User login
Randomized placebo-controlled trial comparing efficacy and safety of valdecoxib with naproxen in patients with osteoarthritis
OBJECTIVE: We compared the efficacy and upper gastrointestinal safety of the cyclooxygenase-2–specific inhibitor valdecoxib with naproxen and placebo in treating moderate to severe osteoarthritis of the knee.
STUDY DESIGN: This multicenter, randomized, double-blind, placebo-controlled study compared the efficacy and upper gastrointestinal tract safety of valdecoxib at dosages of 5, 10, and 20 mg once daily with placebo and naproxen at the dosage of 500 mg twice daily.
POPULATION: We included patients who had been diagnosed with moderate to severe osteoarthritis of the knee according to the modified criteria of the American College of Rheumatology.
OUTCOMES MEASURED: The Patient’s and Physician’s Global Assessment of Arthritis (PaGAA, PhGAA), Patient’s Assessment of Arthritis Pain–Visual Analog Scale (PAAP-VAS), and Western Ontario and McMaster’s Universities (WOMAC) Osteoarthritis indices were assessed at baseline and at weeks 2, 6, and 12. Upper gastrointestinal ulceration was assessed by pre- and posttreatment endoscopies.
RESULTS: Valdecoxib 10 and 20 mg once daily (but not 5 mg once daily) demonstrated similar efficacy to naproxen at 500 mg twice daily, and all 3 dosages were superior to placebo for the PaGAA, PhGAA, PAAP-VAS, and WOMAC Osteoarthritis indices at most assessments throughout the 12-week study (P < .05). The incidence of endoscopically proven ulcers was significantly higher in the naproxen group than in the 5- and 10-mg valdecoxib groups, but not in the 20-mg valdecoxib group. All 3 valdecoxib doses were comparable to placebo in ulcer incidence.
CONCLUSIONS: Valdecoxib (10 and 20 mg once daily) is significantly superior to placebo and as effective as naproxen (500 mg twice daily) in improving moderate to severe osteoarthritis of the knee. Upper gastrointestinal tract safety of valdecoxib (5 and 10 mg) was comparable to that of placebo and significantly better than that of naproxen.
- The cyclooxygenase-2–specific inhibitor valdecoxib 10 or 20 mg once daily is as effective as naproxen 500 mg twice daily.
- Valdecoxib at the recommended dose for treatment of osteoarthritis (10 mg once daily) had better upper gastrointestinal safety than naproxen.
Current medical therapies for osteoarthritis include conventional nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, glucosamine sulfate, and intra-articular injections of corticosteroids and hyaluronic acid. However, long-term use of corticosteroid injections can exacerbate damage to the affected joints.1,2 Conventional NSAIDs are associated with upper gastrointestinal tract ulceration and inhibition of platelet function.3
Cyclooxygenase-2 (COX-2)–specific inhibitors have demonstrated equivalent efficacy to conventional NSAIDs in treating pain and inflammation associated with osteoarthritis and rheumatoid arthritis. Further, COX-2–specific inhibitors significantly reduce the incidence of gastrointestinal ulceration and bleeding side effects caused by conventional NSAIDs.4,5 Valdecoxib (Bextra; Pharmacia Corporation and Pfizer Corporation) is a novel COX-2–specific inhibitor that is approximately 28,000-fold more selective against COX-2 than against COX-1. As a potent COX-2–specific inhibitor, valdecoxib is expected to provide efficacy equivalent to conventional NSAIDs for treatment of arthritis and spare the COX-1–related side effects. This randomized, placebo-controlled, double-blind, 12-week study was designed to test this hypothesis by comparing the efficacy and upper gastrointestinal tract safety of valdecoxib with that of naproxen, a leading conventional NSAID comparator.
Methods
Study population
Ambulatory adults who had been diagnosed with moderate to severe osteoarthritis of the knee according to the modified criteria of the American College of Rheumatology6,7 were eligible to participate in the trial. Patients were recruited from primary care and rheumatology specialty settings. Patients who had baseline scores of at least 40 mm on the Patient’s Assessment of Arthritis Pain–Visual Analog Scale (PAAP-VAS) and baseline categorical scores of poor to very poor on the Patient’s (PaGAA) and Physician’s (PhGAA) Global Assessments of Arthritis were included.8,9 Any patient suffering from inflammatory arthritis, gout, pseudogout, Paget disease, or any chronic pain syndrome that might interfere with assessment of the Index Knee was excluded from the trial. Patients diagnosed with osteoarthritis of the hip ipsilateral to the Index Knee, severe anserine bursitis, acute joint trauma, or complete loss of articular cartilage on the Index Knee also were excluded. Patients were not eligible if they had active gastrointestinal disease, gastrointestinal tract ulceration 30 days before the trial, a significant bleeding disorder, or a history of gastric or duodenal surgery. Patients with an esophageal, gastric, pyloric channel, or duodenal ulcer or a score of at least 10 for esophageal, gastric, or duodenal erosions at the pretreatment endoscopy examination also were excluded.
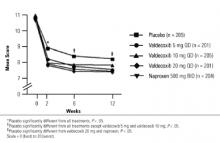
FIGURE 1
Patient’s global assessment of arthritis
Study design
This multicenter, randomized, double-blind, placebo-controlled study compared the efficacy and upper gastrointestinal tract safety of valdecoxib at dosages of 5, 10, and 20 mg once daily with placebo and naproxen at a dosage of 500 mg twice daily in relieving moderate to severe osteoarthritis of the knee. The trial was conducted in 85 centers in the United States and Canada, in accordance with the principles of good clinical practice and the Declaration of Helsinki. Eligible patients were randomized to treatment groups and self-administered oral study medication. Patients were randomized to study treatment in the order in which they were enrolled into the study by using a treatment sequence that was determined by a Searle-prepared computer-generated randomization schedule. Patients received their allocated study medications in bottles labeled A and B according to the randomization schedule. Personnel at the study centers carried out the assessments and remained blinded throughout the study. Eligible patients were enrolled and discontinued regular pain medication. Patients discontinued their normal medications at the following specified times before the baseline endoscopy: NSAIDs (including full-dose aspirin at a dosage of ≥325 mg/day) at 48 hours, corticosteroid injections at 4 weeks, and intra-articular injections of corticosteroid or hyaluronic acid preparations at 3 and 6 months, respectively. The use of antiulcer drugs, including H2 blockers, proton pump inhibitors, misoprostol, and sucralfate, was discontinued at least 24 hours before the baseline endoscopy.
Efficacy assessments
The following arthritis assessments were made at baseline and at 2, 6, and 12 weeks or at early termination after study drug administration. PaGAA or PhGAA was measured on a 5-point categorical scale, where 1 = very good, 2 = good, 3 = fair, 4 = poor, and 5 = very poor. The PAAP-VAS was measured on a scale of 0 to 100 mm, where 0 = no pain and 100 = most severe pain. The Western Ontario and McMaster’s Universities (WOMAC) Osteoarthritis indices including Pain, Stiffness, Physical Function, and Composite were measured as described previously.10
Upper gastrointestinal assessments
Upper gastrointestinal tract endoscopy was performed within 7 days before the first study dose and at the 12-week assessment or at early termination if the patient withdrew. An endoscopy could be performed at any time if the patient experienced symptoms suggestive of an ulcer. The endoscopists performing baseline and 12-week (early termination) assessments remained blinded throughout the study.
General safety assessments
Clinical laboratory tests were performed at screening, baseline, weeks 2, 6, and 12, or at early termination, and a complete physical examination was performed at screening and final visits. The incidence of adverse events occurring in each treatment arm was monitored throughout the study. Adverse events occurring within 7 days and serious adverse events occurring within 30 days of the last study dosage of medication were included in the safety analyses.
Statistical analyses
A sample size of 200 patients per treatment group was deemed sufficient to detect a difference in ulcer rates of 5% for valdecoxib vs 16% for naproxen, with 80% power and type 1 error at .017 (adjusted for 3 primary comparisons against placebo). Homogeneity of treatment groups at baseline with respect to age, height, weight, duration of osteoarthritis, PAAP-VAS, and WOMAC Osteoarthritis Index scores was assessed with 2-way analysis of variance, with treatment group and center as factors. All other demographics and baseline characteristics were compared with the Cochran-Mantel-Haenszel (CMH) test, stratified by center.
All efficacy assessments were performed on the modified intent-to-treat (ITT) cohort by using the last observation carried forward approach. The ITT cohort comprised all patients who were randomized and had taken at least 1 dose of study medication. Analyses of mean change from baseline for PaGAA, PhGAA, PAAP-VAS, and WOMAC Osteoarthritis indices were performed by using analysis of covariance, with treatment and center as factors and the corresponding baseline score as the covariate. Pairwise comparisons of valdecoxib at dosages of 10 and 20 mg once daily vs placebo were interpreted with the Hochberg procedure.11 Primary pairwise comparisons were amended in the statistical analysis plan before data unblinding to compare placebo with 10 and 20 mg valdecoxib, but not with the 5-mg dose. For all other comparisons, including 5 mg valdecoxib and naproxen vs placebo, differences were considered significant if the pairwise P values were less than .05. The incidence of withdrawal due to treatment failure was analyzed by the Fisher exact test, and the time to withdrawal in each treatment group was analyzed by log-rank test and plotted with the Kaplan-Meier product limit.12,13
Upper gastrointestinal tract endoscopic analyses were performed on the upper gastrointestinal tract ITT population. Randomized patients were included in this cohort if they received at least 1 dose of study medication and had undergone pretreatment and posttreatment endoscopies. Overall and pairwise comparisons of gastroduodenal, gastric, and duodenal ulcers and erosions were assessed with the CMH test stratified by center. The incidence of adverse events was compared between treatment groups with the Fisher exact test. Changes in vital signs were compared between treatment groups with an analysis of covariance using pairwise treatment comparisons, with treatment group as a factor and baseline value as a covariate.
Results
Patient baseline characteristics
Of the 1019 eligible randomized patients, 1 patient randomized to 10 mg/day valdecoxib, 1 to 20 mg/day valdecoxib, and 1 to 500 mg naproxen twice daily did not take the study medication and were excluded from efficacy and safety analyses. The remaining 1016 randomized patients received study medication and were included in the ITT cohort on which analyses of all efficacy end points were based. A total of 269 patients withdrew before the end of the study due to treatment failure, preexisting protocol violations, noncompliance, or adverse signs and symptoms, or were lost to follow-up: 74 patients in the placebo group, 39 in the 5-mg valdecoxib group, 56 in the 10-mg valdecoxib group, 44 in the 20-mg valdecoxib group, and 56 in the naproxen group. The upper gastrointestinal tract ITT cohort comprised 908 patients who were included in the upper gastrointestinal tract safety analyses. More than 90% of patients included in the study evaluated their osteoarthritis as poor to very poor as assessed by baseline PaGAA scores. Treatment groups were homogeneous with respect to demographics, vital signs, medical history, and all baseline arthritis assessments (Table 1).
TABLE 1
Patient baseline characteristics
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 205) | 5 mg qd (n = 201) | 10 mg qd (n = 206) | 20 mg qd (n = 202) | 500 mg bid (n = 205) | |
| Mean (SD) age, y | 60.3 (10.5) | 58.7 (11.9) | 59.8 (11.0) | 59.6 (10.4) | 60.4 (10.7) |
| Mean (SD) weight, kg | 87.5 (21.2) | 91.4 (22.6) | 89.3 (21.4) | 92.6 (23.7) | 88.1 (21.7) |
| Race, n (%) | |||||
| White | 162 (79) | 155 (77) | 154 (75) | 160 (79) | 163 (80) |
| Black | 21 (10) | 26 (13) | 24 (12) | 24 (12) | 23 (11) |
| Asian | 1 (0) | 1 (0) | 1 (0) | 1 (0) | 2 (1) |
| Hispanic | 19 (9) | 18 (9) | 25 (12) | 15 (7) | 15 (7) |
| Male sex, n (%) | 73 (36) | 73 (36) | 72 (35) | 66 (33) | 76 (37) |
| Mean (SD) disease duration, y | 8.3 (8.0) | 9.8 (9.5) | 8.7 (8.0) | 9.2 (8.0) | 9.4 (8.7) |
| History of GI bleeding, n (%) | 2 (1) | 0 (0) | 3 (1) | 2 (1) | 3 (1) |
| History of gastroduodenal ulcer, n (%) | 20 (10) | 21 (10) | 24 (12) | 28 (14) | 31 (15) |
| PaGAA, n (%) | |||||
| Poor | 168 (82) | 175 (87) | 168 (82) | 162 (80) | 169 (82) |
| Very poor | 33 (16) | 23 (11) | 32 (16) | 36 (18) | 31 (15) |
| PhGAA, n (%) | |||||
| Poor | 179 (87) | 181 (90) | 176 (85) | 173 (86) | 175 (85) |
| Very poor | 24 (12) | 18 (9) | 25 (12) | 24 (12) | 25 (12) |
| No significant differences were observed between treatment groups at any baseline characteristic. | |||||
| bid, twice daily; GI, gastrointestinal; PaGAA, Patient’s Global Assessment of Arthritis; PhGAA, Physician’s Global Assessment of Arthritis; qd, once daily. | |||||
Efficacy
The least square mean change in the PaGAA was significantly improved at most assessments in response to valdecoxib (10 and 20 mg/day) and 500 mg naproxen twice daily compared with placebo (Table 2). However, the improvement in response to valdecoxib 5 mg qd did not reach statistical significance (Table 2). Significant improvements in the PhGAA were observed in response to valdecoxib and naproxen at all assessments (Table 2).
