User login
Electromagnetic vs Self‐Advancing Tube
Enteral nutrition is an essential component of the care plan for critically ill and injured patients. There is consensus that critically ill patients are at risk for malnutrition, and those who will be unable to consume adequate oral nutrition within 3 days should receive specialized enteral and/or parenteral nutrition therapy.[1] Multiple studies and reputable scientific societies support early initiation of enteral feedings within 24 to 48 hours of admission to the intensive care unit (ICU) to promote tolerance, minimize the risk of intestinal barrier dysfunction and infectious complications, and reduce the length of mechanical ventilation and hospital stay, as well as mortality.[2, 3, 4, 5] Although nasogastric feeding is appropriate for the majority of patients requiring short‐term nutrition support, there is a large group of patients in whom impaired gastric emptying presents challenges to feeding. The American Society for Parenteral and Enteral Nutrition, the American Thoracic Society, as well as the Infectious Diseases Society of America (IDSA), have published guidelines in support of postpyloric feeding in the ICU setting due to its association with reduced incidence of healthcare‐associated infections, specifically ventilator‐associated pneumonia (VAP).[2, 3, 6, 7] Four randomized clinical trials in the last 5 years have attempted to end the debate on the benefits of postpyloric feeding compared to intragastric feeding[5, 8, 9, 10]; 2 trials demonstrated an increase in calorie and protein intake and lower incidence of VAP in patients fed via the postpyloric route.[8, 10] One recent article[11] has suggested that severity of illness may play a role in the optimal selection of feeding route. Huang et al. randomly assigned patients to the nasogastric or nasoduodenal feeding route and documented the Acute Physiology and Chronic Health Evaluation II score as less than or greater than 20. Among more severely ill patients, those fed by the gastric route experienced longer ICU stay, more feeding complications, and lower calorie and protein intake than patients fed by the postpyloric route.[11] In an article comparing nutrition therapy recommendations among 3 major North American nutrition societies, the consensus was that critically ill patients at high risk for aspiration or feeding intolerance should be fed using small bowel access.[12] The Canadian Critical Care Guidelines Committee had the strongest recommendation for small bowel feeding stating that, if feasible, all critically ill patients should be fed via this route, based on the reduction in pneumonia.[12, 13]
When the decision is made to use postpyloric tube placement for nutrition therapy, the next decision is how to safely place the tube, ensure its postpyloric location, and minimize delays in feeding. Initiation of enteral formulas and timely advancement to nutrition goals is often delayed by unsuccessful feeding tube placement. Insertion of an enteral feeding tube into the postpyloric position is often done at the bedside by trained medical personnel without endoscopic or fluoroscopic guidance; however, the blind bedside approach is not without challenges. Success rates of this approach vary greatly depending on the patient population and provider expertise. The most challenging insertions may occur in patients who are endotracheally intubated, have depressed mental status, or impaired cough reflex.[14] Procedural complications from placement of nasoenteral feeding tubes by all methods can be as high as 10%,[15] with complication rates of 1% to 3%[16] for inadvertent placement of the feeding tube in the airway alone. The most common and serious complication is intubation of the bronchial tree with resulting pneumonitis, pneumonia, and pneumothorax, which reportedly occurs in 2.4% to 3.2% of tube insertions.[17, 18] It is recommended that radiographic confirmation of tube placement by any method occur prior to initiating feeding, thus eliminating any possibility of misplacement and administration of formula into the lungs.[18]
Historically, our institution advocated blind bedside placement of small bowel feeding tubes by trained ICU nurses, residents, and housestaff. Although not without risks, this method avoids the difficulty of coordinating endoscopic or fluoroscopic interventions that often necessitate transfer out of the ICU, with potential complications such as patient deterioration, and result in delays in initiating feeding.[19] However, like many other institutions, our level II medical center was interested in purchasing the Cortrak Enteral Access System (C‐EAS) (Viasys Medsystems, Wheeling, IL), which allows tracking of the small bowel feeding tube tip during placement. The C‐EAS uses an electromagnetic guide with a bedside monitor display to help providers observe the progress of the tube as it passes through the gastrointestinal tract. A receiver is placed on the patient's xiphoid process to detect the signal from the stylet that has an electromagnetic transmitter in the tip. The monitor displays the exact position of the postpyloric placement prior to removal of the tube guidewire. One early study by Ackerman and colleagues[20] found that the C‐EAS had a 100% success rate in avoiding lung placement and improves patient safety. The ability to monitor the location of the feeding tube tip in real time provides a safety feature for the clinician performing bedside insertions. In a recent study, the C‐EAS system was reported as not inferior to direct visualization of postpyloric placement via upper endoscopy.[21] In addition, several studies reported a reduction in mean time from physician order for tube placement to feeding initiation and fewer x‐rays for confirmation, thereby decreasing cost.[22] Not long after the C‐EAS system was purchased, Tiger 2 tubes (T2T) (Cook Inc., Bloomington, IN) were introduced in our facility for use in postpyloric feeding of ICU patients. The T2T system is a self‐advancing nasal jejunal feeding tube that uses a combination of intrinsic and stimulated gastric peristalsis with soft cilia‐like flaps in the side of the tube to propel the tube forward into the small bowel. Both tube systems have been studied over the past decade, with Gray et al.[22] reporting a 78% rate of successful small bowel placement using the electromagnetic‐guided device and Holzinger et al.[21] reporting an 89% success rate for jejunal placement using the same device. Davies and Bellomo[23] reported that their institution experienced a 100% success rate with small bowel placement of the T2T. Armed with 2 reputable, reliable modes of postpyloric tube placement, we encouraged all ICU staff to use these approaches for short‐term feeding for ICU patients whenever possible in conjunction with the ICU protocol for insertion and maintenance of small bowel feeding tubes. Both systems are preferred by our ICU physicians and nurses over other blind intubation systems (eg, Dobhoff tubes) and anecdotally, both appeared to have good success at initial postpyloric placement. However, having no objective data to support these observations, a clinical study was in order. The purpose of this retrospective review of small bowel feeding tube insertions was to determine which system achieves the objective of small bowel placement with the greatest accuracy on initial placement attempt, thus potentially improving patient outcomes and patient comfort for all future ICU patients.
METHODS
We conducted a retrospective chart review, examining the success of small bowel feeding tube placement in all ICU patients who received either a C‐EAS or a T2T from December 2009 through July 2013. Institutional review board approval was obtained, and due to the retrospective nature of the study, informed consent was waived. Neither manufacturer played any role in this study; the authors have no financial interests in either product and do not serve as consultants for either manufacturer.
Our ICU is a 20‐bed, mixed surgical and medical unit in a tertiary academic military medical center. To insert the C‐EAS tube, providers were required to take a three hour in‐service training session with the manufacturer's device representative. After initial training, providers were required to attempt 3 placements under the direct supervision of an expert user before they could independently place C‐EAS tubes. Competency was reviewed quarterly with hands‐on training. There are no designated tube insertion teams at our institution. All feeding tubes were inserted according to a current approved institutional protocol. Patients received a gastric motility agent (erythromycin 200 mg orally or intravenously) 30 minutes prior to tube insertion. C‐EAS patients received a confirmatory x‐ray, either anterior‐posterior (AP) portable chest x‐ray or portable abdominal film, when the provider felt the C‐EAS monitor tracing was consistent with postpyloric placement per the manufacturer's instructions. T2T patients received an AP portable chest x‐ray once the T2T had been inserted to 50 cm to ensure the tube was in the gastric system. The tube was advanced 10 cm every 30 to 60 minutes thereafter to a total distance of 90 cm per the manufacturer's recommendations, at which point a confirmatory portable abdominal film was taken for final location determination.
