User login
Autonomic Dysfunction in the Setting of CADASIL Syndrome
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) syndrome is the most common monogenic inherited cause of stroke. CADASIL syndrome is a nonsclerotic angiopathy resulting from a mutation of the NOTCH3 gene on chromosome 19p13, encoding a receptor expressed by vascular smooth muscle cells.1 This mutation results in migraine, recurrent ischemic stroke, affective disorders, and dementia, with migraine often manifesting earliest.2,3
The onset of stroke symptoms occurs typically in ages ≥ 60 years with some patients experiencing stroke as early as in their 30s.1,4 Presentation varies among patients even within the same family.5 CADASIL syndrome is frequently mistaken for other more common neurologic conditions due to the low prevalence of CADASIL syndrome, reported to be between 2 and 5 per 100,000.3,6 The cumulative nature of multiple ischemic episodes seen in 85% of symptomatic individuals leads to disability. Dementia is often hallmarked as one of the features of end-stage CADASIL syndrome.7 Extent and severity of brain tissue damage are shown to be the most critical factors of clinical symptoms.8 There is no specific treatment for CADASIL syndrome other than addressing risk factors.9
Symptoms are traditionally described to be limited to the central nervous system (CNS); however, reports of other organ system effects exist. Twenty-six percent of premature mortality relating to CADASIL syndrome is sudden unexpected death, which several authors have postulated could be attributed to cardiac events.10,11
The NOTCH3 gene encodes a protein expressed during gastrulation and in the CNS during embryological development. The expression of this protein decreases with time and has limited expression in adulthood.12 The pathophysiology of CADASIL syndrome includes myriad changes, including cerebral vessels narrowed by intimal thickening due to expansion of the extracellular matrix, degeneration of smooth muscle cells of the cerebral vessel walls, and osmiophilic material deposition in patients with CADASIL syndrome.13 Granular osmiophilic material in the vascular basal lamina can be observed on electron microscopy of patients with CADASIL syndrome and are used for diagnostic purposes.14
CADASIL syndrome often presents a diagnostic dilemma for physicians and is easy to misdiagnose in the early stages. The diagnostic dilemma arises given the subacute onset of CADASIL syndrome with vague early presenting symptoms, such as headache, prior to more specific findings (ie, multiple early strokes or transient ischemic attacks [TIA]). Patients presenting with CADASIL syndrome may be misdiagnosed with other neurologic conditions, including migraine or multiple sclerosis (MS).15 Especially in the case of MS, lesions visible on magnetic resonance imaging (MRI) may be differentiated by the higher rates of temporo polar lesions seen in CADASIL syndrome in comparison with those in MS.3
It is important to consider CADASIL syndrome in patients presenting at a young age with stroke due to the compounding effects of multiple ischemic episodes and subsequent motor/sensory and neuropsychologic deficits. This necessitates increasing awareness of CADASIL syndrome in the neurologic and radiologic community and the importance of educating families of patients on the importance of being evaluated. This diagnostic dilemma can lead to delay in appropriate therapy and control of related modifiable risk factors, including hypertension, hyperlipidemia, etc. Delays in initiation of anti-stroke pharmacotherapy can lead to additional morbidity and mortality in these patients.
The radiology of CADASIL syndrome is unique and particularly important due to the possible confusion with MS. MRI is an important tool in the evaluation of the cerebral pathology of CADASIL syndrome, revealing white matter and microangiopathic signal abnormalities, indicative of ischemic infarcts, lacunar strokes, and diffuse leukoencephalopathy.13,16 MRI lesions are often seen in the basal ganglia, thalamus, external capsule, and pons.7 The lesions also are seen in the periventricular region, explaining its misperception as MS.17 In addition, cerebral microhemorrhages have been seen. To further differentiate these lesions, the anterior temporal lobe should be observed for gliosis or hyperintensities, which correlates with CADASIL syndrome.18 Location of hyperintensity in the temporal lobes, relative sparing of the occipital/orbitofrontal white matter, corpus callosum, subcortical u-fibers, and cortex is helpful in differentiating from other etiologies, such as microvascular white matter ischemic disease, MS, and mitochondrial encephalopathy with lactic acidosis and strokelike symptoms (MELAS).
Case Presentation
A patient aged > 50 years presented to the emergency department (ED) due to numbness of the right perioral area, gait difficulties, difficulty speaking, and increasing right lower extremity weakness with no numbness or paresthesia. The patient’s medical history is relevant for CADASIL syndrome, hypertension, prior cerebrovascular accident, recurrent TIAs, multinodular goiter with a history of radioactive iodine treatment, and neurogenic bladder controlled with oxybutynin since age 30 years. The patient had a significant stroke history: the first stroke occurred at age 36 years and 3 more strokes at ages 38, 44, and 53 years and 4 TIAs over that period. This patient reported no recent headache or memory changes and had no history of smoking, alcohol, or recreational drug use. Family history was pertinent for the mother’s death secondary to stroke, with a history of multiple strokes beginning at a young, undetermined age and no major motor, sensory, or neuropsychologic deficits prior to her death. A sister and first cousin had been diagnosed with MS.
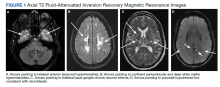
On triage in the ED, stroke alert was called but tissue plasminogen activator was not given due to time eligibility. The patient’s numbness and weakness were improved within 7 hours, but she continued to have difficulty with dysarthric speech and unsteady gait following this incident. Antihypertensive medications were discontinued on admission to allow for permissive hypertension to improve cerebral blood flow. A brain MRI revealed bilateral increased T2 fluid-attenuated inversion recovery (FLAIR) signal in the anterior temporal lobes, confluent increased T2 FLAIR signal in the periventricular/deep white matter, bilateral basal ganglia chronic lacunar infarcts, and several chronic microbleeds (Figure 1). There was no evidence for an acute infarct on the MRI. Recrudescence of prior stroke symptoms secondary to CADASIL syndrome was suspected as a primary diagnosis with a differential of TIA.
Starting the second day of admission, the patient had intermittent sinus bradycardia with the lowest heart rate (HR) in the range of 40 beats per minute (bpm) while awake with an unchanged neurologic examination. Each episode was transient, lasting less than an hour per staff documentation. The electrocardiogram (ECG) on admission demonstrated normal sinus rhythm in the range of 70 to 80 bpm.
The patient was asymptomatic and normotensive during the episodes of bradycardia. The patient had not yet resumed any antihypertensives. An echocardiogram was unremarkable with a left ventricular ejection fraction of 55 to 60%, normal anatomy, and no significant pericardial effusion. Carotid artery duplex examination demonstrated patent vessels with anterograde vertebral flow bilaterally. Due to the unknown cause of the bradycardia, the patient was discharged with a 14-day ambulatory cardiac monitor, advised to continue statin, aspirin, and lisinopril, and given a referral to continue with outpatient physical therapy and occupational therapy.
