User login
Sim and Learn: Simulation and its Value in Neurology Education
Sim and Learn: Simulation and its Value in Neurology Education
Clinical simulation is a technique, not a technology, used to replace or amplify real experiences with guided experiences that evoke or replicate substantial aspects of the real world in a fully interactive fashion.1 Simulation is widely used in medical education and spans a spectrum of sophistication, from simple reproduction of isolated body parts to high-fidelity human patient simulators that replicate whole body appearance and variable physiological parameters.2,3
Simulation-based medical education can be a valuable tool for safe health care delivery.4Simulation-based education is typically provided via 5 modalities: mannequins, computer-based mannequins, standardized patients, computer-based simulators, and software-based simulations. Simulation technology increases procedural skill by allowing for deliberate practice in a safe environment.5 Mastery learning is a stringent form of competency-based education that requires trainees to acquire clinical skill measured against a fixed achievement standard.6 In mastery learning, educational practice time varies but results are uniform. This approach improves patient outcomes and is more effective than clinical training alone.7-9
Advanced simulation models are helpful tools for neurologic education and training, especially for emergency department encounters.10 In recent years, advanced simulation models have been applied in various fields of medicine, especially emergency medicine and anesthesia.11-14
Acute neurology
In acute neurologic conditions (eg, stroke, intracerebral hemorrhage, status epilepticus, and neuromuscular respiratory failure) clinical outcomes are highly time dependent; consequently, a reduction in treatment delays can improve patient care. The application of simulation methodology allows trainees to address acute and potentially life-threatening emergencies in a safe, controlled, and reproducible environment. In addition to improving trainees’ knowledge base, simulation also helps to enhance team skills, communication, multidisciplinary collaboration, and leadership. Research has shown that deliberate practice leads to a decrease in clinical errors and improved procedural performance in the operating room.8,15 These results can be extrapolated to acute neurology settings to improve adherence to set protocols, thus streamlining management in acute settings.
Scenarios can be built to teach skills such as eliciting an appropriate history, establishing inclusion or exclusion criteria for the use of certain medications, evaluating neuroimaging and laboratory studies (while avoiding related common pitfalls), and managing treatment complications. Simulation also provides an opportunity for interprofessional education by training nurses and collaborative staff. It can be used to enhance nontechnical skills (eg, communication, situation awareness, decision making, and leadership) that further contribute to patient safety.
Simulation can be performed with the help of mannequins such as the SimMan 3G(Laerdal), which can display neurologic symptoms and physiological findings, or live actors who portray a patient by mimicking focal neurologic deficits.16,17 A briefing familiarizes the trainees with the equipment and explains the simulation process. The documentation and equipment are the same as that which is used in emergency departments or intensive care units.
Once the simulation is completed, a trainee’s performance is checked against a critical action checklist before a debriefing process during which the scenario is reviewed and learning goals are assessed. Immediate feedback is given to trainees to identify weaknesses and the simulation is repeated if multiple critical action items are missed. (Figure).17
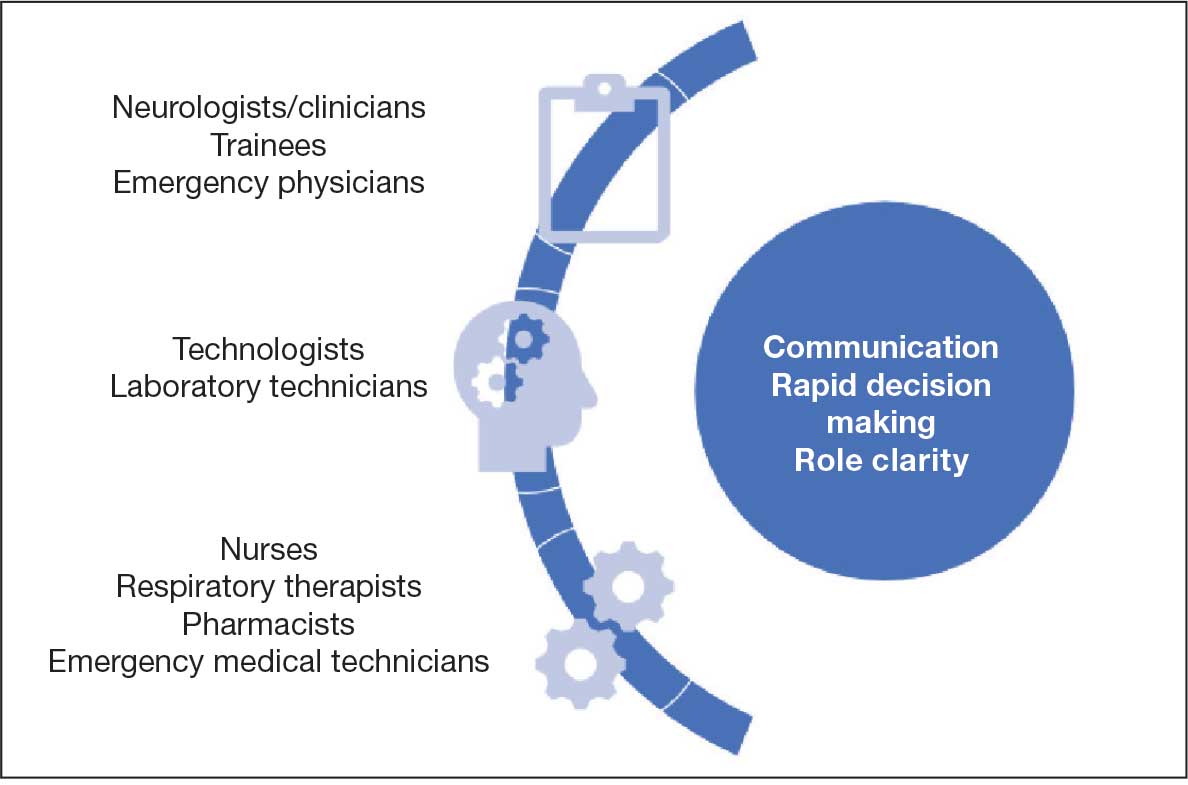
RESIDENCY TRAINING
Simulation training in stroke is mandatory in some residency programs for neurology postgraduate year (PGY) 2 residents.18 These simulations are a part of a boot camp for incoming neurology residents after completing an internal medicine internship. The simulation program is not standardized across various training programs. The European Stroke Organization Simulation Committee has published an opinion paper with a consensus of experts about the implementation of simulation techniques in the stroke field.19,20 Residents participating in these mandatory programs are required to complete certification in the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale, including a pretest that assesses their knowledge of acute stroke protocols prior to live simulation.17 A stepwise algorithm that incorporates faculty specialized in the field is used to evaluate and debrief the simulation.
Stroke vignettes are typically selected by the vascular neurology attending physician to cover thrombolytic therapy (indications and contraindications), mechanical thrombectomy, early arterial blood pressure management, anticoagulant reversal protocols, and management of thrombolytic complications (eg, neurologic worsening). Nursing staff is educated on the acute stroke protocol. Computed tomography (CT) and CT angiography scans are retrieved from teaching files. These are provided as live responses along with pertinent laboratory work, vital signs, and electrocardiogram tracings. Trainee performance is based on adherence to a critical action checklist, which includes (but is not limited to) identification of relative and absolute contraindications of thrombolytic treatments, estimation of NIHSS within 5 minutes of arrival, and consideration of candidacy for endovascular intervention.17
EVIDENCE FOR SIMULATION TRAINING
Simulations for acute ischemic stroke also improve cohesive teamwork to improve the door-to-needle and door-to-puncture time. A retrospective analysis involving first-year neurology residents at a comprehensive stroke center that compared patient cohort data before and after implementation of simulation training found that there was an improvement in door-to-needle time after implementation of stroke simulation training program by nearly 10 minutes.17 This was likely due to improvement in the comfort of the flow of management across multidisciplinary teams.
Discussing goals of care, communicating poor prognosis or complex decisions with distraught family members or patients requires practice. Simulation programs with video playback help focus on trainee’s body language, avoiding medical jargon and handling ethical dilemmas while adjusting the communication style to the patient’s personality.20 Enhanced communication skills improve patient satisfaction, trust, and adherence to treatments, all of which lead to better outcomes.21
Simulation has been effectively used as a training tool for recognizing and managing acute neuromuscular respiratory failure. These scenarios emphasize the importance of obtaining a focused clinical history, performing key neurological assessments (such as neck flexion strength and breath counting), evaluating pulmonary function tests, and identifying when to initiate ventilatory support.22 In a study designed as a simulation-based learning curriculum for status epilepticus, there was an improvement in the performance of PGY-2 residents after completing the curriculum from a median of 44.2% at pretest to 94.2% at posttest.23 In this curriculum, an emphasis was placed on the following: recognizing the delay in identification and treatment of status epilepticus; evaluating contraindications of certain antiseizure medication (ASM) based on history or laboratory work; giving first-line ASM within 5 minutes of seizure onset; airway and blood pressure assessment; suctioning the patient; use of second-line ASMs after first-line has failed; ordering a head CT and re-evaluating the case with postload ASM level; ordering a stat electroencephalography (EEG); and communicating the decision regarding patient disposition/level of care.24
There is a growing need for well designed simulation education programs targeted at the management of disorders requiring acute neurologic care, including not only stroke and status epilepticus, but also traumatic brain injury, subarachnoid hemorrhage, neuromuscular respiratory failure, flare of multiple sclerosis, acutely elevated intracranial pressure, malignant cerebral infarction, deterioration of Parkinson disease, and brain death evaluation with family counseling.25 This novel approach to teaching provides an opportunity to learn in addition to remediation with repetition of scenario and might be used for maintenance of recertification programs.
PROCEDURAL SKILLs
Perhaps one of the most studied uses for simulation in neurology is in procedural skills. This extends beyond neurology trainees and can include pulmonary critical care fellows, pediatric residents, and internal medicine residents receiving training in neurology-based procedures such as lumbar punctures (LPs). Other examples of neurology procedures and protocols in which simulation has been studied include fundoscopy, brain death evaluation, EEG interpretation in context of status epilepticus, and simulated stroke code responses. Additional procedures that lack research but may benefit from simulation-based training include the use of Doppler ultrasound and botulinum toxin injections practiced on mannequins.
Proficiency in LP procedural skills has been extensively studied by multiple institutions, with trainee levels ranging from medical students to fellows. One study in France enrolled 115 medical students without prior LP experience and randomized them to either a simulation or a control group.26 Those in the simulation group received instruction using a mannequin, and those in the control group received clinical training through hospital rotations. Both groups received an email containing literature-based information on the procedure as well as a self-assessment questionnaire before participating in either educational program.
The study showed that those students who received simulation training had a success rate of 67% on their first LP on a live patient compared with a success rate of 14% in those with traditional training. Students receiving simulation training required less assistance during the procedure from a supervisor and had higher satisfaction rates and confidence in their procedural skills.26
Another study of 128 medical students at the University of Pittsburgh found that a hybrid LP simulation significantly improved students’ confidence and perceived skill in performing LPs, obtaining informed consent, and electronic order entry. For example, confidence with LP increased from 5.95% presimulation to 90% postsimulation, with 58.24% of students reporting an improvement from minimal or no confidence to average or better (P < .001). Similarly, the proportion of students who felt able to perform LP with minimal or no assistance rose from 0% to 38.57% (P < .001). Confidence and perceived skill in obtaining informed consent and electronic order entry also saw significant gains. Although real-world skill assessments were limited by low survey response rates, preceptor evaluations and follow-up surveys suggested that students who participated in the simulation were more likely to perform these tasks independently or with minimal supervision during clinical rotations.27
Research on simulation training involving nonneurology residents is also encouraging. One study compared the LP skills of traditionally trained neurology residents (PGY-2 to PGY-4) to internal medicine residents (PGY-1) who underwent simulation on a mannequin.28 The internal medicine residents first underwent a pretest on LP performance, watched an educational video, underwent an LP demonstration, and practiced on a mannequin with feedback. The neurology residents completed the checklist-style pretest and performed an LP on a mannequin. Internal medicine residents were found to increase their pretest scores from a mean of 46.3% to 95.7% following training, whereas neurology residents scored a mean of 65.4%. More than half of neurology residents were unable to identify the correct anatomic location or standard cerebrospinal fluid (CSF) tests to be ordered on a routine LP.28
A pediatric resident study in Canada found that following simulation-based training, LP procedural skill improved in 15 of 16 residents tested, and PGY-1 residents showed a reduction in anxiety related to performing the procedure.29
Virtual Reality
An additional tool for simulation is the use of virtual reality (VR) in combination with mannequins. A French study used videos of LPs on actual patients, from equipment set up to final CSF collection and termination of the procedure.30 These videos included a 360-degree view of the procedure. The short video was administered through a VR device (the Oculus Go headset by Microsoft) or by a YouTube video (if VR was not desired).
Participants in the study watched the video then performed an LP on a mannequin. Those who used the VR option had minimal adverse effects (eg, low rates of cybersickness, blurred vision, nausea) and high satisfaction regarding their training environment.30Another VR-based program is the vascular intervention system trainer, which allows clinicians to use endovascular devices and simulate procedures such as thrombectomies. VR simulation is used for trainees and to retrain experienced physicians in performance of high-risk procedures.31
Fundoscopic and Ultrasound Simulations
The AR403 eye stimulator device for fundoscopic examinations is a mannequin-based simulation.32 In a single-center, prospective, single-blind study of neurology and pediatric neurology residents, trainees were split into control and intervention groups, with the intervention group receiving simulator training. Both groups received video lectures on fundoscopy techniques. Pre- and postintervention measurements included knowledge, skill, and total scores on the skills assessment. Of the 48 trainees who participated, the intervention group demonstrated significantly higher increases in skills (P = .01) and total (P = .02) scores, although knowledge scores did not improve. The intervention group also reported higher comfort levels, higher confidence, and higher success rates.
Areas that would benefit from simulation training and development include ultrasound training, such as transcranial Doppler evaluation. In a national survey of residents in anesthesia and critical care, trainees reported that simulation was not frequently used in ultrasound training and that bedside teaching was more common. Interestingly, there was a discrepancy between the opinions of residents and program directors. The program directors felt simulation was in fact used (18.2% of program directors reported this vs 5.3% of trainees).33
A new program, the NewroSim (Gaumard), is a computer-based model of cerebral perfusion that may be a useful tool in this setting. It can simulate blood flow velocities, including pathologic ones, both with a mannequin or without.34
Another potential area for development is the use of mannequins to teach botulinum toxin injections for migraine, dystonia and spasticity in a training environment This is typically led by pharmaceutical representatives who are not necessarily clinicians. Residents and fellows may benefit instead from clinician-led education during their training programs.
Simulation in Patient Communication
Simulation provides a realistic environment for teaching rapid decision-making, leadership, and appropriate management of acutely ill neurologic patients; this includes the communication skills needed in response to neurologic injury.35 Simulation can be particularly useful in situations involving brain death determination, where the communication techniques differ significantly from those used in shared decision-making. Simulation provides a low-stakes setting for clinicians to practice the process of brain death determination and communication, leading to improved confidence and knowledge.36
In the context of acute neurologic emergencies, simulation exercises have been used to investigate the consistency of prognostication across a spectrum of neurology physicians. These exercises revealed that acute neuroprognostication is highly variable and often inaccurate among neurology clinicians, suggesting a potential area for improvement through further simulation training.37
FUTURE DIRECTIONS
Simulation education in neurology can be directed towards learners at all levels, including medical students, residents, fellows, nurses, and medical technologists. In addition, simulation has great value to different disciplines, including emergency medicine, intensive care, and psychiatry. In our view simulation is not being used to full potential in neurology.
