User login
Virtual Care Expansion in VA Health System Tied to 12% Drop in ED Visits for Low-Acuity Conditions
TOPLINE:
Emergency department (ED) visits by veterans for low-acuity conditions declined following the US Department of Veterans Affairs (VA) virtual care expansion in March 2020 and remained 12% below the baseline rate through February 2023.
METHODOLOGY:
- Researchers conducted a retrospective cross-sectional analysis using data from the VA Corporate Data Warehouse, including 10,364,893 ED visits (54.3% low-acuity visits) by about 2.6 million veterans (mean age, 60.8 years; 89.6% men; 63.7% White individuals) between March 2017 and February 2023.
- They evaluated the impact of the virtual care expansion — defined as the transition to virtual visits, including telephone and video care — which was implemented from March to May 2020, and assessed outcomes through that period.
- The primary outcome was the change in monthly counts of low-acuity visits to VA EDs, assessed using an interrupted time series analysis. The analysis focused on two intervention points: March 2020 (the start of the pandemic and virtual care scale-up) and May 2020 (when virtual care plateaued).
- A secondary analysis assessed the characteristics of ED users with low-acuity visits before and after the virtual care expansion, using 2 years of data — baseline pre-expansion year 3 (March 2019 to February 2020) and post-expansion year 3 (March 2022 to February 2023).
TAKEAWAY:
- Low-acuity ED utilization dropped by 24,514 visits (P < .001) in March 2020, followed by a modest increase of 7863 visits per month (P = .047) after May 2020, but remained 12.4% below the baseline rate by the end of February 2023.
- High-acuity visits showed similar patterns, with an initial decrease of 22,197 visits in March 2020 (P < .001) and a subsequent increase of 4180 visits per month in the post-expansion period (P = .05).
- Increased virtual care utilization was not significantly associated with reduced ED use for selected low-acuity conditions. The largest relative reductions were observed for major depression (42.4%), gastroenteritis (38.3%), and conjunctivitis (35.6%), whereas the largest absolute reductions occurred in low back pain, knee pain, and cellulitis.
- ED users with low-acuity ED visits in the post-expansion period were more likely to have 100% VA service connection (20.2% vs 14.6%), less medically complex (mean Elixhauser comorbidity score, 3.8 vs 4.2), and more likely to be classified as highly disabled (55.0% vs 48.1%) compared with those in the pre-expansion period.
IN PRACTICE:
“In this national, cross-sectional study, low-acuity ED utilization declined after the VA’s expansion of virtual care. While shifting low-acuity care away from ED settings toward virtual options may improve the value and efficiency of services, questions remain about the effects on quality and patient satisfaction,” the authors wrote. “Further research should be directed at exploring patient- and system-level factors that influence care-seeking decisions for low-acuity conditions,” they added.
SOURCE:
The study was led by Anu Ramachandran, MD, MPH, VA Palo Alto Health Care System, Menlo Park, California. It was published online on JAMA Network Open.
LIMITATIONS:
The classification of visits as low acuity in this study was based on International Classification of Diseases, Tenth Revision codes and discharge disposition; this classification did not imply inappropriate ED use as factors such as symptom severity, medical comorbidities, and access to care — which can influence care-seeking decisions — were not captured. The study did not assess all potential alternatives, including VA Urgent Care centers. Additionally, although virtual care use increased as ED visits declined, the models did not provide evidence of direct substitution.
DISCLOSURES:
The study received support from grants from the Department of VA, Veterans Health Administration, Office of Health Systems Research and Development. One author reported receiving research support through Department of VA Office of Health Systems Research and Development interagency agreement, whereas another reported receiving grant support from the VA Health Services Research program and being employed by the Veterans Affairs during the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Emergency department (ED) visits by veterans for low-acuity conditions declined following the US Department of Veterans Affairs (VA) virtual care expansion in March 2020 and remained 12% below the baseline rate through February 2023.
METHODOLOGY:
- Researchers conducted a retrospective cross-sectional analysis using data from the VA Corporate Data Warehouse, including 10,364,893 ED visits (54.3% low-acuity visits) by about 2.6 million veterans (mean age, 60.8 years; 89.6% men; 63.7% White individuals) between March 2017 and February 2023.
- They evaluated the impact of the virtual care expansion — defined as the transition to virtual visits, including telephone and video care — which was implemented from March to May 2020, and assessed outcomes through that period.
- The primary outcome was the change in monthly counts of low-acuity visits to VA EDs, assessed using an interrupted time series analysis. The analysis focused on two intervention points: March 2020 (the start of the pandemic and virtual care scale-up) and May 2020 (when virtual care plateaued).
- A secondary analysis assessed the characteristics of ED users with low-acuity visits before and after the virtual care expansion, using 2 years of data — baseline pre-expansion year 3 (March 2019 to February 2020) and post-expansion year 3 (March 2022 to February 2023).
TAKEAWAY:
- Low-acuity ED utilization dropped by 24,514 visits (P < .001) in March 2020, followed by a modest increase of 7863 visits per month (P = .047) after May 2020, but remained 12.4% below the baseline rate by the end of February 2023.
- High-acuity visits showed similar patterns, with an initial decrease of 22,197 visits in March 2020 (P < .001) and a subsequent increase of 4180 visits per month in the post-expansion period (P = .05).
- Increased virtual care utilization was not significantly associated with reduced ED use for selected low-acuity conditions. The largest relative reductions were observed for major depression (42.4%), gastroenteritis (38.3%), and conjunctivitis (35.6%), whereas the largest absolute reductions occurred in low back pain, knee pain, and cellulitis.
- ED users with low-acuity ED visits in the post-expansion period were more likely to have 100% VA service connection (20.2% vs 14.6%), less medically complex (mean Elixhauser comorbidity score, 3.8 vs 4.2), and more likely to be classified as highly disabled (55.0% vs 48.1%) compared with those in the pre-expansion period.
IN PRACTICE:
“In this national, cross-sectional study, low-acuity ED utilization declined after the VA’s expansion of virtual care. While shifting low-acuity care away from ED settings toward virtual options may improve the value and efficiency of services, questions remain about the effects on quality and patient satisfaction,” the authors wrote. “Further research should be directed at exploring patient- and system-level factors that influence care-seeking decisions for low-acuity conditions,” they added.
SOURCE:
The study was led by Anu Ramachandran, MD, MPH, VA Palo Alto Health Care System, Menlo Park, California. It was published online on JAMA Network Open.
LIMITATIONS:
The classification of visits as low acuity in this study was based on International Classification of Diseases, Tenth Revision codes and discharge disposition; this classification did not imply inappropriate ED use as factors such as symptom severity, medical comorbidities, and access to care — which can influence care-seeking decisions — were not captured. The study did not assess all potential alternatives, including VA Urgent Care centers. Additionally, although virtual care use increased as ED visits declined, the models did not provide evidence of direct substitution.
DISCLOSURES:
The study received support from grants from the Department of VA, Veterans Health Administration, Office of Health Systems Research and Development. One author reported receiving research support through Department of VA Office of Health Systems Research and Development interagency agreement, whereas another reported receiving grant support from the VA Health Services Research program and being employed by the Veterans Affairs during the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Emergency department (ED) visits by veterans for low-acuity conditions declined following the US Department of Veterans Affairs (VA) virtual care expansion in March 2020 and remained 12% below the baseline rate through February 2023.
METHODOLOGY:
- Researchers conducted a retrospective cross-sectional analysis using data from the VA Corporate Data Warehouse, including 10,364,893 ED visits (54.3% low-acuity visits) by about 2.6 million veterans (mean age, 60.8 years; 89.6% men; 63.7% White individuals) between March 2017 and February 2023.
- They evaluated the impact of the virtual care expansion — defined as the transition to virtual visits, including telephone and video care — which was implemented from March to May 2020, and assessed outcomes through that period.
- The primary outcome was the change in monthly counts of low-acuity visits to VA EDs, assessed using an interrupted time series analysis. The analysis focused on two intervention points: March 2020 (the start of the pandemic and virtual care scale-up) and May 2020 (when virtual care plateaued).
- A secondary analysis assessed the characteristics of ED users with low-acuity visits before and after the virtual care expansion, using 2 years of data — baseline pre-expansion year 3 (March 2019 to February 2020) and post-expansion year 3 (March 2022 to February 2023).
TAKEAWAY:
- Low-acuity ED utilization dropped by 24,514 visits (P < .001) in March 2020, followed by a modest increase of 7863 visits per month (P = .047) after May 2020, but remained 12.4% below the baseline rate by the end of February 2023.
- High-acuity visits showed similar patterns, with an initial decrease of 22,197 visits in March 2020 (P < .001) and a subsequent increase of 4180 visits per month in the post-expansion period (P = .05).
- Increased virtual care utilization was not significantly associated with reduced ED use for selected low-acuity conditions. The largest relative reductions were observed for major depression (42.4%), gastroenteritis (38.3%), and conjunctivitis (35.6%), whereas the largest absolute reductions occurred in low back pain, knee pain, and cellulitis.
- ED users with low-acuity ED visits in the post-expansion period were more likely to have 100% VA service connection (20.2% vs 14.6%), less medically complex (mean Elixhauser comorbidity score, 3.8 vs 4.2), and more likely to be classified as highly disabled (55.0% vs 48.1%) compared with those in the pre-expansion period.
IN PRACTICE:
“In this national, cross-sectional study, low-acuity ED utilization declined after the VA’s expansion of virtual care. While shifting low-acuity care away from ED settings toward virtual options may improve the value and efficiency of services, questions remain about the effects on quality and patient satisfaction,” the authors wrote. “Further research should be directed at exploring patient- and system-level factors that influence care-seeking decisions for low-acuity conditions,” they added.
SOURCE:
The study was led by Anu Ramachandran, MD, MPH, VA Palo Alto Health Care System, Menlo Park, California. It was published online on JAMA Network Open.
LIMITATIONS:
The classification of visits as low acuity in this study was based on International Classification of Diseases, Tenth Revision codes and discharge disposition; this classification did not imply inappropriate ED use as factors such as symptom severity, medical comorbidities, and access to care — which can influence care-seeking decisions — were not captured. The study did not assess all potential alternatives, including VA Urgent Care centers. Additionally, although virtual care use increased as ED visits declined, the models did not provide evidence of direct substitution.
DISCLOSURES:
The study received support from grants from the Department of VA, Veterans Health Administration, Office of Health Systems Research and Development. One author reported receiving research support through Department of VA Office of Health Systems Research and Development interagency agreement, whereas another reported receiving grant support from the VA Health Services Research program and being employed by the Veterans Affairs during the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
Veterans experiencing homelessness are at an elevated risk for adverse health outcomes, including suicide. This population also experiences chronic health conditions (eg, cardiovascular disease and sexually transmitted infections) and psychiatric conditions (eg, substance use disorders and posttraumatic stress disorder) with a greater propensity than veterans without history of homelessness.1,2 Similarly, veterans experiencing homelessness often report concurrent stressors, such as justice involvement and unemployment, which further impact social functioning.3
The US Department of Veterans Affairs (VA) offers a range of health and social services to veterans experiencing homelessness. These programs are designed to respond to the multifactorial challenges faced by this population and are aimed at achieving sustained, permanent housing.4 To facilitate this effort, these programs provide targeted and tailored health (eg, primary care) and social (eg, case management and vocational rehabilitation) services to address barriers to housing stability (eg, substance use, serious mental illness, interacting with the criminal legal system, and unemployment).
Despite the availability of these programs, engaging veterans in VA services—whether in general or tailored for those experiencing or at risk for homelessness—remains challenging. Many veterans at risk for or experiencing homelessness overuse service settings that provide immediate care, such as urgent care or emergency departments (EDs).5,6 These individuals often visit an ED to augment or complement medical care they received in an outpatient setting, which can result in an elevated health care burden as well as impacted provision of treatment, especially surrounding care for chronic conditions (eg, cardiovascular health or serious mental illness).7-9
VA EDs offer urgent care and emergency services and often serve as a point of entry for veterans experiencing homelessness.10 They offer veterans expedient access to care that can address immediate needs (eg, substance use withdrawal, pain management, and suicide risk). EDs may be easier to access given they have longer hours of operation and patients can present without a scheduled appointment. VA EDs are an important point to identify homelessness and connect individuals to social service resources and outpatient health care referrals (eg, primary care and mental health).4,11
Some clinicians experience uncertainty in navigating or providing care for veterans experiencing or at risk for homelessness. A qualitative study conducted outside the VA found many clinicians did not know how to approach clinical conversations among unstably housed individuals, particularly when they discussed how to manage care for complex health conditions in the context of ongoing case management challenges, such as discharge planning.12 Another study found that clinicians working with individuals experiencing homelessness may have limited prior training or experience treating these patients.13 As a result, these clinicians may be unaware of available social services or unknowingly have biases that negatively impact care. Research remains limited surrounding beliefs about and methods of enhancing care among VA clinicians working with veterans experiencing homelessness in the ED.
This multiphase pilot study sought to understand service delivery processes and gaps in VA ED settings. Phase 1 examined ED clinician perceptions of care, facilitators, and barriers to providing care (including suicide risk assessments) and making postdischarge outpatient referrals among VA ED clinicians who regularly work with veterans experiencing homelessness. Phase 2 used this information to develop a clinical psychoeducational resource to enhance post-ED access to care for veterans experiencing or at risk for homelessness.
QUALITATIVE INTERVIEWS
Semistructured qualitative interviews were conducted with 11 VA ED clinicians from 6 Veteran Integrated Service Networks between August 2022 and February 2023. Clinicians were eligible if they currently worked within a VA ED setting (including urgent care) and indicated that some of their patients were veterans experiencing homelessness. All health care practitioners (HCPs) participated in an interview and a postinterview self-report survey that assessed demographic and job-related characteristics. Eight HCPs identified as female and 3 identified as male. All clinicians identified as White and 3 as Hispanic or Latino. Eight clinicians were licensed clinical social workers, 2 were ED nurses, and 1 was an ED physician.
After each clinician provided informed consent, they were invited to complete a telephone or Microsoft Teams interview. All interviews were recorded and subsequently transcribed. Interviews explored clinicians’ experiences caring for veterans experiencing homelessness, with a focus on services provided within the ED, as well as mandated ED screenings such as a suicide risk assessment. Interview questions also addressed postdischarge knowledge and experiences with referrals to VA health services (eg, primary care, mental health) and social services (eg, housing programs). Interviews lasted 30 to 90 minutes.
Recruitment ended after attaining sufficient thematic data, accomplished via an information power approach to sampling. This occurred when the study aims, sample characteristics, existing theory, and depth and quality of interviews dynamically informed the decision to cease recruitment of additional participants.14,15 Given the scope of study (examining service delivery and knowledge gaps), the specificity of the targeted sample (VA ED clinicians providing care to veterans experiencing homelessness), the level of pre-existing theoretical background informing the study aims, and depth and quality of interview dialogue, this information power approach provides justification for attaining small sample sizes. Following the interview, HCPs completed a demographic questionnaire. Participants were not compensated.
Data Analysis
Directed content analysis was used to analyze qualitative data, with the framework method employed as an analytic instrument to facilitate analysis.16-18 Analysts engaged in bracketing and discussed reflexivity before data analysis to reflect on personal subjectivities and reduce potential bias.19,20
A prototype coding framework was developed that enabled coders to meaningfully summarize and condense data within transcripts into varying domains, categories, or topics found within the interview guide. Domain examples included clinical backgrounds, suicide risk and assessment protocols among veterans experiencing homelessness, beliefs about service delivery for veterans experiencing homelessness, and barriers and facilitators that may impact their ability to provide post-ED discharge care. Coders discussed the findings and if there was a need to modify templates. All transcripts were double coded. Once complete, individual templates were merged into a unified Microsoft Excel sheet, which allowed for more discrete analyses, enabling analysts to examine trends across content areas within the dataset.
Clinical Resource Development
HCPs were queried regarding available outpatient resources for post-ED care (eg, printed discharge paperwork and best practice alerts or automated workflows within the electronic health record). Resources used by participants were examined, as well as which resources clinicians thought would help them care for veterans experiencing homelessness. Noted gaps were used to develop a tailored resource for clinicians who treat veterans experiencing homelessness in the ED. This resource was created with the intention it could inform all ED clinicians, with the option for personalization to align with the needs of local services, based on needed content areas identified (eg, emergency shelters and suicide prevention resources).
Resource development followed an information systems research (ISR) framework that used a 3-pronged process of identifying circumstances for how a tool is developed, the problems it aims to address, and the knowledge that informs its development, implementation, and evaluation.21,22 Initial wireframes of the resource were provided via email to 10 subject matter experts (SMEs) in veteran suicide prevention, emergency medicine, and homeless programs. SMEs were identified via professional listservs, VA program office leadership, literature searches of similar research, and snowball sampling. Solicited feedback on the resource from the SMEs included its design, language, tone, flow, format, and content (ideation and prototyping). The feedback was collated and used to revise the resource. SMEs then reviewed and provided feedback on the revised resource. This iterative cycle (prototype review, commentary, ideation, prototype review) continued until the SMEs offered no additional edits to the resource. In total, 7 iterations of the resource were developed, critiqued, and revised.
INTERVIEW RESULTS
Compassion Fatigue
Many participants expressed concerns about compassion fatigue among VA ED clinicians. Those interviewed indicated that treating veterans experiencing homelessness sometimes led to the development of what they described as a “callus,” a “sixth sense,” or an inherent sense of “suspicion” or distrust. These feelings resulted from concerns about an individual’s secondary gain or potential hidden agenda (eg, a veteran reporting suicidal ideation to attain shelter on a cold night), with clinicians not wanting to feel as if they were taken advantage of or deceived.
Many clinicians noted that compassion fatigue resulted from witnessing the same veterans experiencing homelessness routinely use emergency services for nonemergent or nonmedical needs. Some also expressed that over time this may result in them becoming less empathetic when caring for veterans experiencing homelessness. They hypothesized that clinicians may experience burnout, which could potentially result in a lack of curiosity and concern about a veteran’s risk for suicide or need for social services. Others may “take things for granted,” leading them to discount stressors that are “very real to the patient, this person.”
Clinicians indicated that such sentiments may impact overall care. Potential negative consequences included stigmatization of veterans experiencing homelessness, incomplete or partial suicide risk screenings with this population, inattentive or impersonal care, and expedited discharge from the ED without appropriate safety planning or social service referrals. Clinicians interviewed intended to find ways to combat compassion fatigue and maintain a commitment to provide comprehensive care to all veterans, including those experiencing homelessness. They felt conflict between a lack of empathy for individuals experiencing homelessness and becoming numb to the problem due to overexposure. However, these clinicians remained committed to providing care to these veterans and fighting to maintain the purpose of recovery-focused care.
Knowledge Gaps on Available Services
While many clinicians knew of general resources available to veterans experiencing homelessness, few had detailed information on where to seek consults for other homeless programs, who to contact regarding these services, when they were available, or how to refer to them. Many reported feeling uneasy when discharging veterans experiencing homelessness from care, often being unable to provide local, comprehensive referrals to support their needs and ensure their well-being. These sentiments were compounded when the veteran reported suicidal thoughts or recent suicidal behavior; clinicians felt concerned about the methods to engage these individuals into evidence-based mental health care within the context of unstable housing arrangements.
Some clinicians appeared to lack awareness of the wide array of VA homeless programming. Most could acknowledge at least some aspects of available programming (eg, the US Department of Housing and Urban Development– VA Supportive Housing program), while others were unaware of services tailored to the needs of those experiencing homelessness (eg, homeless patient aligned care teams), or of services targeting concurrent psychosocial stressors (eg, Veterans Justice Programs). Interviewees hypothesized this as being particularly notable among clinicians who are new to the VA or those who work in VA settings as part of their graduate or medical school training. Those aware of the services were uncertain of the referral process, relying on a single social worker or nurse to connect individuals experiencing homelessness to health and social services.
Interviewed clinicians noted that suicide risk screening of veterans experiencing homelessness was only performed by a limited number of individuals within the ED. Some did not feel sufficiently trained, comfortable, or knowledgeable about how to navigate care for veterans experiencing homelessness and at risk of suicide. Clinicians described “an uncomfortableness about suicidal ideation, where people just freeze up” and “don’t know what to do and don’t know what to say.”
Lack of Tangible Resources, Trainings, and Referrals
HCPs reported occasionally lacking the necessary clinical resources and information in the ED to properly support veterans experiencing homelessness and suicidal ideation. Common concerns included case management and discharge planning, as well as navigating health factors, such as elevated suicide risk. Some HCPs felt the local resources they do have access to—discharge packets or other forms of patient information—were not always tailored for the needs (eg, transportation) or abilities of veterans experiencing homelessness. One noted: “We give them a sheet of paper with some resources, which they don’t have the skills to follow up [with] anyway.”
