User login
Shortness of breath: Looking beyond the usual suspects
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
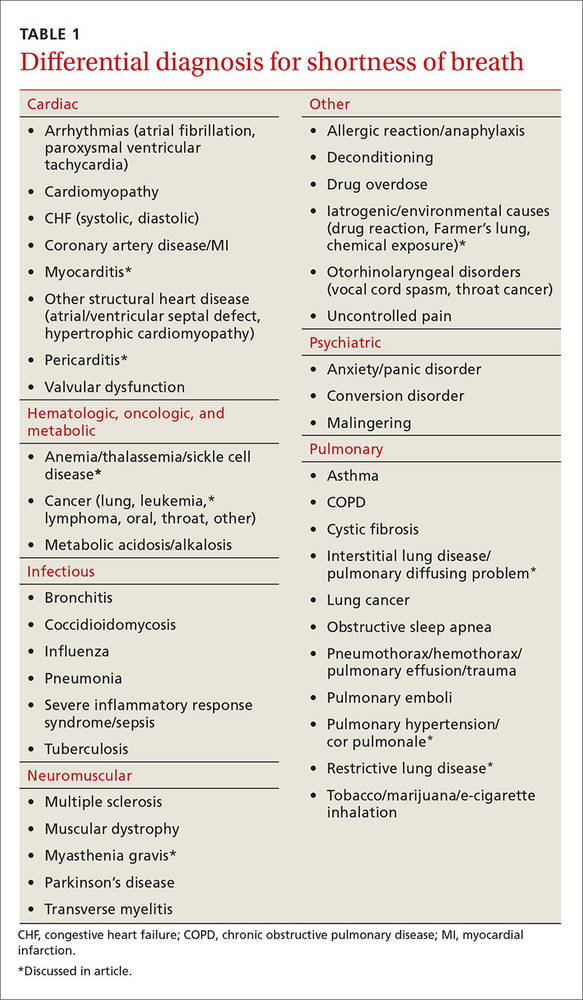
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.
Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.

Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
› Consider diagnoses other than asthma, COPD, heart failure, and pneumonia in patients with persistent or progressive dyspnea. C
› Avoid steroids in patients with acute pericarditis because research shows that they increase the risk of recurrence. B
› Consider anticoagulation with warfarin in patients with pulmonary arterial hypertension and cor pulmonale. Evidence shows that it improves survival and quality of life. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joan C is a 68-year-old woman who presents to the office complaining of an enlarging left chest wall mass that appeared within the past month. She was treated for small-cell lung cancer 11 years ago. She has a 45 pack-year smoking history (she quit when she received the diagnosis) and has heart failure, which is controlled. Your examination reveals a large (5 cm) firm mass on her left chest wall. There is no erythema or tenderness. She has no other complaints. You recommend surgical biopsy and refer her to surgery.
Ms. C returns to your office several days later complaining of new and worsening shortness of breath with exertion that began the previous day. The presentation is similar to prior asthma exacerbation episodes. She denies any cough, fever, chest pain, symptoms at rest, or hemoptysis. On exam she appears comfortable and not in any acute distress. You refill her albuterol.
The next day you learn that she is being admitted to the hospital with respiratory distress. An x-ray of her chest shows a concerning mass in her right upper lung.
Dyspnea is an uncomfortable awareness of breathing that occurs when complex neurochemical pathways used to maintain oxygenation and ventilation are disrupted. (See "The variable, and subjective, process of dyspnea"1-5). Sometimes described as air hunger, increased work of breathing, chest tightness, or chest constriction, the symptom is usually disproportionate to the patient’s level of exertion.
The variable, and subjective, process of dyspnea
The mechanism of action of shortness of breath is a complex and incompletely understood one that involves the central and peripheral nervous systems and neurochemical modulators. In the central nervous system, the medullary respiratory center likely relays increased oxygen demand to the anterior insula. The anterior insula, which is where dyspnea is perceived as unpleasant, then simultaneously disseminates this information to the cerebral cortex and the respiratory muscles to increase respiration and oxygen.1-3
The peripheral nervous system measures current oxygen flux and lung mechanics through pulmonary stretch mechanoreceptors, pulmonary irritant receptors, and alveolar C fibers. Input from all of these receptors ascends the respiratory pathway and affects how dyspnea is perceived. For example, a patient may complain of shortness of breath because the medullary respiratory center interprets input from activated pulmonary muscular stretch receptors in the setting of discordant oxygen (measured via peripheral chemoreceptors) and carbon dioxide levels (measured by medullary chemoreceptors) as an increased work of breathing.2,4,5
Neurochemical dissociation, which is the difference between the brain’s desired oxygen level and the amount it gets, is one potential hypothesis to explain why dyspnea is subjective and variable.2,5 One patient may complain of moderate or severe shortness of breath because he or she has a large dissociation between desired and actual oxygenation despite having only mild to moderate disease severity. However, another patient may report mild dyspnea despite having severe disease because his or her dissociation is small.