The patient’s ambulatory cardiac monitoring showed dominant sinus rhythm, with the HR in the range of 40 to 170 bpm with an overall average 70 to 80 bpm. The patient’s HR spent 5% of the recording time under 50 bpm and 14% of the time > 100. There was no evidence of heart block. No symptoms were recorded per the patient’s symptom diary during the entire 2 weeks of monitoring. Further follow-up showed that the patient presented to a primary care practitioner 1 month later with similar symptoms and was sent to the ED of an outside hospital without admission. The ECG was again unremarkable, demonstrating only sinus bradycardia with normal T waves, QT interval, without ST elevations or depressions. About 3 weeks later, the patient presented to the ED again with chest pain and was discharged with a diagnosis of atypical chest pain possibly related to anxiety without findings consistent with acute coronary syndrome (ACS).
Discussion
This patient with CADASIL syndrome and significant stroke history with cardiac symptoms demonstrates 3 important discussion points: the difficulty of early diagnosis, high rates of morbidity/mortality, and the need for further research into the cardiac effects of CADASIL syndrome. Due to this patient’s bradycardic episodes while being monitored on telemetry, it is possible that the cause of the strokelike symptoms was a TIA, secondary to decreased perfusion pressure, explaining the lack of acute ischemia on imaging. With regards to the history of thyroid dysfunction, this particular episode of bradycardia was unlikely to be related as the thyroid-stimulating hormone was reflective of subclinical hyperthyroidism with T4 levels within normal limits.
This case demonstrates a potential link between CADASIL syndrome and autonomic dysfunction. Similar to general stroke patients, patients with CADASIL syndrome are at an increased risk of hypoperfusion injury secondary to cardiovascular and autonomic dysfunction. This raises a question of initial and surveillance screening tests on diagnosis of CADASIL syndrome. It may be appropriate to obtain routine echocardiogram and ECG and other arrhythmia screening tests in these patients, especially during or following an ischemic episode. However, more evidence is required to support creation of a formal recommendation.
In a study of cardiac rhythm abnormalities in a half-million adults, 1.57% of women aged 55 to 64 years were found to have rhythm abnormality with 0.27% having a bradyarrhythmia.19 In the setting of neurologic disease, ECG changes such as arrhythmias and repolarization changes are regularly noted.20 However, it is unlikely that the bradycardia would be causing the brain lesions. In CADASIL syndrome, there is relative sparing of the occipital, orbitofrontal subcortical white matter, subcortical fibers, and cortex. Specifically, within CADASIL syndrome, a study of 23 patients showed no ECG changes regarding infarction/ischemia, conduction disturbances, or arrhythmias compared with that of controls.21
Further research into the cardiac effects of CADASIL syndrome is needed. As CADASIL syndrome is primarily a disorder of the vasculature, the disease has potential to affect the heart in addition to the brain.1 This theory is well supported by the embryologic effects of the NOTCH3 receptor pathways, which are responsible for the development of the cardiovascular system.22 Anecdotal evidence supports this theory as few case reports have been published that describe various cardiac abnormalities in patients with CADASIL syndrome, including myocardial infarction (MI), conduction abnormalities, and arrhythmias.2, 23-25
There have only been 2 published studies regarding investigations into CADASIL syndrome and cardiac disease. The first paper was a case-control study that investigated ECG changes in the setting of CADASIL syndrome. The study found no evidence for MI, ischemia, conduction disorder, or arrhythmias in patients with CADASIL syndrome.21 Unfortunately, this study was underpowered and limited in scope, only investigating a single ECG recording from 23 patients with CADASIL syndrome in a single clinic.21 Other cardiac markers, such as echocardiogram, stress test, and contractility, and longitudinal cardiac outcomes were not investigated in this study.21 The second paper was also a case-control study by Rufa and colleagues that investigated HR variability and other ECG changes during a 10-minute rest recording on 23 patients with CADASIL syndrome and compared the results to 22 age- and gender-matched patients in good health.11
This study found reduced HR variability and an increased ratio of low-frequency to high-frequency variability, which the authors claimed demonstrates autonomic dysfunction in patients with CADASIL syndrome.11 Rufa and colleagues concluded that patients with CADASIL syndrome are at higher risk for cardiac arrhythmias.11 This study also found no evidence for MI, ischemia, conduction disorder, or arrhythmias in the patients with CADASIL syndrome compared with that of age-matched controls.11 Similar to the first paper, this study is underpowered, only looks at a single timepoint recording, and uses incomplete and indirect measurements of cardiac function.
There is a need for a longitudinal review of cardiac outcomes in the CADASIL syndrome population to determine whether these patients require additional surveillance or prophylaxis. While the variability in HR of our patient cannot be definitively attributed solely to CADASIL syndrome, the subsequent admissions demonstrate that long-term monitoring may be warranted.
Conclusions
CADASIL syndrome is an autosomal dominant NOTCH3 signaling disease that affects the small vessel vasculature and leads to early ischemic events, headache, dementia, and death. CADASIL syndrome is frequently misdiagnosed due to insidious onset and vague presenting symptoms. Delay in diagnosis often results in nonoptimized medical management. Current guidelines recommend following poststroke protocol and minimizing individual risk factors by using antiplatelet, antihypertensive, and dyslipidemia medications. This case demonstrates a classic presentation of CADASIL syndrome with lesser described cardiac symptoms. Few cases of unusual cardiac symptoms in the setting of CADASIL syndrome have been reported. The relationship between cardiovascular disease and CADASIL syndrome is not well described. Further research is needed to elucidate any links between CADASIL syndrome and cardiovascular disease and to optimize management for these patients.
1. Moreton FC, Razvi SS, Davidson R, Muir KW. Changing clinical patterns and increasing prevalence in CADASIL. Acta Neurol Scand. 2014;130(3):197-203. doi:10.1111/ane.12266
2. Lesnik Oberstein SA, Jukema JW, Van Duinen SG, Macfarlane PW, van Houwelingen HC, Breuning MH, et al. Myocardial infarction in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Medicine (Baltimore). 2003;82(4):251-256. doi:10.1097/01.md.0000085054.63483.40
3. Di Donato I, Bianchi S, De Stefano N, Dichgans M, Dotti MT, Duering M, et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med. 2017;15(1):41. doi:10.1186/s12916-017-0778-8
4. Dunphy L, Rani A, Duodu Y, Behnam Y. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) presenting with stroke in a young man. BMJ Case Rep. 2019 ;12(7):e229609. doi:10.1136/bcr-2019-229609
5. Bianchi S, Zicari E, Carluccio A, Di Donato I, Pescini F, Nannucci S, et al. CADASIL in central Italy: a retrospective clinical and genetic study in 229 patients. J Neurol. 2015;262(1):134-141. doi:10.1007/s00415-014-7533-2
6. Phillips CD, Zuckerman SJ, Medical Education Commission. CADASIL can mimic multiple sclerosis. J La State Med Soc. 2010 May-Jun;162(3):174.
