User login
Basic research models for the study of underlying mechanisms of electrical neuromodulation and ischemic heart-brain interactions
RATIONALE
In the industrialized world, average life expectancy has nearly doubled since the 19th century. One of the consequences of this increase in life span is that the sequelae of diseases also have increased. For coronary artery disease (CAD), one of the most prevalent diseases in the western world, this has resulted in an amplification of the number of patients suffering from heart failure, arrhythmias, and refractory angina. Much progress has recently been made in nonpharmacologic therapies for these deleterious consequences of CAD, such as cardiac resynchronization for heart failure, implantable defibrillators for ventricular arrhythmias, and electrical neuromodulation by means of spinal cord stimulation for chronic angina that is refractory to conventional strategies.
For patients suffering from severe angina secondary to end-stage CAD who have no other options to alleviate their complaints, electrical neuromodulation may be the preferred adjunctive treatment.1 Although spinal cord stimulation is still not approved by the US Food and Drug Administration for treatment of refractory angina, it is is accepted in the American College of Cardiology/American Heart Association guidelines for chronic stable angina, with a class II indication, and is frequently used for this indication in Europe.2
However, to understand underlying mechanisms of therapies such as electrical neuromodulation—executed through either transcutaneous electrical nerve stimulation or spinal cord stimulation—for angina pectoris and to improve the effect and safety of these therapies, clinical questions concerning neuromodulation must be evaluated in experimental models. The outcomes of these preclinical experimental studies subsequently need to be assessed in humans.
Although therapeutic improvements from implantable devices would not have been possible without experimental work, any experimentation must be avoided if it is not approved by the relevant ethics committee(s) or is not conducted in keeping with standard guidelines. For this reason it is sometimes more feasible, when appropriate, to make use of simulation models—for instance, to study regularization of atrial fibrillation by means of a device.3,4
PROJECT 1: EMOTIONS AND MYOCARDIAL ISCHEMIA
In 1772, Heberden described to physicians in England the clinical symptoms of exercise-induced chest discomfort, with its emotional component and vaguely distributed projection on the chest, as follows: “The seat of it, and sense of strangling and anxiety with which it is attended, may make it not improperly be called angina pectoris.”5 Since then, it has been demonstrated repeatedly that strong emotional distress frequently precedes or is associated with complaints of pain in the chest. Further, emotional suffering has been associated with increased mortality in patients with CAD. We and others, unfortunately, were confronted with very limited knowledge of the precise locations of the origin of emotions in the limbic structures of the forebrain. Even less was known about the relationship of these brain structures and the heart, owing to technical limitations in the field of neuroanatomical tract tracing, among other reasons. As a result, the nervous pathways from the heart, through which signals are propagated to the brain in order to activate emotional components, were not accurately identified. We therefore initiated Project 1 to study, in a rat model, neuroanatomical characterization of the neuronal circuitry controlling cardiac activity, specifically during cardiac distress.
In the area of identifying efferent neural pathways from the heart, we were the first to publish an experimental setup making use of a neurotropic herpesvirus from the Bartha strain of the pseudorabies virus (PRV).6 Following injections of PRV into the left and right myocardium or into atrial tissue, PRV infects the neurons that innervate the injection site and is then transported in the neural network, where the virus may cross at least four synapses. This transneuronal retrograde viral pathway labeling method with PRV provided us the opportunity to study cardiovascular controlling networks. The distribution of the PRV-infected cells was studied immunocytochemically after survival times of 3 to 6 days. Right ventricular infection showed labeling in the same nuclei as left ventricular labeling, but the number of PRV-positive cells was always higher and the localization of PRV within the nuclei differed. These obvious signs for differentiation within the nuclei suggest differential neuronal pathways to various parts of the heart.
Following injection of PRV at different cardiac sites, differences in density and localization of PRV-positive cells were found predominantly in higher-order neurons that are known to be involved in cardiac control. Transection of the spinal cord at Th1, performed to reveal selectively the parasympathetic neuronal networks, reduced the number of labeled cells, specifically in the periaqueductal gray matter. Virus-labeled sympathetic preganglionic cells were found in the Th1–Th7 thoracic intermediolateral cell groups, with some additional infections at Th8–Th11 after inoculations of the ventricular myocardium. The rostral parts of the insular cortex appeared to be linked selectively to sympathetic innervation of the heart.6
From the experiments we hypothesized that, according to the type of lesion, the pattern of cardiac innervation may account for a specific malfunctioning. Subsequently, the subendocardial clustered parasympathetic nerves make these nerves more vulnerable for myocardial damage than the superficial spread of sympathetic nerves. In this respect, the identification of three preganglionic parasympathetic nuclei in cardiac control—ie, the dorsal motor nucleus of the vagus (20% labeling), the nucleus ambiguus, and the periambiguus—constituted the most striking findings.
PROJECTS 2 AND 3: CARDIAC NOCICEPTOR ACTIVATION
The cortical structures and their related output pathways also serve as effector systems for initiation of autonomic and behavioral responses by forebrain neuronal networks that make us aware of cardiac pain. However, these cortical and subcortical structures involved in cardiac pain perception were more or less terra incognita. In addition, we studied fundamental aspects of cardiac nociceptor activation (Project 2) and transduction of cardiac pain (Project 3). Unfortunately, there was no experimental animal model for angina pectoris. The aim of these projects was to obtain, both in patients and in animals, knowledge about cardiac nociceptor activation mechanisms, the transmission and perception of cardiac pain, and behavioral and autonomic responses.
To enable the study of mechanisms of neurostimulation during episodes of acute cardiac pain, we worked out an animal model for angina pectoris. For that reason we experimented with models in which we created an acute myocardial infarction. We had to reject this model since surgery and, more importantly, anesthesia interfered with the patterns of cerebral expression of immediate early genes (c-fos, c-jun) triggered by cardiac pain and/or neurostimulation. However, a spinoff from this project was the observation that cardiac tissue damage causes a reproducible and selective cerebral endothelial leakage of immunoglobulin G (IgG) molecules. Follow-up experiments showed that proinflammatory cytokines, which are released into the circulation after cardiac tissue damage, can generate the same pattern of blood-brain barrier dysfunction7 (see Project 4).
We then experimented with infusions of capsaicin into the pericardial space of unrestrained and unanesthetized rats to induce acute cardiac pain. This model appeared to be very promising and allows visualization of the behavioral and autonomic responses to cardiac pain. Cerebral c-fos expression patterns, a marker for structures involved in cardiac pain transmission and perception, were studied and validated with positron emission tomography (PET) imaging in patients.8
Project 2: Nociception of cardiac pain in patients
To study relationships between neurotransmitters and other molecules that contribute to pain and psychological variables, we studied cardiac tissues obtained from 22 patients with angina during coronary artery bypass graft surgery (CABG). Cardiac nociceptor activation mechanisms were investigated in heart biopsies from these 22 CABG patients; reverse transcriptase polymerase chain reaction analysis (RT-PCR) was conducted for adenosine and bradykinin receptor mRNA.9,10
An age-related decrease was observed in the adenosine A1 mRNA density but not in the bradykinin receptor mRNA levels. The adenosine A1 receptor density also correlated with pain characteristics reported in a questionnaire. Making use of semiquantitative RT-PCR, cardiac tissue substrates were assessed to determine the expression of adenosine A1 and bradykinin B1/2 receptor mRNA densities. The outcomes were associated with the quality of pain, age, gender, medication, and duration of disease.9,10
For evaluation of pain characteristics, we used questionnaires and objective pain scores. We found that qualitative age-related alterations in angina perception correlated with the development of the more “strangling” component of angina at older age. This observation may be explained, in part, by a reduction in adenosine A1 receptor mRNA expression in the heart, since bradykinin B1/2 receptor densities remain the same.9,10
Project 3: Nociception of cardiac pain in unrestrained rats
Having identified neural pathways, we studied neurons that were activated during electrical neuromodulation. 11 In search of a putative mechanism of action of electrical neuromodulation, we hypothesized that neuromodulation affects processing of nociceptive information within the central nervous system (CNS). To characterize neural activity we used expression of both the immediate early gene c-fos and the “late gene” or stress protein known as heat shock protein 72 (HSP72). c-fos was used to identify structures in the CNS affected by spinal cord stimulation. HSP72 was applied to ascertain whether spinal cord stimulation might operate as a stressor.12
Animal experiments were conducted on unrestrained unanesthetized rats implanted with a permanent catheter in the pericardial space; acute cardiac pain was triggered in this space using capsaicin as the algogenic substance.13 The autonomic cardiovascular responses were recorded with implantable telemetric devices. Behavioral responses were recorded on videotapes taken from the same animals in which the involved cerebral structures were characterized by analyzing cerebral immediate early gene expression. Quantification of data makes it possible to study the effects of electrical neuromodulation and analgesic drugs on perception of cardiac pain. To apply electrical neuromodulation, two electrodes were positioned and sutured epidurally at the spinal cord of the rats. One electrode was fixated at spinal nerve C7 and the other at T2. Furthermore, we studied the effect of spinal cord stimulation on behavior. Three hours after stimulation, the rats were sacrificed and their brains and spinal cords were removed.
The treated group showed regional increased c-fos expression in a select group of regions of the limbic system—periaqueductal gray, paraventricular hypothalamic nucleus, paraventricular thalamic nucleus, central amygdala, agranular and dysgranular insular cortex, (peri)ambiguus, nucleus tractus solitarius, and spinal cord—involved in the processing of pain and cardiovascular regulation, among other functions. Moreover, in both treated rats and controls, HSP72 expression was found in the endothelium of the enthorhinal cortex, the amygdala, and the ventral hypothalamus, but not in the neurons. The treated animals were significantly more alert and active than were the controls.
Thus, the rat model we developed appears to be suitable for studying potential mechanisms through which neuromodulation may act. Moreover, neuromodulation affects c-fos expression in specific parts of the brain known to be involved in regulation of pain and emotions. HSP72 expression is limited to the endothelium of certain parts of the CNS, and thus physical stress effects were excluded as a potential mechanism of neuromodulation. Finally, our experimental model identified regions corresponding with regional cerebral blood flow changes during neurostimulation in patients.8
PROJECT 4: BIDIRECTIONAL HUMORAL AND NERVOUS HEART-BRAIN INTERACTIONS
With respect to the emotional component of angina, we thought to study alternative pathways of communication between the heart and the brain. This idea occurred as a consequence of observations that many patients who suffer serious cardiac events, such as CABG or myocardial infarction, are confronted with a period of emotional problems following these events. So, from our experimental projects, the question became relevant as to whether emotional alterations in behavior following a cardiac life event may be executed by a humoral pathway from the heart to the brain, since, vice versa, the brain controls the heart through both nervous and humoral pathways. In other words, is it feasible that both humoral and neural pathways are involved, bidirectionally, in interactions between the brain and the heart?
Cardiac disease, proinflammatory cytokines, and blood-brain barrier damage
Cardiac ischemia, the underlying cause of cardiac pain in angina pectoris, triggers a cascade of events that release numerous substances in the myocardium and circulation, all of which are potential candidates for nociceptor activation and initiation of behavioral and autonomic responses to cardiac pain. Some of the substances that are released into the circulation may play a role in the humoral communication between heart and brain, but when released chronically, these substances may induce neuropathological modifications. Anxiety disorders and depression are cerebral disorders that are frequently comorbid with ischemic heart diseases. The latter are attributed to noncoping behavior, but our own experiments (as part of the program) showed that immune activation after tissue damage in the heart generates regional blood-brain barrier damage (Project 4) that could be an underlying organic basis for comorbid neuropsychiatric disorders. The incentive for this project in general was the observation that myocardial infarction is accompanied by behavioral and neuronal abnormalities.
In this project we established whether release of proinflammatory cytokines after tissue damage in the heart is a possible inducer of comorbid neuropsychiatric diseases.
As a model for immune activation, we studied the effects of intravenous injections of the proinflammatory recombinant tumor necrosis factor–alpha (TNF-α) on cerebral endothelial leakage, induction of neuronal damage, and motor and cognitive function in rats. Determinants of selectivity of blood-brain barrier damage were assessed with a molecular biological approach in which we studied regional differences of TNF-α–induced expression in the cerebral endothelial cells of the immediate early gene c-fos and proteins involved in leukocyte docking (intercellular adhesion molecules [ICAMs]) and TNF-α receptors.
