User login
Pseudobulbar affect: When patients laugh or cry, but don’t know why
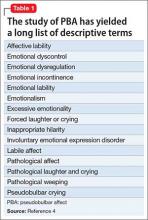
Pseudobulbar affect (PBA) is a disorder of affective expression that manifests as stereotyped and frequent outbursts of crying (not limited to lacrimation) or laughter. Symptoms are involuntary, uncontrolled, and exaggerated or incongruent with current mood. Episodes, lasting a few seconds to several minutes, may be unprovoked or occur in response to a mild stimulus, and patients typically display a normal affect between episodes.1 PBA is estimated to affect 1 to 2 million people in the United States, although some studies suggest as many as 7 million,1,2 depending on the evaluation method and threshold criteria used.3
Where to look for pseudobulbar affect
PBA has been most commonly described in 6 major neurologic disorders:
- Alzheimer’s disease
- amyotrophic lateral sclerosis (ALS)
- multiple sclerosis (MS)
- Parkinson’s disease
- stroke
- traumatic brain injury (TBI).
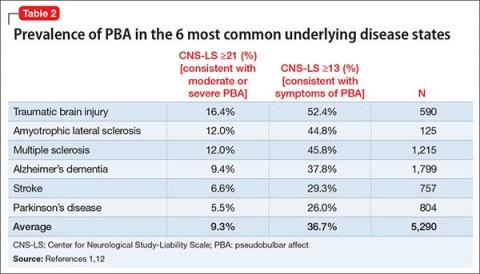
Of these disorders, most studies have found the highest PBA prevalence in patients with ALS and TBI, with lesser (although significant) prevalence in Parkinson’s disease (Table 2).1,12 These “big 6” diagnoses are not a comprehensive list, as many other disease states are associated with PBA (Table 3).12-14
2 Pathways: ‘Generator’ and ‘governor’
Despite the many and varied injuries and illnesses associated with PBA, Lauterbach et al10 noted patterns that suggest dysregulation of 2 distinct but interconnected brain pathways: an emotional pathway controlled by a separate volitional pathway. Lesions to the volitional pathway (or its associated feedback or processing circuits) are thought to cause PBA symptoms.
To borrow an analogy from engineering, the emotional pathway is the “generator” of affect, whereas the volitional pathway is the “governor” of affect. Thus, injury to the “governor” results in overspill, or overflow, of affect that usually would be suppressed.
The emotional pathway, which coordinates the motor aspect of reflex laughing or crying, originates at the frontotemporal cortex, relaying to the amygdala and hypothalamus, then projecting to the dorsal brainstem, which includes the midbrain-pontine periaqueductal gray (PAG), dorsal tegmentum, and related brainstem.
The volitional pathway, which regulates the emotional pathway, originates in the dorsal and lateral frontoparietal cortex, projects through the internal capsule and midbrain basis pedunculi, and continues on to the anteroventral basis pontis. The basis pontis then serves as an afferent relay center for cerebellar activity. Projections from the pons then regulate the emotional circuitry primarily at the level of the PAG.10
Lesions of the volitional pathway have been correlated with conditions of PBA, whereas direct activation of the emotional pathway tended to lead to emotional lability or the crying and laughing behaviors observed in dacrystic or gelastic epilepsy.10 The pivotal nature of the regulation occurring at the PAG has guided treatment options. Neurotransmitter receptors most closely associated with this region include glutamatergic N-methyl-
When to screen for PBA
Ask the right question. PBA as a disease state likely has been widely under-reported, under-recognized, and misdiagnosed (typically, as a primary mood disorder).9 Three factors underscore this problem:
- Patients do not specifically report symptoms of affective disturbance (perhaps because they lack a vocabulary to separate concepts of mood and affect)
- Physicians do not ask patients about separations of mood and affect
- Perhaps most importantly, PBA lacks a general awareness and understanding.
Co-occurring mood disorders also may thwart PBA detection. One study of PBA in Alzheimer’s dementia found that 53% of patients with symptoms consistent with PBA also had a distinct mood disorder.17 This suggests that a PBA-specific screening test is needed for accurate diagnosis.
A single question might best refine the likelihood that a patient has PBA: “Do you ever cry for no reason?” In primary psychiatric illness, crying typically is associated with a specific trigger (eg, depressed mood, despair, anxiety). A patient’s inability to identify a trigger for crying suggests the pathological separation of mood and affect—the core of PBA, and worthy of further investigation.
Clinical rating scales that correlate to disease severity appear to be the most effective in identifying PBA. The PRISM study, to date the largest clinic-based study of PBA symptoms, used the Center for Neurologic Study-Liability Scale (CNS-LS) to gauge the presence and severity of PBA symptoms.1 A 7-question, patient self-administered tool, the CNS-LS is graded on a 5-point Likert scale. A score ≥13 has high sensitivity and specificity for diagnosis of PBA, compared with physician diagnosis.
