User login
Effect of an RRT on Resident Perceptions
Rapid response teams (RRTs) have been promoted by patient safety and quality‐improvement organizations as a strategy to reduce preventable in‐hospital deaths.[1] To date, critical analysis of RRTs has focused primarily on their impact on quality‐of‐care metrics.[2, 3, 4] Comparatively few studies have examined the cultural and educational impact of RRTs, particularly at academic medical centers, and those that do exist have focused almost exclusively on perceptions of nurses rather than resident physicians.[5, 6, 7, 8, 9, 10]
Although a prior study found that internal medicine and general surgery residents believed that RRTs improved patient safety, they were largely ambivalent about the RRT's impact on education and training.[11] To date, there has been no focused assessment of resident physician impressions of an RRT across years of training and medical specialty to inform the use of this multidisciplinary team as a component of their residency education.
We sought to determine whether resident physicians at a tertiary care academic medical center perceive educational benefit from collaboration with the RRT and whether they feel that the RRT adversely affects clinical autonomy.
METHODS
The Hospital
Moffitt‐Long Hospital, the tertiary academic medical center of the University of California, San Francisco (UCSF), is a 600‐bed acute care hospital that provides comprehensive critical care services and serves as a major referral center in northern California. There are roughly 5000 admissions to the hospital annually. At the time the study was conducted, there were approximately 200 RRT calls per 1000 adult hospital discharges.
The Rapid Response Team
The RRT is called to assess, triage, and treat patients who have experienced a decline in their clinical status short of a cardiopulmonary arrest. The RRT has been operational at UCSF since June 1, 2007, and is composed of a dedicated critical care nurse and respiratory therapist available 24 hours a day, 7 days a week. The RRT can be activated by any concerned staff member based on vital sign abnormalities, decreased urine output, changes in mental status, or any significant concern about the trajectory of the patient's clinical course.
When the RRT is called on a given patient, the patient's primary physician (at our institution, a resident) is also called to the bedside and works alongside the RRT to address the patient's acute clinical needs. The primary physician, bedside nurse, and RRT discuss the plan of care for the patient, including clinical evaluation, management, and the need for additional monitoring or a transition to a higher level of care. Residents at our institution receive no formal instruction regarding the role of the RRT or curriculum on interfacing with the RRT, and they do not serve as members of the RRT as part of a clinical rotation.
The Survey Process
Study subjects were asked via e‐mail to participate in a brief online survey. Subjects were offered the opportunity to win a $100 gift certificate in return for their participation. Weekly e‐mail reminders were sent for a period of 3 months or until a given subject had completed the survey. The survey was administered over a 3‐month period, from March through May, to allow time for residents to work with the RRT during the academic year. The Committee on Human Research at the University of California San Francisco Medical Center approved the study.
Target Population
All residents in specialties that involved direct patient care and the potential to use the adult RRT were included in the study. This included residents in the fields of internal medicine, neurology, general surgery, orthopedic surgery, neurosurgery, plastic surgery, urology, and otolaryngology (Table 1). Residents in pediatrics and obstetrics and gynecology were excluded, as emergencies in their patients are addressed by a pediatric RRT and an obstetric anesthesiologist, respectively. Residents in anesthesiology were excluded as they do not care for nonintensive care unit (ICU) patients as part of the primary team and are not involved in RRT encounters.
| Demographic | No. (%) |
|---|---|
| |
| Medical specialty | |
| Internal medicine | 145 (61.4) |
| Neurology | 18 (7.6) |
| General surgery | 31 (13.1) |
| Orthopedic surgery | 17 (7.2) |
| Neurosurgery | 4 (1.7) |
| Plastic surgery | 2 (0.8) |
| Urology | 9 (3.8) |
| Otolaryngology | 10 (4.2) |
| Years of postgraduate training | Average 2.34 (SD 1.41) |
| 1 | 83 (35.2) |
| 2 | 60 (25.4) |
| 3 | 55 (23.3) |
| 4 | 20 (8.5) |
| 5 | 8 (3.4) |
| 6 | 5 (2.1) |
| 7 | 5 (2.1) |
| Gender | |
| Male | 133 (56.4) |
| Female | 102 (43.2) |
| Had exposure to RRT during training | |
| Yes | 106 (44.9) |
| No | 127 (53.8) |
| Had previously initiated a call to the RRT | |
| Yes | 106 (44.9) |
| No | 128 (54.2) |
Survey Design
The resident survey contained 20 RRT‐related items and 7 demographic and practice items. Responses for RRT‐related questions utilized a 5‐point Likert scale ranging from strongly disagree to strongly agree. The survey was piloted prior to administration to check comprehension and interpretation by physicians with experience in survey writing (for the full survey, see Supporting Information, Appendix, in the online version of this article).
Survey Objectives
The survey was designed to capture the experiences of residents who had cared for a patient for whom the RRT had been activated. Data collected included residents' perceptions of the impact of the RRT on their residency education and clinical autonomy, the quality of care provided, patient safety, and hospital‐wide culture. Potential barriers to use of the RRT were also examined.
Outcomes
The study's primary outcomes included the perceived educational benefit of the RRT and its perceived impact on clinical autonomy. Secondary outcomes included the effect of years of training and resident specialty on both the perceived educational benefit and impact on clinical autonomy among our study group.
Statistical Analysis
Responses to each survey item were described for each specialty, and subgroup analysis was conducted. For years of training, that item was dichotomized into either 1 year (henceforth referred to as interns) or greater than 1 year (henceforth referred to as upper‐level residents). Resident specialty was dichotomized into medical fields (internal medicine and neurology) or surgical fields. For statistical analysis, agreement statements were collapsed to either disagree (strongly disagree/disagree), neutral, or agree (strongly agree/agree). The influence of years of resident training and resident specialty was assessed for all items in the survey using 2 or Fisher exact tests as appropriate for the 3 agreement categories. Analysis was conducted using SPSS 21.0 (IBM Corp., Armonk, NY).
RESULTS
There were 246 responses to the survey of a possible 342, yielding a response rate of 72% (Table 2). Ten respondents stated that they had never cared for a patient where the RRT had been activated. Given their lack of exposure to the RRT, these respondents were excluded from the analysis, yielding a final sample size of 236. The demographic and clinical practice characteristics of respondents are shown in Table 1.
| The resident | Strongly Disagree/Disagree, n (%) | Neutral, n (%) | Agree/ Strongly Agree, n (%) |
|---|---|---|---|
| |||
| Is comfortable managing the unstable patient without the RRT | 104 (44.1) | 64 (27.1) | 66 (28.0) |
| And RRT work together to make treatment decisions | 10 (4.2) | 13 (5.5) | 208 (88.1) |
| Believes there are fewer opportunities to care for unstable floor patients due to the RRT | 188 (79.7) | 26 (11.0) | 17 (7.2) |
| Feels less prepared to care for unstable patients due to the RRT | 201 (85.2) | 22 (9.3) | 13 (5.5) |
| Feels that working with the RRT creates a valuable educational experience | 9 (3.8) | 39 (16.5) | 184 (78.0) |
| Feels that nurses caring for the unstable patient should always contact them prior to contacting the RRT | 123 (52.1) | 33 (14.0) | 76 (32.2) |
| Would be unhappy with nurses calling RRT prior to contacting them | 141 (59.7) | 44 (18.6) | 51 (21.6) |
| Perceives that the presence of RRT decreases residents' autonomy | 179 (75.8) | 25 (10.6) | 28 (11.9) |
Demographics and Primary Outcomes
Interns comprised 83 (35%) of the respondents; the average time in postgraduate training was 2.34 years (standard deviation=1.41). Of respondents, 163 (69%) were in medical fields, and 73 (31%) were in surgical fields. Overall responses to the survey are shown in Table 2, and subgroup analysis is shown in Table 3.
| The resident | 1 Year, n=83, n (%) | >1 Year, n=153, n (%) | P Value | Medical, n=163, n (%) | Surgical, n=73, n (%) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Is comfortable managing the unstable patient without the RRT | 0.01 | <0.01 | ||||
| Strongly disagree/disagree | 39 (47.6) | 65 (42.8) | 67 (41.6) | 37 (50.7) | ||
| Neutral | 29 (35.4) | 35 (23.0) | 56 (34.8) | 8 (11.0) | ||
| Agree/strongly agree | 14 (17.1) | 52 (34.2) | 38 (23.6) | 28 (38.4) | ||
| And RRT work together to make treatment decisions | 0.61 | 0.04 | ||||
| Strongly disagree/disagree | 2 (2.4) | 8 (5.4) | 4 (2.5) | 6 (8.7) | ||
| Neutral | 5 (6.1) | 8 (5.4) | 7 (4.3) | 6 (8.7) | ||
| Agree/strongly agree | 75 (91.5) | 137 (89.3) | 151 (93.2) | 57 (82.6) | ||
| Believes there are fewer opportunities to care for unstable floor patients due to the RRT | 0.05 | 0.04 | ||||
| Strongly disagree/disagree | 59 (72.8) | 129 (86.0) | 136 (85.5) | 52 (72.2) | ||
| Neutral | 13 (16.0) | 13 (8.7) | 15 (9.4) | 11 (15.3) | ||
| Agree/strongly agree | 9 (11.1) | 8 (5.3) | 8 (5.0) | 9 (12.5) | ||
| Feels less prepared to care for unstable patients due to the RRT | <0.01 | 0.79 | ||||
| Strongly disagree/disagree | 62 (74.7) | 139 (90.8) | 140 (85.9) | 61 (83.6) | ||
| Neutral | 14 (16.9) | 8 (5.2) | 15 (9.2) | 7 (9.6) | ||
| Agree/Strongly agree | 7 (8.4) | 6 (3.9) | 8 (4.9) | 5 (6.8) | ||
| Feels working with the RRT is a valuable educational experience | 0.61 | 0.01 | ||||
| Strongly disagree/disagree | 2 (2.4) | 7 (4.7) | 2 (1.2) | 7 (9.9) | ||
| Neutral | 12 (14.6) | 27 (18.0) | 25 (15.5) | 14 (19.7) | ||
| Agree/strongly agree | 68 (82.9) | 116 (77.3) | 134 (83.2) | 50 (70.4) | ||
| Feels nurses caring for unstable patients should always contact the resident prior to contacting the RRT | 0.49 | <0.01 | ||||
| Strongly disagree/disagree | 47 (57.3) | 76 (50.7) | 97 (60.2) | 26 (36.6) | ||
| Neutral | 9 (11.0) | 24 (16.0) | 26 (16.1) | 7 (9.9) | ||
| Agree/strongly agree | 26 (31.7) | 50 (33.3) | 38 (23.6) | 38 (53.5) | ||
| Would be unhappy with nurses calling RRT prior to contacting them | 0.81 | <0.01 | ||||
| Strongly disagree/disagree | 51 (61.4) | 90 (58.8) | 109 (66.9) | 32 (43.8) | ||
| Neutral | 16 (19.3) | 28 (18.3) | 30 (18.4) | 14 (19.2) | ||
| Agree/strongly agree | 16 (19.3) | 35 (22.9) | 24 (14.7) | 27 (37.0) | ||
| Perceives that the presence of the RRT decreases autonomy as a physician | 0.95 | 0.18 | ||||
| Strongly disagree/disagree | 63 (77.8) | 116 (76.8) | 127 (79.9) | 52 (71.2) | ||
| Neutral | 9 (11.1) | 16 (10.6) | 17 (10.7) | 8 (11.0) | ||
| Agree/strongly agree | 9 (11.1) | 19 (12.6) | 15 (9.4) | 13 (17.8) | ||
Effect of the RRT on Resident Education
Of all residents, 66 (28%) agreed that they felt comfortable managing an unstable patient without the assistance of the RRT. Surgical residents felt more comfortable managing an unstable patient alone (38%) compared medical residents (24%) (P<0.01). Interns felt less comfortable caring for unstable patients without the RRT's assistance (17%) compared with upper‐level residents (34%) (P=0.01).
Residents overall disagreed with the statement that the RRT left them feeling less prepared to care for unstable patients (n=201; 85%). More upper‐level residents disagreed with this assertion (91%) compared with interns (75%) (P<0.01). Responses to this question did not differ significantly between medical and surgical residents.
Upper‐level residents were more likely to disagree with the statement that the RRT resulted in fewer opportunities to care for unstable patients (n=129; 86%) compared with interns (n=59; 73%) (P=0.05). Medical residents were also more likely to disagree with this statement (n=136; 86%) compared with surgical residents (n=52; 72%) (P=0.04).
With respect to residents' overall impressions of the educational value of the RRT, 68 (83%) interns and 116 (77%) upper‐level residents agreed that it provided a valuable educational experience (P=0.61). Medical and surgical residents differed in this regard, with 134 (83%) medical residents and 50 (70%) surgical residents agreeing that the RRT provided a valuable educational experience (P=0.01).
Effect of the RRT on Clinical Autonomy
Of all residents, 123 (52%) disagreed that the bedside nurse should always contact the primary resident prior to calling the RRT; 76 (32%) agreed with this statement. Medicine residents were more likely to disagree with this approach (n=97; 60%) than were surgical residents (n=26; 36%) (P<0.01). There was no difference between interns and upper‐level residents in response to this question. Most of those who disagreed with this statement were medical residents, whereas most surgical residents (n=38; 54%) agreed that they should be contacted first (P<0.01).
There were no differences between interns and upper‐level residents with respect to perceptions of the RRT's impact on clinical autonomy: 11% of interns and 13% of residents agreed that the RRT decreased their clinical autonomy as a physician. There was no significant difference between medical and surgical residents' responses to this question.
The majority of residents (n=208; 88%) agreed that they and the RRT work together to make treatment decisions for patients. This was true regardless of year of training (P=0.61), but it was expressed more often among medical residents than surgical residents (n=151, 93% vs n=57, 83%; P=0.04).
DISCUSSION
Most studies examining the educational and cultural impact of RRTs exist in the nursing literature. These studies demonstrate that medical and surgical nurses are often reluctant to call the RRT for fear of criticism by the patient's physician.[5, 8, 9, 10, 11, 12, 13] In contrast, our data demonstrate that resident physicians across all levels of training and specialties have a positive view of the RRT and its role in patient care. The data support our hypothesis that although most residents perceive educational benefit from their interactions with the RRT, this perception is greater for less‐experienced residents and for those residents who routinely provide care for critically ill patients and serve as code team leaders. In addition, a minority of residents, irrespective of years of training or medical specialty, felt that the RRT negatively impacted their clinical autonomy.
Our data have several important implications. First, although over half of the residents surveyed had not been exposed to RRTs during medical school, and despite having no formal training on the role of the RRT during residency, most residents identified their interactions with the RRT as potential learning opportunities. This finding differs from that of Benin and colleagues, who suggested that RRTs might negatively impact residents' educational development and decrease opportunities for high‐stakes clinical reasoning by allowing the clinical decision‐making process to be driven by the RRT staff rather than the resident.[5] One possible explanation for this discrepancy is the variable makeup of the RRT at different institutions. At our medical center, the RRT is comprised of a critical care nurse and respiratory therapist, whereas at other institutions, the RRT may be led by a resident, fellow, attending hospitalist, or intensivist, any of whom might supersede the primary resident once the RRT is engaged.
In our study, the perceived educational benefit of the RRT was most pronounced with interns. Interns likely derive incrementally greater benefit from each encounter with an acutely decompensating patient than do senior residents, whether the RRT is present or not. Observing the actions of seasoned nurses and respiratory therapists may demonstrate new tools for interns to use in their management of such situations; for example, the RRT may suggest different modes of oxygen delivery or new diagnostic tests. The RRT also likely helps interns navigate the hospital system by assisting with decisions around escalation of care and serving as a liaison to ICU staff.
Our data also have implications for resident perceptions of clinical autonomy. Interns, far less experienced caring for unstable patients than upper‐level residents, expressed more concern about the RRT stripping them of opportunities to do so and about feeling less prepared to handle clinically deteriorating patients. Part of this perception may be due to interns feeling less comfortable taking charge of a patient's care in the presence of an experienced critical care nurse and respiratory therapist, both for reasons related to clinical experience and to a cultural hierarchy that often places the intern at the bottom of the authority spectrum. In addition, when the RRT is called on an intern's patient, the senior resident may accompany the intern to the bedside and guide the intern on his or her approach to the situation; in some cases, the senior resident may take charge, leaving the intern feeling less autonomous.
