User login
In reply: Submassive pulmonary embolism
In Reply: We thank Dr. Katyal for his thoughtful comments.
Dr. Katyal points out that the grade of recommendation for thrombolysis in patients with massive pulmonary embolism was upgraded from 2C to 2B in the 2016 American College of Chest Physicians (ACCP) guidelines1 compared with the 2012 guidelines2 that we cited. The upgrade in this recommendation was owing to 2 small trials and 1 large randomized controlled trial that included patients with submassive pulmonary embolism.3–5 Interestingly, these 3 studies led to an upgrade in the level of recommendation for thrombolysis in the treatment of massive pulmonary embolism, perhaps more from a safety aspect (in view of the incidence of major bleeding vs mortality). Regardless, Dr. Katyal is correct in highlighting that the new 2016 ACCP guidelines now give a grade of 2B for thrombolytic therapy in the treatment of massive pulmonary embolism. These guidelines had not been published at the time of submission of our manuscript.
Dr. Katyal is also correct that patients were not required to have right ventricular dysfunction to be enrolled in the MOPETT trial.3 As we pointed out, “Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.”6
Regarding catheter-directed therapy, patients who received low-dose catheter-directed alteplase were also concurrently anticoagulated with systemic unfractionated heparin in the Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism (ULTIMA) trial.7 The ULTIMA trial authors commented that unfractionated heparin was started with an 80-U/kg bolus followed by an 18-U/kg/hour infusion to target an anti-factor Xa level of 0.3 to 0.7 μg/mL, which is considered therapeutic anticoagulation. The investigators in the SEATTLE II trial8 continued systemic unfractionated heparin but targeted a lower “intermediate” anticoagulation target (an augmented partial thromboplastin time of 40–60 seconds), so these patients weren’t completely without systemic anticoagulation either. At our institution, the current practice is to target an anti-Xa level of 0.3 to 0.7 μg/mL in patients receiving catheter-directed therapy for large-volume pulmonary embolism.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
In Reply: We thank Dr. Katyal for his thoughtful comments.
Dr. Katyal points out that the grade of recommendation for thrombolysis in patients with massive pulmonary embolism was upgraded from 2C to 2B in the 2016 American College of Chest Physicians (ACCP) guidelines1 compared with the 2012 guidelines2 that we cited. The upgrade in this recommendation was owing to 2 small trials and 1 large randomized controlled trial that included patients with submassive pulmonary embolism.3–5 Interestingly, these 3 studies led to an upgrade in the level of recommendation for thrombolysis in the treatment of massive pulmonary embolism, perhaps more from a safety aspect (in view of the incidence of major bleeding vs mortality). Regardless, Dr. Katyal is correct in highlighting that the new 2016 ACCP guidelines now give a grade of 2B for thrombolytic therapy in the treatment of massive pulmonary embolism. These guidelines had not been published at the time of submission of our manuscript.
Dr. Katyal is also correct that patients were not required to have right ventricular dysfunction to be enrolled in the MOPETT trial.3 As we pointed out, “Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.”6
Regarding catheter-directed therapy, patients who received low-dose catheter-directed alteplase were also concurrently anticoagulated with systemic unfractionated heparin in the Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism (ULTIMA) trial.7 The ULTIMA trial authors commented that unfractionated heparin was started with an 80-U/kg bolus followed by an 18-U/kg/hour infusion to target an anti-factor Xa level of 0.3 to 0.7 μg/mL, which is considered therapeutic anticoagulation. The investigators in the SEATTLE II trial8 continued systemic unfractionated heparin but targeted a lower “intermediate” anticoagulation target (an augmented partial thromboplastin time of 40–60 seconds), so these patients weren’t completely without systemic anticoagulation either. At our institution, the current practice is to target an anti-Xa level of 0.3 to 0.7 μg/mL in patients receiving catheter-directed therapy for large-volume pulmonary embolism.
In Reply: We thank Dr. Katyal for his thoughtful comments.
Dr. Katyal points out that the grade of recommendation for thrombolysis in patients with massive pulmonary embolism was upgraded from 2C to 2B in the 2016 American College of Chest Physicians (ACCP) guidelines1 compared with the 2012 guidelines2 that we cited. The upgrade in this recommendation was owing to 2 small trials and 1 large randomized controlled trial that included patients with submassive pulmonary embolism.3–5 Interestingly, these 3 studies led to an upgrade in the level of recommendation for thrombolysis in the treatment of massive pulmonary embolism, perhaps more from a safety aspect (in view of the incidence of major bleeding vs mortality). Regardless, Dr. Katyal is correct in highlighting that the new 2016 ACCP guidelines now give a grade of 2B for thrombolytic therapy in the treatment of massive pulmonary embolism. These guidelines had not been published at the time of submission of our manuscript.
Dr. Katyal is also correct that patients were not required to have right ventricular dysfunction to be enrolled in the MOPETT trial.3 As we pointed out, “Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.”6
Regarding catheter-directed therapy, patients who received low-dose catheter-directed alteplase were also concurrently anticoagulated with systemic unfractionated heparin in the Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism (ULTIMA) trial.7 The ULTIMA trial authors commented that unfractionated heparin was started with an 80-U/kg bolus followed by an 18-U/kg/hour infusion to target an anti-factor Xa level of 0.3 to 0.7 μg/mL, which is considered therapeutic anticoagulation. The investigators in the SEATTLE II trial8 continued systemic unfractionated heparin but targeted a lower “intermediate” anticoagulation target (an augmented partial thromboplastin time of 40–60 seconds), so these patients weren’t completely without systemic anticoagulation either. At our institution, the current practice is to target an anti-Xa level of 0.3 to 0.7 μg/mL in patients receiving catheter-directed therapy for large-volume pulmonary embolism.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
Do patients with submassive pulmonary embolism benefit from thrombolytic therapy?
For patients with submassive acute pulmonary embolism—the intermediate category between massive and low-risk—whether to give thrombolytic therapy is controversial. In general, patients with massive pulmonary embolism need this therapy, whereas those with low-risk pulmonary embolism do not—and neither do most of those with submassive embolism. But where should we draw the line?
More than 600,000 patients suffer pulmonary embolisms every year in the United States, and 50,000 to 200,000 people die of them.1–3 In various studies,4–6 within 1 year, 12.9% of patients had another pulmonary embolism, 7.3% developed chronic venous insufficiency, and 3.8% developed chronic thromboembolic pulmonary hypertension.
THREE CATEGORIES OF RISK
Episodes of acute pulmonary embolism are classified as low-risk (about 70% of cases), hemodynamically unstable or massive (5%), or submassive (25%).7,8
Low-risk acute pulmonary embolism is defined by the absence of right ventricular dysfunction and the absence of myocardial necrosis. The death rate in such cases is less than 1%.9 Its pharmacologic management includes parenteral anticoagulation and early initiation of long-term anticoagulation therapy, which the American College of Chest Physicians (ACCP) gives a grade IB recommendation (strong, based on moderate-quality evidence).10
Massive or hemodynamically unstable pulmonary embolism is characterized by any of the following, in the absence of other causes8:
- Sustained hypotension (systolic blood pressure < 90 mm Hg for ≥ 15 minutes)
- An absolute decrease in systolic blood pressure of 40 mm Hg or more
- Need for inotropic support
- Cardiac arrest
- Bradycardia (heart rate < 40 beats per minute).
The death rate is more than 30% in patients presenting with shock and approaches 70% in those presenting with cardiac arrest.11,12 Therefore, the consensus is that this category of pulmonary embolism merits aggressive treatment. Systemic thrombolytic therapy is recommended in those who are not at high risk of major bleeding, though the ACCP gives it only a grade 2C recommendation (weak, based on low-quality evidence).10
Submassive pulmonary embolism is defined by evidence of right ventricular dysfunction with normal blood pressure. According to the ACCP guidelines, thrombolytic therapy should be considered (grade 2C recommendation) for patients with acute pulmonary embolism without hypotension and with a low bleeding risk (with no renal failure and not on dual antiplatelet therapy), but at high risk of developing hypotension.10
DIAGNOSING SUBMASSIVE PULMONARY EMBOLISM, DELINEATING ITS SEVERITY
In managing acute pulmonary embolism, it is critical to recognize whether a patient is at high risk of clinical deterioration.
Blood pressure
The systolic blood pressure not only indicates whether the patient has hypotension (systolic blood pressure < 90 mm Hg) and therefore massive rather than submassive or low-risk pulmonary embolism; it is also important as a baseline value. A decrease in systolic blood pressure of 40 mm Hg or more is associated with worse outcomes.12
Right ventricular dysfunction
The physiologic response to occlusion of the pulmonary arteries can result in early myocardial injury and right ventricular dysfunction, which can be assessed by various methods (Table 1).
Electrocardiographic signs. Right heart strain may be recognized on electrocardiography as:
- Evidence of new complete or incomplete right bundle branch block
- T-wave inversion in the anterolateral leads V1 to V4
- S1Q3T3 (a large S wave in lead I, a Q wave in lead III, and an inverted T wave in lead III, the classic pattern of acute cor pulmonale).13
These findings add incremental prognostic value to echocardiographic findings in patients with submassive pulmonary embolism.14
Cardiac biomarkers such as B-type natriuretic peptide (BNP), N-terminal-pro-BNP (NT-pro-BNP), cardiac troponins, and heart-type fatty acid-binding protein (H-FABP) are also markers of right-sided myocardial damage and strain and can be used to identify patients with submassive pulmonary embolism.15 Abnormal levels of these substances are as follows:
- Troponin T greater than 0.1 ng/mL
- Troponin I greater than 0.4 ng/mL
- BNP greater than 90 pg/mL
- NT-pro-BNP greater than 500 pg/mL
- H-FABP less than 6 ng/mL.
