User login
Fluids or vasopressors: Is sepsis management that simple?
In recent months, we have seen the results of the much awaited Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial showing that a restrictive fluid and early vasopressor strategy initiated on arrival of patients with sepsis and hypotension in the ED did not result in decreased mortality compared with a liberal fluid approach (PETAL Network. www.nejm.org/doi/10.1056/NEJMoa2202707). The March 2023 issue of CHEST Physician provided a synopsis of the trial highlighting several limitations (Splete H. CHEST Physician. 2023;18[3]:1). Last year in 2022, the Conservative versus Liberal Approach to Fluid Therapy in Septic Shock (CLASSIC) trial also showed no difference in mortality with restrictive fluid compared with standard fluid in patients with septic shock in the ICU already receiving vasopressor therapy (Meyhoff TS, et al. N Engl J Med. 2022;386[26]:2459). Did their results suggest a “you can do what you want” approach? Is the management of sepsis and septic shock limited to fluids vs vasopressors? Hopefully, the ongoing studies ARISE FLUIDS (NCT04569942), EVIS (NCT05179499), FRESHLY (NCT05453565), 1BED (NCT05273034), and REDUCE (NCT04931485) will further address these questions.
In the meantime, I continue to admit and care for patients with sepsis in the ICU. One example was a 72-year-old woman with a history of stroke, coronary artery disease, diabetes, and chronic kidney disease presenting with 3 days of progressive cough and dyspnea. In the ED, temperature was 38.2° C, heart rate 120 beats per min, respiratory rate 28/min, blood pressure 82/48 mm Hg, and weight 92 kg. She had audible crackles in the left lower lung. Her laboratory and imaging results supported a diagnosis of sepsis due to severe community-acquired pneumonia, including the following values: white blood cell 18.2 million/mm3; lactate 3.8 mmol/L; and creatinine 4.3 mg/dL.
While in the ED, the patient received 1 liter of crystalloid fluids and appropriate broad spectrum antibiotics. Repeat lactate value was 2.8 mmol/L. Patient’s blood pressure then decreased to 85/42 mm Hg. Norepinephrine was started peripherally and titrated to 6 mcg/min to achieve blood pressure 104/56 mm Hg. No further fluid administration was given, and the patient was admitted to the medical ICU. On admission, a repeat lactate had increased to 3.4 mmol/L with blood pressure of 80/45 mm Hg. Instead of further escalating vasopressor administration, she received 2 L of fluid and continued at 150 mL/h. Shortly after, norepinephrine was titrated off. Fluid resuscitation was then deescalated. We transfered the patient to the general ward within 12 hours of ICU admission.
Could we have avoided ICU admission and critical care resource utilization if the patient had received more optimal fluid resuscitation in the ED?
While our fear of fluids (or hydrophobia) may be unwarranted, the management of this patient was a common example of fluid restriction in sepsis (Jaehne AK, et al. Crit Care Med. 2016;44[12]:2263). By clinical criteria, she was in septic shock (requiring vasopressor) and appropriately required ICU admission. But, I would posit that the patient had severe sepsis based on pre-Sepsis 3 criteria. Optimal initial fluid resuscitation would have prevented her from requiring vasopressor and progressing to septic shock with ICU admission. Unfortunately, the patient’s care reflected the objective of CLOVERS and its results. Other than the lack of decreased mortality, decreased ventilator use, decreased renal replacement therapy, and decreased hospital length of stay, restricting fluids resulted in an increase of 8.1% (95% confidence interval 3.3 to 12.8) ICU utilization. Furthermore, the data and safety monitoring committee halted the trial for futility at two-thirds of enrollment. One must wonder if CLOVERS had completed its intended enrollment of 2,320 patients, negative outcomes would have occurred.
Should an astute clinician interpret the results of the CLOVERS and CLASSIC trials as “Fluids, it doesn’t matter, so I can do what I want?” Absolutely not! The literature is abundant with studies showing that increasing dose and/or number of vasopressors is associated with higher mortality in septic shock. One example is a recent multicenter prospective cohort study examining the association of vasopressor dosing during the first 24 hours and 30-day mortality in septic shock over 33 hospitals (Roberts RJ, et al. Crit Care Med. 2020;48[10]:1445).
Six hundred and sixteen patients were enrolled with 31% 30-day mortality. In 24 hours after shock diagnosis, patients received a median of 3.4 (1.9-5.3) L of fluids and 8.5 mcg/min norepinephrine equivalent. During the first 6 hours, increasing vasopressor dosing was associated with increased odds of mortality. Every 10 mcg/min increase in norepinephrine over the 24-hour period was associated with a 33% increased odds of mortality. Patients who received no fluids but 35 mcg/min norepinephrine in 6 hours had the highest mortality of 50%. As fluid volume increased, the association between vasopressor dosing and mortality decreased, such that at least 2 L of fluid during the first 6 hours was required for this association to become nonsignificant. Based on these results and a number of past studies, we should be cautious in believing that a resuscitation strategy favoring vasopressors would result in a better outcome.
Shock resuscitation is complex, and there is no one-size-fits-all approach. With the present climate, the success of resuscitation has been simplified to assessing fluid responsiveness. Trainees learn to identify the inferior vena cava and lung B-lines by ultrasound. With more advanced technology, stroke volume variation is considered. And, let us not forget the passive leg raise. Rarely can our fellows and residents recite the components of oxygen delivery as targets of shock resuscitation: preload, afterload, contractility, hemoglobin, and oxygen saturation. Another patient example comes to mind when fluid responsiveness alone is inadequate.
Our patient was a 46-year-old man now day 4 in the ICU with Klebsiella bacteremia and acute cholecystitis undergoing medical management. His comorbidities included diabetes, obesity, hypertension, and cardiomyopathy with ejection fraction 35%. He was supported sson mechanical ventilation, norepinephrine 20 mcg/min, and receiving appropriate antibiotics. For hemodynamic monitoring, a central venous and arterial catheter have been placed. The patient had a heart rate 92 beats per min, mean arterial pressure (MAP) 57 mm Hg, central venous pressure (CVP) 26 mm Hg, stroke volume variation (SVV) 9%, cardiac output (CO) 2.5 L/min, and central venous oxygen saturation (ScvO2) 42%.
Based on these parameters, we initiated dobutamine at 2.5 mcg/kg/min, which was then titrated to 20 mcg/kg/min over 2 hours to achieve ScvO2 72%. Interestingly, CVP had decreased to 18 mm Hg, SVV increased to 16%, with CO 4.5 L/min. MAP also increased to 68 mm Hg. We then administered 1-L fluid bolus with the elevated SVV. Given the patient’s underlying cardiomyopathy, CVP < 20 mm Hg appeared to indicate a state of fluid responsiveness. After our fluid administration, heart rate 98 beats per min, MAP 70 mm Hg, CVP increased to 21 mm Hg, SVV 12%, CO 4.7 L/min, and ScvO2 74%. In acknowledging a mixed hypovolemic, cardiogenic, and septic shock, we had optimized his hemodynamic state. Importantly, during this exercise of hemodynamic manipulation, we were able to decrease norepinephrine to 8 mcg/min, maintaining dobutamine at 20 mcg/kg/min.
The above case illustrates that the hemodynamic perturbations in sepsis and septic shock are not simple. Patients do not present with a single shock state. An infection progressing to shock often is confounded by hypovolemia and underlying comorbidities, such as cardiac dysfunction. Without considering the complex physiology, our desire to continue the debate of fluids vs vasopressors is on the brink of taking us back several decades when the management of sepsis was to start a fluid bolus, administer “Rocephin,” and initiate dopamine. But I remind myself that we have made advances – now it’s 1 L lactated Ringer’s, administer “vanco and zosyn,” and initiate norepinephrine.
In recent months, we have seen the results of the much awaited Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial showing that a restrictive fluid and early vasopressor strategy initiated on arrival of patients with sepsis and hypotension in the ED did not result in decreased mortality compared with a liberal fluid approach (PETAL Network. www.nejm.org/doi/10.1056/NEJMoa2202707). The March 2023 issue of CHEST Physician provided a synopsis of the trial highlighting several limitations (Splete H. CHEST Physician. 2023;18[3]:1). Last year in 2022, the Conservative versus Liberal Approach to Fluid Therapy in Septic Shock (CLASSIC) trial also showed no difference in mortality with restrictive fluid compared with standard fluid in patients with septic shock in the ICU already receiving vasopressor therapy (Meyhoff TS, et al. N Engl J Med. 2022;386[26]:2459). Did their results suggest a “you can do what you want” approach? Is the management of sepsis and septic shock limited to fluids vs vasopressors? Hopefully, the ongoing studies ARISE FLUIDS (NCT04569942), EVIS (NCT05179499), FRESHLY (NCT05453565), 1BED (NCT05273034), and REDUCE (NCT04931485) will further address these questions.
In the meantime, I continue to admit and care for patients with sepsis in the ICU. One example was a 72-year-old woman with a history of stroke, coronary artery disease, diabetes, and chronic kidney disease presenting with 3 days of progressive cough and dyspnea. In the ED, temperature was 38.2° C, heart rate 120 beats per min, respiratory rate 28/min, blood pressure 82/48 mm Hg, and weight 92 kg. She had audible crackles in the left lower lung. Her laboratory and imaging results supported a diagnosis of sepsis due to severe community-acquired pneumonia, including the following values: white blood cell 18.2 million/mm3; lactate 3.8 mmol/L; and creatinine 4.3 mg/dL.
While in the ED, the patient received 1 liter of crystalloid fluids and appropriate broad spectrum antibiotics. Repeat lactate value was 2.8 mmol/L. Patient’s blood pressure then decreased to 85/42 mm Hg. Norepinephrine was started peripherally and titrated to 6 mcg/min to achieve blood pressure 104/56 mm Hg. No further fluid administration was given, and the patient was admitted to the medical ICU. On admission, a repeat lactate had increased to 3.4 mmol/L with blood pressure of 80/45 mm Hg. Instead of further escalating vasopressor administration, she received 2 L of fluid and continued at 150 mL/h. Shortly after, norepinephrine was titrated off. Fluid resuscitation was then deescalated. We transfered the patient to the general ward within 12 hours of ICU admission.
Could we have avoided ICU admission and critical care resource utilization if the patient had received more optimal fluid resuscitation in the ED?
While our fear of fluids (or hydrophobia) may be unwarranted, the management of this patient was a common example of fluid restriction in sepsis (Jaehne AK, et al. Crit Care Med. 2016;44[12]:2263). By clinical criteria, she was in septic shock (requiring vasopressor) and appropriately required ICU admission. But, I would posit that the patient had severe sepsis based on pre-Sepsis 3 criteria. Optimal initial fluid resuscitation would have prevented her from requiring vasopressor and progressing to septic shock with ICU admission. Unfortunately, the patient’s care reflected the objective of CLOVERS and its results. Other than the lack of decreased mortality, decreased ventilator use, decreased renal replacement therapy, and decreased hospital length of stay, restricting fluids resulted in an increase of 8.1% (95% confidence interval 3.3 to 12.8) ICU utilization. Furthermore, the data and safety monitoring committee halted the trial for futility at two-thirds of enrollment. One must wonder if CLOVERS had completed its intended enrollment of 2,320 patients, negative outcomes would have occurred.
Should an astute clinician interpret the results of the CLOVERS and CLASSIC trials as “Fluids, it doesn’t matter, so I can do what I want?” Absolutely not! The literature is abundant with studies showing that increasing dose and/or number of vasopressors is associated with higher mortality in septic shock. One example is a recent multicenter prospective cohort study examining the association of vasopressor dosing during the first 24 hours and 30-day mortality in septic shock over 33 hospitals (Roberts RJ, et al. Crit Care Med. 2020;48[10]:1445).
Six hundred and sixteen patients were enrolled with 31% 30-day mortality. In 24 hours after shock diagnosis, patients received a median of 3.4 (1.9-5.3) L of fluids and 8.5 mcg/min norepinephrine equivalent. During the first 6 hours, increasing vasopressor dosing was associated with increased odds of mortality. Every 10 mcg/min increase in norepinephrine over the 24-hour period was associated with a 33% increased odds of mortality. Patients who received no fluids but 35 mcg/min norepinephrine in 6 hours had the highest mortality of 50%. As fluid volume increased, the association between vasopressor dosing and mortality decreased, such that at least 2 L of fluid during the first 6 hours was required for this association to become nonsignificant. Based on these results and a number of past studies, we should be cautious in believing that a resuscitation strategy favoring vasopressors would result in a better outcome.
Shock resuscitation is complex, and there is no one-size-fits-all approach. With the present climate, the success of resuscitation has been simplified to assessing fluid responsiveness. Trainees learn to identify the inferior vena cava and lung B-lines by ultrasound. With more advanced technology, stroke volume variation is considered. And, let us not forget the passive leg raise. Rarely can our fellows and residents recite the components of oxygen delivery as targets of shock resuscitation: preload, afterload, contractility, hemoglobin, and oxygen saturation. Another patient example comes to mind when fluid responsiveness alone is inadequate.
Our patient was a 46-year-old man now day 4 in the ICU with Klebsiella bacteremia and acute cholecystitis undergoing medical management. His comorbidities included diabetes, obesity, hypertension, and cardiomyopathy with ejection fraction 35%. He was supported sson mechanical ventilation, norepinephrine 20 mcg/min, and receiving appropriate antibiotics. For hemodynamic monitoring, a central venous and arterial catheter have been placed. The patient had a heart rate 92 beats per min, mean arterial pressure (MAP) 57 mm Hg, central venous pressure (CVP) 26 mm Hg, stroke volume variation (SVV) 9%, cardiac output (CO) 2.5 L/min, and central venous oxygen saturation (ScvO2) 42%.
