User login
Antimicrobial Prescription in Pneumonia
Pneumonia is the second most common infection in nursing home residents after urinary tract infection, and is the most common reason for transfer to the hospital.1 Although it remains difficult to determine the incidence of pneumonia in institutionalized elderly patients, an estimated 4 million cases of nursing homeacquired pneumonia (NHAP) occur annually in the United States and result in more than 600,000 emergency department visits.2 In the past 2 decades, multiple studies have documented the rapid rise in drug resistance among common pathogens responsible for pneumonia in the elderly and the acquisition of multidrug‐resistant organisms in residents of long‐term care facilities.3, 4 Health care practitioners are faced with the dilemma of attempting to limit broad‐spectrum antimicrobial drug use while striving to maximize therapeutic efficacy in individual patients.5 The current practice guidelines for the management of NHAP from various professional societies provide mixed messages on the class of antibiotics for patients requiring hospitalization.2, 68 While the 2000 Canadian and the 2003 Infectious Disease Society of America (IDSA) guidelines advocate a community‐acquired pneumonia‐like approach to therapy, the 2005 American Thoracic Society (ATS)/IDSA guidelines and the 2007 IDSA/ATS guidelines consider drug‐resistant pathogens (DRPs) (ie, methicillin‐resistant Staphylococcus aureus [MRSA] and Pseudomonas aeruginosa) to be major etiologic agents in NHAP and thus the empiric treatment recommendations focus specifically on these pathogens (Table 1).
|
| 2003 IDSA |
| 1. Parenteral third‐generation cephalosporin or ampicillin sulbactam + macrolide; or |
| 2. Parenteral fluoroquinolone alone |
| 2000 Canadian |
| 1. Parenteral fluoroquinolone alone; or |
| 2. Parenteral third‐generation, or fourth‐generation cephalosporin + macrolide |
| 2005 ATS/IDSA |
| 1. Antipseudomonal cephalosporin or antipseudomonal carbapenem or antipseudomonal penicillin + antipseudomonal fluoroquinolone or aminoglycoside + anti‐methicillin‐resistant Staphylococcus agents |
Given these differences in antibiotic recommendations among the various guidelines, we sought to examine the antimicrobial prescription patterns in hospitalized non‐critically‐ill patients with NHAP in multiple tertiary care facilities vis‐‐vis the population demographics and clinical characteristics.
Methods
Study Population
This retrospective study was conducted in 3 tertiary‐care hospitals (Erie County Medical Center, Millard Fillmore Hospital, and Buffalo General Hospital) in the city of Buffalo, New York. These hospitals account for 96% of admissions from nursing homes in Erie County. The Institutional Review Board approved the study and certified that it met the criteria for a waiver of the requirement to obtain informed consent. All medical charts of adult patients with pneumonia listed under admission diagnosis or discharge diagnosis (International Classification of Diseases, ninth revision, Clinical Modification Codes [ICD‐9‐CM] [35] codes 480.0480.9, 481, 482.0482.9, 483.0483.8, 485, 486, 487.0, and 507.0) between April 2005 and December 2007 were abstracted. The records were searched for place of residence prior to admission and all patients residing in nursing homes for 30 days or more were selected for review. Inclusion criteria included the presence of new or increased radiographic abnormalities plus 2 or more of the following symptoms and signs: new or increased cough, new or increased sputum production, and temperature greater than 38C. Patients who met at least one of the following criteria were excluded: (1) admission to a critical care unit from the emergency department; (2) discharge within 24 hours; (3) human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) or immunocompromised; (4) transfer from another hospital; or (5) receiving active chemotherapy. Patients with multiple admissions were included only once to ensure independence of observations.
Data Collection
Data collected included information on sociodemographic characteristics, admitting service (University‐affiliated or private service), comorbidities, preadmission functional status, do not resuscitate (DNR) order, and prior antibiotic therapy. Antibiotic information was comprised of the name of the antibiotic, start and stop dates (including postdischarge), monotherapy or combination therapy, and route of administration. Antimicrobials were assigned to 1 of the following categories: macrolides (azithromycin, clarithromycin), lincosamide (clindamycin), fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin), carbapenems (ertapenem, imipenem), cephalosporins (ceftriaxone, cefpodoxime, cefepime), and ureidopenicillins (piperacillin‐tazobactam). Patients who died during the hospital stay before completion of therapy were assigned 14 days of antibiotic therapy.
The burden of comorbidities was assessed by the Charlson Index.9 The Activity of Daily Living (ADL) score was abstracted from a standardized patient‐review instrument included in all patients' charts.10 Patients were assigned an ADL score in each of the 6 major areas of activity: eating, toileting, feeding, bathing, mobility, and continence; ranging from 1 if they were fully independent, 2 if they were partially independent, and 3 if they were completely dependent. The ADL score was calculated by adding the points assigned for each activity, and it ranged from 6 to 18. Three categories were arbitrarily created: ADL I, corresponding to ADL scores from 6 to 8; ADL II, scores from 9 to 13; and ADL III, scores from 14 to 18.4
The Pneumonia Severity Index Score (PSI)11 was also calculated. The PSI is a validated disease‐severity classification system based on age, sex, nursing home residence, 5 comorbid illnesses, vital signs on admission, mental status, 7 laboratory values, and the findings on chest roentgenograms. Based on the scoring system, patients were stratified into 5 categories or classes of risk for in‐hospital mortality. Class I patients have the lowest disease severity while class V have the highest disease severity.
Statistics
Data were analyzed using the NCSS 2000 Statistical Analysis System (NCSS, Kaysville, UT). Continuous variables were tested for normal distribution using the Kolmogorov‐Smirnov test. Results are expressed as means standard deviation (SD). Univariate analysis was carried out using the chi‐square test and Fisher's exact test for categorical data and the t test for independent samples for continuous variables. Missing values for ADL and Charlson scores were encountered at <3% of the total population sample. Multiple regression models of available variables were utilized to predict missing values as described by Little and Rubin.12 All tests were 2‐tailed and statistical significance was determined at the 5% level.
Results
A total of 397 subjects with NHAP were included in the study. The mean age of the cohort group was 76.8 13.5 years. Eighty percent had 2 or more chronic diseases. Degenerative nervous system, cardiac, and pulmonary diseases accounted for the majority of underlying comorbidities. Demographic and clinical characteristics of the study population are presented in Table 2. At the time of admission, 17% of patients had received antimicrobial therapy for a respiratory ailment within the last week prior to transfer to an acute care facility. The most commonly prescribed agents at the nursing home were an oral fluoroquinolone (81%), a cephalosporin (14%), or a macrolide (3%).
| |
| Characteristic (n = 397) | |
| Age (years), mean (SD) | 76.8 (13.5) |
| Male, n (%) | 162 (41) |
| Underlying comorbidities, n (%) | |
| Cardiac diseases | 135 (34) |
| Pulmonary diseases | 129 (32) |
| Cerebrovascular accident | 98 (25) |
| Diabetes mellitus | 138 (35) |
| Dementia | 179 (45) |
| DNR, n (%) | 42 (11) |
| Activity of daily living, n (%) | |
| ADL I | 57 (14) |
| ADL II | 150 (38) |
| ADL III | 190 (48) |
| Pneumonia Severity Index, n (%) | |
| Class II | 13 (3) |
| Class III | 34 (8) |
| Class IV | 177 (45) |
| Class V | 173 (44) |
| Bacteremia, n (%) | 48 (12) |
Of the 397 patients who met the criteria for NHAP, all but 5 patients received antimicrobial therapy. The 3 most commonly used antimicrobial compounds for inpatient treatment were fluoroquinolones (51.4%), ceftriaxone (45.0%), and azithromycin (42.1%). None of the participating hospitals had an antibiotic restriction policy for the use of fluoroquinolones or vancomycin.
