User login
Patient with newly diagnosed type 2 diabetes? Remember these steps
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8
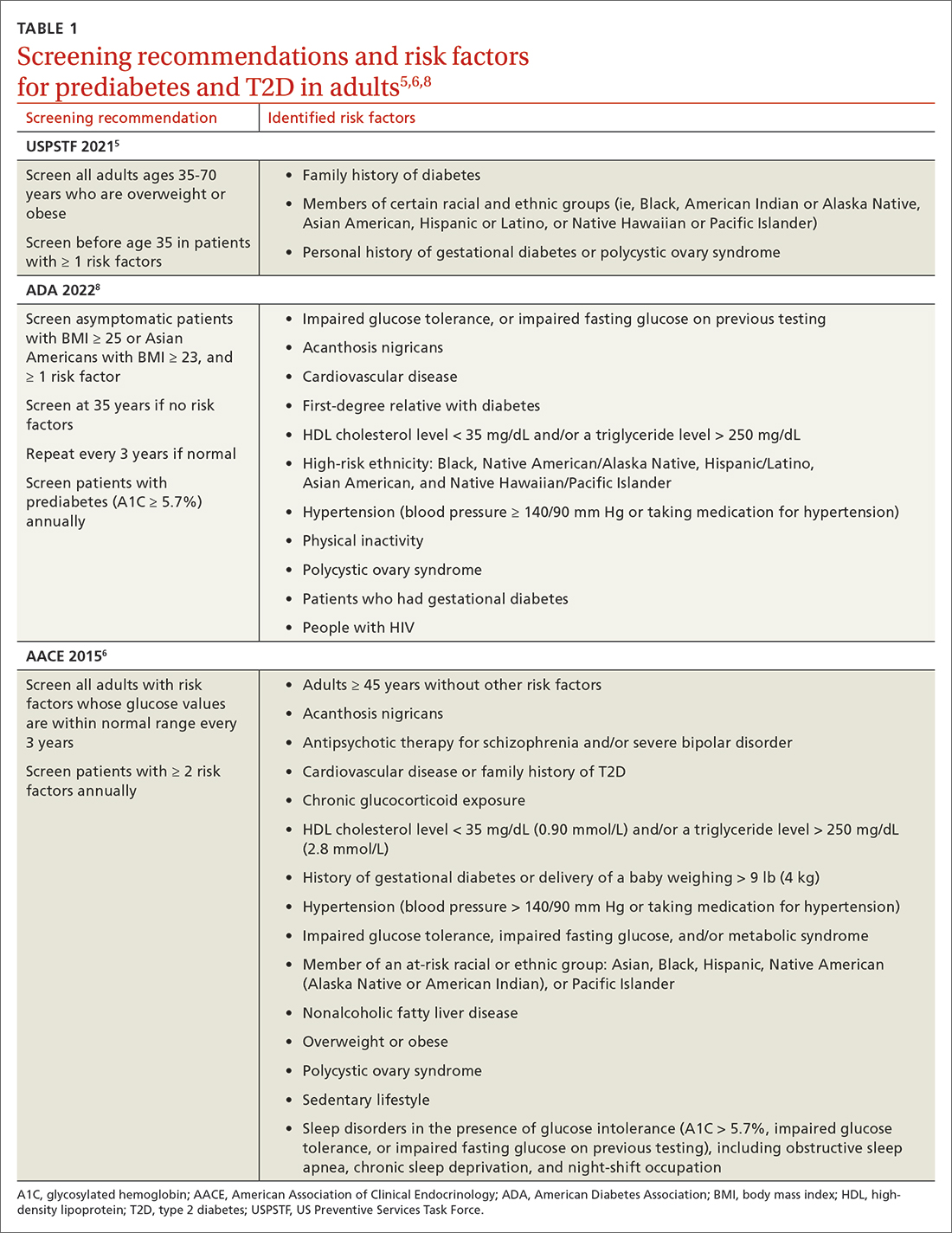
Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.
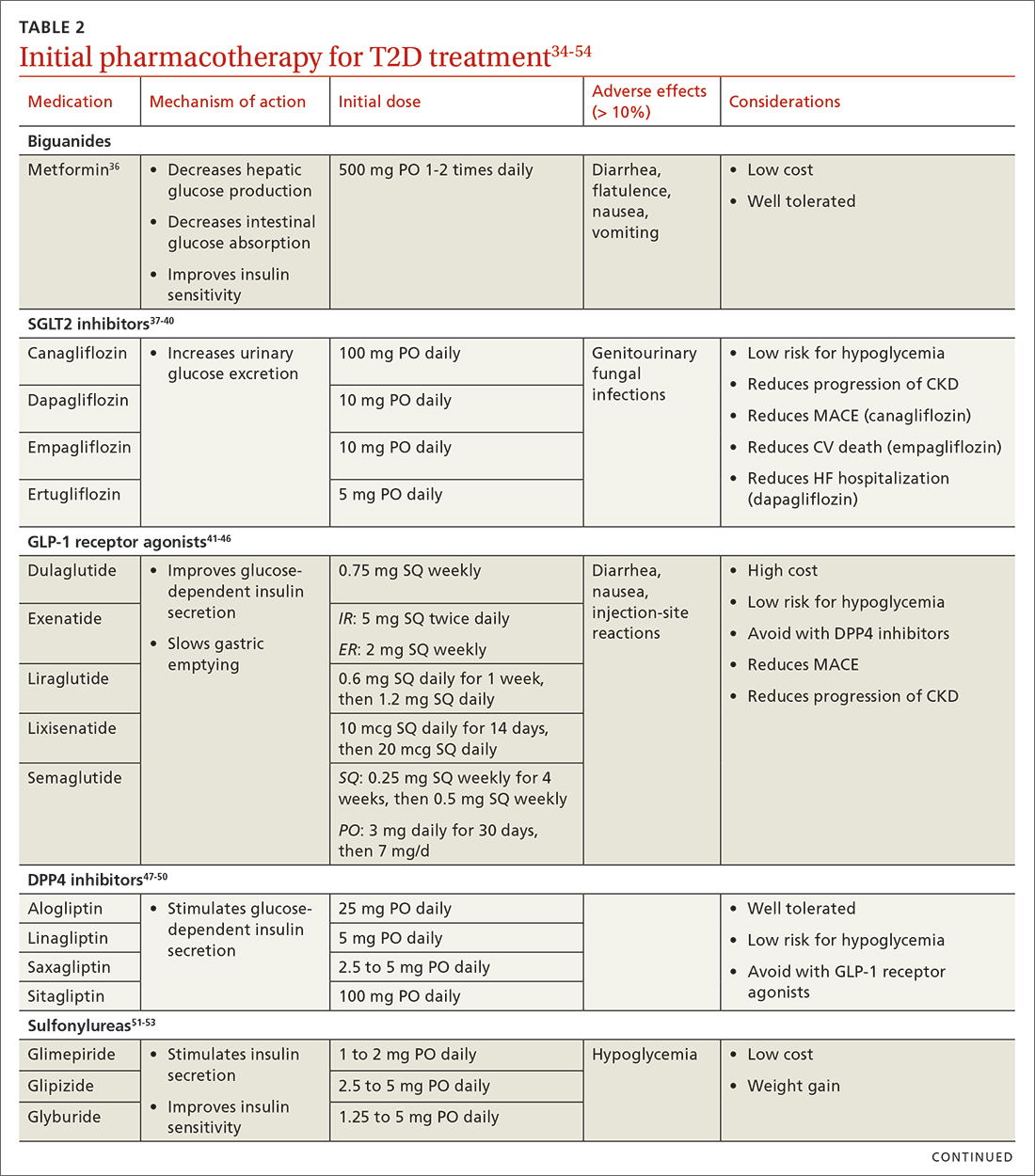
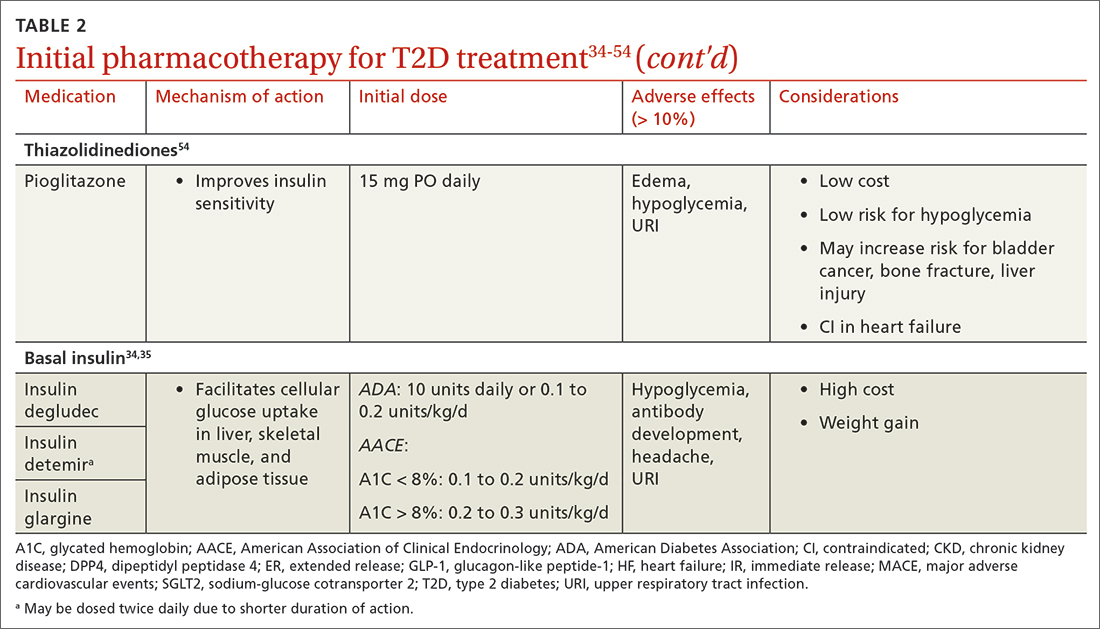
Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.
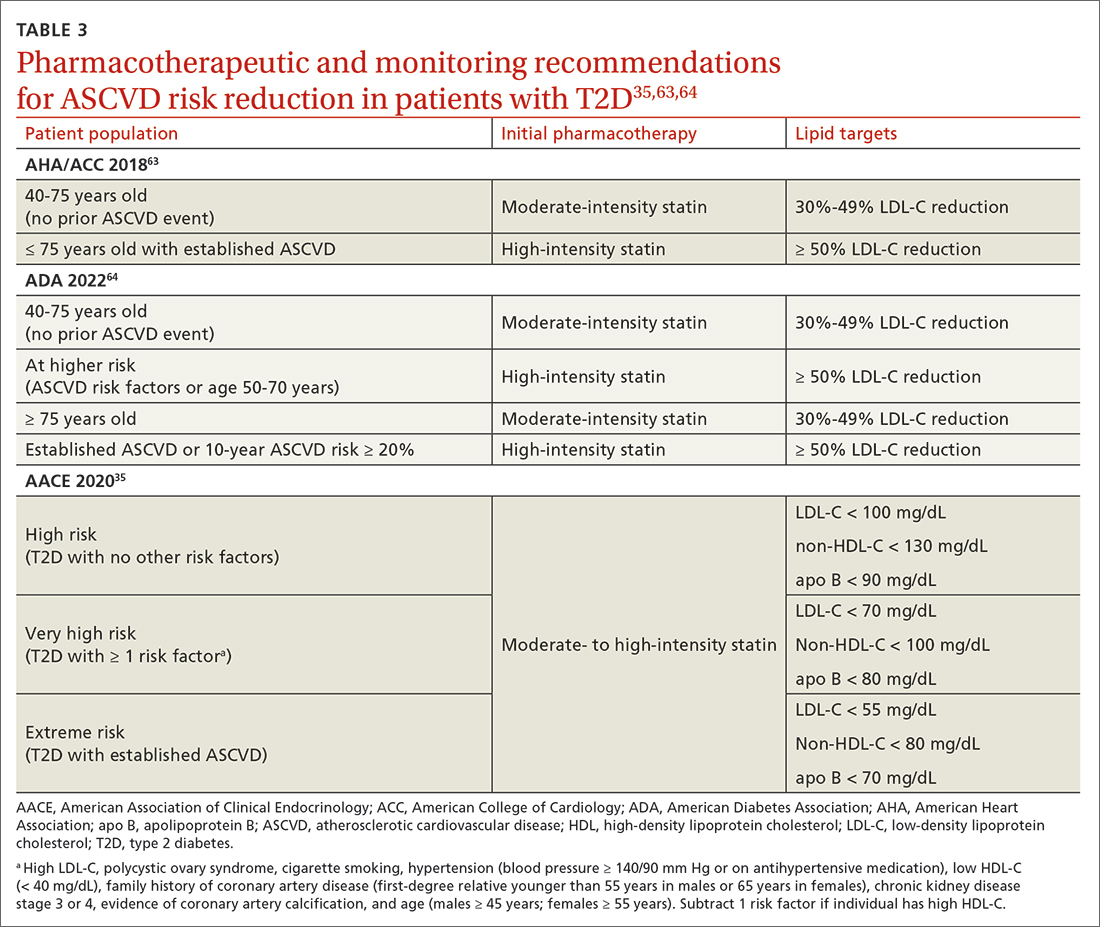
It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8

Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.


Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.

It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8

Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.


Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.

It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
PRACTICE RECOMMENDATIONS
› Individualize lifestyle modifications, considering personal and cultural experiences, health literacy, access to healthy foods, willingness and ability to make behavior changes, and barriers to change. C
› Initiate medication therapy at diagnosis, considering medication efficacy and cost, hypoglycemia risk, weight effects, benefits in cardiovascular and kidney disease, and patient-specific comorbidities. C
› Start basal insulin as first-line therapy in patients with severe baseline hyperglycemia, symptoms of hyperglycemia, or evidence of catabolism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
