User login
How Does Your PICCOMPARE? A Pilot Randomized Controlled Trial Comparing Various PICC Materials in Pediatrics
Peripherally inserted central catheters (PICCs) have evolved since their inception in the early 1970s and are used with increasing frequency for pediatric inpatients and outpatients.1-3 Emerging literature, including a meta-analysis of international observational studies,4 reports PICC failure (complications necessitating premature removal) occurs in up to 30% of PICCs, most commonly due to infection, thrombosis, occlusion, and fracture.4-7 Raffini et al.7 report the increasing incidence of pediatric PICC-related thrombosis increases morbidity and mortality8 and negatively impacts future vessel health and preservation.9
PICCs have progressed from relatively simple, silicone-based catheters with an external clamp to chemically engineered polyurethane with pressure-activated valves placed at the proximal or distal catheter hub with the intent to reduce occlusion.10 Further modernization of PICC material occurred with the incorporation of antithrombogenic (AT) material (Endexo®). These PICCs are designed to contain a nonstick polymer, which is designed to reduce the adherence of blood components (platelets and clotting factors) and inhibit thrombus formation (and hence prevent deep vein thrombosis andocclusion, as well as inhibit microbial biofilm attachment [and subsequent infection]).11
In addition to new materials, other aspects of this PICC design have been the addition of a pressure-activated safety valve (PASV®) built into the proximal hub. Pressure-activated valve technology promises to prevent catheter occlusion by reducing blood reflux into the PICC; the valve opens with pressure during infusion and aspiration and remains closed with normal venous pressure, circumventing the need for clinicians to manually clamp the PICC and reducing human error and the potential for thrombosis, occlusion, and fracture development.12 Hoffer et al.13 reported half as many occlusions of valved PICCs (3.3%) compared with nonvalved or clamped PICCs (7.1%); although not statistically significant (P = .10), perhaps due to the small sample, overall complications, including occlusion and infection, were significantly lessened with the valved PICC (35% vs 79%; P = .02). Comparatively, Pittiruti et al.14 conducted a trial of 2 types of valved PICCs with an open-ended, nonvalved PICC and found no reduction in PICC occlusion or catheter malfunction.
Today, PICC use is common for patients who require short-to-medium intravenous therapy. PICCs are increasingly recognized for their significant complications, including thrombosis and infection.15 Novel PICC technology, including the incorporation of AT material such as Endexo® and PASV®, may reduce complications; however, the clinical efficacy, cost-effectiveness, and acceptability of these innovations have not been tested through randomized trials in pediatric patients. In accordance with Medical Research Council guidelines16 for developing interventions, we pilot tested the feasibility of the BioFlo® PICC, including intervention acceptability, compliance, recruitment, and initial estimates of effect, in anticipation of a subsequent full-scale efficacy randomized controlled trial. Our secondary aim was to compare the effectiveness of the BioFlo® PICC with Endexo® and PASV® technology in reducing PICC complications and failure.
METHODS
Design
We undertook a pilot randomized controlled trial comparing the standard polyurethane PICC (with external clamp) with the BioFlo® PICC (with internal valve) in preventing catheter failure in pediatric patients. The study was prospectively registered with the Australian Clinical Trials Registry (ACTRN12615001290583), and the research protocol was published.17
Study Setting
The study commenced in March 2016 at the Lady Cilento Children’s Hospital in South Brisbane, Australia, a tertiary-level, specialist, pediatric teaching hospital in Queensland, Australia, providing full-spectrum health services to children and young people from birth to 18 years of age. Recruitment, including data collection, was completed in November 2016.
Sample
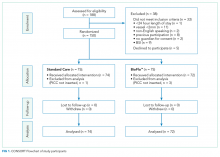
The target sample size was 110 participants, 50 participants per group plus 10% for potential attrition, as determined by standard pilot trial sample size recommendations.18 With ethics approval, the sample size was later increased to 150 participants in order to adequately pilot a microbiological substudy method (published separately).17 Participants were consecutively recruited if they met the inclusion criteria: PICC insertion, age <18 years, predicted hospital stay >24 hours, single-lumen PICC, and written informed consent by an English-speaking, legal parent or guardian. Patients were excluded if they had a current (<48 hours) blood stream infection (BSI), vessel size <2 mm, could not speak English without an interpreter, required a multilumen PICC, or were previously enrolled in the study.
Interventions
Participants were randomized to receive either of the following PICCs: (1) standard care: Cook™ polyurethane, turbo-ject, power-injectable PICC (Cook Medical, Bloomington, IN) or (2) comparison: BioFlo® polyurethane with Endexo® technology (AngioDynamics Inc, Queensbury, NY).
Outcomes
The primary outcome was feasibility of a full-efficacy trial established by composite analysis of the elements of eligibility (>70% of patients will be eligible), recruitment (>70% of patients will agree to enroll), retention and attrition (<15% of participants are lost to follow-up or withdraw from the study), protocol adherence (>80% of participants receive their allocated, randomly assigned study product), missing data (<10% of data are missed during data collection), parent and healthcare staff satisfaction, and PICC failure effect size estimates to allow sample size calculations.18,19 PICC failure was defined as the following complications associated with PICC removal: (1) catheter-associated BSI,8,20-22 (2) local site infection,22 (3) venous thrombosis,23 (4) occlusion,24,25 (5) PICC fracture, or (6) PICC dislodgement.25,26 Parents (or caregivers) and healthcare staff were asked to rate their level of confidence with the study product and ease of PICC removal by using a 0 to 100 numeric rating scale (NRS) of increasing confidence and/or ease. These data were collected at the time of PICC removal. Operators were also asked to rate their levels of satisfaction with the insertion equipment and ease of PICC insertion immediately upon completion of the insertion procedure (both 0-100 NRS of increasing satisfaction and/or ease). Secondary outcomes included individual PICC complications (eg, occlusion) occurring at any time point during the PICC dwell (including at removal), adverse events, pain, redness at the insertion site, and overall PICC dwell.
Study Procedures
The research nurse (ReN) screened operating theater lists for patients, obtained written informed consent, and initiated the randomization. Randomization was computer generated, and web based via Griffith University (https://www151.griffith.edu.au/random) to ensure allocation concealment until study entry. Patients were randomly assigned in a 1:1 ratio with computer-generated and randomly varied block sizes of 2 and 4. Data were collected by the ReN on the day of insertion, at day 1 postinsertion, then every 2 to 3 days thereafter so that PICCs were checked at least twice per week until study completion. Participants were included in the trial until 12 weeks post-PICC insertion, study withdrawal or PICC removal (whichever came first), with an additional 48 hours follow-up for infection outcomes. Patient review was face to face during the inpatient stay, with discharged patients’ follow-up occurring via outpatient clinics, hospital-in-the-home service, or telephone.
Data collection was via Research Electronic Data Capture (http://project-redcap.org/). The ReN collected data on primary and secondary outcomes by using the predefined criteria. Demographic and clinical data were collected to assess the success of randomization, describe the participant group, and display characteristics known to increase the risk of PICC complication and thrombosis. A blinded radiologist and infectious disease specialist reviewed and diagnosed thrombosis of deep veins and catheter-associated BSI outcomes, respectively.
PICC Procedures
Extensive prestudy education for 2 months prior to trial commencement was provided to all clinicians involved with the insertion and care of PICCs, including the study products. PICCs were inserted in an operating theater environment by a qualified consultant pediatric anesthetist, a senior anesthetic registrar or fellow in an approved anesthetic training program, or pediatric vascular access nurse practitioner. Ultrasound guidance was used to assess a patient’s vasculature and puncture the vessel. The operator chose the PICC size on the basis of clinical judgment of vessel size and patient needs and then inserted the allocated PICC.27 Preferred PICC tip location was the cavoatrial junction. All PICC tip positions were confirmed with a chest x-ray before use.
Postinsertion, PICCs were managed by local interdisciplinary clinicians in accordance with local practice guidelines.27-31 PICC care and management includes the use of 2% chlorhexidine gluconate in 70% alcohol for site antisepsis and neutral displacement needleless connectors (TUTA Pulse; Medical Australia Limited, Lidcombe, New South Wales, Australia); normal saline was used to flush after medication administration, and if the device was not in use for 6 hours or longer, heparin is instilled with securement via bordered polyurethane dressing (Tegaderm 1616; 3M, St Paul, Minnesota) and a sutureless securement device (Statlock VPPCSP; Bard, Georgia).
Statistical Analyses
Data were exported to Stata 1532 for cleaning and analysis. Data cleaning of outlying figures and missing and implausible data was undertaken prior to analysis. Missing data were not imputed. The PICC was the unit of measurement, and all randomly assigned patients were analyzed on an intention-to-treat basis.33 Descriptive statistics (frequencies and percentages) were used to ascertain the primary outcome of feasibility for the larger trial. Incidence rates (per 1,000 catheter days) and rate ratios, including 95% confidence intervals (CIs), were calculated. The comparability of groups at baseline was described across demographic, clinical, and device characteristics. Kaplan-Meier survival curves (with log-rank tests) were used to compare PICC failure between study groups over time. Associations between baseline characteristics and failure were described by calculating hazard ratios (HRs). Univariable Cox regression was performed only due to the relatively low number of outcomes. P values of <.05 were considered statistically significant.
Ethics
The Children’s Health Service District, Queensland (Human Research Ethics Committee/15/QRCH/164), and Griffith University (2016/077) Human Research Ethics Committees provided ethics and governance approval. Informed consent was obtained from parents or legal guardians, with children providing youth assent if they were 7 years or older, dependent upon cognitive ability.
RESULTS
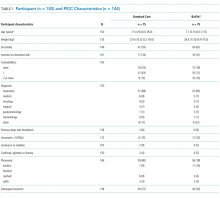
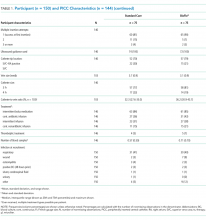
Participant and PICC Characteristics
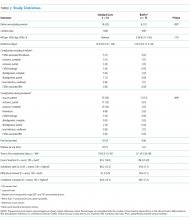
Feasibility Outcomes
PICC Failure and Complications
As per supplementary Table 1, univariate Cox regression identified PICC failure as significantly associated with tip placement in the proximal superior vena cava (SVC) compared to the SVC–right atrium junction (HR 2.61; 95% CI, 1.17-5.82; P = .024). Reduced risk of PICC failure was significantly associated with any infusion during the dwell (continuous fluid infusion, P = .007; continuous antibiotic, P = .042; or intermittent infusion, P = .046) compared to no infusion. Other variables potentially influencing the risk of failure included PICC insertion by nurse specialist compared to consultant anesthetist (HR 2.61; 95% CI, 0.85-5.44) or registrar (HR 1.97; 95% CI, 0.57-6.77). These differences were not statistically significant; however, baseline imbalance between study groups for this variable and the feasibility design preclude absolute conclusions.
DISCUSSION
This is the first pilot feasibility trial of new PICC materials and valve design incorporated in the BioFlo® PICC in the pediatric population. The trial incorporated best practice for randomized trials, including using a concurrent control group, centralized and concealed randomization, predetermined feasibility criteria, and a registered and published trial protocol.17 As in other studies,15,24,34 PICC failure and complication prevalence was unacceptably high for this essential device. Standard care PICCs failed twice as often as the new BioFlo® PICCs (22% vs 11%), which is a clinically important difference. As researchers in a pilot study, we did not expect to detect statistically significant differences; however, we found that overall complications during the dwell occurred significantly more with the standard care than BioFlo® PICCs (P = .009).
BioFlo® PICC material offers a major advancement in PICC material through the incorporation of AT technologies into catheter materials, such as PICCs. Endexo® is a low molecular–weight, fluoro-oligomeric additive that self-locates to the top few nanometers of the material surface. When added to power-injectable polyurethane, the additive results in a strong but passive, nonstick, fluorinated surface in the base PICC material. This inhibits platelet adhesion, suppresses protein procoagulant conformation, and thereby reduces thrombus formation in medical devices. Additionally, Endexo® is not a catheter coating; rather, it is incorporated within the polyurethane of the PICC, thereby ensuring these AT properties are present on the internal, external, and cut surfaces of the PICC. If this technology can reduce complication during treatment and reduce failure from infection, thrombosis, occlusion, fracture, and dislodgement, it will improve patient outcomes considerably and lower health system costs. Previous studies investigating valve technology in PICC design to reduce occlusion have been inconclusive.12-14,35,36 Occlusion (both partial and complete) was less frequent in our study with the BioFlo® group (n = 3; 4%) compared to the standard care group (n = 6; 8%). The results of this pilot study suggest that either the Endexo® material or PASV® technology has a positive association with occlusion reduction during PICC treatment.
Thrombosis was the primary failure type for the standard care PICCs, comprising one-third of failures. All but one patient with radiologically confirmed thrombosis required the removal of the PICC prior to completion of treatment. The decision to remove the PICC or retain and treat conservatively remained with the treating team. Raffini et al.7 found thrombosis to increase in patients with one or more coexisting chronic medical condition. Slightly more standard care than BioFlo® patients were free of such comorbidities (25% vs 16%), yet standard care patients still had the higher number of thromboses (7% vs 3%). Morgenthaler and Rodriguez37 reported vascular access-associated thrombosis in pediatrics to be less common than in adults but higher in medically complex children. Worryingly, Menendez et al.38 reported pediatric thrombosis to be largely asymptomatic, so the true incidence in our study is likely higher because only radiologically confirmed thromboses were recorded.
Occlusion (partial or complete) was the predominant complication across the study, being associated with one-third of all failures. When occlusion complications during the dwell (some of which were resolved with treatment), in addition to those causing failure, were considered, this number was even greater. Occlusion complications are prevalent and costly. Smith et al.24 reported that occlusion was the most common reason for PICC removal and the most likely complication to delay treatment. Both the BioFlo® and standard care PICCs are pressure rated with good tensile strength; however, fracture occurred in 4% (n = 3) of standard care PICCs compared to no fractures in BioFlo® PICCs. Although the numbers are small, it may suggest a superior tensile strength of the BioFlo® material.
This study reinforces previously published results24,38 that PICC tip position is important and can influence complications, such as occlusion and thrombosis. In addition, we found a significant association with failure when PICCs did not have a continuous infusion. These findings reinforce the need for optimal tip location at insertion and ongoing flushing and maintenance of PICCs not used for infusions.
Limitations of this study include the small sample size, which was not designed to detect statistical differences in the primary outcome between groups. Despite randomization, there were slight imbalances at baseline for inserter type and leukocyte count, although these were not significantly associated with PICC failure in the Cox regression (data not shown), and thus were unlikely to influence findings. Additionally, a difference of <10% was associated with PICC tip position, favoring the BioFlo® group. PICC tip position outside the cavoatrial junction was positively associated with failure; therefore, the effect of tip positioning on outcomes is difficult to ascertain given the small sample size and feasibility nature of the study. Further study is warranted to further explore this effect. The population sampled was pediatric medical and surgical inpatients with a vessel size >2 mm attending the operating theater suite for PICC insertion, thereby limiting the study’s generalizability to adults and other populations, including neonates and those with PICCs inserted in the pediatric intensive care unit. The study could not be blinded because study products had to be visible to the clinical and research staff. However, it is unlikely that staff would intentionally sabotage PICCs to bias the study. Blinding was possible for the assessment of blood culture and ultrasound reports to diagnose infection and thrombosis. Strengths of this study included 100% protocol adherence, and no patients were lost to follow-up.
CONCLUSION
These results confirm that PICC failure is unacceptably high and suggest that the innovative BioFlo® PICC material and design holds promise to improve PICC outcomes by reducing complications and overall PICC failure. Trials of this technology are feasible, safe, and acceptable to healthcare staff and parents. Further trials are required, including in other patient populations, to definitively identify clinical, cost-effective methods to prevent PICC failure and improve reliability during treatment.
Acknowledgments
The authors thank the children and parents of Lady Cilento Children’s Hospital for participating in this important research. A special thank you goes to the nurses within the Vascular Assessment and Management Service and to Karen Turner, Julieta Woosley, and Anna Dean for their efforts in data collecting and ensuring protocol adherence.
Disclosure
Griffith University has received unrestricted, investigator-initiated research or educational grants to support the research of T. K., A. J. U., and C. R. M. from product manufacturers 3M, Adhezion Inc, AngioDynamics, Bard Medical, Baxter, B. Braun Medical Inc, Becton Dickinson, CareFusion, Centurion Medical Products, Cook Medical, Entrotech, FloMedical, ICU Medical Inc, Medical Australia Limited, Medtronic, Smiths Medical, and Teleflex. Griffith University has received consultancy payments on behalf of C. R. M., A. J. U., and T. K. from manufacturers 3M, AngioDynamics, Bard Medical, B. Braun Medical Inc, Becton Dickinson, CareFusion, Mayo Healthcare Inc, ResQDevices, and Smiths Medical. AngioDynamics (the BioFlo® PICC manufacturer) provided partial funds to undertake this research via an unrestricted donation to Griffith University (but not the study authors). Queensland Health provided in-kind support to fund the remainder of the trial. The funders had no role in the study design, collection, analysis, or interpretation of the data, writing of the report, or decision to submit the article for publication.
1. Chopra V, Flanders SA, Saint S. The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527-1528. PubMed
2. Gibson C, Connolly BL, Moineddin R, Mahant S, Filipescu D, Amaral JG. Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323-1331. PubMed
3. Ullman AJ, Cooke M, Kleidon T, Rickard CM. Road map for improvement: point prevalence audit and survey of central venous access devices in paediatric acute care. J Paediatr Child Health. 2017;53(2):123-130. PubMed
4. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. PubMed
5. Barrier A, Williams DJ, Connelly M, Creech CB. Frequency of peripherally inserted central catheter complications in children. Pediatr Infect Dis J. 2012;31(5):519-521. PubMed
6. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. PubMed
7. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. PubMed
8. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. PubMed
9. Moureau NL, Trick N, Nifong T, et al. Vessel health and preservation (part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13(3):351-356. PubMed
10. Poli P, Scocca A, Di Puccio F, Gallone G, Angelini L, Calabro EM. A comparative study on the mechanical behavior of polyurethane PICCs. J Vasc Access. 2016;17(2):175-181. PubMed
11. Interface Biologics. Surface modification technology platform. 2017. http://www.interfacebiologics.com/endexo.htm. Accessed April 5, 2017.
12. Hoffer EK, Bloch RD, Borsa JJ, Santulli P, Fontaine AB, Francoeur N. Peripherally inserted central catheters with distal versus proximal valves: prospective randomized trial. J Vasc Interv Radiol. 2001;12(10):1173-1177. PubMed
13. Hoffer EK, Borsa J, Santulli P, Bloch R, Fontaine AB. Prospective randomized comparison of valved versus nonvalved peripherally inserted central vein catheters. AJR Am J Roentgenol. 1999;173(5):1393-1398. PubMed
14. Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and nonvalved peripherally inserted central catheters. J Vasc Access. 2014;15(6):519-523.
15. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
16. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337. PubMed
17. Kleidon TM, Ullman AJ, Zhang L, Mihala G, Rickard CM. How does your PICCOMPARE? A pilot randomized controlled trial comparing PICC materials in pediatrics. J Hosp Med. 2017;(under review). PubMed
18. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180-191. PubMed
19. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. PubMed
20. Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908-918. PubMed
21. Kramer RD, Rogers MA, Conte M, Mann J, Saint S, Chopra V. Are antimicrobial peripherally inserted central catheters associated with reduction in central line-associated bloodstream infection? A systematic review and meta-analysis. Am J Infect Control. 2017;45(2):108-114. PubMed
22. Centers for Disease Control and Prevention. National Healthcare Safety Network Device Associated Module: CLABSI. 2014.
23. Lobo BL, Vaidean G, Broyles J, Reaves AB, Shorr RI. Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417-422. PubMed
24. Smith SN, Moureau N, Vaughn VM, et al. Patterns and Predictors of Peripherally Inserted Central Catheter Occlusion: The 3P-O Study. J Vasc Interv Radiol. 28(5):749.e742-756.e742. PubMed
25. Chow LML, Friedman JN, MacArthur C, et al. Peripherally inserted central catheter (PICC) fracture and embolozation in the pediatric population. Pediatrics. 2003;142(2):141-144. PubMed
26. Chopra V, Kuhn L, Ratz D, Flanders SA, Krein SL. Vascular nursing experience, practice knowledge, and beliefs: Results from the Michigan PICC1 survey. J Hosp Med. 2016;11(4):269-275. PubMed
27. Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14(2):212. PubMed
28. Centre for Healthcare Related Infection Surveilance and Prevention and Tuberculosis Control. Guideline: Peripherally inserted central catheter (PICC). 2013.
29. Services Children’s Health Service. Central venous catheters: nursing care and management of peripherally inserted central catheter (PICC) in paediatric patients. 2011. http://qheps.health.qld.gov.au/childrenshealth/resources/nursestand/docs/ns_03452.pdf. Accessed Februrary 1, 2016.
30. Services CsH. Central Venous Access Device Insertion and Management. 2014.
31. Central venous access device insertion and management. Queensland Government; 2014. http://qheps.health.qld.gov.au/childrenshealth/resources/proc/docs/proc_03450.pdf Accessed March 13, 2014.
32. StatCorp. Stata Statistical Software: Release 12.1 College Station. 2006.
33. Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(1):e9. PubMed
34. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. PubMed
35. Alport B, Burbridge B, Lim H. Bard PowerPICC Solo2 vs Cook Turbo-Ject: A Tale of Two PICCs. Can Assoc Radiol J. 2012;63(4):323-328. PubMed
36. Johnston AJ, Streater CT, Noorani R, Crofts JL, Del Mundo AB, Parker RA. The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates—the ‘ELeCTRiC’ study. J Vasc Access. 2012;13(4):421-425. PubMed
37. Morgenthaler TI, Rodriguez V. Preventing acute care-associated venous thromboembolism in adult and pediatric patients across a large healthcare system. J Hosp Med. 2016;11(Suppl 2):S15-S21. PubMed
38. Menendez JJ, Verdu C, Calderon B, et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J Thromb Haemost. 2016;14(11):2158-2168. PubMed
Peripherally inserted central catheters (PICCs) have evolved since their inception in the early 1970s and are used with increasing frequency for pediatric inpatients and outpatients.1-3 Emerging literature, including a meta-analysis of international observational studies,4 reports PICC failure (complications necessitating premature removal) occurs in up to 30% of PICCs, most commonly due to infection, thrombosis, occlusion, and fracture.4-7 Raffini et al.7 report the increasing incidence of pediatric PICC-related thrombosis increases morbidity and mortality8 and negatively impacts future vessel health and preservation.9
PICCs have progressed from relatively simple, silicone-based catheters with an external clamp to chemically engineered polyurethane with pressure-activated valves placed at the proximal or distal catheter hub with the intent to reduce occlusion.10 Further modernization of PICC material occurred with the incorporation of antithrombogenic (AT) material (Endexo®). These PICCs are designed to contain a nonstick polymer, which is designed to reduce the adherence of blood components (platelets and clotting factors) and inhibit thrombus formation (and hence prevent deep vein thrombosis andocclusion, as well as inhibit microbial biofilm attachment [and subsequent infection]).11
In addition to new materials, other aspects of this PICC design have been the addition of a pressure-activated safety valve (PASV®) built into the proximal hub. Pressure-activated valve technology promises to prevent catheter occlusion by reducing blood reflux into the PICC; the valve opens with pressure during infusion and aspiration and remains closed with normal venous pressure, circumventing the need for clinicians to manually clamp the PICC and reducing human error and the potential for thrombosis, occlusion, and fracture development.12 Hoffer et al.13 reported half as many occlusions of valved PICCs (3.3%) compared with nonvalved or clamped PICCs (7.1%); although not statistically significant (P = .10), perhaps due to the small sample, overall complications, including occlusion and infection, were significantly lessened with the valved PICC (35% vs 79%; P = .02). Comparatively, Pittiruti et al.14 conducted a trial of 2 types of valved PICCs with an open-ended, nonvalved PICC and found no reduction in PICC occlusion or catheter malfunction.
Today, PICC use is common for patients who require short-to-medium intravenous therapy. PICCs are increasingly recognized for their significant complications, including thrombosis and infection.15 Novel PICC technology, including the incorporation of AT material such as Endexo® and PASV®, may reduce complications; however, the clinical efficacy, cost-effectiveness, and acceptability of these innovations have not been tested through randomized trials in pediatric patients. In accordance with Medical Research Council guidelines16 for developing interventions, we pilot tested the feasibility of the BioFlo® PICC, including intervention acceptability, compliance, recruitment, and initial estimates of effect, in anticipation of a subsequent full-scale efficacy randomized controlled trial. Our secondary aim was to compare the effectiveness of the BioFlo® PICC with Endexo® and PASV® technology in reducing PICC complications and failure.
METHODS
Design
We undertook a pilot randomized controlled trial comparing the standard polyurethane PICC (with external clamp) with the BioFlo® PICC (with internal valve) in preventing catheter failure in pediatric patients. The study was prospectively registered with the Australian Clinical Trials Registry (ACTRN12615001290583), and the research protocol was published.17
Study Setting
The study commenced in March 2016 at the Lady Cilento Children’s Hospital in South Brisbane, Australia, a tertiary-level, specialist, pediatric teaching hospital in Queensland, Australia, providing full-spectrum health services to children and young people from birth to 18 years of age. Recruitment, including data collection, was completed in November 2016.
Sample
The target sample size was 110 participants, 50 participants per group plus 10% for potential attrition, as determined by standard pilot trial sample size recommendations.18 With ethics approval, the sample size was later increased to 150 participants in order to adequately pilot a microbiological substudy method (published separately).17 Participants were consecutively recruited if they met the inclusion criteria: PICC insertion, age <18 years, predicted hospital stay >24 hours, single-lumen PICC, and written informed consent by an English-speaking, legal parent or guardian. Patients were excluded if they had a current (<48 hours) blood stream infection (BSI), vessel size <2 mm, could not speak English without an interpreter, required a multilumen PICC, or were previously enrolled in the study.
Interventions
Participants were randomized to receive either of the following PICCs: (1) standard care: Cook™ polyurethane, turbo-ject, power-injectable PICC (Cook Medical, Bloomington, IN) or (2) comparison: BioFlo® polyurethane with Endexo® technology (AngioDynamics Inc, Queensbury, NY).
Outcomes
The primary outcome was feasibility of a full-efficacy trial established by composite analysis of the elements of eligibility (>70% of patients will be eligible), recruitment (>70% of patients will agree to enroll), retention and attrition (<15% of participants are lost to follow-up or withdraw from the study), protocol adherence (>80% of participants receive their allocated, randomly assigned study product), missing data (<10% of data are missed during data collection), parent and healthcare staff satisfaction, and PICC failure effect size estimates to allow sample size calculations.18,19 PICC failure was defined as the following complications associated with PICC removal: (1) catheter-associated BSI,8,20-22 (2) local site infection,22 (3) venous thrombosis,23 (4) occlusion,24,25 (5) PICC fracture, or (6) PICC dislodgement.25,26 Parents (or caregivers) and healthcare staff were asked to rate their level of confidence with the study product and ease of PICC removal by using a 0 to 100 numeric rating scale (NRS) of increasing confidence and/or ease. These data were collected at the time of PICC removal. Operators were also asked to rate their levels of satisfaction with the insertion equipment and ease of PICC insertion immediately upon completion of the insertion procedure (both 0-100 NRS of increasing satisfaction and/or ease). Secondary outcomes included individual PICC complications (eg, occlusion) occurring at any time point during the PICC dwell (including at removal), adverse events, pain, redness at the insertion site, and overall PICC dwell.
Study Procedures
The research nurse (ReN) screened operating theater lists for patients, obtained written informed consent, and initiated the randomization. Randomization was computer generated, and web based via Griffith University (https://www151.griffith.edu.au/random) to ensure allocation concealment until study entry. Patients were randomly assigned in a 1:1 ratio with computer-generated and randomly varied block sizes of 2 and 4. Data were collected by the ReN on the day of insertion, at day 1 postinsertion, then every 2 to 3 days thereafter so that PICCs were checked at least twice per week until study completion. Participants were included in the trial until 12 weeks post-PICC insertion, study withdrawal or PICC removal (whichever came first), with an additional 48 hours follow-up for infection outcomes. Patient review was face to face during the inpatient stay, with discharged patients’ follow-up occurring via outpatient clinics, hospital-in-the-home service, or telephone.
Data collection was via Research Electronic Data Capture (http://project-redcap.org/). The ReN collected data on primary and secondary outcomes by using the predefined criteria. Demographic and clinical data were collected to assess the success of randomization, describe the participant group, and display characteristics known to increase the risk of PICC complication and thrombosis. A blinded radiologist and infectious disease specialist reviewed and diagnosed thrombosis of deep veins and catheter-associated BSI outcomes, respectively.
PICC Procedures
Extensive prestudy education for 2 months prior to trial commencement was provided to all clinicians involved with the insertion and care of PICCs, including the study products. PICCs were inserted in an operating theater environment by a qualified consultant pediatric anesthetist, a senior anesthetic registrar or fellow in an approved anesthetic training program, or pediatric vascular access nurse practitioner. Ultrasound guidance was used to assess a patient’s vasculature and puncture the vessel. The operator chose the PICC size on the basis of clinical judgment of vessel size and patient needs and then inserted the allocated PICC.27 Preferred PICC tip location was the cavoatrial junction. All PICC tip positions were confirmed with a chest x-ray before use.
Postinsertion, PICCs were managed by local interdisciplinary clinicians in accordance with local practice guidelines.27-31 PICC care and management includes the use of 2% chlorhexidine gluconate in 70% alcohol for site antisepsis and neutral displacement needleless connectors (TUTA Pulse; Medical Australia Limited, Lidcombe, New South Wales, Australia); normal saline was used to flush after medication administration, and if the device was not in use for 6 hours or longer, heparin is instilled with securement via bordered polyurethane dressing (Tegaderm 1616; 3M, St Paul, Minnesota) and a sutureless securement device (Statlock VPPCSP; Bard, Georgia).
Statistical Analyses
Data were exported to Stata 1532 for cleaning and analysis. Data cleaning of outlying figures and missing and implausible data was undertaken prior to analysis. Missing data were not imputed. The PICC was the unit of measurement, and all randomly assigned patients were analyzed on an intention-to-treat basis.33 Descriptive statistics (frequencies and percentages) were used to ascertain the primary outcome of feasibility for the larger trial. Incidence rates (per 1,000 catheter days) and rate ratios, including 95% confidence intervals (CIs), were calculated. The comparability of groups at baseline was described across demographic, clinical, and device characteristics. Kaplan-Meier survival curves (with log-rank tests) were used to compare PICC failure between study groups over time. Associations between baseline characteristics and failure were described by calculating hazard ratios (HRs). Univariable Cox regression was performed only due to the relatively low number of outcomes. P values of <.05 were considered statistically significant.
Ethics
The Children’s Health Service District, Queensland (Human Research Ethics Committee/15/QRCH/164), and Griffith University (2016/077) Human Research Ethics Committees provided ethics and governance approval. Informed consent was obtained from parents or legal guardians, with children providing youth assent if they were 7 years or older, dependent upon cognitive ability.
RESULTS
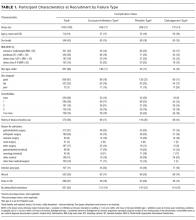
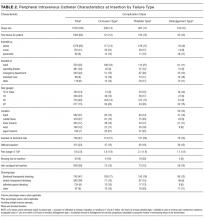
Participant and PICC Characteristics
Feasibility Outcomes
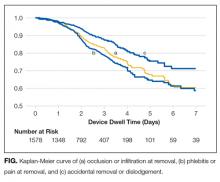
PICC Failure and Complications
As per supplementary Table 1, univariate Cox regression identified PICC failure as significantly associated with tip placement in the proximal superior vena cava (SVC) compared to the SVC–right atrium junction (HR 2.61; 95% CI, 1.17-5.82; P = .024). Reduced risk of PICC failure was significantly associated with any infusion during the dwell (continuous fluid infusion, P = .007; continuous antibiotic, P = .042; or intermittent infusion, P = .046) compared to no infusion. Other variables potentially influencing the risk of failure included PICC insertion by nurse specialist compared to consultant anesthetist (HR 2.61; 95% CI, 0.85-5.44) or registrar (HR 1.97; 95% CI, 0.57-6.77). These differences were not statistically significant; however, baseline imbalance between study groups for this variable and the feasibility design preclude absolute conclusions.
DISCUSSION
This is the first pilot feasibility trial of new PICC materials and valve design incorporated in the BioFlo® PICC in the pediatric population. The trial incorporated best practice for randomized trials, including using a concurrent control group, centralized and concealed randomization, predetermined feasibility criteria, and a registered and published trial protocol.17 As in other studies,15,24,34 PICC failure and complication prevalence was unacceptably high for this essential device. Standard care PICCs failed twice as often as the new BioFlo® PICCs (22% vs 11%), which is a clinically important difference. As researchers in a pilot study, we did not expect to detect statistically significant differences; however, we found that overall complications during the dwell occurred significantly more with the standard care than BioFlo® PICCs (P = .009).
BioFlo® PICC material offers a major advancement in PICC material through the incorporation of AT technologies into catheter materials, such as PICCs. Endexo® is a low molecular–weight, fluoro-oligomeric additive that self-locates to the top few nanometers of the material surface. When added to power-injectable polyurethane, the additive results in a strong but passive, nonstick, fluorinated surface in the base PICC material. This inhibits platelet adhesion, suppresses protein procoagulant conformation, and thereby reduces thrombus formation in medical devices. Additionally, Endexo® is not a catheter coating; rather, it is incorporated within the polyurethane of the PICC, thereby ensuring these AT properties are present on the internal, external, and cut surfaces of the PICC. If this technology can reduce complication during treatment and reduce failure from infection, thrombosis, occlusion, fracture, and dislodgement, it will improve patient outcomes considerably and lower health system costs. Previous studies investigating valve technology in PICC design to reduce occlusion have been inconclusive.12-14,35,36 Occlusion (both partial and complete) was less frequent in our study with the BioFlo® group (n = 3; 4%) compared to the standard care group (n = 6; 8%). The results of this pilot study suggest that either the Endexo® material or PASV® technology has a positive association with occlusion reduction during PICC treatment.
Thrombosis was the primary failure type for the standard care PICCs, comprising one-third of failures. All but one patient with radiologically confirmed thrombosis required the removal of the PICC prior to completion of treatment. The decision to remove the PICC or retain and treat conservatively remained with the treating team. Raffini et al.7 found thrombosis to increase in patients with one or more coexisting chronic medical condition. Slightly more standard care than BioFlo® patients were free of such comorbidities (25% vs 16%), yet standard care patients still had the higher number of thromboses (7% vs 3%). Morgenthaler and Rodriguez37 reported vascular access-associated thrombosis in pediatrics to be less common than in adults but higher in medically complex children. Worryingly, Menendez et al.38 reported pediatric thrombosis to be largely asymptomatic, so the true incidence in our study is likely higher because only radiologically confirmed thromboses were recorded.
