User login
Effects of blood conservation on the incidence of anemia and transfusions in pediatric parapneumonic effusion: A hospitalist perspective
Pediatric patients with pneumonia frequently develop parapneumonic effusion (PNE),1 which account for 0.4 to 6 cases per 1000 pediatric admissions.2 The effusion initially is in a transudative, free‐flowing phase that may evolve to fibrino‐purulent phase and a later organizing phase.3 Hospital management includes antibiotic therapy, pain control, fluids, nutritional support, diagnostic imaging and most important is fluid drainage. Fluid drainage could be through thoracentesis, chest tubes,4 fibrinolysis,5 video‐assisted thoracoscopic surgery (VATS),6 or thoracotomy.7
During hospitalization, repeated phlebotomy and surgical procedures result in ongoing blood losses8, 9 while systemic inflammation and nutritional compromise may blunt erythropoiesis.10, 11 Both mechanisms may result in developing anemia and subsequent need for red blood cell transfusion (RBCT). Blood transfusions are associated with multiple complications including transfusion transmitted infections, acute lung injury, hemodynamic compromise, volume overload, hemolysis, and immune compromise.12, 13 Furthermore, suboptimal benefits,14 increased resource utilization,15 and risk of mortality16 have also been reported in Pediatric intensive care unit (ICU) patients who received blood transfusions.
A Pediatric Blood Conservation program was launched at Helen DeVos Children's Hospital in 1999, and since its inception the pediatric hospitalists have implemented blood conservation guidelines (BCG) in the management of some of the patients with PNE. The BCG incorporated into the care of the intervention group (group I) were orders for:
Minimizing phlebotomy draws (without stating a specific frequency).
Use of micro sampling blood collection tubes and reinfusion of any blood drawn prior to obtaining a blood sample (waste return).
Hematinics use at managing physician's discretion (see Supporting Appendices 1 and 2 in the online version of this article).
No previous studies have addressed the impact of blood conservation strategies on the development of anemia in pediatric PNE. We hypothesized that BCG implementation resulted in smaller phlebotomy losses, lesser incidence of anemia, and lower transfusion requirements.
Methods
After obtaining an approval from the institutional review board, a retrospective medical records review was conducted of all pediatric patients (1 month‐18 years) admitted to Helen DeVos Children's Hospital with diagnoses of PNE from the period of January 1997 to December 2004.
Study Groups
Intervention group (group I) included all patients who were admitted with a diagnosis of PNE between the year 2000 to 2004 and had orders for BCG (as outlined above) written on or after admission to the hospital. That group included patients that were either solely managed by the hospitalists or comanaged by both the general pediatrician and the Hospitalist.
Simultaneous nonintervention group (group S) included patients with PNE who were admitted between 2000 to 2004 and did not have BCG orders on their record. Those patients were either managed solely by the general pediatricians or the Hospitalist may have been involved but no BCG orders were written. It was assumed that no intervention was implemented.
Historical nonintervention group (group H) included patients with PNE admitted between 1997 and 2000 prior to the implementation of the blood conservation program or the hospitalists service. Those patients were managed by the general pediatricians with the Intensivists help at times and no blood conservation measures were implemented.
Phlebotomy frequency and volume data were collected from the patient's medical record. When volume was not documented, an estimate was made based on the hospital actual practice and labs reported. If the child had central vascular access, a standard of 2 mL of blood was removed to clear the line prior to drawing the blood sample. In the S and H groups that volume was discarded and recorded as a blood loss. In Group I the blood used to clear the line was returned to the patients. Data regarding the patient's hemoglobin (Hgb) levels on admission and during hospitalization as well as RBCT frequency and volume were also collected. Other background data collected included, hospital stay, ICU admission, antibiotic use, isolated organisms, and PNE‐related interventions (thoracentesis, VATS and chest tube placement). A pediatric risk of mortality score (PRISM score17) was assigned for every patient based on data collected on the day of admission.
Statistical analysis was done using a Fisher exact test to compare qualitative data. For quantitative data Kruskal‐Wallis was used for comparison between the three groups while the Mann Whitney test was used for the pair wise comparisons. Odds Ratios and Confidence Intervals were calculated for variables associated with needing RBCTs.
Results
A total of 81 patients who were admitted to the hospital for medical and surgical management with PNE were included in the study. During the study period 24 patients with blood conservation orders on the chart were assigned to the intervention group. Another 28 patients were identified during the same period but did not have blood conservation orders and were assigned to the simultaneous no intervention group. A historical no intervention group of 29 patients were identified from a 3‐year period prior to the study period. Groups were similar in age, weight, and an overall low PRISM score. Group H tended to have lower acuity, longer hospital stay, more frequent pediatric intensive care unit (PICU) admissions, and tended to have more chest tube days compared to the (I) and (S) groups (Table 1).
| Group I (n = 24) | Group S (n = 28) | Group H (n = 29) | Kruskal‐Wallis P Value | |
|---|---|---|---|---|
| ||||
| Age, years | 6 (4) | 5 (4) | 6 (5) | 0.75 |
| Weight, kg | 26 (19) | 22 (13) | 27 (17) | 0.77 |
| PRISM Score | 2 (3) | 3 (5) | (1) | 0.007* |
| PICU admission number (%) | 3 (12) | 8 (29) | 14 (48) | 0.018 |
| Chest tube days | 7 (4) | 6 (4) | 9 (5) | 0.054 |
| Hospital stay days | 10 (4) | 13 (6) | 15 (6) | 0.008 |
| Initial Hgb, gm | 11.2 (1.7) | 11.2 (1.6) | 11.1 (1.9) | 0.95 |
| Drop in Hgb, gm | 1.7 (1.4) | 2.1 (1.2) | 2 (1.4) | 0.37 |
| Days to Hgb Nadir | 6.1 (37) | 8.5 (5.5) | 6.9 (4.3) | 0.31 |
| Number of blood draws | 7.6 (4) | 11 (9) | 12.6 (12) | 0.36 |
| Phlebotomy volume, mL/kg/day | 0.08 (0.05) | 0.14 (0.33) | 0.22 (0.24) | 0.006 |
| Number of patients transfused (%) | 2 (8.3) | 5 (17.9) | 9 (31) | 0.11 |
All groups (I, S and H) had similar initial Hgb, in‐hospital decline in Hgb levels and time to reach a Hgb nadir. There was a trend toward a lower frequency of phlebotomy and significant difference in the phlebotomy volumes drawn even when corrected for patient weight and hospital stay, (I < S < H; P = 0.006), (Table 1).
