User login
Implementation of a Process for Initiating Naltrexone in Patients Hospitalized for Alcohol Detoxification or Withdrawal
Alcohol use disorders (AUDs) are common, with an estimated lifetime prevalence of 17.8% for alcohol dependence.1 Alcohol misuse is costly, accounting for $24.6 billion in annual healthcare expenditures, including $5.1 billion for alcohol-related hospitalizations.2 A number of trials have demonstrated that naltrexone can help patients with AUDs maintain abstinence or diminish heavy drinking.3-10 A recent meta-analysis of pharmacotherapy trials for patients with AUDs reported that for patients using 50 mg of naltrexone daily, the number needed to treat was 12 to prevent a return to heavy drinking and 20 to prevent a return to any drinking.11 Despite good evidence for its effectiveness, naltrexone is not prescribed to the majority of patients with AUDs. In a study of veterans with AUDs cared for in the Veterans Affairs health system, only 1.9% of patients were prescribed naltrexone over the 6-month study period.12 A 2003 survey of 2 professional organizations for addiction treatment specialists reported that a mean of 13% of providers prescribed naltrexone to their patients.13
When naltrexone is prescribed, it is most frequently in the outpatient setting.3-10 Data for initiation of naltrexone in the inpatient setting are more limited. Wei et al.14 reported on the implementation of a discharge protocol, including counseling about naltrexone, for hospitalized patients with AUDs at an urban academic medical center. They reported a significant increase in the prescription of naltrexone to eligible patients by the time of discharge that was associated with a significant decrease in 30-day readmissions. Initiation of naltrexone in the inpatient versus the outpatient setting has some potential advantages. First, patients hospitalized for alcohol withdrawal have AUDs, obviating the need for screening. Second, the outpatient trials of naltrexone typically required 3 days of sobriety before initiation, which is generally achieved during hospitalization for detoxification or withdrawal.
Previous work at our institution centered on standardizing the process of evaluating patients needing alcohol detoxification at the time of referral for admission.15 The use of a standardized protocol reduced the number of inpatient admissions for alcohol-related diagnoses but had no effect on the 30-day readmission rate (28%) for those patients who were hospitalized. Our hospitalist group had no standardized process for discharging hospitalized patients with AUDs, and the discharge process rarely included counseling on medications for maintenance of sobriety. In this manuscript, we describe the implementation and impact of a process for counseling patients hospitalized for alcohol detoxification or withdrawal about naltrexone for maintenance of sobriety by the time of hospital discharge.
METHODS
Study Setting
The University of North Carolina (UNC) Hospitals is an 803-bed tertiary academic center. UNC Hospital Medicine is staffed by 29 physicians and 3 advanced practice providers (APPs). During the study period, there were 3 hospital medicine services at UNC Hospitals with a combined average daily census of approximately 40 patients, and each service was staffed by one attending physician every day of the week and one APP Monday through Friday.
Study Design
We used a pre-post study design, in which we implemented a new process for standardizing the discharge of hospitalized patients with AUDs, including a process for counseling about naltrexone by the time of discharge. We sought and received institutional review board (IRB) approval for this study (UNC IRB 15-1441).
Interventions
We formed an improvement team that included 3 physicians and an APP in hospital medicine, a general internist and a psychiatrist, both with expertise in the use of medications for maintenance of sobriety, the director of UNC’s Alcohol and Substance Abuse Program, and 2 case managers. The team developed a number of interventions, including group education, a process for patient identification, and algorithms for counseling about, prescribing, and documenting the discussion of naltrexone.
Group Education
We presented evidence about medications for the maintenance of sobriety at a regularly scheduled hospitalist meeting. An hour-long session on motivational interviewing techniques was also presented at a separate meeting. All created algorithms were circulated to the group electronically and posted at workstations in the hospitalist work area. As data were generated postimplementation, control charts of process measures were created, posted in the hospitalist work area, and presented at subsequent group meetings.
Identification of Patients
We focused our interventions on patients admitted for alcohol detoxification or withdrawal (including withdrawal seizures). We asked our group to preferentially admit these patients to 1 of our 3 hospitalists services, on which the service APP (K.S.) was also an improvement team member.
Creation of Algorithms and Scripts for Counseling
We created a simple algorithm for evaluating patients for naltrexone. We recommended that all patients admitted for alcohol detoxification or withdrawal be counseled about naltrexone for the maintenance of sobriety before discharge. The contraindications to naltrexone we included were (1) concurrent opioid use, (2) documented cirrhosis, and/or (3) liver function tests greater than 3 times the upper limit of normal by the time of hospital discharge.
We also created a suggested script for motivational interviewing (supplemental Appendix 1). This was presented at a group meeting and circulated via e-mail. The actual counseling technique and process was left up to individual providers. In practice, counseling took place in the course of daily rounds, generally the day before or day of hospital discharge.
Prescription of Medication
For interested patients without contraindications, we recommended a prescription of naltrexone at 50 mg daily for 3 months. For patients prescribed naltrexone without medical insurance (n = 17), we utilized our existing pharmacy assistance program, whereby discharging patients can obtain an initial 14-day supply after applying to the program and then can fill subsequent prescriptions if they meet program financial requirements.
Follow-up Appointments
For patients with established outpatient providers, we asked patients to schedule follow-up appointments within a month of discharge. Patients prescribed naltrexone without primary providers (n = 16) were eligible for an existing program, the UNC Transitions Program, whereby patients identified as having moderate-to-high risk of hospital readmission can receive a follow-up appointment at UNC Internal Medicine or UNC Family Medicine within 2 weeks of discharge.
Creation of “Smart Phrases”
To aid in documentation, we created “smart phrases” (easily accessed, previously created phrases that can be adopted by all users) within the hospital electronic health record. We created one smart phrase for documentation of counseling about naltrexone, which included dropdown menus for contraindications and the patient’s preference and one for discharge instructions for patients started on naltrexone (supplemental Appendix 2).
Implementation
After the presentation of suggested interventions in July 2015 and the subsequent dissemination of educational materials, we implemented our new process on August 1, 2015.
Data Collection
Patients were identified for inclusion in the study analysis by querying UNC Hospitals’ billing database for the inpatient diagnosis codes (diagnosis-related groupings) 896 and 897, “alcohol/drug abuse or dependence without rehabilitation therapy,” with and without major comorbidity or complication, respectively, and with hospital medicine as the discharging service. All encounters were then manually reviewed by 2 investigators (J.S. and C.M.). Encounters were included if the history and physical indicated that the primary reason for admission was alcohol detoxification or withdrawal. Encounters with other primary reasons for admission (eg, pancreatitis, gastrointestinal bleeding) were excluded. For patients with multiple encounters, only the first eligible encounter in the pre- and/or postimplementation period was included. Comorbidities for identified patients were assessed via the search of study encounters for the International Classification of Diseases, 9th Revision-Clinical Modification codes for hypertension, anxiety, depression, cirrhosis, diabetes, and congestive heart failure.
Process, Outcomes, and Balancing Measures
The study process measures included the percentage of patients hospitalized for alcohol detoxification or withdrawal with documentation of counseling about naltrexone by the time of discharge, before and after process intervention. Documentation was defined as the description of counseling about naltrexone in the discharge summary or progress notes of identified encounters. We also measured the percentage of patients started on naltrexone before and after intervention. Lastly, we measured the percentage of patients prescribed naltrexone who filled at least 1 prescription for the medication, assessed by calls to the pharmacy where the medication was prescribed. Prescriptions that could not be confirmed (ie, paper rather than electronic prescriptions) were counted as not filled.
For outcome measures, we recorded the percentages of study patients who returned to the emergency department (ED) and were readmitted to UNC Hospitals (inpatient or observation) for any reason within 30 days of discharge. These outcomes were determined by a manual chart review.
In order to ensure the new process was not associated with delays in patient discharge, we measured the mean length of stay in days for study patient encounters before and after intervention as a balancing measure.
Statistical Analysis
Demographic and clinical characteristics for included patients were compared for the 16 months preimplementation (April 1, 2014 through July 31, 2015) and the 19 months postimplementation (August 1, 2015 through February 28, 2017). Descriptive statistics were calculated by using the Student t test for continuous variables and the χ2 test for dichotomous variables. We used multivariate logistic regression to evaluate the associations between the intervention arms (pre- vs postintervention) and study outcomes, adjusting for age, gender, race, insurance type, and medical comorbidities. We chose these variables for inclusion based on their association with study outcomes at the P ≤ .20 level in bivariate analyses. P < .05 was considered statistically significant. All analyses were performed by using Stata version 13.1 (StataCorp LLC, College Station, TX).
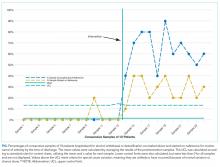
For 2 process measures, the percentages of patients counseled about and started on naltrexone, we plotted consecutive samples of 10 patients before and after intervention on a control chart, using preintervention data to calculate means and control limits.
Subgroup Analysis
We used multivariate logistic regression to evaluate the associations between counseling versus no counseling and prescription of naltrexone versus no prescription for study outcomes in the postintervention subgroup, adjusting for age, gender, race, insurance type, and medical comorbidities.
RESULTS
Patients
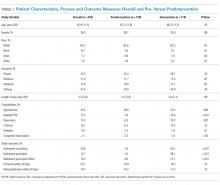
We identified 188 preimplementation encounters and excluded 12 patients (6.4%) for primary admission reasons other than alcohol withdrawal or detoxification and 48 (25.5%) repeat hospitalizations, leaving 128 unique patient encounters. We identified 166 postimplementation encounters and excluded 25 (15.1%) hospitalizations for admission reason and 27 repeat hospitalizations (16.3%), leaving 114 unique patient encounters (flow diagram in supplemental Appendix 3). The most common admission reason for the exclusion of encounters was withdrawal from a substance other than alcohol (supplemental Appendix 4). The percentages of encounters excluded in preimplementation and postimplementation periods were similar at 31.9% and 31.4%, respectively.
The majority of patients were male and white, and almost half were uninsured (Table 1). There were no demographic differences between patients in the pre- versus postimplementation groups. For studied comorbidities, postintervention patients were more likely to have hypertension, anxiety, and depression.
Process Measures
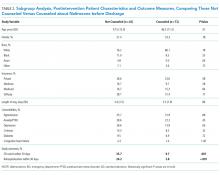
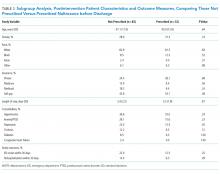
Among those counseled about naltrexone before discharge, 34 of 74 patients (45.9%) had no contraindications to naltrexone and were interested in taking the medication. Among the 40 patients who were counseled about but not prescribed naltrexone, 19 (47.5%) declined, 9 (22.5%) had liver function tests elevated more than 3 times the upper limit of the reference range, 9 (22.5%) had concurrent opiate use, and 3 (7.5%) had multiple contraindications.
Among the 34 patients who were prescribed naltrexone, 25 (73.5%) filled at least 1 prescription as confirmed by phone call to the relevant pharmacy.
Outcome Measures
Subgroup Analysis
Balancing Measure
The mean length of stay for all patient encounters was 3.3 days. There were no differences in length of stay comparing pre- with postintervention patient encounters (Table 1) or those postintervention patients counseled versus not counseled (Table 2).
DISCUSSION
Our study demonstrates that counseling about medications for the maintenance of sobriety can be implemented as part of the routine care of hospitalized patients with AUDs. In our experience, about half of the patients counseled had no contraindications to naltrexone and were willing to take it at discharge. Almost three-fourths of those who were prescribed naltrexone filled the prescription at least once. The counseling process was not associated with increased length of stay. In the adjusted analysis, postintervention patients had significantly lower odds of 30-day ED returns. Additionally, in subgroup analysis, postintervention patients counseled about naltrexone had significantly lower rates of subsequent healthcare utilization compared with those not counseled, with absolute differences of 26% for ED revisits and 22% for rehospitalizations within 30 days.
The failure to demonstrate a difference in adjusted rehospitalization rates in the postintervention versus the preintervention group has several possible explanations. First, we had incomplete fidelity to our interventions, documenting counseling about naltrexone before discharge in over 60% of postintervention patients, raising the possibility that better fidelity may have resulted in improved outcomes. Related to this, only 28% of postintervention patients were prescribed naltrexone, which may be an inadequate sample size to demonstrate positive effects from the medication. Another possible explanation is that the postintervention group had higher rates of some of the comorbidities we assessed, namely, anxiety, depression, and hypertension, which could have negatively impacted the effectiveness of the interventions to prevent rehospitalization; however, after adjusting for comorbidities, the odds of rehospitalization were still not significantly different. It is interesting that the odds of postintervention ED revisits (but not rehospitalizations) were lower in the adjusted analysis. It may be that patients who revisit the ED and are not rehospitalized are different in important ways from those who are readmitted. Alternately, the larger number of ED revisits overall (about twice the rate of rehospitalization) may have made it easier to identify positive effects from the intervention for this outcome than rehospitalization (ie, the study may have been underpowered to detect a relatively small reduction in rehospitalization). It is also possible, however, that the interventions were simply insufficient to prevent rehospitalization.
The subgroup analysis, however, did find significant differences in both outcome measures for postintervention patients counseled versus not counseled about naltrexone before discharge. There are several possible explanations for these results. First, there may have been unmeasured differences in those counseled versus not counseled that explain the reductions observed in subsequent healthcare utilization. For example, the counseled patients could have been more motivated to change and, thus, more readily approached by providers for counseling. The lack of any demographic differences between the 2 groups and the relative simplicity of the counseling part of the intervention occurring as part of daily rounds argue against this hypothesis, but there are many potential unmeasured confounders (eg, homelessness, ability to afford medications), and this possibility remains. A second possible explanation is that patients counseled about naltrexone could have been more likely than those not counseled to seek subsequent care at other institutions. A third possibility is that that the counseling about (and prescribing when appropriate) naltrexone itself led to the observed decreases in subsequent ED visits and hospitalizations. This hypothesis would have been more supported had we been able to demonstrate a statistically significant reduction in healthcare utilization in those prescribed versus not prescribed naltrexone. But there were nonsignificant trends in the reduction of ED revisits and rehospitalizations among those prescribed the medication, suggesting we may have been able to demonstrate statistically significant reductions with a larger sample size.
Comparing our results with existing literature is challenging. The majority of randomized trials of naltrexone for AUDs were conducted in the outpatient setting.3-10 Most of these trials utilized some type of psychosocial intervention in addition to naltrexone.3-5,8-10 The 1 prior naltrexone study we identified conducted in the inpatient setting by Wei et al.14 is the most similar to our study. The authors reported the effects of a new process for assessing hospitalized patients with AUDs, including the use of a discharge planning tool for all patients admitted with alcohol dependence. The discharge tool included prompts for naltrexone in appropriate patients. The measured outcomes included the percentage of eligible patients prescribed naltrexone at discharge and the percentages of ED revisits and rehospitalizations within 30 days. Postintervention, 64% of eligible patients were prescribed naltrexone compared with 0% before, very similar to our results. There were significant decreases among all discharged patients with alcohol dependence for 30-day ED revisits (18.8% pre- vs 6.1% postimplementation) and rehospitalizations (23.4% vs 8.2%). The study differed from ours in a number of important respects, including a location in a large urban setting and implementation on a teaching service rather than an attending-only hospitalist service. Additionally, the authors studied 1 month of process implementation and compared it to another month 1 year before the new process, with an overall smaller sample size of 64 patients before and 49 patients after implementation. Potential reasons why Wei et al.14 were able to document lower rehospitalization rates postintervention when we did not include the differences in patient population (eg, high homeless rate, lower percentage of female patients in Wei study) and secular trends unrelated to interventions in either study.
Limitations of our study include the nonrandomized and uncontrolled design, which introduces the possibility of unmeasured confounding factors leading to the decrease we observed in healthcare utilization. Additionally, the single-center design precludes our ability to assess for healthcare utilization outcomes in other nearby facilities. We had incomplete implementation of our new process, counseling just over 60% of patients. As our primary outcomes relied on documentation in the medical record, both undersampling (not documenting some interventions) and reporting bias (being more likely to record positive sessions from intervention) are possible. Lastly, despite a moderate total sample size of almost 250 patients, the relatively small numbers of patients who were actually prescribed naltrexone in our study lessens our ability to show direct impact.
