User login
Visual hallucinations and severe anxiety in the ICU after surgery
CASE Anxiety in the ICU
Mr. B, age 42, an African American man, is admitted to the inpatient medical unit for surgical treatment of peritoneal carcinomatosis with pelvic exenteration. He has a history of metastatic rectal cancer, chronic pain, and hypertension, but no psychiatric history. Mr. B’s postsurgical hospital stay is complicated by treatment-resistant tachycardia and hypertension, and he requires a lengthy stay in the ICU. In the ICU, Mr. B reports having visual hallucinations where he sees an individual placing a drug in his IV line. Additionally, he reports severe anxiety related to this experience. His anxiety and visual hallucinations are treated with coadministration of IV lorazepam, diphenhydramine, and haloperidol. These medications resolve the hallucinations, but his anxiety worsens and he becomes restless. He receives additional doses of IV haloperidol administered in 5 mg increments and reaching a cumulative 12-hour dose of 50 mg. Mr. B continues to report anxiety, so the psychiatry consultation-liaison (C-L) service is called.
[polldaddy:9970907]
The authors’ observations
Determining the cause of Mr. B’s anxiety is challenging because of his prolonged medical course, com
From a medical perspective, in a post-surgical patient treated in the ICU, the consulting practitioner must pay particular attention to delirium. ICU delirium is common—one report indicated that it occurs in 32.3% of ICU patients1—and frequently confused with psychiatric morbidity.2 Identifying delirium as the cause of impairment is important because delirium has potentially modifiable underlying etiologies. Symptomatically, delirium presents as impairment and fluctuation in attention, awareness, and at least one other cognitive domain, with a clear indication that the impairment occurred over a short period of time and represents a departure from baseline.3 In Mr. B’s case, these symptoms have not been excluded and should be considered by the C-L psychiatrists.
In addition to delirium, the C-L team must consider psychiatric comorbidity. Mr. B has no psychiatric history and a sudden first occurrence of hallucinations; therefore, it is unlikely that he has developed a primary psychotic disorder. Because he reported his symptoms had been present only for several days, he would not meet criteria for schizophrenia, which according to DSM-5 criteria require at least 1 month of ≥2 symptoms (including delusions, hallucinations, disorganized speech, disorganized behavior, or negative symptoms) and 6 months of declining function.3 However, although it is improbable, the C-L team must consider a primary psychotic illness, particularly given the potential devastating consequence of being misdiagnosed and mismanaged for an alternative illness. Unlike psychotic disorders, anxiety disorders are significantly more prevalent in the U.S. general population than primary psychotic disorders.4 Furthermore, the prevalence of anxiety disorders increases in the ICU setting; one study found that up to 61% of ICU patients setting experience “anxiety features.”5 Therefore, anxiety disorders and stress disorders should be considered in ICU patients who exhibit psychiatric symptoms.
Clinicians also should consider medication-induced adverse effects. In the ICU, patients are frequently managed on multiple medications, which increase their risk of developing adverse effects and adverse reactions.6 One potential consequence of polypharmacy is delirium, which remains a relevant potential diagnosis for Mr. B.7 Alternative consequences vary by medication and their respective pharmacodynamics. We take into consideration Mr. B’s exposure to high doses of the high-potency antipsychotic agent, haloperidol. Exposure to haloperidol can result in extrapyramidal symptoms, including akathisia,8,9,10 and the rare, but potentially fatal, NMS.11 These reactions can often be distinguished by taking a thorough history and a physical evaluation. In the case of akathisia, the clinician should look for medication exposure, titration, or taper. Most commonly, akathisia occurs secondary to antipsychotic exposure,12 followed by the onset of a combination of subjective symptoms, such as restlessness, anxiety, and irritability, and an objective symptom of increased motor activity.3 NMS, on the other hand, is distinguished by symptoms that include hyperthermia (>38ºC), diaphoresis, severe rigidity, urinary incontinence, vital instability, alterations in mental health status, and elevations in creatine kinase greater than 4-fold the upper limit, usually in the setting of treatment with antipsychotics.3 Nearly all cases of NMS occur within the first 30 days of antipsychotic exposure.3 While, overtly, NMS may appear to be less subtle than akathisia, clinicians should still be weary to rule out this admittedly rare, though potentially lethal diagnosis, especially in an ICU patient, where the diagnosis can be muddied by medical comorbidities that may mask the syndrome.
Continue to: EVALUATION Focus on akathisia
EVALUATION Focus on akathisia
On interview by the C-L team, Mr. B is visibly restless, moving all 4 extremities. He reports increased anxiety and irritability over the past 2 to 3 days. Mr. B states that he is aware of his increased motor movements and can briefly suppress them. However, after several seconds, he again begins spontaneously fidgeting, moving all 4 extremities and shifting from side to side in bed, saying, “I just feel anxious.” He denies having visual hallucinations, and says that the previous hallucinations had spontaneously presented and remitted after surgery. He denies the use of psychotropics for mental illness, prior similar symptoms to this presentation, a family history of mental illness, recent illicit substance use, or excessive alcohol use prior to presentation. This history is corroborated by collateral information from his brother, who was present in the ICU. On physical examination, Mr. B is afebrile and his vital signs are within normal limits. He does not have muscular rigidity or neck dystonia. His laboratory values, including complete blood count, electrolytes, liver function tests, and creatine phosphokinase, are within normal limits.
His medication administration record includes 46 standing agents, 16 “as-needed” agents, and 8 infusions. Several of the standing agents had psychotropic properties; however, the most salient were several opioids, ketamine, midazolam, lorazepam, dexamethasone, haloperidol, and olanzapine.
[polldaddy:9970908]
The authors’ observations
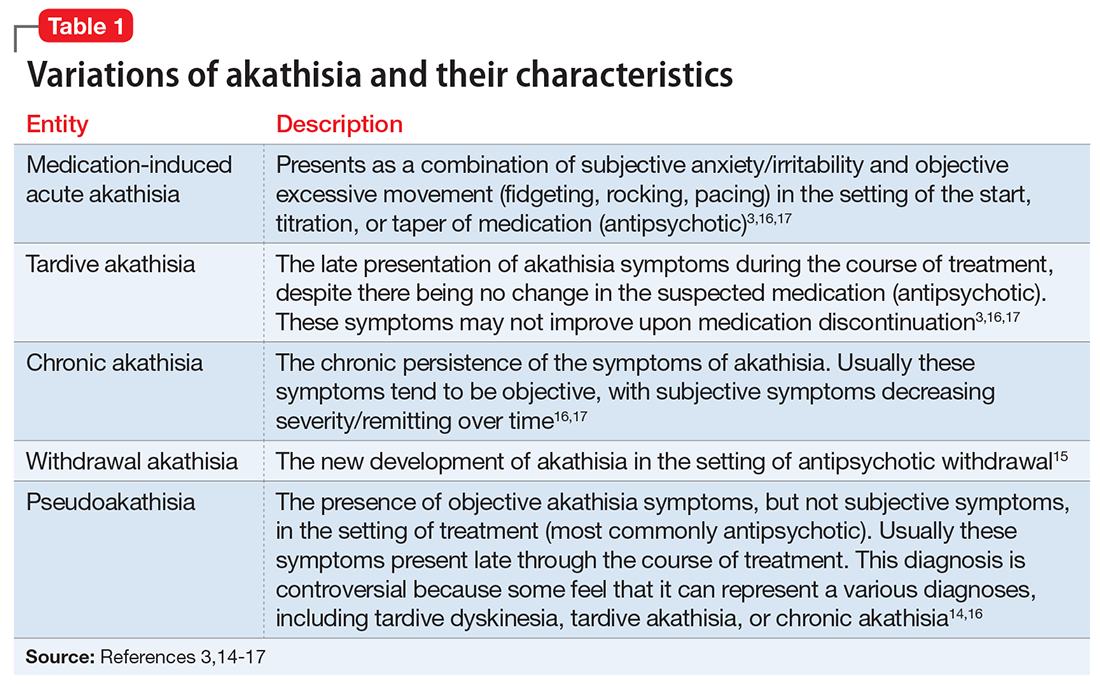
We determined that the most likely diagnosis for Mr. B’s symptoms was medication-induced akathisia secondary to haloperidol. Akathisia, coined by Haskovec in 1901,12,13 is from Greek, meaning an “inability to sit.”12 DSM-5 describes 2 forms of akathisia: medication-induced acute akathisia, and tardive akathisia.3 In the literature, others have described additional classifications, including chronic akathisia, withdrawal akathisia, and pseudoakathisia (Table 13,14-17). In Mr. B’s case, given his sudden development of symptoms and their direct chronologic relationship to antipsychotic treatment, and his combined subjective and objective symptoms, we believed that Mr. B’s symptoms were consistent with medication-induced acute akathisia (MIA). The identification and treatment of this clinical entity is important for several reasons, including reducing patient morbidity and maximizing patient comfort. Additionally, because akathisia has been associated with poor medication adherence, increased agitation/aggression, increased suicidality, and the eventual development of tardive dyskinesia,18 it is a relevant prognostic consideration when deciding to treat a patient with antipsychotics.
Pathophysiologically, we have yet to fully shed light on the exact underpinnings of akathisia. Much of our present knowledge stems from patient response to pharmacologic agents. While dopamine blockade has been linked to akathisia, the exact mechanism is not completely understood. Previous theories linking nigrostriatal pathways have been expanded to include mesocortical and mesolimbic considerations.12,17,18 Similarly surmised from medication effects, the transmitters y-aminobutyric acid, serotonin/5-hydroxytryptamine (5-HT), norepinephrine, and acetylcholine also have been linked to this syndrome, though as of yet, exact gross pathophysiologic mechanisms have not been fully elucidated.12 More recently, Stahl and Loonen19 described a novel mechanism by which they link the shell of the nucleus accumbens to akathisia. In their report, they indicate that the potential reduction in dopaminergic activity, secondary to antipsychotic administration, can result in compensatory noradrenergic activation of the locus coeruleus.19 The increased noradrenergic activity results in the downstream activation of the shell of the nucleus accumbens.19 The activation of the nucleus accumbens shell, which has been linked to unconditioned feeding and fear behavior, can then result in a cascade of effects that would phenotypically present as the syndrome we recognize to be akathisia.19
Numerous etiologies have been linked to MIA. Of these, high-potency antipsychotics are believed to remain the greatest risk factor for akathisia,18 although atypical antipsychotics, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors, have been linked to the disorder.18,19
Continue to: Regarding antipsychotics...
Regarding antipsychotics, risk factors for akathisia include drug potency, dose, and rapidity of titration.20 All of these factors were relevant in our patient’s case. Risk across antipsychotic classes is not well understood; few head-to-head studies have comparing antipsychotics. However, general estimates suggest a 15% to 40% prevalence in patients exposed to typical antipsychotics, as compared with 0% to 12% exposed to atypical antipsychotics.8 The literature-reported difference in risk, as well as our patient’s comparative difference in exposure to large doses of haloperidol (50 mg) as compared with 1 dose of olanzapine (5 mg), led us to believe his akathisia developed primarily due to his exposure to haloperidol. Conclusively linking his symptoms to haloperidol alone, however, is not possible, and we did consider that olanzapine may in fact have had some effect in worsening Mr. B’s akathisia.
[polldaddy:9970909]
The authors’ observations
While there are reports on the efficacy of various agents in the treatment of akathisia, the most commonly evaluated agents are propranolol, anticholinergics, and benzodiazepines.17, 21
Propranolol is a nonselective beta-adrenergic blocker with numerous indications.17 Despite a 2004 Cochrane Review indicating that there is no evidence in support of central acting beta-blockers for treating akathisia,22 propranolol is not yet recognized as an appropriate treatment.17 The reason for this discrepancy is likely due to the Cochrane Review’s restrictive inclusion criteria, which prevented the analysis of much of the literature.22 In fact, several reports cite evidence for the treatment efficacy of propranolol17 and, to date, some reports continue to advocate for its use as a first-line agent in the treatment of akathisia. Admittedly, besides the Cochrane Review,22 other reports have found propranolol to be ineffective for treating akathisia,23 although these tend to be limited by their population size and generalizability.
As with propranolol, a 2006 Cochrane Review found “no reliable evidence to support or refute” using anticholinergic agents in the treatment of akathisia.24 We suspect that the review’s findings were likely secondary to its strict inclusion criteria.24 In fact, several reports support using anticholinergic agents for treating akathisia.25 Here we focus on benztropine and diphenhydramine.
Two reviews—Blaisdell26 (1994) and Poyurovsky27 (2010)—suggest modest benefits from benztropine, primarily in patients with comorbid Parkinson’s disease. Despite these benefits, head-to-head trials seem to either point to the superiority of propranolol or to no difference between these agents for treating akathisia.28,29 In a review, we only found 1 trial demonstrating benztropine’s superiority over propranolol,23 but this trial was constrained by its small population (6 patients). Therefore, the data suggest that, when indicated, clinicians should lean towards using propranolol for treating akathisia.
Continue to: Diphenhydramine, a first-generation antihistamine...
