User login
Cardiac implantable electronic device infection
Cardiac implantable electronic devices (CIEDs) have become common tools to improve the quality of life and longevity of patients with cardiac disease over the last few decades.1–4 CIEDs include implantable cardioverter defibrillators (ICDs), permanent pacemakers, biventricular pacemakers providing cardiac resynchronization therapy with or without a defibrillator, subcutaneous ICDs, and implantable loop recorders. With increasing approved indications, the number of CIEDs implanted each year continues to grow. This, paired with the aging population of patients receiving devices and their medical complexity, has led to a corresponding increase in device-related complications.2,3 One of the most serious complications is CIED infection, which leads to significant morbidity and death. These infections also represent a significant cost burden to the healthcare system, with treatment costs for a CIED infection estimated at over $146,000 in 2008.5
SCOPE OF THE PROBLEM
More than half a million permanent pacemakers and ICDs are implanted each year in the United States, with more than 4 million implanted between 1993 and 2008.5 The risk of infection is 0.5% to 1%, for a first-time implantation and 1% to 5% for a device replacement or upgrade.1,2,5–9 These infections can involve the generator pocket, bloodstream, or cardiac structures, leading to infective endocarditis.10 The timing of CIED infection appears to be bimodal in distribution: early infections usually occur as a result of the implantation procedure itself, whereas late infections occur in patients who are generally unwell or because of an insidious process that eventually crosses a threshold of clinical significance.3,11,12
Incidence and risk factors
Klug et al13 investigated the incidence rate and risk factors of CIED infection prospectively in a large cohort of patients from 44 centers who underwent CIED implantation. Of 6,319 procedures, 4,465 were first implants and the other 1,854 were a replacement or revision; 42 patients (0.68%) developed CIED infection by 12 months after the procedure, and the incidence of infection in replacement or revision cases was nearly twice the rate found in first implants.13 Risk factors for CIED infection included renal failure, heart failure, diabetes, and fever within last 24 hours before CIED implantation.14 The Implantable Cardiac Pulse Generator Replacement (REPLACE) registry found the 6-month incidence rate of CIED infection to be 1.4% after CIED replacement.6
Recently, there has been concern that the rate of newly infected CIEDs has outpaced the rate of newly implanted ones.5,15 Voigt et al15 reported a 12% increase in the rate of CIED implantation from 2004 to 2006 and an out-of-proportion 57% increase in the rate of CIED infection. A review from 2011 confirmed these findings, showing the annual CIED implantation incidence increased an average of 4.7% per year between 1993 and 2008.5 This was probably driven by clinical trials that broadened the indications for ICD implantation for primary prevention.16–19 Between 1993 and 2008, the rate of newly implanted devices increased by 96%, while the rate for newly infected CIEDs increased by 210%; the majority of this increase occurred after 2004.5 The study showed that comorbidities in patients receiving CIEDs increased sharply starting in 2004—alluding to the contribution of comorbid medical conditions such as renal failure, respiratory failure, heart failure, and diabetes to infection risk.5
However, a major obstacle to defining the true incidence rate of CIED infection is the lack of a clear denominator. CIED infection is not limited to the first few months after implantation. In fact, over half of these patients present more than 1 year after the last CIED intervention.12 Therefore, the number of patients at risk continues to grow each year and includes patients who underwent implantation that year or before, making it very difficult to compare infection rates. Additionally, the lack of a clear definition of CIED infection and the variations in duration of follow-up in different studies make it difficult to accurately assess the incidence of CIED infection.
PATHOGENESIS
DIAGNOSIS
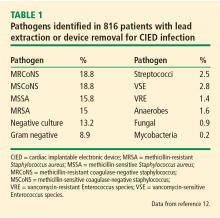
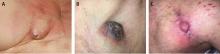
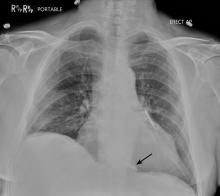
Prompt and accurate diagnosis of CIED infection is critical as it allows for early management with antibiotic therapy and device removal. As the number of CIED implantations increases, providers on the front lines—emergency, family practice, and internal medicine physicians—will play an increasing role in recognizing and diagnosing CIED infection. Patients with CIED infection present with a range of signs and symptoms including fever, chills, erythema, swelling, drainage, tenderness, malaise, erosion, and warmth of the skin overlying the generator pocket.2 In 55% of cases, patients present with localized pocket infection, while the remaining patients have signs of an endovascular infection without obvious pocket involvement.12 Localized pocket infection is more common during the first year after device implantation. CIED-associated endovascular infections occur more commonly in patients with multiple comorbidities including diabetes, renal failure, prior heart valve operation, rheumatic heart disease, and prior bloodstream infection.2 Despite the theoretical divide in CIED infections (endovascular vs pocket), overlap is common: many patients with pocket infection show evidence of bacteremia and vegetations on the leads.
Diagnosing pocket infection from the physical examination can be difficult due to the often subtle manifestations of the underlying pathophysiology and because visible changes to the pocket can occur over weeks and months. Furthermore, differentiating superficial infection, hematoma, seroma, and allergic reactions from deep pocket infection can be challenging. In cases when the diagnosis is not clear and there are no systemic findings of infection, conservative management with close follow-up is reasonable. Similarly, the diagnosis of endovascular infection is sometimes delayed because the symptoms are not very specific or because of a lack of awareness of the presence of a CIED and its role in endovascular infection.
MANAGEMENT
A multidisciplinary approach involving cardiology, infectious disease, electrophysiology, and cardiothoracic surgery teams is required to optimize outcomes in patients with CIED infection. CIED infection is particularly difficult to treat with antibiotic therapy alone because it involves infection of an implanted device and an associated biofilm that is resistant to the effects of antibiotics. Once infection is confirmed, antibiotic therapy serves as an adjunct to the complete removal of the hardware. Most patients receive 2 weeks of intravenous antibiotics after removal of an infected CIED, with longer courses for patients with Staphylococcus aureus infection or documented endocarditis.21
Infectious disease consultation is paramount in order to choose the appropriate type and duration of antibiotic therapy. Conservative approaches that involve using antibiotics alone or incomplete system removal have high failure rates with high rates of morbidity and mortality.13,21–28 However, chronic antibiotic suppressive therapy may be considered as a palliative measure for patients who are not candidates for lead extraction.
