User login
In reply: Prostate cancer prevention
In Reply: Although the two drugs were not compared head to head, the data from randomized trials suggest that they have similar effects on the amelioration of lower urinary tract symptoms due to benign prostatic hypertrophy. The full results of the REDUCE trial are not yet available and until they are published it is not possible to comment any further on whether one or the other is the better choice for prevention of prostate cancer.
In Reply: Although the two drugs were not compared head to head, the data from randomized trials suggest that they have similar effects on the amelioration of lower urinary tract symptoms due to benign prostatic hypertrophy. The full results of the REDUCE trial are not yet available and until they are published it is not possible to comment any further on whether one or the other is the better choice for prevention of prostate cancer.
In Reply: Although the two drugs were not compared head to head, the data from randomized trials suggest that they have similar effects on the amelioration of lower urinary tract symptoms due to benign prostatic hypertrophy. The full results of the REDUCE trial are not yet available and until they are published it is not possible to comment any further on whether one or the other is the better choice for prevention of prostate cancer.
What’s new in prostate cancer screening and prevention?
In spite of some recent studies, or perhaps because of them, we still are unsure about how best to screen for and prevent prostate cancer. Two large trials of screening with prostate-specific antigen (PSA) measurements came to seemingly opposite conclusions.1,2 Furthermore, a large trial of selenium and vitamin E found that these agents have no value as preventive agents.3
Nevertheless, negative studies also advance science, and steady progress is being made in prostate cancer research. In this paper I briefly summarize and comment on some of the recent findings.
TO SCREEN OR NOT TO SCREEN?
All cases of prostate cancer are clinically relevant in that they can cause anxiety or can lead to treatment-related morbidity. The challenge is to detect the minority of cases of cancer that are biologically significant, ie, those that will cause serious illness or death.
Many men have prostate cancer
In the United States, the lifetime probability of developing prostate cancer is 1 in 6, and the probability increases with age. Prostate cancer is primarily a disease of the Western world, but it is becoming more common in other areas as well.
Risk factors for prostate cancer are age, race, and family history. Clinically apparent disease is very rare in men younger than 40 years; until recently, most guidelines suggested that screening for it should begin at age 50. African American men have the highest risk of developing and dying of prostate cancer, for reasons that are not clear. In the past, this finding was attributed to disparities in access and less aggressive therapy in black men, but recent studies suggest the differences persist even in the absence of these factors, suggesting there is a biological difference in cancers between blacks and whites. Having a father or brother who had prostate cancer increases one’s risk twofold (threefold if the father or brother was affected before the age of 60); having a father and a brother with prostate cancer increases one’s risk fourfold, and true hereditary cancer raises the risk fivefold.4
But relatively few men die of it
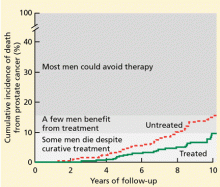
The Scandinavian Prostate Cancer Group5 randomized 695 men with early prostate cancer (mostly discovered by digital rectal examination or by symptoms) to undergo either radical prostatectomy or a program of watchful waiting. In 8.2 years of follow-up, 8.6% of the men in the surgery group and 14.4% of those in the watchful waiting group died of prostate cancer. Thus, we can conclude that surgery is beneficial in this situation.
Despite these calculations, in contemporary practice in the United States, about 90% of men with newly diagnosed low-grade prostate cancer choose to be treated.6 This high level of intervention reflects our current inability to predict which cancers will remain indolent vs which will progress and the lack of validated markers that tell us when to intervene in patients who are managed expectantly and not lose the chance for cure. Most often, patients and their physicians, who are paid to intervene, deal with this uncertainty by choosing the high likelihood of cure with early intervention despite treatment-related morbidity.
What PSA has wrought
When PSA screening was introduced in the late 1980s and early 1990s, it brought about several changes in the epidemiology and clinical profile of this disease that led us to believe that it was making a meaningful difference.
A spike in the apparent incidence of prostate cancer occurred in the late 1980s and early 1990s with the introduction of PSA screening. The spike was temporary, representing detection of preexisting cases. Now, the incidence may have leveled off.7
A shift in the stages of cancers detected. In 1982, half of men with newly diagnosed prostate cancer had incurable disease.8 Five years after the introduction of PSA testing, 95% had curable disease.9
An increase in the rate of cure after radical prostatectomy was seen.
A decrease in the death rate from prostate cancer since the early 1990s has been noted, which is likely due not only to earlier detection but also to earlier and better treatment.
Limitations of PSA screening
PSA screening has low specificity. PSA is more sensitive than digital rectal examination, but most men with “elevated” PSA do not have prostate cancer. Nevertheless, although it is not a perfect screening test, it is still the best cancer marker that we have.
In the Prostate Cancer Prevention Trial (PCPT),10 finasteride (Proscar) decreased the incidence of prostate cancer by about 25% over 7 years. But there were also lessons to be learned from the placebo group, which underwent PSA testing every year and prostate biopsy at the end of the study.
We used to think the cutoff PSA level that had high sensitivity and specificity for finding cancer was 4 ng/mL. However, in the PCPT, 6.6% of men with PSA levels below 0.5 ng/mL were found to have cancer, and 12.5% of those cancers were high-grade. Of those with PSA levels of 3.1 to 4.0 ng/mL, 26.9% had cancer, and 25.0% of the cancers were high-grade. These data demonstrate that there is no PSA level below which risk of cancer is zero, and that there is no PSA cutoff with sufficient sensitivity and specificity to be clinically useful.
The PCPT risk calculator (http://deb.uthscsa.edu/URORiskCalc/Pages/uroriskcalc.jsp) is a wonderful tool that came out of that study. It uses seven variables—race, age, PSA level, family history of prostate cancer, findings on digital rectal examination, whether the patient has ever undergone a prostate biopsy, and whether the patient is taking finasteride—and calculates the patient’s risk of harboring prostate cancer and, more important, the risk of having high-grade prostate cancer. This tool allows estimation of individual risk and helps identify who is at risk of cancer that may require therapy.
Other factors can affect PSA levels. Men with a higher body mass index have lower PSA levels. The reason is not clear; it may be a hormonal effect, or heavier men may simply have higher blood volume, which may dilute the PSA. Furthermore, there are genetic differences that make some men secrete more PSA, but this effect is probably not clinically important. And a study by Hamilton et al11 suggested that statin drugs lower PSA levels. As these findings are confirmed, in the future it may be necessary to adjust PSA levels to account for their effects before deciding on the need for biopsy.
Two new, conflicting studies
Two large trials of PSA screening, published simultaneously in March 2009, came to opposite conclusions.