Patients who received small bowel feeding tubes were identified via electronic medical record data search; confirmation of tube placement was made with direct examination of the electronic medical record. Patients who received other small bowel feeding tubes, such as Dobbhoff tubes or endoscopically placed tubes of any type, were excluded. The date, time, and type of tube for initial insertion attempt were recorded, and radiographs, radiologic reports, and archived real‐time tracings (for C‐EAS) were compared. Tubes were considered successfully placed if the first confirmation film after completion of the procedure noted the tip of the tube in a postpyloric position. Tubes were considered unsuccessfully placed if the tip of the tube was noted anywhere proximal to the gastroduodenal junction. Insertions were excluded if investigator examination of the radiograph and the radiologic report were unable to identify the location of the tip of the tube. Complication rates, including endotracheal insertion, were recorded.
A power analysis was conducted a priori (SamplePower 3.0; IBM SPSS, Armonk, NY) by estimating successful placement with the C‐EAS tube on the first attempt at 80% and the T2T at 95% based on previous reports in the literature. A sample size of 75 patients was required in each group to achieve statistical significance at 0.80 power and at 0.05. The small bowel feeding tube placement success rate was analyzed using [2] and the Kappa coefficient.
RESULTS
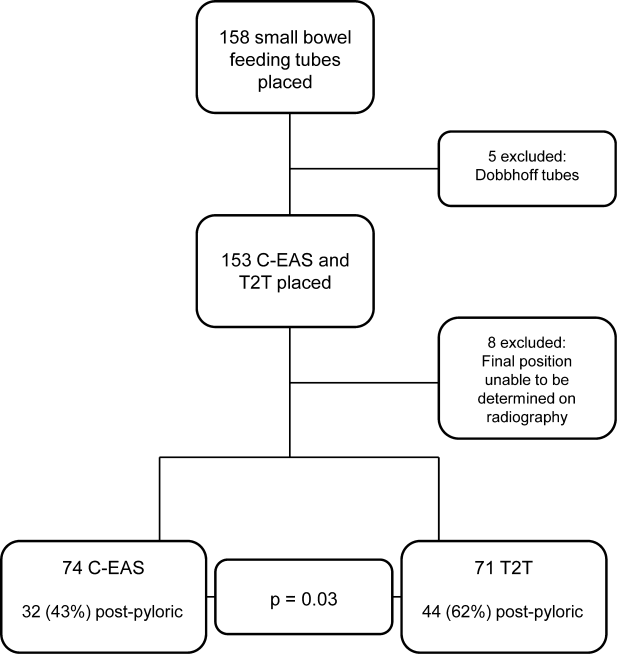
During the 3‐year study period, 158 small bowel feeding tubes were placed in the ICU. Of these, 5 were Dobhoff tubes (3 blind insertions and 2 endoscopic placements), 72 T2T, and 81 C‐EAS tubes. Of the T2T and C‐EAS tubes, final position was unable to be determined via radiograph for 1 T2T (1%) and 7 C‐EAS (8%). These tubes (N=13) were excluded from data analysis, leaving a final study population of 145: 71 T2T and 74 C‐EAS. Demographics of the included patients are found in Table 1. Successful postpyloric placement on the first attempt was achieved in 44 (62%) of T2T and 32 (43%) of C‐EAS (P=0.03) (Figure 1).
| Cortrak, n=74 | Tiger 2, n=71 | |
|---|---|---|
| ||
| Characteristic | ||
| Age (y) | 6719 | 6814 |
| Body mass index | 286 | 308 |
| Female, n (%) | 27 (36) | 33 (46) |
| Male, n (%) | 47 (64) | 38 (54) |
| Patient type, n (%) | ||
| MICU | 54 (73) | 59 (83) |
| SICU | 18 (24) | 10 (14) |
| Trauma | 2 (3) | 2 (3) |
| Airway, n (%) | ||
| Endotracheal tube | 37 (50) | 48 (68) |
| None | 33 (45) | 18 (25) |
| Tracheostomy | 4 (5) | 5 (7) |
| Admission reason, n (%) | ||
| Sepsis | 17 (23) | 16 (23) |
| ARDS | 12 (16) | 8 (11) |
| Respiratory failure | 13 (18) | 15 (21) |
| Surgical | 10 (13) | 4 (6) |
| Pancreatitis | 8 (11) | 8 (11) |
| CVA | 5 (7) | 7 (10) |
| Multitrauma | 2 (3) | 2 (3) |
| Other | 7 (9) | 11 (15) |
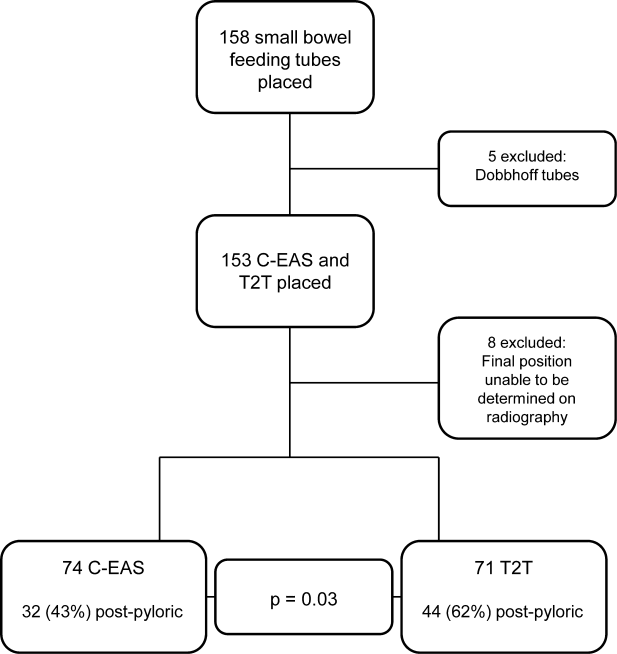
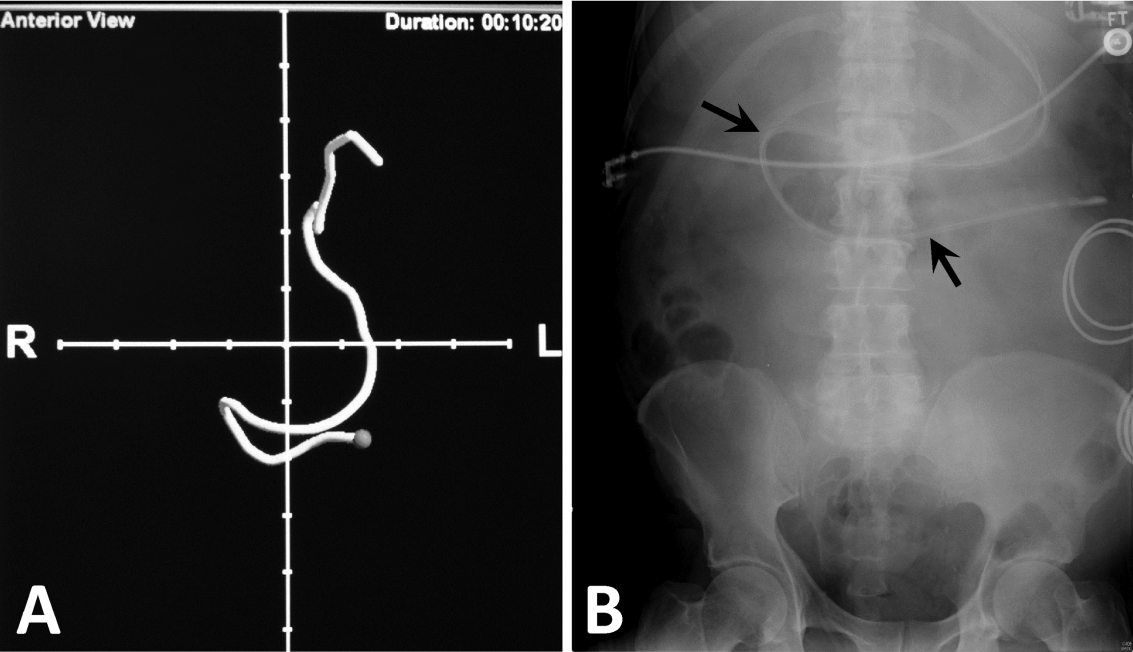
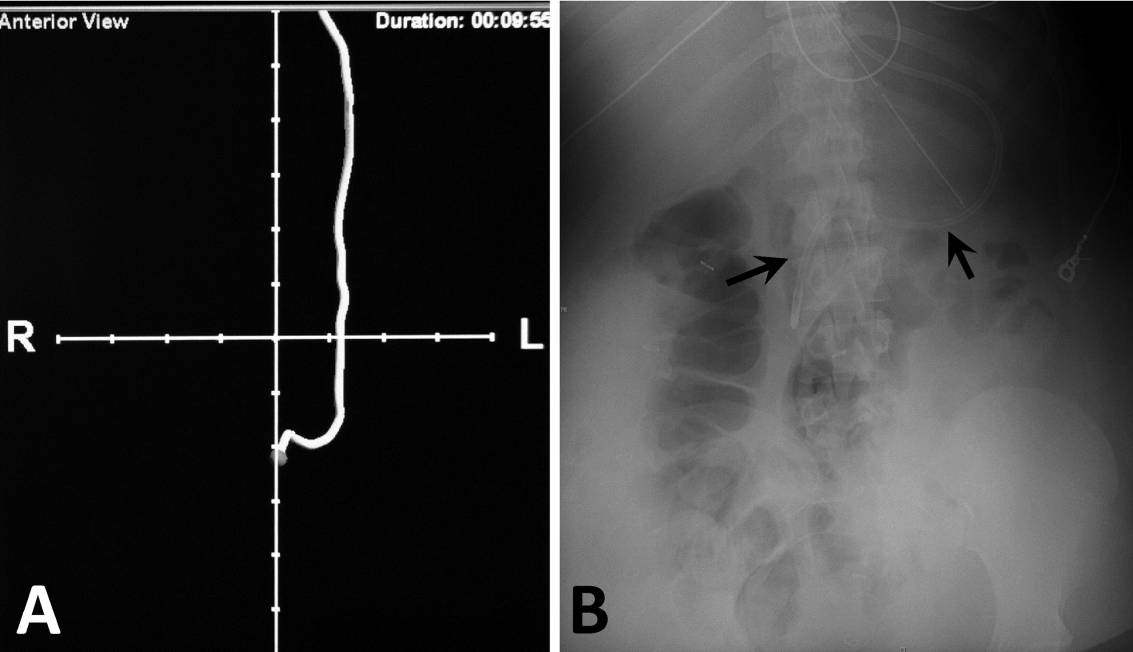
Next, we compared the congruency of the real‐time C‐EAS tracings to the confirmation radiographs (Figures 2 and 3). Of the C‐EAS tracings that indicated postpyloric position (N=29), the radiograph confirmed postpyloric placement 83% (n=24) of the time. Of C‐EAS tracings that indicated a prepyloric position (N=45), the radiograph also demonstrated a prepyloric position 82% (n=37) with a Kappa coefficient of 0.638 (Table 2).
| X‐Ray PP, N=32 | X‐Ray nPP, N=42 | |
|---|---|---|
| ||
| C‐EAS PP, N=29 | 24 (83%) | 5 (17%) |
| C‐EAS nPP, N=45 | 8 (18%) | 37 (82%) |
In addition to real‐time tracing archives, the C‐EAS system allows providers to designate their specialty. Of the 74 tubes placed, registered nurses and physicians placed the most tubes (36 each) and registered dieticians placed 2 tubes. Physicians and registered nurses successfully achieved postpyloric position on 17 (47%) and 14 (39%) initial attempts, respectively. Of the 2 registered dietician‐inserted tubes, only 1 tube was in the correct postpyloric position at the end of the initial attempt.
There were no endotracheal insertions or other complications noted during the study period with either small bowel feeding tube system.
CONCLUSION/DISCUSSION