Simulation can be used to expose clinicians to rare pathology, play an integral role in competency-based evaluations, and serve as the foundation for simulation-based neurology curriculums, teleneurology simulation training programs, and team training for neurologic emergencies.38Another under-recognized aspect of neurology education is teaching interpersonal communication and professionalism. A survey conducted at a neurology department (20 residents and 73 faculty respondents) asked about residents’ comfort level in performing a number of interpersonal communication and professionalism tasks.38 While none of the residents said they were “very uncomfortable” with these tasks, only 1 resident reported being “very comfortable.” In addition, fewer than 50% noted that they had been directly observed by a faculty member while performing these tasks. The results prompted the facility to develop a simulation curriculum that including observation and feedback from 8 objective structured clinical examinations at a simulation center. A standardized professional simulated the role of a patient, caregiver, medical student, or a faculty member. Residents indicated in postsimulation surveys that it was very useful, and a majority voted for the activity to be repeated for future classes.38
Simulation models may also provide a more objective method to evaluate neurology residents. Accreditation Council for Graduate Medical Education has provided Milestones that are used for assessment of neurology residents. Most of the programs rely on end-of-rotation faculty evaluations. These are subjective evaluations, rely on chance evaluations and may not reflect the exact caliber of a trainee in different clinical situations. Simulation models can serve as alternatives to provide an objective and accurate assessment of resident’s competency in different neurologic scenarios.
In a study of PGY-4 neurology residents from 3 tertiary care academic medical centers were evaluated using simulation-based assessment. Their skills in identifying and managing status epilepticus were assessed via a simulation-based model and compared with clinical experience. No graduating neurology residents were able to meet or exceed the minimum passing score during the testing. It was suggested that end-of-rotation evaluations are inadequate for assigning level of Milestones.24 To move forward with use of simulation-based assessments, these models need to be trialed more extensively and validated.
Morris et al developed simulations for assessment in neurocritical care.39 Ten evaluative simulation cases were developed. Researchers reported on 64 trainee participants in 274 evaluative simulation scenarios. The participants were very satisfied with the cases, found them to be very realistic and appropriately difficult. Interrater reliability was acceptable for both checklist action items and global rating scales. The researchers concluded that they were able to demonstrate validity evidence via the 10 simulation cases for assessment in neurologic emergencies.39 It is the authors’ belief that the future of residents’ competency assessment should include more widespread use of similar simulation models.
Finally, VR and augmented reality (AR) have shown promise in various fields, including neurology. In neurology, these technologies are being explored for applications in rehabilitation, therapy, and medical training. Ongoing research aims to leverage these technologies for improved patient outcomes and medical education. Virtual simulations can recreate neurologic scenarios, allowing learners to interact with 3-dimensional (3D) models of the brain or experience virtual patient cases. AR can enhance traditional learning materials by overlaying digital information onto real-world objects, aiding in the understanding of complex neuroanatomy and medical concepts. These technologies contribute to more engaging and effective neurology education.39In a study of 84 graduate medical students divided into 3 groups, the first group attended a traditional lecture on neuroanatomy, the second group was shown VR-based 3D images, and the third group had a VR-based, interactive and stereoscopic session.40 Groups 2 and 3 showed the highest mean scores in evaluations and differed significantly from Group 1 (P < .05). Groups 2 and 3 did not differ significantly from each other. The researchers concluded that VR-based resources for teaching neuroanatomy fostered significantly higher learning when compared to the traditional methods.40
- Corvetto M, Bravo MP, Montaña R, et al. Simulación en educación médica: una sinopsis. Rev Med Chil. 2013;141:70-79. doi:10.4067/S0034-98872013000100010
- Lane JL, Slavin S, Ziv A. Simulation in medical education: a review. Simul Gaming. 2001;32:297-314. doi:10.1177/104687810103200302
- Bradley P. The history of simulation in medical education and possible future directions. Med Educ. 2006;40:254-262. doi:10.1111/j.1365-2929.2006.02394.x
- Jones F, Passos-Neto C, Melro Braghiroli O. Simulation in medical education: brief history and methodology. Princ Pract Clin Res J. 2015;1:46-54. doi:10.21801/ppcrj.2015.12.8
- Issenberg SB. Simulation technology for health care professional skills training and assessment. JAMA. 1999;28:861-866. doi:10.1001/jama.282.9.861
- McGaghie WC, Miller GE, Sajid AW, et al. Competency-based curriculum development on medical education: an introduction. Public Health Pap. 1978;68:11-91.
- Barsuk JH, Cohen ER, Feinglass J, et al. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169:1420-1423. doi:10.1001/archinternmed.2009.215
- Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133:56-61. doi:10.1378/chest.07-0131
- McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86:706-711. doi:10.1097/ACM.0b013e318217e119
- Micieli G, Cavallini A, Santalucia P, et al. Simulation in neurology. Neurol Sci. 2015;36:1967-1971. doi:10.1007/s10072-015-2228-8
- Bond WF, Lammers RL, Spillane LL, et al. The use of simulation in emergency medicine: a research agenda. Acad Emerg Med. 2007;14:353-363. doi:10.1197/j.aem.2006.11.02112.
- McLaughlin SA, Doezema D, Sklar DP. Human simulation in emergency medicine training: a model curriculum. Acad Emerg Med. 2002;9:1310-1318. doi:10.1111/j.1553-2712.2002.tb01593.x
- Howard SK, Gaba DM, Fish KJ, et al. Anesthesia crisis resource management training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63:763-770.
- Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785-788. doi:10.1136/bmj.320.7237.785
- Sedlack RE, Kolars JC. Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study. Am J Gastroenterol. 2004;99:33-37. doi:10.1111/j.1572-0241.2004.04007.x
- Tchopev ZN, Nelson AE, Hunninghake JC, et al. Curriculum innovations: high-fidelity simulation of acute neurology enhances rising resident confidence: results from a multicohort study. Neurol Educ. 2022;1:e200022. doi:10.1212/ne9.0000000000200022
- Mehta T, Strauss S, Beland D, et al. Stroke simulation improves acute stroke management: a systems-based practice experience. J Grad Med Educ. 2018;10:57-62. doi:10.4300/JGME-D-17-00167.1
- Pergakis MB, Chang WTW, Tabatabai A, et al. Simulation-based assessment of graduate neurology trainees’ performance managing acute ischemic stroke. Neurology. 2021;97:e2414-e2422. doi:10.1212/WNL.0000000000012972
- Casolla B. Simulation for neurology training: acute setting and beyond. Rev Neurol (Paris). 2021;177:1207-1213. doi:10.1016/j.neurol.2021.03.008
- Casolla B, de Leciñana MA, Neves R, et al. Simulation training programs for acute stroke care: Objectives and standards of methodology. Eur Stroke J. 2020;5:328-335. doi:10.1177/2396987320971105
- Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826-834.doi:10.1097/MLR.0b013e31819a5acc
- Patel RA, Mohl L, Paetow G, Maiser S. Acute neuromuscular respiratory weakness due to acute inflammatory demyelinating polyneuropathy (AIDP): a simulation scenario for neurology providers. MedEdPORTAL. 2019;15:10811. doi:10.15766/mep_2374-8265.10811
- Mikhaeil-Demo Y, Barsuk JH, Culler GW, et al. Use of a simulation-based mastery learning curriculum for neurology residents to improve the identification and management of status epilepticus. Epilepsy Behav. 2020;111:107247. doi:10.1016/j.yebeh.2020.107247
- Mikhaeil-Demo Y, Holmboe E, Gerard EE, et al. Simulation-based assessments and graduating neurology residents’ milestones: status epilepticus milestones. J Grad Med Educ. 2021;13:223-230. doi:10.4300/JGME-D-20-00832.1
- Hocker S, Wijdicks EFM, Feske SK, et al. Use of simulation in acute neurology training: point and counterpoint. Ann Neurol. 2015;78:337-342. doi:10.1002/ana.24473
- Gaubert S, Blet A, Dib F, et al. Positive effects of lumbar puncture simulation training for medical students in clinical practice. BMC Med Educ. 2021;21:1-6. doi:10.1186/S12909-020-02452-327.
- Yanta C, Knepper L, Van Deusen R, et al. The use of hybrid lumbar puncture simulation to teach entrustable professional activities during a medical student neurology clerkship. MedEdPublish (2016). 2021;9:266. doi:10.15694/mep.2020.000266.2
- Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79:132-137. doi:10.1212/WNL.0B013E31825DD39D
- McMillan HJ, Writer H, Moreau KA, et al. Lumbar puncture simulation in pediatric residency training: improving procedural competence and decreasing anxiety. BMC Med Educ. 2016;16:198. doi:10.1186/S12909-016-0722-1
- Vrillon A, Gonzales-Marabal L, Ceccaldi PF, et al. Using virtual reality in lumbar puncture training improves students learning experience. BMC Med Educ. 2022;22:244. doi:10.1186/S12909-022-03317-7
- Liebig T, Holtmannspötter M, Crossley R, et al. Metric-based virtual reality simulation: a paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018;49:e239-e242.doi:10.1161/STROKEAHA.118.021089
- Gupta DK, Khandker N, Stacy K, et al. Utility of combining a simulation-based method with a lecture-based method for fundoscopy training in neurology residency. JAMA Neurol. 2017;74:1223-1227. doi:10.1001/JAMANEUROL.2017.2073
- Mongodi S, Bonomi F, Vaschetto R, et al. Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ. 2022;22:647. doi:10.1186/S12909-022-03708-W
- Morris NA, Czeisler BM, Sarwal A. Simulation in neurocritical care: past, present, and future. Neurocrit Care. 2019;30:522-533. doi:10.1007/S12028-018-0629-2
- Wijdicks EFM, Hocker SE. A future for simulation in acute neurology. Semin Neurol. 2018;38:465-470. doi:10.1055/s-0038-1666986
- Kramer NM, O’Mahony S, Deamant C. Brain death determination and communication: an innovative approach using simulation and standardized patients. J Pain Symptom Manage. 2022;63:e765-e772. doi:10.1016/j.jpainsymman.2022.01.020
- Sloane KL, Miller JJ, Piquet A, et al. Prognostication in acute neurological emergencies. J Stroke Cerebrovasc Dis. 2022;31:106277. doi:10.1016/J.JSTROKECEREBROVASDIS.2021.106277
- Kurzweil AM, Lewis A, Pleninger P, et al. Education research: teaching and assessing communication and professionalism in neurology residency with simulation. Neurology. 2020;94:229-232. doi:10.1212/WNL.0000000000008895
- Morris NA, Chang WT, Tabatabai A, et al. Development of neurological emergency simulations for assessment: content evidence and response process. Neurocrit Care. 2021;35:389-396. doi:10.1007/S12028-020-01176-Y
- De Faria JWV, Teixeira MJ, De Moura Sousa Júnior L, et al. Virtual and stereoscopic anatomy: when virtual reality meets medical education. J Neurosurg. 2016;125:1105-1111. doi:10.3171/2015.8.JNS141563
Clinical simulation is a technique, not a technology, used to replace or amplify real experiences with guided experiences that evoke or replicate substantial aspects of the real world in a fully interactive fashion.1 Simulation is widely used in medical education and spans a spectrum of sophistication, from simple reproduction of isolated body parts to high-fidelity human patient simulators that replicate whole body appearance and variable physiological parameters.2,3
Simulation-based medical education can be a valuable tool for safe health care delivery.4Simulation-based education is typically provided via 5 modalities: mannequins, computer-based mannequins, standardized patients, computer-based simulators, and software-based simulations. Simulation technology increases procedural skill by allowing for deliberate practice in a safe environment.5 Mastery learning is a stringent form of competency-based education that requires trainees to acquire clinical skill measured against a fixed achievement standard.6 In mastery learning, educational practice time varies but results are uniform. This approach improves patient outcomes and is more effective than clinical training alone.7-9
Advanced simulation models are helpful tools for neurologic education and training, especially for emergency department encounters.10 In recent years, advanced simulation models have been applied in various fields of medicine, especially emergency medicine and anesthesia.11-14
Acute neurology
In acute neurologic conditions (eg, stroke, intracerebral hemorrhage, status epilepticus, and neuromuscular respiratory failure) clinical outcomes are highly time dependent; consequently, a reduction in treatment delays can improve patient care. The application of simulation methodology allows trainees to address acute and potentially life-threatening emergencies in a safe, controlled, and reproducible environment. In addition to improving trainees’ knowledge base, simulation also helps to enhance team skills, communication, multidisciplinary collaboration, and leadership. Research has shown that deliberate practice leads to a decrease in clinical errors and improved procedural performance in the operating room.8,15 These results can be extrapolated to acute neurology settings to improve adherence to set protocols, thus streamlining management in acute settings.
Scenarios can be built to teach skills such as eliciting an appropriate history, establishing inclusion or exclusion criteria for the use of certain medications, evaluating neuroimaging and laboratory studies (while avoiding related common pitfalls), and managing treatment complications. Simulation also provides an opportunity for interprofessional education by training nurses and collaborative staff. It can be used to enhance nontechnical skills (eg, communication, situation awareness, decision making, and leadership) that further contribute to patient safety.
Simulation can be performed with the help of mannequins such as the SimMan 3G(Laerdal), which can display neurologic symptoms and physiological findings, or live actors who portray a patient by mimicking focal neurologic deficits.16,17 A briefing familiarizes the trainees with the equipment and explains the simulation process. The documentation and equipment are the same as that which is used in emergency departments or intensive care units.
Once the simulation is completed, a trainee’s performance is checked against a critical action checklist before a debriefing process during which the scenario is reviewed and learning goals are assessed. Immediate feedback is given to trainees to identify weaknesses and the simulation is repeated if multiple critical action items are missed. (Figure).17

RESIDENCY TRAINING
Simulation training in stroke is mandatory in some residency programs for neurology postgraduate year (PGY) 2 residents.18 These simulations are a part of a boot camp for incoming neurology residents after completing an internal medicine internship. The simulation program is not standardized across various training programs. The European Stroke Organization Simulation Committee has published an opinion paper with a consensus of experts about the implementation of simulation techniques in the stroke field.19,20 Residents participating in these mandatory programs are required to complete certification in the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale, including a pretest that assesses their knowledge of acute stroke protocols prior to live simulation.17 A stepwise algorithm that incorporates faculty specialized in the field is used to evaluate and debrief the simulation.
Stroke vignettes are typically selected by the vascular neurology attending physician to cover thrombolytic therapy (indications and contraindications), mechanical thrombectomy, early arterial blood pressure management, anticoagulant reversal protocols, and management of thrombolytic complications (eg, neurologic worsening). Nursing staff is educated on the acute stroke protocol. Computed tomography (CT) and CT angiography scans are retrieved from teaching files. These are provided as live responses along with pertinent laboratory work, vital signs, and electrocardiogram tracings. Trainee performance is based on adherence to a critical action checklist, which includes (but is not limited to) identification of relative and absolute contraindications of thrombolytic treatments, estimation of NIHSS within 5 minutes of arrival, and consideration of candidacy for endovascular intervention.17
EVIDENCE FOR SIMULATION TRAINING
Simulations for acute ischemic stroke also improve cohesive teamwork to improve the door-to-needle and door-to-puncture time. A retrospective analysis involving first-year neurology residents at a comprehensive stroke center that compared patient cohort data before and after implementation of simulation training found that there was an improvement in door-to-needle time after implementation of stroke simulation training program by nearly 10 minutes.17 This was likely due to improvement in the comfort of the flow of management across multidisciplinary teams.