DEVICE REMOVAL
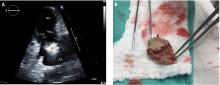
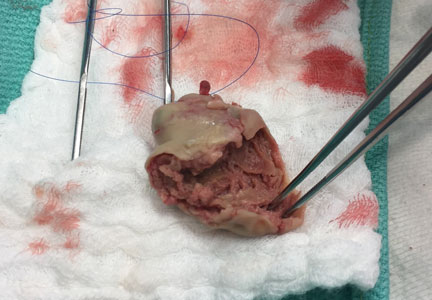
Confirmation of CIED infection is a class I indication for device removal and the patient should be referred to an electrophysiologist. Transvenous lead extraction (TLE) is a percutaneous procedure performed by the electrophysiologist in the electrophysiology laboratory or hybrid operating room with cardiothoracic surgery support, and it is generally performed under general anesthesia with invasive hemodynamic monitoring. After opening and debriding the infected pocket, the generator is disconnected from the leads. After the lead tips are unscrewed from the myocardium, gentle traction is applied to determine if the leads can easily be removed. If traction is unsuccessful, additional tools (both powered or mechanical sheaths) are used to complete the lead extraction29; the goal is to lyse and free the fibrotic attachments between parallel leads and between the leads and vessel wall or the myocardium. Once the lead is freed from the adhesions it can be removed safely.
REIMPLANTATION
The need for reimplantation after removal of an infected CIED should be thought about before the extraction. In general, extracting an infected CIED should be viewed as an opportunity to reassess the need for the device. Almost one-third of patients who undergo extraction of infected CIED do not require immediate reimplantation.2 This could be due to reversal of the initial indication, emergence of new clinical conditions, patient preference, or the lack of an absolute indication. If reimplantation is necessary, the new device is typically placed on the opposite side of the chest from the previously infected pocket site after blood cultures are negative for at least 72 hours.21
CIED INFECTION MORTALITY
Despite proper management with CIED removal supported by antibiotic therapy, CIED infection carries a high risk of death. The 30-day mortality is estimated to be between 5% and 6%.33 In a large case series of 412 CIED extractions, there were 19 in-hospital deaths. Of these 19 deaths, 2 were related to the extraction itself with the other 17 related to sepsis, multiorgan failure, stroke, renal failure, or heart failure.2 The 1-year mortality rate is also increased for this population; recent data show 1-year mortality rates of 8% to 17% despite device removal and antibiotic therapy.2,34,35 This increased mortality rate was also demonstrated in a large cohort of Medicare patients undergoing CIED procedures.36 Medicare patients with CIED infection had double the risk of death at 1 year compared with patients without infection.36
Risk factors for death at 1 year include worse baseline functional status, renal failure, and type of infection; eg, endovascular infection carries a risk of death 2 times higher than pocket infection.37
PREVENTION
Because CIED infection carries significant short-term and long-term mortality rates despite optimal management, the best strategy is prevention. Preventing CIED infection begins with the decision to implant a device with careful assessment of the indication, the timing of the procedure, and the patient’s clinical status. CIED procedures are performed under strict sterile surgical techniques with great attention to the incision and proper closure. Surgical data favor the use of chlorhexidine-alcohol solutions for skin preparation compared with povidone-iodine solutions to prevent both superficial and deep surgical wound infections.38 However, recent studies showed no significant difference between the 2 preparation methods in reducing rates of CIED infection.39,40 In individuals colonized with S aureus, the risk of CIED infection can be reduced using a body wash containing chlorhexidine and a nasal spray containing mupirocin.41,42
Preoperative antibiotics
The use of preoperative antibiotics has been shown to reduce the risk of infection.43 In a large prospective cohort of patients undergoing a de novo or secondary CIED procedure, the use of perioperative antibiotics was negatively associated with the risk of CIED infection.13 This was later confirmed by a double-blind randomized trial of 1,000 patients undergoing permanent pacemaker or ICD initial implantation or generator replacement. This study was stopped prematurely as the use of antibiotics was clearly associated with a lower risk of CIED infection.44 Therefore, prophylaxis with an antibiotic active against staphylococci before the incision is made is a class I indication to prevent infection.1
Currently, no data support giving prophylactic antibiotics after the procedure; however, the Prevention of Arrhythmia Device Infection Trial (PADIT) is currently comparing the risk of infection with conventional preoperative antibiotics vs a regimen of pre- and post-procedure antibiotics (clinicaltrial.gov: NCT01628666).
Hemostasis
Adequate hemostasis is critical, since the risk of CIED infection is 7 times greater with formation of a hematoma.45 Heparin products, especially low-molecular-weight heparin, should be avoided at the time of CIED implantation. In patients at high risk for thromboembolism who are on warfarin therapy, the continuation of warfarin is associated with a lower incidence of hematoma compared with bridging with heparin in patients undergoing CIED procedures.46 Therefore, if anticoagulation can be withheld, it is better to stop the anticoagulant before the procedure. When this is not possible or when it carries significant risk (eg, a patient with a mechanical mitral valve who needs a CIED implantation), it is better to maintain the patient on warfarin therapy with a therapeutic international normalized ratio rather than bridging with heparin products.
Antibacterial envelop and new devices
CONCLUSION
CIED infection is a major complication that carries significant risk of morbidity and death. Early diagnosis and referral to a multidisciplinary treatment team is crucial to increasing the possibility of a cure. While device extraction has risks, it is nevertheless typically required for complete resolution of the infection. Large clinical trials are under way to address current knowledge gaps about CIED infection, including our understanding of the true incidence rate, risk factors, and efficacy of various implantation techniques. Future trends to minimize the risk of CIED infection include better screening, better diagnostic tools, new devices with fewer or no leads, longer battery life to minimize the need for additional procedures, and the use of supportive tools and products to minimize the risk of infection.
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; and the Interdisciplinary Council on Quality of Care and Outcomes Research. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 2010; 7:1043–1047.
- Baddour LM. Cardiac device infection—or not. Circulation 2010; 121:1686–1687.
- Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017; Sept 15. pii: S1547-5271(17)31080-9. doi:10.1016/j.hrthm.2017.09.001. [Epub ahead of print]
- Greenspon AJ, Patel JD, Lau E, et al. 16-Year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J Am Coll Cardiol 2011; 58:1001–1006.
- Poole JE, Gleva MJ, Mela T, et al; REPLACE Registry Investigators. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation 2010; 122:1553–1561.
- Mela T, McGovern BA, Garan H, et al. Long-term infection rates associated with the pectoral versus abdominal approach to cardioverter-defibrillator implants. Am J Cardiol 2001; 88:750–753.
- de Bie MK, van Rees JB, Thijssen J, et al. Cardiac device infections are associated with a significant mortality risk. Heart Rhythm 2012; 9:494–498.
- Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015; 17:767–777.
- Deharo J-C, Quatre A, Mancini J, et al. Long-term outcomes following infection of cardiac implantable electronic devices: a prospective matched cohort study. Heart 2012; 98:724–731.
- Sohail MR, Hussain S, Le KY, et al; Mayo Cardiovascular Infections Study Group. Risk factors associated with early- versus late-onset implantable cardioverter-defibrillator infections. J Interv Card Electrophysiol 2011; 31:171–183.
- Hussein AA, Baghdy Y, Wazni OM, et al. Microbiology of cardiac implantable electronic device infections. JACC Clin Electrophysiol 2016; 2:498–505.
- Klug D, Balde M, Pavin D, et al; PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116:1349–1355.
