User login
Patient with newly diagnosed type 2 diabetes? Remember these steps
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
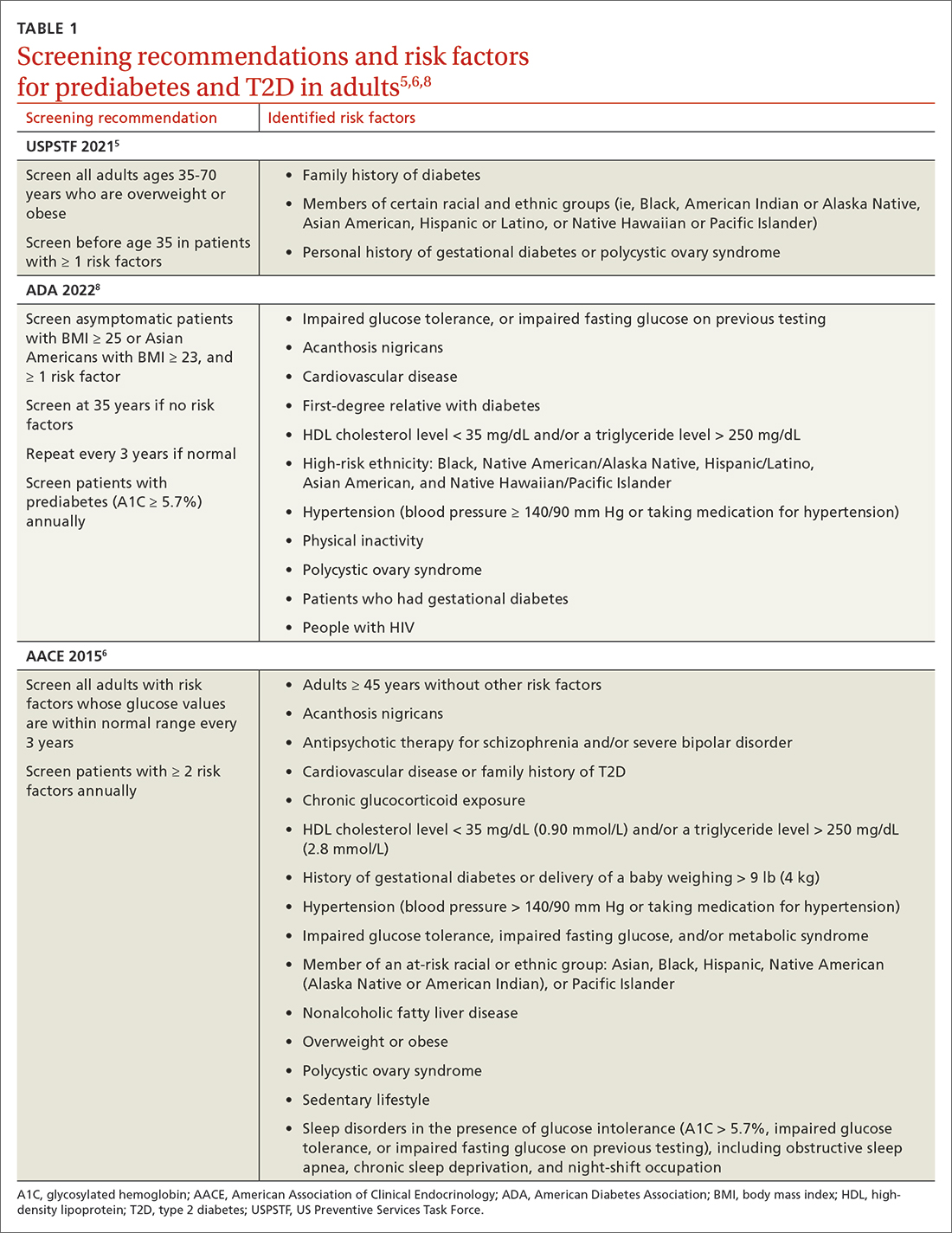
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8
Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
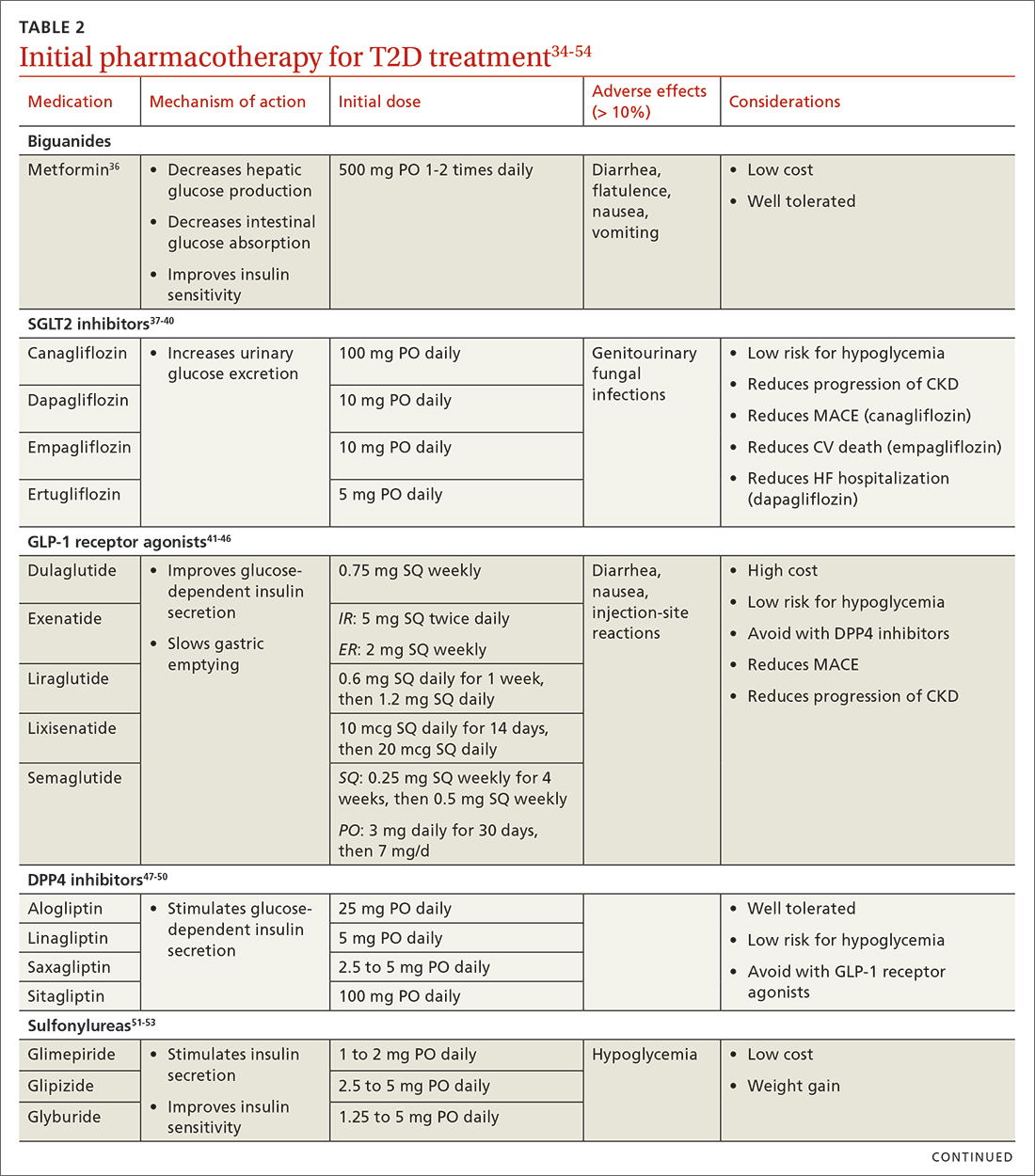
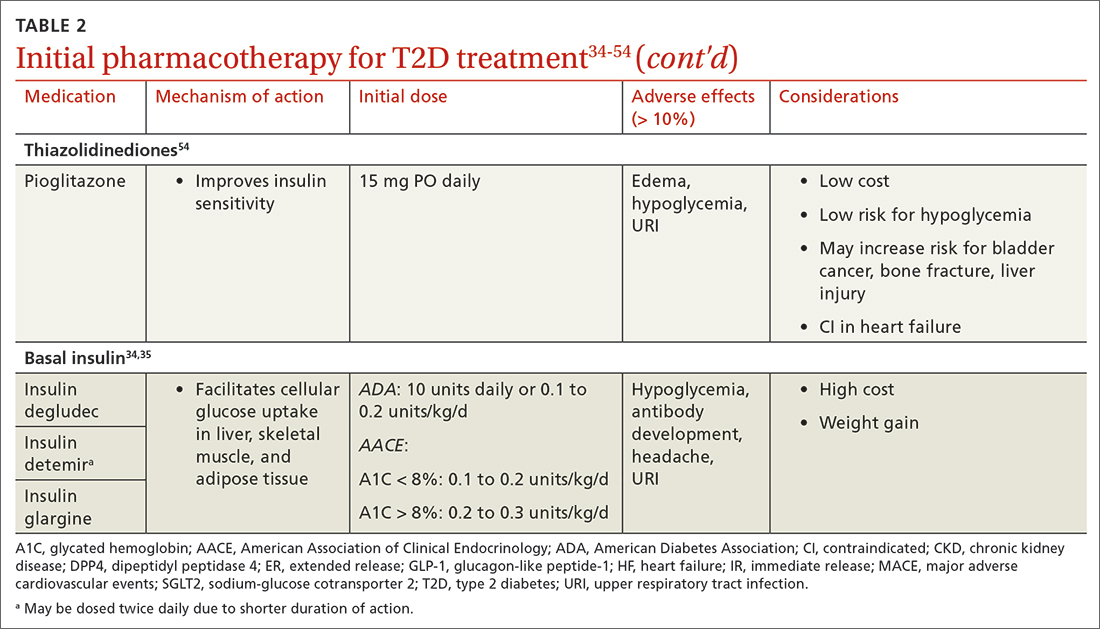
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.
Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
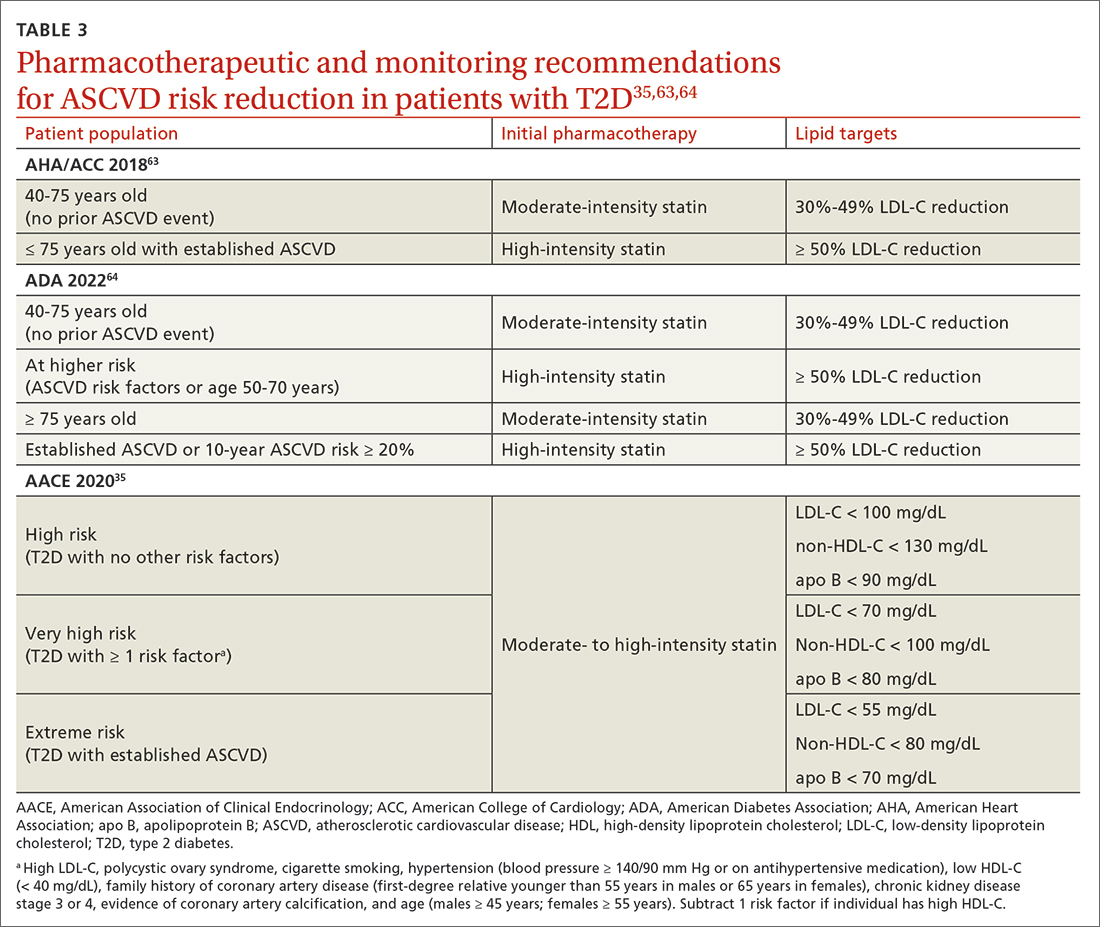
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.
It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8

Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.


Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.

It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8

Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.


Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.

It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
PRACTICE RECOMMENDATIONS
› Individualize lifestyle modifications, considering personal and cultural experiences, health literacy, access to healthy foods, willingness and ability to make behavior changes, and barriers to change. C
› Initiate medication therapy at diagnosis, considering medication efficacy and cost, hypoglycemia risk, weight effects, benefits in cardiovascular and kidney disease, and patient-specific comorbidities. C
› Start basal insulin as first-line therapy in patients with severe baseline hyperglycemia, symptoms of hyperglycemia, or evidence of catabolism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medication-assisted recovery for opioid use disorder: A guide
Medication-assisted recovery (MAR)—the preferred terminology for the service formerly known as medication-assisted treatment—entails a comprehensive set of interventions for managing opioid use disorder (OUD), including medications for opioid use disorder (MOUD). Despite the benefits of MAR—reducing opioid use, opioid-related mortality, and health care costs1-3—only 11% of patients with a diagnosis of OUD received MOUD in 2020.3
Primary care physicians, including family physicians, are well positioned to provide MAR across the patient’s lifespan. However, many family medicine clinicians do not possess the logistical knowledge or resources to implement this service.4 In this article, we describe options for, and barriers to, MAR and societal issues that have an impact on the care of these patients.
Pathophysiology of OUD
Opioids relieve pain by stimulating μ-opioid receptors and activating the brain’s reward system. These pleasurable effects motivate repeated use.5 Frequent opioid exposure causes neuroadaptation, tolerance, and dependence. For patients with OUD who are misusing illicit or prescription opioids, periods of abstinence following neuroadaptation lead to withdrawal symptoms that vary in intensity, depending on the drug, dose, and duration of use. Upregulated noradrenergic tone and dopamine deficiency manifest as numerous signs and symptoms of withdrawal, including5:
- Physiologic: secretory (diaphoresis, rhinorrhea, lacrimation, vomiting, diarrhea) and stimulatory (mydriasis, piloerection, hypertension, tachycardia, insomnia)
- Psychological: pain, cravings, dysphoria, anxiety.
A single episode of opioid withdrawal is not directly life-threatening, but untreated episodes can progressively amplify negative feedback and reinforce continued opioid use.6 Left untreated, withdrawal can be terminal.
Medication-assisted recovery: Effective intervention
MAR services that integrate medical, behavioral, and psychosocial programs can reduce mortality from OUD 2-fold.7,8 A meta-analysis found that, when MAR services are rendered in primary care, treatment retention improves by 25% (number needed to treat [NNT] = 6) and ongoing illicit opioid use is reduced by 50% (NNT = 6), relative to care at a specialty clinic9—highlighting a role for family medicine clinicians in treating OUD.
All 3 US Food and Drug Administration (FDA)–approved MOUD (methadone, buprenorphine, and naltrexone) reduce cravings; 2 (methadone and buprenorphine) mitigate withdrawal symptoms by activating the μ-opioid receptor; and naltrexone diminishes the reinforcing effects of use (TABLE10-12). It is crucial to recognize the pharmacologic distinctions among MOUD because untreated withdrawal syndromes increase dropout from treatment programs and subsequent relapse.13
The Hx of medication-assisted recovery
To understand the landscape of MAR, it is important to understand the history of opioid treatment in the United States. In 1966, Congress passed the Narcotic Addiction Rehabilitation Act (NARA), which secured federal assistance by which state and local governments could develop drug treatment programs.14 NARA permitted legal offenders with OUD to be civilly committed to treatment programs, rather than prosecuted. However, limited resources and a burgeoning population led, instead, to low-cost outpatient programs saddled by strict requirements that lacked a basis for improving clinical outcomes.
Continue to: At the time NARA...
At the time NARA was passed by Congress, OUD was viewed—inaccurately—as a criminal problem, not a medical one. Subsequent legislation was crafted through that lens, which has placed a heavy burden on patients until today.14 Although medical understanding of OUD has advanced tremendously over the past 50 years, treatment remains siloed from mainstream medicine, even in primary care.
There is no one-size-fits-all approach to MAR, and relapse is common. Patient-specific factors and the availability of resources should be considered when designing the most individualized, advantageous plan for MAR.
Methadone
Background. Methadone has the most extensive history for treating OUD and consistently has demonstrated efficacy.13 A meta-analysis of randomized controlled trials comparing methadone to nonpharmacotherapy alone found that methadone improved treatment retention by an absolute 57% (NNT = 2).10
Methadone was approved by the FDA for detoxification and maintenance treatment in the early 1970s, although the Narcotic Addict Treatment Act (NATA) of 1974 restricted dispensing of maintenance treatment to highly regulated clinics known as opioid treatment programs (OTPs).14 NATA required the treating physician to register with the US Drug Enforcement Agency (DEA) and to comply with conservative dosing regimens and observed dosing.
Over time, regulations evolved to give the physician greater flexibility in developing a care plan, allowing “take-home” doses, and improving patients’ access to care. Although access to methadone for the treatment of OUD remains limited to federally certified OTPs, regulations facilitate incorporation of a whole-person approach to care, including counseling, individual and group therapy, and toxicology testing.7
Continue to: Clinical considerations
Clinical considerations. Methadone requires slow titration. For patients starting methadone as an outpatient, federal law15 limits the initial dose to 30 mg and requires physician documentation when the first-day total dosage exceeds 40 mg. This dosing constraint makes it challenging to provide care because a daily dosage ≥ 60 mg has been found to produce, first, higher program retention (relative risk = 1.36; 95% CI, 1.13-1.63) and, second, greater reduction in illicit opioid use (relative risk = 1.59; 95% CI, 1.16-2.18) than is seen in patients who receive a lower daily dosage.16
Due to a prolonged elimination half-life, methadone reaches steady-state in 3 to 5 days. Patients and their families should be educated that withdrawal symptoms might not feel fully managed in the first few days of therapy and that time is required to experience safely the regimen’s full effects.
Aggressive dose-titration during methadone induction can result in drug accumulation and respiratory depression. The risk for methadone-related mortality is highest in the first 2 weeks of therapy, mostly related to overdose potential if the drug is combined with other opioids.17
Buprenorphine
Background. The prescribing rate for buprenorphine, particularly in primary care, is accelerating.18 A meta-analysis of randomized controlled trials found that11:
- compared to placebo, buprenorphine, at any dosage, improves treatment retention by an absolute 21% to 28% (NNT = 4-5)
- patients receiving high-dose buprenorphine (≥ 16 mg/d) had fewer evident cases of illicit opioid use.
Unlike methadone, buprenorphine exerts partial agonism at the μ-opioid receptor, resulting in a so-called ceiling effect that significantly reduces the adverse effect profile, including respiratory depression and euphoria, relative to a full-agonist opioid, such as methadone.19
Continue to: Whereas accessing methadone...
Whereas accessing methadone is limited to OTPs, buprenorphine is available for office-based treatment. By hosting OUD treatment and primary care in the same place, primary care physicians can provide comprehensive medical care including and beyond OUD, thereby improving retention and managing comorbidity.20
Integrated models involving support staff—eg, nurses, behavioral health providers, and pharmacists—have produced the greatest success with office-based treatment models.21 Office-based treatment normalizes OUD as a chronic disease managed by the primary care physician, enabling concurrent harm-reduction strategies; medication reconciliation; and convenient, regular prescribing intervals (eg, every 30 days).22 Nevertheless, access to buprenorphine is limited. Because buprenorphine is a controlled substance, the Ryan Haight Online Pharmacy Consumer Protection Act of 2008 prevents initial prescribing of buprenorphine without in-person evaluation. Telehealth consultations increased access to buprenorphine through temporary exceptions during the COVID-19 pandemic. However, revised rules and regulations for telehealth visits for these controlled substances are forthcoming from the DEA as temporary exceptions for telehealth consultations come to an end. Additionally, prescribing buprenorphine for OUD requires that the treating physician undergo specific training and obtain qualifications, which have evolved over time through federal legislation.
The Drug Addiction Treatment Act of 2000 (DATA 2000) authorized what is known as an X-waiver, which allows physicians to prescribe controlled substances for office-based treatment of OUD, provided that:
- they are registered to do so with the Substance Abuse and Mental Health Services Administration and the DEA
- they have had subspecialty training in addiction or completed an 8-hour training course
- they are able to refer patients to appropriate counseling and ancillary services.
