User login
Can the test for human papillomavirus DNA be used as the stand-alone, first-line screening test for cervical cancer?
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
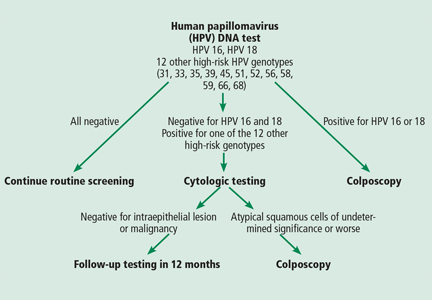
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.
Cervical cancer screening: Less testing, smarter testing
Cervical cancer screening and prevention have evolved rapidly in the last decade, especially in the 5 years since the introduction of the first cancer prevention vaccine, human papillomavirus (HPV) recombinant vaccine.1
Providers need to understand the rationale for the recommendations so that they can explain them to patients. In particular, patients may wonder why we now begin screening for cervical cancer later than we used to, and why some women do not need to be screened as often. Both of these changes result from our enhanced understanding of the role of HPV in cervical cancer genesis.
In this article we will briefly review:
- The current understanding of the natural history of cervical cancer
- Advantages and disadvantages of cervical cytology, ie, the Papanicolaou (Pap) test
- The role of HPV testing in cervical cancer screening
- The latest screening guidelines (the new standard of care)
- A possible future screening strategy
- The impact of HPV vaccination on screening.
500,000 NEW CASES EVERY YEAR
The incidence of cervical cancer and its mortality rate have decreased more than 50% in the United States over the past 3 decades, largely as a result of screening with the Pap test.2 In 2010, there were an estimated 12,200 new cases of invasive cervical cancer in the United States and 4,210 deaths from it,3 which are lower than the historical rates.
However, because most developing countries lack effective screening programs, cervical cancer remains the second-leading cause of death from cancer in women worldwide. According to a recent estimate, there are almost 500,000 new cases and 240,000 deaths from this disease worldwide every year.4 If effective global screening programs could be set up, they would markedly reduce the incidence of cervical cancer and deaths from it.5
HPV IS NECESSARY BUT NOT SUFFICIENT FOR CERVICAL CANCER TO DEVELOP
For cervical cancer to develop, the essential first step is infection of the cervical epithelium with one of the oncogenic (high-risk) types of HPV (see below).6–10 Walboomers et al9 tested cervical tissue samples taken from 932 women with cervical cancer and detected HPV DNA in 930 (99.8%) of them.
Fortunately, most HPV-infected women do not develop cervical cancer, as most young women clear the infection in an average of 8 to 24 months.11,12 However, if the infection persists, and if it is one of the high-risk types of HPV, precursor lesions can develop that can progress to cervical cancer.13 The evidence conclusively supports the association between oncogenic HPV infection and the subsequent development of virtually all cases of cervical cancer.6–10
Known risk factors for HPV persistence and the subsequent development of high-grade lesions are cigarette smoking and a compromised immune system.14,15
Terminology: Results of Pap smears
- Normal
- Atypical squamous cells of undetermined significance (ASC-US)
- Low-grade squamous intraepithelial lesions (LSIL)
- High-grade squamous intraepithelial lesions (HSIL)
- Cancer.
Terminology: Results of cervical biopsy
- Normal
- Cervical intraepithelial neoplasia grade 1 (CIN1)
- CIN2 (previously called moderate dysplasia)
- CIN3 (previously called severe dysplasia)
- Carcinoma in situ
- Invasive cervical cancer.
Lesions that are CIN2 or higher are considered high-grade.13
The 18 high-risk HPV types
More than 40 types of HPV infect the genital tract16; 18 of these (types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) are classified as high-risk because of their causative association with cervical cancer (ie, their oncogenic potential).17
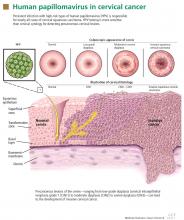
How HPV causes cervical cancer
In laboratory cultures, normal human cells die out after a few generations. However, if human epithelial cells are infected by one of the high-risk types of HPV, they can go on dividing indefinitely.18,19
Two HPV proteins, E6 and E7, induce this cell “immortalization.”20,21 E6 from high-risk HPV binds to the human tumor-suppressor protein p53 and rapidly degrades it in a proteolytic process. The p53 protein normally suppresses cell proliferation by arresting growth in the G1 phase of the cell cycle. Therefore, with less p53, the cell cannot suppress uncontrolled cell growth.22–24
Similarly, E7 from high-risk HPV forms a complex with another human tumor suppressor, the retinoblastoma protein (pRB), and disrupts its binding to a transcriptional factor, E2F-1. The freed E2F-1 then stimulates DNA synthesis and uncontrolled cell growth.25
Furthermore, HPV-16 E6 and E7 proteins can collectively cause cellular genetic instability.26
The carcinogenic mechanism of high-risk HPV is complex. The host immune system and natural tumor suppression play important roles. However, the natural history of cervical intraepithelial neoplasia is not well understood. For example, it remains unclear if low-grade lesions such as CIN1 are necessary precursors to high-grade lesions and invasive cancer.6,7,10
THE PAP TEST: SPECIFIC BUT NOT VERY SENSITIVE, AND PRONE TO ERROR
The principal advantage of cervical cytologic testing (ie, the Pap test) in detecting cervical dysplasia is its overall high specificity. Many studies have found that the specificity of conventional Pap testing can reach approximately 98%.27
However, the conventional Pap test has drawbacks. Contaminants such as blood, discharge, and lubricant can make it difficult to interpret, and artifact can occur with air-drying of the Pap smear as it is transferred to the cell slide (“air-drying artifact”).
Liquid-based cytologic study has replaced the older method
To overcome these disadvantages, a liquid-based method of cervical cytologic study, ThinPrep (Hologic, Bedford, MA), was introduced in the mid-1990s. In this method, cell samples are first transferred to a liquid solution for mechanical separation from contaminants, and a representative sample of cells is then placed on a slide for review.
The liquid-based method filters out most contaminating blood, inflammatory cells, and debris. It also eliminates the air-drying artifact in the conventional Pap collection technique and improves specimen adequacy. Cytotechnologists find liquid-based specimens easier to read because the cells are more evenly distributed on a clearer background. Another advantage is that we can routinely test for HPV in the available residual specimen if the cytologic interpretation is abnormal.
The main disadvantages of the liquid-based method are that its specificity is lower than that of conventional Pap smears (around 78%) and that it costs more.28 Nevertheless, the liquid-based technique has become the main method of cervical cytology, used by nearly 90% of gynecologists in the United States since 2003.1
Cytology is still prone to false-negative results
Despite the success of both conventional Pap testing and liquid-based Pap testing, cervical cytology is inherently prone to sample-quality variation, subjective interpretation error, and false-negative results. False-negative results can be due to failure to transfer dysplastic cells to the slide or failure of the cytologist to recognize abnormal cells. In 30% of new cases of cervical cancer, the patient had recently had a Pap test that was interpreted as negative.1,29
Errors in interpretation are exacerbated by inconsistency among cytopathologists. In one study,6,30 when a group of quality-control pathologists reviewed nearly 5,000 cytology specimens, they came to the same conclusion that the original reviewers did more than 50% of the time only for negative and LSIL readings. Of the specimens initially reported as ASC-US, almost 40% were reclassified as negative on further review. Of those originally interpreted as HSIL, more than 50% were reclassified as LSIL, as ASC-US, or as negative.
Furthermore, many studies found that the sensitivity of conventional Pap testing was only around 50%.27 The new liquid-based Pap test uses computer imaging, which has improved the rate of detection of cervical dysplasia but may still miss 15% to 35% of cases of HSIL (severe dysplasia) or cancer.31 Failure to detect cervical dysplasia or cancer on Pap smear has led to a number of lawsuits.32
Clearly, with its relatively low sensitivity, cervical cytology is no longer good enough to use as a sole screening test in all situations. However, its high specificity is an advantage when it is combined with HPV testing in screening.
HPV TESTING AND PAP TESTING COMPLEMENT EACH OTHER
From 17% to 36% of HPV-infected women develop a cytologic abnormality within 5 years, compared with 4% to 15% of women who are HPV-negative.33,34
The usefulness of testing for HPV in women who have had an abnormal Pap test has been well demonstrated in multiple studies.35–38
The landmark Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS)39 found that 82.9% of women with LSIL were HPV-positive. The investigators concluded that HPV testing has little utility in women with LSIL, as the test would likely be positive and thus would not change the decision to perform colposcopy.
However, in women with ASC-US, the sensitivity of HPV testing for predicting CIN3 or cancer was 96.3% and the negative predictive value was 99.5%. In contrast, the sensitivity of a single repeat Pap test was only 44.1%. This large randomized trial conclusively validates the important role of HPV testing in triaging women with ASC-US.
