User login
I’ve been rethinking my zeal for breast cancer screening
The numbers remain striking in the United States:
- more than 190,000 cases of invasive breast cancer diagnosed each year
- taken together in our practices, more than 2 million survivors of breast cancer.
Furthermore, two decades of screening for breast cancer with mammography have meant a major increase in the detection of early breast tumors.
In 1980, a woman’s lifetime risk of a diagnosis of breast cancer was 1 in 12; today, her risk is 1 in 8. Include cases of ductal carcinoma in situ and the risk of a diagnosis of breast cancer has almost doubled since 1980—an increase that parallels the rise in the rate of screening mammography among US women.
Breast Ca screening hasn’t behaved as expected
in a wider screening paradigm
We know that screening mammography has increased the rate of diagnosis of localized tumors. We also know that, although 5-year survival is very high for women who have localized breast cancer—greater than 98%—survival among those who have advanced tumor is only 27%. The high survival rate associated with small, local tumors has been used to promote the importance of screening—and successfully so: Approximately 70% of women in the United States older than 40 years report having a mammogram recently.1
But, in an article in The Journal of the American Medical Association (JAMA) recently, Esserman and colleagues proposed that, contrary to conventional wisdom, the increase in the detection of early breast cancers that has been associated with a rise in screening mammography may, in fact, be a mixed blessing.2 Why?
The introduction of an optimal screening test, they point out, should be followed by:
- 1) an increase in the rate of detection of early disease, followed by
- 2) a decrease in the rate of detection of regional disease, while
- 3) the rate of detection of cancer overall remains constant.
Such a trend model has been observed with screening programs for colon cancer and cervical cancer, in which precancerous conditions (notably, polyps and cervical intraepithelial neoplasia, respectively) are detected and eliminated. Indeed, screening colonoscopy and Pap testing have resulted in a reduction in the detection of invasive colon cancer and invasive cervical cancer.
But screening mammography has not reduced the incidence of invasive breast tumors. As Esserman’s team points out, ductal carcinoma in situ—a diagnosis that was rare before screening mammography grew widespread—now accounts for more than 25% of all new breast cancers diagnosed (more than 60,000 new cases annually). Yet there is no convincing evidence, they note, of substantial reduction in the incidence of invasive breast cancers from detection of these preinvasive lesions.
Yes, mortality from breast cancer has decreased over the past decades but, again, as the authors of the JAMA report point out, most of the reduction is a result of improvements in adjuvant therapy.
So why has widespread screening had a limited impact on mortality from breast cancer?
- Screening increases the detection of indolent tumors that may not lead to life-threatening disease; some regress even without treatment.
- Annual screening isn’t frequent enough to detect aggressive, rapidly growing tumors at a curable stage.
- We have limited ability to distinguish low-risk cancer from high-risk cancer; Esserman’s perspective is that this knowledge gap has led to substantial overtreatment of women who are given a diagnosis of breast cancer.
Esserman and colleagues strikingly frame the issue this way: For every 1 death from breast cancer that is prevented by screening (even in the age group, 50 to 70 years, in which screening is least controversial), 838 women must be screened for 6 years—at a cost of thousands of screens, hundreds of biopsies that carry their own costs and risk of morbidity, and many cancers treated aggressively even though they are not destined to progress.
What am I going to say to my patients?
Will I continue to recommend screening mammograms?
Yes, unless—until—guidelines change.
Here is how I see it working in my practice. When a woman asks, “With all the controversy I hear about, do you still recommend that I have a mammogram every year?,” I’ll explain that:
- although annual mammography can save lives through early diagnosis of significant cancer, it sometimes detects small tumors that don’t need to be treated.
- she should have a mammogram annually because that’s what current guidelines call for.
I confess: For a long time, I’ve nagged the few women in my practice over 50 years old who refuse annual mammography to get screened. I’m determined to be more accepting of their decision from now on.
Will I encourage patients to perform breast self-examination if they refuse annual mammography?
No—I stopped recommending the self-exam several years ago. First, there’s an absence of data that encouraging breast self-examination save lives.3,4 Second, in some of my patients, I’ve observed anxiety (that they might miss something) or guilt (because they didn’t examine themselves regularly despite the recommendation) about breast self-examination.
I continue to perform breast examinations, of course—regardless of whether a woman has annual screening mammograms—and I continue to encourage patients who become aware of changes in their breasts to contact me for further evaluation.
My view of the bottom line
It’s time to rethink our approach to screening for breast cancer. I look forward to ACOG, The American Cancer Society, other professional associations, and government agencies preparing revised materials that update clinicians and women about screening for breast cancer. That kind of guidance will help us discuss a difficult topic with our patients and choose the best possible strategy with their participation.
- Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528-1540.
- Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685-1692.
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst. 2002;94:1445-1457.
- Harris R, Kinsinger LS. Routinely teaching breast self-examination is dead. What does this mean? J Natl Cancer Inst. 2002;94:1420-1421.
The numbers remain striking in the United States:
- more than 190,000 cases of invasive breast cancer diagnosed each year
- taken together in our practices, more than 2 million survivors of breast cancer.
Furthermore, two decades of screening for breast cancer with mammography have meant a major increase in the detection of early breast tumors.
In 1980, a woman’s lifetime risk of a diagnosis of breast cancer was 1 in 12; today, her risk is 1 in 8. Include cases of ductal carcinoma in situ and the risk of a diagnosis of breast cancer has almost doubled since 1980—an increase that parallels the rise in the rate of screening mammography among US women.
Breast Ca screening hasn’t behaved as expected
in a wider screening paradigm
We know that screening mammography has increased the rate of diagnosis of localized tumors. We also know that, although 5-year survival is very high for women who have localized breast cancer—greater than 98%—survival among those who have advanced tumor is only 27%. The high survival rate associated with small, local tumors has been used to promote the importance of screening—and successfully so: Approximately 70% of women in the United States older than 40 years report having a mammogram recently.1
But, in an article in The Journal of the American Medical Association (JAMA) recently, Esserman and colleagues proposed that, contrary to conventional wisdom, the increase in the detection of early breast cancers that has been associated with a rise in screening mammography may, in fact, be a mixed blessing.2 Why?
The introduction of an optimal screening test, they point out, should be followed by:
- 1) an increase in the rate of detection of early disease, followed by
- 2) a decrease in the rate of detection of regional disease, while
- 3) the rate of detection of cancer overall remains constant.
Such a trend model has been observed with screening programs for colon cancer and cervical cancer, in which precancerous conditions (notably, polyps and cervical intraepithelial neoplasia, respectively) are detected and eliminated. Indeed, screening colonoscopy and Pap testing have resulted in a reduction in the detection of invasive colon cancer and invasive cervical cancer.
But screening mammography has not reduced the incidence of invasive breast tumors. As Esserman’s team points out, ductal carcinoma in situ—a diagnosis that was rare before screening mammography grew widespread—now accounts for more than 25% of all new breast cancers diagnosed (more than 60,000 new cases annually). Yet there is no convincing evidence, they note, of substantial reduction in the incidence of invasive breast cancers from detection of these preinvasive lesions.
Yes, mortality from breast cancer has decreased over the past decades but, again, as the authors of the JAMA report point out, most of the reduction is a result of improvements in adjuvant therapy.
So why has widespread screening had a limited impact on mortality from breast cancer?
- Screening increases the detection of indolent tumors that may not lead to life-threatening disease; some regress even without treatment.
- Annual screening isn’t frequent enough to detect aggressive, rapidly growing tumors at a curable stage.
- We have limited ability to distinguish low-risk cancer from high-risk cancer; Esserman’s perspective is that this knowledge gap has led to substantial overtreatment of women who are given a diagnosis of breast cancer.
Esserman and colleagues strikingly frame the issue this way: For every 1 death from breast cancer that is prevented by screening (even in the age group, 50 to 70 years, in which screening is least controversial), 838 women must be screened for 6 years—at a cost of thousands of screens, hundreds of biopsies that carry their own costs and risk of morbidity, and many cancers treated aggressively even though they are not destined to progress.
What am I going to say to my patients?
Will I continue to recommend screening mammograms?
Yes, unless—until—guidelines change.
Here is how I see it working in my practice. When a woman asks, “With all the controversy I hear about, do you still recommend that I have a mammogram every year?,” I’ll explain that:
- although annual mammography can save lives through early diagnosis of significant cancer, it sometimes detects small tumors that don’t need to be treated.
- she should have a mammogram annually because that’s what current guidelines call for.
I confess: For a long time, I’ve nagged the few women in my practice over 50 years old who refuse annual mammography to get screened. I’m determined to be more accepting of their decision from now on.
Will I encourage patients to perform breast self-examination if they refuse annual mammography?
No—I stopped recommending the self-exam several years ago. First, there’s an absence of data that encouraging breast self-examination save lives.3,4 Second, in some of my patients, I’ve observed anxiety (that they might miss something) or guilt (because they didn’t examine themselves regularly despite the recommendation) about breast self-examination.
I continue to perform breast examinations, of course—regardless of whether a woman has annual screening mammograms—and I continue to encourage patients who become aware of changes in their breasts to contact me for further evaluation.
My view of the bottom line
It’s time to rethink our approach to screening for breast cancer. I look forward to ACOG, The American Cancer Society, other professional associations, and government agencies preparing revised materials that update clinicians and women about screening for breast cancer. That kind of guidance will help us discuss a difficult topic with our patients and choose the best possible strategy with their participation.
The numbers remain striking in the United States:
- more than 190,000 cases of invasive breast cancer diagnosed each year
- taken together in our practices, more than 2 million survivors of breast cancer.
Furthermore, two decades of screening for breast cancer with mammography have meant a major increase in the detection of early breast tumors.
In 1980, a woman’s lifetime risk of a diagnosis of breast cancer was 1 in 12; today, her risk is 1 in 8. Include cases of ductal carcinoma in situ and the risk of a diagnosis of breast cancer has almost doubled since 1980—an increase that parallels the rise in the rate of screening mammography among US women.
Breast Ca screening hasn’t behaved as expected
in a wider screening paradigm
We know that screening mammography has increased the rate of diagnosis of localized tumors. We also know that, although 5-year survival is very high for women who have localized breast cancer—greater than 98%—survival among those who have advanced tumor is only 27%. The high survival rate associated with small, local tumors has been used to promote the importance of screening—and successfully so: Approximately 70% of women in the United States older than 40 years report having a mammogram recently.1
But, in an article in The Journal of the American Medical Association (JAMA) recently, Esserman and colleagues proposed that, contrary to conventional wisdom, the increase in the detection of early breast cancers that has been associated with a rise in screening mammography may, in fact, be a mixed blessing.2 Why?
The introduction of an optimal screening test, they point out, should be followed by:
- 1) an increase in the rate of detection of early disease, followed by
- 2) a decrease in the rate of detection of regional disease, while
- 3) the rate of detection of cancer overall remains constant.
Such a trend model has been observed with screening programs for colon cancer and cervical cancer, in which precancerous conditions (notably, polyps and cervical intraepithelial neoplasia, respectively) are detected and eliminated. Indeed, screening colonoscopy and Pap testing have resulted in a reduction in the detection of invasive colon cancer and invasive cervical cancer.
But screening mammography has not reduced the incidence of invasive breast tumors. As Esserman’s team points out, ductal carcinoma in situ—a diagnosis that was rare before screening mammography grew widespread—now accounts for more than 25% of all new breast cancers diagnosed (more than 60,000 new cases annually). Yet there is no convincing evidence, they note, of substantial reduction in the incidence of invasive breast cancers from detection of these preinvasive lesions.
Yes, mortality from breast cancer has decreased over the past decades but, again, as the authors of the JAMA report point out, most of the reduction is a result of improvements in adjuvant therapy.
So why has widespread screening had a limited impact on mortality from breast cancer?
- Screening increases the detection of indolent tumors that may not lead to life-threatening disease; some regress even without treatment.
- Annual screening isn’t frequent enough to detect aggressive, rapidly growing tumors at a curable stage.
- We have limited ability to distinguish low-risk cancer from high-risk cancer; Esserman’s perspective is that this knowledge gap has led to substantial overtreatment of women who are given a diagnosis of breast cancer.
Esserman and colleagues strikingly frame the issue this way: For every 1 death from breast cancer that is prevented by screening (even in the age group, 50 to 70 years, in which screening is least controversial), 838 women must be screened for 6 years—at a cost of thousands of screens, hundreds of biopsies that carry their own costs and risk of morbidity, and many cancers treated aggressively even though they are not destined to progress.
What am I going to say to my patients?
Will I continue to recommend screening mammograms?
Yes, unless—until—guidelines change.
Here is how I see it working in my practice. When a woman asks, “With all the controversy I hear about, do you still recommend that I have a mammogram every year?,” I’ll explain that:
- although annual mammography can save lives through early diagnosis of significant cancer, it sometimes detects small tumors that don’t need to be treated.
- she should have a mammogram annually because that’s what current guidelines call for.
I confess: For a long time, I’ve nagged the few women in my practice over 50 years old who refuse annual mammography to get screened. I’m determined to be more accepting of their decision from now on.
Will I encourage patients to perform breast self-examination if they refuse annual mammography?
No—I stopped recommending the self-exam several years ago. First, there’s an absence of data that encouraging breast self-examination save lives.3,4 Second, in some of my patients, I’ve observed anxiety (that they might miss something) or guilt (because they didn’t examine themselves regularly despite the recommendation) about breast self-examination.
I continue to perform breast examinations, of course—regardless of whether a woman has annual screening mammograms—and I continue to encourage patients who become aware of changes in their breasts to contact me for further evaluation.
My view of the bottom line
It’s time to rethink our approach to screening for breast cancer. I look forward to ACOG, The American Cancer Society, other professional associations, and government agencies preparing revised materials that update clinicians and women about screening for breast cancer. That kind of guidance will help us discuss a difficult topic with our patients and choose the best possible strategy with their participation.
- Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528-1540.
- Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685-1692.
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst. 2002;94:1445-1457.
- Harris R, Kinsinger LS. Routinely teaching breast self-examination is dead. What does this mean? J Natl Cancer Inst. 2002;94:1420-1421.
- Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528-1540.
- Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685-1692.
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst. 2002;94:1445-1457.
- Harris R, Kinsinger LS. Routinely teaching breast self-examination is dead. What does this mean? J Natl Cancer Inst. 2002;94:1420-1421.
Does benefit always outweigh risk when a SERM is used to prevent primary breast cancer?
In the United States, tamoxifen is usually prescribed as adjuvant endocrine therapy after treatment of estrogen-receptor–positive breast cancer in both premenopausal and post-menopausal women. Raloxifene is most often prescribed for the prevention and treatment of osteoporosis in postmenopausal women. Use of these agents as chemoprophylaxis in women who have no history of breast cancer is less common, largely because of the risks and side effects of these drugs.
The increase in venous thromboembolism is of particular concern for women who have an elevated risk of this outcome, including overweight women and those of advanced age. The elevated risk of malignant and benign gynecologic disease associated with tamoxifen is of concern in all women who have an intact uterus.
Details of the review
Nelson and colleagues performed a systematic review, funded by the Agency for Health-care Research and Quality, that involved the aggregation of findings from seven placebo-controlled trials and one head-to-head, randomized, clinical trial involving women who had no history of preinvasive or invasive breast cancer. In the process, they focused on harms as well as benefits associated with use of these chemoprophylactic agents.
Tamoxifen and raloxifene reduced the risk of invasive breast cancer by 7 to 10 cases in every 1,000 women annually. These agents reduced the risk of estrogen-receptor–positive malignancy, but not estrogen-receptor–negative tumors, noninvasive cancer, or breast cancer–related mortality.
Tamoxifen and raloxifene were similarly effective in premenopausal and postmenopausal populations; both drugs also reduced the rate of osteoporotic fracture.
Most of the participants in the prevention trials were white and relatively healthy. Therefore, the relevance of these findings to women of other racial and ethnic groups and to women who have chronic disease or other morbidity is uncertain.
Aromatase inhibitors are being assessed for chemoprophylaxis, so we should have information on their risk-benefit ratio in the near future.
Clinicians who care for women at high risk of primary breast cancer should thoroughly counsel each candidate for chemoprophylaxis about the potential benefits and risks of these agents in her particular circumstances. It may be that the risks outweigh the benefits in some women—such as those who already have an elevated risk of venous thromboembolism.
In addition, because most of the participants in the studies included in this review were healthy and white, we cannot be certain how generalizable these findings are to other subpopulations.—ANDREW M. KAUNITZ, MD
In the United States, tamoxifen is usually prescribed as adjuvant endocrine therapy after treatment of estrogen-receptor–positive breast cancer in both premenopausal and post-menopausal women. Raloxifene is most often prescribed for the prevention and treatment of osteoporosis in postmenopausal women. Use of these agents as chemoprophylaxis in women who have no history of breast cancer is less common, largely because of the risks and side effects of these drugs.
The increase in venous thromboembolism is of particular concern for women who have an elevated risk of this outcome, including overweight women and those of advanced age. The elevated risk of malignant and benign gynecologic disease associated with tamoxifen is of concern in all women who have an intact uterus.
Details of the review
Nelson and colleagues performed a systematic review, funded by the Agency for Health-care Research and Quality, that involved the aggregation of findings from seven placebo-controlled trials and one head-to-head, randomized, clinical trial involving women who had no history of preinvasive or invasive breast cancer. In the process, they focused on harms as well as benefits associated with use of these chemoprophylactic agents.
Tamoxifen and raloxifene reduced the risk of invasive breast cancer by 7 to 10 cases in every 1,000 women annually. These agents reduced the risk of estrogen-receptor–positive malignancy, but not estrogen-receptor–negative tumors, noninvasive cancer, or breast cancer–related mortality.
Tamoxifen and raloxifene were similarly effective in premenopausal and postmenopausal populations; both drugs also reduced the rate of osteoporotic fracture.
Most of the participants in the prevention trials were white and relatively healthy. Therefore, the relevance of these findings to women of other racial and ethnic groups and to women who have chronic disease or other morbidity is uncertain.
Aromatase inhibitors are being assessed for chemoprophylaxis, so we should have information on their risk-benefit ratio in the near future.
Clinicians who care for women at high risk of primary breast cancer should thoroughly counsel each candidate for chemoprophylaxis about the potential benefits and risks of these agents in her particular circumstances. It may be that the risks outweigh the benefits in some women—such as those who already have an elevated risk of venous thromboembolism.
In addition, because most of the participants in the studies included in this review were healthy and white, we cannot be certain how generalizable these findings are to other subpopulations.—ANDREW M. KAUNITZ, MD
In the United States, tamoxifen is usually prescribed as adjuvant endocrine therapy after treatment of estrogen-receptor–positive breast cancer in both premenopausal and post-menopausal women. Raloxifene is most often prescribed for the prevention and treatment of osteoporosis in postmenopausal women. Use of these agents as chemoprophylaxis in women who have no history of breast cancer is less common, largely because of the risks and side effects of these drugs.
The increase in venous thromboembolism is of particular concern for women who have an elevated risk of this outcome, including overweight women and those of advanced age. The elevated risk of malignant and benign gynecologic disease associated with tamoxifen is of concern in all women who have an intact uterus.
Details of the review
Nelson and colleagues performed a systematic review, funded by the Agency for Health-care Research and Quality, that involved the aggregation of findings from seven placebo-controlled trials and one head-to-head, randomized, clinical trial involving women who had no history of preinvasive or invasive breast cancer. In the process, they focused on harms as well as benefits associated with use of these chemoprophylactic agents.
Tamoxifen and raloxifene reduced the risk of invasive breast cancer by 7 to 10 cases in every 1,000 women annually. These agents reduced the risk of estrogen-receptor–positive malignancy, but not estrogen-receptor–negative tumors, noninvasive cancer, or breast cancer–related mortality.
Tamoxifen and raloxifene were similarly effective in premenopausal and postmenopausal populations; both drugs also reduced the rate of osteoporotic fracture.
Most of the participants in the prevention trials were white and relatively healthy. Therefore, the relevance of these findings to women of other racial and ethnic groups and to women who have chronic disease or other morbidity is uncertain.
Aromatase inhibitors are being assessed for chemoprophylaxis, so we should have information on their risk-benefit ratio in the near future.