If training sessions could be developed to address not only clinical decision making, but also multidisciplinary team interactions and roles in the acute care setting, this may mitigate interns' concerns. Such curricula could also enhance residents' experience in interprofessional care, an aspect of clinical training that has become increasingly important in the age of limited duty hours and higher volume, and higher acuity inpatient censuses. An RRT model, like a code blue model, could be used in simulation‐based training to increase both comfort with use of the RRT and efficiency of the RRTresidentnurse team. Although our study did not address specifically residents' perceptions of multidisciplinary teams, this could be a promising area for further study.
For surgical residents, additional factors are likely at play. Surgical residents spend significant time in the operating room, reducing time present at the bedside and hindering the ability to respond swiftly when an RRT is called on their patient. This could cause surgical residents to feel less involved in the care of that patientsupported by our finding that fewer surgical residents felt able to collaborate with the RRTand also to derive less educational benefit and clinical satisfaction from the experience. Differences between medical and surgical postgraduate training also likely play a role, manifest by varying clinical roles and duration of training, and as such it may not be appropriate to draw direct comparisons between respective postgraduate year levels. In addition, differences in patients' medical complexity, varying allegiance to the traditional hierarchy of medical providers, and degree of familiarity with the RRT itself may impact surgical residents' comfort with the RRT.
Limitations of our study include that it was conducted at a single site and addressed a specific population of residents at our tertiary academic center. Though we achieved an excellent response rate, our subspecialty sample sizes were too small to allow for individual comparisons among those groups. Conducting a larger study at multiple institutions where the makeup of the RRT differs could provide further insight into how different clinical environments and different RRT models impact resident perceptions. Finally, we allowed each respondent to interpret both educational benefit and clinical autonomy in the context of their own level of training and clinical practice rather than providing strict definitions of these terms. There is no standardized definition of autonomy in the context of resident clinical practice, and we did not measure direct educational outcomes. Our study design therefore allowed only for measurement of perceptions of these concepts. Measurement of actual educational value of the RRTfor example, through direct clinical observation or by incorporating the RRT experience into an entrustable professional activitywould provide more quantitative evidence of the RRT's utility for our resident population. Future study in this area would help to support the development and ongoing assessment of RRT‐based curricula moving forward.
CONCLUSION
Our data show that resident physicians have a strongly favorable opinion of the RRT at our institution. Future studies should aim to quantify the educational benefit of RRTs for residents and identify areas for curricular development to enhance resident education as RRTs become more pervasive.
- Institute for Healthcare Improvement. Rapid response teams. Available at: http://www.ihi.org/topics/rapidresponseteams. Accessed May 5, 2014.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34(9):2463–2478.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , , . Defining impact of a rapid response team: qualitative study with nurses, physicians and hospital administrators. BMJ Qual Saf. 2012;21(5):391–398.
- , , . How RNs rescue patients: a qualitative study of RNs' perceived involvement in rapid response teams. Qual Saf Health Care. 2010;19(5):e13.
- , , , . Rapid response team approach to staff satisfaction. Orthop Nurs. 2008;27(5):266–271; quiz 272–273.
- , , , . Voices from the floor: nurses' perceptions of the medical emergency team. Intensive Crit Care Nurs. 2006;22(3):138–143.
- , , . Rapid response teams seen through the eyes of the nurse. Am J Nurs. 2010;110(6):28–34; quiz 35–36.
- , , , et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi‐campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21(7):569–575.
- , , , et al. Resident and RN perceptions of the impact of a medical emergency team on education and patient safety in an academic medical center. Crit Care Med. 2009;37(12):3091–3096.
- , , , et al. Why don't hospital staff activate the rapid response system (RRS)? How frequently is it needed and can the process be improved? Implement Sci. 2011;6:39.
- , , , . Timing and teamwork–an observational pilot study of patients referred to a rapid response team with the aim of identifying factors amenable to re‐design of a rapid response system. Resuscitation. 2012;83(6):782–787.
Rapid response teams (RRTs) have been promoted by patient safety and quality‐improvement organizations as a strategy to reduce preventable in‐hospital deaths.[1] To date, critical analysis of RRTs has focused primarily on their impact on quality‐of‐care metrics.[2, 3, 4] Comparatively few studies have examined the cultural and educational impact of RRTs, particularly at academic medical centers, and those that do exist have focused almost exclusively on perceptions of nurses rather than resident physicians.[5, 6, 7, 8, 9, 10]
Although a prior study found that internal medicine and general surgery residents believed that RRTs improved patient safety, they were largely ambivalent about the RRT's impact on education and training.[11] To date, there has been no focused assessment of resident physician impressions of an RRT across years of training and medical specialty to inform the use of this multidisciplinary team as a component of their residency education.
We sought to determine whether resident physicians at a tertiary care academic medical center perceive educational benefit from collaboration with the RRT and whether they feel that the RRT adversely affects clinical autonomy.
METHODS
The Hospital
Moffitt‐Long Hospital, the tertiary academic medical center of the University of California, San Francisco (UCSF), is a 600‐bed acute care hospital that provides comprehensive critical care services and serves as a major referral center in northern California. There are roughly 5000 admissions to the hospital annually. At the time the study was conducted, there were approximately 200 RRT calls per 1000 adult hospital discharges.
The Rapid Response Team
The RRT is called to assess, triage, and treat patients who have experienced a decline in their clinical status short of a cardiopulmonary arrest. The RRT has been operational at UCSF since June 1, 2007, and is composed of a dedicated critical care nurse and respiratory therapist available 24 hours a day, 7 days a week. The RRT can be activated by any concerned staff member based on vital sign abnormalities, decreased urine output, changes in mental status, or any significant concern about the trajectory of the patient's clinical course.
When the RRT is called on a given patient, the patient's primary physician (at our institution, a resident) is also called to the bedside and works alongside the RRT to address the patient's acute clinical needs. The primary physician, bedside nurse, and RRT discuss the plan of care for the patient, including clinical evaluation, management, and the need for additional monitoring or a transition to a higher level of care. Residents at our institution receive no formal instruction regarding the role of the RRT or curriculum on interfacing with the RRT, and they do not serve as members of the RRT as part of a clinical rotation.
The Survey Process
Study subjects were asked via e‐mail to participate in a brief online survey. Subjects were offered the opportunity to win a $100 gift certificate in return for their participation. Weekly e‐mail reminders were sent for a period of 3 months or until a given subject had completed the survey. The survey was administered over a 3‐month period, from March through May, to allow time for residents to work with the RRT during the academic year. The Committee on Human Research at the University of California San Francisco Medical Center approved the study.
Target Population
All residents in specialties that involved direct patient care and the potential to use the adult RRT were included in the study. This included residents in the fields of internal medicine, neurology, general surgery, orthopedic surgery, neurosurgery, plastic surgery, urology, and otolaryngology (Table 1). Residents in pediatrics and obstetrics and gynecology were excluded, as emergencies in their patients are addressed by a pediatric RRT and an obstetric anesthesiologist, respectively. Residents in anesthesiology were excluded as they do not care for nonintensive care unit (ICU) patients as part of the primary team and are not involved in RRT encounters.
| Demographic | No. (%) |
|---|---|
| |
| Medical specialty | |
| Internal medicine | 145 (61.4) |
| Neurology | 18 (7.6) |
| General surgery | 31 (13.1) |
| Orthopedic surgery | 17 (7.2) |
| Neurosurgery | 4 (1.7) |
| Plastic surgery | 2 (0.8) |
| Urology | 9 (3.8) |
| Otolaryngology | 10 (4.2) |
| Years of postgraduate training | Average 2.34 (SD 1.41) |
| 1 | 83 (35.2) |
| 2 | 60 (25.4) |
| 3 | 55 (23.3) |
| 4 | 20 (8.5) |
| 5 | 8 (3.4) |
| 6 | 5 (2.1) |
| 7 | 5 (2.1) |
| Gender | |
| Male | 133 (56.4) |
| Female | 102 (43.2) |
| Had exposure to RRT during training | |
| Yes | 106 (44.9) |
| No | 127 (53.8) |
| Had previously initiated a call to the RRT | |
| Yes | 106 (44.9) |
| No | 128 (54.2) |
Survey Design
The resident survey contained 20 RRT‐related items and 7 demographic and practice items. Responses for RRT‐related questions utilized a 5‐point Likert scale ranging from strongly disagree to strongly agree. The survey was piloted prior to administration to check comprehension and interpretation by physicians with experience in survey writing (for the full survey, see Supporting Information, Appendix, in the online version of this article).
Survey Objectives
The survey was designed to capture the experiences of residents who had cared for a patient for whom the RRT had been activated. Data collected included residents' perceptions of the impact of the RRT on their residency education and clinical autonomy, the quality of care provided, patient safety, and hospital‐wide culture. Potential barriers to use of the RRT were also examined.
Outcomes
The study's primary outcomes included the perceived educational benefit of the RRT and its perceived impact on clinical autonomy. Secondary outcomes included the effect of years of training and resident specialty on both the perceived educational benefit and impact on clinical autonomy among our study group.
Statistical Analysis
Responses to each survey item were described for each specialty, and subgroup analysis was conducted. For years of training, that item was dichotomized into either 1 year (henceforth referred to as interns) or greater than 1 year (henceforth referred to as upper‐level residents). Resident specialty was dichotomized into medical fields (internal medicine and neurology) or surgical fields. For statistical analysis, agreement statements were collapsed to either disagree (strongly disagree/disagree), neutral, or agree (strongly agree/agree). The influence of years of resident training and resident specialty was assessed for all items in the survey using 2 or Fisher exact tests as appropriate for the 3 agreement categories. Analysis was conducted using SPSS 21.0 (IBM Corp., Armonk, NY).
RESULTS
There were 246 responses to the survey of a possible 342, yielding a response rate of 72% (Table 2). Ten respondents stated that they had never cared for a patient where the RRT had been activated. Given their lack of exposure to the RRT, these respondents were excluded from the analysis, yielding a final sample size of 236. The demographic and clinical practice characteristics of respondents are shown in Table 1.
| The resident | Strongly Disagree/Disagree, n (%) | Neutral, n (%) | Agree/ Strongly Agree, n (%) |
|---|---|---|---|
| |||
| Is comfortable managing the unstable patient without the RRT | 104 (44.1) | 64 (27.1) | 66 (28.0) |
| And RRT work together to make treatment decisions | 10 (4.2) | 13 (5.5) | 208 (88.1) |
| Believes there are fewer opportunities to care for unstable floor patients due to the RRT | 188 (79.7) | 26 (11.0) | 17 (7.2) |
| Feels less prepared to care for unstable patients due to the RRT | 201 (85.2) | 22 (9.3) | 13 (5.5) |
| Feels that working with the RRT creates a valuable educational experience | 9 (3.8) | 39 (16.5) | 184 (78.0) |
| Feels that nurses caring for the unstable patient should always contact them prior to contacting the RRT | 123 (52.1) | 33 (14.0) | 76 (32.2) |
| Would be unhappy with nurses calling RRT prior to contacting them | 141 (59.7) | 44 (18.6) | 51 (21.6) |
| Perceives that the presence of RRT decreases residents' autonomy | 179 (75.8) | 25 (10.6) | 28 (11.9) |
Demographics and Primary Outcomes
Interns comprised 83 (35%) of the respondents; the average time in postgraduate training was 2.34 years (standard deviation=1.41). Of respondents, 163 (69%) were in medical fields, and 73 (31%) were in surgical fields. Overall responses to the survey are shown in Table 2, and subgroup analysis is shown in Table 3.
| The resident | 1 Year, n=83, n (%) | >1 Year, n=153, n (%) | P Value | Medical, n=163, n (%) | Surgical, n=73, n (%) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Is comfortable managing the unstable patient without the RRT | 0.01 | <0.01 | ||||
| Strongly disagree/disagree | 39 (47.6) | 65 (42.8) | 67 (41.6) | 37 (50.7) | ||
| Neutral | 29 (35.4) | 35 (23.0) | 56 (34.8) | 8 (11.0) | ||
| Agree/strongly agree | 14 (17.1) | 52 (34.2) | 38 (23.6) | 28 (38.4) | ||
| And RRT work together to make treatment decisions | 0.61 | 0.04 | ||||
| Strongly disagree/disagree | 2 (2.4) | 8 (5.4) | 4 (2.5) | 6 (8.7) | ||
| Neutral | 5 (6.1) | 8 (5.4) | 7 (4.3) | 6 (8.7) | ||
| Agree/strongly agree | 75 (91.5) | 137 (89.3) | 151 (93.2) | 57 (82.6) | ||
| Believes there are fewer opportunities to care for unstable floor patients due to the RRT | 0.05 | 0.04 | ||||
| Strongly disagree/disagree | 59 (72.8) | 129 (86.0) | 136 (85.5) | 52 (72.2) | ||
| Neutral | 13 (16.0) | 13 (8.7) | 15 (9.4) | 11 (15.3) | ||
| Agree/strongly agree | 9 (11.1) | 8 (5.3) | 8 (5.0) | 9 (12.5) | ||
| Feels less prepared to care for unstable patients due to the RRT | <0.01 | 0.79 | ||||
| Strongly disagree/disagree | 62 (74.7) | 139 (90.8) | 140 (85.9) | 61 (83.6) | ||
| Neutral | 14 (16.9) | 8 (5.2) | 15 (9.2) | 7 (9.6) | ||
| Agree/Strongly agree | 7 (8.4) | 6 (3.9) | 8 (4.9) | 5 (6.8) | ||
| Feels working with the RRT is a valuable educational experience | 0.61 | 0.01 | ||||
| Strongly disagree/disagree | 2 (2.4) | 7 (4.7) | 2 (1.2) | 7 (9.9) | ||
| Neutral | 12 (14.6) | 27 (18.0) | 25 (15.5) | 14 (19.7) | ||
| Agree/strongly agree | 68 (82.9) | 116 (77.3) | 134 (83.2) | 50 (70.4) | ||
| Feels nurses caring for unstable patients should always contact the resident prior to contacting the RRT | 0.49 | <0.01 | ||||
| Strongly disagree/disagree | 47 (57.3) | 76 (50.7) | 97 (60.2) | 26 (36.6) | ||
| Neutral | 9 (11.0) | 24 (16.0) | 26 (16.1) | 7 (9.9) | ||
| Agree/strongly agree | 26 (31.7) | 50 (33.3) | 38 (23.6) | 38 (53.5) | ||
| Would be unhappy with nurses calling RRT prior to contacting them | 0.81 | <0.01 | ||||
| Strongly disagree/disagree | 51 (61.4) | 90 (58.8) | 109 (66.9) | 32 (43.8) | ||
| Neutral | 16 (19.3) | 28 (18.3) | 30 (18.4) | 14 (19.2) | ||
| Agree/strongly agree | 16 (19.3) | 35 (22.9) | 24 (14.7) | 27 (37.0) | ||
| Perceives that the presence of the RRT decreases autonomy as a physician | 0.95 | 0.18 | ||||
| Strongly disagree/disagree | 63 (77.8) | 116 (76.8) | 127 (79.9) | 52 (71.2) | ||
| Neutral | 9 (11.1) | 16 (10.6) | 17 (10.7) | 8 (11.0) | ||
| Agree/strongly agree | 9 (11.1) | 19 (12.6) | 15 (9.4) | 13 (17.8) | ||
Effect of the RRT on Resident Education
Of all residents, 66 (28%) agreed that they felt comfortable managing an unstable patient without the assistance of the RRT. Surgical residents felt more comfortable managing an unstable patient alone (38%) compared medical residents (24%) (P<0.01). Interns felt less comfortable caring for unstable patients without the RRT's assistance (17%) compared with upper‐level residents (34%) (P=0.01).
Residents overall disagreed with the statement that the RRT left them feeling less prepared to care for unstable patients (n=201; 85%). More upper‐level residents disagreed with this assertion (91%) compared with interns (75%) (P<0.01). Responses to this question did not differ significantly between medical and surgical residents.
Upper‐level residents were more likely to disagree with the statement that the RRT resulted in fewer opportunities to care for unstable patients (n=129; 86%) compared with interns (n=59; 73%) (P=0.05). Medical residents were also more likely to disagree with this statement (n=136; 86%) compared with surgical residents (n=52; 72%) (P=0.04).
