User login
Bone density vs bone quality: What’s a clinician to do?
Most clinicians were taught directly or indirectly that bone density is the gauge for assessing bone strength and the response to antiosteoporotic treatment. In recent years, however, the concept of bone strength has moved beyond density alone and has expanded to include a number of characteristics of bone that collectively are called quality.
This paper describes how the notion of quality has emerged and some of the clinical scenarios in which quality applies. It discusses several observations in the clinical literature that challenge our understanding of bone density and strength and provides the practitioner a better understanding of densitometry in clinical practice.
WHAT IS BONE QUALITY?
Bone quality is not precisely defined. It is described operationally as an amalgamation of all the factors that determine how well the skeleton can resist fracturing, such as microarchitecture, accumulated microscopic damage, the quality of collagen, the size of mineral crystals, and the rate of bone turnover. The term became popular in the early 1990s, when paradoxes in the treatment of osteoporosis challenged the generally accepted orthodoxy that bone density itself was the best way to assess strength of bone.
FROM BONE MASS TO T SCORES TO BONE QUALITY
Today’s practitioners appreciate the importance of the T score in diagnosing osteoporosis. It was not always this way, since the early attempts to use bone densitometry focused on a specific cutoff of bone mass as a risk for fracture and not the statistical T scores or Z scores that we know.1–3
The T score concept was originally developed to assess the probability of fragility fractures in postmenopausal white women in their mid to late 60s and older.4 It has been useful because the disease prevalence is high in this age group. The T score as originally used was a surrogate marker for the histologic changes in aged bone that render it weak and susceptible to fractures from low loading forces: the lower the score, the worse the fracture risk. It followed intuitively that a low T score clinched the diagnosis of primary osteoporosis.
But the T score has its problems when used outside this intended population. Practitioners have assumed that all patients with abnormally low scores have primary osteoporosis. However, this number alone is insufficient to accurately make such a diagnosis in patients outside the demographic group in which it was developed, because the low disease prevalence in younger groups makes the score less accurate as a predictive tool. Moreover, reevaluation of data from pivotal clinical trials has brought into question our long-held idea that increases in bone density parallel increases in bone strength and reduction in fractures, and that therapeutic improvement in bone density is the mark of success. Bone strength or resistance to fracture is more complex than density alone. Into this arena enters the concept of bone quality, which attempts to explain the following observations.
DENSER BONE IS NOT ALWAYS STRONGER
NOT ALL LOW BONE MINERAL DENSITY IS OSTEOPOROSIS
The following case describes a clinical scenario in which a patient has low bone density but does not have osteoporosis.
A young healthy woman with low bone density
A 35-year-old healthy woman who has jogged recreationally for decades is evaluated for possible treatment of osteoporosis. She started to feel back pain after doing heavy work in her garden. Spinal radiographs did not show a reason for her pain, but her physician, concerned about osteopenia, sent her for dual-energy x-ray absorptiometry. Her spinal T scores and Z scores were 2.5 standard deviations below the mean.
Should she start pharmacologic therapy?
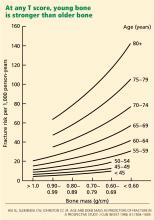
Young bone is stronger than older bone
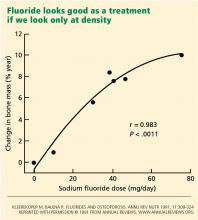
This case shows the other end of the spectrum from the fluoride story. Here, a young healthy person inappropriately underwent a density scan, which led to confusion about how to interpret the results.
As stated above, T scores are not appropriate for young patients—the Z score is used instead. In this case, the low value implied deficiency of bone mass compared with age-matched norms. However, in this patient with no clinical risk factors for fracture, a low T score meant that her bone density was low, but not that she had osteoporosis.
Several factors could account for her low bone density. It could be genetic, if her family is small in stature, or she could be at the extreme end of the distribution curve for normal individuals. Runners tend to be slight in build, and so may have lighter bones. Furthermore, for women, excessive running could lead to lower estrogen activity and therefore lower bone mineral density.
Drug treatment is not warranted for this patient, but standard therapy with exercise, vitamin D, and adequate elemental calcium from the diet or supplements is reasonable.
Thus, the notion of quality entered the clinical arena. Young bone and older bone are qualitatively different in strength, even with similar bone density. This difference was later found to be related to significant qualitative changes within the microscopic architecture of the bone, the collagen, the mineral, and the physiologic activity of the skeletal cells—elements that the T score does not reflect.
Hence, young bone is stronger than older bone across all levels of bone mass or T scores. Its quality is better.
CHANGES IN DENSITY ACCOUNT FOR ONLY PART OF THE DECREASE IN RISK
BONES BECOME STRONGER BEFORE THEY BECOME DENSER
In a number of clinical trials, antiresorptive drugs of various classes started to reduce the risk of fractures before the increases in bone density reached their maximum. Raloxifene significantly reduces the incidence of fractures within 6 to 12 months of starting treatment, whereas the maximal increase in spinal bone density of 2% to 3% is seen at 3 years.15 This type of information further supported the discordance of density and bone strength and underscored the concept that drug therapy affects other factors in bone physiology.