To examine the mechanisms by which this interaction occurs, we induced myocardial infarction in a group of rats and then performed immunohistochemistry of the brain. This experiment revealed regional serum protein extravasation, pointing to leakage of the blood-brain barrier. This process occurred in certain cortical, subcortical, and hindbrain areas in discrete patches. The leakage was colocalized with expression of the immune activation marker ICAM-1. To assess the involvement of the immune system in the effects shown, a second group of rats was injected with TNF-α, as the major proinflammatory cytokine. This procedure rendered the same results. It was concluded that myocardial infarction may interfere with the integrity of the blood-brain barrier and possibly with brain functioning through activation of the immune system. The relevance for pathophysiological processes may provide a substrate for further research in unraveling the emotional consequences of serious cardiac events.
In the state of immune activation that follows myocardial ischemic events, various cytokines are released from the myocardium into the plasma. These cyto kines potentiate the cytotoxicity of TNF-α. In the next experiment we were able to demonstrate that intravenous injection of TNF-α induces a selective and regional neural IgG and endothelial ICAM-1 immunoreactivity. The expression of TNF-α–induced changes in the brain suggests that TNF-α is capable of inducing blood-brain barrier dysfunction. It is hypothesized that through dysfunction of the blood-brain barrier, the released cytokines bind to specific cognitive centers in the brain and thus may lead to emotional disturbances following cardiac events.14
Having identified some specific centers involved in cardiovascular control, we further studied the effects of electrical and chemical stimulation of a specific brain center on the heart.
PROJECT 5: EFFECT OF BRAIN STIMULATION ON CORONARY FLOW
From a clinical PET study performed in patients with end-stage CAD during active spinal cord stimulation therapy, as well as from our PRV experiments and the literature, we concluded that the periaqueductal gray plays a central role in the regulation of different cardiovascular responses and in the integration of motor output from the limbic system.6,7 Subsequently, the peri aqueductal gray has been thought to be one of the pivotal cerebral centers involved in executing electrical neuromodulation effects.
We investigated the function of the periaqueductal gray in regulation of the coronary flow of the heart. Depending on the stimulation site, electrical stimulation in the periaqueductal gray resulted in increases and decreases in coronary flow and conductance. These effects were organized topographically. The sites producing increases in coronary flow and conductance were found in both the dorsolateral and the ventrolateral periaqueductal gray. The sites producing decreases were restricted mainly to the ventrolateral portion. Similar topographic distributions were observed for the sites producing changes in carotid conductance and heart rate, but not for those producing changes in blood pressure and carotid flow. It is hypothesized that the topographic distribution of coronary vasoconstrictive and vasodilatory responses from the periaqueductal gray may enable optimal adjustments of the coronary perfusion. These optimal adjustments can then accommodate variations in myocardial oxygen demands accompanying different behavioral modes.
CONCLUSION
From all our experiments, mainly performed in rats (but sometimes also in a cat model due to the existence of a stereotactic brain atlas for the cat), we have learned about heart-brain communication through the use of electrical neuromodulation. In the last decade we have further studied heart-brain interactions in the International Working Group on Neurocardiology (IWGN), making use of canine and rabbit models. The main focus of the IWGN is on neural hierarchy in cardiac control. These projects are discussed by one of us (R.D.F.) elsewhere in these proceedings. In brief, the importance for the heart of the intracardiac neuron system and controlling centers at the C1 spinal level,15–17 in conjunction with the induction of myocardial ischemia, will be highlighted. For a more extensive overview of recent work performed by the IWGN, see the reviews by Foreman et al18 and Wu et al.19
- Mannheimer C, Camici P, Chester MR, et al. The problem of chronic refractory angina: report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J 2002; 23:355–370.
- Fraker TD, Fihn SD. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol 2007; 50:2264–2274.
- Wittkampf FHM, DeJongste MJL, Meijler FL. Atrioventricular nodal response to retrograde activation in atrial fibrillation. J Cardiovasc Electrophysiol 1990; 1:437–447.
- Wittkampf FHM, DeJongste MJL, Meijler FL. Competitive anterograde and retrograde atrioventricular junctional activation in atrial fibrillation. J Cardiovasc Electrophysiol 1990; 1:448–456.
- Heberden W. Some account of a disorder of the breast. Med Trans 1772; 2:59–67.
- Ter Horst GJ, Hautvast RW, DeJongste MJ, Korf J. Neuroanatomy of cardiac activity-regulating circuitry: a transneuronal retrograde viral labelling study in the rat. Eur J Neurosci 1996; 8:2029–2041.
- Ter Horst GJ, VanderWerf YD, DeJongste MJL Acute myocardial infarction and cytokine-mediated selective blood-brain barrier leakage in the rat. J Neurochem 1996; 66( suppl 2):S54A. Abstract.
- Hautvast RW, Ter Horst GJ, DeJong BM, et al. Relative changes in regional cerebral blood flow during spinal cord stimulation in patients with refractory angina pectoris. Eur J Neurosci 1997; 9:1178–1183.
- DeJongste MJL, Ter Horst GJ. Mediators of inflammation in patients with coronary artery disease. In:Ter Horst GJ, ed. The Nervous System and the Heart. Totowa, NJ: Humana Press; 2000:467–487.
- Van Der Werf YD, TerHorst GJ, DeJongste MJL. Receptor mRNA densities and psychometric measures of determinants of anginal pain. Eur J Neurosci 1996; 8:S77. Abstract 32.35.
- Albutaihi IA, DeJongste MJ, Ter Horst GJ. An integrated study of heart pain and behavior in freely moving rats (using fos as a marker for neuronal activation). Neurosignals 2004; 13:207–226.
- DeJongste MJL, Hautvast RWM, Ruiters MHJ, Ter Horst GJ. Spinal cord stimulation and the induction of c-fos and heat shock protein 72 in the central nervous system of rats. Neuromodulation 1998; 1:73–84.
- Albutaihi IA, Hautvast RW, DeJongste MJ, Ter Horst GJ, Staal MJ. Cardiac nociception in rats: neuronal pathways and the influence of dermal neurostimulation on conveyance to the central nervous system. J Mol Neurosci 2003; 20:43–52.
- Ter Horst GJ, Nagel JG, DeJongste MJL, Van Der Werf YD. Selective blood brain barrier dysfunction after intravenous injections of rTNFα in the rat. In:Teelken A, Korf J, eds. Neurochemistry and Neuroimmunology of EAE: Implications for Therapy of MS. New York, NY: Plenum Press; 1997; Section 5:141–146.
- Foreman RD. Integration of viscerosomatic sensory input at the spinal level. Prog Brain Res 2000; 122:209–221.
- Ding X, Ardell JL, Hua F, et al. Modulation of cardiac ischemiasensitive afferent neuron signaling by preemptive C2 spinal cord stimulation: effect on substance P release from rat spinal cord. Am J Physiol Regul Integr Comp Physiol 2008; 294:R93–R101.
- Qin C, Faber JP, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9:71–78.
- Foreman RD, DeJongste MJ, Linderoth B. Integrative control of cardiac function by cervical and thoracic spinal neurons. In:Armour JA, Ardell JL, eds. Basic and Clinical Neurocardiology. New York, NY: Oxford University Press; 2004:153–186.
- Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci 2008; 138:9–23.
RATIONALE
In the industrialized world, average life expectancy has nearly doubled since the 19th century. One of the consequences of this increase in life span is that the sequelae of diseases also have increased. For coronary artery disease (CAD), one of the most prevalent diseases in the western world, this has resulted in an amplification of the number of patients suffering from heart failure, arrhythmias, and refractory angina. Much progress has recently been made in nonpharmacologic therapies for these deleterious consequences of CAD, such as cardiac resynchronization for heart failure, implantable defibrillators for ventricular arrhythmias, and electrical neuromodulation by means of spinal cord stimulation for chronic angina that is refractory to conventional strategies.
For patients suffering from severe angina secondary to end-stage CAD who have no other options to alleviate their complaints, electrical neuromodulation may be the preferred adjunctive treatment.1 Although spinal cord stimulation is still not approved by the US Food and Drug Administration for treatment of refractory angina, it is is accepted in the American College of Cardiology/American Heart Association guidelines for chronic stable angina, with a class II indication, and is frequently used for this indication in Europe.2
However, to understand underlying mechanisms of therapies such as electrical neuromodulation—executed through either transcutaneous electrical nerve stimulation or spinal cord stimulation—for angina pectoris and to improve the effect and safety of these therapies, clinical questions concerning neuromodulation must be evaluated in experimental models. The outcomes of these preclinical experimental studies subsequently need to be assessed in humans.
Although therapeutic improvements from implantable devices would not have been possible without experimental work, any experimentation must be avoided if it is not approved by the relevant ethics committee(s) or is not conducted in keeping with standard guidelines. For this reason it is sometimes more feasible, when appropriate, to make use of simulation models—for instance, to study regularization of atrial fibrillation by means of a device.3,4
PROJECT 1: EMOTIONS AND MYOCARDIAL ISCHEMIA
In 1772, Heberden described to physicians in England the clinical symptoms of exercise-induced chest discomfort, with its emotional component and vaguely distributed projection on the chest, as follows: “The seat of it, and sense of strangling and anxiety with which it is attended, may make it not improperly be called angina pectoris.”5 Since then, it has been demonstrated repeatedly that strong emotional distress frequently precedes or is associated with complaints of pain in the chest. Further, emotional suffering has been associated with increased mortality in patients with CAD. We and others, unfortunately, were confronted with very limited knowledge of the precise locations of the origin of emotions in the limbic structures of the forebrain. Even less was known about the relationship of these brain structures and the heart, owing to technical limitations in the field of neuroanatomical tract tracing, among other reasons. As a result, the nervous pathways from the heart, through which signals are propagated to the brain in order to activate emotional components, were not accurately identified. We therefore initiated Project 1 to study, in a rat model, neuroanatomical characterization of the neuronal circuitry controlling cardiac activity, specifically during cardiac distress.
In the area of identifying efferent neural pathways from the heart, we were the first to publish an experimental setup making use of a neurotropic herpesvirus from the Bartha strain of the pseudorabies virus (PRV).6 Following injections of PRV into the left and right myocardium or into atrial tissue, PRV infects the neurons that innervate the injection site and is then transported in the neural network, where the virus may cross at least four synapses. This transneuronal retrograde viral pathway labeling method with PRV provided us the opportunity to study cardiovascular controlling networks. The distribution of the PRV-infected cells was studied immunocytochemically after survival times of 3 to 6 days. Right ventricular infection showed labeling in the same nuclei as left ventricular labeling, but the number of PRV-positive cells was always higher and the localization of PRV within the nuclei differed. These obvious signs for differentiation within the nuclei suggest differential neuronal pathways to various parts of the heart.
Following injection of PRV at different cardiac sites, differences in density and localization of PRV-positive cells were found predominantly in higher-order neurons that are known to be involved in cardiac control. Transection of the spinal cord at Th1, performed to reveal selectively the parasympathetic neuronal networks, reduced the number of labeled cells, specifically in the periaqueductal gray matter. Virus-labeled sympathetic preganglionic cells were found in the Th1–Th7 thoracic intermediolateral cell groups, with some additional infections at Th8–Th11 after inoculations of the ventricular myocardium. The rostral parts of the insular cortex appeared to be linked selectively to sympathetic innervation of the heart.6
From the experiments we hypothesized that, according to the type of lesion, the pattern of cardiac innervation may account for a specific malfunctioning. Subsequently, the subendocardial clustered parasympathetic nerves make these nerves more vulnerable for myocardial damage than the superficial spread of sympathetic nerves. In this respect, the identification of three preganglionic parasympathetic nuclei in cardiac control—ie, the dorsal motor nucleus of the vagus (20% labeling), the nucleus ambiguus, and the periambiguus—constituted the most striking findings.