Based on these parameters, we initiated dobutamine at 2.5 mcg/kg/min, which was then titrated to 20 mcg/kg/min over 2 hours to achieve ScvO2 72%. Interestingly, CVP had decreased to 18 mm Hg, SVV increased to 16%, with CO 4.5 L/min. MAP also increased to 68 mm Hg. We then administered 1-L fluid bolus with the elevated SVV. Given the patient’s underlying cardiomyopathy, CVP < 20 mm Hg appeared to indicate a state of fluid responsiveness. After our fluid administration, heart rate 98 beats per min, MAP 70 mm Hg, CVP increased to 21 mm Hg, SVV 12%, CO 4.7 L/min, and ScvO2 74%. In acknowledging a mixed hypovolemic, cardiogenic, and septic shock, we had optimized his hemodynamic state. Importantly, during this exercise of hemodynamic manipulation, we were able to decrease norepinephrine to 8 mcg/min, maintaining dobutamine at 20 mcg/kg/min.
The above case illustrates that the hemodynamic perturbations in sepsis and septic shock are not simple. Patients do not present with a single shock state. An infection progressing to shock often is confounded by hypovolemia and underlying comorbidities, such as cardiac dysfunction. Without considering the complex physiology, our desire to continue the debate of fluids vs vasopressors is on the brink of taking us back several decades when the management of sepsis was to start a fluid bolus, administer “Rocephin,” and initiate dopamine. But I remind myself that we have made advances – now it’s 1 L lactated Ringer’s, administer “vanco and zosyn,” and initiate norepinephrine.
In recent months, we have seen the results of the much awaited Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial showing that a restrictive fluid and early vasopressor strategy initiated on arrival of patients with sepsis and hypotension in the ED did not result in decreased mortality compared with a liberal fluid approach (PETAL Network. www.nejm.org/doi/10.1056/NEJMoa2202707). The March 2023 issue of CHEST Physician provided a synopsis of the trial highlighting several limitations (Splete H. CHEST Physician. 2023;18[3]:1). Last year in 2022, the Conservative versus Liberal Approach to Fluid Therapy in Septic Shock (CLASSIC) trial also showed no difference in mortality with restrictive fluid compared with standard fluid in patients with septic shock in the ICU already receiving vasopressor therapy (Meyhoff TS, et al. N Engl J Med. 2022;386[26]:2459). Did their results suggest a “you can do what you want” approach? Is the management of sepsis and septic shock limited to fluids vs vasopressors? Hopefully, the ongoing studies ARISE FLUIDS (NCT04569942), EVIS (NCT05179499), FRESHLY (NCT05453565), 1BED (NCT05273034), and REDUCE (NCT04931485) will further address these questions.
In the meantime, I continue to admit and care for patients with sepsis in the ICU. One example was a 72-year-old woman with a history of stroke, coronary artery disease, diabetes, and chronic kidney disease presenting with 3 days of progressive cough and dyspnea. In the ED, temperature was 38.2° C, heart rate 120 beats per min, respiratory rate 28/min, blood pressure 82/48 mm Hg, and weight 92 kg. She had audible crackles in the left lower lung. Her laboratory and imaging results supported a diagnosis of sepsis due to severe community-acquired pneumonia, including the following values: white blood cell 18.2 million/mm3; lactate 3.8 mmol/L; and creatinine 4.3 mg/dL.
While in the ED, the patient received 1 liter of crystalloid fluids and appropriate broad spectrum antibiotics. Repeat lactate value was 2.8 mmol/L. Patient’s blood pressure then decreased to 85/42 mm Hg. Norepinephrine was started peripherally and titrated to 6 mcg/min to achieve blood pressure 104/56 mm Hg. No further fluid administration was given, and the patient was admitted to the medical ICU. On admission, a repeat lactate had increased to 3.4 mmol/L with blood pressure of 80/45 mm Hg. Instead of further escalating vasopressor administration, she received 2 L of fluid and continued at 150 mL/h. Shortly after, norepinephrine was titrated off. Fluid resuscitation was then deescalated. We transfered the patient to the general ward within 12 hours of ICU admission.
Could we have avoided ICU admission and critical care resource utilization if the patient had received more optimal fluid resuscitation in the ED?
While our fear of fluids (or hydrophobia) may be unwarranted, the management of this patient was a common example of fluid restriction in sepsis (Jaehne AK, et al. Crit Care Med. 2016;44[12]:2263). By clinical criteria, she was in septic shock (requiring vasopressor) and appropriately required ICU admission. But, I would posit that the patient had severe sepsis based on pre-Sepsis 3 criteria. Optimal initial fluid resuscitation would have prevented her from requiring vasopressor and progressing to septic shock with ICU admission. Unfortunately, the patient’s care reflected the objective of CLOVERS and its results. Other than the lack of decreased mortality, decreased ventilator use, decreased renal replacement therapy, and decreased hospital length of stay, restricting fluids resulted in an increase of 8.1% (95% confidence interval 3.3 to 12.8) ICU utilization. Furthermore, the data and safety monitoring committee halted the trial for futility at two-thirds of enrollment. One must wonder if CLOVERS had completed its intended enrollment of 2,320 patients, negative outcomes would have occurred.
Should an astute clinician interpret the results of the CLOVERS and CLASSIC trials as “Fluids, it doesn’t matter, so I can do what I want?” Absolutely not! The literature is abundant with studies showing that increasing dose and/or number of vasopressors is associated with higher mortality in septic shock. One example is a recent multicenter prospective cohort study examining the association of vasopressor dosing during the first 24 hours and 30-day mortality in septic shock over 33 hospitals (Roberts RJ, et al. Crit Care Med. 2020;48[10]:1445).
Six hundred and sixteen patients were enrolled with 31% 30-day mortality. In 24 hours after shock diagnosis, patients received a median of 3.4 (1.9-5.3) L of fluids and 8.5 mcg/min norepinephrine equivalent. During the first 6 hours, increasing vasopressor dosing was associated with increased odds of mortality. Every 10 mcg/min increase in norepinephrine over the 24-hour period was associated with a 33% increased odds of mortality. Patients who received no fluids but 35 mcg/min norepinephrine in 6 hours had the highest mortality of 50%. As fluid volume increased, the association between vasopressor dosing and mortality decreased, such that at least 2 L of fluid during the first 6 hours was required for this association to become nonsignificant. Based on these results and a number of past studies, we should be cautious in believing that a resuscitation strategy favoring vasopressors would result in a better outcome.
Shock resuscitation is complex, and there is no one-size-fits-all approach. With the present climate, the success of resuscitation has been simplified to assessing fluid responsiveness. Trainees learn to identify the inferior vena cava and lung B-lines by ultrasound. With more advanced technology, stroke volume variation is considered. And, let us not forget the passive leg raise. Rarely can our fellows and residents recite the components of oxygen delivery as targets of shock resuscitation: preload, afterload, contractility, hemoglobin, and oxygen saturation. Another patient example comes to mind when fluid responsiveness alone is inadequate.
Our patient was a 46-year-old man now day 4 in the ICU with Klebsiella bacteremia and acute cholecystitis undergoing medical management. His comorbidities included diabetes, obesity, hypertension, and cardiomyopathy with ejection fraction 35%. He was supported sson mechanical ventilation, norepinephrine 20 mcg/min, and receiving appropriate antibiotics. For hemodynamic monitoring, a central venous and arterial catheter have been placed. The patient had a heart rate 92 beats per min, mean arterial pressure (MAP) 57 mm Hg, central venous pressure (CVP) 26 mm Hg, stroke volume variation (SVV) 9%, cardiac output (CO) 2.5 L/min, and central venous oxygen saturation (ScvO2) 42%.
Based on these parameters, we initiated dobutamine at 2.5 mcg/kg/min, which was then titrated to 20 mcg/kg/min over 2 hours to achieve ScvO2 72%. Interestingly, CVP had decreased to 18 mm Hg, SVV increased to 16%, with CO 4.5 L/min. MAP also increased to 68 mm Hg. We then administered 1-L fluid bolus with the elevated SVV. Given the patient’s underlying cardiomyopathy, CVP < 20 mm Hg appeared to indicate a state of fluid responsiveness. After our fluid administration, heart rate 98 beats per min, MAP 70 mm Hg, CVP increased to 21 mm Hg, SVV 12%, CO 4.7 L/min, and ScvO2 74%. In acknowledging a mixed hypovolemic, cardiogenic, and septic shock, we had optimized his hemodynamic state. Importantly, during this exercise of hemodynamic manipulation, we were able to decrease norepinephrine to 8 mcg/min, maintaining dobutamine at 20 mcg/kg/min.
The above case illustrates that the hemodynamic perturbations in sepsis and septic shock are not simple. Patients do not present with a single shock state. An infection progressing to shock often is confounded by hypovolemia and underlying comorbidities, such as cardiac dysfunction. Without considering the complex physiology, our desire to continue the debate of fluids vs vasopressors is on the brink of taking us back several decades when the management of sepsis was to start a fluid bolus, administer “Rocephin,” and initiate dopamine. But I remind myself that we have made advances – now it’s 1 L lactated Ringer’s, administer “vanco and zosyn,” and initiate norepinephrine.
Management of patients with neuromuscular weakness: The latest CHEST guideline
Patients with neuromuscular diseases (NMD) face an increased risk of respiratory muscle weakness, which can contribute to various health problems. These include chronic respiratory failure, sleep-related breathing disorders, sialorrhea, and reduced cough effectiveness. In collaboration with AASM, AARC, and ATS, CHEST has developed guidelines to help clinicians manage patients with NMD. using the population, intervention, comparator, and outcome (PICO) format using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) methodology.
A few of the key recommendations are as follows:
1. Addressing the use and timing of pulmonary function tests (PFT), the panel suggests measuring vital capacity (FVC or SVC), MIP/MEP, SNIP, or PCF in patients with NMD every 6 months.
2. For the detection of respiratory failure and sleep-related breathing disorders in symptomatic patients with NMD who have normal PFT and overnight oximetry (ONO), the panel suggested that clinicians consider polysomnography (PSG) to assess whether noninvasive ventilation (NIV) would be beneficial. Adult patients do not have to have PSG to manage NMD if the PFT or ONO criteria support using NIV.
3. The panel recommends the use of NIV for the treatment of respiratory failure. To guide the initiation of NIV, clinicians can use any fall in FVC to < 80% of predicted with symptoms or FVC to < 50% of predicted without symptoms or SNIP/MIP to < –40 cm H2O or hypercapnia. The panel recommended individualizing treatment.
4. The panel suggested mouth piece ventilation (MPV) for daytime ventilatory support in patients with preserved bulbar function. Its desirable effects include delaying or avoiding tracheostomy and improving speech, cough effectiveness, and coordination of breathing and swallowing.
5. Invasive home mechanical ventilation (MV) by tracheostomy was identified as an acceptable option for patients with progressive respiratory failure, particularly those who were unable to clear secretions. Because of the high costs and caregiver burden, the guideline highlights the need to consider patient preferences, tolerability, the ability to maintain mouthpiece ventilation, and the availability of resources when choosing an appropriate treatment option.
6. The panel suggested practicing clinicians address the management of sialorrhea and airway clearance techniques in patients with NMD, as they face the risk of aspiration and pneumonia. For sialorrhea, the panel suggests starting with a trial of anticholinergic agents, as they are inexpensive and readily available. The panel also provided advice on botulinum toxin therapy and radiation therapy, which have limited data and should be reserved for experienced centers.
7. The panel reviewed data on airway clearance techniques, including glossopharyngeal breathing (GPB), mechanical insufflation-exsufflation (MI-E), also commonly known as cough-assist device, manually assisted cough, lung volume recruitment (LVR) by air stacking, and high-frequency chest wall oscillation (HFCWO). The panel suggested using airway clearance techniques based on local resources, expertise, and shared decision-making with patients.
The panel stressed the importance of respect for patient preferences, treatment goals, and quality of life considerations. The panel emphasized the need to modernize and improve access to ventilatory support for patients with NMD and the role of shared decision-making in improving quality of life and long-term outcomes. The panel also suggests that randomized controlled trials in patients with NMD would help establish a higher grade of evidence.
Dr. Hubel and Dr. Khan are from the Division of Pulmonary Allergy and Critical Care Medicine, Oregon Health and Science University, Portland.
Reference
Khan A et al. Respiratory management of patients with neuromuscular weakness: An American College of Chest Physicians Clinical Practice Guideline and Expert Panel Report [published online ahead of print, 2023 Mar 13]. Chest. 2023;S0012-3692(23)00353-7. doi: 10.1016/j.chest.2023.03.011.
Patients with neuromuscular diseases (NMD) face an increased risk of respiratory muscle weakness, which can contribute to various health problems. These include chronic respiratory failure, sleep-related breathing disorders, sialorrhea, and reduced cough effectiveness. In collaboration with AASM, AARC, and ATS, CHEST has developed guidelines to help clinicians manage patients with NMD. using the population, intervention, comparator, and outcome (PICO) format using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) methodology.
A few of the key recommendations are as follows:
1. Addressing the use and timing of pulmonary function tests (PFT), the panel suggests measuring vital capacity (FVC or SVC), MIP/MEP, SNIP, or PCF in patients with NMD every 6 months.
2. For the detection of respiratory failure and sleep-related breathing disorders in symptomatic patients with NMD who have normal PFT and overnight oximetry (ONO), the panel suggested that clinicians consider polysomnography (PSG) to assess whether noninvasive ventilation (NIV) would be beneficial. Adult patients do not have to have PSG to manage NMD if the PFT or ONO criteria support using NIV.