- Tarakji KG, Wilkoff BL. Management of cardiac implantable electronic device infections: the challenges of understanding the scope of the problem and its associated mortality. Expert Rev Cardiovasc Ther 2013; 11:607–616.
- Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010; 33:414–419.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Kadish A, Dyer A, Daubert JP, et al; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004; 350:2151–2158.
- Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G; Multicenter Unsustained Tachycardia Trial Investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med 1999; 341:1882–1890.
- Abdulmassih R, Makadia J, Como J, Paulson M, Min Z, Bhanot N. Propionibacterium acnes: Time-to-positivity in standard bacterial culture from different anatomical sites. J Clin Med Res 2016; 8:916–918.
- Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007; 49:1851–1859.
- Cacoub P, Leprince P, Nataf P, et al. Pacemaker infective endocarditis. Am J Cardiol 1998; 82:480–484.
- Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med 2000; 133:604–608.
- Bracke FA, Meijer A, van Gelder LM. Pacemaker lead complications: when is extraction appropriate and what can we learn from published data? Heart 2001; 85:254–259.
- Camus C, Leport C, Raffi F, Michelet C, Cartier F, Vilde JL. Sustained bacteremia in 26 patients with a permanent endocardial pacemaker: assessment of wire removal. Clin Infect Dis 1993; 17:46–55.
- Molina JE. Undertreatment and overtreatment of patients with infected antiarrhythmic implantable devices. Ann Thorac Surg 1997; 63:504–509.
- Viganego F, O’Donoghue S, Eldadah Z, et al. Effect of early diagnosis and treatment with percutaneous lead extraction on survival in patients with cardiac device infections. Am J Cardiol 2012; 109:1466–1471.
- Le KY, Sohail MR, Friedman PA, et al; Mayo Cardiovascular Infections Study Group. Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm 2011; 8:1678–1685.
- Wazni O, Wilkoff BL. Considerations for cardiac device lead extraction. Nat Rev Cardiol 2016; 13:221–229.
- Brunner MP, Cronin EM, Duarte VE, et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm 2014; 11:799–805.
- Wazni O, Epstein LM, Carrillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol 2010; 55:579–586.
- Brunner MP, Cronin EM, Wazni O, et al. Outcomes of patients requiring emergent surgical or endovascular intervention for catastrophic complications during transvenous lead extraction. Heart Rhythm 2014; 11:419–425.
- Habib A, Le KY, Baddour LM, et al; for the Mayo Cardiovascular Infections Study Group. Predictors of mortality in patients with cardiovascular implantable electronic device infections. Am J Cardiol 2013; 111:874–879.
- Baman TS, Gupta SK, Valle JA, Yamada E. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol 2009; 2:129–134.
- Deckx S, Marynissen T, Rega F, et al. Predictors of 30-day and 1-year mortality after transvenous lead extraction: a single-centre experience. Europace 2014; 16:1218–1225.
- Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Increased long-term mortality in patients with cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol 2015; 38:231–239.
- Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace 2014; 16:1490–1495.
- Darouiche RO, Wall MJ Jr., Itani KM, et al. Chlorhexidine—alcohol versus povidone—iodine for surgical-site antisepsis. N Engl J Med 2010; 362:18–26.
- Qintar M, Zardkoohi O, Hammadah M, et al. The impact of changing antiseptic skin preparation agent used for cardiac implantable electronic device (CIED) procedures on the risk of infection. Pacing Clin Electrophysiol 2015; 38:240–246.
- Da Costa A, Tulane C, Dauphinot V, et al. Preoperative skin antiseptics for prevention of cardiac implantable electronic device infections: a historical-controlled interventional trial comparing aqueous against alcoholic povidone-iodine solutions. Europace 2015; 17:1092–1098.
- Padfield GJ, Steinberg C, Bennett MT, et al. Preventing cardiac implantable electronic device infections. Heart Rhythm 2015; 12:2344–2356.
- Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010; 362:9–17.
- Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation 1998; 97:1796–1801.
- de Oliveira JC, Martinelli M, Nishioka SADO, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009; 2:29–34.
- Essebag V, Verma A, Healey JS, et al; BRUISE CONTROL Investigators. Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol 2016; 67:1300–1308.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Henrikson CA, Sohail MR, Acosta H, et al. Antibacterial envelope is associated with low infection rates after implantable cardioverter-defibrillator and cardiac resynchronization therapy device replacement: results of the Citadel and Centurion studies. 2017 http://dx.doi.org/10.1016/j.jacep.2017.02.016
- Mittal S, Shaw RE, Michel K, et al. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm 2014; 11:595–601.
- Tarakji KG, Mittal S, Kennergren C, et al. Worldwide Randomized Antibiotic EnveloPe Infection PrevenTion Trial (WRAP-IT). Am Heart J 2016; 180:12–B21.
- Burke MC, Gold MR, Knight BP, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS registry. J Am Coll Cardiol 2015; 65:1605–1615.
- Reddy VY, Exner DV, Cantillon DJ, et al; LEADLESS II Study Investigators. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med 2015; 373:1125–1135.
- Reynolds D, Duray GZ, Omar R, et al; Micra Transcatheter Pacing Study Group. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016; 374:533–541.
Cardiac implantable electronic devices (CIEDs) have become common tools to improve the quality of life and longevity of patients with cardiac disease over the last few decades.1–4 CIEDs include implantable cardioverter defibrillators (ICDs), permanent pacemakers, biventricular pacemakers providing cardiac resynchronization therapy with or without a defibrillator, subcutaneous ICDs, and implantable loop recorders. With increasing approved indications, the number of CIEDs implanted each year continues to grow. This, paired with the aging population of patients receiving devices and their medical complexity, has led to a corresponding increase in device-related complications.2,3 One of the most serious complications is CIED infection, which leads to significant morbidity and death. These infections also represent a significant cost burden to the healthcare system, with treatment costs for a CIED infection estimated at over $146,000 in 2008.5
SCOPE OF THE PROBLEM
More than half a million permanent pacemakers and ICDs are implanted each year in the United States, with more than 4 million implanted between 1993 and 2008.5 The risk of infection is 0.5% to 1%, for a first-time implantation and 1% to 5% for a device replacement or upgrade.1,2,5–9 These infections can involve the generator pocket, bloodstream, or cardiac structures, leading to infective endocarditis.10 The timing of CIED infection appears to be bimodal in distribution: early infections usually occur as a result of the implantation procedure itself, whereas late infections occur in patients who are generally unwell or because of an insidious process that eventually crosses a threshold of clinical significance.3,11,12
Incidence and risk factors
Klug et al13 investigated the incidence rate and risk factors of CIED infection prospectively in a large cohort of patients from 44 centers who underwent CIED implantation. Of 6,319 procedures, 4,465 were first implants and the other 1,854 were a replacement or revision; 42 patients (0.68%) developed CIED infection by 12 months after the procedure, and the incidence of infection in replacement or revision cases was nearly twice the rate found in first implants.13 Risk factors for CIED infection included renal failure, heart failure, diabetes, and fever within last 24 hours before CIED implantation.14 The Implantable Cardiac Pulse Generator Replacement (REPLACE) registry found the 6-month incidence rate of CIED infection to be 1.4% after CIED replacement.6
Recently, there has been concern that the rate of newly infected CIEDs has outpaced the rate of newly implanted ones.5,15 Voigt et al15 reported a 12% increase in the rate of CIED implantation from 2004 to 2006 and an out-of-proportion 57% increase in the rate of CIED infection. A review from 2011 confirmed these findings, showing the annual CIED implantation incidence increased an average of 4.7% per year between 1993 and 2008.5 This was probably driven by clinical trials that broadened the indications for ICD implantation for primary prevention.16–19 Between 1993 and 2008, the rate of newly implanted devices increased by 96%, while the rate for newly infected CIEDs increased by 210%; the majority of this increase occurred after 2004.5 The study showed that comorbidities in patients receiving CIEDs increased sharply starting in 2004—alluding to the contribution of comorbid medical conditions such as renal failure, respiratory failure, heart failure, and diabetes to infection risk.5
However, a major obstacle to defining the true incidence rate of CIED infection is the lack of a clear denominator. CIED infection is not limited to the first few months after implantation. In fact, over half of these patients present more than 1 year after the last CIED intervention.12 Therefore, the number of patients at risk continues to grow each year and includes patients who underwent implantation that year or before, making it very difficult to compare infection rates. Additionally, the lack of a clear definition of CIED infection and the variations in duration of follow-up in different studies make it difficult to accurately assess the incidence of CIED infection.