Clinicians who care for women at high risk of primary breast cancer should thoroughly counsel each candidate for chemoprophylaxis about the potential benefits and risks of these agents in her particular circumstances. It may be that the risks outweigh the benefits in some women—such as those who already have an elevated risk of venous thromboembolism.
In addition, because most of the participants in the studies included in this review were healthy and white, we cannot be certain how generalizable these findings are to other subpopulations.—ANDREW M. KAUNITZ, MD
Does cognitive function decline during the menopausal transition?
These important findings from SWAN confirm the memory problems reported by many women during the menopausal transition. They also build on the findings of clinical trials of HT in younger menopausal women, which explored the effect of HT on cardiovascular health, to underscore the critical role that timing of such therapy can play.1,2
Other cohort studies of middle-aged women in the United States have found the menopausal transition to be associated with undesirable changes in mood3,4; some experts have described the endocrine shifts that accompany menopause as “hormonal chaos.”5
Details of the study
Participants were 42 to 52 years old and had an intact uterus and at least one ovary at entry. They were followed for 4 years, with delineation of the menopausal stage (i.e., premenopause, early and late perimenopause, and menopause) and assessment of hormone use prior to the final menstrual period and after menopause. The outcome was longitudinal performance in three cognitive domains:
- processing speed—assessed using the Symbol Digit Modalities Test. Premenopausal, early perimenopausal, and postmenopausal women improved with repeated administration of this test, but late perimenopausal women did not. Prior use of HT improved the score, whereas late use of HT reduced it.
- verbal memory—evaluated via the East Boston Memory Test. Test scores increased during premenopause and postmenopause but not during early or late perimenopause. Prior use of HT improved the test score, but late use reduced the score.
- working memory—assessed using the Digit Span Backward test. This domain did not vary by stage of menopause.
Participants who initiated menopausal HT or oral contraceptives prior to the last menstrual period were excluded from the analysis during use of HT. They were allowed to reenter the study, however, once HT or oral contraceptives were discontinued.
As the cognitive component of SWAN began, the mean age of participants was 50 years, and 8% were premenopausal, 49% were early perimenopausal, 12% were late perimenopausal, and 27% were postmenopausal. In addition, 4% were both postmenopausal and current users of HT.
Because perimenopausal HT should include a dosage of progestin adequate to suppress ovulation in order to prevent iatrogenic irregular uterine bleeding, women who are healthy, lean, nonsmoking, and still having menstrual periods can safely use a conventional oral contraceptive. Options for other symptomatic perimenopausal women include a continuous oral menopausal regimen formulated with 5 μg of ethinyl estradiol and 1 mg of norethindrone acetate (Femhrt 1/5) or 1 mg of estradiol and 0.5 mg of norethindrone acetate (Activella 1/0.5 or generic).—ANDREW M. KAUNITZ, MD
1. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477.
2. Manson JE, Allison MA, Rossouw JE, et al. For the WHI and WHI-CACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591-2602.
3. Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with the menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007;110(2 Pt 1):230-240.
4. Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell S. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2008;15:223-232.
5. Berga SL. Disordered folliculogenesis during the menopausal transition: explaining chaos. Menopause. 2009;16:11-12.
These important findings from SWAN confirm the memory problems reported by many women during the menopausal transition. They also build on the findings of clinical trials of HT in younger menopausal women, which explored the effect of HT on cardiovascular health, to underscore the critical role that timing of such therapy can play.1,2
Other cohort studies of middle-aged women in the United States have found the menopausal transition to be associated with undesirable changes in mood3,4; some experts have described the endocrine shifts that accompany menopause as “hormonal chaos.”5
Details of the study
Participants were 42 to 52 years old and had an intact uterus and at least one ovary at entry. They were followed for 4 years, with delineation of the menopausal stage (i.e., premenopause, early and late perimenopause, and menopause) and assessment of hormone use prior to the final menstrual period and after menopause. The outcome was longitudinal performance in three cognitive domains:
- processing speed—assessed using the Symbol Digit Modalities Test. Premenopausal, early perimenopausal, and postmenopausal women improved with repeated administration of this test, but late perimenopausal women did not. Prior use of HT improved the score, whereas late use of HT reduced it.
- verbal memory—evaluated via the East Boston Memory Test. Test scores increased during premenopause and postmenopause but not during early or late perimenopause. Prior use of HT improved the test score, but late use reduced the score.
- working memory—assessed using the Digit Span Backward test. This domain did not vary by stage of menopause.
Participants who initiated menopausal HT or oral contraceptives prior to the last menstrual period were excluded from the analysis during use of HT. They were allowed to reenter the study, however, once HT or oral contraceptives were discontinued.
As the cognitive component of SWAN began, the mean age of participants was 50 years, and 8% were premenopausal, 49% were early perimenopausal, 12% were late perimenopausal, and 27% were postmenopausal. In addition, 4% were both postmenopausal and current users of HT.
Because perimenopausal HT should include a dosage of progestin adequate to suppress ovulation in order to prevent iatrogenic irregular uterine bleeding, women who are healthy, lean, nonsmoking, and still having menstrual periods can safely use a conventional oral contraceptive. Options for other symptomatic perimenopausal women include a continuous oral menopausal regimen formulated with 5 μg of ethinyl estradiol and 1 mg of norethindrone acetate (Femhrt 1/5) or 1 mg of estradiol and 0.5 mg of norethindrone acetate (Activella 1/0.5 or generic).—ANDREW M. KAUNITZ, MD
These important findings from SWAN confirm the memory problems reported by many women during the menopausal transition. They also build on the findings of clinical trials of HT in younger menopausal women, which explored the effect of HT on cardiovascular health, to underscore the critical role that timing of such therapy can play.1,2
Other cohort studies of middle-aged women in the United States have found the menopausal transition to be associated with undesirable changes in mood3,4; some experts have described the endocrine shifts that accompany menopause as “hormonal chaos.”5
Details of the study
Participants were 42 to 52 years old and had an intact uterus and at least one ovary at entry. They were followed for 4 years, with delineation of the menopausal stage (i.e., premenopause, early and late perimenopause, and menopause) and assessment of hormone use prior to the final menstrual period and after menopause. The outcome was longitudinal performance in three cognitive domains:
- processing speed—assessed using the Symbol Digit Modalities Test. Premenopausal, early perimenopausal, and postmenopausal women improved with repeated administration of this test, but late perimenopausal women did not. Prior use of HT improved the score, whereas late use of HT reduced it.
- verbal memory—evaluated via the East Boston Memory Test. Test scores increased during premenopause and postmenopause but not during early or late perimenopause. Prior use of HT improved the test score, but late use reduced the score.
- working memory—assessed using the Digit Span Backward test. This domain did not vary by stage of menopause.
Participants who initiated menopausal HT or oral contraceptives prior to the last menstrual period were excluded from the analysis during use of HT. They were allowed to reenter the study, however, once HT or oral contraceptives were discontinued.
As the cognitive component of SWAN began, the mean age of participants was 50 years, and 8% were premenopausal, 49% were early perimenopausal, 12% were late perimenopausal, and 27% were postmenopausal. In addition, 4% were both postmenopausal and current users of HT.
Because perimenopausal HT should include a dosage of progestin adequate to suppress ovulation in order to prevent iatrogenic irregular uterine bleeding, women who are healthy, lean, nonsmoking, and still having menstrual periods can safely use a conventional oral contraceptive. Options for other symptomatic perimenopausal women include a continuous oral menopausal regimen formulated with 5 μg of ethinyl estradiol and 1 mg of norethindrone acetate (Femhrt 1/5) or 1 mg of estradiol and 0.5 mg of norethindrone acetate (Activella 1/0.5 or generic).—ANDREW M. KAUNITZ, MD
1. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477.
2. Manson JE, Allison MA, Rossouw JE, et al. For the WHI and WHI-CACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591-2602.
3. Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with the menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007;110(2 Pt 1):230-240.
4. Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell S. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2008;15:223-232.
5. Berga SL. Disordered folliculogenesis during the menopausal transition: explaining chaos. Menopause. 2009;16:11-12.
1. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477.
2. Manson JE, Allison MA, Rossouw JE, et al. For the WHI and WHI-CACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591-2602.
3. Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with the menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007;110(2 Pt 1):230-240.
4. Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell S. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2008;15:223-232.
5. Berga SL. Disordered folliculogenesis during the menopausal transition: explaining chaos. Menopause. 2009;16:11-12.
Is ovarian Ca screening effective in postmenopausal women?
Ovarian cancer is relatively rare, but late diagnosis leads to a higher death rate than the death rate observed with other gynecologic malignancies. Detection at an early stage (I or II) is associated with higher survival than detection at a later stage (III or IV).
Earlier studies have suggested that ovarian cancer screening with the serum biomarker CA 125 and transvaginal ultrasonography (TVS) may help the clinician diagnose ovarian cancer at an earlier stage.
British study finds screening to be effective
In the United Kingdom Collaborative Trial of Ovarian Cancer Screening, postmenopausal women 50 to 74 years old were randomized to one of the following:
- no screening (n=101,359)
- annual assessment of CA 125 using a proprietary risk-of-cancer algorithm, with TVS as a second-line test (multimodal screening, or MMS) (n=50,078)
- annual TVS in a 2:1:1 ratio (n=48,230). (A scan was considered abnormal when one or both ovaries had complex morphology or a simple cyst exceeded 60 cm3 in size.)
The mean age at screening was 60.6 years, and 96.5% of participants were white.
Women who underwent MMS were triaged according to their estimated risk of developing ovarian cancer, based on the CA 125 level and age-specific risk estimates. Women in the group with the lowest risk of ovarian cancer continued annual assessment of CA 125, whereas those at highest risk underwent repeat measurement of CA 125, followed by TVS if the repeat assay suggested elevated risk. If TVS findings were also abnormal, the patient underwent clinical evaluation.
In the MMS and TVS groups, 8.7% and 12.0% of subjects, respectively, underwent clinical assessment, and surgery was performed in 0.2% and 1.8%, respectively. A primary ovarian or tubal cancer was diagnosed in 42 and 45 women in the MMS and TVS groups, respectively. In addition, 8 (MMS) and 20 (TVS) borderline malignancies were identified.
Overall, 48.3% of invasive cancers were diagnosed during stage I or II, with no difference in the distribution of stages between MMS and TVS groups. The positive predictive value (PPV) of MMS and TVS for primary invasive epithelial and tubal cancers was 35.1% and 2.8%, respectively. The ratio of surgery to case of invasive ovarian cancer was 2.9:1 (MMS) and 35:1 (TVS).
In the US study, women underwent both CA 125 assessment and TVS
The US study involved four annual rounds of screening in women 55 to 74 years old, who underwent both CA 125 measurement and TVS imaging in each round. A CA 125 level of 35 U/mL or above, or ovarian volume greater than 10 cm3 (or detection of an ovarian cyst with complex morphology) was considered abnormal.
Of 34,621 women randomized to screening, 30,630 underwent at least one screen during the four rounds. Almost two thirds of participants were 55 to 64 years old, and almost 89% were non-Hispanic white.
The percentage of women who had at least one positive screen decreased over the four rounds of screening, from 5.8% in year 1 to 4.9%, 4.6%, and 4.5% in years 2 through 4, respectively. In each round of screening, TVS was more likely to be positive than was CA 125 measurement (e.g., 4.6% vs 1.4% in the first round).
Of the 28,746 women who underwent the initial (prevalence) screen, 1,675 (5.8%) had positive findings, 566 (1.97% of those who were screened) underwent surgery, and 27 neoplasms were detected (0.06% of those who were screened), including 18 ovarian or primary peritoneal invasive cancers. Nine borderline tumors were also identified.
The PPV of a positive screen in the first round was 1.1%. Of the 18 invasive cancers identified in this round, 16.7% were diagnosed during stage I or II, and 83.4% were identified during stage III or IV. The ratio of surgery to cases of invasive ovarian cancer was 31.4:1.
Why did the trials have different findings?
The findings of the UK study suggest that an MMS strategy of CA 125 assessment, followed selectively by TVS, can detect early-stage ovarian cancer with an acceptable PPV and ratio of surgery to case of invasive cancer.
In contrast, the US findings are discouraging because of the low PPV, the large percentage of malignancies detected at an advanced stage, and the high ratio of surgery to cases of invasive cancer.
Although the studies had different primary outcomes—ovarian and tubal cancers in the UK and ovarian and primary peritoneal cancers in the US—a majority of invasive malignancies detected in both studies were ovarian.
In the US study, the poor performance of screening may be due, in part, to universal rather than selective use of TVS; that modality generates substantially more false positives than does CA 125. The US study also defined an ovarian abnormality more broadly (volume greater than 10 cm3) than the British study did (volume greater than 60 cm3), which may have lowered the PPV in the US trial.
As investigators in the UK continue to follow participants and report their findings, we will learn more about the value of MMS, including its impact on cancer mortality, possibly as soon as 2014.
Until we have further data on the value of ovarian cancer screening in postmenopausal women, we should continue the current practice of screening only symptomatic or very-high-risk women for ovarian cancer.—ANDREW M. KAUNITZ, MD
Ovarian cancer is relatively rare, but late diagnosis leads to a higher death rate than the death rate observed with other gynecologic malignancies. Detection at an early stage (I or II) is associated with higher survival than detection at a later stage (III or IV).
Earlier studies have suggested that ovarian cancer screening with the serum biomarker CA 125 and transvaginal ultrasonography (TVS) may help the clinician diagnose ovarian cancer at an earlier stage.
British study finds screening to be effective
In the United Kingdom Collaborative Trial of Ovarian Cancer Screening, postmenopausal women 50 to 74 years old were randomized to one of the following:
- no screening (n=101,359)
- annual assessment of CA 125 using a proprietary risk-of-cancer algorithm, with TVS as a second-line test (multimodal screening, or MMS) (n=50,078)
- annual TVS in a 2:1:1 ratio (n=48,230). (A scan was considered abnormal when one or both ovaries had complex morphology or a simple cyst exceeded 60 cm3 in size.)
The mean age at screening was 60.6 years, and 96.5% of participants were white.
Women who underwent MMS were triaged according to their estimated risk of developing ovarian cancer, based on the CA 125 level and age-specific risk estimates. Women in the group with the lowest risk of ovarian cancer continued annual assessment of CA 125, whereas those at highest risk underwent repeat measurement of CA 125, followed by TVS if the repeat assay suggested elevated risk. If TVS findings were also abnormal, the patient underwent clinical evaluation.
In the MMS and TVS groups, 8.7% and 12.0% of subjects, respectively, underwent clinical assessment, and surgery was performed in 0.2% and 1.8%, respectively. A primary ovarian or tubal cancer was diagnosed in 42 and 45 women in the MMS and TVS groups, respectively. In addition, 8 (MMS) and 20 (TVS) borderline malignancies were identified.
Overall, 48.3% of invasive cancers were diagnosed during stage I or II, with no difference in the distribution of stages between MMS and TVS groups. The positive predictive value (PPV) of MMS and TVS for primary invasive epithelial and tubal cancers was 35.1% and 2.8%, respectively. The ratio of surgery to case of invasive ovarian cancer was 2.9:1 (MMS) and 35:1 (TVS).
In the US study, women underwent both CA 125 assessment and TVS
The US study involved four annual rounds of screening in women 55 to 74 years old, who underwent both CA 125 measurement and TVS imaging in each round. A CA 125 level of 35 U/mL or above, or ovarian volume greater than 10 cm3 (or detection of an ovarian cyst with complex morphology) was considered abnormal.
Of 34,621 women randomized to screening, 30,630 underwent at least one screen during the four rounds. Almost two thirds of participants were 55 to 64 years old, and almost 89% were non-Hispanic white.
The percentage of women who had at least one positive screen decreased over the four rounds of screening, from 5.8% in year 1 to 4.9%, 4.6%, and 4.5% in years 2 through 4, respectively. In each round of screening, TVS was more likely to be positive than was CA 125 measurement (e.g., 4.6% vs 1.4% in the first round).
Of the 28,746 women who underwent the initial (prevalence) screen, 1,675 (5.8%) had positive findings, 566 (1.97% of those who were screened) underwent surgery, and 27 neoplasms were detected (0.06% of those who were screened), including 18 ovarian or primary peritoneal invasive cancers. Nine borderline tumors were also identified.
The PPV of a positive screen in the first round was 1.1%. Of the 18 invasive cancers identified in this round, 16.7% were diagnosed during stage I or II, and 83.4% were identified during stage III or IV. The ratio of surgery to cases of invasive ovarian cancer was 31.4:1.
Why did the trials have different findings?
The findings of the UK study suggest that an MMS strategy of CA 125 assessment, followed selectively by TVS, can detect early-stage ovarian cancer with an acceptable PPV and ratio of surgery to case of invasive cancer.
In contrast, the US findings are discouraging because of the low PPV, the large percentage of malignancies detected at an advanced stage, and the high ratio of surgery to cases of invasive cancer.
Although the studies had different primary outcomes—ovarian and tubal cancers in the UK and ovarian and primary peritoneal cancers in the US—a majority of invasive malignancies detected in both studies were ovarian.
In the US study, the poor performance of screening may be due, in part, to universal rather than selective use of TVS; that modality generates substantially more false positives than does CA 125. The US study also defined an ovarian abnormality more broadly (volume greater than 10 cm3) than the British study did (volume greater than 60 cm3), which may have lowered the PPV in the US trial.
As investigators in the UK continue to follow participants and report their findings, we will learn more about the value of MMS, including its impact on cancer mortality, possibly as soon as 2014.
Until we have further data on the value of ovarian cancer screening in postmenopausal women, we should continue the current practice of screening only symptomatic or very-high-risk women for ovarian cancer.—ANDREW M. KAUNITZ, MD
Ovarian cancer is relatively rare, but late diagnosis leads to a higher death rate than the death rate observed with other gynecologic malignancies. Detection at an early stage (I or II) is associated with higher survival than detection at a later stage (III or IV).
Earlier studies have suggested that ovarian cancer screening with the serum biomarker CA 125 and transvaginal ultrasonography (TVS) may help the clinician diagnose ovarian cancer at an earlier stage.
British study finds screening to be effective
In the United Kingdom Collaborative Trial of Ovarian Cancer Screening, postmenopausal women 50 to 74 years old were randomized to one of the following:
- no screening (n=101,359)
- annual assessment of CA 125 using a proprietary risk-of-cancer algorithm, with TVS as a second-line test (multimodal screening, or MMS) (n=50,078)
- annual TVS in a 2:1:1 ratio (n=48,230). (A scan was considered abnormal when one or both ovaries had complex morphology or a simple cyst exceeded 60 cm3 in size.)
The mean age at screening was 60.6 years, and 96.5% of participants were white.
Women who underwent MMS were triaged according to their estimated risk of developing ovarian cancer, based on the CA 125 level and age-specific risk estimates. Women in the group with the lowest risk of ovarian cancer continued annual assessment of CA 125, whereas those at highest risk underwent repeat measurement of CA 125, followed by TVS if the repeat assay suggested elevated risk. If TVS findings were also abnormal, the patient underwent clinical evaluation.
In the MMS and TVS groups, 8.7% and 12.0% of subjects, respectively, underwent clinical assessment, and surgery was performed in 0.2% and 1.8%, respectively. A primary ovarian or tubal cancer was diagnosed in 42 and 45 women in the MMS and TVS groups, respectively. In addition, 8 (MMS) and 20 (TVS) borderline malignancies were identified.
Overall, 48.3% of invasive cancers were diagnosed during stage I or II, with no difference in the distribution of stages between MMS and TVS groups. The positive predictive value (PPV) of MMS and TVS for primary invasive epithelial and tubal cancers was 35.1% and 2.8%, respectively. The ratio of surgery to case of invasive ovarian cancer was 2.9:1 (MMS) and 35:1 (TVS).
In the US study, women underwent both CA 125 assessment and TVS
The US study involved four annual rounds of screening in women 55 to 74 years old, who underwent both CA 125 measurement and TVS imaging in each round. A CA 125 level of 35 U/mL or above, or ovarian volume greater than 10 cm3 (or detection of an ovarian cyst with complex morphology) was considered abnormal.
