User login
Advancements in nutritional management for critically ill patients
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
New developments on the forefront of intermediate-risk pulmonary embolism
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
Bronchiectasis: A call to action
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
The countdown to CHEST 2024 begins
As we find ourselves in September, I cannot help but dedicate my column to the upcoming CHEST Annual Meeting quickly approaching, October 6 to 9, in Boston.
If you haven’t yet been to a CHEST Annual Meeting, it’s an unmatched experience.
For those who have attended, there’s always something new to see. Every year is different, with the culture of the location guiding the way and new opportunities to network while engaging in activity. No matter how many times you have been, attending the CHEST Annual Meeting never gets old.
Leveraging CHEST 2024’s location, we’ll be hosting a Grand Rounds event days before the meeting starts with pulmonary and critical care medicine fellows from the regional Boston programs to learn from visiting CHEST leadership on a variety of influential topics. These fellowship programs held events like this prepandemic, so I’m truly excited we could help restart the tradition and give the local fellows an opportunity to interact with each other from both an academic and social perspective. Personally, I am very much looking forward to meeting and getting to know the fellows from the Boston area.
The meeting has a lot of notable opportunities lined up (see my official “President’s checklist”), including the third year of CHEST After Hours (Monday, October 7)—a unique storytelling event focusing on the humanities of medicine in partnership with The Nocturnists podcast. And for the first time in recent years, CHEST 2024 will feature a 5K run/walk (Tuesday, October 8) in support of CHEST philanthropy and its work to fuel breakthroughs, empower innovation, and drive toward a future where every patient’s well-being is safeguarded. I encourage you to register in advance of the meeting to secure your space and snag a souvenir T-shirt.
First thing Sunday morning (October 6), the meeting kicks off with the Opening Session where we will be celebrating the new fellows of the college (FCCP), honoring trailblazers in chest medicine, and welcoming this year’s keynote speaker.
This year’s keynote address will come from Vanessa Kerry, MD, who will speak on environmental issues and her work to raise awareness of the impact of climate change on health.
With so many things to look forward to, this meeting will be one to remember for all in attendance.
I look forward to seeing you in Boston,
Jack
As we find ourselves in September, I cannot help but dedicate my column to the upcoming CHEST Annual Meeting quickly approaching, October 6 to 9, in Boston.
If you haven’t yet been to a CHEST Annual Meeting, it’s an unmatched experience.
For those who have attended, there’s always something new to see. Every year is different, with the culture of the location guiding the way and new opportunities to network while engaging in activity. No matter how many times you have been, attending the CHEST Annual Meeting never gets old.
Leveraging CHEST 2024’s location, we’ll be hosting a Grand Rounds event days before the meeting starts with pulmonary and critical care medicine fellows from the regional Boston programs to learn from visiting CHEST leadership on a variety of influential topics. These fellowship programs held events like this prepandemic, so I’m truly excited we could help restart the tradition and give the local fellows an opportunity to interact with each other from both an academic and social perspective. Personally, I am very much looking forward to meeting and getting to know the fellows from the Boston area.
The meeting has a lot of notable opportunities lined up (see my official “President’s checklist”), including the third year of CHEST After Hours (Monday, October 7)—a unique storytelling event focusing on the humanities of medicine in partnership with The Nocturnists podcast. And for the first time in recent years, CHEST 2024 will feature a 5K run/walk (Tuesday, October 8) in support of CHEST philanthropy and its work to fuel breakthroughs, empower innovation, and drive toward a future where every patient’s well-being is safeguarded. I encourage you to register in advance of the meeting to secure your space and snag a souvenir T-shirt.
First thing Sunday morning (October 6), the meeting kicks off with the Opening Session where we will be celebrating the new fellows of the college (FCCP), honoring trailblazers in chest medicine, and welcoming this year’s keynote speaker.
This year’s keynote address will come from Vanessa Kerry, MD, who will speak on environmental issues and her work to raise awareness of the impact of climate change on health.
With so many things to look forward to, this meeting will be one to remember for all in attendance.
I look forward to seeing you in Boston,
Jack
As we find ourselves in September, I cannot help but dedicate my column to the upcoming CHEST Annual Meeting quickly approaching, October 6 to 9, in Boston.
If you haven’t yet been to a CHEST Annual Meeting, it’s an unmatched experience.
For those who have attended, there’s always something new to see. Every year is different, with the culture of the location guiding the way and new opportunities to network while engaging in activity. No matter how many times you have been, attending the CHEST Annual Meeting never gets old.
Leveraging CHEST 2024’s location, we’ll be hosting a Grand Rounds event days before the meeting starts with pulmonary and critical care medicine fellows from the regional Boston programs to learn from visiting CHEST leadership on a variety of influential topics. These fellowship programs held events like this prepandemic, so I’m truly excited we could help restart the tradition and give the local fellows an opportunity to interact with each other from both an academic and social perspective. Personally, I am very much looking forward to meeting and getting to know the fellows from the Boston area.
The meeting has a lot of notable opportunities lined up (see my official “President’s checklist”), including the third year of CHEST After Hours (Monday, October 7)—a unique storytelling event focusing on the humanities of medicine in partnership with The Nocturnists podcast. And for the first time in recent years, CHEST 2024 will feature a 5K run/walk (Tuesday, October 8) in support of CHEST philanthropy and its work to fuel breakthroughs, empower innovation, and drive toward a future where every patient’s well-being is safeguarded. I encourage you to register in advance of the meeting to secure your space and snag a souvenir T-shirt.
First thing Sunday morning (October 6), the meeting kicks off with the Opening Session where we will be celebrating the new fellows of the college (FCCP), honoring trailblazers in chest medicine, and welcoming this year’s keynote speaker.
This year’s keynote address will come from Vanessa Kerry, MD, who will speak on environmental issues and her work to raise awareness of the impact of climate change on health.
With so many things to look forward to, this meeting will be one to remember for all in attendance.
I look forward to seeing you in Boston,
Jack
Top reads from the CHEST journal portfolio
Covering the frailty scale in ILD, diagnosis of peripheral pulmonary nodules, and platelet mitochondrial function in sepsis.
Journal CHEST®
By Guler, MD, and colleagues
Life expectancy is a very important factor for patients with interstitial lung disease (ILD) and their caregivers. The discussion surrounding prognosis is often wrought with uncertainty and is inherently painful for both patients and clinicians when faced with nonmodifiable traits. This study illustrates the significance of employing a method that succinctly and systematically communicates the degree of functional impairment in patients with fibrotic lung disease. The authors have highlighted the importance of identifying and improving health factors associated with frailty to enhance the survival and quality of life of patients with chronic noncurable fibrotic lung disease. It also presents hope that interventions aimed at improving functional capacity may improve frailty and thus modify prognosis. In the future, longitudinal trends of frailty assessments following interventions aimed at improving both exercise and functional capacity, like pulmonary rehab, should be explored.