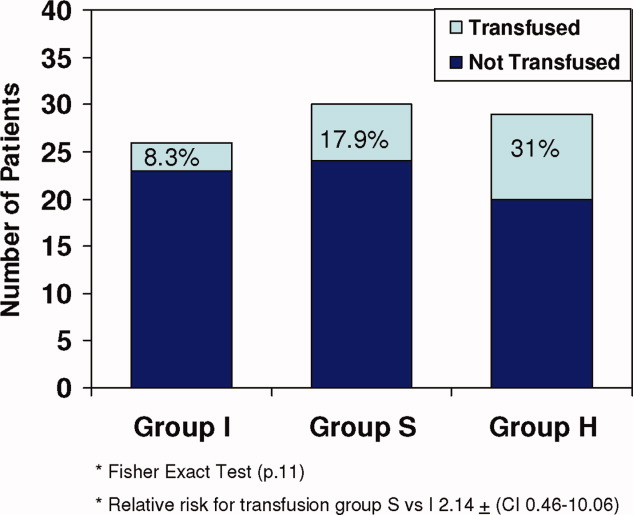
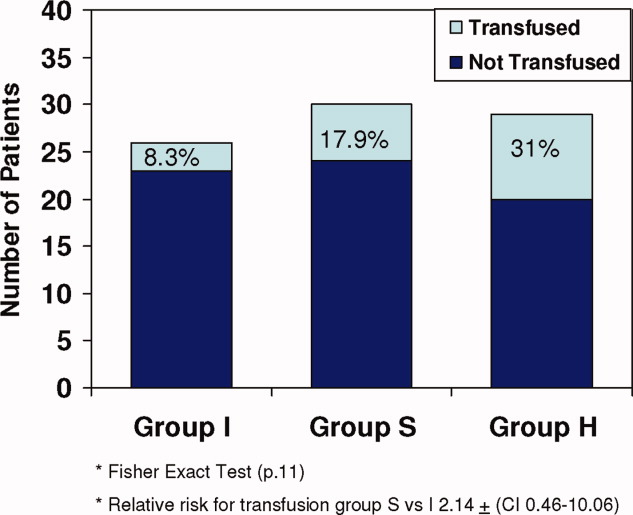
All 3 groups had a similar pretransfusion Hgb trigger (7.7 1 gm/dL), timing of transfusions (7.9 1 day), volume of packed red blood cells (PRBCs) (19 15 mL/kg), and magnitude of Hgb rise following transfusions (3.9 1.5 gm/dL). There was a strong trend toward lower transfusion need in the intervention group though it did not reach statistical significance (8.3% [I], 17.9% [S]; and 31% [H]; P = 0.11) (Table 1 and Figure 1). Being in group (S) compared to (I) carried a relative risk of transfusion of 2.14 (confidence interval [CI], 0.4610.06).
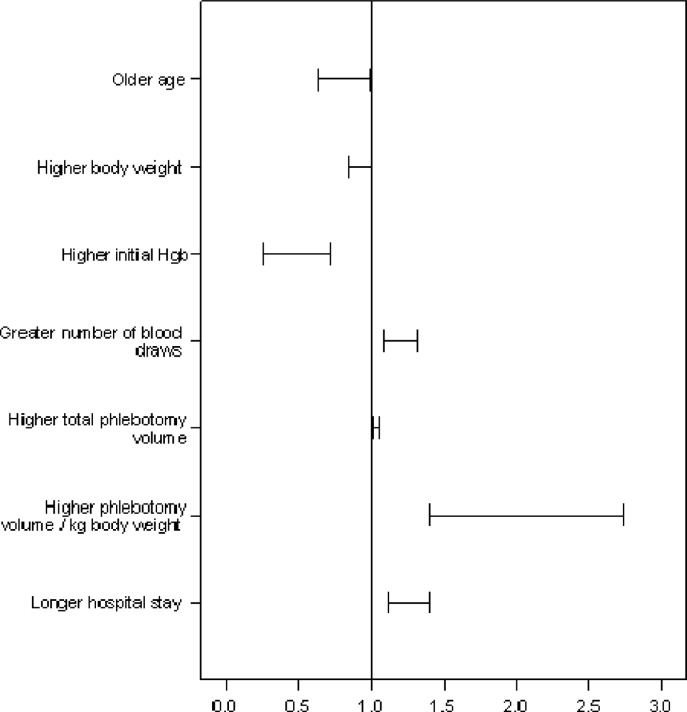
Of all study patients, 19.8% received RBCT. Compared to those who did not require transfusions they were significantly younger, smaller (P = 0.001) and had a higher severity of illness score (PRISM) (P = 0.25). Transfused children had lower initial Hgb levels, more frequent phlebotomy, greater volume of blood drawn, and longer hospital stay (P = 0.001) (Table 2 and Figure 2).
