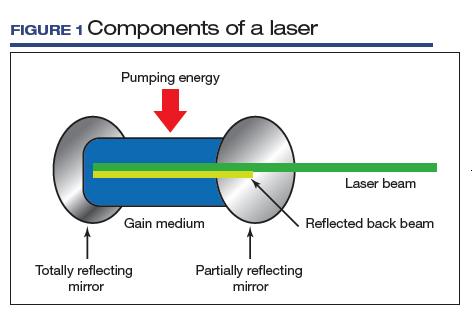
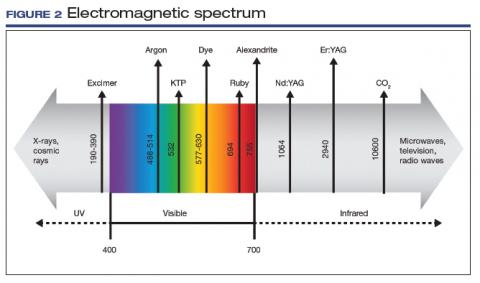
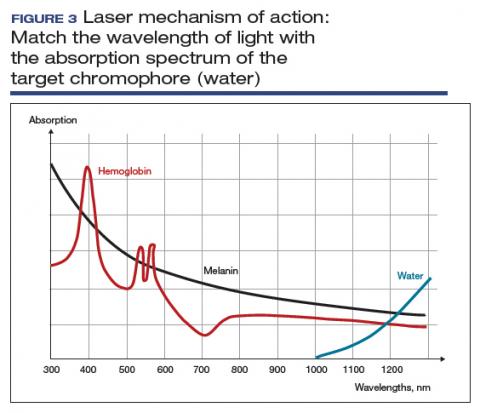
User login
Are single-incision mini-slings the new gold standard for stress urinary incontinence?
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
Best practices for evaluating pelvic pain in patients with Essure tubal occlusion devices
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
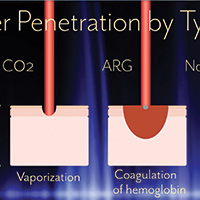
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
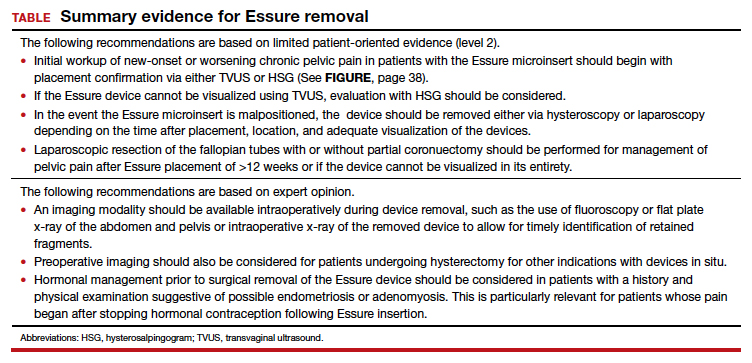
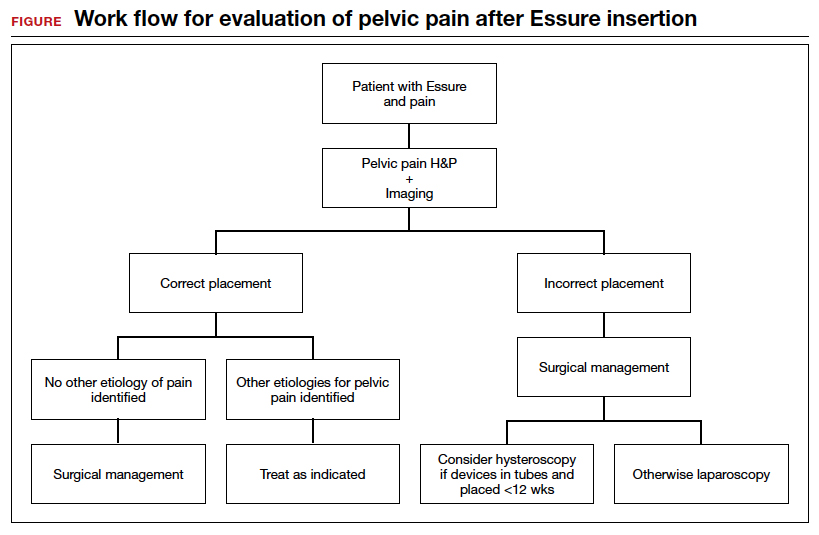
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.
Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.
Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
Is vaginal laser therapy more efficacious in improving vaginal menopausal symptoms compared with sham therapy?
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
Energy-based therapies in female genital cosmetic surgery: Hype, hope, and a way forward
Energy-based therapy use in gynecology dates back to the early 1970s, when ablative carbon dioxide (C02) lasers were employed to treat cervical erosions.1 Soon after, reports were published on laser treatment for diethylstilbestrol-associated vaginal adenosis, laser laparoscopy for adhesiolysis, laser hysteroscopy, and laser genital wart ablation.2 Starting around 2011, the first articles were published on the use of fractional C02 laser treatment for vulvovaginal atrophy.3,4 Use of laser and light-based therapies to treat “vaginal rejuvenation” is now increasing at an annual rate of 26%. In a few years, North America is expected to be the largest market for vaginal laser rejuvenation. In 2016, more than 500,000 feminine rejuvenation procedures were performed in the United States, and it is estimated that more than 27,000 energy-based devices will be in operation by 2021.5
Clearly, there is considerable public interest and intrigue in office-based female genital cosmetic procedures. In 2018, the US Food and Drug Administration contacted 7 manufacturers of energy-based devices to request revision and clarification for marketing of these devices, since these technologies are neither cleared nor approved for cosmetic vulvovaginal conditions.6 The companies responded within 30 days.
In this article, we appraise the existing literature regarding the mechanism of action of energy-based therapies used in gynecology and review outcomes of their use in female genital cosmetic surgery.
Laser technology devices and how they work
LASER is an acronym for Light Amplification by Stimulated Emission of Radiation. Laser devices are composed of 1) an excitable medium (gas, liquid, solid) needed to emit light, 2) an energy source to excite the medium, 3) mirrors to bounce the light back and forth, and 4) a delivery and cooling system (FIGURE 1).
The electromagnetic spectrum is the range of all the wavelengths of light, including visible light, radio waves, infrared light, ultraviolet light, x-rays, and gamma rays (FIGURE 2). Most lasers used for the treatment of vulvovaginal disorders, typically C02 and erbium:yttrium aluminum garnet (Er:YAG) lasers, involve the infrared wavelengths.
The basic principle of laser treatment is to match the wavelength of the laser with the absorption spectrum of the desired target—a chromophore such as hemoglobin, melanin, or water (FIGURE 3). In essence, light is absorbed by the chromophore (which in vulvar and vaginal tissues is mostly water) and transformed into heat, leading to target destruction. In a fractionated (or fractional) laser beam, the laser is broken up into many smaller beams that treat only portions of the treatment area, with areas of intact epithelium in between the treated areas. At appropriately low thermal denaturation temperatures (45° to 50°C), tissue regeneration can occur through activation of heat shock proteins and tissue growth factors, creating neocollagenesis and neovascularization.
The concept of ablative resurfacing versus fractional resurfacing is borrowed from dermatology (FIGURE 4), understanding that tissue ablation and thermal denaturation occur at temperatures greater than 100°C, as occurs with carbonization of vulvar condylomata.
Continue to: In dermatology, fractionated lasers...
In dermatology, fractionated lasers have been used in the treatment of hair removal, vascular and pigmented lesions, scars, wound healing, tattoo removal, warts, and actinic keratoses. For these conditions, the targeted chromophores are water, hemoglobin, melanosomes, and tattoo ink. The laser pulses must be shorter than the target tissue thermal relaxation times in order to avoid excess heating and collateral tissue damage. Choosing appropriate settings is critical to achieve selective heating, or destruction, of the target tissue. These settings include appropriate wavelengths, pulse durations, and fluence, which is energy delivered per unit area (typically, joules per square centimeter).
For gynecologic conditions, the lasers used are most often CO2, Er:YAG, and hybrid (which include ablative and nonablative wavelengths) devices. In the epithelium of the vagina and vulva, these lasers generally have a very shallow depth of optical penetration, on the order of 10 to 200 µm.
Radiofrequency-based devices emit focused electromagnetic waves
Radiofrequency systems use a wand to deliver radiofrequency energy to create heat within the subepithelial layers of vulvar and vaginal tissues, while the surface remains cool. These devices can use monopolar or bipolar energy (current) to create a reverse thermal gradient designed to heat the deeper tissues transepithelially at a higher temperature while a coolant protects the surface epithelium. Some wand technologies require multiple treatments, while others require only a single treatment.
The TABLE lists currently available energy-based technologies.
Therapeutic uses for energy-based devices
Investigators have studied laser devices for treating various gynecologic conditions, including vulvovaginal atrophy, stress urinary incontinence (UI), vaginal laxity, lichen sclerosus, and vulvodynia.
Vulvovaginal atrophy
Genitourinary syndrome of menopause (GSM) includes symptoms of vulvovaginal irritation, burning, itching, discharge, dyspareunia, lower urinary tract symptoms such as frequency and urinary tract infections, and vaginal dryness or vulvovaginal atrophy.7 First-line treatment for vulvovaginal atrophy includes the use of nonhormonal lubricants for intercourse and vaginal moisturizers, which temporarily moisten the vaginal epithelium. Low-dose vaginal estrogen is a second-line therapy for symptomatic vulvovaginal atrophy; newer pharmacologic options include dehydroepiandrosterone (DHEA) suppositories (prasterone), solubilized estradiol capsules, and the selective estrogen receptor modulator (SERM) ospemifene.
Fractionated CO2, Erb:YAG, and hybrid lasers also have been used to treat women with symptomatic vulvovaginal atrophy and GSM through similar mechanisms described in dermatologic conditions with low-temperature laser activation of tissue proteins and growth factors creating new connective tissue and angiogenesis. A number of landmark studies have been published detailing patient outcomes with energy-based treatments for these symptoms.
Three-arm trial. Cruz and colleagues conducted a double-blind randomized trial to evaluate the efficacy of fractional CO2 laser vaginal treatment compared with local estriol therapy and the combination of laser plus estriol.8 The investigators randomly assigned 45 postmenopausal women to treatment with fractional CO2 laser with placebo vaginal cream, estriol with sham laser, or laser plus estriol. Treatment consisted of 2 sessions 4 weeks apart, with 20 consecutive weeks of estriol or placebo 3 times per week.
At weeks 8 and 20, the Vaginal Health Index (VHI) average score was significantly higher in all study arms. At week 20, the laser plus estriol group also showed incremental improvement in the VHI score (P = .01). The laser and the laser plus estriol groups had significant improvement in dyspareunia, burning, and dryness, while the estriol group improved only in dryness (P<.001). The laser plus estriol group had significant improvement in the total Female Sexual Function Index (FSFI) score (P = .02) and in the individual domains of pain, desire, and lubrication. Although the laser-alone group had significant worsening in the FSFI pain domain (P = .04), all treatment arms had comparable FSFI total scores at week 20. No adverse events were recorded during the study period.
Continue to: Retrospective study...
Retrospective study. To assess the efficacy of 3, 4, or 5 treatments with microablative fractional CO2 laser therapy for symptoms of GSM, Athanasiou and colleagues studied outcomes in 94 postmenopausal women.9 The intensity or bothersomeness of GSM symptoms as well as sexual function significantly improved in this cohort. The intensity of dyspareunia and dryness decreased from a median of 9 (minimum–maximum, 5–10) and 8 (0–10), respectively, at baseline to 0 (0–6) and 0 (0–8) at 1 month after the last laser therapy (P<.001 for all). The FSFI score and the frequency of sexual intercourse rose from 10.8 (2–26.9) and 1 (0–8) at baseline to 27.8 (15.2–35.4) and 4 (2–8) at 1 month after the last laser therapy (P<.001 for all).
The positive effects of laser therapy were unchanged throughout the 12 months of follow-up, and the pattern was the same for symptom-free rates. No adverse events were recorded during the study period.
The investigators noted that, based on short- and long-term follow-up, 4 or 5 laser treatments may be superior to 3 treatments for lowering the intensity of GSM symptoms. They found no differences in outcomes between 4 and 5 laser treatments.
Prospective comparative cohort study. Gaspar and colleagues recruited 50 postmenopausal women with GSM and assigned 25 participants to 2 weeks of pretreatment with estriol ovules 3 times per week (for epithelial hydration) followed by 3 sessions of Er:YAG nonablative laser treatments; 25 women in the active control group received treatment with estriol ovules over 8 weeks.10 Pre- and posttreatment biopsies, maturation index, maturation value, pH, and VAS symptom analysis were recorded up to 18 months after treatment.
Up to the 6-month follow-up, both treatment groups had a statistically significant reduction of all GSM symptoms. At all follow-ups, however, symptom relief was more prominent in the laser-treated group. In addition, the effects of the laser therapy remained statistically significant at the 12- and 18-month follow-ups, while the treatment effects of estriol were diminished at 12 months and, at 18 months, this group had some symptoms that were significantly worse than before treatment.
Overall, adverse effects were minimal and transient in both groups, affecting 4% of participants in the laser group, and 12% in the estriol group.
Long-term effectiveness evaluation. To assess the long-term efficacy and acceptability of vaginal laser treatment for the management of GSM, Gambacciani and colleagues treated 205 postmenopausal women with an Er:YAG laser for 3 applications every 30 days, with evaluations performed after 1, 3, 6, 12, 18, and 24 months from the last laser treatment.11 An active control group (n = 49) received 3 months of local treatment with either hormonal (estriol gel twice weekly) or nonhormonal (hyaluronic acid-based preparations or moisturizers and lubricants) agents.
Treatment with the ER:YAG laser induced a significant decrease (P<.01) in scores of the Visual Analog Scale (VAS) for vulvovaginal atrophy symptoms for vaginal dryness and dyspareunia and an increase in the VHI score (P<.01) up to 12 months after the last treatment. After 18 and 24 months, values returned to levels similar to those at baseline.
Women who also had stress UI (n = 114) received additional laser treatment of the anterior vaginal wall specifically designed for UI, with assessment based on the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF). Laser treatment induced a significant decrease (P<.05) in ICIQ-UI SF scores compared with baseline values, and scores remained lower than baseline values after 1, 2, 3, 6, and 12 months after the last laser treatment. Values measured after 18 and 24 months, however, did not differ significantly from baseline.
In the control group, the VAS score showed a similar decrease and comparable pattern during the treatment period. However, after the end of the treatment period, the control group’s VAS scores for vaginal dryness and dyspareunia showed a progressive increase, and after 6 months, the values were significantly different from corresponding values measured in the laser therapy group. The follow-up period in the control group ended after 6 months, because almost all patients started a new local or systemic treatment for their GSM symptoms. No adverse events related to treatment were recorded throughout the study period.
In an earlier pilot study by the same authors, 19 women with GSM who also had mild to moderate stress UI were treated with a vaginal Er:YAG laser.12 Compared with vaginal estriol treatment in the active control group, laser treatment was associated with a significant improvement (P<.01) in ICIQ-SF scores, with rapid and long-lasting effects that persisted up to week 24 of the observation period.
Continue to: Urinary incontinence...
Urinary incontinence
The cause of UI is considered to be multifactorial, including disruption in connective tissue supports of the urethrovesical junction leading to urethral hypermobility, pelvic floor muscle weakness, nerve damage to the urethral rhabdosphincter related to pudendal neuropathy or pelvic plexopathy, and atrophic changes of the urethra mucosa and submucosa. Purported mechanisms of action for energy-based therapies designed for treatment of UI relate to direct effects on connective tissue, blood vessels, and possibly nerves.
In 3 clinical trials designed specifically to treat UI with an Er:YAG laser, women showed subjective symptomatic improvement.
Ogrinc and colleagues followed 175 pre- and postmenopausal women with stress UI or mixed UI in a prospective nonrandomized study.13 They treated women with an Er:YAG laser for an average of 2.5 (0.5) procedures separated by a 2-month period and performed follow-up assessments at 2, 6, and 12 months after treatment.
After treatment, 77% of women with stress UI had significant improvement in symptoms based on the ICIQ SF and the Incontinence Severity Index (ISI), while only 34% of those with mixed UI had no symptoms at 1-year follow-up. No major adverse effects were noted in either group.
Okui compared the effects of Er:YAG laser treatment with those of tension-free vaginal tape (TVT) or transobturator tape (TOT) sling procedures (n = 50 in each group) in women with stress UI or mixed UI.14 At 12 months after treatment, all 3 treatments demonstrated comparable improvements in the women with stress UI. Some patients with mixed UI in the TVT and TOT groups showed exacerbation, while all women in the laser-treated group tended to have symptom improvement.
In another recent study, Blaganje and colleagues randomly assigned 114 premenopausal parous women with stress UI to an Er:YAG laser procedure or sham treatment.15 Three months after treatment, ICIQ-UI SF scores were significantly more improved (P<.001) in the laser-treated group than in the sham group. In addition, 21% of laser-treated patients were dry at follow-up compared with 4% of the sham-treated group.
Key takeaway. While these studies showed promising short-term results for laser treatment of UI, they need to be replicated in appropriately powered clinical trials that include critical subjective and objective outcomes as well as longer-term follow-up for both effectiveness and safety.
Vaginal laxity/pre-prolapse
Vaginal laxity is defined as the symptom of excessive vaginal looseness.16 Also referred to as “pre-prolapse,” this subjective symptom generally refers to a widened vaginal opening (genital hiatus) but with pelvic organ prolapse that is within the vagina or hymen.17 Notably, the definition is ambiguous, and rigorous clinical data based on validated outcomes and prolapse grading are lacking.
Krychman and colleagues conducted the first randomized controlled study comparing monopolar radiofrequency at the vaginal introitus with sham therapy for vaginal laxity in 174 premenopausal women, known as the VIVEVE I trial.18 The primary outcome, the proportion of women reporting no vaginal laxity at 6 months after treatment, was assessed using a vaginal laxity questionnaire, a 7-point rating scale for laxity or tightness ranging from very loose to very tight. With a single radiofrequency treatment, 43.5% of the active group and 19.6% (P = .002) of the sham group obtained the primary outcome.
There were also statistically significant improvements in overall sexual function and decreased sexual distress. The adjusted odds ratio (OR, 3.39; 95% confidence interval, 1.54–7.45) showed that the likelihood of no vaginal laxity at 6 months was more than 3 times greater for women who received the active treatment compared with those who received sham treatment. Adverse events were mild, resolved spontaneously, and were similar in the 2 groups.
Continue to: Outlook for energy-based...
Outlook for energy-based therapies: Cautiously optimistic
Preliminary outcome data on the use of energy-based therapies for female genital cosmetic surgery is largely positive for the treatment of vulvovaginal atrophy, but some case series suggest the potential for scarring, burning, and inefficacy. This prompted the FDA to send “It has come to our attention” letters to a number of device manufacturers in 2018.6
Supportive evidence is weak. Early data are encouraging regarding fractionated laser therapy for the treatment of vulvovaginal atrophy and stress UI and radiofrequency wand therapy for vaginal laxity and stress UI. Unfortunately, the level of evidence to support wide use of these technologies for all pelvic floor disorders is weak. A recent committee opinion from the International Urogynecology Association noted that only 8 studies (1 randomized trial and 7 observational studies) on these conditions fulfilled the criteria of good quality.19 The International Continence Society and the International Society for the Study of Vulvovaginal Disorders recently published a best practice consensus document declaring laser and energy-based treatments in gynecology and urology to be largely experimental.20
Questions persist. Knowledge gaps exist, and recommendations related to subspecialty training—who should perform these procedures (gynecologists, plastic surgeons, urologists, dermatologists, family practitioners) and the level of training needed to safely perform them—are lacking. Patient selection and physician knowledge and experience related to female genital anatomy, female sexual function and dysfunction, multidisciplinary treatment options for various pelvic support problems and UI, as well as psychologic screening for body dysmorphic disorders, need to be considered in terms of treating both the functional and aesthetic aspects related to cosmetic and reconstructive gynecologic surgery.
Special considerations. The use of energy-based therapies in special populations, such as survivors of breast cancer or other gynecologic cancers, as well as women undergoing chemotherapy, radiation therapy, and hormonal manipulation (particularly with antiestrogenic SERMs and aromatase inhibitors) has not been adequately evaluated. A discussion of the risks, benefits, alternatives, and limited long-term outcome data for energy-based therapies in cancer survivors, as for all patients, must be included for adequate informed consent prior to undertaking these treatments.
Guidelines for appropriate tissue priming, laser settings, and concomitant energy-based technology with local hormone treatment (also known as laser-augmented drug delivery) need to be developed. Comparative long-term studies are needed to determine the safety and effectiveness of these technologies.
Caution advised. Given the lack of long-term safety and effectiveness data on energy-based therapies for the vague indications of vaginal laxity, and even for the well-defined conditions of stress UI and vulvovaginal atrophy, clinicians should exercise caution before promoting treatment, which can be expensive and is not without potential complications, such as vaginal pain, adhesive agglutination, and persistent dryness and dyspareunia.21
Fortunately, many randomized trials on various energy-based devices for gynecologic indications (GSM, stress UI, vaginal laxity, lichen sclerosus) are underway, and results from these studies will help inform future clinical practice and guideline development.
- Kaplan I, Goldman J, Ger R. The treatment of erosions of the uterine cervix by means of the CO2 laser. Obstet Gynecol. 1973;41:795-796.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy-based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Gaspar A, Addamo G, Brandi H. Vaginal fractional CO2 laser: a minimally invasive option for vaginal rejuvenation. Am J Cosmetic Surg. 2011;28:156-162.
- Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22:845-849.
- Benedetto AV. What's new in cosmetic dermatology. Dermatol Clin. 2019;37:117-128.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal rejuvenation or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed April 8, 2019.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Athanasiou S, Pitsouni E, Grigoradis T, et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause. 2019;26:248-255.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Russo E, et al. Long-term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148-152.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Ogrinc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47:689-697.
- Okui N. Comparison between erbium-doped yttrium aluminum garnet laser therapy and sling procedures in the treatment of stress and mixed urinary incontinence. World J Urol. 2018. doi:10.1007/s00345-018-2445-x.
