User login
Why placental shape and vasculature matter
The intrauterine environment significantly influences not only fetal and infant health, but adult health risks as well. Yet current efforts in obstetrics to assess the environment and optimize fetal and long-term outcomes are based on diagnostics that focus on and measure fetal signs and symptoms. By and large, the current approach overlooks the placenta – the organ that serves as the principal regulator of fetal growth and health. If the fetus appears free of risk or complications, we assume the placenta must be “okay.”
Yet this isn’t always the case. By assuming the placenta is healthy and not observing and measuring its condition, we are too often too late to effectively alter fetal- and longer-term outcomes once fetal signs and symptoms appear.
Research in recent decades, and particularly in the past 10 years, has demonstrated that placental shape matters, that it’s linked to function, and that quantifying abnormalities in shape and growth can be a meaningful clinical tool for detecting and preventing disease early in pregnancy.
We now know, specifically, that abnormal shapes reflect alterations in placental vascular architecture that lead to reduced placental efficiency. We also now understand that placental weight or size may serve as a proxy for fetoplacental metabolism.
We have more research to do to further develop models, to collect more data, and to more fully understand the placental pathology that precedes detectable fetal and/or maternal disease. We also need to know whether the early detection of placental disease has sufficient positive predictive value to allow for safe and effective intervention.1
The National Institutes of Health is investing more than $40 million in its Human Placenta Project, which aims to develop new technologies to help researchers monitor the placenta in real time. Yet it is possible that the use of ultrasound and Doppler – technologies that we employ routinely and know are safe – may go a long way toward deepening our knowledge that will, in turn, hone our ability to identify early risks.
When I speak to fellow pathologists, my message is, “Let’s stop wasting data.” For ob.gyns., my message is twofold: First, appreciate the potential to predict and alter downstream fetal and/or maternal risks by observing and measuring the placenta. Second, be aware of the value of early in vivo placental images, as well as photographs, and more precise measures of delivered placentas.
Why shape matters
The “average” or “typical” placental shape is round or oval with a centrally inserted umbilical cord. In practice, we see a variety of surface shapes and cord insertion sites, with common variations such as bi- or multi-lobate shapes, or otherwise irregular shapes and cord insertions that are eccentric, marginal, or velamentous. Interestingly, many irregularly shaped placentas display symmetry and have regular, defined geometrical patterns, like snowflakes.2
We have long understood that the microscopic growth of the human placenta involves repeated vascular branching analogous to the roots of a tree. This vascular development, or “placental arborization,” reflects the health of the maternal environment and impacts fetal health.
It is only in recent years, however, that we’ve gained a much better understanding of the relationship of the vascular structure and the shape of the placenta, and an understanding of how early changes in the branching structure of the placenta’s vascular tree drive variation in mature placental shape.
By applying a well-accepted mathematical model for generating highly branched fractals (a model for random growth known in the mathematical physics world as diffusion limited aggregation, or DLA), we have reliably reproduced the variability in placental shapes and related these shapes to the structure of the underlying vascular tree.
When the model is run with unperturbed, random values of a branching growth parameter, we get round-oval fractal shapes. But when the growth parameter is perturbed at a single point in time – when a one-time, early change is introduced – arborization is negatively affected and we get irregular shapes.
The model’s output has explained and verified a clinically observed association between non-round, non-oval placental shapes and smaller newborn birth weight for given placental weight.
This association was evident in an analysis of data collected as part of the National Collaborative Perinatal Project (1959-1974), which included placental measures such as weight, shape, size, and thickness for more than 24,000 women. It also was apparent in an analysis of data and images collected as part of the Pregnancy, Infection, and Nutrition (PIN) Study, conducted in North Carolina.