PROJECTS 2 AND 3: CARDIAC NOCICEPTOR ACTIVATION
The cortical structures and their related output pathways also serve as effector systems for initiation of autonomic and behavioral responses by forebrain neuronal networks that make us aware of cardiac pain. However, these cortical and subcortical structures involved in cardiac pain perception were more or less terra incognita. In addition, we studied fundamental aspects of cardiac nociceptor activation (Project 2) and transduction of cardiac pain (Project 3). Unfortunately, there was no experimental animal model for angina pectoris. The aim of these projects was to obtain, both in patients and in animals, knowledge about cardiac nociceptor activation mechanisms, the transmission and perception of cardiac pain, and behavioral and autonomic responses.
To enable the study of mechanisms of neurostimulation during episodes of acute cardiac pain, we worked out an animal model for angina pectoris. For that reason we experimented with models in which we created an acute myocardial infarction. We had to reject this model since surgery and, more importantly, anesthesia interfered with the patterns of cerebral expression of immediate early genes (c-fos, c-jun) triggered by cardiac pain and/or neurostimulation. However, a spinoff from this project was the observation that cardiac tissue damage causes a reproducible and selective cerebral endothelial leakage of immunoglobulin G (IgG) molecules. Follow-up experiments showed that proinflammatory cytokines, which are released into the circulation after cardiac tissue damage, can generate the same pattern of blood-brain barrier dysfunction7 (see Project 4).
We then experimented with infusions of capsaicin into the pericardial space of unrestrained and unanesthetized rats to induce acute cardiac pain. This model appeared to be very promising and allows visualization of the behavioral and autonomic responses to cardiac pain. Cerebral c-fos expression patterns, a marker for structures involved in cardiac pain transmission and perception, were studied and validated with positron emission tomography (PET) imaging in patients.8
Project 2: Nociception of cardiac pain in patients
To study relationships between neurotransmitters and other molecules that contribute to pain and psychological variables, we studied cardiac tissues obtained from 22 patients with angina during coronary artery bypass graft surgery (CABG). Cardiac nociceptor activation mechanisms were investigated in heart biopsies from these 22 CABG patients; reverse transcriptase polymerase chain reaction analysis (RT-PCR) was conducted for adenosine and bradykinin receptor mRNA.9,10
An age-related decrease was observed in the adenosine A1 mRNA density but not in the bradykinin receptor mRNA levels. The adenosine A1 receptor density also correlated with pain characteristics reported in a questionnaire. Making use of semiquantitative RT-PCR, cardiac tissue substrates were assessed to determine the expression of adenosine A1 and bradykinin B1/2 receptor mRNA densities. The outcomes were associated with the quality of pain, age, gender, medication, and duration of disease.9,10
For evaluation of pain characteristics, we used questionnaires and objective pain scores. We found that qualitative age-related alterations in angina perception correlated with the development of the more “strangling” component of angina at older age. This observation may be explained, in part, by a reduction in adenosine A1 receptor mRNA expression in the heart, since bradykinin B1/2 receptor densities remain the same.9,10
Project 3: Nociception of cardiac pain in unrestrained rats
Having identified neural pathways, we studied neurons that were activated during electrical neuromodulation. 11 In search of a putative mechanism of action of electrical neuromodulation, we hypothesized that neuromodulation affects processing of nociceptive information within the central nervous system (CNS). To characterize neural activity we used expression of both the immediate early gene c-fos and the “late gene” or stress protein known as heat shock protein 72 (HSP72). c-fos was used to identify structures in the CNS affected by spinal cord stimulation. HSP72 was applied to ascertain whether spinal cord stimulation might operate as a stressor.12
Animal experiments were conducted on unrestrained unanesthetized rats implanted with a permanent catheter in the pericardial space; acute cardiac pain was triggered in this space using capsaicin as the algogenic substance.13 The autonomic cardiovascular responses were recorded with implantable telemetric devices. Behavioral responses were recorded on videotapes taken from the same animals in which the involved cerebral structures were characterized by analyzing cerebral immediate early gene expression. Quantification of data makes it possible to study the effects of electrical neuromodulation and analgesic drugs on perception of cardiac pain. To apply electrical neuromodulation, two electrodes were positioned and sutured epidurally at the spinal cord of the rats. One electrode was fixated at spinal nerve C7 and the other at T2. Furthermore, we studied the effect of spinal cord stimulation on behavior. Three hours after stimulation, the rats were sacrificed and their brains and spinal cords were removed.
The treated group showed regional increased c-fos expression in a select group of regions of the limbic system—periaqueductal gray, paraventricular hypothalamic nucleus, paraventricular thalamic nucleus, central amygdala, agranular and dysgranular insular cortex, (peri)ambiguus, nucleus tractus solitarius, and spinal cord—involved in the processing of pain and cardiovascular regulation, among other functions. Moreover, in both treated rats and controls, HSP72 expression was found in the endothelium of the enthorhinal cortex, the amygdala, and the ventral hypothalamus, but not in the neurons. The treated animals were significantly more alert and active than were the controls.
Thus, the rat model we developed appears to be suitable for studying potential mechanisms through which neuromodulation may act. Moreover, neuromodulation affects c-fos expression in specific parts of the brain known to be involved in regulation of pain and emotions. HSP72 expression is limited to the endothelium of certain parts of the CNS, and thus physical stress effects were excluded as a potential mechanism of neuromodulation. Finally, our experimental model identified regions corresponding with regional cerebral blood flow changes during neurostimulation in patients.8
PROJECT 4: BIDIRECTIONAL HUMORAL AND NERVOUS HEART-BRAIN INTERACTIONS
With respect to the emotional component of angina, we thought to study alternative pathways of communication between the heart and the brain. This idea occurred as a consequence of observations that many patients who suffer serious cardiac events, such as CABG or myocardial infarction, are confronted with a period of emotional problems following these events. So, from our experimental projects, the question became relevant as to whether emotional alterations in behavior following a cardiac life event may be executed by a humoral pathway from the heart to the brain, since, vice versa, the brain controls the heart through both nervous and humoral pathways. In other words, is it feasible that both humoral and neural pathways are involved, bidirectionally, in interactions between the brain and the heart?
Cardiac disease, proinflammatory cytokines, and blood-brain barrier damage
Cardiac ischemia, the underlying cause of cardiac pain in angina pectoris, triggers a cascade of events that release numerous substances in the myocardium and circulation, all of which are potential candidates for nociceptor activation and initiation of behavioral and autonomic responses to cardiac pain. Some of the substances that are released into the circulation may play a role in the humoral communication between heart and brain, but when released chronically, these substances may induce neuropathological modifications. Anxiety disorders and depression are cerebral disorders that are frequently comorbid with ischemic heart diseases. The latter are attributed to noncoping behavior, but our own experiments (as part of the program) showed that immune activation after tissue damage in the heart generates regional blood-brain barrier damage (Project 4) that could be an underlying organic basis for comorbid neuropsychiatric disorders. The incentive for this project in general was the observation that myocardial infarction is accompanied by behavioral and neuronal abnormalities.
In this project we established whether release of proinflammatory cytokines after tissue damage in the heart is a possible inducer of comorbid neuropsychiatric diseases.
As a model for immune activation, we studied the effects of intravenous injections of the proinflammatory recombinant tumor necrosis factor–alpha (TNF-α) on cerebral endothelial leakage, induction of neuronal damage, and motor and cognitive function in rats. Determinants of selectivity of blood-brain barrier damage were assessed with a molecular biological approach in which we studied regional differences of TNF-α–induced expression in the cerebral endothelial cells of the immediate early gene c-fos and proteins involved in leukocyte docking (intercellular adhesion molecules [ICAMs]) and TNF-α receptors.
To examine the mechanisms by which this interaction occurs, we induced myocardial infarction in a group of rats and then performed immunohistochemistry of the brain. This experiment revealed regional serum protein extravasation, pointing to leakage of the blood-brain barrier. This process occurred in certain cortical, subcortical, and hindbrain areas in discrete patches. The leakage was colocalized with expression of the immune activation marker ICAM-1. To assess the involvement of the immune system in the effects shown, a second group of rats was injected with TNF-α, as the major proinflammatory cytokine. This procedure rendered the same results. It was concluded that myocardial infarction may interfere with the integrity of the blood-brain barrier and possibly with brain functioning through activation of the immune system. The relevance for pathophysiological processes may provide a substrate for further research in unraveling the emotional consequences of serious cardiac events.
In the state of immune activation that follows myocardial ischemic events, various cytokines are released from the myocardium into the plasma. These cyto kines potentiate the cytotoxicity of TNF-α. In the next experiment we were able to demonstrate that intravenous injection of TNF-α induces a selective and regional neural IgG and endothelial ICAM-1 immunoreactivity. The expression of TNF-α–induced changes in the brain suggests that TNF-α is capable of inducing blood-brain barrier dysfunction. It is hypothesized that through dysfunction of the blood-brain barrier, the released cytokines bind to specific cognitive centers in the brain and thus may lead to emotional disturbances following cardiac events.14
Having identified some specific centers involved in cardiovascular control, we further studied the effects of electrical and chemical stimulation of a specific brain center on the heart.
PROJECT 5: EFFECT OF BRAIN STIMULATION ON CORONARY FLOW
From a clinical PET study performed in patients with end-stage CAD during active spinal cord stimulation therapy, as well as from our PRV experiments and the literature, we concluded that the periaqueductal gray plays a central role in the regulation of different cardiovascular responses and in the integration of motor output from the limbic system.6,7 Subsequently, the peri aqueductal gray has been thought to be one of the pivotal cerebral centers involved in executing electrical neuromodulation effects.
We investigated the function of the periaqueductal gray in regulation of the coronary flow of the heart. Depending on the stimulation site, electrical stimulation in the periaqueductal gray resulted in increases and decreases in coronary flow and conductance. These effects were organized topographically. The sites producing increases in coronary flow and conductance were found in both the dorsolateral and the ventrolateral periaqueductal gray. The sites producing decreases were restricted mainly to the ventrolateral portion. Similar topographic distributions were observed for the sites producing changes in carotid conductance and heart rate, but not for those producing changes in blood pressure and carotid flow. It is hypothesized that the topographic distribution of coronary vasoconstrictive and vasodilatory responses from the periaqueductal gray may enable optimal adjustments of the coronary perfusion. These optimal adjustments can then accommodate variations in myocardial oxygen demands accompanying different behavioral modes.
CONCLUSION
From all our experiments, mainly performed in rats (but sometimes also in a cat model due to the existence of a stereotactic brain atlas for the cat), we have learned about heart-brain communication through the use of electrical neuromodulation. In the last decade we have further studied heart-brain interactions in the International Working Group on Neurocardiology (IWGN), making use of canine and rabbit models. The main focus of the IWGN is on neural hierarchy in cardiac control. These projects are discussed by one of us (R.D.F.) elsewhere in these proceedings. In brief, the importance for the heart of the intracardiac neuron system and controlling centers at the C1 spinal level,15–17 in conjunction with the induction of myocardial ischemia, will be highlighted. For a more extensive overview of recent work performed by the IWGN, see the reviews by Foreman et al18 and Wu et al.19
RATIONALE
In the industrialized world, average life expectancy has nearly doubled since the 19th century. One of the consequences of this increase in life span is that the sequelae of diseases also have increased. For coronary artery disease (CAD), one of the most prevalent diseases in the western world, this has resulted in an amplification of the number of patients suffering from heart failure, arrhythmias, and refractory angina. Much progress has recently been made in nonpharmacologic therapies for these deleterious consequences of CAD, such as cardiac resynchronization for heart failure, implantable defibrillators for ventricular arrhythmias, and electrical neuromodulation by means of spinal cord stimulation for chronic angina that is refractory to conventional strategies.
For patients suffering from severe angina secondary to end-stage CAD who have no other options to alleviate their complaints, electrical neuromodulation may be the preferred adjunctive treatment.1 Although spinal cord stimulation is still not approved by the US Food and Drug Administration for treatment of refractory angina, it is is accepted in the American College of Cardiology/American Heart Association guidelines for chronic stable angina, with a class II indication, and is frequently used for this indication in Europe.2
However, to understand underlying mechanisms of therapies such as electrical neuromodulation—executed through either transcutaneous electrical nerve stimulation or spinal cord stimulation—for angina pectoris and to improve the effect and safety of these therapies, clinical questions concerning neuromodulation must be evaluated in experimental models. The outcomes of these preclinical experimental studies subsequently need to be assessed in humans.
