User login
Confusion recurs 2 weeks after fall
A 77-year-old woman presented to the emergency department complaining of a headache following a syncopal episode (while standing) earlier that day. She said that she’d lost consciousness for several minutes, and then experienced several minutes of mild confusion that resolved spontaneously.
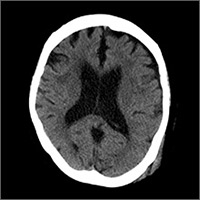
On physical exam, she was oriented to person and place, but not time. She had a contusion in her left occipitoparietal region without extensive bruising or deformity. The patient had normal cardiopulmonary, abdominal, and neurologic exams. Her past medical history included hypertension and normal pressure hydrocephalus, and her vital signs were within normal limits. She was taking aspirin once daily.The patient’s initial head and neck computerized tomography (CT) scans were normal (FIGURE 1), but she was hospitalized because of her confusion. During her hospitalization, the patient had mild episodic headaches that resolved with acetaminophen. The next day, her confusion resolved, and repeat CT scans were unchanged. She was discharged within 24 hours.
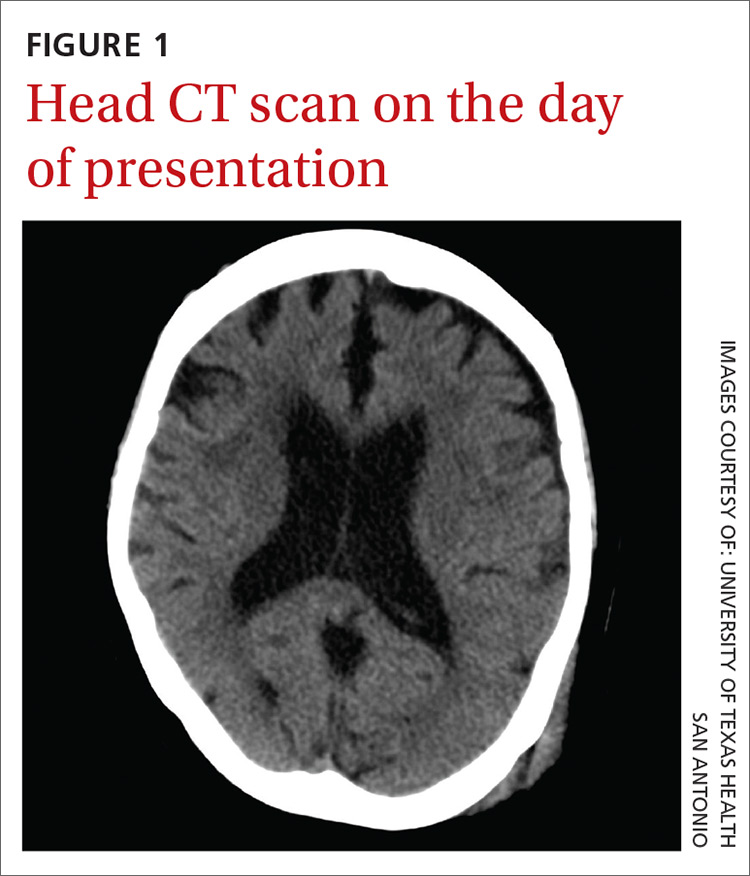
Two weeks later, the patient returned to the hospital after her daughter found her on the toilet, unable to stand up from the sitting position. She was confused and experienced a worsening of headache during transport to the hospital. No recurrent falls or additional episodes of trauma were reported. A CT scan was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Delayed acute subdural hematoma
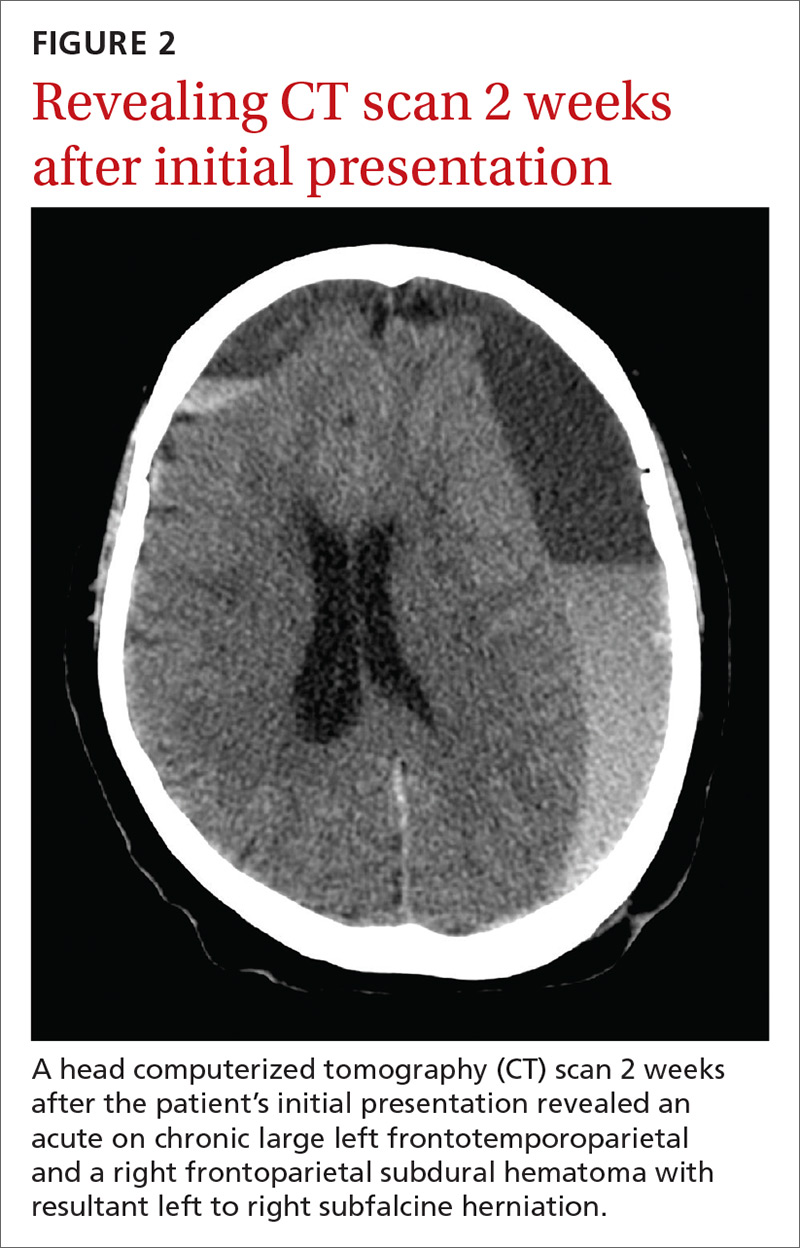
The CT scan (FIGURE 2) revealed an acute on chronic large left frontotemporoparietal and a right frontoparietal subdural hematoma (SDH) with resultant left to right subfalcine herniation. The patient was given a diagnosis of a delayed acute subdural hematoma (DASH)—an acute subdural hematoma that is not apparent on an initial CT scan, but is detected on follow-up CT imaging days or weeks after the injury.1 The incidence of DASH is approximately 0.5% among acute SDH patients who require operative treatment.1
Because DASH is rare, there is a lot of uncertainty surrounding its presentation, pathophysiology, and outcomes. In the few cases that have been described, patients have varied from those who were healthy, and had no coagulation abnormalities, to those who were elderly and taking anticoagulants.2,3 In addition, the period between the head injury and the development of SDH is variable.3