Discussing goals of care, communicating poor prognosis or complex decisions with distraught family members or patients requires practice. Simulation programs with video playback help focus on trainee’s body language, avoiding medical jargon and handling ethical dilemmas while adjusting the communication style to the patient’s personality.20 Enhanced communication skills improve patient satisfaction, trust, and adherence to treatments, all of which lead to better outcomes.21
Simulation has been effectively used as a training tool for recognizing and managing acute neuromuscular respiratory failure. These scenarios emphasize the importance of obtaining a focused clinical history, performing key neurological assessments (such as neck flexion strength and breath counting), evaluating pulmonary function tests, and identifying when to initiate ventilatory support.22 In a study designed as a simulation-based learning curriculum for status epilepticus, there was an improvement in the performance of PGY-2 residents after completing the curriculum from a median of 44.2% at pretest to 94.2% at posttest.23 In this curriculum, an emphasis was placed on the following: recognizing the delay in identification and treatment of status epilepticus; evaluating contraindications of certain antiseizure medication (ASM) based on history or laboratory work; giving first-line ASM within 5 minutes of seizure onset; airway and blood pressure assessment; suctioning the patient; use of second-line ASMs after first-line has failed; ordering a head CT and re-evaluating the case with postload ASM level; ordering a stat electroencephalography (EEG); and communicating the decision regarding patient disposition/level of care.24
There is a growing need for well designed simulation education programs targeted at the management of disorders requiring acute neurologic care, including not only stroke and status epilepticus, but also traumatic brain injury, subarachnoid hemorrhage, neuromuscular respiratory failure, flare of multiple sclerosis, acutely elevated intracranial pressure, malignant cerebral infarction, deterioration of Parkinson disease, and brain death evaluation with family counseling.25 This novel approach to teaching provides an opportunity to learn in addition to remediation with repetition of scenario and might be used for maintenance of recertification programs.
PROCEDURAL SKILLs
Perhaps one of the most studied uses for simulation in neurology is in procedural skills. This extends beyond neurology trainees and can include pulmonary critical care fellows, pediatric residents, and internal medicine residents receiving training in neurology-based procedures such as lumbar punctures (LPs). Other examples of neurology procedures and protocols in which simulation has been studied include fundoscopy, brain death evaluation, EEG interpretation in context of status epilepticus, and simulated stroke code responses. Additional procedures that lack research but may benefit from simulation-based training include the use of Doppler ultrasound and botulinum toxin injections practiced on mannequins.
Proficiency in LP procedural skills has been extensively studied by multiple institutions, with trainee levels ranging from medical students to fellows. One study in France enrolled 115 medical students without prior LP experience and randomized them to either a simulation or a control group.26 Those in the simulation group received instruction using a mannequin, and those in the control group received clinical training through hospital rotations. Both groups received an email containing literature-based information on the procedure as well as a self-assessment questionnaire before participating in either educational program.
The study showed that those students who received simulation training had a success rate of 67% on their first LP on a live patient compared with a success rate of 14% in those with traditional training. Students receiving simulation training required less assistance during the procedure from a supervisor and had higher satisfaction rates and confidence in their procedural skills.26
Another study of 128 medical students at the University of Pittsburgh found that a hybrid LP simulation significantly improved students’ confidence and perceived skill in performing LPs, obtaining informed consent, and electronic order entry. For example, confidence with LP increased from 5.95% presimulation to 90% postsimulation, with 58.24% of students reporting an improvement from minimal or no confidence to average or better (P < .001). Similarly, the proportion of students who felt able to perform LP with minimal or no assistance rose from 0% to 38.57% (P < .001). Confidence and perceived skill in obtaining informed consent and electronic order entry also saw significant gains. Although real-world skill assessments were limited by low survey response rates, preceptor evaluations and follow-up surveys suggested that students who participated in the simulation were more likely to perform these tasks independently or with minimal supervision during clinical rotations.27
Research on simulation training involving nonneurology residents is also encouraging. One study compared the LP skills of traditionally trained neurology residents (PGY-2 to PGY-4) to internal medicine residents (PGY-1) who underwent simulation on a mannequin.28 The internal medicine residents first underwent a pretest on LP performance, watched an educational video, underwent an LP demonstration, and practiced on a mannequin with feedback. The neurology residents completed the checklist-style pretest and performed an LP on a mannequin. Internal medicine residents were found to increase their pretest scores from a mean of 46.3% to 95.7% following training, whereas neurology residents scored a mean of 65.4%. More than half of neurology residents were unable to identify the correct anatomic location or standard cerebrospinal fluid (CSF) tests to be ordered on a routine LP.28
A pediatric resident study in Canada found that following simulation-based training, LP procedural skill improved in 15 of 16 residents tested, and PGY-1 residents showed a reduction in anxiety related to performing the procedure.29
Virtual Reality
An additional tool for simulation is the use of virtual reality (VR) in combination with mannequins. A French study used videos of LPs on actual patients, from equipment set up to final CSF collection and termination of the procedure.30 These videos included a 360-degree view of the procedure. The short video was administered through a VR device (the Oculus Go headset by Microsoft) or by a YouTube video (if VR was not desired).
Participants in the study watched the video then performed an LP on a mannequin. Those who used the VR option had minimal adverse effects (eg, low rates of cybersickness, blurred vision, nausea) and high satisfaction regarding their training environment.30Another VR-based program is the vascular intervention system trainer, which allows clinicians to use endovascular devices and simulate procedures such as thrombectomies. VR simulation is used for trainees and to retrain experienced physicians in performance of high-risk procedures.31
Fundoscopic and Ultrasound Simulations
The AR403 eye stimulator device for fundoscopic examinations is a mannequin-based simulation.32 In a single-center, prospective, single-blind study of neurology and pediatric neurology residents, trainees were split into control and intervention groups, with the intervention group receiving simulator training. Both groups received video lectures on fundoscopy techniques. Pre- and postintervention measurements included knowledge, skill, and total scores on the skills assessment. Of the 48 trainees who participated, the intervention group demonstrated significantly higher increases in skills (P = .01) and total (P = .02) scores, although knowledge scores did not improve. The intervention group also reported higher comfort levels, higher confidence, and higher success rates.
Areas that would benefit from simulation training and development include ultrasound training, such as transcranial Doppler evaluation. In a national survey of residents in anesthesia and critical care, trainees reported that simulation was not frequently used in ultrasound training and that bedside teaching was more common. Interestingly, there was a discrepancy between the opinions of residents and program directors. The program directors felt simulation was in fact used (18.2% of program directors reported this vs 5.3% of trainees).33
A new program, the NewroSim (Gaumard), is a computer-based model of cerebral perfusion that may be a useful tool in this setting. It can simulate blood flow velocities, including pathologic ones, both with a mannequin or without.34
Another potential area for development is the use of mannequins to teach botulinum toxin injections for migraine, dystonia and spasticity in a training environment This is typically led by pharmaceutical representatives who are not necessarily clinicians. Residents and fellows may benefit instead from clinician-led education during their training programs.
Simulation in Patient Communication
Simulation provides a realistic environment for teaching rapid decision-making, leadership, and appropriate management of acutely ill neurologic patients; this includes the communication skills needed in response to neurologic injury.35 Simulation can be particularly useful in situations involving brain death determination, where the communication techniques differ significantly from those used in shared decision-making. Simulation provides a low-stakes setting for clinicians to practice the process of brain death determination and communication, leading to improved confidence and knowledge.36
In the context of acute neurologic emergencies, simulation exercises have been used to investigate the consistency of prognostication across a spectrum of neurology physicians. These exercises revealed that acute neuroprognostication is highly variable and often inaccurate among neurology clinicians, suggesting a potential area for improvement through further simulation training.37
FUTURE DIRECTIONS
Simulation education in neurology can be directed towards learners at all levels, including medical students, residents, fellows, nurses, and medical technologists. In addition, simulation has great value to different disciplines, including emergency medicine, intensive care, and psychiatry. In our view simulation is not being used to full potential in neurology.
Simulation can be used to expose clinicians to rare pathology, play an integral role in competency-based evaluations, and serve as the foundation for simulation-based neurology curriculums, teleneurology simulation training programs, and team training for neurologic emergencies.38Another under-recognized aspect of neurology education is teaching interpersonal communication and professionalism. A survey conducted at a neurology department (20 residents and 73 faculty respondents) asked about residents’ comfort level in performing a number of interpersonal communication and professionalism tasks.38 While none of the residents said they were “very uncomfortable” with these tasks, only 1 resident reported being “very comfortable.” In addition, fewer than 50% noted that they had been directly observed by a faculty member while performing these tasks. The results prompted the facility to develop a simulation curriculum that including observation and feedback from 8 objective structured clinical examinations at a simulation center. A standardized professional simulated the role of a patient, caregiver, medical student, or a faculty member. Residents indicated in postsimulation surveys that it was very useful, and a majority voted for the activity to be repeated for future classes.38
Simulation models may also provide a more objective method to evaluate neurology residents. Accreditation Council for Graduate Medical Education has provided Milestones that are used for assessment of neurology residents. Most of the programs rely on end-of-rotation faculty evaluations. These are subjective evaluations, rely on chance evaluations and may not reflect the exact caliber of a trainee in different clinical situations. Simulation models can serve as alternatives to provide an objective and accurate assessment of resident’s competency in different neurologic scenarios.
In a study of PGY-4 neurology residents from 3 tertiary care academic medical centers were evaluated using simulation-based assessment. Their skills in identifying and managing status epilepticus were assessed via a simulation-based model and compared with clinical experience. No graduating neurology residents were able to meet or exceed the minimum passing score during the testing. It was suggested that end-of-rotation evaluations are inadequate for assigning level of Milestones.24 To move forward with use of simulation-based assessments, these models need to be trialed more extensively and validated.
Morris et al developed simulations for assessment in neurocritical care.39 Ten evaluative simulation cases were developed. Researchers reported on 64 trainee participants in 274 evaluative simulation scenarios. The participants were very satisfied with the cases, found them to be very realistic and appropriately difficult. Interrater reliability was acceptable for both checklist action items and global rating scales. The researchers concluded that they were able to demonstrate validity evidence via the 10 simulation cases for assessment in neurologic emergencies.39 It is the authors’ belief that the future of residents’ competency assessment should include more widespread use of similar simulation models.
Finally, VR and augmented reality (AR) have shown promise in various fields, including neurology. In neurology, these technologies are being explored for applications in rehabilitation, therapy, and medical training. Ongoing research aims to leverage these technologies for improved patient outcomes and medical education. Virtual simulations can recreate neurologic scenarios, allowing learners to interact with 3-dimensional (3D) models of the brain or experience virtual patient cases. AR can enhance traditional learning materials by overlaying digital information onto real-world objects, aiding in the understanding of complex neuroanatomy and medical concepts. These technologies contribute to more engaging and effective neurology education.39In a study of 84 graduate medical students divided into 3 groups, the first group attended a traditional lecture on neuroanatomy, the second group was shown VR-based 3D images, and the third group had a VR-based, interactive and stereoscopic session.40 Groups 2 and 3 showed the highest mean scores in evaluations and differed significantly from Group 1 (P < .05). Groups 2 and 3 did not differ significantly from each other. The researchers concluded that VR-based resources for teaching neuroanatomy fostered significantly higher learning when compared to the traditional methods.40
Clinical simulation is a technique, not a technology, used to replace or amplify real experiences with guided experiences that evoke or replicate substantial aspects of the real world in a fully interactive fashion.1 Simulation is widely used in medical education and spans a spectrum of sophistication, from simple reproduction of isolated body parts to high-fidelity human patient simulators that replicate whole body appearance and variable physiological parameters.2,3
Simulation-based medical education can be a valuable tool for safe health care delivery.4Simulation-based education is typically provided via 5 modalities: mannequins, computer-based mannequins, standardized patients, computer-based simulators, and software-based simulations. Simulation technology increases procedural skill by allowing for deliberate practice in a safe environment.5 Mastery learning is a stringent form of competency-based education that requires trainees to acquire clinical skill measured against a fixed achievement standard.6 In mastery learning, educational practice time varies but results are uniform. This approach improves patient outcomes and is more effective than clinical training alone.7-9
Advanced simulation models are helpful tools for neurologic education and training, especially for emergency department encounters.10 In recent years, advanced simulation models have been applied in various fields of medicine, especially emergency medicine and anesthesia.11-14
Acute neurology
In acute neurologic conditions (eg, stroke, intracerebral hemorrhage, status epilepticus, and neuromuscular respiratory failure) clinical outcomes are highly time dependent; consequently, a reduction in treatment delays can improve patient care. The application of simulation methodology allows trainees to address acute and potentially life-threatening emergencies in a safe, controlled, and reproducible environment. In addition to improving trainees’ knowledge base, simulation also helps to enhance team skills, communication, multidisciplinary collaboration, and leadership. Research has shown that deliberate practice leads to a decrease in clinical errors and improved procedural performance in the operating room.8,15 These results can be extrapolated to acute neurology settings to improve adherence to set protocols, thus streamlining management in acute settings.
Scenarios can be built to teach skills such as eliciting an appropriate history, establishing inclusion or exclusion criteria for the use of certain medications, evaluating neuroimaging and laboratory studies (while avoiding related common pitfalls), and managing treatment complications. Simulation also provides an opportunity for interprofessional education by training nurses and collaborative staff. It can be used to enhance nontechnical skills (eg, communication, situation awareness, decision making, and leadership) that further contribute to patient safety.
Simulation can be performed with the help of mannequins such as the SimMan 3G(Laerdal), which can display neurologic symptoms and physiological findings, or live actors who portray a patient by mimicking focal neurologic deficits.16,17 A briefing familiarizes the trainees with the equipment and explains the simulation process. The documentation and equipment are the same as that which is used in emergency departments or intensive care units.
Once the simulation is completed, a trainee’s performance is checked against a critical action checklist before a debriefing process during which the scenario is reviewed and learning goals are assessed. Immediate feedback is given to trainees to identify weaknesses and the simulation is repeated if multiple critical action items are missed. (Figure).17

RESIDENCY TRAINING
Simulation training in stroke is mandatory in some residency programs for neurology postgraduate year (PGY) 2 residents.18 These simulations are a part of a boot camp for incoming neurology residents after completing an internal medicine internship. The simulation program is not standardized across various training programs. The European Stroke Organization Simulation Committee has published an opinion paper with a consensus of experts about the implementation of simulation techniques in the stroke field.19,20 Residents participating in these mandatory programs are required to complete certification in the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale, including a pretest that assesses their knowledge of acute stroke protocols prior to live simulation.17 A stepwise algorithm that incorporates faculty specialized in the field is used to evaluate and debrief the simulation.
Stroke vignettes are typically selected by the vascular neurology attending physician to cover thrombolytic therapy (indications and contraindications), mechanical thrombectomy, early arterial blood pressure management, anticoagulant reversal protocols, and management of thrombolytic complications (eg, neurologic worsening). Nursing staff is educated on the acute stroke protocol. Computed tomography (CT) and CT angiography scans are retrieved from teaching files. These are provided as live responses along with pertinent laboratory work, vital signs, and electrocardiogram tracings. Trainee performance is based on adherence to a critical action checklist, which includes (but is not limited to) identification of relative and absolute contraindications of thrombolytic treatments, estimation of NIHSS within 5 minutes of arrival, and consideration of candidacy for endovascular intervention.17
EVIDENCE FOR SIMULATION TRAINING
Simulations for acute ischemic stroke also improve cohesive teamwork to improve the door-to-needle and door-to-puncture time. A retrospective analysis involving first-year neurology residents at a comprehensive stroke center that compared patient cohort data before and after implementation of simulation training found that there was an improvement in door-to-needle time after implementation of stroke simulation training program by nearly 10 minutes.17 This was likely due to improvement in the comfort of the flow of management across multidisciplinary teams.
Discussing goals of care, communicating poor prognosis or complex decisions with distraught family members or patients requires practice. Simulation programs with video playback help focus on trainee’s body language, avoiding medical jargon and handling ethical dilemmas while adjusting the communication style to the patient’s personality.20 Enhanced communication skills improve patient satisfaction, trust, and adherence to treatments, all of which lead to better outcomes.21
Simulation has been effectively used as a training tool for recognizing and managing acute neuromuscular respiratory failure. These scenarios emphasize the importance of obtaining a focused clinical history, performing key neurological assessments (such as neck flexion strength and breath counting), evaluating pulmonary function tests, and identifying when to initiate ventilatory support.22 In a study designed as a simulation-based learning curriculum for status epilepticus, there was an improvement in the performance of PGY-2 residents after completing the curriculum from a median of 44.2% at pretest to 94.2% at posttest.23 In this curriculum, an emphasis was placed on the following: recognizing the delay in identification and treatment of status epilepticus; evaluating contraindications of certain antiseizure medication (ASM) based on history or laboratory work; giving first-line ASM within 5 minutes of seizure onset; airway and blood pressure assessment; suctioning the patient; use of second-line ASMs after first-line has failed; ordering a head CT and re-evaluating the case with postload ASM level; ordering a stat electroencephalography (EEG); and communicating the decision regarding patient disposition/level of care.24
There is a growing need for well designed simulation education programs targeted at the management of disorders requiring acute neurologic care, including not only stroke and status epilepticus, but also traumatic brain injury, subarachnoid hemorrhage, neuromuscular respiratory failure, flare of multiple sclerosis, acutely elevated intracranial pressure, malignant cerebral infarction, deterioration of Parkinson disease, and brain death evaluation with family counseling.25 This novel approach to teaching provides an opportunity to learn in addition to remediation with repetition of scenario and might be used for maintenance of recertification programs.