In conclusion, our study demonstrates a practical process for counseling about and prescribing naltrexone to patients hospitalized for alcohol detoxification or withdrawal. We demonstrate that many of these patients will be interested in starting naltrexone at discharge and will reliably fill the prescriptions if written. Counseling was associated with a significant reduction in subsequent healthcare utilization. These results have a wide potential impact given the ubiquitous nature of AUDs among hospitalized patients in community and academic settings.
Disclosure
The authors have no conflicts of interest relevant to this article to disclose. There were no sources of funding for this work.
1. Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842. PubMed
2. Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41(5):516-524. PubMed
3. Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156(11):1758-1764. PubMed
4. Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol. 2005;25(4):349-357. PubMed
5. Guardia J, Caso C, Arias F, et al. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: results from a multicenter clinical trial. Alcohol Clin Exp Res. 2002;26(9):1381-1387. PubMed
6. Kiefer F, Jahn H, Tarnaske T, et al. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60(1):92-99. PubMed
7. Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Aust. 2002;176(11):530-534. PubMed
8. Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: a randomized controlled trial. Addiction. 2001;96(11):1565-1573. PubMed
9. O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49(11):881-887. PubMed
10. O’Malley SS, Robin RW, Levenson AL, et al. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska natives and non-natives residing in rural settings: a randomized controlled trial. Alcohol Clin Exp Res. 2008;32(7):1271-1283. PubMed
11. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 2014;311(18):1889-1900. PubMed
12. Petrakis IL, Leslie D, Rosenheck R. Use of naltrexone in the treatment of alcoholism nationally in the Department of Veterans Affairs. Alcohol Clin Exp Res. 2003;27(11):1780-1784. PubMed
13. Mark TL, Kranzler HR, Song X. Understanding US addiction physicians’ low rate of naltrexone prescription. Drug Alcohol Depend. 2003;71(3):219-228. PubMed
14. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. PubMed
15. Stephens JR, Liles EA, Dancel R, Gilchrist M, Kirsch J, DeWalt DA. Who needs inpatient detox? Development and implementation of a hospitalist protocol for the evaluation of patients for alcohol detoxification. J Gen Intern Med. 2014;29(4):587-593. PubMed
16. Provost LP, Murray SK. The Health Care Data Guide: Learning from Data for Improvement. San Francisco: Jossey-Bass; 2011.
Alcohol use disorders (AUDs) are common, with an estimated lifetime prevalence of 17.8% for alcohol dependence.1 Alcohol misuse is costly, accounting for $24.6 billion in annual healthcare expenditures, including $5.1 billion for alcohol-related hospitalizations.2 A number of trials have demonstrated that naltrexone can help patients with AUDs maintain abstinence or diminish heavy drinking.3-10 A recent meta-analysis of pharmacotherapy trials for patients with AUDs reported that for patients using 50 mg of naltrexone daily, the number needed to treat was 12 to prevent a return to heavy drinking and 20 to prevent a return to any drinking.11 Despite good evidence for its effectiveness, naltrexone is not prescribed to the majority of patients with AUDs. In a study of veterans with AUDs cared for in the Veterans Affairs health system, only 1.9% of patients were prescribed naltrexone over the 6-month study period.12 A 2003 survey of 2 professional organizations for addiction treatment specialists reported that a mean of 13% of providers prescribed naltrexone to their patients.13
When naltrexone is prescribed, it is most frequently in the outpatient setting.3-10 Data for initiation of naltrexone in the inpatient setting are more limited. Wei et al.14 reported on the implementation of a discharge protocol, including counseling about naltrexone, for hospitalized patients with AUDs at an urban academic medical center. They reported a significant increase in the prescription of naltrexone to eligible patients by the time of discharge that was associated with a significant decrease in 30-day readmissions. Initiation of naltrexone in the inpatient versus the outpatient setting has some potential advantages. First, patients hospitalized for alcohol withdrawal have AUDs, obviating the need for screening. Second, the outpatient trials of naltrexone typically required 3 days of sobriety before initiation, which is generally achieved during hospitalization for detoxification or withdrawal.
Previous work at our institution centered on standardizing the process of evaluating patients needing alcohol detoxification at the time of referral for admission.15 The use of a standardized protocol reduced the number of inpatient admissions for alcohol-related diagnoses but had no effect on the 30-day readmission rate (28%) for those patients who were hospitalized. Our hospitalist group had no standardized process for discharging hospitalized patients with AUDs, and the discharge process rarely included counseling on medications for maintenance of sobriety. In this manuscript, we describe the implementation and impact of a process for counseling patients hospitalized for alcohol detoxification or withdrawal about naltrexone for maintenance of sobriety by the time of hospital discharge.
METHODS
Study Setting
The University of North Carolina (UNC) Hospitals is an 803-bed tertiary academic center. UNC Hospital Medicine is staffed by 29 physicians and 3 advanced practice providers (APPs). During the study period, there were 3 hospital medicine services at UNC Hospitals with a combined average daily census of approximately 40 patients, and each service was staffed by one attending physician every day of the week and one APP Monday through Friday.
Study Design
We used a pre-post study design, in which we implemented a new process for standardizing the discharge of hospitalized patients with AUDs, including a process for counseling about naltrexone by the time of discharge. We sought and received institutional review board (IRB) approval for this study (UNC IRB 15-1441).
Interventions
We formed an improvement team that included 3 physicians and an APP in hospital medicine, a general internist and a psychiatrist, both with expertise in the use of medications for maintenance of sobriety, the director of UNC’s Alcohol and Substance Abuse Program, and 2 case managers. The team developed a number of interventions, including group education, a process for patient identification, and algorithms for counseling about, prescribing, and documenting the discussion of naltrexone.
Group Education
We presented evidence about medications for the maintenance of sobriety at a regularly scheduled hospitalist meeting. An hour-long session on motivational interviewing techniques was also presented at a separate meeting. All created algorithms were circulated to the group electronically and posted at workstations in the hospitalist work area. As data were generated postimplementation, control charts of process measures were created, posted in the hospitalist work area, and presented at subsequent group meetings.
Identification of Patients
We focused our interventions on patients admitted for alcohol detoxification or withdrawal (including withdrawal seizures). We asked our group to preferentially admit these patients to 1 of our 3 hospitalists services, on which the service APP (K.S.) was also an improvement team member.
Creation of Algorithms and Scripts for Counseling
We created a simple algorithm for evaluating patients for naltrexone. We recommended that all patients admitted for alcohol detoxification or withdrawal be counseled about naltrexone for the maintenance of sobriety before discharge. The contraindications to naltrexone we included were (1) concurrent opioid use, (2) documented cirrhosis, and/or (3) liver function tests greater than 3 times the upper limit of normal by the time of hospital discharge.
We also created a suggested script for motivational interviewing (supplemental Appendix 1). This was presented at a group meeting and circulated via e-mail. The actual counseling technique and process was left up to individual providers. In practice, counseling took place in the course of daily rounds, generally the day before or day of hospital discharge.
Prescription of Medication
For interested patients without contraindications, we recommended a prescription of naltrexone at 50 mg daily for 3 months. For patients prescribed naltrexone without medical insurance (n = 17), we utilized our existing pharmacy assistance program, whereby discharging patients can obtain an initial 14-day supply after applying to the program and then can fill subsequent prescriptions if they meet program financial requirements.
Follow-up Appointments
For patients with established outpatient providers, we asked patients to schedule follow-up appointments within a month of discharge. Patients prescribed naltrexone without primary providers (n = 16) were eligible for an existing program, the UNC Transitions Program, whereby patients identified as having moderate-to-high risk of hospital readmission can receive a follow-up appointment at UNC Internal Medicine or UNC Family Medicine within 2 weeks of discharge.
Creation of “Smart Phrases”
To aid in documentation, we created “smart phrases” (easily accessed, previously created phrases that can be adopted by all users) within the hospital electronic health record. We created one smart phrase for documentation of counseling about naltrexone, which included dropdown menus for contraindications and the patient’s preference and one for discharge instructions for patients started on naltrexone (supplemental Appendix 2).
Implementation
After the presentation of suggested interventions in July 2015 and the subsequent dissemination of educational materials, we implemented our new process on August 1, 2015.
Data Collection
Patients were identified for inclusion in the study analysis by querying UNC Hospitals’ billing database for the inpatient diagnosis codes (diagnosis-related groupings) 896 and 897, “alcohol/drug abuse or dependence without rehabilitation therapy,” with and without major comorbidity or complication, respectively, and with hospital medicine as the discharging service. All encounters were then manually reviewed by 2 investigators (J.S. and C.M.). Encounters were included if the history and physical indicated that the primary reason for admission was alcohol detoxification or withdrawal. Encounters with other primary reasons for admission (eg, pancreatitis, gastrointestinal bleeding) were excluded. For patients with multiple encounters, only the first eligible encounter in the pre- and/or postimplementation period was included. Comorbidities for identified patients were assessed via the search of study encounters for the International Classification of Diseases, 9th Revision-Clinical Modification codes for hypertension, anxiety, depression, cirrhosis, diabetes, and congestive heart failure.
Process, Outcomes, and Balancing Measures
The study process measures included the percentage of patients hospitalized for alcohol detoxification or withdrawal with documentation of counseling about naltrexone by the time of discharge, before and after process intervention. Documentation was defined as the description of counseling about naltrexone in the discharge summary or progress notes of identified encounters. We also measured the percentage of patients started on naltrexone before and after intervention. Lastly, we measured the percentage of patients prescribed naltrexone who filled at least 1 prescription for the medication, assessed by calls to the pharmacy where the medication was prescribed. Prescriptions that could not be confirmed (ie, paper rather than electronic prescriptions) were counted as not filled.
For outcome measures, we recorded the percentages of study patients who returned to the emergency department (ED) and were readmitted to UNC Hospitals (inpatient or observation) for any reason within 30 days of discharge. These outcomes were determined by a manual chart review.
In order to ensure the new process was not associated with delays in patient discharge, we measured the mean length of stay in days for study patient encounters before and after intervention as a balancing measure.
Statistical Analysis
Demographic and clinical characteristics for included patients were compared for the 16 months preimplementation (April 1, 2014 through July 31, 2015) and the 19 months postimplementation (August 1, 2015 through February 28, 2017). Descriptive statistics were calculated by using the Student t test for continuous variables and the χ2 test for dichotomous variables. We used multivariate logistic regression to evaluate the associations between the intervention arms (pre- vs postintervention) and study outcomes, adjusting for age, gender, race, insurance type, and medical comorbidities. We chose these variables for inclusion based on their association with study outcomes at the P ≤ .20 level in bivariate analyses. P < .05 was considered statistically significant. All analyses were performed by using Stata version 13.1 (StataCorp LLC, College Station, TX).
For 2 process measures, the percentages of patients counseled about and started on naltrexone, we plotted consecutive samples of 10 patients before and after intervention on a control chart, using preintervention data to calculate means and control limits.
Subgroup Analysis
We used multivariate logistic regression to evaluate the associations between counseling versus no counseling and prescription of naltrexone versus no prescription for study outcomes in the postintervention subgroup, adjusting for age, gender, race, insurance type, and medical comorbidities.
RESULTS
Patients
We identified 188 preimplementation encounters and excluded 12 patients (6.4%) for primary admission reasons other than alcohol withdrawal or detoxification and 48 (25.5%) repeat hospitalizations, leaving 128 unique patient encounters. We identified 166 postimplementation encounters and excluded 25 (15.1%) hospitalizations for admission reason and 27 repeat hospitalizations (16.3%), leaving 114 unique patient encounters (flow diagram in supplemental Appendix 3). The most common admission reason for the exclusion of encounters was withdrawal from a substance other than alcohol (supplemental Appendix 4). The percentages of encounters excluded in preimplementation and postimplementation periods were similar at 31.9% and 31.4%, respectively.
The majority of patients were male and white, and almost half were uninsured (Table 1). There were no demographic differences between patients in the pre- versus postimplementation groups. For studied comorbidities, postintervention patients were more likely to have hypertension, anxiety, and depression.
Process Measures
Among those counseled about naltrexone before discharge, 34 of 74 patients (45.9%) had no contraindications to naltrexone and were interested in taking the medication. Among the 40 patients who were counseled about but not prescribed naltrexone, 19 (47.5%) declined, 9 (22.5%) had liver function tests elevated more than 3 times the upper limit of the reference range, 9 (22.5%) had concurrent opiate use, and 3 (7.5%) had multiple contraindications.
Among the 34 patients who were prescribed naltrexone, 25 (73.5%) filled at least 1 prescription as confirmed by phone call to the relevant pharmacy.
Outcome Measures
Subgroup Analysis
Balancing Measure
The mean length of stay for all patient encounters was 3.3 days. There were no differences in length of stay comparing pre- with postintervention patient encounters (Table 1) or those postintervention patients counseled versus not counseled (Table 2).
DISCUSSION
Our study demonstrates that counseling about medications for the maintenance of sobriety can be implemented as part of the routine care of hospitalized patients with AUDs. In our experience, about half of the patients counseled had no contraindications to naltrexone and were willing to take it at discharge. Almost three-fourths of those who were prescribed naltrexone filled the prescription at least once. The counseling process was not associated with increased length of stay. In the adjusted analysis, postintervention patients had significantly lower odds of 30-day ED returns. Additionally, in subgroup analysis, postintervention patients counseled about naltrexone had significantly lower rates of subsequent healthcare utilization compared with those not counseled, with absolute differences of 26% for ED revisits and 22% for rehospitalizations within 30 days.
The failure to demonstrate a difference in adjusted rehospitalization rates in the postintervention versus the preintervention group has several possible explanations. First, we had incomplete fidelity to our interventions, documenting counseling about naltrexone before discharge in over 60% of postintervention patients, raising the possibility that better fidelity may have resulted in improved outcomes. Related to this, only 28% of postintervention patients were prescribed naltrexone, which may be an inadequate sample size to demonstrate positive effects from the medication. Another possible explanation is that the postintervention group had higher rates of some of the comorbidities we assessed, namely, anxiety, depression, and hypertension, which could have negatively impacted the effectiveness of the interventions to prevent rehospitalization; however, after adjusting for comorbidities, the odds of rehospitalization were still not significantly different. It is interesting that the odds of postintervention ED revisits (but not rehospitalizations) were lower in the adjusted analysis. It may be that patients who revisit the ED and are not rehospitalized are different in important ways from those who are readmitted. Alternately, the larger number of ED revisits overall (about twice the rate of rehospitalization) may have made it easier to identify positive effects from the intervention for this outcome than rehospitalization (ie, the study may have been underpowered to detect a relatively small reduction in rehospitalization). It is also possible, however, that the interventions were simply insufficient to prevent rehospitalization.
The subgroup analysis, however, did find significant differences in both outcome measures for postintervention patients counseled versus not counseled about naltrexone before discharge. There are several possible explanations for these results. First, there may have been unmeasured differences in those counseled versus not counseled that explain the reductions observed in subsequent healthcare utilization. For example, the counseled patients could have been more motivated to change and, thus, more readily approached by providers for counseling. The lack of any demographic differences between the 2 groups and the relative simplicity of the counseling part of the intervention occurring as part of daily rounds argue against this hypothesis, but there are many potential unmeasured confounders (eg, homelessness, ability to afford medications), and this possibility remains. A second possible explanation is that patients counseled about naltrexone could have been more likely than those not counseled to seek subsequent care at other institutions. A third possibility is that that the counseling about (and prescribing when appropriate) naltrexone itself led to the observed decreases in subsequent ED visits and hospitalizations. This hypothesis would have been more supported had we been able to demonstrate a statistically significant reduction in healthcare utilization in those prescribed versus not prescribed naltrexone. But there were nonsignificant trends in the reduction of ED revisits and rehospitalizations among those prescribed the medication, suggesting we may have been able to demonstrate statistically significant reductions with a larger sample size.
