User login
The color purple
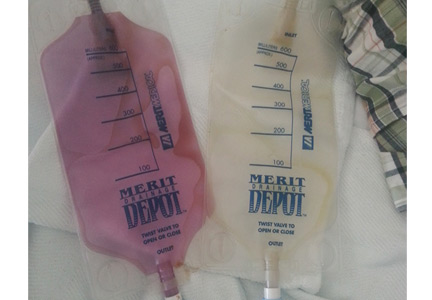
A 58-year-old man with a history of cystoprostatectomy for prostate cancer, end-stage renal disease on hemodialysis, and distal ureteral obstruction requiring bilateral nephrostomy tubes noticed that one of the nephrostomy bags looked “purple” (Figure 1). A specimen collected from one bag was reddish purple (Figure 2). The urine in the other bag was normal. The condition was diagnosed as purple urine bag syndrome.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome, a relatively rare condition that appears after 2 to 3 months of indwelling urinary catheterization, is usually asymptomatic, the only signs being the purplish urine and staining of the urinary bags and catheters. However, it should be considered a sign of underlying urinary tract infection, which can disseminate causing local complications (Fournier gangrene), systemic complications (septicemia), and death.1–3
The syndrome, first described in 1978 in children with spina bifida and urinary diversion,4 is more prevalent in women than in men, possibly because of the shorter urethra and closer proximity to the anus, which predispose women to bacterial colonization of the urinary tract. Predisposing conditions include dementia,5 female sex, increased dietary tryptophan, bacteriuria, urinary tract infection, constipation, older age, immobility, and alkaline urine.6–8
The cause of the discoloration
The purple color is from indigo and indirubin compounds in the urine, the result of the breakdown of dietary tryptophan. The color varies depending on the proportions of the two pigments.
Dietary tryptophan is broken down into indole by colonic bacteria. After reaching the portal circulation, it is excreted into the urine as indoxyl sulfate, which is broken down to indoxyl by sulfatase-producing bacteria (eg, Klebsiella pneumonia, Proteus mirabilis, Pseudomonas aeruginosa, Escherichia coli, Providencia species, Morganella morganii). Indoxyl is then oxidized to indigo and indirubin.
These compounds do not discolor the urine directly, but rather precipitate after interacting with the lining of the urinary catheter and bags, thereby imparting a purple color.1,9–13
Management
Effective initial measures are improved urinary hygiene (eg, frequent, careful changing of the urinary catheter) and management of constipation, as constipation leads to increased colonization of the intestine by bacteria that metabolize dietary tryptophan into indoxyl. Antibiotics should be given for symptomatic urinary tract infection (fever, increased urinary frequency, dysuria, abdominal pain) but not for color change alone. Coverage should be for gram-negative bacilli, although methicillin-resistant Staphylococcus aureus, which is gram-positive, has also been reported to cause purple urine bag syndrome.
In most cases, purple urine bag syndrome is benign and requires no therapy other than that mentioned above.3,13–15 However, in rare cases, immunocompromised patients (eg, people with diabetes) can develop local complications and sepsis from dissemination of bacterial infection, requiring aggressive therapy.14 Therefore, purple urine bag syndrome should be recognized as an indicator of an underlying urinary tract infection and should be treated if symptomatic. Nevertheless, the long-term prognosis is generally good.
OUR PATIENT’S MANAGEMENT
Our patient was confirmed to have urinary colonization with P aeruginosa and E coli, and alkaline urine. He underwent replacement of the nephrostomy tubes and urinary bag during his hospital stay (he was already in the hospital for another indication), but he continued to produce purple-colored urine from his right side and normal-colored urine from his left side. The unilateral involvement was likely from selective colonization of the right-sided nephrostomy tube with gram-negative bacteria.
- Kang KH, Jeong KH, Baik SK, et al. Purple urine bag syndrome: case report and literature review. Clin Nephrol 2011; 75:557–559.
- Ribeiro JP, Marcelino P, Marum S, Fernandes AP, Grilo A. Case report: purple urine bag syndrome. Crit Care 2004; 8:R137.
- Robinson J. Purple urinary bag syndrome: a harmless but alarming problem. Br J Community Nurs 2003; 8:263–266.
- Barlow GB, Dickson JAS. Purple urine bags. Lancet 1978; 1:220–221.
- Ga H, Kojima T. Purple urine bag syndrome. JAMA 2012; 307:1912–1913.
- Ishida T, Ogura S, Kawakami Y. Five cases of purple urine bag syndrome in a geriatric ward. Nihon Ronen Igakkai Zasshi 1999; 36:826–829. Japanese.
- Gautam G, Kothari A, Kumar R, Dogra PN. Purple urine bag syndrome: a rare clinical entity in patients with long term indwelling catheters. Int Urol Nephrol 2007; 39:155–156.
- Shiao CC, Weng CY, Chuang JC, Huang MS, Chen ZY. Purple urine bag syndrome: a community-based study and literature review. Nephrology (Carlton) 2008; 13:554–559.
- Chong VH. Purple urine bag syndrome: it is the urine bag and not the urine that is discolored purple. South Med J 2012; 105:446.
- Chung SD, Liao CH, Sun HD. Purple urine bag syndrome with acidic urine. Int J Infect Dis 2008; 12:526–527.
- Wu HH, Yang WC, Lin CC. Purple urine bag syndrome. Am J Med Sci 2009; 337:368.
- Achtergael W, Michielsen D, Gorus FK, Gerlo E. Indoxyl sulphate and the purple urine bag syndrome: a case report. Acta Clin Belg 2006; 61:38–41.
- Hadano Y, Shimizu T, Takada S, Inoue T, Sorano S. An update on purple urine bag syndrome. Int J Gen Med 2012; 5:707–710.
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Ferrara F, D’Angelo G, Costantino G. Monolateral purple urine bag syndrome in bilateral nephrostomy. Postgrad Med J 2010; 86:627.
A 58-year-old man with a history of cystoprostatectomy for prostate cancer, end-stage renal disease on hemodialysis, and distal ureteral obstruction requiring bilateral nephrostomy tubes noticed that one of the nephrostomy bags looked “purple” (Figure 1). A specimen collected from one bag was reddish purple (Figure 2). The urine in the other bag was normal. The condition was diagnosed as purple urine bag syndrome.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome, a relatively rare condition that appears after 2 to 3 months of indwelling urinary catheterization, is usually asymptomatic, the only signs being the purplish urine and staining of the urinary bags and catheters. However, it should be considered a sign of underlying urinary tract infection, which can disseminate causing local complications (Fournier gangrene), systemic complications (septicemia), and death.1–3
The syndrome, first described in 1978 in children with spina bifida and urinary diversion,4 is more prevalent in women than in men, possibly because of the shorter urethra and closer proximity to the anus, which predispose women to bacterial colonization of the urinary tract. Predisposing conditions include dementia,5 female sex, increased dietary tryptophan, bacteriuria, urinary tract infection, constipation, older age, immobility, and alkaline urine.6–8
The cause of the discoloration
The purple color is from indigo and indirubin compounds in the urine, the result of the breakdown of dietary tryptophan. The color varies depending on the proportions of the two pigments.
Dietary tryptophan is broken down into indole by colonic bacteria. After reaching the portal circulation, it is excreted into the urine as indoxyl sulfate, which is broken down to indoxyl by sulfatase-producing bacteria (eg, Klebsiella pneumonia, Proteus mirabilis, Pseudomonas aeruginosa, Escherichia coli, Providencia species, Morganella morganii). Indoxyl is then oxidized to indigo and indirubin.
These compounds do not discolor the urine directly, but rather precipitate after interacting with the lining of the urinary catheter and bags, thereby imparting a purple color.1,9–13
Management
Effective initial measures are improved urinary hygiene (eg, frequent, careful changing of the urinary catheter) and management of constipation, as constipation leads to increased colonization of the intestine by bacteria that metabolize dietary tryptophan into indoxyl. Antibiotics should be given for symptomatic urinary tract infection (fever, increased urinary frequency, dysuria, abdominal pain) but not for color change alone. Coverage should be for gram-negative bacilli, although methicillin-resistant Staphylococcus aureus, which is gram-positive, has also been reported to cause purple urine bag syndrome.
In most cases, purple urine bag syndrome is benign and requires no therapy other than that mentioned above.3,13–15 However, in rare cases, immunocompromised patients (eg, people with diabetes) can develop local complications and sepsis from dissemination of bacterial infection, requiring aggressive therapy.14 Therefore, purple urine bag syndrome should be recognized as an indicator of an underlying urinary tract infection and should be treated if symptomatic. Nevertheless, the long-term prognosis is generally good.
OUR PATIENT’S MANAGEMENT
Our patient was confirmed to have urinary colonization with P aeruginosa and E coli, and alkaline urine. He underwent replacement of the nephrostomy tubes and urinary bag during his hospital stay (he was already in the hospital for another indication), but he continued to produce purple-colored urine from his right side and normal-colored urine from his left side. The unilateral involvement was likely from selective colonization of the right-sided nephrostomy tube with gram-negative bacteria.
A 58-year-old man with a history of cystoprostatectomy for prostate cancer, end-stage renal disease on hemodialysis, and distal ureteral obstruction requiring bilateral nephrostomy tubes noticed that one of the nephrostomy bags looked “purple” (Figure 1). A specimen collected from one bag was reddish purple (Figure 2). The urine in the other bag was normal. The condition was diagnosed as purple urine bag syndrome.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome, a relatively rare condition that appears after 2 to 3 months of indwelling urinary catheterization, is usually asymptomatic, the only signs being the purplish urine and staining of the urinary bags and catheters. However, it should be considered a sign of underlying urinary tract infection, which can disseminate causing local complications (Fournier gangrene), systemic complications (septicemia), and death.1–3
The syndrome, first described in 1978 in children with spina bifida and urinary diversion,4 is more prevalent in women than in men, possibly because of the shorter urethra and closer proximity to the anus, which predispose women to bacterial colonization of the urinary tract. Predisposing conditions include dementia,5 female sex, increased dietary tryptophan, bacteriuria, urinary tract infection, constipation, older age, immobility, and alkaline urine.6–8
The cause of the discoloration
The purple color is from indigo and indirubin compounds in the urine, the result of the breakdown of dietary tryptophan. The color varies depending on the proportions of the two pigments.
