User login
New tools for detecting occult monoclonal gammopathy, a cause of secondary osteoporosis
Sometimes, osteoporosis can be the presenting sign of a monoclonal gammopathy, which in some people may precede a diagnosis of multiple myeloma.1
In this article, we use two cases to illustrate the challenges of detecting monoclonal gammopathies as the cause of secondary osteoporosis. We also discuss the diagnostic limitations of current tests and the advantages of a newer test—measuring the serum levels of free light chains—in the workup of these patients.
CASE 1: A 55-YEAR-OLD WOMAN WITH BACK PAIN
A 55-year-old woman develops back pain after walking her dog, and the pain worsens despite treatment with a nonsteroidal anti-inflammatory drug for 1 week.
The patient has a history of well-controlled hypertension. She went through menopause 5 years ago, and about 2 years ago she was started on oral calcium and vitamin D for low bone density. At that time she complained of mild fatigue, which she attributed to working overtime and to lack of sleep.
Laboratory data, other tests
- Her white blood cell differential count is normal
- Hemoglobin 11.8 g/dL (normal range 12–15)
- Serum creatinine 1.0 mg/dL (0.5–1.4)
- Calcium 8.2 mg/dL (8.0–10.0)
- Albumin 4.5 g/dL (3.5–5.0)
- Total protein 5.7 g/dL (6.0–8.4)
- Serum and urine protein electrophoreses show no monoclonal spike (M-spike) or bands
- Serum free kappa light chains 5,542 mg/L (normal range 3.3–19.4).
Based on the elevation of serum free kappa light chains, the patient undergoes bone marrow aspiration biopsy. Histologic analysis reveals plasmacytosis (60% of her marrow cells are plasma cells [normal is < 5%]) with kappa light chain restriction.
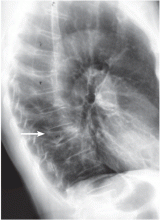
A complete x-ray survey of the skull and long bones reveals widespread lytic lesions, consistent with multiple myeloma.
CASE 2: AN 88-YEAR-OLD MAN WITH MALAISE AND BACK PAIN
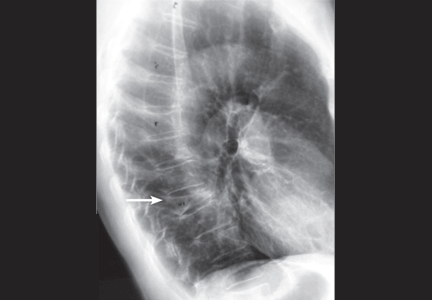
An 88-year-old man sees his family doctor because of malaise and back pain. He was treated for bladder cancer several years ago. He is currently being treated for prostatic hyperplasia, hypertension, and arthritis. Spinal radiography shows a compression deformity at T12, for which he undergoes kyphoplasty.
His complete blood cell count, white blood cell differential count, and kidney and metabolic profiles are normal.
Urine protein electrophoresis is normal, but serum electrophoresis detects an M-spike. On DXA of the hip, his T score is −3.7 (normal ≥ −1.0), and his Z score is −2.4 (normal > −2.0); suspicion of a secondary cause may be raised with Z scores of −1.0 or −1.5. The level of urinary NTX (cross-linked N-telopeptide of type I collagen, a marker of bone turnover) is 190 nmol bone collagen equivalents/nmol creatinine (normal range for men < 75), indicating a high level of bone turnover.
A serum free light chain assay shows twice the normal concentration of kappa light chains. The patient is referred for hematologic study and undergoes bone marrrow aspiration biopsy, which shows an abnormally high number of monoclonal plasma cells.
LESSONS FROM THESE CASES
The cases presented above illustrate several key clinical points:
- Minor back pain can be a symptom of a spinal compression fracture.
- Declining bone density should raise the suspicion of secondary osteoporosis, as should an abnormally low Z score.
- Markers of bone turnover are commonly elevated in secondary osteoporosis.
- Routine laboratory tests often fail to detect multiple myeloma.
BACK PAIN AS A SYMPTOM OF SPINAL COMPRESSION FRACTURE
Back pain is a very common complaint, and fortunately, most cases are due to benign causes. However, serious causes such as cancer, infection, and fractures must be considered. The topic has been reviewed in detail by Siemionow et al.2
Osteoporotic compression fractures are common in the elderly and are associated with loss of height. They can occur spontaneously or from minimal trauma. The workup can start with plain anteroposterior and lateral radiographs and routine laboratory tests, as in the patients described above. This information, as well as DXA testing, may provide clues that suggest that the osteoporosis is secondary to an underlying problem, or that a coexisting bone condition caused the fracture.
DXA CAN SUGGEST SECONDARY OSTEOPOROSIS
Declining bone density
Standard DXA testing is used to identify patients at high risk of fragility fractures from osteoporosis. It is also the accepted way to monitor disease progression and efficacy of treatment.
However, when checking to see if a patient’s bone density has changed over time, one must recognize that variations in technique from center to center or operator to operator can produce false changes in DXA results. 3,4 The testing center should state its own level of variance (referred to as the least significant change) and should indicate whether changes in a patient’s follow-up test results are statistically significant (ie, exceed that level).
A significant decline in bone mineral density over time may indicate that the patient is either not taking his or her medications or is not taking them as directed, as often happens with oral bisphosphonates—which must be taken first thing in the morning, on an empty stomach, with only a glass of water, at least 30 minutes before breakfast, during which time the patient must remain in an upright position.5–7 But a decline also raises the suspicion of an underlying condition instead of or in addition to osteoporosis, as described in the cases above. The normal decline in bone mineral density due to aging is 0.1% to 0.2% per year. For women 5 years after menopause, the rate increases to 1% to 2% and then slows to the rate of decline due to aging. A decline in bone density to the degree seen in case 1 is more than that which could be attributed to primary osteoporosis, and so an underlying cause must be considered.
Abnormally low Z scores also raise the suspicion of secondary osteoporosis
The T score is the difference, in standard deviations, between the patient’s bone density and the mean value in a population of healthy young adults. Since bone density tends to decline with age, so does the T score.
In contrast, the Z score compares a patient’s bone density with the mean value in a population the same age and sex as the patient. When it is abnormally low, it implies greater bone loss than predicted by aging alone or greater than expected from primary disease, so a secondary disorder must be considered.8,9 This was the case in our second patient, who had a Z score of −2.4.
No specific Z score cutoff has been established. Rather, the physician should be suspicious when it is lower than about −1.0 and when something in the patient’s clinical presentation, history, or laboratory evaluation raises suspicion of an underlying condition. In other words, the Z score is useful not by itself, but in context with other information.
In a retrospective analysis of men and women with osteoporosis, Swaminathan et al9 reported that a Z score cutoff of −1.0 had a sensitivity of 87.5% for detecting an underlying cause of osteoporosis.
Again, we want to emphasize that a low Z score alone is not sufficient to make a diagnosis of a secondary cause of osteoporosis. But it is good to be suspicious when a Z score is as low as in our second case and when that suspicion is reinforced by other clinical data.