7. Hervé D, Chabriat H. CADASIL. J Geriatr Psychiatry Neurol. 2010;23(4):269-276. doi:10.1177/0891988710383570
8. Yamamoto Y, Hase Y, Ihara M, Khundakar A, Roeber S, Duering M, et al. Neuronal densities and vascular pathology in the hippocampal formation in CADASIL. Neurobiol Aging. 2021;97:33-40. doi:10.1016/j.neurobiolaging.2020.09.016
9. Ferrante EA, Cudrici CD, Boehm M. CADASIL: new advances in basic science and clinical perspectives. Curr Opin Hematol. 2019;26(3):193-198. doi:10.1097/MOH.0000000000000497
10. Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004;127(pt 11):2533-2539.
11. Rufa A, Guideri F, Acampa M, Cevenini G, Bianchi S, De Stefano N, et al. Cardiac autonomic nervous system and risk of arrhythmias in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Stroke. 2007 Feb;38(2):276-280. doi:10.1093/brain/awh282
12. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707-710. doi:10.1038/383707a0
13. Kalaria RN, Viitanen M, Kalimo H, Dichgans M, Tabira T, CASASIL Group of Vas-Cog. The pathogenesis of CADASIL: an update. J Neurol Sci. 2004;226(1-2):35-39. doi:10.1016/j.jns.2004.09.008
14. Reddy SPK, Vishnu VY, Goyal V, Singh MB, Arora S, Garg A, et al. CADASIL syndrome and stroke in young people. QJM. 2020 Feb 1;113(2):118-119. doi:10.1093/qjmed/hcz243
15. Carone DA. CADASIL and multiple sclerosis: A case report of prolonged misdiagnosis. Applied neuropsychology Adult. 2017;24(3):294-297. doi:10.1080/23279095.2016.1214132
16. Zhu S, Nahas SJ. CADASIL: Imaging characteristics and clinical correlation. Curr Pain Headache Rep. 2016;20(10):57. doi:10.1007/s11916-016-0584-6
17. Kalaria RN, Low WC, Oakley AE, Slade JY, Ince PG, Morris CM, et al. CADASIL and genetics of cerebral ischaemia. J Neural Transm Suppl. 2002;(63):75-90. doi:10.1007/978-3-7091-6137-1_5
18. O’Sullivan M, Jarosz JM, Martin RJ, Deasy N, Powell JF, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56(5):628-634. doi:10.1212/wnl.56.5.628
19. Khurshid S, Choi SH, Weng L-C, Wang EY, Trinquart L, Benjamin EJ, et al. Frequency of cardiac rhythm abnormalities in a half million adults. Circ ArrhythmElectrophysiol. 2018;11(7):e006273. doi:10.1161/CIRCEP.118.006273
20. Samuels MA. The brain–heart connection. Circulation. 2007;116(1):77-84. doi:10.1161/CIRCULATIONAHA. 106.678995
21. Cumurciuc R, Henry P, Gobron C, Vicaut E, Bousser MG, Chabriat H, et al. Electrocardiogram in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy patients without any clinical evidence of coronary artery disease: a case-control study. Stroke. 2006;37(4):1100-1102. doi:10.1161/01.STR.0000209242.68844.20
22. Luxán G, D’Amato G, MacGrogan D, de la Pompa JL. Endocardial notch signaling in cardiac development and disease. Circ Res. 2016;118(1):e1-e18. doi:10.1161/CIRCRESAHA.115.305350
23. Rubin CB, Hahn V, Kobayashi T, Litwack A. A report of accelerated coronary artery disease associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Case Rep Cardiol. 2015;2015:167513. doi:10.1155/2015/167513
24. Langer C, Adukauskaite A, Plank F, Feuchtner G, Cartes-Zumelzu F. Cerebral autosomal dominant arteriopathy (CADASIL) with cardiac involvement (ANOCA) and subcortical leukencephalopathy. J Cardiovasc Comput Tomogr. 2020;14(5):e1-e6. doi:10.1016/j.jcct.2018.08.005
25. Pettersen JA, Keith J, Gao F, Spence JD, Black SE. CADASIL accelerated by acute hypotension: Arterial and venous contribution to leukoaraiosis. Neurology. 2017;88(11):1077-1080. doi:10.1212/WNL.0000000000003717
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) syndrome is the most common monogenic inherited cause of stroke. CADASIL syndrome is a nonsclerotic angiopathy resulting from a mutation of the NOTCH3 gene on chromosome 19p13, encoding a receptor expressed by vascular smooth muscle cells.1 This mutation results in migraine, recurrent ischemic stroke, affective disorders, and dementia, with migraine often manifesting earliest.2,3
The onset of stroke symptoms occurs typically in ages ≥ 60 years with some patients experiencing stroke as early as in their 30s.1,4 Presentation varies among patients even within the same family.5 CADASIL syndrome is frequently mistaken for other more common neurologic conditions due to the low prevalence of CADASIL syndrome, reported to be between 2 and 5 per 100,000.3,6 The cumulative nature of multiple ischemic episodes seen in 85% of symptomatic individuals leads to disability. Dementia is often hallmarked as one of the features of end-stage CADASIL syndrome.7 Extent and severity of brain tissue damage are shown to be the most critical factors of clinical symptoms.8 There is no specific treatment for CADASIL syndrome other than addressing risk factors.9
Symptoms are traditionally described to be limited to the central nervous system (CNS); however, reports of other organ system effects exist. Twenty-six percent of premature mortality relating to CADASIL syndrome is sudden unexpected death, which several authors have postulated could be attributed to cardiac events.10,11
The NOTCH3 gene encodes a protein expressed during gastrulation and in the CNS during embryological development. The expression of this protein decreases with time and has limited expression in adulthood.12 The pathophysiology of CADASIL syndrome includes myriad changes, including cerebral vessels narrowed by intimal thickening due to expansion of the extracellular matrix, degeneration of smooth muscle cells of the cerebral vessel walls, and osmiophilic material deposition in patients with CADASIL syndrome.13 Granular osmiophilic material in the vascular basal lamina can be observed on electron microscopy of patients with CADASIL syndrome and are used for diagnostic purposes.14
CADASIL syndrome often presents a diagnostic dilemma for physicians and is easy to misdiagnose in the early stages. The diagnostic dilemma arises given the subacute onset of CADASIL syndrome with vague early presenting symptoms, such as headache, prior to more specific findings (ie, multiple early strokes or transient ischemic attacks [TIA]). Patients presenting with CADASIL syndrome may be misdiagnosed with other neurologic conditions, including migraine or multiple sclerosis (MS).15 Especially in the case of MS, lesions visible on magnetic resonance imaging (MRI) may be differentiated by the higher rates of temporo polar lesions seen in CADASIL syndrome in comparison with those in MS.3
It is important to consider CADASIL syndrome in patients presenting at a young age with stroke due to the compounding effects of multiple ischemic episodes and subsequent motor/sensory and neuropsychologic deficits. This necessitates increasing awareness of CADASIL syndrome in the neurologic and radiologic community and the importance of educating families of patients on the importance of being evaluated. This diagnostic dilemma can lead to delay in appropriate therapy and control of related modifiable risk factors, including hypertension, hyperlipidemia, etc. Delays in initiation of anti-stroke pharmacotherapy can lead to additional morbidity and mortality in these patients.
