User login
Precision blood test for rheumatoid arthritis receives Medicare coverage
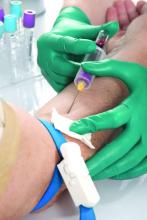
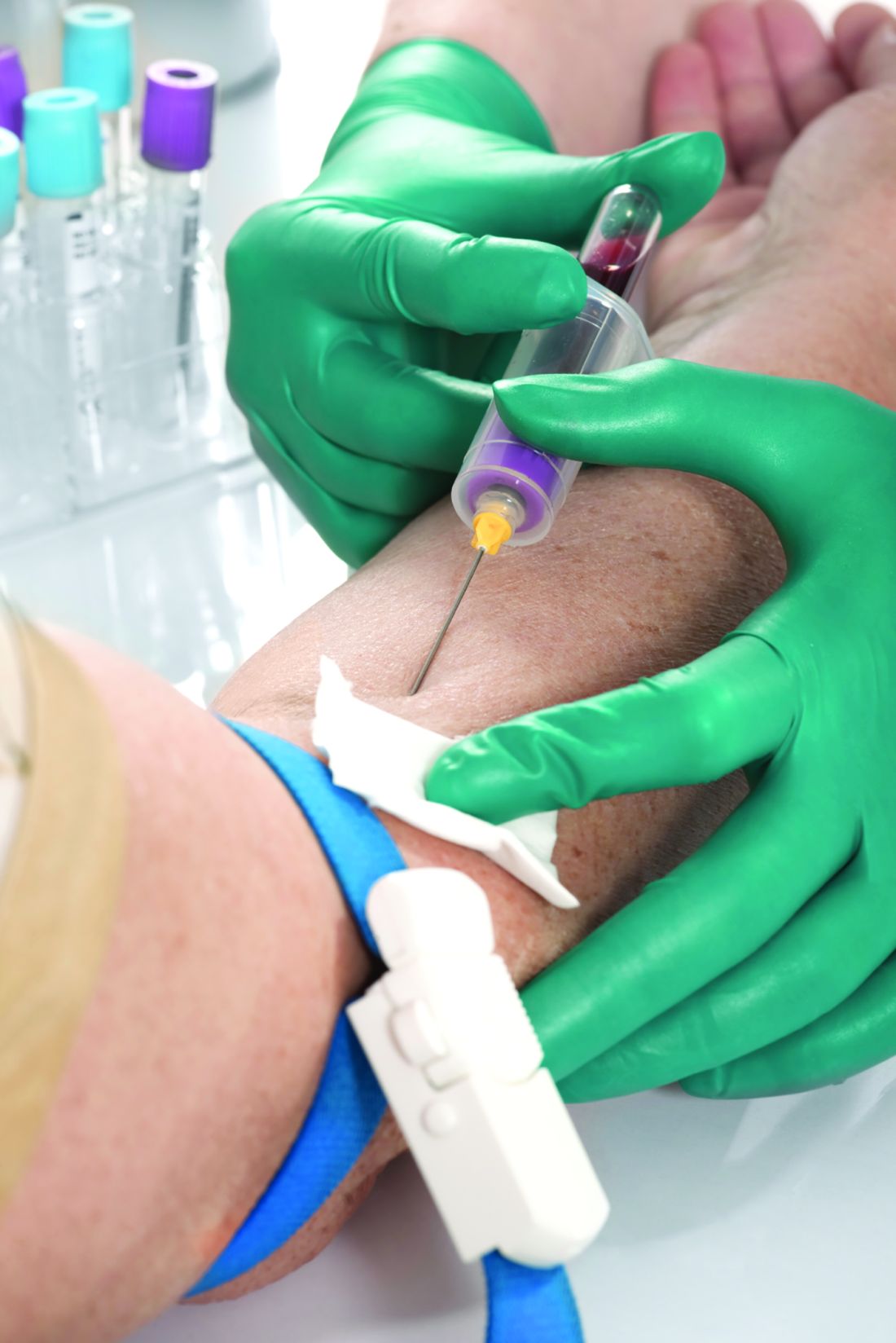
Medicare will now cover a molecular diagnostic test to predict treatment response for certain patients with rheumatoid arthritis.
The blood test, PrismRA, is the first and only commercially available test that can help predict which patients with RA are unlikely to respond to tumor necrosis factor inhibitor therapy, according to the test manufacturer, Scipher Medicine.
“Precision medicine will now be accessible to many patients suffering from RA, a potentially debilitating disease if not treated with the right therapy,” Alif Saleh, the company’s chief executive officer, said in a press release on Sept. 7. “This coverage decision not only represents a significant benefit for patients today but also ushers in a new era of precision medicine in autoimmune diseases.”
PrismRA was first made available in December 2021 for commercial billing. The test costs about $5,000, but most patients with insurance coverage pay less than $75 out-of-pocket after insurance, according to Scipher.
On Sept. 1, 2022, the Medicare administrative contractor Palmetto GBA published a draft recommendation that the test should not be covered by the national health insurance program, stating that biomarker tests “have not yet demonstrated definitive value above the combination of available clinical, laboratory, and demographic data.”
During the comment period, clinicians urged the contractor to reconsider.
“I do not have a test or clinical assessment to inform me of the right biologic for my patients. I utilize the PrismRA test to inform me which biologic is the best start. Without this valuable tool, I am left with prescribing based on what is dictated by the patient’s insurance,” wrote one commenter.
These responses and additional data published during the comment period resulted in Palmetto GBA revising their decision.
“We agree that despite the many limitations of predictive biomarker tests, a review of the evidence supports their limited use given their demonstrated validity and utility,” the company wrote in response. “Specifically, when a nonresponse signature is obtained by the molecular signature response classifier, nearly 90% of those patients will prove to not clinically respond to TNFi therapies using multiple validated disease response criteria including the ACR50 and CDAI. For these patients, a change in management would ultimately serve to avoid time on an unnecessary therapy and shorten the time to an appropriate therapy.”
The local coverage determination provides Medicare coverage nationally for patients who have a confirmed diagnosis of moderate to severely active RA, have failed first-line therapy for RA treatment, and have not started biologic or targeted synthetic therapy for RA or who are being considered for an alternative class of targeted therapy due to failure of an initially targeted therapy despite adequate dosing.
The LCD becomes effective for tests performed on or after Oct. 15, 2023.
A version of this article first appeared on Medscape.com.
Medicare will now cover a molecular diagnostic test to predict treatment response for certain patients with rheumatoid arthritis.
The blood test, PrismRA, is the first and only commercially available test that can help predict which patients with RA are unlikely to respond to tumor necrosis factor inhibitor therapy, according to the test manufacturer, Scipher Medicine.
“Precision medicine will now be accessible to many patients suffering from RA, a potentially debilitating disease if not treated with the right therapy,” Alif Saleh, the company’s chief executive officer, said in a press release on Sept. 7. “This coverage decision not only represents a significant benefit for patients today but also ushers in a new era of precision medicine in autoimmune diseases.”
PrismRA was first made available in December 2021 for commercial billing. The test costs about $5,000, but most patients with insurance coverage pay less than $75 out-of-pocket after insurance, according to Scipher.
On Sept. 1, 2022, the Medicare administrative contractor Palmetto GBA published a draft recommendation that the test should not be covered by the national health insurance program, stating that biomarker tests “have not yet demonstrated definitive value above the combination of available clinical, laboratory, and demographic data.”
During the comment period, clinicians urged the contractor to reconsider.
“I do not have a test or clinical assessment to inform me of the right biologic for my patients. I utilize the PrismRA test to inform me which biologic is the best start. Without this valuable tool, I am left with prescribing based on what is dictated by the patient’s insurance,” wrote one commenter.
These responses and additional data published during the comment period resulted in Palmetto GBA revising their decision.
“We agree that despite the many limitations of predictive biomarker tests, a review of the evidence supports their limited use given their demonstrated validity and utility,” the company wrote in response. “Specifically, when a nonresponse signature is obtained by the molecular signature response classifier, nearly 90% of those patients will prove to not clinically respond to TNFi therapies using multiple validated disease response criteria including the ACR50 and CDAI. For these patients, a change in management would ultimately serve to avoid time on an unnecessary therapy and shorten the time to an appropriate therapy.”
The local coverage determination provides Medicare coverage nationally for patients who have a confirmed diagnosis of moderate to severely active RA, have failed first-line therapy for RA treatment, and have not started biologic or targeted synthetic therapy for RA or who are being considered for an alternative class of targeted therapy due to failure of an initially targeted therapy despite adequate dosing.
The LCD becomes effective for tests performed on or after Oct. 15, 2023.
A version of this article first appeared on Medscape.com.
Medicare will now cover a molecular diagnostic test to predict treatment response for certain patients with rheumatoid arthritis.
The blood test, PrismRA, is the first and only commercially available test that can help predict which patients with RA are unlikely to respond to tumor necrosis factor inhibitor therapy, according to the test manufacturer, Scipher Medicine.
“Precision medicine will now be accessible to many patients suffering from RA, a potentially debilitating disease if not treated with the right therapy,” Alif Saleh, the company’s chief executive officer, said in a press release on Sept. 7. “This coverage decision not only represents a significant benefit for patients today but also ushers in a new era of precision medicine in autoimmune diseases.”
PrismRA was first made available in December 2021 for commercial billing. The test costs about $5,000, but most patients with insurance coverage pay less than $75 out-of-pocket after insurance, according to Scipher.
On Sept. 1, 2022, the Medicare administrative contractor Palmetto GBA published a draft recommendation that the test should not be covered by the national health insurance program, stating that biomarker tests “have not yet demonstrated definitive value above the combination of available clinical, laboratory, and demographic data.”
During the comment period, clinicians urged the contractor to reconsider.
“I do not have a test or clinical assessment to inform me of the right biologic for my patients. I utilize the PrismRA test to inform me which biologic is the best start. Without this valuable tool, I am left with prescribing based on what is dictated by the patient’s insurance,” wrote one commenter.
These responses and additional data published during the comment period resulted in Palmetto GBA revising their decision.
“We agree that despite the many limitations of predictive biomarker tests, a review of the evidence supports their limited use given their demonstrated validity and utility,” the company wrote in response. “Specifically, when a nonresponse signature is obtained by the molecular signature response classifier, nearly 90% of those patients will prove to not clinically respond to TNFi therapies using multiple validated disease response criteria including the ACR50 and CDAI. For these patients, a change in management would ultimately serve to avoid time on an unnecessary therapy and shorten the time to an appropriate therapy.”
