User login
Gap analysis: a strategy to improve the quality of care of head and neck cancer patients
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
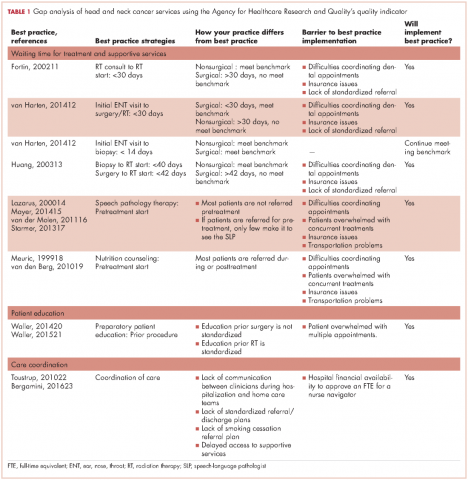
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
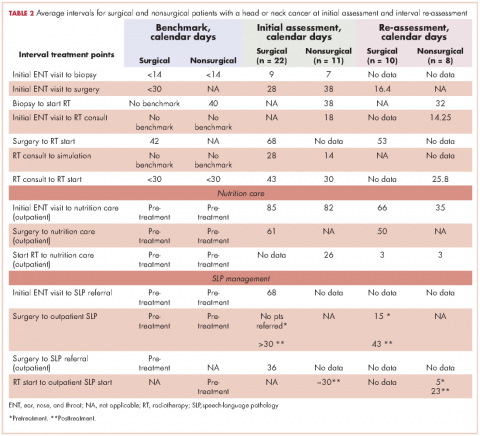
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
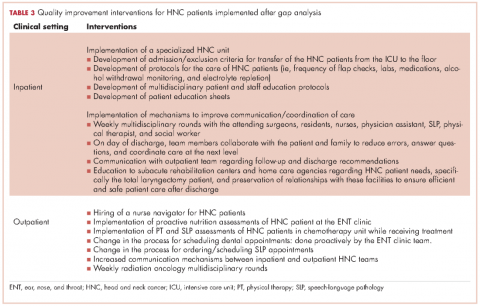
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
In the United States, there will be an estimated 49,670 new cases of head and neck cancer for 2017.1 Head and neck cancer (HNC) is a term used to describe a range of tumors that originate in the area of the body spanning from the lower neck to the upper nasal cavity.2 Specifically, they are malignancies arising in the mouth, larynx, nasal cavity, sinuses, tongue, lips, and numerous glands such as the thyroid and salivary.2 To clarify, HNC, despite the encompassing name, does not include growths of the bones, teeth, skin, brain parenchyma, and eye; therefore, such tumors will not be addressed in this article.
Patients with HNC often experience fragmented and uncoordinated care that leads to delays in cancer treatment, severe distress in patients and families, and dissatisfaction with care. Literature reports that these patients face numerous stressors including aggressive cancer treatments, severe symptoms, body image concerns, loss of speech, difficulty swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis.3,4In addition, patients with HNC and their families are challenged to navigate the health care system and to overcome the difficulties of accessing services within the context of financial constraints. A multidisciplinary team (MDT) approach is the standard of care for HNC patients, as demonstrated in studies reporting better 5-year survival outcomes, increased completion of adjuvant therapy, and higher compliance with speech-language pathologist (SLP) recommendations.5, 6 Furthermore, a recent systematic review of cancer teams concluded that the MDT approach leads to improved clinical outcomes and enhanced communication between the patient and the team.7
The Institute of Medicine (IOM) stated in its 2013 report on cancer care that a high-quality care delivery system requires continuing measurement of cancer care and strategies to carry out performance improvement.8 Following the IOM premise, the cancer center at an academic medical center in Philadelphia made efforts to improve patient access to multidisciplinary services, first, by creating a multidisciplinary Cancer Appetite and Rehabilitation (CARE)clinic to address the symptoms and nutritional needs of HNC patients,9 and second, by using a gap analysis to conduct an assessment of the cancer care services provided to this cancer population. The need to conduct this assessment was generated by the desire to improve access to multidisciplinary care, with the goal of meeting standard benchmarks for completion of treatment while increasing the use of ancillary services. This article describes the process of conducting a gap analysis of cancer services for HNC patients, and includes discussion of the findings, recommendations for improving care, a description of the quality improvement interventions, and a report of the outcomes based on an interval re-assessment 18 months later.