More recently, a meta-analysis of 20 studies of HPV testing in women with ASC-US found that it had a sensitivity of 92.5% and a specificity of 62.5% for detecting CIN2 or worse lesions, and a sensitivity of 95.6% and a specificity of 59.2% for detecting CIN3 or worse lesions.40
Furthermore, HPV testing in primary cervical cancer screening is strongly supported by large cross-sectional studies41–45 and randomized clinical trials.46,47 These studies have conclusively shown that HPV testing is significantly more sensitive than Pap testing for detecting cervical intraepithelial neoplasia, and that, when combined with Pap testing, it can achieve nearly 100% clinical sensitivity and nearly 93% specificity in women age 30 or older. Women who have negative results on both the HPV test and the Pap test can be reassured that their risk of undetected CIN2, CIN3, or cervical cancer is extremely low, since HPV testing has a negative predictive value close to 100%.46
In large multinational European studies involving more than 24,000 women, the risk of CIN3 or cancer after 6 years of follow-up was only 0.28% in women who had negative results on both HPV and Pap testing at baseline. This rate was basically the same as in women who tested negative for HPV alone (0.27%). However, it was significantly lower than that of all women who had negative Pap test results (0.97%). The combination of HPV testing and Pap testing at 6-year intervals offered better protection than Pap testing alone at 3-year intervals.48
NEW STANDARD OF CARE: THE LATEST SCREENING GUIDELINES
Until the mid-1990s, the strategy for cervical cancer screening had remained largely unchanged for many years. Since then, several advances have prompted changes in the standard of care.
1996—The US Food and Drug Administration (FDA) approved liquid-based Thin-Prep for cervical cancer screening, which improved specimen adequacy and reduced ambiguous interpretations compared with the original slide-based method of collection.49
2001—The Bethesda terminology for reporting cervical cytology results was updated. First proposed in 1988 to replace the original Papanicolaou system and revised in 1991, this standardized terminology enabled better clinical decision-making.50
2001—The FDA approved HPV testing for women with ASC-US. This provided a better triage strategy for deciding which women need colposcopy to exclude true intraepithelial lesions. Following the FDA approval, the clinical effectiveness of HPV testing in women with ASC-US was validated by a large randomized clinical trial—the ALTS.51
2003—The FDA approved HPV testing in conjunction with Pap testing for women age 30 or older in routine primary screening.52
Guidelines available
Based on these new developments in technology and reporting terminology, and the incorporation of HPV testing, several organizations issued guidelines.
The American Society for Colposcopy and Cervical Pathology published a consensus guideline on management of abnormal cervical cytology in 2001 and revised it in 2006.53
The American Cancer Society issued its guideline for cervical cancer screening in 2002.54
The US Preventive Services Task Force published its screening guidelines in 2003.55
The American College of Obstetricians and Gynecologists (ACOG) also made new recommendations in 2003 and updated them in December 2009.1
Start screening at age 21
Cervical cancer screening should begin at age 21 regardless of the age of onset of vaginal intercourse, according to the 2009 ACOG guidelines.1 This represents a change from previous recommendations from ACOG, the American Cancer Society, and the US Preventive Services Task Force, which were to start screening within 3 years of the onset of vaginal intercourse.
Rationale. This latest recommendation is based on the high rates of clearance of HPV infection and of spontaneous dysplasia regression and the low incidence of cervical cancer in younger women.57,58 HPV infections are common in young women who have had vaginal intercourse. However, most such HPV infections are cleared by the immune system within 1 to 2 years without causing cervical dysplasia.11,12 Invasive cervical cancer in women younger than 21 years is very rare. The annual incidence is only one to two cases per 1 million women ages 15 to 19.2,55
Another reason for not screening before age 21 is that a positive test result may lead to unnecessary anxiety and potentially harmful evaluations and procedures.
Screening intervals extended
The 2009 ACOG guidelines lengthen the cervical cancer screening interval to every 2 years in women under age 30.1 (The 2003 ACOG guidelines said to screen every year.)
For women age 30 and older, the 2009 ACOG guidelines recommend extending the interval to every 3 years when combined Pap and HPV testing are negative (changed from every 2 to 3 years).1
Rationale. Studies have shown little advantage in screening every year in women under the age of 30, with no higher risk of cervical cancer in women screened at a 2- to 3-year interval.59–62 The absolute risk of cervical cancer in a well-screened population is very low.63 Moreover, the absolute number of cervical cancer cases in women age 30 to 64 years screened at 3-year intervals is only four per 100,000 women.64
HPV-plus-Pap testing for women over 30
Based on convincing evidence of the high sensitivity and the high negative predictive value of HPV testing, since 2003 ACOG had recommended HPV-plus-Pap testing in women over age 30. Its 2009 guidelines upgraded this recommendation to level A, ie, the highest grade, based on good and consistent scientific evidence.1 (Previously the recommendation was level B.)
The American Cancer Society also recommends combined HPV and Pap testing as the optimal screening approach in women age 30 or older, with the subsequent screening interval 3 years if both tests are negative. It also endorses Pap testing alone every 2 to 3 years as an alternative screening strategy in this age group.
The US Preventive Services Task Force recommends Pap testing every 3 years in women age 30 or older, and it does not recommend for or against HPV testing. However, neither the US Preventive Services Task Force nor the American Cancer Society has updated its guidelines in 8 years.
Rationale. Women who undergo HPV-plus-Pap testing and who test negative on both are at very low risk of developing CIN2 or CIN3 during the next 4 to 6 years. The risk is much lower than that for women who have a sole negative Pap test result.39,40 Because of this extremely high negative predictive value, women age 30 and older who had negative results on both Pap and HPV testing should be screened no more often than every 3 years.
We believe that the HPV-plus-Pap testing strategy recommended by the 2009 ACOG guidelines for women age 30 and older is the most effective screening approach. This strategy takes advantage of the high sensitivity and high negative predictive value of HPV testing, as well as the high specificity of Pap testing. It achieves almost 100% clinical sensitivity in detecting cervical dysplasia.46
When to stop screening
The 2009 ACOG guidelines for the first time call for stopping cervical cancer screening in women 65 to 70 years of age who have had three negative Pap tests in a row and no abnormal tests in the previous 10 years.1 The American Cancer Society recommends stopping screening at age 70,65 while the US Preventive Services Task Force recommends stopping at age 65.55
Rationale. Cervical cancer develops slowly, and risk factors tend to decline with age, Also, postmenopausal mucosal atrophy may predispose to false-positive Pap results, which can lead to additional procedures and unnecessary patient anxiety.66
However, it is probably reasonable to continue screening in women age 70 and older who are sexually active with multiple partners and who have a history of abnormal Pap test results.1
Women who have had a hysterectomy
According to the latest American Cancer Society, ACOG, and US Preventive Services Task Force guidelines, cervical cancer screening should be discontinued after total hysterectomy for benign indications in women who have no history of high-grade cervical intraepithelial neoplasia, ie, CIN2 or worse.1
Rationale. If the patient has no cervix, continued vaginal cytology screening is not indicated, since the incidence of primary vaginal cancer is one to two cases per 100,000 women per year, much lower than that of cervical cancer.65
However, before discontinuing screening, clinicians should verify that any Pap tests the patient had before the hysterectomy were all read as normal, that the hysterectomy specimen was normal, and that the cervix was completely removed during hysterectomy.
Be ready to explain the recommendations
It is very important for providers to understand the evidence supporting the latest guidelines, as many patients may not realize the significant technological improvements and improved understanding of the role of HPV in cervical cancer genesis that have resulted in the deferred onset of screening and the longer intervals between screenings. This knowledge gap for patients can result in anxiety when told they no longer need an annual Pap test or can start later, if the issue is not properly and thoroughly explained by a confident provider.
A FUTURE STRATEGY: HPV AS THE SOLE PRIMARY SCREENING TEST?
Since HPV testing is much more sensitive than Pap testing for detecting cervical lesions of grade CIN2 or higher, why not use HPV testing as the primary test and then do Pap testing (which is more specific) only if the HPV test is positive?
Mayrand et al46 conducted the first large randomized trial in which HPV testing was compared directly as a stand-alone test with the Pap test in a North American population with access to quality care. Results were published in 2007. In Canada, a total of 10,154 women ages 30 to 69 years in Montreal and St. John’s were randomly assigned to undergo either conventional Pap testing or HPV testing. The sensitivity of HPV testing for CIN2 or CIN3 was 94.6%, whereas the sensitivity of Pap testing was only 55.4%. The specificity was 94.1% for HPV testing and 96.8% for Pap testing. In addition, HPV screening followed by Pap triage resulted in fewer referrals for colposcopy than did either test alone (1.1% vs 2.9% with Pap testing alone or 6.1% with HPV testing alone). In other words, HPV testing was almost 40% more sensitive and only 2.7% less specific than Pap testing in detecting cervical cancer precursors.
However, more controlled trials are needed to validate such a strategy. Furthermore, it remains unclear if a change from Pap testing to a primary HPV testing screening strategy will further reduce the mortality rate of cervical cancer, since the burden of cervical cancer worldwide lies in less-screened populations in low-resource settings.
Dillner et al,48 in a 2008 European study, further demonstrated that HPV testing offers better long-term (6-year) predictive value for CIN3 or worse lesions than cytology does. These findings suggest that HPV testing, with its higher sensitivity and negative predictive value and its molecular focus on cervical carcinogenesis, may safely permit longer screening intervals in a low-risk population.