Many interviewees wished for additional training in working with veterans experiencing homelessness. They reported that prior training from the VA Talent Management System or through unit-based programming could assist in educating clinicians on homeless services and suicide risk assessment. When queried on what training they had received, many noted there was “no formal training on what the VA offers homeless vets,” leading many to describe it as on-the-job training. This appeared especially among newer clinicians, who reported they were reliant upon learning from other, more senior staff within the ED.
The absence of training further illustrates the issue of institutional knowledge on these services and referrals, which was often confined to a single individual or team. Not having readily accessible resources, training, or information appropriate for all skill levels and positions within the ED hindered the ability of HCPs to connect veterans experiencing homelessness with social services to ensure their health and safety postdischarge: “If we had a better knowledge base of what the VA offers and the steps to go through in order to get the veteran set up for those things, it would be helpful.”
CLINICAL RESOURCE
A psychoeducational resource was developed for HCPs treating veterans experiencing homelessness (Figure). The resource was designed to mitigate compassion fatigue and recenter attention on the VA commitment to care while emphasizing the need to be responsive to the concerns of these individuals. Initial wireframes of the resource were developed by a small group of authors in review and appraisal of qualitative findings (EP, RH). These wireframes were developed to broadly illustrate the arrangement/structure of content, range of resources to potentially include (eg, available VA homeless programs or consultation resources), and to draft initial wording and phrasing. Subject matter expert feedback refined these wireframes, providing commentary on specific programs to include or exclude, changes and alterations to the design and flow of the resource, and edits to language, word choice, and tone over numerous iterations.
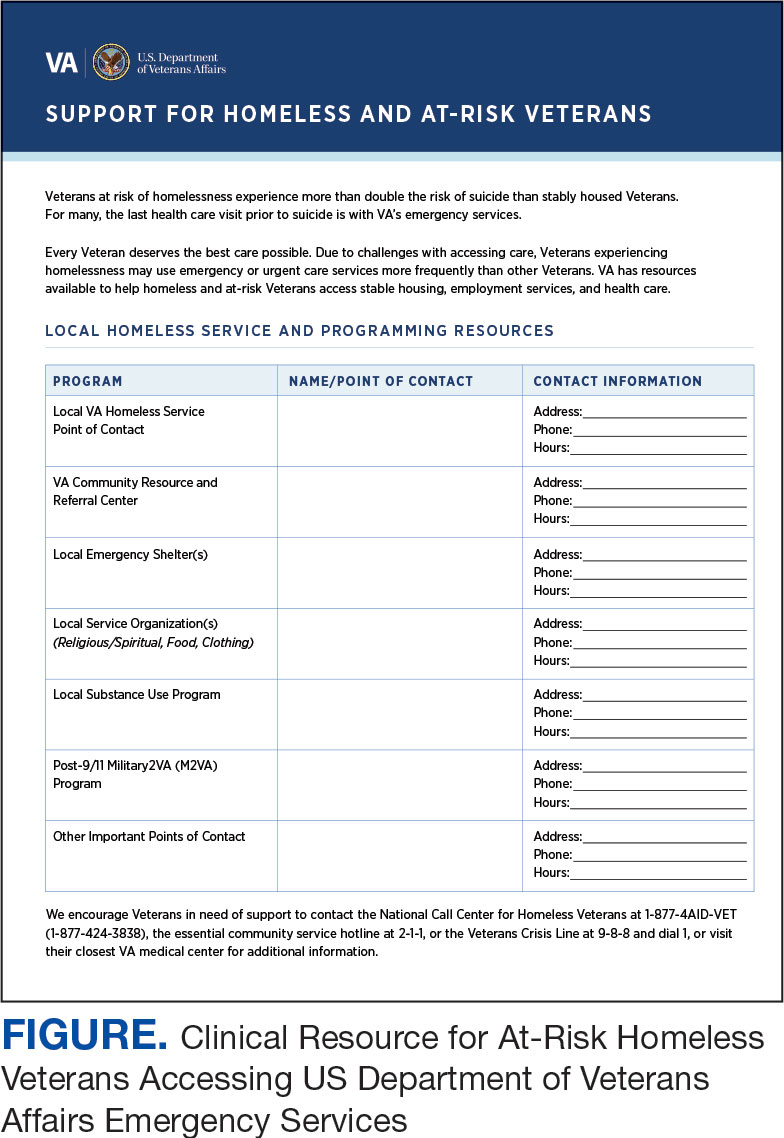
Given that many ED HCPs presented concerns surrounding secondary gain in the context of suicide risk, this resource focused on suicide risk. At the top of the resource, it states “Veterans at risk for homelessness experience more than double the risk for suicide than stably housed veterans.”23 Also at the top, the resource states: “For many, the last health care visit prior to suicide is often with VA emergency services."24 The goal of these statements was to educate users on the elevated risk for suicide in veterans experiencing homelessness and their role in preventing such deaths.
Text in this section emphasizes that every veteran deserves the best care possible and recenters HCP attention on providing quality, comprehensive care regardless of housing status. The inclusion of this material was prioritized given the concerns expressed regarding compassion fatigue and suspicions of secondary gain (eg, a veteran reporting suicidal ideation to attain shelter or respite from outside conditions).
The resource also attempts to address high rates of emergency service by veterans experiencing homelessness: “Due to challenges with accessing care, Veterans experiencing homelessness may use emergency or urgent care services more frequently than other Veterans.”25 The resource also indicates that VA resources are available to help homeless and at-risk veterans to acquire stable housing, employment, and engage in healthcare, which are outlined with specific contact information. Given the breadth of local and VA services, a portion of the resource is dedicated to local health and social services available for veterans experiencing homelessness. HCPs complete the first page, which is devoted to local homeless service and program resources.
Following SME consultation, the list of programs provided underwent a series of iterations. The program types listed are deemed to be of greatest benefit to veterans experiencing homelessness and most consulted by HCPs. Including VA and non-VA emergency shelters allows clinicians flexible options if a particular shelter is full, closed, or would not meet the veteran’s needs or preference (eg, lack of childcare or does not allow pets). The second column of this section is left intentionally blank; here, the HCP is to list a local point-of- contact at each program. This encourages clinical teams to seek out and make direct contact with these programs and establish (in)formal relationships with them. The HCP then completes the third column with contact information.
Once completed, the resource acts as a living document. Clinicians and SMEs consulted for this study expressed the desire to have an easily accessible resource that can be updated based on necessary changes (eg, emergency shelter address or hours of operation). The resource can be housed within each local VA emergency or urgent care service setting alongside other available clinical tools.
While local resources are the primary focus, interviewees also suggested that some HCPs are not aware of the available VA services . This material, found on the back of the resource, provides a general overview of services available through VA homeless programs. SME consultation and discussion led to selecting the 5 listed categories: housing services, health care services, case management, employment services, and justice-related programming, each with a brief description.
Information for the National Call Center for Homeless Veterans, community service hotline, and Veterans Crisis Line are included on the front page. These hotlines and phone numbers are always available for veterans experiencing homelessness, enabling them to make these connections themselves, if desired. Additionally, given the challenges noted by some HCPs in performing suicide risk screening, evaluation, and intervention, a prompt for the VA Suicide Risk Management Consultation service was also included on the back page.
Creating a Shared and Local Resource
This clinical resource was developed to establish a centralized, shared, local resource available to VA ED HCPs who lacked knowledge of available services or reported discomfort conducting suicide risk screening for veterans experiencing homelessness. In many cases, ED referrals to homeless programs and suicide prevention care was assigned to a single individual, often a nurse or social worker. As a result, an undue amount of work and strain was placed on these individuals, as this forced them to act as the sole bridge between care in the ED and postdischarge social (eg, homeless programs) and mental health (eg, suicide prevention) services. The creation of a unified, easily accessible document aimed to distribute this responsibility more equitably across ED staff.
DISCUSSION
This project intended to develop a clinician resource to support VA ED clinicians caring for veterans experiencing homelessness and their access to services postdischarge. Qualitative interviews provided insights into the burnout and compassion fatigue present in these settings, as well as the challenges and needs regarding knowledge of local and VA services. Emphasis was placed on leveraging extant resources and subject matter expertise to develop a resource capable of providing brief and informative guidance.
This resource is particularly relevant for HCPs new to the VA, including trainees and new hires, who may be less aware of VA and local social services. It has the potential to reduce the burden on VA ED staff to provide guidance and recommendations surrounding postdischarge social services. The resource acknowledges homeless programming focused on social determinants of health that can destabilize housing (eg, legal or occupational challenges). This can incentivize clinicians to discuss these programs with veterans to facilitate their ability to navigate complex health and psychosocial challenges.
HCPs interviewed for this study indicated their apprehension regarding suicide risk screening and evaluation, a process currently mandated within VA ED settings.26 This may be compounded among HCPs with minimal mental health training or those who have worked in community-based settings where such screening and evaluation efforts are not required. The resource reminds clinicians of available VA consultation services, which can provide additional training, clinical guidance, and review of existing local ED processes.
While the resource was directly informed by qualitative interviews conducted with VA emergency service HCPs and developed through an iterative process with SMEs, further research is necessary to determine its effectiveness at increasing access to health and social services among veterans experiencing homelessness. The resource has not been used by HCPs working in these settings to examine uptake or sustained use, nor clinicians’ perceptions of its utility, including acceptability and feasibility; these are important next steps to understand if the resource is functioning as intended.
Compassion fatigue, as well as associated sequelae (eg, burnout, distress, and psychiatric symptoms), is well-documented among individuals working with individuals experiencing homelessness, including VA HCPs.27-30 Such experiences are likely driven by several factors, including the clinical complexity and service needs of this veteran population. Although compassion fatigue was noted by many clinicians interviewed for this study, it is unclear if the resource alone would address factors driving compassion fatigue, or if additional programming or services may be necessary.
Limitations
The resource requires local HCPs to routinely update its content (eg, establishment of a new emergency shelter in the community or change in hours or contact information of an existing one), which may be challenging. This is especially true as it relates to community resources, which may be more likely to change than national VA programming.
This resource was initially developed following qualitative interviews with a small sample of VA HCPs (explicitly those working within ED settings) and may not be representative of all HCPs engaged in VA care with veterans experiencing homelessness. The perspectives and experiences of those interviewed do not represent the views of all VA ED HCPs and may differ from the perspectives of those in regions with unique cultural and regional considerations.31
Given that most of the interviewees were social workers in EDs engaged in care for veterans experiencing homelessness, these findings and informational needs may differ among other types of HCPs who provide services for veterans experiencing homelessness in other settings. Content in the resource was included based on clinician input, and may not reflect the perspectives of veterans, who may perceive some resources as more important (eg, access to primary care or dental services).28
CONCLUSIONS
This project represents the culmination of qualitative interviews and SME input to develop a free-to-use clinician resource to facilitate service delivery and connection to services following discharge from VA EDs for veterans experiencing homelessness. Serving as a template, this resource can be customized to increase knowledge of local VA and community resources to support these individuals. Continued refinement and piloting of this resource to evaluate acceptability, implementation barriers, and use remains warranted.
- Holliday R, Kinney AR, Smith AA, et al. A latent class analysis to identify subgroups of VHA using homeless veterans at greater risk for suicide mortality. J Affect Disord. 2022;315:162-167. doi:10.1016/j.jad.2022.07.062
- Weber J, Lee RC, Martsolf D. Understanding the health of veterans who are homeless: a review of the literature. Public Health Nurs. 2017;34(5):505-511. doi:10.1111/phn.12338
- Holliday R, Desai A, Stimmel M, Liu S, Monteith LL, Stewart KE. Meeting the health and social service needs of veterans who interact with the criminal justice system and experience homelessness: a holistic conceptualization and recommendations for tailoring care. Curr Treat Options Psychiatry. 2022;9(3):174-185. doi:10.1007/s40501-022-00275-1
- Holliday R, Desai A, Gerard G, Liu S, Stimmel M. Understanding the intersection of homelessness and justice involvement: enhancing veteran suicide prevention through VA programming. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
- Kushel MB, Perry S, Bangsberg D, Clark R, Moss AR. Emergency department use among the homeless and marginally housed: results from a community-based study. Am J Public Health. 2002;92(5):778-784. doi:10.2105/ajph.92.5.778
- Tsai J, Doran KM, Rosenheck RA. When health insurance is not a factor: national comparison of homeless and nonhomeless US veterans who use Veterans Affairs emergency departments. Am J Public Health. 2013;103(Suppl 2):S225-S231. doi:10.2105/AJPH.2013.301307
- Doran KM, Raven MC, Rosenheck RA. What drives frequent emergency department use in an integrated health system? National data from the Veterans Health Administration. Ann Emerg Med. 2013;62(2):151-159. doi:10.1016/j.annemergmed.2013.02.016
- Tsai J, Rosenheck RA. Risk factors for ED use among homeless veterans. Am J Emerg Med. 2013;31(5):855-858. doi:10.1016/j.ajem.2013.02.046
- Nelson RE, Suo Y, Pettey W, et al. Costs associated with health care services accessed through VA and in the community through Medicare for veterans experiencing homelessness. Health Serv Res. 2018;53(Suppl 3):5352-5374. doi:10.1111/1475-6773.13054
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Decker H, Raguram M, Kanzaria HK, Duke M, Wick E. Provider perceptions of challenges and facilitators to surgical care in unhoused patients: a qualitative analysis. Surgery. 2024;175(4):1095-1102. doi:10.1016/j.surg.2023.11.009
- Panushka KA, Kozlowski Z, Dalessandro C, Sanders JN, Millar MM, Gawron LM. “It’s not a top priority”: a qualitative analysis of provider views on barriers to reproductive healthcare provision for homeless women in the United States. Soc Work Public Health. 2023;38(5 -8):428-436. doi:10.1080/19371918.2024.2315180
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893-1907. doi:10.1007/s11135-017-0574-8
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753-1760. doi:10.1177/1049732315617444
- Assarroudi A, Heshmati Nabavi F, Armat MR, Ebadi A, Vaismoradi M. Directed qualitative content analysis: the description and elaboration of its underpinning methods and data analysis process. J Res Nurs. 2018;23(1):42-55. doi:10.1177/1744987117741667
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
- Goldsmith LJ. Using Framework Analysis in Applied Qualitative Research. Qual Rep. 2021;26(6):2061-2076. doi:10.46743/2160-3715/2021.5011
- Tufford L, Newman P. Bracketing in qualitative research. Qual Soc Work. 2012;11(1):80-96.
- Dodgson JE. Reflexivity in Qualitative Research. J Hum Lact. 2019;35(2):220-222. doi:10.1177/0890334419830990
- Hevner AR. A three cycle view of design science research. Scand J Inf Syst. 2007;19(2):4.
- Farao J, Malila B, Conrad N, Mutsvangwa T, Rangaka MX, Douglas TS. A user-centred design frame work for mHealth. PLOS ONE. 2020;15(8):e0237910. doi:10.1371/journal.pone.0237910
- Hoffberg AS, Spitzer E, Mackelprang JL, Farro SA, Brenner LA. Suicidal Self-Directed Violence Among Homeless US Veterans: A Systematic Review. Suicide Life Threat Behav. 2018;48(4):481-498. doi:10.1111/sltb.12369
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and lowincome Veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Holliday R, Hostetter T, Brenner LA, Bahraini N, Tsai J. Suicide risk screening and evaluation among patients accessing VHA services and identified as being newly homeless. Health Serv Res. 2024;59(5):e14301. doi:10.1111/1475-6773.14301
- Waegemakers Schiff J, Lane AM. PTSD symptoms, vicarious traumatization, and burnout in front line workers in the homeless sector. Community Ment Health J. 2019;55(3):454-462. doi:10.1007/s10597-018-00364-7
- Steenekamp BL, Barker SL. Exploring the experiences of compassion fatigue amongst peer support workers in homelessness services. Community Ment Health J. 2024;60(4):772-783. doi:10.1007/s10597-024-01234-1
- Perez S, Kerman N, Dej E, et al. When I can’t help, I suffer: a scoping review of moral distress in service providers working with persons experiencing homelessness. J Ment Health. Published online 2024:1-16. doi:10.1080/09638237.2024.2426986
- Monteith LL, Holliday R, Christe’An DI, Sherrill A, Brenner LA, Hoffmire CA. Suicide risk and prevention in Guam: clinical and research considerations and a call to action. Asian J Psychiatry. 2023;83:103546. doi:10.1016/j.ajp.2023.103546
- Surís A, Holliday R, Hooshyar D, et al. Development and implementation of a homeless mobile medical/mental veteran intervention. Fed Pract. 2017;34(9):18.
Veterans experiencing homelessness are at an elevated risk for adverse health outcomes, including suicide. This population also experiences chronic health conditions (eg, cardiovascular disease and sexually transmitted infections) and psychiatric conditions (eg, substance use disorders and posttraumatic stress disorder) with a greater propensity than veterans without history of homelessness.1,2 Similarly, veterans experiencing homelessness often report concurrent stressors, such as justice involvement and unemployment, which further impact social functioning.3
The US Department of Veterans Affairs (VA) offers a range of health and social services to veterans experiencing homelessness. These programs are designed to respond to the multifactorial challenges faced by this population and are aimed at achieving sustained, permanent housing.4 To facilitate this effort, these programs provide targeted and tailored health (eg, primary care) and social (eg, case management and vocational rehabilitation) services to address barriers to housing stability (eg, substance use, serious mental illness, interacting with the criminal legal system, and unemployment).
Despite the availability of these programs, engaging veterans in VA services—whether in general or tailored for those experiencing or at risk for homelessness—remains challenging. Many veterans at risk for or experiencing homelessness overuse service settings that provide immediate care, such as urgent care or emergency departments (EDs).5,6 These individuals often visit an ED to augment or complement medical care they received in an outpatient setting, which can result in an elevated health care burden as well as impacted provision of treatment, especially surrounding care for chronic conditions (eg, cardiovascular health or serious mental illness).7-9
VA EDs offer urgent care and emergency services and often serve as a point of entry for veterans experiencing homelessness.10 They offer veterans expedient access to care that can address immediate needs (eg, substance use withdrawal, pain management, and suicide risk). EDs may be easier to access given they have longer hours of operation and patients can present without a scheduled appointment. VA EDs are an important point to identify homelessness and connect individuals to social service resources and outpatient health care referrals (eg, primary care and mental health).4,11
Some clinicians experience uncertainty in navigating or providing care for veterans experiencing or at risk for homelessness. A qualitative study conducted outside the VA found many clinicians did not know how to approach clinical conversations among unstably housed individuals, particularly when they discussed how to manage care for complex health conditions in the context of ongoing case management challenges, such as discharge planning.12 Another study found that clinicians working with individuals experiencing homelessness may have limited prior training or experience treating these patients.13 As a result, these clinicians may be unaware of available social services or unknowingly have biases that negatively impact care. Research remains limited surrounding beliefs about and methods of enhancing care among VA clinicians working with veterans experiencing homelessness in the ED.
This multiphase pilot study sought to understand service delivery processes and gaps in VA ED settings. Phase 1 examined ED clinician perceptions of care, facilitators, and barriers to providing care (including suicide risk assessments) and making postdischarge outpatient referrals among VA ED clinicians who regularly work with veterans experiencing homelessness. Phase 2 used this information to develop a clinical psychoeducational resource to enhance post-ED access to care for veterans experiencing or at risk for homelessness.
QUALITATIVE INTERVIEWS
Semistructured qualitative interviews were conducted with 11 VA ED clinicians from 6 Veteran Integrated Service Networks between August 2022 and February 2023. Clinicians were eligible if they currently worked within a VA ED setting (including urgent care) and indicated that some of their patients were veterans experiencing homelessness. All health care practitioners (HCPs) participated in an interview and a postinterview self-report survey that assessed demographic and job-related characteristics. Eight HCPs identified as female and 3 identified as male. All clinicians identified as White and 3 as Hispanic or Latino. Eight clinicians were licensed clinical social workers, 2 were ED nurses, and 1 was an ED physician.
After each clinician provided informed consent, they were invited to complete a telephone or Microsoft Teams interview. All interviews were recorded and subsequently transcribed. Interviews explored clinicians’ experiences caring for veterans experiencing homelessness, with a focus on services provided within the ED, as well as mandated ED screenings such as a suicide risk assessment. Interview questions also addressed postdischarge knowledge and experiences with referrals to VA health services (eg, primary care, mental health) and social services (eg, housing programs). Interviews lasted 30 to 90 minutes.
