User login
Heart failure treatment: Keeping up with best practices
Heart failure (HF) affects nearly 6 million Americans and accounts for one million hospital admissions each year.1 The condition, which results from a structural or functional disorder that impairs the ventricles’ ability to fill, empty, or both,2 is a major cause of morbidity and mortality. The 5-year mortality rate ranges from 44% to 77%.3,4
Growing evidence demonstrates reduced morbidity and mortality when patients with HF with reduced ejection fraction (HFrEF) are treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a beta-blocker; and a mineralocorticoid/aldosterone receptor antagonist (MRA) in appropriate doses.5 In addition, 2 new medications representing novel drug classes have recently entered the market and are recommended in select patients who remain symptomatic despite standard treatment.
The first is sacubitril, which is available in a combination pill with the ARB valsartan, and the second is ivabradine.6 Additionally, implanted medical devices are proving useful, particularly in the management of patients with refractory symptoms.
This article will briefly review the diagnosis and initial evaluation of the patient with suspected HF and then describe how newer treatments fit within HF management priorities and strategies. But first, a word about what causes HF.
Causes are many and diverse
HF has a variety of cardiac and non-cardiac etiologies.2,7,8 Some important cardiac causes include hypertension (HTN), coronary artery disease (CAD), valvular heart disease, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and postpartum cardiomyopathy. Common and important non-cardiac causes of HF include alcoholic cardiomyopathy, pulmonary embolism, pulmonary hypertension, obstructive sleep apnea, anemia, hemochromatosis, amyloidosis, sarcoidosis, thyroid dysfunction, nephrotic syndrome, and cardiac toxins (especially stimulants and certain chemotherapy drugs).2,7,8
Diagnosing an elusive culprit
HF remains a clinical diagnosis. Common symptoms include dyspnea, cough, pedal edema, and decreased exercise tolerance, but these symptoms are not at all specific. Given the varied causes and manifestations of HF, the diagnosis can be somewhat elusive. Fortunately, there are a number of objective methods to help identify patients with HF.
Framingham criteria. One commonly used tool for making the diagnosis of HF is the Framingham criteria (see https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria),9 which diagnoses HF based on historical and physical exam findings. Another well-validated decision tool is the Heart Failure Diagnostic Rule (see http://circ.ahajournals.org/content/124/25/2865.long),10 which incorporates N-terminal pro–B-type natriuretic peptide (NT-proBNP) results, as well as exam findings.
Measurement of natriuretic peptides, either B-type natriuretic peptide (BNP) or NT-proBNP, aids in the diagnosis of HF.5 Although several factors (including age, weight, and renal function) can affect BNP levels, a normal BNP value effectively rules out HF5,7 and an elevated BNP can help to make the diagnosis in the context of a patient with corresponding symptoms.
The initial evaluation: Necessary lab work and imaging studies
The purpose of the initial evaluation of the patient with suspected HF is to establish the diagnosis, look for underlying etiologies of HF, identify comorbidities, and establish baseline values (eg, of potassium and creatinine) for elements monitored during treatment.5,7 TABLE 15,7 lists the lab work and imaging tests that are commonly ordered in the initial evaluation of the patient with HF.
Echocardiography is useful in determining the ejection fraction (EF), which is essential in guiding treatment. Echocardiography can also identify important structural abnormalities including significant valvular disease. Refer patients with severe valvular disease for evaluation for valve repair/replacement, regardless of EF.8
Noninvasive testing (stress nuclear imaging or echocardiography) to evaluate for underlying CAD is reasonable in patients with unknown CAD status.8,11 Patients for whom there is a high suspicion of obstructive CAD should undergo coronary angiography if they are candidates for revascularization.5,7 Noninvasive testing may also be an acceptable option for assessing ischemia in patients presenting with HF who have known CAD and no angina.5
Classification of HF is determined by ejection fraction
Physicians have traditionally classified patients with HF as having either systolic or diastolic dysfunction. Patients with HF symptoms and a reduced EF were said to have systolic dysfunction; those with a normal EF were said to have diastolic dysfunction.
More recently, researchers have learned that patients with reduced EF and those with preserved EF can have both systolic and diastolic dysfunction simultaneously.8 Therefore, the current preferred terminology is HFpEF (heart failure with preserved ejection fraction) for those with an EF ≥50% and HFrEF (heart failure with reduced ejection fraction) for those with an EF ≤40%.5 Both the American Heart Association (AHA) and the European Society of Cardiology recognize a category of HF with moderately reduced ejection fraction defined as an EF between 40% and 50%.5,7 Practically speaking, this group is treated as per the guidelines for HFrEF.5
Treatment of HFrEF: The evidence is clear
The cornerstone of medical treatment for HFrEF is the combination of an ACE inhibitor or ARB with a beta-blocker.2,5,7,8 Several early trials showed clear benefits of these medications. For example, the Studies Of Left Ventricular Dysfunction trial (SOLVD), compared enalapril to placebo in patients receiving standard therapy (consisting chiefly of digitalis, diuretics, and nitrates). This study demonstrated a reduction in all-cause mortality or first hospitalization for HF (number needed to treat [NNT]=21) in the enalapril group vs the placebo group.12
Similarly, a subgroup analysis of the Valsartan Heart Failure Treatment (Val-HeFT) trial demonstrated morbidity (NNT=10) and all-cause mortality benefits (NNT=6) when valsartan (an ARB) was given to patients who were not receiving an ACE inhibitor.13
MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in congestive Heart Failure) compared the beta-blocker metoprolol succinate to placebo and found fewer deaths from HF and lower all-cause mortality (NNT=26) associated with the treatment group vs the placebo group.14
And a comparison of 2 beta-blockers—carvedilol and metoprolol tartrate—on clinical outcomes in patients with chronic HF in the Carvedilol Or Metoprolol European Trial (COMET) showed that carvedilol extended survival compared with metoprolol tartrate (NNT=19).15
Unlike ACE inhibitors and ARBs, which seem to show a class benefit, only 3 beta-blockers available in the United States have been proven to reduce mortality: sustained-release metoprolol succinate, carvedilol, and bisoprolol.2,7,8
Unless contraindicated, all patients with a reduced EF—even those without symptoms—should receive a beta-blocker and an ACE inhibitor or ARB.5,7,8
Cautionary notes
Remember the following caveats when treating patients with ACE inhibitors, ARBs, and beta-blockers:
- Use ACE inhibitors and ARBs with caution in patients with impaired renal function (serum creatinine >2.5 mg/dL) or elevated serum potassium (>5 mEq/L).16,17
- ARBs are associated with a much lower incidence of cough and angioedema than ACE inhibitors.18
- Although physicians frequently start patients on low doses of beta-blockers and ACE inhibitors or ARBs to minimize hypotension and other adverse effects, the goal of therapy is to titrate up to the therapeutic doses used in clinical trials.5-7 (For dosages of medications commonly used in the treatment of heart failure, see Table 3 in the American College of Cardiology/AHA/Heart Failure Society of America guidelines available at https://www.sciencedirect.com/science/article/pii/S0735109717370870?via%3Dihub#tbl3 and Table 7.2 in the European Society of Cardiology guidelines available at https://academic.oup.com/eurheartj/article/37/27/2129/1748921.)
- Because beta-blockers can exacerbate fluid retention, do not initiate them in patients with fluid overload unless such patients are being treated with diuretics.5,19
When more Tx is needed
For patients who remain symptomatic despite treatment with an ACE inhibitor or ARB and a beta-blocker, consider the following add-on therapies.
Diuretics are the only medications used in the treatment of HF that adequately reduce fluid overload.2,7 While thiazide diuretics confer greater blood pressure control, loop diuretics are generally preferred in the treatment of HF because they are more efficacious.5 Loop diuretics should be prescribed to all patients with fluid overload, as few patients can maintain their target (“dry”) weight without diuretic therapy.5,7 Common adverse effects include hypokalemia, dehydration, and azotemia.