Dietary tryptophan is broken down into indole by colonic bacteria. After reaching the portal circulation, it is excreted into the urine as indoxyl sulfate, which is broken down to indoxyl by sulfatase-producing bacteria (eg, Klebsiella pneumonia, Proteus mirabilis, Pseudomonas aeruginosa, Escherichia coli, Providencia species, Morganella morganii). Indoxyl is then oxidized to indigo and indirubin.
These compounds do not discolor the urine directly, but rather precipitate after interacting with the lining of the urinary catheter and bags, thereby imparting a purple color.1,9–13
Management
Effective initial measures are improved urinary hygiene (eg, frequent, careful changing of the urinary catheter) and management of constipation, as constipation leads to increased colonization of the intestine by bacteria that metabolize dietary tryptophan into indoxyl. Antibiotics should be given for symptomatic urinary tract infection (fever, increased urinary frequency, dysuria, abdominal pain) but not for color change alone. Coverage should be for gram-negative bacilli, although methicillin-resistant Staphylococcus aureus, which is gram-positive, has also been reported to cause purple urine bag syndrome.
In most cases, purple urine bag syndrome is benign and requires no therapy other than that mentioned above.3,13–15 However, in rare cases, immunocompromised patients (eg, people with diabetes) can develop local complications and sepsis from dissemination of bacterial infection, requiring aggressive therapy.14 Therefore, purple urine bag syndrome should be recognized as an indicator of an underlying urinary tract infection and should be treated if symptomatic. Nevertheless, the long-term prognosis is generally good.
OUR PATIENT’S MANAGEMENT
Our patient was confirmed to have urinary colonization with P aeruginosa and E coli, and alkaline urine. He underwent replacement of the nephrostomy tubes and urinary bag during his hospital stay (he was already in the hospital for another indication), but he continued to produce purple-colored urine from his right side and normal-colored urine from his left side. The unilateral involvement was likely from selective colonization of the right-sided nephrostomy tube with gram-negative bacteria.
- Kang KH, Jeong KH, Baik SK, et al. Purple urine bag syndrome: case report and literature review. Clin Nephrol 2011; 75:557–559.
- Ribeiro JP, Marcelino P, Marum S, Fernandes AP, Grilo A. Case report: purple urine bag syndrome. Crit Care 2004; 8:R137.
- Robinson J. Purple urinary bag syndrome: a harmless but alarming problem. Br J Community Nurs 2003; 8:263–266.
- Barlow GB, Dickson JAS. Purple urine bags. Lancet 1978; 1:220–221.
- Ga H, Kojima T. Purple urine bag syndrome. JAMA 2012; 307:1912–1913.
- Ishida T, Ogura S, Kawakami Y. Five cases of purple urine bag syndrome in a geriatric ward. Nihon Ronen Igakkai Zasshi 1999; 36:826–829. Japanese.
- Gautam G, Kothari A, Kumar R, Dogra PN. Purple urine bag syndrome: a rare clinical entity in patients with long term indwelling catheters. Int Urol Nephrol 2007; 39:155–156.
- Shiao CC, Weng CY, Chuang JC, Huang MS, Chen ZY. Purple urine bag syndrome: a community-based study and literature review. Nephrology (Carlton) 2008; 13:554–559.
- Chong VH. Purple urine bag syndrome: it is the urine bag and not the urine that is discolored purple. South Med J 2012; 105:446.
- Chung SD, Liao CH, Sun HD. Purple urine bag syndrome with acidic urine. Int J Infect Dis 2008; 12:526–527.
- Wu HH, Yang WC, Lin CC. Purple urine bag syndrome. Am J Med Sci 2009; 337:368.
- Achtergael W, Michielsen D, Gorus FK, Gerlo E. Indoxyl sulphate and the purple urine bag syndrome: a case report. Acta Clin Belg 2006; 61:38–41.
- Hadano Y, Shimizu T, Takada S, Inoue T, Sorano S. An update on purple urine bag syndrome. Int J Gen Med 2012; 5:707–710.
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Ferrara F, D’Angelo G, Costantino G. Monolateral purple urine bag syndrome in bilateral nephrostomy. Postgrad Med J 2010; 86:627.
- Kang KH, Jeong KH, Baik SK, et al. Purple urine bag syndrome: case report and literature review. Clin Nephrol 2011; 75:557–559.
- Ribeiro JP, Marcelino P, Marum S, Fernandes AP, Grilo A. Case report: purple urine bag syndrome. Crit Care 2004; 8:R137.
- Robinson J. Purple urinary bag syndrome: a harmless but alarming problem. Br J Community Nurs 2003; 8:263–266.
- Barlow GB, Dickson JAS. Purple urine bags. Lancet 1978; 1:220–221.
- Ga H, Kojima T. Purple urine bag syndrome. JAMA 2012; 307:1912–1913.
- Ishida T, Ogura S, Kawakami Y. Five cases of purple urine bag syndrome in a geriatric ward. Nihon Ronen Igakkai Zasshi 1999; 36:826–829. Japanese.
- Gautam G, Kothari A, Kumar R, Dogra PN. Purple urine bag syndrome: a rare clinical entity in patients with long term indwelling catheters. Int Urol Nephrol 2007; 39:155–156.
- Shiao CC, Weng CY, Chuang JC, Huang MS, Chen ZY. Purple urine bag syndrome: a community-based study and literature review. Nephrology (Carlton) 2008; 13:554–559.
- Chong VH. Purple urine bag syndrome: it is the urine bag and not the urine that is discolored purple. South Med J 2012; 105:446.
- Chung SD, Liao CH, Sun HD. Purple urine bag syndrome with acidic urine. Int J Infect Dis 2008; 12:526–527.
- Wu HH, Yang WC, Lin CC. Purple urine bag syndrome. Am J Med Sci 2009; 337:368.
- Achtergael W, Michielsen D, Gorus FK, Gerlo E. Indoxyl sulphate and the purple urine bag syndrome: a case report. Acta Clin Belg 2006; 61:38–41.
- Hadano Y, Shimizu T, Takada S, Inoue T, Sorano S. An update on purple urine bag syndrome. Int J Gen Med 2012; 5:707–710.
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Ferrara F, D’Angelo G, Costantino G. Monolateral purple urine bag syndrome in bilateral nephrostomy. Postgrad Med J 2010; 86:627.
Acute community-acquired bacterial meningitis in adults: An evidence-based review
Although the incidence and rates of morbidity and death from acute community-acquired bacterial meningitis have dramatically declined, probably as a result of vaccination and better antimicrobial and adjuvant therapy, the disease still has a high toll. From 10% to 20% of people who contract it in the United States still die of it.1,2
In the United States, meningitis from all causes accounts for about 72,000 hospitalizations and up to $1.2 billion in hospital costs annually.3 However, the incidence of bacterial meningitis has declined from 3 to 5 per 100,000 per year a few decades ago to 1.3 to 2 per 100,000 per year currently.2 In less-developed countries, rates are much higher.
In the early 1900s in the United States, the death rate from bacterial meningitis was 80% to 100%. The use of intrathecal equine meningococcal antiserum during the first decades of the 1900s dramatically reduced the rate of death from meningococcal meningitis. With the advent of antimicrobial drugs in the 1930s and 1940s, the death rate from bacterial meningitis further declined.
The organisms that cause community-acquired bacterial meningitis differ somewhat by geographic region and by age. In a recent paper based on surveillance data, in the United States, from 1998 to 2007, the most common cause of bacterial meningitis among adults was Streptococcus pneumoniae. Among young adults, Neisseria meningitidis is nearly as common as S pneumoniae. The incidence of Listeria infections increases with age in adults.2
The epidemiologic features of bacterial meningitis have changed dramatically over the past decades with the advent of the Haemophilus influenzae vaccine. In 1986, about half the cases of acute bacterial meningitis were caused by H influenzae, but a decade later the incidence of H influenzae meningitis had been reduced by 94%.4
Meningitis is inflammation of the pia and arachnoid (the inner two layers of the meninges). Acute community-acquired meningitis can develop within hours to days and can be viral or bacterial. Viral meningitis usually has a good prognosis, whereas bacterial meningitis is associated with significant rates of morbidity and death, so it is critical to recognize and differentiate them promptly.
PATHOGENESIS
Most cases of community-acquired bacterial meningitis begin with colonization of the nasopharyngeal mucosa. In certain individuals this leads to mucosal invasion and bacteremia. Not all organisms that cause bacteremia are capable of breaching the blood-cerebrospinal fluid barrier to enter the subarachnoid space to cause meningitis. Very few organisms have this capacity, but N meningitidis and S pneumoniae do.5
Some patients are at higher risk of meningitis because of an abnormal communication between the nasopharynx and the subarachnoid space due either to trauma or a congenital anatomic abnormality. The organisms in these instances can directly spread from the nasopharynx to the meninges. Patients without a spleen or with an immunoglobulin deficiency are also more prone to infections from encapsulated organisms such as pneumococci and meningococci. The opsonizing immunoglobulins coat the capsule, helping phagocytes in the spleen to remove them from the bloodstream. A patient presenting with multiple episodes of bacterial meningitis merits evaluation for these conditions.
In contrast, Listeria spp and, rarely, gramnegative bacteria enter the bloodstream through the gastrointestinal tract and then spread to the meninges.
Once in the subarachnoid space, bacteria elicit a profuse inflammatory response, which can be damaging.5 The inflammation in the subarachnoid space can extend along the Virchow-Robin spaces surrounding the blood vessels deep into the brain parenchyma. This perivascular inflammation can cause thrombosis in both the arterial and venous circulation.
Thus, the inflammation can lead to intracranial complications such as cerebral edema, hydrocephalus, and stroke. The complications of bacterial meningitis can be remembered by the acronym HACTIVE: hydrocephalus, abscess, cerebritis and cranial nerve lesions, thrombosis, infarct, ventriculitis and vasculopathy, and extra-axial collection.5,6
MICROBIOLOGY: WHEN TO SUSPECT DIFFERENT ORGANISMS
S pneumoniae: The most common cause in adults
Patients without a spleen and patients with either a primary or secondary immunoglobulin deficiency, including patients with multiple myeloma or human immunodeficiency virus infection, are at a higher risk of infection with this organism.
