seasonal. The study indicated that the impact of dams on malaria in unstable areas could either lead to intensified malaria transmission or change the nature of transmission from seasonal to perennial.
Explaining the risk
Previous research revealed increases in malaria incidence near major sub-Saharan dams such as the Akosombo Dam in Ghana, the Koka Dam in Ethiopia, and the Kamburu Dam in Kenya. But until now, no attempt has been made to assess the cumulative effect of large dam-building on malaria.
Malaria is transmitted by the Anopheles mosquito, which needs slow-moving or stagnant water in which to breed. Dam reservoirs, particularly shallow puddles that often form along shorelines, provide a perfect environment for the insects to multiply. Thus, dam construction can intensify transmission and shift patterns of malaria infection.
Many African countries are planning new dams to help drive economic growth and increase water security. Improved water storage for growing populations, irrigation, and hydropower generation are needed for a fast-developing continent, but the researchers warn that building new dams has potential costs as well as benefits.
“Dams are an important option for governments anxious to develop,” said study author Matthew McCartney, PhD, of the International Water Management Institute in Vientiane, Laos.
“But it is unethical that people living close to them pay the price of that development through increased suffering and, possibly in extreme cases, loss of life due to disease.”
Lowering the risk
The researchers noted that, despite growing evidence of the impact of dams on malaria, there is scant evidence of their negative impacts being fully offset.
The team therefore made recommendations for managing the increased malaria risk. They said dam reservoirs could be more effectively designed and managed to reduce mosquito breeding. For instance, one option is to adopt operating schedules that, at critical times, dry out shoreline areas where mosquitoes tend to breed.
The researchers said dam developers should also consider increasing investment in integrated malaria intervention programs that include measures such as bed net distribution. Other environmental controls, such as introducing fish that eat mosquito larva in dam reservoirs, could also help reduce malaria cases in some instances.
“The bottom line is that adverse malaria impacts of dams routinely receive recognition in Environmental Impact Assessments, and areas around dams are frequently earmarked for intensive control efforts,” said study author Jonathan Lautze, PhD, of the International Water Management Institute in Pretoria, South Africa.
“The findings of our work hammer home the reality that this recognition and effort—well-intentioned though it may be—is simply not sufficient. Given the need for water resources development in Africa, malaria control around dams requires interdisciplinary cooperation, particularly between water and health communities. Malaria must be addressed while planning, designing, and operating African dams.”

Photo courtesy of the
International Water
Management Institute
More than 1 million people in sub-Saharan Africa will contract malaria this year because they live near a large dam, according to a study published in Malaria Journal.
For the first time, researchers correlated the location of large dams in sub-Saharan Africa with the incidence of malaria.
And they found evidence to suggest that construction of an expected 78 major new dams over the next few years will lead to an additional 56,000 malaria cases annually.
The researchers said these findings have major implications for new dam projects and how health impacts should be assessed prior to construction.
“Dams are at the center of much development planning in Africa,” said study author Solomon Kibret, a graduate student at the University of New England in Armidale, New South Wales, Australia.
“While dams clearly bring many benefits—contributing to economic growth, poverty alleviation, and food security—adverse malaria impacts need to be addressed or they will undermine the sustainability of Africa’s drive for development.”
As part of the CGIAR Research Program on Water, Land, and Ecosystems, Kibret and colleagues looked at 1268 dams in sub-Saharan Africa. Of these, just under two-thirds (n=723) are in malarious areas.
The researchers compared detailed maps of malaria incidence with the dam sites. The number of annual malaria cases associated with the dams was estimated by comparing the number of cases for communities less than 5 kilometers from the dam reservoir with the number of cases for communities further away.
The team found that 15 million people live within 5 kilometers of dam reservoirs and are therefore at risk of contracting malaria. And at least 1.1 million malaria cases annually are linked to the presence of the dams.
“Our study showed that the population at risk of malaria around dams is at least 4 times greater than previously estimated,” Kibret said, noting that the authors were conservative in all their analyses.
The risk is particularly high in areas of sub-Saharan Africa with “unstable” malaria transmission, where malaria is seasonal. The study indicated that the impact of dams on malaria in unstable areas could either lead to intensified malaria transmission or change the nature of transmission from seasonal to perennial.
Explaining the risk
Previous research revealed increases in malaria incidence near major sub-Saharan dams such as the Akosombo Dam in Ghana, the Koka Dam in Ethiopia, and the Kamburu Dam in Kenya. But until now, no attempt has been made to assess the cumulative effect of large dam-building on malaria.
Malaria is transmitted by the Anopheles mosquito, which needs slow-moving or stagnant water in which to breed. Dam reservoirs, particularly shallow puddles that often form along shorelines, provide a perfect environment for the insects to multiply. Thus, dam construction can intensify transmission and shift patterns of malaria infection.
Many African countries are planning new dams to help drive economic growth and increase water security. Improved water storage for growing populations, irrigation, and hydropower generation are needed for a fast-developing continent, but the researchers warn that building new dams has potential costs as well as benefits.
“Dams are an important option for governments anxious to develop,” said study author Matthew McCartney, PhD, of the International Water Management Institute in Vientiane, Laos.
“But it is unethical that people living close to them pay the price of that development through increased suffering and, possibly in extreme cases, loss of life due to disease.”
Lowering the risk
The researchers noted that, despite growing evidence of the impact of dams on malaria, there is scant evidence of their negative impacts being fully offset.
The team therefore made recommendations for managing the increased malaria risk. They said dam reservoirs could be more effectively designed and managed to reduce mosquito breeding. For instance, one option is to adopt operating schedules that, at critical times, dry out shoreline areas where mosquitoes tend to breed.
The researchers said dam developers should also consider increasing investment in integrated malaria intervention programs that include measures such as bed net distribution. Other environmental controls, such as introducing fish that eat mosquito larva in dam reservoirs, could also help reduce malaria cases in some instances.
“The bottom line is that adverse malaria impacts of dams routinely receive recognition in Environmental Impact Assessments, and areas around dams are frequently earmarked for intensive control efforts,” said study author Jonathan Lautze, PhD, of the International Water Management Institute in Pretoria, South Africa.
“The findings of our work hammer home the reality that this recognition and effort—well-intentioned though it may be—is simply not sufficient. Given the need for water resources development in Africa, malaria control around dams requires interdisciplinary cooperation, particularly between water and health communities. Malaria must be addressed while planning, designing, and operating African dams.”