The European Randomized Study of Screening for Prostate Cancer2 randomized 162,243 men between the ages of 55 and 69 to undergo PSA screening at an average of once every 4 years or to a control group. Most of the participating centers used a PSA level of 3.0 ng/mL as an indication for biopsy. At an average follow-up time of 8.8 years, 214 men had died of prostate cancer in the screening group, compared with 326 in the control group, for an adjusted rate ratio of 0.80 (95% confidence interval [CI] 0.65–0.98, P = .04). In other words, screening decreased the risk of death from prostate cancer by 20%.
The Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial,1 conducted in the United States, came to the opposite conclusion, ie, that there is no benefit from PSA screening. This study was smaller, with 76,693 men between ages 55 and 74 randomly assigned to receive PSA testing every year for 6 years and digital rectal examination for 4 years, or usual care. A PSA level of more than 4.0 ng/mL was considered to be positive for prostate cancer. At 7 years, of those who reported undergoing no more than one PSA test at baseline, 48 men had died of prostate cancer in the screening group, compared with 41 in the control group (rate ratio 1.16, 95% CI 0.76–1.76).
Why were the findings different? The PLCO investigators offered several possible explanations for their negative results. The PSA threshold of 4 ng/mL that was used in that study may not be effective. More than half the men in the control group actually had a PSA test in the first 6 years of the study, potentially diluting any effect of testing. (This was the most worrisome flaw in the study, in my opinion.) About 44% of the men in the study had already had one or more PSA tests at baseline, which would have eliminated cancers detectable on screening from the study, and not all men who were advised to undergo biopsy actually did so. The follow-up time may not yet be long enough for the benefit to be apparent. Most important, in their opinion, treatment for prostate cancer improved during the time of the trial, so that fewer men than expected died of prostate cancer in both groups.
Improvements to PSA screening
Derivatives of PSA have been used in an attempt to improve its performance characteristics for detecting cancer.
PSA density, defined as serum PSA divided by prostate volume, has some predictive power but requires performance of transrectal ultrasonography. It is therefore not a good screening test in the primary care setting.
PSA velocity or doubling time, based on the rate of change over time, is predictive of prostate cancer, but is highly dependent on the absolute value of PSA and does not add independent information to the variables defined in the PCPT risk calculator or other standard predictive variables.12
A PSA level between the ages of 44 and 50 may predict the lifetime risk of prostate cancer, according to a study by Lilja et al13 in Sweden. This finding suggests that we should measure PSA early in life and screen men who have higher values more frequently or with better strategies. This recommendation has been adopted by the American Urological Association, which released updated screening guidelines in April 2009 (available at www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf).
New markers under study
A number of new biological markers probably will improve our ability to detect prostate cancer, although they are not yet ready for widespread use.
Urinary PCA3. Prostate cancer gene 3 (PCA3) codes for a messenger RNA that is highly overexpressed in the urine of men with prostate cancer. Urine is collected after prostate massage. Marks et al14 reported that PCA3 scores predicted biopsy outcomes in men with serum PSA levels of 2.5 ng/mL or higher.
Serum EPCA-2 (early prostate cancer antigen 2) is another candidate marker undergoing study.
Gene fusions, specifically of TRMPSS2 and the ETS gene family, are detectable in high levels in the urine of some men with prostate cancer, and appear to be very promising markers for detection.
Metabolomics is a technique that uses mass spectroscopy to detect the metabolic signature of cancer. Sreekumar et al15 identified sarcosine as a potential marker of prostate cancer using this technique.
Genetic tests: Not yet
Some data suggest that we can use genetic tests to screen for prostate cancer, but the tests are not yet as good as we would like.
Zheng et al16 reported that 16 singlenucleotide polymorphisms (SNPs) in five chromosomal regions plus a family history of prostate cancer have a cumulative association with prostate cancer: men who had any five or more of these SNPs had a risk of prostate cancer nearly 10 times as high as men without any of them. However, the number of men who actually fall into this category is so low that routine use in the general population is not cost-effective; it may, however, be useful in men with a family history of prostate cancer.
Other SNPs have been linked to prostate cancer (reviewed by Witte17). Having any one of these loci increases one’s risk only modestly, however. Only about 2% of the population has five or more of these SNPS, and the sensitivity is about only about 16%.
A commercially available DNA test (Decode Genetics, Reykjavik, Iceland) can detect eight variants that, according to the company, account for about half of all cases of prostate cancer.
Prostate cancer screening: My interpretation
I believe the two new studies of PSA screening suggest there is a modest benefit from screening in terms of preventing deaths from prostate cancer. But I also believe we should be more judicious in recommending treatment for men whom we know have biologically indolent tumors, although we cannot yet identify them perfectly.
In the past, we used an arbitrary PSA cutoff to detect prostate cancer of any grade, and men with high levels were advised to have a biopsy. Currently, we use continuous-risk models to look for any cancer and biologically significant cancers. These involve nomograms, a risk calculator, and new markers.
We use the PCPT risk calculator routinely in our practice. I recommend—completely arbitrarily—that a man undergo biopsy if he has a 10% or higher risk of high-grade cancer, but not if the risk is less. I believe this is more accurate than a simple PSA cutoff value.
CAN WE PREVENT PROSTATE CANCER?
Prostate cancer is a significant public health risk, with 186,000 new cases and 26,000 deaths yearly. Its risk factors (age, race, and genes) are not modifiable. The benefit of screening in terms of preventing deaths is not as good as we would like, and therapy is associated with morbidity. That leaves prevention as a potential way to reduce the morbidity and perhaps mortality of prostate cancer and its therapy.
Epidemiologic studies suggest that certain lifestyle factors may increase the risk, ie, consumption of fat, red meat, fried foods, and dairy; high calcium intake; smoking; total calories; and body size. Other factors may decrease the risk: plant-based foods and vegetables, especially lycopene-containing foods such as tomatoes, cruciferous vegetables, soy, and legumes, specific nutrients such as carotenoids, lycopene, total antioxidants, fish oil (omega-3 fatty acids), and moderate to vigorous exercise. However, there have been few randomized trials to determine if any of these agents are beneficial.
Findings of trials of prevention
Selenium and vitamin E do not prevent prostate cancer, lung cancer, colorectal cancer, other primary cancers, or deaths. The Selenium and Vitamin E Cancer Prevention Trial (SELECT)3 involved 35,533 men 55 years of age or older (or 50 and older if they were African American). They were randomized to receive one of four treatments: selenium 200 μg/day plus vitamin E placebo, vitamin E 400 IU/day plus selenium placebo, selenium plus vitamin E, or double placebo. At a median follow-up of 5.46 years, compared with the placebo group, the hazard ratio for prostate cancer was 1.04 in the selenium-only group, 1.13 in the vitamin E-only group, and 1.05 in the selenium-plus-vitamin E group. None of the differences was statistically significant.
The Physician’s Health Study18 also found that vitamin E at the same dose given every other day does not prevent prostate cancer.