Comparing our results with existing literature is challenging. The majority of randomized trials of naltrexone for AUDs were conducted in the outpatient setting.3-10 Most of these trials utilized some type of psychosocial intervention in addition to naltrexone.3-5,8-10 The 1 prior naltrexone study we identified conducted in the inpatient setting by Wei et al.14 is the most similar to our study. The authors reported the effects of a new process for assessing hospitalized patients with AUDs, including the use of a discharge planning tool for all patients admitted with alcohol dependence. The discharge tool included prompts for naltrexone in appropriate patients. The measured outcomes included the percentage of eligible patients prescribed naltrexone at discharge and the percentages of ED revisits and rehospitalizations within 30 days. Postintervention, 64% of eligible patients were prescribed naltrexone compared with 0% before, very similar to our results. There were significant decreases among all discharged patients with alcohol dependence for 30-day ED revisits (18.8% pre- vs 6.1% postimplementation) and rehospitalizations (23.4% vs 8.2%). The study differed from ours in a number of important respects, including a location in a large urban setting and implementation on a teaching service rather than an attending-only hospitalist service. Additionally, the authors studied 1 month of process implementation and compared it to another month 1 year before the new process, with an overall smaller sample size of 64 patients before and 49 patients after implementation. Potential reasons why Wei et al.14 were able to document lower rehospitalization rates postintervention when we did not include the differences in patient population (eg, high homeless rate, lower percentage of female patients in Wei study) and secular trends unrelated to interventions in either study.
Limitations of our study include the nonrandomized and uncontrolled design, which introduces the possibility of unmeasured confounding factors leading to the decrease we observed in healthcare utilization. Additionally, the single-center design precludes our ability to assess for healthcare utilization outcomes in other nearby facilities. We had incomplete implementation of our new process, counseling just over 60% of patients. As our primary outcomes relied on documentation in the medical record, both undersampling (not documenting some interventions) and reporting bias (being more likely to record positive sessions from intervention) are possible. Lastly, despite a moderate total sample size of almost 250 patients, the relatively small numbers of patients who were actually prescribed naltrexone in our study lessens our ability to show direct impact.
In conclusion, our study demonstrates a practical process for counseling about and prescribing naltrexone to patients hospitalized for alcohol detoxification or withdrawal. We demonstrate that many of these patients will be interested in starting naltrexone at discharge and will reliably fill the prescriptions if written. Counseling was associated with a significant reduction in subsequent healthcare utilization. These results have a wide potential impact given the ubiquitous nature of AUDs among hospitalized patients in community and academic settings.
Disclosure
The authors have no conflicts of interest relevant to this article to disclose. There were no sources of funding for this work.
Alcohol use disorders (AUDs) are common, with an estimated lifetime prevalence of 17.8% for alcohol dependence.1 Alcohol misuse is costly, accounting for $24.6 billion in annual healthcare expenditures, including $5.1 billion for alcohol-related hospitalizations.2 A number of trials have demonstrated that naltrexone can help patients with AUDs maintain abstinence or diminish heavy drinking.3-10 A recent meta-analysis of pharmacotherapy trials for patients with AUDs reported that for patients using 50 mg of naltrexone daily, the number needed to treat was 12 to prevent a return to heavy drinking and 20 to prevent a return to any drinking.11 Despite good evidence for its effectiveness, naltrexone is not prescribed to the majority of patients with AUDs. In a study of veterans with AUDs cared for in the Veterans Affairs health system, only 1.9% of patients were prescribed naltrexone over the 6-month study period.12 A 2003 survey of 2 professional organizations for addiction treatment specialists reported that a mean of 13% of providers prescribed naltrexone to their patients.13
When naltrexone is prescribed, it is most frequently in the outpatient setting.3-10 Data for initiation of naltrexone in the inpatient setting are more limited. Wei et al.14 reported on the implementation of a discharge protocol, including counseling about naltrexone, for hospitalized patients with AUDs at an urban academic medical center. They reported a significant increase in the prescription of naltrexone to eligible patients by the time of discharge that was associated with a significant decrease in 30-day readmissions. Initiation of naltrexone in the inpatient versus the outpatient setting has some potential advantages. First, patients hospitalized for alcohol withdrawal have AUDs, obviating the need for screening. Second, the outpatient trials of naltrexone typically required 3 days of sobriety before initiation, which is generally achieved during hospitalization for detoxification or withdrawal.
Previous work at our institution centered on standardizing the process of evaluating patients needing alcohol detoxification at the time of referral for admission.15 The use of a standardized protocol reduced the number of inpatient admissions for alcohol-related diagnoses but had no effect on the 30-day readmission rate (28%) for those patients who were hospitalized. Our hospitalist group had no standardized process for discharging hospitalized patients with AUDs, and the discharge process rarely included counseling on medications for maintenance of sobriety. In this manuscript, we describe the implementation and impact of a process for counseling patients hospitalized for alcohol detoxification or withdrawal about naltrexone for maintenance of sobriety by the time of hospital discharge.
METHODS
Study Setting
The University of North Carolina (UNC) Hospitals is an 803-bed tertiary academic center. UNC Hospital Medicine is staffed by 29 physicians and 3 advanced practice providers (APPs). During the study period, there were 3 hospital medicine services at UNC Hospitals with a combined average daily census of approximately 40 patients, and each service was staffed by one attending physician every day of the week and one APP Monday through Friday.
Study Design
We used a pre-post study design, in which we implemented a new process for standardizing the discharge of hospitalized patients with AUDs, including a process for counseling about naltrexone by the time of discharge. We sought and received institutional review board (IRB) approval for this study (UNC IRB 15-1441).
Interventions
We formed an improvement team that included 3 physicians and an APP in hospital medicine, a general internist and a psychiatrist, both with expertise in the use of medications for maintenance of sobriety, the director of UNC’s Alcohol and Substance Abuse Program, and 2 case managers. The team developed a number of interventions, including group education, a process for patient identification, and algorithms for counseling about, prescribing, and documenting the discussion of naltrexone.
Group Education
We presented evidence about medications for the maintenance of sobriety at a regularly scheduled hospitalist meeting. An hour-long session on motivational interviewing techniques was also presented at a separate meeting. All created algorithms were circulated to the group electronically and posted at workstations in the hospitalist work area. As data were generated postimplementation, control charts of process measures were created, posted in the hospitalist work area, and presented at subsequent group meetings.
Identification of Patients
We focused our interventions on patients admitted for alcohol detoxification or withdrawal (including withdrawal seizures). We asked our group to preferentially admit these patients to 1 of our 3 hospitalists services, on which the service APP (K.S.) was also an improvement team member.
Creation of Algorithms and Scripts for Counseling
We created a simple algorithm for evaluating patients for naltrexone. We recommended that all patients admitted for alcohol detoxification or withdrawal be counseled about naltrexone for the maintenance of sobriety before discharge. The contraindications to naltrexone we included were (1) concurrent opioid use, (2) documented cirrhosis, and/or (3) liver function tests greater than 3 times the upper limit of normal by the time of hospital discharge.
We also created a suggested script for motivational interviewing (supplemental Appendix 1). This was presented at a group meeting and circulated via e-mail. The actual counseling technique and process was left up to individual providers. In practice, counseling took place in the course of daily rounds, generally the day before or day of hospital discharge.
Prescription of Medication
For interested patients without contraindications, we recommended a prescription of naltrexone at 50 mg daily for 3 months. For patients prescribed naltrexone without medical insurance (n = 17), we utilized our existing pharmacy assistance program, whereby discharging patients can obtain an initial 14-day supply after applying to the program and then can fill subsequent prescriptions if they meet program financial requirements.
Follow-up Appointments
For patients with established outpatient providers, we asked patients to schedule follow-up appointments within a month of discharge. Patients prescribed naltrexone without primary providers (n = 16) were eligible for an existing program, the UNC Transitions Program, whereby patients identified as having moderate-to-high risk of hospital readmission can receive a follow-up appointment at UNC Internal Medicine or UNC Family Medicine within 2 weeks of discharge.
Creation of “Smart Phrases”
To aid in documentation, we created “smart phrases” (easily accessed, previously created phrases that can be adopted by all users) within the hospital electronic health record. We created one smart phrase for documentation of counseling about naltrexone, which included dropdown menus for contraindications and the patient’s preference and one for discharge instructions for patients started on naltrexone (supplemental Appendix 2).
Implementation
After the presentation of suggested interventions in July 2015 and the subsequent dissemination of educational materials, we implemented our new process on August 1, 2015.
Data Collection
Patients were identified for inclusion in the study analysis by querying UNC Hospitals’ billing database for the inpatient diagnosis codes (diagnosis-related groupings) 896 and 897, “alcohol/drug abuse or dependence without rehabilitation therapy,” with and without major comorbidity or complication, respectively, and with hospital medicine as the discharging service. All encounters were then manually reviewed by 2 investigators (J.S. and C.M.). Encounters were included if the history and physical indicated that the primary reason for admission was alcohol detoxification or withdrawal. Encounters with other primary reasons for admission (eg, pancreatitis, gastrointestinal bleeding) were excluded. For patients with multiple encounters, only the first eligible encounter in the pre- and/or postimplementation period was included. Comorbidities for identified patients were assessed via the search of study encounters for the International Classification of Diseases, 9th Revision-Clinical Modification codes for hypertension, anxiety, depression, cirrhosis, diabetes, and congestive heart failure.
Process, Outcomes, and Balancing Measures
The study process measures included the percentage of patients hospitalized for alcohol detoxification or withdrawal with documentation of counseling about naltrexone by the time of discharge, before and after process intervention. Documentation was defined as the description of counseling about naltrexone in the discharge summary or progress notes of identified encounters. We also measured the percentage of patients started on naltrexone before and after intervention. Lastly, we measured the percentage of patients prescribed naltrexone who filled at least 1 prescription for the medication, assessed by calls to the pharmacy where the medication was prescribed. Prescriptions that could not be confirmed (ie, paper rather than electronic prescriptions) were counted as not filled.
For outcome measures, we recorded the percentages of study patients who returned to the emergency department (ED) and were readmitted to UNC Hospitals (inpatient or observation) for any reason within 30 days of discharge. These outcomes were determined by a manual chart review.
In order to ensure the new process was not associated with delays in patient discharge, we measured the mean length of stay in days for study patient encounters before and after intervention as a balancing measure.
Statistical Analysis
Demographic and clinical characteristics for included patients were compared for the 16 months preimplementation (April 1, 2014 through July 31, 2015) and the 19 months postimplementation (August 1, 2015 through February 28, 2017). Descriptive statistics were calculated by using the Student t test for continuous variables and the χ2 test for dichotomous variables. We used multivariate logistic regression to evaluate the associations between the intervention arms (pre- vs postintervention) and study outcomes, adjusting for age, gender, race, insurance type, and medical comorbidities. We chose these variables for inclusion based on their association with study outcomes at the P ≤ .20 level in bivariate analyses. P < .05 was considered statistically significant. All analyses were performed by using Stata version 13.1 (StataCorp LLC, College Station, TX).
For 2 process measures, the percentages of patients counseled about and started on naltrexone, we plotted consecutive samples of 10 patients before and after intervention on a control chart, using preintervention data to calculate means and control limits.
Subgroup Analysis
We used multivariate logistic regression to evaluate the associations between counseling versus no counseling and prescription of naltrexone versus no prescription for study outcomes in the postintervention subgroup, adjusting for age, gender, race, insurance type, and medical comorbidities.
RESULTS
Patients
We identified 188 preimplementation encounters and excluded 12 patients (6.4%) for primary admission reasons other than alcohol withdrawal or detoxification and 48 (25.5%) repeat hospitalizations, leaving 128 unique patient encounters. We identified 166 postimplementation encounters and excluded 25 (15.1%) hospitalizations for admission reason and 27 repeat hospitalizations (16.3%), leaving 114 unique patient encounters (flow diagram in supplemental Appendix 3). The most common admission reason for the exclusion of encounters was withdrawal from a substance other than alcohol (supplemental Appendix 4). The percentages of encounters excluded in preimplementation and postimplementation periods were similar at 31.9% and 31.4%, respectively.
The majority of patients were male and white, and almost half were uninsured (Table 1). There were no demographic differences between patients in the pre- versus postimplementation groups. For studied comorbidities, postintervention patients were more likely to have hypertension, anxiety, and depression.
Process Measures
Among those counseled about naltrexone before discharge, 34 of 74 patients (45.9%) had no contraindications to naltrexone and were interested in taking the medication. Among the 40 patients who were counseled about but not prescribed naltrexone, 19 (47.5%) declined, 9 (22.5%) had liver function tests elevated more than 3 times the upper limit of the reference range, 9 (22.5%) had concurrent opiate use, and 3 (7.5%) had multiple contraindications.
Among the 34 patients who were prescribed naltrexone, 25 (73.5%) filled at least 1 prescription as confirmed by phone call to the relevant pharmacy.
Outcome Measures
Subgroup Analysis
Balancing Measure
The mean length of stay for all patient encounters was 3.3 days. There were no differences in length of stay comparing pre- with postintervention patient encounters (Table 1) or those postintervention patients counseled versus not counseled (Table 2).
DISCUSSION
Our study demonstrates that counseling about medications for the maintenance of sobriety can be implemented as part of the routine care of hospitalized patients with AUDs. In our experience, about half of the patients counseled had no contraindications to naltrexone and were willing to take it at discharge. Almost three-fourths of those who were prescribed naltrexone filled the prescription at least once. The counseling process was not associated with increased length of stay. In the adjusted analysis, postintervention patients had significantly lower odds of 30-day ED returns. Additionally, in subgroup analysis, postintervention patients counseled about naltrexone had significantly lower rates of subsequent healthcare utilization compared with those not counseled, with absolute differences of 26% for ED revisits and 22% for rehospitalizations within 30 days.
The failure to demonstrate a difference in adjusted rehospitalization rates in the postintervention versus the preintervention group has several possible explanations. First, we had incomplete fidelity to our interventions, documenting counseling about naltrexone before discharge in over 60% of postintervention patients, raising the possibility that better fidelity may have resulted in improved outcomes. Related to this, only 28% of postintervention patients were prescribed naltrexone, which may be an inadequate sample size to demonstrate positive effects from the medication. Another possible explanation is that the postintervention group had higher rates of some of the comorbidities we assessed, namely, anxiety, depression, and hypertension, which could have negatively impacted the effectiveness of the interventions to prevent rehospitalization; however, after adjusting for comorbidities, the odds of rehospitalization were still not significantly different. It is interesting that the odds of postintervention ED revisits (but not rehospitalizations) were lower in the adjusted analysis. It may be that patients who revisit the ED and are not rehospitalized are different in important ways from those who are readmitted. Alternately, the larger number of ED revisits overall (about twice the rate of rehospitalization) may have made it easier to identify positive effects from the intervention for this outcome than rehospitalization (ie, the study may have been underpowered to detect a relatively small reduction in rehospitalization). It is also possible, however, that the interventions were simply insufficient to prevent rehospitalization.
The subgroup analysis, however, did find significant differences in both outcome measures for postintervention patients counseled versus not counseled about naltrexone before discharge. There are several possible explanations for these results. First, there may have been unmeasured differences in those counseled versus not counseled that explain the reductions observed in subsequent healthcare utilization. For example, the counseled patients could have been more motivated to change and, thus, more readily approached by providers for counseling. The lack of any demographic differences between the 2 groups and the relative simplicity of the counseling part of the intervention occurring as part of daily rounds argue against this hypothesis, but there are many potential unmeasured confounders (eg, homelessness, ability to afford medications), and this possibility remains. A second possible explanation is that patients counseled about naltrexone could have been more likely than those not counseled to seek subsequent care at other institutions. A third possibility is that that the counseling about (and prescribing when appropriate) naltrexone itself led to the observed decreases in subsequent ED visits and hospitalizations. This hypothesis would have been more supported had we been able to demonstrate a statistically significant reduction in healthcare utilization in those prescribed versus not prescribed naltrexone. But there were nonsignificant trends in the reduction of ED revisits and rehospitalizations among those prescribed the medication, suggesting we may have been able to demonstrate statistically significant reductions with a larger sample size.
