User login
Endometrial cancer is the most common gynecologic cancer in the developed world, and its incidence is rising—increasing 50% in the past 10 years in Norway alone. Because endometrial hyperplasia is a precursor to cancer, it is vital that we ensure effective treatment for this prevalent problem. Hysterectomy is one option—used most commonly for complex atypical hyperplasia—but oral progestins have become the norm in women who desire to preserve their uterus when surgery is not the best option.
In this randomized trial from Norway, Orbo and colleagues randomly assigned 170 women aged 30 to 70 years to one of three treatment groups:
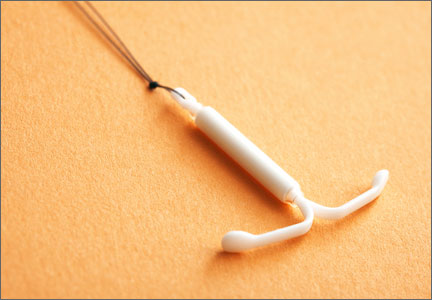
- placement of an LNG-IUS (Mirena)
- medroxyprogesterone acetate (MPA) 10 mg for 10 days per cycle
- continuous MPA 10 mg.
All women in the trial had low- or medium-risk endometrial hyperplasia.
After 6 months, women in the LNG-IUS arm had a 100% response rate, compared with 96% for the continuous MPA group and 69% for cyclic MPA.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; March 2014)
Histologic interpretation of hyperplasia is highly subjective
There are several problems inherent in a study like this. Although Orbo and colleagues address these problems tangentially, the problems affect the interpretation of results.
For example, the histologic interpretation of endometrial hyperplasia is known to be associated with low interobserver agreement.1 Clinical trials that use endometrial safety as an outcome require two primary pathologists to review the histology, with a third pathologist standing by in case of disagreement.
In the current study, two pathologists in the same department independently reviewed the histology. Orbo and colleagues used World Health Organization criteria for hyperplasia. However, as an adjunct, they also used a D-evaluation morphometric assessment.2 When I put in a casual call to local gynecologic pathologists, they told me that neither the D-classification nor the immunochemical-detected PTEN protein is used in routine clinical practice to determine the risk of progression.3
Intermittent use of oral MPA is known to be ineffective
The cyclic use of MPA for only 10 days overlooks epidemiology from estrogen-progestin replacement regimens in postmenopausal women. Use of a progestin for fewer than 12 days during estrogen replacement increases the risk of endometrial cancer.4 Exogenous progestin must be given for more than 12 days to inhibit hyperplasia and neoplasia. The dose itself is not critical; the duration of administration is.
In the current study, both the LNG-IUS and continuous MPA met this criterion. Local delivery of the progestin with the LNG-IUS allows for a reduction of the delivered dose and mitigates side effects even as it uses a more potent progestin than MPA.5–8
Assessment of outcomes was questionable
Although Orbo and colleagues suggest that there is no evidence of progression with the LNG-IUS and continuous MPA, they relied on a Pipelle biopsy of the endometrium performed after 6 months of treatment. The clinical settings that led to the hyperplasia in the first place are poorly characterized as either pre- or postmenopausal, and the cause of the hyperplasia is not identified. This approach overlooks such realities as the increased incidence of simple hyperplasia in many perimenopausal women, which appears to regress with further reduction in ovarian estrogen.9
The final outcomes for women in the cyclic MPA arm are not provided. Hyperplasia without atypia progresses to carcinoma in 1.6% of cases, but when atypia is present, the progression rate is 23%.1
What this evidence means for practice
In low-risk women with simple hyperplasia, the use of targeted low-dose progestins—oral or intrauterine—is appealing. While Orbo and colleagues present an interesting study, they do not definitively establish the optimal intervention.
As we enter a cost-conscious phase of medicine in the United States, we may discover that oral generic MPA (given continuously) may be the most cost-effective treatment despite the option of delivering a low-dose progestin via intrauterine device.
David F. Archer, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001;76(1):25–31.
- Orbo A, Ames M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: A follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008;111(1):68–73.
- Lacey JV Jr, Mutter GL, Ronnett BM, et al. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer Res. 2008;68(14):6014–6020.
- Archer DF. Neoplasia of the female reproductive tract: Effects of hormone therapy. Endocrine. 2004;24(3):259–263.
- Moe BT, Vereide AB, Orbo A, Jaeger R, Sager G. Levonorgestrel, medroxyprogesterone, and progesterone cause a concentration-dependent reduction in endometrial cancer (Ishikawa) cell density, and high concentrations of progesterone and mifepristone act in synergy. Anticancer Res. 2009;29(4):1047–1052.
- Phillips A, Hahn DW, Kimek S, McGuire JL. A comparison of the potencies and activities of progestogens used in contraceptives. Contraception. 1987;36(2):181–192.
- Archer DF. Delivery of therapeutic agents to the target tissue. Menopause. 2011;18(10):1040–1041.
