User login
Atrial fibrillation: Effective strategies using the latest tools
Atrial fibrillation (AF)—the most common supraventricular tachycardia—affects as many as 6.1 million adults in the United States.1 It is associated with a 5-fold increased risk of stroke,2 a 3-fold increased risk of heart failure (HF),3 and about a 2-fold increased risk of dementia4 and mortality.2 The prevalence of AF increases with maturity, from 2% in people <65 years of age to 9% in those ≥65 years,5 and that prevalence is expected to double over the next 25 years as the population ages.1
The primary goals of treatment are to alleviate symptoms and prevent thromboembolism. Strokes related to AF are more likely to result in severe disability or death when compared with those unrelated to AF.6 And yet anticoagulation remains underutilized.7
The net clinical benefit of oral anticoagulation appears to be greatest in patients with the highest risk of bleeding, since these patients are also at the highest risk for stroke.8 Patients at increased risk of stroke are more likely to receive oral anticoagulation; however, for unknown reasons, more than half of people with the highest risk of stroke are not prescribed these important anti-blood-clotting medications.7 One theory is that physicians may be relying on their gut rather than objective risk scores, and underuse of validated schemata leads to poor estimation of risk.
For example, results from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) trial, which involved over 10,000 people with AF, found that although 72% (n=7251) had high-risk CHADS2 scores (≥2), only 16% were assessed as having a high risk of stroke by physicians.9 Along the same lines, a recent study of Canadian primary care physicians showed that stroke risk and bleeding risk were not evaluated with validated tools in 58% and 81% of patients, respectively, leading to both significant underestimation and overestimation of risk.10
This review provides the tools to identify when anticoagulation is indicated, reports the advantages and disadvantages of the currently available anticoagulants, and discusses the selection and implementation of rate- vs rhythm-control strategies. But first, a word about the etiology, classification, and diagnosis of AF.
AF: The result of any number of cardiac and non-cardiac causes
AF is characterized by uncoordinated activation of the atria, which results in ineffective atrial contractions and an irregular, often rapid, ventricular response. It is the ultimate clinical manifestation of multiple diseases that alter atrial tissue through inflammation, fibrosis, or hypertrophy.5 The most common causes are hypertension, coronary artery disease, HF, cardiomyopathies, and valvular heart disease, all of which stimulate the renin-angiotensin-aldosterone system, leading to increased susceptibility to arrhythmia.5 Atrial ectopic tachycardia, Wolff-Parkinson-White (WPW) syndrome, and atrioventricular (AV) nodal reentrant tachycardia also may precipitate AF.5 In these cases, AF usually resolves after catheter ablation (CA) of the primary arrhythmia.11 Unrecognized AF may trigger atrial flutter, and more than 80% of patients who undergo radiofrequency ablation for atrial flutter experience AF at some point in the subsequent 5 years.12
Non-cardiac causes of AF include sleep apnea, obesity, hyperthyroidism, drugs, electrocution, pneumonia, and pulmonary embolism.5 An association between binge drinking and AF (“holiday heart syndrome”) has long been recognized. The evidence now suggests that alcohol increases the risk of AF in a dose-dependent manner with intakes of ≥1 drink per day (12 g per drink).13
Classification schema no longer includes “lone AF”
AF is classified in terms of the duration of episodes:5
- Paroxysmal AF is characterized by brief episodes that terminate spontaneously or with intervention within 7 days of onset. These episodes recur with variable frequency.
- Persistent AF refers to AF that is continuously sustained for more than 7 days.
- Longstanding persistent AF refers to continuous AF that lasts longer than 12 months.
- Permanent AF is not an inherent pathophysiologic attribute of AF, but rather an acceptance of AF where the patient and physician abandon further efforts to restore and/or maintain sinus rhythm.
- Nonvalvular AF occurs in the absence of a valve replacement (mechanical or bioprosthetic), rheumatic mitral stenosis, or mitral valve repair.
Although paroxysmal and persistent AF may occur in the same individual, the distinction is still clinically relevant, as outcomes of certain therapies, such as CA, are superior in patients with paroxysmal AF.14 With a more complete understanding of AF pathophysiology, guidelines now discourage use of the potentially confusing term “lone AF,” which has historically been applied to younger patients with no known clinical risk factors or echocardiographic abnormalities. As a result, therapeutic decisions are no longer based on this nomenclature, according to the 2014 AF practice guideline from the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS).5
Patient complaints—or incidental findings—can prompt a Dx
Fatigue is the most common symptom of AF. Other signs and symptoms include palpitations, dyspnea, HF, hypotension, syncope, chest pain, and stroke. Some patients are asymptomatic, and AF is an incidental finding when an irregular pulse is discovered during a physical examination. The diagnosis is confirmed by electrocardiogram (EKG), telemetry, Holter monitor, event recorder, or an implanted electrocardiographic recording device. A chest x-ray, serum electrolyte levels, a complete blood count, thyroid testing, and renal and hepatic function testing are recommended. Transthoracic echocardiography to measure cardiac function, detect underlying structural heart disease, and evaluate atrial size is essential.5
An electrophysiologic (EP) study may be needed for diagnosis or treatment if another arrhythmia is present. Aberrant conduction may cause AF to present as a wide complex tachycardia and be mislabeled as ventricular tachycardia. The presence of delta waves is an indication for an EP study targeting the WPW accessory pathway. Transesophageal echocardiography (TEE) is the most sensitive and specific test for left atrial thrombi. If you are considering a TEE for a patient with AF of unknown, or >48 hours’, duration who has not been anticoagulated in the preceding 3 weeks, obtain it before performing cardioversion because of the risk of embolism.5
Stroke prevention
The ACC/AHA/HRS AF guideline recommends basing anticoagulation decisions on thromboembolic risk, regardless of AF pattern (paroxysmal, persistent, or permanent) (Class I recommendation).5 For patients with nonvalvular AF and atrial flutter, the guideline recommends using the Birmingham 2009 schema (CHA2DS2-VASc score) (TABLE 115-18) to estimate thromboembolic risk.5,15 CHA2DS2-VASc improves on the older CHADS2 score by significantly reducing the number of patients categorized as having intermediate risk and better identifying truly low-risk patients who are unlikely to benefit from anticoagulation.16,17,19
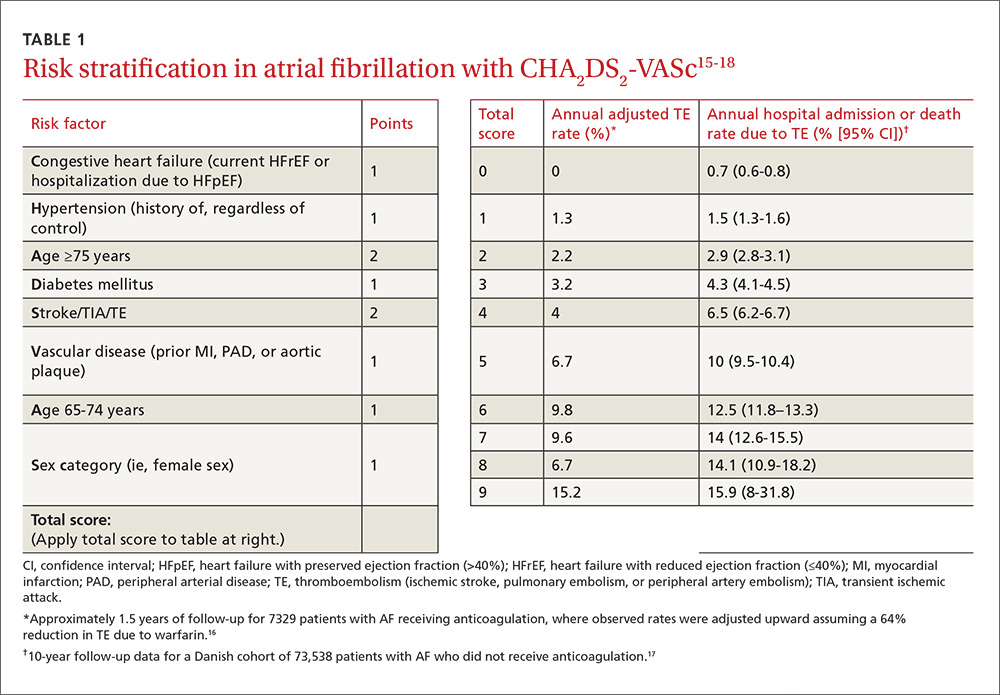
Men with a CHA2DS2-VASc score of zero and women with a score of one do not need anticoagulation.5,20 Discuss the risks and benefits of oral anticoagulation with men who have a score of one. In these intermediate-risk men, antiplatelet therapy with aspirin and/or clopidogrel may be reasonable, especially if there is an indication other than stroke prevention (eg, post-myocardial infarction). Oral anticoagulation is strongly recommended for all patients with a CHA2DS2-VASc score of 2 or higher.5,18,21,22
Anticoagulant considerations: Warfarin vs DOACs
Warfarin was the gold standard for stroke prevention in nonvalvular AF until the direct oral anticoagulants (DOACs) became available in 2010. Guidelines in the United States and the United Kingdom recommend shared decision-making to help patients with AF who do not have a specific indication for warfarin choose between warfarin and the DOACs.5,21 Canadian and European guidelines recommend DOACs as the first-line option for anticoagulation and reserve warfarin for patients who have contraindications to, or are unable to afford, DOACs.18,22 All current guidelines recommend continuing warfarin in patients who are stable, well controlled, and satisfied with warfarin therapy and the monitoring and dietary restrictions it entails.