Comparing our results with existing literature is challenging. The majority of randomized trials of naltrexone for AUDs were conducted in the outpatient setting.3-10 Most of these trials utilized some type of psychosocial intervention in addition to naltrexone.3-5,8-10 The 1 prior naltrexone study we identified conducted in the inpatient setting by Wei et al.14 is the most similar to our study. The authors reported the effects of a new process for assessing hospitalized patients with AUDs, including the use of a discharge planning tool for all patients admitted with alcohol dependence. The discharge tool included prompts for naltrexone in appropriate patients. The measured outcomes included the percentage of eligible patients prescribed naltrexone at discharge and the percentages of ED revisits and rehospitalizations within 30 days. Postintervention, 64% of eligible patients were prescribed naltrexone compared with 0% before, very similar to our results. There were significant decreases among all discharged patients with alcohol dependence for 30-day ED revisits (18.8% pre- vs 6.1% postimplementation) and rehospitalizations (23.4% vs 8.2%). The study differed from ours in a number of important respects, including a location in a large urban setting and implementation on a teaching service rather than an attending-only hospitalist service. Additionally, the authors studied 1 month of process implementation and compared it to another month 1 year before the new process, with an overall smaller sample size of 64 patients before and 49 patients after implementation. Potential reasons why Wei et al.14 were able to document lower rehospitalization rates postintervention when we did not include the differences in patient population (eg, high homeless rate, lower percentage of female patients in Wei study) and secular trends unrelated to interventions in either study.
Limitations of our study include the nonrandomized and uncontrolled design, which introduces the possibility of unmeasured confounding factors leading to the decrease we observed in healthcare utilization. Additionally, the single-center design precludes our ability to assess for healthcare utilization outcomes in other nearby facilities. We had incomplete implementation of our new process, counseling just over 60% of patients. As our primary outcomes relied on documentation in the medical record, both undersampling (not documenting some interventions) and reporting bias (being more likely to record positive sessions from intervention) are possible. Lastly, despite a moderate total sample size of almost 250 patients, the relatively small numbers of patients who were actually prescribed naltrexone in our study lessens our ability to show direct impact.
In conclusion, our study demonstrates a practical process for counseling about and prescribing naltrexone to patients hospitalized for alcohol detoxification or withdrawal. We demonstrate that many of these patients will be interested in starting naltrexone at discharge and will reliably fill the prescriptions if written. Counseling was associated with a significant reduction in subsequent healthcare utilization. These results have a wide potential impact given the ubiquitous nature of AUDs among hospitalized patients in community and academic settings.
Disclosure
The authors have no conflicts of interest relevant to this article to disclose. There were no sources of funding for this work.
1. Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842. PubMed
2. Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41(5):516-524. PubMed
3. Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156(11):1758-1764. PubMed
4. Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol. 2005;25(4):349-357. PubMed
5. Guardia J, Caso C, Arias F, et al. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: results from a multicenter clinical trial. Alcohol Clin Exp Res. 2002;26(9):1381-1387. PubMed
6. Kiefer F, Jahn H, Tarnaske T, et al. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60(1):92-99. PubMed
7. Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Aust. 2002;176(11):530-534. PubMed
8. Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: a randomized controlled trial. Addiction. 2001;96(11):1565-1573. PubMed
9. O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49(11):881-887. PubMed
10. O’Malley SS, Robin RW, Levenson AL, et al. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska natives and non-natives residing in rural settings: a randomized controlled trial. Alcohol Clin Exp Res. 2008;32(7):1271-1283. PubMed
11. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 2014;311(18):1889-1900. PubMed
12. Petrakis IL, Leslie D, Rosenheck R. Use of naltrexone in the treatment of alcoholism nationally in the Department of Veterans Affairs. Alcohol Clin Exp Res. 2003;27(11):1780-1784. PubMed
13. Mark TL, Kranzler HR, Song X. Understanding US addiction physicians’ low rate of naltrexone prescription. Drug Alcohol Depend. 2003;71(3):219-228. PubMed
14. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. PubMed
15. Stephens JR, Liles EA, Dancel R, Gilchrist M, Kirsch J, DeWalt DA. Who needs inpatient detox? Development and implementation of a hospitalist protocol for the evaluation of patients for alcohol detoxification. J Gen Intern Med. 2014;29(4):587-593. PubMed
16. Provost LP, Murray SK. The Health Care Data Guide: Learning from Data for Improvement. San Francisco: Jossey-Bass; 2011.
1. Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842. PubMed
2. Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41(5):516-524. PubMed
3. Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156(11):1758-1764. PubMed
4. Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol. 2005;25(4):349-357. PubMed
5. Guardia J, Caso C, Arias F, et al. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: results from a multicenter clinical trial. Alcohol Clin Exp Res. 2002;26(9):1381-1387. PubMed
6. Kiefer F, Jahn H, Tarnaske T, et al. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60(1):92-99. PubMed
7. Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Aust. 2002;176(11):530-534. PubMed
8. Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: a randomized controlled trial. Addiction. 2001;96(11):1565-1573. PubMed
9. O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49(11):881-887. PubMed
10. O’Malley SS, Robin RW, Levenson AL, et al. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska natives and non-natives residing in rural settings: a randomized controlled trial. Alcohol Clin Exp Res. 2008;32(7):1271-1283. PubMed
11. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 2014;311(18):1889-1900. PubMed
12. Petrakis IL, Leslie D, Rosenheck R. Use of naltrexone in the treatment of alcoholism nationally in the Department of Veterans Affairs. Alcohol Clin Exp Res. 2003;27(11):1780-1784. PubMed
13. Mark TL, Kranzler HR, Song X. Understanding US addiction physicians’ low rate of naltrexone prescription. Drug Alcohol Depend. 2003;71(3):219-228. PubMed
14. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. PubMed
15. Stephens JR, Liles EA, Dancel R, Gilchrist M, Kirsch J, DeWalt DA. Who needs inpatient detox? Development and implementation of a hospitalist protocol for the evaluation of patients for alcohol detoxification. J Gen Intern Med. 2014;29(4):587-593. PubMed
16. Provost LP, Murray SK. The Health Care Data Guide: Learning from Data for Improvement. San Francisco: Jossey-Bass; 2011.
© 2018 Society of Hospital Medicine
Monitoring pulmonary complications in long-term childhood cancer survivors: Guidelines for the primary care physician
Children who undergo radiotherapy, chemotherapy, or surgery for cancer face a risk of complications later in life, including pulmonary fibrosis and pneumonitis.
These long-term cancer survivors need systematic, lifelong surveillance, in a program that takes into account their individual risk (based on therapeutic exposures, genetic predisposition, lifestyle behaviors, and comorbid health conditions).1 Optimally, they would receive their care at multidisciplinary follow-up clinics organized by pediatric oncologists at tertiary care centers. However, access to such centers is limited, making this an option for relatively few. Consequently, as childhood cancer survivors age, internists and family practitioners may need to assume an increasing amount of responsibility for their follow-up care.
Because individual primary care providers are unlikely to follow more than a handful of survivors, specialists have developed guidelines for survivors of pediatric cancer. Working with established multidisciplinary clinics may help ensure appropriate follow-up for this population of patients.
This review summarizes the late effects of cancer therapy on the lungs and an approach to surveillance for the generalist or pulmonologist. We also review the quality of the evidence upon which these recommendations are based.
NUMBERS ON THE RISE
An estimated 1 of every 330 children develops cancer before age 19. With cure rates exceeding 75% for many pediatric malignancies, the number of survivors of childhood cancer, currently in excess of 270,000, will continue to increase.2
THE CHILDREN’S ONCOLOGY GROUP GUIDELINES
The Children’s Oncology Group (COG)3 released its first set of guidelines in 2003 for the follow-up care of patients treated for pediatric malignancies; the current version is available at www.survivorshipguidelines.org. The guidelines contain comprehensive screening recommendations, including those related to pulmonary toxicity, which can be used to standardize care.
Patient education materials accompany the guidelines, offering detailed information on guideline-specific topics in order to promote health maintenance.
HOW WE SEARCHED THE LITERATURE
We performed an extensive review of the literature via MEDLINE for the years 1975–2005. Key search terms were “childhood cancer,” “late effects,” and “pulmonary toxicity,” combined with keywords for each therapeutic exposure. References from selected articles were used to broaden the search. From several hundred citations, fewer than 30 were selected as best illustrating the relevant associations.
RISK IS THREE TIMES HIGHER IN CANCER SURVIVORS
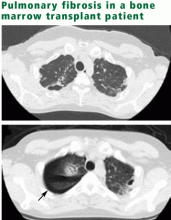
The Childhood Cancer Survivor Study4 is the largest database of late effects, with more than 12,000 survivors of childhood cancer diagnosed between 1970 and 1986. Its data suggest that the risk of pulmonary conditions is more than three times higher in cancer survivors than in their siblings, as manifested by pulmonary signs (abnormal chest wall growth), symptoms (chronic cough, use of supplemental oxygen, exercise-induced shortness of breath), or specific diagnoses (lung fibrosis, recurrent pneumonia, pleurisy, bronchitis, recurrent sinus infection, or tonsillitis). Limitations: these data are retrospective, and the outcomes were detected by self-report and were not validated by review of medical records. Thus, the figures highlight the fact that pulmonary late effects are an important problem but do not give us a way to calculate risk exactly.
Other limitations of the literature: Treatments are constantly evolving, often in attempts to minimize late effects, and newer agents will need to be monitored for pulmonary toxicities. As noted, much of the available information is from studies of survivors of adult cancer; the potential for late effects of similar therapies in children is inferred. Most conclusions—and especially those based upon prospective serial evaluations—derive from small cohorts. For all treatments, the complications in the very long term remain undefined. What we know is summarized below.
CANCER THERAPY CAUSES FIBROSIS, PNEUMONITIS
The courses of these diseases are poorly characterized, since few longitudinal studies have been done. However, like most of the late effects of cancer therapy, pulmonary toxicity may first become apparent during the treatment and persist, or it may not appear until years later. Signs and symptoms may be static, progressive, or reversible.
ANGIOGENESIS MAY CONTRIBUTE TO FIBROSIS
On a microscopic level, pulmonary fibrosis is characterized by epithelial injury, fibroproliferation, and excessive extracellular matrix deposition.6–8
Evidence is mounting that these findings result in part from angiogenesis. Although this has not been studied in long-term cancer survivors, evidence of neovascularization was seen both in an animal model of lung fibrosis and in patients with idiopathic pulmonary fibrosis.6–8 High plasma concentrations of angiogenic cytokines (eg, tumor necrosis factor alpha, interleukin 8, and endothelin 1) have been found in these situations. Antiangiogenic agents and other immune modulators such as thalidomide may be beneficial in patients with lung fibrosis.7
On a macroscopic level, pulmonary fibrosis results in loss of lung volume in older children and in adults. In contrast, in younger children, interference with growth of both the lung and the chest wall may contribute to pulmonary dysfunction.
CANCER TYPES AND TREATMENTS VARY BY AGE
Cancers that commonly involve the thorax are listed in Table 2. Neuroblastoma, hepatoblastoma, extragonadal germ cell tumors, and Wilms tumor typically are diseases of young children; osteosarcoma, Ewing sarcoma, thyroid carcinoma, and Hodgkin disease are most common in older children and adolescents; soft tissue sarcoma and non-Hodgkin lymphoma span all age groups.
Surgery can, in some cases, control the cancer, as with mediastinal neuroblastoma and Ewing sarcoma of the chest wall.
Radiation to the chest remains a major component of treatment for Hodgkin disease, unresected thoracic Ewing sarcoma, soft tissue sarcoma with lung involvement or thyroid carcinoma, and Wilms tumor. Central nervous system tumors and leukemias, the most common pediatric malignancies, may require radiation to the spinal cord—with resulting radiation exposure of the lungs. Total-body irradiation is a component of many preparative regimens for stem cell transplantation.
Chemotherapy remains a mainstay for all types of tumors, and patients with germ cell tumors, Hodgkin disease, and brain tumors are at particular risk of pulmonary toxicity due to heavy reliance on bleomycin (Blenoxane) (for germ cell tumors, Hodgkin disease) and the nitrosoureas (for brain tumors).
RADIATION-INDUCED LUNG DAMAGE
The lungs are particularly sensitive to radiation, and pulmonary problems occur most often in patients with malignant diseases of the chest that are treated with radiation, ie, those involving the mediastinum, the lung parenchyma, or the chest wall.
Abnormal radiographic findings or restrictive changes on pulmonary function testing have been reported in more than 30% of patients who received radiation directly or indirectly to the lung.9–12 These changes have been detected months to years after radiation therapy, most often in patients who suffered radiation pneumonitis as an acute toxicity.
The amount of damage depends on the cumulative dose, how many treatments (“fractions”) this cumulative dose was divided into (dividing the radiation dose into smaller dose fractions can reduce toxicity), the volume of lung tissue involved, and the patient’s age (the younger, the worse) at the time of treatment.
Cumulative dose. Whole-lung irradiation is limited to 12 Gy, although localized areas of cancer can be treated with much higher doses.
Clinically apparent pneumonitis with cough, fever, or dyspnea generally occurs only in survivors who received more than 30 Gy in standard fractions to more than 50% of the lung. However, in 12 survivors of Wilms tumor who received median total doses of approximately 20 Gy to both lungs 7 to 14 years previously, 8 patients had dyspnea on exertion and radiographic evidence of interstitial and pleural thickening.13 Mean total lung volumes and the diffusing capacity of the lung for carbon monoxide (DLCO) were reduced in all patients to approximately 60% of predicted values.
In a prospective study of adults with Hodgkin disease treated after 1980, 145 patients were examined 3 years after receiving more than 44 Gy limited to the mantle area.9 None were experiencing symptoms; however, 30% to 40% had a forced vital capacity (FVC) less than 80% of predicted, and 7% had a reduced DLCO. Some of these patients also had received bleomycin (see below), which may have exacerbated pulmonary toxicity. Asymptomatic restrictive and obstructive lung changes have been detected after lower doses of whole-lung radiation (11–14 Gy) were given for other malignant diseases.11,14
In a recent study of adults and older adolescents with stage I and IIA Hodgkin disease treated with radiation alone (40–45 Gy to involved fields, including the mediastinum), late pulmonary effects observed were minimal.10 While FVC, residual volume, forced expiratory volume in 1 second (FEV1), DLCO, and total lung capacity (TLC) were significantly lower at the end of radiation therapy than before treatment, all except DLCO returned nearly to normal within 1 year. The decrease in DLCO remained stable, but the forced expiratory flow rate between 25% and 75% of FVC (FEF 25%–75%) was significantly lower 3 years after treatment than at baseline.
The high doses of total lung irradiation cited in several of these reports are rarely used in today’s cancer protocols. For example, patients receiving radiation as adjunctive treatment for Hodgkin disease now receive lower doses (< 21 Gy), which are restricted to involved fields, and the pulmonary toxicity is less than in the past.
In one series, 159 children and adolescents with unfavorable Hodgkin disease treated from 1993 to 2000 received six cycles of chemotherapy followed by response-based, involved-field radiation therapy. Patients who achieved a complete response after the first two cycles of chemotherapy got 15 Gy, and those who achieved a partial response got 25.5 Gy to all sites of bulky lymphadenopathy.15 All patients underwent pulmonary function testing. Only 24 (30.8%) of them had pulmonary toxicity, which was limited to asymptomatic deficits of restriction and diffusion.
Nevertheless, the lungs receive some radiation even when they are not the target, such as in patients with malignant brain tumors, and this exposure can contribute to the development of lung disease, although these patients are likely to have no symptoms during day-to-day activities. Innovations in targeted radiation delivery (eg, conformal radiation) should further limit damage to normal lung tissue.