Methods
Methods included a gap analysis, implementation of quality improvement recommendations, and re-assessment of indicators (Figure). A gap analysis “identifies differences between desired and actual practice conditions, including service delivery and quality patient outcomes as measured against evidence-based benchmarks while incorporating key stakeholder concerns and expectations.”10 The gap analysis of cancer care services offered to HNC patients was achieved through the step-by-step process described hereinafter. The implementation of quality improvement recommendations was accomplished by establishing two task force committees focused respectively on education and transitions in care coordination. Re-assessment of indicators related to timeliness of delivery of cancer treatments and collection of additional baseline data regarding supportive services.
Gap analysis
Identification of the scope of the problem. Members of the HNC multidisciplinary team raised concerns about unintended breaches in care for HNC patients that resulted not only in delay of the patients’ cancer treatments, but also in unnecessary distress for the patients and their families. As a result, the HNC team decided to conduct a gap analysis to identify the barriers in care for HNC patients, and by doing this, to determine possible solutions.
Identification of best practice care indicators. The indicators of best practice care (benchmarks) for HNC patients were identified after exhaustive review of the literature11-22(Table 1). For this gap analysis, the indicators focused on waiting time to treatment (surgery, chemotherapy, radiation therapy) and to supportive care interventions (nutrition, speech and language pathology) as follows:
- 9.2Futura StdInitial ear-nose-throat (ENT) visit to surgery: <30 days
- Biopsy to start radiation therapy (RT) for nonsurgical patients: 40 days
- Surgery to RT start: 42 days
- Surgery to nutrition consultation (outpatient), start RT to nutrition: Pretreatment
- Surgery to outpatient SLP, initial ENT visit to SLP referral, surgery to SLP referral, RT start to outpatient SLP start: Pretreatment
Measure gaps against benchmarks. To Gap analysis of measure gaps against benchmarks, the authors used the Agency for Health Care Research and Quality tool that provides a systematic method to compare current practice with best practices and determine the barriers to best practice and the feasibility of implementing best practices by the institution23 (Table 1). For this project, a process map of waiting time to treatment and supportive care interventions was created, so that real-world conditions could be measured against benchmarks.
Process map. The authors identified 67 newly diagnosed HNC patients during January-July 2014 from the surgery, radiation therapy, and nutrition departments, but only 33 patients were able to be tracked from their initial visit at the cancer center until the completion of their treatment through the electronic medical record (EMR) system. Their information was compiled in a spreadsheet based on the EMR information. Data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
To map the typical patient process, the patients were split into two groups: surgical (n = 22) and nonsurgical (n = 11). Surgical patients underwent surgery as their primary treatment and received adjuvant radiation therapy or concurrent chemotherapy. Nonsurgical patients did not require surgery other than biopsy as a part of their treatment. Most of the nonsurgical patients received chemotherapy, and 1 patient received palliative radiation therapy.
SWOT analysis. The SWOT analysis is used to chart institution performance in relation to benchmarks while describing stakeholders’ perceptions.24The stakeholder perspective for this project focused on the views of the health care providers from all disciplines regarding the quality of care provided to the HNC population. In addition, a patient survey was conducted to assess their perception of the care they received.