The dosages of 20 mg/day valdecoxib and 500 mg naproxen twice daily were associated with a reduction in pain, as assessed by the PAAP-VAS scores. Pain reduction associated with 5 and 10 mg/day valdecoxib was significantly better than that with placebo at all assessments except for week 12 (Table 2).
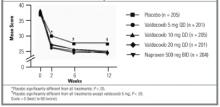
Valdecoxib and naproxen treatments improved the WOMAC Pain, Stiffness, Physical Function, and Composite indices compared with placebo at 2, 6, and 12 weeks. Valdecoxib 20 mg/day and naproxen 500 mg twice daily produced statistically significant changes in all WOMAC Osteoarthritis scores throughout the 12-week study period compared with placebo (P < .05). WOMAC Pain scores for 10 mg valdecoxib were significantly different from those for placebo at 2 weeks (P < .001) but not at 6 or 12 weeks. No significant differences were noted between any of the valdecoxib treatment doses and naproxen in terms of improvement in WOMAC indices.
The incidences of withdrawal due to treatment failure were 20% (95% confidence interval [CI], 15.3–26.8) in the placebo group; 8% (95% CI, 4.8–12.8), 12% (95% CI, 7.8–17.1), and 10% (95% CI, 6.3–15.2) in the 5-, 10-, and 20-mg/day valdecoxib groups; and 6% (95% CI, 3.6–10.9) in the 500-mg naproxen group (P < .05; Table 3). Patients in the placebo group withdrew at a significantly faster rate than those in the 4 active treatment groups (P < .05), but there were no significant differences in withdrawal rates across the 4 active treatment groups.
TABLE 2
Baseline arthritis assessments and mean changes from baseline scores
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 205) | 5 mg qd (n = 201) | 10 mg qd (n = 205) | 20 mg qd (n = 201) | 500 mg bid (n = 204) | |
| PhGAA§ | |||||
| Baseline mean | 4.10 | 4.07 | 4.09 | 4.09 | 4.10 |
| LSM change | |||||
| Week 2 (CI) | -1.04 (-1.16, -0.91) | -1.31‡(-1.44, -1.19) | -1.37‡(-1.50, -1.25) | -1.42‡(-1.54, -1.29) | -1.35‡(-1.48, -1.23) |
| Week 6 (CI) | -1.22 (-1.35, -1.08) | -1.44*(-1.58, -1.31) | -1.50†(-1.63, -1.36) | -1.41* (-1.55, -1.28) | -1.45* (-1.59, -1.32) |
| Week 12 (CI) | -1.22 (-1.36, -1.08) | -1.43* (-1.58, -1.28) | -1.52†(-1.67, -1.38) | -1.45* (-1.60, -1.31) | -1.43* (-1.58, -1.29) |
| PAAP║ | |||||
| Baseline mean | 71.20 | 71.42 | 72.41 | 72.54 | 72.36 |
| LSM change | |||||
| Week 2 (CI) | -21.19 (-24.80, -17.58) | -28.46†(-32.11, -24.82) | -30.21‡(-33.83, -26.59) | -32.07‡(-35.73, -28.41) | -31.03‡(-34.66, -27.40) |
| Week 6 (CI) | -23.92 (-27.72, -20.12) | -30.81†(-34.65, -26.97) | -29.85* (-33.67, -26.04) | -32.28†(-36.13, -28.42) | -31.84†(-35.66, -28.02) |
| Week 12 (CI) | -25.97 (-30.02, -21.92) | -31.33 (-35.42, -27.24) | -30.41 (-34.47, -30.41) | -32.70* (-36.81, -32.70) | -31.83* (-35.90, -27.76) |
| WOMAC OA, Stiffness ¶ | |||||
| Baseline mean | 4.84 | 4.87 | 4.91 | 4.73 | 4.94 |
| LSM change | |||||
| Week 2 (CI) | -0.78 (-0.98, -0.57) | -1.03 (-1.24, -0.82) | -1.20†(-1.41, -0.99) | -1.24†(-1.45, -1.03) | -1.28‡(-1.49, -1.08) |
| Week 6 (CI) | -1.04 (-1.27, -0.82) | -1.25 (-1.48, -1.02) | -1.42* (-1.65, -1.20) | -1.43* (-1.66, -1.20) | -1.40†(-1.62, -1.17) |
| Week 12 (CI) | -1.12 (-1.36, -0.89) | -1.33 (-1.57, -1.09) | -1.41 (-1.65, -1.17) | -1.46* (-1.70, -1.22) | -1.54* (-1.78, -1.30) |
| WOMAC OA, Composite # | |||||
| Baseline mean | 53.49 | 53.03 | 54.73 | 53.42 | 53.67 |
| LSM change | |||||
| Week 2 (CI) | -10.13 (-12.28, -7.99) | -13.26* (-15.42, -11.09) | -15.05‡(-17.20, -12.90) | -15.44‡(-17.63, -13.32) | -15.47‡(-17.63, -13.32) |
| Week 6 (CI) | -12.98 (-15.45, -10.51) | -15.47 (-17.97, -12.98) | -16.74* (-19.22, -14.26) | -17.33* (-19.48, -14.51) | -16.99* (-19.48, -14.51) |
| Week 12 (CI) | -13.48 (-16.07, -10.89) | -16.84 (-19.46, -14.23) | -17.34* (-19.93, -14.74) | -17.22* (-20.64, -15.44) | -18.04* (-20.64, -15.44) |
| *P < .05 vs placebo, significant. | |||||
| † P < .01 vs placebo, significant. | |||||
| ‡ P < .001 vs placebo, significant. | |||||
| § Scale = 1 (very good) to 5 (very poor). | |||||
| ║ Scale = 0 mm (no pain) to 100 mm (most severe pain). | |||||
| ¶ Scale = 0 (no symptoms) to 8 (worse symptoms). | |||||
| # Scale = 0 (no symptoms) to 96 (worse symptoms). | |||||
| bid, twice daily; CI, 95% confidence interval; LSM, least square mean; PAAP, Patient’s Assessment of Arthritis Pain; PhGAA, Physician’s Global Assessment of Arthritis; qd, once daily; WOMAC OA, Western Ontario and McMaster’s Universities Osteoarthritis Index. | |||||
TABLE 3
Incidence of gastroduodenal, gastric, and duodenal ulcers (>5 mm) at final endoscopic evaluation
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 178) | 5 mg qd (n = 188) | 10 mg qd (n = 174) | 20 mg qd (n = 185) | 500 mg bid (n = 183) | |
| Gastroduodenal§ | 8 (4) [2.1, 9.0] | 6 (3)† [1.3, 7.1] | 5 (3)† [1.1, 6.9] | 10 (5) [2.8, 10.0] | 18 (10)* [6.1, 15.3] |
| Gastric§ | 8 (4) [2.1, 9.0] | 4 (2)‡ [0.7, 5.7] | 3 (2)‡ [0.4, 5.4] | 9 (5) [2.4, 9.3] | 16 (9) [5.2, 14.1] |
| Duodenal§ | 0 (0) [0.05, 2.6] | 2 (1) [0.2, 4.2] | 2 (1) [0.2, 4.5] | 1 (1) [0.0, 3.4] | 2 (1) [0.2, 4.3] |
| Symptomatic ulcers (n) | 0 | 1 | 2 | 3 | 7 |
| *P < .05 vs placebo. | |||||
| † P < .05 vs naproxen. | |||||
| ‡ P < .01 vs naproxen. | |||||
| § Data are presented as n (%) [95% confidence interval]. | |||||
| bid, twice daily; qd, once daily. | |||||
Safety
Valdecoxib and placebo had comparable upper gastrointestinal tract ulceration rates, whereas naproxen produced a significantly higher incidence of upper gastrointestinal tract ulcers than did 5 and 10 mg valdecoxib and placebo (P < .05). There were 14 adjudicated symptomatic ulcers during the study: 1 in the 5-mg valdecoxib group, 2 in the 10-mg valdecoxib group, 3 in the 20-mg valdecoxib group, and 7 in the 500-mg naproxen group.
Adverse events with an incidence of at least 5% in any treatment group and adverse events leading to withdrawal from the study are summarized by body system in Table 4. There were no significant differences in the incidence of adverse events between the valdecoxib and placebo groups. In contrast, 500 mg naproxen twice daily was associated with significantly more adverse events than 5 or 10 mg/day valdecoxib (P < .05). The incidence of adverse events was similar in the 20-mg valdecoxib and naproxen groups. Most adverse events were reported in the gastrointestinal system and consisted of abdominal pain, constipation, diarrhea, dyspepsia, flatulence, and nausea. The incidences of constipation, diarrhea, and flatulence were significantly higher in the naproxen group than in the 5-, 10-, and 20-mg valdecoxib groups, respectively. Other adverse events included accidental injury, headache, myalgia, and upper respiratory tract infections. Valdecoxib at 5 mg/day produced a significantly higher incidence of myalgia than did placebo, and valdecoxib at 20 mg/day produced a significantly lower incidence of upper respiratory tract infections than did placebo. Adverse events causing withdrawal with an incidence of at least 1% were accidental injury, abdominal pain, diarrhea, dyspepsia, nausea, abnormal hepatic function, rash, and blurred vision. The proportion of patients in the naproxen group (12.7%) who withdrew from the study was significantly greater than those for the 5-and 20-mg valdecoxib (6.0% and 5.5%) groups (P < .05), although the incidence of withdrawal due to adverse events in the 10-mg valdecoxib and naproxen groups were similar. In addition, gastrointestinal adverse events commonly related to NSAID treatment, such as dyspepsia and constipation, were more frequent in the naproxen group than in the valdecoxib and placebo groups.
TABLE 4
Adverse events
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 178) | 5 mg qd (n = 188) | 10 mg qd (n = 174) | 20 mg qd (n = 185) | 500 mg bid (n = 183) | |
| Incidence ≥ 5% in any treatment group | |||||
| Total | 109 (53.2) | 112 (55.7)† | 113 (55.1)† | 121 (60.2) | 139 (68.1)* |
| Accidental injury | 11 (5.4) | 3 (1.5) | 10 (4.9) | 12 (6.0) | 9 (4.4) |
| Headache | 11 (5.4) | 12 (6.0) | 7 (3.4) | 14 (7.0) | 9 (4.4) |
| Abdominal pain | 19 (9.3) | 14 (7.0) | 18 (8.8) | 13 (6.5) | 25 (12.3) |
| Constipation | 6 (2.9) | 4 (2.0) | 1 (0.5)† | 4 (2.0) | 12 (5.9) |
| Diarrhea | 10 (4.9) | 7 (3.5) | 14 (6.8) | 11 (5.5) | 12 (5.9) |
| Dyspepsia | 15 (7.3) | 22 (10.9) | 22 (10.7) | 20 (9.9)† | 35 (17.2)* |
| Flatulence | 12 (5.9) | 7 (3.5) | 5 (2.4)† | 9 (4.5) | 14 (6.9) |
| Nausea | 10 (4.9) | 18 (9.0) | 17 (8.3) | 9 (4.5) | 10 (4.9) |
| Myalgia | 0 (0.0) | 13 (6.5)*† | 3 (1.5) | 2 (1.0) | 1 (0.5) |
| Upper respiratory tract infections | 18 (8.8) | 9 (4.5) | 10 (4.9) | 7 (3.5)* | 10 (4.9) |
| Incidence ≥ 1% in any treatment group causing withdrawal | |||||
| Total | 17 (8.3) | 12 (6.0)† | 18 (8.8) | 11 (5.5)† | 26 (12.7) |
| Accidental injury | 2 (1.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) |
| Abdominal pain | 5 (2.4) | 2 (1.0) | 6 (2.9) | 2 (1.0) | 7 (3.4) |
| Diarrhea | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) | 3 (1.5) |
| Dyspepsia | 2 (1.0) | 2 (1.0) | 3 (1.5) | 1 (0.5)† | 9 (4.4)* |
| Nausea | 2 (1.0) | 1 (0.5) | 2 (1.0) | 1 (0.5) | 2 (1.0) |
| Abnormal hepatic function | 0 (0.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash | 0 (0.0) | 2 (1.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Blurred vision | 2 (1.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| *P < .05 vs placebo. | |||||
| † P < .05 vs naproxen. | |||||
| ‡ Data are presented as number (%) of patients reporting events. | |||||
| bid, twice daily; qd, once daily. | |||||
FIGURE 2
Western Ontario and McMaster’s Universities Osteoarthritis Pain Index
Discussion
This study confirmed that the novel COX-2–specific inhibitor valdecoxib at a dosage of 10 or 20 mg/day is as effective as naproxen at a dosage of 500 mg twice daily in relieving moderate to severe osteoarthritis of the knee over 12 weeks. In addition, treatment with 10 mg/day valdecoxib orally, the recommended dosage for treatment of osteoarthritis, is associated with a significantly lower gastroduodenal ulceration rate than occurs with the conventional NSAID, naproxen.