The local coverage determination provides Medicare coverage nationally for patients who have a confirmed diagnosis of moderate to severely active RA, have failed first-line therapy for RA treatment, and have not started biologic or targeted synthetic therapy for RA or who are being considered for an alternative class of targeted therapy due to failure of an initially targeted therapy despite adequate dosing.
The LCD becomes effective for tests performed on or after Oct. 15, 2023.
A version of this article first appeared on Medscape.com.
Commentary: Cardiovascular risk, anti-drug antibodies, and prednisolone in RA, September 2023
Anti-drug antibody (ADA) testing for biologics, particularly anti–tumor necrosis factor (TNF) agents, has been commercially available for several years, though its clinical use in rheumatoid arthritis (RA) is not known owing to lack of prospective data. Bitoun and colleagues analyzed data from the European ABI-RA registry to evaluate the association between ADA and the anti–TNF monoclonal antibodies (mAb) etanercept, tocilizumab, and rituximab, and clinical response (as measured by disease activity scores, inflammatory markers, and European Alliance of Associations for Rheumatology [EULAR] response rate). Higher rates of ADA positivity were seen in patients treated with rituximab (50%), anti-TNF mAb (38%), and tocilizumab (20%) compared with etanercept (6%). Patients who had a positive ADA test were less likely to have a EULAR response. In addition, patients treated with methotrexate were less likely to have persistent ADA. Though the study was not powered enough to detect differences between the drug classes, the evidence presented is compelling and suggests a role for measuring ADA in patients with RA who do not respond to treatment.
RA is well-known to be associated with cardiovascular disease, particularly atherosclerotic disease and heart failure, but its association with valvular heart disease and its progression has not been well-explored in the literature. Johnson and colleagues performed a cohort study of over 73,000 patients with RA in the Veterans Health Administration (VHA) system compared with 640,000 patients without RA to evaluate the incidence of aortic stenosis, need for intervention, and risk for death. Though the overall incidence rate was low (about 3%), patients with RA had a higher risk for aortic stenosis, with a hazard ratio of 1.48 compared with those without RA, as well as a higher risk for aortic valve replacement and aortic stenosis–related death. The risk for aortic stenosis was associated with hypertension, stroke, and other cardiovascular disease, as well as a body mass index > 30 kg/m2, although not with a history of smoking or diabetes. Because the study was performed using data from the VHA — that is, from predominantly male patients — this finding may not be generalizable. In addition, the diagnosis of aortic stenosis is generally reliant on echocardiography and may be detected while searching for other conditions not evaluated here (such as pericarditis). As such, these findings would not support routine screening in patients with RA without other reasons for suspicion of valvular heart disease.
In particular, the increase in cardiovascular risk associated with glucocorticoid therapy in patients with RA has received increased scrutiny, along with other side effects of systemic glucocorticoids. In a recent retrospective study, So and colleagues examined the clinical data of over 12,000 patients with RA treated in public hospitals in Hong Kong with a mean of 9 years of follow-up. Consistent with prior studies, systemic glucocorticoid use (prednisolone equivalent > 5 mg daily) was associated with an increased risk for adverse cardiovascular events, whereas lower doses did not increase cardiovascular risk. Because the data on some disease activity measures and traditional cardiovascular risk factors (such as smoking or obesity) were not available in the database, the study supports, but does not expand on, prior evidence regarding cardiovascular risk.
Almayali and colleagues also looked at glucocorticoid therapy in RA in a follow-up study to the previously published pragmatic randomized double-blinded placebo-controlled GLORIA study, which evaluated the effects of 5 mg/d prednisolone added to standard care for 2 years in patients with active RA who were age 65 years or older. In the current study, 191 patients out of the initial 451 were followed for 3 months and prednisolone tapered off. Patients who tapered off prednisolone had, as expected, an increased risk for flare but no evidence of adrenal insufficiency. Although, again, this is not likely to change practice, it does suggest that glucocorticoid tapering is a reasonable goal in RA therapeutic trials.
Anti-drug antibody (ADA) testing for biologics, particularly anti–tumor necrosis factor (TNF) agents, has been commercially available for several years, though its clinical use in rheumatoid arthritis (RA) is not known owing to lack of prospective data. Bitoun and colleagues analyzed data from the European ABI-RA registry to evaluate the association between ADA and the anti–TNF monoclonal antibodies (mAb) etanercept, tocilizumab, and rituximab, and clinical response (as measured by disease activity scores, inflammatory markers, and European Alliance of Associations for Rheumatology [EULAR] response rate). Higher rates of ADA positivity were seen in patients treated with rituximab (50%), anti-TNF mAb (38%), and tocilizumab (20%) compared with etanercept (6%). Patients who had a positive ADA test were less likely to have a EULAR response. In addition, patients treated with methotrexate were less likely to have persistent ADA. Though the study was not powered enough to detect differences between the drug classes, the evidence presented is compelling and suggests a role for measuring ADA in patients with RA who do not respond to treatment.
RA is well-known to be associated with cardiovascular disease, particularly atherosclerotic disease and heart failure, but its association with valvular heart disease and its progression has not been well-explored in the literature. Johnson and colleagues performed a cohort study of over 73,000 patients with RA in the Veterans Health Administration (VHA) system compared with 640,000 patients without RA to evaluate the incidence of aortic stenosis, need for intervention, and risk for death. Though the overall incidence rate was low (about 3%), patients with RA had a higher risk for aortic stenosis, with a hazard ratio of 1.48 compared with those without RA, as well as a higher risk for aortic valve replacement and aortic stenosis–related death. The risk for aortic stenosis was associated with hypertension, stroke, and other cardiovascular disease, as well as a body mass index > 30 kg/m2, although not with a history of smoking or diabetes. Because the study was performed using data from the VHA — that is, from predominantly male patients — this finding may not be generalizable. In addition, the diagnosis of aortic stenosis is generally reliant on echocardiography and may be detected while searching for other conditions not evaluated here (such as pericarditis). As such, these findings would not support routine screening in patients with RA without other reasons for suspicion of valvular heart disease.
In particular, the increase in cardiovascular risk associated with glucocorticoid therapy in patients with RA has received increased scrutiny, along with other side effects of systemic glucocorticoids. In a recent retrospective study, So and colleagues examined the clinical data of over 12,000 patients with RA treated in public hospitals in Hong Kong with a mean of 9 years of follow-up. Consistent with prior studies, systemic glucocorticoid use (prednisolone equivalent > 5 mg daily) was associated with an increased risk for adverse cardiovascular events, whereas lower doses did not increase cardiovascular risk. Because the data on some disease activity measures and traditional cardiovascular risk factors (such as smoking or obesity) were not available in the database, the study supports, but does not expand on, prior evidence regarding cardiovascular risk.
Almayali and colleagues also looked at glucocorticoid therapy in RA in a follow-up study to the previously published pragmatic randomized double-blinded placebo-controlled GLORIA study, which evaluated the effects of 5 mg/d prednisolone added to standard care for 2 years in patients with active RA who were age 65 years or older. In the current study, 191 patients out of the initial 451 were followed for 3 months and prednisolone tapered off. Patients who tapered off prednisolone had, as expected, an increased risk for flare but no evidence of adrenal insufficiency. Although, again, this is not likely to change practice, it does suggest that glucocorticoid tapering is a reasonable goal in RA therapeutic trials.
Anti-drug antibody (ADA) testing for biologics, particularly anti–tumor necrosis factor (TNF) agents, has been commercially available for several years, though its clinical use in rheumatoid arthritis (RA) is not known owing to lack of prospective data. Bitoun and colleagues analyzed data from the European ABI-RA registry to evaluate the association between ADA and the anti–TNF monoclonal antibodies (mAb) etanercept, tocilizumab, and rituximab, and clinical response (as measured by disease activity scores, inflammatory markers, and European Alliance of Associations for Rheumatology [EULAR] response rate). Higher rates of ADA positivity were seen in patients treated with rituximab (50%), anti-TNF mAb (38%), and tocilizumab (20%) compared with etanercept (6%). Patients who had a positive ADA test were less likely to have a EULAR response. In addition, patients treated with methotrexate were less likely to have persistent ADA. Though the study was not powered enough to detect differences between the drug classes, the evidence presented is compelling and suggests a role for measuring ADA in patients with RA who do not respond to treatment.
RA is well-known to be associated with cardiovascular disease, particularly atherosclerotic disease and heart failure, but its association with valvular heart disease and its progression has not been well-explored in the literature. Johnson and colleagues performed a cohort study of over 73,000 patients with RA in the Veterans Health Administration (VHA) system compared with 640,000 patients without RA to evaluate the incidence of aortic stenosis, need for intervention, and risk for death. Though the overall incidence rate was low (about 3%), patients with RA had a higher risk for aortic stenosis, with a hazard ratio of 1.48 compared with those without RA, as well as a higher risk for aortic valve replacement and aortic stenosis–related death. The risk for aortic stenosis was associated with hypertension, stroke, and other cardiovascular disease, as well as a body mass index > 30 kg/m2, although not with a history of smoking or diabetes. Because the study was performed using data from the VHA — that is, from predominantly male patients — this finding may not be generalizable. In addition, the diagnosis of aortic stenosis is generally reliant on echocardiography and may be detected while searching for other conditions not evaluated here (such as pericarditis). As such, these findings would not support routine screening in patients with RA without other reasons for suspicion of valvular heart disease.
In particular, the increase in cardiovascular risk associated with glucocorticoid therapy in patients with RA has received increased scrutiny, along with other side effects of systemic glucocorticoids. In a recent retrospective study, So and colleagues examined the clinical data of over 12,000 patients with RA treated in public hospitals in Hong Kong with a mean of 9 years of follow-up. Consistent with prior studies, systemic glucocorticoid use (prednisolone equivalent > 5 mg daily) was associated with an increased risk for adverse cardiovascular events, whereas lower doses did not increase cardiovascular risk. Because the data on some disease activity measures and traditional cardiovascular risk factors (such as smoking or obesity) were not available in the database, the study supports, but does not expand on, prior evidence regarding cardiovascular risk.
Almayali and colleagues also looked at glucocorticoid therapy in RA in a follow-up study to the previously published pragmatic randomized double-blinded placebo-controlled GLORIA study, which evaluated the effects of 5 mg/d prednisolone added to standard care for 2 years in patients with active RA who were age 65 years or older. In the current study, 191 patients out of the initial 451 were followed for 3 months and prednisolone tapered off. Patients who tapered off prednisolone had, as expected, an increased risk for flare but no evidence of adrenal insufficiency. Although, again, this is not likely to change practice, it does suggest that glucocorticoid tapering is a reasonable goal in RA therapeutic trials.