Another option, the 16-question Pathological Laughing and Crying Scale, is a clinician-administered screening tool. Again, a score ≥13 is consistent with symptoms required for a PBA diagnosis.
Treating PBA symptoms
Until recently, most pharmacotherapeutic interventions for PBA were based on off-label use of tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitors (SSRIs). From 1980 to 2010, only 7 of 22 case reports or trials of TCAs or SSRIs for PBA were randomized, double-blind, and placebo-controlled. Five had 12 to 28 patients, and 2 had 106 and 128 patients, respectively. Only 1 controlled trial included a validated symptom severity scale, and none included a scale validated for PBA.18
In particular, imipramine and nortriptyline were studied for managing PBA in patients with stroke; amitriptyline, in patients with MS; and various SSRIs, in patients with stroke.11 Response of PBA symptoms to antidepressant therapy was greater in all placebo-controlled trials than response to placebo.18 As seen in pharmacotherapy of depression, the lower burden of adverse effects and overall better tolerability of SSRIs resulted in their preferred use over TCAs. In some cases, the side effects of TCAs can be leveraged for therapeutic gain. If insomnia is a problem, a nighttime dose of a TCA could ameliorate this. Similarly, if a patient has sialorrhea, the anticholinergic effect of a TCA may show some benefit.19
Dextromethorphan plus quinidine. Dextromethorphan has long been of interest for a variety of neurodegenerative diseases. Studies of its efficacy were largely unsuccessful, however, because rapid metabolism by cytochrome P450 (CYP) 2D6 prevented CNS penetration.20 Quinidine is an avid inhibitor of CYP2D6, even at very low dosages. Adding quinidine to dextromethorphan limits metabolism, allowing dextromethorphan to accumulate to a plasma concentration sufficient to penetrate the CNS.12 In 2010, the combination agent dextromethorphan hydrobromide (20 mg)/quinidine (10 mg) (DM/Q) became the first treatment to receive FDA approval for managing PBA.11
Mechanism of action. The exact mechanism of DM/Q in PBA remains unknown. Dextromethorphan is an agonist of sigma-1 receptors and a relatively specific noncompetitive antagonist of NMDA receptors. It also has been shown to modulate glutamate and serotonin neurotransmission and ion channel function.20 Sigma-1 receptors are concentrated in the brainstem and parts of the cerebellum that are thought to coordinate motor emotional responses. Agonism of sigma-1 receptors on glutamatergic neurons has been proposed to limit release of glutamate from the presynaptic neuron while also limiting downstream transmission of glutamatergic signal in postsynaptic neurons.
Clinical trials. Two large trials have demonstrated efficacy of DM/Q in PBA. STAR was a 12-week, double-blind, placebo-controlled trial with 326 patients diagnosed with ALS or MS who showed PBA symptoms (CNS-LS score ≥13). Compared with placebo, DM/Q use was associated with significantly reduced (P < .01) daily episodes of PBA at 2, 4, 8, and 12 weeks.20 The effect was rapid, with 30% fewer PBA episodes after the first week (P < .0167). At 12 weeks, 51% of patients on DM/Q had been symptom-free for at least 2 weeks.
The PRISM II study examined the efficacy of DM/Q in managing PBA in 102 individuals with dementia, 92 with stroke, and 67 with TBI. After 30 and 90 days, CNL-LS scores were significantly reduced (P < .001) compared with baseline scores.20
Prescribing information. Dextromethorphan—typically in the form of cough syrup—has been implicated as a substance of abuse. A placebo-controlled trial demonstrated that co-administering quinidine with dextromethorphan limits measures of positive reinforcement, such as euphoria and drug liking. This suggests that quinidine may be used to reduce abuse of dextromethorphan.20 As such, the abuse potential of DM/Q appears to be low.
The most common adverse effects reported with DM/Q are diarrhea, dizziness, and cough.12 Notably, patients who received DM/Q in the STAR trial were more likely to report dizziness than those receiving placebo (10.3% vs 5.5%), but patients receiving placebo were more likely to fall.21,22
Package labeling warns that DM/Q causes dose-dependent QTc prolongation.21 Quinidine can be associated with significant QTc prolongation when dosed at antiarrhythmic levels, although mean plasma concentrations found with the 10 mg of quinidine in the approved DM/Q formulation are 1% to 3% of those associated with typical dosages used in antiarrhythmic therapy. Electrophysiology studies of quinidine 10 mg dosed every 12 hours have demonstrated a mean QTc increase at steady state of 6.8 milliseconds, compared with 9.1 milliseconds for a reference control (moxifloxacin).12,21
Although this would seem to indicate a relatively low risk of clinically significant QTc prolongation at these ultra-low dosages of quinidine, it may be advisable to obtain an initial pre-dose and post-dose ECG and longitudinally monitor the QTc interval in patients with conditions that predispose to cardiac arrhythmias. Because quinidine inhibits CYP2D6, use caution when prescribing and monitoring other medications metabolized by this pathway.
