User login
A 51-year-old woman with dyspnea
A 51-year-old woman presents to the emergency department with dyspnea, which began 4 days ago. She reports no chest pain, palpitations, hemoptysis, fevers, chills, weight loss, recent travel, immobility, or surgery. One week ago she noticed cramping in her right calf, but that has since resolved.
Her history includes hypertension, hypothyroidism, and immune-mediated glomerulonephritis with proteinuria. She is premenopausal. She takes losartan and levothyroxine; she is not taking oral contraceptives or herbal supplements. She is up to date with her cancer screening and has had negative findings on colonoscopy and mammography within the past year.
She has never smoked and she does not drink alcohol or use illicit drugs. Her mother has a history of provoked deep vein thrombosis and colon cancer.
Her temperature is 36.2°C (97.2°F), heart rate 163 beats per minute, blood pressure 158/102 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation by pulse oximetry 80% while breathing room air, corrected to 94% with oxygen 6 L/min via nasal cannula.
On physical examination, she is sitting upright on a stretcher and appears uncomfortable and anxious. She is awake and able to communicate clearly. Examination of the head, ears, eyes, nose, and throat is unremarkable, with moist mucous membranes. Her lungs are clear to auscultation. Her heart beat is very rapid, with a regular rhythm and an accentuated P2 heart sound. A right parasternal heave can be palpated in addition to a rightwardly displaced point of maximal impulse. The abdomen is normal, with no tenderness or organomegaly. She has no pain, edema, or erythema in the legs or feet, and she has strong, symmetric pulses (2+) in all extremities. The neurologic examination is nonfocal.
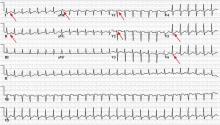
Electrocardiography (ECG) done on arrival (Figure 1) reveals supraventricular tachycardia, a normal axis, and nonspecific ST-segment and T-wave abnormalities, findings commonly seen in pulmonary embolism.1,2 On the other hand, her ECG does not show some of the other signs of right ventricular strain due to pulmonary embolism such as atrial arrhythmias, complete right bundle branch block, or inferior Q-waves.3,4
In view of her ECG findings and her symptoms of dyspnea, calf pain, tachypnea, tachycardia, and a pronounced P2 heart sound, her physician concludes that she very likely has a pulmonary embolism1 and orders an intravenous infusion of unfractionated heparin to be started immediately.
TESTING FOR PULMONARY EMBOLISM
1. Which of the following would be the best initial diagnostic imaging study to perform in this patient, who has a high pretest probability of pulmonary embolism?
- Multidetector computed tomographic (CT) pulmonary angiography
- Transthoracic echocardiography
- Magnetic resonance imaging
- Lower-extremity duplex ultrasonography
- Pulmonary angiography
- Ventilation-perfusion scintigraphy
Multidetector CT angiography is rapid, noninvasive, and highly sensitive (83%–90%) and specific (96%) for pulmonary embolism.5,6 In patients such as ours who have a high pretest probability of having the disease, its positive predictive value is 96%.5 Therefore, it would be the initial diagnostic study to perform in our patient.
Although transthoracic echocardiography is noninvasive and can detect right ventricular strain in the setting of pulmonary embolism, it may miss half of all pulmonary emboli detected by angiography.7,8
When technically adequate images are obtained, the combination of magnetic resonance angiography and magnetic resonance venography is very sensitive (92%) and specific (96%) for pulmonary embolism.9 However, one-fourth of patients undergoing these studies may have technically inadequate results, so this is not the best choice for diagnosis.9
As our patient complained of recent cramping in the right calf, lower-extremity duplex ultrasonography would be a reasonable test to screen for acute deep vein thrombosis as the source of pulmonary embolism. However, given her worrisome vital signs and impending hemodynamic collapse, CT pulmonary angiography would be a better initial test as it may guide more aggressive therapy. Furthermore, even if ultrasonography showed no evidence of deep vein thrombosis, clinical suspicion for pulmonary embolism would remain high enough that therapeutic anticoagulation would be continued until further testing ruled out this diagnosis.
Pulmonary angiography is the gold-standard test for pulmonary embolism. However, it is time-consuming, expensive, and invasive and so is not usually done unless the diagnosis cannot be made with other imaging studies.
Ventilation-perfusion scintigraphy is an established and safe diagnostic test for pulmonary embolism. It is particularly helpful in patients who have renal dysfunction or contrast allergy. The sensitivity of a high-probability scan is 78%, while the specificity of a very-low-probability scan is 97%.10 However, this study is often nondiagnostic (in 26.5% of cases),10 and further imaging may be required.
RESULTS OF CT ANGIOGRAPHY
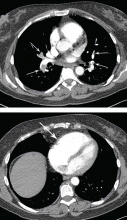
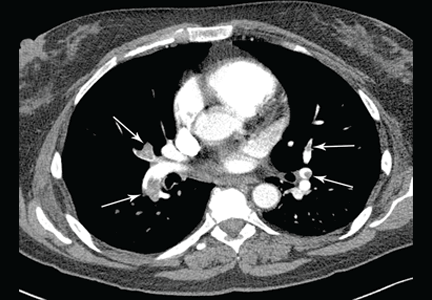
Our patient undergoes CT angiography, which reveals multiple bilateral pulmonary emboli and right ventricular enlargement (Figure 2). Transthoracic echocardiography shows dilation of the right ventricle, with severely reduced systolic function, an underfilled and hyperdynamic left ventricle (ejection fraction 75%), and moderate tricuspid valve regurgitation. Her right ventricular systolic pressure is estimated to be 47 mm Hg.
Doppler ultrasonography of the legs reveals an occlusive thrombus within the right small saphenous vein that bulges and extends into the right popliteal vein. Also noted is a nonocclusive thrombus in the upper right popliteal vein that likely originated from the thrombus in the small saphenous vein.
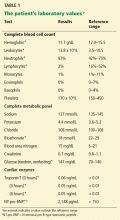
Initial laboratory testing (Table 1) shows elevations of the cardiac enzymes troponin T and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
ESTIMATING PROGNOSIS IN PULMONARY EMBOLISM
2. Which of the following laboratory results at presentation is independently associated with a worse outcome in patients with pulmonary embolism?
- Elevated NT-pro-BNP
- Hypercalcemia
- Thrombocytosis
- Hypernatremia
- Elevated procalcitonin
The Pulmonary Embolism Severity Index11 and the Simplified Pulmonary Embolism Severity Index12 (Table 2) are clinical calculators that help predict 30-day risk of death in patients with pulmonary embolism. Our patient’s Pulmonary Embolism Severity Index score is 60, indicating a very low risk, but her simplified severity index score is 2, indicating a high risk.