One of these other factors is skeletal turnover, which is assessed by measuring the levels of enzymes or collagen fragments released by osteoblasts or osteoclasts in the blood or urine. These substances are markers of bone metabolism. They do not establish the diagnosis of specific diseases, but their concentrations are higher in high-bone-turnover states such as in some cases of primary osteoporosis. The topic has been reviewed in detail by Singer and Eyre.16
Antiresorptive therapy decreases the levels of these markers to normal within weeks of starting therapy. This prompt response is believed to be the reason that fracture risk reduction is seen so early. This effect of therapy represents a reduction in high osteoclastic activity and, secondarily, preservation of the microarchitecture. Meanwhile, osteoblastic activity adds bone to these less-active osteoclastic sites. If the amount is sufficient, bone densitometry may detect it.
LACK OF CHANGE IN DENSITY DOES NOT NECESSARILY MEAN LACK OF RESPONSE
The lack of change in bone density in patients taking bisphosphonates does not necessarily mean a lack of response. The following clinical scenario exemplifies this paradox.
A middle-aged woman on bisphosphonate therapy
A 68-year-old woman is seen because she seems to be having a poor response to oral bisphosphonate therapy, which was started 3 years ago after she had two vertebral fractures. Her bone density has not changed during this time, but the levels of her bone turnover markers have decreased and remain normal.
Should she start another type of therapy?
Bone turnover markers indicate a response
Studies show that patients with osteoporosis can be stratified into those at low or high risk of fractures on the basis of the activity of bone turnover markers. The risk of fractures is two times higher in people who have high levels of these markers than in those with normal levels, and can rise to four to five times as high in people who have both high marker levels and low bone density.17
All antiresorptive treatments lower the levels of these markers to the normal range and keep them low. In the patient described above, her normal levels of bone turnover markers after treatment indicate a good therapeutic response. The treatment should be continued.
WHAT’S A CLINICIAN TO DO?
These cases illustrate some important questions that often arise in the treatment of patients.
How should the risk of fractures be assessed? Bone densitometry is a better marker of fracture risk than of bone strength because it cannot detect the important qualitative elements of strength. The higher prevalence of osteoporosis in the older population gives the T score cutoff of 2.5 standard deviations below the mean a greater predictive power to diagnose osteoporosis than it does in a younger population with a lower disease prevalence. In younger patients, this cutoff at best represents low bone density and is not diagnostic of osteoporosis unless it is present with other risk factors for fracture.
Newer tools for assessing fracture risk are now entering clinical practice. Estimates of absolute fracture risk are being used,18–20 and a fracture risk assessment tool is being implemented worldwide.21–23 Developed by the World Health Organization and called FRAX, it is based on the bone mineral density of the femoral neck combined with other factors: the patient’s age, sex, weight, and height, whether the patient has a personal or family history of fracture, and whether the patient smokes, uses glucocorticoids, has rheumatoid arthritis, has secondary osteoporosis, or consumes alcohol in excess. It is available online (www.shef.ac.uk/FRAX/tool.jsp) and gives an estimate of the 10-year risk of fracture.
How should response to therapy be assessed? In clinical practice, patients who show no changes in bone density may still be responding to therapy, and the response can be detected by the levels of bone turnover markers. Patients using antiresorptive drugs have normal levels of these markers, decreased from a higher baseline value. Patients using anabolic agents show higher levels of these bone markers, indicating enhanced bone building. So therapeutic efficacy is seen as stable or increased bone density coupled with decreased and normal turnover markers with antiresorptive drug use and increased turnover markers with anabolic drug use.
When fractures occur in patients on therapy, however, it becomes difficult to assess good or poor drug response. Patients who have a fracture within the first year of therapy are best left on the treatment, since this may not generate the full response. Patients who start having fractures years into therapy, however, may be experiencing secondary forms of osteoporosis superimposed on the original primary disease.24 Vitamin D deficiency, hyperparathyroidism, and celiac disease are common problems. Or, perhaps, patients may not be adherent to therapy.25–27 Poor compliance, inappropriate use of medications (especially the bisphosphonate drugs), or even problems of malabsorption of oral medication may be a consideration. The intravenous forms of bisphosphonate drugs warrant consideration in this scenario.28–30
In the future, we may have better tests of bone quality. One such test, called finite element analysis, uses computer modeling and three-dimensional imaging. It has been used for years by engineers designing and testing the strength of bridges, airplanes, and other structures and is now being evaluated as a way to estimate bone strength.
In summary, bone physiology and bone strength are very complex issues that have recently attained new and important nuances. The original use of bone densitometry was to assess the risk of fragility fractures and, secondarily, to diagnose primary osteoporosis in the population of patients for which it was originally developed. While the bone densitometry score does bear some relationship to bone strength, it is not a sufficient surrogate marker in many cases. Hence, clinicians need to judiciously use these testing procedures in combination with a number of clinical factors to diagnose osteoporosis and assess the response to therapy.
- Parfitt AM. Interpretation of bone densitometry measurements: disadvantages of a percentage scale and a discussion of some alternatives. J Bone Miner Res 1990; 5:537–540.
- Webber CE. Uncertainties in bone mineral density T-scores. Clin Invest Med 1998; 21:88–93.
- Blake GM, Fogelman I. Interpretation of bone densitometry studies. Semin Nucl Med 1997; 27:248–260.
