User login
Neuromodulation of cardiac pain and cerebral vasculature: Neural mechanisms
The cardinal symptoms of angina pectoris—chest pain and pain that may radiate to either arm or the neck and jaw—are well recognized. The visceral characteristics of anginal pain are also familiar; for example, referral to somatic structures, pain that is diffuse and poorly localized, skin and deep tissue tenderness, enhanced autonomic reflexes such as sweating and vasomotor symptoms, and muscular rigidity.
The neurologic mechanisms that explain the manifestations of angina pectoris are less well clarified, and are targets of active research. Our research into the neuromodulation of cardiovascular function over the last 2 decades has produced results that may have clinical implications and others that have raised new questions. This article summarizes some of our key findings from studies of neural mechanisms of angina pectoris, central sensitization of cardiac nociceptive stimuli, and the neuromodulation of cardiac pain, with a focus on processing in the spinal cord.
NEURAL MECHANISMS OF ANGINA PECTORIS
Cells of the spinothalamic tract form a sensory pathway that transmits afferent information to the thalamus.1 One of our research objectives was to examine how these cells process information when the heart is exposed to noxious stimuli.
Thoracic spinal processing
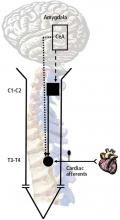
The animal model for our early studies was an anesthetized primate. The afferent nerves were activated in one of two ways: either the coronary artery was occluded or bradykinin and algesic chemicals were injected into the pericardial sac or left atrial appendage. Recorded activity was then made from the spinothalamic tract cells in the T1–T5 and C5–C6 segments.1 We found convergence of visceral and somatic input, generally to the chest and upper arm. The finding was consistent with the observation that pain from angina commonly occurs in proximal somatic fields. No visceral input was evident in cells in C7–C8, where the somatic effects are primarily distal—to the hand, for example.
Upper cervical processing
It is known that some patients experience angina pectoris as neck and jaw pain. The dental literature has shown that what is initially considered to be a toothache occasionally turns out to be angina and coronary artery disease.2 Clinical literature from the late 1940s observed that despite the use of sympathectomy to relieve angina pectoris, neck and jaw pain continued or developed.3,4 This pain was attributed to transmission of nociceptive information in vagal afferent fibers, commonly thought to transmit innocuous cardiac sensory information.
When we recorded activity from spinothalamic tract cells in the C1–C2 region to observe the effect of cardiac nociceptive stimulation, we demonstrated a major role for the vagus nerve.1 Injection of saline into the heart had no effect in the C1–C2 region, but injection of algesic chemicals into the pericardial sac caused significant activity that disappeared after transection of the vagus nerve. This finding suggested that vagal afferent fibers ascend into the nucleus tractus solitarius of the medulla and either directly or indirectly modulate the C1–C2 neurons, which also receive converging somatic information from the neck and jaw region.5
CENTRAL SENSITIZATION OF CARDIAC NOCICEPTIVE STIMULI
Clinical studies suggest that anxiety and depression are prevalent in patients suffering from chest pain with and without underlying cardiac disease.6 Anxiety and/or stress increases circulating levels of corticosteroids, which can act on the glucocorticoid receptors in the amygdala, particularly in the central area.7 The amygdala plays a pivotal role in transforming chronic stressful stimuli into behavioral, visceral, and autonomic responses.8
Previous studies have shown that corticosteroids upregulate expressions of corticotropin-releasing factor in the central nucleus of the amygdala and increase indices of anxiety.7,9 They are also associated with hypersensitivity in visceromotor responses to colorectal distention10 and sensitize lumbosacral spinal neurons to colorectal and urinary bladder distention.11,12 We therefore hypothesized that glucocorticoids manipulate amygdala function, inducing hypersensitivity to nociceptive input from the heart through the modulation of upper thoracic spinal neuronal activity.
To examine the impact of stress on the nervous system when the heart is exposed to noxious stimuli, we assessed the effect of chronic activation of the amygdala on the T3–T4 spinal neurons and on C1–C2 propriospinal neurons. Fisher 344 rats were selected for this study because of their relatively low level of anxiety-related behavior.9 Micropellets of crystalline corticosterone or cholesterol (30 μg, used as a control) were implanted in the central nucleus of the amygdala. After 7 days, the corticosterone-implanted, but not the cholesterol-implanted, animals displayed high-anxiety behavior, as determined with an elevated plus maze.7
The responses of T3–T4 spinal neurons to intrapericardial injections of the algesic chemical bradykinin were compared in the corticosterone- and cholesterol-implanted rats. Compared with cholesterol-implanted animals, the duration of activity in response to the noxious cardiac stimulus was significantly longer in the corticosterone-implanted rats; in addition, activity shifted from the short-lasting (the response lasts only as long as the stimulus is applied) to long-lasting excitatory (the response lasts well beyond the period the stimulus is applied) neurons. Long-lasting excitatory neuronal activity is associated with intense pain and hypersensitivity, while short-lasting neurons are associated with a more acute response. The number of neurons with large field sizes in the corticosterone-implanted animals also increased, which is another indication of sensitization.
