User login
Implications of Vancomycin Troughs Drawn Earlier Than Current Guidelines
Vancomycin was isolated in the 1950s, but due to impurities causing adverse events and semisynthetic penicillin production, its use was greatly reduced.1,2 However, this medication gained in popularity 30 years later as a first-line treatment for methicillin-resistant Staphylococcus aureus infections.
In 2009 the Infectious Diseases Society of America (IDSA), American Society of Health System Pharmacists, and Society of Infectious Diseases Pharmacists developed a consensus review of the therapeutic monitoring and dosing of vancomycin in adult patients.3 Trough serum concentration levels are recommended as the most accurate and convenient method to monitor vancomycin. Per IDSA guidelines, an optimal trough is intended to be high enough to clear infections (> 10 mg/L) and prevent the development of vancomycin intermediate and resistant bacteria. Troughs should be obtained just before the next dose in steady-state conditions (starting just before the fourth dose) in patients with normal renal function.
Since the development of these guidelines, vancomycin trough levels are often drawn early.4-7 This may lead to an overestimation of the true trough concentration. A study by Morrison and colleagues in Boston, Massachusetts, found that 41.3% of vancomycin troughs were drawn early, and this resulted in statistically significant increases in the vancomycin concentrations, the rate of vancomycin regimen adjustments (decrease, discontinuation, or holding of dose), and the repeat vancomycin level orders compared with correctly timed troughs.5 It was noted by the study authors that lowering the daily dose of vancomycin based on early trough levels could lead to an underdosing of vancomycin and an increase in intermediate or resistant bacteria.
Related: IDWEEK: Antibiotic ‘time-out’ cut vancomycin use
The prevalence and implications of early trough samples have been measured at only 1 facility, and it is unknown whether these data can be reproduced elsewhere.5 Thus, this study sought to determine the prevalence and corresponding clinical actions of early trough levels at the Captain James A. Lovell Federal Health Care Center (JALFHCC). This is a unique facility that in 2010 combined a VA hospital with a DoD hospital. This facility cares for 67,000 military and retiree beneficiaries each year from southwestern Wisconsin and northwestern Illinois.The primary objective of this study was to measure the rate of early troughs drawn and their resultant effect on vancomycin regimens compared with correctly timed troughs. Secondarily, this study sought to compare the rate of repeated vancomycin trough levels in early vs correctly timed measurements.
Methods
This retrospective cohort analysis compared the outcomes of early and correctly timed vancomycin troughs. This study was approved by the Edward Hines, Jr. VA Hospital and JALFHCC Institutional Review Board. Veteran patients aged ≥ 18 years, hospitalized at JALFHCC, and receiving IV vancomycin at dosing intervals of 8, 12, 24, and 48 hours with measured trough levels between July 1, 2009, and July 1, 2013, were included in this study. Patients were excluded from analysis if vancomycin was given at any schedule other than the previously stated frequencies, they received hemodialysis during the treatment period, or their insurance coverage was through TRICARE (these patients had either active-duty or retired active-duty status).
Potentially eligible patients were identified via a Computerized Patient Records System (CPRS) search for laboratory vancomycin level measurements. The search supplied the researcher with the patient name, vancomycin level date and time, type of vancomycin level (trough or random), and vancomycin concentration. With this information, further data were gathered through CPRS: demographics, type of clinical infection, desired trough level (inferred if not listed in CPRS note), and vancomycin administration time (through the bar code medication administration system [BCMA] in CPRS). This analysis was of troughs, and multiple troughs may have originated from the same patient.
An early trough was defined as a trough taken more than 2 hours earlier than the next theoretical administration time or anytime before the third dose. After a trough was determined to be early or on time, the clinical actions taken during the dosing interval following sample collection were documented. A dose was considered to be held if stated in the BCMA or in a CPRS provider note. A dose was considered to be decreased with a change in frequency or strength that resulted in an overall daily dose decrease. A recollected vancomycin trough was counted within 24 hours of the trough or per a note in CPRS. Finally, observations that noted trends in vancomycin trough management were recorded.