Recruitment ended after attaining sufficient thematic data, accomplished via an information power approach to sampling. This occurred when the study aims, sample characteristics, existing theory, and depth and quality of interviews dynamically informed the decision to cease recruitment of additional participants.14,15 Given the scope of study (examining service delivery and knowledge gaps), the specificity of the targeted sample (VA ED clinicians providing care to veterans experiencing homelessness), the level of pre-existing theoretical background informing the study aims, and depth and quality of interview dialogue, this information power approach provides justification for attaining small sample sizes. Following the interview, HCPs completed a demographic questionnaire. Participants were not compensated.
Data Analysis
Directed content analysis was used to analyze qualitative data, with the framework method employed as an analytic instrument to facilitate analysis.16-18 Analysts engaged in bracketing and discussed reflexivity before data analysis to reflect on personal subjectivities and reduce potential bias.19,20
A prototype coding framework was developed that enabled coders to meaningfully summarize and condense data within transcripts into varying domains, categories, or topics found within the interview guide. Domain examples included clinical backgrounds, suicide risk and assessment protocols among veterans experiencing homelessness, beliefs about service delivery for veterans experiencing homelessness, and barriers and facilitators that may impact their ability to provide post-ED discharge care. Coders discussed the findings and if there was a need to modify templates. All transcripts were double coded. Once complete, individual templates were merged into a unified Microsoft Excel sheet, which allowed for more discrete analyses, enabling analysts to examine trends across content areas within the dataset.
Clinical Resource Development
HCPs were queried regarding available outpatient resources for post-ED care (eg, printed discharge paperwork and best practice alerts or automated workflows within the electronic health record). Resources used by participants were examined, as well as which resources clinicians thought would help them care for veterans experiencing homelessness. Noted gaps were used to develop a tailored resource for clinicians who treat veterans experiencing homelessness in the ED. This resource was created with the intention it could inform all ED clinicians, with the option for personalization to align with the needs of local services, based on needed content areas identified (eg, emergency shelters and suicide prevention resources).
Resource development followed an information systems research (ISR) framework that used a 3-pronged process of identifying circumstances for how a tool is developed, the problems it aims to address, and the knowledge that informs its development, implementation, and evaluation.21,22 Initial wireframes of the resource were provided via email to 10 subject matter experts (SMEs) in veteran suicide prevention, emergency medicine, and homeless programs. SMEs were identified via professional listservs, VA program office leadership, literature searches of similar research, and snowball sampling. Solicited feedback on the resource from the SMEs included its design, language, tone, flow, format, and content (ideation and prototyping). The feedback was collated and used to revise the resource. SMEs then reviewed and provided feedback on the revised resource. This iterative cycle (prototype review, commentary, ideation, prototype review) continued until the SMEs offered no additional edits to the resource. In total, 7 iterations of the resource were developed, critiqued, and revised.
INTERVIEW RESULTS
Compassion Fatigue
Many participants expressed concerns about compassion fatigue among VA ED clinicians. Those interviewed indicated that treating veterans experiencing homelessness sometimes led to the development of what they described as a “callus,” a “sixth sense,” or an inherent sense of “suspicion” or distrust. These feelings resulted from concerns about an individual’s secondary gain or potential hidden agenda (eg, a veteran reporting suicidal ideation to attain shelter on a cold night), with clinicians not wanting to feel as if they were taken advantage of or deceived.
Many clinicians noted that compassion fatigue resulted from witnessing the same veterans experiencing homelessness routinely use emergency services for nonemergent or nonmedical needs. Some also expressed that over time this may result in them becoming less empathetic when caring for veterans experiencing homelessness. They hypothesized that clinicians may experience burnout, which could potentially result in a lack of curiosity and concern about a veteran’s risk for suicide or need for social services. Others may “take things for granted,” leading them to discount stressors that are “very real to the patient, this person.”
Clinicians indicated that such sentiments may impact overall care. Potential negative consequences included stigmatization of veterans experiencing homelessness, incomplete or partial suicide risk screenings with this population, inattentive or impersonal care, and expedited discharge from the ED without appropriate safety planning or social service referrals. Clinicians interviewed intended to find ways to combat compassion fatigue and maintain a commitment to provide comprehensive care to all veterans, including those experiencing homelessness. They felt conflict between a lack of empathy for individuals experiencing homelessness and becoming numb to the problem due to overexposure. However, these clinicians remained committed to providing care to these veterans and fighting to maintain the purpose of recovery-focused care.
Knowledge Gaps on Available Services
While many clinicians knew of general resources available to veterans experiencing homelessness, few had detailed information on where to seek consults for other homeless programs, who to contact regarding these services, when they were available, or how to refer to them. Many reported feeling uneasy when discharging veterans experiencing homelessness from care, often being unable to provide local, comprehensive referrals to support their needs and ensure their well-being. These sentiments were compounded when the veteran reported suicidal thoughts or recent suicidal behavior; clinicians felt concerned about the methods to engage these individuals into evidence-based mental health care within the context of unstable housing arrangements.
Some clinicians appeared to lack awareness of the wide array of VA homeless programming. Most could acknowledge at least some aspects of available programming (eg, the US Department of Housing and Urban Development– VA Supportive Housing program), while others were unaware of services tailored to the needs of those experiencing homelessness (eg, homeless patient aligned care teams), or of services targeting concurrent psychosocial stressors (eg, Veterans Justice Programs). Interviewees hypothesized this as being particularly notable among clinicians who are new to the VA or those who work in VA settings as part of their graduate or medical school training. Those aware of the services were uncertain of the referral process, relying on a single social worker or nurse to connect individuals experiencing homelessness to health and social services.
Interviewed clinicians noted that suicide risk screening of veterans experiencing homelessness was only performed by a limited number of individuals within the ED. Some did not feel sufficiently trained, comfortable, or knowledgeable about how to navigate care for veterans experiencing homelessness and at risk of suicide. Clinicians described “an uncomfortableness about suicidal ideation, where people just freeze up” and “don’t know what to do and don’t know what to say.”
Lack of Tangible Resources, Trainings, and Referrals
HCPs reported occasionally lacking the necessary clinical resources and information in the ED to properly support veterans experiencing homelessness and suicidal ideation. Common concerns included case management and discharge planning, as well as navigating health factors, such as elevated suicide risk. Some HCPs felt the local resources they do have access to—discharge packets or other forms of patient information—were not always tailored for the needs (eg, transportation) or abilities of veterans experiencing homelessness. One noted: “We give them a sheet of paper with some resources, which they don’t have the skills to follow up [with] anyway.”
Many interviewees wished for additional training in working with veterans experiencing homelessness. They reported that prior training from the VA Talent Management System or through unit-based programming could assist in educating clinicians on homeless services and suicide risk assessment. When queried on what training they had received, many noted there was “no formal training on what the VA offers homeless vets,” leading many to describe it as on-the-job training. This appeared especially among newer clinicians, who reported they were reliant upon learning from other, more senior staff within the ED.
The absence of training further illustrates the issue of institutional knowledge on these services and referrals, which was often confined to a single individual or team. Not having readily accessible resources, training, or information appropriate for all skill levels and positions within the ED hindered the ability of HCPs to connect veterans experiencing homelessness with social services to ensure their health and safety postdischarge: “If we had a better knowledge base of what the VA offers and the steps to go through in order to get the veteran set up for those things, it would be helpful.”
CLINICAL RESOURCE
A psychoeducational resource was developed for HCPs treating veterans experiencing homelessness (Figure). The resource was designed to mitigate compassion fatigue and recenter attention on the VA commitment to care while emphasizing the need to be responsive to the concerns of these individuals. Initial wireframes of the resource were developed by a small group of authors in review and appraisal of qualitative findings (EP, RH). These wireframes were developed to broadly illustrate the arrangement/structure of content, range of resources to potentially include (eg, available VA homeless programs or consultation resources), and to draft initial wording and phrasing. Subject matter expert feedback refined these wireframes, providing commentary on specific programs to include or exclude, changes and alterations to the design and flow of the resource, and edits to language, word choice, and tone over numerous iterations.

Given that many ED HCPs presented concerns surrounding secondary gain in the context of suicide risk, this resource focused on suicide risk. At the top of the resource, it states “Veterans at risk for homelessness experience more than double the risk for suicide than stably housed veterans.”23 Also at the top, the resource states: “For many, the last health care visit prior to suicide is often with VA emergency services."24 The goal of these statements was to educate users on the elevated risk for suicide in veterans experiencing homelessness and their role in preventing such deaths.
Text in this section emphasizes that every veteran deserves the best care possible and recenters HCP attention on providing quality, comprehensive care regardless of housing status. The inclusion of this material was prioritized given the concerns expressed regarding compassion fatigue and suspicions of secondary gain (eg, a veteran reporting suicidal ideation to attain shelter or respite from outside conditions).
The resource also attempts to address high rates of emergency service by veterans experiencing homelessness: “Due to challenges with accessing care, Veterans experiencing homelessness may use emergency or urgent care services more frequently than other Veterans.”25 The resource also indicates that VA resources are available to help homeless and at-risk veterans to acquire stable housing, employment, and engage in healthcare, which are outlined with specific contact information. Given the breadth of local and VA services, a portion of the resource is dedicated to local health and social services available for veterans experiencing homelessness. HCPs complete the first page, which is devoted to local homeless service and program resources.
Following SME consultation, the list of programs provided underwent a series of iterations. The program types listed are deemed to be of greatest benefit to veterans experiencing homelessness and most consulted by HCPs. Including VA and non-VA emergency shelters allows clinicians flexible options if a particular shelter is full, closed, or would not meet the veteran’s needs or preference (eg, lack of childcare or does not allow pets). The second column of this section is left intentionally blank; here, the HCP is to list a local point-of- contact at each program. This encourages clinical teams to seek out and make direct contact with these programs and establish (in)formal relationships with them. The HCP then completes the third column with contact information.
Once completed, the resource acts as a living document. Clinicians and SMEs consulted for this study expressed the desire to have an easily accessible resource that can be updated based on necessary changes (eg, emergency shelter address or hours of operation). The resource can be housed within each local VA emergency or urgent care service setting alongside other available clinical tools.
While local resources are the primary focus, interviewees also suggested that some HCPs are not aware of the available VA services . This material, found on the back of the resource, provides a general overview of services available through VA homeless programs. SME consultation and discussion led to selecting the 5 listed categories: housing services, health care services, case management, employment services, and justice-related programming, each with a brief description.
Information for the National Call Center for Homeless Veterans, community service hotline, and Veterans Crisis Line are included on the front page. These hotlines and phone numbers are always available for veterans experiencing homelessness, enabling them to make these connections themselves, if desired. Additionally, given the challenges noted by some HCPs in performing suicide risk screening, evaluation, and intervention, a prompt for the VA Suicide Risk Management Consultation service was also included on the back page.
Creating a Shared and Local Resource
This clinical resource was developed to establish a centralized, shared, local resource available to VA ED HCPs who lacked knowledge of available services or reported discomfort conducting suicide risk screening for veterans experiencing homelessness. In many cases, ED referrals to homeless programs and suicide prevention care was assigned to a single individual, often a nurse or social worker. As a result, an undue amount of work and strain was placed on these individuals, as this forced them to act as the sole bridge between care in the ED and postdischarge social (eg, homeless programs) and mental health (eg, suicide prevention) services. The creation of a unified, easily accessible document aimed to distribute this responsibility more equitably across ED staff.
DISCUSSION
This project intended to develop a clinician resource to support VA ED clinicians caring for veterans experiencing homelessness and their access to services postdischarge. Qualitative interviews provided insights into the burnout and compassion fatigue present in these settings, as well as the challenges and needs regarding knowledge of local and VA services. Emphasis was placed on leveraging extant resources and subject matter expertise to develop a resource capable of providing brief and informative guidance.
This resource is particularly relevant for HCPs new to the VA, including trainees and new hires, who may be less aware of VA and local social services. It has the potential to reduce the burden on VA ED staff to provide guidance and recommendations surrounding postdischarge social services. The resource acknowledges homeless programming focused on social determinants of health that can destabilize housing (eg, legal or occupational challenges). This can incentivize clinicians to discuss these programs with veterans to facilitate their ability to navigate complex health and psychosocial challenges.
HCPs interviewed for this study indicated their apprehension regarding suicide risk screening and evaluation, a process currently mandated within VA ED settings.26 This may be compounded among HCPs with minimal mental health training or those who have worked in community-based settings where such screening and evaluation efforts are not required. The resource reminds clinicians of available VA consultation services, which can provide additional training, clinical guidance, and review of existing local ED processes.
While the resource was directly informed by qualitative interviews conducted with VA emergency service HCPs and developed through an iterative process with SMEs, further research is necessary to determine its effectiveness at increasing access to health and social services among veterans experiencing homelessness. The resource has not been used by HCPs working in these settings to examine uptake or sustained use, nor clinicians’ perceptions of its utility, including acceptability and feasibility; these are important next steps to understand if the resource is functioning as intended.
Compassion fatigue, as well as associated sequelae (eg, burnout, distress, and psychiatric symptoms), is well-documented among individuals working with individuals experiencing homelessness, including VA HCPs.27-30 Such experiences are likely driven by several factors, including the clinical complexity and service needs of this veteran population. Although compassion fatigue was noted by many clinicians interviewed for this study, it is unclear if the resource alone would address factors driving compassion fatigue, or if additional programming or services may be necessary.
Limitations
The resource requires local HCPs to routinely update its content (eg, establishment of a new emergency shelter in the community or change in hours or contact information of an existing one), which may be challenging. This is especially true as it relates to community resources, which may be more likely to change than national VA programming.
This resource was initially developed following qualitative interviews with a small sample of VA HCPs (explicitly those working within ED settings) and may not be representative of all HCPs engaged in VA care with veterans experiencing homelessness. The perspectives and experiences of those interviewed do not represent the views of all VA ED HCPs and may differ from the perspectives of those in regions with unique cultural and regional considerations.31
Given that most of the interviewees were social workers in EDs engaged in care for veterans experiencing homelessness, these findings and informational needs may differ among other types of HCPs who provide services for veterans experiencing homelessness in other settings. Content in the resource was included based on clinician input, and may not reflect the perspectives of veterans, who may perceive some resources as more important (eg, access to primary care or dental services).28
CONCLUSIONS
This project represents the culmination of qualitative interviews and SME input to develop a free-to-use clinician resource to facilitate service delivery and connection to services following discharge from VA EDs for veterans experiencing homelessness. Serving as a template, this resource can be customized to increase knowledge of local VA and community resources to support these individuals. Continued refinement and piloting of this resource to evaluate acceptability, implementation barriers, and use remains warranted.
Veterans experiencing homelessness are at an elevated risk for adverse health outcomes, including suicide. This population also experiences chronic health conditions (eg, cardiovascular disease and sexually transmitted infections) and psychiatric conditions (eg, substance use disorders and posttraumatic stress disorder) with a greater propensity than veterans without history of homelessness.1,2 Similarly, veterans experiencing homelessness often report concurrent stressors, such as justice involvement and unemployment, which further impact social functioning.3
The US Department of Veterans Affairs (VA) offers a range of health and social services to veterans experiencing homelessness. These programs are designed to respond to the multifactorial challenges faced by this population and are aimed at achieving sustained, permanent housing.4 To facilitate this effort, these programs provide targeted and tailored health (eg, primary care) and social (eg, case management and vocational rehabilitation) services to address barriers to housing stability (eg, substance use, serious mental illness, interacting with the criminal legal system, and unemployment).
Despite the availability of these programs, engaging veterans in VA services—whether in general or tailored for those experiencing or at risk for homelessness—remains challenging. Many veterans at risk for or experiencing homelessness overuse service settings that provide immediate care, such as urgent care or emergency departments (EDs).5,6 These individuals often visit an ED to augment or complement medical care they received in an outpatient setting, which can result in an elevated health care burden as well as impacted provision of treatment, especially surrounding care for chronic conditions (eg, cardiovascular health or serious mental illness).7-9
VA EDs offer urgent care and emergency services and often serve as a point of entry for veterans experiencing homelessness.10 They offer veterans expedient access to care that can address immediate needs (eg, substance use withdrawal, pain management, and suicide risk). EDs may be easier to access given they have longer hours of operation and patients can present without a scheduled appointment. VA EDs are an important point to identify homelessness and connect individuals to social service resources and outpatient health care referrals (eg, primary care and mental health).4,11
Some clinicians experience uncertainty in navigating or providing care for veterans experiencing or at risk for homelessness. A qualitative study conducted outside the VA found many clinicians did not know how to approach clinical conversations among unstably housed individuals, particularly when they discussed how to manage care for complex health conditions in the context of ongoing case management challenges, such as discharge planning.12 Another study found that clinicians working with individuals experiencing homelessness may have limited prior training or experience treating these patients.13 As a result, these clinicians may be unaware of available social services or unknowingly have biases that negatively impact care. Research remains limited surrounding beliefs about and methods of enhancing care among VA clinicians working with veterans experiencing homelessness in the ED.
This multiphase pilot study sought to understand service delivery processes and gaps in VA ED settings. Phase 1 examined ED clinician perceptions of care, facilitators, and barriers to providing care (including suicide risk assessments) and making postdischarge outpatient referrals among VA ED clinicians who regularly work with veterans experiencing homelessness. Phase 2 used this information to develop a clinical psychoeducational resource to enhance post-ED access to care for veterans experiencing or at risk for homelessness.
QUALITATIVE INTERVIEWS
Semistructured qualitative interviews were conducted with 11 VA ED clinicians from 6 Veteran Integrated Service Networks between August 2022 and February 2023. Clinicians were eligible if they currently worked within a VA ED setting (including urgent care) and indicated that some of their patients were veterans experiencing homelessness. All health care practitioners (HCPs) participated in an interview and a postinterview self-report survey that assessed demographic and job-related characteristics. Eight HCPs identified as female and 3 identified as male. All clinicians identified as White and 3 as Hispanic or Latino. Eight clinicians were licensed clinical social workers, 2 were ED nurses, and 1 was an ED physician.
After each clinician provided informed consent, they were invited to complete a telephone or Microsoft Teams interview. All interviews were recorded and subsequently transcribed. Interviews explored clinicians’ experiences caring for veterans experiencing homelessness, with a focus on services provided within the ED, as well as mandated ED screenings such as a suicide risk assessment. Interview questions also addressed postdischarge knowledge and experiences with referrals to VA health services (eg, primary care, mental health) and social services (eg, housing programs). Interviews lasted 30 to 90 minutes.
Recruitment ended after attaining sufficient thematic data, accomplished via an information power approach to sampling. This occurred when the study aims, sample characteristics, existing theory, and depth and quality of interviews dynamically informed the decision to cease recruitment of additional participants.14,15 Given the scope of study (examining service delivery and knowledge gaps), the specificity of the targeted sample (VA ED clinicians providing care to veterans experiencing homelessness), the level of pre-existing theoretical background informing the study aims, and depth and quality of interview dialogue, this information power approach provides justification for attaining small sample sizes. Following the interview, HCPs completed a demographic questionnaire. Participants were not compensated.
Data Analysis
Directed content analysis was used to analyze qualitative data, with the framework method employed as an analytic instrument to facilitate analysis.16-18 Analysts engaged in bracketing and discussed reflexivity before data analysis to reflect on personal subjectivities and reduce potential bias.19,20
A prototype coding framework was developed that enabled coders to meaningfully summarize and condense data within transcripts into varying domains, categories, or topics found within the interview guide. Domain examples included clinical backgrounds, suicide risk and assessment protocols among veterans experiencing homelessness, beliefs about service delivery for veterans experiencing homelessness, and barriers and facilitators that may impact their ability to provide post-ED discharge care. Coders discussed the findings and if there was a need to modify templates. All transcripts were double coded. Once complete, individual templates were merged into a unified Microsoft Excel sheet, which allowed for more discrete analyses, enabling analysts to examine trends across content areas within the dataset.
Clinical Resource Development
HCPs were queried regarding available outpatient resources for post-ED care (eg, printed discharge paperwork and best practice alerts or automated workflows within the electronic health record). Resources used by participants were examined, as well as which resources clinicians thought would help them care for veterans experiencing homelessness. Noted gaps were used to develop a tailored resource for clinicians who treat veterans experiencing homelessness in the ED. This resource was created with the intention it could inform all ED clinicians, with the option for personalization to align with the needs of local services, based on needed content areas identified (eg, emergency shelters and suicide prevention resources).
