User login
Stabilized schizoaffective disorder; later confusion and depression appears
CASE
Disoriented and confused
Mr. D, age 42, presents to our emergency department (ED) accompanied by his family with recent onset of disorientation, confusion, depressive mood with labile affect, sleep disturbances, purposeless movements, and grossly reduced kinetics/verbal output. He has a history of schizoaffective disorder, bipolar type, and recurrent admissions for psychotic mood instability.
A few months earlier, Mr. D was treated at our facility for acute exacerbation of his schizoaffective disorder. He was stabilized and discharged with aripiprazole, 30 mg/d, and mirtazapine, 15 mg/d—he had been taking both medications for some time—and newly started extended-release divalproex, 500 mg in the morning/1000 mg nightly (13.2 mg/kg). His trough valproic acid serum level was 70 µg/mL at discharge. He continued on this medication regimen until he returns to our ED with his family.
Mr. D has several medical problems, such as type 2 diabetes mellitus and hypertension, for which he has been receiving metformin, 1,000 mg/d, lisinopril, 10 mg/d, and simvastatin, 20 mg/d. He has no history of alcohol or substance abuse and does not smoke.
Serum and urine analyses are unremarkable and include finger-stick blood glucose, complete blood count, urinalysis, urine drug screen, comprehensive metabolic panel, magnesium, γ-glutamyl transpeptidase (GGTP), amylase, thyroid-stimulating hormone, and blood alcohol level. Random valproic acid serum level taken in the ED is 64 µg/mL. Non-contrast head CT is interpreted as non-acute. There are no documented abnormal findings during the physical exam.
What could be causing Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected and/or multiple causes
The authors’ observations
The differential diagnosis was broad at the time of Mr. D’s presentation to the ED because his symptoms overlapped across clinical considerations. The initial medical evaluation was negative, which suggested an active primary mental illness. However, Mr. D’s presenting symptoms warranted continued vigilance for concurrent or emergent delirium or catatonia, especially because of the potential morbidity if these conditions are not detected and managed.
EVALUATION
Fluctuating status
Mr. D is admitted to the mental health unit for treatment of presumptive bipolar depression with catatonic features. The initial admitting team continues aripiprazole, increased divalproex extended release to 1,000 mg in the morning/1,500 mg at night, held mirtazapine, and started lorazepam, 2 mg, 3 times daily, for catatonia. Metformin, lisinopril, and simvastatin are continued. Mr. D’s mental status and behavior fluctuates over the next 48 hours prompting the treatment team to consider an emergent delirious process.
On day 3, the primary team assumes care and observes fluctuations in level of arousal with disorientation, inattention, labile affect, disorganized speech and behavior, and responsiveness to internal (visual) stimuli. Finger-stick blood glucose level remains stable. Review of physical symptoms is notable for nausea and examination reveals unsteady gait and asterixis. His family denies that Mr. D used alcohol or drugs before admission. Collateral information from the family and review of Mr. D’s outpatient records is consistent with an acutely fluctuating confusional state that began 10 days before admission.
At this point, what is your differential diagnosis for Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected or multiple causes
TREATMENT
Valproate stopped
Mr. D’s ammonia level is 119 µg/dL (reference range, 15 to 45 μg/dL) on hospital day 3. Divalproex and lorazepam are discontinued, and standing lactulose is started because it is evident that he has active valproate-related hyperammonemic encephalopathy (VHE), also known as delirium due to valproate-related hyperammonemia.
Awake and drowsy EEG within 24 hours reveals “diffuse irregular slow activity” without epileptogenic features. HIV, syphilis, and vitamin B12 and red blood cell folate screening are negative. We confirm that Mr. D is not a vegetarian (dietary carnitine deficiency is a risk factor for VHE). He is not screened for a urea cycle disorder.