- Miller PD. Guidelines for the diagnosis of osteoporosis: T-scores vs fractures. Rev Endocr Metab Disord 2006; 7:75–89.
- Kleerekoper M, Balena R. Fluorides and osteoporosis. Annu Rev Nutr 1991; 11:309–324.
- Riggs BL, Hodgson SF, O'Fallon WM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990; 322:802–809.
- Hui SL, Slemenda CW, Johnston CC. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 1988; 81:1804–1809.
- Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 2006; 354:2250–2261.
- Seeman E. Is a change in bone mineral density a sensitive and specific surrogate of anti-fracture efficacy? Bone 2007; 41:308–317.
- Guyatt GH, Cranney A, Griffith L, et al. Summary of meta-analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures. Endocrinol Metab Clin North Am 2002; 31:659–679.
- Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344:1434–1441.
- Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 2002; 112:281–289.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996; 348:1535–1541.
- Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res 2002; 17:1–10.
- Qu Y, Wong M, Thiebaud D, Stock JL. The effect of raloxifene therapy on the risk of new clinical vertebral fractures at three and six months: a secondary analysis of the MORE trial. Curr Med Res Opin 2005; 21:1955–1959.
- Singer FR, Eyre DR. Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med 2008; 75:739–750.
- Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 2000; 15:1526–1536.
- Siminoski K, Leslie WD, Frame H, et al. Recommendations for bone mineral density reporting in Canada: a shift to absolute fracture risk assessment. J Clin Densitom 2007; 10:120–123.
- Czerwinski E, Badurski JE, Marcinowska-Suchowierska E, Osieleniec J. Current understanding of osteoporosis according to the position of the World Health Organization (WHO) and International Osteoporosis Foundation. Ortop Traumatol Rehabil 2007; 9:337–356.
- Siris ES, Brenneman SK, Barrett-Connor E, et al. The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50–99: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 2006; 17:565–574.
- Borgstrom F, Kanis JA. Health economics of osteoporosis. Best Pract Res Clin Endocrinol Metab 2008; 22:885–900.
- Honig S. Treatment strategies for patients with low bone mass: the younger postmenopausal female. Bull NYU Hosp Jt Dis 2008; 66:240–243.
- Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008; 19:385–397.
- Painter SE, Kleerekoper M, Camacho PM. Secondary osteoporosis: a review of the recent evidence. Endocr Pract 2006; 12:436–445.
- Lau E, Papaioannou A, Dolovich L, et al. Patients' adherence to osteoporosis therapy: exploring the perceptions of postmenopausal women. Can Fam Physician 2008; 54:394–402.
- Zambon A, Baio G, Mazzaglia G, Merlino L, Corrao G. Discontinuity and failures of therapy with bisphosphonates: joint assessment of predictors with multi-state models. Pharmacoepidemiol Drug Saf 2008; 17:260–269.
- Ringe JD, Christodoulakos GE, Mellstrom D, et al. Patient compliance with alendronate, risedronate and raloxifene for the treatment of osteoporosis in postmenopausal women. Curr Med Res Opin 2007; 23:2677–2687.
- Eisman JA, Civitelli R, Adami S, et al. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 2008; 35:488–497.
- Lewiecki EM, Babbitt AM, Piziak VK, Ozturk ZE, Bone HG. Adherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluation. Clin Ther 2008; 30:605–621.
- Lewiecki EM. Intravenous zoledronic acid for the treatment of osteoporosis. Curr Osteoporos Rep 2008; 6:17–23.
Most clinicians were taught directly or indirectly that bone density is the gauge for assessing bone strength and the response to antiosteoporotic treatment. In recent years, however, the concept of bone strength has moved beyond density alone and has expanded to include a number of characteristics of bone that collectively are called quality.
This paper describes how the notion of quality has emerged and some of the clinical scenarios in which quality applies. It discusses several observations in the clinical literature that challenge our understanding of bone density and strength and provides the practitioner a better understanding of densitometry in clinical practice.
WHAT IS BONE QUALITY?
Bone quality is not precisely defined. It is described operationally as an amalgamation of all the factors that determine how well the skeleton can resist fracturing, such as microarchitecture, accumulated microscopic damage, the quality of collagen, the size of mineral crystals, and the rate of bone turnover. The term became popular in the early 1990s, when paradoxes in the treatment of osteoporosis challenged the generally accepted orthodoxy that bone density itself was the best way to assess strength of bone.
FROM BONE MASS TO T SCORES TO BONE QUALITY
Today’s practitioners appreciate the importance of the T score in diagnosing osteoporosis. It was not always this way, since the early attempts to use bone densitometry focused on a specific cutoff of bone mass as a risk for fracture and not the statistical T scores or Z scores that we know.1–3
The T score concept was originally developed to assess the probability of fragility fractures in postmenopausal white women in their mid to late 60s and older.4 It has been useful because the disease prevalence is high in this age group. The T score as originally used was a surrogate marker for the histologic changes in aged bone that render it weak and susceptible to fractures from low loading forces: the lower the score, the worse the fracture risk. It followed intuitively that a low T score clinched the diagnosis of primary osteoporosis.