NEUROMODULATION OF CEREBROVASCULATURE AND CARDIAC PAIN
Neuromodulation of cerebral blood flow
Spinal cord stimulation is used to treat several cerebrovascular disorders, including cerebral ischemia, focal cerebral ischemia, stroke, postapoplectic spastic hemiplegia, and prolonged coma (see Yang et al13 for citations that address these pathologies). There is no clear explanation for its therapeutic effect; mechanisms being investigated include changes in cerebral blood flow and processing of nociceptive information.
To assess the effect of spinal cord stimulation on cerebral blood flow, we exposed the C1–C2 area of an anesthetized rat, stimulated the area with a ball electrode, and used laser Doppler flow probes to measure the blood flow on the surface of the cortex bilaterally. 13 The stimulus parameters were 30%, 60%, and 90% of motor threshold; the threshold was determined by gradually increasing the intensity of spinal cord stimulation until the neck muscles contracted. Blood flow increased on both sides with increasing stimulation intensities.13
Other studies have evaluated cerebral blood flow but did not measure change in cerebrovascular resistance. We observed that spinal cord stimulation—particularly at 60% and 90% of motor threshold—increased blood flow and reduced resistance to spinal cord stimulation on the dorsal columns at C1, both ipsilaterally and contralaterally.
In other tests, cerebral blood flow and vascular resistance to spinal cord stimulation were not changed after transection of the spinal cord at the C6–C7 segments. These results suggested that information was not being transmitted to the sympathetic nervous system via the thoracic spinal cord. We applied ibotenic acid to C1–C2 to assess whether the underlying stimulated neurons affected cerebral blood flow; there was no significant change. On the other hand, a small cut in the dorsal column rostral to the stimulation site caused significantly reduced cerebral blood flow and vascular resistance, indicating that the dorsal columns function in an ascending manner to produce the vasodilation in the cerebral cortex.13
Capsaicin-sensitive sensory nerves, which contain transient receptor potential vanilloid-1 (TRPV1) receptors, may have a role in spinal cord stimulation–induced vasodilation. TRPV1 receptors are nonselective cation channels activated by capsaicin, heat, and hydrogen ions.14 Activation, which causes an influx of cations and release of calcitonin gene-related peptide (CGRP) and substance P, is related to the pathogenesis of inflammation and hypertension. To examine the potential role played by capsaicin-sensitive sensory nerves, we administered resiniferatoxin (RTX), an ultrapotent capsaicin agonist; RTX specifically targets and desensitizes TRPV1-containing sensory fibers.13,15 Administration either intravenously or by direct application to the spinal cord results in a 15- to 20-minute period of sensitization followed by several hours of desensitization; if exposure lasts for several days, the nerves are destroyed.
Intrathecal administration of RTX to the spinal cord resulted in no significant change in cerebral blood flow. However, intravenous administration resulted in significantly decreased cerebral blood flow and decreased resistance, suggesting a role for TRPV1 receptors in cerebral blood flow.13
There may be a connection between spinal cord stimulation at C1 and vasodilation of the cortex. The literature suggests that spinal cord stimulation activates the dorsal column nuclei16; we found evidence of this in our laboratory when we recorded activity from cells in the cuneate and gracilus nuclei after spinal cord stimulation. There is also a possible pathway between the dorsal column, the rostral ventrolateral medulla, and the sphenopalatine ganglion that influences vasodilation.17–20 Although not yet clearly defined, evidence suggests a connection between spinal cord stimulation and transmission of this information through the dorsal columns to influence vasodilation.17–20
Neuromodulation of thoracic spinal processing of cardiac nociceptive information
Using a rat model to assess the effects of spinal stimulation, we recorded T3 activity during dorsal column stimulation of either C8-T1 or C1–C2 segments. Activity was almost completely suppressed with C1–C2 stimulation during bradykinin injection into the pericardial sac. The results suggest that spinal cord stimulation suppresses the processing of nociceptive information.24
Stimulating the spinal cord at C8-T1 also suppresses the effect of bradykinin. One possible mechanism for this effect is that spinal cord stimulation activates large afferent fibers; GABAergic connections in the superficial dorsal horn may suppress the processing of information in the spinothalamic tract neurons.22,25
SUMMARY
Our investigations have generated information about afferent input to the spinothalamic tract cells, the effects of glucocorticoids on amygdala function, and possible therapeutic mechanisms of spinal cord stimulation.
We have demonstrated convergence of viscerosomatic input in spinothalamic cells. There is virtually no viscerocardiac input at the C7–C8 region, but there is input at C5–C6. Vagal afferent activity is the major source of input at the C1–C2 region; in this region. Vagal nerve stimulation may have a major role in processing in the upper cervical spinal cord and may change the balance of processing in the supraspinal nuclei.