Enteral nutrition is important for critically ill patients with early initiation of nutrition leading to decreased length of stay in the ICU and decreased mortality. The IDSA, North American nutrition societies, and Canadian Critical Care Guidelines recommend postpyloric nutrition to prevent frequent interruptions in feeding, allow for earlier feeding initiation, and to reduce the risk of aspiration. We evaluated 2 different enteral feeding tube systemsT2T and C‐EASto determine which system most commonly led to postpyloric placement on initial insertion attempt, thus facilitating postpyloric feeding.
Our results showed that there was a statistically significant difference favoring T2T over C‐EAS. This is in contrast to a study directly comparing the 2 systems, which demonstrated no statistically significant difference between the successful placement of either tube.[24] One reason for this difference may be that C‐EAS relies on user familiarity and dexterity with the electromagnetic guidance system. Our hospital does not have a specific team of trained providers who insert postpyloric tubes and thus may be more facile with this system. It would be interesting to see if a small team of trained providers could improve postpyloric C‐EAS placement over our current ICU staffing model, which allows RNs, physicians, and registered dieticians to place postpyloric feeding tubes. The T2T system is more simplistic in that no further training beyond basic feeding tube insertion is required, and we feel this may be the most important distinction that explains our results.
A reported advantage of the C‐EAS system is that direct visualization via the electromagnetic device replaces the need for confirmatory radiography. As expected, our results demonstrated a high positive predictive value for the C‐EAS tracing, although only 39% of tracings actually predicted postpyloric placement. Given the fact that 57% of C‐EAS tubes were not ultimately located in the postpyloric position, despite the inserting provider's interpretation of the tracing, we feel that confirmatory radiography is still required in our patient population. Again, this result likely points to the need for additional provider training on using the C‐EAS system and interpreting tracings, or a dedicated tube insertion team.
Limitations of this study are those inherent to retrospective research and include an inability to examine individual insertion technique and inability to record the inserting provider's interpretation of the C‐EAS tracing. Additionally, our electronic medical record did not facilitate data gathering regarding the time to completion of each procedure and initiation of enteral nutrition in our patients. It is possible that the speed with which the C‐EAS tube can be inserted and repositioned if prepyloric on initial confirmation, may lead to earlier initiation of enteral nutrition, versus the T2T protocol, which can take several hours until final insertion position is confirmed. In that case, the system that confers higher rates of initial postpyloric placement may be a less important mark than the overall time to completion of the insertion protocol. Finally, we did not perform a cost‐benefit analysis, which may have led to an advantage of 1 system over the other strictly from a resource management perspective.
In conclusion, given 2 small bowel feeding tube systems designed to facilitate postpyloric placement on initial insertion, the T2T tube proved a better system for use in our patient population with our current ICU staffing model. Additional training or designation of a tube insertion team might improve results with the C‐EAS system. Further prospective studies concerning timing of insertion protocols with respect to initiation of enteral nutrition and a complete cost‐benefit analysis comparing the 2 systems should be conducted.
Disclosures
The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the US government. The authors have no financial or other conflicts of interest to disclose.
- , , , . Enteral nutrition in critical care. J Clin Med Res. 2013;5:1–11.
- , , , et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. JPEN J Parenter Enteral Nutr. 2009;33:277–313.
- , , , , ; Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373.
- , , , et al.; Nutrition Guidelines Investigators of the ANZICS Clinical Trials Group. Effect of evidence‐based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300:2731–2741.
- , , , , . A randomised controlled comparison of early post‐pyloric vs early gastric feeding to meet nutritional targets in ventilated intensive care patients. Crit Care. 2009;13:R187.