MARKERS OF BONE TURNOVER
Biochemical markers of bone resorption, such as urinary NTX and the cross-linked C-telopeptide of type I collagen (CTX), have been shown to predict fracture risk independent of bone density measurements. The evidence to date supports the use of these markers in conjunction with bone density measurements to ascertain early on whether osteoporosis is responding to treatment, but their use alone to screen for osteoporosis is not encouraged.10
The markedly high level of NTX in our second patient would be unusual in primary disease—it implies a high degree of bone turnover and, in concert with the clinical information, suggests secondary osteoporosis.
SOME CAUSES OF SECONDARY BONE LOSS
If a patient has a low Z score, a declining T score, or other clues, it is critical to evaluate for causes of secondary bone loss, such as8:
- Endocrine disorders (Cushing syndrome, hyperparathyroidism, hypogonadism)
- Gastrointestinal disorders (malabsorption, cirrhosis, gastric bypass surgery)
- Renal insufficiency and failure
- Pulmonary diseases and their treatment
- Drug use (corticosteroids, antigonadotropins, anticonvulsants, aromatase inhibitors, antirejection drugs)
- Nutritional factors (alcohol abuse, smoking, eating disorders)
- Neurologic disease or its treatment
- Transplantation
- Genetic metabolic disorders
- Malignancy.
As in the scenarios presented above, unexplained changes in bone mineral density and mild anemia may trigger an evaluation for a monoclonal gammopathy.
MULTIPLE MYELOMA
Multiple myeloma is a cancer of the immunoglobulin-producing plasma cells in the bone marrow. Since the cancerous cells are clones, they all produce the same immunoglobulin—thus, the distinctive M-spike on serum or urine protein electrophoresis. It affects about 50,000 people in the United States.
The typical features of multiple myeloma are hypercalcemia, renal insufficiency, anemia, and bone lesions with or without osteoporosis. 11 Most patients have identifiable features of myeloma at the time of diagnosis, but perhaps 20% lack the characteristic symptoms of fatigue, back pain, or bone pain.
Most patients who eventually develop symptomatic multiple myeloma first present with monoclonal gammopathy of undetermined significance (MGUS), a disorder characterized by asymptomatic overproduction of an immunoglobulin. However, MGUS develops into multiple myeloma in only about 15% of cases.11
Widespread osteoporosis, due to cytokine-mediated osteoclast activation, is common in patients with multiple myeloma. As many as 90% of patients have lytic skeletal lesions or osteoporosis at the time of diagnosis.11,12
Myeloma-related osteoporosis can be difficult to differentiate from primary osteoporosis because not all patients secrete a monoclonal protein that standard urine or serum tests can detect.13 But new assays for serum free light chains can help resolve this diagnostic dilemma.14
WHEN IS TESTING FOR MONOCLONAL GAMMOPATHIES WARRANTED?
Screening for MGUS in the general osteoporotic population is not warranted, since its prevalence (2.1%) is similar to that in the general population (2.9%) of women age 50 or older and 5.3% to 7.5% of all persons age 70 years or older.15,16 However, testing for monoclonal gammopathies is warranted when clinical or laboratory findings—eg, subtle hints such as an unexplained elevation in the erythrocyte sedimentation rate or a low anion gap—trigger diagnostic suspicion. Unexplained hypercalcemia, renal insufficiency, unexplained anemia, hypo- and hypergammaglobulinemia, skeletal problems (eg, widespread osteoporosis, unexplained back or bone pain), and distal, symmetric polyneuropathy are the usual signs of underlying plasma cell neoplasia.
Signs of multiple myeloma: the CRAB mnemonic
Patients should be screened for multiple myeloma if they have any of the following presenting features not attributable to another disorder, using the mnemonic CRAB17:
Calcium elevation (serum calcium ≥ 11.5 mg/dL)
Renal insufficiency (serum creatinine > 1.73 mmol/L)
Anemia (normochromic, normocytic anema, with a hemoglobin value lower than 10 g/dL or more than 2 g/dL below the lower limit of normal)
Bone disease (lytic lesions, widespread osteoporosis, or bone fractures on skeletal survey, or a decline in bone mineral density or evidence of osteoporosis on DXA).
For the diagnosis of multiple myeloma to be made, the patient must have at least 10% clonal bone marrow plasma cells, evidence of a monoclonal protein in the serum or urine, and CRAB-related organ damage. When in doubt, referral for a hematologic evaluation is advised. Patients with signs of myeloma-related organ damage warrant prompt treatment.
Electrophoresis is not 100% sensitive
As the clinical cases above illustrate, standard testing for the monoclonal protein is not 100% sensitive for multiple myeloma, as some patients do not secrete the protein in the serum or urine.
In more than 97% of patients, the plasma cells that proliferate clonally produce a measurable monoclonal protein, such as an intact immunoglobulin only (eg, IgG kappa, IgA lambda), a light chain only (kappa or lambda), or intact immunoglobulins and free light chains. In the rest, no detectable monoclonal protein is produced, a disease subtype called nonsecretory multiple myeloma.
Of patients who secrete an intact immunoglobulin, 90% to 95% also produce excess free light chains.18,19 From 15% to 20% of patients with multiple myeloma secrete only light chains.1,20
Classically, serum and urine protein electrophoreses are the diagnostic tools used to evaluate monoclonal gammopathy, but urine electrophoresis detects only about 50% of myelomas.19
WHEN TO CONSIDER FREE LIGHT CHAIN ANALYSIS
While serum and urine protein electrophoreses are still the standard for screening for MGUS or multiple myeloma if one strongly suspects it, additional testing with serum free light chain analysis should be considered if patients exhibit CRAB-related features of myeloma-related organ damage, such as hypercalcemia, renal insufficiency, anemia, or bone loss.
Serum assays for free kappa and free lambda light chains can detect circulating clonal free light chains in most patients with nonsecretory multiple myeloma. In one study,21 elevated concentrations of either kappa or lambda free light chains (and abnormal kappa-lambda ratios) were detected in the sera of 19 of 28 patients with nonsecretory multiple myeloma, such that the diagnosis could be changed to oligosecretory disease.
Several studies have also found serum light chain panels to be highly sensitive for the diagnosis of MGUS or multiple myeloma.22–24 Clonal light chains must be present in a concentration of at least 500 mg/L to be detected by serum protein electrophoresis, or at least 150 mg/L to be detected by serum immunofixation. 25 In contrast, free light chain immunoassays can measure free light chain concentrations of 3 mg/L or lower, and can therefore detect light-chain-related disorders despite negative results on serum protein electrophoresis or immunofixation.14
Cost-effectiveness of free light chain analysis
Serum free light chain assays appear to be more cost-effective than urine tests in screening for monoclonal gammopathy: Medicare reimbursement is $38 for the serum free light chain assay vs $71 for the urine assay, which includes total urine protein, urine protein electrophoresis, and urine immunofixation electrophoresis.22
The kappa-lambda ratio
Normal values for serum free light chains are:
- Kappa 3.3–19.4 mg/L
- Lambda 5.7–26.3 mg/L
- Kappa-lambda ratio 0.26–1.65.
The kappa-lambda ratio is an indication of clonality.26,27 A ratio greater than 1.65 suggests a kappa free light chain monoclonal gammopathy; a ratio less than 0.26 suggests a lambda free light chain monoclonal gammopathy.