Clinician survey. We surveyed 25 clinicians, including physicians, advanced practice providers, nurses, and allied health professionals, from the surgical (n = 3), hospitalization (n = 6), radiation (n = 3), chemotherapy (n = 3) and supportive services teams (n = 10). The survey was conducted face to face and included 7 open-ended questions designed to gain insight about problems encountered with coordination of care, suggestions to improve coordination of care, factors in treatment delays, suggestions to decrease treatment delays, factors in excellent patient outcomes, rate overall patient care, and suggestions for improvement of service. Initial survey responses were filtered by recurring themes in each question among the different patient service teams.
Patient satisfaction survey. The sample of patients was obtained from the surgery, radiation therapy, and nutrition departments during January-July 2014. Sixty-seven initial patients were identified but only 43 were eligible for interview because they had a listed phone number. A six-question nonvalidated survey was developed by the authors to measure patient satisfaction with the scheduling process, waiting time, information provided about treatment and their medical status, emotional support, the coordination of care, and the payment process. Satisfaction was rated on a scale from 1 to 5 (1 = Poor, 2 = Fair, 3 = Satisfactory, 5 = Great).
Analysis and final report. See Results section.
Quality improvement implementation. The transitions and the education committees were created to address the gaps identified during the analysis. The transitions committee developed strategies to improve the coordination of care of HNC patients throughout their cancer treatment and the education committee elaborated new ways to enhance patient education while meeting treatment timeline standards. The implementation of the interventions was developed by the inpatient and outpatient MTD teams caring for the HNC population.
Re-assessment of indicators. During January-December 2015, a total of 58 patients diagnosed with HNC were identified. Of those, 40 patients with recurrent disease were eliminated, leaving 18 patients (10 surgical, 8 nonsurgical). Similar to the initial assessment for the gap analysis, data included patient access to supportive services and number of days between important treatment benchmarks. Tracking data was used to create a treatment flow chart and determine average treatment intervals.
Results
Most of the patients were men (70%), white (70%), and 60% were within the 50-69 years age range at the time of diagnosis.
Clinician survey
The clinicians were surveyed and their responses analyzed by two people, the project leader and the project assistant. The most commonly identified weaknesses in care that the clinicians identified were delayed access to dental referrals, insufficient preoperative patient education, and inefficient discharge planning and/or home care coordination. Dental referrals were identified as a major cause of delay in starting radiation therapy because of scheduling issues, a lack of patient motivation, limited insurance coverage, and difficulty identifying reliable dentists in the patient’s geographic area. Clinicians also identified problems coordinating smoking cessation referrals for patients.
In addition, they identified the hospitalization and/or home care phases as areas for potential improvement. During hospitalization, patients often expressed surprise upon learning that they had a feeding tube and/or tracheostomy despite having received pre-operative education. This misunderstanding by the patient was likely related to the clinicians’ assumptions about the best timing for patient education and the amount of time needed for education before the surgical procedure. The surgical team provided patient education based on individual needs, and it has not been standardized because they felt that patients’ education needs vary from person to person. In contrast, patient education prior radiation therapy is standardized, and all patients received a comprehensive package of information that is re-enforced by direct patient education by the clinicians.
Another gap in care identified by the inpatient team was a prolonged intensive care unit (ICU) stay for the HNC patients. These patients remained in the ICU for the entirety of their stay. Not only was this causing overuse of resources, but patients also felt unprepared for an independent discharge home given the high level of care received in the ICU.
A range of suggestions were made to solve these problems. The most prevalent suggestion was to use a nurse navigator to coordinate referrals, schedule appointments, facilitate interdisciplinary communication, and to address social, financial, and transport needs for HNC patients. Several other suggestions referred to standardizing treatment procedures and pre-operative patient education.
Patient survey
Forty-three patients were identified for the patient satisfaction survey. Each patient was contacted at least three times over the course of 3 weeks. Of the 43 patients, 20 had an invalid phone number, 10 were not available for participation, and 1 declined to participate. A total of 12 patients completed the survey.
