User login
Supportive medications and interventions received by prostate cancer survivors: results from the PiCTure study
Prostate cancer treatments are associated with various physical after-effects, including urinary, sexual, and bowel symptoms.1 These after-effects can have an impact on survivors’ health-related quality of life (HRQoL).2 Pharmaceutical and surgical interventions are available to manage or ameliorate many of these after-effects (eg, sildenafil citrate taken during and after radiotherapy improves sexual function),3 and their receipt has a positive impact on HRQoL.4
However, studies of clinicians suggest that such interventions may not be used widely.5,6 Patient-reported data on this topic is lacking. Therefore, we investigated the use of supportive medications and interventions in this population-based study of prostate cancer survivors.
Methods
The PiCTure (Prostate Cancer Treatment, Your Experience) study methods have been described elsewhere.7 Briefly, 6,559 prostate cancer survivors 2-15 years after diagnosis (diagnosed during January 1, 1995-March 31, 2010, and alive in November 2011), identified from population-based cancer registries in the Republic of Ireland and Northern Ireland, were invited to complete a postal survey. Information was sought on after-effects (incontinence, impotence, gynaecomastia, hot flashes/sweats, bowel problems, depression) that had been experienced at any time after treatment. For each after-effect, men were asked if they had received any medication or interventions to alleviate symptoms, and, if so, what they had received; examples of common interventions were provided. Men were also asked if they had been told they may become infertile and, if so, whether they had preserved their sperm. The Decisional Regret Scale8 was used to measure survivors’ regret over their entire treatment experience. This 5-item scale, rated on a 5-point Likert scale from 1 (strongly agree) to 5 (strongly disagree) was summed and standardized to a value of 0-100, with higher scores reflecting higher levels of decisional regret. 8 This scale has good psychometric properties8 and strong reliability in our sample (Cronbach’s alpha = 0.85). Responders were categorized as having any regret (score ≥1) or no regret (score = 0).
The number of men who reported receiving an intervention was expressed as a percentage of survey responders and of men who reported ever having the relevant after-effect. Chi-square tests were used to investigate variations in receipt by: age at diagnosis (≤59, 60-69, ≥70 years); time since diagnosis (≤5, 5-10, >10 years); jurisdiction (Republic of Ireland, or Northern Ireland); and primary treatment(s) received (radical prostatectomy [RP], external beam radiotherapy [EBRT] with androgen deprivation therapy [ADT], EBRT without ADT, brachytherapy, ADT [without other therapies], and active surveillance/watchful waiting). Among survivors who ever experienced an after-effect, chi-square tests were used to investigate whether the percentage who reported decisional regret differed depending on whether or not they received the relevant supportive intervention.
Ethics approval was from the Irish College of General Practitioners (Republic of Ireland) and the Office for Research Ethics Committee Northern Ireland.