Two MRAs are currently available in the United States: spironolactone and eplerenone. MRAs are used as add-on therapy for symptomatic patients with an EF ≤35% or an EF ≤40% following an acute myocardial infarction (MI).5 They significantly reduce all-cause mortality (NNT=26).20
Because hyperkalemia is a risk with MRAs, do not prescribe them for patients who are already taking both an ACE inhibitor and an ARB.5 Also, do not initiate MRA therapy in patients who have an elevated creatinine level (≥2.5 mg/dL in men; ≥2 mg/dL in women) or a potassium level ≥5 mEq/L.5,7,8 Discontinue MRA therapy if a patient’s potassium level rises to ≥5.5 mEq/L.5
Hydralazine combined with isosorbide dinitrate (H/ID) is an alternative in patients for whom ACE inhibitor/ARB therapy is contraindicated.5,8
H/ID is also an add-on option in African American patients. Trials have demonstrated that H/ID reduces both first hospitalization for HF (NNT=13) and all-cause mortality (NNT=25) when it is used as add-on therapy in African Americans already receiving standard therapy with an ACE inhibitor or ARB, a beta-blocker, and an MRA.21 Headache and dizziness are commonly reported adverse effects.
Digoxin does not reduce mortality, but it does improve both quality of life and exercise tolerance and reduces hospital admissions for patients with HF.5,7 Significant adverse effects of digoxin include anorexia, nausea, visual disturbances, and cardiac arrhythmias.22
Also, hypokalemia can intensify digoxin toxicity.23 Because of these concerns, digoxin is typically dosed at 0.125 mg/d (0.125 mg every other day in patients >70 years or patients with impaired renal function or low body weight) with a target therapeutic range of 0.5 to 0.9 ng/mL.5
New classes, new agents
Sacubitril, a neprilysin inhibitor, is the first drug from this class approved for use in the United States. Neprilysin is the enzyme responsible for the degradation of natriuretic peptides; as such it increases endogenous NPs, promoting diuresis and lowering blood pressure.24,25 Early trials with sacubitril alone showed limited clinical efficacy;25 however, when it was combined with the ARB, valsartan (the combination being called angiotensin receptor blocker + neprilysin inhibitor [ARNI] therapy), it was found to be of significant benefit.6,25
The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial compared outcomes in patients receiving ARNI therapy to those receiving enalapril.26 The authors stopped the trial early due to the overwhelming benefit seen in the ARNI arm.
After a median follow-up of 27 months, the researchers found a reduction in the primary outcomes of either cardiovascular death or first hospitalization for HF (26.5% in the enalapril-treated group vs 21.8% in the ARNI-treated group; NNT=21).26 There were slightly more cases of angioedema in the ARNI arm than in the enalapril arm (0.5% vs 0.2%), although there were no patients in the trial who required endotracheal intubation.26
Because of this increased risk, do not prescribe ARNI therapy for any patient with a history of angioedema.6 Hypotension was more common in the ARNI-treated group than in the enalapril group (14% vs 9.2%), but there were lower rates of hyperkalemia, elevated serum creatinine, and cough in the ARNI-treated group than in the enalapril group.26
Consider ARNI treatment for all patients with an EF ≤40% who remain symptomatic despite appropriate doses of an ACE inhibitor or ARB plus a beta-blocker. Do not administer ARNI therapy concomitantly with an ACE inhibitor or ARB. When switching, do not start ARNI therapy for at least 36 hours after the last dose of an ACE inhibitor or ARB.6
Ivabradine is a sinoatrial node modulator that provides additional heart rate reduction. It does not affect ventricular repolarization or myocardial contractility.27 Early trials with this medication have shown reduced cardiac mortality and an NNT to prevent one first HF hospitalization within one year of 27.28 Adverse effects include symptomatic and asymptomatic bradycardia and luminous phenomena.28
Recommend ivabradine as add-on therapy to all patients with an EF ≤35%, normal sinus rhythm, and resting heart rate ≥70 bpm who remain symptomatic despite taking the maximum-tolerated dose of a beta-blocker.6 The dose is adjusted to achieve a resting heart rate of 50 to 60 bpm.27
Nonpharmacologic options
Implantable cardioverter defibrillators (ICDs) are recommended as primary prevention in select HFrEF patients to reduce the risk of sudden cardiac death and all-cause mortality. The 2013 American College of Cardiology Foundation/AHA Guideline for the Management of Heart Failure recommends an ICD for primary prevention for: 1) patients with symptomatic HF and an LVEF ≤35% despite ≥3 months of optimal medical therapy, and 2) patients at least 40 days post-MI with an LVEF of ≤30%.5,29 ICDs are not recommended for patients who have a life expectancy of less than one year, and the devices are of unclear benefit for patients ≥75 years of age.5
Cardiac resynchronization therapy (CRT), although not new to the field of cardiology, is new to the treatment of heart failure. A number of patients with HFrEF have QRS prolongation and in particular, left bundle branch block (LBBB).5 CRT uses biventricular pacing to restore synchronous contraction of the left and right ventricles.30 It is strongly recommended for patients with an EF ≤35%, sinus rhythm, LBBB, QRS ≥150 ms, and a life expectancy of at least one year.5,7 It is weakly recommended for patients with an EF ≤35% and a QRS ≥150 ms but without LBBB. It’s also weakly recommended for patients with an EF ≤35% and LBBB with a QRS of 120 to 150 ms.5,31
Left ventricular assist devices (LVADs) and cardiac transplantation are considerations for patients with severe symptoms refractory to all other interventions.5 LVADs may be used either while awaiting cardiac transplantation (bridge therapy) or as definitive treatment (destination therapy). Appropriate patient selection for such therapies requires a team of experts that ideally includes HF and transplantation cardiologists, cardiothoracic surgeons, nurses, social workers, and palliative care clinicians.5
Treatment of HFpEF: Evidence is lacking
While HFpEF is common—affecting about half of all patients with HF—ideal treatment remains unclear.32 Some trials have shown promise, but to date no unequivocal evidence exists that any standard therapy reduces mortality in patients with HFpEF.33-37 Underlying mechanisms of action of HFpEF include cardiac rate and rhythm abnormalities, atrial dysfunction, and stiffening of the ventricles. In a sense, it represents an exaggerated expression of the pathophysiology seen with the normal aging of the heart and can be conceptualized as “presbycardia.”37 Indeed, HFpEF is more common in the elderly, but it is also more common in patients of African descent.38,39 Common contributing causes (which we’ll get to in a bit) include HTN, CAD, atrial fibrillation (AF), obesity, and obstructive sleep apnea (OSA).
Trials have failed to show clear benefit for ACE inhibitors, ARBs, or beta-blockers.7,33 The evidence for MRAs is somewhat unclear; however, they have recently been recommended as an option for patients who have been hospitalized in the last year to reduce the risk of subsequent hospitalizations.40 Digoxin is used primarily for rate control in the setting of AF, but otherwise is of unclear benefit.7 A low-sodium diet (ie, ≤2 g/d) may be useful in those patients who are prone to fluid overload.5,7 The cornerstone of treatment of HFpEF is the relief of volume overload with diuretics and the treatment of coexisting conditions.33
Common contributing causes of HFpEF
HTN is not only a common contributing cause, but also the most common comorbid condition affecting patients with HFpEF. As such, treatment of HTN represents the most important management goal.33,34 Based on recent data, the American College of Cardiology, the AHA, and the Heart Failure Society of America have recommended a systolic blood pressure goal <130 mm Hg for patients with HFpEF.40 Most patients with HFpEF and HTN will have some degree of fluid overload and, therefore, should receive a diuretic.
CAD. Patients with HFpEF should be evaluated for CAD and treated with medical management and coronary revascularization, as appropriate.
AF is poorly tolerated by patients with HFpEF.37 Patients with AF should receive anticoagulation and rate control medications, and those with persistent HF symptoms should be evaluated for rhythm control.33
Obesity is more prevalent in patients with HFpEF than in those with HFrEF.41 Although there is indirect evidence that weight loss improves cardiac function,34,42,43 and studies have shown bariatric surgery to improve diastolic function,44,45 there are no studies reporting clinical outcomes.