- Blaganje M, Scepanovic D, Zgur L, et al. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153-158.
- Haylen BT, Maher CF, Barber MD, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecologic J. 2016;27:165-194.
- Garcia B, Pardo J. Academic cosmetic gynecology and energy-based therapies: ambiguities, explorations, and the FDA advisories. Int Urogynecol J. 2019;30:1-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al; IUGA Research and Development Committee. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. Neurourol Urodyn. 2019;38:1009-1023.
- Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26:423-427.
Energy-based therapy use in gynecology dates back to the early 1970s, when ablative carbon dioxide (C02) lasers were employed to treat cervical erosions.1 Soon after, reports were published on laser treatment for diethylstilbestrol-associated vaginal adenosis, laser laparoscopy for adhesiolysis, laser hysteroscopy, and laser genital wart ablation.2 Starting around 2011, the first articles were published on the use of fractional C02 laser treatment for vulvovaginal atrophy.3,4 Use of laser and light-based therapies to treat “vaginal rejuvenation” is now increasing at an annual rate of 26%. In a few years, North America is expected to be the largest market for vaginal laser rejuvenation. In 2016, more than 500,000 feminine rejuvenation procedures were performed in the United States, and it is estimated that more than 27,000 energy-based devices will be in operation by 2021.5
Clearly, there is considerable public interest and intrigue in office-based female genital cosmetic procedures. In 2018, the US Food and Drug Administration contacted 7 manufacturers of energy-based devices to request revision and clarification for marketing of these devices, since these technologies are neither cleared nor approved for cosmetic vulvovaginal conditions.6 The companies responded within 30 days.
In this article, we appraise the existing literature regarding the mechanism of action of energy-based therapies used in gynecology and review outcomes of their use in female genital cosmetic surgery.
Laser technology devices and how they work
LASER is an acronym for Light Amplification by Stimulated Emission of Radiation. Laser devices are composed of 1) an excitable medium (gas, liquid, solid) needed to emit light, 2) an energy source to excite the medium, 3) mirrors to bounce the light back and forth, and 4) a delivery and cooling system (FIGURE 1).
The electromagnetic spectrum is the range of all the wavelengths of light, including visible light, radio waves, infrared light, ultraviolet light, x-rays, and gamma rays (FIGURE 2). Most lasers used for the treatment of vulvovaginal disorders, typically C02 and erbium:yttrium aluminum garnet (Er:YAG) lasers, involve the infrared wavelengths.
The basic principle of laser treatment is to match the wavelength of the laser with the absorption spectrum of the desired target—a chromophore such as hemoglobin, melanin, or water (FIGURE 3). In essence, light is absorbed by the chromophore (which in vulvar and vaginal tissues is mostly water) and transformed into heat, leading to target destruction. In a fractionated (or fractional) laser beam, the laser is broken up into many smaller beams that treat only portions of the treatment area, with areas of intact epithelium in between the treated areas. At appropriately low thermal denaturation temperatures (45° to 50°C), tissue regeneration can occur through activation of heat shock proteins and tissue growth factors, creating neocollagenesis and neovascularization.
The concept of ablative resurfacing versus fractional resurfacing is borrowed from dermatology (FIGURE 4), understanding that tissue ablation and thermal denaturation occur at temperatures greater than 100°C, as occurs with carbonization of vulvar condylomata.
Continue to: In dermatology, fractionated lasers...
In dermatology, fractionated lasers have been used in the treatment of hair removal, vascular and pigmented lesions, scars, wound healing, tattoo removal, warts, and actinic keratoses. For these conditions, the targeted chromophores are water, hemoglobin, melanosomes, and tattoo ink. The laser pulses must be shorter than the target tissue thermal relaxation times in order to avoid excess heating and collateral tissue damage. Choosing appropriate settings is critical to achieve selective heating, or destruction, of the target tissue. These settings include appropriate wavelengths, pulse durations, and fluence, which is energy delivered per unit area (typically, joules per square centimeter).
For gynecologic conditions, the lasers used are most often CO2, Er:YAG, and hybrid (which include ablative and nonablative wavelengths) devices. In the epithelium of the vagina and vulva, these lasers generally have a very shallow depth of optical penetration, on the order of 10 to 200 µm.
Radiofrequency-based devices emit focused electromagnetic waves
Radiofrequency systems use a wand to deliver radiofrequency energy to create heat within the subepithelial layers of vulvar and vaginal tissues, while the surface remains cool. These devices can use monopolar or bipolar energy (current) to create a reverse thermal gradient designed to heat the deeper tissues transepithelially at a higher temperature while a coolant protects the surface epithelium. Some wand technologies require multiple treatments, while others require only a single treatment.
The TABLE lists currently available energy-based technologies.
Therapeutic uses for energy-based devices
Investigators have studied laser devices for treating various gynecologic conditions, including vulvovaginal atrophy, stress urinary incontinence (UI), vaginal laxity, lichen sclerosus, and vulvodynia.
Vulvovaginal atrophy
Genitourinary syndrome of menopause (GSM) includes symptoms of vulvovaginal irritation, burning, itching, discharge, dyspareunia, lower urinary tract symptoms such as frequency and urinary tract infections, and vaginal dryness or vulvovaginal atrophy.7 First-line treatment for vulvovaginal atrophy includes the use of nonhormonal lubricants for intercourse and vaginal moisturizers, which temporarily moisten the vaginal epithelium. Low-dose vaginal estrogen is a second-line therapy for symptomatic vulvovaginal atrophy; newer pharmacologic options include dehydroepiandrosterone (DHEA) suppositories (prasterone), solubilized estradiol capsules, and the selective estrogen receptor modulator (SERM) ospemifene.
Fractionated CO2, Erb:YAG, and hybrid lasers also have been used to treat women with symptomatic vulvovaginal atrophy and GSM through similar mechanisms described in dermatologic conditions with low-temperature laser activation of tissue proteins and growth factors creating new connective tissue and angiogenesis. A number of landmark studies have been published detailing patient outcomes with energy-based treatments for these symptoms.
Three-arm trial. Cruz and colleagues conducted a double-blind randomized trial to evaluate the efficacy of fractional CO2 laser vaginal treatment compared with local estriol therapy and the combination of laser plus estriol.8 The investigators randomly assigned 45 postmenopausal women to treatment with fractional CO2 laser with placebo vaginal cream, estriol with sham laser, or laser plus estriol. Treatment consisted of 2 sessions 4 weeks apart, with 20 consecutive weeks of estriol or placebo 3 times per week.
At weeks 8 and 20, the Vaginal Health Index (VHI) average score was significantly higher in all study arms. At week 20, the laser plus estriol group also showed incremental improvement in the VHI score (P = .01). The laser and the laser plus estriol groups had significant improvement in dyspareunia, burning, and dryness, while the estriol group improved only in dryness (P<.001). The laser plus estriol group had significant improvement in the total Female Sexual Function Index (FSFI) score (P = .02) and in the individual domains of pain, desire, and lubrication. Although the laser-alone group had significant worsening in the FSFI pain domain (P = .04), all treatment arms had comparable FSFI total scores at week 20. No adverse events were recorded during the study period.
Continue to: Retrospective study...
Retrospective study. To assess the efficacy of 3, 4, or 5 treatments with microablative fractional CO2 laser therapy for symptoms of GSM, Athanasiou and colleagues studied outcomes in 94 postmenopausal women.9 The intensity or bothersomeness of GSM symptoms as well as sexual function significantly improved in this cohort. The intensity of dyspareunia and dryness decreased from a median of 9 (minimum–maximum, 5–10) and 8 (0–10), respectively, at baseline to 0 (0–6) and 0 (0–8) at 1 month after the last laser therapy (P<.001 for all). The FSFI score and the frequency of sexual intercourse rose from 10.8 (2–26.9) and 1 (0–8) at baseline to 27.8 (15.2–35.4) and 4 (2–8) at 1 month after the last laser therapy (P<.001 for all).
The positive effects of laser therapy were unchanged throughout the 12 months of follow-up, and the pattern was the same for symptom-free rates. No adverse events were recorded during the study period.
The investigators noted that, based on short- and long-term follow-up, 4 or 5 laser treatments may be superior to 3 treatments for lowering the intensity of GSM symptoms. They found no differences in outcomes between 4 and 5 laser treatments.
Prospective comparative cohort study. Gaspar and colleagues recruited 50 postmenopausal women with GSM and assigned 25 participants to 2 weeks of pretreatment with estriol ovules 3 times per week (for epithelial hydration) followed by 3 sessions of Er:YAG nonablative laser treatments; 25 women in the active control group received treatment with estriol ovules over 8 weeks.10 Pre- and posttreatment biopsies, maturation index, maturation value, pH, and VAS symptom analysis were recorded up to 18 months after treatment.
Up to the 6-month follow-up, both treatment groups had a statistically significant reduction of all GSM symptoms. At all follow-ups, however, symptom relief was more prominent in the laser-treated group. In addition, the effects of the laser therapy remained statistically significant at the 12- and 18-month follow-ups, while the treatment effects of estriol were diminished at 12 months and, at 18 months, this group had some symptoms that were significantly worse than before treatment.
Overall, adverse effects were minimal and transient in both groups, affecting 4% of participants in the laser group, and 12% in the estriol group.
Long-term effectiveness evaluation. To assess the long-term efficacy and acceptability of vaginal laser treatment for the management of GSM, Gambacciani and colleagues treated 205 postmenopausal women with an Er:YAG laser for 3 applications every 30 days, with evaluations performed after 1, 3, 6, 12, 18, and 24 months from the last laser treatment.11 An active control group (n = 49) received 3 months of local treatment with either hormonal (estriol gel twice weekly) or nonhormonal (hyaluronic acid-based preparations or moisturizers and lubricants) agents.
Treatment with the ER:YAG laser induced a significant decrease (P<.01) in scores of the Visual Analog Scale (VAS) for vulvovaginal atrophy symptoms for vaginal dryness and dyspareunia and an increase in the VHI score (P<.01) up to 12 months after the last treatment. After 18 and 24 months, values returned to levels similar to those at baseline.
Women who also had stress UI (n = 114) received additional laser treatment of the anterior vaginal wall specifically designed for UI, with assessment based on the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF). Laser treatment induced a significant decrease (P<.05) in ICIQ-UI SF scores compared with baseline values, and scores remained lower than baseline values after 1, 2, 3, 6, and 12 months after the last laser treatment. Values measured after 18 and 24 months, however, did not differ significantly from baseline.
In the control group, the VAS score showed a similar decrease and comparable pattern during the treatment period. However, after the end of the treatment period, the control group’s VAS scores for vaginal dryness and dyspareunia showed a progressive increase, and after 6 months, the values were significantly different from corresponding values measured in the laser therapy group. The follow-up period in the control group ended after 6 months, because almost all patients started a new local or systemic treatment for their GSM symptoms. No adverse events related to treatment were recorded throughout the study period.
In an earlier pilot study by the same authors, 19 women with GSM who also had mild to moderate stress UI were treated with a vaginal Er:YAG laser.12 Compared with vaginal estriol treatment in the active control group, laser treatment was associated with a significant improvement (P<.01) in ICIQ-SF scores, with rapid and long-lasting effects that persisted up to week 24 of the observation period.
Continue to: Urinary incontinence...
Urinary incontinence
The cause of UI is considered to be multifactorial, including disruption in connective tissue supports of the urethrovesical junction leading to urethral hypermobility, pelvic floor muscle weakness, nerve damage to the urethral rhabdosphincter related to pudendal neuropathy or pelvic plexopathy, and atrophic changes of the urethra mucosa and submucosa. Purported mechanisms of action for energy-based therapies designed for treatment of UI relate to direct effects on connective tissue, blood vessels, and possibly nerves.
In 3 clinical trials designed specifically to treat UI with an Er:YAG laser, women showed subjective symptomatic improvement.
Ogrinc and colleagues followed 175 pre- and postmenopausal women with stress UI or mixed UI in a prospective nonrandomized study.13 They treated women with an Er:YAG laser for an average of 2.5 (0.5) procedures separated by a 2-month period and performed follow-up assessments at 2, 6, and 12 months after treatment.
After treatment, 77% of women with stress UI had significant improvement in symptoms based on the ICIQ SF and the Incontinence Severity Index (ISI), while only 34% of those with mixed UI had no symptoms at 1-year follow-up. No major adverse effects were noted in either group.
Okui compared the effects of Er:YAG laser treatment with those of tension-free vaginal tape (TVT) or transobturator tape (TOT) sling procedures (n = 50 in each group) in women with stress UI or mixed UI.14 At 12 months after treatment, all 3 treatments demonstrated comparable improvements in the women with stress UI. Some patients with mixed UI in the TVT and TOT groups showed exacerbation, while all women in the laser-treated group tended to have symptom improvement.
In another recent study, Blaganje and colleagues randomly assigned 114 premenopausal parous women with stress UI to an Er:YAG laser procedure or sham treatment.15 Three months after treatment, ICIQ-UI SF scores were significantly more improved (P<.001) in the laser-treated group than in the sham group. In addition, 21% of laser-treated patients were dry at follow-up compared with 4% of the sham-treated group.
Key takeaway. While these studies showed promising short-term results for laser treatment of UI, they need to be replicated in appropriately powered clinical trials that include critical subjective and objective outcomes as well as longer-term follow-up for both effectiveness and safety.
Vaginal laxity/pre-prolapse
Vaginal laxity is defined as the symptom of excessive vaginal looseness.16 Also referred to as “pre-prolapse,” this subjective symptom generally refers to a widened vaginal opening (genital hiatus) but with pelvic organ prolapse that is within the vagina or hymen.17 Notably, the definition is ambiguous, and rigorous clinical data based on validated outcomes and prolapse grading are lacking.
Krychman and colleagues conducted the first randomized controlled study comparing monopolar radiofrequency at the vaginal introitus with sham therapy for vaginal laxity in 174 premenopausal women, known as the VIVEVE I trial.18 The primary outcome, the proportion of women reporting no vaginal laxity at 6 months after treatment, was assessed using a vaginal laxity questionnaire, a 7-point rating scale for laxity or tightness ranging from very loose to very tight. With a single radiofrequency treatment, 43.5% of the active group and 19.6% (P = .002) of the sham group obtained the primary outcome.
There were also statistically significant improvements in overall sexual function and decreased sexual distress. The adjusted odds ratio (OR, 3.39; 95% confidence interval, 1.54–7.45) showed that the likelihood of no vaginal laxity at 6 months was more than 3 times greater for women who received the active treatment compared with those who received sham treatment. Adverse events were mild, resolved spontaneously, and were similar in the 2 groups.
Continue to: Outlook for energy-based...
Outlook for energy-based therapies: Cautiously optimistic
Preliminary outcome data on the use of energy-based therapies for female genital cosmetic surgery is largely positive for the treatment of vulvovaginal atrophy, but some case series suggest the potential for scarring, burning, and inefficacy. This prompted the FDA to send “It has come to our attention” letters to a number of device manufacturers in 2018.6
Supportive evidence is weak. Early data are encouraging regarding fractionated laser therapy for the treatment of vulvovaginal atrophy and stress UI and radiofrequency wand therapy for vaginal laxity and stress UI. Unfortunately, the level of evidence to support wide use of these technologies for all pelvic floor disorders is weak. A recent committee opinion from the International Urogynecology Association noted that only 8 studies (1 randomized trial and 7 observational studies) on these conditions fulfilled the criteria of good quality.19 The International Continence Society and the International Society for the Study of Vulvovaginal Disorders recently published a best practice consensus document declaring laser and energy-based treatments in gynecology and urology to be largely experimental.20
Questions persist. Knowledge gaps exist, and recommendations related to subspecialty training—who should perform these procedures (gynecologists, plastic surgeons, urologists, dermatologists, family practitioners) and the level of training needed to safely perform them—are lacking. Patient selection and physician knowledge and experience related to female genital anatomy, female sexual function and dysfunction, multidisciplinary treatment options for various pelvic support problems and UI, as well as psychologic screening for body dysmorphic disorders, need to be considered in terms of treating both the functional and aesthetic aspects related to cosmetic and reconstructive gynecologic surgery.
Special considerations. The use of energy-based therapies in special populations, such as survivors of breast cancer or other gynecologic cancers, as well as women undergoing chemotherapy, radiation therapy, and hormonal manipulation (particularly with antiestrogenic SERMs and aromatase inhibitors) has not been adequately evaluated. A discussion of the risks, benefits, alternatives, and limited long-term outcome data for energy-based therapies in cancer survivors, as for all patients, must be included for adequate informed consent prior to undertaking these treatments.
Guidelines for appropriate tissue priming, laser settings, and concomitant energy-based technology with local hormone treatment (also known as laser-augmented drug delivery) need to be developed. Comparative long-term studies are needed to determine the safety and effectiveness of these technologies.
Caution advised. Given the lack of long-term safety and effectiveness data on energy-based therapies for the vague indications of vaginal laxity, and even for the well-defined conditions of stress UI and vulvovaginal atrophy, clinicians should exercise caution before promoting treatment, which can be expensive and is not without potential complications, such as vaginal pain, adhesive agglutination, and persistent dryness and dyspareunia.21
Fortunately, many randomized trials on various energy-based devices for gynecologic indications (GSM, stress UI, vaginal laxity, lichen sclerosus) are underway, and results from these studies will help inform future clinical practice and guideline development.
Energy-based therapy use in gynecology dates back to the early 1970s, when ablative carbon dioxide (C02) lasers were employed to treat cervical erosions.1 Soon after, reports were published on laser treatment for diethylstilbestrol-associated vaginal adenosis, laser laparoscopy for adhesiolysis, laser hysteroscopy, and laser genital wart ablation.2 Starting around 2011, the first articles were published on the use of fractional C02 laser treatment for vulvovaginal atrophy.3,4 Use of laser and light-based therapies to treat “vaginal rejuvenation” is now increasing at an annual rate of 26%. In a few years, North America is expected to be the largest market for vaginal laser rejuvenation. In 2016, more than 500,000 feminine rejuvenation procedures were performed in the United States, and it is estimated that more than 27,000 energy-based devices will be in operation by 2021.5
Clearly, there is considerable public interest and intrigue in office-based female genital cosmetic procedures. In 2018, the US Food and Drug Administration contacted 7 manufacturers of energy-based devices to request revision and clarification for marketing of these devices, since these technologies are neither cleared nor approved for cosmetic vulvovaginal conditions.6 The companies responded within 30 days.
In this article, we appraise the existing literature regarding the mechanism of action of energy-based therapies used in gynecology and review outcomes of their use in female genital cosmetic surgery.
Laser technology devices and how they work
LASER is an acronym for Light Amplification by Stimulated Emission of Radiation. Laser devices are composed of 1) an excitable medium (gas, liquid, solid) needed to emit light, 2) an energy source to excite the medium, 3) mirrors to bounce the light back and forth, and 4) a delivery and cooling system (FIGURE 1).