A shock index score (the heart rate divided by the systolic blood pressure) greater than 1 is also a sensitive measure of risk.13 (Our patient’s shock index score is 1.03.) Although the simplified version is more accurate,14 the shock index is also helpful when deciding whether patients with suspected pulmonary embolism should receive early fibrinolysis.15
In a large registry of patients with confirmed pulmonary embolism, risk factors for death were age greater than 70, cancer, clinical congestive heart failure, chronic obstructive pulmonary disease, systolic blood pressure lower than 90 mm Hg, respiratory rate less than 20 per minute, and right ventricular hypokinesis.16 Right ventricular dysfunction progressing to right ventricular failure and cardiogenic shock is the most common cause of death in patients with pulmonary embolism.16–18
Post hoc analysis has also shown that elevations of the biomarkers BNP, NT-pro-BNP, and cardiac troponins I and T are associated with a prolonged hospital course and a higher risk of death within 30 days.19 Interestingly, a recent retrospective analysis found hyponatremia to be an independent risk factor for death in the short term.20
Thrombocytopenia, not thrombocytosis, is associated with worse outcomes in patients with pulmonary embolism.16 Procalcitonin is elevated in bacterial pneumonia but is normal in pulmonary embolism and so may be helpful in differentiating between the two.21,22 Hypernatremia, hypercalcemia, and elevated procalcitonin have not been shown to be independently associated with worse outcomes in acute pulmonary embolism.
Thus, of the answer choices shown above, elevated NT-pro-BNP is the correct answer.
Classified as massive, submassive, or low-risk
Pulmonary embolism is often stratified as massive, submassive, or low-risk, reflecting the severity and the degree of cardiovascular collapse. The treatment depends on the classification.
Pulmonary embolism is classified as massive if the patient has a cardiac arrest or a systolic blood pressure lower than 90 mm Hg for more than 15 minutes.23 Nearly half of patients in this category die.24
Pulmonary embolism is submassive if the patient has systolic pressure greater than 90 mm Hg but has right ventricular dysfunction, as evidenced by physical examination, elevated cardiac biomarkers, electrocardiography, transthoracic echocardiography, or computed tomography. The death rate is as high as 15%.24
Pulmonary embolism in a normotensive patient with no right ventricular dysfunction is defined as low-risk.
Our patient so far
Our patient has bilateral pulmonary emboli, most likely originating from a deep vein thrombosis in her right lower leg. Her pulmonary embolism would be classified as submassive, as her systolic pressure is greater than 90 mm Hg and right ventricular dysfunction—significant right ventricular strain—was noted on both transthoracic echocardiography and computed tomography. Also, cardiac biomarkers are elevated, and the physical examination revealed a prominent P2 sound and right parasternal heave, also suggestive of right ventricular dysfunction.
Now, 6 hours have passed, and even though she has been receiving intravenous heparin during this time, her shock index remains greater than 1, indicating hemodynamic instability. Her pulse rate is still markedly high—over 160 bpm—and she still appears quite anxious and uncomfortable.
HOW SHOULD THIS PATIENT BE TREATED?
3. Which of the following is the most appropriate treatment for this patient?
- Start warfarin immediately while bridging with unfractionated heparin, low-molecular-weight heparin, or fondaparinux
- Start fibrinolysis with alteplase
- Give metoprolol intravenously to control her heart rate
- Start dabigatran immediately while bridging with unfractionated heparin
- Place an inferior vena cava filter
- Consult cardiothoracic surgery for emergency pulmonary embolectomy
All patients with confirmed pulmonary embolism and no contraindications to anticoagulation should begin treatment with low-molecular-weight heparin, unfractionated heparin, or fondaparinux.23 In addition, this therapy should be started empirically while the patient is still undergoing diagnostic testing if the pretest probability of pulmonary embolism is intermediate or high.23
Warfarin is indicated for all patients with pulmonary embolism who do not have contraindications to it (Table 3). If unfractionated heparin, low-molecular-weight heparin, or fondaparinux has not already been started, it should be started at the same time as warfarin and should be continued until the international normalized ratio (INR) is within the therapeutic range.
Fibrinolysis. Treatment with a fibrinolytic agent in addition to heparin results in faster improvement of right ventricular function and pulmonary perfusion than with heparin alone.25 It may also decrease the incidence of pulmonary hypertension secondary to chronic thromboembolic disease.26 It should be considered in patients with massive pulmonary embolism.23
Whether fibrinolysis is appropriate for all patients with submassive pulmonary embolism remains controversial. Currently, it is not recommended for minor right ventricular dysfunction or myocardial necrosis if the patient has no signs of overt clinical decline.23 The Pulmonary Embolism Thrombolysis trial27 is an ongoing prospective randomized comparison of tenecteplase in a single bolus plus heparin vs heparin alone in normotensive patients with submassive pulmonary embolism, such as our patient. This trial may elucidate the benefit of fibrinolytic therapy in patients with submassive pulmonary embolism.
Patients at low risk are generally treated with heparin and warfarin anticoagulation alone. Fibrinolysis is not recommended in these patients, as the risk of bleeding outweighs the potential benefits.23
Metoprolol may not be advisable for our patient, as her tachycardia is likely compensatory, and beta-blocker therapy could blunt this compensatory response, leading to inadequate systemic perfusion.
Dabigatran is an oral direct thrombin inhibitor that does not require laboratory monitoring. It is currently approved for the prevention of stroke in patients with atrial fibrillation. It has been shown to be as effective as warfarin in the treatment of acute venous thromboembolism28 and may be a viable option in the future, but as of this writing it has not yet been approved in the United States for this indication. Furthermore, dabigatran inhibits thrombin immediately, so continued heparin bridging would not be necessary.
An inferior vena cava filter may prevent recurrent pulmonary embolism for patients who have absolute contraindications to anticoagulation, most significantly in the short term, ie, in the first few weeks after placement. However, these devices have not yet been shown to improve long-term mortality rates.
Embolectomy, percutaneous or surgical, is also an option. For patients in whom thrombolytic therapy is not effective, “rescue” surgical embolectomy has been associated with better outcomes compared with secondary thrombolysis and so should be considered.29
Back to our patient
An intravenous infusion of alteplase is started, and the patient’s tachycardia improves. Her oxygen requirements normalize, and she is transferred to the general medical floor the next day. She receives subcutaneous dalteparin as a bridge therapy, and warfarin is titrated to a goal INR of 2.0 to 3.0. Because of the acute deep vein thrombosis in her right lower leg, she is instructed to wear knee-high fitted compression hose for primary prevention of postphlebitic syndrome.