The authors’ observations
Divalproex is a commonly used FDA-approved treatment for a variety of neurologic and psychiatric conditions including acute bipolar mania.1-3 It also is used for off-label control of various psychiatric symptoms. It is a stable coordination compound composed of sodium valproate and valproic acid that dissipates into the valproate ion in the gastrointestinal tract.1 (In this article, references to valproate [VPA] include valproic acid and divalproex.) The drug is relatively well-tolerated; however, use may carry teratogenic risk and can adversely impact a variety of body systems, especially hematopoietic, gastrointestinal, and neurologic systems.1-3 Adverse effects can be idiosyncratic or in part related to VPA serum levels.1,4 VPA toxicity increases the likelihood of some adverse health outcomes, such as nausea, diarrhea, and tremors.1
Identifying and treating VHE
Asymptomatic elevations in ammonia without evidence of hepatic injury are common, might be related to valproic serum levels, and may occur in up to one-half of psychiatric patients receiving VPA.2-4 In contrast, VHE is a rare and potentially lethal idiosyncratic event unrelated to duration of VPA treatment, dosage, or valproic serum level.2-4 In addition, prior safe use might not protect against future VHE.3,4
VHE presents as delirium with characteristic acute changes in mental status, including alterations in cognition or level of consciousness ranging from lethargy to coma, along with possible focal neurological findings or vomiting.1,3,4 Although more common among patients with a seizure disorder, VHE also might be associated with new seizure activity in patients who do not have a seizure disorder.5
Although symptomatically acute in onset, emergence is unpredictable and can occur within days or up to years of use with therapeutic VPA dosing and valproic serum levels.2,4 Complicating identification, laboratory transaminase or ammonia elevations may or may not be present2-4; however, VHE typically occurs in the setting of hyperammonemia and normal transaminase levels.2 Reversible EEG findings are nonspecific2 and could show generalized slowing with occasional bursts of frontal intermittent rhythmic delta activity and triphasic waves.2,4
Pathophysiological descriptions of emergent VHE have been hypothesized,2-4 but the definitive causal mechanism remains unclear.6 Published VHE risk factors2-6 include:
- polypharmacy (especially anti-convulsants)
- inherited or dietary-based carnitine deficiency
- urea cycle disorders
- mental retardation.
How would you treat VHE?
a) cholinesterase inhibitors
b) antipsychotic therapy
c) supportive care
d) ammonia-reducing agents such as lactulose, carnitine, and neomycin
e) discontinue valproate
Outcome Normalized ammonia
Four days after discontinuing divalproex and starting lactulose, Mr. D’s fluctuating level of arousal, orientation, attention, and perceptual disturbances resolve along with restoration of environmental relatedness in setting of normalized ammonia level to 39 µg/dL. He is euthymic, non-psychotic, and without cognitive impairment at time of discharge. An “allergy” to divalproex is entered in his electronic medical record in an effort to discourage future retrial.
The authors’ observations
Once identified, management of VHE invariably includes consideration for discontinuation of valproate1,2,4,19; other adjunctive, expediting, ammonia-reducing strategies, including lactulose and carnitine, have also been described.2,4,5,20 Although lactulose is more commonly used, carnitine supplementation might be associated with a preferable dosing schedule and drug interaction and side-effect profile.20 Rapidly deteriorating clinical status could indicate hemodialysis.4
Of critical importance, these management strategies rely on awareness of and prompt identification of the condition, which includes an ability to distinguish emergent VHE from the mental illness VPA is used to treat.
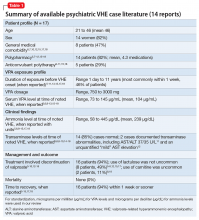
Stopping the offending agent generally results in complete recovery in VHE patients with psychiatric illness.4 Most (>90%, n = 31) psychiatric patients in our and prior5 case series reviews recovered within 2 weeks of intervention.5 Cautious resumption of divalproex could be considered if there is a compelling clinical indication and you suspect that a putative polypharmacy agent such as topiramate has been removed; otherwise future retrial of VPA should be avoided.14
Mr. D’s case was consistent with a valproate-related hyperammonemic delirious event. He had preadmission acute onset, intra-daily fluctuating confusion, and visual perceptual disturbances with nausea, asterixis, gait disturbance, elevated ammonia, and a supportive EEG months after starting divalproex. Similar to our case, some challenging aspects of identifying emergent VHE include:
- earlier safe use of divalproex over extended periods
- lack of elevated VPA serum level
- lack of transaminase elevation
- lack of apparent risk factors
- presence of background serious mental illness, which can distract from VHE detection via misattribution to uncontrolled primary mental illness.
This last point is critical because it can delay VHE identification and treatment or worse, result in misdiagnosis with accompanying continuation or escalation of VPA dosing as has initially occurred in Mr. D’s case. Similar concerns have been raised2,5 and occurred,5,19 which is not surprising given the frequency of VPA use for psychiatric conditions and symptoms.
Providers should have a low threshold for checking an ammonia level in clinical scenarios that involve any alteration in mental status that may resemble delirium in psychiatric patients treated with valproate. From a preventative perspective, it may be prudent to avoid valproate in psychiatric patients with known VHE risk factors. Either way, promotion of VHE awareness and detection across medical disciplines is paramount.
1. Depakote [package insert]. Chicago, IL: AbbVie; 2016.
2. Lewis C, Deshpande A, Tesar G, et al. Valproate-induced hyperammonemic encephalopathy: a brief review. Curr Med Res Opin. 2012;28(6):1039-1042.
3. Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46(15):1323-1338.
4. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
5. Carr RB, Shrewsbury K. Hyperammonemia due to valproic acid in the psychiatric setting. Am J Psychiatry. 2007;164(7):1020-1027.
6. Hung C, Li T, Wei I, et al. The real mechanism of VPA-induced hyperammonemia remains unknown. Gen Hosp Psychiatry. 2011;33(1):84.e3-84.e4.
7. Starer J, Chang G. Hyperammonemic encephalopathy, valproic acid, and benzodiazepine withdrawal: a case series. Am J Drug Alcohol Abuse. 2010;36(2):98-101.
8. Eubanks AL, Aguirre B, Bourgeois JA. Severe acute hyperammonemia after brief exposure to valproate. Psychosomatics. 2008;49(1):82-83.
9. Fan CC, Huang MC, Liu HC. Lamotrigine might potentiate valproic acid-induced hyperammonemic encephalopathy. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1747-1748.
10. Deutsch SI, Burket JA, Rosse RB. Valproate-induced hyperammonemic encephalopathy and normal liver functions: possible synergism with topiramate. Clin Neuropharmacol. 2009;32(6):350-352.
11. Rodrigues-Silva N, Venâncio Ä, Bouça J. Risperidone, a risk for valproate induced encephalopathy? Gen Hosp Psychiatry. 2013;35(4):452.e5-452.e6.
12. Sunkavalli KK, Iqbal FM, Singh B, et al. Valproate-induced hyperammonemic encephalopathy: a case report and brief review of the literature. Am J Ther. 2013;20(5):569-571.
13. Abreu LN, Issler C, Lafer B. Valproate-induced reversible pseudoatrophy of the brain and hyperammonemic encephalopathy in a bipolar patient. Aust N Z J Psychiatry. 2009;43(5):484-485.
14. Hong L, Schutz J, Nance M. A case of valproate-induced encephalopathy. Aust N Z J Psychiatry. 2012;46(12):1200-1201.
15. Kimmel RJ, Irwin SA, Meyer JM. Valproic acid-associated hyperammonemic encephalopathy: a case report from the psychiatric setting. Int Clin Psychopharmacol. 2005;20(1):57-58.
16. Elgudin L, Hall Y, Schubert D. Ammonia induced encephalopathy from valproic acid in a bipolar patient: case report. Int J Psychiatry Med. 2003;33(1):91-96.
17. Stewart JT. A case of hyperammonemic encephalopathy after 11 years of valproate therapy. J Clin Psychopharmacol. 2008;28(3):361-362.
18. Wadzinski J, Franks R, Roane D, et al. Valproate-associated hyperammonemic encephalopathy. J Am Board Fam Med. 2007;20(5):499-502.
19. Chang M, Tang X, Wen S, et al. Valproate (VPA)-associated hyperammonemic encephalopathy independent of elevated serum VPA levels: 21 cases in China from May 2000 to May 2012. Compr Psychiatry. 2013;54(5):562-567.
20. Sonik P, Hilty DM, Rossaro L, et al. Carnitine supplementation for valproate-related hyperammonemia to maintain therapeutic valproate level. J Clin Psychopharmacol. 2011;31(5):680-682.
CASE
Disoriented and confused
Mr. D, age 42, presents to our emergency department (ED) accompanied by his family with recent onset of disorientation, confusion, depressive mood with labile affect, sleep disturbances, purposeless movements, and grossly reduced kinetics/verbal output. He has a history of schizoaffective disorder, bipolar type, and recurrent admissions for psychotic mood instability.
A few months earlier, Mr. D was treated at our facility for acute exacerbation of his schizoaffective disorder. He was stabilized and discharged with aripiprazole, 30 mg/d, and mirtazapine, 15 mg/d—he had been taking both medications for some time—and newly started extended-release divalproex, 500 mg in the morning/1000 mg nightly (13.2 mg/kg). His trough valproic acid serum level was 70 µg/mL at discharge. He continued on this medication regimen until he returns to our ED with his family.
Mr. D has several medical problems, such as type 2 diabetes mellitus and hypertension, for which he has been receiving metformin, 1,000 mg/d, lisinopril, 10 mg/d, and simvastatin, 20 mg/d. He has no history of alcohol or substance abuse and does not smoke.
Serum and urine analyses are unremarkable and include finger-stick blood glucose, complete blood count, urinalysis, urine drug screen, comprehensive metabolic panel, magnesium, γ-glutamyl transpeptidase (GGTP), amylase, thyroid-stimulating hormone, and blood alcohol level. Random valproic acid serum level taken in the ED is 64 µg/mL. Non-contrast head CT is interpreted as non-acute. There are no documented abnormal findings during the physical exam.
