User login
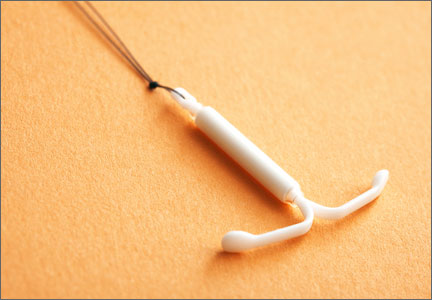
Does the LNG-IUS treat endometrial hyperplasia as effectively as MPA?
Endometrial cancer is the most common gynecologic cancer in the developed world, and its incidence is rising—increasing 50% in the past 10 years in Norway alone. Because endometrial hyperplasia is a precursor to cancer, it is vital that we ensure effective treatment for this prevalent problem. Hysterectomy is one option—used most commonly for complex atypical hyperplasia—but oral progestins have become the norm in women who desire to preserve their uterus when surgery is not the best option.
In this randomized trial from Norway, Orbo and colleagues randomly assigned 170 women aged 30 to 70 years to one of three treatment groups:
- placement of an LNG-IUS (Mirena)
- medroxyprogesterone acetate (MPA) 10 mg for 10 days per cycle
- continuous MPA 10 mg.
All women in the trial had low- or medium-risk endometrial hyperplasia.
After 6 months, women in the LNG-IUS arm had a 100% response rate, compared with 96% for the continuous MPA group and 69% for cyclic MPA.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; March 2014)
Histologic interpretation of hyperplasia is highly subjective
There are several problems inherent in a study like this. Although Orbo and colleagues address these problems tangentially, the problems affect the interpretation of results.
For example, the histologic interpretation of endometrial hyperplasia is known to be associated with low interobserver agreement.1 Clinical trials that use endometrial safety as an outcome require two primary pathologists to review the histology, with a third pathologist standing by in case of disagreement.
In the current study, two pathologists in the same department independently reviewed the histology. Orbo and colleagues used World Health Organization criteria for hyperplasia. However, as an adjunct, they also used a D-evaluation morphometric assessment.2 When I put in a casual call to local gynecologic pathologists, they told me that neither the D-classification nor the immunochemical-detected PTEN protein is used in routine clinical practice to determine the risk of progression.3
Intermittent use of oral MPA is known to be ineffective
The cyclic use of MPA for only 10 days overlooks epidemiology from estrogen-progestin replacement regimens in postmenopausal women. Use of a progestin for fewer than 12 days during estrogen replacement increases the risk of endometrial cancer.4 Exogenous progestin must be given for more than 12 days to inhibit hyperplasia and neoplasia. The dose itself is not critical; the duration of administration is.
In the current study, both the LNG-IUS and continuous MPA met this criterion. Local delivery of the progestin with the LNG-IUS allows for a reduction of the delivered dose and mitigates side effects even as it uses a more potent progestin than MPA.5–8
Assessment of outcomes was questionable
Although Orbo and colleagues suggest that there is no evidence of progression with the LNG-IUS and continuous MPA, they relied on a Pipelle biopsy of the endometrium performed after 6 months of treatment. The clinical settings that led to the hyperplasia in the first place are poorly characterized as either pre- or postmenopausal, and the cause of the hyperplasia is not identified. This approach overlooks such realities as the increased incidence of simple hyperplasia in many perimenopausal women, which appears to regress with further reduction in ovarian estrogen.9
The final outcomes for women in the cyclic MPA arm are not provided. Hyperplasia without atypia progresses to carcinoma in 1.6% of cases, but when atypia is present, the progression rate is 23%.1
What this evidence means for practice
In low-risk women with simple hyperplasia, the use of targeted low-dose progestins—oral or intrauterine—is appealing. While Orbo and colleagues present an interesting study, they do not definitively establish the optimal intervention.
As we enter a cost-conscious phase of medicine in the United States, we may discover that oral generic MPA (given continuously) may be the most cost-effective treatment despite the option of delivering a low-dose progestin via intrauterine device.
David F. Archer, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001;76(1):25–31.
- Orbo A, Ames M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: A follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008;111(1):68–73.
- Lacey JV Jr, Mutter GL, Ronnett BM, et al. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer Res. 2008;68(14):6014–6020.
- Archer DF. Neoplasia of the female reproductive tract: Effects of hormone therapy. Endocrine. 2004;24(3):259–263.
- Moe BT, Vereide AB, Orbo A, Jaeger R, Sager G. Levonorgestrel, medroxyprogesterone, and progesterone cause a concentration-dependent reduction in endometrial cancer (Ishikawa) cell density, and high concentrations of progesterone and mifepristone act in synergy. Anticancer Res. 2009;29(4):1047–1052.
- Phillips A, Hahn DW, Kimek S, McGuire JL. A comparison of the potencies and activities of progestogens used in contraceptives. Contraception. 1987;36(2):181–192.
- Archer DF. Delivery of therapeutic agents to the target tissue. Menopause. 2011;18(10):1040–1041.
- Somboonporn W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause. 2011;18(10):1060–1066.
- Archer DF, McIntyre-Seltman K, Wilborn WW Jr, et al. Endometrial morphology in asymptomatic postmenopausal women. Am J Obstet Gynecol. 1991;165(2):317–322.
Endometrial cancer is the most common gynecologic cancer in the developed world, and its incidence is rising—increasing 50% in the past 10 years in Norway alone. Because endometrial hyperplasia is a precursor to cancer, it is vital that we ensure effective treatment for this prevalent problem. Hysterectomy is one option—used most commonly for complex atypical hyperplasia—but oral progestins have become the norm in women who desire to preserve their uterus when surgery is not the best option.
In this randomized trial from Norway, Orbo and colleagues randomly assigned 170 women aged 30 to 70 years to one of three treatment groups:
- placement of an LNG-IUS (Mirena)
- medroxyprogesterone acetate (MPA) 10 mg for 10 days per cycle
- continuous MPA 10 mg.
All women in the trial had low- or medium-risk endometrial hyperplasia.
After 6 months, women in the LNG-IUS arm had a 100% response rate, compared with 96% for the continuous MPA group and 69% for cyclic MPA.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; March 2014)
Histologic interpretation of hyperplasia is highly subjective
There are several problems inherent in a study like this. Although Orbo and colleagues address these problems tangentially, the problems affect the interpretation of results.
For example, the histologic interpretation of endometrial hyperplasia is known to be associated with low interobserver agreement.1 Clinical trials that use endometrial safety as an outcome require two primary pathologists to review the histology, with a third pathologist standing by in case of disagreement.
In the current study, two pathologists in the same department independently reviewed the histology. Orbo and colleagues used World Health Organization criteria for hyperplasia. However, as an adjunct, they also used a D-evaluation morphometric assessment.2 When I put in a casual call to local gynecologic pathologists, they told me that neither the D-classification nor the immunochemical-detected PTEN protein is used in routine clinical practice to determine the risk of progression.3
Intermittent use of oral MPA is known to be ineffective
The cyclic use of MPA for only 10 days overlooks epidemiology from estrogen-progestin replacement regimens in postmenopausal women. Use of a progestin for fewer than 12 days during estrogen replacement increases the risk of endometrial cancer.4 Exogenous progestin must be given for more than 12 days to inhibit hyperplasia and neoplasia. The dose itself is not critical; the duration of administration is.
In the current study, both the LNG-IUS and continuous MPA met this criterion. Local delivery of the progestin with the LNG-IUS allows for a reduction of the delivered dose and mitigates side effects even as it uses a more potent progestin than MPA.5–8
Assessment of outcomes was questionable
Although Orbo and colleagues suggest that there is no evidence of progression with the LNG-IUS and continuous MPA, they relied on a Pipelle biopsy of the endometrium performed after 6 months of treatment. The clinical settings that led to the hyperplasia in the first place are poorly characterized as either pre- or postmenopausal, and the cause of the hyperplasia is not identified. This approach overlooks such realities as the increased incidence of simple hyperplasia in many perimenopausal women, which appears to regress with further reduction in ovarian estrogen.9
The final outcomes for women in the cyclic MPA arm are not provided. Hyperplasia without atypia progresses to carcinoma in 1.6% of cases, but when atypia is present, the progression rate is 23%.1
What this evidence means for practice
In low-risk women with simple hyperplasia, the use of targeted low-dose progestins—oral or intrauterine—is appealing. While Orbo and colleagues present an interesting study, they do not definitively establish the optimal intervention.
As we enter a cost-conscious phase of medicine in the United States, we may discover that oral generic MPA (given continuously) may be the most cost-effective treatment despite the option of delivering a low-dose progestin via intrauterine device.
David F. Archer, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Endometrial cancer is the most common gynecologic cancer in the developed world, and its incidence is rising—increasing 50% in the past 10 years in Norway alone. Because endometrial hyperplasia is a precursor to cancer, it is vital that we ensure effective treatment for this prevalent problem. Hysterectomy is one option—used most commonly for complex atypical hyperplasia—but oral progestins have become the norm in women who desire to preserve their uterus when surgery is not the best option.
In this randomized trial from Norway, Orbo and colleagues randomly assigned 170 women aged 30 to 70 years to one of three treatment groups:
- placement of an LNG-IUS (Mirena)
- medroxyprogesterone acetate (MPA) 10 mg for 10 days per cycle
- continuous MPA 10 mg.
All women in the trial had low- or medium-risk endometrial hyperplasia.
After 6 months, women in the LNG-IUS arm had a 100% response rate, compared with 96% for the continuous MPA group and 69% for cyclic MPA.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; March 2014)
Histologic interpretation of hyperplasia is highly subjective
There are several problems inherent in a study like this. Although Orbo and colleagues address these problems tangentially, the problems affect the interpretation of results.
For example, the histologic interpretation of endometrial hyperplasia is known to be associated with low interobserver agreement.1 Clinical trials that use endometrial safety as an outcome require two primary pathologists to review the histology, with a third pathologist standing by in case of disagreement.
In the current study, two pathologists in the same department independently reviewed the histology. Orbo and colleagues used World Health Organization criteria for hyperplasia. However, as an adjunct, they also used a D-evaluation morphometric assessment.2 When I put in a casual call to local gynecologic pathologists, they told me that neither the D-classification nor the immunochemical-detected PTEN protein is used in routine clinical practice to determine the risk of progression.3
Intermittent use of oral MPA is known to be ineffective
The cyclic use of MPA for only 10 days overlooks epidemiology from estrogen-progestin replacement regimens in postmenopausal women. Use of a progestin for fewer than 12 days during estrogen replacement increases the risk of endometrial cancer.4 Exogenous progestin must be given for more than 12 days to inhibit hyperplasia and neoplasia. The dose itself is not critical; the duration of administration is.
In the current study, both the LNG-IUS and continuous MPA met this criterion. Local delivery of the progestin with the LNG-IUS allows for a reduction of the delivered dose and mitigates side effects even as it uses a more potent progestin than MPA.5–8
Assessment of outcomes was questionable
Although Orbo and colleagues suggest that there is no evidence of progression with the LNG-IUS and continuous MPA, they relied on a Pipelle biopsy of the endometrium performed after 6 months of treatment. The clinical settings that led to the hyperplasia in the first place are poorly characterized as either pre- or postmenopausal, and the cause of the hyperplasia is not identified. This approach overlooks such realities as the increased incidence of simple hyperplasia in many perimenopausal women, which appears to regress with further reduction in ovarian estrogen.9
The final outcomes for women in the cyclic MPA arm are not provided. Hyperplasia without atypia progresses to carcinoma in 1.6% of cases, but when atypia is present, the progression rate is 23%.1
What this evidence means for practice
In low-risk women with simple hyperplasia, the use of targeted low-dose progestins—oral or intrauterine—is appealing. While Orbo and colleagues present an interesting study, they do not definitively establish the optimal intervention.
As we enter a cost-conscious phase of medicine in the United States, we may discover that oral generic MPA (given continuously) may be the most cost-effective treatment despite the option of delivering a low-dose progestin via intrauterine device.
David F. Archer, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001;76(1):25–31.
- Orbo A, Ames M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: A follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008;111(1):68–73.
- Lacey JV Jr, Mutter GL, Ronnett BM, et al. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer Res. 2008;68(14):6014–6020.
- Archer DF. Neoplasia of the female reproductive tract: Effects of hormone therapy. Endocrine. 2004;24(3):259–263.
- Moe BT, Vereide AB, Orbo A, Jaeger R, Sager G. Levonorgestrel, medroxyprogesterone, and progesterone cause a concentration-dependent reduction in endometrial cancer (Ishikawa) cell density, and high concentrations of progesterone and mifepristone act in synergy. Anticancer Res. 2009;29(4):1047–1052.
- Phillips A, Hahn DW, Kimek S, McGuire JL. A comparison of the potencies and activities of progestogens used in contraceptives. Contraception. 1987;36(2):181–192.
- Archer DF. Delivery of therapeutic agents to the target tissue. Menopause. 2011;18(10):1040–1041.
- Somboonporn W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause. 2011;18(10):1060–1066.
- Archer DF, McIntyre-Seltman K, Wilborn WW Jr, et al. Endometrial morphology in asymptomatic postmenopausal women. Am J Obstet Gynecol. 1991;165(2):317–322.
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001;76(1):25–31.
- Orbo A, Ames M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: A follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008;111(1):68–73.
- Lacey JV Jr, Mutter GL, Ronnett BM, et al. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer Res. 2008;68(14):6014–6020.
- Archer DF. Neoplasia of the female reproductive tract: Effects of hormone therapy. Endocrine. 2004;24(3):259–263.
- Moe BT, Vereide AB, Orbo A, Jaeger R, Sager G. Levonorgestrel, medroxyprogesterone, and progesterone cause a concentration-dependent reduction in endometrial cancer (Ishikawa) cell density, and high concentrations of progesterone and mifepristone act in synergy. Anticancer Res. 2009;29(4):1047–1052.
- Phillips A, Hahn DW, Kimek S, McGuire JL. A comparison of the potencies and activities of progestogens used in contraceptives. Contraception. 1987;36(2):181–192.
- Archer DF. Delivery of therapeutic agents to the target tissue. Menopause. 2011;18(10):1040–1041.
- Somboonporn W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause. 2011;18(10):1060–1066.
- Archer DF, McIntyre-Seltman K, Wilborn WW Jr, et al. Endometrial morphology in asymptomatic postmenopausal women. Am J Obstet Gynecol. 1991;165(2):317–322.
Postmenopausal HRT: What is fact, what is fiction?
HRT stops vaginal atrophy, hot flashes, and bone loss
Three applications form the basis for HRT in postmenopausal women:
- Hot flashes subside. Hot flashes occur with varying intensity in about 85% of women, and are effectively treated with estrogen, whether given orally, transdermally, or vaginally.1,2 As long as an appropriate blood level of the hormone is reached, hot flashes diminish.3-5 This reduction is dose-related.
- Measurable improvements in vaginal atrophy. Estrogen’s efficacy in relieving dryness, itching, burning, and dyspareunia is well demonstrated, regardless of the route of administration.3,6,7 A fall in vaginal pH from 6.0 to 5.0 after estrogen administration has been documented,8 as has the increase in the number of superficial cells of the vagina with exogenous estrogen.9
- HRT maintains or increases bone mineral density (BMD). Most estrogen preparations on the US market have been shown to improve BMD.10-15 “Improvement” means no significant loss, or an increase, in BMD. In the WHI, both vertebral and nonvertebral fractures diminished unequivocally in women using estrogen—alone or with a progestin.16,17 Other clinical trials also have shown increased BMD, as well as decreased urinary and serum markers of bone turnover.
Do new data link progestin to cancer?
Although compelling evidence supports the use of progestational agents in addition to estrogen to prevent endometrial hyperplasia and endometrial cancer,18 a 2005 report19 suggests that chronic, long-term use of estrogen with a progestin may increase the risk of endometrial carcinoma. Because this is the only study in which this risk has been found, corroboration is required.
Until then, give progestin at a sufficient dose and duration to inhibit endometrial hyperplasia.20-25
Effects on heart disease may be age-related
With notable exceptions, the overall conclusion of clinical trials and observational studies to date is that estrogen helps prevent coronary heart disease (CHD).26-30 This finding was first observed in the late 1980s with evidence that estrogen increases high-density lipoprotein (HDL) cholesterol and reduces total and low-density lipoprotein (LDL) cholesterol.31
Some experts argue that these observational trials are biased because many of the women taking estrogen had modified their lifestyles to maintain their weight, control their diet, and exercise regularly.32 Indeed, the randomized, placebo-controlled Heart and Estrogen Replacement Study (HERS) and both arms of the WHI trial found no evidence for a significant increase or decrease in CHD events.33-35
Time from menopause to HRT may be key
Both the HERS and WHI trials enrolled older women who had entered menopause a few months to several years before starting HRT.36 In addition, the estrogen-progestin arm of the WHI trial lacked sufficient power to detect a significant difference in CHD outcomes.37
The WHI findings contrast those of the large, ongoing, observational Nurses Health Study, which has shown a consistent decrease in CHD incidence in women who began HRT with the onset of menopausal symptoms.27-30 The most recent data suggest that the interval between menopause and the start of HRT may explain the different findings in randomized, controlled trials and observational studies.38 The WHI data support this theory: CHD was lower in women who began taking HRT within 5 years of menopause, compared with women who initiated HRT more than 5 years afterward.36 In addition, data from the estrogen-only arm of the WHI show fewer CHD events in women younger than 60.34
Several other studies support this hypothesis:
- The surgically postmenopausal cynomolgus macaque had a lower rate of atherosclerotic plaque development when estrogen was given, with or without a progestin.39,40
- In the Rancho Bernardo study, women who had used HRT had less cardiac calcification documented by computed tomography, compared with nonusers.41
- Estrogen has been shown, by measurement of carotid intimal medial thickness, to inhibit atherosclerotic plaque in humans.42
- Older women with established atherosclerosis do not undergo any significant change in plaque size with the use of exogenous estrogen.43
Although these findings support the use of estrogen or estrogen-progestin early after menopause as a way of preventing CHD, further clinical trials are needed.44
Stroke risk is small but real
Both arms of the WHI found an increased incidence of stroke in women using hormones, compared with nonusers.16,36 The exact mechanisms underlying this increased risk are unclear.