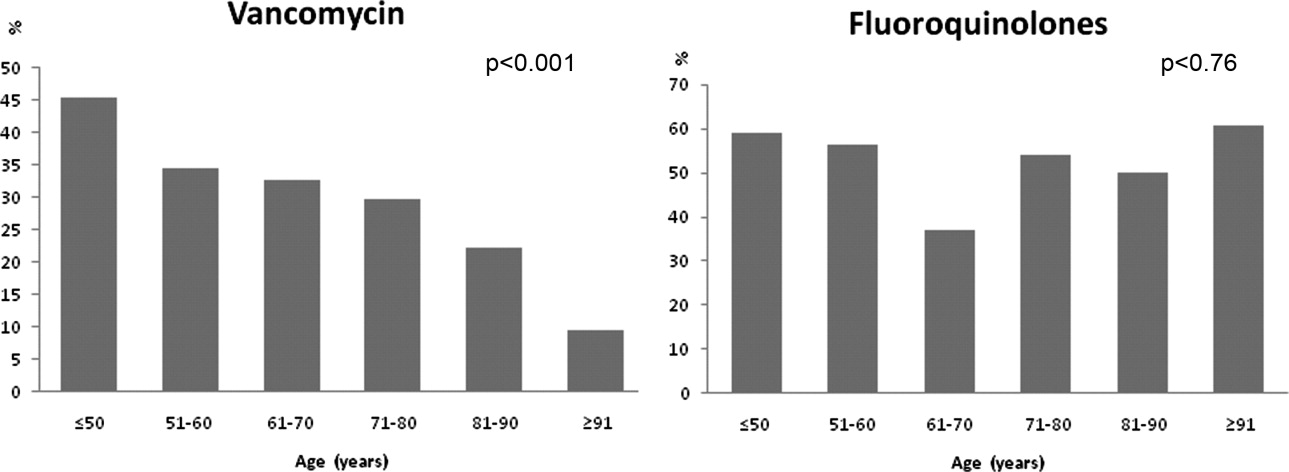
Monotherapy was prescribed in 57.4%. Fluoroquinolones represented 79.5% of these cases. The other monotherapy choices included a third‐generation cephalosporin (10.7%), piperacillin/tazobactam (8%), and vancomycin (0.2%). Combination therapy consisted mainly of a macrolide plus a third‐generation cephalosporin (74/168; 44%). Other combination regimens included vancomycin plus piperacillin/tazobactam plus ciprofloxacin (35%), vancomycin plus imipenem plus ciprofloxacin (9%), vancomycin plus piperacillin/tazobactam plus azithromycin (4%), vancomycin plus piperacillin/tazobactam (7%), and piperacillin/tazobactam plus azithromycin (1%). Figure 1 shows the distribution of vancomycin and fluoroquinolones use across the different age groups. While the use of fluoroquinolones (P = 0.76) was comparable between groups, there was a significant trend in prescribing less vancomycin with increasing age (P < 0.001). As for the rest of the antibiotics, there was no difference in the overall use of macrolides (P = 0.53), cephalosporins (P = 0.84), or carbapenems (P = 0.67) among age groups. Clindamycin was only used in 9 (2%) out of 392 patients. None of the patients had an aminoglycoside or a sulfa drug prescribed. We also found no difference in terms of antibiotic choice or use of combination therapy among the 3 hospitals (P = 0.78, and P = 0.52; respectively).
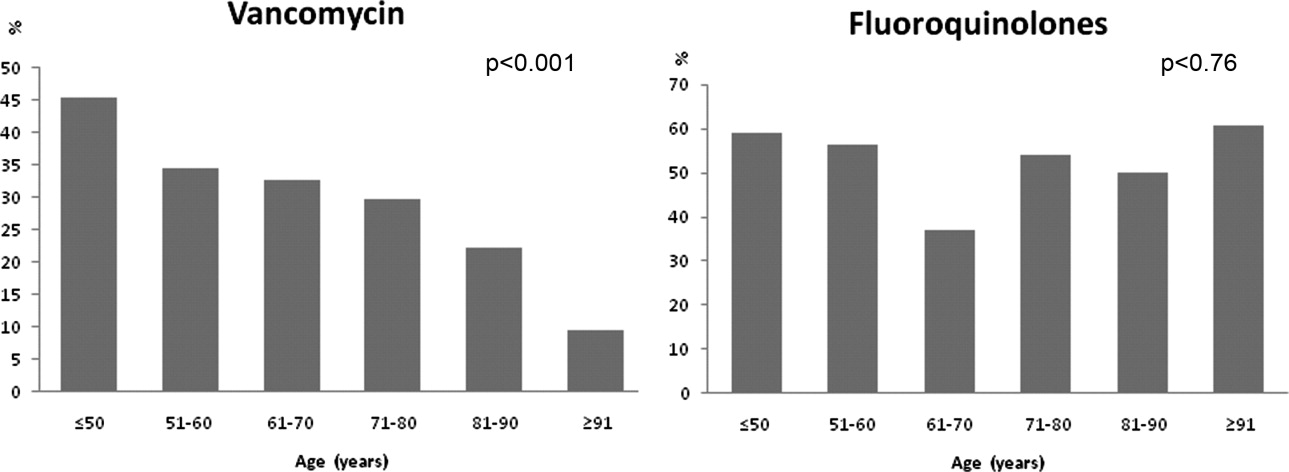
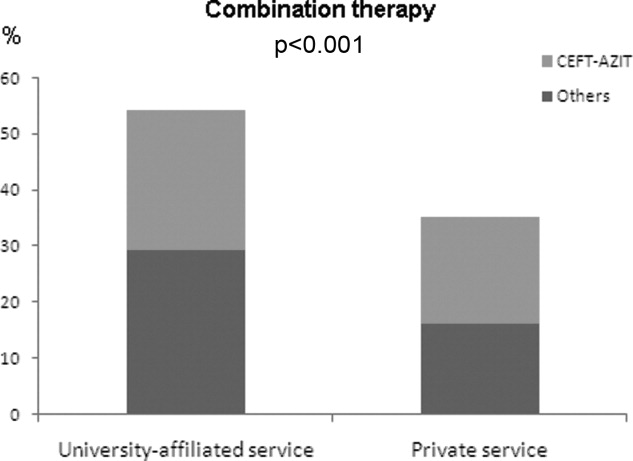
Antibiotic choices were influenced by severity of illness. There was an inverse relationship between PSI classes and the use of either fluoroquinolones or ceftriaxone plus azithromycin (P = 0.02) (Figure 2). Patients with higher acuity of illness were more likely to receive combination regimens that include vancomycin plus piperacillin/tazobactam than those with lower acuity of illness (P < 0.001). Neither the comorbidity index nor the ADL scores had a significant impact on the use of combination therapy (P = 0.49 and P = 0.2; respectively). There was a trend toward association between increasing ADL score and the use of vancomycin plus piperacillin/tazobactam but it did not reach statistical significance (P = 0.06). Of interest, patients who were admitted on the University‐affiliated service were more likely to receive combination therapy than those who were under the care of private service (P < 0.001) (Figure 3). Ceftriaxone plus azithromycin accounted for the majority of combination regimens irrespective of physicians' affiliation.
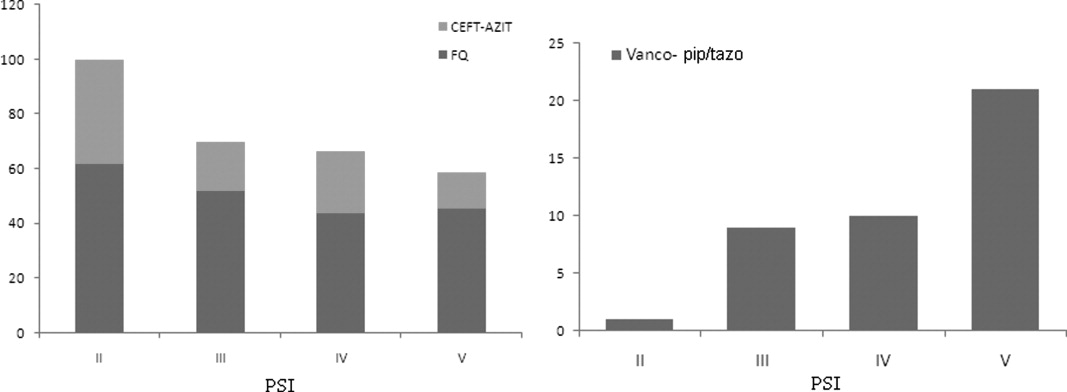
Overall, there were more patients who received antibiotic therapy in compliance with the 2003 IDSA guidelines6 compared with the 2005 ATS/IDSA guidelines7 (65% vs. 19%, respectively; P < 0.001). A positive correlation was noted between severity of illness and adherence to the 2005 ATS/IDSA antimicrobial recommendations (P = 0.02). However, neither the burden of comorbidities nor the functional status was associated with the use of guidelines (P = 0.76 and P = 0.43; respectively).