PROCEDURAL SKILLs
Perhaps one of the most studied uses for simulation in neurology is in procedural skills. This extends beyond neurology trainees and can include pulmonary critical care fellows, pediatric residents, and internal medicine residents receiving training in neurology-based procedures such as lumbar punctures (LPs). Other examples of neurology procedures and protocols in which simulation has been studied include fundoscopy, brain death evaluation, EEG interpretation in context of status epilepticus, and simulated stroke code responses. Additional procedures that lack research but may benefit from simulation-based training include the use of Doppler ultrasound and botulinum toxin injections practiced on mannequins.
Proficiency in LP procedural skills has been extensively studied by multiple institutions, with trainee levels ranging from medical students to fellows. One study in France enrolled 115 medical students without prior LP experience and randomized them to either a simulation or a control group.26 Those in the simulation group received instruction using a mannequin, and those in the control group received clinical training through hospital rotations. Both groups received an email containing literature-based information on the procedure as well as a self-assessment questionnaire before participating in either educational program.
The study showed that those students who received simulation training had a success rate of 67% on their first LP on a live patient compared with a success rate of 14% in those with traditional training. Students receiving simulation training required less assistance during the procedure from a supervisor and had higher satisfaction rates and confidence in their procedural skills.26
Another study of 128 medical students at the University of Pittsburgh found that a hybrid LP simulation significantly improved students’ confidence and perceived skill in performing LPs, obtaining informed consent, and electronic order entry. For example, confidence with LP increased from 5.95% presimulation to 90% postsimulation, with 58.24% of students reporting an improvement from minimal or no confidence to average or better (P < .001). Similarly, the proportion of students who felt able to perform LP with minimal or no assistance rose from 0% to 38.57% (P < .001). Confidence and perceived skill in obtaining informed consent and electronic order entry also saw significant gains. Although real-world skill assessments were limited by low survey response rates, preceptor evaluations and follow-up surveys suggested that students who participated in the simulation were more likely to perform these tasks independently or with minimal supervision during clinical rotations.27
Research on simulation training involving nonneurology residents is also encouraging. One study compared the LP skills of traditionally trained neurology residents (PGY-2 to PGY-4) to internal medicine residents (PGY-1) who underwent simulation on a mannequin.28 The internal medicine residents first underwent a pretest on LP performance, watched an educational video, underwent an LP demonstration, and practiced on a mannequin with feedback. The neurology residents completed the checklist-style pretest and performed an LP on a mannequin. Internal medicine residents were found to increase their pretest scores from a mean of 46.3% to 95.7% following training, whereas neurology residents scored a mean of 65.4%. More than half of neurology residents were unable to identify the correct anatomic location or standard cerebrospinal fluid (CSF) tests to be ordered on a routine LP.28
A pediatric resident study in Canada found that following simulation-based training, LP procedural skill improved in 15 of 16 residents tested, and PGY-1 residents showed a reduction in anxiety related to performing the procedure.29
Virtual Reality
An additional tool for simulation is the use of virtual reality (VR) in combination with mannequins. A French study used videos of LPs on actual patients, from equipment set up to final CSF collection and termination of the procedure.30 These videos included a 360-degree view of the procedure. The short video was administered through a VR device (the Oculus Go headset by Microsoft) or by a YouTube video (if VR was not desired).
Participants in the study watched the video then performed an LP on a mannequin. Those who used the VR option had minimal adverse effects (eg, low rates of cybersickness, blurred vision, nausea) and high satisfaction regarding their training environment.30Another VR-based program is the vascular intervention system trainer, which allows clinicians to use endovascular devices and simulate procedures such as thrombectomies. VR simulation is used for trainees and to retrain experienced physicians in performance of high-risk procedures.31
Fundoscopic and Ultrasound Simulations
The AR403 eye stimulator device for fundoscopic examinations is a mannequin-based simulation.32 In a single-center, prospective, single-blind study of neurology and pediatric neurology residents, trainees were split into control and intervention groups, with the intervention group receiving simulator training. Both groups received video lectures on fundoscopy techniques. Pre- and postintervention measurements included knowledge, skill, and total scores on the skills assessment. Of the 48 trainees who participated, the intervention group demonstrated significantly higher increases in skills (P = .01) and total (P = .02) scores, although knowledge scores did not improve. The intervention group also reported higher comfort levels, higher confidence, and higher success rates.
Areas that would benefit from simulation training and development include ultrasound training, such as transcranial Doppler evaluation. In a national survey of residents in anesthesia and critical care, trainees reported that simulation was not frequently used in ultrasound training and that bedside teaching was more common. Interestingly, there was a discrepancy between the opinions of residents and program directors. The program directors felt simulation was in fact used (18.2% of program directors reported this vs 5.3% of trainees).33
A new program, the NewroSim (Gaumard), is a computer-based model of cerebral perfusion that may be a useful tool in this setting. It can simulate blood flow velocities, including pathologic ones, both with a mannequin or without.34
Another potential area for development is the use of mannequins to teach botulinum toxin injections for migraine, dystonia and spasticity in a training environment This is typically led by pharmaceutical representatives who are not necessarily clinicians. Residents and fellows may benefit instead from clinician-led education during their training programs.
Simulation in Patient Communication
Simulation provides a realistic environment for teaching rapid decision-making, leadership, and appropriate management of acutely ill neurologic patients; this includes the communication skills needed in response to neurologic injury.35 Simulation can be particularly useful in situations involving brain death determination, where the communication techniques differ significantly from those used in shared decision-making. Simulation provides a low-stakes setting for clinicians to practice the process of brain death determination and communication, leading to improved confidence and knowledge.36
In the context of acute neurologic emergencies, simulation exercises have been used to investigate the consistency of prognostication across a spectrum of neurology physicians. These exercises revealed that acute neuroprognostication is highly variable and often inaccurate among neurology clinicians, suggesting a potential area for improvement through further simulation training.37
FUTURE DIRECTIONS
Simulation education in neurology can be directed towards learners at all levels, including medical students, residents, fellows, nurses, and medical technologists. In addition, simulation has great value to different disciplines, including emergency medicine, intensive care, and psychiatry. In our view simulation is not being used to full potential in neurology.
Simulation can be used to expose clinicians to rare pathology, play an integral role in competency-based evaluations, and serve as the foundation for simulation-based neurology curriculums, teleneurology simulation training programs, and team training for neurologic emergencies.38Another under-recognized aspect of neurology education is teaching interpersonal communication and professionalism. A survey conducted at a neurology department (20 residents and 73 faculty respondents) asked about residents’ comfort level in performing a number of interpersonal communication and professionalism tasks.38 While none of the residents said they were “very uncomfortable” with these tasks, only 1 resident reported being “very comfortable.” In addition, fewer than 50% noted that they had been directly observed by a faculty member while performing these tasks. The results prompted the facility to develop a simulation curriculum that including observation and feedback from 8 objective structured clinical examinations at a simulation center. A standardized professional simulated the role of a patient, caregiver, medical student, or a faculty member. Residents indicated in postsimulation surveys that it was very useful, and a majority voted for the activity to be repeated for future classes.38
Simulation models may also provide a more objective method to evaluate neurology residents. Accreditation Council for Graduate Medical Education has provided Milestones that are used for assessment of neurology residents. Most of the programs rely on end-of-rotation faculty evaluations. These are subjective evaluations, rely on chance evaluations and may not reflect the exact caliber of a trainee in different clinical situations. Simulation models can serve as alternatives to provide an objective and accurate assessment of resident’s competency in different neurologic scenarios.
In a study of PGY-4 neurology residents from 3 tertiary care academic medical centers were evaluated using simulation-based assessment. Their skills in identifying and managing status epilepticus were assessed via a simulation-based model and compared with clinical experience. No graduating neurology residents were able to meet or exceed the minimum passing score during the testing. It was suggested that end-of-rotation evaluations are inadequate for assigning level of Milestones.24 To move forward with use of simulation-based assessments, these models need to be trialed more extensively and validated.
Morris et al developed simulations for assessment in neurocritical care.39 Ten evaluative simulation cases were developed. Researchers reported on 64 trainee participants in 274 evaluative simulation scenarios. The participants were very satisfied with the cases, found them to be very realistic and appropriately difficult. Interrater reliability was acceptable for both checklist action items and global rating scales. The researchers concluded that they were able to demonstrate validity evidence via the 10 simulation cases for assessment in neurologic emergencies.39 It is the authors’ belief that the future of residents’ competency assessment should include more widespread use of similar simulation models.
Finally, VR and augmented reality (AR) have shown promise in various fields, including neurology. In neurology, these technologies are being explored for applications in rehabilitation, therapy, and medical training. Ongoing research aims to leverage these technologies for improved patient outcomes and medical education. Virtual simulations can recreate neurologic scenarios, allowing learners to interact with 3-dimensional (3D) models of the brain or experience virtual patient cases. AR can enhance traditional learning materials by overlaying digital information onto real-world objects, aiding in the understanding of complex neuroanatomy and medical concepts. These technologies contribute to more engaging and effective neurology education.39In a study of 84 graduate medical students divided into 3 groups, the first group attended a traditional lecture on neuroanatomy, the second group was shown VR-based 3D images, and the third group had a VR-based, interactive and stereoscopic session.40 Groups 2 and 3 showed the highest mean scores in evaluations and differed significantly from Group 1 (P < .05). Groups 2 and 3 did not differ significantly from each other. The researchers concluded that VR-based resources for teaching neuroanatomy fostered significantly higher learning when compared to the traditional methods.40
- Corvetto M, Bravo MP, Montaña R, et al. Simulación en educación médica: una sinopsis. Rev Med Chil. 2013;141:70-79. doi:10.4067/S0034-98872013000100010
- Lane JL, Slavin S, Ziv A. Simulation in medical education: a review. Simul Gaming. 2001;32:297-314. doi:10.1177/104687810103200302
- Bradley P. The history of simulation in medical education and possible future directions. Med Educ. 2006;40:254-262. doi:10.1111/j.1365-2929.2006.02394.x
- Jones F, Passos-Neto C, Melro Braghiroli O. Simulation in medical education: brief history and methodology. Princ Pract Clin Res J. 2015;1:46-54. doi:10.21801/ppcrj.2015.12.8
- Issenberg SB. Simulation technology for health care professional skills training and assessment. JAMA. 1999;28:861-866. doi:10.1001/jama.282.9.861
- McGaghie WC, Miller GE, Sajid AW, et al. Competency-based curriculum development on medical education: an introduction. Public Health Pap. 1978;68:11-91.
- Barsuk JH, Cohen ER, Feinglass J, et al. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169:1420-1423. doi:10.1001/archinternmed.2009.215
- Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133:56-61. doi:10.1378/chest.07-0131
- McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86:706-711. doi:10.1097/ACM.0b013e318217e119
- Micieli G, Cavallini A, Santalucia P, et al. Simulation in neurology. Neurol Sci. 2015;36:1967-1971. doi:10.1007/s10072-015-2228-8
- Bond WF, Lammers RL, Spillane LL, et al. The use of simulation in emergency medicine: a research agenda. Acad Emerg Med. 2007;14:353-363. doi:10.1197/j.aem.2006.11.02112.
- McLaughlin SA, Doezema D, Sklar DP. Human simulation in emergency medicine training: a model curriculum. Acad Emerg Med. 2002;9:1310-1318. doi:10.1111/j.1553-2712.2002.tb01593.x
- Howard SK, Gaba DM, Fish KJ, et al. Anesthesia crisis resource management training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63:763-770.
- Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785-788. doi:10.1136/bmj.320.7237.785
- Sedlack RE, Kolars JC. Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study. Am J Gastroenterol. 2004;99:33-37. doi:10.1111/j.1572-0241.2004.04007.x
- Tchopev ZN, Nelson AE, Hunninghake JC, et al. Curriculum innovations: high-fidelity simulation of acute neurology enhances rising resident confidence: results from a multicohort study. Neurol Educ. 2022;1:e200022. doi:10.1212/ne9.0000000000200022
- Mehta T, Strauss S, Beland D, et al. Stroke simulation improves acute stroke management: a systems-based practice experience. J Grad Med Educ. 2018;10:57-62. doi:10.4300/JGME-D-17-00167.1
- Pergakis MB, Chang WTW, Tabatabai A, et al. Simulation-based assessment of graduate neurology trainees’ performance managing acute ischemic stroke. Neurology. 2021;97:e2414-e2422. doi:10.1212/WNL.0000000000012972
- Casolla B. Simulation for neurology training: acute setting and beyond. Rev Neurol (Paris). 2021;177:1207-1213. doi:10.1016/j.neurol.2021.03.008
- Casolla B, de Leciñana MA, Neves R, et al. Simulation training programs for acute stroke care: Objectives and standards of methodology. Eur Stroke J. 2020;5:328-335. doi:10.1177/2396987320971105
- Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826-834.doi:10.1097/MLR.0b013e31819a5acc
- Patel RA, Mohl L, Paetow G, Maiser S. Acute neuromuscular respiratory weakness due to acute inflammatory demyelinating polyneuropathy (AIDP): a simulation scenario for neurology providers. MedEdPORTAL. 2019;15:10811. doi:10.15766/mep_2374-8265.10811
- Mikhaeil-Demo Y, Barsuk JH, Culler GW, et al. Use of a simulation-based mastery learning curriculum for neurology residents to improve the identification and management of status epilepticus. Epilepsy Behav. 2020;111:107247. doi:10.1016/j.yebeh.2020.107247
- Mikhaeil-Demo Y, Holmboe E, Gerard EE, et al. Simulation-based assessments and graduating neurology residents’ milestones: status epilepticus milestones. J Grad Med Educ. 2021;13:223-230. doi:10.4300/JGME-D-20-00832.1
- Hocker S, Wijdicks EFM, Feske SK, et al. Use of simulation in acute neurology training: point and counterpoint. Ann Neurol. 2015;78:337-342. doi:10.1002/ana.24473
- Gaubert S, Blet A, Dib F, et al. Positive effects of lumbar puncture simulation training for medical students in clinical practice. BMC Med Educ. 2021;21:1-6. doi:10.1186/S12909-020-02452-327.