Results
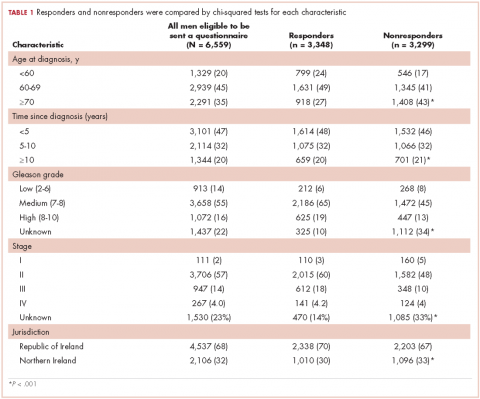
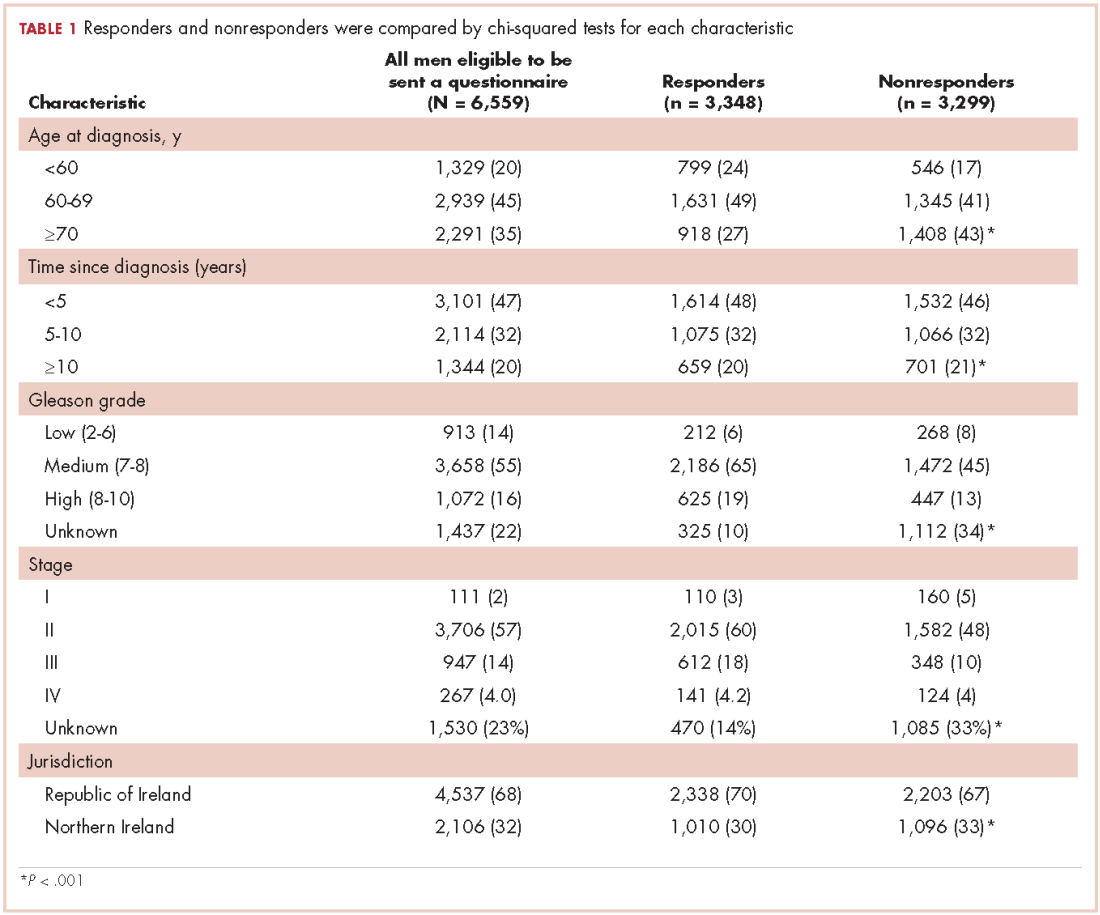
In all, 3,348 survivors participated in the survey (adjusted response rate, 54%). Compared with nonresponders, responders were more often from the Republic of Ireland (P = .007), <70 years at diagnosis (P < .001), 5-10 years post diagnosis (P < .001), with low or medium Gleason grade (Gleason scores of ≤6 [good prognosis] and 7, respectively; P < .001), and clinical stage II-IV (P < .001; Table 1).
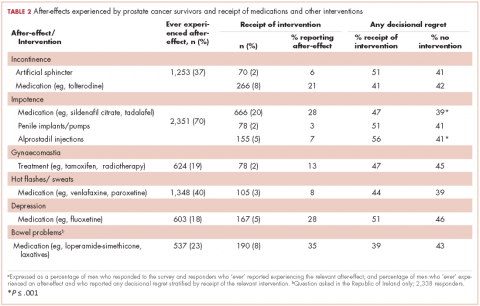
Impotence (70%) was the most commonly reported after-effect, followed by hot flashes/sweats (40%), incontinence (37%), bowel problems (23%), gynaecomastia (19%), and depression (18%; Table 2).