N meningitidis: More common in young adults
N meningitidis is easily transmitted and is associated with crowding, as in school dormitories and military barracks. People with congenital deficiencies of components of terminal complement are at greater risk for both meningococcal and gonococcal infections. Patients with recurrent episodes of Neisseria infection should be evaluated for complement deficiency.
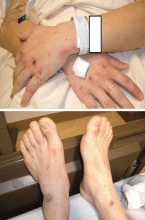
Meningococcal infection is more commonly associated with a rash. The most common rash of meningococcal meningitis is a very transient, maculopapular rash that appears early in the course of the disease. More pathognomonic is a petechial rash (Figure 1) with thrombocytopenia, which can very rapidly progress to purpura, ecchymosis, and disseminated intravascular coagulation. The petechial rash is evident in 60% of adults and up to 90% of children,7 and it is most likely to appear in dependent areas (such as the back of a patient lying down) and in areas of pressure, such as under the elastic band of underwear or stockings.
Listeria
Listeria infection is usually acquired through contaminated food such as raw vegetables, unpasteurized milk and cheese, and deli meats. From the gastrointestinal tract, it spreads to the bloodstream and then to the meninges.
Listeria is an intracellular pathogen; thus, people at greater risk are those with poor cell-mediated immunity due to immunosuppressant medications such as steroids or tumor necrosis factor inhibitors.
The rate of Listeria meningitis starts to increase with age, especially after age 50, probably due to immune senescence or decreased immunity with age.
Aerobic gram-negative bacilli
Gram-negative enteric bacilli usually cause meningitis after head trauma or neurosurgery and are very uncommon causes of community-acquired meningitis. Disseminated strongyloidiasis, also known as hyperinfection syndrome, should be suspected in any patient with community-acquired meningitis caused by enteric gram-negative bacilli.
Strongyloides stercoralis is a parasitic intestinal roundworm that is found in the tropics, in the subtropics, and in certain parts of the United States and Europe. The adult worm lives in the intestines and lays eggs, which hatch in the mucosa; the larvae are excreted in the stool. A small percentage of larvae penetrate the perianal skin and gut mucosa to cause an autoinfection. People may asymptomatically harbor the parasite for decades, then develop the hyperinfection syndrome when treated with immunosuppressive drugs such as steroids. In the hyperinfection syndrome a significant proportion of the larvae penetrate the gut mucosa to enter the bloodstream and travel throughout the body, including into the brain, carrying gram-negative bacteria with them.
The mortality rate of untreated hyperinfection syndrome can sometimes reach 100%.8 Thus, it is important to identify and treat the hyperinfection syndrome in the context of gram-negative bacillary meningitis.
SUSPECTED MENINGITIS: CLINICAL SCENARIO
A 36-year-old man presents to the emergency department with high fever, headache, and lethargy that developed over the past 24 hours. His temperature is 104°F (40°C), pulse 120 beats/min, respiratory rate 30/min, and blood pressure 130/70 mm Hg. He is oriented only to person and has nuchal rigidity. His white blood cell count is 30 × 109/L, with 20% bands.
The clinical questions that arise with such a patient are:
- Does the patient have bacterial or viral meningitis?
- Can we reliably rule out meningitis based on a history and physical examination?
- Is a lumbar puncture for cerebrospinal fluid (CSF) analysis needed? How should these studies be interpreted?
- Should computed tomography of the head be done before lumbar puncture?
- Which antimicrobial drugs should be started empirically at the outset?
- What is the role of steroids in treatment?
CLINICAL SIGNS AND SYMPTOMS
The classic triad of meningitis is fever, neck stiffness, and altered mental status. Other signs and symptoms that have been described are photophobia, headache, nausea, vomiting, focal neurologic symptoms, altered mental status, the Kernig sign (inability to allow full knee extension when the hip is flexed to a 90° angle), and the Brudzinski sign (spontaneous flexion of the hips during attempted passive flexion of the neck).
Can meningitis be ruled out if the patient does not have this classic presentation?
Unfortunately, only a few high-quality studies of the diagnostic accuracy of signs and symptoms of bacterial meningitis have been done. Fourteen retrospective studies examined this issue, but they were heterogeneous with respect to patient age, immunosuppression status, and clinical presentation, as well as to how meningitis was diagnosed (via culture or cerebrospinal fluid analysis), making the results difficult to interpret.9 Retrospective studies are more prone to bias, as they lack a control group, and examiner bias is more likely. Based on retrospective data, the combination of fever, neck stiffness, and altered mental status has a sensitivity of only 0.46.9
Two prospective studies examined symptoms and signs. Thomas et al10 evaluated 297 patients with “clinically suspected meningitis.” Unfortunately, in this study the physical examination was not standardized. In a study by Uchihara and Tsukagoshi,11 the measurement was more reliable, as they used a single examiner to evaluate patients presenting with fever and headache, but only 54 patients were studied.
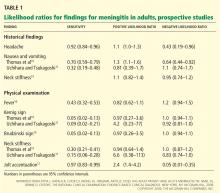
Based on these prospective studies, the presence of nausea and vomiting, headache, or neck stiffness does not reliably rule in meningitis (Table 1).9 Similarly, the absence of these does not rule it out. The 95% confidence intervals (CIs) of the positive and negative likelihood ratios include the value 1. (A simple interpretation of that would be that the likelihood of finding these features is the same in patients with meningitis when compared with those without meningitis.9)
For the physical examination, the presence or absence of fever, the Kernig sign, or the Brudzinski sign were also inconclusive. The CIs of the positive and negative likelihood ratios, like those of the symptoms, included the value 1. Only one test done on physical examination looked promising in having diagnostic utility to rule out meningitis: the jolt accentuation test (performed by asking a patient with a headache to quickly move his or her head twice horizontally; the result is positive if the headache worsens). If the result is negative, meningitis is unlikely (negative likelihood ratio 0.05, 95% CI 0.01–0.35).9 However, a positive test is not useful in making the diagnosis. A caveat is that this is based on a single study.
In summary, the history and physical examination are not sufficient to determine whether a patient has meningitis. If a patient is suspected of having meningitis, a lumbar puncture is needed.
WORKUP AND DIAGNOSTIC TESTS
Which tests are needed?
Blood cultures should be drawn before antimicrobial treatment is started.12–14 Although positive only 19% to 70% of the time, they can help identify the pathogen.15–17
Lumbar puncture with CSF study is essential to make the diagnosis and to identify the organism and its susceptibility to various antibiotics. If lumbar puncture can be performed immediately, it should be done before starting antibiotics, to maximize the yield of cultures. Pediatric studies show that after starting antibiotics, complete sterilization of the cerebrospinal fluid can occur within 2 hours for N meningitides and within 4 hours for S pneumoniae.14 However, starting antimicrobials should not be delayed if a lumbar puncture cannot be done expeditiously.
Is computed tomography of the brain necessary before a lumbar puncture?
The rationale behind performing CT before lumbar puncture is to determine if the patient has elevated intracranial pressure, which would increase the risk of brain herniation due to lowering of the lumbar CSF pressure during lumbar puncture. For ethical and practical reasons, it would be difficult to evaluate this in a randomized clinical trial.
Hasbun et al18 performed a study to evaluate if any features on clinical presentation can predict abnormal findings on CT of the head suggestive of elevated intracranial pressure and thus the risk of herniation. The study included 301 adults with suspected meningitis. It found that abnormal findings on CT were unlikely if all of the following features were absent at baseline:
- Immunocompromised state
- History of central nervous system disease (mass lesion, stroke, or a focal infection)
- New onset of seizure (≤ 1 week from presentation)
- Specific abnormal neurologic findings (eg, an abnormal level of consciousness, inability to answer two consecutive questions correctly or to follow two consecutive commands, gaze palsy, abnormal visual fields, facial palsy, arm drift, leg drift, abnormal language).
Absence of these baseline features made it unlikely that CT would be abnormal (negative likelihood ratio 0.1, 95% CI 0.03–0.31).
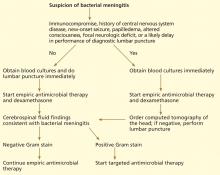
According to the guidelines from the Infectious Diseases Society of America (IDSA),19 if none of those features is present, blood cultures and a lumbar puncture should be done immediately, followed by empiric antimicrobial therapy. If any of the features is present, blood cultures should be obtained first, then empiric antimicrobial therapy started, followed by CT of the brain to look for contraindications to a lumbar puncture (Figure 2).
What can lumbar puncture tell us?
Results of lumbar puncture studies can help determine whether meningitis is present and, if so, whether the cause is likely bacterial or viral.20
The opening pressure is elevated (usually > 180 mm H2O) in acute bacterial meningitis. The CSF white blood cell count is usually more than 1.0 × 109/L, consisting predominantly of neutrophils, in acute bacterial meningitis. In viral meningitis, it is usually less than 0.1 × 109/L, mostly lymphocytes.
Protein shows a mild to marked elevation in bacterial meningitis but is normal to elevated in viral meningitis.
The CSF glucose level is lower in bacterial meningitis than in viral meningitis.
The ratio of CSF glucose to blood glucose. Because the glucose levels in the CSF and the blood equilibrate, the ratio of CSF glucose to serum glucose has better diagnostic accuracy than the CSF glucose level alone. The equilibration takes place within a few hours, so the serum glucose level should be ordered at the same time lumbar puncture is done. The CSF glucose-blood glucose ratio is a better predictor of bacterial meningitis than the CSF white blood cell count. Bacterial meningitis is likely if the ratio is lower than 0.4.
Lactate levels are not usually measured, but a lactate level greater than 31.5 mg/dL (3.5 mmol/L) is predictive of meningitis, and a lower level makes the diagnosis unlikely.