The chi-square test with a significance criterion of 0.05 was used to compare early and on time troughs. Based on the results from the Boston, Massachusetts, study and 1 other study, about 780 vancomycin troughs would be required to meet significance in the primary outcome.5,6
Results
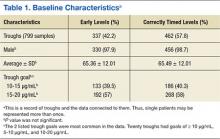
A total of 474 patient charts were reviewed, and 278 met inclusion criteria (196 were excluded). Of the included patients, 799 trough levels were analyzed. Of these, 377 (42.2%) were drawn early. There was no significant difference in the baseline characteristics of the early group vs the correctly timed group (Table 1). Of the early troughs, 190 (56.3%) were drawn prior to the third dose of vancomycin. It was observed that a large portion of these troughs occurred after a vancomycin dose adjustment.
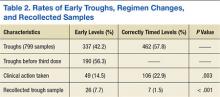
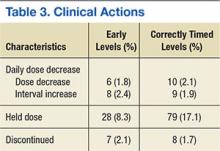
Clinical actions taken after sampling occurred at a rate of 14.5% in the early group and 22.9% in the correctly timed group (P = .003; Table 2). Early troughs led to a 7.7% rate of trough recollection, which was significantly greater than the 1.5% rate in the correctly timed group (P < .001). An analysis of each factor resulting in a clinical action illustrated that the rates of daily dose decrease and discontinued dose were similar between the groups (Table 3). However, the rate of held doses was 8.3% in the early group and 17.1% in the correctly timed group.
This research process yielded some observations. Occasionally a trough was drawn after vancomycin therapy was discontinued and when there was no concern for nephrotoxicity. After the guidelines were published, providers continued to document in CPRS notes to check troughs before the third dose. This incidence decreased over time. Troughs were taken often in patients who were receiving a short course of therapy or who were hemodynamically stable. Finally, documentation of vancomycin regimen changes occasionally did not match the record in the BCMA (in these situations, the BCMA record was used for this study).
Related: Assessment of High Staphylococcus aureus MIC and Poor Patient Outcomes
Discussion
A large portion of trough levels at the JALFHCC were drawn early and did not adhere to the 2009 consensus guidelines. The rates of early troughs in this study and in the Boston, Massachusetts, study are similar.5 However, the 2 studies differed in 1 significant aspect: Clinical actions were taken less often in the early group at JALFHCC, whereas they were taken more often in the early group in the Boston, Massachusetts, study. This dissimilarity could be attributed to a difference in software between the hospitals. In the previous study, trough levels and the time that they were drawn were not displayed together. Thus, clinicians may have been less likely to gauge whether a trough was early. Since this information is available at the JALFHCC, clinicians may have been aware that the trough was early and avoided adjusting treatment (such as holding a dose, as illustrated in the data) based on a falsely elevated trough. This point is further supported by significantly greater amounts of recollected troughs in the early group, suggesting an understanding that the trough was early.
The low trough recollection rate of 7.7% of all early samples could be due to several factors that would prevent a trough redraw. First, medication discontinuation resulting from course completion or sensitivity results would not require further trough monitoring. Second, practitioners may assess the early sample as insignificantly different from a correctly timed one and elect not to redraw the trough. Sometimes a trough was drawn at the correct time, but the time was recorded incorrectly. In this situation, a new trough level would not be necessary. Finally, a lack of sufficient staffing during nights and weekends may result in a delay in interpreting results leading to a missed opportunity for recollection. Additionally, some troughs may not have been redrawn based on a practitioner’s opinion that a trough was not significantly early and did not represent skewed results. Sometimes an incorrect recording of trough draw time reflected that it was taken after vancomycin dosing when it was not.
Specific observations regarding the timing of the trough indicate other possible concerns and areas for improvement. First, providers must cancel future trough orders concurrently with canceling treatment. Second, at the time of publication of the consensus, some providers were slow adopters of the new guidelines. Finally, the IDSA guidelines state that frequent monitoring for short course, lower intensity therapy, or in patients who are hemodynamically stable is not recommended.3 However, troughs were sometimes measured 2 to 3 times weekly in these patients.
Related: Results mixed in hospital efforts to tackle antimicrobial resistance
The data and observations lead to the conclusion that although providers may be able to discern between early and correctly timed troughs, they were not consistently adherent to the 2009 IDSA guidelines. It has been shown that pharmacy involvement of Medicare patients with infections in the intensive care unit has led to better clinical and monetary outcomes.8 Therefore, continued efforts by clinical pharmacists to monitor trough timing can be used to improve adherence and decrease costs (each trough is estimated to cost $16.97).