Although therapeutic improvements from implantable devices would not have been possible without experimental work, any experimentation must be avoided if it is not approved by the relevant ethics committee(s) or is not conducted in keeping with standard guidelines. For this reason it is sometimes more feasible, when appropriate, to make use of simulation models—for instance, to study regularization of atrial fibrillation by means of a device.3,4
PROJECT 1: EMOTIONS AND MYOCARDIAL ISCHEMIA
In 1772, Heberden described to physicians in England the clinical symptoms of exercise-induced chest discomfort, with its emotional component and vaguely distributed projection on the chest, as follows: “The seat of it, and sense of strangling and anxiety with which it is attended, may make it not improperly be called angina pectoris.”5 Since then, it has been demonstrated repeatedly that strong emotional distress frequently precedes or is associated with complaints of pain in the chest. Further, emotional suffering has been associated with increased mortality in patients with CAD. We and others, unfortunately, were confronted with very limited knowledge of the precise locations of the origin of emotions in the limbic structures of the forebrain. Even less was known about the relationship of these brain structures and the heart, owing to technical limitations in the field of neuroanatomical tract tracing, among other reasons. As a result, the nervous pathways from the heart, through which signals are propagated to the brain in order to activate emotional components, were not accurately identified. We therefore initiated Project 1 to study, in a rat model, neuroanatomical characterization of the neuronal circuitry controlling cardiac activity, specifically during cardiac distress.
In the area of identifying efferent neural pathways from the heart, we were the first to publish an experimental setup making use of a neurotropic herpesvirus from the Bartha strain of the pseudorabies virus (PRV).6 Following injections of PRV into the left and right myocardium or into atrial tissue, PRV infects the neurons that innervate the injection site and is then transported in the neural network, where the virus may cross at least four synapses. This transneuronal retrograde viral pathway labeling method with PRV provided us the opportunity to study cardiovascular controlling networks. The distribution of the PRV-infected cells was studied immunocytochemically after survival times of 3 to 6 days. Right ventricular infection showed labeling in the same nuclei as left ventricular labeling, but the number of PRV-positive cells was always higher and the localization of PRV within the nuclei differed. These obvious signs for differentiation within the nuclei suggest differential neuronal pathways to various parts of the heart.
Following injection of PRV at different cardiac sites, differences in density and localization of PRV-positive cells were found predominantly in higher-order neurons that are known to be involved in cardiac control. Transection of the spinal cord at Th1, performed to reveal selectively the parasympathetic neuronal networks, reduced the number of labeled cells, specifically in the periaqueductal gray matter. Virus-labeled sympathetic preganglionic cells were found in the Th1–Th7 thoracic intermediolateral cell groups, with some additional infections at Th8–Th11 after inoculations of the ventricular myocardium. The rostral parts of the insular cortex appeared to be linked selectively to sympathetic innervation of the heart.6
From the experiments we hypothesized that, according to the type of lesion, the pattern of cardiac innervation may account for a specific malfunctioning. Subsequently, the subendocardial clustered parasympathetic nerves make these nerves more vulnerable for myocardial damage than the superficial spread of sympathetic nerves. In this respect, the identification of three preganglionic parasympathetic nuclei in cardiac control—ie, the dorsal motor nucleus of the vagus (20% labeling), the nucleus ambiguus, and the periambiguus—constituted the most striking findings.
PROJECTS 2 AND 3: CARDIAC NOCICEPTOR ACTIVATION
The cortical structures and their related output pathways also serve as effector systems for initiation of autonomic and behavioral responses by forebrain neuronal networks that make us aware of cardiac pain. However, these cortical and subcortical structures involved in cardiac pain perception were more or less terra incognita. In addition, we studied fundamental aspects of cardiac nociceptor activation (Project 2) and transduction of cardiac pain (Project 3). Unfortunately, there was no experimental animal model for angina pectoris. The aim of these projects was to obtain, both in patients and in animals, knowledge about cardiac nociceptor activation mechanisms, the transmission and perception of cardiac pain, and behavioral and autonomic responses.
To enable the study of mechanisms of neurostimulation during episodes of acute cardiac pain, we worked out an animal model for angina pectoris. For that reason we experimented with models in which we created an acute myocardial infarction. We had to reject this model since surgery and, more importantly, anesthesia interfered with the patterns of cerebral expression of immediate early genes (c-fos, c-jun) triggered by cardiac pain and/or neurostimulation. However, a spinoff from this project was the observation that cardiac tissue damage causes a reproducible and selective cerebral endothelial leakage of immunoglobulin G (IgG) molecules. Follow-up experiments showed that proinflammatory cytokines, which are released into the circulation after cardiac tissue damage, can generate the same pattern of blood-brain barrier dysfunction7 (see Project 4).
We then experimented with infusions of capsaicin into the pericardial space of unrestrained and unanesthetized rats to induce acute cardiac pain. This model appeared to be very promising and allows visualization of the behavioral and autonomic responses to cardiac pain. Cerebral c-fos expression patterns, a marker for structures involved in cardiac pain transmission and perception, were studied and validated with positron emission tomography (PET) imaging in patients.8
Project 2: Nociception of cardiac pain in patients
To study relationships between neurotransmitters and other molecules that contribute to pain and psychological variables, we studied cardiac tissues obtained from 22 patients with angina during coronary artery bypass graft surgery (CABG). Cardiac nociceptor activation mechanisms were investigated in heart biopsies from these 22 CABG patients; reverse transcriptase polymerase chain reaction analysis (RT-PCR) was conducted for adenosine and bradykinin receptor mRNA.9,10
An age-related decrease was observed in the adenosine A1 mRNA density but not in the bradykinin receptor mRNA levels. The adenosine A1 receptor density also correlated with pain characteristics reported in a questionnaire. Making use of semiquantitative RT-PCR, cardiac tissue substrates were assessed to determine the expression of adenosine A1 and bradykinin B1/2 receptor mRNA densities. The outcomes were associated with the quality of pain, age, gender, medication, and duration of disease.9,10
For evaluation of pain characteristics, we used questionnaires and objective pain scores. We found that qualitative age-related alterations in angina perception correlated with the development of the more “strangling” component of angina at older age. This observation may be explained, in part, by a reduction in adenosine A1 receptor mRNA expression in the heart, since bradykinin B1/2 receptor densities remain the same.9,10
Project 3: Nociception of cardiac pain in unrestrained rats
Having identified neural pathways, we studied neurons that were activated during electrical neuromodulation. 11 In search of a putative mechanism of action of electrical neuromodulation, we hypothesized that neuromodulation affects processing of nociceptive information within the central nervous system (CNS). To characterize neural activity we used expression of both the immediate early gene c-fos and the “late gene” or stress protein known as heat shock protein 72 (HSP72). c-fos was used to identify structures in the CNS affected by spinal cord stimulation. HSP72 was applied to ascertain whether spinal cord stimulation might operate as a stressor.12
Animal experiments were conducted on unrestrained unanesthetized rats implanted with a permanent catheter in the pericardial space; acute cardiac pain was triggered in this space using capsaicin as the algogenic substance.13 The autonomic cardiovascular responses were recorded with implantable telemetric devices. Behavioral responses were recorded on videotapes taken from the same animals in which the involved cerebral structures were characterized by analyzing cerebral immediate early gene expression. Quantification of data makes it possible to study the effects of electrical neuromodulation and analgesic drugs on perception of cardiac pain. To apply electrical neuromodulation, two electrodes were positioned and sutured epidurally at the spinal cord of the rats. One electrode was fixated at spinal nerve C7 and the other at T2. Furthermore, we studied the effect of spinal cord stimulation on behavior. Three hours after stimulation, the rats were sacrificed and their brains and spinal cords were removed.
The treated group showed regional increased c-fos expression in a select group of regions of the limbic system—periaqueductal gray, paraventricular hypothalamic nucleus, paraventricular thalamic nucleus, central amygdala, agranular and dysgranular insular cortex, (peri)ambiguus, nucleus tractus solitarius, and spinal cord—involved in the processing of pain and cardiovascular regulation, among other functions. Moreover, in both treated rats and controls, HSP72 expression was found in the endothelium of the enthorhinal cortex, the amygdala, and the ventral hypothalamus, but not in the neurons. The treated animals were significantly more alert and active than were the controls.
Thus, the rat model we developed appears to be suitable for studying potential mechanisms through which neuromodulation may act. Moreover, neuromodulation affects c-fos expression in specific parts of the brain known to be involved in regulation of pain and emotions. HSP72 expression is limited to the endothelium of certain parts of the CNS, and thus physical stress effects were excluded as a potential mechanism of neuromodulation. Finally, our experimental model identified regions corresponding with regional cerebral blood flow changes during neurostimulation in patients.8
PROJECT 4: BIDIRECTIONAL HUMORAL AND NERVOUS HEART-BRAIN INTERACTIONS
With respect to the emotional component of angina, we thought to study alternative pathways of communication between the heart and the brain. This idea occurred as a consequence of observations that many patients who suffer serious cardiac events, such as CABG or myocardial infarction, are confronted with a period of emotional problems following these events. So, from our experimental projects, the question became relevant as to whether emotional alterations in behavior following a cardiac life event may be executed by a humoral pathway from the heart to the brain, since, vice versa, the brain controls the heart through both nervous and humoral pathways. In other words, is it feasible that both humoral and neural pathways are involved, bidirectionally, in interactions between the brain and the heart?
Cardiac disease, proinflammatory cytokines, and blood-brain barrier damage
Cardiac ischemia, the underlying cause of cardiac pain in angina pectoris, triggers a cascade of events that release numerous substances in the myocardium and circulation, all of which are potential candidates for nociceptor activation and initiation of behavioral and autonomic responses to cardiac pain. Some of the substances that are released into the circulation may play a role in the humoral communication between heart and brain, but when released chronically, these substances may induce neuropathological modifications. Anxiety disorders and depression are cerebral disorders that are frequently comorbid with ischemic heart diseases. The latter are attributed to noncoping behavior, but our own experiments (as part of the program) showed that immune activation after tissue damage in the heart generates regional blood-brain barrier damage (Project 4) that could be an underlying organic basis for comorbid neuropsychiatric disorders. The incentive for this project in general was the observation that myocardial infarction is accompanied by behavioral and neuronal abnormalities.
In this project we established whether release of proinflammatory cytokines after tissue damage in the heart is a possible inducer of comorbid neuropsychiatric diseases.
As a model for immune activation, we studied the effects of intravenous injections of the proinflammatory recombinant tumor necrosis factor–alpha (TNF-α) on cerebral endothelial leakage, induction of neuronal damage, and motor and cognitive function in rats. Determinants of selectivity of blood-brain barrier damage were assessed with a molecular biological approach in which we studied regional differences of TNF-α–induced expression in the cerebral endothelial cells of the immediate early gene c-fos and proteins involved in leukocyte docking (intercellular adhesion molecules [ICAMs]) and TNF-α receptors.
To examine the mechanisms by which this interaction occurs, we induced myocardial infarction in a group of rats and then performed immunohistochemistry of the brain. This experiment revealed regional serum protein extravasation, pointing to leakage of the blood-brain barrier. This process occurred in certain cortical, subcortical, and hindbrain areas in discrete patches. The leakage was colocalized with expression of the immune activation marker ICAM-1. To assess the involvement of the immune system in the effects shown, a second group of rats was injected with TNF-α, as the major proinflammatory cytokine. This procedure rendered the same results. It was concluded that myocardial infarction may interfere with the integrity of the blood-brain barrier and possibly with brain functioning through activation of the immune system. The relevance for pathophysiological processes may provide a substrate for further research in unraveling the emotional consequences of serious cardiac events.
In the state of immune activation that follows myocardial ischemic events, various cytokines are released from the myocardium into the plasma. These cyto kines potentiate the cytotoxicity of TNF-α. In the next experiment we were able to demonstrate that intravenous injection of TNF-α induces a selective and regional neural IgG and endothelial ICAM-1 immunoreactivity. The expression of TNF-α–induced changes in the brain suggests that TNF-α is capable of inducing blood-brain barrier dysfunction. It is hypothesized that through dysfunction of the blood-brain barrier, the released cytokines bind to specific cognitive centers in the brain and thus may lead to emotional disturbances following cardiac events.14
Having identified some specific centers involved in cardiovascular control, we further studied the effects of electrical and chemical stimulation of a specific brain center on the heart.