Occlusion (partial or complete) was the predominant complication across the study, being associated with one-third of all failures. When occlusion complications during the dwell (some of which were resolved with treatment), in addition to those causing failure, were considered, this number was even greater. Occlusion complications are prevalent and costly. Smith et al.24 reported that occlusion was the most common reason for PICC removal and the most likely complication to delay treatment. Both the BioFlo® and standard care PICCs are pressure rated with good tensile strength; however, fracture occurred in 4% (n = 3) of standard care PICCs compared to no fractures in BioFlo® PICCs. Although the numbers are small, it may suggest a superior tensile strength of the BioFlo® material.
This study reinforces previously published results24,38 that PICC tip position is important and can influence complications, such as occlusion and thrombosis. In addition, we found a significant association with failure when PICCs did not have a continuous infusion. These findings reinforce the need for optimal tip location at insertion and ongoing flushing and maintenance of PICCs not used for infusions.
Limitations of this study include the small sample size, which was not designed to detect statistical differences in the primary outcome between groups. Despite randomization, there were slight imbalances at baseline for inserter type and leukocyte count, although these were not significantly associated with PICC failure in the Cox regression (data not shown), and thus were unlikely to influence findings. Additionally, a difference of <10% was associated with PICC tip position, favoring the BioFlo® group. PICC tip position outside the cavoatrial junction was positively associated with failure; therefore, the effect of tip positioning on outcomes is difficult to ascertain given the small sample size and feasibility nature of the study. Further study is warranted to further explore this effect. The population sampled was pediatric medical and surgical inpatients with a vessel size >2 mm attending the operating theater suite for PICC insertion, thereby limiting the study’s generalizability to adults and other populations, including neonates and those with PICCs inserted in the pediatric intensive care unit. The study could not be blinded because study products had to be visible to the clinical and research staff. However, it is unlikely that staff would intentionally sabotage PICCs to bias the study. Blinding was possible for the assessment of blood culture and ultrasound reports to diagnose infection and thrombosis. Strengths of this study included 100% protocol adherence, and no patients were lost to follow-up.
CONCLUSION
These results confirm that PICC failure is unacceptably high and suggest that the innovative BioFlo® PICC material and design holds promise to improve PICC outcomes by reducing complications and overall PICC failure. Trials of this technology are feasible, safe, and acceptable to healthcare staff and parents. Further trials are required, including in other patient populations, to definitively identify clinical, cost-effective methods to prevent PICC failure and improve reliability during treatment.
Acknowledgments
The authors thank the children and parents of Lady Cilento Children’s Hospital for participating in this important research. A special thank you goes to the nurses within the Vascular Assessment and Management Service and to Karen Turner, Julieta Woosley, and Anna Dean for their efforts in data collecting and ensuring protocol adherence.
Disclosure
Griffith University has received unrestricted, investigator-initiated research or educational grants to support the research of T. K., A. J. U., and C. R. M. from product manufacturers 3M, Adhezion Inc, AngioDynamics, Bard Medical, Baxter, B. Braun Medical Inc, Becton Dickinson, CareFusion, Centurion Medical Products, Cook Medical, Entrotech, FloMedical, ICU Medical Inc, Medical Australia Limited, Medtronic, Smiths Medical, and Teleflex. Griffith University has received consultancy payments on behalf of C. R. M., A. J. U., and T. K. from manufacturers 3M, AngioDynamics, Bard Medical, B. Braun Medical Inc, Becton Dickinson, CareFusion, Mayo Healthcare Inc, ResQDevices, and Smiths Medical. AngioDynamics (the BioFlo® PICC manufacturer) provided partial funds to undertake this research via an unrestricted donation to Griffith University (but not the study authors). Queensland Health provided in-kind support to fund the remainder of the trial. The funders had no role in the study design, collection, analysis, or interpretation of the data, writing of the report, or decision to submit the article for publication.
Peripherally inserted central catheters (PICCs) have evolved since their inception in the early 1970s and are used with increasing frequency for pediatric inpatients and outpatients.1-3 Emerging literature, including a meta-analysis of international observational studies,4 reports PICC failure (complications necessitating premature removal) occurs in up to 30% of PICCs, most commonly due to infection, thrombosis, occlusion, and fracture.4-7 Raffini et al.7 report the increasing incidence of pediatric PICC-related thrombosis increases morbidity and mortality8 and negatively impacts future vessel health and preservation.9
PICCs have progressed from relatively simple, silicone-based catheters with an external clamp to chemically engineered polyurethane with pressure-activated valves placed at the proximal or distal catheter hub with the intent to reduce occlusion.10 Further modernization of PICC material occurred with the incorporation of antithrombogenic (AT) material (Endexo®). These PICCs are designed to contain a nonstick polymer, which is designed to reduce the adherence of blood components (platelets and clotting factors) and inhibit thrombus formation (and hence prevent deep vein thrombosis andocclusion, as well as inhibit microbial biofilm attachment [and subsequent infection]).11
In addition to new materials, other aspects of this PICC design have been the addition of a pressure-activated safety valve (PASV®) built into the proximal hub. Pressure-activated valve technology promises to prevent catheter occlusion by reducing blood reflux into the PICC; the valve opens with pressure during infusion and aspiration and remains closed with normal venous pressure, circumventing the need for clinicians to manually clamp the PICC and reducing human error and the potential for thrombosis, occlusion, and fracture development.12 Hoffer et al.13 reported half as many occlusions of valved PICCs (3.3%) compared with nonvalved or clamped PICCs (7.1%); although not statistically significant (P = .10), perhaps due to the small sample, overall complications, including occlusion and infection, were significantly lessened with the valved PICC (35% vs 79%; P = .02). Comparatively, Pittiruti et al.14 conducted a trial of 2 types of valved PICCs with an open-ended, nonvalved PICC and found no reduction in PICC occlusion or catheter malfunction.
Today, PICC use is common for patients who require short-to-medium intravenous therapy. PICCs are increasingly recognized for their significant complications, including thrombosis and infection.15 Novel PICC technology, including the incorporation of AT material such as Endexo® and PASV®, may reduce complications; however, the clinical efficacy, cost-effectiveness, and acceptability of these innovations have not been tested through randomized trials in pediatric patients. In accordance with Medical Research Council guidelines16 for developing interventions, we pilot tested the feasibility of the BioFlo® PICC, including intervention acceptability, compliance, recruitment, and initial estimates of effect, in anticipation of a subsequent full-scale efficacy randomized controlled trial. Our secondary aim was to compare the effectiveness of the BioFlo® PICC with Endexo® and PASV® technology in reducing PICC complications and failure.
METHODS
Design
We undertook a pilot randomized controlled trial comparing the standard polyurethane PICC (with external clamp) with the BioFlo® PICC (with internal valve) in preventing catheter failure in pediatric patients. The study was prospectively registered with the Australian Clinical Trials Registry (ACTRN12615001290583), and the research protocol was published.17
Study Setting
The study commenced in March 2016 at the Lady Cilento Children’s Hospital in South Brisbane, Australia, a tertiary-level, specialist, pediatric teaching hospital in Queensland, Australia, providing full-spectrum health services to children and young people from birth to 18 years of age. Recruitment, including data collection, was completed in November 2016.
Sample
The target sample size was 110 participants, 50 participants per group plus 10% for potential attrition, as determined by standard pilot trial sample size recommendations.18 With ethics approval, the sample size was later increased to 150 participants in order to adequately pilot a microbiological substudy method (published separately).17 Participants were consecutively recruited if they met the inclusion criteria: PICC insertion, age <18 years, predicted hospital stay >24 hours, single-lumen PICC, and written informed consent by an English-speaking, legal parent or guardian. Patients were excluded if they had a current (<48 hours) blood stream infection (BSI), vessel size <2 mm, could not speak English without an interpreter, required a multilumen PICC, or were previously enrolled in the study.
Interventions
Participants were randomized to receive either of the following PICCs: (1) standard care: Cook™ polyurethane, turbo-ject, power-injectable PICC (Cook Medical, Bloomington, IN) or (2) comparison: BioFlo® polyurethane with Endexo® technology (AngioDynamics Inc, Queensbury, NY).
Outcomes
The primary outcome was feasibility of a full-efficacy trial established by composite analysis of the elements of eligibility (>70% of patients will be eligible), recruitment (>70% of patients will agree to enroll), retention and attrition (<15% of participants are lost to follow-up or withdraw from the study), protocol adherence (>80% of participants receive their allocated, randomly assigned study product), missing data (<10% of data are missed during data collection), parent and healthcare staff satisfaction, and PICC failure effect size estimates to allow sample size calculations.18,19 PICC failure was defined as the following complications associated with PICC removal: (1) catheter-associated BSI,8,20-22 (2) local site infection,22 (3) venous thrombosis,23 (4) occlusion,24,25 (5) PICC fracture, or (6) PICC dislodgement.25,26 Parents (or caregivers) and healthcare staff were asked to rate their level of confidence with the study product and ease of PICC removal by using a 0 to 100 numeric rating scale (NRS) of increasing confidence and/or ease. These data were collected at the time of PICC removal. Operators were also asked to rate their levels of satisfaction with the insertion equipment and ease of PICC insertion immediately upon completion of the insertion procedure (both 0-100 NRS of increasing satisfaction and/or ease). Secondary outcomes included individual PICC complications (eg, occlusion) occurring at any time point during the PICC dwell (including at removal), adverse events, pain, redness at the insertion site, and overall PICC dwell.
Study Procedures
The research nurse (ReN) screened operating theater lists for patients, obtained written informed consent, and initiated the randomization. Randomization was computer generated, and web based via Griffith University (https://www151.griffith.edu.au/random) to ensure allocation concealment until study entry. Patients were randomly assigned in a 1:1 ratio with computer-generated and randomly varied block sizes of 2 and 4. Data were collected by the ReN on the day of insertion, at day 1 postinsertion, then every 2 to 3 days thereafter so that PICCs were checked at least twice per week until study completion. Participants were included in the trial until 12 weeks post-PICC insertion, study withdrawal or PICC removal (whichever came first), with an additional 48 hours follow-up for infection outcomes. Patient review was face to face during the inpatient stay, with discharged patients’ follow-up occurring via outpatient clinics, hospital-in-the-home service, or telephone.
Data collection was via Research Electronic Data Capture (http://project-redcap.org/). The ReN collected data on primary and secondary outcomes by using the predefined criteria. Demographic and clinical data were collected to assess the success of randomization, describe the participant group, and display characteristics known to increase the risk of PICC complication and thrombosis. A blinded radiologist and infectious disease specialist reviewed and diagnosed thrombosis of deep veins and catheter-associated BSI outcomes, respectively.
PICC Procedures
Extensive prestudy education for 2 months prior to trial commencement was provided to all clinicians involved with the insertion and care of PICCs, including the study products. PICCs were inserted in an operating theater environment by a qualified consultant pediatric anesthetist, a senior anesthetic registrar or fellow in an approved anesthetic training program, or pediatric vascular access nurse practitioner. Ultrasound guidance was used to assess a patient’s vasculature and puncture the vessel. The operator chose the PICC size on the basis of clinical judgment of vessel size and patient needs and then inserted the allocated PICC.27 Preferred PICC tip location was the cavoatrial junction. All PICC tip positions were confirmed with a chest x-ray before use.
Postinsertion, PICCs were managed by local interdisciplinary clinicians in accordance with local practice guidelines.27-31 PICC care and management includes the use of 2% chlorhexidine gluconate in 70% alcohol for site antisepsis and neutral displacement needleless connectors (TUTA Pulse; Medical Australia Limited, Lidcombe, New South Wales, Australia); normal saline was used to flush after medication administration, and if the device was not in use for 6 hours or longer, heparin is instilled with securement via bordered polyurethane dressing (Tegaderm 1616; 3M, St Paul, Minnesota) and a sutureless securement device (Statlock VPPCSP; Bard, Georgia).
Statistical Analyses
Data were exported to Stata 1532 for cleaning and analysis. Data cleaning of outlying figures and missing and implausible data was undertaken prior to analysis. Missing data were not imputed. The PICC was the unit of measurement, and all randomly assigned patients were analyzed on an intention-to-treat basis.33 Descriptive statistics (frequencies and percentages) were used to ascertain the primary outcome of feasibility for the larger trial. Incidence rates (per 1,000 catheter days) and rate ratios, including 95% confidence intervals (CIs), were calculated. The comparability of groups at baseline was described across demographic, clinical, and device characteristics. Kaplan-Meier survival curves (with log-rank tests) were used to compare PICC failure between study groups over time. Associations between baseline characteristics and failure were described by calculating hazard ratios (HRs). Univariable Cox regression was performed only due to the relatively low number of outcomes. P values of <.05 were considered statistically significant.
Ethics
The Children’s Health Service District, Queensland (Human Research Ethics Committee/15/QRCH/164), and Griffith University (2016/077) Human Research Ethics Committees provided ethics and governance approval. Informed consent was obtained from parents or legal guardians, with children providing youth assent if they were 7 years or older, dependent upon cognitive ability.
RESULTS
Participant and PICC Characteristics
Feasibility Outcomes
PICC Failure and Complications
As per supplementary Table 1, univariate Cox regression identified PICC failure as significantly associated with tip placement in the proximal superior vena cava (SVC) compared to the SVC–right atrium junction (HR 2.61; 95% CI, 1.17-5.82; P = .024). Reduced risk of PICC failure was significantly associated with any infusion during the dwell (continuous fluid infusion, P = .007; continuous antibiotic, P = .042; or intermittent infusion, P = .046) compared to no infusion. Other variables potentially influencing the risk of failure included PICC insertion by nurse specialist compared to consultant anesthetist (HR 2.61; 95% CI, 0.85-5.44) or registrar (HR 1.97; 95% CI, 0.57-6.77). These differences were not statistically significant; however, baseline imbalance between study groups for this variable and the feasibility design preclude absolute conclusions.
DISCUSSION
This is the first pilot feasibility trial of new PICC materials and valve design incorporated in the BioFlo® PICC in the pediatric population. The trial incorporated best practice for randomized trials, including using a concurrent control group, centralized and concealed randomization, predetermined feasibility criteria, and a registered and published trial protocol.17 As in other studies,15,24,34 PICC failure and complication prevalence was unacceptably high for this essential device. Standard care PICCs failed twice as often as the new BioFlo® PICCs (22% vs 11%), which is a clinically important difference. As researchers in a pilot study, we did not expect to detect statistically significant differences; however, we found that overall complications during the dwell occurred significantly more with the standard care than BioFlo® PICCs (P = .009).
BioFlo® PICC material offers a major advancement in PICC material through the incorporation of AT technologies into catheter materials, such as PICCs. Endexo® is a low molecular–weight, fluoro-oligomeric additive that self-locates to the top few nanometers of the material surface. When added to power-injectable polyurethane, the additive results in a strong but passive, nonstick, fluorinated surface in the base PICC material. This inhibits platelet adhesion, suppresses protein procoagulant conformation, and thereby reduces thrombus formation in medical devices. Additionally, Endexo® is not a catheter coating; rather, it is incorporated within the polyurethane of the PICC, thereby ensuring these AT properties are present on the internal, external, and cut surfaces of the PICC. If this technology can reduce complication during treatment and reduce failure from infection, thrombosis, occlusion, fracture, and dislodgement, it will improve patient outcomes considerably and lower health system costs. Previous studies investigating valve technology in PICC design to reduce occlusion have been inconclusive.12-14,35,36 Occlusion (both partial and complete) was less frequent in our study with the BioFlo® group (n = 3; 4%) compared to the standard care group (n = 6; 8%). The results of this pilot study suggest that either the Endexo® material or PASV® technology has a positive association with occlusion reduction during PICC treatment.
Thrombosis was the primary failure type for the standard care PICCs, comprising one-third of failures. All but one patient with radiologically confirmed thrombosis required the removal of the PICC prior to completion of treatment. The decision to remove the PICC or retain and treat conservatively remained with the treating team. Raffini et al.7 found thrombosis to increase in patients with one or more coexisting chronic medical condition. Slightly more standard care than BioFlo® patients were free of such comorbidities (25% vs 16%), yet standard care patients still had the higher number of thromboses (7% vs 3%). Morgenthaler and Rodriguez37 reported vascular access-associated thrombosis in pediatrics to be less common than in adults but higher in medically complex children. Worryingly, Menendez et al.38 reported pediatric thrombosis to be largely asymptomatic, so the true incidence in our study is likely higher because only radiologically confirmed thromboses were recorded.
Occlusion (partial or complete) was the predominant complication across the study, being associated with one-third of all failures. When occlusion complications during the dwell (some of which were resolved with treatment), in addition to those causing failure, were considered, this number was even greater. Occlusion complications are prevalent and costly. Smith et al.24 reported that occlusion was the most common reason for PICC removal and the most likely complication to delay treatment. Both the BioFlo® and standard care PICCs are pressure rated with good tensile strength; however, fracture occurred in 4% (n = 3) of standard care PICCs compared to no fractures in BioFlo® PICCs. Although the numbers are small, it may suggest a superior tensile strength of the BioFlo® material.
This study reinforces previously published results24,38 that PICC tip position is important and can influence complications, such as occlusion and thrombosis. In addition, we found a significant association with failure when PICCs did not have a continuous infusion. These findings reinforce the need for optimal tip location at insertion and ongoing flushing and maintenance of PICCs not used for infusions.
Limitations of this study include the small sample size, which was not designed to detect statistical differences in the primary outcome between groups. Despite randomization, there were slight imbalances at baseline for inserter type and leukocyte count, although these were not significantly associated with PICC failure in the Cox regression (data not shown), and thus were unlikely to influence findings. Additionally, a difference of <10% was associated with PICC tip position, favoring the BioFlo® group. PICC tip position outside the cavoatrial junction was positively associated with failure; therefore, the effect of tip positioning on outcomes is difficult to ascertain given the small sample size and feasibility nature of the study. Further study is warranted to further explore this effect. The population sampled was pediatric medical and surgical inpatients with a vessel size >2 mm attending the operating theater suite for PICC insertion, thereby limiting the study’s generalizability to adults and other populations, including neonates and those with PICCs inserted in the pediatric intensive care unit. The study could not be blinded because study products had to be visible to the clinical and research staff. However, it is unlikely that staff would intentionally sabotage PICCs to bias the study. Blinding was possible for the assessment of blood culture and ultrasound reports to diagnose infection and thrombosis. Strengths of this study included 100% protocol adherence, and no patients were lost to follow-up.
CONCLUSION
These results confirm that PICC failure is unacceptably high and suggest that the innovative BioFlo® PICC material and design holds promise to improve PICC outcomes by reducing complications and overall PICC failure. Trials of this technology are feasible, safe, and acceptable to healthcare staff and parents. Further trials are required, including in other patient populations, to definitively identify clinical, cost-effective methods to prevent PICC failure and improve reliability during treatment.
Acknowledgments
The authors thank the children and parents of Lady Cilento Children’s Hospital for participating in this important research. A special thank you goes to the nurses within the Vascular Assessment and Management Service and to Karen Turner, Julieta Woosley, and Anna Dean for their efforts in data collecting and ensuring protocol adherence.
Disclosure
Griffith University has received unrestricted, investigator-initiated research or educational grants to support the research of T. K., A. J. U., and C. R. M. from product manufacturers 3M, Adhezion Inc, AngioDynamics, Bard Medical, Baxter, B. Braun Medical Inc, Becton Dickinson, CareFusion, Centurion Medical Products, Cook Medical, Entrotech, FloMedical, ICU Medical Inc, Medical Australia Limited, Medtronic, Smiths Medical, and Teleflex. Griffith University has received consultancy payments on behalf of C. R. M., A. J. U., and T. K. from manufacturers 3M, AngioDynamics, Bard Medical, B. Braun Medical Inc, Becton Dickinson, CareFusion, Mayo Healthcare Inc, ResQDevices, and Smiths Medical. AngioDynamics (the BioFlo® PICC manufacturer) provided partial funds to undertake this research via an unrestricted donation to Griffith University (but not the study authors). Queensland Health provided in-kind support to fund the remainder of the trial. The funders had no role in the study design, collection, analysis, or interpretation of the data, writing of the report, or decision to submit the article for publication.
1. Chopra V, Flanders SA, Saint S. The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527-1528. PubMed
2. Gibson C, Connolly BL, Moineddin R, Mahant S, Filipescu D, Amaral JG. Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323-1331. PubMed
3. Ullman AJ, Cooke M, Kleidon T, Rickard CM. Road map for improvement: point prevalence audit and survey of central venous access devices in paediatric acute care. J Paediatr Child Health. 2017;53(2):123-130. PubMed
4. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. PubMed
5. Barrier A, Williams DJ, Connelly M, Creech CB. Frequency of peripherally inserted central catheter complications in children. Pediatr Infect Dis J. 2012;31(5):519-521. PubMed
6. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. PubMed
7. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. PubMed
8. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. PubMed
9. Moureau NL, Trick N, Nifong T, et al. Vessel health and preservation (part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13(3):351-356. PubMed
10. Poli P, Scocca A, Di Puccio F, Gallone G, Angelini L, Calabro EM. A comparative study on the mechanical behavior of polyurethane PICCs. J Vasc Access. 2016;17(2):175-181. PubMed
11. Interface Biologics. Surface modification technology platform. 2017. http://www.interfacebiologics.com/endexo.htm. Accessed April 5, 2017.
12. Hoffer EK, Bloch RD, Borsa JJ, Santulli P, Fontaine AB, Francoeur N. Peripherally inserted central catheters with distal versus proximal valves: prospective randomized trial. J Vasc Interv Radiol. 2001;12(10):1173-1177. PubMed
13. Hoffer EK, Borsa J, Santulli P, Bloch R, Fontaine AB. Prospective randomized comparison of valved versus nonvalved peripherally inserted central vein catheters. AJR Am J Roentgenol. 1999;173(5):1393-1398. PubMed
14. Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and nonvalved peripherally inserted central catheters. J Vasc Access. 2014;15(6):519-523.
15. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
16. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337. PubMed
17. Kleidon TM, Ullman AJ, Zhang L, Mihala G, Rickard CM. How does your PICCOMPARE? A pilot randomized controlled trial comparing PICC materials in pediatrics. J Hosp Med. 2017;(under review). PubMed
18. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180-191. PubMed
19. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. PubMed
20. Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908-918. PubMed
21. Kramer RD, Rogers MA, Conte M, Mann J, Saint S, Chopra V. Are antimicrobial peripherally inserted central catheters associated with reduction in central line-associated bloodstream infection? A systematic review and meta-analysis. Am J Infect Control. 2017;45(2):108-114. PubMed
22. Centers for Disease Control and Prevention. National Healthcare Safety Network Device Associated Module: CLABSI. 2014.
23. Lobo BL, Vaidean G, Broyles J, Reaves AB, Shorr RI. Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417-422. PubMed
24. Smith SN, Moureau N, Vaughn VM, et al. Patterns and Predictors of Peripherally Inserted Central Catheter Occlusion: The 3P-O Study. J Vasc Interv Radiol. 28(5):749.e742-756.e742. PubMed
25. Chow LML, Friedman JN, MacArthur C, et al. Peripherally inserted central catheter (PICC) fracture and embolozation in the pediatric population. Pediatrics. 2003;142(2):141-144. PubMed
26. Chopra V, Kuhn L, Ratz D, Flanders SA, Krein SL. Vascular nursing experience, practice knowledge, and beliefs: Results from the Michigan PICC1 survey. J Hosp Med. 2016;11(4):269-275. PubMed
27. Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14(2):212. PubMed
28. Centre for Healthcare Related Infection Surveilance and Prevention and Tuberculosis Control. Guideline: Peripherally inserted central catheter (PICC). 2013.
29. Services Children’s Health Service. Central venous catheters: nursing care and management of peripherally inserted central catheter (PICC) in paediatric patients. 2011. http://qheps.health.qld.gov.au/childrenshealth/resources/nursestand/docs/ns_03452.pdf. Accessed Februrary 1, 2016.
30. Services CsH. Central Venous Access Device Insertion and Management. 2014.
31. Central venous access device insertion and management. Queensland Government; 2014. http://qheps.health.qld.gov.au/childrenshealth/resources/proc/docs/proc_03450.pdf Accessed March 13, 2014.
32. StatCorp. Stata Statistical Software: Release 12.1 College Station. 2006.
33. Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(1):e9. PubMed
34. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. PubMed
35. Alport B, Burbridge B, Lim H. Bard PowerPICC Solo2 vs Cook Turbo-Ject: A Tale of Two PICCs. Can Assoc Radiol J. 2012;63(4):323-328. PubMed
36. Johnston AJ, Streater CT, Noorani R, Crofts JL, Del Mundo AB, Parker RA. The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates—the ‘ELeCTRiC’ study. J Vasc Access. 2012;13(4):421-425. PubMed
37. Morgenthaler TI, Rodriguez V. Preventing acute care-associated venous thromboembolism in adult and pediatric patients across a large healthcare system. J Hosp Med. 2016;11(Suppl 2):S15-S21. PubMed
38. Menendez JJ, Verdu C, Calderon B, et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J Thromb Haemost. 2016;14(11):2158-2168. PubMed
1. Chopra V, Flanders SA, Saint S. The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527-1528. PubMed
2. Gibson C, Connolly BL, Moineddin R, Mahant S, Filipescu D, Amaral JG. Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323-1331. PubMed
3. Ullman AJ, Cooke M, Kleidon T, Rickard CM. Road map for improvement: point prevalence audit and survey of central venous access devices in paediatric acute care. J Paediatr Child Health. 2017;53(2):123-130. PubMed
4. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. PubMed
5. Barrier A, Williams DJ, Connelly M, Creech CB. Frequency of peripherally inserted central catheter complications in children. Pediatr Infect Dis J. 2012;31(5):519-521. PubMed
6. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. PubMed
7. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. PubMed
8. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. PubMed
9. Moureau NL, Trick N, Nifong T, et al. Vessel health and preservation (part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13(3):351-356. PubMed
10. Poli P, Scocca A, Di Puccio F, Gallone G, Angelini L, Calabro EM. A comparative study on the mechanical behavior of polyurethane PICCs. J Vasc Access. 2016;17(2):175-181. PubMed
11. Interface Biologics. Surface modification technology platform. 2017. http://www.interfacebiologics.com/endexo.htm. Accessed April 5, 2017.
12. Hoffer EK, Bloch RD, Borsa JJ, Santulli P, Fontaine AB, Francoeur N. Peripherally inserted central catheters with distal versus proximal valves: prospective randomized trial. J Vasc Interv Radiol. 2001;12(10):1173-1177. PubMed
13. Hoffer EK, Borsa J, Santulli P, Bloch R, Fontaine AB. Prospective randomized comparison of valved versus nonvalved peripherally inserted central vein catheters. AJR Am J Roentgenol. 1999;173(5):1393-1398. PubMed
14. Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and nonvalved peripherally inserted central catheters. J Vasc Access. 2014;15(6):519-523.
15. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
16. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337. PubMed
17. Kleidon TM, Ullman AJ, Zhang L, Mihala G, Rickard CM. How does your PICCOMPARE? A pilot randomized controlled trial comparing PICC materials in pediatrics. J Hosp Med. 2017;(under review). PubMed
18. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180-191. PubMed
19. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. PubMed
20. Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908-918. PubMed
21. Kramer RD, Rogers MA, Conte M, Mann J, Saint S, Chopra V. Are antimicrobial peripherally inserted central catheters associated with reduction in central line-associated bloodstream infection? A systematic review and meta-analysis. Am J Infect Control. 2017;45(2):108-114. PubMed
22. Centers for Disease Control and Prevention. National Healthcare Safety Network Device Associated Module: CLABSI. 2014.
23. Lobo BL, Vaidean G, Broyles J, Reaves AB, Shorr RI. Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417-422. PubMed
24. Smith SN, Moureau N, Vaughn VM, et al. Patterns and Predictors of Peripherally Inserted Central Catheter Occlusion: The 3P-O Study. J Vasc Interv Radiol. 28(5):749.e742-756.e742. PubMed
25. Chow LML, Friedman JN, MacArthur C, et al. Peripherally inserted central catheter (PICC) fracture and embolozation in the pediatric population. Pediatrics. 2003;142(2):141-144. PubMed
26. Chopra V, Kuhn L, Ratz D, Flanders SA, Krein SL. Vascular nursing experience, practice knowledge, and beliefs: Results from the Michigan PICC1 survey. J Hosp Med. 2016;11(4):269-275. PubMed
27. Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14(2):212. PubMed
28. Centre for Healthcare Related Infection Surveilance and Prevention and Tuberculosis Control. Guideline: Peripherally inserted central catheter (PICC). 2013.
29. Services Children’s Health Service. Central venous catheters: nursing care and management of peripherally inserted central catheter (PICC) in paediatric patients. 2011. http://qheps.health.qld.gov.au/childrenshealth/resources/nursestand/docs/ns_03452.pdf. Accessed Februrary 1, 2016.
30. Services CsH. Central Venous Access Device Insertion and Management. 2014.
31. Central venous access device insertion and management. Queensland Government; 2014. http://qheps.health.qld.gov.au/childrenshealth/resources/proc/docs/proc_03450.pdf Accessed March 13, 2014.
32. StatCorp. Stata Statistical Software: Release 12.1 College Station. 2006.
33. Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(1):e9. PubMed
34. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. PubMed
35. Alport B, Burbridge B, Lim H. Bard PowerPICC Solo2 vs Cook Turbo-Ject: A Tale of Two PICCs. Can Assoc Radiol J. 2012;63(4):323-328. PubMed
36. Johnston AJ, Streater CT, Noorani R, Crofts JL, Del Mundo AB, Parker RA. The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates—the ‘ELeCTRiC’ study. J Vasc Access. 2012;13(4):421-425. PubMed
37. Morgenthaler TI, Rodriguez V. Preventing acute care-associated venous thromboembolism in adult and pediatric patients across a large healthcare system. J Hosp Med. 2016;11(Suppl 2):S15-S21. PubMed
38. Menendez JJ, Verdu C, Calderon B, et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J Thromb Haemost. 2016;14(11):2158-2168. PubMed
© 2018 Society of Hospital Medicine
Observational Study of Peripheral Intravenous Catheter Outcomes in Adult Hospitalized Patients: A Multivariable Analysis of Peripheral Intravenous Catheter Failure
INTRODUCTION
Peripheral intravenous catheter (PIV) insertion is the fastest, simplest, and most cost-effective method to gain vascular access, and it is used for short-term intravenous (IV) fluids, medications, blood products, and contrast media.1 It is the most common invasive device in hospitalized patients,2 with up to 70% of hospital patients receiving a PIV.3 Unacceptable PIV failure rates have been reported as high as 69%.4-7 Failure is most frequently due to phlebitis (vein wall irritation/inflammation), occlusion (blockage), infiltration or extravasation (IV fluids/vesicant therapy entering surrounding tissue), partial dislodgement or accidental removal, leakage, and infection.4,6,8 These failures have important implications for patients, who endure the discomfort of PIV complications and catheter replacements, and healthcare staff and budgets.
To reduce the incidence of catheter failure and avoid preventable PIV replacements, a clear understanding of why catheters fail is required. Previous research has identified that catheter gauge,9-11 insertion site,12-14 and inserter skill10,15 have an impact on PIV failure. Limitations of existing research are small study sizes,16-18 retrospective design,19 or secondary analysis of an existing data set; all potentially introduce sampling bias.10,20
To overcome these potential biases, we developed a data collection instrument based on the catheter-associated risk factors described in the literature,9-11,13 and other potential insertion and maintenance risks for PIV failure (eg, multiple insertion attempts, medications administered), with data collected prospectively. The study aim was to improve patient outcomes by identifying PIV insertion and maintenance risk factors amenable to modification through education or alternative clinical interventions, such as catheter gauge selection or insertion site.
METHODS
Study Design and Participants
We conducted this prospective cohort study in a large tertiary hospital in Queensland, Australia. Ethics committee approval was obtained from the hospital (HREC/14/QRBW/76) and Griffith University (NRS/26/14/HREC). The study was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12615000738527). Patients in medical and surgical wards were screened Monday, Wednesday, and Friday between October 2014 and December 2015. Patients over 18 years with a PIV (BD InsyteTM AutoguardTM BC; Becton Dickinson, Franklin Lakes, NJ) inserted within 24 hours, and who were able to provide written informed consent, were eligible and recruited sequentially. Patients classified as palliative by the treating clinical team were excluded.
Sample Size Calculation
The “10 events per variable” rule was used to determine the sample size required to study 50 potential risk factors.21,22 This determined that 1000 patients, with an average of 1.5 PIVs each and an expected PIV failure of 30% (500 events), were required.
Data Collection
At recruitment, baseline patient information was collected by a research nurse (ReNs) (demographics, admitting diagnosis, comorbidities, skin type,23 and vein condition) and entered into an electronic data platform supported by Research Electronic Data Capture (REDCap).24 Baseline data also included catheter variables (eg, gauge, insertion site, catheterized vein) and insertion details (eg, department of insertion, inserting clinician, number of insertion attempts). We included every PIV the participant had during their admission until hospital discharge or insertion of a central venous access device. PIV sites were reviewed Monday, Wednesday, and Friday by ReNs for site complications (eg, redness, pain, swelling, palpable cord). Potential risk factors for failure were also recorded (eg, infusates and additives, antibiotic type and dosage, flushing regimen, number of times the PIV was accessed each day for administration of IV medications or fluids, dressing type and condition, securement method for the catheter and tubing, presence of extension tubing or 3-way taps, patient mobility status, and delirium). A project manager trained and supervised ReNs for protocol compliance and audited study data quality. We considered PIV failure to have occurred if the catheter had complications at removal identified by the ReNs assessment, from medical charts, or by speaking to the patient and beside nurse. We grouped the failures in 1 of 3 types: (1) occlusion or infiltration, defined as blockage, IV fluids moving into surrounding tissue, induration, or swelling greater than 1 cm from the insertion site at or within 24 hours of removal; (2) phlebitis, defined as per clinicians’ definitions or one or more of the following signs and symptoms: pain or tenderness scored at 2 or more on a 1 to 10 increasing severity pain scale, or redness or a palpable cord (either extending greater than 1 cm from the insertion site) at or within 24 hours of PIV removal; and (3) dislodgement (partial or complete). If multiple complications were present, all were recorded.
Statistical Analysis
Data were downloaded from REDcap to Stata 14.2 (StataCorp., College Station, TX) for data management and analysis. Missing data were not imputed. Nominal data observations were collapsed into a single observation per device. Patient and device variables were described as frequencies and proportions, means and standard deviations, or medians and interquartile ranges. Failure incidence rates were calculated, and a Kaplan-Meier survival curve was plotted. In general, Cox proportional hazards models were fitted (Efron method) to handle tied failures (clustering by patient). Variables significant at P < 0.20 on univariable analyses were subjected to multivariable regression. Generally, the largest category was set as referent. Correlations between variables were checked (Spearman’s rank for binary variables, R-squared value of linear regressions for continuous/categorical or continuous/continuous variables). Correlations were considered significant if r > 0.5 and the lower bound of the 95% confidence interval (CI) was >0.5 (where calculated). Covariate interactions were explored, and effects at P < 0.05 noted. The 4 steps of multivariable model building were (1) baseline covariates only with manual stepwise removal of covariates at P ≥ 0.05, (2) treatment covariates only with manual stepwise removal of covariates at P ≥ 0.05, (3) a combination of the derived models from (1) and (2) and manual stepwise removal of covariates at P ≥ 0.05, and (4) manual stepwise addition and removal (at P ≥ 0.05) of variables dropped during the previous steps and interaction testing. Final models were checked as follows: global proportional-hazards assumption test, concordance probability (that predictions and outcomes were in agreement), and Nelson-Aalen cumulative hazard function plotted against the Cox-Snell residuals.