ACR releases guideline for managing ILD in patients with rheumatic disease
The American College of Rheumatology has released a summary of upcoming guidelines on screening, monitoring, and treatment for interstitial lung disease (ILD) in patients with systemic autoimmune rheumatic disease.
The recommendations apply to adults with rheumatic diseases at greater risk for ILD: rheumatoid arthritis, systemic sclerosis (SSc), mixed connective tissue disease (MCTD), Sjögren’s disease (SjD), and idiopathic inflammatory myopathies (IIM).
“Interstitial lung disease is a major cause of morbidity and mortality across several systemic autoimmune rheumatic diseases,” Sindhu R. Johnson, MD, PhD, lead author of the new guidelines and director of the clinical epidemiology and health care research program at the University of Toronto, said in an ACR press release. “Guidance was needed for which tests to use for screening and monitoring this particular disease.”
The two documents are summaries of part of a larger manuscript currently awaiting peer review, according to the ACR, and the final guidelines are anticipated to be published by early 2024.
The recommendations were developed using “the best available evidence and consensus across a range of expert opinions and incorporated patient values and preferences,” according to the press release.
Highlights of recommendations for screening and monitoring ILD are:
- Providers can screen patients at higher risk for ILD with pulmonary function tests (PFTs) and high-resolution CT of the chest.
- PFTs, chest high-resolution CT, and ambulatory desaturation testing are conditionally recommended for monitoring ILD progression.
- It is conditionally recommended that providers do not use 6-minute walk test distance, chest radiography, or bronchoscopy for screening or monitoring disease.
- It is suggested that patients with IIM-ILD and SSc-ILD receive PFTs for monitoring every 3-6 months during the first year, then less frequently once stable.
- It is suggested that patients with RA-ILD, SjD-ILD, and MCTD-ILD receive PFTs every 3-12 months for the first year, then less frequently once stable.
Suggestions on how often to screen for ILD were not present in the summary documents, but will be made available in the larger manuscript, said Elana Bernstein, MD, director of the Columbia University Medical Center/New York–Presbyterian Hospital scleroderma program, New York. She is co–first author of the guidelines.
Nearly all recommendations are conditional, primarily because the certainty of evidence behind many of these recommendations is low or very low, she said in an interview. More clinical data on ILD in patients with rheumatic disease would help strengthen evidence, she said, particularly for best practices in frequency of testing. “We need more research on how often patients should be screened for ILD and how often they should be monitored for ILD progression,” she said. “That would enable us to provide recommendations, rather than just suggestions.”
Highlights of recommendations for ILD treatment are:
- The guidelines strongly recommend against using glucocorticoids for first-line ILD treatment in patients with SSc-ILD.
- Short-term glucocorticoids are conditionally recommended as a first-line ILD treatment for patients with systemic autoimmune rheumatic disease–related ILD (SARD-ILD), excluding SSc-ILD.
- Mycophenolate, azathioprine, rituximab, and cyclophosphamide are all potential first-line ILD treatment options for patients with SARD-ILD.
- It is conditionally recommended that patients with SARD-ILD do not receive leflunomide, methotrexate, tumor necrosis factor inhibitors, or abatacept as first-line ILD treatment.
- If SARD-ILD progresses despite first-line therapy, mycophenolate, rituximab, cyclophosphamide, and nintedanib are potential secondary treatment options.
- If RA-ILD progresses following initial therapy, pirfenidone is a treatment option.
- The guidelines conditionally recommend against pirfenidone as a secondary treatment option for SARD-ILD other than RA-ILD.
These summary guidelines appear “comprehensive,” but there has yet to be information published on the basis of these recommendations, Elizabeth Volkmann, MD, said in an interview.
“It’s important to understand that we don’t know whether most of these recommendations were just driven by expert opinion versus actual evidence from randomized, controlled clinical trials,” said Dr. Volkmann, who codirects the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles. She was not involved with creating the guidelines.
She expects that many of the recommendations for first- and second-line ILD treatment options were based on expert opinion, as there have been no randomized clinical trials looking at that specific topic, she said. For example, nintedanib is conditionally recommended as a first-line treatment option for SSc-ILD, but as a second-line treatment for SjD-ILD, IIM-ILD, and MCTD-ILD. “There’s no literature to support one or the other – whether nintedanib is first-line or second-line [treatment].”
The decision to publish the summary recommendations online prior to peer review is unusual, she said, as these recommendations could be altered during that process; however, Dr. Bernstein noted that was not likely.
By releasing the summary guideline now, the ACR can “get the needed information to clinicians earlier as the manuscript goes through its remaining stages and is finalized,” an ACR representative explained.
Prior to the expected publication of these guidelines in early 2024, Dr. Volkmann noted that the American Thoracic Society will be publishing guidelines on the treatment of SSc-ILD in the American Journal of Respiratory and Critical Care Medicine in September.
Dr. Bernstein reported grants/contracts with the Department of Defense, the Scleroderma Research Foundation, the National Institutes of Health, Eicos, Boehringer Ingelheim, Kadmon, and Pfizer. Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and GlaxoSmithKline and institutional support for performing studies on systemic sclerosis for Kadmon, Boehringer Ingelheim, Horizon, and Prometheus.
A version of this article first appeared on Medscape.com.
The American College of Rheumatology has released a summary of upcoming guidelines on screening, monitoring, and treatment for interstitial lung disease (ILD) in patients with systemic autoimmune rheumatic disease.
The recommendations apply to adults with rheumatic diseases at greater risk for ILD: rheumatoid arthritis, systemic sclerosis (SSc), mixed connective tissue disease (MCTD), Sjögren’s disease (SjD), and idiopathic inflammatory myopathies (IIM).
“Interstitial lung disease is a major cause of morbidity and mortality across several systemic autoimmune rheumatic diseases,” Sindhu R. Johnson, MD, PhD, lead author of the new guidelines and director of the clinical epidemiology and health care research program at the University of Toronto, said in an ACR press release. “Guidance was needed for which tests to use for screening and monitoring this particular disease.”
The two documents are summaries of part of a larger manuscript currently awaiting peer review, according to the ACR, and the final guidelines are anticipated to be published by early 2024.
The recommendations were developed using “the best available evidence and consensus across a range of expert opinions and incorporated patient values and preferences,” according to the press release.
Highlights of recommendations for screening and monitoring ILD are:
- Providers can screen patients at higher risk for ILD with pulmonary function tests (PFTs) and high-resolution CT of the chest.
- PFTs, chest high-resolution CT, and ambulatory desaturation testing are conditionally recommended for monitoring ILD progression.
- It is conditionally recommended that providers do not use 6-minute walk test distance, chest radiography, or bronchoscopy for screening or monitoring disease.
- It is suggested that patients with IIM-ILD and SSc-ILD receive PFTs for monitoring every 3-6 months during the first year, then less frequently once stable.
- It is suggested that patients with RA-ILD, SjD-ILD, and MCTD-ILD receive PFTs every 3-12 months for the first year, then less frequently once stable.
Suggestions on how often to screen for ILD were not present in the summary documents, but will be made available in the larger manuscript, said Elana Bernstein, MD, director of the Columbia University Medical Center/New York–Presbyterian Hospital scleroderma program, New York. She is co–first author of the guidelines.
Nearly all recommendations are conditional, primarily because the certainty of evidence behind many of these recommendations is low or very low, she said in an interview. More clinical data on ILD in patients with rheumatic disease would help strengthen evidence, she said, particularly for best practices in frequency of testing. “We need more research on how often patients should be screened for ILD and how often they should be monitored for ILD progression,” she said. “That would enable us to provide recommendations, rather than just suggestions.”
Highlights of recommendations for ILD treatment are:
- The guidelines strongly recommend against using glucocorticoids for first-line ILD treatment in patients with SSc-ILD.
- Short-term glucocorticoids are conditionally recommended as a first-line ILD treatment for patients with systemic autoimmune rheumatic disease–related ILD (SARD-ILD), excluding SSc-ILD.
- Mycophenolate, azathioprine, rituximab, and cyclophosphamide are all potential first-line ILD treatment options for patients with SARD-ILD.
- It is conditionally recommended that patients with SARD-ILD do not receive leflunomide, methotrexate, tumor necrosis factor inhibitors, or abatacept as first-line ILD treatment.
- If SARD-ILD progresses despite first-line therapy, mycophenolate, rituximab, cyclophosphamide, and nintedanib are potential secondary treatment options.
- If RA-ILD progresses following initial therapy, pirfenidone is a treatment option.
- The guidelines conditionally recommend against pirfenidone as a secondary treatment option for SARD-ILD other than RA-ILD.
These summary guidelines appear “comprehensive,” but there has yet to be information published on the basis of these recommendations, Elizabeth Volkmann, MD, said in an interview.
“It’s important to understand that we don’t know whether most of these recommendations were just driven by expert opinion versus actual evidence from randomized, controlled clinical trials,” said Dr. Volkmann, who codirects the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles. She was not involved with creating the guidelines.
She expects that many of the recommendations for first- and second-line ILD treatment options were based on expert opinion, as there have been no randomized clinical trials looking at that specific topic, she said. For example, nintedanib is conditionally recommended as a first-line treatment option for SSc-ILD, but as a second-line treatment for SjD-ILD, IIM-ILD, and MCTD-ILD. “There’s no literature to support one or the other – whether nintedanib is first-line or second-line [treatment].”
The decision to publish the summary recommendations online prior to peer review is unusual, she said, as these recommendations could be altered during that process; however, Dr. Bernstein noted that was not likely.
By releasing the summary guideline now, the ACR can “get the needed information to clinicians earlier as the manuscript goes through its remaining stages and is finalized,” an ACR representative explained.
Prior to the expected publication of these guidelines in early 2024, Dr. Volkmann noted that the American Thoracic Society will be publishing guidelines on the treatment of SSc-ILD in the American Journal of Respiratory and Critical Care Medicine in September.
Dr. Bernstein reported grants/contracts with the Department of Defense, the Scleroderma Research Foundation, the National Institutes of Health, Eicos, Boehringer Ingelheim, Kadmon, and Pfizer. Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and GlaxoSmithKline and institutional support for performing studies on systemic sclerosis for Kadmon, Boehringer Ingelheim, Horizon, and Prometheus.