While not much is known about DASH, the mechanism of acute SDH has been widely studied and researched. Acute SDH, which typically follows a head trauma, results from the tearing of bridging veins that lack supporting structures and are most vulnerable to injury when crossing the subdural space.4 The potential pathophysiology for DASH is not completely understood, but is likely to involve subtle damage to the bridging veins of the brain that continue to leak over a matter of hours and days.1,5
Two risk factors to consider. Increasing age and use of oral anticoagulants can increase the risk of developing an intracranial lesion after head injury.3 Due to the infrequency of DASH, the same risk factors for SDH should be considered for DASH. These factors make it increasingly important to establish guidelines on how to approach mild traumatic brain injury (TBI) in both DASH and SDH, especially for those who are elderly or have been on anticoagulation therapy.
Differential Dx
The differential diagnosis for our patient’s decline and altered mentation weeks after the initial event included worsening normal pressure hydrocephalus, cerebrovascular accident, and seizure.
Normal pressure hydrocephalus typically has a more chronic onset than DASH. It manifests with the classic triad of dementia, incontinence, and magnetic or festinating gait (“wild, wet, and wobbly”).
Cerebrovascular accidents are most often associated with focal neurologic deficits, which can be ischemic or hemorrhagic. If hemorrhagic, the hemorrhage is typically parenchymal and not subdural.
Seizure, especially partial complex seizure, can arise after trauma and may not involve obvious motor movements. Symptoms generally abate over a few minutes to hours with treatment. Electroencephalogram and CT scan can differentiate seizure from a subdural hematoma.
Keep DASH on your radar screen
The American College of Emergency Physicians states that a non-contrast head CT scan is indicated in head trauma patients with loss of consciousness if one or more of the following is present: age >60 years, vomiting, headache, drug or alcohol intoxication, short-term memory deficits, posttraumatic seizure, Glasgow Coma Scale score of <15, focal neurologic deficits, and coagulopathy.6
Some have suggested that the initial head CT scan be delayed by up to 8 hours to prevent missing a slowly developing intracranial hemorrhage. Others suggest that the CT scan be repeated at 24 hours. Still others have suggested that patients with even mild TBI be admitted for a period of observation if any risk factors, such as age or history of anticoagulation therapy, are noted.