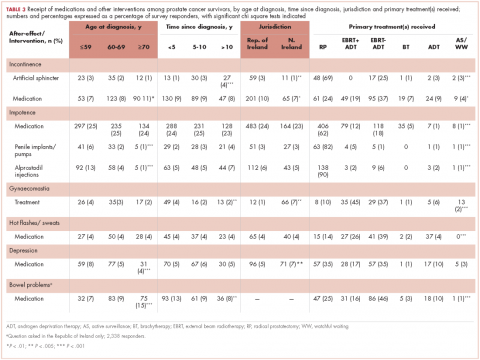
Of responders, 2% received an artificial sphincter, representing 6% of men who ever experienced incontinence post diagnosis (Table 2). This percentage was significantly higher in participants diagnosed longer ago, from the Republic of Ireland, and who received RP (Table 3).
Incontinence medication was received by 8% of participants (21% of those who experienced incontinence). Use varied significantly by age, jurisdiction, and treatment. For impotence, medications were more commonly used (20% of participants; 28% with impotence) than were injections (5% and 7%, respectively) or penile implants/pumps (2% and 3%, respectively). Use of all 3 types of intervention was highest in men who had RP; injections and implants/pumps were significantly more common among younger men. Of those experiencing gynaecomastia, 13% received interventions; receipt was highest in men who had EBRT with ADT, were <5 years post diagnosis and from Northern Ireland. For hot flashes/sweats, 3% of participants (8% who experienced symptoms) received mediations; this was higher in men who had EBRT. Of those who reported depression, 28% received medication; receipt was highest in younger men and in Northern Ireland. Medication for bowel problems was used by 35% of men who experienced these; use was highest in older men, those diagnosed more recently, and those who had EBRT. Sixty percent of men reported having been told they would become infertile; 11 (0.3% of participants) preserved their sperm, 7 from the Republic of Ireland and 4 from Northern Ireland.
A total of 35.6% of survivors reported any decisional regret. Among survivors who ever had an after-effect, a higher percentage of those who used a supportive intervention reported decisional regret compared with those who did not; this was only statistically significant for those using medication or alprostadil injections for impotence (Table 2).
Discussion
This study documents, for the first time, population-based data on patient-reported use of supportive medications and interventions to alleviate adverse effects of prostate cancer and its treatment. Among survivors who experienced after-effects, use was highest for bowel problems, impotence, and depression, but even for those, only 28%-35% of men took medication. Although it is possible that some survivors declined medications or other interventions, these low levels of use strongly suggest that not all survivors who might benefit from supports receive them.
There was little evidence that utilisation was higher in survivors diagnosed more recently. This suggests that, although the number of prostate cancer survivors has grown, and there is greater focus on survivorship issues in clinical practice, this has not translated into more men receiving support to manage after-effects. Care is needed to ensure that the newer models of post-cancer follow-up being considered or adopted in many settings,9 do not exacerbate this issue.
As expected, patterns of utilisation varied by treatment(s) received. Higher use of surgical and pharmaceutical interventions to alleviate incontinence among survivors in the Republic of Ireland than in Northern Ireland is likely owing to the higher rate of radical prostatectomy in the Republic of Ireland, whereas greater use of treatments for gynaecomastia in Northern Ireland reflects higher use of hormone therapy there.10 Other variations in intervention use were more surprising. Younger men were significantly more likely to report using supportive interventions for depression and impotence, the latter finding being consistent with findings in a Swedish population-based study.11 Older men were significantly more likely to report interventions for incontinence and bowel problems. Although those trends could be explained by differences in treatment receipt by age, it is possible that men of different ages may be more likely to seek, or be offered, help for certain types of after-effects. With the exception of interventions for bowel problems, a higher percentage of men who received intervention(s) for an after-effect reported decisional regret. There are a number of possible explanations: these men may have experienced more severe after-effects, which required interventions; they may have been less satisfied with their posttreatment function and/or more proactive about recovering or treating their after-effects. This requires further investigation.