Of 34,621 women randomized to screening, 30,630 underwent at least one screen during the four rounds. Almost two thirds of participants were 55 to 64 years old, and almost 89% were non-Hispanic white.
The percentage of women who had at least one positive screen decreased over the four rounds of screening, from 5.8% in year 1 to 4.9%, 4.6%, and 4.5% in years 2 through 4, respectively. In each round of screening, TVS was more likely to be positive than was CA 125 measurement (e.g., 4.6% vs 1.4% in the first round).
Of the 28,746 women who underwent the initial (prevalence) screen, 1,675 (5.8%) had positive findings, 566 (1.97% of those who were screened) underwent surgery, and 27 neoplasms were detected (0.06% of those who were screened), including 18 ovarian or primary peritoneal invasive cancers. Nine borderline tumors were also identified.
The PPV of a positive screen in the first round was 1.1%. Of the 18 invasive cancers identified in this round, 16.7% were diagnosed during stage I or II, and 83.4% were identified during stage III or IV. The ratio of surgery to cases of invasive ovarian cancer was 31.4:1.
Why did the trials have different findings?
The findings of the UK study suggest that an MMS strategy of CA 125 assessment, followed selectively by TVS, can detect early-stage ovarian cancer with an acceptable PPV and ratio of surgery to case of invasive cancer.
In contrast, the US findings are discouraging because of the low PPV, the large percentage of malignancies detected at an advanced stage, and the high ratio of surgery to cases of invasive cancer.
Although the studies had different primary outcomes—ovarian and tubal cancers in the UK and ovarian and primary peritoneal cancers in the US—a majority of invasive malignancies detected in both studies were ovarian.
In the US study, the poor performance of screening may be due, in part, to universal rather than selective use of TVS; that modality generates substantially more false positives than does CA 125. The US study also defined an ovarian abnormality more broadly (volume greater than 10 cm3) than the British study did (volume greater than 60 cm3), which may have lowered the PPV in the US trial.
As investigators in the UK continue to follow participants and report their findings, we will learn more about the value of MMS, including its impact on cancer mortality, possibly as soon as 2014.
Until we have further data on the value of ovarian cancer screening in postmenopausal women, we should continue the current practice of screening only symptomatic or very-high-risk women for ovarian cancer.—ANDREW M. KAUNITZ, MD
UPDATE: MENOPAUSE
Baby Boomers have transformed attitudes toward many aspects of aging. Menopause is no exception. Once a taboo topic, menopause is now openly discussed among women who seek information about vasomotor symptoms, hormones and their alternatives, and ways to maintain health as they move past midlife. ObGyns are treating more and more of these women, and fielding their many questions.
In this Update, I examine recent data on three important aspects of menopause:
- how to reduce the risk of cardiovascular disease among women who enter menopause surgically, through oophorectomy
- what to offer women who ask for nonhormonal relief from vasomotor symptoms
- a new drug on the horizon to combat osteoporosis.
Bilateral oophorectomy raises young women’s risk of cardiovascular death
Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23.
Parker WH, Manson JE. Oophorectomy and cardiovascular mortality: is there a link? Menopause. 2009;16:1–2.
Cardiovascular mortality does not increase among women who undergo unilateral oophorectomy, but it does rise among women who undergo bilateral oophorectomy before 45 years of age. However, among women who initiate estrogen therapy at the time of bilateral oophorectomy and continue that therapy through at least 45 years of age, no excess cardiovascular mortality occurs.
Those are the findings of a unique retrospective cohort study performed by investigators from the Mayo Clinic. In the study, investigators reviewed the death certificates of more than 2,300 women who underwent unilateral or bilateral oophorectomy for benign disease before menopause in Olmstead County, Minnesota, from 1950 to 1987. They also followed a similar number of age-matched women for several decades.
These results support the findings of other studies that have observed that menopausal hormone therapy is associated with a lower incidence of cardiovascular death in “young” menopausal women, including those in their 50s or within one decade of the onset of menopause.1,2
More than 500,000 women undergo bilateral oophorectomy each year in the United States, usually in association with hysterectomy for benign disease.
Induced menopause merits special attention
Spontaneous menopause is physiologic. In contrast, induced menopause (whether associated with surgery, radiation therapy, or chemotherapy) and premature ovarian failure are pathologic conditions.3 Unless they are managed appropriately, induced menopause and premature ovarian failure raise the risk of cardiovascular disease.
Since the initial findings of the Women’s Health Initiative trial of estrogen–progestin therapy were published in 2002, many women and clinicians have become wary about the use of hormone therapy, even among young women who have no ovarian function and who lack a contraindication to hormone therapy.4 Unless hormonal management is contraindicated, it is recommended in this setting.
In addition, Parker and Manson recommend that gynecologic surgeons who routinely perform bilateral oophorectomy at the time of hysterectomy for benign disease in premenopausal women who do not have an elevated risk of ovarian cancer should consider updating their therapeutic recommendations and, whenever possible, preserving the ovaries.
Interest in nonhormonal therapies for hot flashes remains high
Bair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopausal transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause. 2008;15:32–43.
Butt DA, Lock M, Lewis JE, Ross S, Moineddin R. Gabapentin for the treatment of menopausal hot flashes: a randomized trial. Menopause. 2008;15:310–318.
More than three quarters of women use some type of complementary and alternative medicine (CAM) during the menopausal transition. So found a survey conducted as part of the Study of Women’s Health Across the Nation (SWAN), which involved more than 2,000 premenopausal and perimenopausal women.
More than one third of all US women use one or more forms of CAM, spending more than $600 million a year.
In the SWAN survey, Japanese and white women were significantly more likely to report use of CAM than were Chinese, African-American, and Hispanic women during menopause.
Some women use both CAM and hormones
A notable finding of this report from SWAN is that concomitant use of menopausal hormone therapy and CAM is common among symptomatic women, even though herbal therapies have not been proved to be more effective than placebo in the treatment of vasomotor symptoms.5
Given the high prevalence of use of CAM, ObGyns should recognize that symptomatic patients especially bothered by vasomotor symptoms may seek relief with both CAM and a prescription medication. For this reason, it is wise to ask perimenopausal and postmenopausal women to list all the remedies they use—both prescription and over the counter.
Gabapentin eases symptoms in some women
Along with the antidepressants paroxetine and venlafaxine, gabapentin is the most widely studied prescription agent used in the nonhormonal treatment of menopausal vasomotor symptoms.
Canadian investigators performed a double-blind, placebo-controlled trial involving 197 symptomatic, spontaneously menopausal women (age range: 45 to 65 years). Participants were randomized to gabapentin (300 mg) or placebo, given three times daily for 4 weeks.
Ten of the 99 women who were randomized to gabapentin and six of the 98 women who were randomized to placebo dropped out of the study because of adverse effects. Hot-flash scores decreased by 51% among women taking gabapentin and by 26% among women in the placebo arm (p<.001). Women who used gabapentin were significantly more likely to report dizziness, drowsiness, and unsteadiness than those who received placebo.
Despite its proven efficacy, gabapentin does not appear to equal estrogen in its ability to alleviate vasomotor symptoms. Further, gabapentin’s side-effect profile and the need for thrice-daily administration also limit its appeal. Nevertheless, symptomatic women who are interested in nonhormonal treatment may wish to consider this option.
Anti-osteoporosis arsenal may gain a new weapon
Cummings S, McClung M, Christiansen C, et al. A phase III study of the effects of denosumab on vertebral, nonvertebral, and hip fracture in women with osteoporosis: results from the FREEDOM Trial. Paper presented at: Annual Meeting of the American Society for Bone and Mineral Research; September 16, 2008; Montreal, Canada.
Miller PD, Bolognese MA, Lewiecki EM, et al; AMG Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized, blinded phase 2 clinical trial. Bone. 2008;43:222–229.
In a 3-year study in which more than 7,800 women 60 to 90 years old were randomized to denosumab (60 mg) or placebo, denosumab reduced the risk of vertebral fracture by 68% and the risk of hip fracture by 40%.
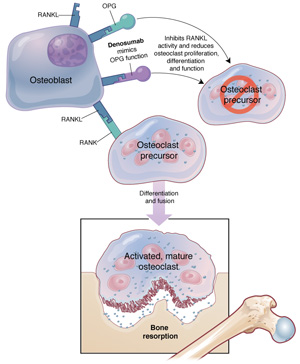
FIGURE A new agent to inhibit bone resorption
RankL is a protein that controls osteoclast maturation, activity, and survival. Denosumab mimics the endogenous decoy receptor OPG to inhibit RankL and reduce osteoclast proliferation, differentiation, and function. Denosumab is a monoclonal antibody to RankL, a mediator of osteoclast function ( FIGURE ). In contrast to alendronate, which enhances trabecular bone density (e.g., that found in the spine), denosumab increases cortical (as in long bones) as well as trabecular bone density.6 If the agent obtains approval by the US Food and Drug Administration, it will be an important new approach to the treatment of osteoporosis in menopausal women.7
The drug is administered every 6 months by subcutaneous injection.
Like estrogen, denosumab has rapidly reversible effects
The trial by Miller and associates found that the effects of denosumab are rapidly reversible after discontinuation of therapy, in contrast to alendronate. As Goldstein noted in “Update on Osteoporosis” in the November 2008 issue of OBG Management, clinicians are still exploring the relationships between alendronate and osteonecrosis of the jaw and proximal femoral-shaft fracture.7 Only further research and surveillance will clarify the clinical pros and cons of denosumab’s swift reversibility.
1. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477.
2. Manson JE, Allison MA, Rossouw JE, et al. WHI and WHICACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591-2602.
3. Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606-614.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
5. Kaunitz AM. Effective herbal treatment of vasomotor symptoms—are we any closer? Menopause. 2009; Jan 21 [Epub ahead of print].
6. McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821-832.
7. Goldstein SR. Update on osteoporosis. OBG Management. 2008;20(11):41-46.
Baby Boomers have transformed attitudes toward many aspects of aging. Menopause is no exception. Once a taboo topic, menopause is now openly discussed among women who seek information about vasomotor symptoms, hormones and their alternatives, and ways to maintain health as they move past midlife. ObGyns are treating more and more of these women, and fielding their many questions.
In this Update, I examine recent data on three important aspects of menopause:
- how to reduce the risk of cardiovascular disease among women who enter menopause surgically, through oophorectomy
- what to offer women who ask for nonhormonal relief from vasomotor symptoms
- a new drug on the horizon to combat osteoporosis.
Bilateral oophorectomy raises young women’s risk of cardiovascular death
Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23.
Parker WH, Manson JE. Oophorectomy and cardiovascular mortality: is there a link? Menopause. 2009;16:1–2.
Cardiovascular mortality does not increase among women who undergo unilateral oophorectomy, but it does rise among women who undergo bilateral oophorectomy before 45 years of age. However, among women who initiate estrogen therapy at the time of bilateral oophorectomy and continue that therapy through at least 45 years of age, no excess cardiovascular mortality occurs.
Those are the findings of a unique retrospective cohort study performed by investigators from the Mayo Clinic. In the study, investigators reviewed the death certificates of more than 2,300 women who underwent unilateral or bilateral oophorectomy for benign disease before menopause in Olmstead County, Minnesota, from 1950 to 1987. They also followed a similar number of age-matched women for several decades.
These results support the findings of other studies that have observed that menopausal hormone therapy is associated with a lower incidence of cardiovascular death in “young” menopausal women, including those in their 50s or within one decade of the onset of menopause.1,2
More than 500,000 women undergo bilateral oophorectomy each year in the United States, usually in association with hysterectomy for benign disease.
Induced menopause merits special attention
Spontaneous menopause is physiologic. In contrast, induced menopause (whether associated with surgery, radiation therapy, or chemotherapy) and premature ovarian failure are pathologic conditions.3 Unless they are managed appropriately, induced menopause and premature ovarian failure raise the risk of cardiovascular disease.
Since the initial findings of the Women’s Health Initiative trial of estrogen–progestin therapy were published in 2002, many women and clinicians have become wary about the use of hormone therapy, even among young women who have no ovarian function and who lack a contraindication to hormone therapy.4 Unless hormonal management is contraindicated, it is recommended in this setting.
In addition, Parker and Manson recommend that gynecologic surgeons who routinely perform bilateral oophorectomy at the time of hysterectomy for benign disease in premenopausal women who do not have an elevated risk of ovarian cancer should consider updating their therapeutic recommendations and, whenever possible, preserving the ovaries.
Interest in nonhormonal therapies for hot flashes remains high
Bair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopausal transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause. 2008;15:32–43.
Butt DA, Lock M, Lewis JE, Ross S, Moineddin R. Gabapentin for the treatment of menopausal hot flashes: a randomized trial. Menopause. 2008;15:310–318.
More than three quarters of women use some type of complementary and alternative medicine (CAM) during the menopausal transition. So found a survey conducted as part of the Study of Women’s Health Across the Nation (SWAN), which involved more than 2,000 premenopausal and perimenopausal women.
More than one third of all US women use one or more forms of CAM, spending more than $600 million a year.
In the SWAN survey, Japanese and white women were significantly more likely to report use of CAM than were Chinese, African-American, and Hispanic women during menopause.
Some women use both CAM and hormones
A notable finding of this report from SWAN is that concomitant use of menopausal hormone therapy and CAM is common among symptomatic women, even though herbal therapies have not been proved to be more effective than placebo in the treatment of vasomotor symptoms.5
Given the high prevalence of use of CAM, ObGyns should recognize that symptomatic patients especially bothered by vasomotor symptoms may seek relief with both CAM and a prescription medication. For this reason, it is wise to ask perimenopausal and postmenopausal women to list all the remedies they use—both prescription and over the counter.
Gabapentin eases symptoms in some women
Along with the antidepressants paroxetine and venlafaxine, gabapentin is the most widely studied prescription agent used in the nonhormonal treatment of menopausal vasomotor symptoms.
Canadian investigators performed a double-blind, placebo-controlled trial involving 197 symptomatic, spontaneously menopausal women (age range: 45 to 65 years). Participants were randomized to gabapentin (300 mg) or placebo, given three times daily for 4 weeks.
Ten of the 99 women who were randomized to gabapentin and six of the 98 women who were randomized to placebo dropped out of the study because of adverse effects. Hot-flash scores decreased by 51% among women taking gabapentin and by 26% among women in the placebo arm (p<.001). Women who used gabapentin were significantly more likely to report dizziness, drowsiness, and unsteadiness than those who received placebo.
Despite its proven efficacy, gabapentin does not appear to equal estrogen in its ability to alleviate vasomotor symptoms. Further, gabapentin’s side-effect profile and the need for thrice-daily administration also limit its appeal. Nevertheless, symptomatic women who are interested in nonhormonal treatment may wish to consider this option.
Anti-osteoporosis arsenal may gain a new weapon
Cummings S, McClung M, Christiansen C, et al. A phase III study of the effects of denosumab on vertebral, nonvertebral, and hip fracture in women with osteoporosis: results from the FREEDOM Trial. Paper presented at: Annual Meeting of the American Society for Bone and Mineral Research; September 16, 2008; Montreal, Canada.
Miller PD, Bolognese MA, Lewiecki EM, et al; AMG Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized, blinded phase 2 clinical trial. Bone. 2008;43:222–229.
In a 3-year study in which more than 7,800 women 60 to 90 years old were randomized to denosumab (60 mg) or placebo, denosumab reduced the risk of vertebral fracture by 68% and the risk of hip fracture by 40%.

FIGURE A new agent to inhibit bone resorption
RankL is a protein that controls osteoclast maturation, activity, and survival. Denosumab mimics the endogenous decoy receptor OPG to inhibit RankL and reduce osteoclast proliferation, differentiation, and function. Denosumab is a monoclonal antibody to RankL, a mediator of osteoclast function ( FIGURE ). In contrast to alendronate, which enhances trabecular bone density (e.g., that found in the spine), denosumab increases cortical (as in long bones) as well as trabecular bone density.6 If the agent obtains approval by the US Food and Drug Administration, it will be an important new approach to the treatment of osteoporosis in menopausal women.7
The drug is administered every 6 months by subcutaneous injection.
Like estrogen, denosumab has rapidly reversible effects
The trial by Miller and associates found that the effects of denosumab are rapidly reversible after discontinuation of therapy, in contrast to alendronate. As Goldstein noted in “Update on Osteoporosis” in the November 2008 issue of OBG Management, clinicians are still exploring the relationships between alendronate and osteonecrosis of the jaw and proximal femoral-shaft fracture.7 Only further research and surveillance will clarify the clinical pros and cons of denosumab’s swift reversibility.
Baby Boomers have transformed attitudes toward many aspects of aging. Menopause is no exception. Once a taboo topic, menopause is now openly discussed among women who seek information about vasomotor symptoms, hormones and their alternatives, and ways to maintain health as they move past midlife. ObGyns are treating more and more of these women, and fielding their many questions.
In this Update, I examine recent data on three important aspects of menopause:
- how to reduce the risk of cardiovascular disease among women who enter menopause surgically, through oophorectomy
- what to offer women who ask for nonhormonal relief from vasomotor symptoms
- a new drug on the horizon to combat osteoporosis.
Bilateral oophorectomy raises young women’s risk of cardiovascular death
Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23.
Parker WH, Manson JE. Oophorectomy and cardiovascular mortality: is there a link? Menopause. 2009;16:1–2.
Cardiovascular mortality does not increase among women who undergo unilateral oophorectomy, but it does rise among women who undergo bilateral oophorectomy before 45 years of age. However, among women who initiate estrogen therapy at the time of bilateral oophorectomy and continue that therapy through at least 45 years of age, no excess cardiovascular mortality occurs.
Those are the findings of a unique retrospective cohort study performed by investigators from the Mayo Clinic. In the study, investigators reviewed the death certificates of more than 2,300 women who underwent unilateral or bilateral oophorectomy for benign disease before menopause in Olmstead County, Minnesota, from 1950 to 1987. They also followed a similar number of age-matched women for several decades.
These results support the findings of other studies that have observed that menopausal hormone therapy is associated with a lower incidence of cardiovascular death in “young” menopausal women, including those in their 50s or within one decade of the onset of menopause.1,2
More than 500,000 women undergo bilateral oophorectomy each year in the United States, usually in association with hysterectomy for benign disease.
Induced menopause merits special attention
Spontaneous menopause is physiologic. In contrast, induced menopause (whether associated with surgery, radiation therapy, or chemotherapy) and premature ovarian failure are pathologic conditions.3 Unless they are managed appropriately, induced menopause and premature ovarian failure raise the risk of cardiovascular disease.
Since the initial findings of the Women’s Health Initiative trial of estrogen–progestin therapy were published in 2002, many women and clinicians have become wary about the use of hormone therapy, even among young women who have no ovarian function and who lack a contraindication to hormone therapy.4 Unless hormonal management is contraindicated, it is recommended in this setting.
In addition, Parker and Manson recommend that gynecologic surgeons who routinely perform bilateral oophorectomy at the time of hysterectomy for benign disease in premenopausal women who do not have an elevated risk of ovarian cancer should consider updating their therapeutic recommendations and, whenever possible, preserving the ovaries.
Interest in nonhormonal therapies for hot flashes remains high
Bair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopausal transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause. 2008;15:32–43.
Butt DA, Lock M, Lewis JE, Ross S, Moineddin R. Gabapentin for the treatment of menopausal hot flashes: a randomized trial. Menopause. 2008;15:310–318.
More than three quarters of women use some type of complementary and alternative medicine (CAM) during the menopausal transition. So found a survey conducted as part of the Study of Women’s Health Across the Nation (SWAN), which involved more than 2,000 premenopausal and perimenopausal women.
More than one third of all US women use one or more forms of CAM, spending more than $600 million a year.
In the SWAN survey, Japanese and white women were significantly more likely to report use of CAM than were Chinese, African-American, and Hispanic women during menopause.
Some women use both CAM and hormones
A notable finding of this report from SWAN is that concomitant use of menopausal hormone therapy and CAM is common among symptomatic women, even though herbal therapies have not been proved to be more effective than placebo in the treatment of vasomotor symptoms.5
Given the high prevalence of use of CAM, ObGyns should recognize that symptomatic patients especially bothered by vasomotor symptoms may seek relief with both CAM and a prescription medication. For this reason, it is wise to ask perimenopausal and postmenopausal women to list all the remedies they use—both prescription and over the counter.