The electromagnetic spectrum is the range of all the wavelengths of light, including visible light, radio waves, infrared light, ultraviolet light, x-rays, and gamma rays (FIGURE 2). Most lasers used for the treatment of vulvovaginal disorders, typically C02 and erbium:yttrium aluminum garnet (Er:YAG) lasers, involve the infrared wavelengths.
The basic principle of laser treatment is to match the wavelength of the laser with the absorption spectrum of the desired target—a chromophore such as hemoglobin, melanin, or water (FIGURE 3). In essence, light is absorbed by the chromophore (which in vulvar and vaginal tissues is mostly water) and transformed into heat, leading to target destruction. In a fractionated (or fractional) laser beam, the laser is broken up into many smaller beams that treat only portions of the treatment area, with areas of intact epithelium in between the treated areas. At appropriately low thermal denaturation temperatures (45° to 50°C), tissue regeneration can occur through activation of heat shock proteins and tissue growth factors, creating neocollagenesis and neovascularization.
The concept of ablative resurfacing versus fractional resurfacing is borrowed from dermatology (FIGURE 4), understanding that tissue ablation and thermal denaturation occur at temperatures greater than 100°C, as occurs with carbonization of vulvar condylomata.
Continue to: In dermatology, fractionated lasers...
In dermatology, fractionated lasers have been used in the treatment of hair removal, vascular and pigmented lesions, scars, wound healing, tattoo removal, warts, and actinic keratoses. For these conditions, the targeted chromophores are water, hemoglobin, melanosomes, and tattoo ink. The laser pulses must be shorter than the target tissue thermal relaxation times in order to avoid excess heating and collateral tissue damage. Choosing appropriate settings is critical to achieve selective heating, or destruction, of the target tissue. These settings include appropriate wavelengths, pulse durations, and fluence, which is energy delivered per unit area (typically, joules per square centimeter).
For gynecologic conditions, the lasers used are most often CO2, Er:YAG, and hybrid (which include ablative and nonablative wavelengths) devices. In the epithelium of the vagina and vulva, these lasers generally have a very shallow depth of optical penetration, on the order of 10 to 200 µm.
Radiofrequency-based devices emit focused electromagnetic waves
Radiofrequency systems use a wand to deliver radiofrequency energy to create heat within the subepithelial layers of vulvar and vaginal tissues, while the surface remains cool. These devices can use monopolar or bipolar energy (current) to create a reverse thermal gradient designed to heat the deeper tissues transepithelially at a higher temperature while a coolant protects the surface epithelium. Some wand technologies require multiple treatments, while others require only a single treatment.
The TABLE lists currently available energy-based technologies.
Therapeutic uses for energy-based devices
Investigators have studied laser devices for treating various gynecologic conditions, including vulvovaginal atrophy, stress urinary incontinence (UI), vaginal laxity, lichen sclerosus, and vulvodynia.
Vulvovaginal atrophy
Genitourinary syndrome of menopause (GSM) includes symptoms of vulvovaginal irritation, burning, itching, discharge, dyspareunia, lower urinary tract symptoms such as frequency and urinary tract infections, and vaginal dryness or vulvovaginal atrophy.7 First-line treatment for vulvovaginal atrophy includes the use of nonhormonal lubricants for intercourse and vaginal moisturizers, which temporarily moisten the vaginal epithelium. Low-dose vaginal estrogen is a second-line therapy for symptomatic vulvovaginal atrophy; newer pharmacologic options include dehydroepiandrosterone (DHEA) suppositories (prasterone), solubilized estradiol capsules, and the selective estrogen receptor modulator (SERM) ospemifene.
Fractionated CO2, Erb:YAG, and hybrid lasers also have been used to treat women with symptomatic vulvovaginal atrophy and GSM through similar mechanisms described in dermatologic conditions with low-temperature laser activation of tissue proteins and growth factors creating new connective tissue and angiogenesis. A number of landmark studies have been published detailing patient outcomes with energy-based treatments for these symptoms.
Three-arm trial. Cruz and colleagues conducted a double-blind randomized trial to evaluate the efficacy of fractional CO2 laser vaginal treatment compared with local estriol therapy and the combination of laser plus estriol.8 The investigators randomly assigned 45 postmenopausal women to treatment with fractional CO2 laser with placebo vaginal cream, estriol with sham laser, or laser plus estriol. Treatment consisted of 2 sessions 4 weeks apart, with 20 consecutive weeks of estriol or placebo 3 times per week.
At weeks 8 and 20, the Vaginal Health Index (VHI) average score was significantly higher in all study arms. At week 20, the laser plus estriol group also showed incremental improvement in the VHI score (P = .01). The laser and the laser plus estriol groups had significant improvement in dyspareunia, burning, and dryness, while the estriol group improved only in dryness (P<.001). The laser plus estriol group had significant improvement in the total Female Sexual Function Index (FSFI) score (P = .02) and in the individual domains of pain, desire, and lubrication. Although the laser-alone group had significant worsening in the FSFI pain domain (P = .04), all treatment arms had comparable FSFI total scores at week 20. No adverse events were recorded during the study period.
Continue to: Retrospective study...
Retrospective study. To assess the efficacy of 3, 4, or 5 treatments with microablative fractional CO2 laser therapy for symptoms of GSM, Athanasiou and colleagues studied outcomes in 94 postmenopausal women.9 The intensity or bothersomeness of GSM symptoms as well as sexual function significantly improved in this cohort. The intensity of dyspareunia and dryness decreased from a median of 9 (minimum–maximum, 5–10) and 8 (0–10), respectively, at baseline to 0 (0–6) and 0 (0–8) at 1 month after the last laser therapy (P<.001 for all). The FSFI score and the frequency of sexual intercourse rose from 10.8 (2–26.9) and 1 (0–8) at baseline to 27.8 (15.2–35.4) and 4 (2–8) at 1 month after the last laser therapy (P<.001 for all).
The positive effects of laser therapy were unchanged throughout the 12 months of follow-up, and the pattern was the same for symptom-free rates. No adverse events were recorded during the study period.
The investigators noted that, based on short- and long-term follow-up, 4 or 5 laser treatments may be superior to 3 treatments for lowering the intensity of GSM symptoms. They found no differences in outcomes between 4 and 5 laser treatments.
Prospective comparative cohort study. Gaspar and colleagues recruited 50 postmenopausal women with GSM and assigned 25 participants to 2 weeks of pretreatment with estriol ovules 3 times per week (for epithelial hydration) followed by 3 sessions of Er:YAG nonablative laser treatments; 25 women in the active control group received treatment with estriol ovules over 8 weeks.10 Pre- and posttreatment biopsies, maturation index, maturation value, pH, and VAS symptom analysis were recorded up to 18 months after treatment.
Up to the 6-month follow-up, both treatment groups had a statistically significant reduction of all GSM symptoms. At all follow-ups, however, symptom relief was more prominent in the laser-treated group. In addition, the effects of the laser therapy remained statistically significant at the 12- and 18-month follow-ups, while the treatment effects of estriol were diminished at 12 months and, at 18 months, this group had some symptoms that were significantly worse than before treatment.
Overall, adverse effects were minimal and transient in both groups, affecting 4% of participants in the laser group, and 12% in the estriol group.
Long-term effectiveness evaluation. To assess the long-term efficacy and acceptability of vaginal laser treatment for the management of GSM, Gambacciani and colleagues treated 205 postmenopausal women with an Er:YAG laser for 3 applications every 30 days, with evaluations performed after 1, 3, 6, 12, 18, and 24 months from the last laser treatment.11 An active control group (n = 49) received 3 months of local treatment with either hormonal (estriol gel twice weekly) or nonhormonal (hyaluronic acid-based preparations or moisturizers and lubricants) agents.
Treatment with the ER:YAG laser induced a significant decrease (P<.01) in scores of the Visual Analog Scale (VAS) for vulvovaginal atrophy symptoms for vaginal dryness and dyspareunia and an increase in the VHI score (P<.01) up to 12 months after the last treatment. After 18 and 24 months, values returned to levels similar to those at baseline.
Women who also had stress UI (n = 114) received additional laser treatment of the anterior vaginal wall specifically designed for UI, with assessment based on the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF). Laser treatment induced a significant decrease (P<.05) in ICIQ-UI SF scores compared with baseline values, and scores remained lower than baseline values after 1, 2, 3, 6, and 12 months after the last laser treatment. Values measured after 18 and 24 months, however, did not differ significantly from baseline.
In the control group, the VAS score showed a similar decrease and comparable pattern during the treatment period. However, after the end of the treatment period, the control group’s VAS scores for vaginal dryness and dyspareunia showed a progressive increase, and after 6 months, the values were significantly different from corresponding values measured in the laser therapy group. The follow-up period in the control group ended after 6 months, because almost all patients started a new local or systemic treatment for their GSM symptoms. No adverse events related to treatment were recorded throughout the study period.
In an earlier pilot study by the same authors, 19 women with GSM who also had mild to moderate stress UI were treated with a vaginal Er:YAG laser.12 Compared with vaginal estriol treatment in the active control group, laser treatment was associated with a significant improvement (P<.01) in ICIQ-SF scores, with rapid and long-lasting effects that persisted up to week 24 of the observation period.
Continue to: Urinary incontinence...
Urinary incontinence
The cause of UI is considered to be multifactorial, including disruption in connective tissue supports of the urethrovesical junction leading to urethral hypermobility, pelvic floor muscle weakness, nerve damage to the urethral rhabdosphincter related to pudendal neuropathy or pelvic plexopathy, and atrophic changes of the urethra mucosa and submucosa. Purported mechanisms of action for energy-based therapies designed for treatment of UI relate to direct effects on connective tissue, blood vessels, and possibly nerves.
In 3 clinical trials designed specifically to treat UI with an Er:YAG laser, women showed subjective symptomatic improvement.
Ogrinc and colleagues followed 175 pre- and postmenopausal women with stress UI or mixed UI in a prospective nonrandomized study.13 They treated women with an Er:YAG laser for an average of 2.5 (0.5) procedures separated by a 2-month period and performed follow-up assessments at 2, 6, and 12 months after treatment.
After treatment, 77% of women with stress UI had significant improvement in symptoms based on the ICIQ SF and the Incontinence Severity Index (ISI), while only 34% of those with mixed UI had no symptoms at 1-year follow-up. No major adverse effects were noted in either group.
Okui compared the effects of Er:YAG laser treatment with those of tension-free vaginal tape (TVT) or transobturator tape (TOT) sling procedures (n = 50 in each group) in women with stress UI or mixed UI.14 At 12 months after treatment, all 3 treatments demonstrated comparable improvements in the women with stress UI. Some patients with mixed UI in the TVT and TOT groups showed exacerbation, while all women in the laser-treated group tended to have symptom improvement.
In another recent study, Blaganje and colleagues randomly assigned 114 premenopausal parous women with stress UI to an Er:YAG laser procedure or sham treatment.15 Three months after treatment, ICIQ-UI SF scores were significantly more improved (P<.001) in the laser-treated group than in the sham group. In addition, 21% of laser-treated patients were dry at follow-up compared with 4% of the sham-treated group.
Key takeaway. While these studies showed promising short-term results for laser treatment of UI, they need to be replicated in appropriately powered clinical trials that include critical subjective and objective outcomes as well as longer-term follow-up for both effectiveness and safety.
Vaginal laxity/pre-prolapse
Vaginal laxity is defined as the symptom of excessive vaginal looseness.16 Also referred to as “pre-prolapse,” this subjective symptom generally refers to a widened vaginal opening (genital hiatus) but with pelvic organ prolapse that is within the vagina or hymen.17 Notably, the definition is ambiguous, and rigorous clinical data based on validated outcomes and prolapse grading are lacking.
Krychman and colleagues conducted the first randomized controlled study comparing monopolar radiofrequency at the vaginal introitus with sham therapy for vaginal laxity in 174 premenopausal women, known as the VIVEVE I trial.18 The primary outcome, the proportion of women reporting no vaginal laxity at 6 months after treatment, was assessed using a vaginal laxity questionnaire, a 7-point rating scale for laxity or tightness ranging from very loose to very tight. With a single radiofrequency treatment, 43.5% of the active group and 19.6% (P = .002) of the sham group obtained the primary outcome.
There were also statistically significant improvements in overall sexual function and decreased sexual distress. The adjusted odds ratio (OR, 3.39; 95% confidence interval, 1.54–7.45) showed that the likelihood of no vaginal laxity at 6 months was more than 3 times greater for women who received the active treatment compared with those who received sham treatment. Adverse events were mild, resolved spontaneously, and were similar in the 2 groups.
Continue to: Outlook for energy-based...
Outlook for energy-based therapies: Cautiously optimistic
Preliminary outcome data on the use of energy-based therapies for female genital cosmetic surgery is largely positive for the treatment of vulvovaginal atrophy, but some case series suggest the potential for scarring, burning, and inefficacy. This prompted the FDA to send “It has come to our attention” letters to a number of device manufacturers in 2018.6
Supportive evidence is weak. Early data are encouraging regarding fractionated laser therapy for the treatment of vulvovaginal atrophy and stress UI and radiofrequency wand therapy for vaginal laxity and stress UI. Unfortunately, the level of evidence to support wide use of these technologies for all pelvic floor disorders is weak. A recent committee opinion from the International Urogynecology Association noted that only 8 studies (1 randomized trial and 7 observational studies) on these conditions fulfilled the criteria of good quality.19 The International Continence Society and the International Society for the Study of Vulvovaginal Disorders recently published a best practice consensus document declaring laser and energy-based treatments in gynecology and urology to be largely experimental.20
Questions persist. Knowledge gaps exist, and recommendations related to subspecialty training—who should perform these procedures (gynecologists, plastic surgeons, urologists, dermatologists, family practitioners) and the level of training needed to safely perform them—are lacking. Patient selection and physician knowledge and experience related to female genital anatomy, female sexual function and dysfunction, multidisciplinary treatment options for various pelvic support problems and UI, as well as psychologic screening for body dysmorphic disorders, need to be considered in terms of treating both the functional and aesthetic aspects related to cosmetic and reconstructive gynecologic surgery.
Special considerations. The use of energy-based therapies in special populations, such as survivors of breast cancer or other gynecologic cancers, as well as women undergoing chemotherapy, radiation therapy, and hormonal manipulation (particularly with antiestrogenic SERMs and aromatase inhibitors) has not been adequately evaluated. A discussion of the risks, benefits, alternatives, and limited long-term outcome data for energy-based therapies in cancer survivors, as for all patients, must be included for adequate informed consent prior to undertaking these treatments.
Guidelines for appropriate tissue priming, laser settings, and concomitant energy-based technology with local hormone treatment (also known as laser-augmented drug delivery) need to be developed. Comparative long-term studies are needed to determine the safety and effectiveness of these technologies.
Caution advised. Given the lack of long-term safety and effectiveness data on energy-based therapies for the vague indications of vaginal laxity, and even for the well-defined conditions of stress UI and vulvovaginal atrophy, clinicians should exercise caution before promoting treatment, which can be expensive and is not without potential complications, such as vaginal pain, adhesive agglutination, and persistent dryness and dyspareunia.21
Fortunately, many randomized trials on various energy-based devices for gynecologic indications (GSM, stress UI, vaginal laxity, lichen sclerosus) are underway, and results from these studies will help inform future clinical practice and guideline development.
- Kaplan I, Goldman J, Ger R. The treatment of erosions of the uterine cervix by means of the CO2 laser. Obstet Gynecol. 1973;41:795-796.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy-based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Gaspar A, Addamo G, Brandi H. Vaginal fractional CO2 laser: a minimally invasive option for vaginal rejuvenation. Am J Cosmetic Surg. 2011;28:156-162.
- Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22:845-849.
- Benedetto AV. What's new in cosmetic dermatology. Dermatol Clin. 2019;37:117-128.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal rejuvenation or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed April 8, 2019.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Athanasiou S, Pitsouni E, Grigoradis T, et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause. 2019;26:248-255.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Russo E, et al. Long-term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148-152.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Ogrinc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47:689-697.
- Okui N. Comparison between erbium-doped yttrium aluminum garnet laser therapy and sling procedures in the treatment of stress and mixed urinary incontinence. World J Urol. 2018. doi:10.1007/s00345-018-2445-x.
- Blaganje M, Scepanovic D, Zgur L, et al. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153-158.
- Haylen BT, Maher CF, Barber MD, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecologic J. 2016;27:165-194.
- Garcia B, Pardo J. Academic cosmetic gynecology and energy-based therapies: ambiguities, explorations, and the FDA advisories. Int Urogynecol J. 2019;30:1-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al; IUGA Research and Development Committee. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. Neurourol Urodyn. 2019;38:1009-1023.
- Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26:423-427.
- Kaplan I, Goldman J, Ger R. The treatment of erosions of the uterine cervix by means of the CO2 laser. Obstet Gynecol. 1973;41:795-796.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy-based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Gaspar A, Addamo G, Brandi H. Vaginal fractional CO2 laser: a minimally invasive option for vaginal rejuvenation. Am J Cosmetic Surg. 2011;28:156-162.
- Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22:845-849.
- Benedetto AV. What's new in cosmetic dermatology. Dermatol Clin. 2019;37:117-128.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal rejuvenation or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed April 8, 2019.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Athanasiou S, Pitsouni E, Grigoradis T, et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause. 2019;26:248-255.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Russo E, et al. Long-term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148-152.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Ogrinc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47:689-697.
- Okui N. Comparison between erbium-doped yttrium aluminum garnet laser therapy and sling procedures in the treatment of stress and mixed urinary incontinence. World J Urol. 2018. doi:10.1007/s00345-018-2445-x.
- Blaganje M, Scepanovic D, Zgur L, et al. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153-158.
- Haylen BT, Maher CF, Barber MD, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecologic J. 2016;27:165-194.
- Garcia B, Pardo J. Academic cosmetic gynecology and energy-based therapies: ambiguities, explorations, and the FDA advisories. Int Urogynecol J. 2019;30:1-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al; IUGA Research and Development Committee. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. Neurourol Urodyn. 2019;38:1009-1023.
- Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26:423-427.
What works best for genitourinary syndrome of menopause: vaginal estrogen, vaginal laser, or combined laser and estrogen therapy?
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.