- Somboonporn W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause. 2011;18(10):1060–1066.
- Archer DF, McIntyre-Seltman K, Wilborn WW Jr, et al. Endometrial morphology in asymptomatic postmenopausal women. Am J Obstet Gynecol. 1991;165(2):317–322.
Endometrial cancer is the most common gynecologic cancer in the developed world, and its incidence is rising—increasing 50% in the past 10 years in Norway alone. Because endometrial hyperplasia is a precursor to cancer, it is vital that we ensure effective treatment for this prevalent problem. Hysterectomy is one option—used most commonly for complex atypical hyperplasia—but oral progestins have become the norm in women who desire to preserve their uterus when surgery is not the best option.
In this randomized trial from Norway, Orbo and colleagues randomly assigned 170 women aged 30 to 70 years to one of three treatment groups:
- placement of an LNG-IUS (Mirena)
- medroxyprogesterone acetate (MPA) 10 mg for 10 days per cycle
- continuous MPA 10 mg.
All women in the trial had low- or medium-risk endometrial hyperplasia.
After 6 months, women in the LNG-IUS arm had a 100% response rate, compared with 96% for the continuous MPA group and 69% for cyclic MPA.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; March 2014)
Histologic interpretation of hyperplasia is highly subjective
There are several problems inherent in a study like this. Although Orbo and colleagues address these problems tangentially, the problems affect the interpretation of results.
For example, the histologic interpretation of endometrial hyperplasia is known to be associated with low interobserver agreement.1 Clinical trials that use endometrial safety as an outcome require two primary pathologists to review the histology, with a third pathologist standing by in case of disagreement.
In the current study, two pathologists in the same department independently reviewed the histology. Orbo and colleagues used World Health Organization criteria for hyperplasia. However, as an adjunct, they also used a D-evaluation morphometric assessment.2 When I put in a casual call to local gynecologic pathologists, they told me that neither the D-classification nor the immunochemical-detected PTEN protein is used in routine clinical practice to determine the risk of progression.3
Intermittent use of oral MPA is known to be ineffective
The cyclic use of MPA for only 10 days overlooks epidemiology from estrogen-progestin replacement regimens in postmenopausal women. Use of a progestin for fewer than 12 days during estrogen replacement increases the risk of endometrial cancer.4 Exogenous progestin must be given for more than 12 days to inhibit hyperplasia and neoplasia. The dose itself is not critical; the duration of administration is.
In the current study, both the LNG-IUS and continuous MPA met this criterion. Local delivery of the progestin with the LNG-IUS allows for a reduction of the delivered dose and mitigates side effects even as it uses a more potent progestin than MPA.5–8
Assessment of outcomes was questionable
Although Orbo and colleagues suggest that there is no evidence of progression with the LNG-IUS and continuous MPA, they relied on a Pipelle biopsy of the endometrium performed after 6 months of treatment. The clinical settings that led to the hyperplasia in the first place are poorly characterized as either pre- or postmenopausal, and the cause of the hyperplasia is not identified. This approach overlooks such realities as the increased incidence of simple hyperplasia in many perimenopausal women, which appears to regress with further reduction in ovarian estrogen.9
The final outcomes for women in the cyclic MPA arm are not provided. Hyperplasia without atypia progresses to carcinoma in 1.6% of cases, but when atypia is present, the progression rate is 23%.1
What this evidence means for practice
In low-risk women with simple hyperplasia, the use of targeted low-dose progestins—oral or intrauterine—is appealing. While Orbo and colleagues present an interesting study, they do not definitively establish the optimal intervention.
As we enter a cost-conscious phase of medicine in the United States, we may discover that oral generic MPA (given continuously) may be the most cost-effective treatment despite the option of delivering a low-dose progestin via intrauterine device.
David F. Archer, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Endometrial cancer is the most common gynecologic cancer in the developed world, and its incidence is rising—increasing 50% in the past 10 years in Norway alone. Because endometrial hyperplasia is a precursor to cancer, it is vital that we ensure effective treatment for this prevalent problem. Hysterectomy is one option—used most commonly for complex atypical hyperplasia—but oral progestins have become the norm in women who desire to preserve their uterus when surgery is not the best option.
In this randomized trial from Norway, Orbo and colleagues randomly assigned 170 women aged 30 to 70 years to one of three treatment groups:
- placement of an LNG-IUS (Mirena)
- medroxyprogesterone acetate (MPA) 10 mg for 10 days per cycle
- continuous MPA 10 mg.
All women in the trial had low- or medium-risk endometrial hyperplasia.
After 6 months, women in the LNG-IUS arm had a 100% response rate, compared with 96% for the continuous MPA group and 69% for cyclic MPA.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; March 2014)
Histologic interpretation of hyperplasia is highly subjective
There are several problems inherent in a study like this. Although Orbo and colleagues address these problems tangentially, the problems affect the interpretation of results.
