User login
2020 Update on pelvic floor dysfunction
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
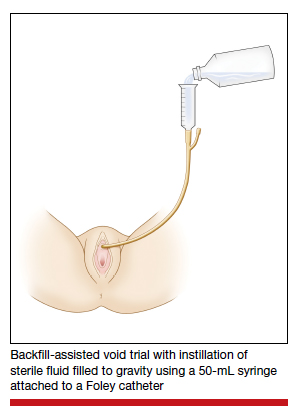
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).
Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
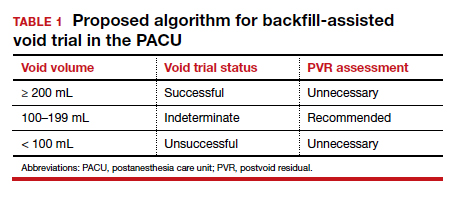
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.
A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
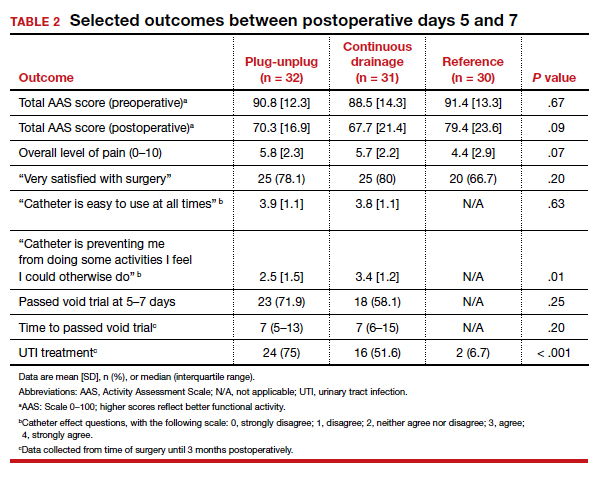
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).
Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).

Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.

A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).

Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).

Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.

A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).

Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
2019 Update on pelvic floor dysfunction
Fecal incontinence (FI), also known as accidental bowel leakage, is the involuntary loss of feces, which includes both liquid and solid stool as defined by the International Continence Society (ICS) and the International Urogynecological Association (IUGA).1,2 Fecal incontinence is common, occurring in 7% to 25% of community-dwelling women, and it increases with age.2-6 The condition is rarely addressed, with only 30% of women seeking care.6-8 This is due to patient embarrassment and the lack of a reliable screening tool. However, FI affects quality of life and mental health, and the associated economic burden likely will rise given the increased prevalence of FI among older women.2,4,7,9
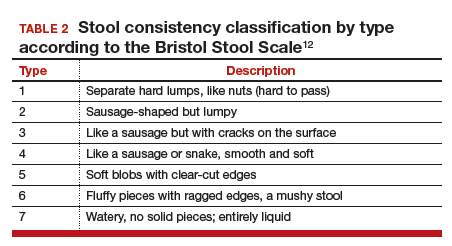
Fecal incontinence occurs due to poor stool consistency, anal and pelvic muscle weakness, reduced rectal compliance, reduced or increased rectal sensation, or bowel inflammation or dysfunction. Many conditions can cause FI (TABLE 1).5,10,11 It is therefore important to elicit a full medical history with a focus on specific bowel symptoms, such as stool consistency type (TABLE 2),12 FI frequency, and duration of symptoms, as well as to perform a complete examination to identify any readily reversible or malignant causes. A colonoscopy is recommended for individuals who meet screening criteria or present with a change in bowel symptoms, such as diarrhea, bleeding, or obstruction.13,14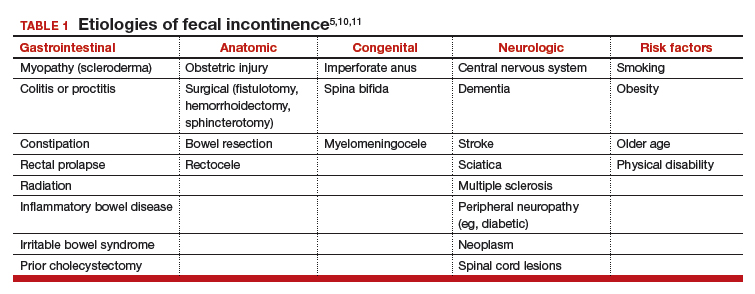
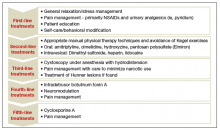
Fecal incontinence treatments include a range of approaches categorized from conservative, or first-line therapy, to fourth-line surgical managements (FIGURE 1).1,10,13,14 In this Update, we review the results of 3 well-designed trials that enrolled women with frequent nonneurogenic FI.
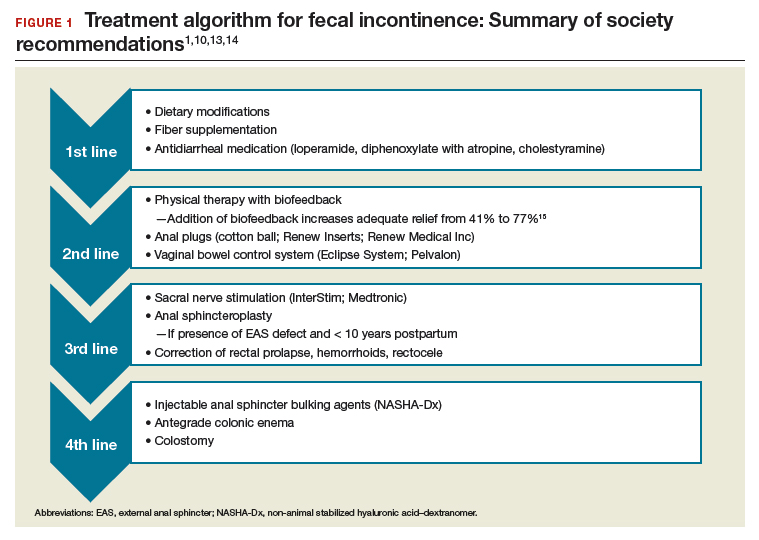
Common first- and second-line treatments produce equivalent improvements in FI symptoms at
6 months
Jelovsek JE, Markland AD, Whitehead WE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomized clinical trial. Lancet Gastroenterol Hepatol. 2019;4:698-710.
In a multicenter, randomized trial of first- and second-line treatments for FI, Jelovsek and colleagues evaluated the efficacy of oral placebo, loperamide, pelvic floor physical therapy (PFPT) with biofeedback using anorectal manometry, or combination therapy over a 24-week period.
Continue to: Four treatments compared...
Four treatments compared
Three hundred women with FI occurring monthly for 3 months were included in the trial. Women were excluded if they had a stool classification of type 1 or type 7 on the Bristol Stool Scale, inflammatory bowel disease (IBD), history of rectovaginal fistula or cloacal defect, rectal prolapse, prior bowel diversion, fecal impaction, neurologic disorder leading to incontinence, use of loperamide or diphenoxylate within the last 30 days, childbirth within the last 3 months, need for antiretroviral drugs, hepatic impairment, or chronic abdominal pain without diarrhea.
Baseline characteristics and symptoms severity were similar among participants. The average age of the women was 63 years, with 79% white and 85% postmenopausal. Participants had a mean (SD) of 1.6 (1.8) leaks per day.
Participants were randomly assigned in a 0.5:1:1:1 fashion to receive oral placebo, loperamide, oral placebo with PFPT/biofeedback, or loperamide with PFPT/biofeedback. All participants received a standardized educational pamphlet that outlined dietary and behavioral recommendations.
Women assigned to PFPT/biofeedback received 6 sessions every other week. Loperamide was started at a dosage of 2 mg per day with the possibility of dose maintenance, escalation, reduction, or discontinuation.
Study outcomes. The primary outcome was a change from baseline to 24 weeks in the Vaizey FI symptom severity score, which assesses fecal frequency, urgency, and use of pads and medications. Secondary outcomes included assessment of a 7-day bowel diary and other quality-of-life measures. Data at 24 weeks were available for 89% of the women.
All treatment groups experienced improved FI symptoms
Based on changes in Vaizey scores after 24 weeks of treatment, women in all treatment groups had similar improvement in symptoms severity. However, those who received loperamide and PFPT/biofeedback had decreased pad changes per week and more accident-free days compared with women treated with placebo and biofeedback. Quality of life at 24 weeks was not statistically different between treatment groups as improvement was seen in all groups, including those who received oral placebo and patient education.
Adverse events. The proportion of gastrointestinal adverse effects was similar between treatment groups, ranging from 45% to 63%. Constipation was the most common adverse event overall and was more common in those taking loperamide, occurring in 51% of the loperamide plus PFPT/biofeedback group, 38% of those who received loperamide alone, 23% of the biofeedback with placebo group, and 12% of the placebo-alone group.
Strengths and limitations. Strengths of this study include its multisite, large sample size, low dropout rate, and sufficiently powered design to compare various combinations of first- and second-line therapies in women with a mean baseline FI of 1.6 leaks per day. Another strength is the robustness of the PFPT/biofeedback sessions that used anorectal manometry. This may, however, limit the study's external validity given that clinical use of this device is likely rare. Additionally, the population was comprised largely of postmenopausal and white women, which may make the findings less generalizable to other populations.
Women who suffer from frequent FI may require both loperamide and PFPT/biofeedback if they want to increase the likelihood of accident-free days and use of fewer pads. Should they note increased constipation or are not amenable to scheduled PFPT sessions, formalized education about dietary modifications, according to this study, will provide improvement in symptom severity.
Continue to: Novel vaginal bowel control system...
Novel vaginal bowel control system is effective, durable over 12 months for FI treatment
Richter HE, Dunivan G, Brown HW, et al. A 12-month clinical durability of effectiveness and safety evaluation of a vaginal bowel control system for the nonsurgical treatment of fecal incontinence. Female Pelvic Med Reconstr Surg. 2019;25:113-119.
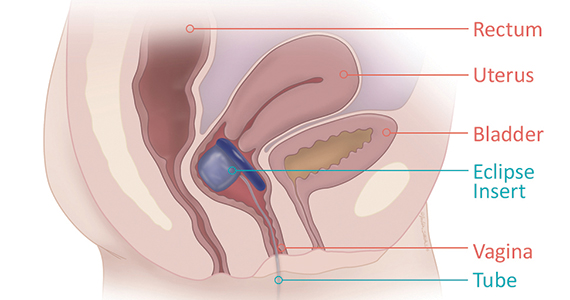
Richter and colleagues characterized clinical success, effect on quality of life, and durability over 12 months of a novel vaginal bowel control device (Eclipse System; Pelvalon) for FI in a prospective cohort study. The device is a silicone-coated vaginal insert with a detachable pump and balloon that deflects the rectovaginal septum posteriorly, thus impeding the passage of stool in the rectum (FIGURE 2).
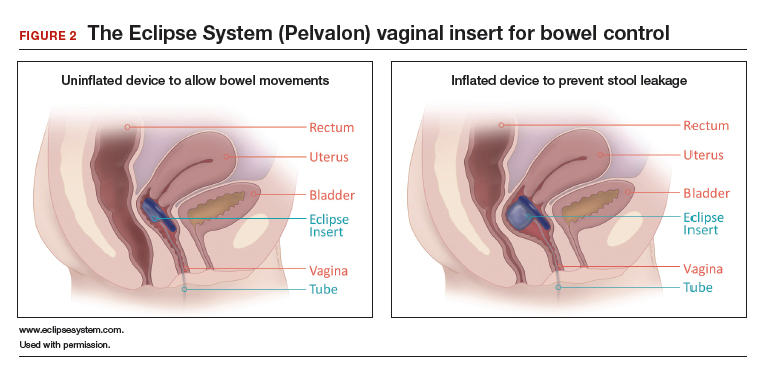
Study eligibility criteria and treatment protocol
Women were eligible for the study if they had 4 or more episodes of fecal soiling on a 2-week bowel diary and had FI for at least 6 months. Participants were excluded if they had prolapse outside the hymen, rectovaginal fistula, IBD, congenital anorectal malformation, urinary or colorectal infection, chronic pelvic or anorectal pain, pregnancy or planning pregnancy in the next 5 months, unmanaged chronic watery diarrhea, presence of an open wound or tear in the vagina, significant urogenital atrophy, or any psychiatric or neurologic disorder that would hinder the ability to participate.
Participants successfully fitted with the device (3 attempts were allowed) were entered into the study's run-in phase. Those who were successfully fitted and had a 50% or greater reduction in FI continued into the treatment phase with 12 months of follow-up.
Of the 137 women eligible for device fitting, 62% were successfully fitted. The 73 (86%) women who had a 50% or greater reduction in FI during the run-in period comprised the intent-to-treat study population. On average, these women were 61.3 years of age, with 70% white and 82% postmenopausal. At baseline, they had a mean of 14.1 episodes of FI over 2 weeks. (Prior to enrollment, 97.3% of women attempted self-management strategies, 17.8% to 23% failed conservative therapy, and 7.8% to 13.7% failed surgical therapy.) The follow-up rate at 12 months was 74%.
Study outcomes. The primary outcome was treatment success, defined as proportion of subjects with a 50% or greater reduction in FI episodes at 3 months; this outcome also was evaluated at 6 and 12 months. Secondary outcomes were the number of FI episodes and quality-of-life measures at 3, 6, and 12 months.
Treatment success, patient satisfaction high
In the treatment phase, women had sustained improvements in symptom severity and quality-of-life measures over 12 months. Treatment success was 73% at 3 months, 71% at 6 months, and 70% at 12 months. Complete continence was achieved in 46% of participants at 12 months, and major FI episodes (requiring immediate change of undergarments) decreased from 5.0 at baseline to 0.5 at 12 months. Quality-of-life measures were improved at 3 months, and improvement was sustained over 12 months. Satisfaction was 94% at 12 months.
Adverse events. No serious device-related adverse events occurred. Mild device-related adverse events were experienced by 45% of women during the fitting process and by 38% during treatment period. These included vaginal wall injury such as hyperemia and erosion; vaginal or pelvic discomfort; vaginal infection; constipation; and lower urinary tract issues such as urinary tract infection, urinary incontinence, and voiding dysfunction. No adverse events led to treatment discontinuation.
Strengths and limitations. Strengths of this study include that it was conducted at multiple clinical sites, had a large sample size, and had a 1-year follow-up period in a population with daily FI. A limitation was that only women who had a 50% or greater reduction in FI episodes during the run-in period were followed for 12 months; however, this was 86% of the original cohort. The use of a comparative group using other devices, such as anal plugs, would have strengthened this study.
The Eclipse intravaginal bowel control device (approved by the US Food and Drug Administration in 2015) provided a sustained 50% or greater reduction in FI episodes in more than 70% of women wearing the device for 1 year, with high patient satisfaction. Thus, for women who fail conservative treatment methods for FI, clinicians should consider referring them to a urogynecologist or specialist who is knowledgeable in fitting this vaginal bowel control device.
Continue to: Sacroneuromodulation for FI…
Sacral neuromodulation for FI is effective long-term
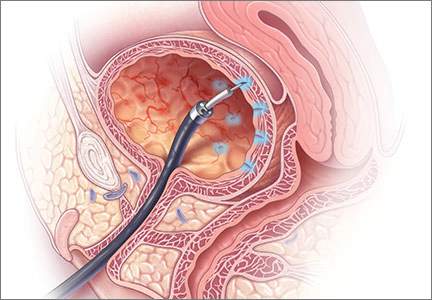
Hull T, Giese C, Wexner SD, et al; for the SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234-245.
In this multicenter, prospective cohort study, Hull and colleagues evaluated the 5-year efficacy of sacral neuromodulation (SNM), also known as sacral nerve stimulation, for treatment of FI. This study followed an earlier investigation by Wexner and colleagues, which reported that 83% of 120 patients treated with SNM had a 50% or greater improvement in FI episodes at 12 months.16
Details of the study
The investigators enrolled 133 participants (92% female) who had more than 2 episodes of FI per week for longer than 6 months (12 months after vaginal delivery). Participants were excluded if they had congenital anorectal malformations, prior rectal surgery within the past 12 months (or 24 months if due to cancer), defects greater than 120° of the external anal sphincter (EAS), IBD, unmanaged chronic watery diarrhea, stool consistency type 6 or type 7 on the Bristol Stool Scale, sequela of pelvic radiation, active anal abscess or fistula, pregnancy, or planned pregnancy.
Eligible participants underwent a 2-stage procedure with the InterStim bowel control device (Medtronic). If participants experienced a 50% or greater reduction in incontinence episodes with a wearable external SNM device in the test stimulation (stage 1), they received the chronic SNM implant device (stage 2).
Participants who underwent device implantation were followed at 1, 3, and 6 months and annually for 5 years or until they exited the study. Bowel diaries and quality of life assessments were completed at baseline and at follow-up.
The primary outcome was therapeutic success, defined as 50% or greater improvement in FI episodes per week.
A total of 120 participants (90%) underwent implantation of the chronic lead and neuromodulator, and 76 (63%) were followed for 5 years. Baseline characteristics available in the initial study of 133 participants showed that the mean age was 60.5 years; 25% had undergone a prior anal sphincteroplasty; and 16.5% and 10.5% had EAS or internal anal sphincter (IAS) defects, respectively, on endoanal ultrasonography.16
Therapeutic success was high at 5 years
At the 5-year follow-up, 89% (64/72) of participants met therapeutic success, with a reduction in weekly FI episodes from 9.1 at baseline to 1.7 at 5 years. The number of incontinence pads required decreased, and more participants wore no pads at 5 years. In the intention-to-treat analysis, carrying forward the baseline FI rate in participants who lacked follow-up data, the therapeutic success rate was 69%. Quality-of-life measures improved at 5 years, both statistically and by minimal clinical difference.
Adverse events. Sixty-eight percent of participants experienced device-related adverse events, including implant site pain, change in sensation of stimulation, change in efficacy, implant site infection, or neurostimulator battery depletion (neurostimulator use commonly expires after 3 to 5 years). Of these events, 80% were successfully treated with medications, reprogramming, or no intervention. The 5-year probability of device revision or replacement was 24.4%, and the 5-year probability of device explant was 19.0%.
Strengths and limitations. Overall, this study was a well-designed, multicenter trial with long-term follow-up that showed significant improvement in FI with the use of SNM. Its strengths include the enrollment of postmenopausal women who had current defects in EAS and/or IAS on endoanal ultrasonography and 25% who had a prior sphincteroplasty. The findings therefore are relevant to the gynecologic population in whom anal sphincteroplasty would not be recommended. The study also accounted for dropouts and reported the adjusted success rate of 69% at 5 years in that group.
The lack of a control arm to rule out the placebo effect is a limitation of this study, although randomized trials comparing the effect of SNM "on" versus "off" showed greater improvement with the device "on."17
Sacral neuromodulation is an excellent therapy for women with daily FI who have failed noninvasive options and desire to proceed to a more durable, long-lasting device therapy. Although adverse events may occur, they are mild and most often resolve with device reprogramming.
- Sultan AH, Monga A, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Neurourol Urodyn. 2017;36:10-34.
- Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127-136.
- Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004-1008.
- Perry S, Shaw C, McGrother C, et al; Leicestershire MRC Incontinence Study Team. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480-484.
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014;12:636-643.e1-2.
- Brown HW, Wexner SD, Lukacz ES. Factors associated with care seeking among women with accidental bowel leakage. Female Pelvic Med Reconstr Surg. 2013;19:66-71.
- Norton NJ. The perspective of the patient. Gastroenterology. 2004;126(1 suppl 1):S175-S179.
- Guan W, Schmuhl NB, Brown HW. Response re: If we don't ask, they won't tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2019;32:119.3-120.
- Whitehead WE, Borrud L, Goode PS, et al; Pelvic Floor Disorders Network. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
- Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157.
- Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559-1566.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924.
- Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623-636.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 210: Fecal incontinence. Obstet Gynecol. 2019;133:e260-e273.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730-1737.
- Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441-449.
- Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662-669.
Fecal incontinence (FI), also known as accidental bowel leakage, is the involuntary loss of feces, which includes both liquid and solid stool as defined by the International Continence Society (ICS) and the International Urogynecological Association (IUGA).1,2 Fecal incontinence is common, occurring in 7% to 25% of community-dwelling women, and it increases with age.2-6 The condition is rarely addressed, with only 30% of women seeking care.6-8 This is due to patient embarrassment and the lack of a reliable screening tool. However, FI affects quality of life and mental health, and the associated economic burden likely will rise given the increased prevalence of FI among older women.2,4,7,9
Fecal incontinence occurs due to poor stool consistency, anal and pelvic muscle weakness, reduced rectal compliance, reduced or increased rectal sensation, or bowel inflammation or dysfunction. Many conditions can cause FI (TABLE 1).5,10,11 It is therefore important to elicit a full medical history with a focus on specific bowel symptoms, such as stool consistency type (TABLE 2),12 FI frequency, and duration of symptoms, as well as to perform a complete examination to identify any readily reversible or malignant causes. A colonoscopy is recommended for individuals who meet screening criteria or present with a change in bowel symptoms, such as diarrhea, bleeding, or obstruction.13,14

Fecal incontinence treatments include a range of approaches categorized from conservative, or first-line therapy, to fourth-line surgical managements (FIGURE 1).1,10,13,14 In this Update, we review the results of 3 well-designed trials that enrolled women with frequent nonneurogenic FI.

Common first- and second-line treatments produce equivalent improvements in FI symptoms at
6 months
Jelovsek JE, Markland AD, Whitehead WE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomized clinical trial. Lancet Gastroenterol Hepatol. 2019;4:698-710.
In a multicenter, randomized trial of first- and second-line treatments for FI, Jelovsek and colleagues evaluated the efficacy of oral placebo, loperamide, pelvic floor physical therapy (PFPT) with biofeedback using anorectal manometry, or combination therapy over a 24-week period.
Continue to: Four treatments compared...
Four treatments compared
Three hundred women with FI occurring monthly for 3 months were included in the trial. Women were excluded if they had a stool classification of type 1 or type 7 on the Bristol Stool Scale, inflammatory bowel disease (IBD), history of rectovaginal fistula or cloacal defect, rectal prolapse, prior bowel diversion, fecal impaction, neurologic disorder leading to incontinence, use of loperamide or diphenoxylate within the last 30 days, childbirth within the last 3 months, need for antiretroviral drugs, hepatic impairment, or chronic abdominal pain without diarrhea.
Baseline characteristics and symptoms severity were similar among participants. The average age of the women was 63 years, with 79% white and 85% postmenopausal. Participants had a mean (SD) of 1.6 (1.8) leaks per day.
Participants were randomly assigned in a 0.5:1:1:1 fashion to receive oral placebo, loperamide, oral placebo with PFPT/biofeedback, or loperamide with PFPT/biofeedback. All participants received a standardized educational pamphlet that outlined dietary and behavioral recommendations.
Women assigned to PFPT/biofeedback received 6 sessions every other week. Loperamide was started at a dosage of 2 mg per day with the possibility of dose maintenance, escalation, reduction, or discontinuation.
Study outcomes. The primary outcome was a change from baseline to 24 weeks in the Vaizey FI symptom severity score, which assesses fecal frequency, urgency, and use of pads and medications. Secondary outcomes included assessment of a 7-day bowel diary and other quality-of-life measures. Data at 24 weeks were available for 89% of the women.
All treatment groups experienced improved FI symptoms
Based on changes in Vaizey scores after 24 weeks of treatment, women in all treatment groups had similar improvement in symptoms severity. However, those who received loperamide and PFPT/biofeedback had decreased pad changes per week and more accident-free days compared with women treated with placebo and biofeedback. Quality of life at 24 weeks was not statistically different between treatment groups as improvement was seen in all groups, including those who received oral placebo and patient education.
Adverse events. The proportion of gastrointestinal adverse effects was similar between treatment groups, ranging from 45% to 63%. Constipation was the most common adverse event overall and was more common in those taking loperamide, occurring in 51% of the loperamide plus PFPT/biofeedback group, 38% of those who received loperamide alone, 23% of the biofeedback with placebo group, and 12% of the placebo-alone group.
Strengths and limitations. Strengths of this study include its multisite, large sample size, low dropout rate, and sufficiently powered design to compare various combinations of first- and second-line therapies in women with a mean baseline FI of 1.6 leaks per day. Another strength is the robustness of the PFPT/biofeedback sessions that used anorectal manometry. This may, however, limit the study's external validity given that clinical use of this device is likely rare. Additionally, the population was comprised largely of postmenopausal and white women, which may make the findings less generalizable to other populations.
Women who suffer from frequent FI may require both loperamide and PFPT/biofeedback if they want to increase the likelihood of accident-free days and use of fewer pads. Should they note increased constipation or are not amenable to scheduled PFPT sessions, formalized education about dietary modifications, according to this study, will provide improvement in symptom severity.
Continue to: Novel vaginal bowel control system...
Novel vaginal bowel control system is effective, durable over 12 months for FI treatment
Richter HE, Dunivan G, Brown HW, et al. A 12-month clinical durability of effectiveness and safety evaluation of a vaginal bowel control system for the nonsurgical treatment of fecal incontinence. Female Pelvic Med Reconstr Surg. 2019;25:113-119.
Richter and colleagues characterized clinical success, effect on quality of life, and durability over 12 months of a novel vaginal bowel control device (Eclipse System; Pelvalon) for FI in a prospective cohort study. The device is a silicone-coated vaginal insert with a detachable pump and balloon that deflects the rectovaginal septum posteriorly, thus impeding the passage of stool in the rectum (FIGURE 2).

Study eligibility criteria and treatment protocol
Women were eligible for the study if they had 4 or more episodes of fecal soiling on a 2-week bowel diary and had FI for at least 6 months. Participants were excluded if they had prolapse outside the hymen, rectovaginal fistula, IBD, congenital anorectal malformation, urinary or colorectal infection, chronic pelvic or anorectal pain, pregnancy or planning pregnancy in the next 5 months, unmanaged chronic watery diarrhea, presence of an open wound or tear in the vagina, significant urogenital atrophy, or any psychiatric or neurologic disorder that would hinder the ability to participate.
Participants successfully fitted with the device (3 attempts were allowed) were entered into the study's run-in phase. Those who were successfully fitted and had a 50% or greater reduction in FI continued into the treatment phase with 12 months of follow-up.
Of the 137 women eligible for device fitting, 62% were successfully fitted. The 73 (86%) women who had a 50% or greater reduction in FI during the run-in period comprised the intent-to-treat study population. On average, these women were 61.3 years of age, with 70% white and 82% postmenopausal. At baseline, they had a mean of 14.1 episodes of FI over 2 weeks. (Prior to enrollment, 97.3% of women attempted self-management strategies, 17.8% to 23% failed conservative therapy, and 7.8% to 13.7% failed surgical therapy.) The follow-up rate at 12 months was 74%.
Study outcomes. The primary outcome was treatment success, defined as proportion of subjects with a 50% or greater reduction in FI episodes at 3 months; this outcome also was evaluated at 6 and 12 months. Secondary outcomes were the number of FI episodes and quality-of-life measures at 3, 6, and 12 months.
Treatment success, patient satisfaction high
In the treatment phase, women had sustained improvements in symptom severity and quality-of-life measures over 12 months. Treatment success was 73% at 3 months, 71% at 6 months, and 70% at 12 months. Complete continence was achieved in 46% of participants at 12 months, and major FI episodes (requiring immediate change of undergarments) decreased from 5.0 at baseline to 0.5 at 12 months. Quality-of-life measures were improved at 3 months, and improvement was sustained over 12 months. Satisfaction was 94% at 12 months.
Adverse events. No serious device-related adverse events occurred. Mild device-related adverse events were experienced by 45% of women during the fitting process and by 38% during treatment period. These included vaginal wall injury such as hyperemia and erosion; vaginal or pelvic discomfort; vaginal infection; constipation; and lower urinary tract issues such as urinary tract infection, urinary incontinence, and voiding dysfunction. No adverse events led to treatment discontinuation.
Strengths and limitations. Strengths of this study include that it was conducted at multiple clinical sites, had a large sample size, and had a 1-year follow-up period in a population with daily FI. A limitation was that only women who had a 50% or greater reduction in FI episodes during the run-in period were followed for 12 months; however, this was 86% of the original cohort. The use of a comparative group using other devices, such as anal plugs, would have strengthened this study.
The Eclipse intravaginal bowel control device (approved by the US Food and Drug Administration in 2015) provided a sustained 50% or greater reduction in FI episodes in more than 70% of women wearing the device for 1 year, with high patient satisfaction. Thus, for women who fail conservative treatment methods for FI, clinicians should consider referring them to a urogynecologist or specialist who is knowledgeable in fitting this vaginal bowel control device.
Continue to: Sacroneuromodulation for FI…
Sacral neuromodulation for FI is effective long-term
Hull T, Giese C, Wexner SD, et al; for the SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234-245.
In this multicenter, prospective cohort study, Hull and colleagues evaluated the 5-year efficacy of sacral neuromodulation (SNM), also known as sacral nerve stimulation, for treatment of FI. This study followed an earlier investigation by Wexner and colleagues, which reported that 83% of 120 patients treated with SNM had a 50% or greater improvement in FI episodes at 12 months.16
Details of the study
The investigators enrolled 133 participants (92% female) who had more than 2 episodes of FI per week for longer than 6 months (12 months after vaginal delivery). Participants were excluded if they had congenital anorectal malformations, prior rectal surgery within the past 12 months (or 24 months if due to cancer), defects greater than 120° of the external anal sphincter (EAS), IBD, unmanaged chronic watery diarrhea, stool consistency type 6 or type 7 on the Bristol Stool Scale, sequela of pelvic radiation, active anal abscess or fistula, pregnancy, or planned pregnancy.
Eligible participants underwent a 2-stage procedure with the InterStim bowel control device (Medtronic). If participants experienced a 50% or greater reduction in incontinence episodes with a wearable external SNM device in the test stimulation (stage 1), they received the chronic SNM implant device (stage 2).
Participants who underwent device implantation were followed at 1, 3, and 6 months and annually for 5 years or until they exited the study. Bowel diaries and quality of life assessments were completed at baseline and at follow-up.
The primary outcome was therapeutic success, defined as 50% or greater improvement in FI episodes per week.
A total of 120 participants (90%) underwent implantation of the chronic lead and neuromodulator, and 76 (63%) were followed for 5 years. Baseline characteristics available in the initial study of 133 participants showed that the mean age was 60.5 years; 25% had undergone a prior anal sphincteroplasty; and 16.5% and 10.5% had EAS or internal anal sphincter (IAS) defects, respectively, on endoanal ultrasonography.16
Therapeutic success was high at 5 years
At the 5-year follow-up, 89% (64/72) of participants met therapeutic success, with a reduction in weekly FI episodes from 9.1 at baseline to 1.7 at 5 years. The number of incontinence pads required decreased, and more participants wore no pads at 5 years. In the intention-to-treat analysis, carrying forward the baseline FI rate in participants who lacked follow-up data, the therapeutic success rate was 69%. Quality-of-life measures improved at 5 years, both statistically and by minimal clinical difference.
Adverse events. Sixty-eight percent of participants experienced device-related adverse events, including implant site pain, change in sensation of stimulation, change in efficacy, implant site infection, or neurostimulator battery depletion (neurostimulator use commonly expires after 3 to 5 years). Of these events, 80% were successfully treated with medications, reprogramming, or no intervention. The 5-year probability of device revision or replacement was 24.4%, and the 5-year probability of device explant was 19.0%.
Strengths and limitations. Overall, this study was a well-designed, multicenter trial with long-term follow-up that showed significant improvement in FI with the use of SNM. Its strengths include the enrollment of postmenopausal women who had current defects in EAS and/or IAS on endoanal ultrasonography and 25% who had a prior sphincteroplasty. The findings therefore are relevant to the gynecologic population in whom anal sphincteroplasty would not be recommended. The study also accounted for dropouts and reported the adjusted success rate of 69% at 5 years in that group.
The lack of a control arm to rule out the placebo effect is a limitation of this study, although randomized trials comparing the effect of SNM "on" versus "off" showed greater improvement with the device "on."17
Sacral neuromodulation is an excellent therapy for women with daily FI who have failed noninvasive options and desire to proceed to a more durable, long-lasting device therapy. Although adverse events may occur, they are mild and most often resolve with device reprogramming.
Fecal incontinence (FI), also known as accidental bowel leakage, is the involuntary loss of feces, which includes both liquid and solid stool as defined by the International Continence Society (ICS) and the International Urogynecological Association (IUGA).1,2 Fecal incontinence is common, occurring in 7% to 25% of community-dwelling women, and it increases with age.2-6 The condition is rarely addressed, with only 30% of women seeking care.6-8 This is due to patient embarrassment and the lack of a reliable screening tool. However, FI affects quality of life and mental health, and the associated economic burden likely will rise given the increased prevalence of FI among older women.2,4,7,9
Fecal incontinence occurs due to poor stool consistency, anal and pelvic muscle weakness, reduced rectal compliance, reduced or increased rectal sensation, or bowel inflammation or dysfunction. Many conditions can cause FI (TABLE 1).5,10,11 It is therefore important to elicit a full medical history with a focus on specific bowel symptoms, such as stool consistency type (TABLE 2),12 FI frequency, and duration of symptoms, as well as to perform a complete examination to identify any readily reversible or malignant causes. A colonoscopy is recommended for individuals who meet screening criteria or present with a change in bowel symptoms, such as diarrhea, bleeding, or obstruction.13,14

Fecal incontinence treatments include a range of approaches categorized from conservative, or first-line therapy, to fourth-line surgical managements (FIGURE 1).1,10,13,14 In this Update, we review the results of 3 well-designed trials that enrolled women with frequent nonneurogenic FI.