3. The panel recommends the use of NIV for the treatment of respiratory failure. To guide the initiation of NIV, clinicians can use any fall in FVC to < 80% of predicted with symptoms or FVC to < 50% of predicted without symptoms or SNIP/MIP to < –40 cm H2O or hypercapnia. The panel recommended individualizing treatment.
4. The panel suggested mouth piece ventilation (MPV) for daytime ventilatory support in patients with preserved bulbar function. Its desirable effects include delaying or avoiding tracheostomy and improving speech, cough effectiveness, and coordination of breathing and swallowing.
5. Invasive home mechanical ventilation (MV) by tracheostomy was identified as an acceptable option for patients with progressive respiratory failure, particularly those who were unable to clear secretions. Because of the high costs and caregiver burden, the guideline highlights the need to consider patient preferences, tolerability, the ability to maintain mouthpiece ventilation, and the availability of resources when choosing an appropriate treatment option.
6. The panel suggested practicing clinicians address the management of sialorrhea and airway clearance techniques in patients with NMD, as they face the risk of aspiration and pneumonia. For sialorrhea, the panel suggests starting with a trial of anticholinergic agents, as they are inexpensive and readily available. The panel also provided advice on botulinum toxin therapy and radiation therapy, which have limited data and should be reserved for experienced centers.
7. The panel reviewed data on airway clearance techniques, including glossopharyngeal breathing (GPB), mechanical insufflation-exsufflation (MI-E), also commonly known as cough-assist device, manually assisted cough, lung volume recruitment (LVR) by air stacking, and high-frequency chest wall oscillation (HFCWO). The panel suggested using airway clearance techniques based on local resources, expertise, and shared decision-making with patients.
The panel stressed the importance of respect for patient preferences, treatment goals, and quality of life considerations. The panel emphasized the need to modernize and improve access to ventilatory support for patients with NMD and the role of shared decision-making in improving quality of life and long-term outcomes. The panel also suggests that randomized controlled trials in patients with NMD would help establish a higher grade of evidence.
Dr. Hubel and Dr. Khan are from the Division of Pulmonary Allergy and Critical Care Medicine, Oregon Health and Science University, Portland.
Reference
Khan A et al. Respiratory management of patients with neuromuscular weakness: An American College of Chest Physicians Clinical Practice Guideline and Expert Panel Report [published online ahead of print, 2023 Mar 13]. Chest. 2023;S0012-3692(23)00353-7. doi: 10.1016/j.chest.2023.03.011.
Patients with neuromuscular diseases (NMD) face an increased risk of respiratory muscle weakness, which can contribute to various health problems. These include chronic respiratory failure, sleep-related breathing disorders, sialorrhea, and reduced cough effectiveness. In collaboration with AASM, AARC, and ATS, CHEST has developed guidelines to help clinicians manage patients with NMD. using the population, intervention, comparator, and outcome (PICO) format using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) methodology.
A few of the key recommendations are as follows:
1. Addressing the use and timing of pulmonary function tests (PFT), the panel suggests measuring vital capacity (FVC or SVC), MIP/MEP, SNIP, or PCF in patients with NMD every 6 months.
2. For the detection of respiratory failure and sleep-related breathing disorders in symptomatic patients with NMD who have normal PFT and overnight oximetry (ONO), the panel suggested that clinicians consider polysomnography (PSG) to assess whether noninvasive ventilation (NIV) would be beneficial. Adult patients do not have to have PSG to manage NMD if the PFT or ONO criteria support using NIV.
3. The panel recommends the use of NIV for the treatment of respiratory failure. To guide the initiation of NIV, clinicians can use any fall in FVC to < 80% of predicted with symptoms or FVC to < 50% of predicted without symptoms or SNIP/MIP to < –40 cm H2O or hypercapnia. The panel recommended individualizing treatment.
4. The panel suggested mouth piece ventilation (MPV) for daytime ventilatory support in patients with preserved bulbar function. Its desirable effects include delaying or avoiding tracheostomy and improving speech, cough effectiveness, and coordination of breathing and swallowing.
5. Invasive home mechanical ventilation (MV) by tracheostomy was identified as an acceptable option for patients with progressive respiratory failure, particularly those who were unable to clear secretions. Because of the high costs and caregiver burden, the guideline highlights the need to consider patient preferences, tolerability, the ability to maintain mouthpiece ventilation, and the availability of resources when choosing an appropriate treatment option.
6. The panel suggested practicing clinicians address the management of sialorrhea and airway clearance techniques in patients with NMD, as they face the risk of aspiration and pneumonia. For sialorrhea, the panel suggests starting with a trial of anticholinergic agents, as they are inexpensive and readily available. The panel also provided advice on botulinum toxin therapy and radiation therapy, which have limited data and should be reserved for experienced centers.
7. The panel reviewed data on airway clearance techniques, including glossopharyngeal breathing (GPB), mechanical insufflation-exsufflation (MI-E), also commonly known as cough-assist device, manually assisted cough, lung volume recruitment (LVR) by air stacking, and high-frequency chest wall oscillation (HFCWO). The panel suggested using airway clearance techniques based on local resources, expertise, and shared decision-making with patients.
The panel stressed the importance of respect for patient preferences, treatment goals, and quality of life considerations. The panel emphasized the need to modernize and improve access to ventilatory support for patients with NMD and the role of shared decision-making in improving quality of life and long-term outcomes. The panel also suggests that randomized controlled trials in patients with NMD would help establish a higher grade of evidence.
Dr. Hubel and Dr. Khan are from the Division of Pulmonary Allergy and Critical Care Medicine, Oregon Health and Science University, Portland.
Reference
Khan A et al. Respiratory management of patients with neuromuscular weakness: An American College of Chest Physicians Clinical Practice Guideline and Expert Panel Report [published online ahead of print, 2023 Mar 13]. Chest. 2023;S0012-3692(23)00353-7. doi: 10.1016/j.chest.2023.03.011.
The AGA Research Foundation awards $2.66 million in research funding
The American Gastroenterological Association (AGA) is proud to announce the 71 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“This year’s recipients are determined to make an impact on digestive health care through their research,” said Michael Camilleri, MD, AGAF, chair, AGA Research Foundation. “We are honored to support these talented individuals at a critical stage in their careers and research projects. We look forward to seeing their great accomplishments.”

Treatment options for digestive diseases begin with vigorous research. The AGA Research Foundation supports medical investigators as they advance our understanding of gastrointestinal and liver conditions. The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at foundation.gastro.org.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Alexander Nguyen, MD, PhD, The Regent of the University of California, Los Angeles
Jeffrey W. Patterson-Fortin, MD, PhD, Dana-Farber Cancer Institute, Boston, Massachusetts
Sean Spencer, MD, PhD, Stanford Medicine, California
Ken Y. Hui, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
Martina Molgora, PhD, Washington University School of Medicine, St. Louis, Missouri
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Brooke R. Druliner, PhD, Mayo Clinic, Rochester, Minnesota
Specialty Awards
AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Simon Schwörer, PhD, University of Chicago, Illinois
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
Bryson W. Katona, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia
AGA-Amgen Fellowship-to-Faculty Transition Award
Cynthia Hsu, MD, PhD, University of California, San Diego
AGA-Bristol Myers Squibb Fellowship-to-Faculty Transition Award
Siyan Cao, MD, PhD, Washington University in St. Louis
Amit Ringel, MD, Brigham and Women’s Hospital, Boston, Massachusetts
Pilot Awards
AGA Pilot Research Award In Digestive Disease Health Disparities
Sharad Wadhwani, MD, MPH, University of California, San Francisco
AGA Pilot Research Award in Health Disparities
Enrique Soto Pérez de Celis, MD, PhD, MS, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
AGA Pilot Research Award
Diana L. Snyder, MD, Mayo Clinic, Rochester, Minnesota
Michael Li, MD, MPH, University of California, San Francisco
Patricia Bloom, MD, University of Michigan, Ann Arbor
Edward Barnes, MD, MPH, University of North Carolina School of Medicine, Chapel Hill
AGA-Amgen Pilot Research Award In Digestive Disease Health Disparities
Laura Targownik, MD, MSHS, University of Toronto/Mount Sinai Hospital, Toronto, ON
Undergraduate Research Awards
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gwyneth Garramone, Loyola Marymount University, Los Angeles, California
Ella McLaren, University of California, San Diego
Nathan Moy, University of Southern California, Los Angeles
Hussein Elfayoumy, Johns Hopkins University, Baltimore, Maryland
Isabelle Garcia-Fischer, Tufts University, Medford, Massachusetts
Lidia Appell, University of New Mexico, Albuquerque
Katherine Burkman, Duke University, Durham, North Carolina
Alexa Boylan, Spelman College, Atlanta, Georgia
AGA-Dr. Harvey Young Education and Development Foundation’s Young Guts Scholar Program
Lucy Zhao, Massachusetts Institute of Technology Koch Institute for Integrative Cancer Research, Cambridge
Andrew Tran, Duke University, Durham, North Carolina
Sohaib Hassan, Rutgers University – Verzi Lab, New Brunswick, New Jersey
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor
Daniella Montalvo, University of Miami, Coral Gables, Florida
Sara Chough, Columbia University Irving Medical Center, New York, New York
Abstract Awards
Fellow Abstract Awards
David Flores Marin, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Jesse Platt, MD, PhD, Massachusetts General Hospital, Boston
Devika Gandhi, MD, Loma Linda University, California
Amanda Krause, MD, University of California, San Diego
Cynthia Tsay, MD, Mphil, Johns Hopkins Hospital, Baltimore, Maryland
Suha Abushamma, MD, Cleveland Clinic Foundation, Ohio
Md Obaidul Islam, PhD, University of Miami, Coral Gables, Florida
Sakteesh Gurunathan, MD, New York University School of Medicine, New York
Aaron Yeoh, MD, Stanford Hospital & Clinics, California
Yang Xiao, PhD, Mayo Clinic, Rochester, Minnesota
Jacques Gonzales, PhD, MS, Michigan State University, East Lansing
Kai Wang, MD, PhD, Harvard T.H. Chan School of Public Health, Cambridge, Massachusetts
Hoyeol Kim, PhD, Cedars Sinai Medical Center, New York, New York
Babajide Ojo, PhD, MS, Stanford University, California
AGA Fellow Abstract of the Year Award
Stefania Tocci, PhD, MS, University of Massachusetts, Cambridge
Student Abstract Awards
Pritha Chatterjee, MS, University of California, Riverside
Ela Contreras Panta, Vanderbilt University, Nashville, Tennessee
Mihir Shah, MD, MBBS, John H. Stroger Hospital of Cook County, Chicago, Illinois
Yuhan Fu, DO, Metrohealth Medical Center, Cleveland, Ohio
Raissa Nana Sede Mbakop, MD, Piedmont Athens Regional Medical Center, Athens, Georgia
Eleazar Montalvan-Sanchez, MD, Indiana University School of Medicine, Bloomington
Sarang Gupta, MD, St. Michael’s Hospital, Toronto, Ontario
Daniel Kim, Harvard Medical School, Cambridge, Massachusetts
Hannah Hrncir, Emory University, Decatur, Georgia
Zarwa Saqib, McMaster University, Hamilton, Ontario
Ying Zhu, MD, PhD, University of Michigan, Ann Arbor
Lizeth Cifuentes, MD, University of Pittsburgh Medical Center, Pennsylvania
Sharvani Dhandibhotla, MBBS, MS, Massachusetts General Hospital, Boston
Lauren Lynch, Baylor College of Medicine, Houston, Texas
AGA Student Abstract of The Year Award
Gabrielle Waclawik, MD, MPH, University of Wisconsin, Madison
AGA Abstract Award for Health Disparities Research
Soyoun Min, PhD, Lerner Research Institute (fellow), Cleveland, Ohio
Xiaobei Zhang, PhD , David Geffen School of Medicine at University of California, Los Angeles (fellow)
Matthew Zhao, David Geffen School of Medicine at University of California, Los Angeles (student)
Hannah Fiske, MD, Brown University/Rhode Island Hospital (student), Providence
AGA-APFED Abstract Award in Eosinophilic GI Diseases
Matthew Buendia, MD, Vanderbilt University Medical Center – Monroe Carell Jr. Children’s Hospital, Nashville, Tennessee
Alexandra L. Strauss, MD, University of Pennsylvania Health System, Philadelphia
Mira Yang, Northwestern Feinberg School of Medicine, Chicago, Illinois
AGA-Moti L. & Kamla Rustgi International Travel Award
Aviv Pudipeddi, MBBS, Concord Repatriation General Hospital, Sydney, Australia
Dianqin Sun, MBBS, Mmed, Erasmus University Medical Center, Rotterdam, Netherlands
The American Gastroenterological Association (AGA) is proud to announce the 71 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“This year’s recipients are determined to make an impact on digestive health care through their research,” said Michael Camilleri, MD, AGAF, chair, AGA Research Foundation. “We are honored to support these talented individuals at a critical stage in their careers and research projects. We look forward to seeing their great accomplishments.”