One take-away from both of these studies has been that increased variability of placental shape is associated with lower placental functional efficiency. Moreover, in the University of North Carolina cohort, the impact of placental vascular pathology (either maternal uteroplacental or fetoplacental) on placental efficiency and function was shown to be dependent on shape. Only in the case of irregularly shaped chorionic plates did each of the two pathologies have a significant association with placental inefficiency.3
The realization that placental size (weight/mass/volume) may serve as a proxy for the fetoplacental basal metabolic rate came after it was shown that Kleiber’s law, which states that basal metabolic rate (BMR) is proportional to the body mass to the 3/4 power, can be applied to the newborn’s birth weight by substituting placental weight for BMR.
This fetal-placental version (placenta weight = .75 birth weight) of Kleiber’s law was validated through an analysis of the sets of placental measures and birth weights stored in the Collaborative Perinatal Project. It has implications for our ability to use ultrasound and Doppler measures to predict risk and to understand pathologic pregnancies, such as those complicated by diabetes or fetal growth restriction.
Research also has shed light on the timing of shape variants. We now know that abnormalities of placental surface shape result mainly from early influences – perturbations of placental growth that occur no later than mid-gestation – rather than from trophotropism (the placenta “grows where it can and does not grow where it can’t”) and passive uterine remodeling later in pregnancy, as has traditionally been believed.4
With respect to the umbilical cord, the location of cord insertion is independent of eventual disk shape, but is to a large degree determined by the end of the first trimester. In addition, cord insertion does influence and is correlated with chorionic vascular density and with disk thickness. Greater eccentricity of cord insertion appears to be linked to increased placental disk thickness, each of which is independently associated with reduced placental functional efficiency.5,6
We have worked with placentas from newborns in families with an older child diagnosed with autism and have found significant differences between these placentas and the placentas of low-risk newborns. In particular, we have measured a reduction in the number or chorionic surface vessel branch points of more than 40%.
Current implications
Irregularities in placental surface shape, disk thickness, and various descriptors of placental size may all be determined from ultrasound and Doppler imaging. We can also assess cord insertion and chorionic surface vessel distribution, track patterns and rates of placental growth, and use various placental measures to understand placental efficiency and to improve the specificity of placental histopathologic diagnoses.
At this point, our use of in vivo imaging of the placenta has mainly involved grayscale ultrasound, but with color or power Doppler and improved surface network tracing protocols, we could save the red and blue areas we visualize as a “shape” and assess the density of surface vessel branching, for instance, and the degree of uniformity in vessel distribution.
We currently have quantitative markers of placental shape and mathematical models to help us identify at-risk pregnancies. What we need are more data from early ultrasounds (from all pregnancies and not only complicated ones) and more comprehensive and precise models of placental growth and function. This will enable us to better identify preclinical fetoplacental pathophysiology and predict downstream risks.
In the meantime, the delivered placenta can be a valuable source of information – an extra dimension for looking back in time. With a paradigm shift toward more thorough pathologic analysis, the delivered placenta can provide unique insights into how placental growth evolved during the pregnancy.
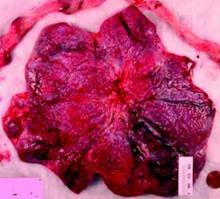
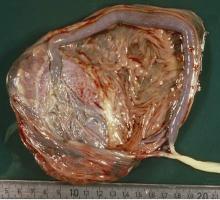
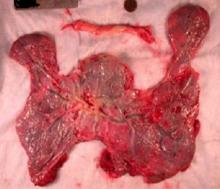
Do not throw away the placenta, and do not just weigh it. Take a photograph, because even with a photograph we can assess vascular density, disk thickness, and other placental characteristics.
In the case of pregnancy complications or suboptimal outcomes, the knowledge we can gain from the delivered placenta can help the physician and patient to understand recurrence risks and to better target evaluation, monitoring, and management in the next pregnancy.