Patients receiving 10 and 20 mg/day valdecoxib experienced significant improvements in the signs and symptoms of osteoarthritis, and in all assessments the efficacies of valdecoxib 10 and 20 mg/day were numerically similar to that of naproxen. This finding is consistent with the inhibition of prostaglandin production in inflamed synovial tissue and in the central pain pathway. Increased COX-2 activity in the spinal cord in response to tissue damage and in the synovial membrane of osteoarthritis patients is at least partly responsible for joint inflammation and sensitization to inflammatory pain.14–16 The efficacy of valdecoxib in treating moderate to severe osteoarthritis of the knee was consistent with reports of other COX-2–specific inhibitors that are comparable to conventional NSAIDs in relieving chronic pain and inflammation.17,18 These data confirmed that 10 mg/day valdecoxib is as effective as 500 mg naproxen twice daily in treating the pain and inflammation associated with osteoarthritis. The efficacy of 10 mg/day valdecoxib makes it one of the most potent COX-2–specific inhibitors for treating moderate to severe osteoarthritis.
Conventional NSAIDs were associated with a significant risk of serious gastrointestinal complications such as ulceration and perforation and low gastrointestinal tolerability.20–22 Naproxen treatment of osteoarthritis and rheumatoid arthritis demonstrated a higher rate of endoscopically proven gastrointestinal ulceration than did COX-2–specific inhibitors,17,23 and that finding was confirmed in this study for 10 mg valdecoxib. Naproxen treatment was associated with significantly more gastroduodenal ulcers than 5 or 10 mg valdecoxib. We found no significant difference between 20 mg valdecoxib and naproxen, which might be explained by a lower incidence of ulcers with naproxen than reported in previous studies.24 In terms of numbers needed to treat, 14 patients would be needed to observe a difference in endoscopic ulcer rates between valdecoxib (5 or 10 mg) and naproxen compared with 20 patients to observe a difference between 20 mg valdecoxib and naproxen and 16 to observe a difference in ulcer rates between naproxen and placebo.
Valdecoxib at a dosage of 10 mg/day also demonstrated overall improved gastrointestinal tolerability, with significantly fewer adverse events and withdrawals due to adverse events, in particular gastrointestinal-related events such as constipation and dyspepsia, than did naproxen. The improved upper gastrointestinal tract safety of valdecoxib was as expected because the COX-1–sparing nature of this agent allows effective inhibition of COX-2 without inhibiting COX-1 in the gastric mucosa and platelets. An improved gastrointestinal safety profile is an important consideration in the treatment of osteoarthritis because the moderate to severe gastrointestinal complications associated with conventional NSAID therapy frequently lead to poor patient compliance or discontinuation of the medication.25,26
Overall, this study suggests clinical benefits of single daily doses of 10 and 20 mg valdecoxib and improved upper gastrointestinal tract safety for the 10-mg dose, compared with 500 mg/day naproxen. No additional efficacy benefit was obtained from a 20-mg dose as opposed to a 10-mg dose. Valdecoxib (10 mg) is a potent and effective once-daily alternative to conventional NSAIDs, with a gastrointestinal safety advantage that will be of value to rheumatologists and primary care physicians alike.
FIGURE 3
Western Ontario and Western Universities Osteoarthritis Physical Function Index
1. Klippel J, et al. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001.
2. Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev 1988;10:1-28.
3. Borda IT, Koff R. NSAIDs: A Profile of Adverse Effects. Philadelphia: Hanley and Belfus; 1995.
4. Bensen WG, Fiechtner JJ, McMillen JI, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999;74:1095-105.
5. Geis GS. Update on clinical developments with celecoxib, a new specific COX-2 inhibitor: what can we expect? J Rheumatol 1999;26(suppl 56):31-6.
6. Altman R, Asch E, Bloch G, et al. The American College of Rheumatology criteria for the classification and reportings of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039-49.
7. Schumacher HR. Primer on the Rheumatic Diseases. Atlanta, GA: Arthritis Foundation; 1986.
8. Cooperating Clinics Committee of American Rheumatism Association. A seven day variability study of 499 patients with peripheral rheumatoid arthritis. Arthritis Rheum 1965;8:302-34.
9. Ward JR, Williams HJ, Boyce E, et al. Comparison of auranofin, gold sodium thiomalate, and placebo in the treatment of rheumatoid arthritis. Subsets of responses. Am J Med 1983;75:133-7.
10. Bellamy N. WOMAC Osteoarthritis Index: A User’s Guide. London, Ontario, Canada: The Western Ontario and McMaster Universities; 1995.
11. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1998;75:800-2.
12. Miller R. Survival Analyses. New York: John Wiley & Sons; 1998.
13. Simon R, Lee YJ. Nonparametric confidence limits for survival probabilities and median survival time. Cancer Treat Rep 1982;66:37-42.
14. Amin AR, Attur M, Patel RN, et al. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest 1997;99:1231-7.
15. Hay C, de Belleroche J. Carrageenan-induced hyperalgesia is associated with increased cyclooxygenase-2 expression in spinal cord. Neuroreport 1997;8:1249-51.
16. Kang RY, Freire Moar, Sigal E, et al. Expression of cyclooxygenase-2 in human and an animal model of rheumatoid arthritis. Br J Rheumatol 1996;35:711-8.
17. Bensen WG, Zhao SZ, Burke TA, et al. Upper gastrointestinal tolerability of celecoxib, a COX-2 specific inhibitor, compared to naproxen and placebo. J Rheumatol 2000;27:1876-83.
18. Day R, Morrison B, Luza A, et al. A randomized trial of the efficacy and tolerability of the COX-2 inhibitor rofecoxib vs ibuprofen in patients with osteoarthritis. Rofecoxib/Ibuprofen Comparator Study Group. Arch Intern Med 2000;160:1781-7.
19. Fiechtner J, Sikes D, Recker D. A double-blind, placebo-controlled dose ranging study to evaluate the efficacy of valdecoxib, a novel COX-2 specific inhibitor, in treating the signs and symptoms of osteoarthritis of the knee. Paper presented at: European League Against Rheumatism (EULAR); May 13–16, 2001; Prague, Czech Republic.
20. Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual nonsteroidal anti-inflammatory drugs. Lancet 1994;343:769-72.
21. Singh G, Ramey DR, Morfeld D, et al. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Intern Med 1996;156:1530-6.
22. Singh G, Rosen Ramey D. NSAID induced gastrointestinal complications: the ARAMIS perspective-1997. Arthritis, Rheumatism, and Aging Medical Information System. J Rheumatol 1998;51(suppl):8-16.
23. Watson DJ, Harper SE, Zhao PL, et al. Gastrointestinal tolerability of the selective cyclooxygenase-2 (COX-2) inhibitor rofecoxib compared with nonselective COX-1 and COX-2 inhibitors in osteoarthritis. Arch Intern Med 2000;160:2998-3003.
24. Simon LS, Weaver AL, Graham DY, et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA 1999;282:1921-8.
25. Langman MJ, Jensen DM, Watson DJ, et al. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA 1999;282:1929-33.
26. Scholes D, Stergachis A, Penna P, Normand E, Hansten P. Nonsteroidal anti-inflammatory drug discontinuation in patients with osteoarthritis. J Rheumatol 1995;22:708-12.
OBJECTIVE: We compared the efficacy and upper gastrointestinal safety of the cyclooxygenase-2–specific inhibitor valdecoxib with naproxen and placebo in treating moderate to severe osteoarthritis of the knee.
STUDY DESIGN: This multicenter, randomized, double-blind, placebo-controlled study compared the efficacy and upper gastrointestinal tract safety of valdecoxib at dosages of 5, 10, and 20 mg once daily with placebo and naproxen at the dosage of 500 mg twice daily.
POPULATION: We included patients who had been diagnosed with moderate to severe osteoarthritis of the knee according to the modified criteria of the American College of Rheumatology.
OUTCOMES MEASURED: The Patient’s and Physician’s Global Assessment of Arthritis (PaGAA, PhGAA), Patient’s Assessment of Arthritis Pain–Visual Analog Scale (PAAP-VAS), and Western Ontario and McMaster’s Universities (WOMAC) Osteoarthritis indices were assessed at baseline and at weeks 2, 6, and 12. Upper gastrointestinal ulceration was assessed by pre- and posttreatment endoscopies.
RESULTS: Valdecoxib 10 and 20 mg once daily (but not 5 mg once daily) demonstrated similar efficacy to naproxen at 500 mg twice daily, and all 3 dosages were superior to placebo for the PaGAA, PhGAA, PAAP-VAS, and WOMAC Osteoarthritis indices at most assessments throughout the 12-week study (P < .05). The incidence of endoscopically proven ulcers was significantly higher in the naproxen group than in the 5- and 10-mg valdecoxib groups, but not in the 20-mg valdecoxib group. All 3 valdecoxib doses were comparable to placebo in ulcer incidence.
CONCLUSIONS: Valdecoxib (10 and 20 mg once daily) is significantly superior to placebo and as effective as naproxen (500 mg twice daily) in improving moderate to severe osteoarthritis of the knee. Upper gastrointestinal tract safety of valdecoxib (5 and 10 mg) was comparable to that of placebo and significantly better than that of naproxen.
- The cyclooxygenase-2–specific inhibitor valdecoxib 10 or 20 mg once daily is as effective as naproxen 500 mg twice daily.
- Valdecoxib at the recommended dose for treatment of osteoarthritis (10 mg once daily) had better upper gastrointestinal safety than naproxen.
Current medical therapies for osteoarthritis include conventional nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, glucosamine sulfate, and intra-articular injections of corticosteroids and hyaluronic acid. However, long-term use of corticosteroid injections can exacerbate damage to the affected joints.1,2 Conventional NSAIDs are associated with upper gastrointestinal tract ulceration and inhibition of platelet function.3
Cyclooxygenase-2 (COX-2)–specific inhibitors have demonstrated equivalent efficacy to conventional NSAIDs in treating pain and inflammation associated with osteoarthritis and rheumatoid arthritis. Further, COX-2–specific inhibitors significantly reduce the incidence of gastrointestinal ulceration and bleeding side effects caused by conventional NSAIDs.4,5 Valdecoxib (Bextra; Pharmacia Corporation and Pfizer Corporation) is a novel COX-2–specific inhibitor that is approximately 28,000-fold more selective against COX-2 than against COX-1. As a potent COX-2–specific inhibitor, valdecoxib is expected to provide efficacy equivalent to conventional NSAIDs for treatment of arthritis and spare the COX-1–related side effects. This randomized, placebo-controlled, double-blind, 12-week study was designed to test this hypothesis by comparing the efficacy and upper gastrointestinal tract safety of valdecoxib with that of naproxen, a leading conventional NSAID comparator.