RESULTS
Patient Characteristics
In total, 1000 patients with 1578 PIVs were recruited. The average age was 54 years and the majority were surgical patients (673; 67%). Almost half of patients (455; 46%) had 2 or more comorbidities, and 334 (33%) were obese (body mass index greater than 30). Sample characteristics are shown by the type of catheter failure in Table 1.
PIV Characteristics
All 1578 PIVs were followed until removal, with only 7 PIVs (0.44%) having missing data for the 3 outcomes of interest (these were coded as nonfailures for analysis). Sixty percent of participants had more than 1 PIV followed in the study. Doctors and physicians inserted 1278 (83%) catheters. A total of 550 (35%) were placed in the ward, with 428 (28%) inserted in the emergency department or ambulance. A third of the catheters (540; 34%) were 18-gauge or larger in diameter, and 1000 (64%) were located in the cubital fossa or hand. Multiple insertion attempts were required to place 315 (23%) PIVs. No PIVs were inserted with ultrasound, as this is rarely used in this hospital. The flushing policy was for the administration of 9% sodium chloride every 8 hours if no IV medications or fluids were ordered. Table 2 contains further details of device-related characteristics. Although the hospital policy was for catheter removal by 72 hours, dwell time ranged from <1 to 14 days, with an average of 2.4 days.
PIV Complications
Catheter failure (any cause) occurred in 512 (32%) catheters, which is a failure rate of 136 per 1000 catheter days (95% CI, 125-148). A total of 346 patients out of 1000 (35%) had at least 1 failed PIV during the study. Failures were 267 phlebitis (17%), 228 occlusion/infiltration (14%), and/or 154 dislodgement (10%; Figure), with some PIVs exhibiting multiple concurrent complications (Table 2).
Multivariable AnalysisOcclusion/Infiltration
The multivariable analysis (Table 3) showed occlusion or infiltration was statistically significantly associated with female patients (hazard ratio [HR], 1.48; 95% CI, 1.10-2.00), with a 22-gauge catheter (HR, 1.43; 95% CI, 1.02-2.00), IV flucloxacillin (HR, 1.98; 95% CI, 1.19-3.31), and with frequent PIV access (HR, 1.12; 95% CI, 1.04-1.21; ie, with each increase of 1 in the mean medications/fluids administrations per day, relative PIV failure increased 112%). Less occlusion and infiltration were statistically significantly associated with securement by using additional nonsterile tape (HR, 0.46; 95% CI, 0.33-0.63), elasticized tubular bandages (HR, 0.49; 95% CI, 0.35-0.70 ), or other types of additional securement for the PIV (HR, 0.35; 95% CI, 0.26-0.47).
Phlebitis
Phlebitis was statistically significantly associated with female patients (HR, 1.81; 95% CI, 1.40-2.35), bruising at the insertion site (HR, 2.16; 95% CI, 1.26-3.71), insertion in patients’ dominant side (HR, 1.39; 95% CI, 1.09-1.77), IV flucloxicillin (HR, 2.01; 95% CI, 1.26-3.21), or with frequent PIV access (HR, 1.14; 95% CI, 1.08-1.21). Older age, (HR, 0.99; 95% CI, 0.98-0.99; ie, each year older was associated with 1% less phlebitis), securement with additional nonsterile tape (HR, 0.63; 95% CI, 0.48-0.82) or with any other additional securement (HR, 0.53; 95% CI, 0.39-0.70), or the administration of IV cephazolin (HR, 0.63; 95% CI, 0.44-0.89) were associated with lower phlebitis risk.
Dislodgement
Statistically significant predictors associated with an increased risk of PIV dislodgement included paramedic insertion (HR, 1.78; 95% CI, 1.03-3.06) and frequent PIV access (HR, 1.11; 95% CI, 1.03-1.20). A decreased risk was associated with the additional securement of the PIV, including nonsterile tape (HR, 0.44; 95% CI, 0.31-0.63) or other forms of additional securement (HR, 0.32; 95% CI, 0.22-0.46).
DISCUSSION
One in 3 PIVs failed in this study, with phlebitis as the most common cause of PIV failure. The 17% phlebitis rate reflected clinician-reported phlebitis or phlebitis observed by research staff using a 1-criteria definition because any sign or symptom can trigger PIV removal (eg, pain), even if other signs or symptoms are not present. Reported phlebitis rates are lower if definitions require 2 signs or symptoms.4,6 With over 71 different phlebitis assessment scales in use, and none well validated, the best method for diagnosing phlebitis remains unclear and explains the variation in reported rates.25 Occlusion/infiltration and dislodgement were also highly prevalent forms of PIV failure at 14% and 10%, respectively. Occlusion and infiltration were combined because clinical staff use these terms interchangeably, and differential diagnostic tools are not used in practice. Both result in the same outcome (therapy interruption and PIV removal), and this combination of outcomes has been used previously.23 No PIV-associated bloodstream infections occurred, despite the heightened awareness of these infections in the literature.3
Females had significantly more occlusion/infiltration and phlebitis than males, in keeping with previous studies.7,9,10 This could be because of females’ smaller vein caliber, although the effect remained after adjustment for PIV gauge.7,26 The effect of aging on vascular endothelium and structural integrity may explain the observed decrease in phlebitis of 1% with each older year of age.27 However, gender and age effects could be explained by psychosocial factors (eg, older people may be less likely to admit pain, or we may question them less sympathetically), but, regardless, women and younger patients should be monitored more closely.
We found 22-gauge catheters were more likely to fail from occlusion/infiltration than other sizes. This confirms similar findings from Abolfotouh et al.9 PIV gauge selection for this study was made at the inserter’s discretion and may be confounded by smaller vein size, which was not measured. In addition, risk may be because of smaller gauge alone or also more influenced by the shorter length of the studied 22-gauge (25 mm) than the <20-gauge catheters (30 mm). These results question international guidelines, which currently recommend the smallest gauge peripheral catheter possible,28,29 and randomized trials are needed. Although practice varies between inserters, some preferentially cannulate the nondominant limb. We are not aware of previous studies on this practice; however, our results support this approach.
Flucloxacillin was associated with a 2-fold increase in occlusion/infiltration and phlebitis. Although multiple studies have reported IV medications9,11 and IV antibiotics10,30,31 as risk factors for PIV failure, none have identified flucloxacillin as an independent risk factor. IV flucloxacillin is recommended for reconstitution as 1 g in 15 mL to 20 mL of sterile water, and injection over 3 to 4 minutes, although this may not be adhered to in practice. Alternative administration regimes or improved adherence to current policy may be needed. An exception to the relationship between IV antibiotics and catheter failure was IV cephazolin, associated with 40% relatively less phlebitis. This may be a spurious finding because the administration, pH, and osmolality of cephazolin are similar to other IV antibiotics.
The more PIVs that were accessed per day, whether for infusions or medications, the more failure occurred from occlusion/infiltration, phlebitis, and dislodgement. This suggests that peripheral veins are easily damaged and/or inflamed by the influx of fluids or medications. Lower injection pressures or the timely transfer to oral medications may limit this problem. Flushing regimens may also assist because practice varies greatly, and questions on whether slow continuous flush infusion or intermittent manual flushing are more vein-protective, and the optimal flush volume, frequency, and technique (eg, pulsatile) remain.32,33 Manual handling for frequent access may loosen dressings and securement, thus explaining the observed association between frequent access and catheter dislodgement. Finally, the association between use and failure may indicate that many of these patients were not suitable for a PIV, and different approaches (eg, ultrasound-guided insertion) or a midline may have been a superior option. There is growing emphasis on the need for better preinsertion assessment and selection of the most appropriate device for the patient and the IV treatment required.34
Suboptimal dressings or securements are not unusual in hospitals.35 Despite our policy of PIV securement with bordered transparent dressings, we found 4 dressing types in use. In addition, we found almost 50% of PIVs had an additional (secondary) securement, and this was associated with significantly less PIV failure of all 3 types. This suggests that 1 or more of nonsterile tape, elasticized tubular bandages, or other securement (eg, bandage or second transparent dressing) can reduce PIV failure, although a randomized trial is lacking.36 Whether the dressing was failing and required reinforcement or hospital staff lacked confidence in the dressing and placed additional securement preventatively is unclear. Both PIV failure and PIV dressing failure are common, and further research into superior PIV products and practices is urgently needed. Paramedic insertions had a higher risk of dislodgement, suggesting that the increased emphasis on securement should start in the prehospital setting.
While multiple or difficult insertion attempts were not associated with PIV failure, insertions were not directly observed, and clinicians may have underreported attempts. In contrast, insertion-related bruising (a surrogate for difficult insertion) was associated with more than double the incidence of phlebitis. The long-term implications of multiple insertion attempts on patient’s vasculature are unclear, but we believe first time PIV insertion is important to patients and of interest to clinicians. A recent systematic review of strategies associated with first attempt PIV insertion success in an emergency department found little evidence for effective strategies and recommended further research.37
The overall PIV failure rate in our study was 32%, lower than the 35% to 40% failure observed in our previous randomized controlled trials, which had more stringent inclusion and exclusion criteria (eg, longer predicted duration of therapy).6,38 The implications for patients and costs to the organization of frequent catheter replacement demonstrate urgent need for further research in this area of practice.39 A strength of this study is that all PIVs, regardless of the expected length of dwell time or reason for insertion, were eligible for inclusion, providing more generalizable results. The PIV failure rate of 32% is concerning because these failures trigger treatment delays and replacement insertions, with significant increased labor and equipment costs. The mean cost of PIV replacement has been costed at AUD $69.30 or US $51.92 (as per 2010 $ value) per episode of IV treatment.40 For our hospital, which uses 200,000 PIVs per year, the current level of PIV failure suggests almost AU $5.5 (US $4.1) million in waste annually at this site alone.
The additional strengths of this study include the extensive information collected prospectively about PIV insertion and maintenance, including information on who inserted the PIV, IV medications administered, and PIV dressings used. Limitations were the population of surgical and medical patients in 1 tertiary hospital, which may not be generalizable to other settings.
CONCLUSION
Our study confirms the high rate of catheter failure in acute care hospitals, validates existing evidence related to PIV failure, and identifies new, potentially modifiable risk factors to improve PIV insertion and management. Implications for future research were also identified.
Acknowledgments
The researchers acknowledge and thank the nurses and patients involved in this study. The authors would also like to acknowledge Becton Dickinson for partly funding this study in the form of an unrestricted grant-in-aid paid to Griffith University. Becton Dickinson did not design the study protocol, collect or analyze data, and did not prepare or review the manuscript.
Disclosure
On behalf of NM and CMR, Griffith University has received unrestricted educational and research grants and consultancy payment for lectures from 3M and Becton Dickinson. On behalf of NM, MC, and CMR, Griffith University has received unrestricted investigator-initiated research grants from Centurion Medical Products and Entrotech Lifesciences (manufacturers of PIV dressings) and Becton Dickinson (manufacturer of PIVs). On behalf of MC, Griffith University has received a consultancy payment to develop education material from Baxter. On behalf of CMR, Griffith University has received unrestricted donations or investigator initiated research grants unrelated to this research from Adhezion, Angiodynamics, Baxter, Carefusion, Cook Medical, Hospira, Mayo, Smiths Medical, and Vygon. On behalf of CMR, Griffith University has received consultancy payments for educational lectures or professional opinion from B. Braun, Bard, Carefusion, Mayo, ResQDevices, and Smiths Medical. On behalf of EL, Griffith University has received consultancy payments for educational lecture from 3M. On behalf of MC, Griffith University has received a consultancy payment to develop education material from Baxter. As this was an observational study, no products were trialed in this study. JW and GM have no conflicts of interest.
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
1
2
2
2
2
2
2
2
2
2
2
3
3
3
33. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once
daily maintain peripheral intravenous catheter patency: a randomised controlled
trial. Arch Dis Child. 2015;100(7):700-703. PubMed
34. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide
for Intravenous Catheters (MAGIC): results from a multispecialty panel using
the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):
S1-S40. PubMed
35. New KA, Webster J, Marsh NM, Hewer B. Intravascular device use, management,
documentation and complications: a point prevalence survey. Aust Health Rev.
2014;38(3):345-349. PubMed
36. Marsh N, Webster J, Mihala G, Rickard C. Devices and dressings to secure peripheral
venous catheters to prevent complications. Cochrane Database Syst Rev.
2015(6):CD11070. PubMed
37. Parker SI, Benzies KM, Hayden KA, Lang ES. Effectiveness of interventions for
adult peripheral intravenous catheterization: A systematic review and meta-analysis
of randomized controlled trials. Int Emerg Nurs. 2016;31:15-21. PubMed
38. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base
for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled
trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
39. Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable:
peripheral IV catheter failure. J Infus Nurs. 2015;38(3):189-203. PubMed
40. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically
indicated versus routine replacement of peripheral intravenous catheters. Appl
Health Econ Health Policy. 2014;12(1):51-58. PubMed
INTRODUCTION
Peripheral intravenous catheter (PIV) insertion is the fastest, simplest, and most cost-effective method to gain vascular access, and it is used for short-term intravenous (IV) fluids, medications, blood products, and contrast media.1 It is the most common invasive device in hospitalized patients,2 with up to 70% of hospital patients receiving a PIV.3 Unacceptable PIV failure rates have been reported as high as 69%.4-7 Failure is most frequently due to phlebitis (vein wall irritation/inflammation), occlusion (blockage), infiltration or extravasation (IV fluids/vesicant therapy entering surrounding tissue), partial dislodgement or accidental removal, leakage, and infection.4,6,8 These failures have important implications for patients, who endure the discomfort of PIV complications and catheter replacements, and healthcare staff and budgets.
To reduce the incidence of catheter failure and avoid preventable PIV replacements, a clear understanding of why catheters fail is required. Previous research has identified that catheter gauge,9-11 insertion site,12-14 and inserter skill10,15 have an impact on PIV failure. Limitations of existing research are small study sizes,16-18 retrospective design,19 or secondary analysis of an existing data set; all potentially introduce sampling bias.10,20
To overcome these potential biases, we developed a data collection instrument based on the catheter-associated risk factors described in the literature,9-11,13 and other potential insertion and maintenance risks for PIV failure (eg, multiple insertion attempts, medications administered), with data collected prospectively. The study aim was to improve patient outcomes by identifying PIV insertion and maintenance risk factors amenable to modification through education or alternative clinical interventions, such as catheter gauge selection or insertion site.
METHODS
Study Design and Participants
We conducted this prospective cohort study in a large tertiary hospital in Queensland, Australia. Ethics committee approval was obtained from the hospital (HREC/14/QRBW/76) and Griffith University (NRS/26/14/HREC). The study was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12615000738527). Patients in medical and surgical wards were screened Monday, Wednesday, and Friday between October 2014 and December 2015. Patients over 18 years with a PIV (BD InsyteTM AutoguardTM BC; Becton Dickinson, Franklin Lakes, NJ) inserted within 24 hours, and who were able to provide written informed consent, were eligible and recruited sequentially. Patients classified as palliative by the treating clinical team were excluded.
Sample Size Calculation
The “10 events per variable” rule was used to determine the sample size required to study 50 potential risk factors.21,22 This determined that 1000 patients, with an average of 1.5 PIVs each and an expected PIV failure of 30% (500 events), were required.
Data Collection
At recruitment, baseline patient information was collected by a research nurse (ReNs) (demographics, admitting diagnosis, comorbidities, skin type,23 and vein condition) and entered into an electronic data platform supported by Research Electronic Data Capture (REDCap).24 Baseline data also included catheter variables (eg, gauge, insertion site, catheterized vein) and insertion details (eg, department of insertion, inserting clinician, number of insertion attempts). We included every PIV the participant had during their admission until hospital discharge or insertion of a central venous access device. PIV sites were reviewed Monday, Wednesday, and Friday by ReNs for site complications (eg, redness, pain, swelling, palpable cord). Potential risk factors for failure were also recorded (eg, infusates and additives, antibiotic type and dosage, flushing regimen, number of times the PIV was accessed each day for administration of IV medications or fluids, dressing type and condition, securement method for the catheter and tubing, presence of extension tubing or 3-way taps, patient mobility status, and delirium). A project manager trained and supervised ReNs for protocol compliance and audited study data quality. We considered PIV failure to have occurred if the catheter had complications at removal identified by the ReNs assessment, from medical charts, or by speaking to the patient and beside nurse. We grouped the failures in 1 of 3 types: (1) occlusion or infiltration, defined as blockage, IV fluids moving into surrounding tissue, induration, or swelling greater than 1 cm from the insertion site at or within 24 hours of removal; (2) phlebitis, defined as per clinicians’ definitions or one or more of the following signs and symptoms: pain or tenderness scored at 2 or more on a 1 to 10 increasing severity pain scale, or redness or a palpable cord (either extending greater than 1 cm from the insertion site) at or within 24 hours of PIV removal; and (3) dislodgement (partial or complete). If multiple complications were present, all were recorded.
Statistical Analysis
Data were downloaded from REDcap to Stata 14.2 (StataCorp., College Station, TX) for data management and analysis. Missing data were not imputed. Nominal data observations were collapsed into a single observation per device. Patient and device variables were described as frequencies and proportions, means and standard deviations, or medians and interquartile ranges. Failure incidence rates were calculated, and a Kaplan-Meier survival curve was plotted. In general, Cox proportional hazards models were fitted (Efron method) to handle tied failures (clustering by patient). Variables significant at P < 0.20 on univariable analyses were subjected to multivariable regression. Generally, the largest category was set as referent. Correlations between variables were checked (Spearman’s rank for binary variables, R-squared value of linear regressions for continuous/categorical or continuous/continuous variables). Correlations were considered significant if r > 0.5 and the lower bound of the 95% confidence interval (CI) was >0.5 (where calculated). Covariate interactions were explored, and effects at P < 0.05 noted. The 4 steps of multivariable model building were (1) baseline covariates only with manual stepwise removal of covariates at P ≥ 0.05, (2) treatment covariates only with manual stepwise removal of covariates at P ≥ 0.05, (3) a combination of the derived models from (1) and (2) and manual stepwise removal of covariates at P ≥ 0.05, and (4) manual stepwise addition and removal (at P ≥ 0.05) of variables dropped during the previous steps and interaction testing. Final models were checked as follows: global proportional-hazards assumption test, concordance probability (that predictions and outcomes were in agreement), and Nelson-Aalen cumulative hazard function plotted against the Cox-Snell residuals.
RESULTS
Patient Characteristics
In total, 1000 patients with 1578 PIVs were recruited. The average age was 54 years and the majority were surgical patients (673; 67%). Almost half of patients (455; 46%) had 2 or more comorbidities, and 334 (33%) were obese (body mass index greater than 30). Sample characteristics are shown by the type of catheter failure in Table 1.
PIV Characteristics
All 1578 PIVs were followed until removal, with only 7 PIVs (0.44%) having missing data for the 3 outcomes of interest (these were coded as nonfailures for analysis). Sixty percent of participants had more than 1 PIV followed in the study. Doctors and physicians inserted 1278 (83%) catheters. A total of 550 (35%) were placed in the ward, with 428 (28%) inserted in the emergency department or ambulance. A third of the catheters (540; 34%) were 18-gauge or larger in diameter, and 1000 (64%) were located in the cubital fossa or hand. Multiple insertion attempts were required to place 315 (23%) PIVs. No PIVs were inserted with ultrasound, as this is rarely used in this hospital. The flushing policy was for the administration of 9% sodium chloride every 8 hours if no IV medications or fluids were ordered. Table 2 contains further details of device-related characteristics. Although the hospital policy was for catheter removal by 72 hours, dwell time ranged from <1 to 14 days, with an average of 2.4 days.
PIV Complications
Catheter failure (any cause) occurred in 512 (32%) catheters, which is a failure rate of 136 per 1000 catheter days (95% CI, 125-148). A total of 346 patients out of 1000 (35%) had at least 1 failed PIV during the study. Failures were 267 phlebitis (17%), 228 occlusion/infiltration (14%), and/or 154 dislodgement (10%; Figure), with some PIVs exhibiting multiple concurrent complications (Table 2).
Multivariable AnalysisOcclusion/Infiltration
The multivariable analysis (Table 3) showed occlusion or infiltration was statistically significantly associated with female patients (hazard ratio [HR], 1.48; 95% CI, 1.10-2.00), with a 22-gauge catheter (HR, 1.43; 95% CI, 1.02-2.00), IV flucloxacillin (HR, 1.98; 95% CI, 1.19-3.31), and with frequent PIV access (HR, 1.12; 95% CI, 1.04-1.21; ie, with each increase of 1 in the mean medications/fluids administrations per day, relative PIV failure increased 112%). Less occlusion and infiltration were statistically significantly associated with securement by using additional nonsterile tape (HR, 0.46; 95% CI, 0.33-0.63), elasticized tubular bandages (HR, 0.49; 95% CI, 0.35-0.70 ), or other types of additional securement for the PIV (HR, 0.35; 95% CI, 0.26-0.47).
Phlebitis
Phlebitis was statistically significantly associated with female patients (HR, 1.81; 95% CI, 1.40-2.35), bruising at the insertion site (HR, 2.16; 95% CI, 1.26-3.71), insertion in patients’ dominant side (HR, 1.39; 95% CI, 1.09-1.77), IV flucloxicillin (HR, 2.01; 95% CI, 1.26-3.21), or with frequent PIV access (HR, 1.14; 95% CI, 1.08-1.21). Older age, (HR, 0.99; 95% CI, 0.98-0.99; ie, each year older was associated with 1% less phlebitis), securement with additional nonsterile tape (HR, 0.63; 95% CI, 0.48-0.82) or with any other additional securement (HR, 0.53; 95% CI, 0.39-0.70), or the administration of IV cephazolin (HR, 0.63; 95% CI, 0.44-0.89) were associated with lower phlebitis risk.
Dislodgement
Statistically significant predictors associated with an increased risk of PIV dislodgement included paramedic insertion (HR, 1.78; 95% CI, 1.03-3.06) and frequent PIV access (HR, 1.11; 95% CI, 1.03-1.20). A decreased risk was associated with the additional securement of the PIV, including nonsterile tape (HR, 0.44; 95% CI, 0.31-0.63) or other forms of additional securement (HR, 0.32; 95% CI, 0.22-0.46).
DISCUSSION
One in 3 PIVs failed in this study, with phlebitis as the most common cause of PIV failure. The 17% phlebitis rate reflected clinician-reported phlebitis or phlebitis observed by research staff using a 1-criteria definition because any sign or symptom can trigger PIV removal (eg, pain), even if other signs or symptoms are not present. Reported phlebitis rates are lower if definitions require 2 signs or symptoms.4,6 With over 71 different phlebitis assessment scales in use, and none well validated, the best method for diagnosing phlebitis remains unclear and explains the variation in reported rates.25 Occlusion/infiltration and dislodgement were also highly prevalent forms of PIV failure at 14% and 10%, respectively. Occlusion and infiltration were combined because clinical staff use these terms interchangeably, and differential diagnostic tools are not used in practice. Both result in the same outcome (therapy interruption and PIV removal), and this combination of outcomes has been used previously.23 No PIV-associated bloodstream infections occurred, despite the heightened awareness of these infections in the literature.3
Females had significantly more occlusion/infiltration and phlebitis than males, in keeping with previous studies.7,9,10 This could be because of females’ smaller vein caliber, although the effect remained after adjustment for PIV gauge.7,26 The effect of aging on vascular endothelium and structural integrity may explain the observed decrease in phlebitis of 1% with each older year of age.27 However, gender and age effects could be explained by psychosocial factors (eg, older people may be less likely to admit pain, or we may question them less sympathetically), but, regardless, women and younger patients should be monitored more closely.
We found 22-gauge catheters were more likely to fail from occlusion/infiltration than other sizes. This confirms similar findings from Abolfotouh et al.9 PIV gauge selection for this study was made at the inserter’s discretion and may be confounded by smaller vein size, which was not measured. In addition, risk may be because of smaller gauge alone or also more influenced by the shorter length of the studied 22-gauge (25 mm) than the <20-gauge catheters (30 mm). These results question international guidelines, which currently recommend the smallest gauge peripheral catheter possible,28,29 and randomized trials are needed. Although practice varies between inserters, some preferentially cannulate the nondominant limb. We are not aware of previous studies on this practice; however, our results support this approach.
Flucloxacillin was associated with a 2-fold increase in occlusion/infiltration and phlebitis. Although multiple studies have reported IV medications9,11 and IV antibiotics10,30,31 as risk factors for PIV failure, none have identified flucloxacillin as an independent risk factor. IV flucloxacillin is recommended for reconstitution as 1 g in 15 mL to 20 mL of sterile water, and injection over 3 to 4 minutes, although this may not be adhered to in practice. Alternative administration regimes or improved adherence to current policy may be needed. An exception to the relationship between IV antibiotics and catheter failure was IV cephazolin, associated with 40% relatively less phlebitis. This may be a spurious finding because the administration, pH, and osmolality of cephazolin are similar to other IV antibiotics.
The more PIVs that were accessed per day, whether for infusions or medications, the more failure occurred from occlusion/infiltration, phlebitis, and dislodgement. This suggests that peripheral veins are easily damaged and/or inflamed by the influx of fluids or medications. Lower injection pressures or the timely transfer to oral medications may limit this problem. Flushing regimens may also assist because practice varies greatly, and questions on whether slow continuous flush infusion or intermittent manual flushing are more vein-protective, and the optimal flush volume, frequency, and technique (eg, pulsatile) remain.32,33 Manual handling for frequent access may loosen dressings and securement, thus explaining the observed association between frequent access and catheter dislodgement. Finally, the association between use and failure may indicate that many of these patients were not suitable for a PIV, and different approaches (eg, ultrasound-guided insertion) or a midline may have been a superior option. There is growing emphasis on the need for better preinsertion assessment and selection of the most appropriate device for the patient and the IV treatment required.34
Suboptimal dressings or securements are not unusual in hospitals.35 Despite our policy of PIV securement with bordered transparent dressings, we found 4 dressing types in use. In addition, we found almost 50% of PIVs had an additional (secondary) securement, and this was associated with significantly less PIV failure of all 3 types. This suggests that 1 or more of nonsterile tape, elasticized tubular bandages, or other securement (eg, bandage or second transparent dressing) can reduce PIV failure, although a randomized trial is lacking.36 Whether the dressing was failing and required reinforcement or hospital staff lacked confidence in the dressing and placed additional securement preventatively is unclear. Both PIV failure and PIV dressing failure are common, and further research into superior PIV products and practices is urgently needed. Paramedic insertions had a higher risk of dislodgement, suggesting that the increased emphasis on securement should start in the prehospital setting.
While multiple or difficult insertion attempts were not associated with PIV failure, insertions were not directly observed, and clinicians may have underreported attempts. In contrast, insertion-related bruising (a surrogate for difficult insertion) was associated with more than double the incidence of phlebitis. The long-term implications of multiple insertion attempts on patient’s vasculature are unclear, but we believe first time PIV insertion is important to patients and of interest to clinicians. A recent systematic review of strategies associated with first attempt PIV insertion success in an emergency department found little evidence for effective strategies and recommended further research.37
The overall PIV failure rate in our study was 32%, lower than the 35% to 40% failure observed in our previous randomized controlled trials, which had more stringent inclusion and exclusion criteria (eg, longer predicted duration of therapy).6,38 The implications for patients and costs to the organization of frequent catheter replacement demonstrate urgent need for further research in this area of practice.39 A strength of this study is that all PIVs, regardless of the expected length of dwell time or reason for insertion, were eligible for inclusion, providing more generalizable results. The PIV failure rate of 32% is concerning because these failures trigger treatment delays and replacement insertions, with significant increased labor and equipment costs. The mean cost of PIV replacement has been costed at AUD $69.30 or US $51.92 (as per 2010 $ value) per episode of IV treatment.40 For our hospital, which uses 200,000 PIVs per year, the current level of PIV failure suggests almost AU $5.5 (US $4.1) million in waste annually at this site alone.
The additional strengths of this study include the extensive information collected prospectively about PIV insertion and maintenance, including information on who inserted the PIV, IV medications administered, and PIV dressings used. Limitations were the population of surgical and medical patients in 1 tertiary hospital, which may not be generalizable to other settings.
CONCLUSION
Our study confirms the high rate of catheter failure in acute care hospitals, validates existing evidence related to PIV failure, and identifies new, potentially modifiable risk factors to improve PIV insertion and management. Implications for future research were also identified.
Acknowledgments
The researchers acknowledge and thank the nurses and patients involved in this study. The authors would also like to acknowledge Becton Dickinson for partly funding this study in the form of an unrestricted grant-in-aid paid to Griffith University. Becton Dickinson did not design the study protocol, collect or analyze data, and did not prepare or review the manuscript.
Disclosure
On behalf of NM and CMR, Griffith University has received unrestricted educational and research grants and consultancy payment for lectures from 3M and Becton Dickinson. On behalf of NM, MC, and CMR, Griffith University has received unrestricted investigator-initiated research grants from Centurion Medical Products and Entrotech Lifesciences (manufacturers of PIV dressings) and Becton Dickinson (manufacturer of PIVs). On behalf of MC, Griffith University has received a consultancy payment to develop education material from Baxter. On behalf of CMR, Griffith University has received unrestricted donations or investigator initiated research grants unrelated to this research from Adhezion, Angiodynamics, Baxter, Carefusion, Cook Medical, Hospira, Mayo, Smiths Medical, and Vygon. On behalf of CMR, Griffith University has received consultancy payments for educational lectures or professional opinion from B. Braun, Bard, Carefusion, Mayo, ResQDevices, and Smiths Medical. On behalf of EL, Griffith University has received consultancy payments for educational lecture from 3M. On behalf of MC, Griffith University has received a consultancy payment to develop education material from Baxter. As this was an observational study, no products were trialed in this study. JW and GM have no conflicts of interest.
INTRODUCTION
Peripheral intravenous catheter (PIV) insertion is the fastest, simplest, and most cost-effective method to gain vascular access, and it is used for short-term intravenous (IV) fluids, medications, blood products, and contrast media.1 It is the most common invasive device in hospitalized patients,2 with up to 70% of hospital patients receiving a PIV.3 Unacceptable PIV failure rates have been reported as high as 69%.4-7 Failure is most frequently due to phlebitis (vein wall irritation/inflammation), occlusion (blockage), infiltration or extravasation (IV fluids/vesicant therapy entering surrounding tissue), partial dislodgement or accidental removal, leakage, and infection.4,6,8 These failures have important implications for patients, who endure the discomfort of PIV complications and catheter replacements, and healthcare staff and budgets.
To reduce the incidence of catheter failure and avoid preventable PIV replacements, a clear understanding of why catheters fail is required. Previous research has identified that catheter gauge,9-11 insertion site,12-14 and inserter skill10,15 have an impact on PIV failure. Limitations of existing research are small study sizes,16-18 retrospective design,19 or secondary analysis of an existing data set; all potentially introduce sampling bias.10,20
To overcome these potential biases, we developed a data collection instrument based on the catheter-associated risk factors described in the literature,9-11,13 and other potential insertion and maintenance risks for PIV failure (eg, multiple insertion attempts, medications administered), with data collected prospectively. The study aim was to improve patient outcomes by identifying PIV insertion and maintenance risk factors amenable to modification through education or alternative clinical interventions, such as catheter gauge selection or insertion site.
METHODS
Study Design and Participants
We conducted this prospective cohort study in a large tertiary hospital in Queensland, Australia. Ethics committee approval was obtained from the hospital (HREC/14/QRBW/76) and Griffith University (NRS/26/14/HREC). The study was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12615000738527). Patients in medical and surgical wards were screened Monday, Wednesday, and Friday between October 2014 and December 2015. Patients over 18 years with a PIV (BD InsyteTM AutoguardTM BC; Becton Dickinson, Franklin Lakes, NJ) inserted within 24 hours, and who were able to provide written informed consent, were eligible and recruited sequentially. Patients classified as palliative by the treating clinical team were excluded.
Sample Size Calculation
The “10 events per variable” rule was used to determine the sample size required to study 50 potential risk factors.21,22 This determined that 1000 patients, with an average of 1.5 PIVs each and an expected PIV failure of 30% (500 events), were required.
Data Collection
At recruitment, baseline patient information was collected by a research nurse (ReNs) (demographics, admitting diagnosis, comorbidities, skin type,23 and vein condition) and entered into an electronic data platform supported by Research Electronic Data Capture (REDCap).24 Baseline data also included catheter variables (eg, gauge, insertion site, catheterized vein) and insertion details (eg, department of insertion, inserting clinician, number of insertion attempts). We included every PIV the participant had during their admission until hospital discharge or insertion of a central venous access device. PIV sites were reviewed Monday, Wednesday, and Friday by ReNs for site complications (eg, redness, pain, swelling, palpable cord). Potential risk factors for failure were also recorded (eg, infusates and additives, antibiotic type and dosage, flushing regimen, number of times the PIV was accessed each day for administration of IV medications or fluids, dressing type and condition, securement method for the catheter and tubing, presence of extension tubing or 3-way taps, patient mobility status, and delirium). A project manager trained and supervised ReNs for protocol compliance and audited study data quality. We considered PIV failure to have occurred if the catheter had complications at removal identified by the ReNs assessment, from medical charts, or by speaking to the patient and beside nurse. We grouped the failures in 1 of 3 types: (1) occlusion or infiltration, defined as blockage, IV fluids moving into surrounding tissue, induration, or swelling greater than 1 cm from the insertion site at or within 24 hours of removal; (2) phlebitis, defined as per clinicians’ definitions or one or more of the following signs and symptoms: pain or tenderness scored at 2 or more on a 1 to 10 increasing severity pain scale, or redness or a palpable cord (either extending greater than 1 cm from the insertion site) at or within 24 hours of PIV removal; and (3) dislodgement (partial or complete). If multiple complications were present, all were recorded.
Statistical Analysis
Data were downloaded from REDcap to Stata 14.2 (StataCorp., College Station, TX) for data management and analysis. Missing data were not imputed. Nominal data observations were collapsed into a single observation per device. Patient and device variables were described as frequencies and proportions, means and standard deviations, or medians and interquartile ranges. Failure incidence rates were calculated, and a Kaplan-Meier survival curve was plotted. In general, Cox proportional hazards models were fitted (Efron method) to handle tied failures (clustering by patient). Variables significant at P < 0.20 on univariable analyses were subjected to multivariable regression. Generally, the largest category was set as referent. Correlations between variables were checked (Spearman’s rank for binary variables, R-squared value of linear regressions for continuous/categorical or continuous/continuous variables). Correlations were considered significant if r > 0.5 and the lower bound of the 95% confidence interval (CI) was >0.5 (where calculated). Covariate interactions were explored, and effects at P < 0.05 noted. The 4 steps of multivariable model building were (1) baseline covariates only with manual stepwise removal of covariates at P ≥ 0.05, (2) treatment covariates only with manual stepwise removal of covariates at P ≥ 0.05, (3) a combination of the derived models from (1) and (2) and manual stepwise removal of covariates at P ≥ 0.05, and (4) manual stepwise addition and removal (at P ≥ 0.05) of variables dropped during the previous steps and interaction testing. Final models were checked as follows: global proportional-hazards assumption test, concordance probability (that predictions and outcomes were in agreement), and Nelson-Aalen cumulative hazard function plotted against the Cox-Snell residuals.