This is a large, international, population-based study, the first such study to describe patient-reported use of supportive care following a range of prostate cancer treatments. Although this study is novel, there are a number of limitations. It is a cross-sectional, descriptive study. We did not ask survivors whether the supportive interventions received matched their needs and wants, and whether they were satisfied with the supportive care received. Furthermore, although the response rate is comparable with other similar studies,12,13 it is possible that the supportive care of nonresponders was different to that of responders.
Our study included men from 2 jurisdictions with separate health care systems, suggesting that low use of supportive interventions may be common across systems. There is a need for further research into patient and health care system factors associated with the receipt of supportive interventions and how satisfied men are with these, in this and other health care settings. Presently, it is clear that more needs to be done in the clinical setting to support prostate cancer survivors manage treatment after-effects; this in turn could improve survivors’ HRQoL.
1. Drummond FJ, Kinnear H, O’Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9(2):361-72.
2. Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009; 339:b4817.
3. Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192(3):868-874.
4. Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of follow-up. J Urol. 1997;158(2):435-439.
5. Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237-241.
6. Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126-131,
7. Drummond FJ, Kinnear H, Donnelly C, et al. Establishing a population-based patient reported outcomes study (PROMs) using national cancer registries across two jurisdictions: Prostate Cancer Treatment, your experience (PiCTure) Study. BMJ Open 2015;5:e006851.
8. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-92.
9. Howell D, Hack TF, Oliver et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
10. Donnelly DW, Gavin AT, Comber H. Cancer in Ireland 1994-2004. A comprehensive report. Northern Ireland Cancer Registry/National Cancer Registry, Ireland, 2009.
11. Plym A, Folkvaljon Y, Garmo H, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med. 2014;11(8):2100-2108.
12. Hervouet S, Savard J, Simard S, et al. Psychological functioning associated with prostate cancer: cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage. 2005;30(5):474-484.
13. Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1-5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3(4). pii: e002317.
Prostate cancer treatments are associated with various physical after-effects, including urinary, sexual, and bowel symptoms.1 These after-effects can have an impact on survivors’ health-related quality of life (HRQoL).2 Pharmaceutical and surgical interventions are available to manage or ameliorate many of these after-effects (eg, sildenafil citrate taken during and after radiotherapy improves sexual function),3 and their receipt has a positive impact on HRQoL.4
However, studies of clinicians suggest that such interventions may not be used widely.5,6 Patient-reported data on this topic is lacking. Therefore, we investigated the use of supportive medications and interventions in this population-based study of prostate cancer survivors.
Methods
The PiCTure (Prostate Cancer Treatment, Your Experience) study methods have been described elsewhere.7 Briefly, 6,559 prostate cancer survivors 2-15 years after diagnosis (diagnosed during January 1, 1995-March 31, 2010, and alive in November 2011), identified from population-based cancer registries in the Republic of Ireland and Northern Ireland, were invited to complete a postal survey. Information was sought on after-effects (incontinence, impotence, gynaecomastia, hot flashes/sweats, bowel problems, depression) that had been experienced at any time after treatment. For each after-effect, men were asked if they had received any medication or interventions to alleviate symptoms, and, if so, what they had received; examples of common interventions were provided. Men were also asked if they had been told they may become infertile and, if so, whether they had preserved their sperm. The Decisional Regret Scale8 was used to measure survivors’ regret over their entire treatment experience. This 5-item scale, rated on a 5-point Likert scale from 1 (strongly agree) to 5 (strongly disagree) was summed and standardized to a value of 0-100, with higher scores reflecting higher levels of decisional regret. 8 This scale has good psychometric properties8 and strong reliability in our sample (Cronbach’s alpha = 0.85). Responders were categorized as having any regret (score ≥1) or no regret (score = 0).