References
1. Am J Perinatol. 2016 Aug 4. doi: 10.1055/s-0036-1586508.
2. Placenta. 2008 Sep;29(9):790-7.
3. Placenta. 2010 Nov;31(11):958-62.
4. Placenta. 2012 Mar;33(3):164-70.
5. J Dev Orig Health Dis. 2011 Aug;2(4):205-11.
6. Placenta. 2009 Dec;30(12):1058-64.
Dr. Salafia leads the Placental Modulation Laboratory at New York State’s Institute for Basic Research in Developmental Disabilities, Staten Island, N.Y. She reported that she has no relevant financial disclosures.
The intrauterine environment significantly influences not only fetal and infant health, but adult health risks as well. Yet current efforts in obstetrics to assess the environment and optimize fetal and long-term outcomes are based on diagnostics that focus on and measure fetal signs and symptoms. By and large, the current approach overlooks the placenta – the organ that serves as the principal regulator of fetal growth and health. If the fetus appears free of risk or complications, we assume the placenta must be “okay.”
Yet this isn’t always the case. By assuming the placenta is healthy and not observing and measuring its condition, we are too often too late to effectively alter fetal- and longer-term outcomes once fetal signs and symptoms appear.
Research in recent decades, and particularly in the past 10 years, has demonstrated that placental shape matters, that it’s linked to function, and that quantifying abnormalities in shape and growth can be a meaningful clinical tool for detecting and preventing disease early in pregnancy.
We now know, specifically, that abnormal shapes reflect alterations in placental vascular architecture that lead to reduced placental efficiency. We also now understand that placental weight or size may serve as a proxy for fetoplacental metabolism.
We have more research to do to further develop models, to collect more data, and to more fully understand the placental pathology that precedes detectable fetal and/or maternal disease. We also need to know whether the early detection of placental disease has sufficient positive predictive value to allow for safe and effective intervention.1
The National Institutes of Health is investing more than $40 million in its Human Placenta Project, which aims to develop new technologies to help researchers monitor the placenta in real time. Yet it is possible that the use of ultrasound and Doppler – technologies that we employ routinely and know are safe – may go a long way toward deepening our knowledge that will, in turn, hone our ability to identify early risks.
When I speak to fellow pathologists, my message is, “Let’s stop wasting data.” For ob.gyns., my message is twofold: First, appreciate the potential to predict and alter downstream fetal and/or maternal risks by observing and measuring the placenta. Second, be aware of the value of early in vivo placental images, as well as photographs, and more precise measures of delivered placentas.
Why shape matters
The “average” or “typical” placental shape is round or oval with a centrally inserted umbilical cord. In practice, we see a variety of surface shapes and cord insertion sites, with common variations such as bi- or multi-lobate shapes, or otherwise irregular shapes and cord insertions that are eccentric, marginal, or velamentous. Interestingly, many irregularly shaped placentas display symmetry and have regular, defined geometrical patterns, like snowflakes.2
We have long understood that the microscopic growth of the human placenta involves repeated vascular branching analogous to the roots of a tree. This vascular development, or “placental arborization,” reflects the health of the maternal environment and impacts fetal health.
It is only in recent years, however, that we’ve gained a much better understanding of the relationship of the vascular structure and the shape of the placenta, and an understanding of how early changes in the branching structure of the placenta’s vascular tree drive variation in mature placental shape.
By applying a well-accepted mathematical model for generating highly branched fractals (a model for random growth known in the mathematical physics world as diffusion limited aggregation, or DLA), we have reliably reproduced the variability in placental shapes and related these shapes to the structure of the underlying vascular tree.
When the model is run with unperturbed, random values of a branching growth parameter, we get round-oval fractal shapes. But when the growth parameter is perturbed at a single point in time – when a one-time, early change is introduced – arborization is negatively affected and we get irregular shapes.
The model’s output has explained and verified a clinically observed association between non-round, non-oval placental shapes and smaller newborn birth weight for given placental weight.
This association was evident in an analysis of data collected as part of the National Collaborative Perinatal Project (1959-1974), which included placental measures such as weight, shape, size, and thickness for more than 24,000 women. It also was apparent in an analysis of data and images collected as part of the Pregnancy, Infection, and Nutrition (PIN) Study, conducted in North Carolina.