With respect to residents' overall impressions of the educational value of the RRT, 68 (83%) interns and 116 (77%) upper‐level residents agreed that it provided a valuable educational experience (P=0.61). Medical and surgical residents differed in this regard, with 134 (83%) medical residents and 50 (70%) surgical residents agreeing that the RRT provided a valuable educational experience (P=0.01).
Effect of the RRT on Clinical Autonomy
Of all residents, 123 (52%) disagreed that the bedside nurse should always contact the primary resident prior to calling the RRT; 76 (32%) agreed with this statement. Medicine residents were more likely to disagree with this approach (n=97; 60%) than were surgical residents (n=26; 36%) (P<0.01). There was no difference between interns and upper‐level residents in response to this question. Most of those who disagreed with this statement were medical residents, whereas most surgical residents (n=38; 54%) agreed that they should be contacted first (P<0.01).
There were no differences between interns and upper‐level residents with respect to perceptions of the RRT's impact on clinical autonomy: 11% of interns and 13% of residents agreed that the RRT decreased their clinical autonomy as a physician. There was no significant difference between medical and surgical residents' responses to this question.
The majority of residents (n=208; 88%) agreed that they and the RRT work together to make treatment decisions for patients. This was true regardless of year of training (P=0.61), but it was expressed more often among medical residents than surgical residents (n=151, 93% vs n=57, 83%; P=0.04).
DISCUSSION
Most studies examining the educational and cultural impact of RRTs exist in the nursing literature. These studies demonstrate that medical and surgical nurses are often reluctant to call the RRT for fear of criticism by the patient's physician.[5, 8, 9, 10, 11, 12, 13] In contrast, our data demonstrate that resident physicians across all levels of training and specialties have a positive view of the RRT and its role in patient care. The data support our hypothesis that although most residents perceive educational benefit from their interactions with the RRT, this perception is greater for less‐experienced residents and for those residents who routinely provide care for critically ill patients and serve as code team leaders. In addition, a minority of residents, irrespective of years of training or medical specialty, felt that the RRT negatively impacted their clinical autonomy.
Our data have several important implications. First, although over half of the residents surveyed had not been exposed to RRTs during medical school, and despite having no formal training on the role of the RRT during residency, most residents identified their interactions with the RRT as potential learning opportunities. This finding differs from that of Benin and colleagues, who suggested that RRTs might negatively impact residents' educational development and decrease opportunities for high‐stakes clinical reasoning by allowing the clinical decision‐making process to be driven by the RRT staff rather than the resident.[5] One possible explanation for this discrepancy is the variable makeup of the RRT at different institutions. At our medical center, the RRT is comprised of a critical care nurse and respiratory therapist, whereas at other institutions, the RRT may be led by a resident, fellow, attending hospitalist, or intensivist, any of whom might supersede the primary resident once the RRT is engaged.
In our study, the perceived educational benefit of the RRT was most pronounced with interns. Interns likely derive incrementally greater benefit from each encounter with an acutely decompensating patient than do senior residents, whether the RRT is present or not. Observing the actions of seasoned nurses and respiratory therapists may demonstrate new tools for interns to use in their management of such situations; for example, the RRT may suggest different modes of oxygen delivery or new diagnostic tests. The RRT also likely helps interns navigate the hospital system by assisting with decisions around escalation of care and serving as a liaison to ICU staff.
Our data also have implications for resident perceptions of clinical autonomy. Interns, far less experienced caring for unstable patients than upper‐level residents, expressed more concern about the RRT stripping them of opportunities to do so and about feeling less prepared to handle clinically deteriorating patients. Part of this perception may be due to interns feeling less comfortable taking charge of a patient's care in the presence of an experienced critical care nurse and respiratory therapist, both for reasons related to clinical experience and to a cultural hierarchy that often places the intern at the bottom of the authority spectrum. In addition, when the RRT is called on an intern's patient, the senior resident may accompany the intern to the bedside and guide the intern on his or her approach to the situation; in some cases, the senior resident may take charge, leaving the intern feeling less autonomous.
If training sessions could be developed to address not only clinical decision making, but also multidisciplinary team interactions and roles in the acute care setting, this may mitigate interns' concerns. Such curricula could also enhance residents' experience in interprofessional care, an aspect of clinical training that has become increasingly important in the age of limited duty hours and higher volume, and higher acuity inpatient censuses. An RRT model, like a code blue model, could be used in simulation‐based training to increase both comfort with use of the RRT and efficiency of the RRTresidentnurse team. Although our study did not address specifically residents' perceptions of multidisciplinary teams, this could be a promising area for further study.
For surgical residents, additional factors are likely at play. Surgical residents spend significant time in the operating room, reducing time present at the bedside and hindering the ability to respond swiftly when an RRT is called on their patient. This could cause surgical residents to feel less involved in the care of that patientsupported by our finding that fewer surgical residents felt able to collaborate with the RRTand also to derive less educational benefit and clinical satisfaction from the experience. Differences between medical and surgical postgraduate training also likely play a role, manifest by varying clinical roles and duration of training, and as such it may not be appropriate to draw direct comparisons between respective postgraduate year levels. In addition, differences in patients' medical complexity, varying allegiance to the traditional hierarchy of medical providers, and degree of familiarity with the RRT itself may impact surgical residents' comfort with the RRT.
Limitations of our study include that it was conducted at a single site and addressed a specific population of residents at our tertiary academic center. Though we achieved an excellent response rate, our subspecialty sample sizes were too small to allow for individual comparisons among those groups. Conducting a larger study at multiple institutions where the makeup of the RRT differs could provide further insight into how different clinical environments and different RRT models impact resident perceptions. Finally, we allowed each respondent to interpret both educational benefit and clinical autonomy in the context of their own level of training and clinical practice rather than providing strict definitions of these terms. There is no standardized definition of autonomy in the context of resident clinical practice, and we did not measure direct educational outcomes. Our study design therefore allowed only for measurement of perceptions of these concepts. Measurement of actual educational value of the RRTfor example, through direct clinical observation or by incorporating the RRT experience into an entrustable professional activitywould provide more quantitative evidence of the RRT's utility for our resident population. Future study in this area would help to support the development and ongoing assessment of RRT‐based curricula moving forward.
CONCLUSION
Our data show that resident physicians have a strongly favorable opinion of the RRT at our institution. Future studies should aim to quantify the educational benefit of RRTs for residents and identify areas for curricular development to enhance resident education as RRTs become more pervasive.
Rapid response teams (RRTs) have been promoted by patient safety and quality‐improvement organizations as a strategy to reduce preventable in‐hospital deaths.[1] To date, critical analysis of RRTs has focused primarily on their impact on quality‐of‐care metrics.[2, 3, 4] Comparatively few studies have examined the cultural and educational impact of RRTs, particularly at academic medical centers, and those that do exist have focused almost exclusively on perceptions of nurses rather than resident physicians.[5, 6, 7, 8, 9, 10]
Although a prior study found that internal medicine and general surgery residents believed that RRTs improved patient safety, they were largely ambivalent about the RRT's impact on education and training.[11] To date, there has been no focused assessment of resident physician impressions of an RRT across years of training and medical specialty to inform the use of this multidisciplinary team as a component of their residency education.
We sought to determine whether resident physicians at a tertiary care academic medical center perceive educational benefit from collaboration with the RRT and whether they feel that the RRT adversely affects clinical autonomy.
METHODS
The Hospital
Moffitt‐Long Hospital, the tertiary academic medical center of the University of California, San Francisco (UCSF), is a 600‐bed acute care hospital that provides comprehensive critical care services and serves as a major referral center in northern California. There are roughly 5000 admissions to the hospital annually. At the time the study was conducted, there were approximately 200 RRT calls per 1000 adult hospital discharges.
The Rapid Response Team
The RRT is called to assess, triage, and treat patients who have experienced a decline in their clinical status short of a cardiopulmonary arrest. The RRT has been operational at UCSF since June 1, 2007, and is composed of a dedicated critical care nurse and respiratory therapist available 24 hours a day, 7 days a week. The RRT can be activated by any concerned staff member based on vital sign abnormalities, decreased urine output, changes in mental status, or any significant concern about the trajectory of the patient's clinical course.
When the RRT is called on a given patient, the patient's primary physician (at our institution, a resident) is also called to the bedside and works alongside the RRT to address the patient's acute clinical needs. The primary physician, bedside nurse, and RRT discuss the plan of care for the patient, including clinical evaluation, management, and the need for additional monitoring or a transition to a higher level of care. Residents at our institution receive no formal instruction regarding the role of the RRT or curriculum on interfacing with the RRT, and they do not serve as members of the RRT as part of a clinical rotation.
The Survey Process
Study subjects were asked via e‐mail to participate in a brief online survey. Subjects were offered the opportunity to win a $100 gift certificate in return for their participation. Weekly e‐mail reminders were sent for a period of 3 months or until a given subject had completed the survey. The survey was administered over a 3‐month period, from March through May, to allow time for residents to work with the RRT during the academic year. The Committee on Human Research at the University of California San Francisco Medical Center approved the study.
Target Population
All residents in specialties that involved direct patient care and the potential to use the adult RRT were included in the study. This included residents in the fields of internal medicine, neurology, general surgery, orthopedic surgery, neurosurgery, plastic surgery, urology, and otolaryngology (Table 1). Residents in pediatrics and obstetrics and gynecology were excluded, as emergencies in their patients are addressed by a pediatric RRT and an obstetric anesthesiologist, respectively. Residents in anesthesiology were excluded as they do not care for nonintensive care unit (ICU) patients as part of the primary team and are not involved in RRT encounters.
| Demographic | No. (%) |
|---|---|
| |
| Medical specialty | |
| Internal medicine | 145 (61.4) |
| Neurology | 18 (7.6) |
| General surgery | 31 (13.1) |
| Orthopedic surgery | 17 (7.2) |
| Neurosurgery | 4 (1.7) |
| Plastic surgery | 2 (0.8) |
| Urology | 9 (3.8) |
| Otolaryngology | 10 (4.2) |
| Years of postgraduate training | Average 2.34 (SD 1.41) |
| 1 | 83 (35.2) |
| 2 | 60 (25.4) |
| 3 | 55 (23.3) |
| 4 | 20 (8.5) |
| 5 | 8 (3.4) |
| 6 | 5 (2.1) |
| 7 | 5 (2.1) |
| Gender | |
| Male | 133 (56.4) |
| Female | 102 (43.2) |
| Had exposure to RRT during training | |
| Yes | 106 (44.9) |
| No | 127 (53.8) |
| Had previously initiated a call to the RRT | |
| Yes | 106 (44.9) |
| No | 128 (54.2) |
Survey Design
The resident survey contained 20 RRT‐related items and 7 demographic and practice items. Responses for RRT‐related questions utilized a 5‐point Likert scale ranging from strongly disagree to strongly agree. The survey was piloted prior to administration to check comprehension and interpretation by physicians with experience in survey writing (for the full survey, see Supporting Information, Appendix, in the online version of this article).
Survey Objectives
The survey was designed to capture the experiences of residents who had cared for a patient for whom the RRT had been activated. Data collected included residents' perceptions of the impact of the RRT on their residency education and clinical autonomy, the quality of care provided, patient safety, and hospital‐wide culture. Potential barriers to use of the RRT were also examined.
Outcomes
The study's primary outcomes included the perceived educational benefit of the RRT and its perceived impact on clinical autonomy. Secondary outcomes included the effect of years of training and resident specialty on both the perceived educational benefit and impact on clinical autonomy among our study group.
Statistical Analysis
Responses to each survey item were described for each specialty, and subgroup analysis was conducted. For years of training, that item was dichotomized into either 1 year (henceforth referred to as interns) or greater than 1 year (henceforth referred to as upper‐level residents). Resident specialty was dichotomized into medical fields (internal medicine and neurology) or surgical fields. For statistical analysis, agreement statements were collapsed to either disagree (strongly disagree/disagree), neutral, or agree (strongly agree/agree). The influence of years of resident training and resident specialty was assessed for all items in the survey using 2 or Fisher exact tests as appropriate for the 3 agreement categories. Analysis was conducted using SPSS 21.0 (IBM Corp., Armonk, NY).
RESULTS
There were 246 responses to the survey of a possible 342, yielding a response rate of 72% (Table 2). Ten respondents stated that they had never cared for a patient where the RRT had been activated. Given their lack of exposure to the RRT, these respondents were excluded from the analysis, yielding a final sample size of 236. The demographic and clinical practice characteristics of respondents are shown in Table 1.
| The resident | Strongly Disagree/Disagree, n (%) | Neutral, n (%) | Agree/ Strongly Agree, n (%) |
|---|---|---|---|
| |||
| Is comfortable managing the unstable patient without the RRT | 104 (44.1) | 64 (27.1) | 66 (28.0) |
| And RRT work together to make treatment decisions | 10 (4.2) | 13 (5.5) | 208 (88.1) |
| Believes there are fewer opportunities to care for unstable floor patients due to the RRT | 188 (79.7) | 26 (11.0) | 17 (7.2) |
| Feels less prepared to care for unstable patients due to the RRT | 201 (85.2) | 22 (9.3) | 13 (5.5) |
| Feels that working with the RRT creates a valuable educational experience | 9 (3.8) | 39 (16.5) | 184 (78.0) |
| Feels that nurses caring for the unstable patient should always contact them prior to contacting the RRT | 123 (52.1) | 33 (14.0) | 76 (32.2) |
| Would be unhappy with nurses calling RRT prior to contacting them | 141 (59.7) | 44 (18.6) | 51 (21.6) |
| Perceives that the presence of RRT decreases residents' autonomy | 179 (75.8) | 25 (10.6) | 28 (11.9) |
Demographics and Primary Outcomes
Interns comprised 83 (35%) of the respondents; the average time in postgraduate training was 2.34 years (standard deviation=1.41). Of respondents, 163 (69%) were in medical fields, and 73 (31%) were in surgical fields. Overall responses to the survey are shown in Table 2, and subgroup analysis is shown in Table 3.
| The resident | 1 Year, n=83, n (%) | >1 Year, n=153, n (%) | P Value | Medical, n=163, n (%) | Surgical, n=73, n (%) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Is comfortable managing the unstable patient without the RRT | 0.01 | <0.01 | ||||
| Strongly disagree/disagree | 39 (47.6) | 65 (42.8) | 67 (41.6) | 37 (50.7) | ||
| Neutral | 29 (35.4) | 35 (23.0) | 56 (34.8) | 8 (11.0) | ||
| Agree/strongly agree | 14 (17.1) | 52 (34.2) | 38 (23.6) | 28 (38.4) | ||
| And RRT work together to make treatment decisions | 0.61 | 0.04 | ||||
| Strongly disagree/disagree | 2 (2.4) | 8 (5.4) | 4 (2.5) | 6 (8.7) | ||
| Neutral | 5 (6.1) | 8 (5.4) | 7 (4.3) | 6 (8.7) | ||
| Agree/strongly agree | 75 (91.5) | 137 (89.3) | 151 (93.2) | 57 (82.6) | ||
| Believes there are fewer opportunities to care for unstable floor patients due to the RRT | 0.05 | 0.04 | ||||
| Strongly disagree/disagree | 59 (72.8) | 129 (86.0) | 136 (85.5) | 52 (72.2) | ||
| Neutral | 13 (16.0) | 13 (8.7) | 15 (9.4) | 11 (15.3) | ||
| Agree/strongly agree | 9 (11.1) | 8 (5.3) | 8 (5.0) | 9 (12.5) | ||
| Feels less prepared to care for unstable patients due to the RRT | <0.01 | 0.79 | ||||
| Strongly disagree/disagree | 62 (74.7) | 139 (90.8) | 140 (85.9) | 61 (83.6) | ||
| Neutral | 14 (16.9) | 8 (5.2) | 15 (9.2) | 7 (9.6) | ||
| Agree/Strongly agree | 7 (8.4) | 6 (3.9) | 8 (4.9) | 5 (6.8) | ||
| Feels working with the RRT is a valuable educational experience | 0.61 | 0.01 | ||||
| Strongly disagree/disagree | 2 (2.4) | 7 (4.7) | 2 (1.2) | 7 (9.9) | ||
| Neutral | 12 (14.6) | 27 (18.0) | 25 (15.5) | 14 (19.7) | ||
| Agree/strongly agree | 68 (82.9) | 116 (77.3) | 134 (83.2) | 50 (70.4) | ||
| Feels nurses caring for unstable patients should always contact the resident prior to contacting the RRT | 0.49 | <0.01 | ||||
| Strongly disagree/disagree | 47 (57.3) | 76 (50.7) | 97 (60.2) | 26 (36.6) | ||
| Neutral | 9 (11.0) | 24 (16.0) | 26 (16.1) | 7 (9.9) | ||
| Agree/strongly agree | 26 (31.7) | 50 (33.3) | 38 (23.6) | 38 (53.5) | ||
| Would be unhappy with nurses calling RRT prior to contacting them | 0.81 | <0.01 | ||||
| Strongly disagree/disagree | 51 (61.4) | 90 (58.8) | 109 (66.9) | 32 (43.8) | ||
| Neutral | 16 (19.3) | 28 (18.3) | 30 (18.4) | 14 (19.2) | ||
| Agree/strongly agree | 16 (19.3) | 35 (22.9) | 24 (14.7) | 27 (37.0) | ||
| Perceives that the presence of the RRT decreases autonomy as a physician | 0.95 | 0.18 | ||||
| Strongly disagree/disagree | 63 (77.8) | 116 (76.8) | 127 (79.9) | 52 (71.2) | ||
| Neutral | 9 (11.1) | 16 (10.6) | 17 (10.7) | 8 (11.0) | ||
| Agree/strongly agree | 9 (11.1) | 19 (12.6) | 15 (9.4) | 13 (17.8) | ||
Effect of the RRT on Resident Education
Of all residents, 66 (28%) agreed that they felt comfortable managing an unstable patient without the assistance of the RRT. Surgical residents felt more comfortable managing an unstable patient alone (38%) compared medical residents (24%) (P<0.01). Interns felt less comfortable caring for unstable patients without the RRT's assistance (17%) compared with upper‐level residents (34%) (P=0.01).