Finasteride prevents prostate cancer. The PCPT19 included 18,882 men, 55 years of age or older, who had PSA levels of 3.0 ng/mL or less and normal findings on digital rectal examination. Treatment was with finasteride 5 mg/day or placebo. At 7 years, prostate cancer had been discovered in 18.4% of the finasteride group vs 24.4% of the placebo group, a 24.8% reduction (95% CI 18.6–30.6, P < .001). Sexual side effects were more common in the men who received finasteride, while urinary symptoms were more common in the placebo group.
At the time of the original PCPT report in 2003,19 tumors of Gleason grade 7 or higher were more common in the finasteride group, accounting for 37.0% of the tumors discovered, than in the placebo group (22.2%), creating concern that finasteride might somehow cause the tumors that occurred to be more aggressive. However, a subsequent analysis20 found the opposite to be true, ie, that finasteride decreases the risk of high-grade cancers. A companion quality-of-life study showed that chronic use of finasteride had clinically insignificant effects on sexual function, and the PCPT and other studies have shown benefits of finasteride in reducing lower urinary tract symptoms due to benign prostatic hyperplasia (BPH), reducing the risk of acute urinary retention and the need for surgical intervention for BPH, and reducing the risk of prostatitis.
Dutasteride also prevents prostate cancer. A large-scale trial of another 5-alpha reductase inhibitor, dutasteride (Avodart), was reported by Andriole at the annual meeting of the American Urological Association in April 2009.21 The Reduction by Dutasteride of Prostate Events (REDUCE) trial included men who were 50 to 75 years old, inclusively, and who had PSA levels between 2.5 and 10 ng/mL, prostate volume less than 80 cc, and one prior negative prostate biopsy within 6 months of enrollment, representing a group at high risk for cancer on a subsequent biopsy. The trial accrued 8,231 men. At 4 years, prostate cancer had occurred in 659 men in the dutasteride group vs 857 in the placebo group, a 23% reduction (P < .0001). Interestingly, no significant increase in Gleason grade 8 to grade 10 tumors was observed in the study.
Preliminary analyses also suggest that dutasteride enhanced the utility of PSA as a diagnostic test for prostate cancer, had beneficial effects on BPH, and was generally well tolerated. The fact that the results of REDUCE were congruent with those of the PCPT with respect to the magnitude of risk reduction, beneficial effects on benign prostatic hypertrophy, minimal toxicity, and no issues related to tumor grade suggests a class effect for 5-alpha reductase inhibitors, and suggests that these agents should be used more liberally for the prevention of prostate cancer.
There is current debate about whether 5-alpha reductase inhibitors should be used by all men at risk of prostate cancer or only by those at high risk. However, the American Urological Association and the American Society of Clinical Oncology have issued guidelines stating that men at risk should consider this intervention.22
- Andriole GL, Grubb RL, Buys SS, et al; PLCO Project Team. Mortality results from a randomized prostate cancer screening trial. N Engl J Med 2009; 360:1310–1319.
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality in a randomized European study. N Engl J Med 2009; 360:1351–1354.
- Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009; 301:39–51.
- Bratt O. Hereditary prostate cancer: clinical aspects. J Urol 2002; 168:906–913.
- Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005; 352:1977–1984.
- Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol 2007; 178:S14–S19.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009. Accessed 6/28/2009.
- Murphy GP, Natarajan N, Pontes JE, et al. The national survey of prostate cancer in the United States by the American College of Surgeons. J Urol 1982; 127:928–934.
- Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organconfined prostate cancer is increased through prostate-specific antigen-based screening. JAMA 1993; 270:948–954.
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004; 350:2239–2246.
- Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. N Natl Cancer Inst 2008; 100:1487–1488.
- Vickers AJ, Savage C, O’Brien MF, Lilja H. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol 2009; 27:398–403.
- Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer 2008; 8:268–278.
- Marks LS, Fradet Y, Deras IL, et al. PCA molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology 2007; 69:532–535.
- Sreekumar A, Poisson LM, Thekkelnaycke M, et al. Metabolomic profile delineates potential role for sarcosine in prostate cancer progression. Nature 2009; 457:910–914.
- Zheng SL, Sun J, Wiklund F, et al. Cumulative association of five genetic variants with prostate cancer. N Engl J Med 2008; 358:910–919.
- Witte JS. Prostate cancer genomics: toward a new understanding. Nat Rev Genet 2009; 10:77–82.
- Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2009; 301:52–62.
- Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003; 349:215–224.
- Lucia MS, Darke AK, Goodman PJ, et al. Pathologic characteristics of cancers detected in the Prostate Cancer Prevention Trial: implications for prostate cancer detection and chemoprevention. Cancer Prev Res (Phila PA) 2008; 1:167–173.
- Andriole G, Bostwick D, Brawley O, et al. Further analyses from the REDUCE prostate cancer risk reduction trial [abstract]. J Urol 2009; 181:( suppl):555.
- Kramer BS, Hagerty KL, Justman S, et al; American Society of Clinical Oncology/American Urological Association. Use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Urol 2009; 181:1642–1657.
In spite of some recent studies, or perhaps because of them, we still are unsure about how best to screen for and prevent prostate cancer. Two large trials of screening with prostate-specific antigen (PSA) measurements came to seemingly opposite conclusions.1,2 Furthermore, a large trial of selenium and vitamin E found that these agents have no value as preventive agents.3
Nevertheless, negative studies also advance science, and steady progress is being made in prostate cancer research. In this paper I briefly summarize and comment on some of the recent findings.
TO SCREEN OR NOT TO SCREEN?
All cases of prostate cancer are clinically relevant in that they can cause anxiety or can lead to treatment-related morbidity. The challenge is to detect the minority of cases of cancer that are biologically significant, ie, those that will cause serious illness or death.
Many men have prostate cancer
In the United States, the lifetime probability of developing prostate cancer is 1 in 6, and the probability increases with age. Prostate cancer is primarily a disease of the Western world, but it is becoming more common in other areas as well.
Risk factors for prostate cancer are age, race, and family history. Clinically apparent disease is very rare in men younger than 40 years; until recently, most guidelines suggested that screening for it should begin at age 50. African American men have the highest risk of developing and dying of prostate cancer, for reasons that are not clear. In the past, this finding was attributed to disparities in access and less aggressive therapy in black men, but recent studies suggest the differences persist even in the absence of these factors, suggesting there is a biological difference in cancers between blacks and whites. Having a father or brother who had prostate cancer increases one’s risk twofold (threefold if the father or brother was affected before the age of 60); having a father and a brother with prostate cancer increases one’s risk fourfold, and true hereditary cancer raises the risk fivefold.4
But relatively few men die of it
The Scandinavian Prostate Cancer Group5 randomized 695 men with early prostate cancer (mostly discovered by digital rectal examination or by symptoms) to undergo either radical prostatectomy or a program of watchful waiting. In 8.2 years of follow-up, 8.6% of the men in the surgery group and 14.4% of those in the watchful waiting group died of prostate cancer. Thus, we can conclude that surgery is beneficial in this situation.