Age at the time of treatment also may influence the type and incidence of pulmonary sequelae. In older children and adults, radiation for thoracic malignancy results in pulmonary fibrosis with loss of lung volume. Similar injury can occur in younger children, but pulmonary function may also be compromised by inhibited growth of the supportive structures and the chest wall. One report suggests that children younger than 3 years at the time of therapy experience more chronic toxicity.11
In contrast, after bone marrow transplantation, children seem to be at less risk of significant late pulmonary dysfunction than adults are, despite similar preparatory regimens.16 This may in part reflect a lower incidence of severe graft-vs-host disease involving the lung. Nonetheless, in a recent report of children treated with fractionated total-body irradiation between 1985 and 1993, restrictive pulmonary diseases were found in 30 of 42 patients at a median of 3.1 years after treatment (range 0.5–17 years).17 Most of these patients had asymptomatic mild restrictive disease, and the one patient with severe changes had previously received thoracic radiation for treatment of neuroblastoma. In about half of the cases, pulmonary function abnormalities were permanent although not progressive.
CHEMOTHERAPY-RELATED LUNG DAMAGE
A growing list of chemotherapeutic agents appears to cause pulmonary disease in long-term survivors.5
Bleomycin
Bleomycin toxicity is the prototype for chemotherapy-related lung injury: bleomycin was the first chemotherapy drug shown to cause lung injury, this effect is suggested by a large database, and the mechanism is typical.5,18 Preclinical studies have attributed bleomycin’s toxicity to its tendency to promote free radicals.
Although interstitial pneumonitis and pulmonary fibrosis have been reported in children, clinically apparent bleomycin pneumonopathy is most frequent in older adults. Usually, the abnormalities began within 3 months of therapy and persisted or progressed. Like the acute toxicity, it is dose-dependent and more common above a threshold cumulative dose of 400 units/m2. Above this dose, 10% of adult patients without other risk factors develop fibrosis; data are not available for these doses in children. At lower doses, fibrosis occurs sporadically in fewer than 5% of patients, with a 1% to 2% mortality rate. In some series, bleomycin toxicity was anticipated on the basis of DLCO abnormalities.
Bleomycin pulmonary toxicity is variably exacerbated by concurrent or previous radiation therapy.
Increased oxygen concentrations associated with general anesthesia have also been found to exacerbate prior bleomycin-induced pulmonary injury.19,20 In one instance (reviewed by Zaniboni et al20), the patient recovered with corticosteroid treatment.
Alkylating agents
Carmustine (also called BCNU; brand names BiCNU, Gliadel) and lomustine (CCNU; CeeNU) are thought to cause dose-related lung injury. When cumulative carmustine doses are greater than 1,500 mg/m2, more than 50% of patients develop symptoms.21
In a careful clinicopathologic review of 31 children with brain tumors, restrictive changes with lung fibrosis were reported up to 17 years after treatment, most often with carmustine 100 mg/m2 every 6 to 8 weeks for up to 2 years.22 Four of the 8 patients still alive at the time of study experienced shortness of breath and coughing; 6 showed a characteristic pattern of upper zone fibrosis on chest radiography and computed tomography; all 8 survivors had restrictive findings on pulmonary function testing, with vital capacities of about 50% of normal. Toxicity increases with more intensive dose-scheduling.
Cyclophosphamide (Cytoxan) may cause delayed-onset pulmonary fibrosis with severe restrictive lung disease in association with marked reductions in the anteroposterior diameter of the chest, although the evidence is less convincing than with carmustine and lomustine, coming from case reports and small series.23
Melphalan (Alkeran), generally in doses used in stem cell transplant conditioning regimens, is also thought to cause pulmonary fibrosis.24
Busulfan most predictably causes toxicity when it is used in transplantation doses (ie, more than 500 mg), and may be associated with a progressive, potentially fatal restrictive lung disease.25 A current trend is to adjust the dose on the basis of pharmacokinetic analysis, which we hope will reduce toxicity.
Other agents
Methotrexate (MTX; Trexall) also has been associated with chronic pneumonitis and fibrosis.26 This probably occurs with an incidence well below 1% and may be idiosyncratic and not dose-related. Asymptomatic changes in pulmonary function tests that do not predict clinically significant problems have most frequently been associated with low-dose oral administration during more than 3 years.27 This is a treatment approach used in patients with psoriasis or rheumatoid arthritis, but is now obsolete for pediatric cancer. Intravenous and, rarely, intrathecal administration also have been associated with pulmonary toxicity.28 A single report of two patients who developed diffuse interstitial pulmonary infiltrates and chronic pulmonary changes links vinblastine to these sequelae.29
LUNG INJURY AFTER BONE MARROW TRANSPLANTATION
Hematopoietic stem cell transplantation is associated with various late pulmonary complications. The factors that influence these complications are similar to those discussed earlier. However, the intensity of therapy in patients undergoing transplantation and the additive effects of previous therapies magnify the risks.
Patients undergoing total-body irradiation as part of their preparation for transplantation have a high incidence of late pulmonary complications.25,30–32 Busulfan, carmustine, bleomycin, and cyclophosphamide, also commonly used conditioning chemotherapies, are known to cause pneumonitis and fibrosis after transplantation.33
Some acute pulmonary toxicities can have long-standing effects, including serious pulmonary infections, idiopathic pneumonia syndrome, bronchiolitis obliterans, acute respiratory distress syndrome, or other damage related to graft-vs-host disease.
OTHER RISK FACTORS FOR LUNG DAMAGE
Additional factors contributing to chronic pulmonary toxicity include superimposed infection, underlying pneumonopathy (eg, asthma), cigarette use, respirator toxicity, chronic graft-vs-host disease, and the effects of chronic pulmonary involvement by tumor or reaction to tumor. For example, a subset of patients with Langerhans cell histiocytosis can develop histiocytic pulmonary infiltrates or honeycombing with severe chronic restrictive lung disease unrelated to therapy or the presence of active tumor.
Although not well documented, scuba diving also has been said to exacerbate pulmonary fibrosis through increased underwater pressures and high oxygen levels.34
Lung lobectomy during childhood appears to have no significant impact on long-term pulmonary function,35–37 but the effect of lung surgery for children with cancer is not well defined.
GET THE PATIENT’S TREATMENT SUMMARY
Regardless of the setting for follow-up, the first step in any evaluation is to obtain the patient’s medical history and especially a treatment summary. The treatment summary should outline the cancer diagnosis, involved sites of disease, age at diagnosis, specific treatments (surgery, chemotherapy, radiation), and other key interventions and events during and after cancer therapy. Sample forms for physicians and patients are available at www.survivorshipguidelines.org.
Before the long-term survivor of childhood cancer graduates from the care of a pediatric oncologist, this treatment record and possible long-term problems should be reviewed with the family and, in the case of an adolescent, with the patient. Correspondence between the pediatric oncologist and subsequent caregivers should also include a treatment summary. The treatment summary allows the survivor or his health care provider to interface with the COG guidelines to determine recommended follow-up care. The primary care physician and the patient both should have copies of this document.
We are developing an interactive Web-based version of a standardized summary form, designed to interface with an automated version of the COG guidelines in order to generate individualized follow-up recommendations.
ASK ABOUT LUNG SYMPTOMS
We recommend that health care providers investigate symptoms of pulmonary dysfunction, and specifically ask about chronic cough with or without fever, shortness of breath, and dyspnea on exertion during yearly health care visits.
Baseline pulmonary function testing (including DLCO and spirometry) and chest radiography are recommended 2 or more years after completion of therapy to document persistent deficits and determine the need for continued monitoring. Reevaluation of pulmonary function should be considered in patients with established deficits who require general anesthesia and for those treated with bleomycin.
Scuba diving remains controversial for long-term survivors. Consequently, patients with risk factors for lung disease should be encouraged to consult with a pulmonary specialist to determine if diving poses a health threat to their pulmonary status. If clinical pulmonary dysfunction is identified, referral to colleagues in pulmonology for additional evaluation and treatment is essential. Increasing familiarity of primary care providers with surveillance concepts is a key element in survivorship care.
Smoking cessation can enhance the health of all patients and is particularly important among long-term survivors, especially those who received treatments predisposing to pulmonary injury. Strategies for cessation and patient information can be found at www.cdc.gov/tobacco/how2quit.htm.
Clinicians can take advantage of every patient interaction to assess readiness for smoking cessation and assist patients in this goal. Following the principles of patient-centered counseling, physicians can guide patients into considering a change of behavior with advice and encouragement. Whenever possible, physicians should personalize the risks of smoking as well as the short-term and long-term benefits. As smokers prepare to quit, their physicians can assist in developing a plan that includes a quit date.
Many pharmacologic agents are available to assist patients, ie, nicotine inhalers, sprays, gum, and transdermal patches; the antidepressants bupropion (Wellbutrin) and nortriptiline (Pamelor); the alpha-2 adrenergic agonist clonidine (Catapres); and, most recently, the nicotine receptor partial agonist varenicline (Chantix).38
Follow-up to prevent relapse is an important part of this process.
Acknowledgment. This work was supported in part by the Swim Across America Foundation and the Campini Foundation.
- Hewitt M, Weiner S, Simone J. The epidemiology of childhood cancer. Washington DC: The National Academies Press, 2003:20–36.
- Ries L, Eisner M, Kosary C, et al. SEER cancer statistics review, 1975–2001. Bethesda, MD: National Cancer Institute; 2004.
- Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and nursing discipline. J Clin Oncol 2004; 22:4979–4990.
- Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer 2002; 95:2431–2441.
- Bhatia S, Blatt J, Meadows A. Late effects of childhood cancer and its treatment. In:Pizzo P, Poplack D. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Anscher MS, Chen L, Rabbani Z, et al. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int J Radiat Oncol Biol Phys 2005; 62:255–259.
- Bhatt N, Baran CP, Allen J, et al. Promising pharmacologic innovations in treating pulmonary fibrosis. Curr Opin Pharmacol 2006; 6:284–292.
- Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol 2001: 13:242–248.
- Horning SJ, Adhikari A, Rizk N, et al. Effect of treatment for Hodgkin’s disease on pulmonary function: results of a prospective study. J Clin Oncol 1994; 12:297–305.
- Villani F, Viviani S, Bonfante V, et al. Late pulmonary effects in favorable stage I and IIA Hodgkin’s disease treated with radiotherapy alone. Am J Clin Oncol 2000; 23:18–21.
- Miller RW, Fusner JE, Fink RJ, et al. Pulmonary function abnormalities in long-term survivors of childhood cancer. Med Pediatr Oncol 1986; 14:202–207.
- Weiner DJ, Maity A, Carlson CA, et al. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer 2006; 46:222–227.
- Wohl ME, Griscom NT, Traggis DG, et al. Effects of therapeutic irradiation delivered in early childhood upon subsequent lung function. Pediatrics 1975; 55:507–516.
- Hudson MM, Greenwald C, Thompson E, et al. Efficacy and toxicity of multiagent chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin’s disease. J Clin Oncol 1993; 11:100–108.
- Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin’s disease. J Clin Oncol 2004; 22:4541–4550.
- Quigley PM, Yeager AM, Loughlin GM. The effects of bone marrow transplantation on pulmonary function in children. Pediatr Pulmonol 1994; 18:361–367.
- Faraci M, Barra S, Cohen A, et al. Very late nonfatal consequences of fractionated TBI in children undergoing bone marrow transplant. Int J Radiat Oncol Biol Phys 2005; 63:1568–1575.
- Eigen H, Wyszomierski D. Bleomycin lung injury in children. Pathophysiology and guidelines for management. Am J Pediatr Hematol Oncol 1985; 7:71–78.
- Goldiner PL, Schweizer O. The hazards of anesthesia and surgery in bleomycin-treated patients. Semin Oncol 1979; 6:121–124.
- Zaniboni A, Prabhu S, Audisio RA. Chemotherapy and anaesthetic drugs: too little is known. Lancet Oncol 2005; 6:176–181.
- Aronin PA, Mahaley MS, Rudnick SA, et al. Prediction of BCNU pulmonary toxicity in patients with malignant gliomas: an assessment of risk factors. N Engl J Med 1980; 303:183–188.
- O’Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med 1990; 323:378–382.
- Alvarado CS, Boat TF, Newman AJ. Late-onset pulmonary fibrosis and chest deformity in two children treated with cyclophosphamide. J Pediatr 1978; 92:443–446.
- Codling BW, Chakera TM. Pulmonary fibrosis following therapy with melphalan for multiple myeloma. J Clin Pathol 1972; 25:668–673.
- Bruno B, Souillet G, Bertrand Y, et al. Effects of allogeneic bone marrow transplantation on pulmonary function in 80 children in a single paediatric centre. Bone Marrow Transplant 2004; 34:143–147.
- Lateef O, Shakoor N, Balk RA. Methotrexate pulmonary toxicity. Expert Opin Drug Saf 2005; 4:723–730.
- Cottin V, Tebob J, Massonnet B, et al. Pulmonary function in patients receiving long-term methotrexate. Chest 1996; 109:933–938.
- Gutin PH, Green MR, Bleyer WA, et al. Methotrexate pneumonitis induced by intrathecal methotrexate therapy: a case report with pharmacokinetic data. Cancer 1976; 38:1529–1534.
- Konits PH, Aisner J, Sutherland JC, et al. Possible pulmonary toxicity secondary to vinblastine. Cancer 1982; 50:2771–2774.
- Cerveri I, Zoia MC, Fulgoni P, et al. Late pulmonary sequelae after childhood bone marrow transplantation. Thorax 1999; 54:131–135.
- Cerveri I, Fulgoni P, Giorgiani G, et al. Lung function abnormalities after bone marrow transplantation in children: has the trend recently changed? Chest 2001; 120:1900–1906.
- Neve V, Foot AB, Michon J, et al. Longitudinal clinical and functional pulmonary follow-up after megatherapy, fractionated total body irradiation, and autologous bone marrow transplantation for metastatic neuroblastoma. Med Pediatr Oncol 1999; 32:170–176.
- Nenadov Beck M, Meresse V, Hartmann O, et al. Long-term pulmonary sequelae after autologous bone marrow transplantation in children without total body irradiation. Bone Marrow Transplant 1995; 16:771–775.
- Huls G, ten Bokkel Huinink D. Bleomycin and scuba diving: to dive or not to dive? Neth J Med 2003; 61:50–53.
- Kreisel D, Krupnick AS, Huddleston CB. Outcomes and late complications after pulmonary resections in the pediatric population. Semin Thorac Cardiovasc Surg 2004; 16:215–219.
- Lezama-del Valle Valle P, Blakely ML, Lobe TE. Physiologic consequences of pneumonectomy. Long-term consequences of pneumonectomy done in children. Chest Surg Clin North Am 1999; 9:485–495.
- Tobias JD, Bozeman PM, Mackert PW, et al. Postoperative outcome following thoracotomy in the pediatric oncology patient with diminished pulmonary function. J Surg Oncol 1993; 52:105–109.
- Drugs for Tobacco Dependence. Treatment Guidelines from the Medical Letter. June, 2003: 1(10):65–68.
Children who undergo radiotherapy, chemotherapy, or surgery for cancer face a risk of complications later in life, including pulmonary fibrosis and pneumonitis.
These long-term cancer survivors need systematic, lifelong surveillance, in a program that takes into account their individual risk (based on therapeutic exposures, genetic predisposition, lifestyle behaviors, and comorbid health conditions).1 Optimally, they would receive their care at multidisciplinary follow-up clinics organized by pediatric oncologists at tertiary care centers. However, access to such centers is limited, making this an option for relatively few. Consequently, as childhood cancer survivors age, internists and family practitioners may need to assume an increasing amount of responsibility for their follow-up care.
Because individual primary care providers are unlikely to follow more than a handful of survivors, specialists have developed guidelines for survivors of pediatric cancer. Working with established multidisciplinary clinics may help ensure appropriate follow-up for this population of patients.
This review summarizes the late effects of cancer therapy on the lungs and an approach to surveillance for the generalist or pulmonologist. We also review the quality of the evidence upon which these recommendations are based.
