User login
In Reference to: “Cost and Utility of Thrombophilia Testing”
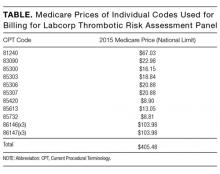
The article by Petrilli et al. points to the important but complicated issue of ordering laboratory testing for thrombophilia despite multiple guidelines that dispute the clinical utility of such testing for many indications.1 We question the basis of these authors’ assertion that Medicare spends $300 to $672 million for thrombophilia testing annually. They arrived at this figure by multiplying the price of a thrombophilia test panel (between $1100 and $2400) by the number of annual Medicare claims for thrombophilia analysis, which they estimated at 280,000. The price of the panel is derived from two papers: (1) a 2001 review2 that lists prices of various thrombophilia-related tests adding up to $1782, and (2) a 2006 evaluation by Somma et al.3 of thrombophilia screening at one hospital in New York in 2005. The latter paper refers to various thrombophilia panels from Quest Diagnostics with list prices ranging from $1311 to $2429. However, the repertoire of available test panels and their prices have changed over the last decade. The cost evaluation of thrombophilia testing should be based on actual current payments for tests, and not on list prices for laboratory offerings from over a decade ago. Several laboratories offer mutational analysis of 3 genes—F5, F2, and MTHFR—as a thrombophilia risk panel. Based on the Current Procedural Terminology (CPT) codes listed by the test suppliers (81240, 81241, and 81291), the average Medicare payment for the combination of these 3 markers in 2013 was $172.4 A broader panel of several biochemical, immunological, and genetic assays had a maximum Medicare payment in 2015 of $405 (Table).5
In conclusion, the cost evaluation of thrombophilia screening is more challenging than the calculation by Petrilli et al. suggests.1 Even if Medicare paid as much as $400 per individual tested and assuming up to 200,000 individuals underwent thrombophilia testing per year, the aggregate Medicare expenditure would have been no more than roughly $80 million. Thus, the estimated range in the article appears to have overstated actual Medicare expenditures by an order of magnitude. This does not take away from their overall conclusion that payers are burdened with significant expenditures for laboratory testing that may not present clinical value for many patients.6 We need research into the patterns of utilization as well as improvements in documentation of expenditures associated with these tests.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the United States government. The autho
1. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
2. Abramson N, Abramson S. Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013-1020. PubMed
3. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
4. Lynch JA, Berse B, Dotson WD, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: Analysis of gene-specific billing in Medicare claims data [Published online ahead of print January 26, 2017]. Genet Med. 2017. doi: 10.1038/gim.2016.209. PubMed
5. Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule 2016. https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html. Accessed on December 20, 2016.
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
The article by Petrilli et al. points to the important but complicated issue of ordering laboratory testing for thrombophilia despite multiple guidelines that dispute the clinical utility of such testing for many indications.1 We question the basis of these authors’ assertion that Medicare spends $300 to $672 million for thrombophilia testing annually. They arrived at this figure by multiplying the price of a thrombophilia test panel (between $1100 and $2400) by the number of annual Medicare claims for thrombophilia analysis, which they estimated at 280,000. The price of the panel is derived from two papers: (1) a 2001 review2 that lists prices of various thrombophilia-related tests adding up to $1782, and (2) a 2006 evaluation by Somma et al.3 of thrombophilia screening at one hospital in New York in 2005. The latter paper refers to various thrombophilia panels from Quest Diagnostics with list prices ranging from $1311 to $2429. However, the repertoire of available test panels and their prices have changed over the last decade. The cost evaluation of thrombophilia testing should be based on actual current payments for tests, and not on list prices for laboratory offerings from over a decade ago. Several laboratories offer mutational analysis of 3 genes—F5, F2, and MTHFR—as a thrombophilia risk panel. Based on the Current Procedural Terminology (CPT) codes listed by the test suppliers (81240, 81241, and 81291), the average Medicare payment for the combination of these 3 markers in 2013 was $172.4 A broader panel of several biochemical, immunological, and genetic assays had a maximum Medicare payment in 2015 of $405 (Table).5
In conclusion, the cost evaluation of thrombophilia screening is more challenging than the calculation by Petrilli et al. suggests.1 Even if Medicare paid as much as $400 per individual tested and assuming up to 200,000 individuals underwent thrombophilia testing per year, the aggregate Medicare expenditure would have been no more than roughly $80 million. Thus, the estimated range in the article appears to have overstated actual Medicare expenditures by an order of magnitude. This does not take away from their overall conclusion that payers are burdened with significant expenditures for laboratory testing that may not present clinical value for many patients.6 We need research into the patterns of utilization as well as improvements in documentation of expenditures associated with these tests.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the United States government. The autho
The article by Petrilli et al. points to the important but complicated issue of ordering laboratory testing for thrombophilia despite multiple guidelines that dispute the clinical utility of such testing for many indications.1 We question the basis of these authors’ assertion that Medicare spends $300 to $672 million for thrombophilia testing annually. They arrived at this figure by multiplying the price of a thrombophilia test panel (between $1100 and $2400) by the number of annual Medicare claims for thrombophilia analysis, which they estimated at 280,000. The price of the panel is derived from two papers: (1) a 2001 review2 that lists prices of various thrombophilia-related tests adding up to $1782, and (2) a 2006 evaluation by Somma et al.3 of thrombophilia screening at one hospital in New York in 2005. The latter paper refers to various thrombophilia panels from Quest Diagnostics with list prices ranging from $1311 to $2429. However, the repertoire of available test panels and their prices have changed over the last decade. The cost evaluation of thrombophilia testing should be based on actual current payments for tests, and not on list prices for laboratory offerings from over a decade ago. Several laboratories offer mutational analysis of 3 genes—F5, F2, and MTHFR—as a thrombophilia risk panel. Based on the Current Procedural Terminology (CPT) codes listed by the test suppliers (81240, 81241, and 81291), the average Medicare payment for the combination of these 3 markers in 2013 was $172.4 A broader panel of several biochemical, immunological, and genetic assays had a maximum Medicare payment in 2015 of $405 (Table).5
In conclusion, the cost evaluation of thrombophilia screening is more challenging than the calculation by Petrilli et al. suggests.1 Even if Medicare paid as much as $400 per individual tested and assuming up to 200,000 individuals underwent thrombophilia testing per year, the aggregate Medicare expenditure would have been no more than roughly $80 million. Thus, the estimated range in the article appears to have overstated actual Medicare expenditures by an order of magnitude. This does not take away from their overall conclusion that payers are burdened with significant expenditures for laboratory testing that may not present clinical value for many patients.6 We need research into the patterns of utilization as well as improvements in documentation of expenditures associated with these tests.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the United States government. The autho
1. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
2. Abramson N, Abramson S. Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013-1020. PubMed
3. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
4. Lynch JA, Berse B, Dotson WD, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: Analysis of gene-specific billing in Medicare claims data [Published online ahead of print January 26, 2017]. Genet Med. 2017. doi: 10.1038/gim.2016.209. PubMed
5. Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule 2016. https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html. Accessed on December 20, 2016.
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
1. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
2. Abramson N, Abramson S. Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013-1020. PubMed
3. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
4. Lynch JA, Berse B, Dotson WD, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: Analysis of gene-specific billing in Medicare claims data [Published online ahead of print January 26, 2017]. Genet Med. 2017. doi: 10.1038/gim.2016.209. PubMed
5. Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule 2016. https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html. Accessed on December 20, 2016.