The actual attributable risk was an increase of 0.7 cases of stroke per 1,000 women per year over placebo in the estrogen-progestin arm,36 and 1.2 cases per 1,000 in the estrogen-only arm.16 The relative hazards were 1.31 (95% confidence interval [CI] 1.02–1.68) and 1.30 (95% CI 1.10–1.77), respectively.
Note that women in the estrogen-only arm had a greater incidence of hypertension and diabetes mellitus—known risk factors for stroke—than did women in the estrogen-progestin arm.16,36
VTE risk is twice as high in HRT users
Postmenopausal women who take estrogen have a higher risk of venous thromboembolism (VTE) than those who do not. This risk translated into a relative hazard of 2.06 (1.57–2.70) in the WHI estrogen-progestin arm, or an attributable risk of 3.6 cases per 1,000 women, compared with 1.8 cases per thousand in the control group.36
The absolute increased risk is 1.8 cases per 1,000 women, or, as expressed in the study itself, 18 cases per 10,000 women per year.
I have deliberately reduced the attributable risk to the number of cases per thousand because I believe this number is more easily understood by the patient and accurately demonstrates the low risk.
In the estrogen-only arm of the WHI, the hazard ratio for VTE was 1.33 (0.99–1.79), or an absolute increased risk of 0.7 cases per thousand—although this finding was not significant. The attributable risk was 2.7 cases per 1,000 women, compared with 2.0 cases per thousand among controls.16
Like stroke, the risk of VTE may be confounded by other factors besides use of exogenous estrogen.
No cause and effect for HRT and breast cancer
Nothing frightens women as much as breast cancer, and articles focusing on the relationship between breast cancer and HRT have drawn widespread attention. However, despite voluminous literature, the etiology of breast cancer remains elusive—and there is no evidence that either estrogen or progestins cause the disease.45,46 Rather, there is only an association between the use of estrogen, progestin, and breast cancer. Linking the finding of an increased risk with an implication of causality would be inappropriate.
Breast cancer risk with HRT is not consistently elevated, in studies
In fact, a qualitative review of observational studies from 1975 to 2000 found no significant increase or decrease in the risk of breast cancer with estrogen or estrogen-progestin in 80% of the reports.47
Risk factors for breast cancer (TABLE 1) include family history, obesity, late childbirth, and hormone therapy—but obesity and family history have higher relative risks than the use of HRT.48
TABLE 1
Relative risk of breast cancer
| CHARACTERISTIC | RELATIVE RISK |
|---|---|
| 2 family members with breast cancer | 14 |
| 1 family member with breast cancer | 2.2 |
| Obesity | 1.8 |
| Young age at menarche | 1.6 |
| Hormone therapy | 1.3 |
| >30 years of age at birth of first child | 1.3 |
| Menopause | 0.7 |
WHI arms find different risks
In the widely publicized WHI, women in the estrogen-progestin arm had an overall relative hazard for breast cancer of 1.24 (95% CI 1.01–1.54), but there was no increased risk in women who had never before used hormones.36 Women who had previously used hormones for 5 years or more did have an increased risk.36 The incidence of breast cancer in the study population was 3 cases per 1,000 women, and the excess number was 0.7 more cases with the use of estrogen-progestin (TABLE 2).
Conversely, in the estrogen-only arm of the WHI,16 the relative hazard for breast cancer was 0.77 (95% CI 0.59–1.01), and the reduction in risk was almost statistically significant. There are at least 2 potential explanations for the lower incidence of breast cancer in this arm:
- Without a progestin, estrogen increases breast density only minimally, allowing for easier mammographic interpretation.
- Women susceptible to breast cancer because of their previous use of estrogen may not have been present in the at-risk population in sufficient numbers to cause an increase.
TABLE 2
Extra cases of breast cancer, by risk factor
| RISK FACTOR | BREAST CANCERS DIAGNOSED OVER 20 YEARS FROM AGES 50 TO 70 (PER 1,000) | EXTRA BREAST CANCERS (PER 1,000) |
|---|---|---|
| Never used HRT | 45 | - |
| >5 years HRT | 47 | 2 |
| >10 years HRT | 51 | 6 |
| >15 years HRT | 57 | 12 |
| Late menopause (age 60) | 59 | 14 |
| Alcohol (2 drinks/day) | 72 | 27 |
| No daily exercise | 72 | 27 |
| Weight gain (>20 kg) | 90 | 45 |
| Reprinted from THE LANCET, Vol. 350: 1047–1059, Collaborative Group on Hormonal Factors in Breast Cancer, Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Copyright 1997, with permission from Elsevier | ||
HRT may promote, rather than induce, breast cancer
The role of hormones in the etiology of breast cancer is difficult to assess. The Million Women Study49 found that the elevated risk of breast cancer disappeared within 1 year of stopping HRT. This finding implies that hormones may be a promoter, rather than inducer, of neoplasms in the breast.
Breast cancer may be present in many women, but apparent in few
When autopsies were performed on women in their 40s who had died from other diseases, the incidence of breast cancer was 39%, but the clinical detection rate was only 1% for this population.50 This discrepancy suggests that neoplastic cells may be present in the body at any time, but become clinically apparent only under certain conditions.51
More recent data suggest that undifferentiated stem cells in the breast become dysfunctional and result in cancer.52 This theory is supported by the various histologic types of cancer found in the breast.
A weak link
Although it may be compelling to link hormone use with breast cancer, the association is weak and the incidence is lower than in other known relationships such as obesity. At present, the cause of breast neoplasia appears to be multifactorial.
1. Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:11-25.
2. Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3:47.-
3. Archer DF. Percutaneous 17beta-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
4. Archer DF. Low-dose hormone therapy for postmenopausal women. Clin Obstet Gynecol. 2003;46:317-324.
5. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610-1620.
6. Ballagh SA. Vaginal rings for menopausal symptom relief. Drugs Aging. 2004;21:757-766.
7. Speroff L. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstet Gynecol. 2003;102:823-834.
8. Notelovitz M. Urogenital atrophy and low-dose vaginal estrogen therapy. Menopause. 2000;7:140-142.
9. Utian WH, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. The Esclim Study Group. Am J Obstet Gynecol. 1999;181:71-79.
10. Christiansen C. Effects of drospirenone/estrogen combinations on bone metabolism. Climacteric. 2005;8(suppl 3):35-41.
11. Delmas PD, Confavreux E, Garnero P, et al. A combination of low doses of 17 beta-estradiol and norethisterone acetate prevents bone loss and normalizes bone turnover in postmenopausal women. Osteoporos Int. 2000;11:177-187.
12. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
13. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Bone response to treatment with lower doses of conjugated estrogens with and without medroxyprogesterone acetate in early postmenopausal women. Osteoporos Int. 2005;16:372-379.
14. Ravn P, Bidstrup M, Wasnich RD, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med. 1999;131:935-942.
15. Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Ann Intern Med. 1999;130:897-904.
16. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
17. Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729-1738.
18. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
19. Lacey JV, Jr, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:1724-1731.
20. Archer DF, Furst K, Tipping D, Dain MP, Vandepol C. A randomized comparison of continuous combined transdermal delivery of estradiol-norethindrone acetate and estradiol alone for menopause. CombiPatch Study Group. Obstet Gynecol. 1999;94:498-503.
21. Bouchard P, De Cicco-Nardone F, Spielmann D, Garcea N. Bleeding profile and endometrial safety of continuous combined regimens 1mg 17beta-estradiol/trimegestone versus 1or 2 mg 17beta-estradiol/norethisterone acetate in postmenopausal women. Gynecol Endocrinol. 2005;21:142-148.
22. Kurman RJ, Felix JC, Archer DF, Nanavati N, Arce J, Moyer DL. Norethindrone acetate and estradiol-induced endometrial hyperplasia. Obstet Gynecol. 2000;96:373-379.
23. Speroff L, Rowan J, Symons J, Genant H, Wilborn W. The comparative effect on bone density, endometrium, and lipids of continuous hormones as replacement therapy (CHART study). A randomized controlled trial. JAMA. 1996;276:1397-1403.
24. Sturdee DW, Ulrich LG, Barlow DH, et al. The endometrial response to sequential and continuous combined oestrogen-progestogen replacement therapy. BJOG. 2000;107:1392-1400.
25. Ylikorkala O, Wahlstrom T, Caubel P, Lane R. Intermittent progestin administration as part of hormone replacement therapy: long-term comparison between estradiol 1mg combined with intermittent norgestimate and estradiol 2 mg combined with constant norethisterone acetate. Acta Obstet Gynecol Scand. 2002;81:654-660.
26. Espeland MA, Bush TL, Mebane-Sims I, et al. Rationale, design, and conduct of the PEPI Trial. Postmenopausal Estrogen/Progestin Interventions. Control Clin Trials. 1995;16(suppl):3S-19S.
27. Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453-461.
28. Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756-762.
29. Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941.
30. Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study. a prospective, observational study. Ann Intern Med. 2001;135:1-8.
31. Bush TL, Cowan LD, Barrett-Connor E, et al. Estrogen use and all-cause mortality. Preliminary results from the Lipid Research Clinics Program Follow-Up Study. JAMA. 1983;249:903-906.
32. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55-72.
33. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49-57.
34. Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357-365.
35. Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
36. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
37. Naftolin F, Taylor HS, Karas R, et al. The Women’s Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition. Fertil Steril. 2004;81:1498-1501.
38. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35-44.
39. Clarkson TB, Appt SE. Controversies about HRT-lessons from monkey models. Maturitas. 2005;51:64-74.
40. Wagner JD, Clarkson TB. The applicability of hormonal effects on atherosclerosis in animals to heart disease in postmenopausal women. Semin Reprod Med. 2005;23:149-156.
41. Barrett-Connor E, Laughlin GA. Hormone therapy and coronary artery calcification in symptomatic postmenopausal women: the Rancho Bernardo Study. Menopause. 2005;12:40-48.
42. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939-953.
43. Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522-529.
44. Harman SM, Brinton EA, Cedars M, et al. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3-12.
45. Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276-285.
46. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270-282.
47. Bush TL, Whiteman M, Flaws JA. Hormone replacement therapy and breast cancer: a qualitative review. Obstet Gynecol. 2001;98:498-508.
48. Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control. 2002;13:741-751.
49. Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427.
50. Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237-1243.
51. Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787.-
52. Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 Suppl 1:59-72.
HRT stops vaginal atrophy, hot flashes, and bone loss
Three applications form the basis for HRT in postmenopausal women:
- Hot flashes subside. Hot flashes occur with varying intensity in about 85% of women, and are effectively treated with estrogen, whether given orally, transdermally, or vaginally.1,2 As long as an appropriate blood level of the hormone is reached, hot flashes diminish.3-5 This reduction is dose-related.
- Measurable improvements in vaginal atrophy. Estrogen’s efficacy in relieving dryness, itching, burning, and dyspareunia is well demonstrated, regardless of the route of administration.3,6,7 A fall in vaginal pH from 6.0 to 5.0 after estrogen administration has been documented,8 as has the increase in the number of superficial cells of the vagina with exogenous estrogen.9
- HRT maintains or increases bone mineral density (BMD). Most estrogen preparations on the US market have been shown to improve BMD.10-15 “Improvement” means no significant loss, or an increase, in BMD. In the WHI, both vertebral and nonvertebral fractures diminished unequivocally in women using estrogen—alone or with a progestin.16,17 Other clinical trials also have shown increased BMD, as well as decreased urinary and serum markers of bone turnover.
Do new data link progestin to cancer?
Although compelling evidence supports the use of progestational agents in addition to estrogen to prevent endometrial hyperplasia and endometrial cancer,18 a 2005 report19 suggests that chronic, long-term use of estrogen with a progestin may increase the risk of endometrial carcinoma. Because this is the only study in which this risk has been found, corroboration is required.
Until then, give progestin at a sufficient dose and duration to inhibit endometrial hyperplasia.20-25
Effects on heart disease may be age-related
With notable exceptions, the overall conclusion of clinical trials and observational studies to date is that estrogen helps prevent coronary heart disease (CHD).26-30 This finding was first observed in the late 1980s with evidence that estrogen increases high-density lipoprotein (HDL) cholesterol and reduces total and low-density lipoprotein (LDL) cholesterol.31
Some experts argue that these observational trials are biased because many of the women taking estrogen had modified their lifestyles to maintain their weight, control their diet, and exercise regularly.32 Indeed, the randomized, placebo-controlled Heart and Estrogen Replacement Study (HERS) and both arms of the WHI trial found no evidence for a significant increase or decrease in CHD events.33-35
Time from menopause to HRT may be key
Both the HERS and WHI trials enrolled older women who had entered menopause a few months to several years before starting HRT.36 In addition, the estrogen-progestin arm of the WHI trial lacked sufficient power to detect a significant difference in CHD outcomes.37
The WHI findings contrast those of the large, ongoing, observational Nurses Health Study, which has shown a consistent decrease in CHD incidence in women who began HRT with the onset of menopausal symptoms.27-30 The most recent data suggest that the interval between menopause and the start of HRT may explain the different findings in randomized, controlled trials and observational studies.38 The WHI data support this theory: CHD was lower in women who began taking HRT within 5 years of menopause, compared with women who initiated HRT more than 5 years afterward.36 In addition, data from the estrogen-only arm of the WHI show fewer CHD events in women younger than 60.34
Several other studies support this hypothesis:
- The surgically postmenopausal cynomolgus macaque had a lower rate of atherosclerotic plaque development when estrogen was given, with or without a progestin.39,40
- In the Rancho Bernardo study, women who had used HRT had less cardiac calcification documented by computed tomography, compared with nonusers.41
- Estrogen has been shown, by measurement of carotid intimal medial thickness, to inhibit atherosclerotic plaque in humans.42
- Older women with established atherosclerosis do not undergo any significant change in plaque size with the use of exogenous estrogen.43
Although these findings support the use of estrogen or estrogen-progestin early after menopause as a way of preventing CHD, further clinical trials are needed.44
Stroke risk is small but real
Both arms of the WHI found an increased incidence of stroke in women using hormones, compared with nonusers.16,36 The exact mechanisms underlying this increased risk are unclear.
The actual attributable risk was an increase of 0.7 cases of stroke per 1,000 women per year over placebo in the estrogen-progestin arm,36 and 1.2 cases per 1,000 in the estrogen-only arm.16 The relative hazards were 1.31 (95% confidence interval [CI] 1.02–1.68) and 1.30 (95% CI 1.10–1.77), respectively.
Note that women in the estrogen-only arm had a greater incidence of hypertension and diabetes mellitus—known risk factors for stroke—than did women in the estrogen-progestin arm.16,36
VTE risk is twice as high in HRT users
Postmenopausal women who take estrogen have a higher risk of venous thromboembolism (VTE) than those who do not. This risk translated into a relative hazard of 2.06 (1.57–2.70) in the WHI estrogen-progestin arm, or an attributable risk of 3.6 cases per 1,000 women, compared with 1.8 cases per thousand in the control group.36
The absolute increased risk is 1.8 cases per 1,000 women, or, as expressed in the study itself, 18 cases per 10,000 women per year.
I have deliberately reduced the attributable risk to the number of cases per thousand because I believe this number is more easily understood by the patient and accurately demonstrates the low risk.
In the estrogen-only arm of the WHI, the hazard ratio for VTE was 1.33 (0.99–1.79), or an absolute increased risk of 0.7 cases per thousand—although this finding was not significant. The attributable risk was 2.7 cases per 1,000 women, compared with 2.0 cases per thousand among controls.16
Like stroke, the risk of VTE may be confounded by other factors besides use of exogenous estrogen.
No cause and effect for HRT and breast cancer
Nothing frightens women as much as breast cancer, and articles focusing on the relationship between breast cancer and HRT have drawn widespread attention. However, despite voluminous literature, the etiology of breast cancer remains elusive—and there is no evidence that either estrogen or progestins cause the disease.45,46 Rather, there is only an association between the use of estrogen, progestin, and breast cancer. Linking the finding of an increased risk with an implication of causality would be inappropriate.