– Commentary by Priya Balakrishnan, MD, MS, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Pulmonary
By Michael V. Brown, MD, and colleagues
Brown and colleagues provide a systemic review and meta-analysis of the diagnostic yield of cone beam computed tomography (CBCT) scan combined with radial-endobronchial ultrasound (r-EBUS) for the diagnosis of peripheral pulmonary nodules. They included 14 studies (865 patients with 882 lesions) with pooled diagnostic yield from CBCT scan and r-EBUS for peripheral pulmonary nodules of 80% (95% CI, 76% to 84%) with complication rates of 2.01% for pneumothorax and 1.08% for bleeding. Amongst the studies selected, confounders (including study design, definition of diagnostic yield, use of ROSE, additional equipment, etc) existed. The important takeaway is that 3D imaging guidance with CBCT scan can corroborate “tool in lesion” and thus potentially improve the outcomes of the different bronchoscopic modalities utilized to diagnose peripheral pulmonary nodules. Future prospective investigations with less heterogeneity in study design and outcomes, as well as comparison with newer technologies such as robotic bronchoscopy, are necessary to corroborate these findings.
– Commentary by Saadia A. Faiz, MD, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Critical Care
Platelet Bioenergetics and Associations With Delirium and Coma in Patients With Sepsis
By Chukwudi A. Onyemekwu, DO, and colleagues
The study by Onyemekwu and colleagues explores the link between platelet mitochondrial function and acute brain dysfunction (delirium and coma) in patients with sepsis. The investigators measured various parameters of platelet mitochondrial respiratory bioenergetics and found that increased spare respiratory capacity was significantly associated with coma but not delirium. These findings suggest that systemic mitochondrial function could influence brain health and indicate a potential link between mitochondrial bioenergetics and coma during sepsis. The study did not find a significant association between platelet bioenergetics and delirium, suggesting that coma and delirium may have different underlying pathophysiologic mechanisms. We must interpret the results with caution, as the associations identified in this observational study do not prove causation. It is possible that the changes seen in platelet mitochondria may be a result of coma rather than a mechanism. Nonetheless, the study provides a foundation for future research to explore the mechanistic role of mitochondria in acute brain dysfunction during sepsis and the potential for developing mitochondrial-targeted therapies as a possible treatment approach for patients with sepsis-induced coma.
– Commentary by Angel O. Coz, MD, FCCP, Editor in Chief of CHEST Physician
Covering the frailty scale in ILD, diagnosis of peripheral pulmonary nodules, and platelet mitochondrial function in sepsis.
Covering the frailty scale in ILD, diagnosis of peripheral pulmonary nodules, and platelet mitochondrial function in sepsis.
Journal CHEST®
By Guler, MD, and colleagues
Life expectancy is a very important factor for patients with interstitial lung disease (ILD) and their caregivers. The discussion surrounding prognosis is often wrought with uncertainty and is inherently painful for both patients and clinicians when faced with nonmodifiable traits. This study illustrates the significance of employing a method that succinctly and systematically communicates the degree of functional impairment in patients with fibrotic lung disease. The authors have highlighted the importance of identifying and improving health factors associated with frailty to enhance the survival and quality of life of patients with chronic noncurable fibrotic lung disease. It also presents hope that interventions aimed at improving functional capacity may improve frailty and thus modify prognosis. In the future, longitudinal trends of frailty assessments following interventions aimed at improving both exercise and functional capacity, like pulmonary rehab, should be explored.
– Commentary by Priya Balakrishnan, MD, MS, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Pulmonary
By Michael V. Brown, MD, and colleagues
Brown and colleagues provide a systemic review and meta-analysis of the diagnostic yield of cone beam computed tomography (CBCT) scan combined with radial-endobronchial ultrasound (r-EBUS) for the diagnosis of peripheral pulmonary nodules. They included 14 studies (865 patients with 882 lesions) with pooled diagnostic yield from CBCT scan and r-EBUS for peripheral pulmonary nodules of 80% (95% CI, 76% to 84%) with complication rates of 2.01% for pneumothorax and 1.08% for bleeding. Amongst the studies selected, confounders (including study design, definition of diagnostic yield, use of ROSE, additional equipment, etc) existed. The important takeaway is that 3D imaging guidance with CBCT scan can corroborate “tool in lesion” and thus potentially improve the outcomes of the different bronchoscopic modalities utilized to diagnose peripheral pulmonary nodules. Future prospective investigations with less heterogeneity in study design and outcomes, as well as comparison with newer technologies such as robotic bronchoscopy, are necessary to corroborate these findings.
– Commentary by Saadia A. Faiz, MD, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Critical Care
Platelet Bioenergetics and Associations With Delirium and Coma in Patients With Sepsis
By Chukwudi A. Onyemekwu, DO, and colleagues
The study by Onyemekwu and colleagues explores the link between platelet mitochondrial function and acute brain dysfunction (delirium and coma) in patients with sepsis. The investigators measured various parameters of platelet mitochondrial respiratory bioenergetics and found that increased spare respiratory capacity was significantly associated with coma but not delirium. These findings suggest that systemic mitochondrial function could influence brain health and indicate a potential link between mitochondrial bioenergetics and coma during sepsis. The study did not find a significant association between platelet bioenergetics and delirium, suggesting that coma and delirium may have different underlying pathophysiologic mechanisms. We must interpret the results with caution, as the associations identified in this observational study do not prove causation. It is possible that the changes seen in platelet mitochondria may be a result of coma rather than a mechanism. Nonetheless, the study provides a foundation for future research to explore the mechanistic role of mitochondria in acute brain dysfunction during sepsis and the potential for developing mitochondrial-targeted therapies as a possible treatment approach for patients with sepsis-induced coma.
– Commentary by Angel O. Coz, MD, FCCP, Editor in Chief of CHEST Physician
Journal CHEST®
By Guler, MD, and colleagues
Life expectancy is a very important factor for patients with interstitial lung disease (ILD) and their caregivers. The discussion surrounding prognosis is often wrought with uncertainty and is inherently painful for both patients and clinicians when faced with nonmodifiable traits. This study illustrates the significance of employing a method that succinctly and systematically communicates the degree of functional impairment in patients with fibrotic lung disease. The authors have highlighted the importance of identifying and improving health factors associated with frailty to enhance the survival and quality of life of patients with chronic noncurable fibrotic lung disease. It also presents hope that interventions aimed at improving functional capacity may improve frailty and thus modify prognosis. In the future, longitudinal trends of frailty assessments following interventions aimed at improving both exercise and functional capacity, like pulmonary rehab, should be explored.