Because there is no evidence to support delaying the initial head CT scan, physicians should be thorough in their evaluation of head trauma patients with loss of consciousness and consider a repeat CT scan of the head if worsening of any symptoms occurs. Physicians should also consider a repeat CT scan of the head for patients at high risk, including the elderly and those who have taken anticoagulants.
In addition, patients with traumatic head injuries must be properly counseled to return if they experience repeated vomiting, worsening headache, memory loss, confusion, focal neurologic deficit, abnormal behavior, increased sleepiness, or seizures.6 An extra precaution for high-risk patients includes suggesting adequate follow-up with a primary care physician to help monitor recovery and prevent any occurrences of DASH from going unnoticed.
Our patient underwent a mini-craniotomy. Postoperatively, she was discharged to a skilled nursing facility and ultimately made a complete recovery.
CORRESPONDENCE
Andrew Muck, MD, Department of Emergency Medicine, University of Texas Health San Antonio, 7703 Floyd Curl Drive, MSC 7736, San Antonio, TX 78229; [email protected].
1. Cohen T, Gudeman S. In: Narayan RK, ed. Delayed Traumatic Intracranial Hematoma. Neurotrauma. New York, NY: McGraw-Hill;1995:689-701.
2. Matsuda W, Sugimoto K, Sato N, et al. Delayed onset of posttraumatic acute subdural hematoma after mild head injury with normal computed tomography: a case report and brief review. J Trauma. 2008;65:461-463.
3. Itshayek E, Rosenthal G, Fraifeld S, et al. Delayed posttraumatic acute subdural hematoma in elderly patients on anticoagulation. Neurosurgery. 2006;58:E851-E856.
4. Culotta VP, Sermentilli ME, Gerold K, et al. Clinicopathological heterogeneity in the classification of mild head injury. Neurosurgery. 1996;38:245-250.
5. Shabani S, Nguyen HS, Doan N, et al. Case Report and Review of Literature of Delayed Acute Subdural Hematoma. World Neurosurg. 2016;96:66-71.
6. Jagoda AS, Bazarian JJ, Bruns Jr JJ, et al. Clinical Policy: Neuroimaging and Decisionmaking in Adult Mild Traumatic Brain Injury in the Acute Setting. Ann Emerg Med. 2008;52:714-748.
A 77-year-old woman presented to the emergency department complaining of a headache following a syncopal episode (while standing) earlier that day. She said that she’d lost consciousness for several minutes, and then experienced several minutes of mild confusion that resolved spontaneously.
On physical exam, she was oriented to person and place, but not time. She had a contusion in her left occipitoparietal region without extensive bruising or deformity. The patient had normal cardiopulmonary, abdominal, and neurologic exams. Her past medical history included hypertension and normal pressure hydrocephalus, and her vital signs were within normal limits. She was taking aspirin once daily.The patient’s initial head and neck computerized tomography (CT) scans were normal (FIGURE 1), but she was hospitalized because of her confusion. During her hospitalization, the patient had mild episodic headaches that resolved with acetaminophen. The next day, her confusion resolved, and repeat CT scans were unchanged. She was discharged within 24 hours.

Two weeks later, the patient returned to the hospital after her daughter found her on the toilet, unable to stand up from the sitting position. She was confused and experienced a worsening of headache during transport to the hospital. No recurrent falls or additional episodes of trauma were reported. A CT scan was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Delayed acute subdural hematoma
The CT scan (FIGURE 2) revealed an acute on chronic large left frontotemporoparietal and a right frontoparietal subdural hematoma (SDH) with resultant left to right subfalcine herniation. The patient was given a diagnosis of a delayed acute subdural hematoma (DASH)—an acute subdural hematoma that is not apparent on an initial CT scan, but is detected on follow-up CT imaging days or weeks after the injury.1 The incidence of DASH is approximately 0.5% among acute SDH patients who require operative treatment.1

Because DASH is rare, there is a lot of uncertainty surrounding its presentation, pathophysiology, and outcomes. In the few cases that have been described, patients have varied from those who were healthy, and had no coagulation abnormalities, to those who were elderly and taking anticoagulants.2,3 In addition, the period between the head injury and the development of SDH is variable.3
While not much is known about DASH, the mechanism of acute SDH has been widely studied and researched. Acute SDH, which typically follows a head trauma, results from the tearing of bridging veins that lack supporting structures and are most vulnerable to injury when crossing the subdural space.4 The potential pathophysiology for DASH is not completely understood, but is likely to involve subtle damage to the bridging veins of the brain that continue to leak over a matter of hours and days.1,5
Two risk factors to consider. Increasing age and use of oral anticoagulants can increase the risk of developing an intracranial lesion after head injury.3 Due to the infrequency of DASH, the same risk factors for SDH should be considered for DASH. These factors make it increasingly important to establish guidelines on how to approach mild traumatic brain injury (TBI) in both DASH and SDH, especially for those who are elderly or have been on anticoagulation therapy.
