User login
Efficacy of daily hypertonic saline nasal irrigation among patients with sinusitis: A randomized controlled trial
- Nasal irrigation improved sinus symptoms and decreased sinus medication use.
- Patient satisfaction and compliance were high for nasal irrigation.
- Patient training in nasal irrigation technique should be provided.
- OBJECTIVES: To test whether daily hypertonic saline nasal irrigation improves sinus symptoms and quality of life and decreases medication use in adult subjects with a history of sinusitis.
- STUDY DESIGN: Randomized controlled trial. Experimental subjects used nasal irrigation daily for 6 months.
- POPULATION: Seventy-six subjects from primary care (n = 70) and otolaryngology (n = 6) clinics with histories of frequent sinusitis were randomized to experimental (n = 52) and control (n = 24) groups.
- OUTCOMES MEASURED: Primary outcome measures included the Medical Outcomes Survey Short Form (SF-12), the Rhinosinusitis Disability Index (RSDI), and a Single-Item Sinus-Symptom Severity Assessment (SIA); all 3 were completed at baseline, 1.5, 3, and 6 months. Secondary outcomes included daily assessment of compliance and biweekly assessment of symptoms and medication use. At 6 months, subjects reported on side effects, satisfaction with nasal irrigation, and the percentage of change in their sinus-related quality of life.
- RESULTS: No significant baseline differences existed between the 2 groups. Sixty-nine subjects (90.8%) completed the study. Compliance averaged 87%. Experimental group RSDI scores improved from 58.4 ± 2.0 to 72.8 ± 2.2 (P ≤ .05) compared with those of the control group (from 59.6 ± 3.0 to 60.4 ± 1.1); experimental group SIA scores improved from 3.9 ± 0.1 to 2.4 ± 0.1 (P ≤.05) compared with those of the control group (from 4.08 ± 0.15 to 4.07 ± 0.27). The number needed to treat to achieve 10% improvement on RSDI at 6 months was 2.0. Experimental subjects reported fewer 2-week periods with sinus-related symptoms (P < .05), used less antibiotics (P < .05), and used less nasal spray (P = .06). On the exit questionnaire 93% of experimental subjects reported overall improvement of sinus-related quality of life, and none reported worsening (P < .001); on average, experimental subjects reported 57 ± 4.5% improvement. Side effects were minor and infrequent. Satisfaction was high. We found no statistically significant improvement on the SF-12.
- CONCLUSIONS: Daily hypertonic saline nasal irrigation improves sinus-related quality of life, decreases symptoms, and decreases medication use in patients with frequent sinusitis. Primary care physicians can feel comfortable recommending this therapy.
Sinusitis is a common clinical problem with significant morbidity and often refractory symptoms that accounted for approximately 26.7 million office and emergency visits and resulted in $5.8 billion spent in direct costs in 1996.1 Sinusitis was the fifth most common diagnosis for which antibiotics were prescribed from 1985 to 1992.2 In 1992, 13 million prescriptions were written for sinusitis, up from 5.8 million in 1985.2 The number of US chronic sinusitis cases in 1994 was estimated at 35 million, for a prevalence of 134 per 1000 patients.3 The effect of sinusitis on patients’ quality of life (QOL) is significant and can rate as high as back pain, congestive heart disease, and chronic obstructive pulmonary disease on some measures.4
Hypertonic nasal irrigation is a therapy that flushes the nasal cavity with saline solution, facilitating a wash of the structures within. Originally part of the Yogic tradition, this technique is anecdotally regarded as safe and effective; it has been suggested as adjunctive therapy for sinusitis and sinus symptoms.5-7 Potential efficacy is supported by the observation that hypertonic saline improves mucociliary clearance,8 thins mucus,9,10 and may decrease inflammation.8 Optimal irrigant salinity and pH are unclear.10,11 Several small trials examining nasal irrigation have suggested that nasal irrigation is safe, improves nasal symptoms, and is physically tolerable, but inclusion criteria, intervention protocols, and methodological quality vary.12-18 Improvement of QOL scores12-14 and several surrogate measures14-16 have been reported. No study has rigorously evaluated nasal irrigation over a longer period for its effect on QOL, antibiotic and nasal medication use, symptom severity, compliance, and side effects.
We conducted a randomized controlled trial to test the hypotheses that daily hypertonic saline nasal irrigation improves symptoms, decreases antibiotic and nasal medication use, and improves QOL in adult subjects with a history of sinusitis.
Methods
The study protocol was approved by the University of Wisconsin Health Sciences Human Subjects Committee. Subjects were enrolled from May to August 2000 and, after a study period of 6 months, were exited from November 2000 to February 2001. No prior studies existed at inception to guide sample size estimation. Power calculations performed before study initiation indicated that a sample size of 60 subjects would provide 80% power to detect a 10% difference in the Rhinosinusitis Disability Index (RSDI) between study groups. Due to the high patient burden of this study, we assumed a 25% dropout rate.
Randomization
The randomization scheme was prepared by the Investigational Drug Services of the University of Wisconsin Hospital and Clinics. Subjects were stratified by smoking status and then randomized by using an approximate 2:1 block design, with 10 subjects per block. Therefore 68% of subjects were assigned to the experimental group and 32% to the control group. A 2:1 scheme favoring the experimental group was selected due to resource limitations.
Eligibility criteria and subject recruitment
The recruitment and subject participation scheme is shown in Figure W1 (available on the JFP Web site: http://www.jfponline.com). The billing databases for the University of Wisconsin primary care and Ear, Nose, and Throat (ENT) practices were screened for acute and chronic sinusitis (codes 461 and 473, respectively, from the International Classification of Diseases, Ninth Revision). Patients 18 to 65 years old with 2 episodes of acute sinusitis or 1 episode of chronic sinusitis per year for 2 consecutive years (n = 602) were sent a letter explaining the study and inviting participation, along with an opt-out postcard. If no card was returned, potential subjects were phoned. Exclusion criteria included pregnancy and comorbidity significant enough to preclude travel to an informational meeting or performance of the nasal irrigation technique. Patients indicating “moderate to severe” impact of sinus symptoms on their QOL on a Likert scale of 1 to 7 were invited to attend an informational meeting involving enrollment, randomization, and training (n = 128). Of those potential subjects, 44 declined the meeting or were ineligible; 84 agreed to attend the meeting, 77 attended, and 76 enrolled. Of the initial group of 602 potential subjects, 375 were not contacted because the study census reached intended sample size.
One of us (D.R., R.M., or A.Z.) facilitated each informational meeting of 1 to 6 persons. Sealed envelopes containing the patient’s randomized group assignment were distributed to subjects in the order they entered the room. The group assignment was unknown to the investigator. Subjects broke the seal and learned their assignment. Thereafter, investigators were not blind to subjects’ group assignment. Persons managing and analyzing data also saw unblinded data but had no contact with subjects. Participants heard a brief presentation about sinus disease and its treatment. Nasal irrigation theory and technique were explained. Seventy-six subjects consented and were allocated by their randomized group assignments to experimental (n = 52) or control (n = 24) groups. Control subjects continued treatment of sinus disease in their usual manner. Experimental subjects saw a brief demonstration film, witnessed nasal irrigation by the facilitator, and demonstrated proficiency with the nasal irrigation technique before departure. Subjects were provided all ingredients and materials for 6 months of daily nasal irrigation. Experimental subjects also continued usual care for sinus disease.
Intervention
Subjects in the experimental group were asked to irrigate the nose (150 mL through each nostril) daily for 6 months with the SinuCleanse19 nasal cup containing 2.0% saline buffered with baking soda (1 heaping teaspoon of canning salt, one half teaspoon of baking soda, and 1 pint of tap water; (Table 1). Solution was mixed fresh every 1 to 2 days. All subjects were phoned at 2 weeks to assess initial compliance with study protocols and thereafter if assessment instruments were not returned promptly.
TABLE 1
Baseline patient characteristics*
| Variable | Control group (n = 24) | Experimental group (n = 52) |
|---|---|---|
| Age, y† | 41.4 ± 2.4 | 42.4 ± 1.4 |
| RSDI score† | 59.6 ± 3.0 | 58.4 ± 2.0 |
| SF-12 score† | 59.3 ± 4.0 | 60.3 ± 3.0 |
| SIA score† | 4.1 ± 0.2 | 3.9 ± 0.1 |
| Female‡ | 18 (75) | 37 (71) |
| Caucasian race‡ | 23 (96) | 49 (94) |
| Smokers‡ | 1 (4) | 3 (6) |
| Education‡ | ||
| ≤High school | 6 (25) | 11 (21) |
| Some college | 10 (42) | 18 (35) |
| ≥College degree | 8 (33) | 23 (44) |
| Seasonal allergies‡ | 17 (71) | 34 (66) |
| Medication allergies‡ | 12 (50) | 29 (56) |
| ENT history‡ | ||
| Nasal surgery | 7 (29) | 19 (37) |
| Nasal polyps | 3 (13) | 9 (17) |
| Deviated septum | 7 (29) | 12 (23) |
| Nasal fracture | 4 (17) | 7 (13) |
| Asthma‡ | 4 (17) | 14 (27) |
| ICD-9 code‡ | ||
| 461 (acute sinusitis) | 20 (83) | 34 (65) |
| 473 (chronic sinusitis) | 2 (8) | 11 (21) |
| Both (acute and chronic sinusitis) | 2 (8) | 7 (14) |
| Clinic type‡ | ||
| Primary care | 24 (100) | 46 (89) |
| ENT | 0 (0) | 6 (12) |
| *At baseline, there were no statistically significant (P > .05) differences between the experimental and control groups. | ||
| †Data are presented as mean ± standard error. | ||
| ‡Data are presented as number (%) of subjects. | ||
| ENT, Ear, Nose, and Throat; ICD-9, International Classification of Diseases, Ninth Revision; RSDI, Rhinosinusitis Disability Index; SF-12, Medical Outcomes Survey Short Form 12; SIA, Single-Item Symptom Severity Assessment. | ||
Outcome measures
The primary outcomes were QOL scores from 2 validated questionnaires: the general health assessment Medical Outcomes Survey Short Form (SF-12)20 and the RSDI,21 a disease-specific instrument assessing QOL in emotional, functional, and physical domains. We reworded the phrase my problem to my sinus symptoms on several RSDI items. Consensus within the research group and among consulted experts was that this minor change facilitated more accurate reading and reporting. We also measured overall sinus symptom severity with a Single-Item Symptom Severity Assessment (SIA): “Please evaluate the overall severity of your sinus symptoms since you enrolled in the study”; higher scores on the Likert scale SIA indicated increased severity. Scales for RSDI and SF-12 ranged from 0 to 100 points, with higher scores indicating better overall QOL. Each was completed at baseline and at 1.5, 3, and 6 months; at the 6-month assessment, subjects were shown their baseline answers for comparison because they had told us they needed to recall answers to past questions. They believed they knew whether they felt better or worse and wanted their later answers to reflect this change. Allowing subjects to view previous scores is an accepted research practice.22 However, because we did not allow subjects to see their baseline answers at 1.5 and 3 months, scores must be interpreted in light of the availability of the baseline data to the subjects.
