User login
Which Patients Should Receive Bridging Anticoagulation?
Case
A 77-year-old woman with a history of stroke five months prior, bileaflet aortic valve prosthesis, hypertension, and insulin-dependent diabetes is admitted for laparoscopy with lysis of adhesions. The patient stopped her warfarin 10 days prior to admission and initiated enoxaparin five days later. When should the enoxaparin be discontinued?
Intra-operatively, the surgeon converted the case to an open laparotomy for a bowel resection with re-anastomosis; post-operatively, when should the hospitalist reinitiate warfarin and enoxaparin?
Background
Many patients receive chronic oral anticoagulant therapy to minimize their long-term risk of thromboembolic disease. Hospitalists and outpatient providers often care for such patients who need to undergo a medical procedure or operation. The risk of bleeding associated with the medical procedure necessitates an interruption in the patient’s chronic oral anticoagulant therapy. In this scenario, providers are faced with several therapeutic decisions:
- How soon before the procedure should patients stop taking oral anticoagulant?
- During the period of time when the patient is not taking chronic oral anticoagulant, should the patient receive parenteral bridging anticoagulant therapy?
- After the procedure, when should patients restart chronic oral anticoagulant therapy?
‘Bridge’ anticoagulant therapy is the administration of a short-acting parenteral anticoagulant during the peri-operative period, when the patient is not taking chronic oral anticoagulant.1 The intent of bridge anticoagulant therapy is to minimize both the risk of thromboembolic events and the risk of bleeding during the peri-operative period. Bridging anticoagulant therapy is appropriate for some but not all patients undergoing medical procedures.
The Data
When to discontinue warfarin? Warfarin, the most commonly prescribed oral anticoagulant, achieves its therapeutic effects by antagonizing the actions of endogenous vitamin K-dependent coagulation factors. The decision on when to stop warfarin prior to surgery is dependent on the regeneration time of coagulation factors following the discontinuation of warfarin therapy. Although warfarin’s half-life is typically 36-42 hours, its therapeutic effects typically last up to five days in healthy subjects and often longer in elderly patients.2
Current guidelines recommend the discontinuation of warfarin at least five days prior to surgery (Grade 1C recommendation).3 Despite this recommendation, approximately 7% of patients will still have an international normalized ratio (INR) >1.5 after not taking warfarin for five days.4 For this reason, the guidelines recommend that all patients have their INR checked on the day of surgery. For those patients with an INR of 1.5 to 1.9 on the day prior to surgery, there is evidence to show that administration of 1 mg of vitamin K will lower the INR to 1.4 in greater than 90% of cases.5
Assessment of peri-procedural thrombotic risk. Knowledge of a patient’s past medical history is critical in helping providers stratify the patient’s peri-procedural thrombotic risk. According to the 2012 American College of Chest Physicians (ACCP) guidelines, a history of atrial fibrillation (Afib), mechanical heart valve(s), and previous VTE are independent risk factors for peri-procedural thrombotic events.3 Hospitalists may risk-stratify their patients based on the anticipated annualized rate of thrombosis or embolization: <5%, 5%-10%, or >15% for the respective low, medium, and high-risk groups.6
Patients with Afib history. For these patients, the CHADS2 score helps to stratify the risk of peri-procedural thrombosis. Low risk is defined as a CHADS2 score of zero to two, assuming that the two points were not scored for transient ischemic attack (TIA) or cerebrovascular accident (CVA). Any patient with a TIA or CVA within the previous three months is automatically considered high risk. Medium risk is a score of three or four.
In addition to the aforementioned TIA or CVA within the prior three months, high-risk patients also include those with a CHADS2 score of five or six or any patient with a history of rheumatic heart disease.3 Patients with CHADS2 scores less than five but with a TIA or CVA greater than three months in the past are high risk.7
Presence of mechanical heart valve(s). For patients with a mechanical heart valve, knowledge of the valve type and location is essential to assist hospitalists in stratifying the risk of peri-procedural thrombosis. The current ACCP guidelines consider patients with bileaflet aortic valve prostheses without additional risk factors for stroke or atrial fibrillation to be low risk.3
The guidelines define the following characteristics as medium risk for patients: the presence of a bileaflet valve with additional risk factors for stroke such as atrial fibrillation, age greater than 75, prior CVA (more than six months prior), hypertension, diabetes mellitus, or congestive heart failure.
Patients at high risk include those with aortic valve prosthesis with a caged-ball or tilting disc, patients with mitral valve prosthesis, and those with a mechanical valve with CVA or TIA during the prior six months.7
History of previous VTE. For these patients, the duration of time that has passed since their last VTE event is an important factor in helping to stratify their risk for peri-procedural thrombosis. Hospitalists should consider patients low risk if they had VTE more than one year prior to the procedure.
Medium-risk patients are those with VTE events in the preceding three to twelve months, those with recurrent VTE, those with active cancer who have received cancer therapy within six months, or patients with non-severe thrombophilias (e.g. heterogenous factor V Leiden or prothrombin gene mutation).
Hospitalists should identify high-risk patients as those with VTE that has occurred within three months or those with severe thrombophilias such as Protein C or S deficiency, antithrombin III deficiency, or antiphospholipid antibody syndrome.
Assessment of procedure-related thrombotic risk. The type of anticipated procedure itself conveys peri-procedural thrombotic risk. For example, heart valve replacement, carotid endarterectomy, or other major vascular surgeries automatically stratify patients in the high-risk category, regardless of underlying medication condition.
Assessment of bleeding risk. Hospitalists must identify any preexisting bleeding risk factors (i.e., hemophilias or thrombocytopenia) in addition to the post-procedural bleeding risks. Risk factors for increased post-procedural bleeding include: major surgery with extensive tissue injury, procedures involving highly vascularized organs, removal of large colonic polyps, urological procedures, placement of implantable cardioverter-defibrillator/pacemakers, and procedures at sites where minor bleeding would be clinically devastating, such as the brain or spine.3
Thus, communication with the proceduralist or surgeon regarding the anticipated bleeding risk is vital.
Should the patient receive bridging anticoagulation? Patients considered high risk for peri-procedural thrombosis should receive peri-procedural bridging anticoagulation therapy, while those considered low risk should not. For patients with a moderate peri-procedural risk of thrombosis, hospitalists should base the decision on individual and anticipated pre-surgical/procedural thrombotic risks.
Recent evidence suggests that bridging anticoagulation should be avoided in patients undergoing procedures with high bleeding risk who are not at high thromboembolic risk.8
Selection and pre-operative discontinuation of bridging medication. Current ACCP guidelines only support the use of unfractionated heparin (UFH) or low molecular weight heparin (LMWH) as bridging anticoagulants.3 Evidence supports the use of either intravenous UFH (goal aPTT 1.5 to two times control aPTT) or enoxaparin (1 mg/kg BID or 1.5 mg/kg once daily).9 UFH is preferred over LMWH in patients with chronic kidney disease stage IV or V due to a more predictable pharmacokinetic profile.
Clinicians should initiate a bridge when a patient’s INR falls to less than 2.0 and discontinue the UFH bridge four to six hours prior to the procedure.10 The recent update to the guidelines now states that LMWH should be discontinued 24, instead of 12, hours prior to the procedure.3
When to restart UHF or LMWH bridge post-procedure. The type of procedure being performed dictates when bridging anticoagulation should resume. In patients who have undergone surgeries that involve high bleeding risk, LMWH should not be administered until 48-72 hours post-surgery (Grade 2C evidence).3 For those patients undergoing surgeries with low bleeding risk, bridging should be resumed approximately 24 hours after the procedure.
Of note, enoxaparin administered in one single daily dose, as compared to divided doses, is associated with a greater risk of post-operative bleeding. UFH bridging should resume post-operatively without a bolus dose at 24 hours in low-risk bleeding cases or 48-72 hours in high-risk bleeding cases (Grade 2C evidence).3
On occasion, unanticipated adjustments to surgical cases—or complications—change the previously determined post-operative bleeding risk. In these instances, the hospitalist and surgeon/proceduralist should review the case and reassess the bleeding risk prior to employing bridging anticoagulation protocols.