HOW LONG TO TREAT? IS GENETIC TESTING INDICATED?
Patients with a first episode of unprovoked venous thromboembolism should receive oral anticoagulants for 6 months, while those with recurrent unprovoked venous thromboembolism require lifelong oral anticoagulation.23
Whether to test for inherited thrombophilia after a first episode of venous thromboembolism to guide the duration of anticoagulation is controversial.30 Indiscriminate testing has not been recommended in these patients,31 but the American College of Medical Genetics recommends genetic screening for factor V Leiden in patients who have an unprovoked incident of venous thromboembolism before age 50.32
No randomized controlled trial has assessed whether thrombophilia testing decreases the recurrence rate of venous thromboembolism.33 One uncontrolled study suggested that testing for inherited thrombophilias in patients with a first episode does not affect the risk of recurrence.34 Testing is costly and may cause psychological distress for patients and family members.
Our patient is discharged home on warfarin for 6 months with subsequent follow-up evaluation in the thrombophilia clinic.
WHEN SHOULD WARFARIN BE RESTARTED?
4. If our patient were to discontinue oral anticoagulation in 6 months, which of the following, if present 1 month afterwards, would be a reason to restart oral anticoagulation?
- Elevated serum cotinine
- Positive pregnancy test
- Elevated follicle-stimulating hormone and luteinizing hormone and low estradiol levels
- Elevated D-dimer
Cotinine is a nicotine metabolite, and serum levels are elevated in smokers. Smoking and pregnancy both increase the risk of venous thromboembolism. However, smoking or pregnancy alone would not be a reason to increase the duration of anticoagulation.
Warfarin is contraindicated in pregnancy because of its teratogenic effects, particularly in the first trimester. Follicle-stimulating hormone and luteinizing hormone levels increase in response to decreased estradiol at menopause. Postmenopausal women are not at increased risk of venous thromboembolism unless they are taking oral estrogen hormone replacement therapy.
An elevated D-dimer level 1 month after stopping of oral anticoagulation has been associated with a higher rate of recurrence of venous thromboembolism, which is reduced by a resumption of anticoagulation.35 Therefore, consideration should be given to resuming anticoagulation in patients who have an elevated D-dimer level.
TAKE-HOME POINTS
It is important to distinguish between massive, submassive, and low-risk pulmonary embolism, since each type has a different treatment and prognosis.
Fibrinolytic therapy is indicated in patients with massive pulmonary embolism when no contraindication is present, whereas it is not indicated in those with low-risk pulmonary embolism.
Management of submassive pulmonary embolism continues to be an area of considerable debate. Current American Heart Association guidelines recommend consideration of fibrinolysis initially in patients with submassive acute pulmonary embolism if there is concurrent clinical evidence of adverse prognosis—eg, new hemodynamic instability, worsening respiratory insufficiency, severe right ventricular dysfunction, or major myocardial necrosis.23 On the other hand, the American College of Chest Physicians recommends against initial systemic fibrinolysis in submassive acute pulmonary embolism based on biomarkers or findings on ECG, transthoracic echocardiography, or CT, recommending it only in therapeutically anticoagulated patients deemed to be at high risk of hypotension.36
Since the optimal treatment of submassive pulmonary embolism is still not known, it is important that clinicians remain up to date on the evidence and guidelines.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. Am J Cardiol 1991; 68:1723–1724.
- Ferrari E, Imbert A, Chevalier T, Mihoubi A, Morand P, Baudouy M. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads—80 case reports. Chest 1997; 111:537–543.
- Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005; 25:843–848.
- Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 2005; 352:1760–1768.
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000; 217:447–455.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001; 110:528–535.
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003; 21:180–183.
- Stein PD, Chenevert TL, Fowler SE, et al; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med 2010; 152:434–443,W142–W143.
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 2008; 246:941–946.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Otero R, Trujillo-Santos J, Cayuela A, et al; Registro Informatizado de la Enfermedad Tromboembólica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007; 30:1111–1116.
- Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J 2011; 37:762–766.
- Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003; 24:366–376.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–1389.
- ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004; 164:1685–1689.
- Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008; 29:1569–1577.
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003; 108:2191–2194.
- Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, Aujesky D. Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 2010; 182:1178–1183.
- Delèvaux I, André M, Aumaître O, Bègue RJ, Colombier M, Piette JC. Procalcitonin measurement for differential diagnosis between pulmonary embolism and pneumonia. Crit Care Med 2003; 31:661.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community-acquired pneumonia. Clin Appl Thromb Hemost 2011; 17:519–525.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341:507–511.
- Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009; 136:1202–1210.
- Steering Committee of PEITHO Investigators. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J 2012; 163:33–38.e1.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006; 129:1043–1050.
- Ho WK, Hankey GJ, Eikelboom JW. Should adult patients be routinely tested for heritable thrombophilia after an episode of venous thromboembolism? Med J Aust 2011; 195:139–142.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Cohn D, Vansenne F, de Borgie C, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev 2009; ( 1):CD007069.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008; 6:1474–1477.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. Ddimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):7S–47S.
A 51-year-old woman presents to the emergency department with dyspnea, which began 4 days ago. She reports no chest pain, palpitations, hemoptysis, fevers, chills, weight loss, recent travel, immobility, or surgery. One week ago she noticed cramping in her right calf, but that has since resolved.
Her history includes hypertension, hypothyroidism, and immune-mediated glomerulonephritis with proteinuria. She is premenopausal. She takes losartan and levothyroxine; she is not taking oral contraceptives or herbal supplements. She is up to date with her cancer screening and has had negative findings on colonoscopy and mammography within the past year.
She has never smoked and she does not drink alcohol or use illicit drugs. Her mother has a history of provoked deep vein thrombosis and colon cancer.
Her temperature is 36.2°C (97.2°F), heart rate 163 beats per minute, blood pressure 158/102 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation by pulse oximetry 80% while breathing room air, corrected to 94% with oxygen 6 L/min via nasal cannula.
On physical examination, she is sitting upright on a stretcher and appears uncomfortable and anxious. She is awake and able to communicate clearly. Examination of the head, ears, eyes, nose, and throat is unremarkable, with moist mucous membranes. Her lungs are clear to auscultation. Her heart beat is very rapid, with a regular rhythm and an accentuated P2 heart sound. A right parasternal heave can be palpated in addition to a rightwardly displaced point of maximal impulse. The abdomen is normal, with no tenderness or organomegaly. She has no pain, edema, or erythema in the legs or feet, and she has strong, symmetric pulses (2+) in all extremities. The neurologic examination is nonfocal.