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
© 2017 Society of Hospital Medicine
Caring for Patients With Prostate Cancer Who Are BRCA Positive
There are several risk assessment tools and clinical practice guidelines used in the management of localized prostate cancer (PCa). These include the D’Amico classification, the Cancer of the Prostate Risk Assessment (CAPRA) score, the National Comprehensive Cancer Network (NCCN) risk criteria, and the American Urological Association (AUA) clinical practice guidelines.1-4 None of these tools incorporate the BRCA1 and BRCA2 genes in the risk assessment or treatment recommendations for localized PCa.5 The BRCA mutations are most strongly associated with breast and ovarian cancer risk. However, BRCA mutations also increase susceptibility and disease progression in PCa.6 This article illustrates the current knowledge gap in PCa treatment algorithms for the BRCA2-positive patient population.
Traditional risk assessment tools use clinical and pathologic features of PCa, including prostate-specific antigen (PSA) level, Gleason score, tumor stage, and disease burden to measure cancer aggressiveness.1,7,8 These criteria are the basis of the AUA and NCCN guidelines for management of clinically localized PCa, which recognize 3 categories of clinically localized disease (low, intermediate, and high risk).3,4 The NCCN guidelines (version 1.2016) include a fourth category (very low risk or pathologically insignificant PCa) among some stage T1c patients, based on additional criteria, including PSA density. Both the AUA and NCCN recommend active surveillance as a treatment option for men with low-risk PCa. The NCCN recently revised its guidelines to state that intermediate-risk patients with PCa with favorable features (Gleason grade 3 and < 50% of positive biopsy cores) may also be considered for active surveillance.3
BRCA Mutations in Prostate Cancer
Estimates of the relative risk of PCa for men with BRCA1 and BRCA2 mutations have varied, but recent data suggest that it is 3.75-fold for BRCA1 mutations and 8.6-fold for BRCA2 by age 65 years.9-11 Moreover, PCas associated with BRCA1/2 mutations, particularly those in the BRCA2 gene, are often more aggressive and characterized by poor outcomes.12,13 The presence of a BRCA2 mutation is a negative prognostic factor in PCa, independent of tumor grade, stage, and PSA levels.14 Both PCa-specific survival and metastasis-free survival rates following surgical or radiation therapy are significantly lower in the BRCA mutation carriers than in noncarriers.15 Preliminary results of the IMPACT study demonstrate that targeted PCa screening in men with BRCA1 or BRCA2 mutations may result in identification of tumors more likely to require treatment.16
As a result of these increased risks, it is recommended that men with BRCA2 mutations begin PCa screening at age 40 years; however, there are no clear guidelines for clinical management of PCa in this group of patients.5 The lack of guidelines presents a challenge for clinical management of BRCA1/2 mutation carriers with localized PCa who otherwise qualify for active surveillance. A recent editorial by Bratt and Loman specifically
calls for aggressive therapy for patients who are BRCA positive, particularly BRCA2 carriers, suggesting the need to combine early radical local treatment with adjuvant systemic therapy.17 However, data on the effectiveness of aggressive therapies in patients with PCa who carry BRCA2 mutations are sparse.5
Genomic Test for Risk
There is growing recognition of the need to include molecular testing to improve risk assessment in PCa. Using traditional risk assessment tools, about 8% of low-risk patients are found to have progressive disease postoperatively.3 Current AUA guidelines from 2007 are silent on the issue of molecular testing. The 2015 and 2016 NCCN guidelines include molecular testing for better risk stratification of patients with PCa, specifically naming Oncotype DX Prostate Cancer Assay (Genomic Health, Redwood City, CA) and Prolaris (Myriad Genetics, Salt Lake City, UT).3 However, they do not address molecular BRCA mutation testing.
There are several genomic tests aimed at improving PCa risk assessment. These include Oncotype DX PCa Assay; Prolaris; Decipher Prostate Cancer Classifier (GenomeDx Biosciences, San Diego, CA); and ProMark (Metamark Laboratories, Cambridge, MA). These assays are tissuebased and measure gene expression on the RNA or protein level to identify low- or intermediate-risk patients who may be candidates for active surveillance, as well as patients at higher risk who may benefit from closer monitoring or additional therapy after their initial treatment. By 2015, the Centers for Medicare and Medicaid Services had issued positive coverage decisions for several tests.18
The Oncotype DX test is a quantitative real-time polymerase chain reaction assay that measures the expression of 17 genes (12 cancer-related genes and 5 reference genes) representing 4 biologic pathways, including from the androgen signaling, stromal response, cellular organization, and cellular proliferation (Table). Prolaris focuses on a larger number of genes in the cell-cycle progression (CCP) pathway (31 cell-cycle-related genes and 15 reference genes). There is no overlap between the 2 gene sets. Both tests integrate genomic data with clinical and histopathologic characteristics of the tumor to arrive at prognostic information. The Oncotype DX test yields a specific Genomic Prostate Score (GPS; scaled 0-100) that is integrated with the patient’s NCCN clinical risk group to quantify the likelihood of favorable pathology, which is defined as low-grade organ-confined disease.19 The Prolaris test uses the patient’s AUA risk category and then evaluates the patient’s risk based on the cell-cycle progression
gene panel compared with that risk category. It also provides an estimate of disease-specific mortality as validated by 2 independent cohorts that were managed conservatively initially with watchful waiting.
In this article, the authors present a case report of a BRCA2-positive veteran with newly diagnosed lowrisk PCa and a history of breast cancer. In addition to evaluating clinical criteria, Oncotype DX and Prolaris gene expression tests were ordered for this patient. The authors obtained veteran and institutional review board permission. To protect the identity of the patient, minor changes were made to patient demographics.
Case Presentation
A 68-year-old white man with a history of coronary artery disease, dyslipidemia, and hypertension, was recently diagnosed with PCa. He presented to Genomic Medicine Service to discuss how his BRCA2 mutation status might impact management decisions for PCa. Priorto the PCa diagnosis, the veteran had a history of breast and skin cancer. He was diagnosed with invasive ductal carcinoma of the right breast (ER+/PR+/Her2+) at age 62 years and treated with mastectomy and tamoxifen. He had testing at that time, which revealed a BRCA2 mutation: 3773delTT. Squamous cell carcinoma was detected on his right leg and removed at age 64 years. Basal cell carcinoma was removed from his left forehead first at age 65 years, and then residual basal cell carcinoma was removed from the forehead 2 months later.
The veteran was diagnosed with PCa at age 67 years at a non-VA clinic. The urology consult note reported a sudden increase of his PSA level to 5.9. A prostate needle biopsy was performed. The Gleason score was 3 + 3 = 6 in 2 of 12, with < 1% PCa involvement and focal highgrade prostatic intraepithelial neoplasia. The patient was asymptomatic, and his cancer was identified by needle biopsy due to elevated PSA. His clinical stage was T1c. According to AUA and NCCN guidelines, the patient was categorized as low risk, defined as Gleason Score ≤ 6, PSA < 10 ng/mL, and clinical stage up to T2a.3,4 Additionally, the veteran met 3 criteria for the NCCN very low-risk category (stage T1c, < 3 positive biopsy cores and ≤ 50% cancer in any core). However, because he was initially diagnosed at a non-VA clinic, his PSA density (the remaining criterion) was not available to the VA urologist. Therefore, the low-risk category was assumed for molecular test interpretation.
The non-VA urologist recommended active surveillance. The VA urologist agreed that active surveillance was an appropriate treatment recommendation at this time. However, the veteran and his family members remained concerned that his PCa might be more aggressive due to his BRCA2 mutation, and they worried that active surveillance would result in a worse outcome. Their concern was exacerbated by the veteran’s comorbidities, which could have potential implications on the timing of surgical options. The patient expressed these concerns to his VA primary care physician, who then referred him to the VA Genomic Medicine Services.
