User login
Inverted Appendix in a Patient With Weakness and Occult Bleeding
Appendiceal mucinous neoplasms (AMNs) are rare tumors of the appendix that can be asymptomatic or present with acute right lower quadrant (RLQ) pain mimicking appendicitis. Due to their potential to cause either no symptoms or nonspecific symptoms, such as abdominal pain, nausea, or vomiting, AMNs are often found incidentally during appendectomies or, even more rarely, colonoscopies. Most AMNs grow slowly and have little metastatic potential. However, due to potential complications, such as bowel obstruction and rupture, timely detection and removal of AMN is essential. We describe the case of a patient who appeared to have acute appendicitis complicated by rupture on imaging who was found instead to have a perforated low-grade AMN during surgery.
Case Presentation
A male patient aged 72 years with a history of type 2 diabetes mellitus, hypertension, and aortic stenosis, but no prior abdominal surgery, presented with a chief concern of generalized weakness. As part of the workup for his weakness, a computed tomography (CT) scan of the abdomen was performed which showed an RLQ phlegmon and mild fat stranding in the area. Imaging also revealed an asymptomatic gallstone measuring 1.5 cm with no evidence of cholecystitis. The patient had no fever and reported no abdominal pain, nausea, vomiting, or change in bowel habits. On physical examination, the patient’s abdomen was soft, nontender, and nondistended with normoactive bowel sounds and no rebound or guarding.
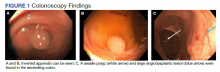
To manage the appendicitis, the patient started a 2-week course of amoxicillin clavulanate 875 mg twice daily and was instructed to schedule an interval appendectomy in the coming months. Four days later, during a follow-up with his primary care physician, he was found to be asymptomatic. However, at this visit his stool was found to be positive for occult blood. Given this finding and the lack of a previous colonoscopy, the patient underwent a colonoscopy, which revealed bulging at the appendiceal orifice, consistent with an inverted appendix. Portions of the appendix were biopsied (Figure 1). Histologic analysis of the appendiceal biopsies revealed no dysplasia or malignancy. The colonoscopy also revealed an 8-mm sessile polyp in the ascending colon which was resected, and histologic analysis of this polyp revealed a low-grade tubular adenoma. Additionally, a large angiodysplastic lesion was found in the ascending colon as well as external and medium-sized internal hemorrhoids.
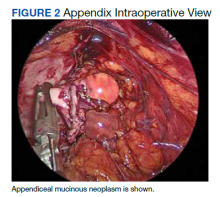
Six weeks after the colonoscopy, the patient was taken to the operating room for a laparoscopic appendectomy. Upon entry of the abdomen, extensive adhesions throughout the RLQ were found which required adhesiolysis. A calcified fecalith adherent to the mesentery of the small intestine in the RLQ was also found and resected. After lysis of the adhesions, the appendix and fibrotic tissue surrounding it could be seen (Figure 2). The appendix was dilated and the tip showed perforation. During dissection of the appendix, clear gelatinous material was found coming from the appendiceal lumen as well as from the fibrotic tissue around the appendix. On postoperative day 1 the appendix was resected and the patient was discharged.
Histologic specimens of the appendix were notable for evidence of perforation and neoplasia leading to a diagnosis of low-grade AMN. The presence of atypical mucinous epithelial cells on the serosal surface of the appendix, confirmed with a positive pancytokeratin stain, provided histologic evidence of appendiceal perforation (Figure 3). The presence of nuclear atypia demonstrated that the appendix was involved by a neoplastic process. Additionally, attenuation of the normal appendiceal epithelium, evidence of a chronic process, further helped to differentiate the AMN from complicated appendicitis. The presence of mucin involving the serosa of the appendix led to the classification of this patient’s neoplasm as grade pT4a. Of note, histologic examination demonstrated that the surgical margins contained tumor cells.
Given the positive margins of the resected AMN and the relatively large size of the neoplasm, a laparoscopic right hemicolectomy was performed 2 months later. Although multiple adhesions were found in the terminal ileum, cecum, and ascending colon during the hemicolectomy, no mucinous lesions were observed grossly. Histologic analysis showed no residual neoplasm as well as no lymph node involvement. On postoperative day 3 the patient was discharged and had an uneventful recovery. At his first surveillance visit 6 months after his hemicolectomy, the patient appeared to be doing well and reported no abdominal pain, nausea, vomiting, change in bowel habits, or any blood in the stool.