- Critical Illness Update Evidence‐Based Nutrition Practice Guideline. 2012. Available at: http://andevidencelibrary.com/topic.cfm?format_tables=0171:388–416.
- , , , , , . Duodenal vs gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med. 2009;37:866–872.
- , , , et al. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med. 2012;40:2342–2348.
- , , , et al. Gastric versus transpyloric feeding in severe traumatic brain injury: a prospective, randomized trial. Intens Care Med. 2010;36:1532–1539.
- , , , , , . Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. J Acad Nutr Diet. 2012;112:1138–1146.
- , , , et al. Guidelines, guidelines, guidelines: what are we to do with all these North American guidelines? J Parent Ent Nutr. 2010;34:625–643.
- Critical Care Nutrition. Nutrition Clinical Practice Guidelines. 2009. Available at: http://www.criticalcarenutrition.com/docs/cpg/5.3Smallbowel_FINAL.pdf. Accessed July 21, 2013.
- , , , et al. Nasoenteric tube complications. Scand J Surg. 2012;101:147–155.
- , , , . Feeding tube placement: errors and complications. Nutr Clin Pract. 2007;27:738–748.
- , . Use of small‐bore feeding tubes: successes and failures. Curr Opin Clin Nutr Metab Care. 2007;10:291–296.
- , . Enhancing patient safety during feeding tube insertion: a review of more than 2,000 insertions. J Parent Ent Nutr. 2006;30:440–445.
- , , . Patient safety: effect of institutional protocols on adverse events related to feeding tube placement in the critically ill. J Am Coll Surg. 2004;199:39–50.
- . Acute complications associated with bedside placement of feeding tubes. Nutr Clin Pract. 2006;21:40–55.
- , , , , . The effectiveness of the CORTRAKTM device in avoiding lung placement of small bore enteral feeding tubes [abstract]. Am J Crit Care. 2004;13:268.
- , , . Jejunal tube placement in critically ill patients: a prospective, randomized trial comparing the endoscopic technique with the electromagnetically visualized method. Crit Care Med. 2011;39:73–77.
- , , , et al. Bedside electromagnetic‐guided feeding tube placement: an improvement over traditional placement technique? Nutr Clin Pract. 2007;22:436–444.
- , . Establishment of enteral nutrition: prokinetic agents and small bowel feeding tubes. Curr Opin Crit Care. 2004;10:156–161.
- , , , et al. Postpyloric feeding tubes for surgical intensive care patients. Pilot series to evaluate two methods for bedside placement [abstract]. Anaesthesist. 2011;60(3):214–220.
Enteral nutrition is an essential component of the care plan for critically ill and injured patients. There is consensus that critically ill patients are at risk for malnutrition, and those who will be unable to consume adequate oral nutrition within 3 days should receive specialized enteral and/or parenteral nutrition therapy.[1] Multiple studies and reputable scientific societies support early initiation of enteral feedings within 24 to 48 hours of admission to the intensive care unit (ICU) to promote tolerance, minimize the risk of intestinal barrier dysfunction and infectious complications, and reduce the length of mechanical ventilation and hospital stay, as well as mortality.[2, 3, 4, 5] Although nasogastric feeding is appropriate for the majority of patients requiring short‐term nutrition support, there is a large group of patients in whom impaired gastric emptying presents challenges to feeding. The American Society for Parenteral and Enteral Nutrition, the American Thoracic Society, as well as the Infectious Diseases Society of America (IDSA), have published guidelines in support of postpyloric feeding in the ICU setting due to its association with reduced incidence of healthcare‐associated infections, specifically ventilator‐associated pneumonia (VAP).[2, 3, 6, 7] Four randomized clinical trials in the last 5 years have attempted to end the debate on the benefits of postpyloric feeding compared to intragastric feeding[5, 8, 9, 10]; 2 trials demonstrated an increase in calorie and protein intake and lower incidence of VAP in patients fed via the postpyloric route.[8, 10] One recent article[11] has suggested that severity of illness may play a role in the optimal selection of feeding route. Huang et al. randomly assigned patients to the nasogastric or nasoduodenal feeding route and documented the Acute Physiology and Chronic Health Evaluation II score as less than or greater than 20. Among more severely ill patients, those fed by the gastric route experienced longer ICU stay, more feeding complications, and lower calorie and protein intake than patients fed by the postpyloric route.[11] In an article comparing nutrition therapy recommendations among 3 major North American nutrition societies, the consensus was that critically ill patients at high risk for aspiration or feeding intolerance should be fed using small bowel access.[12] The Canadian Critical Care Guidelines Committee had the strongest recommendation for small bowel feeding stating that, if feasible, all critically ill patients should be fed via this route, based on the reduction in pneumonia.[12, 13]
When the decision is made to use postpyloric tube placement for nutrition therapy, the next decision is how to safely place the tube, ensure its postpyloric location, and minimize delays in feeding. Initiation of enteral formulas and timely advancement to nutrition goals is often delayed by unsuccessful feeding tube placement. Insertion of an enteral feeding tube into the postpyloric position is often done at the bedside by trained medical personnel without endoscopic or fluoroscopic guidance; however, the blind bedside approach is not without challenges. Success rates of this approach vary greatly depending on the patient population and provider expertise. The most challenging insertions may occur in patients who are endotracheally intubated, have depressed mental status, or impaired cough reflex.[14] Procedural complications from placement of nasoenteral feeding tubes by all methods can be as high as 10%,[15] with complication rates of 1% to 3%[16] for inadvertent placement of the feeding tube in the airway alone. The most common and serious complication is intubation of the bronchial tree with resulting pneumonitis, pneumonia, and pneumothorax, which reportedly occurs in 2.4% to 3.2% of tube insertions.[17, 18] It is recommended that radiographic confirmation of tube placement by any method occur prior to initiating feeding, thus eliminating any possibility of misplacement and administration of formula into the lungs.[18]
Historically, our institution advocated blind bedside placement of small bowel feeding tubes by trained ICU nurses, residents, and housestaff. Although not without risks, this method avoids the difficulty of coordinating endoscopic or fluoroscopic interventions that often necessitate transfer out of the ICU, with potential complications such as patient deterioration, and result in delays in initiating feeding.[19] However, like many other institutions, our level II medical center was interested in purchasing the Cortrak Enteral Access System (C‐EAS) (Viasys Medsystems, Wheeling, IL), which allows tracking of the small bowel feeding tube tip during placement. The C‐EAS uses an electromagnetic guide with a bedside monitor display to help providers observe the progress of the tube as it passes through the gastrointestinal tract. A receiver is placed on the patient's xiphoid process to detect the signal from the stylet that has an electromagnetic transmitter in the tip. The monitor displays the exact position of the postpyloric placement prior to removal of the tube guidewire. One early study by Ackerman and colleagues[20] found that the C‐EAS had a 100% success rate in avoiding lung placement and improves patient safety. The ability to monitor the location of the feeding tube tip in real time provides a safety feature for the clinician performing bedside insertions. In a recent study, the C‐EAS system was reported as not inferior to direct visualization of postpyloric placement via upper endoscopy.[21] In addition, several studies reported a reduction in mean time from physician order for tube placement to feeding initiation and fewer x‐rays for confirmation, thereby decreasing cost.[22] Not long after the C‐EAS system was purchased, Tiger 2 tubes (T2T) (Cook Inc., Bloomington, IN) were introduced in our facility for use in postpyloric feeding of ICU patients. The T2T system is a self‐advancing nasal jejunal feeding tube that uses a combination of intrinsic and stimulated gastric peristalsis with soft cilia‐like flaps in the side of the tube to propel the tube forward into the small bowel. Both tube systems have been studied over the past decade, with Gray et al.[22] reporting a 78% rate of successful small bowel placement using the electromagnetic‐guided device and Holzinger et al.[21] reporting an 89% success rate for jejunal placement using the same device. Davies and Bellomo[23] reported that their institution experienced a 100% success rate with small bowel placement of the T2T. Armed with 2 reputable, reliable modes of postpyloric tube placement, we encouraged all ICU staff to use these approaches for short‐term feeding for ICU patients whenever possible in conjunction with the ICU protocol for insertion and maintenance of small bowel feeding tubes. Both systems are preferred by our ICU physicians and nurses over other blind intubation systems (eg, Dobhoff tubes) and anecdotally, both appeared to have good success at initial postpyloric placement. However, having no objective data to support these observations, a clinical study was in order. The purpose of this retrospective review of small bowel feeding tube insertions was to determine which system achieves the objective of small bowel placement with the greatest accuracy on initial placement attempt, thus potentially improving patient outcomes and patient comfort for all future ICU patients.