Take, for example, a patient who has had an acute myocardial infarction. Such patients often complain of significant difficulty breathing, likely because of the acute and sudden neurochemical dissociation that occurs with the infarction. On the other hand, a patient with gradually worsening moderate heart failure may complain of only mild dyspnea because the change in the patient’s perception of the ability to breathe is slow and small.
Most of the time dyspnea is due to either a primary lung or cardiovascular problem such as chronic obstructive pulmonary disease (COPD), asthma, pulmonary embolism (PE), pneumonia, congestive heart failure (CHF), or myocardial infarction. However, many other illnesses can also produce this symptom (TABLE 1). This article will review the uncommon etiologies of dyspnea that should be considered when the usual suspects have been eliminated.

Cardiovascular culprits
Dyspnea is a common symptom with cardiovascular diseases because cardiac output relates directly to tissue oxygenation. Any pathology that decreases the ability of the heart and blood vessels to transport oxygen will likely trigger discord between the central, peripheral, and neurochemical respiratory centers. Two uncommon cardiovascular etiologies of dyspnea are pericarditis and myocarditis.
Pericarditis
Pericarditis is generally a self-limited condition that responds promptly to initial treatment, although it can cause significant morbidity and mortality. One study showed that acute pericarditis accounted for 5% of patients presenting to the emergency department with non-ischemic chest pain.6 Another study found that the in-hospital mortality rate for acute pericarditis was 1.1%.7
Pericarditis causes dyspnea by restricting the heart’s ability to relax, thus decreasing preload and cardiac output. This occurs with large effusions (>20 mm in width on echocardiography) and can lead to cardiac tamponade—a medical emergency that should be suspected in patients with muffled heart sounds, hypotension, and increased jugular venous distention (Beck’s triad).
Pericarditis etiologies include:
- infectious causes (viral and bacterial entities, myocarditis),
- rheumatologic causes (gout, systemic lupus erythematosus, tumor necrosis factor receptor-associated periodic syndrome [TRAPS], familial Mediterranean fever),
- post-cardiac injury syndromes (either of the acute [2-4 days post injury] or late [Dressler syndrome] variety),
- metabolic disorders (hypothyroid disease, dialysis-related conditions), and
- malignancy.
More than 80% of pericarditis cases in developed countries are idiopathic and are assumed to have a viral source.8
Diagnosis. Acute pericarditis is diagnosed when 2 or more of the following symptoms are present:
- pleuritic chest pain radiating to the trapezius that is relieved by leaning forward
- pericardial friction rub
- electrocardiographic changes showing ST segment elevation in all leads but aVR and V1 and diffuse PR interval depression
- pericardial effusion on echocardiography.
Treatment. Treat non-severe and non-life threatening pericarditis with nonsteroidal anti-inflammatory drugs (NSAIDs). Avoid steroids because research has shown that they increase the risk for developing recurrent pericarditis.8 Hospitalize patients with large pericardial effusions and consider them for pericardiocentesis. Treat cardiac tamponade with urgent pericardiocentesis and hospitalization.
Myocarditis
Myocarditis can have a variety of etiologies (TABLE 29,10). Myocarditis causes dyspnea either by causing pericardial effusion or heart failure.
Diagnosis. Myocarditis can be difficult to diagnose. Suspect it in any patient with cardiogenic shock, acute or subacute left ventricular dysfunction, or myocardial damage from a non-coronary artery disease source. Echocardiography and cardiac serum biomarkers can help diagnose myocarditis, but the diagnostic gold standard remains myocardial biopsy.
Treatment. Treatment is focused on 2 goals: treating the specific etiology suspected and stabilizing any hemodynamic instability. Patients with mild cases can be treated and monitored in the outpatient setting.
Immunosuppressive therapy with immunoglobulin or steroids is not routinely recommended, but a trial may be considered in children, patients with severe hemodynamic compromise, or patients with giant cell arteritis, another autoimmune condition, sarcoidosis, or eosinophilic or non-viral myocarditis.