A study conducted in Australia demonstrated that pharmacist-led education of vancomycin dosing and monitoring (including when to measure a trough level) among prescribers and nurses led to improved adherence to the current guidelines and a greater number of patients treated within desired therapeutic ranges.9 In addition, a small study at the Atlanta VAMC in Georgia demonstrated that education of nurses, lab personnel, residents, ward clerks, and pharmacists led to a greater number of appropriately timed vancomycin and aminoglycoside levels.10 Thus, an interdisciplinary review of the current IDSA guidelines and review on the publication of the anticipated updated vancomycin guidelines should be provided to hospital personnel to aid in adoption of current dosing and monitoring recommendations.11
Limitations
This study is limited by the 4-year span of time that it encompassed, which may give a skewed depiction of current practices. Another limitation is that patients with fluctuating renal function were included in the analysis. Instead of selecting a random level order, a trough level order was sometimes selected for these patients. This could lead to a lower actual rate of early troughs. A third limitation is that this was a small and unblinded study. Also, the actual trough levels and the resulting changes that were made to specific regimens were not recorded. Thus, these data do not indicate whether the changes that were made reflected guideline recommendations. Finally, some clinical actions were taken after the dosing interval following the trough. This was often a result of off-hours lab results or waiting on attending physician or infectious disease guidance. These data were not included in the analysis.
Conclusion
Vancomycin troughs were often drawn too early and resulted in an increased rate of trough recollection. In an attempt to improve adherence to the current and the upcoming revised version of the IDSA consensus statement, it is recommended to educate and reeducate providers through interdisciplinary-led review sessions.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Moellering RC Jr. Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42(suppl 1):S3-S4.
2. Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(suppl 1):S5-S12.
3. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009;29(11):1275-1279.
4. Davis SL, Scheetz MH, Bosso JA, Goff DA, Rybak MJ. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: a cross-sectional survey of U.S. hospitals. Pharmacotherapy. 2013;33(12):1256-1263.
5. Morrison AP, Melanson SEF, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early? Frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472-478.
6. Traugott KA, Maxwell PR, Green K, Frei C, Lewis JS 2nd. Effects of therapeutic drug monitoring criteria in a computerized prescriber-order-entry system on the appropriateness of vancomycin level orders. Am J Health Syst Pharm. 2011;68(4):347-352.
7. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801-806.
8. MacLaren R, Bond CA, Martin SJ, Fike D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 2008;36(12):3184-3189.
9. Phillips CJ, Doan H, Quinn S, Kirkpatrick CM, Gordon DL, Doogue MP. An educational intervention to improve vancomycin prescribing and monitoring. Int J Antimicrob Agents. 2013;41(4):393-394.
10. Carroll DJ, Austin GE, Stajich GV, Miyrhaya RK, Murphy JE, Ward ES. Effect of education on the appropriateness of serum drug concentration determination. Ther Drug Monit. 1992;14(1):81-84.
11. Infectious Diseases Society of America (IDSA). IDSA practice guidelines: antimicrobial agent use. IDSA Website. 2015. http://www.idsociety.org/Antimicrobial_Agents. Accessed November 16, 2015.
Vancomycin was isolated in the 1950s, but due to impurities causing adverse events and semisynthetic penicillin production, its use was greatly reduced.1,2 However, this medication gained in popularity 30 years later as a first-line treatment for methicillin-resistant Staphylococcus aureus infections.
In 2009 the Infectious Diseases Society of America (IDSA), American Society of Health System Pharmacists, and Society of Infectious Diseases Pharmacists developed a consensus review of the therapeutic monitoring and dosing of vancomycin in adult patients.3 Trough serum concentration levels are recommended as the most accurate and convenient method to monitor vancomycin. Per IDSA guidelines, an optimal trough is intended to be high enough to clear infections (> 10 mg/L) and prevent the development of vancomycin intermediate and resistant bacteria. Troughs should be obtained just before the next dose in steady-state conditions (starting just before the fourth dose) in patients with normal renal function.