Despite these calculations, in contemporary practice in the United States, about 90% of men with newly diagnosed low-grade prostate cancer choose to be treated.6 This high level of intervention reflects our current inability to predict which cancers will remain indolent vs which will progress and the lack of validated markers that tell us when to intervene in patients who are managed expectantly and not lose the chance for cure. Most often, patients and their physicians, who are paid to intervene, deal with this uncertainty by choosing the high likelihood of cure with early intervention despite treatment-related morbidity.
What PSA has wrought
When PSA screening was introduced in the late 1980s and early 1990s, it brought about several changes in the epidemiology and clinical profile of this disease that led us to believe that it was making a meaningful difference.
A spike in the apparent incidence of prostate cancer occurred in the late 1980s and early 1990s with the introduction of PSA screening. The spike was temporary, representing detection of preexisting cases. Now, the incidence may have leveled off.7
A shift in the stages of cancers detected. In 1982, half of men with newly diagnosed prostate cancer had incurable disease.8 Five years after the introduction of PSA testing, 95% had curable disease.9
An increase in the rate of cure after radical prostatectomy was seen.
A decrease in the death rate from prostate cancer since the early 1990s has been noted, which is likely due not only to earlier detection but also to earlier and better treatment.
Limitations of PSA screening
PSA screening has low specificity. PSA is more sensitive than digital rectal examination, but most men with “elevated” PSA do not have prostate cancer. Nevertheless, although it is not a perfect screening test, it is still the best cancer marker that we have.
In the Prostate Cancer Prevention Trial (PCPT),10 finasteride (Proscar) decreased the incidence of prostate cancer by about 25% over 7 years. But there were also lessons to be learned from the placebo group, which underwent PSA testing every year and prostate biopsy at the end of the study.
We used to think the cutoff PSA level that had high sensitivity and specificity for finding cancer was 4 ng/mL. However, in the PCPT, 6.6% of men with PSA levels below 0.5 ng/mL were found to have cancer, and 12.5% of those cancers were high-grade. Of those with PSA levels of 3.1 to 4.0 ng/mL, 26.9% had cancer, and 25.0% of the cancers were high-grade. These data demonstrate that there is no PSA level below which risk of cancer is zero, and that there is no PSA cutoff with sufficient sensitivity and specificity to be clinically useful.
The PCPT risk calculator (http://deb.uthscsa.edu/URORiskCalc/Pages/uroriskcalc.jsp) is a wonderful tool that came out of that study. It uses seven variables—race, age, PSA level, family history of prostate cancer, findings on digital rectal examination, whether the patient has ever undergone a prostate biopsy, and whether the patient is taking finasteride—and calculates the patient’s risk of harboring prostate cancer and, more important, the risk of having high-grade prostate cancer. This tool allows estimation of individual risk and helps identify who is at risk of cancer that may require therapy.
Other factors can affect PSA levels. Men with a higher body mass index have lower PSA levels. The reason is not clear; it may be a hormonal effect, or heavier men may simply have higher blood volume, which may dilute the PSA. Furthermore, there are genetic differences that make some men secrete more PSA, but this effect is probably not clinically important. And a study by Hamilton et al11 suggested that statin drugs lower PSA levels. As these findings are confirmed, in the future it may be necessary to adjust PSA levels to account for their effects before deciding on the need for biopsy.
Two new, conflicting studies
Two large trials of PSA screening, published simultaneously in March 2009, came to opposite conclusions.
The European Randomized Study of Screening for Prostate Cancer2 randomized 162,243 men between the ages of 55 and 69 to undergo PSA screening at an average of once every 4 years or to a control group. Most of the participating centers used a PSA level of 3.0 ng/mL as an indication for biopsy. At an average follow-up time of 8.8 years, 214 men had died of prostate cancer in the screening group, compared with 326 in the control group, for an adjusted rate ratio of 0.80 (95% confidence interval [CI] 0.65–0.98, P = .04). In other words, screening decreased the risk of death from prostate cancer by 20%.
The Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial,1 conducted in the United States, came to the opposite conclusion, ie, that there is no benefit from PSA screening. This study was smaller, with 76,693 men between ages 55 and 74 randomly assigned to receive PSA testing every year for 6 years and digital rectal examination for 4 years, or usual care. A PSA level of more than 4.0 ng/mL was considered to be positive for prostate cancer. At 7 years, of those who reported undergoing no more than one PSA test at baseline, 48 men had died of prostate cancer in the screening group, compared with 41 in the control group (rate ratio 1.16, 95% CI 0.76–1.76).
Why were the findings different? The PLCO investigators offered several possible explanations for their negative results. The PSA threshold of 4 ng/mL that was used in that study may not be effective. More than half the men in the control group actually had a PSA test in the first 6 years of the study, potentially diluting any effect of testing. (This was the most worrisome flaw in the study, in my opinion.) About 44% of the men in the study had already had one or more PSA tests at baseline, which would have eliminated cancers detectable on screening from the study, and not all men who were advised to undergo biopsy actually did so. The follow-up time may not yet be long enough for the benefit to be apparent. Most important, in their opinion, treatment for prostate cancer improved during the time of the trial, so that fewer men than expected died of prostate cancer in both groups.
Improvements to PSA screening
Derivatives of PSA have been used in an attempt to improve its performance characteristics for detecting cancer.
PSA density, defined as serum PSA divided by prostate volume, has some predictive power but requires performance of transrectal ultrasonography. It is therefore not a good screening test in the primary care setting.
PSA velocity or doubling time, based on the rate of change over time, is predictive of prostate cancer, but is highly dependent on the absolute value of PSA and does not add independent information to the variables defined in the PCPT risk calculator or other standard predictive variables.12
A PSA level between the ages of 44 and 50 may predict the lifetime risk of prostate cancer, according to a study by Lilja et al13 in Sweden. This finding suggests that we should measure PSA early in life and screen men who have higher values more frequently or with better strategies. This recommendation has been adopted by the American Urological Association, which released updated screening guidelines in April 2009 (available at www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf).
New markers under study
A number of new biological markers probably will improve our ability to detect prostate cancer, although they are not yet ready for widespread use.
Urinary PCA3. Prostate cancer gene 3 (PCA3) codes for a messenger RNA that is highly overexpressed in the urine of men with prostate cancer. Urine is collected after prostate massage. Marks et al14 reported that PCA3 scores predicted biopsy outcomes in men with serum PSA levels of 2.5 ng/mL or higher.