For example, the histologic interpretation of endometrial hyperplasia is known to be associated with low interobserver agreement.1 Clinical trials that use endometrial safety as an outcome require two primary pathologists to review the histology, with a third pathologist standing by in case of disagreement.
In the current study, two pathologists in the same department independently reviewed the histology. Orbo and colleagues used World Health Organization criteria for hyperplasia. However, as an adjunct, they also used a D-evaluation morphometric assessment.2 When I put in a casual call to local gynecologic pathologists, they told me that neither the D-classification nor the immunochemical-detected PTEN protein is used in routine clinical practice to determine the risk of progression.3
Intermittent use of oral MPA is known to be ineffective
The cyclic use of MPA for only 10 days overlooks epidemiology from estrogen-progestin replacement regimens in postmenopausal women. Use of a progestin for fewer than 12 days during estrogen replacement increases the risk of endometrial cancer.4 Exogenous progestin must be given for more than 12 days to inhibit hyperplasia and neoplasia. The dose itself is not critical; the duration of administration is.
In the current study, both the LNG-IUS and continuous MPA met this criterion. Local delivery of the progestin with the LNG-IUS allows for a reduction of the delivered dose and mitigates side effects even as it uses a more potent progestin than MPA.5–8
Assessment of outcomes was questionable
Although Orbo and colleagues suggest that there is no evidence of progression with the LNG-IUS and continuous MPA, they relied on a Pipelle biopsy of the endometrium performed after 6 months of treatment. The clinical settings that led to the hyperplasia in the first place are poorly characterized as either pre- or postmenopausal, and the cause of the hyperplasia is not identified. This approach overlooks such realities as the increased incidence of simple hyperplasia in many perimenopausal women, which appears to regress with further reduction in ovarian estrogen.9
The final outcomes for women in the cyclic MPA arm are not provided. Hyperplasia without atypia progresses to carcinoma in 1.6% of cases, but when atypia is present, the progression rate is 23%.1
What this evidence means for practice
In low-risk women with simple hyperplasia, the use of targeted low-dose progestins—oral or intrauterine—is appealing. While Orbo and colleagues present an interesting study, they do not definitively establish the optimal intervention.
As we enter a cost-conscious phase of medicine in the United States, we may discover that oral generic MPA (given continuously) may be the most cost-effective treatment despite the option of delivering a low-dose progestin via intrauterine device.
David F. Archer, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001;76(1):25–31.
- Orbo A, Ames M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: A follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008;111(1):68–73.
- Lacey JV Jr, Mutter GL, Ronnett BM, et al. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer Res. 2008;68(14):6014–6020.
- Archer DF. Neoplasia of the female reproductive tract: Effects of hormone therapy. Endocrine. 2004;24(3):259–263.
- Moe BT, Vereide AB, Orbo A, Jaeger R, Sager G. Levonorgestrel, medroxyprogesterone, and progesterone cause a concentration-dependent reduction in endometrial cancer (Ishikawa) cell density, and high concentrations of progesterone and mifepristone act in synergy. Anticancer Res. 2009;29(4):1047–1052.
- Phillips A, Hahn DW, Kimek S, McGuire JL. A comparison of the potencies and activities of progestogens used in contraceptives. Contraception. 1987;36(2):181–192.
- Archer DF. Delivery of therapeutic agents to the target tissue. Menopause. 2011;18(10):1040–1041.
- Somboonporn W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause. 2011;18(10):1060–1066.
- Archer DF, McIntyre-Seltman K, Wilborn WW Jr, et al. Endometrial morphology in asymptomatic postmenopausal women. Am J Obstet Gynecol. 1991;165(2):317–322.
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril. 2001;76(1):25–31.
- Orbo A, Ames M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: A follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008;111(1):68–73.
- Lacey JV Jr, Mutter GL, Ronnett BM, et al. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer Res. 2008;68(14):6014–6020.
- Archer DF. Neoplasia of the female reproductive tract: Effects of hormone therapy. Endocrine. 2004;24(3):259–263.
- Moe BT, Vereide AB, Orbo A, Jaeger R, Sager G. Levonorgestrel, medroxyprogesterone, and progesterone cause a concentration-dependent reduction in endometrial cancer (Ishikawa) cell density, and high concentrations of progesterone and mifepristone act in synergy. Anticancer Res. 2009;29(4):1047–1052.
- Phillips A, Hahn DW, Kimek S, McGuire JL. A comparison of the potencies and activities of progestogens used in contraceptives. Contraception. 1987;36(2):181–192.
- Archer DF. Delivery of therapeutic agents to the target tissue. Menopause. 2011;18(10):1040–1041.
- Somboonporn W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause. 2011;18(10):1060–1066.
- Archer DF, McIntyre-Seltman K, Wilborn WW Jr, et al. Endometrial morphology in asymptomatic postmenopausal women. Am J Obstet Gynecol. 1991;165(2):317–322.