Bottom Line
1. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
2. Cruz MP. Nuedexta for the treatment of pseudobulbar affect: a condition of involuntary laughing and crying. P T. 2013;38(6):325-328.
3. Work SS, Colamonico JA, Bradley WG, et al. Pseudobulbar affect: an under-recognized and under-treated neurological disorder. Adv Ther. 2011;28(7):586-601.
4. Arciniegas DB, Lauterbach EC, Anderson KE, et al. The differential diagnosis of pseudobulbar affect (PBA). Distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting. CNS Spectr. 2005;10(5):1-14; quiz 15-16.
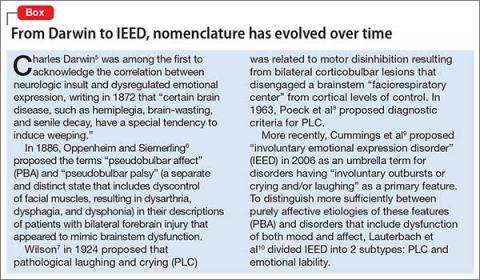
5. Darwin C. The expression of the emotions in man and animals. London, United Kingdom: John Murray; 1872.
6. Oppenheim H, Siemerling E. Mitteilungen über Pseudobulbärparalyse und akute Bulbärparalyse. Berl Kli Woch. 1886;46.
7. Wilson SA. Original papers: some problems in neurology. J Neurol Psychopathol. 1924;4(16):299-333.
8. Poeck K, Risso M, Pilleri G. Contribution to the pathophysiology and clinical systematology of pathological laughing and crying [in German]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1963;204:181-198.
9. Cummings JL, Gilbart J, Andersen G. Pseudobulbar affect - a disabling but under-recognised consequence of neurological disease and brain injury. Eur Neurol Rev. 2013;8(2):74-81.
10. Lauterbach EC, Cummings JL, Kuppuswamy PS. Toward a more precise, clinically–informed pathophysiology of pathological laughing and crying. Neurosci Biobehav Rev. 2013;37(8):1893-1916.
11. Pioro EP. Review of dextromethorphan 20 mg/quinidine 10 mg (Nuedexta(®)) for pseudobulbar affect. Neurol Ther. 2014;3(1):15-28.
12. Schoedel KA, Morrow SA, Sellers EM. Evaluating the safety and efficacy of dextromethorphan/quinidine in the treatment of pseudobulbar affect. Neuropsychiatr Dis Treat. 2014;10:1161-1174.
13. Li Z, Luo S, Ou J, et al. Persistent pseudobulbar affect secondary to acute disseminated encephalomyelitis. Socioaffect Neurosci Psychol. 2015;5:26210. doi: 10.3402/snp.v5.26210.
14. Pattee GL, Wymer JP, Lomen-Hoerth C, et al. An open-label multicenter study to assess the safety of dextromethorphan/quinidine in patients with pseudobulbar affect associated with a range of underlying neurological conditions. Curr Med Res Opin. 2014;30(11):2255-2265.
15. Strowd RE, Cartwright MS, Okun MS, et al. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J Neurol. 2010;257(8):1382-1387.
16. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
17. Starkstein SE, Migliorelli R, Tesón A, et al. Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59(1):55-60.
18. Pioro EP. Current concepts in the pharmacotherapy of pseudobulbar affect. Drugs. 2011;71(9):1193-1207.
19. Ahmed A, Simmons A. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag. 2013;9:483-489.
20. Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs. 2015;75(1):83-90.
21. Nuedexta [package insert]. Aliso Viejo, CA: Avanir Pharmaceuticals, Inc.; 2015.
22. Pioro EP, Brooks BR, Cummings J, et al; Safety, Tolerability, and Efficacy trial of AVP-923 in PBA Investigators. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010;68(5):693-702.
Pseudobulbar affect (PBA) is a disorder of affective expression that manifests as stereotyped and frequent outbursts of crying (not limited to lacrimation) or laughter. Symptoms are involuntary, uncontrolled, and exaggerated or incongruent with current mood. Episodes, lasting a few seconds to several minutes, may be unprovoked or occur in response to a mild stimulus, and patients typically display a normal affect between episodes.1 PBA is estimated to affect 1 to 2 million people in the United States, although some studies suggest as many as 7 million,1,2 depending on the evaluation method and threshold criteria used.3
Where to look for pseudobulbar affect
PBA has been most commonly described in 6 major neurologic disorders:
- Alzheimer’s disease
- amyotrophic lateral sclerosis (ALS)
- multiple sclerosis (MS)
- Parkinson’s disease
- stroke
- traumatic brain injury (TBI).