Residents overall disagreed with the statement that the RRT left them feeling less prepared to care for unstable patients (n=201; 85%). More upper‐level residents disagreed with this assertion (91%) compared with interns (75%) (P<0.01). Responses to this question did not differ significantly between medical and surgical residents.
Upper‐level residents were more likely to disagree with the statement that the RRT resulted in fewer opportunities to care for unstable patients (n=129; 86%) compared with interns (n=59; 73%) (P=0.05). Medical residents were also more likely to disagree with this statement (n=136; 86%) compared with surgical residents (n=52; 72%) (P=0.04).
With respect to residents' overall impressions of the educational value of the RRT, 68 (83%) interns and 116 (77%) upper‐level residents agreed that it provided a valuable educational experience (P=0.61). Medical and surgical residents differed in this regard, with 134 (83%) medical residents and 50 (70%) surgical residents agreeing that the RRT provided a valuable educational experience (P=0.01).
Effect of the RRT on Clinical Autonomy
Of all residents, 123 (52%) disagreed that the bedside nurse should always contact the primary resident prior to calling the RRT; 76 (32%) agreed with this statement. Medicine residents were more likely to disagree with this approach (n=97; 60%) than were surgical residents (n=26; 36%) (P<0.01). There was no difference between interns and upper‐level residents in response to this question. Most of those who disagreed with this statement were medical residents, whereas most surgical residents (n=38; 54%) agreed that they should be contacted first (P<0.01).
There were no differences between interns and upper‐level residents with respect to perceptions of the RRT's impact on clinical autonomy: 11% of interns and 13% of residents agreed that the RRT decreased their clinical autonomy as a physician. There was no significant difference between medical and surgical residents' responses to this question.
The majority of residents (n=208; 88%) agreed that they and the RRT work together to make treatment decisions for patients. This was true regardless of year of training (P=0.61), but it was expressed more often among medical residents than surgical residents (n=151, 93% vs n=57, 83%; P=0.04).
DISCUSSION
Most studies examining the educational and cultural impact of RRTs exist in the nursing literature. These studies demonstrate that medical and surgical nurses are often reluctant to call the RRT for fear of criticism by the patient's physician.[5, 8, 9, 10, 11, 12, 13] In contrast, our data demonstrate that resident physicians across all levels of training and specialties have a positive view of the RRT and its role in patient care. The data support our hypothesis that although most residents perceive educational benefit from their interactions with the RRT, this perception is greater for less‐experienced residents and for those residents who routinely provide care for critically ill patients and serve as code team leaders. In addition, a minority of residents, irrespective of years of training or medical specialty, felt that the RRT negatively impacted their clinical autonomy.
Our data have several important implications. First, although over half of the residents surveyed had not been exposed to RRTs during medical school, and despite having no formal training on the role of the RRT during residency, most residents identified their interactions with the RRT as potential learning opportunities. This finding differs from that of Benin and colleagues, who suggested that RRTs might negatively impact residents' educational development and decrease opportunities for high‐stakes clinical reasoning by allowing the clinical decision‐making process to be driven by the RRT staff rather than the resident.[5] One possible explanation for this discrepancy is the variable makeup of the RRT at different institutions. At our medical center, the RRT is comprised of a critical care nurse and respiratory therapist, whereas at other institutions, the RRT may be led by a resident, fellow, attending hospitalist, or intensivist, any of whom might supersede the primary resident once the RRT is engaged.
In our study, the perceived educational benefit of the RRT was most pronounced with interns. Interns likely derive incrementally greater benefit from each encounter with an acutely decompensating patient than do senior residents, whether the RRT is present or not. Observing the actions of seasoned nurses and respiratory therapists may demonstrate new tools for interns to use in their management of such situations; for example, the RRT may suggest different modes of oxygen delivery or new diagnostic tests. The RRT also likely helps interns navigate the hospital system by assisting with decisions around escalation of care and serving as a liaison to ICU staff.
Our data also have implications for resident perceptions of clinical autonomy. Interns, far less experienced caring for unstable patients than upper‐level residents, expressed more concern about the RRT stripping them of opportunities to do so and about feeling less prepared to handle clinically deteriorating patients. Part of this perception may be due to interns feeling less comfortable taking charge of a patient's care in the presence of an experienced critical care nurse and respiratory therapist, both for reasons related to clinical experience and to a cultural hierarchy that often places the intern at the bottom of the authority spectrum. In addition, when the RRT is called on an intern's patient, the senior resident may accompany the intern to the bedside and guide the intern on his or her approach to the situation; in some cases, the senior resident may take charge, leaving the intern feeling less autonomous.
If training sessions could be developed to address not only clinical decision making, but also multidisciplinary team interactions and roles in the acute care setting, this may mitigate interns' concerns. Such curricula could also enhance residents' experience in interprofessional care, an aspect of clinical training that has become increasingly important in the age of limited duty hours and higher volume, and higher acuity inpatient censuses. An RRT model, like a code blue model, could be used in simulation‐based training to increase both comfort with use of the RRT and efficiency of the RRTresidentnurse team. Although our study did not address specifically residents' perceptions of multidisciplinary teams, this could be a promising area for further study.
For surgical residents, additional factors are likely at play. Surgical residents spend significant time in the operating room, reducing time present at the bedside and hindering the ability to respond swiftly when an RRT is called on their patient. This could cause surgical residents to feel less involved in the care of that patientsupported by our finding that fewer surgical residents felt able to collaborate with the RRTand also to derive less educational benefit and clinical satisfaction from the experience. Differences between medical and surgical postgraduate training also likely play a role, manifest by varying clinical roles and duration of training, and as such it may not be appropriate to draw direct comparisons between respective postgraduate year levels. In addition, differences in patients' medical complexity, varying allegiance to the traditional hierarchy of medical providers, and degree of familiarity with the RRT itself may impact surgical residents' comfort with the RRT.
Limitations of our study include that it was conducted at a single site and addressed a specific population of residents at our tertiary academic center. Though we achieved an excellent response rate, our subspecialty sample sizes were too small to allow for individual comparisons among those groups. Conducting a larger study at multiple institutions where the makeup of the RRT differs could provide further insight into how different clinical environments and different RRT models impact resident perceptions. Finally, we allowed each respondent to interpret both educational benefit and clinical autonomy in the context of their own level of training and clinical practice rather than providing strict definitions of these terms. There is no standardized definition of autonomy in the context of resident clinical practice, and we did not measure direct educational outcomes. Our study design therefore allowed only for measurement of perceptions of these concepts. Measurement of actual educational value of the RRTfor example, through direct clinical observation or by incorporating the RRT experience into an entrustable professional activitywould provide more quantitative evidence of the RRT's utility for our resident population. Future study in this area would help to support the development and ongoing assessment of RRT‐based curricula moving forward.
CONCLUSION
Our data show that resident physicians have a strongly favorable opinion of the RRT at our institution. Future studies should aim to quantify the educational benefit of RRTs for residents and identify areas for curricular development to enhance resident education as RRTs become more pervasive.
- Institute for Healthcare Improvement. Rapid response teams. Available at: http://www.ihi.org/topics/rapidresponseteams. Accessed May 5, 2014.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34(9):2463–2478.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , , . Defining impact of a rapid response team: qualitative study with nurses, physicians and hospital administrators. BMJ Qual Saf. 2012;21(5):391–398.
- , , . How RNs rescue patients: a qualitative study of RNs' perceived involvement in rapid response teams. Qual Saf Health Care. 2010;19(5):e13.
- , , , . Rapid response team approach to staff satisfaction. Orthop Nurs. 2008;27(5):266–271; quiz 272–273.
- , , , . Voices from the floor: nurses' perceptions of the medical emergency team. Intensive Crit Care Nurs. 2006;22(3):138–143.
- , , . Rapid response teams seen through the eyes of the nurse. Am J Nurs. 2010;110(6):28–34; quiz 35–36.
- , , , et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi‐campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21(7):569–575.
- , , , et al. Resident and RN perceptions of the impact of a medical emergency team on education and patient safety in an academic medical center. Crit Care Med. 2009;37(12):3091–3096.
- , , , et al. Why don't hospital staff activate the rapid response system (RRS)? How frequently is it needed and can the process be improved? Implement Sci. 2011;6:39.
- , , , . Timing and teamwork–an observational pilot study of patients referred to a rapid response team with the aim of identifying factors amenable to re‐design of a rapid response system. Resuscitation. 2012;83(6):782–787.
- Institute for Healthcare Improvement. Rapid response teams. Available at: http://www.ihi.org/topics/rapidresponseteams. Accessed May 5, 2014.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34(9):2463–2478.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , , . Defining impact of a rapid response team: qualitative study with nurses, physicians and hospital administrators. BMJ Qual Saf. 2012;21(5):391–398.
- , , . How RNs rescue patients: a qualitative study of RNs' perceived involvement in rapid response teams. Qual Saf Health Care. 2010;19(5):e13.
- , , , . Rapid response team approach to staff satisfaction. Orthop Nurs. 2008;27(5):266–271; quiz 272–273.
- , , , . Voices from the floor: nurses' perceptions of the medical emergency team. Intensive Crit Care Nurs. 2006;22(3):138–143.
- , , . Rapid response teams seen through the eyes of the nurse. Am J Nurs. 2010;110(6):28–34; quiz 35–36.
- , , , et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi‐campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21(7):569–575.
- , , , et al. Resident and RN perceptions of the impact of a medical emergency team on education and patient safety in an academic medical center. Crit Care Med. 2009;37(12):3091–3096.
- , , , et al. Why don't hospital staff activate the rapid response system (RRS)? How frequently is it needed and can the process be improved? Implement Sci. 2011;6:39.
- , , , . Timing and teamwork–an observational pilot study of patients referred to a rapid response team with the aim of identifying factors amenable to re‐design of a rapid response system. Resuscitation. 2012;83(6):782–787.
© 2015 Society of Hospital Medicine
Proactive Rounding by RRT
Rapid response teams (RRT) have been promoted by numerous patient safety organizations to reduce preventable in‐hospital deaths.14 Initial studies of RRTs were promising,57 but recent literature,811 including systematic reviews and meta‐analyses, has called these findings into question. Nevertheless, RRTs remain popular in academic and community hospitals worldwide, and many have expanded their roles beyond solely responding to the deteriorating patient.12
Some RRTs, for example, proactively round on seriously ill ward patients and patients recently discharged from the intensive care unit (ICU) in an effort to prevent transitions to higher levels of care. Priestley and colleagues demonstrated that institution of such a team, referred to as a critical care outreach team (CCOT), decreased in‐hospital mortality while possibly increasing hospital length of stay (LOS).13 Three additional single‐center studies from the United Kingdom, where CCOTs are common, specifically examined proactive rounding by CCOTs on the ICU readmission rate: 2 observed no improvement,14, 15 while the third, limited by a small sample size, demonstrated a modest reduction in ICU readmissions.16
We sought to determine the impact of proactive rounding by an RRT on patients discharged from intensive care on the ICU readmission rate, ICU LOS, and in‐hospital mortality of patients discharged from the ICU. We hypothesized that proactive rounding by an RRT would decrease the ICU readmission rate, ICU LOS, and the in‐hospital mortality of patients discharged from the ICU.
MATERIALS AND METHODS
Site and Subjects
We carried out a retrospective, observational study of adult patients discharged from the ICU at University of California San Francisco (UCSF) Medical Center between January 2006 and June 2009. UCSF is a 790‐bed quaternary care academic hospital that admits approximately 17,000 patients annually and has 5 adult ICUs, with 62 beds and 3500 to 4000 ICU admissions annually. Our study was approved by the UCSF Medical Center Committee on Human Research; need for informed consent was waived.
Description of the RRT Before June 1, 2007
Throughout the study, the goal of the RRT was unchanged: to assess, triage, and institute early treatment in patients who experienced an acute decline in their clinical status. From November 2005 to October 2006, the RRT was staffed by an attending hospitalist and medicine resident during daytime, and by a critical care fellow at nighttime and on weekends. The RRT could be activated by any concerned staff member in response to a set of predetermined vital sign abnormalities, decreased urine output, or altered mental status, or simply if the staff member was concerned about the patient's clinical status. Despite extensive educational efforts, utilization of the team was low (2.7 calls per 1000 admissions) and, accordingly, it was discontinued in October 2006. After this time, staff would contact the primary team caring for the patient, should concerns regarding the patient's condition arise.
Description of the RRT After June 1, 2007
In an effort to expand its scope and utility, the RRT was reinstated on June 1, 2007 with a new composition and increased responsibilities. After this date, physician roles were eliminated, and the team composition changed to a dedicated critical care nurse and respiratory therapist, available 24 hours a day. Criteria for calling the team remained unchanged. In addition to responding to acute deteriorations in patients' clinical courses, the RRT began to proactively assess all patients within 12 hours of discharge from the ICU and would continue to round on these patients daily until it was felt that they were clinically stable. During these rounds, the RRT would provide consultation expertise to the bedside nurse and contact the patient's clinicians if concern existed about a patient's clinical trajectory; decisions to transfer a patient back to the ICU ultimately rested with the patient's primary team. During this time period, the RRT received an average of 110.6 calls per 1000 admissions.
Data Sources
Data collected included: demographics, clinical information (all patient refined [APR] severity of illness, APR risk of mortality, and the presence of 29 comorbidities), whether there was a readmission to the ICU, the total ICU LOS, and the vital status at the time of hospital discharge.
Outcomes
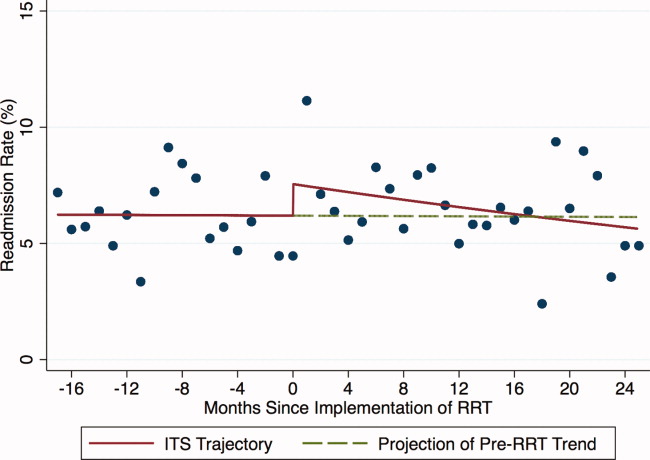
Outcomes included: readmission to the ICU, defined as 2 noncontiguous ICU stays during a single hospitalization; ICU LOS, defined as the total number of ICU days accrued during hospitalization; and in‐hospital mortality of patients discharged from the ICU.