Although the sample size was small, the patients surveyed were very satisfied with their care. Of the 12 patients, 5 patients rated all of the services relevant to their treatment as a 5 (Great). Areas of particular concern for the patients included the waiting time to see a physician in the ENT clinic, the explanation/collection of charges, and the accessibility of support groups. Services rated 3 (Satisfactory) included waiting time to schedule appointments; the amount of information and patient education provided by about radiation, nutrition, physical therapy (PT), occupational therapy (OT), and SLP; and overall satisfaction with care.
Surgical patients. The Danish Head and Neck Society guidelines state that the interval between the initial visit diagnosis and surgery should be within 30 days.12A comparison of the average intervals between important treatment points for the surgical sample patients with the benchmark timing recommended in the literature are shown in Table 2. The mean time from initial visit to surgery was 28 days in the cancer center sample; 67% of patients (n = 14) had surgery within 30 days, and 33% of patients (n = 7) had surgery beyond 30 days. The interval re-assessment showed improvements in this area: the mean time from initial visit to surgery went from 28 to 18 days, and 100% of patients
n = 10) had surgery within 30 days.
Huang and colleagues have indicated that postoperative radiation therapy should ideally occur within 42 days of surgery;13 however, in the present study, 79% (n = 11) of the sample surgical patients undergoing radiation began their therapy on average more than 63 days after surgery. The interval re-assessment found the same results with 80% of patients starting radiation over 42 days after surgery although the average time lag decreased from 68 days to 53 days.
Nonsurgical patients. Huang and colleagues have indicated that for patients undergoing radiation as their primary form of treatment, an interval of 40 days between biopsy and the start of radiation is ideal.13 The average intervals between important time points of treatment for patients who did not require surgery in their treatment are shown in Table 2. The cancer center met the benchmark at baseline with an average of 38 days (n = 11 patients). The re-assessment showed improvement in this area with 100% of cases (n = 10) meeting the benchmark with an average of 32 days. Likewise, the benchmark waiting time from RT consultation to RT start of less than 30 days11 was met by the cancer center for the nonsurgical group (n = 11).
Access to supportive services
Nutrition care. Studies have shown that standard nutritional care for HNC patients should start before treatment.18,19 In the present study, the waiting time from surgery to outpatient nutrition assessment improved from 61 days to 50 days (Table 2). For patients in the surgical group, the time interval between the initial ENT visit to the outpatient nutrition assessment decreased from 85 days at baseline to 66 days at reassessment, and 82 days to 35 days, respectively, for the nonsurgical group. The time interval from surgery to nutrition assessment has not reached the recommended pretreatment benchmark, but data showed a trend of improvement from 61 days at baseline to 50 days at reassessment for patients in the group.
Patients were typically referred to outpatient nutrition at the start of radiation therapy. In the initial assessment, all patients (n = 33) had access to nutrition services, but 21% (n = 7) never spoke to the nutritionist. The re-assessment found all but one (n = 7) of the patients had been seen by a nutritionist at some point during the treatment period. The benchmark of preradiation nutrition assessment was met by 2 postsurgical patients, with the remainder of the patients being seen within 3 days of the initiation of radiation.
Speech-language pathology management. The literature recommends that patients receive SLP management before the surgery.14-17 In this gap analysis, a difference in access to SLP services was identified between inpatient and outpatient settings. On average, patients within the sample were referred to outpatient SLP over a month after their surgery. In contrast, inpatient surgical patients had access to rapid consultations with SLP (eg, 1 day after surgery for total laryngectomy, and 4 days after surgery for oropharyngeal and oral surgery patients; T Hogan, unpublished data, June 2014). Overall, the benchmark was not met, as patients were not seen by the SPL prior to treatment.
New baseline data was collected about SLP services and showed that 70% of patients had contact with the outpatient SLP at some point during their treatment. Of those, only 29% of patients saw SLP before surgery, meeting the benchmark. The baseline waiting time was an average of 15 days before surgery and 43 days after surgery. Overall, the trend is moving toward the benchmark of care.