These levels have prognostic value, identifying patients with submassive pulmonary embolism at risk of deterioration or death,14,16,17
Echocardiographic signs. Right ventricular dysfunction can be assessed quickly at the bedside with portable transthoracic echocardiography. A meta-analysis showed that close to 37% of hemodynamically stable patients with pulmonary embolism had echocardiographic evidence of right ventricular dysfunction on presentation and a higher short-term mortality rate.18 Evidence of right ventricular dysfunction includes the following:
- New-onset hypokinesis or akinesis
- Right ventricular dilation
- Right ventricular free-wall hypokinesis with apical sparing (the McConnell sign)
- Paradoxical movement of the interventricular septum
- Newly increased right ventricular systolic pressure
- Pulmonary hypertension, defined as tricuspid regurgitation jet velocity greater than 2.8 m/s.15,19
Computed tomographic (CT) angiography is widely available. Findings that have prognostic value in determining those at higher risk of death include the following20,21:
- A dilated right ventricle—ratio of right ventricle to left ventricle diameter (RV:LV ratio) greater than 0.9
- Interventricular septal bowing.
PESI and sPESI scores. The European Society of Cardiology 2014 guidelines stratify the risk in normotensive patients with pulmonary embolism according to their score on the Pulmonary Embolism Severity Index (PESI) or the simplified PESI (sPESI). There are five PESI classes. Those in PESI class III or IV or with an sPESI score of 1 or more (on a scale of 0 to 6) are considered at intermediate risk of clinical deterioration and are then further risk-stratified according to whether they have right ventricular dysfunction (based on echocardiography or computed tomography) and elevated cardiac biomarkers. These scoring systems are based on easily obtainable clinical information such as age, male sex, history of cancer, history of heart failure, history of chronic lung disease, heart rate, systolic blood pressure, respiratory rate, temperature, and altered mental status, and calculators are readily available.
Anticoagulation for all, plus thrombolysis for some
Patients with neither right ventricular dysfunction nor elevated cardiac biomarkers are at intermediate to low risk of clinical deterioration, and it is recommended that they be given anticoagulation therapy in an inpatient setting.
On the other hand, patients with both right ventricular dysfunction and elevated cardiac biomarkers are considered at intermediate to high risk of clinical deterioration; they should also be managed with anticoagulation and monitored closely for the need for rescue reperfusion therapy with thrombolytics.22
THROMBOLYTIC AGENTS
Thrombolytic agents are the cornerstone of management for patients presenting with pulmonary embolism who are at high risk. As noted above, these agents are recommended in massive pulmonary embolism, but their role in submassive pulmonary embolism remains controversial.
Thrombolytics work by activating endogenous plasminogen. The resulting plasmin promotes clot lysis, reducing the size of the thrombus, decreasing pulmonary vasculature resistance, and improving right ventricular function.23
To date, three thrombolytic agents have received US Food and Drug Administration approval for use in massive pulmonary embolism: alteplase, urokinase, and streptokinase. But only alteplase is still available in the United States. Alteplase is also the best tolerated, whereas streptokinase is highly antigenic and may cause further deterioration in an already unstable patient. Alteplase is also the most fibrin-specific and is considered the most potent of the three agents.24
Additional thrombolytic agents under investigation for use in acute pulmonary embolism include reteplase, tenecteplase, and desmoteplase. These agents are more fibrin-specific than alteplase. Reteplase is a second-generation recombinant tissue-type plasminogen activator with a quicker onset of action and longer half-life than alteplase, allowing for bolus dosing. Tenecteplase, a variant of alteplase, is cleared more slowly and is 14 times more fibrin-specific than alteplase, also allowing for bolus dosing. Desmoteplase, a fibrin-specific agent currently in phase 2 trials, also has a longer half-life and appears to be more potent than alteplase. Table 2 lists the dosing and the degree of fibrin specificity of these agents.
Complications of thrombolytic therapy
Submassive pulmonary embolism has a low death rate, and the benefit of systemic thrombolytic therapy for this condition is controversial. Therefore, risk stratification is very important before pursuing this therapy.
A meta-analysis25 of 16 randomized controlled trials included 2,125 patients with pulmonary embolism:
- 210 (9.88%) in the low-risk category
- 1,499 (70.54%) in the submassive category
- 31 (1.46%) in the massive category
- 385 (18.11%) whose disease severity could not be determined.
Major bleeding occurred in:
- 98 (9.24%) of 1,061 patients receiving anticoagulation plus thrombolytics
- 36 (3.42%) of 1,054 patients receiving anticoagulation without thrombolytics (odds ratio [OR] 2.73, 95% confidence interval [CI] 1.91–3.91; number needed to harm [NNH] 18, 95% CI 13–27).
Intracranial hemorrhage occurred in:
- 15 (1.46%) of 2,014 patients on thrombolytic therapy
- 2 (0.19%) of 1,019 patients not on thrombolytic therapy (OR 4.63, 95% CI 1.78–12.04; NNH 78, 95% CI 48–206).
Of note, the incidence of major bleeding was not significantly increased in those age 65 or younger receiving thrombolytics (OR 1.25, 95% CI 0.5–3.14).
Comments. Definitions of major bleeding varied in the individual trials. Additionally, intracranial hemorrhage was included as a major bleeding end point in any trial in which it was not prespecified.
These findings emphasize the importance of risk stratification before pursuing thrombolytic therapy in submassive pulmonary embolism.
Table 3 lists absolute and relative contraindications to thrombolytic therapy.
MAJOR STUDIES IN SUBMASSIVE PULMONARY EMBOLISM
The MAPPET-3 trial
The Management Strategies and Prognosis of Pulmonary Embolism-3 (MAPPET-3) trial,26 in 2002, was the first major trial to study thrombolytic therapy in submassive pulmonary embolism.
In this prospective, randomized, double-blinded trial conducted in Germany, 118 patients received heparin with alteplase (100 mg over 2 hours) and 138 received heparin with placebo. The primary end point was in-hospital death or clinical deterioration requiring escalation of treatment. Secondary outcomes included recurrent pulmonary embolism, major bleeding, and stroke. Major bleeding was defined as fatal bleeding, hemorrhagic stroke, or drop in the hemoglobin concentration by more than 4 g/dL, with or without the need for red blood cell transfusion.
Right ventricular dysfunction was diagnosed by echocardiography in 30% of the participants, and the rest of the patients were classified as having submassive pulmonary embolism on the basis of electrocardiographic criteria alone. It is likely that the latter group had a less severe form of the disease and did not benefit from thrombolytic therapy as much as patients with echocardiographic findings of right ventricular dysfunction and elevated serum cardiac biomarkers.
Results. At 30 days, 11% of the alteplase-plus-heparin group had met the primary end point, compared with 24.6% of the placebo-plus-heparin group (P = .006). The difference was mostly driven by the need for secondary thrombolysis (7.6% vs 23.2%, P = .001), since 32 (23.2%) of the 138 patients in the control group required secondary thrombolysis, accounting for 94% of the 34 patients in this group who required escalation of treatment. Most cases of clinical deterioration in this group occurred within the first 5 days.
Mortality rates were 3.4% in the heparin-plus-alteplase group and 2.2% in the heparin-plus-placebo group, but the difference was not statistically significant (P = .71).
Major bleeding occurred in 1 patient in the heparin-plus-alteplase group and 5 patients in the heparin-plus-placebo group, but the trial’s definition of major bleeding may have resulted in underestimation of this event. The definition put forth by the International Society on Thrombosis and Haemostasis is less strict, defining bleeding in nonsurgical patients as major if it is fatal, symptomatic in a critical area or organ, or causing a fall in hemoglobin level of 2.0 g/dL or more, leading to transfusion of two or more units of whole blood or red cells.27
MOPETT trial
The Moderate Pulmonary Embolism Treated with Thrombolysis (MOPETT) trial28 was a single-center, randomized trial in 121 normotensive patients with “moderate” pulmonary embolism and right ventricular dysfunction. Moderate pulmonary embolism was defined as signs and symptoms of pulmonary embolism with evidence on computed tomographic angiography of greater than 70% involvement with thrombus in two or more lobes or left or right main pulmonary arteries, or by a high-probability ventilation-perfusion scan showing a mismatch in two or more lobes.
The authors defined right ventricular dysfunction by elevated cardiac markers or by findings on echocardiography. Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.
The primary outcome was the development of pulmonary hypertension, based on echocardiography.
Patients were randomized to either anticoagulation alone (unfractionated heparin or low-molecular-weight heparin) or anticoagulation plus half-dose alteplase (0.5 mg/kg, to a maximum of 50 mg). Echocardiography was performed within 2 hours of study entry, at 48 hours, and every 6 months thereafter. The mean duration of follow-up was 28 months.
Results. Pulmonary hypertension developed in 16% of the anticoagulation-plus-alteplase group vs 57% of the anticoagulation-only group (P < .001). However, the clinical relevance of elevated right-sided pressures observed by echocardiography in asymptomatic patients is uncertain. Alteplase had no impact on the rates of death or recurrent pulmonary embolism.
PEITHO trial
The 2014 Pulmonary Embolism Thrombolysis (PEITHO) trial29 was a prospective, randomized, double-blinded, placebo-controlled trial conducted in 13 countries between 2007 and 2012. A total of 1,005 patients with submassive pulmonary embolism received unfractionated heparin and were randomized to also receive either tenecteplase or placebo.
The primary end point was death from any cause or hemodynamic compromise within 7 days of randomization. Secondary end points included death within 30 days, recurrence of pulmonary embolism, major bleeding, and stroke.