Secondary outcomes were assessed with multiple methods. Compliance with nasal irrigation was recorded in a daily diary. The presence or absence of sinus symptoms (headache, congestion, facial pressure, facial pain, nasal discharge), antibiotic use, and nasal-spray use was assessed every 2 weeks. An exit questionnaire asked subjects to report categorically whether their sinus-related QOL had gotten worse, stayed the same, or improved, and to estimate the percentage of change (scale from 0 to ±100%). Overall satisfaction and side effects were reported at 6 months.
Statistical methods
Baseline characteristics of experimental and control groups were compared to assess randomization. Analysis, performed on an intention-to-treat basis, involved all 76 subjects randomized into the study. As dictated by the intention-to-treat model, the few missing values were imputed with multiple regression. Repeated measures analysis of variance contrasted the primary outcomes, that is, QOL status and sinus symptom scores within each group at baseline and subsequent periods. Differences between experimental and control groups were analyzed at each point in the repeated measures model and comprehensively for the entire time frame of the study. Statistical significance was assessed with 2-tailed tests. Data are presented as mean values with range of standard error, unless otherwise indicated.
Results
The study sample (Table 1) consisted of 76 subjects (55 female) randomized to experimental (n = 52) and control (n = 24) groups. Subjects’ ages ranged from 19 to 62 years, with a mean age of 42 years. Sixty-nine subjects (46 experimental and 23 control) completed the study. Seven subjects dropped out of the study at 1.5 months or earlier. A phone questionnaire was completed by 3 experimental dropouts; 2 of the 3 identified “lack of time” as the main reason for leaving the study; the remaining subject did not specify a reason. All 3 identified nasal irrigation as “helpful,” and none identified side effects as significant. The remaining 4 subjects were lost to follow-up. Dropouts tended to have slightly better baseline RSDI scores than nondropouts, 66.8 vs 58.1 points, but this difference was not significant (P = .15). No significant baseline differences were found between the groups of mostly white, female, well-educated subjects (Table 1). Baseline RSDI, SF-12, and SIA scores were similar in both groups. Although ENT subjects tended to have slightly worse baseline RSDI and SIA scores and improved slightly more during the study than other experimental subjects, the effect of clinic type (ENT vs primary care) was not statistically significant. By chance all subjects from ENT clinics (n = 6) and a disproportionate percentage of subjects with chronic sinusitis were randomized to the experimental group. Neither variable was statistically significant.
Experimental subjects showed a significant improvement in RSDI scores: 58.4 ± 2.0, 66.6 ± 2.2, 72.4 ± 2.2, and 72.8 ± 2.2 points at baseline, 1.5, 3, and 6 months, respectively (Table 2, Figure 2). Although the difference was not significant (P = .08), experimental subjects whose initial RSDI score was less than 50 points improved the most, with an average score change of 17.8 ± 4.4, and comparable control subjects had an average RSDI score change of 8.8 ± 2.9 points. Emotional and functional RSDI domains were not significantly related to score change; however, the physical domain of the survey was significant (P = .05).
SIA scores for experimental subjects improved (P < .05) at all follow-up points compared with control subjects; scores for the experimental group were 3.9 ± 0.1, 3.1 ± 0.2, 2.7 ± 0.2, and 2.4 ± 0.1 points at baseline, 1.5, 3, and 6 months, respectively (Table 2, Figure 2).
SF-12 score showed no significant differences between groups at any follow-up point but by 6 months trended toward significance (P = .06; Table 2).
Forty-one (93%) experimental subjects completing the exit questionnaire reported improvement. Most (n = 16, 73%) control subjects reported no change, but 18% reported worsening (P < .001; Table 3). Experimental subjects reported an average of 57 ± 4.5% improvement (range, 0–100%), whereas control subjects reported an average of 7 ± 5.9% worsening (range, -80% to 50%; P < .001).
Experimental subjects reported using nasal irrigation on 87% of days during the study; 31 subjects reported using nasal irrigation on 91% or more days, 13 subjects on 76% to 90% of days, and 5 subjects on 51% to 75% of days. Only 3 subjects used nasal irrigation on 50% or fewer days; these 3 subjects had relatively good baseline RSDI and SIA scores compared with other experimental subjects. Compliance was not significantly associated with changes in SIA or RSDI scores. The average survey completion rate was 96% at each assessment by each group.
Experimental subjects spent fewer 2-week blocks with nasal congestion, sinus headache, and frontal pain and pressure and used antibiotics and nasal sprays in fewer blocks (Table 3).
Forty-four experimental subjects answered questions about satisfaction and side effects. Forty-two stated they “will continue to use” nasal irrigation; the remaining 2 subjects found nasal irrigation less helpful but did not experience side effects. All 44 subjects “would recommend” nasal irrigation to friends or family with sinus problems. Ten subjects (23%) experienced side effects; 8 identified nasal irritation, nasal burning, tearing, nosebleeds, headache, or nasal drainage as occurring but “not significant.” Two subjects identified nasal burning, irritation, and headache as “significant,” but this did not change their high satisfaction rating. Of the 10 subjects who experienced side effects, 4 reduced or eliminated the side effects by temporarily alternating treatment days or decreasing salinity by 50%.
TABLE 2
Primary outcomes: RSDI, SF-12, and SIA baseline scores and mean score changes*
| Status | Baseline score | Baseline vs score change at | ||
|---|---|---|---|---|
| 1.5 mo | 3 mo | 6 mo | ||
| RSDI | ||||
| Experimental | 58.4 ± 2.0 | 8.2 ± 1.2 | 14.0 ± 2.0† | 14.4 ± 1.7‡ |
| Control | 59.6 ± 3.0 | 5.6 ± 1.4 | 7.7 ± 1.9 | 0.9 ± 1.0 |
| SF-12 | ||||
| Experimental | 60.3 ± 3.0 | 6.7 ± 2.1 | 8.2 ± 2.9 | 12.7 ± 3.6 |
| Control | 59.3 ± 3.9 | 5.4 ± 3.9 | 2.9 ± 4.0 | 2.2 ± 3.5 |
| SIA | ||||
| Experimental | 3.9 ± 0.1 | -0.8 ± 0.2† | -1.2 ± 0.2† | -1.6 ± 0.2‡ |
| Control | 4.1 ± 0.2 | -0.02 ± 0.21 | -0.3 ± 0.2 | -0.005 ± 0.2 |
| *Data are presented as mean ± standard error. | ||||
| †Statistically significant at P < .05. | ||||
| ‡Statistically significant at P < .001. | ||||
| RSDI, Rhinosinusitis Disability Index; SF-12, Medical Outcomes Survey Short Form 12; SIA, Single-Item Symptom Severity Assessment. | ||||
TABLE 3
Secondary outcomes
| Secondary outcome | Experimental | Control |
|---|---|---|
| Sinus symptoms* | ||
| Sinus headache† | 57 ± 0.05 | 76 ± 0.06 |
| Frontal pain‡ | 55 ± 0.05 | 82 ± 0.05 |
| Frontal pressure‡ | 53 ± 0.05 | 86 ± 0.05 |
| Nasal congestion† | 67 ± 0.04 | 83 ± 0.05 |
| Nasal discharge | 65 ± 0.05 | 69 ± 0.07 |
| Medication use* | ||
| Antibiotics† | 10 ± 0.02 | 19 ± 0.04 |
| Nasal sprays§ | 4 ± 0.01 | 8 ± 0.02 |
| EQ: sinus symptoms related to QOL|| | ||
| Better‡ | 41 (93) | 2 (9) |
| Same‡ | 3 (7) | 16 (73) |
| Worse‡ | 0 (0) | 4 (18) |
| *Data are presented as the percentage of 2-week blocks ± standard error during the study. | ||
| †Statistically significant difference between groups: P < .05. | ||
| ‡Statistically significant difference between groups: P < .001. | ||
| §Not statistically significant, difference between groups: P = .06. | ||
| || Data are presented as number (%) of subjects. | ||
| EQ, exit questionnaire (Is your quality of life with respect to sinus symptoms better or worse since the beginning of the study?); QOL, quality of life. | ||
FIGURE 1
Position of nasal cup for nasal irrigation therapy A B
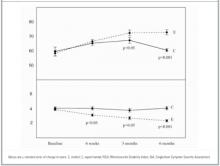
FIGURE 2
Mean RSDI and SIA scores in control and experimental subjects
DISCUSSION
Our trial of daily hypertonic nasal irrigation produced several significant findings. We found consistent, statistically significant improvements in QOL (RSDI) and overall symptom severity (SIA). This was consistent with QOL improvement previously reported over short periods with the use of disease-specific measures.12-14 The RSDI is a moderately well-developed and validated disease-specific QOL instrument.21-23 The “minimal clinically important difference,” defined as the average score improvement needed to justify costs and risks,24-26 has not been established for sinusitis. However, it has been estimated for other disease states. For example, a half-point change on a 7-point Likert scale corresponds to estimates of important change in patients with chronic heart and lung disease.22,27 Others have found similar relationships.28-31 In our study, RSDI scores among treated subjects averaged 6.0 and 15.5 points better than controls at 3 and 6 months, respectively. On the SIA, treated subjects averaged 0.6, 0.9, and 1.6 points better. Extrapolating from these findings, these differences appear to be clinically significant. By using 10% improvement of the RSDI, our data showed numbers needed to treat of 9, 5, and 2 at 1.5, 3, and 6 months, respectively (95% confidence interval at 6 months, 1.4–2.6). Numbers needed to treat for SIA, symptom frequency, and medication use were similar. SF-12 improvement, although not statistically significant in this small trial, may represent clinically significant improvements in general health-related QOL.