Electrocardiography (ECG) done on arrival (Figure 1) reveals supraventricular tachycardia, a normal axis, and nonspecific ST-segment and T-wave abnormalities, findings commonly seen in pulmonary embolism.1,2 On the other hand, her ECG does not show some of the other signs of right ventricular strain due to pulmonary embolism such as atrial arrhythmias, complete right bundle branch block, or inferior Q-waves.3,4
In view of her ECG findings and her symptoms of dyspnea, calf pain, tachypnea, tachycardia, and a pronounced P2 heart sound, her physician concludes that she very likely has a pulmonary embolism1 and orders an intravenous infusion of unfractionated heparin to be started immediately.
TESTING FOR PULMONARY EMBOLISM
1. Which of the following would be the best initial diagnostic imaging study to perform in this patient, who has a high pretest probability of pulmonary embolism?
- Multidetector computed tomographic (CT) pulmonary angiography
- Transthoracic echocardiography
- Magnetic resonance imaging
- Lower-extremity duplex ultrasonography
- Pulmonary angiography
- Ventilation-perfusion scintigraphy
Multidetector CT angiography is rapid, noninvasive, and highly sensitive (83%–90%) and specific (96%) for pulmonary embolism.5,6 In patients such as ours who have a high pretest probability of having the disease, its positive predictive value is 96%.5 Therefore, it would be the initial diagnostic study to perform in our patient.
Although transthoracic echocardiography is noninvasive and can detect right ventricular strain in the setting of pulmonary embolism, it may miss half of all pulmonary emboli detected by angiography.7,8
When technically adequate images are obtained, the combination of magnetic resonance angiography and magnetic resonance venography is very sensitive (92%) and specific (96%) for pulmonary embolism.9 However, one-fourth of patients undergoing these studies may have technically inadequate results, so this is not the best choice for diagnosis.9
As our patient complained of recent cramping in the right calf, lower-extremity duplex ultrasonography would be a reasonable test to screen for acute deep vein thrombosis as the source of pulmonary embolism. However, given her worrisome vital signs and impending hemodynamic collapse, CT pulmonary angiography would be a better initial test as it may guide more aggressive therapy. Furthermore, even if ultrasonography showed no evidence of deep vein thrombosis, clinical suspicion for pulmonary embolism would remain high enough that therapeutic anticoagulation would be continued until further testing ruled out this diagnosis.
Pulmonary angiography is the gold-standard test for pulmonary embolism. However, it is time-consuming, expensive, and invasive and so is not usually done unless the diagnosis cannot be made with other imaging studies.
Ventilation-perfusion scintigraphy is an established and safe diagnostic test for pulmonary embolism. It is particularly helpful in patients who have renal dysfunction or contrast allergy. The sensitivity of a high-probability scan is 78%, while the specificity of a very-low-probability scan is 97%.10 However, this study is often nondiagnostic (in 26.5% of cases),10 and further imaging may be required.
RESULTS OF CT ANGIOGRAPHY
Our patient undergoes CT angiography, which reveals multiple bilateral pulmonary emboli and right ventricular enlargement (Figure 2). Transthoracic echocardiography shows dilation of the right ventricle, with severely reduced systolic function, an underfilled and hyperdynamic left ventricle (ejection fraction 75%), and moderate tricuspid valve regurgitation. Her right ventricular systolic pressure is estimated to be 47 mm Hg.
Doppler ultrasonography of the legs reveals an occlusive thrombus within the right small saphenous vein that bulges and extends into the right popliteal vein. Also noted is a nonocclusive thrombus in the upper right popliteal vein that likely originated from the thrombus in the small saphenous vein.
Initial laboratory testing (Table 1) shows elevations of the cardiac enzymes troponin T and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
ESTIMATING PROGNOSIS IN PULMONARY EMBOLISM
2. Which of the following laboratory results at presentation is independently associated with a worse outcome in patients with pulmonary embolism?
- Elevated NT-pro-BNP
- Hypercalcemia
- Thrombocytosis
- Hypernatremia
- Elevated procalcitonin
The Pulmonary Embolism Severity Index11 and the Simplified Pulmonary Embolism Severity Index12 (Table 2) are clinical calculators that help predict 30-day risk of death in patients with pulmonary embolism. Our patient’s Pulmonary Embolism Severity Index score is 60, indicating a very low risk, but her simplified severity index score is 2, indicating a high risk.
A shock index score (the heart rate divided by the systolic blood pressure) greater than 1 is also a sensitive measure of risk.13 (Our patient’s shock index score is 1.03.) Although the simplified version is more accurate,14 the shock index is also helpful when deciding whether patients with suspected pulmonary embolism should receive early fibrinolysis.15
In a large registry of patients with confirmed pulmonary embolism, risk factors for death were age greater than 70, cancer, clinical congestive heart failure, chronic obstructive pulmonary disease, systolic blood pressure lower than 90 mm Hg, respiratory rate less than 20 per minute, and right ventricular hypokinesis.16 Right ventricular dysfunction progressing to right ventricular failure and cardiogenic shock is the most common cause of death in patients with pulmonary embolism.16–18
Post hoc analysis has also shown that elevations of the biomarkers BNP, NT-pro-BNP, and cardiac troponins I and T are associated with a prolonged hospital course and a higher risk of death within 30 days.19 Interestingly, a recent retrospective analysis found hyponatremia to be an independent risk factor for death in the short term.20
Thrombocytopenia, not thrombocytosis, is associated with worse outcomes in patients with pulmonary embolism.16 Procalcitonin is elevated in bacterial pneumonia but is normal in pulmonary embolism and so may be helpful in differentiating between the two.21,22 Hypernatremia, hypercalcemia, and elevated procalcitonin have not been shown to be independently associated with worse outcomes in acute pulmonary embolism.
Thus, of the answer choices shown above, elevated NT-pro-BNP is the correct answer.
Classified as massive, submassive, or low-risk
Pulmonary embolism is often stratified as massive, submassive, or low-risk, reflecting the severity and the degree of cardiovascular collapse. The treatment depends on the classification.
Pulmonary embolism is classified as massive if the patient has a cardiac arrest or a systolic blood pressure lower than 90 mm Hg for more than 15 minutes.23 Nearly half of patients in this category die.24
Pulmonary embolism is submassive if the patient has systolic pressure greater than 90 mm Hg but has right ventricular dysfunction, as evidenced by physical examination, elevated cardiac biomarkers, electrocardiography, transthoracic echocardiography, or computed tomography. The death rate is as high as 15%.24
Pulmonary embolism in a normotensive patient with no right ventricular dysfunction is defined as low-risk.