Treatment of OSA with continuous positive airway pressure appears to alleviate some symptoms of HF and to reduce all-cause mortality.46,47
Keeping HF patients out of the hospital
Many readmissions to the hospital for HF exacerbation are preventable. Patients often do not understand hospital discharge instructions or the nature of their chronic disease and its management.48-51 Routine follow-up in the office or clinic provides an opportunity to improve quality of life for patients and decrease admissions.7,52
A major role for the family physician is in the co-creation of, and adherence to, an individualized, comprehensive care plan. Make sure such a plan is easily understood not only by the patient with HF, but also by his or her care team. In addition, it should be evidence-based and reflect the patient’s culture, values, and goals of treatment.5,7
At each visit, the family physician or a member of the health care team should assess adherence to guideline-directed medical therapy, measure weight, evaluate fluid status, and provide ongoing patient education including information on the importance of activity, monitoring weight daily, and moderating fluid, salt, and alcohol intake.5,52
Research shows that cardiac rehabilitation improves functional capacity, exercise duration, quality of life, and mortality. Therefore, recommend it to all symptomatic patients with HF who are clinically stable.2
Consider collaboration with a subspecialist. Patients who remain symptomatic despite optimal medical management and patients with recurrent hospitalizations are best managed in conjunction with a subspecialist in HF treatment.2,5
CORRESPONDENCE
Darin Brink, MD, 420 Delaware St. SE, MMC 381, Minneapolis, MN 55455; [email protected].
1. Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief. 2012;(108):1-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23102190. Accessed April 26, 2017.
2. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
3. Passantino A, Guida P, Lagioia R, et al. Predictors of long-term mortality in older patients hospitalized for acutely decompensated heart failure: clinical relevance of natriuretic peptides. J Am Geriatr Soc. 2017;65:822-826.
4. Lassus JP, Siirilä-Waris K, Nieminen MS, et al. Long-term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168:458-462.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
6. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2016;68:1476-1488.
7. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200.
8. Pinkerman CP, Sander JE, Breeding D, et al. Institute for Clinical Systems Improvement. Heart failure in adults. Available at: https://www.scribd.com/document/310893227/HeartFailure-pdf. Accessed December 6, 2017.
9. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441-1446.
10. Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011;124:2865-2873.
11. Heart Failure Society of America, Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194.
12. Pouleur H, The SOLVD Investigators. Results of the treatment trial of the studies of left ventricular dysfunction (SOLVD). Am J Cardiol. 1992;70:135-136.
13. Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414-1421.
14. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
15. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13.
16. Gehr TW, Sica DA. Pharmacotherapy in congestive heart failure: Hyperkalemia in congestive heart failure. Congest Heart Fail. 2001;7:97-100.
17. National Institute for Health and Clinical Excellence (NICE). Chronic heart failure in adults: management. 2010. Available at: https://www.nice.org.uk/guidance/cg108. Accessed November 27, 2017.
18. Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent). 2003;16:123-126.
19. Epstein SE, Braunwald E. The effect of beta adrenergic blockade on patterns of urinary sodium excretion: studies in normal subjects and in patients with heart disease. Ann Intern Med. 1966;65:20-27.
20. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:246.
21. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
22. Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992;69:108G-118G.
23. Sundar S, Burma DP, Vaish SK. Digoxin toxicity and electrolytes: a correlative study. Acta Cardiol. 1983;38:115-123.
24. McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79-84.
25. Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18:48.
26. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
27. Corlanor package insert. Amgen Inc., Thousand Oaks, CA. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/corlanor/corlanor_pi.pdf. Accessed November 28, 2017.
28. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
29. Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94-125.
30. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047-1058.
31. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283-e352.
32. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-679.
33. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868-1877.
34. Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186-2198.
35. Cleland JG, Pellicori P, Dierckx R. Clinical trials in patients with heart failure and preserved left ventricular ejection fraction. Heart Fail Clin. 2014;10:511-523.
36. Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665-671.
37. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507-515.
38. Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015-1021.
39. Zile MR. Heart failure with a preserved ejection fraction. In: Mann DL, Zipes D, Libby P BR, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Phila
40. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803.
41. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281-2293.
42. de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376-2381.
43. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315:36-46.
44. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718-726.
45. Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198-202.
46. Yoshihisa A, Suzuki S, Yamauchi H, et al. Beneficial effects of positive airway pressure therapy for sleep-disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol. 2015;38:413-421.
47. Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620-626.
48. Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141-1163.
49. Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984.
50. Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459-467.
51. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407-413.
52. Cowie MR, Anker SD, Cleland JG, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. Available at: http://www.oxfordhealthpolicyforum.org/files/reports/ahf-report.pdf. Accessed November 27, 2017.
Heart failure (HF) affects nearly 6 million Americans and accounts for one million hospital admissions each year.1 The condition, which results from a structural or functional disorder that impairs the ventricles’ ability to fill, empty, or both,2 is a major cause of morbidity and mortality. The 5-year mortality rate ranges from 44% to 77%.3,4
Growing evidence demonstrates reduced morbidity and mortality when patients with HF with reduced ejection fraction (HFrEF) are treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a beta-blocker; and a mineralocorticoid/aldosterone receptor antagonist (MRA) in appropriate doses.5 In addition, 2 new medications representing novel drug classes have recently entered the market and are recommended in select patients who remain symptomatic despite standard treatment.
The first is sacubitril, which is available in a combination pill with the ARB valsartan, and the second is ivabradine.6 Additionally, implanted medical devices are proving useful, particularly in the management of patients with refractory symptoms.
This article will briefly review the diagnosis and initial evaluation of the patient with suspected HF and then describe how newer treatments fit within HF management priorities and strategies. But first, a word about what causes HF.
Causes are many and diverse
HF has a variety of cardiac and non-cardiac etiologies.2,7,8 Some important cardiac causes include hypertension (HTN), coronary artery disease (CAD), valvular heart disease, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and postpartum cardiomyopathy. Common and important non-cardiac causes of HF include alcoholic cardiomyopathy, pulmonary embolism, pulmonary hypertension, obstructive sleep apnea, anemia, hemochromatosis, amyloidosis, sarcoidosis, thyroid dysfunction, nephrotic syndrome, and cardiac toxins (especially stimulants and certain chemotherapy drugs).2,7,8
Diagnosing an elusive culprit
HF remains a clinical diagnosis. Common symptoms include dyspnea, cough, pedal edema, and decreased exercise tolerance, but these symptoms are not at all specific. Given the varied causes and manifestations of HF, the diagnosis can be somewhat elusive. Fortunately, there are a number of objective methods to help identify patients with HF.
Framingham criteria. One commonly used tool for making the diagnosis of HF is the Framingham criteria (see https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria),9 which diagnoses HF based on historical and physical exam findings. Another well-validated decision tool is the Heart Failure Diagnostic Rule (see http://circ.ahajournals.org/content/124/25/2865.long),10 which incorporates N-terminal pro–B-type natriuretic peptide (NT-proBNP) results, as well as exam findings.
Measurement of natriuretic peptides, either B-type natriuretic peptide (BNP) or NT-proBNP, aids in the diagnosis of HF.5 Although several factors (including age, weight, and renal function) can affect BNP levels, a normal BNP value effectively rules out HF5,7 and an elevated BNP can help to make the diagnosis in the context of a patient with corresponding symptoms.