Breast cancer risk with HRT is not consistently elevated, in studies
In fact, a qualitative review of observational studies from 1975 to 2000 found no significant increase or decrease in the risk of breast cancer with estrogen or estrogen-progestin in 80% of the reports.47
Risk factors for breast cancer (TABLE 1) include family history, obesity, late childbirth, and hormone therapy—but obesity and family history have higher relative risks than the use of HRT.48
TABLE 1
Relative risk of breast cancer
| CHARACTERISTIC | RELATIVE RISK |
|---|---|
| 2 family members with breast cancer | 14 |
| 1 family member with breast cancer | 2.2 |
| Obesity | 1.8 |
| Young age at menarche | 1.6 |
| Hormone therapy | 1.3 |
| >30 years of age at birth of first child | 1.3 |
| Menopause | 0.7 |
WHI arms find different risks
In the widely publicized WHI, women in the estrogen-progestin arm had an overall relative hazard for breast cancer of 1.24 (95% CI 1.01–1.54), but there was no increased risk in women who had never before used hormones.36 Women who had previously used hormones for 5 years or more did have an increased risk.36 The incidence of breast cancer in the study population was 3 cases per 1,000 women, and the excess number was 0.7 more cases with the use of estrogen-progestin (TABLE 2).
Conversely, in the estrogen-only arm of the WHI,16 the relative hazard for breast cancer was 0.77 (95% CI 0.59–1.01), and the reduction in risk was almost statistically significant. There are at least 2 potential explanations for the lower incidence of breast cancer in this arm:
- Without a progestin, estrogen increases breast density only minimally, allowing for easier mammographic interpretation.
- Women susceptible to breast cancer because of their previous use of estrogen may not have been present in the at-risk population in sufficient numbers to cause an increase.
TABLE 2
Extra cases of breast cancer, by risk factor
| RISK FACTOR | BREAST CANCERS DIAGNOSED OVER 20 YEARS FROM AGES 50 TO 70 (PER 1,000) | EXTRA BREAST CANCERS (PER 1,000) |
|---|---|---|
| Never used HRT | 45 | - |
| >5 years HRT | 47 | 2 |
| >10 years HRT | 51 | 6 |
| >15 years HRT | 57 | 12 |
| Late menopause (age 60) | 59 | 14 |
| Alcohol (2 drinks/day) | 72 | 27 |
| No daily exercise | 72 | 27 |
| Weight gain (>20 kg) | 90 | 45 |
| Reprinted from THE LANCET, Vol. 350: 1047–1059, Collaborative Group on Hormonal Factors in Breast Cancer, Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Copyright 1997, with permission from Elsevier | ||
HRT may promote, rather than induce, breast cancer
The role of hormones in the etiology of breast cancer is difficult to assess. The Million Women Study49 found that the elevated risk of breast cancer disappeared within 1 year of stopping HRT. This finding implies that hormones may be a promoter, rather than inducer, of neoplasms in the breast.
Breast cancer may be present in many women, but apparent in few
When autopsies were performed on women in their 40s who had died from other diseases, the incidence of breast cancer was 39%, but the clinical detection rate was only 1% for this population.50 This discrepancy suggests that neoplastic cells may be present in the body at any time, but become clinically apparent only under certain conditions.51
More recent data suggest that undifferentiated stem cells in the breast become dysfunctional and result in cancer.52 This theory is supported by the various histologic types of cancer found in the breast.
A weak link
Although it may be compelling to link hormone use with breast cancer, the association is weak and the incidence is lower than in other known relationships such as obesity. At present, the cause of breast neoplasia appears to be multifactorial.
HRT stops vaginal atrophy, hot flashes, and bone loss
Three applications form the basis for HRT in postmenopausal women:
- Hot flashes subside. Hot flashes occur with varying intensity in about 85% of women, and are effectively treated with estrogen, whether given orally, transdermally, or vaginally.1,2 As long as an appropriate blood level of the hormone is reached, hot flashes diminish.3-5 This reduction is dose-related.
- Measurable improvements in vaginal atrophy. Estrogen’s efficacy in relieving dryness, itching, burning, and dyspareunia is well demonstrated, regardless of the route of administration.3,6,7 A fall in vaginal pH from 6.0 to 5.0 after estrogen administration has been documented,8 as has the increase in the number of superficial cells of the vagina with exogenous estrogen.9
- HRT maintains or increases bone mineral density (BMD). Most estrogen preparations on the US market have been shown to improve BMD.10-15 “Improvement” means no significant loss, or an increase, in BMD. In the WHI, both vertebral and nonvertebral fractures diminished unequivocally in women using estrogen—alone or with a progestin.16,17 Other clinical trials also have shown increased BMD, as well as decreased urinary and serum markers of bone turnover.
Do new data link progestin to cancer?
Although compelling evidence supports the use of progestational agents in addition to estrogen to prevent endometrial hyperplasia and endometrial cancer,18 a 2005 report19 suggests that chronic, long-term use of estrogen with a progestin may increase the risk of endometrial carcinoma. Because this is the only study in which this risk has been found, corroboration is required.
Until then, give progestin at a sufficient dose and duration to inhibit endometrial hyperplasia.20-25
Effects on heart disease may be age-related
With notable exceptions, the overall conclusion of clinical trials and observational studies to date is that estrogen helps prevent coronary heart disease (CHD).26-30 This finding was first observed in the late 1980s with evidence that estrogen increases high-density lipoprotein (HDL) cholesterol and reduces total and low-density lipoprotein (LDL) cholesterol.31
Some experts argue that these observational trials are biased because many of the women taking estrogen had modified their lifestyles to maintain their weight, control their diet, and exercise regularly.32 Indeed, the randomized, placebo-controlled Heart and Estrogen Replacement Study (HERS) and both arms of the WHI trial found no evidence for a significant increase or decrease in CHD events.33-35
Time from menopause to HRT may be key
Both the HERS and WHI trials enrolled older women who had entered menopause a few months to several years before starting HRT.36 In addition, the estrogen-progestin arm of the WHI trial lacked sufficient power to detect a significant difference in CHD outcomes.37
The WHI findings contrast those of the large, ongoing, observational Nurses Health Study, which has shown a consistent decrease in CHD incidence in women who began HRT with the onset of menopausal symptoms.27-30 The most recent data suggest that the interval between menopause and the start of HRT may explain the different findings in randomized, controlled trials and observational studies.38 The WHI data support this theory: CHD was lower in women who began taking HRT within 5 years of menopause, compared with women who initiated HRT more than 5 years afterward.36 In addition, data from the estrogen-only arm of the WHI show fewer CHD events in women younger than 60.34
Several other studies support this hypothesis:
- The surgically postmenopausal cynomolgus macaque had a lower rate of atherosclerotic plaque development when estrogen was given, with or without a progestin.39,40
- In the Rancho Bernardo study, women who had used HRT had less cardiac calcification documented by computed tomography, compared with nonusers.41
- Estrogen has been shown, by measurement of carotid intimal medial thickness, to inhibit atherosclerotic plaque in humans.42
- Older women with established atherosclerosis do not undergo any significant change in plaque size with the use of exogenous estrogen.43
Although these findings support the use of estrogen or estrogen-progestin early after menopause as a way of preventing CHD, further clinical trials are needed.44
Stroke risk is small but real
Both arms of the WHI found an increased incidence of stroke in women using hormones, compared with nonusers.16,36 The exact mechanisms underlying this increased risk are unclear.
The actual attributable risk was an increase of 0.7 cases of stroke per 1,000 women per year over placebo in the estrogen-progestin arm,36 and 1.2 cases per 1,000 in the estrogen-only arm.16 The relative hazards were 1.31 (95% confidence interval [CI] 1.02–1.68) and 1.30 (95% CI 1.10–1.77), respectively.
Note that women in the estrogen-only arm had a greater incidence of hypertension and diabetes mellitus—known risk factors for stroke—than did women in the estrogen-progestin arm.16,36
VTE risk is twice as high in HRT users
Postmenopausal women who take estrogen have a higher risk of venous thromboembolism (VTE) than those who do not. This risk translated into a relative hazard of 2.06 (1.57–2.70) in the WHI estrogen-progestin arm, or an attributable risk of 3.6 cases per 1,000 women, compared with 1.8 cases per thousand in the control group.36
The absolute increased risk is 1.8 cases per 1,000 women, or, as expressed in the study itself, 18 cases per 10,000 women per year.
I have deliberately reduced the attributable risk to the number of cases per thousand because I believe this number is more easily understood by the patient and accurately demonstrates the low risk.
In the estrogen-only arm of the WHI, the hazard ratio for VTE was 1.33 (0.99–1.79), or an absolute increased risk of 0.7 cases per thousand—although this finding was not significant. The attributable risk was 2.7 cases per 1,000 women, compared with 2.0 cases per thousand among controls.16
Like stroke, the risk of VTE may be confounded by other factors besides use of exogenous estrogen.
No cause and effect for HRT and breast cancer
Nothing frightens women as much as breast cancer, and articles focusing on the relationship between breast cancer and HRT have drawn widespread attention. However, despite voluminous literature, the etiology of breast cancer remains elusive—and there is no evidence that either estrogen or progestins cause the disease.45,46 Rather, there is only an association between the use of estrogen, progestin, and breast cancer. Linking the finding of an increased risk with an implication of causality would be inappropriate.
Breast cancer risk with HRT is not consistently elevated, in studies
In fact, a qualitative review of observational studies from 1975 to 2000 found no significant increase or decrease in the risk of breast cancer with estrogen or estrogen-progestin in 80% of the reports.47
Risk factors for breast cancer (TABLE 1) include family history, obesity, late childbirth, and hormone therapy—but obesity and family history have higher relative risks than the use of HRT.48
TABLE 1
Relative risk of breast cancer
| CHARACTERISTIC | RELATIVE RISK |
|---|---|
| 2 family members with breast cancer | 14 |
| 1 family member with breast cancer | 2.2 |
| Obesity | 1.8 |
| Young age at menarche | 1.6 |
| Hormone therapy | 1.3 |
| >30 years of age at birth of first child | 1.3 |
| Menopause | 0.7 |
WHI arms find different risks
In the widely publicized WHI, women in the estrogen-progestin arm had an overall relative hazard for breast cancer of 1.24 (95% CI 1.01–1.54), but there was no increased risk in women who had never before used hormones.36 Women who had previously used hormones for 5 years or more did have an increased risk.36 The incidence of breast cancer in the study population was 3 cases per 1,000 women, and the excess number was 0.7 more cases with the use of estrogen-progestin (TABLE 2).
Conversely, in the estrogen-only arm of the WHI,16 the relative hazard for breast cancer was 0.77 (95% CI 0.59–1.01), and the reduction in risk was almost statistically significant. There are at least 2 potential explanations for the lower incidence of breast cancer in this arm:
- Without a progestin, estrogen increases breast density only minimally, allowing for easier mammographic interpretation.
- Women susceptible to breast cancer because of their previous use of estrogen may not have been present in the at-risk population in sufficient numbers to cause an increase.
TABLE 2
Extra cases of breast cancer, by risk factor
| RISK FACTOR | BREAST CANCERS DIAGNOSED OVER 20 YEARS FROM AGES 50 TO 70 (PER 1,000) | EXTRA BREAST CANCERS (PER 1,000) |
|---|---|---|
| Never used HRT | 45 | - |
| >5 years HRT | 47 | 2 |
| >10 years HRT | 51 | 6 |
| >15 years HRT | 57 | 12 |
| Late menopause (age 60) | 59 | 14 |
| Alcohol (2 drinks/day) | 72 | 27 |
| No daily exercise | 72 | 27 |
| Weight gain (>20 kg) | 90 | 45 |
| Reprinted from THE LANCET, Vol. 350: 1047–1059, Collaborative Group on Hormonal Factors in Breast Cancer, Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Copyright 1997, with permission from Elsevier | ||
HRT may promote, rather than induce, breast cancer
The role of hormones in the etiology of breast cancer is difficult to assess. The Million Women Study49 found that the elevated risk of breast cancer disappeared within 1 year of stopping HRT. This finding implies that hormones may be a promoter, rather than inducer, of neoplasms in the breast.
Breast cancer may be present in many women, but apparent in few
When autopsies were performed on women in their 40s who had died from other diseases, the incidence of breast cancer was 39%, but the clinical detection rate was only 1% for this population.50 This discrepancy suggests that neoplastic cells may be present in the body at any time, but become clinically apparent only under certain conditions.51
More recent data suggest that undifferentiated stem cells in the breast become dysfunctional and result in cancer.52 This theory is supported by the various histologic types of cancer found in the breast.
A weak link
Although it may be compelling to link hormone use with breast cancer, the association is weak and the incidence is lower than in other known relationships such as obesity. At present, the cause of breast neoplasia appears to be multifactorial.
1. Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:11-25.
2. Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3:47.-
3. Archer DF. Percutaneous 17beta-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
4. Archer DF. Low-dose hormone therapy for postmenopausal women. Clin Obstet Gynecol. 2003;46:317-324.
5. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610-1620.
6. Ballagh SA. Vaginal rings for menopausal symptom relief. Drugs Aging. 2004;21:757-766.
7. Speroff L. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstet Gynecol. 2003;102:823-834.
8. Notelovitz M. Urogenital atrophy and low-dose vaginal estrogen therapy. Menopause. 2000;7:140-142.
9. Utian WH, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. The Esclim Study Group. Am J Obstet Gynecol. 1999;181:71-79.
10. Christiansen C. Effects of drospirenone/estrogen combinations on bone metabolism. Climacteric. 2005;8(suppl 3):35-41.
11. Delmas PD, Confavreux E, Garnero P, et al. A combination of low doses of 17 beta-estradiol and norethisterone acetate prevents bone loss and normalizes bone turnover in postmenopausal women. Osteoporos Int. 2000;11:177-187.
12. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
13. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Bone response to treatment with lower doses of conjugated estrogens with and without medroxyprogesterone acetate in early postmenopausal women. Osteoporos Int. 2005;16:372-379.
14. Ravn P, Bidstrup M, Wasnich RD, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med. 1999;131:935-942.
15. Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Ann Intern Med. 1999;130:897-904.
16. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
17. Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729-1738.
18. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
19. Lacey JV, Jr, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:1724-1731.
20. Archer DF, Furst K, Tipping D, Dain MP, Vandepol C. A randomized comparison of continuous combined transdermal delivery of estradiol-norethindrone acetate and estradiol alone for menopause. CombiPatch Study Group. Obstet Gynecol. 1999;94:498-503.
21. Bouchard P, De Cicco-Nardone F, Spielmann D, Garcea N. Bleeding profile and endometrial safety of continuous combined regimens 1mg 17beta-estradiol/trimegestone versus 1or 2 mg 17beta-estradiol/norethisterone acetate in postmenopausal women. Gynecol Endocrinol. 2005;21:142-148.
22. Kurman RJ, Felix JC, Archer DF, Nanavati N, Arce J, Moyer DL. Norethindrone acetate and estradiol-induced endometrial hyperplasia. Obstet Gynecol. 2000;96:373-379.
23. Speroff L, Rowan J, Symons J, Genant H, Wilborn W. The comparative effect on bone density, endometrium, and lipids of continuous hormones as replacement therapy (CHART study). A randomized controlled trial. JAMA. 1996;276:1397-1403.
24. Sturdee DW, Ulrich LG, Barlow DH, et al. The endometrial response to sequential and continuous combined oestrogen-progestogen replacement therapy. BJOG. 2000;107:1392-1400.
25. Ylikorkala O, Wahlstrom T, Caubel P, Lane R. Intermittent progestin administration as part of hormone replacement therapy: long-term comparison between estradiol 1mg combined with intermittent norgestimate and estradiol 2 mg combined with constant norethisterone acetate. Acta Obstet Gynecol Scand. 2002;81:654-660.
26. Espeland MA, Bush TL, Mebane-Sims I, et al. Rationale, design, and conduct of the PEPI Trial. Postmenopausal Estrogen/Progestin Interventions. Control Clin Trials. 1995;16(suppl):3S-19S.
27. Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453-461.
28. Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756-762.
29. Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941.
30. Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study. a prospective, observational study. Ann Intern Med. 2001;135:1-8.
31. Bush TL, Cowan LD, Barrett-Connor E, et al. Estrogen use and all-cause mortality. Preliminary results from the Lipid Research Clinics Program Follow-Up Study. JAMA. 1983;249:903-906.
32. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55-72.
33. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49-57.
34. Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357-365.
35. Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
36. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
37. Naftolin F, Taylor HS, Karas R, et al. The Women’s Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition. Fertil Steril. 2004;81:1498-1501.
38. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35-44.
39. Clarkson TB, Appt SE. Controversies about HRT-lessons from monkey models. Maturitas. 2005;51:64-74.
40. Wagner JD, Clarkson TB. The applicability of hormonal effects on atherosclerosis in animals to heart disease in postmenopausal women. Semin Reprod Med. 2005;23:149-156.
41. Barrett-Connor E, Laughlin GA. Hormone therapy and coronary artery calcification in symptomatic postmenopausal women: the Rancho Bernardo Study. Menopause. 2005;12:40-48.
42. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939-953.
43. Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522-529.
44. Harman SM, Brinton EA, Cedars M, et al. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3-12.
45. Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276-285.
46. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270-282.
47. Bush TL, Whiteman M, Flaws JA. Hormone replacement therapy and breast cancer: a qualitative review. Obstet Gynecol. 2001;98:498-508.
48. Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control. 2002;13:741-751.
49. Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427.
50. Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237-1243.
51. Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787.-
52. Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 Suppl 1:59-72.
1. Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:11-25.
2. Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes. 2005;3:47.-
3. Archer DF. Percutaneous 17beta-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
4. Archer DF. Low-dose hormone therapy for postmenopausal women. Clin Obstet Gynecol. 2003;46:317-324.
5. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610-1620.
6. Ballagh SA. Vaginal rings for menopausal symptom relief. Drugs Aging. 2004;21:757-766.
7. Speroff L. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstet Gynecol. 2003;102:823-834.
8. Notelovitz M. Urogenital atrophy and low-dose vaginal estrogen therapy. Menopause. 2000;7:140-142.
9. Utian WH, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. The Esclim Study Group. Am J Obstet Gynecol. 1999;181:71-79.
10. Christiansen C. Effects of drospirenone/estrogen combinations on bone metabolism. Climacteric. 2005;8(suppl 3):35-41.
11. Delmas PD, Confavreux E, Garnero P, et al. A combination of low doses of 17 beta-estradiol and norethisterone acetate prevents bone loss and normalizes bone turnover in postmenopausal women. Osteoporos Int. 2000;11:177-187.
12. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
13. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Bone response to treatment with lower doses of conjugated estrogens with and without medroxyprogesterone acetate in early postmenopausal women. Osteoporos Int. 2005;16:372-379.
14. Ravn P, Bidstrup M, Wasnich RD, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med. 1999;131:935-942.
15. Recker RR, Davies KM, Dowd RM, Heaney RP. The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized, controlled trial. Ann Intern Med. 1999;130:897-904.
16. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712.
17. Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729-1738.
18. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
19. Lacey JV, Jr, Brinton LA, Lubin JH, Sherman ME, Schatzkin A, Schairer C. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:1724-1731.
20. Archer DF, Furst K, Tipping D, Dain MP, Vandepol C. A randomized comparison of continuous combined transdermal delivery of estradiol-norethindrone acetate and estradiol alone for menopause. CombiPatch Study Group. Obstet Gynecol. 1999;94:498-503.
21. Bouchard P, De Cicco-Nardone F, Spielmann D, Garcea N. Bleeding profile and endometrial safety of continuous combined regimens 1mg 17beta-estradiol/trimegestone versus 1or 2 mg 17beta-estradiol/norethisterone acetate in postmenopausal women. Gynecol Endocrinol. 2005;21:142-148.
22. Kurman RJ, Felix JC, Archer DF, Nanavati N, Arce J, Moyer DL. Norethindrone acetate and estradiol-induced endometrial hyperplasia. Obstet Gynecol. 2000;96:373-379.
23. Speroff L, Rowan J, Symons J, Genant H, Wilborn W. The comparative effect on bone density, endometrium, and lipids of continuous hormones as replacement therapy (CHART study). A randomized controlled trial. JAMA. 1996;276:1397-1403.
24. Sturdee DW, Ulrich LG, Barlow DH, et al. The endometrial response to sequential and continuous combined oestrogen-progestogen replacement therapy. BJOG. 2000;107:1392-1400.
25. Ylikorkala O, Wahlstrom T, Caubel P, Lane R. Intermittent progestin administration as part of hormone replacement therapy: long-term comparison between estradiol 1mg combined with intermittent norgestimate and estradiol 2 mg combined with constant norethisterone acetate. Acta Obstet Gynecol Scand. 2002;81:654-660.
26. Espeland MA, Bush TL, Mebane-Sims I, et al. Rationale, design, and conduct of the PEPI Trial. Postmenopausal Estrogen/Progestin Interventions. Control Clin Trials. 1995;16(suppl):3S-19S.
27. Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453-461.
28. Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756-762.
29. Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941.
30. Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study. a prospective, observational study. Ann Intern Med. 2001;135:1-8.
31. Bush TL, Cowan LD, Barrett-Connor E, et al. Estrogen use and all-cause mortality. Preliminary results from the Lipid Research Clinics Program Follow-Up Study. JAMA. 1983;249:903-906.
32. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55-72.
33. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49-57.
34. Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357-365.
35. Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523-534.
36. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
37. Naftolin F, Taylor HS, Karas R, et al. The Women’s Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition. Fertil Steril. 2004;81:1498-1501.
38. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35-44.
39. Clarkson TB, Appt SE. Controversies about HRT-lessons from monkey models. Maturitas. 2005;51:64-74.
40. Wagner JD, Clarkson TB. The applicability of hormonal effects on atherosclerosis in animals to heart disease in postmenopausal women. Semin Reprod Med. 2005;23:149-156.
41. Barrett-Connor E, Laughlin GA. Hormone therapy and coronary artery calcification in symptomatic postmenopausal women: the Rancho Bernardo Study. Menopause. 2005;12:40-48.
42. Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939-953.
43. Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522-529.
44. Harman SM, Brinton EA, Cedars M, et al. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3-12.
45. Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276-285.
46. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270-282.
47. Bush TL, Whiteman M, Flaws JA. Hormone replacement therapy and breast cancer: a qualitative review. Obstet Gynecol. 2001;98:498-508.
48. Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control. 2002;13:741-751.
49. Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427.
50. Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237-1243.
51. Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787.-
52. Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 Suppl 1:59-72.
Estradiol gel: A new option in hormone replacement therapy
- Percutaneous estradiol gel can be prescribed at low doses.
- Relief of menopausal symptoms can begin as early as 2 weeks after starting treatment.
- Treatment with estradiol gel maintains or increases bone mineral density.
- No large randomized, controlled trials have explored the effect of estradiol gel on coronary artery disease. It appears to have metabolic effects similar to those of oral estradiol.
- Because percutaneous estradiol stimulates the endometrium, women with an intact uterus should also take a progestogen.
Although the Women’s Health Initiative1 discouraged many menopausal women from using oral estrogen, it failed to address the risks of treatment with other dosages or forms of estrogen and progestin.
Nor has any other trial of similar size and complexity taken up the issue. However, numerous smaller studies have been published.
This article summarizes findings on percutaneous delivery of estradiol gel (EstroGel), the most recently FDA-approved estrogen option for treatment of menopausal symptoms.
It describes the overall safety of estradiol gel, as well as its effectson:
- menopausal symptoms,
- bone,
- metabolism, and
- endometrium.
(In this article, “percutaneous delivery” refers to estradiol gel applied to the skin, and “transdermal estradiol” indicates delivery via a transdermal reservoir or matrix system, otherwise known as “the patch.” I have used an arbitrary definition to distinguish the gel from other methods of delivering estradiol across the skin. For example, Estrasorb is a liposomal formulation that is applied to the skin of the thigh. Although it is a percutaneous estradiol similar to the gel, this article focuses only on the latter option.)
Easy to apply, few skin reactions
Percutaneous estradiol gel formulations have been available for almost 30 years in Europe, where they are utilized by a majority of women on hormone therapy. In the United States, the hydroalcoholic gel is packaged in a pump that delivers 64 standardized 1.25-g doses, which contain 0.75 mg of 17ß-estradiol. Once it is applied, the gel is absorbed into an intradermal reservoir (FIGURE) and dries in 2 to 5 minutes, leaving no residue.
Patient selection. Estradiol gel is well-suited for patients who are concerned about the risks of oral estrogen (as portrayed in the mainstream press following the Women’s Health Initiative) and want to avoid that route of administration, as well as women who dislike or have difficulty swallowing pills. Percutaneous administration also is appropriate for physically active women who may have adhesion problems or skin irritation with the transdermal patch, or those who have had reactions to local adhesives in the past. The patient should be motivated to apply the gel on a daily basis.
Indications are moderate to severe vasomotor symptoms in menopausal women, and moderate to severe symptoms of vulvar and vaginal atrophy, although topical vaginal products should also be considered for the latter indication.
Contraindications are undiagnosed abnormal vaginal bleeding; history of breast cancer, other estrogen-dependent malignancy, stroke, heart attack, or liver disease or dysfunction; active thrombophlebitis or thromboembolic disorders (or a history of these); and known or suspected pregnancy.
Common side effects include headache, breast pain, irregular vaginal bleeding or spotting, stomach cramps or bloating, nausea and vomiting, and hair loss.
- Skin reactions are infrequent, but should be taken into account when discussing per-cutaneous or transdermal delivery of any drug. However, estradiol gel appears to cause fewer skin reactions than the patch. In a study over more than 5 years, 0 of 157 women treated with percutaneous estradiol reported skin irritation.2 Other comparisons found similar outcomes.3,4
Onset of action is rapid, as it is with the transdermal patch.5 Dose variability is minimized when the gel is applied at the same time every day to a large area of skin, preferably the arm, although all application sites appear to produce similar results: abdomen, shoulders, arms, and inner thigh.6 The gel should not be applied to the breast or vagina.
Diminished effect with skin washing. In 1 trial, site washing 1/2 hour after application significantly decreased bioavailability and time to reach peak plasma concentrations.7 For this reason, the gel should be not applied before a bath, shower, or sauna.
Dosing options. The initial dose is 1.25 mg of gel, which is 1 pump of the bottle. The gel is collected in the palm of 1 hand and applied to the skin of the opposite arm from the wrist to the shoulder. The dose can be titrated by adding a second pump of the gel and applying it to the opposite arm. The dose can be lowered by using less than a full depression of the pump.
Stable, physiologic estrogen levels
Estradiol gel produces relatively stable serum estradiol levels, and therapeutic estradiol levels similar to those seen with other formulations, routes of administration, and dosages.
Percutaneous administration produces serum estrone to estradiol ratios close to 1, in contrast to higher ratios (5:1) with oral administration.8-11 The lower ratio approximates levels during the menstrual cycle of premenopausal women. (TABLE 1 gives estradiol and estrone levels from different studies following administration of estradiol as a gel, oral formulation, and transdermal patch.)
The percutaneous route also allows for delivery directly to the systemic circulation, avoiding gastrointestinal and first-pass hepatic metabolism and elimination. In contrast, oral micronized estradiol causes large fluctuations in serum estradiol and estrone levels due to absorption and metabolism.8
More stable serum levels than with the patch. One study12 comparing percutaneous and transdermal estradiol found similar interindividual variability but less stable serum levels in women using the transdermal system. A separate study13 also reported greater fluctuation of serum estradiol levels in women using a transdermal system than in those using the gel.
TABLE 1
Serum estradiol and estrone levels for various routes of estradiol
| AUTHOR | THERAPY | ESTRADIOL LEVEL (PG/ML) | ESTRONE LEVEL (PG/ML) |
|---|---|---|---|
| Scott et al8 | E2gel 3 mg/day | 102.9±39.9 | 120.0±50.5 |
| E2gel 1.5 mg/day | 68.1±27.4 | 90.6±45.7 | |
| Transdermal estradiol 50 μg/day | 41.1±13.5 | 45.0±15.9 | |
| Oral micronized estradiol 2 mg/day | 114.0±65.2 | 575.2±279.9 | |
| Palacios et al10 | E2gel 1.5 mg/day | 75.7±1.0 | 58.5±2.9 |
| Oral conjugated estrogen 0.625 mg/day | 39.6±4.6 | 126.0±7.6 | |
| Basdevant et al11 | E2gel 3 mg/day | 221.0±35.0 | 146.0±19.0 |
| Oral micronized estradiol 2 mg/day | 121.0±27.0 | 811.0±370.0 | |
| Archer15 | E2gel 0.75 mg/day | 33.5* | 49.0* |
| E2gel 1.5 mg/day | 65.0* | 58.0* | |
| Placebo | 5.0* | 23.0* | |
| *Median value | |||
| E2gel = percutaneous estradiol gel | |||
Studies compared relief of menopausal symptoms
Estradiol gel effectively relieved meno-pausal symptoms in randomized, double-blind studies, open label comparisons, and observational trials in postmenopausal women, with and without the addition of various progestins.
Symptom relief in comparison with baseline values is statistically significant as early as 2 weeks after initiating treatment. Relief of up to 2 years’ duration has been reported.3,14
Several studies have compared symptom relief achieved with estradiol gel, oral estrogen, transdermal delivery systems, and placebo3,9,14-18; findings are summarized in TABLE 2.
Same efficacy when progestogen is added. The following studies, and other studies,14 demonstrated that estradiol gel relieves menopausal symptoms whether it is administered alone or in combination with a progestogen, with efficacy similar to other estrogen formulations:
Climacteric symptoms decreased to the same extent when estradiol gel was combined with a levonorgestrel-releasing intrauterine device, oral micronized progesterone, or vaginal micronized progesterone.19
A study20 that added lynestrenol decreased the frequency of hot flushes and night sweats more than in women using estradiol gel alone. However, negative mood symptoms were more pronounced in the progestin-treated group.
Estradiol gel 1 mg/day in combination with monthly or quarterly oral medroxyprog-esterone acetate reduced the severity of hot flushes, sweating, and vaginal dryness, according to a 12-month trial.21
Symptoms decreased the same whether a levonorgestrel-releasing IUD or oral or vaginal progesterone was added.
Bone mineral density maintained or increased
Several randomized, controlled trials have documented the effects of estradiol gel on bone mineral density (BMD) and various markers of bone metabolism. In these studies, BMD remained steady22,23 or increased10,24,25 following treatment, and estradiol gel remained effective for up to 4 years.25 Estradiol gel maintained or increased BMD with or without addition of progestins.22,23,26
These investigations involved measurement of BMD at the lumbar spine, forearms, or hip, as well as biologic markers of bone turnover such as urinary hydroxyproline/creatinine ratio, serum alkaline phosphatase, and serum osteocalcin.
Serum estradiol and skeletal uptake of a bone-seeking agent also were determined. Estradiol gel regimens ranged from 0.75 mg/day to 3 mg/day, and populations included both surgical and natural menopausal subjects in several countries.
Effects comparable to oral estrogen. Compared with oral conjugated estrogen, which increased BMD at the lumbar spine by 4.3% (±3.2%), estradiol gel produced increases of 5.6% (±2.9%) at 24 months and 4.7% (±3.2%) at 36 months.10
The case. A 52-year-old woman with no menses for 8 months presents for management complaining of disabling hot flushes. Although she is moderately obese, with a body mass index of 29, she is normotensive without any other significant medical history except for hysterectomy at age 44 for excessive bleeding.
Counseling. During her 20s, the patient tried to use oral contraceptives on 3 separate occasions, but was unable to continue them because of nausea. Although she is interested in estrogen therapy for her vasomotor symptoms, she is concerned about the possibility of experiencing nausea again. You explain that one of the benefits of percutaneous estradiol is that it avoids the gastrointestinal tract.
Physical findings. Her physical examination is within normal limits, and gynecologic examination confirms no uterus and finds no palpable adnexal masses.
Outcome. After weighing the pros and cons, she elects to use estradiol gel. At her 3-month follow-up, she reports effective relief and good compliance.
Minimum level of protection achieved. Following a comparison of oral and percutaneous estradiol, Reginster et al27 suggested that a minimum estradiol level of 60 pg/mL is necessary to prevent postmenopausal bone loss. Mean serum estradiol levels in women receiving 1.5 mg/day estradiol gel were 75.7 pg/mL and 78.4 pg/mL at 24 and 36 months, respectively, in a study by Palacios et al,10 and 85.8 pg/mL in a study by Devogelaer and colleagues.24 In these trials, BMD increased, and it remained steady in other investigations.28,29
More recent trials suggest that lower serum estradiol levels secondary to smaller estrogen doses have the capacity to maintain BMD.30,31 A 1.9% mean increase of BMD at the lumbar spine was reported in women with a mean serum concentration of 17 pg/mL in a 2-year study30 of a transdermal estradiol delivery system. The percutaneous route does not appear to limit these beneficial effects.
How are metabolic factors affected?
In general, oral estrogens produce beneficial changes in lipid metabolism, particularly higher levels of high-density lipoprotein (HDL) cholesterol. However, they also elevate triglyceride and glucose levels. How this plays out clinically is unclear. Both the Women’s Health Initiative and the Heart and Estrogen/Progestin Replacement Study (HERS) found no cardioprotective effects of oral estrogen despite increases in HDL and decreases in low-density lipoprotein (LDL) levels.1,32
Oral versus percutaneous estradiol. No long-term studies of this magnitude have investigated the effect of percutaneous estradiol on coronary artery disease, although numerous clinical trials have shown that the route of estrogen delivery affects many of the metabolic variables used to estimate the risk of negative cardiac outcomes. It remains to be determined whether percutaneous estradiol affects these metabolic factors in ways that influence morbidity and mortality.
In 1 trial,33 oral conjugated estrogen led to the following significant changes in lipids:
- increase in very low density lipoprotein (VLDL) cholesterol,
- decrease in LDL cholesterol (but not the LDL apoprotein B),
- increase in HDL and apoprotein A1, and
- significant increase in HDL2 cholesterol.