Resource development followed an information systems research (ISR) framework that used a 3-pronged process of identifying circumstances for how a tool is developed, the problems it aims to address, and the knowledge that informs its development, implementation, and evaluation.21,22 Initial wireframes of the resource were provided via email to 10 subject matter experts (SMEs) in veteran suicide prevention, emergency medicine, and homeless programs. SMEs were identified via professional listservs, VA program office leadership, literature searches of similar research, and snowball sampling. Solicited feedback on the resource from the SMEs included its design, language, tone, flow, format, and content (ideation and prototyping). The feedback was collated and used to revise the resource. SMEs then reviewed and provided feedback on the revised resource. This iterative cycle (prototype review, commentary, ideation, prototype review) continued until the SMEs offered no additional edits to the resource. In total, 7 iterations of the resource were developed, critiqued, and revised.
INTERVIEW RESULTS
Compassion Fatigue
Many participants expressed concerns about compassion fatigue among VA ED clinicians. Those interviewed indicated that treating veterans experiencing homelessness sometimes led to the development of what they described as a “callus,” a “sixth sense,” or an inherent sense of “suspicion” or distrust. These feelings resulted from concerns about an individual’s secondary gain or potential hidden agenda (eg, a veteran reporting suicidal ideation to attain shelter on a cold night), with clinicians not wanting to feel as if they were taken advantage of or deceived.
Many clinicians noted that compassion fatigue resulted from witnessing the same veterans experiencing homelessness routinely use emergency services for nonemergent or nonmedical needs. Some also expressed that over time this may result in them becoming less empathetic when caring for veterans experiencing homelessness. They hypothesized that clinicians may experience burnout, which could potentially result in a lack of curiosity and concern about a veteran’s risk for suicide or need for social services. Others may “take things for granted,” leading them to discount stressors that are “very real to the patient, this person.”
Clinicians indicated that such sentiments may impact overall care. Potential negative consequences included stigmatization of veterans experiencing homelessness, incomplete or partial suicide risk screenings with this population, inattentive or impersonal care, and expedited discharge from the ED without appropriate safety planning or social service referrals. Clinicians interviewed intended to find ways to combat compassion fatigue and maintain a commitment to provide comprehensive care to all veterans, including those experiencing homelessness. They felt conflict between a lack of empathy for individuals experiencing homelessness and becoming numb to the problem due to overexposure. However, these clinicians remained committed to providing care to these veterans and fighting to maintain the purpose of recovery-focused care.
Knowledge Gaps on Available Services
While many clinicians knew of general resources available to veterans experiencing homelessness, few had detailed information on where to seek consults for other homeless programs, who to contact regarding these services, when they were available, or how to refer to them. Many reported feeling uneasy when discharging veterans experiencing homelessness from care, often being unable to provide local, comprehensive referrals to support their needs and ensure their well-being. These sentiments were compounded when the veteran reported suicidal thoughts or recent suicidal behavior; clinicians felt concerned about the methods to engage these individuals into evidence-based mental health care within the context of unstable housing arrangements.
Some clinicians appeared to lack awareness of the wide array of VA homeless programming. Most could acknowledge at least some aspects of available programming (eg, the US Department of Housing and Urban Development– VA Supportive Housing program), while others were unaware of services tailored to the needs of those experiencing homelessness (eg, homeless patient aligned care teams), or of services targeting concurrent psychosocial stressors (eg, Veterans Justice Programs). Interviewees hypothesized this as being particularly notable among clinicians who are new to the VA or those who work in VA settings as part of their graduate or medical school training. Those aware of the services were uncertain of the referral process, relying on a single social worker or nurse to connect individuals experiencing homelessness to health and social services.
Interviewed clinicians noted that suicide risk screening of veterans experiencing homelessness was only performed by a limited number of individuals within the ED. Some did not feel sufficiently trained, comfortable, or knowledgeable about how to navigate care for veterans experiencing homelessness and at risk of suicide. Clinicians described “an uncomfortableness about suicidal ideation, where people just freeze up” and “don’t know what to do and don’t know what to say.”
Lack of Tangible Resources, Trainings, and Referrals
HCPs reported occasionally lacking the necessary clinical resources and information in the ED to properly support veterans experiencing homelessness and suicidal ideation. Common concerns included case management and discharge planning, as well as navigating health factors, such as elevated suicide risk. Some HCPs felt the local resources they do have access to—discharge packets or other forms of patient information—were not always tailored for the needs (eg, transportation) or abilities of veterans experiencing homelessness. One noted: “We give them a sheet of paper with some resources, which they don’t have the skills to follow up [with] anyway.”
Many interviewees wished for additional training in working with veterans experiencing homelessness. They reported that prior training from the VA Talent Management System or through unit-based programming could assist in educating clinicians on homeless services and suicide risk assessment. When queried on what training they had received, many noted there was “no formal training on what the VA offers homeless vets,” leading many to describe it as on-the-job training. This appeared especially among newer clinicians, who reported they were reliant upon learning from other, more senior staff within the ED.
The absence of training further illustrates the issue of institutional knowledge on these services and referrals, which was often confined to a single individual or team. Not having readily accessible resources, training, or information appropriate for all skill levels and positions within the ED hindered the ability of HCPs to connect veterans experiencing homelessness with social services to ensure their health and safety postdischarge: “If we had a better knowledge base of what the VA offers and the steps to go through in order to get the veteran set up for those things, it would be helpful.”
CLINICAL RESOURCE
A psychoeducational resource was developed for HCPs treating veterans experiencing homelessness (Figure). The resource was designed to mitigate compassion fatigue and recenter attention on the VA commitment to care while emphasizing the need to be responsive to the concerns of these individuals. Initial wireframes of the resource were developed by a small group of authors in review and appraisal of qualitative findings (EP, RH). These wireframes were developed to broadly illustrate the arrangement/structure of content, range of resources to potentially include (eg, available VA homeless programs or consultation resources), and to draft initial wording and phrasing. Subject matter expert feedback refined these wireframes, providing commentary on specific programs to include or exclude, changes and alterations to the design and flow of the resource, and edits to language, word choice, and tone over numerous iterations.

Given that many ED HCPs presented concerns surrounding secondary gain in the context of suicide risk, this resource focused on suicide risk. At the top of the resource, it states “Veterans at risk for homelessness experience more than double the risk for suicide than stably housed veterans.”23 Also at the top, the resource states: “For many, the last health care visit prior to suicide is often with VA emergency services."24 The goal of these statements was to educate users on the elevated risk for suicide in veterans experiencing homelessness and their role in preventing such deaths.
Text in this section emphasizes that every veteran deserves the best care possible and recenters HCP attention on providing quality, comprehensive care regardless of housing status. The inclusion of this material was prioritized given the concerns expressed regarding compassion fatigue and suspicions of secondary gain (eg, a veteran reporting suicidal ideation to attain shelter or respite from outside conditions).
The resource also attempts to address high rates of emergency service by veterans experiencing homelessness: “Due to challenges with accessing care, Veterans experiencing homelessness may use emergency or urgent care services more frequently than other Veterans.”25 The resource also indicates that VA resources are available to help homeless and at-risk veterans to acquire stable housing, employment, and engage in healthcare, which are outlined with specific contact information. Given the breadth of local and VA services, a portion of the resource is dedicated to local health and social services available for veterans experiencing homelessness. HCPs complete the first page, which is devoted to local homeless service and program resources.
Following SME consultation, the list of programs provided underwent a series of iterations. The program types listed are deemed to be of greatest benefit to veterans experiencing homelessness and most consulted by HCPs. Including VA and non-VA emergency shelters allows clinicians flexible options if a particular shelter is full, closed, or would not meet the veteran’s needs or preference (eg, lack of childcare or does not allow pets). The second column of this section is left intentionally blank; here, the HCP is to list a local point-of- contact at each program. This encourages clinical teams to seek out and make direct contact with these programs and establish (in)formal relationships with them. The HCP then completes the third column with contact information.
Once completed, the resource acts as a living document. Clinicians and SMEs consulted for this study expressed the desire to have an easily accessible resource that can be updated based on necessary changes (eg, emergency shelter address or hours of operation). The resource can be housed within each local VA emergency or urgent care service setting alongside other available clinical tools.
While local resources are the primary focus, interviewees also suggested that some HCPs are not aware of the available VA services . This material, found on the back of the resource, provides a general overview of services available through VA homeless programs. SME consultation and discussion led to selecting the 5 listed categories: housing services, health care services, case management, employment services, and justice-related programming, each with a brief description.
Information for the National Call Center for Homeless Veterans, community service hotline, and Veterans Crisis Line are included on the front page. These hotlines and phone numbers are always available for veterans experiencing homelessness, enabling them to make these connections themselves, if desired. Additionally, given the challenges noted by some HCPs in performing suicide risk screening, evaluation, and intervention, a prompt for the VA Suicide Risk Management Consultation service was also included on the back page.
Creating a Shared and Local Resource
This clinical resource was developed to establish a centralized, shared, local resource available to VA ED HCPs who lacked knowledge of available services or reported discomfort conducting suicide risk screening for veterans experiencing homelessness. In many cases, ED referrals to homeless programs and suicide prevention care was assigned to a single individual, often a nurse or social worker. As a result, an undue amount of work and strain was placed on these individuals, as this forced them to act as the sole bridge between care in the ED and postdischarge social (eg, homeless programs) and mental health (eg, suicide prevention) services. The creation of a unified, easily accessible document aimed to distribute this responsibility more equitably across ED staff.
DISCUSSION
This project intended to develop a clinician resource to support VA ED clinicians caring for veterans experiencing homelessness and their access to services postdischarge. Qualitative interviews provided insights into the burnout and compassion fatigue present in these settings, as well as the challenges and needs regarding knowledge of local and VA services. Emphasis was placed on leveraging extant resources and subject matter expertise to develop a resource capable of providing brief and informative guidance.
This resource is particularly relevant for HCPs new to the VA, including trainees and new hires, who may be less aware of VA and local social services. It has the potential to reduce the burden on VA ED staff to provide guidance and recommendations surrounding postdischarge social services. The resource acknowledges homeless programming focused on social determinants of health that can destabilize housing (eg, legal or occupational challenges). This can incentivize clinicians to discuss these programs with veterans to facilitate their ability to navigate complex health and psychosocial challenges.
HCPs interviewed for this study indicated their apprehension regarding suicide risk screening and evaluation, a process currently mandated within VA ED settings.26 This may be compounded among HCPs with minimal mental health training or those who have worked in community-based settings where such screening and evaluation efforts are not required. The resource reminds clinicians of available VA consultation services, which can provide additional training, clinical guidance, and review of existing local ED processes.
While the resource was directly informed by qualitative interviews conducted with VA emergency service HCPs and developed through an iterative process with SMEs, further research is necessary to determine its effectiveness at increasing access to health and social services among veterans experiencing homelessness. The resource has not been used by HCPs working in these settings to examine uptake or sustained use, nor clinicians’ perceptions of its utility, including acceptability and feasibility; these are important next steps to understand if the resource is functioning as intended.
Compassion fatigue, as well as associated sequelae (eg, burnout, distress, and psychiatric symptoms), is well-documented among individuals working with individuals experiencing homelessness, including VA HCPs.27-30 Such experiences are likely driven by several factors, including the clinical complexity and service needs of this veteran population. Although compassion fatigue was noted by many clinicians interviewed for this study, it is unclear if the resource alone would address factors driving compassion fatigue, or if additional programming or services may be necessary.
Limitations
The resource requires local HCPs to routinely update its content (eg, establishment of a new emergency shelter in the community or change in hours or contact information of an existing one), which may be challenging. This is especially true as it relates to community resources, which may be more likely to change than national VA programming.
This resource was initially developed following qualitative interviews with a small sample of VA HCPs (explicitly those working within ED settings) and may not be representative of all HCPs engaged in VA care with veterans experiencing homelessness. The perspectives and experiences of those interviewed do not represent the views of all VA ED HCPs and may differ from the perspectives of those in regions with unique cultural and regional considerations.31
Given that most of the interviewees were social workers in EDs engaged in care for veterans experiencing homelessness, these findings and informational needs may differ among other types of HCPs who provide services for veterans experiencing homelessness in other settings. Content in the resource was included based on clinician input, and may not reflect the perspectives of veterans, who may perceive some resources as more important (eg, access to primary care or dental services).28
CONCLUSIONS
This project represents the culmination of qualitative interviews and SME input to develop a free-to-use clinician resource to facilitate service delivery and connection to services following discharge from VA EDs for veterans experiencing homelessness. Serving as a template, this resource can be customized to increase knowledge of local VA and community resources to support these individuals. Continued refinement and piloting of this resource to evaluate acceptability, implementation barriers, and use remains warranted.
- Holliday R, Kinney AR, Smith AA, et al. A latent class analysis to identify subgroups of VHA using homeless veterans at greater risk for suicide mortality. J Affect Disord. 2022;315:162-167. doi:10.1016/j.jad.2022.07.062
- Weber J, Lee RC, Martsolf D. Understanding the health of veterans who are homeless: a review of the literature. Public Health Nurs. 2017;34(5):505-511. doi:10.1111/phn.12338
- Holliday R, Desai A, Stimmel M, Liu S, Monteith LL, Stewart KE. Meeting the health and social service needs of veterans who interact with the criminal justice system and experience homelessness: a holistic conceptualization and recommendations for tailoring care. Curr Treat Options Psychiatry. 2022;9(3):174-185. doi:10.1007/s40501-022-00275-1
- Holliday R, Desai A, Gerard G, Liu S, Stimmel M. Understanding the intersection of homelessness and justice involvement: enhancing veteran suicide prevention through VA programming. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
- Kushel MB, Perry S, Bangsberg D, Clark R, Moss AR. Emergency department use among the homeless and marginally housed: results from a community-based study. Am J Public Health. 2002;92(5):778-784. doi:10.2105/ajph.92.5.778
- Tsai J, Doran KM, Rosenheck RA. When health insurance is not a factor: national comparison of homeless and nonhomeless US veterans who use Veterans Affairs emergency departments. Am J Public Health. 2013;103(Suppl 2):S225-S231. doi:10.2105/AJPH.2013.301307
- Doran KM, Raven MC, Rosenheck RA. What drives frequent emergency department use in an integrated health system? National data from the Veterans Health Administration. Ann Emerg Med. 2013;62(2):151-159. doi:10.1016/j.annemergmed.2013.02.016
- Tsai J, Rosenheck RA. Risk factors for ED use among homeless veterans. Am J Emerg Med. 2013;31(5):855-858. doi:10.1016/j.ajem.2013.02.046
- Nelson RE, Suo Y, Pettey W, et al. Costs associated with health care services accessed through VA and in the community through Medicare for veterans experiencing homelessness. Health Serv Res. 2018;53(Suppl 3):5352-5374. doi:10.1111/1475-6773.13054
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Decker H, Raguram M, Kanzaria HK, Duke M, Wick E. Provider perceptions of challenges and facilitators to surgical care in unhoused patients: a qualitative analysis. Surgery. 2024;175(4):1095-1102. doi:10.1016/j.surg.2023.11.009
- Panushka KA, Kozlowski Z, Dalessandro C, Sanders JN, Millar MM, Gawron LM. “It’s not a top priority”: a qualitative analysis of provider views on barriers to reproductive healthcare provision for homeless women in the United States. Soc Work Public Health. 2023;38(5 -8):428-436. doi:10.1080/19371918.2024.2315180
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893-1907. doi:10.1007/s11135-017-0574-8
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753-1760. doi:10.1177/1049732315617444
- Assarroudi A, Heshmati Nabavi F, Armat MR, Ebadi A, Vaismoradi M. Directed qualitative content analysis: the description and elaboration of its underpinning methods and data analysis process. J Res Nurs. 2018;23(1):42-55. doi:10.1177/1744987117741667
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
- Goldsmith LJ. Using Framework Analysis in Applied Qualitative Research. Qual Rep. 2021;26(6):2061-2076. doi:10.46743/2160-3715/2021.5011
- Tufford L, Newman P. Bracketing in qualitative research. Qual Soc Work. 2012;11(1):80-96.
- Dodgson JE. Reflexivity in Qualitative Research. J Hum Lact. 2019;35(2):220-222. doi:10.1177/0890334419830990
- Hevner AR. A three cycle view of design science research. Scand J Inf Syst. 2007;19(2):4.
- Farao J, Malila B, Conrad N, Mutsvangwa T, Rangaka MX, Douglas TS. A user-centred design frame work for mHealth. PLOS ONE. 2020;15(8):e0237910. doi:10.1371/journal.pone.0237910
- Hoffberg AS, Spitzer E, Mackelprang JL, Farro SA, Brenner LA. Suicidal Self-Directed Violence Among Homeless US Veterans: A Systematic Review. Suicide Life Threat Behav. 2018;48(4):481-498. doi:10.1111/sltb.12369
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and lowincome Veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Holliday R, Hostetter T, Brenner LA, Bahraini N, Tsai J. Suicide risk screening and evaluation among patients accessing VHA services and identified as being newly homeless. Health Serv Res. 2024;59(5):e14301. doi:10.1111/1475-6773.14301
- Waegemakers Schiff J, Lane AM. PTSD symptoms, vicarious traumatization, and burnout in front line workers in the homeless sector. Community Ment Health J. 2019;55(3):454-462. doi:10.1007/s10597-018-00364-7
- Steenekamp BL, Barker SL. Exploring the experiences of compassion fatigue amongst peer support workers in homelessness services. Community Ment Health J. 2024;60(4):772-783. doi:10.1007/s10597-024-01234-1
- Perez S, Kerman N, Dej E, et al. When I can’t help, I suffer: a scoping review of moral distress in service providers working with persons experiencing homelessness. J Ment Health. Published online 2024:1-16. doi:10.1080/09638237.2024.2426986
- Monteith LL, Holliday R, Christe’An DI, Sherrill A, Brenner LA, Hoffmire CA. Suicide risk and prevention in Guam: clinical and research considerations and a call to action. Asian J Psychiatry. 2023;83:103546. doi:10.1016/j.ajp.2023.103546
- Surís A, Holliday R, Hooshyar D, et al. Development and implementation of a homeless mobile medical/mental veteran intervention. Fed Pract. 2017;34(9):18.
- Holliday R, Kinney AR, Smith AA, et al. A latent class analysis to identify subgroups of VHA using homeless veterans at greater risk for suicide mortality. J Affect Disord. 2022;315:162-167. doi:10.1016/j.jad.2022.07.062
- Weber J, Lee RC, Martsolf D. Understanding the health of veterans who are homeless: a review of the literature. Public Health Nurs. 2017;34(5):505-511. doi:10.1111/phn.12338
- Holliday R, Desai A, Stimmel M, Liu S, Monteith LL, Stewart KE. Meeting the health and social service needs of veterans who interact with the criminal justice system and experience homelessness: a holistic conceptualization and recommendations for tailoring care. Curr Treat Options Psychiatry. 2022;9(3):174-185. doi:10.1007/s40501-022-00275-1
- Holliday R, Desai A, Gerard G, Liu S, Stimmel M. Understanding the intersection of homelessness and justice involvement: enhancing veteran suicide prevention through VA programming. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
- Kushel MB, Perry S, Bangsberg D, Clark R, Moss AR. Emergency department use among the homeless and marginally housed: results from a community-based study. Am J Public Health. 2002;92(5):778-784. doi:10.2105/ajph.92.5.778
- Tsai J, Doran KM, Rosenheck RA. When health insurance is not a factor: national comparison of homeless and nonhomeless US veterans who use Veterans Affairs emergency departments. Am J Public Health. 2013;103(Suppl 2):S225-S231. doi:10.2105/AJPH.2013.301307
- Doran KM, Raven MC, Rosenheck RA. What drives frequent emergency department use in an integrated health system? National data from the Veterans Health Administration. Ann Emerg Med. 2013;62(2):151-159. doi:10.1016/j.annemergmed.2013.02.016
- Tsai J, Rosenheck RA. Risk factors for ED use among homeless veterans. Am J Emerg Med. 2013;31(5):855-858. doi:10.1016/j.ajem.2013.02.046
- Nelson RE, Suo Y, Pettey W, et al. Costs associated with health care services accessed through VA and in the community through Medicare for veterans experiencing homelessness. Health Serv Res. 2018;53(Suppl 3):5352-5374. doi:10.1111/1475-6773.13054
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Decker H, Raguram M, Kanzaria HK, Duke M, Wick E. Provider perceptions of challenges and facilitators to surgical care in unhoused patients: a qualitative analysis. Surgery. 2024;175(4):1095-1102. doi:10.1016/j.surg.2023.11.009
- Panushka KA, Kozlowski Z, Dalessandro C, Sanders JN, Millar MM, Gawron LM. “It’s not a top priority”: a qualitative analysis of provider views on barriers to reproductive healthcare provision for homeless women in the United States. Soc Work Public Health. 2023;38(5 -8):428-436. doi:10.1080/19371918.2024.2315180
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893-1907. doi:10.1007/s11135-017-0574-8
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753-1760. doi:10.1177/1049732315617444
- Assarroudi A, Heshmati Nabavi F, Armat MR, Ebadi A, Vaismoradi M. Directed qualitative content analysis: the description and elaboration of its underpinning methods and data analysis process. J Res Nurs. 2018;23(1):42-55. doi:10.1177/1744987117741667
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
- Goldsmith LJ. Using Framework Analysis in Applied Qualitative Research. Qual Rep. 2021;26(6):2061-2076. doi:10.46743/2160-3715/2021.5011
- Tufford L, Newman P. Bracketing in qualitative research. Qual Soc Work. 2012;11(1):80-96.