The initial evaluation: Necessary lab work and imaging studies
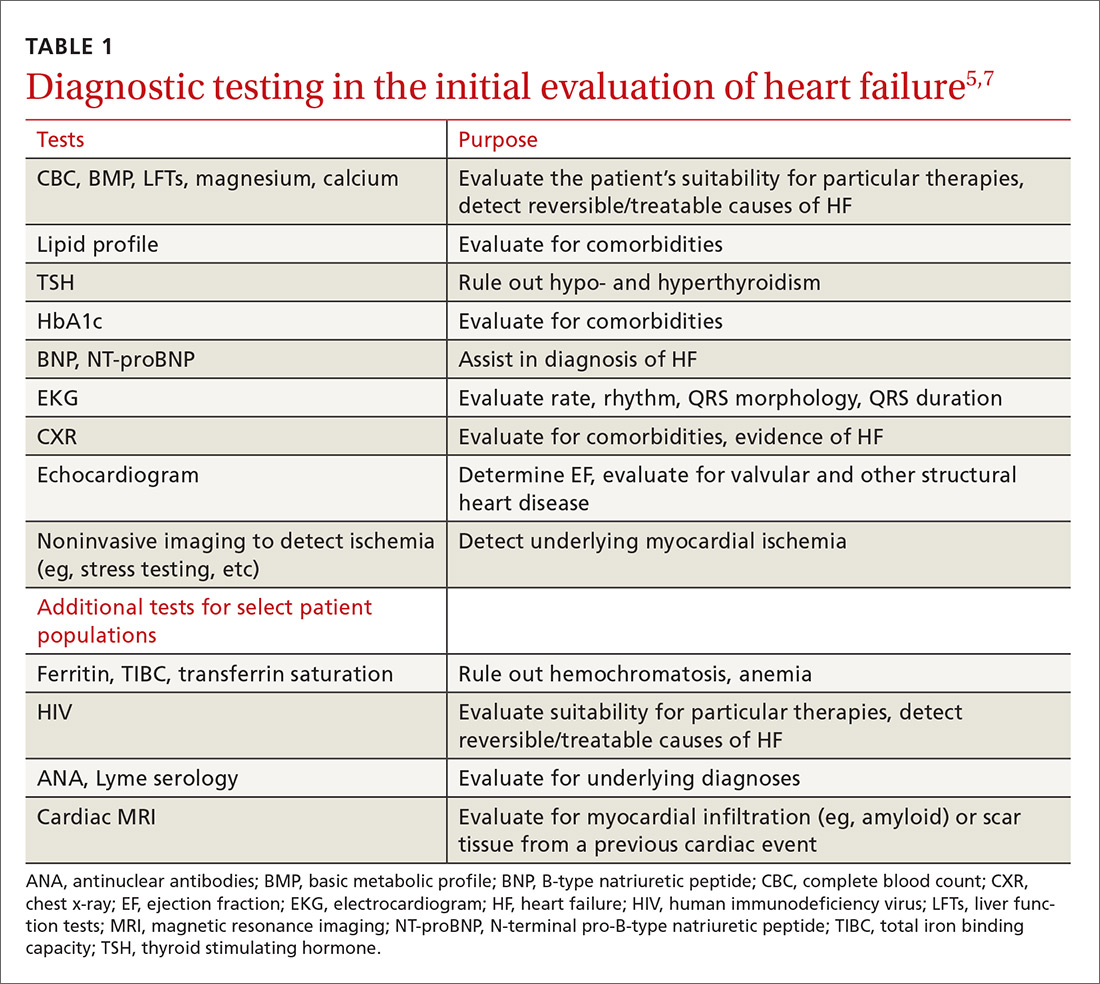
The purpose of the initial evaluation of the patient with suspected HF is to establish the diagnosis, look for underlying etiologies of HF, identify comorbidities, and establish baseline values (eg, of potassium and creatinine) for elements monitored during treatment.5,7 TABLE 15,7 lists the lab work and imaging tests that are commonly ordered in the initial evaluation of the patient with HF.

Echocardiography is useful in determining the ejection fraction (EF), which is essential in guiding treatment. Echocardiography can also identify important structural abnormalities including significant valvular disease. Refer patients with severe valvular disease for evaluation for valve repair/replacement, regardless of EF.8
Noninvasive testing (stress nuclear imaging or echocardiography) to evaluate for underlying CAD is reasonable in patients with unknown CAD status.8,11 Patients for whom there is a high suspicion of obstructive CAD should undergo coronary angiography if they are candidates for revascularization.5,7 Noninvasive testing may also be an acceptable option for assessing ischemia in patients presenting with HF who have known CAD and no angina.5
Classification of HF is determined by ejection fraction
Physicians have traditionally classified patients with HF as having either systolic or diastolic dysfunction. Patients with HF symptoms and a reduced EF were said to have systolic dysfunction; those with a normal EF were said to have diastolic dysfunction.
More recently, researchers have learned that patients with reduced EF and those with preserved EF can have both systolic and diastolic dysfunction simultaneously.8 Therefore, the current preferred terminology is HFpEF (heart failure with preserved ejection fraction) for those with an EF ≥50% and HFrEF (heart failure with reduced ejection fraction) for those with an EF ≤40%.5 Both the American Heart Association (AHA) and the European Society of Cardiology recognize a category of HF with moderately reduced ejection fraction defined as an EF between 40% and 50%.5,7 Practically speaking, this group is treated as per the guidelines for HFrEF.5
Treatment of HFrEF: The evidence is clear
The cornerstone of medical treatment for HFrEF is the combination of an ACE inhibitor or ARB with a beta-blocker.2,5,7,8 Several early trials showed clear benefits of these medications. For example, the Studies Of Left Ventricular Dysfunction trial (SOLVD), compared enalapril to placebo in patients receiving standard therapy (consisting chiefly of digitalis, diuretics, and nitrates). This study demonstrated a reduction in all-cause mortality or first hospitalization for HF (number needed to treat [NNT]=21) in the enalapril group vs the placebo group.12
Similarly, a subgroup analysis of the Valsartan Heart Failure Treatment (Val-HeFT) trial demonstrated morbidity (NNT=10) and all-cause mortality benefits (NNT=6) when valsartan (an ARB) was given to patients who were not receiving an ACE inhibitor.13
MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in congestive Heart Failure) compared the beta-blocker metoprolol succinate to placebo and found fewer deaths from HF and lower all-cause mortality (NNT=26) associated with the treatment group vs the placebo group.14
And a comparison of 2 beta-blockers—carvedilol and metoprolol tartrate—on clinical outcomes in patients with chronic HF in the Carvedilol Or Metoprolol European Trial (COMET) showed that carvedilol extended survival compared with metoprolol tartrate (NNT=19).15
Unlike ACE inhibitors and ARBs, which seem to show a class benefit, only 3 beta-blockers available in the United States have been proven to reduce mortality: sustained-release metoprolol succinate, carvedilol, and bisoprolol.2,7,8
Unless contraindicated, all patients with a reduced EF—even those without symptoms—should receive a beta-blocker and an ACE inhibitor or ARB.5,7,8
Cautionary notes
Remember the following caveats when treating patients with ACE inhibitors, ARBs, and beta-blockers:
- Use ACE inhibitors and ARBs with caution in patients with impaired renal function (serum creatinine >2.5 mg/dL) or elevated serum potassium (>5 mEq/L).16,17
- ARBs are associated with a much lower incidence of cough and angioedema than ACE inhibitors.18
- Although physicians frequently start patients on low doses of beta-blockers and ACE inhibitors or ARBs to minimize hypotension and other adverse effects, the goal of therapy is to titrate up to the therapeutic doses used in clinical trials.5-7 (For dosages of medications commonly used in the treatment of heart failure, see Table 3 in the American College of Cardiology/AHA/Heart Failure Society of America guidelines available at https://www.sciencedirect.com/science/article/pii/S0735109717370870?via%3Dihub#tbl3 and Table 7.2 in the European Society of Cardiology guidelines available at https://academic.oup.com/eurheartj/article/37/27/2129/1748921.)
- Because beta-blockers can exacerbate fluid retention, do not initiate them in patients with fluid overload unless such patients are being treated with diuretics.5,19
When more Tx is needed
For patients who remain symptomatic despite treatment with an ACE inhibitor or ARB and a beta-blocker, consider the following add-on therapies.
Diuretics are the only medications used in the treatment of HF that adequately reduce fluid overload.2,7 While thiazide diuretics confer greater blood pressure control, loop diuretics are generally preferred in the treatment of HF because they are more efficacious.5 Loop diuretics should be prescribed to all patients with fluid overload, as few patients can maintain their target (“dry”) weight without diuretic therapy.5,7 Common adverse effects include hypokalemia, dehydration, and azotemia.
Two MRAs are currently available in the United States: spironolactone and eplerenone. MRAs are used as add-on therapy for symptomatic patients with an EF ≤35% or an EF ≤40% following an acute myocardial infarction (MI).5 They significantly reduce all-cause mortality (NNT=26).20
Because hyperkalemia is a risk with MRAs, do not prescribe them for patients who are already taking both an ACE inhibitor and an ARB.5 Also, do not initiate MRA therapy in patients who have an elevated creatinine level (≥2.5 mg/dL in men; ≥2 mg/dL in women) or a potassium level ≥5 mEq/L.5,7,8 Discontinue MRA therapy if a patient’s potassium level rises to ≥5.5 mEq/L.5
Hydralazine combined with isosorbide dinitrate (H/ID) is an alternative in patients for whom ACE inhibitor/ARB therapy is contraindicated.5,8
H/ID is also an add-on option in African American patients. Trials have demonstrated that H/ID reduces both first hospitalization for HF (NNT=13) and all-cause mortality (NNT=25) when it is used as add-on therapy in African Americans already receiving standard therapy with an ACE inhibitor or ARB, a beta-blocker, and an MRA.21 Headache and dizziness are commonly reported adverse effects.