Since the development of these guidelines, vancomycin trough levels are often drawn early.4-7 This may lead to an overestimation of the true trough concentration. A study by Morrison and colleagues in Boston, Massachusetts, found that 41.3% of vancomycin troughs were drawn early, and this resulted in statistically significant increases in the vancomycin concentrations, the rate of vancomycin regimen adjustments (decrease, discontinuation, or holding of dose), and the repeat vancomycin level orders compared with correctly timed troughs.5 It was noted by the study authors that lowering the daily dose of vancomycin based on early trough levels could lead to an underdosing of vancomycin and an increase in intermediate or resistant bacteria.
Related: IDWEEK: Antibiotic ‘time-out’ cut vancomycin use
The prevalence and implications of early trough samples have been measured at only 1 facility, and it is unknown whether these data can be reproduced elsewhere.5 Thus, this study sought to determine the prevalence and corresponding clinical actions of early trough levels at the Captain James A. Lovell Federal Health Care Center (JALFHCC). This is a unique facility that in 2010 combined a VA hospital with a DoD hospital. This facility cares for 67,000 military and retiree beneficiaries each year from southwestern Wisconsin and northwestern Illinois.The primary objective of this study was to measure the rate of early troughs drawn and their resultant effect on vancomycin regimens compared with correctly timed troughs. Secondarily, this study sought to compare the rate of repeated vancomycin trough levels in early vs correctly timed measurements.
Methods
This retrospective cohort analysis compared the outcomes of early and correctly timed vancomycin troughs. This study was approved by the Edward Hines, Jr. VA Hospital and JALFHCC Institutional Review Board. Veteran patients aged ≥ 18 years, hospitalized at JALFHCC, and receiving IV vancomycin at dosing intervals of 8, 12, 24, and 48 hours with measured trough levels between July 1, 2009, and July 1, 2013, were included in this study. Patients were excluded from analysis if vancomycin was given at any schedule other than the previously stated frequencies, they received hemodialysis during the treatment period, or their insurance coverage was through TRICARE (these patients had either active-duty or retired active-duty status).
Potentially eligible patients were identified via a Computerized Patient Records System (CPRS) search for laboratory vancomycin level measurements. The search supplied the researcher with the patient name, vancomycin level date and time, type of vancomycin level (trough or random), and vancomycin concentration. With this information, further data were gathered through CPRS: demographics, type of clinical infection, desired trough level (inferred if not listed in CPRS note), and vancomycin administration time (through the bar code medication administration system [BCMA] in CPRS). This analysis was of troughs, and multiple troughs may have originated from the same patient.
An early trough was defined as a trough taken more than 2 hours earlier than the next theoretical administration time or anytime before the third dose. After a trough was determined to be early or on time, the clinical actions taken during the dosing interval following sample collection were documented. A dose was considered to be held if stated in the BCMA or in a CPRS provider note. A dose was considered to be decreased with a change in frequency or strength that resulted in an overall daily dose decrease. A recollected vancomycin trough was counted within 24 hours of the trough or per a note in CPRS. Finally, observations that noted trends in vancomycin trough management were recorded.
The chi-square test with a significance criterion of 0.05 was used to compare early and on time troughs. Based on the results from the Boston, Massachusetts, study and 1 other study, about 780 vancomycin troughs would be required to meet significance in the primary outcome.5,6
Results
A total of 474 patient charts were reviewed, and 278 met inclusion criteria (196 were excluded). Of the included patients, 799 trough levels were analyzed. Of these, 377 (42.2%) were drawn early. There was no significant difference in the baseline characteristics of the early group vs the correctly timed group (Table 1). Of the early troughs, 190 (56.3%) were drawn prior to the third dose of vancomycin. It was observed that a large portion of these troughs occurred after a vancomycin dose adjustment.
Clinical actions taken after sampling occurred at a rate of 14.5% in the early group and 22.9% in the correctly timed group (P = .003; Table 2). Early troughs led to a 7.7% rate of trough recollection, which was significantly greater than the 1.5% rate in the correctly timed group (P < .001). An analysis of each factor resulting in a clinical action illustrated that the rates of daily dose decrease and discontinued dose were similar between the groups (Table 3). However, the rate of held doses was 8.3% in the early group and 17.1% in the correctly timed group.
This research process yielded some observations. Occasionally a trough was drawn after vancomycin therapy was discontinued and when there was no concern for nephrotoxicity. After the guidelines were published, providers continued to document in CPRS notes to check troughs before the third dose. This incidence decreased over time. Troughs were taken often in patients who were receiving a short course of therapy or who were hemodynamically stable. Finally, documentation of vancomycin regimen changes occasionally did not match the record in the BCMA (in these situations, the BCMA record was used for this study).