Sankaranarayanan et al72 performed a randomized trial in rural India in which 131,746 women age 30 to 59 years were randomly assigned to four groups: screening by HPV testing, screening by Pap testing, screening by visual inspection with acetic acid, and counseling only (the control group). At 8 years of follow-up, the numbers of cases of cervical cancer and of cervical cancer deaths were as follows:
- With HPV testing: 127 cases, 34 deaths
- With Pap testing: 152 cases, 54 deaths
- With visual inspection: 157 cases, 56 deaths
- With counseling only: 118 cases, 64 deaths.
The authors concluded that in a low-resource setting, a single round of HPV testing was associated with a significant reduction in the number of deaths from cervical cancer. Not only did the HPV testing group have a lower incidence of cancer-related deaths, there were no cancer deaths among the women in this group who tested negative for HPV. This is the first randomized trial to suggest that using HPV testing as the sole primary cervical cancer screening test may have a benefit in terms of the mortality rate.
At present, to the best of our knowledge, there are no US data validating the role of HPV testing as a stand-alone screening test for cervical cancer.
HPV VACCINATION DOES NOT MEAN THE END OF SCREENING
The development of an effective HPV vaccine and FDA approval of the first quadrivalent (active against HPV 6, 11, 16, and 18) recombinant vaccine (Gardasil) in 2006 has opened a new era of cervical cancer prevention.73,74 At present, the Advisory Committee on Immunization Practices75 recommends vaccination for females 9 to 26 years old.
However, HPV vaccination will not make screening obsolete, since not all women will be vaccinated, and those who have already contracted one of these high-risk HPV types will not benefit.76,77 In addition, the current HPV vaccine does not protect against infection with other oncogenic HPV types. The experts estimate that the initial impact of the HPV vaccine on cervical cancer will not likely be apparent until at least 20 to 30 years after a nationwide vaccination program is implemented.78,79 Therefore, the HPV vaccine certainly does not portend the end of screening. Vaccination combined with continued screening will provide added benefit for cervical cancer prevention.80
The last decade has been an exciting period in the field of cervical cancer screening and prevention, with advances in technology, newly acquired knowledge, and the development of the HPV vaccine. As a result, our clinical practice has become a work in progress, continuing to evolve as we continue to discover more information. The possibility of eradicating cervical cancer has never been greater. The implementation of the most sensitive and effective screening strategy and of a worldwide HPV vaccination program will help us to eventually eradicate cervical cancer and make it a disease of the past.81
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24( suppl 3):S3/11–S3/25.
- Herrero R. Epidemiology of cervical cancer. J Natl Cancer Inst Monogr 1996; 21:1–6.
- Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer 2007; 111:145–153.
- Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 2006; 24(suppl 3):S3/42–S3/51.
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244–265.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol 1996; 175:1099–1104.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–428.
- Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709–715.
- Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002; 325:572.
- Sycuro LK, Xi LF, Hughes JP, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis 2008; 198:971–978.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907.
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–1278.
- Stöppler H, Stöppler MC, Schlegel R. Transforming proteins of the papillomaviruses. Intervirology 1994; 37:168–179.
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994; 48:427–447.
- Scheffner M, Romanczuk H, Münger K, Huibregtse JM, Mietz JA, Howley PM. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol 1994; 186:83–99.
- Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 1994; 68:4358–4368.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248:76–79.
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75:495–505.
- Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995; 55:4420–4424.
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 1989; 8:4099–4105.
- Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 2000; 97:10002–10007.
- Agency for Healthcare Research and Quality. Evaluation of Cervical Cytology. Summary: Evidence Report/Technology Assessment: Number 5. http://archive.ahrq.gov/clinic/epcsums/cervsumm.htm. Accessed August 18, 2011.
- Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001; 83:439–444.
- Shingleton HM, Patrick RL, Johnston WW, Smith RA. The current status of the Papanicolaou smear. CA Cancer J Clin 1995; 45:305–320.
- Stoler MH, Schiffman M; Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001; 285:1500–1505.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Allen KA, Zaleski S, Cohen MB. Review of negative Papanicolaou tests. Is the retrospective 5-year review necessary? Am J Clin Pathol 1994; 101:19–21.
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–3114.
- Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal Pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 2002; 95:2145–2151.
- Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999; 281:1605–1610.
- Wright TC, Lorincz A, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. Am J Obstet Gynecol 1998; 178:962–966.
- Shlay JC, Dunn T, Byers T, Barón AE, Douglas JM. Prediction of cervical intraepithelial neoplasia grade 2–3 using risk assessment and human papillomavirus testing in women with atypia on Papanicolaou smears. Obstet Gynecol 2000; 96:410–416.
- Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstet Gynecol 2000; 95:821–827.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003; 188:1383–1392.
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006; 24(suppl 3):S3/78–S3/89.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003; 362:1871–1876.
- Salmerón J, Lazcano-Ponce E, Lorincz A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003; 14:505–512.
- Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000; 92:464–474.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006; 119:1095–1101.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008; 100:492–501.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Noller KL, Bettes B, Zinberg S, Schulkin J. Cervical cytology screening practices among obstetrician-gynecologists. Obstet Gynecol 2003; 102:259–265.
- Solomon D, Davey D, Kurman R, et al; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–2119.
- The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst 2000; 92:397–402.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol 2008; 112:1419–1444.
- Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol 2007; 197:340–345.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Systematic Evidence Review No. 25. http://www.ahrq.gov/downloads/pub/prevent/pdfser/cervcanser.pdf. Accessed October 9, 2011.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132:277–284.
- Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008; 113(suppl 10):2855–2864.
- IARC Working Group on evaluation of cervical cancer screening programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J (Clin Res Ed) 1986; 293:659–664.
- Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol 2000; 96:219–223.
- Eddy DM. The frequency of cervical cancer screening. Comparison of a mathematical model with empirical data. Cancer 1987; 60:1117–1122.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol 2003; 101:29–37.
- Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med 2003; 349:1501–1509.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS). Ann Intern Med 2000; 133:942–950.
- Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M. Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. Br J Cancer 2005; 93:862–867.
- Ronco G, Segnan N, Giorgi-Rossi P, et al; New Technologies for Cervical Cancer Working Group. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst 2006; 98:765–774.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360:1385–1394.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271–278.
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007; 56(RR02):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm?s_cid=rr5602a1_e. Accessed 8/30/2011.
- Koutsky LA, Harper DM. Chapter 13: Current findings from prophylactic HPV vaccine trials. Vaccine 2006; 24( suppl 3):S3/114–S3/121.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24( suppl 3):S3/178–S3/186.
- Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res 2002; 89:285–293.
- Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 2006; 24(suppl 3):S3/171–S3/177.
- Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine 2006; 24(suppl 3:S3/90–S3/97.
Cervical cancer screening and prevention have evolved rapidly in the last decade, especially in the 5 years since the introduction of the first cancer prevention vaccine, human papillomavirus (HPV) recombinant vaccine.1
Providers need to understand the rationale for the recommendations so that they can explain them to patients. In particular, patients may wonder why we now begin screening for cervical cancer later than we used to, and why some women do not need to be screened as often. Both of these changes result from our enhanced understanding of the role of HPV in cervical cancer genesis.
In this article we will briefly review:
- The current understanding of the natural history of cervical cancer
- Advantages and disadvantages of cervical cytology, ie, the Papanicolaou (Pap) test
- The role of HPV testing in cervical cancer screening
- The latest screening guidelines (the new standard of care)
- A possible future screening strategy
- The impact of HPV vaccination on screening.
500,000 NEW CASES EVERY YEAR
The incidence of cervical cancer and its mortality rate have decreased more than 50% in the United States over the past 3 decades, largely as a result of screening with the Pap test.2 In 2010, there were an estimated 12,200 new cases of invasive cervical cancer in the United States and 4,210 deaths from it,3 which are lower than the historical rates.
However, because most developing countries lack effective screening programs, cervical cancer remains the second-leading cause of death from cancer in women worldwide. According to a recent estimate, there are almost 500,000 new cases and 240,000 deaths from this disease worldwide every year.4 If effective global screening programs could be set up, they would markedly reduce the incidence of cervical cancer and deaths from it.5
HPV IS NECESSARY BUT NOT SUFFICIENT FOR CERVICAL CANCER TO DEVELOP
For cervical cancer to develop, the essential first step is infection of the cervical epithelium with one of the oncogenic (high-risk) types of HPV (see below).6–10 Walboomers et al9 tested cervical tissue samples taken from 932 women with cervical cancer and detected HPV DNA in 930 (99.8%) of them.
Fortunately, most HPV-infected women do not develop cervical cancer, as most young women clear the infection in an average of 8 to 24 months.11,12 However, if the infection persists, and if it is one of the high-risk types of HPV, precursor lesions can develop that can progress to cervical cancer.13 The evidence conclusively supports the association between oncogenic HPV infection and the subsequent development of virtually all cases of cervical cancer.6–10
Known risk factors for HPV persistence and the subsequent development of high-grade lesions are cigarette smoking and a compromised immune system.14,15
Terminology: Results of Pap smears
- Normal
- Atypical squamous cells of undetermined significance (ASC-US)
- Low-grade squamous intraepithelial lesions (LSIL)
- High-grade squamous intraepithelial lesions (HSIL)
- Cancer.
Terminology: Results of cervical biopsy
- Normal
- Cervical intraepithelial neoplasia grade 1 (CIN1)
- CIN2 (previously called moderate dysplasia)
- CIN3 (previously called severe dysplasia)
- Carcinoma in situ
- Invasive cervical cancer.