– Commentary by Priya Balakrishnan, MD, MS, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Pulmonary
By Michael V. Brown, MD, and colleagues
Brown and colleagues provide a systemic review and meta-analysis of the diagnostic yield of cone beam computed tomography (CBCT) scan combined with radial-endobronchial ultrasound (r-EBUS) for the diagnosis of peripheral pulmonary nodules. They included 14 studies (865 patients with 882 lesions) with pooled diagnostic yield from CBCT scan and r-EBUS for peripheral pulmonary nodules of 80% (95% CI, 76% to 84%) with complication rates of 2.01% for pneumothorax and 1.08% for bleeding. Amongst the studies selected, confounders (including study design, definition of diagnostic yield, use of ROSE, additional equipment, etc) existed. The important takeaway is that 3D imaging guidance with CBCT scan can corroborate “tool in lesion” and thus potentially improve the outcomes of the different bronchoscopic modalities utilized to diagnose peripheral pulmonary nodules. Future prospective investigations with less heterogeneity in study design and outcomes, as well as comparison with newer technologies such as robotic bronchoscopy, are necessary to corroborate these findings.
– Commentary by Saadia A. Faiz, MD, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Critical Care
Platelet Bioenergetics and Associations With Delirium and Coma in Patients With Sepsis
By Chukwudi A. Onyemekwu, DO, and colleagues
The study by Onyemekwu and colleagues explores the link between platelet mitochondrial function and acute brain dysfunction (delirium and coma) in patients with sepsis. The investigators measured various parameters of platelet mitochondrial respiratory bioenergetics and found that increased spare respiratory capacity was significantly associated with coma but not delirium. These findings suggest that systemic mitochondrial function could influence brain health and indicate a potential link between mitochondrial bioenergetics and coma during sepsis. The study did not find a significant association between platelet bioenergetics and delirium, suggesting that coma and delirium may have different underlying pathophysiologic mechanisms. We must interpret the results with caution, as the associations identified in this observational study do not prove causation. It is possible that the changes seen in platelet mitochondria may be a result of coma rather than a mechanism. Nonetheless, the study provides a foundation for future research to explore the mechanistic role of mitochondria in acute brain dysfunction during sepsis and the potential for developing mitochondrial-targeted therapies as a possible treatment approach for patients with sepsis-induced coma.
– Commentary by Angel O. Coz, MD, FCCP, Editor in Chief of CHEST Physician
The language of AI and its applications in health care
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.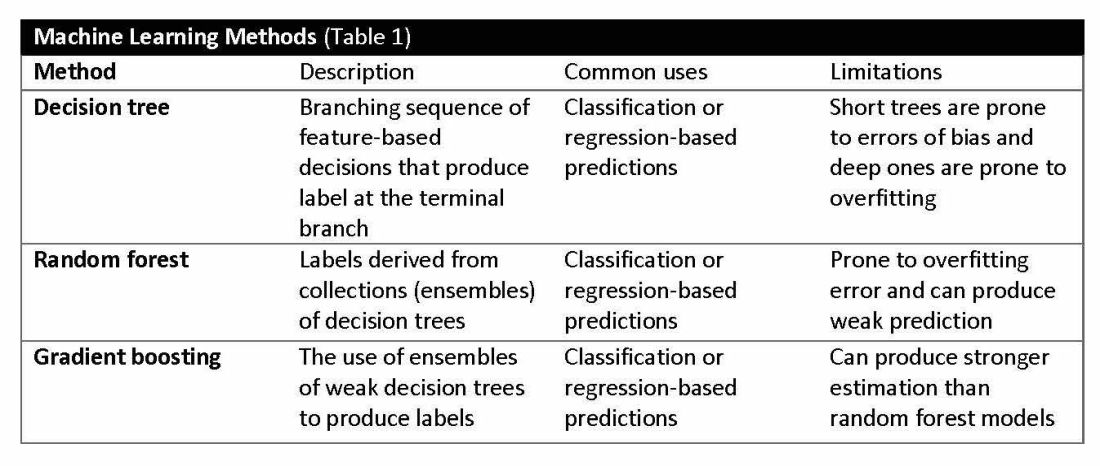
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
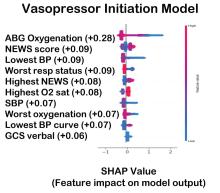
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
CHEST releases new guideline on management of central airway obstruction
CHEST recently released a new clinical guideline on central airway obstruction (CAO).
“Central airway obstruction is associated with a poor prognosis, and the management of CAO is highly variable dependent on the provider expertise and local resources. By releasing this guideline, the panel hopes to standardize the definition of CAO and provide guidance for the management of patients to optimize care and improve outcomes,” said Kamran Mahmood, MD, MPH, FCCP, lead author on the guideline. “The guideline recommendations are developed using GRADE methodology and based on thorough evidence review and expert input. But the quality of overall evidence is very low, and the panel calls for well-designed studies and randomized controlled trials for the management of CAO.”
CAO can be caused by a variety of malignant and nonmalignant disorders, and multiple specialists may be involved in the care, including pulmonologists, interventional pulmonologists, radiologists, anesthesiologists, oncologists, radiation oncologists, thoracic surgeons and otolaryngologists, etc. The panel recommends shared decision-making with the patients and a multidisciplinary approach to manage CAO.