DATA 2000 restricted patient panel sizes to 30 patients in the first year, expanding thereafter upon appropriate certification.
The Comprehensive Addiction and Recovery Act of 2016 (CARA) and the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act of 2018 (the SUPPORT Act) collectively extended prescribing authority for MOUD to other qualifying practitioners (eg, advanced practice clinicians). Despite these attempts to expand access to services, the overdose death rate has continued to increase.
Continue to: To further expand access to MAR...
To further expand access to MAR, the US Department of Health and Human Services updated its practice guidelines in April 2021, allowing clinicians to bypass X-waiver training requirements by applying for a notification-of-intent (NOI) buprenorphine waiver.a However, clinicians are still limited to prescribing buprenorphine for 30 patients at a time. Clinicians who undergo complete X-waiver training may prescribe for 100 patients in the first year and, if eligible, 275 patients thereafter.
In addition, as a component of the Consolidation Appropriations Act of 2023, Congress passed the Mainstreaming Addiction Treatment Act of 2021, or MAT 2021, and Medication Access and Training Expansion Act of 2021, or MATE 2021. MAT eliminated the X-waiver, NOI, and restrictions on the number of patients for whom a provider could prescribe buprenorphine, under federal authority; however, restrictions within one’s state might limit the ability to prescribe buprenorphine. MATE 2021 is an educational requirement for licensing by the DEA (at application and renewal) that will require prescribers to complete 8 hours of training in substance use disorders starting in June 2023.
Use of the monthly injectable extended-release buprenorphine productb is limited by an FDA Risk Evaluation and Mitigation Strategy (REMS) program, which requires specialized training and certification by the prescriber, distributor, and administering clinician. REMS reduces buprenorphine accessibility due to time, cost, and regulatory barriers; although such restrictions have been instituted with the patient’s safety in mind, any limitation to buprenorphine prescribing, apart from controlled substance licensure, serves only to limit access to a primary component of MAR.
Clinical considerations. Due to the competitive nature of buprenorphine and its high affinity for the μ-opioid receptor, the drug can displace other opioid agonists and precipitate acute withdrawal. The withdrawal experience can thereby condition fear and disfavor toward buprenorphine among patients.
It is vital, therefore, that (1) patients’ expectations for treatment be managed appropriately and (2) the treating physician be prepared to provide additional buprenorphine for adequate maintenance doses and utilize adjunct comfort agents (clonidine, nonsteroidal anti-inflammatory drugs, ondansetron) to manage acute withdrawal symptoms. Newer buprenorphine dosing strategies, such as micro-induction and macro-induction, have emerged to curtail these risks.23,24 This is an evolving area of MAR; newer low-threshold initiation strategies25 (see “Low-threshold MOUD prescribing models,” in the text that follows) and evidence that supports micro-induction26 might eliminate the practice of requiring active withdrawal for treatment.
Continue to: Regardless of the strategy...
Regardless of the strategy for dosing buprenorphine, it’s critical that patients be educated on how to initiate treatment outside a clinical setting, such as at home, where they occupy a familiar haven during a potentially uncomfortable time and can be as effective at initiation as they would be in a clinical setting, with no difference in precipitation of adverse effects.
At-home induction might be more appropriate for patients who are not yet in significant enough withdrawal while in the physician's office.27 Guidance should be provided on dosing instructions, self-assessment of withdrawal symptoms, and, if applicable, patience with the slow-dissolving sublingual tablet or film formulation.
Naltrexone
Background. Naltrexone is available as an oral tablet and an extended-release, once-monthly intramuscular injection; the latter has demonstrated superiority in MAR.28 Oral naltrexone has limited supporting evidence, is inferior to other MOUD options, and should not be used to treat OUD.7 Altogether, approval of naltrexone for OUD is controversial, due to potentially unethical trials and approval processes,29 although a multicenter randomized controlled trial demonstrated the drug’s noninferiority with respect to treatment retention relative to buprenorphine.30 Used over time, naltrexone does not relieve withdrawal symptoms but can reduce cravings.
Clinical considerations. There are numerous clinical barriers that limit the use of naltrexone.
First, patients should be abstinent from opioids for 7 to 14 days prior to starting therapy; usually, this means undergoing medically supervised withdrawal in a controlled environment. This is an obvious limitation for patients who are constrained financially—those who lack, or have inadequate, health insurance or are unable to be away from their job for an extended time.
Continue to: Second, because naltrexone...
Second, because naltrexone does not address withdrawal symptoms, supportive therapies should be incorporated into the treatment plan, including:
- clonidine for hyperadrenergic symptoms (anxiety, diaphoresis, hypertension)
- nonopioid analgesics for pain
- antiemetics, such as ondansetron and metoclopramide, for nausea or vomiting
- loperamide for diarrhea
- diphenhydramine for insomnia.
Third, patients taking naltrexone have a diminished response to opioids. This complicates pain management in the event of an emergent surgical procedure.
Last, when naltrexone wears off, patients are effectively opioid-naïve, which increases the risk for overdose in those who stop therapy abruptly.29 The increased risk for overdose should be communicated to all patients with OUD who are being treated with naltrexone.
This nonopioid option is appealing to policymakers and is often prioritized in the criminal justice system; however, the decreased efficacy of naltrexone (compared to methadone and buprenorphine), potential for overdose, and challenges in initiating treatment are concerning and limit the drug’s use in many real-world settings.
Because naltrexone is not a controlled substance, regulations regarding maintaining inventory and distribution are more flexible.
Continue to: Overall, the cost-effectiveness...
Overall, the cost-effectiveness of intramuscular naltrexone is unclear. State-administered insurance programs vary in their requirements for coverage of naltrexone treatment.31
Comprehensive medication reconciliation is vital
Overall fragmentation of care within OTPs places patients at risk for adverse events, such as drug interactions.32 Under Title 42 of the US Code,33 patients must provide written consent for an OTP provider to disclose their history of a substance use disorder. Allowing the patient to decide which medical providers can access their treatment records for an OUD benefits patient confidentiality but poses numerous issues worth exploring.
All prescribed controlled substances are recorded in the prescription drug monitoring program, or PDMP, a state-level electronic database accessible to health care professionals to inform prescribing decisions and identify drug interactions. The PDMP has substantially reduced opioid overprescribing and improved identification of patients at risk for overdose or misuse of opioids.
Unlike all other controlled substances, however, prescriptions ordered by an OTP are not recorded in the PDMP (although there are recent exceptions to this scenario). Without such information, a physician might not have important information about the patient when making medical decisions—placing the patient at risk for harmful outcomes, such as drug–drug and drug–disease interactions.
For example: Methadone is associated with a prolonged QT interval,34 increasing the risk for a fatal arrhythmia. Concurrent QT-prolonging medications, such as azithromycin and citalopram, further increase this risk.35 Because methadone dispensing is isolated from the patient’s medical record, the clinician who prescribes MOUD has an incomplete patient history and could make a potentially fatal treatment decision.
Continue to: Diversion is unlikely
Diversion is unlikely
Health care providers often express concern about diversion in MOUD. However, misuse and diversion rates of methadone and buprenorphine have declined steadily since 2011, and, in fact, are actually lower than the diversion rate of prescription antibiotics.36
Regardless, diversion of buprenorphine should not be a concern for physicians prescribing MOUD. Although a prescriber might worry about manipulation of the formulation of buprenorphine for intravenous administration, addition of naloxone to buprenorphine in tablet form diminishes the potential for overdose. Additionally, the ceiling effect of buprenorphine limits the likelihood of significant respiratory depression and euphoria.
Should buprenorphine reach a patient for whom it was not prescribed, it is highly unlikely that an overdose would result. Rather, the medication would protect against the effects of illicit opioids and relieve withdrawal symptoms. Most people with OUD who have misused buprenorphine have done so to relieve withdrawal symptoms,37 not to experience intoxication.
Health care deserts
So-called health care deserts in parts of the United States are an ongoing problem that disproportionately affects lower-income and segregated Black and Hispanic communities38—communities that shoulder the highest burden of OUD and OUD-related mortality39 and whose populace is in greatest need of MAR. Even when health care is accessible in such a desert, some clinicians and pharmacies refuse to prescribe or dispense MOUD because of the accompanying stigma of OUD.
A MAR desert, like a pharmacy desert, is a geographic region—one without access to a MAR or an OTP provider, thereby preventing patients from reaching appropriate care; for some patients, having to travel to the nearest provider can render treatment inaccessible.40
Continue to: Efforts are in place to identify...
Efforts are in place to identify areas at greatest need of OUD-related medical services, such as heat maps that identify areas of increased utilization of emergency medical services for opioid overdose. State-run programs have been implemented to increase access, such as the Illinois Helpline (https://helplineil.org) that provides support and resources for patients, friends, family, and providers.
Novel solutions
Key strategies to increase access to care and slow the opioid epidemic include low-threshold prescribing of MOUD and mobile OTPs.41
Low-threshold MOUD prescribing models. Adoption of one of these models in a medical practice that provides MAR might increase absolute enrollment. A low-threshold prescribing model involves42:
- same-day treatment
- leniency with respect to abstinence periods and a concomitant substance use disorder
- enhanced accessibility to MOUD through nontraditional medical settings.
Low-threshold prescribing is flexible in regard to patients’ needs and bypasses many of the barriers discussed in this article. Impressive multicenter success has been achieved by the CA Bridge program in California (https://cabridge.org), including an increase in recognition of OUD, treatment initiations, and outpatient engagement.25
The cost-effectiveness of low-threshold MOUD prescribing programs remains to be determined.
Mobile OTPs. In July 2021, the DEA authorized a mobile component to existing OTP registrants that is permitted to dispense methadone and buprenorphine. Mobile units are physically separate from the OTP but have similar functions, depending on available space. Services that cannot be provided on the mobile unit of an OTP must be available at its brick-and-mortar location.7 Logistically, OTP registrants no longer need a separate registration to implement a mobile unit, thus expanding care to patients in underserved or remote areas who often encounter barriers to access.43
Conclusion
Understanding the distinct clinical and accessibility benefits and limitations among available MOUD is essential for prescribing clinicians. Accessing treatment is limited by federal regulation, stigma, and the existence of health care deserts that limit access to necessary care for patients with OUD. Newer harm-reduction models, such as low-threshold prescribing and mobile OTPs, represent progress, but many patients remain untreated.
a At buprenorphine.samhsa.gov/forms/select-practitioner-type.php
b Sold under the brand name Sublocade.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, Department of Pharmacy Practice, University of Illinois Chicago College of Pharmacy, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235-S248.
2. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462-468. doi: 10.1111/j.1360-0443.2007.02090.x
3. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. HHS Publication PEP21-07-01-003, NSDUH Series H-56. 2021. Accessed March 19, 2023. www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
4. Haffajee RL, Andraka-Christou B, Attermann J, et al. A mixed-method comparison of physician-reported beliefs about and barriers to treatment with medications for opioid use disorder. Subst Abuse Treat Prev Policy. 2020;15:69. doi: 10.1186/s13011-020-00312-3
5. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20. doi: 10.1151/spp021113
6. Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44-53. doi: 10.1016/j.biopsych.2019.05.023
7. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. For Health care and Addiction Professionals, Policymakers, Patients, and Families. Treatment Improvement Protocol TIP 63. Publication No. PEP21-02-01-002. 2021. Accessed March 19, 2023. https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf
8. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550
9. Korownyk C, Perry D, Ton J, et al. Opioid use disorder in primary care: PEER umbrella systematic review of systematic reviews. Can Fam Physician. 2019;65:e194-e206.
10. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2
11. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4
12. Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513. doi: 10.1016/S0140-6736(11)60358-9
13. Soyka M, Zingg C, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641-653. doi: 10.1017/S146114570700836X
14. Institute of Medicine Committee on Federal Regulation of Methadone Treatment; Rettig R, Yarmolinsky A, eds. Federal Regulation of Methadone Treatment. National Academies Press; 1995.
15. 42 eCFR §8. Medication assisted treatment for opioid use disorders. Revised March 15, 2023. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8?toc=1
16. Faggiano F, Vigna-Taglianti F, Versino E, et al. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;(3):CD002208. doi: 10.1002/14651858.CD002208
17. Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377-386. doi: 10.1097/01.ADM.0000435321.39251.d7
18. Olfson M, Zhang VS, Schoenbaum M, et al. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323:276-277. doi: 10.1001/jama.2019.18913
19. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580. doi: 10.1038/clpt.1994.71
20. Walley AY, Palmisano J, Sorensen-Alawad A, et al. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J Subst Abuse Treat. 2015;59:59-66. doi: 10.1016/j.jsat.2015.07.007
21. Lagisetty P, Klasa K, Bush C, et al. Primary care models for treating opioid use disorders: what actually works? A systematic review. PloS One. 2017;12:e0186315. doi: 10.1371/journal.pone.0186315
22. Du CX, Shi J, Tetrault JM, et al. Primary care and medication management characteristics among patients receiving office-based opioid treatment with buprenorphine. Fam Pract. 2022;39:234-240. doi: 10.1093/fampra/cmab166
23. Herring AA, Vosooghi AA, Luftig J, et al. High-dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4:e2117128. doi: 10.1001/jamanetworkopen.2021.17128
24. Hämmig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105. doi: 10.2147/SAR.S109919
25. Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA Bridge Program. Ann Emerg Med. 2021;78:759-772. doi: 10.1016/j.annemergmed.2021.05.024
26. Wong JSH, Nikoo M, Westenberg JN, et al. Comparing rapid micro-induction and standard induction of buprenorphine/naloxone for treatment of opioid use disorder: protocol for an open-label, parallel-group, superiority, randomized controlled trial. Addict Sci Clin Pract. 2021;16:11. doi: 10.1186/s13722-021-00220-2
27. Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med. 2014;8:299-308. doi: 10.1097/ADM.0000000000000059
28. Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973-981. doi: 10.1001/archgenpsychiatry.2012.1a
29. Wolfe D, Carrieri MP, Dasgupta N, et al. Concerns about injectable naltrexone for opioid dependence. Lancet. 2011;377:1468-1470. doi: 10.1016/S0140-6736(10)62056-9
30. Tanum L, Solli KK, Latif ZEH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine–naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205. doi: 10.1001/jamapsychiatry.2017.3206
31. Murphy SM, Polsky D, Lee JD, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal justice-involved individuals with a history of opioid use disorder. Addiction. 2017;112:1440-1450. doi: 10.1111/add.13807
32. Ferrari A, Coccia CPR, Bertolini A, et al. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551-559. doi: 10.1016/j.phrs.2004.05.002
33. 42 eCFR Part 2. Confidentiality of substance use disorder patient records. January 18, 2017. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-2
34. Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015;110:1468-1475. doi: 10.1111/add.13013
35. Tisdale JE, Chung MK, Campbell KB, et al; . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214-e233. doi: 10.1161/CIR.0000000000000905
36. Leshner AI, Mancher M, eds. Barriers to broader use of medications to treat opioid use disorder. In: Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019:109-136.
37. Chilcoat HD, Amick HR, Sherwood MR, et al. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157. doi: 10.1016/j.jsat. 2019.07.005
38. Qato DM, Daviglus ML, Wilder J, et al. “Pharmacy deserts” are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33:1958-1965. doi: 10.1377/hlthaff.2013.1397
39. Mason M, Soliman R, Kim HS, et al. Disparities by sex and race and ethnicity in death rates due to opioid overdose among adults 55 years or older, 1999 to 2019. JAMA Netw Open. 2022;5:e2142982. doi: 10.1001/jamanetworkopen.2021.42982
40. Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789
41. Chan B, Hoffman KA, Bougatsos C, et al. Mobile methadone medication units: a brief history, scoping review and research opportunity. J Subst Abuse Treat. 2021;129:108483. doi: 10.1016/j.jsat.2021.108483
42. Jakubowski A, Fox A. Defining low-threshold buprenorphine treatment. J Addict Med. 2020;14:95-98. doi: 10.1097/ADM.0000000000000555
43. Messmer SE, Elmes AT, Jimenez AD, et al. Outcomes of a mobile medical unit for low-threshold buprenorphine access targeting opioid overdose hot spots in Chicago. J Subst Use Addict Treat. 2023;209054. doi: 10.1016/j.josat.2023.209054
Medication-assisted recovery (MAR)—the preferred terminology for the service formerly known as medication-assisted treatment—entails a comprehensive set of interventions for managing opioid use disorder (OUD), including medications for opioid use disorder (MOUD). Despite the benefits of MAR—reducing opioid use, opioid-related mortality, and health care costs1-3—only 11% of patients with a diagnosis of OUD received MOUD in 2020.3
Primary care physicians, including family physicians, are well positioned to provide MAR across the patient’s lifespan. However, many family medicine clinicians do not possess the logistical knowledge or resources to implement this service.4 In this article, we describe options for, and barriers to, MAR and societal issues that have an impact on the care of these patients.
Pathophysiology of OUD
Opioids relieve pain by stimulating μ-opioid receptors and activating the brain’s reward system. These pleasurable effects motivate repeated use.5 Frequent opioid exposure causes neuroadaptation, tolerance, and dependence. For patients with OUD who are misusing illicit or prescription opioids, periods of abstinence following neuroadaptation lead to withdrawal symptoms that vary in intensity, depending on the drug, dose, and duration of use. Upregulated noradrenergic tone and dopamine deficiency manifest as numerous signs and symptoms of withdrawal, including5:
- Physiologic: secretory (diaphoresis, rhinorrhea, lacrimation, vomiting, diarrhea) and stimulatory (mydriasis, piloerection, hypertension, tachycardia, insomnia)
- Psychological: pain, cravings, dysphoria, anxiety.
A single episode of opioid withdrawal is not directly life-threatening, but untreated episodes can progressively amplify negative feedback and reinforce continued opioid use.6 Left untreated, withdrawal can be terminal.

Medication-assisted recovery: Effective intervention
MAR services that integrate medical, behavioral, and psychosocial programs can reduce mortality from OUD 2-fold.7,8 A meta-analysis found that, when MAR services are rendered in primary care, treatment retention improves by 25% (number needed to treat [NNT] = 6) and ongoing illicit opioid use is reduced by 50% (NNT = 6), relative to care at a specialty clinic9—highlighting a role for family medicine clinicians in treating OUD.
All 3 US Food and Drug Administration (FDA)–approved MOUD (methadone, buprenorphine, and naltrexone) reduce cravings; 2 (methadone and buprenorphine) mitigate withdrawal symptoms by activating the μ-opioid receptor; and naltrexone diminishes the reinforcing effects of use (TABLE10-12). It is crucial to recognize the pharmacologic distinctions among MOUD because untreated withdrawal syndromes increase dropout from treatment programs and subsequent relapse.13

The Hx of medication-assisted recovery
To understand the landscape of MAR, it is important to understand the history of opioid treatment in the United States. In 1966, Congress passed the Narcotic Addiction Rehabilitation Act (NARA), which secured federal assistance by which state and local governments could develop drug treatment programs.14 NARA permitted legal offenders with OUD to be civilly committed to treatment programs, rather than prosecuted. However, limited resources and a burgeoning population led, instead, to low-cost outpatient programs saddled by strict requirements that lacked a basis for improving clinical outcomes.
Continue to: At the time NARA...
At the time NARA was passed by Congress, OUD was viewed—inaccurately—as a criminal problem, not a medical one. Subsequent legislation was crafted through that lens, which has placed a heavy burden on patients until today.14 Although medical understanding of OUD has advanced tremendously over the past 50 years, treatment remains siloed from mainstream medicine, even in primary care.
There is no one-size-fits-all approach to MAR, and relapse is common. Patient-specific factors and the availability of resources should be considered when designing the most individualized, advantageous plan for MAR.
Methadone
Background. Methadone has the most extensive history for treating OUD and consistently has demonstrated efficacy.13 A meta-analysis of randomized controlled trials comparing methadone to nonpharmacotherapy alone found that methadone improved treatment retention by an absolute 57% (NNT = 2).10
Methadone was approved by the FDA for detoxification and maintenance treatment in the early 1970s, although the Narcotic Addict Treatment Act (NATA) of 1974 restricted dispensing of maintenance treatment to highly regulated clinics known as opioid treatment programs (OTPs).14 NATA required the treating physician to register with the US Drug Enforcement Agency (DEA) and to comply with conservative dosing regimens and observed dosing.
Over time, regulations evolved to give the physician greater flexibility in developing a care plan, allowing “take-home” doses, and improving patients’ access to care. Although access to methadone for the treatment of OUD remains limited to federally certified OTPs, regulations facilitate incorporation of a whole-person approach to care, including counseling, individual and group therapy, and toxicology testing.7
Continue to: Clinical considerations
Clinical considerations. Methadone requires slow titration. For patients starting methadone as an outpatient, federal law15 limits the initial dose to 30 mg and requires physician documentation when the first-day total dosage exceeds 40 mg. This dosing constraint makes it challenging to provide care because a daily dosage ≥ 60 mg has been found to produce, first, higher program retention (relative risk = 1.36; 95% CI, 1.13-1.63) and, second, greater reduction in illicit opioid use (relative risk = 1.59; 95% CI, 1.16-2.18) than is seen in patients who receive a lower daily dosage.16
Due to a prolonged elimination half-life, methadone reaches steady-state in 3 to 5 days. Patients and their families should be educated that withdrawal symptoms might not feel fully managed in the first few days of therapy and that time is required to experience safely the regimen’s full effects.