Discussion
AMNs are rare tumors with an annual age-adjusted incidence of approximately 0.12 per 1,000,000 people.1 These neoplasms can present as acute or chronic abdominal pain, gastrointestinal bleeding, intestinal obstruction, or acute abdomen.2-4 Most AMNs, however, are asymptomatic and are usually found incidentally during appendectomies for appendicitis, and can even be found during colonoscopies,such as in this case.5,6
Low-grade AMNs are distinguished from appendiceal mucinous adenocarcinomas by their lack of wall invasion.7 Additionally, low-grade AMNs have a very good prognosis as even neoplasms that have spread outside of the appendix have a 5-year overall survival rate of 79 to 86%.8 These low-grade neoplasms also have extremely low rates of recurrence after resection.9 In contrast, appendiceal mucinous adenocarcinomas have a much worse prognosis with a 5-year overall survival rate of 53.6%.10
Treatment of AMNs depends on the extent of their spread. Neoplasms that are confined to the appendix can typically be treated with appendectomy alone, while those that have spread beyond the appendix may require cytoreductive surgery and chemotherapy, namely, hyperthermic intraperitoneal chemotherapy (HIPEC), in addition to appendectomy.11 Cases in which neoplasms are not confined to the appendix also require more frequent surveillance for recurrence as compared to appendix-restricted neoplasms.11
Appendiceal inversion is a rare finding in adults with an estimated prevalence of 0.01%.6 Not only is appendiceal inversion rare in and of itself, it is even more rarely found in combination with appendiceal neoplasms.6 Other causes of appendiceal inversion include intussusception, acute appendicitis, appendiceal nodule, or even iatrogenic due to appendectomy.12-14 While appendiceal inversion can be completely benign, because these morphological changes of the appendix can resemble a polyp, these lesions are often biopsied and/or resected.15 However, lesion resection may be quite problematic due to high risk of bleeding and perforation.15 In order to avoid the risks associated with resection of a potentially benign finding, biopsy should be performed prior to any attempted resection of inverted appendices.15
Another interesting aspect of this case is the finding of fecal occult blood. The differential for fecal occult blood is quite broad and the patient had multiple conditions that could have led to the finding of occult blood in his stool. Hemorrhoids can cause a positive result on a fecal occult blood test (FOBT) although this is relatively uncommon, and hemorrhoids are more likely to cause frank blood to be seen.16 The sessile polyp found in the patient’s colon may also have caused the FOBT to be positive. This patient was also found to have an angiodysplasia (a finding that is associated with aortic stenosis, which this patient has a history of) which can also cause gastrointestinal bleeding.17 Lastly, AMNs may also cause gastrointestinal bleeding and thus a positive FOBT, although bleeding is a relatively uncommon presentation of AMNs, especially those that are low-grade as in this case.18
This case also highlights the association between appendiceal neoplasms and colonic neoplastic lesions. Patients with appendiceal neoplasms are more likely to have colonic neoplastic lesions than patients without appendiceal neoplasms.19 Studies have found that approximately 13 to 42% of patients with appendiceal neoplasms also have colonic neoplastic lesions.19 The majority of these lesions in the colon were right-sided and this finding was also seen in this case as the patient’s polyp was located in the ascending colon.19 Due to this association between appendiceal and colorectal neoplasia, the American Society of Colon and Rectal Surgeons strongly recommends that patients with appendiceal neoplasms or who are suspected of having them receive a colonoscopy.19
Additionally, perforation of an AMN, as was seen in this case, is a finding that should raise significant concern. Perforation of an AMN allows for the spread of malignant mucinous epithelial cells throughout the abdomen. The finding of extensive adhesions throughout the patient’s RLQ was unexpected as abdominal adhesions are most often seen in patients with a history of abdominal surgeries. Considering the lack of any prior abdominal surgeries in this patient, these adhesions were most likely the result of the spread and proliferation of malignant mucinous epithelial cells from the perforated AMN in the RLQ.20 The adhesiolysis performed in this case was thus not only important in order to visualize the appendix, but also for preventing future complications of abdominal adhesions such as bowel obstruction.20 Perforated AMN is also so concerning because it can potentially lead to pseudomyxoma peritonei—a condition in which malignant mucinous epithelial cells accumulate in the abdomen.21 Pseudomyxoma peritonei is extremely rare with an incidence of approximately 1 to 2 cases per million per year.22 Early recognition of AMNs and surgical referral are critically important as pseudomyxoma peritonei is difficult to treat, has a high rate of recurrence, and can be fatal.23
Lastly, this case highlights how findings of a ruptured appendix and/or mucin surrounding the appendix on imaging should warrant laparoscopy because only pathologic analysis of the appendix can definitively rule out AMNs. The utility of laparoscopic evaluation of the appendix is especially apparent as nonsurgical treatment of appendicitis using antibiotics is gaining favor for treating even complicated appendicitis.24 Appendicitis is much more common than AMNs. However, had the patient in this case only been given antibiotics for his suspected complicated appendicitis without any colonoscopy or appendectomy, the neoplasm in his appendix would have gone undetected and continued to grow, causing significant complications. The patient’s age at presentation in this case also necessitated laparoscopic evaluation of the appendix as the incidence of AMNs is highest among patients aged > 60 years.25 Additionally, because appendiceal inversion may be seen with AMNs,the patient’s inverted appendix seen during his colonoscopy was another compelling reason for laparoscopic evaluation of his appendix.6
Conclusions
AMNs can present with nonspecific symptoms or can be completely asymptomatic and are often found incidentally during colonoscopies or appendectomies for acute appendicitis. While it is true that AMNs have low metastatic potential and grow slowly, AMNs can rupture leading to pseudomyxoma peritonei or even cause bowel obstruction warranting timely identification and removal of these neoplasms. Laparoscopic evaluation in cases of ruptured appendices is critical not only for treatment, but also for determining the presence of a potential underlying appendiceal malignancy. Although AMNs are a rare pathology, physicians should still consider the possibility of these neoplasms even when imaging findings suggest appendicitis. Having AMNs as part of the differential diagnosis is especially necessary in cases, such as this one, in which the patient has appendiceal inversion, is aged > 50 years, and has concurrent colorectal neoplasms.
1. Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol. 2017;40(6):569-573. doi:10.1097/COC.0000000000000210
2. Kehagias I, Zygomalas A, Markopoulos G, Papandreou T, Kraniotis P. Diagnosis and treatment of mucinous appendiceal neoplasm presented as acute appendicitis. Case Rep Oncol Med. 2016;3:1-6. doi:10.1155/2016/2161952
3. Karatas M, Simsek C, Gunay S, et al. Acute lower gastrointestinal bleeding due to low-grade mucinous neoplasm of appendix. Acta Chir Belg. 2020;120(4):1-4. doi:10.1080/00015458.2020.1860397
4. Mourad FH, Hussein M, Bahlawan M, Haddad M, Tawil A. Intestinal obstruction secondary to appendiceal mucocele. Dig Dis Sci. 1999;44(8):1594-1599. doi:10.1023/a:1026615010989
5. Benabe SH, Leeman R, Brady AC, Hirzel A, Langshaw AH. Low-grade appendiceal mucinous neoplasm in an adolescent patient with untreated Crohn’s disease. ACG Case Reports J. 2020;7(3). doi:10.14309/crj.0000000000000338
6. Liu X, Liu G, Liu Y, et al. Complete appendiceal inversion with local high-grade intraepithelial neoplasia in an adult female: A case report. BMC Surg. 2019;19(1). doi:10.1186/s12893-019-0632-3
7. Gündog˘ar ÖS, Kımılog˘lu ES, Komut NS, et al. The evaluation of appendiceal mucinous neoplasms with a new classification system. Turk J Gastroenterol. 2018;29(5):532-542. doi:10.5152/tjg.2018.17605
8. Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089-1103. doi:10.1097/00000478-200308000-00006
9. Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33(10):1425-1439. doi:10.1097/PAS.0b013e3181af6067
10. Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2015;122(2):213-221. doi:10.1002/cncr.29744
11. Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. 2018;23(1):137. doi:10.1634/theoncologist.2017-0081erratum
12. Tran C, Sakioka J, Nguyen E, Beutler BD, Hsu J. An inverted appendix found on routine colonoscopy: a case report with discussion of imaging findings. Radiol Case Rep. 2019;14(8):952-955. doi:10.1016/j.radcr.2019.05.022
13. Shafi A, Azab M. A case of everted appendix with benign appendiceal nodule masquerading as appendiceal mucocele: a case report. Am J Gastroenterol. 2018;113:S1436. doi:10.14309/00000434-201810001-02585
14. Pokhrel B, Chang M, Anand G, Savides T, Fehmi S. Appendiceal mucinous neoplasm in an inverted appendix found on prior colonoscopy. VideoGIE. 2020;5(1):34-36. doi:10.1016/j.vgie.2019.09.013
15. Johnson EK, Arcila ME, Steele SR. Appendiceal inversion: a diagnostic and therapeutic dilemma. JSLS. 2009;13(1):92-95.
16. van Turenhout ST, Oort FA, sive Droste JST, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc. 2012;76(1):136-143. doi:10.1016/j.gie.2012.03.169
17. Hudzik B, Wilczek K, Gasior M. Heyde syndrome: Gastrointestinal bleeding and aortic stenosis. CMAJ. 2016;188(2):135-138. doi:10.1503/cmaj.150194
18. Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. RadioGraphics. 2017;37(4):1059-1083. doi:10.1148/rg.2017160150
19. Glasgow SC, Gaertner W, Stewart D, et al. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62(12):1425-1438. doi:10.1097/DCR.0000000000001530
20. Panagopoulos P, Tsokaki T, Misiakos E, et al. Low-grade appendiceal mucinous neoplasm presenting as an adnexal mass. Case Reports in Obstetrics and Gynecology. 2017;2017:1-3. doi:10.1155/2017/7165321
21. Ramaswamy V. Pathology of mucinous appendiceal tumors and pseudomyxoma peritonei. Indian J Surg Oncol. 2016;7(2):258-267. doi:10.1007/s13193-016-0516-2.
22. Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44-50. doi:10.4251/wjgo.v2.i1.44
23. Mercier F, Dagbert F, Pocard M, et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open. 2018;3(2):195-202. doi:10.1002/bjs5.97
24. David A, Dodgion C, Eddine SBZ, Davila D, Webb TP, Trevino CM. Perforated appendicitis: Short duration antibiotics are noninferior to traditional long duration antibiotics. Surgery. 2020;167(2):475-477. doi:10.1016/j.surg.2019.08.007
25. Raijman I, Leong S, Hassaram S, Marcon NE. Appendiceal mucocele: Endoscopic appearance. Endoscopy. 1994;26(3):326-328. doi:10.1055/s-2007-1008979
Appendiceal mucinous neoplasms (AMNs) are rare tumors of the appendix that can be asymptomatic or present with acute right lower quadrant (RLQ) pain mimicking appendicitis. Due to their potential to cause either no symptoms or nonspecific symptoms, such as abdominal pain, nausea, or vomiting, AMNs are often found incidentally during appendectomies or, even more rarely, colonoscopies. Most AMNs grow slowly and have little metastatic potential. However, due to potential complications, such as bowel obstruction and rupture, timely detection and removal of AMN is essential. We describe the case of a patient who appeared to have acute appendicitis complicated by rupture on imaging who was found instead to have a perforated low-grade AMN during surgery.
Case Presentation
A male patient aged 72 years with a history of type 2 diabetes mellitus, hypertension, and aortic stenosis, but no prior abdominal surgery, presented with a chief concern of generalized weakness. As part of the workup for his weakness, a computed tomography (CT) scan of the abdomen was performed which showed an RLQ phlegmon and mild fat stranding in the area. Imaging also revealed an asymptomatic gallstone measuring 1.5 cm with no evidence of cholecystitis. The patient had no fever and reported no abdominal pain, nausea, vomiting, or change in bowel habits. On physical examination, the patient’s abdomen was soft, nontender, and nondistended with normoactive bowel sounds and no rebound or guarding.