Gabapentin eases symptoms in some women
Along with the antidepressants paroxetine and venlafaxine, gabapentin is the most widely studied prescription agent used in the nonhormonal treatment of menopausal vasomotor symptoms.
Canadian investigators performed a double-blind, placebo-controlled trial involving 197 symptomatic, spontaneously menopausal women (age range: 45 to 65 years). Participants were randomized to gabapentin (300 mg) or placebo, given three times daily for 4 weeks.
Ten of the 99 women who were randomized to gabapentin and six of the 98 women who were randomized to placebo dropped out of the study because of adverse effects. Hot-flash scores decreased by 51% among women taking gabapentin and by 26% among women in the placebo arm (p<.001). Women who used gabapentin were significantly more likely to report dizziness, drowsiness, and unsteadiness than those who received placebo.
Despite its proven efficacy, gabapentin does not appear to equal estrogen in its ability to alleviate vasomotor symptoms. Further, gabapentin’s side-effect profile and the need for thrice-daily administration also limit its appeal. Nevertheless, symptomatic women who are interested in nonhormonal treatment may wish to consider this option.
Anti-osteoporosis arsenal may gain a new weapon
Cummings S, McClung M, Christiansen C, et al. A phase III study of the effects of denosumab on vertebral, nonvertebral, and hip fracture in women with osteoporosis: results from the FREEDOM Trial. Paper presented at: Annual Meeting of the American Society for Bone and Mineral Research; September 16, 2008; Montreal, Canada.
Miller PD, Bolognese MA, Lewiecki EM, et al; AMG Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized, blinded phase 2 clinical trial. Bone. 2008;43:222–229.
In a 3-year study in which more than 7,800 women 60 to 90 years old were randomized to denosumab (60 mg) or placebo, denosumab reduced the risk of vertebral fracture by 68% and the risk of hip fracture by 40%.

FIGURE A new agent to inhibit bone resorption
RankL is a protein that controls osteoclast maturation, activity, and survival. Denosumab mimics the endogenous decoy receptor OPG to inhibit RankL and reduce osteoclast proliferation, differentiation, and function. Denosumab is a monoclonal antibody to RankL, a mediator of osteoclast function ( FIGURE ). In contrast to alendronate, which enhances trabecular bone density (e.g., that found in the spine), denosumab increases cortical (as in long bones) as well as trabecular bone density.6 If the agent obtains approval by the US Food and Drug Administration, it will be an important new approach to the treatment of osteoporosis in menopausal women.7
The drug is administered every 6 months by subcutaneous injection.
Like estrogen, denosumab has rapidly reversible effects
The trial by Miller and associates found that the effects of denosumab are rapidly reversible after discontinuation of therapy, in contrast to alendronate. As Goldstein noted in “Update on Osteoporosis” in the November 2008 issue of OBG Management, clinicians are still exploring the relationships between alendronate and osteonecrosis of the jaw and proximal femoral-shaft fracture.7 Only further research and surveillance will clarify the clinical pros and cons of denosumab’s swift reversibility.
1. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477.
2. Manson JE, Allison MA, Rossouw JE, et al. WHI and WHICACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591-2602.
3. Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606-614.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
5. Kaunitz AM. Effective herbal treatment of vasomotor symptoms—are we any closer? Menopause. 2009; Jan 21 [Epub ahead of print].
6. McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821-832.
7. Goldstein SR. Update on osteoporosis. OBG Management. 2008;20(11):41-46.
1. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477.
2. Manson JE, Allison MA, Rossouw JE, et al. WHI and WHICACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591-2602.
3. Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606-614.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
5. Kaunitz AM. Effective herbal treatment of vasomotor symptoms—are we any closer? Menopause. 2009; Jan 21 [Epub ahead of print].
6. McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821-832.
7. Goldstein SR. Update on osteoporosis. OBG Management. 2008;20(11):41-46.
What is the optimal timing of elective cesarean delivery at term?
Unless fetal lung maturity has been confirmed, elective cesarean delivery before 39 weeks’ gestation is associated with a higher rate of neonatal respiratory problems. In this observational study, performed at 19 US centers from 1999 to 2002, and funded by the National Institutes of Health, infants of women who underwent elective cesarean delivery at 37 or more weeks’ gestation were assessed for the primary outcome—a composite neonatal outcome that included the occurrence of any of the following:
- death
- respiratory distress syndrome
- transient tachypnea of the newborn
- hypoglycemia
- newborn sepsis
- confirmed seizures
- necrotizing enterocolitis
- hypoxic–ischemic encephalopathy
- cardiopulmonary resuscitation or ventilator support within 24 hours of birth
- umbilical cord–blood arterial pH
- 5-minute Apgar score of 3 or below
- admission to NICU
- hospitalization for 5 or more days.
Of 13,258 elective cesarean deliveries performed at term, 35.8% occurred before 39 completed weeks of gestation (6.3% at 37 weeks, 29.5% at 38 weeks) and 49.1% at 39 weeks. Women who delivered before 39 weeks were more likely to be married, white, and to have initiated prenatal care early.
Compared with infants delivered at 39 weeks, those born at 37 to 38 weeks’ gestation had a greater risk of the primary (composite) outcome. At 37 weeks, the adjusted odds ratio (OR) was 2.1 (95% confidence interval [CI], 1.7–2.5). At 38 weeks, the adjusted OR was 1.5 (1.3–1.7).
The authors estimated that, at 37 weeks’ gestation, postponing elective delivery until 39 weeks might prevent 48% of cases of the primary outcome; this percentage was estimated to be 27% at 38 weeks’ gestation.
Patient preference determines timing in some cases
Cesarean delivery accounts for almost one third of US births, and most women who have had such a delivery opt to repeat it in their next pregnancy. As an editorial accompanying this article points out, women in this study who delivered before 39 weeks were more likely to be private patients and had likely asked their own obstetrician to perform the delivery.1 Obstetricians who wish to promote patient satisfaction are likely to honor such a request, recognizing that waiting until later would increase the likelihood of labor, which would exclude the possibility of an elective procedure.
Limitations of the study
Because this study lacked data about testing for fetal lung maturity, it is unclear whether the higher rate of adverse outcomes with elective cesarean delivery before 39 weeks could be explained by failure to assess for fetal lung maturity.
It also appears that the delay of delivery to 39 weeks or beyond may be associated with an increased risk of stillbirth. In other populations, this risk has been estimated to be roughly 0.5 of every 1,000 births for each advancing week of gestation.
Obstetricians and their patients should weigh the known risks of elective cesarean delivery before 39 weeks’ gestation against the small risk of late stillbirth. At the same time, it is important to factor in the patient’s preferences about when delivery occurs and who performs it.—ANDREW M. KAUNITZ, MD
1. Greene MF. Making small risks even smaller [editorial]. N Engl J Med. 2009;360:183-184.
Unless fetal lung maturity has been confirmed, elective cesarean delivery before 39 weeks’ gestation is associated with a higher rate of neonatal respiratory problems. In this observational study, performed at 19 US centers from 1999 to 2002, and funded by the National Institutes of Health, infants of women who underwent elective cesarean delivery at 37 or more weeks’ gestation were assessed for the primary outcome—a composite neonatal outcome that included the occurrence of any of the following:
- death
- respiratory distress syndrome
- transient tachypnea of the newborn
- hypoglycemia
- newborn sepsis
- confirmed seizures
- necrotizing enterocolitis
- hypoxic–ischemic encephalopathy
- cardiopulmonary resuscitation or ventilator support within 24 hours of birth
- umbilical cord–blood arterial pH
- 5-minute Apgar score of 3 or below
- admission to NICU
- hospitalization for 5 or more days.
Of 13,258 elective cesarean deliveries performed at term, 35.8% occurred before 39 completed weeks of gestation (6.3% at 37 weeks, 29.5% at 38 weeks) and 49.1% at 39 weeks. Women who delivered before 39 weeks were more likely to be married, white, and to have initiated prenatal care early.
Compared with infants delivered at 39 weeks, those born at 37 to 38 weeks’ gestation had a greater risk of the primary (composite) outcome. At 37 weeks, the adjusted odds ratio (OR) was 2.1 (95% confidence interval [CI], 1.7–2.5). At 38 weeks, the adjusted OR was 1.5 (1.3–1.7).
The authors estimated that, at 37 weeks’ gestation, postponing elective delivery until 39 weeks might prevent 48% of cases of the primary outcome; this percentage was estimated to be 27% at 38 weeks’ gestation.
Patient preference determines timing in some cases
Cesarean delivery accounts for almost one third of US births, and most women who have had such a delivery opt to repeat it in their next pregnancy. As an editorial accompanying this article points out, women in this study who delivered before 39 weeks were more likely to be private patients and had likely asked their own obstetrician to perform the delivery.1 Obstetricians who wish to promote patient satisfaction are likely to honor such a request, recognizing that waiting until later would increase the likelihood of labor, which would exclude the possibility of an elective procedure.
Limitations of the study
Because this study lacked data about testing for fetal lung maturity, it is unclear whether the higher rate of adverse outcomes with elective cesarean delivery before 39 weeks could be explained by failure to assess for fetal lung maturity.
It also appears that the delay of delivery to 39 weeks or beyond may be associated with an increased risk of stillbirth. In other populations, this risk has been estimated to be roughly 0.5 of every 1,000 births for each advancing week of gestation.
Obstetricians and their patients should weigh the known risks of elective cesarean delivery before 39 weeks’ gestation against the small risk of late stillbirth. At the same time, it is important to factor in the patient’s preferences about when delivery occurs and who performs it.—ANDREW M. KAUNITZ, MD
Unless fetal lung maturity has been confirmed, elective cesarean delivery before 39 weeks’ gestation is associated with a higher rate of neonatal respiratory problems. In this observational study, performed at 19 US centers from 1999 to 2002, and funded by the National Institutes of Health, infants of women who underwent elective cesarean delivery at 37 or more weeks’ gestation were assessed for the primary outcome—a composite neonatal outcome that included the occurrence of any of the following:
- death
- respiratory distress syndrome
- transient tachypnea of the newborn
- hypoglycemia
- newborn sepsis
- confirmed seizures
- necrotizing enterocolitis
- hypoxic–ischemic encephalopathy
- cardiopulmonary resuscitation or ventilator support within 24 hours of birth
- umbilical cord–blood arterial pH
- 5-minute Apgar score of 3 or below
- admission to NICU
- hospitalization for 5 or more days.
Of 13,258 elective cesarean deliveries performed at term, 35.8% occurred before 39 completed weeks of gestation (6.3% at 37 weeks, 29.5% at 38 weeks) and 49.1% at 39 weeks. Women who delivered before 39 weeks were more likely to be married, white, and to have initiated prenatal care early.
Compared with infants delivered at 39 weeks, those born at 37 to 38 weeks’ gestation had a greater risk of the primary (composite) outcome. At 37 weeks, the adjusted odds ratio (OR) was 2.1 (95% confidence interval [CI], 1.7–2.5). At 38 weeks, the adjusted OR was 1.5 (1.3–1.7).
The authors estimated that, at 37 weeks’ gestation, postponing elective delivery until 39 weeks might prevent 48% of cases of the primary outcome; this percentage was estimated to be 27% at 38 weeks’ gestation.
Patient preference determines timing in some cases
Cesarean delivery accounts for almost one third of US births, and most women who have had such a delivery opt to repeat it in their next pregnancy. As an editorial accompanying this article points out, women in this study who delivered before 39 weeks were more likely to be private patients and had likely asked their own obstetrician to perform the delivery.1 Obstetricians who wish to promote patient satisfaction are likely to honor such a request, recognizing that waiting until later would increase the likelihood of labor, which would exclude the possibility of an elective procedure.
Limitations of the study
Because this study lacked data about testing for fetal lung maturity, it is unclear whether the higher rate of adverse outcomes with elective cesarean delivery before 39 weeks could be explained by failure to assess for fetal lung maturity.
It also appears that the delay of delivery to 39 weeks or beyond may be associated with an increased risk of stillbirth. In other populations, this risk has been estimated to be roughly 0.5 of every 1,000 births for each advancing week of gestation.
Obstetricians and their patients should weigh the known risks of elective cesarean delivery before 39 weeks’ gestation against the small risk of late stillbirth. At the same time, it is important to factor in the patient’s preferences about when delivery occurs and who performs it.—ANDREW M. KAUNITZ, MD
1. Greene MF. Making small risks even smaller [editorial]. N Engl J Med. 2009;360:183-184.
1. Greene MF. Making small risks even smaller [editorial]. N Engl J Med. 2009;360:183-184.
Is personal distress an important measure when assessing sexual dysfunction?
In the survey described by this study, which had a response rate of 63% (n=31,581), female heads of household 18 years of age and older were asked to evaluate their sexual function using the female short-form Changes in Sexual Functioning Questionnaire and the Female Sexual Distress Scale. The prevalence of any sexual problem was 44.2%, with the most common problems being:
- low desire (38.7%)
- low arousal (26.1%)
- orgasmic dysfunction (20.5%).
Dyspareunia was not assessed in this survey because a physical examination is required.
How the findings broke down by age
The prevalence of sexual problems increased with age:
- Among women 18 to 44 years old, 27.2% reported a problem with desire, arousal, orgasm, or a combination of the three.
- Among women 45 to 64 years old, the prevalence was 44.6%.
- Among women 65 years and older, the prevalence of one or more of these problems was 80.1%.
- highest (14.8%) among respondents 45 to 64 years old
- lowest (8.9%) among women 65 years or older
- intermediate (10.8%) in women 18 to 44 years old.
Medical problems associated with a higher prevalence of distressing problems of desire were depression, thyroid dysfunction, anxiety, and urinary incontinence.
Strengths and weaknesses of the study
The large sample size, wide age range, and use of verified instruments to measure sexual problems and related distress were all strengths of this study.
However, to increase response rates to “sensitive” questions, the authors used a research panel that was not randomly chosen. As a result, respondents may have been more health-conscious and self-aware than otherwise would have been the case; they also may have had more time to answer mailed questionnaires.
The fact that sexual problems and distress were self-reported without clinical evaluation also may have biased the findings slightly. Because the study was cross-sectional, cause and effect could not be established.
Although sexual problems are common among women in the United States, this survey confirms that distress caused by these problems is considerably less widespread. Sexual problems increase with age, but related distress is most common in women at midlife (45 to 64 years old).
Women’s health clinicians who elicit a history of sexual dysfunction should determine the level of distress that is present before deciding to address the problem.—ANDREW M. KAUNITZ, MD
1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors [published erratum appears in JAMA. 1999;281:1174]. JAMA. 1999;281:537-544.
In the survey described by this study, which had a response rate of 63% (n=31,581), female heads of household 18 years of age and older were asked to evaluate their sexual function using the female short-form Changes in Sexual Functioning Questionnaire and the Female Sexual Distress Scale. The prevalence of any sexual problem was 44.2%, with the most common problems being:
- low desire (38.7%)
- low arousal (26.1%)
- orgasmic dysfunction (20.5%).
Dyspareunia was not assessed in this survey because a physical examination is required.
How the findings broke down by age
The prevalence of sexual problems increased with age:
- Among women 18 to 44 years old, 27.2% reported a problem with desire, arousal, orgasm, or a combination of the three.
- Among women 45 to 64 years old, the prevalence was 44.6%.
- Among women 65 years and older, the prevalence of one or more of these problems was 80.1%.
- highest (14.8%) among respondents 45 to 64 years old
- lowest (8.9%) among women 65 years or older
- intermediate (10.8%) in women 18 to 44 years old.
Medical problems associated with a higher prevalence of distressing problems of desire were depression, thyroid dysfunction, anxiety, and urinary incontinence.
Strengths and weaknesses of the study
The large sample size, wide age range, and use of verified instruments to measure sexual problems and related distress were all strengths of this study.
However, to increase response rates to “sensitive” questions, the authors used a research panel that was not randomly chosen. As a result, respondents may have been more health-conscious and self-aware than otherwise would have been the case; they also may have had more time to answer mailed questionnaires.
The fact that sexual problems and distress were self-reported without clinical evaluation also may have biased the findings slightly. Because the study was cross-sectional, cause and effect could not be established.
Although sexual problems are common among women in the United States, this survey confirms that distress caused by these problems is considerably less widespread. Sexual problems increase with age, but related distress is most common in women at midlife (45 to 64 years old).
Women’s health clinicians who elicit a history of sexual dysfunction should determine the level of distress that is present before deciding to address the problem.—ANDREW M. KAUNITZ, MD
In the survey described by this study, which had a response rate of 63% (n=31,581), female heads of household 18 years of age and older were asked to evaluate their sexual function using the female short-form Changes in Sexual Functioning Questionnaire and the Female Sexual Distress Scale. The prevalence of any sexual problem was 44.2%, with the most common problems being:
- low desire (38.7%)
- low arousal (26.1%)
- orgasmic dysfunction (20.5%).
Dyspareunia was not assessed in this survey because a physical examination is required.
How the findings broke down by age
The prevalence of sexual problems increased with age:
- Among women 18 to 44 years old, 27.2% reported a problem with desire, arousal, orgasm, or a combination of the three.
- Among women 45 to 64 years old, the prevalence was 44.6%.
- Among women 65 years and older, the prevalence of one or more of these problems was 80.1%.
- highest (14.8%) among respondents 45 to 64 years old
- lowest (8.9%) among women 65 years or older
- intermediate (10.8%) in women 18 to 44 years old.
Medical problems associated with a higher prevalence of distressing problems of desire were depression, thyroid dysfunction, anxiety, and urinary incontinence.
Strengths and weaknesses of the study
The large sample size, wide age range, and use of verified instruments to measure sexual problems and related distress were all strengths of this study.
However, to increase response rates to “sensitive” questions, the authors used a research panel that was not randomly chosen. As a result, respondents may have been more health-conscious and self-aware than otherwise would have been the case; they also may have had more time to answer mailed questionnaires.
The fact that sexual problems and distress were self-reported without clinical evaluation also may have biased the findings slightly. Because the study was cross-sectional, cause and effect could not be established.
Although sexual problems are common among women in the United States, this survey confirms that distress caused by these problems is considerably less widespread. Sexual problems increase with age, but related distress is most common in women at midlife (45 to 64 years old).
Women’s health clinicians who elicit a history of sexual dysfunction should determine the level of distress that is present before deciding to address the problem.—ANDREW M. KAUNITZ, MD
1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors [published erratum appears in JAMA. 1999;281:1174]. JAMA. 1999;281:537-544.
1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors [published erratum appears in JAMA. 1999;281:1174]. JAMA. 1999;281:537-544.
Does menopausal estrogen therapy increase the risk of benign proliferative breast disease?
EXPERT COMMENTARY
Benign proliferative breast disease (BPBD) is a relatively common condition that is diagnosed by biopsy. It is important because of the economic and psychological burdens of biopsy and because BPBD may be a precursor to breast cancer. Some experts believe that the earliest phase of a continuum leading to invasive cancer is BPBD without atypia, followed by BPBD with atypia, in situ cancer, and then invasive disease.
Population was derived from WHI study
In this report, the risk of incident BPBD was compared among hysterectomized women 50 to 79 years old who participated in the Women’s Health Initiative (WHI). These women were randomized to conjugated equine estrogen (CEE) or placebo and monitored for, on average, 6.9 years of follow-up. They underwent baseline and annual clinical breast examination and mammography.
Overall, 232 new cases of BPBD were diagnosed among 10,739 women—approximately two thirds of them in the group randomized to CEE. Compared with women randomized to placebo, women taking CEE had twice the risk of being diagnosed with BPBD without atypia (hazard ratio [HR], 2.34; 95% confidence interval [CI], 1.71–3.20). For the subgroup of women with BPBD in whom histopathology was moderately extensive or florid (but lacking atypia), risk was similarly elevated (HR, 2.22).
Use of CEE was not associated with a substantially increased risk of atypical hyperplasia.
Limitations of the study
This trial tested only one estrogen regimen and dosage. It also was terminated earlier than expected, which may have rendered the risk estimates less precise. In addition, a significant number of participants stopped taking CEE during the trial.