Aggressive dose-titration during methadone induction can result in drug accumulation and respiratory depression. The risk for methadone-related mortality is highest in the first 2 weeks of therapy, mostly related to overdose potential if the drug is combined with other opioids.17
Buprenorphine
Background. The prescribing rate for buprenorphine, particularly in primary care, is accelerating.18 A meta-analysis of randomized controlled trials found that11:
- compared to placebo, buprenorphine, at any dosage, improves treatment retention by an absolute 21% to 28% (NNT = 4-5)
- patients receiving high-dose buprenorphine (≥ 16 mg/d) had fewer evident cases of illicit opioid use.
Unlike methadone, buprenorphine exerts partial agonism at the μ-opioid receptor, resulting in a so-called ceiling effect that significantly reduces the adverse effect profile, including respiratory depression and euphoria, relative to a full-agonist opioid, such as methadone.19
Continue to: Whereas accessing methadone...
Whereas accessing methadone is limited to OTPs, buprenorphine is available for office-based treatment. By hosting OUD treatment and primary care in the same place, primary care physicians can provide comprehensive medical care including and beyond OUD, thereby improving retention and managing comorbidity.20
Integrated models involving support staff—eg, nurses, behavioral health providers, and pharmacists—have produced the greatest success with office-based treatment models.21 Office-based treatment normalizes OUD as a chronic disease managed by the primary care physician, enabling concurrent harm-reduction strategies; medication reconciliation; and convenient, regular prescribing intervals (eg, every 30 days).22 Nevertheless, access to buprenorphine is limited. Because buprenorphine is a controlled substance, the Ryan Haight Online Pharmacy Consumer Protection Act of 2008 prevents initial prescribing of buprenorphine without in-person evaluation. Telehealth consultations increased access to buprenorphine through temporary exceptions during the COVID-19 pandemic. However, revised rules and regulations for telehealth visits for these controlled substances are forthcoming from the DEA as temporary exceptions for telehealth consultations come to an end. Additionally, prescribing buprenorphine for OUD requires that the treating physician undergo specific training and obtain qualifications, which have evolved over time through federal legislation.
The Drug Addiction Treatment Act of 2000 (DATA 2000) authorized what is known as an X-waiver, which allows physicians to prescribe controlled substances for office-based treatment of OUD, provided that:
- they are registered to do so with the Substance Abuse and Mental Health Services Administration and the DEA
- they have had subspecialty training in addiction or completed an 8-hour training course
- they are able to refer patients to appropriate counseling and ancillary services.
DATA 2000 restricted patient panel sizes to 30 patients in the first year, expanding thereafter upon appropriate certification.
The Comprehensive Addiction and Recovery Act of 2016 (CARA) and the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act of 2018 (the SUPPORT Act) collectively extended prescribing authority for MOUD to other qualifying practitioners (eg, advanced practice clinicians). Despite these attempts to expand access to services, the overdose death rate has continued to increase.
Continue to: To further expand access to MAR...
To further expand access to MAR, the US Department of Health and Human Services updated its practice guidelines in April 2021, allowing clinicians to bypass X-waiver training requirements by applying for a notification-of-intent (NOI) buprenorphine waiver.a However, clinicians are still limited to prescribing buprenorphine for 30 patients at a time. Clinicians who undergo complete X-waiver training may prescribe for 100 patients in the first year and, if eligible, 275 patients thereafter.
In addition, as a component of the Consolidation Appropriations Act of 2023, Congress passed the Mainstreaming Addiction Treatment Act of 2021, or MAT 2021, and Medication Access and Training Expansion Act of 2021, or MATE 2021. MAT eliminated the X-waiver, NOI, and restrictions on the number of patients for whom a provider could prescribe buprenorphine, under federal authority; however, restrictions within one’s state might limit the ability to prescribe buprenorphine. MATE 2021 is an educational requirement for licensing by the DEA (at application and renewal) that will require prescribers to complete 8 hours of training in substance use disorders starting in June 2023.
Use of the monthly injectable extended-release buprenorphine productb is limited by an FDA Risk Evaluation and Mitigation Strategy (REMS) program, which requires specialized training and certification by the prescriber, distributor, and administering clinician. REMS reduces buprenorphine accessibility due to time, cost, and regulatory barriers; although such restrictions have been instituted with the patient’s safety in mind, any limitation to buprenorphine prescribing, apart from controlled substance licensure, serves only to limit access to a primary component of MAR.
Clinical considerations. Due to the competitive nature of buprenorphine and its high affinity for the μ-opioid receptor, the drug can displace other opioid agonists and precipitate acute withdrawal. The withdrawal experience can thereby condition fear and disfavor toward buprenorphine among patients.
It is vital, therefore, that (1) patients’ expectations for treatment be managed appropriately and (2) the treating physician be prepared to provide additional buprenorphine for adequate maintenance doses and utilize adjunct comfort agents (clonidine, nonsteroidal anti-inflammatory drugs, ondansetron) to manage acute withdrawal symptoms. Newer buprenorphine dosing strategies, such as micro-induction and macro-induction, have emerged to curtail these risks.23,24 This is an evolving area of MAR; newer low-threshold initiation strategies25 (see “Low-threshold MOUD prescribing models,” in the text that follows) and evidence that supports micro-induction26 might eliminate the practice of requiring active withdrawal for treatment.
Continue to: Regardless of the strategy...
Regardless of the strategy for dosing buprenorphine, it’s critical that patients be educated on how to initiate treatment outside a clinical setting, such as at home, where they occupy a familiar haven during a potentially uncomfortable time and can be as effective at initiation as they would be in a clinical setting, with no difference in precipitation of adverse effects.
At-home induction might be more appropriate for patients who are not yet in significant enough withdrawal while in the physician's office.27 Guidance should be provided on dosing instructions, self-assessment of withdrawal symptoms, and, if applicable, patience with the slow-dissolving sublingual tablet or film formulation.
Naltrexone
Background. Naltrexone is available as an oral tablet and an extended-release, once-monthly intramuscular injection; the latter has demonstrated superiority in MAR.28 Oral naltrexone has limited supporting evidence, is inferior to other MOUD options, and should not be used to treat OUD.7 Altogether, approval of naltrexone for OUD is controversial, due to potentially unethical trials and approval processes,29 although a multicenter randomized controlled trial demonstrated the drug’s noninferiority with respect to treatment retention relative to buprenorphine.30 Used over time, naltrexone does not relieve withdrawal symptoms but can reduce cravings.
Clinical considerations. There are numerous clinical barriers that limit the use of naltrexone.
First, patients should be abstinent from opioids for 7 to 14 days prior to starting therapy; usually, this means undergoing medically supervised withdrawal in a controlled environment. This is an obvious limitation for patients who are constrained financially—those who lack, or have inadequate, health insurance or are unable to be away from their job for an extended time.
Continue to: Second, because naltrexone...
Second, because naltrexone does not address withdrawal symptoms, supportive therapies should be incorporated into the treatment plan, including:
- clonidine for hyperadrenergic symptoms (anxiety, diaphoresis, hypertension)
- nonopioid analgesics for pain
- antiemetics, such as ondansetron and metoclopramide, for nausea or vomiting
- loperamide for diarrhea
- diphenhydramine for insomnia.
Third, patients taking naltrexone have a diminished response to opioids. This complicates pain management in the event of an emergent surgical procedure.
Last, when naltrexone wears off, patients are effectively opioid-naïve, which increases the risk for overdose in those who stop therapy abruptly.29 The increased risk for overdose should be communicated to all patients with OUD who are being treated with naltrexone.
This nonopioid option is appealing to policymakers and is often prioritized in the criminal justice system; however, the decreased efficacy of naltrexone (compared to methadone and buprenorphine), potential for overdose, and challenges in initiating treatment are concerning and limit the drug’s use in many real-world settings.
Because naltrexone is not a controlled substance, regulations regarding maintaining inventory and distribution are more flexible.
Continue to: Overall, the cost-effectiveness...
Overall, the cost-effectiveness of intramuscular naltrexone is unclear. State-administered insurance programs vary in their requirements for coverage of naltrexone treatment.31
Comprehensive medication reconciliation is vital
Overall fragmentation of care within OTPs places patients at risk for adverse events, such as drug interactions.32 Under Title 42 of the US Code,33 patients must provide written consent for an OTP provider to disclose their history of a substance use disorder. Allowing the patient to decide which medical providers can access their treatment records for an OUD benefits patient confidentiality but poses numerous issues worth exploring.
All prescribed controlled substances are recorded in the prescription drug monitoring program, or PDMP, a state-level electronic database accessible to health care professionals to inform prescribing decisions and identify drug interactions. The PDMP has substantially reduced opioid overprescribing and improved identification of patients at risk for overdose or misuse of opioids.
Unlike all other controlled substances, however, prescriptions ordered by an OTP are not recorded in the PDMP (although there are recent exceptions to this scenario). Without such information, a physician might not have important information about the patient when making medical decisions—placing the patient at risk for harmful outcomes, such as drug–drug and drug–disease interactions.
For example: Methadone is associated with a prolonged QT interval,34 increasing the risk for a fatal arrhythmia. Concurrent QT-prolonging medications, such as azithromycin and citalopram, further increase this risk.35 Because methadone dispensing is isolated from the patient’s medical record, the clinician who prescribes MOUD has an incomplete patient history and could make a potentially fatal treatment decision.
Continue to: Diversion is unlikely
Diversion is unlikely
Health care providers often express concern about diversion in MOUD. However, misuse and diversion rates of methadone and buprenorphine have declined steadily since 2011, and, in fact, are actually lower than the diversion rate of prescription antibiotics.36
Regardless, diversion of buprenorphine should not be a concern for physicians prescribing MOUD. Although a prescriber might worry about manipulation of the formulation of buprenorphine for intravenous administration, addition of naloxone to buprenorphine in tablet form diminishes the potential for overdose. Additionally, the ceiling effect of buprenorphine limits the likelihood of significant respiratory depression and euphoria.
Should buprenorphine reach a patient for whom it was not prescribed, it is highly unlikely that an overdose would result. Rather, the medication would protect against the effects of illicit opioids and relieve withdrawal symptoms. Most people with OUD who have misused buprenorphine have done so to relieve withdrawal symptoms,37 not to experience intoxication.
Health care deserts
So-called health care deserts in parts of the United States are an ongoing problem that disproportionately affects lower-income and segregated Black and Hispanic communities38—communities that shoulder the highest burden of OUD and OUD-related mortality39 and whose populace is in greatest need of MAR. Even when health care is accessible in such a desert, some clinicians and pharmacies refuse to prescribe or dispense MOUD because of the accompanying stigma of OUD.
A MAR desert, like a pharmacy desert, is a geographic region—one without access to a MAR or an OTP provider, thereby preventing patients from reaching appropriate care; for some patients, having to travel to the nearest provider can render treatment inaccessible.40
Continue to: Efforts are in place to identify...