PROJECT 5: EFFECT OF BRAIN STIMULATION ON CORONARY FLOW
From a clinical PET study performed in patients with end-stage CAD during active spinal cord stimulation therapy, as well as from our PRV experiments and the literature, we concluded that the periaqueductal gray plays a central role in the regulation of different cardiovascular responses and in the integration of motor output from the limbic system.6,7 Subsequently, the peri aqueductal gray has been thought to be one of the pivotal cerebral centers involved in executing electrical neuromodulation effects.
We investigated the function of the periaqueductal gray in regulation of the coronary flow of the heart. Depending on the stimulation site, electrical stimulation in the periaqueductal gray resulted in increases and decreases in coronary flow and conductance. These effects were organized topographically. The sites producing increases in coronary flow and conductance were found in both the dorsolateral and the ventrolateral periaqueductal gray. The sites producing decreases were restricted mainly to the ventrolateral portion. Similar topographic distributions were observed for the sites producing changes in carotid conductance and heart rate, but not for those producing changes in blood pressure and carotid flow. It is hypothesized that the topographic distribution of coronary vasoconstrictive and vasodilatory responses from the periaqueductal gray may enable optimal adjustments of the coronary perfusion. These optimal adjustments can then accommodate variations in myocardial oxygen demands accompanying different behavioral modes.
CONCLUSION
From all our experiments, mainly performed in rats (but sometimes also in a cat model due to the existence of a stereotactic brain atlas for the cat), we have learned about heart-brain communication through the use of electrical neuromodulation. In the last decade we have further studied heart-brain interactions in the International Working Group on Neurocardiology (IWGN), making use of canine and rabbit models. The main focus of the IWGN is on neural hierarchy in cardiac control. These projects are discussed by one of us (R.D.F.) elsewhere in these proceedings. In brief, the importance for the heart of the intracardiac neuron system and controlling centers at the C1 spinal level,15–17 in conjunction with the induction of myocardial ischemia, will be highlighted. For a more extensive overview of recent work performed by the IWGN, see the reviews by Foreman et al18 and Wu et al.19
- Mannheimer C, Camici P, Chester MR, et al. The problem of chronic refractory angina: report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J 2002; 23:355–370.
- Fraker TD, Fihn SD. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol 2007; 50:2264–2274.
- Wittkampf FHM, DeJongste MJL, Meijler FL. Atrioventricular nodal response to retrograde activation in atrial fibrillation. J Cardiovasc Electrophysiol 1990; 1:437–447.
- Wittkampf FHM, DeJongste MJL, Meijler FL. Competitive anterograde and retrograde atrioventricular junctional activation in atrial fibrillation. J Cardiovasc Electrophysiol 1990; 1:448–456.
- Heberden W. Some account of a disorder of the breast. Med Trans 1772; 2:59–67.
- Ter Horst GJ, Hautvast RW, DeJongste MJ, Korf J. Neuroanatomy of cardiac activity-regulating circuitry: a transneuronal retrograde viral labelling study in the rat. Eur J Neurosci 1996; 8:2029–2041.
- Ter Horst GJ, VanderWerf YD, DeJongste MJL Acute myocardial infarction and cytokine-mediated selective blood-brain barrier leakage in the rat. J Neurochem 1996; 66( suppl 2):S54A. Abstract.
- Hautvast RW, Ter Horst GJ, DeJong BM, et al. Relative changes in regional cerebral blood flow during spinal cord stimulation in patients with refractory angina pectoris. Eur J Neurosci 1997; 9:1178–1183.
- DeJongste MJL, Ter Horst GJ. Mediators of inflammation in patients with coronary artery disease. In:Ter Horst GJ, ed. The Nervous System and the Heart. Totowa, NJ: Humana Press; 2000:467–487.
- Van Der Werf YD, TerHorst GJ, DeJongste MJL. Receptor mRNA densities and psychometric measures of determinants of anginal pain. Eur J Neurosci 1996; 8:S77. Abstract 32.35.
- Albutaihi IA, DeJongste MJ, Ter Horst GJ. An integrated study of heart pain and behavior in freely moving rats (using fos as a marker for neuronal activation). Neurosignals 2004; 13:207–226.
- DeJongste MJL, Hautvast RWM, Ruiters MHJ, Ter Horst GJ. Spinal cord stimulation and the induction of c-fos and heat shock protein 72 in the central nervous system of rats. Neuromodulation 1998; 1:73–84.
- Albutaihi IA, Hautvast RW, DeJongste MJ, Ter Horst GJ, Staal MJ. Cardiac nociception in rats: neuronal pathways and the influence of dermal neurostimulation on conveyance to the central nervous system. J Mol Neurosci 2003; 20:43–52.
- Ter Horst GJ, Nagel JG, DeJongste MJL, Van Der Werf YD. Selective blood brain barrier dysfunction after intravenous injections of rTNFα in the rat. In:Teelken A, Korf J, eds. Neurochemistry and Neuroimmunology of EAE: Implications for Therapy of MS. New York, NY: Plenum Press; 1997; Section 5:141–146.
- Foreman RD. Integration of viscerosomatic sensory input at the spinal level. Prog Brain Res 2000; 122:209–221.
- Ding X, Ardell JL, Hua F, et al. Modulation of cardiac ischemiasensitive afferent neuron signaling by preemptive C2 spinal cord stimulation: effect on substance P release from rat spinal cord. Am J Physiol Regul Integr Comp Physiol 2008; 294:R93–R101.
- Qin C, Faber JP, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9:71–78.
- Foreman RD, DeJongste MJ, Linderoth B. Integrative control of cardiac function by cervical and thoracic spinal neurons. In:Armour JA, Ardell JL, eds. Basic and Clinical Neurocardiology. New York, NY: Oxford University Press; 2004:153–186.
- Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci 2008; 138:9–23.
- Mannheimer C, Camici P, Chester MR, et al. The problem of chronic refractory angina: report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J 2002; 23:355–370.
- Fraker TD, Fihn SD. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol 2007; 50:2264–2274.
- Wittkampf FHM, DeJongste MJL, Meijler FL. Atrioventricular nodal response to retrograde activation in atrial fibrillation. J Cardiovasc Electrophysiol 1990; 1:437–447.
- Wittkampf FHM, DeJongste MJL, Meijler FL. Competitive anterograde and retrograde atrioventricular junctional activation in atrial fibrillation. J Cardiovasc Electrophysiol 1990; 1:448–456.
- Heberden W. Some account of a disorder of the breast. Med Trans 1772; 2:59–67.
- Ter Horst GJ, Hautvast RW, DeJongste MJ, Korf J. Neuroanatomy of cardiac activity-regulating circuitry: a transneuronal retrograde viral labelling study in the rat. Eur J Neurosci 1996; 8:2029–2041.
- Ter Horst GJ, VanderWerf YD, DeJongste MJL Acute myocardial infarction and cytokine-mediated selective blood-brain barrier leakage in the rat. J Neurochem 1996; 66( suppl 2):S54A. Abstract.
- Hautvast RW, Ter Horst GJ, DeJong BM, et al. Relative changes in regional cerebral blood flow during spinal cord stimulation in patients with refractory angina pectoris. Eur J Neurosci 1997; 9:1178–1183.
- DeJongste MJL, Ter Horst GJ. Mediators of inflammation in patients with coronary artery disease. In:Ter Horst GJ, ed. The Nervous System and the Heart. Totowa, NJ: Humana Press; 2000:467–487.
- Van Der Werf YD, TerHorst GJ, DeJongste MJL. Receptor mRNA densities and psychometric measures of determinants of anginal pain. Eur J Neurosci 1996; 8:S77. Abstract 32.35.
- Albutaihi IA, DeJongste MJ, Ter Horst GJ. An integrated study of heart pain and behavior in freely moving rats (using fos as a marker for neuronal activation). Neurosignals 2004; 13:207–226.
- DeJongste MJL, Hautvast RWM, Ruiters MHJ, Ter Horst GJ. Spinal cord stimulation and the induction of c-fos and heat shock protein 72 in the central nervous system of rats. Neuromodulation 1998; 1:73–84.
- Albutaihi IA, Hautvast RW, DeJongste MJ, Ter Horst GJ, Staal MJ. Cardiac nociception in rats: neuronal pathways and the influence of dermal neurostimulation on conveyance to the central nervous system. J Mol Neurosci 2003; 20:43–52.
- Ter Horst GJ, Nagel JG, DeJongste MJL, Van Der Werf YD. Selective blood brain barrier dysfunction after intravenous injections of rTNFα in the rat. In:Teelken A, Korf J, eds. Neurochemistry and Neuroimmunology of EAE: Implications for Therapy of MS. New York, NY: Plenum Press; 1997; Section 5:141–146.
- Foreman RD. Integration of viscerosomatic sensory input at the spinal level. Prog Brain Res 2000; 122:209–221.
- Ding X, Ardell JL, Hua F, et al. Modulation of cardiac ischemiasensitive afferent neuron signaling by preemptive C2 spinal cord stimulation: effect on substance P release from rat spinal cord. Am J Physiol Regul Integr Comp Physiol 2008; 294:R93–R101.
- Qin C, Faber JP, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9:71–78.
- Foreman RD, DeJongste MJ, Linderoth B. Integrative control of cardiac function by cervical and thoracic spinal neurons. In:Armour JA, Ardell JL, eds. Basic and Clinical Neurocardiology. New York, NY: Oxford University Press; 2004:153–186.
- Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci 2008; 138:9–23.
Neuromodulation of cardiac pain and cerebral vasculature: Neural mechanisms
The cardinal symptoms of angina pectoris—chest pain and pain that may radiate to either arm or the neck and jaw—are well recognized. The visceral characteristics of anginal pain are also familiar; for example, referral to somatic structures, pain that is diffuse and poorly localized, skin and deep tissue tenderness, enhanced autonomic reflexes such as sweating and vasomotor symptoms, and muscular rigidity.
The neurologic mechanisms that explain the manifestations of angina pectoris are less well clarified, and are targets of active research. Our research into the neuromodulation of cardiovascular function over the last 2 decades has produced results that may have clinical implications and others that have raised new questions. This article summarizes some of our key findings from studies of neural mechanisms of angina pectoris, central sensitization of cardiac nociceptive stimuli, and the neuromodulation of cardiac pain, with a focus on processing in the spinal cord.
NEURAL MECHANISMS OF ANGINA PECTORIS
Cells of the spinothalamic tract form a sensory pathway that transmits afferent information to the thalamus.1 One of our research objectives was to examine how these cells process information when the heart is exposed to noxious stimuli.
Thoracic spinal processing
The animal model for our early studies was an anesthetized primate. The afferent nerves were activated in one of two ways: either the coronary artery was occluded or bradykinin and algesic chemicals were injected into the pericardial sac or left atrial appendage. Recorded activity was then made from the spinothalamic tract cells in the T1–T5 and C5–C6 segments.1 We found convergence of visceral and somatic input, generally to the chest and upper arm. The finding was consistent with the observation that pain from angina commonly occurs in proximal somatic fields. No visceral input was evident in cells in C7–C8, where the somatic effects are primarily distal—to the hand, for example.
Upper cervical processing
It is known that some patients experience angina pectoris as neck and jaw pain. The dental literature has shown that what is initially considered to be a toothache occasionally turns out to be angina and coronary artery disease.2 Clinical literature from the late 1940s observed that despite the use of sympathectomy to relieve angina pectoris, neck and jaw pain continued or developed.3,4 This pain was attributed to transmission of nociceptive information in vagal afferent fibers, commonly thought to transmit innocuous cardiac sensory information.