Below, you’ll find flowcharts outlining the suggested treatments for patients with a malignant or nonmalignant CAO.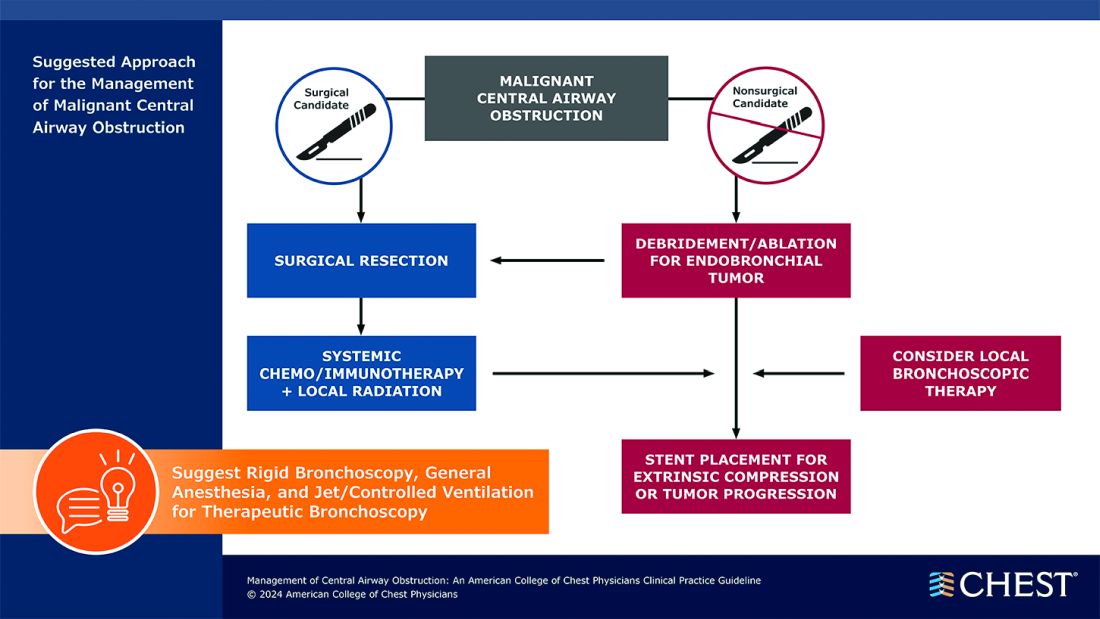
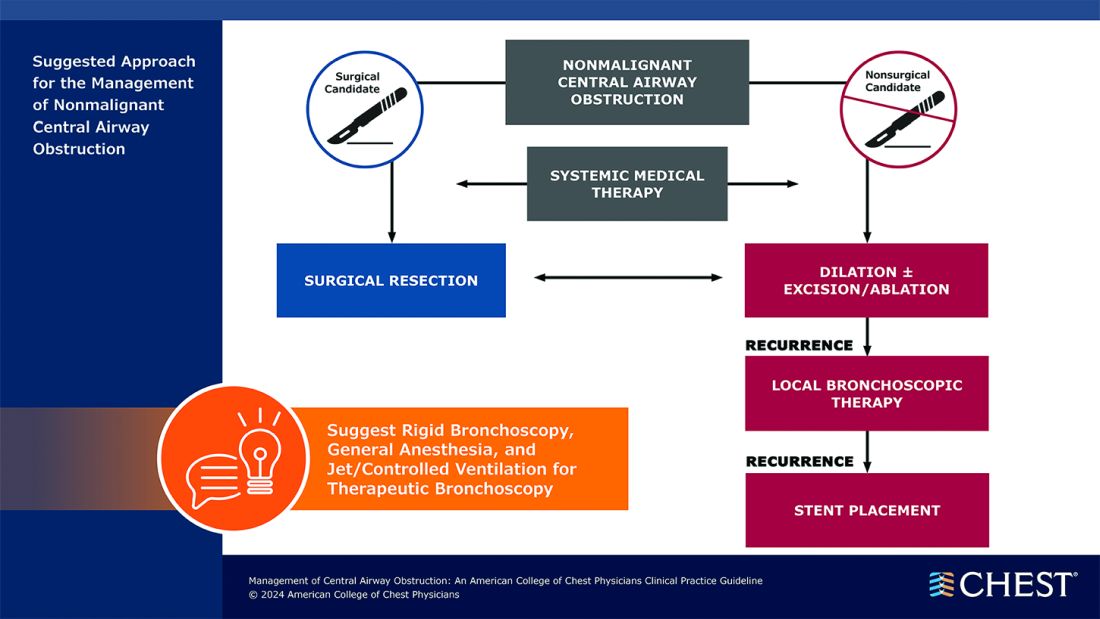
Visit www.chestnet.org/CAO-guideline to download the flowcharts and access the complete guideline.
CHEST recently released a new clinical guideline on central airway obstruction (CAO).
“Central airway obstruction is associated with a poor prognosis, and the management of CAO is highly variable dependent on the provider expertise and local resources. By releasing this guideline, the panel hopes to standardize the definition of CAO and provide guidance for the management of patients to optimize care and improve outcomes,” said Kamran Mahmood, MD, MPH, FCCP, lead author on the guideline. “The guideline recommendations are developed using GRADE methodology and based on thorough evidence review and expert input. But the quality of overall evidence is very low, and the panel calls for well-designed studies and randomized controlled trials for the management of CAO.”
CAO can be caused by a variety of malignant and nonmalignant disorders, and multiple specialists may be involved in the care, including pulmonologists, interventional pulmonologists, radiologists, anesthesiologists, oncologists, radiation oncologists, thoracic surgeons and otolaryngologists, etc. The panel recommends shared decision-making with the patients and a multidisciplinary approach to manage CAO.
Below, you’ll find flowcharts outlining the suggested treatments for patients with a malignant or nonmalignant CAO.

Visit www.chestnet.org/CAO-guideline to download the flowcharts and access the complete guideline.
CHEST recently released a new clinical guideline on central airway obstruction (CAO).
“Central airway obstruction is associated with a poor prognosis, and the management of CAO is highly variable dependent on the provider expertise and local resources. By releasing this guideline, the panel hopes to standardize the definition of CAO and provide guidance for the management of patients to optimize care and improve outcomes,” said Kamran Mahmood, MD, MPH, FCCP, lead author on the guideline. “The guideline recommendations are developed using GRADE methodology and based on thorough evidence review and expert input. But the quality of overall evidence is very low, and the panel calls for well-designed studies and randomized controlled trials for the management of CAO.”
CAO can be caused by a variety of malignant and nonmalignant disorders, and multiple specialists may be involved in the care, including pulmonologists, interventional pulmonologists, radiologists, anesthesiologists, oncologists, radiation oncologists, thoracic surgeons and otolaryngologists, etc. The panel recommends shared decision-making with the patients and a multidisciplinary approach to manage CAO.
Below, you’ll find flowcharts outlining the suggested treatments for patients with a malignant or nonmalignant CAO.

Visit www.chestnet.org/CAO-guideline to download the flowcharts and access the complete guideline.
Obesity Is Not a Moral Failing, GI Physician Says
It takes a long time for some patients with obesity to acknowledge that they need help, said Dr. Laster, a bariatric endoscopist who specializes in gastroenterology, nutrition, and obesity medicine at Gut Theory Total Digestive Care, and Georgetown University Hospital in Washington. “If somebody has high blood pressure or has a cut or has chest pain, you don’t wait for things. You would go seek help immediately. I wish more patients reached out sooner and didn’t struggle.”
Another big challenge is making sure patients have insurance coverage for things like medications, surgery, and bariatric endoscopy, she added.
Her response: education and advocacy. “I’m giving as many talks as I can to fellows, creating courses for residents and fellows and medical students” to change the way physicians talk about obesity and excess weight, she said. Patients need to understand that physicians care about them, that “we’re not judging and we’re changing that perspective.”
Dr. Laster is also working with members of Congress to get bills passed for coverage of obesity medication and procedures. In an interview with GI & Hepatology News, she spoke more about the intersection between nutrition, medicine and bariatric procedures and the importance of offering patients multiple solutions.
Q: Why did you choose GI?
It allowed me a little bit of everything. You have clinic, where you can really interact with patients and get to the root of their problem. You have preventative care with routine colonoscopies and upper endoscopies to prevent for cancer. But then you also have fun stuff — which my mom told me to stop saying out loud — ‘bleeders’ and acute things that you get to fix immediately. So, you get the adrenaline rush too. I like it because you get the best of all worlds and it’s really hard to get bored.
Q: How did you become interested in nutrition and bariatric endoscopy?