Echocardiography was strongly recommended for diagnosing right ventricular dysfunction in all patients. When this was unavailable, computed tomographic images were used to assess right ventricular dysfunction. Major bleeding was characterized as moderate or severe, and bleeding events were reported using the International Society on Thrombosis and Haemostasis criteria.
Results. The tenecteplase group had a lower rate of the primary end point at 7 days (2.6% vs 5.6%, P = .02), but no significant reduction in all-cause mortality at 30 days (2.4% vs 3.2%, P = .42). In addition, the tenecteplase group had higher rates of major extracranial bleeding (6.3% vs 1.2%, P < .001) and stroke (2.4% vs 0.2%, P = .004) at 7 days.
Although the PEITHO trial showed no reduction in mortality rates and showed a higher rate of major bleeding, this may have been related to using a higher dose of tenecteplase than needed in this population. Further studies should be conducted to confirm this theory.
TOPCOAT trial
The Tenecteplase or Placebo, Cardiopulmonary Outcomes at Three months (TOPCOAT) trial,30 published in 2014, was a multicenter, double-blind, intention-to-treat, randomized trial carried out in eight centers in the United States. The authors evaluated a composite outcome (death, circulatory shock, intubation, major bleeding, recurrent pulmonary embolism, and functional capacity) with the use of tenecteplase in submassive pulmonary embolism.
A total of 83 patients received low-molecular-weight heparin and were randomized to also receive either tenecteplase or placebo. Submassive pulmonary embolism was defined as evidence of right ventricular strain based on echocardiographic findings and elevated cardiac markers (troponin, BNP, or NT-pro-BNP).
Results. Adverse outcomes occurred in 37% of the patients in the placebo group compared with 15% of those in the tenecteplase group (P = .017). The study was terminated early because the lead author relocated to another institution.
Wang et al
In a prospective, randomized, open-label, multicenter study31 conducted in China between 2002 and 2006, 118 patients received low-molecular-weight heparin plus alteplase in a dose of either 100 mg or 50 mg over 2 hours.
Results. There were significantly fewer bleeding episodes in patients receiving half-dose alteplase in the subgroups that weighed less than 65 kg (14.8% vs 41.2%, P = .049) or who had a body mass index less than 24 kg/m2 (8.7% vs 42.9%, P = .014).
Meta-analysis
A subgroup analysis25 of patients with submassive pulmonary embolism from a 2014 meta-analysis of randomized controlled trials of thrombolytic therapy in pulmonary embolism found that thrombolysis was associated with a lower mortality rate (OR 0.48; 95% CI 0.25–0.92) but a higher rate of major bleeding (OR 3.19, 95% CI 2.07–4.92).
Is there a role for low-dose thrombolytic therapy?
The MOPETT study, discussed above, evaluated the effect of thrombolysis in a low (“safe”) dose in reducing pulmonary artery pressure in moderate pulmonary embolism.28 The primary end points were pulmonary hypertension and the composite end point of pulmonary hypertension and recurrent pulmonary embolism. In the thrombolysis group, the pulmonary arterial pressure fell immediately and was still lower at 28 months. As mentioned, although the incidence of pulmonary hypertension was lower with thrombolysis, no significant differences were noted in the rate of individual outcomes of death and recurrent pulmonary embolism when assessed independently. Furthermore, the definition of moderate pulmonary embolism used in this study is different from the submassive criteria.
Wang et al31 enrolled patients to receive low-molecular-weight heparin plus alteplase in a dose of either 50 or 100 mg. The rate of bleeding was lower with the 50-mg dose, but only in the subset of patients with lower weight and body mass index.
What is the role of catheter-guided therapy?
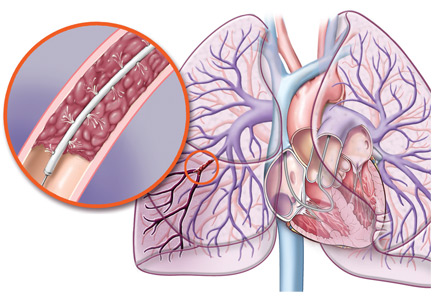
Catheter-directed therapy involves infusing thrombolytic agents directly into the pulmonary arteries where the clots are. The idea is to expose the patient to lower doses of systemic thrombolytics and thus decrease the risk of bleeding.
The ULTIMA study32 (Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism) evaluated whether this treatment would reverse right ventricular dilation in intermediate-risk patients, compared with anticoagulation. Intermediate-risk pulmonary embolism was defined as an embolus located in at least one main or proximal lower lobe pulmonary artery and an RV:LV ratio of at least 1.0 obtained from the echocardiographic apical four-chamber view.
The study showed hemodynamic improvement as evidenced by a lower RV:LV ratio. However, at 90 days the mortality rate was not significantly lower in the treatment group than in the control group. Of note, no major bleeding events were reported in the treatment group.
The SEATTLE II trial,33 a nonrandomized study completed in April 2013, evaluated the efficacy and safety of ultrasonographically guided, catheter-based, low-dose fibrinolysis for patients with massive and submassive pulmonary embolism. Patients had CT evidence of proximal pulmonary embolism and a dilated right ventricle (RV:LV ratio ≥ 0.9). Patients received alteplase 24 mg, either as 1 mg/hour for 24 hours with a unilateral catheter or 1 mg/hour in each of two catheters for 12 hours.
At 48 hours after the procedure, the mean RV:LV ratio had decreased from 1.55 to 1.13, the mean pulmonary arterial systolic pressure had fallen, and the anatomical clot burden had decreased. A total of 15 patients (10%) experienced major bleeding but there were no reports of any fatal or intracranial bleeding. Patients with massive pulmonary embolism were more likely to experience major bleeding episodes than those with submassive pulmonary embolism (23% vs 7%, P = .02).
The weakness of this study is that it was a single-arm study and therefore limits comparisons with other therapies such as tissue plasminogen activator for massive pulmonary embolism or anticoagulation. Also, although there was an acute improvement in hemodynamics, it is unclear if that translates to improvement in mortality rate.
Based on the available literature,29,31,33 patients presenting with submassive pulmonary embolism who are of low body weight (body mass index < 24 kg/m2 or weight < 65 kg) or are over age 75 may benefit from low-dose catheter-guided thrombolysis therapy or low-dose systemic alteplase (50 mg). Further studies should be conducted comparing these two therapeutic strategies.
SURGICAL EMBOLECTOMY: STILL THE LAST RESORT
Surgery has been the last resort for patients with pulmonary embolism. Although recent reports show a decrease in mortality from advances in surgical embolectomy, the mortality rate is greater than 10%.34
- Indications for surgical embolectomy are35:
- Failure of or contraindications to thrombolytic therapy
- Continued hemodynamic instability despite maximal medical therapy
- Associated cardiac pathology such as patent foramen ovale, atrial septal defect, and free-floating right heart thrombi
- Inadequate time for systemic thrombolytics to take effect.
No large or randomized controlled trials of surgical embolectomy for submassive pulmonary embolism have been done. In one study, of 47 patients undergoing surgical embolectomy, 15 (32%) met the criteria for submassive pulmonary embolism based on right ventricular hemodynamic dysfunction. The report did not mention if biomarkers such as troponin and BNP were considered in the decision to operate.36
At this time, surgical embolectomy remains a last resort for patients with acute massive pulmonary embolism who have contraindications to thrombolysis or for whom it has failed. Given the risk of death associated with surgical embolectomy, large randomized controlled trials need to be done to see if there is any benefit in the submassive pulmonary embolism population.
ONE TREATMENT DOES NOT FIT ALL
Given the evidence to date, we do not recommend thrombolytic therapy for all patients with submassive pulmonary embolism. The risk of complications (hemorrhage) is significant, and the benefit is unclear. A one-treatment-for-all approach cannot be applied in this situation.
Furthermore, each of the trials performed so far defined submassive pulmonary embolism slightly differently (Table 4), and many were underpowered to detect a difference in mortality rates between the treatment groups. Further studies are needed to determine the exact subset of patients with submassive pulmonary embolism that may truly benefit from thrombolytic therapy.
As such, patients with submassive pulmonary embolism should be managed by a multidisciplinary team to determine the need for thrombolytic therapy, especially in low doses, on a case-by-case basis according to the patient’s risk of further clinical deterioration.
- Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998; 158:585–593.
- Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med 2012; 125:478–484.
- Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest 2002; 121:877–905.
- Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ 3rd. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med 2000; 160:761–768.
- Mohr DN, Silverstein MD, Heit JA, Petterson TM, O’Fallon WM, Melton LJ. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: a population-based study. Mayo Clin Proc 2000; 75:1249–1256.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest 2004; 125:1539–1545.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Comess KA, DeRook FA, Russell ML, Tognazzi-Evans TA, Beach KW. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med 2000; 109:351–356.
- Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 1997; 30:1165–1171.
- Piazza G. Submassive pulmonary embolism. JAMA 2013; 309:171–180.
- Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008; 178:425–430.
- Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med 2009; 122:257–264.
- Amorim S, Dias P, Rodrigues RA, et al. Troponin I as a marker of right ventricular dysfunction and severity of pulmonary embolism. Rev Port Cardiol 2006; 25:181–186.
- Dellas C, Puls M, Lankeit M, et al. Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol 2010; 55:2150–2157.
- Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord 2014; 14:64.
- Nazeyrollas P, Metz D, Jolly D, et al. Use of transthoracic Doppler echocardiography combined with clinical and electrocardiographic data to predict acute pulmonary embolism. Eur Heart J 1996;17: 779–786.
- Wake N, Kumamaru KK, George E, et al. Computed tomography and echocardiography in patients with acute pulmonary embolism: part 1: correlation of findings of right ventricular enlargement. J Thorac Imaging 2014; 29:W1–W6.
- Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J 2014; 43:1678–1690.
- Konstantinides SV, Torbicki A, Agnelli G, et al; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069, 69a–69k.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Daley MJ, Lat I. Clinical controversies in thrombolytic therapy for the management of acute pulmonary embolism. Pharmacotherapy 2012; 32:158–172.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W; Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002; 347:1143–1150.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Wang C, Zhai Z, Yang Y, et al; China Venous Thromboembolism (VTE) Study Group. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest 2010; 137:254–262.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
- Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99:421–423.
- He C, Von Segesser LK, Kappetein PA, Mestres CA, Smith JA, Choong CK. Acute pulmonary embolectomy. Eur J Cardiothorac Surg 2013; 43:1087–1095.
- Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005; 129:1018–1023.
For patients with submassive acute pulmonary embolism—the intermediate category between massive and low-risk—whether to give thrombolytic therapy is controversial. In general, patients with massive pulmonary embolism need this therapy, whereas those with low-risk pulmonary embolism do not—and neither do most of those with submassive embolism. But where should we draw the line?
More than 600,000 patients suffer pulmonary embolisms every year in the United States, and 50,000 to 200,000 people die of them.1–3 In various studies,4–6 within 1 year, 12.9% of patients had another pulmonary embolism, 7.3% developed chronic venous insufficiency, and 3.8% developed chronic thromboembolic pulmonary hypertension.
THREE CATEGORIES OF RISK
Episodes of acute pulmonary embolism are classified as low-risk (about 70% of cases), hemodynamically unstable or massive (5%), or submassive (25%).7,8
Low-risk acute pulmonary embolism is defined by the absence of right ventricular dysfunction and the absence of myocardial necrosis. The death rate in such cases is less than 1%.9 Its pharmacologic management includes parenteral anticoagulation and early initiation of long-term anticoagulation therapy, which the American College of Chest Physicians (ACCP) gives a grade IB recommendation (strong, based on moderate-quality evidence).10
Massive or hemodynamically unstable pulmonary embolism is characterized by any of the following, in the absence of other causes8:
- Sustained hypotension (systolic blood pressure < 90 mm Hg for ≥ 15 minutes)
- An absolute decrease in systolic blood pressure of 40 mm Hg or more
- Need for inotropic support
- Cardiac arrest
- Bradycardia (heart rate < 40 beats per minute).
The death rate is more than 30% in patients presenting with shock and approaches 70% in those presenting with cardiac arrest.11,12 Therefore, the consensus is that this category of pulmonary embolism merits aggressive treatment. Systemic thrombolytic therapy is recommended in those who are not at high risk of major bleeding, though the ACCP gives it only a grade 2C recommendation (weak, based on low-quality evidence).10
Submassive pulmonary embolism is defined by evidence of right ventricular dysfunction with normal blood pressure. According to the ACCP guidelines, thrombolytic therapy should be considered (grade 2C recommendation) for patients with acute pulmonary embolism without hypotension and with a low bleeding risk (with no renal failure and not on dual antiplatelet therapy), but at high risk of developing hypotension.10
DIAGNOSING SUBMASSIVE PULMONARY EMBOLISM, DELINEATING ITS SEVERITY
In managing acute pulmonary embolism, it is critical to recognize whether a patient is at high risk of clinical deterioration.
Blood pressure
The systolic blood pressure not only indicates whether the patient has hypotension (systolic blood pressure < 90 mm Hg) and therefore massive rather than submassive or low-risk pulmonary embolism; it is also important as a baseline value. A decrease in systolic blood pressure of 40 mm Hg or more is associated with worse outcomes.12
Right ventricular dysfunction
The physiologic response to occlusion of the pulmonary arteries can result in early myocardial injury and right ventricular dysfunction, which can be assessed by various methods (Table 1).
Electrocardiographic signs. Right heart strain may be recognized on electrocardiography as:
- Evidence of new complete or incomplete right bundle branch block
- T-wave inversion in the anterolateral leads V1 to V4
- S1Q3T3 (a large S wave in lead I, a Q wave in lead III, and an inverted T wave in lead III, the classic pattern of acute cor pulmonale).13
These findings add incremental prognostic value to echocardiographic findings in patients with submassive pulmonary embolism.14
Cardiac biomarkers such as B-type natriuretic peptide (BNP), N-terminal-pro-BNP (NT-pro-BNP), cardiac troponins, and heart-type fatty acid-binding protein (H-FABP) are also markers of right-sided myocardial damage and strain and can be used to identify patients with submassive pulmonary embolism.15 Abnormal levels of these substances are as follows:
- Troponin T greater than 0.1 ng/mL
- Troponin I greater than 0.4 ng/mL
- BNP greater than 90 pg/mL
- NT-pro-BNP greater than 500 pg/mL
- H-FABP less than 6 ng/mL.
These levels have prognostic value, identifying patients with submassive pulmonary embolism at risk of deterioration or death,14,16,17
Echocardiographic signs. Right ventricular dysfunction can be assessed quickly at the bedside with portable transthoracic echocardiography. A meta-analysis showed that close to 37% of hemodynamically stable patients with pulmonary embolism had echocardiographic evidence of right ventricular dysfunction on presentation and a higher short-term mortality rate.18 Evidence of right ventricular dysfunction includes the following:
- New-onset hypokinesis or akinesis
- Right ventricular dilation
- Right ventricular free-wall hypokinesis with apical sparing (the McConnell sign)
- Paradoxical movement of the interventricular septum
- Newly increased right ventricular systolic pressure
- Pulmonary hypertension, defined as tricuspid regurgitation jet velocity greater than 2.8 m/s.15,19
Computed tomographic (CT) angiography is widely available. Findings that have prognostic value in determining those at higher risk of death include the following20,21:
- A dilated right ventricle—ratio of right ventricle to left ventricle diameter (RV:LV ratio) greater than 0.9
- Interventricular septal bowing.
PESI and sPESI scores. The European Society of Cardiology 2014 guidelines stratify the risk in normotensive patients with pulmonary embolism according to their score on the Pulmonary Embolism Severity Index (PESI) or the simplified PESI (sPESI). There are five PESI classes. Those in PESI class III or IV or with an sPESI score of 1 or more (on a scale of 0 to 6) are considered at intermediate risk of clinical deterioration and are then further risk-stratified according to whether they have right ventricular dysfunction (based on echocardiography or computed tomography) and elevated cardiac biomarkers. These scoring systems are based on easily obtainable clinical information such as age, male sex, history of cancer, history of heart failure, history of chronic lung disease, heart rate, systolic blood pressure, respiratory rate, temperature, and altered mental status, and calculators are readily available.
Anticoagulation for all, plus thrombolysis for some
Patients with neither right ventricular dysfunction nor elevated cardiac biomarkers are at intermediate to low risk of clinical deterioration, and it is recommended that they be given anticoagulation therapy in an inpatient setting.
On the other hand, patients with both right ventricular dysfunction and elevated cardiac biomarkers are considered at intermediate to high risk of clinical deterioration; they should also be managed with anticoagulation and monitored closely for the need for rescue reperfusion therapy with thrombolytics.22
THROMBOLYTIC AGENTS
Thrombolytic agents are the cornerstone of management for patients presenting with pulmonary embolism who are at high risk. As noted above, these agents are recommended in massive pulmonary embolism, but their role in submassive pulmonary embolism remains controversial.
Thrombolytics work by activating endogenous plasminogen. The resulting plasmin promotes clot lysis, reducing the size of the thrombus, decreasing pulmonary vasculature resistance, and improving right ventricular function.23
To date, three thrombolytic agents have received US Food and Drug Administration approval for use in massive pulmonary embolism: alteplase, urokinase, and streptokinase. But only alteplase is still available in the United States. Alteplase is also the best tolerated, whereas streptokinase is highly antigenic and may cause further deterioration in an already unstable patient. Alteplase is also the most fibrin-specific and is considered the most potent of the three agents.24
Additional thrombolytic agents under investigation for use in acute pulmonary embolism include reteplase, tenecteplase, and desmoteplase. These agents are more fibrin-specific than alteplase. Reteplase is a second-generation recombinant tissue-type plasminogen activator with a quicker onset of action and longer half-life than alteplase, allowing for bolus dosing. Tenecteplase, a variant of alteplase, is cleared more slowly and is 14 times more fibrin-specific than alteplase, also allowing for bolus dosing. Desmoteplase, a fibrin-specific agent currently in phase 2 trials, also has a longer half-life and appears to be more potent than alteplase. Table 2 lists the dosing and the degree of fibrin specificity of these agents.
Complications of thrombolytic therapy
Submassive pulmonary embolism has a low death rate, and the benefit of systemic thrombolytic therapy for this condition is controversial. Therefore, risk stratification is very important before pursuing this therapy.
A meta-analysis25 of 16 randomized controlled trials included 2,125 patients with pulmonary embolism:
- 210 (9.88%) in the low-risk category
- 1,499 (70.54%) in the submassive category
- 31 (1.46%) in the massive category
- 385 (18.11%) whose disease severity could not be determined.
Major bleeding occurred in:
- 98 (9.24%) of 1,061 patients receiving anticoagulation plus thrombolytics
- 36 (3.42%) of 1,054 patients receiving anticoagulation without thrombolytics (odds ratio [OR] 2.73, 95% confidence interval [CI] 1.91–3.91; number needed to harm [NNH] 18, 95% CI 13–27).
Intracranial hemorrhage occurred in:
- 15 (1.46%) of 2,014 patients on thrombolytic therapy
- 2 (0.19%) of 1,019 patients not on thrombolytic therapy (OR 4.63, 95% CI 1.78–12.04; NNH 78, 95% CI 48–206).