Common first- and second-line treatments produce equivalent improvements in FI symptoms at
6 months
Jelovsek JE, Markland AD, Whitehead WE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomized clinical trial. Lancet Gastroenterol Hepatol. 2019;4:698-710.
In a multicenter, randomized trial of first- and second-line treatments for FI, Jelovsek and colleagues evaluated the efficacy of oral placebo, loperamide, pelvic floor physical therapy (PFPT) with biofeedback using anorectal manometry, or combination therapy over a 24-week period.
Continue to: Four treatments compared...
Four treatments compared
Three hundred women with FI occurring monthly for 3 months were included in the trial. Women were excluded if they had a stool classification of type 1 or type 7 on the Bristol Stool Scale, inflammatory bowel disease (IBD), history of rectovaginal fistula or cloacal defect, rectal prolapse, prior bowel diversion, fecal impaction, neurologic disorder leading to incontinence, use of loperamide or diphenoxylate within the last 30 days, childbirth within the last 3 months, need for antiretroviral drugs, hepatic impairment, or chronic abdominal pain without diarrhea.
Baseline characteristics and symptoms severity were similar among participants. The average age of the women was 63 years, with 79% white and 85% postmenopausal. Participants had a mean (SD) of 1.6 (1.8) leaks per day.
Participants were randomly assigned in a 0.5:1:1:1 fashion to receive oral placebo, loperamide, oral placebo with PFPT/biofeedback, or loperamide with PFPT/biofeedback. All participants received a standardized educational pamphlet that outlined dietary and behavioral recommendations.
Women assigned to PFPT/biofeedback received 6 sessions every other week. Loperamide was started at a dosage of 2 mg per day with the possibility of dose maintenance, escalation, reduction, or discontinuation.
Study outcomes. The primary outcome was a change from baseline to 24 weeks in the Vaizey FI symptom severity score, which assesses fecal frequency, urgency, and use of pads and medications. Secondary outcomes included assessment of a 7-day bowel diary and other quality-of-life measures. Data at 24 weeks were available for 89% of the women.
All treatment groups experienced improved FI symptoms
Based on changes in Vaizey scores after 24 weeks of treatment, women in all treatment groups had similar improvement in symptoms severity. However, those who received loperamide and PFPT/biofeedback had decreased pad changes per week and more accident-free days compared with women treated with placebo and biofeedback. Quality of life at 24 weeks was not statistically different between treatment groups as improvement was seen in all groups, including those who received oral placebo and patient education.
Adverse events. The proportion of gastrointestinal adverse effects was similar between treatment groups, ranging from 45% to 63%. Constipation was the most common adverse event overall and was more common in those taking loperamide, occurring in 51% of the loperamide plus PFPT/biofeedback group, 38% of those who received loperamide alone, 23% of the biofeedback with placebo group, and 12% of the placebo-alone group.
Strengths and limitations. Strengths of this study include its multisite, large sample size, low dropout rate, and sufficiently powered design to compare various combinations of first- and second-line therapies in women with a mean baseline FI of 1.6 leaks per day. Another strength is the robustness of the PFPT/biofeedback sessions that used anorectal manometry. This may, however, limit the study's external validity given that clinical use of this device is likely rare. Additionally, the population was comprised largely of postmenopausal and white women, which may make the findings less generalizable to other populations.
Women who suffer from frequent FI may require both loperamide and PFPT/biofeedback if they want to increase the likelihood of accident-free days and use of fewer pads. Should they note increased constipation or are not amenable to scheduled PFPT sessions, formalized education about dietary modifications, according to this study, will provide improvement in symptom severity.
Continue to: Novel vaginal bowel control system...
Novel vaginal bowel control system is effective, durable over 12 months for FI treatment
Richter HE, Dunivan G, Brown HW, et al. A 12-month clinical durability of effectiveness and safety evaluation of a vaginal bowel control system for the nonsurgical treatment of fecal incontinence. Female Pelvic Med Reconstr Surg. 2019;25:113-119.
Richter and colleagues characterized clinical success, effect on quality of life, and durability over 12 months of a novel vaginal bowel control device (Eclipse System; Pelvalon) for FI in a prospective cohort study. The device is a silicone-coated vaginal insert with a detachable pump and balloon that deflects the rectovaginal septum posteriorly, thus impeding the passage of stool in the rectum (FIGURE 2).

Study eligibility criteria and treatment protocol
Women were eligible for the study if they had 4 or more episodes of fecal soiling on a 2-week bowel diary and had FI for at least 6 months. Participants were excluded if they had prolapse outside the hymen, rectovaginal fistula, IBD, congenital anorectal malformation, urinary or colorectal infection, chronic pelvic or anorectal pain, pregnancy or planning pregnancy in the next 5 months, unmanaged chronic watery diarrhea, presence of an open wound or tear in the vagina, significant urogenital atrophy, or any psychiatric or neurologic disorder that would hinder the ability to participate.
Participants successfully fitted with the device (3 attempts were allowed) were entered into the study's run-in phase. Those who were successfully fitted and had a 50% or greater reduction in FI continued into the treatment phase with 12 months of follow-up.
Of the 137 women eligible for device fitting, 62% were successfully fitted. The 73 (86%) women who had a 50% or greater reduction in FI during the run-in period comprised the intent-to-treat study population. On average, these women were 61.3 years of age, with 70% white and 82% postmenopausal. At baseline, they had a mean of 14.1 episodes of FI over 2 weeks. (Prior to enrollment, 97.3% of women attempted self-management strategies, 17.8% to 23% failed conservative therapy, and 7.8% to 13.7% failed surgical therapy.) The follow-up rate at 12 months was 74%.
Study outcomes. The primary outcome was treatment success, defined as proportion of subjects with a 50% or greater reduction in FI episodes at 3 months; this outcome also was evaluated at 6 and 12 months. Secondary outcomes were the number of FI episodes and quality-of-life measures at 3, 6, and 12 months.
Treatment success, patient satisfaction high
In the treatment phase, women had sustained improvements in symptom severity and quality-of-life measures over 12 months. Treatment success was 73% at 3 months, 71% at 6 months, and 70% at 12 months. Complete continence was achieved in 46% of participants at 12 months, and major FI episodes (requiring immediate change of undergarments) decreased from 5.0 at baseline to 0.5 at 12 months. Quality-of-life measures were improved at 3 months, and improvement was sustained over 12 months. Satisfaction was 94% at 12 months.
Adverse events. No serious device-related adverse events occurred. Mild device-related adverse events were experienced by 45% of women during the fitting process and by 38% during treatment period. These included vaginal wall injury such as hyperemia and erosion; vaginal or pelvic discomfort; vaginal infection; constipation; and lower urinary tract issues such as urinary tract infection, urinary incontinence, and voiding dysfunction. No adverse events led to treatment discontinuation.
Strengths and limitations. Strengths of this study include that it was conducted at multiple clinical sites, had a large sample size, and had a 1-year follow-up period in a population with daily FI. A limitation was that only women who had a 50% or greater reduction in FI episodes during the run-in period were followed for 12 months; however, this was 86% of the original cohort. The use of a comparative group using other devices, such as anal plugs, would have strengthened this study.
The Eclipse intravaginal bowel control device (approved by the US Food and Drug Administration in 2015) provided a sustained 50% or greater reduction in FI episodes in more than 70% of women wearing the device for 1 year, with high patient satisfaction. Thus, for women who fail conservative treatment methods for FI, clinicians should consider referring them to a urogynecologist or specialist who is knowledgeable in fitting this vaginal bowel control device.
Continue to: Sacroneuromodulation for FI…
Sacral neuromodulation for FI is effective long-term
Hull T, Giese C, Wexner SD, et al; for the SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234-245.
In this multicenter, prospective cohort study, Hull and colleagues evaluated the 5-year efficacy of sacral neuromodulation (SNM), also known as sacral nerve stimulation, for treatment of FI. This study followed an earlier investigation by Wexner and colleagues, which reported that 83% of 120 patients treated with SNM had a 50% or greater improvement in FI episodes at 12 months.16
Details of the study
The investigators enrolled 133 participants (92% female) who had more than 2 episodes of FI per week for longer than 6 months (12 months after vaginal delivery). Participants were excluded if they had congenital anorectal malformations, prior rectal surgery within the past 12 months (or 24 months if due to cancer), defects greater than 120° of the external anal sphincter (EAS), IBD, unmanaged chronic watery diarrhea, stool consistency type 6 or type 7 on the Bristol Stool Scale, sequela of pelvic radiation, active anal abscess or fistula, pregnancy, or planned pregnancy.
Eligible participants underwent a 2-stage procedure with the InterStim bowel control device (Medtronic). If participants experienced a 50% or greater reduction in incontinence episodes with a wearable external SNM device in the test stimulation (stage 1), they received the chronic SNM implant device (stage 2).
Participants who underwent device implantation were followed at 1, 3, and 6 months and annually for 5 years or until they exited the study. Bowel diaries and quality of life assessments were completed at baseline and at follow-up.
The primary outcome was therapeutic success, defined as 50% or greater improvement in FI episodes per week.
A total of 120 participants (90%) underwent implantation of the chronic lead and neuromodulator, and 76 (63%) were followed for 5 years. Baseline characteristics available in the initial study of 133 participants showed that the mean age was 60.5 years; 25% had undergone a prior anal sphincteroplasty; and 16.5% and 10.5% had EAS or internal anal sphincter (IAS) defects, respectively, on endoanal ultrasonography.16
Therapeutic success was high at 5 years
At the 5-year follow-up, 89% (64/72) of participants met therapeutic success, with a reduction in weekly FI episodes from 9.1 at baseline to 1.7 at 5 years. The number of incontinence pads required decreased, and more participants wore no pads at 5 years. In the intention-to-treat analysis, carrying forward the baseline FI rate in participants who lacked follow-up data, the therapeutic success rate was 69%. Quality-of-life measures improved at 5 years, both statistically and by minimal clinical difference.
Adverse events. Sixty-eight percent of participants experienced device-related adverse events, including implant site pain, change in sensation of stimulation, change in efficacy, implant site infection, or neurostimulator battery depletion (neurostimulator use commonly expires after 3 to 5 years). Of these events, 80% were successfully treated with medications, reprogramming, or no intervention. The 5-year probability of device revision or replacement was 24.4%, and the 5-year probability of device explant was 19.0%.
Strengths and limitations. Overall, this study was a well-designed, multicenter trial with long-term follow-up that showed significant improvement in FI with the use of SNM. Its strengths include the enrollment of postmenopausal women who had current defects in EAS and/or IAS on endoanal ultrasonography and 25% who had a prior sphincteroplasty. The findings therefore are relevant to the gynecologic population in whom anal sphincteroplasty would not be recommended. The study also accounted for dropouts and reported the adjusted success rate of 69% at 5 years in that group.
The lack of a control arm to rule out the placebo effect is a limitation of this study, although randomized trials comparing the effect of SNM "on" versus "off" showed greater improvement with the device "on."17
Sacral neuromodulation is an excellent therapy for women with daily FI who have failed noninvasive options and desire to proceed to a more durable, long-lasting device therapy. Although adverse events may occur, they are mild and most often resolve with device reprogramming.
- Sultan AH, Monga A, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Neurourol Urodyn. 2017;36:10-34.
- Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127-136.
- Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004-1008.
- Perry S, Shaw C, McGrother C, et al; Leicestershire MRC Incontinence Study Team. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480-484.
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014;12:636-643.e1-2.
- Brown HW, Wexner SD, Lukacz ES. Factors associated with care seeking among women with accidental bowel leakage. Female Pelvic Med Reconstr Surg. 2013;19:66-71.
- Norton NJ. The perspective of the patient. Gastroenterology. 2004;126(1 suppl 1):S175-S179.
- Guan W, Schmuhl NB, Brown HW. Response re: If we don't ask, they won't tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2019;32:119.3-120.
- Whitehead WE, Borrud L, Goode PS, et al; Pelvic Floor Disorders Network. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
- Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157.
- Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559-1566.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924.
- Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623-636.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 210: Fecal incontinence. Obstet Gynecol. 2019;133:e260-e273.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730-1737.
- Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441-449.
- Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662-669.
- Sultan AH, Monga A, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Neurourol Urodyn. 2017;36:10-34.
- Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127-136.
- Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004-1008.
- Perry S, Shaw C, McGrother C, et al; Leicestershire MRC Incontinence Study Team. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480-484.
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014;12:636-643.e1-2.
- Brown HW, Wexner SD, Lukacz ES. Factors associated with care seeking among women with accidental bowel leakage. Female Pelvic Med Reconstr Surg. 2013;19:66-71.
- Norton NJ. The perspective of the patient. Gastroenterology. 2004;126(1 suppl 1):S175-S179.
- Guan W, Schmuhl NB, Brown HW. Response re: If we don't ask, they won't tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2019;32:119.3-120.
- Whitehead WE, Borrud L, Goode PS, et al; Pelvic Floor Disorders Network. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
- Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157.
- Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559-1566.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924.
- Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623-636.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 210: Fecal incontinence. Obstet Gynecol. 2019;133:e260-e273.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730-1737.
- Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441-449.
- Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662-669.
2018 Update on pelvic floor dysfunction
Using cystoscopy to evaluate ureteral efflux and bladder integrity following benign gynecologic surgery increases the detection rate of urinary tract injuries.1 Currently, it is standard of care to perform a cystoscopy following anti-incontinence procedures, but there is no consensus among ObGyns regarding the use of universal cystoscopy following benign gynecologic surgery.2 A number of studies, however, have suggested potential best practices for evaluating urinary tract injury during pelvic surgery for benign gynecologic conditions.
Pelvic surgeries for benign gynecologic conditions, including fibroids, menorrhagia, and pelvic organ prolapse (POP), are common. More than 500,000 hysterectomies are performed annually in the United States, and up to 11% of women will undergo at least one surgery for POP or urinary incontinence in their lifetime.3,4 During gynecologic surgery, the urinary tract is at risk, and the injury rate ranges from 0.02% to 2% for ureteral injury and from 1% to 5% for bladder injury.5,6
In a recent large randomized controlled trial, the rate of intraoperative ureteral obstruction following uterosacral ligament suspension (USLS) was 3.2%.7 Vaginal vault suspensions, as well as other vaginal cuff closure techniques, are common procedures associated with urinary tract injury.8 Additionally, ureteral injury during surgery for POP occurs in as many as 2% of anterior vaginal wall repairs.9
It is well documented that a delay in diagnosis of ureteral and/or bladder injuries is associated with increased morbidity, including the need for additional surgery to repair the injury; in addition, significant delay in identifying an injury may lead to subsequent sequela, such as renal injury and fistula formation.8
A large study in California found that 36.5% of hysterectomies performed for POP were performed by general gynecologists.10 General ObGyns performing these surgeries therefore must understand the risk of urinary tract injury during hysterectomy and reconstructive pelvic procedures so that they can appropriately identify, evaluate, and repair injuries in a timely fashion.
The best way to identify urinary tract injury at the time of gynecologic surgery is by cystoscopy, including a bladder survey and ureteral efflux evaluation. When should a cystoscopy be performed, and what is the best method for visualizing ureteral efflux? Can instituting universal cystoscopy for all gynecologic procedures make a difference in the rate of injury detection? In this Update, we summarize the data from 4 studies that help to answer these questions.
Continue to: About 30% of urinary tract injuries...
About 30% of urinary tract injuries identified prior to cystoscopy at hysterectomy (which detected 5 of 6 injuries)
Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192(5):1599–1604.
Vakili and colleagues conducted a multicenter prospective cohort study of women undergoing hysterectomy for benign indications; cystoscopy was performed in all cases. The 3 hospitals involved were all part of the Louisiana State University Health system. The investigators’ goal was to determine the rate of urinary tract injury in this patient population at the time of intraoperative cystoscopy.
Intraoperative cystoscopy beats visual evaluation
Four hundred and seventy-one women underwent hysterectomy and had intraoperative cystoscopy, including evaluation of ureteral patency with administration of intravenous (IV) indigo carmine. Patients underwent abdominal, vaginal, or laparoscopic hysterectomy, and 54 (11.4%) had concurrent POP or anti-incontinence procedures. The majority underwent an abdominal hysterectomy (59%), 31% had a vaginal hysterectomy, and 10% had a laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy.
Rate of urinary tract injuries. The total urinary tract injury rate detected by cystoscopy was 4.8%. The ureteral injury rate was 1.7%, and the bladder injury rate was 3.6%. A combined ureteral and bladder injury occurred in 2 women.
Surgery for POP significantly increased the risk of ureteral injury (7.3% vs 1.2%; P = .025). All cases of ureteral injury during POP surgery occurred during USLS. There was a trend toward a higher rate of bladder injury in the group with concurrent anti-incontinence surgery (12.5% vs 3.1%; P = .049). Regarding the route of hysterectomy, the vaginal approach had the highest rate of ureteral injury; however, when prolapse cases were removed from the analysis, there were no differences between the abdominal, vaginal, and laparoscopic approaches for ureteral or bladder injuries.
Injury detection with cystoscopy. Importantly, the authors found that only 30% of injuries were identified prior to performing intraoperative cystoscopy. The majority of these were bladder injuries. In addition, despite visual confirmation of ureteral peristalsis during abdominal hysterectomy, when intraoperative cystoscopy was performed with evaluation for ureteral efflux, 5 of 6 ureteral injury cases were identified. The authors reported 1 postoperative vesicovaginal fistula and concluded that it was likely due to an unrecognized bladder injury. No other undetected injuries were identified.
Notably, no complications occurred as a result of cystoscopy.
Multiple surgical indications reflect real-world scenario
The study included physicians from 3 different hospitals and all routes of hysterectomy for multiple benign gynecologic indications as well as concomitant pelvic reconstructive procedures. While this enhances the generalizability of the study results, all study sites were located in Louisiana at hospitals with resident trainee involvement. Additionally, this study confirms previous retrospective studies that reported higher rates of injury with pelvic reconstructive procedures.
The study is limited by the inability to blind surgeons, which may have resulted in the surgeons altering their techniques and/or having a heightened awareness of the urinary tract. However, their rates of ureteral and bladder injuries were slightly higher than previously reported rates, suggesting that the procedures inherently carry risk. The study is further limited by the lack of a retrospective comparison group of hysterectomy without routine cystoscopy and a longer follow-up period that may have revealed additional missed delayed urologic injuries.
The rate of urinary tract injury, including both bladder and ureteral injuries, was more than 4% at the time of hysterectomy for benign conditions. Using intraoperative peristalsis or normal ureteral caliber could result in a false sense of security since these are not reliable signs of ureteral integrity. The majority of urinary tract injuries will not be identified without cystoscopic evaluation.
Continue to: Universal cystoscopy policy...
Universal cystoscopy policy proves protective, surgeon adherence is high
Chi AM, Curran DS, Morgan DM, Fenner DE, Swenson CW. Universal cystoscopy after benign hysterectomy: examining the effects of an institutional policy. Obstet Gynecol. 2016;127(2):369–375.
In a retrospective cohort study, Chi and colleagues evaluated urinary tract injuries at the time of hysterectomy before and after the institution of a universal cystoscopy policy. At the time of policy implementation at the University of Michigan, all faculty who performed hysterectomies attended a cystoscopy workshop. Attending physicians without prior cystoscopy training also were proctored in the operating room for 3 sessions and were required to demonstrate competency with bladder survey, visualizing ureteral efflux, and urethral assessment. Indigo carmine was used to visualize ureteral efflux.
Detection of urologic injury almost doubled with cystoscopy
A total of 2,822 hysterectomies were included in the study, with 973 in the pre–universal cystoscopy group and 1,849 in the post–universal cystoscopy group. The study period was 7 years, and data on complications were abstracted for 1 year after the completion of the study period.
The primary outcome had 3 components:
- the rate of urologic injury before and after the policy
- the cystoscopy detection rate of urologic injury
- the adherence rate to the policy.
The overall rate of bladder and ureteral injury was 2.1%; the rate of injury during pre–universal screening was 2.6%, and during post–universal screening was 1.8%. The intraoperative detection rate of injury nearly doubled, from 24% to 47%, when intraoperative cystoscopy was utilized. In addition, the percentage of delayed urologic complications decreased from 28% to 5.9% (P = .03) following implementation of the universal cystoscopy policy. With regard to surgeon adherence, cystoscopy was documented in 86.1% of the hysterectomy cases after the policy was implemented compared with 35.7% of cases before the policy.
The investigators performed a cost analysis and found that hospital costs were nearly twice as much if a delayed urologic injury was diagnosed.
Study had many strengths
This study evaluated aspects of implementing quality initiatives after proper training and proctoring of a procedure. The authors compared very large cohorts from a busy academic medical center in which surgeon adherence with routine cystoscopy was high. The majority of patient outcomes were tracked for an extended period following surgery, thereby minimizing the risk of missing delayed urologic injuries. Notably, however, there was shorter follow-up time for the post–universal cystoscopy group, which could result in underestimating the rate of delayed urologic injuries in this cohort.
Instituting a universal cystoscopy policy for hysterectomy was associated with a significant decrease in delayed postoperative urinary tract complications and an increase in the intraoperative detection rate of urologic injuries. Intraoperative detection and repair of a urinary tract injury is cost-effective compared with a delayed diagnosis.
Continue to: Cystoscopy reveals ureteral obstruction...
Cystoscopy reveals ureteral obstruction during various vaginal POP repair procedures
Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194(5):1478–1485.
To determine the rate of ureteral obstruction and ureteral injury during vaginal surgery for POP and the accuracy of using intraoperative cystoscopy to prevent upper urinary tract morbidity, Gustilo-Ashby and colleagues performed a retrospective review study of a large patient cohort.
Cystoscopy with indigo carmine is highly sensitive
The study included 700 patients who underwent vaginal surgery for anterior and/or apical POP. Patients had 1 or more of the following anterior and apical prolapse repair procedures: USLS (51%), distal McCall culdeplasty (26%), proximal McCall culdeplasty (29%), anterior colporrhaphy (82%), and colpocleisis (1.4%). Of note, distal McCall culdeplasty was defined as incorporation of the “vaginal epithelium into the uterosacral plication,” while proximal McCall culdeplasty involved plication of “the uterosacral ligaments in the midline proximal to the vaginal cuff.” All patients were given IV indigo carmine to aid in visualizing ureteral efflux.
The majority of patients had a hysterectomy (56%). When accounting for rare false-positive and negative cystoscopy results, the overall ureteral obstruction rate was 5.1% and the ureteral injury rate was 0.9%. The majority of obstructions occurred with USLS (5.9%), proximal McCall culdeplasty (4.4%), and colpocleisis (4.2%). Ureteral injuries occurred only in 6 cases: 3 USLS and 3 proximal McCall culdeplasty procedures.
Based on these findings, the authors calculated that cystoscopy at the time of vaginal surgery for anterior and/or apical prolapse has a sensitivity of 94.4% and a specificity of 99.5% for detecting ureteral obstruction. The positive predictive value of cystoscopy with the use of indigo carmine for detection of ureteral obstruction is 91.9% and the negative predictive value is 99.7%.
Impact of indigo carmine’s unavailability
This study’s strengths include its large sample size and the variety of surgical approaches used for repair of anterior vaginal wall and apical prolapse. Its retrospective design, however, is a limitation; this could result in underreporting of ureteral injuries if patients received care at another institution after surgery. Furthermore, it is unclear if cystoscopy would be as predictive of ureteral injury without the use of indigo carmine, which is no longer available at most institutions.
The utility of cystoscopy with IV indigo carmine as a screening test for ureteral obstruction is highlighted by the fact that most obstructions were relieved by intraoperative suture removal following positive cystoscopy. McCall culdeplasty procedures are commonly performed by general ObGyns at the time of vaginal hysterectomy. It is therefore important to note that rates of ureteral obstruction after proximal McCall culdeplasty were only slightly lower than those after USLS.
Continue to: Sodium fluorescein and 10% dextrose...
Sodium fluorescein and 10% dextrose provide clear visibility of ureteral jets in cystoscopy
Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
In a multicenter randomized controlled trial, Espaillat-Rijo and colleagues compared various methods for visualizing ureteral efflux in participants who underwent gynecologic or urogynecologic procedures in which cystoscopy was performed.
Study compared 4 media
The investigators enrolled 176 participants (174 completed the trial) and randomly assigned them to receive 1 of 4 modalities: 1) normal saline as a bladder distention medium (control), 2) 10% dextrose as a bladder distention medium, 3) 200 mg oral phenazopyridine given 30 minutes prior to cystoscopy, or 4) 50 mg IV sodium fluorescein at the start of cystoscopy. Indigo carmine was not included in this study because it has not been routinely available since 2014.
Surgeons were asked to categorize the ureteral jets as “clearly visible,” “somewhat visible,” or “not visible.”
The primary outcome was subjective visibility of the ureteral jet with each modality during cystoscopy. Secondary outcomes included surgeon satisfaction, adverse reactions to the modality used, postoperative urinary tract infection, postoperative urinary retention, and delayed diagnosis of ureteral injury.
Visibility assessment results. Overall, ureteral jets were “clearly visible” in 125 cases (71%) compared with “somewhat visible” in 45 (25.6%) and “not visible” in 4 (2.3%) cases. There was a statistically significant difference between the 4 groups. Use of sodium fluorescein and 10% dextrose resulted in significantly better visualization of ureteral jets (P < .001 and P = .004, respectively) compared with the control group. Visibility with phenazopyridine was not significantly different from that in the control group or in the 10% dextrose group (FIGURE).
Surgeon satisfaction was highest with 10% dextrose and sodium fluorescein. In 6 cases, the surgeon was not satisfied with visualization of the ureteral jets and relied on fluorescein (5 times) or 10% dextrose (1 time) to ultimately see efflux. No significant adverse events occurred; the rate of urinary tract infection was 24.1% and did not differ between groups.
Results are widely generalizable
This was a well-designed randomized multicenter trial that included both benign gynecologic and urogynecologic procedures, thus strengthening the generalizability of the study. The study was timely since proven methods for visualization of ureteral patency became limited with the withdrawal of commercially available indigo carmine, the previous gold standard.
Intravenous sodium fluorescein and 10% dextrose as bladder distention media can both safely be used to visualize ureteral efflux and result in high surgeon satisfaction. Although 10% dextrose has been associated with higher rates of postoperative urinary tract infection,11 this was not found to be the case in this study. Preoperative administration of oral phenazopyridine was no different from the control modality with regard to visibility and surgeon satisfaction.
Continue to: The cost-effectiveness consideration
The cost-effectiveness consideration
The debate around universal cystoscopy following benign gynecologic surgery is ongoing.
The studies discussed in this Update demonstrate that cystoscopy following hysterectomy for benign indications:
- is superior to visualizing ureteral peristalsis
- increases detection of urinary tract injuries, and
- decreases delayed urologic injuries.
Although these articles emphasize the importance of detecting urologic injury, the picture would not be complete without mention of cost-effectiveness. Only one study, from 2001, has evaluated the cost-effectiveness of universal cystoscopy.1 Those authors concluded that universal cystoscopy is cost-effective only when the rate of urologic injury is 1.5% to 2%, but this conclusion, admittedly, was limited by the lack of data on medicolegal settlements, outpatient expenses, and nonmedical-related economic loss from decreased productivity. Given the extensive changes that have occurred in medical practice over the last 17 years and the emphasis on quality metrics and safety, an updated analysis would be needed to make definitive conclusions about cost-effectiveness.
While this Update cannot settle the ongoing debate of universal cystoscopy in gynecology, it is important to remember that the American College of Obstetricians and Gynecologists and the American Urogynecologic Society recommend cystoscopy following all surgeries for pelvic organ prolapse and stress urinary incontinence.2
References
- Visco AG, Taber KH, Weidner AD, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97(5 pt 1):685–692.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ibeanu OA, Chesson RR, Echols KT, Nieves M, Busangu F, Nolan TE. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet Gynecol. 2009;113(1):6–10.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83(4):549–555.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506.
- Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10,110 hysterectomies by type of approach. Hum Reprod. 2001;16(7):1473–1478.
- Gilmour DT, Dwyer PL, Carey MP. Lower urinary tract injury during gynecologic surgery and its detection by intraoperative cystoscopy. Obstet Gynecol. 1999;94(5 pt 2):883–889.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289.
- Kwon CH, Goldberg RP, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187(6):1466–1471.
- Adams-Piper ER, Guaderrama NM, Chen Q, Whitcomb EL. Impact of surgical training on the performance of proposed quality measures for hysterectomy for pelvic organ prolapse. Am J Obstet Gynecol. 2017;216(6):588.e1–588.e5.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–74.e6.
- Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
Using cystoscopy to evaluate ureteral efflux and bladder integrity following benign gynecologic surgery increases the detection rate of urinary tract injuries.1 Currently, it is standard of care to perform a cystoscopy following anti-incontinence procedures, but there is no consensus among ObGyns regarding the use of universal cystoscopy following benign gynecologic surgery.2 A number of studies, however, have suggested potential best practices for evaluating urinary tract injury during pelvic surgery for benign gynecologic conditions.
Pelvic surgeries for benign gynecologic conditions, including fibroids, menorrhagia, and pelvic organ prolapse (POP), are common. More than 500,000 hysterectomies are performed annually in the United States, and up to 11% of women will undergo at least one surgery for POP or urinary incontinence in their lifetime.3,4 During gynecologic surgery, the urinary tract is at risk, and the injury rate ranges from 0.02% to 2% for ureteral injury and from 1% to 5% for bladder injury.5,6
In a recent large randomized controlled trial, the rate of intraoperative ureteral obstruction following uterosacral ligament suspension (USLS) was 3.2%.7 Vaginal vault suspensions, as well as other vaginal cuff closure techniques, are common procedures associated with urinary tract injury.8 Additionally, ureteral injury during surgery for POP occurs in as many as 2% of anterior vaginal wall repairs.9
It is well documented that a delay in diagnosis of ureteral and/or bladder injuries is associated with increased morbidity, including the need for additional surgery to repair the injury; in addition, significant delay in identifying an injury may lead to subsequent sequela, such as renal injury and fistula formation.8
A large study in California found that 36.5% of hysterectomies performed for POP were performed by general gynecologists.10 General ObGyns performing these surgeries therefore must understand the risk of urinary tract injury during hysterectomy and reconstructive pelvic procedures so that they can appropriately identify, evaluate, and repair injuries in a timely fashion.
The best way to identify urinary tract injury at the time of gynecologic surgery is by cystoscopy, including a bladder survey and ureteral efflux evaluation. When should a cystoscopy be performed, and what is the best method for visualizing ureteral efflux? Can instituting universal cystoscopy for all gynecologic procedures make a difference in the rate of injury detection? In this Update, we summarize the data from 4 studies that help to answer these questions.
Continue to: About 30% of urinary tract injuries...
About 30% of urinary tract injuries identified prior to cystoscopy at hysterectomy (which detected 5 of 6 injuries)
Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192(5):1599–1604.
Vakili and colleagues conducted a multicenter prospective cohort study of women undergoing hysterectomy for benign indications; cystoscopy was performed in all cases. The 3 hospitals involved were all part of the Louisiana State University Health system. The investigators’ goal was to determine the rate of urinary tract injury in this patient population at the time of intraoperative cystoscopy.
Intraoperative cystoscopy beats visual evaluation
Four hundred and seventy-one women underwent hysterectomy and had intraoperative cystoscopy, including evaluation of ureteral patency with administration of intravenous (IV) indigo carmine. Patients underwent abdominal, vaginal, or laparoscopic hysterectomy, and 54 (11.4%) had concurrent POP or anti-incontinence procedures. The majority underwent an abdominal hysterectomy (59%), 31% had a vaginal hysterectomy, and 10% had a laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy.
Rate of urinary tract injuries. The total urinary tract injury rate detected by cystoscopy was 4.8%. The ureteral injury rate was 1.7%, and the bladder injury rate was 3.6%. A combined ureteral and bladder injury occurred in 2 women.
Surgery for POP significantly increased the risk of ureteral injury (7.3% vs 1.2%; P = .025). All cases of ureteral injury during POP surgery occurred during USLS. There was a trend toward a higher rate of bladder injury in the group with concurrent anti-incontinence surgery (12.5% vs 3.1%; P = .049). Regarding the route of hysterectomy, the vaginal approach had the highest rate of ureteral injury; however, when prolapse cases were removed from the analysis, there were no differences between the abdominal, vaginal, and laparoscopic approaches for ureteral or bladder injuries.
Injury detection with cystoscopy. Importantly, the authors found that only 30% of injuries were identified prior to performing intraoperative cystoscopy. The majority of these were bladder injuries. In addition, despite visual confirmation of ureteral peristalsis during abdominal hysterectomy, when intraoperative cystoscopy was performed with evaluation for ureteral efflux, 5 of 6 ureteral injury cases were identified. The authors reported 1 postoperative vesicovaginal fistula and concluded that it was likely due to an unrecognized bladder injury. No other undetected injuries were identified.
Notably, no complications occurred as a result of cystoscopy.
Multiple surgical indications reflect real-world scenario
The study included physicians from 3 different hospitals and all routes of hysterectomy for multiple benign gynecologic indications as well as concomitant pelvic reconstructive procedures. While this enhances the generalizability of the study results, all study sites were located in Louisiana at hospitals with resident trainee involvement. Additionally, this study confirms previous retrospective studies that reported higher rates of injury with pelvic reconstructive procedures.
The study is limited by the inability to blind surgeons, which may have resulted in the surgeons altering their techniques and/or having a heightened awareness of the urinary tract. However, their rates of ureteral and bladder injuries were slightly higher than previously reported rates, suggesting that the procedures inherently carry risk. The study is further limited by the lack of a retrospective comparison group of hysterectomy without routine cystoscopy and a longer follow-up period that may have revealed additional missed delayed urologic injuries.
The rate of urinary tract injury, including both bladder and ureteral injuries, was more than 4% at the time of hysterectomy for benign conditions. Using intraoperative peristalsis or normal ureteral caliber could result in a false sense of security since these are not reliable signs of ureteral integrity. The majority of urinary tract injuries will not be identified without cystoscopic evaluation.
Continue to: Universal cystoscopy policy...
Universal cystoscopy policy proves protective, surgeon adherence is high
Chi AM, Curran DS, Morgan DM, Fenner DE, Swenson CW. Universal cystoscopy after benign hysterectomy: examining the effects of an institutional policy. Obstet Gynecol. 2016;127(2):369–375.
In a retrospective cohort study, Chi and colleagues evaluated urinary tract injuries at the time of hysterectomy before and after the institution of a universal cystoscopy policy. At the time of policy implementation at the University of Michigan, all faculty who performed hysterectomies attended a cystoscopy workshop. Attending physicians without prior cystoscopy training also were proctored in the operating room for 3 sessions and were required to demonstrate competency with bladder survey, visualizing ureteral efflux, and urethral assessment. Indigo carmine was used to visualize ureteral efflux.
Detection of urologic injury almost doubled with cystoscopy
A total of 2,822 hysterectomies were included in the study, with 973 in the pre–universal cystoscopy group and 1,849 in the post–universal cystoscopy group. The study period was 7 years, and data on complications were abstracted for 1 year after the completion of the study period.
The primary outcome had 3 components:
- the rate of urologic injury before and after the policy
- the cystoscopy detection rate of urologic injury
- the adherence rate to the policy.
The overall rate of bladder and ureteral injury was 2.1%; the rate of injury during pre–universal screening was 2.6%, and during post–universal screening was 1.8%. The intraoperative detection rate of injury nearly doubled, from 24% to 47%, when intraoperative cystoscopy was utilized. In addition, the percentage of delayed urologic complications decreased from 28% to 5.9% (P = .03) following implementation of the universal cystoscopy policy. With regard to surgeon adherence, cystoscopy was documented in 86.1% of the hysterectomy cases after the policy was implemented compared with 35.7% of cases before the policy.
The investigators performed a cost analysis and found that hospital costs were nearly twice as much if a delayed urologic injury was diagnosed.
Study had many strengths
This study evaluated aspects of implementing quality initiatives after proper training and proctoring of a procedure. The authors compared very large cohorts from a busy academic medical center in which surgeon adherence with routine cystoscopy was high. The majority of patient outcomes were tracked for an extended period following surgery, thereby minimizing the risk of missing delayed urologic injuries. Notably, however, there was shorter follow-up time for the post–universal cystoscopy group, which could result in underestimating the rate of delayed urologic injuries in this cohort.
Instituting a universal cystoscopy policy for hysterectomy was associated with a significant decrease in delayed postoperative urinary tract complications and an increase in the intraoperative detection rate of urologic injuries. Intraoperative detection and repair of a urinary tract injury is cost-effective compared with a delayed diagnosis.
Continue to: Cystoscopy reveals ureteral obstruction...
Cystoscopy reveals ureteral obstruction during various vaginal POP repair procedures
Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194(5):1478–1485.
To determine the rate of ureteral obstruction and ureteral injury during vaginal surgery for POP and the accuracy of using intraoperative cystoscopy to prevent upper urinary tract morbidity, Gustilo-Ashby and colleagues performed a retrospective review study of a large patient cohort.
Cystoscopy with indigo carmine is highly sensitive
The study included 700 patients who underwent vaginal surgery for anterior and/or apical POP. Patients had 1 or more of the following anterior and apical prolapse repair procedures: USLS (51%), distal McCall culdeplasty (26%), proximal McCall culdeplasty (29%), anterior colporrhaphy (82%), and colpocleisis (1.4%). Of note, distal McCall culdeplasty was defined as incorporation of the “vaginal epithelium into the uterosacral plication,” while proximal McCall culdeplasty involved plication of “the uterosacral ligaments in the midline proximal to the vaginal cuff.” All patients were given IV indigo carmine to aid in visualizing ureteral efflux.
The majority of patients had a hysterectomy (56%). When accounting for rare false-positive and negative cystoscopy results, the overall ureteral obstruction rate was 5.1% and the ureteral injury rate was 0.9%. The majority of obstructions occurred with USLS (5.9%), proximal McCall culdeplasty (4.4%), and colpocleisis (4.2%). Ureteral injuries occurred only in 6 cases: 3 USLS and 3 proximal McCall culdeplasty procedures.
Based on these findings, the authors calculated that cystoscopy at the time of vaginal surgery for anterior and/or apical prolapse has a sensitivity of 94.4% and a specificity of 99.5% for detecting ureteral obstruction. The positive predictive value of cystoscopy with the use of indigo carmine for detection of ureteral obstruction is 91.9% and the negative predictive value is 99.7%.
Impact of indigo carmine’s unavailability
This study’s strengths include its large sample size and the variety of surgical approaches used for repair of anterior vaginal wall and apical prolapse. Its retrospective design, however, is a limitation; this could result in underreporting of ureteral injuries if patients received care at another institution after surgery. Furthermore, it is unclear if cystoscopy would be as predictive of ureteral injury without the use of indigo carmine, which is no longer available at most institutions.
The utility of cystoscopy with IV indigo carmine as a screening test for ureteral obstruction is highlighted by the fact that most obstructions were relieved by intraoperative suture removal following positive cystoscopy. McCall culdeplasty procedures are commonly performed by general ObGyns at the time of vaginal hysterectomy. It is therefore important to note that rates of ureteral obstruction after proximal McCall culdeplasty were only slightly lower than those after USLS.
Continue to: Sodium fluorescein and 10% dextrose...
Sodium fluorescein and 10% dextrose provide clear visibility of ureteral jets in cystoscopy
Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
In a multicenter randomized controlled trial, Espaillat-Rijo and colleagues compared various methods for visualizing ureteral efflux in participants who underwent gynecologic or urogynecologic procedures in which cystoscopy was performed.
Study compared 4 media
The investigators enrolled 176 participants (174 completed the trial) and randomly assigned them to receive 1 of 4 modalities: 1) normal saline as a bladder distention medium (control), 2) 10% dextrose as a bladder distention medium, 3) 200 mg oral phenazopyridine given 30 minutes prior to cystoscopy, or 4) 50 mg IV sodium fluorescein at the start of cystoscopy. Indigo carmine was not included in this study because it has not been routinely available since 2014.
Surgeons were asked to categorize the ureteral jets as “clearly visible,” “somewhat visible,” or “not visible.”
The primary outcome was subjective visibility of the ureteral jet with each modality during cystoscopy. Secondary outcomes included surgeon satisfaction, adverse reactions to the modality used, postoperative urinary tract infection, postoperative urinary retention, and delayed diagnosis of ureteral injury.
Visibility assessment results. Overall, ureteral jets were “clearly visible” in 125 cases (71%) compared with “somewhat visible” in 45 (25.6%) and “not visible” in 4 (2.3%) cases. There was a statistically significant difference between the 4 groups. Use of sodium fluorescein and 10% dextrose resulted in significantly better visualization of ureteral jets (P < .001 and P = .004, respectively) compared with the control group. Visibility with phenazopyridine was not significantly different from that in the control group or in the 10% dextrose group (FIGURE).