While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.

While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
Is mannitol a good alternative agent for evaluating ureteral patency after gynecologic surgery?
EXPERT COMMENTARY
Although the incidence of lower urinary tract and ureteral injury following gynecologic surgery is low, intraoperative identification of ureteral patency can prevent serious long-term sequelae. Since the indigo carmine shortage in 2014, US surgeons have searched for multiple alternative agents. Intravenous methylene blue is suboptimal due to its systemic adverse effects and the length of time for dye excretion in the urine.
Grimes and colleagues conducted a study to determine if there was any significant difference in surgeon satisfaction among 4 different alternatives to indigo carmine for intraoperative ureteral patency evaluation.
Related article:
Farewell to indigo carmine
Details of the study
The investigators conducted a randomized clinical trial of 130 women undergoing benign gynecologic or pelvic reconstructive surgery. Four different regimens were used for intraoperative ureteral evaluation: 1) oral phenazopyridine 200 mg, 2) intravenous sodium fluorescein 25 mg, 3) mannitol bladder distention, and 4) normal saline bladder distention.
Study outcomes. The primary outcome was surgeon satisfaction based on a 0 to 100 point visual analog scale rating (with 0 indicating strong agreement, 100 indicating disagreement). Secondary outcomes included ease of ureteral jet visualization, time to surgeon confidence of ureteral patency, and occurrence of adverse events over 6 weeks.
Surgeon satisfaction rating. The investigators found statistically significant physician satisfaction with the use of mannitol as a bladder distention medium over oral phenazopyridine, and slightly better satisfaction compared with the use of intravenous sodium fluorescein or normal saline distention. The median (range) visual analog scores for ureteral patency were phenazopyridine, 48 (0–83); sodium fluorescein 20 (0–82); mannitol, 0 (0–44); and normal saline, 23 (3–96) (P<.001).
There was no difference across the 4 groups in the timing to surgeon confidence of ureteral patency, length of cystoscopy (on average, 3 minutes), and development of postoperative urinary tract infections (UTIs).
Most dissatisfaction related to phenazopyridine is the fact that the resulting orange-stained urine can obscure the bladder mucosa.
One significant adverse event was a protocol deviation in which 1 patient received an incorrect dose of IV sodium fluorescein (500 mg) instead of the recommended 25-mg dose.
Related article:
Alternative options for visualizing ureteral patency during intraoperative cystoscopy
Study strengths and weaknesses
The strength of this study is in its randomized design and power. Its major weakness is surgeon bias, since the surgeons could not possibly be blinded to the method used.
The study confirms the problem that phenazopyridine makes the urine so orange that bladder mucosal lesions and de novo hematuria could be difficult to detect. Recommending mannitol as a hypertonic distending medium (as it is used in hysteroscopy procedures), however, may be premature. Prior studies have shown increased postoperative UTIs when 50% and 10% dextrose was used versus normal saline for cystoscopy.1,2 Since the Grimes study protocol did not include postoperative urine collection for cultures, more research on UTIs after mannitol use would be needed before surgeons confidently could use it routinely.
In our practice, surgeons prefer that intravenous sodium fluorescein be administered just prior to cystoscopy and oral phenazopyridine en route to the operating room. I agree that a major disadvantage to phenazopyridine is the heavy orange staining that obscures visualization.
Finally, this study did not account for cost of the various methods; standard normal saline would be cheapest, followed by phenazopyridine.
This study showed that surgeon satisfaction was greatest with the use of mannitol as a distending medium for intraoperative evaluation of ureteral patency compared with oral phenazopyridine, intravenous sodium fluorescein, and normal saline distention. However, time to surgeon confidence of ureteral patency was similar with all 4 methods. More data are needed related to UTIs and the cost of mannitol compared with the other 3 methods.
-- Cheryl B. Iglesia, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Narasimhulu DM, Prabakar C, Tang N, Bral P. 50% dextrose versus normal saline as distention media during cystoscopy for assessment of ureteric patency. Eur J Obstet Gynecol Reprod Biol. 2016;199:38–41.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM, Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–e6.
EXPERT COMMENTARY
Although the incidence of lower urinary tract and ureteral injury following gynecologic surgery is low, intraoperative identification of ureteral patency can prevent serious long-term sequelae. Since the indigo carmine shortage in 2014, US surgeons have searched for multiple alternative agents. Intravenous methylene blue is suboptimal due to its systemic adverse effects and the length of time for dye excretion in the urine.
Grimes and colleagues conducted a study to determine if there was any significant difference in surgeon satisfaction among 4 different alternatives to indigo carmine for intraoperative ureteral patency evaluation.
Related article:
Farewell to indigo carmine
Details of the study
The investigators conducted a randomized clinical trial of 130 women undergoing benign gynecologic or pelvic reconstructive surgery. Four different regimens were used for intraoperative ureteral evaluation: 1) oral phenazopyridine 200 mg, 2) intravenous sodium fluorescein 25 mg, 3) mannitol bladder distention, and 4) normal saline bladder distention.
Study outcomes. The primary outcome was surgeon satisfaction based on a 0 to 100 point visual analog scale rating (with 0 indicating strong agreement, 100 indicating disagreement). Secondary outcomes included ease of ureteral jet visualization, time to surgeon confidence of ureteral patency, and occurrence of adverse events over 6 weeks.
Surgeon satisfaction rating. The investigators found statistically significant physician satisfaction with the use of mannitol as a bladder distention medium over oral phenazopyridine, and slightly better satisfaction compared with the use of intravenous sodium fluorescein or normal saline distention. The median (range) visual analog scores for ureteral patency were phenazopyridine, 48 (0–83); sodium fluorescein 20 (0–82); mannitol, 0 (0–44); and normal saline, 23 (3–96) (P<.001).
There was no difference across the 4 groups in the timing to surgeon confidence of ureteral patency, length of cystoscopy (on average, 3 minutes), and development of postoperative urinary tract infections (UTIs).
Most dissatisfaction related to phenazopyridine is the fact that the resulting orange-stained urine can obscure the bladder mucosa.
One significant adverse event was a protocol deviation in which 1 patient received an incorrect dose of IV sodium fluorescein (500 mg) instead of the recommended 25-mg dose.
Related article:
Alternative options for visualizing ureteral patency during intraoperative cystoscopy
Study strengths and weaknesses
The strength of this study is in its randomized design and power. Its major weakness is surgeon bias, since the surgeons could not possibly be blinded to the method used.
The study confirms the problem that phenazopyridine makes the urine so orange that bladder mucosal lesions and de novo hematuria could be difficult to detect. Recommending mannitol as a hypertonic distending medium (as it is used in hysteroscopy procedures), however, may be premature. Prior studies have shown increased postoperative UTIs when 50% and 10% dextrose was used versus normal saline for cystoscopy.1,2 Since the Grimes study protocol did not include postoperative urine collection for cultures, more research on UTIs after mannitol use would be needed before surgeons confidently could use it routinely.
In our practice, surgeons prefer that intravenous sodium fluorescein be administered just prior to cystoscopy and oral phenazopyridine en route to the operating room. I agree that a major disadvantage to phenazopyridine is the heavy orange staining that obscures visualization.
Finally, this study did not account for cost of the various methods; standard normal saline would be cheapest, followed by phenazopyridine.
This study showed that surgeon satisfaction was greatest with the use of mannitol as a distending medium for intraoperative evaluation of ureteral patency compared with oral phenazopyridine, intravenous sodium fluorescein, and normal saline distention. However, time to surgeon confidence of ureteral patency was similar with all 4 methods. More data are needed related to UTIs and the cost of mannitol compared with the other 3 methods.
-- Cheryl B. Iglesia, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Although the incidence of lower urinary tract and ureteral injury following gynecologic surgery is low, intraoperative identification of ureteral patency can prevent serious long-term sequelae. Since the indigo carmine shortage in 2014, US surgeons have searched for multiple alternative agents. Intravenous methylene blue is suboptimal due to its systemic adverse effects and the length of time for dye excretion in the urine.
Grimes and colleagues conducted a study to determine if there was any significant difference in surgeon satisfaction among 4 different alternatives to indigo carmine for intraoperative ureteral patency evaluation.
Related article:
Farewell to indigo carmine
Details of the study
The investigators conducted a randomized clinical trial of 130 women undergoing benign gynecologic or pelvic reconstructive surgery. Four different regimens were used for intraoperative ureteral evaluation: 1) oral phenazopyridine 200 mg, 2) intravenous sodium fluorescein 25 mg, 3) mannitol bladder distention, and 4) normal saline bladder distention.
Study outcomes. The primary outcome was surgeon satisfaction based on a 0 to 100 point visual analog scale rating (with 0 indicating strong agreement, 100 indicating disagreement). Secondary outcomes included ease of ureteral jet visualization, time to surgeon confidence of ureteral patency, and occurrence of adverse events over 6 weeks.
Surgeon satisfaction rating. The investigators found statistically significant physician satisfaction with the use of mannitol as a bladder distention medium over oral phenazopyridine, and slightly better satisfaction compared with the use of intravenous sodium fluorescein or normal saline distention. The median (range) visual analog scores for ureteral patency were phenazopyridine, 48 (0–83); sodium fluorescein 20 (0–82); mannitol, 0 (0–44); and normal saline, 23 (3–96) (P<.001).
There was no difference across the 4 groups in the timing to surgeon confidence of ureteral patency, length of cystoscopy (on average, 3 minutes), and development of postoperative urinary tract infections (UTIs).
Most dissatisfaction related to phenazopyridine is the fact that the resulting orange-stained urine can obscure the bladder mucosa.
One significant adverse event was a protocol deviation in which 1 patient received an incorrect dose of IV sodium fluorescein (500 mg) instead of the recommended 25-mg dose.
Related article:
Alternative options for visualizing ureteral patency during intraoperative cystoscopy
Study strengths and weaknesses
The strength of this study is in its randomized design and power. Its major weakness is surgeon bias, since the surgeons could not possibly be blinded to the method used.
The study confirms the problem that phenazopyridine makes the urine so orange that bladder mucosal lesions and de novo hematuria could be difficult to detect. Recommending mannitol as a hypertonic distending medium (as it is used in hysteroscopy procedures), however, may be premature. Prior studies have shown increased postoperative UTIs when 50% and 10% dextrose was used versus normal saline for cystoscopy.1,2 Since the Grimes study protocol did not include postoperative urine collection for cultures, more research on UTIs after mannitol use would be needed before surgeons confidently could use it routinely.
In our practice, surgeons prefer that intravenous sodium fluorescein be administered just prior to cystoscopy and oral phenazopyridine en route to the operating room. I agree that a major disadvantage to phenazopyridine is the heavy orange staining that obscures visualization.
Finally, this study did not account for cost of the various methods; standard normal saline would be cheapest, followed by phenazopyridine.
This study showed that surgeon satisfaction was greatest with the use of mannitol as a distending medium for intraoperative evaluation of ureteral patency compared with oral phenazopyridine, intravenous sodium fluorescein, and normal saline distention. However, time to surgeon confidence of ureteral patency was similar with all 4 methods. More data are needed related to UTIs and the cost of mannitol compared with the other 3 methods.
-- Cheryl B. Iglesia, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Narasimhulu DM, Prabakar C, Tang N, Bral P. 50% dextrose versus normal saline as distention media during cystoscopy for assessment of ureteric patency. Eur J Obstet Gynecol Reprod Biol. 2016;199:38–41.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM, Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–e6.
- Narasimhulu DM, Prabakar C, Tang N, Bral P. 50% dextrose versus normal saline as distention media during cystoscopy for assessment of ureteric patency. Eur J Obstet Gynecol Reprod Biol. 2016;199:38–41.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM, Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–e6.
Advances in ablative and non-ablative lasers in gynecology: A clinician’s guide