But the T score has its problems when used outside this intended population. Practitioners have assumed that all patients with abnormally low scores have primary osteoporosis. However, this number alone is insufficient to accurately make such a diagnosis in patients outside the demographic group in which it was developed, because the low disease prevalence in younger groups makes the score less accurate as a predictive tool. Moreover, reevaluation of data from pivotal clinical trials has brought into question our long-held idea that increases in bone density parallel increases in bone strength and reduction in fractures, and that therapeutic improvement in bone density is the mark of success. Bone strength or resistance to fracture is more complex than density alone. Into this arena enters the concept of bone quality, which attempts to explain the following observations.
DENSER BONE IS NOT ALWAYS STRONGER
NOT ALL LOW BONE MINERAL DENSITY IS OSTEOPOROSIS
The following case describes a clinical scenario in which a patient has low bone density but does not have osteoporosis.
A young healthy woman with low bone density
A 35-year-old healthy woman who has jogged recreationally for decades is evaluated for possible treatment of osteoporosis. She started to feel back pain after doing heavy work in her garden. Spinal radiographs did not show a reason for her pain, but her physician, concerned about osteopenia, sent her for dual-energy x-ray absorptiometry. Her spinal T scores and Z scores were 2.5 standard deviations below the mean.
Should she start pharmacologic therapy?
Young bone is stronger than older bone
This case shows the other end of the spectrum from the fluoride story. Here, a young healthy person inappropriately underwent a density scan, which led to confusion about how to interpret the results.
As stated above, T scores are not appropriate for young patients—the Z score is used instead. In this case, the low value implied deficiency of bone mass compared with age-matched norms. However, in this patient with no clinical risk factors for fracture, a low T score meant that her bone density was low, but not that she had osteoporosis.
Several factors could account for her low bone density. It could be genetic, if her family is small in stature, or she could be at the extreme end of the distribution curve for normal individuals. Runners tend to be slight in build, and so may have lighter bones. Furthermore, for women, excessive running could lead to lower estrogen activity and therefore lower bone mineral density.
Drug treatment is not warranted for this patient, but standard therapy with exercise, vitamin D, and adequate elemental calcium from the diet or supplements is reasonable.
Thus, the notion of quality entered the clinical arena. Young bone and older bone are qualitatively different in strength, even with similar bone density. This difference was later found to be related to significant qualitative changes within the microscopic architecture of the bone, the collagen, the mineral, and the physiologic activity of the skeletal cells—elements that the T score does not reflect.
Hence, young bone is stronger than older bone across all levels of bone mass or T scores. Its quality is better.
CHANGES IN DENSITY ACCOUNT FOR ONLY PART OF THE DECREASE IN RISK
BONES BECOME STRONGER BEFORE THEY BECOME DENSER
In a number of clinical trials, antiresorptive drugs of various classes started to reduce the risk of fractures before the increases in bone density reached their maximum. Raloxifene significantly reduces the incidence of fractures within 6 to 12 months of starting treatment, whereas the maximal increase in spinal bone density of 2% to 3% is seen at 3 years.15 This type of information further supported the discordance of density and bone strength and underscored the concept that drug therapy affects other factors in bone physiology.
One of these other factors is skeletal turnover, which is assessed by measuring the levels of enzymes or collagen fragments released by osteoblasts or osteoclasts in the blood or urine. These substances are markers of bone metabolism. They do not establish the diagnosis of specific diseases, but their concentrations are higher in high-bone-turnover states such as in some cases of primary osteoporosis. The topic has been reviewed in detail by Singer and Eyre.16
Antiresorptive therapy decreases the levels of these markers to normal within weeks of starting therapy. This prompt response is believed to be the reason that fracture risk reduction is seen so early. This effect of therapy represents a reduction in high osteoclastic activity and, secondarily, preservation of the microarchitecture. Meanwhile, osteoblastic activity adds bone to these less-active osteoclastic sites. If the amount is sufficient, bone densitometry may detect it.
LACK OF CHANGE IN DENSITY DOES NOT NECESSARILY MEAN LACK OF RESPONSE
The lack of change in bone density in patients taking bisphosphonates does not necessarily mean a lack of response. The following clinical scenario exemplifies this paradox.
A middle-aged woman on bisphosphonate therapy
A 68-year-old woman is seen because she seems to be having a poor response to oral bisphosphonate therapy, which was started 3 years ago after she had two vertebral fractures. Her bone density has not changed during this time, but the levels of her bone turnover markers have decreased and remain normal.
Should she start another type of therapy?
Bone turnover markers indicate a response
Studies show that patients with osteoporosis can be stratified into those at low or high risk of fractures on the basis of the activity of bone turnover markers. The risk of fractures is two times higher in people who have high levels of these markers than in those with normal levels, and can rise to four to five times as high in people who have both high marker levels and low bone density.17
All antiresorptive treatments lower the levels of these markers to the normal range and keep them low. In the patient described above, her normal levels of bone turnover markers after treatment indicate a good therapeutic response. The treatment should be continued.
WHAT’S A CLINICIAN TO DO?
These cases illustrate some important questions that often arise in the treatment of patients.