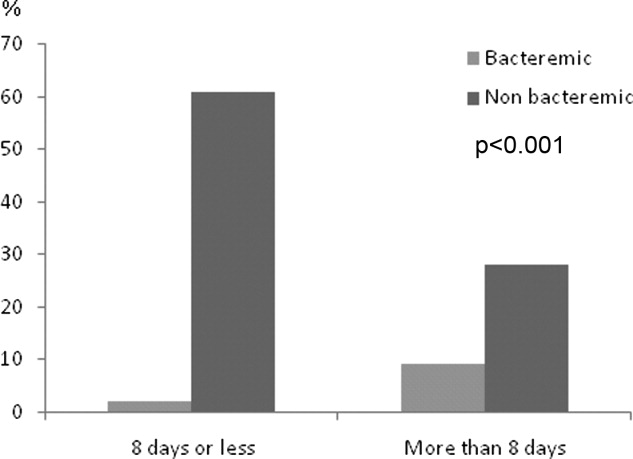
Duration of therapy ranged from 3 to 21 days with a median of 8 days. The choice of antibiotics, burden of comorbidities, DNR status, or PSI scores had no correlation with antibiotic duration. Only the presence of bacteremia was associated with more than 8 days of antibiotic duration (P < 0.001) (Figure 4). On average, bacteremic patients received 10.1 3.3 (range, 6‐21) days of antimicrobial therapy compared to 7.8 4.1 (range, 319) days for nonbacteremic cases (P < 0.001). During the course of hospitalization, change in antibiotics occurred in 35 (9%) out of the 392 patients, with the majority of substitutions affecting those who were initially prescribed a regimen that included vancomycin plus piperacillin/tazobactam. In these cases, patients were most commonly switched to fluoroquinolones (n = 20), followed by cephalosporins (n = 11).
Discussion
Our study suggests that antimicrobial selection among hospitalized nursing homes patients with pneumonia is influenced by patients' age, severity of illness, and provider's academic affiliation.
This is the first comprehensive study, to our knowledge, to report on the type, distribution, and pattern of antimicrobials prescribed among institutionalized patients requiring hospital admission. Various treatment regimens have been investigated in the last 2 decades using both retrospective and prospective randomized clinical trials to examine the efficacy and safety of parenteral and oral antibiotics in nursing homes.1316 However, there are no randomized controlled clinical trials for the treatment of hospitalized NHAP on which to base treatment recommendations. For some healthcare providers, the treatment parallels the coverage of patients with community‐acquired pneumonia; for others, broad‐spectrum coverage is the norm. In the absence of validated guidelines, the present investigation shows that prescription patterns varied across demographic and clinical characteristics. Fluoroquinolones were the preferred agents for the initial therapy of NHAP across all age groups, probably because of their single daily dosing, broad spectrum coverage against typical and atypical pathogens, and favorable side effect profile. Conversely, the use of vancomycin tended to decline in older age groups. This decline could be attributed to the need for frequent monitoring of trough levels when venous access can be difficult, lack of oral formulation, or potential toxicity. Further studies are needed to examine the validity of this pattern.
To our knowledge, compliance with guidelines regarding treatment of NHAP has not been previously reported. Despite recent studies suggesting that adherence to community‐acquired pneumonia guidelines resulted in reduced need for hospitalization, shorter stays, and lower mortality,1721 our findings indicated a rather low compliance with the most recently published guidelines. Potential reasons for the low levels of compliance include lack of awareness, time lag for the information to be disseminated in the medical community, lack of endorsement by local opinion leaders, or local barriers to implementation of these guidelines. Unfortunately, little is known about physicians' familiarity and attitude toward NHAP guidelines use. Efforts to improve the effectiveness of pneumonia care will depend on future studies aiming at identifying factors that influence nonadherence.
Severity of illness had a significant influence on the prescription pattern of antimicrobial therapy. As the PSI increased, treatment with a fluoroquinolone or with combination therapy of nonpseudomonal third‐generation cephalosporin plus macrolide was replaced by a broader spectrum of antimicrobial coverage. We believe that healthcare providers' prescriptions may be influenced by the recommendations of the ATS guidelines for the treatment of health care associated pneumonia,7 in which antimicrobial therapy for severely ill patients admitted from long‐term care facilities is directed toward multidrug resistant pathogens. The validity of this practice, however, remains the subject of intense debate,2225 driven by the absence of randomized trials showing improved morbidity and mortality.
Few formal clinical trials exist to guide the length of therapy of hospitalized patients with NHAP. The usual recommendation ranges from 7 to 14 days.16, 26 The median duration of 8 days observed in the current study is consistent with length of therapy advocated in the literature.16 Yet, prolonging antibiotic duration has been suggested when clinical severity of illness is high, comorbid illnesses are multiple, and expected resolution is delayed.27 Arguing against such a practice is evidence from meta‐analysis,28 expert reviews,29 and clinical investigation.30 Prescribing principles are nevertheless unlikely to induce substantial change unless their dissemination and promotion is sustained through intensive continuing educational programs for physicians and pharmacists.3133
Our study has a number of limitations. First, the cohort group described in this investigation consists of institutionalized patients in Western New York and hence the antibiotic prescribing patterns may vary in other locations. Second, we did not have adequate microbial information to fully assess the appropriateness of antimicrobial therapy. Third, the absence of microbial etiology may have resulted in incorrect identification of patients with pneumonia. Further, retrospective data extraction is notoriously imperfect, and pneumonia cases may have been missed because of either coding errors or atypical manifestations. However, we have used strict inclusion criteria in to minimize any potential bias. Fourth, the results of this study describe patterns of antibiotic utilization in the treatment of NHAP but do not provide reasoning for such a practice. The rationale behind these practices can only be discerned by a survey of healthcare providers.
In conclusion, we have observed in this study a poor compliance with the current guidelines for the treatment of NHAP. It is generally accepted that physicians' prescribing habits are influenced by their understanding of the pathophysiology and epidemiology of the infection being treated, as well as the pharmacology and spectrum of available antimicrobials. In the absence of outcome data, translation of this knowledge into practice may be influenced by a number of factors, such as the physician's preference, the academic milieu in which the practice occurs, and more importantly, by the patients' clinical condition.
- .Pneumonia in the long‐term‐care facility.Infect Control Hosp Epidemiol.2002;23:159–164.
- ,,,,.Canadian guidelines for the initial management of an evidence based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Pneumonia Working Group.Clin Infect Dis.2000;31:383–421.
- ,,, et al.Antimicrobial resistance in long‐term care facilities.Infect Control Hosp Epidemiol.1996;17:129–140.
- ,,,.Etiology of severe pneumonia in the very elderly.Am J Respir Crit Care Med.2001;163:645–651.
- ,,,.Tensions in antibiotic prescribing: pitting social concerns against the interests of individual patients.J Gen Intern Med.2002;17:87–94.
- ,,,,,.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital acquired, ventilator‐associated, and health care associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,, et al.Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the management of community‐acquired pneumonia in adults.Clin Infect Dis.2007;44:S27–S72.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chron Dis.1987;40:373–383.
- New York State Department of Health. Hospital and Community Patient Review Instrument. DOH‐694.Albany, NY:Department of Health;1989.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,.Statistical Analysis with Missing Data.New York:John Wiley 1987.
- ,,,,.A randomized study of ciprofloxacin versus ceftriaxone in the treatment of nursing home‐acquired lower respiratory tract infections.J Am Geriatr Soc.1991;39:979–985.
- ,,, et al.Prospective study of lower respiratory tract infections in an extended‐care nursing home program: potential role of oral ciprofloxacin.Am J Med.1988;85:164–171.
- ,,.Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial.JAMA2006;295:2503–2510.
- ,.Treatment guideline for nursing‐home acquired pneumonia based on community practice.J Am Geriatr Soc.2000;48:82–88.
- ,,,,,.Decreased mortality after implementation of a treatment guideline for community‐acquired pneumonia.Am J Med.2001;110:451–457.
- ,,,,,.A controlled trial of a critical pathway for treatment of community‐acquired pneumonia. CAPITAL Study Investigators. Community‐Acquired Pneumonia Intervention Trial Assessing Levofloxacin.JAMA.2000;283:749–755.