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
The pelvic exam revisited
More than 44 million pelvic examinations are performed annually in the United States.1 In March 2017, the United States Preventive Services Task Force (USPSTF) published an updated recommendation statement regarding the need for routine screening pelvic examinations in asymptomatic adult women (18 years and older) receiving primary care: “The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of performing screening pelvic examinations in asymptomatic, nonpregnant adult women.”2
That statement, however, was assigned a grade of I, which means that evidence is lacking, of poor quality, or conflicting, and that the balance of benefits and harms cannot be determined. This USPSTF recommendation statement thus will not change practice for ObGyn providers but likely will renew our commitment to provide individualized well-woman care. There was inadequate or poor quality evidence for benefits related to all-cause mortality, disease-specific morbidity, and quality of life, as well as inadequate evidence on harms related to false-positive findings and anxiety stemming from screening pelvic exams.
Read about coding and billing for a standard pelvic exam
Melanie Witt, RN, MA
Coding and billing for the care provided at a well-woman visit can be uncomplicated if you know the right codes for the right program. The information presented here concerns straightforward preventive care and assumes that the patient also has not presented with a significant problem at the same visit.
First, a patient who is not Medicare-eligible might have insurance coverage for an annual preventive care examination every year. Normally, this service would be billed using the Current Procedural Terminology (CPT) preventive medicine codes, but some insurers require the use of special codes for an annual gynecologic exam. These special codes are:
- S0610, Annual gynecological examination, new patient
- S0612, Annual gynecological examination, established patient
- S0613, Annual gynecological examination; clinical breast examination without pelvic evaluation.
Notably, Aetna, Cigna, and UnitedHealthcare require these codes to signify that a pelvic examination has been performed (except for code S0613), but many Blue Cross Blue Shield programs, for whom these codes were originally created, are now reverting to the CPT preventive medicine codes for all preventive care.
CPT outlines the requirements for use of the preventive medicine codes as: an initial or periodic comprehensive preventive medicine evaluation or reevaluation and management (E/M) service, which includes an age- and gender-appropriate history, examination, counseling/anticipatory guidance/risk factor reduction interventions, and the ordering of laboratory/diagnostic procedures. The codes are divided into new or established patient categories by age range as follows:
The Medicare E/M documentation guidelines do not apply to preventive services, and a head-to-toe examination also is not required. CPT recognizes the American College of Obstetricians and Gynecologists (ACOG) as an authoritative body to make recommendations for the expected preventive service for women, and if such a service is provided and documented, the preventive care codes are to be reported. The payers who use the S codes for a gynecologic exam will require that a pelvic examination has been performed, but such an examination would not be required when using the CPT codes or ACOG's guidelines if the physician and patient agreed that such an exam was not warranted every year. The other components of a preventive service applicable to the female patient's age, however, should be documented in order to report the CPT codes for preventive medicine services.
If a pelvic examination is not performed, say because the patient is young and not sexually active, but an examination of other areas is carried out, the diagnosis code would change from Z01.411, Encounter for gynecological examination (general) (routine) with abnormal findings, or Z01.419, Encounter for gynecological examination (general) (routine) without abnormal findings, to a general health exam: Z00.00, Encounter for general adult medical examination without abnormal findings, or Z00.01, Encounter for general adult medical examination with abnormal findings.
What about Medicare?
Medicare requirements are somewhat different. First, Medicare covers only a small portion of the preventive care service; that is, it covers a physical examination of the genital organs and breasts and the collection and conveyance of a Pap specimen to the laboratory every 2 years for a low-risk patient. Second, the codes required to get reimbursed for the examination are:
- G0101, Cervical or vaginal cancer screening; pelvic and clinical breast examination
- Q0091, Screening Papanicolaou smear; obtaining, preparing, and conveyance of cervical or vaginal smear to laboratory.
It is not necessary to perform both of these services every 2 years (for instance, the patient may not need a Pap smear every 2 years based on her age and history), but the benefit is available if the service is performed. If the woman is at high risk for developing cervical or vaginal cancer, Medicare will cover this portion of the encounter every year so long as the Medicare-defined criteria for high risk have been documented at the time of the exam.
Related article:
GYN coding changes to note for your maximized reimbursement

Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read the authors’ interpretation of the new USPSTF statement
Interpreting the new USPSTF statement
We understand the USPSTF statement to mean that pelvic exams should not be abandoned, but rather should be individualized to each patient for her specific visit. We agree that for visits focused on counseling and routine screening in asymptomatic, nonpregnant women, pelvic exams likely will not increase the early detection and treatment of disease and more benefit likely would be derived by performing and discussing evidence-based and age-appropriate health services. A classic example would be for initiation or maintenance of oral contraception in an 18-year-old patient for whom an exam could cause unnecessary trauma, pain, or psychological distress leading to future avoidance or barriers to seeking health care. For long-acting reversible contraception placement, however, a pelvic exam clearly would be necessary for insertion of an intrauterine device.
Related article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Indications for pelvic examination
Remember that the pelvic examination has 3 distinct parts (and that not all parts need to be routinely conducted)3:
- general inspection of the external genitalia and vulva
- speculum examination and evaluation of the vagina and cervix
- bimanual examination with possible rectovaginal examination in age-appropriate or symptomatic women.
According to the Well-Woman Task Force of the American College of Obstetricians and Gynecologists (ACOG), “For women 21 years and older, external exam may be performed annually and that inclusion of speculum examination, bimanual examination, or both in otherwise healthy women should be a shared, informed decision between patient and provider.”4
Indications for performing certain parts of the pelvic exam include4:
- routine screening for cervical cancer (Pap test)
- routine screening for gonorrhea, chlamydia infection, and other sexually transmitted infections
- evaluation of abnormal vaginal discharge
- evaluation of abnormal bleeding, pelvic pain, and pelvic floor disorders, such as prolapse, urinary incontinence, and accidental bowel leakage
- evaluation of menopausal symptoms, such as dryness, dyspareunia, and the genitourinary syndrome of menopause
- evaluation of women at increased risk for gynecologic malignancy, such as women with known hereditary breast–ovarian cancer syndromes.
In 2016, ACOG launched the Women’s Preventive Services Initiative (WPSI) in conjunction with the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services. In this 5-year collaboration, the agencies are endeavoring to review and update the recommendations for women’s preventive health care services, including well-woman visits, human papillomavirus testing, and contraception, among many others.5 Once the HRSA adopts these recommendations, women will be able to access comprehensive preventive health services without incurring any out-of-pocket expenses.
Roshanak Mansouri Zinn, MD, and Rebekah L. Williams, MD, MS
No literature addresses the utility of screening pelvic examination in the pediatric and adolescent population. According to the American College of Obstetricians and Gynecologists Committee on Adolescent Health Care opinion on the initial reproductive health visit for screening and preventive reproductive health care (reaffirmed in 2016), a screening internal exam is not necessary, but an external genital exam may be indicated and may vary depending on the patient's concerns and prior clinical encounters.1 The American Academy of Pediatrics promotes annual screening external genital examination for all female patients as part of routine primary care, with internal examinations only as indicated.2
Age-appropriate pelvic examination for girls and nonsexually active adolescents usually is limited to an external genital exam to evaluate the anatomy and note the sexual maturity rating (Tanner stage), an important indicator of normal pubertal development. As in adults, the potential benefits of screening examination in this population include detection of benign gynecologic conditions (including vulvar skin conditions and abnormalities of hymenal or vaginal development). Additionally, early reproductive health visits are an important time for clinicians to build rapport with younger patients and to provide anticipatory education on menstruation, hygiene, and anatomy. These visits can destigmatize and demystify the pelvic examination and help young women seek care more appropriately and more comfortably if problems do arise.
Even when a pelvic exam is indicated, a patient's young age can give providers pause as to what type of exam to perform. Patients with vulvovaginal symptoms, abnormal vaginal bleeding, vaginal discharge, or pelvic or abdominal pain should receive complete evaluation with external genital examination. If external vaginal examination does not allow for complete assessment of the problem, the patient and provider can assess the likelihood of her tolerating an internal exam in the clinic versus undergoing vaginoscopy under sedation. Limited laboratory evaluation and transabdominal pelvic ultrasonography may provide sufficient information for appropriate clinical decision making and management without internal examination. If symptoms persist or do not respond to first-line treatment, an internal exam should be performed.
Patients of any age may experience anxiety or physical discomfort or may even delay or avoid seeking care because of fear of a pelvic exam. However, providers of reproductive health care for children and adolescents can offer early education, reassurance, and a more comfortable experience when pelvic examination is necessary in this population.
References
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care. Committee Opinion No. 598: Committee on Adolescent Health Care: the initial reproductive health visit. Obstet Gynecol. 2014;123(5):1143-1147.
- Braverman PK, Breech L; Committee on Adolescence. American Academy of Pediatrics. Clinical report: gynecologic examination for adolescents in the pediatric office setting. Pediatrics. 2010;126(3):583-590.