In a separate study,9 oral conjugated estrogen had a 2.5-fold increase in serum angiotensin, and percutaneous estradiol gel, no effect.
Same effects when progestin is added. Beneficial effects were not diminished when oral micronized progesterone was added to percutaneous estradiol.34 Estradiol gel significantly reduced total serum cholesterol and LDL cholesterol in the first year of treatment, compared with placebo.
Coagulation effects. Percutaneous estradiol has fewer negative effects on coagulation factors than its oral counterpart. One study35 investigating combination therapy with micronized progesterone compared the effects of percutaneous estradiol and oral estradiol valerate. The group receiving percutaneous estradiol gel/micronized progesterone had no significant changes in plasminogen activator inhibitor, tissue-type plasminogen concentration, and global fibrinolytic capacity. The other group had a significant decrease in mean tissue-type plasminogen concentration and plasminogen activator inhibitor activity and a significant rise in global fibrinolytic capacity.
The oral estradiol—but not the percutaneous formulation—significantly increased the mean value of prothrombin activation peptide and decreased mean antithrombin activity compared with no treatment.
Poor glycemic control may increase the risk of cardiovascular disease, but oral and percutaneous estradiol appear to have similar glycemic effects. A comparative trial36 of oral estradiol valerate 2 mg/day and percutaneous estradiol 1 mg/day found no differences in glycosylated hemoglobin A1c levels (declined in both groups) or in fasting and 2-hour post-prandial blood glucose levels (constant in both groups) or insulin sensitivity.
Treatment duration may also influence how percutaneous estradiol affects metabolic factors. An open-label longitudinal prospective study37 of 30 women receiving estradiol gel 1.5 mg/day for 6 months found a significant decrease in lipoprotein (a), apoprotein A-I, apoprotein B, HDL cholesterol, and HDL3 cholesterol. At 1 year, however, these changes were not significant.
No association with venous thromboembolism. Transdermal estradiol administered as a patch or percutaneous gel had no effect on the risk of venous thromboembolism in a multicenter case-control investigation.38
In contrast, a recent retrospective study found a risk of venous thromboembolism that was at least 4 times greater with oral estrogen than with transdermal estradiol.38
Use a progestogen to prevent endometrial hyperplasia
Like other forms of estrogen, percutaneous estradiol stimulates the endometrium. For this reason, women who have an intact uterus should use a progestogen in an adequate dose to prevent hyperplastic changes.39 Although numerous regimens appear to be effective, the optimal route, dose, and duration of progestin in women using percutaneous estrogen remain to be determined.
Percutaneous estradiol versus other routes of administration. Endometrial thickness and amenorrhea rates over 6 months were not statistically different among 54 women treated with estradiol gel 1.5 mg/day, transdermal estradiol 50 μg/day, or oral estradiol valerate 2 mg/day—all in combination with nomegestrol acetate 2.5 mg/day.40 The overall rates of no bleeding or spotting over 6 cycles of treatment were 78% in the percutaneous estradiol group, 48% in the transdermal group, and 60% in the oral group.
Although the percutaneous estradiol group had a lower overall incidence of no bleeding or spotting than the other groups, the difference was not significant. Nor were there significant differences in the variation of endometrial thickness from baseline: A mean increase of 1.5 mm (±0.4 mm) was reported in the percutaneous group, compared with 1.5 mm (±0.7 mm) and 1.7 mm (±0.6 mm) in the transdermal and oral estrogen groups, respectively.
Estrogenic and progestogenic effects were similar for transdermal and percutaneous estradiol (with dydrogesterone 10 mg/day for days 1 to 12) after a baseline atropic endometrium was identified.3
Estradiol gel produces stable, physiologic serum estradiol levels and has a serum estrone to estradiol ratio close to 1.
The most effective progestogen dosage and duration are unknown, although many regimens have been studied:
- Percutaneous estradiol 1.5 mg/day for the first 24 days of the month in combination with nomegestrol acetate 5 mg/day for days 11 to 24: Over 6 months, researchers found a secretory pattern in the majority of women and no evidence of hyperplasia.18
- Percutaneous estradiol 3 mg/day for 3 of every 4 weeks in combination with nomegestrol acetate 5 mg/day for 10 days: No reduction in estrogenic endometrial effects.41,42
- Estradiol gel 3 mg/day for 3 of every 4 weeks in combination with 200 mg or 300 mg oral micronized progesterone for the last 10 days of treatment: Dose was too low or treatment too short to produce a complete secretory transformation of the endometrium.43 However, the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial44 found no increased occurrence of hyperplasia in women using micronized progesterone 200 mg for 12 days of each cycle for more than 3 years in combination with oral conjugated estrogen 0.625 mg.
- Percutaneous estradiol 1.5 mg/day combined with micronized progesterone 100 mg daily (orally or vaginally) for the first 25 days of the month: Fully inhibited mitoses and induced amenorrhea in most of the women studied, with amenorrhea rates of 93.3% at 3 months and 91.6% at 6 months.45
- Estradiol gel 1.5 mg/day with 100 mg vaginal micronized progesterone for 21 days per cycle: Stable endometrial thickness and no endometrial hyperplasia over 12 months.46 However, breakthrough bleeding was reported in up to 30% of subjects, principally in the second 3 months of treatment.
- Percutaneous estradiol 1 mg/day for 3 months with oral medroxyprogesterone acetate 20 mg/day for the last 14 days, followed by a 7-day free interval: No reports of endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 2 mg/day for 21 days with 10 mg oral medroxyprogesterone acetate for the last 14 days, followed by a 7-day free interval: No endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 1 mg/day with medroxyprogesterone acetate 10 mg on days 1-12 every month or every 3 months: Endometrial hyperplasia was found in 1 woman (0.3%) in the group receiving the progestogen every 3 months. Endometrial histology did not differ between women taking medroxyprogesterone monthly and those taking it every 3 months.21
- Estradiol gel 3 mg/day with oral micronized progesterone 200 mg for 12 days of each cycle: Regular withdrawal bleeding in 70% of women.34
- Percutaneous estradiol 1.5 mg/day in combination with a levonorgestrel-releasing intrauterine device: 80% of women were amenorrheic at 1 year, with a mean endometrial thickness 3 mm; at 5 years, 100% of women had epithelial atrophy.47
- Estradiol gel 1.5 mg for 21 days with 200 mg oral progesterone for 14 days (126 women); 3 mg percutaneous estradiol for 21 days with 300 mg oral progesterone for 10 days (23 women); 1.5 mg estradiol gel with 300 mg oral progesterone (3 women); or 3 mg estradiol gel for 28 days with 200 mg progesterone for 14 days (5 women): No evidence of hyperplasia after 5 years of treatment.2
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
2. Moyer DL, de Lignieres B, Driguez P, et al. Prevention of endometrial hyperplasia by progesterone during long-term estradiol replacement: influence of bleeding pattern and secretory changes. Fertil Steril. 1993;59:992-997.
3. Hirvonen E, Cacciatore B, Wahlström T, et al. Effects of transdermal oestrogen therapy in postmenopausal women: a comparative study of an oestradiol gel and an oestradiol delivering patch. BJOG. 1997;104(Suppl 16):26-31.
4. Travassos de Figueiredo Alves S, et al. Comparison of gel and patch estradiol replacement in Brazil, a tropical country. Maturitas. 2000;36:69-74.
5. Henzl MR, Loomba PK. Transdermal delivery of sex steroids for hormone replacement therapy and contraception. A review of principles and practice. J Reprod Med. 2003;48:525-540.
6. Holst J, et al. Serum estrogen levels after topic application of estradiol-17 beta on two different cutaneous areas. Acta Obstet Gynecol Scand. 1987;662:151-152.
7. Jarvinen A, Granander M, Nykanen S, Laine T, Geurts P, Viitanen A. Steady-state pharmacokinetics of oestradiol gel in post-menopausal women: effects of application area and washing. Br J Obstet Gynaecol. 1997;104(Suppl 16):14-18.
8. Scott RT, Ross B, Anderson C, Archer DF. Pharmacokinetics of percutaneous estradiol: a crossover study using a gel and a transdermal system in comparison with oral micronized estradiol. Obstet Gynecol. 1991;77:758-764.
9. Dupont A, Dupont P, Cusan M, et al. Comparative endocrinological and clinical effects of percutaneous estradiol and oral conjugated estrogens as replacement therapy in menopausal women. Maturitas. 1991;13:297-311.
10. Palacios S, Menéndez C, Jurado AR, Vargas JC. Effects of percutaneous oestradiol versus oral oestrogens on bone density. Maturitas. 1995;20:209-213.
11. Basdevant A, de Lignieres B, Simon P, et al. Hepatic lipase activity during oral and parenteral 17ß-estradiol replacement therapy: high-density lipoprotein increase may not be antiatherogenic. Fertil Steril. 1991;55:1112-1117.
12. Simon JA, et al. Are there significant differences between patch and gel cutaneous estradiol therapy? In: Genazzani AR, Petraglia F, Volpe A, Facchinetti F, eds. Recent Research on Gynecological Endocrinology. Casterton Hall, Carnforth, Lancashire, UK: Parthenon Publishing, Casterton Hall; 1998;317-324.
13. Paoletti AM, et al. Comparison of pharmacokinetic profiles of 17ß-estradiol gel 0.6mg/g (Gelestra) with a transdermal delivery system (Estraderm TTS 50) in postmenopausal women at steady state. Maturitas. 2001;40:203-209.
14. Jensen PB, Jensen J, Riis BJ, et al. Climacteric symptoms after oral and percutaneous hormone replacement therapy. Maturitas. 1987;9:207-215.
15. Archer DF. for the EstroGel Study Group. Percutaneous 17ß-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
16. Kornafel KL, March CM. Estradiol gel in the treatment of menopausal symptoms: a placebo-controlled double-blind case study of efficacy and safety. South Med J. 1992;85:270-273.
17. Hirvonen E, Lamberg-Allardt C, Lankinen KS, Geurts P, Wilén-Rosenqvist G. Transdermal oestradiol gel in the treatment of the climacterium: a comparison with oral therapy. BJOG. 1997;104(Suppl 16):19-25.
18. Foidart JM, Béliard A, Hedon B, et al. Impact of percutaneous oestradiol gels in postmenopausal hormone replacement therapy on clinical symptoms and endometrium. BJOG. 1997;104:305-310.
19. Suvanto-Luukkonen E, Sundström H, Penttinen J, et al. Percutaneous estradiol gel with an intrauterine levonorgestrel releasing device or natural progesterone in hormone replacement therapy. Maturitas. 1997;26:211-17.
20. Holst J, et al. Progestogen addition during oestrogen replacement therapy—effects on vasomotor symptoms and mood. Maturitas. 1989;11:13-20.
21. Hirvonen E, et al. Effect of an estradiol gel with monthly or quarterly progestogen on menopausal symptoms and bleeding. Climacteric. 2000;3:262-270.
22. Ng HT, Chang SP, Yang TS, Cho MP, Wei TC. Estradiol administered in a percutaneous gel for the prevention of postmenopausal bone loss. Asia-Oceania J Obstet Gynaecol. 1993;19(2):115-119.
23. Riis B, Thomsen K, et al. Does calcium supplementation prevent postmenopausal bone loss? A double blind, controlled clinical study. N Engl J Med. 1987;316:173-177.
24. Devogelaer JP, Lecart C, Dupret P, De Nayer P, Nagant De Deuxchaisnes C. Long-term effects of percutaneous estradiol on bone loss and bone metabolism in post-menopausal hysterectomized women. Maturitas. 1998;28:243-249.
25. Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four year randomized study. Am J Med. 1995;99:36-42.
26. Sun A, Lin S, Yu W, et al. Percutaneous estrogen in prevention of early post-menopausal bone loss in Chinese women. Chin Med J. 2002;115:1790-1795.
27. Reginster JY, Sarlet N, Deroisy R, et al. Minimum levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
28. Cicinelli E, Cantatore FP, Galantino P, et al. Effects of continuous percutaneous estradiol administration on skeletal turnover in postmenopausal women: a 1-year prospective controlled study. Eur J Obstet Gynecol Reprod Biol. 1996;69(2):109-113.
29. Riis B, et al. The effect of percutaneous estradiol and natural progesterone on post-menopausal bone loss. Am J Obstet Gynecol. 1987;156:61-65.
30. Notelvitz M, John VA, Good WR. Effectiveness of Alora estradiol matrix transdermal delivery system in improving lumbar bone mineral density in healthy, post-menopausal women. Menopause. 2002;9:343-353.
31. Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17ß-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042-1048.
32. Hully S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
33. Moorjani S, Dupont A, Labrie F, et al. Changes in plasma lipoprotein and apolipoprotein composition in relation to oral versus percutaneous administration of estrogen alone or in cyclic association with Utrogestan in menopausal women. J Clin Endocrinol Metab. 1991;73:373-379.
34. Jensen J, Riis BJ, Strøm V, Nilas L, Christiansen C. Long-term effects of percutaneous estrogens and oral progesterone on serum lipoproteins in postmenopausal women. Am J Obstet Gyencol. 1987;156:66-71.
35. Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17:3071-3078.
36. Karjalainen A, Paassilta M, Heikkinen J, et al. Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clin Endocrin. 2001;54:165-173.
37. Haines CJ, Chung TKH, Masarei JRL, Tomlinson B, Lau JTF. The effect of per-cutaneous oestrogen replacement therapy on Lp(a) and other lipoproteins. Maturitas. 1995;22:219-225.
38. Scarabin PY, Oger E, Plu-Bureau G. EStrogen and THromboEmbolism Risk (ESTHER) Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428-432.
39. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
40. Blanc B, Cravello L, Micheletti MC, et al. Continuous hormone replacement therapy for menopause combining nomegestrol acetate and gel, patch, or oral estrogen: a comparison of amenorrhea rates. Clin Therap. 1998;20(5):901-912.
41. Holst J. Percutaneous estrogen therapy. Endometrial response and metabolic effects. Acta Obstet Gynecol Scand Suppl. 1983;115:1-30
42. Holst J, Cajander S, von Schoultz B. Cellular morphometric analysis of the post-menopausal endometrium during treatment with percutaneous estradiol-17ß with and without oral gestagen. Acta Obstet Gynecol Scand. 1983;62:267-270.
43. Holst J, Cajander S, von Schoultz B. Endometrial response in post-menopausal women during treatment with percutaneous 17ß-oestradiol opposed by oral progesterone. Maturitas. 1986;8:201-207.
44. Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370-375.
45. Gillet JY, Andre G, Faguer B, et al. Induction of amenorrhea during hormone replacement therapy: optimized micronized progesterone dose. A multicenter study. Maturitas. 1994;19:103-115.
46. Vilodre LC, et al. Endometrial response to a cyclic regimen of percutaneous 17ß-estradiol and low-dose vaginal micronized progesterone in women with mild-to-moderate hypertension. Gynecol Endocrinol. 2003;17:323-328.
47. Suvanto-Luukkonen E, Kauppila A. The levonorgestrel intrauterine system in menopausal hormone replacement therapy: five-year experience. Fertil Steril. 1999;72:161-163.
- Percutaneous estradiol gel can be prescribed at low doses.
- Relief of menopausal symptoms can begin as early as 2 weeks after starting treatment.
- Treatment with estradiol gel maintains or increases bone mineral density.
- No large randomized, controlled trials have explored the effect of estradiol gel on coronary artery disease. It appears to have metabolic effects similar to those of oral estradiol.
- Because percutaneous estradiol stimulates the endometrium, women with an intact uterus should also take a progestogen.
Although the Women’s Health Initiative1 discouraged many menopausal women from using oral estrogen, it failed to address the risks of treatment with other dosages or forms of estrogen and progestin.
Nor has any other trial of similar size and complexity taken up the issue. However, numerous smaller studies have been published.
This article summarizes findings on percutaneous delivery of estradiol gel (EstroGel), the most recently FDA-approved estrogen option for treatment of menopausal symptoms.
It describes the overall safety of estradiol gel, as well as its effectson:
- menopausal symptoms,
- bone,
- metabolism, and
- endometrium.
(In this article, “percutaneous delivery” refers to estradiol gel applied to the skin, and “transdermal estradiol” indicates delivery via a transdermal reservoir or matrix system, otherwise known as “the patch.” I have used an arbitrary definition to distinguish the gel from other methods of delivering estradiol across the skin. For example, Estrasorb is a liposomal formulation that is applied to the skin of the thigh. Although it is a percutaneous estradiol similar to the gel, this article focuses only on the latter option.)
Easy to apply, few skin reactions
Percutaneous estradiol gel formulations have been available for almost 30 years in Europe, where they are utilized by a majority of women on hormone therapy. In the United States, the hydroalcoholic gel is packaged in a pump that delivers 64 standardized 1.25-g doses, which contain 0.75 mg of 17ß-estradiol. Once it is applied, the gel is absorbed into an intradermal reservoir (FIGURE) and dries in 2 to 5 minutes, leaving no residue.