Of these disorders, most studies have found the highest PBA prevalence in patients with ALS and TBI, with lesser (although significant) prevalence in Parkinson’s disease (Table 2).1,12 These “big 6” diagnoses are not a comprehensive list, as many other disease states are associated with PBA (Table 3).12-14
2 Pathways: ‘Generator’ and ‘governor’
Despite the many and varied injuries and illnesses associated with PBA, Lauterbach et al10 noted patterns that suggest dysregulation of 2 distinct but interconnected brain pathways: an emotional pathway controlled by a separate volitional pathway. Lesions to the volitional pathway (or its associated feedback or processing circuits) are thought to cause PBA symptoms.
To borrow an analogy from engineering, the emotional pathway is the “generator” of affect, whereas the volitional pathway is the “governor” of affect. Thus, injury to the “governor” results in overspill, or overflow, of affect that usually would be suppressed.
The emotional pathway, which coordinates the motor aspect of reflex laughing or crying, originates at the frontotemporal cortex, relaying to the amygdala and hypothalamus, then projecting to the dorsal brainstem, which includes the midbrain-pontine periaqueductal gray (PAG), dorsal tegmentum, and related brainstem.
The volitional pathway, which regulates the emotional pathway, originates in the dorsal and lateral frontoparietal cortex, projects through the internal capsule and midbrain basis pedunculi, and continues on to the anteroventral basis pontis. The basis pontis then serves as an afferent relay center for cerebellar activity. Projections from the pons then regulate the emotional circuitry primarily at the level of the PAG.10
Lesions of the volitional pathway have been correlated with conditions of PBA, whereas direct activation of the emotional pathway tended to lead to emotional lability or the crying and laughing behaviors observed in dacrystic or gelastic epilepsy.10 The pivotal nature of the regulation occurring at the PAG has guided treatment options. Neurotransmitter receptors most closely associated with this region include glutamatergic N-methyl-
When to screen for PBA
Ask the right question. PBA as a disease state likely has been widely under-reported, under-recognized, and misdiagnosed (typically, as a primary mood disorder).9 Three factors underscore this problem:
- Patients do not specifically report symptoms of affective disturbance (perhaps because they lack a vocabulary to separate concepts of mood and affect)
- Physicians do not ask patients about separations of mood and affect
- Perhaps most importantly, PBA lacks a general awareness and understanding.
Co-occurring mood disorders also may thwart PBA detection. One study of PBA in Alzheimer’s dementia found that 53% of patients with symptoms consistent with PBA also had a distinct mood disorder.17 This suggests that a PBA-specific screening test is needed for accurate diagnosis.
A single question might best refine the likelihood that a patient has PBA: “Do you ever cry for no reason?” In primary psychiatric illness, crying typically is associated with a specific trigger (eg, depressed mood, despair, anxiety). A patient’s inability to identify a trigger for crying suggests the pathological separation of mood and affect—the core of PBA, and worthy of further investigation.
Clinical rating scales that correlate to disease severity appear to be the most effective in identifying PBA. The PRISM study, to date the largest clinic-based study of PBA symptoms, used the Center for Neurologic Study-Liability Scale (CNS-LS) to gauge the presence and severity of PBA symptoms.1 A 7-question, patient self-administered tool, the CNS-LS is graded on a 5-point Likert scale. A score ≥13 has high sensitivity and specificity for diagnosis of PBA, compared with physician diagnosis.
Another option, the 16-question Pathological Laughing and Crying Scale, is a clinician-administered screening tool. Again, a score ≥13 is consistent with symptoms required for a PBA diagnosis.
Treating PBA symptoms
Until recently, most pharmacotherapeutic interventions for PBA were based on off-label use of tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitors (SSRIs). From 1980 to 2010, only 7 of 22 case reports or trials of TCAs or SSRIs for PBA were randomized, double-blind, and placebo-controlled. Five had 12 to 28 patients, and 2 had 106 and 128 patients, respectively. Only 1 controlled trial included a validated symptom severity scale, and none included a scale validated for PBA.18
In particular, imipramine and nortriptyline were studied for managing PBA in patients with stroke; amitriptyline, in patients with MS; and various SSRIs, in patients with stroke.11 Response of PBA symptoms to antidepressant therapy was greater in all placebo-controlled trials than response to placebo.18 As seen in pharmacotherapy of depression, the lower burden of adverse effects and overall better tolerability of SSRIs resulted in their preferred use over TCAs. In some cases, the side effects of TCAs can be leveraged for therapeutic gain. If insomnia is a problem, a nighttime dose of a TCA could ameliorate this. Similarly, if a patient has sialorrhea, the anticholinergic effect of a TCA may show some benefit.19
Dextromethorphan plus quinidine. Dextromethorphan has long been of interest for a variety of neurodegenerative diseases. Studies of its efficacy were largely unsuccessful, however, because rapid metabolism by cytochrome P450 (CYP) 2D6 prevented CNS penetration.20 Quinidine is an avid inhibitor of CYP2D6, even at very low dosages. Adding quinidine to dextromethorphan limits metabolism, allowing dextromethorphan to accumulate to a plasma concentration sufficient to penetrate the CNS.12 In 2010, the combination agent dextromethorphan hydrobromide (20 mg)/quinidine (10 mg) (DM/Q) became the first treatment to receive FDA approval for managing PBA.11
Mechanism of action. The exact mechanism of DM/Q in PBA remains unknown. Dextromethorphan is an agonist of sigma-1 receptors and a relatively specific noncompetitive antagonist of NMDA receptors. It also has been shown to modulate glutamate and serotonin neurotransmission and ion channel function.20 Sigma-1 receptors are concentrated in the brainstem and parts of the cerebellum that are thought to coordinate motor emotional responses. Agonism of sigma-1 receptors on glutamatergic neurons has been proposed to limit release of glutamate from the presynaptic neuron while also limiting downstream transmission of glutamatergic signal in postsynaptic neurons.