This study found a significantly heightened risk of BPBD with use of unopposed estrogen, raising concerns about the potential for subsequent breast cancer. However, the same WHI estrogen-only trial from which this study1 derives, as well as the Nurses Health Study,2 found no increased risk of invasive breast cancer with estrogen therapy.
The authors of this WHI report suggest that longer follow-up of participants may help resolve this contradiction. In the meantime, some clinicians may choose to advise women who are beginning unopposed estrogen therapy that they face an elevated risk of breast biopsy for benign breast disease.—ANDREW M. KAUNITZ, MD
1. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291:1701-1712.
2. Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032.
EXPERT COMMENTARY
Benign proliferative breast disease (BPBD) is a relatively common condition that is diagnosed by biopsy. It is important because of the economic and psychological burdens of biopsy and because BPBD may be a precursor to breast cancer. Some experts believe that the earliest phase of a continuum leading to invasive cancer is BPBD without atypia, followed by BPBD with atypia, in situ cancer, and then invasive disease.
Population was derived from WHI study
In this report, the risk of incident BPBD was compared among hysterectomized women 50 to 79 years old who participated in the Women’s Health Initiative (WHI). These women were randomized to conjugated equine estrogen (CEE) or placebo and monitored for, on average, 6.9 years of follow-up. They underwent baseline and annual clinical breast examination and mammography.
Overall, 232 new cases of BPBD were diagnosed among 10,739 women—approximately two thirds of them in the group randomized to CEE. Compared with women randomized to placebo, women taking CEE had twice the risk of being diagnosed with BPBD without atypia (hazard ratio [HR], 2.34; 95% confidence interval [CI], 1.71–3.20). For the subgroup of women with BPBD in whom histopathology was moderately extensive or florid (but lacking atypia), risk was similarly elevated (HR, 2.22).
Use of CEE was not associated with a substantially increased risk of atypical hyperplasia.
Limitations of the study
This trial tested only one estrogen regimen and dosage. It also was terminated earlier than expected, which may have rendered the risk estimates less precise. In addition, a significant number of participants stopped taking CEE during the trial.
This study found a significantly heightened risk of BPBD with use of unopposed estrogen, raising concerns about the potential for subsequent breast cancer. However, the same WHI estrogen-only trial from which this study1 derives, as well as the Nurses Health Study,2 found no increased risk of invasive breast cancer with estrogen therapy.
The authors of this WHI report suggest that longer follow-up of participants may help resolve this contradiction. In the meantime, some clinicians may choose to advise women who are beginning unopposed estrogen therapy that they face an elevated risk of breast biopsy for benign breast disease.—ANDREW M. KAUNITZ, MD
EXPERT COMMENTARY
Benign proliferative breast disease (BPBD) is a relatively common condition that is diagnosed by biopsy. It is important because of the economic and psychological burdens of biopsy and because BPBD may be a precursor to breast cancer. Some experts believe that the earliest phase of a continuum leading to invasive cancer is BPBD without atypia, followed by BPBD with atypia, in situ cancer, and then invasive disease.
Population was derived from WHI study
In this report, the risk of incident BPBD was compared among hysterectomized women 50 to 79 years old who participated in the Women’s Health Initiative (WHI). These women were randomized to conjugated equine estrogen (CEE) or placebo and monitored for, on average, 6.9 years of follow-up. They underwent baseline and annual clinical breast examination and mammography.
Overall, 232 new cases of BPBD were diagnosed among 10,739 women—approximately two thirds of them in the group randomized to CEE. Compared with women randomized to placebo, women taking CEE had twice the risk of being diagnosed with BPBD without atypia (hazard ratio [HR], 2.34; 95% confidence interval [CI], 1.71–3.20). For the subgroup of women with BPBD in whom histopathology was moderately extensive or florid (but lacking atypia), risk was similarly elevated (HR, 2.22).
Use of CEE was not associated with a substantially increased risk of atypical hyperplasia.
Limitations of the study
This trial tested only one estrogen regimen and dosage. It also was terminated earlier than expected, which may have rendered the risk estimates less precise. In addition, a significant number of participants stopped taking CEE during the trial.
This study found a significantly heightened risk of BPBD with use of unopposed estrogen, raising concerns about the potential for subsequent breast cancer. However, the same WHI estrogen-only trial from which this study1 derives, as well as the Nurses Health Study,2 found no increased risk of invasive breast cancer with estrogen therapy.
The authors of this WHI report suggest that longer follow-up of participants may help resolve this contradiction. In the meantime, some clinicians may choose to advise women who are beginning unopposed estrogen therapy that they face an elevated risk of breast biopsy for benign breast disease.—ANDREW M. KAUNITZ, MD
1. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291:1701-1712.
2. Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032.
1. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291:1701-1712.
2. Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027-1032.
Do aromatase inhibitors extend disease-free survival after tamoxifen therapy in breast cancer survivors?
YES. In postmenopausal women treated for early-stage hormone receptor-positive breast cancer who have completed therapy with tamoxifen, treatment with the aromatase inhibitors letrozole or exemestane increased disease-free survival.
EXPERT COMMENTARY
Survival clearly improves in postmenopausal women with early-stage receptor-positive breast cancer who take tamoxifen or an aromatase inhibitor for 5 years after treatment. The risk of recurrence remains heightened, however, for many years after adjuvant endocrine therapy ends. These three studies explore the effects of extended hormonal adjuvant therapy in women who have completed tamoxifen therapy.
Canadian trial focused on letrozole
In the first trial, known as MA.17 and sponsored by the National Cancer Institute of Canada, more than 5,000 women who had taken tamoxifen for 5 years were randomized to letrozole or placebo. At a median follow-up of 30 months, letrozole significantly increased disease-free survival.
This study analyzed the risks and benefits of letrozole by age group:
- younger than 60 years
- 60 to 69 years
- 70 years and older.
After 4 years of letrozole, disease-free survival increased to a similar degree in all groups, but achieved statistical significance in the youngest group.
Compared with placebo, the youngest women experienced a lower incidence of vaginal bleeding and a greater incidence of arthralgias. Women in the 60-to-69-year group experienced more insomnia, hot flushes, arthralgias, and alopecia. In contrast, women 70 years or older had a side effect profile that was similar to that of the placebo group.
Both treated women and those randomized to placebo had a similar rate of diagnosis of new osteoporosis or fracture. One reason for this finding may be enhanced bone density from the 5 years of tamoxifen that preceded letrozole.
Letrozole is effective even long after tamoxifen therapy has ended
The study by Goss and colleagues explored the use of letrozole among women originally assigned to the placebo group in the MA.17 trial. After that trial was unblinded, roughly 66% of placebo-assigned women opted for open-label use of letrozole. The median time since completion of 5 years of tamoxifen therapy among these women was 2.8 years.
Although women who chose not to take letrozole had a lower baseline risk of disease recurrence, women who did choose letrozole had greater disease-free survival at a median follow-up of 5.3 years (hazard ratio, 0.39; P=.004), demonstrating that letrozole is effective even when it is not initiated for several years after discontinuation of tamoxifen.
Exemestane also improved survival
The National Surgical Adjuvant Breast and Bowel Project (NSABP), funded by the US National Cancer Institute, randomized postmenopausal women to exemestane or placebo. All women had receptor-positive breast cancer and had taken tamoxifen for 5 years. When the MA.17 trial was unblinded, accrual to the NSABP was halted, and all women randomized to placebo were offered exemestane. At a median follow-up of 30 months, disease-free survival improved marginally (P=.07) in the 560 women originally assigned to exemestane, compared with the 344 women originally randomized to placebo.
An editorial accompanying these studies describes trials still under way to assess the benefits and risks of aromatase inhibitors beyond 5 years of therapy.1 The findings of those trials will help determine whether extended use is beneficial.
Overall, these three studies demonstrate that the extension of adjuvant endocrine therapy beyond the initial 5 years (in tamoxifen users) improves disease-free survival without impairing quality of life or causing major toxicity. Younger postmenopausal women are more likely than older women to experience menopausal symptoms when taking an aromatase inhibitor.
To prevent fractures, assess bone mineral density at baseline and prescribe bisphosphonates when necessary.
The good news? Aromatase inhibitors are easily tolerated in most women. Significant arthralgias or other bother-some side effects in some subgroups, however, may make it necessary to weigh the benefits of aromatase inhibitors against quality of life.—ANDREW M. KAUNITZ, MD
At the moment, there is no adequate way to capture data on the many women who receive long-term pharmacotherapy to prevent a recurrence of estrogen receptor-positive breast cancer: The recommended code, V58.69 (long-term [current] use of other medications), does not help you identify the type of treatment.
That dilemma will be resolved on October 1, however, when three new codes are added:
- V07.51 Prophylactic use of selective estrogen-receptor modulators (SERMs)
- V07.52 Prophylactic use of inhibitors
- V07.59 Prophylactic use of agents affecting estrogen receptors and estrogen levels.
When using the new codes, you should report a secondary code that identifies the patient’s:
- status as estrogen receptor-positive (V86.0)
- family history of breast cancer, if any (V16.3)
- genetic susceptibility to cancer (V84.01–V84.09)
- personal history of breast cancer (V10.3)
- postmenopausal status (V49.81).
In addition, the new V07.5 series of codes may also be used with neoplasm codes if the patient is still in active treatment for cancer.
An “includes” note with each of these codes indicates the most typical drugs that would be reported. For example: SERMs include raloxifene, tamoxifen, and toremifene; aromatase inhibitors include anastrozole, exemestane, and letrozole; and drugs that act on estrogen receptors and estrogen levels include such estrogen-receptor down-regulators as fulvestrant, gonadotropin-releasing hormones, the agonist goserelin, leuprolide, and megestrol.—MELANIE WITT, RN, CPC-OGS, MS
Reference
1. Lin NU, Winer EP. Optimizing endocrine therapy for estrogen receptor-positive breast cancer: treating the right patients for the right length of time. J Clin Oncol. 2008;26:1919-1921.
YES. In postmenopausal women treated for early-stage hormone receptor-positive breast cancer who have completed therapy with tamoxifen, treatment with the aromatase inhibitors letrozole or exemestane increased disease-free survival.
EXPERT COMMENTARY
Survival clearly improves in postmenopausal women with early-stage receptor-positive breast cancer who take tamoxifen or an aromatase inhibitor for 5 years after treatment. The risk of recurrence remains heightened, however, for many years after adjuvant endocrine therapy ends. These three studies explore the effects of extended hormonal adjuvant therapy in women who have completed tamoxifen therapy.
Canadian trial focused on letrozole
In the first trial, known as MA.17 and sponsored by the National Cancer Institute of Canada, more than 5,000 women who had taken tamoxifen for 5 years were randomized to letrozole or placebo. At a median follow-up of 30 months, letrozole significantly increased disease-free survival.
This study analyzed the risks and benefits of letrozole by age group:
- younger than 60 years
- 60 to 69 years
- 70 years and older.
After 4 years of letrozole, disease-free survival increased to a similar degree in all groups, but achieved statistical significance in the youngest group.
Compared with placebo, the youngest women experienced a lower incidence of vaginal bleeding and a greater incidence of arthralgias. Women in the 60-to-69-year group experienced more insomnia, hot flushes, arthralgias, and alopecia. In contrast, women 70 years or older had a side effect profile that was similar to that of the placebo group.
Both treated women and those randomized to placebo had a similar rate of diagnosis of new osteoporosis or fracture. One reason for this finding may be enhanced bone density from the 5 years of tamoxifen that preceded letrozole.
Letrozole is effective even long after tamoxifen therapy has ended
The study by Goss and colleagues explored the use of letrozole among women originally assigned to the placebo group in the MA.17 trial. After that trial was unblinded, roughly 66% of placebo-assigned women opted for open-label use of letrozole. The median time since completion of 5 years of tamoxifen therapy among these women was 2.8 years.
Although women who chose not to take letrozole had a lower baseline risk of disease recurrence, women who did choose letrozole had greater disease-free survival at a median follow-up of 5.3 years (hazard ratio, 0.39; P=.004), demonstrating that letrozole is effective even when it is not initiated for several years after discontinuation of tamoxifen.
Exemestane also improved survival
The National Surgical Adjuvant Breast and Bowel Project (NSABP), funded by the US National Cancer Institute, randomized postmenopausal women to exemestane or placebo. All women had receptor-positive breast cancer and had taken tamoxifen for 5 years. When the MA.17 trial was unblinded, accrual to the NSABP was halted, and all women randomized to placebo were offered exemestane. At a median follow-up of 30 months, disease-free survival improved marginally (P=.07) in the 560 women originally assigned to exemestane, compared with the 344 women originally randomized to placebo.
An editorial accompanying these studies describes trials still under way to assess the benefits and risks of aromatase inhibitors beyond 5 years of therapy.1 The findings of those trials will help determine whether extended use is beneficial.
Overall, these three studies demonstrate that the extension of adjuvant endocrine therapy beyond the initial 5 years (in tamoxifen users) improves disease-free survival without impairing quality of life or causing major toxicity. Younger postmenopausal women are more likely than older women to experience menopausal symptoms when taking an aromatase inhibitor.
To prevent fractures, assess bone mineral density at baseline and prescribe bisphosphonates when necessary.
The good news? Aromatase inhibitors are easily tolerated in most women. Significant arthralgias or other bother-some side effects in some subgroups, however, may make it necessary to weigh the benefits of aromatase inhibitors against quality of life.—ANDREW M. KAUNITZ, MD
At the moment, there is no adequate way to capture data on the many women who receive long-term pharmacotherapy to prevent a recurrence of estrogen receptor-positive breast cancer: The recommended code, V58.69 (long-term [current] use of other medications), does not help you identify the type of treatment.
That dilemma will be resolved on October 1, however, when three new codes are added:
- V07.51 Prophylactic use of selective estrogen-receptor modulators (SERMs)
- V07.52 Prophylactic use of inhibitors
- V07.59 Prophylactic use of agents affecting estrogen receptors and estrogen levels.
When using the new codes, you should report a secondary code that identifies the patient’s:
- status as estrogen receptor-positive (V86.0)
- family history of breast cancer, if any (V16.3)
- genetic susceptibility to cancer (V84.01–V84.09)
- personal history of breast cancer (V10.3)
- postmenopausal status (V49.81).
In addition, the new V07.5 series of codes may also be used with neoplasm codes if the patient is still in active treatment for cancer.
An “includes” note with each of these codes indicates the most typical drugs that would be reported. For example: SERMs include raloxifene, tamoxifen, and toremifene; aromatase inhibitors include anastrozole, exemestane, and letrozole; and drugs that act on estrogen receptors and estrogen levels include such estrogen-receptor down-regulators as fulvestrant, gonadotropin-releasing hormones, the agonist goserelin, leuprolide, and megestrol.—MELANIE WITT, RN, CPC-OGS, MS
YES. In postmenopausal women treated for early-stage hormone receptor-positive breast cancer who have completed therapy with tamoxifen, treatment with the aromatase inhibitors letrozole or exemestane increased disease-free survival.
EXPERT COMMENTARY
Survival clearly improves in postmenopausal women with early-stage receptor-positive breast cancer who take tamoxifen or an aromatase inhibitor for 5 years after treatment. The risk of recurrence remains heightened, however, for many years after adjuvant endocrine therapy ends. These three studies explore the effects of extended hormonal adjuvant therapy in women who have completed tamoxifen therapy.
Canadian trial focused on letrozole
In the first trial, known as MA.17 and sponsored by the National Cancer Institute of Canada, more than 5,000 women who had taken tamoxifen for 5 years were randomized to letrozole or placebo. At a median follow-up of 30 months, letrozole significantly increased disease-free survival.
This study analyzed the risks and benefits of letrozole by age group:
- younger than 60 years
- 60 to 69 years
- 70 years and older.
After 4 years of letrozole, disease-free survival increased to a similar degree in all groups, but achieved statistical significance in the youngest group.
Compared with placebo, the youngest women experienced a lower incidence of vaginal bleeding and a greater incidence of arthralgias. Women in the 60-to-69-year group experienced more insomnia, hot flushes, arthralgias, and alopecia. In contrast, women 70 years or older had a side effect profile that was similar to that of the placebo group.
Both treated women and those randomized to placebo had a similar rate of diagnosis of new osteoporosis or fracture. One reason for this finding may be enhanced bone density from the 5 years of tamoxifen that preceded letrozole.
Letrozole is effective even long after tamoxifen therapy has ended
The study by Goss and colleagues explored the use of letrozole among women originally assigned to the placebo group in the MA.17 trial. After that trial was unblinded, roughly 66% of placebo-assigned women opted for open-label use of letrozole. The median time since completion of 5 years of tamoxifen therapy among these women was 2.8 years.
Although women who chose not to take letrozole had a lower baseline risk of disease recurrence, women who did choose letrozole had greater disease-free survival at a median follow-up of 5.3 years (hazard ratio, 0.39; P=.004), demonstrating that letrozole is effective even when it is not initiated for several years after discontinuation of tamoxifen.
Exemestane also improved survival
The National Surgical Adjuvant Breast and Bowel Project (NSABP), funded by the US National Cancer Institute, randomized postmenopausal women to exemestane or placebo. All women had receptor-positive breast cancer and had taken tamoxifen for 5 years. When the MA.17 trial was unblinded, accrual to the NSABP was halted, and all women randomized to placebo were offered exemestane. At a median follow-up of 30 months, disease-free survival improved marginally (P=.07) in the 560 women originally assigned to exemestane, compared with the 344 women originally randomized to placebo.
An editorial accompanying these studies describes trials still under way to assess the benefits and risks of aromatase inhibitors beyond 5 years of therapy.1 The findings of those trials will help determine whether extended use is beneficial.
Overall, these three studies demonstrate that the extension of adjuvant endocrine therapy beyond the initial 5 years (in tamoxifen users) improves disease-free survival without impairing quality of life or causing major toxicity. Younger postmenopausal women are more likely than older women to experience menopausal symptoms when taking an aromatase inhibitor.
To prevent fractures, assess bone mineral density at baseline and prescribe bisphosphonates when necessary.
The good news? Aromatase inhibitors are easily tolerated in most women. Significant arthralgias or other bother-some side effects in some subgroups, however, may make it necessary to weigh the benefits of aromatase inhibitors against quality of life.—ANDREW M. KAUNITZ, MD
At the moment, there is no adequate way to capture data on the many women who receive long-term pharmacotherapy to prevent a recurrence of estrogen receptor-positive breast cancer: The recommended code, V58.69 (long-term [current] use of other medications), does not help you identify the type of treatment.
That dilemma will be resolved on October 1, however, when three new codes are added:
- V07.51 Prophylactic use of selective estrogen-receptor modulators (SERMs)
- V07.52 Prophylactic use of inhibitors
- V07.59 Prophylactic use of agents affecting estrogen receptors and estrogen levels.
When using the new codes, you should report a secondary code that identifies the patient’s:
- status as estrogen receptor-positive (V86.0)
- family history of breast cancer, if any (V16.3)
- genetic susceptibility to cancer (V84.01–V84.09)
- personal history of breast cancer (V10.3)
- postmenopausal status (V49.81).
In addition, the new V07.5 series of codes may also be used with neoplasm codes if the patient is still in active treatment for cancer.
An “includes” note with each of these codes indicates the most typical drugs that would be reported. For example: SERMs include raloxifene, tamoxifen, and toremifene; aromatase inhibitors include anastrozole, exemestane, and letrozole; and drugs that act on estrogen receptors and estrogen levels include such estrogen-receptor down-regulators as fulvestrant, gonadotropin-releasing hormones, the agonist goserelin, leuprolide, and megestrol.—MELANIE WITT, RN, CPC-OGS, MS
Reference
1. Lin NU, Winer EP. Optimizing endocrine therapy for estrogen receptor-positive breast cancer: treating the right patients for the right length of time. J Clin Oncol. 2008;26:1919-1921.
Reference
1. Lin NU, Winer EP. Optimizing endocrine therapy for estrogen receptor-positive breast cancer: treating the right patients for the right length of time. J Clin Oncol. 2008;26:1919-1921.
Is hormonal contraception right for your perimenopausal patient?