How should the risk of fractures be assessed? Bone densitometry is a better marker of fracture risk than of bone strength because it cannot detect the important qualitative elements of strength. The higher prevalence of osteoporosis in the older population gives the T score cutoff of 2.5 standard deviations below the mean a greater predictive power to diagnose osteoporosis than it does in a younger population with a lower disease prevalence. In younger patients, this cutoff at best represents low bone density and is not diagnostic of osteoporosis unless it is present with other risk factors for fracture.
Newer tools for assessing fracture risk are now entering clinical practice. Estimates of absolute fracture risk are being used,18–20 and a fracture risk assessment tool is being implemented worldwide.21–23 Developed by the World Health Organization and called FRAX, it is based on the bone mineral density of the femoral neck combined with other factors: the patient’s age, sex, weight, and height, whether the patient has a personal or family history of fracture, and whether the patient smokes, uses glucocorticoids, has rheumatoid arthritis, has secondary osteoporosis, or consumes alcohol in excess. It is available online (www.shef.ac.uk/FRAX/tool.jsp) and gives an estimate of the 10-year risk of fracture.
How should response to therapy be assessed? In clinical practice, patients who show no changes in bone density may still be responding to therapy, and the response can be detected by the levels of bone turnover markers. Patients using antiresorptive drugs have normal levels of these markers, decreased from a higher baseline value. Patients using anabolic agents show higher levels of these bone markers, indicating enhanced bone building. So therapeutic efficacy is seen as stable or increased bone density coupled with decreased and normal turnover markers with antiresorptive drug use and increased turnover markers with anabolic drug use.
When fractures occur in patients on therapy, however, it becomes difficult to assess good or poor drug response. Patients who have a fracture within the first year of therapy are best left on the treatment, since this may not generate the full response. Patients who start having fractures years into therapy, however, may be experiencing secondary forms of osteoporosis superimposed on the original primary disease.24 Vitamin D deficiency, hyperparathyroidism, and celiac disease are common problems. Or, perhaps, patients may not be adherent to therapy.25–27 Poor compliance, inappropriate use of medications (especially the bisphosphonate drugs), or even problems of malabsorption of oral medication may be a consideration. The intravenous forms of bisphosphonate drugs warrant consideration in this scenario.28–30
In the future, we may have better tests of bone quality. One such test, called finite element analysis, uses computer modeling and three-dimensional imaging. It has been used for years by engineers designing and testing the strength of bridges, airplanes, and other structures and is now being evaluated as a way to estimate bone strength.
In summary, bone physiology and bone strength are very complex issues that have recently attained new and important nuances. The original use of bone densitometry was to assess the risk of fragility fractures and, secondarily, to diagnose primary osteoporosis in the population of patients for which it was originally developed. While the bone densitometry score does bear some relationship to bone strength, it is not a sufficient surrogate marker in many cases. Hence, clinicians need to judiciously use these testing procedures in combination with a number of clinical factors to diagnose osteoporosis and assess the response to therapy.
Most clinicians were taught directly or indirectly that bone density is the gauge for assessing bone strength and the response to antiosteoporotic treatment. In recent years, however, the concept of bone strength has moved beyond density alone and has expanded to include a number of characteristics of bone that collectively are called quality.
This paper describes how the notion of quality has emerged and some of the clinical scenarios in which quality applies. It discusses several observations in the clinical literature that challenge our understanding of bone density and strength and provides the practitioner a better understanding of densitometry in clinical practice.
WHAT IS BONE QUALITY?
Bone quality is not precisely defined. It is described operationally as an amalgamation of all the factors that determine how well the skeleton can resist fracturing, such as microarchitecture, accumulated microscopic damage, the quality of collagen, the size of mineral crystals, and the rate of bone turnover. The term became popular in the early 1990s, when paradoxes in the treatment of osteoporosis challenged the generally accepted orthodoxy that bone density itself was the best way to assess strength of bone.
FROM BONE MASS TO T SCORES TO BONE QUALITY
Today’s practitioners appreciate the importance of the T score in diagnosing osteoporosis. It was not always this way, since the early attempts to use bone densitometry focused on a specific cutoff of bone mass as a risk for fracture and not the statistical T scores or Z scores that we know.1–3
The T score concept was originally developed to assess the probability of fragility fractures in postmenopausal white women in their mid to late 60s and older.4 It has been useful because the disease prevalence is high in this age group. The T score as originally used was a surrogate marker for the histologic changes in aged bone that render it weak and susceptible to fractures from low loading forces: the lower the score, the worse the fracture risk. It followed intuitively that a low T score clinched the diagnosis of primary osteoporosis.
But the T score has its problems when used outside this intended population. Practitioners have assumed that all patients with abnormally low scores have primary osteoporosis. However, this number alone is insufficient to accurately make such a diagnosis in patients outside the demographic group in which it was developed, because the low disease prevalence in younger groups makes the score less accurate as a predictive tool. Moreover, reevaluation of data from pivotal clinical trials has brought into question our long-held idea that increases in bone density parallel increases in bone strength and reduction in fractures, and that therapeutic improvement in bone density is the mark of success. Bone strength or resistance to fracture is more complex than density alone. Into this arena enters the concept of bone quality, which attempts to explain the following observations.
DENSER BONE IS NOT ALWAYS STRONGER
NOT ALL LOW BONE MINERAL DENSITY IS OSTEOPOROSIS
The following case describes a clinical scenario in which a patient has low bone density but does not have osteoporosis.