Differential Dx
The differential diagnosis for our patient’s decline and altered mentation weeks after the initial event included worsening normal pressure hydrocephalus, cerebrovascular accident, and seizure.
Normal pressure hydrocephalus typically has a more chronic onset than DASH. It manifests with the classic triad of dementia, incontinence, and magnetic or festinating gait (“wild, wet, and wobbly”).
Cerebrovascular accidents are most often associated with focal neurologic deficits, which can be ischemic or hemorrhagic. If hemorrhagic, the hemorrhage is typically parenchymal and not subdural.
Seizure, especially partial complex seizure, can arise after trauma and may not involve obvious motor movements. Symptoms generally abate over a few minutes to hours with treatment. Electroencephalogram and CT scan can differentiate seizure from a subdural hematoma.
Keep DASH on your radar screen
The American College of Emergency Physicians states that a non-contrast head CT scan is indicated in head trauma patients with loss of consciousness if one or more of the following is present: age >60 years, vomiting, headache, drug or alcohol intoxication, short-term memory deficits, posttraumatic seizure, Glasgow Coma Scale score of <15, focal neurologic deficits, and coagulopathy.6
Some have suggested that the initial head CT scan be delayed by up to 8 hours to prevent missing a slowly developing intracranial hemorrhage. Others suggest that the CT scan be repeated at 24 hours. Still others have suggested that patients with even mild TBI be admitted for a period of observation if any risk factors, such as age or history of anticoagulation therapy, are noted.
Because there is no evidence to support delaying the initial head CT scan, physicians should be thorough in their evaluation of head trauma patients with loss of consciousness and consider a repeat CT scan of the head if worsening of any symptoms occurs. Physicians should also consider a repeat CT scan of the head for patients at high risk, including the elderly and those who have taken anticoagulants.
In addition, patients with traumatic head injuries must be properly counseled to return if they experience repeated vomiting, worsening headache, memory loss, confusion, focal neurologic deficit, abnormal behavior, increased sleepiness, or seizures.6 An extra precaution for high-risk patients includes suggesting adequate follow-up with a primary care physician to help monitor recovery and prevent any occurrences of DASH from going unnoticed.
Our patient underwent a mini-craniotomy. Postoperatively, she was discharged to a skilled nursing facility and ultimately made a complete recovery.
CORRESPONDENCE
Andrew Muck, MD, Department of Emergency Medicine, University of Texas Health San Antonio, 7703 Floyd Curl Drive, MSC 7736, San Antonio, TX 78229; [email protected].
A 77-year-old woman presented to the emergency department complaining of a headache following a syncopal episode (while standing) earlier that day. She said that she’d lost consciousness for several minutes, and then experienced several minutes of mild confusion that resolved spontaneously.
On physical exam, she was oriented to person and place, but not time. She had a contusion in her left occipitoparietal region without extensive bruising or deformity. The patient had normal cardiopulmonary, abdominal, and neurologic exams. Her past medical history included hypertension and normal pressure hydrocephalus, and her vital signs were within normal limits. She was taking aspirin once daily.The patient’s initial head and neck computerized tomography (CT) scans were normal (FIGURE 1), but she was hospitalized because of her confusion. During her hospitalization, the patient had mild episodic headaches that resolved with acetaminophen. The next day, her confusion resolved, and repeat CT scans were unchanged. She was discharged within 24 hours.

Two weeks later, the patient returned to the hospital after her daughter found her on the toilet, unable to stand up from the sitting position. She was confused and experienced a worsening of headache during transport to the hospital. No recurrent falls or additional episodes of trauma were reported. A CT scan was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Delayed acute subdural hematoma
The CT scan (FIGURE 2) revealed an acute on chronic large left frontotemporoparietal and a right frontoparietal subdural hematoma (SDH) with resultant left to right subfalcine herniation. The patient was given a diagnosis of a delayed acute subdural hematoma (DASH)—an acute subdural hematoma that is not apparent on an initial CT scan, but is detected on follow-up CT imaging days or weeks after the injury.1 The incidence of DASH is approximately 0.5% among acute SDH patients who require operative treatment.1

Because DASH is rare, there is a lot of uncertainty surrounding its presentation, pathophysiology, and outcomes. In the few cases that have been described, patients have varied from those who were healthy, and had no coagulation abnormalities, to those who were elderly and taking anticoagulants.2,3 In addition, the period between the head injury and the development of SDH is variable.3
While not much is known about DASH, the mechanism of acute SDH has been widely studied and researched. Acute SDH, which typically follows a head trauma, results from the tearing of bridging veins that lack supporting structures and are most vulnerable to injury when crossing the subdural space.4 The potential pathophysiology for DASH is not completely understood, but is likely to involve subtle damage to the bridging veins of the brain that continue to leak over a matter of hours and days.1,5
Two risk factors to consider. Increasing age and use of oral anticoagulants can increase the risk of developing an intracranial lesion after head injury.3 Due to the infrequency of DASH, the same risk factors for SDH should be considered for DASH. These factors make it increasingly important to establish guidelines on how to approach mild traumatic brain injury (TBI) in both DASH and SDH, especially for those who are elderly or have been on anticoagulation therapy.