Glucocorticoids manipulate amygdala function by inducing hypersensitivity to nociceptive input from the heart through central sensitization of upper thoracic spinal neuronal activity. Descending information from the amygdala depends, in part, on the C1–C2 propriospinal pathway.
Spinal cord stimulation at C1–C2 or C8-T1 can activate inner neuronal mechanisms that may involve GABA, modulating the wide dynamic range of neurons that are part of the spinothalamic tract.
- Foreman RD. Mechanisms of cardiac pain. Annu Rev Physiol 1999; 61:143–167.
- Myers DE. Vagus nerve pain referred to the craniofacial region. A case report and literature review with implications for referred cardiac pain. Br Dent J 2008; 204:187–189.
- Lindgren I, Olivecrona H. Surgical treatment of angina pectoris. J Neurosurg 1947; 4:19–39.
- White JC, Bland EF. The surgical relief of severe angina pectoris: methods employed and end results in 83 patients. Medicine 1948; 27:1–42.
- McNeill DL, Chandler MJ, Fu QG, Foreman RD. Projection of nodose ganglion cells to the upper cervical spinal cord in the rat. Brain Res Bull 1991; 27:151–155.
- Sheps DS, Creed F, Clouse RE. Chest pain in patients with cardiac and noncardiac disease. Psychosom Med 2004; 66:861–867.
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 2000; 861:288–295.
- Rozen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev 1998; 105:325–350.
- Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav 2000; 69:379–382.
- Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 2001; 893:135–142.
- Qin C, Greenwood-Van Meerveld B, Myers DA, Foreman RD. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol 2003; 89:1343–1352.
- Qin C, Greenwood-Van Meerveld B, Foreman RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J Neurophysiol 2003; 90:2180–2189.
- Yang X, Farber JP, Wu M, Foreman RD, Qin C. Roles of dorsal column pathway and transient receptor potential vanilloid type 1 in augmentation of cerebral blood flow by upper cervical spinal cord stimulation in rats. Neuroscience 2008; 152:950–958.
- Steenland HW, Ko SW, Wu LJ, Zhuo M. Hot receptors in the brain. Mol Pain 2006; 2:34.
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Roles of peripheral terminals of transient receptor potential vanil-loid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res 2007; 1156:80–92.
- Sagher O, Huang DL. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg 2000; 93( 1 suppl):71–76.
- Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab 1988; 8:875–878.
- Suzuki N, Hardebo JE, Kåhrström J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab 1990; 10:383–391.
- Kamiya H, Itoh K, Yasui Y, Ino T, Mizuno N. Somatosensory and auditory relay nucleus in the rostral part of the ventrolateral medulla: a morphological study in the cat. J Comp Neurol 1988; 273:421–435.
- Patel S, Huang DL, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery 2004; 55:201–206.
- Meyerson BA, Linderoth B. Spinal cord stimulation: mechanisms of action in neuropathic and ischemic pain. In:Simpson BA, ed. Electrical Stimulation and the Relief of Pain. Vol. 15. New York, NY: Elsevier Publishers; 2003:161–182.
- Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med 2006; 7:S14–S26.
- González-Darder JM, Canela P, González-Martinez V. High cervical spinal cord stimulation for unstable angina pectoris. Stereotact Funct Neurosurg 1991; 56:20–27.
- Qin C, Farber JP, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9:71–78.
- Linderoth B, Meyerson B. Spinal cord stimulation: mechanisms of action. In:Burchiel K. Surgical Management of Pain. New York, NY: Thieme Medical Publishers Inc; 2002:505–526.
The cardinal symptoms of angina pectoris—chest pain and pain that may radiate to either arm or the neck and jaw—are well recognized. The visceral characteristics of anginal pain are also familiar; for example, referral to somatic structures, pain that is diffuse and poorly localized, skin and deep tissue tenderness, enhanced autonomic reflexes such as sweating and vasomotor symptoms, and muscular rigidity.
The neurologic mechanisms that explain the manifestations of angina pectoris are less well clarified, and are targets of active research. Our research into the neuromodulation of cardiovascular function over the last 2 decades has produced results that may have clinical implications and others that have raised new questions. This article summarizes some of our key findings from studies of neural mechanisms of angina pectoris, central sensitization of cardiac nociceptive stimuli, and the neuromodulation of cardiac pain, with a focus on processing in the spinal cord.
NEURAL MECHANISMS OF ANGINA PECTORIS
Cells of the spinothalamic tract form a sensory pathway that transmits afferent information to the thalamus.1 One of our research objectives was to examine how these cells process information when the heart is exposed to noxious stimuli.
Thoracic spinal processing
The animal model for our early studies was an anesthetized primate. The afferent nerves were activated in one of two ways: either the coronary artery was occluded or bradykinin and algesic chemicals were injected into the pericardial sac or left atrial appendage. Recorded activity was then made from the spinothalamic tract cells in the T1–T5 and C5–C6 segments.1 We found convergence of visceral and somatic input, generally to the chest and upper arm. The finding was consistent with the observation that pain from angina commonly occurs in proximal somatic fields. No visceral input was evident in cells in C7–C8, where the somatic effects are primarily distal—to the hand, for example.