- Dodgson JE. Reflexivity in Qualitative Research. J Hum Lact. 2019;35(2):220-222. doi:10.1177/0890334419830990
- Hevner AR. A three cycle view of design science research. Scand J Inf Syst. 2007;19(2):4.
- Farao J, Malila B, Conrad N, Mutsvangwa T, Rangaka MX, Douglas TS. A user-centred design frame work for mHealth. PLOS ONE. 2020;15(8):e0237910. doi:10.1371/journal.pone.0237910
- Hoffberg AS, Spitzer E, Mackelprang JL, Farro SA, Brenner LA. Suicidal Self-Directed Violence Among Homeless US Veterans: A Systematic Review. Suicide Life Threat Behav. 2018;48(4):481-498. doi:10.1111/sltb.12369
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and lowincome Veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Holliday R, Hostetter T, Brenner LA, Bahraini N, Tsai J. Suicide risk screening and evaluation among patients accessing VHA services and identified as being newly homeless. Health Serv Res. 2024;59(5):e14301. doi:10.1111/1475-6773.14301
- Waegemakers Schiff J, Lane AM. PTSD symptoms, vicarious traumatization, and burnout in front line workers in the homeless sector. Community Ment Health J. 2019;55(3):454-462. doi:10.1007/s10597-018-00364-7
- Steenekamp BL, Barker SL. Exploring the experiences of compassion fatigue amongst peer support workers in homelessness services. Community Ment Health J. 2024;60(4):772-783. doi:10.1007/s10597-024-01234-1
- Perez S, Kerman N, Dej E, et al. When I can’t help, I suffer: a scoping review of moral distress in service providers working with persons experiencing homelessness. J Ment Health. Published online 2024:1-16. doi:10.1080/09638237.2024.2426986
- Monteith LL, Holliday R, Christe’An DI, Sherrill A, Brenner LA, Hoffmire CA. Suicide risk and prevention in Guam: clinical and research considerations and a call to action. Asian J Psychiatry. 2023;83:103546. doi:10.1016/j.ajp.2023.103546
- Surís A, Holliday R, Hooshyar D, et al. Development and implementation of a homeless mobile medical/mental veteran intervention. Fed Pract. 2017;34(9):18.
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
VA Pays Billions for Costs Shifted From Medicare
In Fiscal Year (FY) 2023, > 40% of veterans enrolled by the US Department of Veterans Affairs (VA) received care from private practice, mainly for emergency services. Costs associated with that care have shifted from Medicare to the VA to the tune of billions of dollars, according to a recent study published in JAMA Health Forum.
The expenses are a result of the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act of 2018, which established the Veterans Community Care Program (VCCP) and allowed the VA to contract with private clinicians. This provided veterans enrolled in both the Veterans Health Administration (VHA) and Medicare to have 2 government sources of health care financing. The VHA is billed if the veteran receives care at one of its facilities or is referred to a community facility; Medicare is billed only if the veteran is treated for a service not covered by VHA.
These shifts are concerning, according to Kenneth W. Kizer, MD, MPH, and Said Ibrahim, MD, MPH. In an accompanying editorial, they outline how the changes affect whether VHA care will have adequate funding to provide care for the additional 740,000 enrollees who have entered the system in the past 2 years.
“This has created a $12 billion medical care budget shortfall for FY 2024,” Kizer and Ibrahim argue. The resulting “substantial budgetary tumult … is adversely impacting the front lines of care delivery at individual VA facilities, leading to delays in hiring caregivers and impeding access to VA care and timely care delivery, as well as greatly straining the traditional roles of VA staff and clinicians trying to manage the challenging cross-system referral processes.”
The study calculated the number of yearly emergency department (ED) visits per 1000 veterans in Medicare overall and by VA ED visits, VA-purchased community ED visits, and Medicare-purchased community ED visits. Estimated total costs shifted from Medicare to the VA after the MISSION Act between 2016 and 2021 were then calculated.
Of the 4,960,189 VA and Medicare enrollees in 2016, 37.0% presented to the ED at least once. Of the 4,837,436 dual enrollees in 2021, 37.6% presented to the ED at least once. ED visits increased 8%, from 820 per 1000 veterans in 2016, to 886 per 1000 veterans in 2019. The COVID-19 pandemic caused a dip in ED visits in 2020 by veterans (769 per 1000), but the number rose 2021 (852 per 1000 veterans).
Between 2016 and 2021, the percentage of VA-purchased community ED visits more than doubled, from 8.0% to 21.1%, while Medicare-purchased community ED visits dropped from 65.2% to 52.6%. Patterns were similar among veterans enrolled in traditional Medicare vs Medicare Advantage (MA). The study estimated that in 2021 at least $2 billion of VA community ED spending was due to payer shift from Medicare.
The shift is “particularly concerning” among veterans enrolled in MA since insurance plans receive capitated payments regardless of actual use of VA- or Medicare-covered services. However, the study’s observational design “limited our ability to infer causality between MISSION Act implementation and payer change.”
The cost shifting is “symptomatic of the fiscally undisciplined implementation of the VCCP and the lack of financially sound policy on payment for VA-Medicare dual enrollees,” according to Drs. Kizer and Ibrahim. “Addressing this matter seems especially important in light of numerous studies showing that the quality of community care often may be inferior to VA care, as well as less timely.”
Kizer and Ibrahim point out that when a veteran who is jointly enrolled in VA and MA plans receives care from the VA, the VA incurs the cost of providing those services even though the MA plan is being paid to provide them. The VA is not allowed to recoup its costs from Medicare. Thus, the government pays twice for the care of the same person.
A recent study reported > $78 billion in duplicate VA-MA spending between 2011 and 2020, with $12 billion in FY 2020. Kizer and Ibrahim suggest the current VA-MA duplicate spending is likely to be significantly more than the reported amounts.
“[No] evidence shows that this duplicate spending yields a demonstrable health benefit for veterans, although undoubtedly it benefits the financial well-being of the MA plans,” they write.
It’s a “challenging policy and programmatic conundrum,” the co-authors say, noting that eligible veterans often have military service-related conditions that the VA is uniquely experienced in treating.
“Policies and programs need to be designed and aligned to ensure that veterans have timely access to emergency and other services and that rising community care costs do not jeopardize veterans’ choice to access and use VA services, nor compromise the nationally vital roles of the VA in graduate medical education and other health professional training, research, and emergency preparedness.”
In Fiscal Year (FY) 2023, > 40% of veterans enrolled by the US Department of Veterans Affairs (VA) received care from private practice, mainly for emergency services. Costs associated with that care have shifted from Medicare to the VA to the tune of billions of dollars, according to a recent study published in JAMA Health Forum.
The expenses are a result of the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act of 2018, which established the Veterans Community Care Program (VCCP) and allowed the VA to contract with private clinicians. This provided veterans enrolled in both the Veterans Health Administration (VHA) and Medicare to have 2 government sources of health care financing. The VHA is billed if the veteran receives care at one of its facilities or is referred to a community facility; Medicare is billed only if the veteran is treated for a service not covered by VHA.
These shifts are concerning, according to Kenneth W. Kizer, MD, MPH, and Said Ibrahim, MD, MPH. In an accompanying editorial, they outline how the changes affect whether VHA care will have adequate funding to provide care for the additional 740,000 enrollees who have entered the system in the past 2 years.
“This has created a $12 billion medical care budget shortfall for FY 2024,” Kizer and Ibrahim argue. The resulting “substantial budgetary tumult … is adversely impacting the front lines of care delivery at individual VA facilities, leading to delays in hiring caregivers and impeding access to VA care and timely care delivery, as well as greatly straining the traditional roles of VA staff and clinicians trying to manage the challenging cross-system referral processes.”
The study calculated the number of yearly emergency department (ED) visits per 1000 veterans in Medicare overall and by VA ED visits, VA-purchased community ED visits, and Medicare-purchased community ED visits. Estimated total costs shifted from Medicare to the VA after the MISSION Act between 2016 and 2021 were then calculated.
Of the 4,960,189 VA and Medicare enrollees in 2016, 37.0% presented to the ED at least once. Of the 4,837,436 dual enrollees in 2021, 37.6% presented to the ED at least once. ED visits increased 8%, from 820 per 1000 veterans in 2016, to 886 per 1000 veterans in 2019. The COVID-19 pandemic caused a dip in ED visits in 2020 by veterans (769 per 1000), but the number rose 2021 (852 per 1000 veterans).
Between 2016 and 2021, the percentage of VA-purchased community ED visits more than doubled, from 8.0% to 21.1%, while Medicare-purchased community ED visits dropped from 65.2% to 52.6%. Patterns were similar among veterans enrolled in traditional Medicare vs Medicare Advantage (MA). The study estimated that in 2021 at least $2 billion of VA community ED spending was due to payer shift from Medicare.
The shift is “particularly concerning” among veterans enrolled in MA since insurance plans receive capitated payments regardless of actual use of VA- or Medicare-covered services. However, the study’s observational design “limited our ability to infer causality between MISSION Act implementation and payer change.”
The cost shifting is “symptomatic of the fiscally undisciplined implementation of the VCCP and the lack of financially sound policy on payment for VA-Medicare dual enrollees,” according to Drs. Kizer and Ibrahim. “Addressing this matter seems especially important in light of numerous studies showing that the quality of community care often may be inferior to VA care, as well as less timely.”
Kizer and Ibrahim point out that when a veteran who is jointly enrolled in VA and MA plans receives care from the VA, the VA incurs the cost of providing those services even though the MA plan is being paid to provide them. The VA is not allowed to recoup its costs from Medicare. Thus, the government pays twice for the care of the same person.
A recent study reported > $78 billion in duplicate VA-MA spending between 2011 and 2020, with $12 billion in FY 2020. Kizer and Ibrahim suggest the current VA-MA duplicate spending is likely to be significantly more than the reported amounts.
“[No] evidence shows that this duplicate spending yields a demonstrable health benefit for veterans, although undoubtedly it benefits the financial well-being of the MA plans,” they write.
It’s a “challenging policy and programmatic conundrum,” the co-authors say, noting that eligible veterans often have military service-related conditions that the VA is uniquely experienced in treating.
“Policies and programs need to be designed and aligned to ensure that veterans have timely access to emergency and other services and that rising community care costs do not jeopardize veterans’ choice to access and use VA services, nor compromise the nationally vital roles of the VA in graduate medical education and other health professional training, research, and emergency preparedness.”
In Fiscal Year (FY) 2023, > 40% of veterans enrolled by the US Department of Veterans Affairs (VA) received care from private practice, mainly for emergency services. Costs associated with that care have shifted from Medicare to the VA to the tune of billions of dollars, according to a recent study published in JAMA Health Forum.
The expenses are a result of the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act of 2018, which established the Veterans Community Care Program (VCCP) and allowed the VA to contract with private clinicians. This provided veterans enrolled in both the Veterans Health Administration (VHA) and Medicare to have 2 government sources of health care financing. The VHA is billed if the veteran receives care at one of its facilities or is referred to a community facility; Medicare is billed only if the veteran is treated for a service not covered by VHA.
These shifts are concerning, according to Kenneth W. Kizer, MD, MPH, and Said Ibrahim, MD, MPH. In an accompanying editorial, they outline how the changes affect whether VHA care will have adequate funding to provide care for the additional 740,000 enrollees who have entered the system in the past 2 years.
“This has created a $12 billion medical care budget shortfall for FY 2024,” Kizer and Ibrahim argue. The resulting “substantial budgetary tumult … is adversely impacting the front lines of care delivery at individual VA facilities, leading to delays in hiring caregivers and impeding access to VA care and timely care delivery, as well as greatly straining the traditional roles of VA staff and clinicians trying to manage the challenging cross-system referral processes.”
The study calculated the number of yearly emergency department (ED) visits per 1000 veterans in Medicare overall and by VA ED visits, VA-purchased community ED visits, and Medicare-purchased community ED visits. Estimated total costs shifted from Medicare to the VA after the MISSION Act between 2016 and 2021 were then calculated.
Of the 4,960,189 VA and Medicare enrollees in 2016, 37.0% presented to the ED at least once. Of the 4,837,436 dual enrollees in 2021, 37.6% presented to the ED at least once. ED visits increased 8%, from 820 per 1000 veterans in 2016, to 886 per 1000 veterans in 2019. The COVID-19 pandemic caused a dip in ED visits in 2020 by veterans (769 per 1000), but the number rose 2021 (852 per 1000 veterans).
Between 2016 and 2021, the percentage of VA-purchased community ED visits more than doubled, from 8.0% to 21.1%, while Medicare-purchased community ED visits dropped from 65.2% to 52.6%. Patterns were similar among veterans enrolled in traditional Medicare vs Medicare Advantage (MA). The study estimated that in 2021 at least $2 billion of VA community ED spending was due to payer shift from Medicare.
The shift is “particularly concerning” among veterans enrolled in MA since insurance plans receive capitated payments regardless of actual use of VA- or Medicare-covered services. However, the study’s observational design “limited our ability to infer causality between MISSION Act implementation and payer change.”
The cost shifting is “symptomatic of the fiscally undisciplined implementation of the VCCP and the lack of financially sound policy on payment for VA-Medicare dual enrollees,” according to Drs. Kizer and Ibrahim. “Addressing this matter seems especially important in light of numerous studies showing that the quality of community care often may be inferior to VA care, as well as less timely.”
Kizer and Ibrahim point out that when a veteran who is jointly enrolled in VA and MA plans receives care from the VA, the VA incurs the cost of providing those services even though the MA plan is being paid to provide them. The VA is not allowed to recoup its costs from Medicare. Thus, the government pays twice for the care of the same person.
A recent study reported > $78 billion in duplicate VA-MA spending between 2011 and 2020, with $12 billion in FY 2020. Kizer and Ibrahim suggest the current VA-MA duplicate spending is likely to be significantly more than the reported amounts.
“[No] evidence shows that this duplicate spending yields a demonstrable health benefit for veterans, although undoubtedly it benefits the financial well-being of the MA plans,” they write.
It’s a “challenging policy and programmatic conundrum,” the co-authors say, noting that eligible veterans often have military service-related conditions that the VA is uniquely experienced in treating.
“Policies and programs need to be designed and aligned to ensure that veterans have timely access to emergency and other services and that rising community care costs do not jeopardize veterans’ choice to access and use VA services, nor compromise the nationally vital roles of the VA in graduate medical education and other health professional training, research, and emergency preparedness.”
Meet the JCOM Author with Dr. Barkoudah: The Hospitalist Triage Role for Reducing Admission Delays
The Hospitalist Triage Role for Reducing Admission Delays: Impacts on Throughput, Quality, Interprofessional Practice, and Clinician Experience of Care
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).
There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.
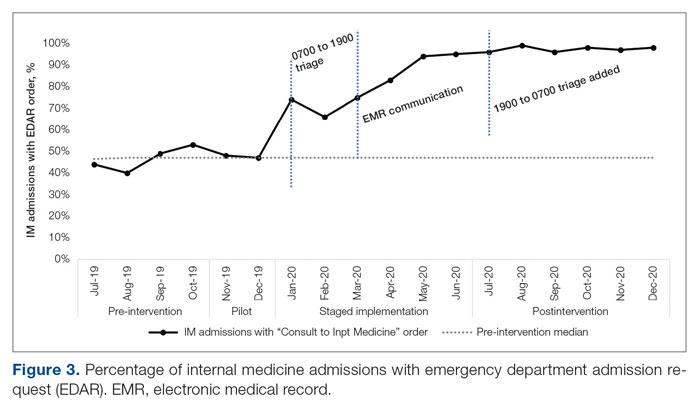
Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions
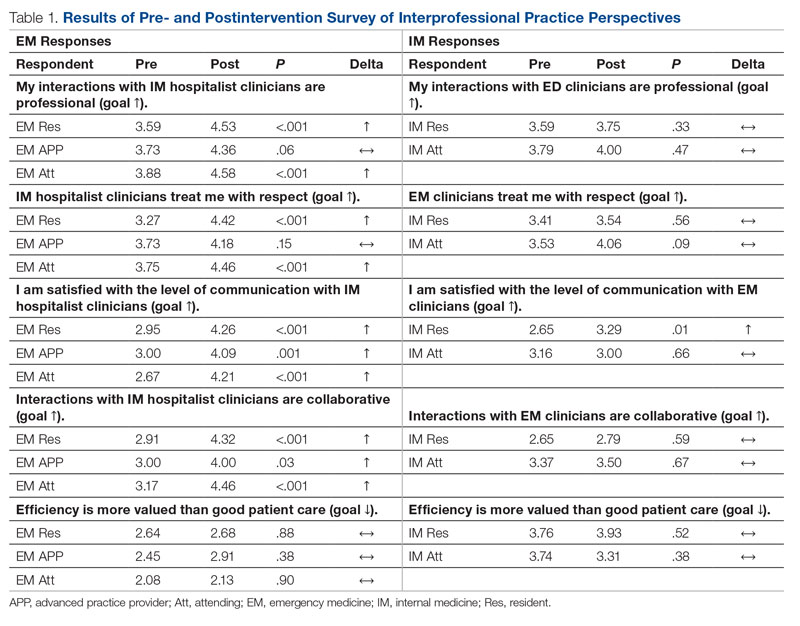
For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.
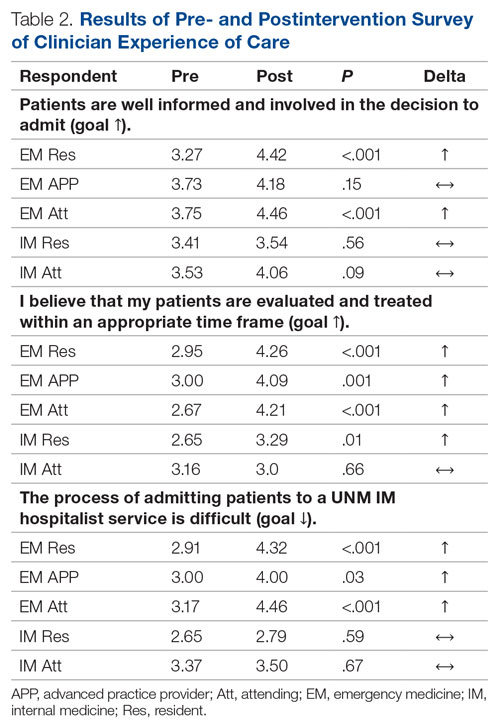
Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
Redesign of Health Care Systems to Reduce Diagnostic Errors: Leveraging Human Experience and Artificial Intelligence
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).
Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; [email protected]
Disclosures: None reported.
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).

Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; [email protected]
Disclosures: None reported.
From the Institute for Healthcare Improvement, Boston, MA (Dr. Abid); Continuous Quality Improvement and Patient Safety Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Abid); Primary and Secondary Healthcare Department, Government of Punjab, Lahore, Pakistan (Dr. Ahmed); Infection Prevention and Control Department, Armed Forces Hospitals Taif Region, Taif, Saudi Arabia (Dr. Din); Internal Medicine Department, Greater Baltimore Medical Center, Baltimore, MD (Dr. Abid); Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX (Dr. Ratnani).