Digoxin does not reduce mortality, but it does improve both quality of life and exercise tolerance and reduces hospital admissions for patients with HF.5,7 Significant adverse effects of digoxin include anorexia, nausea, visual disturbances, and cardiac arrhythmias.22
Also, hypokalemia can intensify digoxin toxicity.23 Because of these concerns, digoxin is typically dosed at 0.125 mg/d (0.125 mg every other day in patients >70 years or patients with impaired renal function or low body weight) with a target therapeutic range of 0.5 to 0.9 ng/mL.5
New classes, new agents
Sacubitril, a neprilysin inhibitor, is the first drug from this class approved for use in the United States. Neprilysin is the enzyme responsible for the degradation of natriuretic peptides; as such it increases endogenous NPs, promoting diuresis and lowering blood pressure.24,25 Early trials with sacubitril alone showed limited clinical efficacy;25 however, when it was combined with the ARB, valsartan (the combination being called angiotensin receptor blocker + neprilysin inhibitor [ARNI] therapy), it was found to be of significant benefit.6,25
The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial compared outcomes in patients receiving ARNI therapy to those receiving enalapril.26 The authors stopped the trial early due to the overwhelming benefit seen in the ARNI arm.
After a median follow-up of 27 months, the researchers found a reduction in the primary outcomes of either cardiovascular death or first hospitalization for HF (26.5% in the enalapril-treated group vs 21.8% in the ARNI-treated group; NNT=21).26 There were slightly more cases of angioedema in the ARNI arm than in the enalapril arm (0.5% vs 0.2%), although there were no patients in the trial who required endotracheal intubation.26
Because of this increased risk, do not prescribe ARNI therapy for any patient with a history of angioedema.6 Hypotension was more common in the ARNI-treated group than in the enalapril group (14% vs 9.2%), but there were lower rates of hyperkalemia, elevated serum creatinine, and cough in the ARNI-treated group than in the enalapril group.26
Consider ARNI treatment for all patients with an EF ≤40% who remain symptomatic despite appropriate doses of an ACE inhibitor or ARB plus a beta-blocker. Do not administer ARNI therapy concomitantly with an ACE inhibitor or ARB. When switching, do not start ARNI therapy for at least 36 hours after the last dose of an ACE inhibitor or ARB.6
Ivabradine is a sinoatrial node modulator that provides additional heart rate reduction. It does not affect ventricular repolarization or myocardial contractility.27 Early trials with this medication have shown reduced cardiac mortality and an NNT to prevent one first HF hospitalization within one year of 27.28 Adverse effects include symptomatic and asymptomatic bradycardia and luminous phenomena.28
Recommend ivabradine as add-on therapy to all patients with an EF ≤35%, normal sinus rhythm, and resting heart rate ≥70 bpm who remain symptomatic despite taking the maximum-tolerated dose of a beta-blocker.6 The dose is adjusted to achieve a resting heart rate of 50 to 60 bpm.27
Nonpharmacologic options
Implantable cardioverter defibrillators (ICDs) are recommended as primary prevention in select HFrEF patients to reduce the risk of sudden cardiac death and all-cause mortality. The 2013 American College of Cardiology Foundation/AHA Guideline for the Management of Heart Failure recommends an ICD for primary prevention for: 1) patients with symptomatic HF and an LVEF ≤35% despite ≥3 months of optimal medical therapy, and 2) patients at least 40 days post-MI with an LVEF of ≤30%.5,29 ICDs are not recommended for patients who have a life expectancy of less than one year, and the devices are of unclear benefit for patients ≥75 years of age.5
Cardiac resynchronization therapy (CRT), although not new to the field of cardiology, is new to the treatment of heart failure. A number of patients with HFrEF have QRS prolongation and in particular, left bundle branch block (LBBB).5 CRT uses biventricular pacing to restore synchronous contraction of the left and right ventricles.30 It is strongly recommended for patients with an EF ≤35%, sinus rhythm, LBBB, QRS ≥150 ms, and a life expectancy of at least one year.5,7 It is weakly recommended for patients with an EF ≤35% and a QRS ≥150 ms but without LBBB. It’s also weakly recommended for patients with an EF ≤35% and LBBB with a QRS of 120 to 150 ms.5,31
Left ventricular assist devices (LVADs) and cardiac transplantation are considerations for patients with severe symptoms refractory to all other interventions.5 LVADs may be used either while awaiting cardiac transplantation (bridge therapy) or as definitive treatment (destination therapy). Appropriate patient selection for such therapies requires a team of experts that ideally includes HF and transplantation cardiologists, cardiothoracic surgeons, nurses, social workers, and palliative care clinicians.5
Treatment of HFpEF: Evidence is lacking
While HFpEF is common—affecting about half of all patients with HF—ideal treatment remains unclear.32 Some trials have shown promise, but to date no unequivocal evidence exists that any standard therapy reduces mortality in patients with HFpEF.33-37 Underlying mechanisms of action of HFpEF include cardiac rate and rhythm abnormalities, atrial dysfunction, and stiffening of the ventricles. In a sense, it represents an exaggerated expression of the pathophysiology seen with the normal aging of the heart and can be conceptualized as “presbycardia.”37 Indeed, HFpEF is more common in the elderly, but it is also more common in patients of African descent.38,39 Common contributing causes (which we’ll get to in a bit) include HTN, CAD, atrial fibrillation (AF), obesity, and obstructive sleep apnea (OSA).
Trials have failed to show clear benefit for ACE inhibitors, ARBs, or beta-blockers.7,33 The evidence for MRAs is somewhat unclear; however, they have recently been recommended as an option for patients who have been hospitalized in the last year to reduce the risk of subsequent hospitalizations.40 Digoxin is used primarily for rate control in the setting of AF, but otherwise is of unclear benefit.7 A low-sodium diet (ie, ≤2 g/d) may be useful in those patients who are prone to fluid overload.5,7 The cornerstone of treatment of HFpEF is the relief of volume overload with diuretics and the treatment of coexisting conditions.33
Common contributing causes of HFpEF
HTN is not only a common contributing cause, but also the most common comorbid condition affecting patients with HFpEF. As such, treatment of HTN represents the most important management goal.33,34 Based on recent data, the American College of Cardiology, the AHA, and the Heart Failure Society of America have recommended a systolic blood pressure goal <130 mm Hg for patients with HFpEF.40 Most patients with HFpEF and HTN will have some degree of fluid overload and, therefore, should receive a diuretic.
CAD. Patients with HFpEF should be evaluated for CAD and treated with medical management and coronary revascularization, as appropriate.
AF is poorly tolerated by patients with HFpEF.37 Patients with AF should receive anticoagulation and rate control medications, and those with persistent HF symptoms should be evaluated for rhythm control.33
Obesity is more prevalent in patients with HFpEF than in those with HFrEF.41 Although there is indirect evidence that weight loss improves cardiac function,34,42,43 and studies have shown bariatric surgery to improve diastolic function,44,45 there are no studies reporting clinical outcomes.
Treatment of OSA with continuous positive airway pressure appears to alleviate some symptoms of HF and to reduce all-cause mortality.46,47
Keeping HF patients out of the hospital
Many readmissions to the hospital for HF exacerbation are preventable. Patients often do not understand hospital discharge instructions or the nature of their chronic disease and its management.48-51 Routine follow-up in the office or clinic provides an opportunity to improve quality of life for patients and decrease admissions.7,52
A major role for the family physician is in the co-creation of, and adherence to, an individualized, comprehensive care plan. Make sure such a plan is easily understood not only by the patient with HF, but also by his or her care team. In addition, it should be evidence-based and reflect the patient’s culture, values, and goals of treatment.5,7
At each visit, the family physician or a member of the health care team should assess adherence to guideline-directed medical therapy, measure weight, evaluate fluid status, and provide ongoing patient education including information on the importance of activity, monitoring weight daily, and moderating fluid, salt, and alcohol intake.5,52
Research shows that cardiac rehabilitation improves functional capacity, exercise duration, quality of life, and mortality. Therefore, recommend it to all symptomatic patients with HF who are clinically stable.2
Consider collaboration with a subspecialist. Patients who remain symptomatic despite optimal medical management and patients with recurrent hospitalizations are best managed in conjunction with a subspecialist in HF treatment.2,5
CORRESPONDENCE
Darin Brink, MD, 420 Delaware St. SE, MMC 381, Minneapolis, MN 55455; [email protected].