Patient selection. Estradiol gel is well-suited for patients who are concerned about the risks of oral estrogen (as portrayed in the mainstream press following the Women’s Health Initiative) and want to avoid that route of administration, as well as women who dislike or have difficulty swallowing pills. Percutaneous administration also is appropriate for physically active women who may have adhesion problems or skin irritation with the transdermal patch, or those who have had reactions to local adhesives in the past. The patient should be motivated to apply the gel on a daily basis.
Indications are moderate to severe vasomotor symptoms in menopausal women, and moderate to severe symptoms of vulvar and vaginal atrophy, although topical vaginal products should also be considered for the latter indication.
Contraindications are undiagnosed abnormal vaginal bleeding; history of breast cancer, other estrogen-dependent malignancy, stroke, heart attack, or liver disease or dysfunction; active thrombophlebitis or thromboembolic disorders (or a history of these); and known or suspected pregnancy.
Common side effects include headache, breast pain, irregular vaginal bleeding or spotting, stomach cramps or bloating, nausea and vomiting, and hair loss.
- Skin reactions are infrequent, but should be taken into account when discussing per-cutaneous or transdermal delivery of any drug. However, estradiol gel appears to cause fewer skin reactions than the patch. In a study over more than 5 years, 0 of 157 women treated with percutaneous estradiol reported skin irritation.2 Other comparisons found similar outcomes.3,4
Onset of action is rapid, as it is with the transdermal patch.5 Dose variability is minimized when the gel is applied at the same time every day to a large area of skin, preferably the arm, although all application sites appear to produce similar results: abdomen, shoulders, arms, and inner thigh.6 The gel should not be applied to the breast or vagina.
Diminished effect with skin washing. In 1 trial, site washing 1/2 hour after application significantly decreased bioavailability and time to reach peak plasma concentrations.7 For this reason, the gel should be not applied before a bath, shower, or sauna.
Dosing options. The initial dose is 1.25 mg of gel, which is 1 pump of the bottle. The gel is collected in the palm of 1 hand and applied to the skin of the opposite arm from the wrist to the shoulder. The dose can be titrated by adding a second pump of the gel and applying it to the opposite arm. The dose can be lowered by using less than a full depression of the pump.
Stable, physiologic estrogen levels
Estradiol gel produces relatively stable serum estradiol levels, and therapeutic estradiol levels similar to those seen with other formulations, routes of administration, and dosages.
Percutaneous administration produces serum estrone to estradiol ratios close to 1, in contrast to higher ratios (5:1) with oral administration.8-11 The lower ratio approximates levels during the menstrual cycle of premenopausal women. (TABLE 1 gives estradiol and estrone levels from different studies following administration of estradiol as a gel, oral formulation, and transdermal patch.)
The percutaneous route also allows for delivery directly to the systemic circulation, avoiding gastrointestinal and first-pass hepatic metabolism and elimination. In contrast, oral micronized estradiol causes large fluctuations in serum estradiol and estrone levels due to absorption and metabolism.8
More stable serum levels than with the patch. One study12 comparing percutaneous and transdermal estradiol found similar interindividual variability but less stable serum levels in women using the transdermal system. A separate study13 also reported greater fluctuation of serum estradiol levels in women using a transdermal system than in those using the gel.
TABLE 1
Serum estradiol and estrone levels for various routes of estradiol
| AUTHOR | THERAPY | ESTRADIOL LEVEL (PG/ML) | ESTRONE LEVEL (PG/ML) |
|---|---|---|---|
| Scott et al8 | E2gel 3 mg/day | 102.9±39.9 | 120.0±50.5 |
| E2gel 1.5 mg/day | 68.1±27.4 | 90.6±45.7 | |
| Transdermal estradiol 50 μg/day | 41.1±13.5 | 45.0±15.9 | |
| Oral micronized estradiol 2 mg/day | 114.0±65.2 | 575.2±279.9 | |
| Palacios et al10 | E2gel 1.5 mg/day | 75.7±1.0 | 58.5±2.9 |
| Oral conjugated estrogen 0.625 mg/day | 39.6±4.6 | 126.0±7.6 | |
| Basdevant et al11 | E2gel 3 mg/day | 221.0±35.0 | 146.0±19.0 |
| Oral micronized estradiol 2 mg/day | 121.0±27.0 | 811.0±370.0 | |
| Archer15 | E2gel 0.75 mg/day | 33.5* | 49.0* |
| E2gel 1.5 mg/day | 65.0* | 58.0* | |
| Placebo | 5.0* | 23.0* | |
| *Median value | |||
| E2gel = percutaneous estradiol gel | |||
Studies compared relief of menopausal symptoms
Estradiol gel effectively relieved meno-pausal symptoms in randomized, double-blind studies, open label comparisons, and observational trials in postmenopausal women, with and without the addition of various progestins.
Symptom relief in comparison with baseline values is statistically significant as early as 2 weeks after initiating treatment. Relief of up to 2 years’ duration has been reported.3,14
Several studies have compared symptom relief achieved with estradiol gel, oral estrogen, transdermal delivery systems, and placebo3,9,14-18; findings are summarized in TABLE 2.
Same efficacy when progestogen is added. The following studies, and other studies,14 demonstrated that estradiol gel relieves menopausal symptoms whether it is administered alone or in combination with a progestogen, with efficacy similar to other estrogen formulations:
Climacteric symptoms decreased to the same extent when estradiol gel was combined with a levonorgestrel-releasing intrauterine device, oral micronized progesterone, or vaginal micronized progesterone.19
A study20 that added lynestrenol decreased the frequency of hot flushes and night sweats more than in women using estradiol gel alone. However, negative mood symptoms were more pronounced in the progestin-treated group.
Estradiol gel 1 mg/day in combination with monthly or quarterly oral medroxyprog-esterone acetate reduced the severity of hot flushes, sweating, and vaginal dryness, according to a 12-month trial.21
Symptoms decreased the same whether a levonorgestrel-releasing IUD or oral or vaginal progesterone was added.
Bone mineral density maintained or increased
Several randomized, controlled trials have documented the effects of estradiol gel on bone mineral density (BMD) and various markers of bone metabolism. In these studies, BMD remained steady22,23 or increased10,24,25 following treatment, and estradiol gel remained effective for up to 4 years.25 Estradiol gel maintained or increased BMD with or without addition of progestins.22,23,26
These investigations involved measurement of BMD at the lumbar spine, forearms, or hip, as well as biologic markers of bone turnover such as urinary hydroxyproline/creatinine ratio, serum alkaline phosphatase, and serum osteocalcin.
Serum estradiol and skeletal uptake of a bone-seeking agent also were determined. Estradiol gel regimens ranged from 0.75 mg/day to 3 mg/day, and populations included both surgical and natural menopausal subjects in several countries.
Effects comparable to oral estrogen. Compared with oral conjugated estrogen, which increased BMD at the lumbar spine by 4.3% (±3.2%), estradiol gel produced increases of 5.6% (±2.9%) at 24 months and 4.7% (±3.2%) at 36 months.10
The case. A 52-year-old woman with no menses for 8 months presents for management complaining of disabling hot flushes. Although she is moderately obese, with a body mass index of 29, she is normotensive without any other significant medical history except for hysterectomy at age 44 for excessive bleeding.
Counseling. During her 20s, the patient tried to use oral contraceptives on 3 separate occasions, but was unable to continue them because of nausea. Although she is interested in estrogen therapy for her vasomotor symptoms, she is concerned about the possibility of experiencing nausea again. You explain that one of the benefits of percutaneous estradiol is that it avoids the gastrointestinal tract.
Physical findings. Her physical examination is within normal limits, and gynecologic examination confirms no uterus and finds no palpable adnexal masses.
Outcome. After weighing the pros and cons, she elects to use estradiol gel. At her 3-month follow-up, she reports effective relief and good compliance.
Minimum level of protection achieved. Following a comparison of oral and percutaneous estradiol, Reginster et al27 suggested that a minimum estradiol level of 60 pg/mL is necessary to prevent postmenopausal bone loss. Mean serum estradiol levels in women receiving 1.5 mg/day estradiol gel were 75.7 pg/mL and 78.4 pg/mL at 24 and 36 months, respectively, in a study by Palacios et al,10 and 85.8 pg/mL in a study by Devogelaer and colleagues.24 In these trials, BMD increased, and it remained steady in other investigations.28,29
More recent trials suggest that lower serum estradiol levels secondary to smaller estrogen doses have the capacity to maintain BMD.30,31 A 1.9% mean increase of BMD at the lumbar spine was reported in women with a mean serum concentration of 17 pg/mL in a 2-year study30 of a transdermal estradiol delivery system. The percutaneous route does not appear to limit these beneficial effects.
How are metabolic factors affected?
In general, oral estrogens produce beneficial changes in lipid metabolism, particularly higher levels of high-density lipoprotein (HDL) cholesterol. However, they also elevate triglyceride and glucose levels. How this plays out clinically is unclear. Both the Women’s Health Initiative and the Heart and Estrogen/Progestin Replacement Study (HERS) found no cardioprotective effects of oral estrogen despite increases in HDL and decreases in low-density lipoprotein (LDL) levels.1,32
Oral versus percutaneous estradiol. No long-term studies of this magnitude have investigated the effect of percutaneous estradiol on coronary artery disease, although numerous clinical trials have shown that the route of estrogen delivery affects many of the metabolic variables used to estimate the risk of negative cardiac outcomes. It remains to be determined whether percutaneous estradiol affects these metabolic factors in ways that influence morbidity and mortality.
In 1 trial,33 oral conjugated estrogen led to the following significant changes in lipids:
- increase in very low density lipoprotein (VLDL) cholesterol,
- decrease in LDL cholesterol (but not the LDL apoprotein B),
- increase in HDL and apoprotein A1, and
- significant increase in HDL2 cholesterol.
In a separate study,9 oral conjugated estrogen had a 2.5-fold increase in serum angiotensin, and percutaneous estradiol gel, no effect.
Same effects when progestin is added. Beneficial effects were not diminished when oral micronized progesterone was added to percutaneous estradiol.34 Estradiol gel significantly reduced total serum cholesterol and LDL cholesterol in the first year of treatment, compared with placebo.
Coagulation effects. Percutaneous estradiol has fewer negative effects on coagulation factors than its oral counterpart. One study35 investigating combination therapy with micronized progesterone compared the effects of percutaneous estradiol and oral estradiol valerate. The group receiving percutaneous estradiol gel/micronized progesterone had no significant changes in plasminogen activator inhibitor, tissue-type plasminogen concentration, and global fibrinolytic capacity. The other group had a significant decrease in mean tissue-type plasminogen concentration and plasminogen activator inhibitor activity and a significant rise in global fibrinolytic capacity.
The oral estradiol—but not the percutaneous formulation—significantly increased the mean value of prothrombin activation peptide and decreased mean antithrombin activity compared with no treatment.
Poor glycemic control may increase the risk of cardiovascular disease, but oral and percutaneous estradiol appear to have similar glycemic effects. A comparative trial36 of oral estradiol valerate 2 mg/day and percutaneous estradiol 1 mg/day found no differences in glycosylated hemoglobin A1c levels (declined in both groups) or in fasting and 2-hour post-prandial blood glucose levels (constant in both groups) or insulin sensitivity.
Treatment duration may also influence how percutaneous estradiol affects metabolic factors. An open-label longitudinal prospective study37 of 30 women receiving estradiol gel 1.5 mg/day for 6 months found a significant decrease in lipoprotein (a), apoprotein A-I, apoprotein B, HDL cholesterol, and HDL3 cholesterol. At 1 year, however, these changes were not significant.
No association with venous thromboembolism. Transdermal estradiol administered as a patch or percutaneous gel had no effect on the risk of venous thromboembolism in a multicenter case-control investigation.38
In contrast, a recent retrospective study found a risk of venous thromboembolism that was at least 4 times greater with oral estrogen than with transdermal estradiol.38
Use a progestogen to prevent endometrial hyperplasia
Like other forms of estrogen, percutaneous estradiol stimulates the endometrium. For this reason, women who have an intact uterus should use a progestogen in an adequate dose to prevent hyperplastic changes.39 Although numerous regimens appear to be effective, the optimal route, dose, and duration of progestin in women using percutaneous estrogen remain to be determined.
Percutaneous estradiol versus other routes of administration. Endometrial thickness and amenorrhea rates over 6 months were not statistically different among 54 women treated with estradiol gel 1.5 mg/day, transdermal estradiol 50 μg/day, or oral estradiol valerate 2 mg/day—all in combination with nomegestrol acetate 2.5 mg/day.40 The overall rates of no bleeding or spotting over 6 cycles of treatment were 78% in the percutaneous estradiol group, 48% in the transdermal group, and 60% in the oral group.
Although the percutaneous estradiol group had a lower overall incidence of no bleeding or spotting than the other groups, the difference was not significant. Nor were there significant differences in the variation of endometrial thickness from baseline: A mean increase of 1.5 mm (±0.4 mm) was reported in the percutaneous group, compared with 1.5 mm (±0.7 mm) and 1.7 mm (±0.6 mm) in the transdermal and oral estrogen groups, respectively.
Estrogenic and progestogenic effects were similar for transdermal and percutaneous estradiol (with dydrogesterone 10 mg/day for days 1 to 12) after a baseline atropic endometrium was identified.3
Estradiol gel produces stable, physiologic serum estradiol levels and has a serum estrone to estradiol ratio close to 1.
The most effective progestogen dosage and duration are unknown, although many regimens have been studied:
- Percutaneous estradiol 1.5 mg/day for the first 24 days of the month in combination with nomegestrol acetate 5 mg/day for days 11 to 24: Over 6 months, researchers found a secretory pattern in the majority of women and no evidence of hyperplasia.18
- Percutaneous estradiol 3 mg/day for 3 of every 4 weeks in combination with nomegestrol acetate 5 mg/day for 10 days: No reduction in estrogenic endometrial effects.41,42
- Estradiol gel 3 mg/day for 3 of every 4 weeks in combination with 200 mg or 300 mg oral micronized progesterone for the last 10 days of treatment: Dose was too low or treatment too short to produce a complete secretory transformation of the endometrium.43 However, the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial44 found no increased occurrence of hyperplasia in women using micronized progesterone 200 mg for 12 days of each cycle for more than 3 years in combination with oral conjugated estrogen 0.625 mg.
- Percutaneous estradiol 1.5 mg/day combined with micronized progesterone 100 mg daily (orally or vaginally) for the first 25 days of the month: Fully inhibited mitoses and induced amenorrhea in most of the women studied, with amenorrhea rates of 93.3% at 3 months and 91.6% at 6 months.45
- Estradiol gel 1.5 mg/day with 100 mg vaginal micronized progesterone for 21 days per cycle: Stable endometrial thickness and no endometrial hyperplasia over 12 months.46 However, breakthrough bleeding was reported in up to 30% of subjects, principally in the second 3 months of treatment.
- Percutaneous estradiol 1 mg/day for 3 months with oral medroxyprogesterone acetate 20 mg/day for the last 14 days, followed by a 7-day free interval: No reports of endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 2 mg/day for 21 days with 10 mg oral medroxyprogesterone acetate for the last 14 days, followed by a 7-day free interval: No endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 1 mg/day with medroxyprogesterone acetate 10 mg on days 1-12 every month or every 3 months: Endometrial hyperplasia was found in 1 woman (0.3%) in the group receiving the progestogen every 3 months. Endometrial histology did not differ between women taking medroxyprogesterone monthly and those taking it every 3 months.21
- Estradiol gel 3 mg/day with oral micronized progesterone 200 mg for 12 days of each cycle: Regular withdrawal bleeding in 70% of women.34
- Percutaneous estradiol 1.5 mg/day in combination with a levonorgestrel-releasing intrauterine device: 80% of women were amenorrheic at 1 year, with a mean endometrial thickness 3 mm; at 5 years, 100% of women had epithelial atrophy.47
- Estradiol gel 1.5 mg for 21 days with 200 mg oral progesterone for 14 days (126 women); 3 mg percutaneous estradiol for 21 days with 300 mg oral progesterone for 10 days (23 women); 1.5 mg estradiol gel with 300 mg oral progesterone (3 women); or 3 mg estradiol gel for 28 days with 200 mg progesterone for 14 days (5 women): No evidence of hyperplasia after 5 years of treatment.2
- Percutaneous estradiol gel can be prescribed at low doses.
- Relief of menopausal symptoms can begin as early as 2 weeks after starting treatment.
- Treatment with estradiol gel maintains or increases bone mineral density.
- No large randomized, controlled trials have explored the effect of estradiol gel on coronary artery disease. It appears to have metabolic effects similar to those of oral estradiol.
- Because percutaneous estradiol stimulates the endometrium, women with an intact uterus should also take a progestogen.
Although the Women’s Health Initiative1 discouraged many menopausal women from using oral estrogen, it failed to address the risks of treatment with other dosages or forms of estrogen and progestin.
Nor has any other trial of similar size and complexity taken up the issue. However, numerous smaller studies have been published.
This article summarizes findings on percutaneous delivery of estradiol gel (EstroGel), the most recently FDA-approved estrogen option for treatment of menopausal symptoms.
It describes the overall safety of estradiol gel, as well as its effectson:
- menopausal symptoms,
- bone,
- metabolism, and
- endometrium.