“Percentage change” is used often by clinicians to gauge therapeutic progress. Ours is the first study to document such change in sinusitis patients using nasal irrigation. Ours is also the first trial to show decreased symptom frequency over a 6-month period. Shorter trials have documented improvement in patients with nasal symptoms12,13,17,18 or with chronic sinusitis in adult14,15 and pediatric16 populations. Consistent with improved symptoms and QOL, experimental subjects decreased their use of antibiotics and nasal sprays, as previously reported in a short trial.12
Side effects have not been carefully assessed in previous trials. Although generally safe, daily hypertonic nasal irrigation was associated with some clinically minor side effects. Interestingly, subjects were able to decrease side effects by adjusting irrigation schedule or salinity. Side effects were not sufficiently bothersome to stop therapy. Compliance with daily therapy was very high and is previously unreported. Although this was consistent with a positive effect on relatively severe symptoms, we believe high compliance also was related to teaching, demonstrated proficiency with nasal irrigation, and close telephone follow-up. One prior study reported subjects’ observation of the first nasal irrigation15; several studies reported providing some education.1214,18
Our study has several limitations. It was not blinded or placebo controlled. Blinding subjects to a physical therapy is inherently difficult. Investigators who have tried to use normal saline placebos probably affected outcomes.14-16 One trial using a fresh water (0% saline) placebo was stopped early when several control subjects developed otitis media.32 The investigators also were unblinded, possibly creating observer bias.
Methodologic and recruitment strengths of this study included effective randomization, matched control group, intention-to-treat analysis, low missing data rates, high compliance rate, and low dropout rate. Clinical strengths included significant findings on most parameters assessed. Particularly intriguing was the decreased use of antibiotics in the experimental group. This study offered strong evidence that nasal irrigation is a safe, effective, and inexpensive (nasal pot, $15; daily therapy, <$1/month) therapy for sinus disease that properly trained patients will use. Although questions about the protocol (schedule, concentration, and buffering) and indications require further study in a more diverse patient population, clinicians may confidently recommend nasal irrigation; it offers significant hope for symptomatic relief and QOL improvement for millions of individuals with sinus disease who often have few therapeutic options.
CONCLUSIONS
Daily hypertonic saline nasal irrigation improves sinus-related QOL, decreases symptoms, and decreases medication use in patients with frequent sinusitis. Primary care physicians can feel comfortable recommending this therapy.
Acknowledgments
We thank Thomas Pasic, MD, Michael McDonald, MD, and Diane Heatley, MD, Department of Otolaryngology, University of Wisconsin, Madison.
1. Ray NF, Baraniuk JN, Thamer M, et al. Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis and other airway disorders. J Allergy Clin Immunol 1999;103:408-14.
2. McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA 1995;273:214-9.
3. Centers for Disease Control.Vital and Health Statistics.Current Estimates From the National Health Interview Survey, 1994. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Center for Health Statistics, 1995. DHHS publication PHS 96-1521.
4. Glicklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg 1995;113:104-9.
5. Kaliner MA, Osuguthorpe JD, Fireman P, et al. Sinusitis bench to bedside: current findings, future directions. J Allergy Clin Immunol 1997;99:S829-47.
6. Druce HM. Adjuncts to medical management of sinusitis. Otolaryngol Head Neck Surg 1990;103:880-3.
7. Zieger RS. Prospects for ancillary treatment of sinusitis in the 1990’s. J Allergy Clin Immunol 1992;90:478-93.
8. Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope 1997;107:500-3.
9. Robinson M, Hemming AL, Regnis JA, et al. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax 1997;52:900-3.
10. Homer JJ, Dowley AC, Condon L, El-Jassar P, Sood S. The effect of hypertonicity on nasal mucociliary clearance. Clin Otolaryngol 2000;25:558-60.
11. Homer JJ, England RJ, Wilde AD, Harwood GRJ, Stafford ND. The effect of pH of douching solution on mucociliary clearance. Clin Otolaryngol 1999;24:312-5.
12. Heatley DG, McConnell KE, Kille TL, Leverson GE. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngol Head Neck Surg 200l;125:44-8.
13. Tamooka LT, Murphy C, Davidson TM. Clinical study and literature review of nasal irrigation. Laryngoscope 2000;110:1189-93.
14. Taccariello M, Parikh A, Darby Y, Scadding G. Nasal douching as a valuable adjunct in the management of chronic rhinosinusitis. Rhinology 1999;37:29-32.
15. Bachmann G, Hommel G, Michel O. Effect of irrigation of the nose with isotonic salt solution on patients with chronic paranasal sinus disease. Eur Arch Otorhinolaryngol 2000;257:537-41.
16. Shoseyov D, Bibi H, Shai P, et al. Treatment with hypertonic saline versus normal saline wash of pediatric chronic sinusitis. J Allergy Clin Immunol 1998;101:602-5.
17. Rabone SJ, Saraswati SB. Acceptance and effects of nasal lavage in volunteer woodworkers. Occupat Med 1999;49:365-9.
18. Holmstrom M, Rosen G, Walander L. Effect of nasal lavage on nasal symptoms and physiology in wood industry workers. Rhinology 1997;35:108-12.
19. SinuCleanse Med Systems, April 22 2002. Available at: http://www.sinucleanse.com. Accessed October 7, 2002.
20. Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary test of reliability and validity. Med Care 1996;34:220-6.
21. Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolyryngol Head Neck Surg 1997;123:1175-9.
22. Guyatt GH, Bombardier C, Tugwell P. Measuring disease-specific quality of life in clinical trials. CMAJ 1986;134:889-95.
23. Senior BA, Glaze C, Benninger MS. Use of the Rhinosinusitis Disability Score (RSDI) in rhinologic disease. Am J Rhinol 2001;15:15-20.
24. Deyo RA, Patrick DL. The significance of treatment effects: the clinical perspective. Med Care 1995;33:AS286-91.
25. Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol 1996;49:1215-9.
26. Samsa G. How should the minimum important difference for a health-related quality-of-life instrument be estimated? Med Care 2001;39:1037-8.
27. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15.
28. Bellamy N, Carr A, Dougados M, Shea B, Wells G. Towards a definition of “difference” in osteoarthritis. J Rheumatol 2001;28:427-30.
29. Powell CV, Kelly A-M. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med 2001;37:28-31.
30. Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog pain scores. Acad Emerg Med 1996;3:142-6.
31. Wells GA, Tugwell P, Kraag GR, et al. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol 1993;20:557-60.
32. Wendeler HM, Muller J, Dieler R, Helms J. Nasenspuling mit isotoner Emser-Salz-Losung bei chronischer rhinosinusitis. Otorhinolaryngol Nova 1997;7:254-8.
- Nasal irrigation improved sinus symptoms and decreased sinus medication use.
- Patient satisfaction and compliance were high for nasal irrigation.
- Patient training in nasal irrigation technique should be provided.
- OBJECTIVES: To test whether daily hypertonic saline nasal irrigation improves sinus symptoms and quality of life and decreases medication use in adult subjects with a history of sinusitis.
- STUDY DESIGN: Randomized controlled trial. Experimental subjects used nasal irrigation daily for 6 months.
- POPULATION: Seventy-six subjects from primary care (n = 70) and otolaryngology (n = 6) clinics with histories of frequent sinusitis were randomized to experimental (n = 52) and control (n = 24) groups.
- OUTCOMES MEASURED: Primary outcome measures included the Medical Outcomes Survey Short Form (SF-12), the Rhinosinusitis Disability Index (RSDI), and a Single-Item Sinus-Symptom Severity Assessment (SIA); all 3 were completed at baseline, 1.5, 3, and 6 months. Secondary outcomes included daily assessment of compliance and biweekly assessment of symptoms and medication use. At 6 months, subjects reported on side effects, satisfaction with nasal irrigation, and the percentage of change in their sinus-related quality of life.
- RESULTS: No significant baseline differences existed between the 2 groups. Sixty-nine subjects (90.8%) completed the study. Compliance averaged 87%. Experimental group RSDI scores improved from 58.4 ± 2.0 to 72.8 ± 2.2 (P ≤ .05) compared with those of the control group (from 59.6 ± 3.0 to 60.4 ± 1.1); experimental group SIA scores improved from 3.9 ± 0.1 to 2.4 ± 0.1 (P ≤.05) compared with those of the control group (from 4.08 ± 0.15 to 4.07 ± 0.27). The number needed to treat to achieve 10% improvement on RSDI at 6 months was 2.0. Experimental subjects reported fewer 2-week periods with sinus-related symptoms (P < .05), used less antibiotics (P < .05), and used less nasal spray (P = .06). On the exit questionnaire 93% of experimental subjects reported overall improvement of sinus-related quality of life, and none reported worsening (P < .001); on average, experimental subjects reported 57 ± 4.5% improvement. Side effects were minor and infrequent. Satisfaction was high. We found no statistically significant improvement on the SF-12.
- CONCLUSIONS: Daily hypertonic saline nasal irrigation improves sinus-related quality of life, decreases symptoms, and decreases medication use in patients with frequent sinusitis. Primary care physicians can feel comfortable recommending this therapy.