Lesions that are CIN2 or higher are considered high-grade.13
The 18 high-risk HPV types
More than 40 types of HPV infect the genital tract16; 18 of these (types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) are classified as high-risk because of their causative association with cervical cancer (ie, their oncogenic potential).17
How HPV causes cervical cancer
In laboratory cultures, normal human cells die out after a few generations. However, if human epithelial cells are infected by one of the high-risk types of HPV, they can go on dividing indefinitely.18,19
Two HPV proteins, E6 and E7, induce this cell “immortalization.”20,21 E6 from high-risk HPV binds to the human tumor-suppressor protein p53 and rapidly degrades it in a proteolytic process. The p53 protein normally suppresses cell proliferation by arresting growth in the G1 phase of the cell cycle. Therefore, with less p53, the cell cannot suppress uncontrolled cell growth.22–24
Similarly, E7 from high-risk HPV forms a complex with another human tumor suppressor, the retinoblastoma protein (pRB), and disrupts its binding to a transcriptional factor, E2F-1. The freed E2F-1 then stimulates DNA synthesis and uncontrolled cell growth.25
Furthermore, HPV-16 E6 and E7 proteins can collectively cause cellular genetic instability.26
The carcinogenic mechanism of high-risk HPV is complex. The host immune system and natural tumor suppression play important roles. However, the natural history of cervical intraepithelial neoplasia is not well understood. For example, it remains unclear if low-grade lesions such as CIN1 are necessary precursors to high-grade lesions and invasive cancer.6,7,10
THE PAP TEST: SPECIFIC BUT NOT VERY SENSITIVE, AND PRONE TO ERROR
The principal advantage of cervical cytologic testing (ie, the Pap test) in detecting cervical dysplasia is its overall high specificity. Many studies have found that the specificity of conventional Pap testing can reach approximately 98%.27
However, the conventional Pap test has drawbacks. Contaminants such as blood, discharge, and lubricant can make it difficult to interpret, and artifact can occur with air-drying of the Pap smear as it is transferred to the cell slide (“air-drying artifact”).
Liquid-based cytologic study has replaced the older method
To overcome these disadvantages, a liquid-based method of cervical cytologic study, ThinPrep (Hologic, Bedford, MA), was introduced in the mid-1990s. In this method, cell samples are first transferred to a liquid solution for mechanical separation from contaminants, and a representative sample of cells is then placed on a slide for review.
The liquid-based method filters out most contaminating blood, inflammatory cells, and debris. It also eliminates the air-drying artifact in the conventional Pap collection technique and improves specimen adequacy. Cytotechnologists find liquid-based specimens easier to read because the cells are more evenly distributed on a clearer background. Another advantage is that we can routinely test for HPV in the available residual specimen if the cytologic interpretation is abnormal.
The main disadvantages of the liquid-based method are that its specificity is lower than that of conventional Pap smears (around 78%) and that it costs more.28 Nevertheless, the liquid-based technique has become the main method of cervical cytology, used by nearly 90% of gynecologists in the United States since 2003.1
Cytology is still prone to false-negative results
Despite the success of both conventional Pap testing and liquid-based Pap testing, cervical cytology is inherently prone to sample-quality variation, subjective interpretation error, and false-negative results. False-negative results can be due to failure to transfer dysplastic cells to the slide or failure of the cytologist to recognize abnormal cells. In 30% of new cases of cervical cancer, the patient had recently had a Pap test that was interpreted as negative.1,29
Errors in interpretation are exacerbated by inconsistency among cytopathologists. In one study,6,30 when a group of quality-control pathologists reviewed nearly 5,000 cytology specimens, they came to the same conclusion that the original reviewers did more than 50% of the time only for negative and LSIL readings. Of the specimens initially reported as ASC-US, almost 40% were reclassified as negative on further review. Of those originally interpreted as HSIL, more than 50% were reclassified as LSIL, as ASC-US, or as negative.
Furthermore, many studies found that the sensitivity of conventional Pap testing was only around 50%.27 The new liquid-based Pap test uses computer imaging, which has improved the rate of detection of cervical dysplasia but may still miss 15% to 35% of cases of HSIL (severe dysplasia) or cancer.31 Failure to detect cervical dysplasia or cancer on Pap smear has led to a number of lawsuits.32
Clearly, with its relatively low sensitivity, cervical cytology is no longer good enough to use as a sole screening test in all situations. However, its high specificity is an advantage when it is combined with HPV testing in screening.
HPV TESTING AND PAP TESTING COMPLEMENT EACH OTHER
From 17% to 36% of HPV-infected women develop a cytologic abnormality within 5 years, compared with 4% to 15% of women who are HPV-negative.33,34
The usefulness of testing for HPV in women who have had an abnormal Pap test has been well demonstrated in multiple studies.35–38
The landmark Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS)39 found that 82.9% of women with LSIL were HPV-positive. The investigators concluded that HPV testing has little utility in women with LSIL, as the test would likely be positive and thus would not change the decision to perform colposcopy.
However, in women with ASC-US, the sensitivity of HPV testing for predicting CIN3 or cancer was 96.3% and the negative predictive value was 99.5%. In contrast, the sensitivity of a single repeat Pap test was only 44.1%. This large randomized trial conclusively validates the important role of HPV testing in triaging women with ASC-US.
More recently, a meta-analysis of 20 studies of HPV testing in women with ASC-US found that it had a sensitivity of 92.5% and a specificity of 62.5% for detecting CIN2 or worse lesions, and a sensitivity of 95.6% and a specificity of 59.2% for detecting CIN3 or worse lesions.40
Furthermore, HPV testing in primary cervical cancer screening is strongly supported by large cross-sectional studies41–45 and randomized clinical trials.46,47 These studies have conclusively shown that HPV testing is significantly more sensitive than Pap testing for detecting cervical intraepithelial neoplasia, and that, when combined with Pap testing, it can achieve nearly 100% clinical sensitivity and nearly 93% specificity in women age 30 or older. Women who have negative results on both the HPV test and the Pap test can be reassured that their risk of undetected CIN2, CIN3, or cervical cancer is extremely low, since HPV testing has a negative predictive value close to 100%.46
In large multinational European studies involving more than 24,000 women, the risk of CIN3 or cancer after 6 years of follow-up was only 0.28% in women who had negative results on both HPV and Pap testing at baseline. This rate was basically the same as in women who tested negative for HPV alone (0.27%). However, it was significantly lower than that of all women who had negative Pap test results (0.97%). The combination of HPV testing and Pap testing at 6-year intervals offered better protection than Pap testing alone at 3-year intervals.48
NEW STANDARD OF CARE: THE LATEST SCREENING GUIDELINES
Until the mid-1990s, the strategy for cervical cancer screening had remained largely unchanged for many years. Since then, several advances have prompted changes in the standard of care.
1996—The US Food and Drug Administration (FDA) approved liquid-based Thin-Prep for cervical cancer screening, which improved specimen adequacy and reduced ambiguous interpretations compared with the original slide-based method of collection.49
2001—The Bethesda terminology for reporting cervical cytology results was updated. First proposed in 1988 to replace the original Papanicolaou system and revised in 1991, this standardized terminology enabled better clinical decision-making.50
2001—The FDA approved HPV testing for women with ASC-US. This provided a better triage strategy for deciding which women need colposcopy to exclude true intraepithelial lesions. Following the FDA approval, the clinical effectiveness of HPV testing in women with ASC-US was validated by a large randomized clinical trial—the ALTS.51
2003—The FDA approved HPV testing in conjunction with Pap testing for women age 30 or older in routine primary screening.52
Guidelines available
Based on these new developments in technology and reporting terminology, and the incorporation of HPV testing, several organizations issued guidelines.
The American Society for Colposcopy and Cervical Pathology published a consensus guideline on management of abnormal cervical cytology in 2001 and revised it in 2006.53
The American Cancer Society issued its guideline for cervical cancer screening in 2002.54
The US Preventive Services Task Force published its screening guidelines in 2003.55
The American College of Obstetricians and Gynecologists (ACOG) also made new recommendations in 2003 and updated them in December 2009.1
Start screening at age 21
Cervical cancer screening should begin at age 21 regardless of the age of onset of vaginal intercourse, according to the 2009 ACOG guidelines.1 This represents a change from previous recommendations from ACOG, the American Cancer Society, and the US Preventive Services Task Force, which were to start screening within 3 years of the onset of vaginal intercourse.
Rationale. This latest recommendation is based on the high rates of clearance of HPV infection and of spontaneous dysplasia regression and the low incidence of cervical cancer in younger women.57,58 HPV infections are common in young women who have had vaginal intercourse. However, most such HPV infections are cleared by the immune system within 1 to 2 years without causing cervical dysplasia.11,12 Invasive cervical cancer in women younger than 21 years is very rare. The annual incidence is only one to two cases per 1 million women ages 15 to 19.2,55
Another reason for not screening before age 21 is that a positive test result may lead to unnecessary anxiety and potentially harmful evaluations and procedures.
Screening intervals extended
The 2009 ACOG guidelines lengthen the cervical cancer screening interval to every 2 years in women under age 30.1 (The 2003 ACOG guidelines said to screen every year.)