When to restart long-term vitamin K antagonists (VKA) post-procedure. In most instances, regardless of pre-operative bleeding risk stratification, the resumption of VKA may occur once post-operative hemostasis has been achieved and the patient has been instructed to resume eating by the proceduralist or surgeon. This most often occurs on the calendar day following surgery, because it takes approximately five days for an INR to achieve therapeutic levels.
Back to the Case
The patient’s history of prosthetic valve with stroke within the preceding six months stratified her to a high thrombotic risk category. Given the high risk of thrombosis, the decision was made to bridge with LMWH. The hospitalist discontinued LMWH 24 hours prior to surgery, and INR was checked on the morning of the procedure.
Although the patient underwent the operation without significant bleeding, the adjustment from an exploratory laparoscopy to an open laparotomy increased her post-operative bleeding risk from medium to high. Therefore, bridging anticoagulation with LMWH was resumed no sooner than 48 hours after the operation. Her warfarin was restarted on the day following surgery, once she resumed her diet.
Bottom Line
Hospitalists must understand both the pre- and post-procedure thrombotic risks, as well as the pre- and post-procedural bleeding risks, when determining the selection and logistics of initiation and cessation of antithrombotic bridging for inpatients.
Drs. McCormick, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center in Boston. Dr. Kerbel is a hospitalist at the University of California Los Angeles.
References
- BRIDGE Study Investigators. Bridging anticoagulation: is it needed when warfarin is interrupted around the time of a surgery or procedure? Circulation. 2012;125(12):e496-498.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
- Kovacs MJ, Kearon C, Rodger M, et al. Single-arm study of bridging therapy with low-molecular-weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin. Circulation. 2004;110(12):1658-1663.
- Woods K, Douketis JD, Kathirgamanathan K, Yi Q, Crowther MA. Low-dose oral vitamin K to normalize the international normalized ratio prior to surgery in patients who require temporary interruption of warfarin. J Thromb Thrombolysis. 2007;24(2):93-97.
- Ortel TL. Perioperative management of patients on chronic antithrombotic therapy. Blood. 2012;120(24):4699-4705.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost. 2010;8(5):884-890.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630-1639.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):188S-203S.
Case
A 77-year-old woman with a history of stroke five months prior, bileaflet aortic valve prosthesis, hypertension, and insulin-dependent diabetes is admitted for laparoscopy with lysis of adhesions. The patient stopped her warfarin 10 days prior to admission and initiated enoxaparin five days later. When should the enoxaparin be discontinued?
Intra-operatively, the surgeon converted the case to an open laparotomy for a bowel resection with re-anastomosis; post-operatively, when should the hospitalist reinitiate warfarin and enoxaparin?
Background
Many patients receive chronic oral anticoagulant therapy to minimize their long-term risk of thromboembolic disease. Hospitalists and outpatient providers often care for such patients who need to undergo a medical procedure or operation. The risk of bleeding associated with the medical procedure necessitates an interruption in the patient’s chronic oral anticoagulant therapy. In this scenario, providers are faced with several therapeutic decisions:
- How soon before the procedure should patients stop taking oral anticoagulant?
- During the period of time when the patient is not taking chronic oral anticoagulant, should the patient receive parenteral bridging anticoagulant therapy?
- After the procedure, when should patients restart chronic oral anticoagulant therapy?
‘Bridge’ anticoagulant therapy is the administration of a short-acting parenteral anticoagulant during the peri-operative period, when the patient is not taking chronic oral anticoagulant.1 The intent of bridge anticoagulant therapy is to minimize both the risk of thromboembolic events and the risk of bleeding during the peri-operative period. Bridging anticoagulant therapy is appropriate for some but not all patients undergoing medical procedures.
The Data
When to discontinue warfarin? Warfarin, the most commonly prescribed oral anticoagulant, achieves its therapeutic effects by antagonizing the actions of endogenous vitamin K-dependent coagulation factors. The decision on when to stop warfarin prior to surgery is dependent on the regeneration time of coagulation factors following the discontinuation of warfarin therapy. Although warfarin’s half-life is typically 36-42 hours, its therapeutic effects typically last up to five days in healthy subjects and often longer in elderly patients.2
Current guidelines recommend the discontinuation of warfarin at least five days prior to surgery (Grade 1C recommendation).3 Despite this recommendation, approximately 7% of patients will still have an international normalized ratio (INR) >1.5 after not taking warfarin for five days.4 For this reason, the guidelines recommend that all patients have their INR checked on the day of surgery. For those patients with an INR of 1.5 to 1.9 on the day prior to surgery, there is evidence to show that administration of 1 mg of vitamin K will lower the INR to 1.4 in greater than 90% of cases.5
Assessment of peri-procedural thrombotic risk. Knowledge of a patient’s past medical history is critical in helping providers stratify the patient’s peri-procedural thrombotic risk. According to the 2012 American College of Chest Physicians (ACCP) guidelines, a history of atrial fibrillation (Afib), mechanical heart valve(s), and previous VTE are independent risk factors for peri-procedural thrombotic events.3 Hospitalists may risk-stratify their patients based on the anticipated annualized rate of thrombosis or embolization: <5%, 5%-10%, or >15% for the respective low, medium, and high-risk groups.6
Patients with Afib history. For these patients, the CHADS2 score helps to stratify the risk of peri-procedural thrombosis. Low risk is defined as a CHADS2 score of zero to two, assuming that the two points were not scored for transient ischemic attack (TIA) or cerebrovascular accident (CVA). Any patient with a TIA or CVA within the previous three months is automatically considered high risk. Medium risk is a score of three or four.
In addition to the aforementioned TIA or CVA within the prior three months, high-risk patients also include those with a CHADS2 score of five or six or any patient with a history of rheumatic heart disease.3 Patients with CHADS2 scores less than five but with a TIA or CVA greater than three months in the past are high risk.7
Presence of mechanical heart valve(s). For patients with a mechanical heart valve, knowledge of the valve type and location is essential to assist hospitalists in stratifying the risk of peri-procedural thrombosis. The current ACCP guidelines consider patients with bileaflet aortic valve prostheses without additional risk factors for stroke or atrial fibrillation to be low risk.3
The guidelines define the following characteristics as medium risk for patients: the presence of a bileaflet valve with additional risk factors for stroke such as atrial fibrillation, age greater than 75, prior CVA (more than six months prior), hypertension, diabetes mellitus, or congestive heart failure.
Patients at high risk include those with aortic valve prosthesis with a caged-ball or tilting disc, patients with mitral valve prosthesis, and those with a mechanical valve with CVA or TIA during the prior six months.7
History of previous VTE. For these patients, the duration of time that has passed since their last VTE event is an important factor in helping to stratify their risk for peri-procedural thrombosis. Hospitalists should consider patients low risk if they had VTE more than one year prior to the procedure.
Medium-risk patients are those with VTE events in the preceding three to twelve months, those with recurrent VTE, those with active cancer who have received cancer therapy within six months, or patients with non-severe thrombophilias (e.g. heterogenous factor V Leiden or prothrombin gene mutation).
Hospitalists should identify high-risk patients as those with VTE that has occurred within three months or those with severe thrombophilias such as Protein C or S deficiency, antithrombin III deficiency, or antiphospholipid antibody syndrome.
Assessment of procedure-related thrombotic risk. The type of anticipated procedure itself conveys peri-procedural thrombotic risk. For example, heart valve replacement, carotid endarterectomy, or other major vascular surgeries automatically stratify patients in the high-risk category, regardless of underlying medication condition.