NUMBERS ON THE RISE
An estimated 1 of every 330 children develops cancer before age 19. With cure rates exceeding 75% for many pediatric malignancies, the number of survivors of childhood cancer, currently in excess of 270,000, will continue to increase.2
THE CHILDREN’S ONCOLOGY GROUP GUIDELINES
The Children’s Oncology Group (COG)3 released its first set of guidelines in 2003 for the follow-up care of patients treated for pediatric malignancies; the current version is available at www.survivorshipguidelines.org. The guidelines contain comprehensive screening recommendations, including those related to pulmonary toxicity, which can be used to standardize care.
Patient education materials accompany the guidelines, offering detailed information on guideline-specific topics in order to promote health maintenance.
HOW WE SEARCHED THE LITERATURE
We performed an extensive review of the literature via MEDLINE for the years 1975–2005. Key search terms were “childhood cancer,” “late effects,” and “pulmonary toxicity,” combined with keywords for each therapeutic exposure. References from selected articles were used to broaden the search. From several hundred citations, fewer than 30 were selected as best illustrating the relevant associations.
RISK IS THREE TIMES HIGHER IN CANCER SURVIVORS
The Childhood Cancer Survivor Study4 is the largest database of late effects, with more than 12,000 survivors of childhood cancer diagnosed between 1970 and 1986. Its data suggest that the risk of pulmonary conditions is more than three times higher in cancer survivors than in their siblings, as manifested by pulmonary signs (abnormal chest wall growth), symptoms (chronic cough, use of supplemental oxygen, exercise-induced shortness of breath), or specific diagnoses (lung fibrosis, recurrent pneumonia, pleurisy, bronchitis, recurrent sinus infection, or tonsillitis). Limitations: these data are retrospective, and the outcomes were detected by self-report and were not validated by review of medical records. Thus, the figures highlight the fact that pulmonary late effects are an important problem but do not give us a way to calculate risk exactly.
Other limitations of the literature: Treatments are constantly evolving, often in attempts to minimize late effects, and newer agents will need to be monitored for pulmonary toxicities. As noted, much of the available information is from studies of survivors of adult cancer; the potential for late effects of similar therapies in children is inferred. Most conclusions—and especially those based upon prospective serial evaluations—derive from small cohorts. For all treatments, the complications in the very long term remain undefined. What we know is summarized below.
CANCER THERAPY CAUSES FIBROSIS, PNEUMONITIS
The courses of these diseases are poorly characterized, since few longitudinal studies have been done. However, like most of the late effects of cancer therapy, pulmonary toxicity may first become apparent during the treatment and persist, or it may not appear until years later. Signs and symptoms may be static, progressive, or reversible.
ANGIOGENESIS MAY CONTRIBUTE TO FIBROSIS
On a microscopic level, pulmonary fibrosis is characterized by epithelial injury, fibroproliferation, and excessive extracellular matrix deposition.6–8
Evidence is mounting that these findings result in part from angiogenesis. Although this has not been studied in long-term cancer survivors, evidence of neovascularization was seen both in an animal model of lung fibrosis and in patients with idiopathic pulmonary fibrosis.6–8 High plasma concentrations of angiogenic cytokines (eg, tumor necrosis factor alpha, interleukin 8, and endothelin 1) have been found in these situations. Antiangiogenic agents and other immune modulators such as thalidomide may be beneficial in patients with lung fibrosis.7
On a macroscopic level, pulmonary fibrosis results in loss of lung volume in older children and in adults. In contrast, in younger children, interference with growth of both the lung and the chest wall may contribute to pulmonary dysfunction.
CANCER TYPES AND TREATMENTS VARY BY AGE
Cancers that commonly involve the thorax are listed in Table 2. Neuroblastoma, hepatoblastoma, extragonadal germ cell tumors, and Wilms tumor typically are diseases of young children; osteosarcoma, Ewing sarcoma, thyroid carcinoma, and Hodgkin disease are most common in older children and adolescents; soft tissue sarcoma and non-Hodgkin lymphoma span all age groups.
Surgery can, in some cases, control the cancer, as with mediastinal neuroblastoma and Ewing sarcoma of the chest wall.
Radiation to the chest remains a major component of treatment for Hodgkin disease, unresected thoracic Ewing sarcoma, soft tissue sarcoma with lung involvement or thyroid carcinoma, and Wilms tumor. Central nervous system tumors and leukemias, the most common pediatric malignancies, may require radiation to the spinal cord—with resulting radiation exposure of the lungs. Total-body irradiation is a component of many preparative regimens for stem cell transplantation.
Chemotherapy remains a mainstay for all types of tumors, and patients with germ cell tumors, Hodgkin disease, and brain tumors are at particular risk of pulmonary toxicity due to heavy reliance on bleomycin (Blenoxane) (for germ cell tumors, Hodgkin disease) and the nitrosoureas (for brain tumors).
RADIATION-INDUCED LUNG DAMAGE
The lungs are particularly sensitive to radiation, and pulmonary problems occur most often in patients with malignant diseases of the chest that are treated with radiation, ie, those involving the mediastinum, the lung parenchyma, or the chest wall.
Abnormal radiographic findings or restrictive changes on pulmonary function testing have been reported in more than 30% of patients who received radiation directly or indirectly to the lung.9–12 These changes have been detected months to years after radiation therapy, most often in patients who suffered radiation pneumonitis as an acute toxicity.
The amount of damage depends on the cumulative dose, how many treatments (“fractions”) this cumulative dose was divided into (dividing the radiation dose into smaller dose fractions can reduce toxicity), the volume of lung tissue involved, and the patient’s age (the younger, the worse) at the time of treatment.
Cumulative dose. Whole-lung irradiation is limited to 12 Gy, although localized areas of cancer can be treated with much higher doses.
Clinically apparent pneumonitis with cough, fever, or dyspnea generally occurs only in survivors who received more than 30 Gy in standard fractions to more than 50% of the lung. However, in 12 survivors of Wilms tumor who received median total doses of approximately 20 Gy to both lungs 7 to 14 years previously, 8 patients had dyspnea on exertion and radiographic evidence of interstitial and pleural thickening.13 Mean total lung volumes and the diffusing capacity of the lung for carbon monoxide (DLCO) were reduced in all patients to approximately 60% of predicted values.
In a prospective study of adults with Hodgkin disease treated after 1980, 145 patients were examined 3 years after receiving more than 44 Gy limited to the mantle area.9 None were experiencing symptoms; however, 30% to 40% had a forced vital capacity (FVC) less than 80% of predicted, and 7% had a reduced DLCO. Some of these patients also had received bleomycin (see below), which may have exacerbated pulmonary toxicity. Asymptomatic restrictive and obstructive lung changes have been detected after lower doses of whole-lung radiation (11–14 Gy) were given for other malignant diseases.11,14
In a recent study of adults and older adolescents with stage I and IIA Hodgkin disease treated with radiation alone (40–45 Gy to involved fields, including the mediastinum), late pulmonary effects observed were minimal.10 While FVC, residual volume, forced expiratory volume in 1 second (FEV1), DLCO, and total lung capacity (TLC) were significantly lower at the end of radiation therapy than before treatment, all except DLCO returned nearly to normal within 1 year. The decrease in DLCO remained stable, but the forced expiratory flow rate between 25% and 75% of FVC (FEF 25%–75%) was significantly lower 3 years after treatment than at baseline.
The high doses of total lung irradiation cited in several of these reports are rarely used in today’s cancer protocols. For example, patients receiving radiation as adjunctive treatment for Hodgkin disease now receive lower doses (< 21 Gy), which are restricted to involved fields, and the pulmonary toxicity is less than in the past.
In one series, 159 children and adolescents with unfavorable Hodgkin disease treated from 1993 to 2000 received six cycles of chemotherapy followed by response-based, involved-field radiation therapy. Patients who achieved a complete response after the first two cycles of chemotherapy got 15 Gy, and those who achieved a partial response got 25.5 Gy to all sites of bulky lymphadenopathy.15 All patients underwent pulmonary function testing. Only 24 (30.8%) of them had pulmonary toxicity, which was limited to asymptomatic deficits of restriction and diffusion.
Nevertheless, the lungs receive some radiation even when they are not the target, such as in patients with malignant brain tumors, and this exposure can contribute to the development of lung disease, although these patients are likely to have no symptoms during day-to-day activities. Innovations in targeted radiation delivery (eg, conformal radiation) should further limit damage to normal lung tissue.
Age at the time of treatment also may influence the type and incidence of pulmonary sequelae. In older children and adults, radiation for thoracic malignancy results in pulmonary fibrosis with loss of lung volume. Similar injury can occur in younger children, but pulmonary function may also be compromised by inhibited growth of the supportive structures and the chest wall. One report suggests that children younger than 3 years at the time of therapy experience more chronic toxicity.11
In contrast, after bone marrow transplantation, children seem to be at less risk of significant late pulmonary dysfunction than adults are, despite similar preparatory regimens.16 This may in part reflect a lower incidence of severe graft-vs-host disease involving the lung. Nonetheless, in a recent report of children treated with fractionated total-body irradiation between 1985 and 1993, restrictive pulmonary diseases were found in 30 of 42 patients at a median of 3.1 years after treatment (range 0.5–17 years).17 Most of these patients had asymptomatic mild restrictive disease, and the one patient with severe changes had previously received thoracic radiation for treatment of neuroblastoma. In about half of the cases, pulmonary function abnormalities were permanent although not progressive.
CHEMOTHERAPY-RELATED LUNG DAMAGE
A growing list of chemotherapeutic agents appears to cause pulmonary disease in long-term survivors.5
Bleomycin
Bleomycin toxicity is the prototype for chemotherapy-related lung injury: bleomycin was the first chemotherapy drug shown to cause lung injury, this effect is suggested by a large database, and the mechanism is typical.5,18 Preclinical studies have attributed bleomycin’s toxicity to its tendency to promote free radicals.
Although interstitial pneumonitis and pulmonary fibrosis have been reported in children, clinically apparent bleomycin pneumonopathy is most frequent in older adults. Usually, the abnormalities began within 3 months of therapy and persisted or progressed. Like the acute toxicity, it is dose-dependent and more common above a threshold cumulative dose of 400 units/m2. Above this dose, 10% of adult patients without other risk factors develop fibrosis; data are not available for these doses in children. At lower doses, fibrosis occurs sporadically in fewer than 5% of patients, with a 1% to 2% mortality rate. In some series, bleomycin toxicity was anticipated on the basis of DLCO abnormalities.
Bleomycin pulmonary toxicity is variably exacerbated by concurrent or previous radiation therapy.
Increased oxygen concentrations associated with general anesthesia have also been found to exacerbate prior bleomycin-induced pulmonary injury.19,20 In one instance (reviewed by Zaniboni et al20), the patient recovered with corticosteroid treatment.
Alkylating agents
Carmustine (also called BCNU; brand names BiCNU, Gliadel) and lomustine (CCNU; CeeNU) are thought to cause dose-related lung injury. When cumulative carmustine doses are greater than 1,500 mg/m2, more than 50% of patients develop symptoms.21
In a careful clinicopathologic review of 31 children with brain tumors, restrictive changes with lung fibrosis were reported up to 17 years after treatment, most often with carmustine 100 mg/m2 every 6 to 8 weeks for up to 2 years.22 Four of the 8 patients still alive at the time of study experienced shortness of breath and coughing; 6 showed a characteristic pattern of upper zone fibrosis on chest radiography and computed tomography; all 8 survivors had restrictive findings on pulmonary function testing, with vital capacities of about 50% of normal. Toxicity increases with more intensive dose-scheduling.
Cyclophosphamide (Cytoxan) may cause delayed-onset pulmonary fibrosis with severe restrictive lung disease in association with marked reductions in the anteroposterior diameter of the chest, although the evidence is less convincing than with carmustine and lomustine, coming from case reports and small series.23
Melphalan (Alkeran), generally in doses used in stem cell transplant conditioning regimens, is also thought to cause pulmonary fibrosis.24
Busulfan most predictably causes toxicity when it is used in transplantation doses (ie, more than 500 mg), and may be associated with a progressive, potentially fatal restrictive lung disease.25 A current trend is to adjust the dose on the basis of pharmacokinetic analysis, which we hope will reduce toxicity.
Other agents
Methotrexate (MTX; Trexall) also has been associated with chronic pneumonitis and fibrosis.26 This probably occurs with an incidence well below 1% and may be idiosyncratic and not dose-related. Asymptomatic changes in pulmonary function tests that do not predict clinically significant problems have most frequently been associated with low-dose oral administration during more than 3 years.27 This is a treatment approach used in patients with psoriasis or rheumatoid arthritis, but is now obsolete for pediatric cancer. Intravenous and, rarely, intrathecal administration also have been associated with pulmonary toxicity.28 A single report of two patients who developed diffuse interstitial pulmonary infiltrates and chronic pulmonary changes links vinblastine to these sequelae.29
LUNG INJURY AFTER BONE MARROW TRANSPLANTATION
Hematopoietic stem cell transplantation is associated with various late pulmonary complications. The factors that influence these complications are similar to those discussed earlier. However, the intensity of therapy in patients undergoing transplantation and the additive effects of previous therapies magnify the risks.
Patients undergoing total-body irradiation as part of their preparation for transplantation have a high incidence of late pulmonary complications.25,30–32 Busulfan, carmustine, bleomycin, and cyclophosphamide, also commonly used conditioning chemotherapies, are known to cause pneumonitis and fibrosis after transplantation.33
Some acute pulmonary toxicities can have long-standing effects, including serious pulmonary infections, idiopathic pneumonia syndrome, bronchiolitis obliterans, acute respiratory distress syndrome, or other damage related to graft-vs-host disease.
OTHER RISK FACTORS FOR LUNG DAMAGE
Additional factors contributing to chronic pulmonary toxicity include superimposed infection, underlying pneumonopathy (eg, asthma), cigarette use, respirator toxicity, chronic graft-vs-host disease, and the effects of chronic pulmonary involvement by tumor or reaction to tumor. For example, a subset of patients with Langerhans cell histiocytosis can develop histiocytic pulmonary infiltrates or honeycombing with severe chronic restrictive lung disease unrelated to therapy or the presence of active tumor.
Although not well documented, scuba diving also has been said to exacerbate pulmonary fibrosis through increased underwater pressures and high oxygen levels.34
Lung lobectomy during childhood appears to have no significant impact on long-term pulmonary function,35–37 but the effect of lung surgery for children with cancer is not well defined.
GET THE PATIENT’S TREATMENT SUMMARY
Regardless of the setting for follow-up, the first step in any evaluation is to obtain the patient’s medical history and especially a treatment summary. The treatment summary should outline the cancer diagnosis, involved sites of disease, age at diagnosis, specific treatments (surgery, chemotherapy, radiation), and other key interventions and events during and after cancer therapy. Sample forms for physicians and patients are available at www.survivorshipguidelines.org.
Before the long-term survivor of childhood cancer graduates from the care of a pediatric oncologist, this treatment record and possible long-term problems should be reviewed with the family and, in the case of an adolescent, with the patient. Correspondence between the pediatric oncologist and subsequent caregivers should also include a treatment summary. The treatment summary allows the survivor or his health care provider to interface with the COG guidelines to determine recommended follow-up care. The primary care physician and the patient both should have copies of this document.
We are developing an interactive Web-based version of a standardized summary form, designed to interface with an automated version of the COG guidelines in order to generate individualized follow-up recommendations.
ASK ABOUT LUNG SYMPTOMS
We recommend that health care providers investigate symptoms of pulmonary dysfunction, and specifically ask about chronic cough with or without fever, shortness of breath, and dyspnea on exertion during yearly health care visits.
Baseline pulmonary function testing (including DLCO and spirometry) and chest radiography are recommended 2 or more years after completion of therapy to document persistent deficits and determine the need for continued monitoring. Reevaluation of pulmonary function should be considered in patients with established deficits who require general anesthesia and for those treated with bleomycin.
Scuba diving remains controversial for long-term survivors. Consequently, patients with risk factors for lung disease should be encouraged to consult with a pulmonary specialist to determine if diving poses a health threat to their pulmonary status. If clinical pulmonary dysfunction is identified, referral to colleagues in pulmonology for additional evaluation and treatment is essential. Increasing familiarity of primary care providers with surveillance concepts is a key element in survivorship care.