Treatment options for digestive diseases begin with vigorous research. The AGA Research Foundation supports medical investigators as they advance our understanding of gastrointestinal and liver conditions. The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at foundation.gastro.org.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Alexander Nguyen, MD, PhD, The Regent of the University of California, Los Angeles
Jeffrey W. Patterson-Fortin, MD, PhD, Dana-Farber Cancer Institute, Boston, Massachusetts
Sean Spencer, MD, PhD, Stanford Medicine, California
Ken Y. Hui, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
Martina Molgora, PhD, Washington University School of Medicine, St. Louis, Missouri
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Brooke R. Druliner, PhD, Mayo Clinic, Rochester, Minnesota
Specialty Awards
AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Simon Schwörer, PhD, University of Chicago, Illinois
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
Bryson W. Katona, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia
AGA-Amgen Fellowship-to-Faculty Transition Award
Cynthia Hsu, MD, PhD, University of California, San Diego
AGA-Bristol Myers Squibb Fellowship-to-Faculty Transition Award
Siyan Cao, MD, PhD, Washington University in St. Louis
Amit Ringel, MD, Brigham and Women’s Hospital, Boston, Massachusetts
Pilot Awards
AGA Pilot Research Award In Digestive Disease Health Disparities
Sharad Wadhwani, MD, MPH, University of California, San Francisco
AGA Pilot Research Award in Health Disparities
Enrique Soto Pérez de Celis, MD, PhD, MS, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
AGA Pilot Research Award
Diana L. Snyder, MD, Mayo Clinic, Rochester, Minnesota
Michael Li, MD, MPH, University of California, San Francisco
Patricia Bloom, MD, University of Michigan, Ann Arbor
Edward Barnes, MD, MPH, University of North Carolina School of Medicine, Chapel Hill
AGA-Amgen Pilot Research Award In Digestive Disease Health Disparities
Laura Targownik, MD, MSHS, University of Toronto/Mount Sinai Hospital, Toronto, ON
Undergraduate Research Awards
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gwyneth Garramone, Loyola Marymount University, Los Angeles, California
Ella McLaren, University of California, San Diego
Nathan Moy, University of Southern California, Los Angeles
Hussein Elfayoumy, Johns Hopkins University, Baltimore, Maryland
Isabelle Garcia-Fischer, Tufts University, Medford, Massachusetts
Lidia Appell, University of New Mexico, Albuquerque
Katherine Burkman, Duke University, Durham, North Carolina
Alexa Boylan, Spelman College, Atlanta, Georgia
AGA-Dr. Harvey Young Education and Development Foundation’s Young Guts Scholar Program
Lucy Zhao, Massachusetts Institute of Technology Koch Institute for Integrative Cancer Research, Cambridge
Andrew Tran, Duke University, Durham, North Carolina
Sohaib Hassan, Rutgers University – Verzi Lab, New Brunswick, New Jersey
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor
Daniella Montalvo, University of Miami, Coral Gables, Florida
Sara Chough, Columbia University Irving Medical Center, New York, New York
Abstract Awards
Fellow Abstract Awards
David Flores Marin, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Jesse Platt, MD, PhD, Massachusetts General Hospital, Boston
Devika Gandhi, MD, Loma Linda University, California
Amanda Krause, MD, University of California, San Diego
Cynthia Tsay, MD, Mphil, Johns Hopkins Hospital, Baltimore, Maryland
Suha Abushamma, MD, Cleveland Clinic Foundation, Ohio
Md Obaidul Islam, PhD, University of Miami, Coral Gables, Florida
Sakteesh Gurunathan, MD, New York University School of Medicine, New York
Aaron Yeoh, MD, Stanford Hospital & Clinics, California
Yang Xiao, PhD, Mayo Clinic, Rochester, Minnesota
Jacques Gonzales, PhD, MS, Michigan State University, East Lansing
Kai Wang, MD, PhD, Harvard T.H. Chan School of Public Health, Cambridge, Massachusetts
Hoyeol Kim, PhD, Cedars Sinai Medical Center, New York, New York
Babajide Ojo, PhD, MS, Stanford University, California
AGA Fellow Abstract of the Year Award
Stefania Tocci, PhD, MS, University of Massachusetts, Cambridge
Student Abstract Awards
Pritha Chatterjee, MS, University of California, Riverside
Ela Contreras Panta, Vanderbilt University, Nashville, Tennessee
Mihir Shah, MD, MBBS, John H. Stroger Hospital of Cook County, Chicago, Illinois
Yuhan Fu, DO, Metrohealth Medical Center, Cleveland, Ohio
Raissa Nana Sede Mbakop, MD, Piedmont Athens Regional Medical Center, Athens, Georgia
Eleazar Montalvan-Sanchez, MD, Indiana University School of Medicine, Bloomington
Sarang Gupta, MD, St. Michael’s Hospital, Toronto, Ontario
Daniel Kim, Harvard Medical School, Cambridge, Massachusetts
Hannah Hrncir, Emory University, Decatur, Georgia
Zarwa Saqib, McMaster University, Hamilton, Ontario
Ying Zhu, MD, PhD, University of Michigan, Ann Arbor
Lizeth Cifuentes, MD, University of Pittsburgh Medical Center, Pennsylvania
Sharvani Dhandibhotla, MBBS, MS, Massachusetts General Hospital, Boston
Lauren Lynch, Baylor College of Medicine, Houston, Texas
AGA Student Abstract of The Year Award
Gabrielle Waclawik, MD, MPH, University of Wisconsin, Madison
AGA Abstract Award for Health Disparities Research
Soyoun Min, PhD, Lerner Research Institute (fellow), Cleveland, Ohio
Xiaobei Zhang, PhD , David Geffen School of Medicine at University of California, Los Angeles (fellow)
Matthew Zhao, David Geffen School of Medicine at University of California, Los Angeles (student)
Hannah Fiske, MD, Brown University/Rhode Island Hospital (student), Providence
AGA-APFED Abstract Award in Eosinophilic GI Diseases
Matthew Buendia, MD, Vanderbilt University Medical Center – Monroe Carell Jr. Children’s Hospital, Nashville, Tennessee
Alexandra L. Strauss, MD, University of Pennsylvania Health System, Philadelphia
Mira Yang, Northwestern Feinberg School of Medicine, Chicago, Illinois
AGA-Moti L. & Kamla Rustgi International Travel Award
Aviv Pudipeddi, MBBS, Concord Repatriation General Hospital, Sydney, Australia
Dianqin Sun, MBBS, Mmed, Erasmus University Medical Center, Rotterdam, Netherlands
The American Gastroenterological Association (AGA) is proud to announce the 71 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“This year’s recipients are determined to make an impact on digestive health care through their research,” said Michael Camilleri, MD, AGAF, chair, AGA Research Foundation. “We are honored to support these talented individuals at a critical stage in their careers and research projects. We look forward to seeing their great accomplishments.”

Treatment options for digestive diseases begin with vigorous research. The AGA Research Foundation supports medical investigators as they advance our understanding of gastrointestinal and liver conditions. The AGA Research Awards Program is made possible thanks to generous donors and funders. Learn more about the AGA Research Foundation at foundation.gastro.org.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Alexander Nguyen, MD, PhD, The Regent of the University of California, Los Angeles
Jeffrey W. Patterson-Fortin, MD, PhD, Dana-Farber Cancer Institute, Boston, Massachusetts
Sean Spencer, MD, PhD, Stanford Medicine, California
Ken Y. Hui, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, Maryland
AGA-Gastric Cancer Foundation Ben Feinstein Memorial Research Scholar Award in Gastric Cancer
Martina Molgora, PhD, Washington University School of Medicine, St. Louis, Missouri
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Brooke R. Druliner, PhD, Mayo Clinic, Rochester, Minnesota
Specialty Awards
AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Simon Schwörer, PhD, University of Chicago, Illinois
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
Bryson W. Katona, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia
AGA-Amgen Fellowship-to-Faculty Transition Award
Cynthia Hsu, MD, PhD, University of California, San Diego
AGA-Bristol Myers Squibb Fellowship-to-Faculty Transition Award
Siyan Cao, MD, PhD, Washington University in St. Louis
Amit Ringel, MD, Brigham and Women’s Hospital, Boston, Massachusetts
Pilot Awards
AGA Pilot Research Award In Digestive Disease Health Disparities
Sharad Wadhwani, MD, MPH, University of California, San Francisco
AGA Pilot Research Award in Health Disparities
Enrique Soto Pérez de Celis, MD, PhD, MS, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán
AGA Pilot Research Award
Diana L. Snyder, MD, Mayo Clinic, Rochester, Minnesota
Michael Li, MD, MPH, University of California, San Francisco
Patricia Bloom, MD, University of Michigan, Ann Arbor
Edward Barnes, MD, MPH, University of North Carolina School of Medicine, Chapel Hill
AGA-Amgen Pilot Research Award In Digestive Disease Health Disparities
Laura Targownik, MD, MSHS, University of Toronto/Mount Sinai Hospital, Toronto, ON
Undergraduate Research Awards
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gwyneth Garramone, Loyola Marymount University, Los Angeles, California
Ella McLaren, University of California, San Diego
Nathan Moy, University of Southern California, Los Angeles
Hussein Elfayoumy, Johns Hopkins University, Baltimore, Maryland
Isabelle Garcia-Fischer, Tufts University, Medford, Massachusetts
Lidia Appell, University of New Mexico, Albuquerque
Katherine Burkman, Duke University, Durham, North Carolina
Alexa Boylan, Spelman College, Atlanta, Georgia
AGA-Dr. Harvey Young Education and Development Foundation’s Young Guts Scholar Program
Lucy Zhao, Massachusetts Institute of Technology Koch Institute for Integrative Cancer Research, Cambridge
Andrew Tran, Duke University, Durham, North Carolina
Sohaib Hassan, Rutgers University – Verzi Lab, New Brunswick, New Jersey
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor
Daniella Montalvo, University of Miami, Coral Gables, Florida
Sara Chough, Columbia University Irving Medical Center, New York, New York
Abstract Awards
Fellow Abstract Awards
David Flores Marin, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Jesse Platt, MD, PhD, Massachusetts General Hospital, Boston
Devika Gandhi, MD, Loma Linda University, California
Amanda Krause, MD, University of California, San Diego
Cynthia Tsay, MD, Mphil, Johns Hopkins Hospital, Baltimore, Maryland
Suha Abushamma, MD, Cleveland Clinic Foundation, Ohio
Md Obaidul Islam, PhD, University of Miami, Coral Gables, Florida
Sakteesh Gurunathan, MD, New York University School of Medicine, New York
Aaron Yeoh, MD, Stanford Hospital & Clinics, California
Yang Xiao, PhD, Mayo Clinic, Rochester, Minnesota
Jacques Gonzales, PhD, MS, Michigan State University, East Lansing
Kai Wang, MD, PhD, Harvard T.H. Chan School of Public Health, Cambridge, Massachusetts
Hoyeol Kim, PhD, Cedars Sinai Medical Center, New York, New York
Babajide Ojo, PhD, MS, Stanford University, California
AGA Fellow Abstract of the Year Award
Stefania Tocci, PhD, MS, University of Massachusetts, Cambridge
Student Abstract Awards
Pritha Chatterjee, MS, University of California, Riverside
Ela Contreras Panta, Vanderbilt University, Nashville, Tennessee
Mihir Shah, MD, MBBS, John H. Stroger Hospital of Cook County, Chicago, Illinois
Yuhan Fu, DO, Metrohealth Medical Center, Cleveland, Ohio
Raissa Nana Sede Mbakop, MD, Piedmont Athens Regional Medical Center, Athens, Georgia
Eleazar Montalvan-Sanchez, MD, Indiana University School of Medicine, Bloomington
Sarang Gupta, MD, St. Michael’s Hospital, Toronto, Ontario
Daniel Kim, Harvard Medical School, Cambridge, Massachusetts
Hannah Hrncir, Emory University, Decatur, Georgia
Zarwa Saqib, McMaster University, Hamilton, Ontario
Ying Zhu, MD, PhD, University of Michigan, Ann Arbor
Lizeth Cifuentes, MD, University of Pittsburgh Medical Center, Pennsylvania
Sharvani Dhandibhotla, MBBS, MS, Massachusetts General Hospital, Boston
Lauren Lynch, Baylor College of Medicine, Houston, Texas
AGA Student Abstract of The Year Award
Gabrielle Waclawik, MD, MPH, University of Wisconsin, Madison
AGA Abstract Award for Health Disparities Research
Soyoun Min, PhD, Lerner Research Institute (fellow), Cleveland, Ohio
Xiaobei Zhang, PhD , David Geffen School of Medicine at University of California, Los Angeles (fellow)
Matthew Zhao, David Geffen School of Medicine at University of California, Los Angeles (student)
Hannah Fiske, MD, Brown University/Rhode Island Hospital (student), Providence
AGA-APFED Abstract Award in Eosinophilic GI Diseases
Matthew Buendia, MD, Vanderbilt University Medical Center – Monroe Carell Jr. Children’s Hospital, Nashville, Tennessee
Alexandra L. Strauss, MD, University of Pennsylvania Health System, Philadelphia
Mira Yang, Northwestern Feinberg School of Medicine, Chicago, Illinois
AGA-Moti L. & Kamla Rustgi International Travel Award
Aviv Pudipeddi, MBBS, Concord Repatriation General Hospital, Sydney, Australia
Dianqin Sun, MBBS, Mmed, Erasmus University Medical Center, Rotterdam, Netherlands
Membership priorities shape the AGA advocacy agenda
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).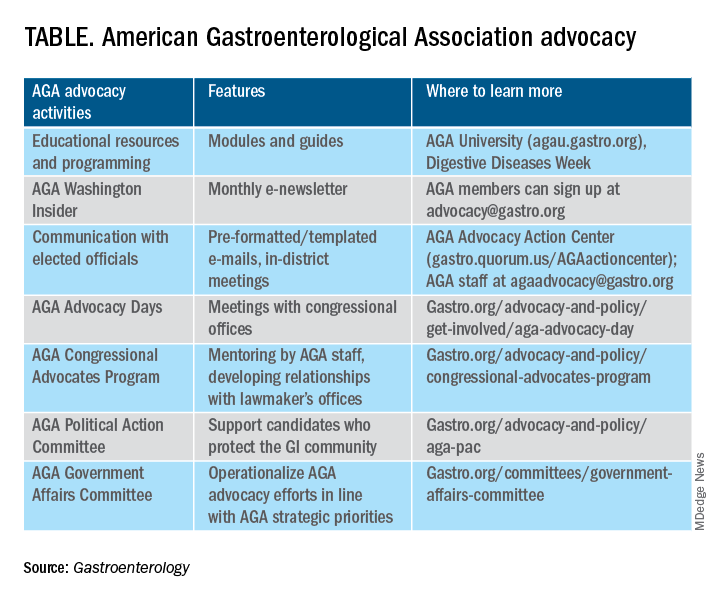
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
Here, we present key highlights from the survey findings and share opportunities for members to engage in GI advocacy.