Because of the risk of sudden death from ventricular arrhythmias, any patient with cardiac symptoms such as chest pain, dyspnea, or palpitations should be admitted for cardiopulmonary monitoring. Patients with heart failure secondary to myocarditis should be treated according to the American Heart Association treatment guidelines for heart failure (available at: http://circ.ahajournals.org/content/128/16/e240.extract). Some patients may benefit from surgical interventions such as percutaneous cardiopulmonary support, extracorporeal membrane oxygenation, mechanical circulatory support, and left ventricular assistive devices. Ventricular arrhythmias may require implantable defibrillators or pacemakers.10

Pulmonary causes
Shortness of breath is common with most pulmonary diseases, although it may not be an initial symptom and may have an insidious onset. It occurs once oxygenation of blood becomes inadequate, resulting in peripheral nervous system activation and neurochemical dissociation. Most patients with a pulmonary infection, asthma exacerbation, or COPD will have dyspnea. Once infection, asthma, and COPD have been ruled out, other pathologic processes that interrupt oxygenation should be considered. Unlike COPD and infections, patients with lung cancer may not have dyspnea until the end stages of their disease.11 The following entities should be considered in patients with dyspnea when more common causes have been eliminated.
Restrictive lung diseases
Restrictive lung disease occurs when functional lung volume is decreased, either by an intrinsic or extrinsic source. As a result, these lung diseases cover a wide variety of pathologies and disease processes including interstitial lung diseases (which we’ll discuss here), environmental exposures, neuromuscular diseases, and other forms of chest wall dysfunction.
Interstitial lung disease occurs in the presence of lung parenchymal scarring or thickening, which can have many causes including pulmonary fibrosis, connective tissue diseases (eg, sarcoidosis or rheumatoid arthritis), and inflammatory processes (eg, hypersensitivity pneumonitis and coal worker's pneumoconiosis). Dyspnea results because parenchymal thickening decreases oxygen diffusion between the alveolar and capillary endothelium. Additionally, the lung’s ability to exchange air is restricted by parenchymal stiffness and decreased total lung and functional lung capacity. Treatment is disease specific.
Idiopathic pulmonary fibrosis is the most common interstitial pneumonia with a prevalence of 13 to 20 per 100,000 people.12 It commonly affects men between the ages of 50 and 75 years. Risk factors include cigarette smoking, dust exposure (to metals, woods, vegetables), and exposure to livestock or other animals.12 Suspect it when you have a middle-aged farmer or mill worker who complains of shortness of breath.
Treatment recommendations have changed recently and now consist of using only nintedanib (a tyrosine-kinase inhibitor), antacid medication, and pirfenidone. Anticoagulation (with warfarin), steroids, other immunologic agents including azathioprine, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are not recommended.13
Pulmonary arterial hypertension and cor pulmonale
Pulmonary arterial hypertension (PAH) is defined as a mean resting precapillary pulmonary artery pressure >25 mm Hg or >30 mm Hg with activity. It can be idiopathic or caused by a variety of agents, diseases, and conditions (TABLE 314). PAH is rare (15 in one million adults) and underdiagnosed, and more often occurs in 20- to 30-year-old black women.14
Suspect PAH in younger, otherwise healthy patients who complain of exertional dyspnea, fatigue, chest pain, or palpitations who do not have any other heart or lung disease signs or symptoms. A diagnosis of PAH is often delayed because patients are worked up for other etiologies such as CHF, coronary artery disease, PE, and COPD.
Diagnosis. When PAH is suspected, the initial work-up should include:
- an echocardiogram with a possible bubble study,
- arterial blood gas measurements,
- complete blood count,
- complete metabolic panel,
- human immunodeficiency virus (HIV) testing,
- thyroid-stimulating hormone levels,
- chest x-ray (which is abnormal in 90% of patients and shows right ventricular enlargement, a prominent central pulmonary artery, or peripheral hypovascularity),14
- electrocardiogram (to rule out other acute cardiac etiologies, but not to diagnosis PAH because of poor sensitivity and specificity),
- liver ultrasound, and
- pulmonary function tests.
If clinically suggested, tests for anticentromere antibody, antinuclear antibodies, anti-Scl-70 antibodies, and ribonucleoprotein antibodies should be ordered, as well as sickle cell screening, cardiac magnetic resonance imaging, and chest computed tomography. A right heart catheterization is required to confirm PAH and determine disease severity.