Serum EPCA-2 (early prostate cancer antigen 2) is another candidate marker undergoing study.
Gene fusions, specifically of TRMPSS2 and the ETS gene family, are detectable in high levels in the urine of some men with prostate cancer, and appear to be very promising markers for detection.
Metabolomics is a technique that uses mass spectroscopy to detect the metabolic signature of cancer. Sreekumar et al15 identified sarcosine as a potential marker of prostate cancer using this technique.
Genetic tests: Not yet
Some data suggest that we can use genetic tests to screen for prostate cancer, but the tests are not yet as good as we would like.
Zheng et al16 reported that 16 singlenucleotide polymorphisms (SNPs) in five chromosomal regions plus a family history of prostate cancer have a cumulative association with prostate cancer: men who had any five or more of these SNPs had a risk of prostate cancer nearly 10 times as high as men without any of them. However, the number of men who actually fall into this category is so low that routine use in the general population is not cost-effective; it may, however, be useful in men with a family history of prostate cancer.
Other SNPs have been linked to prostate cancer (reviewed by Witte17). Having any one of these loci increases one’s risk only modestly, however. Only about 2% of the population has five or more of these SNPS, and the sensitivity is about only about 16%.
A commercially available DNA test (Decode Genetics, Reykjavik, Iceland) can detect eight variants that, according to the company, account for about half of all cases of prostate cancer.
Prostate cancer screening: My interpretation
I believe the two new studies of PSA screening suggest there is a modest benefit from screening in terms of preventing deaths from prostate cancer. But I also believe we should be more judicious in recommending treatment for men whom we know have biologically indolent tumors, although we cannot yet identify them perfectly.
In the past, we used an arbitrary PSA cutoff to detect prostate cancer of any grade, and men with high levels were advised to have a biopsy. Currently, we use continuous-risk models to look for any cancer and biologically significant cancers. These involve nomograms, a risk calculator, and new markers.
We use the PCPT risk calculator routinely in our practice. I recommend—completely arbitrarily—that a man undergo biopsy if he has a 10% or higher risk of high-grade cancer, but not if the risk is less. I believe this is more accurate than a simple PSA cutoff value.
CAN WE PREVENT PROSTATE CANCER?
Prostate cancer is a significant public health risk, with 186,000 new cases and 26,000 deaths yearly. Its risk factors (age, race, and genes) are not modifiable. The benefit of screening in terms of preventing deaths is not as good as we would like, and therapy is associated with morbidity. That leaves prevention as a potential way to reduce the morbidity and perhaps mortality of prostate cancer and its therapy.
Epidemiologic studies suggest that certain lifestyle factors may increase the risk, ie, consumption of fat, red meat, fried foods, and dairy; high calcium intake; smoking; total calories; and body size. Other factors may decrease the risk: plant-based foods and vegetables, especially lycopene-containing foods such as tomatoes, cruciferous vegetables, soy, and legumes, specific nutrients such as carotenoids, lycopene, total antioxidants, fish oil (omega-3 fatty acids), and moderate to vigorous exercise. However, there have been few randomized trials to determine if any of these agents are beneficial.
Findings of trials of prevention
Selenium and vitamin E do not prevent prostate cancer, lung cancer, colorectal cancer, other primary cancers, or deaths. The Selenium and Vitamin E Cancer Prevention Trial (SELECT)3 involved 35,533 men 55 years of age or older (or 50 and older if they were African American). They were randomized to receive one of four treatments: selenium 200 μg/day plus vitamin E placebo, vitamin E 400 IU/day plus selenium placebo, selenium plus vitamin E, or double placebo. At a median follow-up of 5.46 years, compared with the placebo group, the hazard ratio for prostate cancer was 1.04 in the selenium-only group, 1.13 in the vitamin E-only group, and 1.05 in the selenium-plus-vitamin E group. None of the differences was statistically significant.
The Physician’s Health Study18 also found that vitamin E at the same dose given every other day does not prevent prostate cancer.
Finasteride prevents prostate cancer. The PCPT19 included 18,882 men, 55 years of age or older, who had PSA levels of 3.0 ng/mL or less and normal findings on digital rectal examination. Treatment was with finasteride 5 mg/day or placebo. At 7 years, prostate cancer had been discovered in 18.4% of the finasteride group vs 24.4% of the placebo group, a 24.8% reduction (95% CI 18.6–30.6, P < .001). Sexual side effects were more common in the men who received finasteride, while urinary symptoms were more common in the placebo group.
At the time of the original PCPT report in 2003,19 tumors of Gleason grade 7 or higher were more common in the finasteride group, accounting for 37.0% of the tumors discovered, than in the placebo group (22.2%), creating concern that finasteride might somehow cause the tumors that occurred to be more aggressive. However, a subsequent analysis20 found the opposite to be true, ie, that finasteride decreases the risk of high-grade cancers. A companion quality-of-life study showed that chronic use of finasteride had clinically insignificant effects on sexual function, and the PCPT and other studies have shown benefits of finasteride in reducing lower urinary tract symptoms due to benign prostatic hyperplasia (BPH), reducing the risk of acute urinary retention and the need for surgical intervention for BPH, and reducing the risk of prostatitis.
Dutasteride also prevents prostate cancer. A large-scale trial of another 5-alpha reductase inhibitor, dutasteride (Avodart), was reported by Andriole at the annual meeting of the American Urological Association in April 2009.21 The Reduction by Dutasteride of Prostate Events (REDUCE) trial included men who were 50 to 75 years old, inclusively, and who had PSA levels between 2.5 and 10 ng/mL, prostate volume less than 80 cc, and one prior negative prostate biopsy within 6 months of enrollment, representing a group at high risk for cancer on a subsequent biopsy. The trial accrued 8,231 men. At 4 years, prostate cancer had occurred in 659 men in the dutasteride group vs 857 in the placebo group, a 23% reduction (P < .0001). Interestingly, no significant increase in Gleason grade 8 to grade 10 tumors was observed in the study.
Preliminary analyses also suggest that dutasteride enhanced the utility of PSA as a diagnostic test for prostate cancer, had beneficial effects on BPH, and was generally well tolerated. The fact that the results of REDUCE were congruent with those of the PCPT with respect to the magnitude of risk reduction, beneficial effects on benign prostatic hypertrophy, minimal toxicity, and no issues related to tumor grade suggests a class effect for 5-alpha reductase inhibitors, and suggests that these agents should be used more liberally for the prevention of prostate cancer.
There is current debate about whether 5-alpha reductase inhibitors should be used by all men at risk of prostate cancer or only by those at high risk. However, the American Urological Association and the American Society of Clinical Oncology have issued guidelines stating that men at risk should consider this intervention.22
In spite of some recent studies, or perhaps because of them, we still are unsure about how best to screen for and prevent prostate cancer. Two large trials of screening with prostate-specific antigen (PSA) measurements came to seemingly opposite conclusions.1,2 Furthermore, a large trial of selenium and vitamin E found that these agents have no value as preventive agents.3
Nevertheless, negative studies also advance science, and steady progress is being made in prostate cancer research. In this paper I briefly summarize and comment on some of the recent findings.