- Yanta C, Knepper L, Van Deusen R, et al. The use of hybrid lumbar puncture simulation to teach entrustable professional activities during a medical student neurology clerkship. MedEdPublish (2016). 2021;9:266. doi:10.15694/mep.2020.000266.2
- Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79:132-137. doi:10.1212/WNL.0B013E31825DD39D
- McMillan HJ, Writer H, Moreau KA, et al. Lumbar puncture simulation in pediatric residency training: improving procedural competence and decreasing anxiety. BMC Med Educ. 2016;16:198. doi:10.1186/S12909-016-0722-1
- Vrillon A, Gonzales-Marabal L, Ceccaldi PF, et al. Using virtual reality in lumbar puncture training improves students learning experience. BMC Med Educ. 2022;22:244. doi:10.1186/S12909-022-03317-7
- Liebig T, Holtmannspötter M, Crossley R, et al. Metric-based virtual reality simulation: a paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018;49:e239-e242.doi:10.1161/STROKEAHA.118.021089
- Gupta DK, Khandker N, Stacy K, et al. Utility of combining a simulation-based method with a lecture-based method for fundoscopy training in neurology residency. JAMA Neurol. 2017;74:1223-1227. doi:10.1001/JAMANEUROL.2017.2073
- Mongodi S, Bonomi F, Vaschetto R, et al. Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ. 2022;22:647. doi:10.1186/S12909-022-03708-W
- Morris NA, Czeisler BM, Sarwal A. Simulation in neurocritical care: past, present, and future. Neurocrit Care. 2019;30:522-533. doi:10.1007/S12028-018-0629-2
- Wijdicks EFM, Hocker SE. A future for simulation in acute neurology. Semin Neurol. 2018;38:465-470. doi:10.1055/s-0038-1666986
- Kramer NM, O’Mahony S, Deamant C. Brain death determination and communication: an innovative approach using simulation and standardized patients. J Pain Symptom Manage. 2022;63:e765-e772. doi:10.1016/j.jpainsymman.2022.01.020
- Sloane KL, Miller JJ, Piquet A, et al. Prognostication in acute neurological emergencies. J Stroke Cerebrovasc Dis. 2022;31:106277. doi:10.1016/J.JSTROKECEREBROVASDIS.2021.106277
- Kurzweil AM, Lewis A, Pleninger P, et al. Education research: teaching and assessing communication and professionalism in neurology residency with simulation. Neurology. 2020;94:229-232. doi:10.1212/WNL.0000000000008895
- Morris NA, Chang WT, Tabatabai A, et al. Development of neurological emergency simulations for assessment: content evidence and response process. Neurocrit Care. 2021;35:389-396. doi:10.1007/S12028-020-01176-Y
- De Faria JWV, Teixeira MJ, De Moura Sousa Júnior L, et al. Virtual and stereoscopic anatomy: when virtual reality meets medical education. J Neurosurg. 2016;125:1105-1111. doi:10.3171/2015.8.JNS141563
- Corvetto M, Bravo MP, Montaña R, et al. Simulación en educación médica: una sinopsis. Rev Med Chil. 2013;141:70-79. doi:10.4067/S0034-98872013000100010
- Lane JL, Slavin S, Ziv A. Simulation in medical education: a review. Simul Gaming. 2001;32:297-314. doi:10.1177/104687810103200302
- Bradley P. The history of simulation in medical education and possible future directions. Med Educ. 2006;40:254-262. doi:10.1111/j.1365-2929.2006.02394.x
- Jones F, Passos-Neto C, Melro Braghiroli O. Simulation in medical education: brief history and methodology. Princ Pract Clin Res J. 2015;1:46-54. doi:10.21801/ppcrj.2015.12.8
- Issenberg SB. Simulation technology for health care professional skills training and assessment. JAMA. 1999;28:861-866. doi:10.1001/jama.282.9.861
- McGaghie WC, Miller GE, Sajid AW, et al. Competency-based curriculum development on medical education: an introduction. Public Health Pap. 1978;68:11-91.
- Barsuk JH, Cohen ER, Feinglass J, et al. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169:1420-1423. doi:10.1001/archinternmed.2009.215
- Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133:56-61. doi:10.1378/chest.07-0131
- McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86:706-711. doi:10.1097/ACM.0b013e318217e119
- Micieli G, Cavallini A, Santalucia P, et al. Simulation in neurology. Neurol Sci. 2015;36:1967-1971. doi:10.1007/s10072-015-2228-8
- Bond WF, Lammers RL, Spillane LL, et al. The use of simulation in emergency medicine: a research agenda. Acad Emerg Med. 2007;14:353-363. doi:10.1197/j.aem.2006.11.02112.
- McLaughlin SA, Doezema D, Sklar DP. Human simulation in emergency medicine training: a model curriculum. Acad Emerg Med. 2002;9:1310-1318. doi:10.1111/j.1553-2712.2002.tb01593.x
- Howard SK, Gaba DM, Fish KJ, et al. Anesthesia crisis resource management training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63:763-770.
- Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785-788. doi:10.1136/bmj.320.7237.785
- Sedlack RE, Kolars JC. Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study. Am J Gastroenterol. 2004;99:33-37. doi:10.1111/j.1572-0241.2004.04007.x
- Tchopev ZN, Nelson AE, Hunninghake JC, et al. Curriculum innovations: high-fidelity simulation of acute neurology enhances rising resident confidence: results from a multicohort study. Neurol Educ. 2022;1:e200022. doi:10.1212/ne9.0000000000200022
- Mehta T, Strauss S, Beland D, et al. Stroke simulation improves acute stroke management: a systems-based practice experience. J Grad Med Educ. 2018;10:57-62. doi:10.4300/JGME-D-17-00167.1
- Pergakis MB, Chang WTW, Tabatabai A, et al. Simulation-based assessment of graduate neurology trainees’ performance managing acute ischemic stroke. Neurology. 2021;97:e2414-e2422. doi:10.1212/WNL.0000000000012972
- Casolla B. Simulation for neurology training: acute setting and beyond. Rev Neurol (Paris). 2021;177:1207-1213. doi:10.1016/j.neurol.2021.03.008
- Casolla B, de Leciñana MA, Neves R, et al. Simulation training programs for acute stroke care: Objectives and standards of methodology. Eur Stroke J. 2020;5:328-335. doi:10.1177/2396987320971105
- Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826-834.doi:10.1097/MLR.0b013e31819a5acc
- Patel RA, Mohl L, Paetow G, Maiser S. Acute neuromuscular respiratory weakness due to acute inflammatory demyelinating polyneuropathy (AIDP): a simulation scenario for neurology providers. MedEdPORTAL. 2019;15:10811. doi:10.15766/mep_2374-8265.10811
- Mikhaeil-Demo Y, Barsuk JH, Culler GW, et al. Use of a simulation-based mastery learning curriculum for neurology residents to improve the identification and management of status epilepticus. Epilepsy Behav. 2020;111:107247. doi:10.1016/j.yebeh.2020.107247
- Mikhaeil-Demo Y, Holmboe E, Gerard EE, et al. Simulation-based assessments and graduating neurology residents’ milestones: status epilepticus milestones. J Grad Med Educ. 2021;13:223-230. doi:10.4300/JGME-D-20-00832.1
- Hocker S, Wijdicks EFM, Feske SK, et al. Use of simulation in acute neurology training: point and counterpoint. Ann Neurol. 2015;78:337-342. doi:10.1002/ana.24473
- Gaubert S, Blet A, Dib F, et al. Positive effects of lumbar puncture simulation training for medical students in clinical practice. BMC Med Educ. 2021;21:1-6. doi:10.1186/S12909-020-02452-327.
- Yanta C, Knepper L, Van Deusen R, et al. The use of hybrid lumbar puncture simulation to teach entrustable professional activities during a medical student neurology clerkship. MedEdPublish (2016). 2021;9:266. doi:10.15694/mep.2020.000266.2
- Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79:132-137. doi:10.1212/WNL.0B013E31825DD39D
- McMillan HJ, Writer H, Moreau KA, et al. Lumbar puncture simulation in pediatric residency training: improving procedural competence and decreasing anxiety. BMC Med Educ. 2016;16:198. doi:10.1186/S12909-016-0722-1
- Vrillon A, Gonzales-Marabal L, Ceccaldi PF, et al. Using virtual reality in lumbar puncture training improves students learning experience. BMC Med Educ. 2022;22:244. doi:10.1186/S12909-022-03317-7
- Liebig T, Holtmannspötter M, Crossley R, et al. Metric-based virtual reality simulation: a paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018;49:e239-e242.doi:10.1161/STROKEAHA.118.021089
- Gupta DK, Khandker N, Stacy K, et al. Utility of combining a simulation-based method with a lecture-based method for fundoscopy training in neurology residency. JAMA Neurol. 2017;74:1223-1227. doi:10.1001/JAMANEUROL.2017.2073
- Mongodi S, Bonomi F, Vaschetto R, et al. Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ. 2022;22:647. doi:10.1186/S12909-022-03708-W
- Morris NA, Czeisler BM, Sarwal A. Simulation in neurocritical care: past, present, and future. Neurocrit Care. 2019;30:522-533. doi:10.1007/S12028-018-0629-2
- Wijdicks EFM, Hocker SE. A future for simulation in acute neurology. Semin Neurol. 2018;38:465-470. doi:10.1055/s-0038-1666986
- Kramer NM, O’Mahony S, Deamant C. Brain death determination and communication: an innovative approach using simulation and standardized patients. J Pain Symptom Manage. 2022;63:e765-e772. doi:10.1016/j.jpainsymman.2022.01.020
- Sloane KL, Miller JJ, Piquet A, et al. Prognostication in acute neurological emergencies. J Stroke Cerebrovasc Dis. 2022;31:106277. doi:10.1016/J.JSTROKECEREBROVASDIS.2021.106277
- Kurzweil AM, Lewis A, Pleninger P, et al. Education research: teaching and assessing communication and professionalism in neurology residency with simulation. Neurology. 2020;94:229-232. doi:10.1212/WNL.0000000000008895
- Morris NA, Chang WT, Tabatabai A, et al. Development of neurological emergency simulations for assessment: content evidence and response process. Neurocrit Care. 2021;35:389-396. doi:10.1007/S12028-020-01176-Y
- De Faria JWV, Teixeira MJ, De Moura Sousa Júnior L, et al. Virtual and stereoscopic anatomy: when virtual reality meets medical education. J Neurosurg. 2016;125:1105-1111. doi:10.3171/2015.8.JNS141563
Sim and Learn: Simulation and its Value in Neurology Education
Sim and Learn: Simulation and its Value in Neurology Education
Improving Interprofessional Neurology Training Using Tele-Education
Improving Interprofessional Neurology Training Using Tele-Education
Neurologic disorders are major causes of death and disability. Globally, the burden of neurologic disorders continues to increase. The prevalence of disabling neurologic disorders significantly increases with age. As people live longer, health care systems will face increasing demands for treatment, rehabilitation, and support services for neurologic disorders. The scarcity of established modifiable risks for most of the neurologic burden demonstrates how new knowledge is required to develop effective prevention and treatment strategies.1
A single-center study for chronic headache at a rural institution found that, when combined with public education, clinician education not only can increase access to care but also reduce specialist overuse, hospitalizations, polypharmacy, and emergency department visits.2 A predicted shortage of neurologists has sparked increased interest in the field and individual neurology educators are helping fuel its popularity.3-5
TELE-EDUCATION
Educating the next generation of health professionals is 1 of 4 statutory missions of the US Department of Veterans Affairs (VA).6 Tele-education (also known as telelearning and distance learning) deviates from traditional in-person classroom settings, in which the lecture has been a core pedagogic method.7 Audio, video, and online technologies provide health education and can overcome geographic barriers for rural and remote clinicians.8 Recent technological improvements have allowed for inexpensive and efficient dissemination of educational materials, including video lectures, podcasts, online modules, assessment materials, and even entire curricula.9
There has been an increase in the awareness of the parallel curriculum involving self-directed and asynchronous learning opportunities. 10 Several studies report knowledge gained via tele-education is comparable to conventional classroom learning.11-13 A systematic review of e-learning perceptions among health care students suggested benefits (eg, learning flexibility, pedagogical design, online interactions, basic computer skills, and access to technology) and drawbacks (eg, limited acquisition of clinical skills, internet connection problems, and issues with using educational platforms).1
The COVID-19 pandemic forced an abrupt cessation of traditional in-person education, forcing educational institutions and medical organizations to transition to telelearning. Solutions in the education field appeared during the pandemic, such as videoconferencing, social media, and telemedicine, that effectively addressed the sudden cessation of in-person medical education.15
Graduate medical education in neurology residency programs served as an experimental set up for tele-education during the pandemic. Residents from neurology training programs outlined the benefits of a volunteer lecturer-based online didactic program that was established to meet this need, which included exposure to subspeciality topics, access to subspecialist experts not available within the department, exposure to different pedagogic methods, interaction with members of other educational institutions and training programs, career development opportunities, and the potential for forming a community of learning.16
Not all recent educational developments are technology-based. For example, instruction focused on specific patient experiences, and learning processes that emphasize problem solving and personal responsibility over specific knowledge have been successful in neurology.17,18 Departments and institutions must be creative in finding ways to fund continuing education, especially when budgets are limited.19
ANNUAL NEUROLOGY SEMINAR
An annual Veterans Health Administration (VHA) neurology seminar began in 2019 as a 1-day in-person event. Neurologists at the Michael E. DeBakey VA Medical Center in Houston presented in 50-minute sessions. Nonspecialist clinical personnel and neurology clinicians attended the event. Attendees requested making the presentations widely available and regularly repeating the seminar.
The second neurology seminar took place during the COVID-19 pandemic. It was conducted online and advertised across the Veterans Integrated Services Network (VISN) 16. The 1-day program had 204 participants who were primarily nurses (59%) and physicians (21%); 94% agreed with the program objectives (Table 1). Participants could earn CME credits for the 7 presentations primarily by VHA experts.
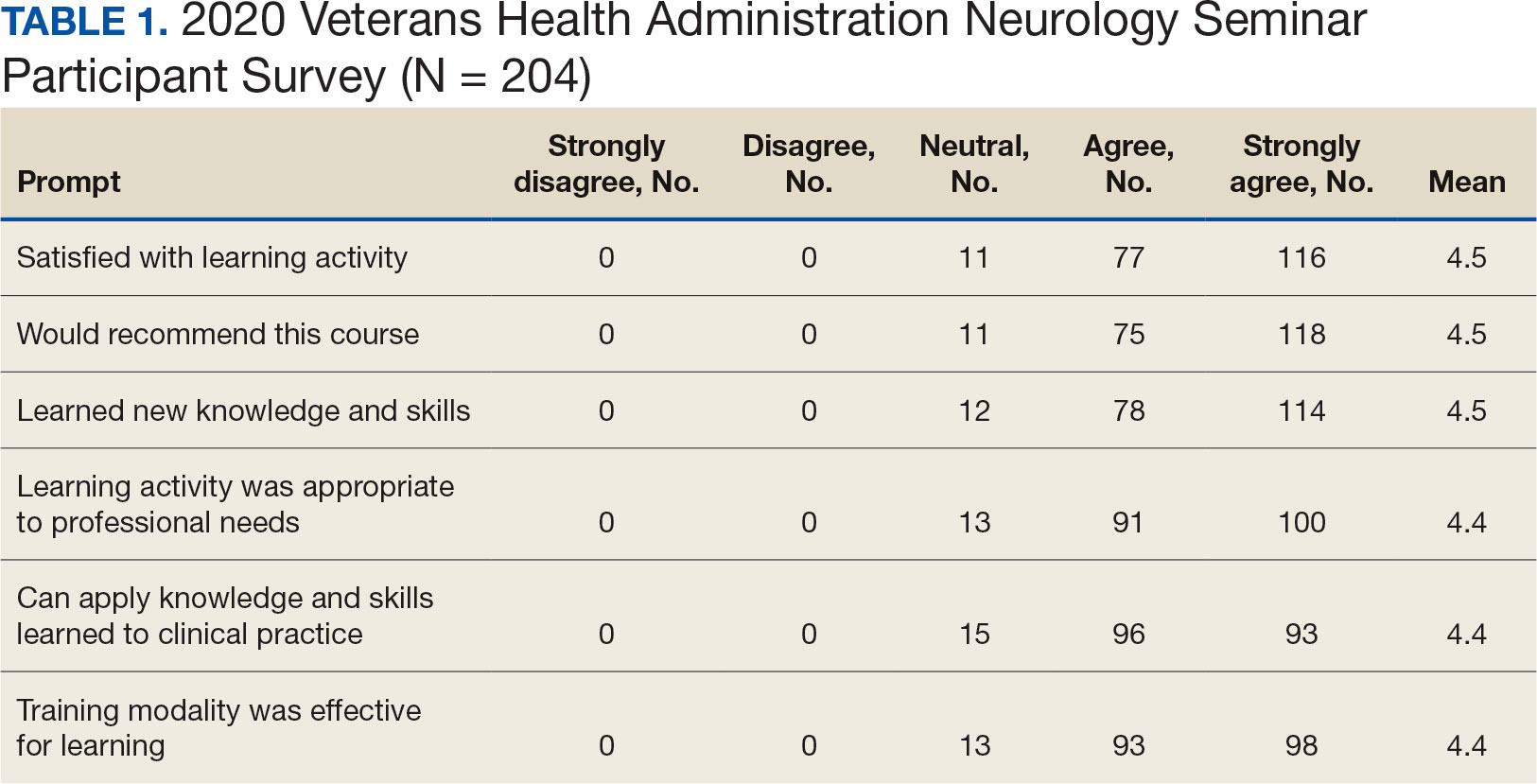
Based on feedback and a needs assessment, the program expanded in 2021 and 2022. With support from the national VHA neurology office and VHA Employee Education System (EES), the Institute for Learning, Education, and Development (ILEAD), the feedback identified topics that resonate with VHA clinicians. Neurological disorders in the fields of stroke, dementia, and headache were included since veterans with these disorders regularly visit primary care, geriatrics, mental health, and other clinical offices. Updates provided in the diagnosis and treatment of common neurological disorders were well received. Almost all speakers were VHA clinicians, which allowed them to focus on topics relevant to clinical practice at the VHA.