- ,,, et al.Improvement of process‐of‐care and outcomes after implementing a guideline for the management of community‐acquired pneumonia: a controlled before‐and‐after design study.Clin Infect Dis.2004;39:955–963.
- ,,, et al.Guidelines for the treatment of community‐acquired pneumonia: predictors of adherence and outcome.Am J Respir Crit Care Med.2005;172:757–762.
- ,,, et al.Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival.Clin Infect Dis.2005;41:1709–1716.
- ,,.Nosocomial or healthcare facility‐related pneumonia in adults.Curr Infect Dis Rep.2000;2:215–223.
- ,.Pneumonia in the older patient.Clin Chest Med.2007;28:751–771.
- .Guidelines for the management of adults with health care‐associated pneumonia: implications for nursing facility residents.Consult Pharm.2006;21:719–725.
- ,,, et al.Health‐care associated pneumonia: a critical appraisal to improve identification, management, and outcomes‐proceedings of the HCAP summit.Clin Infect Dis.2008;46:S296–S334.
- ,,,.Pneumonia in a long term care facility: a prospective study of outcome.Arch Intern Med.1996;156:2365–2370.
- .Understanding the natural history of community‐acquired pneumonia resolution: vital information for optimizing duration of therapy.Clin Infect Dis.2004;39:1791–1793.
- ,,,.Efficacy of short course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis.Am J Med.2007;120:783–790.
- ,.How long should we treat community‐acquired pneumonia?Curr Opin Infect Dis.2007;20:177–181.
- ,,.High‐dose, short course levofloxacin for community‐acquired pneumonia: a new treatment paradigm.Clin Infect Dis.2003;37:752–760.
- ,,,,.Improving the quality of antibiotic prescription patterns in general practice: the role of educational intervention.Med J Aust.1994;160:502–505.
- ,,,,,.Drug prescription attitudes and behaviour of general practitioners: effects of a problem oriented educational programme.Eur J Clin Pharmacol.1995;47:381–387.
- ,,, et al.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;CD003543.
Pneumonia is the second most common infection in nursing home residents after urinary tract infection, and is the most common reason for transfer to the hospital.1 Although it remains difficult to determine the incidence of pneumonia in institutionalized elderly patients, an estimated 4 million cases of nursing homeacquired pneumonia (NHAP) occur annually in the United States and result in more than 600,000 emergency department visits.2 In the past 2 decades, multiple studies have documented the rapid rise in drug resistance among common pathogens responsible for pneumonia in the elderly and the acquisition of multidrug‐resistant organisms in residents of long‐term care facilities.3, 4 Health care practitioners are faced with the dilemma of attempting to limit broad‐spectrum antimicrobial drug use while striving to maximize therapeutic efficacy in individual patients.5 The current practice guidelines for the management of NHAP from various professional societies provide mixed messages on the class of antibiotics for patients requiring hospitalization.2, 68 While the 2000 Canadian and the 2003 Infectious Disease Society of America (IDSA) guidelines advocate a community‐acquired pneumonia‐like approach to therapy, the 2005 American Thoracic Society (ATS)/IDSA guidelines and the 2007 IDSA/ATS guidelines consider drug‐resistant pathogens (DRPs) (ie, methicillin‐resistant Staphylococcus aureus [MRSA] and Pseudomonas aeruginosa) to be major etiologic agents in NHAP and thus the empiric treatment recommendations focus specifically on these pathogens (Table 1).
|
| 2003 IDSA |
| 1. Parenteral third‐generation cephalosporin or ampicillin sulbactam + macrolide; or |
| 2. Parenteral fluoroquinolone alone |
| 2000 Canadian |
| 1. Parenteral fluoroquinolone alone; or |
| 2. Parenteral third‐generation, or fourth‐generation cephalosporin + macrolide |
| 2005 ATS/IDSA |
| 1. Antipseudomonal cephalosporin or antipseudomonal carbapenem or antipseudomonal penicillin + antipseudomonal fluoroquinolone or aminoglycoside + anti‐methicillin‐resistant Staphylococcus agents |
Given these differences in antibiotic recommendations among the various guidelines, we sought to examine the antimicrobial prescription patterns in hospitalized non‐critically‐ill patients with NHAP in multiple tertiary care facilities vis‐‐vis the population demographics and clinical characteristics.
Methods
Study Population
This retrospective study was conducted in 3 tertiary‐care hospitals (Erie County Medical Center, Millard Fillmore Hospital, and Buffalo General Hospital) in the city of Buffalo, New York. These hospitals account for 96% of admissions from nursing homes in Erie County. The Institutional Review Board approved the study and certified that it met the criteria for a waiver of the requirement to obtain informed consent. All medical charts of adult patients with pneumonia listed under admission diagnosis or discharge diagnosis (International Classification of Diseases, ninth revision, Clinical Modification Codes [ICD‐9‐CM] [35] codes 480.0480.9, 481, 482.0482.9, 483.0483.8, 485, 486, 487.0, and 507.0) between April 2005 and December 2007 were abstracted. The records were searched for place of residence prior to admission and all patients residing in nursing homes for 30 days or more were selected for review. Inclusion criteria included the presence of new or increased radiographic abnormalities plus 2 or more of the following symptoms and signs: new or increased cough, new or increased sputum production, and temperature greater than 38C. Patients who met at least one of the following criteria were excluded: (1) admission to a critical care unit from the emergency department; (2) discharge within 24 hours; (3) human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) or immunocompromised; (4) transfer from another hospital; or (5) receiving active chemotherapy. Patients with multiple admissions were included only once to ensure independence of observations.
Data Collection
Data collected included information on sociodemographic characteristics, admitting service (University‐affiliated or private service), comorbidities, preadmission functional status, do not resuscitate (DNR) order, and prior antibiotic therapy. Antibiotic information was comprised of the name of the antibiotic, start and stop dates (including postdischarge), monotherapy or combination therapy, and route of administration. Antimicrobials were assigned to 1 of the following categories: macrolides (azithromycin, clarithromycin), lincosamide (clindamycin), fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin), carbapenems (ertapenem, imipenem), cephalosporins (ceftriaxone, cefpodoxime, cefepime), and ureidopenicillins (piperacillin‐tazobactam). Patients who died during the hospital stay before completion of therapy were assigned 14 days of antibiotic therapy.
The burden of comorbidities was assessed by the Charlson Index.9 The Activity of Daily Living (ADL) score was abstracted from a standardized patient‐review instrument included in all patients' charts.10 Patients were assigned an ADL score in each of the 6 major areas of activity: eating, toileting, feeding, bathing, mobility, and continence; ranging from 1 if they were fully independent, 2 if they were partially independent, and 3 if they were completely dependent. The ADL score was calculated by adding the points assigned for each activity, and it ranged from 6 to 18. Three categories were arbitrarily created: ADL I, corresponding to ADL scores from 6 to 8; ADL II, scores from 9 to 13; and ADL III, scores from 14 to 18.4
The Pneumonia Severity Index Score (PSI)11 was also calculated. The PSI is a validated disease‐severity classification system based on age, sex, nursing home residence, 5 comorbid illnesses, vital signs on admission, mental status, 7 laboratory values, and the findings on chest roentgenograms. Based on the scoring system, patients were stratified into 5 categories or classes of risk for in‐hospital mortality. Class I patients have the lowest disease severity while class V have the highest disease severity.
Statistics
Data were analyzed using the NCSS 2000 Statistical Analysis System (NCSS, Kaysville, UT). Continuous variables were tested for normal distribution using the Kolmogorov‐Smirnov test. Results are expressed as means standard deviation (SD). Univariate analysis was carried out using the chi‐square test and Fisher's exact test for categorical data and the t test for independent samples for continuous variables. Missing values for ADL and Charlson scores were encountered at <3% of the total population sample. Multiple regression models of available variables were utilized to predict missing values as described by Little and Rubin.12 All tests were 2‐tailed and statistical significance was determined at the 5% level.