METHODS
We conducted a retrospective chart review, examining the success of small bowel feeding tube placement in all ICU patients who received either a C‐EAS or a T2T from December 2009 through July 2013. Institutional review board approval was obtained, and due to the retrospective nature of the study, informed consent was waived. Neither manufacturer played any role in this study; the authors have no financial interests in either product and do not serve as consultants for either manufacturer.
Our ICU is a 20‐bed, mixed surgical and medical unit in a tertiary academic military medical center. To insert the C‐EAS tube, providers were required to take a three hour in‐service training session with the manufacturer's device representative. After initial training, providers were required to attempt 3 placements under the direct supervision of an expert user before they could independently place C‐EAS tubes. Competency was reviewed quarterly with hands‐on training. There are no designated tube insertion teams at our institution. All feeding tubes were inserted according to a current approved institutional protocol. Patients received a gastric motility agent (erythromycin 200 mg orally or intravenously) 30 minutes prior to tube insertion. C‐EAS patients received a confirmatory x‐ray, either anterior‐posterior (AP) portable chest x‐ray or portable abdominal film, when the provider felt the C‐EAS monitor tracing was consistent with postpyloric placement per the manufacturer's instructions. T2T patients received an AP portable chest x‐ray once the T2T had been inserted to 50 cm to ensure the tube was in the gastric system. The tube was advanced 10 cm every 30 to 60 minutes thereafter to a total distance of 90 cm per the manufacturer's recommendations, at which point a confirmatory portable abdominal film was taken for final location determination.
Patients who received small bowel feeding tubes were identified via electronic medical record data search; confirmation of tube placement was made with direct examination of the electronic medical record. Patients who received other small bowel feeding tubes, such as Dobbhoff tubes or endoscopically placed tubes of any type, were excluded. The date, time, and type of tube for initial insertion attempt were recorded, and radiographs, radiologic reports, and archived real‐time tracings (for C‐EAS) were compared. Tubes were considered successfully placed if the first confirmation film after completion of the procedure noted the tip of the tube in a postpyloric position. Tubes were considered unsuccessfully placed if the tip of the tube was noted anywhere proximal to the gastroduodenal junction. Insertions were excluded if investigator examination of the radiograph and the radiologic report were unable to identify the location of the tip of the tube. Complication rates, including endotracheal insertion, were recorded.
A power analysis was conducted a priori (SamplePower 3.0; IBM SPSS, Armonk, NY) by estimating successful placement with the C‐EAS tube on the first attempt at 80% and the T2T at 95% based on previous reports in the literature. A sample size of 75 patients was required in each group to achieve statistical significance at 0.80 power and at 0.05. The small bowel feeding tube placement success rate was analyzed using [2] and the Kappa coefficient.
RESULTS
During the 3‐year study period, 158 small bowel feeding tubes were placed in the ICU. Of these, 5 were Dobhoff tubes (3 blind insertions and 2 endoscopic placements), 72 T2T, and 81 C‐EAS tubes. Of the T2T and C‐EAS tubes, final position was unable to be determined via radiograph for 1 T2T (1%) and 7 C‐EAS (8%). These tubes (N=13) were excluded from data analysis, leaving a final study population of 145: 71 T2T and 74 C‐EAS. Demographics of the included patients are found in Table 1. Successful postpyloric placement on the first attempt was achieved in 44 (62%) of T2T and 32 (43%) of C‐EAS (P=0.03) (Figure 1).

| Cortrak, n=74 | Tiger 2, n=71 | |
|---|---|---|
| ||
| Characteristic | ||
| Age (y) | 6719 | 6814 |
| Body mass index | 286 | 308 |
| Female, n (%) | 27 (36) | 33 (46) |
| Male, n (%) | 47 (64) | 38 (54) |
| Patient type, n (%) | ||
| MICU | 54 (73) | 59 (83) |
| SICU | 18 (24) | 10 (14) |
| Trauma | 2 (3) | 2 (3) |
| Airway, n (%) | ||
| Endotracheal tube | 37 (50) | 48 (68) |
| None | 33 (45) | 18 (25) |
| Tracheostomy | 4 (5) | 5 (7) |
| Admission reason, n (%) | ||
| Sepsis | 17 (23) | 16 (23) |
| ARDS | 12 (16) | 8 (11) |
| Respiratory failure | 13 (18) | 15 (21) |
| Surgical | 10 (13) | 4 (6) |
| Pancreatitis | 8 (11) | 8 (11) |
| CVA | 5 (7) | 7 (10) |
| Multitrauma | 2 (3) | 2 (3) |
| Other | 7 (9) | 11 (15) |
Next, we compared the congruency of the real‐time C‐EAS tracings to the confirmation radiographs (Figures 2 and 3). Of the C‐EAS tracings that indicated postpyloric position (N=29), the radiograph confirmed postpyloric placement 83% (n=24) of the time. Of C‐EAS tracings that indicated a prepyloric position (N=45), the radiograph also demonstrated a prepyloric position 82% (n=37) with a Kappa coefficient of 0.638 (Table 2).


| X‐Ray PP, N=32 | X‐Ray nPP, N=42 | |
|---|---|---|
| ||
| C‐EAS PP, N=29 | 24 (83%) | 5 (17%) |
| C‐EAS nPP, N=45 | 8 (18%) | 37 (82%) |
In addition to real‐time tracing archives, the C‐EAS system allows providers to designate their specialty. Of the 74 tubes placed, registered nurses and physicians placed the most tubes (36 each) and registered dieticians placed 2 tubes. Physicians and registered nurses successfully achieved postpyloric position on 17 (47%) and 14 (39%) initial attempts, respectively. Of the 2 registered dietician‐inserted tubes, only 1 tube was in the correct postpyloric position at the end of the initial attempt.
There were no endotracheal insertions or other complications noted during the study period with either small bowel feeding tube system.
CONCLUSION/DISCUSSION
Enteral nutrition is important for critically ill patients with early initiation of nutrition leading to decreased length of stay in the ICU and decreased mortality. The IDSA, North American nutrition societies, and Canadian Critical Care Guidelines recommend postpyloric nutrition to prevent frequent interruptions in feeding, allow for earlier feeding initiation, and to reduce the risk of aspiration. We evaluated 2 different enteral feeding tube systemsT2T and C‐EASto determine which system most commonly led to postpyloric placement on initial insertion attempt, thus facilitating postpyloric feeding.
Our results showed that there was a statistically significant difference favoring T2T over C‐EAS. This is in contrast to a study directly comparing the 2 systems, which demonstrated no statistically significant difference between the successful placement of either tube.[24] One reason for this difference may be that C‐EAS relies on user familiarity and dexterity with the electromagnetic guidance system. Our hospital does not have a specific team of trained providers who insert postpyloric tubes and thus may be more facile with this system. It would be interesting to see if a small team of trained providers could improve postpyloric C‐EAS placement over our current ICU staffing model, which allows RNs, physicians, and registered dieticians to place postpyloric feeding tubes. The T2T system is more simplistic in that no further training beyond basic feeding tube insertion is required, and we feel this may be the most important distinction that explains our results.