My parents had a garden and never let us eat processed foods. In residency, I kept seeing the same medical problems over and over again. Everybody had high blood pressure, high cholesterol, and diabetes. Then in GI clinic, everybody had abdominal pain, bloating, constipation, heartburn, and a million GI appointments for these same things. Everyone’s upper endoscopy or colonoscopy was negative. Something else had to be going on.
And that’s sort of where it came from; figuring out the common denominator. It had to be what people were eating. There’s also the prevalence of patients with obesity going up every single year. Correlating all these other medical problems with people’s diets led me down the rabbit hole of: What else can we be doing?
Most people don’t want to undergo surgery. Only 2% of people eligible for surgery actually do it, even though it works. The reasons are because it’s invasive or there’s shame behind it. Bariatric endoscopy is another option that’s out here, that’s less invasive. I’m an endoscopist and gastroenterologist. I should be able to offer all those things and I should know more about nutrition. We don’t talk about it enough.
Q: Do you think more GI doctors should become better educated about nutrition?
100%. Every patient I see has seen a GI doctor before and says, ‘No one has ever told me that if I have carbonated beverages and cheese every day, I’m going to be bloated and constipated.’ And that shouldn’t be the case.
Q: Why do you think that more GI doctors don’t get the education on nutrition during their medical training?
I think it’s our healthcare system. It’s very much focused on secondary treatment rather than preventative care. There’s no emphasis on preventing things from happening.
We’re really good at reactionary medicine. People who have an ulcer, big polyps and colon cancer, esophageal cancer — we do those things really well. But I think because there’s no ICD-10 codes for preventive care via nutrition education, and no good reimbursement, then there’s no incentive for hospital systems to pay for these things. It’s a system based on RVUs (relative value units) and numbers. That’s been our trajectory. We’ve been so focused on reactionary medicine rather than saying, ‘Okay, let’s stop this from happening.’
We just didn’t talk about nutrition in medical school, in residency, or in fellowship. It was looked at as a soft science. When I was in school, people would also say, ‘No one’s going to change. So it’s a waste of your time essentially to talk to people about making dietary changes.’ I feel like if you give people the opportunity, you have to give them the chance. You can’t just write everybody off. Some people won’t change, but that’s okay. They should at least have the opportunity to do so.
Q: How do you determine whether a patient is a good candidate for bariatric surgery?
It’s based on the guidelines: If they meet the BMI requirements, if they have obesity-associated comorbidities, their risk for surgery is low. But it’s also whether they want to do it or not. A patient has to be in the mindset and be ready for it. They need to want to have surgery or bariatric endoscopy, or to use medications, or start to make a change. Some people aren’t there yet — that preemptive stage of making a change. They want the solution, but they’re not ready to do that legwork yet.
And all of it is work. I tell patients, ‘Whether it’s medication or bariatric endoscopy or bariatric surgery, you still have work to do. None of it is going to just magically happen where you could just continue to do the same thing you’re doing now and you’re going to lose weight and keep it off.’
Q: What advances in obesity prevention are you excited about?
I’m excited that bariatric endoscopy came about in the first place, because in every other field there are less invasive approaches that have become available. I’m also excited about the emergence of weight loss medication, like GLP-1s. I think they are a tool that we need.
Q: Do you think the weight loss medications may negate the need for surgery?
I don’t think they necessarily reduce the need for surgery. There’s still a lot we don’t know about why they work in some patients and why they don’t work in others.
Some of our colleagues have come up with phenotypes and blood tests so we can better understand which things will work in different patients. Surgery doesn’t work for some patients. People may need a combination of both after they reach a plateau. I’m excited that people see this as something that we should be researching and putting more effort into — that obesity isn’t a disease of moral failure, that people with excess weight just need to ‘move more’ or ‘eat less’ and it’s their fault. I’m glad people are starting to understand that.
Q: What teacher or mentor had the greatest impact on you?
Probably two. One of them is Andrea E Reid, MD, MPH, a dean of medicine at Harvard. She gives you such motivation to achieve things, no matter how big your idea is or how crazy it may seem. If you have something that you think is important, you go after it. Another person is Christopher C. Thompson, MD, at Brigham and Women’s Hospital, the father of bariatric endoscopy in a sense. He embodies what Dr. Reid talks about: crazy big ideas. And he goes after them and he succeeds. Having him push me and giving me that type of encouragement was invaluable.
Q: Describe how you would spend a free Saturday afternoon.
Every Saturday is yoga or some type of movement. Spending some time outside doing something, whether it’s messing around with plants that I’m not very good at, or going for a walk.
Lightning Round
Texting or talking?
Text
Favorite city in U.S. besides the one you live in?
New York
Favorite breakfast?
Avocado toast
Place you most want to travel to?
Istanbul
Favorite season?
Fall
Favorite ice cream flavor?
Raspberry sorbet
How many cups of coffee do you drink per day?
One
Best place you ever went on vacation?
Greece
If you weren’t a gastroenterologist, what would you be?
Own a clothing store
Favorite type of music?
Old school R&B
Favorite movie genre?
Romantic comedy or drama
Cat person or dog person?
Dog
Favorite sport?
Football
Favorite holiday?
Christmas
Optimist or pessimist?
Optimist
It takes a long time for some patients with obesity to acknowledge that they need help, said Dr. Laster, a bariatric endoscopist who specializes in gastroenterology, nutrition, and obesity medicine at Gut Theory Total Digestive Care, and Georgetown University Hospital in Washington. “If somebody has high blood pressure or has a cut or has chest pain, you don’t wait for things. You would go seek help immediately. I wish more patients reached out sooner and didn’t struggle.”
Another big challenge is making sure patients have insurance coverage for things like medications, surgery, and bariatric endoscopy, she added.
Her response: education and advocacy. “I’m giving as many talks as I can to fellows, creating courses for residents and fellows and medical students” to change the way physicians talk about obesity and excess weight, she said. Patients need to understand that physicians care about them, that “we’re not judging and we’re changing that perspective.”
Dr. Laster is also working with members of Congress to get bills passed for coverage of obesity medication and procedures. In an interview with GI & Hepatology News, she spoke more about the intersection between nutrition, medicine and bariatric procedures and the importance of offering patients multiple solutions.
Q: Why did you choose GI?
It allowed me a little bit of everything. You have clinic, where you can really interact with patients and get to the root of their problem. You have preventative care with routine colonoscopies and upper endoscopies to prevent for cancer. But then you also have fun stuff — which my mom told me to stop saying out loud — ‘bleeders’ and acute things that you get to fix immediately. So, you get the adrenaline rush too. I like it because you get the best of all worlds and it’s really hard to get bored.
Q: How did you become interested in nutrition and bariatric endoscopy?