Diphenhydramine, a first-generation antihistamine with antimuscarinic properties, has been studied for its efficacy in treating metoclopramide-induced akathisia in the emergency setting.30 There are several reports on the efficacy of this agent, including a large randomized study involving 281 patients that found it effective for preventing metoclopramide-induced akathisia.30 Another head-to-head trial reported the benefit of the diphenhydramine vs midazolam.31 Both agents were efficacious for treating akathisia; however, midazolam had a more rapid onset. Despite these positive reports, double-blind trials have found diphenhydramine to be ineffective,17 which suggests propranolol should be the first-line agent, assuming it is not contraindicated.
Benzodiazepines have also been found to be efficacious for treating akathisia. A 1999 Cochrane Review included 2 randomized controlled trials that assessed the efficacy of clonazepam vs placebo for treating akathisia.32 It found evidence of benefit for clonazepam, but questioned the generalizability of these studies.32 This review did not include several other reports that suggest benefits of other benzodiazepines for treating akathisia. Other than clonazepam, reports suggest benefit for diazepam, lorazepam, and midazolam for treating akathisia.17 Despite this evidence and the findings from this Cochrane Review, the literature does not appear to point to clear dominance of these agents over propranolol. Given the safety concerns when prescribing benzodiazepines, it would be prudent to utilize propranolol as a first-line agent for treating akathisia.
Finally, other reports have cited treatment efficacy linked to serotonin 2A receptor (5-HT2A) antagonists (mianserin, mirtazapine, and trazodone), clonidine, gabapentin, amantadine, and other agents.17 If treatment with propranolol is ineffective or contraindicated, clinicians should utilize their clinical judgement in deciding on the use of one agent over another.
OUTCOME Complete resolution
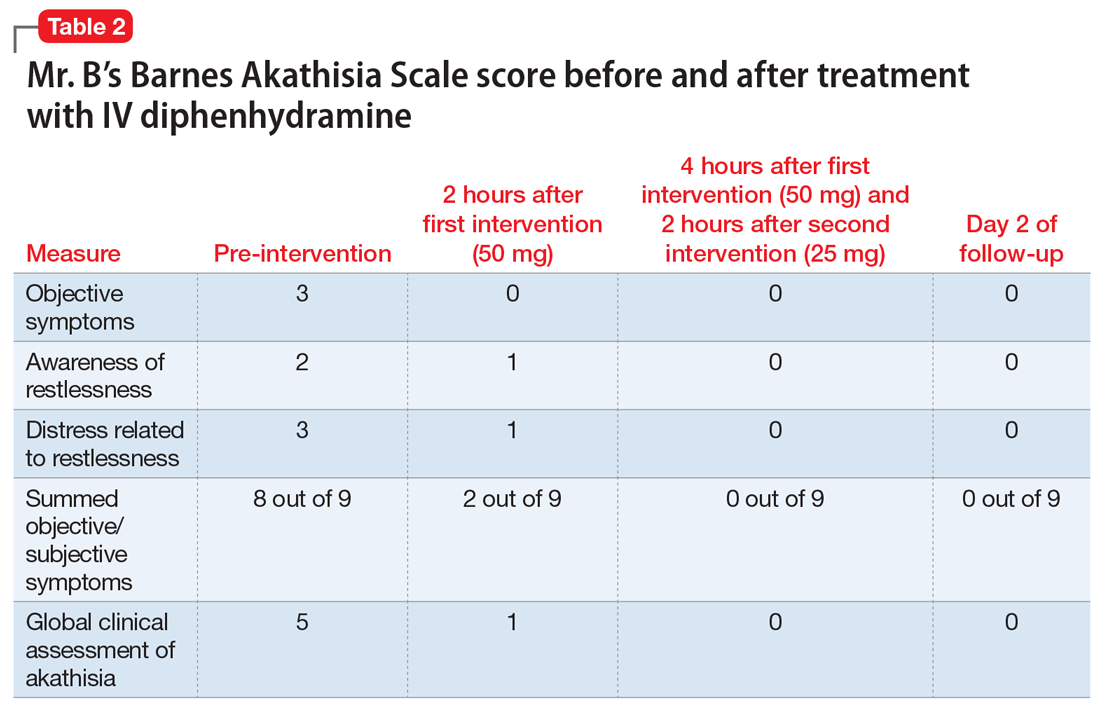
Haloperidol is discontinued and diphenhydramine, 50 mg IV, is administered. (Diphenhydramine was used instead of propranolol due to immediacy of availability.) Most of Mr. B’s signs and symptoms resolve on a repeat interview 3 hours later. He receives another dose of diphenhydramine, 25 mg IV, for persistent mild irritability. By Day 2 of follow-up, his symptoms completely resolve as measured on the Barnes Akathisia Scale33 (Table 2).
Continue to: Bottom Line
Bottom Line
Akathisia is an elusive adverse effect of antipsychotics and can be misdiagnosed as anxiety. Close consideration should be given to potential medical, psychiatric, and drug-related etiologies in patients who have a prolonged medical course, comorbidities, and exposure to multiple pharmacologic agents.
Related Resources
- Factor SA, Leffler JB, Murray CF. Drug-induced movement disorders: a clinical review. Medscape. http://www.medscape.org/viewarticle/586881.
- Marder S, Stroup TS. Pharmacotherapy for schizophrenia: side effect management. UpToDate. https://www.uptodate.com/contents/pharmacotherapy-for-schizophrenia-side-effect-management.
Drug Brand Names
Amantadine • Symmetrel
Benztropine • Cogentin
Clonazepam • Klonopin
Clonidine • Catapres
Dexamethasone • Decadron
Diazepam • Valium
Diphenhydramine • Benadryl
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Metoclopramide • Reglan
Mianserin • Tolvon
Midazolam • Versed
Mirtazapine • Remeron
Olanzapine • Zyprexa
Propranolol • Inderal
Rivastigmine • Exelon
Trazodone • Oleptro
1. Cavallazzi R, Saad M, Marik PE. Delirium in the ICU: an overview. Ann Intensive Care. 2012;2:49.
2. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. National Alliance on Mental Illness. Mental health by the numbers. https://www.nami.org/learn-more/mental-health-by-the-numbers. Accessed March 4, 2018.
5. Jacka MJ, Mitchell N, Perez-Parada J. Incidence and prevalence of anxiety, depression, and post-traumatic stress disorder among critical care patients, families, and practitioners. J Anest & Inten Care Med. 2016;1(1):55555. doi: 10.19080/JAICM.2016.01.555555.
6. Reis AM, Cassiani SH. Adverse drug events in an intensive care unit of a university hospital. Eur J Clin Pharmacol. 2011;67(6):625-632.
7. Garpestad E, Devlin JW. Polypharmacy and delirium in critically ill older adults: recognition and prevention. Clin Geriatr Med. 2017;33(2):189-203.
8. Caroff SN, Hurford I, Lybrand J, et al. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127-148.
9. Van Putten T, Marder SR. Toward a more reliable diagnosis of akathisia. Arch Gen Psychiatry. 1986;43(10):1015-1016.
10. Penders TM, Agarwal S, Rohaidy R. Persistent akathisia masquerading as agitated depression after use of ziprasidone in the treatment of bipolar depression. Neuropsychiatr Dis Treat. 2013;9:463-465.
11. Naganuma H, Fujii I. Incidence and risk factors in neuroleptic malignant syndrome. Acta Psychiatr Scand. 1994;90(6):424-426.
12. Forcen FE, Matsoukas K, Alici Y. Antipsychotic-induced akathisia in delirium: a systematic review. Palliat Support Care. 2016;14(1):77-84.
13. Brune M, Sachdev, PS. Ladislav Haskovec and 100 years of akathisia. American Journal of Psychiatry. 2002;159(5):727-727.
14. Havaki-Kontaxaki BJ, Kontaxakis VP, Christodoulou GN. Prevalence and characteristics of patients with pseudoakathisia. Eur Neuropsychopharmacol. 2000;10(5):333-336.
15. Lang AE. Withdrawal akathisia: case reports and a proposed classification of chronic akathisia. Mov Disord. 1994;9(2):188-192.
16. Sachdev P. The epidemiology of drug-induced akathisia: Part II. Chronic, tardive, and withdrawal akathisias. Schizophr Bull. 1995;21(3):451-461.
17. Kern DS, Lang AE. Acute akathisia. In: Friedman JH, ed. Medication-induced movement disorders. Cambridge, United Kingdom: Cambridge University Press; 2015:12-24.
18. Adler LA, Angrist B, Reiter S, et al. Neuroleptic-induced akathisia: a review. Psychopharmacology (Berl). 1989;97(1):1-11.
19. Stahl SM, Loonen AJM. The mechanism of drug-induced akathisia. CNS Spectr. 2011;16(1):7-10.
20. Sachdev P, Kruk J. Clinical characteristics and predisposing factors in acute drug-induced akathisia. Arch Gen Psychiatry. 1994;51(12):963-974.
21. Laoutidis ZG, Luckhaus C. 5-HT2A receptor antagonists for the treatment of neuroleptic-induced akathisia: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17(5):823-832.
22. Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(4):CD001946.
23. Sachdev P, Loneragan C. Intravenous benztropine and propranolol challenges in acute neuroleptic-induced akathisia. Clin Neuropharmacol. 1993;16(4):324-331.
24. Lima AR, Weiser KV, Bacaltchuk J, et al. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(1):CD003727.
25. Fleischhacker WW, Roth SD, Kane JM. The pharmacologic treatment of neuroleptic-induced akathisia. J Clin Psychopharmacol. 1990;10(1):12-21.
26. Blaisdell GD. Akathisia: a comprehensive review and treatment summary. Pharmacopsychiatry. 1994;27(4):139-146.
27. Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry. 2010;196(2):89-91.
28. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
29. Adler LA, Peselow E, Rosenthal M, et al. A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis. Psychopharmacol Bull. 1993;29(2):283-286.
30. Bender B, Friedman B, Davitt M, et al. 118: metoclopramide in the emergency department: a randomized factorial design study to determine the influence of dose and diphenhydramine on akathisia. Ann of Emerg Med. 2008;52(4):S78.
31. Parlak I, Erdur B, Parlak M, et al. Midazolam vs. diphenhydramine for the treatment of metoclopramide-induced akathisia: a randomized controlled trial. Acad Emerg Med. 2007;14(8):715-721.
32. Lima AR, Soares-Weiser K, Bacaltchuk J, et al. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 1999;(4):CD001950.
33. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676..
CASE Anxiety in the ICU
Mr. B, age 42, an African American man, is admitted to the inpatient medical unit for surgical treatment of peritoneal carcinomatosis with pelvic exenteration. He has a history of metastatic rectal cancer, chronic pain, and hypertension, but no psychiatric history. Mr. B’s postsurgical hospital stay is complicated by treatment-resistant tachycardia and hypertension, and he requires a lengthy stay in the ICU. In the ICU, Mr. B reports having visual hallucinations where he sees an individual placing a drug in his IV line. Additionally, he reports severe anxiety related to this experience. His anxiety and visual hallucinations are treated with coadministration of IV lorazepam, diphenhydramine, and haloperidol. These medications resolve the hallucinations, but his anxiety worsens and he becomes restless. He receives additional doses of IV haloperidol administered in 5 mg increments and reaching a cumulative 12-hour dose of 50 mg. Mr. B continues to report anxiety, so the psychiatry consultation-liaison (C-L) service is called.
[polldaddy:9970907]
The authors’ observations
Determining the cause of Mr. B’s anxiety is challenging because of his prolonged medical course, com
From a medical perspective, in a post-surgical patient treated in the ICU, the consulting practitioner must pay particular attention to delirium. ICU delirium is common—one report indicated that it occurs in 32.3% of ICU patients1—and frequently confused with psychiatric morbidity.2 Identifying delirium as the cause of impairment is important because delirium has potentially modifiable underlying etiologies. Symptomatically, delirium presents as impairment and fluctuation in attention, awareness, and at least one other cognitive domain, with a clear indication that the impairment occurred over a short period of time and represents a departure from baseline.3 In Mr. B’s case, these symptoms have not been excluded and should be considered by the C-L psychiatrists.
In addition to delirium, the C-L team must consider psychiatric comorbidity. Mr. B has no psychiatric history and a sudden first occurrence of hallucinations; therefore, it is unlikely that he has developed a primary psychotic disorder. Because he reported his symptoms had been present only for several days, he would not meet criteria for schizophrenia, which according to DSM-5 criteria require at least 1 month of ≥2 symptoms (including delusions, hallucinations, disorganized speech, disorganized behavior, or negative symptoms) and 6 months of declining function.3 However, although it is improbable, the C-L team must consider a primary psychotic illness, particularly given the potential devastating consequence of being misdiagnosed and mismanaged for an alternative illness. Unlike psychotic disorders, anxiety disorders are significantly more prevalent in the U.S. general population than primary psychotic disorders.4 Furthermore, the prevalence of anxiety disorders increases in the ICU setting; one study found that up to 61% of ICU patients setting experience “anxiety features.”5 Therefore, anxiety disorders and stress disorders should be considered in ICU patients who exhibit psychiatric symptoms.