DOACs are as effective as warfarin. All of the DOACs are approved for stroke prevention based on individual phase III non-inferiority trials in which they were compared to warfarin.23-26 In addition, a meta-analysis of these 4 trials involving a total of 71,683 patients (mean age 70-73 years; median follow-up, 1.8-2.8 years) evaluated the benefits and risks of the 4 DOACs against the former gold standard.27
Higher doses of the DOACs (dabigatran 150 mg BID, rivaroxaban 20 mg/d, edoxaban 60 mg/d, and apixaban 5 mg BID) reduced the rates of stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; P<.0001; number needed to treat [NNT]=147), hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; P<.0001; NNT=219), and all-cause mortality (RR=0.90; 95% CI, 0.85-0.95; P=.0003; NNT=128), compared with warfarin.27 It is important to note that while lower doses of some DOACs (dabigatran 110 mg BID and edoxaban 30 mg/d) were not as effective at preventing ischemic stroke when compared with warfarin (RR=1.3; 95% CI, 1-1.6; P=.045), they still significantly reduced hemorrhagic stroke (RR=0.33; 95% CI, 0.23-0.46; P<.0001) and all-cause mortality (RR=0.89; 95% CI, 0.83-0.96; P=.003).
Of course, the biggest concern is bleeding. In that same meta-analysis, the difference in major bleeding events with DOACs vs warfarin was not statistically significant (RR=0.86; 95% CI, 0.73-1; P=.06). While DOACs likely lower rates of intracranial hemorrhage (RR=0.48; 95% CI, 0.39-0.59; P<.0001; NNT=132), they seem to increase the risk of gastrointestinal (GI) bleeding (RR=1.3; 95% CI, 1-1.6; P=.043; number needed to harm [NNH]=185).27
There was significant heterogeneity in the GI bleeding outcome, however. When compared with warfarin, GI bleeding was increased by dabigatran 150 mg BID (RR=1.5; 95% CI, 1.2-1.9; P<.001) and edoxaban 60 mg/d (HR=1.2; 95% CI, 1.02-1.5; P=.03), but there were no significant differences for dabigatran 110 mg BID or apixaban 5 mg BID.23,25,26
On the other hand, edoxaban 30 mg/d had a lower risk of GI bleeding when compared with warfarin (HR=0.67; 95% CI, 0.53-0.83; P<.001).25 Without head-to-head trials, it is impossible to know if one DOAC is superior to another. Apixaban 5 mg BID appears to offer the best overall balance between efficacy and safety. Other DOACs may be better options for patients who have specific concerns regarding efficacy or safety.28,29
Convenience, interactions, and cost may be the deciding factors. Since all DOACs are fairly comparable in efficacy and safety, other factors such as convenience, interactions with other medications, and cost should be considered when deciding on a medication for an individual patient (TABLE 230,31). The DOACs require no lab monitoring or dose titration, and all 4 have fewer potential drug interactions than warfarin.30 Due to their relatively short half-lives, strict adherence is critical; DOACs are not suitable for patients who frequently miss doses.5 (For more information on starting or switching to DOACs, see, “Is a novel anticoagulant right for your patient?” J Fam Pract. 2014;63:22-28.)

A word about DOACs and renal impairment. Another concern with DOACs is their reliance on renal metabolism and excretion. A meta-analysis of the 4 phase III trials of the DOACs, this time involving 58,338 patients, evaluated DOAC efficacy and safety compared to warfarin in the presence of kidney dysfunction.32 Renal function was categorized as normal (estimated glomerular filtration rate [eGFR] >80 mL/min/1.73 m2), mildly impaired (eGFR 50-80 mL/min/1.73 m2), or moderately impaired (eGFR <50 mL/min/1.73m2). Compared with warfarin, DOACs lowered stroke risk in patients with mild (RR=0.71; 95% CI, 0.62-0.81) or moderate (RR=0.79; 95% CI, 0.66-0.94) renal impairment. DOACs also reduced major bleeding compared to warfarin in patients with mild (RR=0.88; 95% CI, 0.80-0.97) or moderate (RR=0.80; 95% CI, 0.66-0.94) renal impairment. How the DOACs fare in patients with severe renal dysfunction could not be determined because such patients were excluded from the trials.
Keep in mind that the DOACs require dose adjustment at different levels of renal impairment (TABLE 230,31), and warfarin remains the only recommended treatment for patients with severe renal impairment, according to both AHA/ACC/HRS and European Society of Cardiology guidelines.5,18
Tools to help assess patients’ bleeding risk
Of the available scoring mechanisms to identify risk factors for bleeding, 3 have been specifically validated in AF populations (ie, ATRIA,33 HEMORR2HAGES,34 and HAS-BLED35). Of the 3, HAS-BLED is superior,36 the most practical, and recommended by expert guidelines.18,21,22 Additionally, HAS-BLED has good correlation with intracranial hemorrhage risk. The HAS-BLED score ranges from 0 to 9 points with one point assigned for each of the following:35
- Hypertension–uncontrolled with systolic BP >160 mm Hg
- Abnormal liver function–cirrhosis, bilirubin >2× normal, or liver enzymes >3× normal
- Abnormal renal function–dialysis, transplant, or serum creatinine >2.26 mg/dL
- Stroke history–including lacunar infarcts
- Bleeding predisposition–history of major bleeding due to any cause
- Labile international normalized ratio (INR)–time in therapeutic range <60%
- Elderly–age >65 years
- Drug–antiplatelet agents, including nonsteroidal anti-inflammatory drugs
- Alcohol usage–>8 drinks per week.
Patients with a HAS-BLED score ≥3 warrant additional monitoring and attempts to reduce bleeding risk by addressing modifiable risk factors. Bleeding risk scores should not be used to exclude patients from anticoagulation therapy.5 In fact, the British National Institute for Health and Clinical Excellence (NICE) guidelines state that anticoagulation should not be withheld solely due to fall risk.21
Also, anticoagulation with warfarin should not be permanently discontinued because of a single GI bleed, since restarting warfarin is associated with decreased risks of thromboembolism and mortality and a statistically insignificant increase in recurrent GI bleeding.37 Restarting DOAC therapy following a GI bleed has not been evaluated in clinical trials; however, it may be reasonable to use one of the DOAC doses with a lower risk of GI bleeding (dabigatran 110 mg BID, apixaban 5 mg BID, or edoxaban 30 mg/d) in patients who have experienced a GI bleed on warfarin or another DOAC.18,22
An online calculator is available that uses CHA2DS2-VASc and HAS-BLED scores to determine an individual’s risk/benefit profile with the various anticoagulation strategies available (http://www.sparctool.com). Consider percutaneous left atrial appendage occlusion if the risks of anticoagulation truly exceed the benefits.38
Rate control vs rhythm control
Most patients who present with AF require immediate ventricular rate control to reduce symptoms. In the acute setting, this can be accomplished with intravenous (IV) beta-blockers or IV calcium channel antagonists.5,39 If the patient is hemodynamically unstable, urgent direct-current cardioversion is the preferred treatment strategy and should not be delayed pending anticoagulation. IV amiodarone can be used in the ICU patient who does not require cardioversion, but is unable to tolerate beta-blockers or calcium channel antagonists.40 Once the patient is stable, long-term treatment focuses on ventricular rate control or restoration and maintenance of sinus rhythm.
The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial enrolled 4060 patients (mean age 70 years, mean follow-up 3.5 years) with paroxysmal and persistent AF and randomized them to either pharmacologic rate control or rhythm control.41 No significant differences were found in all-cause mortality or in the composite secondary endpoint of death, ischemic stroke, anoxic encephalopathy, major bleeding, or cardiac arrest. In addition, no significant differences emerged in quality of life or global functional status. The number of patients requiring hospitalization during follow-up was significantly lower in the rate-control group vs the rhythm-control group (73% vs 80%; P<.001). Anticoagulation was encouraged but not mandated in the rhythm-control group after 4 weeks in sinus rhythm, and there was a trend toward higher mortality in the rhythm-control group (27% vs 26%; P=.08).