The number of men who reported receiving an intervention was expressed as a percentage of survey responders and of men who reported ever having the relevant after-effect. Chi-square tests were used to investigate variations in receipt by: age at diagnosis (≤59, 60-69, ≥70 years); time since diagnosis (≤5, 5-10, >10 years); jurisdiction (Republic of Ireland, or Northern Ireland); and primary treatment(s) received (radical prostatectomy [RP], external beam radiotherapy [EBRT] with androgen deprivation therapy [ADT], EBRT without ADT, brachytherapy, ADT [without other therapies], and active surveillance/watchful waiting). Among survivors who ever experienced an after-effect, chi-square tests were used to investigate whether the percentage who reported decisional regret differed depending on whether or not they received the relevant supportive intervention.
Ethics approval was from the Irish College of General Practitioners (Republic of Ireland) and the Office for Research Ethics Committee Northern Ireland.
Results
In all, 3,348 survivors participated in the survey (adjusted response rate, 54%). Compared with nonresponders, responders were more often from the Republic of Ireland (P = .007), <70 years at diagnosis (P < .001), 5-10 years post diagnosis (P < .001), with low or medium Gleason grade (Gleason scores of ≤6 [good prognosis] and 7, respectively; P < .001), and clinical stage II-IV (P < .001; Table 1).
Impotence (70%) was the most commonly reported after-effect, followed by hot flashes/sweats (40%), incontinence (37%), bowel problems (23%), gynaecomastia (19%), and depression (18%; Table 2).
Of responders, 2% received an artificial sphincter, representing 6% of men who ever experienced incontinence post diagnosis (Table 2). This percentage was significantly higher in participants diagnosed longer ago, from the Republic of Ireland, and who received RP (Table 3).
Incontinence medication was received by 8% of participants (21% of those who experienced incontinence). Use varied significantly by age, jurisdiction, and treatment. For impotence, medications were more commonly used (20% of participants; 28% with impotence) than were injections (5% and 7%, respectively) or penile implants/pumps (2% and 3%, respectively). Use of all 3 types of intervention was highest in men who had RP; injections and implants/pumps were significantly more common among younger men. Of those experiencing gynaecomastia, 13% received interventions; receipt was highest in men who had EBRT with ADT, were <5 years post diagnosis and from Northern Ireland. For hot flashes/sweats, 3% of participants (8% who experienced symptoms) received mediations; this was higher in men who had EBRT. Of those who reported depression, 28% received medication; receipt was highest in younger men and in Northern Ireland. Medication for bowel problems was used by 35% of men who experienced these; use was highest in older men, those diagnosed more recently, and those who had EBRT. Sixty percent of men reported having been told they would become infertile; 11 (0.3% of participants) preserved their sperm, 7 from the Republic of Ireland and 4 from Northern Ireland.
A total of 35.6% of survivors reported any decisional regret. Among survivors who ever had an after-effect, a higher percentage of those who used a supportive intervention reported decisional regret compared with those who did not; this was only statistically significant for those using medication or alprostadil injections for impotence (Table 2).
Discussion
This study documents, for the first time, population-based data on patient-reported use of supportive medications and interventions to alleviate adverse effects of prostate cancer and its treatment. Among survivors who experienced after-effects, use was highest for bowel problems, impotence, and depression, but even for those, only 28%-35% of men took medication. Although it is possible that some survivors declined medications or other interventions, these low levels of use strongly suggest that not all survivors who might benefit from supports receive them.
There was little evidence that utilisation was higher in survivors diagnosed more recently. This suggests that, although the number of prostate cancer survivors has grown, and there is greater focus on survivorship issues in clinical practice, this has not translated into more men receiving support to manage after-effects. Care is needed to ensure that the newer models of post-cancer follow-up being considered or adopted in many settings,9 do not exacerbate this issue.