Importantly, in patients with renal impairment but no monoclonal gammopathy, the kappa-lambda ratio is often slightly higher—up to 3:1 because of reduced renal light chain clearance.26
However, not all patients with a monoclonal gammopathy have an abnormal free light chain ratio. Only one-third of patients with MGUS do, and these patients are at greater risk of progression to other plasma cell dyscrasias. 28 The free light chain ratio is normal in 5% to 10% of patients with intact immunoglobulin multiple myeloma.29,30 In a study of 116 patients with plasmacytoma, serum protein electrophoresis demonstrated an M-spike in half of patients, serum immunofixation was abnormal in two-thirds, and the kappa-lambda ratio was abnormal in half.31
A risk exists that MGUS will progress to multiple myeloma in patients who have an abnormal free light chain ratio. Thus, patients should be referred to a hematologist-oncologist for evaluation and monitoring if an abnormal kappa-lambda ratio is detected by serum free light chain assay.
Patients with abnormalities in the kappa-lambda ratio and no other evidence of monoclonal protein may harbor light-chain-related diseases only (eg, light chain multiple myeloma, primary amyloidosis, or light chain deposition disease) or a newly described entity, free light chain MGUS.14,19,27 An abnormal kappa-lambda ratio has also been noted in variable percentages of patients with chronic lymphocytic leukemia and malignant lymphoma.32
- Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009; 113:5418–5422.
- Siemionow K, Steinmetz M, Bell G, Ilaslan H, McLain RF. Identifying serious causes of back pain: cancer, infection, fracture. Cleve Clin J Med 2008; 75:557–566.
- Binkley N, Krueger D. What should DXA reports contain? P of ordering health care providers. J Clin Densitom 2009; 12:5–10.
- Bonnick SL, Johnston CC, Kleerekoper M, et al Importance of precision in bone density measurements. J Clin Densitom 2001; 4:105–110.
- Gold DE, Alexander IM, Ettinger MP. How can osteoporosis patients benefit more from their therapy? Adherence issues with bisphosphonate therapy. Ann Pharmacother 2006; 40:1143–1150.
- Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 2005; 44:551–570.
- Lin JT, Lane JM. Bisphosphonates. J Am Acad Orthop Surg 2003; 11:1–4.
- Licata AA. Diagnosing primary osteoporosis: it’s more than a T score. Cleve Clin J Med 2006; 73:473–476.
- Swaminathan K, Flynn K, Garton M, Paterson C, Leese G. Search for secondary osteoporosis: are Z scores useful predictors? Postgrad Med J 2009; 85:38–39.
- Clowes JA, Eastell R. The role of bone turnover markers and risk factors in the assessment of osteoporosis and fracture risk. Baillieres Best Pract Res Clin Endocrinol Metab 2000; 14:213–232.
- Kyle RA, Gertz MA, Witzig TE, et al Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78:21–33.
- Hussein MA, Vrionis FD, Allison R, et al., International Myeloma Working Group. The role of vertebral augmentation in multiple myeloma: International Myeloma Working Group Consensus Statement. Leukemia 2008; 22:1479–1484.
- Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am 1999; 13:1259–1272.
- Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet 2003; 361:489–491.
- Tannenbaum C, Clark J, Schwartzman K, et al Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Kyle RA, Therneau TM, Rajkumar SV, et al Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006; 354:1362–1369.
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121:749–757.
- Pepe J, Petrucci MT, Nofroni I, et al Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol 2006; 134:485–490.
- Berenson JR, Yellin O, Boccia RV, et al Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res 2008; 14:6289–6295.
- Pepe J, Petrucci MT, Mascia ML, et al The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int 2008; 82:418–426.
- Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood 2001; 97:2900–2902.
- Katzmann JA, Dispenzieri A, Kyle RA, et al Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 2006; 81:1575–1578.
- Abadie JM, van Hoeven KH, Wells JM. Are renal reference intervals required when screening for plasma cell disorders with serum free light chains and serum protein electrophoresis? Am J Clin Pathol 2009; 131:166–171.
- Abadie JM, Bankson DD. Assessment of serum free light chain assays for plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab Sci 2006; 36:157–162.
- Shaw GR. Nonsecretory plasma cell myeloma—becoming even more rare with serum free light-chain assay: a brief review. Arch Pathol Lab Med 2006; 130:1212–1215.
- Hutchison CA, Harding S, Hewins P, et al Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3:1684–1690.
- Katzmann JA, Clark RJ, Abraham RS, et al Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem 2002; 48:1437–1444.
- Rajkumar SV, Kyle RA, Therneau TM, et al Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106:812–817.
- Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol 2004; 126:348–354.
- Dispenzieri A, Zhang L, Katzmann JA, et al Appraisal of immunoglobulin free light chain as a marker of response. Blood 2008; 111:4908–4915.
- Dingli D, Kyle RA, Rajkumar SV, et al Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood 2006; 108:1979–1983.
- Martin W, Abraham R, Shanafelt T, et al Serum-free light chain—a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res 2007; 149:231–235.
Sometimes, osteoporosis can be the presenting sign of a monoclonal gammopathy, which in some people may precede a diagnosis of multiple myeloma.1
In this article, we use two cases to illustrate the challenges of detecting monoclonal gammopathies as the cause of secondary osteoporosis. We also discuss the diagnostic limitations of current tests and the advantages of a newer test—measuring the serum levels of free light chains—in the workup of these patients.
CASE 1: A 55-YEAR-OLD WOMAN WITH BACK PAIN
A 55-year-old woman develops back pain after walking her dog, and the pain worsens despite treatment with a nonsteroidal anti-inflammatory drug for 1 week.
The patient has a history of well-controlled hypertension. She went through menopause 5 years ago, and about 2 years ago she was started on oral calcium and vitamin D for low bone density. At that time she complained of mild fatigue, which she attributed to working overtime and to lack of sleep.
Laboratory data, other tests
- Her white blood cell differential count is normal
- Hemoglobin 11.8 g/dL (normal range 12–15)
- Serum creatinine 1.0 mg/dL (0.5–1.4)
- Calcium 8.2 mg/dL (8.0–10.0)
- Albumin 4.5 g/dL (3.5–5.0)
- Total protein 5.7 g/dL (6.0–8.4)
- Serum and urine protein electrophoreses show no monoclonal spike (M-spike) or bands
- Serum free kappa light chains 5,542 mg/L (normal range 3.3–19.4).
Based on the elevation of serum free kappa light chains, the patient undergoes bone marrow aspiration biopsy. Histologic analysis reveals plasmacytosis (60% of her marrow cells are plasma cells [normal is < 5%]) with kappa light chain restriction.
A complete x-ray survey of the skull and long bones reveals widespread lytic lesions, consistent with multiple myeloma.
CASE 2: AN 88-YEAR-OLD MAN WITH MALAISE AND BACK PAIN
An 88-year-old man sees his family doctor because of malaise and back pain. He was treated for bladder cancer several years ago. He is currently being treated for prostatic hyperplasia, hypertension, and arthritis. Spinal radiography shows a compression deformity at T12, for which he undergoes kyphoplasty.
His complete blood cell count, white blood cell differential count, and kidney and metabolic profiles are normal.