The radiology of CADASIL syndrome is unique and particularly important due to the possible confusion with MS. MRI is an important tool in the evaluation of the cerebral pathology of CADASIL syndrome, revealing white matter and microangiopathic signal abnormalities, indicative of ischemic infarcts, lacunar strokes, and diffuse leukoencephalopathy.13,16 MRI lesions are often seen in the basal ganglia, thalamus, external capsule, and pons.7 The lesions also are seen in the periventricular region, explaining its misperception as MS.17 In addition, cerebral microhemorrhages have been seen. To further differentiate these lesions, the anterior temporal lobe should be observed for gliosis or hyperintensities, which correlates with CADASIL syndrome.18 Location of hyperintensity in the temporal lobes, relative sparing of the occipital/orbitofrontal white matter, corpus callosum, subcortical u-fibers, and cortex is helpful in differentiating from other etiologies, such as microvascular white matter ischemic disease, MS, and mitochondrial encephalopathy with lactic acidosis and strokelike symptoms (MELAS).
Case Presentation
A patient aged > 50 years presented to the emergency department (ED) due to numbness of the right perioral area, gait difficulties, difficulty speaking, and increasing right lower extremity weakness with no numbness or paresthesia. The patient’s medical history is relevant for CADASIL syndrome, hypertension, prior cerebrovascular accident, recurrent TIAs, multinodular goiter with a history of radioactive iodine treatment, and neurogenic bladder controlled with oxybutynin since age 30 years. The patient had a significant stroke history: the first stroke occurred at age 36 years and 3 more strokes at ages 38, 44, and 53 years and 4 TIAs over that period. This patient reported no recent headache or memory changes and had no history of smoking, alcohol, or recreational drug use. Family history was pertinent for the mother’s death secondary to stroke, with a history of multiple strokes beginning at a young, undetermined age and no major motor, sensory, or neuropsychologic deficits prior to her death. A sister and first cousin had been diagnosed with MS.
On triage in the ED, stroke alert was called but tissue plasminogen activator was not given due to time eligibility. The patient’s numbness and weakness were improved within 7 hours, but she continued to have difficulty with dysarthric speech and unsteady gait following this incident. Antihypertensive medications were discontinued on admission to allow for permissive hypertension to improve cerebral blood flow. A brain MRI revealed bilateral increased T2 fluid-attenuated inversion recovery (FLAIR) signal in the anterior temporal lobes, confluent increased T2 FLAIR signal in the periventricular/deep white matter, bilateral basal ganglia chronic lacunar infarcts, and several chronic microbleeds (Figure 1). There was no evidence for an acute infarct on the MRI. Recrudescence of prior stroke symptoms secondary to CADASIL syndrome was suspected as a primary diagnosis with a differential of TIA.
Starting the second day of admission, the patient had intermittent sinus bradycardia with the lowest heart rate (HR) in the range of 40 beats per minute (bpm) while awake with an unchanged neurologic examination. Each episode was transient, lasting less than an hour per staff documentation. The electrocardiogram (ECG) on admission demonstrated normal sinus rhythm in the range of 70 to 80 bpm.
The patient was asymptomatic and normotensive during the episodes of bradycardia. The patient had not yet resumed any antihypertensives. An echocardiogram was unremarkable with a left ventricular ejection fraction of 55 to 60%, normal anatomy, and no significant pericardial effusion. Carotid artery duplex examination demonstrated patent vessels with anterograde vertebral flow bilaterally. Due to the unknown cause of the bradycardia, the patient was discharged with a 14-day ambulatory cardiac monitor, advised to continue statin, aspirin, and lisinopril, and given a referral to continue with outpatient physical therapy and occupational therapy.
The patient’s ambulatory cardiac monitoring showed dominant sinus rhythm, with the HR in the range of 40 to 170 bpm with an overall average 70 to 80 bpm. The patient’s HR spent 5% of the recording time under 50 bpm and 14% of the time > 100. There was no evidence of heart block. No symptoms were recorded per the patient’s symptom diary during the entire 2 weeks of monitoring. Further follow-up showed that the patient presented to a primary care practitioner 1 month later with similar symptoms and was sent to the ED of an outside hospital without admission. The ECG was again unremarkable, demonstrating only sinus bradycardia with normal T waves, QT interval, without ST elevations or depressions. About 3 weeks later, the patient presented to the ED again with chest pain and was discharged with a diagnosis of atypical chest pain possibly related to anxiety without findings consistent with acute coronary syndrome (ACS).
Discussion
This patient with CADASIL syndrome and significant stroke history with cardiac symptoms demonstrates 3 important discussion points: the difficulty of early diagnosis, high rates of morbidity/mortality, and the need for further research into the cardiac effects of CADASIL syndrome. Due to this patient’s bradycardic episodes while being monitored on telemetry, it is possible that the cause of the strokelike symptoms was a TIA, secondary to decreased perfusion pressure, explaining the lack of acute ischemia on imaging. With regards to the history of thyroid dysfunction, this particular episode of bradycardia was unlikely to be related as the thyroid-stimulating hormone was reflective of subclinical hyperthyroidism with T4 levels within normal limits.
This case demonstrates a potential link between CADASIL syndrome and autonomic dysfunction. Similar to general stroke patients, patients with CADASIL syndrome are at an increased risk of hypoperfusion injury secondary to cardiovascular and autonomic dysfunction. This raises a question of initial and surveillance screening tests on diagnosis of CADASIL syndrome. It may be appropriate to obtain routine echocardiogram and ECG and other arrhythmia screening tests in these patients, especially during or following an ischemic episode. However, more evidence is required to support creation of a formal recommendation.