Sinusitis is a common clinical problem with significant morbidity and often refractory symptoms that accounted for approximately 26.7 million office and emergency visits and resulted in $5.8 billion spent in direct costs in 1996.1 Sinusitis was the fifth most common diagnosis for which antibiotics were prescribed from 1985 to 1992.2 In 1992, 13 million prescriptions were written for sinusitis, up from 5.8 million in 1985.2 The number of US chronic sinusitis cases in 1994 was estimated at 35 million, for a prevalence of 134 per 1000 patients.3 The effect of sinusitis on patients’ quality of life (QOL) is significant and can rate as high as back pain, congestive heart disease, and chronic obstructive pulmonary disease on some measures.4
Hypertonic nasal irrigation is a therapy that flushes the nasal cavity with saline solution, facilitating a wash of the structures within. Originally part of the Yogic tradition, this technique is anecdotally regarded as safe and effective; it has been suggested as adjunctive therapy for sinusitis and sinus symptoms.5-7 Potential efficacy is supported by the observation that hypertonic saline improves mucociliary clearance,8 thins mucus,9,10 and may decrease inflammation.8 Optimal irrigant salinity and pH are unclear.10,11 Several small trials examining nasal irrigation have suggested that nasal irrigation is safe, improves nasal symptoms, and is physically tolerable, but inclusion criteria, intervention protocols, and methodological quality vary.12-18 Improvement of QOL scores12-14 and several surrogate measures14-16 have been reported. No study has rigorously evaluated nasal irrigation over a longer period for its effect on QOL, antibiotic and nasal medication use, symptom severity, compliance, and side effects.
We conducted a randomized controlled trial to test the hypotheses that daily hypertonic saline nasal irrigation improves symptoms, decreases antibiotic and nasal medication use, and improves QOL in adult subjects with a history of sinusitis.
Methods
The study protocol was approved by the University of Wisconsin Health Sciences Human Subjects Committee. Subjects were enrolled from May to August 2000 and, after a study period of 6 months, were exited from November 2000 to February 2001. No prior studies existed at inception to guide sample size estimation. Power calculations performed before study initiation indicated that a sample size of 60 subjects would provide 80% power to detect a 10% difference in the Rhinosinusitis Disability Index (RSDI) between study groups. Due to the high patient burden of this study, we assumed a 25% dropout rate.
Randomization
The randomization scheme was prepared by the Investigational Drug Services of the University of Wisconsin Hospital and Clinics. Subjects were stratified by smoking status and then randomized by using an approximate 2:1 block design, with 10 subjects per block. Therefore 68% of subjects were assigned to the experimental group and 32% to the control group. A 2:1 scheme favoring the experimental group was selected due to resource limitations.
Eligibility criteria and subject recruitment
The recruitment and subject participation scheme is shown in Figure W1 (available on the JFP Web site: http://www.jfponline.com). The billing databases for the University of Wisconsin primary care and Ear, Nose, and Throat (ENT) practices were screened for acute and chronic sinusitis (codes 461 and 473, respectively, from the International Classification of Diseases, Ninth Revision). Patients 18 to 65 years old with 2 episodes of acute sinusitis or 1 episode of chronic sinusitis per year for 2 consecutive years (n = 602) were sent a letter explaining the study and inviting participation, along with an opt-out postcard. If no card was returned, potential subjects were phoned. Exclusion criteria included pregnancy and comorbidity significant enough to preclude travel to an informational meeting or performance of the nasal irrigation technique. Patients indicating “moderate to severe” impact of sinus symptoms on their QOL on a Likert scale of 1 to 7 were invited to attend an informational meeting involving enrollment, randomization, and training (n = 128). Of those potential subjects, 44 declined the meeting or were ineligible; 84 agreed to attend the meeting, 77 attended, and 76 enrolled. Of the initial group of 602 potential subjects, 375 were not contacted because the study census reached intended sample size.
One of us (D.R., R.M., or A.Z.) facilitated each informational meeting of 1 to 6 persons. Sealed envelopes containing the patient’s randomized group assignment were distributed to subjects in the order they entered the room. The group assignment was unknown to the investigator. Subjects broke the seal and learned their assignment. Thereafter, investigators were not blind to subjects’ group assignment. Persons managing and analyzing data also saw unblinded data but had no contact with subjects. Participants heard a brief presentation about sinus disease and its treatment. Nasal irrigation theory and technique were explained. Seventy-six subjects consented and were allocated by their randomized group assignments to experimental (n = 52) or control (n = 24) groups. Control subjects continued treatment of sinus disease in their usual manner. Experimental subjects saw a brief demonstration film, witnessed nasal irrigation by the facilitator, and demonstrated proficiency with the nasal irrigation technique before departure. Subjects were provided all ingredients and materials for 6 months of daily nasal irrigation. Experimental subjects also continued usual care for sinus disease.
Intervention
Subjects in the experimental group were asked to irrigate the nose (150 mL through each nostril) daily for 6 months with the SinuCleanse19 nasal cup containing 2.0% saline buffered with baking soda (1 heaping teaspoon of canning salt, one half teaspoon of baking soda, and 1 pint of tap water; (Table 1). Solution was mixed fresh every 1 to 2 days. All subjects were phoned at 2 weeks to assess initial compliance with study protocols and thereafter if assessment instruments were not returned promptly.
TABLE 1
Baseline patient characteristics*
| Variable | Control group (n = 24) | Experimental group (n = 52) |
|---|---|---|
| Age, y† | 41.4 ± 2.4 | 42.4 ± 1.4 |
| RSDI score† | 59.6 ± 3.0 | 58.4 ± 2.0 |
| SF-12 score† | 59.3 ± 4.0 | 60.3 ± 3.0 |
| SIA score† | 4.1 ± 0.2 | 3.9 ± 0.1 |
| Female‡ | 18 (75) | 37 (71) |
| Caucasian race‡ | 23 (96) | 49 (94) |
| Smokers‡ | 1 (4) | 3 (6) |
| Education‡ | ||
| ≤High school | 6 (25) | 11 (21) |
| Some college | 10 (42) | 18 (35) |
| ≥College degree | 8 (33) | 23 (44) |
| Seasonal allergies‡ | 17 (71) | 34 (66) |
| Medication allergies‡ | 12 (50) | 29 (56) |
| ENT history‡ | ||
| Nasal surgery | 7 (29) | 19 (37) |
| Nasal polyps | 3 (13) | 9 (17) |
| Deviated septum | 7 (29) | 12 (23) |
| Nasal fracture | 4 (17) | 7 (13) |
| Asthma‡ | 4 (17) | 14 (27) |
| ICD-9 code‡ | ||
| 461 (acute sinusitis) | 20 (83) | 34 (65) |
| 473 (chronic sinusitis) | 2 (8) | 11 (21) |
| Both (acute and chronic sinusitis) | 2 (8) | 7 (14) |
| Clinic type‡ | ||
| Primary care | 24 (100) | 46 (89) |
| ENT | 0 (0) | 6 (12) |
| *At baseline, there were no statistically significant (P > .05) differences between the experimental and control groups. | ||
| †Data are presented as mean ± standard error. | ||
| ‡Data are presented as number (%) of subjects. | ||
| ENT, Ear, Nose, and Throat; ICD-9, International Classification of Diseases, Ninth Revision; RSDI, Rhinosinusitis Disability Index; SF-12, Medical Outcomes Survey Short Form 12; SIA, Single-Item Symptom Severity Assessment. | ||
Outcome measures
The primary outcomes were QOL scores from 2 validated questionnaires: the general health assessment Medical Outcomes Survey Short Form (SF-12)20 and the RSDI,21 a disease-specific instrument assessing QOL in emotional, functional, and physical domains. We reworded the phrase my problem to my sinus symptoms on several RSDI items. Consensus within the research group and among consulted experts was that this minor change facilitated more accurate reading and reporting. We also measured overall sinus symptom severity with a Single-Item Symptom Severity Assessment (SIA): “Please evaluate the overall severity of your sinus symptoms since you enrolled in the study”; higher scores on the Likert scale SIA indicated increased severity. Scales for RSDI and SF-12 ranged from 0 to 100 points, with higher scores indicating better overall QOL. Each was completed at baseline and at 1.5, 3, and 6 months; at the 6-month assessment, subjects were shown their baseline answers for comparison because they had told us they needed to recall answers to past questions. They believed they knew whether they felt better or worse and wanted their later answers to reflect this change. Allowing subjects to view previous scores is an accepted research practice.22 However, because we did not allow subjects to see their baseline answers at 1.5 and 3 months, scores must be interpreted in light of the availability of the baseline data to the subjects.
Secondary outcomes were assessed with multiple methods. Compliance with nasal irrigation was recorded in a daily diary. The presence or absence of sinus symptoms (headache, congestion, facial pressure, facial pain, nasal discharge), antibiotic use, and nasal-spray use was assessed every 2 weeks. An exit questionnaire asked subjects to report categorically whether their sinus-related QOL had gotten worse, stayed the same, or improved, and to estimate the percentage of change (scale from 0 to ±100%). Overall satisfaction and side effects were reported at 6 months.
Statistical methods
Baseline characteristics of experimental and control groups were compared to assess randomization. Analysis, performed on an intention-to-treat basis, involved all 76 subjects randomized into the study. As dictated by the intention-to-treat model, the few missing values were imputed with multiple regression. Repeated measures analysis of variance contrasted the primary outcomes, that is, QOL status and sinus symptom scores within each group at baseline and subsequent periods. Differences between experimental and control groups were analyzed at each point in the repeated measures model and comprehensively for the entire time frame of the study. Statistical significance was assessed with 2-tailed tests. Data are presented as mean values with range of standard error, unless otherwise indicated.
Results
The study sample (Table 1) consisted of 76 subjects (55 female) randomized to experimental (n = 52) and control (n = 24) groups. Subjects’ ages ranged from 19 to 62 years, with a mean age of 42 years. Sixty-nine subjects (46 experimental and 23 control) completed the study. Seven subjects dropped out of the study at 1.5 months or earlier. A phone questionnaire was completed by 3 experimental dropouts; 2 of the 3 identified “lack of time” as the main reason for leaving the study; the remaining subject did not specify a reason. All 3 identified nasal irrigation as “helpful,” and none identified side effects as significant. The remaining 4 subjects were lost to follow-up. Dropouts tended to have slightly better baseline RSDI scores than nondropouts, 66.8 vs 58.1 points, but this difference was not significant (P = .15). No significant baseline differences were found between the groups of mostly white, female, well-educated subjects (Table 1). Baseline RSDI, SF-12, and SIA scores were similar in both groups. Although ENT subjects tended to have slightly worse baseline RSDI and SIA scores and improved slightly more during the study than other experimental subjects, the effect of clinic type (ENT vs primary care) was not statistically significant. By chance all subjects from ENT clinics (n = 6) and a disproportionate percentage of subjects with chronic sinusitis were randomized to the experimental group. Neither variable was statistically significant.