(In this article, “percutaneous delivery” refers to estradiol gel applied to the skin, and “transdermal estradiol” indicates delivery via a transdermal reservoir or matrix system, otherwise known as “the patch.” I have used an arbitrary definition to distinguish the gel from other methods of delivering estradiol across the skin. For example, Estrasorb is a liposomal formulation that is applied to the skin of the thigh. Although it is a percutaneous estradiol similar to the gel, this article focuses only on the latter option.)
Easy to apply, few skin reactions
Percutaneous estradiol gel formulations have been available for almost 30 years in Europe, where they are utilized by a majority of women on hormone therapy. In the United States, the hydroalcoholic gel is packaged in a pump that delivers 64 standardized 1.25-g doses, which contain 0.75 mg of 17ß-estradiol. Once it is applied, the gel is absorbed into an intradermal reservoir (FIGURE) and dries in 2 to 5 minutes, leaving no residue.
Patient selection. Estradiol gel is well-suited for patients who are concerned about the risks of oral estrogen (as portrayed in the mainstream press following the Women’s Health Initiative) and want to avoid that route of administration, as well as women who dislike or have difficulty swallowing pills. Percutaneous administration also is appropriate for physically active women who may have adhesion problems or skin irritation with the transdermal patch, or those who have had reactions to local adhesives in the past. The patient should be motivated to apply the gel on a daily basis.
Indications are moderate to severe vasomotor symptoms in menopausal women, and moderate to severe symptoms of vulvar and vaginal atrophy, although topical vaginal products should also be considered for the latter indication.
Contraindications are undiagnosed abnormal vaginal bleeding; history of breast cancer, other estrogen-dependent malignancy, stroke, heart attack, or liver disease or dysfunction; active thrombophlebitis or thromboembolic disorders (or a history of these); and known or suspected pregnancy.
Common side effects include headache, breast pain, irregular vaginal bleeding or spotting, stomach cramps or bloating, nausea and vomiting, and hair loss.
- Skin reactions are infrequent, but should be taken into account when discussing per-cutaneous or transdermal delivery of any drug. However, estradiol gel appears to cause fewer skin reactions than the patch. In a study over more than 5 years, 0 of 157 women treated with percutaneous estradiol reported skin irritation.2 Other comparisons found similar outcomes.3,4
Onset of action is rapid, as it is with the transdermal patch.5 Dose variability is minimized when the gel is applied at the same time every day to a large area of skin, preferably the arm, although all application sites appear to produce similar results: abdomen, shoulders, arms, and inner thigh.6 The gel should not be applied to the breast or vagina.
Diminished effect with skin washing. In 1 trial, site washing 1/2 hour after application significantly decreased bioavailability and time to reach peak plasma concentrations.7 For this reason, the gel should be not applied before a bath, shower, or sauna.
Dosing options. The initial dose is 1.25 mg of gel, which is 1 pump of the bottle. The gel is collected in the palm of 1 hand and applied to the skin of the opposite arm from the wrist to the shoulder. The dose can be titrated by adding a second pump of the gel and applying it to the opposite arm. The dose can be lowered by using less than a full depression of the pump.
Stable, physiologic estrogen levels
Estradiol gel produces relatively stable serum estradiol levels, and therapeutic estradiol levels similar to those seen with other formulations, routes of administration, and dosages.
Percutaneous administration produces serum estrone to estradiol ratios close to 1, in contrast to higher ratios (5:1) with oral administration.8-11 The lower ratio approximates levels during the menstrual cycle of premenopausal women. (TABLE 1 gives estradiol and estrone levels from different studies following administration of estradiol as a gel, oral formulation, and transdermal patch.)
The percutaneous route also allows for delivery directly to the systemic circulation, avoiding gastrointestinal and first-pass hepatic metabolism and elimination. In contrast, oral micronized estradiol causes large fluctuations in serum estradiol and estrone levels due to absorption and metabolism.8
More stable serum levels than with the patch. One study12 comparing percutaneous and transdermal estradiol found similar interindividual variability but less stable serum levels in women using the transdermal system. A separate study13 also reported greater fluctuation of serum estradiol levels in women using a transdermal system than in those using the gel.
TABLE 1
Serum estradiol and estrone levels for various routes of estradiol
| AUTHOR | THERAPY | ESTRADIOL LEVEL (PG/ML) | ESTRONE LEVEL (PG/ML) |
|---|---|---|---|
| Scott et al8 | E2gel 3 mg/day | 102.9±39.9 | 120.0±50.5 |
| E2gel 1.5 mg/day | 68.1±27.4 | 90.6±45.7 | |
| Transdermal estradiol 50 μg/day | 41.1±13.5 | 45.0±15.9 | |
| Oral micronized estradiol 2 mg/day | 114.0±65.2 | 575.2±279.9 | |
| Palacios et al10 | E2gel 1.5 mg/day | 75.7±1.0 | 58.5±2.9 |
| Oral conjugated estrogen 0.625 mg/day | 39.6±4.6 | 126.0±7.6 | |
| Basdevant et al11 | E2gel 3 mg/day | 221.0±35.0 | 146.0±19.0 |
| Oral micronized estradiol 2 mg/day | 121.0±27.0 | 811.0±370.0 | |
| Archer15 | E2gel 0.75 mg/day | 33.5* | 49.0* |
| E2gel 1.5 mg/day | 65.0* | 58.0* | |
| Placebo | 5.0* | 23.0* | |
| *Median value | |||
| E2gel = percutaneous estradiol gel | |||
Studies compared relief of menopausal symptoms
Estradiol gel effectively relieved meno-pausal symptoms in randomized, double-blind studies, open label comparisons, and observational trials in postmenopausal women, with and without the addition of various progestins.
Symptom relief in comparison with baseline values is statistically significant as early as 2 weeks after initiating treatment. Relief of up to 2 years’ duration has been reported.3,14
Several studies have compared symptom relief achieved with estradiol gel, oral estrogen, transdermal delivery systems, and placebo3,9,14-18; findings are summarized in TABLE 2.
Same efficacy when progestogen is added. The following studies, and other studies,14 demonstrated that estradiol gel relieves menopausal symptoms whether it is administered alone or in combination with a progestogen, with efficacy similar to other estrogen formulations:
Climacteric symptoms decreased to the same extent when estradiol gel was combined with a levonorgestrel-releasing intrauterine device, oral micronized progesterone, or vaginal micronized progesterone.19
A study20 that added lynestrenol decreased the frequency of hot flushes and night sweats more than in women using estradiol gel alone. However, negative mood symptoms were more pronounced in the progestin-treated group.
Estradiol gel 1 mg/day in combination with monthly or quarterly oral medroxyprog-esterone acetate reduced the severity of hot flushes, sweating, and vaginal dryness, according to a 12-month trial.21
Symptoms decreased the same whether a levonorgestrel-releasing IUD or oral or vaginal progesterone was added.
Bone mineral density maintained or increased
Several randomized, controlled trials have documented the effects of estradiol gel on bone mineral density (BMD) and various markers of bone metabolism. In these studies, BMD remained steady22,23 or increased10,24,25 following treatment, and estradiol gel remained effective for up to 4 years.25 Estradiol gel maintained or increased BMD with or without addition of progestins.22,23,26
These investigations involved measurement of BMD at the lumbar spine, forearms, or hip, as well as biologic markers of bone turnover such as urinary hydroxyproline/creatinine ratio, serum alkaline phosphatase, and serum osteocalcin.
Serum estradiol and skeletal uptake of a bone-seeking agent also were determined. Estradiol gel regimens ranged from 0.75 mg/day to 3 mg/day, and populations included both surgical and natural menopausal subjects in several countries.
Effects comparable to oral estrogen. Compared with oral conjugated estrogen, which increased BMD at the lumbar spine by 4.3% (±3.2%), estradiol gel produced increases of 5.6% (±2.9%) at 24 months and 4.7% (±3.2%) at 36 months.10
The case. A 52-year-old woman with no menses for 8 months presents for management complaining of disabling hot flushes. Although she is moderately obese, with a body mass index of 29, she is normotensive without any other significant medical history except for hysterectomy at age 44 for excessive bleeding.
Counseling. During her 20s, the patient tried to use oral contraceptives on 3 separate occasions, but was unable to continue them because of nausea. Although she is interested in estrogen therapy for her vasomotor symptoms, she is concerned about the possibility of experiencing nausea again. You explain that one of the benefits of percutaneous estradiol is that it avoids the gastrointestinal tract.
Physical findings. Her physical examination is within normal limits, and gynecologic examination confirms no uterus and finds no palpable adnexal masses.
Outcome. After weighing the pros and cons, she elects to use estradiol gel. At her 3-month follow-up, she reports effective relief and good compliance.
Minimum level of protection achieved. Following a comparison of oral and percutaneous estradiol, Reginster et al27 suggested that a minimum estradiol level of 60 pg/mL is necessary to prevent postmenopausal bone loss. Mean serum estradiol levels in women receiving 1.5 mg/day estradiol gel were 75.7 pg/mL and 78.4 pg/mL at 24 and 36 months, respectively, in a study by Palacios et al,10 and 85.8 pg/mL in a study by Devogelaer and colleagues.24 In these trials, BMD increased, and it remained steady in other investigations.28,29
More recent trials suggest that lower serum estradiol levels secondary to smaller estrogen doses have the capacity to maintain BMD.30,31 A 1.9% mean increase of BMD at the lumbar spine was reported in women with a mean serum concentration of 17 pg/mL in a 2-year study30 of a transdermal estradiol delivery system. The percutaneous route does not appear to limit these beneficial effects.
How are metabolic factors affected?
In general, oral estrogens produce beneficial changes in lipid metabolism, particularly higher levels of high-density lipoprotein (HDL) cholesterol. However, they also elevate triglyceride and glucose levels. How this plays out clinically is unclear. Both the Women’s Health Initiative and the Heart and Estrogen/Progestin Replacement Study (HERS) found no cardioprotective effects of oral estrogen despite increases in HDL and decreases in low-density lipoprotein (LDL) levels.1,32
Oral versus percutaneous estradiol. No long-term studies of this magnitude have investigated the effect of percutaneous estradiol on coronary artery disease, although numerous clinical trials have shown that the route of estrogen delivery affects many of the metabolic variables used to estimate the risk of negative cardiac outcomes. It remains to be determined whether percutaneous estradiol affects these metabolic factors in ways that influence morbidity and mortality.
In 1 trial,33 oral conjugated estrogen led to the following significant changes in lipids:
- increase in very low density lipoprotein (VLDL) cholesterol,
- decrease in LDL cholesterol (but not the LDL apoprotein B),
- increase in HDL and apoprotein A1, and
- significant increase in HDL2 cholesterol.
In a separate study,9 oral conjugated estrogen had a 2.5-fold increase in serum angiotensin, and percutaneous estradiol gel, no effect.
Same effects when progestin is added. Beneficial effects were not diminished when oral micronized progesterone was added to percutaneous estradiol.34 Estradiol gel significantly reduced total serum cholesterol and LDL cholesterol in the first year of treatment, compared with placebo.
Coagulation effects. Percutaneous estradiol has fewer negative effects on coagulation factors than its oral counterpart. One study35 investigating combination therapy with micronized progesterone compared the effects of percutaneous estradiol and oral estradiol valerate. The group receiving percutaneous estradiol gel/micronized progesterone had no significant changes in plasminogen activator inhibitor, tissue-type plasminogen concentration, and global fibrinolytic capacity. The other group had a significant decrease in mean tissue-type plasminogen concentration and plasminogen activator inhibitor activity and a significant rise in global fibrinolytic capacity.
The oral estradiol—but not the percutaneous formulation—significantly increased the mean value of prothrombin activation peptide and decreased mean antithrombin activity compared with no treatment.
Poor glycemic control may increase the risk of cardiovascular disease, but oral and percutaneous estradiol appear to have similar glycemic effects. A comparative trial36 of oral estradiol valerate 2 mg/day and percutaneous estradiol 1 mg/day found no differences in glycosylated hemoglobin A1c levels (declined in both groups) or in fasting and 2-hour post-prandial blood glucose levels (constant in both groups) or insulin sensitivity.
Treatment duration may also influence how percutaneous estradiol affects metabolic factors. An open-label longitudinal prospective study37 of 30 women receiving estradiol gel 1.5 mg/day for 6 months found a significant decrease in lipoprotein (a), apoprotein A-I, apoprotein B, HDL cholesterol, and HDL3 cholesterol. At 1 year, however, these changes were not significant.
No association with venous thromboembolism. Transdermal estradiol administered as a patch or percutaneous gel had no effect on the risk of venous thromboembolism in a multicenter case-control investigation.38
In contrast, a recent retrospective study found a risk of venous thromboembolism that was at least 4 times greater with oral estrogen than with transdermal estradiol.38
Use a progestogen to prevent endometrial hyperplasia
Like other forms of estrogen, percutaneous estradiol stimulates the endometrium. For this reason, women who have an intact uterus should use a progestogen in an adequate dose to prevent hyperplastic changes.39 Although numerous regimens appear to be effective, the optimal route, dose, and duration of progestin in women using percutaneous estrogen remain to be determined.
Percutaneous estradiol versus other routes of administration. Endometrial thickness and amenorrhea rates over 6 months were not statistically different among 54 women treated with estradiol gel 1.5 mg/day, transdermal estradiol 50 μg/day, or oral estradiol valerate 2 mg/day—all in combination with nomegestrol acetate 2.5 mg/day.40 The overall rates of no bleeding or spotting over 6 cycles of treatment were 78% in the percutaneous estradiol group, 48% in the transdermal group, and 60% in the oral group.
Although the percutaneous estradiol group had a lower overall incidence of no bleeding or spotting than the other groups, the difference was not significant. Nor were there significant differences in the variation of endometrial thickness from baseline: A mean increase of 1.5 mm (±0.4 mm) was reported in the percutaneous group, compared with 1.5 mm (±0.7 mm) and 1.7 mm (±0.6 mm) in the transdermal and oral estrogen groups, respectively.
Estrogenic and progestogenic effects were similar for transdermal and percutaneous estradiol (with dydrogesterone 10 mg/day for days 1 to 12) after a baseline atropic endometrium was identified.3
Estradiol gel produces stable, physiologic serum estradiol levels and has a serum estrone to estradiol ratio close to 1.
The most effective progestogen dosage and duration are unknown, although many regimens have been studied:
- Percutaneous estradiol 1.5 mg/day for the first 24 days of the month in combination with nomegestrol acetate 5 mg/day for days 11 to 24: Over 6 months, researchers found a secretory pattern in the majority of women and no evidence of hyperplasia.18
- Percutaneous estradiol 3 mg/day for 3 of every 4 weeks in combination with nomegestrol acetate 5 mg/day for 10 days: No reduction in estrogenic endometrial effects.41,42
- Estradiol gel 3 mg/day for 3 of every 4 weeks in combination with 200 mg or 300 mg oral micronized progesterone for the last 10 days of treatment: Dose was too low or treatment too short to produce a complete secretory transformation of the endometrium.43 However, the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial44 found no increased occurrence of hyperplasia in women using micronized progesterone 200 mg for 12 days of each cycle for more than 3 years in combination with oral conjugated estrogen 0.625 mg.
- Percutaneous estradiol 1.5 mg/day combined with micronized progesterone 100 mg daily (orally or vaginally) for the first 25 days of the month: Fully inhibited mitoses and induced amenorrhea in most of the women studied, with amenorrhea rates of 93.3% at 3 months and 91.6% at 6 months.45
- Estradiol gel 1.5 mg/day with 100 mg vaginal micronized progesterone for 21 days per cycle: Stable endometrial thickness and no endometrial hyperplasia over 12 months.46 However, breakthrough bleeding was reported in up to 30% of subjects, principally in the second 3 months of treatment.
- Percutaneous estradiol 1 mg/day for 3 months with oral medroxyprogesterone acetate 20 mg/day for the last 14 days, followed by a 7-day free interval: No reports of endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 2 mg/day for 21 days with 10 mg oral medroxyprogesterone acetate for the last 14 days, followed by a 7-day free interval: No endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 1 mg/day with medroxyprogesterone acetate 10 mg on days 1-12 every month or every 3 months: Endometrial hyperplasia was found in 1 woman (0.3%) in the group receiving the progestogen every 3 months. Endometrial histology did not differ between women taking medroxyprogesterone monthly and those taking it every 3 months.21
- Estradiol gel 3 mg/day with oral micronized progesterone 200 mg for 12 days of each cycle: Regular withdrawal bleeding in 70% of women.34
- Percutaneous estradiol 1.5 mg/day in combination with a levonorgestrel-releasing intrauterine device: 80% of women were amenorrheic at 1 year, with a mean endometrial thickness 3 mm; at 5 years, 100% of women had epithelial atrophy.47
- Estradiol gel 1.5 mg for 21 days with 200 mg oral progesterone for 14 days (126 women); 3 mg percutaneous estradiol for 21 days with 300 mg oral progesterone for 10 days (23 women); 1.5 mg estradiol gel with 300 mg oral progesterone (3 women); or 3 mg estradiol gel for 28 days with 200 mg progesterone for 14 days (5 women): No evidence of hyperplasia after 5 years of treatment.2
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
2. Moyer DL, de Lignieres B, Driguez P, et al. Prevention of endometrial hyperplasia by progesterone during long-term estradiol replacement: influence of bleeding pattern and secretory changes. Fertil Steril. 1993;59:992-997.