AGA advocacy has contributed to significant recent successes that include lowering the average-risk of colorectal cancer screening age from 50 to 45 years, phasing out cost-sharing burdens associated with polypectomy at screening colonoscopy, encouraging federal support to focus on GI cancer disparities, ensuring coverage for telehealth services, expanding colonoscopy coverage after positive noninvasive colorectal cancer screening tests, and mitigating scheduled cuts in Medicare reimbursement for GI services.
Despite these important successes, the GI community faces significant challenges that include persisting GI health disparities; declines in reimbursement and increased prior authorization burdens for GI procedures and clinic visits, limited research funding to address the burden of GI disease, climate change, provider burnout, and increasing administrative burdens (such as insurance prior authorizations and step therapy policies.
The AGA sought to better understand policy priorities of the GI community by disseminating a 34-question policy priority survey to AGA members in December 2022. A total of 251 members responded to the survey with career stage and primary practice setting varying among respondents (Figure 1). The AGA vetted and selected 10 health policy issues of highest interest with 95% of survey respondents agreeing these 10 selected topics covered the top priority issues impacting gastroenterology (Figure 2).
From these 10 policy issues, members were asked to identify the top 5 issues that AGA advocacy efforts should address.
The issues most frequently identified included reducing administrative burdens and patient delays in care because of increased prior authorizations (78%), ensuring fair reimbursement for GI providers (68%), reducing insurance-initiated switching of patient treatments for nonmedical reasons (58%), maintaining coverage of video and telephone evaluation and management visits (55%), and reducing delays in clinical care resulting from step therapy protocols (53%).
Other important issues included ensuring patients with pre-existing conditions have access to essential benefits and quality specialty care (43%); protecting providers from medical licensing restrictions and liability to deliver care across state lines (35%); addressing Medicare Quality Payment Program reporting requirements and lack of specialty advanced payment models (27%); increasing funding for GI health disparities (24%); and, increasing federal research funding to ensure greater opportunities for diverse early career investigators (20%).
Most problematic burdens
Survey respondents identified insurer prior authorization and step therapy burdens as especially problematic. 93% of respondents described the impact of prior authorization on their practices as “significantly burdensome” (61%) or “somewhat burdensome” (32%).
About 95% noted that prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes “significantly” (56%) or “somewhat” (39%) negatively. 84% described the burdens associated with prior authorization policies as having increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
Likewise, step therapy protocols were perceived by 84% of respondents as burdensome; by 88% as negatively impactful on patient access to clinically appropriate treatments; and, by 88% as negatively impactful on patient clinical outcomes.
About 84% of respondents noted increases in the frequency of nonmedical switching and dosing restrictions over the last 5 years, with 90% perceiving negative impacts on patient clinical outcomes. 73% of respondents reported increased burdens associated with compliance in the Medicare QPP over the last 5 years.
AGA’s advocacy work
About 76% of respondents were interested in learning more about the AGA’s advocacy work. We presented some of the various opportunities and resources for members to engage with and contribute to AGA advocacy efforts (see pie chart). Based on the tremendous efforts and dedication of AGA staff, some of these opportunities include educational modules on AGA University, DDW programming, the AGA Washington Insider monthly policy newsletter, preformatted communications available through the AGA Advocacy Action Center, participation in AGA Advocacy Days or the AGA Congressional Advocates Program, service on the AGA Government Affairs Committee, and/or contributing to the AGA Political Action Committee.
Overall, the survey respondents illustrate the diversity and enthusiasm of AGA membership. Importantly, 95% of AGA members responding to the survey agreed these 10 selected policy issues are inclusive of the current top priority issues of the GI community. Amidst an ever-shifting health care landscape, we – the AGA community – must remain vigilant and adaptable to best address expected and unexpected changes and challenges to our patients and colleagues. In this respect, we should encourage constructive communication and dialogue between AGA membership, leadership, other issue stakeholders, government representatives and entities, and payers.
Amit Patel, MD, is a gastroenterologist and associate professor of medicine at Duke University and the Durham Veterans Affairs Medical Center, both in Durham, N.C. He serves on the editorial review board of Gastroenterology. Rotonya McCants Carr, MD, is the Cyrus E. Rubin Chair and division head of gastroenterology at the University of Washington, Seattle. Both Dr. Patel and Dr. Carr serve on the AGA Government Affairs Committee. The contents of this article do not represent the views of the Department of Veterans Affairs.
Reference
Patel A et al. Gastroenterology. 2023 May;164[6]:847-50.
Counting electric sheep: Dreaming of AI in sleep medicine
“Artificial intelligence (AI) in healthcare refers to the use of machine learning (ML), deep learning, natural language processing, and computer vision to process and analyze large amounts of health care data.”
The preceding line is a direct quote from ChatGPT when prompted with the question “What is AI in health care?” (OpenAI, 2022). AI has rapidly infiltrated our lives. From using facial recognition software to unlock our cellphones to scrolling through targeted media suggested by streaming services, our daily existence is interwoven with algorithms. With the recent introduction of GPT-3 (the model that powers ChatGPT) in late 2022 and its even more capable successor, GPT-4, in March 2023, AI will continue to dominate our everyday environment in even more complex and meaningful ways.
For sleep medicine, the initial applications of AI in this field have been innovative and promising. (OSA) (Bandyopadhyay A, et al. Sleep Breath. 2023;27[1]:39). Pépin and colleagues (JAMA Netw Open. 2020;3[1]:e1919657) combined ML with mandibular movement to diagnose OSA with a reasonable agreement to polysomnography as a novel home-based alternative for diagnosis. AI has also been used to predict adherence to positive airway pressure (PAP) therapy in OSA (Scioscia G, et al. Inform Health Soc Care. 2022;47[3]:274) and as a digital intervention tool accessed via a smartphone app for people with insomnia (Philip P, et al, J Med Internet Res. 2020;22[12]:e24268). The data-rich field of sleep medicine is primed for further advancements through AI, albeit with a few hurdles and regulations to overcome before becoming mainstream.
Future promise
Sleep medicine is uniquely positioned to develop robust AI algorithms because of its vast data trove. Using AI, scientists can efficiently analyze the raw data from polysomnography, consumer sleep technology (CST), and nightly remote monitoring (from PAP devices) to substantially improve comprehension and management of sleep disorders.
AI can redefine OSA through analysis of the big data available, rather than solely relying on the apnea-hypopnea index. In addition, novel variables such as facial structure; snoring index; temperature trends; and sleep environment, position, and timing using a camera-based contactless technology may be incorporated to enhance the diagnostic accuracy for OSA or better describe sleep quality. AI algorithms can also be embedded into the electronic health record (EHR) to facilitate screening for sleep disorders using patient characteristics, thus accelerating the recognition and evaluation of possible sleep disorders.
New ways of collecting data may deliver deeper insights into sleep health, as well. CST such as wearables, nearables, and phone applications are improving with each iteration, resulting in more data about sleep for millions of people over thousands of nights.
AI can help achieve precision medicine by integrating multimodal data to establish endotypes and phenotypes of various sleep disorders. Delineating endotypes and phenotypes allows for personalized treatment recommendations, which may improve patient adherence and health outcomes.
Treatment personalization can also be achieved through AI by predicting compliance to various therapies and responses, as well as by discovering alternative forms of delivery to accomplish desired health outcomes. For example, to predict PAP compliance, we can record a patient encounter and use natural language processing to analyze their opinion of their treatment, extracting relevant keywords and combining such processing with other available data, such as environmental factors, sleep schedule, medical history, and other information extracted from the EHR. As another example, AI can determine the optimal time for cancer therapy by predicting a patient’s circadian timing (Hesse J, et al. Cancers (Basel). 2020;12[11]:3103). Circadian timing of drug delivery may be relevant in other specialties including cardiovascular disease, endocrine disorders, and psychiatric conditions due to its associations with sleep. Integration of the various “-omics” (eg, proteomics, genomics, and transcriptomics) with physiologic, behavioral, and environmental data can offer opportunities for drug discovery and possible prediction of sleep disorders and sleep-related morbidity. Although generative pretrained transformers are currently used to predict text (ie, ChatGPT), it is theoretically possible to also apply this technique to identify patients at risk for future sleep disorders from an earlier age.
Challenges to an AI renaissance
Despite making strides in numerous specialties such as radiology, ophthalmology, pathology, oncology, and dermatology, AI has not yet gained mainstream usage. Why isn’t AI as ubiquitous and heavily entrenched in health care as it is in other industries? According to the National Academy of Medicine’s AI in Healthcare: The Hope, The Hype, The Promise, The Peril, there are several realities to address before we fully embrace the AI revolution (Matheny M, et al. 2019).
First, AI algorithms should be trained on quality data that are representative of the population. Interoperability between health care systems and standardization across platforms is required to access large volumes of quality data. The current framework for data gathering is limited due to regulations, patient privacy concerns, and organizational preferences. The challenges to data acquisition and standardization of information will continue to snarl progress unless there are legislative remedies.
Furthermore, datasets should be diverse enough to avoid introducing bias into the AI algorithm. If the dataset is limited and health inequities (eg, societal bias and social determinants of health) are excluded from the training set, then the outcome will perpetuate further explicit and implicit biases.
The Food and Drug Administration (FDA) reviews and authorizes AI/ML-enabled devices. Its current regulatory structure treats AI as a static process and does not allow for exercise of its intrinsic ability to continuously learn from additional data, thereby preventing it from becoming more accurate and evolving with the population over time. A more flexible approach is needed.
Lastly, recent advanced AI algorithms including deep learning and neural network methodology function like a “black box.” The models are not explainable or transparent. Without clear comprehension of its methods, acceptance in clinical practice will be guarded and further risk of inherent biases may ensue.
A path forward
But these challenges, like any, can be overcome. Research in the area of differential privacy and the adoption of recent data-sharing standards (eg, HL7 FHIR) can facilitate access to training data (Saripalle R, et al. J Biomed Inform. 2019;94:103188). Regulators are also open to incorporating feedback from the AI research community and industry in favor of innovation in this frenetic domain. The FDA developed the AI/ML Software as a Medical Device Action Plan in response to stakeholder feedback for oversight (FDA, 2021). Specifically, the “Good Machine Learning Practice” will be developed to describe AI/ML best practices (eg, data management, training, interpretability, evaluation, and documentation) to guide product development and standardization.
Sleep medicine has significantly progressed over the last several decades. Rather than maintain the status quo, AI can help fill the existing knowledge gaps, augment clinical practice, and streamline operations by analyzing and processing data at a volume and efficiency beyond human capacity. Fallibility is inevitable in machines and humans; however, like humans, machines can improve with continued training and exposure.
We asked ChatGPT about the future of AI in sleep medicine. It states that AI could have a “significant impact” on sleep disorders diagnosis, treatment, prevention, and sleep tracking and monitoring. Only time will tell if its claims are accurate.
Dr. Tan is Clinical Associate Professor with the Division of Sleep Medicine at the Stanford University School of Medicine. Dr. Bhargava is Clinical Professor with the Division of Pediatric Pulmonary, Asthma, and Sleep Medicine at the Stanford University School of Medicine.
“Artificial intelligence (AI) in healthcare refers to the use of machine learning (ML), deep learning, natural language processing, and computer vision to process and analyze large amounts of health care data.”
The preceding line is a direct quote from ChatGPT when prompted with the question “What is AI in health care?” (OpenAI, 2022). AI has rapidly infiltrated our lives. From using facial recognition software to unlock our cellphones to scrolling through targeted media suggested by streaming services, our daily existence is interwoven with algorithms. With the recent introduction of GPT-3 (the model that powers ChatGPT) in late 2022 and its even more capable successor, GPT-4, in March 2023, AI will continue to dominate our everyday environment in even more complex and meaningful ways.
For sleep medicine, the initial applications of AI in this field have been innovative and promising. (OSA) (Bandyopadhyay A, et al. Sleep Breath. 2023;27[1]:39). Pépin and colleagues (JAMA Netw Open. 2020;3[1]:e1919657) combined ML with mandibular movement to diagnose OSA with a reasonable agreement to polysomnography as a novel home-based alternative for diagnosis. AI has also been used to predict adherence to positive airway pressure (PAP) therapy in OSA (Scioscia G, et al. Inform Health Soc Care. 2022;47[3]:274) and as a digital intervention tool accessed via a smartphone app for people with insomnia (Philip P, et al, J Med Internet Res. 2020;22[12]:e24268). The data-rich field of sleep medicine is primed for further advancements through AI, albeit with a few hurdles and regulations to overcome before becoming mainstream.