Results
A total of 397 subjects with NHAP were included in the study. The mean age of the cohort group was 76.8 13.5 years. Eighty percent had 2 or more chronic diseases. Degenerative nervous system, cardiac, and pulmonary diseases accounted for the majority of underlying comorbidities. Demographic and clinical characteristics of the study population are presented in Table 2. At the time of admission, 17% of patients had received antimicrobial therapy for a respiratory ailment within the last week prior to transfer to an acute care facility. The most commonly prescribed agents at the nursing home were an oral fluoroquinolone (81%), a cephalosporin (14%), or a macrolide (3%).
| |
| Characteristic (n = 397) | |
| Age (years), mean (SD) | 76.8 (13.5) |
| Male, n (%) | 162 (41) |
| Underlying comorbidities, n (%) | |
| Cardiac diseases | 135 (34) |
| Pulmonary diseases | 129 (32) |
| Cerebrovascular accident | 98 (25) |
| Diabetes mellitus | 138 (35) |
| Dementia | 179 (45) |
| DNR, n (%) | 42 (11) |
| Activity of daily living, n (%) | |
| ADL I | 57 (14) |
| ADL II | 150 (38) |
| ADL III | 190 (48) |
| Pneumonia Severity Index, n (%) | |
| Class II | 13 (3) |
| Class III | 34 (8) |
| Class IV | 177 (45) |
| Class V | 173 (44) |
| Bacteremia, n (%) | 48 (12) |
Of the 397 patients who met the criteria for NHAP, all but 5 patients received antimicrobial therapy. The 3 most commonly used antimicrobial compounds for inpatient treatment were fluoroquinolones (51.4%), ceftriaxone (45.0%), and azithromycin (42.1%). None of the participating hospitals had an antibiotic restriction policy for the use of fluoroquinolones or vancomycin.
Monotherapy was prescribed in 57.4%. Fluoroquinolones represented 79.5% of these cases. The other monotherapy choices included a third‐generation cephalosporin (10.7%), piperacillin/tazobactam (8%), and vancomycin (0.2%). Combination therapy consisted mainly of a macrolide plus a third‐generation cephalosporin (74/168; 44%). Other combination regimens included vancomycin plus piperacillin/tazobactam plus ciprofloxacin (35%), vancomycin plus imipenem plus ciprofloxacin (9%), vancomycin plus piperacillin/tazobactam plus azithromycin (4%), vancomycin plus piperacillin/tazobactam (7%), and piperacillin/tazobactam plus azithromycin (1%). Figure 1 shows the distribution of vancomycin and fluoroquinolones use across the different age groups. While the use of fluoroquinolones (P = 0.76) was comparable between groups, there was a significant trend in prescribing less vancomycin with increasing age (P < 0.001). As for the rest of the antibiotics, there was no difference in the overall use of macrolides (P = 0.53), cephalosporins (P = 0.84), or carbapenems (P = 0.67) among age groups. Clindamycin was only used in 9 (2%) out of 392 patients. None of the patients had an aminoglycoside or a sulfa drug prescribed. We also found no difference in terms of antibiotic choice or use of combination therapy among the 3 hospitals (P = 0.78, and P = 0.52; respectively).

Antibiotic choices were influenced by severity of illness. There was an inverse relationship between PSI classes and the use of either fluoroquinolones or ceftriaxone plus azithromycin (P = 0.02) (Figure 2). Patients with higher acuity of illness were more likely to receive combination regimens that include vancomycin plus piperacillin/tazobactam than those with lower acuity of illness (P < 0.001). Neither the comorbidity index nor the ADL scores had a significant impact on the use of combination therapy (P = 0.49 and P = 0.2; respectively). There was a trend toward association between increasing ADL score and the use of vancomycin plus piperacillin/tazobactam but it did not reach statistical significance (P = 0.06). Of interest, patients who were admitted on the University‐affiliated service were more likely to receive combination therapy than those who were under the care of private service (P < 0.001) (Figure 3). Ceftriaxone plus azithromycin accounted for the majority of combination regimens irrespective of physicians' affiliation.


Overall, there were more patients who received antibiotic therapy in compliance with the 2003 IDSA guidelines6 compared with the 2005 ATS/IDSA guidelines7 (65% vs. 19%, respectively; P < 0.001). A positive correlation was noted between severity of illness and adherence to the 2005 ATS/IDSA antimicrobial recommendations (P = 0.02). However, neither the burden of comorbidities nor the functional status was associated with the use of guidelines (P = 0.76 and P = 0.43; respectively).
Duration of therapy ranged from 3 to 21 days with a median of 8 days. The choice of antibiotics, burden of comorbidities, DNR status, or PSI scores had no correlation with antibiotic duration. Only the presence of bacteremia was associated with more than 8 days of antibiotic duration (P < 0.001) (Figure 4). On average, bacteremic patients received 10.1 3.3 (range, 6‐21) days of antimicrobial therapy compared to 7.8 4.1 (range, 319) days for nonbacteremic cases (P < 0.001). During the course of hospitalization, change in antibiotics occurred in 35 (9%) out of the 392 patients, with the majority of substitutions affecting those who were initially prescribed a regimen that included vancomycin plus piperacillin/tazobactam. In these cases, patients were most commonly switched to fluoroquinolones (n = 20), followed by cephalosporins (n = 11).

Discussion
Our study suggests that antimicrobial selection among hospitalized nursing homes patients with pneumonia is influenced by patients' age, severity of illness, and provider's academic affiliation.
This is the first comprehensive study, to our knowledge, to report on the type, distribution, and pattern of antimicrobials prescribed among institutionalized patients requiring hospital admission. Various treatment regimens have been investigated in the last 2 decades using both retrospective and prospective randomized clinical trials to examine the efficacy and safety of parenteral and oral antibiotics in nursing homes.1316 However, there are no randomized controlled clinical trials for the treatment of hospitalized NHAP on which to base treatment recommendations. For some healthcare providers, the treatment parallels the coverage of patients with community‐acquired pneumonia; for others, broad‐spectrum coverage is the norm. In the absence of validated guidelines, the present investigation shows that prescription patterns varied across demographic and clinical characteristics. Fluoroquinolones were the preferred agents for the initial therapy of NHAP across all age groups, probably because of their single daily dosing, broad spectrum coverage against typical and atypical pathogens, and favorable side effect profile. Conversely, the use of vancomycin tended to decline in older age groups. This decline could be attributed to the need for frequent monitoring of trough levels when venous access can be difficult, lack of oral formulation, or potential toxicity. Further studies are needed to examine the validity of this pattern.
To our knowledge, compliance with guidelines regarding treatment of NHAP has not been previously reported. Despite recent studies suggesting that adherence to community‐acquired pneumonia guidelines resulted in reduced need for hospitalization, shorter stays, and lower mortality,1721 our findings indicated a rather low compliance with the most recently published guidelines. Potential reasons for the low levels of compliance include lack of awareness, time lag for the information to be disseminated in the medical community, lack of endorsement by local opinion leaders, or local barriers to implementation of these guidelines. Unfortunately, little is known about physicians' familiarity and attitude toward NHAP guidelines use. Efforts to improve the effectiveness of pneumonia care will depend on future studies aiming at identifying factors that influence nonadherence.
Severity of illness had a significant influence on the prescription pattern of antimicrobial therapy. As the PSI increased, treatment with a fluoroquinolone or with combination therapy of nonpseudomonal third‐generation cephalosporin plus macrolide was replaced by a broader spectrum of antimicrobial coverage. We believe that healthcare providers' prescriptions may be influenced by the recommendations of the ATS guidelines for the treatment of health care associated pneumonia,7 in which antimicrobial therapy for severely ill patients admitted from long‐term care facilities is directed toward multidrug resistant pathogens. The validity of this practice, however, remains the subject of intense debate,2225 driven by the absence of randomized trials showing improved morbidity and mortality.
Few formal clinical trials exist to guide the length of therapy of hospitalized patients with NHAP. The usual recommendation ranges from 7 to 14 days.16, 26 The median duration of 8 days observed in the current study is consistent with length of therapy advocated in the literature.16 Yet, prolonging antibiotic duration has been suggested when clinical severity of illness is high, comorbid illnesses are multiple, and expected resolution is delayed.27 Arguing against such a practice is evidence from meta‐analysis,28 expert reviews,29 and clinical investigation.30 Prescribing principles are nevertheless unlikely to induce substantial change unless their dissemination and promotion is sustained through intensive continuing educational programs for physicians and pharmacists.3133
Our study has a number of limitations. First, the cohort group described in this investigation consists of institutionalized patients in Western New York and hence the antibiotic prescribing patterns may vary in other locations. Second, we did not have adequate microbial information to fully assess the appropriateness of antimicrobial therapy. Third, the absence of microbial etiology may have resulted in incorrect identification of patients with pneumonia. Further, retrospective data extraction is notoriously imperfect, and pneumonia cases may have been missed because of either coding errors or atypical manifestations. However, we have used strict inclusion criteria in to minimize any potential bias. Fourth, the results of this study describe patterns of antibiotic utilization in the treatment of NHAP but do not provide reasoning for such a practice. The rationale behind these practices can only be discerned by a survey of healthcare providers.