For women age 30 and older, the 2009 ACOG guidelines recommend extending the interval to every 3 years when combined Pap and HPV testing are negative (changed from every 2 to 3 years).1
Rationale. Studies have shown little advantage in screening every year in women under the age of 30, with no higher risk of cervical cancer in women screened at a 2- to 3-year interval.59–62 The absolute risk of cervical cancer in a well-screened population is very low.63 Moreover, the absolute number of cervical cancer cases in women age 30 to 64 years screened at 3-year intervals is only four per 100,000 women.64
HPV-plus-Pap testing for women over 30
Based on convincing evidence of the high sensitivity and the high negative predictive value of HPV testing, since 2003 ACOG had recommended HPV-plus-Pap testing in women over age 30. Its 2009 guidelines upgraded this recommendation to level A, ie, the highest grade, based on good and consistent scientific evidence.1 (Previously the recommendation was level B.)
The American Cancer Society also recommends combined HPV and Pap testing as the optimal screening approach in women age 30 or older, with the subsequent screening interval 3 years if both tests are negative. It also endorses Pap testing alone every 2 to 3 years as an alternative screening strategy in this age group.
The US Preventive Services Task Force recommends Pap testing every 3 years in women age 30 or older, and it does not recommend for or against HPV testing. However, neither the US Preventive Services Task Force nor the American Cancer Society has updated its guidelines in 8 years.
Rationale. Women who undergo HPV-plus-Pap testing and who test negative on both are at very low risk of developing CIN2 or CIN3 during the next 4 to 6 years. The risk is much lower than that for women who have a sole negative Pap test result.39,40 Because of this extremely high negative predictive value, women age 30 and older who had negative results on both Pap and HPV testing should be screened no more often than every 3 years.
We believe that the HPV-plus-Pap testing strategy recommended by the 2009 ACOG guidelines for women age 30 and older is the most effective screening approach. This strategy takes advantage of the high sensitivity and high negative predictive value of HPV testing, as well as the high specificity of Pap testing. It achieves almost 100% clinical sensitivity in detecting cervical dysplasia.46
When to stop screening
The 2009 ACOG guidelines for the first time call for stopping cervical cancer screening in women 65 to 70 years of age who have had three negative Pap tests in a row and no abnormal tests in the previous 10 years.1 The American Cancer Society recommends stopping screening at age 70,65 while the US Preventive Services Task Force recommends stopping at age 65.55
Rationale. Cervical cancer develops slowly, and risk factors tend to decline with age, Also, postmenopausal mucosal atrophy may predispose to false-positive Pap results, which can lead to additional procedures and unnecessary patient anxiety.66
However, it is probably reasonable to continue screening in women age 70 and older who are sexually active with multiple partners and who have a history of abnormal Pap test results.1
Women who have had a hysterectomy
According to the latest American Cancer Society, ACOG, and US Preventive Services Task Force guidelines, cervical cancer screening should be discontinued after total hysterectomy for benign indications in women who have no history of high-grade cervical intraepithelial neoplasia, ie, CIN2 or worse.1
Rationale. If the patient has no cervix, continued vaginal cytology screening is not indicated, since the incidence of primary vaginal cancer is one to two cases per 100,000 women per year, much lower than that of cervical cancer.65
However, before discontinuing screening, clinicians should verify that any Pap tests the patient had before the hysterectomy were all read as normal, that the hysterectomy specimen was normal, and that the cervix was completely removed during hysterectomy.
Be ready to explain the recommendations
It is very important for providers to understand the evidence supporting the latest guidelines, as many patients may not realize the significant technological improvements and improved understanding of the role of HPV in cervical cancer genesis that have resulted in the deferred onset of screening and the longer intervals between screenings. This knowledge gap for patients can result in anxiety when told they no longer need an annual Pap test or can start later, if the issue is not properly and thoroughly explained by a confident provider.
A FUTURE STRATEGY: HPV AS THE SOLE PRIMARY SCREENING TEST?
Since HPV testing is much more sensitive than Pap testing for detecting cervical lesions of grade CIN2 or higher, why not use HPV testing as the primary test and then do Pap testing (which is more specific) only if the HPV test is positive?
Mayrand et al46 conducted the first large randomized trial in which HPV testing was compared directly as a stand-alone test with the Pap test in a North American population with access to quality care. Results were published in 2007. In Canada, a total of 10,154 women ages 30 to 69 years in Montreal and St. John’s were randomly assigned to undergo either conventional Pap testing or HPV testing. The sensitivity of HPV testing for CIN2 or CIN3 was 94.6%, whereas the sensitivity of Pap testing was only 55.4%. The specificity was 94.1% for HPV testing and 96.8% for Pap testing. In addition, HPV screening followed by Pap triage resulted in fewer referrals for colposcopy than did either test alone (1.1% vs 2.9% with Pap testing alone or 6.1% with HPV testing alone). In other words, HPV testing was almost 40% more sensitive and only 2.7% less specific than Pap testing in detecting cervical cancer precursors.
However, more controlled trials are needed to validate such a strategy. Furthermore, it remains unclear if a change from Pap testing to a primary HPV testing screening strategy will further reduce the mortality rate of cervical cancer, since the burden of cervical cancer worldwide lies in less-screened populations in low-resource settings.
Dillner et al,48 in a 2008 European study, further demonstrated that HPV testing offers better long-term (6-year) predictive value for CIN3 or worse lesions than cytology does. These findings suggest that HPV testing, with its higher sensitivity and negative predictive value and its molecular focus on cervical carcinogenesis, may safely permit longer screening intervals in a low-risk population.
Sankaranarayanan et al72 performed a randomized trial in rural India in which 131,746 women age 30 to 59 years were randomly assigned to four groups: screening by HPV testing, screening by Pap testing, screening by visual inspection with acetic acid, and counseling only (the control group). At 8 years of follow-up, the numbers of cases of cervical cancer and of cervical cancer deaths were as follows:
- With HPV testing: 127 cases, 34 deaths
- With Pap testing: 152 cases, 54 deaths
- With visual inspection: 157 cases, 56 deaths
- With counseling only: 118 cases, 64 deaths.
The authors concluded that in a low-resource setting, a single round of HPV testing was associated with a significant reduction in the number of deaths from cervical cancer. Not only did the HPV testing group have a lower incidence of cancer-related deaths, there were no cancer deaths among the women in this group who tested negative for HPV. This is the first randomized trial to suggest that using HPV testing as the sole primary cervical cancer screening test may have a benefit in terms of the mortality rate.
At present, to the best of our knowledge, there are no US data validating the role of HPV testing as a stand-alone screening test for cervical cancer.
HPV VACCINATION DOES NOT MEAN THE END OF SCREENING
The development of an effective HPV vaccine and FDA approval of the first quadrivalent (active against HPV 6, 11, 16, and 18) recombinant vaccine (Gardasil) in 2006 has opened a new era of cervical cancer prevention.73,74 At present, the Advisory Committee on Immunization Practices75 recommends vaccination for females 9 to 26 years old.
However, HPV vaccination will not make screening obsolete, since not all women will be vaccinated, and those who have already contracted one of these high-risk HPV types will not benefit.76,77 In addition, the current HPV vaccine does not protect against infection with other oncogenic HPV types. The experts estimate that the initial impact of the HPV vaccine on cervical cancer will not likely be apparent until at least 20 to 30 years after a nationwide vaccination program is implemented.78,79 Therefore, the HPV vaccine certainly does not portend the end of screening. Vaccination combined with continued screening will provide added benefit for cervical cancer prevention.80
The last decade has been an exciting period in the field of cervical cancer screening and prevention, with advances in technology, newly acquired knowledge, and the development of the HPV vaccine. As a result, our clinical practice has become a work in progress, continuing to evolve as we continue to discover more information. The possibility of eradicating cervical cancer has never been greater. The implementation of the most sensitive and effective screening strategy and of a worldwide HPV vaccination program will help us to eventually eradicate cervical cancer and make it a disease of the past.81
Cervical cancer screening and prevention have evolved rapidly in the last decade, especially in the 5 years since the introduction of the first cancer prevention vaccine, human papillomavirus (HPV) recombinant vaccine.1
Providers need to understand the rationale for the recommendations so that they can explain them to patients. In particular, patients may wonder why we now begin screening for cervical cancer later than we used to, and why some women do not need to be screened as often. Both of these changes result from our enhanced understanding of the role of HPV in cervical cancer genesis.
In this article we will briefly review:
- The current understanding of the natural history of cervical cancer
- Advantages and disadvantages of cervical cytology, ie, the Papanicolaou (Pap) test
- The role of HPV testing in cervical cancer screening
- The latest screening guidelines (the new standard of care)
- A possible future screening strategy
- The impact of HPV vaccination on screening.
500,000 NEW CASES EVERY YEAR
The incidence of cervical cancer and its mortality rate have decreased more than 50% in the United States over the past 3 decades, largely as a result of screening with the Pap test.2 In 2010, there were an estimated 12,200 new cases of invasive cervical cancer in the United States and 4,210 deaths from it,3 which are lower than the historical rates.