Assessment of bleeding risk. Hospitalists must identify any preexisting bleeding risk factors (i.e., hemophilias or thrombocytopenia) in addition to the post-procedural bleeding risks. Risk factors for increased post-procedural bleeding include: major surgery with extensive tissue injury, procedures involving highly vascularized organs, removal of large colonic polyps, urological procedures, placement of implantable cardioverter-defibrillator/pacemakers, and procedures at sites where minor bleeding would be clinically devastating, such as the brain or spine.3
Thus, communication with the proceduralist or surgeon regarding the anticipated bleeding risk is vital.
Should the patient receive bridging anticoagulation? Patients considered high risk for peri-procedural thrombosis should receive peri-procedural bridging anticoagulation therapy, while those considered low risk should not. For patients with a moderate peri-procedural risk of thrombosis, hospitalists should base the decision on individual and anticipated pre-surgical/procedural thrombotic risks.
Recent evidence suggests that bridging anticoagulation should be avoided in patients undergoing procedures with high bleeding risk who are not at high thromboembolic risk.8
Selection and pre-operative discontinuation of bridging medication. Current ACCP guidelines only support the use of unfractionated heparin (UFH) or low molecular weight heparin (LMWH) as bridging anticoagulants.3 Evidence supports the use of either intravenous UFH (goal aPTT 1.5 to two times control aPTT) or enoxaparin (1 mg/kg BID or 1.5 mg/kg once daily).9 UFH is preferred over LMWH in patients with chronic kidney disease stage IV or V due to a more predictable pharmacokinetic profile.
Clinicians should initiate a bridge when a patient’s INR falls to less than 2.0 and discontinue the UFH bridge four to six hours prior to the procedure.10 The recent update to the guidelines now states that LMWH should be discontinued 24, instead of 12, hours prior to the procedure.3
When to restart UHF or LMWH bridge post-procedure. The type of procedure being performed dictates when bridging anticoagulation should resume. In patients who have undergone surgeries that involve high bleeding risk, LMWH should not be administered until 48-72 hours post-surgery (Grade 2C evidence).3 For those patients undergoing surgeries with low bleeding risk, bridging should be resumed approximately 24 hours after the procedure.
Of note, enoxaparin administered in one single daily dose, as compared to divided doses, is associated with a greater risk of post-operative bleeding. UFH bridging should resume post-operatively without a bolus dose at 24 hours in low-risk bleeding cases or 48-72 hours in high-risk bleeding cases (Grade 2C evidence).3
On occasion, unanticipated adjustments to surgical cases—or complications—change the previously determined post-operative bleeding risk. In these instances, the hospitalist and surgeon/proceduralist should review the case and reassess the bleeding risk prior to employing bridging anticoagulation protocols.
When to restart long-term vitamin K antagonists (VKA) post-procedure. In most instances, regardless of pre-operative bleeding risk stratification, the resumption of VKA may occur once post-operative hemostasis has been achieved and the patient has been instructed to resume eating by the proceduralist or surgeon. This most often occurs on the calendar day following surgery, because it takes approximately five days for an INR to achieve therapeutic levels.
Back to the Case
The patient’s history of prosthetic valve with stroke within the preceding six months stratified her to a high thrombotic risk category. Given the high risk of thrombosis, the decision was made to bridge with LMWH. The hospitalist discontinued LMWH 24 hours prior to surgery, and INR was checked on the morning of the procedure.
Although the patient underwent the operation without significant bleeding, the adjustment from an exploratory laparoscopy to an open laparotomy increased her post-operative bleeding risk from medium to high. Therefore, bridging anticoagulation with LMWH was resumed no sooner than 48 hours after the operation. Her warfarin was restarted on the day following surgery, once she resumed her diet.
Bottom Line
Hospitalists must understand both the pre- and post-procedure thrombotic risks, as well as the pre- and post-procedural bleeding risks, when determining the selection and logistics of initiation and cessation of antithrombotic bridging for inpatients.
Drs. McCormick, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center in Boston. Dr. Kerbel is a hospitalist at the University of California Los Angeles.
References
- BRIDGE Study Investigators. Bridging anticoagulation: is it needed when warfarin is interrupted around the time of a surgery or procedure? Circulation. 2012;125(12):e496-498.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
- Kovacs MJ, Kearon C, Rodger M, et al. Single-arm study of bridging therapy with low-molecular-weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin. Circulation. 2004;110(12):1658-1663.
- Woods K, Douketis JD, Kathirgamanathan K, Yi Q, Crowther MA. Low-dose oral vitamin K to normalize the international normalized ratio prior to surgery in patients who require temporary interruption of warfarin. J Thromb Thrombolysis. 2007;24(2):93-97.
- Ortel TL. Perioperative management of patients on chronic antithrombotic therapy. Blood. 2012;120(24):4699-4705.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost. 2010;8(5):884-890.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630-1639.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):188S-203S.
Case
A 77-year-old woman with a history of stroke five months prior, bileaflet aortic valve prosthesis, hypertension, and insulin-dependent diabetes is admitted for laparoscopy with lysis of adhesions. The patient stopped her warfarin 10 days prior to admission and initiated enoxaparin five days later. When should the enoxaparin be discontinued?
Intra-operatively, the surgeon converted the case to an open laparotomy for a bowel resection with re-anastomosis; post-operatively, when should the hospitalist reinitiate warfarin and enoxaparin?
Background
Many patients receive chronic oral anticoagulant therapy to minimize their long-term risk of thromboembolic disease. Hospitalists and outpatient providers often care for such patients who need to undergo a medical procedure or operation. The risk of bleeding associated with the medical procedure necessitates an interruption in the patient’s chronic oral anticoagulant therapy. In this scenario, providers are faced with several therapeutic decisions:
- How soon before the procedure should patients stop taking oral anticoagulant?
- During the period of time when the patient is not taking chronic oral anticoagulant, should the patient receive parenteral bridging anticoagulant therapy?
- After the procedure, when should patients restart chronic oral anticoagulant therapy?
‘Bridge’ anticoagulant therapy is the administration of a short-acting parenteral anticoagulant during the peri-operative period, when the patient is not taking chronic oral anticoagulant.1 The intent of bridge anticoagulant therapy is to minimize both the risk of thromboembolic events and the risk of bleeding during the peri-operative period. Bridging anticoagulant therapy is appropriate for some but not all patients undergoing medical procedures.
The Data
When to discontinue warfarin? Warfarin, the most commonly prescribed oral anticoagulant, achieves its therapeutic effects by antagonizing the actions of endogenous vitamin K-dependent coagulation factors. The decision on when to stop warfarin prior to surgery is dependent on the regeneration time of coagulation factors following the discontinuation of warfarin therapy. Although warfarin’s half-life is typically 36-42 hours, its therapeutic effects typically last up to five days in healthy subjects and often longer in elderly patients.2
Current guidelines recommend the discontinuation of warfarin at least five days prior to surgery (Grade 1C recommendation).3 Despite this recommendation, approximately 7% of patients will still have an international normalized ratio (INR) >1.5 after not taking warfarin for five days.4 For this reason, the guidelines recommend that all patients have their INR checked on the day of surgery. For those patients with an INR of 1.5 to 1.9 on the day prior to surgery, there is evidence to show that administration of 1 mg of vitamin K will lower the INR to 1.4 in greater than 90% of cases.5
Assessment of peri-procedural thrombotic risk. Knowledge of a patient’s past medical history is critical in helping providers stratify the patient’s peri-procedural thrombotic risk. According to the 2012 American College of Chest Physicians (ACCP) guidelines, a history of atrial fibrillation (Afib), mechanical heart valve(s), and previous VTE are independent risk factors for peri-procedural thrombotic events.3 Hospitalists may risk-stratify their patients based on the anticipated annualized rate of thrombosis or embolization: <5%, 5%-10%, or >15% for the respective low, medium, and high-risk groups.6
Patients with Afib history. For these patients, the CHADS2 score helps to stratify the risk of peri-procedural thrombosis. Low risk is defined as a CHADS2 score of zero to two, assuming that the two points were not scored for transient ischemic attack (TIA) or cerebrovascular accident (CVA). Any patient with a TIA or CVA within the previous three months is automatically considered high risk. Medium risk is a score of three or four.