One take-away from both of these studies has been that increased variability of placental shape is associated with lower placental functional efficiency. Moreover, in the University of North Carolina cohort, the impact of placental vascular pathology (either maternal uteroplacental or fetoplacental) on placental efficiency and function was shown to be dependent on shape. Only in the case of irregularly shaped chorionic plates did each of the two pathologies have a significant association with placental inefficiency.3
The realization that placental size (weight/mass/volume) may serve as a proxy for the fetoplacental basal metabolic rate came after it was shown that Kleiber’s law, which states that basal metabolic rate (BMR) is proportional to the body mass to the 3/4 power, can be applied to the newborn’s birth weight by substituting placental weight for BMR.
This fetal-placental version (placenta weight = .75 birth weight) of Kleiber’s law was validated through an analysis of the sets of placental measures and birth weights stored in the Collaborative Perinatal Project. It has implications for our ability to use ultrasound and Doppler measures to predict risk and to understand pathologic pregnancies, such as those complicated by diabetes or fetal growth restriction.
Research also has shed light on the timing of shape variants. We now know that abnormalities of placental surface shape result mainly from early influences – perturbations of placental growth that occur no later than mid-gestation – rather than from trophotropism (the placenta “grows where it can and does not grow where it can’t”) and passive uterine remodeling later in pregnancy, as has traditionally been believed.4
With respect to the umbilical cord, the location of cord insertion is independent of eventual disk shape, but is to a large degree determined by the end of the first trimester. In addition, cord insertion does influence and is correlated with chorionic vascular density and with disk thickness. Greater eccentricity of cord insertion appears to be linked to increased placental disk thickness, each of which is independently associated with reduced placental functional efficiency.5,6
We have worked with placentas from newborns in families with an older child diagnosed with autism and have found significant differences between these placentas and the placentas of low-risk newborns. In particular, we have measured a reduction in the number or chorionic surface vessel branch points of more than 40%.
Current implications
Irregularities in placental surface shape, disk thickness, and various descriptors of placental size may all be determined from ultrasound and Doppler imaging. We can also assess cord insertion and chorionic surface vessel distribution, track patterns and rates of placental growth, and use various placental measures to understand placental efficiency and to improve the specificity of placental histopathologic diagnoses.
At this point, our use of in vivo imaging of the placenta has mainly involved grayscale ultrasound, but with color or power Doppler and improved surface network tracing protocols, we could save the red and blue areas we visualize as a “shape” and assess the density of surface vessel branching, for instance, and the degree of uniformity in vessel distribution.
We currently have quantitative markers of placental shape and mathematical models to help us identify at-risk pregnancies. What we need are more data from early ultrasounds (from all pregnancies and not only complicated ones) and more comprehensive and precise models of placental growth and function. This will enable us to better identify preclinical fetoplacental pathophysiology and predict downstream risks.
In the meantime, the delivered placenta can be a valuable source of information – an extra dimension for looking back in time. With a paradigm shift toward more thorough pathologic analysis, the delivered placenta can provide unique insights into how placental growth evolved during the pregnancy.
Do not throw away the placenta, and do not just weigh it. Take a photograph, because even with a photograph we can assess vascular density, disk thickness, and other placental characteristics.
In the case of pregnancy complications or suboptimal outcomes, the knowledge we can gain from the delivered placenta can help the physician and patient to understand recurrence risks and to better target evaluation, monitoring, and management in the next pregnancy.
References
1. Am J Perinatol. 2016 Aug 4. doi: 10.1055/s-0036-1586508.
2. Placenta. 2008 Sep;29(9):790-7.
3. Placenta. 2010 Nov;31(11):958-62.
4. Placenta. 2012 Mar;33(3):164-70.
5. J Dev Orig Health Dis. 2011 Aug;2(4):205-11.
6. Placenta. 2009 Dec;30(12):1058-64.
Dr. Salafia leads the Placental Modulation Laboratory at New York State’s Institute for Basic Research in Developmental Disabilities, Staten Island, N.Y. She reported that she has no relevant financial disclosures.