PATHOGENESIS
DIAGNOSIS
Prompt and accurate diagnosis of CIED infection is critical as it allows for early management with antibiotic therapy and device removal. As the number of CIED implantations increases, providers on the front lines—emergency, family practice, and internal medicine physicians—will play an increasing role in recognizing and diagnosing CIED infection. Patients with CIED infection present with a range of signs and symptoms including fever, chills, erythema, swelling, drainage, tenderness, malaise, erosion, and warmth of the skin overlying the generator pocket.2 In 55% of cases, patients present with localized pocket infection, while the remaining patients have signs of an endovascular infection without obvious pocket involvement.12 Localized pocket infection is more common during the first year after device implantation. CIED-associated endovascular infections occur more commonly in patients with multiple comorbidities including diabetes, renal failure, prior heart valve operation, rheumatic heart disease, and prior bloodstream infection.2 Despite the theoretical divide in CIED infections (endovascular vs pocket), overlap is common: many patients with pocket infection show evidence of bacteremia and vegetations on the leads.
Diagnosing pocket infection from the physical examination can be difficult due to the often subtle manifestations of the underlying pathophysiology and because visible changes to the pocket can occur over weeks and months. Furthermore, differentiating superficial infection, hematoma, seroma, and allergic reactions from deep pocket infection can be challenging. In cases when the diagnosis is not clear and there are no systemic findings of infection, conservative management with close follow-up is reasonable. Similarly, the diagnosis of endovascular infection is sometimes delayed because the symptoms are not very specific or because of a lack of awareness of the presence of a CIED and its role in endovascular infection.
MANAGEMENT
A multidisciplinary approach involving cardiology, infectious disease, electrophysiology, and cardiothoracic surgery teams is required to optimize outcomes in patients with CIED infection. CIED infection is particularly difficult to treat with antibiotic therapy alone because it involves infection of an implanted device and an associated biofilm that is resistant to the effects of antibiotics. Once infection is confirmed, antibiotic therapy serves as an adjunct to the complete removal of the hardware. Most patients receive 2 weeks of intravenous antibiotics after removal of an infected CIED, with longer courses for patients with Staphylococcus aureus infection or documented endocarditis.21
Infectious disease consultation is paramount in order to choose the appropriate type and duration of antibiotic therapy. Conservative approaches that involve using antibiotics alone or incomplete system removal have high failure rates with high rates of morbidity and mortality.13,21–28 However, chronic antibiotic suppressive therapy may be considered as a palliative measure for patients who are not candidates for lead extraction.
DEVICE REMOVAL
Confirmation of CIED infection is a class I indication for device removal and the patient should be referred to an electrophysiologist. Transvenous lead extraction (TLE) is a percutaneous procedure performed by the electrophysiologist in the electrophysiology laboratory or hybrid operating room with cardiothoracic surgery support, and it is generally performed under general anesthesia with invasive hemodynamic monitoring. After opening and debriding the infected pocket, the generator is disconnected from the leads. After the lead tips are unscrewed from the myocardium, gentle traction is applied to determine if the leads can easily be removed. If traction is unsuccessful, additional tools (both powered or mechanical sheaths) are used to complete the lead extraction29; the goal is to lyse and free the fibrotic attachments between parallel leads and between the leads and vessel wall or the myocardium. Once the lead is freed from the adhesions it can be removed safely.
REIMPLANTATION
The need for reimplantation after removal of an infected CIED should be thought about before the extraction. In general, extracting an infected CIED should be viewed as an opportunity to reassess the need for the device. Almost one-third of patients who undergo extraction of infected CIED do not require immediate reimplantation.2 This could be due to reversal of the initial indication, emergence of new clinical conditions, patient preference, or the lack of an absolute indication. If reimplantation is necessary, the new device is typically placed on the opposite side of the chest from the previously infected pocket site after blood cultures are negative for at least 72 hours.21
CIED INFECTION MORTALITY
Despite proper management with CIED removal supported by antibiotic therapy, CIED infection carries a high risk of death. The 30-day mortality is estimated to be between 5% and 6%.33 In a large case series of 412 CIED extractions, there were 19 in-hospital deaths. Of these 19 deaths, 2 were related to the extraction itself with the other 17 related to sepsis, multiorgan failure, stroke, renal failure, or heart failure.2 The 1-year mortality rate is also increased for this population; recent data show 1-year mortality rates of 8% to 17% despite device removal and antibiotic therapy.2,34,35 This increased mortality rate was also demonstrated in a large cohort of Medicare patients undergoing CIED procedures.36 Medicare patients with CIED infection had double the risk of death at 1 year compared with patients without infection.36
Risk factors for death at 1 year include worse baseline functional status, renal failure, and type of infection; eg, endovascular infection carries a risk of death 2 times higher than pocket infection.37
PREVENTION
Because CIED infection carries significant short-term and long-term mortality rates despite optimal management, the best strategy is prevention. Preventing CIED infection begins with the decision to implant a device with careful assessment of the indication, the timing of the procedure, and the patient’s clinical status. CIED procedures are performed under strict sterile surgical techniques with great attention to the incision and proper closure. Surgical data favor the use of chlorhexidine-alcohol solutions for skin preparation compared with povidone-iodine solutions to prevent both superficial and deep surgical wound infections.38 However, recent studies showed no significant difference between the 2 preparation methods in reducing rates of CIED infection.39,40 In individuals colonized with S aureus, the risk of CIED infection can be reduced using a body wash containing chlorhexidine and a nasal spray containing mupirocin.41,42
Preoperative antibiotics
The use of preoperative antibiotics has been shown to reduce the risk of infection.43 In a large prospective cohort of patients undergoing a de novo or secondary CIED procedure, the use of perioperative antibiotics was negatively associated with the risk of CIED infection.13 This was later confirmed by a double-blind randomized trial of 1,000 patients undergoing permanent pacemaker or ICD initial implantation or generator replacement. This study was stopped prematurely as the use of antibiotics was clearly associated with a lower risk of CIED infection.44 Therefore, prophylaxis with an antibiotic active against staphylococci before the incision is made is a class I indication to prevent infection.1
Currently, no data support giving prophylactic antibiotics after the procedure; however, the Prevention of Arrhythmia Device Infection Trial (PADIT) is currently comparing the risk of infection with conventional preoperative antibiotics vs a regimen of pre- and post-procedure antibiotics (clinicaltrial.gov: NCT01628666).