Vasoreactivity testing helps guide treatment because it identifies which patients will benefit from calcium channel blockers. The 6-minute walk test is the best way to estimate prognosis and disease severity. It is a simple test you can perform in the office by measuring how far your patient can walk in 6 minutes. Miyamoto et al showed the test to be predictive of survival in idiopathic PAH.15 A lung biopsy is never indicated or needed for diagnosis, disease severity classification, or prognosis.
Treatment. Collaboration between primary and subspecialty physicians is usually recommended because PAH treatment requires advanced testing such as right heart catheterization or vasoreactivity testing. Research has shown anticoagulation with warfarin prolongs survival and improves quality of life.16 Oxygen may improve symptomatic control and should be started for anyone with saturation less than 90%.
Newer medications that target various pathways resulting in vasodilation include prostacyclin analogues (epoprostenol, iloprost, treprostinil), endothelin receptor antagonists (ambrisentan, bosentan), and phosphodiesterase type 5 inhibitors (sildenafil, tadalafil).14

Hematologic diseases
Hematologic diseases, including sickle cell disease, gammopathies, and malignancies, can cause dyspnea primarily by decreasing the body’s ability to transport oxygen. This usually is due to anemia, but it also can be caused by increased viscosity or sickling. Suspect a hematologic cause of dyspnea when a patient repeatedly returns to your office complaining of progressive dyspnea on exertion and possible Raynaud’s-like symptoms.
Sickle cell disease
Sickle cell disease is a heterogeneous genetic disease with varied physical manifestations. The sickling phenomenon occurs in patients who inherit the homozygous hemoglobin S trait or heterozygous hemoglobin S and C (hemoglobin SC) disease. Sickle cell patients develop dyspnea due to comorbid anemia, infectious processes, or cardiopulmonary disease.
Cardiac disease is common and an often unrecognized comorbidity. It is the leading cause of mortality in adults with sickle cell disease, resulting in 26% of deaths (usually from pulseless electrical activity, pulmonary emboli, multiorgan failure, or stroke).17 Nonfatal cardiac complications may also develop, including chronic heart disease from prolonged increased cardiac output (leading to ventricular hypertrophy), heart failure, or arrhythmias; non-atherosclerotic MI;18 and hemosiderosis-induced cardiomyopathy from repeat blood transfusions.
Pulmonary-related complications may be chronic or acute and may include restrictive lung disease, chronic hypoxemia, pulmonary hypertension, and interstitial fibrosis. Acute chest syndrome and cor pulmonale cause sudden pulmonary disease. Acute chest syndrome is often caused by pneumonia, in situ thrombosis infarction of the lung, or embolic infarction from fat or bone marrow. It is a medical emergency that should be considered in any patient with pulmonary symptoms, fever, chest pain, or cough and an infiltrate on chest x-ray.
Treatment for acute chest syndrome consists of oxygen, aggressive analgesia, antibiotics (if infection is suspected), and transfusions. Research has shown that steroids provide improvement, but result in more hospital readmissions.19
Multiple myeloma and other hematologic malignancies
Multiple myeloma and Waldenstrom macroglobulinemia (discussed here), as well as leukemia, and other hematologic malignancies, can cause dyspnea or dyspnea on exertion through anemia, increasing blood viscosity, or direct lung involvement.
Multiple myeloma, a plasma cell neoplasm, is associated with anemia in 73% of patients at time of diagnosis.20 This is because of bone marrow destruction. Anemia prevalence increases in patients treated with chemotherapy because of the agent's adverse effects. The decision to treat with irradiated, leukoreduced red cell transfusion is based on anemia severity, the presence of symptoms, and whether the patient is currently undergoing chemotherapy.
Waldenstrom macroglobulinemia is an IgM-specific monoclonal gammopathy associated with a lymphoplasmacytic lymphoma in the bone marrow. Dyspnea results from hyperviscosity syndrome, hemolytic or other anemias, and/or direct lung involvement including pleural effusion, pulmonary infiltrates, or a mass.
Hyperviscosity syndrome usually results in neurologic symptoms such as vision changes, headaches, vertigo, dizziness, dementia, or other changes in consciousness. Heart failure, which is often associated with comorbid anemia, can develop in severe cases.