Attendance has increased annually. In 2021, 550 clinicians registered (52% nurses) and 433 completed the postseminar survey (Table 2). In 2022, 635 participants registered and 342 completed evaluations, including attendees from other federal agencies who were invited to participate via EES TRAIN (Training Finder Real-time Affiliate Integrated Network). Forty-seven participants from other federal agencies, including the US Department of Defense, National Institute of Health, and Centers for Disease Control and Prevention, completed the feedback evaluation via TRAIN (Table 3). Participants report high levels of satisfaction each year (mean of 4.5 on a 5-point scale). Respondents preferred conventional lecture presentation and case-based discussions for the teaching format and dementia was the most requested topic for future seminars (Table 4).
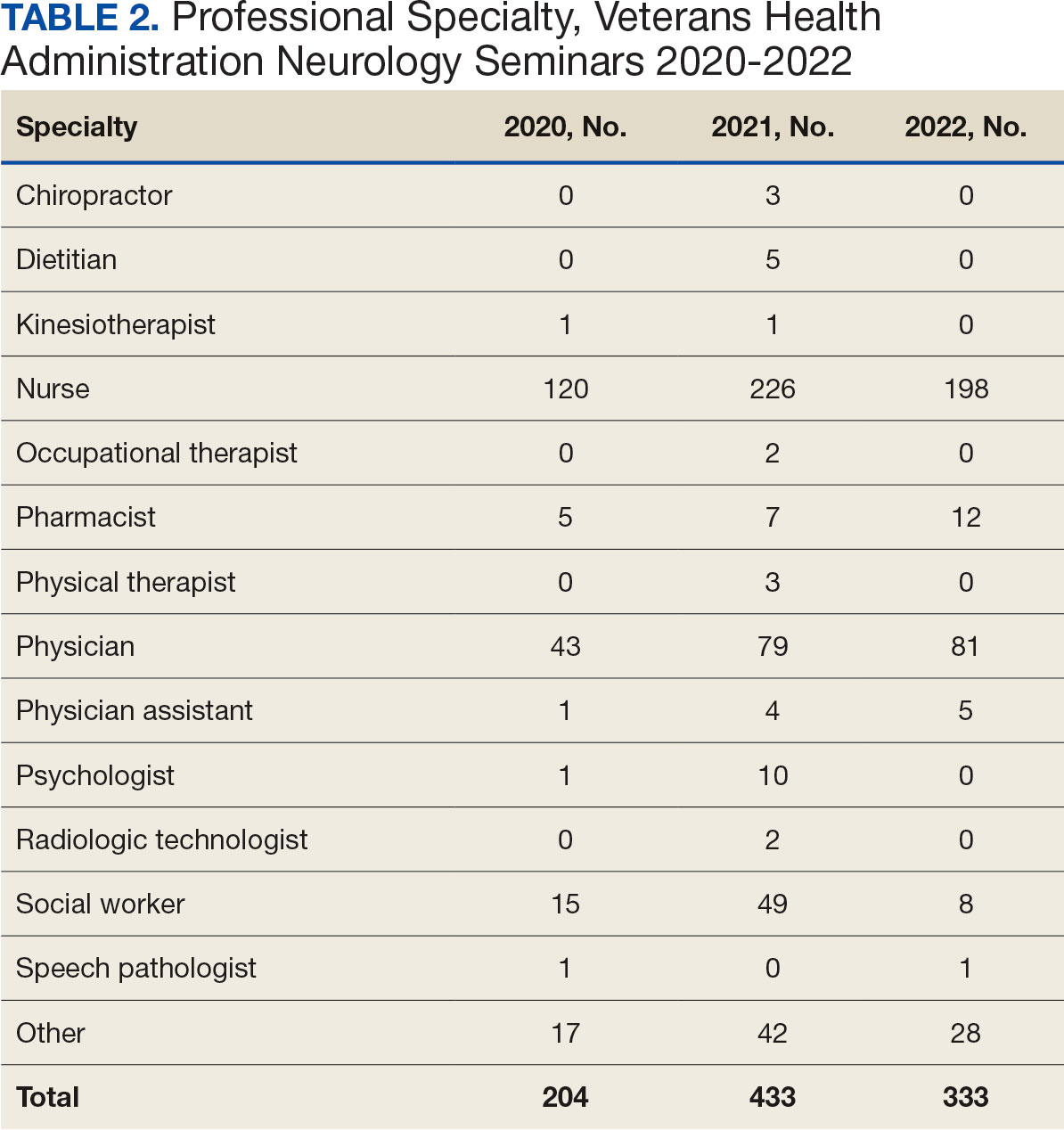

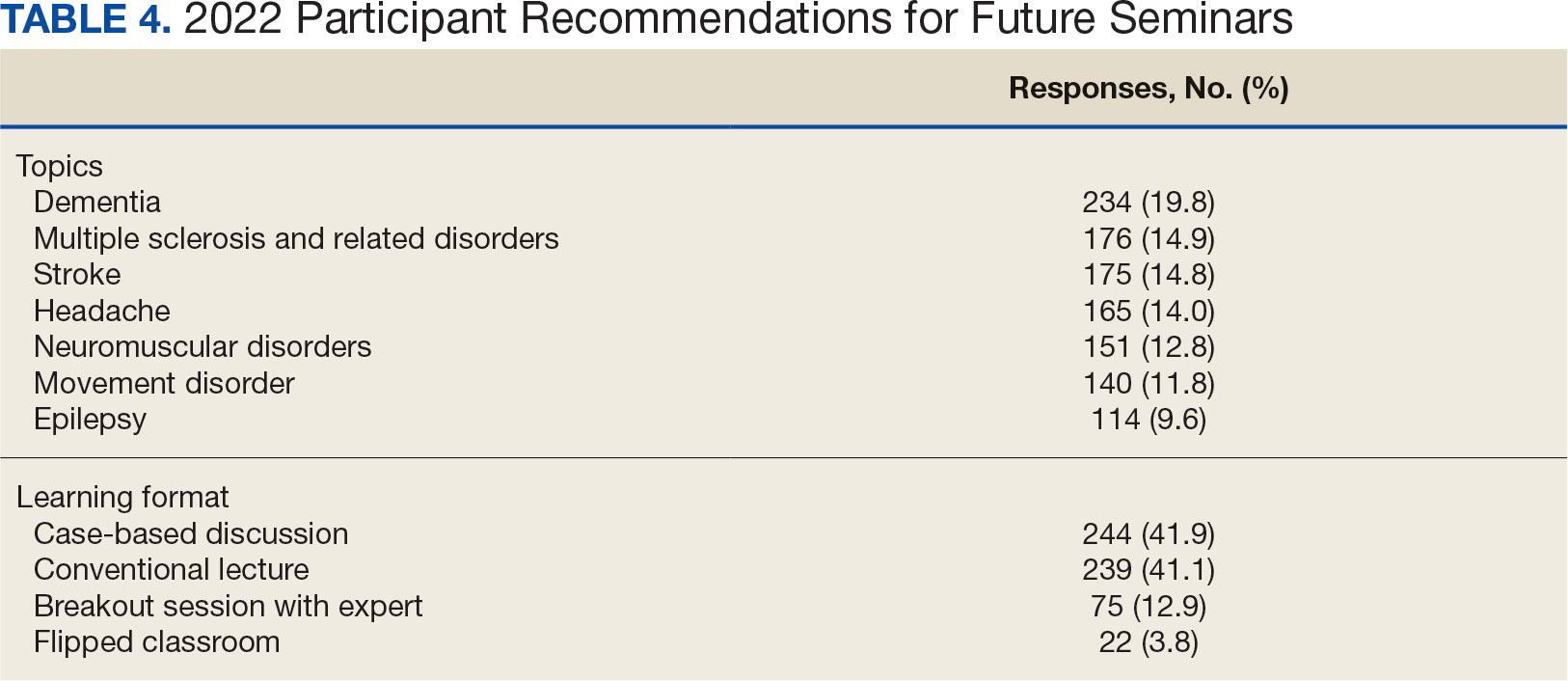
The content of each seminar was designed to include . 1 topic relevant to current clinical practice. The 2020 seminar covered topics of cerebrovascular complications of COVID- 19 and living well with neurodegenerative disease in the COVID-19 era. In 2021, the seminar included COVID-19 and neurologic manifestations. In 2022, topics included trends in stroke rehabilitation. In addition, ≥ 1 session addressed neurologic issues within the VHA. In 2020, the VA Deputy National Director of Neurology presented on the VHA stroke systems of care. In 2021, there was a presentation on traumatic brain injury (TBI) in the military. In 2022, sessions covered long term neurologic consequences of TBI and use of telemedicine for neurologic disorders. Feedback on the sessions were positive (eAppendix, available at doi:10.12788/fp.0545).

At the request of the participants, individual presentations were shared via email by the course director and speakers. In collaboration with the EES, each session was recorded and the 2022 seminar was made available to registrants in TMS and EES TRAIN and via the VHA Neurology SharePoint.
DISCUSSION
The annual VHA neurology seminar is a 1-day neurology conference that provides education to general neurologists and other clinicians caring for patients with neurologic disorders. It is the first of its kind neurology education program in the VHA covering most subspecialties in neurology and aims at improving neurologic patient care and access through education. Sessions have covered stroke, epilepsy, sleep, amyotrophic lateral sclerosis, neuropathy, dementia, movement disorders and Parkinson disease, headaches, multiple sclerosis, neurorehabilitation, and telehealth.
The seminar has transitioned from an inperson meeting to a virtual format, making neurology education more convenient and accessible. The virtual format provides the means to increase educational collaborations and share lecture platforms with other federal agencies. The program offers CME credits at no cost to government employees. Recorded lectures can also be asynchronously viewed from the Neurology SharePoint without the ability to earn CME credits. These recordings may be used to educate trainees as well.
The seminar aims to educate all health care professionals caring for patients with neurologic disorders. It aims to eliminate neurophobia, the fear of neural sciences and clinical neurology, and help general practitioners, especially in rural areas, take care of patients with neurologic disorders. The seminars introduce general practitioners to VHA neurology experts; the epilepsy, headache multiple sclerosis, and Parkinson disease centers of excellence; and the national programs for telestroke and teleneurology.
Education Support in the VHA
The EES/ILEAD provides a wide variety of learning opportunities to VHA employees on a broad range of topics, making it one of the largest medical education programs in the country. Pharmacists, social workers, psychologists, therapists, nurses, physician assistants, and physicians have access to certified training opportunities to gain knowledge and skills needed to provide high-quality, veteran-centered care.
A review of geriatrics learning activities through the EES found > 15,000 lectures from 1999 to 2009 for > 300,000 attendees.20 To our knowledge, a review of neurology-related learning activities offered by the EES/ILEAD has not been completed, but the study on geriatrics shows that a similar review would be feasible, given the integrated education system, and helpful in identifying what topics are covered, formats are used, and participants are engaged in neurology education at the VHA. This is a future project planned by the neurology education workgroup.
The EES/ILEAD arranged CME credit for the VHA Neurology Seminar and assisted in organizing an online event with > 500 attendees. Technology support and tools provided by EES during the virtual seminar, such as polling and chat features, kept the audience engaged. Other specialties may similarly value a virtual, all-day seminar format that is efficient and can encourage increased participation from practitioners, nurses, and clinicians.
Future Growth
We plan to increase future participation in the annual neurology seminar with primary care, geriatrics, neurology, and other specialties by instituting an improved and earlier marketing strategy. This includes working with the VHA neurology office to inform neurology practitioners as well as other program offices in the VHA. We intend to host the seminar the same day every year to make it easy for attendees to plan accordingly. In the future we may consider hybrid in-person and virtual modalities if feasible. We plan to focus on reaching out to other government agencies through platforms like TRAIN and the American Academy of Neurology government sections. Securing funding, administrative staff, and protected time in the future may help expand the program further.
Limitations
While a virtual format offers several advantages, using it removes the feel of an in-person meeting, which could be viewed by some attendees as a limitation. The other challenges and drawbacks of transitioning to the virtual platform for a national meeting are similar to those reported in the literature: time zone differences, internet issues, and participants having difficulty using certain online platforms. Attendance could also be limited by scheduling conflicts.16 Despite a large audience attending the seminar, many clinicians do not get protected time from their institutions. Institutional and leadership support at national and local levels will likely improve participation and help participants earn CME credits. While we are still doing a preliminary needs assessment, a formal needs assessment across federal governmental organizations will be helpful.
CONCLUSIONS
The annual VHA neurology seminar promotes interprofessional education, introduces neurology subspecialty centers of excellence, improves access to renowned neurology experts, and provides neurology-related updates through a VHA lens. The program not only provides educational updates to neurology clinicians, but also increases the confidence of non-neurology clinicians called to care for veterans with neurological disorders in their respective clinics.
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990- 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. doi:10.1016/S1474-4422(18)30499-X
- Baker V, Hack N. Improving access to care for patients with migraine in a remote Pacific population. Neurol Clin Pract. 2020;10(5):444-448. doi:10.1212/CPJ.0000000000000774
- Gutmann L, Cahill C, Jordan JT, et al. Characteristics of graduating US allopathic medical students pursuing a career in neurology. Neurology. 2019;92(17):e2051-e2063. doi:10.1212/WNL.0000000000007369
- Jordan JT, Cahill C, Ostendorf T, et al. Attracting neurology’s next generation: a qualitative study of specialty choice and perceptions. Neurology. 2020;95(8):e1080- e1090. doi:10.1212/WNL.0000000000009461
- Minen MT, Kaplan K, Akter S, et al. Understanding how to strengthen the neurology pipeline with insights from undergraduate neuroscience students. Neurology 2022;98(8):314-323. doi:10.1212/WNL.0000000000013259
- US Department of Veterans Affairs, Office of Academic Affiliations. To Educate for VA and the Nation. Updated August 1, 2024. Accessed August 15, 2024. https://www.va.gov/oaa/
- Schaefer SM, Dominguez M, Moeller JJ. The future of the lecture in neurology education. Semin Neurol. 2018;38(4):418-427. doi:10.1055/s-0038-1667042
- Curran VR. Tele-education. J Telemed Telecare. 2006;12(2):57-63. doi:10.1258/135763306776084400
- Lau KHV, Lakhan SE, Achike F. New media, technology and neurology education. Semin Neurol. 2018;38(4):457- 464. doi:10.1055/s-0038-1666985
- Quirk M, Chumley H. The adaptive medical curriculum: a model for continuous improvement. Med Teach. 2018;40(8):786-790. doi:10.1080/0142159X.2018.1484896
- Brockfeld T, Müller B, de Laffolie J. Video versus live lecture courses: a comparative evaluation of lecture types and results. Med Educ Online. 2018;23(1):1555434. doi:10.1080/10872981.2018.1555434
- Davis J, Crabb S, Rogers E, Zamora J, Khan K. Computer-based teaching is as good as face to face lecture-based teaching of evidence based medicine: a randomized controlled trial. Med Teach. 2008;30(3):302-307. doi:10.1080/01421590701784349
- Markova T, Roth LM, Monsur J. Synchronous distance learning as an effective and feasible method for delivering residency didactics. Fam Med. 2005;37(8):570-575.
