User login
Audit and Feedback: A Quality Improvement Study to Improve Antimicrobial Stewardship
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
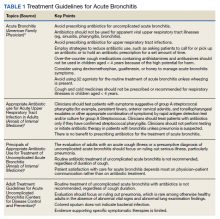
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
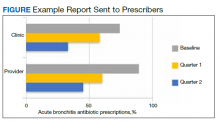
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
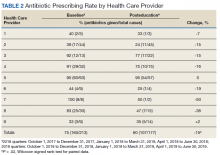
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
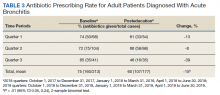
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275