Efforts are in place to identify areas at greatest need of OUD-related medical services, such as heat maps that identify areas of increased utilization of emergency medical services for opioid overdose. State-run programs have been implemented to increase access, such as the Illinois Helpline (https://helplineil.org) that provides support and resources for patients, friends, family, and providers.
Novel solutions
Key strategies to increase access to care and slow the opioid epidemic include low-threshold prescribing of MOUD and mobile OTPs.41
Low-threshold MOUD prescribing models. Adoption of one of these models in a medical practice that provides MAR might increase absolute enrollment. A low-threshold prescribing model involves42:
- same-day treatment
- leniency with respect to abstinence periods and a concomitant substance use disorder
- enhanced accessibility to MOUD through nontraditional medical settings.
Low-threshold prescribing is flexible in regard to patients’ needs and bypasses many of the barriers discussed in this article. Impressive multicenter success has been achieved by the CA Bridge program in California (https://cabridge.org), including an increase in recognition of OUD, treatment initiations, and outpatient engagement.25
The cost-effectiveness of low-threshold MOUD prescribing programs remains to be determined.
Mobile OTPs. In July 2021, the DEA authorized a mobile component to existing OTP registrants that is permitted to dispense methadone and buprenorphine. Mobile units are physically separate from the OTP but have similar functions, depending on available space. Services that cannot be provided on the mobile unit of an OTP must be available at its brick-and-mortar location.7 Logistically, OTP registrants no longer need a separate registration to implement a mobile unit, thus expanding care to patients in underserved or remote areas who often encounter barriers to access.43
Conclusion
Understanding the distinct clinical and accessibility benefits and limitations among available MOUD is essential for prescribing clinicians. Accessing treatment is limited by federal regulation, stigma, and the existence of health care deserts that limit access to necessary care for patients with OUD. Newer harm-reduction models, such as low-threshold prescribing and mobile OTPs, represent progress, but many patients remain untreated.
a At buprenorphine.samhsa.gov/forms/select-practitioner-type.php
b Sold under the brand name Sublocade.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, Department of Pharmacy Practice, University of Illinois Chicago College of Pharmacy, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
Medication-assisted recovery (MAR)—the preferred terminology for the service formerly known as medication-assisted treatment—entails a comprehensive set of interventions for managing opioid use disorder (OUD), including medications for opioid use disorder (MOUD). Despite the benefits of MAR—reducing opioid use, opioid-related mortality, and health care costs1-3—only 11% of patients with a diagnosis of OUD received MOUD in 2020.3
Primary care physicians, including family physicians, are well positioned to provide MAR across the patient’s lifespan. However, many family medicine clinicians do not possess the logistical knowledge or resources to implement this service.4 In this article, we describe options for, and barriers to, MAR and societal issues that have an impact on the care of these patients.
Pathophysiology of OUD
Opioids relieve pain by stimulating μ-opioid receptors and activating the brain’s reward system. These pleasurable effects motivate repeated use.5 Frequent opioid exposure causes neuroadaptation, tolerance, and dependence. For patients with OUD who are misusing illicit or prescription opioids, periods of abstinence following neuroadaptation lead to withdrawal symptoms that vary in intensity, depending on the drug, dose, and duration of use. Upregulated noradrenergic tone and dopamine deficiency manifest as numerous signs and symptoms of withdrawal, including5:
- Physiologic: secretory (diaphoresis, rhinorrhea, lacrimation, vomiting, diarrhea) and stimulatory (mydriasis, piloerection, hypertension, tachycardia, insomnia)
- Psychological: pain, cravings, dysphoria, anxiety.
A single episode of opioid withdrawal is not directly life-threatening, but untreated episodes can progressively amplify negative feedback and reinforce continued opioid use.6 Left untreated, withdrawal can be terminal.

Medication-assisted recovery: Effective intervention
MAR services that integrate medical, behavioral, and psychosocial programs can reduce mortality from OUD 2-fold.7,8 A meta-analysis found that, when MAR services are rendered in primary care, treatment retention improves by 25% (number needed to treat [NNT] = 6) and ongoing illicit opioid use is reduced by 50% (NNT = 6), relative to care at a specialty clinic9—highlighting a role for family medicine clinicians in treating OUD.
All 3 US Food and Drug Administration (FDA)–approved MOUD (methadone, buprenorphine, and naltrexone) reduce cravings; 2 (methadone and buprenorphine) mitigate withdrawal symptoms by activating the μ-opioid receptor; and naltrexone diminishes the reinforcing effects of use (TABLE10-12). It is crucial to recognize the pharmacologic distinctions among MOUD because untreated withdrawal syndromes increase dropout from treatment programs and subsequent relapse.13

The Hx of medication-assisted recovery
To understand the landscape of MAR, it is important to understand the history of opioid treatment in the United States. In 1966, Congress passed the Narcotic Addiction Rehabilitation Act (NARA), which secured federal assistance by which state and local governments could develop drug treatment programs.14 NARA permitted legal offenders with OUD to be civilly committed to treatment programs, rather than prosecuted. However, limited resources and a burgeoning population led, instead, to low-cost outpatient programs saddled by strict requirements that lacked a basis for improving clinical outcomes.
Continue to: At the time NARA...
At the time NARA was passed by Congress, OUD was viewed—inaccurately—as a criminal problem, not a medical one. Subsequent legislation was crafted through that lens, which has placed a heavy burden on patients until today.14 Although medical understanding of OUD has advanced tremendously over the past 50 years, treatment remains siloed from mainstream medicine, even in primary care.
There is no one-size-fits-all approach to MAR, and relapse is common. Patient-specific factors and the availability of resources should be considered when designing the most individualized, advantageous plan for MAR.
Methadone
Background. Methadone has the most extensive history for treating OUD and consistently has demonstrated efficacy.13 A meta-analysis of randomized controlled trials comparing methadone to nonpharmacotherapy alone found that methadone improved treatment retention by an absolute 57% (NNT = 2).10
Methadone was approved by the FDA for detoxification and maintenance treatment in the early 1970s, although the Narcotic Addict Treatment Act (NATA) of 1974 restricted dispensing of maintenance treatment to highly regulated clinics known as opioid treatment programs (OTPs).14 NATA required the treating physician to register with the US Drug Enforcement Agency (DEA) and to comply with conservative dosing regimens and observed dosing.
Over time, regulations evolved to give the physician greater flexibility in developing a care plan, allowing “take-home” doses, and improving patients’ access to care. Although access to methadone for the treatment of OUD remains limited to federally certified OTPs, regulations facilitate incorporation of a whole-person approach to care, including counseling, individual and group therapy, and toxicology testing.7
Continue to: Clinical considerations
Clinical considerations. Methadone requires slow titration. For patients starting methadone as an outpatient, federal law15 limits the initial dose to 30 mg and requires physician documentation when the first-day total dosage exceeds 40 mg. This dosing constraint makes it challenging to provide care because a daily dosage ≥ 60 mg has been found to produce, first, higher program retention (relative risk = 1.36; 95% CI, 1.13-1.63) and, second, greater reduction in illicit opioid use (relative risk = 1.59; 95% CI, 1.16-2.18) than is seen in patients who receive a lower daily dosage.16
Due to a prolonged elimination half-life, methadone reaches steady-state in 3 to 5 days. Patients and their families should be educated that withdrawal symptoms might not feel fully managed in the first few days of therapy and that time is required to experience safely the regimen’s full effects.
Aggressive dose-titration during methadone induction can result in drug accumulation and respiratory depression. The risk for methadone-related mortality is highest in the first 2 weeks of therapy, mostly related to overdose potential if the drug is combined with other opioids.17
Buprenorphine
Background. The prescribing rate for buprenorphine, particularly in primary care, is accelerating.18 A meta-analysis of randomized controlled trials found that11:
- compared to placebo, buprenorphine, at any dosage, improves treatment retention by an absolute 21% to 28% (NNT = 4-5)
- patients receiving high-dose buprenorphine (≥ 16 mg/d) had fewer evident cases of illicit opioid use.
Unlike methadone, buprenorphine exerts partial agonism at the μ-opioid receptor, resulting in a so-called ceiling effect that significantly reduces the adverse effect profile, including respiratory depression and euphoria, relative to a full-agonist opioid, such as methadone.19
Continue to: Whereas accessing methadone...
Whereas accessing methadone is limited to OTPs, buprenorphine is available for office-based treatment. By hosting OUD treatment and primary care in the same place, primary care physicians can provide comprehensive medical care including and beyond OUD, thereby improving retention and managing comorbidity.20
Integrated models involving support staff—eg, nurses, behavioral health providers, and pharmacists—have produced the greatest success with office-based treatment models.21 Office-based treatment normalizes OUD as a chronic disease managed by the primary care physician, enabling concurrent harm-reduction strategies; medication reconciliation; and convenient, regular prescribing intervals (eg, every 30 days).22 Nevertheless, access to buprenorphine is limited. Because buprenorphine is a controlled substance, the Ryan Haight Online Pharmacy Consumer Protection Act of 2008 prevents initial prescribing of buprenorphine without in-person evaluation. Telehealth consultations increased access to buprenorphine through temporary exceptions during the COVID-19 pandemic. However, revised rules and regulations for telehealth visits for these controlled substances are forthcoming from the DEA as temporary exceptions for telehealth consultations come to an end. Additionally, prescribing buprenorphine for OUD requires that the treating physician undergo specific training and obtain qualifications, which have evolved over time through federal legislation.
The Drug Addiction Treatment Act of 2000 (DATA 2000) authorized what is known as an X-waiver, which allows physicians to prescribe controlled substances for office-based treatment of OUD, provided that:
- they are registered to do so with the Substance Abuse and Mental Health Services Administration and the DEA
- they have had subspecialty training in addiction or completed an 8-hour training course
- they are able to refer patients to appropriate counseling and ancillary services.
DATA 2000 restricted patient panel sizes to 30 patients in the first year, expanding thereafter upon appropriate certification.
The Comprehensive Addiction and Recovery Act of 2016 (CARA) and the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act of 2018 (the SUPPORT Act) collectively extended prescribing authority for MOUD to other qualifying practitioners (eg, advanced practice clinicians). Despite these attempts to expand access to services, the overdose death rate has continued to increase.
Continue to: To further expand access to MAR...
To further expand access to MAR, the US Department of Health and Human Services updated its practice guidelines in April 2021, allowing clinicians to bypass X-waiver training requirements by applying for a notification-of-intent (NOI) buprenorphine waiver.a However, clinicians are still limited to prescribing buprenorphine for 30 patients at a time. Clinicians who undergo complete X-waiver training may prescribe for 100 patients in the first year and, if eligible, 275 patients thereafter.