Methods
Study population
Ambulatory adults who had been diagnosed with moderate to severe osteoarthritis of the knee according to the modified criteria of the American College of Rheumatology6,7 were eligible to participate in the trial. Patients were recruited from primary care and rheumatology specialty settings. Patients who had baseline scores of at least 40 mm on the Patient’s Assessment of Arthritis Pain–Visual Analog Scale (PAAP-VAS) and baseline categorical scores of poor to very poor on the Patient’s (PaGAA) and Physician’s (PhGAA) Global Assessments of Arthritis were included.8,9 Any patient suffering from inflammatory arthritis, gout, pseudogout, Paget disease, or any chronic pain syndrome that might interfere with assessment of the Index Knee was excluded from the trial. Patients diagnosed with osteoarthritis of the hip ipsilateral to the Index Knee, severe anserine bursitis, acute joint trauma, or complete loss of articular cartilage on the Index Knee also were excluded. Patients were not eligible if they had active gastrointestinal disease, gastrointestinal tract ulceration 30 days before the trial, a significant bleeding disorder, or a history of gastric or duodenal surgery. Patients with an esophageal, gastric, pyloric channel, or duodenal ulcer or a score of at least 10 for esophageal, gastric, or duodenal erosions at the pretreatment endoscopy examination also were excluded.
FIGURE 1
Patient’s global assessment of arthritis
Study design
This multicenter, randomized, double-blind, placebo-controlled study compared the efficacy and upper gastrointestinal tract safety of valdecoxib at dosages of 5, 10, and 20 mg once daily with placebo and naproxen at a dosage of 500 mg twice daily in relieving moderate to severe osteoarthritis of the knee. The trial was conducted in 85 centers in the United States and Canada, in accordance with the principles of good clinical practice and the Declaration of Helsinki. Eligible patients were randomized to treatment groups and self-administered oral study medication. Patients were randomized to study treatment in the order in which they were enrolled into the study by using a treatment sequence that was determined by a Searle-prepared computer-generated randomization schedule. Patients received their allocated study medications in bottles labeled A and B according to the randomization schedule. Personnel at the study centers carried out the assessments and remained blinded throughout the study. Eligible patients were enrolled and discontinued regular pain medication. Patients discontinued their normal medications at the following specified times before the baseline endoscopy: NSAIDs (including full-dose aspirin at a dosage of ≥325 mg/day) at 48 hours, corticosteroid injections at 4 weeks, and intra-articular injections of corticosteroid or hyaluronic acid preparations at 3 and 6 months, respectively. The use of antiulcer drugs, including H2 blockers, proton pump inhibitors, misoprostol, and sucralfate, was discontinued at least 24 hours before the baseline endoscopy.
Efficacy assessments
The following arthritis assessments were made at baseline and at 2, 6, and 12 weeks or at early termination after study drug administration. PaGAA or PhGAA was measured on a 5-point categorical scale, where 1 = very good, 2 = good, 3 = fair, 4 = poor, and 5 = very poor. The PAAP-VAS was measured on a scale of 0 to 100 mm, where 0 = no pain and 100 = most severe pain. The Western Ontario and McMaster’s Universities (WOMAC) Osteoarthritis indices including Pain, Stiffness, Physical Function, and Composite were measured as described previously.10
Upper gastrointestinal assessments
Upper gastrointestinal tract endoscopy was performed within 7 days before the first study dose and at the 12-week assessment or at early termination if the patient withdrew. An endoscopy could be performed at any time if the patient experienced symptoms suggestive of an ulcer. The endoscopists performing baseline and 12-week (early termination) assessments remained blinded throughout the study.
General safety assessments
Clinical laboratory tests were performed at screening, baseline, weeks 2, 6, and 12, or at early termination, and a complete physical examination was performed at screening and final visits. The incidence of adverse events occurring in each treatment arm was monitored throughout the study. Adverse events occurring within 7 days and serious adverse events occurring within 30 days of the last study dosage of medication were included in the safety analyses.
Statistical analyses
A sample size of 200 patients per treatment group was deemed sufficient to detect a difference in ulcer rates of 5% for valdecoxib vs 16% for naproxen, with 80% power and type 1 error at .017 (adjusted for 3 primary comparisons against placebo). Homogeneity of treatment groups at baseline with respect to age, height, weight, duration of osteoarthritis, PAAP-VAS, and WOMAC Osteoarthritis Index scores was assessed with 2-way analysis of variance, with treatment group and center as factors. All other demographics and baseline characteristics were compared with the Cochran-Mantel-Haenszel (CMH) test, stratified by center.
All efficacy assessments were performed on the modified intent-to-treat (ITT) cohort by using the last observation carried forward approach. The ITT cohort comprised all patients who were randomized and had taken at least 1 dose of study medication. Analyses of mean change from baseline for PaGAA, PhGAA, PAAP-VAS, and WOMAC Osteoarthritis indices were performed by using analysis of covariance, with treatment and center as factors and the corresponding baseline score as the covariate. Pairwise comparisons of valdecoxib at dosages of 10 and 20 mg once daily vs placebo were interpreted with the Hochberg procedure.11 Primary pairwise comparisons were amended in the statistical analysis plan before data unblinding to compare placebo with 10 and 20 mg valdecoxib, but not with the 5-mg dose. For all other comparisons, including 5 mg valdecoxib and naproxen vs placebo, differences were considered significant if the pairwise P values were less than .05. The incidence of withdrawal due to treatment failure was analyzed by the Fisher exact test, and the time to withdrawal in each treatment group was analyzed by log-rank test and plotted with the Kaplan-Meier product limit.12,13
Upper gastrointestinal tract endoscopic analyses were performed on the upper gastrointestinal tract ITT population. Randomized patients were included in this cohort if they received at least 1 dose of study medication and had undergone pretreatment and posttreatment endoscopies. Overall and pairwise comparisons of gastroduodenal, gastric, and duodenal ulcers and erosions were assessed with the CMH test stratified by center. The incidence of adverse events was compared between treatment groups with the Fisher exact test. Changes in vital signs were compared between treatment groups with an analysis of covariance using pairwise treatment comparisons, with treatment group as a factor and baseline value as a covariate.
Results
Patient baseline characteristics
Of the 1019 eligible randomized patients, 1 patient randomized to 10 mg/day valdecoxib, 1 to 20 mg/day valdecoxib, and 1 to 500 mg naproxen twice daily did not take the study medication and were excluded from efficacy and safety analyses. The remaining 1016 randomized patients received study medication and were included in the ITT cohort on which analyses of all efficacy end points were based. A total of 269 patients withdrew before the end of the study due to treatment failure, preexisting protocol violations, noncompliance, or adverse signs and symptoms, or were lost to follow-up: 74 patients in the placebo group, 39 in the 5-mg valdecoxib group, 56 in the 10-mg valdecoxib group, 44 in the 20-mg valdecoxib group, and 56 in the naproxen group. The upper gastrointestinal tract ITT cohort comprised 908 patients who were included in the upper gastrointestinal tract safety analyses. More than 90% of patients included in the study evaluated their osteoarthritis as poor to very poor as assessed by baseline PaGAA scores. Treatment groups were homogeneous with respect to demographics, vital signs, medical history, and all baseline arthritis assessments (Table 1).
TABLE 1
Patient baseline characteristics
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 205) | 5 mg qd (n = 201) | 10 mg qd (n = 206) | 20 mg qd (n = 202) | 500 mg bid (n = 205) | |
| Mean (SD) age, y | 60.3 (10.5) | 58.7 (11.9) | 59.8 (11.0) | 59.6 (10.4) | 60.4 (10.7) |
| Mean (SD) weight, kg | 87.5 (21.2) | 91.4 (22.6) | 89.3 (21.4) | 92.6 (23.7) | 88.1 (21.7) |
| Race, n (%) | |||||
| White | 162 (79) | 155 (77) | 154 (75) | 160 (79) | 163 (80) |
| Black | 21 (10) | 26 (13) | 24 (12) | 24 (12) | 23 (11) |
| Asian | 1 (0) | 1 (0) | 1 (0) | 1 (0) | 2 (1) |
| Hispanic | 19 (9) | 18 (9) | 25 (12) | 15 (7) | 15 (7) |
| Male sex, n (%) | 73 (36) | 73 (36) | 72 (35) | 66 (33) | 76 (37) |
| Mean (SD) disease duration, y | 8.3 (8.0) | 9.8 (9.5) | 8.7 (8.0) | 9.2 (8.0) | 9.4 (8.7) |
| History of GI bleeding, n (%) | 2 (1) | 0 (0) | 3 (1) | 2 (1) | 3 (1) |
| History of gastroduodenal ulcer, n (%) | 20 (10) | 21 (10) | 24 (12) | 28 (14) | 31 (15) |
| PaGAA, n (%) | |||||
| Poor | 168 (82) | 175 (87) | 168 (82) | 162 (80) | 169 (82) |
| Very poor | 33 (16) | 23 (11) | 32 (16) | 36 (18) | 31 (15) |
| PhGAA, n (%) | |||||
| Poor | 179 (87) | 181 (90) | 176 (85) | 173 (86) | 175 (85) |
| Very poor | 24 (12) | 18 (9) | 25 (12) | 24 (12) | 25 (12) |
| No significant differences were observed between treatment groups at any baseline characteristic. | |||||
| bid, twice daily; GI, gastrointestinal; PaGAA, Patient’s Global Assessment of Arthritis; PhGAA, Physician’s Global Assessment of Arthritis; qd, once daily. | |||||
Efficacy
The least square mean change in the PaGAA was significantly improved at most assessments in response to valdecoxib (10 and 20 mg/day) and 500 mg naproxen twice daily compared with placebo (Table 2). However, the improvement in response to valdecoxib 5 mg qd did not reach statistical significance (Table 2). Significant improvements in the PhGAA were observed in response to valdecoxib and naproxen at all assessments (Table 2).
The dosages of 20 mg/day valdecoxib and 500 mg naproxen twice daily were associated with a reduction in pain, as assessed by the PAAP-VAS scores. Pain reduction associated with 5 and 10 mg/day valdecoxib was significantly better than that with placebo at all assessments except for week 12 (Table 2).
Valdecoxib and naproxen treatments improved the WOMAC Pain, Stiffness, Physical Function, and Composite indices compared with placebo at 2, 6, and 12 weeks. Valdecoxib 20 mg/day and naproxen 500 mg twice daily produced statistically significant changes in all WOMAC Osteoarthritis scores throughout the 12-week study period compared with placebo (P < .05). WOMAC Pain scores for 10 mg valdecoxib were significantly different from those for placebo at 2 weeks (P < .001) but not at 6 or 12 weeks. No significant differences were noted between any of the valdecoxib treatment doses and naproxen in terms of improvement in WOMAC indices.
The incidences of withdrawal due to treatment failure were 20% (95% confidence interval [CI], 15.3–26.8) in the placebo group; 8% (95% CI, 4.8–12.8), 12% (95% CI, 7.8–17.1), and 10% (95% CI, 6.3–15.2) in the 5-, 10-, and 20-mg/day valdecoxib groups; and 6% (95% CI, 3.6–10.9) in the 500-mg naproxen group (P < .05; Table 3). Patients in the placebo group withdrew at a significantly faster rate than those in the 4 active treatment groups (P < .05), but there were no significant differences in withdrawal rates across the 4 active treatment groups.