Genetic Consult
The genetic counselor scheduled a telegenetics consult and conducted an assessment of the veteran, which included a review of his medical history, mutation status, and relevant family history. The family history was consistent with hereditary breast/ovarian cancer. However, the primary reason the veteran underwent genetic testing was the diagnosis of breast cancer in a male. The genetic counselor provided the patient with information relevant to his mutation carrier status, including that men with BRCA2 mutations are at increased risk of developing more aggressive PCa, have higher rates of lymph node involvement, and greater mortality compared with men without BRCA2 mutations. The veteran was informed that there were no published guidelines that suggest PCa in BRCA2 carriers should be treated differently from sporadic PCa.
Tumor Testing Strategy
Although the veteran was comfortable with active surveillance at the time of consultation, he was concerned that, given his comorbidities, it would be better to pursue surgery sooner. The veteran asked the genetic counselor for more information about his prognosis given his BRCA2 status. The genetic counselor discussed possible use of tumor gene expression profiling and informed him about 2 active studies within the VA that are evaluating the clinical utility of gene expression tests for PCa risk stratification (Oncotype DX at Genomic Health and Prolaris at Myriad Genetic Laboratories). The veteran expressed an interest in having his biopsy tissue tested by both assays. Tumor biopsy tissue was obtained and sent to both Genomic Health and Myriad Genetics for testing. Neither test incorporated the veteran’s other health conditions or his BRCA mutation status into risk stratification results or the patient report.
Test Results
The Oncotype DX GPS result for this NCCN low-risk patient was 31 (Figure 1). This score corresponds to a likelihood of favorable pathology at radical prostatectomy of 71% (95% confidence interval [CI]: 63%-78%). Favorable pathology is defined as freedom from highgrade (Gleason score > 4+3) and/or nonorgan-confined (pT3) disease. This GPS result was consistent with the range of risk expected for NCCN low-risk patients based on the validation cohorts for the assay. The estimate of likelihood of favorable pathology would be modified if the PSA density result were available and if it placed the patient in the NCCN very low-risk category.
The Prolaris report demonstrated a score of 0.4 (Figure 2). This puts the veteran in the 94th percentile of contemporary U.S. men who are AUA low risk. The CCP score makes his cancer more aggressive than most AUA low-risk men, and the projected 10-year disease-specific mortality is 3%. In conjunction with the patient’s BRCA2 status, he may benefit from definitive intervention. If active surveillance is chosen, careful and regular follow-up for disease progression is mandated.
Interpretation of Genomic Testing in PCa
For both tests, the results are derived from 2-tiered calculations. For Oncotype DX, the gene expression measurement yields the GPS, which is then integrated with the patient’s clinical and pathologic information to yield the likelihood of favorable pathology. Although the Oncotype DX GPS is an independent measure of disease aggressiveness, on the patient report, the GPS is combined with the NCCN clinical risk group to provide a likelihood of favorable pathology. Therefore, 2 patients with the same GPS but different levels of clinical risk will have different likelihoods of favorable pathology.
The Prolaris test provides the Prolaris CCP score as well as the percentile group of patients with a lower score within the same risk category. Also, the Prolaris test yields a numerical 10-year PCa-specific mortality risk. The Prolaris score has been shown to impact therapeutic decisions in patients with newly diagnosed PCa.20
Recently, Myriad defined a threshold for active surveillance combining the CCP and CAPRA scores.21 Myriad validated this cutoff in 2 cohorts of men initially managed conservatively. Although the model predicts up to 3.2% disease-specific mortality, there were no observed deaths during a decade of follow-up. Myriad reports that by using this cutoff in contemporary patients tested commercially with Prolaris, a health care system could increase the percentage of men who would fit current criteria for active surveillance from 36% to 60% with no increase in risk of disease-specific mortality.
The results of these 2 tests are presented in 2 different formats and provide risk estimates for different clinical endpoints, making it challenging for a clinician to directly compare them. Moreover, each genomic test is based on a different set of genes and uses different clinical risk criteria (AUA vs NCCN), which may result in different test output. Finally, and most relevant to the case described here, there is no evidence-based consensus on how to interpret these test results in the context of a BRCA2 mutation.
Based on the published literature reporting that BRCA2 mutations are associated with more aggressive disease, one prediction would be that test scores from genomic assays such as Oncotype DX and Prolaris would tend to be higher in BRCA2 carriers than those of the overall population of PCa patients. This has, in fact, been reported for the Oncotype DX Breast Cancer Assay recurrence score in women who are BRCA carriers.22 Further research is required to ascertain whether this will be true for Oncotype DX GPS and Prolaris CCP score in PCa. The mechanism of action that predisposes BRCA2 mutation carriers to develop a more aggressive variant of PCa may not be detectable by the genomic markers included in the Oncotype DX PCa and Prolaris tests. The degree to which a mutated BRCA2 gene may interact with the genes comprising these assays and the reported tumor aggressiveness is not yet understood but deserving of future study.
Treatment Recommendation and Patient’s Decision
After considering his test results, the veteran chose active surveillance. The sum of clinical, pathologic, and molecular factors, combined with the patient’s preference, determined his course of treatment. Because prostatectomy was not performed, it has not been positively determined whether or not the patient harbors aggressive disease. As the molecular test results place the patient at the high end of the low-risk group, the VA urologist recommended close monitoring and suggested a follow-up biopsy with magnetic resonance-ultrasound fusion guidance.
Conclusions
Molecular testing found that the patient’s PCa stage and grade are consistent with NCCN low risk (Oncotype DX) and that the disease-specific mortality risk is slightly higher than predicted by clinical features alone (Prolaris). Previous studies have shown that molecular testing in men with PCa provides information that influences clinical decisions. The findings reported here suggest that molecular testing may also be a vital component in the medical management of patients with complex clinical phenotypes and common chronic conditions. Additional studies are necessary to evaluate whether the finding reported here is typical of individuals diagnosed with PCa who also have a BRCA2 mutation.
For any new genomic test to be clinically useful, its results must have clinical actionability. In this case, the clinical decision point was whether to recommend immediate definitive treatment or active surveillance. For this patient, the Oncotype DX assay provided a likelihood of favorable surgical pathology of 71% (or conversely a 29% risk of unfavorable pathology); by comparison, the Prolaris CCP score provided a 3% estimate of PCaspecific
mortality at 10 years. A key question is: How do clinicians perceive the actionability of risk estimates for these different endpoints?
The current case illustrates the challenges that rapidly developing genomic medicine pose for physicians trying to optimize care and communicate results to patients in a meaningful and consistent manner. For example, some urologists find the different 2-tiered calculations confusing. When laboratories use proprietary scalesbased on internally develop algorithms, differing interpretations are to be expected. The risk-assessment tests described here use different algorithms, and their interpretations are based on clinical categories from different sets of guidelines. This underscores the need for better standardization of PCa care.23
Oncology and urology professional associations should collaborate to develop consistent guidelines for use of new technologies in the management of PCa. A positive example is the evolution of testing recommendations in lung cancer, which initially varied between professional entities. In April 2013, the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology jointly issued a unified clinical practice guideline on molecular testing in patients with lung cancer.24 In October 2014, the American Society of Clinical Oncology issued an endorsement of the CAP/IASLC/AMP guideline.25 As the number of complex tests being used in PCa increases, it will be important for professional associations such as AUA and NCCN to collaborate in evaluating utility of innovations to make consistent recommendations
Author disclosures
Myriad Genetics and Genomic Health provided funding for research on their tests within the VA. Dr. Dash and Dr. Lynch are principal investigators of the Genomic Health study. Dr. Lowrance is the principal investigator of the Myriad Genetics study.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969-974.
2. Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938-1942.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: prostate cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated November 10, 2015. Accessed December 8, 2016.