Adjustment Variables
Patient age, gender, race, and ethnicity were available from administrative data. We used admission diagnosis code data to classify comorbidities using the method of Elixhauser et al.17
Statistical Analysis
For each of the 3 study outcomes, we assessed the effects of the intervention using multivariable models adjusting for patient‐ and service‐level factors, including a gamma model for ICU LOS and logistic models for ICU readmission and in‐hospital mortality of patients discharged from the ICU. We first compared unadjusted outcome levels before and after implementation. We then used an interrupted time series (ITS) framework to assess the effects of the intervention in terms of 5 measures: 1) the secular trend in the mean of the outcome before the intervention; 2) the change in the mean at the start of the implementation, or immediate effects; 3) the secular trend in the mean after implementation; 4) the change in secular trend, reflecting cumulative intervention effects; and 5) the net effect of the intervention, estimated as the adjusted difference between the fitted mean at the end of the postintervention period and the expected mean if the preintervention trend had continued without interruption or change.
Secondary Analyses
Given the heterogeneity of the RRT in the preintervention period, we assessed potential changes in trend at October 2006, the month in which the RRT was discontinued. We also examined changes in trend midway through the postimplementation period to evaluate for increased efficacy of the RRT with time.
Selection of Covariates
Age, race, and admitting service were included in both the prepost and ITS models by default for face validity. Additional covariates were selected for each outcome using backwards deletion with a retention criterion of P < 0.05, based on models that allowed the outcome rate to vary freely month to month. Because these data were obtained from administrative billing datasets, and the presence of comorbidities could not be definitively linked with time points during hospitalization, only those comorbidities that were likely present prior at ICU discharge were included. For similar reasons, APR severity of illness and risk of mortality scores, which were calculated from billing diagnoses at the end of hospitalization, were excluded from the models.
RESULTS
Patient Characteristics
During the study period, 11,687 patients were admitted to the ICU; 10,288 were discharged from the ICU alive and included in the analysis. In the 17 months prior to the introduction of proactive rounding by the RRT, 4902 (41.9%) patients were admitted, and during the 25 months afterwards, 6785 (58.1%) patients. Patients admitted in the 2 time periods were similar, although there were clinically small but statistically significant differences in race, APR severity of illness, APR risk of mortality, and certain comorbidities between the 2 groups (Table 1).
| Pre‐RRT (n = 4305) N (%) | Post‐RRT (n = 5983) N (%) | P Value | |
|---|---|---|---|
| |||
| Age, mean (y [SD]) | 57.7 [16.6] | 57.9 [16.5] | 0.50 |
| Female gender | 2,005 (46.6) | 2,824 (47.2) | 0.53 |
| Race | 0.0013 | ||
| White | 2,538 (59.0) | 3,520 (58.8) | |
| Black | 327 (7.6) | 436 (7.3) | |
| Asian | 642 (14.9) | 842 (14.1) | |
| Other | 719 (16.7) | 1,121 (18.7) | |
| Unknown | 79 (1.8) | 64 (1.1) | |
| Ethnicity | 0.87 | ||
| Hispanic | 480 (11.2) | 677 (11.3%) | |
| Non‐Hispanic | 3,547 (82.4) | 4,907 (82.0%) | |
| Unknown | 278 (6.5) | 399 (6.7) | |
| Insurance | 0.50 | ||
| Medicare | 1,788 (41.5) | 2,415 (40.4) | |
| Medicaid/Medi‐Cal | 699 (16.2) | 968 (16.2) | |
| Private | 1,642 (38.1) | 2,329 (38.9) | |
| Other | 176 (4.1) | 271 (4.5) | |
| Admission source | 0.41 | ||
| ED | 1,621 (37.7) | 2,244 (37.5) | |
| Outside hospital | 652 (15.2) | 855 (14.3) | |
| Direct admit | 2,032 (47.2) | 2,884 (48.2) | |
| Major surgery | 0.99 | ||
| Yes | 3,107 (72.2) | 4,319 (72.2) | |
| APR severity of illness | 0.0001 | ||
| Mild | 622 (14.5) | 828 (13.8) | |
| Moderate | 1,328 (30.9) | 1,626 (27.2) | |
| Major | 1,292 (30.0) | 1,908 (31.9) | |
| Extreme | 1,063 (24.7) | 1,621 (27.1) | |
| APR risk of mortality | 0.0109 | ||
| Mild | 1,422 (33.0) | 1,821 (30.4) | |
| Moderate | 1,074 (25.0) | 1,467 (24.5) | |
| Major | 947 (22.0) | 1,437 (24.0) | |
| Extreme | 862 (20.0) | 1,258 (21.0) | |
| Admitting service | 0.11 | ||
| Adult general surgery | 190 (4.4) | 260 (4.4) | |
| Cardiology | 347 (8.1) | 424 (7.1) | |
| Cardiothoracic surgery | 671 (15.6) | 930 (15.5) | |
| Kidney transplant surgery | 105 (2.4) | 112 (1.9) | |
| Liver transplant surgery | 298 (6.9) | 379 (6.3) | |
| Medicine | 683 (15.9) | 958 (16.0) | |
| Neurology | 420 (9.8) | 609 (10.2) | |
| Neurosurgery | 1,345 (31.2) | 1,995 (33.3) | |
| Vascular surgery | 246 (5.7) | 316 (5.3) | |
| Comorbidities | |||
| Hypertension | 2,054 (47.7) | 2,886 (48.2) | 0.60 |
| Fluid and electrolyte disorders | 998 (23.2) | 1,723 (28.8) | <0.0001 |
| Diabetes | 708 (16.5) | 880 (14.7) | 0.02 |
| Chronic obstructive pulmonary disease | 632 (14.7) | 849 (14.2) | 0.48 |
| Iron deficiency anemia | 582 (13.5) | 929 (15.5) | 0.005 |
| Renal failure | 541 (12.6) | 744 (12.4) | 0.84 |
| Coagulopathy | 418 (9.7) | 712 (11.9) | 0.0005 |
| Liver disease | 400 (9.3) | 553 (9.2) | 0.93 |
| Hypothyroidism | 330 (7.7) | 500 (8.4) | 0.20 |
| Depression | 306 (7.1) | 508 (8.5) | 0.01 |
| Peripheral vascular disease | 304 (7.1) | 422 (7.1) | 0.99 |
| Congestive heart failure | 263 (6.1) | 360 (6.0) | 0.85 |
| Weight loss | 236 (5.5) | 425 (7.1) | 0.0009 |
| Paralysis | 225 (5.2) | 328 (5.5) | 0.57 |
| Neurological disorders | 229 (5.3) | 276 (4.6) | 0.10 |
| Valvular disease | 210 (4.9) | 329 (5.5) | 0.16 |
| Drug abuse | 198 (4.6) | 268 (4.5) | 0.77 |
| Metastatic cancer | 198 (4.6) | 296 (5.0) | 0.42 |
| Obesity | 201 (4.7) | 306 (5.1) | 0.30 |
| Alcohol abuse | 178 (4.1) | 216 (3.6) | 0.17 |
| Diabetes with complications | 175 (4.1) | 218 (3.6) | 0.27 |
| Solid tumor without metastasis | 146 (3.4) | 245 (4.1) | 0.07 |
| Psychoses | 115 (2.7) | 183 (3.1) | 0.25 |
| Rheumatoid arthritis/collagen vascular disease | 96 (2.2) | 166 (2.8) | 0.08 |
| Pulmonary circulation disease | 83 (1.9) | 181 (3.0) | 0.0005 |
| Outcomes | |||
| Readmission to ICU | 288 (6.7) | 433 (7.3) | 0.24 |
| ICU length of stay, mean [SD] | 5.1 [9.7] | 4.9 [8.3] | 0.24 |
| In‐hospital mortality of patients discharged from the ICU | 260 (6.0) | 326 (5.5) | 0.24 |
ICU Readmission Rate
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the ICU readmission rate (6.7% preintervention vs 7.3% postintervention, P = 0.24; Table 1). In the adjusted ITS model, the intervention had no net effect on the odds of ICU readmission (adjusted odds ratio [AOR] for net intervention effect 0.98, 95% confidence interval [CI] 0.42, 2.28), with similar secular trends both preintervention (AOR 1.00 per year, 95% CI 0.97, 1.03), and afterwards (AOR 0.99 per year, 95% CI 0.98, 01.00), and a nonsignificant increase at implementation (Table 2). Figure 1 uses solid lines to show the fitted readmission rates, a hatched line to show the projection of the preintervention secular trend into the postintervention period, and circles to represent adjusted monthly means. The lack of a net intervention effect is indicated by the convergence of the solid and hatched lines 24 months postintervention.
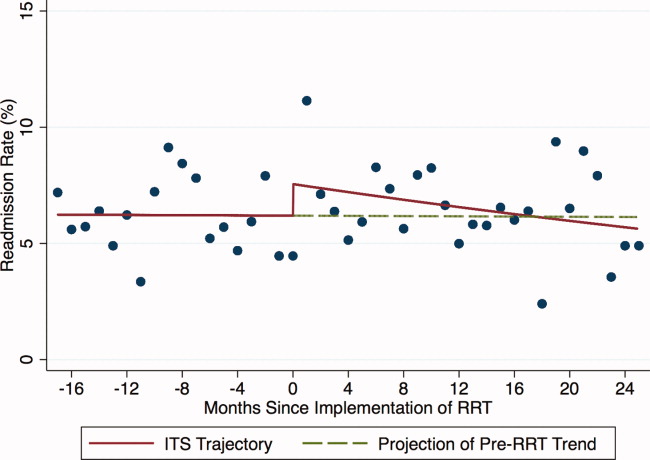
| Outcome: Summary Effect Measure | Value (95% CI) | P Value |
|---|---|---|
| ||
| ICU readmission rateadjusted odds ratio | ||
| Pre‐RRT trend | 1.00 (0.97, 1.03) | 0.98 |
| Change at RRT implementation | 1.24 (0.94, 1.63) | 0.13 |
| Post‐RRT trend | 0.98 (0.97, 1.00) | 0.06 |
| Change in trend | 0.98 (0.96, 1.02) | 0.39 |
| Net intervention effect | 0.92 (0.40, 2.12) | 0.85 |
| ICU average length of stayadjusted ratio of means | ||
| Trend at 9 mo pre‐RRT | 0.98 (0.96, 1.00) | 0.05 |
| Trend at 3 mo pre‐RRT | 1.02 (0.99, 1.04) | 0.19 |
| Change in trend at 3 mo pre‐RRT | 1.03 (1.00, 1.07) | 0.07 |
| Change at RRT implementation | 0.92 (0.80, 1.06) | 0.27 |
| Post‐RRT trend | 1.00 (0.99, 1.00) | 0.35 |
| Change in trend at RRT implementation | 0.98 (0.96, 1.01) | 0.14 |
| Net intervention effect | 0.60 (0.31, 1.18) | 0.14 |
| In‐hospital mortality of patients discharged from the ICUadjusted odds ratio | ||
| Pre‐RRT trend | 1.02 (0.99, 1.06) | 0.15 |
| Change at RRT implementation | 0.74 (0.51, 1.08) | 0.12 |
| Post‐RRT trend | 1.00 (0.98, 1.01) | 0.68 |
| Change in trend | 0.97 (0.94, 1.01) | 0.14 |
| Net intervention effect | 0.39 (0.14, 1.10) | 0.08 |
ICU Average LOS
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in ICU average LOS (5.1 days preintervention vs 4.9 days postintervention, P = 0.24; Table 1). Trends in ICU LOS may have changed in October 2006 (P = 0.07), decreasing in the first half of the study period (adjusted rate ratio [ARR] 0.98 per year, 95% CI 0.961.00), but did not change significantly thereafter. As with the ICU readmission rate, neither the change in estimated secular trend after implementation (ARR 0.98, 95% CI 0.961.01), nor the net effect of the intervention (ARR 0.62, 95% CI 0.321.22) was statistically significant (Table 2); these results are depicted graphically in Figure 2.
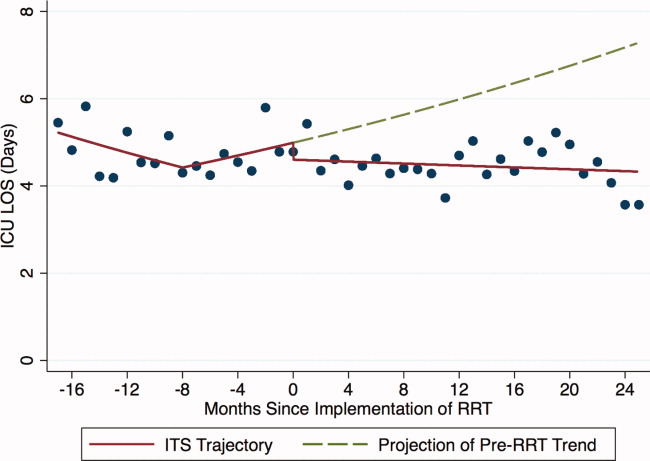
In‐Hospital Mortality of Patients Discharged From the ICU
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the mortality of patients discharged from the ICU (6.0% preintervention vs 5.5% postintervention, P = 0.24; Table 1). Similarly, in the adjusted ITS model, the intervention had no statistically significant net effect on the mortality outcome (Table 2 and Figure 3).
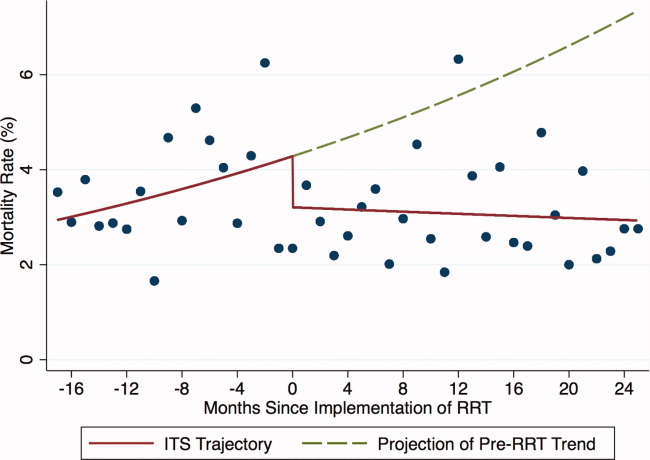
Secondary Analyses
Apart from weak evidence for a change in trend in ICU LOS in October 2006, no other changes in trend were found within the preintervention or postintervention periods (data not shown). This suggests that the heterogeneity of the preintervention RRT had no significant impact on the 3 outcomes examined, and that the RRT intervention failed to gain efficacy with time in the postintervention period. Additionally, we saw no outcome benefit in sensitivity analyses among all ICU patients or in service‐defined analyses (eg, surgical services), where ability to control for illness severity was improved.
DISCUSSION
In this single center study, introduction of an RRT that proactively rounded on patients discharged from the ICU did not reduce the ICU readmission rate, ICU LOS, or mortality of patients discharged from the ICU, after accounting for secular trends using robust ITS methods and adjusting for patient level factors.
Our study is consistent with 2 smaller studies that assessed the impact of proactive rounding by a CCOT on ICU readmission rate. Leary and Ridley14 found that proactively rounding by a CCOT did not reduce ICU readmissions or shorten the ICU LOS, although this study was limited by a surprisingly low ICU readmission rate and short ICU LOS prior to the intervention. Another study15 also observed no change in the ICU readmission rate following introduction of a proactively rounding CCOT but noted small reductions in both ICU and hospital mortality. The sole study showing an effect16 observed a lower ICU readmission rate and increased survival to hospital discharge (after excluding do not resuscitate [DNR] patients) with implementation of a CCOT, although some of their findings may be explained by their CCOT's use of palliative care services, a function not featured in our model.
Our study adds to the meta‐analyses and systematic reviews810 that have questioned the hypothesis that a trained and proactive team of caregivers should be able to prevent patients from returning to the ICU. Perhaps one reason why this is not true is that proactive rounding by RRTs may have minimal effect in systems where step‐down beds are readily available. At UCSF, nearly every patient transferred out of the ICU is triaged to a step‐down unit, where telemetry and pulse oximetry are continuously monitored. Despite this, however, our institution's 2 step‐down units generate more calls to our RRT than any other units in the hospital.