Urine protein electrophoresis is normal, but serum electrophoresis detects an M-spike. On DXA of the hip, his T score is −3.7 (normal ≥ −1.0), and his Z score is −2.4 (normal > −2.0); suspicion of a secondary cause may be raised with Z scores of −1.0 or −1.5. The level of urinary NTX (cross-linked N-telopeptide of type I collagen, a marker of bone turnover) is 190 nmol bone collagen equivalents/nmol creatinine (normal range for men < 75), indicating a high level of bone turnover.
A serum free light chain assay shows twice the normal concentration of kappa light chains. The patient is referred for hematologic study and undergoes bone marrrow aspiration biopsy, which shows an abnormally high number of monoclonal plasma cells.
LESSONS FROM THESE CASES
The cases presented above illustrate several key clinical points:
- Minor back pain can be a symptom of a spinal compression fracture.
- Declining bone density should raise the suspicion of secondary osteoporosis, as should an abnormally low Z score.
- Markers of bone turnover are commonly elevated in secondary osteoporosis.
- Routine laboratory tests often fail to detect multiple myeloma.
BACK PAIN AS A SYMPTOM OF SPINAL COMPRESSION FRACTURE
Back pain is a very common complaint, and fortunately, most cases are due to benign causes. However, serious causes such as cancer, infection, and fractures must be considered. The topic has been reviewed in detail by Siemionow et al.2
Osteoporotic compression fractures are common in the elderly and are associated with loss of height. They can occur spontaneously or from minimal trauma. The workup can start with plain anteroposterior and lateral radiographs and routine laboratory tests, as in the patients described above. This information, as well as DXA testing, may provide clues that suggest that the osteoporosis is secondary to an underlying problem, or that a coexisting bone condition caused the fracture.
DXA CAN SUGGEST SECONDARY OSTEOPOROSIS
Declining bone density
Standard DXA testing is used to identify patients at high risk of fragility fractures from osteoporosis. It is also the accepted way to monitor disease progression and efficacy of treatment.
However, when checking to see if a patient’s bone density has changed over time, one must recognize that variations in technique from center to center or operator to operator can produce false changes in DXA results. 3,4 The testing center should state its own level of variance (referred to as the least significant change) and should indicate whether changes in a patient’s follow-up test results are statistically significant (ie, exceed that level).
A significant decline in bone mineral density over time may indicate that the patient is either not taking his or her medications or is not taking them as directed, as often happens with oral bisphosphonates—which must be taken first thing in the morning, on an empty stomach, with only a glass of water, at least 30 minutes before breakfast, during which time the patient must remain in an upright position.5–7 But a decline also raises the suspicion of an underlying condition instead of or in addition to osteoporosis, as described in the cases above. The normal decline in bone mineral density due to aging is 0.1% to 0.2% per year. For women 5 years after menopause, the rate increases to 1% to 2% and then slows to the rate of decline due to aging. A decline in bone density to the degree seen in case 1 is more than that which could be attributed to primary osteoporosis, and so an underlying cause must be considered.
Abnormally low Z scores also raise the suspicion of secondary osteoporosis
The T score is the difference, in standard deviations, between the patient’s bone density and the mean value in a population of healthy young adults. Since bone density tends to decline with age, so does the T score.
In contrast, the Z score compares a patient’s bone density with the mean value in a population the same age and sex as the patient. When it is abnormally low, it implies greater bone loss than predicted by aging alone or greater than expected from primary disease, so a secondary disorder must be considered.8,9 This was the case in our second patient, who had a Z score of −2.4.
No specific Z score cutoff has been established. Rather, the physician should be suspicious when it is lower than about −1.0 and when something in the patient’s clinical presentation, history, or laboratory evaluation raises suspicion of an underlying condition. In other words, the Z score is useful not by itself, but in context with other information.
In a retrospective analysis of men and women with osteoporosis, Swaminathan et al9 reported that a Z score cutoff of −1.0 had a sensitivity of 87.5% for detecting an underlying cause of osteoporosis.
Again, we want to emphasize that a low Z score alone is not sufficient to make a diagnosis of a secondary cause of osteoporosis. But it is good to be suspicious when a Z score is as low as in our second case and when that suspicion is reinforced by other clinical data.
MARKERS OF BONE TURNOVER
Biochemical markers of bone resorption, such as urinary NTX and the cross-linked C-telopeptide of type I collagen (CTX), have been shown to predict fracture risk independent of bone density measurements. The evidence to date supports the use of these markers in conjunction with bone density measurements to ascertain early on whether osteoporosis is responding to treatment, but their use alone to screen for osteoporosis is not encouraged.10
The markedly high level of NTX in our second patient would be unusual in primary disease—it implies a high degree of bone turnover and, in concert with the clinical information, suggests secondary osteoporosis.
SOME CAUSES OF SECONDARY BONE LOSS
If a patient has a low Z score, a declining T score, or other clues, it is critical to evaluate for causes of secondary bone loss, such as8:
- Endocrine disorders (Cushing syndrome, hyperparathyroidism, hypogonadism)
- Gastrointestinal disorders (malabsorption, cirrhosis, gastric bypass surgery)
- Renal insufficiency and failure
- Pulmonary diseases and their treatment
- Drug use (corticosteroids, antigonadotropins, anticonvulsants, aromatase inhibitors, antirejection drugs)
- Nutritional factors (alcohol abuse, smoking, eating disorders)
- Neurologic disease or its treatment
- Transplantation
- Genetic metabolic disorders
- Malignancy.
As in the scenarios presented above, unexplained changes in bone mineral density and mild anemia may trigger an evaluation for a monoclonal gammopathy.
MULTIPLE MYELOMA
Multiple myeloma is a cancer of the immunoglobulin-producing plasma cells in the bone marrow. Since the cancerous cells are clones, they all produce the same immunoglobulin—thus, the distinctive M-spike on serum or urine protein electrophoresis. It affects about 50,000 people in the United States.
The typical features of multiple myeloma are hypercalcemia, renal insufficiency, anemia, and bone lesions with or without osteoporosis. 11 Most patients have identifiable features of myeloma at the time of diagnosis, but perhaps 20% lack the characteristic symptoms of fatigue, back pain, or bone pain.
Most patients who eventually develop symptomatic multiple myeloma first present with monoclonal gammopathy of undetermined significance (MGUS), a disorder characterized by asymptomatic overproduction of an immunoglobulin. However, MGUS develops into multiple myeloma in only about 15% of cases.11
Widespread osteoporosis, due to cytokine-mediated osteoclast activation, is common in patients with multiple myeloma. As many as 90% of patients have lytic skeletal lesions or osteoporosis at the time of diagnosis.11,12
Myeloma-related osteoporosis can be difficult to differentiate from primary osteoporosis because not all patients secrete a monoclonal protein that standard urine or serum tests can detect.13 But new assays for serum free light chains can help resolve this diagnostic dilemma.14
WHEN IS TESTING FOR MONOCLONAL GAMMOPATHIES WARRANTED?