However, because most developing countries lack effective screening programs, cervical cancer remains the second-leading cause of death from cancer in women worldwide. According to a recent estimate, there are almost 500,000 new cases and 240,000 deaths from this disease worldwide every year.4 If effective global screening programs could be set up, they would markedly reduce the incidence of cervical cancer and deaths from it.5
HPV IS NECESSARY BUT NOT SUFFICIENT FOR CERVICAL CANCER TO DEVELOP
For cervical cancer to develop, the essential first step is infection of the cervical epithelium with one of the oncogenic (high-risk) types of HPV (see below).6–10 Walboomers et al9 tested cervical tissue samples taken from 932 women with cervical cancer and detected HPV DNA in 930 (99.8%) of them.
Fortunately, most HPV-infected women do not develop cervical cancer, as most young women clear the infection in an average of 8 to 24 months.11,12 However, if the infection persists, and if it is one of the high-risk types of HPV, precursor lesions can develop that can progress to cervical cancer.13 The evidence conclusively supports the association between oncogenic HPV infection and the subsequent development of virtually all cases of cervical cancer.6–10
Known risk factors for HPV persistence and the subsequent development of high-grade lesions are cigarette smoking and a compromised immune system.14,15
Terminology: Results of Pap smears
- Normal
- Atypical squamous cells of undetermined significance (ASC-US)
- Low-grade squamous intraepithelial lesions (LSIL)
- High-grade squamous intraepithelial lesions (HSIL)
- Cancer.
Terminology: Results of cervical biopsy
- Normal
- Cervical intraepithelial neoplasia grade 1 (CIN1)
- CIN2 (previously called moderate dysplasia)
- CIN3 (previously called severe dysplasia)
- Carcinoma in situ
- Invasive cervical cancer.
Lesions that are CIN2 or higher are considered high-grade.13
The 18 high-risk HPV types
More than 40 types of HPV infect the genital tract16; 18 of these (types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) are classified as high-risk because of their causative association with cervical cancer (ie, their oncogenic potential).17
How HPV causes cervical cancer
In laboratory cultures, normal human cells die out after a few generations. However, if human epithelial cells are infected by one of the high-risk types of HPV, they can go on dividing indefinitely.18,19
Two HPV proteins, E6 and E7, induce this cell “immortalization.”20,21 E6 from high-risk HPV binds to the human tumor-suppressor protein p53 and rapidly degrades it in a proteolytic process. The p53 protein normally suppresses cell proliferation by arresting growth in the G1 phase of the cell cycle. Therefore, with less p53, the cell cannot suppress uncontrolled cell growth.22–24
Similarly, E7 from high-risk HPV forms a complex with another human tumor suppressor, the retinoblastoma protein (pRB), and disrupts its binding to a transcriptional factor, E2F-1. The freed E2F-1 then stimulates DNA synthesis and uncontrolled cell growth.25
Furthermore, HPV-16 E6 and E7 proteins can collectively cause cellular genetic instability.26
The carcinogenic mechanism of high-risk HPV is complex. The host immune system and natural tumor suppression play important roles. However, the natural history of cervical intraepithelial neoplasia is not well understood. For example, it remains unclear if low-grade lesions such as CIN1 are necessary precursors to high-grade lesions and invasive cancer.6,7,10
THE PAP TEST: SPECIFIC BUT NOT VERY SENSITIVE, AND PRONE TO ERROR
The principal advantage of cervical cytologic testing (ie, the Pap test) in detecting cervical dysplasia is its overall high specificity. Many studies have found that the specificity of conventional Pap testing can reach approximately 98%.27
However, the conventional Pap test has drawbacks. Contaminants such as blood, discharge, and lubricant can make it difficult to interpret, and artifact can occur with air-drying of the Pap smear as it is transferred to the cell slide (“air-drying artifact”).
Liquid-based cytologic study has replaced the older method
To overcome these disadvantages, a liquid-based method of cervical cytologic study, ThinPrep (Hologic, Bedford, MA), was introduced in the mid-1990s. In this method, cell samples are first transferred to a liquid solution for mechanical separation from contaminants, and a representative sample of cells is then placed on a slide for review.
The liquid-based method filters out most contaminating blood, inflammatory cells, and debris. It also eliminates the air-drying artifact in the conventional Pap collection technique and improves specimen adequacy. Cytotechnologists find liquid-based specimens easier to read because the cells are more evenly distributed on a clearer background. Another advantage is that we can routinely test for HPV in the available residual specimen if the cytologic interpretation is abnormal.
The main disadvantages of the liquid-based method are that its specificity is lower than that of conventional Pap smears (around 78%) and that it costs more.28 Nevertheless, the liquid-based technique has become the main method of cervical cytology, used by nearly 90% of gynecologists in the United States since 2003.1
Cytology is still prone to false-negative results
Despite the success of both conventional Pap testing and liquid-based Pap testing, cervical cytology is inherently prone to sample-quality variation, subjective interpretation error, and false-negative results. False-negative results can be due to failure to transfer dysplastic cells to the slide or failure of the cytologist to recognize abnormal cells. In 30% of new cases of cervical cancer, the patient had recently had a Pap test that was interpreted as negative.1,29
Errors in interpretation are exacerbated by inconsistency among cytopathologists. In one study,6,30 when a group of quality-control pathologists reviewed nearly 5,000 cytology specimens, they came to the same conclusion that the original reviewers did more than 50% of the time only for negative and LSIL readings. Of the specimens initially reported as ASC-US, almost 40% were reclassified as negative on further review. Of those originally interpreted as HSIL, more than 50% were reclassified as LSIL, as ASC-US, or as negative.
Furthermore, many studies found that the sensitivity of conventional Pap testing was only around 50%.27 The new liquid-based Pap test uses computer imaging, which has improved the rate of detection of cervical dysplasia but may still miss 15% to 35% of cases of HSIL (severe dysplasia) or cancer.31 Failure to detect cervical dysplasia or cancer on Pap smear has led to a number of lawsuits.32
Clearly, with its relatively low sensitivity, cervical cytology is no longer good enough to use as a sole screening test in all situations. However, its high specificity is an advantage when it is combined with HPV testing in screening.
HPV TESTING AND PAP TESTING COMPLEMENT EACH OTHER
From 17% to 36% of HPV-infected women develop a cytologic abnormality within 5 years, compared with 4% to 15% of women who are HPV-negative.33,34
The usefulness of testing for HPV in women who have had an abnormal Pap test has been well demonstrated in multiple studies.35–38
The landmark Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS)39 found that 82.9% of women with LSIL were HPV-positive. The investigators concluded that HPV testing has little utility in women with LSIL, as the test would likely be positive and thus would not change the decision to perform colposcopy.
However, in women with ASC-US, the sensitivity of HPV testing for predicting CIN3 or cancer was 96.3% and the negative predictive value was 99.5%. In contrast, the sensitivity of a single repeat Pap test was only 44.1%. This large randomized trial conclusively validates the important role of HPV testing in triaging women with ASC-US.
More recently, a meta-analysis of 20 studies of HPV testing in women with ASC-US found that it had a sensitivity of 92.5% and a specificity of 62.5% for detecting CIN2 or worse lesions, and a sensitivity of 95.6% and a specificity of 59.2% for detecting CIN3 or worse lesions.40
Furthermore, HPV testing in primary cervical cancer screening is strongly supported by large cross-sectional studies41–45 and randomized clinical trials.46,47 These studies have conclusively shown that HPV testing is significantly more sensitive than Pap testing for detecting cervical intraepithelial neoplasia, and that, when combined with Pap testing, it can achieve nearly 100% clinical sensitivity and nearly 93% specificity in women age 30 or older. Women who have negative results on both the HPV test and the Pap test can be reassured that their risk of undetected CIN2, CIN3, or cervical cancer is extremely low, since HPV testing has a negative predictive value close to 100%.46
In large multinational European studies involving more than 24,000 women, the risk of CIN3 or cancer after 6 years of follow-up was only 0.28% in women who had negative results on both HPV and Pap testing at baseline. This rate was basically the same as in women who tested negative for HPV alone (0.27%). However, it was significantly lower than that of all women who had negative Pap test results (0.97%). The combination of HPV testing and Pap testing at 6-year intervals offered better protection than Pap testing alone at 3-year intervals.48
NEW STANDARD OF CARE: THE LATEST SCREENING GUIDELINES
Until the mid-1990s, the strategy for cervical cancer screening had remained largely unchanged for many years. Since then, several advances have prompted changes in the standard of care.
1996—The US Food and Drug Administration (FDA) approved liquid-based Thin-Prep for cervical cancer screening, which improved specimen adequacy and reduced ambiguous interpretations compared with the original slide-based method of collection.49
2001—The Bethesda terminology for reporting cervical cytology results was updated. First proposed in 1988 to replace the original Papanicolaou system and revised in 1991, this standardized terminology enabled better clinical decision-making.50
2001—The FDA approved HPV testing for women with ASC-US. This provided a better triage strategy for deciding which women need colposcopy to exclude true intraepithelial lesions. Following the FDA approval, the clinical effectiveness of HPV testing in women with ASC-US was validated by a large randomized clinical trial—the ALTS.51
2003—The FDA approved HPV testing in conjunction with Pap testing for women age 30 or older in routine primary screening.52
Guidelines available
Based on these new developments in technology and reporting terminology, and the incorporation of HPV testing, several organizations issued guidelines.