My parents had a garden and never let us eat processed foods. In residency, I kept seeing the same medical problems over and over again. Everybody had high blood pressure, high cholesterol, and diabetes. Then in GI clinic, everybody had abdominal pain, bloating, constipation, heartburn, and a million GI appointments for these same things. Everyone’s upper endoscopy or colonoscopy was negative. Something else had to be going on.
And that’s sort of where it came from; figuring out the common denominator. It had to be what people were eating. There’s also the prevalence of patients with obesity going up every single year. Correlating all these other medical problems with people’s diets led me down the rabbit hole of: What else can we be doing?
Most people don’t want to undergo surgery. Only 2% of people eligible for surgery actually do it, even though it works. The reasons are because it’s invasive or there’s shame behind it. Bariatric endoscopy is another option that’s out here, that’s less invasive. I’m an endoscopist and gastroenterologist. I should be able to offer all those things and I should know more about nutrition. We don’t talk about it enough.
Q: Do you think more GI doctors should become better educated about nutrition?
100%. Every patient I see has seen a GI doctor before and says, ‘No one has ever told me that if I have carbonated beverages and cheese every day, I’m going to be bloated and constipated.’ And that shouldn’t be the case.
Q: Why do you think that more GI doctors don’t get the education on nutrition during their medical training?
I think it’s our healthcare system. It’s very much focused on secondary treatment rather than preventative care. There’s no emphasis on preventing things from happening.
We’re really good at reactionary medicine. People who have an ulcer, big polyps and colon cancer, esophageal cancer — we do those things really well. But I think because there’s no ICD-10 codes for preventive care via nutrition education, and no good reimbursement, then there’s no incentive for hospital systems to pay for these things. It’s a system based on RVUs (relative value units) and numbers. That’s been our trajectory. We’ve been so focused on reactionary medicine rather than saying, ‘Okay, let’s stop this from happening.’
We just didn’t talk about nutrition in medical school, in residency, or in fellowship. It was looked at as a soft science. When I was in school, people would also say, ‘No one’s going to change. So it’s a waste of your time essentially to talk to people about making dietary changes.’ I feel like if you give people the opportunity, you have to give them the chance. You can’t just write everybody off. Some people won’t change, but that’s okay. They should at least have the opportunity to do so.
Q: How do you determine whether a patient is a good candidate for bariatric surgery?
It’s based on the guidelines: If they meet the BMI requirements, if they have obesity-associated comorbidities, their risk for surgery is low. But it’s also whether they want to do it or not. A patient has to be in the mindset and be ready for it. They need to want to have surgery or bariatric endoscopy, or to use medications, or start to make a change. Some people aren’t there yet — that preemptive stage of making a change. They want the solution, but they’re not ready to do that legwork yet.
And all of it is work. I tell patients, ‘Whether it’s medication or bariatric endoscopy or bariatric surgery, you still have work to do. None of it is going to just magically happen where you could just continue to do the same thing you’re doing now and you’re going to lose weight and keep it off.’
Q: What advances in obesity prevention are you excited about?
I’m excited that bariatric endoscopy came about in the first place, because in every other field there are less invasive approaches that have become available. I’m also excited about the emergence of weight loss medication, like GLP-1s. I think they are a tool that we need.
Q: Do you think the weight loss medications may negate the need for surgery?
I don’t think they necessarily reduce the need for surgery. There’s still a lot we don’t know about why they work in some patients and why they don’t work in others.
Some of our colleagues have come up with phenotypes and blood tests so we can better understand which things will work in different patients. Surgery doesn’t work for some patients. People may need a combination of both after they reach a plateau. I’m excited that people see this as something that we should be researching and putting more effort into — that obesity isn’t a disease of moral failure, that people with excess weight just need to ‘move more’ or ‘eat less’ and it’s their fault. I’m glad people are starting to understand that.
Q: What teacher or mentor had the greatest impact on you?
Probably two. One of them is Andrea E Reid, MD, MPH, a dean of medicine at Harvard. She gives you such motivation to achieve things, no matter how big your idea is or how crazy it may seem. If you have something that you think is important, you go after it. Another person is Christopher C. Thompson, MD, at Brigham and Women’s Hospital, the father of bariatric endoscopy in a sense. He embodies what Dr. Reid talks about: crazy big ideas. And he goes after them and he succeeds. Having him push me and giving me that type of encouragement was invaluable.
Q: Describe how you would spend a free Saturday afternoon.
Every Saturday is yoga or some type of movement. Spending some time outside doing something, whether it’s messing around with plants that I’m not very good at, or going for a walk.
Lightning Round
Texting or talking?
Text
Favorite city in U.S. besides the one you live in?
New York
Favorite breakfast?
Avocado toast
Place you most want to travel to?
Istanbul
Favorite season?
Fall
Favorite ice cream flavor?
Raspberry sorbet
How many cups of coffee do you drink per day?
One
Best place you ever went on vacation?
Greece
If you weren’t a gastroenterologist, what would you be?
Own a clothing store
Favorite type of music?
Old school R&B
Favorite movie genre?
Romantic comedy or drama
Cat person or dog person?
Dog
Favorite sport?
Football
Favorite holiday?
Christmas
Optimist or pessimist?
Optimist
It takes a long time for some patients with obesity to acknowledge that they need help, said Dr. Laster, a bariatric endoscopist who specializes in gastroenterology, nutrition, and obesity medicine at Gut Theory Total Digestive Care, and Georgetown University Hospital in Washington. “If somebody has high blood pressure or has a cut or has chest pain, you don’t wait for things. You would go seek help immediately. I wish more patients reached out sooner and didn’t struggle.”
Another big challenge is making sure patients have insurance coverage for things like medications, surgery, and bariatric endoscopy, she added.
Her response: education and advocacy. “I’m giving as many talks as I can to fellows, creating courses for residents and fellows and medical students” to change the way physicians talk about obesity and excess weight, she said. Patients need to understand that physicians care about them, that “we’re not judging and we’re changing that perspective.”
Dr. Laster is also working with members of Congress to get bills passed for coverage of obesity medication and procedures. In an interview with GI & Hepatology News, she spoke more about the intersection between nutrition, medicine and bariatric procedures and the importance of offering patients multiple solutions.
Q: Why did you choose GI?
It allowed me a little bit of everything. You have clinic, where you can really interact with patients and get to the root of their problem. You have preventative care with routine colonoscopies and upper endoscopies to prevent for cancer. But then you also have fun stuff — which my mom told me to stop saying out loud — ‘bleeders’ and acute things that you get to fix immediately. So, you get the adrenaline rush too. I like it because you get the best of all worlds and it’s really hard to get bored.
Q: How did you become interested in nutrition and bariatric endoscopy?
My parents had a garden and never let us eat processed foods. In residency, I kept seeing the same medical problems over and over again. Everybody had high blood pressure, high cholesterol, and diabetes. Then in GI clinic, everybody had abdominal pain, bloating, constipation, heartburn, and a million GI appointments for these same things. Everyone’s upper endoscopy or colonoscopy was negative. Something else had to be going on.