TABLE 2
Baseline arthritis assessments and mean changes from baseline scores
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 205) | 5 mg qd (n = 201) | 10 mg qd (n = 205) | 20 mg qd (n = 201) | 500 mg bid (n = 204) | |
| PhGAA§ | |||||
| Baseline mean | 4.10 | 4.07 | 4.09 | 4.09 | 4.10 |
| LSM change | |||||
| Week 2 (CI) | -1.04 (-1.16, -0.91) | -1.31‡(-1.44, -1.19) | -1.37‡(-1.50, -1.25) | -1.42‡(-1.54, -1.29) | -1.35‡(-1.48, -1.23) |
| Week 6 (CI) | -1.22 (-1.35, -1.08) | -1.44*(-1.58, -1.31) | -1.50†(-1.63, -1.36) | -1.41* (-1.55, -1.28) | -1.45* (-1.59, -1.32) |
| Week 12 (CI) | -1.22 (-1.36, -1.08) | -1.43* (-1.58, -1.28) | -1.52†(-1.67, -1.38) | -1.45* (-1.60, -1.31) | -1.43* (-1.58, -1.29) |
| PAAP║ | |||||
| Baseline mean | 71.20 | 71.42 | 72.41 | 72.54 | 72.36 |
| LSM change | |||||
| Week 2 (CI) | -21.19 (-24.80, -17.58) | -28.46†(-32.11, -24.82) | -30.21‡(-33.83, -26.59) | -32.07‡(-35.73, -28.41) | -31.03‡(-34.66, -27.40) |
| Week 6 (CI) | -23.92 (-27.72, -20.12) | -30.81†(-34.65, -26.97) | -29.85* (-33.67, -26.04) | -32.28†(-36.13, -28.42) | -31.84†(-35.66, -28.02) |
| Week 12 (CI) | -25.97 (-30.02, -21.92) | -31.33 (-35.42, -27.24) | -30.41 (-34.47, -30.41) | -32.70* (-36.81, -32.70) | -31.83* (-35.90, -27.76) |
| WOMAC OA, Stiffness ¶ | |||||
| Baseline mean | 4.84 | 4.87 | 4.91 | 4.73 | 4.94 |
| LSM change | |||||
| Week 2 (CI) | -0.78 (-0.98, -0.57) | -1.03 (-1.24, -0.82) | -1.20†(-1.41, -0.99) | -1.24†(-1.45, -1.03) | -1.28‡(-1.49, -1.08) |
| Week 6 (CI) | -1.04 (-1.27, -0.82) | -1.25 (-1.48, -1.02) | -1.42* (-1.65, -1.20) | -1.43* (-1.66, -1.20) | -1.40†(-1.62, -1.17) |
| Week 12 (CI) | -1.12 (-1.36, -0.89) | -1.33 (-1.57, -1.09) | -1.41 (-1.65, -1.17) | -1.46* (-1.70, -1.22) | -1.54* (-1.78, -1.30) |
| WOMAC OA, Composite # | |||||
| Baseline mean | 53.49 | 53.03 | 54.73 | 53.42 | 53.67 |
| LSM change | |||||
| Week 2 (CI) | -10.13 (-12.28, -7.99) | -13.26* (-15.42, -11.09) | -15.05‡(-17.20, -12.90) | -15.44‡(-17.63, -13.32) | -15.47‡(-17.63, -13.32) |
| Week 6 (CI) | -12.98 (-15.45, -10.51) | -15.47 (-17.97, -12.98) | -16.74* (-19.22, -14.26) | -17.33* (-19.48, -14.51) | -16.99* (-19.48, -14.51) |
| Week 12 (CI) | -13.48 (-16.07, -10.89) | -16.84 (-19.46, -14.23) | -17.34* (-19.93, -14.74) | -17.22* (-20.64, -15.44) | -18.04* (-20.64, -15.44) |
| *P < .05 vs placebo, significant. | |||||
| † P < .01 vs placebo, significant. | |||||
| ‡ P < .001 vs placebo, significant. | |||||
| § Scale = 1 (very good) to 5 (very poor). | |||||
| ║ Scale = 0 mm (no pain) to 100 mm (most severe pain). | |||||
| ¶ Scale = 0 (no symptoms) to 8 (worse symptoms). | |||||
| # Scale = 0 (no symptoms) to 96 (worse symptoms). | |||||
| bid, twice daily; CI, 95% confidence interval; LSM, least square mean; PAAP, Patient’s Assessment of Arthritis Pain; PhGAA, Physician’s Global Assessment of Arthritis; qd, once daily; WOMAC OA, Western Ontario and McMaster’s Universities Osteoarthritis Index. | |||||
TABLE 3
Incidence of gastroduodenal, gastric, and duodenal ulcers (>5 mm) at final endoscopic evaluation
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 178) | 5 mg qd (n = 188) | 10 mg qd (n = 174) | 20 mg qd (n = 185) | 500 mg bid (n = 183) | |
| Gastroduodenal§ | 8 (4) [2.1, 9.0] | 6 (3)† [1.3, 7.1] | 5 (3)† [1.1, 6.9] | 10 (5) [2.8, 10.0] | 18 (10)* [6.1, 15.3] |
| Gastric§ | 8 (4) [2.1, 9.0] | 4 (2)‡ [0.7, 5.7] | 3 (2)‡ [0.4, 5.4] | 9 (5) [2.4, 9.3] | 16 (9) [5.2, 14.1] |
| Duodenal§ | 0 (0) [0.05, 2.6] | 2 (1) [0.2, 4.2] | 2 (1) [0.2, 4.5] | 1 (1) [0.0, 3.4] | 2 (1) [0.2, 4.3] |
| Symptomatic ulcers (n) | 0 | 1 | 2 | 3 | 7 |
| *P < .05 vs placebo. | |||||
| † P < .05 vs naproxen. | |||||
| ‡ P < .01 vs naproxen. | |||||
| § Data are presented as n (%) [95% confidence interval]. | |||||
| bid, twice daily; qd, once daily. | |||||
Safety
Valdecoxib and placebo had comparable upper gastrointestinal tract ulceration rates, whereas naproxen produced a significantly higher incidence of upper gastrointestinal tract ulcers than did 5 and 10 mg valdecoxib and placebo (P < .05). There were 14 adjudicated symptomatic ulcers during the study: 1 in the 5-mg valdecoxib group, 2 in the 10-mg valdecoxib group, 3 in the 20-mg valdecoxib group, and 7 in the 500-mg naproxen group.
Adverse events with an incidence of at least 5% in any treatment group and adverse events leading to withdrawal from the study are summarized by body system in Table 4. There were no significant differences in the incidence of adverse events between the valdecoxib and placebo groups. In contrast, 500 mg naproxen twice daily was associated with significantly more adverse events than 5 or 10 mg/day valdecoxib (P < .05). The incidence of adverse events was similar in the 20-mg valdecoxib and naproxen groups. Most adverse events were reported in the gastrointestinal system and consisted of abdominal pain, constipation, diarrhea, dyspepsia, flatulence, and nausea. The incidences of constipation, diarrhea, and flatulence were significantly higher in the naproxen group than in the 5-, 10-, and 20-mg valdecoxib groups, respectively. Other adverse events included accidental injury, headache, myalgia, and upper respiratory tract infections. Valdecoxib at 5 mg/day produced a significantly higher incidence of myalgia than did placebo, and valdecoxib at 20 mg/day produced a significantly lower incidence of upper respiratory tract infections than did placebo. Adverse events causing withdrawal with an incidence of at least 1% were accidental injury, abdominal pain, diarrhea, dyspepsia, nausea, abnormal hepatic function, rash, and blurred vision. The proportion of patients in the naproxen group (12.7%) who withdrew from the study was significantly greater than those for the 5-and 20-mg valdecoxib (6.0% and 5.5%) groups (P < .05), although the incidence of withdrawal due to adverse events in the 10-mg valdecoxib and naproxen groups were similar. In addition, gastrointestinal adverse events commonly related to NSAID treatment, such as dyspepsia and constipation, were more frequent in the naproxen group than in the valdecoxib and placebo groups.
TABLE 4
Adverse events
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 178) | 5 mg qd (n = 188) | 10 mg qd (n = 174) | 20 mg qd (n = 185) | 500 mg bid (n = 183) | |
| Incidence ≥ 5% in any treatment group | |||||
| Total | 109 (53.2) | 112 (55.7)† | 113 (55.1)† | 121 (60.2) | 139 (68.1)* |
| Accidental injury | 11 (5.4) | 3 (1.5) | 10 (4.9) | 12 (6.0) | 9 (4.4) |
| Headache | 11 (5.4) | 12 (6.0) | 7 (3.4) | 14 (7.0) | 9 (4.4) |
| Abdominal pain | 19 (9.3) | 14 (7.0) | 18 (8.8) | 13 (6.5) | 25 (12.3) |
| Constipation | 6 (2.9) | 4 (2.0) | 1 (0.5)† | 4 (2.0) | 12 (5.9) |
| Diarrhea | 10 (4.9) | 7 (3.5) | 14 (6.8) | 11 (5.5) | 12 (5.9) |
| Dyspepsia | 15 (7.3) | 22 (10.9) | 22 (10.7) | 20 (9.9)† | 35 (17.2)* |
| Flatulence | 12 (5.9) | 7 (3.5) | 5 (2.4)† | 9 (4.5) | 14 (6.9) |
| Nausea | 10 (4.9) | 18 (9.0) | 17 (8.3) | 9 (4.5) | 10 (4.9) |
| Myalgia | 0 (0.0) | 13 (6.5)*† | 3 (1.5) | 2 (1.0) | 1 (0.5) |
| Upper respiratory tract infections | 18 (8.8) | 9 (4.5) | 10 (4.9) | 7 (3.5)* | 10 (4.9) |
| Incidence ≥ 1% in any treatment group causing withdrawal | |||||
| Total | 17 (8.3) | 12 (6.0)† | 18 (8.8) | 11 (5.5)† | 26 (12.7) |
| Accidental injury | 2 (1.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) |
| Abdominal pain | 5 (2.4) | 2 (1.0) | 6 (2.9) | 2 (1.0) | 7 (3.4) |
| Diarrhea | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) | 3 (1.5) |
| Dyspepsia | 2 (1.0) | 2 (1.0) | 3 (1.5) | 1 (0.5)† | 9 (4.4)* |
| Nausea | 2 (1.0) | 1 (0.5) | 2 (1.0) | 1 (0.5) | 2 (1.0) |
| Abnormal hepatic function | 0 (0.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash | 0 (0.0) | 2 (1.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Blurred vision | 2 (1.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| *P < .05 vs placebo. | |||||
| † P < .05 vs naproxen. | |||||
| ‡ Data are presented as number (%) of patients reporting events. | |||||
| bid, twice daily; qd, once daily. | |||||
FIGURE 2
Western Ontario and McMaster’s Universities Osteoarthritis Pain Index
Discussion
This study confirmed that the novel COX-2–specific inhibitor valdecoxib at a dosage of 10 or 20 mg/day is as effective as naproxen at a dosage of 500 mg twice daily in relieving moderate to severe osteoarthritis of the knee over 12 weeks. In addition, treatment with 10 mg/day valdecoxib orally, the recommended dosage for treatment of osteoarthritis, is associated with a significantly lower gastroduodenal ulceration rate than occurs with the conventional NSAID, naproxen.
Patients receiving 10 and 20 mg/day valdecoxib experienced significant improvements in the signs and symptoms of osteoarthritis, and in all assessments the efficacies of valdecoxib 10 and 20 mg/day were numerically similar to that of naproxen. This finding is consistent with the inhibition of prostaglandin production in inflamed synovial tissue and in the central pain pathway. Increased COX-2 activity in the spinal cord in response to tissue damage and in the synovial membrane of osteoarthritis patients is at least partly responsible for joint inflammation and sensitization to inflammatory pain.14–16 The efficacy of valdecoxib in treating moderate to severe osteoarthritis of the knee was consistent with reports of other COX-2–specific inhibitors that are comparable to conventional NSAIDs in relieving chronic pain and inflammation.17,18 These data confirmed that 10 mg/day valdecoxib is as effective as 500 mg naproxen twice daily in treating the pain and inflammation associated with osteoarthritis. The efficacy of 10 mg/day valdecoxib makes it one of the most potent COX-2–specific inhibitors for treating moderate to severe osteoarthritis.
Conventional NSAIDs were associated with a significant risk of serious gastrointestinal complications such as ulceration and perforation and low gastrointestinal tolerability.20–22 Naproxen treatment of osteoarthritis and rheumatoid arthritis demonstrated a higher rate of endoscopically proven gastrointestinal ulceration than did COX-2–specific inhibitors,17,23 and that finding was confirmed in this study for 10 mg valdecoxib. Naproxen treatment was associated with significantly more gastroduodenal ulcers than 5 or 10 mg valdecoxib. We found no significant difference between 20 mg valdecoxib and naproxen, which might be explained by a lower incidence of ulcers with naproxen than reported in previous studies.24 In terms of numbers needed to treat, 14 patients would be needed to observe a difference in endoscopic ulcer rates between valdecoxib (5 or 10 mg) and naproxen compared with 20 patients to observe a difference between 20 mg valdecoxib and naproxen and 16 to observe a difference in ulcer rates between naproxen and placebo.
Valdecoxib at a dosage of 10 mg/day also demonstrated overall improved gastrointestinal tolerability, with significantly fewer adverse events and withdrawals due to adverse events, in particular gastrointestinal-related events such as constipation and dyspepsia, than did naproxen. The improved upper gastrointestinal tract safety of valdecoxib was as expected because the COX-1–sparing nature of this agent allows effective inhibition of COX-2 without inhibiting COX-1 in the gastric mucosa and platelets. An improved gastrointestinal safety profile is an important consideration in the treatment of osteoarthritis because the moderate to severe gastrointestinal complications associated with conventional NSAID therapy frequently lead to poor patient compliance or discontinuation of the medication.25,26
Overall, this study suggests clinical benefits of single daily doses of 10 and 20 mg valdecoxib and improved upper gastrointestinal tract safety for the 10-mg dose, compared with 500 mg/day naproxen. No additional efficacy benefit was obtained from a 20-mg dose as opposed to a 10-mg dose. Valdecoxib (10 mg) is a potent and effective once-daily alternative to conventional NSAIDs, with a gastrointestinal safety advantage that will be of value to rheumatologists and primary care physicians alike.