Screening for MGUS in the general osteoporotic population is not warranted, since its prevalence (2.1%) is similar to that in the general population (2.9%) of women age 50 or older and 5.3% to 7.5% of all persons age 70 years or older.15,16 However, testing for monoclonal gammopathies is warranted when clinical or laboratory findings—eg, subtle hints such as an unexplained elevation in the erythrocyte sedimentation rate or a low anion gap—trigger diagnostic suspicion. Unexplained hypercalcemia, renal insufficiency, unexplained anemia, hypo- and hypergammaglobulinemia, skeletal problems (eg, widespread osteoporosis, unexplained back or bone pain), and distal, symmetric polyneuropathy are the usual signs of underlying plasma cell neoplasia.
Signs of multiple myeloma: the CRAB mnemonic
Patients should be screened for multiple myeloma if they have any of the following presenting features not attributable to another disorder, using the mnemonic CRAB17:
Calcium elevation (serum calcium ≥ 11.5 mg/dL)
Renal insufficiency (serum creatinine > 1.73 mmol/L)
Anemia (normochromic, normocytic anema, with a hemoglobin value lower than 10 g/dL or more than 2 g/dL below the lower limit of normal)
Bone disease (lytic lesions, widespread osteoporosis, or bone fractures on skeletal survey, or a decline in bone mineral density or evidence of osteoporosis on DXA).
For the diagnosis of multiple myeloma to be made, the patient must have at least 10% clonal bone marrow plasma cells, evidence of a monoclonal protein in the serum or urine, and CRAB-related organ damage. When in doubt, referral for a hematologic evaluation is advised. Patients with signs of myeloma-related organ damage warrant prompt treatment.
Electrophoresis is not 100% sensitive
As the clinical cases above illustrate, standard testing for the monoclonal protein is not 100% sensitive for multiple myeloma, as some patients do not secrete the protein in the serum or urine.
In more than 97% of patients, the plasma cells that proliferate clonally produce a measurable monoclonal protein, such as an intact immunoglobulin only (eg, IgG kappa, IgA lambda), a light chain only (kappa or lambda), or intact immunoglobulins and free light chains. In the rest, no detectable monoclonal protein is produced, a disease subtype called nonsecretory multiple myeloma.
Of patients who secrete an intact immunoglobulin, 90% to 95% also produce excess free light chains.18,19 From 15% to 20% of patients with multiple myeloma secrete only light chains.1,20
Classically, serum and urine protein electrophoreses are the diagnostic tools used to evaluate monoclonal gammopathy, but urine electrophoresis detects only about 50% of myelomas.19
WHEN TO CONSIDER FREE LIGHT CHAIN ANALYSIS
While serum and urine protein electrophoreses are still the standard for screening for MGUS or multiple myeloma if one strongly suspects it, additional testing with serum free light chain analysis should be considered if patients exhibit CRAB-related features of myeloma-related organ damage, such as hypercalcemia, renal insufficiency, anemia, or bone loss.
Serum assays for free kappa and free lambda light chains can detect circulating clonal free light chains in most patients with nonsecretory multiple myeloma. In one study,21 elevated concentrations of either kappa or lambda free light chains (and abnormal kappa-lambda ratios) were detected in the sera of 19 of 28 patients with nonsecretory multiple myeloma, such that the diagnosis could be changed to oligosecretory disease.
Several studies have also found serum light chain panels to be highly sensitive for the diagnosis of MGUS or multiple myeloma.22–24 Clonal light chains must be present in a concentration of at least 500 mg/L to be detected by serum protein electrophoresis, or at least 150 mg/L to be detected by serum immunofixation. 25 In contrast, free light chain immunoassays can measure free light chain concentrations of 3 mg/L or lower, and can therefore detect light-chain-related disorders despite negative results on serum protein electrophoresis or immunofixation.14
Cost-effectiveness of free light chain analysis
Serum free light chain assays appear to be more cost-effective than urine tests in screening for monoclonal gammopathy: Medicare reimbursement is $38 for the serum free light chain assay vs $71 for the urine assay, which includes total urine protein, urine protein electrophoresis, and urine immunofixation electrophoresis.22
The kappa-lambda ratio
Normal values for serum free light chains are:
- Kappa 3.3–19.4 mg/L
- Lambda 5.7–26.3 mg/L
- Kappa-lambda ratio 0.26–1.65.
The kappa-lambda ratio is an indication of clonality.26,27 A ratio greater than 1.65 suggests a kappa free light chain monoclonal gammopathy; a ratio less than 0.26 suggests a lambda free light chain monoclonal gammopathy.
Importantly, in patients with renal impairment but no monoclonal gammopathy, the kappa-lambda ratio is often slightly higher—up to 3:1 because of reduced renal light chain clearance.26
However, not all patients with a monoclonal gammopathy have an abnormal free light chain ratio. Only one-third of patients with MGUS do, and these patients are at greater risk of progression to other plasma cell dyscrasias. 28 The free light chain ratio is normal in 5% to 10% of patients with intact immunoglobulin multiple myeloma.29,30 In a study of 116 patients with plasmacytoma, serum protein electrophoresis demonstrated an M-spike in half of patients, serum immunofixation was abnormal in two-thirds, and the kappa-lambda ratio was abnormal in half.31
A risk exists that MGUS will progress to multiple myeloma in patients who have an abnormal free light chain ratio. Thus, patients should be referred to a hematologist-oncologist for evaluation and monitoring if an abnormal kappa-lambda ratio is detected by serum free light chain assay.
Patients with abnormalities in the kappa-lambda ratio and no other evidence of monoclonal protein may harbor light-chain-related diseases only (eg, light chain multiple myeloma, primary amyloidosis, or light chain deposition disease) or a newly described entity, free light chain MGUS.14,19,27 An abnormal kappa-lambda ratio has also been noted in variable percentages of patients with chronic lymphocytic leukemia and malignant lymphoma.32
Sometimes, osteoporosis can be the presenting sign of a monoclonal gammopathy, which in some people may precede a diagnosis of multiple myeloma.1
In this article, we use two cases to illustrate the challenges of detecting monoclonal gammopathies as the cause of secondary osteoporosis. We also discuss the diagnostic limitations of current tests and the advantages of a newer test—measuring the serum levels of free light chains—in the workup of these patients.
CASE 1: A 55-YEAR-OLD WOMAN WITH BACK PAIN
A 55-year-old woman develops back pain after walking her dog, and the pain worsens despite treatment with a nonsteroidal anti-inflammatory drug for 1 week.
The patient has a history of well-controlled hypertension. She went through menopause 5 years ago, and about 2 years ago she was started on oral calcium and vitamin D for low bone density. At that time she complained of mild fatigue, which she attributed to working overtime and to lack of sleep.
Laboratory data, other tests
- Her white blood cell differential count is normal
- Hemoglobin 11.8 g/dL (normal range 12–15)
- Serum creatinine 1.0 mg/dL (0.5–1.4)
- Calcium 8.2 mg/dL (8.0–10.0)
- Albumin 4.5 g/dL (3.5–5.0)
- Total protein 5.7 g/dL (6.0–8.4)
- Serum and urine protein electrophoreses show no monoclonal spike (M-spike) or bands
- Serum free kappa light chains 5,542 mg/L (normal range 3.3–19.4).