| Transfused (n = 16) | Not Transfused (n = 65) | Odds Ratio (95% CI) | |
|---|---|---|---|
| |||
| Ages, years | 3.5 (4) | 6.4 (4) | 0.8 (0.640.99)* |
| Weight, kg | 16.1 (9) | 26.9 (17) | 0.92 (0.851)* |
| Initial Hgb, gm | 9.9 (1) | 11.4 (1) | 0.43 (0.260.71)* |
| Number of blood draws | 20 (12) | 8 (6) | 1.2 (1.091.32)* |
| Total phlebotomy volume, mL | 82 (75) | 23 (25) | 1.03 (1.011.05)* |
| Total phlebotomy volume, mL/kg | 5.9 (7) | 1.1 (1) | 1.97 (1.412.74)* |
| Hospital stay days | 19 (5) | 11 (5) | 1.25 (1.121.4)* |
| PRISM score | 3.4 (6) | 1.6 (3) | P. 25 |
A total of 36% of the patients were pretreated with antibiotics prior to obtaining pleural fluid cultures. Forty‐eight patients (59%) had negative cultures, 22 (27%) grew pneumococcus, 5 patients (6%) had streptococcus A, 2 patients had streptococcus viridans, 3 patients had staphylococci aureus, and 1 patient had Haemophilus influenzae. There was no difference between the study groups regarding the site of effusion, prior antibiotics therapy or culture results.
Discussion
This retrospective study showed that children with PNE had low Hgb upon admission to the hospital and after dropping an average of 2 gm/dL over the first week of hospitalization one‐fifth of the patients required transfusion. The phlebotomy volumes significantly decreased with BCG implementation, and transfusion frequency showed a strong trend toward decline. The fact that all 3 groups had similar pretransfusion Hgb and similar Hgb decline, despite differences in the phlebotomy volumes, may implicate other factors like bone marrow suppression, malnutrition, and procedural blood losses. All of these factors have been shown to contribute to the development of anemia in the critically ill patients10 and were not accounted for in this limited retrospective review.
Transfused patients were significantly smaller, younger, and had higher illness severity scores. They had lower initial Hgb levels, more phlebotomy, and longer hospital stay than nontransfused patients.
Pretransfusion Hgb was 7.5 gm/dL to 7.7 gm/dL in all groups, consistent with Lacroix et al's18 report on the safety of lower transfusion threshold in stable PICU patients. BCG resulted in a strong trend toward less transfusion but did not reach statistical significance likely due to the small sample size. It is tempting to hypothesize that aggressive erythropoietin therapy might augment that trend; however, given the relatively short hospital stay (1014 days), erythropoietin therapy may be less efficacious in the milder case with shorter hospital stay than those with longer hospitalization. After encouraging smaller studies19 Corwin et al.20 did not find a beneficial effect for erythropoietin in adult ICU patients nor did Jacobs et al.21 in ventilated children with bronchiolitis who had comparable length of hospitalization.
A specific benefit could not be attributed to iron/folate or erythropoietin therapy as neither the dosing, duration nor timing was controlled. Furthermore, given the limitations of retrospective reviews, the relative importance of the beneficial effect of limiting phlebotomy vs. hematinics use could not be determined.
The Hospitalist is focused on improving the quality and efficiency of caring for the inpatient.2225 The initiation of the hospitalist's service at our institution coincided with that of the blood conservation service. Consequently, the Hospitalist contributed to patient care as well as daily house staff and nursing education. For those children whose care was coordinated by the hospitalists, the study data showed a trend toward lesser admissions to the PICU and lower blood utilization. This trend further emphasizes the role a Hospitalist could play in adopting and implementing useful medical strategies lowering the cost of care. In this study, a change in patient care was observed over time with the hospitalists tending to employ more blood conservation measures when compared to the pediatrician.
In summary, children with PNE are at risk for developing severe anemia requiring transfusion. This retrospective study identified the characteristics of those likely to require transfusions. Blood conservation strategies seem to decrease the need for transfusions. The hospitalists played an important role in implementing the BCG. A prospective controlled study with adequate power is needed to examine both the various mechanisms for developing anemia and the impact of the individual components of the blood conservation strategies.
- ,.Thoracic empyema.Surg Clin North Am.2002;82:643–671.
- ,.Parapneumonic Pleural effusion and empyema in children: review of 19 year experience.Clin Pediatr (Phila).1983;22:414–419.
- American Thoracic Society.Management of nontuberculosis empyema.Am Rev Respir Dis.1962;85:935–936.
- ,,, et al.Short‐term course and outcome of treatments of pleural empyema in pediatric patients: repeated ultrasound‐guided needle thoracentesis vs. chest tube drainage.Chest.2002;121:836–840.
- ,,, et al.Randomized trial of Intrapleural Urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,.Therapy of parapneumonic effusion in children: video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,,,.Is open thoracotomy still a good treatment option for the management of Empyema in children?Ann Thorac Surg.2003;76:1854–1858.
- ,,, et al.Anemia, blood loss, and blood transfusions in North American children in the intensive care unit.Am J Respir Crit Care Med.2008;178:25–33.
- .Medical vampires.N Engl J Med.1983;314:1250–1251.
- ,,, et al.Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients.Crit Care Med.1999;27:2630–2639.
- ,,, et al.Erythropoietin response is blunted in critically ill patients.Intensive Care Med.1997;23:159–162.
- ,.The continuing risk of transfusion‐transmitted infections.N Engl J Med.2006;355:1303–1305.
- ,.Immunomodulation by blood transfusion: an evolving scientific and clinical challenge.Am J Med.1996;101:299–308.
- ,,,.Pediatric red blood cell transfusions increase resource use.J Pediatr.2003;142:95–97.
- ,.Effect of blood transfusion on oxygen consumption in pediatric septic shock.Crit Care Med.1990;18:1087–1091.
- ,,,,.Red blood cell transfusion in critically ill children is independently associated with increased mortality.Intensive Care Med.2007;33:1414–1422.
- ,,.PRISM III: an updated pediatric risk of mortality.Crit Care Med.1996;24(5):743–752.