Dr. Mansouri Zinn is Assistant Professor, Department of Women's Health, University of Texas at Austin.

Dr. Williams is Assistant Professor, Clinical Pediatrics, Section of Adolescent Medicine, Indiana University School of Medicine, Indianapolis.

Developed in collaboration with the North American Society for Pediatric and Adolescent Gynecology
The authors report no financial relationships relevant to this article.
How will the USPSTF statement affect practice?
In an editorial in the Journal of the American Medical Association commenting on the USPSTF statement, McNicholas and Peipert stated, “Based on the recommendation from the task force, clinicians may ask whether the pelvic examination should be abandoned. The answer is not found in this recommendation statement, but instead in a renewed commitment to shared decision making.”6 We wholeheartedly agree with this statement. The health care provider and the patient should make the decision, taking into consideration the patient’s risk factors for gynecologic cancers and other conditions, her personal preferences, and her overall values.
This new USPSTF recommendation statement will not change how we currently practice, and the statement’s grade I rating should not impact insurance coverage for pelvic exams. Additionally, further research is needed to better elucidate the role of the pelvic exam at well-woman visits, with hopes of obtaining more precise guidelines from the USPSTF and ACOG.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2012 state and national summary tables. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2012_namcs_web_tables.pdf. Accessed May 11, 2017.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317(9):947–953.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 534: Well-woman visit. Obstet Gynecol. 2012;120(2 pt 1):421–424.
- Conry JA, Brown H. Well-Woman Task Force: components of the well-woman visit. Obstet Gynecol. 2015;126(4):697–701.
- American College of Obstetricians and Gynecologists. The Women’s Preventive Services Initiative (WPSI). https://www.womenspreventivehealth.org. Accessed May 11, 2017.
- McNicholas C, Peipert JF. Is it time to abandon the routine pelvic examination in asymptomatic nonpregnant women? JAMA. 2017;317(9):910–911.
More than 44 million pelvic examinations are performed annually in the United States.1 In March 2017, the United States Preventive Services Task Force (USPSTF) published an updated recommendation statement regarding the need for routine screening pelvic examinations in asymptomatic adult women (18 years and older) receiving primary care: “The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of performing screening pelvic examinations in asymptomatic, nonpregnant adult women.”2
That statement, however, was assigned a grade of I, which means that evidence is lacking, of poor quality, or conflicting, and that the balance of benefits and harms cannot be determined. This USPSTF recommendation statement thus will not change practice for ObGyn providers but likely will renew our commitment to provide individualized well-woman care. There was inadequate or poor quality evidence for benefits related to all-cause mortality, disease-specific morbidity, and quality of life, as well as inadequate evidence on harms related to false-positive findings and anxiety stemming from screening pelvic exams.
Read about coding and billing for a standard pelvic exam
Melanie Witt, RN, MA
Coding and billing for the care provided at a well-woman visit can be uncomplicated if you know the right codes for the right program. The information presented here concerns straightforward preventive care and assumes that the patient also has not presented with a significant problem at the same visit.
First, a patient who is not Medicare-eligible might have insurance coverage for an annual preventive care examination every year. Normally, this service would be billed using the Current Procedural Terminology (CPT) preventive medicine codes, but some insurers require the use of special codes for an annual gynecologic exam. These special codes are:
- S0610, Annual gynecological examination, new patient
- S0612, Annual gynecological examination, established patient
- S0613, Annual gynecological examination; clinical breast examination without pelvic evaluation.
Notably, Aetna, Cigna, and UnitedHealthcare require these codes to signify that a pelvic examination has been performed (except for code S0613), but many Blue Cross Blue Shield programs, for whom these codes were originally created, are now reverting to the CPT preventive medicine codes for all preventive care.
CPT outlines the requirements for use of the preventive medicine codes as: an initial or periodic comprehensive preventive medicine evaluation or reevaluation and management (E/M) service, which includes an age- and gender-appropriate history, examination, counseling/anticipatory guidance/risk factor reduction interventions, and the ordering of laboratory/diagnostic procedures. The codes are divided into new or established patient categories by age range as follows:

The Medicare E/M documentation guidelines do not apply to preventive services, and a head-to-toe examination also is not required. CPT recognizes the American College of Obstetricians and Gynecologists (ACOG) as an authoritative body to make recommendations for the expected preventive service for women, and if such a service is provided and documented, the preventive care codes are to be reported. The payers who use the S codes for a gynecologic exam will require that a pelvic examination has been performed, but such an examination would not be required when using the CPT codes or ACOG's guidelines if the physician and patient agreed that such an exam was not warranted every year. The other components of a preventive service applicable to the female patient's age, however, should be documented in order to report the CPT codes for preventive medicine services.
If a pelvic examination is not performed, say because the patient is young and not sexually active, but an examination of other areas is carried out, the diagnosis code would change from Z01.411, Encounter for gynecological examination (general) (routine) with abnormal findings, or Z01.419, Encounter for gynecological examination (general) (routine) without abnormal findings, to a general health exam: Z00.00, Encounter for general adult medical examination without abnormal findings, or Z00.01, Encounter for general adult medical examination with abnormal findings.
What about Medicare?
Medicare requirements are somewhat different. First, Medicare covers only a small portion of the preventive care service; that is, it covers a physical examination of the genital organs and breasts and the collection and conveyance of a Pap specimen to the laboratory every 2 years for a low-risk patient. Second, the codes required to get reimbursed for the examination are:
- G0101, Cervical or vaginal cancer screening; pelvic and clinical breast examination
- Q0091, Screening Papanicolaou smear; obtaining, preparing, and conveyance of cervical or vaginal smear to laboratory.
It is not necessary to perform both of these services every 2 years (for instance, the patient may not need a Pap smear every 2 years based on her age and history), but the benefit is available if the service is performed. If the woman is at high risk for developing cervical or vaginal cancer, Medicare will cover this portion of the encounter every year so long as the Medicare-defined criteria for high risk have been documented at the time of the exam.
Related article:
GYN coding changes to note for your maximized reimbursement

Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read the authors’ interpretation of the new USPSTF statement
Interpreting the new USPSTF statement
We understand the USPSTF statement to mean that pelvic exams should not be abandoned, but rather should be individualized to each patient for her specific visit. We agree that for visits focused on counseling and routine screening in asymptomatic, nonpregnant women, pelvic exams likely will not increase the early detection and treatment of disease and more benefit likely would be derived by performing and discussing evidence-based and age-appropriate health services. A classic example would be for initiation or maintenance of oral contraception in an 18-year-old patient for whom an exam could cause unnecessary trauma, pain, or psychological distress leading to future avoidance or barriers to seeking health care. For long-acting reversible contraception placement, however, a pelvic exam clearly would be necessary for insertion of an intrauterine device.
Related article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Indications for pelvic examination
Remember that the pelvic examination has 3 distinct parts (and that not all parts need to be routinely conducted)3:
- general inspection of the external genitalia and vulva
- speculum examination and evaluation of the vagina and cervix
- bimanual examination with possible rectovaginal examination in age-appropriate or symptomatic women.
According to the Well-Woman Task Force of the American College of Obstetricians and Gynecologists (ACOG), “For women 21 years and older, external exam may be performed annually and that inclusion of speculum examination, bimanual examination, or both in otherwise healthy women should be a shared, informed decision between patient and provider.”4
Indications for performing certain parts of the pelvic exam include4:
- routine screening for cervical cancer (Pap test)
- routine screening for gonorrhea, chlamydia infection, and other sexually transmitted infections
- evaluation of abnormal vaginal discharge
- evaluation of abnormal bleeding, pelvic pain, and pelvic floor disorders, such as prolapse, urinary incontinence, and accidental bowel leakage
- evaluation of menopausal symptoms, such as dryness, dyspareunia, and the genitourinary syndrome of menopause
- evaluation of women at increased risk for gynecologic malignancy, such as women with known hereditary breast–ovarian cancer syndromes.
In 2016, ACOG launched the Women’s Preventive Services Initiative (WPSI) in conjunction with the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services. In this 5-year collaboration, the agencies are endeavoring to review and update the recommendations for women’s preventive health care services, including well-woman visits, human papillomavirus testing, and contraception, among many others.5 Once the HRSA adopts these recommendations, women will be able to access comprehensive preventive health services without incurring any out-of-pocket expenses.
Roshanak Mansouri Zinn, MD, and Rebekah L. Williams, MD, MS
No literature addresses the utility of screening pelvic examination in the pediatric and adolescent population. According to the American College of Obstetricians and Gynecologists Committee on Adolescent Health Care opinion on the initial reproductive health visit for screening and preventive reproductive health care (reaffirmed in 2016), a screening internal exam is not necessary, but an external genital exam may be indicated and may vary depending on the patient's concerns and prior clinical encounters.1 The American Academy of Pediatrics promotes annual screening external genital examination for all female patients as part of routine primary care, with internal examinations only as indicated.2
Age-appropriate pelvic examination for girls and nonsexually active adolescents usually is limited to an external genital exam to evaluate the anatomy and note the sexual maturity rating (Tanner stage), an important indicator of normal pubertal development. As in adults, the potential benefits of screening examination in this population include detection of benign gynecologic conditions (including vulvar skin conditions and abnormalities of hymenal or vaginal development). Additionally, early reproductive health visits are an important time for clinicians to build rapport with younger patients and to provide anticipatory education on menstruation, hygiene, and anatomy. These visits can destigmatize and demystify the pelvic examination and help young women seek care more appropriately and more comfortably if problems do arise.
Even when a pelvic exam is indicated, a patient's young age can give providers pause as to what type of exam to perform. Patients with vulvovaginal symptoms, abnormal vaginal bleeding, vaginal discharge, or pelvic or abdominal pain should receive complete evaluation with external genital examination. If external vaginal examination does not allow for complete assessment of the problem, the patient and provider can assess the likelihood of her tolerating an internal exam in the clinic versus undergoing vaginoscopy under sedation. Limited laboratory evaluation and transabdominal pelvic ultrasonography may provide sufficient information for appropriate clinical decision making and management without internal examination. If symptoms persist or do not respond to first-line treatment, an internal exam should be performed.
Patients of any age may experience anxiety or physical discomfort or may even delay or avoid seeking care because of fear of a pelvic exam. However, providers of reproductive health care for children and adolescents can offer early education, reassurance, and a more comfortable experience when pelvic examination is necessary in this population.
References
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care. Committee Opinion No. 598: Committee on Adolescent Health Care: the initial reproductive health visit. Obstet Gynecol. 2014;123(5):1143-1147.
- Braverman PK, Breech L; Committee on Adolescence. American Academy of Pediatrics. Clinical report: gynecologic examination for adolescents in the pediatric office setting. Pediatrics. 2010;126(3):583-590.

Dr. Mansouri Zinn is Assistant Professor, Department of Women's Health, University of Texas at Austin.

Dr. Williams is Assistant Professor, Clinical Pediatrics, Section of Adolescent Medicine, Indiana University School of Medicine, Indianapolis.