Related: Assessment of High Staphylococcus aureus MIC and Poor Patient Outcomes
Discussion
A large portion of trough levels at the JALFHCC were drawn early and did not adhere to the 2009 consensus guidelines. The rates of early troughs in this study and in the Boston, Massachusetts, study are similar.5 However, the 2 studies differed in 1 significant aspect: Clinical actions were taken less often in the early group at JALFHCC, whereas they were taken more often in the early group in the Boston, Massachusetts, study. This dissimilarity could be attributed to a difference in software between the hospitals. In the previous study, trough levels and the time that they were drawn were not displayed together. Thus, clinicians may have been less likely to gauge whether a trough was early. Since this information is available at the JALFHCC, clinicians may have been aware that the trough was early and avoided adjusting treatment (such as holding a dose, as illustrated in the data) based on a falsely elevated trough. This point is further supported by significantly greater amounts of recollected troughs in the early group, suggesting an understanding that the trough was early.
The low trough recollection rate of 7.7% of all early samples could be due to several factors that would prevent a trough redraw. First, medication discontinuation resulting from course completion or sensitivity results would not require further trough monitoring. Second, practitioners may assess the early sample as insignificantly different from a correctly timed one and elect not to redraw the trough. Sometimes a trough was drawn at the correct time, but the time was recorded incorrectly. In this situation, a new trough level would not be necessary. Finally, a lack of sufficient staffing during nights and weekends may result in a delay in interpreting results leading to a missed opportunity for recollection. Additionally, some troughs may not have been redrawn based on a practitioner’s opinion that a trough was not significantly early and did not represent skewed results. Sometimes an incorrect recording of trough draw time reflected that it was taken after vancomycin dosing when it was not.
Specific observations regarding the timing of the trough indicate other possible concerns and areas for improvement. First, providers must cancel future trough orders concurrently with canceling treatment. Second, at the time of publication of the consensus, some providers were slow adopters of the new guidelines. Finally, the IDSA guidelines state that frequent monitoring for short course, lower intensity therapy, or in patients who are hemodynamically stable is not recommended.3 However, troughs were sometimes measured 2 to 3 times weekly in these patients.
Related: Results mixed in hospital efforts to tackle antimicrobial resistance
The data and observations lead to the conclusion that although providers may be able to discern between early and correctly timed troughs, they were not consistently adherent to the 2009 IDSA guidelines. It has been shown that pharmacy involvement of Medicare patients with infections in the intensive care unit has led to better clinical and monetary outcomes.8 Therefore, continued efforts by clinical pharmacists to monitor trough timing can be used to improve adherence and decrease costs (each trough is estimated to cost $16.97).
A study conducted in Australia demonstrated that pharmacist-led education of vancomycin dosing and monitoring (including when to measure a trough level) among prescribers and nurses led to improved adherence to the current guidelines and a greater number of patients treated within desired therapeutic ranges.9 In addition, a small study at the Atlanta VAMC in Georgia demonstrated that education of nurses, lab personnel, residents, ward clerks, and pharmacists led to a greater number of appropriately timed vancomycin and aminoglycoside levels.10 Thus, an interdisciplinary review of the current IDSA guidelines and review on the publication of the anticipated updated vancomycin guidelines should be provided to hospital personnel to aid in adoption of current dosing and monitoring recommendations.11
Limitations
This study is limited by the 4-year span of time that it encompassed, which may give a skewed depiction of current practices. Another limitation is that patients with fluctuating renal function were included in the analysis. Instead of selecting a random level order, a trough level order was sometimes selected for these patients. This could lead to a lower actual rate of early troughs. A third limitation is that this was a small and unblinded study. Also, the actual trough levels and the resulting changes that were made to specific regimens were not recorded. Thus, these data do not indicate whether the changes that were made reflected guideline recommendations. Finally, some clinical actions were taken after the dosing interval following the trough. This was often a result of off-hours lab results or waiting on attending physician or infectious disease guidance. These data were not included in the analysis.