Hemostasis
Adequate hemostasis is critical, since the risk of CIED infection is 7 times greater with formation of a hematoma.45 Heparin products, especially low-molecular-weight heparin, should be avoided at the time of CIED implantation. In patients at high risk for thromboembolism who are on warfarin therapy, the continuation of warfarin is associated with a lower incidence of hematoma compared with bridging with heparin in patients undergoing CIED procedures.46 Therefore, if anticoagulation can be withheld, it is better to stop the anticoagulant before the procedure. When this is not possible or when it carries significant risk (eg, a patient with a mechanical mitral valve who needs a CIED implantation), it is better to maintain the patient on warfarin therapy with a therapeutic international normalized ratio rather than bridging with heparin products.
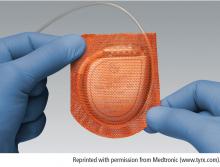
Antibacterial envelop and new devices
CONCLUSION
CIED infection is a major complication that carries significant risk of morbidity and death. Early diagnosis and referral to a multidisciplinary treatment team is crucial to increasing the possibility of a cure. While device extraction has risks, it is nevertheless typically required for complete resolution of the infection. Large clinical trials are under way to address current knowledge gaps about CIED infection, including our understanding of the true incidence rate, risk factors, and efficacy of various implantation techniques. Future trends to minimize the risk of CIED infection include better screening, better diagnostic tools, new devices with fewer or no leads, longer battery life to minimize the need for additional procedures, and the use of supportive tools and products to minimize the risk of infection.
Cardiac implantable electronic devices (CIEDs) have become common tools to improve the quality of life and longevity of patients with cardiac disease over the last few decades.1–4 CIEDs include implantable cardioverter defibrillators (ICDs), permanent pacemakers, biventricular pacemakers providing cardiac resynchronization therapy with or without a defibrillator, subcutaneous ICDs, and implantable loop recorders. With increasing approved indications, the number of CIEDs implanted each year continues to grow. This, paired with the aging population of patients receiving devices and their medical complexity, has led to a corresponding increase in device-related complications.2,3 One of the most serious complications is CIED infection, which leads to significant morbidity and death. These infections also represent a significant cost burden to the healthcare system, with treatment costs for a CIED infection estimated at over $146,000 in 2008.5
SCOPE OF THE PROBLEM
More than half a million permanent pacemakers and ICDs are implanted each year in the United States, with more than 4 million implanted between 1993 and 2008.5 The risk of infection is 0.5% to 1%, for a first-time implantation and 1% to 5% for a device replacement or upgrade.1,2,5–9 These infections can involve the generator pocket, bloodstream, or cardiac structures, leading to infective endocarditis.10 The timing of CIED infection appears to be bimodal in distribution: early infections usually occur as a result of the implantation procedure itself, whereas late infections occur in patients who are generally unwell or because of an insidious process that eventually crosses a threshold of clinical significance.3,11,12
Incidence and risk factors
Klug et al13 investigated the incidence rate and risk factors of CIED infection prospectively in a large cohort of patients from 44 centers who underwent CIED implantation. Of 6,319 procedures, 4,465 were first implants and the other 1,854 were a replacement or revision; 42 patients (0.68%) developed CIED infection by 12 months after the procedure, and the incidence of infection in replacement or revision cases was nearly twice the rate found in first implants.13 Risk factors for CIED infection included renal failure, heart failure, diabetes, and fever within last 24 hours before CIED implantation.14 The Implantable Cardiac Pulse Generator Replacement (REPLACE) registry found the 6-month incidence rate of CIED infection to be 1.4% after CIED replacement.6
Recently, there has been concern that the rate of newly infected CIEDs has outpaced the rate of newly implanted ones.5,15 Voigt et al15 reported a 12% increase in the rate of CIED implantation from 2004 to 2006 and an out-of-proportion 57% increase in the rate of CIED infection. A review from 2011 confirmed these findings, showing the annual CIED implantation incidence increased an average of 4.7% per year between 1993 and 2008.5 This was probably driven by clinical trials that broadened the indications for ICD implantation for primary prevention.16–19 Between 1993 and 2008, the rate of newly implanted devices increased by 96%, while the rate for newly infected CIEDs increased by 210%; the majority of this increase occurred after 2004.5 The study showed that comorbidities in patients receiving CIEDs increased sharply starting in 2004—alluding to the contribution of comorbid medical conditions such as renal failure, respiratory failure, heart failure, and diabetes to infection risk.5
However, a major obstacle to defining the true incidence rate of CIED infection is the lack of a clear denominator. CIED infection is not limited to the first few months after implantation. In fact, over half of these patients present more than 1 year after the last CIED intervention.12 Therefore, the number of patients at risk continues to grow each year and includes patients who underwent implantation that year or before, making it very difficult to compare infection rates. Additionally, the lack of a clear definition of CIED infection and the variations in duration of follow-up in different studies make it difficult to accurately assess the incidence of CIED infection.
PATHOGENESIS
DIAGNOSIS
Prompt and accurate diagnosis of CIED infection is critical as it allows for early management with antibiotic therapy and device removal. As the number of CIED implantations increases, providers on the front lines—emergency, family practice, and internal medicine physicians—will play an increasing role in recognizing and diagnosing CIED infection. Patients with CIED infection present with a range of signs and symptoms including fever, chills, erythema, swelling, drainage, tenderness, malaise, erosion, and warmth of the skin overlying the generator pocket.2 In 55% of cases, patients present with localized pocket infection, while the remaining patients have signs of an endovascular infection without obvious pocket involvement.12 Localized pocket infection is more common during the first year after device implantation. CIED-associated endovascular infections occur more commonly in patients with multiple comorbidities including diabetes, renal failure, prior heart valve operation, rheumatic heart disease, and prior bloodstream infection.2 Despite the theoretical divide in CIED infections (endovascular vs pocket), overlap is common: many patients with pocket infection show evidence of bacteremia and vegetations on the leads.