- ,,, et al.Transfusion strategies for patients in pediatric intensive care units.Nw Engl J Med.2007;356:1609–1619.
- ,,, et al.Efficacy of recombinant human erythropoietin in critically ill patients.JAMA.2002;288:2827–2835.
- ,,, et al.EPO Critical Care Trials Group.Efficacy and safety of epoetin alfa in criticall ill patients.Nw Engl J Med.2007;357:965–976.
- ,,.Erythropoietin therapy in children with Bronchiolitis and anemia.Pediatr Crit Care Med.2003;4(1):44–47.
- .The state of hospital medicine in 2008.Med Clin North Am.2008;92:265–273.
- ,,, et al.Impact of a Hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120:267–274.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
Pediatric patients with pneumonia frequently develop parapneumonic effusion (PNE),1 which account for 0.4 to 6 cases per 1000 pediatric admissions.2 The effusion initially is in a transudative, free‐flowing phase that may evolve to fibrino‐purulent phase and a later organizing phase.3 Hospital management includes antibiotic therapy, pain control, fluids, nutritional support, diagnostic imaging and most important is fluid drainage. Fluid drainage could be through thoracentesis, chest tubes,4 fibrinolysis,5 video‐assisted thoracoscopic surgery (VATS),6 or thoracotomy.7
During hospitalization, repeated phlebotomy and surgical procedures result in ongoing blood losses8, 9 while systemic inflammation and nutritional compromise may blunt erythropoiesis.10, 11 Both mechanisms may result in developing anemia and subsequent need for red blood cell transfusion (RBCT). Blood transfusions are associated with multiple complications including transfusion transmitted infections, acute lung injury, hemodynamic compromise, volume overload, hemolysis, and immune compromise.12, 13 Furthermore, suboptimal benefits,14 increased resource utilization,15 and risk of mortality16 have also been reported in Pediatric intensive care unit (ICU) patients who received blood transfusions.
A Pediatric Blood Conservation program was launched at Helen DeVos Children's Hospital in 1999, and since its inception the pediatric hospitalists have implemented blood conservation guidelines (BCG) in the management of some of the patients with PNE. The BCG incorporated into the care of the intervention group (group I) were orders for:
Minimizing phlebotomy draws (without stating a specific frequency).
Use of micro sampling blood collection tubes and reinfusion of any blood drawn prior to obtaining a blood sample (waste return).
Hematinics use at managing physician's discretion (see Supporting Appendices 1 and 2 in the online version of this article).
No previous studies have addressed the impact of blood conservation strategies on the development of anemia in pediatric PNE. We hypothesized that BCG implementation resulted in smaller phlebotomy losses, lesser incidence of anemia, and lower transfusion requirements.
Methods
After obtaining an approval from the institutional review board, a retrospective medical records review was conducted of all pediatric patients (1 month‐18 years) admitted to Helen DeVos Children's Hospital with diagnoses of PNE from the period of January 1997 to December 2004.
Study Groups
Intervention group (group I) included all patients who were admitted with a diagnosis of PNE between the year 2000 to 2004 and had orders for BCG (as outlined above) written on or after admission to the hospital. That group included patients that were either solely managed by the hospitalists or comanaged by both the general pediatrician and the Hospitalist.
Simultaneous nonintervention group (group S) included patients with PNE who were admitted between 2000 to 2004 and did not have BCG orders on their record. Those patients were either managed solely by the general pediatricians or the Hospitalist may have been involved but no BCG orders were written. It was assumed that no intervention was implemented.
Historical nonintervention group (group H) included patients with PNE admitted between 1997 and 2000 prior to the implementation of the blood conservation program or the hospitalists service. Those patients were managed by the general pediatricians with the Intensivists help at times and no blood conservation measures were implemented.
Phlebotomy frequency and volume data were collected from the patient's medical record. When volume was not documented, an estimate was made based on the hospital actual practice and labs reported. If the child had central vascular access, a standard of 2 mL of blood was removed to clear the line prior to drawing the blood sample. In the S and H groups that volume was discarded and recorded as a blood loss. In Group I the blood used to clear the line was returned to the patients. Data regarding the patient's hemoglobin (Hgb) levels on admission and during hospitalization as well as RBCT frequency and volume were also collected. Other background data collected included, hospital stay, ICU admission, antibiotic use, isolated organisms, and PNE‐related interventions (thoracentesis, VATS and chest tube placement). A pediatric risk of mortality score (PRISM score17) was assigned for every patient based on data collected on the day of admission.
Statistical analysis was done using a Fisher exact test to compare qualitative data. For quantitative data Kruskal‐Wallis was used for comparison between the three groups while the Mann Whitney test was used for the pair wise comparisons. Odds Ratios and Confidence Intervals were calculated for variables associated with needing RBCTs.
Results
A total of 81 patients who were admitted to the hospital for medical and surgical management with PNE were included in the study. During the study period 24 patients with blood conservation orders on the chart were assigned to the intervention group. Another 28 patients were identified during the same period but did not have blood conservation orders and were assigned to the simultaneous no intervention group. A historical no intervention group of 29 patients were identified from a 3‐year period prior to the study period. Groups were similar in age, weight, and an overall low PRISM score. Group H tended to have lower acuity, longer hospital stay, more frequent pediatric intensive care unit (PICU) admissions, and tended to have more chest tube days compared to the (I) and (S) groups (Table 1).