TO SCREEN OR NOT TO SCREEN?
All cases of prostate cancer are clinically relevant in that they can cause anxiety or can lead to treatment-related morbidity. The challenge is to detect the minority of cases of cancer that are biologically significant, ie, those that will cause serious illness or death.
Many men have prostate cancer
In the United States, the lifetime probability of developing prostate cancer is 1 in 6, and the probability increases with age. Prostate cancer is primarily a disease of the Western world, but it is becoming more common in other areas as well.
Risk factors for prostate cancer are age, race, and family history. Clinically apparent disease is very rare in men younger than 40 years; until recently, most guidelines suggested that screening for it should begin at age 50. African American men have the highest risk of developing and dying of prostate cancer, for reasons that are not clear. In the past, this finding was attributed to disparities in access and less aggressive therapy in black men, but recent studies suggest the differences persist even in the absence of these factors, suggesting there is a biological difference in cancers between blacks and whites. Having a father or brother who had prostate cancer increases one’s risk twofold (threefold if the father or brother was affected before the age of 60); having a father and a brother with prostate cancer increases one’s risk fourfold, and true hereditary cancer raises the risk fivefold.4
But relatively few men die of it
The Scandinavian Prostate Cancer Group5 randomized 695 men with early prostate cancer (mostly discovered by digital rectal examination or by symptoms) to undergo either radical prostatectomy or a program of watchful waiting. In 8.2 years of follow-up, 8.6% of the men in the surgery group and 14.4% of those in the watchful waiting group died of prostate cancer. Thus, we can conclude that surgery is beneficial in this situation.
Despite these calculations, in contemporary practice in the United States, about 90% of men with newly diagnosed low-grade prostate cancer choose to be treated.6 This high level of intervention reflects our current inability to predict which cancers will remain indolent vs which will progress and the lack of validated markers that tell us when to intervene in patients who are managed expectantly and not lose the chance for cure. Most often, patients and their physicians, who are paid to intervene, deal with this uncertainty by choosing the high likelihood of cure with early intervention despite treatment-related morbidity.
What PSA has wrought
When PSA screening was introduced in the late 1980s and early 1990s, it brought about several changes in the epidemiology and clinical profile of this disease that led us to believe that it was making a meaningful difference.
A spike in the apparent incidence of prostate cancer occurred in the late 1980s and early 1990s with the introduction of PSA screening. The spike was temporary, representing detection of preexisting cases. Now, the incidence may have leveled off.7
A shift in the stages of cancers detected. In 1982, half of men with newly diagnosed prostate cancer had incurable disease.8 Five years after the introduction of PSA testing, 95% had curable disease.9
An increase in the rate of cure after radical prostatectomy was seen.
A decrease in the death rate from prostate cancer since the early 1990s has been noted, which is likely due not only to earlier detection but also to earlier and better treatment.
Limitations of PSA screening
PSA screening has low specificity. PSA is more sensitive than digital rectal examination, but most men with “elevated” PSA do not have prostate cancer. Nevertheless, although it is not a perfect screening test, it is still the best cancer marker that we have.
In the Prostate Cancer Prevention Trial (PCPT),10 finasteride (Proscar) decreased the incidence of prostate cancer by about 25% over 7 years. But there were also lessons to be learned from the placebo group, which underwent PSA testing every year and prostate biopsy at the end of the study.
We used to think the cutoff PSA level that had high sensitivity and specificity for finding cancer was 4 ng/mL. However, in the PCPT, 6.6% of men with PSA levels below 0.5 ng/mL were found to have cancer, and 12.5% of those cancers were high-grade. Of those with PSA levels of 3.1 to 4.0 ng/mL, 26.9% had cancer, and 25.0% of the cancers were high-grade. These data demonstrate that there is no PSA level below which risk of cancer is zero, and that there is no PSA cutoff with sufficient sensitivity and specificity to be clinically useful.
The PCPT risk calculator (http://deb.uthscsa.edu/URORiskCalc/Pages/uroriskcalc.jsp) is a wonderful tool that came out of that study. It uses seven variables—race, age, PSA level, family history of prostate cancer, findings on digital rectal examination, whether the patient has ever undergone a prostate biopsy, and whether the patient is taking finasteride—and calculates the patient’s risk of harboring prostate cancer and, more important, the risk of having high-grade prostate cancer. This tool allows estimation of individual risk and helps identify who is at risk of cancer that may require therapy.
Other factors can affect PSA levels. Men with a higher body mass index have lower PSA levels. The reason is not clear; it may be a hormonal effect, or heavier men may simply have higher blood volume, which may dilute the PSA. Furthermore, there are genetic differences that make some men secrete more PSA, but this effect is probably not clinically important. And a study by Hamilton et al11 suggested that statin drugs lower PSA levels. As these findings are confirmed, in the future it may be necessary to adjust PSA levels to account for their effects before deciding on the need for biopsy.
Two new, conflicting studies
Two large trials of PSA screening, published simultaneously in March 2009, came to opposite conclusions.
The European Randomized Study of Screening for Prostate Cancer2 randomized 162,243 men between the ages of 55 and 69 to undergo PSA screening at an average of once every 4 years or to a control group. Most of the participating centers used a PSA level of 3.0 ng/mL as an indication for biopsy. At an average follow-up time of 8.8 years, 214 men had died of prostate cancer in the screening group, compared with 326 in the control group, for an adjusted rate ratio of 0.80 (95% confidence interval [CI] 0.65–0.98, P = .04). In other words, screening decreased the risk of death from prostate cancer by 20%.
The Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial,1 conducted in the United States, came to the opposite conclusion, ie, that there is no benefit from PSA screening. This study was smaller, with 76,693 men between ages 55 and 74 randomly assigned to receive PSA testing every year for 6 years and digital rectal examination for 4 years, or usual care. A PSA level of more than 4.0 ng/mL was considered to be positive for prostate cancer. At 7 years, of those who reported undergoing no more than one PSA test at baseline, 48 men had died of prostate cancer in the screening group, compared with 41 in the control group (rate ratio 1.16, 95% CI 0.76–1.76).
Why were the findings different? The PLCO investigators offered several possible explanations for their negative results. The PSA threshold of 4 ng/mL that was used in that study may not be effective. More than half the men in the control group actually had a PSA test in the first 6 years of the study, potentially diluting any effect of testing. (This was the most worrisome flaw in the study, in my opinion.) About 44% of the men in the study had already had one or more PSA tests at baseline, which would have eliminated cancers detectable on screening from the study, and not all men who were advised to undergo biopsy actually did so. The follow-up time may not yet be long enough for the benefit to be apparent. Most important, in their opinion, treatment for prostate cancer improved during the time of the trial, so that fewer men than expected died of prostate cancer in both groups.