Conclusion
Vancomycin troughs were often drawn too early and resulted in an increased rate of trough recollection. In an attempt to improve adherence to the current and the upcoming revised version of the IDSA consensus statement, it is recommended to educate and reeducate providers through interdisciplinary-led review sessions.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Vancomycin was isolated in the 1950s, but due to impurities causing adverse events and semisynthetic penicillin production, its use was greatly reduced.1,2 However, this medication gained in popularity 30 years later as a first-line treatment for methicillin-resistant Staphylococcus aureus infections.
In 2009 the Infectious Diseases Society of America (IDSA), American Society of Health System Pharmacists, and Society of Infectious Diseases Pharmacists developed a consensus review of the therapeutic monitoring and dosing of vancomycin in adult patients.3 Trough serum concentration levels are recommended as the most accurate and convenient method to monitor vancomycin. Per IDSA guidelines, an optimal trough is intended to be high enough to clear infections (> 10 mg/L) and prevent the development of vancomycin intermediate and resistant bacteria. Troughs should be obtained just before the next dose in steady-state conditions (starting just before the fourth dose) in patients with normal renal function.
Since the development of these guidelines, vancomycin trough levels are often drawn early.4-7 This may lead to an overestimation of the true trough concentration. A study by Morrison and colleagues in Boston, Massachusetts, found that 41.3% of vancomycin troughs were drawn early, and this resulted in statistically significant increases in the vancomycin concentrations, the rate of vancomycin regimen adjustments (decrease, discontinuation, or holding of dose), and the repeat vancomycin level orders compared with correctly timed troughs.5 It was noted by the study authors that lowering the daily dose of vancomycin based on early trough levels could lead to an underdosing of vancomycin and an increase in intermediate or resistant bacteria.
Related: IDWEEK: Antibiotic ‘time-out’ cut vancomycin use
The prevalence and implications of early trough samples have been measured at only 1 facility, and it is unknown whether these data can be reproduced elsewhere.5 Thus, this study sought to determine the prevalence and corresponding clinical actions of early trough levels at the Captain James A. Lovell Federal Health Care Center (JALFHCC). This is a unique facility that in 2010 combined a VA hospital with a DoD hospital. This facility cares for 67,000 military and retiree beneficiaries each year from southwestern Wisconsin and northwestern Illinois.The primary objective of this study was to measure the rate of early troughs drawn and their resultant effect on vancomycin regimens compared with correctly timed troughs. Secondarily, this study sought to compare the rate of repeated vancomycin trough levels in early vs correctly timed measurements.
Methods
This retrospective cohort analysis compared the outcomes of early and correctly timed vancomycin troughs. This study was approved by the Edward Hines, Jr. VA Hospital and JALFHCC Institutional Review Board. Veteran patients aged ≥ 18 years, hospitalized at JALFHCC, and receiving IV vancomycin at dosing intervals of 8, 12, 24, and 48 hours with measured trough levels between July 1, 2009, and July 1, 2013, were included in this study. Patients were excluded from analysis if vancomycin was given at any schedule other than the previously stated frequencies, they received hemodialysis during the treatment period, or their insurance coverage was through TRICARE (these patients had either active-duty or retired active-duty status).
Potentially eligible patients were identified via a Computerized Patient Records System (CPRS) search for laboratory vancomycin level measurements. The search supplied the researcher with the patient name, vancomycin level date and time, type of vancomycin level (trough or random), and vancomycin concentration. With this information, further data were gathered through CPRS: demographics, type of clinical infection, desired trough level (inferred if not listed in CPRS note), and vancomycin administration time (through the bar code medication administration system [BCMA] in CPRS). This analysis was of troughs, and multiple troughs may have originated from the same patient.
An early trough was defined as a trough taken more than 2 hours earlier than the next theoretical administration time or anytime before the third dose. After a trough was determined to be early or on time, the clinical actions taken during the dosing interval following sample collection were documented. A dose was considered to be held if stated in the BCMA or in a CPRS provider note. A dose was considered to be decreased with a change in frequency or strength that resulted in an overall daily dose decrease. A recollected vancomycin trough was counted within 24 hours of the trough or per a note in CPRS. Finally, observations that noted trends in vancomycin trough management were recorded.