We were surprised to see that proactive rounding failed to shorten ICU LOS, hypothesizing that clinicians would be more comfortable discharging patients from the ICU knowing that the RRT would be closely monitoring them afterwards. Although we have no data to support this hypothesis, increased use of the RRT may have also increased step‐down bed use, as patients on the general medicalsurgical floors were transferred to a higher level of care upon recommendation of the RRT, thereby delaying transfers out of the ICU. Moreover, the opening of an additional 16‐bed ICU in October 2008 might have encouraged clinicians to transfer patients back to the ICU simply because beds were more easily accessible than before.
Introduction of proactive rounding by the RRT was also not associated with differences in the mortality rate of patients discharged from the ICU. This finding conflicts with the results of Garcea et al,15 Ball et al,16 and Priestley et al13, all of which found that implementation of a CCOT led to small but statistically significant reductions in in‐hospital mortality. All 3 of these studies, however, examined smaller patient populations (1380, 470, and 2903 patients, respectively), and both the Priestley and Ball studies13, 16 had significantly shorter periods of data collection (24 months and 32 weeks, respectively). Our results are based on models with confidence intervals and P values that account for variability in all 3 underlying effect estimates but assume a linear extrapolation of the preintervention trend. This approach allowed us to flexibly deal with changes related to the intervention, while relying on our large sample size to define time trends not dealt with adequately (or at all) in previous research.
The lack of improvement in outcomes cannot be attributed to immaturity of the RRT or failure of the clinical staff to use the RRT adequately. A prespecified secondary data analysis midway through the postintervention time period demonstrated that the RRT failed to gain efficacy with time with respect to all 3 outcomes. The postintervention RRT was also utilized far more frequently than its predecessor (110.6 vs 2.7 calls per 1000 admissions, respectively), and this degree of RRT utilization far surpasses the dose considered to be indicative of a mature RRT system.12
Our study has several limitations. First, we relied on administrative rather than chart‐collected data to determine the reason for ICU admission, and the APR severity of illness and risk of mortality scores. It seems unlikely, however, that coding deficiencies or biases affected the preintervention and postintervention patient populations differently. Even though we adjusted for all available measures, it is possible that we were not able to account for time trends in all potential confounders. Second, we did not have detailed clinical information on reasons for ICU readmission and whether readmissions occurred before or after the RRT proactively rounded on the patient. Therefore, potential readmissions to the ICU that might have been planned or which would have happened regardless of the presence of the RRT, such as for antibiotic desensitization, could not be accounted for. Third, introduction of proactive rounding by the RRT in June 2007 was accompanied by a change in the RRT's composition, from a physician‐led model to a nurse‐led model. Therefore, inherent differences in the way that physicians and nurses might assess and triage patients could not have been adjusted for. Lastly, this was a retrospective study conducted at a single academic medical center with a specific RRT model, and our results may not be directly applicable to nonteaching settings or to different RRT models.
Our findings raise further questions about the benefits of RRTs as they assume additional roles, such as proactive rounding on patients recently discharged from the ICU. The failure of our RRT to reduce the ICU readmission rate, the ICU average LOS, and the mortality of patients discharged from the ICU raises concerns that the benefits of our RRT are not commensurate with its cost. While defining the degree of impact and underlying mechanisms are worthy of prospective study, hospitals seeking to improve their RRT models should consider how to develop systems that achieve the RRT's promise in measurable ways.
Acknowledgements
The authors acknowledge Heather Leicester, MSPH, Senior Performance Improvement Analyst for Patient Safety and Quality Services at the University of California San Francisco for her work in data acquisition.
Disclosures: Dr Vittinghoff received salary support from an NIH grant during the time of this work for statistical consulting. He receives textbook royalties from Springer Verlag. Dr Auerbach was supported by 5K24HL098372‐02 from the National Heart Lung and Blood Institute during the period of this study although not specifically for this study; they had no role in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The other authors have no financial conflicts of interest.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Clinical Governance Unit, Quality and Safety Branch, Rural and Regional Health and Aged Care Services Division Safer Systems, Department of Human Services, State Government of Victoria. Safer Systems—Saving Lives Campaign. Available at: http://www.health.vic.gov.au/sssl. Accessed April 5, 2012.
- Canadian Patient Safety Institute. Safer Healthcare Now! Campaign. Available at: http://www.saferhealthcarenow.ca. Accessed April 5, 2012.
- , . The growth of rapid response systems. Jt Comm J Qual Patient Saf. 2008;34:489–495.
- , , , . The medical emergency team. Anaesth Intensive Care. 1995;23(2):183–186.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admission: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , , . The patient‐at‐risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54:853–860.
- , , , , . Effects of rapid response systems on clinical outcomes: systemic review and meta‐analysis. J Hosp Med. 2007;2:422–432.
- , , , , . Rapid response teams: a systemic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , . Rapid response teams. N Engl J Med. 2011;365:139–146.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , . Impact of an outreach team on re‐admissions to a critical care unit. Anaesthesia. 2003;58:328–332.
- , , , , . Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand. 2004;48:1096–1100.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1017.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Rapid response teams (RRT) have been promoted by numerous patient safety organizations to reduce preventable in‐hospital deaths.14 Initial studies of RRTs were promising,57 but recent literature,811 including systematic reviews and meta‐analyses, has called these findings into question. Nevertheless, RRTs remain popular in academic and community hospitals worldwide, and many have expanded their roles beyond solely responding to the deteriorating patient.12
Some RRTs, for example, proactively round on seriously ill ward patients and patients recently discharged from the intensive care unit (ICU) in an effort to prevent transitions to higher levels of care. Priestley and colleagues demonstrated that institution of such a team, referred to as a critical care outreach team (CCOT), decreased in‐hospital mortality while possibly increasing hospital length of stay (LOS).13 Three additional single‐center studies from the United Kingdom, where CCOTs are common, specifically examined proactive rounding by CCOTs on the ICU readmission rate: 2 observed no improvement,14, 15 while the third, limited by a small sample size, demonstrated a modest reduction in ICU readmissions.16
We sought to determine the impact of proactive rounding by an RRT on patients discharged from intensive care on the ICU readmission rate, ICU LOS, and in‐hospital mortality of patients discharged from the ICU. We hypothesized that proactive rounding by an RRT would decrease the ICU readmission rate, ICU LOS, and the in‐hospital mortality of patients discharged from the ICU.
MATERIALS AND METHODS
Site and Subjects
We carried out a retrospective, observational study of adult patients discharged from the ICU at University of California San Francisco (UCSF) Medical Center between January 2006 and June 2009. UCSF is a 790‐bed quaternary care academic hospital that admits approximately 17,000 patients annually and has 5 adult ICUs, with 62 beds and 3500 to 4000 ICU admissions annually. Our study was approved by the UCSF Medical Center Committee on Human Research; need for informed consent was waived.
Description of the RRT Before June 1, 2007
Throughout the study, the goal of the RRT was unchanged: to assess, triage, and institute early treatment in patients who experienced an acute decline in their clinical status. From November 2005 to October 2006, the RRT was staffed by an attending hospitalist and medicine resident during daytime, and by a critical care fellow at nighttime and on weekends. The RRT could be activated by any concerned staff member in response to a set of predetermined vital sign abnormalities, decreased urine output, or altered mental status, or simply if the staff member was concerned about the patient's clinical status. Despite extensive educational efforts, utilization of the team was low (2.7 calls per 1000 admissions) and, accordingly, it was discontinued in October 2006. After this time, staff would contact the primary team caring for the patient, should concerns regarding the patient's condition arise.
Description of the RRT After June 1, 2007
In an effort to expand its scope and utility, the RRT was reinstated on June 1, 2007 with a new composition and increased responsibilities. After this date, physician roles were eliminated, and the team composition changed to a dedicated critical care nurse and respiratory therapist, available 24 hours a day. Criteria for calling the team remained unchanged. In addition to responding to acute deteriorations in patients' clinical courses, the RRT began to proactively assess all patients within 12 hours of discharge from the ICU and would continue to round on these patients daily until it was felt that they were clinically stable. During these rounds, the RRT would provide consultation expertise to the bedside nurse and contact the patient's clinicians if concern existed about a patient's clinical trajectory; decisions to transfer a patient back to the ICU ultimately rested with the patient's primary team. During this time period, the RRT received an average of 110.6 calls per 1000 admissions.
Data Sources
Data collected included: demographics, clinical information (all patient refined [APR] severity of illness, APR risk of mortality, and the presence of 29 comorbidities), whether there was a readmission to the ICU, the total ICU LOS, and the vital status at the time of hospital discharge.
Outcomes
Outcomes included: readmission to the ICU, defined as 2 noncontiguous ICU stays during a single hospitalization; ICU LOS, defined as the total number of ICU days accrued during hospitalization; and in‐hospital mortality of patients discharged from the ICU.
Adjustment Variables
Patient age, gender, race, and ethnicity were available from administrative data. We used admission diagnosis code data to classify comorbidities using the method of Elixhauser et al.17
Statistical Analysis
For each of the 3 study outcomes, we assessed the effects of the intervention using multivariable models adjusting for patient‐ and service‐level factors, including a gamma model for ICU LOS and logistic models for ICU readmission and in‐hospital mortality of patients discharged from the ICU. We first compared unadjusted outcome levels before and after implementation. We then used an interrupted time series (ITS) framework to assess the effects of the intervention in terms of 5 measures: 1) the secular trend in the mean of the outcome before the intervention; 2) the change in the mean at the start of the implementation, or immediate effects; 3) the secular trend in the mean after implementation; 4) the change in secular trend, reflecting cumulative intervention effects; and 5) the net effect of the intervention, estimated as the adjusted difference between the fitted mean at the end of the postintervention period and the expected mean if the preintervention trend had continued without interruption or change.
Secondary Analyses
Given the heterogeneity of the RRT in the preintervention period, we assessed potential changes in trend at October 2006, the month in which the RRT was discontinued. We also examined changes in trend midway through the postimplementation period to evaluate for increased efficacy of the RRT with time.
Selection of Covariates
Age, race, and admitting service were included in both the prepost and ITS models by default for face validity. Additional covariates were selected for each outcome using backwards deletion with a retention criterion of P < 0.05, based on models that allowed the outcome rate to vary freely month to month. Because these data were obtained from administrative billing datasets, and the presence of comorbidities could not be definitively linked with time points during hospitalization, only those comorbidities that were likely present prior at ICU discharge were included. For similar reasons, APR severity of illness and risk of mortality scores, which were calculated from billing diagnoses at the end of hospitalization, were excluded from the models.
RESULTS
Patient Characteristics
During the study period, 11,687 patients were admitted to the ICU; 10,288 were discharged from the ICU alive and included in the analysis. In the 17 months prior to the introduction of proactive rounding by the RRT, 4902 (41.9%) patients were admitted, and during the 25 months afterwards, 6785 (58.1%) patients. Patients admitted in the 2 time periods were similar, although there were clinically small but statistically significant differences in race, APR severity of illness, APR risk of mortality, and certain comorbidities between the 2 groups (Table 1).
| Pre‐RRT (n = 4305) N (%) | Post‐RRT (n = 5983) N (%) | P Value | |
|---|---|---|---|
| |||
| Age, mean (y [SD]) | 57.7 [16.6] | 57.9 [16.5] | 0.50 |
| Female gender | 2,005 (46.6) | 2,824 (47.2) | 0.53 |
| Race | 0.0013 | ||
| White | 2,538 (59.0) | 3,520 (58.8) | |
| Black | 327 (7.6) | 436 (7.3) | |
| Asian | 642 (14.9) | 842 (14.1) | |
| Other | 719 (16.7) | 1,121 (18.7) | |
| Unknown | 79 (1.8) | 64 (1.1) | |
| Ethnicity | 0.87 | ||
| Hispanic | 480 (11.2) | 677 (11.3%) | |
| Non‐Hispanic | 3,547 (82.4) | 4,907 (82.0%) | |
| Unknown | 278 (6.5) | 399 (6.7) | |
| Insurance | 0.50 | ||
| Medicare | 1,788 (41.5) | 2,415 (40.4) | |
| Medicaid/Medi‐Cal | 699 (16.2) | 968 (16.2) | |
| Private | 1,642 (38.1) | 2,329 (38.9) | |
| Other | 176 (4.1) | 271 (4.5) | |
| Admission source | 0.41 | ||
| ED | 1,621 (37.7) | 2,244 (37.5) | |
| Outside hospital | 652 (15.2) | 855 (14.3) | |
| Direct admit | 2,032 (47.2) | 2,884 (48.2) | |
| Major surgery | 0.99 | ||
| Yes | 3,107 (72.2) | 4,319 (72.2) | |
| APR severity of illness | 0.0001 | ||
| Mild | 622 (14.5) | 828 (13.8) | |
| Moderate | 1,328 (30.9) | 1,626 (27.2) | |
| Major | 1,292 (30.0) | 1,908 (31.9) | |
| Extreme | 1,063 (24.7) | 1,621 (27.1) | |
| APR risk of mortality | 0.0109 | ||
| Mild | 1,422 (33.0) | 1,821 (30.4) | |
| Moderate | 1,074 (25.0) | 1,467 (24.5) | |
| Major | 947 (22.0) | 1,437 (24.0) | |
| Extreme | 862 (20.0) | 1,258 (21.0) | |
| Admitting service | 0.11 | ||
| Adult general surgery | 190 (4.4) | 260 (4.4) | |
| Cardiology | 347 (8.1) | 424 (7.1) | |
| Cardiothoracic surgery | 671 (15.6) | 930 (15.5) | |
| Kidney transplant surgery | 105 (2.4) | 112 (1.9) | |
| Liver transplant surgery | 298 (6.9) | 379 (6.3) | |
| Medicine | 683 (15.9) | 958 (16.0) | |
| Neurology | 420 (9.8) | 609 (10.2) | |
| Neurosurgery | 1,345 (31.2) | 1,995 (33.3) | |
| Vascular surgery | 246 (5.7) | 316 (5.3) | |
| Comorbidities | |||
| Hypertension | 2,054 (47.7) | 2,886 (48.2) | 0.60 |
| Fluid and electrolyte disorders | 998 (23.2) | 1,723 (28.8) | <0.0001 |
| Diabetes | 708 (16.5) | 880 (14.7) | 0.02 |
| Chronic obstructive pulmonary disease | 632 (14.7) | 849 (14.2) | 0.48 |
| Iron deficiency anemia | 582 (13.5) | 929 (15.5) | 0.005 |
| Renal failure | 541 (12.6) | 744 (12.4) | 0.84 |
| Coagulopathy | 418 (9.7) | 712 (11.9) | 0.0005 |
| Liver disease | 400 (9.3) | 553 (9.2) | 0.93 |
| Hypothyroidism | 330 (7.7) | 500 (8.4) | 0.20 |
| Depression | 306 (7.1) | 508 (8.5) | 0.01 |
| Peripheral vascular disease | 304 (7.1) | 422 (7.1) | 0.99 |
| Congestive heart failure | 263 (6.1) | 360 (6.0) | 0.85 |
| Weight loss | 236 (5.5) | 425 (7.1) | 0.0009 |
| Paralysis | 225 (5.2) | 328 (5.5) | 0.57 |
| Neurological disorders | 229 (5.3) | 276 (4.6) | 0.10 |
| Valvular disease | 210 (4.9) | 329 (5.5) | 0.16 |
| Drug abuse | 198 (4.6) | 268 (4.5) | 0.77 |
| Metastatic cancer | 198 (4.6) | 296 (5.0) | 0.42 |
| Obesity | 201 (4.7) | 306 (5.1) | 0.30 |
| Alcohol abuse | 178 (4.1) | 216 (3.6) | 0.17 |
| Diabetes with complications | 175 (4.1) | 218 (3.6) | 0.27 |
| Solid tumor without metastasis | 146 (3.4) | 245 (4.1) | 0.07 |
| Psychoses | 115 (2.7) | 183 (3.1) | 0.25 |
| Rheumatoid arthritis/collagen vascular disease | 96 (2.2) | 166 (2.8) | 0.08 |
| Pulmonary circulation disease | 83 (1.9) | 181 (3.0) | 0.0005 |
| Outcomes | |||
| Readmission to ICU | 288 (6.7) | 433 (7.3) | 0.24 |
| ICU length of stay, mean [SD] | 5.1 [9.7] | 4.9 [8.3] | 0.24 |
| In‐hospital mortality of patients discharged from the ICU | 260 (6.0) | 326 (5.5) | 0.24 |
ICU Readmission Rate
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the ICU readmission rate (6.7% preintervention vs 7.3% postintervention, P = 0.24; Table 1). In the adjusted ITS model, the intervention had no net effect on the odds of ICU readmission (adjusted odds ratio [AOR] for net intervention effect 0.98, 95% confidence interval [CI] 0.42, 2.28), with similar secular trends both preintervention (AOR 1.00 per year, 95% CI 0.97, 1.03), and afterwards (AOR 0.99 per year, 95% CI 0.98, 01.00), and a nonsignificant increase at implementation (Table 2). Figure 1 uses solid lines to show the fitted readmission rates, a hatched line to show the projection of the preintervention secular trend into the postintervention period, and circles to represent adjusted monthly means. The lack of a net intervention effect is indicated by the convergence of the solid and hatched lines 24 months postintervention.