| Group I (n = 24) | Group S (n = 28) | Group H (n = 29) | Kruskal‐Wallis P Value | |
|---|---|---|---|---|
| ||||
| Age, years | 6 (4) | 5 (4) | 6 (5) | 0.75 |
| Weight, kg | 26 (19) | 22 (13) | 27 (17) | 0.77 |
| PRISM Score | 2 (3) | 3 (5) | (1) | 0.007* |
| PICU admission number (%) | 3 (12) | 8 (29) | 14 (48) | 0.018 |
| Chest tube days | 7 (4) | 6 (4) | 9 (5) | 0.054 |
| Hospital stay days | 10 (4) | 13 (6) | 15 (6) | 0.008 |
| Initial Hgb, gm | 11.2 (1.7) | 11.2 (1.6) | 11.1 (1.9) | 0.95 |
| Drop in Hgb, gm | 1.7 (1.4) | 2.1 (1.2) | 2 (1.4) | 0.37 |
| Days to Hgb Nadir | 6.1 (37) | 8.5 (5.5) | 6.9 (4.3) | 0.31 |
| Number of blood draws | 7.6 (4) | 11 (9) | 12.6 (12) | 0.36 |
| Phlebotomy volume, mL/kg/day | 0.08 (0.05) | 0.14 (0.33) | 0.22 (0.24) | 0.006 |
| Number of patients transfused (%) | 2 (8.3) | 5 (17.9) | 9 (31) | 0.11 |
All groups (I, S and H) had similar initial Hgb, in‐hospital decline in Hgb levels and time to reach a Hgb nadir. There was a trend toward a lower frequency of phlebotomy and significant difference in the phlebotomy volumes drawn even when corrected for patient weight and hospital stay, (I < S < H; P = 0.006), (Table 1).
All 3 groups had a similar pretransfusion Hgb trigger (7.7 1 gm/dL), timing of transfusions (7.9 1 day), volume of packed red blood cells (PRBCs) (19 15 mL/kg), and magnitude of Hgb rise following transfusions (3.9 1.5 gm/dL). There was a strong trend toward lower transfusion need in the intervention group though it did not reach statistical significance (8.3% [I], 17.9% [S]; and 31% [H]; P = 0.11) (Table 1 and Figure 1). Being in group (S) compared to (I) carried a relative risk of transfusion of 2.14 (confidence interval [CI], 0.4610.06).

Of all study patients, 19.8% received RBCT. Compared to those who did not require transfusions they were significantly younger, smaller (P = 0.001) and had a higher severity of illness score (PRISM) (P = 0.25). Transfused children had lower initial Hgb levels, more frequent phlebotomy, greater volume of blood drawn, and longer hospital stay (P = 0.001) (Table 2 and Figure 2).

| Transfused (n = 16) | Not Transfused (n = 65) | Odds Ratio (95% CI) | |
|---|---|---|---|
| |||
| Ages, years | 3.5 (4) | 6.4 (4) | 0.8 (0.640.99)* |
| Weight, kg | 16.1 (9) | 26.9 (17) | 0.92 (0.851)* |
| Initial Hgb, gm | 9.9 (1) | 11.4 (1) | 0.43 (0.260.71)* |
| Number of blood draws | 20 (12) | 8 (6) | 1.2 (1.091.32)* |
| Total phlebotomy volume, mL | 82 (75) | 23 (25) | 1.03 (1.011.05)* |
| Total phlebotomy volume, mL/kg | 5.9 (7) | 1.1 (1) | 1.97 (1.412.74)* |
| Hospital stay days | 19 (5) | 11 (5) | 1.25 (1.121.4)* |
| PRISM score | 3.4 (6) | 1.6 (3) | P. 25 |
A total of 36% of the patients were pretreated with antibiotics prior to obtaining pleural fluid cultures. Forty‐eight patients (59%) had negative cultures, 22 (27%) grew pneumococcus, 5 patients (6%) had streptococcus A, 2 patients had streptococcus viridans, 3 patients had staphylococci aureus, and 1 patient had Haemophilus influenzae. There was no difference between the study groups regarding the site of effusion, prior antibiotics therapy or culture results.
Discussion
This retrospective study showed that children with PNE had low Hgb upon admission to the hospital and after dropping an average of 2 gm/dL over the first week of hospitalization one‐fifth of the patients required transfusion. The phlebotomy volumes significantly decreased with BCG implementation, and transfusion frequency showed a strong trend toward decline. The fact that all 3 groups had similar pretransfusion Hgb and similar Hgb decline, despite differences in the phlebotomy volumes, may implicate other factors like bone marrow suppression, malnutrition, and procedural blood losses. All of these factors have been shown to contribute to the development of anemia in the critically ill patients10 and were not accounted for in this limited retrospective review.
Transfused patients were significantly smaller, younger, and had higher illness severity scores. They had lower initial Hgb levels, more phlebotomy, and longer hospital stay than nontransfused patients.
Pretransfusion Hgb was 7.5 gm/dL to 7.7 gm/dL in all groups, consistent with Lacroix et al's18 report on the safety of lower transfusion threshold in stable PICU patients. BCG resulted in a strong trend toward less transfusion but did not reach statistical significance likely due to the small sample size. It is tempting to hypothesize that aggressive erythropoietin therapy might augment that trend; however, given the relatively short hospital stay (1014 days), erythropoietin therapy may be less efficacious in the milder case with shorter hospital stay than those with longer hospitalization. After encouraging smaller studies19 Corwin et al.20 did not find a beneficial effect for erythropoietin in adult ICU patients nor did Jacobs et al.21 in ventilated children with bronchiolitis who had comparable length of hospitalization.
A specific benefit could not be attributed to iron/folate or erythropoietin therapy as neither the dosing, duration nor timing was controlled. Furthermore, given the limitations of retrospective reviews, the relative importance of the beneficial effect of limiting phlebotomy vs. hematinics use could not be determined.
The Hospitalist is focused on improving the quality and efficiency of caring for the inpatient.2225 The initiation of the hospitalist's service at our institution coincided with that of the blood conservation service. Consequently, the Hospitalist contributed to patient care as well as daily house staff and nursing education. For those children whose care was coordinated by the hospitalists, the study data showed a trend toward lesser admissions to the PICU and lower blood utilization. This trend further emphasizes the role a Hospitalist could play in adopting and implementing useful medical strategies lowering the cost of care. In this study, a change in patient care was observed over time with the hospitalists tending to employ more blood conservation measures when compared to the pediatrician.