Clinicians also should consider medication-induced adverse effects. In the ICU, patients are frequently managed on multiple medications, which increase their risk of developing adverse effects and adverse reactions.6 One potential consequence of polypharmacy is delirium, which remains a relevant potential diagnosis for Mr. B.7 Alternative consequences vary by medication and their respective pharmacodynamics. We take into consideration Mr. B’s exposure to high doses of the high-potency antipsychotic agent, haloperidol. Exposure to haloperidol can result in extrapyramidal symptoms, including akathisia,8,9,10 and the rare, but potentially fatal, NMS.11 These reactions can often be distinguished by taking a thorough history and a physical evaluation. In the case of akathisia, the clinician should look for medication exposure, titration, or taper. Most commonly, akathisia occurs secondary to antipsychotic exposure,12 followed by the onset of a combination of subjective symptoms, such as restlessness, anxiety, and irritability, and an objective symptom of increased motor activity.3 NMS, on the other hand, is distinguished by symptoms that include hyperthermia (>38ºC), diaphoresis, severe rigidity, urinary incontinence, vital instability, alterations in mental health status, and elevations in creatine kinase greater than 4-fold the upper limit, usually in the setting of treatment with antipsychotics.3 Nearly all cases of NMS occur within the first 30 days of antipsychotic exposure.3 While, overtly, NMS may appear to be less subtle than akathisia, clinicians should still be weary to rule out this admittedly rare, though potentially lethal diagnosis, especially in an ICU patient, where the diagnosis can be muddied by medical comorbidities that may mask the syndrome.
Continue to: EVALUATION Focus on akathisia
EVALUATION Focus on akathisia
On interview by the C-L team, Mr. B is visibly restless, moving all 4 extremities. He reports increased anxiety and irritability over the past 2 to 3 days. Mr. B states that he is aware of his increased motor movements and can briefly suppress them. However, after several seconds, he again begins spontaneously fidgeting, moving all 4 extremities and shifting from side to side in bed, saying, “I just feel anxious.” He denies having visual hallucinations, and says that the previous hallucinations had spontaneously presented and remitted after surgery. He denies the use of psychotropics for mental illness, prior similar symptoms to this presentation, a family history of mental illness, recent illicit substance use, or excessive alcohol use prior to presentation. This history is corroborated by collateral information from his brother, who was present in the ICU. On physical examination, Mr. B is afebrile and his vital signs are within normal limits. He does not have muscular rigidity or neck dystonia. His laboratory values, including complete blood count, electrolytes, liver function tests, and creatine phosphokinase, are within normal limits.
His medication administration record includes 46 standing agents, 16 “as-needed” agents, and 8 infusions. Several of the standing agents had psychotropic properties; however, the most salient were several opioids, ketamine, midazolam, lorazepam, dexamethasone, haloperidol, and olanzapine.
[polldaddy:9970908]
The authors’ observations
We determined that the most likely diagnosis for Mr. B’s symptoms was medication-induced akathisia secondary to haloperidol. Akathisia, coined by Haskovec in 1901,12,13 is from Greek, meaning an “inability to sit.”12 DSM-5 describes 2 forms of akathisia: medication-induced acute akathisia, and tardive akathisia.3 In the literature, others have described additional classifications, including chronic akathisia, withdrawal akathisia, and pseudoakathisia (Table 13,14-17). In Mr. B’s case, given his sudden development of symptoms and their direct chronologic relationship to antipsychotic treatment, and his combined subjective and objective symptoms, we believed that Mr. B’s symptoms were consistent with medication-induced acute akathisia (MIA). The identification and treatment of this clinical entity is important for several reasons, including reducing patient morbidity and maximizing patient comfort. Additionally, because akathisia has been associated with poor medication adherence, increased agitation/aggression, increased suicidality, and the eventual development of tardive dyskinesia,18 it is a relevant prognostic consideration when deciding to treat a patient with antipsychotics.

Pathophysiologically, we have yet to fully shed light on the exact underpinnings of akathisia. Much of our present knowledge stems from patient response to pharmacologic agents. While dopamine blockade has been linked to akathisia, the exact mechanism is not completely understood. Previous theories linking nigrostriatal pathways have been expanded to include mesocortical and mesolimbic considerations.12,17,18 Similarly surmised from medication effects, the transmitters y-aminobutyric acid, serotonin/5-hydroxytryptamine (5-HT), norepinephrine, and acetylcholine also have been linked to this syndrome, though as of yet, exact gross pathophysiologic mechanisms have not been fully elucidated.12 More recently, Stahl and Loonen19 described a novel mechanism by which they link the shell of the nucleus accumbens to akathisia. In their report, they indicate that the potential reduction in dopaminergic activity, secondary to antipsychotic administration, can result in compensatory noradrenergic activation of the locus coeruleus.19 The increased noradrenergic activity results in the downstream activation of the shell of the nucleus accumbens.19 The activation of the nucleus accumbens shell, which has been linked to unconditioned feeding and fear behavior, can then result in a cascade of effects that would phenotypically present as the syndrome we recognize to be akathisia.19
Numerous etiologies have been linked to MIA. Of these, high-potency antipsychotics are believed to remain the greatest risk factor for akathisia,18 although atypical antipsychotics, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors, have been linked to the disorder.18,19
Continue to: Regarding antipsychotics...
Regarding antipsychotics, risk factors for akathisia include drug potency, dose, and rapidity of titration.20 All of these factors were relevant in our patient’s case. Risk across antipsychotic classes is not well understood; few head-to-head studies have comparing antipsychotics. However, general estimates suggest a 15% to 40% prevalence in patients exposed to typical antipsychotics, as compared with 0% to 12% exposed to atypical antipsychotics.8 The literature-reported difference in risk, as well as our patient’s comparative difference in exposure to large doses of haloperidol (50 mg) as compared with 1 dose of olanzapine (5 mg), led us to believe his akathisia developed primarily due to his exposure to haloperidol. Conclusively linking his symptoms to haloperidol alone, however, is not possible, and we did consider that olanzapine may in fact have had some effect in worsening Mr. B’s akathisia.
[polldaddy:9970909]
The authors’ observations
While there are reports on the efficacy of various agents in the treatment of akathisia, the most commonly evaluated agents are propranolol, anticholinergics, and benzodiazepines.17, 21
Propranolol is a nonselective beta-adrenergic blocker with numerous indications.17 Despite a 2004 Cochrane Review indicating that there is no evidence in support of central acting beta-blockers for treating akathisia,22 propranolol is not yet recognized as an appropriate treatment.17 The reason for this discrepancy is likely due to the Cochrane Review’s restrictive inclusion criteria, which prevented the analysis of much of the literature.22 In fact, several reports cite evidence for the treatment efficacy of propranolol17 and, to date, some reports continue to advocate for its use as a first-line agent in the treatment of akathisia. Admittedly, besides the Cochrane Review,22 other reports have found propranolol to be ineffective for treating akathisia,23 although these tend to be limited by their population size and generalizability.
As with propranolol, a 2006 Cochrane Review found “no reliable evidence to support or refute” using anticholinergic agents in the treatment of akathisia.24 We suspect that the review’s findings were likely secondary to its strict inclusion criteria.24 In fact, several reports support using anticholinergic agents for treating akathisia.25 Here we focus on benztropine and diphenhydramine.
Two reviews—Blaisdell26 (1994) and Poyurovsky27 (2010)—suggest modest benefits from benztropine, primarily in patients with comorbid Parkinson’s disease. Despite these benefits, head-to-head trials seem to either point to the superiority of propranolol or to no difference between these agents for treating akathisia.28,29 In a review, we only found 1 trial demonstrating benztropine’s superiority over propranolol,23 but this trial was constrained by its small population (6 patients). Therefore, the data suggest that, when indicated, clinicians should lean towards using propranolol for treating akathisia.
Continue to: Diphenhydramine, a first-generation antihistamine...
Diphenhydramine, a first-generation antihistamine with antimuscarinic properties, has been studied for its efficacy in treating metoclopramide-induced akathisia in the emergency setting.30 There are several reports on the efficacy of this agent, including a large randomized study involving 281 patients that found it effective for preventing metoclopramide-induced akathisia.30 Another head-to-head trial reported the benefit of the diphenhydramine vs midazolam.31 Both agents were efficacious for treating akathisia; however, midazolam had a more rapid onset. Despite these positive reports, double-blind trials have found diphenhydramine to be ineffective,17 which suggests propranolol should be the first-line agent, assuming it is not contraindicated.
Benzodiazepines have also been found to be efficacious for treating akathisia. A 1999 Cochrane Review included 2 randomized controlled trials that assessed the efficacy of clonazepam vs placebo for treating akathisia.32 It found evidence of benefit for clonazepam, but questioned the generalizability of these studies.32 This review did not include several other reports that suggest benefits of other benzodiazepines for treating akathisia. Other than clonazepam, reports suggest benefit for diazepam, lorazepam, and midazolam for treating akathisia.17 Despite this evidence and the findings from this Cochrane Review, the literature does not appear to point to clear dominance of these agents over propranolol. Given the safety concerns when prescribing benzodiazepines, it would be prudent to utilize propranolol as a first-line agent for treating akathisia.
Finally, other reports have cited treatment efficacy linked to serotonin 2A receptor (5-HT2A) antagonists (mianserin, mirtazapine, and trazodone), clonidine, gabapentin, amantadine, and other agents.17 If treatment with propranolol is ineffective or contraindicated, clinicians should utilize their clinical judgement in deciding on the use of one agent over another.
OUTCOME Complete resolution
Haloperidol is discontinued and diphenhydramine, 50 mg IV, is administered. (Diphenhydramine was used instead of propranolol due to immediacy of availability.) Most of Mr. B’s signs and symptoms resolve on a repeat interview 3 hours later. He receives another dose of diphenhydramine, 25 mg IV, for persistent mild irritability. By Day 2 of follow-up, his symptoms completely resolve as measured on the Barnes Akathisia Scale33 (Table 2).

Continue to: Bottom Line
Bottom Line
Akathisia is an elusive adverse effect of antipsychotics and can be misdiagnosed as anxiety. Close consideration should be given to potential medical, psychiatric, and drug-related etiologies in patients who have a prolonged medical course, comorbidities, and exposure to multiple pharmacologic agents.
Related Resources
- Factor SA, Leffler JB, Murray CF. Drug-induced movement disorders: a clinical review. Medscape. http://www.medscape.org/viewarticle/586881.
- Marder S, Stroup TS. Pharmacotherapy for schizophrenia: side effect management. UpToDate. https://www.uptodate.com/contents/pharmacotherapy-for-schizophrenia-side-effect-management.
Drug Brand Names
Amantadine • Symmetrel
Benztropine • Cogentin
Clonazepam • Klonopin
Clonidine • Catapres
Dexamethasone • Decadron
Diazepam • Valium
Diphenhydramine • Benadryl
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Metoclopramide • Reglan
Mianserin • Tolvon
Midazolam • Versed
Mirtazapine • Remeron
Olanzapine • Zyprexa
Propranolol • Inderal
Rivastigmine • Exelon
Trazodone • Oleptro
CASE Anxiety in the ICU
Mr. B, age 42, an African American man, is admitted to the inpatient medical unit for surgical treatment of peritoneal carcinomatosis with pelvic exenteration. He has a history of metastatic rectal cancer, chronic pain, and hypertension, but no psychiatric history. Mr. B’s postsurgical hospital stay is complicated by treatment-resistant tachycardia and hypertension, and he requires a lengthy stay in the ICU. In the ICU, Mr. B reports having visual hallucinations where he sees an individual placing a drug in his IV line. Additionally, he reports severe anxiety related to this experience. His anxiety and visual hallucinations are treated with coadministration of IV lorazepam, diphenhydramine, and haloperidol. These medications resolve the hallucinations, but his anxiety worsens and he becomes restless. He receives additional doses of IV haloperidol administered in 5 mg increments and reaching a cumulative 12-hour dose of 50 mg. Mr. B continues to report anxiety, so the psychiatry consultation-liaison (C-L) service is called.
[polldaddy:9970907]
The authors’ observations
Determining the cause of Mr. B’s anxiety is challenging because of his prolonged medical course, com
From a medical perspective, in a post-surgical patient treated in the ICU, the consulting practitioner must pay particular attention to delirium. ICU delirium is common—one report indicated that it occurs in 32.3% of ICU patients1—and frequently confused with psychiatric morbidity.2 Identifying delirium as the cause of impairment is important because delirium has potentially modifiable underlying etiologies. Symptomatically, delirium presents as impairment and fluctuation in attention, awareness, and at least one other cognitive domain, with a clear indication that the impairment occurred over a short period of time and represents a departure from baseline.3 In Mr. B’s case, these symptoms have not been excluded and should be considered by the C-L psychiatrists.