Experimental subjects showed a significant improvement in RSDI scores: 58.4 ± 2.0, 66.6 ± 2.2, 72.4 ± 2.2, and 72.8 ± 2.2 points at baseline, 1.5, 3, and 6 months, respectively (Table 2, Figure 2). Although the difference was not significant (P = .08), experimental subjects whose initial RSDI score was less than 50 points improved the most, with an average score change of 17.8 ± 4.4, and comparable control subjects had an average RSDI score change of 8.8 ± 2.9 points. Emotional and functional RSDI domains were not significantly related to score change; however, the physical domain of the survey was significant (P = .05).
SIA scores for experimental subjects improved (P < .05) at all follow-up points compared with control subjects; scores for the experimental group were 3.9 ± 0.1, 3.1 ± 0.2, 2.7 ± 0.2, and 2.4 ± 0.1 points at baseline, 1.5, 3, and 6 months, respectively (Table 2, Figure 2).
SF-12 score showed no significant differences between groups at any follow-up point but by 6 months trended toward significance (P = .06; Table 2).
Forty-one (93%) experimental subjects completing the exit questionnaire reported improvement. Most (n = 16, 73%) control subjects reported no change, but 18% reported worsening (P < .001; Table 3). Experimental subjects reported an average of 57 ± 4.5% improvement (range, 0–100%), whereas control subjects reported an average of 7 ± 5.9% worsening (range, -80% to 50%; P < .001).
Experimental subjects reported using nasal irrigation on 87% of days during the study; 31 subjects reported using nasal irrigation on 91% or more days, 13 subjects on 76% to 90% of days, and 5 subjects on 51% to 75% of days. Only 3 subjects used nasal irrigation on 50% or fewer days; these 3 subjects had relatively good baseline RSDI and SIA scores compared with other experimental subjects. Compliance was not significantly associated with changes in SIA or RSDI scores. The average survey completion rate was 96% at each assessment by each group.
Experimental subjects spent fewer 2-week blocks with nasal congestion, sinus headache, and frontal pain and pressure and used antibiotics and nasal sprays in fewer blocks (Table 3).
Forty-four experimental subjects answered questions about satisfaction and side effects. Forty-two stated they “will continue to use” nasal irrigation; the remaining 2 subjects found nasal irrigation less helpful but did not experience side effects. All 44 subjects “would recommend” nasal irrigation to friends or family with sinus problems. Ten subjects (23%) experienced side effects; 8 identified nasal irritation, nasal burning, tearing, nosebleeds, headache, or nasal drainage as occurring but “not significant.” Two subjects identified nasal burning, irritation, and headache as “significant,” but this did not change their high satisfaction rating. Of the 10 subjects who experienced side effects, 4 reduced or eliminated the side effects by temporarily alternating treatment days or decreasing salinity by 50%.
TABLE 2
Primary outcomes: RSDI, SF-12, and SIA baseline scores and mean score changes*
| Status | Baseline score | Baseline vs score change at | ||
|---|---|---|---|---|
| 1.5 mo | 3 mo | 6 mo | ||
| RSDI | ||||
| Experimental | 58.4 ± 2.0 | 8.2 ± 1.2 | 14.0 ± 2.0† | 14.4 ± 1.7‡ |
| Control | 59.6 ± 3.0 | 5.6 ± 1.4 | 7.7 ± 1.9 | 0.9 ± 1.0 |
| SF-12 | ||||
| Experimental | 60.3 ± 3.0 | 6.7 ± 2.1 | 8.2 ± 2.9 | 12.7 ± 3.6 |
| Control | 59.3 ± 3.9 | 5.4 ± 3.9 | 2.9 ± 4.0 | 2.2 ± 3.5 |
| SIA | ||||
| Experimental | 3.9 ± 0.1 | -0.8 ± 0.2† | -1.2 ± 0.2† | -1.6 ± 0.2‡ |
| Control | 4.1 ± 0.2 | -0.02 ± 0.21 | -0.3 ± 0.2 | -0.005 ± 0.2 |
| *Data are presented as mean ± standard error. | ||||
| †Statistically significant at P < .05. | ||||
| ‡Statistically significant at P < .001. | ||||
| RSDI, Rhinosinusitis Disability Index; SF-12, Medical Outcomes Survey Short Form 12; SIA, Single-Item Symptom Severity Assessment. | ||||
TABLE 3
Secondary outcomes
| Secondary outcome | Experimental | Control |
|---|---|---|
| Sinus symptoms* | ||
| Sinus headache† | 57 ± 0.05 | 76 ± 0.06 |
| Frontal pain‡ | 55 ± 0.05 | 82 ± 0.05 |
| Frontal pressure‡ | 53 ± 0.05 | 86 ± 0.05 |
| Nasal congestion† | 67 ± 0.04 | 83 ± 0.05 |
| Nasal discharge | 65 ± 0.05 | 69 ± 0.07 |
| Medication use* | ||
| Antibiotics† | 10 ± 0.02 | 19 ± 0.04 |
| Nasal sprays§ | 4 ± 0.01 | 8 ± 0.02 |
| EQ: sinus symptoms related to QOL|| | ||
| Better‡ | 41 (93) | 2 (9) |
| Same‡ | 3 (7) | 16 (73) |
| Worse‡ | 0 (0) | 4 (18) |
| *Data are presented as the percentage of 2-week blocks ± standard error during the study. | ||
| †Statistically significant difference between groups: P < .05. | ||
| ‡Statistically significant difference between groups: P < .001. | ||
| §Not statistically significant, difference between groups: P = .06. | ||
| || Data are presented as number (%) of subjects. | ||
| EQ, exit questionnaire (Is your quality of life with respect to sinus symptoms better or worse since the beginning of the study?); QOL, quality of life. | ||
FIGURE 1
Position of nasal cup for nasal irrigation therapy A B
FIGURE 2
Mean RSDI and SIA scores in control and experimental subjects
DISCUSSION
Our trial of daily hypertonic nasal irrigation produced several significant findings. We found consistent, statistically significant improvements in QOL (RSDI) and overall symptom severity (SIA). This was consistent with QOL improvement previously reported over short periods with the use of disease-specific measures.12-14 The RSDI is a moderately well-developed and validated disease-specific QOL instrument.21-23 The “minimal clinically important difference,” defined as the average score improvement needed to justify costs and risks,24-26 has not been established for sinusitis. However, it has been estimated for other disease states. For example, a half-point change on a 7-point Likert scale corresponds to estimates of important change in patients with chronic heart and lung disease.22,27 Others have found similar relationships.28-31 In our study, RSDI scores among treated subjects averaged 6.0 and 15.5 points better than controls at 3 and 6 months, respectively. On the SIA, treated subjects averaged 0.6, 0.9, and 1.6 points better. Extrapolating from these findings, these differences appear to be clinically significant. By using 10% improvement of the RSDI, our data showed numbers needed to treat of 9, 5, and 2 at 1.5, 3, and 6 months, respectively (95% confidence interval at 6 months, 1.4–2.6). Numbers needed to treat for SIA, symptom frequency, and medication use were similar. SF-12 improvement, although not statistically significant in this small trial, may represent clinically significant improvements in general health-related QOL.
“Percentage change” is used often by clinicians to gauge therapeutic progress. Ours is the first study to document such change in sinusitis patients using nasal irrigation. Ours is also the first trial to show decreased symptom frequency over a 6-month period. Shorter trials have documented improvement in patients with nasal symptoms12,13,17,18 or with chronic sinusitis in adult14,15 and pediatric16 populations. Consistent with improved symptoms and QOL, experimental subjects decreased their use of antibiotics and nasal sprays, as previously reported in a short trial.12
Side effects have not been carefully assessed in previous trials. Although generally safe, daily hypertonic nasal irrigation was associated with some clinically minor side effects. Interestingly, subjects were able to decrease side effects by adjusting irrigation schedule or salinity. Side effects were not sufficiently bothersome to stop therapy. Compliance with daily therapy was very high and is previously unreported. Although this was consistent with a positive effect on relatively severe symptoms, we believe high compliance also was related to teaching, demonstrated proficiency with nasal irrigation, and close telephone follow-up. One prior study reported subjects’ observation of the first nasal irrigation15; several studies reported providing some education.1214,18
Our study has several limitations. It was not blinded or placebo controlled. Blinding subjects to a physical therapy is inherently difficult. Investigators who have tried to use normal saline placebos probably affected outcomes.14-16 One trial using a fresh water (0% saline) placebo was stopped early when several control subjects developed otitis media.32 The investigators also were unblinded, possibly creating observer bias.
Methodologic and recruitment strengths of this study included effective randomization, matched control group, intention-to-treat analysis, low missing data rates, high compliance rate, and low dropout rate. Clinical strengths included significant findings on most parameters assessed. Particularly intriguing was the decreased use of antibiotics in the experimental group. This study offered strong evidence that nasal irrigation is a safe, effective, and inexpensive (nasal pot, $15; daily therapy, <$1/month) therapy for sinus disease that properly trained patients will use. Although questions about the protocol (schedule, concentration, and buffering) and indications require further study in a more diverse patient population, clinicians may confidently recommend nasal irrigation; it offers significant hope for symptomatic relief and QOL improvement for millions of individuals with sinus disease who often have few therapeutic options.
CONCLUSIONS
Daily hypertonic saline nasal irrigation improves sinus-related QOL, decreases symptoms, and decreases medication use in patients with frequent sinusitis. Primary care physicians can feel comfortable recommending this therapy.
Acknowledgments
We thank Thomas Pasic, MD, Michael McDonald, MD, and Diane Heatley, MD, Department of Otolaryngology, University of Wisconsin, Madison.
- Nasal irrigation improved sinus symptoms and decreased sinus medication use.
- Patient satisfaction and compliance were high for nasal irrigation.
- Patient training in nasal irrigation technique should be provided.
- OBJECTIVES: To test whether daily hypertonic saline nasal irrigation improves sinus symptoms and quality of life and decreases medication use in adult subjects with a history of sinusitis.
- STUDY DESIGN: Randomized controlled trial. Experimental subjects used nasal irrigation daily for 6 months.
- POPULATION: Seventy-six subjects from primary care (n = 70) and otolaryngology (n = 6) clinics with histories of frequent sinusitis were randomized to experimental (n = 52) and control (n = 24) groups.