Our patient so far
Our patient has bilateral pulmonary emboli, most likely originating from a deep vein thrombosis in her right lower leg. Her pulmonary embolism would be classified as submassive, as her systolic pressure is greater than 90 mm Hg and right ventricular dysfunction—significant right ventricular strain—was noted on both transthoracic echocardiography and computed tomography. Also, cardiac biomarkers are elevated, and the physical examination revealed a prominent P2 sound and right parasternal heave, also suggestive of right ventricular dysfunction.
Now, 6 hours have passed, and even though she has been receiving intravenous heparin during this time, her shock index remains greater than 1, indicating hemodynamic instability. Her pulse rate is still markedly high—over 160 bpm—and she still appears quite anxious and uncomfortable.
HOW SHOULD THIS PATIENT BE TREATED?
3. Which of the following is the most appropriate treatment for this patient?
- Start warfarin immediately while bridging with unfractionated heparin, low-molecular-weight heparin, or fondaparinux
- Start fibrinolysis with alteplase
- Give metoprolol intravenously to control her heart rate
- Start dabigatran immediately while bridging with unfractionated heparin
- Place an inferior vena cava filter
- Consult cardiothoracic surgery for emergency pulmonary embolectomy
All patients with confirmed pulmonary embolism and no contraindications to anticoagulation should begin treatment with low-molecular-weight heparin, unfractionated heparin, or fondaparinux.23 In addition, this therapy should be started empirically while the patient is still undergoing diagnostic testing if the pretest probability of pulmonary embolism is intermediate or high.23
Warfarin is indicated for all patients with pulmonary embolism who do not have contraindications to it (Table 3). If unfractionated heparin, low-molecular-weight heparin, or fondaparinux has not already been started, it should be started at the same time as warfarin and should be continued until the international normalized ratio (INR) is within the therapeutic range.
Fibrinolysis. Treatment with a fibrinolytic agent in addition to heparin results in faster improvement of right ventricular function and pulmonary perfusion than with heparin alone.25 It may also decrease the incidence of pulmonary hypertension secondary to chronic thromboembolic disease.26 It should be considered in patients with massive pulmonary embolism.23
Whether fibrinolysis is appropriate for all patients with submassive pulmonary embolism remains controversial. Currently, it is not recommended for minor right ventricular dysfunction or myocardial necrosis if the patient has no signs of overt clinical decline.23 The Pulmonary Embolism Thrombolysis trial27 is an ongoing prospective randomized comparison of tenecteplase in a single bolus plus heparin vs heparin alone in normotensive patients with submassive pulmonary embolism, such as our patient. This trial may elucidate the benefit of fibrinolytic therapy in patients with submassive pulmonary embolism.
Patients at low risk are generally treated with heparin and warfarin anticoagulation alone. Fibrinolysis is not recommended in these patients, as the risk of bleeding outweighs the potential benefits.23
Metoprolol may not be advisable for our patient, as her tachycardia is likely compensatory, and beta-blocker therapy could blunt this compensatory response, leading to inadequate systemic perfusion.
Dabigatran is an oral direct thrombin inhibitor that does not require laboratory monitoring. It is currently approved for the prevention of stroke in patients with atrial fibrillation. It has been shown to be as effective as warfarin in the treatment of acute venous thromboembolism28 and may be a viable option in the future, but as of this writing it has not yet been approved in the United States for this indication. Furthermore, dabigatran inhibits thrombin immediately, so continued heparin bridging would not be necessary.
An inferior vena cava filter may prevent recurrent pulmonary embolism for patients who have absolute contraindications to anticoagulation, most significantly in the short term, ie, in the first few weeks after placement. However, these devices have not yet been shown to improve long-term mortality rates.
Embolectomy, percutaneous or surgical, is also an option. For patients in whom thrombolytic therapy is not effective, “rescue” surgical embolectomy has been associated with better outcomes compared with secondary thrombolysis and so should be considered.29
Back to our patient
An intravenous infusion of alteplase is started, and the patient’s tachycardia improves. Her oxygen requirements normalize, and she is transferred to the general medical floor the next day. She receives subcutaneous dalteparin as a bridge therapy, and warfarin is titrated to a goal INR of 2.0 to 3.0. Because of the acute deep vein thrombosis in her right lower leg, she is instructed to wear knee-high fitted compression hose for primary prevention of postphlebitic syndrome.
HOW LONG TO TREAT? IS GENETIC TESTING INDICATED?
Patients with a first episode of unprovoked venous thromboembolism should receive oral anticoagulants for 6 months, while those with recurrent unprovoked venous thromboembolism require lifelong oral anticoagulation.23
Whether to test for inherited thrombophilia after a first episode of venous thromboembolism to guide the duration of anticoagulation is controversial.30 Indiscriminate testing has not been recommended in these patients,31 but the American College of Medical Genetics recommends genetic screening for factor V Leiden in patients who have an unprovoked incident of venous thromboembolism before age 50.32
No randomized controlled trial has assessed whether thrombophilia testing decreases the recurrence rate of venous thromboembolism.33 One uncontrolled study suggested that testing for inherited thrombophilias in patients with a first episode does not affect the risk of recurrence.34 Testing is costly and may cause psychological distress for patients and family members.
Our patient is discharged home on warfarin for 6 months with subsequent follow-up evaluation in the thrombophilia clinic.
WHEN SHOULD WARFARIN BE RESTARTED?
4. If our patient were to discontinue oral anticoagulation in 6 months, which of the following, if present 1 month afterwards, would be a reason to restart oral anticoagulation?
- Elevated serum cotinine
- Positive pregnancy test
- Elevated follicle-stimulating hormone and luteinizing hormone and low estradiol levels
- Elevated D-dimer
Cotinine is a nicotine metabolite, and serum levels are elevated in smokers. Smoking and pregnancy both increase the risk of venous thromboembolism. However, smoking or pregnancy alone would not be a reason to increase the duration of anticoagulation.
Warfarin is contraindicated in pregnancy because of its teratogenic effects, particularly in the first trimester. Follicle-stimulating hormone and luteinizing hormone levels increase in response to decreased estradiol at menopause. Postmenopausal women are not at increased risk of venous thromboembolism unless they are taking oral estrogen hormone replacement therapy.
An elevated D-dimer level 1 month after stopping of oral anticoagulation has been associated with a higher rate of recurrence of venous thromboembolism, which is reduced by a resumption of anticoagulation.35 Therefore, consideration should be given to resuming anticoagulation in patients who have an elevated D-dimer level.
TAKE-HOME POINTS
It is important to distinguish between massive, submassive, and low-risk pulmonary embolism, since each type has a different treatment and prognosis.
Fibrinolytic therapy is indicated in patients with massive pulmonary embolism when no contraindication is present, whereas it is not indicated in those with low-risk pulmonary embolism.