Heart failure (HF) affects nearly 6 million Americans and accounts for one million hospital admissions each year.1 The condition, which results from a structural or functional disorder that impairs the ventricles’ ability to fill, empty, or both,2 is a major cause of morbidity and mortality. The 5-year mortality rate ranges from 44% to 77%.3,4
Growing evidence demonstrates reduced morbidity and mortality when patients with HF with reduced ejection fraction (HFrEF) are treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a beta-blocker; and a mineralocorticoid/aldosterone receptor antagonist (MRA) in appropriate doses.5 In addition, 2 new medications representing novel drug classes have recently entered the market and are recommended in select patients who remain symptomatic despite standard treatment.
The first is sacubitril, which is available in a combination pill with the ARB valsartan, and the second is ivabradine.6 Additionally, implanted medical devices are proving useful, particularly in the management of patients with refractory symptoms.
This article will briefly review the diagnosis and initial evaluation of the patient with suspected HF and then describe how newer treatments fit within HF management priorities and strategies. But first, a word about what causes HF.
Causes are many and diverse
HF has a variety of cardiac and non-cardiac etiologies.2,7,8 Some important cardiac causes include hypertension (HTN), coronary artery disease (CAD), valvular heart disease, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and postpartum cardiomyopathy. Common and important non-cardiac causes of HF include alcoholic cardiomyopathy, pulmonary embolism, pulmonary hypertension, obstructive sleep apnea, anemia, hemochromatosis, amyloidosis, sarcoidosis, thyroid dysfunction, nephrotic syndrome, and cardiac toxins (especially stimulants and certain chemotherapy drugs).2,7,8
Diagnosing an elusive culprit
HF remains a clinical diagnosis. Common symptoms include dyspnea, cough, pedal edema, and decreased exercise tolerance, but these symptoms are not at all specific. Given the varied causes and manifestations of HF, the diagnosis can be somewhat elusive. Fortunately, there are a number of objective methods to help identify patients with HF.
Framingham criteria. One commonly used tool for making the diagnosis of HF is the Framingham criteria (see https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria),9 which diagnoses HF based on historical and physical exam findings. Another well-validated decision tool is the Heart Failure Diagnostic Rule (see http://circ.ahajournals.org/content/124/25/2865.long),10 which incorporates N-terminal pro–B-type natriuretic peptide (NT-proBNP) results, as well as exam findings.
Measurement of natriuretic peptides, either B-type natriuretic peptide (BNP) or NT-proBNP, aids in the diagnosis of HF.5 Although several factors (including age, weight, and renal function) can affect BNP levels, a normal BNP value effectively rules out HF5,7 and an elevated BNP can help to make the diagnosis in the context of a patient with corresponding symptoms.
The initial evaluation: Necessary lab work and imaging studies
The purpose of the initial evaluation of the patient with suspected HF is to establish the diagnosis, look for underlying etiologies of HF, identify comorbidities, and establish baseline values (eg, of potassium and creatinine) for elements monitored during treatment.5,7 TABLE 15,7 lists the lab work and imaging tests that are commonly ordered in the initial evaluation of the patient with HF.

Echocardiography is useful in determining the ejection fraction (EF), which is essential in guiding treatment. Echocardiography can also identify important structural abnormalities including significant valvular disease. Refer patients with severe valvular disease for evaluation for valve repair/replacement, regardless of EF.8
Noninvasive testing (stress nuclear imaging or echocardiography) to evaluate for underlying CAD is reasonable in patients with unknown CAD status.8,11 Patients for whom there is a high suspicion of obstructive CAD should undergo coronary angiography if they are candidates for revascularization.5,7 Noninvasive testing may also be an acceptable option for assessing ischemia in patients presenting with HF who have known CAD and no angina.5
Classification of HF is determined by ejection fraction
Physicians have traditionally classified patients with HF as having either systolic or diastolic dysfunction. Patients with HF symptoms and a reduced EF were said to have systolic dysfunction; those with a normal EF were said to have diastolic dysfunction.
More recently, researchers have learned that patients with reduced EF and those with preserved EF can have both systolic and diastolic dysfunction simultaneously.8 Therefore, the current preferred terminology is HFpEF (heart failure with preserved ejection fraction) for those with an EF ≥50% and HFrEF (heart failure with reduced ejection fraction) for those with an EF ≤40%.5 Both the American Heart Association (AHA) and the European Society of Cardiology recognize a category of HF with moderately reduced ejection fraction defined as an EF between 40% and 50%.5,7 Practically speaking, this group is treated as per the guidelines for HFrEF.5
Treatment of HFrEF: The evidence is clear
The cornerstone of medical treatment for HFrEF is the combination of an ACE inhibitor or ARB with a beta-blocker.2,5,7,8 Several early trials showed clear benefits of these medications. For example, the Studies Of Left Ventricular Dysfunction trial (SOLVD), compared enalapril to placebo in patients receiving standard therapy (consisting chiefly of digitalis, diuretics, and nitrates). This study demonstrated a reduction in all-cause mortality or first hospitalization for HF (number needed to treat [NNT]=21) in the enalapril group vs the placebo group.12
Similarly, a subgroup analysis of the Valsartan Heart Failure Treatment (Val-HeFT) trial demonstrated morbidity (NNT=10) and all-cause mortality benefits (NNT=6) when valsartan (an ARB) was given to patients who were not receiving an ACE inhibitor.13
MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in congestive Heart Failure) compared the beta-blocker metoprolol succinate to placebo and found fewer deaths from HF and lower all-cause mortality (NNT=26) associated with the treatment group vs the placebo group.14
And a comparison of 2 beta-blockers—carvedilol and metoprolol tartrate—on clinical outcomes in patients with chronic HF in the Carvedilol Or Metoprolol European Trial (COMET) showed that carvedilol extended survival compared with metoprolol tartrate (NNT=19).15
Unlike ACE inhibitors and ARBs, which seem to show a class benefit, only 3 beta-blockers available in the United States have been proven to reduce mortality: sustained-release metoprolol succinate, carvedilol, and bisoprolol.2,7,8
Unless contraindicated, all patients with a reduced EF—even those without symptoms—should receive a beta-blocker and an ACE inhibitor or ARB.5,7,8
Cautionary notes
Remember the following caveats when treating patients with ACE inhibitors, ARBs, and beta-blockers:
- Use ACE inhibitors and ARBs with caution in patients with impaired renal function (serum creatinine >2.5 mg/dL) or elevated serum potassium (>5 mEq/L).16,17
- ARBs are associated with a much lower incidence of cough and angioedema than ACE inhibitors.18
- Although physicians frequently start patients on low doses of beta-blockers and ACE inhibitors or ARBs to minimize hypotension and other adverse effects, the goal of therapy is to titrate up to the therapeutic doses used in clinical trials.5-7 (For dosages of medications commonly used in the treatment of heart failure, see Table 3 in the American College of Cardiology/AHA/Heart Failure Society of America guidelines available at https://www.sciencedirect.com/science/article/pii/S0735109717370870?via%3Dihub#tbl3 and Table 7.2 in the European Society of Cardiology guidelines available at https://academic.oup.com/eurheartj/article/37/27/2129/1748921.)
- Because beta-blockers can exacerbate fluid retention, do not initiate them in patients with fluid overload unless such patients are being treated with diuretics.5,19
When more Tx is needed
For patients who remain symptomatic despite treatment with an ACE inhibitor or ARB and a beta-blocker, consider the following add-on therapies.
Diuretics are the only medications used in the treatment of HF that adequately reduce fluid overload.2,7 While thiazide diuretics confer greater blood pressure control, loop diuretics are generally preferred in the treatment of HF because they are more efficacious.5 Loop diuretics should be prescribed to all patients with fluid overload, as few patients can maintain their target (“dry”) weight without diuretic therapy.5,7 Common adverse effects include hypokalemia, dehydration, and azotemia.