Based on the elevation of serum free kappa light chains, the patient undergoes bone marrow aspiration biopsy. Histologic analysis reveals plasmacytosis (60% of her marrow cells are plasma cells [normal is < 5%]) with kappa light chain restriction.
A complete x-ray survey of the skull and long bones reveals widespread lytic lesions, consistent with multiple myeloma.
CASE 2: AN 88-YEAR-OLD MAN WITH MALAISE AND BACK PAIN
An 88-year-old man sees his family doctor because of malaise and back pain. He was treated for bladder cancer several years ago. He is currently being treated for prostatic hyperplasia, hypertension, and arthritis. Spinal radiography shows a compression deformity at T12, for which he undergoes kyphoplasty.
His complete blood cell count, white blood cell differential count, and kidney and metabolic profiles are normal.
Urine protein electrophoresis is normal, but serum electrophoresis detects an M-spike. On DXA of the hip, his T score is −3.7 (normal ≥ −1.0), and his Z score is −2.4 (normal > −2.0); suspicion of a secondary cause may be raised with Z scores of −1.0 or −1.5. The level of urinary NTX (cross-linked N-telopeptide of type I collagen, a marker of bone turnover) is 190 nmol bone collagen equivalents/nmol creatinine (normal range for men < 75), indicating a high level of bone turnover.
A serum free light chain assay shows twice the normal concentration of kappa light chains. The patient is referred for hematologic study and undergoes bone marrrow aspiration biopsy, which shows an abnormally high number of monoclonal plasma cells.
LESSONS FROM THESE CASES
The cases presented above illustrate several key clinical points:
- Minor back pain can be a symptom of a spinal compression fracture.
- Declining bone density should raise the suspicion of secondary osteoporosis, as should an abnormally low Z score.
- Markers of bone turnover are commonly elevated in secondary osteoporosis.
- Routine laboratory tests often fail to detect multiple myeloma.
BACK PAIN AS A SYMPTOM OF SPINAL COMPRESSION FRACTURE
Back pain is a very common complaint, and fortunately, most cases are due to benign causes. However, serious causes such as cancer, infection, and fractures must be considered. The topic has been reviewed in detail by Siemionow et al.2
Osteoporotic compression fractures are common in the elderly and are associated with loss of height. They can occur spontaneously or from minimal trauma. The workup can start with plain anteroposterior and lateral radiographs and routine laboratory tests, as in the patients described above. This information, as well as DXA testing, may provide clues that suggest that the osteoporosis is secondary to an underlying problem, or that a coexisting bone condition caused the fracture.
DXA CAN SUGGEST SECONDARY OSTEOPOROSIS
Declining bone density
Standard DXA testing is used to identify patients at high risk of fragility fractures from osteoporosis. It is also the accepted way to monitor disease progression and efficacy of treatment.
However, when checking to see if a patient’s bone density has changed over time, one must recognize that variations in technique from center to center or operator to operator can produce false changes in DXA results. 3,4 The testing center should state its own level of variance (referred to as the least significant change) and should indicate whether changes in a patient’s follow-up test results are statistically significant (ie, exceed that level).
A significant decline in bone mineral density over time may indicate that the patient is either not taking his or her medications or is not taking them as directed, as often happens with oral bisphosphonates—which must be taken first thing in the morning, on an empty stomach, with only a glass of water, at least 30 minutes before breakfast, during which time the patient must remain in an upright position.5–7 But a decline also raises the suspicion of an underlying condition instead of or in addition to osteoporosis, as described in the cases above. The normal decline in bone mineral density due to aging is 0.1% to 0.2% per year. For women 5 years after menopause, the rate increases to 1% to 2% and then slows to the rate of decline due to aging. A decline in bone density to the degree seen in case 1 is more than that which could be attributed to primary osteoporosis, and so an underlying cause must be considered.
Abnormally low Z scores also raise the suspicion of secondary osteoporosis
The T score is the difference, in standard deviations, between the patient’s bone density and the mean value in a population of healthy young adults. Since bone density tends to decline with age, so does the T score.
In contrast, the Z score compares a patient’s bone density with the mean value in a population the same age and sex as the patient. When it is abnormally low, it implies greater bone loss than predicted by aging alone or greater than expected from primary disease, so a secondary disorder must be considered.8,9 This was the case in our second patient, who had a Z score of −2.4.
No specific Z score cutoff has been established. Rather, the physician should be suspicious when it is lower than about −1.0 and when something in the patient’s clinical presentation, history, or laboratory evaluation raises suspicion of an underlying condition. In other words, the Z score is useful not by itself, but in context with other information.
In a retrospective analysis of men and women with osteoporosis, Swaminathan et al9 reported that a Z score cutoff of −1.0 had a sensitivity of 87.5% for detecting an underlying cause of osteoporosis.
Again, we want to emphasize that a low Z score alone is not sufficient to make a diagnosis of a secondary cause of osteoporosis. But it is good to be suspicious when a Z score is as low as in our second case and when that suspicion is reinforced by other clinical data.
MARKERS OF BONE TURNOVER
Biochemical markers of bone resorption, such as urinary NTX and the cross-linked C-telopeptide of type I collagen (CTX), have been shown to predict fracture risk independent of bone density measurements. The evidence to date supports the use of these markers in conjunction with bone density measurements to ascertain early on whether osteoporosis is responding to treatment, but their use alone to screen for osteoporosis is not encouraged.10
The markedly high level of NTX in our second patient would be unusual in primary disease—it implies a high degree of bone turnover and, in concert with the clinical information, suggests secondary osteoporosis.
SOME CAUSES OF SECONDARY BONE LOSS
If a patient has a low Z score, a declining T score, or other clues, it is critical to evaluate for causes of secondary bone loss, such as8:
- Endocrine disorders (Cushing syndrome, hyperparathyroidism, hypogonadism)
- Gastrointestinal disorders (malabsorption, cirrhosis, gastric bypass surgery)
- Renal insufficiency and failure
- Pulmonary diseases and their treatment
- Drug use (corticosteroids, antigonadotropins, anticonvulsants, aromatase inhibitors, antirejection drugs)
- Nutritional factors (alcohol abuse, smoking, eating disorders)
- Neurologic disease or its treatment
- Transplantation
- Genetic metabolic disorders
- Malignancy.
As in the scenarios presented above, unexplained changes in bone mineral density and mild anemia may trigger an evaluation for a monoclonal gammopathy.
MULTIPLE MYELOMA
Multiple myeloma is a cancer of the immunoglobulin-producing plasma cells in the bone marrow. Since the cancerous cells are clones, they all produce the same immunoglobulin—thus, the distinctive M-spike on serum or urine protein electrophoresis. It affects about 50,000 people in the United States.
The typical features of multiple myeloma are hypercalcemia, renal insufficiency, anemia, and bone lesions with or without osteoporosis. 11 Most patients have identifiable features of myeloma at the time of diagnosis, but perhaps 20% lack the characteristic symptoms of fatigue, back pain, or bone pain.