Of note, the incidence of major bleeding was not significantly increased in those age 65 or younger receiving thrombolytics (OR 1.25, 95% CI 0.5–3.14).
Comments. Definitions of major bleeding varied in the individual trials. Additionally, intracranial hemorrhage was included as a major bleeding end point in any trial in which it was not prespecified.
These findings emphasize the importance of risk stratification before pursuing thrombolytic therapy in submassive pulmonary embolism.
Table 3 lists absolute and relative contraindications to thrombolytic therapy.
MAJOR STUDIES IN SUBMASSIVE PULMONARY EMBOLISM
The MAPPET-3 trial
The Management Strategies and Prognosis of Pulmonary Embolism-3 (MAPPET-3) trial,26 in 2002, was the first major trial to study thrombolytic therapy in submassive pulmonary embolism.
In this prospective, randomized, double-blinded trial conducted in Germany, 118 patients received heparin with alteplase (100 mg over 2 hours) and 138 received heparin with placebo. The primary end point was in-hospital death or clinical deterioration requiring escalation of treatment. Secondary outcomes included recurrent pulmonary embolism, major bleeding, and stroke. Major bleeding was defined as fatal bleeding, hemorrhagic stroke, or drop in the hemoglobin concentration by more than 4 g/dL, with or without the need for red blood cell transfusion.
Right ventricular dysfunction was diagnosed by echocardiography in 30% of the participants, and the rest of the patients were classified as having submassive pulmonary embolism on the basis of electrocardiographic criteria alone. It is likely that the latter group had a less severe form of the disease and did not benefit from thrombolytic therapy as much as patients with echocardiographic findings of right ventricular dysfunction and elevated serum cardiac biomarkers.
Results. At 30 days, 11% of the alteplase-plus-heparin group had met the primary end point, compared with 24.6% of the placebo-plus-heparin group (P = .006). The difference was mostly driven by the need for secondary thrombolysis (7.6% vs 23.2%, P = .001), since 32 (23.2%) of the 138 patients in the control group required secondary thrombolysis, accounting for 94% of the 34 patients in this group who required escalation of treatment. Most cases of clinical deterioration in this group occurred within the first 5 days.
Mortality rates were 3.4% in the heparin-plus-alteplase group and 2.2% in the heparin-plus-placebo group, but the difference was not statistically significant (P = .71).
Major bleeding occurred in 1 patient in the heparin-plus-alteplase group and 5 patients in the heparin-plus-placebo group, but the trial’s definition of major bleeding may have resulted in underestimation of this event. The definition put forth by the International Society on Thrombosis and Haemostasis is less strict, defining bleeding in nonsurgical patients as major if it is fatal, symptomatic in a critical area or organ, or causing a fall in hemoglobin level of 2.0 g/dL or more, leading to transfusion of two or more units of whole blood or red cells.27
MOPETT trial
The Moderate Pulmonary Embolism Treated with Thrombolysis (MOPETT) trial28 was a single-center, randomized trial in 121 normotensive patients with “moderate” pulmonary embolism and right ventricular dysfunction. Moderate pulmonary embolism was defined as signs and symptoms of pulmonary embolism with evidence on computed tomographic angiography of greater than 70% involvement with thrombus in two or more lobes or left or right main pulmonary arteries, or by a high-probability ventilation-perfusion scan showing a mismatch in two or more lobes.
The authors defined right ventricular dysfunction by elevated cardiac markers or by findings on echocardiography. Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.
The primary outcome was the development of pulmonary hypertension, based on echocardiography.
Patients were randomized to either anticoagulation alone (unfractionated heparin or low-molecular-weight heparin) or anticoagulation plus half-dose alteplase (0.5 mg/kg, to a maximum of 50 mg). Echocardiography was performed within 2 hours of study entry, at 48 hours, and every 6 months thereafter. The mean duration of follow-up was 28 months.
Results. Pulmonary hypertension developed in 16% of the anticoagulation-plus-alteplase group vs 57% of the anticoagulation-only group (P < .001). However, the clinical relevance of elevated right-sided pressures observed by echocardiography in asymptomatic patients is uncertain. Alteplase had no impact on the rates of death or recurrent pulmonary embolism.
PEITHO trial
The 2014 Pulmonary Embolism Thrombolysis (PEITHO) trial29 was a prospective, randomized, double-blinded, placebo-controlled trial conducted in 13 countries between 2007 and 2012. A total of 1,005 patients with submassive pulmonary embolism received unfractionated heparin and were randomized to also receive either tenecteplase or placebo.
The primary end point was death from any cause or hemodynamic compromise within 7 days of randomization. Secondary end points included death within 30 days, recurrence of pulmonary embolism, major bleeding, and stroke.
Echocardiography was strongly recommended for diagnosing right ventricular dysfunction in all patients. When this was unavailable, computed tomographic images were used to assess right ventricular dysfunction. Major bleeding was characterized as moderate or severe, and bleeding events were reported using the International Society on Thrombosis and Haemostasis criteria.
Results. The tenecteplase group had a lower rate of the primary end point at 7 days (2.6% vs 5.6%, P = .02), but no significant reduction in all-cause mortality at 30 days (2.4% vs 3.2%, P = .42). In addition, the tenecteplase group had higher rates of major extracranial bleeding (6.3% vs 1.2%, P < .001) and stroke (2.4% vs 0.2%, P = .004) at 7 days.
Although the PEITHO trial showed no reduction in mortality rates and showed a higher rate of major bleeding, this may have been related to using a higher dose of tenecteplase than needed in this population. Further studies should be conducted to confirm this theory.
TOPCOAT trial
The Tenecteplase or Placebo, Cardiopulmonary Outcomes at Three months (TOPCOAT) trial,30 published in 2014, was a multicenter, double-blind, intention-to-treat, randomized trial carried out in eight centers in the United States. The authors evaluated a composite outcome (death, circulatory shock, intubation, major bleeding, recurrent pulmonary embolism, and functional capacity) with the use of tenecteplase in submassive pulmonary embolism.
A total of 83 patients received low-molecular-weight heparin and were randomized to also receive either tenecteplase or placebo. Submassive pulmonary embolism was defined as evidence of right ventricular strain based on echocardiographic findings and elevated cardiac markers (troponin, BNP, or NT-pro-BNP).
Results. Adverse outcomes occurred in 37% of the patients in the placebo group compared with 15% of those in the tenecteplase group (P = .017). The study was terminated early because the lead author relocated to another institution.
Wang et al
In a prospective, randomized, open-label, multicenter study31 conducted in China between 2002 and 2006, 118 patients received low-molecular-weight heparin plus alteplase in a dose of either 100 mg or 50 mg over 2 hours.
Results. There were significantly fewer bleeding episodes in patients receiving half-dose alteplase in the subgroups that weighed less than 65 kg (14.8% vs 41.2%, P = .049) or who had a body mass index less than 24 kg/m2 (8.7% vs 42.9%, P = .014).
Meta-analysis
A subgroup analysis25 of patients with submassive pulmonary embolism from a 2014 meta-analysis of randomized controlled trials of thrombolytic therapy in pulmonary embolism found that thrombolysis was associated with a lower mortality rate (OR 0.48; 95% CI 0.25–0.92) but a higher rate of major bleeding (OR 3.19, 95% CI 2.07–4.92).
Is there a role for low-dose thrombolytic therapy?
The MOPETT study, discussed above, evaluated the effect of thrombolysis in a low (“safe”) dose in reducing pulmonary artery pressure in moderate pulmonary embolism.28 The primary end points were pulmonary hypertension and the composite end point of pulmonary hypertension and recurrent pulmonary embolism. In the thrombolysis group, the pulmonary arterial pressure fell immediately and was still lower at 28 months. As mentioned, although the incidence of pulmonary hypertension was lower with thrombolysis, no significant differences were noted in the rate of individual outcomes of death and recurrent pulmonary embolism when assessed independently. Furthermore, the definition of moderate pulmonary embolism used in this study is different from the submassive criteria.
Wang et al31 enrolled patients to receive low-molecular-weight heparin plus alteplase in a dose of either 50 or 100 mg. The rate of bleeding was lower with the 50-mg dose, but only in the subset of patients with lower weight and body mass index.
What is the role of catheter-guided therapy?
Catheter-directed therapy involves infusing thrombolytic agents directly into the pulmonary arteries where the clots are. The idea is to expose the patient to lower doses of systemic thrombolytics and thus decrease the risk of bleeding.
The ULTIMA study32 (Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism) evaluated whether this treatment would reverse right ventricular dilation in intermediate-risk patients, compared with anticoagulation. Intermediate-risk pulmonary embolism was defined as an embolus located in at least one main or proximal lower lobe pulmonary artery and an RV:LV ratio of at least 1.0 obtained from the echocardiographic apical four-chamber view.
The study showed hemodynamic improvement as evidenced by a lower RV:LV ratio. However, at 90 days the mortality rate was not significantly lower in the treatment group than in the control group. Of note, no major bleeding events were reported in the treatment group.
The SEATTLE II trial,33 a nonrandomized study completed in April 2013, evaluated the efficacy and safety of ultrasonographically guided, catheter-based, low-dose fibrinolysis for patients with massive and submassive pulmonary embolism. Patients had CT evidence of proximal pulmonary embolism and a dilated right ventricle (RV:LV ratio ≥ 0.9). Patients received alteplase 24 mg, either as 1 mg/hour for 24 hours with a unilateral catheter or 1 mg/hour in each of two catheters for 12 hours.