Clinical trials. Two large trials have demonstrated efficacy of DM/Q in PBA. STAR was a 12-week, double-blind, placebo-controlled trial with 326 patients diagnosed with ALS or MS who showed PBA symptoms (CNS-LS score ≥13). Compared with placebo, DM/Q use was associated with significantly reduced (P < .01) daily episodes of PBA at 2, 4, 8, and 12 weeks.20 The effect was rapid, with 30% fewer PBA episodes after the first week (P < .0167). At 12 weeks, 51% of patients on DM/Q had been symptom-free for at least 2 weeks.
The PRISM II study examined the efficacy of DM/Q in managing PBA in 102 individuals with dementia, 92 with stroke, and 67 with TBI. After 30 and 90 days, CNL-LS scores were significantly reduced (P < .001) compared with baseline scores.20
Prescribing information. Dextromethorphan—typically in the form of cough syrup—has been implicated as a substance of abuse. A placebo-controlled trial demonstrated that co-administering quinidine with dextromethorphan limits measures of positive reinforcement, such as euphoria and drug liking. This suggests that quinidine may be used to reduce abuse of dextromethorphan.20 As such, the abuse potential of DM/Q appears to be low.
The most common adverse effects reported with DM/Q are diarrhea, dizziness, and cough.12 Notably, patients who received DM/Q in the STAR trial were more likely to report dizziness than those receiving placebo (10.3% vs 5.5%), but patients receiving placebo were more likely to fall.21,22
Package labeling warns that DM/Q causes dose-dependent QTc prolongation.21 Quinidine can be associated with significant QTc prolongation when dosed at antiarrhythmic levels, although mean plasma concentrations found with the 10 mg of quinidine in the approved DM/Q formulation are 1% to 3% of those associated with typical dosages used in antiarrhythmic therapy. Electrophysiology studies of quinidine 10 mg dosed every 12 hours have demonstrated a mean QTc increase at steady state of 6.8 milliseconds, compared with 9.1 milliseconds for a reference control (moxifloxacin).12,21
Although this would seem to indicate a relatively low risk of clinically significant QTc prolongation at these ultra-low dosages of quinidine, it may be advisable to obtain an initial pre-dose and post-dose ECG and longitudinally monitor the QTc interval in patients with conditions that predispose to cardiac arrhythmias. Because quinidine inhibits CYP2D6, use caution when prescribing and monitoring other medications metabolized by this pathway.
Bottom Line
Pseudobulbar affect (PBA) is a disorder of affective expression that manifests as stereotyped and frequent outbursts of crying (not limited to lacrimation) or laughter. Symptoms are involuntary, uncontrolled, and exaggerated or incongruent with current mood. Episodes, lasting a few seconds to several minutes, may be unprovoked or occur in response to a mild stimulus, and patients typically display a normal affect between episodes.1 PBA is estimated to affect 1 to 2 million people in the United States, although some studies suggest as many as 7 million,1,2 depending on the evaluation method and threshold criteria used.3
Where to look for pseudobulbar affect
PBA has been most commonly described in 6 major neurologic disorders:
- Alzheimer’s disease
- amyotrophic lateral sclerosis (ALS)
- multiple sclerosis (MS)
- Parkinson’s disease
- stroke
- traumatic brain injury (TBI).
Of these disorders, most studies have found the highest PBA prevalence in patients with ALS and TBI, with lesser (although significant) prevalence in Parkinson’s disease (Table 2).1,12 These “big 6” diagnoses are not a comprehensive list, as many other disease states are associated with PBA (Table 3).12-14
2 Pathways: ‘Generator’ and ‘governor’
Despite the many and varied injuries and illnesses associated with PBA, Lauterbach et al10 noted patterns that suggest dysregulation of 2 distinct but interconnected brain pathways: an emotional pathway controlled by a separate volitional pathway. Lesions to the volitional pathway (or its associated feedback or processing circuits) are thought to cause PBA symptoms.