Upper cervical processing
It is known that some patients experience angina pectoris as neck and jaw pain. The dental literature has shown that what is initially considered to be a toothache occasionally turns out to be angina and coronary artery disease.2 Clinical literature from the late 1940s observed that despite the use of sympathectomy to relieve angina pectoris, neck and jaw pain continued or developed.3,4 This pain was attributed to transmission of nociceptive information in vagal afferent fibers, commonly thought to transmit innocuous cardiac sensory information.
When we recorded activity from spinothalamic tract cells in the C1–C2 region to observe the effect of cardiac nociceptive stimulation, we demonstrated a major role for the vagus nerve.1 Injection of saline into the heart had no effect in the C1–C2 region, but injection of algesic chemicals into the pericardial sac caused significant activity that disappeared after transection of the vagus nerve. This finding suggested that vagal afferent fibers ascend into the nucleus tractus solitarius of the medulla and either directly or indirectly modulate the C1–C2 neurons, which also receive converging somatic information from the neck and jaw region.5
CENTRAL SENSITIZATION OF CARDIAC NOCICEPTIVE STIMULI
Clinical studies suggest that anxiety and depression are prevalent in patients suffering from chest pain with and without underlying cardiac disease.6 Anxiety and/or stress increases circulating levels of corticosteroids, which can act on the glucocorticoid receptors in the amygdala, particularly in the central area.7 The amygdala plays a pivotal role in transforming chronic stressful stimuli into behavioral, visceral, and autonomic responses.8
Previous studies have shown that corticosteroids upregulate expressions of corticotropin-releasing factor in the central nucleus of the amygdala and increase indices of anxiety.7,9 They are also associated with hypersensitivity in visceromotor responses to colorectal distention10 and sensitize lumbosacral spinal neurons to colorectal and urinary bladder distention.11,12 We therefore hypothesized that glucocorticoids manipulate amygdala function, inducing hypersensitivity to nociceptive input from the heart through the modulation of upper thoracic spinal neuronal activity.
To examine the impact of stress on the nervous system when the heart is exposed to noxious stimuli, we assessed the effect of chronic activation of the amygdala on the T3–T4 spinal neurons and on C1–C2 propriospinal neurons. Fisher 344 rats were selected for this study because of their relatively low level of anxiety-related behavior.9 Micropellets of crystalline corticosterone or cholesterol (30 μg, used as a control) were implanted in the central nucleus of the amygdala. After 7 days, the corticosterone-implanted, but not the cholesterol-implanted, animals displayed high-anxiety behavior, as determined with an elevated plus maze.7
The responses of T3–T4 spinal neurons to intrapericardial injections of the algesic chemical bradykinin were compared in the corticosterone- and cholesterol-implanted rats. Compared with cholesterol-implanted animals, the duration of activity in response to the noxious cardiac stimulus was significantly longer in the corticosterone-implanted rats; in addition, activity shifted from the short-lasting (the response lasts only as long as the stimulus is applied) to long-lasting excitatory (the response lasts well beyond the period the stimulus is applied) neurons. Long-lasting excitatory neuronal activity is associated with intense pain and hypersensitivity, while short-lasting neurons are associated with a more acute response. The number of neurons with large field sizes in the corticosterone-implanted animals also increased, which is another indication of sensitization.
NEUROMODULATION OF CEREBROVASCULATURE AND CARDIAC PAIN
Neuromodulation of cerebral blood flow
Spinal cord stimulation is used to treat several cerebrovascular disorders, including cerebral ischemia, focal cerebral ischemia, stroke, postapoplectic spastic hemiplegia, and prolonged coma (see Yang et al13 for citations that address these pathologies). There is no clear explanation for its therapeutic effect; mechanisms being investigated include changes in cerebral blood flow and processing of nociceptive information.
To assess the effect of spinal cord stimulation on cerebral blood flow, we exposed the C1–C2 area of an anesthetized rat, stimulated the area with a ball electrode, and used laser Doppler flow probes to measure the blood flow on the surface of the cortex bilaterally. 13 The stimulus parameters were 30%, 60%, and 90% of motor threshold; the threshold was determined by gradually increasing the intensity of spinal cord stimulation until the neck muscles contracted. Blood flow increased on both sides with increasing stimulation intensities.13
Other studies have evaluated cerebral blood flow but did not measure change in cerebrovascular resistance. We observed that spinal cord stimulation—particularly at 60% and 90% of motor threshold—increased blood flow and reduced resistance to spinal cord stimulation on the dorsal columns at C1, both ipsilaterally and contralaterally.