In addition to delirium, the C-L team must consider psychiatric comorbidity. Mr. B has no psychiatric history and a sudden first occurrence of hallucinations; therefore, it is unlikely that he has developed a primary psychotic disorder. Because he reported his symptoms had been present only for several days, he would not meet criteria for schizophrenia, which according to DSM-5 criteria require at least 1 month of ≥2 symptoms (including delusions, hallucinations, disorganized speech, disorganized behavior, or negative symptoms) and 6 months of declining function.3 However, although it is improbable, the C-L team must consider a primary psychotic illness, particularly given the potential devastating consequence of being misdiagnosed and mismanaged for an alternative illness. Unlike psychotic disorders, anxiety disorders are significantly more prevalent in the U.S. general population than primary psychotic disorders.4 Furthermore, the prevalence of anxiety disorders increases in the ICU setting; one study found that up to 61% of ICU patients setting experience “anxiety features.”5 Therefore, anxiety disorders and stress disorders should be considered in ICU patients who exhibit psychiatric symptoms.
Clinicians also should consider medication-induced adverse effects. In the ICU, patients are frequently managed on multiple medications, which increase their risk of developing adverse effects and adverse reactions.6 One potential consequence of polypharmacy is delirium, which remains a relevant potential diagnosis for Mr. B.7 Alternative consequences vary by medication and their respective pharmacodynamics. We take into consideration Mr. B’s exposure to high doses of the high-potency antipsychotic agent, haloperidol. Exposure to haloperidol can result in extrapyramidal symptoms, including akathisia,8,9,10 and the rare, but potentially fatal, NMS.11 These reactions can often be distinguished by taking a thorough history and a physical evaluation. In the case of akathisia, the clinician should look for medication exposure, titration, or taper. Most commonly, akathisia occurs secondary to antipsychotic exposure,12 followed by the onset of a combination of subjective symptoms, such as restlessness, anxiety, and irritability, and an objective symptom of increased motor activity.3 NMS, on the other hand, is distinguished by symptoms that include hyperthermia (>38ºC), diaphoresis, severe rigidity, urinary incontinence, vital instability, alterations in mental health status, and elevations in creatine kinase greater than 4-fold the upper limit, usually in the setting of treatment with antipsychotics.3 Nearly all cases of NMS occur within the first 30 days of antipsychotic exposure.3 While, overtly, NMS may appear to be less subtle than akathisia, clinicians should still be weary to rule out this admittedly rare, though potentially lethal diagnosis, especially in an ICU patient, where the diagnosis can be muddied by medical comorbidities that may mask the syndrome.
Continue to: EVALUATION Focus on akathisia
EVALUATION Focus on akathisia
On interview by the C-L team, Mr. B is visibly restless, moving all 4 extremities. He reports increased anxiety and irritability over the past 2 to 3 days. Mr. B states that he is aware of his increased motor movements and can briefly suppress them. However, after several seconds, he again begins spontaneously fidgeting, moving all 4 extremities and shifting from side to side in bed, saying, “I just feel anxious.” He denies having visual hallucinations, and says that the previous hallucinations had spontaneously presented and remitted after surgery. He denies the use of psychotropics for mental illness, prior similar symptoms to this presentation, a family history of mental illness, recent illicit substance use, or excessive alcohol use prior to presentation. This history is corroborated by collateral information from his brother, who was present in the ICU. On physical examination, Mr. B is afebrile and his vital signs are within normal limits. He does not have muscular rigidity or neck dystonia. His laboratory values, including complete blood count, electrolytes, liver function tests, and creatine phosphokinase, are within normal limits.
His medication administration record includes 46 standing agents, 16 “as-needed” agents, and 8 infusions. Several of the standing agents had psychotropic properties; however, the most salient were several opioids, ketamine, midazolam, lorazepam, dexamethasone, haloperidol, and olanzapine.
[polldaddy:9970908]
The authors’ observations
We determined that the most likely diagnosis for Mr. B’s symptoms was medication-induced akathisia secondary to haloperidol. Akathisia, coined by Haskovec in 1901,12,13 is from Greek, meaning an “inability to sit.”12 DSM-5 describes 2 forms of akathisia: medication-induced acute akathisia, and tardive akathisia.3 In the literature, others have described additional classifications, including chronic akathisia, withdrawal akathisia, and pseudoakathisia (Table 13,14-17). In Mr. B’s case, given his sudden development of symptoms and their direct chronologic relationship to antipsychotic treatment, and his combined subjective and objective symptoms, we believed that Mr. B’s symptoms were consistent with medication-induced acute akathisia (MIA). The identification and treatment of this clinical entity is important for several reasons, including reducing patient morbidity and maximizing patient comfort. Additionally, because akathisia has been associated with poor medication adherence, increased agitation/aggression, increased suicidality, and the eventual development of tardive dyskinesia,18 it is a relevant prognostic consideration when deciding to treat a patient with antipsychotics.

Pathophysiologically, we have yet to fully shed light on the exact underpinnings of akathisia. Much of our present knowledge stems from patient response to pharmacologic agents. While dopamine blockade has been linked to akathisia, the exact mechanism is not completely understood. Previous theories linking nigrostriatal pathways have been expanded to include mesocortical and mesolimbic considerations.12,17,18 Similarly surmised from medication effects, the transmitters y-aminobutyric acid, serotonin/5-hydroxytryptamine (5-HT), norepinephrine, and acetylcholine also have been linked to this syndrome, though as of yet, exact gross pathophysiologic mechanisms have not been fully elucidated.12 More recently, Stahl and Loonen19 described a novel mechanism by which they link the shell of the nucleus accumbens to akathisia. In their report, they indicate that the potential reduction in dopaminergic activity, secondary to antipsychotic administration, can result in compensatory noradrenergic activation of the locus coeruleus.19 The increased noradrenergic activity results in the downstream activation of the shell of the nucleus accumbens.19 The activation of the nucleus accumbens shell, which has been linked to unconditioned feeding and fear behavior, can then result in a cascade of effects that would phenotypically present as the syndrome we recognize to be akathisia.19
Numerous etiologies have been linked to MIA. Of these, high-potency antipsychotics are believed to remain the greatest risk factor for akathisia,18 although atypical antipsychotics, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors, have been linked to the disorder.18,19
Continue to: Regarding antipsychotics...
Regarding antipsychotics, risk factors for akathisia include drug potency, dose, and rapidity of titration.20 All of these factors were relevant in our patient’s case. Risk across antipsychotic classes is not well understood; few head-to-head studies have comparing antipsychotics. However, general estimates suggest a 15% to 40% prevalence in patients exposed to typical antipsychotics, as compared with 0% to 12% exposed to atypical antipsychotics.8 The literature-reported difference in risk, as well as our patient’s comparative difference in exposure to large doses of haloperidol (50 mg) as compared with 1 dose of olanzapine (5 mg), led us to believe his akathisia developed primarily due to his exposure to haloperidol. Conclusively linking his symptoms to haloperidol alone, however, is not possible, and we did consider that olanzapine may in fact have had some effect in worsening Mr. B’s akathisia.
[polldaddy:9970909]
The authors’ observations
While there are reports on the efficacy of various agents in the treatment of akathisia, the most commonly evaluated agents are propranolol, anticholinergics, and benzodiazepines.17, 21
Propranolol is a nonselective beta-adrenergic blocker with numerous indications.17 Despite a 2004 Cochrane Review indicating that there is no evidence in support of central acting beta-blockers for treating akathisia,22 propranolol is not yet recognized as an appropriate treatment.17 The reason for this discrepancy is likely due to the Cochrane Review’s restrictive inclusion criteria, which prevented the analysis of much of the literature.22 In fact, several reports cite evidence for the treatment efficacy of propranolol17 and, to date, some reports continue to advocate for its use as a first-line agent in the treatment of akathisia. Admittedly, besides the Cochrane Review,22 other reports have found propranolol to be ineffective for treating akathisia,23 although these tend to be limited by their population size and generalizability.
As with propranolol, a 2006 Cochrane Review found “no reliable evidence to support or refute” using anticholinergic agents in the treatment of akathisia.24 We suspect that the review’s findings were likely secondary to its strict inclusion criteria.24 In fact, several reports support using anticholinergic agents for treating akathisia.25 Here we focus on benztropine and diphenhydramine.
Two reviews—Blaisdell26 (1994) and Poyurovsky27 (2010)—suggest modest benefits from benztropine, primarily in patients with comorbid Parkinson’s disease. Despite these benefits, head-to-head trials seem to either point to the superiority of propranolol or to no difference between these agents for treating akathisia.28,29 In a review, we only found 1 trial demonstrating benztropine’s superiority over propranolol,23 but this trial was constrained by its small population (6 patients). Therefore, the data suggest that, when indicated, clinicians should lean towards using propranolol for treating akathisia.
Continue to: Diphenhydramine, a first-generation antihistamine...
Diphenhydramine, a first-generation antihistamine with antimuscarinic properties, has been studied for its efficacy in treating metoclopramide-induced akathisia in the emergency setting.30 There are several reports on the efficacy of this agent, including a large randomized study involving 281 patients that found it effective for preventing metoclopramide-induced akathisia.30 Another head-to-head trial reported the benefit of the diphenhydramine vs midazolam.31 Both agents were efficacious for treating akathisia; however, midazolam had a more rapid onset. Despite these positive reports, double-blind trials have found diphenhydramine to be ineffective,17 which suggests propranolol should be the first-line agent, assuming it is not contraindicated.
Benzodiazepines have also been found to be efficacious for treating akathisia. A 1999 Cochrane Review included 2 randomized controlled trials that assessed the efficacy of clonazepam vs placebo for treating akathisia.32 It found evidence of benefit for clonazepam, but questioned the generalizability of these studies.32 This review did not include several other reports that suggest benefits of other benzodiazepines for treating akathisia. Other than clonazepam, reports suggest benefit for diazepam, lorazepam, and midazolam for treating akathisia.17 Despite this evidence and the findings from this Cochrane Review, the literature does not appear to point to clear dominance of these agents over propranolol. Given the safety concerns when prescribing benzodiazepines, it would be prudent to utilize propranolol as a first-line agent for treating akathisia.
Finally, other reports have cited treatment efficacy linked to serotonin 2A receptor (5-HT2A) antagonists (mianserin, mirtazapine, and trazodone), clonidine, gabapentin, amantadine, and other agents.17 If treatment with propranolol is ineffective or contraindicated, clinicians should utilize their clinical judgement in deciding on the use of one agent over another.
OUTCOME Complete resolution
Haloperidol is discontinued and diphenhydramine, 50 mg IV, is administered. (Diphenhydramine was used instead of propranolol due to immediacy of availability.) Most of Mr. B’s signs and symptoms resolve on a repeat interview 3 hours later. He receives another dose of diphenhydramine, 25 mg IV, for persistent mild irritability. By Day 2 of follow-up, his symptoms completely resolve as measured on the Barnes Akathisia Scale33 (Table 2).

Continue to: Bottom Line
Bottom Line
Akathisia is an elusive adverse effect of antipsychotics and can be misdiagnosed as anxiety. Close consideration should be given to potential medical, psychiatric, and drug-related etiologies in patients who have a prolonged medical course, comorbidities, and exposure to multiple pharmacologic agents.
Related Resources
- Factor SA, Leffler JB, Murray CF. Drug-induced movement disorders: a clinical review. Medscape. http://www.medscape.org/viewarticle/586881.
- Marder S, Stroup TS. Pharmacotherapy for schizophrenia: side effect management. UpToDate. https://www.uptodate.com/contents/pharmacotherapy-for-schizophrenia-side-effect-management.
Drug Brand Names
Amantadine • Symmetrel
Benztropine • Cogentin
Clonazepam • Klonopin
Clonidine • Catapres
Dexamethasone • Decadron
Diazepam • Valium
Diphenhydramine • Benadryl
Gabapentin • Neurontin
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Metoclopramide • Reglan
Mianserin • Tolvon
Midazolam • Versed
Mirtazapine • Remeron
Olanzapine • Zyprexa
Propranolol • Inderal
Rivastigmine • Exelon
Trazodone • Oleptro
1. Cavallazzi R, Saad M, Marik PE. Delirium in the ICU: an overview. Ann Intensive Care. 2012;2:49.
2. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. National Alliance on Mental Illness. Mental health by the numbers. https://www.nami.org/learn-more/mental-health-by-the-numbers. Accessed March 4, 2018.
5. Jacka MJ, Mitchell N, Perez-Parada J. Incidence and prevalence of anxiety, depression, and post-traumatic stress disorder among critical care patients, families, and practitioners. J Anest & Inten Care Med. 2016;1(1):55555. doi: 10.19080/JAICM.2016.01.555555.
6. Reis AM, Cassiani SH. Adverse drug events in an intensive care unit of a university hospital. Eur J Clin Pharmacol. 2011;67(6):625-632.
7. Garpestad E, Devlin JW. Polypharmacy and delirium in critically ill older adults: recognition and prevention. Clin Geriatr Med. 2017;33(2):189-203.