Surgeon satisfaction was highest with 10% dextrose and sodium fluorescein. In 6 cases, the surgeon was not satisfied with visualization of the ureteral jets and relied on fluorescein (5 times) or 10% dextrose (1 time) to ultimately see efflux. No significant adverse events occurred; the rate of urinary tract infection was 24.1% and did not differ between groups.
Results are widely generalizable
This was a well-designed randomized multicenter trial that included both benign gynecologic and urogynecologic procedures, thus strengthening the generalizability of the study. The study was timely since proven methods for visualization of ureteral patency became limited with the withdrawal of commercially available indigo carmine, the previous gold standard.
Intravenous sodium fluorescein and 10% dextrose as bladder distention media can both safely be used to visualize ureteral efflux and result in high surgeon satisfaction. Although 10% dextrose has been associated with higher rates of postoperative urinary tract infection,11 this was not found to be the case in this study. Preoperative administration of oral phenazopyridine was no different from the control modality with regard to visibility and surgeon satisfaction.
Continue to: The cost-effectiveness consideration
The cost-effectiveness consideration
The debate around universal cystoscopy following benign gynecologic surgery is ongoing.
The studies discussed in this Update demonstrate that cystoscopy following hysterectomy for benign indications:
- is superior to visualizing ureteral peristalsis
- increases detection of urinary tract injuries, and
- decreases delayed urologic injuries.
Although these articles emphasize the importance of detecting urologic injury, the picture would not be complete without mention of cost-effectiveness. Only one study, from 2001, has evaluated the cost-effectiveness of universal cystoscopy.1 Those authors concluded that universal cystoscopy is cost-effective only when the rate of urologic injury is 1.5% to 2%, but this conclusion, admittedly, was limited by the lack of data on medicolegal settlements, outpatient expenses, and nonmedical-related economic loss from decreased productivity. Given the extensive changes that have occurred in medical practice over the last 17 years and the emphasis on quality metrics and safety, an updated analysis would be needed to make definitive conclusions about cost-effectiveness.
While this Update cannot settle the ongoing debate of universal cystoscopy in gynecology, it is important to remember that the American College of Obstetricians and Gynecologists and the American Urogynecologic Society recommend cystoscopy following all surgeries for pelvic organ prolapse and stress urinary incontinence.2
References
- Visco AG, Taber KH, Weidner AD, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97(5 pt 1):685–692.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Using cystoscopy to evaluate ureteral efflux and bladder integrity following benign gynecologic surgery increases the detection rate of urinary tract injuries.1 Currently, it is standard of care to perform a cystoscopy following anti-incontinence procedures, but there is no consensus among ObGyns regarding the use of universal cystoscopy following benign gynecologic surgery.2 A number of studies, however, have suggested potential best practices for evaluating urinary tract injury during pelvic surgery for benign gynecologic conditions.
Pelvic surgeries for benign gynecologic conditions, including fibroids, menorrhagia, and pelvic organ prolapse (POP), are common. More than 500,000 hysterectomies are performed annually in the United States, and up to 11% of women will undergo at least one surgery for POP or urinary incontinence in their lifetime.3,4 During gynecologic surgery, the urinary tract is at risk, and the injury rate ranges from 0.02% to 2% for ureteral injury and from 1% to 5% for bladder injury.5,6
In a recent large randomized controlled trial, the rate of intraoperative ureteral obstruction following uterosacral ligament suspension (USLS) was 3.2%.7 Vaginal vault suspensions, as well as other vaginal cuff closure techniques, are common procedures associated with urinary tract injury.8 Additionally, ureteral injury during surgery for POP occurs in as many as 2% of anterior vaginal wall repairs.9
It is well documented that a delay in diagnosis of ureteral and/or bladder injuries is associated with increased morbidity, including the need for additional surgery to repair the injury; in addition, significant delay in identifying an injury may lead to subsequent sequela, such as renal injury and fistula formation.8
A large study in California found that 36.5% of hysterectomies performed for POP were performed by general gynecologists.10 General ObGyns performing these surgeries therefore must understand the risk of urinary tract injury during hysterectomy and reconstructive pelvic procedures so that they can appropriately identify, evaluate, and repair injuries in a timely fashion.
The best way to identify urinary tract injury at the time of gynecologic surgery is by cystoscopy, including a bladder survey and ureteral efflux evaluation. When should a cystoscopy be performed, and what is the best method for visualizing ureteral efflux? Can instituting universal cystoscopy for all gynecologic procedures make a difference in the rate of injury detection? In this Update, we summarize the data from 4 studies that help to answer these questions.
Continue to: About 30% of urinary tract injuries...
About 30% of urinary tract injuries identified prior to cystoscopy at hysterectomy (which detected 5 of 6 injuries)
Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192(5):1599–1604.
Vakili and colleagues conducted a multicenter prospective cohort study of women undergoing hysterectomy for benign indications; cystoscopy was performed in all cases. The 3 hospitals involved were all part of the Louisiana State University Health system. The investigators’ goal was to determine the rate of urinary tract injury in this patient population at the time of intraoperative cystoscopy.
Intraoperative cystoscopy beats visual evaluation
Four hundred and seventy-one women underwent hysterectomy and had intraoperative cystoscopy, including evaluation of ureteral patency with administration of intravenous (IV) indigo carmine. Patients underwent abdominal, vaginal, or laparoscopic hysterectomy, and 54 (11.4%) had concurrent POP or anti-incontinence procedures. The majority underwent an abdominal hysterectomy (59%), 31% had a vaginal hysterectomy, and 10% had a laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy.
Rate of urinary tract injuries. The total urinary tract injury rate detected by cystoscopy was 4.8%. The ureteral injury rate was 1.7%, and the bladder injury rate was 3.6%. A combined ureteral and bladder injury occurred in 2 women.
Surgery for POP significantly increased the risk of ureteral injury (7.3% vs 1.2%; P = .025). All cases of ureteral injury during POP surgery occurred during USLS. There was a trend toward a higher rate of bladder injury in the group with concurrent anti-incontinence surgery (12.5% vs 3.1%; P = .049). Regarding the route of hysterectomy, the vaginal approach had the highest rate of ureteral injury; however, when prolapse cases were removed from the analysis, there were no differences between the abdominal, vaginal, and laparoscopic approaches for ureteral or bladder injuries.
Injury detection with cystoscopy. Importantly, the authors found that only 30% of injuries were identified prior to performing intraoperative cystoscopy. The majority of these were bladder injuries. In addition, despite visual confirmation of ureteral peristalsis during abdominal hysterectomy, when intraoperative cystoscopy was performed with evaluation for ureteral efflux, 5 of 6 ureteral injury cases were identified. The authors reported 1 postoperative vesicovaginal fistula and concluded that it was likely due to an unrecognized bladder injury. No other undetected injuries were identified.
Notably, no complications occurred as a result of cystoscopy.
Multiple surgical indications reflect real-world scenario
The study included physicians from 3 different hospitals and all routes of hysterectomy for multiple benign gynecologic indications as well as concomitant pelvic reconstructive procedures. While this enhances the generalizability of the study results, all study sites were located in Louisiana at hospitals with resident trainee involvement. Additionally, this study confirms previous retrospective studies that reported higher rates of injury with pelvic reconstructive procedures.
The study is limited by the inability to blind surgeons, which may have resulted in the surgeons altering their techniques and/or having a heightened awareness of the urinary tract. However, their rates of ureteral and bladder injuries were slightly higher than previously reported rates, suggesting that the procedures inherently carry risk. The study is further limited by the lack of a retrospective comparison group of hysterectomy without routine cystoscopy and a longer follow-up period that may have revealed additional missed delayed urologic injuries.
The rate of urinary tract injury, including both bladder and ureteral injuries, was more than 4% at the time of hysterectomy for benign conditions. Using intraoperative peristalsis or normal ureteral caliber could result in a false sense of security since these are not reliable signs of ureteral integrity. The majority of urinary tract injuries will not be identified without cystoscopic evaluation.
Continue to: Universal cystoscopy policy...
Universal cystoscopy policy proves protective, surgeon adherence is high
Chi AM, Curran DS, Morgan DM, Fenner DE, Swenson CW. Universal cystoscopy after benign hysterectomy: examining the effects of an institutional policy. Obstet Gynecol. 2016;127(2):369–375.
In a retrospective cohort study, Chi and colleagues evaluated urinary tract injuries at the time of hysterectomy before and after the institution of a universal cystoscopy policy. At the time of policy implementation at the University of Michigan, all faculty who performed hysterectomies attended a cystoscopy workshop. Attending physicians without prior cystoscopy training also were proctored in the operating room for 3 sessions and were required to demonstrate competency with bladder survey, visualizing ureteral efflux, and urethral assessment. Indigo carmine was used to visualize ureteral efflux.
Detection of urologic injury almost doubled with cystoscopy
A total of 2,822 hysterectomies were included in the study, with 973 in the pre–universal cystoscopy group and 1,849 in the post–universal cystoscopy group. The study period was 7 years, and data on complications were abstracted for 1 year after the completion of the study period.
The primary outcome had 3 components:
- the rate of urologic injury before and after the policy
- the cystoscopy detection rate of urologic injury
- the adherence rate to the policy.
The overall rate of bladder and ureteral injury was 2.1%; the rate of injury during pre–universal screening was 2.6%, and during post–universal screening was 1.8%. The intraoperative detection rate of injury nearly doubled, from 24% to 47%, when intraoperative cystoscopy was utilized. In addition, the percentage of delayed urologic complications decreased from 28% to 5.9% (P = .03) following implementation of the universal cystoscopy policy. With regard to surgeon adherence, cystoscopy was documented in 86.1% of the hysterectomy cases after the policy was implemented compared with 35.7% of cases before the policy.
The investigators performed a cost analysis and found that hospital costs were nearly twice as much if a delayed urologic injury was diagnosed.
Study had many strengths
This study evaluated aspects of implementing quality initiatives after proper training and proctoring of a procedure. The authors compared very large cohorts from a busy academic medical center in which surgeon adherence with routine cystoscopy was high. The majority of patient outcomes were tracked for an extended period following surgery, thereby minimizing the risk of missing delayed urologic injuries. Notably, however, there was shorter follow-up time for the post–universal cystoscopy group, which could result in underestimating the rate of delayed urologic injuries in this cohort.
Instituting a universal cystoscopy policy for hysterectomy was associated with a significant decrease in delayed postoperative urinary tract complications and an increase in the intraoperative detection rate of urologic injuries. Intraoperative detection and repair of a urinary tract injury is cost-effective compared with a delayed diagnosis.
Continue to: Cystoscopy reveals ureteral obstruction...
Cystoscopy reveals ureteral obstruction during various vaginal POP repair procedures
Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194(5):1478–1485.
To determine the rate of ureteral obstruction and ureteral injury during vaginal surgery for POP and the accuracy of using intraoperative cystoscopy to prevent upper urinary tract morbidity, Gustilo-Ashby and colleagues performed a retrospective review study of a large patient cohort.
Cystoscopy with indigo carmine is highly sensitive
The study included 700 patients who underwent vaginal surgery for anterior and/or apical POP. Patients had 1 or more of the following anterior and apical prolapse repair procedures: USLS (51%), distal McCall culdeplasty (26%), proximal McCall culdeplasty (29%), anterior colporrhaphy (82%), and colpocleisis (1.4%). Of note, distal McCall culdeplasty was defined as incorporation of the “vaginal epithelium into the uterosacral plication,” while proximal McCall culdeplasty involved plication of “the uterosacral ligaments in the midline proximal to the vaginal cuff.” All patients were given IV indigo carmine to aid in visualizing ureteral efflux.
The majority of patients had a hysterectomy (56%). When accounting for rare false-positive and negative cystoscopy results, the overall ureteral obstruction rate was 5.1% and the ureteral injury rate was 0.9%. The majority of obstructions occurred with USLS (5.9%), proximal McCall culdeplasty (4.4%), and colpocleisis (4.2%). Ureteral injuries occurred only in 6 cases: 3 USLS and 3 proximal McCall culdeplasty procedures.
Based on these findings, the authors calculated that cystoscopy at the time of vaginal surgery for anterior and/or apical prolapse has a sensitivity of 94.4% and a specificity of 99.5% for detecting ureteral obstruction. The positive predictive value of cystoscopy with the use of indigo carmine for detection of ureteral obstruction is 91.9% and the negative predictive value is 99.7%.
Impact of indigo carmine’s unavailability
This study’s strengths include its large sample size and the variety of surgical approaches used for repair of anterior vaginal wall and apical prolapse. Its retrospective design, however, is a limitation; this could result in underreporting of ureteral injuries if patients received care at another institution after surgery. Furthermore, it is unclear if cystoscopy would be as predictive of ureteral injury without the use of indigo carmine, which is no longer available at most institutions.
The utility of cystoscopy with IV indigo carmine as a screening test for ureteral obstruction is highlighted by the fact that most obstructions were relieved by intraoperative suture removal following positive cystoscopy. McCall culdeplasty procedures are commonly performed by general ObGyns at the time of vaginal hysterectomy. It is therefore important to note that rates of ureteral obstruction after proximal McCall culdeplasty were only slightly lower than those after USLS.
Continue to: Sodium fluorescein and 10% dextrose...
Sodium fluorescein and 10% dextrose provide clear visibility of ureteral jets in cystoscopy
Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
In a multicenter randomized controlled trial, Espaillat-Rijo and colleagues compared various methods for visualizing ureteral efflux in participants who underwent gynecologic or urogynecologic procedures in which cystoscopy was performed.
Study compared 4 media
The investigators enrolled 176 participants (174 completed the trial) and randomly assigned them to receive 1 of 4 modalities: 1) normal saline as a bladder distention medium (control), 2) 10% dextrose as a bladder distention medium, 3) 200 mg oral phenazopyridine given 30 minutes prior to cystoscopy, or 4) 50 mg IV sodium fluorescein at the start of cystoscopy. Indigo carmine was not included in this study because it has not been routinely available since 2014.
Surgeons were asked to categorize the ureteral jets as “clearly visible,” “somewhat visible,” or “not visible.”
The primary outcome was subjective visibility of the ureteral jet with each modality during cystoscopy. Secondary outcomes included surgeon satisfaction, adverse reactions to the modality used, postoperative urinary tract infection, postoperative urinary retention, and delayed diagnosis of ureteral injury.
Visibility assessment results. Overall, ureteral jets were “clearly visible” in 125 cases (71%) compared with “somewhat visible” in 45 (25.6%) and “not visible” in 4 (2.3%) cases. There was a statistically significant difference between the 4 groups. Use of sodium fluorescein and 10% dextrose resulted in significantly better visualization of ureteral jets (P < .001 and P = .004, respectively) compared with the control group. Visibility with phenazopyridine was not significantly different from that in the control group or in the 10% dextrose group (FIGURE).

Surgeon satisfaction was highest with 10% dextrose and sodium fluorescein. In 6 cases, the surgeon was not satisfied with visualization of the ureteral jets and relied on fluorescein (5 times) or 10% dextrose (1 time) to ultimately see efflux. No significant adverse events occurred; the rate of urinary tract infection was 24.1% and did not differ between groups.
Results are widely generalizable
This was a well-designed randomized multicenter trial that included both benign gynecologic and urogynecologic procedures, thus strengthening the generalizability of the study. The study was timely since proven methods for visualization of ureteral patency became limited with the withdrawal of commercially available indigo carmine, the previous gold standard.
Intravenous sodium fluorescein and 10% dextrose as bladder distention media can both safely be used to visualize ureteral efflux and result in high surgeon satisfaction. Although 10% dextrose has been associated with higher rates of postoperative urinary tract infection,11 this was not found to be the case in this study. Preoperative administration of oral phenazopyridine was no different from the control modality with regard to visibility and surgeon satisfaction.
Continue to: The cost-effectiveness consideration
The cost-effectiveness consideration
The debate around universal cystoscopy following benign gynecologic surgery is ongoing.
The studies discussed in this Update demonstrate that cystoscopy following hysterectomy for benign indications:
- is superior to visualizing ureteral peristalsis
- increases detection of urinary tract injuries, and
- decreases delayed urologic injuries.
Although these articles emphasize the importance of detecting urologic injury, the picture would not be complete without mention of cost-effectiveness. Only one study, from 2001, has evaluated the cost-effectiveness of universal cystoscopy.1 Those authors concluded that universal cystoscopy is cost-effective only when the rate of urologic injury is 1.5% to 2%, but this conclusion, admittedly, was limited by the lack of data on medicolegal settlements, outpatient expenses, and nonmedical-related economic loss from decreased productivity. Given the extensive changes that have occurred in medical practice over the last 17 years and the emphasis on quality metrics and safety, an updated analysis would be needed to make definitive conclusions about cost-effectiveness.
While this Update cannot settle the ongoing debate of universal cystoscopy in gynecology, it is important to remember that the American College of Obstetricians and Gynecologists and the American Urogynecologic Society recommend cystoscopy following all surgeries for pelvic organ prolapse and stress urinary incontinence.2
References
- Visco AG, Taber KH, Weidner AD, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97(5 pt 1):685–692.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ibeanu OA, Chesson RR, Echols KT, Nieves M, Busangu F, Nolan TE. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet Gynecol. 2009;113(1):6–10.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83(4):549–555.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506.
- Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10,110 hysterectomies by type of approach. Hum Reprod. 2001;16(7):1473–1478.
- Gilmour DT, Dwyer PL, Carey MP. Lower urinary tract injury during gynecologic surgery and its detection by intraoperative cystoscopy. Obstet Gynecol. 1999;94(5 pt 2):883–889.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289.
- Kwon CH, Goldberg RP, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187(6):1466–1471.
- Adams-Piper ER, Guaderrama NM, Chen Q, Whitcomb EL. Impact of surgical training on the performance of proposed quality measures for hysterectomy for pelvic organ prolapse. Am J Obstet Gynecol. 2017;216(6):588.e1–588.e5.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–74.e6.
- Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
- Ibeanu OA, Chesson RR, Echols KT, Nieves M, Busangu F, Nolan TE. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet Gynecol. 2009;113(1):6–10.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83(4):549–555.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506.
- Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10,110 hysterectomies by type of approach. Hum Reprod. 2001;16(7):1473–1478.
- Gilmour DT, Dwyer PL, Carey MP. Lower urinary tract injury during gynecologic surgery and its detection by intraoperative cystoscopy. Obstet Gynecol. 1999;94(5 pt 2):883–889.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289.
- Kwon CH, Goldberg RP, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187(6):1466–1471.
- Adams-Piper ER, Guaderrama NM, Chen Q, Whitcomb EL. Impact of surgical training on the performance of proposed quality measures for hysterectomy for pelvic organ prolapse. Am J Obstet Gynecol. 2017;216(6):588.e1–588.e5.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–74.e6.
- Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
2017 Update on pelvic floor dysfunction
The International Continence Society (ICS) defines overactive bladder (OAB) as a syndrome of "urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence (UUI), in the absence of urinary tract infection [UTI] or obvious pathology."1 The Agency for Healthcare Research and Quality (AHRQ) reported OAB prevalence to be 15% in US women, with 11% reporting UUI.2 OAB represents a significant health care burden that impacts nearly every aspect of life, including physical, emotional, and psychological domains.3,4 The economic impact is notable; the projected cost is estimated to reach $82.6 billion annually by 2020.5
The American Urological Association (AUA) and the Society for Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) have endorsed an algorithm for use in the evaluation of idiopathic OAB (FIGURE).6 If the patient's symptoms are certain, minimal evaluation is needed and it is reasonable to proceed with first-line therapy, which includes fluid management (decreasing caffeine intake and limiting evening fluid intake), bladder retraining drills such as timed voiding, and improving pelvic floor muscles with the use of biofeedback and functional electrical stimulation.6,7 Pelvic floor muscle training can be facilitated with a referral to a physical therapist trained in pelvic floor muscle education.