Improvements to PSA screening
Derivatives of PSA have been used in an attempt to improve its performance characteristics for detecting cancer.
PSA density, defined as serum PSA divided by prostate volume, has some predictive power but requires performance of transrectal ultrasonography. It is therefore not a good screening test in the primary care setting.
PSA velocity or doubling time, based on the rate of change over time, is predictive of prostate cancer, but is highly dependent on the absolute value of PSA and does not add independent information to the variables defined in the PCPT risk calculator or other standard predictive variables.12
A PSA level between the ages of 44 and 50 may predict the lifetime risk of prostate cancer, according to a study by Lilja et al13 in Sweden. This finding suggests that we should measure PSA early in life and screen men who have higher values more frequently or with better strategies. This recommendation has been adopted by the American Urological Association, which released updated screening guidelines in April 2009 (available at www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/psa09.pdf).
New markers under study
A number of new biological markers probably will improve our ability to detect prostate cancer, although they are not yet ready for widespread use.
Urinary PCA3. Prostate cancer gene 3 (PCA3) codes for a messenger RNA that is highly overexpressed in the urine of men with prostate cancer. Urine is collected after prostate massage. Marks et al14 reported that PCA3 scores predicted biopsy outcomes in men with serum PSA levels of 2.5 ng/mL or higher.
Serum EPCA-2 (early prostate cancer antigen 2) is another candidate marker undergoing study.
Gene fusions, specifically of TRMPSS2 and the ETS gene family, are detectable in high levels in the urine of some men with prostate cancer, and appear to be very promising markers for detection.
Metabolomics is a technique that uses mass spectroscopy to detect the metabolic signature of cancer. Sreekumar et al15 identified sarcosine as a potential marker of prostate cancer using this technique.
Genetic tests: Not yet
Some data suggest that we can use genetic tests to screen for prostate cancer, but the tests are not yet as good as we would like.
Zheng et al16 reported that 16 singlenucleotide polymorphisms (SNPs) in five chromosomal regions plus a family history of prostate cancer have a cumulative association with prostate cancer: men who had any five or more of these SNPs had a risk of prostate cancer nearly 10 times as high as men without any of them. However, the number of men who actually fall into this category is so low that routine use in the general population is not cost-effective; it may, however, be useful in men with a family history of prostate cancer.
Other SNPs have been linked to prostate cancer (reviewed by Witte17). Having any one of these loci increases one’s risk only modestly, however. Only about 2% of the population has five or more of these SNPS, and the sensitivity is about only about 16%.
A commercially available DNA test (Decode Genetics, Reykjavik, Iceland) can detect eight variants that, according to the company, account for about half of all cases of prostate cancer.
Prostate cancer screening: My interpretation
I believe the two new studies of PSA screening suggest there is a modest benefit from screening in terms of preventing deaths from prostate cancer. But I also believe we should be more judicious in recommending treatment for men whom we know have biologically indolent tumors, although we cannot yet identify them perfectly.
In the past, we used an arbitrary PSA cutoff to detect prostate cancer of any grade, and men with high levels were advised to have a biopsy. Currently, we use continuous-risk models to look for any cancer and biologically significant cancers. These involve nomograms, a risk calculator, and new markers.
We use the PCPT risk calculator routinely in our practice. I recommend—completely arbitrarily—that a man undergo biopsy if he has a 10% or higher risk of high-grade cancer, but not if the risk is less. I believe this is more accurate than a simple PSA cutoff value.
CAN WE PREVENT PROSTATE CANCER?
Prostate cancer is a significant public health risk, with 186,000 new cases and 26,000 deaths yearly. Its risk factors (age, race, and genes) are not modifiable. The benefit of screening in terms of preventing deaths is not as good as we would like, and therapy is associated with morbidity. That leaves prevention as a potential way to reduce the morbidity and perhaps mortality of prostate cancer and its therapy.
Epidemiologic studies suggest that certain lifestyle factors may increase the risk, ie, consumption of fat, red meat, fried foods, and dairy; high calcium intake; smoking; total calories; and body size. Other factors may decrease the risk: plant-based foods and vegetables, especially lycopene-containing foods such as tomatoes, cruciferous vegetables, soy, and legumes, specific nutrients such as carotenoids, lycopene, total antioxidants, fish oil (omega-3 fatty acids), and moderate to vigorous exercise. However, there have been few randomized trials to determine if any of these agents are beneficial.
Findings of trials of prevention
Selenium and vitamin E do not prevent prostate cancer, lung cancer, colorectal cancer, other primary cancers, or deaths. The Selenium and Vitamin E Cancer Prevention Trial (SELECT)3 involved 35,533 men 55 years of age or older (or 50 and older if they were African American). They were randomized to receive one of four treatments: selenium 200 μg/day plus vitamin E placebo, vitamin E 400 IU/day plus selenium placebo, selenium plus vitamin E, or double placebo. At a median follow-up of 5.46 years, compared with the placebo group, the hazard ratio for prostate cancer was 1.04 in the selenium-only group, 1.13 in the vitamin E-only group, and 1.05 in the selenium-plus-vitamin E group. None of the differences was statistically significant.
The Physician’s Health Study18 also found that vitamin E at the same dose given every other day does not prevent prostate cancer.
Finasteride prevents prostate cancer. The PCPT19 included 18,882 men, 55 years of age or older, who had PSA levels of 3.0 ng/mL or less and normal findings on digital rectal examination. Treatment was with finasteride 5 mg/day or placebo. At 7 years, prostate cancer had been discovered in 18.4% of the finasteride group vs 24.4% of the placebo group, a 24.8% reduction (95% CI 18.6–30.6, P < .001). Sexual side effects were more common in the men who received finasteride, while urinary symptoms were more common in the placebo group.
At the time of the original PCPT report in 2003,19 tumors of Gleason grade 7 or higher were more common in the finasteride group, accounting for 37.0% of the tumors discovered, than in the placebo group (22.2%), creating concern that finasteride might somehow cause the tumors that occurred to be more aggressive. However, a subsequent analysis20 found the opposite to be true, ie, that finasteride decreases the risk of high-grade cancers. A companion quality-of-life study showed that chronic use of finasteride had clinically insignificant effects on sexual function, and the PCPT and other studies have shown benefits of finasteride in reducing lower urinary tract symptoms due to benign prostatic hyperplasia (BPH), reducing the risk of acute urinary retention and the need for surgical intervention for BPH, and reducing the risk of prostatitis.