Patients <65 years were excluded from the AFFIRM trial. When younger patients experience significant symptoms, early referral to Cardiology should be considered to discuss the long-term benefits and risks of a rhythm-control strategy. Regardless of age, when patients remain symptomatic despite rate- or rhythm-control management, the strategy should be changed.5
Rate-control targets and options
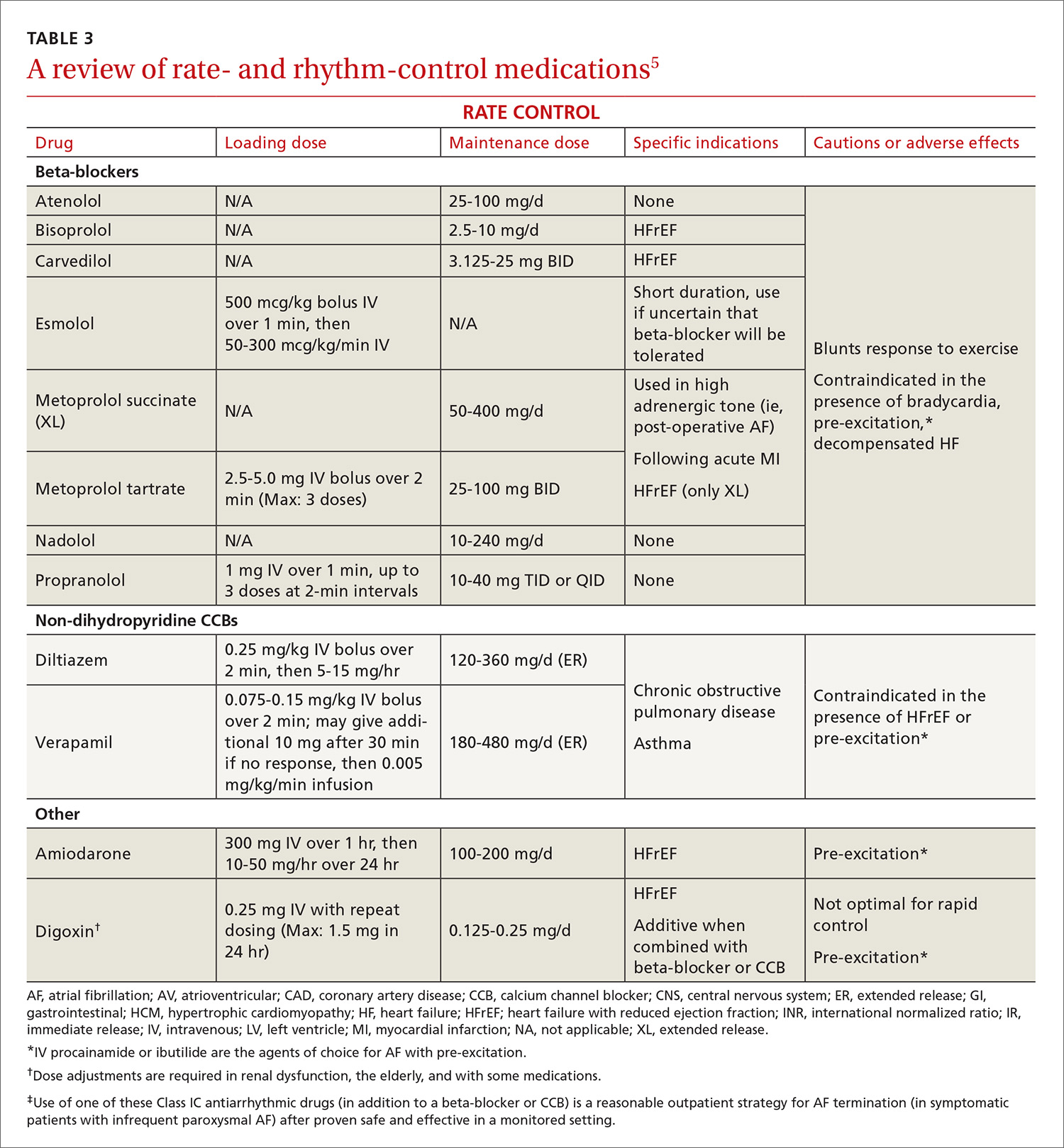
Target heart rates should be individualized. The 2014 ACC/AHA/HRS guideline recommends a resting target heart rate <80 beats per minute (bpm) in symptomatic patients.5 In patients with permanent AF who remain asymptomatic at higher resting heart rates, a more lenient rate-control strategy (resting heart rate <110 bpm) has demonstrated outcomes equivalent to those of a more strict approach (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm).42 Pharmacologic rate-control options include beta-blockers, non-dihydropyridine calcium channel antagonists, and digoxin (TABLE 35). Digoxin is associated with increased all-cause mortality in patients with AF regardless of HF status (HR=1.4; 95% CI, 1.2-1.6, P=.0001).43 Digoxin should be reserved for patients who are sedentary or have inadequate control with first-line medications.5
Indications for rhythm control
The NICE guidelines, which are consistent with the ACC/AHA/HRS guidelines, recommend rate control as the first-line strategy for AF management, except in people:21
- whose AF has a reversible cause
- who have HF believed to be primarily caused by AF
- with new-onset AF
- with atrial flutter that is considered suitable for an ablation strategy to restore sinus rhythm
- for whom a rhythm-control strategy would be more suitable based on clinical judgment.
In addition, patients who continue to experience symptomatic AF despite an adequate trial of rate control should be offered rhythm control.5
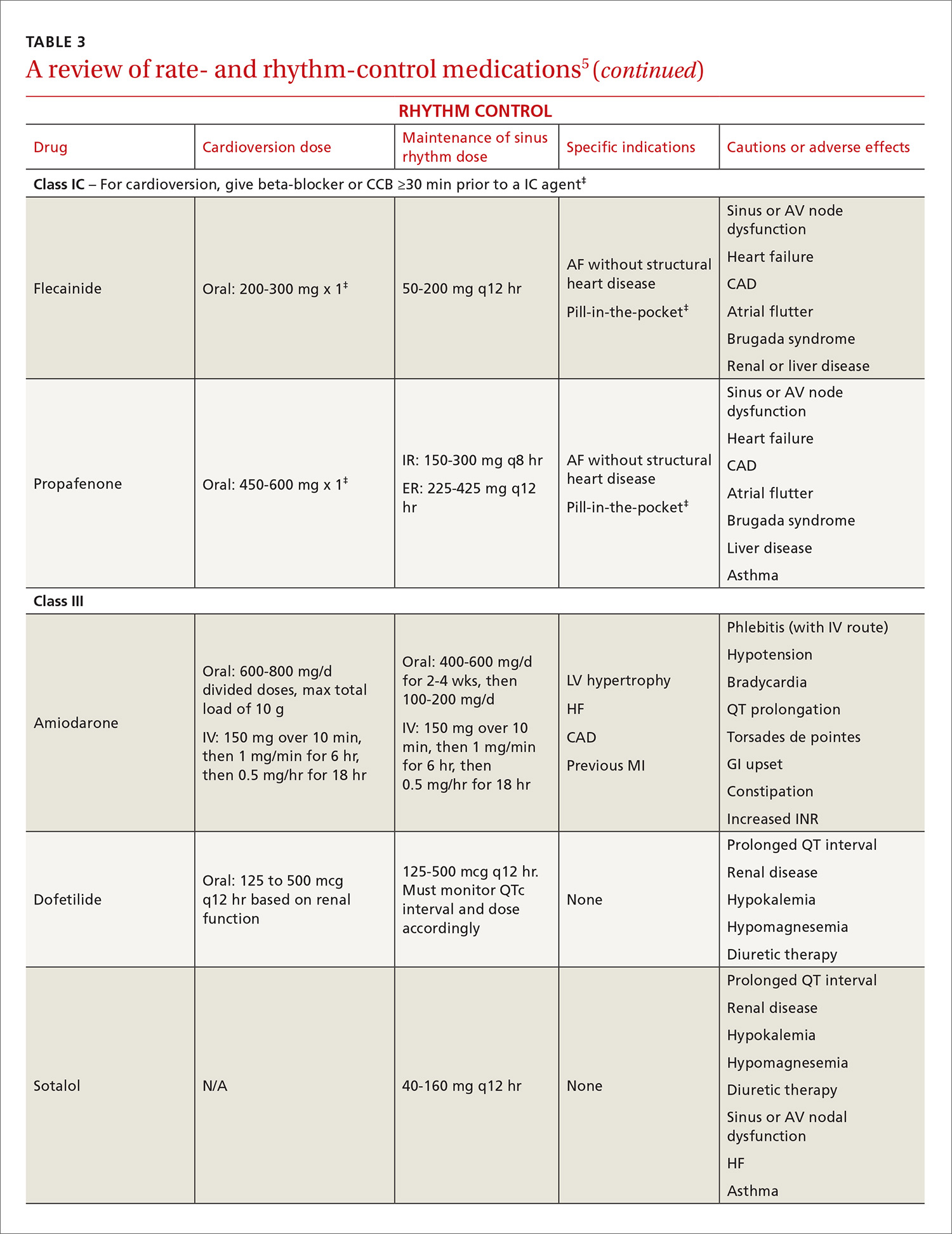
Pharmacologic rhythm-control strategies. Antiarrhythmic drugs can be used for chemical cardioversion, reduction of paroxysms, and long-term maintenance of sinus rhythm. The most commonly used antiarrhythmic drugs are Class IC and Class III agents (TABLE 3).5 Tailored drug selection for each patient is key. Patients with left atrial diameters >4.5 cm are less likely to remain in sinus rhythm, and patients with left ventricular hypertrophy are at increased risk for proarrhythmic adverse effects.44 Patients with paroxysmal AF may be candidates for a “pill-in-the-pocket” strategy using propafenone or flecainide.5
AF frequently progresses from paroxysmal to persistent and can subsequently result in electrical and structural remodeling that becomes irreversible over time.45 The patient with uncontrolled symptoms despite attempts at rate control and rhythm control should be promptly referred to an electrophysiologist.
Surgical interventions for rate or rhythm control
Electrophysiology interventions include AV nodal ablation with pacemaker placement for rate control, or catheter-directed ablation (radiofrequency or cryotherapy) for rhythm control. CA appears to be more effective than pharmacologic rhythm control.46,47 Treatment with CA is indicated for symptomatic paroxysmal AF when a rhythm-control strategy is desired and the AF is refractory to, or the patient is intolerant of, at least one class I or III antiarrhythmic medication.5 With these same caveats, CA is a reasonable strategy for symptomatic persistent AF.