To borrow an analogy from engineering, the emotional pathway is the “generator” of affect, whereas the volitional pathway is the “governor” of affect. Thus, injury to the “governor” results in overspill, or overflow, of affect that usually would be suppressed.
The emotional pathway, which coordinates the motor aspect of reflex laughing or crying, originates at the frontotemporal cortex, relaying to the amygdala and hypothalamus, then projecting to the dorsal brainstem, which includes the midbrain-pontine periaqueductal gray (PAG), dorsal tegmentum, and related brainstem.
The volitional pathway, which regulates the emotional pathway, originates in the dorsal and lateral frontoparietal cortex, projects through the internal capsule and midbrain basis pedunculi, and continues on to the anteroventral basis pontis. The basis pontis then serves as an afferent relay center for cerebellar activity. Projections from the pons then regulate the emotional circuitry primarily at the level of the PAG.10
Lesions of the volitional pathway have been correlated with conditions of PBA, whereas direct activation of the emotional pathway tended to lead to emotional lability or the crying and laughing behaviors observed in dacrystic or gelastic epilepsy.10 The pivotal nature of the regulation occurring at the PAG has guided treatment options. Neurotransmitter receptors most closely associated with this region include glutamatergic N-methyl-
When to screen for PBA
Ask the right question. PBA as a disease state likely has been widely under-reported, under-recognized, and misdiagnosed (typically, as a primary mood disorder).9 Three factors underscore this problem:
- Patients do not specifically report symptoms of affective disturbance (perhaps because they lack a vocabulary to separate concepts of mood and affect)
- Physicians do not ask patients about separations of mood and affect
- Perhaps most importantly, PBA lacks a general awareness and understanding.
Co-occurring mood disorders also may thwart PBA detection. One study of PBA in Alzheimer’s dementia found that 53% of patients with symptoms consistent with PBA also had a distinct mood disorder.17 This suggests that a PBA-specific screening test is needed for accurate diagnosis.
A single question might best refine the likelihood that a patient has PBA: “Do you ever cry for no reason?” In primary psychiatric illness, crying typically is associated with a specific trigger (eg, depressed mood, despair, anxiety). A patient’s inability to identify a trigger for crying suggests the pathological separation of mood and affect—the core of PBA, and worthy of further investigation.
Clinical rating scales that correlate to disease severity appear to be the most effective in identifying PBA. The PRISM study, to date the largest clinic-based study of PBA symptoms, used the Center for Neurologic Study-Liability Scale (CNS-LS) to gauge the presence and severity of PBA symptoms.1 A 7-question, patient self-administered tool, the CNS-LS is graded on a 5-point Likert scale. A score ≥13 has high sensitivity and specificity for diagnosis of PBA, compared with physician diagnosis.
Another option, the 16-question Pathological Laughing and Crying Scale, is a clinician-administered screening tool. Again, a score ≥13 is consistent with symptoms required for a PBA diagnosis.
Treating PBA symptoms
Until recently, most pharmacotherapeutic interventions for PBA were based on off-label use of tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitors (SSRIs). From 1980 to 2010, only 7 of 22 case reports or trials of TCAs or SSRIs for PBA were randomized, double-blind, and placebo-controlled. Five had 12 to 28 patients, and 2 had 106 and 128 patients, respectively. Only 1 controlled trial included a validated symptom severity scale, and none included a scale validated for PBA.18
In particular, imipramine and nortriptyline were studied for managing PBA in patients with stroke; amitriptyline, in patients with MS; and various SSRIs, in patients with stroke.11 Response of PBA symptoms to antidepressant therapy was greater in all placebo-controlled trials than response to placebo.18 As seen in pharmacotherapy of depression, the lower burden of adverse effects and overall better tolerability of SSRIs resulted in their preferred use over TCAs. In some cases, the side effects of TCAs can be leveraged for therapeutic gain. If insomnia is a problem, a nighttime dose of a TCA could ameliorate this. Similarly, if a patient has sialorrhea, the anticholinergic effect of a TCA may show some benefit.19
Dextromethorphan plus quinidine. Dextromethorphan has long been of interest for a variety of neurodegenerative diseases. Studies of its efficacy were largely unsuccessful, however, because rapid metabolism by cytochrome P450 (CYP) 2D6 prevented CNS penetration.20 Quinidine is an avid inhibitor of CYP2D6, even at very low dosages. Adding quinidine to dextromethorphan limits metabolism, allowing dextromethorphan to accumulate to a plasma concentration sufficient to penetrate the CNS.12 In 2010, the combination agent dextromethorphan hydrobromide (20 mg)/quinidine (10 mg) (DM/Q) became the first treatment to receive FDA approval for managing PBA.11
Mechanism of action. The exact mechanism of DM/Q in PBA remains unknown. Dextromethorphan is an agonist of sigma-1 receptors and a relatively specific noncompetitive antagonist of NMDA receptors. It also has been shown to modulate glutamate and serotonin neurotransmission and ion channel function.20 Sigma-1 receptors are concentrated in the brainstem and parts of the cerebellum that are thought to coordinate motor emotional responses. Agonism of sigma-1 receptors on glutamatergic neurons has been proposed to limit release of glutamate from the presynaptic neuron while also limiting downstream transmission of glutamatergic signal in postsynaptic neurons.