In summary, children with PNE are at risk for developing severe anemia requiring transfusion. This retrospective study identified the characteristics of those likely to require transfusions. Blood conservation strategies seem to decrease the need for transfusions. The hospitalists played an important role in implementing the BCG. A prospective controlled study with adequate power is needed to examine both the various mechanisms for developing anemia and the impact of the individual components of the blood conservation strategies.
Pediatric patients with pneumonia frequently develop parapneumonic effusion (PNE),1 which account for 0.4 to 6 cases per 1000 pediatric admissions.2 The effusion initially is in a transudative, free‐flowing phase that may evolve to fibrino‐purulent phase and a later organizing phase.3 Hospital management includes antibiotic therapy, pain control, fluids, nutritional support, diagnostic imaging and most important is fluid drainage. Fluid drainage could be through thoracentesis, chest tubes,4 fibrinolysis,5 video‐assisted thoracoscopic surgery (VATS),6 or thoracotomy.7
During hospitalization, repeated phlebotomy and surgical procedures result in ongoing blood losses8, 9 while systemic inflammation and nutritional compromise may blunt erythropoiesis.10, 11 Both mechanisms may result in developing anemia and subsequent need for red blood cell transfusion (RBCT). Blood transfusions are associated with multiple complications including transfusion transmitted infections, acute lung injury, hemodynamic compromise, volume overload, hemolysis, and immune compromise.12, 13 Furthermore, suboptimal benefits,14 increased resource utilization,15 and risk of mortality16 have also been reported in Pediatric intensive care unit (ICU) patients who received blood transfusions.
A Pediatric Blood Conservation program was launched at Helen DeVos Children's Hospital in 1999, and since its inception the pediatric hospitalists have implemented blood conservation guidelines (BCG) in the management of some of the patients with PNE. The BCG incorporated into the care of the intervention group (group I) were orders for:
Minimizing phlebotomy draws (without stating a specific frequency).
Use of micro sampling blood collection tubes and reinfusion of any blood drawn prior to obtaining a blood sample (waste return).
Hematinics use at managing physician's discretion (see Supporting Appendices 1 and 2 in the online version of this article).
No previous studies have addressed the impact of blood conservation strategies on the development of anemia in pediatric PNE. We hypothesized that BCG implementation resulted in smaller phlebotomy losses, lesser incidence of anemia, and lower transfusion requirements.
Methods
After obtaining an approval from the institutional review board, a retrospective medical records review was conducted of all pediatric patients (1 month‐18 years) admitted to Helen DeVos Children's Hospital with diagnoses of PNE from the period of January 1997 to December 2004.
Study Groups
Intervention group (group I) included all patients who were admitted with a diagnosis of PNE between the year 2000 to 2004 and had orders for BCG (as outlined above) written on or after admission to the hospital. That group included patients that were either solely managed by the hospitalists or comanaged by both the general pediatrician and the Hospitalist.
Simultaneous nonintervention group (group S) included patients with PNE who were admitted between 2000 to 2004 and did not have BCG orders on their record. Those patients were either managed solely by the general pediatricians or the Hospitalist may have been involved but no BCG orders were written. It was assumed that no intervention was implemented.
Historical nonintervention group (group H) included patients with PNE admitted between 1997 and 2000 prior to the implementation of the blood conservation program or the hospitalists service. Those patients were managed by the general pediatricians with the Intensivists help at times and no blood conservation measures were implemented.
Phlebotomy frequency and volume data were collected from the patient's medical record. When volume was not documented, an estimate was made based on the hospital actual practice and labs reported. If the child had central vascular access, a standard of 2 mL of blood was removed to clear the line prior to drawing the blood sample. In the S and H groups that volume was discarded and recorded as a blood loss. In Group I the blood used to clear the line was returned to the patients. Data regarding the patient's hemoglobin (Hgb) levels on admission and during hospitalization as well as RBCT frequency and volume were also collected. Other background data collected included, hospital stay, ICU admission, antibiotic use, isolated organisms, and PNE‐related interventions (thoracentesis, VATS and chest tube placement). A pediatric risk of mortality score (PRISM score17) was assigned for every patient based on data collected on the day of admission.
Statistical analysis was done using a Fisher exact test to compare qualitative data. For quantitative data Kruskal‐Wallis was used for comparison between the three groups while the Mann Whitney test was used for the pair wise comparisons. Odds Ratios and Confidence Intervals were calculated for variables associated with needing RBCTs.
Results
A total of 81 patients who were admitted to the hospital for medical and surgical management with PNE were included in the study. During the study period 24 patients with blood conservation orders on the chart were assigned to the intervention group. Another 28 patients were identified during the same period but did not have blood conservation orders and were assigned to the simultaneous no intervention group. A historical no intervention group of 29 patients were identified from a 3‐year period prior to the study period. Groups were similar in age, weight, and an overall low PRISM score. Group H tended to have lower acuity, longer hospital stay, more frequent pediatric intensive care unit (PICU) admissions, and tended to have more chest tube days compared to the (I) and (S) groups (Table 1).