The American Society for Colposcopy and Cervical Pathology published a consensus guideline on management of abnormal cervical cytology in 2001 and revised it in 2006.53
The American Cancer Society issued its guideline for cervical cancer screening in 2002.54
The US Preventive Services Task Force published its screening guidelines in 2003.55
The American College of Obstetricians and Gynecologists (ACOG) also made new recommendations in 2003 and updated them in December 2009.1
Start screening at age 21
Cervical cancer screening should begin at age 21 regardless of the age of onset of vaginal intercourse, according to the 2009 ACOG guidelines.1 This represents a change from previous recommendations from ACOG, the American Cancer Society, and the US Preventive Services Task Force, which were to start screening within 3 years of the onset of vaginal intercourse.
Rationale. This latest recommendation is based on the high rates of clearance of HPV infection and of spontaneous dysplasia regression and the low incidence of cervical cancer in younger women.57,58 HPV infections are common in young women who have had vaginal intercourse. However, most such HPV infections are cleared by the immune system within 1 to 2 years without causing cervical dysplasia.11,12 Invasive cervical cancer in women younger than 21 years is very rare. The annual incidence is only one to two cases per 1 million women ages 15 to 19.2,55
Another reason for not screening before age 21 is that a positive test result may lead to unnecessary anxiety and potentially harmful evaluations and procedures.
Screening intervals extended
The 2009 ACOG guidelines lengthen the cervical cancer screening interval to every 2 years in women under age 30.1 (The 2003 ACOG guidelines said to screen every year.)
For women age 30 and older, the 2009 ACOG guidelines recommend extending the interval to every 3 years when combined Pap and HPV testing are negative (changed from every 2 to 3 years).1
Rationale. Studies have shown little advantage in screening every year in women under the age of 30, with no higher risk of cervical cancer in women screened at a 2- to 3-year interval.59–62 The absolute risk of cervical cancer in a well-screened population is very low.63 Moreover, the absolute number of cervical cancer cases in women age 30 to 64 years screened at 3-year intervals is only four per 100,000 women.64
HPV-plus-Pap testing for women over 30
Based on convincing evidence of the high sensitivity and the high negative predictive value of HPV testing, since 2003 ACOG had recommended HPV-plus-Pap testing in women over age 30. Its 2009 guidelines upgraded this recommendation to level A, ie, the highest grade, based on good and consistent scientific evidence.1 (Previously the recommendation was level B.)
The American Cancer Society also recommends combined HPV and Pap testing as the optimal screening approach in women age 30 or older, with the subsequent screening interval 3 years if both tests are negative. It also endorses Pap testing alone every 2 to 3 years as an alternative screening strategy in this age group.
The US Preventive Services Task Force recommends Pap testing every 3 years in women age 30 or older, and it does not recommend for or against HPV testing. However, neither the US Preventive Services Task Force nor the American Cancer Society has updated its guidelines in 8 years.
Rationale. Women who undergo HPV-plus-Pap testing and who test negative on both are at very low risk of developing CIN2 or CIN3 during the next 4 to 6 years. The risk is much lower than that for women who have a sole negative Pap test result.39,40 Because of this extremely high negative predictive value, women age 30 and older who had negative results on both Pap and HPV testing should be screened no more often than every 3 years.
We believe that the HPV-plus-Pap testing strategy recommended by the 2009 ACOG guidelines for women age 30 and older is the most effective screening approach. This strategy takes advantage of the high sensitivity and high negative predictive value of HPV testing, as well as the high specificity of Pap testing. It achieves almost 100% clinical sensitivity in detecting cervical dysplasia.46
When to stop screening
The 2009 ACOG guidelines for the first time call for stopping cervical cancer screening in women 65 to 70 years of age who have had three negative Pap tests in a row and no abnormal tests in the previous 10 years.1 The American Cancer Society recommends stopping screening at age 70,65 while the US Preventive Services Task Force recommends stopping at age 65.55
Rationale. Cervical cancer develops slowly, and risk factors tend to decline with age, Also, postmenopausal mucosal atrophy may predispose to false-positive Pap results, which can lead to additional procedures and unnecessary patient anxiety.66
However, it is probably reasonable to continue screening in women age 70 and older who are sexually active with multiple partners and who have a history of abnormal Pap test results.1
Women who have had a hysterectomy
According to the latest American Cancer Society, ACOG, and US Preventive Services Task Force guidelines, cervical cancer screening should be discontinued after total hysterectomy for benign indications in women who have no history of high-grade cervical intraepithelial neoplasia, ie, CIN2 or worse.1
Rationale. If the patient has no cervix, continued vaginal cytology screening is not indicated, since the incidence of primary vaginal cancer is one to two cases per 100,000 women per year, much lower than that of cervical cancer.65
However, before discontinuing screening, clinicians should verify that any Pap tests the patient had before the hysterectomy were all read as normal, that the hysterectomy specimen was normal, and that the cervix was completely removed during hysterectomy.
Be ready to explain the recommendations
It is very important for providers to understand the evidence supporting the latest guidelines, as many patients may not realize the significant technological improvements and improved understanding of the role of HPV in cervical cancer genesis that have resulted in the deferred onset of screening and the longer intervals between screenings. This knowledge gap for patients can result in anxiety when told they no longer need an annual Pap test or can start later, if the issue is not properly and thoroughly explained by a confident provider.
A FUTURE STRATEGY: HPV AS THE SOLE PRIMARY SCREENING TEST?
Since HPV testing is much more sensitive than Pap testing for detecting cervical lesions of grade CIN2 or higher, why not use HPV testing as the primary test and then do Pap testing (which is more specific) only if the HPV test is positive?
Mayrand et al46 conducted the first large randomized trial in which HPV testing was compared directly as a stand-alone test with the Pap test in a North American population with access to quality care. Results were published in 2007. In Canada, a total of 10,154 women ages 30 to 69 years in Montreal and St. John’s were randomly assigned to undergo either conventional Pap testing or HPV testing. The sensitivity of HPV testing for CIN2 or CIN3 was 94.6%, whereas the sensitivity of Pap testing was only 55.4%. The specificity was 94.1% for HPV testing and 96.8% for Pap testing. In addition, HPV screening followed by Pap triage resulted in fewer referrals for colposcopy than did either test alone (1.1% vs 2.9% with Pap testing alone or 6.1% with HPV testing alone). In other words, HPV testing was almost 40% more sensitive and only 2.7% less specific than Pap testing in detecting cervical cancer precursors.
However, more controlled trials are needed to validate such a strategy. Furthermore, it remains unclear if a change from Pap testing to a primary HPV testing screening strategy will further reduce the mortality rate of cervical cancer, since the burden of cervical cancer worldwide lies in less-screened populations in low-resource settings.
Dillner et al,48 in a 2008 European study, further demonstrated that HPV testing offers better long-term (6-year) predictive value for CIN3 or worse lesions than cytology does. These findings suggest that HPV testing, with its higher sensitivity and negative predictive value and its molecular focus on cervical carcinogenesis, may safely permit longer screening intervals in a low-risk population.
Sankaranarayanan et al72 performed a randomized trial in rural India in which 131,746 women age 30 to 59 years were randomly assigned to four groups: screening by HPV testing, screening by Pap testing, screening by visual inspection with acetic acid, and counseling only (the control group). At 8 years of follow-up, the numbers of cases of cervical cancer and of cervical cancer deaths were as follows:
- With HPV testing: 127 cases, 34 deaths
- With Pap testing: 152 cases, 54 deaths
- With visual inspection: 157 cases, 56 deaths
- With counseling only: 118 cases, 64 deaths.
The authors concluded that in a low-resource setting, a single round of HPV testing was associated with a significant reduction in the number of deaths from cervical cancer. Not only did the HPV testing group have a lower incidence of cancer-related deaths, there were no cancer deaths among the women in this group who tested negative for HPV. This is the first randomized trial to suggest that using HPV testing as the sole primary cervical cancer screening test may have a benefit in terms of the mortality rate.
At present, to the best of our knowledge, there are no US data validating the role of HPV testing as a stand-alone screening test for cervical cancer.
HPV VACCINATION DOES NOT MEAN THE END OF SCREENING
The development of an effective HPV vaccine and FDA approval of the first quadrivalent (active against HPV 6, 11, 16, and 18) recombinant vaccine (Gardasil) in 2006 has opened a new era of cervical cancer prevention.73,74 At present, the Advisory Committee on Immunization Practices75 recommends vaccination for females 9 to 26 years old.
However, HPV vaccination will not make screening obsolete, since not all women will be vaccinated, and those who have already contracted one of these high-risk HPV types will not benefit.76,77 In addition, the current HPV vaccine does not protect against infection with other oncogenic HPV types. The experts estimate that the initial impact of the HPV vaccine on cervical cancer will not likely be apparent until at least 20 to 30 years after a nationwide vaccination program is implemented.78,79 Therefore, the HPV vaccine certainly does not portend the end of screening. Vaccination combined with continued screening will provide added benefit for cervical cancer prevention.80
The last decade has been an exciting period in the field of cervical cancer screening and prevention, with advances in technology, newly acquired knowledge, and the development of the HPV vaccine. As a result, our clinical practice has become a work in progress, continuing to evolve as we continue to discover more information. The possibility of eradicating cervical cancer has never been greater. The implementation of the most sensitive and effective screening strategy and of a worldwide HPV vaccination program will help us to eventually eradicate cervical cancer and make it a disease of the past.81
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24( suppl 3):S3/11–S3/25.