When we recorded activity from spinothalamic tract cells in the C1–C2 region to observe the effect of cardiac nociceptive stimulation, we demonstrated a major role for the vagus nerve.1 Injection of saline into the heart had no effect in the C1–C2 region, but injection of algesic chemicals into the pericardial sac caused significant activity that disappeared after transection of the vagus nerve. This finding suggested that vagal afferent fibers ascend into the nucleus tractus solitarius of the medulla and either directly or indirectly modulate the C1–C2 neurons, which also receive converging somatic information from the neck and jaw region.5
CENTRAL SENSITIZATION OF CARDIAC NOCICEPTIVE STIMULI
Clinical studies suggest that anxiety and depression are prevalent in patients suffering from chest pain with and without underlying cardiac disease.6 Anxiety and/or stress increases circulating levels of corticosteroids, which can act on the glucocorticoid receptors in the amygdala, particularly in the central area.7 The amygdala plays a pivotal role in transforming chronic stressful stimuli into behavioral, visceral, and autonomic responses.8
Previous studies have shown that corticosteroids upregulate expressions of corticotropin-releasing factor in the central nucleus of the amygdala and increase indices of anxiety.7,9 They are also associated with hypersensitivity in visceromotor responses to colorectal distention10 and sensitize lumbosacral spinal neurons to colorectal and urinary bladder distention.11,12 We therefore hypothesized that glucocorticoids manipulate amygdala function, inducing hypersensitivity to nociceptive input from the heart through the modulation of upper thoracic spinal neuronal activity.
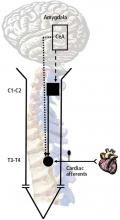
To examine the impact of stress on the nervous system when the heart is exposed to noxious stimuli, we assessed the effect of chronic activation of the amygdala on the T3–T4 spinal neurons and on C1–C2 propriospinal neurons. Fisher 344 rats were selected for this study because of their relatively low level of anxiety-related behavior.9 Micropellets of crystalline corticosterone or cholesterol (30 μg, used as a control) were implanted in the central nucleus of the amygdala. After 7 days, the corticosterone-implanted, but not the cholesterol-implanted, animals displayed high-anxiety behavior, as determined with an elevated plus maze.7
The responses of T3–T4 spinal neurons to intrapericardial injections of the algesic chemical bradykinin were compared in the corticosterone- and cholesterol-implanted rats. Compared with cholesterol-implanted animals, the duration of activity in response to the noxious cardiac stimulus was significantly longer in the corticosterone-implanted rats; in addition, activity shifted from the short-lasting (the response lasts only as long as the stimulus is applied) to long-lasting excitatory (the response lasts well beyond the period the stimulus is applied) neurons. Long-lasting excitatory neuronal activity is associated with intense pain and hypersensitivity, while short-lasting neurons are associated with a more acute response. The number of neurons with large field sizes in the corticosterone-implanted animals also increased, which is another indication of sensitization.
NEUROMODULATION OF CEREBROVASCULATURE AND CARDIAC PAIN
Neuromodulation of cerebral blood flow
Spinal cord stimulation is used to treat several cerebrovascular disorders, including cerebral ischemia, focal cerebral ischemia, stroke, postapoplectic spastic hemiplegia, and prolonged coma (see Yang et al13 for citations that address these pathologies). There is no clear explanation for its therapeutic effect; mechanisms being investigated include changes in cerebral blood flow and processing of nociceptive information.
To assess the effect of spinal cord stimulation on cerebral blood flow, we exposed the C1–C2 area of an anesthetized rat, stimulated the area with a ball electrode, and used laser Doppler flow probes to measure the blood flow on the surface of the cortex bilaterally. 13 The stimulus parameters were 30%, 60%, and 90% of motor threshold; the threshold was determined by gradually increasing the intensity of spinal cord stimulation until the neck muscles contracted. Blood flow increased on both sides with increasing stimulation intensities.13
Other studies have evaluated cerebral blood flow but did not measure change in cerebrovascular resistance. We observed that spinal cord stimulation—particularly at 60% and 90% of motor threshold—increased blood flow and reduced resistance to spinal cord stimulation on the dorsal columns at C1, both ipsilaterally and contralaterally.
In other tests, cerebral blood flow and vascular resistance to spinal cord stimulation were not changed after transection of the spinal cord at the C6–C7 segments. These results suggested that information was not being transmitted to the sympathetic nervous system via the thoracic spinal cord. We applied ibotenic acid to C1–C2 to assess whether the underlying stimulated neurons affected cerebral blood flow; there was no significant change. On the other hand, a small cut in the dorsal column rostral to the stimulation site caused significantly reduced cerebral blood flow and vascular resistance, indicating that the dorsal columns function in an ascending manner to produce the vasodilation in the cerebral cortex.13
Capsaicin-sensitive sensory nerves, which contain transient receptor potential vanilloid-1 (TRPV1) receptors, may have a role in spinal cord stimulation–induced vasodilation. TRPV1 receptors are nonselective cation channels activated by capsaicin, heat, and hydrogen ions.14 Activation, which causes an influx of cations and release of calcitonin gene-related peptide (CGRP) and substance P, is related to the pathogenesis of inflammation and hypertension. To examine the potential role played by capsaicin-sensitive sensory nerves, we administered resiniferatoxin (RTX), an ultrapotent capsaicin agonist; RTX specifically targets and desensitizes TRPV1-containing sensory fibers.13,15 Administration either intravenously or by direct application to the spinal cord results in a 15- to 20-minute period of sensitization followed by several hours of desensitization; if exposure lasts for several days, the nerves are destroyed.
Intrathecal administration of RTX to the spinal cord resulted in no significant change in cerebral blood flow. However, intravenous administration resulted in significantly decreased cerebral blood flow and decreased resistance, suggesting a role for TRPV1 receptors in cerebral blood flow.13
There may be a connection between spinal cord stimulation at C1 and vasodilation of the cortex. The literature suggests that spinal cord stimulation activates the dorsal column nuclei16; we found evidence of this in our laboratory when we recorded activity from cells in the cuneate and gracilus nuclei after spinal cord stimulation. There is also a possible pathway between the dorsal column, the rostral ventrolateral medulla, and the sphenopalatine ganglion that influences vasodilation.17–20 Although not yet clearly defined, evidence suggests a connection between spinal cord stimulation and transmission of this information through the dorsal columns to influence vasodilation.17–20
Neuromodulation of thoracic spinal processing of cardiac nociceptive information
Using a rat model to assess the effects of spinal stimulation, we recorded T3 activity during dorsal column stimulation of either C8-T1 or C1–C2 segments. Activity was almost completely suppressed with C1–C2 stimulation during bradykinin injection into the pericardial sac. The results suggest that spinal cord stimulation suppresses the processing of nociceptive information.24
Stimulating the spinal cord at C8-T1 also suppresses the effect of bradykinin. One possible mechanism for this effect is that spinal cord stimulation activates large afferent fibers; GABAergic connections in the superficial dorsal horn may suppress the processing of information in the spinothalamic tract neurons.22,25
SUMMARY
Our investigations have generated information about afferent input to the spinothalamic tract cells, the effects of glucocorticoids on amygdala function, and possible therapeutic mechanisms of spinal cord stimulation.
We have demonstrated convergence of viscerosomatic input in spinothalamic cells. There is virtually no viscerocardiac input at the C7–C8 region, but there is input at C5–C6. Vagal afferent activity is the major source of input at the C1–C2 region; in this region. Vagal nerve stimulation may have a major role in processing in the upper cervical spinal cord and may change the balance of processing in the supraspinal nuclei.
Glucocorticoids manipulate amygdala function by inducing hypersensitivity to nociceptive input from the heart through central sensitization of upper thoracic spinal neuronal activity. Descending information from the amygdala depends, in part, on the C1–C2 propriospinal pathway.
Spinal cord stimulation at C1–C2 or C8-T1 can activate inner neuronal mechanisms that may involve GABA, modulating the wide dynamic range of neurons that are part of the spinothalamic tract.
- Foreman RD. Mechanisms of cardiac pain. Annu Rev Physiol 1999; 61:143–167.
- Myers DE. Vagus nerve pain referred to the craniofacial region. A case report and literature review with implications for referred cardiac pain. Br Dent J 2008; 204:187–189.
- Lindgren I, Olivecrona H. Surgical treatment of angina pectoris. J Neurosurg 1947; 4:19–39.
- White JC, Bland EF. The surgical relief of severe angina pectoris: methods employed and end results in 83 patients. Medicine 1948; 27:1–42.
- McNeill DL, Chandler MJ, Fu QG, Foreman RD. Projection of nodose ganglion cells to the upper cervical spinal cord in the rat. Brain Res Bull 1991; 27:151–155.
- Sheps DS, Creed F, Clouse RE. Chest pain in patients with cardiac and noncardiac disease. Psychosom Med 2004; 66:861–867.
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 2000; 861:288–295.
- Rozen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev 1998; 105:325–350.
- Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav 2000; 69:379–382.
- Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 2001; 893:135–142.
- Qin C, Greenwood-Van Meerveld B, Myers DA, Foreman RD. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol 2003; 89:1343–1352.
- Qin C, Greenwood-Van Meerveld B, Foreman RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J Neurophysiol 2003; 90:2180–2189.
- Yang X, Farber JP, Wu M, Foreman RD, Qin C. Roles of dorsal column pathway and transient receptor potential vanilloid type 1 in augmentation of cerebral blood flow by upper cervical spinal cord stimulation in rats. Neuroscience 2008; 152:950–958.
- Steenland HW, Ko SW, Wu LJ, Zhuo M. Hot receptors in the brain. Mol Pain 2006; 2:34.
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Roles of peripheral terminals of transient receptor potential vanil-loid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res 2007; 1156:80–92.
- Sagher O, Huang DL. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg 2000; 93( 1 suppl):71–76.
- Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab 1988; 8:875–878.
- Suzuki N, Hardebo JE, Kåhrström J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab 1990; 10:383–391.
- Kamiya H, Itoh K, Yasui Y, Ino T, Mizuno N. Somatosensory and auditory relay nucleus in the rostral part of the ventrolateral medulla: a morphological study in the cat. J Comp Neurol 1988; 273:421–435.
- Patel S, Huang DL, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery 2004; 55:201–206.
- Meyerson BA, Linderoth B. Spinal cord stimulation: mechanisms of action in neuropathic and ischemic pain. In:Simpson BA, ed. Electrical Stimulation and the Relief of Pain. Vol. 15. New York, NY: Elsevier Publishers; 2003:161–182.
- Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med 2006; 7:S14–S26.
- González-Darder JM, Canela P, González-Martinez V. High cervical spinal cord stimulation for unstable angina pectoris. Stereotact Funct Neurosurg 1991; 56:20–27.
- Qin C, Farber JP, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9:71–78.
- Linderoth B, Meyerson B. Spinal cord stimulation: mechanisms of action. In:Burchiel K. Surgical Management of Pain. New York, NY: Thieme Medical Publishers Inc; 2002:505–526.
The cardinal symptoms of angina pectoris—chest pain and pain that may radiate to either arm or the neck and jaw—are well recognized. The visceral characteristics of anginal pain are also familiar; for example, referral to somatic structures, pain that is diffuse and poorly localized, skin and deep tissue tenderness, enhanced autonomic reflexes such as sweating and vasomotor symptoms, and muscular rigidity.
The neurologic mechanisms that explain the manifestations of angina pectoris are less well clarified, and are targets of active research. Our research into the neuromodulation of cardiovascular function over the last 2 decades has produced results that may have clinical implications and others that have raised new questions. This article summarizes some of our key findings from studies of neural mechanisms of angina pectoris, central sensitization of cardiac nociceptive stimuli, and the neuromodulation of cardiac pain, with a focus on processing in the spinal cord.
NEURAL MECHANISMS OF ANGINA PECTORIS
Cells of the spinothalamic tract form a sensory pathway that transmits afferent information to the thalamus.1 One of our research objectives was to examine how these cells process information when the heart is exposed to noxious stimuli.
Thoracic spinal processing
The animal model for our early studies was an anesthetized primate. The afferent nerves were activated in one of two ways: either the coronary artery was occluded or bradykinin and algesic chemicals were injected into the pericardial sac or left atrial appendage. Recorded activity was then made from the spinothalamic tract cells in the T1–T5 and C5–C6 segments.1 We found convergence of visceral and somatic input, generally to the chest and upper arm. The finding was consistent with the observation that pain from angina commonly occurs in proximal somatic fields. No visceral input was evident in cells in C7–C8, where the somatic effects are primarily distal—to the hand, for example.