A reported advantage of the C‐EAS system is that direct visualization via the electromagnetic device replaces the need for confirmatory radiography. As expected, our results demonstrated a high positive predictive value for the C‐EAS tracing, although only 39% of tracings actually predicted postpyloric placement. Given the fact that 57% of C‐EAS tubes were not ultimately located in the postpyloric position, despite the inserting provider's interpretation of the tracing, we feel that confirmatory radiography is still required in our patient population. Again, this result likely points to the need for additional provider training on using the C‐EAS system and interpreting tracings, or a dedicated tube insertion team.
Limitations of this study are those inherent to retrospective research and include an inability to examine individual insertion technique and inability to record the inserting provider's interpretation of the C‐EAS tracing. Additionally, our electronic medical record did not facilitate data gathering regarding the time to completion of each procedure and initiation of enteral nutrition in our patients. It is possible that the speed with which the C‐EAS tube can be inserted and repositioned if prepyloric on initial confirmation, may lead to earlier initiation of enteral nutrition, versus the T2T protocol, which can take several hours until final insertion position is confirmed. In that case, the system that confers higher rates of initial postpyloric placement may be a less important mark than the overall time to completion of the insertion protocol. Finally, we did not perform a cost‐benefit analysis, which may have led to an advantage of 1 system over the other strictly from a resource management perspective.
In conclusion, given 2 small bowel feeding tube systems designed to facilitate postpyloric placement on initial insertion, the T2T tube proved a better system for use in our patient population with our current ICU staffing model. Additional training or designation of a tube insertion team might improve results with the C‐EAS system. Further prospective studies concerning timing of insertion protocols with respect to initiation of enteral nutrition and a complete cost‐benefit analysis comparing the 2 systems should be conducted.
Disclosures
The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the US government. The authors have no financial or other conflicts of interest to disclose.
Enteral nutrition is an essential component of the care plan for critically ill and injured patients. There is consensus that critically ill patients are at risk for malnutrition, and those who will be unable to consume adequate oral nutrition within 3 days should receive specialized enteral and/or parenteral nutrition therapy.[1] Multiple studies and reputable scientific societies support early initiation of enteral feedings within 24 to 48 hours of admission to the intensive care unit (ICU) to promote tolerance, minimize the risk of intestinal barrier dysfunction and infectious complications, and reduce the length of mechanical ventilation and hospital stay, as well as mortality.[2, 3, 4, 5] Although nasogastric feeding is appropriate for the majority of patients requiring short‐term nutrition support, there is a large group of patients in whom impaired gastric emptying presents challenges to feeding. The American Society for Parenteral and Enteral Nutrition, the American Thoracic Society, as well as the Infectious Diseases Society of America (IDSA), have published guidelines in support of postpyloric feeding in the ICU setting due to its association with reduced incidence of healthcare‐associated infections, specifically ventilator‐associated pneumonia (VAP).[2, 3, 6, 7] Four randomized clinical trials in the last 5 years have attempted to end the debate on the benefits of postpyloric feeding compared to intragastric feeding[5, 8, 9, 10]; 2 trials demonstrated an increase in calorie and protein intake and lower incidence of VAP in patients fed via the postpyloric route.[8, 10] One recent article[11] has suggested that severity of illness may play a role in the optimal selection of feeding route. Huang et al. randomly assigned patients to the nasogastric or nasoduodenal feeding route and documented the Acute Physiology and Chronic Health Evaluation II score as less than or greater than 20. Among more severely ill patients, those fed by the gastric route experienced longer ICU stay, more feeding complications, and lower calorie and protein intake than patients fed by the postpyloric route.[11] In an article comparing nutrition therapy recommendations among 3 major North American nutrition societies, the consensus was that critically ill patients at high risk for aspiration or feeding intolerance should be fed using small bowel access.[12] The Canadian Critical Care Guidelines Committee had the strongest recommendation for small bowel feeding stating that, if feasible, all critically ill patients should be fed via this route, based on the reduction in pneumonia.[12, 13]
When the decision is made to use postpyloric tube placement for nutrition therapy, the next decision is how to safely place the tube, ensure its postpyloric location, and minimize delays in feeding. Initiation of enteral formulas and timely advancement to nutrition goals is often delayed by unsuccessful feeding tube placement. Insertion of an enteral feeding tube into the postpyloric position is often done at the bedside by trained medical personnel without endoscopic or fluoroscopic guidance; however, the blind bedside approach is not without challenges. Success rates of this approach vary greatly depending on the patient population and provider expertise. The most challenging insertions may occur in patients who are endotracheally intubated, have depressed mental status, or impaired cough reflex.[14] Procedural complications from placement of nasoenteral feeding tubes by all methods can be as high as 10%,[15] with complication rates of 1% to 3%[16] for inadvertent placement of the feeding tube in the airway alone. The most common and serious complication is intubation of the bronchial tree with resulting pneumonitis, pneumonia, and pneumothorax, which reportedly occurs in 2.4% to 3.2% of tube insertions.[17, 18] It is recommended that radiographic confirmation of tube placement by any method occur prior to initiating feeding, thus eliminating any possibility of misplacement and administration of formula into the lungs.[18]
Historically, our institution advocated blind bedside placement of small bowel feeding tubes by trained ICU nurses, residents, and housestaff. Although not without risks, this method avoids the difficulty of coordinating endoscopic or fluoroscopic interventions that often necessitate transfer out of the ICU, with potential complications such as patient deterioration, and result in delays in initiating feeding.[19] However, like many other institutions, our level II medical center was interested in purchasing the Cortrak Enteral Access System (C‐EAS) (Viasys Medsystems, Wheeling, IL), which allows tracking of the small bowel feeding tube tip during placement. The C‐EAS uses an electromagnetic guide with a bedside monitor display to help providers observe the progress of the tube as it passes through the gastrointestinal tract. A receiver is placed on the patient's xiphoid process to detect the signal from the stylet that has an electromagnetic transmitter in the tip. The monitor displays the exact position of the postpyloric placement prior to removal of the tube guidewire. One early study by Ackerman and colleagues[20] found that the C‐EAS had a 100% success rate in avoiding lung placement and improves patient safety. The ability to monitor the location of the feeding tube tip in real time provides a safety feature for the clinician performing bedside insertions. In a recent study, the C‐EAS system was reported as not inferior to direct visualization of postpyloric placement via upper endoscopy.[21] In addition, several studies reported a reduction in mean time from physician order for tube placement to feeding initiation and fewer x‐rays for confirmation, thereby decreasing cost.[22] Not long after the C‐EAS system was purchased, Tiger 2 tubes (T2T) (Cook Inc., Bloomington, IN) were introduced in our facility for use in postpyloric feeding of ICU patients. The T2T system is a self‐advancing nasal jejunal feeding tube that uses a combination of intrinsic and stimulated gastric peristalsis with soft cilia‐like flaps in the side of the tube to propel the tube forward into the small bowel. Both tube systems have been studied over the past decade, with Gray et al.[22] reporting a 78% rate of successful small bowel placement using the electromagnetic‐guided device and Holzinger et al.[21] reporting an 89% success rate for jejunal placement using the same device. Davies and Bellomo[23] reported that their institution experienced a 100% success rate with small bowel placement of the T2T. Armed with 2 reputable, reliable modes of postpyloric tube placement, we encouraged all ICU staff to use these approaches for short‐term feeding for ICU patients whenever possible in conjunction with the ICU protocol for insertion and maintenance of small bowel feeding tubes. Both systems are preferred by our ICU physicians and nurses over other blind intubation systems (eg, Dobhoff tubes) and anecdotally, both appeared to have good success at initial postpyloric placement. However, having no objective data to support these observations, a clinical study was in order. The purpose of this retrospective review of small bowel feeding tube insertions was to determine which system achieves the objective of small bowel placement with the greatest accuracy on initial placement attempt, thus potentially improving patient outcomes and patient comfort for all future ICU patients.