In addition, as a component of the Consolidation Appropriations Act of 2023, Congress passed the Mainstreaming Addiction Treatment Act of 2021, or MAT 2021, and Medication Access and Training Expansion Act of 2021, or MATE 2021. MAT eliminated the X-waiver, NOI, and restrictions on the number of patients for whom a provider could prescribe buprenorphine, under federal authority; however, restrictions within one’s state might limit the ability to prescribe buprenorphine. MATE 2021 is an educational requirement for licensing by the DEA (at application and renewal) that will require prescribers to complete 8 hours of training in substance use disorders starting in June 2023.
Use of the monthly injectable extended-release buprenorphine productb is limited by an FDA Risk Evaluation and Mitigation Strategy (REMS) program, which requires specialized training and certification by the prescriber, distributor, and administering clinician. REMS reduces buprenorphine accessibility due to time, cost, and regulatory barriers; although such restrictions have been instituted with the patient’s safety in mind, any limitation to buprenorphine prescribing, apart from controlled substance licensure, serves only to limit access to a primary component of MAR.
Clinical considerations. Due to the competitive nature of buprenorphine and its high affinity for the μ-opioid receptor, the drug can displace other opioid agonists and precipitate acute withdrawal. The withdrawal experience can thereby condition fear and disfavor toward buprenorphine among patients.
It is vital, therefore, that (1) patients’ expectations for treatment be managed appropriately and (2) the treating physician be prepared to provide additional buprenorphine for adequate maintenance doses and utilize adjunct comfort agents (clonidine, nonsteroidal anti-inflammatory drugs, ondansetron) to manage acute withdrawal symptoms. Newer buprenorphine dosing strategies, such as micro-induction and macro-induction, have emerged to curtail these risks.23,24 This is an evolving area of MAR; newer low-threshold initiation strategies25 (see “Low-threshold MOUD prescribing models,” in the text that follows) and evidence that supports micro-induction26 might eliminate the practice of requiring active withdrawal for treatment.
Continue to: Regardless of the strategy...
Regardless of the strategy for dosing buprenorphine, it’s critical that patients be educated on how to initiate treatment outside a clinical setting, such as at home, where they occupy a familiar haven during a potentially uncomfortable time and can be as effective at initiation as they would be in a clinical setting, with no difference in precipitation of adverse effects.
At-home induction might be more appropriate for patients who are not yet in significant enough withdrawal while in the physician's office.27 Guidance should be provided on dosing instructions, self-assessment of withdrawal symptoms, and, if applicable, patience with the slow-dissolving sublingual tablet or film formulation.
Naltrexone
Background. Naltrexone is available as an oral tablet and an extended-release, once-monthly intramuscular injection; the latter has demonstrated superiority in MAR.28 Oral naltrexone has limited supporting evidence, is inferior to other MOUD options, and should not be used to treat OUD.7 Altogether, approval of naltrexone for OUD is controversial, due to potentially unethical trials and approval processes,29 although a multicenter randomized controlled trial demonstrated the drug’s noninferiority with respect to treatment retention relative to buprenorphine.30 Used over time, naltrexone does not relieve withdrawal symptoms but can reduce cravings.
Clinical considerations. There are numerous clinical barriers that limit the use of naltrexone.
First, patients should be abstinent from opioids for 7 to 14 days prior to starting therapy; usually, this means undergoing medically supervised withdrawal in a controlled environment. This is an obvious limitation for patients who are constrained financially—those who lack, or have inadequate, health insurance or are unable to be away from their job for an extended time.
Continue to: Second, because naltrexone...
Second, because naltrexone does not address withdrawal symptoms, supportive therapies should be incorporated into the treatment plan, including:
- clonidine for hyperadrenergic symptoms (anxiety, diaphoresis, hypertension)
- nonopioid analgesics for pain
- antiemetics, such as ondansetron and metoclopramide, for nausea or vomiting
- loperamide for diarrhea
- diphenhydramine for insomnia.
Third, patients taking naltrexone have a diminished response to opioids. This complicates pain management in the event of an emergent surgical procedure.
Last, when naltrexone wears off, patients are effectively opioid-naïve, which increases the risk for overdose in those who stop therapy abruptly.29 The increased risk for overdose should be communicated to all patients with OUD who are being treated with naltrexone.
This nonopioid option is appealing to policymakers and is often prioritized in the criminal justice system; however, the decreased efficacy of naltrexone (compared to methadone and buprenorphine), potential for overdose, and challenges in initiating treatment are concerning and limit the drug’s use in many real-world settings.
Because naltrexone is not a controlled substance, regulations regarding maintaining inventory and distribution are more flexible.
Continue to: Overall, the cost-effectiveness...
Overall, the cost-effectiveness of intramuscular naltrexone is unclear. State-administered insurance programs vary in their requirements for coverage of naltrexone treatment.31
Comprehensive medication reconciliation is vital
Overall fragmentation of care within OTPs places patients at risk for adverse events, such as drug interactions.32 Under Title 42 of the US Code,33 patients must provide written consent for an OTP provider to disclose their history of a substance use disorder. Allowing the patient to decide which medical providers can access their treatment records for an OUD benefits patient confidentiality but poses numerous issues worth exploring.
All prescribed controlled substances are recorded in the prescription drug monitoring program, or PDMP, a state-level electronic database accessible to health care professionals to inform prescribing decisions and identify drug interactions. The PDMP has substantially reduced opioid overprescribing and improved identification of patients at risk for overdose or misuse of opioids.
Unlike all other controlled substances, however, prescriptions ordered by an OTP are not recorded in the PDMP (although there are recent exceptions to this scenario). Without such information, a physician might not have important information about the patient when making medical decisions—placing the patient at risk for harmful outcomes, such as drug–drug and drug–disease interactions.
For example: Methadone is associated with a prolonged QT interval,34 increasing the risk for a fatal arrhythmia. Concurrent QT-prolonging medications, such as azithromycin and citalopram, further increase this risk.35 Because methadone dispensing is isolated from the patient’s medical record, the clinician who prescribes MOUD has an incomplete patient history and could make a potentially fatal treatment decision.
Continue to: Diversion is unlikely
Diversion is unlikely
Health care providers often express concern about diversion in MOUD. However, misuse and diversion rates of methadone and buprenorphine have declined steadily since 2011, and, in fact, are actually lower than the diversion rate of prescription antibiotics.36
Regardless, diversion of buprenorphine should not be a concern for physicians prescribing MOUD. Although a prescriber might worry about manipulation of the formulation of buprenorphine for intravenous administration, addition of naloxone to buprenorphine in tablet form diminishes the potential for overdose. Additionally, the ceiling effect of buprenorphine limits the likelihood of significant respiratory depression and euphoria.
Should buprenorphine reach a patient for whom it was not prescribed, it is highly unlikely that an overdose would result. Rather, the medication would protect against the effects of illicit opioids and relieve withdrawal symptoms. Most people with OUD who have misused buprenorphine have done so to relieve withdrawal symptoms,37 not to experience intoxication.
Health care deserts
So-called health care deserts in parts of the United States are an ongoing problem that disproportionately affects lower-income and segregated Black and Hispanic communities38—communities that shoulder the highest burden of OUD and OUD-related mortality39 and whose populace is in greatest need of MAR. Even when health care is accessible in such a desert, some clinicians and pharmacies refuse to prescribe or dispense MOUD because of the accompanying stigma of OUD.
A MAR desert, like a pharmacy desert, is a geographic region—one without access to a MAR or an OTP provider, thereby preventing patients from reaching appropriate care; for some patients, having to travel to the nearest provider can render treatment inaccessible.40
Continue to: Efforts are in place to identify...
Efforts are in place to identify areas at greatest need of OUD-related medical services, such as heat maps that identify areas of increased utilization of emergency medical services for opioid overdose. State-run programs have been implemented to increase access, such as the Illinois Helpline (https://helplineil.org) that provides support and resources for patients, friends, family, and providers.
Novel solutions
Key strategies to increase access to care and slow the opioid epidemic include low-threshold prescribing of MOUD and mobile OTPs.41
Low-threshold MOUD prescribing models. Adoption of one of these models in a medical practice that provides MAR might increase absolute enrollment. A low-threshold prescribing model involves42:
- same-day treatment
- leniency with respect to abstinence periods and a concomitant substance use disorder
- enhanced accessibility to MOUD through nontraditional medical settings.
Low-threshold prescribing is flexible in regard to patients’ needs and bypasses many of the barriers discussed in this article. Impressive multicenter success has been achieved by the CA Bridge program in California (https://cabridge.org), including an increase in recognition of OUD, treatment initiations, and outpatient engagement.25
The cost-effectiveness of low-threshold MOUD prescribing programs remains to be determined.
Mobile OTPs. In July 2021, the DEA authorized a mobile component to existing OTP registrants that is permitted to dispense methadone and buprenorphine. Mobile units are physically separate from the OTP but have similar functions, depending on available space. Services that cannot be provided on the mobile unit of an OTP must be available at its brick-and-mortar location.7 Logistically, OTP registrants no longer need a separate registration to implement a mobile unit, thus expanding care to patients in underserved or remote areas who often encounter barriers to access.43
Conclusion
Understanding the distinct clinical and accessibility benefits and limitations among available MOUD is essential for prescribing clinicians. Accessing treatment is limited by federal regulation, stigma, and the existence of health care deserts that limit access to necessary care for patients with OUD. Newer harm-reduction models, such as low-threshold prescribing and mobile OTPs, represent progress, but many patients remain untreated.
a At buprenorphine.samhsa.gov/forms/select-practitioner-type.php
b Sold under the brand name Sublocade.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, Department of Pharmacy Practice, University of Illinois Chicago College of Pharmacy, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235-S248.
2. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462-468. doi: 10.1111/j.1360-0443.2007.02090.x
3. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. HHS Publication PEP21-07-01-003, NSDUH Series H-56. 2021. Accessed March 19, 2023. www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
4. Haffajee RL, Andraka-Christou B, Attermann J, et al. A mixed-method comparison of physician-reported beliefs about and barriers to treatment with medications for opioid use disorder. Subst Abuse Treat Prev Policy. 2020;15:69. doi: 10.1186/s13011-020-00312-3
5. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20. doi: 10.1151/spp021113
6. Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44-53. doi: 10.1016/j.biopsych.2019.05.023
7. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. For Health care and Addiction Professionals, Policymakers, Patients, and Families. Treatment Improvement Protocol TIP 63. Publication No. PEP21-02-01-002. 2021. Accessed March 19, 2023. https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf
8. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550
9. Korownyk C, Perry D, Ton J, et al. Opioid use disorder in primary care: PEER umbrella systematic review of systematic reviews. Can Fam Physician. 2019;65:e194-e206.
10. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2
11. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4
12. Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513. doi: 10.1016/S0140-6736(11)60358-9
13. Soyka M, Zingg C, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641-653. doi: 10.1017/S146114570700836X
14. Institute of Medicine Committee on Federal Regulation of Methadone Treatment; Rettig R, Yarmolinsky A, eds. Federal Regulation of Methadone Treatment. National Academies Press; 1995.