A version of this article first appeared on Medscape.com.
The American College of Rheumatology has released a summary of upcoming guidelines on screening, monitoring, and treatment for interstitial lung disease (ILD) in patients with systemic autoimmune rheumatic disease.
The recommendations apply to adults with rheumatic diseases at greater risk for ILD: rheumatoid arthritis, systemic sclerosis (SSc), mixed connective tissue disease (MCTD), Sjögren’s disease (SjD), and idiopathic inflammatory myopathies (IIM).
“Interstitial lung disease is a major cause of morbidity and mortality across several systemic autoimmune rheumatic diseases,” Sindhu R. Johnson, MD, PhD, lead author of the new guidelines and director of the clinical epidemiology and health care research program at the University of Toronto, said in an ACR press release. “Guidance was needed for which tests to use for screening and monitoring this particular disease.”
The two documents are summaries of part of a larger manuscript currently awaiting peer review, according to the ACR, and the final guidelines are anticipated to be published by early 2024.
The recommendations were developed using “the best available evidence and consensus across a range of expert opinions and incorporated patient values and preferences,” according to the press release.
Highlights of recommendations for screening and monitoring ILD are:
- Providers can screen patients at higher risk for ILD with pulmonary function tests (PFTs) and high-resolution CT of the chest.
- PFTs, chest high-resolution CT, and ambulatory desaturation testing are conditionally recommended for monitoring ILD progression.
- It is conditionally recommended that providers do not use 6-minute walk test distance, chest radiography, or bronchoscopy for screening or monitoring disease.
- It is suggested that patients with IIM-ILD and SSc-ILD receive PFTs for monitoring every 3-6 months during the first year, then less frequently once stable.
- It is suggested that patients with RA-ILD, SjD-ILD, and MCTD-ILD receive PFTs every 3-12 months for the first year, then less frequently once stable.
Suggestions on how often to screen for ILD were not present in the summary documents, but will be made available in the larger manuscript, said Elana Bernstein, MD, director of the Columbia University Medical Center/New York–Presbyterian Hospital scleroderma program, New York. She is co–first author of the guidelines.
Nearly all recommendations are conditional, primarily because the certainty of evidence behind many of these recommendations is low or very low, she said in an interview. More clinical data on ILD in patients with rheumatic disease would help strengthen evidence, she said, particularly for best practices in frequency of testing. “We need more research on how often patients should be screened for ILD and how often they should be monitored for ILD progression,” she said. “That would enable us to provide recommendations, rather than just suggestions.”
Highlights of recommendations for ILD treatment are:
- The guidelines strongly recommend against using glucocorticoids for first-line ILD treatment in patients with SSc-ILD.
- Short-term glucocorticoids are conditionally recommended as a first-line ILD treatment for patients with systemic autoimmune rheumatic disease–related ILD (SARD-ILD), excluding SSc-ILD.
- Mycophenolate, azathioprine, rituximab, and cyclophosphamide are all potential first-line ILD treatment options for patients with SARD-ILD.
- It is conditionally recommended that patients with SARD-ILD do not receive leflunomide, methotrexate, tumor necrosis factor inhibitors, or abatacept as first-line ILD treatment.
- If SARD-ILD progresses despite first-line therapy, mycophenolate, rituximab, cyclophosphamide, and nintedanib are potential secondary treatment options.
- If RA-ILD progresses following initial therapy, pirfenidone is a treatment option.
- The guidelines conditionally recommend against pirfenidone as a secondary treatment option for SARD-ILD other than RA-ILD.
These summary guidelines appear “comprehensive,” but there has yet to be information published on the basis of these recommendations, Elizabeth Volkmann, MD, said in an interview.
“It’s important to understand that we don’t know whether most of these recommendations were just driven by expert opinion versus actual evidence from randomized, controlled clinical trials,” said Dr. Volkmann, who codirects the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles. She was not involved with creating the guidelines.
She expects that many of the recommendations for first- and second-line ILD treatment options were based on expert opinion, as there have been no randomized clinical trials looking at that specific topic, she said. For example, nintedanib is conditionally recommended as a first-line treatment option for SSc-ILD, but as a second-line treatment for SjD-ILD, IIM-ILD, and MCTD-ILD. “There’s no literature to support one or the other – whether nintedanib is first-line or second-line [treatment].”
The decision to publish the summary recommendations online prior to peer review is unusual, she said, as these recommendations could be altered during that process; however, Dr. Bernstein noted that was not likely.
By releasing the summary guideline now, the ACR can “get the needed information to clinicians earlier as the manuscript goes through its remaining stages and is finalized,” an ACR representative explained.
Prior to the expected publication of these guidelines in early 2024, Dr. Volkmann noted that the American Thoracic Society will be publishing guidelines on the treatment of SSc-ILD in the American Journal of Respiratory and Critical Care Medicine in September.
Dr. Bernstein reported grants/contracts with the Department of Defense, the Scleroderma Research Foundation, the National Institutes of Health, Eicos, Boehringer Ingelheim, Kadmon, and Pfizer. Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and GlaxoSmithKline and institutional support for performing studies on systemic sclerosis for Kadmon, Boehringer Ingelheim, Horizon, and Prometheus.
A version of this article first appeared on Medscape.com.
Medicare announces 10 drugs targeted for price cuts in 2026
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
Slight weight gain, no blood pressure increase with low-dose steroids for RA
Patients taking long-term, low-dose glucocorticoids for rheumatoid arthritis over 2 years had a very modest relative weight gain but no relative increase in blood pressure when compared with patients who did not take the drugs, according to findings from a combined study of randomized, controlled trials (RCTs).
“This pooled analysis of five RCTs in RA found that 2 years of low-dose glucocorticoid treatment [at 7.5 mg/day or less] leads to a modest weight gain of about 1 kg but has no effect on blood pressure,” lead study author Andriko Palmowski, MD, a physician and researcher in rheumatology and clinical immunology at Charité-Universitätsmedizin Berlin, and colleagues wrote in the study, published in Annals of Internal Medicine.
“Many clinicians fear using even low-dose glucocorticoids because of the adverse effects associated with their long-term use at higher doses,” noted Leslie J. Crofford, MD, professor of medicine, pathology, microbiology, and immunology, and director of the division of rheumatology and immunology at Vanderbilt University, Nashville, Tenn.
“Indeed, long-term use of even these low doses increases risk for many significant adverse effects, including osteoporosis and cataracts in observational cohorts,” added Dr. Crofford, who was not involved in the study.
Studies were combined for stronger results
Observational studies are prone to confounding, and the RCTs in the literature have been small, resulting in low statistical power, the authors explained.
To overcome these limitations, Dr. Palmowski and associates combined individual participant data from five RCTs of glucocorticoid treatment for RA in 12 countries in Europe. The 1,112 participants had early and established RA, averaged 61.4 years of age, and 68% were women. The GLORIA trial, an RCT that contributed about 40% of the overall study population, “explicitly included elderly patients and patients with multimorbidity who are often excluded from RA trials,” the authors wrote.
Participants in the intervention group took low-dose glucocorticoids (prednisone equivalent, ≤ 7.5 mg/day; three trials used a dose of 5 mg prednisone equivalent per day); and patients in the control groups took placebo, disease-modifying antirheumatic drugs, or both. The researchers compared change over 2 years in body weight and mean arterial pressure between the groups.
At 2 years, both groups gained weight, but participants who took glucocorticoids gained an average of 1.1 kg (P < .001) more than the controls. Mean arterial pressure increased by around 2 mm Hg in both groups, with a –0.4 mm Hg between-group difference (P = .187).
Daniel G. Arkfeld MD, DDS, professor of clinical medicine in the division of rheumatology at the University of Southern California, Los Angeles, found this “a fascinating analysis” and called the lack of change in blood pressure important.
“Steroids are used less in RA due to perceived side effects. Yet many patients have ongoing synovitis and need steroids to enable them to work and perform other activities,” said Dr. Arkfeld, who also was not involved in the study. “NSAIDs are more of an issue, with up to 10% raising blood pressure. Should we be using more steroids and less NSAIDs?”
Dr. Arkfeld also was concerned that the small 2-year weight gain may become significant over time.
Kim Marie Huffman, MD, PhD, associate professor of medicine at Duke University, Durham, N.C., agreed.
“More investigations and longer (or shorter) time periods may have yielded additional findings,” said Dr. Huffman, who also was not involved in the study. “Efforts should be made to minimize long-term prednisone use to minimize impact on weight gain and resulting consequences.”
Are these results applicable to U.S. patients?
“Low-dose prednisone is commonly used in the U.S.,” Dr. Huffman said. “Extrapolating the results to a U.S. population is probably fine.”
Dr. Arkfeld agreed that the results can be used to treat U.S. patients because of the large number of study participants.
According to Rebecca B. Blank, MD, PhD, rheumatologist and instructor of medicine at NYU Langone Health, New York, this is an important study. But she cautioned that the literature does not contain good data for other potential harmful effects of long-term, low-dose glucocorticoid use. “Therefore, as per both ACR [American College of Rheumatology] and EULAR [European Alliance of Associations for Rheumatology] recommendations, we should still try to limit glucocorticoids to the lowest dose and shortest duration possible in our RA patients,” advised Dr. Blank, who was not an author in the study.
Strengths, weaknesses, and thoughts on further research
“Pooling trials can be tricky, but these investigators used individual-level data, which increases the rigor of the analyses,” Dr. Crofford noted. “There were differences in patient populations and with the glucocorticoid doses and routes of administration. The fact that the patients in each of the studies were randomized is very important in determining if the outcomes can be attributed to the drugs or could be the results of other exposures.”
Dr. Arkfeld would like to know whether early versus late RA patients may have different results because they may have different pathophysiologies.
Dr. Huffman is interested in low-dose glucocorticoids’ impacts on glucose homeostasis, bone density, infection, and other common adverse effects.
In an accompanying editorial, David Fernandez, MD, PhD, of Hospital for Special Surgery, New York, wrote: “These findings provide a more quantifiable assessment of the potential adverse effects of steroid therapy than had existed previously and will be helpful to providers and patients as they decide on the relative risks and benefits of glucocorticoids as part of their therapy plan in rheumatoid arthritis.”
The study received no specific funding. Four of the study’s 13 authors reported financial relationships with pharmaceutical companies. Dr. Fernandez and all outside experts who commented on the study reported no relevant financial relationships.