Clinical trials. Two large trials have demonstrated efficacy of DM/Q in PBA. STAR was a 12-week, double-blind, placebo-controlled trial with 326 patients diagnosed with ALS or MS who showed PBA symptoms (CNS-LS score ≥13). Compared with placebo, DM/Q use was associated with significantly reduced (P < .01) daily episodes of PBA at 2, 4, 8, and 12 weeks.20 The effect was rapid, with 30% fewer PBA episodes after the first week (P < .0167). At 12 weeks, 51% of patients on DM/Q had been symptom-free for at least 2 weeks.
The PRISM II study examined the efficacy of DM/Q in managing PBA in 102 individuals with dementia, 92 with stroke, and 67 with TBI. After 30 and 90 days, CNL-LS scores were significantly reduced (P < .001) compared with baseline scores.20
Prescribing information. Dextromethorphan—typically in the form of cough syrup—has been implicated as a substance of abuse. A placebo-controlled trial demonstrated that co-administering quinidine with dextromethorphan limits measures of positive reinforcement, such as euphoria and drug liking. This suggests that quinidine may be used to reduce abuse of dextromethorphan.20 As such, the abuse potential of DM/Q appears to be low.
The most common adverse effects reported with DM/Q are diarrhea, dizziness, and cough.12 Notably, patients who received DM/Q in the STAR trial were more likely to report dizziness than those receiving placebo (10.3% vs 5.5%), but patients receiving placebo were more likely to fall.21,22
Package labeling warns that DM/Q causes dose-dependent QTc prolongation.21 Quinidine can be associated with significant QTc prolongation when dosed at antiarrhythmic levels, although mean plasma concentrations found with the 10 mg of quinidine in the approved DM/Q formulation are 1% to 3% of those associated with typical dosages used in antiarrhythmic therapy. Electrophysiology studies of quinidine 10 mg dosed every 12 hours have demonstrated a mean QTc increase at steady state of 6.8 milliseconds, compared with 9.1 milliseconds for a reference control (moxifloxacin).12,21
Although this would seem to indicate a relatively low risk of clinically significant QTc prolongation at these ultra-low dosages of quinidine, it may be advisable to obtain an initial pre-dose and post-dose ECG and longitudinally monitor the QTc interval in patients with conditions that predispose to cardiac arrhythmias. Because quinidine inhibits CYP2D6, use caution when prescribing and monitoring other medications metabolized by this pathway.
Bottom Line
1. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
2. Cruz MP. Nuedexta for the treatment of pseudobulbar affect: a condition of involuntary laughing and crying. P T. 2013;38(6):325-328.
3. Work SS, Colamonico JA, Bradley WG, et al. Pseudobulbar affect: an under-recognized and under-treated neurological disorder. Adv Ther. 2011;28(7):586-601.
4. Arciniegas DB, Lauterbach EC, Anderson KE, et al. The differential diagnosis of pseudobulbar affect (PBA). Distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting. CNS Spectr. 2005;10(5):1-14; quiz 15-16.
5. Darwin C. The expression of the emotions in man and animals. London, United Kingdom: John Murray; 1872.
6. Oppenheim H, Siemerling E. Mitteilungen über Pseudobulbärparalyse und akute Bulbärparalyse. Berl Kli Woch. 1886;46.
7. Wilson SA. Original papers: some problems in neurology. J Neurol Psychopathol. 1924;4(16):299-333.
8. Poeck K, Risso M, Pilleri G. Contribution to the pathophysiology and clinical systematology of pathological laughing and crying [in German]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1963;204:181-198.
9. Cummings JL, Gilbart J, Andersen G. Pseudobulbar affect - a disabling but under-recognised consequence of neurological disease and brain injury. Eur Neurol Rev. 2013;8(2):74-81.
10. Lauterbach EC, Cummings JL, Kuppuswamy PS. Toward a more precise, clinically–informed pathophysiology of pathological laughing and crying. Neurosci Biobehav Rev. 2013;37(8):1893-1916.
11. Pioro EP. Review of dextromethorphan 20 mg/quinidine 10 mg (Nuedexta(®)) for pseudobulbar affect. Neurol Ther. 2014;3(1):15-28.