Smoking cessation can enhance the health of all patients and is particularly important among long-term survivors, especially those who received treatments predisposing to pulmonary injury. Strategies for cessation and patient information can be found at www.cdc.gov/tobacco/how2quit.htm.
Clinicians can take advantage of every patient interaction to assess readiness for smoking cessation and assist patients in this goal. Following the principles of patient-centered counseling, physicians can guide patients into considering a change of behavior with advice and encouragement. Whenever possible, physicians should personalize the risks of smoking as well as the short-term and long-term benefits. As smokers prepare to quit, their physicians can assist in developing a plan that includes a quit date.
Many pharmacologic agents are available to assist patients, ie, nicotine inhalers, sprays, gum, and transdermal patches; the antidepressants bupropion (Wellbutrin) and nortriptiline (Pamelor); the alpha-2 adrenergic agonist clonidine (Catapres); and, most recently, the nicotine receptor partial agonist varenicline (Chantix).38
Follow-up to prevent relapse is an important part of this process.
Acknowledgment. This work was supported in part by the Swim Across America Foundation and the Campini Foundation.
Children who undergo radiotherapy, chemotherapy, or surgery for cancer face a risk of complications later in life, including pulmonary fibrosis and pneumonitis.
These long-term cancer survivors need systematic, lifelong surveillance, in a program that takes into account their individual risk (based on therapeutic exposures, genetic predisposition, lifestyle behaviors, and comorbid health conditions).1 Optimally, they would receive their care at multidisciplinary follow-up clinics organized by pediatric oncologists at tertiary care centers. However, access to such centers is limited, making this an option for relatively few. Consequently, as childhood cancer survivors age, internists and family practitioners may need to assume an increasing amount of responsibility for their follow-up care.
Because individual primary care providers are unlikely to follow more than a handful of survivors, specialists have developed guidelines for survivors of pediatric cancer. Working with established multidisciplinary clinics may help ensure appropriate follow-up for this population of patients.
This review summarizes the late effects of cancer therapy on the lungs and an approach to surveillance for the generalist or pulmonologist. We also review the quality of the evidence upon which these recommendations are based.
NUMBERS ON THE RISE
An estimated 1 of every 330 children develops cancer before age 19. With cure rates exceeding 75% for many pediatric malignancies, the number of survivors of childhood cancer, currently in excess of 270,000, will continue to increase.2
THE CHILDREN’S ONCOLOGY GROUP GUIDELINES
The Children’s Oncology Group (COG)3 released its first set of guidelines in 2003 for the follow-up care of patients treated for pediatric malignancies; the current version is available at www.survivorshipguidelines.org. The guidelines contain comprehensive screening recommendations, including those related to pulmonary toxicity, which can be used to standardize care.
Patient education materials accompany the guidelines, offering detailed information on guideline-specific topics in order to promote health maintenance.
HOW WE SEARCHED THE LITERATURE
We performed an extensive review of the literature via MEDLINE for the years 1975–2005. Key search terms were “childhood cancer,” “late effects,” and “pulmonary toxicity,” combined with keywords for each therapeutic exposure. References from selected articles were used to broaden the search. From several hundred citations, fewer than 30 were selected as best illustrating the relevant associations.
RISK IS THREE TIMES HIGHER IN CANCER SURVIVORS
The Childhood Cancer Survivor Study4 is the largest database of late effects, with more than 12,000 survivors of childhood cancer diagnosed between 1970 and 1986. Its data suggest that the risk of pulmonary conditions is more than three times higher in cancer survivors than in their siblings, as manifested by pulmonary signs (abnormal chest wall growth), symptoms (chronic cough, use of supplemental oxygen, exercise-induced shortness of breath), or specific diagnoses (lung fibrosis, recurrent pneumonia, pleurisy, bronchitis, recurrent sinus infection, or tonsillitis). Limitations: these data are retrospective, and the outcomes were detected by self-report and were not validated by review of medical records. Thus, the figures highlight the fact that pulmonary late effects are an important problem but do not give us a way to calculate risk exactly.
Other limitations of the literature: Treatments are constantly evolving, often in attempts to minimize late effects, and newer agents will need to be monitored for pulmonary toxicities. As noted, much of the available information is from studies of survivors of adult cancer; the potential for late effects of similar therapies in children is inferred. Most conclusions—and especially those based upon prospective serial evaluations—derive from small cohorts. For all treatments, the complications in the very long term remain undefined. What we know is summarized below.
CANCER THERAPY CAUSES FIBROSIS, PNEUMONITIS
The courses of these diseases are poorly characterized, since few longitudinal studies have been done. However, like most of the late effects of cancer therapy, pulmonary toxicity may first become apparent during the treatment and persist, or it may not appear until years later. Signs and symptoms may be static, progressive, or reversible.
ANGIOGENESIS MAY CONTRIBUTE TO FIBROSIS
On a microscopic level, pulmonary fibrosis is characterized by epithelial injury, fibroproliferation, and excessive extracellular matrix deposition.6–8
Evidence is mounting that these findings result in part from angiogenesis. Although this has not been studied in long-term cancer survivors, evidence of neovascularization was seen both in an animal model of lung fibrosis and in patients with idiopathic pulmonary fibrosis.6–8 High plasma concentrations of angiogenic cytokines (eg, tumor necrosis factor alpha, interleukin 8, and endothelin 1) have been found in these situations. Antiangiogenic agents and other immune modulators such as thalidomide may be beneficial in patients with lung fibrosis.7
On a macroscopic level, pulmonary fibrosis results in loss of lung volume in older children and in adults. In contrast, in younger children, interference with growth of both the lung and the chest wall may contribute to pulmonary dysfunction.
CANCER TYPES AND TREATMENTS VARY BY AGE
Cancers that commonly involve the thorax are listed in Table 2. Neuroblastoma, hepatoblastoma, extragonadal germ cell tumors, and Wilms tumor typically are diseases of young children; osteosarcoma, Ewing sarcoma, thyroid carcinoma, and Hodgkin disease are most common in older children and adolescents; soft tissue sarcoma and non-Hodgkin lymphoma span all age groups.
Surgery can, in some cases, control the cancer, as with mediastinal neuroblastoma and Ewing sarcoma of the chest wall.
Radiation to the chest remains a major component of treatment for Hodgkin disease, unresected thoracic Ewing sarcoma, soft tissue sarcoma with lung involvement or thyroid carcinoma, and Wilms tumor. Central nervous system tumors and leukemias, the most common pediatric malignancies, may require radiation to the spinal cord—with resulting radiation exposure of the lungs. Total-body irradiation is a component of many preparative regimens for stem cell transplantation.
Chemotherapy remains a mainstay for all types of tumors, and patients with germ cell tumors, Hodgkin disease, and brain tumors are at particular risk of pulmonary toxicity due to heavy reliance on bleomycin (Blenoxane) (for germ cell tumors, Hodgkin disease) and the nitrosoureas (for brain tumors).
RADIATION-INDUCED LUNG DAMAGE
The lungs are particularly sensitive to radiation, and pulmonary problems occur most often in patients with malignant diseases of the chest that are treated with radiation, ie, those involving the mediastinum, the lung parenchyma, or the chest wall.
Abnormal radiographic findings or restrictive changes on pulmonary function testing have been reported in more than 30% of patients who received radiation directly or indirectly to the lung.9–12 These changes have been detected months to years after radiation therapy, most often in patients who suffered radiation pneumonitis as an acute toxicity.
The amount of damage depends on the cumulative dose, how many treatments (“fractions”) this cumulative dose was divided into (dividing the radiation dose into smaller dose fractions can reduce toxicity), the volume of lung tissue involved, and the patient’s age (the younger, the worse) at the time of treatment.
Cumulative dose. Whole-lung irradiation is limited to 12 Gy, although localized areas of cancer can be treated with much higher doses.
Clinically apparent pneumonitis with cough, fever, or dyspnea generally occurs only in survivors who received more than 30 Gy in standard fractions to more than 50% of the lung. However, in 12 survivors of Wilms tumor who received median total doses of approximately 20 Gy to both lungs 7 to 14 years previously, 8 patients had dyspnea on exertion and radiographic evidence of interstitial and pleural thickening.13 Mean total lung volumes and the diffusing capacity of the lung for carbon monoxide (DLCO) were reduced in all patients to approximately 60% of predicted values.
In a prospective study of adults with Hodgkin disease treated after 1980, 145 patients were examined 3 years after receiving more than 44 Gy limited to the mantle area.9 None were experiencing symptoms; however, 30% to 40% had a forced vital capacity (FVC) less than 80% of predicted, and 7% had a reduced DLCO. Some of these patients also had received bleomycin (see below), which may have exacerbated pulmonary toxicity. Asymptomatic restrictive and obstructive lung changes have been detected after lower doses of whole-lung radiation (11–14 Gy) were given for other malignant diseases.11,14
In a recent study of adults and older adolescents with stage I and IIA Hodgkin disease treated with radiation alone (40–45 Gy to involved fields, including the mediastinum), late pulmonary effects observed were minimal.10 While FVC, residual volume, forced expiratory volume in 1 second (FEV1), DLCO, and total lung capacity (TLC) were significantly lower at the end of radiation therapy than before treatment, all except DLCO returned nearly to normal within 1 year. The decrease in DLCO remained stable, but the forced expiratory flow rate between 25% and 75% of FVC (FEF 25%–75%) was significantly lower 3 years after treatment than at baseline.
The high doses of total lung irradiation cited in several of these reports are rarely used in today’s cancer protocols. For example, patients receiving radiation as adjunctive treatment for Hodgkin disease now receive lower doses (< 21 Gy), which are restricted to involved fields, and the pulmonary toxicity is less than in the past.
In one series, 159 children and adolescents with unfavorable Hodgkin disease treated from 1993 to 2000 received six cycles of chemotherapy followed by response-based, involved-field radiation therapy. Patients who achieved a complete response after the first two cycles of chemotherapy got 15 Gy, and those who achieved a partial response got 25.5 Gy to all sites of bulky lymphadenopathy.15 All patients underwent pulmonary function testing. Only 24 (30.8%) of them had pulmonary toxicity, which was limited to asymptomatic deficits of restriction and diffusion.
Nevertheless, the lungs receive some radiation even when they are not the target, such as in patients with malignant brain tumors, and this exposure can contribute to the development of lung disease, although these patients are likely to have no symptoms during day-to-day activities. Innovations in targeted radiation delivery (eg, conformal radiation) should further limit damage to normal lung tissue.
Age at the time of treatment also may influence the type and incidence of pulmonary sequelae. In older children and adults, radiation for thoracic malignancy results in pulmonary fibrosis with loss of lung volume. Similar injury can occur in younger children, but pulmonary function may also be compromised by inhibited growth of the supportive structures and the chest wall. One report suggests that children younger than 3 years at the time of therapy experience more chronic toxicity.11
In contrast, after bone marrow transplantation, children seem to be at less risk of significant late pulmonary dysfunction than adults are, despite similar preparatory regimens.16 This may in part reflect a lower incidence of severe graft-vs-host disease involving the lung. Nonetheless, in a recent report of children treated with fractionated total-body irradiation between 1985 and 1993, restrictive pulmonary diseases were found in 30 of 42 patients at a median of 3.1 years after treatment (range 0.5–17 years).17 Most of these patients had asymptomatic mild restrictive disease, and the one patient with severe changes had previously received thoracic radiation for treatment of neuroblastoma. In about half of the cases, pulmonary function abnormalities were permanent although not progressive.
CHEMOTHERAPY-RELATED LUNG DAMAGE
A growing list of chemotherapeutic agents appears to cause pulmonary disease in long-term survivors.5
Bleomycin
Bleomycin toxicity is the prototype for chemotherapy-related lung injury: bleomycin was the first chemotherapy drug shown to cause lung injury, this effect is suggested by a large database, and the mechanism is typical.5,18 Preclinical studies have attributed bleomycin’s toxicity to its tendency to promote free radicals.
Although interstitial pneumonitis and pulmonary fibrosis have been reported in children, clinically apparent bleomycin pneumonopathy is most frequent in older adults. Usually, the abnormalities began within 3 months of therapy and persisted or progressed. Like the acute toxicity, it is dose-dependent and more common above a threshold cumulative dose of 400 units/m2. Above this dose, 10% of adult patients without other risk factors develop fibrosis; data are not available for these doses in children. At lower doses, fibrosis occurs sporadically in fewer than 5% of patients, with a 1% to 2% mortality rate. In some series, bleomycin toxicity was anticipated on the basis of DLCO abnormalities.
Bleomycin pulmonary toxicity is variably exacerbated by concurrent or previous radiation therapy.
Increased oxygen concentrations associated with general anesthesia have also been found to exacerbate prior bleomycin-induced pulmonary injury.19,20 In one instance (reviewed by Zaniboni et al20), the patient recovered with corticosteroid treatment.
Alkylating agents
Carmustine (also called BCNU; brand names BiCNU, Gliadel) and lomustine (CCNU; CeeNU) are thought to cause dose-related lung injury. When cumulative carmustine doses are greater than 1,500 mg/m2, more than 50% of patients develop symptoms.21
In a careful clinicopathologic review of 31 children with brain tumors, restrictive changes with lung fibrosis were reported up to 17 years after treatment, most often with carmustine 100 mg/m2 every 6 to 8 weeks for up to 2 years.22 Four of the 8 patients still alive at the time of study experienced shortness of breath and coughing; 6 showed a characteristic pattern of upper zone fibrosis on chest radiography and computed tomography; all 8 survivors had restrictive findings on pulmonary function testing, with vital capacities of about 50% of normal. Toxicity increases with more intensive dose-scheduling.
Cyclophosphamide (Cytoxan) may cause delayed-onset pulmonary fibrosis with severe restrictive lung disease in association with marked reductions in the anteroposterior diameter of the chest, although the evidence is less convincing than with carmustine and lomustine, coming from case reports and small series.23
Melphalan (Alkeran), generally in doses used in stem cell transplant conditioning regimens, is also thought to cause pulmonary fibrosis.24
Busulfan most predictably causes toxicity when it is used in transplantation doses (ie, more than 500 mg), and may be associated with a progressive, potentially fatal restrictive lung disease.25 A current trend is to adjust the dose on the basis of pharmacokinetic analysis, which we hope will reduce toxicity.
Other agents
Methotrexate (MTX; Trexall) also has been associated with chronic pneumonitis and fibrosis.26 This probably occurs with an incidence well below 1% and may be idiosyncratic and not dose-related. Asymptomatic changes in pulmonary function tests that do not predict clinically significant problems have most frequently been associated with low-dose oral administration during more than 3 years.27 This is a treatment approach used in patients with psoriasis or rheumatoid arthritis, but is now obsolete for pediatric cancer. Intravenous and, rarely, intrathecal administration also have been associated with pulmonary toxicity.28 A single report of two patients who developed diffuse interstitial pulmonary infiltrates and chronic pulmonary changes links vinblastine to these sequelae.29
LUNG INJURY AFTER BONE MARROW TRANSPLANTATION
Hematopoietic stem cell transplantation is associated with various late pulmonary complications. The factors that influence these complications are similar to those discussed earlier. However, the intensity of therapy in patients undergoing transplantation and the additive effects of previous therapies magnify the risks.
Patients undergoing total-body irradiation as part of their preparation for transplantation have a high incidence of late pulmonary complications.25,30–32 Busulfan, carmustine, bleomycin, and cyclophosphamide, also commonly used conditioning chemotherapies, are known to cause pneumonitis and fibrosis after transplantation.33
Some acute pulmonary toxicities can have long-standing effects, including serious pulmonary infections, idiopathic pneumonia syndrome, bronchiolitis obliterans, acute respiratory distress syndrome, or other damage related to graft-vs-host disease.
OTHER RISK FACTORS FOR LUNG DAMAGE
Additional factors contributing to chronic pulmonary toxicity include superimposed infection, underlying pneumonopathy (eg, asthma), cigarette use, respirator toxicity, chronic graft-vs-host disease, and the effects of chronic pulmonary involvement by tumor or reaction to tumor. For example, a subset of patients with Langerhans cell histiocytosis can develop histiocytic pulmonary infiltrates or honeycombing with severe chronic restrictive lung disease unrelated to therapy or the presence of active tumor.