CASE Perimenopausal complaints, and a request for contraception
At her annual visit, M.B., a healthy 48-year-old divorced woman, reports that her periods are increasingly erratic and that she has begun experiencing occasional hot flushes. Although her previous husband had a vasectomy, she has started to date and is concerned about contraception. A close friend became pregnant at the age of 46 and chose to have an abortion. M.B. hopes to avoid the same fate and asks specifically about birth control pills. Is this an appropriate option for her? What do you tell her?
Although only 11% of women 40 to 44 years old reported using oral contraceptives (OCs) in 2002 in the United States, that figure represents a 5% increase over 1995,1,2 and all indications are that the percentage is still rising.
In lean, nonsmoking, healthy perimenopausal women, OCs offer users not only effective contraception, but also benefits that include a reduction in heavy menstrual bleeding; regularization of the menstrual cycle; protection against ovarian, endometrial, and colorectal cancer; prevention of bone loss (with possible prevention of postmenopausal osteoporotic fractures); and some degree of relief from vasomotor symptoms. Although an increased risk of venous thromboembolism (VTE) is well documented in OC users, concerns also exist that use of the pill might increase the risk of myocardial infarction (MI), stroke, and breast cancer in older reproductive-age women.
To explore the range of hormonal contraceptive options and their risks and benefits in perimenopausal women in more depth, OBG Management recently caught up with Andrew M. Kaunitz, MD, an expert in both contraception and menopause and a member of the OBG Management Board of Editors. He describes and interprets the robust data in this field to answer our many questions—although he points out that perimenopausal women have been underrepresented in studies of OC use in particular and hormonal contraception in general.
Why hormonal contraception?
OBG Management: Why is effective contraception important in this age group? Aren’t perimenopausal women less fertile than younger women?
Kaunitz: Older women are less fecund, but irregular menstrual cycles make it difficult to predict when ovulation is occurring, making unplanned pregnancy a real possibility in sexually active women.
Pregnancy itself is fraught with risks in this age group. Pregnancy-related mortality among women 40 years or older in the United States is five times higher than among 25- to 29-year-olds. Older women are also more likely to have comorbidities such as hypertension and diabetes, further increasing the risks of pregnancy.3,4 In addition, perimenopausal women are more likely than any reproductive age group except adolescents to opt for induced abortion when they do become pregnant, with 304 abortions for every 1,000 live births in women 40 years or older in the United States.5
OBG Management: Why should a perimenopausal woman consider hormonal contraception?
Kaunitz: It is highly effective and offers a range of noncontraceptive benefits, and older women are more likely to use it properly, making contraceptive failure less likely than in younger patients.
Nor are combination OCs the only option for this age group. Progestin-only OCs, the levonorgestrel-releasing intrauterine system, the etonogestrel implant, and injectable depot medroxyprogesterone acetate (DMPA) are alternatives. Although the vaginal patch and ring have not been studied extensively, they may be appropriate in some instances. Until further data specific to these combination estrogen–progestin methods are available, let’s assume for our discussion that they carry the same risk–benefit profile as combination OCs.
Thromboembolism is the greatest risk
OBG Management: What is the greatest risk of OC use in perimenopausal women?
Kaunitz: That would be VTE. The risk rises sharply after 39 years of age among users of combination OCs, with approximately 100 cases for every 100,000 person-years, compared with 25 cases for every 100,000 person-years among adolescents.6 This already elevated risk almost doubles among obese women older than 39 years.7 In these women, progestin-only or intrauterine contraceptives are better options than combination OCs.8
Also, avoid prescribing combination OCs for women with a known thrombophilic defect. However, because screening for thrombophilia is not cost-effective, routinely evaluating candidates for combination contraception with testing for familial thrombophilic disorders is not recommended.
OBG Management: Does the dosage of estrogen determine the risk of VTE?
Kaunitz: That is the general assumption—that higher dosages of estrogen pose a greater risk—but we lack definitive evidence that OCs formulated with 20 μg of estrogen are any safer in this regard than those that contain 30 to 35 μg.7,9
There is some evidence that the progestin plays a role. OCs that contain desogestrel appear to carry almost twice the risk of VTE as those formulated with levonorgestrel or norgestimate.10
TABLE
How selected health conditions affect choice of contraceptive in women ≥35 years
| Condition | Recommendation* |
|---|---|
| Obesity | Avoid combination contraceptives (OCs, patch, and ring) |
| Smoking | |
| Diabetes | |
| Migraine | |
| Hypertension | |
| * Based on guidelines from the American College of Obstetricians and Gynecologists8 | |
| † Includes progestin-only OCs, progestin implants, depot medroxyprogesterone acetate, and copper and progestin-releasing intrauterine devices | |
Risk of MI, stroke may rise in some older women
OBG Management: Do perimenopausal women who take combination OCs face a heightened risk of MI or stroke?
Kaunitz: Yes, if they smoke or have hypertension. The reason: In women who use combination OCs, smoking and hypertension are synergistic risk factors for MI and stroke. That means perimenopausal women who smoke or have high blood pressure should avoid combination contraceptives.
Although it is limited, available evidence supports the safety of OCs in older women who do not smoke or have hypertension. One large case-control study from the United States found no increased risk of MI or stroke among this population when they used OCs containing less than 50 μg of ethinyl estradiol.11,12 However, this study included few women older than 35 years who used OCs and smoked or had hypertension.
A large, prospective study from Sweden that included 1,761 current OC users between 40 and 49 years of age found no increased risk of MI among former or current OC users.13 It also found that the initiation of OC use in women 30 years of age or older carried no higher risk of MI than did initiation at age 29 or younger.
Avoid OCs in older women who have diabetes
OBG Management: What about women 35 years of age or older who have diabetes? Is hormonal contraception appropriate for them?
Kaunitz: Both premenopausal and postmenopausal women who have diabetes have a higher risk of cardiovascular disease, so combination contraceptives are a bad idea when the woman has diabetes and is 35 years of age or older. OCs also should be avoided in women younger than 35 years who have diabetes, unless they are normotensive and free of nephropathy and other vascular disease. Intrauterine contraception and progestin-only formulations tend to be better options for diabetic women.
Avoid combination OCs in perimenopausal migraineurs
OBG Management: Isn’t there evidence that women who have migraine headaches have an elevated stroke risk? How does this affect their choice of contraceptive?
Kaunitz: One case-control study from a large US health maintenance organization found twice the risk of stroke among OC users who had migraines as among those who did not.12 However, this study did not distinguish between women who had migraines with aura and those who had migraines without aura.
Another study found an increased risk of stroke among OC users who had migraines with aura, but not among those who had migraines without aura.14
Accordingly, both the American College of Obstetricians and Gynecologists (ACOG) and the World Health Organization recommend that older women who experience migraines use progestin-only or intrauterine contraception.8,15
Does estrogen use increase the risk of breast cancer?
OBG Management: It’s a common assumption that hormonal contraceptives that contain estrogen increase the risk of breast cancer. Is that assumption backed by data?
Kaunitz: Long-term use of combination estrogen–progestin menopausal hormone therapy modestly increases the risk of breast cancer. Accordingly, many clinicians and women assume that use of hormonal contraception must likewise increase risk. In fact, the evidence does not indicate that combination OCs or progestin-only contraceptives increase the risk of breast cancer. However, studies to date have involved a relatively small number of women older than 45 years.
For example, a large cohort study from the United Kingdom that involved more than 1 million person-years of follow-up found no association between use of OCs and breast cancer, even among long-term users.16 Most cases of OC use in this study involved OCs formulated with 50 μg or more of ethinyl estradiol. However, this study did not indicate the age at which women used OCs.
In the Women’s Contraceptive and Reproductive Experiences (CARE) study, current or previous users of OCs had no increased risk of invasive or in situ breast cancer, compared with never-users.17,18 This study did include a subgroup of women who had started using OCs after age 40. Nor did the CARE study find an association between progestin-only injectable DMPA or implantable contraceptives and breast cancer.19
Last, a population-based case-control study in the United States found no increased risk of death from breast cancer among previous users of OCs, compared with women who had never used them.20 This study included an analysis limited to women who had begun using OCs at 30 years of age or older.
OBG Management: What about women who have a family history of breast cancer? Do OCs and other hormonal contraceptives elevate their risk further?
Kaunitz: Women who have a family history of breast cancer are often cautioned that it would be unsafe for them to use hormonal contraception. However, use of hormonal contraception does not appear to impact the risk of breast cancer in women who have a family history of the disease.
A large prospective study from Canada involving women who had a family history of breast cancer and a mean age of 49 found no increased risk of breast cancer among former or current OC users.21 This study did not assess risk by BRCA mutation status.
A separate study found that the risk of breast cancer increased slightly among women who had a BRCA1 mutation, with an odds ratio of 1.20 (95% confidence interval, 1.02–1.40), but not among women who had a BRCA2 mutation.22 Another study found no significant increase in the risk of breast cancer among women who had either a BRCA1 or BRCA2 mutation.23
Benefits include improved bleeding patterns
OBG Management: Many perimenopausal women who have fibroids or adenomyosis experience menorrhagia or dysfunctional uterine bleeding (DUB) and opt for surgery such as endometrial ablation or hysterectomy. Can OCs or other hormonal contraceptives alleviate these patterns without the need for surgery?
Kaunitz: Yes. OCs can restore physiologic bleeding in older women who have DUB. One study involving women 15 to 50 years of age who had DUB found improved bleeding patterns in more than 80% of women randomized to OCs, compared with less than 50% of women randomized to placebo.24 In addition, women who have menorrhagia have reported a significant reduction of blood loss after using OCs.25
Another effective option for women who have menorrhagia is the levonorgestrel-releasing intrauterine system (LNG-IUS), even in women who have menorrhagia associated with fibroids and adenomyosis.26-28
Because long-term use of injectable forms of contraception tends to lead to amenorrhea, some physicians recommend DMPA as a treatment for menorrhagia. Data supporting this strategy are scant, however.29
OCs reduce the risk of three cancers
OBG Management: Oral contraceptives are known to reduce the risk of ovarian, endometrial, and colorectal cancer to varying degrees. Does this benefit hold up for older women, too?
Kaunitz: Yes. And because the incidence of ovarian cancer, in particular, increases with age, the protection afforded by combination OCs may be especially beneficial for women of older reproductive age.
OBG Management: Just how much protection against ovarian cancer does OC use afford?
Kaunitz: Among users of low-dose combination OCs, the risk of epithelial ovarian cancer declines by at least 50%, compared with women who have never used the pill—and, the longer the use, the greater the protection.16,30,31 Once OCs are discontinued, the protection diminishes over time, but some degree of reduced risk persists for three decades or longer.31
OBG Management: What about endometrial cancer?
Kaunitz: Not just OCs, but also DMPA, are associated with a significant reduction in the risk of endometrial cancer: 50% with use of OCs formulated with 30 μg or more of estrogen, and 80% with use of DMPA. In the case of OCs, the reduced risk is greater with longer use, and it persists after discontinuation for at least 20 years.25,32
OBG Management: Is the protection against colorectal cancer as great as the protection against these other cancers?
Kaunitz: No, it isn’t, but the protection is still significant. OC use reduces the risk of colorectal cancer by approximately 20%, but the protection against colorectal cancer does not appear to increase with duration of use.16,33 It also may be that more recent OC use (past 5 years) affords greater protection than use in the more distant past.16,33
OCs may reduce fracture risk postmenopausally
OBG Management: What effect do combination OCs and other forms of hormonal contraception have on the bone loss that accelerates around the time of menopause?
Kaunitz: One randomized trial found that OC use increases bone mineral density (BMD) in women of older reproductive age.34 And a population-based, case-control trial from Sweden found a 25% reduction in the risk of hip fracture among postmenopausal women who had a history of OC use. The reduction in risk was even greater when the women had used OCs in their 40s or for an extended duration.35
The Women’s Health Initiative found no reduction in the risk of fracture among previous users of OCs, but failed to stratify women by the age at which they used OCs.
OBG Management: Are any hormonal contraceptives associated with bone loss?
Kaunitz: Yes. Use of intramuscular DMPA (150 mg) or subcutaneous DMPA (104 mg) is linked to a loss of BMD. The good news is that BMD recovers after discontinuation of the drug, even in women who begin to use it after 40 years of age.29,36 However, we lack data on the risk of fracture among postmenopausal women with a history of DMPA use.
OCs may ease hot flushes and other menopausal symptoms
OBG Management: Is there any evidence that use of combination OCs by perimenopausal women relieves vasomotor symptoms?
Kaunitz: Yes, but the number of studies demonstrating this association so far has been limited. One small double-blind trial randomly assigned women to use of an OC containing 20 μg of estradiol or to placebo.37 Although the number and severity of symptoms diminished by about 50% in those taking the OC, the difference was not statistically significant.
A prospective observational study found that 90% of perimenopausal women experienced complete relief after taking an OC containing 30 μg of ethinyl estradiol, compared with only 40% of nonusers.38
OBG Management: What about other forms of hormonal contraception? Are any effective against vasomotor symptoms?
Kaunitz: One interesting option is to use menopausal doses of estrogen to treat vasomotor symptoms along with an LNG-IUS to prevent endometrial hyperplasia and provide contraception, if needed. This combination produced substantial improvement in a trial involving perimenopausal women who were experiencing vasomotor symptoms.39 Most of the women became amenorrheic, and there was no endometrial hyperplasia.
DMPA in contraceptive dosages also has relieved vasomotor symptoms in menopausal women, compared with placebo.40
OBG Management: What about women who experience vasomotor symptoms during the 7 placebo days of a 28-pill cycle? What options do they have?
Kaunitz: Some physicians either switch to a 24/4 OC formulation (Yaz or Lo-Estrin 24), an extended OC formulation with no placebo days (Seasonique), a continuous OC formulation (Lybrel), or simply prescribe pills from a traditional 21/7 pack in a continuous fashion so as to eliminate the hormone-free interval. However, this strategy has been studied to only a limited degree.
At what age should an OC be discontinued?
OBG Management: Perimenopausal women are, obviously, going to become menopausal at some point. How do you know when that transition occurs if they are taking OCs?
Kaunitz: It turns out that testing is not useful in this clinical setting. Some people have advocated measuring the follicle-stimulating hormone (FSH) level, but this strategy is unreliable. An elevated FSH level—thought to be indicative of menopause—has been found in older ovulatory women,41 and a depressed FSH level has been found in postmenopausal women for weeks after discontinuation of OCs.42
Rather than use this imperfect science to try and predict the point of menopause, I recommend discontinuing OCs once the woman has attained age 55, arbitrarily assuming that she is menopausal at this age. I use the same approach for women using other hormonal contraceptives.8,43
1. Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Advance data from vital and health statistics. No. 350. Hyattsville, MD: National Center for Health Statistics, December 10, 2004.
2. Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital and health statistics. Series 23. No. 19. Hyattsville, MD: National Center for Health Statistics, May 1997:1–114. (DHHS publication no. (PHS) 97–1995.)
3. Callaghan WM, Berg CJ. Pregnancy-related mortality among women aged 35 years and older, United States, 1991–1997. Obstet Gynecol. 2003;102:1015-1021.
4. Viegas OA, Leong WP, Ahmed S, Ratnam SS. Obstetrical outcome with increasing maternal age. J Biosoc Sci. 1994;26:261-267.
5. Strauss LT, Herndon J, Chang J, et al. Abortion surveillance—United States, 2001. MMWR Surveill Summ. 2004;53(SS–9):1-32.
6. Nightingale AL, Lawrenson RA, Simpson EL, Williams TJ, MacRae KD, Farmer RDT. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur J Contracept Reprod Health Care. 2000;5:265-274.
7. Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP, Jr. Venous thromboembolic disease in users of low-estrogen combined estrogen–progestin oral contraceptives. Contraception. 2004;70:3-10.
8. ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 73: Use of hormonal contraceptive in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
9. Gallo MF, Nanda K, Grimes DA, Schulz KF. Twenty micrograms vs. >20 microg estrogen oral contraceptives for contraception: systematic review of randomized controlled trials. Contraception. 2005;71:162-169.
10. Jick SS, Kaye JA, Russman S, Jick H. Risk of nonfatal venous thromboembolism with oral contraceptives containing levonorgestrel. Contraception. 2006;73:566-570.
11. Sidney S, Siscovick DS, Petitti DB, et al. Myocardial infarction and use of low-dose oral contraceptives: a pooled analysis of 2 US studies. Circulation. 1998;98:1058-1063.
12. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29:2277-2284.
13. Margolis KL, Adami H-O, Luo J, Ye W, Weider-pass E. A prospective study of oral contraceptive use and risk of myocardial infarction among Swedish women. Fertil Steril. 2007;88:310-316.
14. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2345.
15. Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: World Health Organization, 2004.
16. Hannaford PC, Selvaraj S, Elliott AM, Angus V, Iversen L, Lee AJ. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioners’ oral contraception study. BMJ. 2007;335:651.-
17. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
18. Gill JK, Press MF, Patel AV, Bernstein L. Oral contraceptive use and risk of breast carcinoma in situ (United States). Cancer Causes Control. 2006;17:1155-1162.
19. Strom BL, Berlin JA, Weber AL, et al. Absence of an effect of injectable and implantable progestin-only contraceptives on subsequent risk of breast cancer. Contraception. 2004;69:353-360.
20. Wingo PA, Austin A, Marchbanks PA, et al. Oral contraceptives and the risk of death from breast cancer. Obstet Gynecol. 2007;110:793-800.
21. Silvera SA, Miller AB, Rohan TE. Oral contraceptive use and risk of breast cancer among women with a family history of breast cancer: a prospective cohort study. Cancer Causes Control. 2005;16:1059-1063.
22. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
23. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
24. Davis A, Godwin A, Lippman J, Olson W, Kafrissen M. Triphasic norgestimate-ethinyl estradiol for treating dysfunctional uterine bleeding. Obstet Gynecol. 2000;96:913-920.
25. Kaunitz AM. Noncontraceptive health benefits of oral contraceptives. Rev Endocr Metab Disord. 2002;3:277-283.
26. Hurskainen R, Reperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456-1463.
27. Kaunitz AM. Progestin-releasing intrauterine systems and leiomyoma. Contraception. 2007;75 (6 Suppl):S130-S133.
28. Bragheto AM, Caserta N, Bahamondes L, Petta CA. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
29. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. In: Rose BD, ed. UpToDate. Wellesley, MA: UpToDate, 2008.
30. Petitti DB. Combination estrogen–progestin oral contraceptives. N Engl J Med. 2003;349:1443-1450.[Erratum, N Engl J Med. 2004;350:92.]
31. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303-314.
32. Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives: a practitioner’s guide to meta-analysis. Hum Reprod. 1997;12:1851-1863.
33. Fernandez E, LaVecchia C, Balducci A, Chatenoud L, Francheschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722-727.
34. Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54:176-180.
35. Michaëlsson K, Baron JA, Farahmand BY, Persson I, Ljunghall S. Oral-contraceptive use and risk of hip fracture: a case-control study. Lancet. 1999;353:1481-1484.
36. Rosenberg L, Zhang Y, Constant D, et al. Bone status after cessation of use of injectable progestin contraceptives. Contraception. 2007;76:425-431.
37. Casper RF, Dodin S, Reid RL. The effect of 20 μg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes, and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
38. Shargil AA. Hormone replacement therapy in perimenopausal women with a triphasic contraceptive compound: a three-year prospective study. Int J Fertil. 1985;30:15,18-28.
39. Hampton NRE, Rees MCP, Lowe DG, Rauramo I, Barlow D, Guillebaud J. Levonorgestrel intrauterine system (LNG-IUS) with conjugated equine estrogen: a successful regimen for HRT in perimenopausal women. Hum Reprod. 2005;20:2653-2669.
40. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
41. Gebbie AE, Glasier A, Sweeting V. Incidence of ovulation in perimenopausal women before and during hormone replacement therapy. Contraception. 1995;52:221-222.
42. Creinin MD. Laboratory criteria for menopause in women using oral contraceptives. Fertil Steril. 1996;66:101-104.
43. Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270.