In other tests, cerebral blood flow and vascular resistance to spinal cord stimulation were not changed after transection of the spinal cord at the C6–C7 segments. These results suggested that information was not being transmitted to the sympathetic nervous system via the thoracic spinal cord. We applied ibotenic acid to C1–C2 to assess whether the underlying stimulated neurons affected cerebral blood flow; there was no significant change. On the other hand, a small cut in the dorsal column rostral to the stimulation site caused significantly reduced cerebral blood flow and vascular resistance, indicating that the dorsal columns function in an ascending manner to produce the vasodilation in the cerebral cortex.13
Capsaicin-sensitive sensory nerves, which contain transient receptor potential vanilloid-1 (TRPV1) receptors, may have a role in spinal cord stimulation–induced vasodilation. TRPV1 receptors are nonselective cation channels activated by capsaicin, heat, and hydrogen ions.14 Activation, which causes an influx of cations and release of calcitonin gene-related peptide (CGRP) and substance P, is related to the pathogenesis of inflammation and hypertension. To examine the potential role played by capsaicin-sensitive sensory nerves, we administered resiniferatoxin (RTX), an ultrapotent capsaicin agonist; RTX specifically targets and desensitizes TRPV1-containing sensory fibers.13,15 Administration either intravenously or by direct application to the spinal cord results in a 15- to 20-minute period of sensitization followed by several hours of desensitization; if exposure lasts for several days, the nerves are destroyed.
Intrathecal administration of RTX to the spinal cord resulted in no significant change in cerebral blood flow. However, intravenous administration resulted in significantly decreased cerebral blood flow and decreased resistance, suggesting a role for TRPV1 receptors in cerebral blood flow.13
There may be a connection between spinal cord stimulation at C1 and vasodilation of the cortex. The literature suggests that spinal cord stimulation activates the dorsal column nuclei16; we found evidence of this in our laboratory when we recorded activity from cells in the cuneate and gracilus nuclei after spinal cord stimulation. There is also a possible pathway between the dorsal column, the rostral ventrolateral medulla, and the sphenopalatine ganglion that influences vasodilation.17–20 Although not yet clearly defined, evidence suggests a connection between spinal cord stimulation and transmission of this information through the dorsal columns to influence vasodilation.17–20
Neuromodulation of thoracic spinal processing of cardiac nociceptive information
Using a rat model to assess the effects of spinal stimulation, we recorded T3 activity during dorsal column stimulation of either C8-T1 or C1–C2 segments. Activity was almost completely suppressed with C1–C2 stimulation during bradykinin injection into the pericardial sac. The results suggest that spinal cord stimulation suppresses the processing of nociceptive information.24
Stimulating the spinal cord at C8-T1 also suppresses the effect of bradykinin. One possible mechanism for this effect is that spinal cord stimulation activates large afferent fibers; GABAergic connections in the superficial dorsal horn may suppress the processing of information in the spinothalamic tract neurons.22,25
SUMMARY
Our investigations have generated information about afferent input to the spinothalamic tract cells, the effects of glucocorticoids on amygdala function, and possible therapeutic mechanisms of spinal cord stimulation.
We have demonstrated convergence of viscerosomatic input in spinothalamic cells. There is virtually no viscerocardiac input at the C7–C8 region, but there is input at C5–C6. Vagal afferent activity is the major source of input at the C1–C2 region; in this region. Vagal nerve stimulation may have a major role in processing in the upper cervical spinal cord and may change the balance of processing in the supraspinal nuclei.
Glucocorticoids manipulate amygdala function by inducing hypersensitivity to nociceptive input from the heart through central sensitization of upper thoracic spinal neuronal activity. Descending information from the amygdala depends, in part, on the C1–C2 propriospinal pathway.
Spinal cord stimulation at C1–C2 or C8-T1 can activate inner neuronal mechanisms that may involve GABA, modulating the wide dynamic range of neurons that are part of the spinothalamic tract.
The cardinal symptoms of angina pectoris—chest pain and pain that may radiate to either arm or the neck and jaw—are well recognized. The visceral characteristics of anginal pain are also familiar; for example, referral to somatic structures, pain that is diffuse and poorly localized, skin and deep tissue tenderness, enhanced autonomic reflexes such as sweating and vasomotor symptoms, and muscular rigidity.
The neurologic mechanisms that explain the manifestations of angina pectoris are less well clarified, and are targets of active research. Our research into the neuromodulation of cardiovascular function over the last 2 decades has produced results that may have clinical implications and others that have raised new questions. This article summarizes some of our key findings from studies of neural mechanisms of angina pectoris, central sensitization of cardiac nociceptive stimuli, and the neuromodulation of cardiac pain, with a focus on processing in the spinal cord.
NEURAL MECHANISMS OF ANGINA PECTORIS
Cells of the spinothalamic tract form a sensory pathway that transmits afferent information to the thalamus.1 One of our research objectives was to examine how these cells process information when the heart is exposed to noxious stimuli.
Thoracic spinal processing
The animal model for our early studies was an anesthetized primate. The afferent nerves were activated in one of two ways: either the coronary artery was occluded or bradykinin and algesic chemicals were injected into the pericardial sac or left atrial appendage. Recorded activity was then made from the spinothalamic tract cells in the T1–T5 and C5–C6 segments.1 We found convergence of visceral and somatic input, generally to the chest and upper arm. The finding was consistent with the observation that pain from angina commonly occurs in proximal somatic fields. No visceral input was evident in cells in C7–C8, where the somatic effects are primarily distal—to the hand, for example.