- Herrero R. Epidemiology of cervical cancer. J Natl Cancer Inst Monogr 1996; 21:1–6.
- Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer 2007; 111:145–153.
- Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 2006; 24(suppl 3):S3/42–S3/51.
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244–265.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol 1996; 175:1099–1104.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–428.
- Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709–715.
- Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002; 325:572.
- Sycuro LK, Xi LF, Hughes JP, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis 2008; 198:971–978.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907.
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–1278.
- Stöppler H, Stöppler MC, Schlegel R. Transforming proteins of the papillomaviruses. Intervirology 1994; 37:168–179.
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994; 48:427–447.
- Scheffner M, Romanczuk H, Münger K, Huibregtse JM, Mietz JA, Howley PM. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol 1994; 186:83–99.
- Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 1994; 68:4358–4368.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248:76–79.
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75:495–505.
- Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995; 55:4420–4424.
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 1989; 8:4099–4105.
- Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 2000; 97:10002–10007.
- Agency for Healthcare Research and Quality. Evaluation of Cervical Cytology. Summary: Evidence Report/Technology Assessment: Number 5. http://archive.ahrq.gov/clinic/epcsums/cervsumm.htm. Accessed August 18, 2011.
- Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001; 83:439–444.
- Shingleton HM, Patrick RL, Johnston WW, Smith RA. The current status of the Papanicolaou smear. CA Cancer J Clin 1995; 45:305–320.
- Stoler MH, Schiffman M; Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001; 285:1500–1505.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Allen KA, Zaleski S, Cohen MB. Review of negative Papanicolaou tests. Is the retrospective 5-year review necessary? Am J Clin Pathol 1994; 101:19–21.
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–3114.
- Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal Pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 2002; 95:2145–2151.
- Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999; 281:1605–1610.
- Wright TC, Lorincz A, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. Am J Obstet Gynecol 1998; 178:962–966.
- Shlay JC, Dunn T, Byers T, Barón AE, Douglas JM. Prediction of cervical intraepithelial neoplasia grade 2–3 using risk assessment and human papillomavirus testing in women with atypia on Papanicolaou smears. Obstet Gynecol 2000; 96:410–416.
- Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstet Gynecol 2000; 95:821–827.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003; 188:1383–1392.
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006; 24(suppl 3):S3/78–S3/89.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003; 362:1871–1876.
- Salmerón J, Lazcano-Ponce E, Lorincz A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003; 14:505–512.
- Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000; 92:464–474.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006; 119:1095–1101.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008; 100:492–501.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Noller KL, Bettes B, Zinberg S, Schulkin J. Cervical cytology screening practices among obstetrician-gynecologists. Obstet Gynecol 2003; 102:259–265.
- Solomon D, Davey D, Kurman R, et al; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–2119.
- The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst 2000; 92:397–402.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol 2008; 112:1419–1444.
- Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol 2007; 197:340–345.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Systematic Evidence Review No. 25. http://www.ahrq.gov/downloads/pub/prevent/pdfser/cervcanser.pdf. Accessed October 9, 2011.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132:277–284.
- Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008; 113(suppl 10):2855–2864.
- IARC Working Group on evaluation of cervical cancer screening programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J (Clin Res Ed) 1986; 293:659–664.
- Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol 2000; 96:219–223.
- Eddy DM. The frequency of cervical cancer screening. Comparison of a mathematical model with empirical data. Cancer 1987; 60:1117–1122.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol 2003; 101:29–37.
- Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med 2003; 349:1501–1509.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS). Ann Intern Med 2000; 133:942–950.
- Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M. Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. Br J Cancer 2005; 93:862–867.
- Ronco G, Segnan N, Giorgi-Rossi P, et al; New Technologies for Cervical Cancer Working Group. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst 2006; 98:765–774.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360:1385–1394.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271–278.
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007; 56(RR02):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm?s_cid=rr5602a1_e. Accessed 8/30/2011.
- Koutsky LA, Harper DM. Chapter 13: Current findings from prophylactic HPV vaccine trials. Vaccine 2006; 24( suppl 3):S3/114–S3/121.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24( suppl 3):S3/178–S3/186.
- Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res 2002; 89:285–293.
- Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 2006; 24(suppl 3):S3/171–S3/177.
- Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine 2006; 24(suppl 3:S3/90–S3/97.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24( suppl 3):S3/11–S3/25.
- Herrero R. Epidemiology of cervical cancer. J Natl Cancer Inst Monogr 1996; 21:1–6.
- Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer 2007; 111:145–153.
- Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 2006; 24(suppl 3):S3/42–S3/51.
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244–265.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol 1996; 175:1099–1104.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–428.
- Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709–715.
- Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002; 325:572.
- Sycuro LK, Xi LF, Hughes JP, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis 2008; 198:971–978.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907.
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–1278.
- Stöppler H, Stöppler MC, Schlegel R. Transforming proteins of the papillomaviruses. Intervirology 1994; 37:168–179.
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994; 48:427–447.
- Scheffner M, Romanczuk H, Münger K, Huibregtse JM, Mietz JA, Howley PM. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol 1994; 186:83–99.
- Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 1994; 68:4358–4368.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248:76–79.
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75:495–505.
- Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995; 55:4420–4424.
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 1989; 8:4099–4105.
- Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 2000; 97:10002–10007.
- Agency for Healthcare Research and Quality. Evaluation of Cervical Cytology. Summary: Evidence Report/Technology Assessment: Number 5. http://archive.ahrq.gov/clinic/epcsums/cervsumm.htm. Accessed August 18, 2011.
- Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001; 83:439–444.
- Shingleton HM, Patrick RL, Johnston WW, Smith RA. The current status of the Papanicolaou smear. CA Cancer J Clin 1995; 45:305–320.
- Stoler MH, Schiffman M; Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001; 285:1500–1505.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Allen KA, Zaleski S, Cohen MB. Review of negative Papanicolaou tests. Is the retrospective 5-year review necessary? Am J Clin Pathol 1994; 101:19–21.
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–3114.
- Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal Pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 2002; 95:2145–2151.
- Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999; 281:1605–1610.
- Wright TC, Lorincz A, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. Am J Obstet Gynecol 1998; 178:962–966.
- Shlay JC, Dunn T, Byers T, Barón AE, Douglas JM. Prediction of cervical intraepithelial neoplasia grade 2–3 using risk assessment and human papillomavirus testing in women with atypia on Papanicolaou smears. Obstet Gynecol 2000; 96:410–416.
- Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstet Gynecol 2000; 95:821–827.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003; 188:1383–1392.
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006; 24(suppl 3):S3/78–S3/89.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003; 362:1871–1876.
- Salmerón J, Lazcano-Ponce E, Lorincz A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003; 14:505–512.
- Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000; 92:464–474.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006; 119:1095–1101.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008; 100:492–501.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Noller KL, Bettes B, Zinberg S, Schulkin J. Cervical cytology screening practices among obstetrician-gynecologists. Obstet Gynecol 2003; 102:259–265.
- Solomon D, Davey D, Kurman R, et al; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–2119.
- The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst 2000; 92:397–402.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol 2008; 112:1419–1444.
- Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol 2007; 197:340–345.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Systematic Evidence Review No. 25. http://www.ahrq.gov/downloads/pub/prevent/pdfser/cervcanser.pdf. Accessed October 9, 2011.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132:277–284.
- Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008; 113(suppl 10):2855–2864.
- IARC Working Group on evaluation of cervical cancer screening programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J (Clin Res Ed) 1986; 293:659–664.
- Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol 2000; 96:219–223.
- Eddy DM. The frequency of cervical cancer screening. Comparison of a mathematical model with empirical data. Cancer 1987; 60:1117–1122.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol 2003; 101:29–37.
- Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med 2003; 349:1501–1509.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS). Ann Intern Med 2000; 133:942–950.
- Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M. Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. Br J Cancer 2005; 93:862–867.
- Ronco G, Segnan N, Giorgi-Rossi P, et al; New Technologies for Cervical Cancer Working Group. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst 2006; 98:765–774.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360:1385–1394.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271–278.
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007; 56(RR02):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm?s_cid=rr5602a1_e. Accessed 8/30/2011.
- Koutsky LA, Harper DM. Chapter 13: Current findings from prophylactic HPV vaccine trials. Vaccine 2006; 24( suppl 3):S3/114–S3/121.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24( suppl 3):S3/178–S3/186.
- Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res 2002; 89:285–293.
- Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 2006; 24(suppl 3):S3/171–S3/177.
- Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine 2006; 24(suppl 3:S3/90–S3/97.
KEY POINTS
- Persistent infection with one of the 18 high-risk types of HPV is associated with the development of nearly all cases of cervical cancer.
- The 2009 ACOG guidelines recommend starting to screen with the Pap test at an older age (21 years) than in the past, and they recommend a longer screening interval for women in their 20s, ie, every 2 years instead of yearly.
- Women age 30 and older should undergo both Pap and HPV testing. If both tests are negative, screening should be done again no sooner than 3 years. Alternatively, women age 30 or older who have had three consecutive negative Pap tests can be screened by Pap testing every 3 years.
- Although vaccination can prevent most primary infections with high-risk HPV, it does not eliminate the need for continuing cervical cancer screening, as it does not protect against all high-risk HPV subtypes.
- Screening can stop at age 65 to 70 in women who have had three negative Pap tests in a row and no abnormal tests within the past 10 years.