Consider more invasive interventions, such as an atrial maze procedure, when patients require cardiac surgery for another indication. Patients with an increased risk of thromboembolism (based on CHA2DS2-VASc) remain at high risk even after successful ablation.48 As a result, some guidelines recommend continued long-term anticoagulation following CA.18,22
CORRESPONDENCE
Philip Dooley, MD, University of Kansas School of Medicine–Wichita Family Medicine Residency at Via Christi, 707 North Emporia, Wichita, KS 67207; [email protected].
ACKNOWLEDGMENTS
We thank Professor Anne Walling, MB, ChB, FFPHM, Department of Family and Community Medicine, University of Kansas School of Medicine–Wichita for her suggestions and critical review of an earlier version of this manuscript.
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375.
2. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N.
3. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476-484.
4. Ott A, Breteler MMB, de Bruyne MC, et al. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke. 1997;28:316-321.
5. January CT, Wann L, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76.
6. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. Stroke. 1996;27:1760-1764.
7. Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55-62.
8. Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739-749.
9. Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005-2012.
10. Angaran P, Dorian P, Tan MK, et al. The risk stratification and stroke prevention therapy care gap in Canadian atrial fibrillation patients. Can J Cardiol. 2016;32:336-343.
11. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779-786.
12. Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799-802.
13. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281-289.
14. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171-257.
15. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263-272.
16. Lip GYH, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731-2738.
17. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124.
18. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747.
19. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172-1179.
20. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522.
21. National Institute for Health and Clinical Excellence (NICE). Atrial fibrillation: the management of atrial fibrillation [CG180]. 2014. Available at: https://www.nice.org.uk/guidance/cg180. Accessed July 31, 2016.
22. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114-1130.
23. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
24. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
25. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
26. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
27. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
28. Morimoto T, Crawford B, Wada K, et al. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466-474.
29. Verdecchia P, Angeli F, Bartolini C, et al. Safety and efficacy of non-vitamin K oral anticoagulants in non-valvular atrial fibrillation: a Bayesian meta-analysis approach. Expert Opin Drug Saf. 2015;14:7-20.
30. Micromedex® 2.0 (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com. Accessed August 18, 2016.
31. GoodRx. Available at: https://www.goodrx.com. Accessed August 18, 2016.
32. Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69-75.
33. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401.
34. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719.
35. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100.
36. Zhu W, He W, Guo L, et al. The HAS-BLED Score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561.
37. Chai-Adisaksopha C, Hillis C, Monreal M, et al. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819-825.
38. Xu H, Xie X, Wang B, et al. Efficacy and safety of percutaneous left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation: a meta-analysis of contemporary studies. Heart Lung Circ. 2016;25:1107-1117.
39. Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174-2179.
40. Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594-598.
41. The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
42. Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
43. Wang ZQ, Zhang R, Chen MT, et al. Digoxin is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: a meta-analysis. J Cardiovasc Pharmacol. 2015;66:270-275.
44. Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026-2033.
45. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation: clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725-731.
46. Cheng X, Li X, He Y, et al. Catheter ablation versus anti-arrhythmic drug therapy for the management of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. 2014;41:267-272.
47. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637-1644.
48.
Atrial fibrillation (AF)—the most common supraventricular tachycardia—affects as many as 6.1 million adults in the United States.1 It is associated with a 5-fold increased risk of stroke,2 a 3-fold increased risk of heart failure (HF),3 and about a 2-fold increased risk of dementia4 and mortality.2 The prevalence of AF increases with maturity, from 2% in people <65 years of age to 9% in those ≥65 years,5 and that prevalence is expected to double over the next 25 years as the population ages.1
The primary goals of treatment are to alleviate symptoms and prevent thromboembolism. Strokes related to AF are more likely to result in severe disability or death when compared with those unrelated to AF.6 And yet anticoagulation remains underutilized.7
The net clinical benefit of oral anticoagulation appears to be greatest in patients with the highest risk of bleeding, since these patients are also at the highest risk for stroke.8 Patients at increased risk of stroke are more likely to receive oral anticoagulation; however, for unknown reasons, more than half of people with the highest risk of stroke are not prescribed these important anti-blood-clotting medications.7 One theory is that physicians may be relying on their gut rather than objective risk scores, and underuse of validated schemata leads to poor estimation of risk.

For example, results from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) trial, which involved over 10,000 people with AF, found that although 72% (n=7251) had high-risk CHADS2 scores (≥2), only 16% were assessed as having a high risk of stroke by physicians.9 Along the same lines, a recent study of Canadian primary care physicians showed that stroke risk and bleeding risk were not evaluated with validated tools in 58% and 81% of patients, respectively, leading to both significant underestimation and overestimation of risk.10
This review provides the tools to identify when anticoagulation is indicated, reports the advantages and disadvantages of the currently available anticoagulants, and discusses the selection and implementation of rate- vs rhythm-control strategies. But first, a word about the etiology, classification, and diagnosis of AF.
AF: The result of any number of cardiac and non-cardiac causes
AF is characterized by uncoordinated activation of the atria, which results in ineffective atrial contractions and an irregular, often rapid, ventricular response. It is the ultimate clinical manifestation of multiple diseases that alter atrial tissue through inflammation, fibrosis, or hypertrophy.5 The most common causes are hypertension, coronary artery disease, HF, cardiomyopathies, and valvular heart disease, all of which stimulate the renin-angiotensin-aldosterone system, leading to increased susceptibility to arrhythmia.5 Atrial ectopic tachycardia, Wolff-Parkinson-White (WPW) syndrome, and atrioventricular (AV) nodal reentrant tachycardia also may precipitate AF.5 In these cases, AF usually resolves after catheter ablation (CA) of the primary arrhythmia.11 Unrecognized AF may trigger atrial flutter, and more than 80% of patients who undergo radiofrequency ablation for atrial flutter experience AF at some point in the subsequent 5 years.12
Non-cardiac causes of AF include sleep apnea, obesity, hyperthyroidism, drugs, electrocution, pneumonia, and pulmonary embolism.5 An association between binge drinking and AF (“holiday heart syndrome”) has long been recognized. The evidence now suggests that alcohol increases the risk of AF in a dose-dependent manner with intakes of ≥1 drink per day (12 g per drink).13
Classification schema no longer includes “lone AF”
AF is classified in terms of the duration of episodes:5
- Paroxysmal AF is characterized by brief episodes that terminate spontaneously or with intervention within 7 days of onset. These episodes recur with variable frequency.
- Persistent AF refers to AF that is continuously sustained for more than 7 days.
- Longstanding persistent AF refers to continuous AF that lasts longer than 12 months.
- Permanent AF is not an inherent pathophysiologic attribute of AF, but rather an acceptance of AF where the patient and physician abandon further efforts to restore and/or maintain sinus rhythm.
- Nonvalvular AF occurs in the absence of a valve replacement (mechanical or bioprosthetic), rheumatic mitral stenosis, or mitral valve repair.
Although paroxysmal and persistent AF may occur in the same individual, the distinction is still clinically relevant, as outcomes of certain therapies, such as CA, are superior in patients with paroxysmal AF.14 With a more complete understanding of AF pathophysiology, guidelines now discourage use of the potentially confusing term “lone AF,” which has historically been applied to younger patients with no known clinical risk factors or echocardiographic abnormalities. As a result, therapeutic decisions are no longer based on this nomenclature, according to the 2014 AF practice guideline from the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS).5
Patient complaints—or incidental findings—can prompt a Dx
Fatigue is the most common symptom of AF. Other signs and symptoms include palpitations, dyspnea, HF, hypotension, syncope, chest pain, and stroke. Some patients are asymptomatic, and AF is an incidental finding when an irregular pulse is discovered during a physical examination. The diagnosis is confirmed by electrocardiogram (EKG), telemetry, Holter monitor, event recorder, or an implanted electrocardiographic recording device. A chest x-ray, serum electrolyte levels, a complete blood count, thyroid testing, and renal and hepatic function testing are recommended. Transthoracic echocardiography to measure cardiac function, detect underlying structural heart disease, and evaluate atrial size is essential.5
An electrophysiologic (EP) study may be needed for diagnosis or treatment if another arrhythmia is present. Aberrant conduction may cause AF to present as a wide complex tachycardia and be mislabeled as ventricular tachycardia. The presence of delta waves is an indication for an EP study targeting the WPW accessory pathway. Transesophageal echocardiography (TEE) is the most sensitive and specific test for left atrial thrombi. If you are considering a TEE for a patient with AF of unknown, or >48 hours’, duration who has not been anticoagulated in the preceding 3 weeks, obtain it before performing cardioversion because of the risk of embolism.5
Stroke prevention
The ACC/AHA/HRS AF guideline recommends basing anticoagulation decisions on thromboembolic risk, regardless of AF pattern (paroxysmal, persistent, or permanent) (Class I recommendation).5 For patients with nonvalvular AF and atrial flutter, the guideline recommends using the Birmingham 2009 schema (CHA2DS2-VASc score) (TABLE 115-18) to estimate thromboembolic risk.5,15 CHA2DS2-VASc improves on the older CHADS2 score by significantly reducing the number of patients categorized as having intermediate risk and better identifying truly low-risk patients who are unlikely to benefit from anticoagulation.16,17,19

Men with a CHA2DS2-VASc score of zero and women with a score of one do not need anticoagulation.5,20 Discuss the risks and benefits of oral anticoagulation with men who have a score of one. In these intermediate-risk men, antiplatelet therapy with aspirin and/or clopidogrel may be reasonable, especially if there is an indication other than stroke prevention (eg, post-myocardial infarction). Oral anticoagulation is strongly recommended for all patients with a CHA2DS2-VASc score of 2 or higher.5,18,21,22
Anticoagulant considerations: Warfarin vs DOACs
Warfarin was the gold standard for stroke prevention in nonvalvular AF until the direct oral anticoagulants (DOACs) became available in 2010. Guidelines in the United States and the United Kingdom recommend shared decision-making to help patients with AF who do not have a specific indication for warfarin choose between warfarin and the DOACs.5,21 Canadian and European guidelines recommend DOACs as the first-line option for anticoagulation and reserve warfarin for patients who have contraindications to, or are unable to afford, DOACs.18,22 All current guidelines recommend continuing warfarin in patients who are stable, well controlled, and satisfied with warfarin therapy and the monitoring and dietary restrictions it entails.