What could be causing Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected and/or multiple causes
The authors’ observations
The differential diagnosis was broad at the time of Mr. D’s presentation to the ED because his symptoms overlapped across clinical considerations. The initial medical evaluation was negative, which suggested an active primary mental illness. However, Mr. D’s presenting symptoms warranted continued vigilance for concurrent or emergent delirium or catatonia, especially because of the potential morbidity if these conditions are not detected and managed.
EVALUATION
Fluctuating status
Mr. D is admitted to the mental health unit for treatment of presumptive bipolar depression with catatonic features. The initial admitting team continues aripiprazole, increased divalproex extended release to 1,000 mg in the morning/1,500 mg at night, held mirtazapine, and started lorazepam, 2 mg, 3 times daily, for catatonia. Metformin, lisinopril, and simvastatin are continued. Mr. D’s mental status and behavior fluctuates over the next 48 hours prompting the treatment team to consider an emergent delirious process.
On day 3, the primary team assumes care and observes fluctuations in level of arousal with disorientation, inattention, labile affect, disorganized speech and behavior, and responsiveness to internal (visual) stimuli. Finger-stick blood glucose level remains stable. Review of physical symptoms is notable for nausea and examination reveals unsteady gait and asterixis. His family denies that Mr. D used alcohol or drugs before admission. Collateral information from the family and review of Mr. D’s outpatient records is consistent with an acutely fluctuating confusional state that began 10 days before admission.
At this point, what is your differential diagnosis for Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected or multiple causes
TREATMENT
Valproate stopped
Mr. D’s ammonia level is 119 µg/dL (reference range, 15 to 45 μg/dL) on hospital day 3. Divalproex and lorazepam are discontinued, and standing lactulose is started because it is evident that he has active valproate-related hyperammonemic encephalopathy (VHE), also known as delirium due to valproate-related hyperammonemia.
Awake and drowsy EEG within 24 hours reveals “diffuse irregular slow activity” without epileptogenic features. HIV, syphilis, and vitamin B12 and red blood cell folate screening are negative. We confirm that Mr. D is not a vegetarian (dietary carnitine deficiency is a risk factor for VHE). He is not screened for a urea cycle disorder.
The authors’ observations
Divalproex is a commonly used FDA-approved treatment for a variety of neurologic and psychiatric conditions including acute bipolar mania.1-3 It also is used for off-label control of various psychiatric symptoms. It is a stable coordination compound composed of sodium valproate and valproic acid that dissipates into the valproate ion in the gastrointestinal tract.1 (In this article, references to valproate [VPA] include valproic acid and divalproex.) The drug is relatively well-tolerated; however, use may carry teratogenic risk and can adversely impact a variety of body systems, especially hematopoietic, gastrointestinal, and neurologic systems.1-3 Adverse effects can be idiosyncratic or in part related to VPA serum levels.1,4 VPA toxicity increases the likelihood of some adverse health outcomes, such as nausea, diarrhea, and tremors.1
Identifying and treating VHE
Asymptomatic elevations in ammonia without evidence of hepatic injury are common, might be related to valproic serum levels, and may occur in up to one-half of psychiatric patients receiving VPA.2-4 In contrast, VHE is a rare and potentially lethal idiosyncratic event unrelated to duration of VPA treatment, dosage, or valproic serum level.2-4 In addition, prior safe use might not protect against future VHE.3,4
VHE presents as delirium with characteristic acute changes in mental status, including alterations in cognition or level of consciousness ranging from lethargy to coma, along with possible focal neurological findings or vomiting.1,3,4 Although more common among patients with a seizure disorder, VHE also might be associated with new seizure activity in patients who do not have a seizure disorder.5
Although symptomatically acute in onset, emergence is unpredictable and can occur within days or up to years of use with therapeutic VPA dosing and valproic serum levels.2,4 Complicating identification, laboratory transaminase or ammonia elevations may or may not be present2-4; however, VHE typically occurs in the setting of hyperammonemia and normal transaminase levels.2 Reversible EEG findings are nonspecific2 and could show generalized slowing with occasional bursts of frontal intermittent rhythmic delta activity and triphasic waves.2,4
Pathophysiological descriptions of emergent VHE have been hypothesized,2-4 but the definitive causal mechanism remains unclear.6 Published VHE risk factors2-6 include:
- polypharmacy (especially anti-convulsants)
- inherited or dietary-based carnitine deficiency
- urea cycle disorders
- mental retardation.