| Outcome: Summary Effect Measure | Value (95% CI) | P Value |
|---|---|---|
| ||
| ICU readmission rateadjusted odds ratio | ||
| Pre‐RRT trend | 1.00 (0.97, 1.03) | 0.98 |
| Change at RRT implementation | 1.24 (0.94, 1.63) | 0.13 |
| Post‐RRT trend | 0.98 (0.97, 1.00) | 0.06 |
| Change in trend | 0.98 (0.96, 1.02) | 0.39 |
| Net intervention effect | 0.92 (0.40, 2.12) | 0.85 |
| ICU average length of stayadjusted ratio of means | ||
| Trend at 9 mo pre‐RRT | 0.98 (0.96, 1.00) | 0.05 |
| Trend at 3 mo pre‐RRT | 1.02 (0.99, 1.04) | 0.19 |
| Change in trend at 3 mo pre‐RRT | 1.03 (1.00, 1.07) | 0.07 |
| Change at RRT implementation | 0.92 (0.80, 1.06) | 0.27 |
| Post‐RRT trend | 1.00 (0.99, 1.00) | 0.35 |
| Change in trend at RRT implementation | 0.98 (0.96, 1.01) | 0.14 |
| Net intervention effect | 0.60 (0.31, 1.18) | 0.14 |
| In‐hospital mortality of patients discharged from the ICUadjusted odds ratio | ||
| Pre‐RRT trend | 1.02 (0.99, 1.06) | 0.15 |
| Change at RRT implementation | 0.74 (0.51, 1.08) | 0.12 |
| Post‐RRT trend | 1.00 (0.98, 1.01) | 0.68 |
| Change in trend | 0.97 (0.94, 1.01) | 0.14 |
| Net intervention effect | 0.39 (0.14, 1.10) | 0.08 |
ICU Average LOS
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in ICU average LOS (5.1 days preintervention vs 4.9 days postintervention, P = 0.24; Table 1). Trends in ICU LOS may have changed in October 2006 (P = 0.07), decreasing in the first half of the study period (adjusted rate ratio [ARR] 0.98 per year, 95% CI 0.961.00), but did not change significantly thereafter. As with the ICU readmission rate, neither the change in estimated secular trend after implementation (ARR 0.98, 95% CI 0.961.01), nor the net effect of the intervention (ARR 0.62, 95% CI 0.321.22) was statistically significant (Table 2); these results are depicted graphically in Figure 2.

In‐Hospital Mortality of Patients Discharged From the ICU
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the mortality of patients discharged from the ICU (6.0% preintervention vs 5.5% postintervention, P = 0.24; Table 1). Similarly, in the adjusted ITS model, the intervention had no statistically significant net effect on the mortality outcome (Table 2 and Figure 3).

Secondary Analyses
Apart from weak evidence for a change in trend in ICU LOS in October 2006, no other changes in trend were found within the preintervention or postintervention periods (data not shown). This suggests that the heterogeneity of the preintervention RRT had no significant impact on the 3 outcomes examined, and that the RRT intervention failed to gain efficacy with time in the postintervention period. Additionally, we saw no outcome benefit in sensitivity analyses among all ICU patients or in service‐defined analyses (eg, surgical services), where ability to control for illness severity was improved.
DISCUSSION
In this single center study, introduction of an RRT that proactively rounded on patients discharged from the ICU did not reduce the ICU readmission rate, ICU LOS, or mortality of patients discharged from the ICU, after accounting for secular trends using robust ITS methods and adjusting for patient level factors.
Our study is consistent with 2 smaller studies that assessed the impact of proactive rounding by a CCOT on ICU readmission rate. Leary and Ridley14 found that proactively rounding by a CCOT did not reduce ICU readmissions or shorten the ICU LOS, although this study was limited by a surprisingly low ICU readmission rate and short ICU LOS prior to the intervention. Another study15 also observed no change in the ICU readmission rate following introduction of a proactively rounding CCOT but noted small reductions in both ICU and hospital mortality. The sole study showing an effect16 observed a lower ICU readmission rate and increased survival to hospital discharge (after excluding do not resuscitate [DNR] patients) with implementation of a CCOT, although some of their findings may be explained by their CCOT's use of palliative care services, a function not featured in our model.
Our study adds to the meta‐analyses and systematic reviews810 that have questioned the hypothesis that a trained and proactive team of caregivers should be able to prevent patients from returning to the ICU. Perhaps one reason why this is not true is that proactive rounding by RRTs may have minimal effect in systems where step‐down beds are readily available. At UCSF, nearly every patient transferred out of the ICU is triaged to a step‐down unit, where telemetry and pulse oximetry are continuously monitored. Despite this, however, our institution's 2 step‐down units generate more calls to our RRT than any other units in the hospital.
We were surprised to see that proactive rounding failed to shorten ICU LOS, hypothesizing that clinicians would be more comfortable discharging patients from the ICU knowing that the RRT would be closely monitoring them afterwards. Although we have no data to support this hypothesis, increased use of the RRT may have also increased step‐down bed use, as patients on the general medicalsurgical floors were transferred to a higher level of care upon recommendation of the RRT, thereby delaying transfers out of the ICU. Moreover, the opening of an additional 16‐bed ICU in October 2008 might have encouraged clinicians to transfer patients back to the ICU simply because beds were more easily accessible than before.
Introduction of proactive rounding by the RRT was also not associated with differences in the mortality rate of patients discharged from the ICU. This finding conflicts with the results of Garcea et al,15 Ball et al,16 and Priestley et al13, all of which found that implementation of a CCOT led to small but statistically significant reductions in in‐hospital mortality. All 3 of these studies, however, examined smaller patient populations (1380, 470, and 2903 patients, respectively), and both the Priestley and Ball studies13, 16 had significantly shorter periods of data collection (24 months and 32 weeks, respectively). Our results are based on models with confidence intervals and P values that account for variability in all 3 underlying effect estimates but assume a linear extrapolation of the preintervention trend. This approach allowed us to flexibly deal with changes related to the intervention, while relying on our large sample size to define time trends not dealt with adequately (or at all) in previous research.
The lack of improvement in outcomes cannot be attributed to immaturity of the RRT or failure of the clinical staff to use the RRT adequately. A prespecified secondary data analysis midway through the postintervention time period demonstrated that the RRT failed to gain efficacy with time with respect to all 3 outcomes. The postintervention RRT was also utilized far more frequently than its predecessor (110.6 vs 2.7 calls per 1000 admissions, respectively), and this degree of RRT utilization far surpasses the dose considered to be indicative of a mature RRT system.12
Our study has several limitations. First, we relied on administrative rather than chart‐collected data to determine the reason for ICU admission, and the APR severity of illness and risk of mortality scores. It seems unlikely, however, that coding deficiencies or biases affected the preintervention and postintervention patient populations differently. Even though we adjusted for all available measures, it is possible that we were not able to account for time trends in all potential confounders. Second, we did not have detailed clinical information on reasons for ICU readmission and whether readmissions occurred before or after the RRT proactively rounded on the patient. Therefore, potential readmissions to the ICU that might have been planned or which would have happened regardless of the presence of the RRT, such as for antibiotic desensitization, could not be accounted for. Third, introduction of proactive rounding by the RRT in June 2007 was accompanied by a change in the RRT's composition, from a physician‐led model to a nurse‐led model. Therefore, inherent differences in the way that physicians and nurses might assess and triage patients could not have been adjusted for. Lastly, this was a retrospective study conducted at a single academic medical center with a specific RRT model, and our results may not be directly applicable to nonteaching settings or to different RRT models.
Our findings raise further questions about the benefits of RRTs as they assume additional roles, such as proactive rounding on patients recently discharged from the ICU. The failure of our RRT to reduce the ICU readmission rate, the ICU average LOS, and the mortality of patients discharged from the ICU raises concerns that the benefits of our RRT are not commensurate with its cost. While defining the degree of impact and underlying mechanisms are worthy of prospective study, hospitals seeking to improve their RRT models should consider how to develop systems that achieve the RRT's promise in measurable ways.
Acknowledgements
The authors acknowledge Heather Leicester, MSPH, Senior Performance Improvement Analyst for Patient Safety and Quality Services at the University of California San Francisco for her work in data acquisition.
Disclosures: Dr Vittinghoff received salary support from an NIH grant during the time of this work for statistical consulting. He receives textbook royalties from Springer Verlag. Dr Auerbach was supported by 5K24HL098372‐02 from the National Heart Lung and Blood Institute during the period of this study although not specifically for this study; they had no role in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The other authors have no financial conflicts of interest.
Rapid response teams (RRT) have been promoted by numerous patient safety organizations to reduce preventable in‐hospital deaths.14 Initial studies of RRTs were promising,57 but recent literature,811 including systematic reviews and meta‐analyses, has called these findings into question. Nevertheless, RRTs remain popular in academic and community hospitals worldwide, and many have expanded their roles beyond solely responding to the deteriorating patient.12
Some RRTs, for example, proactively round on seriously ill ward patients and patients recently discharged from the intensive care unit (ICU) in an effort to prevent transitions to higher levels of care. Priestley and colleagues demonstrated that institution of such a team, referred to as a critical care outreach team (CCOT), decreased in‐hospital mortality while possibly increasing hospital length of stay (LOS).13 Three additional single‐center studies from the United Kingdom, where CCOTs are common, specifically examined proactive rounding by CCOTs on the ICU readmission rate: 2 observed no improvement,14, 15 while the third, limited by a small sample size, demonstrated a modest reduction in ICU readmissions.16
We sought to determine the impact of proactive rounding by an RRT on patients discharged from intensive care on the ICU readmission rate, ICU LOS, and in‐hospital mortality of patients discharged from the ICU. We hypothesized that proactive rounding by an RRT would decrease the ICU readmission rate, ICU LOS, and the in‐hospital mortality of patients discharged from the ICU.
MATERIALS AND METHODS
Site and Subjects
We carried out a retrospective, observational study of adult patients discharged from the ICU at University of California San Francisco (UCSF) Medical Center between January 2006 and June 2009. UCSF is a 790‐bed quaternary care academic hospital that admits approximately 17,000 patients annually and has 5 adult ICUs, with 62 beds and 3500 to 4000 ICU admissions annually. Our study was approved by the UCSF Medical Center Committee on Human Research; need for informed consent was waived.
Description of the RRT Before June 1, 2007
Throughout the study, the goal of the RRT was unchanged: to assess, triage, and institute early treatment in patients who experienced an acute decline in their clinical status. From November 2005 to October 2006, the RRT was staffed by an attending hospitalist and medicine resident during daytime, and by a critical care fellow at nighttime and on weekends. The RRT could be activated by any concerned staff member in response to a set of predetermined vital sign abnormalities, decreased urine output, or altered mental status, or simply if the staff member was concerned about the patient's clinical status. Despite extensive educational efforts, utilization of the team was low (2.7 calls per 1000 admissions) and, accordingly, it was discontinued in October 2006. After this time, staff would contact the primary team caring for the patient, should concerns regarding the patient's condition arise.
Description of the RRT After June 1, 2007
In an effort to expand its scope and utility, the RRT was reinstated on June 1, 2007 with a new composition and increased responsibilities. After this date, physician roles were eliminated, and the team composition changed to a dedicated critical care nurse and respiratory therapist, available 24 hours a day. Criteria for calling the team remained unchanged. In addition to responding to acute deteriorations in patients' clinical courses, the RRT began to proactively assess all patients within 12 hours of discharge from the ICU and would continue to round on these patients daily until it was felt that they were clinically stable. During these rounds, the RRT would provide consultation expertise to the bedside nurse and contact the patient's clinicians if concern existed about a patient's clinical trajectory; decisions to transfer a patient back to the ICU ultimately rested with the patient's primary team. During this time period, the RRT received an average of 110.6 calls per 1000 admissions.
Data Sources
Data collected included: demographics, clinical information (all patient refined [APR] severity of illness, APR risk of mortality, and the presence of 29 comorbidities), whether there was a readmission to the ICU, the total ICU LOS, and the vital status at the time of hospital discharge.
Outcomes
Outcomes included: readmission to the ICU, defined as 2 noncontiguous ICU stays during a single hospitalization; ICU LOS, defined as the total number of ICU days accrued during hospitalization; and in‐hospital mortality of patients discharged from the ICU.
Adjustment Variables
Patient age, gender, race, and ethnicity were available from administrative data. We used admission diagnosis code data to classify comorbidities using the method of Elixhauser et al.17
Statistical Analysis
For each of the 3 study outcomes, we assessed the effects of the intervention using multivariable models adjusting for patient‐ and service‐level factors, including a gamma model for ICU LOS and logistic models for ICU readmission and in‐hospital mortality of patients discharged from the ICU. We first compared unadjusted outcome levels before and after implementation. We then used an interrupted time series (ITS) framework to assess the effects of the intervention in terms of 5 measures: 1) the secular trend in the mean of the outcome before the intervention; 2) the change in the mean at the start of the implementation, or immediate effects; 3) the secular trend in the mean after implementation; 4) the change in secular trend, reflecting cumulative intervention effects; and 5) the net effect of the intervention, estimated as the adjusted difference between the fitted mean at the end of the postintervention period and the expected mean if the preintervention trend had continued without interruption or change.
Secondary Analyses
Given the heterogeneity of the RRT in the preintervention period, we assessed potential changes in trend at October 2006, the month in which the RRT was discontinued. We also examined changes in trend midway through the postimplementation period to evaluate for increased efficacy of the RRT with time.
Selection of Covariates
Age, race, and admitting service were included in both the prepost and ITS models by default for face validity. Additional covariates were selected for each outcome using backwards deletion with a retention criterion of P < 0.05, based on models that allowed the outcome rate to vary freely month to month. Because these data were obtained from administrative billing datasets, and the presence of comorbidities could not be definitively linked with time points during hospitalization, only those comorbidities that were likely present prior at ICU discharge were included. For similar reasons, APR severity of illness and risk of mortality scores, which were calculated from billing diagnoses at the end of hospitalization, were excluded from the models.