DOACs are as effective as warfarin. All of the DOACs are approved for stroke prevention based on individual phase III non-inferiority trials in which they were compared to warfarin.23-26 In addition, a meta-analysis of these 4 trials involving a total of 71,683 patients (mean age 70-73 years; median follow-up, 1.8-2.8 years) evaluated the benefits and risks of the 4 DOACs against the former gold standard.27
Higher doses of the DOACs (dabigatran 150 mg BID, rivaroxaban 20 mg/d, edoxaban 60 mg/d, and apixaban 5 mg BID) reduced the rates of stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; P<.0001; number needed to treat [NNT]=147), hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; P<.0001; NNT=219), and all-cause mortality (RR=0.90; 95% CI, 0.85-0.95; P=.0003; NNT=128), compared with warfarin.27 It is important to note that while lower doses of some DOACs (dabigatran 110 mg BID and edoxaban 30 mg/d) were not as effective at preventing ischemic stroke when compared with warfarin (RR=1.3; 95% CI, 1-1.6; P=.045), they still significantly reduced hemorrhagic stroke (RR=0.33; 95% CI, 0.23-0.46; P<.0001) and all-cause mortality (RR=0.89; 95% CI, 0.83-0.96; P=.003).
Of course, the biggest concern is bleeding. In that same meta-analysis, the difference in major bleeding events with DOACs vs warfarin was not statistically significant (RR=0.86; 95% CI, 0.73-1; P=.06). While DOACs likely lower rates of intracranial hemorrhage (RR=0.48; 95% CI, 0.39-0.59; P<.0001; NNT=132), they seem to increase the risk of gastrointestinal (GI) bleeding (RR=1.3; 95% CI, 1-1.6; P=.043; number needed to harm [NNH]=185).27
There was significant heterogeneity in the GI bleeding outcome, however. When compared with warfarin, GI bleeding was increased by dabigatran 150 mg BID (RR=1.5; 95% CI, 1.2-1.9; P<.001) and edoxaban 60 mg/d (HR=1.2; 95% CI, 1.02-1.5; P=.03), but there were no significant differences for dabigatran 110 mg BID or apixaban 5 mg BID.23,25,26
On the other hand, edoxaban 30 mg/d had a lower risk of GI bleeding when compared with warfarin (HR=0.67; 95% CI, 0.53-0.83; P<.001).25 Without head-to-head trials, it is impossible to know if one DOAC is superior to another. Apixaban 5 mg BID appears to offer the best overall balance between efficacy and safety. Other DOACs may be better options for patients who have specific concerns regarding efficacy or safety.28,29
Convenience, interactions, and cost may be the deciding factors. Since all DOACs are fairly comparable in efficacy and safety, other factors such as convenience, interactions with other medications, and cost should be considered when deciding on a medication for an individual patient (TABLE 230,31). The DOACs require no lab monitoring or dose titration, and all 4 have fewer potential drug interactions than warfarin.30 Due to their relatively short half-lives, strict adherence is critical; DOACs are not suitable for patients who frequently miss doses.5 (For more information on starting or switching to DOACs, see, “Is a novel anticoagulant right for your patient?” J Fam Pract. 2014;63:22-28.)

A word about DOACs and renal impairment. Another concern with DOACs is their reliance on renal metabolism and excretion. A meta-analysis of the 4 phase III trials of the DOACs, this time involving 58,338 patients, evaluated DOAC efficacy and safety compared to warfarin in the presence of kidney dysfunction.32 Renal function was categorized as normal (estimated glomerular filtration rate [eGFR] >80 mL/min/1.73 m2), mildly impaired (eGFR 50-80 mL/min/1.73 m2), or moderately impaired (eGFR <50 mL/min/1.73m2). Compared with warfarin, DOACs lowered stroke risk in patients with mild (RR=0.71; 95% CI, 0.62-0.81) or moderate (RR=0.79; 95% CI, 0.66-0.94) renal impairment. DOACs also reduced major bleeding compared to warfarin in patients with mild (RR=0.88; 95% CI, 0.80-0.97) or moderate (RR=0.80; 95% CI, 0.66-0.94) renal impairment. How the DOACs fare in patients with severe renal dysfunction could not be determined because such patients were excluded from the trials.
Keep in mind that the DOACs require dose adjustment at different levels of renal impairment (TABLE 230,31), and warfarin remains the only recommended treatment for patients with severe renal impairment, according to both AHA/ACC/HRS and European Society of Cardiology guidelines.5,18
Tools to help assess patients’ bleeding risk
Of the available scoring mechanisms to identify risk factors for bleeding, 3 have been specifically validated in AF populations (ie, ATRIA,33 HEMORR2HAGES,34 and HAS-BLED35). Of the 3, HAS-BLED is superior,36 the most practical, and recommended by expert guidelines.18,21,22 Additionally, HAS-BLED has good correlation with intracranial hemorrhage risk. The HAS-BLED score ranges from 0 to 9 points with one point assigned for each of the following:35
- Hypertension–uncontrolled with systolic BP >160 mm Hg
- Abnormal liver function–cirrhosis, bilirubin >2× normal, or liver enzymes >3× normal
- Abnormal renal function–dialysis, transplant, or serum creatinine >2.26 mg/dL
- Stroke history–including lacunar infarcts
- Bleeding predisposition–history of major bleeding due to any cause
- Labile international normalized ratio (INR)–time in therapeutic range <60%
- Elderly–age >65 years
- Drug–antiplatelet agents, including nonsteroidal anti-inflammatory drugs
- Alcohol usage–>8 drinks per week.
Patients with a HAS-BLED score ≥3 warrant additional monitoring and attempts to reduce bleeding risk by addressing modifiable risk factors. Bleeding risk scores should not be used to exclude patients from anticoagulation therapy.5 In fact, the British National Institute for Health and Clinical Excellence (NICE) guidelines state that anticoagulation should not be withheld solely due to fall risk.21
Also, anticoagulation with warfarin should not be permanently discontinued because of a single GI bleed, since restarting warfarin is associated with decreased risks of thromboembolism and mortality and a statistically insignificant increase in recurrent GI bleeding.37 Restarting DOAC therapy following a GI bleed has not been evaluated in clinical trials; however, it may be reasonable to use one of the DOAC doses with a lower risk of GI bleeding (dabigatran 110 mg BID, apixaban 5 mg BID, or edoxaban 30 mg/d) in patients who have experienced a GI bleed on warfarin or another DOAC.18,22
An online calculator is available that uses CHA2DS2-VASc and HAS-BLED scores to determine an individual’s risk/benefit profile with the various anticoagulation strategies available (http://www.sparctool.com). Consider percutaneous left atrial appendage occlusion if the risks of anticoagulation truly exceed the benefits.38
Rate control vs rhythm control
Most patients who present with AF require immediate ventricular rate control to reduce symptoms. In the acute setting, this can be accomplished with intravenous (IV) beta-blockers or IV calcium channel antagonists.5,39 If the patient is hemodynamically unstable, urgent direct-current cardioversion is the preferred treatment strategy and should not be delayed pending anticoagulation. IV amiodarone can be used in the ICU patient who does not require cardioversion, but is unable to tolerate beta-blockers or calcium channel antagonists.40 Once the patient is stable, long-term treatment focuses on ventricular rate control or restoration and maintenance of sinus rhythm.
The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial enrolled 4060 patients (mean age 70 years, mean follow-up 3.5 years) with paroxysmal and persistent AF and randomized them to either pharmacologic rate control or rhythm control.41 No significant differences were found in all-cause mortality or in the composite secondary endpoint of death, ischemic stroke, anoxic encephalopathy, major bleeding, or cardiac arrest. In addition, no significant differences emerged in quality of life or global functional status. The number of patients requiring hospitalization during follow-up was significantly lower in the rate-control group vs the rhythm-control group (73% vs 80%; P<.001). Anticoagulation was encouraged but not mandated in the rhythm-control group after 4 weeks in sinus rhythm, and there was a trend toward higher mortality in the rhythm-control group (27% vs 26%; P=.08).