As expected, patterns of utilisation varied by treatment(s) received. Higher use of surgical and pharmaceutical interventions to alleviate incontinence among survivors in the Republic of Ireland than in Northern Ireland is likely owing to the higher rate of radical prostatectomy in the Republic of Ireland, whereas greater use of treatments for gynaecomastia in Northern Ireland reflects higher use of hormone therapy there.10 Other variations in intervention use were more surprising. Younger men were significantly more likely to report using supportive interventions for depression and impotence, the latter finding being consistent with findings in a Swedish population-based study.11 Older men were significantly more likely to report interventions for incontinence and bowel problems. Although those trends could be explained by differences in treatment receipt by age, it is possible that men of different ages may be more likely to seek, or be offered, help for certain types of after-effects. With the exception of interventions for bowel problems, a higher percentage of men who received intervention(s) for an after-effect reported decisional regret. There are a number of possible explanations: these men may have experienced more severe after-effects, which required interventions; they may have been less satisfied with their posttreatment function and/or more proactive about recovering or treating their after-effects. This requires further investigation.
This is a large, international, population-based study, the first such study to describe patient-reported use of supportive care following a range of prostate cancer treatments. Although this study is novel, there are a number of limitations. It is a cross-sectional, descriptive study. We did not ask survivors whether the supportive interventions received matched their needs and wants, and whether they were satisfied with the supportive care received. Furthermore, although the response rate is comparable with other similar studies,12,13 it is possible that the supportive care of nonresponders was different to that of responders.
Our study included men from 2 jurisdictions with separate health care systems, suggesting that low use of supportive interventions may be common across systems. There is a need for further research into patient and health care system factors associated with the receipt of supportive interventions and how satisfied men are with these, in this and other health care settings. Presently, it is clear that more needs to be done in the clinical setting to support prostate cancer survivors manage treatment after-effects; this in turn could improve survivors’ HRQoL.
Prostate cancer treatments are associated with various physical after-effects, including urinary, sexual, and bowel symptoms.1 These after-effects can have an impact on survivors’ health-related quality of life (HRQoL).2 Pharmaceutical and surgical interventions are available to manage or ameliorate many of these after-effects (eg, sildenafil citrate taken during and after radiotherapy improves sexual function),3 and their receipt has a positive impact on HRQoL.4
However, studies of clinicians suggest that such interventions may not be used widely.5,6 Patient-reported data on this topic is lacking. Therefore, we investigated the use of supportive medications and interventions in this population-based study of prostate cancer survivors.
Methods
The PiCTure (Prostate Cancer Treatment, Your Experience) study methods have been described elsewhere.7 Briefly, 6,559 prostate cancer survivors 2-15 years after diagnosis (diagnosed during January 1, 1995-March 31, 2010, and alive in November 2011), identified from population-based cancer registries in the Republic of Ireland and Northern Ireland, were invited to complete a postal survey. Information was sought on after-effects (incontinence, impotence, gynaecomastia, hot flashes/sweats, bowel problems, depression) that had been experienced at any time after treatment. For each after-effect, men were asked if they had received any medication or interventions to alleviate symptoms, and, if so, what they had received; examples of common interventions were provided. Men were also asked if they had been told they may become infertile and, if so, whether they had preserved their sperm. The Decisional Regret Scale8 was used to measure survivors’ regret over their entire treatment experience. This 5-item scale, rated on a 5-point Likert scale from 1 (strongly agree) to 5 (strongly disagree) was summed and standardized to a value of 0-100, with higher scores reflecting higher levels of decisional regret. 8 This scale has good psychometric properties8 and strong reliability in our sample (Cronbach’s alpha = 0.85). Responders were categorized as having any regret (score ≥1) or no regret (score = 0).