RESULTS
Patient Characteristics
During the study period, 11,687 patients were admitted to the ICU; 10,288 were discharged from the ICU alive and included in the analysis. In the 17 months prior to the introduction of proactive rounding by the RRT, 4902 (41.9%) patients were admitted, and during the 25 months afterwards, 6785 (58.1%) patients. Patients admitted in the 2 time periods were similar, although there were clinically small but statistically significant differences in race, APR severity of illness, APR risk of mortality, and certain comorbidities between the 2 groups (Table 1).
| Pre‐RRT (n = 4305) N (%) | Post‐RRT (n = 5983) N (%) | P Value | |
|---|---|---|---|
| |||
| Age, mean (y [SD]) | 57.7 [16.6] | 57.9 [16.5] | 0.50 |
| Female gender | 2,005 (46.6) | 2,824 (47.2) | 0.53 |
| Race | 0.0013 | ||
| White | 2,538 (59.0) | 3,520 (58.8) | |
| Black | 327 (7.6) | 436 (7.3) | |
| Asian | 642 (14.9) | 842 (14.1) | |
| Other | 719 (16.7) | 1,121 (18.7) | |
| Unknown | 79 (1.8) | 64 (1.1) | |
| Ethnicity | 0.87 | ||
| Hispanic | 480 (11.2) | 677 (11.3%) | |
| Non‐Hispanic | 3,547 (82.4) | 4,907 (82.0%) | |
| Unknown | 278 (6.5) | 399 (6.7) | |
| Insurance | 0.50 | ||
| Medicare | 1,788 (41.5) | 2,415 (40.4) | |
| Medicaid/Medi‐Cal | 699 (16.2) | 968 (16.2) | |
| Private | 1,642 (38.1) | 2,329 (38.9) | |
| Other | 176 (4.1) | 271 (4.5) | |
| Admission source | 0.41 | ||
| ED | 1,621 (37.7) | 2,244 (37.5) | |
| Outside hospital | 652 (15.2) | 855 (14.3) | |
| Direct admit | 2,032 (47.2) | 2,884 (48.2) | |
| Major surgery | 0.99 | ||
| Yes | 3,107 (72.2) | 4,319 (72.2) | |
| APR severity of illness | 0.0001 | ||
| Mild | 622 (14.5) | 828 (13.8) | |
| Moderate | 1,328 (30.9) | 1,626 (27.2) | |
| Major | 1,292 (30.0) | 1,908 (31.9) | |
| Extreme | 1,063 (24.7) | 1,621 (27.1) | |
| APR risk of mortality | 0.0109 | ||
| Mild | 1,422 (33.0) | 1,821 (30.4) | |
| Moderate | 1,074 (25.0) | 1,467 (24.5) | |
| Major | 947 (22.0) | 1,437 (24.0) | |
| Extreme | 862 (20.0) | 1,258 (21.0) | |
| Admitting service | 0.11 | ||
| Adult general surgery | 190 (4.4) | 260 (4.4) | |
| Cardiology | 347 (8.1) | 424 (7.1) | |
| Cardiothoracic surgery | 671 (15.6) | 930 (15.5) | |
| Kidney transplant surgery | 105 (2.4) | 112 (1.9) | |
| Liver transplant surgery | 298 (6.9) | 379 (6.3) | |
| Medicine | 683 (15.9) | 958 (16.0) | |
| Neurology | 420 (9.8) | 609 (10.2) | |
| Neurosurgery | 1,345 (31.2) | 1,995 (33.3) | |
| Vascular surgery | 246 (5.7) | 316 (5.3) | |
| Comorbidities | |||
| Hypertension | 2,054 (47.7) | 2,886 (48.2) | 0.60 |
| Fluid and electrolyte disorders | 998 (23.2) | 1,723 (28.8) | <0.0001 |
| Diabetes | 708 (16.5) | 880 (14.7) | 0.02 |
| Chronic obstructive pulmonary disease | 632 (14.7) | 849 (14.2) | 0.48 |
| Iron deficiency anemia | 582 (13.5) | 929 (15.5) | 0.005 |
| Renal failure | 541 (12.6) | 744 (12.4) | 0.84 |
| Coagulopathy | 418 (9.7) | 712 (11.9) | 0.0005 |
| Liver disease | 400 (9.3) | 553 (9.2) | 0.93 |
| Hypothyroidism | 330 (7.7) | 500 (8.4) | 0.20 |
| Depression | 306 (7.1) | 508 (8.5) | 0.01 |
| Peripheral vascular disease | 304 (7.1) | 422 (7.1) | 0.99 |
| Congestive heart failure | 263 (6.1) | 360 (6.0) | 0.85 |
| Weight loss | 236 (5.5) | 425 (7.1) | 0.0009 |
| Paralysis | 225 (5.2) | 328 (5.5) | 0.57 |
| Neurological disorders | 229 (5.3) | 276 (4.6) | 0.10 |
| Valvular disease | 210 (4.9) | 329 (5.5) | 0.16 |
| Drug abuse | 198 (4.6) | 268 (4.5) | 0.77 |
| Metastatic cancer | 198 (4.6) | 296 (5.0) | 0.42 |
| Obesity | 201 (4.7) | 306 (5.1) | 0.30 |
| Alcohol abuse | 178 (4.1) | 216 (3.6) | 0.17 |
| Diabetes with complications | 175 (4.1) | 218 (3.6) | 0.27 |
| Solid tumor without metastasis | 146 (3.4) | 245 (4.1) | 0.07 |
| Psychoses | 115 (2.7) | 183 (3.1) | 0.25 |
| Rheumatoid arthritis/collagen vascular disease | 96 (2.2) | 166 (2.8) | 0.08 |
| Pulmonary circulation disease | 83 (1.9) | 181 (3.0) | 0.0005 |
| Outcomes | |||
| Readmission to ICU | 288 (6.7) | 433 (7.3) | 0.24 |
| ICU length of stay, mean [SD] | 5.1 [9.7] | 4.9 [8.3] | 0.24 |
| In‐hospital mortality of patients discharged from the ICU | 260 (6.0) | 326 (5.5) | 0.24 |
ICU Readmission Rate
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the ICU readmission rate (6.7% preintervention vs 7.3% postintervention, P = 0.24; Table 1). In the adjusted ITS model, the intervention had no net effect on the odds of ICU readmission (adjusted odds ratio [AOR] for net intervention effect 0.98, 95% confidence interval [CI] 0.42, 2.28), with similar secular trends both preintervention (AOR 1.00 per year, 95% CI 0.97, 1.03), and afterwards (AOR 0.99 per year, 95% CI 0.98, 01.00), and a nonsignificant increase at implementation (Table 2). Figure 1 uses solid lines to show the fitted readmission rates, a hatched line to show the projection of the preintervention secular trend into the postintervention period, and circles to represent adjusted monthly means. The lack of a net intervention effect is indicated by the convergence of the solid and hatched lines 24 months postintervention.

| Outcome: Summary Effect Measure | Value (95% CI) | P Value |
|---|---|---|
| ||
| ICU readmission rateadjusted odds ratio | ||
| Pre‐RRT trend | 1.00 (0.97, 1.03) | 0.98 |
| Change at RRT implementation | 1.24 (0.94, 1.63) | 0.13 |
| Post‐RRT trend | 0.98 (0.97, 1.00) | 0.06 |
| Change in trend | 0.98 (0.96, 1.02) | 0.39 |
| Net intervention effect | 0.92 (0.40, 2.12) | 0.85 |
| ICU average length of stayadjusted ratio of means | ||
| Trend at 9 mo pre‐RRT | 0.98 (0.96, 1.00) | 0.05 |
| Trend at 3 mo pre‐RRT | 1.02 (0.99, 1.04) | 0.19 |
| Change in trend at 3 mo pre‐RRT | 1.03 (1.00, 1.07) | 0.07 |
| Change at RRT implementation | 0.92 (0.80, 1.06) | 0.27 |
| Post‐RRT trend | 1.00 (0.99, 1.00) | 0.35 |
| Change in trend at RRT implementation | 0.98 (0.96, 1.01) | 0.14 |
| Net intervention effect | 0.60 (0.31, 1.18) | 0.14 |
| In‐hospital mortality of patients discharged from the ICUadjusted odds ratio | ||
| Pre‐RRT trend | 1.02 (0.99, 1.06) | 0.15 |
| Change at RRT implementation | 0.74 (0.51, 1.08) | 0.12 |
| Post‐RRT trend | 1.00 (0.98, 1.01) | 0.68 |
| Change in trend | 0.97 (0.94, 1.01) | 0.14 |
| Net intervention effect | 0.39 (0.14, 1.10) | 0.08 |
ICU Average LOS
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in ICU average LOS (5.1 days preintervention vs 4.9 days postintervention, P = 0.24; Table 1). Trends in ICU LOS may have changed in October 2006 (P = 0.07), decreasing in the first half of the study period (adjusted rate ratio [ARR] 0.98 per year, 95% CI 0.961.00), but did not change significantly thereafter. As with the ICU readmission rate, neither the change in estimated secular trend after implementation (ARR 0.98, 95% CI 0.961.01), nor the net effect of the intervention (ARR 0.62, 95% CI 0.321.22) was statistically significant (Table 2); these results are depicted graphically in Figure 2.

In‐Hospital Mortality of Patients Discharged From the ICU
Introduction of proactive rounding by the RRT was not associated with unadjusted differences in the mortality of patients discharged from the ICU (6.0% preintervention vs 5.5% postintervention, P = 0.24; Table 1). Similarly, in the adjusted ITS model, the intervention had no statistically significant net effect on the mortality outcome (Table 2 and Figure 3).

Secondary Analyses
Apart from weak evidence for a change in trend in ICU LOS in October 2006, no other changes in trend were found within the preintervention or postintervention periods (data not shown). This suggests that the heterogeneity of the preintervention RRT had no significant impact on the 3 outcomes examined, and that the RRT intervention failed to gain efficacy with time in the postintervention period. Additionally, we saw no outcome benefit in sensitivity analyses among all ICU patients or in service‐defined analyses (eg, surgical services), where ability to control for illness severity was improved.
DISCUSSION
In this single center study, introduction of an RRT that proactively rounded on patients discharged from the ICU did not reduce the ICU readmission rate, ICU LOS, or mortality of patients discharged from the ICU, after accounting for secular trends using robust ITS methods and adjusting for patient level factors.
Our study is consistent with 2 smaller studies that assessed the impact of proactive rounding by a CCOT on ICU readmission rate. Leary and Ridley14 found that proactively rounding by a CCOT did not reduce ICU readmissions or shorten the ICU LOS, although this study was limited by a surprisingly low ICU readmission rate and short ICU LOS prior to the intervention. Another study15 also observed no change in the ICU readmission rate following introduction of a proactively rounding CCOT but noted small reductions in both ICU and hospital mortality. The sole study showing an effect16 observed a lower ICU readmission rate and increased survival to hospital discharge (after excluding do not resuscitate [DNR] patients) with implementation of a CCOT, although some of their findings may be explained by their CCOT's use of palliative care services, a function not featured in our model.
Our study adds to the meta‐analyses and systematic reviews810 that have questioned the hypothesis that a trained and proactive team of caregivers should be able to prevent patients from returning to the ICU. Perhaps one reason why this is not true is that proactive rounding by RRTs may have minimal effect in systems where step‐down beds are readily available. At UCSF, nearly every patient transferred out of the ICU is triaged to a step‐down unit, where telemetry and pulse oximetry are continuously monitored. Despite this, however, our institution's 2 step‐down units generate more calls to our RRT than any other units in the hospital.
We were surprised to see that proactive rounding failed to shorten ICU LOS, hypothesizing that clinicians would be more comfortable discharging patients from the ICU knowing that the RRT would be closely monitoring them afterwards. Although we have no data to support this hypothesis, increased use of the RRT may have also increased step‐down bed use, as patients on the general medicalsurgical floors were transferred to a higher level of care upon recommendation of the RRT, thereby delaying transfers out of the ICU. Moreover, the opening of an additional 16‐bed ICU in October 2008 might have encouraged clinicians to transfer patients back to the ICU simply because beds were more easily accessible than before.
Introduction of proactive rounding by the RRT was also not associated with differences in the mortality rate of patients discharged from the ICU. This finding conflicts with the results of Garcea et al,15 Ball et al,16 and Priestley et al13, all of which found that implementation of a CCOT led to small but statistically significant reductions in in‐hospital mortality. All 3 of these studies, however, examined smaller patient populations (1380, 470, and 2903 patients, respectively), and both the Priestley and Ball studies13, 16 had significantly shorter periods of data collection (24 months and 32 weeks, respectively). Our results are based on models with confidence intervals and P values that account for variability in all 3 underlying effect estimates but assume a linear extrapolation of the preintervention trend. This approach allowed us to flexibly deal with changes related to the intervention, while relying on our large sample size to define time trends not dealt with adequately (or at all) in previous research.
The lack of improvement in outcomes cannot be attributed to immaturity of the RRT or failure of the clinical staff to use the RRT adequately. A prespecified secondary data analysis midway through the postintervention time period demonstrated that the RRT failed to gain efficacy with time with respect to all 3 outcomes. The postintervention RRT was also utilized far more frequently than its predecessor (110.6 vs 2.7 calls per 1000 admissions, respectively), and this degree of RRT utilization far surpasses the dose considered to be indicative of a mature RRT system.12
Our study has several limitations. First, we relied on administrative rather than chart‐collected data to determine the reason for ICU admission, and the APR severity of illness and risk of mortality scores. It seems unlikely, however, that coding deficiencies or biases affected the preintervention and postintervention patient populations differently. Even though we adjusted for all available measures, it is possible that we were not able to account for time trends in all potential confounders. Second, we did not have detailed clinical information on reasons for ICU readmission and whether readmissions occurred before or after the RRT proactively rounded on the patient. Therefore, potential readmissions to the ICU that might have been planned or which would have happened regardless of the presence of the RRT, such as for antibiotic desensitization, could not be accounted for. Third, introduction of proactive rounding by the RRT in June 2007 was accompanied by a change in the RRT's composition, from a physician‐led model to a nurse‐led model. Therefore, inherent differences in the way that physicians and nurses might assess and triage patients could not have been adjusted for. Lastly, this was a retrospective study conducted at a single academic medical center with a specific RRT model, and our results may not be directly applicable to nonteaching settings or to different RRT models.
Our findings raise further questions about the benefits of RRTs as they assume additional roles, such as proactive rounding on patients recently discharged from the ICU. The failure of our RRT to reduce the ICU readmission rate, the ICU average LOS, and the mortality of patients discharged from the ICU raises concerns that the benefits of our RRT are not commensurate with its cost. While defining the degree of impact and underlying mechanisms are worthy of prospective study, hospitals seeking to improve their RRT models should consider how to develop systems that achieve the RRT's promise in measurable ways.
Acknowledgements
The authors acknowledge Heather Leicester, MSPH, Senior Performance Improvement Analyst for Patient Safety and Quality Services at the University of California San Francisco for her work in data acquisition.
Disclosures: Dr Vittinghoff received salary support from an NIH grant during the time of this work for statistical consulting. He receives textbook royalties from Springer Verlag. Dr Auerbach was supported by 5K24HL098372‐02 from the National Heart Lung and Blood Institute during the period of this study although not specifically for this study; they had no role in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The other authors have no financial conflicts of interest.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Clinical Governance Unit, Quality and Safety Branch, Rural and Regional Health and Aged Care Services Division Safer Systems, Department of Human Services, State Government of Victoria. Safer Systems—Saving Lives Campaign. Available at: http://www.health.vic.gov.au/sssl. Accessed April 5, 2012.
- Canadian Patient Safety Institute. Safer Healthcare Now! Campaign. Available at: http://www.saferhealthcarenow.ca. Accessed April 5, 2012.
- , . The growth of rapid response systems. Jt Comm J Qual Patient Saf. 2008;34:489–495.
- , , , . The medical emergency team. Anaesth Intensive Care. 1995;23(2):183–186.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admission: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , , . The patient‐at‐risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54:853–860.
- , , , , . Effects of rapid response systems on clinical outcomes: systemic review and meta‐analysis. J Hosp Med. 2007;2:422–432.
- , , , , . Rapid response teams: a systemic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , . Rapid response teams. N Engl J Med. 2011;365:139–146.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , . Impact of an outreach team on re‐admissions to a critical care unit. Anaesthesia. 2003;58:328–332.
- , , , , . Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand. 2004;48:1096–1100.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1017.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Clinical Governance Unit, Quality and Safety Branch, Rural and Regional Health and Aged Care Services Division Safer Systems, Department of Human Services, State Government of Victoria. Safer Systems—Saving Lives Campaign. Available at: http://www.health.vic.gov.au/sssl. Accessed April 5, 2012.
- Canadian Patient Safety Institute. Safer Healthcare Now! Campaign. Available at: http://www.saferhealthcarenow.ca. Accessed April 5, 2012.
- , . The growth of rapid response systems. Jt Comm J Qual Patient Saf. 2008;34:489–495.
- , , , . The medical emergency team. Anaesth Intensive Care. 1995;23(2):183–186.
- , , , et al. Rates of in‐hospital arrests, deaths, and intensive care admission: the effect of a medical emergency team. Med J Aust. 2000;173:236–240.
- , , , , . The patient‐at‐risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54:853–860.
- , , , , . Effects of rapid response systems on clinical outcomes: systemic review and meta‐analysis. J Hosp Med. 2007;2:422–432.
- , , , , . Rapid response teams: a systemic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35(5):1238–1243.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , . Rapid response teams. N Engl J Med. 2011;365:139–146.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , . Impact of an outreach team on re‐admissions to a critical care unit. Anaesthesia. 2003;58:328–332.
- , , , , . Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand. 2004;48:1096–1100.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1017.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Copyright © 2012 Society of Hospital Medicine