In addition to the aforementioned TIA or CVA within the prior three months, high-risk patients also include those with a CHADS2 score of five or six or any patient with a history of rheumatic heart disease.3 Patients with CHADS2 scores less than five but with a TIA or CVA greater than three months in the past are high risk.7
Presence of mechanical heart valve(s). For patients with a mechanical heart valve, knowledge of the valve type and location is essential to assist hospitalists in stratifying the risk of peri-procedural thrombosis. The current ACCP guidelines consider patients with bileaflet aortic valve prostheses without additional risk factors for stroke or atrial fibrillation to be low risk.3
The guidelines define the following characteristics as medium risk for patients: the presence of a bileaflet valve with additional risk factors for stroke such as atrial fibrillation, age greater than 75, prior CVA (more than six months prior), hypertension, diabetes mellitus, or congestive heart failure.
Patients at high risk include those with aortic valve prosthesis with a caged-ball or tilting disc, patients with mitral valve prosthesis, and those with a mechanical valve with CVA or TIA during the prior six months.7
History of previous VTE. For these patients, the duration of time that has passed since their last VTE event is an important factor in helping to stratify their risk for peri-procedural thrombosis. Hospitalists should consider patients low risk if they had VTE more than one year prior to the procedure.
Medium-risk patients are those with VTE events in the preceding three to twelve months, those with recurrent VTE, those with active cancer who have received cancer therapy within six months, or patients with non-severe thrombophilias (e.g. heterogenous factor V Leiden or prothrombin gene mutation).
Hospitalists should identify high-risk patients as those with VTE that has occurred within three months or those with severe thrombophilias such as Protein C or S deficiency, antithrombin III deficiency, or antiphospholipid antibody syndrome.
Assessment of procedure-related thrombotic risk. The type of anticipated procedure itself conveys peri-procedural thrombotic risk. For example, heart valve replacement, carotid endarterectomy, or other major vascular surgeries automatically stratify patients in the high-risk category, regardless of underlying medication condition.
Assessment of bleeding risk. Hospitalists must identify any preexisting bleeding risk factors (i.e., hemophilias or thrombocytopenia) in addition to the post-procedural bleeding risks. Risk factors for increased post-procedural bleeding include: major surgery with extensive tissue injury, procedures involving highly vascularized organs, removal of large colonic polyps, urological procedures, placement of implantable cardioverter-defibrillator/pacemakers, and procedures at sites where minor bleeding would be clinically devastating, such as the brain or spine.3
Thus, communication with the proceduralist or surgeon regarding the anticipated bleeding risk is vital.
Should the patient receive bridging anticoagulation? Patients considered high risk for peri-procedural thrombosis should receive peri-procedural bridging anticoagulation therapy, while those considered low risk should not. For patients with a moderate peri-procedural risk of thrombosis, hospitalists should base the decision on individual and anticipated pre-surgical/procedural thrombotic risks.
Recent evidence suggests that bridging anticoagulation should be avoided in patients undergoing procedures with high bleeding risk who are not at high thromboembolic risk.8
Selection and pre-operative discontinuation of bridging medication. Current ACCP guidelines only support the use of unfractionated heparin (UFH) or low molecular weight heparin (LMWH) as bridging anticoagulants.3 Evidence supports the use of either intravenous UFH (goal aPTT 1.5 to two times control aPTT) or enoxaparin (1 mg/kg BID or 1.5 mg/kg once daily).9 UFH is preferred over LMWH in patients with chronic kidney disease stage IV or V due to a more predictable pharmacokinetic profile.
Clinicians should initiate a bridge when a patient’s INR falls to less than 2.0 and discontinue the UFH bridge four to six hours prior to the procedure.10 The recent update to the guidelines now states that LMWH should be discontinued 24, instead of 12, hours prior to the procedure.3
When to restart UHF or LMWH bridge post-procedure. The type of procedure being performed dictates when bridging anticoagulation should resume. In patients who have undergone surgeries that involve high bleeding risk, LMWH should not be administered until 48-72 hours post-surgery (Grade 2C evidence).3 For those patients undergoing surgeries with low bleeding risk, bridging should be resumed approximately 24 hours after the procedure.
Of note, enoxaparin administered in one single daily dose, as compared to divided doses, is associated with a greater risk of post-operative bleeding. UFH bridging should resume post-operatively without a bolus dose at 24 hours in low-risk bleeding cases or 48-72 hours in high-risk bleeding cases (Grade 2C evidence).3
On occasion, unanticipated adjustments to surgical cases—or complications—change the previously determined post-operative bleeding risk. In these instances, the hospitalist and surgeon/proceduralist should review the case and reassess the bleeding risk prior to employing bridging anticoagulation protocols.
When to restart long-term vitamin K antagonists (VKA) post-procedure. In most instances, regardless of pre-operative bleeding risk stratification, the resumption of VKA may occur once post-operative hemostasis has been achieved and the patient has been instructed to resume eating by the proceduralist or surgeon. This most often occurs on the calendar day following surgery, because it takes approximately five days for an INR to achieve therapeutic levels.
Back to the Case
The patient’s history of prosthetic valve with stroke within the preceding six months stratified her to a high thrombotic risk category. Given the high risk of thrombosis, the decision was made to bridge with LMWH. The hospitalist discontinued LMWH 24 hours prior to surgery, and INR was checked on the morning of the procedure.
Although the patient underwent the operation without significant bleeding, the adjustment from an exploratory laparoscopy to an open laparotomy increased her post-operative bleeding risk from medium to high. Therefore, bridging anticoagulation with LMWH was resumed no sooner than 48 hours after the operation. Her warfarin was restarted on the day following surgery, once she resumed her diet.
Bottom Line
Hospitalists must understand both the pre- and post-procedure thrombotic risks, as well as the pre- and post-procedural bleeding risks, when determining the selection and logistics of initiation and cessation of antithrombotic bridging for inpatients.
Drs. McCormick, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center in Boston. Dr. Kerbel is a hospitalist at the University of California Los Angeles.
References
- BRIDGE Study Investigators. Bridging anticoagulation: is it needed when warfarin is interrupted around the time of a surgery or procedure? Circulation. 2012;125(12):e496-498.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
- Kovacs MJ, Kearon C, Rodger M, et al. Single-arm study of bridging therapy with low-molecular-weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin. Circulation. 2004;110(12):1658-1663.
- Woods K, Douketis JD, Kathirgamanathan K, Yi Q, Crowther MA. Low-dose oral vitamin K to normalize the international normalized ratio prior to surgery in patients who require temporary interruption of warfarin. J Thromb Thrombolysis. 2007;24(2):93-97.
- Ortel TL. Perioperative management of patients on chronic antithrombotic therapy. Blood. 2012;120(24):4699-4705.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost. 2010;8(5):884-890.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630-1639.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):188S-203S.
Which Patients Should be Screened for Hepatitis C Virus Infection?
Case
A 65-year-old male with a history of a motor vehicle accident that required emergency surgery in 1982 is hospitalized for acute renal failure. He reports a distant history of IV heroin use and a brief incarceration. He does not currently use illicit drugs. He has no signs or symptoms of liver disease. Should this patient be screened for chronic hepatitis C virus (HCV) infection?