8. Caroff SN, Hurford I, Lybrand J, et al. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127-148.
9. Van Putten T, Marder SR. Toward a more reliable diagnosis of akathisia. Arch Gen Psychiatry. 1986;43(10):1015-1016.
10. Penders TM, Agarwal S, Rohaidy R. Persistent akathisia masquerading as agitated depression after use of ziprasidone in the treatment of bipolar depression. Neuropsychiatr Dis Treat. 2013;9:463-465.
11. Naganuma H, Fujii I. Incidence and risk factors in neuroleptic malignant syndrome. Acta Psychiatr Scand. 1994;90(6):424-426.
12. Forcen FE, Matsoukas K, Alici Y. Antipsychotic-induced akathisia in delirium: a systematic review. Palliat Support Care. 2016;14(1):77-84.
13. Brune M, Sachdev, PS. Ladislav Haskovec and 100 years of akathisia. American Journal of Psychiatry. 2002;159(5):727-727.
14. Havaki-Kontaxaki BJ, Kontaxakis VP, Christodoulou GN. Prevalence and characteristics of patients with pseudoakathisia. Eur Neuropsychopharmacol. 2000;10(5):333-336.
15. Lang AE. Withdrawal akathisia: case reports and a proposed classification of chronic akathisia. Mov Disord. 1994;9(2):188-192.
16. Sachdev P. The epidemiology of drug-induced akathisia: Part II. Chronic, tardive, and withdrawal akathisias. Schizophr Bull. 1995;21(3):451-461.
17. Kern DS, Lang AE. Acute akathisia. In: Friedman JH, ed. Medication-induced movement disorders. Cambridge, United Kingdom: Cambridge University Press; 2015:12-24.
18. Adler LA, Angrist B, Reiter S, et al. Neuroleptic-induced akathisia: a review. Psychopharmacology (Berl). 1989;97(1):1-11.
19. Stahl SM, Loonen AJM. The mechanism of drug-induced akathisia. CNS Spectr. 2011;16(1):7-10.
20. Sachdev P, Kruk J. Clinical characteristics and predisposing factors in acute drug-induced akathisia. Arch Gen Psychiatry. 1994;51(12):963-974.
21. Laoutidis ZG, Luckhaus C. 5-HT2A receptor antagonists for the treatment of neuroleptic-induced akathisia: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17(5):823-832.
22. Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(4):CD001946.
23. Sachdev P, Loneragan C. Intravenous benztropine and propranolol challenges in acute neuroleptic-induced akathisia. Clin Neuropharmacol. 1993;16(4):324-331.
24. Lima AR, Weiser KV, Bacaltchuk J, et al. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(1):CD003727.
25. Fleischhacker WW, Roth SD, Kane JM. The pharmacologic treatment of neuroleptic-induced akathisia. J Clin Psychopharmacol. 1990;10(1):12-21.
26. Blaisdell GD. Akathisia: a comprehensive review and treatment summary. Pharmacopsychiatry. 1994;27(4):139-146.
27. Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry. 2010;196(2):89-91.
28. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
29. Adler LA, Peselow E, Rosenthal M, et al. A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis. Psychopharmacol Bull. 1993;29(2):283-286.
30. Bender B, Friedman B, Davitt M, et al. 118: metoclopramide in the emergency department: a randomized factorial design study to determine the influence of dose and diphenhydramine on akathisia. Ann of Emerg Med. 2008;52(4):S78.
31. Parlak I, Erdur B, Parlak M, et al. Midazolam vs. diphenhydramine for the treatment of metoclopramide-induced akathisia: a randomized controlled trial. Acad Emerg Med. 2007;14(8):715-721.
32. Lima AR, Soares-Weiser K, Bacaltchuk J, et al. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 1999;(4):CD001950.
33. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676..
1. Cavallazzi R, Saad M, Marik PE. Delirium in the ICU: an overview. Ann Intensive Care. 2012;2:49.
2. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. National Alliance on Mental Illness. Mental health by the numbers. https://www.nami.org/learn-more/mental-health-by-the-numbers. Accessed March 4, 2018.
5. Jacka MJ, Mitchell N, Perez-Parada J. Incidence and prevalence of anxiety, depression, and post-traumatic stress disorder among critical care patients, families, and practitioners. J Anest & Inten Care Med. 2016;1(1):55555. doi: 10.19080/JAICM.2016.01.555555.
6. Reis AM, Cassiani SH. Adverse drug events in an intensive care unit of a university hospital. Eur J Clin Pharmacol. 2011;67(6):625-632.
7. Garpestad E, Devlin JW. Polypharmacy and delirium in critically ill older adults: recognition and prevention. Clin Geriatr Med. 2017;33(2):189-203.
8. Caroff SN, Hurford I, Lybrand J, et al. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127-148.
9. Van Putten T, Marder SR. Toward a more reliable diagnosis of akathisia. Arch Gen Psychiatry. 1986;43(10):1015-1016.
10. Penders TM, Agarwal S, Rohaidy R. Persistent akathisia masquerading as agitated depression after use of ziprasidone in the treatment of bipolar depression. Neuropsychiatr Dis Treat. 2013;9:463-465.
11. Naganuma H, Fujii I. Incidence and risk factors in neuroleptic malignant syndrome. Acta Psychiatr Scand. 1994;90(6):424-426.
12. Forcen FE, Matsoukas K, Alici Y. Antipsychotic-induced akathisia in delirium: a systematic review. Palliat Support Care. 2016;14(1):77-84.
13. Brune M, Sachdev, PS. Ladislav Haskovec and 100 years of akathisia. American Journal of Psychiatry. 2002;159(5):727-727.
14. Havaki-Kontaxaki BJ, Kontaxakis VP, Christodoulou GN. Prevalence and characteristics of patients with pseudoakathisia. Eur Neuropsychopharmacol. 2000;10(5):333-336.
15. Lang AE. Withdrawal akathisia: case reports and a proposed classification of chronic akathisia. Mov Disord. 1994;9(2):188-192.
16. Sachdev P. The epidemiology of drug-induced akathisia: Part II. Chronic, tardive, and withdrawal akathisias. Schizophr Bull. 1995;21(3):451-461.
17. Kern DS, Lang AE. Acute akathisia. In: Friedman JH, ed. Medication-induced movement disorders. Cambridge, United Kingdom: Cambridge University Press; 2015:12-24.
18. Adler LA, Angrist B, Reiter S, et al. Neuroleptic-induced akathisia: a review. Psychopharmacology (Berl). 1989;97(1):1-11.
19. Stahl SM, Loonen AJM. The mechanism of drug-induced akathisia. CNS Spectr. 2011;16(1):7-10.
20. Sachdev P, Kruk J. Clinical characteristics and predisposing factors in acute drug-induced akathisia. Arch Gen Psychiatry. 1994;51(12):963-974.
21. Laoutidis ZG, Luckhaus C. 5-HT2A receptor antagonists for the treatment of neuroleptic-induced akathisia: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17(5):823-832.
22. Lima AR, Bacalcthuk J, Barnes TR, et al. Central action beta-blockers versus placebo for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(4):CD001946.
23. Sachdev P, Loneragan C. Intravenous benztropine and propranolol challenges in acute neuroleptic-induced akathisia. Clin Neuropharmacol. 1993;16(4):324-331.
24. Lima AR, Weiser KV, Bacaltchuk J, et al. Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 2004;(1):CD003727.
25. Fleischhacker WW, Roth SD, Kane JM. The pharmacologic treatment of neuroleptic-induced akathisia. J Clin Psychopharmacol. 1990;10(1):12-21.
26. Blaisdell GD. Akathisia: a comprehensive review and treatment summary. Pharmacopsychiatry. 1994;27(4):139-146.
27. Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry. 2010;196(2):89-91.
28. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
29. Adler LA, Peselow E, Rosenthal M, et al. A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis. Psychopharmacol Bull. 1993;29(2):283-286.
30. Bender B, Friedman B, Davitt M, et al. 118: metoclopramide in the emergency department: a randomized factorial design study to determine the influence of dose and diphenhydramine on akathisia. Ann of Emerg Med. 2008;52(4):S78.
31. Parlak I, Erdur B, Parlak M, et al. Midazolam vs. diphenhydramine for the treatment of metoclopramide-induced akathisia: a randomized controlled trial. Acad Emerg Med. 2007;14(8):715-721.
32. Lima AR, Soares-Weiser K, Bacaltchuk J, et al. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev. 1999;(4):CD001950.
33. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676..
Pregnant and moving involuntarily
CASE: Abnormal movements
Pregnant and unsure of her due date, Ms. A, age 35, presents to the emergency room complaining of hourly uterine contractions for the last 3 days and new onset vaginal bleeding. Ms. A is admitted to the obstetrics (OB) service for preterm labor at 34 and 3/7 weeks as dated by a triage ultrasound.
During initial examination by the OB service, Ms. A’s blood pressure is 155/112 mm Hg with a pulse of 126. Her cervix is dilated to 4 centimeters. Her physical exam is notable for rapid, repetitive, involuntary movements in her upper extremities and to a lesser degree in lower extremities. Ms. A is started on IV fluids and hydralazine, 10 mg/d, for elevated blood pressure. Later that day, she delivers a preterm female weighing 2,360 grams in a spontaneous vaginal delivery without any complications.
After delivery, the OB service requests a psychiatric consultation to evaluate Ms. A’s “blunted affect,” history of heavy alcohol use, and abnormal movements. During examination, Ms. A is alert and oriented to her surroundings. She states that this was her eleventh pregnancy; however, she is unable to recall details of most previous pregnancies. She also cannot remember any significant medical, surgical, or mental health history. Ms. A appears distracted, has difficulty participating in the interview, and gives contradictory histories to different team members. She is well groomed but shows repetitive circular movements of her hands, feet, and jaw that are nearly continuous. In addition, Ms. A has intermittent lip biting and smacking. Her speech is delayed, with increased latency of her responses to basic questions.
Her mood is neutral, her affect is blunted, and she denies any current suicidal or homicidal ideations, delusions, and auditory or visual hallucinations. Although her chart indicates a history of alcohol abuse, she denies this history and current drug or alcohol use. Her Mini-Mental State Exam score is a 22/30, missing points in her ability to copy shapes and write a sentence, complicated by her chorea-like upper body movements. She also demonstrates marked inattentiveness and is unwilling to cooperate with spelling “world.” On physical exam, her gait is wide-based but steady.
The authors’ observations
Determining the cause of Ms. A’s abnormal movements, delayed speech, and neutral mood initially proves difficult because she is minimally cooperative with the interview and we find discrepancies between information she provides and her medical records from previous OB admissions. It is unclear whether these inconsistencies are because of her faltering memory—which she admits has worsened in the last year—or unwillingness to provide a complete medical history.
We consider possible substance intoxication given her documented history of substance use. However, an extended drug screen is negative and her laboratory values do not suggest heavy alcohol use.
HISTORY: Depression and confusion
The next day, Ms. A is more cooperative with the interview. She says that she began feeling depressed 8 years ago, around the time her brother was killed in a violent crime. She denies previous psychiatric hospitalizations, but says she attempted suicide 4 years ago by stabbing herself in the throat with a fork. After that attempt, she was referred to an outpatient psychiatrist whom she continues to see intermittently. She says that her abnormal movements started 2 years before she first saw her outpatient psychiatrist.
She says she has been prescribed several medications, but remembers only taking quetiapine for depressive symptoms and insomnia. After a discussion with her psychiatrist about the possible effects of quetiapine on the fetus, she discontinued the drug approximately 8 weeks into her pregnancy. Quetiapine decreased her movement symptoms slightly, and she feels her movements have become uncontrollable since discontinuing it.
She reports increased feelings of sadness, worthlessness, guilt, decreased energy, irritability, and difficulty sleeping during her pregnancy. She denies current or past psychotic symptoms or mania. Ms. A says she has noticed problems with her memory as well as increased confusion over recent months. She often gets lost and cannot remember where she lives after leaving her home.
Based on hospital records, we learn that an MRI of the brain without contrast was completed 1 year ago to “evaluate choreiform movements.” The scan showed mild atrophy and abnormal signal within the caudate and putamen, as well as volume loss. We consult with the neurology service to evaluate Ms. A’s abnormal movements and her previous abnormal brain imaging. The neurologic exam notes that Ms. A has orofacial dyskinesias and near-continuous choreiform movements in her arms and hands. Her gait remains wide-based and she is unable to tandem walk. Because Ms. A shows no new neurologic symptoms, the neurology service does not feel that additional neuroimaging is indicated.
The authors’ observations
In consultation with neurology, the leading differential diagnoses include tardive dyskinesia, chorea gravidarum, and Huntington’s disease. See the Table1,2 for the differential diagnosis of chorea.
Ms. A reports taking quetiapine for 3 years, which suggests possible tardive dyskinesia. Although second-generation antipsychotics have a lower incidence of movement disorders than first-generation antipsychotics, the risk still exists. Withdrawal dyskinesias can occur after suddenly stopping or tapering antipsychotics and appear as extrapyramidal symptoms, including choreoathetosis similar to what Ms. A experienced.3,4 This type of dyskinesia is thought to be secondary to chronic dopamine antagonism leading to increased postsynaptic receptors and dopamine hypersensitivity.5 Because Ms. A discontinued quetiapine early in her pregnancy, withdrawal dyskinesias are less likely.