How would you treat VHE?
a) cholinesterase inhibitors
b) antipsychotic therapy
c) supportive care
d) ammonia-reducing agents such as lactulose, carnitine, and neomycin
e) discontinue valproate
Outcome Normalized ammonia
Four days after discontinuing divalproex and starting lactulose, Mr. D’s fluctuating level of arousal, orientation, attention, and perceptual disturbances resolve along with restoration of environmental relatedness in setting of normalized ammonia level to 39 µg/dL. He is euthymic, non-psychotic, and without cognitive impairment at time of discharge. An “allergy” to divalproex is entered in his electronic medical record in an effort to discourage future retrial.
The authors’ observations
Once identified, management of VHE invariably includes consideration for discontinuation of valproate1,2,4,19; other adjunctive, expediting, ammonia-reducing strategies, including lactulose and carnitine, have also been described.2,4,5,20 Although lactulose is more commonly used, carnitine supplementation might be associated with a preferable dosing schedule and drug interaction and side-effect profile.20 Rapidly deteriorating clinical status could indicate hemodialysis.4
Of critical importance, these management strategies rely on awareness of and prompt identification of the condition, which includes an ability to distinguish emergent VHE from the mental illness VPA is used to treat.
Stopping the offending agent generally results in complete recovery in VHE patients with psychiatric illness.4 Most (>90%, n = 31) psychiatric patients in our and prior5 case series reviews recovered within 2 weeks of intervention.5 Cautious resumption of divalproex could be considered if there is a compelling clinical indication and you suspect that a putative polypharmacy agent such as topiramate has been removed; otherwise future retrial of VPA should be avoided.14
Mr. D’s case was consistent with a valproate-related hyperammonemic delirious event. He had preadmission acute onset, intra-daily fluctuating confusion, and visual perceptual disturbances with nausea, asterixis, gait disturbance, elevated ammonia, and a supportive EEG months after starting divalproex. Similar to our case, some challenging aspects of identifying emergent VHE include:
- earlier safe use of divalproex over extended periods
- lack of elevated VPA serum level
- lack of transaminase elevation
- lack of apparent risk factors
- presence of background serious mental illness, which can distract from VHE detection via misattribution to uncontrolled primary mental illness.
This last point is critical because it can delay VHE identification and treatment or worse, result in misdiagnosis with accompanying continuation or escalation of VPA dosing as has initially occurred in Mr. D’s case. Similar concerns have been raised2,5 and occurred,5,19 which is not surprising given the frequency of VPA use for psychiatric conditions and symptoms.
Providers should have a low threshold for checking an ammonia level in clinical scenarios that involve any alteration in mental status that may resemble delirium in psychiatric patients treated with valproate. From a preventative perspective, it may be prudent to avoid valproate in psychiatric patients with known VHE risk factors. Either way, promotion of VHE awareness and detection across medical disciplines is paramount.
CASE
Disoriented and confused
Mr. D, age 42, presents to our emergency department (ED) accompanied by his family with recent onset of disorientation, confusion, depressive mood with labile affect, sleep disturbances, purposeless movements, and grossly reduced kinetics/verbal output. He has a history of schizoaffective disorder, bipolar type, and recurrent admissions for psychotic mood instability.
A few months earlier, Mr. D was treated at our facility for acute exacerbation of his schizoaffective disorder. He was stabilized and discharged with aripiprazole, 30 mg/d, and mirtazapine, 15 mg/d—he had been taking both medications for some time—and newly started extended-release divalproex, 500 mg in the morning/1000 mg nightly (13.2 mg/kg). His trough valproic acid serum level was 70 µg/mL at discharge. He continued on this medication regimen until he returns to our ED with his family.
Mr. D has several medical problems, such as type 2 diabetes mellitus and hypertension, for which he has been receiving metformin, 1,000 mg/d, lisinopril, 10 mg/d, and simvastatin, 20 mg/d. He has no history of alcohol or substance abuse and does not smoke.
Serum and urine analyses are unremarkable and include finger-stick blood glucose, complete blood count, urinalysis, urine drug screen, comprehensive metabolic panel, magnesium, γ-glutamyl transpeptidase (GGTP), amylase, thyroid-stimulating hormone, and blood alcohol level. Random valproic acid serum level taken in the ED is 64 µg/mL. Non-contrast head CT is interpreted as non-acute. There are no documented abnormal findings during the physical exam.
What could be causing Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected and/or multiple causes
The authors’ observations
The differential diagnosis was broad at the time of Mr. D’s presentation to the ED because his symptoms overlapped across clinical considerations. The initial medical evaluation was negative, which suggested an active primary mental illness. However, Mr. D’s presenting symptoms warranted continued vigilance for concurrent or emergent delirium or catatonia, especially because of the potential morbidity if these conditions are not detected and managed.