In a study of cardiac rhythm abnormalities in a half-million adults, 1.57% of women aged 55 to 64 years were found to have rhythm abnormality with 0.27% having a bradyarrhythmia.19 In the setting of neurologic disease, ECG changes such as arrhythmias and repolarization changes are regularly noted.20 However, it is unlikely that the bradycardia would be causing the brain lesions. In CADASIL syndrome, there is relative sparing of the occipital, orbitofrontal subcortical white matter, subcortical fibers, and cortex. Specifically, within CADASIL syndrome, a study of 23 patients showed no ECG changes regarding infarction/ischemia, conduction disturbances, or arrhythmias compared with that of controls.21
Further research into the cardiac effects of CADASIL syndrome is needed. As CADASIL syndrome is primarily a disorder of the vasculature, the disease has potential to affect the heart in addition to the brain.1 This theory is well supported by the embryologic effects of the NOTCH3 receptor pathways, which are responsible for the development of the cardiovascular system.22 Anecdotal evidence supports this theory as few case reports have been published that describe various cardiac abnormalities in patients with CADASIL syndrome, including myocardial infarction (MI), conduction abnormalities, and arrhythmias.2, 23-25
There have only been 2 published studies regarding investigations into CADASIL syndrome and cardiac disease. The first paper was a case-control study that investigated ECG changes in the setting of CADASIL syndrome. The study found no evidence for MI, ischemia, conduction disorder, or arrhythmias in patients with CADASIL syndrome.21 Unfortunately, this study was underpowered and limited in scope, only investigating a single ECG recording from 23 patients with CADASIL syndrome in a single clinic.21 Other cardiac markers, such as echocardiogram, stress test, and contractility, and longitudinal cardiac outcomes were not investigated in this study.21 The second paper was also a case-control study by Rufa and colleagues that investigated HR variability and other ECG changes during a 10-minute rest recording on 23 patients with CADASIL syndrome and compared the results to 22 age- and gender-matched patients in good health.11
This study found reduced HR variability and an increased ratio of low-frequency to high-frequency variability, which the authors claimed demonstrates autonomic dysfunction in patients with CADASIL syndrome.11 Rufa and colleagues concluded that patients with CADASIL syndrome are at higher risk for cardiac arrhythmias.11 This study also found no evidence for MI, ischemia, conduction disorder, or arrhythmias in the patients with CADASIL syndrome compared with that of age-matched controls.11 Similar to the first paper, this study is underpowered, only looks at a single timepoint recording, and uses incomplete and indirect measurements of cardiac function.
There is a need for a longitudinal review of cardiac outcomes in the CADASIL syndrome population to determine whether these patients require additional surveillance or prophylaxis. While the variability in HR of our patient cannot be definitively attributed solely to CADASIL syndrome, the subsequent admissions demonstrate that long-term monitoring may be warranted.
Conclusions
CADASIL syndrome is an autosomal dominant NOTCH3 signaling disease that affects the small vessel vasculature and leads to early ischemic events, headache, dementia, and death. CADASIL syndrome is frequently misdiagnosed due to insidious onset and vague presenting symptoms. Delay in diagnosis often results in nonoptimized medical management. Current guidelines recommend following poststroke protocol and minimizing individual risk factors by using antiplatelet, antihypertensive, and dyslipidemia medications. This case demonstrates a classic presentation of CADASIL syndrome with lesser described cardiac symptoms. Few cases of unusual cardiac symptoms in the setting of CADASIL syndrome have been reported. The relationship between cardiovascular disease and CADASIL syndrome is not well described. Further research is needed to elucidate any links between CADASIL syndrome and cardiovascular disease and to optimize management for these patients.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) syndrome is the most common monogenic inherited cause of stroke. CADASIL syndrome is a nonsclerotic angiopathy resulting from a mutation of the NOTCH3 gene on chromosome 19p13, encoding a receptor expressed by vascular smooth muscle cells.1 This mutation results in migraine, recurrent ischemic stroke, affective disorders, and dementia, with migraine often manifesting earliest.2,3
The onset of stroke symptoms occurs typically in ages ≥ 60 years with some patients experiencing stroke as early as in their 30s.1,4 Presentation varies among patients even within the same family.5 CADASIL syndrome is frequently mistaken for other more common neurologic conditions due to the low prevalence of CADASIL syndrome, reported to be between 2 and 5 per 100,000.3,6 The cumulative nature of multiple ischemic episodes seen in 85% of symptomatic individuals leads to disability. Dementia is often hallmarked as one of the features of end-stage CADASIL syndrome.7 Extent and severity of brain tissue damage are shown to be the most critical factors of clinical symptoms.8 There is no specific treatment for CADASIL syndrome other than addressing risk factors.9
Symptoms are traditionally described to be limited to the central nervous system (CNS); however, reports of other organ system effects exist. Twenty-six percent of premature mortality relating to CADASIL syndrome is sudden unexpected death, which several authors have postulated could be attributed to cardiac events.10,11
The NOTCH3 gene encodes a protein expressed during gastrulation and in the CNS during embryological development. The expression of this protein decreases with time and has limited expression in adulthood.12 The pathophysiology of CADASIL syndrome includes myriad changes, including cerebral vessels narrowed by intimal thickening due to expansion of the extracellular matrix, degeneration of smooth muscle cells of the cerebral vessel walls, and osmiophilic material deposition in patients with CADASIL syndrome.13 Granular osmiophilic material in the vascular basal lamina can be observed on electron microscopy of patients with CADASIL syndrome and are used for diagnostic purposes.14
CADASIL syndrome often presents a diagnostic dilemma for physicians and is easy to misdiagnose in the early stages. The diagnostic dilemma arises given the subacute onset of CADASIL syndrome with vague early presenting symptoms, such as headache, prior to more specific findings (ie, multiple early strokes or transient ischemic attacks [TIA]). Patients presenting with CADASIL syndrome may be misdiagnosed with other neurologic conditions, including migraine or multiple sclerosis (MS).15 Especially in the case of MS, lesions visible on magnetic resonance imaging (MRI) may be differentiated by the higher rates of temporo polar lesions seen in CADASIL syndrome in comparison with those in MS.3
It is important to consider CADASIL syndrome in patients presenting at a young age with stroke due to the compounding effects of multiple ischemic episodes and subsequent motor/sensory and neuropsychologic deficits. This necessitates increasing awareness of CADASIL syndrome in the neurologic and radiologic community and the importance of educating families of patients on the importance of being evaluated. This diagnostic dilemma can lead to delay in appropriate therapy and control of related modifiable risk factors, including hypertension, hyperlipidemia, etc. Delays in initiation of anti-stroke pharmacotherapy can lead to additional morbidity and mortality in these patients.