Similarly, studies determined that the gold standard of care for nonsurgical patients is that SLPs begin pretreatment management of HNC.16Patients in the baseline sample were typically referred to outpatient SLP about a month after biopsy (presumably diagnosis), but before the start of chemo-radiation. There were no data available for the number of patients who were actually seen by the outpatient SLP before the start of chemo-radiation.
The new baseline data found that 100% of nonsurgical patients were referred to SLP, but only25% (n = 2) were seen before they started chemo-radiation therapy (an average 5 days before) and 75% (n = 6) were seen after starting chemo-radiation therapy (an average 23 days after).
SWOT analysis
The SWOT analysis included strengths, weaknesses, opportunities and threats of the care provided to HNC patients at the cancer center. The gap analysis based on the results of the clinician surveys, process mapping, and patient satisfaction survey is summarized in Table 3. Three main gaps were identified: waiting time to treatment, education, and coordination of and transitions in care.
Quality improvement actions
Interventions by the outpatient MTD team included changing the process of scheduling dental appointments, creating a new approach to outpatient nutrition by proactively meeting patients in the ENT clinic, and conducting PT and SLP assessments to patients in the chemotherapy unit while receiving their treatment. A nurse navigator position for this patient population was approved and an expedited referral system was initiated. At the same time, the inpatient team implemented a specialized HNC unit in the medical-surgical floor, developed the protocols for the management of postsurgery HNC patients, educated nursing staff, and standardized patient education to facilitate transition to the next level of care (Table 3).
Discussion
The gap analysis of services provided to HNC patients at the cancer center identified three gaps in care: delay in treatment and supportive services, nonstandardized patient education, and lack of care coordination.
All patients should have access to a timely treatment initiation. In this analysis, surgical patients encountered a delay between surgery and the start of radiation therapy, about 3 weeks beyond the recommended in the literature.12 Clinicians mentioned delays in ensuring preradiation dental consultations as a significant issue affecting the patient treatment process. Re-assessment data reported that despite interventions for early dental referrals, 80% of patients still started radiation over 6 weeks after surgery; however, the average time lag decreased from 68 days to 53 days.
RT delays in HNC patients not only affect patients’ emotional state but may also impact clinical outcomes. Treatment delays have the potential to harm patients by: allowing tumor growth that impact on the curative outcomes of RT; postponing the benefits of palliative RT on symptom relief; and causing psychological distress.25 In addition, delay in starting treatment has shown to increase the risk for local recurrence,13,26 and decrease survival.27
Higher demand for advanced RT modalities has been linked to treatment delays. Waiting times from initial RT evaluation to start RT have increased over time, from <14 days in 1989 to 31 days in 1997.11 This is explained by the complexity of the pretreatment evaluations and the increasing demand of radiation services, especially in high volume institutions.25,27A fast-track program to reduce waiting time in the treatment of HNC patients reported to be effective.22 This program includes a patient coordinator, a hotline for referral procedures, prebooked slots for ENT and RT clinics, faster pathology and imaging reports, and the establishment of an MTD team.
The clinician survey identified patient needs classified in three categories: pre-operative education, hospitalization process, and access to support services. Regarding pre-operative education, clinicians acknowledged that although patients were educated about their surgical options and possible outcomes prior to hospitalization, they often could not fully understand this information at the time of the instruction. The high need for education particularly in the pretreatment phase was documented in a needs assessment survey for HNC patients conducted at the cancer center D DeMille, RD, unpublished data, August 2013).