Future promise
Sleep medicine is uniquely positioned to develop robust AI algorithms because of its vast data trove. Using AI, scientists can efficiently analyze the raw data from polysomnography, consumer sleep technology (CST), and nightly remote monitoring (from PAP devices) to substantially improve comprehension and management of sleep disorders.
AI can redefine OSA through analysis of the big data available, rather than solely relying on the apnea-hypopnea index. In addition, novel variables such as facial structure; snoring index; temperature trends; and sleep environment, position, and timing using a camera-based contactless technology may be incorporated to enhance the diagnostic accuracy for OSA or better describe sleep quality. AI algorithms can also be embedded into the electronic health record (EHR) to facilitate screening for sleep disorders using patient characteristics, thus accelerating the recognition and evaluation of possible sleep disorders.
New ways of collecting data may deliver deeper insights into sleep health, as well. CST such as wearables, nearables, and phone applications are improving with each iteration, resulting in more data about sleep for millions of people over thousands of nights.
AI can help achieve precision medicine by integrating multimodal data to establish endotypes and phenotypes of various sleep disorders. Delineating endotypes and phenotypes allows for personalized treatment recommendations, which may improve patient adherence and health outcomes.
Treatment personalization can also be achieved through AI by predicting compliance to various therapies and responses, as well as by discovering alternative forms of delivery to accomplish desired health outcomes. For example, to predict PAP compliance, we can record a patient encounter and use natural language processing to analyze their opinion of their treatment, extracting relevant keywords and combining such processing with other available data, such as environmental factors, sleep schedule, medical history, and other information extracted from the EHR. As another example, AI can determine the optimal time for cancer therapy by predicting a patient’s circadian timing (Hesse J, et al. Cancers (Basel). 2020;12[11]:3103). Circadian timing of drug delivery may be relevant in other specialties including cardiovascular disease, endocrine disorders, and psychiatric conditions due to its associations with sleep. Integration of the various “-omics” (eg, proteomics, genomics, and transcriptomics) with physiologic, behavioral, and environmental data can offer opportunities for drug discovery and possible prediction of sleep disorders and sleep-related morbidity. Although generative pretrained transformers are currently used to predict text (ie, ChatGPT), it is theoretically possible to also apply this technique to identify patients at risk for future sleep disorders from an earlier age.
Challenges to an AI renaissance
Despite making strides in numerous specialties such as radiology, ophthalmology, pathology, oncology, and dermatology, AI has not yet gained mainstream usage. Why isn’t AI as ubiquitous and heavily entrenched in health care as it is in other industries? According to the National Academy of Medicine’s AI in Healthcare: The Hope, The Hype, The Promise, The Peril, there are several realities to address before we fully embrace the AI revolution (Matheny M, et al. 2019).
First, AI algorithms should be trained on quality data that are representative of the population. Interoperability between health care systems and standardization across platforms is required to access large volumes of quality data. The current framework for data gathering is limited due to regulations, patient privacy concerns, and organizational preferences. The challenges to data acquisition and standardization of information will continue to snarl progress unless there are legislative remedies.
Furthermore, datasets should be diverse enough to avoid introducing bias into the AI algorithm. If the dataset is limited and health inequities (eg, societal bias and social determinants of health) are excluded from the training set, then the outcome will perpetuate further explicit and implicit biases.
The Food and Drug Administration (FDA) reviews and authorizes AI/ML-enabled devices. Its current regulatory structure treats AI as a static process and does not allow for exercise of its intrinsic ability to continuously learn from additional data, thereby preventing it from becoming more accurate and evolving with the population over time. A more flexible approach is needed.
Lastly, recent advanced AI algorithms including deep learning and neural network methodology function like a “black box.” The models are not explainable or transparent. Without clear comprehension of its methods, acceptance in clinical practice will be guarded and further risk of inherent biases may ensue.
A path forward
But these challenges, like any, can be overcome. Research in the area of differential privacy and the adoption of recent data-sharing standards (eg, HL7 FHIR) can facilitate access to training data (Saripalle R, et al. J Biomed Inform. 2019;94:103188). Regulators are also open to incorporating feedback from the AI research community and industry in favor of innovation in this frenetic domain. The FDA developed the AI/ML Software as a Medical Device Action Plan in response to stakeholder feedback for oversight (FDA, 2021). Specifically, the “Good Machine Learning Practice” will be developed to describe AI/ML best practices (eg, data management, training, interpretability, evaluation, and documentation) to guide product development and standardization.
Sleep medicine has significantly progressed over the last several decades. Rather than maintain the status quo, AI can help fill the existing knowledge gaps, augment clinical practice, and streamline operations by analyzing and processing data at a volume and efficiency beyond human capacity. Fallibility is inevitable in machines and humans; however, like humans, machines can improve with continued training and exposure.
We asked ChatGPT about the future of AI in sleep medicine. It states that AI could have a “significant impact” on sleep disorders diagnosis, treatment, prevention, and sleep tracking and monitoring. Only time will tell if its claims are accurate.
Dr. Tan is Clinical Associate Professor with the Division of Sleep Medicine at the Stanford University School of Medicine. Dr. Bhargava is Clinical Professor with the Division of Pediatric Pulmonary, Asthma, and Sleep Medicine at the Stanford University School of Medicine.
“Artificial intelligence (AI) in healthcare refers to the use of machine learning (ML), deep learning, natural language processing, and computer vision to process and analyze large amounts of health care data.”
The preceding line is a direct quote from ChatGPT when prompted with the question “What is AI in health care?” (OpenAI, 2022). AI has rapidly infiltrated our lives. From using facial recognition software to unlock our cellphones to scrolling through targeted media suggested by streaming services, our daily existence is interwoven with algorithms. With the recent introduction of GPT-3 (the model that powers ChatGPT) in late 2022 and its even more capable successor, GPT-4, in March 2023, AI will continue to dominate our everyday environment in even more complex and meaningful ways.
For sleep medicine, the initial applications of AI in this field have been innovative and promising. (OSA) (Bandyopadhyay A, et al. Sleep Breath. 2023;27[1]:39). Pépin and colleagues (JAMA Netw Open. 2020;3[1]:e1919657) combined ML with mandibular movement to diagnose OSA with a reasonable agreement to polysomnography as a novel home-based alternative for diagnosis. AI has also been used to predict adherence to positive airway pressure (PAP) therapy in OSA (Scioscia G, et al. Inform Health Soc Care. 2022;47[3]:274) and as a digital intervention tool accessed via a smartphone app for people with insomnia (Philip P, et al, J Med Internet Res. 2020;22[12]:e24268). The data-rich field of sleep medicine is primed for further advancements through AI, albeit with a few hurdles and regulations to overcome before becoming mainstream.
Future promise
Sleep medicine is uniquely positioned to develop robust AI algorithms because of its vast data trove. Using AI, scientists can efficiently analyze the raw data from polysomnography, consumer sleep technology (CST), and nightly remote monitoring (from PAP devices) to substantially improve comprehension and management of sleep disorders.
AI can redefine OSA through analysis of the big data available, rather than solely relying on the apnea-hypopnea index. In addition, novel variables such as facial structure; snoring index; temperature trends; and sleep environment, position, and timing using a camera-based contactless technology may be incorporated to enhance the diagnostic accuracy for OSA or better describe sleep quality. AI algorithms can also be embedded into the electronic health record (EHR) to facilitate screening for sleep disorders using patient characteristics, thus accelerating the recognition and evaluation of possible sleep disorders.
New ways of collecting data may deliver deeper insights into sleep health, as well. CST such as wearables, nearables, and phone applications are improving with each iteration, resulting in more data about sleep for millions of people over thousands of nights.
AI can help achieve precision medicine by integrating multimodal data to establish endotypes and phenotypes of various sleep disorders. Delineating endotypes and phenotypes allows for personalized treatment recommendations, which may improve patient adherence and health outcomes.
Treatment personalization can also be achieved through AI by predicting compliance to various therapies and responses, as well as by discovering alternative forms of delivery to accomplish desired health outcomes. For example, to predict PAP compliance, we can record a patient encounter and use natural language processing to analyze their opinion of their treatment, extracting relevant keywords and combining such processing with other available data, such as environmental factors, sleep schedule, medical history, and other information extracted from the EHR. As another example, AI can determine the optimal time for cancer therapy by predicting a patient’s circadian timing (Hesse J, et al. Cancers (Basel). 2020;12[11]:3103). Circadian timing of drug delivery may be relevant in other specialties including cardiovascular disease, endocrine disorders, and psychiatric conditions due to its associations with sleep. Integration of the various “-omics” (eg, proteomics, genomics, and transcriptomics) with physiologic, behavioral, and environmental data can offer opportunities for drug discovery and possible prediction of sleep disorders and sleep-related morbidity. Although generative pretrained transformers are currently used to predict text (ie, ChatGPT), it is theoretically possible to also apply this technique to identify patients at risk for future sleep disorders from an earlier age.
Challenges to an AI renaissance
Despite making strides in numerous specialties such as radiology, ophthalmology, pathology, oncology, and dermatology, AI has not yet gained mainstream usage. Why isn’t AI as ubiquitous and heavily entrenched in health care as it is in other industries? According to the National Academy of Medicine’s AI in Healthcare: The Hope, The Hype, The Promise, The Peril, there are several realities to address before we fully embrace the AI revolution (Matheny M, et al. 2019).
First, AI algorithms should be trained on quality data that are representative of the population. Interoperability between health care systems and standardization across platforms is required to access large volumes of quality data. The current framework for data gathering is limited due to regulations, patient privacy concerns, and organizational preferences. The challenges to data acquisition and standardization of information will continue to snarl progress unless there are legislative remedies.
Furthermore, datasets should be diverse enough to avoid introducing bias into the AI algorithm. If the dataset is limited and health inequities (eg, societal bias and social determinants of health) are excluded from the training set, then the outcome will perpetuate further explicit and implicit biases.
The Food and Drug Administration (FDA) reviews and authorizes AI/ML-enabled devices. Its current regulatory structure treats AI as a static process and does not allow for exercise of its intrinsic ability to continuously learn from additional data, thereby preventing it from becoming more accurate and evolving with the population over time. A more flexible approach is needed.
Lastly, recent advanced AI algorithms including deep learning and neural network methodology function like a “black box.” The models are not explainable or transparent. Without clear comprehension of its methods, acceptance in clinical practice will be guarded and further risk of inherent biases may ensue.
A path forward
But these challenges, like any, can be overcome. Research in the area of differential privacy and the adoption of recent data-sharing standards (eg, HL7 FHIR) can facilitate access to training data (Saripalle R, et al. J Biomed Inform. 2019;94:103188). Regulators are also open to incorporating feedback from the AI research community and industry in favor of innovation in this frenetic domain. The FDA developed the AI/ML Software as a Medical Device Action Plan in response to stakeholder feedback for oversight (FDA, 2021). Specifically, the “Good Machine Learning Practice” will be developed to describe AI/ML best practices (eg, data management, training, interpretability, evaluation, and documentation) to guide product development and standardization.
Sleep medicine has significantly progressed over the last several decades. Rather than maintain the status quo, AI can help fill the existing knowledge gaps, augment clinical practice, and streamline operations by analyzing and processing data at a volume and efficiency beyond human capacity. Fallibility is inevitable in machines and humans; however, like humans, machines can improve with continued training and exposure.
We asked ChatGPT about the future of AI in sleep medicine. It states that AI could have a “significant impact” on sleep disorders diagnosis, treatment, prevention, and sleep tracking and monitoring. Only time will tell if its claims are accurate.
Dr. Tan is Clinical Associate Professor with the Division of Sleep Medicine at the Stanford University School of Medicine. Dr. Bhargava is Clinical Professor with the Division of Pediatric Pulmonary, Asthma, and Sleep Medicine at the Stanford University School of Medicine.
American Gastroenterological Association invests in unsedated transnasal endoscopy medical device company EvoEndo®
, a medical device company developing platforms for unsedated transnasal endoscopy (TNE).
“AGA is proud to support EvoEndo® and its innovative technology that has the potential to improve care, save time, resources, and cost for hospitals and the GI community at large,” said Michael L. Kochman, MD, AGAF, MASGE, Wilmott Family Professor of Medicine and Surgery, Center for Endoscopic Innovation, Research and Training, gastroenterology division, University of Pennsylvania Health System; fund manager and adviser, AGA GI Opportunity Fund.
The EvoEndo® Single-Use Endoscopy System received FDA 510(k) clearance in February 2022. The EvoEndo System includes a sterile, single-use, flexible gastroscope designed for unsedated transnasal upper endoscopy and a small portable video controller. The EvoEndo Comfort Kit (not part of the cleared EvoEndo System) includes virtual reality (VR) goggles for patient distraction during the unsedated transnasal endoscopy procedure. Unsedated TNE can be used to evaluate and diagnose a wide range of upper GI conditions that may require frequent monitoring, including eosinophilic esophagitis (EoE), dysphagia, celiac disease, gastroesophageal reflux disease, Barrett’s esophagus, malabsorption, and abdominal pain.
“We are grateful for the support of the AGA, which is a testament to our ongoing commitment to improving GI outcomes with our technology,” said Jonathan T. Hartmann, CEO at EvoEndo. “The AGA has always been at the forefront of improving GI care. Our team could not be more excited that they have recognized EvoEndo, and we look forward to continuing to expand adoption of our technology to the GI community, its physicians, and their patients.”