The diagnostic accuracies (likelihood ratios) of the CSF tests were analyzed by Straus et al.21 The positive likelihood ratios for the CSF white blood cell count and for the CSF glucose-blood glucose ratio are greater than 10, but these tests have negative likelihood ratios of more than 0.1. (It is generally thought that a test with a positive likelihood ratio of more than 10 is considered good for ruling in a diagnosis, whereas one with a negative likelihood ratio of less than 0.1 is good for ruling out a diagnosis.) Thus, these tests are good to rule in bacterial meningitis, but not as good to rule it out. There are some data to show that CSF lactate and procalcitonin might be more sensitive in ruling out bacterial meningitis, but more studies are needed.22
Gram stain of the cerebrospinal fluid can be done quickly. If no bacteria are seen, the information is not helpful in ruling out bacterial meningitis (negative likelihood ratio 0.14, 95% CI 0.08–0.27). If it is positive, it is almost 100% specific for meningitis due to the organism seen (positive likelihood ratio 735, 95% CI 230–2,295).21
MANAGEMENT
Empiric antimicrobial therapy must be started as soon as feasible
Most studies of the timing of antimicrobial drugs were retrospective and included a very heterogeneous population. They were thus more prone to bias and confounding.23,24 Proulx et al,23 in a retrospective study, found that if antibiotics were given within 6 hours of the time the patient presented to the emergency department, the case fatality rate was only 5% to 6%. If treatment started 6 to 8 hours after presentation, the death rate was 45%, and if it started from 8 to 10 hours after presentation, the death rate was 75%. Most physicians would agree that starting antimicrobials early would be beneficial.
CSF concentrations of most antimicrobial drugs are considerably less than in the serum due to poor penetration of the blood-CSF barrier. Thus, the dose for treating meningitis is usually higher than the regular dose. For example, for the treatment of pneumococcal pneumonia, ceftriaxone (Rocephin) is used at a dose of 1 g every 24 hours, but for pneumococcal meningitis the dose is 2 g every 12 hours.
Empiric treatment of community-acquired bacterial meningitis in immunocompetent adults up to 50 years of age consists of a third-generation cephalosporin such as cefotaxime (Claforan) 2 g intravenously every 4 hours or ceftriaxone 2 g intravenously every 12 hours, which covers most S pneumoniae and N meningitides strains.19 The IDSA guidelines recommend adding vancomycin (Vancocin) empirically in suspected S pneumoniae meningitis due to concerns about drug-resistant pneumococcal strains.19 For vancomycin, 45 to 60 mg/kg intravenously per day divided into every-6-hour or every-8-hour doses would achieve better CSF concentrations.25
In patients over age 50 or those with a cell-mediated immunodeficiency, empiric therapy should also include ampicillin 2 g intravenously every 4 hours to cover Listeria.
It is important to tailor therapy to the results of Gram stain, culture, and susceptibility as they become available.
Role of corticosteroids
Glucocorticoids, especially dexamethasone (Decadron), have been well studied as adjunctive therapies in bacterial meningitis. The rationale behind their use is that the profuse inflammatory response to the bacterial components in the CSF by itself has deleterious effects, and steroids can reduce that.
In 2004, a Cochrane meta-analysis26 of five randomized clinical trials, including 623 adults with bacterial meningitis (234 with pneumococcal meningitis and 232 with meningococcal meningitis), found a significant reduction in the death rate for patients who received steroids: the death rate was 12% in patients who received steroids vs 22% in those who did not (odds ratio 0.6; 95% CI 0.40–0.81). This led to an IDSA practice guideline recommendation that in adults with suspected or proven pneumococcal meningitis, dexamethasone would be beneficial.19
But since then, many more studies have emerged from Europe, South America, Malawi, and Vietnam, and another Cochrane metaanalysis27 incorporated the new studies. Twenty-four studies involving 4,041 participants were included. Similar numbers of participants died in the corticosteroid and placebo groups (18% vs 20%; risk ratio [RR] 0.92, 95% CI 0.82–1.04, P = .18). A trend towards a lower mortality rate was noticed in adults receiving corticosteroids (RR 0.74, 95% CI 0.53–1.05, P = .09). In adults, corticosteroids were associated with lower rates of hearing loss (RR 0.74, 95% CI 0.56–0.98), and there was a trend towards fewer neurologic sequelae (RR 0.72, 95% CI 0.51–1.01). The benefits were shown in studies in adults in high-income countries, but the studies from low-income countries showed neither harm nor benefit. Based on these findings, the authors recommended the use of steroids in high-income countries, though the strength of the evidence was not optimal. The recommended steroid was dexamethasone 0.15 mg/kg intravenously every 6 hours for 4 days.
- Swartz MN. Bacterial meningitis—a view of the past 90 years. N Engl J Med 2004; 351:1826–1828.
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2010–2025.
- Holmquist L, Russo CA, Elixhauser A. Meningitis-related hospitalizations in the United States, 2006. Statistical Brief #57. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD, 2008. www.hcup-us.ahrq.gov/reports/statbriefs/sb57.jsp. Accessed May 4, 2012.
- Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. N Engl J Med 1997; 337:970–976.
- Koedel U, Scheld WM, Pfister H-W. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis 2002; 2:721–736.
- Hughes DC, Raghavan A, Mordekar SR, Griffiths PD, Connolly DJ. Role of imaging in the diagnosis of acute bacterial meningitis and its complications. Postgrad Med J 2010; 86:478–485.
- Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010; 23:467–492.
- Maguire JH. Intestinal nematodes (roundworms). In:Mandell G, Bennett J, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Elsevier, 2009:3577–3586.
- Attia J, Hatala R, Cook DJ, Wong JG. Original article: does this adult patient have acute meningitis?In:Simel DL, Rennie D, editors. The Rational Clinical Examinatino: Evidence-Based Clinical Diagnosis. New York, NY: McGraw-Hill; 2009.
- Thomas KE, Hasbun R, Jekel J, Quagliarello VJ. The diagnostic accuracy of Kernig’s sign, Brudzinski’s sign, and nuchal rigidity in adults with suspected meningitis. Clin Infect Dis 2002; 35:46–52.
- Uchihara T, Tsukagoshi H. Jolt accenulation of headache: the most sensitive sign of CSF pleocytosis. Headache 1991; 31:167–171.
- Geiseler PJ, Nelson KE, Levin S, Reddi KT, Moses VK. Community-acquired purulent meningitis: a review of 1,316 cases during the antibiotic era, 1954–1976. Rev Infect Dis 1980; 2:725–745.
- Talan DA, Hoffman JR, Yoshikawa TT, Overturf GD. Role of empiric parenteral antibiotics prior to lumbar puncture in suspected bacterial meningitis: state of the art. Rev Infect Dis 1988; 10:365–376.
- Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics 2001; 108:1169–1174.
- Sigurdardóttir B, Björnsson OM, Jónsdóttir KE, Erlendsdóttir H, Gudmundsson S. Acute bacterial meningitis in adults. A 20-year overview. Arch Intern Med 1997; 157:425–430.
- Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med 1998; 129:862–869.
- Andersen J, Backer V, Voldsgaard P, Skinhój P, Wandall JH. Acute meningococcal meningitis: analysis of features of the disease according to the age of 255 patients. Copenhagen Meningitis Study Group. J Infect 1997; 34:227–235.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician 2003; 68:1103–1108.
- Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA 2006; 296:2010–2022.
- Viallon A, Desseigne N, Marjollet O, et al. Meningitis in adult patients with a negative direct cerebrospinal fluid examination: value of cytochemical markers for differential diagnosis. Crit Care 2011; 15:R136.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Radetsky M. Duration of symptoms and outcome in bacterial meningitis: an analysis of causation and the implications of a delay in diagnosis. Pediatr Infect Dis J 1992; 11:694–698.
- Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis 2007; 44:250–255.
- van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis 2004; 4:139–143.
- van de Beek D, Farrar JJ, de Gans J, et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol 2010; 9:254–263.
Although the incidence and rates of morbidity and death from acute community-acquired bacterial meningitis have dramatically declined, probably as a result of vaccination and better antimicrobial and adjuvant therapy, the disease still has a high toll. From 10% to 20% of people who contract it in the United States still die of it.1,2
In the United States, meningitis from all causes accounts for about 72,000 hospitalizations and up to $1.2 billion in hospital costs annually.3 However, the incidence of bacterial meningitis has declined from 3 to 5 per 100,000 per year a few decades ago to 1.3 to 2 per 100,000 per year currently.2 In less-developed countries, rates are much higher.
In the early 1900s in the United States, the death rate from bacterial meningitis was 80% to 100%. The use of intrathecal equine meningococcal antiserum during the first decades of the 1900s dramatically reduced the rate of death from meningococcal meningitis. With the advent of antimicrobial drugs in the 1930s and 1940s, the death rate from bacterial meningitis further declined.
The organisms that cause community-acquired bacterial meningitis differ somewhat by geographic region and by age. In a recent paper based on surveillance data, in the United States, from 1998 to 2007, the most common cause of bacterial meningitis among adults was Streptococcus pneumoniae. Among young adults, Neisseria meningitidis is nearly as common as S pneumoniae. The incidence of Listeria infections increases with age in adults.2
The epidemiologic features of bacterial meningitis have changed dramatically over the past decades with the advent of the Haemophilus influenzae vaccine. In 1986, about half the cases of acute bacterial meningitis were caused by H influenzae, but a decade later the incidence of H influenzae meningitis had been reduced by 94%.4
Meningitis is inflammation of the pia and arachnoid (the inner two layers of the meninges). Acute community-acquired meningitis can develop within hours to days and can be viral or bacterial. Viral meningitis usually has a good prognosis, whereas bacterial meningitis is associated with significant rates of morbidity and death, so it is critical to recognize and differentiate them promptly.
PATHOGENESIS
Most cases of community-acquired bacterial meningitis begin with colonization of the nasopharyngeal mucosa. In certain individuals this leads to mucosal invasion and bacteremia. Not all organisms that cause bacteremia are capable of breaching the blood-cerebrospinal fluid barrier to enter the subarachnoid space to cause meningitis. Very few organisms have this capacity, but N meningitidis and S pneumoniae do.5
Some patients are at higher risk of meningitis because of an abnormal communication between the nasopharynx and the subarachnoid space due either to trauma or a congenital anatomic abnormality. The organisms in these instances can directly spread from the nasopharynx to the meninges. Patients without a spleen or with an immunoglobulin deficiency are also more prone to infections from encapsulated organisms such as pneumococci and meningococci. The opsonizing immunoglobulins coat the capsule, helping phagocytes in the spleen to remove them from the bloodstream. A patient presenting with multiple episodes of bacterial meningitis merits evaluation for these conditions.