- OUTCOMES MEASURED: Primary outcome measures included the Medical Outcomes Survey Short Form (SF-12), the Rhinosinusitis Disability Index (RSDI), and a Single-Item Sinus-Symptom Severity Assessment (SIA); all 3 were completed at baseline, 1.5, 3, and 6 months. Secondary outcomes included daily assessment of compliance and biweekly assessment of symptoms and medication use. At 6 months, subjects reported on side effects, satisfaction with nasal irrigation, and the percentage of change in their sinus-related quality of life.
- RESULTS: No significant baseline differences existed between the 2 groups. Sixty-nine subjects (90.8%) completed the study. Compliance averaged 87%. Experimental group RSDI scores improved from 58.4 ± 2.0 to 72.8 ± 2.2 (P ≤ .05) compared with those of the control group (from 59.6 ± 3.0 to 60.4 ± 1.1); experimental group SIA scores improved from 3.9 ± 0.1 to 2.4 ± 0.1 (P ≤.05) compared with those of the control group (from 4.08 ± 0.15 to 4.07 ± 0.27). The number needed to treat to achieve 10% improvement on RSDI at 6 months was 2.0. Experimental subjects reported fewer 2-week periods with sinus-related symptoms (P < .05), used less antibiotics (P < .05), and used less nasal spray (P = .06). On the exit questionnaire 93% of experimental subjects reported overall improvement of sinus-related quality of life, and none reported worsening (P < .001); on average, experimental subjects reported 57 ± 4.5% improvement. Side effects were minor and infrequent. Satisfaction was high. We found no statistically significant improvement on the SF-12.
- CONCLUSIONS: Daily hypertonic saline nasal irrigation improves sinus-related quality of life, decreases symptoms, and decreases medication use in patients with frequent sinusitis. Primary care physicians can feel comfortable recommending this therapy.
Sinusitis is a common clinical problem with significant morbidity and often refractory symptoms that accounted for approximately 26.7 million office and emergency visits and resulted in $5.8 billion spent in direct costs in 1996.1 Sinusitis was the fifth most common diagnosis for which antibiotics were prescribed from 1985 to 1992.2 In 1992, 13 million prescriptions were written for sinusitis, up from 5.8 million in 1985.2 The number of US chronic sinusitis cases in 1994 was estimated at 35 million, for a prevalence of 134 per 1000 patients.3 The effect of sinusitis on patients’ quality of life (QOL) is significant and can rate as high as back pain, congestive heart disease, and chronic obstructive pulmonary disease on some measures.4
Hypertonic nasal irrigation is a therapy that flushes the nasal cavity with saline solution, facilitating a wash of the structures within. Originally part of the Yogic tradition, this technique is anecdotally regarded as safe and effective; it has been suggested as adjunctive therapy for sinusitis and sinus symptoms.5-7 Potential efficacy is supported by the observation that hypertonic saline improves mucociliary clearance,8 thins mucus,9,10 and may decrease inflammation.8 Optimal irrigant salinity and pH are unclear.10,11 Several small trials examining nasal irrigation have suggested that nasal irrigation is safe, improves nasal symptoms, and is physically tolerable, but inclusion criteria, intervention protocols, and methodological quality vary.12-18 Improvement of QOL scores12-14 and several surrogate measures14-16 have been reported. No study has rigorously evaluated nasal irrigation over a longer period for its effect on QOL, antibiotic and nasal medication use, symptom severity, compliance, and side effects.
We conducted a randomized controlled trial to test the hypotheses that daily hypertonic saline nasal irrigation improves symptoms, decreases antibiotic and nasal medication use, and improves QOL in adult subjects with a history of sinusitis.
Methods
The study protocol was approved by the University of Wisconsin Health Sciences Human Subjects Committee. Subjects were enrolled from May to August 2000 and, after a study period of 6 months, were exited from November 2000 to February 2001. No prior studies existed at inception to guide sample size estimation. Power calculations performed before study initiation indicated that a sample size of 60 subjects would provide 80% power to detect a 10% difference in the Rhinosinusitis Disability Index (RSDI) between study groups. Due to the high patient burden of this study, we assumed a 25% dropout rate.
Randomization
The randomization scheme was prepared by the Investigational Drug Services of the University of Wisconsin Hospital and Clinics. Subjects were stratified by smoking status and then randomized by using an approximate 2:1 block design, with 10 subjects per block. Therefore 68% of subjects were assigned to the experimental group and 32% to the control group. A 2:1 scheme favoring the experimental group was selected due to resource limitations.
Eligibility criteria and subject recruitment
The recruitment and subject participation scheme is shown in Figure W1 (available on the JFP Web site: http://www.jfponline.com). The billing databases for the University of Wisconsin primary care and Ear, Nose, and Throat (ENT) practices were screened for acute and chronic sinusitis (codes 461 and 473, respectively, from the International Classification of Diseases, Ninth Revision). Patients 18 to 65 years old with 2 episodes of acute sinusitis or 1 episode of chronic sinusitis per year for 2 consecutive years (n = 602) were sent a letter explaining the study and inviting participation, along with an opt-out postcard. If no card was returned, potential subjects were phoned. Exclusion criteria included pregnancy and comorbidity significant enough to preclude travel to an informational meeting or performance of the nasal irrigation technique. Patients indicating “moderate to severe” impact of sinus symptoms on their QOL on a Likert scale of 1 to 7 were invited to attend an informational meeting involving enrollment, randomization, and training (n = 128). Of those potential subjects, 44 declined the meeting or were ineligible; 84 agreed to attend the meeting, 77 attended, and 76 enrolled. Of the initial group of 602 potential subjects, 375 were not contacted because the study census reached intended sample size.
One of us (D.R., R.M., or A.Z.) facilitated each informational meeting of 1 to 6 persons. Sealed envelopes containing the patient’s randomized group assignment were distributed to subjects in the order they entered the room. The group assignment was unknown to the investigator. Subjects broke the seal and learned their assignment. Thereafter, investigators were not blind to subjects’ group assignment. Persons managing and analyzing data also saw unblinded data but had no contact with subjects. Participants heard a brief presentation about sinus disease and its treatment. Nasal irrigation theory and technique were explained. Seventy-six subjects consented and were allocated by their randomized group assignments to experimental (n = 52) or control (n = 24) groups. Control subjects continued treatment of sinus disease in their usual manner. Experimental subjects saw a brief demonstration film, witnessed nasal irrigation by the facilitator, and demonstrated proficiency with the nasal irrigation technique before departure. Subjects were provided all ingredients and materials for 6 months of daily nasal irrigation. Experimental subjects also continued usual care for sinus disease.
Intervention
Subjects in the experimental group were asked to irrigate the nose (150 mL through each nostril) daily for 6 months with the SinuCleanse19 nasal cup containing 2.0% saline buffered with baking soda (1 heaping teaspoon of canning salt, one half teaspoon of baking soda, and 1 pint of tap water; (Table 1). Solution was mixed fresh every 1 to 2 days. All subjects were phoned at 2 weeks to assess initial compliance with study protocols and thereafter if assessment instruments were not returned promptly.
TABLE 1
Baseline patient characteristics*
| Variable | Control group (n = 24) | Experimental group (n = 52) |
|---|---|---|
| Age, y† | 41.4 ± 2.4 | 42.4 ± 1.4 |
| RSDI score† | 59.6 ± 3.0 | 58.4 ± 2.0 |
| SF-12 score† | 59.3 ± 4.0 | 60.3 ± 3.0 |
| SIA score† | 4.1 ± 0.2 | 3.9 ± 0.1 |
| Female‡ | 18 (75) | 37 (71) |
| Caucasian race‡ | 23 (96) | 49 (94) |
| Smokers‡ | 1 (4) | 3 (6) |
| Education‡ | ||
| ≤High school | 6 (25) | 11 (21) |
| Some college | 10 (42) | 18 (35) |
| ≥College degree | 8 (33) | 23 (44) |
| Seasonal allergies‡ | 17 (71) | 34 (66) |
| Medication allergies‡ | 12 (50) | 29 (56) |
| ENT history‡ | ||
| Nasal surgery | 7 (29) | 19 (37) |
| Nasal polyps | 3 (13) | 9 (17) |
| Deviated septum | 7 (29) | 12 (23) |
| Nasal fracture | 4 (17) | 7 (13) |
| Asthma‡ | 4 (17) | 14 (27) |
| ICD-9 code‡ | ||
| 461 (acute sinusitis) | 20 (83) | 34 (65) |
| 473 (chronic sinusitis) | 2 (8) | 11 (21) |
| Both (acute and chronic sinusitis) | 2 (8) | 7 (14) |
| Clinic type‡ | ||
| Primary care | 24 (100) | 46 (89) |
| ENT | 0 (0) | 6 (12) |
| *At baseline, there were no statistically significant (P > .05) differences between the experimental and control groups. | ||
| †Data are presented as mean ± standard error. | ||
| ‡Data are presented as number (%) of subjects. | ||
| ENT, Ear, Nose, and Throat; ICD-9, International Classification of Diseases, Ninth Revision; RSDI, Rhinosinusitis Disability Index; SF-12, Medical Outcomes Survey Short Form 12; SIA, Single-Item Symptom Severity Assessment. | ||
Outcome measures
The primary outcomes were QOL scores from 2 validated questionnaires: the general health assessment Medical Outcomes Survey Short Form (SF-12)20 and the RSDI,21 a disease-specific instrument assessing QOL in emotional, functional, and physical domains. We reworded the phrase my problem to my sinus symptoms on several RSDI items. Consensus within the research group and among consulted experts was that this minor change facilitated more accurate reading and reporting. We also measured overall sinus symptom severity with a Single-Item Symptom Severity Assessment (SIA): “Please evaluate the overall severity of your sinus symptoms since you enrolled in the study”; higher scores on the Likert scale SIA indicated increased severity. Scales for RSDI and SF-12 ranged from 0 to 100 points, with higher scores indicating better overall QOL. Each was completed at baseline and at 1.5, 3, and 6 months; at the 6-month assessment, subjects were shown their baseline answers for comparison because they had told us they needed to recall answers to past questions. They believed they knew whether they felt better or worse and wanted their later answers to reflect this change. Allowing subjects to view previous scores is an accepted research practice.22 However, because we did not allow subjects to see their baseline answers at 1.5 and 3 months, scores must be interpreted in light of the availability of the baseline data to the subjects.