Diagnosing pocket infection from the physical examination can be difficult due to the often subtle manifestations of the underlying pathophysiology and because visible changes to the pocket can occur over weeks and months. Furthermore, differentiating superficial infection, hematoma, seroma, and allergic reactions from deep pocket infection can be challenging. In cases when the diagnosis is not clear and there are no systemic findings of infection, conservative management with close follow-up is reasonable. Similarly, the diagnosis of endovascular infection is sometimes delayed because the symptoms are not very specific or because of a lack of awareness of the presence of a CIED and its role in endovascular infection.
MANAGEMENT
A multidisciplinary approach involving cardiology, infectious disease, electrophysiology, and cardiothoracic surgery teams is required to optimize outcomes in patients with CIED infection. CIED infection is particularly difficult to treat with antibiotic therapy alone because it involves infection of an implanted device and an associated biofilm that is resistant to the effects of antibiotics. Once infection is confirmed, antibiotic therapy serves as an adjunct to the complete removal of the hardware. Most patients receive 2 weeks of intravenous antibiotics after removal of an infected CIED, with longer courses for patients with Staphylococcus aureus infection or documented endocarditis.21
Infectious disease consultation is paramount in order to choose the appropriate type and duration of antibiotic therapy. Conservative approaches that involve using antibiotics alone or incomplete system removal have high failure rates with high rates of morbidity and mortality.13,21–28 However, chronic antibiotic suppressive therapy may be considered as a palliative measure for patients who are not candidates for lead extraction.
DEVICE REMOVAL
Confirmation of CIED infection is a class I indication for device removal and the patient should be referred to an electrophysiologist. Transvenous lead extraction (TLE) is a percutaneous procedure performed by the electrophysiologist in the electrophysiology laboratory or hybrid operating room with cardiothoracic surgery support, and it is generally performed under general anesthesia with invasive hemodynamic monitoring. After opening and debriding the infected pocket, the generator is disconnected from the leads. After the lead tips are unscrewed from the myocardium, gentle traction is applied to determine if the leads can easily be removed. If traction is unsuccessful, additional tools (both powered or mechanical sheaths) are used to complete the lead extraction29; the goal is to lyse and free the fibrotic attachments between parallel leads and between the leads and vessel wall or the myocardium. Once the lead is freed from the adhesions it can be removed safely.
REIMPLANTATION
The need for reimplantation after removal of an infected CIED should be thought about before the extraction. In general, extracting an infected CIED should be viewed as an opportunity to reassess the need for the device. Almost one-third of patients who undergo extraction of infected CIED do not require immediate reimplantation.2 This could be due to reversal of the initial indication, emergence of new clinical conditions, patient preference, or the lack of an absolute indication. If reimplantation is necessary, the new device is typically placed on the opposite side of the chest from the previously infected pocket site after blood cultures are negative for at least 72 hours.21
CIED INFECTION MORTALITY
Despite proper management with CIED removal supported by antibiotic therapy, CIED infection carries a high risk of death. The 30-day mortality is estimated to be between 5% and 6%.33 In a large case series of 412 CIED extractions, there were 19 in-hospital deaths. Of these 19 deaths, 2 were related to the extraction itself with the other 17 related to sepsis, multiorgan failure, stroke, renal failure, or heart failure.2 The 1-year mortality rate is also increased for this population; recent data show 1-year mortality rates of 8% to 17% despite device removal and antibiotic therapy.2,34,35 This increased mortality rate was also demonstrated in a large cohort of Medicare patients undergoing CIED procedures.36 Medicare patients with CIED infection had double the risk of death at 1 year compared with patients without infection.36
Risk factors for death at 1 year include worse baseline functional status, renal failure, and type of infection; eg, endovascular infection carries a risk of death 2 times higher than pocket infection.37
PREVENTION
Because CIED infection carries significant short-term and long-term mortality rates despite optimal management, the best strategy is prevention. Preventing CIED infection begins with the decision to implant a device with careful assessment of the indication, the timing of the procedure, and the patient’s clinical status. CIED procedures are performed under strict sterile surgical techniques with great attention to the incision and proper closure. Surgical data favor the use of chlorhexidine-alcohol solutions for skin preparation compared with povidone-iodine solutions to prevent both superficial and deep surgical wound infections.38 However, recent studies showed no significant difference between the 2 preparation methods in reducing rates of CIED infection.39,40 In individuals colonized with S aureus, the risk of CIED infection can be reduced using a body wash containing chlorhexidine and a nasal spray containing mupirocin.41,42
Preoperative antibiotics
The use of preoperative antibiotics has been shown to reduce the risk of infection.43 In a large prospective cohort of patients undergoing a de novo or secondary CIED procedure, the use of perioperative antibiotics was negatively associated with the risk of CIED infection.13 This was later confirmed by a double-blind randomized trial of 1,000 patients undergoing permanent pacemaker or ICD initial implantation or generator replacement. This study was stopped prematurely as the use of antibiotics was clearly associated with a lower risk of CIED infection.44 Therefore, prophylaxis with an antibiotic active against staphylococci before the incision is made is a class I indication to prevent infection.1
Currently, no data support giving prophylactic antibiotics after the procedure; however, the Prevention of Arrhythmia Device Infection Trial (PADIT) is currently comparing the risk of infection with conventional preoperative antibiotics vs a regimen of pre- and post-procedure antibiotics (clinicaltrial.gov: NCT01628666).
Hemostasis
Adequate hemostasis is critical, since the risk of CIED infection is 7 times greater with formation of a hematoma.45 Heparin products, especially low-molecular-weight heparin, should be avoided at the time of CIED implantation. In patients at high risk for thromboembolism who are on warfarin therapy, the continuation of warfarin is associated with a lower incidence of hematoma compared with bridging with heparin in patients undergoing CIED procedures.46 Therefore, if anticoagulation can be withheld, it is better to stop the anticoagulant before the procedure. When this is not possible or when it carries significant risk (eg, a patient with a mechanical mitral valve who needs a CIED implantation), it is better to maintain the patient on warfarin therapy with a therapeutic international normalized ratio rather than bridging with heparin products.
Antibacterial envelop and new devices
CONCLUSION
CIED infection is a major complication that carries significant risk of morbidity and death. Early diagnosis and referral to a multidisciplinary treatment team is crucial to increasing the possibility of a cure. While device extraction has risks, it is nevertheless typically required for complete resolution of the infection. Large clinical trials are under way to address current knowledge gaps about CIED infection, including our understanding of the true incidence rate, risk factors, and efficacy of various implantation techniques. Future trends to minimize the risk of CIED infection include better screening, better diagnostic tools, new devices with fewer or no leads, longer battery life to minimize the need for additional procedures, and the use of supportive tools and products to minimize the risk of infection.
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; and the Interdisciplinary Council on Quality of Care and Outcomes Research. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 2010; 7:1043–1047.
- Baddour LM. Cardiac device infection—or not. Circulation 2010; 121:1686–1687.
- Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017; Sept 15. pii: S1547-5271(17)31080-9. doi:10.1016/j.hrthm.2017.09.001. [Epub ahead of print]
- Greenspon AJ, Patel JD, Lau E, et al. 16-Year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J Am Coll Cardiol 2011; 58:1001–1006.
- Poole JE, Gleva MJ, Mela T, et al; REPLACE Registry Investigators. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation 2010; 122:1553–1561.
- Mela T, McGovern BA, Garan H, et al. Long-term infection rates associated with the pectoral versus abdominal approach to cardioverter-defibrillator implants. Am J Cardiol 2001; 88:750–753.
- de Bie MK, van Rees JB, Thijssen J, et al. Cardiac device infections are associated with a significant mortality risk. Heart Rhythm 2012; 9:494–498.
- Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015; 17:767–777.
- Deharo J-C, Quatre A, Mancini J, et al. Long-term outcomes following infection of cardiac implantable electronic devices: a prospective matched cohort study. Heart 2012; 98:724–731.
- Sohail MR, Hussain S, Le KY, et al; Mayo Cardiovascular Infections Study Group. Risk factors associated with early- versus late-onset implantable cardioverter-defibrillator infections. J Interv Card Electrophysiol 2011; 31:171–183.
- Hussein AA, Baghdy Y, Wazni OM, et al. Microbiology of cardiac implantable electronic device infections. JACC Clin Electrophysiol 2016; 2:498–505.
- Klug D, Balde M, Pavin D, et al; PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116:1349–1355.
- Tarakji KG, Wilkoff BL. Management of cardiac implantable electronic device infections: the challenges of understanding the scope of the problem and its associated mortality. Expert Rev Cardiovasc Ther 2013; 11:607–616.
- Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010; 33:414–419.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Kadish A, Dyer A, Daubert JP, et al; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004; 350:2151–2158.
- Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G; Multicenter Unsustained Tachycardia Trial Investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med 1999; 341:1882–1890.
- Abdulmassih R, Makadia J, Como J, Paulson M, Min Z, Bhanot N. Propionibacterium acnes: Time-to-positivity in standard bacterial culture from different anatomical sites. J Clin Med Res 2016; 8:916–918.
- Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007; 49:1851–1859.
- Cacoub P, Leprince P, Nataf P, et al. Pacemaker infective endocarditis. Am J Cardiol 1998; 82:480–484.
- Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med 2000; 133:604–608.
- Bracke FA, Meijer A, van Gelder LM. Pacemaker lead complications: when is extraction appropriate and what can we learn from published data? Heart 2001; 85:254–259.
- Camus C, Leport C, Raffi F, Michelet C, Cartier F, Vilde JL. Sustained bacteremia in 26 patients with a permanent endocardial pacemaker: assessment of wire removal. Clin Infect Dis 1993; 17:46–55.
- Molina JE. Undertreatment and overtreatment of patients with infected antiarrhythmic implantable devices. Ann Thorac Surg 1997; 63:504–509.
- Viganego F, O’Donoghue S, Eldadah Z, et al. Effect of early diagnosis and treatment with percutaneous lead extraction on survival in patients with cardiac device infections. Am J Cardiol 2012; 109:1466–1471.
- Le KY, Sohail MR, Friedman PA, et al; Mayo Cardiovascular Infections Study Group. Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm 2011; 8:1678–1685.
- Wazni O, Wilkoff BL. Considerations for cardiac device lead extraction. Nat Rev Cardiol 2016; 13:221–229.
- Brunner MP, Cronin EM, Duarte VE, et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm 2014; 11:799–805.
- Wazni O, Epstein LM, Carrillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol 2010; 55:579–586.
- Brunner MP, Cronin EM, Wazni O, et al. Outcomes of patients requiring emergent surgical or endovascular intervention for catastrophic complications during transvenous lead extraction. Heart Rhythm 2014; 11:419–425.
- Habib A, Le KY, Baddour LM, et al; for the Mayo Cardiovascular Infections Study Group. Predictors of mortality in patients with cardiovascular implantable electronic device infections. Am J Cardiol 2013; 111:874–879.
- Baman TS, Gupta SK, Valle JA, Yamada E. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol 2009; 2:129–134.
- Deckx S, Marynissen T, Rega F, et al. Predictors of 30-day and 1-year mortality after transvenous lead extraction: a single-centre experience. Europace 2014; 16:1218–1225.
- Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Increased long-term mortality in patients with cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol 2015; 38:231–239.
- Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace 2014; 16:1490–1495.
- Darouiche RO, Wall MJ Jr., Itani KM, et al. Chlorhexidine—alcohol versus povidone—iodine for surgical-site antisepsis. N Engl J Med 2010; 362:18–26.
- Qintar M, Zardkoohi O, Hammadah M, et al. The impact of changing antiseptic skin preparation agent used for cardiac implantable electronic device (CIED) procedures on the risk of infection. Pacing Clin Electrophysiol 2015; 38:240–246.
- Da Costa A, Tulane C, Dauphinot V, et al. Preoperative skin antiseptics for prevention of cardiac implantable electronic device infections: a historical-controlled interventional trial comparing aqueous against alcoholic povidone-iodine solutions. Europace 2015; 17:1092–1098.
- Padfield GJ, Steinberg C, Bennett MT, et al. Preventing cardiac implantable electronic device infections. Heart Rhythm 2015; 12:2344–2356.
- Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010; 362:9–17.
- Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation 1998; 97:1796–1801.
- de Oliveira JC, Martinelli M, Nishioka SADO, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009; 2:29–34.
- Essebag V, Verma A, Healey JS, et al; BRUISE CONTROL Investigators. Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol 2016; 67:1300–1308.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Henrikson CA, Sohail MR, Acosta H, et al. Antibacterial envelope is associated with low infection rates after implantable cardioverter-defibrillator and cardiac resynchronization therapy device replacement: results of the Citadel and Centurion studies. 2017 http://dx.doi.org/10.1016/j.jacep.2017.02.016
- Mittal S, Shaw RE, Michel K, et al. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm 2014; 11:595–601.
- Tarakji KG, Mittal S, Kennergren C, et al. Worldwide Randomized Antibiotic EnveloPe Infection PrevenTion Trial (WRAP-IT). Am Heart J 2016; 180:12–B21.
- Burke MC, Gold MR, Knight BP, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS registry. J Am Coll Cardiol 2015; 65:1605–1615.
- Reddy VY, Exner DV, Cantillon DJ, et al; LEADLESS II Study Investigators. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med 2015; 373:1125–1135.
- Reynolds D, Duray GZ, Omar R, et al; Micra Transcatheter Pacing Study Group. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016; 374:533–541.
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; and the Interdisciplinary Council on Quality of Care and Outcomes Research. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 2010; 7:1043–1047.
- Baddour LM. Cardiac device infection—or not. Circulation 2010; 121:1686–1687.
- Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017; Sept 15. pii: S1547-5271(17)31080-9. doi:10.1016/j.hrthm.2017.09.001. [Epub ahead of print]
- Greenspon AJ, Patel JD, Lau E, et al. 16-Year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J Am Coll Cardiol 2011; 58:1001–1006.
- Poole JE, Gleva MJ, Mela T, et al; REPLACE Registry Investigators. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation 2010; 122:1553–1561.
- Mela T, McGovern BA, Garan H, et al. Long-term infection rates associated with the pectoral versus abdominal approach to cardioverter-defibrillator implants. Am J Cardiol 2001; 88:750–753.
- de Bie MK, van Rees JB, Thijssen J, et al. Cardiac device infections are associated with a significant mortality risk. Heart Rhythm 2012; 9:494–498.
- Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015; 17:767–777.
- Deharo J-C, Quatre A, Mancini J, et al. Long-term outcomes following infection of cardiac implantable electronic devices: a prospective matched cohort study. Heart 2012; 98:724–731.
- Sohail MR, Hussain S, Le KY, et al; Mayo Cardiovascular Infections Study Group. Risk factors associated with early- versus late-onset implantable cardioverter-defibrillator infections. J Interv Card Electrophysiol 2011; 31:171–183.
- Hussein AA, Baghdy Y, Wazni OM, et al. Microbiology of cardiac implantable electronic device infections. JACC Clin Electrophysiol 2016; 2:498–505.
- Klug D, Balde M, Pavin D, et al; PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116:1349–1355.
- Tarakji KG, Wilkoff BL. Management of cardiac implantable electronic device infections: the challenges of understanding the scope of the problem and its associated mortality. Expert Rev Cardiovasc Ther 2013; 11:607–616.
- Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010; 33:414–419.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Kadish A, Dyer A, Daubert JP, et al; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004; 350:2151–2158.
- Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G; Multicenter Unsustained Tachycardia Trial Investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med 1999; 341:1882–1890.
- Abdulmassih R, Makadia J, Como J, Paulson M, Min Z, Bhanot N. Propionibacterium acnes: Time-to-positivity in standard bacterial culture from different anatomical sites. J Clin Med Res 2016; 8:916–918.
- Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007; 49:1851–1859.
- Cacoub P, Leprince P, Nataf P, et al. Pacemaker infective endocarditis. Am J Cardiol 1998; 82:480–484.
- Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med 2000; 133:604–608.
- Bracke FA, Meijer A, van Gelder LM. Pacemaker lead complications: when is extraction appropriate and what can we learn from published data? Heart 2001; 85:254–259.
- Camus C, Leport C, Raffi F, Michelet C, Cartier F, Vilde JL. Sustained bacteremia in 26 patients with a permanent endocardial pacemaker: assessment of wire removal. Clin Infect Dis 1993; 17:46–55.
- Molina JE. Undertreatment and overtreatment of patients with infected antiarrhythmic implantable devices. Ann Thorac Surg 1997; 63:504–509.
- Viganego F, O’Donoghue S, Eldadah Z, et al. Effect of early diagnosis and treatment with percutaneous lead extraction on survival in patients with cardiac device infections. Am J Cardiol 2012; 109:1466–1471.
- Le KY, Sohail MR, Friedman PA, et al; Mayo Cardiovascular Infections Study Group. Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm 2011; 8:1678–1685.
- Wazni O, Wilkoff BL. Considerations for cardiac device lead extraction. Nat Rev Cardiol 2016; 13:221–229.
- Brunner MP, Cronin EM, Duarte VE, et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm 2014; 11:799–805.
- Wazni O, Epstein LM, Carrillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol 2010; 55:579–586.
- Brunner MP, Cronin EM, Wazni O, et al. Outcomes of patients requiring emergent surgical or endovascular intervention for catastrophic complications during transvenous lead extraction. Heart Rhythm 2014; 11:419–425.
- Habib A, Le KY, Baddour LM, et al; for the Mayo Cardiovascular Infections Study Group. Predictors of mortality in patients with cardiovascular implantable electronic device infections. Am J Cardiol 2013; 111:874–879.
- Baman TS, Gupta SK, Valle JA, Yamada E. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol 2009; 2:129–134.
- Deckx S, Marynissen T, Rega F, et al. Predictors of 30-day and 1-year mortality after transvenous lead extraction: a single-centre experience. Europace 2014; 16:1218–1225.
- Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Increased long-term mortality in patients with cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol 2015; 38:231–239.
- Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace 2014; 16:1490–1495.
- Darouiche RO, Wall MJ Jr., Itani KM, et al. Chlorhexidine—alcohol versus povidone—iodine for surgical-site antisepsis. N Engl J Med 2010; 362:18–26.
- Qintar M, Zardkoohi O, Hammadah M, et al. The impact of changing antiseptic skin preparation agent used for cardiac implantable electronic device (CIED) procedures on the risk of infection. Pacing Clin Electrophysiol 2015; 38:240–246.
- Da Costa A, Tulane C, Dauphinot V, et al. Preoperative skin antiseptics for prevention of cardiac implantable electronic device infections: a historical-controlled interventional trial comparing aqueous against alcoholic povidone-iodine solutions. Europace 2015; 17:1092–1098.
- Padfield GJ, Steinberg C, Bennett MT, et al. Preventing cardiac implantable electronic device infections. Heart Rhythm 2015; 12:2344–2356.
- Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010; 362:9–17.
- Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation 1998; 97:1796–1801.
- de Oliveira JC, Martinelli M, Nishioka SADO, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009; 2:29–34.
- Essebag V, Verma A, Healey JS, et al; BRUISE CONTROL Investigators. Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol 2016; 67:1300–1308.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Henrikson CA, Sohail MR, Acosta H, et al. Antibacterial envelope is associated with low infection rates after implantable cardioverter-defibrillator and cardiac resynchronization therapy device replacement: results of the Citadel and Centurion studies. 2017 http://dx.doi.org/10.1016/j.jacep.2017.02.016
- Mittal S, Shaw RE, Michel K, et al. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm 2014; 11:595–601.
- Tarakji KG, Mittal S, Kennergren C, et al. Worldwide Randomized Antibiotic EnveloPe Infection PrevenTion Trial (WRAP-IT). Am Heart J 2016; 180:12–B21.
- Burke MC, Gold MR, Knight BP, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS registry. J Am Coll Cardiol 2015; 65:1605–1615.
- Reddy VY, Exner DV, Cantillon DJ, et al; LEADLESS II Study Investigators. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med 2015; 373:1125–1135.
- Reynolds D, Duray GZ, Omar R, et al; Micra Transcatheter Pacing Study Group. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016; 374:533–541.
KEY POINTS
- CIED use is increasing, as are the number of CIED infections, which are associated with significant morbidity and mortality.
- Prompt diagnosis of CIED infection allows for early management with antibiotics and device removal, which is typically needed for resolution of the infection.
- Prevention of CIED infection is an important strategy, and more research is needed to inform the incidence of CIED infection, risk factors, and devices and techniques to minimize the risk of infection.