Management of submassive pulmonary embolism continues to be an area of considerable debate. Current American Heart Association guidelines recommend consideration of fibrinolysis initially in patients with submassive acute pulmonary embolism if there is concurrent clinical evidence of adverse prognosis—eg, new hemodynamic instability, worsening respiratory insufficiency, severe right ventricular dysfunction, or major myocardial necrosis.23 On the other hand, the American College of Chest Physicians recommends against initial systemic fibrinolysis in submassive acute pulmonary embolism based on biomarkers or findings on ECG, transthoracic echocardiography, or CT, recommending it only in therapeutically anticoagulated patients deemed to be at high risk of hypotension.36
Since the optimal treatment of submassive pulmonary embolism is still not known, it is important that clinicians remain up to date on the evidence and guidelines.
A 51-year-old woman presents to the emergency department with dyspnea, which began 4 days ago. She reports no chest pain, palpitations, hemoptysis, fevers, chills, weight loss, recent travel, immobility, or surgery. One week ago she noticed cramping in her right calf, but that has since resolved.
Her history includes hypertension, hypothyroidism, and immune-mediated glomerulonephritis with proteinuria. She is premenopausal. She takes losartan and levothyroxine; she is not taking oral contraceptives or herbal supplements. She is up to date with her cancer screening and has had negative findings on colonoscopy and mammography within the past year.
She has never smoked and she does not drink alcohol or use illicit drugs. Her mother has a history of provoked deep vein thrombosis and colon cancer.
Her temperature is 36.2°C (97.2°F), heart rate 163 beats per minute, blood pressure 158/102 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation by pulse oximetry 80% while breathing room air, corrected to 94% with oxygen 6 L/min via nasal cannula.
On physical examination, she is sitting upright on a stretcher and appears uncomfortable and anxious. She is awake and able to communicate clearly. Examination of the head, ears, eyes, nose, and throat is unremarkable, with moist mucous membranes. Her lungs are clear to auscultation. Her heart beat is very rapid, with a regular rhythm and an accentuated P2 heart sound. A right parasternal heave can be palpated in addition to a rightwardly displaced point of maximal impulse. The abdomen is normal, with no tenderness or organomegaly. She has no pain, edema, or erythema in the legs or feet, and she has strong, symmetric pulses (2+) in all extremities. The neurologic examination is nonfocal.
Electrocardiography (ECG) done on arrival (Figure 1) reveals supraventricular tachycardia, a normal axis, and nonspecific ST-segment and T-wave abnormalities, findings commonly seen in pulmonary embolism.1,2 On the other hand, her ECG does not show some of the other signs of right ventricular strain due to pulmonary embolism such as atrial arrhythmias, complete right bundle branch block, or inferior Q-waves.3,4
In view of her ECG findings and her symptoms of dyspnea, calf pain, tachypnea, tachycardia, and a pronounced P2 heart sound, her physician concludes that she very likely has a pulmonary embolism1 and orders an intravenous infusion of unfractionated heparin to be started immediately.
TESTING FOR PULMONARY EMBOLISM
1. Which of the following would be the best initial diagnostic imaging study to perform in this patient, who has a high pretest probability of pulmonary embolism?
- Multidetector computed tomographic (CT) pulmonary angiography
- Transthoracic echocardiography
- Magnetic resonance imaging
- Lower-extremity duplex ultrasonography
- Pulmonary angiography
- Ventilation-perfusion scintigraphy
Multidetector CT angiography is rapid, noninvasive, and highly sensitive (83%–90%) and specific (96%) for pulmonary embolism.5,6 In patients such as ours who have a high pretest probability of having the disease, its positive predictive value is 96%.5 Therefore, it would be the initial diagnostic study to perform in our patient.
Although transthoracic echocardiography is noninvasive and can detect right ventricular strain in the setting of pulmonary embolism, it may miss half of all pulmonary emboli detected by angiography.7,8
When technically adequate images are obtained, the combination of magnetic resonance angiography and magnetic resonance venography is very sensitive (92%) and specific (96%) for pulmonary embolism.9 However, one-fourth of patients undergoing these studies may have technically inadequate results, so this is not the best choice for diagnosis.9
As our patient complained of recent cramping in the right calf, lower-extremity duplex ultrasonography would be a reasonable test to screen for acute deep vein thrombosis as the source of pulmonary embolism. However, given her worrisome vital signs and impending hemodynamic collapse, CT pulmonary angiography would be a better initial test as it may guide more aggressive therapy. Furthermore, even if ultrasonography showed no evidence of deep vein thrombosis, clinical suspicion for pulmonary embolism would remain high enough that therapeutic anticoagulation would be continued until further testing ruled out this diagnosis.
Pulmonary angiography is the gold-standard test for pulmonary embolism. However, it is time-consuming, expensive, and invasive and so is not usually done unless the diagnosis cannot be made with other imaging studies.
Ventilation-perfusion scintigraphy is an established and safe diagnostic test for pulmonary embolism. It is particularly helpful in patients who have renal dysfunction or contrast allergy. The sensitivity of a high-probability scan is 78%, while the specificity of a very-low-probability scan is 97%.10 However, this study is often nondiagnostic (in 26.5% of cases),10 and further imaging may be required.
RESULTS OF CT ANGIOGRAPHY
Our patient undergoes CT angiography, which reveals multiple bilateral pulmonary emboli and right ventricular enlargement (Figure 2). Transthoracic echocardiography shows dilation of the right ventricle, with severely reduced systolic function, an underfilled and hyperdynamic left ventricle (ejection fraction 75%), and moderate tricuspid valve regurgitation. Her right ventricular systolic pressure is estimated to be 47 mm Hg.
Doppler ultrasonography of the legs reveals an occlusive thrombus within the right small saphenous vein that bulges and extends into the right popliteal vein. Also noted is a nonocclusive thrombus in the upper right popliteal vein that likely originated from the thrombus in the small saphenous vein.
Initial laboratory testing (Table 1) shows elevations of the cardiac enzymes troponin T and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
ESTIMATING PROGNOSIS IN PULMONARY EMBOLISM
2. Which of the following laboratory results at presentation is independently associated with a worse outcome in patients with pulmonary embolism?
- Elevated NT-pro-BNP
- Hypercalcemia
- Thrombocytosis
- Hypernatremia
- Elevated procalcitonin
The Pulmonary Embolism Severity Index11 and the Simplified Pulmonary Embolism Severity Index12 (Table 2) are clinical calculators that help predict 30-day risk of death in patients with pulmonary embolism. Our patient’s Pulmonary Embolism Severity Index score is 60, indicating a very low risk, but her simplified severity index score is 2, indicating a high risk.