4. Thompson I, Thrasher JB, Aus G, et al; AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
5. Alanee SR, Glogowski EA, Schrader KA, Eastham JA, Offit K. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Front Biosci (Elite Ed). 2014;6:15-30.
6. Castro E, Eeles R. The role of BRCA1 and BRCA2 in prostate cancer. Asian J Androl. 2012;14(3):409-414.
7. Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D’amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008;179(4):1354-1360.
8. Lowrance WT, Scardino PT. Predictive models for newly diagnosed prostate cancer patients. Rev Urol. 2009;11(3):117-126.
9. Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96(1):11-15.
10. Leongamornlert D, Mahmud N, Tymrakiewicz M, et al; UKGPCS Collaborators. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106(10):1697-1701.
11. Kote-Jarai Z, Leongamornlert D, Saunders E, et al; UKGPCS Collaborators. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230-1234.
12. Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(12):929-935.
13. Gallagher DJ, Gaudet MM, Pal P, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16(7):2115-2121.
14. Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748-1757.
15. Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. 2015;68(2):186-193.
16. Bancroft EK, Page EC, Castro E, et al; IMPACT Collaborators. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol. 2014;66(3):489-499.
17. Bratt O, Loman N. Clinical management of prostate cancer in men with BRCA mutations. Eur Urol. 2015;68(2):194-195.
18. Centers for Medicare & Medicaid Services (CMS). MCD archive site. CMS Website. http://localcoverage.cms.gov/mcd_archive/overview.aspx. Accessed January 8, 2016.
19. Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66(3):550-560.
20. Shore ND, Kella N, Moran B, et al. Impact of the cell cycle progression test on physician and patient treatment selection for localized prostate cancer. J Urol. 2015;pii:S0022-5347(15)04811-9 [epub ahead of print].
21. Stone S, Cuzick JM, Fisher G, et al. Validation of an active surveillance threshold for the CCP score in conservatively managed men with localized prostate cancer. J Clin Oncol. 2015;33(suppl 15):e16040.
22. Lewin R, Rizel S, Hendler D, et al. Oncotype-DX recurrence score distribution among breast cancer patients harboring a germline mutation in the BRCA1/2 genes. J Clin Oncol. 2015;33(suppl; abstr 564).
23. Dahm P, Yeung LL, Chang SS, Cookson MS. A critical review of clinical practice guidelines for the management of clinically localized prostate cancer. J Urol. 2008;180(2):451-459.
24. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
25. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
Note: Page numbers differ between the print issue and digital edition.
There are several risk assessment tools and clinical practice guidelines used in the management of localized prostate cancer (PCa). These include the D’Amico classification, the Cancer of the Prostate Risk Assessment (CAPRA) score, the National Comprehensive Cancer Network (NCCN) risk criteria, and the American Urological Association (AUA) clinical practice guidelines.1-4 None of these tools incorporate the BRCA1 and BRCA2 genes in the risk assessment or treatment recommendations for localized PCa.5 The BRCA mutations are most strongly associated with breast and ovarian cancer risk. However, BRCA mutations also increase susceptibility and disease progression in PCa.6 This article illustrates the current knowledge gap in PCa treatment algorithms for the BRCA2-positive patient population.
Traditional risk assessment tools use clinical and pathologic features of PCa, including prostate-specific antigen (PSA) level, Gleason score, tumor stage, and disease burden to measure cancer aggressiveness.1,7,8 These criteria are the basis of the AUA and NCCN guidelines for management of clinically localized PCa, which recognize 3 categories of clinically localized disease (low, intermediate, and high risk).3,4 The NCCN guidelines (version 1.2016) include a fourth category (very low risk or pathologically insignificant PCa) among some stage T1c patients, based on additional criteria, including PSA density. Both the AUA and NCCN recommend active surveillance as a treatment option for men with low-risk PCa. The NCCN recently revised its guidelines to state that intermediate-risk patients with PCa with favorable features (Gleason grade 3 and < 50% of positive biopsy cores) may also be considered for active surveillance.3
BRCA Mutations in Prostate Cancer
Estimates of the relative risk of PCa for men with BRCA1 and BRCA2 mutations have varied, but recent data suggest that it is 3.75-fold for BRCA1 mutations and 8.6-fold for BRCA2 by age 65 years.9-11 Moreover, PCas associated with BRCA1/2 mutations, particularly those in the BRCA2 gene, are often more aggressive and characterized by poor outcomes.12,13 The presence of a BRCA2 mutation is a negative prognostic factor in PCa, independent of tumor grade, stage, and PSA levels.14 Both PCa-specific survival and metastasis-free survival rates following surgical or radiation therapy are significantly lower in the BRCA mutation carriers than in noncarriers.15 Preliminary results of the IMPACT study demonstrate that targeted PCa screening in men with BRCA1 or BRCA2 mutations may result in identification of tumors more likely to require treatment.16
As a result of these increased risks, it is recommended that men with BRCA2 mutations begin PCa screening at age 40 years; however, there are no clear guidelines for clinical management of PCa in this group of patients.5 The lack of guidelines presents a challenge for clinical management of BRCA1/2 mutation carriers with localized PCa who otherwise qualify for active surveillance. A recent editorial by Bratt and Loman specifically
calls for aggressive therapy for patients who are BRCA positive, particularly BRCA2 carriers, suggesting the need to combine early radical local treatment with adjuvant systemic therapy.17 However, data on the effectiveness of aggressive therapies in patients with PCa who carry BRCA2 mutations are sparse.5
Genomic Test for Risk
There is growing recognition of the need to include molecular testing to improve risk assessment in PCa. Using traditional risk assessment tools, about 8% of low-risk patients are found to have progressive disease postoperatively.3 Current AUA guidelines from 2007 are silent on the issue of molecular testing. The 2015 and 2016 NCCN guidelines include molecular testing for better risk stratification of patients with PCa, specifically naming Oncotype DX Prostate Cancer Assay (Genomic Health, Redwood City, CA) and Prolaris (Myriad Genetics, Salt Lake City, UT).3 However, they do not address molecular BRCA mutation testing.
There are several genomic tests aimed at improving PCa risk assessment. These include Oncotype DX PCa Assay; Prolaris; Decipher Prostate Cancer Classifier (GenomeDx Biosciences, San Diego, CA); and ProMark (Metamark Laboratories, Cambridge, MA). These assays are tissuebased and measure gene expression on the RNA or protein level to identify low- or intermediate-risk patients who may be candidates for active surveillance, as well as patients at higher risk who may benefit from closer monitoring or additional therapy after their initial treatment. By 2015, the Centers for Medicare and Medicaid Services had issued positive coverage decisions for several tests.18
The Oncotype DX test is a quantitative real-time polymerase chain reaction assay that measures the expression of 17 genes (12 cancer-related genes and 5 reference genes) representing 4 biologic pathways, including from the androgen signaling, stromal response, cellular organization, and cellular proliferation (Table). Prolaris focuses on a larger number of genes in the cell-cycle progression (CCP) pathway (31 cell-cycle-related genes and 15 reference genes). There is no overlap between the 2 gene sets. Both tests integrate genomic data with clinical and histopathologic characteristics of the tumor to arrive at prognostic information. The Oncotype DX test yields a specific Genomic Prostate Score (GPS; scaled 0-100) that is integrated with the patient’s NCCN clinical risk group to quantify the likelihood of favorable pathology, which is defined as low-grade organ-confined disease.19 The Prolaris test uses the patient’s AUA risk category and then evaluates the patient’s risk based on the cell-cycle progression
gene panel compared with that risk category. It also provides an estimate of disease-specific mortality as validated by 2 independent cohorts that were managed conservatively initially with watchful waiting.