Dutasteride also prevents prostate cancer. A large-scale trial of another 5-alpha reductase inhibitor, dutasteride (Avodart), was reported by Andriole at the annual meeting of the American Urological Association in April 2009.21 The Reduction by Dutasteride of Prostate Events (REDUCE) trial included men who were 50 to 75 years old, inclusively, and who had PSA levels between 2.5 and 10 ng/mL, prostate volume less than 80 cc, and one prior negative prostate biopsy within 6 months of enrollment, representing a group at high risk for cancer on a subsequent biopsy. The trial accrued 8,231 men. At 4 years, prostate cancer had occurred in 659 men in the dutasteride group vs 857 in the placebo group, a 23% reduction (P < .0001). Interestingly, no significant increase in Gleason grade 8 to grade 10 tumors was observed in the study.
Preliminary analyses also suggest that dutasteride enhanced the utility of PSA as a diagnostic test for prostate cancer, had beneficial effects on BPH, and was generally well tolerated. The fact that the results of REDUCE were congruent with those of the PCPT with respect to the magnitude of risk reduction, beneficial effects on benign prostatic hypertrophy, minimal toxicity, and no issues related to tumor grade suggests a class effect for 5-alpha reductase inhibitors, and suggests that these agents should be used more liberally for the prevention of prostate cancer.
There is current debate about whether 5-alpha reductase inhibitors should be used by all men at risk of prostate cancer or only by those at high risk. However, the American Urological Association and the American Society of Clinical Oncology have issued guidelines stating that men at risk should consider this intervention.22
- Andriole GL, Grubb RL, Buys SS, et al; PLCO Project Team. Mortality results from a randomized prostate cancer screening trial. N Engl J Med 2009; 360:1310–1319.
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality in a randomized European study. N Engl J Med 2009; 360:1351–1354.
- Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009; 301:39–51.
- Bratt O. Hereditary prostate cancer: clinical aspects. J Urol 2002; 168:906–913.
- Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005; 352:1977–1984.
- Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol 2007; 178:S14–S19.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009. Accessed 6/28/2009.
- Murphy GP, Natarajan N, Pontes JE, et al. The national survey of prostate cancer in the United States by the American College of Surgeons. J Urol 1982; 127:928–934.
- Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organconfined prostate cancer is increased through prostate-specific antigen-based screening. JAMA 1993; 270:948–954.
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004; 350:2239–2246.
- Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. N Natl Cancer Inst 2008; 100:1487–1488.
- Vickers AJ, Savage C, O’Brien MF, Lilja H. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol 2009; 27:398–403.
- Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer 2008; 8:268–278.
- Marks LS, Fradet Y, Deras IL, et al. PCA molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology 2007; 69:532–535.
- Sreekumar A, Poisson LM, Thekkelnaycke M, et al. Metabolomic profile delineates potential role for sarcosine in prostate cancer progression. Nature 2009; 457:910–914.
- Zheng SL, Sun J, Wiklund F, et al. Cumulative association of five genetic variants with prostate cancer. N Engl J Med 2008; 358:910–919.
- Witte JS. Prostate cancer genomics: toward a new understanding. Nat Rev Genet 2009; 10:77–82.
- Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2009; 301:52–62.
- Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003; 349:215–224.
- Lucia MS, Darke AK, Goodman PJ, et al. Pathologic characteristics of cancers detected in the Prostate Cancer Prevention Trial: implications for prostate cancer detection and chemoprevention. Cancer Prev Res (Phila PA) 2008; 1:167–173.
- Andriole G, Bostwick D, Brawley O, et al. Further analyses from the REDUCE prostate cancer risk reduction trial [abstract]. J Urol 2009; 181:( suppl):555.
- Kramer BS, Hagerty KL, Justman S, et al; American Society of Clinical Oncology/American Urological Association. Use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Urol 2009; 181:1642–1657.
- Andriole GL, Grubb RL, Buys SS, et al; PLCO Project Team. Mortality results from a randomized prostate cancer screening trial. N Engl J Med 2009; 360:1310–1319.
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality in a randomized European study. N Engl J Med 2009; 360:1351–1354.
- Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009; 301:39–51.
- Bratt O. Hereditary prostate cancer: clinical aspects. J Urol 2002; 168:906–913.
- Bill-Axelson A, Holmberg L, Ruutu M, et al; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005; 352:1977–1984.
- Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol 2007; 178:S14–S19.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009. Accessed 6/28/2009.
- Murphy GP, Natarajan N, Pontes JE, et al. The national survey of prostate cancer in the United States by the American College of Surgeons. J Urol 1982; 127:928–934.
- Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organconfined prostate cancer is increased through prostate-specific antigen-based screening. JAMA 1993; 270:948–954.
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004; 350:2239–2246.
- Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. N Natl Cancer Inst 2008; 100:1487–1488.
- Vickers AJ, Savage C, O’Brien MF, Lilja H. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol 2009; 27:398–403.
- Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer 2008; 8:268–278.
- Marks LS, Fradet Y, Deras IL, et al. PCA molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology 2007; 69:532–535.
- Sreekumar A, Poisson LM, Thekkelnaycke M, et al. Metabolomic profile delineates potential role for sarcosine in prostate cancer progression. Nature 2009; 457:910–914.
- Zheng SL, Sun J, Wiklund F, et al. Cumulative association of five genetic variants with prostate cancer. N Engl J Med 2008; 358:910–919.
- Witte JS. Prostate cancer genomics: toward a new understanding. Nat Rev Genet 2009; 10:77–82.
- Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2009; 301:52–62.
- Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003; 349:215–224.
- Lucia MS, Darke AK, Goodman PJ, et al. Pathologic characteristics of cancers detected in the Prostate Cancer Prevention Trial: implications for prostate cancer detection and chemoprevention. Cancer Prev Res (Phila PA) 2008; 1:167–173.
- Andriole G, Bostwick D, Brawley O, et al. Further analyses from the REDUCE prostate cancer risk reduction trial [abstract]. J Urol 2009; 181:( suppl):555.
- Kramer BS, Hagerty KL, Justman S, et al; American Society of Clinical Oncology/American Urological Association. Use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Urol 2009; 181:1642–1657.
KEY POINTS
- An elevated PSA level lacks specificity as a test for prostate cancer, but PSA measurements can be useful in combination with clinical risk factors or to measure changes in PSA over time.
- Rather than relying on PSA screening alone, we should stratify the risk of prostate cancer on the basis of race, age, PSA level, family history, findings on digital rectal examination, whether the patient has ever undergone a prostate biopsy, and whether the patient is taking finasteride (Proscar). A simple online tool is available to do this.
- There is no PSA level below which the risk of cancer is zero.
- Finasteride has been found in a randomized trial to decrease the risk of prostate cancer, but vitamin E and selenium supplements have failed to show a benefit.