12. Schoedel KA, Morrow SA, Sellers EM. Evaluating the safety and efficacy of dextromethorphan/quinidine in the treatment of pseudobulbar affect. Neuropsychiatr Dis Treat. 2014;10:1161-1174.
13. Li Z, Luo S, Ou J, et al. Persistent pseudobulbar affect secondary to acute disseminated encephalomyelitis. Socioaffect Neurosci Psychol. 2015;5:26210. doi: 10.3402/snp.v5.26210.
14. Pattee GL, Wymer JP, Lomen-Hoerth C, et al. An open-label multicenter study to assess the safety of dextromethorphan/quinidine in patients with pseudobulbar affect associated with a range of underlying neurological conditions. Curr Med Res Opin. 2014;30(11):2255-2265.
15. Strowd RE, Cartwright MS, Okun MS, et al. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J Neurol. 2010;257(8):1382-1387.
16. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
17. Starkstein SE, Migliorelli R, Tesón A, et al. Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59(1):55-60.
18. Pioro EP. Current concepts in the pharmacotherapy of pseudobulbar affect. Drugs. 2011;71(9):1193-1207.
19. Ahmed A, Simmons A. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag. 2013;9:483-489.
20. Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs. 2015;75(1):83-90.
21. Nuedexta [package insert]. Aliso Viejo, CA: Avanir Pharmaceuticals, Inc.; 2015.
22. Pioro EP, Brooks BR, Cummings J, et al; Safety, Tolerability, and Efficacy trial of AVP-923 in PBA Investigators. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010;68(5):693-702.
1. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
2. Cruz MP. Nuedexta for the treatment of pseudobulbar affect: a condition of involuntary laughing and crying. P T. 2013;38(6):325-328.
3. Work SS, Colamonico JA, Bradley WG, et al. Pseudobulbar affect: an under-recognized and under-treated neurological disorder. Adv Ther. 2011;28(7):586-601.
4. Arciniegas DB, Lauterbach EC, Anderson KE, et al. The differential diagnosis of pseudobulbar affect (PBA). Distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting. CNS Spectr. 2005;10(5):1-14; quiz 15-16.
5. Darwin C. The expression of the emotions in man and animals. London, United Kingdom: John Murray; 1872.
6. Oppenheim H, Siemerling E. Mitteilungen über Pseudobulbärparalyse und akute Bulbärparalyse. Berl Kli Woch. 1886;46.
7. Wilson SA. Original papers: some problems in neurology. J Neurol Psychopathol. 1924;4(16):299-333.
8. Poeck K, Risso M, Pilleri G. Contribution to the pathophysiology and clinical systematology of pathological laughing and crying [in German]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1963;204:181-198.
9. Cummings JL, Gilbart J, Andersen G. Pseudobulbar affect - a disabling but under-recognised consequence of neurological disease and brain injury. Eur Neurol Rev. 2013;8(2):74-81.
10. Lauterbach EC, Cummings JL, Kuppuswamy PS. Toward a more precise, clinically–informed pathophysiology of pathological laughing and crying. Neurosci Biobehav Rev. 2013;37(8):1893-1916.
11. Pioro EP. Review of dextromethorphan 20 mg/quinidine 10 mg (Nuedexta(®)) for pseudobulbar affect. Neurol Ther. 2014;3(1):15-28.
12. Schoedel KA, Morrow SA, Sellers EM. Evaluating the safety and efficacy of dextromethorphan/quinidine in the treatment of pseudobulbar affect. Neuropsychiatr Dis Treat. 2014;10:1161-1174.
13. Li Z, Luo S, Ou J, et al. Persistent pseudobulbar affect secondary to acute disseminated encephalomyelitis. Socioaffect Neurosci Psychol. 2015;5:26210. doi: 10.3402/snp.v5.26210.
14. Pattee GL, Wymer JP, Lomen-Hoerth C, et al. An open-label multicenter study to assess the safety of dextromethorphan/quinidine in patients with pseudobulbar affect associated with a range of underlying neurological conditions. Curr Med Res Opin. 2014;30(11):2255-2265.
15. Strowd RE, Cartwright MS, Okun MS, et al. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J Neurol. 2010;257(8):1382-1387.
16. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
17. Starkstein SE, Migliorelli R, Tesón A, et al. Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1995;59(1):55-60.
18. Pioro EP. Current concepts in the pharmacotherapy of pseudobulbar affect. Drugs. 2011;71(9):1193-1207.
19. Ahmed A, Simmons A. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag. 2013;9:483-489.
20. Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs. 2015;75(1):83-90.
21. Nuedexta [package insert]. Aliso Viejo, CA: Avanir Pharmaceuticals, Inc.; 2015.
22. Pioro EP, Brooks BR, Cummings J, et al; Safety, Tolerability, and Efficacy trial of AVP-923 in PBA Investigators. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010;68(5):693-702.