At 48 hours after the procedure, the mean RV:LV ratio had decreased from 1.55 to 1.13, the mean pulmonary arterial systolic pressure had fallen, and the anatomical clot burden had decreased. A total of 15 patients (10%) experienced major bleeding but there were no reports of any fatal or intracranial bleeding. Patients with massive pulmonary embolism were more likely to experience major bleeding episodes than those with submassive pulmonary embolism (23% vs 7%, P = .02).
The weakness of this study is that it was a single-arm study and therefore limits comparisons with other therapies such as tissue plasminogen activator for massive pulmonary embolism or anticoagulation. Also, although there was an acute improvement in hemodynamics, it is unclear if that translates to improvement in mortality rate.
Based on the available literature,29,31,33 patients presenting with submassive pulmonary embolism who are of low body weight (body mass index < 24 kg/m2 or weight < 65 kg) or are over age 75 may benefit from low-dose catheter-guided thrombolysis therapy or low-dose systemic alteplase (50 mg). Further studies should be conducted comparing these two therapeutic strategies.
SURGICAL EMBOLECTOMY: STILL THE LAST RESORT
Surgery has been the last resort for patients with pulmonary embolism. Although recent reports show a decrease in mortality from advances in surgical embolectomy, the mortality rate is greater than 10%.34
- Indications for surgical embolectomy are35:
- Failure of or contraindications to thrombolytic therapy
- Continued hemodynamic instability despite maximal medical therapy
- Associated cardiac pathology such as patent foramen ovale, atrial septal defect, and free-floating right heart thrombi
- Inadequate time for systemic thrombolytics to take effect.
No large or randomized controlled trials of surgical embolectomy for submassive pulmonary embolism have been done. In one study, of 47 patients undergoing surgical embolectomy, 15 (32%) met the criteria for submassive pulmonary embolism based on right ventricular hemodynamic dysfunction. The report did not mention if biomarkers such as troponin and BNP were considered in the decision to operate.36
At this time, surgical embolectomy remains a last resort for patients with acute massive pulmonary embolism who have contraindications to thrombolysis or for whom it has failed. Given the risk of death associated with surgical embolectomy, large randomized controlled trials need to be done to see if there is any benefit in the submassive pulmonary embolism population.
ONE TREATMENT DOES NOT FIT ALL
Given the evidence to date, we do not recommend thrombolytic therapy for all patients with submassive pulmonary embolism. The risk of complications (hemorrhage) is significant, and the benefit is unclear. A one-treatment-for-all approach cannot be applied in this situation.
Furthermore, each of the trials performed so far defined submassive pulmonary embolism slightly differently (Table 4), and many were underpowered to detect a difference in mortality rates between the treatment groups. Further studies are needed to determine the exact subset of patients with submassive pulmonary embolism that may truly benefit from thrombolytic therapy.
As such, patients with submassive pulmonary embolism should be managed by a multidisciplinary team to determine the need for thrombolytic therapy, especially in low doses, on a case-by-case basis according to the patient’s risk of further clinical deterioration.
For patients with submassive acute pulmonary embolism—the intermediate category between massive and low-risk—whether to give thrombolytic therapy is controversial. In general, patients with massive pulmonary embolism need this therapy, whereas those with low-risk pulmonary embolism do not—and neither do most of those with submassive embolism. But where should we draw the line?
More than 600,000 patients suffer pulmonary embolisms every year in the United States, and 50,000 to 200,000 people die of them.1–3 In various studies,4–6 within 1 year, 12.9% of patients had another pulmonary embolism, 7.3% developed chronic venous insufficiency, and 3.8% developed chronic thromboembolic pulmonary hypertension.
THREE CATEGORIES OF RISK
Episodes of acute pulmonary embolism are classified as low-risk (about 70% of cases), hemodynamically unstable or massive (5%), or submassive (25%).7,8
Low-risk acute pulmonary embolism is defined by the absence of right ventricular dysfunction and the absence of myocardial necrosis. The death rate in such cases is less than 1%.9 Its pharmacologic management includes parenteral anticoagulation and early initiation of long-term anticoagulation therapy, which the American College of Chest Physicians (ACCP) gives a grade IB recommendation (strong, based on moderate-quality evidence).10
Massive or hemodynamically unstable pulmonary embolism is characterized by any of the following, in the absence of other causes8:
- Sustained hypotension (systolic blood pressure < 90 mm Hg for ≥ 15 minutes)
- An absolute decrease in systolic blood pressure of 40 mm Hg or more
- Need for inotropic support
- Cardiac arrest
- Bradycardia (heart rate < 40 beats per minute).
The death rate is more than 30% in patients presenting with shock and approaches 70% in those presenting with cardiac arrest.11,12 Therefore, the consensus is that this category of pulmonary embolism merits aggressive treatment. Systemic thrombolytic therapy is recommended in those who are not at high risk of major bleeding, though the ACCP gives it only a grade 2C recommendation (weak, based on low-quality evidence).10
Submassive pulmonary embolism is defined by evidence of right ventricular dysfunction with normal blood pressure. According to the ACCP guidelines, thrombolytic therapy should be considered (grade 2C recommendation) for patients with acute pulmonary embolism without hypotension and with a low bleeding risk (with no renal failure and not on dual antiplatelet therapy), but at high risk of developing hypotension.10
DIAGNOSING SUBMASSIVE PULMONARY EMBOLISM, DELINEATING ITS SEVERITY
In managing acute pulmonary embolism, it is critical to recognize whether a patient is at high risk of clinical deterioration.
Blood pressure
The systolic blood pressure not only indicates whether the patient has hypotension (systolic blood pressure < 90 mm Hg) and therefore massive rather than submassive or low-risk pulmonary embolism; it is also important as a baseline value. A decrease in systolic blood pressure of 40 mm Hg or more is associated with worse outcomes.12
Right ventricular dysfunction
The physiologic response to occlusion of the pulmonary arteries can result in early myocardial injury and right ventricular dysfunction, which can be assessed by various methods (Table 1).
Electrocardiographic signs. Right heart strain may be recognized on electrocardiography as:
- Evidence of new complete or incomplete right bundle branch block
- T-wave inversion in the anterolateral leads V1 to V4
- S1Q3T3 (a large S wave in lead I, a Q wave in lead III, and an inverted T wave in lead III, the classic pattern of acute cor pulmonale).13
These findings add incremental prognostic value to echocardiographic findings in patients with submassive pulmonary embolism.14
Cardiac biomarkers such as B-type natriuretic peptide (BNP), N-terminal-pro-BNP (NT-pro-BNP), cardiac troponins, and heart-type fatty acid-binding protein (H-FABP) are also markers of right-sided myocardial damage and strain and can be used to identify patients with submassive pulmonary embolism.15 Abnormal levels of these substances are as follows:
- Troponin T greater than 0.1 ng/mL
- Troponin I greater than 0.4 ng/mL
- BNP greater than 90 pg/mL
- NT-pro-BNP greater than 500 pg/mL
- H-FABP less than 6 ng/mL.
These levels have prognostic value, identifying patients with submassive pulmonary embolism at risk of deterioration or death,14,16,17
Echocardiographic signs. Right ventricular dysfunction can be assessed quickly at the bedside with portable transthoracic echocardiography. A meta-analysis showed that close to 37% of hemodynamically stable patients with pulmonary embolism had echocardiographic evidence of right ventricular dysfunction on presentation and a higher short-term mortality rate.18 Evidence of right ventricular dysfunction includes the following:
- New-onset hypokinesis or akinesis
- Right ventricular dilation
- Right ventricular free-wall hypokinesis with apical sparing (the McConnell sign)
- Paradoxical movement of the interventricular septum
- Newly increased right ventricular systolic pressure
- Pulmonary hypertension, defined as tricuspid regurgitation jet velocity greater than 2.8 m/s.15,19
Computed tomographic (CT) angiography is widely available. Findings that have prognostic value in determining those at higher risk of death include the following20,21:
- A dilated right ventricle—ratio of right ventricle to left ventricle diameter (RV:LV ratio) greater than 0.9
- Interventricular septal bowing.
PESI and sPESI scores. The European Society of Cardiology 2014 guidelines stratify the risk in normotensive patients with pulmonary embolism according to their score on the Pulmonary Embolism Severity Index (PESI) or the simplified PESI (sPESI). There are five PESI classes. Those in PESI class III or IV or with an sPESI score of 1 or more (on a scale of 0 to 6) are considered at intermediate risk of clinical deterioration and are then further risk-stratified according to whether they have right ventricular dysfunction (based on echocardiography or computed tomography) and elevated cardiac biomarkers. These scoring systems are based on easily obtainable clinical information such as age, male sex, history of cancer, history of heart failure, history of chronic lung disease, heart rate, systolic blood pressure, respiratory rate, temperature, and altered mental status, and calculators are readily available.
Anticoagulation for all, plus thrombolysis for some
Patients with neither right ventricular dysfunction nor elevated cardiac biomarkers are at intermediate to low risk of clinical deterioration, and it is recommended that they be given anticoagulation therapy in an inpatient setting.
On the other hand, patients with both right ventricular dysfunction and elevated cardiac biomarkers are considered at intermediate to high risk of clinical deterioration; they should also be managed with anticoagulation and monitored closely for the need for rescue reperfusion therapy with thrombolytics.22
THROMBOLYTIC AGENTS
Thrombolytic agents are the cornerstone of management for patients presenting with pulmonary embolism who are at high risk. As noted above, these agents are recommended in massive pulmonary embolism, but their role in submassive pulmonary embolism remains controversial.