In this article, the authors present a case report of a BRCA2-positive veteran with newly diagnosed lowrisk PCa and a history of breast cancer. In addition to evaluating clinical criteria, Oncotype DX and Prolaris gene expression tests were ordered for this patient. The authors obtained veteran and institutional review board permission. To protect the identity of the patient, minor changes were made to patient demographics.
Case Presentation
A 68-year-old white man with a history of coronary artery disease, dyslipidemia, and hypertension, was recently diagnosed with PCa. He presented to Genomic Medicine Service to discuss how his BRCA2 mutation status might impact management decisions for PCa. Priorto the PCa diagnosis, the veteran had a history of breast and skin cancer. He was diagnosed with invasive ductal carcinoma of the right breast (ER+/PR+/Her2+) at age 62 years and treated with mastectomy and tamoxifen. He had testing at that time, which revealed a BRCA2 mutation: 3773delTT. Squamous cell carcinoma was detected on his right leg and removed at age 64 years. Basal cell carcinoma was removed from his left forehead first at age 65 years, and then residual basal cell carcinoma was removed from the forehead 2 months later.
The veteran was diagnosed with PCa at age 67 years at a non-VA clinic. The urology consult note reported a sudden increase of his PSA level to 5.9. A prostate needle biopsy was performed. The Gleason score was 3 + 3 = 6 in 2 of 12, with < 1% PCa involvement and focal highgrade prostatic intraepithelial neoplasia. The patient was asymptomatic, and his cancer was identified by needle biopsy due to elevated PSA. His clinical stage was T1c. According to AUA and NCCN guidelines, the patient was categorized as low risk, defined as Gleason Score ≤ 6, PSA < 10 ng/mL, and clinical stage up to T2a.3,4 Additionally, the veteran met 3 criteria for the NCCN very low-risk category (stage T1c, < 3 positive biopsy cores and ≤ 50% cancer in any core). However, because he was initially diagnosed at a non-VA clinic, his PSA density (the remaining criterion) was not available to the VA urologist. Therefore, the low-risk category was assumed for molecular test interpretation.
The non-VA urologist recommended active surveillance. The VA urologist agreed that active surveillance was an appropriate treatment recommendation at this time. However, the veteran and his family members remained concerned that his PCa might be more aggressive due to his BRCA2 mutation, and they worried that active surveillance would result in a worse outcome. Their concern was exacerbated by the veteran’s comorbidities, which could have potential implications on the timing of surgical options. The patient expressed these concerns to his VA primary care physician, who then referred him to the VA Genomic Medicine Services.
Genetic Consult
The genetic counselor scheduled a telegenetics consult and conducted an assessment of the veteran, which included a review of his medical history, mutation status, and relevant family history. The family history was consistent with hereditary breast/ovarian cancer. However, the primary reason the veteran underwent genetic testing was the diagnosis of breast cancer in a male. The genetic counselor provided the patient with information relevant to his mutation carrier status, including that men with BRCA2 mutations are at increased risk of developing more aggressive PCa, have higher rates of lymph node involvement, and greater mortality compared with men without BRCA2 mutations. The veteran was informed that there were no published guidelines that suggest PCa in BRCA2 carriers should be treated differently from sporadic PCa.
Tumor Testing Strategy
Although the veteran was comfortable with active surveillance at the time of consultation, he was concerned that, given his comorbidities, it would be better to pursue surgery sooner. The veteran asked the genetic counselor for more information about his prognosis given his BRCA2 status. The genetic counselor discussed possible use of tumor gene expression profiling and informed him about 2 active studies within the VA that are evaluating the clinical utility of gene expression tests for PCa risk stratification (Oncotype DX at Genomic Health and Prolaris at Myriad Genetic Laboratories). The veteran expressed an interest in having his biopsy tissue tested by both assays. Tumor biopsy tissue was obtained and sent to both Genomic Health and Myriad Genetics for testing. Neither test incorporated the veteran’s other health conditions or his BRCA mutation status into risk stratification results or the patient report.
Test Results
The Oncotype DX GPS result for this NCCN low-risk patient was 31 (Figure 1). This score corresponds to a likelihood of favorable pathology at radical prostatectomy of 71% (95% confidence interval [CI]: 63%-78%). Favorable pathology is defined as freedom from highgrade (Gleason score > 4+3) and/or nonorgan-confined (pT3) disease. This GPS result was consistent with the range of risk expected for NCCN low-risk patients based on the validation cohorts for the assay. The estimate of likelihood of favorable pathology would be modified if the PSA density result were available and if it placed the patient in the NCCN very low-risk category.
The Prolaris report demonstrated a score of 0.4 (Figure 2). This puts the veteran in the 94th percentile of contemporary U.S. men who are AUA low risk. The CCP score makes his cancer more aggressive than most AUA low-risk men, and the projected 10-year disease-specific mortality is 3%. In conjunction with the patient’s BRCA2 status, he may benefit from definitive intervention. If active surveillance is chosen, careful and regular follow-up for disease progression is mandated.
Interpretation of Genomic Testing in PCa
For both tests, the results are derived from 2-tiered calculations. For Oncotype DX, the gene expression measurement yields the GPS, which is then integrated with the patient’s clinical and pathologic information to yield the likelihood of favorable pathology. Although the Oncotype DX GPS is an independent measure of disease aggressiveness, on the patient report, the GPS is combined with the NCCN clinical risk group to provide a likelihood of favorable pathology. Therefore, 2 patients with the same GPS but different levels of clinical risk will have different likelihoods of favorable pathology.
The Prolaris test provides the Prolaris CCP score as well as the percentile group of patients with a lower score within the same risk category. Also, the Prolaris test yields a numerical 10-year PCa-specific mortality risk. The Prolaris score has been shown to impact therapeutic decisions in patients with newly diagnosed PCa.20
Recently, Myriad defined a threshold for active surveillance combining the CCP and CAPRA scores.21 Myriad validated this cutoff in 2 cohorts of men initially managed conservatively. Although the model predicts up to 3.2% disease-specific mortality, there were no observed deaths during a decade of follow-up. Myriad reports that by using this cutoff in contemporary patients tested commercially with Prolaris, a health care system could increase the percentage of men who would fit current criteria for active surveillance from 36% to 60% with no increase in risk of disease-specific mortality.
The results of these 2 tests are presented in 2 different formats and provide risk estimates for different clinical endpoints, making it challenging for a clinician to directly compare them. Moreover, each genomic test is based on a different set of genes and uses different clinical risk criteria (AUA vs NCCN), which may result in different test output. Finally, and most relevant to the case described here, there is no evidence-based consensus on how to interpret these test results in the context of a BRCA2 mutation.
Based on the published literature reporting that BRCA2 mutations are associated with more aggressive disease, one prediction would be that test scores from genomic assays such as Oncotype DX and Prolaris would tend to be higher in BRCA2 carriers than those of the overall population of PCa patients. This has, in fact, been reported for the Oncotype DX Breast Cancer Assay recurrence score in women who are BRCA carriers.22 Further research is required to ascertain whether this will be true for Oncotype DX GPS and Prolaris CCP score in PCa. The mechanism of action that predisposes BRCA2 mutation carriers to develop a more aggressive variant of PCa may not be detectable by the genomic markers included in the Oncotype DX PCa and Prolaris tests. The degree to which a mutated BRCA2 gene may interact with the genes comprising these assays and the reported tumor aggressiveness is not yet understood but deserving of future study.
Treatment Recommendation and Patient’s Decision
After considering his test results, the veteran chose active surveillance. The sum of clinical, pathologic, and molecular factors, combined with the patient’s preference, determined his course of treatment. Because prostatectomy was not performed, it has not been positively determined whether or not the patient harbors aggressive disease. As the molecular test results place the patient at the high end of the low-risk group, the VA urologist recommended close monitoring and suggested a follow-up biopsy with magnetic resonance-ultrasound fusion guidance.