The radiology of CADASIL syndrome is unique and particularly important due to the possible confusion with MS. MRI is an important tool in the evaluation of the cerebral pathology of CADASIL syndrome, revealing white matter and microangiopathic signal abnormalities, indicative of ischemic infarcts, lacunar strokes, and diffuse leukoencephalopathy.13,16 MRI lesions are often seen in the basal ganglia, thalamus, external capsule, and pons.7 The lesions also are seen in the periventricular region, explaining its misperception as MS.17 In addition, cerebral microhemorrhages have been seen. To further differentiate these lesions, the anterior temporal lobe should be observed for gliosis or hyperintensities, which correlates with CADASIL syndrome.18 Location of hyperintensity in the temporal lobes, relative sparing of the occipital/orbitofrontal white matter, corpus callosum, subcortical u-fibers, and cortex is helpful in differentiating from other etiologies, such as microvascular white matter ischemic disease, MS, and mitochondrial encephalopathy with lactic acidosis and strokelike symptoms (MELAS).
Case Presentation
A patient aged > 50 years presented to the emergency department (ED) due to numbness of the right perioral area, gait difficulties, difficulty speaking, and increasing right lower extremity weakness with no numbness or paresthesia. The patient’s medical history is relevant for CADASIL syndrome, hypertension, prior cerebrovascular accident, recurrent TIAs, multinodular goiter with a history of radioactive iodine treatment, and neurogenic bladder controlled with oxybutynin since age 30 years. The patient had a significant stroke history: the first stroke occurred at age 36 years and 3 more strokes at ages 38, 44, and 53 years and 4 TIAs over that period. This patient reported no recent headache or memory changes and had no history of smoking, alcohol, or recreational drug use. Family history was pertinent for the mother’s death secondary to stroke, with a history of multiple strokes beginning at a young, undetermined age and no major motor, sensory, or neuropsychologic deficits prior to her death. A sister and first cousin had been diagnosed with MS.
On triage in the ED, stroke alert was called but tissue plasminogen activator was not given due to time eligibility. The patient’s numbness and weakness were improved within 7 hours, but she continued to have difficulty with dysarthric speech and unsteady gait following this incident. Antihypertensive medications were discontinued on admission to allow for permissive hypertension to improve cerebral blood flow. A brain MRI revealed bilateral increased T2 fluid-attenuated inversion recovery (FLAIR) signal in the anterior temporal lobes, confluent increased T2 FLAIR signal in the periventricular/deep white matter, bilateral basal ganglia chronic lacunar infarcts, and several chronic microbleeds (Figure 1). There was no evidence for an acute infarct on the MRI. Recrudescence of prior stroke symptoms secondary to CADASIL syndrome was suspected as a primary diagnosis with a differential of TIA.
Starting the second day of admission, the patient had intermittent sinus bradycardia with the lowest heart rate (HR) in the range of 40 beats per minute (bpm) while awake with an unchanged neurologic examination. Each episode was transient, lasting less than an hour per staff documentation. The electrocardiogram (ECG) on admission demonstrated normal sinus rhythm in the range of 70 to 80 bpm.
The patient was asymptomatic and normotensive during the episodes of bradycardia. The patient had not yet resumed any antihypertensives. An echocardiogram was unremarkable with a left ventricular ejection fraction of 55 to 60%, normal anatomy, and no significant pericardial effusion. Carotid artery duplex examination demonstrated patent vessels with anterograde vertebral flow bilaterally. Due to the unknown cause of the bradycardia, the patient was discharged with a 14-day ambulatory cardiac monitor, advised to continue statin, aspirin, and lisinopril, and given a referral to continue with outpatient physical therapy and occupational therapy.
The patient’s ambulatory cardiac monitoring showed dominant sinus rhythm, with the HR in the range of 40 to 170 bpm with an overall average 70 to 80 bpm. The patient’s HR spent 5% of the recording time under 50 bpm and 14% of the time > 100. There was no evidence of heart block. No symptoms were recorded per the patient’s symptom diary during the entire 2 weeks of monitoring. Further follow-up showed that the patient presented to a primary care practitioner 1 month later with similar symptoms and was sent to the ED of an outside hospital without admission. The ECG was again unremarkable, demonstrating only sinus bradycardia with normal T waves, QT interval, without ST elevations or depressions. About 3 weeks later, the patient presented to the ED again with chest pain and was discharged with a diagnosis of atypical chest pain possibly related to anxiety without findings consistent with acute coronary syndrome (ACS).
Discussion
This patient with CADASIL syndrome and significant stroke history with cardiac symptoms demonstrates 3 important discussion points: the difficulty of early diagnosis, high rates of morbidity/mortality, and the need for further research into the cardiac effects of CADASIL syndrome. Due to this patient’s bradycardic episodes while being monitored on telemetry, it is possible that the cause of the strokelike symptoms was a TIA, secondary to decreased perfusion pressure, explaining the lack of acute ischemia on imaging. With regards to the history of thyroid dysfunction, this particular episode of bradycardia was unlikely to be related as the thyroid-stimulating hormone was reflective of subclinical hyperthyroidism with T4 levels within normal limits.
This case demonstrates a potential link between CADASIL syndrome and autonomic dysfunction. Similar to general stroke patients, patients with CADASIL syndrome are at an increased risk of hypoperfusion injury secondary to cardiovascular and autonomic dysfunction. This raises a question of initial and surveillance screening tests on diagnosis of CADASIL syndrome. It may be appropriate to obtain routine echocardiogram and ECG and other arrhythmia screening tests in these patients, especially during or following an ischemic episode. However, more evidence is required to support creation of a formal recommendation.
In a study of cardiac rhythm abnormalities in a half-million adults, 1.57% of women aged 55 to 64 years were found to have rhythm abnormality with 0.27% having a bradyarrhythmia.19 In the setting of neurologic disease, ECG changes such as arrhythmias and repolarization changes are regularly noted.20 However, it is unlikely that the bradycardia would be causing the brain lesions. In CADASIL syndrome, there is relative sparing of the occipital, orbitofrontal subcortical white matter, subcortical fibers, and cortex. Specifically, within CADASIL syndrome, a study of 23 patients showed no ECG changes regarding infarction/ischemia, conduction disturbances, or arrhythmias compared with that of controls.21
Further research into the cardiac effects of CADASIL syndrome is needed. As CADASIL syndrome is primarily a disorder of the vasculature, the disease has potential to affect the heart in addition to the brain.1 This theory is well supported by the embryologic effects of the NOTCH3 receptor pathways, which are responsible for the development of the cardiovascular system.22 Anecdotal evidence supports this theory as few case reports have been published that describe various cardiac abnormalities in patients with CADASIL syndrome, including myocardial infarction (MI), conduction abnormalities, and arrhythmias.2, 23-25
There have only been 2 published studies regarding investigations into CADASIL syndrome and cardiac disease. The first paper was a case-control study that investigated ECG changes in the setting of CADASIL syndrome. The study found no evidence for MI, ischemia, conduction disorder, or arrhythmias in patients with CADASIL syndrome.21 Unfortunately, this study was underpowered and limited in scope, only investigating a single ECG recording from 23 patients with CADASIL syndrome in a single clinic.21 Other cardiac markers, such as echocardiogram, stress test, and contractility, and longitudinal cardiac outcomes were not investigated in this study.21 The second paper was also a case-control study by Rufa and colleagues that investigated HR variability and other ECG changes during a 10-minute rest recording on 23 patients with CADASIL syndrome and compared the results to 22 age- and gender-matched patients in good health.11
This study found reduced HR variability and an increased ratio of low-frequency to high-frequency variability, which the authors claimed demonstrates autonomic dysfunction in patients with CADASIL syndrome.11 Rufa and colleagues concluded that patients with CADASIL syndrome are at higher risk for cardiac arrhythmias.11 This study also found no evidence for MI, ischemia, conduction disorder, or arrhythmias in the patients with CADASIL syndrome compared with that of age-matched controls.11 Similar to the first paper, this study is underpowered, only looks at a single timepoint recording, and uses incomplete and indirect measurements of cardiac function.