EVALUATION
Fluctuating status
Mr. D is admitted to the mental health unit for treatment of presumptive bipolar depression with catatonic features. The initial admitting team continues aripiprazole, increased divalproex extended release to 1,000 mg in the morning/1,500 mg at night, held mirtazapine, and started lorazepam, 2 mg, 3 times daily, for catatonia. Metformin, lisinopril, and simvastatin are continued. Mr. D’s mental status and behavior fluctuates over the next 48 hours prompting the treatment team to consider an emergent delirious process.
On day 3, the primary team assumes care and observes fluctuations in level of arousal with disorientation, inattention, labile affect, disorganized speech and behavior, and responsiveness to internal (visual) stimuli. Finger-stick blood glucose level remains stable. Review of physical symptoms is notable for nausea and examination reveals unsteady gait and asterixis. His family denies that Mr. D used alcohol or drugs before admission. Collateral information from the family and review of Mr. D’s outpatient records is consistent with an acutely fluctuating confusional state that began 10 days before admission.
At this point, what is your differential diagnosis for Mr. D’s altered mental status?
a) symptoms of a medical illness
b) medication, undetected substance intoxication, or withdrawal-related symptoms
c) acute exacerbation of schizoaffective disorder
d) delirium
e) catatonia of undetected or multiple causes
TREATMENT
Valproate stopped
Mr. D’s ammonia level is 119 µg/dL (reference range, 15 to 45 μg/dL) on hospital day 3. Divalproex and lorazepam are discontinued, and standing lactulose is started because it is evident that he has active valproate-related hyperammonemic encephalopathy (VHE), also known as delirium due to valproate-related hyperammonemia.
Awake and drowsy EEG within 24 hours reveals “diffuse irregular slow activity” without epileptogenic features. HIV, syphilis, and vitamin B12 and red blood cell folate screening are negative. We confirm that Mr. D is not a vegetarian (dietary carnitine deficiency is a risk factor for VHE). He is not screened for a urea cycle disorder.
The authors’ observations
Divalproex is a commonly used FDA-approved treatment for a variety of neurologic and psychiatric conditions including acute bipolar mania.1-3 It also is used for off-label control of various psychiatric symptoms. It is a stable coordination compound composed of sodium valproate and valproic acid that dissipates into the valproate ion in the gastrointestinal tract.1 (In this article, references to valproate [VPA] include valproic acid and divalproex.) The drug is relatively well-tolerated; however, use may carry teratogenic risk and can adversely impact a variety of body systems, especially hematopoietic, gastrointestinal, and neurologic systems.1-3 Adverse effects can be idiosyncratic or in part related to VPA serum levels.1,4 VPA toxicity increases the likelihood of some adverse health outcomes, such as nausea, diarrhea, and tremors.1
Identifying and treating VHE
Asymptomatic elevations in ammonia without evidence of hepatic injury are common, might be related to valproic serum levels, and may occur in up to one-half of psychiatric patients receiving VPA.2-4 In contrast, VHE is a rare and potentially lethal idiosyncratic event unrelated to duration of VPA treatment, dosage, or valproic serum level.2-4 In addition, prior safe use might not protect against future VHE.3,4
VHE presents as delirium with characteristic acute changes in mental status, including alterations in cognition or level of consciousness ranging from lethargy to coma, along with possible focal neurological findings or vomiting.1,3,4 Although more common among patients with a seizure disorder, VHE also might be associated with new seizure activity in patients who do not have a seizure disorder.5
Although symptomatically acute in onset, emergence is unpredictable and can occur within days or up to years of use with therapeutic VPA dosing and valproic serum levels.2,4 Complicating identification, laboratory transaminase or ammonia elevations may or may not be present2-4; however, VHE typically occurs in the setting of hyperammonemia and normal transaminase levels.2 Reversible EEG findings are nonspecific2 and could show generalized slowing with occasional bursts of frontal intermittent rhythmic delta activity and triphasic waves.2,4
Pathophysiological descriptions of emergent VHE have been hypothesized,2-4 but the definitive causal mechanism remains unclear.6 Published VHE risk factors2-6 include:
- polypharmacy (especially anti-convulsants)
- inherited or dietary-based carnitine deficiency
- urea cycle disorders
- mental retardation.
How would you treat VHE?
a) cholinesterase inhibitors
b) antipsychotic therapy
c) supportive care
d) ammonia-reducing agents such as lactulose, carnitine, and neomycin
e) discontinue valproate
Outcome Normalized ammonia
Four days after discontinuing divalproex and starting lactulose, Mr. D’s fluctuating level of arousal, orientation, attention, and perceptual disturbances resolve along with restoration of environmental relatedness in setting of normalized ammonia level to 39 µg/dL. He is euthymic, non-psychotic, and without cognitive impairment at time of discharge. An “allergy” to divalproex is entered in his electronic medical record in an effort to discourage future retrial.