Because Ms. A presented with a movement disorder while pregnant, the neurology service considers chorea gravidarum, the term given to chorea occurring during pregnancy. This syndrome is thought to be caused by the effects of pregnancy on the basal ganglia.6 Historically, chorea gravidarum was associated with rheumatic fever (RF); however, with the decline in prevalence of RF, most choreiform movements that appear during pregnancy typically are caused by other diseases, such as systemic lupus erythematosus or Huntington’s disease. Approximately one-half of chorea gravidarum cases are idiopathic, with RF and antiphospholipid syndrome accounting for the remainder.7 Huntington’s disease during pregnancy is rare because it tends to present in women beyond childbearing age.
Based on Ms. A’s symptoms and previous MRI findings, we ask her if she has a known family history of Huntington’s disease. She denies this, but says she has not seen her father since she was very young and is uncertain of his medical history.
Table
Differential diagnosis for chorea
| Genetic | Huntington’s disease, benign hereditary chorea, neuroacanthocytosis, dentatorubral-pallidoluysian atrophy, Wilson’s disease, spinocerebellar ataxia, Friedreich’s ataxia |
| Rheumatic disorders | Sydenham’s chorea, chorea gravidarum |
| Drug-induced/toxicity | Neuroleptic drugs, steroids, anticonvulsants, antiparkinson agents, stimulants (amphetamines, cocaine), lithium, dopamine agonists |
| Systemic disorders | Systemic lupus erythematosus, thyrotoxicosis, polycythemia vera, hyperglycemia, AIDS, paraneoplastic syndrome |
| Vascular/trauma | Cerebral hemorrhage, vasculitis, stroke, antiphospholipid antibody syndrome |
| AIDS: acquired immune deficiency syndrome Source: References 1,2 | |
TREATMENT: Restart medication
Ms. A’s laboratory results show a slightly low hemoglobin of 10.5 g/dL and hematocrit of 32.8%. Her mean corpuscular volume is slightly decreased at 77 fL. Her urinalysis is negative, and blood glucose and thyroid-stimulating hormone are within normal limits. Rapid plasma regain, anti-nuclear antibody, and human immunodeficiency virus (HIV) are negative. Based on hospital records, we learn that during the previous admission a year ago a serum ceruloplasmin and serum copper were drawn and were normal.
We contact Ms. A’s outpatient psychiatrist for collateral information. The psychiatrist says he first evaluated Ms. A 3 years ago after a friend brought her in because of strange behavior, including talking to herself, making odd facial gestures, and laughing inappropriately. Although Ms. A denies past psychiatric hospitalizations, her psychiatrist states that she was hospitalized for 1 week after the suicide attempt 4 years ago and prescribed lorazepam and sertraline during that admission. He speculates that the suicide attempt may have been related to 5 of her children being taken from her by the Department of Family and Child Services after police raided her home to search for drugs. Custody was awarded to their respective fathers, causing Ms. A to “snap,” according to her friend.
Since then, neither Ms. A nor her psychiatrist have reported any further psychotic symptoms. Her psychiatrist confirms that Ms. A’s abnormal movements were present before her first appointment with him. He says that he referred Ms. A to a local hospital for a neurology work-up, but she did not schedule an appointment.
When we follow up with Ms. A 2 days after delivery, she continues to deny depressive symptoms, although her affect remains blunted. She says she is looking forward to going home with the baby, whom she plans to bottle feed. Her choreiform movements appear unchanged. She also continues to experience lip smacking. Although Ms. A recognizes that she has some movements, she minimizes them and says they do not bother her. She continues to demonstrate latency in her verbal responses to questions. Based on the collateral history and positive response with quetiapine, we recommend that Ms. A be restarted on quetiapine, 200 mg/d.
The authors’ observations
Ms. A’s choreiform movements started before her psychotic symptoms and subsequent usage of neuroleptic medication, which makes tardive dyskinesia less likely. Laboratory studies rule out systemic lupus erythematosus, HIV, and Wilson’s disease as the cause of her abnormal movements.
Ms. A’s history is highly suggestive of Huntington’s disease. She exhibits classic motor signs, including involuntary choreiform movements in her extremities. She also has psychiatric symptoms that are commonly associated with Huntington’s disease, including depression—which preceded her motor symptoms—cognitive decline, apathy, and psychotic symptoms. In addition, her MRI findings of volume changes in the caudate nucleus and the putamen and inability to rule out a family history make Huntington’s disease more likely (Box).1,8-11
Huntington’s disease is an autosomal dominant disorder characterized by progressive motor, cognitive, and psychiatric disturbances and is the most common genetic cause of chorea. The underlying genetic mutation is a CAG repeat expansion in the Huntington’s disease gene. A Huntington’s disease diagnosis generally is considered in the presence of the characteristic choreiform movements and slowly progressive cognitive decline.8 Physical symptoms can present at any age, although they usually begin between age 35 and 44. In early stages of the disease, patients may experience subtle changes in personality, cognition, and physical skills. Although most Huntington’s disease patients eventually exhibit similar physical symptoms, the onset, progression, and extent of cognitive and psychiatric symptoms vary among individuals. However, psychiatric symptoms frequently are present during the early stages of the disease, often before motor symptoms begin and can include personality changes, irritability, agitation, apathy, and depression. In addition, up to 23% of patients with Huntington’s disease develop psychotic symptoms.1,9 There is no cure for Huntington’s disease, and mean disease duration is 17 to 20 years. The most common cause of death among Huntington’s disease patients is pneumonia, followed by suicide.1
A Huntington’s disease diagnosis is based on clinical symptoms and signs in an individual who has a parent with proven Huntington’s disease and is confirmed by DNA tests.1 Typical neuroanatomic findings include initial neuronal loss in the striatum followed by a diffuse involvement of cortical and subcortical areas.10 Volume changes in the caudate nucleus and the putamen may be a reliable measure of Huntington’s disease and potentially serve as a biomarker.11
Psychiatric symptoms
Psychiatric symptoms frequently are evident in the early stages of Huntington’s disease, often before onset of motor symptoms.1 Depression is the most common sign, and can be difficult to diagnose because weight loss, apathy, and inactivity also occur in Huntington’s disease. Feelings of low self-esteem, guilt, and anxiety can help distinguish depression from symptoms of Huntington’s disease. Cognitive decline also may present before the first motor symptoms occur. Cognitive changes typically are related to executive functions and affected individuals may develop impairments in organization and planning. Psychotic symptoms may be present, but are more common in later stages of the disease.1
Ms. A reported that quetiapine seemed to lessen her choreiform movements, and dopamine receptor blocking agents (ie, antipsychotics) often are considered for managing chorea and psychosis in Huntington’s disease. However, there are few double-blind, placebo-controlled studies evaluating the efficacy of these agents.12 Small, uncontrolled, nonrandomized trials found quetiapine has some efficacy for both motor and psychiatric symptoms in Huntington’s disease.12-15
OUTCOME: Lost to follow-up
Ms. A is discharged from the hospital 3 days after she delivers her daughter and is given an appointment in 6 weeks at an affiliated movement disorders clinic. Before discharge, she is tested for the Huntington’s disease gene mutation with a plan to receive her results during her follow-up visit. During the informed consent process for the genetic testing, Ms. A states that she was tested previously and was quite sure that the test was positive for Huntington’s disease, although she could not recall where or when this testing was completed.
Ms. A also is scheduled to follow up with her obstetrician for a 6-week postpartum check-up and tubal ligation. We encourage Ms. A to make an appointment with her psychiatrist soon after discharge. We also make a referral to the Department of Family and Children Services to provide adequate support and resources to her and her children because of her physical and psychiatric issues.
Ms. A does not show up for her follow-up appointment at the movement disorders clinic. The genetic test is not completed during this admission because of a clerical error, and the serum sample subsequently expires.
The authors’ observations
Although Huntington’s disease is the most likely cause of Ms. A’s presentation, we were unable to confirm the diagnosis with genetic testing. If Ms. A returns to the neurology service and the genetic test is negative for Huntington’s disease, other causes of chorea must be investigated.
Related Resources
- De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000; 7(5):278-289.
- Revilla FJ, Grutzendler J, Larsh TR. Huntington disease. Medscape. http://emedicine.medscape.com/article/1150165-overview.
Drug Brand Names
- Hydralazine • Apresoline
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5(1):40.-
2. Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7:360-373.
3. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
4. Kafantaris V, Hirsch J, Saito E, et al. Treatment of withdrawal dyskinesia. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1102-1103.
5. Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197(4303):596-598.
6. Kranick SM, Mowry EM, Colcher A, et al. Movement disorders and pregnancy: a review of the literature. Mov Disord. 2010;25(6):665-671.
7. Ramachandran TS. Chorea gravidarum. Medscape. Available at: http://emedicine.medscape.com/article/1149725-overview. Accessed May 4 2011.
8. Panegyres PK, Goh JG. The neurology and natural history of patients with indeterminate CAG repeat length mutations of the Huntington disease gene. J Neurol Sci. 2011;301(1-2):14-20.
9. Shiwach R. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand. 1994;90:241-246.
10. De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000;7:278-289.
11. van den Bogaard SJ, Dumas EM, Acharya TP, et al. and the TRACK-HD Investigator Group. Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. J Neurol. 2011;258(3):412-420.
12. Frank S, Jankovic J. Advances in the pharmacological management of Huntington’s disease. Drugs. 2010;70(5):561-571.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Seitz DP, Millson RC. Quetiapine in the management of psychosis secondary to Huntington’s disease: a case report. Can J Psychiatry. 2004;49(6):413.-
15. Bonelli RM, Niederwieser G. Quetiapine in Huntington’s disease: a first case report. J Neurol. 2002;249(8):1114-1115.
CASE: Abnormal movements
Pregnant and unsure of her due date, Ms. A, age 35, presents to the emergency room complaining of hourly uterine contractions for the last 3 days and new onset vaginal bleeding. Ms. A is admitted to the obstetrics (OB) service for preterm labor at 34 and 3/7 weeks as dated by a triage ultrasound.
During initial examination by the OB service, Ms. A’s blood pressure is 155/112 mm Hg with a pulse of 126. Her cervix is dilated to 4 centimeters. Her physical exam is notable for rapid, repetitive, involuntary movements in her upper extremities and to a lesser degree in lower extremities. Ms. A is started on IV fluids and hydralazine, 10 mg/d, for elevated blood pressure. Later that day, she delivers a preterm female weighing 2,360 grams in a spontaneous vaginal delivery without any complications.
After delivery, the OB service requests a psychiatric consultation to evaluate Ms. A’s “blunted affect,” history of heavy alcohol use, and abnormal movements. During examination, Ms. A is alert and oriented to her surroundings. She states that this was her eleventh pregnancy; however, she is unable to recall details of most previous pregnancies. She also cannot remember any significant medical, surgical, or mental health history. Ms. A appears distracted, has difficulty participating in the interview, and gives contradictory histories to different team members. She is well groomed but shows repetitive circular movements of her hands, feet, and jaw that are nearly continuous. In addition, Ms. A has intermittent lip biting and smacking. Her speech is delayed, with increased latency of her responses to basic questions.
Her mood is neutral, her affect is blunted, and she denies any current suicidal or homicidal ideations, delusions, and auditory or visual hallucinations. Although her chart indicates a history of alcohol abuse, she denies this history and current drug or alcohol use. Her Mini-Mental State Exam score is a 22/30, missing points in her ability to copy shapes and write a sentence, complicated by her chorea-like upper body movements. She also demonstrates marked inattentiveness and is unwilling to cooperate with spelling “world.” On physical exam, her gait is wide-based but steady.
The authors’ observations
Determining the cause of Ms. A’s abnormal movements, delayed speech, and neutral mood initially proves difficult because she is minimally cooperative with the interview and we find discrepancies between information she provides and her medical records from previous OB admissions. It is unclear whether these inconsistencies are because of her faltering memory—which she admits has worsened in the last year—or unwillingness to provide a complete medical history.
We consider possible substance intoxication given her documented history of substance use. However, an extended drug screen is negative and her laboratory values do not suggest heavy alcohol use.
HISTORY: Depression and confusion
The next day, Ms. A is more cooperative with the interview. She says that she began feeling depressed 8 years ago, around the time her brother was killed in a violent crime. She denies previous psychiatric hospitalizations, but says she attempted suicide 4 years ago by stabbing herself in the throat with a fork. After that attempt, she was referred to an outpatient psychiatrist whom she continues to see intermittently. She says that her abnormal movements started 2 years before she first saw her outpatient psychiatrist.
She says she has been prescribed several medications, but remembers only taking quetiapine for depressive symptoms and insomnia. After a discussion with her psychiatrist about the possible effects of quetiapine on the fetus, she discontinued the drug approximately 8 weeks into her pregnancy. Quetiapine decreased her movement symptoms slightly, and she feels her movements have become uncontrollable since discontinuing it.