Thrombolytics work by activating endogenous plasminogen. The resulting plasmin promotes clot lysis, reducing the size of the thrombus, decreasing pulmonary vasculature resistance, and improving right ventricular function.23
To date, three thrombolytic agents have received US Food and Drug Administration approval for use in massive pulmonary embolism: alteplase, urokinase, and streptokinase. But only alteplase is still available in the United States. Alteplase is also the best tolerated, whereas streptokinase is highly antigenic and may cause further deterioration in an already unstable patient. Alteplase is also the most fibrin-specific and is considered the most potent of the three agents.24
Additional thrombolytic agents under investigation for use in acute pulmonary embolism include reteplase, tenecteplase, and desmoteplase. These agents are more fibrin-specific than alteplase. Reteplase is a second-generation recombinant tissue-type plasminogen activator with a quicker onset of action and longer half-life than alteplase, allowing for bolus dosing. Tenecteplase, a variant of alteplase, is cleared more slowly and is 14 times more fibrin-specific than alteplase, also allowing for bolus dosing. Desmoteplase, a fibrin-specific agent currently in phase 2 trials, also has a longer half-life and appears to be more potent than alteplase. Table 2 lists the dosing and the degree of fibrin specificity of these agents.
Complications of thrombolytic therapy
Submassive pulmonary embolism has a low death rate, and the benefit of systemic thrombolytic therapy for this condition is controversial. Therefore, risk stratification is very important before pursuing this therapy.
A meta-analysis25 of 16 randomized controlled trials included 2,125 patients with pulmonary embolism:
- 210 (9.88%) in the low-risk category
- 1,499 (70.54%) in the submassive category
- 31 (1.46%) in the massive category
- 385 (18.11%) whose disease severity could not be determined.
Major bleeding occurred in:
- 98 (9.24%) of 1,061 patients receiving anticoagulation plus thrombolytics
- 36 (3.42%) of 1,054 patients receiving anticoagulation without thrombolytics (odds ratio [OR] 2.73, 95% confidence interval [CI] 1.91–3.91; number needed to harm [NNH] 18, 95% CI 13–27).
Intracranial hemorrhage occurred in:
- 15 (1.46%) of 2,014 patients on thrombolytic therapy
- 2 (0.19%) of 1,019 patients not on thrombolytic therapy (OR 4.63, 95% CI 1.78–12.04; NNH 78, 95% CI 48–206).
Of note, the incidence of major bleeding was not significantly increased in those age 65 or younger receiving thrombolytics (OR 1.25, 95% CI 0.5–3.14).
Comments. Definitions of major bleeding varied in the individual trials. Additionally, intracranial hemorrhage was included as a major bleeding end point in any trial in which it was not prespecified.
These findings emphasize the importance of risk stratification before pursuing thrombolytic therapy in submassive pulmonary embolism.
Table 3 lists absolute and relative contraindications to thrombolytic therapy.
MAJOR STUDIES IN SUBMASSIVE PULMONARY EMBOLISM
The MAPPET-3 trial
The Management Strategies and Prognosis of Pulmonary Embolism-3 (MAPPET-3) trial,26 in 2002, was the first major trial to study thrombolytic therapy in submassive pulmonary embolism.
In this prospective, randomized, double-blinded trial conducted in Germany, 118 patients received heparin with alteplase (100 mg over 2 hours) and 138 received heparin with placebo. The primary end point was in-hospital death or clinical deterioration requiring escalation of treatment. Secondary outcomes included recurrent pulmonary embolism, major bleeding, and stroke. Major bleeding was defined as fatal bleeding, hemorrhagic stroke, or drop in the hemoglobin concentration by more than 4 g/dL, with or without the need for red blood cell transfusion.
Right ventricular dysfunction was diagnosed by echocardiography in 30% of the participants, and the rest of the patients were classified as having submassive pulmonary embolism on the basis of electrocardiographic criteria alone. It is likely that the latter group had a less severe form of the disease and did not benefit from thrombolytic therapy as much as patients with echocardiographic findings of right ventricular dysfunction and elevated serum cardiac biomarkers.
Results. At 30 days, 11% of the alteplase-plus-heparin group had met the primary end point, compared with 24.6% of the placebo-plus-heparin group (P = .006). The difference was mostly driven by the need for secondary thrombolysis (7.6% vs 23.2%, P = .001), since 32 (23.2%) of the 138 patients in the control group required secondary thrombolysis, accounting for 94% of the 34 patients in this group who required escalation of treatment. Most cases of clinical deterioration in this group occurred within the first 5 days.
Mortality rates were 3.4% in the heparin-plus-alteplase group and 2.2% in the heparin-plus-placebo group, but the difference was not statistically significant (P = .71).
Major bleeding occurred in 1 patient in the heparin-plus-alteplase group and 5 patients in the heparin-plus-placebo group, but the trial’s definition of major bleeding may have resulted in underestimation of this event. The definition put forth by the International Society on Thrombosis and Haemostasis is less strict, defining bleeding in nonsurgical patients as major if it is fatal, symptomatic in a critical area or organ, or causing a fall in hemoglobin level of 2.0 g/dL or more, leading to transfusion of two or more units of whole blood or red cells.27
MOPETT trial
The Moderate Pulmonary Embolism Treated with Thrombolysis (MOPETT) trial28 was a single-center, randomized trial in 121 normotensive patients with “moderate” pulmonary embolism and right ventricular dysfunction. Moderate pulmonary embolism was defined as signs and symptoms of pulmonary embolism with evidence on computed tomographic angiography of greater than 70% involvement with thrombus in two or more lobes or left or right main pulmonary arteries, or by a high-probability ventilation-perfusion scan showing a mismatch in two or more lobes.
The authors defined right ventricular dysfunction by elevated cardiac markers or by findings on echocardiography. Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.
The primary outcome was the development of pulmonary hypertension, based on echocardiography.
Patients were randomized to either anticoagulation alone (unfractionated heparin or low-molecular-weight heparin) or anticoagulation plus half-dose alteplase (0.5 mg/kg, to a maximum of 50 mg). Echocardiography was performed within 2 hours of study entry, at 48 hours, and every 6 months thereafter. The mean duration of follow-up was 28 months.
Results. Pulmonary hypertension developed in 16% of the anticoagulation-plus-alteplase group vs 57% of the anticoagulation-only group (P < .001). However, the clinical relevance of elevated right-sided pressures observed by echocardiography in asymptomatic patients is uncertain. Alteplase had no impact on the rates of death or recurrent pulmonary embolism.
PEITHO trial
The 2014 Pulmonary Embolism Thrombolysis (PEITHO) trial29 was a prospective, randomized, double-blinded, placebo-controlled trial conducted in 13 countries between 2007 and 2012. A total of 1,005 patients with submassive pulmonary embolism received unfractionated heparin and were randomized to also receive either tenecteplase or placebo.
The primary end point was death from any cause or hemodynamic compromise within 7 days of randomization. Secondary end points included death within 30 days, recurrence of pulmonary embolism, major bleeding, and stroke.
Echocardiography was strongly recommended for diagnosing right ventricular dysfunction in all patients. When this was unavailable, computed tomographic images were used to assess right ventricular dysfunction. Major bleeding was characterized as moderate or severe, and bleeding events were reported using the International Society on Thrombosis and Haemostasis criteria.
Results. The tenecteplase group had a lower rate of the primary end point at 7 days (2.6% vs 5.6%, P = .02), but no significant reduction in all-cause mortality at 30 days (2.4% vs 3.2%, P = .42). In addition, the tenecteplase group had higher rates of major extracranial bleeding (6.3% vs 1.2%, P < .001) and stroke (2.4% vs 0.2%, P = .004) at 7 days.
Although the PEITHO trial showed no reduction in mortality rates and showed a higher rate of major bleeding, this may have been related to using a higher dose of tenecteplase than needed in this population. Further studies should be conducted to confirm this theory.
TOPCOAT trial
The Tenecteplase or Placebo, Cardiopulmonary Outcomes at Three months (TOPCOAT) trial,30 published in 2014, was a multicenter, double-blind, intention-to-treat, randomized trial carried out in eight centers in the United States. The authors evaluated a composite outcome (death, circulatory shock, intubation, major bleeding, recurrent pulmonary embolism, and functional capacity) with the use of tenecteplase in submassive pulmonary embolism.
A total of 83 patients received low-molecular-weight heparin and were randomized to also receive either tenecteplase or placebo. Submassive pulmonary embolism was defined as evidence of right ventricular strain based on echocardiographic findings and elevated cardiac markers (troponin, BNP, or NT-pro-BNP).
Results. Adverse outcomes occurred in 37% of the patients in the placebo group compared with 15% of those in the tenecteplase group (P = .017). The study was terminated early because the lead author relocated to another institution.
Wang et al
In a prospective, randomized, open-label, multicenter study31 conducted in China between 2002 and 2006, 118 patients received low-molecular-weight heparin plus alteplase in a dose of either 100 mg or 50 mg over 2 hours.
Results. There were significantly fewer bleeding episodes in patients receiving half-dose alteplase in the subgroups that weighed less than 65 kg (14.8% vs 41.2%, P = .049) or who had a body mass index less than 24 kg/m2 (8.7% vs 42.9%, P = .014).
Meta-analysis
A subgroup analysis25 of patients with submassive pulmonary embolism from a 2014 meta-analysis of randomized controlled trials of thrombolytic therapy in pulmonary embolism found that thrombolysis was associated with a lower mortality rate (OR 0.48; 95% CI 0.25–0.92) but a higher rate of major bleeding (OR 3.19, 95% CI 2.07–4.92).
Is there a role for low-dose thrombolytic therapy?