In conclusion, we have observed in this study a poor compliance with the current guidelines for the treatment of NHAP. It is generally accepted that physicians' prescribing habits are influenced by their understanding of the pathophysiology and epidemiology of the infection being treated, as well as the pharmacology and spectrum of available antimicrobials. In the absence of outcome data, translation of this knowledge into practice may be influenced by a number of factors, such as the physician's preference, the academic milieu in which the practice occurs, and more importantly, by the patients' clinical condition.
Pneumonia is the second most common infection in nursing home residents after urinary tract infection, and is the most common reason for transfer to the hospital.1 Although it remains difficult to determine the incidence of pneumonia in institutionalized elderly patients, an estimated 4 million cases of nursing homeacquired pneumonia (NHAP) occur annually in the United States and result in more than 600,000 emergency department visits.2 In the past 2 decades, multiple studies have documented the rapid rise in drug resistance among common pathogens responsible for pneumonia in the elderly and the acquisition of multidrug‐resistant organisms in residents of long‐term care facilities.3, 4 Health care practitioners are faced with the dilemma of attempting to limit broad‐spectrum antimicrobial drug use while striving to maximize therapeutic efficacy in individual patients.5 The current practice guidelines for the management of NHAP from various professional societies provide mixed messages on the class of antibiotics for patients requiring hospitalization.2, 68 While the 2000 Canadian and the 2003 Infectious Disease Society of America (IDSA) guidelines advocate a community‐acquired pneumonia‐like approach to therapy, the 2005 American Thoracic Society (ATS)/IDSA guidelines and the 2007 IDSA/ATS guidelines consider drug‐resistant pathogens (DRPs) (ie, methicillin‐resistant Staphylococcus aureus [MRSA] and Pseudomonas aeruginosa) to be major etiologic agents in NHAP and thus the empiric treatment recommendations focus specifically on these pathogens (Table 1).
|
| 2003 IDSA |
| 1. Parenteral third‐generation cephalosporin or ampicillin sulbactam + macrolide; or |
| 2. Parenteral fluoroquinolone alone |
| 2000 Canadian |
| 1. Parenteral fluoroquinolone alone; or |
| 2. Parenteral third‐generation, or fourth‐generation cephalosporin + macrolide |
| 2005 ATS/IDSA |
| 1. Antipseudomonal cephalosporin or antipseudomonal carbapenem or antipseudomonal penicillin + antipseudomonal fluoroquinolone or aminoglycoside + anti‐methicillin‐resistant Staphylococcus agents |
Given these differences in antibiotic recommendations among the various guidelines, we sought to examine the antimicrobial prescription patterns in hospitalized non‐critically‐ill patients with NHAP in multiple tertiary care facilities vis‐‐vis the population demographics and clinical characteristics.
Methods
Study Population
This retrospective study was conducted in 3 tertiary‐care hospitals (Erie County Medical Center, Millard Fillmore Hospital, and Buffalo General Hospital) in the city of Buffalo, New York. These hospitals account for 96% of admissions from nursing homes in Erie County. The Institutional Review Board approved the study and certified that it met the criteria for a waiver of the requirement to obtain informed consent. All medical charts of adult patients with pneumonia listed under admission diagnosis or discharge diagnosis (International Classification of Diseases, ninth revision, Clinical Modification Codes [ICD‐9‐CM] [35] codes 480.0480.9, 481, 482.0482.9, 483.0483.8, 485, 486, 487.0, and 507.0) between April 2005 and December 2007 were abstracted. The records were searched for place of residence prior to admission and all patients residing in nursing homes for 30 days or more were selected for review. Inclusion criteria included the presence of new or increased radiographic abnormalities plus 2 or more of the following symptoms and signs: new or increased cough, new or increased sputum production, and temperature greater than 38C. Patients who met at least one of the following criteria were excluded: (1) admission to a critical care unit from the emergency department; (2) discharge within 24 hours; (3) human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) or immunocompromised; (4) transfer from another hospital; or (5) receiving active chemotherapy. Patients with multiple admissions were included only once to ensure independence of observations.
Data Collection
Data collected included information on sociodemographic characteristics, admitting service (University‐affiliated or private service), comorbidities, preadmission functional status, do not resuscitate (DNR) order, and prior antibiotic therapy. Antibiotic information was comprised of the name of the antibiotic, start and stop dates (including postdischarge), monotherapy or combination therapy, and route of administration. Antimicrobials were assigned to 1 of the following categories: macrolides (azithromycin, clarithromycin), lincosamide (clindamycin), fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin), carbapenems (ertapenem, imipenem), cephalosporins (ceftriaxone, cefpodoxime, cefepime), and ureidopenicillins (piperacillin‐tazobactam). Patients who died during the hospital stay before completion of therapy were assigned 14 days of antibiotic therapy.
The burden of comorbidities was assessed by the Charlson Index.9 The Activity of Daily Living (ADL) score was abstracted from a standardized patient‐review instrument included in all patients' charts.10 Patients were assigned an ADL score in each of the 6 major areas of activity: eating, toileting, feeding, bathing, mobility, and continence; ranging from 1 if they were fully independent, 2 if they were partially independent, and 3 if they were completely dependent. The ADL score was calculated by adding the points assigned for each activity, and it ranged from 6 to 18. Three categories were arbitrarily created: ADL I, corresponding to ADL scores from 6 to 8; ADL II, scores from 9 to 13; and ADL III, scores from 14 to 18.4
The Pneumonia Severity Index Score (PSI)11 was also calculated. The PSI is a validated disease‐severity classification system based on age, sex, nursing home residence, 5 comorbid illnesses, vital signs on admission, mental status, 7 laboratory values, and the findings on chest roentgenograms. Based on the scoring system, patients were stratified into 5 categories or classes of risk for in‐hospital mortality. Class I patients have the lowest disease severity while class V have the highest disease severity.
Statistics
Data were analyzed using the NCSS 2000 Statistical Analysis System (NCSS, Kaysville, UT). Continuous variables were tested for normal distribution using the Kolmogorov‐Smirnov test. Results are expressed as means standard deviation (SD). Univariate analysis was carried out using the chi‐square test and Fisher's exact test for categorical data and the t test for independent samples for continuous variables. Missing values for ADL and Charlson scores were encountered at <3% of the total population sample. Multiple regression models of available variables were utilized to predict missing values as described by Little and Rubin.12 All tests were 2‐tailed and statistical significance was determined at the 5% level.
Results
A total of 397 subjects with NHAP were included in the study. The mean age of the cohort group was 76.8 13.5 years. Eighty percent had 2 or more chronic diseases. Degenerative nervous system, cardiac, and pulmonary diseases accounted for the majority of underlying comorbidities. Demographic and clinical characteristics of the study population are presented in Table 2. At the time of admission, 17% of patients had received antimicrobial therapy for a respiratory ailment within the last week prior to transfer to an acute care facility. The most commonly prescribed agents at the nursing home were an oral fluoroquinolone (81%), a cephalosporin (14%), or a macrolide (3%).
| |
| Characteristic (n = 397) | |
| Age (years), mean (SD) | 76.8 (13.5) |
| Male, n (%) | 162 (41) |
| Underlying comorbidities, n (%) | |
| Cardiac diseases | 135 (34) |
| Pulmonary diseases | 129 (32) |
| Cerebrovascular accident | 98 (25) |
| Diabetes mellitus | 138 (35) |
| Dementia | 179 (45) |
| DNR, n (%) | 42 (11) |
| Activity of daily living, n (%) | |
| ADL I | 57 (14) |
| ADL II | 150 (38) |
| ADL III | 190 (48) |
| Pneumonia Severity Index, n (%) | |
| Class II | 13 (3) |
| Class III | 34 (8) |
| Class IV | 177 (45) |
| Class V | 173 (44) |
| Bacteremia, n (%) | 48 (12) |
Of the 397 patients who met the criteria for NHAP, all but 5 patients received antimicrobial therapy. The 3 most commonly used antimicrobial compounds for inpatient treatment were fluoroquinolones (51.4%), ceftriaxone (45.0%), and azithromycin (42.1%). None of the participating hospitals had an antibiotic restriction policy for the use of fluoroquinolones or vancomycin.