TNE enabled by EvoEndo’s Single-Use Endoscopy System allows hospitals to move endoscopy procedures from an ambulatory procedural suite to an office-based environment and allows the “traditional” sedation procedure rooms to be used for more complex, therapeutic cases.
“Expanding our fund’s portfolio to include technologies that can transform the pediatric GI landscape is particularly exciting for Varia Ventures,” said Andrea Vossler, cofounder and managing director at Varia Ventures. “EvoEndo® has made significant progress in the TNE category, and we are excited for what’s to come in the future.”
The EvoEndo® Model LE Gastroscope is intended for the visualization of the upper digestive tract in adults and pediatric patients, specifically for the observation, diagnosis, and endoscopic treatment of the esophagus, stomach, and duodenal bulb in patients over the age of five. The gastroscope is a sterile, single-use device and can be inserted orally or transnasally. The EvoEndo® Controller is intended for use with an EvoEndo® Endoscope for endoscopic diagnosis, treatment, and video observation. The EvoEndo System is only intended for use by medical professionals. Physicians and other medical providers interested in learning more about EvoEndo’s TNE system or scheduling demonstrations and training can contact the company here.
, a medical device company developing platforms for unsedated transnasal endoscopy (TNE).
“AGA is proud to support EvoEndo® and its innovative technology that has the potential to improve care, save time, resources, and cost for hospitals and the GI community at large,” said Michael L. Kochman, MD, AGAF, MASGE, Wilmott Family Professor of Medicine and Surgery, Center for Endoscopic Innovation, Research and Training, gastroenterology division, University of Pennsylvania Health System; fund manager and adviser, AGA GI Opportunity Fund.
The EvoEndo® Single-Use Endoscopy System received FDA 510(k) clearance in February 2022. The EvoEndo System includes a sterile, single-use, flexible gastroscope designed for unsedated transnasal upper endoscopy and a small portable video controller. The EvoEndo Comfort Kit (not part of the cleared EvoEndo System) includes virtual reality (VR) goggles for patient distraction during the unsedated transnasal endoscopy procedure. Unsedated TNE can be used to evaluate and diagnose a wide range of upper GI conditions that may require frequent monitoring, including eosinophilic esophagitis (EoE), dysphagia, celiac disease, gastroesophageal reflux disease, Barrett’s esophagus, malabsorption, and abdominal pain.
“We are grateful for the support of the AGA, which is a testament to our ongoing commitment to improving GI outcomes with our technology,” said Jonathan T. Hartmann, CEO at EvoEndo. “The AGA has always been at the forefront of improving GI care. Our team could not be more excited that they have recognized EvoEndo, and we look forward to continuing to expand adoption of our technology to the GI community, its physicians, and their patients.”
TNE enabled by EvoEndo’s Single-Use Endoscopy System allows hospitals to move endoscopy procedures from an ambulatory procedural suite to an office-based environment and allows the “traditional” sedation procedure rooms to be used for more complex, therapeutic cases.
“Expanding our fund’s portfolio to include technologies that can transform the pediatric GI landscape is particularly exciting for Varia Ventures,” said Andrea Vossler, cofounder and managing director at Varia Ventures. “EvoEndo® has made significant progress in the TNE category, and we are excited for what’s to come in the future.”
The EvoEndo® Model LE Gastroscope is intended for the visualization of the upper digestive tract in adults and pediatric patients, specifically for the observation, diagnosis, and endoscopic treatment of the esophagus, stomach, and duodenal bulb in patients over the age of five. The gastroscope is a sterile, single-use device and can be inserted orally or transnasally. The EvoEndo® Controller is intended for use with an EvoEndo® Endoscope for endoscopic diagnosis, treatment, and video observation. The EvoEndo System is only intended for use by medical professionals. Physicians and other medical providers interested in learning more about EvoEndo’s TNE system or scheduling demonstrations and training can contact the company here.
, a medical device company developing platforms for unsedated transnasal endoscopy (TNE).
“AGA is proud to support EvoEndo® and its innovative technology that has the potential to improve care, save time, resources, and cost for hospitals and the GI community at large,” said Michael L. Kochman, MD, AGAF, MASGE, Wilmott Family Professor of Medicine and Surgery, Center for Endoscopic Innovation, Research and Training, gastroenterology division, University of Pennsylvania Health System; fund manager and adviser, AGA GI Opportunity Fund.
The EvoEndo® Single-Use Endoscopy System received FDA 510(k) clearance in February 2022. The EvoEndo System includes a sterile, single-use, flexible gastroscope designed for unsedated transnasal upper endoscopy and a small portable video controller. The EvoEndo Comfort Kit (not part of the cleared EvoEndo System) includes virtual reality (VR) goggles for patient distraction during the unsedated transnasal endoscopy procedure. Unsedated TNE can be used to evaluate and diagnose a wide range of upper GI conditions that may require frequent monitoring, including eosinophilic esophagitis (EoE), dysphagia, celiac disease, gastroesophageal reflux disease, Barrett’s esophagus, malabsorption, and abdominal pain.
“We are grateful for the support of the AGA, which is a testament to our ongoing commitment to improving GI outcomes with our technology,” said Jonathan T. Hartmann, CEO at EvoEndo. “The AGA has always been at the forefront of improving GI care. Our team could not be more excited that they have recognized EvoEndo, and we look forward to continuing to expand adoption of our technology to the GI community, its physicians, and their patients.”
TNE enabled by EvoEndo’s Single-Use Endoscopy System allows hospitals to move endoscopy procedures from an ambulatory procedural suite to an office-based environment and allows the “traditional” sedation procedure rooms to be used for more complex, therapeutic cases.
“Expanding our fund’s portfolio to include technologies that can transform the pediatric GI landscape is particularly exciting for Varia Ventures,” said Andrea Vossler, cofounder and managing director at Varia Ventures. “EvoEndo® has made significant progress in the TNE category, and we are excited for what’s to come in the future.”
The EvoEndo® Model LE Gastroscope is intended for the visualization of the upper digestive tract in adults and pediatric patients, specifically for the observation, diagnosis, and endoscopic treatment of the esophagus, stomach, and duodenal bulb in patients over the age of five. The gastroscope is a sterile, single-use device and can be inserted orally or transnasally. The EvoEndo® Controller is intended for use with an EvoEndo® Endoscope for endoscopic diagnosis, treatment, and video observation. The EvoEndo System is only intended for use by medical professionals. Physicians and other medical providers interested in learning more about EvoEndo’s TNE system or scheduling demonstrations and training can contact the company here.
Sybil – Prophecies for lung cancer risk prediction?
Thoracic Oncology and Chest Procedures Network
Lung Cancer Section
The mortality benefit associated with lung cancer screening (LCS) using low dose CT (LDCT) relies, in large part, on adherence rates to annual screening of ≥90%. However, the first 1 million “real world” patients screened in the US had very low (22%) annual adherence (Silvestri, et al. Chest. 2023;S0012-3692[23]00175-7). Refining how we estimate future lung cancer risk is an important opportunity for personalized medicine to bolster adherence to follow-up after initial LDCT.
(Mikhael, et al. J Clin Oncol. 2023;JCO2201345). The model was developed, trained, and tested in a total of 14,185 National Lung Screening Trial (NLST) participants including all cancer diagnoses. Within these data, Sybil’s accuracy in predicting 1-year lung cancer risk had AUC 0.92 (95% CI, 0.88-0.95) and at 6 years, AUC 0.75 (95% CI, 0.72-0.78).
The model was validated in two large independent LCS datasets, one in the US and one in Taiwan, where an LDCT can be obtained regardless of a personal smoking history. The cancer prevalence in these datasets was 3.4% and 0.9%, respectively. Reassuringly, Sybil’s performance was similar to the NLST data and was maintained in relevant subgroups such as sex, age and smoking history. Furthermore, Sybil reduced the false positive rate in the NLST to 8% at baseline scan, compared with 14% for Lung-RADS 1.0. Sybil’s algorithm, unlike others, has been made publicly available and hopefully will spur further validation and prospective study.
Robert Smyth, MD
Member-at-Large
Thoracic Oncology and Chest Procedures Network
Lung Cancer Section
The mortality benefit associated with lung cancer screening (LCS) using low dose CT (LDCT) relies, in large part, on adherence rates to annual screening of ≥90%. However, the first 1 million “real world” patients screened in the US had very low (22%) annual adherence (Silvestri, et al. Chest. 2023;S0012-3692[23]00175-7). Refining how we estimate future lung cancer risk is an important opportunity for personalized medicine to bolster adherence to follow-up after initial LDCT.
(Mikhael, et al. J Clin Oncol. 2023;JCO2201345). The model was developed, trained, and tested in a total of 14,185 National Lung Screening Trial (NLST) participants including all cancer diagnoses. Within these data, Sybil’s accuracy in predicting 1-year lung cancer risk had AUC 0.92 (95% CI, 0.88-0.95) and at 6 years, AUC 0.75 (95% CI, 0.72-0.78).
The model was validated in two large independent LCS datasets, one in the US and one in Taiwan, where an LDCT can be obtained regardless of a personal smoking history. The cancer prevalence in these datasets was 3.4% and 0.9%, respectively. Reassuringly, Sybil’s performance was similar to the NLST data and was maintained in relevant subgroups such as sex, age and smoking history. Furthermore, Sybil reduced the false positive rate in the NLST to 8% at baseline scan, compared with 14% for Lung-RADS 1.0. Sybil’s algorithm, unlike others, has been made publicly available and hopefully will spur further validation and prospective study.
Robert Smyth, MD
Member-at-Large
Thoracic Oncology and Chest Procedures Network
Lung Cancer Section
The mortality benefit associated with lung cancer screening (LCS) using low dose CT (LDCT) relies, in large part, on adherence rates to annual screening of ≥90%. However, the first 1 million “real world” patients screened in the US had very low (22%) annual adherence (Silvestri, et al. Chest. 2023;S0012-3692[23]00175-7). Refining how we estimate future lung cancer risk is an important opportunity for personalized medicine to bolster adherence to follow-up after initial LDCT.
(Mikhael, et al. J Clin Oncol. 2023;JCO2201345). The model was developed, trained, and tested in a total of 14,185 National Lung Screening Trial (NLST) participants including all cancer diagnoses. Within these data, Sybil’s accuracy in predicting 1-year lung cancer risk had AUC 0.92 (95% CI, 0.88-0.95) and at 6 years, AUC 0.75 (95% CI, 0.72-0.78).
The model was validated in two large independent LCS datasets, one in the US and one in Taiwan, where an LDCT can be obtained regardless of a personal smoking history. The cancer prevalence in these datasets was 3.4% and 0.9%, respectively. Reassuringly, Sybil’s performance was similar to the NLST data and was maintained in relevant subgroups such as sex, age and smoking history. Furthermore, Sybil reduced the false positive rate in the NLST to 8% at baseline scan, compared with 14% for Lung-RADS 1.0. Sybil’s algorithm, unlike others, has been made publicly available and hopefully will spur further validation and prospective study.
Robert Smyth, MD
Member-at-Large
Home sleep apnea test: Peripheral arterial tonometry
Sleep Medicine Network
Respiratory-related Sleep Disorders Section
Home sleep apnea test: Peripheral arterial tonometry
OSA is associated with serious health consequences and increased health care utilization (Kapur V, et al. Sleep. 1999:22[6]:749).
Polysomnography (PSG) is the gold standard for diagnosis, but is expensive, cumbersome, and inconsistently accessible. (Kapur VK, et al. J Clin Sleep Med. 2017;13[3]:479; Skomro RP, et al. Chest. 2010;138[2]:257).
Utilization of HSAT devices has increased in recent years, partly due to the COVID-19 pandemic and limitations in insurance reimbursement for PSG as the initial diagnostic test. But while there are benefits to home testing with respect to convenience and increased access, we must take the clinical context into account.
Peripheral arterial tonometry (PAT) is a commonly used HSAT technology, which measures peripheral arterial vascular tone using plethysmography at the fingertip. It has a sensitivity of 80% and specificity of 83% for detecting OSA in patients without significant comorbidities and high pretest probability of OSA compared to PSG (Ward KL, et al. J Clin Sleep Med. 2015;11[4]:433). But PAT has also been criticized for lacking diagnostic accuracy, particularly when including patients with mild OSA in analysis (Ichikawa M, et al. J Sleep Res. 2022;31[6]:e13682).
HSAT devices using PAT technology have been studied in patients with atrial fibrillation (Tauman R, et al. Nat Sci Sleep. 2020;12:1115), adolescents (Choi JH, et al. J Clin Sleep Med. 2018;14[10]:1741), and pregnant women (O’Brien LM, et al. J Clin Sleep Med. 2012;8[3]:287), and to assess OSA treatment adequacy with varying sensitivity and specificity. Study in special populations may allow for increased access to testing with the benefit of increased recognition of a generally underdiagnosed disorder. But it’s important to use HSAT alongside awareness of its limitations and it should not replace good clinical judgment when making treatment decisions.
Dimple Tejwani, MD
Member-at-Large
Kara Dupuy-McCauley, MD
Member-at-Large
Sleep Medicine Network
Respiratory-related Sleep Disorders Section
Home sleep apnea test: Peripheral arterial tonometry
OSA is associated with serious health consequences and increased health care utilization (Kapur V, et al. Sleep. 1999:22[6]:749).
Polysomnography (PSG) is the gold standard for diagnosis, but is expensive, cumbersome, and inconsistently accessible. (Kapur VK, et al. J Clin Sleep Med. 2017;13[3]:479; Skomro RP, et al. Chest. 2010;138[2]:257).