The authors’ observations
Once identified, management of VHE invariably includes consideration for discontinuation of valproate1,2,4,19; other adjunctive, expediting, ammonia-reducing strategies, including lactulose and carnitine, have also been described.2,4,5,20 Although lactulose is more commonly used, carnitine supplementation might be associated with a preferable dosing schedule and drug interaction and side-effect profile.20 Rapidly deteriorating clinical status could indicate hemodialysis.4
Of critical importance, these management strategies rely on awareness of and prompt identification of the condition, which includes an ability to distinguish emergent VHE from the mental illness VPA is used to treat.
Stopping the offending agent generally results in complete recovery in VHE patients with psychiatric illness.4 Most (>90%, n = 31) psychiatric patients in our and prior5 case series reviews recovered within 2 weeks of intervention.5 Cautious resumption of divalproex could be considered if there is a compelling clinical indication and you suspect that a putative polypharmacy agent such as topiramate has been removed; otherwise future retrial of VPA should be avoided.14
Mr. D’s case was consistent with a valproate-related hyperammonemic delirious event. He had preadmission acute onset, intra-daily fluctuating confusion, and visual perceptual disturbances with nausea, asterixis, gait disturbance, elevated ammonia, and a supportive EEG months after starting divalproex. Similar to our case, some challenging aspects of identifying emergent VHE include:
- earlier safe use of divalproex over extended periods
- lack of elevated VPA serum level
- lack of transaminase elevation
- lack of apparent risk factors
- presence of background serious mental illness, which can distract from VHE detection via misattribution to uncontrolled primary mental illness.
This last point is critical because it can delay VHE identification and treatment or worse, result in misdiagnosis with accompanying continuation or escalation of VPA dosing as has initially occurred in Mr. D’s case. Similar concerns have been raised2,5 and occurred,5,19 which is not surprising given the frequency of VPA use for psychiatric conditions and symptoms.
Providers should have a low threshold for checking an ammonia level in clinical scenarios that involve any alteration in mental status that may resemble delirium in psychiatric patients treated with valproate. From a preventative perspective, it may be prudent to avoid valproate in psychiatric patients with known VHE risk factors. Either way, promotion of VHE awareness and detection across medical disciplines is paramount.
1. Depakote [package insert]. Chicago, IL: AbbVie; 2016.
2. Lewis C, Deshpande A, Tesar G, et al. Valproate-induced hyperammonemic encephalopathy: a brief review. Curr Med Res Opin. 2012;28(6):1039-1042.
3. Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46(15):1323-1338.
4. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
5. Carr RB, Shrewsbury K. Hyperammonemia due to valproic acid in the psychiatric setting. Am J Psychiatry. 2007;164(7):1020-1027.
6. Hung C, Li T, Wei I, et al. The real mechanism of VPA-induced hyperammonemia remains unknown. Gen Hosp Psychiatry. 2011;33(1):84.e3-84.e4.
7. Starer J, Chang G. Hyperammonemic encephalopathy, valproic acid, and benzodiazepine withdrawal: a case series. Am J Drug Alcohol Abuse. 2010;36(2):98-101.
8. Eubanks AL, Aguirre B, Bourgeois JA. Severe acute hyperammonemia after brief exposure to valproate. Psychosomatics. 2008;49(1):82-83.
9. Fan CC, Huang MC, Liu HC. Lamotrigine might potentiate valproic acid-induced hyperammonemic encephalopathy. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1747-1748.
10. Deutsch SI, Burket JA, Rosse RB. Valproate-induced hyperammonemic encephalopathy and normal liver functions: possible synergism with topiramate. Clin Neuropharmacol. 2009;32(6):350-352.
11. Rodrigues-Silva N, Venâncio Ä, Bouça J. Risperidone, a risk for valproate induced encephalopathy? Gen Hosp Psychiatry. 2013;35(4):452.e5-452.e6.
12. Sunkavalli KK, Iqbal FM, Singh B, et al. Valproate-induced hyperammonemic encephalopathy: a case report and brief review of the literature. Am J Ther. 2013;20(5):569-571.
13. Abreu LN, Issler C, Lafer B. Valproate-induced reversible pseudoatrophy of the brain and hyperammonemic encephalopathy in a bipolar patient. Aust N Z J Psychiatry. 2009;43(5):484-485.