| Group I (n = 24) | Group S (n = 28) | Group H (n = 29) | Kruskal‐Wallis P Value | |
|---|---|---|---|---|
| ||||
| Age, years | 6 (4) | 5 (4) | 6 (5) | 0.75 |
| Weight, kg | 26 (19) | 22 (13) | 27 (17) | 0.77 |
| PRISM Score | 2 (3) | 3 (5) | (1) | 0.007* |
| PICU admission number (%) | 3 (12) | 8 (29) | 14 (48) | 0.018 |
| Chest tube days | 7 (4) | 6 (4) | 9 (5) | 0.054 |
| Hospital stay days | 10 (4) | 13 (6) | 15 (6) | 0.008 |
| Initial Hgb, gm | 11.2 (1.7) | 11.2 (1.6) | 11.1 (1.9) | 0.95 |
| Drop in Hgb, gm | 1.7 (1.4) | 2.1 (1.2) | 2 (1.4) | 0.37 |
| Days to Hgb Nadir | 6.1 (37) | 8.5 (5.5) | 6.9 (4.3) | 0.31 |
| Number of blood draws | 7.6 (4) | 11 (9) | 12.6 (12) | 0.36 |
| Phlebotomy volume, mL/kg/day | 0.08 (0.05) | 0.14 (0.33) | 0.22 (0.24) | 0.006 |
| Number of patients transfused (%) | 2 (8.3) | 5 (17.9) | 9 (31) | 0.11 |
All groups (I, S and H) had similar initial Hgb, in‐hospital decline in Hgb levels and time to reach a Hgb nadir. There was a trend toward a lower frequency of phlebotomy and significant difference in the phlebotomy volumes drawn even when corrected for patient weight and hospital stay, (I < S < H; P = 0.006), (Table 1).
All 3 groups had a similar pretransfusion Hgb trigger (7.7 1 gm/dL), timing of transfusions (7.9 1 day), volume of packed red blood cells (PRBCs) (19 15 mL/kg), and magnitude of Hgb rise following transfusions (3.9 1.5 gm/dL). There was a strong trend toward lower transfusion need in the intervention group though it did not reach statistical significance (8.3% [I], 17.9% [S]; and 31% [H]; P = 0.11) (Table 1 and Figure 1). Being in group (S) compared to (I) carried a relative risk of transfusion of 2.14 (confidence interval [CI], 0.4610.06).

Of all study patients, 19.8% received RBCT. Compared to those who did not require transfusions they were significantly younger, smaller (P = 0.001) and had a higher severity of illness score (PRISM) (P = 0.25). Transfused children had lower initial Hgb levels, more frequent phlebotomy, greater volume of blood drawn, and longer hospital stay (P = 0.001) (Table 2 and Figure 2).

| Transfused (n = 16) | Not Transfused (n = 65) | Odds Ratio (95% CI) | |
|---|---|---|---|
| |||
| Ages, years | 3.5 (4) | 6.4 (4) | 0.8 (0.640.99)* |
| Weight, kg | 16.1 (9) | 26.9 (17) | 0.92 (0.851)* |
| Initial Hgb, gm | 9.9 (1) | 11.4 (1) | 0.43 (0.260.71)* |
| Number of blood draws | 20 (12) | 8 (6) | 1.2 (1.091.32)* |
| Total phlebotomy volume, mL | 82 (75) | 23 (25) | 1.03 (1.011.05)* |
| Total phlebotomy volume, mL/kg | 5.9 (7) | 1.1 (1) | 1.97 (1.412.74)* |
| Hospital stay days | 19 (5) | 11 (5) | 1.25 (1.121.4)* |
| PRISM score | 3.4 (6) | 1.6 (3) | P. 25 |
A total of 36% of the patients were pretreated with antibiotics prior to obtaining pleural fluid cultures. Forty‐eight patients (59%) had negative cultures, 22 (27%) grew pneumococcus, 5 patients (6%) had streptococcus A, 2 patients had streptococcus viridans, 3 patients had staphylococci aureus, and 1 patient had Haemophilus influenzae. There was no difference between the study groups regarding the site of effusion, prior antibiotics therapy or culture results.
Discussion
This retrospective study showed that children with PNE had low Hgb upon admission to the hospital and after dropping an average of 2 gm/dL over the first week of hospitalization one‐fifth of the patients required transfusion. The phlebotomy volumes significantly decreased with BCG implementation, and transfusion frequency showed a strong trend toward decline. The fact that all 3 groups had similar pretransfusion Hgb and similar Hgb decline, despite differences in the phlebotomy volumes, may implicate other factors like bone marrow suppression, malnutrition, and procedural blood losses. All of these factors have been shown to contribute to the development of anemia in the critically ill patients10 and were not accounted for in this limited retrospective review.
Transfused patients were significantly smaller, younger, and had higher illness severity scores. They had lower initial Hgb levels, more phlebotomy, and longer hospital stay than nontransfused patients.
Pretransfusion Hgb was 7.5 gm/dL to 7.7 gm/dL in all groups, consistent with Lacroix et al's18 report on the safety of lower transfusion threshold in stable PICU patients. BCG resulted in a strong trend toward less transfusion but did not reach statistical significance likely due to the small sample size. It is tempting to hypothesize that aggressive erythropoietin therapy might augment that trend; however, given the relatively short hospital stay (1014 days), erythropoietin therapy may be less efficacious in the milder case with shorter hospital stay than those with longer hospitalization. After encouraging smaller studies19 Corwin et al.20 did not find a beneficial effect for erythropoietin in adult ICU patients nor did Jacobs et al.21 in ventilated children with bronchiolitis who had comparable length of hospitalization.
A specific benefit could not be attributed to iron/folate or erythropoietin therapy as neither the dosing, duration nor timing was controlled. Furthermore, given the limitations of retrospective reviews, the relative importance of the beneficial effect of limiting phlebotomy vs. hematinics use could not be determined.
The Hospitalist is focused on improving the quality and efficiency of caring for the inpatient.2225 The initiation of the hospitalist's service at our institution coincided with that of the blood conservation service. Consequently, the Hospitalist contributed to patient care as well as daily house staff and nursing education. For those children whose care was coordinated by the hospitalists, the study data showed a trend toward lesser admissions to the PICU and lower blood utilization. This trend further emphasizes the role a Hospitalist could play in adopting and implementing useful medical strategies lowering the cost of care. In this study, a change in patient care was observed over time with the hospitalists tending to employ more blood conservation measures when compared to the pediatrician.