A young healthy woman with low bone density
A 35-year-old healthy woman who has jogged recreationally for decades is evaluated for possible treatment of osteoporosis. She started to feel back pain after doing heavy work in her garden. Spinal radiographs did not show a reason for her pain, but her physician, concerned about osteopenia, sent her for dual-energy x-ray absorptiometry. Her spinal T scores and Z scores were 2.5 standard deviations below the mean.
Should she start pharmacologic therapy?
Young bone is stronger than older bone
This case shows the other end of the spectrum from the fluoride story. Here, a young healthy person inappropriately underwent a density scan, which led to confusion about how to interpret the results.
As stated above, T scores are not appropriate for young patients—the Z score is used instead. In this case, the low value implied deficiency of bone mass compared with age-matched norms. However, in this patient with no clinical risk factors for fracture, a low T score meant that her bone density was low, but not that she had osteoporosis.
Several factors could account for her low bone density. It could be genetic, if her family is small in stature, or she could be at the extreme end of the distribution curve for normal individuals. Runners tend to be slight in build, and so may have lighter bones. Furthermore, for women, excessive running could lead to lower estrogen activity and therefore lower bone mineral density.
Drug treatment is not warranted for this patient, but standard therapy with exercise, vitamin D, and adequate elemental calcium from the diet or supplements is reasonable.
Thus, the notion of quality entered the clinical arena. Young bone and older bone are qualitatively different in strength, even with similar bone density. This difference was later found to be related to significant qualitative changes within the microscopic architecture of the bone, the collagen, the mineral, and the physiologic activity of the skeletal cells—elements that the T score does not reflect.
Hence, young bone is stronger than older bone across all levels of bone mass or T scores. Its quality is better.
CHANGES IN DENSITY ACCOUNT FOR ONLY PART OF THE DECREASE IN RISK
BONES BECOME STRONGER BEFORE THEY BECOME DENSER
In a number of clinical trials, antiresorptive drugs of various classes started to reduce the risk of fractures before the increases in bone density reached their maximum. Raloxifene significantly reduces the incidence of fractures within 6 to 12 months of starting treatment, whereas the maximal increase in spinal bone density of 2% to 3% is seen at 3 years.15 This type of information further supported the discordance of density and bone strength and underscored the concept that drug therapy affects other factors in bone physiology.
One of these other factors is skeletal turnover, which is assessed by measuring the levels of enzymes or collagen fragments released by osteoblasts or osteoclasts in the blood or urine. These substances are markers of bone metabolism. They do not establish the diagnosis of specific diseases, but their concentrations are higher in high-bone-turnover states such as in some cases of primary osteoporosis. The topic has been reviewed in detail by Singer and Eyre.16
Antiresorptive therapy decreases the levels of these markers to normal within weeks of starting therapy. This prompt response is believed to be the reason that fracture risk reduction is seen so early. This effect of therapy represents a reduction in high osteoclastic activity and, secondarily, preservation of the microarchitecture. Meanwhile, osteoblastic activity adds bone to these less-active osteoclastic sites. If the amount is sufficient, bone densitometry may detect it.
LACK OF CHANGE IN DENSITY DOES NOT NECESSARILY MEAN LACK OF RESPONSE
The lack of change in bone density in patients taking bisphosphonates does not necessarily mean a lack of response. The following clinical scenario exemplifies this paradox.
A middle-aged woman on bisphosphonate therapy
A 68-year-old woman is seen because she seems to be having a poor response to oral bisphosphonate therapy, which was started 3 years ago after she had two vertebral fractures. Her bone density has not changed during this time, but the levels of her bone turnover markers have decreased and remain normal.
Should she start another type of therapy?
Bone turnover markers indicate a response
Studies show that patients with osteoporosis can be stratified into those at low or high risk of fractures on the basis of the activity of bone turnover markers. The risk of fractures is two times higher in people who have high levels of these markers than in those with normal levels, and can rise to four to five times as high in people who have both high marker levels and low bone density.17
All antiresorptive treatments lower the levels of these markers to the normal range and keep them low. In the patient described above, her normal levels of bone turnover markers after treatment indicate a good therapeutic response. The treatment should be continued.
WHAT’S A CLINICIAN TO DO?
These cases illustrate some important questions that often arise in the treatment of patients.
How should the risk of fractures be assessed? Bone densitometry is a better marker of fracture risk than of bone strength because it cannot detect the important qualitative elements of strength. The higher prevalence of osteoporosis in the older population gives the T score cutoff of 2.5 standard deviations below the mean a greater predictive power to diagnose osteoporosis than it does in a younger population with a lower disease prevalence. In younger patients, this cutoff at best represents low bone density and is not diagnostic of osteoporosis unless it is present with other risk factors for fracture.
Newer tools for assessing fracture risk are now entering clinical practice. Estimates of absolute fracture risk are being used,18–20 and a fracture risk assessment tool is being implemented worldwide.21–23 Developed by the World Health Organization and called FRAX, it is based on the bone mineral density of the femoral neck combined with other factors: the patient’s age, sex, weight, and height, whether the patient has a personal or family history of fracture, and whether the patient smokes, uses glucocorticoids, has rheumatoid arthritis, has secondary osteoporosis, or consumes alcohol in excess. It is available online (www.shef.ac.uk/FRAX/tool.jsp) and gives an estimate of the 10-year risk of fracture.