Two MRAs are currently available in the United States: spironolactone and eplerenone. MRAs are used as add-on therapy for symptomatic patients with an EF ≤35% or an EF ≤40% following an acute myocardial infarction (MI).5 They significantly reduce all-cause mortality (NNT=26).20
Because hyperkalemia is a risk with MRAs, do not prescribe them for patients who are already taking both an ACE inhibitor and an ARB.5 Also, do not initiate MRA therapy in patients who have an elevated creatinine level (≥2.5 mg/dL in men; ≥2 mg/dL in women) or a potassium level ≥5 mEq/L.5,7,8 Discontinue MRA therapy if a patient’s potassium level rises to ≥5.5 mEq/L.5
Hydralazine combined with isosorbide dinitrate (H/ID) is an alternative in patients for whom ACE inhibitor/ARB therapy is contraindicated.5,8
H/ID is also an add-on option in African American patients. Trials have demonstrated that H/ID reduces both first hospitalization for HF (NNT=13) and all-cause mortality (NNT=25) when it is used as add-on therapy in African Americans already receiving standard therapy with an ACE inhibitor or ARB, a beta-blocker, and an MRA.21 Headache and dizziness are commonly reported adverse effects.
Digoxin does not reduce mortality, but it does improve both quality of life and exercise tolerance and reduces hospital admissions for patients with HF.5,7 Significant adverse effects of digoxin include anorexia, nausea, visual disturbances, and cardiac arrhythmias.22
Also, hypokalemia can intensify digoxin toxicity.23 Because of these concerns, digoxin is typically dosed at 0.125 mg/d (0.125 mg every other day in patients >70 years or patients with impaired renal function or low body weight) with a target therapeutic range of 0.5 to 0.9 ng/mL.5
New classes, new agents
Sacubitril, a neprilysin inhibitor, is the first drug from this class approved for use in the United States. Neprilysin is the enzyme responsible for the degradation of natriuretic peptides; as such it increases endogenous NPs, promoting diuresis and lowering blood pressure.24,25 Early trials with sacubitril alone showed limited clinical efficacy;25 however, when it was combined with the ARB, valsartan (the combination being called angiotensin receptor blocker + neprilysin inhibitor [ARNI] therapy), it was found to be of significant benefit.6,25
The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial compared outcomes in patients receiving ARNI therapy to those receiving enalapril.26 The authors stopped the trial early due to the overwhelming benefit seen in the ARNI arm.
After a median follow-up of 27 months, the researchers found a reduction in the primary outcomes of either cardiovascular death or first hospitalization for HF (26.5% in the enalapril-treated group vs 21.8% in the ARNI-treated group; NNT=21).26 There were slightly more cases of angioedema in the ARNI arm than in the enalapril arm (0.5% vs 0.2%), although there were no patients in the trial who required endotracheal intubation.26
Because of this increased risk, do not prescribe ARNI therapy for any patient with a history of angioedema.6 Hypotension was more common in the ARNI-treated group than in the enalapril group (14% vs 9.2%), but there were lower rates of hyperkalemia, elevated serum creatinine, and cough in the ARNI-treated group than in the enalapril group.26
Consider ARNI treatment for all patients with an EF ≤40% who remain symptomatic despite appropriate doses of an ACE inhibitor or ARB plus a beta-blocker. Do not administer ARNI therapy concomitantly with an ACE inhibitor or ARB. When switching, do not start ARNI therapy for at least 36 hours after the last dose of an ACE inhibitor or ARB.6
Ivabradine is a sinoatrial node modulator that provides additional heart rate reduction. It does not affect ventricular repolarization or myocardial contractility.27 Early trials with this medication have shown reduced cardiac mortality and an NNT to prevent one first HF hospitalization within one year of 27.28 Adverse effects include symptomatic and asymptomatic bradycardia and luminous phenomena.28
Recommend ivabradine as add-on therapy to all patients with an EF ≤35%, normal sinus rhythm, and resting heart rate ≥70 bpm who remain symptomatic despite taking the maximum-tolerated dose of a beta-blocker.6 The dose is adjusted to achieve a resting heart rate of 50 to 60 bpm.27
Nonpharmacologic options
Implantable cardioverter defibrillators (ICDs) are recommended as primary prevention in select HFrEF patients to reduce the risk of sudden cardiac death and all-cause mortality. The 2013 American College of Cardiology Foundation/AHA Guideline for the Management of Heart Failure recommends an ICD for primary prevention for: 1) patients with symptomatic HF and an LVEF ≤35% despite ≥3 months of optimal medical therapy, and 2) patients at least 40 days post-MI with an LVEF of ≤30%.5,29 ICDs are not recommended for patients who have a life expectancy of less than one year, and the devices are of unclear benefit for patients ≥75 years of age.5
Cardiac resynchronization therapy (CRT), although not new to the field of cardiology, is new to the treatment of heart failure. A number of patients with HFrEF have QRS prolongation and in particular, left bundle branch block (LBBB).5 CRT uses biventricular pacing to restore synchronous contraction of the left and right ventricles.30 It is strongly recommended for patients with an EF ≤35%, sinus rhythm, LBBB, QRS ≥150 ms, and a life expectancy of at least one year.5,7 It is weakly recommended for patients with an EF ≤35% and a QRS ≥150 ms but without LBBB. It’s also weakly recommended for patients with an EF ≤35% and LBBB with a QRS of 120 to 150 ms.5,31
Left ventricular assist devices (LVADs) and cardiac transplantation are considerations for patients with severe symptoms refractory to all other interventions.5 LVADs may be used either while awaiting cardiac transplantation (bridge therapy) or as definitive treatment (destination therapy). Appropriate patient selection for such therapies requires a team of experts that ideally includes HF and transplantation cardiologists, cardiothoracic surgeons, nurses, social workers, and palliative care clinicians.5
Treatment of HFpEF: Evidence is lacking
While HFpEF is common—affecting about half of all patients with HF—ideal treatment remains unclear.32 Some trials have shown promise, but to date no unequivocal evidence exists that any standard therapy reduces mortality in patients with HFpEF.33-37 Underlying mechanisms of action of HFpEF include cardiac rate and rhythm abnormalities, atrial dysfunction, and stiffening of the ventricles. In a sense, it represents an exaggerated expression of the pathophysiology seen with the normal aging of the heart and can be conceptualized as “presbycardia.”37 Indeed, HFpEF is more common in the elderly, but it is also more common in patients of African descent.38,39 Common contributing causes (which we’ll get to in a bit) include HTN, CAD, atrial fibrillation (AF), obesity, and obstructive sleep apnea (OSA).
Trials have failed to show clear benefit for ACE inhibitors, ARBs, or beta-blockers.7,33 The evidence for MRAs is somewhat unclear; however, they have recently been recommended as an option for patients who have been hospitalized in the last year to reduce the risk of subsequent hospitalizations.40 Digoxin is used primarily for rate control in the setting of AF, but otherwise is of unclear benefit.7 A low-sodium diet (ie, ≤2 g/d) may be useful in those patients who are prone to fluid overload.5,7 The cornerstone of treatment of HFpEF is the relief of volume overload with diuretics and the treatment of coexisting conditions.33
Common contributing causes of HFpEF
HTN is not only a common contributing cause, but also the most common comorbid condition affecting patients with HFpEF. As such, treatment of HTN represents the most important management goal.33,34 Based on recent data, the American College of Cardiology, the AHA, and the Heart Failure Society of America have recommended a systolic blood pressure goal <130 mm Hg for patients with HFpEF.40 Most patients with HFpEF and HTN will have some degree of fluid overload and, therefore, should receive a diuretic.
CAD. Patients with HFpEF should be evaluated for CAD and treated with medical management and coronary revascularization, as appropriate.
AF is poorly tolerated by patients with HFpEF.37 Patients with AF should receive anticoagulation and rate control medications, and those with persistent HF symptoms should be evaluated for rhythm control.33
Obesity is more prevalent in patients with HFpEF than in those with HFrEF.41 Although there is indirect evidence that weight loss improves cardiac function,34,42,43 and studies have shown bariatric surgery to improve diastolic function,44,45 there are no studies reporting clinical outcomes.