The MOPETT study, discussed above, evaluated the effect of thrombolysis in a low (“safe”) dose in reducing pulmonary artery pressure in moderate pulmonary embolism.28 The primary end points were pulmonary hypertension and the composite end point of pulmonary hypertension and recurrent pulmonary embolism. In the thrombolysis group, the pulmonary arterial pressure fell immediately and was still lower at 28 months. As mentioned, although the incidence of pulmonary hypertension was lower with thrombolysis, no significant differences were noted in the rate of individual outcomes of death and recurrent pulmonary embolism when assessed independently. Furthermore, the definition of moderate pulmonary embolism used in this study is different from the submassive criteria.
Wang et al31 enrolled patients to receive low-molecular-weight heparin plus alteplase in a dose of either 50 or 100 mg. The rate of bleeding was lower with the 50-mg dose, but only in the subset of patients with lower weight and body mass index.
What is the role of catheter-guided therapy?
Catheter-directed therapy involves infusing thrombolytic agents directly into the pulmonary arteries where the clots are. The idea is to expose the patient to lower doses of systemic thrombolytics and thus decrease the risk of bleeding.
The ULTIMA study32 (Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism) evaluated whether this treatment would reverse right ventricular dilation in intermediate-risk patients, compared with anticoagulation. Intermediate-risk pulmonary embolism was defined as an embolus located in at least one main or proximal lower lobe pulmonary artery and an RV:LV ratio of at least 1.0 obtained from the echocardiographic apical four-chamber view.
The study showed hemodynamic improvement as evidenced by a lower RV:LV ratio. However, at 90 days the mortality rate was not significantly lower in the treatment group than in the control group. Of note, no major bleeding events were reported in the treatment group.
The SEATTLE II trial,33 a nonrandomized study completed in April 2013, evaluated the efficacy and safety of ultrasonographically guided, catheter-based, low-dose fibrinolysis for patients with massive and submassive pulmonary embolism. Patients had CT evidence of proximal pulmonary embolism and a dilated right ventricle (RV:LV ratio ≥ 0.9). Patients received alteplase 24 mg, either as 1 mg/hour for 24 hours with a unilateral catheter or 1 mg/hour in each of two catheters for 12 hours.
At 48 hours after the procedure, the mean RV:LV ratio had decreased from 1.55 to 1.13, the mean pulmonary arterial systolic pressure had fallen, and the anatomical clot burden had decreased. A total of 15 patients (10%) experienced major bleeding but there were no reports of any fatal or intracranial bleeding. Patients with massive pulmonary embolism were more likely to experience major bleeding episodes than those with submassive pulmonary embolism (23% vs 7%, P = .02).
The weakness of this study is that it was a single-arm study and therefore limits comparisons with other therapies such as tissue plasminogen activator for massive pulmonary embolism or anticoagulation. Also, although there was an acute improvement in hemodynamics, it is unclear if that translates to improvement in mortality rate.
Based on the available literature,29,31,33 patients presenting with submassive pulmonary embolism who are of low body weight (body mass index < 24 kg/m2 or weight < 65 kg) or are over age 75 may benefit from low-dose catheter-guided thrombolysis therapy or low-dose systemic alteplase (50 mg). Further studies should be conducted comparing these two therapeutic strategies.
SURGICAL EMBOLECTOMY: STILL THE LAST RESORT
Surgery has been the last resort for patients with pulmonary embolism. Although recent reports show a decrease in mortality from advances in surgical embolectomy, the mortality rate is greater than 10%.34
- Indications for surgical embolectomy are35:
- Failure of or contraindications to thrombolytic therapy
- Continued hemodynamic instability despite maximal medical therapy
- Associated cardiac pathology such as patent foramen ovale, atrial septal defect, and free-floating right heart thrombi
- Inadequate time for systemic thrombolytics to take effect.
No large or randomized controlled trials of surgical embolectomy for submassive pulmonary embolism have been done. In one study, of 47 patients undergoing surgical embolectomy, 15 (32%) met the criteria for submassive pulmonary embolism based on right ventricular hemodynamic dysfunction. The report did not mention if biomarkers such as troponin and BNP were considered in the decision to operate.36
At this time, surgical embolectomy remains a last resort for patients with acute massive pulmonary embolism who have contraindications to thrombolysis or for whom it has failed. Given the risk of death associated with surgical embolectomy, large randomized controlled trials need to be done to see if there is any benefit in the submassive pulmonary embolism population.
ONE TREATMENT DOES NOT FIT ALL
Given the evidence to date, we do not recommend thrombolytic therapy for all patients with submassive pulmonary embolism. The risk of complications (hemorrhage) is significant, and the benefit is unclear. A one-treatment-for-all approach cannot be applied in this situation.
Furthermore, each of the trials performed so far defined submassive pulmonary embolism slightly differently (Table 4), and many were underpowered to detect a difference in mortality rates between the treatment groups. Further studies are needed to determine the exact subset of patients with submassive pulmonary embolism that may truly benefit from thrombolytic therapy.
As such, patients with submassive pulmonary embolism should be managed by a multidisciplinary team to determine the need for thrombolytic therapy, especially in low doses, on a case-by-case basis according to the patient’s risk of further clinical deterioration.
- Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998; 158:585–593.
- Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med 2012; 125:478–484.
- Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest 2002; 121:877–905.
- Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ 3rd. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med 2000; 160:761–768.
- Mohr DN, Silverstein MD, Heit JA, Petterson TM, O’Fallon WM, Melton LJ. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: a population-based study. Mayo Clin Proc 2000; 75:1249–1256.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest 2004; 125:1539–1545.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Comess KA, DeRook FA, Russell ML, Tognazzi-Evans TA, Beach KW. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med 2000; 109:351–356.
- Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 1997; 30:1165–1171.
- Piazza G. Submassive pulmonary embolism. JAMA 2013; 309:171–180.
- Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008; 178:425–430.
- Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med 2009; 122:257–264.
- Amorim S, Dias P, Rodrigues RA, et al. Troponin I as a marker of right ventricular dysfunction and severity of pulmonary embolism. Rev Port Cardiol 2006; 25:181–186.
- Dellas C, Puls M, Lankeit M, et al. Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol 2010; 55:2150–2157.
- Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord 2014; 14:64.
- Nazeyrollas P, Metz D, Jolly D, et al. Use of transthoracic Doppler echocardiography combined with clinical and electrocardiographic data to predict acute pulmonary embolism. Eur Heart J 1996;17: 779–786.
- Wake N, Kumamaru KK, George E, et al. Computed tomography and echocardiography in patients with acute pulmonary embolism: part 1: correlation of findings of right ventricular enlargement. J Thorac Imaging 2014; 29:W1–W6.
- Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J 2014; 43:1678–1690.
- Konstantinides SV, Torbicki A, Agnelli G, et al; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069, 69a–69k.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Daley MJ, Lat I. Clinical controversies in thrombolytic therapy for the management of acute pulmonary embolism. Pharmacotherapy 2012; 32:158–172.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W; Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002; 347:1143–1150.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Wang C, Zhai Z, Yang Y, et al; China Venous Thromboembolism (VTE) Study Group. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest 2010; 137:254–262.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
- Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99:421–423.
- He C, Von Segesser LK, Kappetein PA, Mestres CA, Smith JA, Choong CK. Acute pulmonary embolectomy. Eur J Cardiothorac Surg 2013; 43:1087–1095.
- Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005; 129:1018–1023.
- Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998; 158:585–593.
- Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med 2012; 125:478–484.
- Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest 2002; 121:877–905.
- Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ 3rd. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med 2000; 160:761–768.
- Mohr DN, Silverstein MD, Heit JA, Petterson TM, O’Fallon WM, Melton LJ. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: a population-based study. Mayo Clin Proc 2000; 75:1249–1256.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest 2004; 125:1539–1545.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Comess KA, DeRook FA, Russell ML, Tognazzi-Evans TA, Beach KW. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med 2000; 109:351–356.
- Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 1997; 30:1165–1171.
- Piazza G. Submassive pulmonary embolism. JAMA 2013; 309:171–180.
- Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008; 178:425–430.
- Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med 2009; 122:257–264.
- Amorim S, Dias P, Rodrigues RA, et al. Troponin I as a marker of right ventricular dysfunction and severity of pulmonary embolism. Rev Port Cardiol 2006; 25:181–186.
- Dellas C, Puls M, Lankeit M, et al. Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol 2010; 55:2150–2157.
- Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord 2014; 14:64.
- Nazeyrollas P, Metz D, Jolly D, et al. Use of transthoracic Doppler echocardiography combined with clinical and electrocardiographic data to predict acute pulmonary embolism. Eur Heart J 1996;17: 779–786.
- Wake N, Kumamaru KK, George E, et al. Computed tomography and echocardiography in patients with acute pulmonary embolism: part 1: correlation of findings of right ventricular enlargement. J Thorac Imaging 2014; 29:W1–W6.
- Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J 2014; 43:1678–1690.
- Konstantinides SV, Torbicki A, Agnelli G, et al; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069, 69a–69k.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Daley MJ, Lat I. Clinical controversies in thrombolytic therapy for the management of acute pulmonary embolism. Pharmacotherapy 2012; 32:158–172.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W; Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002; 347:1143–1150.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Wang C, Zhai Z, Yang Y, et al; China Venous Thromboembolism (VTE) Study Group. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest 2010; 137:254–262.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
- Stein PD, Alnas M, Beemath A, Patel NR. Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99:421–423.
- He C, Von Segesser LK, Kappetein PA, Mestres CA, Smith JA, Choong CK. Acute pulmonary embolectomy. Eur J Cardiothorac Surg 2013; 43:1087–1095.
- Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005; 129:1018–1023.
KEY POINTS
- Most patients with submassive pulmonary embolism do not need thrombolytic therapy.
- Identifying patients with submassive pulmonary embolism at highest risk of clinical deterioration can guide physicians to consider thrombolytic therapy.
- In clinical trials, thrombolytic therapy reduced the rates of secondary outcomes but did not reduce the rate of death in this patient population.