Differential Dx
The differential diagnosis for our patient’s decline and altered mentation weeks after the initial event included worsening normal pressure hydrocephalus, cerebrovascular accident, and seizure.
Normal pressure hydrocephalus typically has a more chronic onset than DASH. It manifests with the classic triad of dementia, incontinence, and magnetic or festinating gait (“wild, wet, and wobbly”).
Cerebrovascular accidents are most often associated with focal neurologic deficits, which can be ischemic or hemorrhagic. If hemorrhagic, the hemorrhage is typically parenchymal and not subdural.
Seizure, especially partial complex seizure, can arise after trauma and may not involve obvious motor movements. Symptoms generally abate over a few minutes to hours with treatment. Electroencephalogram and CT scan can differentiate seizure from a subdural hematoma.
Keep DASH on your radar screen
The American College of Emergency Physicians states that a non-contrast head CT scan is indicated in head trauma patients with loss of consciousness if one or more of the following is present: age >60 years, vomiting, headache, drug or alcohol intoxication, short-term memory deficits, posttraumatic seizure, Glasgow Coma Scale score of <15, focal neurologic deficits, and coagulopathy.6
Some have suggested that the initial head CT scan be delayed by up to 8 hours to prevent missing a slowly developing intracranial hemorrhage. Others suggest that the CT scan be repeated at 24 hours. Still others have suggested that patients with even mild TBI be admitted for a period of observation if any risk factors, such as age or history of anticoagulation therapy, are noted.
Because there is no evidence to support delaying the initial head CT scan, physicians should be thorough in their evaluation of head trauma patients with loss of consciousness and consider a repeat CT scan of the head if worsening of any symptoms occurs. Physicians should also consider a repeat CT scan of the head for patients at high risk, including the elderly and those who have taken anticoagulants.
In addition, patients with traumatic head injuries must be properly counseled to return if they experience repeated vomiting, worsening headache, memory loss, confusion, focal neurologic deficit, abnormal behavior, increased sleepiness, or seizures.6 An extra precaution for high-risk patients includes suggesting adequate follow-up with a primary care physician to help monitor recovery and prevent any occurrences of DASH from going unnoticed.
Our patient underwent a mini-craniotomy. Postoperatively, she was discharged to a skilled nursing facility and ultimately made a complete recovery.
CORRESPONDENCE
Andrew Muck, MD, Department of Emergency Medicine, University of Texas Health San Antonio, 7703 Floyd Curl Drive, MSC 7736, San Antonio, TX 78229; [email protected].
1. Cohen T, Gudeman S. In: Narayan RK, ed. Delayed Traumatic Intracranial Hematoma. Neurotrauma. New York, NY: McGraw-Hill;1995:689-701.
2. Matsuda W, Sugimoto K, Sato N, et al. Delayed onset of posttraumatic acute subdural hematoma after mild head injury with normal computed tomography: a case report and brief review. J Trauma. 2008;65:461-463.
3. Itshayek E, Rosenthal G, Fraifeld S, et al. Delayed posttraumatic acute subdural hematoma in elderly patients on anticoagulation. Neurosurgery. 2006;58:E851-E856.
4. Culotta VP, Sermentilli ME, Gerold K, et al. Clinicopathological heterogeneity in the classification of mild head injury. Neurosurgery. 1996;38:245-250.
5. Shabani S, Nguyen HS, Doan N, et al. Case Report and Review of Literature of Delayed Acute Subdural Hematoma. World Neurosurg. 2016;96:66-71.
6. Jagoda AS, Bazarian JJ, Bruns Jr JJ, et al. Clinical Policy: Neuroimaging and Decisionmaking in Adult Mild Traumatic Brain Injury in the Acute Setting. Ann Emerg Med. 2008;52:714-748.
1. Cohen T, Gudeman S. In: Narayan RK, ed. Delayed Traumatic Intracranial Hematoma. Neurotrauma. New York, NY: McGraw-Hill;1995:689-701.
2. Matsuda W, Sugimoto K, Sato N, et al. Delayed onset of posttraumatic acute subdural hematoma after mild head injury with normal computed tomography: a case report and brief review. J Trauma. 2008;65:461-463.
3. Itshayek E, Rosenthal G, Fraifeld S, et al. Delayed posttraumatic acute subdural hematoma in elderly patients on anticoagulation. Neurosurgery. 2006;58:E851-E856.