If treatment goals are not met with first-line strategies, second-line therapy may be initiated with anticholinergic or β3-adrenergic receptor agonist medications. If symptoms persist after 4 to 8 weeks of pharmacologic therapy, clinicians are encouraged to reassess or refer the patient to a specialist. Further evaluation may include a bladder diary in which the patient documents voided volumes, voiding frequency, and number of incontinent episodes; symptom-specific questionnaires; and/or urodynamic testing.
Related article:
The latest treatments for urinary and fecal incontinence: Which hold water?
Based on that evaluation, the patient may be a candidate for third-line therapy with either intradetrusor onabotulinumtoxinA, posterior tibial nerve stimulation (PTNS), or sacral neuromodulation.
There is a paucity of information comparing third-line therapies. In this Update, we focus on 4 randomized clinical trials that compare third-line treatment options for idiopathic OAB.
Read about how anticholinergic medication and onabotulinumtoxinA compare for treating UUI.
Anticholinergic therapy and onabotulinumtoxinA produce equivalent reductions in the frequency of daily UUI episodes
Visco AG, Brubaker L, Richter HE, et al; for the Pelvic Floor Disorders Network. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367(19):1803-1813.
In a double-blind, double-placebo-controlled randomized trial, Visco and colleagues compared anticholinergic medication with onabotulinumtoxinA 100 U for the treatment of women with UUI.
Details of the study
Two hundred forty-one women with moderate to severe UUI received either 6 months of oral anticholinergic therapy (solifenacin 5 mg daily with the option of dose escalation to 10 mg daily or change to trospium XR 60 mg daily based on the Patient Global Symptom Control score) plus a single intradetrusor injection of saline, or a single intradetrusor injection of onabotulinumtoxinA 100 U plus a 6-month oral placebo regimen.
Inclusion criteria were 5 or more UUI episodes on a 3-day diary, insufficient resolution of symptoms after 2 medications, or being drug naive. Exclusions included a postvoid residual (PVR) urine volume greater than 150 mL or previous therapy with onabotulinumtoxinA.
Participants were scheduled for follow up every 2 to 6 months post randomization, at which time all study medications were discontinued. The primary outcome was reduction from baseline in the mean number of UUI episodes per day over the 6-month period, as recorded in the monthly 3-day bladder diaries. Secondary outcomes included the proportion of participants with complete resolution of UUI, the proportion of participants with 75% or more reduction in UUI episodes, Overactive Bladder Questionnaire Short Form (OABq-SF) scores, other symptom-specific questionnaire scores, and adverse events.
Related article:
Which treatments for pelvic floor disorders are backed by evidence?
Both treatments significantly reduced UUI episodes
At baseline, participants reported a mean (SD) of 5.0 (2.7) UUI episodes per day, and 41% of participants were drug naive. Both treatment groups experienced significant reductions compared with baseline in mean UUI episodes, and the reductions were similar between the 2 groups (reduction of 3.4 episodes per day in the anticholinergic group, reduction of 3.3 episodes in the onabotulinumtoxinA group; P = .81). Complete resolution of UUI was more common in the onabotulinumtoxinA group (27%) as compared with the anticholinergic group (13%) (P = .003). There were no differences in improvement in OABq-SF scores (37.05 in the anticholinergic group vs 37.13 in the onabotulinumtoxinA group; P = .98) or other quality-of-life measures.
Adverse events. The anticholinergic group experienced a higher rate of dry mouth compared with the onabotulinumtoxinA group (46% vs 31%; P = .02) but had lower rates of intermittent catheterization use at 2 months (0% vs 5%, P = .01) and UTIs (13% vs 33%, P<.001).
Strengths and limitations. This was a well-designed, multicenter, randomized double-blind, double placebo-controlled trial. The study design allowed for dose escalation and change to another medication for inadequate symptom control and included drug-naive participants, which increases the generalizability of the results. However, current guidelines recommend reserving onabotulinumtoxinA therapy for third-line therapy, thus deterring this treatment's use in the drug-naive population. Additionally, the lack of a pure placebo arm makes it difficult to interpret the extent to which a placebo effect contributed to observed improvements in clinical symptoms.
Through 6 months, both a single intradetrusor injection of onabotulinumtoxinA 100 U and anticholinergic therapy reduce UUI episodes and improve quality-of-life measures in women who have failed medications or are drug naive. Use of onabotulinumtoxinA, however, more likely will lead to complete resolution of UUI, although with an increased risk of transient urinary retention and UTI. Even given the study findings supporting the use of onabotulinumtoxinA over anticholinergic therapy for complete resolution of UUI, it is most appropriate to align with current practice, which includes a trial of pharmacotherapy before proceeding with third-line onabotulinumtoxinA.
Read: onabotulinumtoxinA vs PTNS for OAB.
OnabotulinumtoxinA has greater 9-month durability for OAB symptoms compared with12 weeks of PTNS
Sherif H, Khalil M, Omar R. Management of refractory idiopathic overactive bladder: intradetrusor injection of botulinum toxin type A versus posterior tibial nerve stimulation. Can J Urol. 2017;24(3):8838-8846.
In this randomized clinical trial, Sherif and colleagues compared the safety and efficacy of a single intradetrusor injection of onabotulinumtoxinA 100 U with that of PTNS for OAB.
Details of the study
Sixty adult men and women with OAB who did not respond to medical therapy were randomly assigned to treatment with either onabotulinumtoxinA 100 U or PTNS. Criteria for exclusion were current UTI, PVR urine volume of more than 150 mL, previous radiation therapy or chemotherapy, previous incontinence surgery or bladder malignancy, or presence of mixed urinary incontinence.
At baseline, participants completed a 3-day bladder diary, an OAB symptom score (OABSS) questionnaire, and urodynamic testing. The OABSS questionnaire included 7 questions (scoring range, 0-28), with higher scores indicating worse symptoms, and included subscales for urgency and quality-of-life measures. Total OABSS, urgency score, quality-of-life score, bladder diary records, and urodynamic testing parameters were assessed at 6, 12, 24, and 36 weeks, along with adverse events.
OnabotulinumtoxinA injections were performed under spinal anesthesia. If PVR urine volume was greater than 200 mL at any follow-up visit, participants were instructed to begin clean intermittent self-catheterization. PTNS was administered as weekly 30-minute sessions for 12 consecutive weeks.
Participants' baseline demographics and symptoms were similar. Average age was 45 years. Averages (SD) for duration of anticholinergic use was 13 (0.8) weeks, UUI episode score was 4.5 (1) on 3-day bladder diary, and OABSS was 22 (2.7). Nine-month data were available for 29 participants in the onabotulinumtoxinA group and for 8 in the PTNS group.
Related article:
Update on pelvic floor dysfunction: Focus on urinary incontinence
OnabotulinumtoxinA treatment benefits sustained for 9 months
Through 6 months, compared with baseline assessments, both treatment groups had significant improvements in clinical symptoms and OABSS total score, as well as urgency and quality-of-life subscales. At 3 months, urodynamic study parameters were similarly improved from baseline in both groups.
At 9 months, however, only the onabotulinumtoxinA group, compared with the PTNS group, maintained the significant improvement from baseline in 3-day bladder diary voiding episodes (average [SD], 10.7 [1.01] vs 11.6 [1.09]; P = .009), 3-day bladder diary nocturia episodes (average [SD], 3.8 [1.09] vs 4.4 [0.8]; P = .02), and average [SD] UUI episodes over 3 days (3.5 [1.2] vs 4.2 [1.04]; P = .02). Similarly, onabotulinumtoxinA-treated participants, compared with those treated with PTNS, maintained improvements at 9 months in average (SD): OABSS total score (19.2 [2.4] vs 20.4 [1.7]; P = .03), urgency scores (10.9 [1.3] vs 11.8 [1.4]; P = .009), urine volume at first desire (177.8 [9.2] vs 171.8 [7.7]), maximum cystometric capacity (304 [17.6] vs 290 [13.1]), and Qmax (mL/sec) (20.7 [1.6] vs 22.2 [1.2]).
Adverse events. Average PVR urine volumes were higher in the onabotulinumtoxinA group compared with the PTNS group (36.8 [2.7] vs 32.4 [3.03]; P = .0001) at all time points, and self-catheterization was required in 6.6% of onabotulinumtoxinA-treated participants. Urinary tract infection occurred in 6.6% of participants in the onabotulinumtoxinA group and in none of the PTNS group. In the PTNS group, few experienced pain and minor bleeding at the needle site.
Strengths and limitations. This randomized, open-label trial comparing treatment with onabotulinumtoxinA 100 U and PTNS included both men and women with idiopathic OAB symptoms. The participants were assessed at regular intervals with various measures, and follow-up adherence was good. The sample size was small, so the study may not have been powered to see differences prior to 9 months.
Although at 9 months only the onabotulinumtoxinA group maintained significant improvement over baseline levels, the improvement was diminished, and therefore the clinical meaningfulness is uncertain. Further, participants in the PTNS group did not undergo monthly maintenance therapy after 3 months, which is recommended for those with a 12-week therapeutic response; this may have affected 9-month outcomes in this group. Since the one-time onabotulinumtoxinA 100 U injection was performed under spinal anesthesia, cost comparisons should be considered, since future onabotulinumtoxinA injections would be necessary.
A one-time onabotulinumtoxinA 100 U injection and 12 weeks of PTNS therapy are reasonable short-term options for symptomatic OAB relief after unsuccessful therapy with medications. OnabotulinumtoxinA injection may provide more durable OAB symptom control at 9 months but with a risk of UTI and need for self-catheterization.
Read about using different doses of onabotulinumtoxinA for OAB.
OnabotulinumtoxinA 200-U injection provides longer OAB symptom improvement than 100-U injection
Abdelwahab O, Sherif H, Soliman T, Elbarky I, Eshazly A. Efficacy of botulinum toxin type A 100 units versus 200 units for treatment of refractory idiopathic overactive bladder. Int Braz J Urol. 2015;41(6):1132-1140.
Abdelwahab and colleagues conducted a single-center, randomized clinical trial to investigate the safety and efficacy of a single injection of intradetrusor onabotulinumtoxinA in 2 different doses (100 U and 200 U) for treatment of OAB.
Details of the study
Eighty adults (63 women, 17 men) who did not benefit from anticholinergic medication during the previous 3 months were randomly assigned to receive either a 100-U (n = 40) or a 200-U (n = 40) injection of onabotulinumtoxinA. Exclusion criteria were PVR urine volume greater than 150 mL and previous radiation therapy or chemotherapy.
Initial assessments -- completed at baseline and at 1, 3, 6, and 9 months -- included the health-related quality-of-life (HR-QOL) questionnaire (maximum score, 100; higher score indicates better quality of life), an abbreviated OABSS questionnaire (4 questions; score range, 0-15; higher score indicates more severe symptoms), and urodynamic evaluation. Outcomes included OABSS, HR-QOL score, and urodynamic parameters at the various time points.
Related article:
Is there a link between impaired mobility and urinary incontinence in elderly, community-dwelling women?
Higher dose, greater symptom improvement and higher adverse event rate
At baseline, participants (average age, 31 years) had an average (SD) OABSS of 1.7 (1.6). OnabotulinumtoxinA treatment with both a 100-U and a 200-U dose resulted in significant improvements (compared with baseline levels) in frequency, nocturia, UUI episodes, OABSS, and urodynamic parameters throughout the 9 months. At 9 months, however, the group treated with the 200-U dose had greater improvements, compared with the group who received a 100-U dose, in urinary frequency symptom scores (mean [SD], 0.32 [0.47] vs 1.1 [0.51]; P<.05), nocturia symptom scores (mean [SD], 0.13 [0.34] vs 0.36 [0.49]; P<.05), UUI symptom scores (mean [SD], 0.68 [0.16] vs 1.26 [1.1]; P<.05), and mean (SD) total OABSS (2.6 [2.31] vs 5.3 [2.11]; P<.05). Similarly, at 9 months the 200-U dose resulted in greater improvements in volume at first desire (mean [SD], 291.8 [42.8] vs 246.8 [53.8] mL; P<.05), volume at strong desire (mean [SD], 392.1 [37.3] vs 313.1 [67.4] mL; P<.05), detrusor pressure (mean [SD], 10.4 [4.0] vs 19.2 [7.8] cm H2O; P<.05), and maximum cystometric capacity (mean [SD], 430.5 [34.2] vs 350 [69.1] mL; P<.05) compared with the 100-U dose.
Adverse events. No participant had a PVR urine volume greater than 100 mL at any follow-up visit. Postoperative hematuria occurred in 23% of the group treated with onabotulinumtoxinA 200 U versus in 15% of those treated with a 100-U dose. Similarly, UTIs occurred in 17.5% of the 200-U dose group and in 7.5% of the 100-U dose group. Dysuria was reported in 37.5% and 15% of the 200-U and 100-U dose groups, respectively.
Strengths and limitations. This randomized, open-label trial comparing a single injection of 100 U versus 200 U of onabotulinumtoxinA included mostly women. OAB symptoms and urodynamic parameters improved after treatment with both dose levels, but a longer duration of improvement was seen with the 200-U dose. The cohort had a low baseline OAB severity, based on the OABSS questionnaire, and a young average age of participants, which limits the generalizability of the study results to a population with refractory OAB. The 0% rate of clean intermittent self-catheterization postinjection might be based on the study's criteria for requiring clean intermittent catheterization. In addition, the initial postinjection visit occurred at 1 month, possibly missing participants who had symptoms of retention soon after injection.
Two dose levels (100 U and 200 U) of a single injection of onabotulinumtoxinA are associated with comparable OAB symptom and urodyanamic improvements. The benefits of a longer duration of effect with the 200-U dose must be weighed against the possible higher risks of transient hematuria, dysuria, and UTI.
Read: onabotulinumtoxinA vs sacral neuromodulation therapy for UUI.
Treatment with onabotulinumtoxinA may control UUI symptoms better than sacral neuromodulation therapy
Amundsen CL, Richter HE, Menefee SA, et al; Pelvic Floor Disorders Network. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: a randomized clinical trial. JAMA. 2016;316(13):1366-1374.
In this multicenter open-label randomized trial, Amundsen and colleagues compared the efficacy and safety of onabotulinumtoxinA 200 U with that of sacral neuromodulation.
Details of the study
Three hundred sixty-four women with UUI had data available for primary analysis at 6 months. Women were considered eligible for the study if they had 6 or more UUI episodes on a 3-day bladder diary, persistent symptoms despite anticholinergic therapy, a PVR urine volume of less than 150 mL, and had never previously received either study treatment.
There were no differences in baseline characteristics of the participants. The average (SD) age of the study population was 63 (11.6) years, with an average (SD) daily number of UUI episodes of 5.3 (2.8). The average (SD) body mass index was 32 (8) kg/m2.
Participants were randomly assigned to undergo either sacral neuromodulation (n = 174) or intradetrusor injection of onabotulinumtoxinA 200 U (n = 190). The primary outcome was change from baseline in mean number of daily UUI episodes averaged over 6 months as recorded on a monthly 3-day bladder diary. Secondary outcomes included complete resolution of urgency incontinence, 75% or more reduction in UUI episodes, the Overactive Bladder Questionnaire Short Form (SF) score (range, 0-100; higher score indicates higher symptom severity), the Overactive Bladder Satisfaction of Treatment questionnaire (range, 0-100; higher score indicates better satisfaction), other quality-of-life measures, and adverse events.
Related article:
2015 Update on pelvic floor dysfunction: Bladder pain syndrome
Greater symptom bother improvement, treatment satisfaction with onabotulinumtoxinA 200 U
Participants treated with onabotulinumtoxinA had a greater mean reduction of 3.9 UUI episodes per day than the sacral neuromodulation group's reduction of 3.3 UUI episodes per day (mean difference, 0.63; 95% confidence interval [CI], 0.13-1.14; P = .01). In addition, complete UUI resolution was higher in the onabotulinumtoxinA group as compared with the sacral neuromodulation group (20% vs 4%; P<.001). The onabotulinumtoxinA group also had higher rates of 75% or more reduction of UUI episodes compared with the sacral neuromodulation group (46% vs 26%; P<.001). Over 6 months, both groups had improvements in all quality-of-life measures, but the onabotulinumtoxinA group had greater improvement in symptom bother compared with the sacral neuromodulation group (-46.7 vs -38.6; mean difference, 8.1; 95% CI, 3.0-13.3; P = .002). Furthermore, the onabotulinumtoxinA group had greater treatment satisfaction compared with the sacral neuromodulation group (mean difference, 7.8; 95% CI, 1.6-14.1; P = .01).
Adverse events. Six women (3%) underwent sacral neuromodulation device revision or removal. Approximately 8% of onabotulinumtoxinA-treated participants required intermittent self-catheterization at 1 month, 4% at 3 months, and 2% at 6 months. The risk of UTI was higher in the onabotulinumtoxinA group compared with the sacral neuromodulation group (35% vs 11%; risk difference, 23%; 95% CI, -33% to -13%; P<.001).
Strengths and limitations. This is a well-designed randomized clinical trial comparing clinical outcomes and adverse events after treatment with onabotulinumtoxinA 200-U versus sacral neuromodulation. The interventions were standardized across investigators at multiple sites, and the study design required close follow-up to assess efficacy and adverse events. The study used a 200-U dose based on reported durability of effect at that time and findings of equivalency between onabotulinumtoxinA 100 U and anticholinergic therapy. The US Food and Drug Administration's recommendation to use a 100-U dose in all patients with idiopathic OAB might dissuade clinicians from considering the higher dose of onabotulinumtoxinA. The study was limited by the lack of a placebo group.
Both onabotulinumtoxinA 200 U and sacral neuromodulation provide significant improvement in UUI episodes and quality of life over 6 months. However, while treatment with onabotulinumtoxinA has a likelihood of complete UUI resolution, greater improvements in symptom bother and treatment satisfaction, these benefits must be weighed against the risks of transient catheterization and UTI.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al; International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20.
- Hartmann KE, McPheeters ML, Biller DH, et al. Treatment of overactive bladder in women. Evid Rep Technol Assess (Full Rep). 2009;187:1-120.
- Reynolds,WS, Fowke J, Dmochowski, R. The burden of overactive bladder on US public health. Curr Bladder Dysfunct Rep. 2016;11(1):8-13.
- Willis-Gray MG, Dieter AA, Geller EJ. Evaluation and management of overactive bladder: strategies for optimizing care. Res Rep Urol. 2016;8:113-122.
- Ganz ML, Smalarz AM, Krupski TL, et al. Economic costs of overactive bladder in the United States. Urology. 2010;75(3):526-532.
- Gormley EA, Lightner DJ, Faraday M, Vasavada SP; American Urological Association; Society of Urodyndamics, Female Pelvic Medicine. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015; 193(5):1572-1580.
- Gormley EA, Lightner DJ, Burgio KL, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2455-2463.
The International Continence Society (ICS) defines overactive bladder (OAB) as a syndrome of "urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence (UUI), in the absence of urinary tract infection [UTI] or obvious pathology."1 The Agency for Healthcare Research and Quality (AHRQ) reported OAB prevalence to be 15% in US women, with 11% reporting UUI.2 OAB represents a significant health care burden that impacts nearly every aspect of life, including physical, emotional, and psychological domains.3,4 The economic impact is notable; the projected cost is estimated to reach $82.6 billion annually by 2020.5
The American Urological Association (AUA) and the Society for Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) have endorsed an algorithm for use in the evaluation of idiopathic OAB (FIGURE).6 If the patient's symptoms are certain, minimal evaluation is needed and it is reasonable to proceed with first-line therapy, which includes fluid management (decreasing caffeine intake and limiting evening fluid intake), bladder retraining drills such as timed voiding, and improving pelvic floor muscles with the use of biofeedback and functional electrical stimulation.6,7 Pelvic floor muscle training can be facilitated with a referral to a physical therapist trained in pelvic floor muscle education.

If treatment goals are not met with first-line strategies, second-line therapy may be initiated with anticholinergic or β3-adrenergic receptor agonist medications. If symptoms persist after 4 to 8 weeks of pharmacologic therapy, clinicians are encouraged to reassess or refer the patient to a specialist. Further evaluation may include a bladder diary in which the patient documents voided volumes, voiding frequency, and number of incontinent episodes; symptom-specific questionnaires; and/or urodynamic testing.
Related article:
The latest treatments for urinary and fecal incontinence: Which hold water?
Based on that evaluation, the patient may be a candidate for third-line therapy with either intradetrusor onabotulinumtoxinA, posterior tibial nerve stimulation (PTNS), or sacral neuromodulation.
There is a paucity of information comparing third-line therapies. In this Update, we focus on 4 randomized clinical trials that compare third-line treatment options for idiopathic OAB.
Read about how anticholinergic medication and onabotulinumtoxinA compare for treating UUI.
Anticholinergic therapy and onabotulinumtoxinA produce equivalent reductions in the frequency of daily UUI episodes
Visco AG, Brubaker L, Richter HE, et al; for the Pelvic Floor Disorders Network. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367(19):1803-1813.
In a double-blind, double-placebo-controlled randomized trial, Visco and colleagues compared anticholinergic medication with onabotulinumtoxinA 100 U for the treatment of women with UUI.
Details of the study
Two hundred forty-one women with moderate to severe UUI received either 6 months of oral anticholinergic therapy (solifenacin 5 mg daily with the option of dose escalation to 10 mg daily or change to trospium XR 60 mg daily based on the Patient Global Symptom Control score) plus a single intradetrusor injection of saline, or a single intradetrusor injection of onabotulinumtoxinA 100 U plus a 6-month oral placebo regimen.
Inclusion criteria were 5 or more UUI episodes on a 3-day diary, insufficient resolution of symptoms after 2 medications, or being drug naive. Exclusions included a postvoid residual (PVR) urine volume greater than 150 mL or previous therapy with onabotulinumtoxinA.
Participants were scheduled for follow up every 2 to 6 months post randomization, at which time all study medications were discontinued. The primary outcome was reduction from baseline in the mean number of UUI episodes per day over the 6-month period, as recorded in the monthly 3-day bladder diaries. Secondary outcomes included the proportion of participants with complete resolution of UUI, the proportion of participants with 75% or more reduction in UUI episodes, Overactive Bladder Questionnaire Short Form (OABq-SF) scores, other symptom-specific questionnaire scores, and adverse events.
Related article:
Which treatments for pelvic floor disorders are backed by evidence?
Both treatments significantly reduced UUI episodes
At baseline, participants reported a mean (SD) of 5.0 (2.7) UUI episodes per day, and 41% of participants were drug naive. Both treatment groups experienced significant reductions compared with baseline in mean UUI episodes, and the reductions were similar between the 2 groups (reduction of 3.4 episodes per day in the anticholinergic group, reduction of 3.3 episodes in the onabotulinumtoxinA group; P = .81). Complete resolution of UUI was more common in the onabotulinumtoxinA group (27%) as compared with the anticholinergic group (13%) (P = .003). There were no differences in improvement in OABq-SF scores (37.05 in the anticholinergic group vs 37.13 in the onabotulinumtoxinA group; P = .98) or other quality-of-life measures.
Adverse events. The anticholinergic group experienced a higher rate of dry mouth compared with the onabotulinumtoxinA group (46% vs 31%; P = .02) but had lower rates of intermittent catheterization use at 2 months (0% vs 5%, P = .01) and UTIs (13% vs 33%, P<.001).
Strengths and limitations. This was a well-designed, multicenter, randomized double-blind, double placebo-controlled trial. The study design allowed for dose escalation and change to another medication for inadequate symptom control and included drug-naive participants, which increases the generalizability of the results. However, current guidelines recommend reserving onabotulinumtoxinA therapy for third-line therapy, thus deterring this treatment's use in the drug-naive population. Additionally, the lack of a pure placebo arm makes it difficult to interpret the extent to which a placebo effect contributed to observed improvements in clinical symptoms.
Through 6 months, both a single intradetrusor injection of onabotulinumtoxinA 100 U and anticholinergic therapy reduce UUI episodes and improve quality-of-life measures in women who have failed medications or are drug naive. Use of onabotulinumtoxinA, however, more likely will lead to complete resolution of UUI, although with an increased risk of transient urinary retention and UTI. Even given the study findings supporting the use of onabotulinumtoxinA over anticholinergic therapy for complete resolution of UUI, it is most appropriate to align with current practice, which includes a trial of pharmacotherapy before proceeding with third-line onabotulinumtoxinA.
Read: onabotulinumtoxinA vs PTNS for OAB.
OnabotulinumtoxinA has greater 9-month durability for OAB symptoms compared with12 weeks of PTNS
Sherif H, Khalil M, Omar R. Management of refractory idiopathic overactive bladder: intradetrusor injection of botulinum toxin type A versus posterior tibial nerve stimulation. Can J Urol. 2017;24(3):8838-8846.
In this randomized clinical trial, Sherif and colleagues compared the safety and efficacy of a single intradetrusor injection of onabotulinumtoxinA 100 U with that of PTNS for OAB.
Details of the study
Sixty adult men and women with OAB who did not respond to medical therapy were randomly assigned to treatment with either onabotulinumtoxinA 100 U or PTNS. Criteria for exclusion were current UTI, PVR urine volume of more than 150 mL, previous radiation therapy or chemotherapy, previous incontinence surgery or bladder malignancy, or presence of mixed urinary incontinence.
At baseline, participants completed a 3-day bladder diary, an OAB symptom score (OABSS) questionnaire, and urodynamic testing. The OABSS questionnaire included 7 questions (scoring range, 0-28), with higher scores indicating worse symptoms, and included subscales for urgency and quality-of-life measures. Total OABSS, urgency score, quality-of-life score, bladder diary records, and urodynamic testing parameters were assessed at 6, 12, 24, and 36 weeks, along with adverse events.
OnabotulinumtoxinA injections were performed under spinal anesthesia. If PVR urine volume was greater than 200 mL at any follow-up visit, participants were instructed to begin clean intermittent self-catheterization. PTNS was administered as weekly 30-minute sessions for 12 consecutive weeks.
Participants' baseline demographics and symptoms were similar. Average age was 45 years. Averages (SD) for duration of anticholinergic use was 13 (0.8) weeks, UUI episode score was 4.5 (1) on 3-day bladder diary, and OABSS was 22 (2.7). Nine-month data were available for 29 participants in the onabotulinumtoxinA group and for 8 in the PTNS group.
Related article:
Update on pelvic floor dysfunction: Focus on urinary incontinence
OnabotulinumtoxinA treatment benefits sustained for 9 months
Through 6 months, compared with baseline assessments, both treatment groups had significant improvements in clinical symptoms and OABSS total score, as well as urgency and quality-of-life subscales. At 3 months, urodynamic study parameters were similarly improved from baseline in both groups.
At 9 months, however, only the onabotulinumtoxinA group, compared with the PTNS group, maintained the significant improvement from baseline in 3-day bladder diary voiding episodes (average [SD], 10.7 [1.01] vs 11.6 [1.09]; P = .009), 3-day bladder diary nocturia episodes (average [SD], 3.8 [1.09] vs 4.4 [0.8]; P = .02), and average [SD] UUI episodes over 3 days (3.5 [1.2] vs 4.2 [1.04]; P = .02). Similarly, onabotulinumtoxinA-treated participants, compared with those treated with PTNS, maintained improvements at 9 months in average (SD): OABSS total score (19.2 [2.4] vs 20.4 [1.7]; P = .03), urgency scores (10.9 [1.3] vs 11.8 [1.4]; P = .009), urine volume at first desire (177.8 [9.2] vs 171.8 [7.7]), maximum cystometric capacity (304 [17.6] vs 290 [13.1]), and Qmax (mL/sec) (20.7 [1.6] vs 22.2 [1.2]).
Adverse events. Average PVR urine volumes were higher in the onabotulinumtoxinA group compared with the PTNS group (36.8 [2.7] vs 32.4 [3.03]; P = .0001) at all time points, and self-catheterization was required in 6.6% of onabotulinumtoxinA-treated participants. Urinary tract infection occurred in 6.6% of participants in the onabotulinumtoxinA group and in none of the PTNS group. In the PTNS group, few experienced pain and minor bleeding at the needle site.
Strengths and limitations. This randomized, open-label trial comparing treatment with onabotulinumtoxinA 100 U and PTNS included both men and women with idiopathic OAB symptoms. The participants were assessed at regular intervals with various measures, and follow-up adherence was good. The sample size was small, so the study may not have been powered to see differences prior to 9 months.
Although at 9 months only the onabotulinumtoxinA group maintained significant improvement over baseline levels, the improvement was diminished, and therefore the clinical meaningfulness is uncertain. Further, participants in the PTNS group did not undergo monthly maintenance therapy after 3 months, which is recommended for those with a 12-week therapeutic response; this may have affected 9-month outcomes in this group. Since the one-time onabotulinumtoxinA 100 U injection was performed under spinal anesthesia, cost comparisons should be considered, since future onabotulinumtoxinA injections would be necessary.
A one-time onabotulinumtoxinA 100 U injection and 12 weeks of PTNS therapy are reasonable short-term options for symptomatic OAB relief after unsuccessful therapy with medications. OnabotulinumtoxinA injection may provide more durable OAB symptom control at 9 months but with a risk of UTI and need for self-catheterization.
Read about using different doses of onabotulinumtoxinA for OAB.
OnabotulinumtoxinA 200-U injection provides longer OAB symptom improvement than 100-U injection
Abdelwahab O, Sherif H, Soliman T, Elbarky I, Eshazly A. Efficacy of botulinum toxin type A 100 units versus 200 units for treatment of refractory idiopathic overactive bladder. Int Braz J Urol. 2015;41(6):1132-1140.
Abdelwahab and colleagues conducted a single-center, randomized clinical trial to investigate the safety and efficacy of a single injection of intradetrusor onabotulinumtoxinA in 2 different doses (100 U and 200 U) for treatment of OAB.
Details of the study
Eighty adults (63 women, 17 men) who did not benefit from anticholinergic medication during the previous 3 months were randomly assigned to receive either a 100-U (n = 40) or a 200-U (n = 40) injection of onabotulinumtoxinA. Exclusion criteria were PVR urine volume greater than 150 mL and previous radiation therapy or chemotherapy.
Initial assessments -- completed at baseline and at 1, 3, 6, and 9 months -- included the health-related quality-of-life (HR-QOL) questionnaire (maximum score, 100; higher score indicates better quality of life), an abbreviated OABSS questionnaire (4 questions; score range, 0-15; higher score indicates more severe symptoms), and urodynamic evaluation. Outcomes included OABSS, HR-QOL score, and urodynamic parameters at the various time points.
Related article:
Is there a link between impaired mobility and urinary incontinence in elderly, community-dwelling women?
Higher dose, greater symptom improvement and higher adverse event rate
At baseline, participants (average age, 31 years) had an average (SD) OABSS of 1.7 (1.6). OnabotulinumtoxinA treatment with both a 100-U and a 200-U dose resulted in significant improvements (compared with baseline levels) in frequency, nocturia, UUI episodes, OABSS, and urodynamic parameters throughout the 9 months. At 9 months, however, the group treated with the 200-U dose had greater improvements, compared with the group who received a 100-U dose, in urinary frequency symptom scores (mean [SD], 0.32 [0.47] vs 1.1 [0.51]; P<.05), nocturia symptom scores (mean [SD], 0.13 [0.34] vs 0.36 [0.49]; P<.05), UUI symptom scores (mean [SD], 0.68 [0.16] vs 1.26 [1.1]; P<.05), and mean (SD) total OABSS (2.6 [2.31] vs 5.3 [2.11]; P<.05). Similarly, at 9 months the 200-U dose resulted in greater improvements in volume at first desire (mean [SD], 291.8 [42.8] vs 246.8 [53.8] mL; P<.05), volume at strong desire (mean [SD], 392.1 [37.3] vs 313.1 [67.4] mL; P<.05), detrusor pressure (mean [SD], 10.4 [4.0] vs 19.2 [7.8] cm H2O; P<.05), and maximum cystometric capacity (mean [SD], 430.5 [34.2] vs 350 [69.1] mL; P<.05) compared with the 100-U dose.
Adverse events. No participant had a PVR urine volume greater than 100 mL at any follow-up visit. Postoperative hematuria occurred in 23% of the group treated with onabotulinumtoxinA 200 U versus in 15% of those treated with a 100-U dose. Similarly, UTIs occurred in 17.5% of the 200-U dose group and in 7.5% of the 100-U dose group. Dysuria was reported in 37.5% and 15% of the 200-U and 100-U dose groups, respectively.
Strengths and limitations. This randomized, open-label trial comparing a single injection of 100 U versus 200 U of onabotulinumtoxinA included mostly women. OAB symptoms and urodynamic parameters improved after treatment with both dose levels, but a longer duration of improvement was seen with the 200-U dose. The cohort had a low baseline OAB severity, based on the OABSS questionnaire, and a young average age of participants, which limits the generalizability of the study results to a population with refractory OAB. The 0% rate of clean intermittent self-catheterization postinjection might be based on the study's criteria for requiring clean intermittent catheterization. In addition, the initial postinjection visit occurred at 1 month, possibly missing participants who had symptoms of retention soon after injection.
Two dose levels (100 U and 200 U) of a single injection of onabotulinumtoxinA are associated with comparable OAB symptom and urodyanamic improvements. The benefits of a longer duration of effect with the 200-U dose must be weighed against the possible higher risks of transient hematuria, dysuria, and UTI.
Read: onabotulinumtoxinA vs sacral neuromodulation therapy for UUI.
Treatment with onabotulinumtoxinA may control UUI symptoms better than sacral neuromodulation therapy
Amundsen CL, Richter HE, Menefee SA, et al; Pelvic Floor Disorders Network. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: a randomized clinical trial. JAMA. 2016;316(13):1366-1374.
In this multicenter open-label randomized trial, Amundsen and colleagues compared the efficacy and safety of onabotulinumtoxinA 200 U with that of sacral neuromodulation.
Details of the study
Three hundred sixty-four women with UUI had data available for primary analysis at 6 months. Women were considered eligible for the study if they had 6 or more UUI episodes on a 3-day bladder diary, persistent symptoms despite anticholinergic therapy, a PVR urine volume of less than 150 mL, and had never previously received either study treatment.
There were no differences in baseline characteristics of the participants. The average (SD) age of the study population was 63 (11.6) years, with an average (SD) daily number of UUI episodes of 5.3 (2.8). The average (SD) body mass index was 32 (8) kg/m2.
Participants were randomly assigned to undergo either sacral neuromodulation (n = 174) or intradetrusor injection of onabotulinumtoxinA 200 U (n = 190). The primary outcome was change from baseline in mean number of daily UUI episodes averaged over 6 months as recorded on a monthly 3-day bladder diary. Secondary outcomes included complete resolution of urgency incontinence, 75% or more reduction in UUI episodes, the Overactive Bladder Questionnaire Short Form (SF) score (range, 0-100; higher score indicates higher symptom severity), the Overactive Bladder Satisfaction of Treatment questionnaire (range, 0-100; higher score indicates better satisfaction), other quality-of-life measures, and adverse events.
Related article:
2015 Update on pelvic floor dysfunction: Bladder pain syndrome
Greater symptom bother improvement, treatment satisfaction with onabotulinumtoxinA 200 U
Participants treated with onabotulinumtoxinA had a greater mean reduction of 3.9 UUI episodes per day than the sacral neuromodulation group's reduction of 3.3 UUI episodes per day (mean difference, 0.63; 95% confidence interval [CI], 0.13-1.14; P = .01). In addition, complete UUI resolution was higher in the onabotulinumtoxinA group as compared with the sacral neuromodulation group (20% vs 4%; P<.001). The onabotulinumtoxinA group also had higher rates of 75% or more reduction of UUI episodes compared with the sacral neuromodulation group (46% vs 26%; P<.001). Over 6 months, both groups had improvements in all quality-of-life measures, but the onabotulinumtoxinA group had greater improvement in symptom bother compared with the sacral neuromodulation group (-46.7 vs -38.6; mean difference, 8.1; 95% CI, 3.0-13.3; P = .002). Furthermore, the onabotulinumtoxinA group had greater treatment satisfaction compared with the sacral neuromodulation group (mean difference, 7.8; 95% CI, 1.6-14.1; P = .01).
Adverse events. Six women (3%) underwent sacral neuromodulation device revision or removal. Approximately 8% of onabotulinumtoxinA-treated participants required intermittent self-catheterization at 1 month, 4% at 3 months, and 2% at 6 months. The risk of UTI was higher in the onabotulinumtoxinA group compared with the sacral neuromodulation group (35% vs 11%; risk difference, 23%; 95% CI, -33% to -13%; P<.001).
Strengths and limitations. This is a well-designed randomized clinical trial comparing clinical outcomes and adverse events after treatment with onabotulinumtoxinA 200-U versus sacral neuromodulation. The interventions were standardized across investigators at multiple sites, and the study design required close follow-up to assess efficacy and adverse events. The study used a 200-U dose based on reported durability of effect at that time and findings of equivalency between onabotulinumtoxinA 100 U and anticholinergic therapy. The US Food and Drug Administration's recommendation to use a 100-U dose in all patients with idiopathic OAB might dissuade clinicians from considering the higher dose of onabotulinumtoxinA. The study was limited by the lack of a placebo group.
Both onabotulinumtoxinA 200 U and sacral neuromodulation provide significant improvement in UUI episodes and quality of life over 6 months. However, while treatment with onabotulinumtoxinA has a likelihood of complete UUI resolution, greater improvements in symptom bother and treatment satisfaction, these benefits must be weighed against the risks of transient catheterization and UTI.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The International Continence Society (ICS) defines overactive bladder (OAB) as a syndrome of "urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence (UUI), in the absence of urinary tract infection [UTI] or obvious pathology."1 The Agency for Healthcare Research and Quality (AHRQ) reported OAB prevalence to be 15% in US women, with 11% reporting UUI.2 OAB represents a significant health care burden that impacts nearly every aspect of life, including physical, emotional, and psychological domains.3,4 The economic impact is notable; the projected cost is estimated to reach $82.6 billion annually by 2020.5
The American Urological Association (AUA) and the Society for Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) have endorsed an algorithm for use in the evaluation of idiopathic OAB (FIGURE).6 If the patient's symptoms are certain, minimal evaluation is needed and it is reasonable to proceed with first-line therapy, which includes fluid management (decreasing caffeine intake and limiting evening fluid intake), bladder retraining drills such as timed voiding, and improving pelvic floor muscles with the use of biofeedback and functional electrical stimulation.6,7 Pelvic floor muscle training can be facilitated with a referral to a physical therapist trained in pelvic floor muscle education.