CASE Perimenopausal complaints, and a request for contraception
At her annual visit, M.B., a healthy 48-year-old divorced woman, reports that her periods are increasingly erratic and that she has begun experiencing occasional hot flushes. Although her previous husband had a vasectomy, she has started to date and is concerned about contraception. A close friend became pregnant at the age of 46 and chose to have an abortion. M.B. hopes to avoid the same fate and asks specifically about birth control pills. Is this an appropriate option for her? What do you tell her?
Although only 11% of women 40 to 44 years old reported using oral contraceptives (OCs) in 2002 in the United States, that figure represents a 5% increase over 1995,1,2 and all indications are that the percentage is still rising.
In lean, nonsmoking, healthy perimenopausal women, OCs offer users not only effective contraception, but also benefits that include a reduction in heavy menstrual bleeding; regularization of the menstrual cycle; protection against ovarian, endometrial, and colorectal cancer; prevention of bone loss (with possible prevention of postmenopausal osteoporotic fractures); and some degree of relief from vasomotor symptoms. Although an increased risk of venous thromboembolism (VTE) is well documented in OC users, concerns also exist that use of the pill might increase the risk of myocardial infarction (MI), stroke, and breast cancer in older reproductive-age women.
To explore the range of hormonal contraceptive options and their risks and benefits in perimenopausal women in more depth, OBG Management recently caught up with Andrew M. Kaunitz, MD, an expert in both contraception and menopause and a member of the OBG Management Board of Editors. He describes and interprets the robust data in this field to answer our many questions—although he points out that perimenopausal women have been underrepresented in studies of OC use in particular and hormonal contraception in general.
Why hormonal contraception?
OBG Management: Why is effective contraception important in this age group? Aren’t perimenopausal women less fertile than younger women?
Kaunitz: Older women are less fecund, but irregular menstrual cycles make it difficult to predict when ovulation is occurring, making unplanned pregnancy a real possibility in sexually active women.
Pregnancy itself is fraught with risks in this age group. Pregnancy-related mortality among women 40 years or older in the United States is five times higher than among 25- to 29-year-olds. Older women are also more likely to have comorbidities such as hypertension and diabetes, further increasing the risks of pregnancy.3,4 In addition, perimenopausal women are more likely than any reproductive age group except adolescents to opt for induced abortion when they do become pregnant, with 304 abortions for every 1,000 live births in women 40 years or older in the United States.5
OBG Management: Why should a perimenopausal woman consider hormonal contraception?
Kaunitz: It is highly effective and offers a range of noncontraceptive benefits, and older women are more likely to use it properly, making contraceptive failure less likely than in younger patients.
Nor are combination OCs the only option for this age group. Progestin-only OCs, the levonorgestrel-releasing intrauterine system, the etonogestrel implant, and injectable depot medroxyprogesterone acetate (DMPA) are alternatives. Although the vaginal patch and ring have not been studied extensively, they may be appropriate in some instances. Until further data specific to these combination estrogen–progestin methods are available, let’s assume for our discussion that they carry the same risk–benefit profile as combination OCs.
Thromboembolism is the greatest risk
OBG Management: What is the greatest risk of OC use in perimenopausal women?
Kaunitz: That would be VTE. The risk rises sharply after 39 years of age among users of combination OCs, with approximately 100 cases for every 100,000 person-years, compared with 25 cases for every 100,000 person-years among adolescents.6 This already elevated risk almost doubles among obese women older than 39 years.7 In these women, progestin-only or intrauterine contraceptives are better options than combination OCs.8
Also, avoid prescribing combination OCs for women with a known thrombophilic defect. However, because screening for thrombophilia is not cost-effective, routinely evaluating candidates for combination contraception with testing for familial thrombophilic disorders is not recommended.
OBG Management: Does the dosage of estrogen determine the risk of VTE?
Kaunitz: That is the general assumption—that higher dosages of estrogen pose a greater risk—but we lack definitive evidence that OCs formulated with 20 μg of estrogen are any safer in this regard than those that contain 30 to 35 μg.7,9
There is some evidence that the progestin plays a role. OCs that contain desogestrel appear to carry almost twice the risk of VTE as those formulated with levonorgestrel or norgestimate.10
TABLE
How selected health conditions affect choice of contraceptive in women ≥35 years
| Condition | Recommendation* |
|---|---|
| Obesity | Avoid combination contraceptives (OCs, patch, and ring) |
| Smoking | |
| Diabetes | |
| Migraine | |
| Hypertension | |
| * Based on guidelines from the American College of Obstetricians and Gynecologists8 | |
| † Includes progestin-only OCs, progestin implants, depot medroxyprogesterone acetate, and copper and progestin-releasing intrauterine devices | |
Risk of MI, stroke may rise in some older women
OBG Management: Do perimenopausal women who take combination OCs face a heightened risk of MI or stroke?
Kaunitz: Yes, if they smoke or have hypertension. The reason: In women who use combination OCs, smoking and hypertension are synergistic risk factors for MI and stroke. That means perimenopausal women who smoke or have high blood pressure should avoid combination contraceptives.
Although it is limited, available evidence supports the safety of OCs in older women who do not smoke or have hypertension. One large case-control study from the United States found no increased risk of MI or stroke among this population when they used OCs containing less than 50 μg of ethinyl estradiol.11,12 However, this study included few women older than 35 years who used OCs and smoked or had hypertension.
A large, prospective study from Sweden that included 1,761 current OC users between 40 and 49 years of age found no increased risk of MI among former or current OC users.13 It also found that the initiation of OC use in women 30 years of age or older carried no higher risk of MI than did initiation at age 29 or younger.
Avoid OCs in older women who have diabetes
OBG Management: What about women 35 years of age or older who have diabetes? Is hormonal contraception appropriate for them?
Kaunitz: Both premenopausal and postmenopausal women who have diabetes have a higher risk of cardiovascular disease, so combination contraceptives are a bad idea when the woman has diabetes and is 35 years of age or older. OCs also should be avoided in women younger than 35 years who have diabetes, unless they are normotensive and free of nephropathy and other vascular disease. Intrauterine contraception and progestin-only formulations tend to be better options for diabetic women.
Avoid combination OCs in perimenopausal migraineurs
OBG Management: Isn’t there evidence that women who have migraine headaches have an elevated stroke risk? How does this affect their choice of contraceptive?
Kaunitz: One case-control study from a large US health maintenance organization found twice the risk of stroke among OC users who had migraines as among those who did not.12 However, this study did not distinguish between women who had migraines with aura and those who had migraines without aura.
Another study found an increased risk of stroke among OC users who had migraines with aura, but not among those who had migraines without aura.14
Accordingly, both the American College of Obstetricians and Gynecologists (ACOG) and the World Health Organization recommend that older women who experience migraines use progestin-only or intrauterine contraception.8,15
Does estrogen use increase the risk of breast cancer?
OBG Management: It’s a common assumption that hormonal contraceptives that contain estrogen increase the risk of breast cancer. Is that assumption backed by data?
Kaunitz: Long-term use of combination estrogen–progestin menopausal hormone therapy modestly increases the risk of breast cancer. Accordingly, many clinicians and women assume that use of hormonal contraception must likewise increase risk. In fact, the evidence does not indicate that combination OCs or progestin-only contraceptives increase the risk of breast cancer. However, studies to date have involved a relatively small number of women older than 45 years.
For example, a large cohort study from the United Kingdom that involved more than 1 million person-years of follow-up found no association between use of OCs and breast cancer, even among long-term users.16 Most cases of OC use in this study involved OCs formulated with 50 μg or more of ethinyl estradiol. However, this study did not indicate the age at which women used OCs.
In the Women’s Contraceptive and Reproductive Experiences (CARE) study, current or previous users of OCs had no increased risk of invasive or in situ breast cancer, compared with never-users.17,18 This study did include a subgroup of women who had started using OCs after age 40. Nor did the CARE study find an association between progestin-only injectable DMPA or implantable contraceptives and breast cancer.19
Last, a population-based case-control study in the United States found no increased risk of death from breast cancer among previous users of OCs, compared with women who had never used them.20 This study included an analysis limited to women who had begun using OCs at 30 years of age or older.
OBG Management: What about women who have a family history of breast cancer? Do OCs and other hormonal contraceptives elevate their risk further?
Kaunitz: Women who have a family history of breast cancer are often cautioned that it would be unsafe for them to use hormonal contraception. However, use of hormonal contraception does not appear to impact the risk of breast cancer in women who have a family history of the disease.
A large prospective study from Canada involving women who had a family history of breast cancer and a mean age of 49 found no increased risk of breast cancer among former or current OC users.21 This study did not assess risk by BRCA mutation status.
A separate study found that the risk of breast cancer increased slightly among women who had a BRCA1 mutation, with an odds ratio of 1.20 (95% confidence interval, 1.02–1.40), but not among women who had a BRCA2 mutation.22 Another study found no significant increase in the risk of breast cancer among women who had either a BRCA1 or BRCA2 mutation.23
Benefits include improved bleeding patterns
OBG Management: Many perimenopausal women who have fibroids or adenomyosis experience menorrhagia or dysfunctional uterine bleeding (DUB) and opt for surgery such as endometrial ablation or hysterectomy. Can OCs or other hormonal contraceptives alleviate these patterns without the need for surgery?
Kaunitz: Yes. OCs can restore physiologic bleeding in older women who have DUB. One study involving women 15 to 50 years of age who had DUB found improved bleeding patterns in more than 80% of women randomized to OCs, compared with less than 50% of women randomized to placebo.24 In addition, women who have menorrhagia have reported a significant reduction of blood loss after using OCs.25
Another effective option for women who have menorrhagia is the levonorgestrel-releasing intrauterine system (LNG-IUS), even in women who have menorrhagia associated with fibroids and adenomyosis.26-28
Because long-term use of injectable forms of contraception tends to lead to amenorrhea, some physicians recommend DMPA as a treatment for menorrhagia. Data supporting this strategy are scant, however.29
OCs reduce the risk of three cancers
OBG Management: Oral contraceptives are known to reduce the risk of ovarian, endometrial, and colorectal cancer to varying degrees. Does this benefit hold up for older women, too?
Kaunitz: Yes. And because the incidence of ovarian cancer, in particular, increases with age, the protection afforded by combination OCs may be especially beneficial for women of older reproductive age.
OBG Management: Just how much protection against ovarian cancer does OC use afford?
Kaunitz: Among users of low-dose combination OCs, the risk of epithelial ovarian cancer declines by at least 50%, compared with women who have never used the pill—and, the longer the use, the greater the protection.16,30,31 Once OCs are discontinued, the protection diminishes over time, but some degree of reduced risk persists for three decades or longer.31
OBG Management: What about endometrial cancer?
Kaunitz: Not just OCs, but also DMPA, are associated with a significant reduction in the risk of endometrial cancer: 50% with use of OCs formulated with 30 μg or more of estrogen, and 80% with use of DMPA. In the case of OCs, the reduced risk is greater with longer use, and it persists after discontinuation for at least 20 years.25,32
OBG Management: Is the protection against colorectal cancer as great as the protection against these other cancers?
Kaunitz: No, it isn’t, but the protection is still significant. OC use reduces the risk of colorectal cancer by approximately 20%, but the protection against colorectal cancer does not appear to increase with duration of use.16,33 It also may be that more recent OC use (past 5 years) affords greater protection than use in the more distant past.16,33
OCs may reduce fracture risk postmenopausally
OBG Management: What effect do combination OCs and other forms of hormonal contraception have on the bone loss that accelerates around the time of menopause?
Kaunitz: One randomized trial found that OC use increases bone mineral density (BMD) in women of older reproductive age.34 And a population-based, case-control trial from Sweden found a 25% reduction in the risk of hip fracture among postmenopausal women who had a history of OC use. The reduction in risk was even greater when the women had used OCs in their 40s or for an extended duration.35
The Women’s Health Initiative found no reduction in the risk of fracture among previous users of OCs, but failed to stratify women by the age at which they used OCs.
OBG Management: Are any hormonal contraceptives associated with bone loss?
Kaunitz: Yes. Use of intramuscular DMPA (150 mg) or subcutaneous DMPA (104 mg) is linked to a loss of BMD. The good news is that BMD recovers after discontinuation of the drug, even in women who begin to use it after 40 years of age.29,36 However, we lack data on the risk of fracture among postmenopausal women with a history of DMPA use.
OCs may ease hot flushes and other menopausal symptoms
OBG Management: Is there any evidence that use of combination OCs by perimenopausal women relieves vasomotor symptoms?
Kaunitz: Yes, but the number of studies demonstrating this association so far has been limited. One small double-blind trial randomly assigned women to use of an OC containing 20 μg of estradiol or to placebo.37 Although the number and severity of symptoms diminished by about 50% in those taking the OC, the difference was not statistically significant.
A prospective observational study found that 90% of perimenopausal women experienced complete relief after taking an OC containing 30 μg of ethinyl estradiol, compared with only 40% of nonusers.38
OBG Management: What about other forms of hormonal contraception? Are any effective against vasomotor symptoms?
Kaunitz: One interesting option is to use menopausal doses of estrogen to treat vasomotor symptoms along with an LNG-IUS to prevent endometrial hyperplasia and provide contraception, if needed. This combination produced substantial improvement in a trial involving perimenopausal women who were experiencing vasomotor symptoms.39 Most of the women became amenorrheic, and there was no endometrial hyperplasia.
DMPA in contraceptive dosages also has relieved vasomotor symptoms in menopausal women, compared with placebo.40
OBG Management: What about women who experience vasomotor symptoms during the 7 placebo days of a 28-pill cycle? What options do they have?
Kaunitz: Some physicians either switch to a 24/4 OC formulation (Yaz or Lo-Estrin 24), an extended OC formulation with no placebo days (Seasonique), a continuous OC formulation (Lybrel), or simply prescribe pills from a traditional 21/7 pack in a continuous fashion so as to eliminate the hormone-free interval. However, this strategy has been studied to only a limited degree.
At what age should an OC be discontinued?
OBG Management: Perimenopausal women are, obviously, going to become menopausal at some point. How do you know when that transition occurs if they are taking OCs?
Kaunitz: It turns out that testing is not useful in this clinical setting. Some people have advocated measuring the follicle-stimulating hormone (FSH) level, but this strategy is unreliable. An elevated FSH level—thought to be indicative of menopause—has been found in older ovulatory women,41 and a depressed FSH level has been found in postmenopausal women for weeks after discontinuation of OCs.42
Rather than use this imperfect science to try and predict the point of menopause, I recommend discontinuing OCs once the woman has attained age 55, arbitrarily assuming that she is menopausal at this age. I use the same approach for women using other hormonal contraceptives.8,43
CASE Perimenopausal complaints, and a request for contraception
At her annual visit, M.B., a healthy 48-year-old divorced woman, reports that her periods are increasingly erratic and that she has begun experiencing occasional hot flushes. Although her previous husband had a vasectomy, she has started to date and is concerned about contraception. A close friend became pregnant at the age of 46 and chose to have an abortion. M.B. hopes to avoid the same fate and asks specifically about birth control pills. Is this an appropriate option for her? What do you tell her?
Although only 11% of women 40 to 44 years old reported using oral contraceptives (OCs) in 2002 in the United States, that figure represents a 5% increase over 1995,1,2 and all indications are that the percentage is still rising.
In lean, nonsmoking, healthy perimenopausal women, OCs offer users not only effective contraception, but also benefits that include a reduction in heavy menstrual bleeding; regularization of the menstrual cycle; protection against ovarian, endometrial, and colorectal cancer; prevention of bone loss (with possible prevention of postmenopausal osteoporotic fractures); and some degree of relief from vasomotor symptoms. Although an increased risk of venous thromboembolism (VTE) is well documented in OC users, concerns also exist that use of the pill might increase the risk of myocardial infarction (MI), stroke, and breast cancer in older reproductive-age women.
To explore the range of hormonal contraceptive options and their risks and benefits in perimenopausal women in more depth, OBG Management recently caught up with Andrew M. Kaunitz, MD, an expert in both contraception and menopause and a member of the OBG Management Board of Editors. He describes and interprets the robust data in this field to answer our many questions—although he points out that perimenopausal women have been underrepresented in studies of OC use in particular and hormonal contraception in general.
Why hormonal contraception?
OBG Management: Why is effective contraception important in this age group? Aren’t perimenopausal women less fertile than younger women?
Kaunitz: Older women are less fecund, but irregular menstrual cycles make it difficult to predict when ovulation is occurring, making unplanned pregnancy a real possibility in sexually active women.
Pregnancy itself is fraught with risks in this age group. Pregnancy-related mortality among women 40 years or older in the United States is five times higher than among 25- to 29-year-olds. Older women are also more likely to have comorbidities such as hypertension and diabetes, further increasing the risks of pregnancy.3,4 In addition, perimenopausal women are more likely than any reproductive age group except adolescents to opt for induced abortion when they do become pregnant, with 304 abortions for every 1,000 live births in women 40 years or older in the United States.5
OBG Management: Why should a perimenopausal woman consider hormonal contraception?
Kaunitz: It is highly effective and offers a range of noncontraceptive benefits, and older women are more likely to use it properly, making contraceptive failure less likely than in younger patients.
Nor are combination OCs the only option for this age group. Progestin-only OCs, the levonorgestrel-releasing intrauterine system, the etonogestrel implant, and injectable depot medroxyprogesterone acetate (DMPA) are alternatives. Although the vaginal patch and ring have not been studied extensively, they may be appropriate in some instances. Until further data specific to these combination estrogen–progestin methods are available, let’s assume for our discussion that they carry the same risk–benefit profile as combination OCs.
Thromboembolism is the greatest risk
OBG Management: What is the greatest risk of OC use in perimenopausal women?
Kaunitz: That would be VTE. The risk rises sharply after 39 years of age among users of combination OCs, with approximately 100 cases for every 100,000 person-years, compared with 25 cases for every 100,000 person-years among adolescents.6 This already elevated risk almost doubles among obese women older than 39 years.7 In these women, progestin-only or intrauterine contraceptives are better options than combination OCs.8
Also, avoid prescribing combination OCs for women with a known thrombophilic defect. However, because screening for thrombophilia is not cost-effective, routinely evaluating candidates for combination contraception with testing for familial thrombophilic disorders is not recommended.
OBG Management: Does the dosage of estrogen determine the risk of VTE?
Kaunitz: That is the general assumption—that higher dosages of estrogen pose a greater risk—but we lack definitive evidence that OCs formulated with 20 μg of estrogen are any safer in this regard than those that contain 30 to 35 μg.7,9
There is some evidence that the progestin plays a role. OCs that contain desogestrel appear to carry almost twice the risk of VTE as those formulated with levonorgestrel or norgestimate.10
TABLE
How selected health conditions affect choice of contraceptive in women ≥35 years
| Condition | Recommendation* |
|---|---|
| Obesity | Avoid combination contraceptives (OCs, patch, and ring) |
| Smoking | |
| Diabetes | |
| Migraine | |
| Hypertension | |
| * Based on guidelines from the American College of Obstetricians and Gynecologists8 | |
| † Includes progestin-only OCs, progestin implants, depot medroxyprogesterone acetate, and copper and progestin-releasing intrauterine devices | |
Risk of MI, stroke may rise in some older women
OBG Management: Do perimenopausal women who take combination OCs face a heightened risk of MI or stroke?
Kaunitz: Yes, if they smoke or have hypertension. The reason: In women who use combination OCs, smoking and hypertension are synergistic risk factors for MI and stroke. That means perimenopausal women who smoke or have high blood pressure should avoid combination contraceptives.
Although it is limited, available evidence supports the safety of OCs in older women who do not smoke or have hypertension. One large case-control study from the United States found no increased risk of MI or stroke among this population when they used OCs containing less than 50 μg of ethinyl estradiol.11,12 However, this study included few women older than 35 years who used OCs and smoked or had hypertension.
A large, prospective study from Sweden that included 1,761 current OC users between 40 and 49 years of age found no increased risk of MI among former or current OC users.13 It also found that the initiation of OC use in women 30 years of age or older carried no higher risk of MI than did initiation at age 29 or younger.
Avoid OCs in older women who have diabetes
OBG Management: What about women 35 years of age or older who have diabetes? Is hormonal contraception appropriate for them?