To manage the appendicitis, the patient started a 2-week course of amoxicillin clavulanate 875 mg twice daily and was instructed to schedule an interval appendectomy in the coming months. Four days later, during a follow-up with his primary care physician, he was found to be asymptomatic. However, at this visit his stool was found to be positive for occult blood. Given this finding and the lack of a previous colonoscopy, the patient underwent a colonoscopy, which revealed bulging at the appendiceal orifice, consistent with an inverted appendix. Portions of the appendix were biopsied (Figure 1). Histologic analysis of the appendiceal biopsies revealed no dysplasia or malignancy. The colonoscopy also revealed an 8-mm sessile polyp in the ascending colon which was resected, and histologic analysis of this polyp revealed a low-grade tubular adenoma. Additionally, a large angiodysplastic lesion was found in the ascending colon as well as external and medium-sized internal hemorrhoids.
Six weeks after the colonoscopy, the patient was taken to the operating room for a laparoscopic appendectomy. Upon entry of the abdomen, extensive adhesions throughout the RLQ were found which required adhesiolysis. A calcified fecalith adherent to the mesentery of the small intestine in the RLQ was also found and resected. After lysis of the adhesions, the appendix and fibrotic tissue surrounding it could be seen (Figure 2). The appendix was dilated and the tip showed perforation. During dissection of the appendix, clear gelatinous material was found coming from the appendiceal lumen as well as from the fibrotic tissue around the appendix. On postoperative day 1 the appendix was resected and the patient was discharged.
Histologic specimens of the appendix were notable for evidence of perforation and neoplasia leading to a diagnosis of low-grade AMN. The presence of atypical mucinous epithelial cells on the serosal surface of the appendix, confirmed with a positive pancytokeratin stain, provided histologic evidence of appendiceal perforation (Figure 3). The presence of nuclear atypia demonstrated that the appendix was involved by a neoplastic process. Additionally, attenuation of the normal appendiceal epithelium, evidence of a chronic process, further helped to differentiate the AMN from complicated appendicitis. The presence of mucin involving the serosa of the appendix led to the classification of this patient’s neoplasm as grade pT4a. Of note, histologic examination demonstrated that the surgical margins contained tumor cells.
Given the positive margins of the resected AMN and the relatively large size of the neoplasm, a laparoscopic right hemicolectomy was performed 2 months later. Although multiple adhesions were found in the terminal ileum, cecum, and ascending colon during the hemicolectomy, no mucinous lesions were observed grossly. Histologic analysis showed no residual neoplasm as well as no lymph node involvement. On postoperative day 3 the patient was discharged and had an uneventful recovery. At his first surveillance visit 6 months after his hemicolectomy, the patient appeared to be doing well and reported no abdominal pain, nausea, vomiting, change in bowel habits, or any blood in the stool.
Discussion
AMNs are rare tumors with an annual age-adjusted incidence of approximately 0.12 per 1,000,000 people.1 These neoplasms can present as acute or chronic abdominal pain, gastrointestinal bleeding, intestinal obstruction, or acute abdomen.2-4 Most AMNs, however, are asymptomatic and are usually found incidentally during appendectomies for appendicitis, and can even be found during colonoscopies,such as in this case.5,6
Low-grade AMNs are distinguished from appendiceal mucinous adenocarcinomas by their lack of wall invasion.7 Additionally, low-grade AMNs have a very good prognosis as even neoplasms that have spread outside of the appendix have a 5-year overall survival rate of 79 to 86%.8 These low-grade neoplasms also have extremely low rates of recurrence after resection.9 In contrast, appendiceal mucinous adenocarcinomas have a much worse prognosis with a 5-year overall survival rate of 53.6%.10
Treatment of AMNs depends on the extent of their spread. Neoplasms that are confined to the appendix can typically be treated with appendectomy alone, while those that have spread beyond the appendix may require cytoreductive surgery and chemotherapy, namely, hyperthermic intraperitoneal chemotherapy (HIPEC), in addition to appendectomy.11 Cases in which neoplasms are not confined to the appendix also require more frequent surveillance for recurrence as compared to appendix-restricted neoplasms.11
Appendiceal inversion is a rare finding in adults with an estimated prevalence of 0.01%.6 Not only is appendiceal inversion rare in and of itself, it is even more rarely found in combination with appendiceal neoplasms.6 Other causes of appendiceal inversion include intussusception, acute appendicitis, appendiceal nodule, or even iatrogenic due to appendectomy.12-14 While appendiceal inversion can be completely benign, because these morphological changes of the appendix can resemble a polyp, these lesions are often biopsied and/or resected.15 However, lesion resection may be quite problematic due to high risk of bleeding and perforation.15 In order to avoid the risks associated with resection of a potentially benign finding, biopsy should be performed prior to any attempted resection of inverted appendices.15
Another interesting aspect of this case is the finding of fecal occult blood. The differential for fecal occult blood is quite broad and the patient had multiple conditions that could have led to the finding of occult blood in his stool. Hemorrhoids can cause a positive result on a fecal occult blood test (FOBT) although this is relatively uncommon, and hemorrhoids are more likely to cause frank blood to be seen.16 The sessile polyp found in the patient’s colon may also have caused the FOBT to be positive. This patient was also found to have an angiodysplasia (a finding that is associated with aortic stenosis, which this patient has a history of) which can also cause gastrointestinal bleeding.17 Lastly, AMNs may also cause gastrointestinal bleeding and thus a positive FOBT, although bleeding is a relatively uncommon presentation of AMNs, especially those that are low-grade as in this case.18
This case also highlights the association between appendiceal neoplasms and colonic neoplastic lesions. Patients with appendiceal neoplasms are more likely to have colonic neoplastic lesions than patients without appendiceal neoplasms.19 Studies have found that approximately 13 to 42% of patients with appendiceal neoplasms also have colonic neoplastic lesions.19 The majority of these lesions in the colon were right-sided and this finding was also seen in this case as the patient’s polyp was located in the ascending colon.19 Due to this association between appendiceal and colorectal neoplasia, the American Society of Colon and Rectal Surgeons strongly recommends that patients with appendiceal neoplasms or who are suspected of having them receive a colonoscopy.19