- Naciri A, Radid M, Kharbach A, Chemsi G. E-learning in health professions education during the COVID-19 pandemic: a systematic review. J Educ Eval Health Prof. 2021;18:27. doi:10.3352/jeehp.2021.18.27
- Dedeilia A, Sotiropoulos MG, Hanrahan JG, Janga D, Dedeilias P, Sideris M. Medical and surgical education challenges and innovations in the COVID-19 era: a systematic review. In Vivo. 2020;34(3 Suppl):1603-1611. doi:10.21873/invivo.11950
- Weber DJ, Albert DVF, Aravamuthan BR, Bernson-Leung ME, Bhatti D, Milligan TA. Training in neurology: rapid implementation of cross-institutional neurology resident education in the time of COVID-19. Neurology. 2020;95(19):883-886. doi:10.1212/WNL.0000000000010753
- Frey J, Neeley B, Umer A, et al. Training in neurology: neuro day: an innovative curriculum connecting medical students with patients. Neurology. 2021;96(10):e1482- e1486. doi:10.1212/WNL.0000000000010859
- Schwartzstein RM, Dienstag JL, King RW, et al. The Harvard Medical School Pathways Curriculum: reimagining developmentally appropriate medical education for contemporary learners. Acad Med. 2020;95(11):1687-1695. doi:10.1097/ACM.0000000000003270
- Greer DM, Moeller J, Torres DR, et al. Funding the educational mission in neurology. Neurology. 2021;96(12):574- 582. doi:10.1212/WNL.0000000000011635
- Thielke S, Tumosa N, Lindenfeld R, Shay K. Geriatric focused educational offerings in the Department of Veterans Affairs from 1999 to 2009. Gerontol Geriatr Educ. 2011;32(1):38-53. doi:10.1080/02701960.2011.550214
Neurologic disorders are major causes of death and disability. Globally, the burden of neurologic disorders continues to increase. The prevalence of disabling neurologic disorders significantly increases with age. As people live longer, health care systems will face increasing demands for treatment, rehabilitation, and support services for neurologic disorders. The scarcity of established modifiable risks for most of the neurologic burden demonstrates how new knowledge is required to develop effective prevention and treatment strategies.1
A single-center study for chronic headache at a rural institution found that, when combined with public education, clinician education not only can increase access to care but also reduce specialist overuse, hospitalizations, polypharmacy, and emergency department visits.2 A predicted shortage of neurologists has sparked increased interest in the field and individual neurology educators are helping fuel its popularity.3-5
TELE-EDUCATION
Educating the next generation of health professionals is 1 of 4 statutory missions of the US Department of Veterans Affairs (VA).6 Tele-education (also known as telelearning and distance learning) deviates from traditional in-person classroom settings, in which the lecture has been a core pedagogic method.7 Audio, video, and online technologies provide health education and can overcome geographic barriers for rural and remote clinicians.8 Recent technological improvements have allowed for inexpensive and efficient dissemination of educational materials, including video lectures, podcasts, online modules, assessment materials, and even entire curricula.9
There has been an increase in the awareness of the parallel curriculum involving self-directed and asynchronous learning opportunities. 10 Several studies report knowledge gained via tele-education is comparable to conventional classroom learning.11-13 A systematic review of e-learning perceptions among health care students suggested benefits (eg, learning flexibility, pedagogical design, online interactions, basic computer skills, and access to technology) and drawbacks (eg, limited acquisition of clinical skills, internet connection problems, and issues with using educational platforms).1
The COVID-19 pandemic forced an abrupt cessation of traditional in-person education, forcing educational institutions and medical organizations to transition to telelearning. Solutions in the education field appeared during the pandemic, such as videoconferencing, social media, and telemedicine, that effectively addressed the sudden cessation of in-person medical education.15
Graduate medical education in neurology residency programs served as an experimental set up for tele-education during the pandemic. Residents from neurology training programs outlined the benefits of a volunteer lecturer-based online didactic program that was established to meet this need, which included exposure to subspeciality topics, access to subspecialist experts not available within the department, exposure to different pedagogic methods, interaction with members of other educational institutions and training programs, career development opportunities, and the potential for forming a community of learning.16
Not all recent educational developments are technology-based. For example, instruction focused on specific patient experiences, and learning processes that emphasize problem solving and personal responsibility over specific knowledge have been successful in neurology.17,18 Departments and institutions must be creative in finding ways to fund continuing education, especially when budgets are limited.19
ANNUAL NEUROLOGY SEMINAR
An annual Veterans Health Administration (VHA) neurology seminar began in 2019 as a 1-day in-person event. Neurologists at the Michael E. DeBakey VA Medical Center in Houston presented in 50-minute sessions. Nonspecialist clinical personnel and neurology clinicians attended the event. Attendees requested making the presentations widely available and regularly repeating the seminar.
The second neurology seminar took place during the COVID-19 pandemic. It was conducted online and advertised across the Veterans Integrated Services Network (VISN) 16. The 1-day program had 204 participants who were primarily nurses (59%) and physicians (21%); 94% agreed with the program objectives (Table 1). Participants could earn CME credits for the 7 presentations primarily by VHA experts.

Based on feedback and a needs assessment, the program expanded in 2021 and 2022. With support from the national VHA neurology office and VHA Employee Education System (EES), the Institute for Learning, Education, and Development (ILEAD), the feedback identified topics that resonate with VHA clinicians. Neurological disorders in the fields of stroke, dementia, and headache were included since veterans with these disorders regularly visit primary care, geriatrics, mental health, and other clinical offices. Updates provided in the diagnosis and treatment of common neurological disorders were well received. Almost all speakers were VHA clinicians, which allowed them to focus on topics relevant to clinical practice at the VHA.
Attendance has increased annually. In 2021, 550 clinicians registered (52% nurses) and 433 completed the postseminar survey (Table 2). In 2022, 635 participants registered and 342 completed evaluations, including attendees from other federal agencies who were invited to participate via EES TRAIN (Training Finder Real-time Affiliate Integrated Network). Forty-seven participants from other federal agencies, including the US Department of Defense, National Institute of Health, and Centers for Disease Control and Prevention, completed the feedback evaluation via TRAIN (Table 3). Participants report high levels of satisfaction each year (mean of 4.5 on a 5-point scale). Respondents preferred conventional lecture presentation and case-based discussions for the teaching format and dementia was the most requested topic for future seminars (Table 4).



The content of each seminar was designed to include . 1 topic relevant to current clinical practice. The 2020 seminar covered topics of cerebrovascular complications of COVID- 19 and living well with neurodegenerative disease in the COVID-19 era. In 2021, the seminar included COVID-19 and neurologic manifestations. In 2022, topics included trends in stroke rehabilitation. In addition, ≥ 1 session addressed neurologic issues within the VHA. In 2020, the VA Deputy National Director of Neurology presented on the VHA stroke systems of care. In 2021, there was a presentation on traumatic brain injury (TBI) in the military. In 2022, sessions covered long term neurologic consequences of TBI and use of telemedicine for neurologic disorders. Feedback on the sessions were positive (eAppendix, available at doi:10.12788/fp.0545).

At the request of the participants, individual presentations were shared via email by the course director and speakers. In collaboration with the EES, each session was recorded and the 2022 seminar was made available to registrants in TMS and EES TRAIN and via the VHA Neurology SharePoint.
DISCUSSION
The annual VHA neurology seminar is a 1-day neurology conference that provides education to general neurologists and other clinicians caring for patients with neurologic disorders. It is the first of its kind neurology education program in the VHA covering most subspecialties in neurology and aims at improving neurologic patient care and access through education. Sessions have covered stroke, epilepsy, sleep, amyotrophic lateral sclerosis, neuropathy, dementia, movement disorders and Parkinson disease, headaches, multiple sclerosis, neurorehabilitation, and telehealth.
The seminar has transitioned from an inperson meeting to a virtual format, making neurology education more convenient and accessible. The virtual format provides the means to increase educational collaborations and share lecture platforms with other federal agencies. The program offers CME credits at no cost to government employees. Recorded lectures can also be asynchronously viewed from the Neurology SharePoint without the ability to earn CME credits. These recordings may be used to educate trainees as well.
The seminar aims to educate all health care professionals caring for patients with neurologic disorders. It aims to eliminate neurophobia, the fear of neural sciences and clinical neurology, and help general practitioners, especially in rural areas, take care of patients with neurologic disorders. The seminars introduce general practitioners to VHA neurology experts; the epilepsy, headache multiple sclerosis, and Parkinson disease centers of excellence; and the national programs for telestroke and teleneurology.
Education Support in the VHA
The EES/ILEAD provides a wide variety of learning opportunities to VHA employees on a broad range of topics, making it one of the largest medical education programs in the country. Pharmacists, social workers, psychologists, therapists, nurses, physician assistants, and physicians have access to certified training opportunities to gain knowledge and skills needed to provide high-quality, veteran-centered care.
A review of geriatrics learning activities through the EES found > 15,000 lectures from 1999 to 2009 for > 300,000 attendees.20 To our knowledge, a review of neurology-related learning activities offered by the EES/ILEAD has not been completed, but the study on geriatrics shows that a similar review would be feasible, given the integrated education system, and helpful in identifying what topics are covered, formats are used, and participants are engaged in neurology education at the VHA. This is a future project planned by the neurology education workgroup.
The EES/ILEAD arranged CME credit for the VHA Neurology Seminar and assisted in organizing an online event with > 500 attendees. Technology support and tools provided by EES during the virtual seminar, such as polling and chat features, kept the audience engaged. Other specialties may similarly value a virtual, all-day seminar format that is efficient and can encourage increased participation from practitioners, nurses, and clinicians.
Future Growth
We plan to increase future participation in the annual neurology seminar with primary care, geriatrics, neurology, and other specialties by instituting an improved and earlier marketing strategy. This includes working with the VHA neurology office to inform neurology practitioners as well as other program offices in the VHA. We intend to host the seminar the same day every year to make it easy for attendees to plan accordingly. In the future we may consider hybrid in-person and virtual modalities if feasible. We plan to focus on reaching out to other government agencies through platforms like TRAIN and the American Academy of Neurology government sections. Securing funding, administrative staff, and protected time in the future may help expand the program further.
Limitations
While a virtual format offers several advantages, using it removes the feel of an in-person meeting, which could be viewed by some attendees as a limitation. The other challenges and drawbacks of transitioning to the virtual platform for a national meeting are similar to those reported in the literature: time zone differences, internet issues, and participants having difficulty using certain online platforms. Attendance could also be limited by scheduling conflicts.16 Despite a large audience attending the seminar, many clinicians do not get protected time from their institutions. Institutional and leadership support at national and local levels will likely improve participation and help participants earn CME credits. While we are still doing a preliminary needs assessment, a formal needs assessment across federal governmental organizations will be helpful.
CONCLUSIONS
The annual VHA neurology seminar promotes interprofessional education, introduces neurology subspecialty centers of excellence, improves access to renowned neurology experts, and provides neurology-related updates through a VHA lens. The program not only provides educational updates to neurology clinicians, but also increases the confidence of non-neurology clinicians called to care for veterans with neurological disorders in their respective clinics.
Neurologic disorders are major causes of death and disability. Globally, the burden of neurologic disorders continues to increase. The prevalence of disabling neurologic disorders significantly increases with age. As people live longer, health care systems will face increasing demands for treatment, rehabilitation, and support services for neurologic disorders. The scarcity of established modifiable risks for most of the neurologic burden demonstrates how new knowledge is required to develop effective prevention and treatment strategies.1
A single-center study for chronic headache at a rural institution found that, when combined with public education, clinician education not only can increase access to care but also reduce specialist overuse, hospitalizations, polypharmacy, and emergency department visits.2 A predicted shortage of neurologists has sparked increased interest in the field and individual neurology educators are helping fuel its popularity.3-5
TELE-EDUCATION
Educating the next generation of health professionals is 1 of 4 statutory missions of the US Department of Veterans Affairs (VA).6 Tele-education (also known as telelearning and distance learning) deviates from traditional in-person classroom settings, in which the lecture has been a core pedagogic method.7 Audio, video, and online technologies provide health education and can overcome geographic barriers for rural and remote clinicians.8 Recent technological improvements have allowed for inexpensive and efficient dissemination of educational materials, including video lectures, podcasts, online modules, assessment materials, and even entire curricula.9
There has been an increase in the awareness of the parallel curriculum involving self-directed and asynchronous learning opportunities. 10 Several studies report knowledge gained via tele-education is comparable to conventional classroom learning.11-13 A systematic review of e-learning perceptions among health care students suggested benefits (eg, learning flexibility, pedagogical design, online interactions, basic computer skills, and access to technology) and drawbacks (eg, limited acquisition of clinical skills, internet connection problems, and issues with using educational platforms).1
The COVID-19 pandemic forced an abrupt cessation of traditional in-person education, forcing educational institutions and medical organizations to transition to telelearning. Solutions in the education field appeared during the pandemic, such as videoconferencing, social media, and telemedicine, that effectively addressed the sudden cessation of in-person medical education.15
Graduate medical education in neurology residency programs served as an experimental set up for tele-education during the pandemic. Residents from neurology training programs outlined the benefits of a volunteer lecturer-based online didactic program that was established to meet this need, which included exposure to subspeciality topics, access to subspecialist experts not available within the department, exposure to different pedagogic methods, interaction with members of other educational institutions and training programs, career development opportunities, and the potential for forming a community of learning.16
Not all recent educational developments are technology-based. For example, instruction focused on specific patient experiences, and learning processes that emphasize problem solving and personal responsibility over specific knowledge have been successful in neurology.17,18 Departments and institutions must be creative in finding ways to fund continuing education, especially when budgets are limited.19
ANNUAL NEUROLOGY SEMINAR
An annual Veterans Health Administration (VHA) neurology seminar began in 2019 as a 1-day in-person event. Neurologists at the Michael E. DeBakey VA Medical Center in Houston presented in 50-minute sessions. Nonspecialist clinical personnel and neurology clinicians attended the event. Attendees requested making the presentations widely available and regularly repeating the seminar.
The second neurology seminar took place during the COVID-19 pandemic. It was conducted online and advertised across the Veterans Integrated Services Network (VISN) 16. The 1-day program had 204 participants who were primarily nurses (59%) and physicians (21%); 94% agreed with the program objectives (Table 1). Participants could earn CME credits for the 7 presentations primarily by VHA experts.

Based on feedback and a needs assessment, the program expanded in 2021 and 2022. With support from the national VHA neurology office and VHA Employee Education System (EES), the Institute for Learning, Education, and Development (ILEAD), the feedback identified topics that resonate with VHA clinicians. Neurological disorders in the fields of stroke, dementia, and headache were included since veterans with these disorders regularly visit primary care, geriatrics, mental health, and other clinical offices. Updates provided in the diagnosis and treatment of common neurological disorders were well received. Almost all speakers were VHA clinicians, which allowed them to focus on topics relevant to clinical practice at the VHA.
Attendance has increased annually. In 2021, 550 clinicians registered (52% nurses) and 433 completed the postseminar survey (Table 2). In 2022, 635 participants registered and 342 completed evaluations, including attendees from other federal agencies who were invited to participate via EES TRAIN (Training Finder Real-time Affiliate Integrated Network). Forty-seven participants from other federal agencies, including the US Department of Defense, National Institute of Health, and Centers for Disease Control and Prevention, completed the feedback evaluation via TRAIN (Table 3). Participants report high levels of satisfaction each year (mean of 4.5 on a 5-point scale). Respondents preferred conventional lecture presentation and case-based discussions for the teaching format and dementia was the most requested topic for future seminars (Table 4).