She reports increased feelings of sadness, worthlessness, guilt, decreased energy, irritability, and difficulty sleeping during her pregnancy. She denies current or past psychotic symptoms or mania. Ms. A says she has noticed problems with her memory as well as increased confusion over recent months. She often gets lost and cannot remember where she lives after leaving her home.
Based on hospital records, we learn that an MRI of the brain without contrast was completed 1 year ago to “evaluate choreiform movements.” The scan showed mild atrophy and abnormal signal within the caudate and putamen, as well as volume loss. We consult with the neurology service to evaluate Ms. A’s abnormal movements and her previous abnormal brain imaging. The neurologic exam notes that Ms. A has orofacial dyskinesias and near-continuous choreiform movements in her arms and hands. Her gait remains wide-based and she is unable to tandem walk. Because Ms. A shows no new neurologic symptoms, the neurology service does not feel that additional neuroimaging is indicated.
The authors’ observations
In consultation with neurology, the leading differential diagnoses include tardive dyskinesia, chorea gravidarum, and Huntington’s disease. See the Table1,2 for the differential diagnosis of chorea.
Ms. A reports taking quetiapine for 3 years, which suggests possible tardive dyskinesia. Although second-generation antipsychotics have a lower incidence of movement disorders than first-generation antipsychotics, the risk still exists. Withdrawal dyskinesias can occur after suddenly stopping or tapering antipsychotics and appear as extrapyramidal symptoms, including choreoathetosis similar to what Ms. A experienced.3,4 This type of dyskinesia is thought to be secondary to chronic dopamine antagonism leading to increased postsynaptic receptors and dopamine hypersensitivity.5 Because Ms. A discontinued quetiapine early in her pregnancy, withdrawal dyskinesias are less likely.
Because Ms. A presented with a movement disorder while pregnant, the neurology service considers chorea gravidarum, the term given to chorea occurring during pregnancy. This syndrome is thought to be caused by the effects of pregnancy on the basal ganglia.6 Historically, chorea gravidarum was associated with rheumatic fever (RF); however, with the decline in prevalence of RF, most choreiform movements that appear during pregnancy typically are caused by other diseases, such as systemic lupus erythematosus or Huntington’s disease. Approximately one-half of chorea gravidarum cases are idiopathic, with RF and antiphospholipid syndrome accounting for the remainder.7 Huntington’s disease during pregnancy is rare because it tends to present in women beyond childbearing age.
Based on Ms. A’s symptoms and previous MRI findings, we ask her if she has a known family history of Huntington’s disease. She denies this, but says she has not seen her father since she was very young and is uncertain of his medical history.
Table
Differential diagnosis for chorea
| Genetic | Huntington’s disease, benign hereditary chorea, neuroacanthocytosis, dentatorubral-pallidoluysian atrophy, Wilson’s disease, spinocerebellar ataxia, Friedreich’s ataxia |
| Rheumatic disorders | Sydenham’s chorea, chorea gravidarum |
| Drug-induced/toxicity | Neuroleptic drugs, steroids, anticonvulsants, antiparkinson agents, stimulants (amphetamines, cocaine), lithium, dopamine agonists |
| Systemic disorders | Systemic lupus erythematosus, thyrotoxicosis, polycythemia vera, hyperglycemia, AIDS, paraneoplastic syndrome |
| Vascular/trauma | Cerebral hemorrhage, vasculitis, stroke, antiphospholipid antibody syndrome |
| AIDS: acquired immune deficiency syndrome Source: References 1,2 | |
TREATMENT: Restart medication
Ms. A’s laboratory results show a slightly low hemoglobin of 10.5 g/dL and hematocrit of 32.8%. Her mean corpuscular volume is slightly decreased at 77 fL. Her urinalysis is negative, and blood glucose and thyroid-stimulating hormone are within normal limits. Rapid plasma regain, anti-nuclear antibody, and human immunodeficiency virus (HIV) are negative. Based on hospital records, we learn that during the previous admission a year ago a serum ceruloplasmin and serum copper were drawn and were normal.
We contact Ms. A’s outpatient psychiatrist for collateral information. The psychiatrist says he first evaluated Ms. A 3 years ago after a friend brought her in because of strange behavior, including talking to herself, making odd facial gestures, and laughing inappropriately. Although Ms. A denies past psychiatric hospitalizations, her psychiatrist states that she was hospitalized for 1 week after the suicide attempt 4 years ago and prescribed lorazepam and sertraline during that admission. He speculates that the suicide attempt may have been related to 5 of her children being taken from her by the Department of Family and Child Services after police raided her home to search for drugs. Custody was awarded to their respective fathers, causing Ms. A to “snap,” according to her friend.
Since then, neither Ms. A nor her psychiatrist have reported any further psychotic symptoms. Her psychiatrist confirms that Ms. A’s abnormal movements were present before her first appointment with him. He says that he referred Ms. A to a local hospital for a neurology work-up, but she did not schedule an appointment.
When we follow up with Ms. A 2 days after delivery, she continues to deny depressive symptoms, although her affect remains blunted. She says she is looking forward to going home with the baby, whom she plans to bottle feed. Her choreiform movements appear unchanged. She also continues to experience lip smacking. Although Ms. A recognizes that she has some movements, she minimizes them and says they do not bother her. She continues to demonstrate latency in her verbal responses to questions. Based on the collateral history and positive response with quetiapine, we recommend that Ms. A be restarted on quetiapine, 200 mg/d.
The authors’ observations
Ms. A’s choreiform movements started before her psychotic symptoms and subsequent usage of neuroleptic medication, which makes tardive dyskinesia less likely. Laboratory studies rule out systemic lupus erythematosus, HIV, and Wilson’s disease as the cause of her abnormal movements.
Ms. A’s history is highly suggestive of Huntington’s disease. She exhibits classic motor signs, including involuntary choreiform movements in her extremities. She also has psychiatric symptoms that are commonly associated with Huntington’s disease, including depression—which preceded her motor symptoms—cognitive decline, apathy, and psychotic symptoms. In addition, her MRI findings of volume changes in the caudate nucleus and the putamen and inability to rule out a family history make Huntington’s disease more likely (Box).1,8-11
Huntington’s disease is an autosomal dominant disorder characterized by progressive motor, cognitive, and psychiatric disturbances and is the most common genetic cause of chorea. The underlying genetic mutation is a CAG repeat expansion in the Huntington’s disease gene. A Huntington’s disease diagnosis generally is considered in the presence of the characteristic choreiform movements and slowly progressive cognitive decline.8 Physical symptoms can present at any age, although they usually begin between age 35 and 44. In early stages of the disease, patients may experience subtle changes in personality, cognition, and physical skills. Although most Huntington’s disease patients eventually exhibit similar physical symptoms, the onset, progression, and extent of cognitive and psychiatric symptoms vary among individuals. However, psychiatric symptoms frequently are present during the early stages of the disease, often before motor symptoms begin and can include personality changes, irritability, agitation, apathy, and depression. In addition, up to 23% of patients with Huntington’s disease develop psychotic symptoms.1,9 There is no cure for Huntington’s disease, and mean disease duration is 17 to 20 years. The most common cause of death among Huntington’s disease patients is pneumonia, followed by suicide.1
A Huntington’s disease diagnosis is based on clinical symptoms and signs in an individual who has a parent with proven Huntington’s disease and is confirmed by DNA tests.1 Typical neuroanatomic findings include initial neuronal loss in the striatum followed by a diffuse involvement of cortical and subcortical areas.10 Volume changes in the caudate nucleus and the putamen may be a reliable measure of Huntington’s disease and potentially serve as a biomarker.11
Psychiatric symptoms
Psychiatric symptoms frequently are evident in the early stages of Huntington’s disease, often before onset of motor symptoms.1 Depression is the most common sign, and can be difficult to diagnose because weight loss, apathy, and inactivity also occur in Huntington’s disease. Feelings of low self-esteem, guilt, and anxiety can help distinguish depression from symptoms of Huntington’s disease. Cognitive decline also may present before the first motor symptoms occur. Cognitive changes typically are related to executive functions and affected individuals may develop impairments in organization and planning. Psychotic symptoms may be present, but are more common in later stages of the disease.1
Ms. A reported that quetiapine seemed to lessen her choreiform movements, and dopamine receptor blocking agents (ie, antipsychotics) often are considered for managing chorea and psychosis in Huntington’s disease. However, there are few double-blind, placebo-controlled studies evaluating the efficacy of these agents.12 Small, uncontrolled, nonrandomized trials found quetiapine has some efficacy for both motor and psychiatric symptoms in Huntington’s disease.12-15
OUTCOME: Lost to follow-up
Ms. A is discharged from the hospital 3 days after she delivers her daughter and is given an appointment in 6 weeks at an affiliated movement disorders clinic. Before discharge, she is tested for the Huntington’s disease gene mutation with a plan to receive her results during her follow-up visit. During the informed consent process for the genetic testing, Ms. A states that she was tested previously and was quite sure that the test was positive for Huntington’s disease, although she could not recall where or when this testing was completed.
Ms. A also is scheduled to follow up with her obstetrician for a 6-week postpartum check-up and tubal ligation. We encourage Ms. A to make an appointment with her psychiatrist soon after discharge. We also make a referral to the Department of Family and Children Services to provide adequate support and resources to her and her children because of her physical and psychiatric issues.
Ms. A does not show up for her follow-up appointment at the movement disorders clinic. The genetic test is not completed during this admission because of a clerical error, and the serum sample subsequently expires.
The authors’ observations
Although Huntington’s disease is the most likely cause of Ms. A’s presentation, we were unable to confirm the diagnosis with genetic testing. If Ms. A returns to the neurology service and the genetic test is negative for Huntington’s disease, other causes of chorea must be investigated.
Related Resources
- De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000; 7(5):278-289.
- Revilla FJ, Grutzendler J, Larsh TR. Huntington disease. Medscape. http://emedicine.medscape.com/article/1150165-overview.
Drug Brand Names
- Hydralazine • Apresoline
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Abnormal movements
Pregnant and unsure of her due date, Ms. A, age 35, presents to the emergency room complaining of hourly uterine contractions for the last 3 days and new onset vaginal bleeding. Ms. A is admitted to the obstetrics (OB) service for preterm labor at 34 and 3/7 weeks as dated by a triage ultrasound.
During initial examination by the OB service, Ms. A’s blood pressure is 155/112 mm Hg with a pulse of 126. Her cervix is dilated to 4 centimeters. Her physical exam is notable for rapid, repetitive, involuntary movements in her upper extremities and to a lesser degree in lower extremities. Ms. A is started on IV fluids and hydralazine, 10 mg/d, for elevated blood pressure. Later that day, she delivers a preterm female weighing 2,360 grams in a spontaneous vaginal delivery without any complications.
After delivery, the OB service requests a psychiatric consultation to evaluate Ms. A’s “blunted affect,” history of heavy alcohol use, and abnormal movements. During examination, Ms. A is alert and oriented to her surroundings. She states that this was her eleventh pregnancy; however, she is unable to recall details of most previous pregnancies. She also cannot remember any significant medical, surgical, or mental health history. Ms. A appears distracted, has difficulty participating in the interview, and gives contradictory histories to different team members. She is well groomed but shows repetitive circular movements of her hands, feet, and jaw that are nearly continuous. In addition, Ms. A has intermittent lip biting and smacking. Her speech is delayed, with increased latency of her responses to basic questions.
Her mood is neutral, her affect is blunted, and she denies any current suicidal or homicidal ideations, delusions, and auditory or visual hallucinations. Although her chart indicates a history of alcohol abuse, she denies this history and current drug or alcohol use. Her Mini-Mental State Exam score is a 22/30, missing points in her ability to copy shapes and write a sentence, complicated by her chorea-like upper body movements. She also demonstrates marked inattentiveness and is unwilling to cooperate with spelling “world.” On physical exam, her gait is wide-based but steady.
The authors’ observations
Determining the cause of Ms. A’s abnormal movements, delayed speech, and neutral mood initially proves difficult because she is minimally cooperative with the interview and we find discrepancies between information she provides and her medical records from previous OB admissions. It is unclear whether these inconsistencies are because of her faltering memory—which she admits has worsened in the last year—or unwillingness to provide a complete medical history.
We consider possible substance intoxication given her documented history of substance use. However, an extended drug screen is negative and her laboratory values do not suggest heavy alcohol use.
HISTORY: Depression and confusion
The next day, Ms. A is more cooperative with the interview. She says that she began feeling depressed 8 years ago, around the time her brother was killed in a violent crime. She denies previous psychiatric hospitalizations, but says she attempted suicide 4 years ago by stabbing herself in the throat with a fork. After that attempt, she was referred to an outpatient psychiatrist whom she continues to see intermittently. She says that her abnormal movements started 2 years before she first saw her outpatient psychiatrist.
She says she has been prescribed several medications, but remembers only taking quetiapine for depressive symptoms and insomnia. After a discussion with her psychiatrist about the possible effects of quetiapine on the fetus, she discontinued the drug approximately 8 weeks into her pregnancy. Quetiapine decreased her movement symptoms slightly, and she feels her movements have become uncontrollable since discontinuing it.