Secondary outcomes were assessed with multiple methods. Compliance with nasal irrigation was recorded in a daily diary. The presence or absence of sinus symptoms (headache, congestion, facial pressure, facial pain, nasal discharge), antibiotic use, and nasal-spray use was assessed every 2 weeks. An exit questionnaire asked subjects to report categorically whether their sinus-related QOL had gotten worse, stayed the same, or improved, and to estimate the percentage of change (scale from 0 to ±100%). Overall satisfaction and side effects were reported at 6 months.
Statistical methods
Baseline characteristics of experimental and control groups were compared to assess randomization. Analysis, performed on an intention-to-treat basis, involved all 76 subjects randomized into the study. As dictated by the intention-to-treat model, the few missing values were imputed with multiple regression. Repeated measures analysis of variance contrasted the primary outcomes, that is, QOL status and sinus symptom scores within each group at baseline and subsequent periods. Differences between experimental and control groups were analyzed at each point in the repeated measures model and comprehensively for the entire time frame of the study. Statistical significance was assessed with 2-tailed tests. Data are presented as mean values with range of standard error, unless otherwise indicated.
Results
The study sample (Table 1) consisted of 76 subjects (55 female) randomized to experimental (n = 52) and control (n = 24) groups. Subjects’ ages ranged from 19 to 62 years, with a mean age of 42 years. Sixty-nine subjects (46 experimental and 23 control) completed the study. Seven subjects dropped out of the study at 1.5 months or earlier. A phone questionnaire was completed by 3 experimental dropouts; 2 of the 3 identified “lack of time” as the main reason for leaving the study; the remaining subject did not specify a reason. All 3 identified nasal irrigation as “helpful,” and none identified side effects as significant. The remaining 4 subjects were lost to follow-up. Dropouts tended to have slightly better baseline RSDI scores than nondropouts, 66.8 vs 58.1 points, but this difference was not significant (P = .15). No significant baseline differences were found between the groups of mostly white, female, well-educated subjects (Table 1). Baseline RSDI, SF-12, and SIA scores were similar in both groups. Although ENT subjects tended to have slightly worse baseline RSDI and SIA scores and improved slightly more during the study than other experimental subjects, the effect of clinic type (ENT vs primary care) was not statistically significant. By chance all subjects from ENT clinics (n = 6) and a disproportionate percentage of subjects with chronic sinusitis were randomized to the experimental group. Neither variable was statistically significant.
Experimental subjects showed a significant improvement in RSDI scores: 58.4 ± 2.0, 66.6 ± 2.2, 72.4 ± 2.2, and 72.8 ± 2.2 points at baseline, 1.5, 3, and 6 months, respectively (Table 2, Figure 2). Although the difference was not significant (P = .08), experimental subjects whose initial RSDI score was less than 50 points improved the most, with an average score change of 17.8 ± 4.4, and comparable control subjects had an average RSDI score change of 8.8 ± 2.9 points. Emotional and functional RSDI domains were not significantly related to score change; however, the physical domain of the survey was significant (P = .05).
SIA scores for experimental subjects improved (P < .05) at all follow-up points compared with control subjects; scores for the experimental group were 3.9 ± 0.1, 3.1 ± 0.2, 2.7 ± 0.2, and 2.4 ± 0.1 points at baseline, 1.5, 3, and 6 months, respectively (Table 2, Figure 2).
SF-12 score showed no significant differences between groups at any follow-up point but by 6 months trended toward significance (P = .06; Table 2).
Forty-one (93%) experimental subjects completing the exit questionnaire reported improvement. Most (n = 16, 73%) control subjects reported no change, but 18% reported worsening (P < .001; Table 3). Experimental subjects reported an average of 57 ± 4.5% improvement (range, 0–100%), whereas control subjects reported an average of 7 ± 5.9% worsening (range, -80% to 50%; P < .001).
Experimental subjects reported using nasal irrigation on 87% of days during the study; 31 subjects reported using nasal irrigation on 91% or more days, 13 subjects on 76% to 90% of days, and 5 subjects on 51% to 75% of days. Only 3 subjects used nasal irrigation on 50% or fewer days; these 3 subjects had relatively good baseline RSDI and SIA scores compared with other experimental subjects. Compliance was not significantly associated with changes in SIA or RSDI scores. The average survey completion rate was 96% at each assessment by each group.
Experimental subjects spent fewer 2-week blocks with nasal congestion, sinus headache, and frontal pain and pressure and used antibiotics and nasal sprays in fewer blocks (Table 3).
Forty-four experimental subjects answered questions about satisfaction and side effects. Forty-two stated they “will continue to use” nasal irrigation; the remaining 2 subjects found nasal irrigation less helpful but did not experience side effects. All 44 subjects “would recommend” nasal irrigation to friends or family with sinus problems. Ten subjects (23%) experienced side effects; 8 identified nasal irritation, nasal burning, tearing, nosebleeds, headache, or nasal drainage as occurring but “not significant.” Two subjects identified nasal burning, irritation, and headache as “significant,” but this did not change their high satisfaction rating. Of the 10 subjects who experienced side effects, 4 reduced or eliminated the side effects by temporarily alternating treatment days or decreasing salinity by 50%.
TABLE 2
Primary outcomes: RSDI, SF-12, and SIA baseline scores and mean score changes*
| Status | Baseline score | Baseline vs score change at | ||
|---|---|---|---|---|
| 1.5 mo | 3 mo | 6 mo | ||
| RSDI | ||||
| Experimental | 58.4 ± 2.0 | 8.2 ± 1.2 | 14.0 ± 2.0† | 14.4 ± 1.7‡ |
| Control | 59.6 ± 3.0 | 5.6 ± 1.4 | 7.7 ± 1.9 | 0.9 ± 1.0 |
| SF-12 | ||||
| Experimental | 60.3 ± 3.0 | 6.7 ± 2.1 | 8.2 ± 2.9 | 12.7 ± 3.6 |
| Control | 59.3 ± 3.9 | 5.4 ± 3.9 | 2.9 ± 4.0 | 2.2 ± 3.5 |
| SIA | ||||
| Experimental | 3.9 ± 0.1 | -0.8 ± 0.2† | -1.2 ± 0.2† | -1.6 ± 0.2‡ |
| Control | 4.1 ± 0.2 | -0.02 ± 0.21 | -0.3 ± 0.2 | -0.005 ± 0.2 |
| *Data are presented as mean ± standard error. | ||||
| †Statistically significant at P < .05. | ||||
| ‡Statistically significant at P < .001. | ||||
| RSDI, Rhinosinusitis Disability Index; SF-12, Medical Outcomes Survey Short Form 12; SIA, Single-Item Symptom Severity Assessment. | ||||
TABLE 3
Secondary outcomes
| Secondary outcome | Experimental | Control |
|---|---|---|
| Sinus symptoms* | ||
| Sinus headache† | 57 ± 0.05 | 76 ± 0.06 |
| Frontal pain‡ | 55 ± 0.05 | 82 ± 0.05 |
| Frontal pressure‡ | 53 ± 0.05 | 86 ± 0.05 |
| Nasal congestion† | 67 ± 0.04 | 83 ± 0.05 |
| Nasal discharge | 65 ± 0.05 | 69 ± 0.07 |
| Medication use* | ||
| Antibiotics† | 10 ± 0.02 | 19 ± 0.04 |
| Nasal sprays§ | 4 ± 0.01 | 8 ± 0.02 |
| EQ: sinus symptoms related to QOL|| | ||
| Better‡ | 41 (93) | 2 (9) |
| Same‡ | 3 (7) | 16 (73) |
| Worse‡ | 0 (0) | 4 (18) |
| *Data are presented as the percentage of 2-week blocks ± standard error during the study. | ||
| †Statistically significant difference between groups: P < .05. | ||
| ‡Statistically significant difference between groups: P < .001. | ||
| §Not statistically significant, difference between groups: P = .06. | ||
| || Data are presented as number (%) of subjects. | ||
| EQ, exit questionnaire (Is your quality of life with respect to sinus symptoms better or worse since the beginning of the study?); QOL, quality of life. | ||
FIGURE 1
Position of nasal cup for nasal irrigation therapy A B
FIGURE 2
Mean RSDI and SIA scores in control and experimental subjects
DISCUSSION
Our trial of daily hypertonic nasal irrigation produced several significant findings. We found consistent, statistically significant improvements in QOL (RSDI) and overall symptom severity (SIA). This was consistent with QOL improvement previously reported over short periods with the use of disease-specific measures.12-14 The RSDI is a moderately well-developed and validated disease-specific QOL instrument.21-23 The “minimal clinically important difference,” defined as the average score improvement needed to justify costs and risks,24-26 has not been established for sinusitis. However, it has been estimated for other disease states. For example, a half-point change on a 7-point Likert scale corresponds to estimates of important change in patients with chronic heart and lung disease.22,27 Others have found similar relationships.28-31 In our study, RSDI scores among treated subjects averaged 6.0 and 15.5 points better than controls at 3 and 6 months, respectively. On the SIA, treated subjects averaged 0.6, 0.9, and 1.6 points better. Extrapolating from these findings, these differences appear to be clinically significant. By using 10% improvement of the RSDI, our data showed numbers needed to treat of 9, 5, and 2 at 1.5, 3, and 6 months, respectively (95% confidence interval at 6 months, 1.4–2.6). Numbers needed to treat for SIA, symptom frequency, and medication use were similar. SF-12 improvement, although not statistically significant in this small trial, may represent clinically significant improvements in general health-related QOL.