Conclusions
Molecular testing found that the patient’s PCa stage and grade are consistent with NCCN low risk (Oncotype DX) and that the disease-specific mortality risk is slightly higher than predicted by clinical features alone (Prolaris). Previous studies have shown that molecular testing in men with PCa provides information that influences clinical decisions. The findings reported here suggest that molecular testing may also be a vital component in the medical management of patients with complex clinical phenotypes and common chronic conditions. Additional studies are necessary to evaluate whether the finding reported here is typical of individuals diagnosed with PCa who also have a BRCA2 mutation.
For any new genomic test to be clinically useful, its results must have clinical actionability. In this case, the clinical decision point was whether to recommend immediate definitive treatment or active surveillance. For this patient, the Oncotype DX assay provided a likelihood of favorable surgical pathology of 71% (or conversely a 29% risk of unfavorable pathology); by comparison, the Prolaris CCP score provided a 3% estimate of PCaspecific
mortality at 10 years. A key question is: How do clinicians perceive the actionability of risk estimates for these different endpoints?
The current case illustrates the challenges that rapidly developing genomic medicine pose for physicians trying to optimize care and communicate results to patients in a meaningful and consistent manner. For example, some urologists find the different 2-tiered calculations confusing. When laboratories use proprietary scalesbased on internally develop algorithms, differing interpretations are to be expected. The risk-assessment tests described here use different algorithms, and their interpretations are based on clinical categories from different sets of guidelines. This underscores the need for better standardization of PCa care.23
Oncology and urology professional associations should collaborate to develop consistent guidelines for use of new technologies in the management of PCa. A positive example is the evolution of testing recommendations in lung cancer, which initially varied between professional entities. In April 2013, the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology jointly issued a unified clinical practice guideline on molecular testing in patients with lung cancer.24 In October 2014, the American Society of Clinical Oncology issued an endorsement of the CAP/IASLC/AMP guideline.25 As the number of complex tests being used in PCa increases, it will be important for professional associations such as AUA and NCCN to collaborate in evaluating utility of innovations to make consistent recommendations
Author disclosures
Myriad Genetics and Genomic Health provided funding for research on their tests within the VA. Dr. Dash and Dr. Lynch are principal investigators of the Genomic Health study. Dr. Lowrance is the principal investigator of the Myriad Genetics study.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
There are several risk assessment tools and clinical practice guidelines used in the management of localized prostate cancer (PCa). These include the D’Amico classification, the Cancer of the Prostate Risk Assessment (CAPRA) score, the National Comprehensive Cancer Network (NCCN) risk criteria, and the American Urological Association (AUA) clinical practice guidelines.1-4 None of these tools incorporate the BRCA1 and BRCA2 genes in the risk assessment or treatment recommendations for localized PCa.5 The BRCA mutations are most strongly associated with breast and ovarian cancer risk. However, BRCA mutations also increase susceptibility and disease progression in PCa.6 This article illustrates the current knowledge gap in PCa treatment algorithms for the BRCA2-positive patient population.
Traditional risk assessment tools use clinical and pathologic features of PCa, including prostate-specific antigen (PSA) level, Gleason score, tumor stage, and disease burden to measure cancer aggressiveness.1,7,8 These criteria are the basis of the AUA and NCCN guidelines for management of clinically localized PCa, which recognize 3 categories of clinically localized disease (low, intermediate, and high risk).3,4 The NCCN guidelines (version 1.2016) include a fourth category (very low risk or pathologically insignificant PCa) among some stage T1c patients, based on additional criteria, including PSA density. Both the AUA and NCCN recommend active surveillance as a treatment option for men with low-risk PCa. The NCCN recently revised its guidelines to state that intermediate-risk patients with PCa with favorable features (Gleason grade 3 and < 50% of positive biopsy cores) may also be considered for active surveillance.3
BRCA Mutations in Prostate Cancer
Estimates of the relative risk of PCa for men with BRCA1 and BRCA2 mutations have varied, but recent data suggest that it is 3.75-fold for BRCA1 mutations and 8.6-fold for BRCA2 by age 65 years.9-11 Moreover, PCas associated with BRCA1/2 mutations, particularly those in the BRCA2 gene, are often more aggressive and characterized by poor outcomes.12,13 The presence of a BRCA2 mutation is a negative prognostic factor in PCa, independent of tumor grade, stage, and PSA levels.14 Both PCa-specific survival and metastasis-free survival rates following surgical or radiation therapy are significantly lower in the BRCA mutation carriers than in noncarriers.15 Preliminary results of the IMPACT study demonstrate that targeted PCa screening in men with BRCA1 or BRCA2 mutations may result in identification of tumors more likely to require treatment.16
As a result of these increased risks, it is recommended that men with BRCA2 mutations begin PCa screening at age 40 years; however, there are no clear guidelines for clinical management of PCa in this group of patients.5 The lack of guidelines presents a challenge for clinical management of BRCA1/2 mutation carriers with localized PCa who otherwise qualify for active surveillance. A recent editorial by Bratt and Loman specifically
calls for aggressive therapy for patients who are BRCA positive, particularly BRCA2 carriers, suggesting the need to combine early radical local treatment with adjuvant systemic therapy.17 However, data on the effectiveness of aggressive therapies in patients with PCa who carry BRCA2 mutations are sparse.5
Genomic Test for Risk
There is growing recognition of the need to include molecular testing to improve risk assessment in PCa. Using traditional risk assessment tools, about 8% of low-risk patients are found to have progressive disease postoperatively.3 Current AUA guidelines from 2007 are silent on the issue of molecular testing. The 2015 and 2016 NCCN guidelines include molecular testing for better risk stratification of patients with PCa, specifically naming Oncotype DX Prostate Cancer Assay (Genomic Health, Redwood City, CA) and Prolaris (Myriad Genetics, Salt Lake City, UT).3 However, they do not address molecular BRCA mutation testing.
There are several genomic tests aimed at improving PCa risk assessment. These include Oncotype DX PCa Assay; Prolaris; Decipher Prostate Cancer Classifier (GenomeDx Biosciences, San Diego, CA); and ProMark (Metamark Laboratories, Cambridge, MA). These assays are tissuebased and measure gene expression on the RNA or protein level to identify low- or intermediate-risk patients who may be candidates for active surveillance, as well as patients at higher risk who may benefit from closer monitoring or additional therapy after their initial treatment. By 2015, the Centers for Medicare and Medicaid Services had issued positive coverage decisions for several tests.18
The Oncotype DX test is a quantitative real-time polymerase chain reaction assay that measures the expression of 17 genes (12 cancer-related genes and 5 reference genes) representing 4 biologic pathways, including from the androgen signaling, stromal response, cellular organization, and cellular proliferation (Table). Prolaris focuses on a larger number of genes in the cell-cycle progression (CCP) pathway (31 cell-cycle-related genes and 15 reference genes). There is no overlap between the 2 gene sets. Both tests integrate genomic data with clinical and histopathologic characteristics of the tumor to arrive at prognostic information. The Oncotype DX test yields a specific Genomic Prostate Score (GPS; scaled 0-100) that is integrated with the patient’s NCCN clinical risk group to quantify the likelihood of favorable pathology, which is defined as low-grade organ-confined disease.19 The Prolaris test uses the patient’s AUA risk category and then evaluates the patient’s risk based on the cell-cycle progression
gene panel compared with that risk category. It also provides an estimate of disease-specific mortality as validated by 2 independent cohorts that were managed conservatively initially with watchful waiting.