Patients taking long-term, low-dose glucocorticoids for rheumatoid arthritis over 2 years had a very modest relative weight gain but no relative increase in blood pressure when compared with patients who did not take the drugs, according to findings from a combined study of randomized, controlled trials (RCTs).
“This pooled analysis of five RCTs in RA found that 2 years of low-dose glucocorticoid treatment [at 7.5 mg/day or less] leads to a modest weight gain of about 1 kg but has no effect on blood pressure,” lead study author Andriko Palmowski, MD, a physician and researcher in rheumatology and clinical immunology at Charité-Universitätsmedizin Berlin, and colleagues wrote in the study, published in Annals of Internal Medicine.
“Many clinicians fear using even low-dose glucocorticoids because of the adverse effects associated with their long-term use at higher doses,” noted Leslie J. Crofford, MD, professor of medicine, pathology, microbiology, and immunology, and director of the division of rheumatology and immunology at Vanderbilt University, Nashville, Tenn.
“Indeed, long-term use of even these low doses increases risk for many significant adverse effects, including osteoporosis and cataracts in observational cohorts,” added Dr. Crofford, who was not involved in the study.
Studies were combined for stronger results
Observational studies are prone to confounding, and the RCTs in the literature have been small, resulting in low statistical power, the authors explained.
To overcome these limitations, Dr. Palmowski and associates combined individual participant data from five RCTs of glucocorticoid treatment for RA in 12 countries in Europe. The 1,112 participants had early and established RA, averaged 61.4 years of age, and 68% were women. The GLORIA trial, an RCT that contributed about 40% of the overall study population, “explicitly included elderly patients and patients with multimorbidity who are often excluded from RA trials,” the authors wrote.
Participants in the intervention group took low-dose glucocorticoids (prednisone equivalent, ≤ 7.5 mg/day; three trials used a dose of 5 mg prednisone equivalent per day); and patients in the control groups took placebo, disease-modifying antirheumatic drugs, or both. The researchers compared change over 2 years in body weight and mean arterial pressure between the groups.
At 2 years, both groups gained weight, but participants who took glucocorticoids gained an average of 1.1 kg (P < .001) more than the controls. Mean arterial pressure increased by around 2 mm Hg in both groups, with a –0.4 mm Hg between-group difference (P = .187).
Daniel G. Arkfeld MD, DDS, professor of clinical medicine in the division of rheumatology at the University of Southern California, Los Angeles, found this “a fascinating analysis” and called the lack of change in blood pressure important.
“Steroids are used less in RA due to perceived side effects. Yet many patients have ongoing synovitis and need steroids to enable them to work and perform other activities,” said Dr. Arkfeld, who also was not involved in the study. “NSAIDs are more of an issue, with up to 10% raising blood pressure. Should we be using more steroids and less NSAIDs?”
Dr. Arkfeld also was concerned that the small 2-year weight gain may become significant over time.
Kim Marie Huffman, MD, PhD, associate professor of medicine at Duke University, Durham, N.C., agreed.
“More investigations and longer (or shorter) time periods may have yielded additional findings,” said Dr. Huffman, who also was not involved in the study. “Efforts should be made to minimize long-term prednisone use to minimize impact on weight gain and resulting consequences.”
Are these results applicable to U.S. patients?
“Low-dose prednisone is commonly used in the U.S.,” Dr. Huffman said. “Extrapolating the results to a U.S. population is probably fine.”
Dr. Arkfeld agreed that the results can be used to treat U.S. patients because of the large number of study participants.
According to Rebecca B. Blank, MD, PhD, rheumatologist and instructor of medicine at NYU Langone Health, New York, this is an important study. But she cautioned that the literature does not contain good data for other potential harmful effects of long-term, low-dose glucocorticoid use. “Therefore, as per both ACR [American College of Rheumatology] and EULAR [European Alliance of Associations for Rheumatology] recommendations, we should still try to limit glucocorticoids to the lowest dose and shortest duration possible in our RA patients,” advised Dr. Blank, who was not an author in the study.
Strengths, weaknesses, and thoughts on further research
“Pooling trials can be tricky, but these investigators used individual-level data, which increases the rigor of the analyses,” Dr. Crofford noted. “There were differences in patient populations and with the glucocorticoid doses and routes of administration. The fact that the patients in each of the studies were randomized is very important in determining if the outcomes can be attributed to the drugs or could be the results of other exposures.”
Dr. Arkfeld would like to know whether early versus late RA patients may have different results because they may have different pathophysiologies.
Dr. Huffman is interested in low-dose glucocorticoids’ impacts on glucose homeostasis, bone density, infection, and other common adverse effects.
In an accompanying editorial, David Fernandez, MD, PhD, of Hospital for Special Surgery, New York, wrote: “These findings provide a more quantifiable assessment of the potential adverse effects of steroid therapy than had existed previously and will be helpful to providers and patients as they decide on the relative risks and benefits of glucocorticoids as part of their therapy plan in rheumatoid arthritis.”
The study received no specific funding. Four of the study’s 13 authors reported financial relationships with pharmaceutical companies. Dr. Fernandez and all outside experts who commented on the study reported no relevant financial relationships.
Patients taking long-term, low-dose glucocorticoids for rheumatoid arthritis over 2 years had a very modest relative weight gain but no relative increase in blood pressure when compared with patients who did not take the drugs, according to findings from a combined study of randomized, controlled trials (RCTs).
“This pooled analysis of five RCTs in RA found that 2 years of low-dose glucocorticoid treatment [at 7.5 mg/day or less] leads to a modest weight gain of about 1 kg but has no effect on blood pressure,” lead study author Andriko Palmowski, MD, a physician and researcher in rheumatology and clinical immunology at Charité-Universitätsmedizin Berlin, and colleagues wrote in the study, published in Annals of Internal Medicine.
“Many clinicians fear using even low-dose glucocorticoids because of the adverse effects associated with their long-term use at higher doses,” noted Leslie J. Crofford, MD, professor of medicine, pathology, microbiology, and immunology, and director of the division of rheumatology and immunology at Vanderbilt University, Nashville, Tenn.
“Indeed, long-term use of even these low doses increases risk for many significant adverse effects, including osteoporosis and cataracts in observational cohorts,” added Dr. Crofford, who was not involved in the study.
Studies were combined for stronger results
Observational studies are prone to confounding, and the RCTs in the literature have been small, resulting in low statistical power, the authors explained.
To overcome these limitations, Dr. Palmowski and associates combined individual participant data from five RCTs of glucocorticoid treatment for RA in 12 countries in Europe. The 1,112 participants had early and established RA, averaged 61.4 years of age, and 68% were women. The GLORIA trial, an RCT that contributed about 40% of the overall study population, “explicitly included elderly patients and patients with multimorbidity who are often excluded from RA trials,” the authors wrote.
Participants in the intervention group took low-dose glucocorticoids (prednisone equivalent, ≤ 7.5 mg/day; three trials used a dose of 5 mg prednisone equivalent per day); and patients in the control groups took placebo, disease-modifying antirheumatic drugs, or both. The researchers compared change over 2 years in body weight and mean arterial pressure between the groups.
At 2 years, both groups gained weight, but participants who took glucocorticoids gained an average of 1.1 kg (P < .001) more than the controls. Mean arterial pressure increased by around 2 mm Hg in both groups, with a –0.4 mm Hg between-group difference (P = .187).
Daniel G. Arkfeld MD, DDS, professor of clinical medicine in the division of rheumatology at the University of Southern California, Los Angeles, found this “a fascinating analysis” and called the lack of change in blood pressure important.
“Steroids are used less in RA due to perceived side effects. Yet many patients have ongoing synovitis and need steroids to enable them to work and perform other activities,” said Dr. Arkfeld, who also was not involved in the study. “NSAIDs are more of an issue, with up to 10% raising blood pressure. Should we be using more steroids and less NSAIDs?”
Dr. Arkfeld also was concerned that the small 2-year weight gain may become significant over time.
Kim Marie Huffman, MD, PhD, associate professor of medicine at Duke University, Durham, N.C., agreed.
“More investigations and longer (or shorter) time periods may have yielded additional findings,” said Dr. Huffman, who also was not involved in the study. “Efforts should be made to minimize long-term prednisone use to minimize impact on weight gain and resulting consequences.”
Are these results applicable to U.S. patients?
“Low-dose prednisone is commonly used in the U.S.,” Dr. Huffman said. “Extrapolating the results to a U.S. population is probably fine.”
Dr. Arkfeld agreed that the results can be used to treat U.S. patients because of the large number of study participants.
According to Rebecca B. Blank, MD, PhD, rheumatologist and instructor of medicine at NYU Langone Health, New York, this is an important study. But she cautioned that the literature does not contain good data for other potential harmful effects of long-term, low-dose glucocorticoid use. “Therefore, as per both ACR [American College of Rheumatology] and EULAR [European Alliance of Associations for Rheumatology] recommendations, we should still try to limit glucocorticoids to the lowest dose and shortest duration possible in our RA patients,” advised Dr. Blank, who was not an author in the study.
Strengths, weaknesses, and thoughts on further research
“Pooling trials can be tricky, but these investigators used individual-level data, which increases the rigor of the analyses,” Dr. Crofford noted. “There were differences in patient populations and with the glucocorticoid doses and routes of administration. The fact that the patients in each of the studies were randomized is very important in determining if the outcomes can be attributed to the drugs or could be the results of other exposures.”
Dr. Arkfeld would like to know whether early versus late RA patients may have different results because they may have different pathophysiologies.
Dr. Huffman is interested in low-dose glucocorticoids’ impacts on glucose homeostasis, bone density, infection, and other common adverse effects.
In an accompanying editorial, David Fernandez, MD, PhD, of Hospital for Special Surgery, New York, wrote: “These findings provide a more quantifiable assessment of the potential adverse effects of steroid therapy than had existed previously and will be helpful to providers and patients as they decide on the relative risks and benefits of glucocorticoids as part of their therapy plan in rheumatoid arthritis.”
The study received no specific funding. Four of the study’s 13 authors reported financial relationships with pharmaceutical companies. Dr. Fernandez and all outside experts who commented on the study reported no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Rheumatology trials seem vulnerable to unblinding: Report
Until more is known about the potential for unblinding, clinicians need to keep in mind that patients and physicians could often guess accurately who was getting placebo or active drug, first author Cody Bruggemeyer, MD, a resident at the Medical College of Wisconsin, Milwaukee, said in an interview.