Patients <65 years were excluded from the AFFIRM trial. When younger patients experience significant symptoms, early referral to Cardiology should be considered to discuss the long-term benefits and risks of a rhythm-control strategy. Regardless of age, when patients remain symptomatic despite rate- or rhythm-control management, the strategy should be changed.5
Rate-control targets and options
Target heart rates should be individualized. The 2014 ACC/AHA/HRS guideline recommends a resting target heart rate <80 beats per minute (bpm) in symptomatic patients.5 In patients with permanent AF who remain asymptomatic at higher resting heart rates, a more lenient rate-control strategy (resting heart rate <110 bpm) has demonstrated outcomes equivalent to those of a more strict approach (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm).42 Pharmacologic rate-control options include beta-blockers, non-dihydropyridine calcium channel antagonists, and digoxin (TABLE 35). Digoxin is associated with increased all-cause mortality in patients with AF regardless of HF status (HR=1.4; 95% CI, 1.2-1.6, P=.0001).43 Digoxin should be reserved for patients who are sedentary or have inadequate control with first-line medications.5


Indications for rhythm control
The NICE guidelines, which are consistent with the ACC/AHA/HRS guidelines, recommend rate control as the first-line strategy for AF management, except in people:21
- whose AF has a reversible cause
- who have HF believed to be primarily caused by AF
- with new-onset AF
- with atrial flutter that is considered suitable for an ablation strategy to restore sinus rhythm
- for whom a rhythm-control strategy would be more suitable based on clinical judgment.
In addition, patients who continue to experience symptomatic AF despite an adequate trial of rate control should be offered rhythm control.5
Pharmacologic rhythm-control strategies. Antiarrhythmic drugs can be used for chemical cardioversion, reduction of paroxysms, and long-term maintenance of sinus rhythm. The most commonly used antiarrhythmic drugs are Class IC and Class III agents (TABLE 3).5 Tailored drug selection for each patient is key. Patients with left atrial diameters >4.5 cm are less likely to remain in sinus rhythm, and patients with left ventricular hypertrophy are at increased risk for proarrhythmic adverse effects.44 Patients with paroxysmal AF may be candidates for a “pill-in-the-pocket” strategy using propafenone or flecainide.5
AF frequently progresses from paroxysmal to persistent and can subsequently result in electrical and structural remodeling that becomes irreversible over time.45 The patient with uncontrolled symptoms despite attempts at rate control and rhythm control should be promptly referred to an electrophysiologist.
Surgical interventions for rate or rhythm control
Electrophysiology interventions include AV nodal ablation with pacemaker placement for rate control, or catheter-directed ablation (radiofrequency or cryotherapy) for rhythm control. CA appears to be more effective than pharmacologic rhythm control.46,47 Treatment with CA is indicated for symptomatic paroxysmal AF when a rhythm-control strategy is desired and the AF is refractory to, or the patient is intolerant of, at least one class I or III antiarrhythmic medication.5 With these same caveats, CA is a reasonable strategy for symptomatic persistent AF.
Consider more invasive interventions, such as an atrial maze procedure, when patients require cardiac surgery for another indication. Patients with an increased risk of thromboembolism (based on CHA2DS2-VASc) remain at high risk even after successful ablation.48 As a result, some guidelines recommend continued long-term anticoagulation following CA.18,22
CORRESPONDENCE
Philip Dooley, MD, University of Kansas School of Medicine–Wichita Family Medicine Residency at Via Christi, 707 North Emporia, Wichita, KS 67207; [email protected].
ACKNOWLEDGMENTS
We thank Professor Anne Walling, MB, ChB, FFPHM, Department of Family and Community Medicine, University of Kansas School of Medicine–Wichita for her suggestions and critical review of an earlier version of this manuscript.
Atrial fibrillation (AF)—the most common supraventricular tachycardia—affects as many as 6.1 million adults in the United States.1 It is associated with a 5-fold increased risk of stroke,2 a 3-fold increased risk of heart failure (HF),3 and about a 2-fold increased risk of dementia4 and mortality.2 The prevalence of AF increases with maturity, from 2% in people <65 years of age to 9% in those ≥65 years,5 and that prevalence is expected to double over the next 25 years as the population ages.1
The primary goals of treatment are to alleviate symptoms and prevent thromboembolism. Strokes related to AF are more likely to result in severe disability or death when compared with those unrelated to AF.6 And yet anticoagulation remains underutilized.7
The net clinical benefit of oral anticoagulation appears to be greatest in patients with the highest risk of bleeding, since these patients are also at the highest risk for stroke.8 Patients at increased risk of stroke are more likely to receive oral anticoagulation; however, for unknown reasons, more than half of people with the highest risk of stroke are not prescribed these important anti-blood-clotting medications.7 One theory is that physicians may be relying on their gut rather than objective risk scores, and underuse of validated schemata leads to poor estimation of risk.

For example, results from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) trial, which involved over 10,000 people with AF, found that although 72% (n=7251) had high-risk CHADS2 scores (≥2), only 16% were assessed as having a high risk of stroke by physicians.9 Along the same lines, a recent study of Canadian primary care physicians showed that stroke risk and bleeding risk were not evaluated with validated tools in 58% and 81% of patients, respectively, leading to both significant underestimation and overestimation of risk.10
This review provides the tools to identify when anticoagulation is indicated, reports the advantages and disadvantages of the currently available anticoagulants, and discusses the selection and implementation of rate- vs rhythm-control strategies. But first, a word about the etiology, classification, and diagnosis of AF.
AF: The result of any number of cardiac and non-cardiac causes
AF is characterized by uncoordinated activation of the atria, which results in ineffective atrial contractions and an irregular, often rapid, ventricular response. It is the ultimate clinical manifestation of multiple diseases that alter atrial tissue through inflammation, fibrosis, or hypertrophy.5 The most common causes are hypertension, coronary artery disease, HF, cardiomyopathies, and valvular heart disease, all of which stimulate the renin-angiotensin-aldosterone system, leading to increased susceptibility to arrhythmia.5 Atrial ectopic tachycardia, Wolff-Parkinson-White (WPW) syndrome, and atrioventricular (AV) nodal reentrant tachycardia also may precipitate AF.5 In these cases, AF usually resolves after catheter ablation (CA) of the primary arrhythmia.11 Unrecognized AF may trigger atrial flutter, and more than 80% of patients who undergo radiofrequency ablation for atrial flutter experience AF at some point in the subsequent 5 years.12
Non-cardiac causes of AF include sleep apnea, obesity, hyperthyroidism, drugs, electrocution, pneumonia, and pulmonary embolism.5 An association between binge drinking and AF (“holiday heart syndrome”) has long been recognized. The evidence now suggests that alcohol increases the risk of AF in a dose-dependent manner with intakes of ≥1 drink per day (12 g per drink).13
Classification schema no longer includes “lone AF”
AF is classified in terms of the duration of episodes:5
- Paroxysmal AF is characterized by brief episodes that terminate spontaneously or with intervention within 7 days of onset. These episodes recur with variable frequency.
- Persistent AF refers to AF that is continuously sustained for more than 7 days.
- Longstanding persistent AF refers to continuous AF that lasts longer than 12 months.
- Permanent AF is not an inherent pathophysiologic attribute of AF, but rather an acceptance of AF where the patient and physician abandon further efforts to restore and/or maintain sinus rhythm.
- Nonvalvular AF occurs in the absence of a valve replacement (mechanical or bioprosthetic), rheumatic mitral stenosis, or mitral valve repair.
Although paroxysmal and persistent AF may occur in the same individual, the distinction is still clinically relevant, as outcomes of certain therapies, such as CA, are superior in patients with paroxysmal AF.14 With a more complete understanding of AF pathophysiology, guidelines now discourage use of the potentially confusing term “lone AF,” which has historically been applied to younger patients with no known clinical risk factors or echocardiographic abnormalities. As a result, therapeutic decisions are no longer based on this nomenclature, according to the 2014 AF practice guideline from the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS).5
Patient complaints—or incidental findings—can prompt a Dx
Fatigue is the most common symptom of AF. Other signs and symptoms include palpitations, dyspnea, HF, hypotension, syncope, chest pain, and stroke. Some patients are asymptomatic, and AF is an incidental finding when an irregular pulse is discovered during a physical examination. The diagnosis is confirmed by electrocardiogram (EKG), telemetry, Holter monitor, event recorder, or an implanted electrocardiographic recording device. A chest x-ray, serum electrolyte levels, a complete blood count, thyroid testing, and renal and hepatic function testing are recommended. Transthoracic echocardiography to measure cardiac function, detect underlying structural heart disease, and evaluate atrial size is essential.5
An electrophysiologic (EP) study may be needed for diagnosis or treatment if another arrhythmia is present. Aberrant conduction may cause AF to present as a wide complex tachycardia and be mislabeled as ventricular tachycardia. The presence of delta waves is an indication for an EP study targeting the WPW accessory pathway. Transesophageal echocardiography (TEE) is the most sensitive and specific test for left atrial thrombi. If you are considering a TEE for a patient with AF of unknown, or >48 hours’, duration who has not been anticoagulated in the preceding 3 weeks, obtain it before performing cardioversion because of the risk of embolism.5
Stroke prevention
The ACC/AHA/HRS AF guideline recommends basing anticoagulation decisions on thromboembolic risk, regardless of AF pattern (paroxysmal, persistent, or permanent) (Class I recommendation).5 For patients with nonvalvular AF and atrial flutter, the guideline recommends using the Birmingham 2009 schema (CHA2DS2-VASc score) (TABLE 115-18) to estimate thromboembolic risk.5,15 CHA2DS2-VASc improves on the older CHADS2 score by significantly reducing the number of patients categorized as having intermediate risk and better identifying truly low-risk patients who are unlikely to benefit from anticoagulation.16,17,19

Men with a CHA2DS2-VASc score of zero and women with a score of one do not need anticoagulation.5,20 Discuss the risks and benefits of oral anticoagulation with men who have a score of one. In these intermediate-risk men, antiplatelet therapy with aspirin and/or clopidogrel may be reasonable, especially if there is an indication other than stroke prevention (eg, post-myocardial infarction). Oral anticoagulation is strongly recommended for all patients with a CHA2DS2-VASc score of 2 or higher.5,18,21,22
Anticoagulant considerations: Warfarin vs DOACs
Warfarin was the gold standard for stroke prevention in nonvalvular AF until the direct oral anticoagulants (DOACs) became available in 2010. Guidelines in the United States and the United Kingdom recommend shared decision-making to help patients with AF who do not have a specific indication for warfarin choose between warfarin and the DOACs.5,21 Canadian and European guidelines recommend DOACs as the first-line option for anticoagulation and reserve warfarin for patients who have contraindications to, or are unable to afford, DOACs.18,22 All current guidelines recommend continuing warfarin in patients who are stable, well controlled, and satisfied with warfarin therapy and the monitoring and dietary restrictions it entails.