RESULTS
Patient Characteristics
In total, 1000 patients with 1578 PIVs were recruited. The average age was 54 years and the majority were surgical patients (673; 67%). Almost half of patients (455; 46%) had 2 or more comorbidities, and 334 (33%) were obese (body mass index greater than 30). Sample characteristics are shown by the type of catheter failure in Table 1.
PIV Characteristics
All 1578 PIVs were followed until removal, with only 7 PIVs (0.44%) having missing data for the 3 outcomes of interest (these were coded as nonfailures for analysis). Sixty percent of participants had more than 1 PIV followed in the study. Doctors and physicians inserted 1278 (83%) catheters. A total of 550 (35%) were placed in the ward, with 428 (28%) inserted in the emergency department or ambulance. A third of the catheters (540; 34%) were 18-gauge or larger in diameter, and 1000 (64%) were located in the cubital fossa or hand. Multiple insertion attempts were required to place 315 (23%) PIVs. No PIVs were inserted with ultrasound, as this is rarely used in this hospital. The flushing policy was for the administration of 9% sodium chloride every 8 hours if no IV medications or fluids were ordered. Table 2 contains further details of device-related characteristics. Although the hospital policy was for catheter removal by 72 hours, dwell time ranged from <1 to 14 days, with an average of 2.4 days.
PIV Complications
Catheter failure (any cause) occurred in 512 (32%) catheters, which is a failure rate of 136 per 1000 catheter days (95% CI, 125-148). A total of 346 patients out of 1000 (35%) had at least 1 failed PIV during the study. Failures were 267 phlebitis (17%), 228 occlusion/infiltration (14%), and/or 154 dislodgement (10%; Figure), with some PIVs exhibiting multiple concurrent complications (Table 2).
Multivariable AnalysisOcclusion/Infiltration
The multivariable analysis (Table 3) showed occlusion or infiltration was statistically significantly associated with female patients (hazard ratio [HR], 1.48; 95% CI, 1.10-2.00), with a 22-gauge catheter (HR, 1.43; 95% CI, 1.02-2.00), IV flucloxacillin (HR, 1.98; 95% CI, 1.19-3.31), and with frequent PIV access (HR, 1.12; 95% CI, 1.04-1.21; ie, with each increase of 1 in the mean medications/fluids administrations per day, relative PIV failure increased 112%). Less occlusion and infiltration were statistically significantly associated with securement by using additional nonsterile tape (HR, 0.46; 95% CI, 0.33-0.63), elasticized tubular bandages (HR, 0.49; 95% CI, 0.35-0.70 ), or other types of additional securement for the PIV (HR, 0.35; 95% CI, 0.26-0.47).
Phlebitis
Phlebitis was statistically significantly associated with female patients (HR, 1.81; 95% CI, 1.40-2.35), bruising at the insertion site (HR, 2.16; 95% CI, 1.26-3.71), insertion in patients’ dominant side (HR, 1.39; 95% CI, 1.09-1.77), IV flucloxicillin (HR, 2.01; 95% CI, 1.26-3.21), or with frequent PIV access (HR, 1.14; 95% CI, 1.08-1.21). Older age, (HR, 0.99; 95% CI, 0.98-0.99; ie, each year older was associated with 1% less phlebitis), securement with additional nonsterile tape (HR, 0.63; 95% CI, 0.48-0.82) or with any other additional securement (HR, 0.53; 95% CI, 0.39-0.70), or the administration of IV cephazolin (HR, 0.63; 95% CI, 0.44-0.89) were associated with lower phlebitis risk.
Dislodgement
Statistically significant predictors associated with an increased risk of PIV dislodgement included paramedic insertion (HR, 1.78; 95% CI, 1.03-3.06) and frequent PIV access (HR, 1.11; 95% CI, 1.03-1.20). A decreased risk was associated with the additional securement of the PIV, including nonsterile tape (HR, 0.44; 95% CI, 0.31-0.63) or other forms of additional securement (HR, 0.32; 95% CI, 0.22-0.46).
DISCUSSION
One in 3 PIVs failed in this study, with phlebitis as the most common cause of PIV failure. The 17% phlebitis rate reflected clinician-reported phlebitis or phlebitis observed by research staff using a 1-criteria definition because any sign or symptom can trigger PIV removal (eg, pain), even if other signs or symptoms are not present. Reported phlebitis rates are lower if definitions require 2 signs or symptoms.4,6 With over 71 different phlebitis assessment scales in use, and none well validated, the best method for diagnosing phlebitis remains unclear and explains the variation in reported rates.25 Occlusion/infiltration and dislodgement were also highly prevalent forms of PIV failure at 14% and 10%, respectively. Occlusion and infiltration were combined because clinical staff use these terms interchangeably, and differential diagnostic tools are not used in practice. Both result in the same outcome (therapy interruption and PIV removal), and this combination of outcomes has been used previously.23 No PIV-associated bloodstream infections occurred, despite the heightened awareness of these infections in the literature.3
Females had significantly more occlusion/infiltration and phlebitis than males, in keeping with previous studies.7,9,10 This could be because of females’ smaller vein caliber, although the effect remained after adjustment for PIV gauge.7,26 The effect of aging on vascular endothelium and structural integrity may explain the observed decrease in phlebitis of 1% with each older year of age.27 However, gender and age effects could be explained by psychosocial factors (eg, older people may be less likely to admit pain, or we may question them less sympathetically), but, regardless, women and younger patients should be monitored more closely.
We found 22-gauge catheters were more likely to fail from occlusion/infiltration than other sizes. This confirms similar findings from Abolfotouh et al.9 PIV gauge selection for this study was made at the inserter’s discretion and may be confounded by smaller vein size, which was not measured. In addition, risk may be because of smaller gauge alone or also more influenced by the shorter length of the studied 22-gauge (25 mm) than the <20-gauge catheters (30 mm). These results question international guidelines, which currently recommend the smallest gauge peripheral catheter possible,28,29 and randomized trials are needed. Although practice varies between inserters, some preferentially cannulate the nondominant limb. We are not aware of previous studies on this practice; however, our results support this approach.
Flucloxacillin was associated with a 2-fold increase in occlusion/infiltration and phlebitis. Although multiple studies have reported IV medications9,11 and IV antibiotics10,30,31 as risk factors for PIV failure, none have identified flucloxacillin as an independent risk factor. IV flucloxacillin is recommended for reconstitution as 1 g in 15 mL to 20 mL of sterile water, and injection over 3 to 4 minutes, although this may not be adhered to in practice. Alternative administration regimes or improved adherence to current policy may be needed. An exception to the relationship between IV antibiotics and catheter failure was IV cephazolin, associated with 40% relatively less phlebitis. This may be a spurious finding because the administration, pH, and osmolality of cephazolin are similar to other IV antibiotics.
The more PIVs that were accessed per day, whether for infusions or medications, the more failure occurred from occlusion/infiltration, phlebitis, and dislodgement. This suggests that peripheral veins are easily damaged and/or inflamed by the influx of fluids or medications. Lower injection pressures or the timely transfer to oral medications may limit this problem. Flushing regimens may also assist because practice varies greatly, and questions on whether slow continuous flush infusion or intermittent manual flushing are more vein-protective, and the optimal flush volume, frequency, and technique (eg, pulsatile) remain.32,33 Manual handling for frequent access may loosen dressings and securement, thus explaining the observed association between frequent access and catheter dislodgement. Finally, the association between use and failure may indicate that many of these patients were not suitable for a PIV, and different approaches (eg, ultrasound-guided insertion) or a midline may have been a superior option. There is growing emphasis on the need for better preinsertion assessment and selection of the most appropriate device for the patient and the IV treatment required.34
Suboptimal dressings or securements are not unusual in hospitals.35 Despite our policy of PIV securement with bordered transparent dressings, we found 4 dressing types in use. In addition, we found almost 50% of PIVs had an additional (secondary) securement, and this was associated with significantly less PIV failure of all 3 types. This suggests that 1 or more of nonsterile tape, elasticized tubular bandages, or other securement (eg, bandage or second transparent dressing) can reduce PIV failure, although a randomized trial is lacking.36 Whether the dressing was failing and required reinforcement or hospital staff lacked confidence in the dressing and placed additional securement preventatively is unclear. Both PIV failure and PIV dressing failure are common, and further research into superior PIV products and practices is urgently needed. Paramedic insertions had a higher risk of dislodgement, suggesting that the increased emphasis on securement should start in the prehospital setting.
While multiple or difficult insertion attempts were not associated with PIV failure, insertions were not directly observed, and clinicians may have underreported attempts. In contrast, insertion-related bruising (a surrogate for difficult insertion) was associated with more than double the incidence of phlebitis. The long-term implications of multiple insertion attempts on patient’s vasculature are unclear, but we believe first time PIV insertion is important to patients and of interest to clinicians. A recent systematic review of strategies associated with first attempt PIV insertion success in an emergency department found little evidence for effective strategies and recommended further research.37
The overall PIV failure rate in our study was 32%, lower than the 35% to 40% failure observed in our previous randomized controlled trials, which had more stringent inclusion and exclusion criteria (eg, longer predicted duration of therapy).6,38 The implications for patients and costs to the organization of frequent catheter replacement demonstrate urgent need for further research in this area of practice.39 A strength of this study is that all PIVs, regardless of the expected length of dwell time or reason for insertion, were eligible for inclusion, providing more generalizable results. The PIV failure rate of 32% is concerning because these failures trigger treatment delays and replacement insertions, with significant increased labor and equipment costs. The mean cost of PIV replacement has been costed at AUD $69.30 or US $51.92 (as per 2010 $ value) per episode of IV treatment.40 For our hospital, which uses 200,000 PIVs per year, the current level of PIV failure suggests almost AU $5.5 (US $4.1) million in waste annually at this site alone.
The additional strengths of this study include the extensive information collected prospectively about PIV insertion and maintenance, including information on who inserted the PIV, IV medications administered, and PIV dressings used. Limitations were the population of surgical and medical patients in 1 tertiary hospital, which may not be generalizable to other settings.
CONCLUSION
Our study confirms the high rate of catheter failure in acute care hospitals, validates existing evidence related to PIV failure, and identifies new, potentially modifiable risk factors to improve PIV insertion and management. Implications for future research were also identified.
Acknowledgments
The researchers acknowledge and thank the nurses and patients involved in this study. The authors would also like to acknowledge Becton Dickinson for partly funding this study in the form of an unrestricted grant-in-aid paid to Griffith University. Becton Dickinson did not design the study protocol, collect or analyze data, and did not prepare or review the manuscript.
Disclosure
On behalf of NM and CMR, Griffith University has received unrestricted educational and research grants and consultancy payment for lectures from 3M and Becton Dickinson. On behalf of NM, MC, and CMR, Griffith University has received unrestricted investigator-initiated research grants from Centurion Medical Products and Entrotech Lifesciences (manufacturers of PIV dressings) and Becton Dickinson (manufacturer of PIVs). On behalf of MC, Griffith University has received a consultancy payment to develop education material from Baxter. On behalf of CMR, Griffith University has received unrestricted donations or investigator initiated research grants unrelated to this research from Adhezion, Angiodynamics, Baxter, Carefusion, Cook Medical, Hospira, Mayo, Smiths Medical, and Vygon. On behalf of CMR, Griffith University has received consultancy payments for educational lectures or professional opinion from B. Braun, Bard, Carefusion, Mayo, ResQDevices, and Smiths Medical. On behalf of EL, Griffith University has received consultancy payments for educational lecture from 3M. On behalf of MC, Griffith University has received a consultancy payment to develop education material from Baxter. As this was an observational study, no products were trialed in this study. JW and GM have no conflicts of interest.
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
1
2
2
2
2
2
2
2
2
2
2
3
3
3
33. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once
daily maintain peripheral intravenous catheter patency: a randomised controlled
trial. Arch Dis Child. 2015;100(7):700-703. PubMed
34. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide
for Intravenous Catheters (MAGIC): results from a multispecialty panel using
the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):
S1-S40. PubMed
35. New KA, Webster J, Marsh NM, Hewer B. Intravascular device use, management,
documentation and complications: a point prevalence survey. Aust Health Rev.
2014;38(3):345-349. PubMed
36. Marsh N, Webster J, Mihala G, Rickard C. Devices and dressings to secure peripheral
venous catheters to prevent complications. Cochrane Database Syst Rev.
2015(6):CD11070. PubMed
37. Parker SI, Benzies KM, Hayden KA, Lang ES. Effectiveness of interventions for
adult peripheral intravenous catheterization: A systematic review and meta-analysis
of randomized controlled trials. Int Emerg Nurs. 2016;31:15-21. PubMed
38. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base
for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled
trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
39. Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable:
peripheral IV catheter failure. J Infus Nurs. 2015;38(3):189-203. PubMed
40. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically
indicated versus routine replacement of peripheral intravenous catheters. Appl
Health Econ Health Policy. 2014;12(1):51-58. PubMed
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
1
2
2
2
2
2
2
2
2
2
2
3
3
3
33. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once
daily maintain peripheral intravenous catheter patency: a randomised controlled
trial. Arch Dis Child. 2015;100(7):700-703. PubMed
34. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide
for Intravenous Catheters (MAGIC): results from a multispecialty panel using
the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):
S1-S40. PubMed
35. New KA, Webster J, Marsh NM, Hewer B. Intravascular device use, management,
documentation and complications: a point prevalence survey. Aust Health Rev.
2014;38(3):345-349. PubMed
36. Marsh N, Webster J, Mihala G, Rickard C. Devices and dressings to secure peripheral
venous catheters to prevent complications. Cochrane Database Syst Rev.
2015(6):CD11070. PubMed
37. Parker SI, Benzies KM, Hayden KA, Lang ES. Effectiveness of interventions for
adult peripheral intravenous catheterization: A systematic review and meta-analysis
of randomized controlled trials. Int Emerg Nurs. 2016;31:15-21. PubMed
38. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base
for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled
trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
39. Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable:
peripheral IV catheter failure. J Infus Nurs. 2015;38(3):189-203. PubMed
40. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically
indicated versus routine replacement of peripheral intravenous catheters. Appl
Health Econ Health Policy. 2014;12(1):51-58. PubMed
© 2017 Society of Hospital Medicine
Tools, Clinical Prediction Rules, and Algorithms for the Insertion of Peripheral Intravenous Catheters in Adult Hospitalized Patients: A Systematic Scoping Review of Literature
Up to a billion peripheral intravenous catheters (PIVCs) are inserted annually; therefore, the importance of this invasive device in modern medicine cannot be argued.1 The insertion of a PIVC is a clinical procedure undertaken by a range of clinical staff and in a variety of patient populations and settings. In many clinical environments (for example, the emergency department [ED]), PIVCs are the predominant first-choice vascular access device (VAD).2,3 Researchers in one study estimated over 25 million PIVCs are used in French EDs each year,3 and intravenous therapy is the leading ED treatment in the United States.4
The purpose of this systematic scoping review was to investigate what PIVC decision-making approaches exist to facilitate FTIS of PIVCs in adult hospitalized patients. Our intention was to systematically synthesize the research on TRAs, to review significant associations identified with these TRAs, and to critique TRA validity and reliability.
METHODS
Scoping Review
We selected a scoping review method that, by definition, maps the evidence to identify gaps,13,14 set research agendas, and identify implications for decision making. This allowed a targeted approach to answering our 3 research questions:
- What published clinical TRAs exist to facilitate PIVC insertion in adults?
- What clinical, patient and/or product variables have been identified using TRAs as having significant associations with FTIS for PIVCs in adult patients?
- What is the reported reliability, validity, responsiveness, clinical feasibility, and utility of existing TRAs for PIVC insertion in adults?
Our aim was to identify the amount, variety and essential qualities of TRA literature rather than to critically appraise and evaluate the effectiveness of TRAs, a process reserved for systematic review and meta-analysis of interventional studies.13,14 We followed scoping review guidelines published by members and collaborators of the Joanna Briggs Institute, an internationally recognized leader in research synthesis, evidence use, and implementation. The guidance is based on 5 steps: (i) scoping review objective and question, (ii) background of the topic to support scoping review, (iii) study selection, (iv) charting the results, and (v) collating and summarizing results.15 Clinicometric assessment of a TRA or any clinical prediction rule requires 4 specific phases: (i) development (identification of predictors from data), (ii) validation (testing the rule in a separate population for reliability), (iii) impact analysis or responsiveness (How clinically useful is the rule in the clinical setting? Is it resource heavy or light? Is it cost effective?), and (iv) implementation and adoption (uptake into clinical practice).16
Search Strategy
We included studies that described the use or development of any TRA regarding PIVC insertion in the adult hospitalized population.
Inclusion Criteria
Studies were included if they were published in the English Language, included TRAs for PIVC insertion in adult hospital patients, and prospectively assessed a clinical category of patient for PIVC insertion using a traditional approach. We defined a traditional PIVC insertion approach as an assessment and/or insertion with touch and feel, therefore, without vessel-locating technology such as ultrasound and/or near infrared technology.
Exclusion Criteria
Exclusion criteria included pediatric studies, authors’ personal (nonresearch) experience of tools, TRAs focused on postinsertion assessment of the cannula (such as phlebitis, infiltration, and/or dressing failure), and papers with a focus on VADs other than PIVCs. We excluded studies using PIVC ultrasound and/or near infrared technology because these are not standard in all insertions and greatly change the information available for pre-insertion assessment as well as the likelihood of insertion success.
In June 2016, a systematic search of the Cochrane library, Ovid Medline® In-process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>, EBSCO CINAHL databases, and Google Scholar with specific keywords to identify publications that identified or defined TRAs was undertaken. Medical subject headings were created with assistance from a research librarian using tailored functions within individual databases. With key search terms, we limited studies to those related to our inclusion criteria. See Appendix 1 for our search strategy for Medline and CINAHL.
We used Covidence, a web-based application specifically designed for systematic reviews to screen and evaluate eligible publications.17 Two authors (PJC and NSH) screened the initial retrieved searches based upon the predetermined inclusion and exclusion criteria.
Data Extraction
A paper template was developed and used by 2 reviewers (P.J.C. and N.S.H.). Data included the following: study sample, aim(s), design, setting and country in which the study took place, clinical and patient variables, and how the TRAs were developed and tested. Studies were categorized by TRA type. We also sought to identify if clinical trial registration (where appropriate) was evidenced, in addition to evidence of protocol publication and what standardized reporting guidelines were used (such as those outlined by the EQUATOR Network).18
Data Synthesis
Formal meta-analysis was beyond the scope and intention of this review. However, we provide the FTIS rate and the ranges of odds ratios (ORs) with 95% confidence intervals (CIs) for certain independent predictors.
RESULTS
Thirty-six references were imported for screening against title and abstract content, with 11 studies excluded and 25 studies assessed for full-text eligibility (see Figure, PRISMA Flowchart). We then excluded a further 12 studies (6 did not meet inclusion criteria, 2 were focused on the prehospital setting, 2 were personal correspondence and focused on another type of VAD, 1 was a protocol to establish a TRA, and 1 was a framework for all device types), leaving 13 studies included in the final review (see Figure). These studies presented data on 4 tools,19-22 4 predictive models3,23-25 (of which 3 present receiver operating characteristic/area under the curve scores),3,23,24 2 framed as risk factor studies,26,27 and 1 of each of the following: a scale,28 a score,29 and an estimation of the incidence report rate (Table 1).30 Seven studies had “difficult” or “difficulty” in their title as a term to use to describe insertion failure.3,19,24-27,30 One study was titled exclusively for the nursing profession,20 5 studies were reported in medical journals,3,24,26,29,30 and 6 were reported in nursing journals,19-22,25,27 with the remainder published in a vascular access journal.23,28
General Characteristics of Included Studies
One TRA which was registered as a clinical trial24 involved a standardized reporting tool as is recommended by the EQUATOR Network.18
Nine of the 13 papers reported that TRA components were chosen based on identified predictors of successful insertion from observational data3,19,23-28,30, with 5 papers using multivariate logistic regression to identify independent predictors.3,23,24,26,2 At least 4330 insertion attempts on patients were reported. Seven papers reported FTIS, which ranged from 61%-90%.3,23-27,30
Two clinical settings accounted for 10 of the 13 included studies. We identified 5 papers from the ED setting3,23,26,29,30 and 5 studies specific to cancer settings.19-22,28 Two ED papers identified clinical predictors of insertion difficulty, with 1 identifying an existing medical diagnosis (such as sickle cell disease, diabetes, or intravenous drug abuse) and the other reporting a pragmatic patient self-report of difficulty.26,30 Three studies focused on patient-exclusive variables (such as vein characteristics)19,21,28 and some with a combined clinician and patient focus.3,23-25,27,30Relatively few studies reported interobserver measurements to describe the reliability of clinical assessments made.3,19,21,28 Webster et al. in Australia assessed interrater reliability of a vein assessment tool (VAT) and found high agreement (kappa 0.83 for medical imaging nurses and 0.93 for oncology nurses).21 Wells compared reliability with Altman’s K scores obtained from a different VAT when compared with the Deciding on Intravenous Access tool and found good agreement.22 Vein deterioration was proposed as a variable for inclusion when developing an assessment tool within an oncological context.31 In Spain, de la Torre and colleagues28 demonstrated good interrater agreement (with kappa, 0.77) for the Venous International Assessment (VIA) tool. The VIA offers a grading system scale to predict the patient’s declining vessel size while undergoing chemotherapy via peripheral veins with PIVCs. Grade I suggests little or no insertion failure, whereas a Grade V should predict insertion failure.
Patient Variables
Vein characteristics were significant independent factors associated with insertion success in a number of studies.3,19,23,24,27,28 These included the number of veins, descriptive quality (eg, small, medium, large), size, location, visible veins, and palpable veins. Other factors appear to be patient specific (such as chronic conditions), including diabetes (OR, 2.1 [adjusted to identify demographic risk factors]; 95% CI, 1.3-3.4), sickle cell disease (OR, 3.5; 95% CI, 1.4-4.8), and intravenous drug abuse (OR, 2.4; 95% CI, 1.1-5.3).26 It is unclear if a consistent relationship between weight classification and insertion outcomes exists. Despite a finding that BMI was not independently associated with insertion difficulty,26 one study reports that BMI was independently associated with insertion failure (BMI <18.5 [OR, 2.24; 95% CI, 1.07-4.67], BMI >30 [OR, 1.98; 95% CI, 1.9-3.60])3 and another reports emaciated patients were associated with greater failure when compared to normal weight patients (OR, 0.07; 95% CI, 0.02-0.34).23 Consequently, extremes of BMI appear to be associated with insertion outcomes despite 1 study reporting no significant association with BMI as an independent factor of insertion failure.26 A history of difficult intravenous access (DIVA) was reported in 1 study and independently associated with insertion failure (OR, 3.86; 95% CI, 2.39-6.25; see Table 2). DIVA appears to be the motivating factor in the title of 7 studies. When defined, the definitions of DIVA are heterogeneous and varied and include the following: >1 minute to insert a PIVC and requiring >1 attempt27; 2 failed attempts30; 3 or more PIVC attempts.26 In the remaining 4 studies, variables associated with difficulty are identified and, therefore, TRAs to target those in future with predicted difficulty prior to any attempts are proposed.3,19,24,25
Clinician Variables
Specialist nurse certification, years of experience, and self-report skill level (P < 0.001) appear to be significantly associated with successful insertions.25 This is in part validated in another study reporting greater procedural inserting PIVCs as an independent predictor of success (OR, 4.404; 95% CI, 1.61-12-06; see Table 2).23 Two studies involved simple pragmatic percentage cut offs for PIVCs: likelihood of use29 and likelihood of insertion success.23 One paper using a cross-sectional design that surveyed ED clinicians suggested if the clinician’s predicted likelihood of the patient needing a PIVC was >80%, this was a reasonable trigger for PIVC insertion.29 The other, in a self-report cohort study, reported that a clinician’s likelihood estimation of PIVC FTIS prior to insertion is independently associated with FTIS (OR, 1.06; 95% CI, 1.04-1.07).23
Product Variables
In this review, higher failure rates were identified in smaller sizes (22-24 g).26 One study revealed gauge size was significantly associated with a failed first attempt in a univariate analysis (OR, 0.44; 95% CI, 0.34-0.58), but this was not retained in a multivariate model.24 Matching the PIVC size with vein assessment is considered in the VIA tool.28 It suggests a large PIVC (18 g) can be considered in patients with at least 6 vein options; smaller PIVCs of 22 to 24 g are recommended when 3 or fewer veins are found.28 One paper describes a greater proportion of success between PIVC brands.25
DISCUSSION
The published evidence for TRAs for PIVCs is limited, with few studies using 2 or more reliability, validity, responsiveness, clinical feasibility, or utility measurements in their development. There is a clear need to assess the clinical utility and clinical feasibility of these approaches so they can be externally validated prior to clinical adoption.16 For this reason, a validated TRA is likely required but must be appropriate for the capability of the healthcare services to use it. We suggest the consistent absence of all of these phases is owing to the variety of healthcare practitioners who are responsible for the insertion, the care and surveillance of peripheral cannulae, and the fragmentation of clinical approaches that exist.32
Previously, a comprehensive systematic review on the subject of PIVCs found that the presence of a visible and/or palpable vein is usually associated with FTIS.33 This current review found evidence of simple scores or cutoff percentage estimates in 2 TRA reports to predict either appropriate PIVC insertion or FTIS.23,29 If such methods are supported by future experimental trials, then such simple approaches could initiate huge clinical return, particularly given that idle or unused PIVCs are of substantial clinical concern.34-36 PIVCs transcend a variety of clinical environments with excessive use identified in the ED, where it may be performed for blood sampling alone and, hence, are labeled as “just in case” PIVCs and contribute to the term “idle PIVC.”23,34 Therefore, a clinical indication to perform PIVC insertion in the first instance must be embedded into any TRA; for example, clinical deterioration is likely and the risks are outweighed by benefit, intravenous fluids and/or medicines are required, and/or diagnostic or clinical procedures are requested (such as contrast scans or procedural sedation).
In the majority of papers reviewed, researchers described how to categorize patients into levels of anticipated and predicted difficulty, but none offered corresponding detailed recommendations for strategies to increase insertion success, such as insertion with ultrasound or vascular access expert. Hypothetically, adopting a TRA may assist with the early identification of difficult to cannulate patients who may require a more expert vascular access clinician. However, in this review, we identify that a uniform definition for DIVA is lacking. Both Webster et al.21 and Wells22 suggest that an expert inserter is required if difficult access is identified by their tools, but there is no clear description of the qualities of an expert inserter in the literature.37 Recently, consensus recommendations for the definition of vascular access specialist add to discussions about defining vascular access as an interdisciplinary specialist role.38 This is supported by other publications that highlight the association between PIVC procedural experience and increased insertion success.6,23,39-41With regards to products, PIVC gauge size may or may not be significantly associated with insertion success. For identifying a relationship of PIVC gauge with vein quality, both the vein diameter and description will help with the clinical interpretation of results. For example, it may be the case that bigger veins are easier to insert a PIVC and, thus, larger PIVCs are inserted. The opposite can occur when the veins are small and poorly visualized; hence, one may select a small gauge catheter. This argument is supported by Prottengeier et al.42 in a prehospital study that excluded PIVC size in a multivariate analysis because of confounding. However, gauge size is very likely to influence postinsertion complications. Prospective studies are contradictory and suggest 16 to 18 g PIVCs are more likely to contribute to superficial thrombus,43 phlebitis, and, thus, device failure, in contrast to others reporting more frequent dislodgement with smaller 22 g PIVCs.6,44Finally, the studies included did not assess survival times of the inserted PIVCs, given postinsertion failure in the hospitalized patient is prevalent45 and, importantly, modifiable.46 A TRA may yield initial insertion success, but if postinsertion the PIVC fails because of a modifiable reason that the TRA has not acknowledged, then it may be of negligible overall benefit. Therefore, TRAs for PIVC insertion need calibration, further development, and ongoing refinement prior to external validation testing.24 Future research should also examine the role of TRAs in settings where ultrasound or other insertion technology is routinely used.
CONCLUSION
This review identifies a clinically significant gap in vascular access science. The findings of this review support recent work on vessel health and preservation47-49 and appropriate device insertion.50 It also points to the need for further research on the development and testing of an appropriate clinical TRA to improve vascular access outcomes in clinical practice.
Acknowledgments
The authors thank Ms. Kylie Black and Mr. Simon Lewis, who are medical research librarians at The University of Western Australia.
Disclosure
Mr. Carr has received “speakers bureau” payment form CareFusion in 2013 and Becton Dickinson (BD) in 2014 for lectures on the subject of vascular access. He received a grant from CareFusion (facilitated by his institution at the time) to attend a scientific meeting on vascular access in the USA in 2012. Griffith University has received unrestricted investigator initiated research or educational grants on Marie Cooke’s behalf from product manufacturers: Baxter; Becton, Dickinson and Company; Centurion Medical Products and Entrotech Lifesciences. Griffith University has received unrestricted investigator initiated research or educational grants on Claire M. Rickard’s behalf from product manufacturers: 3M; Adhezion Biomedical, AngioDynamics; Bard, Baxter; B.Braun; Becton, Dickinson and Company; Centurion Medical Products; Cook Medical; Entrotech, Flomedical; ICU Medical; Medtronic; Smiths Medical, Teleflex. Griffith University has received consultancy payments on Claire M. Rickard’s behalf from product manufacturers: 3M, Bard; BBraun, BD, ResQDevices, Smiths Medical. Dr. Higgins and Dr. Rippey have nothing to disclose. All of the aforementioned have not biased or influenced this review.
All authors have made substantial contributions with this review. Each author has contributed to drafting and editing the manuscript and approves the final version for publishing.
1. Alexandrou E, Ray-Barruel G, Carr PJ, et al. International prevalence of the use of peripheral intravenous catheters. J Hosp Med. 2015;10(8):530-533. PubMed
2. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. PubMed
3. Sebbane M, Claret PG, Lefebvre S, et al. Predicting peripheral venous access difficulty in the emergency department using body mass index and a clinical evaluation of venous accessibility. J Emerg Med. 2013;44(2):299-305. PubMed
4. Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report. 2010;(26):1-31. PubMed
5. Aulagnier J, Hoc C, Mathieu E, Dreyfus JF, Fischler M, Le Guen M. Efficacy of AccuVein to Facilitate Peripheral Intravenous Placement in Adults Presenting to an Emergency Department: A Randomized Clinical Trial. Acad Emerg Med. 2014;21(8):858-863. PubMed
6. Carr PJ, Glynn RW, Dineen B, Kropmans TJ. A pilot intravenous cannulation team: an Irish perspective. Br J Nurs. 2010;19(10):S19-S27. PubMed
7. Conaghan PG. Predicting outcomes in rheumatoid arthritis. Clin Rheumatol. 2011;30(Suppl 1):S41-S47. PubMed
8. Hendriksen JM, Geersing GJ, Moons KG, de Groot JA. Diagnostic and prognostic prediction models. J Thromb Haemost. 2013;11(Suppl 1):129-141. PubMed
9. Hodgson C, Needham D, Haines K, et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung. 2014;43(1):19-24. PubMed
10. Pace NL, Eberhart LHJ, Kranke PR. Quantifying prognosis with risk predictions. Eur J Anaesthesiol. 2012;29(1):7-16. PubMed
11. Yen K, Riegert A, Gorelick MH. Derivation of the DIVA score: A clinical prediction rule for the identification of children with difficult intravenous access. Pediatr Emerg Care. 2008;24(3):143-147. PubMed
12. Manuel DG, Rosella LC, Hennessy D, Sanmartin C, Wilson K. Predictive risk algorithms in a population setting: An overview. J Epidemiol Community Health. 2012;66(10):859-865. PubMed
13. Tricco AC, Lillie E, Zarin W, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16:15. PubMed
14. Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5(4):371-385. PubMed
15. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. PubMed
16. Adams ST, Leveson SH. Clinical prediction rules. BMJ. 2012;344:d8312 PubMed
17. Babineau J. Product Review: Covidence (Systematic Review Software). J Can Health Libr Assoc. 2014;35(2):68-71.
18. Morris C. The EQUATOR network: Promoting the transparent and accurate reporting of research. Dev Med Child Neurol. 2008;50(10):723. PubMed
19. Pagnutti L, Bin A, Donato R, et al. Difficult intravenous access tool in patients receiving peripheral chemotherapy: A pilot-validation study. Eur J Oncol Nurs. 2016;20:58-63. PubMed
20. Ung L, Cook S, Edwards B, Hocking L, Osmond F, Buttergieg H. Peripheral intravenous cannulation in nursing: performance predictors. J Infus Nurs. 2002;25(3):189-195. PubMed
21. Webster J, Morris H-L, Robinson K, Sanderson U. Development and validation of a Vein Assessment Tool (VAT). Aust J Adv Nurs. 2007;24(4):5-7. PubMed
22. Wells S. Venous access in oncology and haematology patients: Part two. Nurs Stand. 2008;23(1):35–42. PubMed
23. Carr PJ, Rippey JA, Budgeon CA, Cooke ML, Higgins NS, Rickard Claire M. Insertion of peripheral intravenous cannulae in the Emergency Department: factors associated with first-time insertion success. J Vasc Access. 2016;17(2):182-190. PubMed
24. Loon FHJ van, Puijn LAPM, Houterman S, Bouwman ARA. Development of the A-DIVA Scale: A Clinical Predictive Scale to Identify Difficult Intravenous Access in Adult Patients Based on Clinical Observations. Medicine (Baltimore). 2016;95(16):e3428. PubMed
25. Jacobson AF, Winslow EH. Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005;34(5):345-359. PubMed
26. Fields MJ, Piela NE, Au AK, Ku BS. Risk factors associated with difficult venous access in adult ED patients. Am J Emerg Med. 2014;32(10):1179-1182 PubMed
27. Piredda M, Biagioli V, Barrella B, et al. Factors Affecting Difficult Peripheral Intravenous Cannulation in Adults: A Prospective Observational Study. J Clin Nurs. 2017;26(7-8):1074-1084 PubMed
28. de la Torre-Montero J-C, Montealegre-Sanz M, Faraldo-Cabana A, et al. Venous International Assessment, VIA scale, validated classification procedure for the peripheral venous system. J Vasc Access. 2014;15(1):45-50. PubMed
29. Kelly AM, Egerton-Warburton D. When is peripheral intravenous catheter insertion indicated in the emergency department? Emerg Med Australas. 2014;26(5):515–516. PubMed
30. Witting MD. IV access difficulty: Incidence and delays in an urban emergency department. J Emerg Med. 2012;42(4):483-487. PubMed
31. McGowan D, Wood S. Developing a venous assessment tool in IV chemotherapy administration. Br J Nurs. 2008;17(3):158-164. PubMed
32. Castro-Sánchez E, Charani E, Drumright LN, Sevdalis N, Shah N, Holmes AH. Fragmentation of Care Threatens Patient Safety in Peripheral Vascular Catheter Management in Acute Care–A Qualitative Study. PLoS One. 2014;9(1):e86167. PubMed
33. Sabri A, Szalas J, Holmes KS, Labib L, Mussivand T. Failed attempts and improvement strategies in peripheral intravenous catheterization. Biomed Mater Eng. 2013;23(1-2):93-108. PubMed
34. Limm EI, Fang X, Dendle C, Stuart RL, Egerton Warburton D. Half of All Peripheral Intravenous Lines in an Australian Tertiary Emergency Department Are Unused: Pain With No Gain? Ann Emerg Med. 2013;62(5):521-525 PubMed
35. Egerton-Warburton D, Ieraci S. First do no harm: In fact, first do nothing, at least not a cannula. Emerg Med Australas. 2013;25(4):289-290.