Brief Overview
HCV is a major public health concern in the United States and worldwide. It is estimated that more than 4.1 million people in the U.S. (1.6% prevalence) and more than 180 million worldwide (2.8% prevalence) are HCV antibody-positive.1,2 The acute infection is most often asymptomatic, and 80% to 100% of patients will remain HCV RNA-positive, 60% to 80% will have persistently elevated liver enzymes, and 16% will develop evidence of cirrhosis at 20 years after initial infection.3
A number of organizations in the United States have released HCV screening guidelines, including the CDC, the American Association for the Study of Liver Disease (AASLD), and the U.S. Preventive Services Task Force (USPSTF); however, despite these established recommendations, an estimated 50% of individuals with chronic HCV infection are unscreened and unaware of their infection status.4 Furthermore, in a recent study of one managed care network, even when one or more risk factors were present, only 29% of individuals underwent screening for HCV antibodies detection.5 The importance of detecting chronic HCV infection will have greater significance as newer and better-tolerated treatment options become available.6
Multiple organizations recommend screening for chronic HCV infection. This screening is recommended for patients with known risk factors and those in populations with a high prevalence of HCV infection.
Risk Factors and High-Prevalence Populations
IV or intranasal drug use. IV drug use is the main identifiable source of HCV infection in the U.S. It is estimated that 60% of new HCV infections occur in people who have injected drugs in the past six months.7 The prevalence of HCV antibodies in current IV drug users is between 72% and 96%.8 Intranasal cocaine use is also associated with a higher prevalence of HCV antibodies than the general population.8
Blood transfusion prior to July 1992. Testing of donor blood was not routinely done until 1990, and more sensitive testing was not implemented until July 1992.8 The prevalence of HCV antibodies in people who received blood transfusions prior to 1990 is 6%.8 Prior to 1990, the risk for transfusion-associated HCV infection was one in 526 units transfused.9 Since implementation of highly sensitive screening techniques, the risk of infection has dropped to less than one in 1.9 million units transfused.10
Clotting factors prior to 1987 or transplanted tissue prior to 1992. Individuals who have received clotting factors, other blood product transfusions, or transplanted tissue prior to 1987 are at an increased risk for developing HCV infection. For instance, individuals with hemophilia treated with clotting factors prior to 1987 had chronic HCV infection rates of up to 90%.8 In 1987, widespread use of protocols to inactivate HCV in clotting factors and other blood products was adopted.8 In addition, widespread screening of potential tissue donors and the use of HCV antibody-negative donors became routine.8
Alanine aminotransferase elevation. This can be considered screening or part of the diagnostic work-up of transaminitis. Regardless of the classification, this is a cohort of people with a high prevalence of HCV antibody. For individuals with one isolated alanine aminotransferase elevation, the prevalence is 3.2%.4 With two or more elevated aminotransferase results, the prevalence rises to 8.2%.4
Hemodialysis. Two major studies have estimated the prevalence of HCV antibody-positive in end-stage renal disease individuals on hemodialysis to be 7.8% and 10.4%.11,12 This prevalence can reach 64% at some dialysis centers.11 The risk of HCV infection has been associated with blood transfusions, longer duration of hemodialysis, and higher rates of HCV infection in the dialysis unit.13 With implementation of infection control practices in dialysis units, the incidence and prevalence of HCV infection are declining.13
Born in the U.S. between 1945 and 1965. The CDC and USPSTF recommend a one-time screening for HCV infection for people born in the U.S. between 1945 and 1965, regardless of the presence or absence of risk factors.6,14 This age group has an increased prevalence of HCV antibodies, at 3.25%.6
Human immunodeficiency virus (HIV). HCV has a prevalence of 30% in people infected with HIV.15 The rate of co-infection is likely secondary to shared routes of transmission. For example, 72.7% of HIV-infected individuals who used IV drugs had HCV antibodies, but only 3.5% of “low-risk” HIV-infected individuals had HCV antibodies.16
Born in a high prevalence country. In the U.S., a significant number of immigrants are from areas with a high endemic rate of HCV infection. High prevalence areas (greater than 3.5%) include Central Asia and East Asia, North Africa, and the Middle East.7 Of note, Egypt is thought to have the highest prevalence of chronic HCV infection in the world, with well over 10% of the population being antibody-positive.17 Although major guidelines do not currently recommend it, the high prevalence of chronic HCV infection in this population may warrant screening.
Other high-risk or high-prevalence populations. The prevalence of HCV infection in people who have had over 10 lifetime sexual partners (3% to 9%), those with a history of sexually transmitted disease (6%), men who have had sex with men (5%), and children born to HCV-infected mothers (5%) is increased compared with the general population.8 Incarcerated people in the U.S. have an HCV antibody prevalence of 16% to 41%.18 In addition, people who have sustained needle-stick injury or mucosal exposure, or those with potential exposures in unregulated tattoo or piercing salons, may also benefit from HCV antibody screening.14
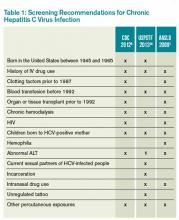
Table 1 reviews HCV screening recommendations for the CDC, AASLD, and USPSTF.1,6,8,14
CDC=Centers for Disease Control and Prevention; USPSTF=U.S. Preventive Services Task Force; AASLD=American Association for the Study of Liver Disease; ALT=alanine aminotransferase; 1=considered diagnostic and not screening test
Screening Method
The most common initial screening test for the diagnosis of chronic HCV infection is the HCV antibody test. A positive antibody test should be followed by an HCV RNA test. In an individual with recent exposure, it takes between four and 10 weeks for the antibody to be detectable. HCV RNA testing can be positive as soon as two to three weeks after infection.8
Hospitalist Role in HCV Screening
None of the U.S.-based guidelines make recommendations on the preferred setting for HCV screening. According to the CDC, 60.4% of HCV screening was done in a physician office and 5.9% was done as a hospital inpatient.19 Traditionally, the PCP is responsible for screening for chronic diseases, including HCV infection; however, the current screening rate is insufficient, as 50% of people with chronic HCV infection remain unscreened.4
Given the insufficient rate of HCV screening at present, hospital medicine (HM) physicians have an opportunity to help improve this rate. Currently, there is no established standard of care for HCV screening in hospitalized patients. HM physicians could use the following strategies:
- Continue the current system and defer screening to outpatient providers;
- Offer screening to selected inpatients at high risk for chronic HCV infection; or
- Offer screening to all inpatients who meet screening criteria based on current guidelines.
Given the shortcomings of the current screening strategies, these authors would recommend widespread screening for chronic HCV infection in hospitalized people who meet screening criteria per current guidelines.
If HM physicians are to take an increased role in HCV screening, there are a number of important considerations. Because hospitalized patients have a limited length of stay, it would be unreasonable to expect HM physicians to test for HCV RNA viral load or genotype for all patients with a positive antibody test, because the duration of the inpatient stay may be shorter than the time it takes for these test results to return. These tests are often indicated after a positive HCV antibody test, however. Thus, communication of HCV antibody results to PCPs or other responsible providers is essential. If no follow-up is available or there are no responsible outpatient providers, HM physicians should continue with a limited screening strategy.
Back to the Case
This individual has multiple indications for chronic HCV infection screening. His risk factors include date of birth between 1945 and 1965, a history of IV drug use, and a history of incarceration. He also notes a history of emergency surgery, for which he may have received blood products prior to 1987. These factors significantly raise the likelihood of chronic HCV infection when compared with the general population. He was screened and found to be HCV antibody-positive. A follow-up HCV RNA viral load was also positive. He did not have any evidence of liver disease but did have a mild transaminitis. He has followed up as an outpatient with plans to start therapy.
Bottom Line
The current screening strategies for individuals with high prevalence of chronic HCV infection are insufficient. HM physicians have an opportunity to improve the rates of screening in this population.
Dr. Theisen-Toupal is an internist, Dr. Rosenthal is a clinical fellow in medicine, and Dr. Carbo is an assistant professor of medicine, all at Beth Israel Deaconess Medical Center in Boston. Dr. Li is an internist and associate professor of medicine at Harvard Medical School and director of the hospital medicine division at Beth Israel Deaconess Medical Center.
References
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333-1342.