The intrauterine environment significantly influences not only fetal and infant health, but adult health risks as well. Yet current efforts in obstetrics to assess the environment and optimize fetal and long-term outcomes are based on diagnostics that focus on and measure fetal signs and symptoms. By and large, the current approach overlooks the placenta – the organ that serves as the principal regulator of fetal growth and health. If the fetus appears free of risk or complications, we assume the placenta must be “okay.”
Yet this isn’t always the case. By assuming the placenta is healthy and not observing and measuring its condition, we are too often too late to effectively alter fetal- and longer-term outcomes once fetal signs and symptoms appear.
Research in recent decades, and particularly in the past 10 years, has demonstrated that placental shape matters, that it’s linked to function, and that quantifying abnormalities in shape and growth can be a meaningful clinical tool for detecting and preventing disease early in pregnancy.
We now know, specifically, that abnormal shapes reflect alterations in placental vascular architecture that lead to reduced placental efficiency. We also now understand that placental weight or size may serve as a proxy for fetoplacental metabolism.
We have more research to do to further develop models, to collect more data, and to more fully understand the placental pathology that precedes detectable fetal and/or maternal disease. We also need to know whether the early detection of placental disease has sufficient positive predictive value to allow for safe and effective intervention.1
The National Institutes of Health is investing more than $40 million in its Human Placenta Project, which aims to develop new technologies to help researchers monitor the placenta in real time. Yet it is possible that the use of ultrasound and Doppler – technologies that we employ routinely and know are safe – may go a long way toward deepening our knowledge that will, in turn, hone our ability to identify early risks.
When I speak to fellow pathologists, my message is, “Let’s stop wasting data.” For ob.gyns., my message is twofold: First, appreciate the potential to predict and alter downstream fetal and/or maternal risks by observing and measuring the placenta. Second, be aware of the value of early in vivo placental images, as well as photographs, and more precise measures of delivered placentas.
Why shape matters
The “average” or “typical” placental shape is round or oval with a centrally inserted umbilical cord. In practice, we see a variety of surface shapes and cord insertion sites, with common variations such as bi- or multi-lobate shapes, or otherwise irregular shapes and cord insertions that are eccentric, marginal, or velamentous. Interestingly, many irregularly shaped placentas display symmetry and have regular, defined geometrical patterns, like snowflakes.2
We have long understood that the microscopic growth of the human placenta involves repeated vascular branching analogous to the roots of a tree. This vascular development, or “placental arborization,” reflects the health of the maternal environment and impacts fetal health.
It is only in recent years, however, that we’ve gained a much better understanding of the relationship of the vascular structure and the shape of the placenta, and an understanding of how early changes in the branching structure of the placenta’s vascular tree drive variation in mature placental shape.
By applying a well-accepted mathematical model for generating highly branched fractals (a model for random growth known in the mathematical physics world as diffusion limited aggregation, or DLA), we have reliably reproduced the variability in placental shapes and related these shapes to the structure of the underlying vascular tree.
When the model is run with unperturbed, random values of a branching growth parameter, we get round-oval fractal shapes. But when the growth parameter is perturbed at a single point in time – when a one-time, early change is introduced – arborization is negatively affected and we get irregular shapes.
The model’s output has explained and verified a clinically observed association between non-round, non-oval placental shapes and smaller newborn birth weight for given placental weight.
This association was evident in an analysis of data collected as part of the National Collaborative Perinatal Project (1959-1974), which included placental measures such as weight, shape, size, and thickness for more than 24,000 women. It also was apparent in an analysis of data and images collected as part of the Pregnancy, Infection, and Nutrition (PIN) Study, conducted in North Carolina.