36. Becerra MB, Shirley D, Safdar N. Prevalence, risk factors, and outcomes of idle intravenous catheters: An integrative review. Am J Infect Control. 2016;44(10):e167-e172. PubMed
37. Carr PJ, Higgins NS, Cooke ML, Mihala G, Rickard CM. Vascular access specialist teams for device insertion and prevention of failure. Cochrane Library. John Wiley & Sons, Ltd; 2014.
38. Davis L, Owens AK, Thompson J. Defining the Specialty of Vascular Access through Consensus: Shaping the Future of Vascular Access. J Assoc Vasc Access. 2016;21(3):125-130.
39. Da Silva GA, Priebe S, Dias FN. Benefits of establishing an intravenous team and the standardization of peripheral intravenous catheters. J Infus Nurs. 2010;33(3):156-160. PubMed
40. Soifer NE, Borzak S, Edlin BR, Weinstein RA. Prevention of peripheral venous catheter complications with an intravenous therapy team: A randomized controlled trial. Arch Intern Med. 1998;158(5):473-477. PubMed
41. Cuper NJ, de Graaff JC, van Dijk AT, Verdaasdonk RM, van der Werff DB, Kalkman CJ. Predictive factors for difficult intravenous cannulation in pediatric patients at a tertiary pediatric hospital. Paediatr Anaesth. 2012;22(3):223-229. PubMed
42. Prottengeier J, Albermann M, Heinrich S, Birkholz T, Gall C, Schmidt J. The prehospital intravenous access assessment: a prospective study on intravenous access failure and access delay in prehospital emergency medicine. Eur J Emerg Med. 2016; 23(6)442-447. PubMed
43. Cicolini G, Bonghi AP, Di Labio L, Di Mascio R. Position of peripheral venous cannulae and the incidence of thrombophlebitis: an observational study. J Adv Nurs. 2009;65(6):1268-1273. PubMed
44. Wallis MC, McGrail M, Webster J, et al. Risk factors for peripheral intravenous catheter failure: a multivariate analysis of data from a randomized controlled trial. Infect Control Hosp Epidemiol. 2014;35(1):63-68. PubMed
45. Carr PJ, Rippey J, Moore T, et al. Reasons for Removal of Emergency Department-Inserted Peripheral Intravenous Cannulae in Admitted Patients: A Retrospective Medical Chart Audit in Australia. Infect Control Hosp Epidemiol. 2016;37(7):874-876. PubMed
46. Bugden S, Shean K, Scott M, et al. Skin Glue Reduces the Failure Rate of Emergency Department-Inserted Peripheral Intravenous Catheters: A Randomized Controlled Trial. Ann Emerg Med. 2016;68(2):196-201. PubMed
47. Moureau N, Trick N, Nifong T, Perry C, Kelley C, Carrico R, et al. Vessel health and preservation (Part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13(3):351-356. PubMed
48. Jackson T, Hallam C, Corner T, Hill S. Right line, right patient, right time: Every choice matters. Br J Nurs. 2013;22(8):S24-S28. PubMed
49. Hallam C, Weston V, Denton A, et al. Development of the UK Vessel Health and Preservation (VHP) framework: a multi-organisational collaborative. J Infect Prev. 2016;17(2):65-72.
50. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
Up to a billion peripheral intravenous catheters (PIVCs) are inserted annually; therefore, the importance of this invasive device in modern medicine cannot be argued.1 The insertion of a PIVC is a clinical procedure undertaken by a range of clinical staff and in a variety of patient populations and settings. In many clinical environments (for example, the emergency department [ED]), PIVCs are the predominant first-choice vascular access device (VAD).2,3 Researchers in one study estimated over 25 million PIVCs are used in French EDs each year,3 and intravenous therapy is the leading ED treatment in the United States.4
The purpose of this systematic scoping review was to investigate what PIVC decision-making approaches exist to facilitate FTIS of PIVCs in adult hospitalized patients. Our intention was to systematically synthesize the research on TRAs, to review significant associations identified with these TRAs, and to critique TRA validity and reliability.
METHODS
Scoping Review
We selected a scoping review method that, by definition, maps the evidence to identify gaps,13,14 set research agendas, and identify implications for decision making. This allowed a targeted approach to answering our 3 research questions:
- What published clinical TRAs exist to facilitate PIVC insertion in adults?
- What clinical, patient and/or product variables have been identified using TRAs as having significant associations with FTIS for PIVCs in adult patients?
- What is the reported reliability, validity, responsiveness, clinical feasibility, and utility of existing TRAs for PIVC insertion in adults?
Our aim was to identify the amount, variety and essential qualities of TRA literature rather than to critically appraise and evaluate the effectiveness of TRAs, a process reserved for systematic review and meta-analysis of interventional studies.13,14 We followed scoping review guidelines published by members and collaborators of the Joanna Briggs Institute, an internationally recognized leader in research synthesis, evidence use, and implementation. The guidance is based on 5 steps: (i) scoping review objective and question, (ii) background of the topic to support scoping review, (iii) study selection, (iv) charting the results, and (v) collating and summarizing results.15 Clinicometric assessment of a TRA or any clinical prediction rule requires 4 specific phases: (i) development (identification of predictors from data), (ii) validation (testing the rule in a separate population for reliability), (iii) impact analysis or responsiveness (How clinically useful is the rule in the clinical setting? Is it resource heavy or light? Is it cost effective?), and (iv) implementation and adoption (uptake into clinical practice).16
Search Strategy
We included studies that described the use or development of any TRA regarding PIVC insertion in the adult hospitalized population.
Inclusion Criteria
Studies were included if they were published in the English Language, included TRAs for PIVC insertion in adult hospital patients, and prospectively assessed a clinical category of patient for PIVC insertion using a traditional approach. We defined a traditional PIVC insertion approach as an assessment and/or insertion with touch and feel, therefore, without vessel-locating technology such as ultrasound and/or near infrared technology.
Exclusion Criteria
Exclusion criteria included pediatric studies, authors’ personal (nonresearch) experience of tools, TRAs focused on postinsertion assessment of the cannula (such as phlebitis, infiltration, and/or dressing failure), and papers with a focus on VADs other than PIVCs. We excluded studies using PIVC ultrasound and/or near infrared technology because these are not standard in all insertions and greatly change the information available for pre-insertion assessment as well as the likelihood of insertion success.
In June 2016, a systematic search of the Cochrane library, Ovid Medline® In-process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>, EBSCO CINAHL databases, and Google Scholar with specific keywords to identify publications that identified or defined TRAs was undertaken. Medical subject headings were created with assistance from a research librarian using tailored functions within individual databases. With key search terms, we limited studies to those related to our inclusion criteria. See Appendix 1 for our search strategy for Medline and CINAHL.
We used Covidence, a web-based application specifically designed for systematic reviews to screen and evaluate eligible publications.17 Two authors (PJC and NSH) screened the initial retrieved searches based upon the predetermined inclusion and exclusion criteria.
Data Extraction
A paper template was developed and used by 2 reviewers (P.J.C. and N.S.H.). Data included the following: study sample, aim(s), design, setting and country in which the study took place, clinical and patient variables, and how the TRAs were developed and tested. Studies were categorized by TRA type. We also sought to identify if clinical trial registration (where appropriate) was evidenced, in addition to evidence of protocol publication and what standardized reporting guidelines were used (such as those outlined by the EQUATOR Network).18
Data Synthesis
Formal meta-analysis was beyond the scope and intention of this review. However, we provide the FTIS rate and the ranges of odds ratios (ORs) with 95% confidence intervals (CIs) for certain independent predictors.
RESULTS
Thirty-six references were imported for screening against title and abstract content, with 11 studies excluded and 25 studies assessed for full-text eligibility (see Figure, PRISMA Flowchart). We then excluded a further 12 studies (6 did not meet inclusion criteria, 2 were focused on the prehospital setting, 2 were personal correspondence and focused on another type of VAD, 1 was a protocol to establish a TRA, and 1 was a framework for all device types), leaving 13 studies included in the final review (see Figure). These studies presented data on 4 tools,19-22 4 predictive models3,23-25 (of which 3 present receiver operating characteristic/area under the curve scores),3,23,24 2 framed as risk factor studies,26,27 and 1 of each of the following: a scale,28 a score,29 and an estimation of the incidence report rate (Table 1).30 Seven studies had “difficult” or “difficulty” in their title as a term to use to describe insertion failure.3,19,24-27,30 One study was titled exclusively for the nursing profession,20 5 studies were reported in medical journals,3,24,26,29,30 and 6 were reported in nursing journals,19-22,25,27 with the remainder published in a vascular access journal.23,28
General Characteristics of Included Studies
One TRA which was registered as a clinical trial24 involved a standardized reporting tool as is recommended by the EQUATOR Network.18
Nine of the 13 papers reported that TRA components were chosen based on identified predictors of successful insertion from observational data3,19,23-28,30, with 5 papers using multivariate logistic regression to identify independent predictors.3,23,24,26,2 At least 4330 insertion attempts on patients were reported. Seven papers reported FTIS, which ranged from 61%-90%.3,23-27,30
Two clinical settings accounted for 10 of the 13 included studies. We identified 5 papers from the ED setting3,23,26,29,30 and 5 studies specific to cancer settings.19-22,28 Two ED papers identified clinical predictors of insertion difficulty, with 1 identifying an existing medical diagnosis (such as sickle cell disease, diabetes, or intravenous drug abuse) and the other reporting a pragmatic patient self-report of difficulty.26,30 Three studies focused on patient-exclusive variables (such as vein characteristics)19,21,28 and some with a combined clinician and patient focus.3,23-25,27,30Relatively few studies reported interobserver measurements to describe the reliability of clinical assessments made.3,19,21,28 Webster et al. in Australia assessed interrater reliability of a vein assessment tool (VAT) and found high agreement (kappa 0.83 for medical imaging nurses and 0.93 for oncology nurses).21 Wells compared reliability with Altman’s K scores obtained from a different VAT when compared with the Deciding on Intravenous Access tool and found good agreement.22 Vein deterioration was proposed as a variable for inclusion when developing an assessment tool within an oncological context.31 In Spain, de la Torre and colleagues28 demonstrated good interrater agreement (with kappa, 0.77) for the Venous International Assessment (VIA) tool. The VIA offers a grading system scale to predict the patient’s declining vessel size while undergoing chemotherapy via peripheral veins with PIVCs. Grade I suggests little or no insertion failure, whereas a Grade V should predict insertion failure.
Patient Variables
Vein characteristics were significant independent factors associated with insertion success in a number of studies.3,19,23,24,27,28 These included the number of veins, descriptive quality (eg, small, medium, large), size, location, visible veins, and palpable veins. Other factors appear to be patient specific (such as chronic conditions), including diabetes (OR, 2.1 [adjusted to identify demographic risk factors]; 95% CI, 1.3-3.4), sickle cell disease (OR, 3.5; 95% CI, 1.4-4.8), and intravenous drug abuse (OR, 2.4; 95% CI, 1.1-5.3).26 It is unclear if a consistent relationship between weight classification and insertion outcomes exists. Despite a finding that BMI was not independently associated with insertion difficulty,26 one study reports that BMI was independently associated with insertion failure (BMI <18.5 [OR, 2.24; 95% CI, 1.07-4.67], BMI >30 [OR, 1.98; 95% CI, 1.9-3.60])3 and another reports emaciated patients were associated with greater failure when compared to normal weight patients (OR, 0.07; 95% CI, 0.02-0.34).23 Consequently, extremes of BMI appear to be associated with insertion outcomes despite 1 study reporting no significant association with BMI as an independent factor of insertion failure.26 A history of difficult intravenous access (DIVA) was reported in 1 study and independently associated with insertion failure (OR, 3.86; 95% CI, 2.39-6.25; see Table 2). DIVA appears to be the motivating factor in the title of 7 studies. When defined, the definitions of DIVA are heterogeneous and varied and include the following: >1 minute to insert a PIVC and requiring >1 attempt27; 2 failed attempts30; 3 or more PIVC attempts.26 In the remaining 4 studies, variables associated with difficulty are identified and, therefore, TRAs to target those in future with predicted difficulty prior to any attempts are proposed.3,19,24,25
Clinician Variables
Specialist nurse certification, years of experience, and self-report skill level (P < 0.001) appear to be significantly associated with successful insertions.25 This is in part validated in another study reporting greater procedural inserting PIVCs as an independent predictor of success (OR, 4.404; 95% CI, 1.61-12-06; see Table 2).23 Two studies involved simple pragmatic percentage cut offs for PIVCs: likelihood of use29 and likelihood of insertion success.23 One paper using a cross-sectional design that surveyed ED clinicians suggested if the clinician’s predicted likelihood of the patient needing a PIVC was >80%, this was a reasonable trigger for PIVC insertion.29 The other, in a self-report cohort study, reported that a clinician’s likelihood estimation of PIVC FTIS prior to insertion is independently associated with FTIS (OR, 1.06; 95% CI, 1.04-1.07).23
Product Variables
In this review, higher failure rates were identified in smaller sizes (22-24 g).26 One study revealed gauge size was significantly associated with a failed first attempt in a univariate analysis (OR, 0.44; 95% CI, 0.34-0.58), but this was not retained in a multivariate model.24 Matching the PIVC size with vein assessment is considered in the VIA tool.28 It suggests a large PIVC (18 g) can be considered in patients with at least 6 vein options; smaller PIVCs of 22 to 24 g are recommended when 3 or fewer veins are found.28 One paper describes a greater proportion of success between PIVC brands.25
DISCUSSION
The published evidence for TRAs for PIVCs is limited, with few studies using 2 or more reliability, validity, responsiveness, clinical feasibility, or utility measurements in their development. There is a clear need to assess the clinical utility and clinical feasibility of these approaches so they can be externally validated prior to clinical adoption.16 For this reason, a validated TRA is likely required but must be appropriate for the capability of the healthcare services to use it. We suggest the consistent absence of all of these phases is owing to the variety of healthcare practitioners who are responsible for the insertion, the care and surveillance of peripheral cannulae, and the fragmentation of clinical approaches that exist.32
Previously, a comprehensive systematic review on the subject of PIVCs found that the presence of a visible and/or palpable vein is usually associated with FTIS.33 This current review found evidence of simple scores or cutoff percentage estimates in 2 TRA reports to predict either appropriate PIVC insertion or FTIS.23,29 If such methods are supported by future experimental trials, then such simple approaches could initiate huge clinical return, particularly given that idle or unused PIVCs are of substantial clinical concern.34-36 PIVCs transcend a variety of clinical environments with excessive use identified in the ED, where it may be performed for blood sampling alone and, hence, are labeled as “just in case” PIVCs and contribute to the term “idle PIVC.”23,34 Therefore, a clinical indication to perform PIVC insertion in the first instance must be embedded into any TRA; for example, clinical deterioration is likely and the risks are outweighed by benefit, intravenous fluids and/or medicines are required, and/or diagnostic or clinical procedures are requested (such as contrast scans or procedural sedation).
In the majority of papers reviewed, researchers described how to categorize patients into levels of anticipated and predicted difficulty, but none offered corresponding detailed recommendations for strategies to increase insertion success, such as insertion with ultrasound or vascular access expert. Hypothetically, adopting a TRA may assist with the early identification of difficult to cannulate patients who may require a more expert vascular access clinician. However, in this review, we identify that a uniform definition for DIVA is lacking. Both Webster et al.21 and Wells22 suggest that an expert inserter is required if difficult access is identified by their tools, but there is no clear description of the qualities of an expert inserter in the literature.37 Recently, consensus recommendations for the definition of vascular access specialist add to discussions about defining vascular access as an interdisciplinary specialist role.38 This is supported by other publications that highlight the association between PIVC procedural experience and increased insertion success.6,23,39-41With regards to products, PIVC gauge size may or may not be significantly associated with insertion success. For identifying a relationship of PIVC gauge with vein quality, both the vein diameter and description will help with the clinical interpretation of results. For example, it may be the case that bigger veins are easier to insert a PIVC and, thus, larger PIVCs are inserted. The opposite can occur when the veins are small and poorly visualized; hence, one may select a small gauge catheter. This argument is supported by Prottengeier et al.42 in a prehospital study that excluded PIVC size in a multivariate analysis because of confounding. However, gauge size is very likely to influence postinsertion complications. Prospective studies are contradictory and suggest 16 to 18 g PIVCs are more likely to contribute to superficial thrombus,43 phlebitis, and, thus, device failure, in contrast to others reporting more frequent dislodgement with smaller 22 g PIVCs.6,44Finally, the studies included did not assess survival times of the inserted PIVCs, given postinsertion failure in the hospitalized patient is prevalent45 and, importantly, modifiable.46 A TRA may yield initial insertion success, but if postinsertion the PIVC fails because of a modifiable reason that the TRA has not acknowledged, then it may be of negligible overall benefit. Therefore, TRAs for PIVC insertion need calibration, further development, and ongoing refinement prior to external validation testing.24 Future research should also examine the role of TRAs in settings where ultrasound or other insertion technology is routinely used.
CONCLUSION
This review identifies a clinically significant gap in vascular access science. The findings of this review support recent work on vessel health and preservation47-49 and appropriate device insertion.50 It also points to the need for further research on the development and testing of an appropriate clinical TRA to improve vascular access outcomes in clinical practice.
Acknowledgments
The authors thank Ms. Kylie Black and Mr. Simon Lewis, who are medical research librarians at The University of Western Australia.
Disclosure
Mr. Carr has received “speakers bureau” payment form CareFusion in 2013 and Becton Dickinson (BD) in 2014 for lectures on the subject of vascular access. He received a grant from CareFusion (facilitated by his institution at the time) to attend a scientific meeting on vascular access in the USA in 2012. Griffith University has received unrestricted investigator initiated research or educational grants on Marie Cooke’s behalf from product manufacturers: Baxter; Becton, Dickinson and Company; Centurion Medical Products and Entrotech Lifesciences. Griffith University has received unrestricted investigator initiated research or educational grants on Claire M. Rickard’s behalf from product manufacturers: 3M; Adhezion Biomedical, AngioDynamics; Bard, Baxter; B.Braun; Becton, Dickinson and Company; Centurion Medical Products; Cook Medical; Entrotech, Flomedical; ICU Medical; Medtronic; Smiths Medical, Teleflex. Griffith University has received consultancy payments on Claire M. Rickard’s behalf from product manufacturers: 3M, Bard; BBraun, BD, ResQDevices, Smiths Medical. Dr. Higgins and Dr. Rippey have nothing to disclose. All of the aforementioned have not biased or influenced this review.
All authors have made substantial contributions with this review. Each author has contributed to drafting and editing the manuscript and approves the final version for publishing.
Up to a billion peripheral intravenous catheters (PIVCs) are inserted annually; therefore, the importance of this invasive device in modern medicine cannot be argued.1 The insertion of a PIVC is a clinical procedure undertaken by a range of clinical staff and in a variety of patient populations and settings. In many clinical environments (for example, the emergency department [ED]), PIVCs are the predominant first-choice vascular access device (VAD).2,3 Researchers in one study estimated over 25 million PIVCs are used in French EDs each year,3 and intravenous therapy is the leading ED treatment in the United States.4
The purpose of this systematic scoping review was to investigate what PIVC decision-making approaches exist to facilitate FTIS of PIVCs in adult hospitalized patients. Our intention was to systematically synthesize the research on TRAs, to review significant associations identified with these TRAs, and to critique TRA validity and reliability.
METHODS
Scoping Review
We selected a scoping review method that, by definition, maps the evidence to identify gaps,13,14 set research agendas, and identify implications for decision making. This allowed a targeted approach to answering our 3 research questions:
- What published clinical TRAs exist to facilitate PIVC insertion in adults?
- What clinical, patient and/or product variables have been identified using TRAs as having significant associations with FTIS for PIVCs in adult patients?
- What is the reported reliability, validity, responsiveness, clinical feasibility, and utility of existing TRAs for PIVC insertion in adults?
Our aim was to identify the amount, variety and essential qualities of TRA literature rather than to critically appraise and evaluate the effectiveness of TRAs, a process reserved for systematic review and meta-analysis of interventional studies.13,14 We followed scoping review guidelines published by members and collaborators of the Joanna Briggs Institute, an internationally recognized leader in research synthesis, evidence use, and implementation. The guidance is based on 5 steps: (i) scoping review objective and question, (ii) background of the topic to support scoping review, (iii) study selection, (iv) charting the results, and (v) collating and summarizing results.15 Clinicometric assessment of a TRA or any clinical prediction rule requires 4 specific phases: (i) development (identification of predictors from data), (ii) validation (testing the rule in a separate population for reliability), (iii) impact analysis or responsiveness (How clinically useful is the rule in the clinical setting? Is it resource heavy or light? Is it cost effective?), and (iv) implementation and adoption (uptake into clinical practice).16
Search Strategy
We included studies that described the use or development of any TRA regarding PIVC insertion in the adult hospitalized population.
Inclusion Criteria
Studies were included if they were published in the English Language, included TRAs for PIVC insertion in adult hospital patients, and prospectively assessed a clinical category of patient for PIVC insertion using a traditional approach. We defined a traditional PIVC insertion approach as an assessment and/or insertion with touch and feel, therefore, without vessel-locating technology such as ultrasound and/or near infrared technology.
Exclusion Criteria
Exclusion criteria included pediatric studies, authors’ personal (nonresearch) experience of tools, TRAs focused on postinsertion assessment of the cannula (such as phlebitis, infiltration, and/or dressing failure), and papers with a focus on VADs other than PIVCs. We excluded studies using PIVC ultrasound and/or near infrared technology because these are not standard in all insertions and greatly change the information available for pre-insertion assessment as well as the likelihood of insertion success.
In June 2016, a systematic search of the Cochrane library, Ovid Medline® In-process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>, EBSCO CINAHL databases, and Google Scholar with specific keywords to identify publications that identified or defined TRAs was undertaken. Medical subject headings were created with assistance from a research librarian using tailored functions within individual databases. With key search terms, we limited studies to those related to our inclusion criteria. See Appendix 1 for our search strategy for Medline and CINAHL.
We used Covidence, a web-based application specifically designed for systematic reviews to screen and evaluate eligible publications.17 Two authors (PJC and NSH) screened the initial retrieved searches based upon the predetermined inclusion and exclusion criteria.
Data Extraction
A paper template was developed and used by 2 reviewers (P.J.C. and N.S.H.). Data included the following: study sample, aim(s), design, setting and country in which the study took place, clinical and patient variables, and how the TRAs were developed and tested. Studies were categorized by TRA type. We also sought to identify if clinical trial registration (where appropriate) was evidenced, in addition to evidence of protocol publication and what standardized reporting guidelines were used (such as those outlined by the EQUATOR Network).18
Data Synthesis
Formal meta-analysis was beyond the scope and intention of this review. However, we provide the FTIS rate and the ranges of odds ratios (ORs) with 95% confidence intervals (CIs) for certain independent predictors.
RESULTS
Thirty-six references were imported for screening against title and abstract content, with 11 studies excluded and 25 studies assessed for full-text eligibility (see Figure, PRISMA Flowchart). We then excluded a further 12 studies (6 did not meet inclusion criteria, 2 were focused on the prehospital setting, 2 were personal correspondence and focused on another type of VAD, 1 was a protocol to establish a TRA, and 1 was a framework for all device types), leaving 13 studies included in the final review (see Figure). These studies presented data on 4 tools,19-22 4 predictive models3,23-25 (of which 3 present receiver operating characteristic/area under the curve scores),3,23,24 2 framed as risk factor studies,26,27 and 1 of each of the following: a scale,28 a score,29 and an estimation of the incidence report rate (Table 1).30 Seven studies had “difficult” or “difficulty” in their title as a term to use to describe insertion failure.3,19,24-27,30 One study was titled exclusively for the nursing profession,20 5 studies were reported in medical journals,3,24,26,29,30 and 6 were reported in nursing journals,19-22,25,27 with the remainder published in a vascular access journal.23,28
General Characteristics of Included Studies
One TRA which was registered as a clinical trial24 involved a standardized reporting tool as is recommended by the EQUATOR Network.18
Nine of the 13 papers reported that TRA components were chosen based on identified predictors of successful insertion from observational data3,19,23-28,30, with 5 papers using multivariate logistic regression to identify independent predictors.3,23,24,26,2 At least 4330 insertion attempts on patients were reported. Seven papers reported FTIS, which ranged from 61%-90%.3,23-27,30
Two clinical settings accounted for 10 of the 13 included studies. We identified 5 papers from the ED setting3,23,26,29,30 and 5 studies specific to cancer settings.19-22,28 Two ED papers identified clinical predictors of insertion difficulty, with 1 identifying an existing medical diagnosis (such as sickle cell disease, diabetes, or intravenous drug abuse) and the other reporting a pragmatic patient self-report of difficulty.26,30 Three studies focused on patient-exclusive variables (such as vein characteristics)19,21,28 and some with a combined clinician and patient focus.3,23-25,27,30Relatively few studies reported interobserver measurements to describe the reliability of clinical assessments made.3,19,21,28 Webster et al. in Australia assessed interrater reliability of a vein assessment tool (VAT) and found high agreement (kappa 0.83 for medical imaging nurses and 0.93 for oncology nurses).21 Wells compared reliability with Altman’s K scores obtained from a different VAT when compared with the Deciding on Intravenous Access tool and found good agreement.22 Vein deterioration was proposed as a variable for inclusion when developing an assessment tool within an oncological context.31 In Spain, de la Torre and colleagues28 demonstrated good interrater agreement (with kappa, 0.77) for the Venous International Assessment (VIA) tool. The VIA offers a grading system scale to predict the patient’s declining vessel size while undergoing chemotherapy via peripheral veins with PIVCs. Grade I suggests little or no insertion failure, whereas a Grade V should predict insertion failure.
Patient Variables
Vein characteristics were significant independent factors associated with insertion success in a number of studies.3,19,23,24,27,28 These included the number of veins, descriptive quality (eg, small, medium, large), size, location, visible veins, and palpable veins. Other factors appear to be patient specific (such as chronic conditions), including diabetes (OR, 2.1 [adjusted to identify demographic risk factors]; 95% CI, 1.3-3.4), sickle cell disease (OR, 3.5; 95% CI, 1.4-4.8), and intravenous drug abuse (OR, 2.4; 95% CI, 1.1-5.3).26 It is unclear if a consistent relationship between weight classification and insertion outcomes exists. Despite a finding that BMI was not independently associated with insertion difficulty,26 one study reports that BMI was independently associated with insertion failure (BMI <18.5 [OR, 2.24; 95% CI, 1.07-4.67], BMI >30 [OR, 1.98; 95% CI, 1.9-3.60])3 and another reports emaciated patients were associated with greater failure when compared to normal weight patients (OR, 0.07; 95% CI, 0.02-0.34).23 Consequently, extremes of BMI appear to be associated with insertion outcomes despite 1 study reporting no significant association with BMI as an independent factor of insertion failure.26 A history of difficult intravenous access (DIVA) was reported in 1 study and independently associated with insertion failure (OR, 3.86; 95% CI, 2.39-6.25; see Table 2). DIVA appears to be the motivating factor in the title of 7 studies. When defined, the definitions of DIVA are heterogeneous and varied and include the following: >1 minute to insert a PIVC and requiring >1 attempt27; 2 failed attempts30; 3 or more PIVC attempts.26 In the remaining 4 studies, variables associated with difficulty are identified and, therefore, TRAs to target those in future with predicted difficulty prior to any attempts are proposed.3,19,24,25
Clinician Variables
Specialist nurse certification, years of experience, and self-report skill level (P < 0.001) appear to be significantly associated with successful insertions.25 This is in part validated in another study reporting greater procedural inserting PIVCs as an independent predictor of success (OR, 4.404; 95% CI, 1.61-12-06; see Table 2).23 Two studies involved simple pragmatic percentage cut offs for PIVCs: likelihood of use29 and likelihood of insertion success.23 One paper using a cross-sectional design that surveyed ED clinicians suggested if the clinician’s predicted likelihood of the patient needing a PIVC was >80%, this was a reasonable trigger for PIVC insertion.29 The other, in a self-report cohort study, reported that a clinician’s likelihood estimation of PIVC FTIS prior to insertion is independently associated with FTIS (OR, 1.06; 95% CI, 1.04-1.07).23
Product Variables
In this review, higher failure rates were identified in smaller sizes (22-24 g).26 One study revealed gauge size was significantly associated with a failed first attempt in a univariate analysis (OR, 0.44; 95% CI, 0.34-0.58), but this was not retained in a multivariate model.24 Matching the PIVC size with vein assessment is considered in the VIA tool.28 It suggests a large PIVC (18 g) can be considered in patients with at least 6 vein options; smaller PIVCs of 22 to 24 g are recommended when 3 or fewer veins are found.28 One paper describes a greater proportion of success between PIVC brands.25
DISCUSSION
The published evidence for TRAs for PIVCs is limited, with few studies using 2 or more reliability, validity, responsiveness, clinical feasibility, or utility measurements in their development. There is a clear need to assess the clinical utility and clinical feasibility of these approaches so they can be externally validated prior to clinical adoption.16 For this reason, a validated TRA is likely required but must be appropriate for the capability of the healthcare services to use it. We suggest the consistent absence of all of these phases is owing to the variety of healthcare practitioners who are responsible for the insertion, the care and surveillance of peripheral cannulae, and the fragmentation of clinical approaches that exist.32
Previously, a comprehensive systematic review on the subject of PIVCs found that the presence of a visible and/or palpable vein is usually associated with FTIS.33 This current review found evidence of simple scores or cutoff percentage estimates in 2 TRA reports to predict either appropriate PIVC insertion or FTIS.23,29 If such methods are supported by future experimental trials, then such simple approaches could initiate huge clinical return, particularly given that idle or unused PIVCs are of substantial clinical concern.34-36 PIVCs transcend a variety of clinical environments with excessive use identified in the ED, where it may be performed for blood sampling alone and, hence, are labeled as “just in case” PIVCs and contribute to the term “idle PIVC.”23,34 Therefore, a clinical indication to perform PIVC insertion in the first instance must be embedded into any TRA; for example, clinical deterioration is likely and the risks are outweighed by benefit, intravenous fluids and/or medicines are required, and/or diagnostic or clinical procedures are requested (such as contrast scans or procedural sedation).
In the majority of papers reviewed, researchers described how to categorize patients into levels of anticipated and predicted difficulty, but none offered corresponding detailed recommendations for strategies to increase insertion success, such as insertion with ultrasound or vascular access expert. Hypothetically, adopting a TRA may assist with the early identification of difficult to cannulate patients who may require a more expert vascular access clinician. However, in this review, we identify that a uniform definition for DIVA is lacking. Both Webster et al.21 and Wells22 suggest that an expert inserter is required if difficult access is identified by their tools, but there is no clear description of the qualities of an expert inserter in the literature.37 Recently, consensus recommendations for the definition of vascular access specialist add to discussions about defining vascular access as an interdisciplinary specialist role.38 This is supported by other publications that highlight the association between PIVC procedural experience and increased insertion success.6,23,39-41With regards to products, PIVC gauge size may or may not be significantly associated with insertion success. For identifying a relationship of PIVC gauge with vein quality, both the vein diameter and description will help with the clinical interpretation of results. For example, it may be the case that bigger veins are easier to insert a PIVC and, thus, larger PIVCs are inserted. The opposite can occur when the veins are small and poorly visualized; hence, one may select a small gauge catheter. This argument is supported by Prottengeier et al.42 in a prehospital study that excluded PIVC size in a multivariate analysis because of confounding. However, gauge size is very likely to influence postinsertion complications. Prospective studies are contradictory and suggest 16 to 18 g PIVCs are more likely to contribute to superficial thrombus,43 phlebitis, and, thus, device failure, in contrast to others reporting more frequent dislodgement with smaller 22 g PIVCs.6,44Finally, the studies included did not assess survival times of the inserted PIVCs, given postinsertion failure in the hospitalized patient is prevalent45 and, importantly, modifiable.46 A TRA may yield initial insertion success, but if postinsertion the PIVC fails because of a modifiable reason that the TRA has not acknowledged, then it may be of negligible overall benefit. Therefore, TRAs for PIVC insertion need calibration, further development, and ongoing refinement prior to external validation testing.24 Future research should also examine the role of TRAs in settings where ultrasound or other insertion technology is routinely used.
CONCLUSION
This review identifies a clinically significant gap in vascular access science. The findings of this review support recent work on vessel health and preservation47-49 and appropriate device insertion.50 It also points to the need for further research on the development and testing of an appropriate clinical TRA to improve vascular access outcomes in clinical practice.
Acknowledgments
The authors thank Ms. Kylie Black and Mr. Simon Lewis, who are medical research librarians at The University of Western Australia.
Disclosure
Mr. Carr has received “speakers bureau” payment form CareFusion in 2013 and Becton Dickinson (BD) in 2014 for lectures on the subject of vascular access. He received a grant from CareFusion (facilitated by his institution at the time) to attend a scientific meeting on vascular access in the USA in 2012. Griffith University has received unrestricted investigator initiated research or educational grants on Marie Cooke’s behalf from product manufacturers: Baxter; Becton, Dickinson and Company; Centurion Medical Products and Entrotech Lifesciences. Griffith University has received unrestricted investigator initiated research or educational grants on Claire M. Rickard’s behalf from product manufacturers: 3M; Adhezion Biomedical, AngioDynamics; Bard, Baxter; B.Braun; Becton, Dickinson and Company; Centurion Medical Products; Cook Medical; Entrotech, Flomedical; ICU Medical; Medtronic; Smiths Medical, Teleflex. Griffith University has received consultancy payments on Claire M. Rickard’s behalf from product manufacturers: 3M, Bard; BBraun, BD, ResQDevices, Smiths Medical. Dr. Higgins and Dr. Rippey have nothing to disclose. All of the aforementioned have not biased or influenced this review.
All authors have made substantial contributions with this review. Each author has contributed to drafting and editing the manuscript and approves the final version for publishing.