METHODS
We conducted a retrospective chart review, examining the success of small bowel feeding tube placement in all ICU patients who received either a C‐EAS or a T2T from December 2009 through July 2013. Institutional review board approval was obtained, and due to the retrospective nature of the study, informed consent was waived. Neither manufacturer played any role in this study; the authors have no financial interests in either product and do not serve as consultants for either manufacturer.
Our ICU is a 20‐bed, mixed surgical and medical unit in a tertiary academic military medical center. To insert the C‐EAS tube, providers were required to take a three hour in‐service training session with the manufacturer's device representative. After initial training, providers were required to attempt 3 placements under the direct supervision of an expert user before they could independently place C‐EAS tubes. Competency was reviewed quarterly with hands‐on training. There are no designated tube insertion teams at our institution. All feeding tubes were inserted according to a current approved institutional protocol. Patients received a gastric motility agent (erythromycin 200 mg orally or intravenously) 30 minutes prior to tube insertion. C‐EAS patients received a confirmatory x‐ray, either anterior‐posterior (AP) portable chest x‐ray or portable abdominal film, when the provider felt the C‐EAS monitor tracing was consistent with postpyloric placement per the manufacturer's instructions. T2T patients received an AP portable chest x‐ray once the T2T had been inserted to 50 cm to ensure the tube was in the gastric system. The tube was advanced 10 cm every 30 to 60 minutes thereafter to a total distance of 90 cm per the manufacturer's recommendations, at which point a confirmatory portable abdominal film was taken for final location determination.
Patients who received small bowel feeding tubes were identified via electronic medical record data search; confirmation of tube placement was made with direct examination of the electronic medical record. Patients who received other small bowel feeding tubes, such as Dobbhoff tubes or endoscopically placed tubes of any type, were excluded. The date, time, and type of tube for initial insertion attempt were recorded, and radiographs, radiologic reports, and archived real‐time tracings (for C‐EAS) were compared. Tubes were considered successfully placed if the first confirmation film after completion of the procedure noted the tip of the tube in a postpyloric position. Tubes were considered unsuccessfully placed if the tip of the tube was noted anywhere proximal to the gastroduodenal junction. Insertions were excluded if investigator examination of the radiograph and the radiologic report were unable to identify the location of the tip of the tube. Complication rates, including endotracheal insertion, were recorded.
A power analysis was conducted a priori (SamplePower 3.0; IBM SPSS, Armonk, NY) by estimating successful placement with the C‐EAS tube on the first attempt at 80% and the T2T at 95% based on previous reports in the literature. A sample size of 75 patients was required in each group to achieve statistical significance at 0.80 power and at 0.05. The small bowel feeding tube placement success rate was analyzed using [2] and the Kappa coefficient.
RESULTS
During the 3‐year study period, 158 small bowel feeding tubes were placed in the ICU. Of these, 5 were Dobhoff tubes (3 blind insertions and 2 endoscopic placements), 72 T2T, and 81 C‐EAS tubes. Of the T2T and C‐EAS tubes, final position was unable to be determined via radiograph for 1 T2T (1%) and 7 C‐EAS (8%). These tubes (N=13) were excluded from data analysis, leaving a final study population of 145: 71 T2T and 74 C‐EAS. Demographics of the included patients are found in Table 1. Successful postpyloric placement on the first attempt was achieved in 44 (62%) of T2T and 32 (43%) of C‐EAS (P=0.03) (Figure 1).

| Cortrak, n=74 | Tiger 2, n=71 | |
|---|---|---|
| ||
| Characteristic | ||
| Age (y) | 6719 | 6814 |
| Body mass index | 286 | 308 |
| Female, n (%) | 27 (36) | 33 (46) |
| Male, n (%) | 47 (64) | 38 (54) |
| Patient type, n (%) | ||
| MICU | 54 (73) | 59 (83) |
| SICU | 18 (24) | 10 (14) |
| Trauma | 2 (3) | 2 (3) |
| Airway, n (%) | ||
| Endotracheal tube | 37 (50) | 48 (68) |
| None | 33 (45) | 18 (25) |
| Tracheostomy | 4 (5) | 5 (7) |
| Admission reason, n (%) | ||
| Sepsis | 17 (23) | 16 (23) |
| ARDS | 12 (16) | 8 (11) |
| Respiratory failure | 13 (18) | 15 (21) |
| Surgical | 10 (13) | 4 (6) |
| Pancreatitis | 8 (11) | 8 (11) |
| CVA | 5 (7) | 7 (10) |
| Multitrauma | 2 (3) | 2 (3) |
| Other | 7 (9) | 11 (15) |
Next, we compared the congruency of the real‐time C‐EAS tracings to the confirmation radiographs (Figures 2 and 3). Of the C‐EAS tracings that indicated postpyloric position (N=29), the radiograph confirmed postpyloric placement 83% (n=24) of the time. Of C‐EAS tracings that indicated a prepyloric position (N=45), the radiograph also demonstrated a prepyloric position 82% (n=37) with a Kappa coefficient of 0.638 (Table 2).


| X‐Ray PP, N=32 | X‐Ray nPP, N=42 | |
|---|---|---|
| ||
| C‐EAS PP, N=29 | 24 (83%) | 5 (17%) |
| C‐EAS nPP, N=45 | 8 (18%) | 37 (82%) |
In addition to real‐time tracing archives, the C‐EAS system allows providers to designate their specialty. Of the 74 tubes placed, registered nurses and physicians placed the most tubes (36 each) and registered dieticians placed 2 tubes. Physicians and registered nurses successfully achieved postpyloric position on 17 (47%) and 14 (39%) initial attempts, respectively. Of the 2 registered dietician‐inserted tubes, only 1 tube was in the correct postpyloric position at the end of the initial attempt.
There were no endotracheal insertions or other complications noted during the study period with either small bowel feeding tube system.
CONCLUSION/DISCUSSION
Enteral nutrition is important for critically ill patients with early initiation of nutrition leading to decreased length of stay in the ICU and decreased mortality. The IDSA, North American nutrition societies, and Canadian Critical Care Guidelines recommend postpyloric nutrition to prevent frequent interruptions in feeding, allow for earlier feeding initiation, and to reduce the risk of aspiration. We evaluated 2 different enteral feeding tube systemsT2T and C‐EASto determine which system most commonly led to postpyloric placement on initial insertion attempt, thus facilitating postpyloric feeding.
Our results showed that there was a statistically significant difference favoring T2T over C‐EAS. This is in contrast to a study directly comparing the 2 systems, which demonstrated no statistically significant difference between the successful placement of either tube.[24] One reason for this difference may be that C‐EAS relies on user familiarity and dexterity with the electromagnetic guidance system. Our hospital does not have a specific team of trained providers who insert postpyloric tubes and thus may be more facile with this system. It would be interesting to see if a small team of trained providers could improve postpyloric C‐EAS placement over our current ICU staffing model, which allows RNs, physicians, and registered dieticians to place postpyloric feeding tubes. The T2T system is more simplistic in that no further training beyond basic feeding tube insertion is required, and we feel this may be the most important distinction that explains our results.
A reported advantage of the C‐EAS system is that direct visualization via the electromagnetic device replaces the need for confirmatory radiography. As expected, our results demonstrated a high positive predictive value for the C‐EAS tracing, although only 39% of tracings actually predicted postpyloric placement. Given the fact that 57% of C‐EAS tubes were not ultimately located in the postpyloric position, despite the inserting provider's interpretation of the tracing, we feel that confirmatory radiography is still required in our patient population. Again, this result likely points to the need for additional provider training on using the C‐EAS system and interpreting tracings, or a dedicated tube insertion team.