Patients are generally asymptomatic if serum viscosity is <3 centipoises (cP). Symptoms increase in frequency and severity with increasing serum viscosity so that about two-thirds (67%) of patients have symptoms when viscosity is >4 cP and 75% have symptoms when viscosity is >5 cP.21
Neuromuscular diseases
Dyspnea occurs when respiratory muscles are weakened by neuromuscular diseases such as myasthenia gravis (discussed here), multiple sclerosis, or muscular dystrophy. Such diseases can cause respiratory insufficiency, increased rates of infection, or complete respiratory failure. Respiratory involvement is usually a manifestation of advanced disease. Suspect neuromuscular causes of dyspnea when you are seeing a patient admitted to the nursing home for long-term care because of profound weakness affecting their ability to do activities of daily living.
Myasthenia gravis
Myasthenia gravis, an autoimmune-mediated destruction of the postsynaptic acetylcholine receptors of the neuromuscular junction, is the most common disorder of neuromuscular transmission. It often affects the ocular (>50%; ptosis, diplopia), bulbar (15%; dysarthria, dysphagia, fatigable chewing), limb (<5%; usually proximal weakness), and respiratory muscles. Weakness typically fluctuates and worsens with muscle fatigue. Myasthenic crisis, an acute respiratory failure that occurs in 15% to 20% of patients, is often precipitated by an event such as surgery, an infection, or a medication change.22
Diagnosis. Myasthenia gravis is diagnosed by a clinical history and exam suggestive of the disease. Suspect it if signs and symptoms include weakness worse with fatigue especially of the ocular muscles (ptosis or diplopia), dysphagia, dysphonia, chewing difficulty, or limb weakness. Consider laboratory testing with an anti-acetylcholine receptor (AChR) antibody assay, an assay for muscle-specific kinase (MuSK) antibody, or an anti-striated muscle (anti-SM) antibody assay if the history and exam are suggestive of the disorder.
The most reliable test is the anti-AChR antibody assay, which is positive in 50% to 90% of patients with the disease.22 Less reliable is the anti-MuSK antibody assay, which can be positive in 40% to 60% of patients who are AChR-seronegative.23 An anti-striated muscle antibody assay is only helpful in patients with thymoma or onset of disease after age 40 years.24
Consider electrophysiologic tests, including repetitive nerve stimulation studies and single-fiber electromyography, if the above laboratory tests are inconclusive.25
Treatment depends on symptom severity and frequency. It can range from observation for mild occasional symptoms to chronic steroids and immunosuppressant medications in severe cases.
CASE › You see Ms. C in the intensive care unit the next day. She is intubated and has been responding poorly to the diuresis and breathing treatments used overnight. Her biopsy pathology results return and show recurrence of her small-cell lung cancer. She begins chemotherapy immediately and is extubated a few days later. She is discharged from the hospital a week later. Her shortness of breath is mild at this time, although she does require 2 liters of continuous oxygen.
CORRESPONDENCE
Christopher Taggart, MD, St. Mary’s Medical Center, Department of Family Medicine, 2698 Patterson Rd, Grand Junction, CO 81506; [email protected].
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
1. von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Resp Crit Care Med. 2008;177:1026-1032.
2. Thoma J, Gunten CV. Dyspnea. In: Bruera E, Higginson IJ, Ripamonti C, et al, eds. Textbook of Palliative Medicine. London: Hodder Arnold; 2009.
3. Manning H, Schwartzstein R. Pathophysiology of dyspnea. N Engl J Med. 1995;333;1547-1553.
4. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155:530-535.
5. O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148:1351-1357.
6. Seferovic PM, Ristic AD, Maksimovic R, et al. Pericardial syndromes: an update after the ESC guidelines 2004. Heart Fail Rev. 2013;18:255-266.
7. Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601-1606.
8. LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410-2416.
9. Pursnani A, Yee H, Slater W, et al. Hypersensitivity myocarditis associated with azithromycin exposure. Ann Intern Med. 2009;150:225-226.
10. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747.
11. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55:1000-1006.
12. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949-1961.
13. Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3-e19.
14. Stringham R, Shah NR. Pulmonary arterial hypertension: an update on diagnosis and treatment. Am Fam Physician. 2010;82:370-377.
15. Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlate and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487-492.
16. Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 1997;112:714-721.
17. Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patient with sickle cell disease. Am J Hematol. 2010;85:36-40.
18. Martin CR, Johnson CS, Cobb C, et al. D. Myocardial infarction in sickle cell disease. J Natl Med Assoc. 1996;88:428-432.
19. Paul RN, Castro OL, Aggarwal A, et al. Acute chest syndrome: sickle cell disease. Eur J Haematol. 2011;87:191-207.
20. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060.
21. Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
22. Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32;215-226.
23. Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530-535.
24. Skeie GO, Mygland A, Aarli JA, et al. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99-104.
25. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006;16:459-467.