Additionally, perforation of an AMN, as was seen in this case, is a finding that should raise significant concern. Perforation of an AMN allows for the spread of malignant mucinous epithelial cells throughout the abdomen. The finding of extensive adhesions throughout the patient’s RLQ was unexpected as abdominal adhesions are most often seen in patients with a history of abdominal surgeries. Considering the lack of any prior abdominal surgeries in this patient, these adhesions were most likely the result of the spread and proliferation of malignant mucinous epithelial cells from the perforated AMN in the RLQ.20 The adhesiolysis performed in this case was thus not only important in order to visualize the appendix, but also for preventing future complications of abdominal adhesions such as bowel obstruction.20 Perforated AMN is also so concerning because it can potentially lead to pseudomyxoma peritonei—a condition in which malignant mucinous epithelial cells accumulate in the abdomen.21 Pseudomyxoma peritonei is extremely rare with an incidence of approximately 1 to 2 cases per million per year.22 Early recognition of AMNs and surgical referral are critically important as pseudomyxoma peritonei is difficult to treat, has a high rate of recurrence, and can be fatal.23
Lastly, this case highlights how findings of a ruptured appendix and/or mucin surrounding the appendix on imaging should warrant laparoscopy because only pathologic analysis of the appendix can definitively rule out AMNs. The utility of laparoscopic evaluation of the appendix is especially apparent as nonsurgical treatment of appendicitis using antibiotics is gaining favor for treating even complicated appendicitis.24 Appendicitis is much more common than AMNs. However, had the patient in this case only been given antibiotics for his suspected complicated appendicitis without any colonoscopy or appendectomy, the neoplasm in his appendix would have gone undetected and continued to grow, causing significant complications. The patient’s age at presentation in this case also necessitated laparoscopic evaluation of the appendix as the incidence of AMNs is highest among patients aged > 60 years.25 Additionally, because appendiceal inversion may be seen with AMNs,the patient’s inverted appendix seen during his colonoscopy was another compelling reason for laparoscopic evaluation of his appendix.6
Conclusions
AMNs can present with nonspecific symptoms or can be completely asymptomatic and are often found incidentally during colonoscopies or appendectomies for acute appendicitis. While it is true that AMNs have low metastatic potential and grow slowly, AMNs can rupture leading to pseudomyxoma peritonei or even cause bowel obstruction warranting timely identification and removal of these neoplasms. Laparoscopic evaluation in cases of ruptured appendices is critical not only for treatment, but also for determining the presence of a potential underlying appendiceal malignancy. Although AMNs are a rare pathology, physicians should still consider the possibility of these neoplasms even when imaging findings suggest appendicitis. Having AMNs as part of the differential diagnosis is especially necessary in cases, such as this one, in which the patient has appendiceal inversion, is aged > 50 years, and has concurrent colorectal neoplasms.
Appendiceal mucinous neoplasms (AMNs) are rare tumors of the appendix that can be asymptomatic or present with acute right lower quadrant (RLQ) pain mimicking appendicitis. Due to their potential to cause either no symptoms or nonspecific symptoms, such as abdominal pain, nausea, or vomiting, AMNs are often found incidentally during appendectomies or, even more rarely, colonoscopies. Most AMNs grow slowly and have little metastatic potential. However, due to potential complications, such as bowel obstruction and rupture, timely detection and removal of AMN is essential. We describe the case of a patient who appeared to have acute appendicitis complicated by rupture on imaging who was found instead to have a perforated low-grade AMN during surgery.
Case Presentation
A male patient aged 72 years with a history of type 2 diabetes mellitus, hypertension, and aortic stenosis, but no prior abdominal surgery, presented with a chief concern of generalized weakness. As part of the workup for his weakness, a computed tomography (CT) scan of the abdomen was performed which showed an RLQ phlegmon and mild fat stranding in the area. Imaging also revealed an asymptomatic gallstone measuring 1.5 cm with no evidence of cholecystitis. The patient had no fever and reported no abdominal pain, nausea, vomiting, or change in bowel habits. On physical examination, the patient’s abdomen was soft, nontender, and nondistended with normoactive bowel sounds and no rebound or guarding.
To manage the appendicitis, the patient started a 2-week course of amoxicillin clavulanate 875 mg twice daily and was instructed to schedule an interval appendectomy in the coming months. Four days later, during a follow-up with his primary care physician, he was found to be asymptomatic. However, at this visit his stool was found to be positive for occult blood. Given this finding and the lack of a previous colonoscopy, the patient underwent a colonoscopy, which revealed bulging at the appendiceal orifice, consistent with an inverted appendix. Portions of the appendix were biopsied (Figure 1). Histologic analysis of the appendiceal biopsies revealed no dysplasia or malignancy. The colonoscopy also revealed an 8-mm sessile polyp in the ascending colon which was resected, and histologic analysis of this polyp revealed a low-grade tubular adenoma. Additionally, a large angiodysplastic lesion was found in the ascending colon as well as external and medium-sized internal hemorrhoids.
Six weeks after the colonoscopy, the patient was taken to the operating room for a laparoscopic appendectomy. Upon entry of the abdomen, extensive adhesions throughout the RLQ were found which required adhesiolysis. A calcified fecalith adherent to the mesentery of the small intestine in the RLQ was also found and resected. After lysis of the adhesions, the appendix and fibrotic tissue surrounding it could be seen (Figure 2). The appendix was dilated and the tip showed perforation. During dissection of the appendix, clear gelatinous material was found coming from the appendiceal lumen as well as from the fibrotic tissue around the appendix. On postoperative day 1 the appendix was resected and the patient was discharged.