FIGURE 3
Western Ontario and Western Universities Osteoarthritis Physical Function Index
OBJECTIVE: We compared the efficacy and upper gastrointestinal safety of the cyclooxygenase-2–specific inhibitor valdecoxib with naproxen and placebo in treating moderate to severe osteoarthritis of the knee.
STUDY DESIGN: This multicenter, randomized, double-blind, placebo-controlled study compared the efficacy and upper gastrointestinal tract safety of valdecoxib at dosages of 5, 10, and 20 mg once daily with placebo and naproxen at the dosage of 500 mg twice daily.
POPULATION: We included patients who had been diagnosed with moderate to severe osteoarthritis of the knee according to the modified criteria of the American College of Rheumatology.
OUTCOMES MEASURED: The Patient’s and Physician’s Global Assessment of Arthritis (PaGAA, PhGAA), Patient’s Assessment of Arthritis Pain–Visual Analog Scale (PAAP-VAS), and Western Ontario and McMaster’s Universities (WOMAC) Osteoarthritis indices were assessed at baseline and at weeks 2, 6, and 12. Upper gastrointestinal ulceration was assessed by pre- and posttreatment endoscopies.
RESULTS: Valdecoxib 10 and 20 mg once daily (but not 5 mg once daily) demonstrated similar efficacy to naproxen at 500 mg twice daily, and all 3 dosages were superior to placebo for the PaGAA, PhGAA, PAAP-VAS, and WOMAC Osteoarthritis indices at most assessments throughout the 12-week study (P < .05). The incidence of endoscopically proven ulcers was significantly higher in the naproxen group than in the 5- and 10-mg valdecoxib groups, but not in the 20-mg valdecoxib group. All 3 valdecoxib doses were comparable to placebo in ulcer incidence.
CONCLUSIONS: Valdecoxib (10 and 20 mg once daily) is significantly superior to placebo and as effective as naproxen (500 mg twice daily) in improving moderate to severe osteoarthritis of the knee. Upper gastrointestinal tract safety of valdecoxib (5 and 10 mg) was comparable to that of placebo and significantly better than that of naproxen.
- The cyclooxygenase-2–specific inhibitor valdecoxib 10 or 20 mg once daily is as effective as naproxen 500 mg twice daily.
- Valdecoxib at the recommended dose for treatment of osteoarthritis (10 mg once daily) had better upper gastrointestinal safety than naproxen.
Current medical therapies for osteoarthritis include conventional nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, glucosamine sulfate, and intra-articular injections of corticosteroids and hyaluronic acid. However, long-term use of corticosteroid injections can exacerbate damage to the affected joints.1,2 Conventional NSAIDs are associated with upper gastrointestinal tract ulceration and inhibition of platelet function.3
Cyclooxygenase-2 (COX-2)–specific inhibitors have demonstrated equivalent efficacy to conventional NSAIDs in treating pain and inflammation associated with osteoarthritis and rheumatoid arthritis. Further, COX-2–specific inhibitors significantly reduce the incidence of gastrointestinal ulceration and bleeding side effects caused by conventional NSAIDs.4,5 Valdecoxib (Bextra; Pharmacia Corporation and Pfizer Corporation) is a novel COX-2–specific inhibitor that is approximately 28,000-fold more selective against COX-2 than against COX-1. As a potent COX-2–specific inhibitor, valdecoxib is expected to provide efficacy equivalent to conventional NSAIDs for treatment of arthritis and spare the COX-1–related side effects. This randomized, placebo-controlled, double-blind, 12-week study was designed to test this hypothesis by comparing the efficacy and upper gastrointestinal tract safety of valdecoxib with that of naproxen, a leading conventional NSAID comparator.
Methods
Study population
Ambulatory adults who had been diagnosed with moderate to severe osteoarthritis of the knee according to the modified criteria of the American College of Rheumatology6,7 were eligible to participate in the trial. Patients were recruited from primary care and rheumatology specialty settings. Patients who had baseline scores of at least 40 mm on the Patient’s Assessment of Arthritis Pain–Visual Analog Scale (PAAP-VAS) and baseline categorical scores of poor to very poor on the Patient’s (PaGAA) and Physician’s (PhGAA) Global Assessments of Arthritis were included.8,9 Any patient suffering from inflammatory arthritis, gout, pseudogout, Paget disease, or any chronic pain syndrome that might interfere with assessment of the Index Knee was excluded from the trial. Patients diagnosed with osteoarthritis of the hip ipsilateral to the Index Knee, severe anserine bursitis, acute joint trauma, or complete loss of articular cartilage on the Index Knee also were excluded. Patients were not eligible if they had active gastrointestinal disease, gastrointestinal tract ulceration 30 days before the trial, a significant bleeding disorder, or a history of gastric or duodenal surgery. Patients with an esophageal, gastric, pyloric channel, or duodenal ulcer or a score of at least 10 for esophageal, gastric, or duodenal erosions at the pretreatment endoscopy examination also were excluded.
FIGURE 1
Patient’s global assessment of arthritis
Study design
This multicenter, randomized, double-blind, placebo-controlled study compared the efficacy and upper gastrointestinal tract safety of valdecoxib at dosages of 5, 10, and 20 mg once daily with placebo and naproxen at a dosage of 500 mg twice daily in relieving moderate to severe osteoarthritis of the knee. The trial was conducted in 85 centers in the United States and Canada, in accordance with the principles of good clinical practice and the Declaration of Helsinki. Eligible patients were randomized to treatment groups and self-administered oral study medication. Patients were randomized to study treatment in the order in which they were enrolled into the study by using a treatment sequence that was determined by a Searle-prepared computer-generated randomization schedule. Patients received their allocated study medications in bottles labeled A and B according to the randomization schedule. Personnel at the study centers carried out the assessments and remained blinded throughout the study. Eligible patients were enrolled and discontinued regular pain medication. Patients discontinued their normal medications at the following specified times before the baseline endoscopy: NSAIDs (including full-dose aspirin at a dosage of ≥325 mg/day) at 48 hours, corticosteroid injections at 4 weeks, and intra-articular injections of corticosteroid or hyaluronic acid preparations at 3 and 6 months, respectively. The use of antiulcer drugs, including H2 blockers, proton pump inhibitors, misoprostol, and sucralfate, was discontinued at least 24 hours before the baseline endoscopy.
Efficacy assessments
The following arthritis assessments were made at baseline and at 2, 6, and 12 weeks or at early termination after study drug administration. PaGAA or PhGAA was measured on a 5-point categorical scale, where 1 = very good, 2 = good, 3 = fair, 4 = poor, and 5 = very poor. The PAAP-VAS was measured on a scale of 0 to 100 mm, where 0 = no pain and 100 = most severe pain. The Western Ontario and McMaster’s Universities (WOMAC) Osteoarthritis indices including Pain, Stiffness, Physical Function, and Composite were measured as described previously.10
Upper gastrointestinal assessments
Upper gastrointestinal tract endoscopy was performed within 7 days before the first study dose and at the 12-week assessment or at early termination if the patient withdrew. An endoscopy could be performed at any time if the patient experienced symptoms suggestive of an ulcer. The endoscopists performing baseline and 12-week (early termination) assessments remained blinded throughout the study.
General safety assessments
Clinical laboratory tests were performed at screening, baseline, weeks 2, 6, and 12, or at early termination, and a complete physical examination was performed at screening and final visits. The incidence of adverse events occurring in each treatment arm was monitored throughout the study. Adverse events occurring within 7 days and serious adverse events occurring within 30 days of the last study dosage of medication were included in the safety analyses.
Statistical analyses
A sample size of 200 patients per treatment group was deemed sufficient to detect a difference in ulcer rates of 5% for valdecoxib vs 16% for naproxen, with 80% power and type 1 error at .017 (adjusted for 3 primary comparisons against placebo). Homogeneity of treatment groups at baseline with respect to age, height, weight, duration of osteoarthritis, PAAP-VAS, and WOMAC Osteoarthritis Index scores was assessed with 2-way analysis of variance, with treatment group and center as factors. All other demographics and baseline characteristics were compared with the Cochran-Mantel-Haenszel (CMH) test, stratified by center.
All efficacy assessments were performed on the modified intent-to-treat (ITT) cohort by using the last observation carried forward approach. The ITT cohort comprised all patients who were randomized and had taken at least 1 dose of study medication. Analyses of mean change from baseline for PaGAA, PhGAA, PAAP-VAS, and WOMAC Osteoarthritis indices were performed by using analysis of covariance, with treatment and center as factors and the corresponding baseline score as the covariate. Pairwise comparisons of valdecoxib at dosages of 10 and 20 mg once daily vs placebo were interpreted with the Hochberg procedure.11 Primary pairwise comparisons were amended in the statistical analysis plan before data unblinding to compare placebo with 10 and 20 mg valdecoxib, but not with the 5-mg dose. For all other comparisons, including 5 mg valdecoxib and naproxen vs placebo, differences were considered significant if the pairwise P values were less than .05. The incidence of withdrawal due to treatment failure was analyzed by the Fisher exact test, and the time to withdrawal in each treatment group was analyzed by log-rank test and plotted with the Kaplan-Meier product limit.12,13
Upper gastrointestinal tract endoscopic analyses were performed on the upper gastrointestinal tract ITT population. Randomized patients were included in this cohort if they received at least 1 dose of study medication and had undergone pretreatment and posttreatment endoscopies. Overall and pairwise comparisons of gastroduodenal, gastric, and duodenal ulcers and erosions were assessed with the CMH test stratified by center. The incidence of adverse events was compared between treatment groups with the Fisher exact test. Changes in vital signs were compared between treatment groups with an analysis of covariance using pairwise treatment comparisons, with treatment group as a factor and baseline value as a covariate.
Results
Patient baseline characteristics
Of the 1019 eligible randomized patients, 1 patient randomized to 10 mg/day valdecoxib, 1 to 20 mg/day valdecoxib, and 1 to 500 mg naproxen twice daily did not take the study medication and were excluded from efficacy and safety analyses. The remaining 1016 randomized patients received study medication and were included in the ITT cohort on which analyses of all efficacy end points were based. A total of 269 patients withdrew before the end of the study due to treatment failure, preexisting protocol violations, noncompliance, or adverse signs and symptoms, or were lost to follow-up: 74 patients in the placebo group, 39 in the 5-mg valdecoxib group, 56 in the 10-mg valdecoxib group, 44 in the 20-mg valdecoxib group, and 56 in the naproxen group. The upper gastrointestinal tract ITT cohort comprised 908 patients who were included in the upper gastrointestinal tract safety analyses. More than 90% of patients included in the study evaluated their osteoarthritis as poor to very poor as assessed by baseline PaGAA scores. Treatment groups were homogeneous with respect to demographics, vital signs, medical history, and all baseline arthritis assessments (Table 1).
TABLE 1
Patient baseline characteristics
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 205) | 5 mg qd (n = 201) | 10 mg qd (n = 206) | 20 mg qd (n = 202) | 500 mg bid (n = 205) | |
| Mean (SD) age, y | 60.3 (10.5) | 58.7 (11.9) | 59.8 (11.0) | 59.6 (10.4) | 60.4 (10.7) |
| Mean (SD) weight, kg | 87.5 (21.2) | 91.4 (22.6) | 89.3 (21.4) | 92.6 (23.7) | 88.1 (21.7) |
| Race, n (%) | |||||
| White | 162 (79) | 155 (77) | 154 (75) | 160 (79) | 163 (80) |
| Black | 21 (10) | 26 (13) | 24 (12) | 24 (12) | 23 (11) |
| Asian | 1 (0) | 1 (0) | 1 (0) | 1 (0) | 2 (1) |
| Hispanic | 19 (9) | 18 (9) | 25 (12) | 15 (7) | 15 (7) |
| Male sex, n (%) | 73 (36) | 73 (36) | 72 (35) | 66 (33) | 76 (37) |
| Mean (SD) disease duration, y | 8.3 (8.0) | 9.8 (9.5) | 8.7 (8.0) | 9.2 (8.0) | 9.4 (8.7) |
| History of GI bleeding, n (%) | 2 (1) | 0 (0) | 3 (1) | 2 (1) | 3 (1) |
| History of gastroduodenal ulcer, n (%) | 20 (10) | 21 (10) | 24 (12) | 28 (14) | 31 (15) |
| PaGAA, n (%) | |||||
| Poor | 168 (82) | 175 (87) | 168 (82) | 162 (80) | 169 (82) |
| Very poor | 33 (16) | 23 (11) | 32 (16) | 36 (18) | 31 (15) |
| PhGAA, n (%) | |||||
| Poor | 179 (87) | 181 (90) | 176 (85) | 173 (86) | 175 (85) |
| Very poor | 24 (12) | 18 (9) | 25 (12) | 24 (12) | 25 (12) |
| No significant differences were observed between treatment groups at any baseline characteristic. | |||||
| bid, twice daily; GI, gastrointestinal; PaGAA, Patient’s Global Assessment of Arthritis; PhGAA, Physician’s Global Assessment of Arthritis; qd, once daily. | |||||
Efficacy
The least square mean change in the PaGAA was significantly improved at most assessments in response to valdecoxib (10 and 20 mg/day) and 500 mg naproxen twice daily compared with placebo (Table 2). However, the improvement in response to valdecoxib 5 mg qd did not reach statistical significance (Table 2). Significant improvements in the PhGAA were observed in response to valdecoxib and naproxen at all assessments (Table 2).