Most patients who eventually develop symptomatic multiple myeloma first present with monoclonal gammopathy of undetermined significance (MGUS), a disorder characterized by asymptomatic overproduction of an immunoglobulin. However, MGUS develops into multiple myeloma in only about 15% of cases.11
Widespread osteoporosis, due to cytokine-mediated osteoclast activation, is common in patients with multiple myeloma. As many as 90% of patients have lytic skeletal lesions or osteoporosis at the time of diagnosis.11,12
Myeloma-related osteoporosis can be difficult to differentiate from primary osteoporosis because not all patients secrete a monoclonal protein that standard urine or serum tests can detect.13 But new assays for serum free light chains can help resolve this diagnostic dilemma.14
WHEN IS TESTING FOR MONOCLONAL GAMMOPATHIES WARRANTED?
Screening for MGUS in the general osteoporotic population is not warranted, since its prevalence (2.1%) is similar to that in the general population (2.9%) of women age 50 or older and 5.3% to 7.5% of all persons age 70 years or older.15,16 However, testing for monoclonal gammopathies is warranted when clinical or laboratory findings—eg, subtle hints such as an unexplained elevation in the erythrocyte sedimentation rate or a low anion gap—trigger diagnostic suspicion. Unexplained hypercalcemia, renal insufficiency, unexplained anemia, hypo- and hypergammaglobulinemia, skeletal problems (eg, widespread osteoporosis, unexplained back or bone pain), and distal, symmetric polyneuropathy are the usual signs of underlying plasma cell neoplasia.
Signs of multiple myeloma: the CRAB mnemonic
Patients should be screened for multiple myeloma if they have any of the following presenting features not attributable to another disorder, using the mnemonic CRAB17:
Calcium elevation (serum calcium ≥ 11.5 mg/dL)
Renal insufficiency (serum creatinine > 1.73 mmol/L)
Anemia (normochromic, normocytic anema, with a hemoglobin value lower than 10 g/dL or more than 2 g/dL below the lower limit of normal)
Bone disease (lytic lesions, widespread osteoporosis, or bone fractures on skeletal survey, or a decline in bone mineral density or evidence of osteoporosis on DXA).
For the diagnosis of multiple myeloma to be made, the patient must have at least 10% clonal bone marrow plasma cells, evidence of a monoclonal protein in the serum or urine, and CRAB-related organ damage. When in doubt, referral for a hematologic evaluation is advised. Patients with signs of myeloma-related organ damage warrant prompt treatment.
Electrophoresis is not 100% sensitive
As the clinical cases above illustrate, standard testing for the monoclonal protein is not 100% sensitive for multiple myeloma, as some patients do not secrete the protein in the serum or urine.
In more than 97% of patients, the plasma cells that proliferate clonally produce a measurable monoclonal protein, such as an intact immunoglobulin only (eg, IgG kappa, IgA lambda), a light chain only (kappa or lambda), or intact immunoglobulins and free light chains. In the rest, no detectable monoclonal protein is produced, a disease subtype called nonsecretory multiple myeloma.
Of patients who secrete an intact immunoglobulin, 90% to 95% also produce excess free light chains.18,19 From 15% to 20% of patients with multiple myeloma secrete only light chains.1,20
Classically, serum and urine protein electrophoreses are the diagnostic tools used to evaluate monoclonal gammopathy, but urine electrophoresis detects only about 50% of myelomas.19
WHEN TO CONSIDER FREE LIGHT CHAIN ANALYSIS
While serum and urine protein electrophoreses are still the standard for screening for MGUS or multiple myeloma if one strongly suspects it, additional testing with serum free light chain analysis should be considered if patients exhibit CRAB-related features of myeloma-related organ damage, such as hypercalcemia, renal insufficiency, anemia, or bone loss.
Serum assays for free kappa and free lambda light chains can detect circulating clonal free light chains in most patients with nonsecretory multiple myeloma. In one study,21 elevated concentrations of either kappa or lambda free light chains (and abnormal kappa-lambda ratios) were detected in the sera of 19 of 28 patients with nonsecretory multiple myeloma, such that the diagnosis could be changed to oligosecretory disease.
Several studies have also found serum light chain panels to be highly sensitive for the diagnosis of MGUS or multiple myeloma.22–24 Clonal light chains must be present in a concentration of at least 500 mg/L to be detected by serum protein electrophoresis, or at least 150 mg/L to be detected by serum immunofixation. 25 In contrast, free light chain immunoassays can measure free light chain concentrations of 3 mg/L or lower, and can therefore detect light-chain-related disorders despite negative results on serum protein electrophoresis or immunofixation.14
Cost-effectiveness of free light chain analysis
Serum free light chain assays appear to be more cost-effective than urine tests in screening for monoclonal gammopathy: Medicare reimbursement is $38 for the serum free light chain assay vs $71 for the urine assay, which includes total urine protein, urine protein electrophoresis, and urine immunofixation electrophoresis.22
The kappa-lambda ratio
Normal values for serum free light chains are:
- Kappa 3.3–19.4 mg/L
- Lambda 5.7–26.3 mg/L
- Kappa-lambda ratio 0.26–1.65.
The kappa-lambda ratio is an indication of clonality.26,27 A ratio greater than 1.65 suggests a kappa free light chain monoclonal gammopathy; a ratio less than 0.26 suggests a lambda free light chain monoclonal gammopathy.
Importantly, in patients with renal impairment but no monoclonal gammopathy, the kappa-lambda ratio is often slightly higher—up to 3:1 because of reduced renal light chain clearance.26
However, not all patients with a monoclonal gammopathy have an abnormal free light chain ratio. Only one-third of patients with MGUS do, and these patients are at greater risk of progression to other plasma cell dyscrasias. 28 The free light chain ratio is normal in 5% to 10% of patients with intact immunoglobulin multiple myeloma.29,30 In a study of 116 patients with plasmacytoma, serum protein electrophoresis demonstrated an M-spike in half of patients, serum immunofixation was abnormal in two-thirds, and the kappa-lambda ratio was abnormal in half.31
A risk exists that MGUS will progress to multiple myeloma in patients who have an abnormal free light chain ratio. Thus, patients should be referred to a hematologist-oncologist for evaluation and monitoring if an abnormal kappa-lambda ratio is detected by serum free light chain assay.
Patients with abnormalities in the kappa-lambda ratio and no other evidence of monoclonal protein may harbor light-chain-related diseases only (eg, light chain multiple myeloma, primary amyloidosis, or light chain deposition disease) or a newly described entity, free light chain MGUS.14,19,27 An abnormal kappa-lambda ratio has also been noted in variable percentages of patients with chronic lymphocytic leukemia and malignant lymphoma.32
- Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009; 113:5418–5422.
- Siemionow K, Steinmetz M, Bell G, Ilaslan H, McLain RF. Identifying serious causes of back pain: cancer, infection, fracture. Cleve Clin J Med 2008; 75:557–566.
- Binkley N, Krueger D. What should DXA reports contain? P of ordering health care providers. J Clin Densitom 2009; 12:5–10.
- Bonnick SL, Johnston CC, Kleerekoper M, et al Importance of precision in bone density measurements. J Clin Densitom 2001; 4:105–110.
- Gold DE, Alexander IM, Ettinger MP. How can osteoporosis patients benefit more from their therapy? Adherence issues with bisphosphonate therapy. Ann Pharmacother 2006; 40:1143–1150.
- Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 2005; 44:551–570.
- Lin JT, Lane JM. Bisphosphonates. J Am Acad Orthop Surg 2003; 11:1–4.
- Licata AA. Diagnosing primary osteoporosis: it’s more than a T score. Cleve Clin J Med 2006; 73:473–476.
- Swaminathan K, Flynn K, Garton M, Paterson C, Leese G. Search for secondary osteoporosis: are Z scores useful predictors? Postgrad Med J 2009; 85:38–39.
- Clowes JA, Eastell R. The role of bone turnover markers and risk factors in the assessment of osteoporosis and fracture risk. Baillieres Best Pract Res Clin Endocrinol Metab 2000; 14:213–232.
- Kyle RA, Gertz MA, Witzig TE, et al Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78:21–33.
- Hussein MA, Vrionis FD, Allison R, et al., International Myeloma Working Group. The role of vertebral augmentation in multiple myeloma: International Myeloma Working Group Consensus Statement. Leukemia 2008; 22:1479–1484.
- Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am 1999; 13:1259–1272.
- Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet 2003; 361:489–491.
- Tannenbaum C, Clark J, Schwartzman K, et al Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Kyle RA, Therneau TM, Rajkumar SV, et al Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006; 354:1362–1369.
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121:749–757.
- Pepe J, Petrucci MT, Nofroni I, et al Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol 2006; 134:485–490.
- Berenson JR, Yellin O, Boccia RV, et al Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res 2008; 14:6289–6295.
- Pepe J, Petrucci MT, Mascia ML, et al The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int 2008; 82:418–426.
- Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood 2001; 97:2900–2902.
- Katzmann JA, Dispenzieri A, Kyle RA, et al Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 2006; 81:1575–1578.
- Abadie JM, van Hoeven KH, Wells JM. Are renal reference intervals required when screening for plasma cell disorders with serum free light chains and serum protein electrophoresis? Am J Clin Pathol 2009; 131:166–171.
- Abadie JM, Bankson DD. Assessment of serum free light chain assays for plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab Sci 2006; 36:157–162.
- Shaw GR. Nonsecretory plasma cell myeloma—becoming even more rare with serum free light-chain assay: a brief review. Arch Pathol Lab Med 2006; 130:1212–1215.
- Hutchison CA, Harding S, Hewins P, et al Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3:1684–1690.
- Katzmann JA, Clark RJ, Abraham RS, et al Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem 2002; 48:1437–1444.
- Rajkumar SV, Kyle RA, Therneau TM, et al Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106:812–817.
- Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol 2004; 126:348–354.
- Dispenzieri A, Zhang L, Katzmann JA, et al Appraisal of immunoglobulin free light chain as a marker of response. Blood 2008; 111:4908–4915.
- Dingli D, Kyle RA, Rajkumar SV, et al Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood 2006; 108:1979–1983.
- Martin W, Abraham R, Shanafelt T, et al Serum-free light chain—a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res 2007; 149:231–235.
- Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009; 113:5418–5422.
- Siemionow K, Steinmetz M, Bell G, Ilaslan H, McLain RF. Identifying serious causes of back pain: cancer, infection, fracture. Cleve Clin J Med 2008; 75:557–566.
- Binkley N, Krueger D. What should DXA reports contain? P of ordering health care providers. J Clin Densitom 2009; 12:5–10.
- Bonnick SL, Johnston CC, Kleerekoper M, et al Importance of precision in bone density measurements. J Clin Densitom 2001; 4:105–110.
- Gold DE, Alexander IM, Ettinger MP. How can osteoporosis patients benefit more from their therapy? Adherence issues with bisphosphonate therapy. Ann Pharmacother 2006; 40:1143–1150.
- Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet 2005; 44:551–570.
- Lin JT, Lane JM. Bisphosphonates. J Am Acad Orthop Surg 2003; 11:1–4.
- Licata AA. Diagnosing primary osteoporosis: it’s more than a T score. Cleve Clin J Med 2006; 73:473–476.
- Swaminathan K, Flynn K, Garton M, Paterson C, Leese G. Search for secondary osteoporosis: are Z scores useful predictors? Postgrad Med J 2009; 85:38–39.
- Clowes JA, Eastell R. The role of bone turnover markers and risk factors in the assessment of osteoporosis and fracture risk. Baillieres Best Pract Res Clin Endocrinol Metab 2000; 14:213–232.
- Kyle RA, Gertz MA, Witzig TE, et al Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78:21–33.
- Hussein MA, Vrionis FD, Allison R, et al., International Myeloma Working Group. The role of vertebral augmentation in multiple myeloma: International Myeloma Working Group Consensus Statement. Leukemia 2008; 22:1479–1484.
- Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am 1999; 13:1259–1272.
- Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet 2003; 361:489–491.
- Tannenbaum C, Clark J, Schwartzman K, et al Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Kyle RA, Therneau TM, Rajkumar SV, et al Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006; 354:1362–1369.
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121:749–757.
- Pepe J, Petrucci MT, Nofroni I, et al Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol 2006; 134:485–490.
- Berenson JR, Yellin O, Boccia RV, et al Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res 2008; 14:6289–6295.
- Pepe J, Petrucci MT, Mascia ML, et al The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int 2008; 82:418–426.
- Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood 2001; 97:2900–2902.
- Katzmann JA, Dispenzieri A, Kyle RA, et al Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 2006; 81:1575–1578.
- Abadie JM, van Hoeven KH, Wells JM. Are renal reference intervals required when screening for plasma cell disorders with serum free light chains and serum protein electrophoresis? Am J Clin Pathol 2009; 131:166–171.
- Abadie JM, Bankson DD. Assessment of serum free light chain assays for plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab Sci 2006; 36:157–162.
- Shaw GR. Nonsecretory plasma cell myeloma—becoming even more rare with serum free light-chain assay: a brief review. Arch Pathol Lab Med 2006; 130:1212–1215.
- Hutchison CA, Harding S, Hewins P, et al Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3:1684–1690.
- Katzmann JA, Clark RJ, Abraham RS, et al Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem 2002; 48:1437–1444.
- Rajkumar SV, Kyle RA, Therneau TM, et al Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106:812–817.
- Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol 2004; 126:348–354.
- Dispenzieri A, Zhang L, Katzmann JA, et al Appraisal of immunoglobulin free light chain as a marker of response. Blood 2008; 111:4908–4915.
- Dingli D, Kyle RA, Rajkumar SV, et al Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood 2006; 108:1979–1983.
- Martin W, Abraham R, Shanafelt T, et al Serum-free light chain—a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res 2007; 149:231–235.
KEY POINTS
- Minor back pain can be a symptom of spinal compression fracture.
- Rapidly declining bone density or a low Z score on dual-energy x-ray absorptiometry suggests that osteoporosis is secondary to another condition.
- The evidence to date supports the use of bone turnover markers in conjunction with density measurements to ascertain early on whether osteoporosis is responding to treatment, but the use of biochemical markers by themselves to screen for osteoporosis is not encouraged.
- Standard tests may fail to detect myeloma in the presence of worsening bone density.
- While serum and urine protein electrophoreses are still the standard screening tests for multiple myeloma, additional testing with serum free light chain analysis should be considered if the suspicion is high.