DOACs are as effective as warfarin. All of the DOACs are approved for stroke prevention based on individual phase III non-inferiority trials in which they were compared to warfarin.23-26 In addition, a meta-analysis of these 4 trials involving a total of 71,683 patients (mean age 70-73 years; median follow-up, 1.8-2.8 years) evaluated the benefits and risks of the 4 DOACs against the former gold standard.27
Higher doses of the DOACs (dabigatran 150 mg BID, rivaroxaban 20 mg/d, edoxaban 60 mg/d, and apixaban 5 mg BID) reduced the rates of stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; P<.0001; number needed to treat [NNT]=147), hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; P<.0001; NNT=219), and all-cause mortality (RR=0.90; 95% CI, 0.85-0.95; P=.0003; NNT=128), compared with warfarin.27 It is important to note that while lower doses of some DOACs (dabigatran 110 mg BID and edoxaban 30 mg/d) were not as effective at preventing ischemic stroke when compared with warfarin (RR=1.3; 95% CI, 1-1.6; P=.045), they still significantly reduced hemorrhagic stroke (RR=0.33; 95% CI, 0.23-0.46; P<.0001) and all-cause mortality (RR=0.89; 95% CI, 0.83-0.96; P=.003).
Of course, the biggest concern is bleeding. In that same meta-analysis, the difference in major bleeding events with DOACs vs warfarin was not statistically significant (RR=0.86; 95% CI, 0.73-1; P=.06). While DOACs likely lower rates of intracranial hemorrhage (RR=0.48; 95% CI, 0.39-0.59; P<.0001; NNT=132), they seem to increase the risk of gastrointestinal (GI) bleeding (RR=1.3; 95% CI, 1-1.6; P=.043; number needed to harm [NNH]=185).27
There was significant heterogeneity in the GI bleeding outcome, however. When compared with warfarin, GI bleeding was increased by dabigatran 150 mg BID (RR=1.5; 95% CI, 1.2-1.9; P<.001) and edoxaban 60 mg/d (HR=1.2; 95% CI, 1.02-1.5; P=.03), but there were no significant differences for dabigatran 110 mg BID or apixaban 5 mg BID.23,25,26
On the other hand, edoxaban 30 mg/d had a lower risk of GI bleeding when compared with warfarin (HR=0.67; 95% CI, 0.53-0.83; P<.001).25 Without head-to-head trials, it is impossible to know if one DOAC is superior to another. Apixaban 5 mg BID appears to offer the best overall balance between efficacy and safety. Other DOACs may be better options for patients who have specific concerns regarding efficacy or safety.28,29
Convenience, interactions, and cost may be the deciding factors. Since all DOACs are fairly comparable in efficacy and safety, other factors such as convenience, interactions with other medications, and cost should be considered when deciding on a medication for an individual patient (TABLE 230,31). The DOACs require no lab monitoring or dose titration, and all 4 have fewer potential drug interactions than warfarin.30 Due to their relatively short half-lives, strict adherence is critical; DOACs are not suitable for patients who frequently miss doses.5 (For more information on starting or switching to DOACs, see, “Is a novel anticoagulant right for your patient?” J Fam Pract. 2014;63:22-28.)

A word about DOACs and renal impairment. Another concern with DOACs is their reliance on renal metabolism and excretion. A meta-analysis of the 4 phase III trials of the DOACs, this time involving 58,338 patients, evaluated DOAC efficacy and safety compared to warfarin in the presence of kidney dysfunction.32 Renal function was categorized as normal (estimated glomerular filtration rate [eGFR] >80 mL/min/1.73 m2), mildly impaired (eGFR 50-80 mL/min/1.73 m2), or moderately impaired (eGFR <50 mL/min/1.73m2). Compared with warfarin, DOACs lowered stroke risk in patients with mild (RR=0.71; 95% CI, 0.62-0.81) or moderate (RR=0.79; 95% CI, 0.66-0.94) renal impairment. DOACs also reduced major bleeding compared to warfarin in patients with mild (RR=0.88; 95% CI, 0.80-0.97) or moderate (RR=0.80; 95% CI, 0.66-0.94) renal impairment. How the DOACs fare in patients with severe renal dysfunction could not be determined because such patients were excluded from the trials.
Keep in mind that the DOACs require dose adjustment at different levels of renal impairment (TABLE 230,31), and warfarin remains the only recommended treatment for patients with severe renal impairment, according to both AHA/ACC/HRS and European Society of Cardiology guidelines.5,18
Tools to help assess patients’ bleeding risk
Of the available scoring mechanisms to identify risk factors for bleeding, 3 have been specifically validated in AF populations (ie, ATRIA,33 HEMORR2HAGES,34 and HAS-BLED35). Of the 3, HAS-BLED is superior,36 the most practical, and recommended by expert guidelines.18,21,22 Additionally, HAS-BLED has good correlation with intracranial hemorrhage risk. The HAS-BLED score ranges from 0 to 9 points with one point assigned for each of the following:35
- Hypertension–uncontrolled with systolic BP >160 mm Hg
- Abnormal liver function–cirrhosis, bilirubin >2× normal, or liver enzymes >3× normal
- Abnormal renal function–dialysis, transplant, or serum creatinine >2.26 mg/dL
- Stroke history–including lacunar infarcts
- Bleeding predisposition–history of major bleeding due to any cause
- Labile international normalized ratio (INR)–time in therapeutic range <60%
- Elderly–age >65 years
- Drug–antiplatelet agents, including nonsteroidal anti-inflammatory drugs
- Alcohol usage–>8 drinks per week.
Patients with a HAS-BLED score ≥3 warrant additional monitoring and attempts to reduce bleeding risk by addressing modifiable risk factors. Bleeding risk scores should not be used to exclude patients from anticoagulation therapy.5 In fact, the British National Institute for Health and Clinical Excellence (NICE) guidelines state that anticoagulation should not be withheld solely due to fall risk.21
Also, anticoagulation with warfarin should not be permanently discontinued because of a single GI bleed, since restarting warfarin is associated with decreased risks of thromboembolism and mortality and a statistically insignificant increase in recurrent GI bleeding.37 Restarting DOAC therapy following a GI bleed has not been evaluated in clinical trials; however, it may be reasonable to use one of the DOAC doses with a lower risk of GI bleeding (dabigatran 110 mg BID, apixaban 5 mg BID, or edoxaban 30 mg/d) in patients who have experienced a GI bleed on warfarin or another DOAC.18,22
An online calculator is available that uses CHA2DS2-VASc and HAS-BLED scores to determine an individual’s risk/benefit profile with the various anticoagulation strategies available (http://www.sparctool.com). Consider percutaneous left atrial appendage occlusion if the risks of anticoagulation truly exceed the benefits.38
Rate control vs rhythm control
Most patients who present with AF require immediate ventricular rate control to reduce symptoms. In the acute setting, this can be accomplished with intravenous (IV) beta-blockers or IV calcium channel antagonists.5,39 If the patient is hemodynamically unstable, urgent direct-current cardioversion is the preferred treatment strategy and should not be delayed pending anticoagulation. IV amiodarone can be used in the ICU patient who does not require cardioversion, but is unable to tolerate beta-blockers or calcium channel antagonists.40 Once the patient is stable, long-term treatment focuses on ventricular rate control or restoration and maintenance of sinus rhythm.
The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial enrolled 4060 patients (mean age 70 years, mean follow-up 3.5 years) with paroxysmal and persistent AF and randomized them to either pharmacologic rate control or rhythm control.41 No significant differences were found in all-cause mortality or in the composite secondary endpoint of death, ischemic stroke, anoxic encephalopathy, major bleeding, or cardiac arrest. In addition, no significant differences emerged in quality of life or global functional status. The number of patients requiring hospitalization during follow-up was significantly lower in the rate-control group vs the rhythm-control group (73% vs 80%; P<.001). Anticoagulation was encouraged but not mandated in the rhythm-control group after 4 weeks in sinus rhythm, and there was a trend toward higher mortality in the rhythm-control group (27% vs 26%; P=.08).