And that’s sort of where it came from; figuring out the common denominator. It had to be what people were eating. There’s also the prevalence of patients with obesity going up every single year. Correlating all these other medical problems with people’s diets led me down the rabbit hole of: What else can we be doing?
Most people don’t want to undergo surgery. Only 2% of people eligible for surgery actually do it, even though it works. The reasons are because it’s invasive or there’s shame behind it. Bariatric endoscopy is another option that’s out here, that’s less invasive. I’m an endoscopist and gastroenterologist. I should be able to offer all those things and I should know more about nutrition. We don’t talk about it enough.
Q: Do you think more GI doctors should become better educated about nutrition?
100%. Every patient I see has seen a GI doctor before and says, ‘No one has ever told me that if I have carbonated beverages and cheese every day, I’m going to be bloated and constipated.’ And that shouldn’t be the case.
Q: Why do you think that more GI doctors don’t get the education on nutrition during their medical training?
I think it’s our healthcare system. It’s very much focused on secondary treatment rather than preventative care. There’s no emphasis on preventing things from happening.
We’re really good at reactionary medicine. People who have an ulcer, big polyps and colon cancer, esophageal cancer — we do those things really well. But I think because there’s no ICD-10 codes for preventive care via nutrition education, and no good reimbursement, then there’s no incentive for hospital systems to pay for these things. It’s a system based on RVUs (relative value units) and numbers. That’s been our trajectory. We’ve been so focused on reactionary medicine rather than saying, ‘Okay, let’s stop this from happening.’
We just didn’t talk about nutrition in medical school, in residency, or in fellowship. It was looked at as a soft science. When I was in school, people would also say, ‘No one’s going to change. So it’s a waste of your time essentially to talk to people about making dietary changes.’ I feel like if you give people the opportunity, you have to give them the chance. You can’t just write everybody off. Some people won’t change, but that’s okay. They should at least have the opportunity to do so.
Q: How do you determine whether a patient is a good candidate for bariatric surgery?
It’s based on the guidelines: If they meet the BMI requirements, if they have obesity-associated comorbidities, their risk for surgery is low. But it’s also whether they want to do it or not. A patient has to be in the mindset and be ready for it. They need to want to have surgery or bariatric endoscopy, or to use medications, or start to make a change. Some people aren’t there yet — that preemptive stage of making a change. They want the solution, but they’re not ready to do that legwork yet.
And all of it is work. I tell patients, ‘Whether it’s medication or bariatric endoscopy or bariatric surgery, you still have work to do. None of it is going to just magically happen where you could just continue to do the same thing you’re doing now and you’re going to lose weight and keep it off.’
Q: What advances in obesity prevention are you excited about?
I’m excited that bariatric endoscopy came about in the first place, because in every other field there are less invasive approaches that have become available. I’m also excited about the emergence of weight loss medication, like GLP-1s. I think they are a tool that we need.
Q: Do you think the weight loss medications may negate the need for surgery?
I don’t think they necessarily reduce the need for surgery. There’s still a lot we don’t know about why they work in some patients and why they don’t work in others.
Some of our colleagues have come up with phenotypes and blood tests so we can better understand which things will work in different patients. Surgery doesn’t work for some patients. People may need a combination of both after they reach a plateau. I’m excited that people see this as something that we should be researching and putting more effort into — that obesity isn’t a disease of moral failure, that people with excess weight just need to ‘move more’ or ‘eat less’ and it’s their fault. I’m glad people are starting to understand that.
Q: What teacher or mentor had the greatest impact on you?
Probably two. One of them is Andrea E Reid, MD, MPH, a dean of medicine at Harvard. She gives you such motivation to achieve things, no matter how big your idea is or how crazy it may seem. If you have something that you think is important, you go after it. Another person is Christopher C. Thompson, MD, at Brigham and Women’s Hospital, the father of bariatric endoscopy in a sense. He embodies what Dr. Reid talks about: crazy big ideas. And he goes after them and he succeeds. Having him push me and giving me that type of encouragement was invaluable.
Q: Describe how you would spend a free Saturday afternoon.
Every Saturday is yoga or some type of movement. Spending some time outside doing something, whether it’s messing around with plants that I’m not very good at, or going for a walk.
Lightning Round
Texting or talking?
Text
Favorite city in U.S. besides the one you live in?
New York
Favorite breakfast?
Avocado toast
Place you most want to travel to?
Istanbul
Favorite season?
Fall
Favorite ice cream flavor?
Raspberry sorbet
How many cups of coffee do you drink per day?
One
Best place you ever went on vacation?
Greece
If you weren’t a gastroenterologist, what would you be?
Own a clothing store
Favorite type of music?
Old school R&B
Favorite movie genre?
Romantic comedy or drama
Cat person or dog person?
Dog
Favorite sport?
Football
Favorite holiday?
Christmas
Optimist or pessimist?
Optimist
Support Researchers with a Donation to the AGA Research Foundation
By joining others in donating to the AGA Research Foundation, you will ensure that researchers have opportunities to continue their lifesaving work.
The AGA Research Foundation remains committed to providing young researchers with unprecedented research opportunities. Each year, we receive a caliber of nominations for AGA research awards. You can help gifted investigators as they work to advance the understanding of digestive diseases through their novel research objectives.
As an AGA member, you can help fund discoveries that will continue to improve GI practice and better patient care.
AGA grants have led to discoveries, including new approaches to down-regulate intestinal inflammation, a test for genetic predisposition to colon cancer and autoimmune liver disease treatments. The importance of these awards is evidenced by the fact that virtually every major advance leading to the understanding, prevention, treatment, and cure of digestive diseases has been made in the research laboratory of a talented young investigator.
Donate to the AGA Research Foundation to ensure that researchers have opportunities to continue their lifesaving work.
Three Easy Ways To Give
Online: Donate at www.foundation.gastro.org.
Through the mail:
AGA Research Foundation
4930 Del Ray Avenue
Bethesda, MD 20814
Over the phone: 301-222-4002
All gifts are tax-deductible to the fullest extent of U.S. law.
By joining others in donating to the AGA Research Foundation, you will ensure that researchers have opportunities to continue their lifesaving work.
The AGA Research Foundation remains committed to providing young researchers with unprecedented research opportunities. Each year, we receive a caliber of nominations for AGA research awards. You can help gifted investigators as they work to advance the understanding of digestive diseases through their novel research objectives.
As an AGA member, you can help fund discoveries that will continue to improve GI practice and better patient care.
AGA grants have led to discoveries, including new approaches to down-regulate intestinal inflammation, a test for genetic predisposition to colon cancer and autoimmune liver disease treatments. The importance of these awards is evidenced by the fact that virtually every major advance leading to the understanding, prevention, treatment, and cure of digestive diseases has been made in the research laboratory of a talented young investigator.