How should response to therapy be assessed? In clinical practice, patients who show no changes in bone density may still be responding to therapy, and the response can be detected by the levels of bone turnover markers. Patients using antiresorptive drugs have normal levels of these markers, decreased from a higher baseline value. Patients using anabolic agents show higher levels of these bone markers, indicating enhanced bone building. So therapeutic efficacy is seen as stable or increased bone density coupled with decreased and normal turnover markers with antiresorptive drug use and increased turnover markers with anabolic drug use.
When fractures occur in patients on therapy, however, it becomes difficult to assess good or poor drug response. Patients who have a fracture within the first year of therapy are best left on the treatment, since this may not generate the full response. Patients who start having fractures years into therapy, however, may be experiencing secondary forms of osteoporosis superimposed on the original primary disease.24 Vitamin D deficiency, hyperparathyroidism, and celiac disease are common problems. Or, perhaps, patients may not be adherent to therapy.25–27 Poor compliance, inappropriate use of medications (especially the bisphosphonate drugs), or even problems of malabsorption of oral medication may be a consideration. The intravenous forms of bisphosphonate drugs warrant consideration in this scenario.28–30
In the future, we may have better tests of bone quality. One such test, called finite element analysis, uses computer modeling and three-dimensional imaging. It has been used for years by engineers designing and testing the strength of bridges, airplanes, and other structures and is now being evaluated as a way to estimate bone strength.
In summary, bone physiology and bone strength are very complex issues that have recently attained new and important nuances. The original use of bone densitometry was to assess the risk of fragility fractures and, secondarily, to diagnose primary osteoporosis in the population of patients for which it was originally developed. While the bone densitometry score does bear some relationship to bone strength, it is not a sufficient surrogate marker in many cases. Hence, clinicians need to judiciously use these testing procedures in combination with a number of clinical factors to diagnose osteoporosis and assess the response to therapy.
- Parfitt AM. Interpretation of bone densitometry measurements: disadvantages of a percentage scale and a discussion of some alternatives. J Bone Miner Res 1990; 5:537–540.
- Webber CE. Uncertainties in bone mineral density T-scores. Clin Invest Med 1998; 21:88–93.
- Blake GM, Fogelman I. Interpretation of bone densitometry studies. Semin Nucl Med 1997; 27:248–260.
- Miller PD. Guidelines for the diagnosis of osteoporosis: T-scores vs fractures. Rev Endocr Metab Disord 2006; 7:75–89.
- Kleerekoper M, Balena R. Fluorides and osteoporosis. Annu Rev Nutr 1991; 11:309–324.
- Riggs BL, Hodgson SF, O'Fallon WM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990; 322:802–809.
- Hui SL, Slemenda CW, Johnston CC. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 1988; 81:1804–1809.
- Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 2006; 354:2250–2261.
- Seeman E. Is a change in bone mineral density a sensitive and specific surrogate of anti-fracture efficacy? Bone 2007; 41:308–317.
- Guyatt GH, Cranney A, Griffith L, et al. Summary of meta-analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures. Endocrinol Metab Clin North Am 2002; 31:659–679.
- Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344:1434–1441.
- Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 2002; 112:281–289.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996; 348:1535–1541.
- Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res 2002; 17:1–10.
- Qu Y, Wong M, Thiebaud D, Stock JL. The effect of raloxifene therapy on the risk of new clinical vertebral fractures at three and six months: a secondary analysis of the MORE trial. Curr Med Res Opin 2005; 21:1955–1959.
- Singer FR, Eyre DR. Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med 2008; 75:739–750.
- Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 2000; 15:1526–1536.
- Siminoski K, Leslie WD, Frame H, et al. Recommendations for bone mineral density reporting in Canada: a shift to absolute fracture risk assessment. J Clin Densitom 2007; 10:120–123.
- Czerwinski E, Badurski JE, Marcinowska-Suchowierska E, Osieleniec J. Current understanding of osteoporosis according to the position of the World Health Organization (WHO) and International Osteoporosis Foundation. Ortop Traumatol Rehabil 2007; 9:337–356.
- Siris ES, Brenneman SK, Barrett-Connor E, et al. The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50–99: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 2006; 17:565–574.
- Borgstrom F, Kanis JA. Health economics of osteoporosis. Best Pract Res Clin Endocrinol Metab 2008; 22:885–900.
- Honig S. Treatment strategies for patients with low bone mass: the younger postmenopausal female. Bull NYU Hosp Jt Dis 2008; 66:240–243.
- Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008; 19:385–397.
- Painter SE, Kleerekoper M, Camacho PM. Secondary osteoporosis: a review of the recent evidence. Endocr Pract 2006; 12:436–445.
- Lau E, Papaioannou A, Dolovich L, et al. Patients' adherence to osteoporosis therapy: exploring the perceptions of postmenopausal women. Can Fam Physician 2008; 54:394–402.
- Zambon A, Baio G, Mazzaglia G, Merlino L, Corrao G. Discontinuity and failures of therapy with bisphosphonates: joint assessment of predictors with multi-state models. Pharmacoepidemiol Drug Saf 2008; 17:260–269.