Studies have looked at the effectiveness of education in cancer patients. The use of teaching interventions (written information, audiotapes, videotapes, and computer programs) has proven to be valuable for educating patients prior to experiencing cancer treatments.20Further, a systematic review of preparatory education for cancer patients undergoing surgery reported that face-to-face discussions appear to be effective at improving patient outcomes with regards to increasing knowledge and decreasing anxiety.21 However, it was stated that the timing of the delivery of education is critical to be efficient. For example, an education session provided one day prior the day of surgery is not useful as it may place additional stress on a patient who is already highly anxious and decreases the likelihood for the information to be managed. It is recommended to deliver education early enough prior surgery to allow time for the patient to process the information. Also, a study reported that presurgical education on potential side effects; the assessment of patients’ needs by an SLP, physical therapist, nutritionist, and social worker; and pre-operative nutritional support decrease postoperative complications.4
The education committee was created in response to the gap on patient education. The inpatient team took the lead and provided intense education on the care of HNC patients to the nursing staff and to HNC patients and their families about postoperative care at home. Education was also extended to rehabilitation facilities caring for this cancer population at discharge from the hospital.
Clinicians identified a gap during the hospitalization process. The gap included prolonged stay of patients in the ICU postsurgery, inefficient interclinician communication, lack of standardization of postsurgical care, and difficulty communicating with external home care teams. A major intervention was implemented that included the creation of a HNC specialized unit that offered a structured setting for standardized care and communication between patients and clinicians. Dedicated units for the management of HNC patients highly enhance the quality of care provided because it enables the MTD team to work properly by clearly defining roles and responsibilities, delineating evidence-based clinical interventions, and promoting expert care for this patient population.23In addition, several key steps have been recommended to reduce the fragmentation of care for hospital patients, including developing a referral/transition tracking system, organizing and training staff members to coordinate transition/referrals, and identifying and creating agreements with key care providers.28
Early patient access to supportive services was a concern to most clinicians. HNC providers were not consulting the CARE clinic about patients’ nutritional, physical and SLP needs until the patient was having serious problems. Patient tracking found that the minority of patients met the standard of having a presurgical speech referral. Most patients had access to outpatient nutrition services during radiation therapy but the majority of patients in the sample did not attend CARE clinic. The literature strongly supports early management of HNC patients by the SPL and nutrition counselor. Van der Molen and colleagues demonstrated that a pretreatment SPL rehabilitation program is feasible and offers reasonable patient compliance despite of the burden caused by ongoing chemo-radiation therapy for HNC patients.16Similarly, early nutrition counseling for HNC patients undergoing RT has reported to decrease unintended weight loss and malnutrition compared with late nutrition intervention.19
Although there are clear gaps in care for HNC patients from the clinicians’ perspective, the patients surveyed indicated a clear satisfaction with their care at the cancer center. Almost all patients were satisfied with their relationships with clinicians in the team. Some patients mentioned complaints of insufficient pre-operative education and waiting time, but there were not significant complaints about coordination, which clinicians had identified as a major issue. This is likely explained by the small sample size and the patients’ inability to see the background interclinician communication.
A crucial suggestion to address all of these gaps in care was the implementation of a nurse navigator. With the support of hospital and cancer center administration, a nurse navigator was hired to address the needs of HNC patients throughout their disease trajectory. The team agreed that the nurse navigator should make contact with HNC patients during their initial appointment at the surgical ENT office. This initial contact allows the nurse navigator to provide support and connection to resources. Thereafter, early contact with this patient population allows the nurse navigator to follow the patient through the continuum of care from biopsy and diagnosis to survivorship. The nurse navigator facilitates communication between clinicians, patients and their families; and provides emotional support to patients while helping to manage their financial and transportation needs.29
Limitations
This is a quality improvement project with a small sample size of HNC cases. Data from this gap analysis are not statistically significant; yet, are clinically relevant in the management of the HNC population at the cancer center. Likewise, the patient sample size was small, making definitive generalizations about patient experience difficult; however, the data are helpful in highlighting possible problems for patients.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7-30.
2. National Cancer Institute. Head and neck cancers. https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet. Reviewed February 1, 2013. Accessed January 26, 2017
3. Weiderholt PA, Connor NP, Hartig GK, Harari PM. Bridging gaps in multidisciplinary head and neck cancer care: nursing coordination and case management. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl):S88-S91.