Although not well documented, scuba diving also has been said to exacerbate pulmonary fibrosis through increased underwater pressures and high oxygen levels.34
Lung lobectomy during childhood appears to have no significant impact on long-term pulmonary function,35–37 but the effect of lung surgery for children with cancer is not well defined.
GET THE PATIENT’S TREATMENT SUMMARY
Regardless of the setting for follow-up, the first step in any evaluation is to obtain the patient’s medical history and especially a treatment summary. The treatment summary should outline the cancer diagnosis, involved sites of disease, age at diagnosis, specific treatments (surgery, chemotherapy, radiation), and other key interventions and events during and after cancer therapy. Sample forms for physicians and patients are available at www.survivorshipguidelines.org.
Before the long-term survivor of childhood cancer graduates from the care of a pediatric oncologist, this treatment record and possible long-term problems should be reviewed with the family and, in the case of an adolescent, with the patient. Correspondence between the pediatric oncologist and subsequent caregivers should also include a treatment summary. The treatment summary allows the survivor or his health care provider to interface with the COG guidelines to determine recommended follow-up care. The primary care physician and the patient both should have copies of this document.
We are developing an interactive Web-based version of a standardized summary form, designed to interface with an automated version of the COG guidelines in order to generate individualized follow-up recommendations.
ASK ABOUT LUNG SYMPTOMS
We recommend that health care providers investigate symptoms of pulmonary dysfunction, and specifically ask about chronic cough with or without fever, shortness of breath, and dyspnea on exertion during yearly health care visits.
Baseline pulmonary function testing (including DLCO and spirometry) and chest radiography are recommended 2 or more years after completion of therapy to document persistent deficits and determine the need for continued monitoring. Reevaluation of pulmonary function should be considered in patients with established deficits who require general anesthesia and for those treated with bleomycin.
Scuba diving remains controversial for long-term survivors. Consequently, patients with risk factors for lung disease should be encouraged to consult with a pulmonary specialist to determine if diving poses a health threat to their pulmonary status. If clinical pulmonary dysfunction is identified, referral to colleagues in pulmonology for additional evaluation and treatment is essential. Increasing familiarity of primary care providers with surveillance concepts is a key element in survivorship care.
Smoking cessation can enhance the health of all patients and is particularly important among long-term survivors, especially those who received treatments predisposing to pulmonary injury. Strategies for cessation and patient information can be found at www.cdc.gov/tobacco/how2quit.htm.
Clinicians can take advantage of every patient interaction to assess readiness for smoking cessation and assist patients in this goal. Following the principles of patient-centered counseling, physicians can guide patients into considering a change of behavior with advice and encouragement. Whenever possible, physicians should personalize the risks of smoking as well as the short-term and long-term benefits. As smokers prepare to quit, their physicians can assist in developing a plan that includes a quit date.
Many pharmacologic agents are available to assist patients, ie, nicotine inhalers, sprays, gum, and transdermal patches; the antidepressants bupropion (Wellbutrin) and nortriptiline (Pamelor); the alpha-2 adrenergic agonist clonidine (Catapres); and, most recently, the nicotine receptor partial agonist varenicline (Chantix).38
Follow-up to prevent relapse is an important part of this process.
Acknowledgment. This work was supported in part by the Swim Across America Foundation and the Campini Foundation.
- Hewitt M, Weiner S, Simone J. The epidemiology of childhood cancer. Washington DC: The National Academies Press, 2003:20–36.
- Ries L, Eisner M, Kosary C, et al. SEER cancer statistics review, 1975–2001. Bethesda, MD: National Cancer Institute; 2004.
- Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and nursing discipline. J Clin Oncol 2004; 22:4979–4990.
- Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer 2002; 95:2431–2441.
- Bhatia S, Blatt J, Meadows A. Late effects of childhood cancer and its treatment. In:Pizzo P, Poplack D. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Anscher MS, Chen L, Rabbani Z, et al. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int J Radiat Oncol Biol Phys 2005; 62:255–259.
- Bhatt N, Baran CP, Allen J, et al. Promising pharmacologic innovations in treating pulmonary fibrosis. Curr Opin Pharmacol 2006; 6:284–292.
- Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol 2001: 13:242–248.
- Horning SJ, Adhikari A, Rizk N, et al. Effect of treatment for Hodgkin’s disease on pulmonary function: results of a prospective study. J Clin Oncol 1994; 12:297–305.
- Villani F, Viviani S, Bonfante V, et al. Late pulmonary effects in favorable stage I and IIA Hodgkin’s disease treated with radiotherapy alone. Am J Clin Oncol 2000; 23:18–21.
- Miller RW, Fusner JE, Fink RJ, et al. Pulmonary function abnormalities in long-term survivors of childhood cancer. Med Pediatr Oncol 1986; 14:202–207.
- Weiner DJ, Maity A, Carlson CA, et al. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer 2006; 46:222–227.
- Wohl ME, Griscom NT, Traggis DG, et al. Effects of therapeutic irradiation delivered in early childhood upon subsequent lung function. Pediatrics 1975; 55:507–516.
- Hudson MM, Greenwald C, Thompson E, et al. Efficacy and toxicity of multiagent chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin’s disease. J Clin Oncol 1993; 11:100–108.
- Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin’s disease. J Clin Oncol 2004; 22:4541–4550.
- Quigley PM, Yeager AM, Loughlin GM. The effects of bone marrow transplantation on pulmonary function in children. Pediatr Pulmonol 1994; 18:361–367.
- Faraci M, Barra S, Cohen A, et al. Very late nonfatal consequences of fractionated TBI in children undergoing bone marrow transplant. Int J Radiat Oncol Biol Phys 2005; 63:1568–1575.
- Eigen H, Wyszomierski D. Bleomycin lung injury in children. Pathophysiology and guidelines for management. Am J Pediatr Hematol Oncol 1985; 7:71–78.
- Goldiner PL, Schweizer O. The hazards of anesthesia and surgery in bleomycin-treated patients. Semin Oncol 1979; 6:121–124.
- Zaniboni A, Prabhu S, Audisio RA. Chemotherapy and anaesthetic drugs: too little is known. Lancet Oncol 2005; 6:176–181.
- Aronin PA, Mahaley MS, Rudnick SA, et al. Prediction of BCNU pulmonary toxicity in patients with malignant gliomas: an assessment of risk factors. N Engl J Med 1980; 303:183–188.
- O’Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med 1990; 323:378–382.
- Alvarado CS, Boat TF, Newman AJ. Late-onset pulmonary fibrosis and chest deformity in two children treated with cyclophosphamide. J Pediatr 1978; 92:443–446.
- Codling BW, Chakera TM. Pulmonary fibrosis following therapy with melphalan for multiple myeloma. J Clin Pathol 1972; 25:668–673.
- Bruno B, Souillet G, Bertrand Y, et al. Effects of allogeneic bone marrow transplantation on pulmonary function in 80 children in a single paediatric centre. Bone Marrow Transplant 2004; 34:143–147.
- Lateef O, Shakoor N, Balk RA. Methotrexate pulmonary toxicity. Expert Opin Drug Saf 2005; 4:723–730.
- Cottin V, Tebob J, Massonnet B, et al. Pulmonary function in patients receiving long-term methotrexate. Chest 1996; 109:933–938.
- Gutin PH, Green MR, Bleyer WA, et al. Methotrexate pneumonitis induced by intrathecal methotrexate therapy: a case report with pharmacokinetic data. Cancer 1976; 38:1529–1534.
- Konits PH, Aisner J, Sutherland JC, et al. Possible pulmonary toxicity secondary to vinblastine. Cancer 1982; 50:2771–2774.
- Cerveri I, Zoia MC, Fulgoni P, et al. Late pulmonary sequelae after childhood bone marrow transplantation. Thorax 1999; 54:131–135.
- Cerveri I, Fulgoni P, Giorgiani G, et al. Lung function abnormalities after bone marrow transplantation in children: has the trend recently changed? Chest 2001; 120:1900–1906.
- Neve V, Foot AB, Michon J, et al. Longitudinal clinical and functional pulmonary follow-up after megatherapy, fractionated total body irradiation, and autologous bone marrow transplantation for metastatic neuroblastoma. Med Pediatr Oncol 1999; 32:170–176.
- Nenadov Beck M, Meresse V, Hartmann O, et al. Long-term pulmonary sequelae after autologous bone marrow transplantation in children without total body irradiation. Bone Marrow Transplant 1995; 16:771–775.
- Huls G, ten Bokkel Huinink D. Bleomycin and scuba diving: to dive or not to dive? Neth J Med 2003; 61:50–53.
- Kreisel D, Krupnick AS, Huddleston CB. Outcomes and late complications after pulmonary resections in the pediatric population. Semin Thorac Cardiovasc Surg 2004; 16:215–219.
- Lezama-del Valle Valle P, Blakely ML, Lobe TE. Physiologic consequences of pneumonectomy. Long-term consequences of pneumonectomy done in children. Chest Surg Clin North Am 1999; 9:485–495.
- Tobias JD, Bozeman PM, Mackert PW, et al. Postoperative outcome following thoracotomy in the pediatric oncology patient with diminished pulmonary function. J Surg Oncol 1993; 52:105–109.
- Drugs for Tobacco Dependence. Treatment Guidelines from the Medical Letter. June, 2003: 1(10):65–68.
- Hewitt M, Weiner S, Simone J. The epidemiology of childhood cancer. Washington DC: The National Academies Press, 2003:20–36.
- Ries L, Eisner M, Kosary C, et al. SEER cancer statistics review, 1975–2001. Bethesda, MD: National Cancer Institute; 2004.
- Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and nursing discipline. J Clin Oncol 2004; 22:4979–4990.
- Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer 2002; 95:2431–2441.
- Bhatia S, Blatt J, Meadows A. Late effects of childhood cancer and its treatment. In:Pizzo P, Poplack D. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Anscher MS, Chen L, Rabbani Z, et al. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int J Radiat Oncol Biol Phys 2005; 62:255–259.
- Bhatt N, Baran CP, Allen J, et al. Promising pharmacologic innovations in treating pulmonary fibrosis. Curr Opin Pharmacol 2006; 6:284–292.
- Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol 2001: 13:242–248.
- Horning SJ, Adhikari A, Rizk N, et al. Effect of treatment for Hodgkin’s disease on pulmonary function: results of a prospective study. J Clin Oncol 1994; 12:297–305.
- Villani F, Viviani S, Bonfante V, et al. Late pulmonary effects in favorable stage I and IIA Hodgkin’s disease treated with radiotherapy alone. Am J Clin Oncol 2000; 23:18–21.
- Miller RW, Fusner JE, Fink RJ, et al. Pulmonary function abnormalities in long-term survivors of childhood cancer. Med Pediatr Oncol 1986; 14:202–207.
- Weiner DJ, Maity A, Carlson CA, et al. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer 2006; 46:222–227.
- Wohl ME, Griscom NT, Traggis DG, et al. Effects of therapeutic irradiation delivered in early childhood upon subsequent lung function. Pediatrics 1975; 55:507–516.
- Hudson MM, Greenwald C, Thompson E, et al. Efficacy and toxicity of multiagent chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin’s disease. J Clin Oncol 1993; 11:100–108.
- Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin’s disease. J Clin Oncol 2004; 22:4541–4550.
- Quigley PM, Yeager AM, Loughlin GM. The effects of bone marrow transplantation on pulmonary function in children. Pediatr Pulmonol 1994; 18:361–367.
- Faraci M, Barra S, Cohen A, et al. Very late nonfatal consequences of fractionated TBI in children undergoing bone marrow transplant. Int J Radiat Oncol Biol Phys 2005; 63:1568–1575.
- Eigen H, Wyszomierski D. Bleomycin lung injury in children. Pathophysiology and guidelines for management. Am J Pediatr Hematol Oncol 1985; 7:71–78.
- Goldiner PL, Schweizer O. The hazards of anesthesia and surgery in bleomycin-treated patients. Semin Oncol 1979; 6:121–124.
- Zaniboni A, Prabhu S, Audisio RA. Chemotherapy and anaesthetic drugs: too little is known. Lancet Oncol 2005; 6:176–181.
- Aronin PA, Mahaley MS, Rudnick SA, et al. Prediction of BCNU pulmonary toxicity in patients with malignant gliomas: an assessment of risk factors. N Engl J Med 1980; 303:183–188.
- O’Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med 1990; 323:378–382.
- Alvarado CS, Boat TF, Newman AJ. Late-onset pulmonary fibrosis and chest deformity in two children treated with cyclophosphamide. J Pediatr 1978; 92:443–446.
- Codling BW, Chakera TM. Pulmonary fibrosis following therapy with melphalan for multiple myeloma. J Clin Pathol 1972; 25:668–673.
- Bruno B, Souillet G, Bertrand Y, et al. Effects of allogeneic bone marrow transplantation on pulmonary function in 80 children in a single paediatric centre. Bone Marrow Transplant 2004; 34:143–147.
- Lateef O, Shakoor N, Balk RA. Methotrexate pulmonary toxicity. Expert Opin Drug Saf 2005; 4:723–730.
- Cottin V, Tebob J, Massonnet B, et al. Pulmonary function in patients receiving long-term methotrexate. Chest 1996; 109:933–938.
- Gutin PH, Green MR, Bleyer WA, et al. Methotrexate pneumonitis induced by intrathecal methotrexate therapy: a case report with pharmacokinetic data. Cancer 1976; 38:1529–1534.
- Konits PH, Aisner J, Sutherland JC, et al. Possible pulmonary toxicity secondary to vinblastine. Cancer 1982; 50:2771–2774.
- Cerveri I, Zoia MC, Fulgoni P, et al. Late pulmonary sequelae after childhood bone marrow transplantation. Thorax 1999; 54:131–135.
- Cerveri I, Fulgoni P, Giorgiani G, et al. Lung function abnormalities after bone marrow transplantation in children: has the trend recently changed? Chest 2001; 120:1900–1906.
- Neve V, Foot AB, Michon J, et al. Longitudinal clinical and functional pulmonary follow-up after megatherapy, fractionated total body irradiation, and autologous bone marrow transplantation for metastatic neuroblastoma. Med Pediatr Oncol 1999; 32:170–176.
- Nenadov Beck M, Meresse V, Hartmann O, et al. Long-term pulmonary sequelae after autologous bone marrow transplantation in children without total body irradiation. Bone Marrow Transplant 1995; 16:771–775.
- Huls G, ten Bokkel Huinink D. Bleomycin and scuba diving: to dive or not to dive? Neth J Med 2003; 61:50–53.
- Kreisel D, Krupnick AS, Huddleston CB. Outcomes and late complications after pulmonary resections in the pediatric population. Semin Thorac Cardiovasc Surg 2004; 16:215–219.
- Lezama-del Valle Valle P, Blakely ML, Lobe TE. Physiologic consequences of pneumonectomy. Long-term consequences of pneumonectomy done in children. Chest Surg Clin North Am 1999; 9:485–495.
- Tobias JD, Bozeman PM, Mackert PW, et al. Postoperative outcome following thoracotomy in the pediatric oncology patient with diminished pulmonary function. J Surg Oncol 1993; 52:105–109.
- Drugs for Tobacco Dependence. Treatment Guidelines from the Medical Letter. June, 2003: 1(10):65–68.
KEY POINTS
- Radiation therapy causes pulmonary fibrosis, interstitial pneumonitis, and restrictive or obstructive lung disease. The risk is dose-dependent and increases with concomitant chemotherapy, younger age at treatment, atopic history, and smoking.
- Alkylating agents cause pulmonary fibrosis. Bleomycin can cause interstitial pneumonitis, pulmonary fibrosis, or, very rarely, acute respiratory distress syndrome.
- Cancer survivors should have a yearly history and physical examination, plus pulmonary function testing and radiography at baseline and repeated as clinically indicated.
- All patients who smoke should be encouraged to quit.