Diagnostic errors are defined by the National Academies of Sciences, Engineering, and Medicine (NASEM) as the failure to either establish an accurate and timely explanation of the patient’s health problem(s) or communicate that explanation to the patient.1 According to a report by the Institute of Medicine, diagnostic errors account for a substantial number of adverse events in health care, affecting an estimated 12 million Americans each year.1 Diagnostic errors are a common and serious issue in health care systems, with studies estimating that 5% to 15% of all diagnoses are incorrect.1 Such errors can result in unnecessary treatments, delays in necessary treatments, and harm to patients. The high prevalence of diagnostic errors in primary care has been identified as a global issue.2 While many factors contribute to diagnostic errors, the complex nature of health care systems, the limited processing capacity of human cognition, and deficiencies in interpersonal patient-clinician communication are primary contributors.3,4
Discussions around the redesign of health care systems to reduce diagnostic errors have been at the forefront of medical research for years.2,4 To decrease diagnostic errors in health care, a comprehensive strategy is necessary. This strategy should focus on utilizing both human experience (HX) in health care and artificial intelligence (AI) technologies to transform health care systems into proactive, patient-centered, and safer systems, specifically concerning diagnostic errors.1
Human Experience and Diagnostic Errors
The role of HX in health care cannot be overstated. The HX in health care integrates the sum of all interactions, every encounter among patients, families and care partners, and the health care workforce.5 Patients and their families have a unique perspective on their health care experiences that can provide valuable insight into potential diagnostic errors.6 The new definition of diagnostic errors introduced in the 2015 NASEM report emphasized the significance of effective communication during the diagnostic procedure.1 Engaging patients and their families in the diagnostic process can improve communication, improve diagnostic accuracy, and help to identify errors before they cause harm.7 However, many patients and families feel that they are not listened to or taken seriously by health care providers, and may not feel comfortable sharing information that they feel is important.8 To address this, health care systems can implement programs that encourage patients and families to be more engaged in the diagnostic process, such as shared decision-making, patient portals, and patient and family advisory councils.9 Health care systems must prioritize patient-centered care, teamwork, and communication. Patients and their families must be actively engaged in their care, and health care providers must be willing to work collaboratively and listen to patients’ concerns.6,10
Health care providers also bring their own valuable experiences and expertise to the diagnostic process, as they are often the ones on the front lines of patient care. However, health care providers may not always feel comfortable reporting errors or near misses, and may not have the time or resources to participate in quality improvement initiatives. To address this, health care systems can implement programs that encourage providers to report errors and near misses, such as anonymous reporting systems, just-culture initiatives, and peer review.11 Creating a culture of teamwork and collaboration among health care providers can improve the accuracy of diagnoses and reduce the risk of errors.12
A key factor in utilizing HX to reduce diagnostic errors is effective communication. Communication breakdowns among health care providers, patients, and their families are a common contributing factor resulting in diagnostic errors.2 Strategies to improve communication include using clear and concise language, involving patients and their families in the decision-making process, and utilizing electronic health records (EHRs) to ensure that all health care providers have access to relevant, accurate, and up-to-date patient information.4,13,14
Another important aspect of utilizing HX in health care to reduce diagnostic errors is the need to recognize and address cognitive biases that may influence diagnostic decisions.3 Cognitive biases are common in health care and can lead to errors in diagnosis. For example, confirmation bias, which is the tendency to look for information that confirms preexisting beliefs, can lead providers to overlook important diagnostic information.15 Biases such as anchoring bias, premature closure, and confirmation bias can lead to incorrect diagnoses and can be difficult to recognize and overcome. Addressing cognitive biases requires a commitment to self-reflection and self-awareness among health care providers as well as structured training of health care providers to improve their diagnostic reasoning skills and reduce the risk of cognitive errors.15 By implementing these strategies around HX in health care, health care systems can become more patient-centered and reduce the likelihood of diagnostic errors (Figure).

Artificial Intelligence and Diagnostic Errors
Artificial intelligence has the potential to significantly reduce diagnostic errors in health care (Figure), and its role in health care is rapidly expanding. AI technologies such as machine learning (ML) and natural language processing (NLP) have the potential to significantly reduce diagnostic errors by augmenting human cognition and improving access to relevant patient data.1,16 Machine learning algorithms can analyze large amounts of patient data sets to identify patterns and risk factors and predict patient outcomes, which can aid health care providers in making accurate diagnoses.17 Artificial intelligence can also help to address some of the communication breakdowns that contribute to diagnostic errors.18 Natural language processing can improve the accuracy of EHR documentation and reduce the associated clinician burden, making it easier for providers to access relevant patient information and communicate more effectively with each other.18
In health care, AI can be used to analyze medical images, laboratory results, genomic data, and EHRs to identify potential diagnoses and flag patients who may be at risk for diagnostic errors. One of the primary benefits of AI in health care is its ability to process large amounts of data quickly and accurately.19 This can be particularly valuable in diagnosing rare or complex conditions. Machine learning algorithms can analyze patient data to identify subtle patterns that may not be apparent to human providers.16 This can lead to earlier and more accurate diagnoses, which can reduce diagnostic errors and improve patient outcomes.17 One example of the application of AI in health care is the use of computer-aided detection (CAD) software to analyze medical images. This software can help radiologists detect abnormalities in medical images that may be missed by the human eye, such as early-stage breast cancer.20 Another example is the use of NLP and ML to analyze unstructured data in EHRs, such as physician notes, to identify potential diagnoses and flag patients who may be at risk for diagnostic errors.21 A recent study showed that using NLP on EHRs for screening and detecting individuals at risk for psychosis can considerably enhance the prognostic accuracy of psychosis risk calculators.22 This can help identify patients who require assessment and specialized care, facilitating earlier detection and potentially improving patient outcomes. On the same note, ML-based severe sepsis prediction algorithms have been shown to reduce the average length of stay and in-hospital mortality rate.23
However, there are also concerns about the use of AI in health care, including the potential for bias and the risk of overreliance on AI. Bias can occur when AI algorithms are trained on data that is not representative of the population being analyzed, leading to inaccurate or unfair results, hence, perpetuating and exacerbating existing biases in health care.24 Over-reliance on AI can occur when health care providers rely too heavily on AI algorithms and fail to consider other important information, such as the lived experience of patients, families, and health care providers. Addressing these concerns will require ongoing efforts to ensure that AI technologies are developed and implemented in an ethical and responsible manner.25
Conclusion
Reducing diagnostic errors is a critical goal for health care systems, and requires a comprehensive approach that utilizes both HX and AI technologies. Engaging patients and their families in the diagnostic process, promoting teamwork and collaboration among health care providers, addressing cognitive biases, and harnessing the power of AI can all contribute to more accurate diagnoses and better patient outcomes. By integrating the lived experience of patients, families, and health care providers with AI technologies, health care systems can be redesigned to become more proactive, safer, and patient-centered in identifying potential health problems and reducing the risk of diagnostic errors, ensuring that patients receive the care they need and deserve.
Corresponding author: Iqbal Ratnani, Department of Anesthesiology and Critical Care, DeBakey Heart and Vascular Center, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030; [email protected]
Disclosures: None reported.
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
1. National Academy of Medicine. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR, eds. National Academies Press; 2015. doi:10.17226/21794
2. Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
3. Croskerry P, Campbell SG, Petrie DA. The challenge of cognitive science for medical diagnosis. Cogn Res Princ Implic. 2023;8(1):13. doi:10.1186/s41235-022-00460-z
4. Dahm MR, Williams M, Crock C. ‘More than words’ - interpersonal communication, cogntive bias and diagnostic errors. Patient Educ Couns. 2022;105(1):252-256. doi:10.1016/j.pec.2021.05.012
5. Wolf JA, Niederhauser V, Marshburn D, LaVela SL. Reexamining “defining patient experience”: The human experience in Healthcare. Patient Experience J. 2021;8(1):16-29. doi:10.35680/2372-0247.1594
6. Sacco AY, Self QR, Worswick EL, et al. Patients’ perspectives of diagnostic error: A qualitative study. J Patient Saf. 2021;17(8):e1759-e1764. doi:10.1097/PTS.0000000000000642
7. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
8. Austin E, LeRouge C, Hartzler AL, Segal C, Lavallee DC. Capturing the patient voice: implementing patient-reported outcomes across the health system. Qual Life Res. 2020;29(2):347-355. doi:10.1007/s11136-019-02320-8
9. Waddell A, Lennox A, Spassova G, Bragge P. Barriers and facilitators to shared decision-making in hospitals from policy to practice: a systematic review. Implement Sci. 2021;16(1):74. doi: 10.1186/s13012-021-01142-y
10. US Preventive Services Task Force. Collaboration and shared decision-making between patients and clinicians in preventive health care decisions and US Preventive Services Task Force Recommendations. JAMA. 2022;327(12):1171-1176. doi:10.1001/jama.2022.3267
11. Reporting patient safety events. Patient Safety Network. Published September 7, 2019. Accessed April 29, 2023. https://psnet.ahrq.gov/primer/reporting-patient-safety-events
12. McLaney E, Morassaei S, Hughes L, et al. A framework for interprofessional team collaboration in a hospital setting: Advancing team competencies and behaviours. Healthc Manage Forum. 2022;35(2):112-117. doi:10.1177/08404704211063584
13. Abid MH, Abid MM, Shahid R, et al. Patient and family engagement during challenging times: what works and what does not? Cureus. 2021;13(5):e14814. doi:10.7759/cureus.14814
14. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, et al. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
15. Watari T, Tokuda Y, Amano Y, et al. Cognitive bias and diagnostic errors among physicians in Japan: A self-reflection survey. Int J Environ Res Public Health. 2022;19(8):4645. doi:10.3390/ijerph19084645
16. Rajkomar A, Oren E, Chen K et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. https://doi.org/10.1038/s41746-018-0029-1
17. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94-98. doi:10.7861/futurehosp.6-2-94
18. Dymek C, Kim B, Melton GB, et al. Building the evidence-base to reduce electronic health record-related clinician burden. J Am Med Inform Assoc. 2021;28(5):1057-1061. doi:10.1093/jamia/ocaa238
19. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi:10.1001/jama.2017.18391
20. Lehman CD, Wellman RD, Buist DS, et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med. 2015;175(11):1828-1837. doi:10.1001/jamainternmed.2015.5231
21. Liao KP, Cai T, Savova GK, et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. BMJ. 2015;350:h1885. doi:10.1136/bmj.h1885
22. Irving J, Patel R, Oliver D, et al. Using natural language processing on electronic health records to enhance detection and prediction of psychosis risk. Schizophr Bull. 2021;47(2):405-414. doi:10.1093/schbul/sbaa126. Erratum in: Schizophr Bull. 2021;47(2):575.
23. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4(1):e000234. doi:10.1136/bmjresp-2017-000234
24. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. doi:10.1126/science.aax2342
25. Ibrahim SA, Pronovost PJ. Diagnostic errors, health disparities, and artificial intelligence: a combination for health or harm? JAMA Health Forum. 2021;2(9):e212430. doi:10.1001/jamahealthforum.2021.2430
Meet the JCOM Author with Dr. Barkoudah: A Multidisciplinary Team–Based Clinical Care Pathway for Infective Endocarditis
Implementation of a Multidisciplinary Team–Based Clinical Care Pathway Is Associated With Increased Surgery Rates for Infective Endocarditis
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.
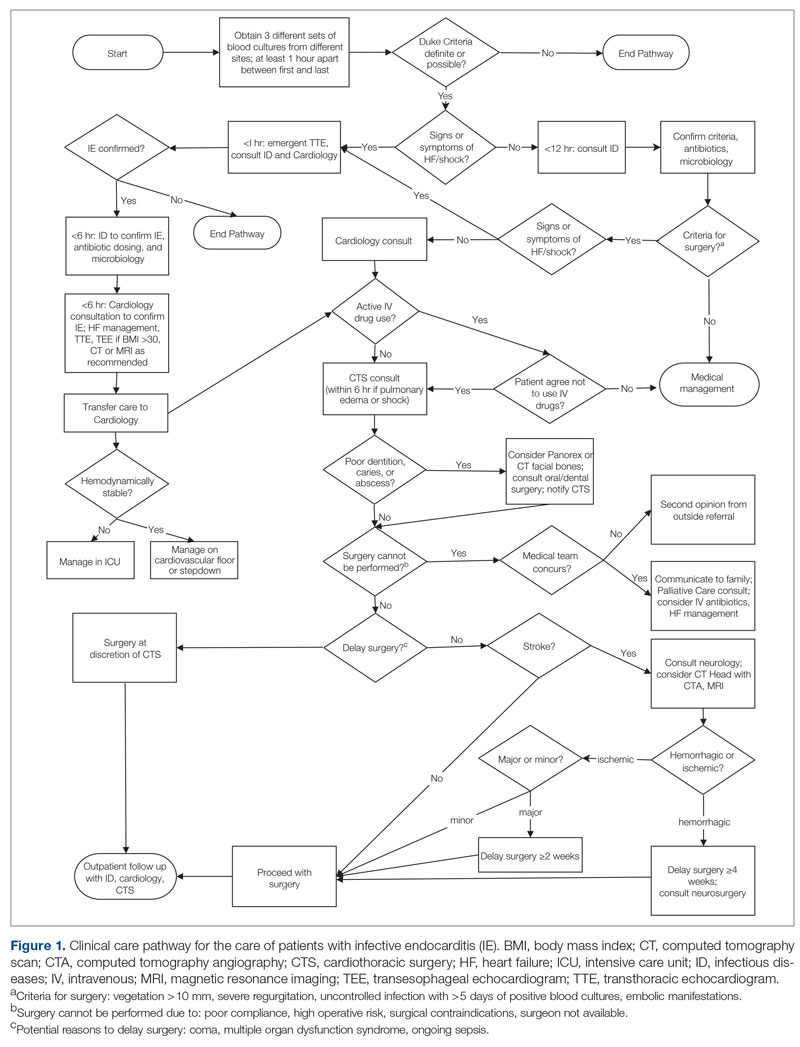
Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.
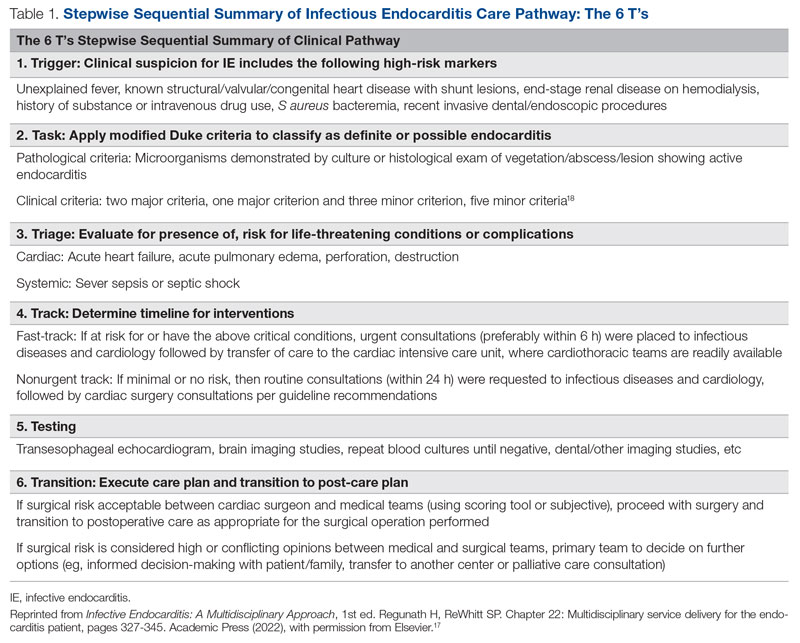
To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.
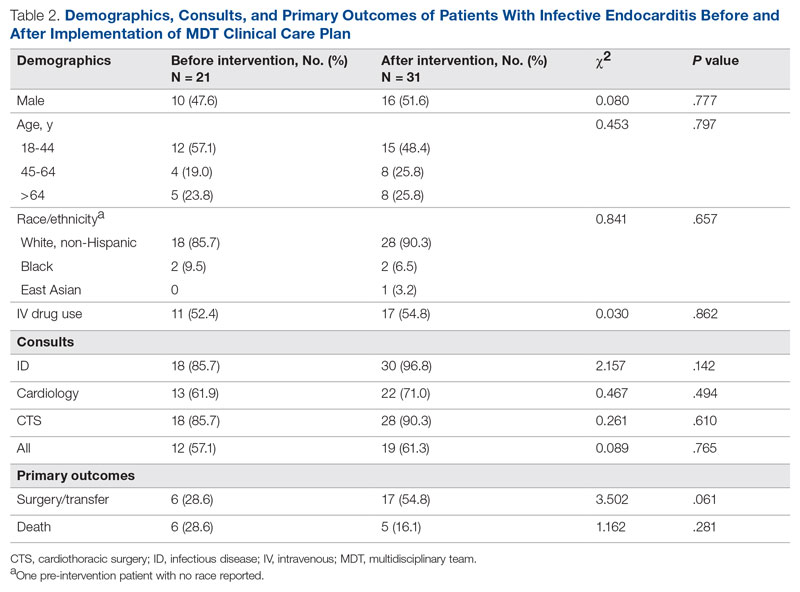
The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).
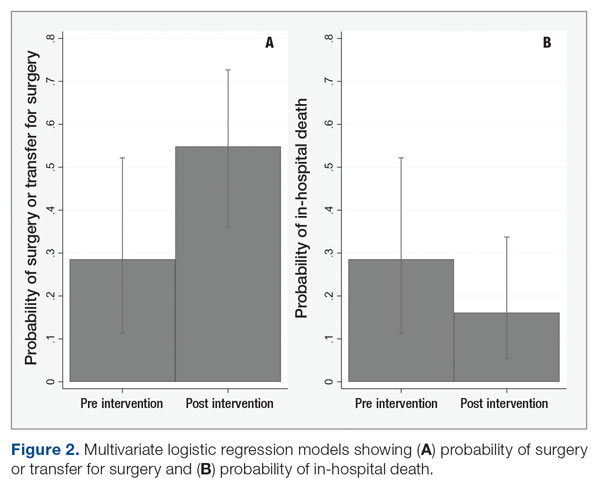
Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
The Shifting Landscape of Thrombolytic Therapy for Acute Ischemic Stroke
Study 1 Overview (Menon et al)
Objective: To determine whether a 0.25 mg/kg dose of intravenous tenecteplase is noninferior to intravenous alteplase 0.9 mg/kg for patients with acute ischemic stroke eligible for thrombolytic therapy.
Design: Multicenter, parallel-group, open-label randomized controlled trial.
Setting and participants: The trial was conducted at 22 primary and comprehensive stroke centers across Canada. A primary stroke center was defined as a hospital capable of offering intravenous thrombolysis to patients with acute ischemic stroke, while a comprehensive stroke center was able to offer thrombectomy services in addition. The involved centers also participated in Canadian quality improvement registries (either Quality Improvement and Clinical Research [QuiCR] or Optimizing Patient Treatment in Major Ischemic Stroke with EVT [OPTIMISE]) that track patient outcomes. Patients were eligible for inclusion if they were aged 18 years or older, had a diagnosis of acute ischemic stroke, presented within 4.5 hours of symptom onset, and were eligible for thrombolysis according to Canadian guidelines.
Patients were randomized in a 1:1 fashion to either intravenous tenecteplase (0.25 mg/kg single dose, maximum of 25 mg) or intravenous alteplase (0.9 mg/kg total dose to a maximum of 90 mg, delivered as a bolus followed by a continuous infusion). A total of 1600 patients were enrolled, with 816 randomly assigned to the tenecteplase arm and 784 to the alteplase arm; 1577 patients were included in the intention-to-treat (ITT) analysis (n = 806 tenecteplase; n = 771 alteplase). The median age of enrollees was 74 years, and 52.1% of the ITT population were men.
Main outcome measures: In the ITT population, the primary outcome measure was a modified Rankin score (mRS) of 0 or 1 at 90 to 120 days post treatment. Safety outcomes included symptomatic intracerebral hemorrhage, orolingual angioedema, extracranial bleeding that required blood transfusion (all within 24 hours of thrombolytic administration), and all-cause mortality at 90 days. The noninferiority threshold for intravenous tenecteplase was set as the lower 95% CI of the difference between the tenecteplase and alteplase groups in the proportion of patients who met the primary outcome exceeding –5%.
Main results: The primary outcome of mRS of either 0 or 1 at 90 to 120 days of treatment occurred in 296 (36.9%) of the 802 patients assigned to tenecteplase and 266 (34.8%) of the 765 patients assigned to alteplase (unadjusted risk difference, 2.1%; 95% CI, –2.6 to 6.9). The prespecified noninferiority threshold was met. There were no significant differences between the groups in rates of intracerebral hemorrhage at 24 hours or 90-day all-cause mortality.