Histologic specimens of the appendix were notable for evidence of perforation and neoplasia leading to a diagnosis of low-grade AMN. The presence of atypical mucinous epithelial cells on the serosal surface of the appendix, confirmed with a positive pancytokeratin stain, provided histologic evidence of appendiceal perforation (Figure 3). The presence of nuclear atypia demonstrated that the appendix was involved by a neoplastic process. Additionally, attenuation of the normal appendiceal epithelium, evidence of a chronic process, further helped to differentiate the AMN from complicated appendicitis. The presence of mucin involving the serosa of the appendix led to the classification of this patient’s neoplasm as grade pT4a. Of note, histologic examination demonstrated that the surgical margins contained tumor cells.
Given the positive margins of the resected AMN and the relatively large size of the neoplasm, a laparoscopic right hemicolectomy was performed 2 months later. Although multiple adhesions were found in the terminal ileum, cecum, and ascending colon during the hemicolectomy, no mucinous lesions were observed grossly. Histologic analysis showed no residual neoplasm as well as no lymph node involvement. On postoperative day 3 the patient was discharged and had an uneventful recovery. At his first surveillance visit 6 months after his hemicolectomy, the patient appeared to be doing well and reported no abdominal pain, nausea, vomiting, change in bowel habits, or any blood in the stool.
Discussion
AMNs are rare tumors with an annual age-adjusted incidence of approximately 0.12 per 1,000,000 people.1 These neoplasms can present as acute or chronic abdominal pain, gastrointestinal bleeding, intestinal obstruction, or acute abdomen.2-4 Most AMNs, however, are asymptomatic and are usually found incidentally during appendectomies for appendicitis, and can even be found during colonoscopies,such as in this case.5,6
Low-grade AMNs are distinguished from appendiceal mucinous adenocarcinomas by their lack of wall invasion.7 Additionally, low-grade AMNs have a very good prognosis as even neoplasms that have spread outside of the appendix have a 5-year overall survival rate of 79 to 86%.8 These low-grade neoplasms also have extremely low rates of recurrence after resection.9 In contrast, appendiceal mucinous adenocarcinomas have a much worse prognosis with a 5-year overall survival rate of 53.6%.10
Treatment of AMNs depends on the extent of their spread. Neoplasms that are confined to the appendix can typically be treated with appendectomy alone, while those that have spread beyond the appendix may require cytoreductive surgery and chemotherapy, namely, hyperthermic intraperitoneal chemotherapy (HIPEC), in addition to appendectomy.11 Cases in which neoplasms are not confined to the appendix also require more frequent surveillance for recurrence as compared to appendix-restricted neoplasms.11
Appendiceal inversion is a rare finding in adults with an estimated prevalence of 0.01%.6 Not only is appendiceal inversion rare in and of itself, it is even more rarely found in combination with appendiceal neoplasms.6 Other causes of appendiceal inversion include intussusception, acute appendicitis, appendiceal nodule, or even iatrogenic due to appendectomy.12-14 While appendiceal inversion can be completely benign, because these morphological changes of the appendix can resemble a polyp, these lesions are often biopsied and/or resected.15 However, lesion resection may be quite problematic due to high risk of bleeding and perforation.15 In order to avoid the risks associated with resection of a potentially benign finding, biopsy should be performed prior to any attempted resection of inverted appendices.15
Another interesting aspect of this case is the finding of fecal occult blood. The differential for fecal occult blood is quite broad and the patient had multiple conditions that could have led to the finding of occult blood in his stool. Hemorrhoids can cause a positive result on a fecal occult blood test (FOBT) although this is relatively uncommon, and hemorrhoids are more likely to cause frank blood to be seen.16 The sessile polyp found in the patient’s colon may also have caused the FOBT to be positive. This patient was also found to have an angiodysplasia (a finding that is associated with aortic stenosis, which this patient has a history of) which can also cause gastrointestinal bleeding.17 Lastly, AMNs may also cause gastrointestinal bleeding and thus a positive FOBT, although bleeding is a relatively uncommon presentation of AMNs, especially those that are low-grade as in this case.18
This case also highlights the association between appendiceal neoplasms and colonic neoplastic lesions. Patients with appendiceal neoplasms are more likely to have colonic neoplastic lesions than patients without appendiceal neoplasms.19 Studies have found that approximately 13 to 42% of patients with appendiceal neoplasms also have colonic neoplastic lesions.19 The majority of these lesions in the colon were right-sided and this finding was also seen in this case as the patient’s polyp was located in the ascending colon.19 Due to this association between appendiceal and colorectal neoplasia, the American Society of Colon and Rectal Surgeons strongly recommends that patients with appendiceal neoplasms or who are suspected of having them receive a colonoscopy.19
Additionally, perforation of an AMN, as was seen in this case, is a finding that should raise significant concern. Perforation of an AMN allows for the spread of malignant mucinous epithelial cells throughout the abdomen. The finding of extensive adhesions throughout the patient’s RLQ was unexpected as abdominal adhesions are most often seen in patients with a history of abdominal surgeries. Considering the lack of any prior abdominal surgeries in this patient, these adhesions were most likely the result of the spread and proliferation of malignant mucinous epithelial cells from the perforated AMN in the RLQ.20 The adhesiolysis performed in this case was thus not only important in order to visualize the appendix, but also for preventing future complications of abdominal adhesions such as bowel obstruction.20 Perforated AMN is also so concerning because it can potentially lead to pseudomyxoma peritonei—a condition in which malignant mucinous epithelial cells accumulate in the abdomen.21 Pseudomyxoma peritonei is extremely rare with an incidence of approximately 1 to 2 cases per million per year.22 Early recognition of AMNs and surgical referral are critically important as pseudomyxoma peritonei is difficult to treat, has a high rate of recurrence, and can be fatal.23