1. Alexandrou E, Ray-Barruel G, Carr PJ, et al. International prevalence of the use of peripheral intravenous catheters. J Hosp Med. 2015;10(8):530-533. PubMed
2. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. PubMed
3. Sebbane M, Claret PG, Lefebvre S, et al. Predicting peripheral venous access difficulty in the emergency department using body mass index and a clinical evaluation of venous accessibility. J Emerg Med. 2013;44(2):299-305. PubMed
4. Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report. 2010;(26):1-31. PubMed
5. Aulagnier J, Hoc C, Mathieu E, Dreyfus JF, Fischler M, Le Guen M. Efficacy of AccuVein to Facilitate Peripheral Intravenous Placement in Adults Presenting to an Emergency Department: A Randomized Clinical Trial. Acad Emerg Med. 2014;21(8):858-863. PubMed
6. Carr PJ, Glynn RW, Dineen B, Kropmans TJ. A pilot intravenous cannulation team: an Irish perspective. Br J Nurs. 2010;19(10):S19-S27. PubMed
7. Conaghan PG. Predicting outcomes in rheumatoid arthritis. Clin Rheumatol. 2011;30(Suppl 1):S41-S47. PubMed
8. Hendriksen JM, Geersing GJ, Moons KG, de Groot JA. Diagnostic and prognostic prediction models. J Thromb Haemost. 2013;11(Suppl 1):129-141. PubMed
9. Hodgson C, Needham D, Haines K, et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung. 2014;43(1):19-24. PubMed
10. Pace NL, Eberhart LHJ, Kranke PR. Quantifying prognosis with risk predictions. Eur J Anaesthesiol. 2012;29(1):7-16. PubMed
11. Yen K, Riegert A, Gorelick MH. Derivation of the DIVA score: A clinical prediction rule for the identification of children with difficult intravenous access. Pediatr Emerg Care. 2008;24(3):143-147. PubMed
12. Manuel DG, Rosella LC, Hennessy D, Sanmartin C, Wilson K. Predictive risk algorithms in a population setting: An overview. J Epidemiol Community Health. 2012;66(10):859-865. PubMed
13. Tricco AC, Lillie E, Zarin W, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16:15. PubMed
14. Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5(4):371-385. PubMed
15. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. PubMed
16. Adams ST, Leveson SH. Clinical prediction rules. BMJ. 2012;344:d8312 PubMed
17. Babineau J. Product Review: Covidence (Systematic Review Software). J Can Health Libr Assoc. 2014;35(2):68-71.
18. Morris C. The EQUATOR network: Promoting the transparent and accurate reporting of research. Dev Med Child Neurol. 2008;50(10):723. PubMed
19. Pagnutti L, Bin A, Donato R, et al. Difficult intravenous access tool in patients receiving peripheral chemotherapy: A pilot-validation study. Eur J Oncol Nurs. 2016;20:58-63. PubMed
20. Ung L, Cook S, Edwards B, Hocking L, Osmond F, Buttergieg H. Peripheral intravenous cannulation in nursing: performance predictors. J Infus Nurs. 2002;25(3):189-195. PubMed
21. Webster J, Morris H-L, Robinson K, Sanderson U. Development and validation of a Vein Assessment Tool (VAT). Aust J Adv Nurs. 2007;24(4):5-7. PubMed
22. Wells S. Venous access in oncology and haematology patients: Part two. Nurs Stand. 2008;23(1):35–42. PubMed
23. Carr PJ, Rippey JA, Budgeon CA, Cooke ML, Higgins NS, Rickard Claire M. Insertion of peripheral intravenous cannulae in the Emergency Department: factors associated with first-time insertion success. J Vasc Access. 2016;17(2):182-190. PubMed
24. Loon FHJ van, Puijn LAPM, Houterman S, Bouwman ARA. Development of the A-DIVA Scale: A Clinical Predictive Scale to Identify Difficult Intravenous Access in Adult Patients Based on Clinical Observations. Medicine (Baltimore). 2016;95(16):e3428. PubMed
25. Jacobson AF, Winslow EH. Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005;34(5):345-359. PubMed
26. Fields MJ, Piela NE, Au AK, Ku BS. Risk factors associated with difficult venous access in adult ED patients. Am J Emerg Med. 2014;32(10):1179-1182 PubMed
27. Piredda M, Biagioli V, Barrella B, et al. Factors Affecting Difficult Peripheral Intravenous Cannulation in Adults: A Prospective Observational Study. J Clin Nurs. 2017;26(7-8):1074-1084 PubMed
28. de la Torre-Montero J-C, Montealegre-Sanz M, Faraldo-Cabana A, et al. Venous International Assessment, VIA scale, validated classification procedure for the peripheral venous system. J Vasc Access. 2014;15(1):45-50. PubMed
29. Kelly AM, Egerton-Warburton D. When is peripheral intravenous catheter insertion indicated in the emergency department? Emerg Med Australas. 2014;26(5):515–516. PubMed
30. Witting MD. IV access difficulty: Incidence and delays in an urban emergency department. J Emerg Med. 2012;42(4):483-487. PubMed
31. McGowan D, Wood S. Developing a venous assessment tool in IV chemotherapy administration. Br J Nurs. 2008;17(3):158-164. PubMed
32. Castro-Sánchez E, Charani E, Drumright LN, Sevdalis N, Shah N, Holmes AH. Fragmentation of Care Threatens Patient Safety in Peripheral Vascular Catheter Management in Acute Care–A Qualitative Study. PLoS One. 2014;9(1):e86167. PubMed
33. Sabri A, Szalas J, Holmes KS, Labib L, Mussivand T. Failed attempts and improvement strategies in peripheral intravenous catheterization. Biomed Mater Eng. 2013;23(1-2):93-108. PubMed
34. Limm EI, Fang X, Dendle C, Stuart RL, Egerton Warburton D. Half of All Peripheral Intravenous Lines in an Australian Tertiary Emergency Department Are Unused: Pain With No Gain? Ann Emerg Med. 2013;62(5):521-525 PubMed
35. Egerton-Warburton D, Ieraci S. First do no harm: In fact, first do nothing, at least not a cannula. Emerg Med Australas. 2013;25(4):289-290.
36. Becerra MB, Shirley D, Safdar N. Prevalence, risk factors, and outcomes of idle intravenous catheters: An integrative review. Am J Infect Control. 2016;44(10):e167-e172. PubMed
37. Carr PJ, Higgins NS, Cooke ML, Mihala G, Rickard CM. Vascular access specialist teams for device insertion and prevention of failure. Cochrane Library. John Wiley & Sons, Ltd; 2014.
38. Davis L, Owens AK, Thompson J. Defining the Specialty of Vascular Access through Consensus: Shaping the Future of Vascular Access. J Assoc Vasc Access. 2016;21(3):125-130.
39. Da Silva GA, Priebe S, Dias FN. Benefits of establishing an intravenous team and the standardization of peripheral intravenous catheters. J Infus Nurs. 2010;33(3):156-160. PubMed
40. Soifer NE, Borzak S, Edlin BR, Weinstein RA. Prevention of peripheral venous catheter complications with an intravenous therapy team: A randomized controlled trial. Arch Intern Med. 1998;158(5):473-477. PubMed
41. Cuper NJ, de Graaff JC, van Dijk AT, Verdaasdonk RM, van der Werff DB, Kalkman CJ. Predictive factors for difficult intravenous cannulation in pediatric patients at a tertiary pediatric hospital. Paediatr Anaesth. 2012;22(3):223-229. PubMed
42. Prottengeier J, Albermann M, Heinrich S, Birkholz T, Gall C, Schmidt J. The prehospital intravenous access assessment: a prospective study on intravenous access failure and access delay in prehospital emergency medicine. Eur J Emerg Med. 2016; 23(6)442-447. PubMed
43. Cicolini G, Bonghi AP, Di Labio L, Di Mascio R. Position of peripheral venous cannulae and the incidence of thrombophlebitis: an observational study. J Adv Nurs. 2009;65(6):1268-1273. PubMed
44. Wallis MC, McGrail M, Webster J, et al. Risk factors for peripheral intravenous catheter failure: a multivariate analysis of data from a randomized controlled trial. Infect Control Hosp Epidemiol. 2014;35(1):63-68. PubMed
45. Carr PJ, Rippey J, Moore T, et al. Reasons for Removal of Emergency Department-Inserted Peripheral Intravenous Cannulae in Admitted Patients: A Retrospective Medical Chart Audit in Australia. Infect Control Hosp Epidemiol. 2016;37(7):874-876. PubMed
46. Bugden S, Shean K, Scott M, et al. Skin Glue Reduces the Failure Rate of Emergency Department-Inserted Peripheral Intravenous Catheters: A Randomized Controlled Trial. Ann Emerg Med. 2016;68(2):196-201. PubMed
47. Moureau N, Trick N, Nifong T, Perry C, Kelley C, Carrico R, et al. Vessel health and preservation (Part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13(3):351-356. PubMed
48. Jackson T, Hallam C, Corner T, Hill S. Right line, right patient, right time: Every choice matters. Br J Nurs. 2013;22(8):S24-S28. PubMed
49. Hallam C, Weston V, Denton A, et al. Development of the UK Vessel Health and Preservation (VHP) framework: a multi-organisational collaborative. J Infect Prev. 2016;17(2):65-72.
50. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
1. Alexandrou E, Ray-Barruel G, Carr PJ, et al. International prevalence of the use of peripheral intravenous catheters. J Hosp Med. 2015;10(8):530-533. PubMed
2. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. PubMed
3. Sebbane M, Claret PG, Lefebvre S, et al. Predicting peripheral venous access difficulty in the emergency department using body mass index and a clinical evaluation of venous accessibility. J Emerg Med. 2013;44(2):299-305. PubMed
4. Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report. 2010;(26):1-31. PubMed
5. Aulagnier J, Hoc C, Mathieu E, Dreyfus JF, Fischler M, Le Guen M. Efficacy of AccuVein to Facilitate Peripheral Intravenous Placement in Adults Presenting to an Emergency Department: A Randomized Clinical Trial. Acad Emerg Med. 2014;21(8):858-863. PubMed
6. Carr PJ, Glynn RW, Dineen B, Kropmans TJ. A pilot intravenous cannulation team: an Irish perspective. Br J Nurs. 2010;19(10):S19-S27. PubMed
7. Conaghan PG. Predicting outcomes in rheumatoid arthritis. Clin Rheumatol. 2011;30(Suppl 1):S41-S47. PubMed
8. Hendriksen JM, Geersing GJ, Moons KG, de Groot JA. Diagnostic and prognostic prediction models. J Thromb Haemost. 2013;11(Suppl 1):129-141. PubMed
9. Hodgson C, Needham D, Haines K, et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung. 2014;43(1):19-24. PubMed
10. Pace NL, Eberhart LHJ, Kranke PR. Quantifying prognosis with risk predictions. Eur J Anaesthesiol. 2012;29(1):7-16. PubMed
11. Yen K, Riegert A, Gorelick MH. Derivation of the DIVA score: A clinical prediction rule for the identification of children with difficult intravenous access. Pediatr Emerg Care. 2008;24(3):143-147. PubMed
12. Manuel DG, Rosella LC, Hennessy D, Sanmartin C, Wilson K. Predictive risk algorithms in a population setting: An overview. J Epidemiol Community Health. 2012;66(10):859-865. PubMed
13. Tricco AC, Lillie E, Zarin W, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16:15. PubMed
14. Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5(4):371-385. PubMed
15. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. PubMed
16. Adams ST, Leveson SH. Clinical prediction rules. BMJ. 2012;344:d8312 PubMed
17. Babineau J. Product Review: Covidence (Systematic Review Software). J Can Health Libr Assoc. 2014;35(2):68-71.
18. Morris C. The EQUATOR network: Promoting the transparent and accurate reporting of research. Dev Med Child Neurol. 2008;50(10):723. PubMed
19. Pagnutti L, Bin A, Donato R, et al. Difficult intravenous access tool in patients receiving peripheral chemotherapy: A pilot-validation study. Eur J Oncol Nurs. 2016;20:58-63. PubMed
20. Ung L, Cook S, Edwards B, Hocking L, Osmond F, Buttergieg H. Peripheral intravenous cannulation in nursing: performance predictors. J Infus Nurs. 2002;25(3):189-195. PubMed
21. Webster J, Morris H-L, Robinson K, Sanderson U. Development and validation of a Vein Assessment Tool (VAT). Aust J Adv Nurs. 2007;24(4):5-7. PubMed
22. Wells S. Venous access in oncology and haematology patients: Part two. Nurs Stand. 2008;23(1):35–42. PubMed
23. Carr PJ, Rippey JA, Budgeon CA, Cooke ML, Higgins NS, Rickard Claire M. Insertion of peripheral intravenous cannulae in the Emergency Department: factors associated with first-time insertion success. J Vasc Access. 2016;17(2):182-190. PubMed
24. Loon FHJ van, Puijn LAPM, Houterman S, Bouwman ARA. Development of the A-DIVA Scale: A Clinical Predictive Scale to Identify Difficult Intravenous Access in Adult Patients Based on Clinical Observations. Medicine (Baltimore). 2016;95(16):e3428. PubMed
25. Jacobson AF, Winslow EH. Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005;34(5):345-359. PubMed
26. Fields MJ, Piela NE, Au AK, Ku BS. Risk factors associated with difficult venous access in adult ED patients. Am J Emerg Med. 2014;32(10):1179-1182 PubMed
27. Piredda M, Biagioli V, Barrella B, et al. Factors Affecting Difficult Peripheral Intravenous Cannulation in Adults: A Prospective Observational Study. J Clin Nurs. 2017;26(7-8):1074-1084 PubMed
28. de la Torre-Montero J-C, Montealegre-Sanz M, Faraldo-Cabana A, et al. Venous International Assessment, VIA scale, validated classification procedure for the peripheral venous system. J Vasc Access. 2014;15(1):45-50. PubMed
29. Kelly AM, Egerton-Warburton D. When is peripheral intravenous catheter insertion indicated in the emergency department? Emerg Med Australas. 2014;26(5):515–516. PubMed
30. Witting MD. IV access difficulty: Incidence and delays in an urban emergency department. J Emerg Med. 2012;42(4):483-487. PubMed
31. McGowan D, Wood S. Developing a venous assessment tool in IV chemotherapy administration. Br J Nurs. 2008;17(3):158-164. PubMed
32. Castro-Sánchez E, Charani E, Drumright LN, Sevdalis N, Shah N, Holmes AH. Fragmentation of Care Threatens Patient Safety in Peripheral Vascular Catheter Management in Acute Care–A Qualitative Study. PLoS One. 2014;9(1):e86167. PubMed
33. Sabri A, Szalas J, Holmes KS, Labib L, Mussivand T. Failed attempts and improvement strategies in peripheral intravenous catheterization. Biomed Mater Eng. 2013;23(1-2):93-108. PubMed
34. Limm EI, Fang X, Dendle C, Stuart RL, Egerton Warburton D. Half of All Peripheral Intravenous Lines in an Australian Tertiary Emergency Department Are Unused: Pain With No Gain? Ann Emerg Med. 2013;62(5):521-525 PubMed
35. Egerton-Warburton D, Ieraci S. First do no harm: In fact, first do nothing, at least not a cannula. Emerg Med Australas. 2013;25(4):289-290.
36. Becerra MB, Shirley D, Safdar N. Prevalence, risk factors, and outcomes of idle intravenous catheters: An integrative review. Am J Infect Control. 2016;44(10):e167-e172. PubMed
37. Carr PJ, Higgins NS, Cooke ML, Mihala G, Rickard CM. Vascular access specialist teams for device insertion and prevention of failure. Cochrane Library. John Wiley & Sons, Ltd; 2014.
38. Davis L, Owens AK, Thompson J. Defining the Specialty of Vascular Access through Consensus: Shaping the Future of Vascular Access. J Assoc Vasc Access. 2016;21(3):125-130.
39. Da Silva GA, Priebe S, Dias FN. Benefits of establishing an intravenous team and the standardization of peripheral intravenous catheters. J Infus Nurs. 2010;33(3):156-160. PubMed
40. Soifer NE, Borzak S, Edlin BR, Weinstein RA. Prevention of peripheral venous catheter complications with an intravenous therapy team: A randomized controlled trial. Arch Intern Med. 1998;158(5):473-477. PubMed
41. Cuper NJ, de Graaff JC, van Dijk AT, Verdaasdonk RM, van der Werff DB, Kalkman CJ. Predictive factors for difficult intravenous cannulation in pediatric patients at a tertiary pediatric hospital. Paediatr Anaesth. 2012;22(3):223-229. PubMed
42. Prottengeier J, Albermann M, Heinrich S, Birkholz T, Gall C, Schmidt J. The prehospital intravenous access assessment: a prospective study on intravenous access failure and access delay in prehospital emergency medicine. Eur J Emerg Med. 2016; 23(6)442-447. PubMed
43. Cicolini G, Bonghi AP, Di Labio L, Di Mascio R. Position of peripheral venous cannulae and the incidence of thrombophlebitis: an observational study. J Adv Nurs. 2009;65(6):1268-1273. PubMed
44. Wallis MC, McGrail M, Webster J, et al. Risk factors for peripheral intravenous catheter failure: a multivariate analysis of data from a randomized controlled trial. Infect Control Hosp Epidemiol. 2014;35(1):63-68. PubMed
45. Carr PJ, Rippey J, Moore T, et al. Reasons for Removal of Emergency Department-Inserted Peripheral Intravenous Cannulae in Admitted Patients: A Retrospective Medical Chart Audit in Australia. Infect Control Hosp Epidemiol. 2016;37(7):874-876. PubMed
46. Bugden S, Shean K, Scott M, et al. Skin Glue Reduces the Failure Rate of Emergency Department-Inserted Peripheral Intravenous Catheters: A Randomized Controlled Trial. Ann Emerg Med. 2016;68(2):196-201. PubMed
47. Moureau N, Trick N, Nifong T, Perry C, Kelley C, Carrico R, et al. Vessel health and preservation (Part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13(3):351-356. PubMed
48. Jackson T, Hallam C, Corner T, Hill S. Right line, right patient, right time: Every choice matters. Br J Nurs. 2013;22(8):S24-S28. PubMed
49. Hallam C, Weston V, Denton A, et al. Development of the UK Vessel Health and Preservation (VHP) framework: a multi-organisational collaborative. J Infect Prev. 2016;17(2):65-72.
50. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
© 2017 Society of Hospital Medicine
Navigating Venous Access
Reliable venous access is fundamental for the safe and effective care of hospitalized patients. Venous access devices (VADs) are conduits for this purpose, providing delivery of intravenous medications, accurate measurement of central venous pressure, or administration of life‐saving blood products. Despite this important role, VADs are also often the source of hospital‐acquired complications. Although inpatient providers must balance the relative risks of VADs against their benefits, the evidence supporting such decisions is often limited. Advances in technology, scattered research, and growing availability of novel devices has only further fragmented provider knowledge in the field of vascular access.[1]
It is not surprising, then, that survey‐based studies of hospitalists reveal important knowledge gaps with regard to practices associated with VADs.[2] In this narrative review, we seek to bridge this gap by providing a concise and pragmatic overview of the fundamentals of venous access. We focus specifically on parameters that influence decisions regarding VAD placement in hospitalized patients, providing key takeaways for practicing hospitalists.
METHODS
To compile this review, we systematically searched Medline (via Ovid) for several keywords, including: peripheral intravenous catheters, ultrasound‐guided peripheral catheter, intraosseous, midline, peripherally inserted central catheter, central venous catheters, and vascular access device complications. We concentrated on full‐length articles in English only; no date restrictions were placed on the search. We reviewed guidelines and consensus statements (eg, from the Center for Disease Control [CDC] or Choosing Wisely criteria) as appropriate. Additional studies of interest were identified through content experts (M.P., C.M.R.) and bibliographies of included studies.
SCIENTIFIC PRINCIPLES UNDERPINNING VENOUS ACCESS
It is useful to begin by reviewing VAD‐related nomenclature and physiology. In the simplest sense, a VAD consists of a hub (providing access to various connectors), a hollow tube divided into 1 or many sections (lumens), and a tip that may terminate within a central or peripheral blood vessel. VADs are classified as central venous catheters (eg, centrally inserted central catheters [CICCs] or peripherally inserted central catheters [PICCs]) or peripheral intravenous catheters (eg, midlines or peripheral intravenous catheters) based on site of entry and location of the catheter tip. Therefore, VADs entering via proximal or distal veins of the arm are often referred to as peripheral lines, as their site of entry and tip both reside within peripheral veins. Conversely, the term central line is often used when VADs enter or terminate in a central vein (eg, subclavian vein insertion with the catheter tip in the lower superior vena cava).
Attention to a host of clinical and theoretical parameters is important when choosing a device for venous access. Some such parameters are summarized in Table 1.
| Parameter | Major Considerations |
|---|---|
| |
| Desired flow rate | Smaller diameter veins susceptible to damage with high flow rates. |
| Short, large‐bore catheters facilitate rapid infusion. | |
| Nature of infusion | pH, viscosity, and temperature may damage vessels. |
| Vesicants and irritants should always be administered into larger, central veins. | |
| Desired duration of vascular access, or dwell time | Vessel thrombosis or phlebitis increase over time with catheter in place. |
| Intermittent infusions increase complications in central catheters; often tunneled catheters are recommended. | |
| Urgency of placement | Access to large caliber vessels is often needed in emergencies. |
| Critically ill or hemodynamically unstable patients may require urgent access for invasive monitoring or rapid infusions. | |
| Patients with trauma often require large volumes of blood products and reliable access to central veins. | |
| Number of device lumens | VADs may have single or multiple lumens. |
| Multilumen allows for multiple functions (eg, infusion of multiple agents, measurement of central venous pressures, blood draws). | |
| Device gauge | In general, use of a smaller‐gauge catheter is preferred to prevent complications. |
| However, larger catheter diameter may be needed for specific clinical needs (eg, blood transfusion). | |
| Device coating | VADs may have antithrombotic or anti‐infective coatings. |
| These devices may be of value in patients at high risk of complications. | |
| Such devices, however, may be more costly than their counterparts. | |
| Self‐care compatibility | VADs that can be cared for by patients are ideal for outpatient care. |
| Conversely, VADs such as peripheral catheters, are highly prone to dislodgement and should be reserved for supervised settings only. | |
VENOUS ACCESS DEVICES
We will organize our discussion of VADs based on whether they terminate in peripheral or central vessels. These anatomical considerations are relevant as they determine physical characteristics, compatibility with particular infusates, dwell time, and risk of complications associated with each VAD discussed in Table 2.
| Complications | Major Considerations |
|---|---|
| |
| Infection | VADs breach the integrity of skin and permit skin pathogens to enter the blood stream (extraluminal infection). |
| Inadequate antisepsis of the VAD hub, including poor hand hygiene, failure to "scrub the hub," and multiple manipulations may also increase the risk of VAD‐related infection (endoluminal infection). | |
| Infections may be local (eg, exit‐site infections) or may spread hematogenously (eg, CLABSI). | |
| Type of VAD, duration of therapy, and host characteristics interact to influence infection risk. | |
| VADs with antiseptic coatings (eg, chlorhexidine) or antibiotic coatings (eg, minocycline) may reduce risk of infection in high‐risk patients. | |
| Antiseptic‐impregnated dressings may reduce risk of extraluminal infection. | |
| Venous thrombosis | VADs predispose to venous stasis and thrombosis. |
| Duration of VAD use, type and care of the VAD, and patient characteristics affect risk of thromboembolism. | |
| VAD tip position is a key determinant of venous thrombosis; central VADs that do not terminate at the cavo‐atrial junction should be repositioned to reduce the risk of thrombosis. | |
| Antithrombotic coated or eluting devices may reduce risk of thrombosis, though definitive data are lacking. | |
| Phlebitis | Inflammation caused by damage to tunica media.[18] |
| 3 types of phlebitis: | |
| Chemical: due to irritation of media from the infusate. | |
| Mechanical: VAD physically damages the vessel. | |
| Infective: bacteria invade vein and inflame vessel wall. | |
| Phlebitis may be limited by close attention to infusate compatibility with peripheral veins, appropriate dilution, and prompt removal of catheters that show signs of inflammation. | |
| Phlebitis may be prevented in PICCs by ensuring at least a 2:1 vein:catheter ratio. | |
| Extravasation | Extravasation (also called infiltration) is defined as leakage of infusate from intravascular to extravascular space. |
| Extravasation of vesicants/emrritants is particularly worrisome. | |
| May result in severe tissue injury, blistering, and tissue necrosis.[11] | |
| VADs should be checked frequently for adequate flushing and position prior to each infusion to minimize risk. | |
| Any VAD with redness, swelling, and tenderness at the entry site or problems with flushing should not be used without further examination and review of position. | |
Peripheral Venous Access
Short Peripheral Intravenous Catheter
Approximately 200 million peripheral intravenous catheters (PIVs) are placed annually in the United States, making them the most common intravenous catheter.[3] PIVs are short devices, 3 to 6 cm in length, that enter and terminate in peripheral veins (Figure 1A). Placement is recommended in forearm veins rather than those of the hand, wrist, or upper arm, as forearm sites are less prone to occlusion, accidental removal, and phlebitis.[4] Additionally, placement in hand veins impedes activities of daily living (eg, hand washing) and is not preferred by patients.[5] PIV size ranges from 24 gauge (smallest) to 14 gauge (largest); larger catheters are often reserved for fluid resuscitation or blood transfusion as they accommodate greater flow and limit hemolysis. To decrease risk of phlebitis and thrombosis, the shortest catheter and smallest diameter should be used. However, unless adequately secured, smaller diameter catheters are also associated with greater rates of accidental removal.[4, 5]
By definition, PIVs are short‐term devices. The CDC currently recommends removal and replacement of these devices no more frequently than every 72 to 96 hours in adults. However, a recent randomized controlled trial found that replacing PIVs when clinically indicated (eg, device failure, phlebitis) rather than on a routine schedule added 30 hours to their lifespan without an increase in complications.[6] A systematic review by the Cochrane Collaboration echoes these findings.[3] These data have thus been incorporated into recommendations from the Infusion Nurses Society (INS) and the National Health Service in the United Kingdom.[5, 7] In hospitalized patients, this approach is relevant, as it preserves venous access sites, maximizes device dwell, and limits additional PIV insertions. In turn, these differences may reduce the need for invasive VADs such as PICCs. Furthermore, the projected 5‐year savings from implementation of clinically indicated PIV removal policies is US$300 million and 1 million health‐worker hours in the United States alone.[4]
PIVs offer many advantages. First, they are minimally invasive and require little training to insert. Second, they can be used for diverse indications in patients requiring short‐term (1 week) venous access. Third, PIVs do not require imaging to ensure correct placement; palpation of superficial veins is sufficient. Fourth, PIVs exhibit a risk of bloodstream infection that is about 40‐fold lower than more invasive, longer‐dwelling VADs[8] (0.06 bacteremia per 1000 catheter‐days).
Despite these advantages, PIVs also have important drawbacks. First, a quarter of all PIVs fail through occlusion or accidental dislodgement.[4] Infiltration, extravasation, and hematoma formation are important adverse events that may occur in such cases. Second, thrombophlebitis (pain and redness at the insertion site) is frequent, and may require device removal, especially in patients with catheters 20 guage.[9] Third, despite their relative safety, PIVs can cause localized or hematogenous infection. Septic thrombophlebitis (superficial thrombosis and bloodstream infection) and catheter‐related bloodstream infection, though rare, have been reported with PIVs and may lead to serious complications.[8, 10] In fact, some suggest that the overall burden of bloodstream infection risk posed by PIVs may be similar to that of CICCs given the substantially greater number of devices used and greater number of device days.[8]
PIVs and other peripheral VADs are not suitable for infusion of vesicants or irritants, which require larger, central veins for delivery. Vesicants (drugs that cause blistering on infusion) include chemotherapeutic agents (eg, dactinomycin, paclitaxel) and commonly used nonchemotherapeutical agents (eg, diazepam, piperacillin, vancomycin, esmolol, or total parenteral nutrition [TPN]).[11] Irritants (phlebitogenic drugs) cause short‐term inflammation and pain, and thus should not be peripherally infused for prolonged durations. Common irritants in the hospital setting include acyclovir, dobutamine, penicillin, and potassium chloride.
Of note, about one‐quarter of PIV insertions fail owing to difficult intravenous access.[12] Ultrasound‐guided peripheral intravenous (USGPIV) catheter placement is emerging as a technique to provide peripheral access for such patients to avoid placement of central venous access devices. Novel, longer devices (>8 cm) with built‐in guide wires have been developed to increase placement success of USGPIVs. These new designs provide easier access into deeper arm veins (brachial or basilic) not otherwise accessible by short PIVs. Although studies comparing the efficacy of USGPIV devices to other VADs are limited, a recent systematic review showed that time to successful cannulation was shorter, and fewer attempts were required to place USGPIVs compared to PIVs.[13] A recent study in France found that USGPIVs met the infusion needs of patients with difficult veins with minimal increase in complications.[14] Despite these encouraging data, future studies are needed to better evaluate this technology.
Midline Catheter
A midline is a VAD that is between 7.5 to 25 cm in length and is typically inserted into veins above the antecubital fossa. The catheter tip resides in a peripheral upper arm vein, often the basilic or cephalic vein, terminating just short of the subclavian vein (Figure 1B). Midline‐like devices were first developed in the 1950s and were initially used as an alternative to PIVs because they were thought to allow longer dwell times.[15] However, because they were originally constructed with a fairly rigid material, infiltration, mechanical phlebitis, and inflammation were common and tempered enthusiasm for their use.[15, 16] Newer midline devices obviate many of these problems and are inserted by ultrasound guidance and modified Seldinger technique.[17] Despite these advances, data regarding comparative efficacy are limited.
Midlines offer longer dwell times than standard PIVs owing to termination in the larger diameter basilic and brachial veins of the arm. Additionally, owing to their length, midlines are less prone to dislodgement. As they are inserted with greater antisepsis than PIVs and better secured to the skin, they are more durable than PIVs.[5, 9, 18] Current INS standards recommend use of midlines for 1 to 4 weeks.[5] Because they terminate in a peripheral vein, medications and infusions compatible with midlines are identical to those that are infused through a PIV. Thus, TPN, vesicants or irritants, or drugs that feature a pH 5 or pH >9, or >500 mOsm should not be infused through a midline.[15] New evidence suggests that diluted solutions of vancomycin (usually pH 5) may be safe to infuse for short durations (6 days) through a midline, and that concentration rather than pH may be more important in this regard.[19] Although it is possible that the use of midlines may extend to agents typically not deemed peripheral access compatible, limited evidence exists to support such a strategy at this time.
Midlines offer several advantages. First, because blood flow is greater in the more proximal veins of the arm, midlines can accommodate infusions at rates of 100 to 150 mL/min compared to 20 to 40 mL/min in smaller peripheral veins. Higher flow rates offer greater hemodilution (dilution of the infusion with blood), decreasing the likelihood of phlebitis and infiltration.[20] Second, midlines do not require x‐ray verification of tip placement; thus, their use is often favored in resource‐depleted settings such as skilled nursing facilities. Third, midlines offer longer dwell times than peripheral intravenous catheters and can thus serve as bridge devices for short‐term intravenous antibiotics or peripheral‐compatible infusions in an outpatient setting. Available evidence suggests that midlines are associated with low rates of bloodstream infection (0.30.8 per 1000 catheter‐days).[17] The most frequent complications include phlebitis (4.2%) and occlusion (3.3%).[20] Given these favorable statistics, midlines may offer a good alternative to PIVs in select patients who require peripheral infusions of intermediate duration.
Intraosseous Vascular Access
Intraosseous (IO) devices access the vascular system by piercing cortical bone. These devices provide access to the intramedullary cavity and venous plexi of long bones such as the tibia, femur, or humerus. Several insertion devices are now commercially available and have enhanced the ease and safety of IO placement. Using these newer devices, IO access may be obtained in 1 to 2 minutes with minimal training. By comparison, a central venous catheter often requires 10 to 15 minutes to insert with substantial training efforts for providers.[21, 22, 23]
IO devices thus offer several advantages. First, given the rapidity with which they can be inserted, they are often preferred in emergency settings (eg, trauma). Second, these devices are versatile and can accommodate both central and peripheral infusates.[24] Third, a recent meta‐analysis found that IOs have a low complication rate of 0.8%, with extravasation of infusate through the cortical entry site being the most common adverse event.[21] Of note, this study also reported zero local or distal infectious complications, a finding that may relate to the shorter dwell of these devices.[21] Some animal studies suggest that fat embolism from bone may occur at high rates with IO VADs.[25] However, death or significant morbidity from fat emboli in humans following IO access has not been described. Whether such emboli occur or are clinically significant in the context of IO devices remains unclear at this time.[21]
Central Venous Access Devices
Central venous access devices (CVADs) share in common tip termination in the cavo‐atrial junction, either in the lower portion of the superior vena cava or in the upper portion of the right atrium. CVADs can be safely used for irritant or vesicant medications as well as for blood withdrawal, blood exchange procedures (eg, dialysis), and hemodynamic monitoring. Traditionally, these devices are 15 to 25 cm in length and are directly inserted in the deep veins of the supra‐ or infraclavicular area, including the internal jugular, brachiocephalic, subclavian, or axillary veins. PICCs are unique CVADs in that they enter through peripheral veins but terminate in the proximity of the cavoatrial junction. Regarding nomenclature, CICC will be used to denote devices that enter directly into veins of the neck or chest, whereas PICC will be used for devices that are inserted peripherally but terminate centrally.
Peripherally Inserted Central Catheter
PICCs are inserted into peripheral veins of the upper arm (eg, brachial, basilica, or cephalic vein) and advanced such that the tip resides at the cavoatrial junction (Figure 1C). PICCs offer prolonged dwell times and are thus indicated when patients require venous access for weeks or months.[26] Additionally, they can accommodate a variety of infusates and are safer to insert than CICCs, given placement in peripheral veins of the arm rather than central veins of the chest/neck. Thus, insertion complications such as pneumothorax, hemothorax, or significant bleeding are rare with PICCs. In fact, a recent study reported that PICC insertion by hospitalists was associated with low rates of insertion or infectious complications.[27]
However, like CICCs, PICCs are associated with central lineassociated bloodstream infection (CLABSI), a serious complication known to prolong length of hospital stay, increase costs, and carry a 12% to 25% associated mortality.[28, 29] In the United States alone, over 250,000 CLASBI cases occur per year drawing considerable attention from the CDC and Joint Commission, who now mandate reporting and nonpayment for hospital‐acquired CLABSI.[30, 31, 32] A recent systematic review and meta‐analysis found that PICCs are associated with a substantial risk of CLABSI in hospitalized patients.[33] Importantly, no difference in CLABSI rates between PICCs and CICCs in hospitalized patients was evident in this meta‐analysis. Therefore, current guidelines specifically recommend against use of PICCs over CICCs as a strategy to reduce CLABSI.[34] Additionally, PICCs are associated with 2.5‐fold greater risk of deep vein thrombosis (DVT) compared to CICCs; thus, they should be used with caution in patients with cancer or those with underlying hypercoagulable states.