If treatment goals are not met with first-line strategies, second-line therapy may be initiated with anticholinergic or β3-adrenergic receptor agonist medications. If symptoms persist after 4 to 8 weeks of pharmacologic therapy, clinicians are encouraged to reassess or refer the patient to a specialist. Further evaluation may include a bladder diary in which the patient documents voided volumes, voiding frequency, and number of incontinent episodes; symptom-specific questionnaires; and/or urodynamic testing.
Related article:
The latest treatments for urinary and fecal incontinence: Which hold water?
Based on that evaluation, the patient may be a candidate for third-line therapy with either intradetrusor onabotulinumtoxinA, posterior tibial nerve stimulation (PTNS), or sacral neuromodulation.
There is a paucity of information comparing third-line therapies. In this Update, we focus on 4 randomized clinical trials that compare third-line treatment options for idiopathic OAB.
Read about how anticholinergic medication and onabotulinumtoxinA compare for treating UUI.
Anticholinergic therapy and onabotulinumtoxinA produce equivalent reductions in the frequency of daily UUI episodes
Visco AG, Brubaker L, Richter HE, et al; for the Pelvic Floor Disorders Network. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367(19):1803-1813.
In a double-blind, double-placebo-controlled randomized trial, Visco and colleagues compared anticholinergic medication with onabotulinumtoxinA 100 U for the treatment of women with UUI.
Details of the study
Two hundred forty-one women with moderate to severe UUI received either 6 months of oral anticholinergic therapy (solifenacin 5 mg daily with the option of dose escalation to 10 mg daily or change to trospium XR 60 mg daily based on the Patient Global Symptom Control score) plus a single intradetrusor injection of saline, or a single intradetrusor injection of onabotulinumtoxinA 100 U plus a 6-month oral placebo regimen.
Inclusion criteria were 5 or more UUI episodes on a 3-day diary, insufficient resolution of symptoms after 2 medications, or being drug naive. Exclusions included a postvoid residual (PVR) urine volume greater than 150 mL or previous therapy with onabotulinumtoxinA.
Participants were scheduled for follow up every 2 to 6 months post randomization, at which time all study medications were discontinued. The primary outcome was reduction from baseline in the mean number of UUI episodes per day over the 6-month period, as recorded in the monthly 3-day bladder diaries. Secondary outcomes included the proportion of participants with complete resolution of UUI, the proportion of participants with 75% or more reduction in UUI episodes, Overactive Bladder Questionnaire Short Form (OABq-SF) scores, other symptom-specific questionnaire scores, and adverse events.
Related article:
Which treatments for pelvic floor disorders are backed by evidence?
Both treatments significantly reduced UUI episodes
At baseline, participants reported a mean (SD) of 5.0 (2.7) UUI episodes per day, and 41% of participants were drug naive. Both treatment groups experienced significant reductions compared with baseline in mean UUI episodes, and the reductions were similar between the 2 groups (reduction of 3.4 episodes per day in the anticholinergic group, reduction of 3.3 episodes in the onabotulinumtoxinA group; P = .81). Complete resolution of UUI was more common in the onabotulinumtoxinA group (27%) as compared with the anticholinergic group (13%) (P = .003). There were no differences in improvement in OABq-SF scores (37.05 in the anticholinergic group vs 37.13 in the onabotulinumtoxinA group; P = .98) or other quality-of-life measures.
Adverse events. The anticholinergic group experienced a higher rate of dry mouth compared with the onabotulinumtoxinA group (46% vs 31%; P = .02) but had lower rates of intermittent catheterization use at 2 months (0% vs 5%, P = .01) and UTIs (13% vs 33%, P<.001).
Strengths and limitations. This was a well-designed, multicenter, randomized double-blind, double placebo-controlled trial. The study design allowed for dose escalation and change to another medication for inadequate symptom control and included drug-naive participants, which increases the generalizability of the results. However, current guidelines recommend reserving onabotulinumtoxinA therapy for third-line therapy, thus deterring this treatment's use in the drug-naive population. Additionally, the lack of a pure placebo arm makes it difficult to interpret the extent to which a placebo effect contributed to observed improvements in clinical symptoms.
Through 6 months, both a single intradetrusor injection of onabotulinumtoxinA 100 U and anticholinergic therapy reduce UUI episodes and improve quality-of-life measures in women who have failed medications or are drug naive. Use of onabotulinumtoxinA, however, more likely will lead to complete resolution of UUI, although with an increased risk of transient urinary retention and UTI. Even given the study findings supporting the use of onabotulinumtoxinA over anticholinergic therapy for complete resolution of UUI, it is most appropriate to align with current practice, which includes a trial of pharmacotherapy before proceeding with third-line onabotulinumtoxinA.
Read: onabotulinumtoxinA vs PTNS for OAB.
OnabotulinumtoxinA has greater 9-month durability for OAB symptoms compared with12 weeks of PTNS
Sherif H, Khalil M, Omar R. Management of refractory idiopathic overactive bladder: intradetrusor injection of botulinum toxin type A versus posterior tibial nerve stimulation. Can J Urol. 2017;24(3):8838-8846.
In this randomized clinical trial, Sherif and colleagues compared the safety and efficacy of a single intradetrusor injection of onabotulinumtoxinA 100 U with that of PTNS for OAB.
Details of the study
Sixty adult men and women with OAB who did not respond to medical therapy were randomly assigned to treatment with either onabotulinumtoxinA 100 U or PTNS. Criteria for exclusion were current UTI, PVR urine volume of more than 150 mL, previous radiation therapy or chemotherapy, previous incontinence surgery or bladder malignancy, or presence of mixed urinary incontinence.
At baseline, participants completed a 3-day bladder diary, an OAB symptom score (OABSS) questionnaire, and urodynamic testing. The OABSS questionnaire included 7 questions (scoring range, 0-28), with higher scores indicating worse symptoms, and included subscales for urgency and quality-of-life measures. Total OABSS, urgency score, quality-of-life score, bladder diary records, and urodynamic testing parameters were assessed at 6, 12, 24, and 36 weeks, along with adverse events.
OnabotulinumtoxinA injections were performed under spinal anesthesia. If PVR urine volume was greater than 200 mL at any follow-up visit, participants were instructed to begin clean intermittent self-catheterization. PTNS was administered as weekly 30-minute sessions for 12 consecutive weeks.
Participants' baseline demographics and symptoms were similar. Average age was 45 years. Averages (SD) for duration of anticholinergic use was 13 (0.8) weeks, UUI episode score was 4.5 (1) on 3-day bladder diary, and OABSS was 22 (2.7). Nine-month data were available for 29 participants in the onabotulinumtoxinA group and for 8 in the PTNS group.
Related article:
Update on pelvic floor dysfunction: Focus on urinary incontinence
OnabotulinumtoxinA treatment benefits sustained for 9 months
Through 6 months, compared with baseline assessments, both treatment groups had significant improvements in clinical symptoms and OABSS total score, as well as urgency and quality-of-life subscales. At 3 months, urodynamic study parameters were similarly improved from baseline in both groups.
At 9 months, however, only the onabotulinumtoxinA group, compared with the PTNS group, maintained the significant improvement from baseline in 3-day bladder diary voiding episodes (average [SD], 10.7 [1.01] vs 11.6 [1.09]; P = .009), 3-day bladder diary nocturia episodes (average [SD], 3.8 [1.09] vs 4.4 [0.8]; P = .02), and average [SD] UUI episodes over 3 days (3.5 [1.2] vs 4.2 [1.04]; P = .02). Similarly, onabotulinumtoxinA-treated participants, compared with those treated with PTNS, maintained improvements at 9 months in average (SD): OABSS total score (19.2 [2.4] vs 20.4 [1.7]; P = .03), urgency scores (10.9 [1.3] vs 11.8 [1.4]; P = .009), urine volume at first desire (177.8 [9.2] vs 171.8 [7.7]), maximum cystometric capacity (304 [17.6] vs 290 [13.1]), and Qmax (mL/sec) (20.7 [1.6] vs 22.2 [1.2]).
Adverse events. Average PVR urine volumes were higher in the onabotulinumtoxinA group compared with the PTNS group (36.8 [2.7] vs 32.4 [3.03]; P = .0001) at all time points, and self-catheterization was required in 6.6% of onabotulinumtoxinA-treated participants. Urinary tract infection occurred in 6.6% of participants in the onabotulinumtoxinA group and in none of the PTNS group. In the PTNS group, few experienced pain and minor bleeding at the needle site.
Strengths and limitations. This randomized, open-label trial comparing treatment with onabotulinumtoxinA 100 U and PTNS included both men and women with idiopathic OAB symptoms. The participants were assessed at regular intervals with various measures, and follow-up adherence was good. The sample size was small, so the study may not have been powered to see differences prior to 9 months.
Although at 9 months only the onabotulinumtoxinA group maintained significant improvement over baseline levels, the improvement was diminished, and therefore the clinical meaningfulness is uncertain. Further, participants in the PTNS group did not undergo monthly maintenance therapy after 3 months, which is recommended for those with a 12-week therapeutic response; this may have affected 9-month outcomes in this group. Since the one-time onabotulinumtoxinA 100 U injection was performed under spinal anesthesia, cost comparisons should be considered, since future onabotulinumtoxinA injections would be necessary.
A one-time onabotulinumtoxinA 100 U injection and 12 weeks of PTNS therapy are reasonable short-term options for symptomatic OAB relief after unsuccessful therapy with medications. OnabotulinumtoxinA injection may provide more durable OAB symptom control at 9 months but with a risk of UTI and need for self-catheterization.
Read about using different doses of onabotulinumtoxinA for OAB.
OnabotulinumtoxinA 200-U injection provides longer OAB symptom improvement than 100-U injection
Abdelwahab O, Sherif H, Soliman T, Elbarky I, Eshazly A. Efficacy of botulinum toxin type A 100 units versus 200 units for treatment of refractory idiopathic overactive bladder. Int Braz J Urol. 2015;41(6):1132-1140.
Abdelwahab and colleagues conducted a single-center, randomized clinical trial to investigate the safety and efficacy of a single injection of intradetrusor onabotulinumtoxinA in 2 different doses (100 U and 200 U) for treatment of OAB.
Details of the study
Eighty adults (63 women, 17 men) who did not benefit from anticholinergic medication during the previous 3 months were randomly assigned to receive either a 100-U (n = 40) or a 200-U (n = 40) injection of onabotulinumtoxinA. Exclusion criteria were PVR urine volume greater than 150 mL and previous radiation therapy or chemotherapy.
Initial assessments -- completed at baseline and at 1, 3, 6, and 9 months -- included the health-related quality-of-life (HR-QOL) questionnaire (maximum score, 100; higher score indicates better quality of life), an abbreviated OABSS questionnaire (4 questions; score range, 0-15; higher score indicates more severe symptoms), and urodynamic evaluation. Outcomes included OABSS, HR-QOL score, and urodynamic parameters at the various time points.
Related article:
Is there a link between impaired mobility and urinary incontinence in elderly, community-dwelling women?
Higher dose, greater symptom improvement and higher adverse event rate
At baseline, participants (average age, 31 years) had an average (SD) OABSS of 1.7 (1.6). OnabotulinumtoxinA treatment with both a 100-U and a 200-U dose resulted in significant improvements (compared with baseline levels) in frequency, nocturia, UUI episodes, OABSS, and urodynamic parameters throughout the 9 months. At 9 months, however, the group treated with the 200-U dose had greater improvements, compared with the group who received a 100-U dose, in urinary frequency symptom scores (mean [SD], 0.32 [0.47] vs 1.1 [0.51]; P<.05), nocturia symptom scores (mean [SD], 0.13 [0.34] vs 0.36 [0.49]; P<.05), UUI symptom scores (mean [SD], 0.68 [0.16] vs 1.26 [1.1]; P<.05), and mean (SD) total OABSS (2.6 [2.31] vs 5.3 [2.11]; P<.05). Similarly, at 9 months the 200-U dose resulted in greater improvements in volume at first desire (mean [SD], 291.8 [42.8] vs 246.8 [53.8] mL; P<.05), volume at strong desire (mean [SD], 392.1 [37.3] vs 313.1 [67.4] mL; P<.05), detrusor pressure (mean [SD], 10.4 [4.0] vs 19.2 [7.8] cm H2O; P<.05), and maximum cystometric capacity (mean [SD], 430.5 [34.2] vs 350 [69.1] mL; P<.05) compared with the 100-U dose.
Adverse events. No participant had a PVR urine volume greater than 100 mL at any follow-up visit. Postoperative hematuria occurred in 23% of the group treated with onabotulinumtoxinA 200 U versus in 15% of those treated with a 100-U dose. Similarly, UTIs occurred in 17.5% of the 200-U dose group and in 7.5% of the 100-U dose group. Dysuria was reported in 37.5% and 15% of the 200-U and 100-U dose groups, respectively.
Strengths and limitations. This randomized, open-label trial comparing a single injection of 100 U versus 200 U of onabotulinumtoxinA included mostly women. OAB symptoms and urodynamic parameters improved after treatment with both dose levels, but a longer duration of improvement was seen with the 200-U dose. The cohort had a low baseline OAB severity, based on the OABSS questionnaire, and a young average age of participants, which limits the generalizability of the study results to a population with refractory OAB. The 0% rate of clean intermittent self-catheterization postinjection might be based on the study's criteria for requiring clean intermittent catheterization. In addition, the initial postinjection visit occurred at 1 month, possibly missing participants who had symptoms of retention soon after injection.
Two dose levels (100 U and 200 U) of a single injection of onabotulinumtoxinA are associated with comparable OAB symptom and urodyanamic improvements. The benefits of a longer duration of effect with the 200-U dose must be weighed against the possible higher risks of transient hematuria, dysuria, and UTI.
Read: onabotulinumtoxinA vs sacral neuromodulation therapy for UUI.
Treatment with onabotulinumtoxinA may control UUI symptoms better than sacral neuromodulation therapy
Amundsen CL, Richter HE, Menefee SA, et al; Pelvic Floor Disorders Network. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: a randomized clinical trial. JAMA. 2016;316(13):1366-1374.
In this multicenter open-label randomized trial, Amundsen and colleagues compared the efficacy and safety of onabotulinumtoxinA 200 U with that of sacral neuromodulation.
Details of the study
Three hundred sixty-four women with UUI had data available for primary analysis at 6 months. Women were considered eligible for the study if they had 6 or more UUI episodes on a 3-day bladder diary, persistent symptoms despite anticholinergic therapy, a PVR urine volume of less than 150 mL, and had never previously received either study treatment.
There were no differences in baseline characteristics of the participants. The average (SD) age of the study population was 63 (11.6) years, with an average (SD) daily number of UUI episodes of 5.3 (2.8). The average (SD) body mass index was 32 (8) kg/m2.
Participants were randomly assigned to undergo either sacral neuromodulation (n = 174) or intradetrusor injection of onabotulinumtoxinA 200 U (n = 190). The primary outcome was change from baseline in mean number of daily UUI episodes averaged over 6 months as recorded on a monthly 3-day bladder diary. Secondary outcomes included complete resolution of urgency incontinence, 75% or more reduction in UUI episodes, the Overactive Bladder Questionnaire Short Form (SF) score (range, 0-100; higher score indicates higher symptom severity), the Overactive Bladder Satisfaction of Treatment questionnaire (range, 0-100; higher score indicates better satisfaction), other quality-of-life measures, and adverse events.
Related article:
2015 Update on pelvic floor dysfunction: Bladder pain syndrome
Greater symptom bother improvement, treatment satisfaction with onabotulinumtoxinA 200 U
Participants treated with onabotulinumtoxinA had a greater mean reduction of 3.9 UUI episodes per day than the sacral neuromodulation group's reduction of 3.3 UUI episodes per day (mean difference, 0.63; 95% confidence interval [CI], 0.13-1.14; P = .01). In addition, complete UUI resolution was higher in the onabotulinumtoxinA group as compared with the sacral neuromodulation group (20% vs 4%; P<.001). The onabotulinumtoxinA group also had higher rates of 75% or more reduction of UUI episodes compared with the sacral neuromodulation group (46% vs 26%; P<.001). Over 6 months, both groups had improvements in all quality-of-life measures, but the onabotulinumtoxinA group had greater improvement in symptom bother compared with the sacral neuromodulation group (-46.7 vs -38.6; mean difference, 8.1; 95% CI, 3.0-13.3; P = .002). Furthermore, the onabotulinumtoxinA group had greater treatment satisfaction compared with the sacral neuromodulation group (mean difference, 7.8; 95% CI, 1.6-14.1; P = .01).
Adverse events. Six women (3%) underwent sacral neuromodulation device revision or removal. Approximately 8% of onabotulinumtoxinA-treated participants required intermittent self-catheterization at 1 month, 4% at 3 months, and 2% at 6 months. The risk of UTI was higher in the onabotulinumtoxinA group compared with the sacral neuromodulation group (35% vs 11%; risk difference, 23%; 95% CI, -33% to -13%; P<.001).
Strengths and limitations. This is a well-designed randomized clinical trial comparing clinical outcomes and adverse events after treatment with onabotulinumtoxinA 200-U versus sacral neuromodulation. The interventions were standardized across investigators at multiple sites, and the study design required close follow-up to assess efficacy and adverse events. The study used a 200-U dose based on reported durability of effect at that time and findings of equivalency between onabotulinumtoxinA 100 U and anticholinergic therapy. The US Food and Drug Administration's recommendation to use a 100-U dose in all patients with idiopathic OAB might dissuade clinicians from considering the higher dose of onabotulinumtoxinA. The study was limited by the lack of a placebo group.
Both onabotulinumtoxinA 200 U and sacral neuromodulation provide significant improvement in UUI episodes and quality of life over 6 months. However, while treatment with onabotulinumtoxinA has a likelihood of complete UUI resolution, greater improvements in symptom bother and treatment satisfaction, these benefits must be weighed against the risks of transient catheterization and UTI.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al; International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20.
- Hartmann KE, McPheeters ML, Biller DH, et al. Treatment of overactive bladder in women. Evid Rep Technol Assess (Full Rep). 2009;187:1-120.
- Reynolds,WS, Fowke J, Dmochowski, R. The burden of overactive bladder on US public health. Curr Bladder Dysfunct Rep. 2016;11(1):8-13.
- Willis-Gray MG, Dieter AA, Geller EJ. Evaluation and management of overactive bladder: strategies for optimizing care. Res Rep Urol. 2016;8:113-122.
- Ganz ML, Smalarz AM, Krupski TL, et al. Economic costs of overactive bladder in the United States. Urology. 2010;75(3):526-532.
- Gormley EA, Lightner DJ, Faraday M, Vasavada SP; American Urological Association; Society of Urodyndamics, Female Pelvic Medicine. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015; 193(5):1572-1580.
- Gormley EA, Lightner DJ, Burgio KL, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2455-2463.
- Haylen BT, de Ridder D, Freeman RM, et al; International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20.
- Hartmann KE, McPheeters ML, Biller DH, et al. Treatment of overactive bladder in women. Evid Rep Technol Assess (Full Rep). 2009;187:1-120.
- Reynolds,WS, Fowke J, Dmochowski, R. The burden of overactive bladder on US public health. Curr Bladder Dysfunct Rep. 2016;11(1):8-13.
- Willis-Gray MG, Dieter AA, Geller EJ. Evaluation and management of overactive bladder: strategies for optimizing care. Res Rep Urol. 2016;8:113-122.
- Ganz ML, Smalarz AM, Krupski TL, et al. Economic costs of overactive bladder in the United States. Urology. 2010;75(3):526-532.
- Gormley EA, Lightner DJ, Faraday M, Vasavada SP; American Urological Association; Society of Urodyndamics, Female Pelvic Medicine. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015; 193(5):1572-1580.
- Gormley EA, Lightner DJ, Burgio KL, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2455-2463.
2016 Update on pelvic floor dysfunction
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6

Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6


Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The genitourinary syndrome of menopause (GSM) is a constellation of symptoms and signs of a hypoestrogenic state resulting in some or all of the following: vaginal dryness, burning, irritation, dyspareunia, urinary urgency, dysuria, and recurrent urinary tract infections.1 In 2014, the International Society for the Study of Women’s Sexual Health and the North American Menopause Society endorsed “GSM” as a new term to replace the less comprehensive description, vulvovaginal atrophy (VVA).1
The prevalence of GSM is around 50%, but it may increase each year after menopause, reaching up to 84.2%.2,3 Only about half of women affected seek medical care, with the most commonly reported symptoms being vaginal dryness and dyspareunia.3,4
Nonhormonal vaginal moisturizers andlubricants remain first-line treatment. The benefits are temporary and short lived because these options do not change the physiologic makeup of the vaginal wall; these treatments therefore provide relief only if the GSM symptoms are limited or mild.5
In this Update on pelvic floor dysfunction, we review 2 randomized, placebo-controlled trials of hormonal options (vaginal estrogen and oral ospemifene) and examine the latest information regarding fractional CO2 vaginal laser treatment. Also included are evidence-based guidelines for vaginal estrogen use and recommendations and conclusions for use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. (The terms used in the studies described [ie, VVA versus GSM] have been maintained for accuracy of reporting.)
Low-dose estrogen vaginal cream ameliorates moderate to severe VVA with limited adverse events
Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741.
In a multicenter, double-blind, randomized, placebo-controlled study, Freedman and colleagues evaluated the efficacy of a 1-g dose of synthetic conjugated estrogens A (SCE-A) cream versus placebo in postmenopausal women with moderate to severe VVA.
Details of the study
The investigators enrolled 305 participants aged 30 to 80 years (mean [SD] age, 60 [6.6] years) who were naturally or surgically postmenopausal. The enrollment criteria included ≤5% superficial cells on vaginal smear, vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA (vaginal dryness, soreness, irritation/itching, pain with intercourse, or bleeding after intercourse).
Participants were randomly assigned in a 1:1:1:1 ratio to twice-weekly therapy with 1 g (0.625 mg/g) SCE-A vaginal cream, 2 g SCE-A vaginal cream, 1 g placebo, or 2 g placebo. Study visits occurred on days 14, 21, 28, 56, and 84 (12-week end point). The 3 co-primary outcomes were cytology, vaginal pH, and most bothersome symptom (MBS). Primary outcomes and safety/adverse events (AEs) were recorded at each study visit, and transvaginal ultrasound and endometrial biopsy were performed for women with a uterus at the beginning and end of the study.
Mean change and percent change in the 3 primary outcomes were assessed between baseline and each study visit. MBS was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). The principal indicators of efficacy were the changes from baseline to the end of treatment (12 weeks) for each of the 3 end points. Since the 1-g and 2-g SCE-A dose groups showed a similar degree of efficacy on all 3 co-primary end points, approval from the US Food and Drug Administration (FDA) was sought only for the lower dose, in keeping with the use of the lowest effective dose; therefore, results from only the 1-g SCE-A dose group and matching placebo group were presented in the article. A sample size calculation determined that at least 111 participants in each group were needed to provide 90% power for statistical testing.
Estrogen reduced MBS severity, improved vaginal indices
The modified intent-to-treat (MITT) cohort was used for outcome analysis, and data from 275 participants were available at the 12-week end point. At baseline, 132 participants (48%) indicated vaginal dryness and 86 women (31.3%) indicated pain during intercourse as the MBS. In the SCE-A group at baseline, the vaginal maturation index (VMI) was 31.31 compared with 31.84 in the placebo group. At 12 weeks, the SCE-A group had a mean reduction of 1.71 in overall MBS severity compared with the placebo group’s mean reduction of 1.11 (P<.0001). The SCE-A group had a greater increase in the VMI (with a mean change of 31.46 vs 5.16 in the placebo group [P<.0001]) and a greater decrease in the vaginal pH (mean pH at the end of treatment for the SCE-A group was 4.84, a decrease of 1.48, and for the placebo group was 5.96, a decrease of 0.31 [P<.0001]).
Adverse events. The incidence of AEs was similar for the 1-g SCE-A group and the 1-g placebo group, with no AE occurring at a rate of higher than 5%. There were 15 (10%) treatment-related AEs in the estrogen group and 16 (10.3%) in the placebo group. The SCE-A group had 3 AEs (2%) leading to discontinuation, while the placebo group had 2 AEs (1.3%) leading to discontinuation. There were no clinically significant endometrial biopsy findings at the conclusion of the study.
Strengths and limitations. This study evaluated clinical and physiologic outcomes as well as uterine response to transvaginal estrogen. The use of MBS allows symptoms to be scored objectively compared with prior subjective symptom assessment, which varied widely. However, fewer indicated symptoms will permit limited conclusions.
For evidence-based recommended and suggested treatments for various genitourinary symptoms, we recommended as a resource the Society of Gynecologic Surgeons clinical practice guidelines on vaginal estrogen for the treatment of GSM (TABLE 1).5
In addition, for women with a history of estrogen-dependent breast cancer experiencing urogenital symptoms, the American College of Obstetricians and Gynecologists recommends nonhormonal agents as first-line therapy, with vaginal estrogen treatment reserved for woman whose symptoms are unresponsive to nonhormonal therapies (TABLE 2).6