Donate to the AGA Research Foundation to ensure that researchers have opportunities to continue their lifesaving work.
Three Easy Ways To Give
Online: Donate at www.foundation.gastro.org.
Through the mail:
AGA Research Foundation
4930 Del Ray Avenue
Bethesda, MD 20814
Over the phone: 301-222-4002
All gifts are tax-deductible to the fullest extent of U.S. law.
By joining others in donating to the AGA Research Foundation, you will ensure that researchers have opportunities to continue their lifesaving work.
The AGA Research Foundation remains committed to providing young researchers with unprecedented research opportunities. Each year, we receive a caliber of nominations for AGA research awards. You can help gifted investigators as they work to advance the understanding of digestive diseases through their novel research objectives.
As an AGA member, you can help fund discoveries that will continue to improve GI practice and better patient care.
AGA grants have led to discoveries, including new approaches to down-regulate intestinal inflammation, a test for genetic predisposition to colon cancer and autoimmune liver disease treatments. The importance of these awards is evidenced by the fact that virtually every major advance leading to the understanding, prevention, treatment, and cure of digestive diseases has been made in the research laboratory of a talented young investigator.
Donate to the AGA Research Foundation to ensure that researchers have opportunities to continue their lifesaving work.
Three Easy Ways To Give
Online: Donate at www.foundation.gastro.org.
Through the mail:
AGA Research Foundation
4930 Del Ray Avenue
Bethesda, MD 20814
Over the phone: 301-222-4002
All gifts are tax-deductible to the fullest extent of U.S. law.
FDA Approves First Blood Test for Colorectal Cancer
In late July, the US Food and Drug Administration (FDA) approved the first use of a liquid biopsy (blood test) for colorectal cancer (CRC) screening. The test, called Shield, launched commercially the first week of August and is the first blood test to be approved by the FDA as a primary screening option for CRC that meets requirements for Medicare reimbursement.
While the convenience of a blood test could potentially encourage more people to get screened, expert consensus is that blood tests can’t prevent CRC and should not be considered a replacement for a colonoscopy. Modeling studies and expert consensus published earlier this year in Gastroenterology and in Clinical Gastroenterology and Hepatology shed light on the perils of liquid biopsy.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor as cost-effective, and would worsen outcomes,” said David Lieberman, MD, AGAF, chair, AGA CRC Workshop chair and lead author of an expert commentary on liquid biopsy for CRC screening.
Five Key Takeaways
- A blood test for CRC that meets minimal CMS criteria for sensitivity and performed every 3 years would likely result in better outcomes than no screening.
- A blood test for CRC offers a simple process that could encourage more people to participate in screening. Patients who may have declined colonoscopy should understand the need for a colonoscopy if findings are abnormal.
- Because blood tests for CRC are predicted to be less effective and more costly than currently established screening programs, they cannot be recommended to replace established effective screening methods.
- Although blood tests would improve outcomes in currently unscreened people, substituting blood tests for a currently effective test would worsen patient outcomes and increase cost.
- Potential benchmarks that industry might use to assess an effective blood test for CRC going forward would be sensitivity for stage I-III CRC of > 90%, with sensitivity for advanced adenomas of > 40%-50%.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” said John M. Carethers, MD, AGAF, AGA past president and vice chancellor for health sciences at the University of California San Diego.
In late July, the US Food and Drug Administration (FDA) approved the first use of a liquid biopsy (blood test) for colorectal cancer (CRC) screening. The test, called Shield, launched commercially the first week of August and is the first blood test to be approved by the FDA as a primary screening option for CRC that meets requirements for Medicare reimbursement.
While the convenience of a blood test could potentially encourage more people to get screened, expert consensus is that blood tests can’t prevent CRC and should not be considered a replacement for a colonoscopy. Modeling studies and expert consensus published earlier this year in Gastroenterology and in Clinical Gastroenterology and Hepatology shed light on the perils of liquid biopsy.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor as cost-effective, and would worsen outcomes,” said David Lieberman, MD, AGAF, chair, AGA CRC Workshop chair and lead author of an expert commentary on liquid biopsy for CRC screening.
Five Key Takeaways
- A blood test for CRC that meets minimal CMS criteria for sensitivity and performed every 3 years would likely result in better outcomes than no screening.
- A blood test for CRC offers a simple process that could encourage more people to participate in screening. Patients who may have declined colonoscopy should understand the need for a colonoscopy if findings are abnormal.
- Because blood tests for CRC are predicted to be less effective and more costly than currently established screening programs, they cannot be recommended to replace established effective screening methods.
- Although blood tests would improve outcomes in currently unscreened people, substituting blood tests for a currently effective test would worsen patient outcomes and increase cost.
- Potential benchmarks that industry might use to assess an effective blood test for CRC going forward would be sensitivity for stage I-III CRC of > 90%, with sensitivity for advanced adenomas of > 40%-50%.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” said John M. Carethers, MD, AGAF, AGA past president and vice chancellor for health sciences at the University of California San Diego.
In late July, the US Food and Drug Administration (FDA) approved the first use of a liquid biopsy (blood test) for colorectal cancer (CRC) screening. The test, called Shield, launched commercially the first week of August and is the first blood test to be approved by the FDA as a primary screening option for CRC that meets requirements for Medicare reimbursement.
While the convenience of a blood test could potentially encourage more people to get screened, expert consensus is that blood tests can’t prevent CRC and should not be considered a replacement for a colonoscopy. Modeling studies and expert consensus published earlier this year in Gastroenterology and in Clinical Gastroenterology and Hepatology shed light on the perils of liquid biopsy.
“Based on their current characteristics, blood tests should not be recommended to replace established colorectal cancer screening tests, since blood tests are neither as effective nor as cost-effective, and would worsen outcomes,” said David Lieberman, MD, AGAF, chair, AGA CRC Workshop chair and lead author of an expert commentary on liquid biopsy for CRC screening.
Five Key Takeaways
- A blood test for CRC that meets minimal CMS criteria for sensitivity and performed every 3 years would likely result in better outcomes than no screening.
- A blood test for CRC offers a simple process that could encourage more people to participate in screening. Patients who may have declined colonoscopy should understand the need for a colonoscopy if findings are abnormal.
- Because blood tests for CRC are predicted to be less effective and more costly than currently established screening programs, they cannot be recommended to replace established effective screening methods.
- Although blood tests would improve outcomes in currently unscreened people, substituting blood tests for a currently effective test would worsen patient outcomes and increase cost.
- Potential benchmarks that industry might use to assess an effective blood test for CRC going forward would be sensitivity for stage I-III CRC of > 90%, with sensitivity for advanced adenomas of > 40%-50%.
“Unless we have the expectation of high sensitivity and specificity, blood-based colorectal cancer tests could lead to false positive and false negative results, which are both bad for patient outcomes,” said John M. Carethers, MD, AGAF, AGA past president and vice chancellor for health sciences at the University of California San Diego.