Patients <65 years were excluded from the AFFIRM trial. When younger patients experience significant symptoms, early referral to Cardiology should be considered to discuss the long-term benefits and risks of a rhythm-control strategy. Regardless of age, when patients remain symptomatic despite rate- or rhythm-control management, the strategy should be changed.5
Rate-control targets and options
Target heart rates should be individualized. The 2014 ACC/AHA/HRS guideline recommends a resting target heart rate <80 beats per minute (bpm) in symptomatic patients.5 In patients with permanent AF who remain asymptomatic at higher resting heart rates, a more lenient rate-control strategy (resting heart rate <110 bpm) has demonstrated outcomes equivalent to those of a more strict approach (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm).42 Pharmacologic rate-control options include beta-blockers, non-dihydropyridine calcium channel antagonists, and digoxin (TABLE 35). Digoxin is associated with increased all-cause mortality in patients with AF regardless of HF status (HR=1.4; 95% CI, 1.2-1.6, P=.0001).43 Digoxin should be reserved for patients who are sedentary or have inadequate control with first-line medications.5


Indications for rhythm control
The NICE guidelines, which are consistent with the ACC/AHA/HRS guidelines, recommend rate control as the first-line strategy for AF management, except in people:21
- whose AF has a reversible cause
- who have HF believed to be primarily caused by AF
- with new-onset AF
- with atrial flutter that is considered suitable for an ablation strategy to restore sinus rhythm
- for whom a rhythm-control strategy would be more suitable based on clinical judgment.
In addition, patients who continue to experience symptomatic AF despite an adequate trial of rate control should be offered rhythm control.5
Pharmacologic rhythm-control strategies. Antiarrhythmic drugs can be used for chemical cardioversion, reduction of paroxysms, and long-term maintenance of sinus rhythm. The most commonly used antiarrhythmic drugs are Class IC and Class III agents (TABLE 3).5 Tailored drug selection for each patient is key. Patients with left atrial diameters >4.5 cm are less likely to remain in sinus rhythm, and patients with left ventricular hypertrophy are at increased risk for proarrhythmic adverse effects.44 Patients with paroxysmal AF may be candidates for a “pill-in-the-pocket” strategy using propafenone or flecainide.5
AF frequently progresses from paroxysmal to persistent and can subsequently result in electrical and structural remodeling that becomes irreversible over time.45 The patient with uncontrolled symptoms despite attempts at rate control and rhythm control should be promptly referred to an electrophysiologist.
Surgical interventions for rate or rhythm control
Electrophysiology interventions include AV nodal ablation with pacemaker placement for rate control, or catheter-directed ablation (radiofrequency or cryotherapy) for rhythm control. CA appears to be more effective than pharmacologic rhythm control.46,47 Treatment with CA is indicated for symptomatic paroxysmal AF when a rhythm-control strategy is desired and the AF is refractory to, or the patient is intolerant of, at least one class I or III antiarrhythmic medication.5 With these same caveats, CA is a reasonable strategy for symptomatic persistent AF.
Consider more invasive interventions, such as an atrial maze procedure, when patients require cardiac surgery for another indication. Patients with an increased risk of thromboembolism (based on CHA2DS2-VASc) remain at high risk even after successful ablation.48 As a result, some guidelines recommend continued long-term anticoagulation following CA.18,22
CORRESPONDENCE
Philip Dooley, MD, University of Kansas School of Medicine–Wichita Family Medicine Residency at Via Christi, 707 North Emporia, Wichita, KS 67207; [email protected].
ACKNOWLEDGMENTS
We thank Professor Anne Walling, MB, ChB, FFPHM, Department of Family and Community Medicine, University of Kansas School of Medicine–Wichita for her suggestions and critical review of an earlier version of this manuscript.
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375.
2. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N.
3. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476-484.
4. Ott A, Breteler MMB, de Bruyne MC, et al. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke. 1997;28:316-321.
5. January CT, Wann L, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76.
6. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. Stroke. 1996;27:1760-1764.
7. Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55-62.
8. Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739-749.
9. Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005-2012.
10. Angaran P, Dorian P, Tan MK, et al. The risk stratification and stroke prevention therapy care gap in Canadian atrial fibrillation patients. Can J Cardiol. 2016;32:336-343.
11. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779-786.
12. Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799-802.
13. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281-289.
14. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171-257.
15. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263-272.
16. Lip GYH, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731-2738.
17. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124.
18. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747.
19. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172-1179.
20. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522.
21. National Institute for Health and Clinical Excellence (NICE). Atrial fibrillation: the management of atrial fibrillation [CG180]. 2014. Available at: https://www.nice.org.uk/guidance/cg180. Accessed July 31, 2016.
22. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114-1130.
23. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
24. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
25. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
26. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
27. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
28. Morimoto T, Crawford B, Wada K, et al. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466-474.
29. Verdecchia P, Angeli F, Bartolini C, et al. Safety and efficacy of non-vitamin K oral anticoagulants in non-valvular atrial fibrillation: a Bayesian meta-analysis approach. Expert Opin Drug Saf. 2015;14:7-20.
30. Micromedex® 2.0 (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com. Accessed August 18, 2016.
31. GoodRx. Available at: https://www.goodrx.com. Accessed August 18, 2016.
32. Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69-75.
33. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401.
34. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719.
35. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100.
36. Zhu W, He W, Guo L, et al. The HAS-BLED Score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561.
37. Chai-Adisaksopha C, Hillis C, Monreal M, et al. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819-825.
38. Xu H, Xie X, Wang B, et al. Efficacy and safety of percutaneous left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation: a meta-analysis of contemporary studies. Heart Lung Circ. 2016;25:1107-1117.
39. Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174-2179.
40. Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594-598.
41. The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
42. Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
43. Wang ZQ, Zhang R, Chen MT, et al. Digoxin is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: a meta-analysis. J Cardiovasc Pharmacol. 2015;66:270-275.
44. Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026-2033.
45. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation: clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725-731.
46. Cheng X, Li X, He Y, et al. Catheter ablation versus anti-arrhythmic drug therapy for the management of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. 2014;41:267-272.
47. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637-1644.
48.
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375.
2. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N.
3. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476-484.
4. Ott A, Breteler MMB, de Bruyne MC, et al. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke. 1997;28:316-321.
5. January CT, Wann L, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76.
6. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. Stroke. 1996;27:1760-1764.
7. Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55-62.
8. Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739-749.
9. Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005-2012.
10. Angaran P, Dorian P, Tan MK, et al. The risk stratification and stroke prevention therapy care gap in Canadian atrial fibrillation patients. Can J Cardiol. 2016;32:336-343.
11. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779-786.
12. Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799-802.
13. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281-289.
14. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171-257.
15. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263-272.
16. Lip GYH, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731-2738.
17. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124.
18. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747.
19. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172-1179.
20. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522.
21. National Institute for Health and Clinical Excellence (NICE). Atrial fibrillation: the management of atrial fibrillation [CG180]. 2014. Available at: https://www.nice.org.uk/guidance/cg180. Accessed July 31, 2016.
22. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114-1130.
23. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
24. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
25. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
26. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
27. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
28. Morimoto T, Crawford B, Wada K, et al. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466-474.
29. Verdecchia P, Angeli F, Bartolini C, et al. Safety and efficacy of non-vitamin K oral anticoagulants in non-valvular atrial fibrillation: a Bayesian meta-analysis approach. Expert Opin Drug Saf. 2015;14:7-20.
30. Micromedex® 2.0 (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com. Accessed August 18, 2016.
31. GoodRx. Available at: https://www.goodrx.com. Accessed August 18, 2016.
32. Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69-75.
33. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401.
34. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719.
35. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100.
36. Zhu W, He W, Guo L, et al. The HAS-BLED Score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561.
37. Chai-Adisaksopha C, Hillis C, Monreal M, et al. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819-825.
38. Xu H, Xie X, Wang B, et al. Efficacy and safety of percutaneous left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation: a meta-analysis of contemporary studies. Heart Lung Circ. 2016;25:1107-1117.
39. Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174-2179.
40. Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594-598.
41. The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
42. Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
43. Wang ZQ, Zhang R, Chen MT, et al. Digoxin is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: a meta-analysis. J Cardiovasc Pharmacol. 2015;66:270-275.
44. Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026-2033.
45. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation: clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725-731.
46. Cheng X, Li X, He Y, et al. Catheter ablation versus anti-arrhythmic drug therapy for the management of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. 2014;41:267-272.
47. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637-1644.
48.
PRACTICE RECOMMENDATIONS
› Use the CHA2DS2-VASc score to assess the risk of thromboembolism, including ischemic stroke. A
› Consider prescribing a direct oral anticoagulant (DOAC) instead of warfarin for patients with nonvalvular atrial fibrillation (AF) because they are superior at preventing strokes and lowering all-cause mortality in this population. B
› Do not use a DOAC in patients with mechanical heart valves, hemodynamically significant mitral stenosis, or severe chronic kidney disease (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2). A
› Pursue a rate-control strategy for most patients with AF, although rhythm control may be preferable for younger (<65 years) symptomatic patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series