Ospemifene improves vaginal physiology and dyspareunia
Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–486.
Bachmann and colleagues evaluated the efficacy and safety of ospemifene for the treatment of VVA. This is one of the efficacy studies on which FDA approval was based. Ospemifene is a selective estrogen receptor modulator (SERM) that acts as an estrogen agonist/antagonist.
Details of the study
The study included 826 postmenopausal women randomly assigned to 30 mg/day of ospemifene, 60 mg/day of ospemifene, or placebo for 12 weeks. Participants were aged 40 to 80 years and met the criteria for VVA (defined as ≤5% superficial cells on vaginal smear [maturation index], vaginal pH >5.0, and at least 1 moderate or severe symptom of VVA). All women were given a nonhormonal lubricant for use as needed.
There were 4 co-primary end points: percentage of superficial cells on the vaginal smear, percentage of parabasal cells on the vaginal smear, vaginal pH, and self-assessed MBS using a Likert scale (0, none; 1, mild; 2, moderate; 3, severe). The symptom score was calculated as the change from baseline to week 12 for each MBS. Safety was assessed by patient report; if a participant had an intact uterus and cervix, Pap test, endometrial thickness, and endometrial histologic analysis were performed at baseline and at 12 weeks. Baseline characteristics were similar among all treatment groups. A total of 46% of participants reported dyspareunia as their MBS, and 39% reported vaginal dryness.
Two dose levels of ospemifene effectively relieve symptoms
After 12 weeks of treatment, both the 30-mg and the 60-mg dose of ospemifene produced a statistically significant improvement in vaginal dryness and objective results of maturation index and vaginal pH compared with placebo. Vaginal dryness decreased in the ospemifene 30-mg group (1.22) and in the ospemifene 60-mg group (1.26) compared with placebo (0.84) (P = .04 for the 30-mg group and P = .021 for the 60-mg group). The percentage of superficial cells was increased in both treatment groups compared with placebo (7.8% for the 30-mg group, 10.8% for the 60-mg group, 2.2% for the placebo group; P<.001 for both). The percentage of parabasal cells decreased in both treatment groups compared with participants who received placebo (21.9% in the 30-mg group, 30.1% in the 60-mg group, and 3.98% in the placebo group; P<.001 for both). Both treatment groups had a decrease in vaginal pH versus the placebo group as well (0.67 decrease in the 30-mg group, 1.01 decrease in the 60-mg group, and 0.10 decrease in the placebo group; P<.001 for both). The 60-mg/day ospemifene dose improved dyspareunia compared with placebo and was more effective than the 30-mg dose for all end points.
Adverse effects. Hot flashes were reported in 9.6% of the 30-mg ospemifene group and in 8.3% of the 60-mg group, compared with 3.4% in the placebo group. The increased percentage of participants with hot flashes in the ospemifene groups did not lead to increased discontinuation with the study. Urinary tract infections, defined by symptoms only, were more common in the ospemifene groups (4.6% in the 30-mg group, 7.2% in the 60-mg group, and 2.2% in the placebo group). In each group, 5% of patients discontinued the study because of AEs. There were 5 serious AEs in the 30-mg ospemifene group, 4 serious AEs in the placebo group, and none in the 60-mg group. No venous thromboembolic events were reported.
Strengths and limitations. Vaginal physiology as well as common symptoms of GSM were assessed in this large study. However, AEs were self-reported. While ospemifene was found safe and well tolerated when the study was extended for an additional 52 weeks (in women without a uterus) and 40 weeks (in women with a uterus), longer follow-up is needed to determine endometrial safety.7,8
Some patients may prefer an oral agent over a vaginally applied medication. While ospemifene is not an estrogen, it is a SERM that may increase the risk of endometrial cancer and thromboembolic events as stated in the boxed warning of the ospemifene prescribing information.
Fractional CO2 laser for VVA shows efficacy, patient satisfaction
Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23(10):1102–1107.
In this first US pilot study, postmenopausal women received 3 fractional CO2 laser treatments, 6 weeks apart. The investigators evaluated the safety and efficacy of the treatment for GSM.
Details of the study
Thirty women (mean age, 58.6 years) who were nonsmokers, postmenopausal, had less than stage 2 prolapse, no vaginal procedures for the past 6 months, and did not use vaginal creams, moisturizers, lubricants, or homeopathic preparations for the past 3 months were enrolled. Participants received 3 laser treatments with the SmartXide2, MonaLisa Touch (DEKA M.E.L.A. SRL, Florence, Italy) device at 6-week intervals followed by a 3-month follow-up.
The primary outcome was visual analog scale (VAS) change in 6 categories (vaginal pain, burning, itching, dryness, dyspareunia, and dysuria) assessed from baseline to after each treatment, including 3 months after the final treatment, using an 11-point scale with 0 the lowest (no symptoms) and 10 the highest (extreme bother). Secondary outcomes were Vaginal Health Index (VHI) score, maximal tolerable dilator size, Female Sexual Function Index (FSFI) questionnaire score, general quality of life, degree of difficulty performing the procedure, participant satisfaction, vaginal pH, adverse effects, and treatment discomfort assessed using the VAS.
Improved VVA symptoms and vaginal caliber
Twenty-seven women completed the study. There was a statistically significant change in all 6 symptom categories measured with the VAS. Improvement change (SD) on the VAS was 1.7 (3.2) for pain, 1.4 (2.9) for burning, 1.4 (1.9) for itching, 1.0 (2.4) for dysuria, comparing baseline scores to scores after 3 treatments (all with P<.05). A greater improvement was noted for dryness, 6.1 (2.7), and for dyspareunia, 5.4 (2.9) (both P<.001). There was also a statistically significant change in overall improvement on the VHI and the FSFI. The mean (SD) VHI score at baseline was 14.4 (2.9; range, 8 to 20) and the mean (SD) after 3 laser treatments was 21.4 (2.9; range, 16 to 25), with an overall mean (SD) improvement of 7.0 (3.1; P<.001).
Twenty-six participants completed a follow-up FSFI, with a mean (SD) baseline score of 11.3 (7.3; range, 2 to 25) and a follow-up mean (SD) score of 8.8 (7.3; range, −3.7 to 27.2) (P<.001). There was an increase in dilator size of 83% when comparing baseline to follow-up. At baseline, 24 participants (80%) could comfortably accept an XS or S dilator, and at follow-up 23 of 24 women (96%) could comfortably accept an M or L dilator.
Adverse effects. At their follow-up, 96% of participants were satisfied or extremely satisfied with treatment. Two women reported mild-to-moderate pain lasting 2 to 3 days, and 2 had minor bleeding; however, no women withdrew or discontinued treatment because of adverse events.
Study limitations. This study evaluated the majority of GSM symptoms as well as change in vaginal caliber after a nonhormonal therapy. The cohort was small and had no placebo group. In addition, with the limited observation period, it is difficult to determine the duration of effect and long-term safety of repeated treatments.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
- Portman DJ, Gass ML: Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–354.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Palma F, Volpe A, Villa P, Cagnacci A; Writing Groupt of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas. 2016;83:40–44.
- Kingsberg SA, Sysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Rahn DD, Carberry C, Sanses TV, et al; Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstet Gynecol. 2014;124(6):1147–1156.
- Farrell R; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–e96.
- Simon JA, Lin VH, Radovich C, Bachmann GA; Ospemiphene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2013;20(4):418–427.
- Simon J, Portman D, Mabey RG Jr; Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281.
In this Article
- Low-dose estrogen vaginal cream
- Ospemifene therapy
- Fractional CO2 laser treatment
2015 Update on pelvic floor dysfunction: Bladder pain syndrome
Interstitial cystitis (IC) is a debilitating disease that presents with a constellation of symptoms, including pain, urinary urgency, frequency, nocturia, and small voided volumes in the absence of other identifiable etiologies.1 The overall prevalence of IC among US women is between 2.7% and 6.5%—affecting approximately 3.3 to 7.9 million women2—and it results in substantial costs1,3 and impairments in health-related quality of life.4 Unfortunately, there is a lack of consensus on the pathophysiology and etiology of this prevalent and costly disorder. Thus, therapies are often empiric, with limited evidence and variable levels of improvement.5
There has been no clear evidence that bladder inflammation (cystitis) is involved in the etiology or pathophysiology of the condition. As a result, there has been a movement to rename it “bladder pain syndrome.” Current literature refers to the spectrum of symptoms as interstitial cystitis/bladder pain syndrome (IC/BPS).
Currently, the American Urological Association (AUA) defines IC/BPS as an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than 6 weeks’ duration, in the absence of infection or other identifiable causes.6 This is still a broad, clinical diagnosis that has significant overlap with other pain syndromes but allows for treatment to begin after a relatively short symptomatic period.7 Because gynecologists are frequently the main care providers for women, understanding the diagnosis and treatment options for IC/BPS is important to avoid delayed treatment in a difficult to diagnose population.
Recently, the AUA published an amendment to their 2011 management guidelines to provide direction to clinicians and patients regarding how to recognize IC/BPS, conduct valid diagnostic testing, and approach treatment with the goals of maximizing symptom control and patient quality of life.7
In this article, we review the AUA diagnostic and treatment algorithms and the results of recently published randomized trials comparing the efficacy of various treatment modalities for IC/BPS, including pentosoan polysulfate sodium (PPS; Elmiron, Janssen Pharmaceuticals, Titusville, New Jersey) and botulinum toxin (Botox, Allergan, Irvine, California) with hydrodistension.
- Anger JT, Zabihi N, Clemens JQ, Payne CK, Saigal CS, Rodriguez LV. Treatment choice, duration, and cost in patients with interstitial cystitis and painful bladder syndrome. Int Urogynecol J. 2011;22(4):395–400.
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–544.
- Payne CK, Joyce GF, Wise M, Clemens JQ; Urologic Diseases in America Project. Interstitial cystitis and painful bladder syndrome. J Urol. 2007;177(6):2042–2049.
- Nickel JC, Payne CK, Forrest J, Parsons CL, Wan GJ, Xiao X. The relationship among symptoms, sleep disturbances and quality of life in patients with interstitial cystitis. J Urol. 2009;181(6):2555–2561.
- Giannantoni A, Bini V, Dmochowski R, et al. Contemporary management of the painful bladder: a systematic review. Eur Urol. 2012;61(1):29–53.
- Hanno P, Dmochowski R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol Urodyn. 2009;28(4):274–286.
- Hanno PM, Erickson D, Moldwin R, Faraday MM; American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–1553.
- Hanno PM, Burks DA, Clemens JQ, et al; Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162–2170.
- Boudry G, Labat JJ, Riant T, et al. Validation of voiding diary for stratification of bladder pain syndrome according to the presence/absence of cystoscopic abnormalities: a two-centre prospective study. BJU Int. 2013;112(2):E164−168.
- O’Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarish-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(5A suppl):58–63.
- Nickel JC, Herschom S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo-controlled study. J Urol. 2015;193(3):857–862.
- Nickel JC, Barkin J, Forrest J, et al; Elmiron Study Group. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65(4):654–658.
- Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial. Contemp Clin Trials, 2012;33(1):184–196.
Interstitial cystitis (IC) is a debilitating disease that presents with a constellation of symptoms, including pain, urinary urgency, frequency, nocturia, and small voided volumes in the absence of other identifiable etiologies.1 The overall prevalence of IC among US women is between 2.7% and 6.5%—affecting approximately 3.3 to 7.9 million women2—and it results in substantial costs1,3 and impairments in health-related quality of life.4 Unfortunately, there is a lack of consensus on the pathophysiology and etiology of this prevalent and costly disorder. Thus, therapies are often empiric, with limited evidence and variable levels of improvement.5
There has been no clear evidence that bladder inflammation (cystitis) is involved in the etiology or pathophysiology of the condition. As a result, there has been a movement to rename it “bladder pain syndrome.” Current literature refers to the spectrum of symptoms as interstitial cystitis/bladder pain syndrome (IC/BPS).
Currently, the American Urological Association (AUA) defines IC/BPS as an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than 6 weeks’ duration, in the absence of infection or other identifiable causes.6 This is still a broad, clinical diagnosis that has significant overlap with other pain syndromes but allows for treatment to begin after a relatively short symptomatic period.7 Because gynecologists are frequently the main care providers for women, understanding the diagnosis and treatment options for IC/BPS is important to avoid delayed treatment in a difficult to diagnose population.
Recently, the AUA published an amendment to their 2011 management guidelines to provide direction to clinicians and patients regarding how to recognize IC/BPS, conduct valid diagnostic testing, and approach treatment with the goals of maximizing symptom control and patient quality of life.7
In this article, we review the AUA diagnostic and treatment algorithms and the results of recently published randomized trials comparing the efficacy of various treatment modalities for IC/BPS, including pentosoan polysulfate sodium (PPS; Elmiron, Janssen Pharmaceuticals, Titusville, New Jersey) and botulinum toxin (Botox, Allergan, Irvine, California) with hydrodistension.
Interstitial cystitis (IC) is a debilitating disease that presents with a constellation of symptoms, including pain, urinary urgency, frequency, nocturia, and small voided volumes in the absence of other identifiable etiologies.1 The overall prevalence of IC among US women is between 2.7% and 6.5%—affecting approximately 3.3 to 7.9 million women2—and it results in substantial costs1,3 and impairments in health-related quality of life.4 Unfortunately, there is a lack of consensus on the pathophysiology and etiology of this prevalent and costly disorder. Thus, therapies are often empiric, with limited evidence and variable levels of improvement.5
There has been no clear evidence that bladder inflammation (cystitis) is involved in the etiology or pathophysiology of the condition. As a result, there has been a movement to rename it “bladder pain syndrome.” Current literature refers to the spectrum of symptoms as interstitial cystitis/bladder pain syndrome (IC/BPS).
Currently, the American Urological Association (AUA) defines IC/BPS as an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than 6 weeks’ duration, in the absence of infection or other identifiable causes.6 This is still a broad, clinical diagnosis that has significant overlap with other pain syndromes but allows for treatment to begin after a relatively short symptomatic period.7 Because gynecologists are frequently the main care providers for women, understanding the diagnosis and treatment options for IC/BPS is important to avoid delayed treatment in a difficult to diagnose population.
Recently, the AUA published an amendment to their 2011 management guidelines to provide direction to clinicians and patients regarding how to recognize IC/BPS, conduct valid diagnostic testing, and approach treatment with the goals of maximizing symptom control and patient quality of life.7
In this article, we review the AUA diagnostic and treatment algorithms and the results of recently published randomized trials comparing the efficacy of various treatment modalities for IC/BPS, including pentosoan polysulfate sodium (PPS; Elmiron, Janssen Pharmaceuticals, Titusville, New Jersey) and botulinum toxin (Botox, Allergan, Irvine, California) with hydrodistension.
- Anger JT, Zabihi N, Clemens JQ, Payne CK, Saigal CS, Rodriguez LV. Treatment choice, duration, and cost in patients with interstitial cystitis and painful bladder syndrome. Int Urogynecol J. 2011;22(4):395–400.
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–544.
- Payne CK, Joyce GF, Wise M, Clemens JQ; Urologic Diseases in America Project. Interstitial cystitis and painful bladder syndrome. J Urol. 2007;177(6):2042–2049.
- Nickel JC, Payne CK, Forrest J, Parsons CL, Wan GJ, Xiao X. The relationship among symptoms, sleep disturbances and quality of life in patients with interstitial cystitis. J Urol. 2009;181(6):2555–2561.
- Giannantoni A, Bini V, Dmochowski R, et al. Contemporary management of the painful bladder: a systematic review. Eur Urol. 2012;61(1):29–53.
- Hanno P, Dmochowski R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol Urodyn. 2009;28(4):274–286.
- Hanno PM, Erickson D, Moldwin R, Faraday MM; American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–1553.
- Hanno PM, Burks DA, Clemens JQ, et al; Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162–2170.
- Boudry G, Labat JJ, Riant T, et al. Validation of voiding diary for stratification of bladder pain syndrome according to the presence/absence of cystoscopic abnormalities: a two-centre prospective study. BJU Int. 2013;112(2):E164−168.
- O’Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarish-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(5A suppl):58–63.
- Nickel JC, Herschom S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo-controlled study. J Urol. 2015;193(3):857–862.
- Nickel JC, Barkin J, Forrest J, et al; Elmiron Study Group. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65(4):654–658.
- Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial. Contemp Clin Trials, 2012;33(1):184–196.
- Anger JT, Zabihi N, Clemens JQ, Payne CK, Saigal CS, Rodriguez LV. Treatment choice, duration, and cost in patients with interstitial cystitis and painful bladder syndrome. Int Urogynecol J. 2011;22(4):395–400.
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–544.
- Payne CK, Joyce GF, Wise M, Clemens JQ; Urologic Diseases in America Project. Interstitial cystitis and painful bladder syndrome. J Urol. 2007;177(6):2042–2049.
- Nickel JC, Payne CK, Forrest J, Parsons CL, Wan GJ, Xiao X. The relationship among symptoms, sleep disturbances and quality of life in patients with interstitial cystitis. J Urol. 2009;181(6):2555–2561.
- Giannantoni A, Bini V, Dmochowski R, et al. Contemporary management of the painful bladder: a systematic review. Eur Urol. 2012;61(1):29–53.
- Hanno P, Dmochowski R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol Urodyn. 2009;28(4):274–286.
- Hanno PM, Erickson D, Moldwin R, Faraday MM; American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–1553.
- Hanno PM, Burks DA, Clemens JQ, et al; Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162–2170.
- Boudry G, Labat JJ, Riant T, et al. Validation of voiding diary for stratification of bladder pain syndrome according to the presence/absence of cystoscopic abnormalities: a two-centre prospective study. BJU Int. 2013;112(2):E164−168.
- O’Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarish-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(5A suppl):58–63.
- Nickel JC, Herschom S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo-controlled study. J Urol. 2015;193(3):857–862.
- Nickel JC, Barkin J, Forrest J, et al; Elmiron Study Group. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65(4):654–658.
- Visco AG, Brubaker L, Richter HE, et al; Pelvic Floor Disorders Network. Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial. Contemp Clin Trials, 2012;33(1):184–196.
In this Article
- AUA diagnosis guidelines
- Treatment algorithm
- A new FDA-approved oral treatment option
2014 Update on pelvic floor dysfunction
Constipation is estimated to affect up to 27% of the general population and is more common in women, with a 2:1 female-to-male ratio.1 Because gynecologists are frequently the main care provider for many women, understanding the diagnosis and treatment options for constipation is important. Additionally, gynecologists must manage bowel function during the perioperative period.
The diagnosis of constipation is based on the Rome III criteria.2 Besides frequency of bowel movements (BMs), these criteria include evacuation symptoms and the presence of hard stools (TABLE 1). These symptoms can result from delay in colonic transit or outlet dysfunction. Constipation may be secondary to medical illness, such as central or peripheral neurologic disease, diabetes mellitus, hypothyroidism, or medications. Evaluation begins with a careful history and vaginal and perianal/anal examination.3 Initially, a trial of fiber supplementation with or without over-the-counter (OTC) laxatives may be tried (TABLE 2). If patients have an inadequate response to this therapy, further evaluation may be pursued (ALGORITHM).
------
| TABLE1 Rome III criteria for functional constipation in adults* |
1. Must include ≥2 of the following signs
2. Loose stools are rarely present without the use of laxatives 3. Insufficient criteria for irritable bowel syndrome |
| *At least 3 months, with symptoms beginning ≥6 months before diagnosis. |
--------
| TABLE 2 Common treatments for constipation |
Bulk-forming laxatives absorb water, increasing fecal mass
Surfactant agents lower the surface tension of stool, allowing water to enter the stool
Osmotic laxatives contain poorly/nonabsorbed substances, leading to intestinal water secretion
Stimulant laxatives increase colonic transit and alter electrolyte transport across the colonic mucosa
|
In this article, we review the results of randomized trials comparing the efficacy of OTC medical treatments for constipation, including daily, low-dose polyethylene glycol (PEG) and probiotics. Additionally, we review key trials evaluating perioperative bowel management prior to laparoscopic gynecologic and vaginal surgery.
LONG-TERM PEG USAGE SAFE AND EFFECTIVE?
Corazziari E, Badiali D, Bazzocchi G, et al. Long-term efficacy, safety, and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut. 2000;46(4):522–526.
In this multicenter, randomized, double-blind, placebo-controlled, parallel trial, investigators evaluated the safety, efficacy, and tolerability of a daily low-dose PEG-based osmotic diuretic.
Details of the study
Seventy-eight patients (80% of them female) aged 18 to 75 years with chronic constipation, defined by Rome III diagnostic criteria, underwent a 4-week “run-in” period, with a standardized daily diet of fiber 15 g, water 1500 mL, and twice-daily PMF-100 (PEG/osmotic solution). Patients were randomized if they responded to the regimen, with response defined as having at least two BMs per week and no defecatory disturbance or at least three BMs per week with or without defecatory disturbance. Eight patients were not randomized, one due to nonresponsiveness. Study patients completed 20 weeks of either twice-daily PMF-100 or placebo. Patients, at their own discretion, decreased the frequency of the study drug based on the frequency of their BMs. Use of another laxative was not allowed unless a BM had not occurred over a 5-day period.
The combined primary outcome was at least three BMs per week, no defecatory disturbances, and no additional laxative use. Secondary outcomes (frequency of BMs and defecatory disturbances) were assessed using a bowel diary.
No differences were noted in baseline measurements between the two groups. Of the PMF-100 group, 70% completed the study, compared with 30% of the placebo group (P<.01). Nonresponse to treatment was the reason for dropout in 7% and 46% of patients, respectively (P<.005). Other causes of withdrawal did not differ between the groups.
At the end of the 20 weeks, 77% of patients in the PMF-100 group reported remission, compared with 20% in the placebo group (P<.001). During the study, the PMF-100 group reported more BMs per week (7.4 vs 4.3; P<.001). Furthermore, the treatment group was less likely to report straining at defecation, hard/pellet stools, and need for use of additional laxatives. Adverse events (nausea, anal pain/itching, hematochezia, epigastric pain, and fecal incontinence) were similar between groups. There were no differences in laboratory values.
Study strengths
This was a well-designed trial showing the safety, efficacy, and tolerability of a daily low-dose PEG-based osmotic diuretic. The population was mainly women with functional chronic constipation, similar to a gynecologic population. The results of this trial are consistent with what has been shown for other trials various PEG preparations.4,5
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women who fail initial fiber therapy may respond to daily low-dose PEG on a continuous basis. Resolution of constipation and defecatory symptoms is likely and should be seen within 1 month. Therapy can be continued safely for at least 6 months.
--------------
NEW AND TRENDY OTC TREATMENT OPTION
Del Piano M, Carmagnola S, Anderloni A, et al. The use of probiotics in healthy volunteers with evacuation disorders and hard stools: a double-blind, randomized, placebo-controlled study. J Clin Gastroenterol. 2010;44(suppl 1):S30–S34.
Factors such as age, unhealthy diet, and use of prescription drugs alter the intestinal bacterial flora. As patients strive for a more holistic approach to their health, interest is growing in the benefit of probiotics for treating chronic constipation. To explore the value of such probiotics, Del Piano and colleagues conducted a three-armed, randomized, double-blind placebo-controlled trial of two different probiotic preparations and a placebo among patients aged 24 to 71 years with evacuation disorders and constipation.
Details of the study
One probiotic preparation (A) was composed of Lactobacillus plantarum and Bifidobacterium breve at a concentration of 2.5×109 cfu per day; the other (B) was composed of Bifidobacterium animalis subspecies lactis at a concentration of 5×109 cfu per day. Patients took their preparation for 30 days and recorded data on weekly defecations (primary outcome), along with feces consistency, ease of expulsion, sensation emptying, anal itching/burning/pain with defecation, and abdominal bloating (secondary outcomes).
A total of 300 patients were enrolled in the study; 50% were female. No difference was noted in baseline symptoms among the three groups. No change from baseline was noted in BMs per week within the placebo group during the 30 days (5.6 vs 5.8, respectively). However, both probiotic preparations resulted in increased bowel frequency by day 30 (5.3 vs 7.3 BMs per week for probiotic A [P<.001] and 5.8 vs 6.9 BMs per week for probiotic B [P<.001]).
When comparing each probiotic with the placebo at days 15 and 30, a statistically significant increase in bowel frequency was found with each probiotic preparation. Furthermore, all secondary outcomes improved during the 30 days with the probiotic preparations but not the placebo. There was a statistically significant improvement in these variables when either probiotic was compared with placebo. No adverse events were reported.
Strengths and limitations
This randomized, double-blind, placebo-controlled trial showed improvement in bowel frequency, based on a bowel diary, with two different probiotic preparations when compared with placebo. The study population did not have to meet Rome III criteria for constipation, and baseline frequency of BMs was high. Patients did report subjective improvement in their defecatory symptoms with both probiotic preparations, but use of validated questionnaires would have strengthened this finding.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Patients with mild constipation and defecatory complaints may benefit from the addition of a probiotic preparation. However, more thorough studies need to be performed to characterize the true extent of probiotics’ benefits.
--------------
BOWEL PREP BEFORE LAPAROSCOPIC GYNECOLOGIC SURGERY
Siedhoff MT, Clark LH, Hobbs KA, Findley AD, Moulder JK, Garrett JM. Mechanic bowel preparation before laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol. 2014;123(3):562–567.
Over the past decade, extrapolation of data from colorectal surgery literature, showing no benefit from preoperative mechanical bowel preparation,6 has led to less frequent use of mechanical bowel preparations for open benign gynecologic surgery. Nevertheless, there has been slower adoption of this practice with laparoscopic and vaginal surgery. In a recent study, Siedhoff and colleagues explored surgeons’ assessments of surgical field exposure in patients who did and did not complete preoperative mechanical bowel preparation.
Details of the study
This was a single-masked, randomized, controlled trial involving women undergoing laparoscopic hysterectomy for benign indications. Patients were randomly assigned to either a sodium phosphate enema the night before surgery and, if their stool was not clear, another enema on the morning of surgery versus no preparation. All patients had clear liquids the day prior to surgery, then fasted beginning at midnight. The surgeon was blinded to the randomization.
The primary outcome was a questionnaire completed by the surgeon that assessed surgical field exposure. Secondarily, patients completed a questionnaire addressing symptoms (cramps, hunger, bloating, embarrassment, insomnia, weakness, dizziness, thirst, nausea, and incontinence).
Baseline characteristics of the 160 randomized patients did not differ between the two groups. Analysis was on an intent-to-treat basis, but only two patients did not complete the bowel preparation. Overall, the study population had a mean age of 41 and body mass index of 33.5 kg/m2. No differences were noted in surgical characteristics between the two groups, including complication rate. The mean surgery time was 139 minutes with a mean estimated blood loss of 61 mL and a mean uterine weight of 385 g.
The surgeon’s assessment of the surgical field did not differ between the two groups. This finding also held true when subgroup analysis was performed for obesity, endometriosis, irritable bowel syndrome or inflammatory bowel disease, and chronic constipation. Interestingly, the odds of the surgeon guessing whether a patient had had a preparation were 50:50. The only difference in patient symptoms was an increase in insomnia in the no-preparation group.
Minor drawback
This well-performed trial demonstrated no significant value for mechanical bowel preparation before benign laparoscopic hysterectomy in a young population. How these results might extrapolate to an older population who may have a higher rate of prior pelvic surgery or diverticular disease is uncertain.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women undergoing laparoscopic hysterectomy for a benign indication may forego a mechanical bowel preparation as such preparation did not improve the surgical field.
--------------
BOWEL PREP BEFORE VAGINAL SURGERY
Ballard AC, Parker-Autry CY, Markland AD, Varner RE, Huisingh C, Richter HE. Bowel preparation before vaginal prolapse surgery: a randomized controlled trial. Obstet Gynecol. 2014;123(2 pt 1):232–238.
In this single-masked, randomized controlled trial in women undergoing reconstructive vaginal prolapse surgery, Ballard and colleagues randomly assigned patients to either a clear liquid diet with two saline enemas the day before surgery or a regular diet the day before surgery.
Details of the study
All 150 patients were instructed to fast beginning at midnight the night before surgery, and the surgeon was blinded to randomization. The study’s primary outcome was the surgeon’s perception of the operative field assessed by a questionnaire. The secondary outcome was the patient’s satisfaction with their preoperative regimen as reported on validated questionnaires.
An intent-to-treat analysis was performed (mean age, 60 years); 84% of patients assigned to bowel preparation completed more than 50% of the enemas. Baseline characteristics and surgical procedures were similar between groups. Approximately 33% of patients underwent hysterectomy concomitantly with the prolapse repair. Operative time, estimated blood loss, and bowel injury were similar between the two groups.
No difference between groups was noted in the surgeons’ assessment of the surgical field—which was rated as excellent or good in 85% of patients who underwent the bowel preparation compared with 90% in the no-preparation group (P = .3). Additionally, no difference was noted in the presence of rectal stool or gas by inspection and palpation. Patient satisfaction was significantly lower among those who underwent bowel preparation compared with patients who did not. Patients undergoing bowel preparation were more likely to have abdominal fullness or bloating (P = .004), abdominal cramps or pain (P<.001), anal irritation (P<.001), and hunger pains (P<.001).
Prep group saw no benefit and decreased satisfaction
This well-performed clinical trial showed that the use of mechanical bowel preparation did not significantly improve surgeons’ intraoperative acceptability of the operative field during vaginal prolapse surgery. However, approximately 25% of patients underwent sacrospinous suspensions; therefore, intraperitoneal access was not necessary in these patients. The study results demonstrated decreased patient satisfaction and more distressing bowel symptoms in patients who underwent a mechanical bowel preparation with an enema.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Use of a mechanical bowel preparation is not necessary to improve the surgical field in vaginal prolapse surgery. Not having patients undergo a bowel preparation will improve patients’ assessment of their preparation for surgery.
--------------
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–759.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491.
- Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144(1):211–217.
- American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol. 2005;100(suppl 1):S1–S22.
- Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936–971.
- Guenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011;(9):CD001544.
Constipation is estimated to affect up to 27% of the general population and is more common in women, with a 2:1 female-to-male ratio.1 Because gynecologists are frequently the main care provider for many women, understanding the diagnosis and treatment options for constipation is important. Additionally, gynecologists must manage bowel function during the perioperative period.
The diagnosis of constipation is based on the Rome III criteria.2 Besides frequency of bowel movements (BMs), these criteria include evacuation symptoms and the presence of hard stools (TABLE 1). These symptoms can result from delay in colonic transit or outlet dysfunction. Constipation may be secondary to medical illness, such as central or peripheral neurologic disease, diabetes mellitus, hypothyroidism, or medications. Evaluation begins with a careful history and vaginal and perianal/anal examination.3 Initially, a trial of fiber supplementation with or without over-the-counter (OTC) laxatives may be tried (TABLE 2). If patients have an inadequate response to this therapy, further evaluation may be pursued (ALGORITHM).
------
| TABLE1 Rome III criteria for functional constipation in adults* |
1. Must include ≥2 of the following signs
2. Loose stools are rarely present without the use of laxatives 3. Insufficient criteria for irritable bowel syndrome |
| *At least 3 months, with symptoms beginning ≥6 months before diagnosis. |
--------
| TABLE 2 Common treatments for constipation |
Bulk-forming laxatives absorb water, increasing fecal mass
Surfactant agents lower the surface tension of stool, allowing water to enter the stool
Osmotic laxatives contain poorly/nonabsorbed substances, leading to intestinal water secretion
Stimulant laxatives increase colonic transit and alter electrolyte transport across the colonic mucosa
|
In this article, we review the results of randomized trials comparing the efficacy of OTC medical treatments for constipation, including daily, low-dose polyethylene glycol (PEG) and probiotics. Additionally, we review key trials evaluating perioperative bowel management prior to laparoscopic gynecologic and vaginal surgery.
LONG-TERM PEG USAGE SAFE AND EFFECTIVE?
Corazziari E, Badiali D, Bazzocchi G, et al. Long-term efficacy, safety, and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut. 2000;46(4):522–526.
In this multicenter, randomized, double-blind, placebo-controlled, parallel trial, investigators evaluated the safety, efficacy, and tolerability of a daily low-dose PEG-based osmotic diuretic.
Details of the study
Seventy-eight patients (80% of them female) aged 18 to 75 years with chronic constipation, defined by Rome III diagnostic criteria, underwent a 4-week “run-in” period, with a standardized daily diet of fiber 15 g, water 1500 mL, and twice-daily PMF-100 (PEG/osmotic solution). Patients were randomized if they responded to the regimen, with response defined as having at least two BMs per week and no defecatory disturbance or at least three BMs per week with or without defecatory disturbance. Eight patients were not randomized, one due to nonresponsiveness. Study patients completed 20 weeks of either twice-daily PMF-100 or placebo. Patients, at their own discretion, decreased the frequency of the study drug based on the frequency of their BMs. Use of another laxative was not allowed unless a BM had not occurred over a 5-day period.
The combined primary outcome was at least three BMs per week, no defecatory disturbances, and no additional laxative use. Secondary outcomes (frequency of BMs and defecatory disturbances) were assessed using a bowel diary.
No differences were noted in baseline measurements between the two groups. Of the PMF-100 group, 70% completed the study, compared with 30% of the placebo group (P<.01). Nonresponse to treatment was the reason for dropout in 7% and 46% of patients, respectively (P<.005). Other causes of withdrawal did not differ between the groups.
At the end of the 20 weeks, 77% of patients in the PMF-100 group reported remission, compared with 20% in the placebo group (P<.001). During the study, the PMF-100 group reported more BMs per week (7.4 vs 4.3; P<.001). Furthermore, the treatment group was less likely to report straining at defecation, hard/pellet stools, and need for use of additional laxatives. Adverse events (nausea, anal pain/itching, hematochezia, epigastric pain, and fecal incontinence) were similar between groups. There were no differences in laboratory values.
Study strengths
This was a well-designed trial showing the safety, efficacy, and tolerability of a daily low-dose PEG-based osmotic diuretic. The population was mainly women with functional chronic constipation, similar to a gynecologic population. The results of this trial are consistent with what has been shown for other trials various PEG preparations.4,5
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women who fail initial fiber therapy may respond to daily low-dose PEG on a continuous basis. Resolution of constipation and defecatory symptoms is likely and should be seen within 1 month. Therapy can be continued safely for at least 6 months.
--------------
NEW AND TRENDY OTC TREATMENT OPTION
Del Piano M, Carmagnola S, Anderloni A, et al. The use of probiotics in healthy volunteers with evacuation disorders and hard stools: a double-blind, randomized, placebo-controlled study. J Clin Gastroenterol. 2010;44(suppl 1):S30–S34.
Factors such as age, unhealthy diet, and use of prescription drugs alter the intestinal bacterial flora. As patients strive for a more holistic approach to their health, interest is growing in the benefit of probiotics for treating chronic constipation. To explore the value of such probiotics, Del Piano and colleagues conducted a three-armed, randomized, double-blind placebo-controlled trial of two different probiotic preparations and a placebo among patients aged 24 to 71 years with evacuation disorders and constipation.
Details of the study
One probiotic preparation (A) was composed of Lactobacillus plantarum and Bifidobacterium breve at a concentration of 2.5×109 cfu per day; the other (B) was composed of Bifidobacterium animalis subspecies lactis at a concentration of 5×109 cfu per day. Patients took their preparation for 30 days and recorded data on weekly defecations (primary outcome), along with feces consistency, ease of expulsion, sensation emptying, anal itching/burning/pain with defecation, and abdominal bloating (secondary outcomes).
A total of 300 patients were enrolled in the study; 50% were female. No difference was noted in baseline symptoms among the three groups. No change from baseline was noted in BMs per week within the placebo group during the 30 days (5.6 vs 5.8, respectively). However, both probiotic preparations resulted in increased bowel frequency by day 30 (5.3 vs 7.3 BMs per week for probiotic A [P<.001] and 5.8 vs 6.9 BMs per week for probiotic B [P<.001]).
When comparing each probiotic with the placebo at days 15 and 30, a statistically significant increase in bowel frequency was found with each probiotic preparation. Furthermore, all secondary outcomes improved during the 30 days with the probiotic preparations but not the placebo. There was a statistically significant improvement in these variables when either probiotic was compared with placebo. No adverse events were reported.
Strengths and limitations
This randomized, double-blind, placebo-controlled trial showed improvement in bowel frequency, based on a bowel diary, with two different probiotic preparations when compared with placebo. The study population did not have to meet Rome III criteria for constipation, and baseline frequency of BMs was high. Patients did report subjective improvement in their defecatory symptoms with both probiotic preparations, but use of validated questionnaires would have strengthened this finding.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Patients with mild constipation and defecatory complaints may benefit from the addition of a probiotic preparation. However, more thorough studies need to be performed to characterize the true extent of probiotics’ benefits.
--------------
BOWEL PREP BEFORE LAPAROSCOPIC GYNECOLOGIC SURGERY
Siedhoff MT, Clark LH, Hobbs KA, Findley AD, Moulder JK, Garrett JM. Mechanic bowel preparation before laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol. 2014;123(3):562–567.
Over the past decade, extrapolation of data from colorectal surgery literature, showing no benefit from preoperative mechanical bowel preparation,6 has led to less frequent use of mechanical bowel preparations for open benign gynecologic surgery. Nevertheless, there has been slower adoption of this practice with laparoscopic and vaginal surgery. In a recent study, Siedhoff and colleagues explored surgeons’ assessments of surgical field exposure in patients who did and did not complete preoperative mechanical bowel preparation.
Details of the study
This was a single-masked, randomized, controlled trial involving women undergoing laparoscopic hysterectomy for benign indications. Patients were randomly assigned to either a sodium phosphate enema the night before surgery and, if their stool was not clear, another enema on the morning of surgery versus no preparation. All patients had clear liquids the day prior to surgery, then fasted beginning at midnight. The surgeon was blinded to the randomization.
The primary outcome was a questionnaire completed by the surgeon that assessed surgical field exposure. Secondarily, patients completed a questionnaire addressing symptoms (cramps, hunger, bloating, embarrassment, insomnia, weakness, dizziness, thirst, nausea, and incontinence).
Baseline characteristics of the 160 randomized patients did not differ between the two groups. Analysis was on an intent-to-treat basis, but only two patients did not complete the bowel preparation. Overall, the study population had a mean age of 41 and body mass index of 33.5 kg/m2. No differences were noted in surgical characteristics between the two groups, including complication rate. The mean surgery time was 139 minutes with a mean estimated blood loss of 61 mL and a mean uterine weight of 385 g.
The surgeon’s assessment of the surgical field did not differ between the two groups. This finding also held true when subgroup analysis was performed for obesity, endometriosis, irritable bowel syndrome or inflammatory bowel disease, and chronic constipation. Interestingly, the odds of the surgeon guessing whether a patient had had a preparation were 50:50. The only difference in patient symptoms was an increase in insomnia in the no-preparation group.
Minor drawback
This well-performed trial demonstrated no significant value for mechanical bowel preparation before benign laparoscopic hysterectomy in a young population. How these results might extrapolate to an older population who may have a higher rate of prior pelvic surgery or diverticular disease is uncertain.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women undergoing laparoscopic hysterectomy for a benign indication may forego a mechanical bowel preparation as such preparation did not improve the surgical field.
--------------
BOWEL PREP BEFORE VAGINAL SURGERY
Ballard AC, Parker-Autry CY, Markland AD, Varner RE, Huisingh C, Richter HE. Bowel preparation before vaginal prolapse surgery: a randomized controlled trial. Obstet Gynecol. 2014;123(2 pt 1):232–238.
In this single-masked, randomized controlled trial in women undergoing reconstructive vaginal prolapse surgery, Ballard and colleagues randomly assigned patients to either a clear liquid diet with two saline enemas the day before surgery or a regular diet the day before surgery.
Details of the study
All 150 patients were instructed to fast beginning at midnight the night before surgery, and the surgeon was blinded to randomization. The study’s primary outcome was the surgeon’s perception of the operative field assessed by a questionnaire. The secondary outcome was the patient’s satisfaction with their preoperative regimen as reported on validated questionnaires.
An intent-to-treat analysis was performed (mean age, 60 years); 84% of patients assigned to bowel preparation completed more than 50% of the enemas. Baseline characteristics and surgical procedures were similar between groups. Approximately 33% of patients underwent hysterectomy concomitantly with the prolapse repair. Operative time, estimated blood loss, and bowel injury were similar between the two groups.
No difference between groups was noted in the surgeons’ assessment of the surgical field—which was rated as excellent or good in 85% of patients who underwent the bowel preparation compared with 90% in the no-preparation group (P = .3). Additionally, no difference was noted in the presence of rectal stool or gas by inspection and palpation. Patient satisfaction was significantly lower among those who underwent bowel preparation compared with patients who did not. Patients undergoing bowel preparation were more likely to have abdominal fullness or bloating (P = .004), abdominal cramps or pain (P<.001), anal irritation (P<.001), and hunger pains (P<.001).
Prep group saw no benefit and decreased satisfaction
This well-performed clinical trial showed that the use of mechanical bowel preparation did not significantly improve surgeons’ intraoperative acceptability of the operative field during vaginal prolapse surgery. However, approximately 25% of patients underwent sacrospinous suspensions; therefore, intraperitoneal access was not necessary in these patients. The study results demonstrated decreased patient satisfaction and more distressing bowel symptoms in patients who underwent a mechanical bowel preparation with an enema.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Use of a mechanical bowel preparation is not necessary to improve the surgical field in vaginal prolapse surgery. Not having patients undergo a bowel preparation will improve patients’ assessment of their preparation for surgery.
--------------
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Constipation is estimated to affect up to 27% of the general population and is more common in women, with a 2:1 female-to-male ratio.1 Because gynecologists are frequently the main care provider for many women, understanding the diagnosis and treatment options for constipation is important. Additionally, gynecologists must manage bowel function during the perioperative period.
The diagnosis of constipation is based on the Rome III criteria.2 Besides frequency of bowel movements (BMs), these criteria include evacuation symptoms and the presence of hard stools (TABLE 1). These symptoms can result from delay in colonic transit or outlet dysfunction. Constipation may be secondary to medical illness, such as central or peripheral neurologic disease, diabetes mellitus, hypothyroidism, or medications. Evaluation begins with a careful history and vaginal and perianal/anal examination.3 Initially, a trial of fiber supplementation with or without over-the-counter (OTC) laxatives may be tried (TABLE 2). If patients have an inadequate response to this therapy, further evaluation may be pursued (ALGORITHM).
------
| TABLE1 Rome III criteria for functional constipation in adults* |
1. Must include ≥2 of the following signs
2. Loose stools are rarely present without the use of laxatives 3. Insufficient criteria for irritable bowel syndrome |
| *At least 3 months, with symptoms beginning ≥6 months before diagnosis. |
--------
| TABLE 2 Common treatments for constipation |
Bulk-forming laxatives absorb water, increasing fecal mass
Surfactant agents lower the surface tension of stool, allowing water to enter the stool
Osmotic laxatives contain poorly/nonabsorbed substances, leading to intestinal water secretion
Stimulant laxatives increase colonic transit and alter electrolyte transport across the colonic mucosa
|
In this article, we review the results of randomized trials comparing the efficacy of OTC medical treatments for constipation, including daily, low-dose polyethylene glycol (PEG) and probiotics. Additionally, we review key trials evaluating perioperative bowel management prior to laparoscopic gynecologic and vaginal surgery.
LONG-TERM PEG USAGE SAFE AND EFFECTIVE?
Corazziari E, Badiali D, Bazzocchi G, et al. Long-term efficacy, safety, and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut. 2000;46(4):522–526.
In this multicenter, randomized, double-blind, placebo-controlled, parallel trial, investigators evaluated the safety, efficacy, and tolerability of a daily low-dose PEG-based osmotic diuretic.
Details of the study
Seventy-eight patients (80% of them female) aged 18 to 75 years with chronic constipation, defined by Rome III diagnostic criteria, underwent a 4-week “run-in” period, with a standardized daily diet of fiber 15 g, water 1500 mL, and twice-daily PMF-100 (PEG/osmotic solution). Patients were randomized if they responded to the regimen, with response defined as having at least two BMs per week and no defecatory disturbance or at least three BMs per week with or without defecatory disturbance. Eight patients were not randomized, one due to nonresponsiveness. Study patients completed 20 weeks of either twice-daily PMF-100 or placebo. Patients, at their own discretion, decreased the frequency of the study drug based on the frequency of their BMs. Use of another laxative was not allowed unless a BM had not occurred over a 5-day period.
The combined primary outcome was at least three BMs per week, no defecatory disturbances, and no additional laxative use. Secondary outcomes (frequency of BMs and defecatory disturbances) were assessed using a bowel diary.
No differences were noted in baseline measurements between the two groups. Of the PMF-100 group, 70% completed the study, compared with 30% of the placebo group (P<.01). Nonresponse to treatment was the reason for dropout in 7% and 46% of patients, respectively (P<.005). Other causes of withdrawal did not differ between the groups.
At the end of the 20 weeks, 77% of patients in the PMF-100 group reported remission, compared with 20% in the placebo group (P<.001). During the study, the PMF-100 group reported more BMs per week (7.4 vs 4.3; P<.001). Furthermore, the treatment group was less likely to report straining at defecation, hard/pellet stools, and need for use of additional laxatives. Adverse events (nausea, anal pain/itching, hematochezia, epigastric pain, and fecal incontinence) were similar between groups. There were no differences in laboratory values.
Study strengths
This was a well-designed trial showing the safety, efficacy, and tolerability of a daily low-dose PEG-based osmotic diuretic. The population was mainly women with functional chronic constipation, similar to a gynecologic population. The results of this trial are consistent with what has been shown for other trials various PEG preparations.4,5
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women who fail initial fiber therapy may respond to daily low-dose PEG on a continuous basis. Resolution of constipation and defecatory symptoms is likely and should be seen within 1 month. Therapy can be continued safely for at least 6 months.
--------------
NEW AND TRENDY OTC TREATMENT OPTION
Del Piano M, Carmagnola S, Anderloni A, et al. The use of probiotics in healthy volunteers with evacuation disorders and hard stools: a double-blind, randomized, placebo-controlled study. J Clin Gastroenterol. 2010;44(suppl 1):S30–S34.
Factors such as age, unhealthy diet, and use of prescription drugs alter the intestinal bacterial flora. As patients strive for a more holistic approach to their health, interest is growing in the benefit of probiotics for treating chronic constipation. To explore the value of such probiotics, Del Piano and colleagues conducted a three-armed, randomized, double-blind placebo-controlled trial of two different probiotic preparations and a placebo among patients aged 24 to 71 years with evacuation disorders and constipation.
Details of the study
One probiotic preparation (A) was composed of Lactobacillus plantarum and Bifidobacterium breve at a concentration of 2.5×109 cfu per day; the other (B) was composed of Bifidobacterium animalis subspecies lactis at a concentration of 5×109 cfu per day. Patients took their preparation for 30 days and recorded data on weekly defecations (primary outcome), along with feces consistency, ease of expulsion, sensation emptying, anal itching/burning/pain with defecation, and abdominal bloating (secondary outcomes).
A total of 300 patients were enrolled in the study; 50% were female. No difference was noted in baseline symptoms among the three groups. No change from baseline was noted in BMs per week within the placebo group during the 30 days (5.6 vs 5.8, respectively). However, both probiotic preparations resulted in increased bowel frequency by day 30 (5.3 vs 7.3 BMs per week for probiotic A [P<.001] and 5.8 vs 6.9 BMs per week for probiotic B [P<.001]).
When comparing each probiotic with the placebo at days 15 and 30, a statistically significant increase in bowel frequency was found with each probiotic preparation. Furthermore, all secondary outcomes improved during the 30 days with the probiotic preparations but not the placebo. There was a statistically significant improvement in these variables when either probiotic was compared with placebo. No adverse events were reported.
Strengths and limitations
This randomized, double-blind, placebo-controlled trial showed improvement in bowel frequency, based on a bowel diary, with two different probiotic preparations when compared with placebo. The study population did not have to meet Rome III criteria for constipation, and baseline frequency of BMs was high. Patients did report subjective improvement in their defecatory symptoms with both probiotic preparations, but use of validated questionnaires would have strengthened this finding.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Patients with mild constipation and defecatory complaints may benefit from the addition of a probiotic preparation. However, more thorough studies need to be performed to characterize the true extent of probiotics’ benefits.
--------------
BOWEL PREP BEFORE LAPAROSCOPIC GYNECOLOGIC SURGERY
Siedhoff MT, Clark LH, Hobbs KA, Findley AD, Moulder JK, Garrett JM. Mechanic bowel preparation before laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol. 2014;123(3):562–567.
Over the past decade, extrapolation of data from colorectal surgery literature, showing no benefit from preoperative mechanical bowel preparation,6 has led to less frequent use of mechanical bowel preparations for open benign gynecologic surgery. Nevertheless, there has been slower adoption of this practice with laparoscopic and vaginal surgery. In a recent study, Siedhoff and colleagues explored surgeons’ assessments of surgical field exposure in patients who did and did not complete preoperative mechanical bowel preparation.
Details of the study
This was a single-masked, randomized, controlled trial involving women undergoing laparoscopic hysterectomy for benign indications. Patients were randomly assigned to either a sodium phosphate enema the night before surgery and, if their stool was not clear, another enema on the morning of surgery versus no preparation. All patients had clear liquids the day prior to surgery, then fasted beginning at midnight. The surgeon was blinded to the randomization.
The primary outcome was a questionnaire completed by the surgeon that assessed surgical field exposure. Secondarily, patients completed a questionnaire addressing symptoms (cramps, hunger, bloating, embarrassment, insomnia, weakness, dizziness, thirst, nausea, and incontinence).
Baseline characteristics of the 160 randomized patients did not differ between the two groups. Analysis was on an intent-to-treat basis, but only two patients did not complete the bowel preparation. Overall, the study population had a mean age of 41 and body mass index of 33.5 kg/m2. No differences were noted in surgical characteristics between the two groups, including complication rate. The mean surgery time was 139 minutes with a mean estimated blood loss of 61 mL and a mean uterine weight of 385 g.
The surgeon’s assessment of the surgical field did not differ between the two groups. This finding also held true when subgroup analysis was performed for obesity, endometriosis, irritable bowel syndrome or inflammatory bowel disease, and chronic constipation. Interestingly, the odds of the surgeon guessing whether a patient had had a preparation were 50:50. The only difference in patient symptoms was an increase in insomnia in the no-preparation group.
Minor drawback
This well-performed trial demonstrated no significant value for mechanical bowel preparation before benign laparoscopic hysterectomy in a young population. How these results might extrapolate to an older population who may have a higher rate of prior pelvic surgery or diverticular disease is uncertain.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women undergoing laparoscopic hysterectomy for a benign indication may forego a mechanical bowel preparation as such preparation did not improve the surgical field.
--------------
BOWEL PREP BEFORE VAGINAL SURGERY
Ballard AC, Parker-Autry CY, Markland AD, Varner RE, Huisingh C, Richter HE. Bowel preparation before vaginal prolapse surgery: a randomized controlled trial. Obstet Gynecol. 2014;123(2 pt 1):232–238.