“Percentage change” is used often by clinicians to gauge therapeutic progress. Ours is the first study to document such change in sinusitis patients using nasal irrigation. Ours is also the first trial to show decreased symptom frequency over a 6-month period. Shorter trials have documented improvement in patients with nasal symptoms12,13,17,18 or with chronic sinusitis in adult14,15 and pediatric16 populations. Consistent with improved symptoms and QOL, experimental subjects decreased their use of antibiotics and nasal sprays, as previously reported in a short trial.12
Side effects have not been carefully assessed in previous trials. Although generally safe, daily hypertonic nasal irrigation was associated with some clinically minor side effects. Interestingly, subjects were able to decrease side effects by adjusting irrigation schedule or salinity. Side effects were not sufficiently bothersome to stop therapy. Compliance with daily therapy was very high and is previously unreported. Although this was consistent with a positive effect on relatively severe symptoms, we believe high compliance also was related to teaching, demonstrated proficiency with nasal irrigation, and close telephone follow-up. One prior study reported subjects’ observation of the first nasal irrigation15; several studies reported providing some education.1214,18
Our study has several limitations. It was not blinded or placebo controlled. Blinding subjects to a physical therapy is inherently difficult. Investigators who have tried to use normal saline placebos probably affected outcomes.14-16 One trial using a fresh water (0% saline) placebo was stopped early when several control subjects developed otitis media.32 The investigators also were unblinded, possibly creating observer bias.
Methodologic and recruitment strengths of this study included effective randomization, matched control group, intention-to-treat analysis, low missing data rates, high compliance rate, and low dropout rate. Clinical strengths included significant findings on most parameters assessed. Particularly intriguing was the decreased use of antibiotics in the experimental group. This study offered strong evidence that nasal irrigation is a safe, effective, and inexpensive (nasal pot, $15; daily therapy, <$1/month) therapy for sinus disease that properly trained patients will use. Although questions about the protocol (schedule, concentration, and buffering) and indications require further study in a more diverse patient population, clinicians may confidently recommend nasal irrigation; it offers significant hope for symptomatic relief and QOL improvement for millions of individuals with sinus disease who often have few therapeutic options.
CONCLUSIONS
Daily hypertonic saline nasal irrigation improves sinus-related QOL, decreases symptoms, and decreases medication use in patients with frequent sinusitis. Primary care physicians can feel comfortable recommending this therapy.
Acknowledgments
We thank Thomas Pasic, MD, Michael McDonald, MD, and Diane Heatley, MD, Department of Otolaryngology, University of Wisconsin, Madison.
1. Ray NF, Baraniuk JN, Thamer M, et al. Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis and other airway disorders. J Allergy Clin Immunol 1999;103:408-14.
2. McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA 1995;273:214-9.
3. Centers for Disease Control.Vital and Health Statistics.Current Estimates From the National Health Interview Survey, 1994. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Center for Health Statistics, 1995. DHHS publication PHS 96-1521.
4. Glicklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg 1995;113:104-9.
5. Kaliner MA, Osuguthorpe JD, Fireman P, et al. Sinusitis bench to bedside: current findings, future directions. J Allergy Clin Immunol 1997;99:S829-47.
6. Druce HM. Adjuncts to medical management of sinusitis. Otolaryngol Head Neck Surg 1990;103:880-3.
7. Zieger RS. Prospects for ancillary treatment of sinusitis in the 1990’s. J Allergy Clin Immunol 1992;90:478-93.
8. Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope 1997;107:500-3.
9. Robinson M, Hemming AL, Regnis JA, et al. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax 1997;52:900-3.
10. Homer JJ, Dowley AC, Condon L, El-Jassar P, Sood S. The effect of hypertonicity on nasal mucociliary clearance. Clin Otolaryngol 2000;25:558-60.
11. Homer JJ, England RJ, Wilde AD, Harwood GRJ, Stafford ND. The effect of pH of douching solution on mucociliary clearance. Clin Otolaryngol 1999;24:312-5.
12. Heatley DG, McConnell KE, Kille TL, Leverson GE. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngol Head Neck Surg 200l;125:44-8.
13. Tamooka LT, Murphy C, Davidson TM. Clinical study and literature review of nasal irrigation. Laryngoscope 2000;110:1189-93.
14. Taccariello M, Parikh A, Darby Y, Scadding G. Nasal douching as a valuable adjunct in the management of chronic rhinosinusitis. Rhinology 1999;37:29-32.
15. Bachmann G, Hommel G, Michel O. Effect of irrigation of the nose with isotonic salt solution on patients with chronic paranasal sinus disease. Eur Arch Otorhinolaryngol 2000;257:537-41.
16. Shoseyov D, Bibi H, Shai P, et al. Treatment with hypertonic saline versus normal saline wash of pediatric chronic sinusitis. J Allergy Clin Immunol 1998;101:602-5.
17. Rabone SJ, Saraswati SB. Acceptance and effects of nasal lavage in volunteer woodworkers. Occupat Med 1999;49:365-9.
18. Holmstrom M, Rosen G, Walander L. Effect of nasal lavage on nasal symptoms and physiology in wood industry workers. Rhinology 1997;35:108-12.
19. SinuCleanse Med Systems, April 22 2002. Available at: http://www.sinucleanse.com. Accessed October 7, 2002.
20. Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary test of reliability and validity. Med Care 1996;34:220-6.
21. Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolyryngol Head Neck Surg 1997;123:1175-9.
22. Guyatt GH, Bombardier C, Tugwell P. Measuring disease-specific quality of life in clinical trials. CMAJ 1986;134:889-95.
23. Senior BA, Glaze C, Benninger MS. Use of the Rhinosinusitis Disability Score (RSDI) in rhinologic disease. Am J Rhinol 2001;15:15-20.
24. Deyo RA, Patrick DL. The significance of treatment effects: the clinical perspective. Med Care 1995;33:AS286-91.
25. Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol 1996;49:1215-9.
26. Samsa G. How should the minimum important difference for a health-related quality-of-life instrument be estimated? Med Care 2001;39:1037-8.
27. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15.
28. Bellamy N, Carr A, Dougados M, Shea B, Wells G. Towards a definition of “difference” in osteoarthritis. J Rheumatol 2001;28:427-30.
29. Powell CV, Kelly A-M. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med 2001;37:28-31.
30. Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog pain scores. Acad Emerg Med 1996;3:142-6.
31. Wells GA, Tugwell P, Kraag GR, et al. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol 1993;20:557-60.
32. Wendeler HM, Muller J, Dieler R, Helms J. Nasenspuling mit isotoner Emser-Salz-Losung bei chronischer rhinosinusitis. Otorhinolaryngol Nova 1997;7:254-8.
1. Ray NF, Baraniuk JN, Thamer M, et al. Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis and other airway disorders. J Allergy Clin Immunol 1999;103:408-14.
2. McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA 1995;273:214-9.
3. Centers for Disease Control.Vital and Health Statistics.Current Estimates From the National Health Interview Survey, 1994. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Center for Health Statistics, 1995. DHHS publication PHS 96-1521.
4. Glicklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg 1995;113:104-9.
5. Kaliner MA, Osuguthorpe JD, Fireman P, et al. Sinusitis bench to bedside: current findings, future directions. J Allergy Clin Immunol 1997;99:S829-47.
6. Druce HM. Adjuncts to medical management of sinusitis. Otolaryngol Head Neck Surg 1990;103:880-3.
7. Zieger RS. Prospects for ancillary treatment of sinusitis in the 1990’s. J Allergy Clin Immunol 1992;90:478-93.
8. Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope 1997;107:500-3.
9. Robinson M, Hemming AL, Regnis JA, et al. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax 1997;52:900-3.
10. Homer JJ, Dowley AC, Condon L, El-Jassar P, Sood S. The effect of hypertonicity on nasal mucociliary clearance. Clin Otolaryngol 2000;25:558-60.
11. Homer JJ, England RJ, Wilde AD, Harwood GRJ, Stafford ND. The effect of pH of douching solution on mucociliary clearance. Clin Otolaryngol 1999;24:312-5.
12. Heatley DG, McConnell KE, Kille TL, Leverson GE. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngol Head Neck Surg 200l;125:44-8.
13. Tamooka LT, Murphy C, Davidson TM. Clinical study and literature review of nasal irrigation. Laryngoscope 2000;110:1189-93.
14. Taccariello M, Parikh A, Darby Y, Scadding G. Nasal douching as a valuable adjunct in the management of chronic rhinosinusitis. Rhinology 1999;37:29-32.
15. Bachmann G, Hommel G, Michel O. Effect of irrigation of the nose with isotonic salt solution on patients with chronic paranasal sinus disease. Eur Arch Otorhinolaryngol 2000;257:537-41.
16. Shoseyov D, Bibi H, Shai P, et al. Treatment with hypertonic saline versus normal saline wash of pediatric chronic sinusitis. J Allergy Clin Immunol 1998;101:602-5.
17. Rabone SJ, Saraswati SB. Acceptance and effects of nasal lavage in volunteer woodworkers. Occupat Med 1999;49:365-9.
18. Holmstrom M, Rosen G, Walander L. Effect of nasal lavage on nasal symptoms and physiology in wood industry workers. Rhinology 1997;35:108-12.
19. SinuCleanse Med Systems, April 22 2002. Available at: http://www.sinucleanse.com. Accessed October 7, 2002.
20. Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary test of reliability and validity. Med Care 1996;34:220-6.
21. Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolyryngol Head Neck Surg 1997;123:1175-9.
22. Guyatt GH, Bombardier C, Tugwell P. Measuring disease-specific quality of life in clinical trials. CMAJ 1986;134:889-95.
23. Senior BA, Glaze C, Benninger MS. Use of the Rhinosinusitis Disability Score (RSDI) in rhinologic disease. Am J Rhinol 2001;15:15-20.
24. Deyo RA, Patrick DL. The significance of treatment effects: the clinical perspective. Med Care 1995;33:AS286-91.
25. Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol 1996;49:1215-9.
26. Samsa G. How should the minimum important difference for a health-related quality-of-life instrument be estimated? Med Care 2001;39:1037-8.
27. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15.
28. Bellamy N, Carr A, Dougados M, Shea B, Wells G. Towards a definition of “difference” in osteoarthritis. J Rheumatol 2001;28:427-30.
29. Powell CV, Kelly A-M. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med 2001;37:28-31.
30. Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog pain scores. Acad Emerg Med 1996;3:142-6.
31. Wells GA, Tugwell P, Kraag GR, et al. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol 1993;20:557-60.
32. Wendeler HM, Muller J, Dieler R, Helms J. Nasenspuling mit isotoner Emser-Salz-Losung bei chronischer rhinosinusitis. Otorhinolaryngol Nova 1997;7:254-8.