Kaunitz: Both premenopausal and postmenopausal women who have diabetes have a higher risk of cardiovascular disease, so combination contraceptives are a bad idea when the woman has diabetes and is 35 years of age or older. OCs also should be avoided in women younger than 35 years who have diabetes, unless they are normotensive and free of nephropathy and other vascular disease. Intrauterine contraception and progestin-only formulations tend to be better options for diabetic women.
Avoid combination OCs in perimenopausal migraineurs
OBG Management: Isn’t there evidence that women who have migraine headaches have an elevated stroke risk? How does this affect their choice of contraceptive?
Kaunitz: One case-control study from a large US health maintenance organization found twice the risk of stroke among OC users who had migraines as among those who did not.12 However, this study did not distinguish between women who had migraines with aura and those who had migraines without aura.
Another study found an increased risk of stroke among OC users who had migraines with aura, but not among those who had migraines without aura.14
Accordingly, both the American College of Obstetricians and Gynecologists (ACOG) and the World Health Organization recommend that older women who experience migraines use progestin-only or intrauterine contraception.8,15
Does estrogen use increase the risk of breast cancer?
OBG Management: It’s a common assumption that hormonal contraceptives that contain estrogen increase the risk of breast cancer. Is that assumption backed by data?
Kaunitz: Long-term use of combination estrogen–progestin menopausal hormone therapy modestly increases the risk of breast cancer. Accordingly, many clinicians and women assume that use of hormonal contraception must likewise increase risk. In fact, the evidence does not indicate that combination OCs or progestin-only contraceptives increase the risk of breast cancer. However, studies to date have involved a relatively small number of women older than 45 years.
For example, a large cohort study from the United Kingdom that involved more than 1 million person-years of follow-up found no association between use of OCs and breast cancer, even among long-term users.16 Most cases of OC use in this study involved OCs formulated with 50 μg or more of ethinyl estradiol. However, this study did not indicate the age at which women used OCs.
In the Women’s Contraceptive and Reproductive Experiences (CARE) study, current or previous users of OCs had no increased risk of invasive or in situ breast cancer, compared with never-users.17,18 This study did include a subgroup of women who had started using OCs after age 40. Nor did the CARE study find an association between progestin-only injectable DMPA or implantable contraceptives and breast cancer.19
Last, a population-based case-control study in the United States found no increased risk of death from breast cancer among previous users of OCs, compared with women who had never used them.20 This study included an analysis limited to women who had begun using OCs at 30 years of age or older.
OBG Management: What about women who have a family history of breast cancer? Do OCs and other hormonal contraceptives elevate their risk further?
Kaunitz: Women who have a family history of breast cancer are often cautioned that it would be unsafe for them to use hormonal contraception. However, use of hormonal contraception does not appear to impact the risk of breast cancer in women who have a family history of the disease.
A large prospective study from Canada involving women who had a family history of breast cancer and a mean age of 49 found no increased risk of breast cancer among former or current OC users.21 This study did not assess risk by BRCA mutation status.
A separate study found that the risk of breast cancer increased slightly among women who had a BRCA1 mutation, with an odds ratio of 1.20 (95% confidence interval, 1.02–1.40), but not among women who had a BRCA2 mutation.22 Another study found no significant increase in the risk of breast cancer among women who had either a BRCA1 or BRCA2 mutation.23
Benefits include improved bleeding patterns
OBG Management: Many perimenopausal women who have fibroids or adenomyosis experience menorrhagia or dysfunctional uterine bleeding (DUB) and opt for surgery such as endometrial ablation or hysterectomy. Can OCs or other hormonal contraceptives alleviate these patterns without the need for surgery?
Kaunitz: Yes. OCs can restore physiologic bleeding in older women who have DUB. One study involving women 15 to 50 years of age who had DUB found improved bleeding patterns in more than 80% of women randomized to OCs, compared with less than 50% of women randomized to placebo.24 In addition, women who have menorrhagia have reported a significant reduction of blood loss after using OCs.25
Another effective option for women who have menorrhagia is the levonorgestrel-releasing intrauterine system (LNG-IUS), even in women who have menorrhagia associated with fibroids and adenomyosis.26-28
Because long-term use of injectable forms of contraception tends to lead to amenorrhea, some physicians recommend DMPA as a treatment for menorrhagia. Data supporting this strategy are scant, however.29
OCs reduce the risk of three cancers
OBG Management: Oral contraceptives are known to reduce the risk of ovarian, endometrial, and colorectal cancer to varying degrees. Does this benefit hold up for older women, too?
Kaunitz: Yes. And because the incidence of ovarian cancer, in particular, increases with age, the protection afforded by combination OCs may be especially beneficial for women of older reproductive age.
OBG Management: Just how much protection against ovarian cancer does OC use afford?
Kaunitz: Among users of low-dose combination OCs, the risk of epithelial ovarian cancer declines by at least 50%, compared with women who have never used the pill—and, the longer the use, the greater the protection.16,30,31 Once OCs are discontinued, the protection diminishes over time, but some degree of reduced risk persists for three decades or longer.31
OBG Management: What about endometrial cancer?
Kaunitz: Not just OCs, but also DMPA, are associated with a significant reduction in the risk of endometrial cancer: 50% with use of OCs formulated with 30 μg or more of estrogen, and 80% with use of DMPA. In the case of OCs, the reduced risk is greater with longer use, and it persists after discontinuation for at least 20 years.25,32
OBG Management: Is the protection against colorectal cancer as great as the protection against these other cancers?
Kaunitz: No, it isn’t, but the protection is still significant. OC use reduces the risk of colorectal cancer by approximately 20%, but the protection against colorectal cancer does not appear to increase with duration of use.16,33 It also may be that more recent OC use (past 5 years) affords greater protection than use in the more distant past.16,33
OCs may reduce fracture risk postmenopausally
OBG Management: What effect do combination OCs and other forms of hormonal contraception have on the bone loss that accelerates around the time of menopause?
Kaunitz: One randomized trial found that OC use increases bone mineral density (BMD) in women of older reproductive age.34 And a population-based, case-control trial from Sweden found a 25% reduction in the risk of hip fracture among postmenopausal women who had a history of OC use. The reduction in risk was even greater when the women had used OCs in their 40s or for an extended duration.35
The Women’s Health Initiative found no reduction in the risk of fracture among previous users of OCs, but failed to stratify women by the age at which they used OCs.
OBG Management: Are any hormonal contraceptives associated with bone loss?
Kaunitz: Yes. Use of intramuscular DMPA (150 mg) or subcutaneous DMPA (104 mg) is linked to a loss of BMD. The good news is that BMD recovers after discontinuation of the drug, even in women who begin to use it after 40 years of age.29,36 However, we lack data on the risk of fracture among postmenopausal women with a history of DMPA use.
OCs may ease hot flushes and other menopausal symptoms
OBG Management: Is there any evidence that use of combination OCs by perimenopausal women relieves vasomotor symptoms?
Kaunitz: Yes, but the number of studies demonstrating this association so far has been limited. One small double-blind trial randomly assigned women to use of an OC containing 20 μg of estradiol or to placebo.37 Although the number and severity of symptoms diminished by about 50% in those taking the OC, the difference was not statistically significant.
A prospective observational study found that 90% of perimenopausal women experienced complete relief after taking an OC containing 30 μg of ethinyl estradiol, compared with only 40% of nonusers.38
OBG Management: What about other forms of hormonal contraception? Are any effective against vasomotor symptoms?
Kaunitz: One interesting option is to use menopausal doses of estrogen to treat vasomotor symptoms along with an LNG-IUS to prevent endometrial hyperplasia and provide contraception, if needed. This combination produced substantial improvement in a trial involving perimenopausal women who were experiencing vasomotor symptoms.39 Most of the women became amenorrheic, and there was no endometrial hyperplasia.
DMPA in contraceptive dosages also has relieved vasomotor symptoms in menopausal women, compared with placebo.40
OBG Management: What about women who experience vasomotor symptoms during the 7 placebo days of a 28-pill cycle? What options do they have?
Kaunitz: Some physicians either switch to a 24/4 OC formulation (Yaz or Lo-Estrin 24), an extended OC formulation with no placebo days (Seasonique), a continuous OC formulation (Lybrel), or simply prescribe pills from a traditional 21/7 pack in a continuous fashion so as to eliminate the hormone-free interval. However, this strategy has been studied to only a limited degree.
At what age should an OC be discontinued?
OBG Management: Perimenopausal women are, obviously, going to become menopausal at some point. How do you know when that transition occurs if they are taking OCs?
Kaunitz: It turns out that testing is not useful in this clinical setting. Some people have advocated measuring the follicle-stimulating hormone (FSH) level, but this strategy is unreliable. An elevated FSH level—thought to be indicative of menopause—has been found in older ovulatory women,41 and a depressed FSH level has been found in postmenopausal women for weeks after discontinuation of OCs.42
Rather than use this imperfect science to try and predict the point of menopause, I recommend discontinuing OCs once the woman has attained age 55, arbitrarily assuming that she is menopausal at this age. I use the same approach for women using other hormonal contraceptives.8,43
1. Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Advance data from vital and health statistics. No. 350. Hyattsville, MD: National Center for Health Statistics, December 10, 2004.
2. Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital and health statistics. Series 23. No. 19. Hyattsville, MD: National Center for Health Statistics, May 1997:1–114. (DHHS publication no. (PHS) 97–1995.)
3. Callaghan WM, Berg CJ. Pregnancy-related mortality among women aged 35 years and older, United States, 1991–1997. Obstet Gynecol. 2003;102:1015-1021.
4. Viegas OA, Leong WP, Ahmed S, Ratnam SS. Obstetrical outcome with increasing maternal age. J Biosoc Sci. 1994;26:261-267.
5. Strauss LT, Herndon J, Chang J, et al. Abortion surveillance—United States, 2001. MMWR Surveill Summ. 2004;53(SS–9):1-32.
6. Nightingale AL, Lawrenson RA, Simpson EL, Williams TJ, MacRae KD, Farmer RDT. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur J Contracept Reprod Health Care. 2000;5:265-274.
7. Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP, Jr. Venous thromboembolic disease in users of low-estrogen combined estrogen–progestin oral contraceptives. Contraception. 2004;70:3-10.
8. ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 73: Use of hormonal contraceptive in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
9. Gallo MF, Nanda K, Grimes DA, Schulz KF. Twenty micrograms vs. >20 microg estrogen oral contraceptives for contraception: systematic review of randomized controlled trials. Contraception. 2005;71:162-169.
10. Jick SS, Kaye JA, Russman S, Jick H. Risk of nonfatal venous thromboembolism with oral contraceptives containing levonorgestrel. Contraception. 2006;73:566-570.
11. Sidney S, Siscovick DS, Petitti DB, et al. Myocardial infarction and use of low-dose oral contraceptives: a pooled analysis of 2 US studies. Circulation. 1998;98:1058-1063.
12. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29:2277-2284.
13. Margolis KL, Adami H-O, Luo J, Ye W, Weider-pass E. A prospective study of oral contraceptive use and risk of myocardial infarction among Swedish women. Fertil Steril. 2007;88:310-316.
14. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2345.
15. Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: World Health Organization, 2004.
16. Hannaford PC, Selvaraj S, Elliott AM, Angus V, Iversen L, Lee AJ. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioners’ oral contraception study. BMJ. 2007;335:651.-
17. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
18. Gill JK, Press MF, Patel AV, Bernstein L. Oral contraceptive use and risk of breast carcinoma in situ (United States). Cancer Causes Control. 2006;17:1155-1162.
19. Strom BL, Berlin JA, Weber AL, et al. Absence of an effect of injectable and implantable progestin-only contraceptives on subsequent risk of breast cancer. Contraception. 2004;69:353-360.
20. Wingo PA, Austin A, Marchbanks PA, et al. Oral contraceptives and the risk of death from breast cancer. Obstet Gynecol. 2007;110:793-800.
21. Silvera SA, Miller AB, Rohan TE. Oral contraceptive use and risk of breast cancer among women with a family history of breast cancer: a prospective cohort study. Cancer Causes Control. 2005;16:1059-1063.
22. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
23. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
24. Davis A, Godwin A, Lippman J, Olson W, Kafrissen M. Triphasic norgestimate-ethinyl estradiol for treating dysfunctional uterine bleeding. Obstet Gynecol. 2000;96:913-920.
25. Kaunitz AM. Noncontraceptive health benefits of oral contraceptives. Rev Endocr Metab Disord. 2002;3:277-283.
26. Hurskainen R, Reperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456-1463.
27. Kaunitz AM. Progestin-releasing intrauterine systems and leiomyoma. Contraception. 2007;75 (6 Suppl):S130-S133.
28. Bragheto AM, Caserta N, Bahamondes L, Petta CA. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
29. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. In: Rose BD, ed. UpToDate. Wellesley, MA: UpToDate, 2008.
30. Petitti DB. Combination estrogen–progestin oral contraceptives. N Engl J Med. 2003;349:1443-1450.[Erratum, N Engl J Med. 2004;350:92.]
31. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303-314.
32. Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives: a practitioner’s guide to meta-analysis. Hum Reprod. 1997;12:1851-1863.
33. Fernandez E, LaVecchia C, Balducci A, Chatenoud L, Francheschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722-727.
34. Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54:176-180.
35. Michaëlsson K, Baron JA, Farahmand BY, Persson I, Ljunghall S. Oral-contraceptive use and risk of hip fracture: a case-control study. Lancet. 1999;353:1481-1484.
36. Rosenberg L, Zhang Y, Constant D, et al. Bone status after cessation of use of injectable progestin contraceptives. Contraception. 2007;76:425-431.
37. Casper RF, Dodin S, Reid RL. The effect of 20 μg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes, and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
38. Shargil AA. Hormone replacement therapy in perimenopausal women with a triphasic contraceptive compound: a three-year prospective study. Int J Fertil. 1985;30:15,18-28.
39. Hampton NRE, Rees MCP, Lowe DG, Rauramo I, Barlow D, Guillebaud J. Levonorgestrel intrauterine system (LNG-IUS) with conjugated equine estrogen: a successful regimen for HRT in perimenopausal women. Hum Reprod. 2005;20:2653-2669.
40. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
41. Gebbie AE, Glasier A, Sweeting V. Incidence of ovulation in perimenopausal women before and during hormone replacement therapy. Contraception. 1995;52:221-222.
42. Creinin MD. Laboratory criteria for menopause in women using oral contraceptives. Fertil Steril. 1996;66:101-104.
43. Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270.
1. Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Advance data from vital and health statistics. No. 350. Hyattsville, MD: National Center for Health Statistics, December 10, 2004.
2. Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital and health statistics. Series 23. No. 19. Hyattsville, MD: National Center for Health Statistics, May 1997:1–114. (DHHS publication no. (PHS) 97–1995.)
3. Callaghan WM, Berg CJ. Pregnancy-related mortality among women aged 35 years and older, United States, 1991–1997. Obstet Gynecol. 2003;102:1015-1021.
4. Viegas OA, Leong WP, Ahmed S, Ratnam SS. Obstetrical outcome with increasing maternal age. J Biosoc Sci. 1994;26:261-267.
5. Strauss LT, Herndon J, Chang J, et al. Abortion surveillance—United States, 2001. MMWR Surveill Summ. 2004;53(SS–9):1-32.
6. Nightingale AL, Lawrenson RA, Simpson EL, Williams TJ, MacRae KD, Farmer RDT. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur J Contracept Reprod Health Care. 2000;5:265-274.
7. Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP, Jr. Venous thromboembolic disease in users of low-estrogen combined estrogen–progestin oral contraceptives. Contraception. 2004;70:3-10.
8. ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 73: Use of hormonal contraceptive in women with coexisting medical conditions. Obstet Gynecol. 2006;107:1453-1472.
9. Gallo MF, Nanda K, Grimes DA, Schulz KF. Twenty micrograms vs. >20 microg estrogen oral contraceptives for contraception: systematic review of randomized controlled trials. Contraception. 2005;71:162-169.
10. Jick SS, Kaye JA, Russman S, Jick H. Risk of nonfatal venous thromboembolism with oral contraceptives containing levonorgestrel. Contraception. 2006;73:566-570.
11. Sidney S, Siscovick DS, Petitti DB, et al. Myocardial infarction and use of low-dose oral contraceptives: a pooled analysis of 2 US studies. Circulation. 1998;98:1058-1063.
12. Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998;29:2277-2284.
13. Margolis KL, Adami H-O, Luo J, Ye W, Weider-pass E. A prospective study of oral contraceptive use and risk of myocardial infarction among Swedish women. Fertil Steril. 2007;88:310-316.
14. MacClellan LR, Giles W, Cole J, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438-2345.
15. Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: World Health Organization, 2004.
16. Hannaford PC, Selvaraj S, Elliott AM, Angus V, Iversen L, Lee AJ. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioners’ oral contraception study. BMJ. 2007;335:651.-
17. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032.
18. Gill JK, Press MF, Patel AV, Bernstein L. Oral contraceptive use and risk of breast carcinoma in situ (United States). Cancer Causes Control. 2006;17:1155-1162.
19. Strom BL, Berlin JA, Weber AL, et al. Absence of an effect of injectable and implantable progestin-only contraceptives on subsequent risk of breast cancer. Contraception. 2004;69:353-360.
20. Wingo PA, Austin A, Marchbanks PA, et al. Oral contraceptives and the risk of death from breast cancer. Obstet Gynecol. 2007;110:793-800.
21. Silvera SA, Miller AB, Rohan TE. Oral contraceptive use and risk of breast cancer among women with a family history of breast cancer: a prospective cohort study. Cancer Causes Control. 2005;16:1059-1063.
22. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
23. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
24. Davis A, Godwin A, Lippman J, Olson W, Kafrissen M. Triphasic norgestimate-ethinyl estradiol for treating dysfunctional uterine bleeding. Obstet Gynecol. 2000;96:913-920.
25. Kaunitz AM. Noncontraceptive health benefits of oral contraceptives. Rev Endocr Metab Disord. 2002;3:277-283.
26. Hurskainen R, Reperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456-1463.
27. Kaunitz AM. Progestin-releasing intrauterine systems and leiomyoma. Contraception. 2007;75 (6 Suppl):S130-S133.
28. Bragheto AM, Caserta N, Bahamondes L, Petta CA. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
29. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. In: Rose BD, ed. UpToDate. Wellesley, MA: UpToDate, 2008.
30. Petitti DB. Combination estrogen–progestin oral contraceptives. N Engl J Med. 2003;349:1443-1450.[Erratum, N Engl J Med. 2004;350:92.]
31. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303-314.
32. Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives: a practitioner’s guide to meta-analysis. Hum Reprod. 1997;12:1851-1863.
33. Fernandez E, LaVecchia C, Balducci A, Chatenoud L, Francheschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722-727.
34. Gambacciani M, Cappagli B, Lazzarini V, Ciaponi M, Fruzzetti F, Genazzani AR. Longitudinal evaluation of perimenopausal bone loss: effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas. 2006;54:176-180.
35. Michaëlsson K, Baron JA, Farahmand BY, Persson I, Ljunghall S. Oral-contraceptive use and risk of hip fracture: a case-control study. Lancet. 1999;353:1481-1484.
36. Rosenberg L, Zhang Y, Constant D, et al. Bone status after cessation of use of injectable progestin contraceptives. Contraception. 2007;76:425-431.
37. Casper RF, Dodin S, Reid RL. The effect of 20 μg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes, and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
38. Shargil AA. Hormone replacement therapy in perimenopausal women with a triphasic contraceptive compound: a three-year prospective study. Int J Fertil. 1985;30:15,18-28.
39. Hampton NRE, Rees MCP, Lowe DG, Rauramo I, Barlow D, Guillebaud J. Levonorgestrel intrauterine system (LNG-IUS) with conjugated equine estrogen: a successful regimen for HRT in perimenopausal women. Hum Reprod. 2005;20:2653-2669.
40. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
41. Gebbie AE, Glasier A, Sweeting V. Incidence of ovulation in perimenopausal women before and during hormone replacement therapy. Contraception. 1995;52:221-222.
42. Creinin MD. Laboratory criteria for menopause in women using oral contraceptives. Fertil Steril. 1996;66:101-104.
43. Kaunitz AM. Hormonal contraception in women of older reproductive age. N Engl J Med. 2008;358:1262-1270.