Developed in collaboration with the North American Society for Pediatric and Adolescent Gynecology
The authors report no financial relationships relevant to this article.
How will the USPSTF statement affect practice?
In an editorial in the Journal of the American Medical Association commenting on the USPSTF statement, McNicholas and Peipert stated, “Based on the recommendation from the task force, clinicians may ask whether the pelvic examination should be abandoned. The answer is not found in this recommendation statement, but instead in a renewed commitment to shared decision making.”6 We wholeheartedly agree with this statement. The health care provider and the patient should make the decision, taking into consideration the patient’s risk factors for gynecologic cancers and other conditions, her personal preferences, and her overall values.
This new USPSTF recommendation statement will not change how we currently practice, and the statement’s grade I rating should not impact insurance coverage for pelvic exams. Additionally, further research is needed to better elucidate the role of the pelvic exam at well-woman visits, with hopes of obtaining more precise guidelines from the USPSTF and ACOG.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
More than 44 million pelvic examinations are performed annually in the United States.1 In March 2017, the United States Preventive Services Task Force (USPSTF) published an updated recommendation statement regarding the need for routine screening pelvic examinations in asymptomatic adult women (18 years and older) receiving primary care: “The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of performing screening pelvic examinations in asymptomatic, nonpregnant adult women.”2
That statement, however, was assigned a grade of I, which means that evidence is lacking, of poor quality, or conflicting, and that the balance of benefits and harms cannot be determined. This USPSTF recommendation statement thus will not change practice for ObGyn providers but likely will renew our commitment to provide individualized well-woman care. There was inadequate or poor quality evidence for benefits related to all-cause mortality, disease-specific morbidity, and quality of life, as well as inadequate evidence on harms related to false-positive findings and anxiety stemming from screening pelvic exams.
Read about coding and billing for a standard pelvic exam
Melanie Witt, RN, MA
Coding and billing for the care provided at a well-woman visit can be uncomplicated if you know the right codes for the right program. The information presented here concerns straightforward preventive care and assumes that the patient also has not presented with a significant problem at the same visit.
First, a patient who is not Medicare-eligible might have insurance coverage for an annual preventive care examination every year. Normally, this service would be billed using the Current Procedural Terminology (CPT) preventive medicine codes, but some insurers require the use of special codes for an annual gynecologic exam. These special codes are:
- S0610, Annual gynecological examination, new patient
- S0612, Annual gynecological examination, established patient
- S0613, Annual gynecological examination; clinical breast examination without pelvic evaluation.
Notably, Aetna, Cigna, and UnitedHealthcare require these codes to signify that a pelvic examination has been performed (except for code S0613), but many Blue Cross Blue Shield programs, for whom these codes were originally created, are now reverting to the CPT preventive medicine codes for all preventive care.
CPT outlines the requirements for use of the preventive medicine codes as: an initial or periodic comprehensive preventive medicine evaluation or reevaluation and management (E/M) service, which includes an age- and gender-appropriate history, examination, counseling/anticipatory guidance/risk factor reduction interventions, and the ordering of laboratory/diagnostic procedures. The codes are divided into new or established patient categories by age range as follows:

The Medicare E/M documentation guidelines do not apply to preventive services, and a head-to-toe examination also is not required. CPT recognizes the American College of Obstetricians and Gynecologists (ACOG) as an authoritative body to make recommendations for the expected preventive service for women, and if such a service is provided and documented, the preventive care codes are to be reported. The payers who use the S codes for a gynecologic exam will require that a pelvic examination has been performed, but such an examination would not be required when using the CPT codes or ACOG's guidelines if the physician and patient agreed that such an exam was not warranted every year. The other components of a preventive service applicable to the female patient's age, however, should be documented in order to report the CPT codes for preventive medicine services.
If a pelvic examination is not performed, say because the patient is young and not sexually active, but an examination of other areas is carried out, the diagnosis code would change from Z01.411, Encounter for gynecological examination (general) (routine) with abnormal findings, or Z01.419, Encounter for gynecological examination (general) (routine) without abnormal findings, to a general health exam: Z00.00, Encounter for general adult medical examination without abnormal findings, or Z00.01, Encounter for general adult medical examination with abnormal findings.
What about Medicare?
Medicare requirements are somewhat different. First, Medicare covers only a small portion of the preventive care service; that is, it covers a physical examination of the genital organs and breasts and the collection and conveyance of a Pap specimen to the laboratory every 2 years for a low-risk patient. Second, the codes required to get reimbursed for the examination are:
- G0101, Cervical or vaginal cancer screening; pelvic and clinical breast examination
- Q0091, Screening Papanicolaou smear; obtaining, preparing, and conveyance of cervical or vaginal smear to laboratory.
It is not necessary to perform both of these services every 2 years (for instance, the patient may not need a Pap smear every 2 years based on her age and history), but the benefit is available if the service is performed. If the woman is at high risk for developing cervical or vaginal cancer, Medicare will cover this portion of the encounter every year so long as the Medicare-defined criteria for high risk have been documented at the time of the exam.
Related article:
GYN coding changes to note for your maximized reimbursement

Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read the authors’ interpretation of the new USPSTF statement
Interpreting the new USPSTF statement
We understand the USPSTF statement to mean that pelvic exams should not be abandoned, but rather should be individualized to each patient for her specific visit. We agree that for visits focused on counseling and routine screening in asymptomatic, nonpregnant women, pelvic exams likely will not increase the early detection and treatment of disease and more benefit likely would be derived by performing and discussing evidence-based and age-appropriate health services. A classic example would be for initiation or maintenance of oral contraception in an 18-year-old patient for whom an exam could cause unnecessary trauma, pain, or psychological distress leading to future avoidance or barriers to seeking health care. For long-acting reversible contraception placement, however, a pelvic exam clearly would be necessary for insertion of an intrauterine device.
Related article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Indications for pelvic examination
Remember that the pelvic examination has 3 distinct parts (and that not all parts need to be routinely conducted)3:
- general inspection of the external genitalia and vulva
- speculum examination and evaluation of the vagina and cervix
- bimanual examination with possible rectovaginal examination in age-appropriate or symptomatic women.
According to the Well-Woman Task Force of the American College of Obstetricians and Gynecologists (ACOG), “For women 21 years and older, external exam may be performed annually and that inclusion of speculum examination, bimanual examination, or both in otherwise healthy women should be a shared, informed decision between patient and provider.”4
Indications for performing certain parts of the pelvic exam include4:
- routine screening for cervical cancer (Pap test)
- routine screening for gonorrhea, chlamydia infection, and other sexually transmitted infections
- evaluation of abnormal vaginal discharge
- evaluation of abnormal bleeding, pelvic pain, and pelvic floor disorders, such as prolapse, urinary incontinence, and accidental bowel leakage
- evaluation of menopausal symptoms, such as dryness, dyspareunia, and the genitourinary syndrome of menopause
- evaluation of women at increased risk for gynecologic malignancy, such as women with known hereditary breast–ovarian cancer syndromes.
In 2016, ACOG launched the Women’s Preventive Services Initiative (WPSI) in conjunction with the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services. In this 5-year collaboration, the agencies are endeavoring to review and update the recommendations for women’s preventive health care services, including well-woman visits, human papillomavirus testing, and contraception, among many others.5 Once the HRSA adopts these recommendations, women will be able to access comprehensive preventive health services without incurring any out-of-pocket expenses.
Roshanak Mansouri Zinn, MD, and Rebekah L. Williams, MD, MS
No literature addresses the utility of screening pelvic examination in the pediatric and adolescent population. According to the American College of Obstetricians and Gynecologists Committee on Adolescent Health Care opinion on the initial reproductive health visit for screening and preventive reproductive health care (reaffirmed in 2016), a screening internal exam is not necessary, but an external genital exam may be indicated and may vary depending on the patient's concerns and prior clinical encounters.1 The American Academy of Pediatrics promotes annual screening external genital examination for all female patients as part of routine primary care, with internal examinations only as indicated.2
Age-appropriate pelvic examination for girls and nonsexually active adolescents usually is limited to an external genital exam to evaluate the anatomy and note the sexual maturity rating (Tanner stage), an important indicator of normal pubertal development. As in adults, the potential benefits of screening examination in this population include detection of benign gynecologic conditions (including vulvar skin conditions and abnormalities of hymenal or vaginal development). Additionally, early reproductive health visits are an important time for clinicians to build rapport with younger patients and to provide anticipatory education on menstruation, hygiene, and anatomy. These visits can destigmatize and demystify the pelvic examination and help young women seek care more appropriately and more comfortably if problems do arise.
Even when a pelvic exam is indicated, a patient's young age can give providers pause as to what type of exam to perform. Patients with vulvovaginal symptoms, abnormal vaginal bleeding, vaginal discharge, or pelvic or abdominal pain should receive complete evaluation with external genital examination. If external vaginal examination does not allow for complete assessment of the problem, the patient and provider can assess the likelihood of her tolerating an internal exam in the clinic versus undergoing vaginoscopy under sedation. Limited laboratory evaluation and transabdominal pelvic ultrasonography may provide sufficient information for appropriate clinical decision making and management without internal examination. If symptoms persist or do not respond to first-line treatment, an internal exam should be performed.
Patients of any age may experience anxiety or physical discomfort or may even delay or avoid seeking care because of fear of a pelvic exam. However, providers of reproductive health care for children and adolescents can offer early education, reassurance, and a more comfortable experience when pelvic examination is necessary in this population.
References
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care. Committee Opinion No. 598: Committee on Adolescent Health Care: the initial reproductive health visit. Obstet Gynecol. 2014;123(5):1143-1147.
- Braverman PK, Breech L; Committee on Adolescence. American Academy of Pediatrics. Clinical report: gynecologic examination for adolescents in the pediatric office setting. Pediatrics. 2010;126(3):583-590.

Dr. Mansouri Zinn is Assistant Professor, Department of Women's Health, University of Texas at Austin.

Dr. Williams is Assistant Professor, Clinical Pediatrics, Section of Adolescent Medicine, Indiana University School of Medicine, Indianapolis.

Developed in collaboration with the North American Society for Pediatric and Adolescent Gynecology
The authors report no financial relationships relevant to this article.
How will the USPSTF statement affect practice?
In an editorial in the Journal of the American Medical Association commenting on the USPSTF statement, McNicholas and Peipert stated, “Based on the recommendation from the task force, clinicians may ask whether the pelvic examination should be abandoned. The answer is not found in this recommendation statement, but instead in a renewed commitment to shared decision making.”6 We wholeheartedly agree with this statement. The health care provider and the patient should make the decision, taking into consideration the patient’s risk factors for gynecologic cancers and other conditions, her personal preferences, and her overall values.
This new USPSTF recommendation statement will not change how we currently practice, and the statement’s grade I rating should not impact insurance coverage for pelvic exams. Additionally, further research is needed to better elucidate the role of the pelvic exam at well-woman visits, with hopes of obtaining more precise guidelines from the USPSTF and ACOG.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2012 state and national summary tables. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2012_namcs_web_tables.pdf. Accessed May 11, 2017.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317(9):947–953.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 534: Well-woman visit. Obstet Gynecol. 2012;120(2 pt 1):421–424.
- Conry JA, Brown H. Well-Woman Task Force: components of the well-woman visit. Obstet Gynecol. 2015;126(4):697–701.
- American College of Obstetricians and Gynecologists. The Women’s Preventive Services Initiative (WPSI). https://www.womenspreventivehealth.org. Accessed May 11, 2017.
- McNicholas C, Peipert JF. Is it time to abandon the routine pelvic examination in asymptomatic nonpregnant women? JAMA. 2017;317(9):910–911.
- Centers for Disease Control and Prevention. National Center for Health Statistics. National Ambulatory Medical Care Survey: 2012 state and national summary tables. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2012_namcs_web_tables.pdf. Accessed May 11, 2017.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for gynecologic conditions with pelvic examination: US Preventive Services Task Force recommendation statement. JAMA. 2017;317(9):947–953.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 534: Well-woman visit. Obstet Gynecol. 2012;120(2 pt 1):421–424.
- Conry JA, Brown H. Well-Woman Task Force: components of the well-woman visit. Obstet Gynecol. 2015;126(4):697–701.
- American College of Obstetricians and Gynecologists. The Women’s Preventive Services Initiative (WPSI). https://www.womenspreventivehealth.org. Accessed May 11, 2017.
- McNicholas C, Peipert JF. Is it time to abandon the routine pelvic examination in asymptomatic nonpregnant women? JAMA. 2017;317(9):910–911.
Fostering surgical innovation: The path forward

Click here to download the PDF.
Key learning objectives
The faculty for this roundtable aim to:
- Explain the process for bringing an innovation to market, including the roles of surgeon inventor, engineer, manufacturer, and industry
- Discuss best practices, based on lessons learned, when pursuing an innovative idea for patient care
- Articulate ways to improve upon the entire development process for new techniques, devices, etc, being brought to the FDA for possible approval and to market for patient use.

Click here to download the PDF.
Key learning objectives
The faculty for this roundtable aim to:
- Explain the process for bringing an innovation to market, including the roles of surgeon inventor, engineer, manufacturer, and industry
- Discuss best practices, based on lessons learned, when pursuing an innovative idea for patient care
- Articulate ways to improve upon the entire development process for new techniques, devices, etc, being brought to the FDA for possible approval and to market for patient use.

Click here to download the PDF.
Key learning objectives
The faculty for this roundtable aim to:
- Explain the process for bringing an innovation to market, including the roles of surgeon inventor, engineer, manufacturer, and industry
- Discuss best practices, based on lessons learned, when pursuing an innovative idea for patient care
- Articulate ways to improve upon the entire development process for new techniques, devices, etc, being brought to the FDA for possible approval and to market for patient use.
How do you break the ice with patients to ask about their sexual health?
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
In this Article
- Conversation icebreakers
- The sexual status examination
- When to refer