A shock index score (the heart rate divided by the systolic blood pressure) greater than 1 is also a sensitive measure of risk.13 (Our patient’s shock index score is 1.03.) Although the simplified version is more accurate,14 the shock index is also helpful when deciding whether patients with suspected pulmonary embolism should receive early fibrinolysis.15
In a large registry of patients with confirmed pulmonary embolism, risk factors for death were age greater than 70, cancer, clinical congestive heart failure, chronic obstructive pulmonary disease, systolic blood pressure lower than 90 mm Hg, respiratory rate less than 20 per minute, and right ventricular hypokinesis.16 Right ventricular dysfunction progressing to right ventricular failure and cardiogenic shock is the most common cause of death in patients with pulmonary embolism.16–18
Post hoc analysis has also shown that elevations of the biomarkers BNP, NT-pro-BNP, and cardiac troponins I and T are associated with a prolonged hospital course and a higher risk of death within 30 days.19 Interestingly, a recent retrospective analysis found hyponatremia to be an independent risk factor for death in the short term.20
Thrombocytopenia, not thrombocytosis, is associated with worse outcomes in patients with pulmonary embolism.16 Procalcitonin is elevated in bacterial pneumonia but is normal in pulmonary embolism and so may be helpful in differentiating between the two.21,22 Hypernatremia, hypercalcemia, and elevated procalcitonin have not been shown to be independently associated with worse outcomes in acute pulmonary embolism.
Thus, of the answer choices shown above, elevated NT-pro-BNP is the correct answer.
Classified as massive, submassive, or low-risk
Pulmonary embolism is often stratified as massive, submassive, or low-risk, reflecting the severity and the degree of cardiovascular collapse. The treatment depends on the classification.
Pulmonary embolism is classified as massive if the patient has a cardiac arrest or a systolic blood pressure lower than 90 mm Hg for more than 15 minutes.23 Nearly half of patients in this category die.24
Pulmonary embolism is submassive if the patient has systolic pressure greater than 90 mm Hg but has right ventricular dysfunction, as evidenced by physical examination, elevated cardiac biomarkers, electrocardiography, transthoracic echocardiography, or computed tomography. The death rate is as high as 15%.24
Pulmonary embolism in a normotensive patient with no right ventricular dysfunction is defined as low-risk.
Our patient so far
Our patient has bilateral pulmonary emboli, most likely originating from a deep vein thrombosis in her right lower leg. Her pulmonary embolism would be classified as submassive, as her systolic pressure is greater than 90 mm Hg and right ventricular dysfunction—significant right ventricular strain—was noted on both transthoracic echocardiography and computed tomography. Also, cardiac biomarkers are elevated, and the physical examination revealed a prominent P2 sound and right parasternal heave, also suggestive of right ventricular dysfunction.
Now, 6 hours have passed, and even though she has been receiving intravenous heparin during this time, her shock index remains greater than 1, indicating hemodynamic instability. Her pulse rate is still markedly high—over 160 bpm—and she still appears quite anxious and uncomfortable.
HOW SHOULD THIS PATIENT BE TREATED?
3. Which of the following is the most appropriate treatment for this patient?
- Start warfarin immediately while bridging with unfractionated heparin, low-molecular-weight heparin, or fondaparinux
- Start fibrinolysis with alteplase
- Give metoprolol intravenously to control her heart rate
- Start dabigatran immediately while bridging with unfractionated heparin
- Place an inferior vena cava filter
- Consult cardiothoracic surgery for emergency pulmonary embolectomy
All patients with confirmed pulmonary embolism and no contraindications to anticoagulation should begin treatment with low-molecular-weight heparin, unfractionated heparin, or fondaparinux.23 In addition, this therapy should be started empirically while the patient is still undergoing diagnostic testing if the pretest probability of pulmonary embolism is intermediate or high.23
Warfarin is indicated for all patients with pulmonary embolism who do not have contraindications to it (Table 3). If unfractionated heparin, low-molecular-weight heparin, or fondaparinux has not already been started, it should be started at the same time as warfarin and should be continued until the international normalized ratio (INR) is within the therapeutic range.
Fibrinolysis. Treatment with a fibrinolytic agent in addition to heparin results in faster improvement of right ventricular function and pulmonary perfusion than with heparin alone.25 It may also decrease the incidence of pulmonary hypertension secondary to chronic thromboembolic disease.26 It should be considered in patients with massive pulmonary embolism.23
Whether fibrinolysis is appropriate for all patients with submassive pulmonary embolism remains controversial. Currently, it is not recommended for minor right ventricular dysfunction or myocardial necrosis if the patient has no signs of overt clinical decline.23 The Pulmonary Embolism Thrombolysis trial27 is an ongoing prospective randomized comparison of tenecteplase in a single bolus plus heparin vs heparin alone in normotensive patients with submassive pulmonary embolism, such as our patient. This trial may elucidate the benefit of fibrinolytic therapy in patients with submassive pulmonary embolism.
Patients at low risk are generally treated with heparin and warfarin anticoagulation alone. Fibrinolysis is not recommended in these patients, as the risk of bleeding outweighs the potential benefits.23
Metoprolol may not be advisable for our patient, as her tachycardia is likely compensatory, and beta-blocker therapy could blunt this compensatory response, leading to inadequate systemic perfusion.
Dabigatran is an oral direct thrombin inhibitor that does not require laboratory monitoring. It is currently approved for the prevention of stroke in patients with atrial fibrillation. It has been shown to be as effective as warfarin in the treatment of acute venous thromboembolism28 and may be a viable option in the future, but as of this writing it has not yet been approved in the United States for this indication. Furthermore, dabigatran inhibits thrombin immediately, so continued heparin bridging would not be necessary.
An inferior vena cava filter may prevent recurrent pulmonary embolism for patients who have absolute contraindications to anticoagulation, most significantly in the short term, ie, in the first few weeks after placement. However, these devices have not yet been shown to improve long-term mortality rates.
Embolectomy, percutaneous or surgical, is also an option. For patients in whom thrombolytic therapy is not effective, “rescue” surgical embolectomy has been associated with better outcomes compared with secondary thrombolysis and so should be considered.29
Back to our patient
An intravenous infusion of alteplase is started, and the patient’s tachycardia improves. Her oxygen requirements normalize, and she is transferred to the general medical floor the next day. She receives subcutaneous dalteparin as a bridge therapy, and warfarin is titrated to a goal INR of 2.0 to 3.0. Because of the acute deep vein thrombosis in her right lower leg, she is instructed to wear knee-high fitted compression hose for primary prevention of postphlebitic syndrome.
HOW LONG TO TREAT? IS GENETIC TESTING INDICATED?