“It’s important that rheumatologists be aware of this potential issue and use their clinical reasoning and their ability to critically assess papers to evaluate the study design” of research on treatments, he said in an interview.
Dr. Bruggemeyer and coauthors at the Medical College of Wisconsin presented their assessment of the potential for unblinding in a Viewpoint article in The Lancet Rheumatology.
A sample of pivotal clinical trials
The authors selected a sample of pivotal studies of 14 commonly prescribed drugs for rheumatic conditions for which double-blind randomized controlled trials (RCTs) that compared the active ingredient with a placebo were available.
The 14 trials involved treatments classified as disease-modifying antirheumatic drugs (DMARDs), some of which were likely to produce side effects that placebos would not mimic, such as injection site and infusion reactions and difference in readings in lab reports, the authors wrote.
In their analysis, Dr. Bruggemeyer and colleagues evaluated discrepancies in the rates of adverse events reported between active drugs and placebos and classified the 14 studies as follows:
- High unblinding risk: Nine studies had a high estimated risk of unblinding, including trials of adalimumab with citrate (Humira), anakinra (Kineret), anifrolumab (Saphnelo), apremilast (Otezla), ixekizumab (Taltz), leflunomide (Arava), methotrexate, risankizumab (Skyrizi) and tofacitinib (Xeljanz).
- Moderate unblinding risk: Three studies had a moderate estimated risk of unblinding, including trials of azathioprine (Imuran), mycophenolate mofetil and tocilizumab (Actemra).
- Low unblinding risk: Two studies had a low estimated risk of unblinding. These involved tests of belimumab (Benlysta) and rituximab (Rituxan).
Many of the effectiveness measurements of treatments used in rheumatology depend on patients’ reports of relief of pain and other disease symptoms. For example, the widely used American College of Rheumatology 20% response for rheumatoid arthritis includes components that rely on patient and physician assessment of disease activity.
Unblinding risk to clinical trial validity
CTs are the highest level of evidence to establish efficacy, because the study design aims to mask whether the experimental treatment is a drug or placebo. In cases where patients and physicians are more likely to correctly detect use of an active drug, there can be biases that skew results toward reports of symptom improvement. Other patients’ views of their treatment may be distorted by accurate guesses that they have been given placebo, Dr. Bruggemeyer and coauthors wrote.
“The degree of these effects cannot be predicted, but they tend to erroneously inflate the perceived benefit of novel interventions,” they wrote.
The consequences of this unblinding may be minimal in cases where there’s a clear difference between the placebo and active drug, they said. As an example, they cited trials of interleukin-23 inhibitors for psoriasis, where skin clearance as measured by the Psoriatic Area and Severity Index 75 differed by more than 50% in absolute terms between the treatment and placebo groups.
But in other cases, there needs to be more attention paid to the potential role of unblinding, they wrote.
“Studies where effect sizes were small, contradictory, or dependent on subgroup analyses might be especially problematic, but commentary rarely reflects this issue or acknowledges the potential influence of unblinding,” they wrote.
In the paper, they call for more analysis of previous trials to look for unreported assessments of unblinding, while also asking that researchers consider surveying participants in future trials to evaluate the degree to which unblinding occurs.
“Advocacy from professional societies and the U.S. Food and Drug Administration itself might be necessary, but in the interim, rheumatologists should assume unblinding has occurred to some degree in most trials,” they wrote.
Unblinding measure needs validation
In an interview, Roy M. Fleischmann, MD, co–medical director of the Metroplex Clinical Research Center in Dallas, raised some objections to the paper. The paper addresses an interesting question about unblinding, but there should have been more work done, such as finding “a measure that is validated that can say whether you’ve been unblinded or not.”
He added that he was surprised the paper on unblinding in rheumatology trials was published in its current form.
“I would have sent it for a major rewrite” if asked to review this paper before publication, said Dr. Fleischmann, who as a reviewer for Lancet Rheumatology. “I would have said: ‘Okay, 90% of this paper is okay, but your gist is not correct.’ It should be: ‘Is this a problem?’ ”
Dr. Fleischmann said he would have recommended a different perspective to the paper. “That is, this could occur. Should we be looking at this, and how would we look at this?”
In the paper, the authors acknowledge their approach has not been validated, “but it highlights the potential effect of idiosyncratic adverse events,” they wrote.
There’s less funding in general for meta-research than for studies involving treatments, so researchers look for approaches that can be handled without requiring significant funding, and much of the research on the quality of research is conducted like this analysis of rheumatology trials, Michael Putman, MD, the corresponding author and is a rheumatologist and an assistant professor at the Medical College of Wisconsin, said in an interview.
“You’re mostly doing on a shoestring budget with yourself and trainees,” he said. Dr. Putman is an associate editor at the journal Rheumatology and also involved in meta-research, or efforts to understand how studies and trials answer questions about how medical treatments work.
In an Aug. 16 tweet, Dr. Putman said this issue of unintentional unblinding with rheumatology trials was something he’d “been ruminating about for awhile; took two all star trainees to push it over the top!”
One of the barriers to funding of meta-research is a tendency for major funding for medical studies to be focused on specific diseases or targets. With meta-research, it may be more difficult to explain how a specific project will advance efforts to treat or prevent a certain disease, Dr. Putman said.
“It’s a little more esoteric and maybe not quite as clear how these projects will move things forward,” Dr. Putman said.
In addition, the nature of meta-research is to question and often be critical of work that’s already been published, adding another hurdle in attempts to secure funding, he said.
Dr. Putman is supported by a Rheumatology Research Foundation Scientist Development Grant, receives research funding related to clinical trials by AbbVie and AstraZeneca, and consulting fees from Novartis. The other authors declared no competing interests.
Until more is known about the potential for unblinding, clinicians need to keep in mind that patients and physicians could often guess accurately who was getting placebo or active drug, first author Cody Bruggemeyer, MD, a resident at the Medical College of Wisconsin, Milwaukee, said in an interview.
“It’s important that rheumatologists be aware of this potential issue and use their clinical reasoning and their ability to critically assess papers to evaluate the study design” of research on treatments, he said in an interview.
Dr. Bruggemeyer and coauthors at the Medical College of Wisconsin presented their assessment of the potential for unblinding in a Viewpoint article in The Lancet Rheumatology.
A sample of pivotal clinical trials
The authors selected a sample of pivotal studies of 14 commonly prescribed drugs for rheumatic conditions for which double-blind randomized controlled trials (RCTs) that compared the active ingredient with a placebo were available.
The 14 trials involved treatments classified as disease-modifying antirheumatic drugs (DMARDs), some of which were likely to produce side effects that placebos would not mimic, such as injection site and infusion reactions and difference in readings in lab reports, the authors wrote.
In their analysis, Dr. Bruggemeyer and colleagues evaluated discrepancies in the rates of adverse events reported between active drugs and placebos and classified the 14 studies as follows:
- High unblinding risk: Nine studies had a high estimated risk of unblinding, including trials of adalimumab with citrate (Humira), anakinra (Kineret), anifrolumab (Saphnelo), apremilast (Otezla), ixekizumab (Taltz), leflunomide (Arava), methotrexate, risankizumab (Skyrizi) and tofacitinib (Xeljanz).
- Moderate unblinding risk: Three studies had a moderate estimated risk of unblinding, including trials of azathioprine (Imuran), mycophenolate mofetil and tocilizumab (Actemra).
- Low unblinding risk: Two studies had a low estimated risk of unblinding. These involved tests of belimumab (Benlysta) and rituximab (Rituxan).
Many of the effectiveness measurements of treatments used in rheumatology depend on patients’ reports of relief of pain and other disease symptoms. For example, the widely used American College of Rheumatology 20% response for rheumatoid arthritis includes components that rely on patient and physician assessment of disease activity.
Unblinding risk to clinical trial validity
CTs are the highest level of evidence to establish efficacy, because the study design aims to mask whether the experimental treatment is a drug or placebo. In cases where patients and physicians are more likely to correctly detect use of an active drug, there can be biases that skew results toward reports of symptom improvement. Other patients’ views of their treatment may be distorted by accurate guesses that they have been given placebo, Dr. Bruggemeyer and coauthors wrote.
“The degree of these effects cannot be predicted, but they tend to erroneously inflate the perceived benefit of novel interventions,” they wrote.
The consequences of this unblinding may be minimal in cases where there’s a clear difference between the placebo and active drug, they said. As an example, they cited trials of interleukin-23 inhibitors for psoriasis, where skin clearance as measured by the Psoriatic Area and Severity Index 75 differed by more than 50% in absolute terms between the treatment and placebo groups.
But in other cases, there needs to be more attention paid to the potential role of unblinding, they wrote.
“Studies where effect sizes were small, contradictory, or dependent on subgroup analyses might be especially problematic, but commentary rarely reflects this issue or acknowledges the potential influence of unblinding,” they wrote.
In the paper, they call for more analysis of previous trials to look for unreported assessments of unblinding, while also asking that researchers consider surveying participants in future trials to evaluate the degree to which unblinding occurs.
“Advocacy from professional societies and the U.S. Food and Drug Administration itself might be necessary, but in the interim, rheumatologists should assume unblinding has occurred to some degree in most trials,” they wrote.
Unblinding measure needs validation
In an interview, Roy M. Fleischmann, MD, co–medical director of the Metroplex Clinical Research Center in Dallas, raised some objections to the paper. The paper addresses an interesting question about unblinding, but there should have been more work done, such as finding “a measure that is validated that can say whether you’ve been unblinded or not.”
He added that he was surprised the paper on unblinding in rheumatology trials was published in its current form.
“I would have sent it for a major rewrite” if asked to review this paper before publication, said Dr. Fleischmann, who as a reviewer for Lancet Rheumatology. “I would have said: ‘Okay, 90% of this paper is okay, but your gist is not correct.’ It should be: ‘Is this a problem?’ ”
Dr. Fleischmann said he would have recommended a different perspective to the paper. “That is, this could occur. Should we be looking at this, and how would we look at this?”
In the paper, the authors acknowledge their approach has not been validated, “but it highlights the potential effect of idiosyncratic adverse events,” they wrote.
There’s less funding in general for meta-research than for studies involving treatments, so researchers look for approaches that can be handled without requiring significant funding, and much of the research on the quality of research is conducted like this analysis of rheumatology trials, Michael Putman, MD, the corresponding author and is a rheumatologist and an assistant professor at the Medical College of Wisconsin, said in an interview.