In contrast, Listeria spp and, rarely, gramnegative bacteria enter the bloodstream through the gastrointestinal tract and then spread to the meninges.
Once in the subarachnoid space, bacteria elicit a profuse inflammatory response, which can be damaging.5 The inflammation in the subarachnoid space can extend along the Virchow-Robin spaces surrounding the blood vessels deep into the brain parenchyma. This perivascular inflammation can cause thrombosis in both the arterial and venous circulation.
Thus, the inflammation can lead to intracranial complications such as cerebral edema, hydrocephalus, and stroke. The complications of bacterial meningitis can be remembered by the acronym HACTIVE: hydrocephalus, abscess, cerebritis and cranial nerve lesions, thrombosis, infarct, ventriculitis and vasculopathy, and extra-axial collection.5,6
MICROBIOLOGY: WHEN TO SUSPECT DIFFERENT ORGANISMS
S pneumoniae: The most common cause in adults
Patients without a spleen and patients with either a primary or secondary immunoglobulin deficiency, including patients with multiple myeloma or human immunodeficiency virus infection, are at a higher risk of infection with this organism.
N meningitidis: More common in young adults
N meningitidis is easily transmitted and is associated with crowding, as in school dormitories and military barracks. People with congenital deficiencies of components of terminal complement are at greater risk for both meningococcal and gonococcal infections. Patients with recurrent episodes of Neisseria infection should be evaluated for complement deficiency.
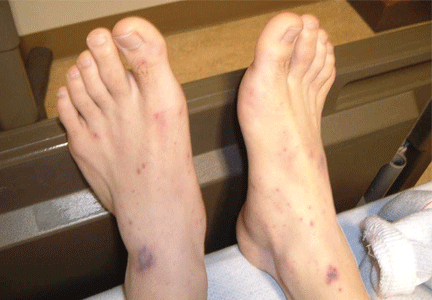
Meningococcal infection is more commonly associated with a rash. The most common rash of meningococcal meningitis is a very transient, maculopapular rash that appears early in the course of the disease. More pathognomonic is a petechial rash (Figure 1) with thrombocytopenia, which can very rapidly progress to purpura, ecchymosis, and disseminated intravascular coagulation. The petechial rash is evident in 60% of adults and up to 90% of children,7 and it is most likely to appear in dependent areas (such as the back of a patient lying down) and in areas of pressure, such as under the elastic band of underwear or stockings.
Listeria
Listeria infection is usually acquired through contaminated food such as raw vegetables, unpasteurized milk and cheese, and deli meats. From the gastrointestinal tract, it spreads to the bloodstream and then to the meninges.
Listeria is an intracellular pathogen; thus, people at greater risk are those with poor cell-mediated immunity due to immunosuppressant medications such as steroids or tumor necrosis factor inhibitors.
The rate of Listeria meningitis starts to increase with age, especially after age 50, probably due to immune senescence or decreased immunity with age.
Aerobic gram-negative bacilli
Gram-negative enteric bacilli usually cause meningitis after head trauma or neurosurgery and are very uncommon causes of community-acquired meningitis. Disseminated strongyloidiasis, also known as hyperinfection syndrome, should be suspected in any patient with community-acquired meningitis caused by enteric gram-negative bacilli.
Strongyloides stercoralis is a parasitic intestinal roundworm that is found in the tropics, in the subtropics, and in certain parts of the United States and Europe. The adult worm lives in the intestines and lays eggs, which hatch in the mucosa; the larvae are excreted in the stool. A small percentage of larvae penetrate the perianal skin and gut mucosa to cause an autoinfection. People may asymptomatically harbor the parasite for decades, then develop the hyperinfection syndrome when treated with immunosuppressive drugs such as steroids. In the hyperinfection syndrome a significant proportion of the larvae penetrate the gut mucosa to enter the bloodstream and travel throughout the body, including into the brain, carrying gram-negative bacteria with them.
The mortality rate of untreated hyperinfection syndrome can sometimes reach 100%.8 Thus, it is important to identify and treat the hyperinfection syndrome in the context of gram-negative bacillary meningitis.
SUSPECTED MENINGITIS: CLINICAL SCENARIO
A 36-year-old man presents to the emergency department with high fever, headache, and lethargy that developed over the past 24 hours. His temperature is 104°F (40°C), pulse 120 beats/min, respiratory rate 30/min, and blood pressure 130/70 mm Hg. He is oriented only to person and has nuchal rigidity. His white blood cell count is 30 × 109/L, with 20% bands.
The clinical questions that arise with such a patient are:
- Does the patient have bacterial or viral meningitis?
- Can we reliably rule out meningitis based on a history and physical examination?
- Is a lumbar puncture for cerebrospinal fluid (CSF) analysis needed? How should these studies be interpreted?
- Should computed tomography of the head be done before lumbar puncture?
- Which antimicrobial drugs should be started empirically at the outset?
- What is the role of steroids in treatment?
CLINICAL SIGNS AND SYMPTOMS
The classic triad of meningitis is fever, neck stiffness, and altered mental status. Other signs and symptoms that have been described are photophobia, headache, nausea, vomiting, focal neurologic symptoms, altered mental status, the Kernig sign (inability to allow full knee extension when the hip is flexed to a 90° angle), and the Brudzinski sign (spontaneous flexion of the hips during attempted passive flexion of the neck).
Can meningitis be ruled out if the patient does not have this classic presentation?
Unfortunately, only a few high-quality studies of the diagnostic accuracy of signs and symptoms of bacterial meningitis have been done. Fourteen retrospective studies examined this issue, but they were heterogeneous with respect to patient age, immunosuppression status, and clinical presentation, as well as to how meningitis was diagnosed (via culture or cerebrospinal fluid analysis), making the results difficult to interpret.9 Retrospective studies are more prone to bias, as they lack a control group, and examiner bias is more likely. Based on retrospective data, the combination of fever, neck stiffness, and altered mental status has a sensitivity of only 0.46.9
Two prospective studies examined symptoms and signs. Thomas et al10 evaluated 297 patients with “clinically suspected meningitis.” Unfortunately, in this study the physical examination was not standardized. In a study by Uchihara and Tsukagoshi,11 the measurement was more reliable, as they used a single examiner to evaluate patients presenting with fever and headache, but only 54 patients were studied.
Based on these prospective studies, the presence of nausea and vomiting, headache, or neck stiffness does not reliably rule in meningitis (Table 1).9 Similarly, the absence of these does not rule it out. The 95% confidence intervals (CIs) of the positive and negative likelihood ratios include the value 1. (A simple interpretation of that would be that the likelihood of finding these features is the same in patients with meningitis when compared with those without meningitis.9)
For the physical examination, the presence or absence of fever, the Kernig sign, or the Brudzinski sign were also inconclusive. The CIs of the positive and negative likelihood ratios, like those of the symptoms, included the value 1. Only one test done on physical examination looked promising in having diagnostic utility to rule out meningitis: the jolt accentuation test (performed by asking a patient with a headache to quickly move his or her head twice horizontally; the result is positive if the headache worsens). If the result is negative, meningitis is unlikely (negative likelihood ratio 0.05, 95% CI 0.01–0.35).9 However, a positive test is not useful in making the diagnosis. A caveat is that this is based on a single study.
In summary, the history and physical examination are not sufficient to determine whether a patient has meningitis. If a patient is suspected of having meningitis, a lumbar puncture is needed.
WORKUP AND DIAGNOSTIC TESTS
Which tests are needed?
Blood cultures should be drawn before antimicrobial treatment is started.12–14 Although positive only 19% to 70% of the time, they can help identify the pathogen.15–17
Lumbar puncture with CSF study is essential to make the diagnosis and to identify the organism and its susceptibility to various antibiotics. If lumbar puncture can be performed immediately, it should be done before starting antibiotics, to maximize the yield of cultures. Pediatric studies show that after starting antibiotics, complete sterilization of the cerebrospinal fluid can occur within 2 hours for N meningitides and within 4 hours for S pneumoniae.14 However, starting antimicrobials should not be delayed if a lumbar puncture cannot be done expeditiously.
Is computed tomography of the brain necessary before a lumbar puncture?
The rationale behind performing CT before lumbar puncture is to determine if the patient has elevated intracranial pressure, which would increase the risk of brain herniation due to lowering of the lumbar CSF pressure during lumbar puncture. For ethical and practical reasons, it would be difficult to evaluate this in a randomized clinical trial.
Hasbun et al18 performed a study to evaluate if any features on clinical presentation can predict abnormal findings on CT of the head suggestive of elevated intracranial pressure and thus the risk of herniation. The study included 301 adults with suspected meningitis. It found that abnormal findings on CT were unlikely if all of the following features were absent at baseline:
- Immunocompromised state
- History of central nervous system disease (mass lesion, stroke, or a focal infection)
- New onset of seizure (≤ 1 week from presentation)
- Specific abnormal neurologic findings (eg, an abnormal level of consciousness, inability to answer two consecutive questions correctly or to follow two consecutive commands, gaze palsy, abnormal visual fields, facial palsy, arm drift, leg drift, abnormal language).
Absence of these baseline features made it unlikely that CT would be abnormal (negative likelihood ratio 0.1, 95% CI 0.03–0.31).
According to the guidelines from the Infectious Diseases Society of America (IDSA),19 if none of those features is present, blood cultures and a lumbar puncture should be done immediately, followed by empiric antimicrobial therapy. If any of the features is present, blood cultures should be obtained first, then empiric antimicrobial therapy started, followed by CT of the brain to look for contraindications to a lumbar puncture (Figure 2).
What can lumbar puncture tell us?
Results of lumbar puncture studies can help determine whether meningitis is present and, if so, whether the cause is likely bacterial or viral.20
The opening pressure is elevated (usually > 180 mm H2O) in acute bacterial meningitis. The CSF white blood cell count is usually more than 1.0 × 109/L, consisting predominantly of neutrophils, in acute bacterial meningitis. In viral meningitis, it is usually less than 0.1 × 109/L, mostly lymphocytes.
Protein shows a mild to marked elevation in bacterial meningitis but is normal to elevated in viral meningitis.