4. Culotta VP, Sermentilli ME, Gerold K, et al. Clinicopathological heterogeneity in the classification of mild head injury. Neurosurgery. 1996;38:245-250.
5. Shabani S, Nguyen HS, Doan N, et al. Case Report and Review of Literature of Delayed Acute Subdural Hematoma. World Neurosurg. 2016;96:66-71.
6. Jagoda AS, Bazarian JJ, Bruns Jr JJ, et al. Clinical Policy: Neuroimaging and Decisionmaking in Adult Mild Traumatic Brain Injury in the Acute Setting. Ann Emerg Med. 2008;52:714-748.
Teenager with shortness of breath and hypoxia
A 19-year-old male complaining of shortness of breath was transferred from our facility’s urgent care unit to our emergency department. He had a 2-week history of hemoptysis and vomiting, and over the previous week, he had developed mild hematemesis. His other symptoms included left thigh, flank, and upper quadrant pain; left chest pain exacerbated by exertion, light-headedness, and palpitations. He said that over the past 8 months, he’d been tired and lost some weight.
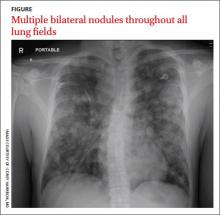
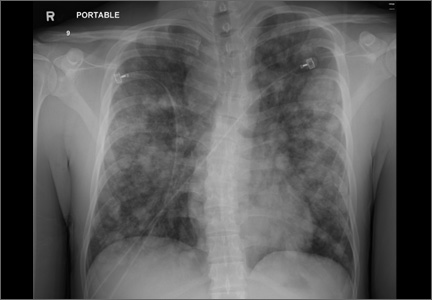
His blood pressure was 138/77 mm Hg, pulse was 142 beats per min, respiratory rate was 22 breaths per min, and oxygen saturation was 93% on room air. The physical exam revealed normal breath sounds and a diffusely tender abdomen. We ordered a chest X-ray (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic testicular cancer
The chest x-ray showed multiple bilateral discrete nodules throughout all of the lung fields. These findings, along with the age of the patient, prompted the radiologist to suspect metastatic testicular cancer. An examination of the patient’s scrotum revealed an 11-cm mass encompassing the patient’s left testicle. When asked about the mass, the patient acknowledged that it had been there for about 8 months.
A rare cancer seen in younger men
Although relatively uncommon, testicular cancer accounts for 1% to 2% of all tumors in men.1 If caught it is highly treatable.
Testicular cancer is classified into germ cell tumors (which our patient had) and sex cord-stromal tumors. Germ cell tumors are the most common malignancy in men ages 15 to 44 years, and have a 95% cure rate when identified early and promptly treated.2,3 Sex cordstromal tumors are more common in pediatric patients and are more often benign.2
Diagnosis usually is made clinically and pathologically at resection. Left untreated, testicular cancer spreads via the lymphatic system to the retroperitoneal lymph nodes and through the bloodstream to the lungs (predominantly),4 as well as to bone, the liver, and the brain. Metastatic testicular cancer to the lungs, liver, and retroperitoneum occurs in advanced disease and has a poor prognosis.4,5
Differential diagnosis includes pneumonia, septic emboli
The differential diagnosis includes atypical pneumonia, septic emboli (ie, endocarditis or Lemierre’s syndrome), or sarcoidosis. Patients with atypical pneumonia often present with a cough, fever, and malaise. Patients with septic emboli will have an x-ray that looks similar to that of our patient. Their signs and symptoms will include malaise, shortness of breath, hypoxia, tachycardia, and tachypnea. Risk factors and physical exam findings might include a history of intravenous drug abuse (endocarditis) or deep tissue neck infection (Lemierre’s syndrome). Sarcoidosis can be a challenging diagnosis without further study.
Successful treatment hinges on early detection
Treatment for testicular cancer often is successful if the condition is localized.
The choice of treatment depends on tumor type and stage. Options include orchiectomy, retroperitoneal lymph node dissection, chemotherapy, and radiation.2-5 After being diagnosed with testicular cancer 95% of patients live for 5 or more years.6 For localized testicular cancer, the 5-year survival rate is 99%.6
An eye toward prevention. The US Preventive Services Task Force recommends against screening with clinical examination or testicular self examination7; however, some clinicians support regular screening and self examinations.
When silence is deadly
Although physicians expect that patients will disclose obvious physical manifestations of disease, we know that this is not always the case. Patients often have barriers to care, including their own reluctance to share certain types of information with a provider.
Our patient. After we diagnosed metastatic testicular cancer in our patient, he was transferred to the medical intensive care unit. His overall clinical status declined and he died 14 days later.
1. Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int. 2009;104(9 pt B):1329-1333.