There is a need for a longitudinal review of cardiac outcomes in the CADASIL syndrome population to determine whether these patients require additional surveillance or prophylaxis. While the variability in HR of our patient cannot be definitively attributed solely to CADASIL syndrome, the subsequent admissions demonstrate that long-term monitoring may be warranted.
Conclusions
CADASIL syndrome is an autosomal dominant NOTCH3 signaling disease that affects the small vessel vasculature and leads to early ischemic events, headache, dementia, and death. CADASIL syndrome is frequently misdiagnosed due to insidious onset and vague presenting symptoms. Delay in diagnosis often results in nonoptimized medical management. Current guidelines recommend following poststroke protocol and minimizing individual risk factors by using antiplatelet, antihypertensive, and dyslipidemia medications. This case demonstrates a classic presentation of CADASIL syndrome with lesser described cardiac symptoms. Few cases of unusual cardiac symptoms in the setting of CADASIL syndrome have been reported. The relationship between cardiovascular disease and CADASIL syndrome is not well described. Further research is needed to elucidate any links between CADASIL syndrome and cardiovascular disease and to optimize management for these patients.
1. Moreton FC, Razvi SS, Davidson R, Muir KW. Changing clinical patterns and increasing prevalence in CADASIL. Acta Neurol Scand. 2014;130(3):197-203. doi:10.1111/ane.12266
2. Lesnik Oberstein SA, Jukema JW, Van Duinen SG, Macfarlane PW, van Houwelingen HC, Breuning MH, et al. Myocardial infarction in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Medicine (Baltimore). 2003;82(4):251-256. doi:10.1097/01.md.0000085054.63483.40
3. Di Donato I, Bianchi S, De Stefano N, Dichgans M, Dotti MT, Duering M, et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med. 2017;15(1):41. doi:10.1186/s12916-017-0778-8
4. Dunphy L, Rani A, Duodu Y, Behnam Y. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) presenting with stroke in a young man. BMJ Case Rep. 2019 ;12(7):e229609. doi:10.1136/bcr-2019-229609
5. Bianchi S, Zicari E, Carluccio A, Di Donato I, Pescini F, Nannucci S, et al. CADASIL in central Italy: a retrospective clinical and genetic study in 229 patients. J Neurol. 2015;262(1):134-141. doi:10.1007/s00415-014-7533-2
6. Phillips CD, Zuckerman SJ, Medical Education Commission. CADASIL can mimic multiple sclerosis. J La State Med Soc. 2010 May-Jun;162(3):174.
7. Hervé D, Chabriat H. CADASIL. J Geriatr Psychiatry Neurol. 2010;23(4):269-276. doi:10.1177/0891988710383570
8. Yamamoto Y, Hase Y, Ihara M, Khundakar A, Roeber S, Duering M, et al. Neuronal densities and vascular pathology in the hippocampal formation in CADASIL. Neurobiol Aging. 2021;97:33-40. doi:10.1016/j.neurobiolaging.2020.09.016
9. Ferrante EA, Cudrici CD, Boehm M. CADASIL: new advances in basic science and clinical perspectives. Curr Opin Hematol. 2019;26(3):193-198. doi:10.1097/MOH.0000000000000497
10. Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004;127(pt 11):2533-2539.
11. Rufa A, Guideri F, Acampa M, Cevenini G, Bianchi S, De Stefano N, et al. Cardiac autonomic nervous system and risk of arrhythmias in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Stroke. 2007 Feb;38(2):276-280. doi:10.1093/brain/awh282
12. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707-710. doi:10.1038/383707a0
13. Kalaria RN, Viitanen M, Kalimo H, Dichgans M, Tabira T, CASASIL Group of Vas-Cog. The pathogenesis of CADASIL: an update. J Neurol Sci. 2004;226(1-2):35-39. doi:10.1016/j.jns.2004.09.008
14. Reddy SPK, Vishnu VY, Goyal V, Singh MB, Arora S, Garg A, et al. CADASIL syndrome and stroke in young people. QJM. 2020 Feb 1;113(2):118-119. doi:10.1093/qjmed/hcz243
15. Carone DA. CADASIL and multiple sclerosis: A case report of prolonged misdiagnosis. Applied neuropsychology Adult. 2017;24(3):294-297. doi:10.1080/23279095.2016.1214132
16. Zhu S, Nahas SJ. CADASIL: Imaging characteristics and clinical correlation. Curr Pain Headache Rep. 2016;20(10):57. doi:10.1007/s11916-016-0584-6
17. Kalaria RN, Low WC, Oakley AE, Slade JY, Ince PG, Morris CM, et al. CADASIL and genetics of cerebral ischaemia. J Neural Transm Suppl. 2002;(63):75-90. doi:10.1007/978-3-7091-6137-1_5
18. O’Sullivan M, Jarosz JM, Martin RJ, Deasy N, Powell JF, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56(5):628-634. doi:10.1212/wnl.56.5.628
19. Khurshid S, Choi SH, Weng L-C, Wang EY, Trinquart L, Benjamin EJ, et al. Frequency of cardiac rhythm abnormalities in a half million adults. Circ ArrhythmElectrophysiol. 2018;11(7):e006273. doi:10.1161/CIRCEP.118.006273
20. Samuels MA. The brain–heart connection. Circulation. 2007;116(1):77-84. doi:10.1161/CIRCULATIONAHA. 106.678995
21. Cumurciuc R, Henry P, Gobron C, Vicaut E, Bousser MG, Chabriat H, et al. Electrocardiogram in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy patients without any clinical evidence of coronary artery disease: a case-control study. Stroke. 2006;37(4):1100-1102. doi:10.1161/01.STR.0000209242.68844.20
22. Luxán G, D’Amato G, MacGrogan D, de la Pompa JL. Endocardial notch signaling in cardiac development and disease. Circ Res. 2016;118(1):e1-e18. doi:10.1161/CIRCRESAHA.115.305350