- Chopra S. Clinical manifestations and natural history of chronic hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/clinical-manifestations-and-natural-history-of-chronic-hepatitis-c-virus-infection. Accessed March 5, 2014.
- Spradling PR, Rupp L, Moorman AC, et al. Hepatitis B and C virus infection among 1.2 million people with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55(8):1047-1055.
- Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000-2007. Am J Manag Care. 2011;17(8):548-555.
- Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among people born during 1945-1965. MMWR. August 17, 2012. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm. Accessed March 5, 2014.
- Chopra S. Epidemiology and transmission of hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/epidemiology-and-transmission-of-hepatitis-c-virus-infection?source=search_result&search=%22Epidemiology+and+transmission+of+hepatitis+C+virus+infection%22&selectedTitle=1~150. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. October 16, 1998. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00055154.htm. Accessed March 5, 2014.
- Donahue JG, Muñoz A, Ness PM, et al. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992;327(6):369-373.
- Pomper GJ, Wu Y, Snyder EL. Risks of transfusion-transmitted infections: 2003. Curr Opin Hematol. 2003;10(6):412-418.
- Tokars JI, Miller ER, Alter MJ, Arduino MJ. National surveillance of dialysis associated diseases in the United States, 1995. ASAIO J. 1998;44(1):98-107.
- Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18(1):52-61.
- Natov S, Pereira BJG. Hepatitis C virus infection in patients on maintenance dialysis. UpToDate. Available at: http://www.uptodate.com/contents/hepatitis-c-virus-infection-in-patients-on-maintenance-dialysis?source=search_result&search=Hepatitis+C+virus+infection+in+patients+on+maintenance+dialysis.&selectedTitle=1~150. Accessed March 5, 2014.
- Moyer VA, U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
- Staples CT II, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29(1):150-154.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the U.S. adult AIDS clinical trials group. Clin Infect Dis. 2002;34(6):831-837.
- Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55 Suppl 1:S10-15.
- Centers for Disease Control and Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR. January 24, 2003. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5201a1.htm. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Locations and reasons for initial testing for hepatitis C infection—chronic hepatitis cohort study, United States, 2006-2010. MMWR. August 16, 2013. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6232a3.htm?s_cid=mm6232a3_w. Accessed March 5, 2014.
Case
A 65-year-old male with a history of a motor vehicle accident that required emergency surgery in 1982 is hospitalized for acute renal failure. He reports a distant history of IV heroin use and a brief incarceration. He does not currently use illicit drugs. He has no signs or symptoms of liver disease. Should this patient be screened for chronic hepatitis C virus (HCV) infection?
Brief Overview
HCV is a major public health concern in the United States and worldwide. It is estimated that more than 4.1 million people in the U.S. (1.6% prevalence) and more than 180 million worldwide (2.8% prevalence) are HCV antibody-positive.1,2 The acute infection is most often asymptomatic, and 80% to 100% of patients will remain HCV RNA-positive, 60% to 80% will have persistently elevated liver enzymes, and 16% will develop evidence of cirrhosis at 20 years after initial infection.3
A number of organizations in the United States have released HCV screening guidelines, including the CDC, the American Association for the Study of Liver Disease (AASLD), and the U.S. Preventive Services Task Force (USPSTF); however, despite these established recommendations, an estimated 50% of individuals with chronic HCV infection are unscreened and unaware of their infection status.4 Furthermore, in a recent study of one managed care network, even when one or more risk factors were present, only 29% of individuals underwent screening for HCV antibodies detection.5 The importance of detecting chronic HCV infection will have greater significance as newer and better-tolerated treatment options become available.6
Multiple organizations recommend screening for chronic HCV infection. This screening is recommended for patients with known risk factors and those in populations with a high prevalence of HCV infection.
Risk Factors and High-Prevalence Populations
IV or intranasal drug use. IV drug use is the main identifiable source of HCV infection in the U.S. It is estimated that 60% of new HCV infections occur in people who have injected drugs in the past six months.7 The prevalence of HCV antibodies in current IV drug users is between 72% and 96%.8 Intranasal cocaine use is also associated with a higher prevalence of HCV antibodies than the general population.8
Blood transfusion prior to July 1992. Testing of donor blood was not routinely done until 1990, and more sensitive testing was not implemented until July 1992.8 The prevalence of HCV antibodies in people who received blood transfusions prior to 1990 is 6%.8 Prior to 1990, the risk for transfusion-associated HCV infection was one in 526 units transfused.9 Since implementation of highly sensitive screening techniques, the risk of infection has dropped to less than one in 1.9 million units transfused.10
Clotting factors prior to 1987 or transplanted tissue prior to 1992. Individuals who have received clotting factors, other blood product transfusions, or transplanted tissue prior to 1987 are at an increased risk for developing HCV infection. For instance, individuals with hemophilia treated with clotting factors prior to 1987 had chronic HCV infection rates of up to 90%.8 In 1987, widespread use of protocols to inactivate HCV in clotting factors and other blood products was adopted.8 In addition, widespread screening of potential tissue donors and the use of HCV antibody-negative donors became routine.8
Alanine aminotransferase elevation. This can be considered screening or part of the diagnostic work-up of transaminitis. Regardless of the classification, this is a cohort of people with a high prevalence of HCV antibody. For individuals with one isolated alanine aminotransferase elevation, the prevalence is 3.2%.4 With two or more elevated aminotransferase results, the prevalence rises to 8.2%.4
Hemodialysis. Two major studies have estimated the prevalence of HCV antibody-positive in end-stage renal disease individuals on hemodialysis to be 7.8% and 10.4%.11,12 This prevalence can reach 64% at some dialysis centers.11 The risk of HCV infection has been associated with blood transfusions, longer duration of hemodialysis, and higher rates of HCV infection in the dialysis unit.13 With implementation of infection control practices in dialysis units, the incidence and prevalence of HCV infection are declining.13
Born in the U.S. between 1945 and 1965. The CDC and USPSTF recommend a one-time screening for HCV infection for people born in the U.S. between 1945 and 1965, regardless of the presence or absence of risk factors.6,14 This age group has an increased prevalence of HCV antibodies, at 3.25%.6
Human immunodeficiency virus (HIV). HCV has a prevalence of 30% in people infected with HIV.15 The rate of co-infection is likely secondary to shared routes of transmission. For example, 72.7% of HIV-infected individuals who used IV drugs had HCV antibodies, but only 3.5% of “low-risk” HIV-infected individuals had HCV antibodies.16
Born in a high prevalence country. In the U.S., a significant number of immigrants are from areas with a high endemic rate of HCV infection. High prevalence areas (greater than 3.5%) include Central Asia and East Asia, North Africa, and the Middle East.7 Of note, Egypt is thought to have the highest prevalence of chronic HCV infection in the world, with well over 10% of the population being antibody-positive.17 Although major guidelines do not currently recommend it, the high prevalence of chronic HCV infection in this population may warrant screening.