Conclusion: Intravenous tenecteplase is a reasonable alternative to alteplase for patients eligible for thrombolytic therapy.
Study 2 Overview (Wang et al)
Objective: To determine whether tenecteplase (dose 0.25 mg/kg) is noninferior to alteplase in patients with acute ischemic stroke who are within 4.5 hours of symptom onset and eligible for thrombolytic therapy but either refused or were ineligible for endovascular thrombectomy.
Design: Multicenter, prospective, open-label, randomized, controlled noninferiority trial.
Setting and participants: This trial was conducted at 53 centers across China and included patients 18 years of age or older who were within 4.5 hours of symptom onset and were thrombolytic eligible, had a mRS ≤ 1 at enrollment, and had a National Institutes of Health Stroke Scale score between 5 and 25. Eligible participants were randomized 1:1 to either tenecteplase 0.25 mg/kg (maximum dose 25 mg) or alteplase 0.9 mg/kg (maximum dose 90 mg, administered as a bolus followed by infusion). During the enrollment period (June 12, 2021, to May 29, 2022), a total of 1430 participants were enrolled, and, of those, 716 were randomly assigned to tenecteplase and 714 to alteplase. Six patients assigned to tenecteplase and 7 assigned to alteplase did not receive drugs. At 90 days, 5 in the tenecteplase group and 11 in the alteplase group were lost to follow up.
Main outcome measures: The primary efficacy outcome was a mRS of 0 or 1 at 90 days. The primary safety outcome was intracranial hemorrhage within 36 hours. Safety outcomes included parenchymal hematoma 2, as defined by the European Cooperative Acute Stroke Study III; any intracranial or significant hemorrhage, as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria; and death from all causes at 90 days. Noninferiority for tenecteplase would be declared if the lower 97.5% 1-sided CI for the relative risk (RR) for the primary outcome did not cross 0.937.
Main results: In the modified ITT population, the primary outcome occurred in 439 (62%) of the tenecteplase group and 405 (68%) of the alteplase group (RR, 1.07; 95% CI, 0.98-1.16). This met the prespecified margin for noninferiority. Intracranial hemorrhage within 36 hours was experienced by 15 (2%) patients in the tenecteplase group and 13 (2%) in the alteplase group (RR, 1.18; 95% CI, 0.56-2.50). Death at 90 days occurred in 46 (7%) patients in the tenecteplase group and 35 (5%) in the alteplase group (RR, 1.31; 95% CI, 0.86-2.01).
Conclusion: Tenecteplase was noninferior to alteplase in patients with acute ischemic stroke who met criteria for thrombolysis and either refused or were ineligible for endovascular thrombectomy.
Commentary
Alteplase has been FDA-approved for managing acute ischemic stroke since 1996 and has demonstrated positive effects on functional outcomes. Drawbacks of alteplase therapy, however, include bleeding risk as well as cumbersome administration of a bolus dose followed by a 60-minute infusion. In recent years, the question of whether or not tenecteplase could replace alteplase as the preferred thrombolytic for acute ischemic stroke has garnered much attention. Several features of tenecteplase make it an attractive option, including increased fibrin specificity, a longer half-life, and ease of administration as a single, rapid bolus dose. In phase 2 trials that compared tenecteplase 0.25 mg/kg with alteplase, findings suggested the potential for early neurological improvement as well as improved outcomes at 90 days. While the role of tenecteplase in acute myocardial infarction has been well established due to ease of use and a favorable adverse-effect profile,1 there is much less evidence from phase 3 randomized controlled clinical trials to secure the role of tenecteplase in acute ischemic stroke.2
Menon et al attempted to close this gap in the literature by conducting a randomized controlled clinical trial (AcT) comparing tenecteplase to alteplase in a Canadian patient population. The trial's patient population mirrors that of real-world data from global registries in terms of age, sex, and baseline stroke severity. In addition, the eligibility window of 4.5 hours from symptom onset as well as the inclusion and exclusion criteria for therapy are common to those utilized in other countries, making the findings generalizable. There were some limitations to the study, however, including the impact of COVID-19 on recruitment efforts as well as limitations of research infrastructure and staffing, which may have limited enrollment efforts at primary stroke centers. Nonetheless, the authors concluded that their results provide evidence that tenecteplase is comparable to alteplase, with similar functional and safety outcomes.
TRACE-2 focused on an Asian patient population and provided follow up to the dose-ranging TRACE-1 phase 2 trial. TRACE-1 showed that tenecteplase 0.25 mg/kg had a similar safety profile to alteplase 0.9 mg/kg in Chinese patients presenting with acute ischemic stroke. TRACE-2 sought to establish noninferiority of tenecteplase and excluded patients who were ineligible for or refused thrombectomy. Interestingly, the tenecteplase arm, as the authors point out, had numerically greater mortality as well as intracranial hemorrhage, but these differences were not statistically significant between the treatment groups at 90 days. The TRACE-2 results parallel those of AcT, and although there were differences in ethnicity between the 2 trials, the authors cite this as evidence that the results are consistent and provide evidence for the role of tenecteplase in the management of acute ischemic stroke. Limitations of this trial include potential bias from its open-label design, as well as exclusion of patients with more severe strokes eligible for thrombectomy, which may limit generalizability to patients with more disabling strokes who could have a higher risk of intracranial hemorrhage.
Application for Clinical Practice and System Implementation
Across the country, many organizations have adopted the off-label use of tenecteplase for managing fibrinolytic-eligible acute ischemic stroke patients. In most cases, the impetus for change is the ease of dosing and administration of tenecteplase compared to alteplase, while the inclusion and exclusion criteria and overall management remain the same. Timely administration of therapy in stroke is critical. This, along with other time constraints in stroke workflows, the weight-based calculation of alteplase doses, and alteplase’s administration method may lead to medication errors when using this agent to treat patients with acute stroke. The rapid, single-dose administration of tenecteplase removes many barriers that hospitals face when patients may need to be treated and then transferred to another site for further care. Without the worry to “drip and ship,” the completion of administration may allow for timely patient transfer and eliminate the need for monitoring of an infusion during transfer. For some organizations, there may be a potential for drug cost-savings as well as improved metrics, such as door-to-needle time, but the overall effects of switching from alteplase to tenecteplase remain to be seen. Currently, tenecteplase is included in stroke guidelines as a “reasonable choice,” though with a low level of evidence.3 However, these 2 studies support the role of tenecteplase in acute ischemic stroke treatment and may provide a foundation for further studies to establish the role of tenecteplase in the acute ischemic stroke population.
Practice Points
- Tenecteplase may be considered as an alternative to alteplase for acute ischemic stroke for patients who meet eligibility criteria for thrombolytics; this recommendation is included in the most recent stroke guidelines, although tenecteplase has not been demonstrated to be superior to alteplase.
- The ease of administration of tenecteplase as a single intravenous bolus dose represents a benefit compared to alteplase; it is an off-label use, however, and further studies are needed to establish the superiority of tenecteplase in terms of functional and safety outcomes.
– Carol Heunisch, PharmD, BCPS, BCCP
Pharmacy Department, NorthShore–Edward-Elmhurst Health, Evanston, IL
1. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators; F Van De Werf, J Adgey, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354(9180):716-722. doi:10.1016/s0140-6736(99)07403-6
2. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischaemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
Study 1 Overview (Menon et al)
Objective: To determine whether a 0.25 mg/kg dose of intravenous tenecteplase is noninferior to intravenous alteplase 0.9 mg/kg for patients with acute ischemic stroke eligible for thrombolytic therapy.
Design: Multicenter, parallel-group, open-label randomized controlled trial.
Setting and participants: The trial was conducted at 22 primary and comprehensive stroke centers across Canada. A primary stroke center was defined as a hospital capable of offering intravenous thrombolysis to patients with acute ischemic stroke, while a comprehensive stroke center was able to offer thrombectomy services in addition. The involved centers also participated in Canadian quality improvement registries (either Quality Improvement and Clinical Research [QuiCR] or Optimizing Patient Treatment in Major Ischemic Stroke with EVT [OPTIMISE]) that track patient outcomes. Patients were eligible for inclusion if they were aged 18 years or older, had a diagnosis of acute ischemic stroke, presented within 4.5 hours of symptom onset, and were eligible for thrombolysis according to Canadian guidelines.
Patients were randomized in a 1:1 fashion to either intravenous tenecteplase (0.25 mg/kg single dose, maximum of 25 mg) or intravenous alteplase (0.9 mg/kg total dose to a maximum of 90 mg, delivered as a bolus followed by a continuous infusion). A total of 1600 patients were enrolled, with 816 randomly assigned to the tenecteplase arm and 784 to the alteplase arm; 1577 patients were included in the intention-to-treat (ITT) analysis (n = 806 tenecteplase; n = 771 alteplase). The median age of enrollees was 74 years, and 52.1% of the ITT population were men.
Main outcome measures: In the ITT population, the primary outcome measure was a modified Rankin score (mRS) of 0 or 1 at 90 to 120 days post treatment. Safety outcomes included symptomatic intracerebral hemorrhage, orolingual angioedema, extracranial bleeding that required blood transfusion (all within 24 hours of thrombolytic administration), and all-cause mortality at 90 days. The noninferiority threshold for intravenous tenecteplase was set as the lower 95% CI of the difference between the tenecteplase and alteplase groups in the proportion of patients who met the primary outcome exceeding –5%.
Main results: The primary outcome of mRS of either 0 or 1 at 90 to 120 days of treatment occurred in 296 (36.9%) of the 802 patients assigned to tenecteplase and 266 (34.8%) of the 765 patients assigned to alteplase (unadjusted risk difference, 2.1%; 95% CI, –2.6 to 6.9). The prespecified noninferiority threshold was met. There were no significant differences between the groups in rates of intracerebral hemorrhage at 24 hours or 90-day all-cause mortality.
Conclusion: Intravenous tenecteplase is a reasonable alternative to alteplase for patients eligible for thrombolytic therapy.
Study 2 Overview (Wang et al)
Objective: To determine whether tenecteplase (dose 0.25 mg/kg) is noninferior to alteplase in patients with acute ischemic stroke who are within 4.5 hours of symptom onset and eligible for thrombolytic therapy but either refused or were ineligible for endovascular thrombectomy.
Design: Multicenter, prospective, open-label, randomized, controlled noninferiority trial.
Setting and participants: This trial was conducted at 53 centers across China and included patients 18 years of age or older who were within 4.5 hours of symptom onset and were thrombolytic eligible, had a mRS ≤ 1 at enrollment, and had a National Institutes of Health Stroke Scale score between 5 and 25. Eligible participants were randomized 1:1 to either tenecteplase 0.25 mg/kg (maximum dose 25 mg) or alteplase 0.9 mg/kg (maximum dose 90 mg, administered as a bolus followed by infusion). During the enrollment period (June 12, 2021, to May 29, 2022), a total of 1430 participants were enrolled, and, of those, 716 were randomly assigned to tenecteplase and 714 to alteplase. Six patients assigned to tenecteplase and 7 assigned to alteplase did not receive drugs. At 90 days, 5 in the tenecteplase group and 11 in the alteplase group were lost to follow up.
Main outcome measures: The primary efficacy outcome was a mRS of 0 or 1 at 90 days. The primary safety outcome was intracranial hemorrhage within 36 hours. Safety outcomes included parenchymal hematoma 2, as defined by the European Cooperative Acute Stroke Study III; any intracranial or significant hemorrhage, as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria; and death from all causes at 90 days. Noninferiority for tenecteplase would be declared if the lower 97.5% 1-sided CI for the relative risk (RR) for the primary outcome did not cross 0.937.
Main results: In the modified ITT population, the primary outcome occurred in 439 (62%) of the tenecteplase group and 405 (68%) of the alteplase group (RR, 1.07; 95% CI, 0.98-1.16). This met the prespecified margin for noninferiority. Intracranial hemorrhage within 36 hours was experienced by 15 (2%) patients in the tenecteplase group and 13 (2%) in the alteplase group (RR, 1.18; 95% CI, 0.56-2.50). Death at 90 days occurred in 46 (7%) patients in the tenecteplase group and 35 (5%) in the alteplase group (RR, 1.31; 95% CI, 0.86-2.01).
Conclusion: Tenecteplase was noninferior to alteplase in patients with acute ischemic stroke who met criteria for thrombolysis and either refused or were ineligible for endovascular thrombectomy.
Commentary
Alteplase has been FDA-approved for managing acute ischemic stroke since 1996 and has demonstrated positive effects on functional outcomes. Drawbacks of alteplase therapy, however, include bleeding risk as well as cumbersome administration of a bolus dose followed by a 60-minute infusion. In recent years, the question of whether or not tenecteplase could replace alteplase as the preferred thrombolytic for acute ischemic stroke has garnered much attention. Several features of tenecteplase make it an attractive option, including increased fibrin specificity, a longer half-life, and ease of administration as a single, rapid bolus dose. In phase 2 trials that compared tenecteplase 0.25 mg/kg with alteplase, findings suggested the potential for early neurological improvement as well as improved outcomes at 90 days. While the role of tenecteplase in acute myocardial infarction has been well established due to ease of use and a favorable adverse-effect profile,1 there is much less evidence from phase 3 randomized controlled clinical trials to secure the role of tenecteplase in acute ischemic stroke.2
Menon et al attempted to close this gap in the literature by conducting a randomized controlled clinical trial (AcT) comparing tenecteplase to alteplase in a Canadian patient population. The trial's patient population mirrors that of real-world data from global registries in terms of age, sex, and baseline stroke severity. In addition, the eligibility window of 4.5 hours from symptom onset as well as the inclusion and exclusion criteria for therapy are common to those utilized in other countries, making the findings generalizable. There were some limitations to the study, however, including the impact of COVID-19 on recruitment efforts as well as limitations of research infrastructure and staffing, which may have limited enrollment efforts at primary stroke centers. Nonetheless, the authors concluded that their results provide evidence that tenecteplase is comparable to alteplase, with similar functional and safety outcomes.
TRACE-2 focused on an Asian patient population and provided follow up to the dose-ranging TRACE-1 phase 2 trial. TRACE-1 showed that tenecteplase 0.25 mg/kg had a similar safety profile to alteplase 0.9 mg/kg in Chinese patients presenting with acute ischemic stroke. TRACE-2 sought to establish noninferiority of tenecteplase and excluded patients who were ineligible for or refused thrombectomy. Interestingly, the tenecteplase arm, as the authors point out, had numerically greater mortality as well as intracranial hemorrhage, but these differences were not statistically significant between the treatment groups at 90 days. The TRACE-2 results parallel those of AcT, and although there were differences in ethnicity between the 2 trials, the authors cite this as evidence that the results are consistent and provide evidence for the role of tenecteplase in the management of acute ischemic stroke. Limitations of this trial include potential bias from its open-label design, as well as exclusion of patients with more severe strokes eligible for thrombectomy, which may limit generalizability to patients with more disabling strokes who could have a higher risk of intracranial hemorrhage.
Application for Clinical Practice and System Implementation
Across the country, many organizations have adopted the off-label use of tenecteplase for managing fibrinolytic-eligible acute ischemic stroke patients. In most cases, the impetus for change is the ease of dosing and administration of tenecteplase compared to alteplase, while the inclusion and exclusion criteria and overall management remain the same. Timely administration of therapy in stroke is critical. This, along with other time constraints in stroke workflows, the weight-based calculation of alteplase doses, and alteplase’s administration method may lead to medication errors when using this agent to treat patients with acute stroke. The rapid, single-dose administration of tenecteplase removes many barriers that hospitals face when patients may need to be treated and then transferred to another site for further care. Without the worry to “drip and ship,” the completion of administration may allow for timely patient transfer and eliminate the need for monitoring of an infusion during transfer. For some organizations, there may be a potential for drug cost-savings as well as improved metrics, such as door-to-needle time, but the overall effects of switching from alteplase to tenecteplase remain to be seen. Currently, tenecteplase is included in stroke guidelines as a “reasonable choice,” though with a low level of evidence.3 However, these 2 studies support the role of tenecteplase in acute ischemic stroke treatment and may provide a foundation for further studies to establish the role of tenecteplase in the acute ischemic stroke population.
Practice Points
- Tenecteplase may be considered as an alternative to alteplase for acute ischemic stroke for patients who meet eligibility criteria for thrombolytics; this recommendation is included in the most recent stroke guidelines, although tenecteplase has not been demonstrated to be superior to alteplase.
- The ease of administration of tenecteplase as a single intravenous bolus dose represents a benefit compared to alteplase; it is an off-label use, however, and further studies are needed to establish the superiority of tenecteplase in terms of functional and safety outcomes.
– Carol Heunisch, PharmD, BCPS, BCCP
Pharmacy Department, NorthShore–Edward-Elmhurst Health, Evanston, IL
Study 1 Overview (Menon et al)
Objective: To determine whether a 0.25 mg/kg dose of intravenous tenecteplase is noninferior to intravenous alteplase 0.9 mg/kg for patients with acute ischemic stroke eligible for thrombolytic therapy.
Design: Multicenter, parallel-group, open-label randomized controlled trial.
Setting and participants: The trial was conducted at 22 primary and comprehensive stroke centers across Canada. A primary stroke center was defined as a hospital capable of offering intravenous thrombolysis to patients with acute ischemic stroke, while a comprehensive stroke center was able to offer thrombectomy services in addition. The involved centers also participated in Canadian quality improvement registries (either Quality Improvement and Clinical Research [QuiCR] or Optimizing Patient Treatment in Major Ischemic Stroke with EVT [OPTIMISE]) that track patient outcomes. Patients were eligible for inclusion if they were aged 18 years or older, had a diagnosis of acute ischemic stroke, presented within 4.5 hours of symptom onset, and were eligible for thrombolysis according to Canadian guidelines.
Patients were randomized in a 1:1 fashion to either intravenous tenecteplase (0.25 mg/kg single dose, maximum of 25 mg) or intravenous alteplase (0.9 mg/kg total dose to a maximum of 90 mg, delivered as a bolus followed by a continuous infusion). A total of 1600 patients were enrolled, with 816 randomly assigned to the tenecteplase arm and 784 to the alteplase arm; 1577 patients were included in the intention-to-treat (ITT) analysis (n = 806 tenecteplase; n = 771 alteplase). The median age of enrollees was 74 years, and 52.1% of the ITT population were men.
Main outcome measures: In the ITT population, the primary outcome measure was a modified Rankin score (mRS) of 0 or 1 at 90 to 120 days post treatment. Safety outcomes included symptomatic intracerebral hemorrhage, orolingual angioedema, extracranial bleeding that required blood transfusion (all within 24 hours of thrombolytic administration), and all-cause mortality at 90 days. The noninferiority threshold for intravenous tenecteplase was set as the lower 95% CI of the difference between the tenecteplase and alteplase groups in the proportion of patients who met the primary outcome exceeding –5%.
Main results: The primary outcome of mRS of either 0 or 1 at 90 to 120 days of treatment occurred in 296 (36.9%) of the 802 patients assigned to tenecteplase and 266 (34.8%) of the 765 patients assigned to alteplase (unadjusted risk difference, 2.1%; 95% CI, –2.6 to 6.9). The prespecified noninferiority threshold was met. There were no significant differences between the groups in rates of intracerebral hemorrhage at 24 hours or 90-day all-cause mortality.
Conclusion: Intravenous tenecteplase is a reasonable alternative to alteplase for patients eligible for thrombolytic therapy.
Study 2 Overview (Wang et al)
Objective: To determine whether tenecteplase (dose 0.25 mg/kg) is noninferior to alteplase in patients with acute ischemic stroke who are within 4.5 hours of symptom onset and eligible for thrombolytic therapy but either refused or were ineligible for endovascular thrombectomy.
Design: Multicenter, prospective, open-label, randomized, controlled noninferiority trial.
Setting and participants: This trial was conducted at 53 centers across China and included patients 18 years of age or older who were within 4.5 hours of symptom onset and were thrombolytic eligible, had a mRS ≤ 1 at enrollment, and had a National Institutes of Health Stroke Scale score between 5 and 25. Eligible participants were randomized 1:1 to either tenecteplase 0.25 mg/kg (maximum dose 25 mg) or alteplase 0.9 mg/kg (maximum dose 90 mg, administered as a bolus followed by infusion). During the enrollment period (June 12, 2021, to May 29, 2022), a total of 1430 participants were enrolled, and, of those, 716 were randomly assigned to tenecteplase and 714 to alteplase. Six patients assigned to tenecteplase and 7 assigned to alteplase did not receive drugs. At 90 days, 5 in the tenecteplase group and 11 in the alteplase group were lost to follow up.