15. 42 eCFR §8. Medication assisted treatment for opioid use disorders. Revised March 15, 2023. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8?toc=1
16. Faggiano F, Vigna-Taglianti F, Versino E, et al. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;(3):CD002208. doi: 10.1002/14651858.CD002208
17. Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377-386. doi: 10.1097/01.ADM.0000435321.39251.d7
18. Olfson M, Zhang VS, Schoenbaum M, et al. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323:276-277. doi: 10.1001/jama.2019.18913
19. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580. doi: 10.1038/clpt.1994.71
20. Walley AY, Palmisano J, Sorensen-Alawad A, et al. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J Subst Abuse Treat. 2015;59:59-66. doi: 10.1016/j.jsat.2015.07.007
21. Lagisetty P, Klasa K, Bush C, et al. Primary care models for treating opioid use disorders: what actually works? A systematic review. PloS One. 2017;12:e0186315. doi: 10.1371/journal.pone.0186315
22. Du CX, Shi J, Tetrault JM, et al. Primary care and medication management characteristics among patients receiving office-based opioid treatment with buprenorphine. Fam Pract. 2022;39:234-240. doi: 10.1093/fampra/cmab166
23. Herring AA, Vosooghi AA, Luftig J, et al. High-dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4:e2117128. doi: 10.1001/jamanetworkopen.2021.17128
24. Hämmig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105. doi: 10.2147/SAR.S109919
25. Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA Bridge Program. Ann Emerg Med. 2021;78:759-772. doi: 10.1016/j.annemergmed.2021.05.024
26. Wong JSH, Nikoo M, Westenberg JN, et al. Comparing rapid micro-induction and standard induction of buprenorphine/naloxone for treatment of opioid use disorder: protocol for an open-label, parallel-group, superiority, randomized controlled trial. Addict Sci Clin Pract. 2021;16:11. doi: 10.1186/s13722-021-00220-2
27. Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med. 2014;8:299-308. doi: 10.1097/ADM.0000000000000059
28. Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973-981. doi: 10.1001/archgenpsychiatry.2012.1a
29. Wolfe D, Carrieri MP, Dasgupta N, et al. Concerns about injectable naltrexone for opioid dependence. Lancet. 2011;377:1468-1470. doi: 10.1016/S0140-6736(10)62056-9
30. Tanum L, Solli KK, Latif ZEH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine–naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205. doi: 10.1001/jamapsychiatry.2017.3206
31. Murphy SM, Polsky D, Lee JD, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal justice-involved individuals with a history of opioid use disorder. Addiction. 2017;112:1440-1450. doi: 10.1111/add.13807
32. Ferrari A, Coccia CPR, Bertolini A, et al. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551-559. doi: 10.1016/j.phrs.2004.05.002
33. 42 eCFR Part 2. Confidentiality of substance use disorder patient records. January 18, 2017. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-2
34. Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015;110:1468-1475. doi: 10.1111/add.13013
35. Tisdale JE, Chung MK, Campbell KB, et al; . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214-e233. doi: 10.1161/CIR.0000000000000905
36. Leshner AI, Mancher M, eds. Barriers to broader use of medications to treat opioid use disorder. In: Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019:109-136.
37. Chilcoat HD, Amick HR, Sherwood MR, et al. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157. doi: 10.1016/j.jsat. 2019.07.005
38. Qato DM, Daviglus ML, Wilder J, et al. “Pharmacy deserts” are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33:1958-1965. doi: 10.1377/hlthaff.2013.1397
39. Mason M, Soliman R, Kim HS, et al. Disparities by sex and race and ethnicity in death rates due to opioid overdose among adults 55 years or older, 1999 to 2019. JAMA Netw Open. 2022;5:e2142982. doi: 10.1001/jamanetworkopen.2021.42982
40. Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789
41. Chan B, Hoffman KA, Bougatsos C, et al. Mobile methadone medication units: a brief history, scoping review and research opportunity. J Subst Abuse Treat. 2021;129:108483. doi: 10.1016/j.jsat.2021.108483
42. Jakubowski A, Fox A. Defining low-threshold buprenorphine treatment. J Addict Med. 2020;14:95-98. doi: 10.1097/ADM.0000000000000555
43. Messmer SE, Elmes AT, Jimenez AD, et al. Outcomes of a mobile medical unit for low-threshold buprenorphine access targeting opioid overdose hot spots in Chicago. J Subst Use Addict Treat. 2023;209054. doi: 10.1016/j.josat.2023.209054
1. Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235-S248.
2. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462-468. doi: 10.1111/j.1360-0443.2007.02090.x
3. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. HHS Publication PEP21-07-01-003, NSDUH Series H-56. 2021. Accessed March 19, 2023. www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
4. Haffajee RL, Andraka-Christou B, Attermann J, et al. A mixed-method comparison of physician-reported beliefs about and barriers to treatment with medications for opioid use disorder. Subst Abuse Treat Prev Policy. 2020;15:69. doi: 10.1186/s13011-020-00312-3
5. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20. doi: 10.1151/spp021113
6. Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44-53. doi: 10.1016/j.biopsych.2019.05.023
7. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. For Health care and Addiction Professionals, Policymakers, Patients, and Families. Treatment Improvement Protocol TIP 63. Publication No. PEP21-02-01-002. 2021. Accessed March 19, 2023. https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf
8. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550
9. Korownyk C, Perry D, Ton J, et al. Opioid use disorder in primary care: PEER umbrella systematic review of systematic reviews. Can Fam Physician. 2019;65:e194-e206.
10. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2
11. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4
12. Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513. doi: 10.1016/S0140-6736(11)60358-9
13. Soyka M, Zingg C, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641-653. doi: 10.1017/S146114570700836X
14. Institute of Medicine Committee on Federal Regulation of Methadone Treatment; Rettig R, Yarmolinsky A, eds. Federal Regulation of Methadone Treatment. National Academies Press; 1995.
15. 42 eCFR §8. Medication assisted treatment for opioid use disorders. Revised March 15, 2023. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8?toc=1
16. Faggiano F, Vigna-Taglianti F, Versino E, et al. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;(3):CD002208. doi: 10.1002/14651858.CD002208
17. Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377-386. doi: 10.1097/01.ADM.0000435321.39251.d7
18. Olfson M, Zhang VS, Schoenbaum M, et al. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323:276-277. doi: 10.1001/jama.2019.18913
19. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580. doi: 10.1038/clpt.1994.71
20. Walley AY, Palmisano J, Sorensen-Alawad A, et al. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J Subst Abuse Treat. 2015;59:59-66. doi: 10.1016/j.jsat.2015.07.007
21. Lagisetty P, Klasa K, Bush C, et al. Primary care models for treating opioid use disorders: what actually works? A systematic review. PloS One. 2017;12:e0186315. doi: 10.1371/journal.pone.0186315
22. Du CX, Shi J, Tetrault JM, et al. Primary care and medication management characteristics among patients receiving office-based opioid treatment with buprenorphine. Fam Pract. 2022;39:234-240. doi: 10.1093/fampra/cmab166
23. Herring AA, Vosooghi AA, Luftig J, et al. High-dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4:e2117128. doi: 10.1001/jamanetworkopen.2021.17128
24. Hämmig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105. doi: 10.2147/SAR.S109919
25. Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA Bridge Program. Ann Emerg Med. 2021;78:759-772. doi: 10.1016/j.annemergmed.2021.05.024
26. Wong JSH, Nikoo M, Westenberg JN, et al. Comparing rapid micro-induction and standard induction of buprenorphine/naloxone for treatment of opioid use disorder: protocol for an open-label, parallel-group, superiority, randomized controlled trial. Addict Sci Clin Pract. 2021;16:11. doi: 10.1186/s13722-021-00220-2
27. Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med. 2014;8:299-308. doi: 10.1097/ADM.0000000000000059
28. Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973-981. doi: 10.1001/archgenpsychiatry.2012.1a
29. Wolfe D, Carrieri MP, Dasgupta N, et al. Concerns about injectable naltrexone for opioid dependence. Lancet. 2011;377:1468-1470. doi: 10.1016/S0140-6736(10)62056-9
30. Tanum L, Solli KK, Latif ZEH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine–naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205. doi: 10.1001/jamapsychiatry.2017.3206
31. Murphy SM, Polsky D, Lee JD, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal justice-involved individuals with a history of opioid use disorder. Addiction. 2017;112:1440-1450. doi: 10.1111/add.13807
32. Ferrari A, Coccia CPR, Bertolini A, et al. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551-559. doi: 10.1016/j.phrs.2004.05.002
33. 42 eCFR Part 2. Confidentiality of substance use disorder patient records. January 18, 2017. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-2
34. Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015;110:1468-1475. doi: 10.1111/add.13013
35. Tisdale JE, Chung MK, Campbell KB, et al; . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214-e233. doi: 10.1161/CIR.0000000000000905
36. Leshner AI, Mancher M, eds. Barriers to broader use of medications to treat opioid use disorder. In: Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019:109-136.
37. Chilcoat HD, Amick HR, Sherwood MR, et al. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157. doi: 10.1016/j.jsat. 2019.07.005
38. Qato DM, Daviglus ML, Wilder J, et al. “Pharmacy deserts” are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33:1958-1965. doi: 10.1377/hlthaff.2013.1397
39. Mason M, Soliman R, Kim HS, et al. Disparities by sex and race and ethnicity in death rates due to opioid overdose among adults 55 years or older, 1999 to 2019. JAMA Netw Open. 2022;5:e2142982. doi: 10.1001/jamanetworkopen.2021.42982
40. Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789
41. Chan B, Hoffman KA, Bougatsos C, et al. Mobile methadone medication units: a brief history, scoping review and research opportunity. J Subst Abuse Treat. 2021;129:108483. doi: 10.1016/j.jsat.2021.108483
42. Jakubowski A, Fox A. Defining low-threshold buprenorphine treatment. J Addict Med. 2020;14:95-98. doi: 10.1097/ADM.0000000000000555
43. Messmer SE, Elmes AT, Jimenez AD, et al. Outcomes of a mobile medical unit for low-threshold buprenorphine access targeting opioid overdose hot spots in Chicago. J Subst Use Addict Treat. 2023;209054. doi: 10.1016/j.josat.2023.209054
PRACTICE RECOMMENDATIONS
› Consider resource availability (eg, treatment programs and regulatory barriers), in addition to patient- and medicationspecific factors, when designing the most individualized, advantageous medication-assisted recovery plan, to reduce the risk for mortality. B
› Schedule early (< 2 weeks) and frequent follow-up with patients who are starting medications for opioid use disorder (particularly methadone), to manage risk when mortality is highest and to support recovery. C
› Set and manage patient expectations for control of withdrawal symptoms when initiating medications for opioid use disorder (particularly buprenorphine). B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series