Lastly, this case highlights how findings of a ruptured appendix and/or mucin surrounding the appendix on imaging should warrant laparoscopy because only pathologic analysis of the appendix can definitively rule out AMNs. The utility of laparoscopic evaluation of the appendix is especially apparent as nonsurgical treatment of appendicitis using antibiotics is gaining favor for treating even complicated appendicitis.24 Appendicitis is much more common than AMNs. However, had the patient in this case only been given antibiotics for his suspected complicated appendicitis without any colonoscopy or appendectomy, the neoplasm in his appendix would have gone undetected and continued to grow, causing significant complications. The patient’s age at presentation in this case also necessitated laparoscopic evaluation of the appendix as the incidence of AMNs is highest among patients aged > 60 years.25 Additionally, because appendiceal inversion may be seen with AMNs,the patient’s inverted appendix seen during his colonoscopy was another compelling reason for laparoscopic evaluation of his appendix.6
Conclusions
AMNs can present with nonspecific symptoms or can be completely asymptomatic and are often found incidentally during colonoscopies or appendectomies for acute appendicitis. While it is true that AMNs have low metastatic potential and grow slowly, AMNs can rupture leading to pseudomyxoma peritonei or even cause bowel obstruction warranting timely identification and removal of these neoplasms. Laparoscopic evaluation in cases of ruptured appendices is critical not only for treatment, but also for determining the presence of a potential underlying appendiceal malignancy. Although AMNs are a rare pathology, physicians should still consider the possibility of these neoplasms even when imaging findings suggest appendicitis. Having AMNs as part of the differential diagnosis is especially necessary in cases, such as this one, in which the patient has appendiceal inversion, is aged > 50 years, and has concurrent colorectal neoplasms.
1. Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol. 2017;40(6):569-573. doi:10.1097/COC.0000000000000210
2. Kehagias I, Zygomalas A, Markopoulos G, Papandreou T, Kraniotis P. Diagnosis and treatment of mucinous appendiceal neoplasm presented as acute appendicitis. Case Rep Oncol Med. 2016;3:1-6. doi:10.1155/2016/2161952
3. Karatas M, Simsek C, Gunay S, et al. Acute lower gastrointestinal bleeding due to low-grade mucinous neoplasm of appendix. Acta Chir Belg. 2020;120(4):1-4. doi:10.1080/00015458.2020.1860397
4. Mourad FH, Hussein M, Bahlawan M, Haddad M, Tawil A. Intestinal obstruction secondary to appendiceal mucocele. Dig Dis Sci. 1999;44(8):1594-1599. doi:10.1023/a:1026615010989
5. Benabe SH, Leeman R, Brady AC, Hirzel A, Langshaw AH. Low-grade appendiceal mucinous neoplasm in an adolescent patient with untreated Crohn’s disease. ACG Case Reports J. 2020;7(3). doi:10.14309/crj.0000000000000338
6. Liu X, Liu G, Liu Y, et al. Complete appendiceal inversion with local high-grade intraepithelial neoplasia in an adult female: A case report. BMC Surg. 2019;19(1). doi:10.1186/s12893-019-0632-3
7. Gündog˘ar ÖS, Kımılog˘lu ES, Komut NS, et al. The evaluation of appendiceal mucinous neoplasms with a new classification system. Turk J Gastroenterol. 2018;29(5):532-542. doi:10.5152/tjg.2018.17605
8. Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089-1103. doi:10.1097/00000478-200308000-00006
9. Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33(10):1425-1439. doi:10.1097/PAS.0b013e3181af6067
10. Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2015;122(2):213-221. doi:10.1002/cncr.29744
11. Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. 2018;23(1):137. doi:10.1634/theoncologist.2017-0081erratum
12. Tran C, Sakioka J, Nguyen E, Beutler BD, Hsu J. An inverted appendix found on routine colonoscopy: a case report with discussion of imaging findings. Radiol Case Rep. 2019;14(8):952-955. doi:10.1016/j.radcr.2019.05.022
13. Shafi A, Azab M. A case of everted appendix with benign appendiceal nodule masquerading as appendiceal mucocele: a case report. Am J Gastroenterol. 2018;113:S1436. doi:10.14309/00000434-201810001-02585
14. Pokhrel B, Chang M, Anand G, Savides T, Fehmi S. Appendiceal mucinous neoplasm in an inverted appendix found on prior colonoscopy. VideoGIE. 2020;5(1):34-36. doi:10.1016/j.vgie.2019.09.013
15. Johnson EK, Arcila ME, Steele SR. Appendiceal inversion: a diagnostic and therapeutic dilemma. JSLS. 2009;13(1):92-95.
16. van Turenhout ST, Oort FA, sive Droste JST, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc. 2012;76(1):136-143. doi:10.1016/j.gie.2012.03.169
17. Hudzik B, Wilczek K, Gasior M. Heyde syndrome: Gastrointestinal bleeding and aortic stenosis. CMAJ. 2016;188(2):135-138. doi:10.1503/cmaj.150194
18. Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. RadioGraphics. 2017;37(4):1059-1083. doi:10.1148/rg.2017160150
19. Glasgow SC, Gaertner W, Stewart D, et al. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62(12):1425-1438. doi:10.1097/DCR.0000000000001530
20. Panagopoulos P, Tsokaki T, Misiakos E, et al. Low-grade appendiceal mucinous neoplasm presenting as an adnexal mass. Case Reports in Obstetrics and Gynecology. 2017;2017:1-3. doi:10.1155/2017/7165321
21. Ramaswamy V. Pathology of mucinous appendiceal tumors and pseudomyxoma peritonei. Indian J Surg Oncol. 2016;7(2):258-267. doi:10.1007/s13193-016-0516-2.
22. Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44-50. doi:10.4251/wjgo.v2.i1.44
23. Mercier F, Dagbert F, Pocard M, et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open. 2018;3(2):195-202. doi:10.1002/bjs5.97
24. David A, Dodgion C, Eddine SBZ, Davila D, Webb TP, Trevino CM. Perforated appendicitis: Short duration antibiotics are noninferior to traditional long duration antibiotics. Surgery. 2020;167(2):475-477. doi:10.1016/j.surg.2019.08.007
25. Raijman I, Leong S, Hassaram S, Marcon NE. Appendiceal mucocele: Endoscopic appearance. Endoscopy. 1994;26(3):326-328. doi:10.1055/s-2007-1008979
1. Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol. 2017;40(6):569-573. doi:10.1097/COC.0000000000000210
2. Kehagias I, Zygomalas A, Markopoulos G, Papandreou T, Kraniotis P. Diagnosis and treatment of mucinous appendiceal neoplasm presented as acute appendicitis. Case Rep Oncol Med. 2016;3:1-6. doi:10.1155/2016/2161952
3. Karatas M, Simsek C, Gunay S, et al. Acute lower gastrointestinal bleeding due to low-grade mucinous neoplasm of appendix. Acta Chir Belg. 2020;120(4):1-4. doi:10.1080/00015458.2020.1860397
4. Mourad FH, Hussein M, Bahlawan M, Haddad M, Tawil A. Intestinal obstruction secondary to appendiceal mucocele. Dig Dis Sci. 1999;44(8):1594-1599. doi:10.1023/a:1026615010989
5. Benabe SH, Leeman R, Brady AC, Hirzel A, Langshaw AH. Low-grade appendiceal mucinous neoplasm in an adolescent patient with untreated Crohn’s disease. ACG Case Reports J. 2020;7(3). doi:10.14309/crj.0000000000000338
6. Liu X, Liu G, Liu Y, et al. Complete appendiceal inversion with local high-grade intraepithelial neoplasia in an adult female: A case report. BMC Surg. 2019;19(1). doi:10.1186/s12893-019-0632-3
7. Gündog˘ar ÖS, Kımılog˘lu ES, Komut NS, et al. The evaluation of appendiceal mucinous neoplasms with a new classification system. Turk J Gastroenterol. 2018;29(5):532-542. doi:10.5152/tjg.2018.17605
8. Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089-1103. doi:10.1097/00000478-200308000-00006
9. Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33(10):1425-1439. doi:10.1097/PAS.0b013e3181af6067
10. Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2015;122(2):213-221. doi:10.1002/cncr.29744
11. Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. 2018;23(1):137. doi:10.1634/theoncologist.2017-0081erratum
12. Tran C, Sakioka J, Nguyen E, Beutler BD, Hsu J. An inverted appendix found on routine colonoscopy: a case report with discussion of imaging findings. Radiol Case Rep. 2019;14(8):952-955. doi:10.1016/j.radcr.2019.05.022
13. Shafi A, Azab M. A case of everted appendix with benign appendiceal nodule masquerading as appendiceal mucocele: a case report. Am J Gastroenterol. 2018;113:S1436. doi:10.14309/00000434-201810001-02585
14. Pokhrel B, Chang M, Anand G, Savides T, Fehmi S. Appendiceal mucinous neoplasm in an inverted appendix found on prior colonoscopy. VideoGIE. 2020;5(1):34-36. doi:10.1016/j.vgie.2019.09.013
15. Johnson EK, Arcila ME, Steele SR. Appendiceal inversion: a diagnostic and therapeutic dilemma. JSLS. 2009;13(1):92-95.
16. van Turenhout ST, Oort FA, sive Droste JST, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc. 2012;76(1):136-143. doi:10.1016/j.gie.2012.03.169
17. Hudzik B, Wilczek K, Gasior M. Heyde syndrome: Gastrointestinal bleeding and aortic stenosis. CMAJ. 2016;188(2):135-138. doi:10.1503/cmaj.150194
18. Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. RadioGraphics. 2017;37(4):1059-1083. doi:10.1148/rg.2017160150
19. Glasgow SC, Gaertner W, Stewart D, et al. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62(12):1425-1438. doi:10.1097/DCR.0000000000001530
20. Panagopoulos P, Tsokaki T, Misiakos E, et al. Low-grade appendiceal mucinous neoplasm presenting as an adnexal mass. Case Reports in Obstetrics and Gynecology. 2017;2017:1-3. doi:10.1155/2017/7165321
21. Ramaswamy V. Pathology of mucinous appendiceal tumors and pseudomyxoma peritonei. Indian J Surg Oncol. 2016;7(2):258-267. doi:10.1007/s13193-016-0516-2.
22. Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44-50. doi:10.4251/wjgo.v2.i1.44
23. Mercier F, Dagbert F, Pocard M, et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open. 2018;3(2):195-202. doi:10.1002/bjs5.97
24. David A, Dodgion C, Eddine SBZ, Davila D, Webb TP, Trevino CM. Perforated appendicitis: Short duration antibiotics are noninferior to traditional long duration antibiotics. Surgery. 2020;167(2):475-477. doi:10.1016/j.surg.2019.08.007
25. Raijman I, Leong S, Hassaram S, Marcon NE. Appendiceal mucocele: Endoscopic appearance. Endoscopy. 1994;26(3):326-328. doi:10.1055/s-2007-1008979