The CSF glucose level is lower in bacterial meningitis than in viral meningitis.
The ratio of CSF glucose to blood glucose. Because the glucose levels in the CSF and the blood equilibrate, the ratio of CSF glucose to serum glucose has better diagnostic accuracy than the CSF glucose level alone. The equilibration takes place within a few hours, so the serum glucose level should be ordered at the same time lumbar puncture is done. The CSF glucose-blood glucose ratio is a better predictor of bacterial meningitis than the CSF white blood cell count. Bacterial meningitis is likely if the ratio is lower than 0.4.
Lactate levels are not usually measured, but a lactate level greater than 31.5 mg/dL (3.5 mmol/L) is predictive of meningitis, and a lower level makes the diagnosis unlikely.
The diagnostic accuracies (likelihood ratios) of the CSF tests were analyzed by Straus et al.21 The positive likelihood ratios for the CSF white blood cell count and for the CSF glucose-blood glucose ratio are greater than 10, but these tests have negative likelihood ratios of more than 0.1. (It is generally thought that a test with a positive likelihood ratio of more than 10 is considered good for ruling in a diagnosis, whereas one with a negative likelihood ratio of less than 0.1 is good for ruling out a diagnosis.) Thus, these tests are good to rule in bacterial meningitis, but not as good to rule it out. There are some data to show that CSF lactate and procalcitonin might be more sensitive in ruling out bacterial meningitis, but more studies are needed.22
Gram stain of the cerebrospinal fluid can be done quickly. If no bacteria are seen, the information is not helpful in ruling out bacterial meningitis (negative likelihood ratio 0.14, 95% CI 0.08–0.27). If it is positive, it is almost 100% specific for meningitis due to the organism seen (positive likelihood ratio 735, 95% CI 230–2,295).21
MANAGEMENT
Empiric antimicrobial therapy must be started as soon as feasible
Most studies of the timing of antimicrobial drugs were retrospective and included a very heterogeneous population. They were thus more prone to bias and confounding.23,24 Proulx et al,23 in a retrospective study, found that if antibiotics were given within 6 hours of the time the patient presented to the emergency department, the case fatality rate was only 5% to 6%. If treatment started 6 to 8 hours after presentation, the death rate was 45%, and if it started from 8 to 10 hours after presentation, the death rate was 75%. Most physicians would agree that starting antimicrobials early would be beneficial.
CSF concentrations of most antimicrobial drugs are considerably less than in the serum due to poor penetration of the blood-CSF barrier. Thus, the dose for treating meningitis is usually higher than the regular dose. For example, for the treatment of pneumococcal pneumonia, ceftriaxone (Rocephin) is used at a dose of 1 g every 24 hours, but for pneumococcal meningitis the dose is 2 g every 12 hours.
Empiric treatment of community-acquired bacterial meningitis in immunocompetent adults up to 50 years of age consists of a third-generation cephalosporin such as cefotaxime (Claforan) 2 g intravenously every 4 hours or ceftriaxone 2 g intravenously every 12 hours, which covers most S pneumoniae and N meningitides strains.19 The IDSA guidelines recommend adding vancomycin (Vancocin) empirically in suspected S pneumoniae meningitis due to concerns about drug-resistant pneumococcal strains.19 For vancomycin, 45 to 60 mg/kg intravenously per day divided into every-6-hour or every-8-hour doses would achieve better CSF concentrations.25
In patients over age 50 or those with a cell-mediated immunodeficiency, empiric therapy should also include ampicillin 2 g intravenously every 4 hours to cover Listeria.
It is important to tailor therapy to the results of Gram stain, culture, and susceptibility as they become available.
Role of corticosteroids
Glucocorticoids, especially dexamethasone (Decadron), have been well studied as adjunctive therapies in bacterial meningitis. The rationale behind their use is that the profuse inflammatory response to the bacterial components in the CSF by itself has deleterious effects, and steroids can reduce that.
In 2004, a Cochrane meta-analysis26 of five randomized clinical trials, including 623 adults with bacterial meningitis (234 with pneumococcal meningitis and 232 with meningococcal meningitis), found a significant reduction in the death rate for patients who received steroids: the death rate was 12% in patients who received steroids vs 22% in those who did not (odds ratio 0.6; 95% CI 0.40–0.81). This led to an IDSA practice guideline recommendation that in adults with suspected or proven pneumococcal meningitis, dexamethasone would be beneficial.19
But since then, many more studies have emerged from Europe, South America, Malawi, and Vietnam, and another Cochrane metaanalysis27 incorporated the new studies. Twenty-four studies involving 4,041 participants were included. Similar numbers of participants died in the corticosteroid and placebo groups (18% vs 20%; risk ratio [RR] 0.92, 95% CI 0.82–1.04, P = .18). A trend towards a lower mortality rate was noticed in adults receiving corticosteroids (RR 0.74, 95% CI 0.53–1.05, P = .09). In adults, corticosteroids were associated with lower rates of hearing loss (RR 0.74, 95% CI 0.56–0.98), and there was a trend towards fewer neurologic sequelae (RR 0.72, 95% CI 0.51–1.01). The benefits were shown in studies in adults in high-income countries, but the studies from low-income countries showed neither harm nor benefit. Based on these findings, the authors recommended the use of steroids in high-income countries, though the strength of the evidence was not optimal. The recommended steroid was dexamethasone 0.15 mg/kg intravenously every 6 hours for 4 days.
Although the incidence and rates of morbidity and death from acute community-acquired bacterial meningitis have dramatically declined, probably as a result of vaccination and better antimicrobial and adjuvant therapy, the disease still has a high toll. From 10% to 20% of people who contract it in the United States still die of it.1,2
In the United States, meningitis from all causes accounts for about 72,000 hospitalizations and up to $1.2 billion in hospital costs annually.3 However, the incidence of bacterial meningitis has declined from 3 to 5 per 100,000 per year a few decades ago to 1.3 to 2 per 100,000 per year currently.2 In less-developed countries, rates are much higher.
In the early 1900s in the United States, the death rate from bacterial meningitis was 80% to 100%. The use of intrathecal equine meningococcal antiserum during the first decades of the 1900s dramatically reduced the rate of death from meningococcal meningitis. With the advent of antimicrobial drugs in the 1930s and 1940s, the death rate from bacterial meningitis further declined.
The organisms that cause community-acquired bacterial meningitis differ somewhat by geographic region and by age. In a recent paper based on surveillance data, in the United States, from 1998 to 2007, the most common cause of bacterial meningitis among adults was Streptococcus pneumoniae. Among young adults, Neisseria meningitidis is nearly as common as S pneumoniae. The incidence of Listeria infections increases with age in adults.2
The epidemiologic features of bacterial meningitis have changed dramatically over the past decades with the advent of the Haemophilus influenzae vaccine. In 1986, about half the cases of acute bacterial meningitis were caused by H influenzae, but a decade later the incidence of H influenzae meningitis had been reduced by 94%.4
Meningitis is inflammation of the pia and arachnoid (the inner two layers of the meninges). Acute community-acquired meningitis can develop within hours to days and can be viral or bacterial. Viral meningitis usually has a good prognosis, whereas bacterial meningitis is associated with significant rates of morbidity and death, so it is critical to recognize and differentiate them promptly.
PATHOGENESIS
Most cases of community-acquired bacterial meningitis begin with colonization of the nasopharyngeal mucosa. In certain individuals this leads to mucosal invasion and bacteremia. Not all organisms that cause bacteremia are capable of breaching the blood-cerebrospinal fluid barrier to enter the subarachnoid space to cause meningitis. Very few organisms have this capacity, but N meningitidis and S pneumoniae do.5
Some patients are at higher risk of meningitis because of an abnormal communication between the nasopharynx and the subarachnoid space due either to trauma or a congenital anatomic abnormality. The organisms in these instances can directly spread from the nasopharynx to the meninges. Patients without a spleen or with an immunoglobulin deficiency are also more prone to infections from encapsulated organisms such as pneumococci and meningococci. The opsonizing immunoglobulins coat the capsule, helping phagocytes in the spleen to remove them from the bloodstream. A patient presenting with multiple episodes of bacterial meningitis merits evaluation for these conditions.
In contrast, Listeria spp and, rarely, gramnegative bacteria enter the bloodstream through the gastrointestinal tract and then spread to the meninges.
Once in the subarachnoid space, bacteria elicit a profuse inflammatory response, which can be damaging.5 The inflammation in the subarachnoid space can extend along the Virchow-Robin spaces surrounding the blood vessels deep into the brain parenchyma. This perivascular inflammation can cause thrombosis in both the arterial and venous circulation.
Thus, the inflammation can lead to intracranial complications such as cerebral edema, hydrocephalus, and stroke. The complications of bacterial meningitis can be remembered by the acronym HACTIVE: hydrocephalus, abscess, cerebritis and cranial nerve lesions, thrombosis, infarct, ventriculitis and vasculopathy, and extra-axial collection.5,6
MICROBIOLOGY: WHEN TO SUSPECT DIFFERENT ORGANISMS
S pneumoniae: The most common cause in adults
Patients without a spleen and patients with either a primary or secondary immunoglobulin deficiency, including patients with multiple myeloma or human immunodeficiency virus infection, are at a higher risk of infection with this organism.
N meningitidis: More common in young adults
N meningitidis is easily transmitted and is associated with crowding, as in school dormitories and military barracks. People with congenital deficiencies of components of terminal complement are at greater risk for both meningococcal and gonococcal infections. Patients with recurrent episodes of Neisseria infection should be evaluated for complement deficiency.
Meningococcal infection is more commonly associated with a rash. The most common rash of meningococcal meningitis is a very transient, maculopapular rash that appears early in the course of the disease. More pathognomonic is a petechial rash (Figure 1) with thrombocytopenia, which can very rapidly progress to purpura, ecchymosis, and disseminated intravascular coagulation. The petechial rash is evident in 60% of adults and up to 90% of children,7 and it is most likely to appear in dependent areas (such as the back of a patient lying down) and in areas of pressure, such as under the elastic band of underwear or stockings.
Listeria
Listeria infection is usually acquired through contaminated food such as raw vegetables, unpasteurized milk and cheese, and deli meats. From the gastrointestinal tract, it spreads to the bloodstream and then to the meninges.