The chi-square test with a significance criterion of 0.05 was used to compare early and on time troughs. Based on the results from the Boston, Massachusetts, study and 1 other study, about 780 vancomycin troughs would be required to meet significance in the primary outcome.5,6
Results
A total of 474 patient charts were reviewed, and 278 met inclusion criteria (196 were excluded). Of the included patients, 799 trough levels were analyzed. Of these, 377 (42.2%) were drawn early. There was no significant difference in the baseline characteristics of the early group vs the correctly timed group (Table 1). Of the early troughs, 190 (56.3%) were drawn prior to the third dose of vancomycin. It was observed that a large portion of these troughs occurred after a vancomycin dose adjustment.
Clinical actions taken after sampling occurred at a rate of 14.5% in the early group and 22.9% in the correctly timed group (P = .003; Table 2). Early troughs led to a 7.7% rate of trough recollection, which was significantly greater than the 1.5% rate in the correctly timed group (P < .001). An analysis of each factor resulting in a clinical action illustrated that the rates of daily dose decrease and discontinued dose were similar between the groups (Table 3). However, the rate of held doses was 8.3% in the early group and 17.1% in the correctly timed group.
This research process yielded some observations. Occasionally a trough was drawn after vancomycin therapy was discontinued and when there was no concern for nephrotoxicity. After the guidelines were published, providers continued to document in CPRS notes to check troughs before the third dose. This incidence decreased over time. Troughs were taken often in patients who were receiving a short course of therapy or who were hemodynamically stable. Finally, documentation of vancomycin regimen changes occasionally did not match the record in the BCMA (in these situations, the BCMA record was used for this study).
Related: Assessment of High Staphylococcus aureus MIC and Poor Patient Outcomes
Discussion
A large portion of trough levels at the JALFHCC were drawn early and did not adhere to the 2009 consensus guidelines. The rates of early troughs in this study and in the Boston, Massachusetts, study are similar.5 However, the 2 studies differed in 1 significant aspect: Clinical actions were taken less often in the early group at JALFHCC, whereas they were taken more often in the early group in the Boston, Massachusetts, study. This dissimilarity could be attributed to a difference in software between the hospitals. In the previous study, trough levels and the time that they were drawn were not displayed together. Thus, clinicians may have been less likely to gauge whether a trough was early. Since this information is available at the JALFHCC, clinicians may have been aware that the trough was early and avoided adjusting treatment (such as holding a dose, as illustrated in the data) based on a falsely elevated trough. This point is further supported by significantly greater amounts of recollected troughs in the early group, suggesting an understanding that the trough was early.
The low trough recollection rate of 7.7% of all early samples could be due to several factors that would prevent a trough redraw. First, medication discontinuation resulting from course completion or sensitivity results would not require further trough monitoring. Second, practitioners may assess the early sample as insignificantly different from a correctly timed one and elect not to redraw the trough. Sometimes a trough was drawn at the correct time, but the time was recorded incorrectly. In this situation, a new trough level would not be necessary. Finally, a lack of sufficient staffing during nights and weekends may result in a delay in interpreting results leading to a missed opportunity for recollection. Additionally, some troughs may not have been redrawn based on a practitioner’s opinion that a trough was not significantly early and did not represent skewed results. Sometimes an incorrect recording of trough draw time reflected that it was taken after vancomycin dosing when it was not.
Specific observations regarding the timing of the trough indicate other possible concerns and areas for improvement. First, providers must cancel future trough orders concurrently with canceling treatment. Second, at the time of publication of the consensus, some providers were slow adopters of the new guidelines. Finally, the IDSA guidelines state that frequent monitoring for short course, lower intensity therapy, or in patients who are hemodynamically stable is not recommended.3 However, troughs were sometimes measured 2 to 3 times weekly in these patients.
Related: Results mixed in hospital efforts to tackle antimicrobial resistance
The data and observations lead to the conclusion that although providers may be able to discern between early and correctly timed troughs, they were not consistently adherent to the 2009 IDSA guidelines. It has been shown that pharmacy involvement of Medicare patients with infections in the intensive care unit has led to better clinical and monetary outcomes.8 Therefore, continued efforts by clinical pharmacists to monitor trough timing can be used to improve adherence and decrease costs (each trough is estimated to cost $16.97).