Patients with a first episode of unprovoked venous thromboembolism should receive oral anticoagulants for 6 months, while those with recurrent unprovoked venous thromboembolism require lifelong oral anticoagulation.23
Whether to test for inherited thrombophilia after a first episode of venous thromboembolism to guide the duration of anticoagulation is controversial.30 Indiscriminate testing has not been recommended in these patients,31 but the American College of Medical Genetics recommends genetic screening for factor V Leiden in patients who have an unprovoked incident of venous thromboembolism before age 50.32
No randomized controlled trial has assessed whether thrombophilia testing decreases the recurrence rate of venous thromboembolism.33 One uncontrolled study suggested that testing for inherited thrombophilias in patients with a first episode does not affect the risk of recurrence.34 Testing is costly and may cause psychological distress for patients and family members.
Our patient is discharged home on warfarin for 6 months with subsequent follow-up evaluation in the thrombophilia clinic.
WHEN SHOULD WARFARIN BE RESTARTED?
4. If our patient were to discontinue oral anticoagulation in 6 months, which of the following, if present 1 month afterwards, would be a reason to restart oral anticoagulation?
- Elevated serum cotinine
- Positive pregnancy test
- Elevated follicle-stimulating hormone and luteinizing hormone and low estradiol levels
- Elevated D-dimer
Cotinine is a nicotine metabolite, and serum levels are elevated in smokers. Smoking and pregnancy both increase the risk of venous thromboembolism. However, smoking or pregnancy alone would not be a reason to increase the duration of anticoagulation.
Warfarin is contraindicated in pregnancy because of its teratogenic effects, particularly in the first trimester. Follicle-stimulating hormone and luteinizing hormone levels increase in response to decreased estradiol at menopause. Postmenopausal women are not at increased risk of venous thromboembolism unless they are taking oral estrogen hormone replacement therapy.
An elevated D-dimer level 1 month after stopping of oral anticoagulation has been associated with a higher rate of recurrence of venous thromboembolism, which is reduced by a resumption of anticoagulation.35 Therefore, consideration should be given to resuming anticoagulation in patients who have an elevated D-dimer level.
TAKE-HOME POINTS
It is important to distinguish between massive, submassive, and low-risk pulmonary embolism, since each type has a different treatment and prognosis.
Fibrinolytic therapy is indicated in patients with massive pulmonary embolism when no contraindication is present, whereas it is not indicated in those with low-risk pulmonary embolism.
Management of submassive pulmonary embolism continues to be an area of considerable debate. Current American Heart Association guidelines recommend consideration of fibrinolysis initially in patients with submassive acute pulmonary embolism if there is concurrent clinical evidence of adverse prognosis—eg, new hemodynamic instability, worsening respiratory insufficiency, severe right ventricular dysfunction, or major myocardial necrosis.23 On the other hand, the American College of Chest Physicians recommends against initial systemic fibrinolysis in submassive acute pulmonary embolism based on biomarkers or findings on ECG, transthoracic echocardiography, or CT, recommending it only in therapeutically anticoagulated patients deemed to be at high risk of hypotension.36
Since the optimal treatment of submassive pulmonary embolism is still not known, it is important that clinicians remain up to date on the evidence and guidelines.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. Am J Cardiol 1991; 68:1723–1724.
- Ferrari E, Imbert A, Chevalier T, Mihoubi A, Morand P, Baudouy M. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads—80 case reports. Chest 1997; 111:537–543.
- Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005; 25:843–848.
- Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 2005; 352:1760–1768.
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000; 217:447–455.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001; 110:528–535.
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003; 21:180–183.
- Stein PD, Chenevert TL, Fowler SE, et al; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med 2010; 152:434–443,W142–W143.
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 2008; 246:941–946.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Otero R, Trujillo-Santos J, Cayuela A, et al; Registro Informatizado de la Enfermedad Tromboembólica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007; 30:1111–1116.
- Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J 2011; 37:762–766.
- Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003; 24:366–376.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–1389.
- ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004; 164:1685–1689.
- Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008; 29:1569–1577.
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003; 108:2191–2194.
- Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, Aujesky D. Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 2010; 182:1178–1183.
- Delèvaux I, André M, Aumaître O, Bègue RJ, Colombier M, Piette JC. Procalcitonin measurement for differential diagnosis between pulmonary embolism and pneumonia. Crit Care Med 2003; 31:661.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community-acquired pneumonia. Clin Appl Thromb Hemost 2011; 17:519–525.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341:507–511.
- Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009; 136:1202–1210.
- Steering Committee of PEITHO Investigators. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J 2012; 163:33–38.e1.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006; 129:1043–1050.
- Ho WK, Hankey GJ, Eikelboom JW. Should adult patients be routinely tested for heritable thrombophilia after an episode of venous thromboembolism? Med J Aust 2011; 195:139–142.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Cohn D, Vansenne F, de Borgie C, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev 2009; ( 1):CD007069.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008; 6:1474–1477.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. Ddimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):7S–47S.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. Am J Cardiol 1991; 68:1723–1724.
- Ferrari E, Imbert A, Chevalier T, Mihoubi A, Morand P, Baudouy M. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads—80 case reports. Chest 1997; 111:537–543.
- Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005; 25:843–848.
- Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 2005; 352:1760–1768.
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000; 217:447–455.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001; 110:528–535.
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003; 21:180–183.
- Stein PD, Chenevert TL, Fowler SE, et al; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med 2010; 152:434–443,W142–W143.
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 2008; 246:941–946.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Otero R, Trujillo-Santos J, Cayuela A, et al; Registro Informatizado de la Enfermedad Tromboembólica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007; 30:1111–1116.
- Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J 2011; 37:762–766.
- Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003; 24:366–376.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–1389.
- ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004; 164:1685–1689.
- Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008; 29:1569–1577.
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003; 108:2191–2194.
- Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, Aujesky D. Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 2010; 182:1178–1183.
- Delèvaux I, André M, Aumaître O, Bègue RJ, Colombier M, Piette JC. Procalcitonin measurement for differential diagnosis between pulmonary embolism and pneumonia. Crit Care Med 2003; 31:661.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community-acquired pneumonia. Clin Appl Thromb Hemost 2011; 17:519–525.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341:507–511.
- Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009; 136:1202–1210.
- Steering Committee of PEITHO Investigators. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J 2012; 163:33–38.e1.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006; 129:1043–1050.
- Ho WK, Hankey GJ, Eikelboom JW. Should adult patients be routinely tested for heritable thrombophilia after an episode of venous thromboembolism? Med J Aust 2011; 195:139–142.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Cohn D, Vansenne F, de Borgie C, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev 2009; ( 1):CD007069.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008; 6:1474–1477.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. Ddimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):7S–47S.