Upper cervical processing
It is known that some patients experience angina pectoris as neck and jaw pain. The dental literature has shown that what is initially considered to be a toothache occasionally turns out to be angina and coronary artery disease.2 Clinical literature from the late 1940s observed that despite the use of sympathectomy to relieve angina pectoris, neck and jaw pain continued or developed.3,4 This pain was attributed to transmission of nociceptive information in vagal afferent fibers, commonly thought to transmit innocuous cardiac sensory information.
When we recorded activity from spinothalamic tract cells in the C1–C2 region to observe the effect of cardiac nociceptive stimulation, we demonstrated a major role for the vagus nerve.1 Injection of saline into the heart had no effect in the C1–C2 region, but injection of algesic chemicals into the pericardial sac caused significant activity that disappeared after transection of the vagus nerve. This finding suggested that vagal afferent fibers ascend into the nucleus tractus solitarius of the medulla and either directly or indirectly modulate the C1–C2 neurons, which also receive converging somatic information from the neck and jaw region.5
CENTRAL SENSITIZATION OF CARDIAC NOCICEPTIVE STIMULI
Clinical studies suggest that anxiety and depression are prevalent in patients suffering from chest pain with and without underlying cardiac disease.6 Anxiety and/or stress increases circulating levels of corticosteroids, which can act on the glucocorticoid receptors in the amygdala, particularly in the central area.7 The amygdala plays a pivotal role in transforming chronic stressful stimuli into behavioral, visceral, and autonomic responses.8
Previous studies have shown that corticosteroids upregulate expressions of corticotropin-releasing factor in the central nucleus of the amygdala and increase indices of anxiety.7,9 They are also associated with hypersensitivity in visceromotor responses to colorectal distention10 and sensitize lumbosacral spinal neurons to colorectal and urinary bladder distention.11,12 We therefore hypothesized that glucocorticoids manipulate amygdala function, inducing hypersensitivity to nociceptive input from the heart through the modulation of upper thoracic spinal neuronal activity.
To examine the impact of stress on the nervous system when the heart is exposed to noxious stimuli, we assessed the effect of chronic activation of the amygdala on the T3–T4 spinal neurons and on C1–C2 propriospinal neurons. Fisher 344 rats were selected for this study because of their relatively low level of anxiety-related behavior.9 Micropellets of crystalline corticosterone or cholesterol (30 μg, used as a control) were implanted in the central nucleus of the amygdala. After 7 days, the corticosterone-implanted, but not the cholesterol-implanted, animals displayed high-anxiety behavior, as determined with an elevated plus maze.7
The responses of T3–T4 spinal neurons to intrapericardial injections of the algesic chemical bradykinin were compared in the corticosterone- and cholesterol-implanted rats. Compared with cholesterol-implanted animals, the duration of activity in response to the noxious cardiac stimulus was significantly longer in the corticosterone-implanted rats; in addition, activity shifted from the short-lasting (the response lasts only as long as the stimulus is applied) to long-lasting excitatory (the response lasts well beyond the period the stimulus is applied) neurons. Long-lasting excitatory neuronal activity is associated with intense pain and hypersensitivity, while short-lasting neurons are associated with a more acute response. The number of neurons with large field sizes in the corticosterone-implanted animals also increased, which is another indication of sensitization.
NEUROMODULATION OF CEREBROVASCULATURE AND CARDIAC PAIN
Neuromodulation of cerebral blood flow
Spinal cord stimulation is used to treat several cerebrovascular disorders, including cerebral ischemia, focal cerebral ischemia, stroke, postapoplectic spastic hemiplegia, and prolonged coma (see Yang et al13 for citations that address these pathologies). There is no clear explanation for its therapeutic effect; mechanisms being investigated include changes in cerebral blood flow and processing of nociceptive information.
To assess the effect of spinal cord stimulation on cerebral blood flow, we exposed the C1–C2 area of an anesthetized rat, stimulated the area with a ball electrode, and used laser Doppler flow probes to measure the blood flow on the surface of the cortex bilaterally. 13 The stimulus parameters were 30%, 60%, and 90% of motor threshold; the threshold was determined by gradually increasing the intensity of spinal cord stimulation until the neck muscles contracted. Blood flow increased on both sides with increasing stimulation intensities.13
Other studies have evaluated cerebral blood flow but did not measure change in cerebrovascular resistance. We observed that spinal cord stimulation—particularly at 60% and 90% of motor threshold—increased blood flow and reduced resistance to spinal cord stimulation on the dorsal columns at C1, both ipsilaterally and contralaterally.