Utilization of HSAT devices has increased in recent years, partly due to the COVID-19 pandemic and limitations in insurance reimbursement for PSG as the initial diagnostic test. But while there are benefits to home testing with respect to convenience and increased access, we must take the clinical context into account.
Peripheral arterial tonometry (PAT) is a commonly used HSAT technology, which measures peripheral arterial vascular tone using plethysmography at the fingertip. It has a sensitivity of 80% and specificity of 83% for detecting OSA in patients without significant comorbidities and high pretest probability of OSA compared to PSG (Ward KL, et al. J Clin Sleep Med. 2015;11[4]:433). But PAT has also been criticized for lacking diagnostic accuracy, particularly when including patients with mild OSA in analysis (Ichikawa M, et al. J Sleep Res. 2022;31[6]:e13682).
HSAT devices using PAT technology have been studied in patients with atrial fibrillation (Tauman R, et al. Nat Sci Sleep. 2020;12:1115), adolescents (Choi JH, et al. J Clin Sleep Med. 2018;14[10]:1741), and pregnant women (O’Brien LM, et al. J Clin Sleep Med. 2012;8[3]:287), and to assess OSA treatment adequacy with varying sensitivity and specificity. Study in special populations may allow for increased access to testing with the benefit of increased recognition of a generally underdiagnosed disorder. But it’s important to use HSAT alongside awareness of its limitations and it should not replace good clinical judgment when making treatment decisions.
Dimple Tejwani, MD
Member-at-Large
Kara Dupuy-McCauley, MD
Member-at-Large
Sleep Medicine Network
Respiratory-related Sleep Disorders Section
Home sleep apnea test: Peripheral arterial tonometry
OSA is associated with serious health consequences and increased health care utilization (Kapur V, et al. Sleep. 1999:22[6]:749).
Polysomnography (PSG) is the gold standard for diagnosis, but is expensive, cumbersome, and inconsistently accessible. (Kapur VK, et al. J Clin Sleep Med. 2017;13[3]:479; Skomro RP, et al. Chest. 2010;138[2]:257).
Utilization of HSAT devices has increased in recent years, partly due to the COVID-19 pandemic and limitations in insurance reimbursement for PSG as the initial diagnostic test. But while there are benefits to home testing with respect to convenience and increased access, we must take the clinical context into account.
Peripheral arterial tonometry (PAT) is a commonly used HSAT technology, which measures peripheral arterial vascular tone using plethysmography at the fingertip. It has a sensitivity of 80% and specificity of 83% for detecting OSA in patients without significant comorbidities and high pretest probability of OSA compared to PSG (Ward KL, et al. J Clin Sleep Med. 2015;11[4]:433). But PAT has also been criticized for lacking diagnostic accuracy, particularly when including patients with mild OSA in analysis (Ichikawa M, et al. J Sleep Res. 2022;31[6]:e13682).
HSAT devices using PAT technology have been studied in patients with atrial fibrillation (Tauman R, et al. Nat Sci Sleep. 2020;12:1115), adolescents (Choi JH, et al. J Clin Sleep Med. 2018;14[10]:1741), and pregnant women (O’Brien LM, et al. J Clin Sleep Med. 2012;8[3]:287), and to assess OSA treatment adequacy with varying sensitivity and specificity. Study in special populations may allow for increased access to testing with the benefit of increased recognition of a generally underdiagnosed disorder. But it’s important to use HSAT alongside awareness of its limitations and it should not replace good clinical judgment when making treatment decisions.
Dimple Tejwani, MD
Member-at-Large
Kara Dupuy-McCauley, MD
Member-at-Large
Emerging role of tele-rehab: Efficacy and challenges
Diffuse Lung Disease and Transplant Network
Pulmonary Physiology and Rehabilitation Section
Pulmonary rehabilitation (PR) is an essential component of the management of chronic pulmonary disease. Interest in alternate PR delivery methods has grown in recent years. The official workshop report of the American Thoracic Society (Holland AE, et al. Ann Am Thorac Soc. 2021;18[5]:e12) identified 13 essential components of PR in response to new program models. They encompass patient assessment, program content, method of delivery, and quality assurance, and serve as a guide for successful implementation of emerging programs.
A recent study reported significant improvement in COPD Assessment Test (CAT) scores after PR in both in-person (n=383) and virtual programs (n=171). Similar improvements were found in health outcomes, attendance, and dropout rate (Huynh VC, et al. Chest. 2023;163[3]:529). Another concurrent 3-year prospective study enrolled COPD patients in standard PR (n=89) or community based tele-PR (n=177) at seven tele-sites and one standard site (Alwakeel AJ, et al. Ann Am Thorac Soc. 2022;19[1]:39).
This study established the accessibility, feasibility, and safety of a community based tele-PR program and noted no differences between groups in 6-minute walk test or CAT score improvement.
Ongoing challenges with tele-PR include standardization of programs and of initial clinical evaluations that determine eligibility for them. Patients on home oxygen and those with exercise desaturation are often excluded, but they have the most potential for improvement. Studies are needed to determine the characteristics of patients who would benefit most from non-traditional models of PR.
Fatima Zeba, MD
Fellow-in-Training
Rania Abdallah, MD
Member-at-Large
Malik Khurram Khan, MD
Member-at-Large
Diffuse Lung Disease and Transplant Network
Pulmonary Physiology and Rehabilitation Section
Pulmonary rehabilitation (PR) is an essential component of the management of chronic pulmonary disease. Interest in alternate PR delivery methods has grown in recent years. The official workshop report of the American Thoracic Society (Holland AE, et al. Ann Am Thorac Soc. 2021;18[5]:e12) identified 13 essential components of PR in response to new program models. They encompass patient assessment, program content, method of delivery, and quality assurance, and serve as a guide for successful implementation of emerging programs.
A recent study reported significant improvement in COPD Assessment Test (CAT) scores after PR in both in-person (n=383) and virtual programs (n=171). Similar improvements were found in health outcomes, attendance, and dropout rate (Huynh VC, et al. Chest. 2023;163[3]:529). Another concurrent 3-year prospective study enrolled COPD patients in standard PR (n=89) or community based tele-PR (n=177) at seven tele-sites and one standard site (Alwakeel AJ, et al. Ann Am Thorac Soc. 2022;19[1]:39).
This study established the accessibility, feasibility, and safety of a community based tele-PR program and noted no differences between groups in 6-minute walk test or CAT score improvement.
Ongoing challenges with tele-PR include standardization of programs and of initial clinical evaluations that determine eligibility for them. Patients on home oxygen and those with exercise desaturation are often excluded, but they have the most potential for improvement. Studies are needed to determine the characteristics of patients who would benefit most from non-traditional models of PR.
Fatima Zeba, MD
Fellow-in-Training
Rania Abdallah, MD
Member-at-Large
Malik Khurram Khan, MD
Member-at-Large
Diffuse Lung Disease and Transplant Network
Pulmonary Physiology and Rehabilitation Section
Pulmonary rehabilitation (PR) is an essential component of the management of chronic pulmonary disease. Interest in alternate PR delivery methods has grown in recent years. The official workshop report of the American Thoracic Society (Holland AE, et al. Ann Am Thorac Soc. 2021;18[5]:e12) identified 13 essential components of PR in response to new program models. They encompass patient assessment, program content, method of delivery, and quality assurance, and serve as a guide for successful implementation of emerging programs.
A recent study reported significant improvement in COPD Assessment Test (CAT) scores after PR in both in-person (n=383) and virtual programs (n=171). Similar improvements were found in health outcomes, attendance, and dropout rate (Huynh VC, et al. Chest. 2023;163[3]:529). Another concurrent 3-year prospective study enrolled COPD patients in standard PR (n=89) or community based tele-PR (n=177) at seven tele-sites and one standard site (Alwakeel AJ, et al. Ann Am Thorac Soc. 2022;19[1]:39).
This study established the accessibility, feasibility, and safety of a community based tele-PR program and noted no differences between groups in 6-minute walk test or CAT score improvement.
Ongoing challenges with tele-PR include standardization of programs and of initial clinical evaluations that determine eligibility for them. Patients on home oxygen and those with exercise desaturation are often excluded, but they have the most potential for improvement. Studies are needed to determine the characteristics of patients who would benefit most from non-traditional models of PR.
Fatima Zeba, MD
Fellow-in-Training
Rania Abdallah, MD
Member-at-Large
Malik Khurram Khan, MD
Member-at-Large
Replacing the Lung Allocation Score
Diffuse Lung Disease and Lung Transplant Network
Lung Transplant Section
In March 2023, the Composite Allocation Score (CAS) will replace the Lung Allocation Score (LAS) for matching donor lungs to transplant candidates in the United States. The LAS was implemented in 2005 to improve lung organ utilization. Its score was determined by two main factors: (1) risk of 1-year waitlist mortality and (2) likelihood of 1-year post-transplant survival, with the first factor having twice the weight. However, LAS did not account for candidate biology attributes, such as pediatric age, blood type, allosensitization, or height. Long-term survival outcomes under LAS may be reduced, given the greater emphasis on waitlist mortality. Candidates were also subjected to strict geographical distributions within a 250-nautical-mile radius, which frequently resulted in those with lower LAS obtaining a transplant. CAS differs from the LAS in that it assigns an allocation score in a continuous distribution based on the following factors: medical urgency, expected survival benefit following transplant, pediatric age, blood type, HLA antibody sensitization, candidate height, and geographical proximity to the donor organ. Each factor has a specific weight, and because donor factors contribute to CAS, a candidate’s score changes with each donor-recipient match run. Continuous distribution removes hard geographical boundaries and aims for more equitable organ allocation. To understand how allocation might change with CAS, Valapour and colleagues created various CAS scenarios using data from individuals on the national transplant waiting list (Am J Transplant. 2022;22[12]:2971).
They found that waitlist deaths decreased by 36%-47%. This effect was greatest in scenarios where there was less weight on placement efficiency (ie, geography) and more weight on post-transplant outcomes. Transplant system equity also improved in their simulation models. It will be exciting to see how candidate and recipient outcomes are affected once CAS is implemented.
Gloria Li, MD
Member-at-Large
Reference
1. United Network for Organ Sharing. www.unos.org.
Diffuse Lung Disease and Lung Transplant Network
Lung Transplant Section
In March 2023, the Composite Allocation Score (CAS) will replace the Lung Allocation Score (LAS) for matching donor lungs to transplant candidates in the United States. The LAS was implemented in 2005 to improve lung organ utilization. Its score was determined by two main factors: (1) risk of 1-year waitlist mortality and (2) likelihood of 1-year post-transplant survival, with the first factor having twice the weight. However, LAS did not account for candidate biology attributes, such as pediatric age, blood type, allosensitization, or height. Long-term survival outcomes under LAS may be reduced, given the greater emphasis on waitlist mortality. Candidates were also subjected to strict geographical distributions within a 250-nautical-mile radius, which frequently resulted in those with lower LAS obtaining a transplant. CAS differs from the LAS in that it assigns an allocation score in a continuous distribution based on the following factors: medical urgency, expected survival benefit following transplant, pediatric age, blood type, HLA antibody sensitization, candidate height, and geographical proximity to the donor organ. Each factor has a specific weight, and because donor factors contribute to CAS, a candidate’s score changes with each donor-recipient match run. Continuous distribution removes hard geographical boundaries and aims for more equitable organ allocation. To understand how allocation might change with CAS, Valapour and colleagues created various CAS scenarios using data from individuals on the national transplant waiting list (Am J Transplant. 2022;22[12]:2971).
They found that waitlist deaths decreased by 36%-47%. This effect was greatest in scenarios where there was less weight on placement efficiency (ie, geography) and more weight on post-transplant outcomes. Transplant system equity also improved in their simulation models. It will be exciting to see how candidate and recipient outcomes are affected once CAS is implemented.
Gloria Li, MD
Member-at-Large
Reference
1. United Network for Organ Sharing. www.unos.org.
Diffuse Lung Disease and Lung Transplant Network
Lung Transplant Section
In March 2023, the Composite Allocation Score (CAS) will replace the Lung Allocation Score (LAS) for matching donor lungs to transplant candidates in the United States. The LAS was implemented in 2005 to improve lung organ utilization. Its score was determined by two main factors: (1) risk of 1-year waitlist mortality and (2) likelihood of 1-year post-transplant survival, with the first factor having twice the weight. However, LAS did not account for candidate biology attributes, such as pediatric age, blood type, allosensitization, or height. Long-term survival outcomes under LAS may be reduced, given the greater emphasis on waitlist mortality. Candidates were also subjected to strict geographical distributions within a 250-nautical-mile radius, which frequently resulted in those with lower LAS obtaining a transplant. CAS differs from the LAS in that it assigns an allocation score in a continuous distribution based on the following factors: medical urgency, expected survival benefit following transplant, pediatric age, blood type, HLA antibody sensitization, candidate height, and geographical proximity to the donor organ. Each factor has a specific weight, and because donor factors contribute to CAS, a candidate’s score changes with each donor-recipient match run. Continuous distribution removes hard geographical boundaries and aims for more equitable organ allocation. To understand how allocation might change with CAS, Valapour and colleagues created various CAS scenarios using data from individuals on the national transplant waiting list (Am J Transplant. 2022;22[12]:2971).
They found that waitlist deaths decreased by 36%-47%. This effect was greatest in scenarios where there was less weight on placement efficiency (ie, geography) and more weight on post-transplant outcomes. Transplant system equity also improved in their simulation models. It will be exciting to see how candidate and recipient outcomes are affected once CAS is implemented.
Gloria Li, MD
Member-at-Large
Reference
1. United Network for Organ Sharing. www.unos.org.