The number of men who reported receiving an intervention was expressed as a percentage of survey responders and of men who reported ever having the relevant after-effect. Chi-square tests were used to investigate variations in receipt by: age at diagnosis (≤59, 60-69, ≥70 years); time since diagnosis (≤5, 5-10, >10 years); jurisdiction (Republic of Ireland, or Northern Ireland); and primary treatment(s) received (radical prostatectomy [RP], external beam radiotherapy [EBRT] with androgen deprivation therapy [ADT], EBRT without ADT, brachytherapy, ADT [without other therapies], and active surveillance/watchful waiting). Among survivors who ever experienced an after-effect, chi-square tests were used to investigate whether the percentage who reported decisional regret differed depending on whether or not they received the relevant supportive intervention.
Ethics approval was from the Irish College of General Practitioners (Republic of Ireland) and the Office for Research Ethics Committee Northern Ireland.
Results
In all, 3,348 survivors participated in the survey (adjusted response rate, 54%). Compared with nonresponders, responders were more often from the Republic of Ireland (P = .007), <70 years at diagnosis (P < .001), 5-10 years post diagnosis (P < .001), with low or medium Gleason grade (Gleason scores of ≤6 [good prognosis] and 7, respectively; P < .001), and clinical stage II-IV (P < .001; Table 1).
Impotence (70%) was the most commonly reported after-effect, followed by hot flashes/sweats (40%), incontinence (37%), bowel problems (23%), gynaecomastia (19%), and depression (18%; Table 2).
Of responders, 2% received an artificial sphincter, representing 6% of men who ever experienced incontinence post diagnosis (Table 2). This percentage was significantly higher in participants diagnosed longer ago, from the Republic of Ireland, and who received RP (Table 3).
Incontinence medication was received by 8% of participants (21% of those who experienced incontinence). Use varied significantly by age, jurisdiction, and treatment. For impotence, medications were more commonly used (20% of participants; 28% with impotence) than were injections (5% and 7%, respectively) or penile implants/pumps (2% and 3%, respectively). Use of all 3 types of intervention was highest in men who had RP; injections and implants/pumps were significantly more common among younger men. Of those experiencing gynaecomastia, 13% received interventions; receipt was highest in men who had EBRT with ADT, were <5 years post diagnosis and from Northern Ireland. For hot flashes/sweats, 3% of participants (8% who experienced symptoms) received mediations; this was higher in men who had EBRT. Of those who reported depression, 28% received medication; receipt was highest in younger men and in Northern Ireland. Medication for bowel problems was used by 35% of men who experienced these; use was highest in older men, those diagnosed more recently, and those who had EBRT. Sixty percent of men reported having been told they would become infertile; 11 (0.3% of participants) preserved their sperm, 7 from the Republic of Ireland and 4 from Northern Ireland.
A total of 35.6% of survivors reported any decisional regret. Among survivors who ever had an after-effect, a higher percentage of those who used a supportive intervention reported decisional regret compared with those who did not; this was only statistically significant for those using medication or alprostadil injections for impotence (Table 2).
Discussion
This study documents, for the first time, population-based data on patient-reported use of supportive medications and interventions to alleviate adverse effects of prostate cancer and its treatment. Among survivors who experienced after-effects, use was highest for bowel problems, impotence, and depression, but even for those, only 28%-35% of men took medication. Although it is possible that some survivors declined medications or other interventions, these low levels of use strongly suggest that not all survivors who might benefit from supports receive them.
There was little evidence that utilisation was higher in survivors diagnosed more recently. This suggests that, although the number of prostate cancer survivors has grown, and there is greater focus on survivorship issues in clinical practice, this has not translated into more men receiving support to manage after-effects. Care is needed to ensure that the newer models of post-cancer follow-up being considered or adopted in many settings,9 do not exacerbate this issue.