2. Schultz KA, Schneider DT, Pashankar F, et al. Management of ovarian and testicular sex cord-stromal tumors in children and adolescents. J Pediatr Hematol Oncol. 2012;34 suppl 2:S55-S63.
3. Sohaib SA, Koh DM, Husband JE. The role of imaging in the diagnosis, staging, and management of testicular cancer. AJR Am J Roentgenol. 2008;191:387-395.
4. Viatori M. Testicular cancer. Semin Oncol Nurs. 2012;28:180-189.
5. Mannuel H, Mitikiri N, Khan M, et al. Testicular germ cell tumors: biology and clinical update. Curr Opin Oncol. 2012;24:266-271.
6. SEER Stat Fact Sheets: Testis Cancer. National Cancer Institute Web site. Available at: http://seer.cancer.gov/statfacts/html/testis.html. Accessed May 20, 2014.
7. Screening for testicular cancer. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf10/testicular/testicuprs.htm. Accessed May 21, 2014.
A 19-year-old male complaining of shortness of breath was transferred from our facility’s urgent care unit to our emergency department. He had a 2-week history of hemoptysis and vomiting, and over the previous week, he had developed mild hematemesis. His other symptoms included left thigh, flank, and upper quadrant pain; left chest pain exacerbated by exertion, light-headedness, and palpitations. He said that over the past 8 months, he’d been tired and lost some weight.
His blood pressure was 138/77 mm Hg, pulse was 142 beats per min, respiratory rate was 22 breaths per min, and oxygen saturation was 93% on room air. The physical exam revealed normal breath sounds and a diffusely tender abdomen. We ordered a chest X-ray (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic testicular cancer
The chest x-ray showed multiple bilateral discrete nodules throughout all of the lung fields. These findings, along with the age of the patient, prompted the radiologist to suspect metastatic testicular cancer. An examination of the patient’s scrotum revealed an 11-cm mass encompassing the patient’s left testicle. When asked about the mass, the patient acknowledged that it had been there for about 8 months.
A rare cancer seen in younger men
Although relatively uncommon, testicular cancer accounts for 1% to 2% of all tumors in men.1 If caught it is highly treatable.
Testicular cancer is classified into germ cell tumors (which our patient had) and sex cord-stromal tumors. Germ cell tumors are the most common malignancy in men ages 15 to 44 years, and have a 95% cure rate when identified early and promptly treated.2,3 Sex cordstromal tumors are more common in pediatric patients and are more often benign.2
Diagnosis usually is made clinically and pathologically at resection. Left untreated, testicular cancer spreads via the lymphatic system to the retroperitoneal lymph nodes and through the bloodstream to the lungs (predominantly),4 as well as to bone, the liver, and the brain. Metastatic testicular cancer to the lungs, liver, and retroperitoneum occurs in advanced disease and has a poor prognosis.4,5
Differential diagnosis includes pneumonia, septic emboli
The differential diagnosis includes atypical pneumonia, septic emboli (ie, endocarditis or Lemierre’s syndrome), or sarcoidosis. Patients with atypical pneumonia often present with a cough, fever, and malaise. Patients with septic emboli will have an x-ray that looks similar to that of our patient. Their signs and symptoms will include malaise, shortness of breath, hypoxia, tachycardia, and tachypnea. Risk factors and physical exam findings might include a history of intravenous drug abuse (endocarditis) or deep tissue neck infection (Lemierre’s syndrome). Sarcoidosis can be a challenging diagnosis without further study.
Successful treatment hinges on early detection
Treatment for testicular cancer often is successful if the condition is localized.
The choice of treatment depends on tumor type and stage. Options include orchiectomy, retroperitoneal lymph node dissection, chemotherapy, and radiation.2-5 After being diagnosed with testicular cancer 95% of patients live for 5 or more years.6 For localized testicular cancer, the 5-year survival rate is 99%.6
An eye toward prevention. The US Preventive Services Task Force recommends against screening with clinical examination or testicular self examination7; however, some clinicians support regular screening and self examinations.
When silence is deadly
Although physicians expect that patients will disclose obvious physical manifestations of disease, we know that this is not always the case. Patients often have barriers to care, including their own reluctance to share certain types of information with a provider.
Our patient. After we diagnosed metastatic testicular cancer in our patient, he was transferred to the medical intensive care unit. His overall clinical status declined and he died 14 days later.
A 19-year-old male complaining of shortness of breath was transferred from our facility’s urgent care unit to our emergency department. He had a 2-week history of hemoptysis and vomiting, and over the previous week, he had developed mild hematemesis. His other symptoms included left thigh, flank, and upper quadrant pain; left chest pain exacerbated by exertion, light-headedness, and palpitations. He said that over the past 8 months, he’d been tired and lost some weight.