“You’re mostly doing on a shoestring budget with yourself and trainees,” he said. Dr. Putman is an associate editor at the journal Rheumatology and also involved in meta-research, or efforts to understand how studies and trials answer questions about how medical treatments work.
In an Aug. 16 tweet, Dr. Putman said this issue of unintentional unblinding with rheumatology trials was something he’d “been ruminating about for awhile; took two all star trainees to push it over the top!”
One of the barriers to funding of meta-research is a tendency for major funding for medical studies to be focused on specific diseases or targets. With meta-research, it may be more difficult to explain how a specific project will advance efforts to treat or prevent a certain disease, Dr. Putman said.
“It’s a little more esoteric and maybe not quite as clear how these projects will move things forward,” Dr. Putman said.
In addition, the nature of meta-research is to question and often be critical of work that’s already been published, adding another hurdle in attempts to secure funding, he said.
Dr. Putman is supported by a Rheumatology Research Foundation Scientist Development Grant, receives research funding related to clinical trials by AbbVie and AstraZeneca, and consulting fees from Novartis. The other authors declared no competing interests.
Until more is known about the potential for unblinding, clinicians need to keep in mind that patients and physicians could often guess accurately who was getting placebo or active drug, first author Cody Bruggemeyer, MD, a resident at the Medical College of Wisconsin, Milwaukee, said in an interview.
“It’s important that rheumatologists be aware of this potential issue and use their clinical reasoning and their ability to critically assess papers to evaluate the study design” of research on treatments, he said in an interview.
Dr. Bruggemeyer and coauthors at the Medical College of Wisconsin presented their assessment of the potential for unblinding in a Viewpoint article in The Lancet Rheumatology.
A sample of pivotal clinical trials
The authors selected a sample of pivotal studies of 14 commonly prescribed drugs for rheumatic conditions for which double-blind randomized controlled trials (RCTs) that compared the active ingredient with a placebo were available.
The 14 trials involved treatments classified as disease-modifying antirheumatic drugs (DMARDs), some of which were likely to produce side effects that placebos would not mimic, such as injection site and infusion reactions and difference in readings in lab reports, the authors wrote.
In their analysis, Dr. Bruggemeyer and colleagues evaluated discrepancies in the rates of adverse events reported between active drugs and placebos and classified the 14 studies as follows:
- High unblinding risk: Nine studies had a high estimated risk of unblinding, including trials of adalimumab with citrate (Humira), anakinra (Kineret), anifrolumab (Saphnelo), apremilast (Otezla), ixekizumab (Taltz), leflunomide (Arava), methotrexate, risankizumab (Skyrizi) and tofacitinib (Xeljanz).
- Moderate unblinding risk: Three studies had a moderate estimated risk of unblinding, including trials of azathioprine (Imuran), mycophenolate mofetil and tocilizumab (Actemra).
- Low unblinding risk: Two studies had a low estimated risk of unblinding. These involved tests of belimumab (Benlysta) and rituximab (Rituxan).
Many of the effectiveness measurements of treatments used in rheumatology depend on patients’ reports of relief of pain and other disease symptoms. For example, the widely used American College of Rheumatology 20% response for rheumatoid arthritis includes components that rely on patient and physician assessment of disease activity.
Unblinding risk to clinical trial validity
CTs are the highest level of evidence to establish efficacy, because the study design aims to mask whether the experimental treatment is a drug or placebo. In cases where patients and physicians are more likely to correctly detect use of an active drug, there can be biases that skew results toward reports of symptom improvement. Other patients’ views of their treatment may be distorted by accurate guesses that they have been given placebo, Dr. Bruggemeyer and coauthors wrote.
“The degree of these effects cannot be predicted, but they tend to erroneously inflate the perceived benefit of novel interventions,” they wrote.
The consequences of this unblinding may be minimal in cases where there’s a clear difference between the placebo and active drug, they said. As an example, they cited trials of interleukin-23 inhibitors for psoriasis, where skin clearance as measured by the Psoriatic Area and Severity Index 75 differed by more than 50% in absolute terms between the treatment and placebo groups.
But in other cases, there needs to be more attention paid to the potential role of unblinding, they wrote.
“Studies where effect sizes were small, contradictory, or dependent on subgroup analyses might be especially problematic, but commentary rarely reflects this issue or acknowledges the potential influence of unblinding,” they wrote.
In the paper, they call for more analysis of previous trials to look for unreported assessments of unblinding, while also asking that researchers consider surveying participants in future trials to evaluate the degree to which unblinding occurs.
“Advocacy from professional societies and the U.S. Food and Drug Administration itself might be necessary, but in the interim, rheumatologists should assume unblinding has occurred to some degree in most trials,” they wrote.
Unblinding measure needs validation
In an interview, Roy M. Fleischmann, MD, co–medical director of the Metroplex Clinical Research Center in Dallas, raised some objections to the paper. The paper addresses an interesting question about unblinding, but there should have been more work done, such as finding “a measure that is validated that can say whether you’ve been unblinded or not.”
He added that he was surprised the paper on unblinding in rheumatology trials was published in its current form.
“I would have sent it for a major rewrite” if asked to review this paper before publication, said Dr. Fleischmann, who as a reviewer for Lancet Rheumatology. “I would have said: ‘Okay, 90% of this paper is okay, but your gist is not correct.’ It should be: ‘Is this a problem?’ ”
Dr. Fleischmann said he would have recommended a different perspective to the paper. “That is, this could occur. Should we be looking at this, and how would we look at this?”
In the paper, the authors acknowledge their approach has not been validated, “but it highlights the potential effect of idiosyncratic adverse events,” they wrote.
There’s less funding in general for meta-research than for studies involving treatments, so researchers look for approaches that can be handled without requiring significant funding, and much of the research on the quality of research is conducted like this analysis of rheumatology trials, Michael Putman, MD, the corresponding author and is a rheumatologist and an assistant professor at the Medical College of Wisconsin, said in an interview.
“You’re mostly doing on a shoestring budget with yourself and trainees,” he said. Dr. Putman is an associate editor at the journal Rheumatology and also involved in meta-research, or efforts to understand how studies and trials answer questions about how medical treatments work.
In an Aug. 16 tweet, Dr. Putman said this issue of unintentional unblinding with rheumatology trials was something he’d “been ruminating about for awhile; took two all star trainees to push it over the top!”
One of the barriers to funding of meta-research is a tendency for major funding for medical studies to be focused on specific diseases or targets. With meta-research, it may be more difficult to explain how a specific project will advance efforts to treat or prevent a certain disease, Dr. Putman said.
“It’s a little more esoteric and maybe not quite as clear how these projects will move things forward,” Dr. Putman said.
In addition, the nature of meta-research is to question and often be critical of work that’s already been published, adding another hurdle in attempts to secure funding, he said.
Dr. Putman is supported by a Rheumatology Research Foundation Scientist Development Grant, receives research funding related to clinical trials by AbbVie and AstraZeneca, and consulting fees from Novartis. The other authors declared no competing interests.
FROM THE LANCET RHEUMATOLOGY
Patients with RA remain at higher risk for COVID-19-related adverse outcomes even in SARS-CoV-2 Omicron era
Key clinical point: Despite improvements in COVID-19 clinical outcomes in the SARS-CoV-2 Omicron era, patients with rheumatoid arthritis (RA) remain at higher risk for COVID-19-associated adverse outcomes compared with the general population, suggesting continued need to follow all prophylactic measures, including receiving vaccinations and antivirals against SARS-CoV-2.
Major finding: Patients with RA vs general population who contracted COVID-19 continue to be at a higher risk for COVID-19-associated hospitalization (adjusted odds ratio [aOR] 2.02; 95% CI 1.79-2.27) and death (aOR 1.73; 95% CI 1.36-2.20) despite better vaccination coverage (88.89% vs 84.07%) and more frequent use of SARS-CoV-2-directed oral antivirals (0.67% vs 0.23%) and monoclonal antibodies (0.10% vs 0.02%).
Study details: This retrospective, population-based study included 34,182 patients with RA matched with 170,910 comparators from the general population.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Bournia VK et al. Outcomes of COVID-19 Omicron variant in patients with rheumatoid arthritis: A nationwide Greek cohort study. Rheumatology (Oxford). 2023 (Jul 19). doi: 10.1093/rheumatology/kead354
Key clinical point: Despite improvements in COVID-19 clinical outcomes in the SARS-CoV-2 Omicron era, patients with rheumatoid arthritis (RA) remain at higher risk for COVID-19-associated adverse outcomes compared with the general population, suggesting continued need to follow all prophylactic measures, including receiving vaccinations and antivirals against SARS-CoV-2.
Major finding: Patients with RA vs general population who contracted COVID-19 continue to be at a higher risk for COVID-19-associated hospitalization (adjusted odds ratio [aOR] 2.02; 95% CI 1.79-2.27) and death (aOR 1.73; 95% CI 1.36-2.20) despite better vaccination coverage (88.89% vs 84.07%) and more frequent use of SARS-CoV-2-directed oral antivirals (0.67% vs 0.23%) and monoclonal antibodies (0.10% vs 0.02%).
Study details: This retrospective, population-based study included 34,182 patients with RA matched with 170,910 comparators from the general population.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Bournia VK et al. Outcomes of COVID-19 Omicron variant in patients with rheumatoid arthritis: A nationwide Greek cohort study. Rheumatology (Oxford). 2023 (Jul 19). doi: 10.1093/rheumatology/kead354
Key clinical point: Despite improvements in COVID-19 clinical outcomes in the SARS-CoV-2 Omicron era, patients with rheumatoid arthritis (RA) remain at higher risk for COVID-19-associated adverse outcomes compared with the general population, suggesting continued need to follow all prophylactic measures, including receiving vaccinations and antivirals against SARS-CoV-2.
Major finding: Patients with RA vs general population who contracted COVID-19 continue to be at a higher risk for COVID-19-associated hospitalization (adjusted odds ratio [aOR] 2.02; 95% CI 1.79-2.27) and death (aOR 1.73; 95% CI 1.36-2.20) despite better vaccination coverage (88.89% vs 84.07%) and more frequent use of SARS-CoV-2-directed oral antivirals (0.67% vs 0.23%) and monoclonal antibodies (0.10% vs 0.02%).