Upper cervical processing
It is known that some patients experience angina pectoris as neck and jaw pain. The dental literature has shown that what is initially considered to be a toothache occasionally turns out to be angina and coronary artery disease.2 Clinical literature from the late 1940s observed that despite the use of sympathectomy to relieve angina pectoris, neck and jaw pain continued or developed.3,4 This pain was attributed to transmission of nociceptive information in vagal afferent fibers, commonly thought to transmit innocuous cardiac sensory information.
When we recorded activity from spinothalamic tract cells in the C1–C2 region to observe the effect of cardiac nociceptive stimulation, we demonstrated a major role for the vagus nerve.1 Injection of saline into the heart had no effect in the C1–C2 region, but injection of algesic chemicals into the pericardial sac caused significant activity that disappeared after transection of the vagus nerve. This finding suggested that vagal afferent fibers ascend into the nucleus tractus solitarius of the medulla and either directly or indirectly modulate the C1–C2 neurons, which also receive converging somatic information from the neck and jaw region.5
CENTRAL SENSITIZATION OF CARDIAC NOCICEPTIVE STIMULI
Clinical studies suggest that anxiety and depression are prevalent in patients suffering from chest pain with and without underlying cardiac disease.6 Anxiety and/or stress increases circulating levels of corticosteroids, which can act on the glucocorticoid receptors in the amygdala, particularly in the central area.7 The amygdala plays a pivotal role in transforming chronic stressful stimuli into behavioral, visceral, and autonomic responses.8
Previous studies have shown that corticosteroids upregulate expressions of corticotropin-releasing factor in the central nucleus of the amygdala and increase indices of anxiety.7,9 They are also associated with hypersensitivity in visceromotor responses to colorectal distention10 and sensitize lumbosacral spinal neurons to colorectal and urinary bladder distention.11,12 We therefore hypothesized that glucocorticoids manipulate amygdala function, inducing hypersensitivity to nociceptive input from the heart through the modulation of upper thoracic spinal neuronal activity.
To examine the impact of stress on the nervous system when the heart is exposed to noxious stimuli, we assessed the effect of chronic activation of the amygdala on the T3–T4 spinal neurons and on C1–C2 propriospinal neurons. Fisher 344 rats were selected for this study because of their relatively low level of anxiety-related behavior.9 Micropellets of crystalline corticosterone or cholesterol (30 μg, used as a control) were implanted in the central nucleus of the amygdala. After 7 days, the corticosterone-implanted, but not the cholesterol-implanted, animals displayed high-anxiety behavior, as determined with an elevated plus maze.7
The responses of T3–T4 spinal neurons to intrapericardial injections of the algesic chemical bradykinin were compared in the corticosterone- and cholesterol-implanted rats. Compared with cholesterol-implanted animals, the duration of activity in response to the noxious cardiac stimulus was significantly longer in the corticosterone-implanted rats; in addition, activity shifted from the short-lasting (the response lasts only as long as the stimulus is applied) to long-lasting excitatory (the response lasts well beyond the period the stimulus is applied) neurons. Long-lasting excitatory neuronal activity is associated with intense pain and hypersensitivity, while short-lasting neurons are associated with a more acute response. The number of neurons with large field sizes in the corticosterone-implanted animals also increased, which is another indication of sensitization.
NEUROMODULATION OF CEREBROVASCULATURE AND CARDIAC PAIN
Neuromodulation of cerebral blood flow
Spinal cord stimulation is used to treat several cerebrovascular disorders, including cerebral ischemia, focal cerebral ischemia, stroke, postapoplectic spastic hemiplegia, and prolonged coma (see Yang et al13 for citations that address these pathologies). There is no clear explanation for its therapeutic effect; mechanisms being investigated include changes in cerebral blood flow and processing of nociceptive information.
To assess the effect of spinal cord stimulation on cerebral blood flow, we exposed the C1–C2 area of an anesthetized rat, stimulated the area with a ball electrode, and used laser Doppler flow probes to measure the blood flow on the surface of the cortex bilaterally. 13 The stimulus parameters were 30%, 60%, and 90% of motor threshold; the threshold was determined by gradually increasing the intensity of spinal cord stimulation until the neck muscles contracted. Blood flow increased on both sides with increasing stimulation intensities.13
Other studies have evaluated cerebral blood flow but did not measure change in cerebrovascular resistance. We observed that spinal cord stimulation—particularly at 60% and 90% of motor threshold—increased blood flow and reduced resistance to spinal cord stimulation on the dorsal columns at C1, both ipsilaterally and contralaterally.
In other tests, cerebral blood flow and vascular resistance to spinal cord stimulation were not changed after transection of the spinal cord at the C6–C7 segments. These results suggested that information was not being transmitted to the sympathetic nervous system via the thoracic spinal cord. We applied ibotenic acid to C1–C2 to assess whether the underlying stimulated neurons affected cerebral blood flow; there was no significant change. On the other hand, a small cut in the dorsal column rostral to the stimulation site caused significantly reduced cerebral blood flow and vascular resistance, indicating that the dorsal columns function in an ascending manner to produce the vasodilation in the cerebral cortex.13
Capsaicin-sensitive sensory nerves, which contain transient receptor potential vanilloid-1 (TRPV1) receptors, may have a role in spinal cord stimulation–induced vasodilation. TRPV1 receptors are nonselective cation channels activated by capsaicin, heat, and hydrogen ions.14 Activation, which causes an influx of cations and release of calcitonin gene-related peptide (CGRP) and substance P, is related to the pathogenesis of inflammation and hypertension. To examine the potential role played by capsaicin-sensitive sensory nerves, we administered resiniferatoxin (RTX), an ultrapotent capsaicin agonist; RTX specifically targets and desensitizes TRPV1-containing sensory fibers.13,15 Administration either intravenously or by direct application to the spinal cord results in a 15- to 20-minute period of sensitization followed by several hours of desensitization; if exposure lasts for several days, the nerves are destroyed.
Intrathecal administration of RTX to the spinal cord resulted in no significant change in cerebral blood flow. However, intravenous administration resulted in significantly decreased cerebral blood flow and decreased resistance, suggesting a role for TRPV1 receptors in cerebral blood flow.13
There may be a connection between spinal cord stimulation at C1 and vasodilation of the cortex. The literature suggests that spinal cord stimulation activates the dorsal column nuclei16; we found evidence of this in our laboratory when we recorded activity from cells in the cuneate and gracilus nuclei after spinal cord stimulation. There is also a possible pathway between the dorsal column, the rostral ventrolateral medulla, and the sphenopalatine ganglion that influences vasodilation.17–20 Although not yet clearly defined, evidence suggests a connection between spinal cord stimulation and transmission of this information through the dorsal columns to influence vasodilation.17–20
Neuromodulation of thoracic spinal processing of cardiac nociceptive information
Using a rat model to assess the effects of spinal stimulation, we recorded T3 activity during dorsal column stimulation of either C8-T1 or C1–C2 segments. Activity was almost completely suppressed with C1–C2 stimulation during bradykinin injection into the pericardial sac. The results suggest that spinal cord stimulation suppresses the processing of nociceptive information.24
Stimulating the spinal cord at C8-T1 also suppresses the effect of bradykinin. One possible mechanism for this effect is that spinal cord stimulation activates large afferent fibers; GABAergic connections in the superficial dorsal horn may suppress the processing of information in the spinothalamic tract neurons.22,25
SUMMARY
Our investigations have generated information about afferent input to the spinothalamic tract cells, the effects of glucocorticoids on amygdala function, and possible therapeutic mechanisms of spinal cord stimulation.
We have demonstrated convergence of viscerosomatic input in spinothalamic cells. There is virtually no viscerocardiac input at the C7–C8 region, but there is input at C5–C6. Vagal afferent activity is the major source of input at the C1–C2 region; in this region. Vagal nerve stimulation may have a major role in processing in the upper cervical spinal cord and may change the balance of processing in the supraspinal nuclei.
Glucocorticoids manipulate amygdala function by inducing hypersensitivity to nociceptive input from the heart through central sensitization of upper thoracic spinal neuronal activity. Descending information from the amygdala depends, in part, on the C1–C2 propriospinal pathway.
Spinal cord stimulation at C1–C2 or C8-T1 can activate inner neuronal mechanisms that may involve GABA, modulating the wide dynamic range of neurons that are part of the spinothalamic tract.
The cardinal symptoms of angina pectoris—chest pain and pain that may radiate to either arm or the neck and jaw—are well recognized. The visceral characteristics of anginal pain are also familiar; for example, referral to somatic structures, pain that is diffuse and poorly localized, skin and deep tissue tenderness, enhanced autonomic reflexes such as sweating and vasomotor symptoms, and muscular rigidity.
The neurologic mechanisms that explain the manifestations of angina pectoris are less well clarified, and are targets of active research. Our research into the neuromodulation of cardiovascular function over the last 2 decades has produced results that may have clinical implications and others that have raised new questions. This article summarizes some of our key findings from studies of neural mechanisms of angina pectoris, central sensitization of cardiac nociceptive stimuli, and the neuromodulation of cardiac pain, with a focus on processing in the spinal cord.
NEURAL MECHANISMS OF ANGINA PECTORIS
Cells of the spinothalamic tract form a sensory pathway that transmits afferent information to the thalamus.1 One of our research objectives was to examine how these cells process information when the heart is exposed to noxious stimuli.
Thoracic spinal processing
The animal model for our early studies was an anesthetized primate. The afferent nerves were activated in one of two ways: either the coronary artery was occluded or bradykinin and algesic chemicals were injected into the pericardial sac or left atrial appendage. Recorded activity was then made from the spinothalamic tract cells in the T1–T5 and C5–C6 segments.1 We found convergence of visceral and somatic input, generally to the chest and upper arm. The finding was consistent with the observation that pain from angina commonly occurs in proximal somatic fields. No visceral input was evident in cells in C7–C8, where the somatic effects are primarily distal—to the hand, for example.
Upper cervical processing
It is known that some patients experience angina pectoris as neck and jaw pain. The dental literature has shown that what is initially considered to be a toothache occasionally turns out to be angina and coronary artery disease.2 Clinical literature from the late 1940s observed that despite the use of sympathectomy to relieve angina pectoris, neck and jaw pain continued or developed.3,4 This pain was attributed to transmission of nociceptive information in vagal afferent fibers, commonly thought to transmit innocuous cardiac sensory information.
When we recorded activity from spinothalamic tract cells in the C1–C2 region to observe the effect of cardiac nociceptive stimulation, we demonstrated a major role for the vagus nerve.1 Injection of saline into the heart had no effect in the C1–C2 region, but injection of algesic chemicals into the pericardial sac caused significant activity that disappeared after transection of the vagus nerve. This finding suggested that vagal afferent fibers ascend into the nucleus tractus solitarius of the medulla and either directly or indirectly modulate the C1–C2 neurons, which also receive converging somatic information from the neck and jaw region.5
CENTRAL SENSITIZATION OF CARDIAC NOCICEPTIVE STIMULI
Clinical studies suggest that anxiety and depression are prevalent in patients suffering from chest pain with and without underlying cardiac disease.6 Anxiety and/or stress increases circulating levels of corticosteroids, which can act on the glucocorticoid receptors in the amygdala, particularly in the central area.7 The amygdala plays a pivotal role in transforming chronic stressful stimuli into behavioral, visceral, and autonomic responses.8
Previous studies have shown that corticosteroids upregulate expressions of corticotropin-releasing factor in the central nucleus of the amygdala and increase indices of anxiety.7,9 They are also associated with hypersensitivity in visceromotor responses to colorectal distention10 and sensitize lumbosacral spinal neurons to colorectal and urinary bladder distention.11,12 We therefore hypothesized that glucocorticoids manipulate amygdala function, inducing hypersensitivity to nociceptive input from the heart through the modulation of upper thoracic spinal neuronal activity.