In this single-masked, randomized controlled trial in women undergoing reconstructive vaginal prolapse surgery, Ballard and colleagues randomly assigned patients to either a clear liquid diet with two saline enemas the day before surgery or a regular diet the day before surgery.
Details of the study
All 150 patients were instructed to fast beginning at midnight the night before surgery, and the surgeon was blinded to randomization. The study’s primary outcome was the surgeon’s perception of the operative field assessed by a questionnaire. The secondary outcome was the patient’s satisfaction with their preoperative regimen as reported on validated questionnaires.
An intent-to-treat analysis was performed (mean age, 60 years); 84% of patients assigned to bowel preparation completed more than 50% of the enemas. Baseline characteristics and surgical procedures were similar between groups. Approximately 33% of patients underwent hysterectomy concomitantly with the prolapse repair. Operative time, estimated blood loss, and bowel injury were similar between the two groups.
No difference between groups was noted in the surgeons’ assessment of the surgical field—which was rated as excellent or good in 85% of patients who underwent the bowel preparation compared with 90% in the no-preparation group (P = .3). Additionally, no difference was noted in the presence of rectal stool or gas by inspection and palpation. Patient satisfaction was significantly lower among those who underwent bowel preparation compared with patients who did not. Patients undergoing bowel preparation were more likely to have abdominal fullness or bloating (P = .004), abdominal cramps or pain (P<.001), anal irritation (P<.001), and hunger pains (P<.001).
Prep group saw no benefit and decreased satisfaction
This well-performed clinical trial showed that the use of mechanical bowel preparation did not significantly improve surgeons’ intraoperative acceptability of the operative field during vaginal prolapse surgery. However, approximately 25% of patients underwent sacrospinous suspensions; therefore, intraperitoneal access was not necessary in these patients. The study results demonstrated decreased patient satisfaction and more distressing bowel symptoms in patients who underwent a mechanical bowel preparation with an enema.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Use of a mechanical bowel preparation is not necessary to improve the surgical field in vaginal prolapse surgery. Not having patients undergo a bowel preparation will improve patients’ assessment of their preparation for surgery.
--------------
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–759.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491.
- Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144(1):211–217.
- American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol. 2005;100(suppl 1):S1–S22.
- Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936–971.
- Guenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011;(9):CD001544.
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–759.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491.
- Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144(1):211–217.
- American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol. 2005;100(suppl 1):S1–S22.
- Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936–971.
- Guenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011;(9):CD001544.
IN THIS ARTICLE
- Long-term PEG usage safe and effective?
- New and trendy OTC treatment option
- Bowel prep before laparoscopic gynecologic surgery
- Bowel prep before vaginal surgery
Update on pelvic floor dysfunction: Focus on urinary incontinence
Urinary incontinence (UI) affects almost half of all women in the United States.1,2 Estimates suggest that the prevalence of UI gradually rises during young adult life, comes to a broad plateau in middle age, and then steadily increases from that plateau after age 65. Therefore, over the next 40 years, as the elderly population expands in size, the number of women affected by UI will significantly grow.3
For patients with UI, a multitude of therapeutic options are available. Which option is the best for your patient? In this article, we aim to answer that question by interpreting the results of four randomized trials, each of which directly compare two available treatment options. The first study examines patients with stress urinary incontinence (SUI), comparing the patients’ subjective improvement in urinary leakage and bladder function at 12 months after randomization to treatment with physiotherapy or midurethral sling surgery.
The three other trials examine patients with overactive bladder (OAB) and urgency urinary incontinence (UUI). Each trial directly compares the use of anticholinergic medications to an alternate treatment modality. Currently, anticholinergic medications and behavioral therapy are the recommended first-line therapies for OAB. Unfortunately, anticholinergic medications have poor patient compliance and significant systemic side effects.4 Caution should be used when considering anticholinergic medications in patients with impaired gastric emptying or a history of urinary retention. They also should be used with caution in elderly patients who are extremely frail. Additionally, clearance from an ophthalmologist must be obtained prior to starting anticholinergic medication in patients with narrow-angle glaucoma.5 Due to poor adherence and potential side effects, there is a growing effort to discover alternative treatment modalities that are safe and effective. Therefore, we chose to examine trials comparing: mirabegron versus tolterodine, percutaneous tibial nerve stimulation versus tolterodine, and onabotulinumtoxinA versus anticholingeric medications.
UI defined
Before discussing treatment options, we want to clarify the main types of UI (FIGURE). UI is defined as the complaint of involuntary loss of urine. UI can be subdivided into SUI, OAB/UUI, or mixed urinary incontinence.6 While there are other less common genitourinary etiologies that can lead to UI, nongenitourinary etiologies are prevalent and can aggravate existing SUI or OAB (TABLE).
SUI is the complaint of involuntary loss of urine on effort or physical exertion (such as during sporting activities) or on sneezing or coughing. Often, SUI can be diagnosed by patient report alone and surgery can be considered in symptomatic patients who demonstrate cough leakage on physical examination and normal postvoid residual volumes.
UUI is the involuntary loss of urine associated with urgency; it often occurs in the setting of OAB, which is defined as the syndrome of urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of urinary tract infection or other obvious pathology (such as neurologic dysfunction, infection, or urologic neoplasm). OAB-dry is present when patients do not have leakage with urgency, but are bothered by urgency, frequency, and/or nocturia. OAB-wet occurs when a patient has urgencyincontinence.
The presence of both SUI and OAB/UUI is known as mixed urinary incontinence. Stress and urgency urinary symptoms often present together. In fact, 10% to 30% of women with stress symptoms are found to have bladder overactivity on subsequent evaluation.2,7 Therefore, it is important to take a good history and consider urodynamic evaluation to confirm the diagnosis of SUI prior to surgery in women with mixed stress and urge symptoms, a history of a previous surgery for incontinence, or when there is a poor correlation of physical examination findings to reported symptoms.
Is surgery a first-line option for patients with SUI?
Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. NEJM. 2013;369(12):1124−1133.
Physiotherapy, including pelvic floor muscle training (“Kegel exercises”), is utilized as a first-line treatment option for women with SUI that carries minimal risk for the patient. Midurethral sling surgery is often recommended if an initial trial of conservative treatment fails.7 Up to 50% of women treated with pelvic floor physiotherapy will ultimately undergo surgery to treat their SUI.8
Related article: Does urodynamic testing before surgery for stress incontinence improve outcomes? G. Willy Davila, MD (Examining the Evidence, December 2012)
Details of the study
This was a randomized, multicenter trial of women aged 35 to 80 years with moderate to severe SUI. After excluding women with previous incontinence surgery and stage 2 or higher pelvic organ prolapse, 460 participants were randomly assigned to undergo either a midurethral sling surgery or physiotherapy (pelvic floor muscle training). The primary outcome was subjective improvement in urinary leakage and bladder function at 12 months, as measured by the Patient Global Impression of Improvement (PGI-I), a 7-point Likert scale ranging from “very much worse” to “very much better.”
In an intention-to-treat analysis, subjective improvement at 12 months was significantly higher in women randomized to midurethral sling surgery than in women randomized to physiotherapy (91% vs 64%, respectively).
Ten percent of patients had adverse events (AEs); all were related to surgery. The most common AEs were hematoma, vaginal epithelial perforation, and bladder perforation.
Notably, women had the option to cross over to the other treatment modality if they desired. In the physiotherapy group, 49% of women elected to cross over to surgery, while 11% of those who underwent midurethral sling surgery elected to cross over to physiotherapy during the 12-month follow-up period. When analyzing results by treatment received, the investigators found that the proportion of women who reported improvement was significantly lower among women who underwent physiotherapy only (32%), versus sling only (94%), or sling after physiotherapy (91%).
This randomized trial was well-designed and included a variety of treatment centers (university and general hospitals) with interventions performed by experienced surgeons (all of whom had performed at least 20 sling surgeries) and physiotherapists educated specifically in pelvic floor physiotherapy. The study population was limited to patients with moderate to severe SUI as defined by the Sandvik severity index.9 Therefore, these results may not be applicable to patients with milder symptoms, for whom physiotherapy has been recommended as first-line therapy with consideration of surgery if physiotherapy fails to sufficiently improve symptoms.7
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women with moderate to severe SUI without significant prolapse or a history of prior incontinence surgery have significantly better outcomes at 12 months after undergoing midurethral sling surgery versus physiotherapy. Physiotherapy carries little to no risk of adverse effects. Women with moderate to severe SUI should be counseled regarding the risks and benefits of both physiotherapy and midurethral sling surgery as initial treatment options.
Because stress and urgency urinary symptoms often present together, it is important to consider urodynamic evaluation to confirm SUI prior to surgery in women with:
• mixed stress and urge symptoms
• a history of a previous surgery for incontinence, or
• poor correlation of physical examination findings to reported symptoms.
Safety and tolerability of mirabegron versus tolterodine for OAB
Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296−305.
In the bladder, beta3-receptors located within the detrusor smooth muscle facilitate urine storage by relaxing the detrusor, enabling the bladder to fill.10 The activation of beta3-receptors is thought to increase the bladder’s ability to store urine, with the goal of decreasing urgency, frequency, nocturia, and urgency incontinence. An alternative to anticholinergic medications, mirabegron is a beta3-agonist approved by the US Food and Drug Administration (FDA) in 2012 for clinical use in the treatment of OAB.
Details of the study
Chapple and colleagues aimed to assess the 12-month efficacy and safety of mirabegron in a randomized, double-blind active controlled trial. The primary outcome was incidence and severity of treatment-emergent adverse effects (TEAEs); the secondary outcome was the change in OAB symptoms from baseline to up to 12 months. Patients experiencing OAB symptoms for more than 3 months were eligible and were subsequently enrolled if they averaged 8 or more voids per day and 3 or more episodes of urgency with or without incontinence on a 3-day bladder diary. A total of 2,444 patients were randomly assigned in a 1:1:1 fashion to mirabegron 50 mg daily, mirabegron 100 mg daily, or tolterodine extended release (ER) 4 mg daily.
There was a similar incidence (60% to 63%) of TEAEs across all three groups. The most common TEAEs were hypertension (defined as average systolic blood pressure [BP] >140 mm Hg or average diastolic BP >90 mm Hg at two consecutive visits), UTI, headache, nasopharyngitis, and constipation. The adjusted mean changes in BP from baseline to final visit were less than 1 mm Hg for both systolic and diastolic BP for patients taking both doses of mirabegron, as well as for patients taking tolterodine. The incidence of dry mouth was higher in the tolterodine group than the mirabegron groups. Mirabegron 50 mg daily and 100 mg daily improved incontinence symptoms within 1 month of starting therapy; the degree of improvement was similar to that seen in the patients taking tolterodine ER 4 mg daily.
Related article: New overactive bladder treatment approved by the FDA (August 2012)
Some caveats
This study was well-designed to assess the safety and tolerability of mirabegron versus tolterodine. The doses utilized in the study were at or above the FDA-approved dosage of 25 mg to 50 mg daily for OAB treatment. Although investigators found mirabegron to be a safe alternative to anticholinergic medication, the study was not designed or powered to examine the efficacy of mirabegron versus tolterodine. No formal comparison of efficacy was made between mirabegron or tolterodine, or between the 50-mg and 100-mg doses of mirabegron. Moreover, 81% of participants had been treated with mirabegron in earlier Phase 3 studies, so most were not treatment naïve, limiting the applicability of results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Mirabegron should be considered as a potential treatment option for patients who demonstrate poor tolerance of or response to anticholinergic medications; however, caution should be used in patients with severe uncontrolled high BP, end-stage kidney disease, or severe liver impairment.
Consider percutaneous tibial nerve stimulation over tolterodine for OAB in select patients
Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. J Urol. 2009;182(3):1055−1061.
Neuromodulation utilizes electrical stimulation to improve bladder function and decrease OAB symptoms. First developed in the early 1980s by McGuire and colleagues, percutaneous tibial nerve stimulation (PTNS) was approved by the FDA in 2000 as Urgent PC and provides an outpatient, nonimplantable neuromodulation alternative to medication therapy for patients with OAB.11,12 By directly stimulating the posterior tibial nerve, PTNS works via the S3 sacral nerve plexus to alter the micturition reflex and improve bladder function.
Details of the study
Patients were eligible for the study if they demonstrated 8 or more voids per day on a 3-day bladder diary (whether or not they had a history of previous anticholinergic drug use). A total of 100 ambulatory adults with OAB symptoms were enrolled and randomly assigned to PTNS 30-minutes per week or tolterodine ER 4 mg daily.
At 12 weeks, both groups demonstrated a significant improvement in OAB measures as well as validated symptom severity and quality-of-life questionnaire scores. Subjective assessment of improvement in OAB symptoms was significantly greater in the PTNS group than in the tolterodine group (79.5% vs 54.8%, respectively; P = .01). However, mean reduction of voids for 24 hours was not significantly different between the two groups.
Both treatments were well tolerated, with only 15% to 16% of patients in both groups reporting mild to moderate side effects. The tolterodine group did have a significantly higher risk of dry mouth; however, the risk of constipation was not significantly different between the groups.
Study limitations
The authors performed an important multicenter, nonblinded, randomized, controlled trial, which was one of the first trials to directly compare two OAB therapies. The generalizability of the findings were limited, as the cohort included mostly patients with dry OAB who had no objective measures on UUI episodes. In addition, this trial had a limited observation period of only 12 weeks. Information regarding the effect of treatment after cessation of weekly PTNS therapy was not examined. Therefore, we are not able to determine whether repeat sessions provide adequate maintenance in the long term.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
PTNS 30 minutes daily is as effective as tolterodine ER 4 mg daily for 12 weeks in reducing OAB symptoms. PTNS is a safe alternative that should be considered in patients with OAB who poorly tolerate or have contraindications to medication therapy.
OnabotulinumtoxinA is an effective therapy for OAB
Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. NEJM. 2012;367(19):1803−1813.
The newest therapy for OAB is onabotulinumtoxinA, or Botox, which was FDA approved this year for the treatment of OAB in adults who cannot use or do not tolerate anticholinergic medications. Recommended doses are 100 U onabotulinumtoxinA in patients with idiopathic refractory OAB and 200 U onabotulinumtoxinA for patients with neurogenic OAB.
OnabotulinumtoxinA is a neurotoxin that blocks synaptic transmission at the neuromuscular junction to cause muscle paralysis and atrophy.13 Injecting onabotulinumtoxinA into the detrusor smooth muscle should relax the bladder and decrease sensations of urgency and frequency to achieve a longer duration of time for bladder filling and reduce the risk of urgency incontinence.
Effects of onabotulinumtoxinA appear to wear off over time, and patients may require repeat injections. Side effects of onabotulinumtoxinA therapy include an increased risk of UTI and the potential for urinary retention requiring intermittent self-catheterization.
Related article: Update on Pelvic Floor Dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
Details of the study
The Anticholinergic Versus Botulinum Toxin Comparison (ABC) study was a multicenter, randomized, double-blind, double-placebo–controlled trial conducted in women without known neurologic disease with moderate to severe UUI (defined as >5 UUI episodes on a 3-day bladder diary). Women were randomly assigned to a single intradetrusor injection of 100 U onabotulinumtoxinA plus oral placebo or to a single intradetrusor injection of saline plus solifenacin 5 mg daily (with the option of dose escalation and then switching to trospium XR if no improvement was seen).
Of the 241 women included in the final analysis, approximately 70% in each group reported adequate control of symptoms at 6 months. Adequate control was defined as a response of “agree strongly” or “agree” to the statement: “This treatment has given me adequate control of my urinary leakage.” Women in the onabotulinumtoxinA group were significantly more likely than women in the anticholinergic medication group to report complete resolution of UUI at 6 months (27% vs 13%, P = .003). However, the mean reduction in episodes of UUI per day and the improvements in quality-of-life questionnaire scores were found to be similar. Interestingly, worse baseline UUI was associated with greater reduction in episodes of UUI for both therapies.
This was a rigorous and well-executed double-blind, double-placebo−controlled randomized trial. By utilizing broad inclusion criteria and enrolling patients both with and without previous exposure to anticholinergic medications, the generalizability of study findings are greatly improved. Because this study did not examine the effect or efficacy of repeat injections, these findings have limited applicability to patients undergoing multiple onabotulinumtoxinA injections.
When considering use in your patient population, keep the possible side effects in mind.There were important differences in the side effects experienced with each therapy. Specifically, while the anticholinergic group had a higher frequency of dry mouth (46% anticholinergic vs 31% onabotulinumtoxinA, P = .02), the onabotulinumtoxinA group demonstrated higher rates of incomplete bladder emptying requiring catheterization (peak of 5% at 2 months) and greater risk of UTI (33% onabotulinumtoxinA vs 13% anticholinergic, P <.001).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This study showed that, among women with UUI, anticholinergic medication and onabotulinumtoxinA are equally effective in reducing UUI episodes and improving quality of life. It is important to consider the side effect profile, determine the patient’s preferences, and weigh the risks and benefits of each therapy when deciding what is the best treatment for your individual patient.
We want to hear from you! Tell us what you think.
- Anger JT, Saigal CS, Litwin MS. The prevalence of urinary incontinence among community dwelling adult women: Results from the National Health and Nutrition Examination Survey. J Urol. 2006;175(2):601–604.
- Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: Results from the National Health and Nutrition Examination Survey. J Urol. 2008;179(2):656–661.
- Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstetr Gynecol. 2009;114(6):1278–1283.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. Americal Urological Association. http://www.auanet.org/common/pdf/education/clinical-guidance/Overactive-Bladder.pdf. Published 2012. Revised June 11, 2013. Accessed October 21, 2013.
- Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health. 2005;8(4):495–505.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- ACOG Practice Bulletin No. 63: Urinary incontinence in women. American College of Obstetricians and Gynecologists. Obstetr Gynecol. 2005;105(6):1533–1545.
- Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstetr Gynecol. 2005;105(5 Pt 1):999–1005.
- Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–499.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129(1):78–79.
- Schiavo G, Santucci A, Dasgupta BR, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99–103.
Urinary incontinence (UI) affects almost half of all women in the United States.1,2 Estimates suggest that the prevalence of UI gradually rises during young adult life, comes to a broad plateau in middle age, and then steadily increases from that plateau after age 65. Therefore, over the next 40 years, as the elderly population expands in size, the number of women affected by UI will significantly grow.3
For patients with UI, a multitude of therapeutic options are available. Which option is the best for your patient? In this article, we aim to answer that question by interpreting the results of four randomized trials, each of which directly compare two available treatment options. The first study examines patients with stress urinary incontinence (SUI), comparing the patients’ subjective improvement in urinary leakage and bladder function at 12 months after randomization to treatment with physiotherapy or midurethral sling surgery.
The three other trials examine patients with overactive bladder (OAB) and urgency urinary incontinence (UUI). Each trial directly compares the use of anticholinergic medications to an alternate treatment modality. Currently, anticholinergic medications and behavioral therapy are the recommended first-line therapies for OAB. Unfortunately, anticholinergic medications have poor patient compliance and significant systemic side effects.4 Caution should be used when considering anticholinergic medications in patients with impaired gastric emptying or a history of urinary retention. They also should be used with caution in elderly patients who are extremely frail. Additionally, clearance from an ophthalmologist must be obtained prior to starting anticholinergic medication in patients with narrow-angle glaucoma.5 Due to poor adherence and potential side effects, there is a growing effort to discover alternative treatment modalities that are safe and effective. Therefore, we chose to examine trials comparing: mirabegron versus tolterodine, percutaneous tibial nerve stimulation versus tolterodine, and onabotulinumtoxinA versus anticholingeric medications.
UI defined
Before discussing treatment options, we want to clarify the main types of UI (FIGURE). UI is defined as the complaint of involuntary loss of urine. UI can be subdivided into SUI, OAB/UUI, or mixed urinary incontinence.6 While there are other less common genitourinary etiologies that can lead to UI, nongenitourinary etiologies are prevalent and can aggravate existing SUI or OAB (TABLE).
SUI is the complaint of involuntary loss of urine on effort or physical exertion (such as during sporting activities) or on sneezing or coughing. Often, SUI can be diagnosed by patient report alone and surgery can be considered in symptomatic patients who demonstrate cough leakage on physical examination and normal postvoid residual volumes.
UUI is the involuntary loss of urine associated with urgency; it often occurs in the setting of OAB, which is defined as the syndrome of urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of urinary tract infection or other obvious pathology (such as neurologic dysfunction, infection, or urologic neoplasm). OAB-dry is present when patients do not have leakage with urgency, but are bothered by urgency, frequency, and/or nocturia. OAB-wet occurs when a patient has urgencyincontinence.
The presence of both SUI and OAB/UUI is known as mixed urinary incontinence. Stress and urgency urinary symptoms often present together. In fact, 10% to 30% of women with stress symptoms are found to have bladder overactivity on subsequent evaluation.2,7 Therefore, it is important to take a good history and consider urodynamic evaluation to confirm the diagnosis of SUI prior to surgery in women with mixed stress and urge symptoms, a history of a previous surgery for incontinence, or when there is a poor correlation of physical examination findings to reported symptoms.
Is surgery a first-line option for patients with SUI?
Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. NEJM. 2013;369(12):1124−1133.
Physiotherapy, including pelvic floor muscle training (“Kegel exercises”), is utilized as a first-line treatment option for women with SUI that carries minimal risk for the patient. Midurethral sling surgery is often recommended if an initial trial of conservative treatment fails.7 Up to 50% of women treated with pelvic floor physiotherapy will ultimately undergo surgery to treat their SUI.8
Related article: Does urodynamic testing before surgery for stress incontinence improve outcomes? G. Willy Davila, MD (Examining the Evidence, December 2012)
Details of the study
This was a randomized, multicenter trial of women aged 35 to 80 years with moderate to severe SUI. After excluding women with previous incontinence surgery and stage 2 or higher pelvic organ prolapse, 460 participants were randomly assigned to undergo either a midurethral sling surgery or physiotherapy (pelvic floor muscle training). The primary outcome was subjective improvement in urinary leakage and bladder function at 12 months, as measured by the Patient Global Impression of Improvement (PGI-I), a 7-point Likert scale ranging from “very much worse” to “very much better.”
In an intention-to-treat analysis, subjective improvement at 12 months was significantly higher in women randomized to midurethral sling surgery than in women randomized to physiotherapy (91% vs 64%, respectively).
Ten percent of patients had adverse events (AEs); all were related to surgery. The most common AEs were hematoma, vaginal epithelial perforation, and bladder perforation.
Notably, women had the option to cross over to the other treatment modality if they desired. In the physiotherapy group, 49% of women elected to cross over to surgery, while 11% of those who underwent midurethral sling surgery elected to cross over to physiotherapy during the 12-month follow-up period. When analyzing results by treatment received, the investigators found that the proportion of women who reported improvement was significantly lower among women who underwent physiotherapy only (32%), versus sling only (94%), or sling after physiotherapy (91%).
This randomized trial was well-designed and included a variety of treatment centers (university and general hospitals) with interventions performed by experienced surgeons (all of whom had performed at least 20 sling surgeries) and physiotherapists educated specifically in pelvic floor physiotherapy. The study population was limited to patients with moderate to severe SUI as defined by the Sandvik severity index.9 Therefore, these results may not be applicable to patients with milder symptoms, for whom physiotherapy has been recommended as first-line therapy with consideration of surgery if physiotherapy fails to sufficiently improve symptoms.7
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women with moderate to severe SUI without significant prolapse or a history of prior incontinence surgery have significantly better outcomes at 12 months after undergoing midurethral sling surgery versus physiotherapy. Physiotherapy carries little to no risk of adverse effects. Women with moderate to severe SUI should be counseled regarding the risks and benefits of both physiotherapy and midurethral sling surgery as initial treatment options.
Because stress and urgency urinary symptoms often present together, it is important to consider urodynamic evaluation to confirm SUI prior to surgery in women with:
• mixed stress and urge symptoms
• a history of a previous surgery for incontinence, or
• poor correlation of physical examination findings to reported symptoms.
Safety and tolerability of mirabegron versus tolterodine for OAB
Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296−305.
In the bladder, beta3-receptors located within the detrusor smooth muscle facilitate urine storage by relaxing the detrusor, enabling the bladder to fill.10 The activation of beta3-receptors is thought to increase the bladder’s ability to store urine, with the goal of decreasing urgency, frequency, nocturia, and urgency incontinence. An alternative to anticholinergic medications, mirabegron is a beta3-agonist approved by the US Food and Drug Administration (FDA) in 2012 for clinical use in the treatment of OAB.
Details of the study
Chapple and colleagues aimed to assess the 12-month efficacy and safety of mirabegron in a randomized, double-blind active controlled trial. The primary outcome was incidence and severity of treatment-emergent adverse effects (TEAEs); the secondary outcome was the change in OAB symptoms from baseline to up to 12 months. Patients experiencing OAB symptoms for more than 3 months were eligible and were subsequently enrolled if they averaged 8 or more voids per day and 3 or more episodes of urgency with or without incontinence on a 3-day bladder diary. A total of 2,444 patients were randomly assigned in a 1:1:1 fashion to mirabegron 50 mg daily, mirabegron 100 mg daily, or tolterodine extended release (ER) 4 mg daily.
There was a similar incidence (60% to 63%) of TEAEs across all three groups. The most common TEAEs were hypertension (defined as average systolic blood pressure [BP] >140 mm Hg or average diastolic BP >90 mm Hg at two consecutive visits), UTI, headache, nasopharyngitis, and constipation. The adjusted mean changes in BP from baseline to final visit were less than 1 mm Hg for both systolic and diastolic BP for patients taking both doses of mirabegron, as well as for patients taking tolterodine. The incidence of dry mouth was higher in the tolterodine group than the mirabegron groups. Mirabegron 50 mg daily and 100 mg daily improved incontinence symptoms within 1 month of starting therapy; the degree of improvement was similar to that seen in the patients taking tolterodine ER 4 mg daily.
Related article: New overactive bladder treatment approved by the FDA (August 2012)
Some caveats
This study was well-designed to assess the safety and tolerability of mirabegron versus tolterodine. The doses utilized in the study were at or above the FDA-approved dosage of 25 mg to 50 mg daily for OAB treatment. Although investigators found mirabegron to be a safe alternative to anticholinergic medication, the study was not designed or powered to examine the efficacy of mirabegron versus tolterodine. No formal comparison of efficacy was made between mirabegron or tolterodine, or between the 50-mg and 100-mg doses of mirabegron. Moreover, 81% of participants had been treated with mirabegron in earlier Phase 3 studies, so most were not treatment naïve, limiting the applicability of results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Mirabegron should be considered as a potential treatment option for patients who demonstrate poor tolerance of or response to anticholinergic medications; however, caution should be used in patients with severe uncontrolled high BP, end-stage kidney disease, or severe liver impairment.
Consider percutaneous tibial nerve stimulation over tolterodine for OAB in select patients
Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. J Urol. 2009;182(3):1055−1061.
Neuromodulation utilizes electrical stimulation to improve bladder function and decrease OAB symptoms. First developed in the early 1980s by McGuire and colleagues, percutaneous tibial nerve stimulation (PTNS) was approved by the FDA in 2000 as Urgent PC and provides an outpatient, nonimplantable neuromodulation alternative to medication therapy for patients with OAB.11,12 By directly stimulating the posterior tibial nerve, PTNS works via the S3 sacral nerve plexus to alter the micturition reflex and improve bladder function.
Details of the study
Patients were eligible for the study if they demonstrated 8 or more voids per day on a 3-day bladder diary (whether or not they had a history of previous anticholinergic drug use). A total of 100 ambulatory adults with OAB symptoms were enrolled and randomly assigned to PTNS 30-minutes per week or tolterodine ER 4 mg daily.
At 12 weeks, both groups demonstrated a significant improvement in OAB measures as well as validated symptom severity and quality-of-life questionnaire scores. Subjective assessment of improvement in OAB symptoms was significantly greater in the PTNS group than in the tolterodine group (79.5% vs 54.8%, respectively; P = .01). However, mean reduction of voids for 24 hours was not significantly different between the two groups.
Both treatments were well tolerated, with only 15% to 16% of patients in both groups reporting mild to moderate side effects. The tolterodine group did have a significantly higher risk of dry mouth; however, the risk of constipation was not significantly different between the groups.
Study limitations
The authors performed an important multicenter, nonblinded, randomized, controlled trial, which was one of the first trials to directly compare two OAB therapies. The generalizability of the findings were limited, as the cohort included mostly patients with dry OAB who had no objective measures on UUI episodes. In addition, this trial had a limited observation period of only 12 weeks. Information regarding the effect of treatment after cessation of weekly PTNS therapy was not examined. Therefore, we are not able to determine whether repeat sessions provide adequate maintenance in the long term.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
PTNS 30 minutes daily is as effective as tolterodine ER 4 mg daily for 12 weeks in reducing OAB symptoms. PTNS is a safe alternative that should be considered in patients with OAB who poorly tolerate or have contraindications to medication therapy.
OnabotulinumtoxinA is an effective therapy for OAB
Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. NEJM. 2012;367(19):1803−1813.
The newest therapy for OAB is onabotulinumtoxinA, or Botox, which was FDA approved this year for the treatment of OAB in adults who cannot use or do not tolerate anticholinergic medications. Recommended doses are 100 U onabotulinumtoxinA in patients with idiopathic refractory OAB and 200 U onabotulinumtoxinA for patients with neurogenic OAB.
OnabotulinumtoxinA is a neurotoxin that blocks synaptic transmission at the neuromuscular junction to cause muscle paralysis and atrophy.13 Injecting onabotulinumtoxinA into the detrusor smooth muscle should relax the bladder and decrease sensations of urgency and frequency to achieve a longer duration of time for bladder filling and reduce the risk of urgency incontinence.
Effects of onabotulinumtoxinA appear to wear off over time, and patients may require repeat injections. Side effects of onabotulinumtoxinA therapy include an increased risk of UTI and the potential for urinary retention requiring intermittent self-catheterization.
Related article: Update on Pelvic Floor Dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
Details of the study
The Anticholinergic Versus Botulinum Toxin Comparison (ABC) study was a multicenter, randomized, double-blind, double-placebo–controlled trial conducted in women without known neurologic disease with moderate to severe UUI (defined as >5 UUI episodes on a 3-day bladder diary). Women were randomly assigned to a single intradetrusor injection of 100 U onabotulinumtoxinA plus oral placebo or to a single intradetrusor injection of saline plus solifenacin 5 mg daily (with the option of dose escalation and then switching to trospium XR if no improvement was seen).
Of the 241 women included in the final analysis, approximately 70% in each group reported adequate control of symptoms at 6 months. Adequate control was defined as a response of “agree strongly” or “agree” to the statement: “This treatment has given me adequate control of my urinary leakage.” Women in the onabotulinumtoxinA group were significantly more likely than women in the anticholinergic medication group to report complete resolution of UUI at 6 months (27% vs 13%, P = .003). However, the mean reduction in episodes of UUI per day and the improvements in quality-of-life questionnaire scores were found to be similar. Interestingly, worse baseline UUI was associated with greater reduction in episodes of UUI for both therapies.
This was a rigorous and well-executed double-blind, double-placebo−controlled randomized trial. By utilizing broad inclusion criteria and enrolling patients both with and without previous exposure to anticholinergic medications, the generalizability of study findings are greatly improved. Because this study did not examine the effect or efficacy of repeat injections, these findings have limited applicability to patients undergoing multiple onabotulinumtoxinA injections.
When considering use in your patient population, keep the possible side effects in mind.There were important differences in the side effects experienced with each therapy. Specifically, while the anticholinergic group had a higher frequency of dry mouth (46% anticholinergic vs 31% onabotulinumtoxinA, P = .02), the onabotulinumtoxinA group demonstrated higher rates of incomplete bladder emptying requiring catheterization (peak of 5% at 2 months) and greater risk of UTI (33% onabotulinumtoxinA vs 13% anticholinergic, P <.001).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This study showed that, among women with UUI, anticholinergic medication and onabotulinumtoxinA are equally effective in reducing UUI episodes and improving quality of life. It is important to consider the side effect profile, determine the patient’s preferences, and weigh the risks and benefits of each therapy when deciding what is the best treatment for your individual patient.
We want to hear from you! Tell us what you think.
Urinary incontinence (UI) affects almost half of all women in the United States.1,2 Estimates suggest that the prevalence of UI gradually rises during young adult life, comes to a broad plateau in middle age, and then steadily increases from that plateau after age 65. Therefore, over the next 40 years, as the elderly population expands in size, the number of women affected by UI will significantly grow.3
For patients with UI, a multitude of therapeutic options are available. Which option is the best for your patient? In this article, we aim to answer that question by interpreting the results of four randomized trials, each of which directly compare two available treatment options. The first study examines patients with stress urinary incontinence (SUI), comparing the patients’ subjective improvement in urinary leakage and bladder function at 12 months after randomization to treatment with physiotherapy or midurethral sling surgery.
The three other trials examine patients with overactive bladder (OAB) and urgency urinary incontinence (UUI). Each trial directly compares the use of anticholinergic medications to an alternate treatment modality. Currently, anticholinergic medications and behavioral therapy are the recommended first-line therapies for OAB. Unfortunately, anticholinergic medications have poor patient compliance and significant systemic side effects.4 Caution should be used when considering anticholinergic medications in patients with impaired gastric emptying or a history of urinary retention. They also should be used with caution in elderly patients who are extremely frail. Additionally, clearance from an ophthalmologist must be obtained prior to starting anticholinergic medication in patients with narrow-angle glaucoma.5 Due to poor adherence and potential side effects, there is a growing effort to discover alternative treatment modalities that are safe and effective. Therefore, we chose to examine trials comparing: mirabegron versus tolterodine, percutaneous tibial nerve stimulation versus tolterodine, and onabotulinumtoxinA versus anticholingeric medications.
UI defined
Before discussing treatment options, we want to clarify the main types of UI (FIGURE). UI is defined as the complaint of involuntary loss of urine. UI can be subdivided into SUI, OAB/UUI, or mixed urinary incontinence.6 While there are other less common genitourinary etiologies that can lead to UI, nongenitourinary etiologies are prevalent and can aggravate existing SUI or OAB (TABLE).
SUI is the complaint of involuntary loss of urine on effort or physical exertion (such as during sporting activities) or on sneezing or coughing. Often, SUI can be diagnosed by patient report alone and surgery can be considered in symptomatic patients who demonstrate cough leakage on physical examination and normal postvoid residual volumes.
UUI is the involuntary loss of urine associated with urgency; it often occurs in the setting of OAB, which is defined as the syndrome of urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of urinary tract infection or other obvious pathology (such as neurologic dysfunction, infection, or urologic neoplasm). OAB-dry is present when patients do not have leakage with urgency, but are bothered by urgency, frequency, and/or nocturia. OAB-wet occurs when a patient has urgencyincontinence.
The presence of both SUI and OAB/UUI is known as mixed urinary incontinence. Stress and urgency urinary symptoms often present together. In fact, 10% to 30% of women with stress symptoms are found to have bladder overactivity on subsequent evaluation.2,7 Therefore, it is important to take a good history and consider urodynamic evaluation to confirm the diagnosis of SUI prior to surgery in women with mixed stress and urge symptoms, a history of a previous surgery for incontinence, or when there is a poor correlation of physical examination findings to reported symptoms.
Is surgery a first-line option for patients with SUI?
Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. NEJM. 2013;369(12):1124−1133.
Physiotherapy, including pelvic floor muscle training (“Kegel exercises”), is utilized as a first-line treatment option for women with SUI that carries minimal risk for the patient. Midurethral sling surgery is often recommended if an initial trial of conservative treatment fails.7 Up to 50% of women treated with pelvic floor physiotherapy will ultimately undergo surgery to treat their SUI.8
Related article: Does urodynamic testing before surgery for stress incontinence improve outcomes? G. Willy Davila, MD (Examining the Evidence, December 2012)
Details of the study
This was a randomized, multicenter trial of women aged 35 to 80 years with moderate to severe SUI. After excluding women with previous incontinence surgery and stage 2 or higher pelvic organ prolapse, 460 participants were randomly assigned to undergo either a midurethral sling surgery or physiotherapy (pelvic floor muscle training). The primary outcome was subjective improvement in urinary leakage and bladder function at 12 months, as measured by the Patient Global Impression of Improvement (PGI-I), a 7-point Likert scale ranging from “very much worse” to “very much better.”
In an intention-to-treat analysis, subjective improvement at 12 months was significantly higher in women randomized to midurethral sling surgery than in women randomized to physiotherapy (91% vs 64%, respectively).
Ten percent of patients had adverse events (AEs); all were related to surgery. The most common AEs were hematoma, vaginal epithelial perforation, and bladder perforation.
Notably, women had the option to cross over to the other treatment modality if they desired. In the physiotherapy group, 49% of women elected to cross over to surgery, while 11% of those who underwent midurethral sling surgery elected to cross over to physiotherapy during the 12-month follow-up period. When analyzing results by treatment received, the investigators found that the proportion of women who reported improvement was significantly lower among women who underwent physiotherapy only (32%), versus sling only (94%), or sling after physiotherapy (91%).
This randomized trial was well-designed and included a variety of treatment centers (university and general hospitals) with interventions performed by experienced surgeons (all of whom had performed at least 20 sling surgeries) and physiotherapists educated specifically in pelvic floor physiotherapy. The study population was limited to patients with moderate to severe SUI as defined by the Sandvik severity index.9 Therefore, these results may not be applicable to patients with milder symptoms, for whom physiotherapy has been recommended as first-line therapy with consideration of surgery if physiotherapy fails to sufficiently improve symptoms.7
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women with moderate to severe SUI without significant prolapse or a history of prior incontinence surgery have significantly better outcomes at 12 months after undergoing midurethral sling surgery versus physiotherapy. Physiotherapy carries little to no risk of adverse effects. Women with moderate to severe SUI should be counseled regarding the risks and benefits of both physiotherapy and midurethral sling surgery as initial treatment options.
Because stress and urgency urinary symptoms often present together, it is important to consider urodynamic evaluation to confirm SUI prior to surgery in women with:
• mixed stress and urge symptoms
• a history of a previous surgery for incontinence, or
• poor correlation of physical examination findings to reported symptoms.
Safety and tolerability of mirabegron versus tolterodine for OAB
Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296−305.
In the bladder, beta3-receptors located within the detrusor smooth muscle facilitate urine storage by relaxing the detrusor, enabling the bladder to fill.10 The activation of beta3-receptors is thought to increase the bladder’s ability to store urine, with the goal of decreasing urgency, frequency, nocturia, and urgency incontinence. An alternative to anticholinergic medications, mirabegron is a beta3-agonist approved by the US Food and Drug Administration (FDA) in 2012 for clinical use in the treatment of OAB.
Details of the study
Chapple and colleagues aimed to assess the 12-month efficacy and safety of mirabegron in a randomized, double-blind active controlled trial. The primary outcome was incidence and severity of treatment-emergent adverse effects (TEAEs); the secondary outcome was the change in OAB symptoms from baseline to up to 12 months. Patients experiencing OAB symptoms for more than 3 months were eligible and were subsequently enrolled if they averaged 8 or more voids per day and 3 or more episodes of urgency with or without incontinence on a 3-day bladder diary. A total of 2,444 patients were randomly assigned in a 1:1:1 fashion to mirabegron 50 mg daily, mirabegron 100 mg daily, or tolterodine extended release (ER) 4 mg daily.
There was a similar incidence (60% to 63%) of TEAEs across all three groups. The most common TEAEs were hypertension (defined as average systolic blood pressure [BP] >140 mm Hg or average diastolic BP >90 mm Hg at two consecutive visits), UTI, headache, nasopharyngitis, and constipation. The adjusted mean changes in BP from baseline to final visit were less than 1 mm Hg for both systolic and diastolic BP for patients taking both doses of mirabegron, as well as for patients taking tolterodine. The incidence of dry mouth was higher in the tolterodine group than the mirabegron groups. Mirabegron 50 mg daily and 100 mg daily improved incontinence symptoms within 1 month of starting therapy; the degree of improvement was similar to that seen in the patients taking tolterodine ER 4 mg daily.
Related article: New overactive bladder treatment approved by the FDA (August 2012)
Some caveats
This study was well-designed to assess the safety and tolerability of mirabegron versus tolterodine. The doses utilized in the study were at or above the FDA-approved dosage of 25 mg to 50 mg daily for OAB treatment. Although investigators found mirabegron to be a safe alternative to anticholinergic medication, the study was not designed or powered to examine the efficacy of mirabegron versus tolterodine. No formal comparison of efficacy was made between mirabegron or tolterodine, or between the 50-mg and 100-mg doses of mirabegron. Moreover, 81% of participants had been treated with mirabegron in earlier Phase 3 studies, so most were not treatment naïve, limiting the applicability of results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Mirabegron should be considered as a potential treatment option for patients who demonstrate poor tolerance of or response to anticholinergic medications; however, caution should be used in patients with severe uncontrolled high BP, end-stage kidney disease, or severe liver impairment.
Consider percutaneous tibial nerve stimulation over tolterodine for OAB in select patients
Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. J Urol. 2009;182(3):1055−1061.
Neuromodulation utilizes electrical stimulation to improve bladder function and decrease OAB symptoms. First developed in the early 1980s by McGuire and colleagues, percutaneous tibial nerve stimulation (PTNS) was approved by the FDA in 2000 as Urgent PC and provides an outpatient, nonimplantable neuromodulation alternative to medication therapy for patients with OAB.11,12 By directly stimulating the posterior tibial nerve, PTNS works via the S3 sacral nerve plexus to alter the micturition reflex and improve bladder function.
Details of the study
Patients were eligible for the study if they demonstrated 8 or more voids per day on a 3-day bladder diary (whether or not they had a history of previous anticholinergic drug use). A total of 100 ambulatory adults with OAB symptoms were enrolled and randomly assigned to PTNS 30-minutes per week or tolterodine ER 4 mg daily.
At 12 weeks, both groups demonstrated a significant improvement in OAB measures as well as validated symptom severity and quality-of-life questionnaire scores. Subjective assessment of improvement in OAB symptoms was significantly greater in the PTNS group than in the tolterodine group (79.5% vs 54.8%, respectively; P = .01). However, mean reduction of voids for 24 hours was not significantly different between the two groups.
Both treatments were well tolerated, with only 15% to 16% of patients in both groups reporting mild to moderate side effects. The tolterodine group did have a significantly higher risk of dry mouth; however, the risk of constipation was not significantly different between the groups.
Study limitations
The authors performed an important multicenter, nonblinded, randomized, controlled trial, which was one of the first trials to directly compare two OAB therapies. The generalizability of the findings were limited, as the cohort included mostly patients with dry OAB who had no objective measures on UUI episodes. In addition, this trial had a limited observation period of only 12 weeks. Information regarding the effect of treatment after cessation of weekly PTNS therapy was not examined. Therefore, we are not able to determine whether repeat sessions provide adequate maintenance in the long term.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
PTNS 30 minutes daily is as effective as tolterodine ER 4 mg daily for 12 weeks in reducing OAB symptoms. PTNS is a safe alternative that should be considered in patients with OAB who poorly tolerate or have contraindications to medication therapy.
OnabotulinumtoxinA is an effective therapy for OAB
Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. NEJM. 2012;367(19):1803−1813.
The newest therapy for OAB is onabotulinumtoxinA, or Botox, which was FDA approved this year for the treatment of OAB in adults who cannot use or do not tolerate anticholinergic medications. Recommended doses are 100 U onabotulinumtoxinA in patients with idiopathic refractory OAB and 200 U onabotulinumtoxinA for patients with neurogenic OAB.
OnabotulinumtoxinA is a neurotoxin that blocks synaptic transmission at the neuromuscular junction to cause muscle paralysis and atrophy.13 Injecting onabotulinumtoxinA into the detrusor smooth muscle should relax the bladder and decrease sensations of urgency and frequency to achieve a longer duration of time for bladder filling and reduce the risk of urgency incontinence.
Effects of onabotulinumtoxinA appear to wear off over time, and patients may require repeat injections. Side effects of onabotulinumtoxinA therapy include an increased risk of UTI and the potential for urinary retention requiring intermittent self-catheterization.
Related article: Update on Pelvic Floor Dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
Details of the study
The Anticholinergic Versus Botulinum Toxin Comparison (ABC) study was a multicenter, randomized, double-blind, double-placebo–controlled trial conducted in women without known neurologic disease with moderate to severe UUI (defined as >5 UUI episodes on a 3-day bladder diary). Women were randomly assigned to a single intradetrusor injection of 100 U onabotulinumtoxinA plus oral placebo or to a single intradetrusor injection of saline plus solifenacin 5 mg daily (with the option of dose escalation and then switching to trospium XR if no improvement was seen).
Of the 241 women included in the final analysis, approximately 70% in each group reported adequate control of symptoms at 6 months. Adequate control was defined as a response of “agree strongly” or “agree” to the statement: “This treatment has given me adequate control of my urinary leakage.” Women in the onabotulinumtoxinA group were significantly more likely than women in the anticholinergic medication group to report complete resolution of UUI at 6 months (27% vs 13%, P = .003). However, the mean reduction in episodes of UUI per day and the improvements in quality-of-life questionnaire scores were found to be similar. Interestingly, worse baseline UUI was associated with greater reduction in episodes of UUI for both therapies.
This was a rigorous and well-executed double-blind, double-placebo−controlled randomized trial. By utilizing broad inclusion criteria and enrolling patients both with and without previous exposure to anticholinergic medications, the generalizability of study findings are greatly improved. Because this study did not examine the effect or efficacy of repeat injections, these findings have limited applicability to patients undergoing multiple onabotulinumtoxinA injections.
When considering use in your patient population, keep the possible side effects in mind.There were important differences in the side effects experienced with each therapy. Specifically, while the anticholinergic group had a higher frequency of dry mouth (46% anticholinergic vs 31% onabotulinumtoxinA, P = .02), the onabotulinumtoxinA group demonstrated higher rates of incomplete bladder emptying requiring catheterization (peak of 5% at 2 months) and greater risk of UTI (33% onabotulinumtoxinA vs 13% anticholinergic, P <.001).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This study showed that, among women with UUI, anticholinergic medication and onabotulinumtoxinA are equally effective in reducing UUI episodes and improving quality of life. It is important to consider the side effect profile, determine the patient’s preferences, and weigh the risks and benefits of each therapy when deciding what is the best treatment for your individual patient.
We want to hear from you! Tell us what you think.
- Anger JT, Saigal CS, Litwin MS. The prevalence of urinary incontinence among community dwelling adult women: Results from the National Health and Nutrition Examination Survey. J Urol. 2006;175(2):601–604.
- Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: Results from the National Health and Nutrition Examination Survey. J Urol. 2008;179(2):656–661.
- Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstetr Gynecol. 2009;114(6):1278–1283.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. Americal Urological Association. http://www.auanet.org/common/pdf/education/clinical-guidance/Overactive-Bladder.pdf. Published 2012. Revised June 11, 2013. Accessed October 21, 2013.
- Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health. 2005;8(4):495–505.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- ACOG Practice Bulletin No. 63: Urinary incontinence in women. American College of Obstetricians and Gynecologists. Obstetr Gynecol. 2005;105(6):1533–1545.
- Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstetr Gynecol. 2005;105(5 Pt 1):999–1005.
- Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–499.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129(1):78–79.
- Schiavo G, Santucci A, Dasgupta BR, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99–103.
- Anger JT, Saigal CS, Litwin MS. The prevalence of urinary incontinence among community dwelling adult women: Results from the National Health and Nutrition Examination Survey. J Urol. 2006;175(2):601–604.
- Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: Results from the National Health and Nutrition Examination Survey. J Urol. 2008;179(2):656–661.
- Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstetr Gynecol. 2009;114(6):1278–1283.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. Americal Urological Association. http://www.auanet.org/common/pdf/education/clinical-guidance/Overactive-Bladder.pdf. Published 2012. Revised June 11, 2013. Accessed October 21, 2013.
- Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health. 2005;8(4):495–505.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- ACOG Practice Bulletin No. 63: Urinary incontinence in women. American College of Obstetricians and Gynecologists. Obstetr Gynecol. 2005;105(6):1533–1545.
- Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstetr Gynecol. 2005;105(5 Pt 1):999–1005.
- Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–499.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129(1):78–79.
- Schiavo G, Santucci A, Dasgupta BR, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99–103.
Novel strategy to prevent recurrent UTI in premenopausal women
Related article Update on Pelvic Floor Dysfunction (October 2012)
Related article Update on Pelvic Floor Dysfunction (October 2012)
Related article Update on Pelvic Floor Dysfunction (October 2012)
UPDATE ON PELVIC FLOOR DYSFUNCTION
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.
FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.
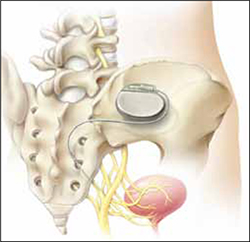
FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.

FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.

FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
Vulvar Pain Syndromes 3-Part Series
- Making the correct diagnosis
(September 2011) - A bounty of treatments-but not all of them are proven
(October 2011) - Provoked vestibulodynia
(Coming in November 2011)
Chronic pelvic pain: 11 critical questions about causes and care
Fred M. Howard, MD (August 2009)
Vague symptoms. Unexpected flares. Inconsistent manifestations. These characteristics can make diagnosis and treatment of chronic pelvic pain frustrating for both patient and physician. Most patients undergo myriad tests and studies to uncover the source of their pain—but a targeted pelvic exam may be all that is necessary to identify a prevalent but commonly overlooked cause of pelvic pain. Levator myalgia, myofascial pelvic pain syndrome, and pelvic floor spasm are all terms that describe a condition that may affect as many as 78% of women who are given a diagnosis of chronic pelvic pain.1 This syndrome may be represented by an array of symptoms, including pelvic pressure, dyspareunia, rectal discomfort, and irritative urinary symptoms such as spasms, frequency, and urgency. It is characterized by the presence of tight, band-like pelvic muscles that reproduce the patient’s pain when palpated.2
Diagnosis of this syndrome often surprises the patient. Although the concept of a muscle spasm is not foreign, the location is unexpected. Patients and physicians alike may forget that there is a large complex of muscles that completely lines the pelvic girdle. To complicate matters, the patient often associates the onset of her symptoms with an acute event such as a “bad” urinary tract infection or pelvic or vaginal surgery, which may divert attention from the musculature. Although a muscle spasm may be the cause of the patient’s pain, it’s important to realize that an underlying process may have triggered the original spasm. To provide effective treatment of pain, therefore, you must identify the fundamental cause, assuming that it is reversible, rather than focus exclusively on symptoms.
Although there are many therapeutic options for levator myalgia, an appraisal of the extensive literature on these medications is beyond the scope of this article. Rather, we will review alternative treatment modalities and summarize the results of five trials that explored physical therapy, trigger-point or chemodenervation injection, and neuromodulation (TABLE).
Weighing the nonpharmaceutical options for treatment
of myofascial pelvic pain
| Treatment | Pros | Cons |
|---|---|---|
| Physical therapy | Minimally invasive Moderate long-term success | Requires highly specialized therapist |
| Trigger-point injection | Minimally invasive Performed in clinic Immediate short-term success | Optimal injectable agent is unknown Botulinum toxin A lacks FDA approval for this indication Limited information on adverse events and long-term efficacy |
| Percutaneous tibial nerve stimulation | Minimally invasive Performed in clinic | Requires numerous office visits for treatment Lacks FDA approval for this indication Limited information on long-term efficacy |
| Sacral neuromodulation | Moderately invasive Permanent implant | Requires implantation in operating room Lacks FDA approval for this indication Limited information on long-term efficacy |
Pelvic myofascial therapy offers relief—but qualified therapists may be scarce
FitzGerald MP, Anderson RU, Potts J, et al; Urological Pelvic Pain Collaborative Research Network. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urology. 2009;182(2):570–580.
Physical therapy of the pelvic floor—otherwise known as pelvic myofascial therapy—requires a therapist who is highly trained and specialized in this technique. It is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers (FIGURE 1).
This pilot study by the Urological Pelvic Pain Collaborative Research Network evaluated the ability of patients to adhere to pelvic myofascial therapy, the response of their pain to therapy, and adverse events associated with manual therapy. It found that patients were willing to undergo the therapy, despite the invasive nature of the maneuvers, because it was significantly effective.
Details of the study
Patients (both men and women) were randomized to myofascial physical therapy or global therapeutic massage. Myofascial therapy consisted of internal or vaginal manipulation of the trigger-point muscle bundles and tissues of the pelvic floor. It also focused on muscles of the hip girdle and abdomen. The comparison group underwent traditional Western full-body massage. In both groups, treatment lasted 1 hour every week, and participants agreed to 10 full treatments.
Patients were eligible for the study if they experienced pelvic pain, urinary frequency, or bladder discomfort in the previous 6 months. In addition, an examiner must have been able to elicit tenderness upon palpation of the pelvic floor during examination. Patients were excluded if they showed signs of urinary tract infection or dysmenorrhea.
A total of 47 patients were randomized—24 to global massage and 23 to myofascial physical therapy. Overall, the myofascial group experienced a significantly higher rate of improvement in the global response at 12 weeks than did patients in the global-massage group (57% vs 21%; P=.03). Patients were willing to engage in myofascial pelvic therapy, and adverse events were minor.

FIGURE 1 Transvaginal myofascial therapy
Physical therapy of the pelvic floor is more invasive than other forms of rehabilitative therapy because of the need to perform transvaginal maneuvers.
Need for specialized training may limit number of therapists
The randomized controlled study design renders these findings fairly reliable. Therapists were unmasked and aware of the treatment arms but were trained to make the different therapy sessions appear as similar as possible.
Although investigators were enthusiastic about their initial findings, additional studies are needed to validate the results. Moreover, these findings may be difficult to generalize because women who volunteer to participate in such a study may differ from the general population.
Nevertheless, patients who suffer from chronic pelvic pain may take heart that there is a nonpharmaceutical alternative to manage their symptoms, although availability is likely limited in many areas. Given the nature of the physical therapy required for this particular location of myofascial pain, specialized training is necessary for therapists. Despite motivated patients and well-informed providers, it may be difficult to find specialized therapists within local vicinities. Referrals to centers where this type of therapy is offered may be necessary.
Pelvic myofascial therapy is an effective and acceptable intervention for the treatment of levator myalgia.
The ideal agent for trigger-point injections remains a mystery
Langford CF, Udvari Nagy S, Ghoniem G M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62.
Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923.
Trigger points are discrete, tender areas within a ridge of contracted muscle. These points may cause focal pain or referred pain upon irritation of the muscle.2 Trigger-point injection therapy aims to anesthetize or relax these points by infiltrating the muscle with medications.
These two studies evaluated the value of trigger-point injections in the treatment of pelvic myofascial pain; they found that the injections provide relief, although the mechanism of action and the ideal agent remain to be determined.
Langford et al: Details of the study
In this prospective study, 18 women who had pelvic pain of at least 6 months’ duration and confirmed trigger points on examination underwent transvaginal injection of a solution of bupivacaine, lidocaine, and triamcinolone. They were assessed by questionnaire at baseline and 3 months after injection. Assessment included a visual analog scale for pain severity. Investigators defined success as a decrease in pain of 50% or more and global-satisfaction and global-cure visual scores of 60% or higher.
Thirteen of the 18 women (72.2%) improved after their first injection, with six women reporting a complete absence of pain. Overall, women reported significant decreases in pain and increases in the rates of satisfaction and cure, meeting the definition of success at 3 months after the injection.
Among the theories proposed to explain the mechanism of action of trigger-point injections are:
- disruption of reflex arcs within skeletal muscle
- release of endorphins
- mechanical changes in abnormally contracted muscle fibers.
This last theory highlights one of the limitations of this study—lack of a placebo arm. Could it be possible that the injection of any fluid produces the same effect?
This study was not designed to investigate the causal relationship between the injection of a particular solution and pain relief, but it does highlight the need for studies to clarify the mechanism of action, including use of a placebo. It also prompts questions about the duration of effect after a single injection.
Goal of chemodenervation is blocking of muscle activity
Botulinum toxin type A (Botox) blocks the release of acetylcholine from presynaptic neurons. The release of acetylcholine stimulates muscle contractions; therefore, blockage of its release reduces muscle activity. This type of chemodenervation has found widespread use, and botulinum toxin A now has approval from the Food and Drug Administration (FDA) for treatment of chronic migraine, limb spasticity, cervical dystonia, strabismus, hyperhidrosis, and facial cosmesis.3 Although it is not approved for pelvic floor levator spasm, its success in treating other myotonic disorders suggests that its application may be relevant.
Abbott et al: Details of the study
Abbott and colleagues performed a double-blind, randomized, controlled trial to compare injection of botulinum toxin A with injection of saline. They measured changes in the pain scale, quality of life, and vaginal pressure.
Women were eligible for the study if they had subjectively reported pelvic pain of more than 2 years’ duration and objective evidence of trigger points (on examination) and elevated vaginal resting pressure (by vaginal manometry). Neither the clinical research staff nor the patient knew the contents of the injections, but all women received a total of four—two at sites in the puborectalis muscle and two in the pubococcygeus muscle.
After periodic assessment by questionnaire and examination through 6 months after injection, no differences were found in the pain score or resting vaginal pressure between the group of women who received botulinum toxin A and the group who received placebo. However, each group experienced a significant reduction in pain and vaginal pressure, compared with baseline. And both groups reported improved quality of life, compared with baseline. Neither group reported voiding dysfunction.
These two studies support the use of trigger-point injection into pelvic floor muscles to reduce pelvic myofascial pain. The findings of Abbott and colleagues, in particular, suggest that the substance that is injected may not be as important as the actual needling of the muscle. Larger studies and comparisons between placebo, botulinum toxin A, and anesthetic solutions are needed to elucidate the therapeutic benefit of these particular medications.
Neuromodulation shows promise as treatment for pelvic myofascial pain
van Balken MR, Vandoninck V, Messelink, BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43(2):158–163.
Zabihi N, Mourtzinos A, Maher MG, Raz S, Rodriguez LV. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):553–557.
Neuromodulation is the science of using electrical impulses to alter neuronal activities. The exact mechanisms of action are unclear, but the technology has been utilized to control symptoms of overactive bladder and urinary retention caused by poor relaxation of the urethral and pelvic floor muscles. While studying the effects of sacral nerve root neuromodulation on the bladder, investigators noted improvements in other symptoms, such as pelvic pain.
Neuromodulation of the sacral nerve roots may be achieved by direct conduction of electrical impulses from a lead implanted in the sacrum (sacral neuromodulation) or by the retrograde conduction of these impulses through the posterior tibial nerve (percutaneous tibial nerve stimulation, or PTNS) (FIGURE 2). The tibial nerve arises from sacral nerves L5 to S3 and is one of the larger branches of the sciatic nerve.

FIGURE 2 InterStim therapy
Stimulation of the sacral nerve has been used successfully to manage overactive bladder and urinary retention and may prove useful in the treatment of pelvic myofascial pain.
Van Balken et al: Details of the study
In this prospective observational study, 33 patients (both male and female) who had chronic pelvic pain by history and examination were treated with weekly, 30-minute outpatient sessions of PTNS for 12 weeks. Participants were asked to provide baseline pain scores and keep a diary of their pain. Quality-of-life questionnaires were also administered at baseline and at 12 weeks.
Investigators considered both subjective and objective success in their outcomes. If a patient elected to continue therapy, he or she was classified as a subjective success. Objective success required a decrease of at least 50% in the pain score. At the end of 12 weeks, although 33 patients (42%) wanted to continue therapy, only seven (21%) met the definition for objective success. Of those seven, six elected to continue therapy.
This study sheds light on a treatment modality that has not been studied adequately for the indication of pelvic pain but that may be promising in patients who have levator myalgia. Limitations of this study include the lack of a placebo arm, short-term outcome, and lack of localization of pain. Furthermore, although PTNS has FDA approval for treatment of urinary urgency, frequency, and urge incontinence, it is not approved for the treatment of pelvic pain. These preliminary findings demonstrate potential but, as with any new indication, long-term comparative studies are needed.
Zabihi et al: Details of the study
Patients in this retrospective study had a diagnosis of interstitial cystitis or chronic pelvic pain. Pelvic myofascial pain and trigger points were not required for eligibility. Thirty patients (21 women and nine men) had temporary placement of a lead containing four small electrodes along the S2 to S4 sacral nerve roots on both sides of the sacrum. They were then followed for a trial period of 2 to 4 weeks. To qualify for the final stage of the study, in which the leads were connected internally to a generator implanted in the buttocks, patients had to report improvement of at least 50% in their symptoms. If their improvement did not meet that threshold, the leads were removed.
Twenty-three patients (77%) met the criteria for permanent implantation. Of these patients, 42% reported improvement of more than 50% at 6 postoperative months. Quality-of-life scores also improved significantly.
Sacral neuromodulation is not FDA-approved for the treatment of chronic pelvic pain; further studies are needed before it can be recommended for this indication.
Neither of these studies required objective evidence of myofascial pain for inclusion. Therefore, although the benefits they demonstrated may be theorized to extend to the relief of myofascial pain, this fact cannot be corroborated.
We want to hear from you! Tell us what you think.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.
1. Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J. 2011;22(4):413-418.
2. Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653-660.
3. Allergan, Inc. Medication Guide: BOTOX. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM176360.pdf. Published October 2010. Accessed August 30, 2011.