Study details: This retrospective, population-based study included 34,182 patients with RA matched with 170,910 comparators from the general population.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Bournia VK et al. Outcomes of COVID-19 Omicron variant in patients with rheumatoid arthritis: A nationwide Greek cohort study. Rheumatology (Oxford). 2023 (Jul 19). doi: 10.1093/rheumatology/kead354
Lung volume loss at acute exacerbation of RA-ILD tied to increased mortality
Key clinical point: Standardized 3-dimensional computed tomography (3D-CT) showed significant loss of lung volume (LV) at acute exacerbation of rheumatoid arthritis-associated interstitial lung disease (RA-ILD), which was associated with a significant increase in the risk for mortality.
Major finding: Patients with lower vs higher 3D-CT LV had shorter survival (median 2.8 vs 41.7 months; P < .001), with standardized 3D-CT LV being significantly associated with mortality at acute exacerbation of RA-ILD (hazard ratio 0.958; P < .001). Loss of LV was predominantly in the lower lobes at diagnosis and extended to the upper and lower lobes at acute exacerbation (P < .001).
Study details: The data come from a retrospective, observational study including 54 patients with a diagnosis of RA-ILD, 53 patients admitted with an acute exacerbation of RA-ILD, and 35 control individuals.
Disclosures: This study was supported by the Japan Society for the Promotion of Science. The authors declared no conflicts of interest.
Source: Tanaka Y et al. Standardised 3D-CT lung volumes for patients with acute exacerbation of rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford). 2023 (Jul 17). doi: 10.1093/rheumatology/kead363
Key clinical point: Standardized 3-dimensional computed tomography (3D-CT) showed significant loss of lung volume (LV) at acute exacerbation of rheumatoid arthritis-associated interstitial lung disease (RA-ILD), which was associated with a significant increase in the risk for mortality.
Major finding: Patients with lower vs higher 3D-CT LV had shorter survival (median 2.8 vs 41.7 months; P < .001), with standardized 3D-CT LV being significantly associated with mortality at acute exacerbation of RA-ILD (hazard ratio 0.958; P < .001). Loss of LV was predominantly in the lower lobes at diagnosis and extended to the upper and lower lobes at acute exacerbation (P < .001).
Study details: The data come from a retrospective, observational study including 54 patients with a diagnosis of RA-ILD, 53 patients admitted with an acute exacerbation of RA-ILD, and 35 control individuals.
Disclosures: This study was supported by the Japan Society for the Promotion of Science. The authors declared no conflicts of interest.
Source: Tanaka Y et al. Standardised 3D-CT lung volumes for patients with acute exacerbation of rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford). 2023 (Jul 17). doi: 10.1093/rheumatology/kead363
Key clinical point: Standardized 3-dimensional computed tomography (3D-CT) showed significant loss of lung volume (LV) at acute exacerbation of rheumatoid arthritis-associated interstitial lung disease (RA-ILD), which was associated with a significant increase in the risk for mortality.
Major finding: Patients with lower vs higher 3D-CT LV had shorter survival (median 2.8 vs 41.7 months; P < .001), with standardized 3D-CT LV being significantly associated with mortality at acute exacerbation of RA-ILD (hazard ratio 0.958; P < .001). Loss of LV was predominantly in the lower lobes at diagnosis and extended to the upper and lower lobes at acute exacerbation (P < .001).
Study details: The data come from a retrospective, observational study including 54 patients with a diagnosis of RA-ILD, 53 patients admitted with an acute exacerbation of RA-ILD, and 35 control individuals.
Disclosures: This study was supported by the Japan Society for the Promotion of Science. The authors declared no conflicts of interest.
Source: Tanaka Y et al. Standardised 3D-CT lung volumes for patients with acute exacerbation of rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford). 2023 (Jul 17). doi: 10.1093/rheumatology/kead363
Opioids not safer than NSAID in patients with RA
Key clinical point: Opioids not only increase the risk for all-cause mortality among patients with rheumatoid arthritis (RA) but also carry a similar risk for major adverse cardiovascular events (MACE) as nonsteroidal anti-inflammatory drugs (NSAID).
Major finding: The risk for MACE was similar among patients initiating opioids and those initiating NSAID (adjusted hazard ratio [aHR] 1.02; 95% CI 0.85-1.22); however, opioid initiators had a 33% higher risk for all-cause mortality compared with NSAID initiators (aHR 1.33; 95% CI 1.06-1.67).
Study details: This new user active comparator cohort study included patients with RA within the FORWARD databank and propensity-score matched patients initiating opioids (n = 6866) to those initiating NSAID (n = 13,689).
Disclosures: This study was supported by the Rheumatology Research Foundation Resident Preceptor Award. The authors declared no conflicts of interest.
Source: Ozen G et al. Major adverse cardiovascular events and mortality with opioids versus NSAIDs initiation in patients with rheumatoid arthritis. Ann Rheum Dis. 2023 (Jul 17). doi: 10.1136/ard-2023-224339
Key clinical point: Opioids not only increase the risk for all-cause mortality among patients with rheumatoid arthritis (RA) but also carry a similar risk for major adverse cardiovascular events (MACE) as nonsteroidal anti-inflammatory drugs (NSAID).
Major finding: The risk for MACE was similar among patients initiating opioids and those initiating NSAID (adjusted hazard ratio [aHR] 1.02; 95% CI 0.85-1.22); however, opioid initiators had a 33% higher risk for all-cause mortality compared with NSAID initiators (aHR 1.33; 95% CI 1.06-1.67).
Study details: This new user active comparator cohort study included patients with RA within the FORWARD databank and propensity-score matched patients initiating opioids (n = 6866) to those initiating NSAID (n = 13,689).
Disclosures: This study was supported by the Rheumatology Research Foundation Resident Preceptor Award. The authors declared no conflicts of interest.
Source: Ozen G et al. Major adverse cardiovascular events and mortality with opioids versus NSAIDs initiation in patients with rheumatoid arthritis. Ann Rheum Dis. 2023 (Jul 17). doi: 10.1136/ard-2023-224339
Key clinical point: Opioids not only increase the risk for all-cause mortality among patients with rheumatoid arthritis (RA) but also carry a similar risk for major adverse cardiovascular events (MACE) as nonsteroidal anti-inflammatory drugs (NSAID).
Major finding: The risk for MACE was similar among patients initiating opioids and those initiating NSAID (adjusted hazard ratio [aHR] 1.02; 95% CI 0.85-1.22); however, opioid initiators had a 33% higher risk for all-cause mortality compared with NSAID initiators (aHR 1.33; 95% CI 1.06-1.67).
Study details: This new user active comparator cohort study included patients with RA within the FORWARD databank and propensity-score matched patients initiating opioids (n = 6866) to those initiating NSAID (n = 13,689).
Disclosures: This study was supported by the Rheumatology Research Foundation Resident Preceptor Award. The authors declared no conflicts of interest.
Source: Ozen G et al. Major adverse cardiovascular events and mortality with opioids versus NSAIDs initiation in patients with rheumatoid arthritis. Ann Rheum Dis. 2023 (Jul 17). doi: 10.1136/ard-2023-224339
Risk for diabetes varies with treatment options in RA
Key clinical point: The use of combination therapy or biologics was associated with a lower risk for diabetes than methotrexate monotherapy in patients with rheumatoid arthritis (RA), with hydroxychloroquine having a significant protective effect on the development of diabetes.
Major finding: The risk for diabetes was significantly lower in the biologic disease-modifying antirheumatic drug (DMARD) periods (adjusted hazard ratio [aHR] 0.51; 95% CI 0.32-0.83), methotrexate combination periods (aHR 0.50; 95% CI 0.32-0.78), and other conventional DMARD periods (aHR 0.56; 95% CI 0.37-0.84) than in the methotrexate monotherapy periods. Hydroxychloroquine (aHR 0.52; P < .001) and sulfasalazine (aHR 0.69; P = .002) had a significant protective effect on diabetes development.
Study details: The data come from a retrospective cohort study that included 5530 adults with RA without diabetes.
Disclosures: This study did not declare any specific funding or conflicts of interest.
Source: Su YJ et al. Disease-modifying anti-rheumatic drugs associated with different diabetes risks in patients with rheumatoid arthritis. RMD Open. 2023;9:e003045 (Jul 17). doi: 10.1136/rmdopen-2023-003045
Key clinical point: The use of combination therapy or biologics was associated with a lower risk for diabetes than methotrexate monotherapy in patients with rheumatoid arthritis (RA), with hydroxychloroquine having a significant protective effect on the development of diabetes.
Major finding: The risk for diabetes was significantly lower in the biologic disease-modifying antirheumatic drug (DMARD) periods (adjusted hazard ratio [aHR] 0.51; 95% CI 0.32-0.83), methotrexate combination periods (aHR 0.50; 95% CI 0.32-0.78), and other conventional DMARD periods (aHR 0.56; 95% CI 0.37-0.84) than in the methotrexate monotherapy periods. Hydroxychloroquine (aHR 0.52; P < .001) and sulfasalazine (aHR 0.69; P = .002) had a significant protective effect on diabetes development.
Study details: The data come from a retrospective cohort study that included 5530 adults with RA without diabetes.
Disclosures: This study did not declare any specific funding or conflicts of interest.
Source: Su YJ et al. Disease-modifying anti-rheumatic drugs associated with different diabetes risks in patients with rheumatoid arthritis. RMD Open. 2023;9:e003045 (Jul 17). doi: 10.1136/rmdopen-2023-003045
Key clinical point: The use of combination therapy or biologics was associated with a lower risk for diabetes than methotrexate monotherapy in patients with rheumatoid arthritis (RA), with hydroxychloroquine having a significant protective effect on the development of diabetes.
Major finding: The risk for diabetes was significantly lower in the biologic disease-modifying antirheumatic drug (DMARD) periods (adjusted hazard ratio [aHR] 0.51; 95% CI 0.32-0.83), methotrexate combination periods (aHR 0.50; 95% CI 0.32-0.78), and other conventional DMARD periods (aHR 0.56; 95% CI 0.37-0.84) than in the methotrexate monotherapy periods. Hydroxychloroquine (aHR 0.52; P < .001) and sulfasalazine (aHR 0.69; P = .002) had a significant protective effect on diabetes development.
Study details: The data come from a retrospective cohort study that included 5530 adults with RA without diabetes.
Disclosures: This study did not declare any specific funding or conflicts of interest.
Source: Su YJ et al. Disease-modifying anti-rheumatic drugs associated with different diabetes risks in patients with rheumatoid arthritis. RMD Open. 2023;9:e003045 (Jul 17). doi: 10.1136/rmdopen-2023-003045