In summary, children with PNE are at risk for developing severe anemia requiring transfusion. This retrospective study identified the characteristics of those likely to require transfusions. Blood conservation strategies seem to decrease the need for transfusions. The hospitalists played an important role in implementing the BCG. A prospective controlled study with adequate power is needed to examine both the various mechanisms for developing anemia and the impact of the individual components of the blood conservation strategies.
- ,.Thoracic empyema.Surg Clin North Am.2002;82:643–671.
- ,.Parapneumonic Pleural effusion and empyema in children: review of 19 year experience.Clin Pediatr (Phila).1983;22:414–419.
- American Thoracic Society.Management of nontuberculosis empyema.Am Rev Respir Dis.1962;85:935–936.
- ,,, et al.Short‐term course and outcome of treatments of pleural empyema in pediatric patients: repeated ultrasound‐guided needle thoracentesis vs. chest tube drainage.Chest.2002;121:836–840.
- ,,, et al.Randomized trial of Intrapleural Urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,.Therapy of parapneumonic effusion in children: video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,,,.Is open thoracotomy still a good treatment option for the management of Empyema in children?Ann Thorac Surg.2003;76:1854–1858.
- ,,, et al.Anemia, blood loss, and blood transfusions in North American children in the intensive care unit.Am J Respir Crit Care Med.2008;178:25–33.
- .Medical vampires.N Engl J Med.1983;314:1250–1251.
- ,,, et al.Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients.Crit Care Med.1999;27:2630–2639.
- ,,, et al.Erythropoietin response is blunted in critically ill patients.Intensive Care Med.1997;23:159–162.
- ,.The continuing risk of transfusion‐transmitted infections.N Engl J Med.2006;355:1303–1305.
- ,.Immunomodulation by blood transfusion: an evolving scientific and clinical challenge.Am J Med.1996;101:299–308.
- ,,,.Pediatric red blood cell transfusions increase resource use.J Pediatr.2003;142:95–97.
- ,.Effect of blood transfusion on oxygen consumption in pediatric septic shock.Crit Care Med.1990;18:1087–1091.
- ,,,,.Red blood cell transfusion in critically ill children is independently associated with increased mortality.Intensive Care Med.2007;33:1414–1422.
- ,,.PRISM III: an updated pediatric risk of mortality.Crit Care Med.1996;24(5):743–752.
- ,,, et al.Transfusion strategies for patients in pediatric intensive care units.Nw Engl J Med.2007;356:1609–1619.
- ,,, et al.Efficacy of recombinant human erythropoietin in critically ill patients.JAMA.2002;288:2827–2835.
- ,,, et al.EPO Critical Care Trials Group.Efficacy and safety of epoetin alfa in criticall ill patients.Nw Engl J Med.2007;357:965–976.
- ,,.Erythropoietin therapy in children with Bronchiolitis and anemia.Pediatr Crit Care Med.2003;4(1):44–47.
- .The state of hospital medicine in 2008.Med Clin North Am.2008;92:265–273.
- ,,, et al.Impact of a Hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120:267–274.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
- ,.Thoracic empyema.Surg Clin North Am.2002;82:643–671.
- ,.Parapneumonic Pleural effusion and empyema in children: review of 19 year experience.Clin Pediatr (Phila).1983;22:414–419.
- American Thoracic Society.Management of nontuberculosis empyema.Am Rev Respir Dis.1962;85:935–936.
- ,,, et al.Short‐term course and outcome of treatments of pleural empyema in pediatric patients: repeated ultrasound‐guided needle thoracentesis vs. chest tube drainage.Chest.2002;121:836–840.
- ,,, et al.Randomized trial of Intrapleural Urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,.Therapy of parapneumonic effusion in children: video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,,,.Is open thoracotomy still a good treatment option for the management of Empyema in children?Ann Thorac Surg.2003;76:1854–1858.
- ,,, et al.Anemia, blood loss, and blood transfusions in North American children in the intensive care unit.Am J Respir Crit Care Med.2008;178:25–33.
- .Medical vampires.N Engl J Med.1983;314:1250–1251.
- ,,, et al.Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients.Crit Care Med.1999;27:2630–2639.
- ,,, et al.Erythropoietin response is blunted in critically ill patients.Intensive Care Med.1997;23:159–162.
- ,.The continuing risk of transfusion‐transmitted infections.N Engl J Med.2006;355:1303–1305.
- ,.Immunomodulation by blood transfusion: an evolving scientific and clinical challenge.Am J Med.1996;101:299–308.
- ,,,.Pediatric red blood cell transfusions increase resource use.J Pediatr.2003;142:95–97.
- ,.Effect of blood transfusion on oxygen consumption in pediatric septic shock.Crit Care Med.1990;18:1087–1091.
- ,,,,.Red blood cell transfusion in critically ill children is independently associated with increased mortality.Intensive Care Med.2007;33:1414–1422.
- ,,.PRISM III: an updated pediatric risk of mortality.Crit Care Med.1996;24(5):743–752.
- ,,, et al.Transfusion strategies for patients in pediatric intensive care units.Nw Engl J Med.2007;356:1609–1619.
- ,,, et al.Efficacy of recombinant human erythropoietin in critically ill patients.JAMA.2002;288:2827–2835.
- ,,, et al.EPO Critical Care Trials Group.Efficacy and safety of epoetin alfa in criticall ill patients.Nw Engl J Med.2007;357:965–976.
- ,,.Erythropoietin therapy in children with Bronchiolitis and anemia.Pediatr Crit Care Med.2003;4(1):44–47.
- .The state of hospital medicine in 2008.Med Clin North Am.2008;92:265–273.
- ,,, et al.Impact of a Hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120:267–274.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
Copyright © 2010 Society of Hospital Medicine