Of particular import to hospitalists is the fact that PICC placement is contraindicated in patients with stage IIIB or greater chronic kidney disease (CKD). In such patients, sequelae of PICC use, such as phlebitis or central vein stenosis, can be devastating in patients with CKD.[35] In a recent study, prior PICC placement was the strongest predictor of subsequent arteriovenous graft failure.[36] For this reason, Choosing Wisely recommendations call for avoidance of PICCs in such patients.[37]
Centrally Inserted Central Catheter
CICCs are CVADs placed by puncture and cannulation of the internal jugular, subclavian, brachiocephalic, or femoral veins (Figure 1D) and compose the vast majority of VADs placed in ICU settings.[38, 39] Central termination of CICCs allows for a variety of infusions, including irritants, vesicants, and vasopressors, as well as blood withdrawal and hemodynamic monitoring. CICCs are typically used for 7 to 14 days, but may remain for longer durations if they remain complication free and clinically necessary.[40] A key advantage of CICCs is that they can be placed in emergent settings to facilitate quick access for rapid infusion or hemodynamic monitoring. In particular, CICCs are inserted in the femoral vein and may be useful in emergency settings. However, owing to risk of infection and inability to monitor central pressures, these femoral devices should be replaced with a proper CICC or PICC when possible. Importantly, although CICCs are almost exclusively used in intensive or emergency care, PICCs may also be considered in such settings.[41, 42] CICCs usually have multiple lumens and often serve several simultaneous functions such as both infusions and hemodynamic monitoring.
Despite their benefits, CICCs have several disadvantages. First, insertion requires an experienced clinician and has historically been a task limited to physicians. However, this is changing rapidly (especially in Europe and Australia) where specially trained nurses are assuming responsibility for CICC placement.[43] Second, these devices are historically more likely to be associated with CLABSI, with estimates of infection rates varying between 2 and 5 infections per 1000 catheter‐days.[44] Third, CICCs pose a significant DVT risk, with rates around 22 DVTs per 1000 catheter‐days.[45] However, compared to PICCs, the DVT risk appears lower, and CICC use may be preferable in patients at high risk of DVT, such as critically ill or cancer populations.[46] An important note to prevent CICC insertion complications relates to use of ultrasound, a practice that has been associated with decreased accidental arterial puncture and hematoma formation. The role of ultrasound guidance with PICCs as well as implications for thrombotic and infectious events remains less characterized at this point.[47]
Tunneled Central Venous Access Devices
Tunneled devices (either CICCs or PICCs) are characterized by the fact that the insertion site on the skin and site of ultimate venipuncture are physically separated (Figure 1E). Tunneling limits bacterial entry from the extraluminal aspect of the CVAD to the bloodstream. For example, internal jugular veins are often ideal sites of puncture but inappropriate sites for catheter placement, as providing care to this area is challenging and may increase risk of infection.[34] Tunneling to the infraclavicular area provides a better option, as it provides an exit site that can be adequately cared for. Importantly, any CVAD (PICCs or CICCs) can be tunneled. Additionally, tunneled CICCs may be used in patients with chronic or impending renal failure where PICCs are contraindicated because entry into dialysis‐relevant vessels is to be avoided.[48] Such devices also allow regular blood sampling in patients who require frequent testing but have limited peripheral access, such as those with hematological malignancies. Additionally, tunneled catheters are more comfortable for patients and viewed as being more socially acceptable than nontunneled devices. However, the more invasive and permanent nature of these devices often requires deliberation prior to insertion.
Of note, tunneled devices and ports may be used as long‐term (>3 months to years) VADs. As our focus in this review is short‐term devices, we will not expand the discussion of these devices as they are almost always used for prolonged durations.[7]
OPERATIONALIZING THE DATA: AN ALGORITHMIC APPROACH TO VENOUS ACCESS
Hospitalists should consider approaching venous access using an algorithm based on a number of parameters. For example, a critically ill patient who requires vasopressor support and hemodynamic monitoring will need a CICC or a PICC. Given the potential greater risk of thromboses from PICCs, a CICC is preferable for critically ill patients provided an experienced inserter is available. Conversely, patients who require short‐term (710 days) venous access for infusion of nonirritant or nonvesicant therapy often only require a PIV. In patients with poor or difficult venous access, USGPIVs or midlines may be ideal and preferred over short PIVs. Finally, patients who require longer‐term or home‐based treatment may benefit from early placement of a midline or a PICC, depending again on the nature of the infusion, duration of treatment, and available venous access sites.
An algorithmic approach considering these parameters is suggested in Figure 2, and a brief overview of the devices and their considerations is shown in Table 3.
| Vascular Access Device | Central/Peripheral | Anatomical Location of Placement | Desired Duration of Placement | Common Uses | BSI Risk (Per 1,000 Catheter‐Days) | Thrombosis Risk | Important Considerations |
|---|---|---|---|---|---|---|---|
| |||||||
| Small peripheral IV | Peripheral | Peripheral veins, usually forearm | 710 days | Fluid resuscitation, most medications, blood products | 0.06[8] | Virtually no risk | Consider necessity of PIV daily and remove unnecessary devices |
| Midline | Peripheral | Inserted around antecubital fossa, reside within basilic or cephalic vein of the arm | 24 weeks | Long‐term medications excluding TPN, vesicants, corrosives | 0.30.8[17] | Insufficient data | Can be used as bridge devices for patients to complete short‐term antibiotics/emnfusions as an outpatient |
| Peripherally inserted central catheter | Central | Inserted into peripheral arm vein and advanced to larger veins (eg, internal jugular or subclavian) to the CAJ | >1 week, 3 months | Large variety of infusates, including TPN, vesicants, corrosives | 2.4[44] | 6.30% | Contraindicated in patients with CKD stage IIIb or higher |
| Centrally inserted central catheters | Central | Inserted above (internal jugular vein, brachiocephalic vein, subclavian vein), or below the clavicle (axillary vein) | >1 week, 3 months | Same infusate variety as PICC, measurement of central venous pressures, common in trauma/emergent settings | 2.3[44] | 1.30% | Given lower rates of DVT than PICC, preferred in ICU and hypercoagulable environments |
| Tunneled CICCs | Central | Placed percutaneously in any large vein in the arm, chest, neck or groin | >3 months to years | Central infusates, as in any CVAD; used for patients with CKD stage IIIb or greater when a PICC is indicated | Insufficient data | Insufficient data | May be superior when insertion site and puncture site are not congruent and may increase risk of infection |
CONCLUSIONS
With strides in technology and progress in medicine, hospitalists have access to an array of options for venous access. However, every VAD has limitations that can be easily overlooked in a perfunctory decision‐making process. The data presented in this review thus provide a first step to improving safety in this evolving science. Studies that further determine appropriateness of VADs in hospitalized settings are necessary. Only through such progressive scientific enquiry will complication‐free venous access be realized.
Disclosure
Nothing to report.
- , . The need for comparative data in vascular access: the rationale and design of the PICC registry. J Vasc Access. 2013; 18(4): 219–224.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013; 8(6): 309–314.
- , , , . Clinically‐indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2013; 4: CD007798.
- , , , et al. Cost‐effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014; 12(1): 51–58.
- Infusion Nurses Society. Infusion Nursing Standards of Practice. Norwood, MA; Infusion Nurses Society; 2011.
- , , , et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012; 380(9847): 1066–1074.
- , , , et al. epic3: national evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospitals in England. J Hosp Infect. 2014; 86(suppl 1): S1–S70.
- . Short peripheral intravenous catheters and infections. J Infus Nurs. 2012; 35(4): 230–240.
- , , , et al. Phlebitis risk varies by peripheral venous catheter site and increases after 96 hours: a large multi‐centre prospective study. J Adv Nurs. 2014; 70(11): 2539–2549.
- , . Intravenous catheter complications in the hand and forearm. J Trauma. 2004; 56(1): 123–127.
- , , , . Vesicant extravasation part I: Mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006; 33(6): 1134–1141.
- , . Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005; 34(5): 345–359.
- , , . Ultrasound‐guided peripheral venous access: a systematic review of randomized‐controlled trials. Eur J Emerg Med. 2014; 21(1): 18–23.
- , , , et al. Difficult peripheral venous access: clinical evaluation of a catheter inserted with the Seldinger method under ultrasound guidance. J Crit Care. 2014; 29(5): 823–827.
- . Choosing the right intravenous catheter. Home Healthc Nurse. 2007; 25(8): 523–531; quiz 532–523.
- Adverse reactions associated with midline catheters—United States, 1992–1995. MMWR Morb Mortal Wkly Rep. 1996; 45(5): 101–103.
- , , . The risk of midline catheterization in hospitalized patients. A prospective study. Ann Intern Med. 1995; 123(11): 841–844.
- . Midline catheters: indications, complications and maintenance. Nurs Stand. 2007; 22(11): 48–57; quiz 58.
- , . Safe administration of vancomycin through a novel midline catheter: a randomized, prospective clinical trial. J Vasc Access. 2014; 15(4): 251–256.
- . Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004; 27(5): 313–321.
- . Vascular access in resuscitation: is there a role for the intraosseous route? Anesthesiology. 2014; 120(4): 1015–1031.
- , , , et al. A new system for sternal intraosseous infusion in adults. Prehosp Emerg Care. 2000; 4(2): 173–177.
- , , , , , . Comparison of two intraosseous access devices in adult patients under resuscitation in the emergency department: a prospective, randomized study. Resuscitation. 2010; 81(8): 994–999.
- , , , , . Comparison study of intraosseous, central intravenous, and peripheral intravenous infusions of emergency drugs. Am J Dis Child. 1990; 144(1): 112–117.
- , , , , . The safety of intraosseous infusions: risks of fat and bone marrow emboli to the lungs. Ann Emerg Med. 1989; 18(10): 1062–1067.
- , , , , . ESPEN guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr. 2009; 28(4): 365–377.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009; 4(6): E1–E4.
- Vital signs: central line‐associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011; 60(8): 243–248.
- , , , . Hospital costs of central line‐associated bloodstream infections and cost‐effectiveness of closed vs. open infusion containers. The case of Intensive Care Units in Italy. Cost Eff Resour Alloc. 2010; 8: 8.
- , , . The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006; 81(9): 1159–1171.
- The Joint Commission. Preventing Central Line‐Associated Bloodstream Infections: A Global Challenge, a Global Perspective. Oak Brook, IL: Joint Commission Resources; 2012.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011; 39(4 suppl 1): S1–S34.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013; 34(9): 908–918.
- Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, American Hospital Association, Association for Professionals in Infection Control and Epidemiology, The Joint Commission. Compendium of Strategies to Prevent Healthcare‐Associated Infections in Acute Care Hospitals: 2014 Updates. Available at: http://www.shea‐online.org. Accessed August 1, 2014.
- , , , , . Guidelines for venous access in patients with chronic kidney disease. A Position Statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access. Semin Dial. 2008; 21(2): 186–191.
- , , , et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case‐control study in hemodialysis patients. Am J Kidney Dis. 2012; 60(4): 601–608.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012; 7(10): 1664–1672.
- , , , et al. Do physicians know which of their patients have central venous catheters? A multi‐center observational study. Ann Intern Med. 2014; 161(8): 562–567.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011; 77(4): 304–308.
- , , , , , . Peripherally inserted central catheter‐related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014; 12(6): 847–854.
- , , , . An in vitro study comparing a peripherally inserted central catheter to a conventional central venous catheter: no difference in static and dynamic pressure transmission. BMC Anesthesiol. 2010; 10: 18.
- , , , et al. Clinical experience with power‐injectable PICCs in intensive care patients. Crit Care. 2012; 16(1): R21.
- , , , et al. Nurse‐led central venous catheter insertion‐procedural characteristics and outcomes of three intensive care based catheter placement services. Int J Nurs Stud. 2012; 49(2): 162–168.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010; 38(2): 149–153.
- , , , et al. Which central venous catheters have the highest rate of catheter‐associated deep venous thrombosis: a prospective analysis of 2,128 catheter days in the surgical intensive care unit. J Trauma Acute Care Surg. 2013; 74(2): 454–460; discussion 461–452.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013; 382(9889): 311–325.
- , , , , . Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015; 1: CD011447.
- , , , et al. Tunneled jugular small‐bore central catheters as an alternative to peripherally inserted central catheters for intermediate‐term venous access in patients with hemodialysis and chronic renal insufficiency. Radiology. 1999; 213(1): 303–306.
Reliable venous access is fundamental for the safe and effective care of hospitalized patients. Venous access devices (VADs) are conduits for this purpose, providing delivery of intravenous medications, accurate measurement of central venous pressure, or administration of life‐saving blood products. Despite this important role, VADs are also often the source of hospital‐acquired complications. Although inpatient providers must balance the relative risks of VADs against their benefits, the evidence supporting such decisions is often limited. Advances in technology, scattered research, and growing availability of novel devices has only further fragmented provider knowledge in the field of vascular access.[1]
It is not surprising, then, that survey‐based studies of hospitalists reveal important knowledge gaps with regard to practices associated with VADs.[2] In this narrative review, we seek to bridge this gap by providing a concise and pragmatic overview of the fundamentals of venous access. We focus specifically on parameters that influence decisions regarding VAD placement in hospitalized patients, providing key takeaways for practicing hospitalists.
METHODS
To compile this review, we systematically searched Medline (via Ovid) for several keywords, including: peripheral intravenous catheters, ultrasound‐guided peripheral catheter, intraosseous, midline, peripherally inserted central catheter, central venous catheters, and vascular access device complications. We concentrated on full‐length articles in English only; no date restrictions were placed on the search. We reviewed guidelines and consensus statements (eg, from the Center for Disease Control [CDC] or Choosing Wisely criteria) as appropriate. Additional studies of interest were identified through content experts (M.P., C.M.R.) and bibliographies of included studies.
SCIENTIFIC PRINCIPLES UNDERPINNING VENOUS ACCESS
It is useful to begin by reviewing VAD‐related nomenclature and physiology. In the simplest sense, a VAD consists of a hub (providing access to various connectors), a hollow tube divided into 1 or many sections (lumens), and a tip that may terminate within a central or peripheral blood vessel. VADs are classified as central venous catheters (eg, centrally inserted central catheters [CICCs] or peripherally inserted central catheters [PICCs]) or peripheral intravenous catheters (eg, midlines or peripheral intravenous catheters) based on site of entry and location of the catheter tip. Therefore, VADs entering via proximal or distal veins of the arm are often referred to as peripheral lines, as their site of entry and tip both reside within peripheral veins. Conversely, the term central line is often used when VADs enter or terminate in a central vein (eg, subclavian vein insertion with the catheter tip in the lower superior vena cava).
Attention to a host of clinical and theoretical parameters is important when choosing a device for venous access. Some such parameters are summarized in Table 1.
| Parameter | Major Considerations |
|---|---|
| |
| Desired flow rate | Smaller diameter veins susceptible to damage with high flow rates. |
| Short, large‐bore catheters facilitate rapid infusion. | |
| Nature of infusion | pH, viscosity, and temperature may damage vessels. |
| Vesicants and irritants should always be administered into larger, central veins. | |
| Desired duration of vascular access, or dwell time | Vessel thrombosis or phlebitis increase over time with catheter in place. |
| Intermittent infusions increase complications in central catheters; often tunneled catheters are recommended. | |
| Urgency of placement | Access to large caliber vessels is often needed in emergencies. |
| Critically ill or hemodynamically unstable patients may require urgent access for invasive monitoring or rapid infusions. | |
| Patients with trauma often require large volumes of blood products and reliable access to central veins. | |
| Number of device lumens | VADs may have single or multiple lumens. |
| Multilumen allows for multiple functions (eg, infusion of multiple agents, measurement of central venous pressures, blood draws). | |
| Device gauge | In general, use of a smaller‐gauge catheter is preferred to prevent complications. |
| However, larger catheter diameter may be needed for specific clinical needs (eg, blood transfusion). | |
| Device coating | VADs may have antithrombotic or anti‐infective coatings. |
| These devices may be of value in patients at high risk of complications. | |
| Such devices, however, may be more costly than their counterparts. | |
| Self‐care compatibility | VADs that can be cared for by patients are ideal for outpatient care. |
| Conversely, VADs such as peripheral catheters, are highly prone to dislodgement and should be reserved for supervised settings only. | |
VENOUS ACCESS DEVICES
We will organize our discussion of VADs based on whether they terminate in peripheral or central vessels. These anatomical considerations are relevant as they determine physical characteristics, compatibility with particular infusates, dwell time, and risk of complications associated with each VAD discussed in Table 2.
| Complications | Major Considerations |
|---|---|
| |
| Infection | VADs breach the integrity of skin and permit skin pathogens to enter the blood stream (extraluminal infection). |
| Inadequate antisepsis of the VAD hub, including poor hand hygiene, failure to "scrub the hub," and multiple manipulations may also increase the risk of VAD‐related infection (endoluminal infection). | |
| Infections may be local (eg, exit‐site infections) or may spread hematogenously (eg, CLABSI). | |
| Type of VAD, duration of therapy, and host characteristics interact to influence infection risk. | |
| VADs with antiseptic coatings (eg, chlorhexidine) or antibiotic coatings (eg, minocycline) may reduce risk of infection in high‐risk patients. | |
| Antiseptic‐impregnated dressings may reduce risk of extraluminal infection. | |
| Venous thrombosis | VADs predispose to venous stasis and thrombosis. |
| Duration of VAD use, type and care of the VAD, and patient characteristics affect risk of thromboembolism. | |
| VAD tip position is a key determinant of venous thrombosis; central VADs that do not terminate at the cavo‐atrial junction should be repositioned to reduce the risk of thrombosis. | |
| Antithrombotic coated or eluting devices may reduce risk of thrombosis, though definitive data are lacking. | |
| Phlebitis | Inflammation caused by damage to tunica media.[18] |
| 3 types of phlebitis: | |
| Chemical: due to irritation of media from the infusate. | |
| Mechanical: VAD physically damages the vessel. | |
| Infective: bacteria invade vein and inflame vessel wall. | |
| Phlebitis may be limited by close attention to infusate compatibility with peripheral veins, appropriate dilution, and prompt removal of catheters that show signs of inflammation. | |
| Phlebitis may be prevented in PICCs by ensuring at least a 2:1 vein:catheter ratio. | |
| Extravasation | Extravasation (also called infiltration) is defined as leakage of infusate from intravascular to extravascular space. |
| Extravasation of vesicants/emrritants is particularly worrisome. | |
| May result in severe tissue injury, blistering, and tissue necrosis.[11] | |
| VADs should be checked frequently for adequate flushing and position prior to each infusion to minimize risk. | |
| Any VAD with redness, swelling, and tenderness at the entry site or problems with flushing should not be used without further examination and review of position. | |
Peripheral Venous Access
Short Peripheral Intravenous Catheter
Approximately 200 million peripheral intravenous catheters (PIVs) are placed annually in the United States, making them the most common intravenous catheter.[3] PIVs are short devices, 3 to 6 cm in length, that enter and terminate in peripheral veins (Figure 1A). Placement is recommended in forearm veins rather than those of the hand, wrist, or upper arm, as forearm sites are less prone to occlusion, accidental removal, and phlebitis.[4] Additionally, placement in hand veins impedes activities of daily living (eg, hand washing) and is not preferred by patients.[5] PIV size ranges from 24 gauge (smallest) to 14 gauge (largest); larger catheters are often reserved for fluid resuscitation or blood transfusion as they accommodate greater flow and limit hemolysis. To decrease risk of phlebitis and thrombosis, the shortest catheter and smallest diameter should be used. However, unless adequately secured, smaller diameter catheters are also associated with greater rates of accidental removal.[4, 5]

By definition, PIVs are short‐term devices. The CDC currently recommends removal and replacement of these devices no more frequently than every 72 to 96 hours in adults. However, a recent randomized controlled trial found that replacing PIVs when clinically indicated (eg, device failure, phlebitis) rather than on a routine schedule added 30 hours to their lifespan without an increase in complications.[6] A systematic review by the Cochrane Collaboration echoes these findings.[3] These data have thus been incorporated into recommendations from the Infusion Nurses Society (INS) and the National Health Service in the United Kingdom.[5, 7] In hospitalized patients, this approach is relevant, as it preserves venous access sites, maximizes device dwell, and limits additional PIV insertions. In turn, these differences may reduce the need for invasive VADs such as PICCs. Furthermore, the projected 5‐year savings from implementation of clinically indicated PIV removal policies is US$300 million and 1 million health‐worker hours in the United States alone.[4]
PIVs offer many advantages. First, they are minimally invasive and require little training to insert. Second, they can be used for diverse indications in patients requiring short‐term (1 week) venous access. Third, PIVs do not require imaging to ensure correct placement; palpation of superficial veins is sufficient. Fourth, PIVs exhibit a risk of bloodstream infection that is about 40‐fold lower than more invasive, longer‐dwelling VADs[8] (0.06 bacteremia per 1000 catheter‐days).
Despite these advantages, PIVs also have important drawbacks. First, a quarter of all PIVs fail through occlusion or accidental dislodgement.[4] Infiltration, extravasation, and hematoma formation are important adverse events that may occur in such cases. Second, thrombophlebitis (pain and redness at the insertion site) is frequent, and may require device removal, especially in patients with catheters 20 guage.[9] Third, despite their relative safety, PIVs can cause localized or hematogenous infection. Septic thrombophlebitis (superficial thrombosis and bloodstream infection) and catheter‐related bloodstream infection, though rare, have been reported with PIVs and may lead to serious complications.[8, 10] In fact, some suggest that the overall burden of bloodstream infection risk posed by PIVs may be similar to that of CICCs given the substantially greater number of devices used and greater number of device days.[8]
PIVs and other peripheral VADs are not suitable for infusion of vesicants or irritants, which require larger, central veins for delivery. Vesicants (drugs that cause blistering on infusion) include chemotherapeutic agents (eg, dactinomycin, paclitaxel) and commonly used nonchemotherapeutical agents (eg, diazepam, piperacillin, vancomycin, esmolol, or total parenteral nutrition [TPN]).[11] Irritants (phlebitogenic drugs) cause short‐term inflammation and pain, and thus should not be peripherally infused for prolonged durations. Common irritants in the hospital setting include acyclovir, dobutamine, penicillin, and potassium chloride.
Of note, about one‐quarter of PIV insertions fail owing to difficult intravenous access.[12] Ultrasound‐guided peripheral intravenous (USGPIV) catheter placement is emerging as a technique to provide peripheral access for such patients to avoid placement of central venous access devices. Novel, longer devices (>8 cm) with built‐in guide wires have been developed to increase placement success of USGPIVs. These new designs provide easier access into deeper arm veins (brachial or basilic) not otherwise accessible by short PIVs. Although studies comparing the efficacy of USGPIV devices to other VADs are limited, a recent systematic review showed that time to successful cannulation was shorter, and fewer attempts were required to place USGPIVs compared to PIVs.[13] A recent study in France found that USGPIVs met the infusion needs of patients with difficult veins with minimal increase in complications.[14] Despite these encouraging data, future studies are needed to better evaluate this technology.
Midline Catheter
A midline is a VAD that is between 7.5 to 25 cm in length and is typically inserted into veins above the antecubital fossa. The catheter tip resides in a peripheral upper arm vein, often the basilic or cephalic vein, terminating just short of the subclavian vein (Figure 1B). Midline‐like devices were first developed in the 1950s and were initially used as an alternative to PIVs because they were thought to allow longer dwell times.[15] However, because they were originally constructed with a fairly rigid material, infiltration, mechanical phlebitis, and inflammation were common and tempered enthusiasm for their use.[15, 16] Newer midline devices obviate many of these problems and are inserted by ultrasound guidance and modified Seldinger technique.[17] Despite these advances, data regarding comparative efficacy are limited.
Midlines offer longer dwell times than standard PIVs owing to termination in the larger diameter basilic and brachial veins of the arm. Additionally, owing to their length, midlines are less prone to dislodgement. As they are inserted with greater antisepsis than PIVs and better secured to the skin, they are more durable than PIVs.[5, 9, 18] Current INS standards recommend use of midlines for 1 to 4 weeks.[5] Because they terminate in a peripheral vein, medications and infusions compatible with midlines are identical to those that are infused through a PIV. Thus, TPN, vesicants or irritants, or drugs that feature a pH 5 or pH >9, or >500 mOsm should not be infused through a midline.[15] New evidence suggests that diluted solutions of vancomycin (usually pH 5) may be safe to infuse for short durations (6 days) through a midline, and that concentration rather than pH may be more important in this regard.[19] Although it is possible that the use of midlines may extend to agents typically not deemed peripheral access compatible, limited evidence exists to support such a strategy at this time.
Midlines offer several advantages. First, because blood flow is greater in the more proximal veins of the arm, midlines can accommodate infusions at rates of 100 to 150 mL/min compared to 20 to 40 mL/min in smaller peripheral veins. Higher flow rates offer greater hemodilution (dilution of the infusion with blood), decreasing the likelihood of phlebitis and infiltration.[20] Second, midlines do not require x‐ray verification of tip placement; thus, their use is often favored in resource‐depleted settings such as skilled nursing facilities. Third, midlines offer longer dwell times than peripheral intravenous catheters and can thus serve as bridge devices for short‐term intravenous antibiotics or peripheral‐compatible infusions in an outpatient setting. Available evidence suggests that midlines are associated with low rates of bloodstream infection (0.30.8 per 1000 catheter‐days).[17] The most frequent complications include phlebitis (4.2%) and occlusion (3.3%).[20] Given these favorable statistics, midlines may offer a good alternative to PIVs in select patients who require peripheral infusions of intermediate duration.
Intraosseous Vascular Access
Intraosseous (IO) devices access the vascular system by piercing cortical bone. These devices provide access to the intramedullary cavity and venous plexi of long bones such as the tibia, femur, or humerus. Several insertion devices are now commercially available and have enhanced the ease and safety of IO placement. Using these newer devices, IO access may be obtained in 1 to 2 minutes with minimal training. By comparison, a central venous catheter often requires 10 to 15 minutes to insert with substantial training efforts for providers.[21, 22, 23]
IO devices thus offer several advantages. First, given the rapidity with which they can be inserted, they are often preferred in emergency settings (eg, trauma). Second, these devices are versatile and can accommodate both central and peripheral infusates.[24] Third, a recent meta‐analysis found that IOs have a low complication rate of 0.8%, with extravasation of infusate through the cortical entry site being the most common adverse event.[21] Of note, this study also reported zero local or distal infectious complications, a finding that may relate to the shorter dwell of these devices.[21] Some animal studies suggest that fat embolism from bone may occur at high rates with IO VADs.[25] However, death or significant morbidity from fat emboli in humans following IO access has not been described. Whether such emboli occur or are clinically significant in the context of IO devices remains unclear at this time.[21]
Central Venous Access Devices
Central venous access devices (CVADs) share in common tip termination in the cavo‐atrial junction, either in the lower portion of the superior vena cava or in the upper portion of the right atrium. CVADs can be safely used for irritant or vesicant medications as well as for blood withdrawal, blood exchange procedures (eg, dialysis), and hemodynamic monitoring. Traditionally, these devices are 15 to 25 cm in length and are directly inserted in the deep veins of the supra‐ or infraclavicular area, including the internal jugular, brachiocephalic, subclavian, or axillary veins. PICCs are unique CVADs in that they enter through peripheral veins but terminate in the proximity of the cavoatrial junction. Regarding nomenclature, CICC will be used to denote devices that enter directly into veins of the neck or chest, whereas PICC will be used for devices that are inserted peripherally but terminate centrally.
Peripherally Inserted Central Catheter
PICCs are inserted into peripheral veins of the upper arm (eg, brachial, basilica, or cephalic vein) and advanced such that the tip resides at the cavoatrial junction (Figure 1C). PICCs offer prolonged dwell times and are thus indicated when patients require venous access for weeks or months.[26] Additionally, they can accommodate a variety of infusates and are safer to insert than CICCs, given placement in peripheral veins of the arm rather than central veins of the chest/neck. Thus, insertion complications such as pneumothorax, hemothorax, or significant bleeding are rare with PICCs. In fact, a recent study reported that PICC insertion by hospitalists was associated with low rates of insertion or infectious complications.[27]
However, like CICCs, PICCs are associated with central lineassociated bloodstream infection (CLABSI), a serious complication known to prolong length of hospital stay, increase costs, and carry a 12% to 25% associated mortality.[28, 29] In the United States alone, over 250,000 CLASBI cases occur per year drawing considerable attention from the CDC and Joint Commission, who now mandate reporting and nonpayment for hospital‐acquired CLABSI.[30, 31, 32] A recent systematic review and meta‐analysis found that PICCs are associated with a substantial risk of CLABSI in hospitalized patients.[33] Importantly, no difference in CLABSI rates between PICCs and CICCs in hospitalized patients was evident in this meta‐analysis. Therefore, current guidelines specifically recommend against use of PICCs over CICCs as a strategy to reduce CLABSI.[34] Additionally, PICCs are associated with 2.5‐fold greater risk of deep vein thrombosis (DVT) compared to CICCs; thus, they should be used with caution in patients with cancer or those with underlying hypercoagulable states.
Of particular import to hospitalists is the fact that PICC placement is contraindicated in patients with stage IIIB or greater chronic kidney disease (CKD). In such patients, sequelae of PICC use, such as phlebitis or central vein stenosis, can be devastating in patients with CKD.[35] In a recent study, prior PICC placement was the strongest predictor of subsequent arteriovenous graft failure.[36] For this reason, Choosing Wisely recommendations call for avoidance of PICCs in such patients.[37]
Centrally Inserted Central Catheter
CICCs are CVADs placed by puncture and cannulation of the internal jugular, subclavian, brachiocephalic, or femoral veins (Figure 1D) and compose the vast majority of VADs placed in ICU settings.[38, 39] Central termination of CICCs allows for a variety of infusions, including irritants, vesicants, and vasopressors, as well as blood withdrawal and hemodynamic monitoring. CICCs are typically used for 7 to 14 days, but may remain for longer durations if they remain complication free and clinically necessary.[40] A key advantage of CICCs is that they can be placed in emergent settings to facilitate quick access for rapid infusion or hemodynamic monitoring. In particular, CICCs are inserted in the femoral vein and may be useful in emergency settings. However, owing to risk of infection and inability to monitor central pressures, these femoral devices should be replaced with a proper CICC or PICC when possible. Importantly, although CICCs are almost exclusively used in intensive or emergency care, PICCs may also be considered in such settings.[41, 42] CICCs usually have multiple lumens and often serve several simultaneous functions such as both infusions and hemodynamic monitoring.
Despite their benefits, CICCs have several disadvantages. First, insertion requires an experienced clinician and has historically been a task limited to physicians. However, this is changing rapidly (especially in Europe and Australia) where specially trained nurses are assuming responsibility for CICC placement.[43] Second, these devices are historically more likely to be associated with CLABSI, with estimates of infection rates varying between 2 and 5 infections per 1000 catheter‐days.[44] Third, CICCs pose a significant DVT risk, with rates around 22 DVTs per 1000 catheter‐days.[45] However, compared to PICCs, the DVT risk appears lower, and CICC use may be preferable in patients at high risk of DVT, such as critically ill or cancer populations.[46] An important note to prevent CICC insertion complications relates to use of ultrasound, a practice that has been associated with decreased accidental arterial puncture and hematoma formation. The role of ultrasound guidance with PICCs as well as implications for thrombotic and infectious events remains less characterized at this point.[47]
Tunneled Central Venous Access Devices
Tunneled devices (either CICCs or PICCs) are characterized by the fact that the insertion site on the skin and site of ultimate venipuncture are physically separated (Figure 1E). Tunneling limits bacterial entry from the extraluminal aspect of the CVAD to the bloodstream. For example, internal jugular veins are often ideal sites of puncture but inappropriate sites for catheter placement, as providing care to this area is challenging and may increase risk of infection.[34] Tunneling to the infraclavicular area provides a better option, as it provides an exit site that can be adequately cared for. Importantly, any CVAD (PICCs or CICCs) can be tunneled. Additionally, tunneled CICCs may be used in patients with chronic or impending renal failure where PICCs are contraindicated because entry into dialysis‐relevant vessels is to be avoided.[48] Such devices also allow regular blood sampling in patients who require frequent testing but have limited peripheral access, such as those with hematological malignancies. Additionally, tunneled catheters are more comfortable for patients and viewed as being more socially acceptable than nontunneled devices. However, the more invasive and permanent nature of these devices often requires deliberation prior to insertion.
Of note, tunneled devices and ports may be used as long‐term (>3 months to years) VADs. As our focus in this review is short‐term devices, we will not expand the discussion of these devices as they are almost always used for prolonged durations.[7]
OPERATIONALIZING THE DATA: AN ALGORITHMIC APPROACH TO VENOUS ACCESS
Hospitalists should consider approaching venous access using an algorithm based on a number of parameters. For example, a critically ill patient who requires vasopressor support and hemodynamic monitoring will need a CICC or a PICC. Given the potential greater risk of thromboses from PICCs, a CICC is preferable for critically ill patients provided an experienced inserter is available. Conversely, patients who require short‐term (710 days) venous access for infusion of nonirritant or nonvesicant therapy often only require a PIV. In patients with poor or difficult venous access, USGPIVs or midlines may be ideal and preferred over short PIVs. Finally, patients who require longer‐term or home‐based treatment may benefit from early placement of a midline or a PICC, depending again on the nature of the infusion, duration of treatment, and available venous access sites.
An algorithmic approach considering these parameters is suggested in Figure 2, and a brief overview of the devices and their considerations is shown in Table 3.
| Vascular Access Device | Central/Peripheral | Anatomical Location of Placement | Desired Duration of Placement | Common Uses | BSI Risk (Per 1,000 Catheter‐Days) | Thrombosis Risk | Important Considerations |
|---|---|---|---|---|---|---|---|
| |||||||
| Small peripheral IV | Peripheral | Peripheral veins, usually forearm | 710 days | Fluid resuscitation, most medications, blood products | 0.06[8] | Virtually no risk | Consider necessity of PIV daily and remove unnecessary devices |
| Midline | Peripheral | Inserted around antecubital fossa, reside within basilic or cephalic vein of the arm | 24 weeks | Long‐term medications excluding TPN, vesicants, corrosives | 0.30.8[17] | Insufficient data | Can be used as bridge devices for patients to complete short‐term antibiotics/emnfusions as an outpatient |
| Peripherally inserted central catheter | Central | Inserted into peripheral arm vein and advanced to larger veins (eg, internal jugular or subclavian) to the CAJ | >1 week, 3 months | Large variety of infusates, including TPN, vesicants, corrosives | 2.4[44] | 6.30% | Contraindicated in patients with CKD stage IIIb or higher |
| Centrally inserted central catheters | Central | Inserted above (internal jugular vein, brachiocephalic vein, subclavian vein), or below the clavicle (axillary vein) | >1 week, 3 months | Same infusate variety as PICC, measurement of central venous pressures, common in trauma/emergent settings | 2.3[44] | 1.30% | Given lower rates of DVT than PICC, preferred in ICU and hypercoagulable environments |
| Tunneled CICCs | Central | Placed percutaneously in any large vein in the arm, chest, neck or groin | >3 months to years | Central infusates, as in any CVAD; used for patients with CKD stage IIIb or greater when a PICC is indicated | Insufficient data | Insufficient data | May be superior when insertion site and puncture site are not congruent and may increase risk of infection |

CONCLUSIONS
With strides in technology and progress in medicine, hospitalists have access to an array of options for venous access. However, every VAD has limitations that can be easily overlooked in a perfunctory decision‐making process. The data presented in this review thus provide a first step to improving safety in this evolving science. Studies that further determine appropriateness of VADs in hospitalized settings are necessary. Only through such progressive scientific enquiry will complication‐free venous access be realized.