- Ringe JD, Christodoulakos GE, Mellstrom D, et al. Patient compliance with alendronate, risedronate and raloxifene for the treatment of osteoporosis in postmenopausal women. Curr Med Res Opin 2007; 23:2677–2687.
- Eisman JA, Civitelli R, Adami S, et al. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 2008; 35:488–497.
- Lewiecki EM, Babbitt AM, Piziak VK, Ozturk ZE, Bone HG. Adherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluation. Clin Ther 2008; 30:605–621.
- Lewiecki EM. Intravenous zoledronic acid for the treatment of osteoporosis. Curr Osteoporos Rep 2008; 6:17–23.
- Parfitt AM. Interpretation of bone densitometry measurements: disadvantages of a percentage scale and a discussion of some alternatives. J Bone Miner Res 1990; 5:537–540.
- Webber CE. Uncertainties in bone mineral density T-scores. Clin Invest Med 1998; 21:88–93.
- Blake GM, Fogelman I. Interpretation of bone densitometry studies. Semin Nucl Med 1997; 27:248–260.
- Miller PD. Guidelines for the diagnosis of osteoporosis: T-scores vs fractures. Rev Endocr Metab Disord 2006; 7:75–89.
- Kleerekoper M, Balena R. Fluorides and osteoporosis. Annu Rev Nutr 1991; 11:309–324.
- Riggs BL, Hodgson SF, O'Fallon WM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990; 322:802–809.
- Hui SL, Slemenda CW, Johnston CC. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 1988; 81:1804–1809.
- Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 2006; 354:2250–2261.
- Seeman E. Is a change in bone mineral density a sensitive and specific surrogate of anti-fracture efficacy? Bone 2007; 41:308–317.
- Guyatt GH, Cranney A, Griffith L, et al. Summary of meta-analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures. Endocrinol Metab Clin North Am 2002; 31:659–679.
- Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344:1434–1441.
- Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 2002; 112:281–289.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996; 348:1535–1541.
- Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res 2002; 17:1–10.
- Qu Y, Wong M, Thiebaud D, Stock JL. The effect of raloxifene therapy on the risk of new clinical vertebral fractures at three and six months: a secondary analysis of the MORE trial. Curr Med Res Opin 2005; 21:1955–1959.
- Singer FR, Eyre DR. Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med 2008; 75:739–750.
- Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 2000; 15:1526–1536.
- Siminoski K, Leslie WD, Frame H, et al. Recommendations for bone mineral density reporting in Canada: a shift to absolute fracture risk assessment. J Clin Densitom 2007; 10:120–123.
- Czerwinski E, Badurski JE, Marcinowska-Suchowierska E, Osieleniec J. Current understanding of osteoporosis according to the position of the World Health Organization (WHO) and International Osteoporosis Foundation. Ortop Traumatol Rehabil 2007; 9:337–356.
- Siris ES, Brenneman SK, Barrett-Connor E, et al. The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50–99: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 2006; 17:565–574.
- Borgstrom F, Kanis JA. Health economics of osteoporosis. Best Pract Res Clin Endocrinol Metab 2008; 22:885–900.
- Honig S. Treatment strategies for patients with low bone mass: the younger postmenopausal female. Bull NYU Hosp Jt Dis 2008; 66:240–243.
- Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008; 19:385–397.
- Painter SE, Kleerekoper M, Camacho PM. Secondary osteoporosis: a review of the recent evidence. Endocr Pract 2006; 12:436–445.
- Lau E, Papaioannou A, Dolovich L, et al. Patients' adherence to osteoporosis therapy: exploring the perceptions of postmenopausal women. Can Fam Physician 2008; 54:394–402.
- Zambon A, Baio G, Mazzaglia G, Merlino L, Corrao G. Discontinuity and failures of therapy with bisphosphonates: joint assessment of predictors with multi-state models. Pharmacoepidemiol Drug Saf 2008; 17:260–269.
- Ringe JD, Christodoulakos GE, Mellstrom D, et al. Patient compliance with alendronate, risedronate and raloxifene for the treatment of osteoporosis in postmenopausal women. Curr Med Res Opin 2007; 23:2677–2687.
- Eisman JA, Civitelli R, Adami S, et al. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 2008; 35:488–497.
- Lewiecki EM, Babbitt AM, Piziak VK, Ozturk ZE, Bone HG. Adherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluation. Clin Ther 2008; 30:605–621.
- Lewiecki EM. Intravenous zoledronic acid for the treatment of osteoporosis. Curr Osteoporos Rep 2008; 6:17–23.
KEY POINTS
- Bone quality is a composite of properties that make bone resist fracture, such as its microarchitecture, accumulated microscopic damage, the quality of collagen, mineral crystal size, and bone turnover.
- The T score was derived from a population of white women in their mid to late 60s and older; in other populations, low T scores do not necessarily reflect the disease state—osteoporosis—with its inherent decreased strength and propensity to fracture.
- In assessing the risk of fractures, clinicians should consider not only the bone mineral density but also clinical risk factors.
- Markers of bone turnover are elevated in some cases of primary osteoporosis and return to normal levels with antiresorptive therapy but not with anabolic therapy.