Listeria is an intracellular pathogen; thus, people at greater risk are those with poor cell-mediated immunity due to immunosuppressant medications such as steroids or tumor necrosis factor inhibitors.
The rate of Listeria meningitis starts to increase with age, especially after age 50, probably due to immune senescence or decreased immunity with age.
Aerobic gram-negative bacilli
Gram-negative enteric bacilli usually cause meningitis after head trauma or neurosurgery and are very uncommon causes of community-acquired meningitis. Disseminated strongyloidiasis, also known as hyperinfection syndrome, should be suspected in any patient with community-acquired meningitis caused by enteric gram-negative bacilli.
Strongyloides stercoralis is a parasitic intestinal roundworm that is found in the tropics, in the subtropics, and in certain parts of the United States and Europe. The adult worm lives in the intestines and lays eggs, which hatch in the mucosa; the larvae are excreted in the stool. A small percentage of larvae penetrate the perianal skin and gut mucosa to cause an autoinfection. People may asymptomatically harbor the parasite for decades, then develop the hyperinfection syndrome when treated with immunosuppressive drugs such as steroids. In the hyperinfection syndrome a significant proportion of the larvae penetrate the gut mucosa to enter the bloodstream and travel throughout the body, including into the brain, carrying gram-negative bacteria with them.
The mortality rate of untreated hyperinfection syndrome can sometimes reach 100%.8 Thus, it is important to identify and treat the hyperinfection syndrome in the context of gram-negative bacillary meningitis.
SUSPECTED MENINGITIS: CLINICAL SCENARIO
A 36-year-old man presents to the emergency department with high fever, headache, and lethargy that developed over the past 24 hours. His temperature is 104°F (40°C), pulse 120 beats/min, respiratory rate 30/min, and blood pressure 130/70 mm Hg. He is oriented only to person and has nuchal rigidity. His white blood cell count is 30 × 109/L, with 20% bands.
The clinical questions that arise with such a patient are:
- Does the patient have bacterial or viral meningitis?
- Can we reliably rule out meningitis based on a history and physical examination?
- Is a lumbar puncture for cerebrospinal fluid (CSF) analysis needed? How should these studies be interpreted?
- Should computed tomography of the head be done before lumbar puncture?
- Which antimicrobial drugs should be started empirically at the outset?
- What is the role of steroids in treatment?
CLINICAL SIGNS AND SYMPTOMS
The classic triad of meningitis is fever, neck stiffness, and altered mental status. Other signs and symptoms that have been described are photophobia, headache, nausea, vomiting, focal neurologic symptoms, altered mental status, the Kernig sign (inability to allow full knee extension when the hip is flexed to a 90° angle), and the Brudzinski sign (spontaneous flexion of the hips during attempted passive flexion of the neck).
Can meningitis be ruled out if the patient does not have this classic presentation?
Unfortunately, only a few high-quality studies of the diagnostic accuracy of signs and symptoms of bacterial meningitis have been done. Fourteen retrospective studies examined this issue, but they were heterogeneous with respect to patient age, immunosuppression status, and clinical presentation, as well as to how meningitis was diagnosed (via culture or cerebrospinal fluid analysis), making the results difficult to interpret.9 Retrospective studies are more prone to bias, as they lack a control group, and examiner bias is more likely. Based on retrospective data, the combination of fever, neck stiffness, and altered mental status has a sensitivity of only 0.46.9
Two prospective studies examined symptoms and signs. Thomas et al10 evaluated 297 patients with “clinically suspected meningitis.” Unfortunately, in this study the physical examination was not standardized. In a study by Uchihara and Tsukagoshi,11 the measurement was more reliable, as they used a single examiner to evaluate patients presenting with fever and headache, but only 54 patients were studied.
Based on these prospective studies, the presence of nausea and vomiting, headache, or neck stiffness does not reliably rule in meningitis (Table 1).9 Similarly, the absence of these does not rule it out. The 95% confidence intervals (CIs) of the positive and negative likelihood ratios include the value 1. (A simple interpretation of that would be that the likelihood of finding these features is the same in patients with meningitis when compared with those without meningitis.9)
For the physical examination, the presence or absence of fever, the Kernig sign, or the Brudzinski sign were also inconclusive. The CIs of the positive and negative likelihood ratios, like those of the symptoms, included the value 1. Only one test done on physical examination looked promising in having diagnostic utility to rule out meningitis: the jolt accentuation test (performed by asking a patient with a headache to quickly move his or her head twice horizontally; the result is positive if the headache worsens). If the result is negative, meningitis is unlikely (negative likelihood ratio 0.05, 95% CI 0.01–0.35).9 However, a positive test is not useful in making the diagnosis. A caveat is that this is based on a single study.
In summary, the history and physical examination are not sufficient to determine whether a patient has meningitis. If a patient is suspected of having meningitis, a lumbar puncture is needed.
WORKUP AND DIAGNOSTIC TESTS
Which tests are needed?
Blood cultures should be drawn before antimicrobial treatment is started.12–14 Although positive only 19% to 70% of the time, they can help identify the pathogen.15–17
Lumbar puncture with CSF study is essential to make the diagnosis and to identify the organism and its susceptibility to various antibiotics. If lumbar puncture can be performed immediately, it should be done before starting antibiotics, to maximize the yield of cultures. Pediatric studies show that after starting antibiotics, complete sterilization of the cerebrospinal fluid can occur within 2 hours for N meningitides and within 4 hours for S pneumoniae.14 However, starting antimicrobials should not be delayed if a lumbar puncture cannot be done expeditiously.
Is computed tomography of the brain necessary before a lumbar puncture?
The rationale behind performing CT before lumbar puncture is to determine if the patient has elevated intracranial pressure, which would increase the risk of brain herniation due to lowering of the lumbar CSF pressure during lumbar puncture. For ethical and practical reasons, it would be difficult to evaluate this in a randomized clinical trial.
Hasbun et al18 performed a study to evaluate if any features on clinical presentation can predict abnormal findings on CT of the head suggestive of elevated intracranial pressure and thus the risk of herniation. The study included 301 adults with suspected meningitis. It found that abnormal findings on CT were unlikely if all of the following features were absent at baseline:
- Immunocompromised state
- History of central nervous system disease (mass lesion, stroke, or a focal infection)
- New onset of seizure (≤ 1 week from presentation)
- Specific abnormal neurologic findings (eg, an abnormal level of consciousness, inability to answer two consecutive questions correctly or to follow two consecutive commands, gaze palsy, abnormal visual fields, facial palsy, arm drift, leg drift, abnormal language).
Absence of these baseline features made it unlikely that CT would be abnormal (negative likelihood ratio 0.1, 95% CI 0.03–0.31).
According to the guidelines from the Infectious Diseases Society of America (IDSA),19 if none of those features is present, blood cultures and a lumbar puncture should be done immediately, followed by empiric antimicrobial therapy. If any of the features is present, blood cultures should be obtained first, then empiric antimicrobial therapy started, followed by CT of the brain to look for contraindications to a lumbar puncture (Figure 2).
What can lumbar puncture tell us?
Results of lumbar puncture studies can help determine whether meningitis is present and, if so, whether the cause is likely bacterial or viral.20
The opening pressure is elevated (usually > 180 mm H2O) in acute bacterial meningitis. The CSF white blood cell count is usually more than 1.0 × 109/L, consisting predominantly of neutrophils, in acute bacterial meningitis. In viral meningitis, it is usually less than 0.1 × 109/L, mostly lymphocytes.
Protein shows a mild to marked elevation in bacterial meningitis but is normal to elevated in viral meningitis.
The CSF glucose level is lower in bacterial meningitis than in viral meningitis.
The ratio of CSF glucose to blood glucose. Because the glucose levels in the CSF and the blood equilibrate, the ratio of CSF glucose to serum glucose has better diagnostic accuracy than the CSF glucose level alone. The equilibration takes place within a few hours, so the serum glucose level should be ordered at the same time lumbar puncture is done. The CSF glucose-blood glucose ratio is a better predictor of bacterial meningitis than the CSF white blood cell count. Bacterial meningitis is likely if the ratio is lower than 0.4.
Lactate levels are not usually measured, but a lactate level greater than 31.5 mg/dL (3.5 mmol/L) is predictive of meningitis, and a lower level makes the diagnosis unlikely.
The diagnostic accuracies (likelihood ratios) of the CSF tests were analyzed by Straus et al.21 The positive likelihood ratios for the CSF white blood cell count and for the CSF glucose-blood glucose ratio are greater than 10, but these tests have negative likelihood ratios of more than 0.1. (It is generally thought that a test with a positive likelihood ratio of more than 10 is considered good for ruling in a diagnosis, whereas one with a negative likelihood ratio of less than 0.1 is good for ruling out a diagnosis.) Thus, these tests are good to rule in bacterial meningitis, but not as good to rule it out. There are some data to show that CSF lactate and procalcitonin might be more sensitive in ruling out bacterial meningitis, but more studies are needed.22
Gram stain of the cerebrospinal fluid can be done quickly. If no bacteria are seen, the information is not helpful in ruling out bacterial meningitis (negative likelihood ratio 0.14, 95% CI 0.08–0.27). If it is positive, it is almost 100% specific for meningitis due to the organism seen (positive likelihood ratio 735, 95% CI 230–2,295).21
MANAGEMENT
Empiric antimicrobial therapy must be started as soon as feasible
Most studies of the timing of antimicrobial drugs were retrospective and included a very heterogeneous population. They were thus more prone to bias and confounding.23,24 Proulx et al,23 in a retrospective study, found that if antibiotics were given within 6 hours of the time the patient presented to the emergency department, the case fatality rate was only 5% to 6%. If treatment started 6 to 8 hours after presentation, the death rate was 45%, and if it started from 8 to 10 hours after presentation, the death rate was 75%. Most physicians would agree that starting antimicrobials early would be beneficial.
CSF concentrations of most antimicrobial drugs are considerably less than in the serum due to poor penetration of the blood-CSF barrier. Thus, the dose for treating meningitis is usually higher than the regular dose. For example, for the treatment of pneumococcal pneumonia, ceftriaxone (Rocephin) is used at a dose of 1 g every 24 hours, but for pneumococcal meningitis the dose is 2 g every 12 hours.