One take-away from both of these studies has been that increased variability of placental shape is associated with lower placental functional efficiency. Moreover, in the University of North Carolina cohort, the impact of placental vascular pathology (either maternal uteroplacental or fetoplacental) on placental efficiency and function was shown to be dependent on shape. Only in the case of irregularly shaped chorionic plates did each of the two pathologies have a significant association with placental inefficiency.3
The realization that placental size (weight/mass/volume) may serve as a proxy for the fetoplacental basal metabolic rate came after it was shown that Kleiber’s law, which states that basal metabolic rate (BMR) is proportional to the body mass to the 3/4 power, can be applied to the newborn’s birth weight by substituting placental weight for BMR.
This fetal-placental version (placenta weight = .75 birth weight) of Kleiber’s law was validated through an analysis of the sets of placental measures and birth weights stored in the Collaborative Perinatal Project. It has implications for our ability to use ultrasound and Doppler measures to predict risk and to understand pathologic pregnancies, such as those complicated by diabetes or fetal growth restriction.
Research also has shed light on the timing of shape variants. We now know that abnormalities of placental surface shape result mainly from early influences – perturbations of placental growth that occur no later than mid-gestation – rather than from trophotropism (the placenta “grows where it can and does not grow where it can’t”) and passive uterine remodeling later in pregnancy, as has traditionally been believed.4
With respect to the umbilical cord, the location of cord insertion is independent of eventual disk shape, but is to a large degree determined by the end of the first trimester. In addition, cord insertion does influence and is correlated with chorionic vascular density and with disk thickness. Greater eccentricity of cord insertion appears to be linked to increased placental disk thickness, each of which is independently associated with reduced placental functional efficiency.5,6
We have worked with placentas from newborns in families with an older child diagnosed with autism and have found significant differences between these placentas and the placentas of low-risk newborns. In particular, we have measured a reduction in the number or chorionic surface vessel branch points of more than 40%.
Current implications
Irregularities in placental surface shape, disk thickness, and various descriptors of placental size may all be determined from ultrasound and Doppler imaging. We can also assess cord insertion and chorionic surface vessel distribution, track patterns and rates of placental growth, and use various placental measures to understand placental efficiency and to improve the specificity of placental histopathologic diagnoses.
At this point, our use of in vivo imaging of the placenta has mainly involved grayscale ultrasound, but with color or power Doppler and improved surface network tracing protocols, we could save the red and blue areas we visualize as a “shape” and assess the density of surface vessel branching, for instance, and the degree of uniformity in vessel distribution.
We currently have quantitative markers of placental shape and mathematical models to help us identify at-risk pregnancies. What we need are more data from early ultrasounds (from all pregnancies and not only complicated ones) and more comprehensive and precise models of placental growth and function. This will enable us to better identify preclinical fetoplacental pathophysiology and predict downstream risks.
In the meantime, the delivered placenta can be a valuable source of information – an extra dimension for looking back in time. With a paradigm shift toward more thorough pathologic analysis, the delivered placenta can provide unique insights into how placental growth evolved during the pregnancy.
Do not throw away the placenta, and do not just weigh it. Take a photograph, because even with a photograph we can assess vascular density, disk thickness, and other placental characteristics.
In the case of pregnancy complications or suboptimal outcomes, the knowledge we can gain from the delivered placenta can help the physician and patient to understand recurrence risks and to better target evaluation, monitoring, and management in the next pregnancy.
References
1. Am J Perinatol. 2016 Aug 4. doi: 10.1055/s-0036-1586508.
2. Placenta. 2008 Sep;29(9):790-7.
3. Placenta. 2010 Nov;31(11):958-62.
4. Placenta. 2012 Mar;33(3):164-70.
5. J Dev Orig Health Dis. 2011 Aug;2(4):205-11.
6. Placenta. 2009 Dec;30(12):1058-64.
Dr. Salafia leads the Placental Modulation Laboratory at New York State’s Institute for Basic Research in Developmental Disabilities, Staten Island, N.Y. She reported that she has no relevant financial disclosures.