Main outcome measures: The primary efficacy outcome was a mRS of 0 or 1 at 90 days. The primary safety outcome was intracranial hemorrhage within 36 hours. Safety outcomes included parenchymal hematoma 2, as defined by the European Cooperative Acute Stroke Study III; any intracranial or significant hemorrhage, as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria; and death from all causes at 90 days. Noninferiority for tenecteplase would be declared if the lower 97.5% 1-sided CI for the relative risk (RR) for the primary outcome did not cross 0.937.
Main results: In the modified ITT population, the primary outcome occurred in 439 (62%) of the tenecteplase group and 405 (68%) of the alteplase group (RR, 1.07; 95% CI, 0.98-1.16). This met the prespecified margin for noninferiority. Intracranial hemorrhage within 36 hours was experienced by 15 (2%) patients in the tenecteplase group and 13 (2%) in the alteplase group (RR, 1.18; 95% CI, 0.56-2.50). Death at 90 days occurred in 46 (7%) patients in the tenecteplase group and 35 (5%) in the alteplase group (RR, 1.31; 95% CI, 0.86-2.01).
Conclusion: Tenecteplase was noninferior to alteplase in patients with acute ischemic stroke who met criteria for thrombolysis and either refused or were ineligible for endovascular thrombectomy.
Commentary
Alteplase has been FDA-approved for managing acute ischemic stroke since 1996 and has demonstrated positive effects on functional outcomes. Drawbacks of alteplase therapy, however, include bleeding risk as well as cumbersome administration of a bolus dose followed by a 60-minute infusion. In recent years, the question of whether or not tenecteplase could replace alteplase as the preferred thrombolytic for acute ischemic stroke has garnered much attention. Several features of tenecteplase make it an attractive option, including increased fibrin specificity, a longer half-life, and ease of administration as a single, rapid bolus dose. In phase 2 trials that compared tenecteplase 0.25 mg/kg with alteplase, findings suggested the potential for early neurological improvement as well as improved outcomes at 90 days. While the role of tenecteplase in acute myocardial infarction has been well established due to ease of use and a favorable adverse-effect profile,1 there is much less evidence from phase 3 randomized controlled clinical trials to secure the role of tenecteplase in acute ischemic stroke.2
Menon et al attempted to close this gap in the literature by conducting a randomized controlled clinical trial (AcT) comparing tenecteplase to alteplase in a Canadian patient population. The trial's patient population mirrors that of real-world data from global registries in terms of age, sex, and baseline stroke severity. In addition, the eligibility window of 4.5 hours from symptom onset as well as the inclusion and exclusion criteria for therapy are common to those utilized in other countries, making the findings generalizable. There were some limitations to the study, however, including the impact of COVID-19 on recruitment efforts as well as limitations of research infrastructure and staffing, which may have limited enrollment efforts at primary stroke centers. Nonetheless, the authors concluded that their results provide evidence that tenecteplase is comparable to alteplase, with similar functional and safety outcomes.
TRACE-2 focused on an Asian patient population and provided follow up to the dose-ranging TRACE-1 phase 2 trial. TRACE-1 showed that tenecteplase 0.25 mg/kg had a similar safety profile to alteplase 0.9 mg/kg in Chinese patients presenting with acute ischemic stroke. TRACE-2 sought to establish noninferiority of tenecteplase and excluded patients who were ineligible for or refused thrombectomy. Interestingly, the tenecteplase arm, as the authors point out, had numerically greater mortality as well as intracranial hemorrhage, but these differences were not statistically significant between the treatment groups at 90 days. The TRACE-2 results parallel those of AcT, and although there were differences in ethnicity between the 2 trials, the authors cite this as evidence that the results are consistent and provide evidence for the role of tenecteplase in the management of acute ischemic stroke. Limitations of this trial include potential bias from its open-label design, as well as exclusion of patients with more severe strokes eligible for thrombectomy, which may limit generalizability to patients with more disabling strokes who could have a higher risk of intracranial hemorrhage.
Application for Clinical Practice and System Implementation
Across the country, many organizations have adopted the off-label use of tenecteplase for managing fibrinolytic-eligible acute ischemic stroke patients. In most cases, the impetus for change is the ease of dosing and administration of tenecteplase compared to alteplase, while the inclusion and exclusion criteria and overall management remain the same. Timely administration of therapy in stroke is critical. This, along with other time constraints in stroke workflows, the weight-based calculation of alteplase doses, and alteplase’s administration method may lead to medication errors when using this agent to treat patients with acute stroke. The rapid, single-dose administration of tenecteplase removes many barriers that hospitals face when patients may need to be treated and then transferred to another site for further care. Without the worry to “drip and ship,” the completion of administration may allow for timely patient transfer and eliminate the need for monitoring of an infusion during transfer. For some organizations, there may be a potential for drug cost-savings as well as improved metrics, such as door-to-needle time, but the overall effects of switching from alteplase to tenecteplase remain to be seen. Currently, tenecteplase is included in stroke guidelines as a “reasonable choice,” though with a low level of evidence.3 However, these 2 studies support the role of tenecteplase in acute ischemic stroke treatment and may provide a foundation for further studies to establish the role of tenecteplase in the acute ischemic stroke population.
Practice Points
- Tenecteplase may be considered as an alternative to alteplase for acute ischemic stroke for patients who meet eligibility criteria for thrombolytics; this recommendation is included in the most recent stroke guidelines, although tenecteplase has not been demonstrated to be superior to alteplase.
- The ease of administration of tenecteplase as a single intravenous bolus dose represents a benefit compared to alteplase; it is an off-label use, however, and further studies are needed to establish the superiority of tenecteplase in terms of functional and safety outcomes.
– Carol Heunisch, PharmD, BCPS, BCCP
Pharmacy Department, NorthShore–Edward-Elmhurst Health, Evanston, IL
1. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators; F Van De Werf, J Adgey, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354(9180):716-722. doi:10.1016/s0140-6736(99)07403-6
2. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischaemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
1. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators; F Van De Werf, J Adgey, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354(9180):716-722. doi:10.1016/s0140-6736(99)07403-6
2. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischaemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
Poor physician access linked with unplanned return ED visits
Difficulty in accessing a family physician is associated with a higher risk for unplanned return visits to the emergency department among patients aged 75 years and older, new data indicate.
In a prospective, observational study that included almost 2,000 patients in this age group, 16% of participants attempted to contact their family physicians before their ED visits. Of this group, more than half reported having difficulty seeing their physicians for urgent problems, more than 40% had difficulty speaking with their family physicians by telephone, and more than one-third had difficulty booking appointments for new health problems.
write study author Marc Afilalo, MD, director of the ED at Jewish General Hospital in Montreal, and colleagues. “Therefore, community-based programs that target patient education and improved access to primary care are necessary not only for reducing return visits to the ED, but also for continuity of care and patient satisfaction.”
The study was published in Canadian Family Physician.
Comorbidities increased risk
Researchers have estimated that half of Canadians aged 75 years or older use emergency services. Data indicate that the number of unplanned return visits to the ED is associated with increased functional decline and death. But the question of how patient access to primary care services affects unplanned ED return visits has received little attention, according to the investigators.
They conducted a multicenter study at three tertiary adult teaching hospitals in Montreal. From 2012 to 2014, they recruited patients aged 75 years and older who had visited the ED and who lived in their own homes or in an autonomous residence.
Investigators collected data through structured interviews, administrative databases, and medical chart reviews. They followed up with participants at 3 months by telephone. The study’s main outcome was return visit to the ED.
The researchers identified 4,577 patients and included 1,998 in their analysis. Of that total, 33% were 85 or older, 34% lived alone, and 91% had a family physician. Within 3 months, 562 patients (28%) had made 894 return visits to the ED.
Among patients aged 85 years or older (relative risk, 0.80), as well as those whose triage score was less severe (RR, 0.83) and those who were admitted during the index ED visit (RR, 0.76), rates of return ED visits were lower. Among patients who had trouble booking appointments with their family doctors to address new problems (RR, 1.19), as well as those who had made ED visits within the previous 6 months (RR, 1.47) or had a higher Charlson comorbidity index score (RR, 1.06 for every 1-unit increase), rates of return visits were higher.
Factors associated with a higher likelihood of return visits were visits to the ED in the previous 6 months (odds ratio, 2.11), increased Charlson comorbidity index score (OR, 1.41 for every 1-unit increase), and having received help from local community services (OR, 3.00).
Primary care access
The study suggests that improvements in primary care access are needed to decrease return visits to the ED, Samir Sinha, MD, DPhil, director of geriatrics at Mount Sinai and the University Health Network Hospitals in Toronto, told this news organization. Dr. Sinha was not involved in the study.
“It reminds us of the importance of having a strong primary care system,” he added. “Of this population, 91% had primary care providers. And what the paper demonstrates is that those who are having trouble accessing their primary care providers are more likely to be readmitted to an ED. We can only imagine how much worse the outcomes are for people who don’t have a primary care provider.”
Patients are frequently advised to visit the ED when they contact their primary care providers, said Mark Rosenberg, PhD, professor of geography and planning and the Canada Research Chair in Aging, Health, and Development at Queens University in Kingston, Ont., said in an interview. He noted that primary care is organized as an appointment-based system. Dr. Rosenberg did not participate in the study.
“If I were to call my primary care provider in the middle of the afternoon and say that I have got chest pains, they are going to simply tell me to go to emergency,” said Dr. Rosenberg. “It is not just older people. Many people end up in the ED because they are told to go to the ED.”
Associations with age
“The higher your Charlson comorbidity index, the more multiple, complex health issues you’re dealing with,” said Sinha. He added that the data suggest the frailty of the study population.
The association between age 85 years or older and a lower rate of a return ED visits might mean that the patient did not return to independent living after the ED visit, Dr. Rosenberg speculated. “If it’s a serious health problem, you’re more likely to end up going into long-term care at that stage, and you are not going back to living in the community in your home,” he said. “You’re likely going into some sort of transition care or alternative care.”
People aged 85 years or older who are hospitalized are more likely not to survive their index hospital admission, compared with patients who are aged 75-85 years. There would be no possibility that such patients would revisit the ED in the future, said Dr. Sinha.
Expanding primary care
The major solution to decreasing reliance on the ED lies in revamping primary health care so that it offers an expanded level of care and 24/7 access, said Dr. Rosenberg.
Providing continuity of care, identifying problems, and managing them in the community before they become urgent or require a hospitalization are priorities for primary care and will help shift away from return visits to the ED, which should be a last resort for patients, said Dr. Sinha.
Moreover, patients must be able to access primary care in various ways, be it a telephone consultation, a video consultation, or a face-to-face consultation, he added. Face-to-face consultations can take place in a provider’s office or even in a patient’s home when warranted, he said. “What we need to make sure of is that all three types of consultations are available, so that people can actually get the most appropriate care at the time they’re calling.”
The study had no external funding. Dr. Afilalo, Dr. Sinha, and Dr. Rosenberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Difficulty in accessing a family physician is associated with a higher risk for unplanned return visits to the emergency department among patients aged 75 years and older, new data indicate.
In a prospective, observational study that included almost 2,000 patients in this age group, 16% of participants attempted to contact their family physicians before their ED visits. Of this group, more than half reported having difficulty seeing their physicians for urgent problems, more than 40% had difficulty speaking with their family physicians by telephone, and more than one-third had difficulty booking appointments for new health problems.
write study author Marc Afilalo, MD, director of the ED at Jewish General Hospital in Montreal, and colleagues. “Therefore, community-based programs that target patient education and improved access to primary care are necessary not only for reducing return visits to the ED, but also for continuity of care and patient satisfaction.”
The study was published in Canadian Family Physician.
Comorbidities increased risk
Researchers have estimated that half of Canadians aged 75 years or older use emergency services. Data indicate that the number of unplanned return visits to the ED is associated with increased functional decline and death. But the question of how patient access to primary care services affects unplanned ED return visits has received little attention, according to the investigators.
They conducted a multicenter study at three tertiary adult teaching hospitals in Montreal. From 2012 to 2014, they recruited patients aged 75 years and older who had visited the ED and who lived in their own homes or in an autonomous residence.
Investigators collected data through structured interviews, administrative databases, and medical chart reviews. They followed up with participants at 3 months by telephone. The study’s main outcome was return visit to the ED.
The researchers identified 4,577 patients and included 1,998 in their analysis. Of that total, 33% were 85 or older, 34% lived alone, and 91% had a family physician. Within 3 months, 562 patients (28%) had made 894 return visits to the ED.
Among patients aged 85 years or older (relative risk, 0.80), as well as those whose triage score was less severe (RR, 0.83) and those who were admitted during the index ED visit (RR, 0.76), rates of return ED visits were lower. Among patients who had trouble booking appointments with their family doctors to address new problems (RR, 1.19), as well as those who had made ED visits within the previous 6 months (RR, 1.47) or had a higher Charlson comorbidity index score (RR, 1.06 for every 1-unit increase), rates of return visits were higher.
Factors associated with a higher likelihood of return visits were visits to the ED in the previous 6 months (odds ratio, 2.11), increased Charlson comorbidity index score (OR, 1.41 for every 1-unit increase), and having received help from local community services (OR, 3.00).
Primary care access
The study suggests that improvements in primary care access are needed to decrease return visits to the ED, Samir Sinha, MD, DPhil, director of geriatrics at Mount Sinai and the University Health Network Hospitals in Toronto, told this news organization. Dr. Sinha was not involved in the study.
“It reminds us of the importance of having a strong primary care system,” he added. “Of this population, 91% had primary care providers. And what the paper demonstrates is that those who are having trouble accessing their primary care providers are more likely to be readmitted to an ED. We can only imagine how much worse the outcomes are for people who don’t have a primary care provider.”
Patients are frequently advised to visit the ED when they contact their primary care providers, said Mark Rosenberg, PhD, professor of geography and planning and the Canada Research Chair in Aging, Health, and Development at Queens University in Kingston, Ont., said in an interview. He noted that primary care is organized as an appointment-based system. Dr. Rosenberg did not participate in the study.
“If I were to call my primary care provider in the middle of the afternoon and say that I have got chest pains, they are going to simply tell me to go to emergency,” said Dr. Rosenberg. “It is not just older people. Many people end up in the ED because they are told to go to the ED.”
Associations with age
“The higher your Charlson comorbidity index, the more multiple, complex health issues you’re dealing with,” said Sinha. He added that the data suggest the frailty of the study population.
The association between age 85 years or older and a lower rate of a return ED visits might mean that the patient did not return to independent living after the ED visit, Dr. Rosenberg speculated. “If it’s a serious health problem, you’re more likely to end up going into long-term care at that stage, and you are not going back to living in the community in your home,” he said. “You’re likely going into some sort of transition care or alternative care.”
People aged 85 years or older who are hospitalized are more likely not to survive their index hospital admission, compared with patients who are aged 75-85 years. There would be no possibility that such patients would revisit the ED in the future, said Dr. Sinha.
Expanding primary care
The major solution to decreasing reliance on the ED lies in revamping primary health care so that it offers an expanded level of care and 24/7 access, said Dr. Rosenberg.
Providing continuity of care, identifying problems, and managing them in the community before they become urgent or require a hospitalization are priorities for primary care and will help shift away from return visits to the ED, which should be a last resort for patients, said Dr. Sinha.
Moreover, patients must be able to access primary care in various ways, be it a telephone consultation, a video consultation, or a face-to-face consultation, he added. Face-to-face consultations can take place in a provider’s office or even in a patient’s home when warranted, he said. “What we need to make sure of is that all three types of consultations are available, so that people can actually get the most appropriate care at the time they’re calling.”
The study had no external funding. Dr. Afilalo, Dr. Sinha, and Dr. Rosenberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Difficulty in accessing a family physician is associated with a higher risk for unplanned return visits to the emergency department among patients aged 75 years and older, new data indicate.
In a prospective, observational study that included almost 2,000 patients in this age group, 16% of participants attempted to contact their family physicians before their ED visits. Of this group, more than half reported having difficulty seeing their physicians for urgent problems, more than 40% had difficulty speaking with their family physicians by telephone, and more than one-third had difficulty booking appointments for new health problems.
write study author Marc Afilalo, MD, director of the ED at Jewish General Hospital in Montreal, and colleagues. “Therefore, community-based programs that target patient education and improved access to primary care are necessary not only for reducing return visits to the ED, but also for continuity of care and patient satisfaction.”
The study was published in Canadian Family Physician.
Comorbidities increased risk
Researchers have estimated that half of Canadians aged 75 years or older use emergency services. Data indicate that the number of unplanned return visits to the ED is associated with increased functional decline and death. But the question of how patient access to primary care services affects unplanned ED return visits has received little attention, according to the investigators.
They conducted a multicenter study at three tertiary adult teaching hospitals in Montreal. From 2012 to 2014, they recruited patients aged 75 years and older who had visited the ED and who lived in their own homes or in an autonomous residence.
Investigators collected data through structured interviews, administrative databases, and medical chart reviews. They followed up with participants at 3 months by telephone. The study’s main outcome was return visit to the ED.
The researchers identified 4,577 patients and included 1,998 in their analysis. Of that total, 33% were 85 or older, 34% lived alone, and 91% had a family physician. Within 3 months, 562 patients (28%) had made 894 return visits to the ED.
Among patients aged 85 years or older (relative risk, 0.80), as well as those whose triage score was less severe (RR, 0.83) and those who were admitted during the index ED visit (RR, 0.76), rates of return ED visits were lower. Among patients who had trouble booking appointments with their family doctors to address new problems (RR, 1.19), as well as those who had made ED visits within the previous 6 months (RR, 1.47) or had a higher Charlson comorbidity index score (RR, 1.06 for every 1-unit increase), rates of return visits were higher.
Factors associated with a higher likelihood of return visits were visits to the ED in the previous 6 months (odds ratio, 2.11), increased Charlson comorbidity index score (OR, 1.41 for every 1-unit increase), and having received help from local community services (OR, 3.00).
Primary care access
The study suggests that improvements in primary care access are needed to decrease return visits to the ED, Samir Sinha, MD, DPhil, director of geriatrics at Mount Sinai and the University Health Network Hospitals in Toronto, told this news organization. Dr. Sinha was not involved in the study.
“It reminds us of the importance of having a strong primary care system,” he added. “Of this population, 91% had primary care providers. And what the paper demonstrates is that those who are having trouble accessing their primary care providers are more likely to be readmitted to an ED. We can only imagine how much worse the outcomes are for people who don’t have a primary care provider.”
Patients are frequently advised to visit the ED when they contact their primary care providers, said Mark Rosenberg, PhD, professor of geography and planning and the Canada Research Chair in Aging, Health, and Development at Queens University in Kingston, Ont., said in an interview. He noted that primary care is organized as an appointment-based system. Dr. Rosenberg did not participate in the study.
“If I were to call my primary care provider in the middle of the afternoon and say that I have got chest pains, they are going to simply tell me to go to emergency,” said Dr. Rosenberg. “It is not just older people. Many people end up in the ED because they are told to go to the ED.”
Associations with age
“The higher your Charlson comorbidity index, the more multiple, complex health issues you’re dealing with,” said Sinha. He added that the data suggest the frailty of the study population.
The association between age 85 years or older and a lower rate of a return ED visits might mean that the patient did not return to independent living after the ED visit, Dr. Rosenberg speculated. “If it’s a serious health problem, you’re more likely to end up going into long-term care at that stage, and you are not going back to living in the community in your home,” he said. “You’re likely going into some sort of transition care or alternative care.”
People aged 85 years or older who are hospitalized are more likely not to survive their index hospital admission, compared with patients who are aged 75-85 years. There would be no possibility that such patients would revisit the ED in the future, said Dr. Sinha.
Expanding primary care
The major solution to decreasing reliance on the ED lies in revamping primary health care so that it offers an expanded level of care and 24/7 access, said Dr. Rosenberg.
Providing continuity of care, identifying problems, and managing them in the community before they become urgent or require a hospitalization are priorities for primary care and will help shift away from return visits to the ED, which should be a last resort for patients, said Dr. Sinha.
Moreover, patients must be able to access primary care in various ways, be it a telephone consultation, a video consultation, or a face-to-face consultation, he added. Face-to-face consultations can take place in a provider’s office or even in a patient’s home when warranted, he said. “What we need to make sure of is that all three types of consultations are available, so that people can actually get the most appropriate care at the time they’re calling.”
The study had no external funding. Dr. Afilalo, Dr. Sinha, and Dr. Rosenberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANADIAN FAMILY PHYSICIAN