23. Rubin CB, Hahn V, Kobayashi T, Litwack A. A report of accelerated coronary artery disease associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Case Rep Cardiol. 2015;2015:167513. doi:10.1155/2015/167513
24. Langer C, Adukauskaite A, Plank F, Feuchtner G, Cartes-Zumelzu F. Cerebral autosomal dominant arteriopathy (CADASIL) with cardiac involvement (ANOCA) and subcortical leukencephalopathy. J Cardiovasc Comput Tomogr. 2020;14(5):e1-e6. doi:10.1016/j.jcct.2018.08.005
25. Pettersen JA, Keith J, Gao F, Spence JD, Black SE. CADASIL accelerated by acute hypotension: Arterial and venous contribution to leukoaraiosis. Neurology. 2017;88(11):1077-1080. doi:10.1212/WNL.0000000000003717
1. Moreton FC, Razvi SS, Davidson R, Muir KW. Changing clinical patterns and increasing prevalence in CADASIL. Acta Neurol Scand. 2014;130(3):197-203. doi:10.1111/ane.12266
2. Lesnik Oberstein SA, Jukema JW, Van Duinen SG, Macfarlane PW, van Houwelingen HC, Breuning MH, et al. Myocardial infarction in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Medicine (Baltimore). 2003;82(4):251-256. doi:10.1097/01.md.0000085054.63483.40
3. Di Donato I, Bianchi S, De Stefano N, Dichgans M, Dotti MT, Duering M, et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med. 2017;15(1):41. doi:10.1186/s12916-017-0778-8
4. Dunphy L, Rani A, Duodu Y, Behnam Y. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) presenting with stroke in a young man. BMJ Case Rep. 2019 ;12(7):e229609. doi:10.1136/bcr-2019-229609
5. Bianchi S, Zicari E, Carluccio A, Di Donato I, Pescini F, Nannucci S, et al. CADASIL in central Italy: a retrospective clinical and genetic study in 229 patients. J Neurol. 2015;262(1):134-141. doi:10.1007/s00415-014-7533-2
6. Phillips CD, Zuckerman SJ, Medical Education Commission. CADASIL can mimic multiple sclerosis. J La State Med Soc. 2010 May-Jun;162(3):174.
7. Hervé D, Chabriat H. CADASIL. J Geriatr Psychiatry Neurol. 2010;23(4):269-276. doi:10.1177/0891988710383570
8. Yamamoto Y, Hase Y, Ihara M, Khundakar A, Roeber S, Duering M, et al. Neuronal densities and vascular pathology in the hippocampal formation in CADASIL. Neurobiol Aging. 2021;97:33-40. doi:10.1016/j.neurobiolaging.2020.09.016
9. Ferrante EA, Cudrici CD, Boehm M. CADASIL: new advances in basic science and clinical perspectives. Curr Opin Hematol. 2019;26(3):193-198. doi:10.1097/MOH.0000000000000497
10. Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004;127(pt 11):2533-2539.
11. Rufa A, Guideri F, Acampa M, Cevenini G, Bianchi S, De Stefano N, et al. Cardiac autonomic nervous system and risk of arrhythmias in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Stroke. 2007 Feb;38(2):276-280. doi:10.1093/brain/awh282
12. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707-710. doi:10.1038/383707a0
13. Kalaria RN, Viitanen M, Kalimo H, Dichgans M, Tabira T, CASASIL Group of Vas-Cog. The pathogenesis of CADASIL: an update. J Neurol Sci. 2004;226(1-2):35-39. doi:10.1016/j.jns.2004.09.008
14. Reddy SPK, Vishnu VY, Goyal V, Singh MB, Arora S, Garg A, et al. CADASIL syndrome and stroke in young people. QJM. 2020 Feb 1;113(2):118-119. doi:10.1093/qjmed/hcz243
15. Carone DA. CADASIL and multiple sclerosis: A case report of prolonged misdiagnosis. Applied neuropsychology Adult. 2017;24(3):294-297. doi:10.1080/23279095.2016.1214132
16. Zhu S, Nahas SJ. CADASIL: Imaging characteristics and clinical correlation. Curr Pain Headache Rep. 2016;20(10):57. doi:10.1007/s11916-016-0584-6
17. Kalaria RN, Low WC, Oakley AE, Slade JY, Ince PG, Morris CM, et al. CADASIL and genetics of cerebral ischaemia. J Neural Transm Suppl. 2002;(63):75-90. doi:10.1007/978-3-7091-6137-1_5
18. O’Sullivan M, Jarosz JM, Martin RJ, Deasy N, Powell JF, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56(5):628-634. doi:10.1212/wnl.56.5.628
19. Khurshid S, Choi SH, Weng L-C, Wang EY, Trinquart L, Benjamin EJ, et al. Frequency of cardiac rhythm abnormalities in a half million adults. Circ ArrhythmElectrophysiol. 2018;11(7):e006273. doi:10.1161/CIRCEP.118.006273
20. Samuels MA. The brain–heart connection. Circulation. 2007;116(1):77-84. doi:10.1161/CIRCULATIONAHA. 106.678995
21. Cumurciuc R, Henry P, Gobron C, Vicaut E, Bousser MG, Chabriat H, et al. Electrocardiogram in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy patients without any clinical evidence of coronary artery disease: a case-control study. Stroke. 2006;37(4):1100-1102. doi:10.1161/01.STR.0000209242.68844.20
22. Luxán G, D’Amato G, MacGrogan D, de la Pompa JL. Endocardial notch signaling in cardiac development and disease. Circ Res. 2016;118(1):e1-e18. doi:10.1161/CIRCRESAHA.115.305350
23. Rubin CB, Hahn V, Kobayashi T, Litwack A. A report of accelerated coronary artery disease associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Case Rep Cardiol. 2015;2015:167513. doi:10.1155/2015/167513
24. Langer C, Adukauskaite A, Plank F, Feuchtner G, Cartes-Zumelzu F. Cerebral autosomal dominant arteriopathy (CADASIL) with cardiac involvement (ANOCA) and subcortical leukencephalopathy. J Cardiovasc Comput Tomogr. 2020;14(5):e1-e6. doi:10.1016/j.jcct.2018.08.005
25. Pettersen JA, Keith J, Gao F, Spence JD, Black SE. CADASIL accelerated by acute hypotension: Arterial and venous contribution to leukoaraiosis. Neurology. 2017;88(11):1077-1080. doi:10.1212/WNL.0000000000003717