Empiric treatment of community-acquired bacterial meningitis in immunocompetent adults up to 50 years of age consists of a third-generation cephalosporin such as cefotaxime (Claforan) 2 g intravenously every 4 hours or ceftriaxone 2 g intravenously every 12 hours, which covers most S pneumoniae and N meningitides strains.19 The IDSA guidelines recommend adding vancomycin (Vancocin) empirically in suspected S pneumoniae meningitis due to concerns about drug-resistant pneumococcal strains.19 For vancomycin, 45 to 60 mg/kg intravenously per day divided into every-6-hour or every-8-hour doses would achieve better CSF concentrations.25
In patients over age 50 or those with a cell-mediated immunodeficiency, empiric therapy should also include ampicillin 2 g intravenously every 4 hours to cover Listeria.
It is important to tailor therapy to the results of Gram stain, culture, and susceptibility as they become available.
Role of corticosteroids
Glucocorticoids, especially dexamethasone (Decadron), have been well studied as adjunctive therapies in bacterial meningitis. The rationale behind their use is that the profuse inflammatory response to the bacterial components in the CSF by itself has deleterious effects, and steroids can reduce that.
In 2004, a Cochrane meta-analysis26 of five randomized clinical trials, including 623 adults with bacterial meningitis (234 with pneumococcal meningitis and 232 with meningococcal meningitis), found a significant reduction in the death rate for patients who received steroids: the death rate was 12% in patients who received steroids vs 22% in those who did not (odds ratio 0.6; 95% CI 0.40–0.81). This led to an IDSA practice guideline recommendation that in adults with suspected or proven pneumococcal meningitis, dexamethasone would be beneficial.19
But since then, many more studies have emerged from Europe, South America, Malawi, and Vietnam, and another Cochrane metaanalysis27 incorporated the new studies. Twenty-four studies involving 4,041 participants were included. Similar numbers of participants died in the corticosteroid and placebo groups (18% vs 20%; risk ratio [RR] 0.92, 95% CI 0.82–1.04, P = .18). A trend towards a lower mortality rate was noticed in adults receiving corticosteroids (RR 0.74, 95% CI 0.53–1.05, P = .09). In adults, corticosteroids were associated with lower rates of hearing loss (RR 0.74, 95% CI 0.56–0.98), and there was a trend towards fewer neurologic sequelae (RR 0.72, 95% CI 0.51–1.01). The benefits were shown in studies in adults in high-income countries, but the studies from low-income countries showed neither harm nor benefit. Based on these findings, the authors recommended the use of steroids in high-income countries, though the strength of the evidence was not optimal. The recommended steroid was dexamethasone 0.15 mg/kg intravenously every 6 hours for 4 days.
- Swartz MN. Bacterial meningitis—a view of the past 90 years. N Engl J Med 2004; 351:1826–1828.
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2010–2025.
- Holmquist L, Russo CA, Elixhauser A. Meningitis-related hospitalizations in the United States, 2006. Statistical Brief #57. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD, 2008. www.hcup-us.ahrq.gov/reports/statbriefs/sb57.jsp. Accessed May 4, 2012.
- Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. N Engl J Med 1997; 337:970–976.
- Koedel U, Scheld WM, Pfister H-W. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis 2002; 2:721–736.
- Hughes DC, Raghavan A, Mordekar SR, Griffiths PD, Connolly DJ. Role of imaging in the diagnosis of acute bacterial meningitis and its complications. Postgrad Med J 2010; 86:478–485.
- Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010; 23:467–492.
- Maguire JH. Intestinal nematodes (roundworms). In:Mandell G, Bennett J, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Elsevier, 2009:3577–3586.
- Attia J, Hatala R, Cook DJ, Wong JG. Original article: does this adult patient have acute meningitis?In:Simel DL, Rennie D, editors. The Rational Clinical Examinatino: Evidence-Based Clinical Diagnosis. New York, NY: McGraw-Hill; 2009.
- Thomas KE, Hasbun R, Jekel J, Quagliarello VJ. The diagnostic accuracy of Kernig’s sign, Brudzinski’s sign, and nuchal rigidity in adults with suspected meningitis. Clin Infect Dis 2002; 35:46–52.
- Uchihara T, Tsukagoshi H. Jolt accenulation of headache: the most sensitive sign of CSF pleocytosis. Headache 1991; 31:167–171.
- Geiseler PJ, Nelson KE, Levin S, Reddi KT, Moses VK. Community-acquired purulent meningitis: a review of 1,316 cases during the antibiotic era, 1954–1976. Rev Infect Dis 1980; 2:725–745.
- Talan DA, Hoffman JR, Yoshikawa TT, Overturf GD. Role of empiric parenteral antibiotics prior to lumbar puncture in suspected bacterial meningitis: state of the art. Rev Infect Dis 1988; 10:365–376.
- Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics 2001; 108:1169–1174.
- Sigurdardóttir B, Björnsson OM, Jónsdóttir KE, Erlendsdóttir H, Gudmundsson S. Acute bacterial meningitis in adults. A 20-year overview. Arch Intern Med 1997; 157:425–430.
- Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med 1998; 129:862–869.
- Andersen J, Backer V, Voldsgaard P, Skinhój P, Wandall JH. Acute meningococcal meningitis: analysis of features of the disease according to the age of 255 patients. Copenhagen Meningitis Study Group. J Infect 1997; 34:227–235.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician 2003; 68:1103–1108.
- Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA 2006; 296:2010–2022.
- Viallon A, Desseigne N, Marjollet O, et al. Meningitis in adult patients with a negative direct cerebrospinal fluid examination: value of cytochemical markers for differential diagnosis. Crit Care 2011; 15:R136.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Radetsky M. Duration of symptoms and outcome in bacterial meningitis: an analysis of causation and the implications of a delay in diagnosis. Pediatr Infect Dis J 1992; 11:694–698.
- Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis 2007; 44:250–255.
- van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis 2004; 4:139–143.
- van de Beek D, Farrar JJ, de Gans J, et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol 2010; 9:254–263.
- Swartz MN. Bacterial meningitis—a view of the past 90 years. N Engl J Med 2004; 351:1826–1828.
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2010–2025.
- Holmquist L, Russo CA, Elixhauser A. Meningitis-related hospitalizations in the United States, 2006. Statistical Brief #57. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD, 2008. www.hcup-us.ahrq.gov/reports/statbriefs/sb57.jsp. Accessed May 4, 2012.
- Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. N Engl J Med 1997; 337:970–976.
- Koedel U, Scheld WM, Pfister H-W. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis 2002; 2:721–736.
- Hughes DC, Raghavan A, Mordekar SR, Griffiths PD, Connolly DJ. Role of imaging in the diagnosis of acute bacterial meningitis and its complications. Postgrad Med J 2010; 86:478–485.
- Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010; 23:467–492.
- Maguire JH. Intestinal nematodes (roundworms). In:Mandell G, Bennett J, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Elsevier, 2009:3577–3586.
- Attia J, Hatala R, Cook DJ, Wong JG. Original article: does this adult patient have acute meningitis?In:Simel DL, Rennie D, editors. The Rational Clinical Examinatino: Evidence-Based Clinical Diagnosis. New York, NY: McGraw-Hill; 2009.
- Thomas KE, Hasbun R, Jekel J, Quagliarello VJ. The diagnostic accuracy of Kernig’s sign, Brudzinski’s sign, and nuchal rigidity in adults with suspected meningitis. Clin Infect Dis 2002; 35:46–52.
- Uchihara T, Tsukagoshi H. Jolt accenulation of headache: the most sensitive sign of CSF pleocytosis. Headache 1991; 31:167–171.
- Geiseler PJ, Nelson KE, Levin S, Reddi KT, Moses VK. Community-acquired purulent meningitis: a review of 1,316 cases during the antibiotic era, 1954–1976. Rev Infect Dis 1980; 2:725–745.
- Talan DA, Hoffman JR, Yoshikawa TT, Overturf GD. Role of empiric parenteral antibiotics prior to lumbar puncture in suspected bacterial meningitis: state of the art. Rev Infect Dis 1988; 10:365–376.
- Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics 2001; 108:1169–1174.
- Sigurdardóttir B, Björnsson OM, Jónsdóttir KE, Erlendsdóttir H, Gudmundsson S. Acute bacterial meningitis in adults. A 20-year overview. Arch Intern Med 1997; 157:425–430.
- Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med 1998; 129:862–869.
- Andersen J, Backer V, Voldsgaard P, Skinhój P, Wandall JH. Acute meningococcal meningitis: analysis of features of the disease according to the age of 255 patients. Copenhagen Meningitis Study Group. J Infect 1997; 34:227–235.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician 2003; 68:1103–1108.
- Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA 2006; 296:2010–2022.
- Viallon A, Desseigne N, Marjollet O, et al. Meningitis in adult patients with a negative direct cerebrospinal fluid examination: value of cytochemical markers for differential diagnosis. Crit Care 2011; 15:R136.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Radetsky M. Duration of symptoms and outcome in bacterial meningitis: an analysis of causation and the implications of a delay in diagnosis. Pediatr Infect Dis J 1992; 11:694–698.
- Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis 2007; 44:250–255.
- van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis 2004; 4:139–143.
- van de Beek D, Farrar JJ, de Gans J, et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol 2010; 9:254–263.
KEY POINTS
- The most common organisms that cause community-acquired bacterial meningitis are Streptococcus pneumoniae and Neisseria meningitidis. The incidence of Listeria infection increases in patients over age 50 and in those with compromised cell-mediated immunity.
- Symptoms and signs are not sensitive or specific enough to diagnose community-acquired bacterial meningitis. A lumbar puncture for cerebrospinal fluid studies is needed to reach the diagnosis, to identify the organism, and to determine antimicrobial susceptibilities.
- Gram stain of cerebrospinal fluid may quickly identify the causative organism. It is not very sensitive, but it is specific.
- Lumbar puncture should be performed as soon as possible. Computed tomography of the head is not necessary in all patients, only in immunocompromised patients and those who have features suggestive of or who are at risk of increased intracranial pressure.
- Try to obtain blood and cerebrospinal fluid cultures before staring antimicrobial therapy, but do not delay therapy if obtaining them is not feasible.