In other tests, cerebral blood flow and vascular resistance to spinal cord stimulation were not changed after transection of the spinal cord at the C6–C7 segments. These results suggested that information was not being transmitted to the sympathetic nervous system via the thoracic spinal cord. We applied ibotenic acid to C1–C2 to assess whether the underlying stimulated neurons affected cerebral blood flow; there was no significant change. On the other hand, a small cut in the dorsal column rostral to the stimulation site caused significantly reduced cerebral blood flow and vascular resistance, indicating that the dorsal columns function in an ascending manner to produce the vasodilation in the cerebral cortex.13
Capsaicin-sensitive sensory nerves, which contain transient receptor potential vanilloid-1 (TRPV1) receptors, may have a role in spinal cord stimulation–induced vasodilation. TRPV1 receptors are nonselective cation channels activated by capsaicin, heat, and hydrogen ions.14 Activation, which causes an influx of cations and release of calcitonin gene-related peptide (CGRP) and substance P, is related to the pathogenesis of inflammation and hypertension. To examine the potential role played by capsaicin-sensitive sensory nerves, we administered resiniferatoxin (RTX), an ultrapotent capsaicin agonist; RTX specifically targets and desensitizes TRPV1-containing sensory fibers.13,15 Administration either intravenously or by direct application to the spinal cord results in a 15- to 20-minute period of sensitization followed by several hours of desensitization; if exposure lasts for several days, the nerves are destroyed.
Intrathecal administration of RTX to the spinal cord resulted in no significant change in cerebral blood flow. However, intravenous administration resulted in significantly decreased cerebral blood flow and decreased resistance, suggesting a role for TRPV1 receptors in cerebral blood flow.13
There may be a connection between spinal cord stimulation at C1 and vasodilation of the cortex. The literature suggests that spinal cord stimulation activates the dorsal column nuclei16; we found evidence of this in our laboratory when we recorded activity from cells in the cuneate and gracilus nuclei after spinal cord stimulation. There is also a possible pathway between the dorsal column, the rostral ventrolateral medulla, and the sphenopalatine ganglion that influences vasodilation.17–20 Although not yet clearly defined, evidence suggests a connection between spinal cord stimulation and transmission of this information through the dorsal columns to influence vasodilation.17–20
Neuromodulation of thoracic spinal processing of cardiac nociceptive information
Using a rat model to assess the effects of spinal stimulation, we recorded T3 activity during dorsal column stimulation of either C8-T1 or C1–C2 segments. Activity was almost completely suppressed with C1–C2 stimulation during bradykinin injection into the pericardial sac. The results suggest that spinal cord stimulation suppresses the processing of nociceptive information.24
Stimulating the spinal cord at C8-T1 also suppresses the effect of bradykinin. One possible mechanism for this effect is that spinal cord stimulation activates large afferent fibers; GABAergic connections in the superficial dorsal horn may suppress the processing of information in the spinothalamic tract neurons.22,25
SUMMARY
Our investigations have generated information about afferent input to the spinothalamic tract cells, the effects of glucocorticoids on amygdala function, and possible therapeutic mechanisms of spinal cord stimulation.
We have demonstrated convergence of viscerosomatic input in spinothalamic cells. There is virtually no viscerocardiac input at the C7–C8 region, but there is input at C5–C6. Vagal afferent activity is the major source of input at the C1–C2 region; in this region. Vagal nerve stimulation may have a major role in processing in the upper cervical spinal cord and may change the balance of processing in the supraspinal nuclei.
Glucocorticoids manipulate amygdala function by inducing hypersensitivity to nociceptive input from the heart through central sensitization of upper thoracic spinal neuronal activity. Descending information from the amygdala depends, in part, on the C1–C2 propriospinal pathway.
Spinal cord stimulation at C1–C2 or C8-T1 can activate inner neuronal mechanisms that may involve GABA, modulating the wide dynamic range of neurons that are part of the spinothalamic tract.
- Foreman RD. Mechanisms of cardiac pain. Annu Rev Physiol 1999; 61:143–167.
- Myers DE. Vagus nerve pain referred to the craniofacial region. A case report and literature review with implications for referred cardiac pain. Br Dent J 2008; 204:187–189.
- Lindgren I, Olivecrona H. Surgical treatment of angina pectoris. J Neurosurg 1947; 4:19–39.
- White JC, Bland EF. The surgical relief of severe angina pectoris: methods employed and end results in 83 patients. Medicine 1948; 27:1–42.
- McNeill DL, Chandler MJ, Fu QG, Foreman RD. Projection of nodose ganglion cells to the upper cervical spinal cord in the rat. Brain Res Bull 1991; 27:151–155.
- Sheps DS, Creed F, Clouse RE. Chest pain in patients with cardiac and noncardiac disease. Psychosom Med 2004; 66:861–867.
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 2000; 861:288–295.
- Rozen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev 1998; 105:325–350.
- Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav 2000; 69:379–382.
- Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 2001; 893:135–142.
- Qin C, Greenwood-Van Meerveld B, Myers DA, Foreman RD. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol 2003; 89:1343–1352.
- Qin C, Greenwood-Van Meerveld B, Foreman RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J Neurophysiol 2003; 90:2180–2189.