His blood pressure was 138/77 mm Hg, pulse was 142 beats per min, respiratory rate was 22 breaths per min, and oxygen saturation was 93% on room air. The physical exam revealed normal breath sounds and a diffusely tender abdomen. We ordered a chest X-ray (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic testicular cancer
The chest x-ray showed multiple bilateral discrete nodules throughout all of the lung fields. These findings, along with the age of the patient, prompted the radiologist to suspect metastatic testicular cancer. An examination of the patient’s scrotum revealed an 11-cm mass encompassing the patient’s left testicle. When asked about the mass, the patient acknowledged that it had been there for about 8 months.
A rare cancer seen in younger men
Although relatively uncommon, testicular cancer accounts for 1% to 2% of all tumors in men.1 If caught it is highly treatable.
Testicular cancer is classified into germ cell tumors (which our patient had) and sex cord-stromal tumors. Germ cell tumors are the most common malignancy in men ages 15 to 44 years, and have a 95% cure rate when identified early and promptly treated.2,3 Sex cordstromal tumors are more common in pediatric patients and are more often benign.2
Diagnosis usually is made clinically and pathologically at resection. Left untreated, testicular cancer spreads via the lymphatic system to the retroperitoneal lymph nodes and through the bloodstream to the lungs (predominantly),4 as well as to bone, the liver, and the brain. Metastatic testicular cancer to the lungs, liver, and retroperitoneum occurs in advanced disease and has a poor prognosis.4,5
Differential diagnosis includes pneumonia, septic emboli
The differential diagnosis includes atypical pneumonia, septic emboli (ie, endocarditis or Lemierre’s syndrome), or sarcoidosis. Patients with atypical pneumonia often present with a cough, fever, and malaise. Patients with septic emboli will have an x-ray that looks similar to that of our patient. Their signs and symptoms will include malaise, shortness of breath, hypoxia, tachycardia, and tachypnea. Risk factors and physical exam findings might include a history of intravenous drug abuse (endocarditis) or deep tissue neck infection (Lemierre’s syndrome). Sarcoidosis can be a challenging diagnosis without further study.
Successful treatment hinges on early detection
Treatment for testicular cancer often is successful if the condition is localized.
The choice of treatment depends on tumor type and stage. Options include orchiectomy, retroperitoneal lymph node dissection, chemotherapy, and radiation.2-5 After being diagnosed with testicular cancer 95% of patients live for 5 or more years.6 For localized testicular cancer, the 5-year survival rate is 99%.6
An eye toward prevention. The US Preventive Services Task Force recommends against screening with clinical examination or testicular self examination7; however, some clinicians support regular screening and self examinations.
When silence is deadly
Although physicians expect that patients will disclose obvious physical manifestations of disease, we know that this is not always the case. Patients often have barriers to care, including their own reluctance to share certain types of information with a provider.
Our patient. After we diagnosed metastatic testicular cancer in our patient, he was transferred to the medical intensive care unit. His overall clinical status declined and he died 14 days later.
1. Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int. 2009;104(9 pt B):1329-1333.
2. Schultz KA, Schneider DT, Pashankar F, et al. Management of ovarian and testicular sex cord-stromal tumors in children and adolescents. J Pediatr Hematol Oncol. 2012;34 suppl 2:S55-S63.
3. Sohaib SA, Koh DM, Husband JE. The role of imaging in the diagnosis, staging, and management of testicular cancer. AJR Am J Roentgenol. 2008;191:387-395.
4. Viatori M. Testicular cancer. Semin Oncol Nurs. 2012;28:180-189.
5. Mannuel H, Mitikiri N, Khan M, et al. Testicular germ cell tumors: biology and clinical update. Curr Opin Oncol. 2012;24:266-271.
6. SEER Stat Fact Sheets: Testis Cancer. National Cancer Institute Web site. Available at: http://seer.cancer.gov/statfacts/html/testis.html. Accessed May 20, 2014.
7. Screening for testicular cancer. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf10/testicular/testicuprs.htm. Accessed May 21, 2014.
1. Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int. 2009;104(9 pt B):1329-1333.
2. Schultz KA, Schneider DT, Pashankar F, et al. Management of ovarian and testicular sex cord-stromal tumors in children and adolescents. J Pediatr Hematol Oncol. 2012;34 suppl 2:S55-S63.
3. Sohaib SA, Koh DM, Husband JE. The role of imaging in the diagnosis, staging, and management of testicular cancer. AJR Am J Roentgenol. 2008;191:387-395.
4. Viatori M. Testicular cancer. Semin Oncol Nurs. 2012;28:180-189.
5. Mannuel H, Mitikiri N, Khan M, et al. Testicular germ cell tumors: biology and clinical update. Curr Opin Oncol. 2012;24:266-271.
6. SEER Stat Fact Sheets: Testis Cancer. National Cancer Institute Web site. Available at: http://seer.cancer.gov/statfacts/html/testis.html. Accessed May 20, 2014.
7. Screening for testicular cancer. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf10/testicular/testicuprs.htm. Accessed May 21, 2014.