She reports increased feelings of sadness, worthlessness, guilt, decreased energy, irritability, and difficulty sleeping during her pregnancy. She denies current or past psychotic symptoms or mania. Ms. A says she has noticed problems with her memory as well as increased confusion over recent months. She often gets lost and cannot remember where she lives after leaving her home.
Based on hospital records, we learn that an MRI of the brain without contrast was completed 1 year ago to “evaluate choreiform movements.” The scan showed mild atrophy and abnormal signal within the caudate and putamen, as well as volume loss. We consult with the neurology service to evaluate Ms. A’s abnormal movements and her previous abnormal brain imaging. The neurologic exam notes that Ms. A has orofacial dyskinesias and near-continuous choreiform movements in her arms and hands. Her gait remains wide-based and she is unable to tandem walk. Because Ms. A shows no new neurologic symptoms, the neurology service does not feel that additional neuroimaging is indicated.
The authors’ observations
In consultation with neurology, the leading differential diagnoses include tardive dyskinesia, chorea gravidarum, and Huntington’s disease. See the Table1,2 for the differential diagnosis of chorea.
Ms. A reports taking quetiapine for 3 years, which suggests possible tardive dyskinesia. Although second-generation antipsychotics have a lower incidence of movement disorders than first-generation antipsychotics, the risk still exists. Withdrawal dyskinesias can occur after suddenly stopping or tapering antipsychotics and appear as extrapyramidal symptoms, including choreoathetosis similar to what Ms. A experienced.3,4 This type of dyskinesia is thought to be secondary to chronic dopamine antagonism leading to increased postsynaptic receptors and dopamine hypersensitivity.5 Because Ms. A discontinued quetiapine early in her pregnancy, withdrawal dyskinesias are less likely.
Because Ms. A presented with a movement disorder while pregnant, the neurology service considers chorea gravidarum, the term given to chorea occurring during pregnancy. This syndrome is thought to be caused by the effects of pregnancy on the basal ganglia.6 Historically, chorea gravidarum was associated with rheumatic fever (RF); however, with the decline in prevalence of RF, most choreiform movements that appear during pregnancy typically are caused by other diseases, such as systemic lupus erythematosus or Huntington’s disease. Approximately one-half of chorea gravidarum cases are idiopathic, with RF and antiphospholipid syndrome accounting for the remainder.7 Huntington’s disease during pregnancy is rare because it tends to present in women beyond childbearing age.
Based on Ms. A’s symptoms and previous MRI findings, we ask her if she has a known family history of Huntington’s disease. She denies this, but says she has not seen her father since she was very young and is uncertain of his medical history.
Table
Differential diagnosis for chorea
| Genetic | Huntington’s disease, benign hereditary chorea, neuroacanthocytosis, dentatorubral-pallidoluysian atrophy, Wilson’s disease, spinocerebellar ataxia, Friedreich’s ataxia |
| Rheumatic disorders | Sydenham’s chorea, chorea gravidarum |
| Drug-induced/toxicity | Neuroleptic drugs, steroids, anticonvulsants, antiparkinson agents, stimulants (amphetamines, cocaine), lithium, dopamine agonists |
| Systemic disorders | Systemic lupus erythematosus, thyrotoxicosis, polycythemia vera, hyperglycemia, AIDS, paraneoplastic syndrome |
| Vascular/trauma | Cerebral hemorrhage, vasculitis, stroke, antiphospholipid antibody syndrome |
| AIDS: acquired immune deficiency syndrome Source: References 1,2 | |
TREATMENT: Restart medication
Ms. A’s laboratory results show a slightly low hemoglobin of 10.5 g/dL and hematocrit of 32.8%. Her mean corpuscular volume is slightly decreased at 77 fL. Her urinalysis is negative, and blood glucose and thyroid-stimulating hormone are within normal limits. Rapid plasma regain, anti-nuclear antibody, and human immunodeficiency virus (HIV) are negative. Based on hospital records, we learn that during the previous admission a year ago a serum ceruloplasmin and serum copper were drawn and were normal.
We contact Ms. A’s outpatient psychiatrist for collateral information. The psychiatrist says he first evaluated Ms. A 3 years ago after a friend brought her in because of strange behavior, including talking to herself, making odd facial gestures, and laughing inappropriately. Although Ms. A denies past psychiatric hospitalizations, her psychiatrist states that she was hospitalized for 1 week after the suicide attempt 4 years ago and prescribed lorazepam and sertraline during that admission. He speculates that the suicide attempt may have been related to 5 of her children being taken from her by the Department of Family and Child Services after police raided her home to search for drugs. Custody was awarded to their respective fathers, causing Ms. A to “snap,” according to her friend.
Since then, neither Ms. A nor her psychiatrist have reported any further psychotic symptoms. Her psychiatrist confirms that Ms. A’s abnormal movements were present before her first appointment with him. He says that he referred Ms. A to a local hospital for a neurology work-up, but she did not schedule an appointment.
When we follow up with Ms. A 2 days after delivery, she continues to deny depressive symptoms, although her affect remains blunted. She says she is looking forward to going home with the baby, whom she plans to bottle feed. Her choreiform movements appear unchanged. She also continues to experience lip smacking. Although Ms. A recognizes that she has some movements, she minimizes them and says they do not bother her. She continues to demonstrate latency in her verbal responses to questions. Based on the collateral history and positive response with quetiapine, we recommend that Ms. A be restarted on quetiapine, 200 mg/d.
The authors’ observations
Ms. A’s choreiform movements started before her psychotic symptoms and subsequent usage of neuroleptic medication, which makes tardive dyskinesia less likely. Laboratory studies rule out systemic lupus erythematosus, HIV, and Wilson’s disease as the cause of her abnormal movements.
Ms. A’s history is highly suggestive of Huntington’s disease. She exhibits classic motor signs, including involuntary choreiform movements in her extremities. She also has psychiatric symptoms that are commonly associated with Huntington’s disease, including depression—which preceded her motor symptoms—cognitive decline, apathy, and psychotic symptoms. In addition, her MRI findings of volume changes in the caudate nucleus and the putamen and inability to rule out a family history make Huntington’s disease more likely (Box).1,8-11
Huntington’s disease is an autosomal dominant disorder characterized by progressive motor, cognitive, and psychiatric disturbances and is the most common genetic cause of chorea. The underlying genetic mutation is a CAG repeat expansion in the Huntington’s disease gene. A Huntington’s disease diagnosis generally is considered in the presence of the characteristic choreiform movements and slowly progressive cognitive decline.8 Physical symptoms can present at any age, although they usually begin between age 35 and 44. In early stages of the disease, patients may experience subtle changes in personality, cognition, and physical skills. Although most Huntington’s disease patients eventually exhibit similar physical symptoms, the onset, progression, and extent of cognitive and psychiatric symptoms vary among individuals. However, psychiatric symptoms frequently are present during the early stages of the disease, often before motor symptoms begin and can include personality changes, irritability, agitation, apathy, and depression. In addition, up to 23% of patients with Huntington’s disease develop psychotic symptoms.1,9 There is no cure for Huntington’s disease, and mean disease duration is 17 to 20 years. The most common cause of death among Huntington’s disease patients is pneumonia, followed by suicide.1
A Huntington’s disease diagnosis is based on clinical symptoms and signs in an individual who has a parent with proven Huntington’s disease and is confirmed by DNA tests.1 Typical neuroanatomic findings include initial neuronal loss in the striatum followed by a diffuse involvement of cortical and subcortical areas.10 Volume changes in the caudate nucleus and the putamen may be a reliable measure of Huntington’s disease and potentially serve as a biomarker.11
Psychiatric symptoms
Psychiatric symptoms frequently are evident in the early stages of Huntington’s disease, often before onset of motor symptoms.1 Depression is the most common sign, and can be difficult to diagnose because weight loss, apathy, and inactivity also occur in Huntington’s disease. Feelings of low self-esteem, guilt, and anxiety can help distinguish depression from symptoms of Huntington’s disease. Cognitive decline also may present before the first motor symptoms occur. Cognitive changes typically are related to executive functions and affected individuals may develop impairments in organization and planning. Psychotic symptoms may be present, but are more common in later stages of the disease.1
Ms. A reported that quetiapine seemed to lessen her choreiform movements, and dopamine receptor blocking agents (ie, antipsychotics) often are considered for managing chorea and psychosis in Huntington’s disease. However, there are few double-blind, placebo-controlled studies evaluating the efficacy of these agents.12 Small, uncontrolled, nonrandomized trials found quetiapine has some efficacy for both motor and psychiatric symptoms in Huntington’s disease.12-15
OUTCOME: Lost to follow-up
Ms. A is discharged from the hospital 3 days after she delivers her daughter and is given an appointment in 6 weeks at an affiliated movement disorders clinic. Before discharge, she is tested for the Huntington’s disease gene mutation with a plan to receive her results during her follow-up visit. During the informed consent process for the genetic testing, Ms. A states that she was tested previously and was quite sure that the test was positive for Huntington’s disease, although she could not recall where or when this testing was completed.
Ms. A also is scheduled to follow up with her obstetrician for a 6-week postpartum check-up and tubal ligation. We encourage Ms. A to make an appointment with her psychiatrist soon after discharge. We also make a referral to the Department of Family and Children Services to provide adequate support and resources to her and her children because of her physical and psychiatric issues.
Ms. A does not show up for her follow-up appointment at the movement disorders clinic. The genetic test is not completed during this admission because of a clerical error, and the serum sample subsequently expires.
The authors’ observations
Although Huntington’s disease is the most likely cause of Ms. A’s presentation, we were unable to confirm the diagnosis with genetic testing. If Ms. A returns to the neurology service and the genetic test is negative for Huntington’s disease, other causes of chorea must be investigated.
Related Resources
- De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000; 7(5):278-289.
- Revilla FJ, Grutzendler J, Larsh TR. Huntington disease. Medscape. http://emedicine.medscape.com/article/1150165-overview.
Drug Brand Names
- Hydralazine • Apresoline
- Lithium • Eskalith, Lithobid, others
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5(1):40.-
2. Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7:360-373.
3. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
4. Kafantaris V, Hirsch J, Saito E, et al. Treatment of withdrawal dyskinesia. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1102-1103.
5. Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197(4303):596-598.
6. Kranick SM, Mowry EM, Colcher A, et al. Movement disorders and pregnancy: a review of the literature. Mov Disord. 2010;25(6):665-671.
7. Ramachandran TS. Chorea gravidarum. Medscape. Available at: http://emedicine.medscape.com/article/1149725-overview. Accessed May 4 2011.
8. Panegyres PK, Goh JG. The neurology and natural history of patients with indeterminate CAG repeat length mutations of the Huntington disease gene. J Neurol Sci. 2011;301(1-2):14-20.
9. Shiwach R. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand. 1994;90:241-246.
10. De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000;7:278-289.
11. van den Bogaard SJ, Dumas EM, Acharya TP, et al. and the TRACK-HD Investigator Group. Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. J Neurol. 2011;258(3):412-420.
12. Frank S, Jankovic J. Advances in the pharmacological management of Huntington’s disease. Drugs. 2010;70(5):561-571.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Seitz DP, Millson RC. Quetiapine in the management of psychosis secondary to Huntington’s disease: a case report. Can J Psychiatry. 2004;49(6):413.-
15. Bonelli RM, Niederwieser G. Quetiapine in Huntington’s disease: a first case report. J Neurol. 2002;249(8):1114-1115.
1. Roos RA. Huntington’s disease: a clinical review. Orphanet J Rare Dis. 2010;5(1):40.-
2. Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7:360-373.
3. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
4. Kafantaris V, Hirsch J, Saito E, et al. Treatment of withdrawal dyskinesia. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1102-1103.
5. Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197(4303):596-598.
6. Kranick SM, Mowry EM, Colcher A, et al. Movement disorders and pregnancy: a review of the literature. Mov Disord. 2010;25(6):665-671.
7. Ramachandran TS. Chorea gravidarum. Medscape. Available at: http://emedicine.medscape.com/article/1149725-overview. Accessed May 4 2011.
8. Panegyres PK, Goh JG. The neurology and natural history of patients with indeterminate CAG repeat length mutations of the Huntington disease gene. J Neurol Sci. 2011;301(1-2):14-20.
9. Shiwach R. Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand. 1994;90:241-246.
10. De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000;7:278-289.
11. van den Bogaard SJ, Dumas EM, Acharya TP, et al. and the TRACK-HD Investigator Group. Early atrophy of pallidum and accumbens nucleus in Huntington’s disease. J Neurol. 2011;258(3):412-420.
12. Frank S, Jankovic J. Advances in the pharmacological management of Huntington’s disease. Drugs. 2010;70(5):561-571.
13. Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47(1):70-72.
14. Seitz DP, Millson RC. Quetiapine in the management of psychosis secondary to Huntington’s disease: a case report. Can J Psychiatry. 2004;49(6):413.-
15. Bonelli RM, Niederwieser G. Quetiapine in Huntington’s disease: a first case report. J Neurol. 2002;249(8):1114-1115.