3. Hirvonen E, Cacciatore B, Wahlström T, et al. Effects of transdermal oestrogen therapy in postmenopausal women: a comparative study of an oestradiol gel and an oestradiol delivering patch. BJOG. 1997;104(Suppl 16):26-31.
4. Travassos de Figueiredo Alves S, et al. Comparison of gel and patch estradiol replacement in Brazil, a tropical country. Maturitas. 2000;36:69-74.
5. Henzl MR, Loomba PK. Transdermal delivery of sex steroids for hormone replacement therapy and contraception. A review of principles and practice. J Reprod Med. 2003;48:525-540.
6. Holst J, et al. Serum estrogen levels after topic application of estradiol-17 beta on two different cutaneous areas. Acta Obstet Gynecol Scand. 1987;662:151-152.
7. Jarvinen A, Granander M, Nykanen S, Laine T, Geurts P, Viitanen A. Steady-state pharmacokinetics of oestradiol gel in post-menopausal women: effects of application area and washing. Br J Obstet Gynaecol. 1997;104(Suppl 16):14-18.
8. Scott RT, Ross B, Anderson C, Archer DF. Pharmacokinetics of percutaneous estradiol: a crossover study using a gel and a transdermal system in comparison with oral micronized estradiol. Obstet Gynecol. 1991;77:758-764.
9. Dupont A, Dupont P, Cusan M, et al. Comparative endocrinological and clinical effects of percutaneous estradiol and oral conjugated estrogens as replacement therapy in menopausal women. Maturitas. 1991;13:297-311.
10. Palacios S, Menéndez C, Jurado AR, Vargas JC. Effects of percutaneous oestradiol versus oral oestrogens on bone density. Maturitas. 1995;20:209-213.
11. Basdevant A, de Lignieres B, Simon P, et al. Hepatic lipase activity during oral and parenteral 17ß-estradiol replacement therapy: high-density lipoprotein increase may not be antiatherogenic. Fertil Steril. 1991;55:1112-1117.
12. Simon JA, et al. Are there significant differences between patch and gel cutaneous estradiol therapy? In: Genazzani AR, Petraglia F, Volpe A, Facchinetti F, eds. Recent Research on Gynecological Endocrinology. Casterton Hall, Carnforth, Lancashire, UK: Parthenon Publishing, Casterton Hall; 1998;317-324.
13. Paoletti AM, et al. Comparison of pharmacokinetic profiles of 17ß-estradiol gel 0.6mg/g (Gelestra) with a transdermal delivery system (Estraderm TTS 50) in postmenopausal women at steady state. Maturitas. 2001;40:203-209.
14. Jensen PB, Jensen J, Riis BJ, et al. Climacteric symptoms after oral and percutaneous hormone replacement therapy. Maturitas. 1987;9:207-215.
15. Archer DF. for the EstroGel Study Group. Percutaneous 17ß-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
16. Kornafel KL, March CM. Estradiol gel in the treatment of menopausal symptoms: a placebo-controlled double-blind case study of efficacy and safety. South Med J. 1992;85:270-273.
17. Hirvonen E, Lamberg-Allardt C, Lankinen KS, Geurts P, Wilén-Rosenqvist G. Transdermal oestradiol gel in the treatment of the climacterium: a comparison with oral therapy. BJOG. 1997;104(Suppl 16):19-25.
18. Foidart JM, Béliard A, Hedon B, et al. Impact of percutaneous oestradiol gels in postmenopausal hormone replacement therapy on clinical symptoms and endometrium. BJOG. 1997;104:305-310.
19. Suvanto-Luukkonen E, Sundström H, Penttinen J, et al. Percutaneous estradiol gel with an intrauterine levonorgestrel releasing device or natural progesterone in hormone replacement therapy. Maturitas. 1997;26:211-17.
20. Holst J, et al. Progestogen addition during oestrogen replacement therapy—effects on vasomotor symptoms and mood. Maturitas. 1989;11:13-20.
21. Hirvonen E, et al. Effect of an estradiol gel with monthly or quarterly progestogen on menopausal symptoms and bleeding. Climacteric. 2000;3:262-270.
22. Ng HT, Chang SP, Yang TS, Cho MP, Wei TC. Estradiol administered in a percutaneous gel for the prevention of postmenopausal bone loss. Asia-Oceania J Obstet Gynaecol. 1993;19(2):115-119.
23. Riis B, Thomsen K, et al. Does calcium supplementation prevent postmenopausal bone loss? A double blind, controlled clinical study. N Engl J Med. 1987;316:173-177.
24. Devogelaer JP, Lecart C, Dupret P, De Nayer P, Nagant De Deuxchaisnes C. Long-term effects of percutaneous estradiol on bone loss and bone metabolism in post-menopausal hysterectomized women. Maturitas. 1998;28:243-249.
25. Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four year randomized study. Am J Med. 1995;99:36-42.
26. Sun A, Lin S, Yu W, et al. Percutaneous estrogen in prevention of early post-menopausal bone loss in Chinese women. Chin Med J. 2002;115:1790-1795.
27. Reginster JY, Sarlet N, Deroisy R, et al. Minimum levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
28. Cicinelli E, Cantatore FP, Galantino P, et al. Effects of continuous percutaneous estradiol administration on skeletal turnover in postmenopausal women: a 1-year prospective controlled study. Eur J Obstet Gynecol Reprod Biol. 1996;69(2):109-113.
29. Riis B, et al. The effect of percutaneous estradiol and natural progesterone on post-menopausal bone loss. Am J Obstet Gynecol. 1987;156:61-65.
30. Notelvitz M, John VA, Good WR. Effectiveness of Alora estradiol matrix transdermal delivery system in improving lumbar bone mineral density in healthy, post-menopausal women. Menopause. 2002;9:343-353.
31. Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17ß-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042-1048.
32. Hully S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
33. Moorjani S, Dupont A, Labrie F, et al. Changes in plasma lipoprotein and apolipoprotein composition in relation to oral versus percutaneous administration of estrogen alone or in cyclic association with Utrogestan in menopausal women. J Clin Endocrinol Metab. 1991;73:373-379.
34. Jensen J, Riis BJ, Strøm V, Nilas L, Christiansen C. Long-term effects of percutaneous estrogens and oral progesterone on serum lipoproteins in postmenopausal women. Am J Obstet Gyencol. 1987;156:66-71.
35. Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17:3071-3078.
36. Karjalainen A, Paassilta M, Heikkinen J, et al. Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clin Endocrin. 2001;54:165-173.
37. Haines CJ, Chung TKH, Masarei JRL, Tomlinson B, Lau JTF. The effect of per-cutaneous oestrogen replacement therapy on Lp(a) and other lipoproteins. Maturitas. 1995;22:219-225.
38. Scarabin PY, Oger E, Plu-Bureau G. EStrogen and THromboEmbolism Risk (ESTHER) Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428-432.
39. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
40. Blanc B, Cravello L, Micheletti MC, et al. Continuous hormone replacement therapy for menopause combining nomegestrol acetate and gel, patch, or oral estrogen: a comparison of amenorrhea rates. Clin Therap. 1998;20(5):901-912.
41. Holst J. Percutaneous estrogen therapy. Endometrial response and metabolic effects. Acta Obstet Gynecol Scand Suppl. 1983;115:1-30
42. Holst J, Cajander S, von Schoultz B. Cellular morphometric analysis of the post-menopausal endometrium during treatment with percutaneous estradiol-17ß with and without oral gestagen. Acta Obstet Gynecol Scand. 1983;62:267-270.
43. Holst J, Cajander S, von Schoultz B. Endometrial response in post-menopausal women during treatment with percutaneous 17ß-oestradiol opposed by oral progesterone. Maturitas. 1986;8:201-207.
44. Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370-375.
45. Gillet JY, Andre G, Faguer B, et al. Induction of amenorrhea during hormone replacement therapy: optimized micronized progesterone dose. A multicenter study. Maturitas. 1994;19:103-115.
46. Vilodre LC, et al. Endometrial response to a cyclic regimen of percutaneous 17ß-estradiol and low-dose vaginal micronized progesterone in women with mild-to-moderate hypertension. Gynecol Endocrinol. 2003;17:323-328.
47. Suvanto-Luukkonen E, Kauppila A. The levonorgestrel intrauterine system in menopausal hormone replacement therapy: five-year experience. Fertil Steril. 1999;72:161-163.
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
2. Moyer DL, de Lignieres B, Driguez P, et al. Prevention of endometrial hyperplasia by progesterone during long-term estradiol replacement: influence of bleeding pattern and secretory changes. Fertil Steril. 1993;59:992-997.
3. Hirvonen E, Cacciatore B, Wahlström T, et al. Effects of transdermal oestrogen therapy in postmenopausal women: a comparative study of an oestradiol gel and an oestradiol delivering patch. BJOG. 1997;104(Suppl 16):26-31.
4. Travassos de Figueiredo Alves S, et al. Comparison of gel and patch estradiol replacement in Brazil, a tropical country. Maturitas. 2000;36:69-74.
5. Henzl MR, Loomba PK. Transdermal delivery of sex steroids for hormone replacement therapy and contraception. A review of principles and practice. J Reprod Med. 2003;48:525-540.
6. Holst J, et al. Serum estrogen levels after topic application of estradiol-17 beta on two different cutaneous areas. Acta Obstet Gynecol Scand. 1987;662:151-152.
7. Jarvinen A, Granander M, Nykanen S, Laine T, Geurts P, Viitanen A. Steady-state pharmacokinetics of oestradiol gel in post-menopausal women: effects of application area and washing. Br J Obstet Gynaecol. 1997;104(Suppl 16):14-18.
8. Scott RT, Ross B, Anderson C, Archer DF. Pharmacokinetics of percutaneous estradiol: a crossover study using a gel and a transdermal system in comparison with oral micronized estradiol. Obstet Gynecol. 1991;77:758-764.
9. Dupont A, Dupont P, Cusan M, et al. Comparative endocrinological and clinical effects of percutaneous estradiol and oral conjugated estrogens as replacement therapy in menopausal women. Maturitas. 1991;13:297-311.
10. Palacios S, Menéndez C, Jurado AR, Vargas JC. Effects of percutaneous oestradiol versus oral oestrogens on bone density. Maturitas. 1995;20:209-213.
11. Basdevant A, de Lignieres B, Simon P, et al. Hepatic lipase activity during oral and parenteral 17ß-estradiol replacement therapy: high-density lipoprotein increase may not be antiatherogenic. Fertil Steril. 1991;55:1112-1117.
12. Simon JA, et al. Are there significant differences between patch and gel cutaneous estradiol therapy? In: Genazzani AR, Petraglia F, Volpe A, Facchinetti F, eds. Recent Research on Gynecological Endocrinology. Casterton Hall, Carnforth, Lancashire, UK: Parthenon Publishing, Casterton Hall; 1998;317-324.
13. Paoletti AM, et al. Comparison of pharmacokinetic profiles of 17ß-estradiol gel 0.6mg/g (Gelestra) with a transdermal delivery system (Estraderm TTS 50) in postmenopausal women at steady state. Maturitas. 2001;40:203-209.
14. Jensen PB, Jensen J, Riis BJ, et al. Climacteric symptoms after oral and percutaneous hormone replacement therapy. Maturitas. 1987;9:207-215.
15. Archer DF. for the EstroGel Study Group. Percutaneous 17ß-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
16. Kornafel KL, March CM. Estradiol gel in the treatment of menopausal symptoms: a placebo-controlled double-blind case study of efficacy and safety. South Med J. 1992;85:270-273.
17. Hirvonen E, Lamberg-Allardt C, Lankinen KS, Geurts P, Wilén-Rosenqvist G. Transdermal oestradiol gel in the treatment of the climacterium: a comparison with oral therapy. BJOG. 1997;104(Suppl 16):19-25.
18. Foidart JM, Béliard A, Hedon B, et al. Impact of percutaneous oestradiol gels in postmenopausal hormone replacement therapy on clinical symptoms and endometrium. BJOG. 1997;104:305-310.
19. Suvanto-Luukkonen E, Sundström H, Penttinen J, et al. Percutaneous estradiol gel with an intrauterine levonorgestrel releasing device or natural progesterone in hormone replacement therapy. Maturitas. 1997;26:211-17.
20. Holst J, et al. Progestogen addition during oestrogen replacement therapy—effects on vasomotor symptoms and mood. Maturitas. 1989;11:13-20.
21. Hirvonen E, et al. Effect of an estradiol gel with monthly or quarterly progestogen on menopausal symptoms and bleeding. Climacteric. 2000;3:262-270.
22. Ng HT, Chang SP, Yang TS, Cho MP, Wei TC. Estradiol administered in a percutaneous gel for the prevention of postmenopausal bone loss. Asia-Oceania J Obstet Gynaecol. 1993;19(2):115-119.
23. Riis B, Thomsen K, et al. Does calcium supplementation prevent postmenopausal bone loss? A double blind, controlled clinical study. N Engl J Med. 1987;316:173-177.
24. Devogelaer JP, Lecart C, Dupret P, De Nayer P, Nagant De Deuxchaisnes C. Long-term effects of percutaneous estradiol on bone loss and bone metabolism in post-menopausal hysterectomized women. Maturitas. 1998;28:243-249.
25. Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four year randomized study. Am J Med. 1995;99:36-42.
26. Sun A, Lin S, Yu W, et al. Percutaneous estrogen in prevention of early post-menopausal bone loss in Chinese women. Chin Med J. 2002;115:1790-1795.
27. Reginster JY, Sarlet N, Deroisy R, et al. Minimum levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
28. Cicinelli E, Cantatore FP, Galantino P, et al. Effects of continuous percutaneous estradiol administration on skeletal turnover in postmenopausal women: a 1-year prospective controlled study. Eur J Obstet Gynecol Reprod Biol. 1996;69(2):109-113.
29. Riis B, et al. The effect of percutaneous estradiol and natural progesterone on post-menopausal bone loss. Am J Obstet Gynecol. 1987;156:61-65.
30. Notelvitz M, John VA, Good WR. Effectiveness of Alora estradiol matrix transdermal delivery system in improving lumbar bone mineral density in healthy, post-menopausal women. Menopause. 2002;9:343-353.
31. Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17ß-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042-1048.
32. Hully S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
33. Moorjani S, Dupont A, Labrie F, et al. Changes in plasma lipoprotein and apolipoprotein composition in relation to oral versus percutaneous administration of estrogen alone or in cyclic association with Utrogestan in menopausal women. J Clin Endocrinol Metab. 1991;73:373-379.
34. Jensen J, Riis BJ, Strøm V, Nilas L, Christiansen C. Long-term effects of percutaneous estrogens and oral progesterone on serum lipoproteins in postmenopausal women. Am J Obstet Gyencol. 1987;156:66-71.
35. Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17:3071-3078.
36. Karjalainen A, Paassilta M, Heikkinen J, et al. Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clin Endocrin. 2001;54:165-173.
37. Haines CJ, Chung TKH, Masarei JRL, Tomlinson B, Lau JTF. The effect of per-cutaneous oestrogen replacement therapy on Lp(a) and other lipoproteins. Maturitas. 1995;22:219-225.
38. Scarabin PY, Oger E, Plu-Bureau G. EStrogen and THromboEmbolism Risk (ESTHER) Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428-432.
39. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
40. Blanc B, Cravello L, Micheletti MC, et al. Continuous hormone replacement therapy for menopause combining nomegestrol acetate and gel, patch, or oral estrogen: a comparison of amenorrhea rates. Clin Therap. 1998;20(5):901-912.
41. Holst J. Percutaneous estrogen therapy. Endometrial response and metabolic effects. Acta Obstet Gynecol Scand Suppl. 1983;115:1-30
42. Holst J, Cajander S, von Schoultz B. Cellular morphometric analysis of the post-menopausal endometrium during treatment with percutaneous estradiol-17ß with and without oral gestagen. Acta Obstet Gynecol Scand. 1983;62:267-270.
43. Holst J, Cajander S, von Schoultz B. Endometrial response in post-menopausal women during treatment with percutaneous 17ß-oestradiol opposed by oral progesterone. Maturitas. 1986;8:201-207.
44. Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370-375.
45. Gillet JY, Andre G, Faguer B, et al. Induction of amenorrhea during hormone replacement therapy: optimized micronized progesterone dose. A multicenter study. Maturitas. 1994;19:103-115.
46. Vilodre LC, et al. Endometrial response to a cyclic regimen of percutaneous 17ß-estradiol and low-dose vaginal micronized progesterone in women with mild-to-moderate hypertension. Gynecol Endocrinol. 2003;17:323-328.
47. Suvanto-Luukkonen E, Kauppila A. The levonorgestrel intrauterine system in menopausal hormone replacement therapy: five-year experience. Fertil Steril. 1999;72:161-163.