Other high-risk or high-prevalence populations. The prevalence of HCV infection in people who have had over 10 lifetime sexual partners (3% to 9%), those with a history of sexually transmitted disease (6%), men who have had sex with men (5%), and children born to HCV-infected mothers (5%) is increased compared with the general population.8 Incarcerated people in the U.S. have an HCV antibody prevalence of 16% to 41%.18 In addition, people who have sustained needle-stick injury or mucosal exposure, or those with potential exposures in unregulated tattoo or piercing salons, may also benefit from HCV antibody screening.14
Table 1 reviews HCV screening recommendations for the CDC, AASLD, and USPSTF.1,6,8,14
CDC=Centers for Disease Control and Prevention; USPSTF=U.S. Preventive Services Task Force; AASLD=American Association for the Study of Liver Disease; ALT=alanine aminotransferase; 1=considered diagnostic and not screening test
Screening Method
The most common initial screening test for the diagnosis of chronic HCV infection is the HCV antibody test. A positive antibody test should be followed by an HCV RNA test. In an individual with recent exposure, it takes between four and 10 weeks for the antibody to be detectable. HCV RNA testing can be positive as soon as two to three weeks after infection.8
Hospitalist Role in HCV Screening
None of the U.S.-based guidelines make recommendations on the preferred setting for HCV screening. According to the CDC, 60.4% of HCV screening was done in a physician office and 5.9% was done as a hospital inpatient.19 Traditionally, the PCP is responsible for screening for chronic diseases, including HCV infection; however, the current screening rate is insufficient, as 50% of people with chronic HCV infection remain unscreened.4
Given the insufficient rate of HCV screening at present, hospital medicine (HM) physicians have an opportunity to help improve this rate. Currently, there is no established standard of care for HCV screening in hospitalized patients. HM physicians could use the following strategies:
- Continue the current system and defer screening to outpatient providers;
- Offer screening to selected inpatients at high risk for chronic HCV infection; or
- Offer screening to all inpatients who meet screening criteria based on current guidelines.
Given the shortcomings of the current screening strategies, these authors would recommend widespread screening for chronic HCV infection in hospitalized people who meet screening criteria per current guidelines.
If HM physicians are to take an increased role in HCV screening, there are a number of important considerations. Because hospitalized patients have a limited length of stay, it would be unreasonable to expect HM physicians to test for HCV RNA viral load or genotype for all patients with a positive antibody test, because the duration of the inpatient stay may be shorter than the time it takes for these test results to return. These tests are often indicated after a positive HCV antibody test, however. Thus, communication of HCV antibody results to PCPs or other responsible providers is essential. If no follow-up is available or there are no responsible outpatient providers, HM physicians should continue with a limited screening strategy.
Back to the Case
This individual has multiple indications for chronic HCV infection screening. His risk factors include date of birth between 1945 and 1965, a history of IV drug use, and a history of incarceration. He also notes a history of emergency surgery, for which he may have received blood products prior to 1987. These factors significantly raise the likelihood of chronic HCV infection when compared with the general population. He was screened and found to be HCV antibody-positive. A follow-up HCV RNA viral load was also positive. He did not have any evidence of liver disease but did have a mild transaminitis. He has followed up as an outpatient with plans to start therapy.
Bottom Line
The current screening strategies for individuals with high prevalence of chronic HCV infection are insufficient. HM physicians have an opportunity to improve the rates of screening in this population.
Dr. Theisen-Toupal is an internist, Dr. Rosenthal is a clinical fellow in medicine, and Dr. Carbo is an assistant professor of medicine, all at Beth Israel Deaconess Medical Center in Boston. Dr. Li is an internist and associate professor of medicine at Harvard Medical School and director of the hospital medicine division at Beth Israel Deaconess Medical Center.
References
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333-1342.
- Chopra S. Clinical manifestations and natural history of chronic hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/clinical-manifestations-and-natural-history-of-chronic-hepatitis-c-virus-infection. Accessed March 5, 2014.
- Spradling PR, Rupp L, Moorman AC, et al. Hepatitis B and C virus infection among 1.2 million people with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55(8):1047-1055.
- Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000-2007. Am J Manag Care. 2011;17(8):548-555.
- Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among people born during 1945-1965. MMWR. August 17, 2012. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm. Accessed March 5, 2014.
- Chopra S. Epidemiology and transmission of hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/epidemiology-and-transmission-of-hepatitis-c-virus-infection?source=search_result&search=%22Epidemiology+and+transmission+of+hepatitis+C+virus+infection%22&selectedTitle=1~150. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. October 16, 1998. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00055154.htm. Accessed March 5, 2014.
- Donahue JG, Muñoz A, Ness PM, et al. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992;327(6):369-373.
- Pomper GJ, Wu Y, Snyder EL. Risks of transfusion-transmitted infections: 2003. Curr Opin Hematol. 2003;10(6):412-418.
- Tokars JI, Miller ER, Alter MJ, Arduino MJ. National surveillance of dialysis associated diseases in the United States, 1995. ASAIO J. 1998;44(1):98-107.
- Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18(1):52-61.
- Natov S, Pereira BJG. Hepatitis C virus infection in patients on maintenance dialysis. UpToDate. Available at: http://www.uptodate.com/contents/hepatitis-c-virus-infection-in-patients-on-maintenance-dialysis?source=search_result&search=Hepatitis+C+virus+infection+in+patients+on+maintenance+dialysis.&selectedTitle=1~150. Accessed March 5, 2014.
- Moyer VA, U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
- Staples CT II, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29(1):150-154.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the U.S. adult AIDS clinical trials group. Clin Infect Dis. 2002;34(6):831-837.
- Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55 Suppl 1:S10-15.
- Centers for Disease Control and Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR. January 24, 2003. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5201a1.htm. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Locations and reasons for initial testing for hepatitis C infection—chronic hepatitis cohort study, United States, 2006-2010. MMWR. August 16, 2013. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6232a3.htm?s_cid=mm6232a3_w. Accessed March 5, 2014.
Case
A 65-year-old male with a history of a motor vehicle accident that required emergency surgery in 1982 is hospitalized for acute renal failure. He reports a distant history of IV heroin use and a brief incarceration. He does not currently use illicit drugs. He has no signs or symptoms of liver disease. Should this patient be screened for chronic hepatitis C virus (HCV) infection?
Brief Overview
HCV is a major public health concern in the United States and worldwide. It is estimated that more than 4.1 million people in the U.S. (1.6% prevalence) and more than 180 million worldwide (2.8% prevalence) are HCV antibody-positive.1,2 The acute infection is most often asymptomatic, and 80% to 100% of patients will remain HCV RNA-positive, 60% to 80% will have persistently elevated liver enzymes, and 16% will develop evidence of cirrhosis at 20 years after initial infection.3
A number of organizations in the United States have released HCV screening guidelines, including the CDC, the American Association for the Study of Liver Disease (AASLD), and the U.S. Preventive Services Task Force (USPSTF); however, despite these established recommendations, an estimated 50% of individuals with chronic HCV infection are unscreened and unaware of their infection status.4 Furthermore, in a recent study of one managed care network, even when one or more risk factors were present, only 29% of individuals underwent screening for HCV antibodies detection.5 The importance of detecting chronic HCV infection will have greater significance as newer and better-tolerated treatment options become available.6
Multiple organizations recommend screening for chronic HCV infection. This screening is recommended for patients with known risk factors and those in populations with a high prevalence of HCV infection.