The dosages of 20 mg/day valdecoxib and 500 mg naproxen twice daily were associated with a reduction in pain, as assessed by the PAAP-VAS scores. Pain reduction associated with 5 and 10 mg/day valdecoxib was significantly better than that with placebo at all assessments except for week 12 (Table 2).
Valdecoxib and naproxen treatments improved the WOMAC Pain, Stiffness, Physical Function, and Composite indices compared with placebo at 2, 6, and 12 weeks. Valdecoxib 20 mg/day and naproxen 500 mg twice daily produced statistically significant changes in all WOMAC Osteoarthritis scores throughout the 12-week study period compared with placebo (P < .05). WOMAC Pain scores for 10 mg valdecoxib were significantly different from those for placebo at 2 weeks (P < .001) but not at 6 or 12 weeks. No significant differences were noted between any of the valdecoxib treatment doses and naproxen in terms of improvement in WOMAC indices.
The incidences of withdrawal due to treatment failure were 20% (95% confidence interval [CI], 15.3–26.8) in the placebo group; 8% (95% CI, 4.8–12.8), 12% (95% CI, 7.8–17.1), and 10% (95% CI, 6.3–15.2) in the 5-, 10-, and 20-mg/day valdecoxib groups; and 6% (95% CI, 3.6–10.9) in the 500-mg naproxen group (P < .05; Table 3). Patients in the placebo group withdrew at a significantly faster rate than those in the 4 active treatment groups (P < .05), but there were no significant differences in withdrawal rates across the 4 active treatment groups.
TABLE 2
Baseline arthritis assessments and mean changes from baseline scores
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 205) | 5 mg qd (n = 201) | 10 mg qd (n = 205) | 20 mg qd (n = 201) | 500 mg bid (n = 204) | |
| PhGAA§ | |||||
| Baseline mean | 4.10 | 4.07 | 4.09 | 4.09 | 4.10 |
| LSM change | |||||
| Week 2 (CI) | -1.04 (-1.16, -0.91) | -1.31‡(-1.44, -1.19) | -1.37‡(-1.50, -1.25) | -1.42‡(-1.54, -1.29) | -1.35‡(-1.48, -1.23) |
| Week 6 (CI) | -1.22 (-1.35, -1.08) | -1.44*(-1.58, -1.31) | -1.50†(-1.63, -1.36) | -1.41* (-1.55, -1.28) | -1.45* (-1.59, -1.32) |
| Week 12 (CI) | -1.22 (-1.36, -1.08) | -1.43* (-1.58, -1.28) | -1.52†(-1.67, -1.38) | -1.45* (-1.60, -1.31) | -1.43* (-1.58, -1.29) |
| PAAP║ | |||||
| Baseline mean | 71.20 | 71.42 | 72.41 | 72.54 | 72.36 |
| LSM change | |||||
| Week 2 (CI) | -21.19 (-24.80, -17.58) | -28.46†(-32.11, -24.82) | -30.21‡(-33.83, -26.59) | -32.07‡(-35.73, -28.41) | -31.03‡(-34.66, -27.40) |
| Week 6 (CI) | -23.92 (-27.72, -20.12) | -30.81†(-34.65, -26.97) | -29.85* (-33.67, -26.04) | -32.28†(-36.13, -28.42) | -31.84†(-35.66, -28.02) |
| Week 12 (CI) | -25.97 (-30.02, -21.92) | -31.33 (-35.42, -27.24) | -30.41 (-34.47, -30.41) | -32.70* (-36.81, -32.70) | -31.83* (-35.90, -27.76) |
| WOMAC OA, Stiffness ¶ | |||||
| Baseline mean | 4.84 | 4.87 | 4.91 | 4.73 | 4.94 |
| LSM change | |||||
| Week 2 (CI) | -0.78 (-0.98, -0.57) | -1.03 (-1.24, -0.82) | -1.20†(-1.41, -0.99) | -1.24†(-1.45, -1.03) | -1.28‡(-1.49, -1.08) |
| Week 6 (CI) | -1.04 (-1.27, -0.82) | -1.25 (-1.48, -1.02) | -1.42* (-1.65, -1.20) | -1.43* (-1.66, -1.20) | -1.40†(-1.62, -1.17) |
| Week 12 (CI) | -1.12 (-1.36, -0.89) | -1.33 (-1.57, -1.09) | -1.41 (-1.65, -1.17) | -1.46* (-1.70, -1.22) | -1.54* (-1.78, -1.30) |
| WOMAC OA, Composite # | |||||
| Baseline mean | 53.49 | 53.03 | 54.73 | 53.42 | 53.67 |
| LSM change | |||||
| Week 2 (CI) | -10.13 (-12.28, -7.99) | -13.26* (-15.42, -11.09) | -15.05‡(-17.20, -12.90) | -15.44‡(-17.63, -13.32) | -15.47‡(-17.63, -13.32) |
| Week 6 (CI) | -12.98 (-15.45, -10.51) | -15.47 (-17.97, -12.98) | -16.74* (-19.22, -14.26) | -17.33* (-19.48, -14.51) | -16.99* (-19.48, -14.51) |
| Week 12 (CI) | -13.48 (-16.07, -10.89) | -16.84 (-19.46, -14.23) | -17.34* (-19.93, -14.74) | -17.22* (-20.64, -15.44) | -18.04* (-20.64, -15.44) |
| *P < .05 vs placebo, significant. | |||||
| † P < .01 vs placebo, significant. | |||||
| ‡ P < .001 vs placebo, significant. | |||||
| § Scale = 1 (very good) to 5 (very poor). | |||||
| ║ Scale = 0 mm (no pain) to 100 mm (most severe pain). | |||||
| ¶ Scale = 0 (no symptoms) to 8 (worse symptoms). | |||||
| # Scale = 0 (no symptoms) to 96 (worse symptoms). | |||||
| bid, twice daily; CI, 95% confidence interval; LSM, least square mean; PAAP, Patient’s Assessment of Arthritis Pain; PhGAA, Physician’s Global Assessment of Arthritis; qd, once daily; WOMAC OA, Western Ontario and McMaster’s Universities Osteoarthritis Index. | |||||
TABLE 3
Incidence of gastroduodenal, gastric, and duodenal ulcers (>5 mm) at final endoscopic evaluation
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 178) | 5 mg qd (n = 188) | 10 mg qd (n = 174) | 20 mg qd (n = 185) | 500 mg bid (n = 183) | |
| Gastroduodenal§ | 8 (4) [2.1, 9.0] | 6 (3)† [1.3, 7.1] | 5 (3)† [1.1, 6.9] | 10 (5) [2.8, 10.0] | 18 (10)* [6.1, 15.3] |
| Gastric§ | 8 (4) [2.1, 9.0] | 4 (2)‡ [0.7, 5.7] | 3 (2)‡ [0.4, 5.4] | 9 (5) [2.4, 9.3] | 16 (9) [5.2, 14.1] |
| Duodenal§ | 0 (0) [0.05, 2.6] | 2 (1) [0.2, 4.2] | 2 (1) [0.2, 4.5] | 1 (1) [0.0, 3.4] | 2 (1) [0.2, 4.3] |
| Symptomatic ulcers (n) | 0 | 1 | 2 | 3 | 7 |
| *P < .05 vs placebo. | |||||
| † P < .05 vs naproxen. | |||||
| ‡ P < .01 vs naproxen. | |||||
| § Data are presented as n (%) [95% confidence interval]. | |||||
| bid, twice daily; qd, once daily. | |||||
Safety
Valdecoxib and placebo had comparable upper gastrointestinal tract ulceration rates, whereas naproxen produced a significantly higher incidence of upper gastrointestinal tract ulcers than did 5 and 10 mg valdecoxib and placebo (P < .05). There were 14 adjudicated symptomatic ulcers during the study: 1 in the 5-mg valdecoxib group, 2 in the 10-mg valdecoxib group, 3 in the 20-mg valdecoxib group, and 7 in the 500-mg naproxen group.
Adverse events with an incidence of at least 5% in any treatment group and adverse events leading to withdrawal from the study are summarized by body system in Table 4. There were no significant differences in the incidence of adverse events between the valdecoxib and placebo groups. In contrast, 500 mg naproxen twice daily was associated with significantly more adverse events than 5 or 10 mg/day valdecoxib (P < .05). The incidence of adverse events was similar in the 20-mg valdecoxib and naproxen groups. Most adverse events were reported in the gastrointestinal system and consisted of abdominal pain, constipation, diarrhea, dyspepsia, flatulence, and nausea. The incidences of constipation, diarrhea, and flatulence were significantly higher in the naproxen group than in the 5-, 10-, and 20-mg valdecoxib groups, respectively. Other adverse events included accidental injury, headache, myalgia, and upper respiratory tract infections. Valdecoxib at 5 mg/day produced a significantly higher incidence of myalgia than did placebo, and valdecoxib at 20 mg/day produced a significantly lower incidence of upper respiratory tract infections than did placebo. Adverse events causing withdrawal with an incidence of at least 1% were accidental injury, abdominal pain, diarrhea, dyspepsia, nausea, abnormal hepatic function, rash, and blurred vision. The proportion of patients in the naproxen group (12.7%) who withdrew from the study was significantly greater than those for the 5-and 20-mg valdecoxib (6.0% and 5.5%) groups (P < .05), although the incidence of withdrawal due to adverse events in the 10-mg valdecoxib and naproxen groups were similar. In addition, gastrointestinal adverse events commonly related to NSAID treatment, such as dyspepsia and constipation, were more frequent in the naproxen group than in the valdecoxib and placebo groups.
TABLE 4
Adverse events
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 178) | 5 mg qd (n = 188) | 10 mg qd (n = 174) | 20 mg qd (n = 185) | 500 mg bid (n = 183) | |
| Incidence ≥ 5% in any treatment group | |||||
| Total | 109 (53.2) | 112 (55.7)† | 113 (55.1)† | 121 (60.2) | 139 (68.1)* |
| Accidental injury | 11 (5.4) | 3 (1.5) | 10 (4.9) | 12 (6.0) | 9 (4.4) |
| Headache | 11 (5.4) | 12 (6.0) | 7 (3.4) | 14 (7.0) | 9 (4.4) |
| Abdominal pain | 19 (9.3) | 14 (7.0) | 18 (8.8) | 13 (6.5) | 25 (12.3) |
| Constipation | 6 (2.9) | 4 (2.0) | 1 (0.5)† | 4 (2.0) | 12 (5.9) |
| Diarrhea | 10 (4.9) | 7 (3.5) | 14 (6.8) | 11 (5.5) | 12 (5.9) |
| Dyspepsia | 15 (7.3) | 22 (10.9) | 22 (10.7) | 20 (9.9)† | 35 (17.2)* |
| Flatulence | 12 (5.9) | 7 (3.5) | 5 (2.4)† | 9 (4.5) | 14 (6.9) |
| Nausea | 10 (4.9) | 18 (9.0) | 17 (8.3) | 9 (4.5) | 10 (4.9) |
| Myalgia | 0 (0.0) | 13 (6.5)*† | 3 (1.5) | 2 (1.0) | 1 (0.5) |
| Upper respiratory tract infections | 18 (8.8) | 9 (4.5) | 10 (4.9) | 7 (3.5)* | 10 (4.9) |
| Incidence ≥ 1% in any treatment group causing withdrawal | |||||
| Total | 17 (8.3) | 12 (6.0)† | 18 (8.8) | 11 (5.5)† | 26 (12.7) |
| Accidental injury | 2 (1.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) |
| Abdominal pain | 5 (2.4) | 2 (1.0) | 6 (2.9) | 2 (1.0) | 7 (3.4) |
| Diarrhea | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) | 3 (1.5) |
| Dyspepsia | 2 (1.0) | 2 (1.0) | 3 (1.5) | 1 (0.5)† | 9 (4.4)* |
| Nausea | 2 (1.0) | 1 (0.5) | 2 (1.0) | 1 (0.5) | 2 (1.0) |
| Abnormal hepatic function | 0 (0.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash | 0 (0.0) | 2 (1.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Blurred vision | 2 (1.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| *P < .05 vs placebo. | |||||
| † P < .05 vs naproxen. | |||||
| ‡ Data are presented as number (%) of patients reporting events. | |||||
| bid, twice daily; qd, once daily. | |||||
FIGURE 2
Western Ontario and McMaster’s Universities Osteoarthritis Pain Index
Discussion
This study confirmed that the novel COX-2–specific inhibitor valdecoxib at a dosage of 10 or 20 mg/day is as effective as naproxen at a dosage of 500 mg twice daily in relieving moderate to severe osteoarthritis of the knee over 12 weeks. In addition, treatment with 10 mg/day valdecoxib orally, the recommended dosage for treatment of osteoarthritis, is associated with a significantly lower gastroduodenal ulceration rate than occurs with the conventional NSAID, naproxen.