To examine the impact of stress on the nervous system when the heart is exposed to noxious stimuli, we assessed the effect of chronic activation of the amygdala on the T3–T4 spinal neurons and on C1–C2 propriospinal neurons. Fisher 344 rats were selected for this study because of their relatively low level of anxiety-related behavior.9 Micropellets of crystalline corticosterone or cholesterol (30 μg, used as a control) were implanted in the central nucleus of the amygdala. After 7 days, the corticosterone-implanted, but not the cholesterol-implanted, animals displayed high-anxiety behavior, as determined with an elevated plus maze.7
The responses of T3–T4 spinal neurons to intrapericardial injections of the algesic chemical bradykinin were compared in the corticosterone- and cholesterol-implanted rats. Compared with cholesterol-implanted animals, the duration of activity in response to the noxious cardiac stimulus was significantly longer in the corticosterone-implanted rats; in addition, activity shifted from the short-lasting (the response lasts only as long as the stimulus is applied) to long-lasting excitatory (the response lasts well beyond the period the stimulus is applied) neurons. Long-lasting excitatory neuronal activity is associated with intense pain and hypersensitivity, while short-lasting neurons are associated with a more acute response. The number of neurons with large field sizes in the corticosterone-implanted animals also increased, which is another indication of sensitization.
NEUROMODULATION OF CEREBROVASCULATURE AND CARDIAC PAIN
Neuromodulation of cerebral blood flow
Spinal cord stimulation is used to treat several cerebrovascular disorders, including cerebral ischemia, focal cerebral ischemia, stroke, postapoplectic spastic hemiplegia, and prolonged coma (see Yang et al13 for citations that address these pathologies). There is no clear explanation for its therapeutic effect; mechanisms being investigated include changes in cerebral blood flow and processing of nociceptive information.
To assess the effect of spinal cord stimulation on cerebral blood flow, we exposed the C1–C2 area of an anesthetized rat, stimulated the area with a ball electrode, and used laser Doppler flow probes to measure the blood flow on the surface of the cortex bilaterally. 13 The stimulus parameters were 30%, 60%, and 90% of motor threshold; the threshold was determined by gradually increasing the intensity of spinal cord stimulation until the neck muscles contracted. Blood flow increased on both sides with increasing stimulation intensities.13
Other studies have evaluated cerebral blood flow but did not measure change in cerebrovascular resistance. We observed that spinal cord stimulation—particularly at 60% and 90% of motor threshold—increased blood flow and reduced resistance to spinal cord stimulation on the dorsal columns at C1, both ipsilaterally and contralaterally.
In other tests, cerebral blood flow and vascular resistance to spinal cord stimulation were not changed after transection of the spinal cord at the C6–C7 segments. These results suggested that information was not being transmitted to the sympathetic nervous system via the thoracic spinal cord. We applied ibotenic acid to C1–C2 to assess whether the underlying stimulated neurons affected cerebral blood flow; there was no significant change. On the other hand, a small cut in the dorsal column rostral to the stimulation site caused significantly reduced cerebral blood flow and vascular resistance, indicating that the dorsal columns function in an ascending manner to produce the vasodilation in the cerebral cortex.13
Capsaicin-sensitive sensory nerves, which contain transient receptor potential vanilloid-1 (TRPV1) receptors, may have a role in spinal cord stimulation–induced vasodilation. TRPV1 receptors are nonselective cation channels activated by capsaicin, heat, and hydrogen ions.14 Activation, which causes an influx of cations and release of calcitonin gene-related peptide (CGRP) and substance P, is related to the pathogenesis of inflammation and hypertension. To examine the potential role played by capsaicin-sensitive sensory nerves, we administered resiniferatoxin (RTX), an ultrapotent capsaicin agonist; RTX specifically targets and desensitizes TRPV1-containing sensory fibers.13,15 Administration either intravenously or by direct application to the spinal cord results in a 15- to 20-minute period of sensitization followed by several hours of desensitization; if exposure lasts for several days, the nerves are destroyed.
Intrathecal administration of RTX to the spinal cord resulted in no significant change in cerebral blood flow. However, intravenous administration resulted in significantly decreased cerebral blood flow and decreased resistance, suggesting a role for TRPV1 receptors in cerebral blood flow.13
There may be a connection between spinal cord stimulation at C1 and vasodilation of the cortex. The literature suggests that spinal cord stimulation activates the dorsal column nuclei16; we found evidence of this in our laboratory when we recorded activity from cells in the cuneate and gracilus nuclei after spinal cord stimulation. There is also a possible pathway between the dorsal column, the rostral ventrolateral medulla, and the sphenopalatine ganglion that influences vasodilation.17–20 Although not yet clearly defined, evidence suggests a connection between spinal cord stimulation and transmission of this information through the dorsal columns to influence vasodilation.17–20
Neuromodulation of thoracic spinal processing of cardiac nociceptive information
Using a rat model to assess the effects of spinal stimulation, we recorded T3 activity during dorsal column stimulation of either C8-T1 or C1–C2 segments. Activity was almost completely suppressed with C1–C2 stimulation during bradykinin injection into the pericardial sac. The results suggest that spinal cord stimulation suppresses the processing of nociceptive information.24
Stimulating the spinal cord at C8-T1 also suppresses the effect of bradykinin. One possible mechanism for this effect is that spinal cord stimulation activates large afferent fibers; GABAergic connections in the superficial dorsal horn may suppress the processing of information in the spinothalamic tract neurons.22,25
SUMMARY
Our investigations have generated information about afferent input to the spinothalamic tract cells, the effects of glucocorticoids on amygdala function, and possible therapeutic mechanisms of spinal cord stimulation.
We have demonstrated convergence of viscerosomatic input in spinothalamic cells. There is virtually no viscerocardiac input at the C7–C8 region, but there is input at C5–C6. Vagal afferent activity is the major source of input at the C1–C2 region; in this region. Vagal nerve stimulation may have a major role in processing in the upper cervical spinal cord and may change the balance of processing in the supraspinal nuclei.
Glucocorticoids manipulate amygdala function by inducing hypersensitivity to nociceptive input from the heart through central sensitization of upper thoracic spinal neuronal activity. Descending information from the amygdala depends, in part, on the C1–C2 propriospinal pathway.
Spinal cord stimulation at C1–C2 or C8-T1 can activate inner neuronal mechanisms that may involve GABA, modulating the wide dynamic range of neurons that are part of the spinothalamic tract.
- Foreman RD. Mechanisms of cardiac pain. Annu Rev Physiol 1999; 61:143–167.
- Myers DE. Vagus nerve pain referred to the craniofacial region. A case report and literature review with implications for referred cardiac pain. Br Dent J 2008; 204:187–189.
- Lindgren I, Olivecrona H. Surgical treatment of angina pectoris. J Neurosurg 1947; 4:19–39.
- White JC, Bland EF. The surgical relief of severe angina pectoris: methods employed and end results in 83 patients. Medicine 1948; 27:1–42.
- McNeill DL, Chandler MJ, Fu QG, Foreman RD. Projection of nodose ganglion cells to the upper cervical spinal cord in the rat. Brain Res Bull 1991; 27:151–155.
- Sheps DS, Creed F, Clouse RE. Chest pain in patients with cardiac and noncardiac disease. Psychosom Med 2004; 66:861–867.
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 2000; 861:288–295.
- Rozen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev 1998; 105:325–350.
- Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav 2000; 69:379–382.
- Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 2001; 893:135–142.
- Qin C, Greenwood-Van Meerveld B, Myers DA, Foreman RD. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol 2003; 89:1343–1352.
- Qin C, Greenwood-Van Meerveld B, Foreman RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J Neurophysiol 2003; 90:2180–2189.
- Yang X, Farber JP, Wu M, Foreman RD, Qin C. Roles of dorsal column pathway and transient receptor potential vanilloid type 1 in augmentation of cerebral blood flow by upper cervical spinal cord stimulation in rats. Neuroscience 2008; 152:950–958.
- Steenland HW, Ko SW, Wu LJ, Zhuo M. Hot receptors in the brain. Mol Pain 2006; 2:34.
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Roles of peripheral terminals of transient receptor potential vanil-loid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res 2007; 1156:80–92.
- Sagher O, Huang DL. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg 2000; 93( 1 suppl):71–76.
- Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab 1988; 8:875–878.
- Suzuki N, Hardebo JE, Kåhrström J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab 1990; 10:383–391.
- Kamiya H, Itoh K, Yasui Y, Ino T, Mizuno N. Somatosensory and auditory relay nucleus in the rostral part of the ventrolateral medulla: a morphological study in the cat. J Comp Neurol 1988; 273:421–435.
- Patel S, Huang DL, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery 2004; 55:201–206.
- Meyerson BA, Linderoth B. Spinal cord stimulation: mechanisms of action in neuropathic and ischemic pain. In:Simpson BA, ed. Electrical Stimulation and the Relief of Pain. Vol. 15. New York, NY: Elsevier Publishers; 2003:161–182.
- Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med 2006; 7:S14–S26.
- González-Darder JM, Canela P, González-Martinez V. High cervical spinal cord stimulation for unstable angina pectoris. Stereotact Funct Neurosurg 1991; 56:20–27.
- Qin C, Farber JP, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9:71–78.
- Linderoth B, Meyerson B. Spinal cord stimulation: mechanisms of action. In:Burchiel K. Surgical Management of Pain. New York, NY: Thieme Medical Publishers Inc; 2002:505–526.
- Foreman RD. Mechanisms of cardiac pain. Annu Rev Physiol 1999; 61:143–167.
- Myers DE. Vagus nerve pain referred to the craniofacial region. A case report and literature review with implications for referred cardiac pain. Br Dent J 2008; 204:187–189.
- Lindgren I, Olivecrona H. Surgical treatment of angina pectoris. J Neurosurg 1947; 4:19–39.
- White JC, Bland EF. The surgical relief of severe angina pectoris: methods employed and end results in 83 patients. Medicine 1948; 27:1–42.
- McNeill DL, Chandler MJ, Fu QG, Foreman RD. Projection of nodose ganglion cells to the upper cervical spinal cord in the rat. Brain Res Bull 1991; 27:151–155.
- Sheps DS, Creed F, Clouse RE. Chest pain in patients with cardiac and noncardiac disease. Psychosom Med 2004; 66:861–867.
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 2000; 861:288–295.
- Rozen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev 1998; 105:325–350.
- Gunter WD, Shepard JD, Foreman RD, Myers DA, Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol Behav 2000; 69:379–382.
- Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 2001; 893:135–142.
- Qin C, Greenwood-Van Meerveld B, Myers DA, Foreman RD. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol 2003; 89:1343–1352.
- Qin C, Greenwood-Van Meerveld B, Foreman RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J Neurophysiol 2003; 90:2180–2189.
- Yang X, Farber JP, Wu M, Foreman RD, Qin C. Roles of dorsal column pathway and transient receptor potential vanilloid type 1 in augmentation of cerebral blood flow by upper cervical spinal cord stimulation in rats. Neuroscience 2008; 152:950–958.
- Steenland HW, Ko SW, Wu LJ, Zhuo M. Hot receptors in the brain. Mol Pain 2006; 2:34.
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Roles of peripheral terminals of transient receptor potential vanil-loid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res 2007; 1156:80–92.
- Sagher O, Huang DL. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg 2000; 93( 1 suppl):71–76.
- Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab 1988; 8:875–878.
- Suzuki N, Hardebo JE, Kåhrström J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab 1990; 10:383–391.
- Kamiya H, Itoh K, Yasui Y, Ino T, Mizuno N. Somatosensory and auditory relay nucleus in the rostral part of the ventrolateral medulla: a morphological study in the cat. J Comp Neurol 1988; 273:421–435.
- Patel S, Huang DL, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery 2004; 55:201–206.
- Meyerson BA, Linderoth B. Spinal cord stimulation: mechanisms of action in neuropathic and ischemic pain. In:Simpson BA, ed. Electrical Stimulation and the Relief of Pain. Vol. 15. New York, NY: Elsevier Publishers; 2003:161–182.
- Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med 2006; 7:S14–S26.
- González-Darder JM, Canela P, González-Martinez V. High cervical spinal cord stimulation for unstable angina pectoris. Stereotact Funct Neurosurg 1991; 56:20–27.
- Qin C, Farber JP, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9:71–78.
- Linderoth B, Meyerson B. Spinal cord stimulation: mechanisms of action. In:Burchiel K. Surgical Management of Pain. New York, NY: Thieme Medical Publishers Inc; 2002:505–526.