As expected, patterns of utilisation varied by treatment(s) received. Higher use of surgical and pharmaceutical interventions to alleviate incontinence among survivors in the Republic of Ireland than in Northern Ireland is likely owing to the higher rate of radical prostatectomy in the Republic of Ireland, whereas greater use of treatments for gynaecomastia in Northern Ireland reflects higher use of hormone therapy there.10 Other variations in intervention use were more surprising. Younger men were significantly more likely to report using supportive interventions for depression and impotence, the latter finding being consistent with findings in a Swedish population-based study.11 Older men were significantly more likely to report interventions for incontinence and bowel problems. Although those trends could be explained by differences in treatment receipt by age, it is possible that men of different ages may be more likely to seek, or be offered, help for certain types of after-effects. With the exception of interventions for bowel problems, a higher percentage of men who received intervention(s) for an after-effect reported decisional regret. There are a number of possible explanations: these men may have experienced more severe after-effects, which required interventions; they may have been less satisfied with their posttreatment function and/or more proactive about recovering or treating their after-effects. This requires further investigation.
This is a large, international, population-based study, the first such study to describe patient-reported use of supportive care following a range of prostate cancer treatments. Although this study is novel, there are a number of limitations. It is a cross-sectional, descriptive study. We did not ask survivors whether the supportive interventions received matched their needs and wants, and whether they were satisfied with the supportive care received. Furthermore, although the response rate is comparable with other similar studies,12,13 it is possible that the supportive care of nonresponders was different to that of responders.
Our study included men from 2 jurisdictions with separate health care systems, suggesting that low use of supportive interventions may be common across systems. There is a need for further research into patient and health care system factors associated with the receipt of supportive interventions and how satisfied men are with these, in this and other health care settings. Presently, it is clear that more needs to be done in the clinical setting to support prostate cancer survivors manage treatment after-effects; this in turn could improve survivors’ HRQoL.
1. Drummond FJ, Kinnear H, O’Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9(2):361-72.
2. Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009; 339:b4817.
3. Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192(3):868-874.
4. Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of follow-up. J Urol. 1997;158(2):435-439.
5. Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237-241.
6. Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126-131,
7. Drummond FJ, Kinnear H, Donnelly C, et al. Establishing a population-based patient reported outcomes study (PROMs) using national cancer registries across two jurisdictions: Prostate Cancer Treatment, your experience (PiCTure) Study. BMJ Open 2015;5:e006851.
8. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-92.
9. Howell D, Hack TF, Oliver et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
10. Donnelly DW, Gavin AT, Comber H. Cancer in Ireland 1994-2004. A comprehensive report. Northern Ireland Cancer Registry/National Cancer Registry, Ireland, 2009.
11. Plym A, Folkvaljon Y, Garmo H, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med. 2014;11(8):2100-2108.
12. Hervouet S, Savard J, Simard S, et al. Psychological functioning associated with prostate cancer: cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage. 2005;30(5):474-484.
13. Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1-5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3(4). pii: e002317.
1. Drummond FJ, Kinnear H, O’Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9(2):361-72.
2. Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009; 339:b4817.
3. Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192(3):868-874.
4. Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of follow-up. J Urol. 1997;158(2):435-439.
5. Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237-241.
6. Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126-131,
7. Drummond FJ, Kinnear H, Donnelly C, et al. Establishing a population-based patient reported outcomes study (PROMs) using national cancer registries across two jurisdictions: Prostate Cancer Treatment, your experience (PiCTure) Study. BMJ Open 2015;5:e006851.
8. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-92.
9. Howell D, Hack TF, Oliver et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
10. Donnelly DW, Gavin AT, Comber H. Cancer in Ireland 1994-2004. A comprehensive report. Northern Ireland Cancer Registry/National Cancer Registry, Ireland, 2009.
11. Plym A, Folkvaljon Y, Garmo H, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med. 2014;11(8):2100-2108.
12. Hervouet S, Savard J, Simard S, et al. Psychological functioning associated with prostate cancer: cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage. 2005;30(5):474-484.
13. Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1-5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3(4). pii: e002317.