A study conducted in Australia demonstrated that pharmacist-led education of vancomycin dosing and monitoring (including when to measure a trough level) among prescribers and nurses led to improved adherence to the current guidelines and a greater number of patients treated within desired therapeutic ranges.9 In addition, a small study at the Atlanta VAMC in Georgia demonstrated that education of nurses, lab personnel, residents, ward clerks, and pharmacists led to a greater number of appropriately timed vancomycin and aminoglycoside levels.10 Thus, an interdisciplinary review of the current IDSA guidelines and review on the publication of the anticipated updated vancomycin guidelines should be provided to hospital personnel to aid in adoption of current dosing and monitoring recommendations.11
Limitations
This study is limited by the 4-year span of time that it encompassed, which may give a skewed depiction of current practices. Another limitation is that patients with fluctuating renal function were included in the analysis. Instead of selecting a random level order, a trough level order was sometimes selected for these patients. This could lead to a lower actual rate of early troughs. A third limitation is that this was a small and unblinded study. Also, the actual trough levels and the resulting changes that were made to specific regimens were not recorded. Thus, these data do not indicate whether the changes that were made reflected guideline recommendations. Finally, some clinical actions were taken after the dosing interval following the trough. This was often a result of off-hours lab results or waiting on attending physician or infectious disease guidance. These data were not included in the analysis.
Conclusion
Vancomycin troughs were often drawn too early and resulted in an increased rate of trough recollection. In an attempt to improve adherence to the current and the upcoming revised version of the IDSA consensus statement, it is recommended to educate and reeducate providers through interdisciplinary-led review sessions.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Moellering RC Jr. Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42(suppl 1):S3-S4.
2. Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(suppl 1):S5-S12.
3. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009;29(11):1275-1279.
4. Davis SL, Scheetz MH, Bosso JA, Goff DA, Rybak MJ. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: a cross-sectional survey of U.S. hospitals. Pharmacotherapy. 2013;33(12):1256-1263.
5. Morrison AP, Melanson SEF, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early? Frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472-478.
6. Traugott KA, Maxwell PR, Green K, Frei C, Lewis JS 2nd. Effects of therapeutic drug monitoring criteria in a computerized prescriber-order-entry system on the appropriateness of vancomycin level orders. Am J Health Syst Pharm. 2011;68(4):347-352.
7. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801-806.
8. MacLaren R, Bond CA, Martin SJ, Fike D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 2008;36(12):3184-3189.
9. Phillips CJ, Doan H, Quinn S, Kirkpatrick CM, Gordon DL, Doogue MP. An educational intervention to improve vancomycin prescribing and monitoring. Int J Antimicrob Agents. 2013;41(4):393-394.
10. Carroll DJ, Austin GE, Stajich GV, Miyrhaya RK, Murphy JE, Ward ES. Effect of education on the appropriateness of serum drug concentration determination. Ther Drug Monit. 1992;14(1):81-84.
11. Infectious Diseases Society of America (IDSA). IDSA practice guidelines: antimicrobial agent use. IDSA Website. 2015. http://www.idsociety.org/Antimicrobial_Agents. Accessed November 16, 2015.
1. Moellering RC Jr. Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42(suppl 1):S3-S4.
2. Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(suppl 1):S5-S12.
3. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009;29(11):1275-1279.
4. Davis SL, Scheetz MH, Bosso JA, Goff DA, Rybak MJ. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: a cross-sectional survey of U.S. hospitals. Pharmacotherapy. 2013;33(12):1256-1263.
5. Morrison AP, Melanson SEF, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early? Frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472-478.
6. Traugott KA, Maxwell PR, Green K, Frei C, Lewis JS 2nd. Effects of therapeutic drug monitoring criteria in a computerized prescriber-order-entry system on the appropriateness of vancomycin level orders. Am J Health Syst Pharm. 2011;68(4):347-352.
7. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801-806.
8. MacLaren R, Bond CA, Martin SJ, Fike D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 2008;36(12):3184-3189.
9. Phillips CJ, Doan H, Quinn S, Kirkpatrick CM, Gordon DL, Doogue MP. An educational intervention to improve vancomycin prescribing and monitoring. Int J Antimicrob Agents. 2013;41(4):393-394.
10. Carroll DJ, Austin GE, Stajich GV, Miyrhaya RK, Murphy JE, Ward ES. Effect of education on the appropriateness of serum drug concentration determination. Ther Drug Monit. 1992;14(1):81-84.
11. Infectious Diseases Society of America (IDSA). IDSA practice guidelines: antimicrobial agent use. IDSA Website. 2015. http://www.idsociety.org/Antimicrobial_Agents. Accessed November 16, 2015.