Monotherapy was prescribed in 57.4%. Fluoroquinolones represented 79.5% of these cases. The other monotherapy choices included a third‐generation cephalosporin (10.7%), piperacillin/tazobactam (8%), and vancomycin (0.2%). Combination therapy consisted mainly of a macrolide plus a third‐generation cephalosporin (74/168; 44%). Other combination regimens included vancomycin plus piperacillin/tazobactam plus ciprofloxacin (35%), vancomycin plus imipenem plus ciprofloxacin (9%), vancomycin plus piperacillin/tazobactam plus azithromycin (4%), vancomycin plus piperacillin/tazobactam (7%), and piperacillin/tazobactam plus azithromycin (1%). Figure 1 shows the distribution of vancomycin and fluoroquinolones use across the different age groups. While the use of fluoroquinolones (P = 0.76) was comparable between groups, there was a significant trend in prescribing less vancomycin with increasing age (P < 0.001). As for the rest of the antibiotics, there was no difference in the overall use of macrolides (P = 0.53), cephalosporins (P = 0.84), or carbapenems (P = 0.67) among age groups. Clindamycin was only used in 9 (2%) out of 392 patients. None of the patients had an aminoglycoside or a sulfa drug prescribed. We also found no difference in terms of antibiotic choice or use of combination therapy among the 3 hospitals (P = 0.78, and P = 0.52; respectively).

Antibiotic choices were influenced by severity of illness. There was an inverse relationship between PSI classes and the use of either fluoroquinolones or ceftriaxone plus azithromycin (P = 0.02) (Figure 2). Patients with higher acuity of illness were more likely to receive combination regimens that include vancomycin plus piperacillin/tazobactam than those with lower acuity of illness (P < 0.001). Neither the comorbidity index nor the ADL scores had a significant impact on the use of combination therapy (P = 0.49 and P = 0.2; respectively). There was a trend toward association between increasing ADL score and the use of vancomycin plus piperacillin/tazobactam but it did not reach statistical significance (P = 0.06). Of interest, patients who were admitted on the University‐affiliated service were more likely to receive combination therapy than those who were under the care of private service (P < 0.001) (Figure 3). Ceftriaxone plus azithromycin accounted for the majority of combination regimens irrespective of physicians' affiliation.


Overall, there were more patients who received antibiotic therapy in compliance with the 2003 IDSA guidelines6 compared with the 2005 ATS/IDSA guidelines7 (65% vs. 19%, respectively; P < 0.001). A positive correlation was noted between severity of illness and adherence to the 2005 ATS/IDSA antimicrobial recommendations (P = 0.02). However, neither the burden of comorbidities nor the functional status was associated with the use of guidelines (P = 0.76 and P = 0.43; respectively).
Duration of therapy ranged from 3 to 21 days with a median of 8 days. The choice of antibiotics, burden of comorbidities, DNR status, or PSI scores had no correlation with antibiotic duration. Only the presence of bacteremia was associated with more than 8 days of antibiotic duration (P < 0.001) (Figure 4). On average, bacteremic patients received 10.1 3.3 (range, 6‐21) days of antimicrobial therapy compared to 7.8 4.1 (range, 319) days for nonbacteremic cases (P < 0.001). During the course of hospitalization, change in antibiotics occurred in 35 (9%) out of the 392 patients, with the majority of substitutions affecting those who were initially prescribed a regimen that included vancomycin plus piperacillin/tazobactam. In these cases, patients were most commonly switched to fluoroquinolones (n = 20), followed by cephalosporins (n = 11).

Discussion
Our study suggests that antimicrobial selection among hospitalized nursing homes patients with pneumonia is influenced by patients' age, severity of illness, and provider's academic affiliation.
This is the first comprehensive study, to our knowledge, to report on the type, distribution, and pattern of antimicrobials prescribed among institutionalized patients requiring hospital admission. Various treatment regimens have been investigated in the last 2 decades using both retrospective and prospective randomized clinical trials to examine the efficacy and safety of parenteral and oral antibiotics in nursing homes.1316 However, there are no randomized controlled clinical trials for the treatment of hospitalized NHAP on which to base treatment recommendations. For some healthcare providers, the treatment parallels the coverage of patients with community‐acquired pneumonia; for others, broad‐spectrum coverage is the norm. In the absence of validated guidelines, the present investigation shows that prescription patterns varied across demographic and clinical characteristics. Fluoroquinolones were the preferred agents for the initial therapy of NHAP across all age groups, probably because of their single daily dosing, broad spectrum coverage against typical and atypical pathogens, and favorable side effect profile. Conversely, the use of vancomycin tended to decline in older age groups. This decline could be attributed to the need for frequent monitoring of trough levels when venous access can be difficult, lack of oral formulation, or potential toxicity. Further studies are needed to examine the validity of this pattern.
To our knowledge, compliance with guidelines regarding treatment of NHAP has not been previously reported. Despite recent studies suggesting that adherence to community‐acquired pneumonia guidelines resulted in reduced need for hospitalization, shorter stays, and lower mortality,1721 our findings indicated a rather low compliance with the most recently published guidelines. Potential reasons for the low levels of compliance include lack of awareness, time lag for the information to be disseminated in the medical community, lack of endorsement by local opinion leaders, or local barriers to implementation of these guidelines. Unfortunately, little is known about physicians' familiarity and attitude toward NHAP guidelines use. Efforts to improve the effectiveness of pneumonia care will depend on future studies aiming at identifying factors that influence nonadherence.
Severity of illness had a significant influence on the prescription pattern of antimicrobial therapy. As the PSI increased, treatment with a fluoroquinolone or with combination therapy of nonpseudomonal third‐generation cephalosporin plus macrolide was replaced by a broader spectrum of antimicrobial coverage. We believe that healthcare providers' prescriptions may be influenced by the recommendations of the ATS guidelines for the treatment of health care associated pneumonia,7 in which antimicrobial therapy for severely ill patients admitted from long‐term care facilities is directed toward multidrug resistant pathogens. The validity of this practice, however, remains the subject of intense debate,2225 driven by the absence of randomized trials showing improved morbidity and mortality.
Few formal clinical trials exist to guide the length of therapy of hospitalized patients with NHAP. The usual recommendation ranges from 7 to 14 days.16, 26 The median duration of 8 days observed in the current study is consistent with length of therapy advocated in the literature.16 Yet, prolonging antibiotic duration has been suggested when clinical severity of illness is high, comorbid illnesses are multiple, and expected resolution is delayed.27 Arguing against such a practice is evidence from meta‐analysis,28 expert reviews,29 and clinical investigation.30 Prescribing principles are nevertheless unlikely to induce substantial change unless their dissemination and promotion is sustained through intensive continuing educational programs for physicians and pharmacists.3133
Our study has a number of limitations. First, the cohort group described in this investigation consists of institutionalized patients in Western New York and hence the antibiotic prescribing patterns may vary in other locations. Second, we did not have adequate microbial information to fully assess the appropriateness of antimicrobial therapy. Third, the absence of microbial etiology may have resulted in incorrect identification of patients with pneumonia. Further, retrospective data extraction is notoriously imperfect, and pneumonia cases may have been missed because of either coding errors or atypical manifestations. However, we have used strict inclusion criteria in to minimize any potential bias. Fourth, the results of this study describe patterns of antibiotic utilization in the treatment of NHAP but do not provide reasoning for such a practice. The rationale behind these practices can only be discerned by a survey of healthcare providers.
In conclusion, we have observed in this study a poor compliance with the current guidelines for the treatment of NHAP. It is generally accepted that physicians' prescribing habits are influenced by their understanding of the pathophysiology and epidemiology of the infection being treated, as well as the pharmacology and spectrum of available antimicrobials. In the absence of outcome data, translation of this knowledge into practice may be influenced by a number of factors, such as the physician's preference, the academic milieu in which the practice occurs, and more importantly, by the patients' clinical condition.