Risk Factors and High-Prevalence Populations
IV or intranasal drug use. IV drug use is the main identifiable source of HCV infection in the U.S. It is estimated that 60% of new HCV infections occur in people who have injected drugs in the past six months.7 The prevalence of HCV antibodies in current IV drug users is between 72% and 96%.8 Intranasal cocaine use is also associated with a higher prevalence of HCV antibodies than the general population.8
Blood transfusion prior to July 1992. Testing of donor blood was not routinely done until 1990, and more sensitive testing was not implemented until July 1992.8 The prevalence of HCV antibodies in people who received blood transfusions prior to 1990 is 6%.8 Prior to 1990, the risk for transfusion-associated HCV infection was one in 526 units transfused.9 Since implementation of highly sensitive screening techniques, the risk of infection has dropped to less than one in 1.9 million units transfused.10
Clotting factors prior to 1987 or transplanted tissue prior to 1992. Individuals who have received clotting factors, other blood product transfusions, or transplanted tissue prior to 1987 are at an increased risk for developing HCV infection. For instance, individuals with hemophilia treated with clotting factors prior to 1987 had chronic HCV infection rates of up to 90%.8 In 1987, widespread use of protocols to inactivate HCV in clotting factors and other blood products was adopted.8 In addition, widespread screening of potential tissue donors and the use of HCV antibody-negative donors became routine.8
Alanine aminotransferase elevation. This can be considered screening or part of the diagnostic work-up of transaminitis. Regardless of the classification, this is a cohort of people with a high prevalence of HCV antibody. For individuals with one isolated alanine aminotransferase elevation, the prevalence is 3.2%.4 With two or more elevated aminotransferase results, the prevalence rises to 8.2%.4
Hemodialysis. Two major studies have estimated the prevalence of HCV antibody-positive in end-stage renal disease individuals on hemodialysis to be 7.8% and 10.4%.11,12 This prevalence can reach 64% at some dialysis centers.11 The risk of HCV infection has been associated with blood transfusions, longer duration of hemodialysis, and higher rates of HCV infection in the dialysis unit.13 With implementation of infection control practices in dialysis units, the incidence and prevalence of HCV infection are declining.13
Born in the U.S. between 1945 and 1965. The CDC and USPSTF recommend a one-time screening for HCV infection for people born in the U.S. between 1945 and 1965, regardless of the presence or absence of risk factors.6,14 This age group has an increased prevalence of HCV antibodies, at 3.25%.6
Human immunodeficiency virus (HIV). HCV has a prevalence of 30% in people infected with HIV.15 The rate of co-infection is likely secondary to shared routes of transmission. For example, 72.7% of HIV-infected individuals who used IV drugs had HCV antibodies, but only 3.5% of “low-risk” HIV-infected individuals had HCV antibodies.16
Born in a high prevalence country. In the U.S., a significant number of immigrants are from areas with a high endemic rate of HCV infection. High prevalence areas (greater than 3.5%) include Central Asia and East Asia, North Africa, and the Middle East.7 Of note, Egypt is thought to have the highest prevalence of chronic HCV infection in the world, with well over 10% of the population being antibody-positive.17 Although major guidelines do not currently recommend it, the high prevalence of chronic HCV infection in this population may warrant screening.
Other high-risk or high-prevalence populations. The prevalence of HCV infection in people who have had over 10 lifetime sexual partners (3% to 9%), those with a history of sexually transmitted disease (6%), men who have had sex with men (5%), and children born to HCV-infected mothers (5%) is increased compared with the general population.8 Incarcerated people in the U.S. have an HCV antibody prevalence of 16% to 41%.18 In addition, people who have sustained needle-stick injury or mucosal exposure, or those with potential exposures in unregulated tattoo or piercing salons, may also benefit from HCV antibody screening.14
Table 1 reviews HCV screening recommendations for the CDC, AASLD, and USPSTF.1,6,8,14
CDC=Centers for Disease Control and Prevention; USPSTF=U.S. Preventive Services Task Force; AASLD=American Association for the Study of Liver Disease; ALT=alanine aminotransferase; 1=considered diagnostic and not screening test
Screening Method
The most common initial screening test for the diagnosis of chronic HCV infection is the HCV antibody test. A positive antibody test should be followed by an HCV RNA test. In an individual with recent exposure, it takes between four and 10 weeks for the antibody to be detectable. HCV RNA testing can be positive as soon as two to three weeks after infection.8
Hospitalist Role in HCV Screening
None of the U.S.-based guidelines make recommendations on the preferred setting for HCV screening. According to the CDC, 60.4% of HCV screening was done in a physician office and 5.9% was done as a hospital inpatient.19 Traditionally, the PCP is responsible for screening for chronic diseases, including HCV infection; however, the current screening rate is insufficient, as 50% of people with chronic HCV infection remain unscreened.4
Given the insufficient rate of HCV screening at present, hospital medicine (HM) physicians have an opportunity to help improve this rate. Currently, there is no established standard of care for HCV screening in hospitalized patients. HM physicians could use the following strategies:
- Continue the current system and defer screening to outpatient providers;
- Offer screening to selected inpatients at high risk for chronic HCV infection; or
- Offer screening to all inpatients who meet screening criteria based on current guidelines.
Given the shortcomings of the current screening strategies, these authors would recommend widespread screening for chronic HCV infection in hospitalized people who meet screening criteria per current guidelines.
If HM physicians are to take an increased role in HCV screening, there are a number of important considerations. Because hospitalized patients have a limited length of stay, it would be unreasonable to expect HM physicians to test for HCV RNA viral load or genotype for all patients with a positive antibody test, because the duration of the inpatient stay may be shorter than the time it takes for these test results to return. These tests are often indicated after a positive HCV antibody test, however. Thus, communication of HCV antibody results to PCPs or other responsible providers is essential. If no follow-up is available or there are no responsible outpatient providers, HM physicians should continue with a limited screening strategy.
Back to the Case
This individual has multiple indications for chronic HCV infection screening. His risk factors include date of birth between 1945 and 1965, a history of IV drug use, and a history of incarceration. He also notes a history of emergency surgery, for which he may have received blood products prior to 1987. These factors significantly raise the likelihood of chronic HCV infection when compared with the general population. He was screened and found to be HCV antibody-positive. A follow-up HCV RNA viral load was also positive. He did not have any evidence of liver disease but did have a mild transaminitis. He has followed up as an outpatient with plans to start therapy.
Bottom Line
The current screening strategies for individuals with high prevalence of chronic HCV infection are insufficient. HM physicians have an opportunity to improve the rates of screening in this population.
Dr. Theisen-Toupal is an internist, Dr. Rosenthal is a clinical fellow in medicine, and Dr. Carbo is an assistant professor of medicine, all at Beth Israel Deaconess Medical Center in Boston. Dr. Li is an internist and associate professor of medicine at Harvard Medical School and director of the hospital medicine division at Beth Israel Deaconess Medical Center.
References
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333-1342.
- Chopra S. Clinical manifestations and natural history of chronic hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/clinical-manifestations-and-natural-history-of-chronic-hepatitis-c-virus-infection. Accessed March 5, 2014.
- Spradling PR, Rupp L, Moorman AC, et al. Hepatitis B and C virus infection among 1.2 million people with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55(8):1047-1055.
- Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000-2007. Am J Manag Care. 2011;17(8):548-555.
- Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among people born during 1945-1965. MMWR. August 17, 2012. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm. Accessed March 5, 2014.
- Chopra S. Epidemiology and transmission of hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/epidemiology-and-transmission-of-hepatitis-c-virus-infection?source=search_result&search=%22Epidemiology+and+transmission+of+hepatitis+C+virus+infection%22&selectedTitle=1~150. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. October 16, 1998. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00055154.htm. Accessed March 5, 2014.
- Donahue JG, Muñoz A, Ness PM, et al. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992;327(6):369-373.
- Pomper GJ, Wu Y, Snyder EL. Risks of transfusion-transmitted infections: 2003. Curr Opin Hematol. 2003;10(6):412-418.
- Tokars JI, Miller ER, Alter MJ, Arduino MJ. National surveillance of dialysis associated diseases in the United States, 1995. ASAIO J. 1998;44(1):98-107.
- Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18(1):52-61.
- Natov S, Pereira BJG. Hepatitis C virus infection in patients on maintenance dialysis. UpToDate. Available at: http://www.uptodate.com/contents/hepatitis-c-virus-infection-in-patients-on-maintenance-dialysis?source=search_result&search=Hepatitis+C+virus+infection+in+patients+on+maintenance+dialysis.&selectedTitle=1~150. Accessed March 5, 2014.
- Moyer VA, U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
- Staples CT II, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29(1):150-154.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the U.S. adult AIDS clinical trials group. Clin Infect Dis. 2002;34(6):831-837.
- Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55 Suppl 1:S10-15.
- Centers for Disease Control and Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR. January 24, 2003. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5201a1.htm. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Locations and reasons for initial testing for hepatitis C infection—chronic hepatitis cohort study, United States, 2006-2010. MMWR. August 16, 2013. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6232a3.htm?s_cid=mm6232a3_w. Accessed March 5, 2014.
What Patients Undergoing Gastrointestinal Endoscopic Procedures Should Receive Antibiotic Prophylaxis?
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.