- Yang X, Farber JP, Wu M, Foreman RD, Qin C. Roles of dorsal column pathway and transient receptor potential vanilloid type 1 in augmentation of cerebral blood flow by upper cervical spinal cord stimulation in rats. Neuroscience 2008; 152:950–958.
- Steenland HW, Ko SW, Wu LJ, Zhuo M. Hot receptors in the brain. Mol Pain 2006; 2:34.
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Roles of peripheral terminals of transient receptor potential vanil-loid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res 2007; 1156:80–92.
- Sagher O, Huang DL. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg 2000; 93( 1 suppl):71–76.
- Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab 1988; 8:875–878.
- Suzuki N, Hardebo JE, Kåhrström J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab 1990; 10:383–391.
- Kamiya H, Itoh K, Yasui Y, Ino T, Mizuno N. Somatosensory and auditory relay nucleus in the rostral part of the ventrolateral medulla: a morphological study in the cat. J Comp Neurol 1988; 273:421–435.
- Patel S, Huang DL, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery 2004; 55:201–206.
- Meyerson BA, Linderoth B. Spinal cord stimulation: mechanisms of action in neuropathic and ischemic pain. In:Simpson BA, ed. Electrical Stimulation and the Relief of Pain. Vol. 15. New York, NY: Elsevier Publishers; 2003:161–182.
- Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med 2006; 7:S14–S26.
- González-Darder JM, Canela P, González-Martinez V. High cervical spinal cord stimulation for unstable angina pectoris. Stereotact Funct Neurosurg 1991; 56:20–27.
- Qin C, Farber JP, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9:71–78.
- Linderoth B, Meyerson B. Spinal cord stimulation: mechanisms of action. In:Burchiel K. Surgical Management of Pain. New York, NY: Thieme Medical Publishers Inc; 2002:505–526.
- Foreman RD. Mechanisms of cardiac pain. Annu Rev Physiol 1999; 61:143–167.
- Myers DE. Vagus nerve pain referred to the craniofacial region. A case report and literature review with implications for referred cardiac pain. Br Dent J 2008; 204:187–189.
- Lindgren I, Olivecrona H. Surgical treatment of angina pectoris. J Neurosurg 1947; 4:19–39.
- White JC, Bland EF. The surgical relief of severe angina pectoris: methods employed and end results in 83 patients. Medicine 1948; 27:1–42.
- McNeill DL, Chandler MJ, Fu QG, Foreman RD. Projection of nodose ganglion cells to the upper cervical spinal cord in the rat. Brain Res Bull 1991; 27:151–155.
- Sheps DS, Creed F, Clouse RE. Chest pain in patients with cardiac and noncardiac disease. Psychosom Med 2004; 66:861–867.
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 2000; 861:288–295.
- Rozen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev 1998; 105:325–350.
- Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav 2000; 69:379–382.
- Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 2001; 893:135–142.
- Qin C, Greenwood-Van Meerveld B, Myers DA, Foreman RD. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol 2003; 89:1343–1352.
- Qin C, Greenwood-Van Meerveld B, Foreman RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J Neurophysiol 2003; 90:2180–2189.
- Yang X, Farber JP, Wu M, Foreman RD, Qin C. Roles of dorsal column pathway and transient receptor potential vanilloid type 1 in augmentation of cerebral blood flow by upper cervical spinal cord stimulation in rats. Neuroscience 2008; 152:950–958.
- Steenland HW, Ko SW, Wu LJ, Zhuo M. Hot receptors in the brain. Mol Pain 2006; 2:34.
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Roles of peripheral terminals of transient receptor potential vanil-loid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res 2007; 1156:80–92.
- Sagher O, Huang DL. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg 2000; 93( 1 suppl):71–76.
- Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab 1988; 8:875–878.
- Suzuki N, Hardebo JE, Kåhrström J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab 1990; 10:383–391.
- Kamiya H, Itoh K, Yasui Y, Ino T, Mizuno N. Somatosensory and auditory relay nucleus in the rostral part of the ventrolateral medulla: a morphological study in the cat. J Comp Neurol 1988; 273:421–435.
- Patel S, Huang DL, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery 2004; 55:201–206.
- Meyerson BA, Linderoth B. Spinal cord stimulation: mechanisms of action in neuropathic and ischemic pain. In:Simpson BA, ed. Electrical Stimulation and the Relief of Pain. Vol. 15. New York, NY: Elsevier Publishers; 2003:161–182.
- Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med 2006; 7:S14–S26.
- González-Darder JM, Canela P, González-Martinez V. High cervical spinal cord stimulation for unstable angina pectoris. Stereotact Funct Neurosurg 1991; 56:20–27.
- Qin C, Farber JP, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9:71–78.
- Linderoth B, Meyerson B. Spinal cord stimulation: mechanisms of action. In:Burchiel K. Surgical Management of Pain. New York, NY: Thieme Medical Publishers Inc; 2002:505–526.