- .Pneumonia in the long‐term‐care facility.Infect Control Hosp Epidemiol.2002;23:159–164.
- ,,,,.Canadian guidelines for the initial management of an evidence based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Pneumonia Working Group.Clin Infect Dis.2000;31:383–421.
- ,,, et al.Antimicrobial resistance in long‐term care facilities.Infect Control Hosp Epidemiol.1996;17:129–140.
- ,,,.Etiology of severe pneumonia in the very elderly.Am J Respir Crit Care Med.2001;163:645–651.
- ,,,.Tensions in antibiotic prescribing: pitting social concerns against the interests of individual patients.J Gen Intern Med.2002;17:87–94.
- ,,,,,.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital acquired, ventilator‐associated, and health care associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,, et al.Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the management of community‐acquired pneumonia in adults.Clin Infect Dis.2007;44:S27–S72.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chron Dis.1987;40:373–383.
- New York State Department of Health. Hospital and Community Patient Review Instrument. DOH‐694.Albany, NY:Department of Health;1989.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,.Statistical Analysis with Missing Data.New York:John Wiley 1987.
- ,,,,.A randomized study of ciprofloxacin versus ceftriaxone in the treatment of nursing home‐acquired lower respiratory tract infections.J Am Geriatr Soc.1991;39:979–985.
- ,,, et al.Prospective study of lower respiratory tract infections in an extended‐care nursing home program: potential role of oral ciprofloxacin.Am J Med.1988;85:164–171.
- ,,.Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial.JAMA2006;295:2503–2510.
- ,.Treatment guideline for nursing‐home acquired pneumonia based on community practice.J Am Geriatr Soc.2000;48:82–88.
- ,,,,,.Decreased mortality after implementation of a treatment guideline for community‐acquired pneumonia.Am J Med.2001;110:451–457.
- ,,,,,.A controlled trial of a critical pathway for treatment of community‐acquired pneumonia. CAPITAL Study Investigators. Community‐Acquired Pneumonia Intervention Trial Assessing Levofloxacin.JAMA.2000;283:749–755.
- ,,, et al.Improvement of process‐of‐care and outcomes after implementing a guideline for the management of community‐acquired pneumonia: a controlled before‐and‐after design study.Clin Infect Dis.2004;39:955–963.
- ,,, et al.Guidelines for the treatment of community‐acquired pneumonia: predictors of adherence and outcome.Am J Respir Crit Care Med.2005;172:757–762.
- ,,, et al.Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival.Clin Infect Dis.2005;41:1709–1716.
- ,,.Nosocomial or healthcare facility‐related pneumonia in adults.Curr Infect Dis Rep.2000;2:215–223.
- ,.Pneumonia in the older patient.Clin Chest Med.2007;28:751–771.
- .Guidelines for the management of adults with health care‐associated pneumonia: implications for nursing facility residents.Consult Pharm.2006;21:719–725.
- ,,, et al.Health‐care associated pneumonia: a critical appraisal to improve identification, management, and outcomes‐proceedings of the HCAP summit.Clin Infect Dis.2008;46:S296–S334.
- ,,,.Pneumonia in a long term care facility: a prospective study of outcome.Arch Intern Med.1996;156:2365–2370.
- .Understanding the natural history of community‐acquired pneumonia resolution: vital information for optimizing duration of therapy.Clin Infect Dis.2004;39:1791–1793.
- ,,,.Efficacy of short course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis.Am J Med.2007;120:783–790.
- ,.How long should we treat community‐acquired pneumonia?Curr Opin Infect Dis.2007;20:177–181.
- ,,.High‐dose, short course levofloxacin for community‐acquired pneumonia: a new treatment paradigm.Clin Infect Dis.2003;37:752–760.
- ,,,,.Improving the quality of antibiotic prescription patterns in general practice: the role of educational intervention.Med J Aust.1994;160:502–505.
- ,,,,,.Drug prescription attitudes and behaviour of general practitioners: effects of a problem oriented educational programme.Eur J Clin Pharmacol.1995;47:381–387.
- ,,, et al.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;CD003543.
- .Pneumonia in the long‐term‐care facility.Infect Control Hosp Epidemiol.2002;23:159–164.
- ,,,,.Canadian guidelines for the initial management of an evidence based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Pneumonia Working Group.Clin Infect Dis.2000;31:383–421.
- ,,, et al.Antimicrobial resistance in long‐term care facilities.Infect Control Hosp Epidemiol.1996;17:129–140.
- ,,,.Etiology of severe pneumonia in the very elderly.Am J Respir Crit Care Med.2001;163:645–651.
- ,,,.Tensions in antibiotic prescribing: pitting social concerns against the interests of individual patients.J Gen Intern Med.2002;17:87–94.
- ,,,,,.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital acquired, ventilator‐associated, and health care associated pneumonia.Am J Respir Crit Care Med.2005;171:388–416.
- ,,, et al.Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the management of community‐acquired pneumonia in adults.Clin Infect Dis.2007;44:S27–S72.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chron Dis.1987;40:373–383.
- New York State Department of Health. Hospital and Community Patient Review Instrument. DOH‐694.Albany, NY:Department of Health;1989.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,.Statistical Analysis with Missing Data.New York:John Wiley 1987.
- ,,,,.A randomized study of ciprofloxacin versus ceftriaxone in the treatment of nursing home‐acquired lower respiratory tract infections.J Am Geriatr Soc.1991;39:979–985.
- ,,, et al.Prospective study of lower respiratory tract infections in an extended‐care nursing home program: potential role of oral ciprofloxacin.Am J Med.1988;85:164–171.
- ,,.Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial.JAMA2006;295:2503–2510.
- ,.Treatment guideline for nursing‐home acquired pneumonia based on community practice.J Am Geriatr Soc.2000;48:82–88.
- ,,,,,.Decreased mortality after implementation of a treatment guideline for community‐acquired pneumonia.Am J Med.2001;110:451–457.
- ,,,,,.A controlled trial of a critical pathway for treatment of community‐acquired pneumonia. CAPITAL Study Investigators. Community‐Acquired Pneumonia Intervention Trial Assessing Levofloxacin.JAMA.2000;283:749–755.
- ,,, et al.Improvement of process‐of‐care and outcomes after implementing a guideline for the management of community‐acquired pneumonia: a controlled before‐and‐after design study.Clin Infect Dis.2004;39:955–963.
- ,,, et al.Guidelines for the treatment of community‐acquired pneumonia: predictors of adherence and outcome.Am J Respir Crit Care Med.2005;172:757–762.
- ,,, et al.Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival.Clin Infect Dis.2005;41:1709–1716.
- ,,.Nosocomial or healthcare facility‐related pneumonia in adults.Curr Infect Dis Rep.2000;2:215–223.
- ,.Pneumonia in the older patient.Clin Chest Med.2007;28:751–771.
- .Guidelines for the management of adults with health care‐associated pneumonia: implications for nursing facility residents.Consult Pharm.2006;21:719–725.
- ,,, et al.Health‐care associated pneumonia: a critical appraisal to improve identification, management, and outcomes‐proceedings of the HCAP summit.Clin Infect Dis.2008;46:S296–S334.
- ,,,.Pneumonia in a long term care facility: a prospective study of outcome.Arch Intern Med.1996;156:2365–2370.
- .Understanding the natural history of community‐acquired pneumonia resolution: vital information for optimizing duration of therapy.Clin Infect Dis.2004;39:1791–1793.
- ,,,.Efficacy of short course antibiotic regimens for community‐acquired pneumonia: a meta‐analysis.Am J Med.2007;120:783–790.
- ,.How long should we treat community‐acquired pneumonia?Curr Opin Infect Dis.2007;20:177–181.
- ,,.High‐dose, short course levofloxacin for community‐acquired pneumonia: a new treatment paradigm.Clin Infect Dis.2003;37:752–760.
- ,,,,.Improving the quality of antibiotic prescription patterns in general practice: the role of educational intervention.Med J Aust.1994;160:502–505.
- ,,,,,.Drug prescription attitudes and behaviour of general practitioners: effects of a problem oriented educational programme.Eur J Clin Pharmacol.1995;47:381–387.
- ,,, et al.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;CD003543.
Copyright © 2010 Society of Hospital Medicine