Limitations of this study are those inherent to retrospective research and include an inability to examine individual insertion technique and inability to record the inserting provider's interpretation of the C‐EAS tracing. Additionally, our electronic medical record did not facilitate data gathering regarding the time to completion of each procedure and initiation of enteral nutrition in our patients. It is possible that the speed with which the C‐EAS tube can be inserted and repositioned if prepyloric on initial confirmation, may lead to earlier initiation of enteral nutrition, versus the T2T protocol, which can take several hours until final insertion position is confirmed. In that case, the system that confers higher rates of initial postpyloric placement may be a less important mark than the overall time to completion of the insertion protocol. Finally, we did not perform a cost‐benefit analysis, which may have led to an advantage of 1 system over the other strictly from a resource management perspective.
In conclusion, given 2 small bowel feeding tube systems designed to facilitate postpyloric placement on initial insertion, the T2T tube proved a better system for use in our patient population with our current ICU staffing model. Additional training or designation of a tube insertion team might improve results with the C‐EAS system. Further prospective studies concerning timing of insertion protocols with respect to initiation of enteral nutrition and a complete cost‐benefit analysis comparing the 2 systems should be conducted.
Disclosures
The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the US government. The authors have no financial or other conflicts of interest to disclose.
- , , , . Enteral nutrition in critical care. J Clin Med Res. 2013;5:1–11.
- , , , et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. JPEN J Parenter Enteral Nutr. 2009;33:277–313.
- , , , , ; Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373.
- , , , et al.; Nutrition Guidelines Investigators of the ANZICS Clinical Trials Group. Effect of evidence‐based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300:2731–2741.
- , , , , . A randomised controlled comparison of early post‐pyloric vs early gastric feeding to meet nutritional targets in ventilated intensive care patients. Crit Care. 2009;13:R187.
- Critical Illness Update Evidence‐Based Nutrition Practice Guideline. 2012. Available at: http://andevidencelibrary.com/topic.cfm?format_tables=0171:388–416.
- , , , , , . Duodenal vs gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med. 2009;37:866–872.
- , , , et al. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med. 2012;40:2342–2348.
- , , , et al. Gastric versus transpyloric feeding in severe traumatic brain injury: a prospective, randomized trial. Intens Care Med. 2010;36:1532–1539.
- , , , , , . Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. J Acad Nutr Diet. 2012;112:1138–1146.
- , , , et al. Guidelines, guidelines, guidelines: what are we to do with all these North American guidelines? J Parent Ent Nutr. 2010;34:625–643.
- Critical Care Nutrition. Nutrition Clinical Practice Guidelines. 2009. Available at: http://www.criticalcarenutrition.com/docs/cpg/5.3Smallbowel_FINAL.pdf. Accessed July 21, 2013.
- , , , et al. Nasoenteric tube complications. Scand J Surg. 2012;101:147–155.
- , , , . Feeding tube placement: errors and complications. Nutr Clin Pract. 2007;27:738–748.
- , . Use of small‐bore feeding tubes: successes and failures. Curr Opin Clin Nutr Metab Care. 2007;10:291–296.
- , . Enhancing patient safety during feeding tube insertion: a review of more than 2,000 insertions. J Parent Ent Nutr. 2006;30:440–445.
- , , . Patient safety: effect of institutional protocols on adverse events related to feeding tube placement in the critically ill. J Am Coll Surg. 2004;199:39–50.
- . Acute complications associated with bedside placement of feeding tubes. Nutr Clin Pract. 2006;21:40–55.
- , , , , . The effectiveness of the CORTRAKTM device in avoiding lung placement of small bore enteral feeding tubes [abstract]. Am J Crit Care. 2004;13:268.
- , , . Jejunal tube placement in critically ill patients: a prospective, randomized trial comparing the endoscopic technique with the electromagnetically visualized method. Crit Care Med. 2011;39:73–77.
- , , , et al. Bedside electromagnetic‐guided feeding tube placement: an improvement over traditional placement technique? Nutr Clin Pract. 2007;22:436–444.
- , . Establishment of enteral nutrition: prokinetic agents and small bowel feeding tubes. Curr Opin Crit Care. 2004;10:156–161.
- , , , et al. Postpyloric feeding tubes for surgical intensive care patients. Pilot series to evaluate two methods for bedside placement [abstract]. Anaesthesist. 2011;60(3):214–220.
- , , , . Enteral nutrition in critical care. J Clin Med Res. 2013;5:1–11.
- , , , et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. JPEN J Parenter Enteral Nutr. 2009;33:277–313.
- , , , , ; Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373.
- , , , et al.; Nutrition Guidelines Investigators of the ANZICS Clinical Trials Group. Effect of evidence‐based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300:2731–2741.
- , , , , . A randomised controlled comparison of early post‐pyloric vs early gastric feeding to meet nutritional targets in ventilated intensive care patients. Crit Care. 2009;13:R187.
- Critical Illness Update Evidence‐Based Nutrition Practice Guideline. 2012. Available at: http://andevidencelibrary.com/topic.cfm?format_tables=0171:388–416.
- , , , , , . Duodenal vs gastric feeding in medical intensive care unit patients: a prospective, randomized, clinical study. Crit Care Med. 2009;37:866–872.
- , , , et al. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med. 2012;40:2342–2348.
- , , , et al. Gastric versus transpyloric feeding in severe traumatic brain injury: a prospective, randomized trial. Intens Care Med. 2010;36:1532–1539.
- , , , , , . Severity of illness influences the efficacy of enteral feeding route on clinical outcomes in patients with critical illness. J Acad Nutr Diet. 2012;112:1138–1146.
- , , , et al. Guidelines, guidelines, guidelines: what are we to do with all these North American guidelines? J Parent Ent Nutr. 2010;34:625–643.
- Critical Care Nutrition. Nutrition Clinical Practice Guidelines. 2009. Available at: http://www.criticalcarenutrition.com/docs/cpg/5.3Smallbowel_FINAL.pdf. Accessed July 21, 2013.
- , , , et al. Nasoenteric tube complications. Scand J Surg. 2012;101:147–155.
- , , , . Feeding tube placement: errors and complications. Nutr Clin Pract. 2007;27:738–748.
- , . Use of small‐bore feeding tubes: successes and failures. Curr Opin Clin Nutr Metab Care. 2007;10:291–296.
- , . Enhancing patient safety during feeding tube insertion: a review of more than 2,000 insertions. J Parent Ent Nutr. 2006;30:440–445.
- , , . Patient safety: effect of institutional protocols on adverse events related to feeding tube placement in the critically ill. J Am Coll Surg. 2004;199:39–50.
- . Acute complications associated with bedside placement of feeding tubes. Nutr Clin Pract. 2006;21:40–55.
- , , , , . The effectiveness of the CORTRAKTM device in avoiding lung placement of small bore enteral feeding tubes [abstract]. Am J Crit Care. 2004;13:268.
- , , . Jejunal tube placement in critically ill patients: a prospective, randomized trial comparing the endoscopic technique with the electromagnetically visualized method. Crit Care Med. 2011;39:73–77.
- , , , et al. Bedside electromagnetic‐guided feeding tube placement: an improvement over traditional placement technique? Nutr Clin Pract. 2007;22:436–444.
- , . Establishment of enteral nutrition: prokinetic agents and small bowel feeding tubes. Curr Opin Crit Care. 2004;10:156–161.
- , , , et al. Postpyloric feeding tubes for surgical intensive care patients. Pilot series to evaluate two methods for bedside placement [abstract]. Anaesthesist. 2011;60(3):214–220.
Published 2013. This article is a U.S. Government work and is in the public domain in the USA