Patients receiving 10 and 20 mg/day valdecoxib experienced significant improvements in the signs and symptoms of osteoarthritis, and in all assessments the efficacies of valdecoxib 10 and 20 mg/day were numerically similar to that of naproxen. This finding is consistent with the inhibition of prostaglandin production in inflamed synovial tissue and in the central pain pathway. Increased COX-2 activity in the spinal cord in response to tissue damage and in the synovial membrane of osteoarthritis patients is at least partly responsible for joint inflammation and sensitization to inflammatory pain.14–16 The efficacy of valdecoxib in treating moderate to severe osteoarthritis of the knee was consistent with reports of other COX-2–specific inhibitors that are comparable to conventional NSAIDs in relieving chronic pain and inflammation.17,18 These data confirmed that 10 mg/day valdecoxib is as effective as 500 mg naproxen twice daily in treating the pain and inflammation associated with osteoarthritis. The efficacy of 10 mg/day valdecoxib makes it one of the most potent COX-2–specific inhibitors for treating moderate to severe osteoarthritis.
Conventional NSAIDs were associated with a significant risk of serious gastrointestinal complications such as ulceration and perforation and low gastrointestinal tolerability.20–22 Naproxen treatment of osteoarthritis and rheumatoid arthritis demonstrated a higher rate of endoscopically proven gastrointestinal ulceration than did COX-2–specific inhibitors,17,23 and that finding was confirmed in this study for 10 mg valdecoxib. Naproxen treatment was associated with significantly more gastroduodenal ulcers than 5 or 10 mg valdecoxib. We found no significant difference between 20 mg valdecoxib and naproxen, which might be explained by a lower incidence of ulcers with naproxen than reported in previous studies.24 In terms of numbers needed to treat, 14 patients would be needed to observe a difference in endoscopic ulcer rates between valdecoxib (5 or 10 mg) and naproxen compared with 20 patients to observe a difference between 20 mg valdecoxib and naproxen and 16 to observe a difference in ulcer rates between naproxen and placebo.
Valdecoxib at a dosage of 10 mg/day also demonstrated overall improved gastrointestinal tolerability, with significantly fewer adverse events and withdrawals due to adverse events, in particular gastrointestinal-related events such as constipation and dyspepsia, than did naproxen. The improved upper gastrointestinal tract safety of valdecoxib was as expected because the COX-1–sparing nature of this agent allows effective inhibition of COX-2 without inhibiting COX-1 in the gastric mucosa and platelets. An improved gastrointestinal safety profile is an important consideration in the treatment of osteoarthritis because the moderate to severe gastrointestinal complications associated with conventional NSAID therapy frequently lead to poor patient compliance or discontinuation of the medication.25,26
Overall, this study suggests clinical benefits of single daily doses of 10 and 20 mg valdecoxib and improved upper gastrointestinal tract safety for the 10-mg dose, compared with 500 mg/day naproxen. No additional efficacy benefit was obtained from a 20-mg dose as opposed to a 10-mg dose. Valdecoxib (10 mg) is a potent and effective once-daily alternative to conventional NSAIDs, with a gastrointestinal safety advantage that will be of value to rheumatologists and primary care physicians alike.
FIGURE 3
Western Ontario and Western Universities Osteoarthritis Physical Function Index
1. Klippel J, et al. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001.
2. Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev 1988;10:1-28.
3. Borda IT, Koff R. NSAIDs: A Profile of Adverse Effects. Philadelphia: Hanley and Belfus; 1995.
4. Bensen WG, Fiechtner JJ, McMillen JI, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999;74:1095-105.
5. Geis GS. Update on clinical developments with celecoxib, a new specific COX-2 inhibitor: what can we expect? J Rheumatol 1999;26(suppl 56):31-6.
6. Altman R, Asch E, Bloch G, et al. The American College of Rheumatology criteria for the classification and reportings of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039-49.
7. Schumacher HR. Primer on the Rheumatic Diseases. Atlanta, GA: Arthritis Foundation; 1986.
8. Cooperating Clinics Committee of American Rheumatism Association. A seven day variability study of 499 patients with peripheral rheumatoid arthritis. Arthritis Rheum 1965;8:302-34.
9. Ward JR, Williams HJ, Boyce E, et al. Comparison of auranofin, gold sodium thiomalate, and placebo in the treatment of rheumatoid arthritis. Subsets of responses. Am J Med 1983;75:133-7.
10. Bellamy N. WOMAC Osteoarthritis Index: A User’s Guide. London, Ontario, Canada: The Western Ontario and McMaster Universities; 1995.
11. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1998;75:800-2.
12. Miller R. Survival Analyses. New York: John Wiley & Sons; 1998.
13. Simon R, Lee YJ. Nonparametric confidence limits for survival probabilities and median survival time. Cancer Treat Rep 1982;66:37-42.
14. Amin AR, Attur M, Patel RN, et al. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest 1997;99:1231-7.
15. Hay C, de Belleroche J. Carrageenan-induced hyperalgesia is associated with increased cyclooxygenase-2 expression in spinal cord. Neuroreport 1997;8:1249-51.
16. Kang RY, Freire Moar, Sigal E, et al. Expression of cyclooxygenase-2 in human and an animal model of rheumatoid arthritis. Br J Rheumatol 1996;35:711-8.
17. Bensen WG, Zhao SZ, Burke TA, et al. Upper gastrointestinal tolerability of celecoxib, a COX-2 specific inhibitor, compared to naproxen and placebo. J Rheumatol 2000;27:1876-83.
18. Day R, Morrison B, Luza A, et al. A randomized trial of the efficacy and tolerability of the COX-2 inhibitor rofecoxib vs ibuprofen in patients with osteoarthritis. Rofecoxib/Ibuprofen Comparator Study Group. Arch Intern Med 2000;160:1781-7.
19. Fiechtner J, Sikes D, Recker D. A double-blind, placebo-controlled dose ranging study to evaluate the efficacy of valdecoxib, a novel COX-2 specific inhibitor, in treating the signs and symptoms of osteoarthritis of the knee. Paper presented at: European League Against Rheumatism (EULAR); May 13–16, 2001; Prague, Czech Republic.
20. Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual nonsteroidal anti-inflammatory drugs. Lancet 1994;343:769-72.
21. Singh G, Ramey DR, Morfeld D, et al. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Intern Med 1996;156:1530-6.
22. Singh G, Rosen Ramey D. NSAID induced gastrointestinal complications: the ARAMIS perspective-1997. Arthritis, Rheumatism, and Aging Medical Information System. J Rheumatol 1998;51(suppl):8-16.
23. Watson DJ, Harper SE, Zhao PL, et al. Gastrointestinal tolerability of the selective cyclooxygenase-2 (COX-2) inhibitor rofecoxib compared with nonselective COX-1 and COX-2 inhibitors in osteoarthritis. Arch Intern Med 2000;160:2998-3003.
24. Simon LS, Weaver AL, Graham DY, et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA 1999;282:1921-8.
25. Langman MJ, Jensen DM, Watson DJ, et al. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA 1999;282:1929-33.
26. Scholes D, Stergachis A, Penna P, Normand E, Hansten P. Nonsteroidal anti-inflammatory drug discontinuation in patients with osteoarthritis. J Rheumatol 1995;22:708-12.
1. Klippel J, et al. Primer on the Rheumatic Diseases. 12th ed. Atlanta, GA: Arthritis Foundation; 2001.
2. Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev 1988;10:1-28.
3. Borda IT, Koff R. NSAIDs: A Profile of Adverse Effects. Philadelphia: Hanley and Belfus; 1995.
4. Bensen WG, Fiechtner JJ, McMillen JI, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999;74:1095-105.
5. Geis GS. Update on clinical developments with celecoxib, a new specific COX-2 inhibitor: what can we expect? J Rheumatol 1999;26(suppl 56):31-6.
6. Altman R, Asch E, Bloch G, et al. The American College of Rheumatology criteria for the classification and reportings of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039-49.
7. Schumacher HR. Primer on the Rheumatic Diseases. Atlanta, GA: Arthritis Foundation; 1986.
8. Cooperating Clinics Committee of American Rheumatism Association. A seven day variability study of 499 patients with peripheral rheumatoid arthritis. Arthritis Rheum 1965;8:302-34.
9. Ward JR, Williams HJ, Boyce E, et al. Comparison of auranofin, gold sodium thiomalate, and placebo in the treatment of rheumatoid arthritis. Subsets of responses. Am J Med 1983;75:133-7.
10. Bellamy N. WOMAC Osteoarthritis Index: A User’s Guide. London, Ontario, Canada: The Western Ontario and McMaster Universities; 1995.
11. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1998;75:800-2.
12. Miller R. Survival Analyses. New York: John Wiley & Sons; 1998.
13. Simon R, Lee YJ. Nonparametric confidence limits for survival probabilities and median survival time. Cancer Treat Rep 1982;66:37-42.
14. Amin AR, Attur M, Patel RN, et al. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest 1997;99:1231-7.
15. Hay C, de Belleroche J. Carrageenan-induced hyperalgesia is associated with increased cyclooxygenase-2 expression in spinal cord. Neuroreport 1997;8:1249-51.
16. Kang RY, Freire Moar, Sigal E, et al. Expression of cyclooxygenase-2 in human and an animal model of rheumatoid arthritis. Br J Rheumatol 1996;35:711-8.
17. Bensen WG, Zhao SZ, Burke TA, et al. Upper gastrointestinal tolerability of celecoxib, a COX-2 specific inhibitor, compared to naproxen and placebo. J Rheumatol 2000;27:1876-83.
18. Day R, Morrison B, Luza A, et al. A randomized trial of the efficacy and tolerability of the COX-2 inhibitor rofecoxib vs ibuprofen in patients with osteoarthritis. Rofecoxib/Ibuprofen Comparator Study Group. Arch Intern Med 2000;160:1781-7.
19. Fiechtner J, Sikes D, Recker D. A double-blind, placebo-controlled dose ranging study to evaluate the efficacy of valdecoxib, a novel COX-2 specific inhibitor, in treating the signs and symptoms of osteoarthritis of the knee. Paper presented at: European League Against Rheumatism (EULAR); May 13–16, 2001; Prague, Czech Republic.
20. Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual nonsteroidal anti-inflammatory drugs. Lancet 1994;343:769-72.
21. Singh G, Ramey DR, Morfeld D, et al. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Intern Med 1996;156:1530-6.
22. Singh G, Rosen Ramey D. NSAID induced gastrointestinal complications: the ARAMIS perspective-1997. Arthritis, Rheumatism, and Aging Medical Information System. J Rheumatol 1998;51(suppl):8-16.
23. Watson DJ, Harper SE, Zhao PL, et al. Gastrointestinal tolerability of the selective cyclooxygenase-2 (COX-2) inhibitor rofecoxib compared with nonselective COX-1 and COX-2 inhibitors in osteoarthritis. Arch Intern Med 2000;160:2998-3003.
24. Simon LS, Weaver AL, Graham DY, et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA 1999;282:1921-8.
25. Langman MJ, Jensen DM, Watson DJ, et al. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA 1999;282:1929-33.
26. Scholes D, Stergachis A, Penna P, Normand E, Hansten P. Nonsteroidal anti-inflammatory drug discontinuation in patients with osteoarthritis. J Rheumatol 1995;22:708-12.