Disclosure
Nothing to report.
Reliable venous access is fundamental for the safe and effective care of hospitalized patients. Venous access devices (VADs) are conduits for this purpose, providing delivery of intravenous medications, accurate measurement of central venous pressure, or administration of life‐saving blood products. Despite this important role, VADs are also often the source of hospital‐acquired complications. Although inpatient providers must balance the relative risks of VADs against their benefits, the evidence supporting such decisions is often limited. Advances in technology, scattered research, and growing availability of novel devices has only further fragmented provider knowledge in the field of vascular access.[1]
It is not surprising, then, that survey‐based studies of hospitalists reveal important knowledge gaps with regard to practices associated with VADs.[2] In this narrative review, we seek to bridge this gap by providing a concise and pragmatic overview of the fundamentals of venous access. We focus specifically on parameters that influence decisions regarding VAD placement in hospitalized patients, providing key takeaways for practicing hospitalists.
METHODS
To compile this review, we systematically searched Medline (via Ovid) for several keywords, including: peripheral intravenous catheters, ultrasound‐guided peripheral catheter, intraosseous, midline, peripherally inserted central catheter, central venous catheters, and vascular access device complications. We concentrated on full‐length articles in English only; no date restrictions were placed on the search. We reviewed guidelines and consensus statements (eg, from the Center for Disease Control [CDC] or Choosing Wisely criteria) as appropriate. Additional studies of interest were identified through content experts (M.P., C.M.R.) and bibliographies of included studies.
SCIENTIFIC PRINCIPLES UNDERPINNING VENOUS ACCESS
It is useful to begin by reviewing VAD‐related nomenclature and physiology. In the simplest sense, a VAD consists of a hub (providing access to various connectors), a hollow tube divided into 1 or many sections (lumens), and a tip that may terminate within a central or peripheral blood vessel. VADs are classified as central venous catheters (eg, centrally inserted central catheters [CICCs] or peripherally inserted central catheters [PICCs]) or peripheral intravenous catheters (eg, midlines or peripheral intravenous catheters) based on site of entry and location of the catheter tip. Therefore, VADs entering via proximal or distal veins of the arm are often referred to as peripheral lines, as their site of entry and tip both reside within peripheral veins. Conversely, the term central line is often used when VADs enter or terminate in a central vein (eg, subclavian vein insertion with the catheter tip in the lower superior vena cava).
Attention to a host of clinical and theoretical parameters is important when choosing a device for venous access. Some such parameters are summarized in Table 1.
| Parameter | Major Considerations |
|---|---|
| |
| Desired flow rate | Smaller diameter veins susceptible to damage with high flow rates. |
| Short, large‐bore catheters facilitate rapid infusion. | |
| Nature of infusion | pH, viscosity, and temperature may damage vessels. |
| Vesicants and irritants should always be administered into larger, central veins. | |
| Desired duration of vascular access, or dwell time | Vessel thrombosis or phlebitis increase over time with catheter in place. |
| Intermittent infusions increase complications in central catheters; often tunneled catheters are recommended. | |
| Urgency of placement | Access to large caliber vessels is often needed in emergencies. |
| Critically ill or hemodynamically unstable patients may require urgent access for invasive monitoring or rapid infusions. | |
| Patients with trauma often require large volumes of blood products and reliable access to central veins. | |
| Number of device lumens | VADs may have single or multiple lumens. |
| Multilumen allows for multiple functions (eg, infusion of multiple agents, measurement of central venous pressures, blood draws). | |
| Device gauge | In general, use of a smaller‐gauge catheter is preferred to prevent complications. |
| However, larger catheter diameter may be needed for specific clinical needs (eg, blood transfusion). | |
| Device coating | VADs may have antithrombotic or anti‐infective coatings. |
| These devices may be of value in patients at high risk of complications. | |
| Such devices, however, may be more costly than their counterparts. | |
| Self‐care compatibility | VADs that can be cared for by patients are ideal for outpatient care. |
| Conversely, VADs such as peripheral catheters, are highly prone to dislodgement and should be reserved for supervised settings only. | |
VENOUS ACCESS DEVICES
We will organize our discussion of VADs based on whether they terminate in peripheral or central vessels. These anatomical considerations are relevant as they determine physical characteristics, compatibility with particular infusates, dwell time, and risk of complications associated with each VAD discussed in Table 2.
| Complications | Major Considerations |
|---|---|
| |
| Infection | VADs breach the integrity of skin and permit skin pathogens to enter the blood stream (extraluminal infection). |
| Inadequate antisepsis of the VAD hub, including poor hand hygiene, failure to "scrub the hub," and multiple manipulations may also increase the risk of VAD‐related infection (endoluminal infection). | |
| Infections may be local (eg, exit‐site infections) or may spread hematogenously (eg, CLABSI). | |
| Type of VAD, duration of therapy, and host characteristics interact to influence infection risk. | |
| VADs with antiseptic coatings (eg, chlorhexidine) or antibiotic coatings (eg, minocycline) may reduce risk of infection in high‐risk patients. | |
| Antiseptic‐impregnated dressings may reduce risk of extraluminal infection. | |
| Venous thrombosis | VADs predispose to venous stasis and thrombosis. |
| Duration of VAD use, type and care of the VAD, and patient characteristics affect risk of thromboembolism. | |
| VAD tip position is a key determinant of venous thrombosis; central VADs that do not terminate at the cavo‐atrial junction should be repositioned to reduce the risk of thrombosis. | |
| Antithrombotic coated or eluting devices may reduce risk of thrombosis, though definitive data are lacking. | |
| Phlebitis | Inflammation caused by damage to tunica media.[18] |
| 3 types of phlebitis: | |
| Chemical: due to irritation of media from the infusate. | |
| Mechanical: VAD physically damages the vessel. | |
| Infective: bacteria invade vein and inflame vessel wall. | |
| Phlebitis may be limited by close attention to infusate compatibility with peripheral veins, appropriate dilution, and prompt removal of catheters that show signs of inflammation. | |
| Phlebitis may be prevented in PICCs by ensuring at least a 2:1 vein:catheter ratio. | |
| Extravasation | Extravasation (also called infiltration) is defined as leakage of infusate from intravascular to extravascular space. |
| Extravasation of vesicants/emrritants is particularly worrisome. | |
| May result in severe tissue injury, blistering, and tissue necrosis.[11] | |
| VADs should be checked frequently for adequate flushing and position prior to each infusion to minimize risk. | |
| Any VAD with redness, swelling, and tenderness at the entry site or problems with flushing should not be used without further examination and review of position. | |
Peripheral Venous Access
Short Peripheral Intravenous Catheter
Approximately 200 million peripheral intravenous catheters (PIVs) are placed annually in the United States, making them the most common intravenous catheter.[3] PIVs are short devices, 3 to 6 cm in length, that enter and terminate in peripheral veins (Figure 1A). Placement is recommended in forearm veins rather than those of the hand, wrist, or upper arm, as forearm sites are less prone to occlusion, accidental removal, and phlebitis.[4] Additionally, placement in hand veins impedes activities of daily living (eg, hand washing) and is not preferred by patients.[5] PIV size ranges from 24 gauge (smallest) to 14 gauge (largest); larger catheters are often reserved for fluid resuscitation or blood transfusion as they accommodate greater flow and limit hemolysis. To decrease risk of phlebitis and thrombosis, the shortest catheter and smallest diameter should be used. However, unless adequately secured, smaller diameter catheters are also associated with greater rates of accidental removal.[4, 5]

By definition, PIVs are short‐term devices. The CDC currently recommends removal and replacement of these devices no more frequently than every 72 to 96 hours in adults. However, a recent randomized controlled trial found that replacing PIVs when clinically indicated (eg, device failure, phlebitis) rather than on a routine schedule added 30 hours to their lifespan without an increase in complications.[6] A systematic review by the Cochrane Collaboration echoes these findings.[3] These data have thus been incorporated into recommendations from the Infusion Nurses Society (INS) and the National Health Service in the United Kingdom.[5, 7] In hospitalized patients, this approach is relevant, as it preserves venous access sites, maximizes device dwell, and limits additional PIV insertions. In turn, these differences may reduce the need for invasive VADs such as PICCs. Furthermore, the projected 5‐year savings from implementation of clinically indicated PIV removal policies is US$300 million and 1 million health‐worker hours in the United States alone.[4]
PIVs offer many advantages. First, they are minimally invasive and require little training to insert. Second, they can be used for diverse indications in patients requiring short‐term (1 week) venous access. Third, PIVs do not require imaging to ensure correct placement; palpation of superficial veins is sufficient. Fourth, PIVs exhibit a risk of bloodstream infection that is about 40‐fold lower than more invasive, longer‐dwelling VADs[8] (0.06 bacteremia per 1000 catheter‐days).
Despite these advantages, PIVs also have important drawbacks. First, a quarter of all PIVs fail through occlusion or accidental dislodgement.[4] Infiltration, extravasation, and hematoma formation are important adverse events that may occur in such cases. Second, thrombophlebitis (pain and redness at the insertion site) is frequent, and may require device removal, especially in patients with catheters 20 guage.[9] Third, despite their relative safety, PIVs can cause localized or hematogenous infection. Septic thrombophlebitis (superficial thrombosis and bloodstream infection) and catheter‐related bloodstream infection, though rare, have been reported with PIVs and may lead to serious complications.[8, 10] In fact, some suggest that the overall burden of bloodstream infection risk posed by PIVs may be similar to that of CICCs given the substantially greater number of devices used and greater number of device days.[8]
PIVs and other peripheral VADs are not suitable for infusion of vesicants or irritants, which require larger, central veins for delivery. Vesicants (drugs that cause blistering on infusion) include chemotherapeutic agents (eg, dactinomycin, paclitaxel) and commonly used nonchemotherapeutical agents (eg, diazepam, piperacillin, vancomycin, esmolol, or total parenteral nutrition [TPN]).[11] Irritants (phlebitogenic drugs) cause short‐term inflammation and pain, and thus should not be peripherally infused for prolonged durations. Common irritants in the hospital setting include acyclovir, dobutamine, penicillin, and potassium chloride.
Of note, about one‐quarter of PIV insertions fail owing to difficult intravenous access.[12] Ultrasound‐guided peripheral intravenous (USGPIV) catheter placement is emerging as a technique to provide peripheral access for such patients to avoid placement of central venous access devices. Novel, longer devices (>8 cm) with built‐in guide wires have been developed to increase placement success of USGPIVs. These new designs provide easier access into deeper arm veins (brachial or basilic) not otherwise accessible by short PIVs. Although studies comparing the efficacy of USGPIV devices to other VADs are limited, a recent systematic review showed that time to successful cannulation was shorter, and fewer attempts were required to place USGPIVs compared to PIVs.[13] A recent study in France found that USGPIVs met the infusion needs of patients with difficult veins with minimal increase in complications.[14] Despite these encouraging data, future studies are needed to better evaluate this technology.
Midline Catheter
A midline is a VAD that is between 7.5 to 25 cm in length and is typically inserted into veins above the antecubital fossa. The catheter tip resides in a peripheral upper arm vein, often the basilic or cephalic vein, terminating just short of the subclavian vein (Figure 1B). Midline‐like devices were first developed in the 1950s and were initially used as an alternative to PIVs because they were thought to allow longer dwell times.[15] However, because they were originally constructed with a fairly rigid material, infiltration, mechanical phlebitis, and inflammation were common and tempered enthusiasm for their use.[15, 16] Newer midline devices obviate many of these problems and are inserted by ultrasound guidance and modified Seldinger technique.[17] Despite these advances, data regarding comparative efficacy are limited.
Midlines offer longer dwell times than standard PIVs owing to termination in the larger diameter basilic and brachial veins of the arm. Additionally, owing to their length, midlines are less prone to dislodgement. As they are inserted with greater antisepsis than PIVs and better secured to the skin, they are more durable than PIVs.[5, 9, 18] Current INS standards recommend use of midlines for 1 to 4 weeks.[5] Because they terminate in a peripheral vein, medications and infusions compatible with midlines are identical to those that are infused through a PIV. Thus, TPN, vesicants or irritants, or drugs that feature a pH 5 or pH >9, or >500 mOsm should not be infused through a midline.[15] New evidence suggests that diluted solutions of vancomycin (usually pH 5) may be safe to infuse for short durations (6 days) through a midline, and that concentration rather than pH may be more important in this regard.[19] Although it is possible that the use of midlines may extend to agents typically not deemed peripheral access compatible, limited evidence exists to support such a strategy at this time.
Midlines offer several advantages. First, because blood flow is greater in the more proximal veins of the arm, midlines can accommodate infusions at rates of 100 to 150 mL/min compared to 20 to 40 mL/min in smaller peripheral veins. Higher flow rates offer greater hemodilution (dilution of the infusion with blood), decreasing the likelihood of phlebitis and infiltration.[20] Second, midlines do not require x‐ray verification of tip placement; thus, their use is often favored in resource‐depleted settings such as skilled nursing facilities. Third, midlines offer longer dwell times than peripheral intravenous catheters and can thus serve as bridge devices for short‐term intravenous antibiotics or peripheral‐compatible infusions in an outpatient setting. Available evidence suggests that midlines are associated with low rates of bloodstream infection (0.30.8 per 1000 catheter‐days).[17] The most frequent complications include phlebitis (4.2%) and occlusion (3.3%).[20] Given these favorable statistics, midlines may offer a good alternative to PIVs in select patients who require peripheral infusions of intermediate duration.
Intraosseous Vascular Access
Intraosseous (IO) devices access the vascular system by piercing cortical bone. These devices provide access to the intramedullary cavity and venous plexi of long bones such as the tibia, femur, or humerus. Several insertion devices are now commercially available and have enhanced the ease and safety of IO placement. Using these newer devices, IO access may be obtained in 1 to 2 minutes with minimal training. By comparison, a central venous catheter often requires 10 to 15 minutes to insert with substantial training efforts for providers.[21, 22, 23]
IO devices thus offer several advantages. First, given the rapidity with which they can be inserted, they are often preferred in emergency settings (eg, trauma). Second, these devices are versatile and can accommodate both central and peripheral infusates.[24] Third, a recent meta‐analysis found that IOs have a low complication rate of 0.8%, with extravasation of infusate through the cortical entry site being the most common adverse event.[21] Of note, this study also reported zero local or distal infectious complications, a finding that may relate to the shorter dwell of these devices.[21] Some animal studies suggest that fat embolism from bone may occur at high rates with IO VADs.[25] However, death or significant morbidity from fat emboli in humans following IO access has not been described. Whether such emboli occur or are clinically significant in the context of IO devices remains unclear at this time.[21]
Central Venous Access Devices
Central venous access devices (CVADs) share in common tip termination in the cavo‐atrial junction, either in the lower portion of the superior vena cava or in the upper portion of the right atrium. CVADs can be safely used for irritant or vesicant medications as well as for blood withdrawal, blood exchange procedures (eg, dialysis), and hemodynamic monitoring. Traditionally, these devices are 15 to 25 cm in length and are directly inserted in the deep veins of the supra‐ or infraclavicular area, including the internal jugular, brachiocephalic, subclavian, or axillary veins. PICCs are unique CVADs in that they enter through peripheral veins but terminate in the proximity of the cavoatrial junction. Regarding nomenclature, CICC will be used to denote devices that enter directly into veins of the neck or chest, whereas PICC will be used for devices that are inserted peripherally but terminate centrally.
Peripherally Inserted Central Catheter
PICCs are inserted into peripheral veins of the upper arm (eg, brachial, basilica, or cephalic vein) and advanced such that the tip resides at the cavoatrial junction (Figure 1C). PICCs offer prolonged dwell times and are thus indicated when patients require venous access for weeks or months.[26] Additionally, they can accommodate a variety of infusates and are safer to insert than CICCs, given placement in peripheral veins of the arm rather than central veins of the chest/neck. Thus, insertion complications such as pneumothorax, hemothorax, or significant bleeding are rare with PICCs. In fact, a recent study reported that PICC insertion by hospitalists was associated with low rates of insertion or infectious complications.[27]
However, like CICCs, PICCs are associated with central lineassociated bloodstream infection (CLABSI), a serious complication known to prolong length of hospital stay, increase costs, and carry a 12% to 25% associated mortality.[28, 29] In the United States alone, over 250,000 CLASBI cases occur per year drawing considerable attention from the CDC and Joint Commission, who now mandate reporting and nonpayment for hospital‐acquired CLABSI.[30, 31, 32] A recent systematic review and meta‐analysis found that PICCs are associated with a substantial risk of CLABSI in hospitalized patients.[33] Importantly, no difference in CLABSI rates between PICCs and CICCs in hospitalized patients was evident in this meta‐analysis. Therefore, current guidelines specifically recommend against use of PICCs over CICCs as a strategy to reduce CLABSI.[34] Additionally, PICCs are associated with 2.5‐fold greater risk of deep vein thrombosis (DVT) compared to CICCs; thus, they should be used with caution in patients with cancer or those with underlying hypercoagulable states.
Of particular import to hospitalists is the fact that PICC placement is contraindicated in patients with stage IIIB or greater chronic kidney disease (CKD). In such patients, sequelae of PICC use, such as phlebitis or central vein stenosis, can be devastating in patients with CKD.[35] In a recent study, prior PICC placement was the strongest predictor of subsequent arteriovenous graft failure.[36] For this reason, Choosing Wisely recommendations call for avoidance of PICCs in such patients.[37]
Centrally Inserted Central Catheter
CICCs are CVADs placed by puncture and cannulation of the internal jugular, subclavian, brachiocephalic, or femoral veins (Figure 1D) and compose the vast majority of VADs placed in ICU settings.[38, 39] Central termination of CICCs allows for a variety of infusions, including irritants, vesicants, and vasopressors, as well as blood withdrawal and hemodynamic monitoring. CICCs are typically used for 7 to 14 days, but may remain for longer durations if they remain complication free and clinically necessary.[40] A key advantage of CICCs is that they can be placed in emergent settings to facilitate quick access for rapid infusion or hemodynamic monitoring. In particular, CICCs are inserted in the femoral vein and may be useful in emergency settings. However, owing to risk of infection and inability to monitor central pressures, these femoral devices should be replaced with a proper CICC or PICC when possible. Importantly, although CICCs are almost exclusively used in intensive or emergency care, PICCs may also be considered in such settings.[41, 42] CICCs usually have multiple lumens and often serve several simultaneous functions such as both infusions and hemodynamic monitoring.
Despite their benefits, CICCs have several disadvantages. First, insertion requires an experienced clinician and has historically been a task limited to physicians. However, this is changing rapidly (especially in Europe and Australia) where specially trained nurses are assuming responsibility for CICC placement.[43] Second, these devices are historically more likely to be associated with CLABSI, with estimates of infection rates varying between 2 and 5 infections per 1000 catheter‐days.[44] Third, CICCs pose a significant DVT risk, with rates around 22 DVTs per 1000 catheter‐days.[45] However, compared to PICCs, the DVT risk appears lower, and CICC use may be preferable in patients at high risk of DVT, such as critically ill or cancer populations.[46] An important note to prevent CICC insertion complications relates to use of ultrasound, a practice that has been associated with decreased accidental arterial puncture and hematoma formation. The role of ultrasound guidance with PICCs as well as implications for thrombotic and infectious events remains less characterized at this point.[47]
Tunneled Central Venous Access Devices
Tunneled devices (either CICCs or PICCs) are characterized by the fact that the insertion site on the skin and site of ultimate venipuncture are physically separated (Figure 1E). Tunneling limits bacterial entry from the extraluminal aspect of the CVAD to the bloodstream. For example, internal jugular veins are often ideal sites of puncture but inappropriate sites for catheter placement, as providing care to this area is challenging and may increase risk of infection.[34] Tunneling to the infraclavicular area provides a better option, as it provides an exit site that can be adequately cared for. Importantly, any CVAD (PICCs or CICCs) can be tunneled. Additionally, tunneled CICCs may be used in patients with chronic or impending renal failure where PICCs are contraindicated because entry into dialysis‐relevant vessels is to be avoided.[48] Such devices also allow regular blood sampling in patients who require frequent testing but have limited peripheral access, such as those with hematological malignancies. Additionally, tunneled catheters are more comfortable for patients and viewed as being more socially acceptable than nontunneled devices. However, the more invasive and permanent nature of these devices often requires deliberation prior to insertion.
Of note, tunneled devices and ports may be used as long‐term (>3 months to years) VADs. As our focus in this review is short‐term devices, we will not expand the discussion of these devices as they are almost always used for prolonged durations.[7]
OPERATIONALIZING THE DATA: AN ALGORITHMIC APPROACH TO VENOUS ACCESS
Hospitalists should consider approaching venous access using an algorithm based on a number of parameters. For example, a critically ill patient who requires vasopressor support and hemodynamic monitoring will need a CICC or a PICC. Given the potential greater risk of thromboses from PICCs, a CICC is preferable for critically ill patients provided an experienced inserter is available. Conversely, patients who require short‐term (710 days) venous access for infusion of nonirritant or nonvesicant therapy often only require a PIV. In patients with poor or difficult venous access, USGPIVs or midlines may be ideal and preferred over short PIVs. Finally, patients who require longer‐term or home‐based treatment may benefit from early placement of a midline or a PICC, depending again on the nature of the infusion, duration of treatment, and available venous access sites.
An algorithmic approach considering these parameters is suggested in Figure 2, and a brief overview of the devices and their considerations is shown in Table 3.
| Vascular Access Device | Central/Peripheral | Anatomical Location of Placement | Desired Duration of Placement | Common Uses | BSI Risk (Per 1,000 Catheter‐Days) | Thrombosis Risk | Important Considerations |
|---|---|---|---|---|---|---|---|
| |||||||
| Small peripheral IV | Peripheral | Peripheral veins, usually forearm | 710 days | Fluid resuscitation, most medications, blood products | 0.06[8] | Virtually no risk | Consider necessity of PIV daily and remove unnecessary devices |
| Midline | Peripheral | Inserted around antecubital fossa, reside within basilic or cephalic vein of the arm | 24 weeks | Long‐term medications excluding TPN, vesicants, corrosives | 0.30.8[17] | Insufficient data | Can be used as bridge devices for patients to complete short‐term antibiotics/emnfusions as an outpatient |
| Peripherally inserted central catheter | Central | Inserted into peripheral arm vein and advanced to larger veins (eg, internal jugular or subclavian) to the CAJ | >1 week, 3 months | Large variety of infusates, including TPN, vesicants, corrosives | 2.4[44] | 6.30% | Contraindicated in patients with CKD stage IIIb or higher |
| Centrally inserted central catheters | Central | Inserted above (internal jugular vein, brachiocephalic vein, subclavian vein), or below the clavicle (axillary vein) | >1 week, 3 months | Same infusate variety as PICC, measurement of central venous pressures, common in trauma/emergent settings | 2.3[44] | 1.30% | Given lower rates of DVT than PICC, preferred in ICU and hypercoagulable environments |
| Tunneled CICCs | Central | Placed percutaneously in any large vein in the arm, chest, neck or groin | >3 months to years | Central infusates, as in any CVAD; used for patients with CKD stage IIIb or greater when a PICC is indicated | Insufficient data | Insufficient data | May be superior when insertion site and puncture site are not congruent and may increase risk of infection |

CONCLUSIONS
With strides in technology and progress in medicine, hospitalists have access to an array of options for venous access. However, every VAD has limitations that can be easily overlooked in a perfunctory decision‐making process. The data presented in this review thus provide a first step to improving safety in this evolving science. Studies that further determine appropriateness of VADs in hospitalized settings are necessary. Only through such progressive scientific enquiry will complication‐free venous access be realized.
Disclosure
Nothing to report.
- , . The need for comparative data in vascular access: the rationale and design of the PICC registry. J Vasc Access. 2013; 18(4): 219–224.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013; 8(6): 309–314.
- , , , . Clinically‐indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2013; 4: CD007798.
- , , , et al. Cost‐effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014; 12(1): 51–58.
- Infusion Nurses Society. Infusion Nursing Standards of Practice. Norwood, MA; Infusion Nurses Society; 2011.
- , , , et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012; 380(9847): 1066–1074.
- , , , et al. epic3: national evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospitals in England. J Hosp Infect. 2014; 86(suppl 1): S1–S70.
- . Short peripheral intravenous catheters and infections. J Infus Nurs. 2012; 35(4): 230–240.
- , , , et al. Phlebitis risk varies by peripheral venous catheter site and increases after 96 hours: a large multi‐centre prospective study. J Adv Nurs. 2014; 70(11): 2539–2549.
- , . Intravenous catheter complications in the hand and forearm. J Trauma. 2004; 56(1): 123–127.
- , , , . Vesicant extravasation part I: Mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006; 33(6): 1134–1141.
- , . Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005; 34(5): 345–359.
- , , . Ultrasound‐guided peripheral venous access: a systematic review of randomized‐controlled trials. Eur J Emerg Med. 2014; 21(1): 18–23.
- , , , et al. Difficult peripheral venous access: clinical evaluation of a catheter inserted with the Seldinger method under ultrasound guidance. J Crit Care. 2014; 29(5): 823–827.
- . Choosing the right intravenous catheter. Home Healthc Nurse. 2007; 25(8): 523–531; quiz 532–523.
- Adverse reactions associated with midline catheters—United States, 1992–1995. MMWR Morb Mortal Wkly Rep. 1996; 45(5): 101–103.
- , , . The risk of midline catheterization in hospitalized patients. A prospective study. Ann Intern Med. 1995; 123(11): 841–844.
- . Midline catheters: indications, complications and maintenance. Nurs Stand. 2007; 22(11): 48–57; quiz 58.
- , . Safe administration of vancomycin through a novel midline catheter: a randomized, prospective clinical trial. J Vasc Access. 2014; 15(4): 251–256.
- . Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004; 27(5): 313–321.
- . Vascular access in resuscitation: is there a role for the intraosseous route? Anesthesiology. 2014; 120(4): 1015–1031.
- , , , et al. A new system for sternal intraosseous infusion in adults. Prehosp Emerg Care. 2000; 4(2): 173–177.
- , , , , , . Comparison of two intraosseous access devices in adult patients under resuscitation in the emergency department: a prospective, randomized study. Resuscitation. 2010; 81(8): 994–999.
- , , , , . Comparison study of intraosseous, central intravenous, and peripheral intravenous infusions of emergency drugs. Am J Dis Child. 1990; 144(1): 112–117.
- , , , , . The safety of intraosseous infusions: risks of fat and bone marrow emboli to the lungs. Ann Emerg Med. 1989; 18(10): 1062–1067.
- , , , , . ESPEN guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr. 2009; 28(4): 365–377.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009; 4(6): E1–E4.
- Vital signs: central line‐associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011; 60(8): 243–248.
- , , , . Hospital costs of central line‐associated bloodstream infections and cost‐effectiveness of closed vs. open infusion containers. The case of Intensive Care Units in Italy. Cost Eff Resour Alloc. 2010; 8: 8.
- , , . The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006; 81(9): 1159–1171.
- The Joint Commission. Preventing Central Line‐Associated Bloodstream Infections: A Global Challenge, a Global Perspective. Oak Brook, IL: Joint Commission Resources; 2012.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011; 39(4 suppl 1): S1–S34.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013; 34(9): 908–918.
- Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, American Hospital Association, Association for Professionals in Infection Control and Epidemiology, The Joint Commission. Compendium of Strategies to Prevent Healthcare‐Associated Infections in Acute Care Hospitals: 2014 Updates. Available at: http://www.shea‐online.org. Accessed August 1, 2014.
- , , , , . Guidelines for venous access in patients with chronic kidney disease. A Position Statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access. Semin Dial. 2008; 21(2): 186–191.
- , , , et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case‐control study in hemodialysis patients. Am J Kidney Dis. 2012; 60(4): 601–608.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012; 7(10): 1664–1672.
- , , , et al. Do physicians know which of their patients have central venous catheters? A multi‐center observational study. Ann Intern Med. 2014; 161(8): 562–567.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011; 77(4): 304–308.
- , , , , , . Peripherally inserted central catheter‐related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014; 12(6): 847–854.
- , , , . An in vitro study comparing a peripherally inserted central catheter to a conventional central venous catheter: no difference in static and dynamic pressure transmission. BMC Anesthesiol. 2010; 10: 18.
- , , , et al. Clinical experience with power‐injectable PICCs in intensive care patients. Crit Care. 2012; 16(1): R21.
- , , , et al. Nurse‐led central venous catheter insertion‐procedural characteristics and outcomes of three intensive care based catheter placement services. Int J Nurs Stud. 2012; 49(2): 162–168.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010; 38(2): 149–153.
- , , , et al. Which central venous catheters have the highest rate of catheter‐associated deep venous thrombosis: a prospective analysis of 2,128 catheter days in the surgical intensive care unit. J Trauma Acute Care Surg. 2013; 74(2): 454–460; discussion 461–452.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013; 382(9889): 311–325.
- , , , , . Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015; 1: CD011447.
- , , , et al. Tunneled jugular small‐bore central catheters as an alternative to peripherally inserted central catheters for intermediate‐term venous access in patients with hemodialysis and chronic renal insufficiency. Radiology. 1999; 213(1): 303–306.
- , . The need for comparative data in vascular access: the rationale and design of the PICC registry. J Vasc Access. 2013; 18(4): 219–224.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013; 8(6): 309–314.
- , , , . Clinically‐indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2013; 4: CD007798.
- , , , et al. Cost‐effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014; 12(1): 51–58.
- Infusion Nurses Society. Infusion Nursing Standards of Practice. Norwood, MA; Infusion Nurses Society; 2011.
- , , , et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012; 380(9847): 1066–1074.
- , , , et al. epic3: national evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospitals in England. J Hosp Infect. 2014; 86(suppl 1): S1–S70.
- . Short peripheral intravenous catheters and infections. J Infus Nurs. 2012; 35(4): 230–240.
- , , , et al. Phlebitis risk varies by peripheral venous catheter site and increases after 96 hours: a large multi‐centre prospective study. J Adv Nurs. 2014; 70(11): 2539–2549.
- , . Intravenous catheter complications in the hand and forearm. J Trauma. 2004; 56(1): 123–127.
- , , , . Vesicant extravasation part I: Mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006; 33(6): 1134–1141.
- , . Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005; 34(5): 345–359.
- , , . Ultrasound‐guided peripheral venous access: a systematic review of randomized‐controlled trials. Eur J Emerg Med. 2014; 21(1): 18–23.
- , , , et al. Difficult peripheral venous access: clinical evaluation of a catheter inserted with the Seldinger method under ultrasound guidance. J Crit Care. 2014; 29(5): 823–827.
- . Choosing the right intravenous catheter. Home Healthc Nurse. 2007; 25(8): 523–531; quiz 532–523.
- Adverse reactions associated with midline catheters—United States, 1992–1995. MMWR Morb Mortal Wkly Rep. 1996; 45(5): 101–103.
- , , . The risk of midline catheterization in hospitalized patients. A prospective study. Ann Intern Med. 1995; 123(11): 841–844.
- . Midline catheters: indications, complications and maintenance. Nurs Stand. 2007; 22(11): 48–57; quiz 58.
- , . Safe administration of vancomycin through a novel midline catheter: a randomized, prospective clinical trial. J Vasc Access. 2014; 15(4): 251–256.
- . Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004; 27(5): 313–321.
- . Vascular access in resuscitation: is there a role for the intraosseous route? Anesthesiology. 2014; 120(4): 1015–1031.
- , , , et al. A new system for sternal intraosseous infusion in adults. Prehosp Emerg Care. 2000; 4(2): 173–177.
- , , , , , . Comparison of two intraosseous access devices in adult patients under resuscitation in the emergency department: a prospective, randomized study. Resuscitation. 2010; 81(8): 994–999.
- , , , , . Comparison study of intraosseous, central intravenous, and peripheral intravenous infusions of emergency drugs. Am J Dis Child. 1990; 144(1): 112–117.
- , , , , . The safety of intraosseous infusions: risks of fat and bone marrow emboli to the lungs. Ann Emerg Med. 1989; 18(10): 1062–1067.
- , , , , . ESPEN guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr. 2009; 28(4): 365–377.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009; 4(6): E1–E4.
- Vital signs: central line‐associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011; 60(8): 243–248.
- , , , . Hospital costs of central line‐associated bloodstream infections and cost‐effectiveness of closed vs. open infusion containers. The case of Intensive Care Units in Italy. Cost Eff Resour Alloc. 2010; 8: 8.
- , , . The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006; 81(9): 1159–1171.
- The Joint Commission. Preventing Central Line‐Associated Bloodstream Infections: A Global Challenge, a Global Perspective. Oak Brook, IL: Joint Commission Resources; 2012.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011; 39(4 suppl 1): S1–S34.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013; 34(9): 908–918.
- Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, American Hospital Association, Association for Professionals in Infection Control and Epidemiology, The Joint Commission. Compendium of Strategies to Prevent Healthcare‐Associated Infections in Acute Care Hospitals: 2014 Updates. Available at: http://www.shea‐online.org. Accessed August 1, 2014.
- , , , , . Guidelines for venous access in patients with chronic kidney disease. A Position Statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access. Semin Dial. 2008; 21(2): 186–191.
- , , , et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case‐control study in hemodialysis patients. Am J Kidney Dis. 2012; 60(4): 601–608.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012; 7(10): 1664–1672.
- , , , et al. Do physicians know which of their patients have central venous catheters? A multi‐center observational study. Ann Intern Med. 2014; 161(8): 562–567.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011; 77(4): 304–308.
- , , , , , . Peripherally inserted central catheter‐related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014; 12(6): 847–854.
- , , , . An in vitro study comparing a peripherally inserted central catheter to a conventional central venous catheter: no difference in static and dynamic pressure transmission. BMC Anesthesiol. 2010; 10: 18.
- , , , et al. Clinical experience with power‐injectable PICCs in intensive care patients. Crit Care. 2012; 16(1): R21.
- , , , et al. Nurse‐led central venous catheter insertion‐procedural characteristics and outcomes of three intensive care based catheter placement services. Int J Nurs Stud. 2012; 49(2): 162–168.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010; 38(2): 149–153.
- , , , et al. Which central venous catheters have the highest rate of catheter‐associated deep venous thrombosis: a prospective analysis of 2,128 catheter days in the surgical intensive care unit. J Trauma Acute Care Surg. 2013; 74(2): 454–460; discussion 461–452.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013; 382(9889): 311–325.
- , , , , . Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015; 1: CD011447.
- , , , et al. Tunneled jugular small‐bore central catheters as an alternative to peripherally inserted central catheters for intermediate‐term venous access in patients with hemodialysis and chronic renal insufficiency. Radiology. 1999; 213(1): 303–306.