Treatment of OSA with continuous positive airway pressure appears to alleviate some symptoms of HF and to reduce all-cause mortality.46,47
Keeping HF patients out of the hospital
Many readmissions to the hospital for HF exacerbation are preventable. Patients often do not understand hospital discharge instructions or the nature of their chronic disease and its management.48-51 Routine follow-up in the office or clinic provides an opportunity to improve quality of life for patients and decrease admissions.7,52
A major role for the family physician is in the co-creation of, and adherence to, an individualized, comprehensive care plan. Make sure such a plan is easily understood not only by the patient with HF, but also by his or her care team. In addition, it should be evidence-based and reflect the patient’s culture, values, and goals of treatment.5,7
At each visit, the family physician or a member of the health care team should assess adherence to guideline-directed medical therapy, measure weight, evaluate fluid status, and provide ongoing patient education including information on the importance of activity, monitoring weight daily, and moderating fluid, salt, and alcohol intake.5,52
Research shows that cardiac rehabilitation improves functional capacity, exercise duration, quality of life, and mortality. Therefore, recommend it to all symptomatic patients with HF who are clinically stable.2
Consider collaboration with a subspecialist. Patients who remain symptomatic despite optimal medical management and patients with recurrent hospitalizations are best managed in conjunction with a subspecialist in HF treatment.2,5
CORRESPONDENCE
Darin Brink, MD, 420 Delaware St. SE, MMC 381, Minneapolis, MN 55455; [email protected].
1. Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief. 2012;(108):1-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23102190. Accessed April 26, 2017.
2. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
3. Passantino A, Guida P, Lagioia R, et al. Predictors of long-term mortality in older patients hospitalized for acutely decompensated heart failure: clinical relevance of natriuretic peptides. J Am Geriatr Soc. 2017;65:822-826.
4. Lassus JP, Siirilä-Waris K, Nieminen MS, et al. Long-term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168:458-462.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
6. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2016;68:1476-1488.
7. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200.
8. Pinkerman CP, Sander JE, Breeding D, et al. Institute for Clinical Systems Improvement. Heart failure in adults. Available at: https://www.scribd.com/document/310893227/HeartFailure-pdf. Accessed December 6, 2017.
9. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441-1446.
10. Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011;124:2865-2873.
11. Heart Failure Society of America, Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194.
12. Pouleur H, The SOLVD Investigators. Results of the treatment trial of the studies of left ventricular dysfunction (SOLVD). Am J Cardiol. 1992;70:135-136.
13. Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414-1421.
14. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
15. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13.
16. Gehr TW, Sica DA. Pharmacotherapy in congestive heart failure: Hyperkalemia in congestive heart failure. Congest Heart Fail. 2001;7:97-100.
17. National Institute for Health and Clinical Excellence (NICE). Chronic heart failure in adults: management. 2010. Available at: https://www.nice.org.uk/guidance/cg108. Accessed November 27, 2017.
18. Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent). 2003;16:123-126.
19. Epstein SE, Braunwald E. The effect of beta adrenergic blockade on patterns of urinary sodium excretion: studies in normal subjects and in patients with heart disease. Ann Intern Med. 1966;65:20-27.
20. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:246.
21. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
22. Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992;69:108G-118G.
23. Sundar S, Burma DP, Vaish SK. Digoxin toxicity and electrolytes: a correlative study. Acta Cardiol. 1983;38:115-123.
24. McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79-84.
25. Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18:48.
26. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
27. Corlanor package insert. Amgen Inc., Thousand Oaks, CA. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/corlanor/corlanor_pi.pdf. Accessed November 28, 2017.
28. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
29. Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94-125.
30. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047-1058.
31. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283-e352.
32. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-679.
33. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868-1877.
34. Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186-2198.
35. Cleland JG, Pellicori P, Dierckx R. Clinical trials in patients with heart failure and preserved left ventricular ejection fraction. Heart Fail Clin. 2014;10:511-523.
36. Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665-671.
37. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507-515.
38. Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015-1021.
39. Zile MR. Heart failure with a preserved ejection fraction. In: Mann DL, Zipes D, Libby P BR, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Phila
40. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803.
41. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281-2293.
42. de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376-2381.
43. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315:36-46.
44. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718-726.
45. Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198-202.
46. Yoshihisa A, Suzuki S, Yamauchi H, et al. Beneficial effects of positive airway pressure therapy for sleep-disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol. 2015;38:413-421.
47. Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620-626.
48. Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141-1163.
49. Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984.
50. Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459-467.
51. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407-413.
52. Cowie MR, Anker SD, Cleland JG, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. Available at: http://www.oxfordhealthpolicyforum.org/files/reports/ahf-report.pdf. Accessed November 27, 2017.
1. Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief. 2012;(108):1-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23102190. Accessed April 26, 2017.
2. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
3. Passantino A, Guida P, Lagioia R, et al. Predictors of long-term mortality in older patients hospitalized for acutely decompensated heart failure: clinical relevance of natriuretic peptides. J Am Geriatr Soc. 2017;65:822-826.
4. Lassus JP, Siirilä-Waris K, Nieminen MS, et al. Long-term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168:458-462.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
6. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2016;68:1476-1488.
7. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200.
8. Pinkerman CP, Sander JE, Breeding D, et al. Institute for Clinical Systems Improvement. Heart failure in adults. Available at: https://www.scribd.com/document/310893227/HeartFailure-pdf. Accessed December 6, 2017.
9. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441-1446.
10. Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011;124:2865-2873.
11. Heart Failure Society of America, Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194.
12. Pouleur H, The SOLVD Investigators. Results of the treatment trial of the studies of left ventricular dysfunction (SOLVD). Am J Cardiol. 1992;70:135-136.
13. Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414-1421.
14. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
15. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13.
16. Gehr TW, Sica DA. Pharmacotherapy in congestive heart failure: Hyperkalemia in congestive heart failure. Congest Heart Fail. 2001;7:97-100.
17. National Institute for Health and Clinical Excellence (NICE). Chronic heart failure in adults: management. 2010. Available at: https://www.nice.org.uk/guidance/cg108. Accessed November 27, 2017.
18. Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent). 2003;16:123-126.
19. Epstein SE, Braunwald E. The effect of beta adrenergic blockade on patterns of urinary sodium excretion: studies in normal subjects and in patients with heart disease. Ann Intern Med. 1966;65:20-27.
20. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:246.
21. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
22. Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992;69:108G-118G.
23. Sundar S, Burma DP, Vaish SK. Digoxin toxicity and electrolytes: a correlative study. Acta Cardiol. 1983;38:115-123.
24. McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79-84.
25. Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18:48.
26. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
27. Corlanor package insert. Amgen Inc., Thousand Oaks, CA. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/corlanor/corlanor_pi.pdf. Accessed November 28, 2017.
28. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
29. Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94-125.
30. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047-1058.
31. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283-e352.
32. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-679.
33. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868-1877.
34. Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186-2198.
35. Cleland JG, Pellicori P, Dierckx R. Clinical trials in patients with heart failure and preserved left ventricular ejection fraction. Heart Fail Clin. 2014;10:511-523.
36. Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665-671.
37. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507-515.
38. Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015-1021.
39. Zile MR. Heart failure with a preserved ejection fraction. In: Mann DL, Zipes D, Libby P BR, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Phila
40. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803.
41. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281-2293.
42. de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376-2381.
43. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315:36-46.
44. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718-726.
45. Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198-202.
46. Yoshihisa A, Suzuki S, Yamauchi H, et al. Beneficial effects of positive airway pressure therapy for sleep-disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol. 2015;38:413-421.
47. Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620-626.
48. Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141-1163.
49. Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984.
50. Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459-467.
51. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407-413.
52. Cowie MR, Anker SD, Cleland JG, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. Available at: http://www.oxfordhealthpolicyforum.org/files/reports/ahf-report.pdf. Accessed November 27, 2017.
From The Journal of Family Practice | 2018;67(1):18-26.
PRACTICE RECOMMENDATIONS
› Order a measurement of B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide in patients with dyspnea to help diagnose and manage heart failure (HF). A
› Refer patients with symptomatic HF and a left ventricular ejection fraction (LVEF) ≤35% that persists despite ≥3 months of optimal medical therapy for an implantable cardioverter defibrillator to reduce the risk of sudden death and all-cause mortality. A
› Consider cardiac resynchronization therapy for patients with an LVEF ≤35%, sinus rhythm, left bundle branch block, and a QRS duration ≥150 ms who remain symptomatic despite optimal medical therapy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