In this article, the authors present a case report of a BRCA2-positive veteran with newly diagnosed lowrisk PCa and a history of breast cancer. In addition to evaluating clinical criteria, Oncotype DX and Prolaris gene expression tests were ordered for this patient. The authors obtained veteran and institutional review board permission. To protect the identity of the patient, minor changes were made to patient demographics.
Case Presentation
A 68-year-old white man with a history of coronary artery disease, dyslipidemia, and hypertension, was recently diagnosed with PCa. He presented to Genomic Medicine Service to discuss how his BRCA2 mutation status might impact management decisions for PCa. Priorto the PCa diagnosis, the veteran had a history of breast and skin cancer. He was diagnosed with invasive ductal carcinoma of the right breast (ER+/PR+/Her2+) at age 62 years and treated with mastectomy and tamoxifen. He had testing at that time, which revealed a BRCA2 mutation: 3773delTT. Squamous cell carcinoma was detected on his right leg and removed at age 64 years. Basal cell carcinoma was removed from his left forehead first at age 65 years, and then residual basal cell carcinoma was removed from the forehead 2 months later.
The veteran was diagnosed with PCa at age 67 years at a non-VA clinic. The urology consult note reported a sudden increase of his PSA level to 5.9. A prostate needle biopsy was performed. The Gleason score was 3 + 3 = 6 in 2 of 12, with < 1% PCa involvement and focal highgrade prostatic intraepithelial neoplasia. The patient was asymptomatic, and his cancer was identified by needle biopsy due to elevated PSA. His clinical stage was T1c. According to AUA and NCCN guidelines, the patient was categorized as low risk, defined as Gleason Score ≤ 6, PSA < 10 ng/mL, and clinical stage up to T2a.3,4 Additionally, the veteran met 3 criteria for the NCCN very low-risk category (stage T1c, < 3 positive biopsy cores and ≤ 50% cancer in any core). However, because he was initially diagnosed at a non-VA clinic, his PSA density (the remaining criterion) was not available to the VA urologist. Therefore, the low-risk category was assumed for molecular test interpretation.
The non-VA urologist recommended active surveillance. The VA urologist agreed that active surveillance was an appropriate treatment recommendation at this time. However, the veteran and his family members remained concerned that his PCa might be more aggressive due to his BRCA2 mutation, and they worried that active surveillance would result in a worse outcome. Their concern was exacerbated by the veteran’s comorbidities, which could have potential implications on the timing of surgical options. The patient expressed these concerns to his VA primary care physician, who then referred him to the VA Genomic Medicine Services.
Genetic Consult
The genetic counselor scheduled a telegenetics consult and conducted an assessment of the veteran, which included a review of his medical history, mutation status, and relevant family history. The family history was consistent with hereditary breast/ovarian cancer. However, the primary reason the veteran underwent genetic testing was the diagnosis of breast cancer in a male. The genetic counselor provided the patient with information relevant to his mutation carrier status, including that men with BRCA2 mutations are at increased risk of developing more aggressive PCa, have higher rates of lymph node involvement, and greater mortality compared with men without BRCA2 mutations. The veteran was informed that there were no published guidelines that suggest PCa in BRCA2 carriers should be treated differently from sporadic PCa.
Tumor Testing Strategy
Although the veteran was comfortable with active surveillance at the time of consultation, he was concerned that, given his comorbidities, it would be better to pursue surgery sooner. The veteran asked the genetic counselor for more information about his prognosis given his BRCA2 status. The genetic counselor discussed possible use of tumor gene expression profiling and informed him about 2 active studies within the VA that are evaluating the clinical utility of gene expression tests for PCa risk stratification (Oncotype DX at Genomic Health and Prolaris at Myriad Genetic Laboratories). The veteran expressed an interest in having his biopsy tissue tested by both assays. Tumor biopsy tissue was obtained and sent to both Genomic Health and Myriad Genetics for testing. Neither test incorporated the veteran’s other health conditions or his BRCA mutation status into risk stratification results or the patient report.
Test Results
The Oncotype DX GPS result for this NCCN low-risk patient was 31 (Figure 1). This score corresponds to a likelihood of favorable pathology at radical prostatectomy of 71% (95% confidence interval [CI]: 63%-78%). Favorable pathology is defined as freedom from highgrade (Gleason score > 4+3) and/or nonorgan-confined (pT3) disease. This GPS result was consistent with the range of risk expected for NCCN low-risk patients based on the validation cohorts for the assay. The estimate of likelihood of favorable pathology would be modified if the PSA density result were available and if it placed the patient in the NCCN very low-risk category.
The Prolaris report demonstrated a score of 0.4 (Figure 2). This puts the veteran in the 94th percentile of contemporary U.S. men who are AUA low risk. The CCP score makes his cancer more aggressive than most AUA low-risk men, and the projected 10-year disease-specific mortality is 3%. In conjunction with the patient’s BRCA2 status, he may benefit from definitive intervention. If active surveillance is chosen, careful and regular follow-up for disease progression is mandated.
Interpretation of Genomic Testing in PCa
For both tests, the results are derived from 2-tiered calculations. For Oncotype DX, the gene expression measurement yields the GPS, which is then integrated with the patient’s clinical and pathologic information to yield the likelihood of favorable pathology. Although the Oncotype DX GPS is an independent measure of disease aggressiveness, on the patient report, the GPS is combined with the NCCN clinical risk group to provide a likelihood of favorable pathology. Therefore, 2 patients with the same GPS but different levels of clinical risk will have different likelihoods of favorable pathology.
The Prolaris test provides the Prolaris CCP score as well as the percentile group of patients with a lower score within the same risk category. Also, the Prolaris test yields a numerical 10-year PCa-specific mortality risk. The Prolaris score has been shown to impact therapeutic decisions in patients with newly diagnosed PCa.20
Recently, Myriad defined a threshold for active surveillance combining the CCP and CAPRA scores.21 Myriad validated this cutoff in 2 cohorts of men initially managed conservatively. Although the model predicts up to 3.2% disease-specific mortality, there were no observed deaths during a decade of follow-up. Myriad reports that by using this cutoff in contemporary patients tested commercially with Prolaris, a health care system could increase the percentage of men who would fit current criteria for active surveillance from 36% to 60% with no increase in risk of disease-specific mortality.
The results of these 2 tests are presented in 2 different formats and provide risk estimates for different clinical endpoints, making it challenging for a clinician to directly compare them. Moreover, each genomic test is based on a different set of genes and uses different clinical risk criteria (AUA vs NCCN), which may result in different test output. Finally, and most relevant to the case described here, there is no evidence-based consensus on how to interpret these test results in the context of a BRCA2 mutation.
Based on the published literature reporting that BRCA2 mutations are associated with more aggressive disease, one prediction would be that test scores from genomic assays such as Oncotype DX and Prolaris would tend to be higher in BRCA2 carriers than those of the overall population of PCa patients. This has, in fact, been reported for the Oncotype DX Breast Cancer Assay recurrence score in women who are BRCA carriers.22 Further research is required to ascertain whether this will be true for Oncotype DX GPS and Prolaris CCP score in PCa. The mechanism of action that predisposes BRCA2 mutation carriers to develop a more aggressive variant of PCa may not be detectable by the genomic markers included in the Oncotype DX PCa and Prolaris tests. The degree to which a mutated BRCA2 gene may interact with the genes comprising these assays and the reported tumor aggressiveness is not yet understood but deserving of future study.