4. Dingman C, Hegedus PD, Likes C, McDowell P, McCarthy E, Zwilling C. A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol. 2008;6(3):125-131.
5. Liao C, Kang CJ, Lee LY, et al. Association between multidisciplinary team care approach and survival rates in patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1544-1553.
6. Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. Laryngoscope. 2011;121(10):2131-2135.
7. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganizing cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organization of MDTs and their impact on patient outcomes. Health Pol. 2015;119(4):464-474.
8. Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. 2013. www.nationalacademies.org/hmd/Reports/2013/Delivering-High-Quality-Cancer-Care-Charting-a-New-Course-for-a-System-in-Crisis.aspx. Published September 10, 2013. Accessed May 29, 2016.
9. Granda-Cameron C, DeMille D, Lynch MP, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72-80.
10. Davis-Ajami ML, Costa L, Kulik S. Gap analysis: synergies and opportunities for effective nursing leadership. Nurs Econ. 2014;32(1):17-25.
11. Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(4):929-936.
12. Van Harten MC, Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282-290.
13. Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J ClinOncol. 2003;21(3):555-563.
14. Lazarus CL. Management of swallowing disorders in head and neck cancer patients: optimal patterns of care. Sem Speech Lang. 2000;21(4):293-310.
15. Mayer KR. Learning to speak after laryngectomy. http://speech-language-pathology-audiology.advanceweb.com/Features/Articles/Learning-to-Speak-After-Laryngectomy.aspx. Posted October 27, 2014. Accessed January 17, 2017.
16. van der Molen L, van Rossum MA, Burkhead LM, et al. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemo-radiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26:155-170.
17. Starmer HM, Gourin CG. Is speech language pathologist evaluation necessary in the nonoperative treatment of head and neck cancer? Laryngoscope. 2013;123(7):1571-1572.
18. [Article in French] Meuric J, Garabige V, Blanc-Vincent MP, et al. Good clinical practice in nutritional management of head and neck cancer patients. Bull Cancer. 1999;86(10):843-854.
19. van den Berg MG, Rasmussen-Conrad EL, Wei KH, et al. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872-877.
20. Waller A, Forshaw K, Bryant J, Mair S. Interventions for preparing patients for chemotherapy and radiotherapy: a systematic review. Supp Care Ca. 2014;22(8):2297-2308.
21. Waller A, Forshaw K, Bryant J, et al. Preparatory education for cancer patients undergoing surgery: a systematic review of volume and quality of research output over time. Patient Educ Couns. 2015;98:1540-1549.
22. Toustrup K, Lambersten K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636-641.
23. Bergamini C, Locati L, Bossi P et al. Does a multidisciplinary team approach in a tertiary referral centre impact on the initial management of head and neck cancer? Oral Oncol. 2016;54:54-57.
24. AHRQ. Pediatric toolkit for using the AHRQ quality indicators. http://www.ahrq.gov/professionals/systems/hospital/qitoolkit/pediatrictoolkit.html . Reviewed July 2016. Accessed January 26, 2017.
25. Mackillop WJ. Killing time: the consequences of delays in radiotherapy. Radiother Oncol. 2007;84:1-4.
26.Chen Z, King, W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3-16.
27. Sharma S, Bekelman J, Lin A et al. Clinical impact of prolonged diagnosis to treatment interval (DTI) among patients with ororpharyngeal squamous cell carcinoma. Oral Oncol. 2016;56:17-24.
28. Improving chronic illness care. Reducing care fragmentation. Care coordination. http://www.improvingchroniccare.org/index.php?p=Care_Coordination&s=326. Published 2010. Accessed May 28, 2016.
29. Fillion L, de Serres M, Cook S, et al. Professional patient navigation in head and neck cancer. Sem Oncol Nurs. 2009;25(3):212-221.