14. Hong L, Schutz J, Nance M. A case of valproate-induced encephalopathy. Aust N Z J Psychiatry. 2012;46(12):1200-1201.
15. Kimmel RJ, Irwin SA, Meyer JM. Valproic acid-associated hyperammonemic encephalopathy: a case report from the psychiatric setting. Int Clin Psychopharmacol. 2005;20(1):57-58.
16. Elgudin L, Hall Y, Schubert D. Ammonia induced encephalopathy from valproic acid in a bipolar patient: case report. Int J Psychiatry Med. 2003;33(1):91-96.
17. Stewart JT. A case of hyperammonemic encephalopathy after 11 years of valproate therapy. J Clin Psychopharmacol. 2008;28(3):361-362.
18. Wadzinski J, Franks R, Roane D, et al. Valproate-associated hyperammonemic encephalopathy. J Am Board Fam Med. 2007;20(5):499-502.
19. Chang M, Tang X, Wen S, et al. Valproate (VPA)-associated hyperammonemic encephalopathy independent of elevated serum VPA levels: 21 cases in China from May 2000 to May 2012. Compr Psychiatry. 2013;54(5):562-567.
20. Sonik P, Hilty DM, Rossaro L, et al. Carnitine supplementation for valproate-related hyperammonemia to maintain therapeutic valproate level. J Clin Psychopharmacol. 2011;31(5):680-682.
1. Depakote [package insert]. Chicago, IL: AbbVie; 2016.
2. Lewis C, Deshpande A, Tesar G, et al. Valproate-induced hyperammonemic encephalopathy: a brief review. Curr Med Res Opin. 2012;28(6):1039-1042.
3. Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46(15):1323-1338.
4. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
5. Carr RB, Shrewsbury K. Hyperammonemia due to valproic acid in the psychiatric setting. Am J Psychiatry. 2007;164(7):1020-1027.
6. Hung C, Li T, Wei I, et al. The real mechanism of VPA-induced hyperammonemia remains unknown. Gen Hosp Psychiatry. 2011;33(1):84.e3-84.e4.
7. Starer J, Chang G. Hyperammonemic encephalopathy, valproic acid, and benzodiazepine withdrawal: a case series. Am J Drug Alcohol Abuse. 2010;36(2):98-101.
8. Eubanks AL, Aguirre B, Bourgeois JA. Severe acute hyperammonemia after brief exposure to valproate. Psychosomatics. 2008;49(1):82-83.
9. Fan CC, Huang MC, Liu HC. Lamotrigine might potentiate valproic acid-induced hyperammonemic encephalopathy. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1747-1748.
10. Deutsch SI, Burket JA, Rosse RB. Valproate-induced hyperammonemic encephalopathy and normal liver functions: possible synergism with topiramate. Clin Neuropharmacol. 2009;32(6):350-352.
11. Rodrigues-Silva N, Venâncio Ä, Bouça J. Risperidone, a risk for valproate induced encephalopathy? Gen Hosp Psychiatry. 2013;35(4):452.e5-452.e6.
12. Sunkavalli KK, Iqbal FM, Singh B, et al. Valproate-induced hyperammonemic encephalopathy: a case report and brief review of the literature. Am J Ther. 2013;20(5):569-571.
13. Abreu LN, Issler C, Lafer B. Valproate-induced reversible pseudoatrophy of the brain and hyperammonemic encephalopathy in a bipolar patient. Aust N Z J Psychiatry. 2009;43(5):484-485.
14. Hong L, Schutz J, Nance M. A case of valproate-induced encephalopathy. Aust N Z J Psychiatry. 2012;46(12):1200-1201.
15. Kimmel RJ, Irwin SA, Meyer JM. Valproic acid-associated hyperammonemic encephalopathy: a case report from the psychiatric setting. Int Clin Psychopharmacol. 2005;20(1):57-58.
16. Elgudin L, Hall Y, Schubert D. Ammonia induced encephalopathy from valproic acid in a bipolar patient: case report. Int J Psychiatry Med. 2003;33(1):91-96.
17. Stewart JT. A case of hyperammonemic encephalopathy after 11 years of valproate therapy. J Clin Psychopharmacol. 2008;28(3):361-362.
18. Wadzinski J, Franks R, Roane D, et al. Valproate-associated hyperammonemic encephalopathy. J Am Board Fam Med. 2007;20(5):499-502.
19. Chang M, Tang X, Wen S, et al. Valproate (VPA)-associated hyperammonemic encephalopathy independent of elevated serum VPA levels: 21 cases in China from May 2000 to May 2012. Compr Psychiatry. 2013;54(5):562-567.
20. Sonik P, Hilty DM, Rossaro L, et al. Carnitine supplementation for valproate-related hyperammonemia to maintain therapeutic valproate level. J Clin Psychopharmacol. 2011;31(5):680-682.