Treatment Recommendation and Patient’s Decision
After considering his test results, the veteran chose active surveillance. The sum of clinical, pathologic, and molecular factors, combined with the patient’s preference, determined his course of treatment. Because prostatectomy was not performed, it has not been positively determined whether or not the patient harbors aggressive disease. As the molecular test results place the patient at the high end of the low-risk group, the VA urologist recommended close monitoring and suggested a follow-up biopsy with magnetic resonance-ultrasound fusion guidance.
Conclusions
Molecular testing found that the patient’s PCa stage and grade are consistent with NCCN low risk (Oncotype DX) and that the disease-specific mortality risk is slightly higher than predicted by clinical features alone (Prolaris). Previous studies have shown that molecular testing in men with PCa provides information that influences clinical decisions. The findings reported here suggest that molecular testing may also be a vital component in the medical management of patients with complex clinical phenotypes and common chronic conditions. Additional studies are necessary to evaluate whether the finding reported here is typical of individuals diagnosed with PCa who also have a BRCA2 mutation.
For any new genomic test to be clinically useful, its results must have clinical actionability. In this case, the clinical decision point was whether to recommend immediate definitive treatment or active surveillance. For this patient, the Oncotype DX assay provided a likelihood of favorable surgical pathology of 71% (or conversely a 29% risk of unfavorable pathology); by comparison, the Prolaris CCP score provided a 3% estimate of PCaspecific
mortality at 10 years. A key question is: How do clinicians perceive the actionability of risk estimates for these different endpoints?
The current case illustrates the challenges that rapidly developing genomic medicine pose for physicians trying to optimize care and communicate results to patients in a meaningful and consistent manner. For example, some urologists find the different 2-tiered calculations confusing. When laboratories use proprietary scalesbased on internally develop algorithms, differing interpretations are to be expected. The risk-assessment tests described here use different algorithms, and their interpretations are based on clinical categories from different sets of guidelines. This underscores the need for better standardization of PCa care.23
Oncology and urology professional associations should collaborate to develop consistent guidelines for use of new technologies in the management of PCa. A positive example is the evolution of testing recommendations in lung cancer, which initially varied between professional entities. In April 2013, the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology jointly issued a unified clinical practice guideline on molecular testing in patients with lung cancer.24 In October 2014, the American Society of Clinical Oncology issued an endorsement of the CAP/IASLC/AMP guideline.25 As the number of complex tests being used in PCa increases, it will be important for professional associations such as AUA and NCCN to collaborate in evaluating utility of innovations to make consistent recommendations
Author disclosures
Myriad Genetics and Genomic Health provided funding for research on their tests within the VA. Dr. Dash and Dr. Lynch are principal investigators of the Genomic Health study. Dr. Lowrance is the principal investigator of the Myriad Genetics study.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969-974.
2. Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938-1942.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: prostate cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated November 10, 2015. Accessed December 8, 2016.
4. Thompson I, Thrasher JB, Aus G, et al; AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
5. Alanee SR, Glogowski EA, Schrader KA, Eastham JA, Offit K. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Front Biosci (Elite Ed). 2014;6:15-30.
6. Castro E, Eeles R. The role of BRCA1 and BRCA2 in prostate cancer. Asian J Androl. 2012;14(3):409-414.
7. Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D’amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008;179(4):1354-1360.
8. Lowrance WT, Scardino PT. Predictive models for newly diagnosed prostate cancer patients. Rev Urol. 2009;11(3):117-126.
9. Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96(1):11-15.
10. Leongamornlert D, Mahmud N, Tymrakiewicz M, et al; UKGPCS Collaborators. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106(10):1697-1701.
11. Kote-Jarai Z, Leongamornlert D, Saunders E, et al; UKGPCS Collaborators. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230-1234.
12. Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(12):929-935.
13. Gallagher DJ, Gaudet MM, Pal P, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16(7):2115-2121.
14. Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748-1757.
15. Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. 2015;68(2):186-193.
16. Bancroft EK, Page EC, Castro E, et al; IMPACT Collaborators. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol. 2014;66(3):489-499.
17. Bratt O, Loman N. Clinical management of prostate cancer in men with BRCA mutations. Eur Urol. 2015;68(2):194-195.
18. Centers for Medicare & Medicaid Services (CMS). MCD archive site. CMS Website. http://localcoverage.cms.gov/mcd_archive/overview.aspx. Accessed January 8, 2016.
19. Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66(3):550-560.
20. Shore ND, Kella N, Moran B, et al. Impact of the cell cycle progression test on physician and patient treatment selection for localized prostate cancer. J Urol. 2015;pii:S0022-5347(15)04811-9 [epub ahead of print].
21. Stone S, Cuzick JM, Fisher G, et al. Validation of an active surveillance threshold for the CCP score in conservatively managed men with localized prostate cancer. J Clin Oncol. 2015;33(suppl 15):e16040.
22. Lewin R, Rizel S, Hendler D, et al. Oncotype-DX recurrence score distribution among breast cancer patients harboring a germline mutation in the BRCA1/2 genes. J Clin Oncol. 2015;33(suppl; abstr 564).
23. Dahm P, Yeung LL, Chang SS, Cookson MS. A critical review of clinical practice guidelines for the management of clinically localized prostate cancer. J Urol. 2008;180(2):451-459.
24. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
25. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
Note: Page numbers differ between the print issue and digital edition.
1. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969-974.
2. Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938-1942.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: prostate cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated November 10, 2015. Accessed December 8, 2016.
4. Thompson I, Thrasher JB, Aus G, et al; AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
5. Alanee SR, Glogowski EA, Schrader KA, Eastham JA, Offit K. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Front Biosci (Elite Ed). 2014;6:15-30.
6. Castro E, Eeles R. The role of BRCA1 and BRCA2 in prostate cancer. Asian J Androl. 2012;14(3):409-414.
7. Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D’amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008;179(4):1354-1360.
8. Lowrance WT, Scardino PT. Predictive models for newly diagnosed prostate cancer patients. Rev Urol. 2009;11(3):117-126.
9. Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96(1):11-15.
10. Leongamornlert D, Mahmud N, Tymrakiewicz M, et al; UKGPCS Collaborators. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106(10):1697-1701.
11. Kote-Jarai Z, Leongamornlert D, Saunders E, et al; UKGPCS Collaborators. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230-1234.
12. Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(12):929-935.
13. Gallagher DJ, Gaudet MM, Pal P, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16(7):2115-2121.
14. Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748-1757.
15. Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. 2015;68(2):186-193.
16. Bancroft EK, Page EC, Castro E, et al; IMPACT Collaborators. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol. 2014;66(3):489-499.
17. Bratt O, Loman N. Clinical management of prostate cancer in men with BRCA mutations. Eur Urol. 2015;68(2):194-195.
18. Centers for Medicare & Medicaid Services (CMS). MCD archive site. CMS Website. http://localcoverage.cms.gov/mcd_archive/overview.aspx. Accessed January 8, 2016.
19. Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66(3):550-560.
20. Shore ND, Kella N, Moran B, et al. Impact of the cell cycle progression test on physician and patient treatment selection for localized prostate cancer. J Urol. 2015;pii:S0022-5347(15)04811-9 [epub ahead of print].
21. Stone S, Cuzick JM, Fisher G, et al. Validation of an active surveillance threshold for the CCP score in conservatively managed men with localized prostate cancer. J Clin Oncol. 2015;33(suppl 15):e16040.
22. Lewin R, Rizel S, Hendler D, et al. Oncotype-DX recurrence score distribution among breast cancer patients harboring a germline mutation in the BRCA1/2 genes. J Clin Oncol. 2015;33(suppl; abstr 564).
23. Dahm P, Yeung LL, Chang SS, Cookson MS. A critical review of clinical practice guidelines for the management of clinically localized prostate cancer. J Urol. 2008;180(2):451-459.
24. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
25. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
Note: Page numbers differ between the print issue and digital edition.