The content of each seminar was designed to include . 1 topic relevant to current clinical practice. The 2020 seminar covered topics of cerebrovascular complications of COVID- 19 and living well with neurodegenerative disease in the COVID-19 era. In 2021, the seminar included COVID-19 and neurologic manifestations. In 2022, topics included trends in stroke rehabilitation. In addition, ≥ 1 session addressed neurologic issues within the VHA. In 2020, the VA Deputy National Director of Neurology presented on the VHA stroke systems of care. In 2021, there was a presentation on traumatic brain injury (TBI) in the military. In 2022, sessions covered long term neurologic consequences of TBI and use of telemedicine for neurologic disorders. Feedback on the sessions were positive (eAppendix, available at doi:10.12788/fp.0545).

At the request of the participants, individual presentations were shared via email by the course director and speakers. In collaboration with the EES, each session was recorded and the 2022 seminar was made available to registrants in TMS and EES TRAIN and via the VHA Neurology SharePoint.
DISCUSSION
The annual VHA neurology seminar is a 1-day neurology conference that provides education to general neurologists and other clinicians caring for patients with neurologic disorders. It is the first of its kind neurology education program in the VHA covering most subspecialties in neurology and aims at improving neurologic patient care and access through education. Sessions have covered stroke, epilepsy, sleep, amyotrophic lateral sclerosis, neuropathy, dementia, movement disorders and Parkinson disease, headaches, multiple sclerosis, neurorehabilitation, and telehealth.
The seminar has transitioned from an inperson meeting to a virtual format, making neurology education more convenient and accessible. The virtual format provides the means to increase educational collaborations and share lecture platforms with other federal agencies. The program offers CME credits at no cost to government employees. Recorded lectures can also be asynchronously viewed from the Neurology SharePoint without the ability to earn CME credits. These recordings may be used to educate trainees as well.
The seminar aims to educate all health care professionals caring for patients with neurologic disorders. It aims to eliminate neurophobia, the fear of neural sciences and clinical neurology, and help general practitioners, especially in rural areas, take care of patients with neurologic disorders. The seminars introduce general practitioners to VHA neurology experts; the epilepsy, headache multiple sclerosis, and Parkinson disease centers of excellence; and the national programs for telestroke and teleneurology.
Education Support in the VHA
The EES/ILEAD provides a wide variety of learning opportunities to VHA employees on a broad range of topics, making it one of the largest medical education programs in the country. Pharmacists, social workers, psychologists, therapists, nurses, physician assistants, and physicians have access to certified training opportunities to gain knowledge and skills needed to provide high-quality, veteran-centered care.
A review of geriatrics learning activities through the EES found > 15,000 lectures from 1999 to 2009 for > 300,000 attendees.20 To our knowledge, a review of neurology-related learning activities offered by the EES/ILEAD has not been completed, but the study on geriatrics shows that a similar review would be feasible, given the integrated education system, and helpful in identifying what topics are covered, formats are used, and participants are engaged in neurology education at the VHA. This is a future project planned by the neurology education workgroup.
The EES/ILEAD arranged CME credit for the VHA Neurology Seminar and assisted in organizing an online event with > 500 attendees. Technology support and tools provided by EES during the virtual seminar, such as polling and chat features, kept the audience engaged. Other specialties may similarly value a virtual, all-day seminar format that is efficient and can encourage increased participation from practitioners, nurses, and clinicians.
Future Growth
We plan to increase future participation in the annual neurology seminar with primary care, geriatrics, neurology, and other specialties by instituting an improved and earlier marketing strategy. This includes working with the VHA neurology office to inform neurology practitioners as well as other program offices in the VHA. We intend to host the seminar the same day every year to make it easy for attendees to plan accordingly. In the future we may consider hybrid in-person and virtual modalities if feasible. We plan to focus on reaching out to other government agencies through platforms like TRAIN and the American Academy of Neurology government sections. Securing funding, administrative staff, and protected time in the future may help expand the program further.
Limitations
While a virtual format offers several advantages, using it removes the feel of an in-person meeting, which could be viewed by some attendees as a limitation. The other challenges and drawbacks of transitioning to the virtual platform for a national meeting are similar to those reported in the literature: time zone differences, internet issues, and participants having difficulty using certain online platforms. Attendance could also be limited by scheduling conflicts.16 Despite a large audience attending the seminar, many clinicians do not get protected time from their institutions. Institutional and leadership support at national and local levels will likely improve participation and help participants earn CME credits. While we are still doing a preliminary needs assessment, a formal needs assessment across federal governmental organizations will be helpful.
CONCLUSIONS
The annual VHA neurology seminar promotes interprofessional education, introduces neurology subspecialty centers of excellence, improves access to renowned neurology experts, and provides neurology-related updates through a VHA lens. The program not only provides educational updates to neurology clinicians, but also increases the confidence of non-neurology clinicians called to care for veterans with neurological disorders in their respective clinics.
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990- 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. doi:10.1016/S1474-4422(18)30499-X
- Baker V, Hack N. Improving access to care for patients with migraine in a remote Pacific population. Neurol Clin Pract. 2020;10(5):444-448. doi:10.1212/CPJ.0000000000000774
- Gutmann L, Cahill C, Jordan JT, et al. Characteristics of graduating US allopathic medical students pursuing a career in neurology. Neurology. 2019;92(17):e2051-e2063. doi:10.1212/WNL.0000000000007369
- Jordan JT, Cahill C, Ostendorf T, et al. Attracting neurology’s next generation: a qualitative study of specialty choice and perceptions. Neurology. 2020;95(8):e1080- e1090. doi:10.1212/WNL.0000000000009461
- Minen MT, Kaplan K, Akter S, et al. Understanding how to strengthen the neurology pipeline with insights from undergraduate neuroscience students. Neurology 2022;98(8):314-323. doi:10.1212/WNL.0000000000013259
- US Department of Veterans Affairs, Office of Academic Affiliations. To Educate for VA and the Nation. Updated August 1, 2024. Accessed August 15, 2024. https://www.va.gov/oaa/
- Schaefer SM, Dominguez M, Moeller JJ. The future of the lecture in neurology education. Semin Neurol. 2018;38(4):418-427. doi:10.1055/s-0038-1667042
- Curran VR. Tele-education. J Telemed Telecare. 2006;12(2):57-63. doi:10.1258/135763306776084400
- Lau KHV, Lakhan SE, Achike F. New media, technology and neurology education. Semin Neurol. 2018;38(4):457- 464. doi:10.1055/s-0038-1666985
- Quirk M, Chumley H. The adaptive medical curriculum: a model for continuous improvement. Med Teach. 2018;40(8):786-790. doi:10.1080/0142159X.2018.1484896
- Brockfeld T, Müller B, de Laffolie J. Video versus live lecture courses: a comparative evaluation of lecture types and results. Med Educ Online. 2018;23(1):1555434. doi:10.1080/10872981.2018.1555434
- Davis J, Crabb S, Rogers E, Zamora J, Khan K. Computer-based teaching is as good as face to face lecture-based teaching of evidence based medicine: a randomized controlled trial. Med Teach. 2008;30(3):302-307. doi:10.1080/01421590701784349
- Markova T, Roth LM, Monsur J. Synchronous distance learning as an effective and feasible method for delivering residency didactics. Fam Med. 2005;37(8):570-575.
- Naciri A, Radid M, Kharbach A, Chemsi G. E-learning in health professions education during the COVID-19 pandemic: a systematic review. J Educ Eval Health Prof. 2021;18:27. doi:10.3352/jeehp.2021.18.27
- Dedeilia A, Sotiropoulos MG, Hanrahan JG, Janga D, Dedeilias P, Sideris M. Medical and surgical education challenges and innovations in the COVID-19 era: a systematic review. In Vivo. 2020;34(3 Suppl):1603-1611. doi:10.21873/invivo.11950
- Weber DJ, Albert DVF, Aravamuthan BR, Bernson-Leung ME, Bhatti D, Milligan TA. Training in neurology: rapid implementation of cross-institutional neurology resident education in the time of COVID-19. Neurology. 2020;95(19):883-886. doi:10.1212/WNL.0000000000010753
- Frey J, Neeley B, Umer A, et al. Training in neurology: neuro day: an innovative curriculum connecting medical students with patients. Neurology. 2021;96(10):e1482- e1486. doi:10.1212/WNL.0000000000010859
- Schwartzstein RM, Dienstag JL, King RW, et al. The Harvard Medical School Pathways Curriculum: reimagining developmentally appropriate medical education for contemporary learners. Acad Med. 2020;95(11):1687-1695. doi:10.1097/ACM.0000000000003270
- Greer DM, Moeller J, Torres DR, et al. Funding the educational mission in neurology. Neurology. 2021;96(12):574- 582. doi:10.1212/WNL.0000000000011635
- Thielke S, Tumosa N, Lindenfeld R, Shay K. Geriatric focused educational offerings in the Department of Veterans Affairs from 1999 to 2009. Gerontol Geriatr Educ. 2011;32(1):38-53. doi:10.1080/02701960.2011.550214
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990- 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. doi:10.1016/S1474-4422(18)30499-X
- Baker V, Hack N. Improving access to care for patients with migraine in a remote Pacific population. Neurol Clin Pract. 2020;10(5):444-448. doi:10.1212/CPJ.0000000000000774
- Gutmann L, Cahill C, Jordan JT, et al. Characteristics of graduating US allopathic medical students pursuing a career in neurology. Neurology. 2019;92(17):e2051-e2063. doi:10.1212/WNL.0000000000007369
- Jordan JT, Cahill C, Ostendorf T, et al. Attracting neurology’s next generation: a qualitative study of specialty choice and perceptions. Neurology. 2020;95(8):e1080- e1090. doi:10.1212/WNL.0000000000009461
- Minen MT, Kaplan K, Akter S, et al. Understanding how to strengthen the neurology pipeline with insights from undergraduate neuroscience students. Neurology 2022;98(8):314-323. doi:10.1212/WNL.0000000000013259
- US Department of Veterans Affairs, Office of Academic Affiliations. To Educate for VA and the Nation. Updated August 1, 2024. Accessed August 15, 2024. https://www.va.gov/oaa/
- Schaefer SM, Dominguez M, Moeller JJ. The future of the lecture in neurology education. Semin Neurol. 2018;38(4):418-427. doi:10.1055/s-0038-1667042
- Curran VR. Tele-education. J Telemed Telecare. 2006;12(2):57-63. doi:10.1258/135763306776084400
- Lau KHV, Lakhan SE, Achike F. New media, technology and neurology education. Semin Neurol. 2018;38(4):457- 464. doi:10.1055/s-0038-1666985
- Quirk M, Chumley H. The adaptive medical curriculum: a model for continuous improvement. Med Teach. 2018;40(8):786-790. doi:10.1080/0142159X.2018.1484896
- Brockfeld T, Müller B, de Laffolie J. Video versus live lecture courses: a comparative evaluation of lecture types and results. Med Educ Online. 2018;23(1):1555434. doi:10.1080/10872981.2018.1555434
- Davis J, Crabb S, Rogers E, Zamora J, Khan K. Computer-based teaching is as good as face to face lecture-based teaching of evidence based medicine: a randomized controlled trial. Med Teach. 2008;30(3):302-307. doi:10.1080/01421590701784349
- Markova T, Roth LM, Monsur J. Synchronous distance learning as an effective and feasible method for delivering residency didactics. Fam Med. 2005;37(8):570-575.
- Naciri A, Radid M, Kharbach A, Chemsi G. E-learning in health professions education during the COVID-19 pandemic: a systematic review. J Educ Eval Health Prof. 2021;18:27. doi:10.3352/jeehp.2021.18.27
- Dedeilia A, Sotiropoulos MG, Hanrahan JG, Janga D, Dedeilias P, Sideris M. Medical and surgical education challenges and innovations in the COVID-19 era: a systematic review. In Vivo. 2020;34(3 Suppl):1603-1611. doi:10.21873/invivo.11950
- Weber DJ, Albert DVF, Aravamuthan BR, Bernson-Leung ME, Bhatti D, Milligan TA. Training in neurology: rapid implementation of cross-institutional neurology resident education in the time of COVID-19. Neurology. 2020;95(19):883-886. doi:10.1212/WNL.0000000000010753
- Frey J, Neeley B, Umer A, et al. Training in neurology: neuro day: an innovative curriculum connecting medical students with patients. Neurology. 2021;96(10):e1482- e1486. doi:10.1212/WNL.0000000000010859
- Schwartzstein RM, Dienstag JL, King RW, et al. The Harvard Medical School Pathways Curriculum: reimagining developmentally appropriate medical education for contemporary learners. Acad Med. 2020;95(11):1687-1695. doi:10.1097/ACM.0000000000003270
- Greer DM, Moeller J, Torres DR, et al. Funding the educational mission in neurology. Neurology. 2021;96(12):574- 582. doi:10.1212/WNL.0000000000011635
- Thielke S, Tumosa N, Lindenfeld R, Shay K. Geriatric focused educational offerings in the Department of Veterans Affairs from 1999 to 2009. Gerontol Geriatr Educ. 2011;32(1):38-53. doi:10.1080/02701960.2011.550214
Improving Interprofessional Neurology Training Using Tele-Education
Improving Interprofessional Neurology Training Using Tele-Education
Immunotherapies Targeting α -Synuclein in Parkinson Disease
Parkinson disease (PD) is a progressive neurodegenerative disorder, characterized by diverse clinical symptoms. PD can present with rest tremor, bradykinesia, rigidity, falls, postural instability, and multiple nonmotor symptoms. Marras and colleagues estimated in a comprehensive meta-analysis that there were 680,000 individuals with PD in the US in 2010; this number is expected to double by 2030 based on the US Census Bureau population projections.1 An estimated 110,000 veterans may be affected by PD; hence, understanding of PD pathology, clinical progression, and effective treatment strategies is of paramount importance to the Veterans Health Administration (VHA).2
The exact pathogenesis underlying clinical features is still being studied. Pathologic diagnosis of PD relies on loss of dopamine neurons in the substantia nigra and accumulation of the abnormal protein, α-synuclein, in the form of Lewy bodies and Lewy neurites. Lewy bodies and neurites accumulate predominantly in the substantia nigra in addition to other brain stem nuclei and cerebral cortex. Lewy bodies are intraneuronal inclusions with a hyaline core and a pale peripheral halo. Central core stains positive for α-synuclein.3,4 Lewy neurites are widespread and are believed to play a larger role in the pathogenesis of PD compared with those of Lewy bodies.5
α-Synuclein
α-synuclein is a small 140 amino-acid protein with a N-terminal region that can interact with cell membranes and a highly acidic unstructured C-terminal region.6 α-synuclein is physiologically present in the presynaptic terminals of neurons and involved in synaptic plasticity and vesicle trafficking.7 There are different hypotheses about the native structure of α-synuclein. The first suggests that it exists in tetrameric form and may be broken down to monomer, which is the pathogenic form of α-synuclein. The second hypothesis suggests that it exists primarily in monomeric form, whereas other studies have shown that both forms exist and with pathologic changes, monomer accumulates in abundance and is neurotoxic.8-11 Work by Burré and colleagues shows that native α-synuclein exists in 2 forms: a soluble, cytosolic α-synuclein, which is monomeric, and a membrane-bound multimeric form.12,13
Alteration in aggregation properties of this protein is believed to play a central role in the pathogenesis of PD.14,15 Pathologic α-synuclein exists in insoluble forms that can aggregate into oligomers and fibrillar structures.16 Lysosomal dysfunction may promote accumulation of insoluble α-synuclein. Prior work has shown that several degradation pathways in lysosomes, including the ubiquitin-proteasome system and autophagy-lysosomal pathway, are down regulated, thus contributing to the accumulation of abnormal α-synuclein.17,18 Accumulation of pathologic α-synuclein leads to mitochondrial dysfunction in PD animal models, contributing further to neurotoxicity.19,20 Aggregates of phosphorylated α-synuclein have been demonstrated in dementia with Lewy body.21
In addition, α-synuclein aggregates may be released into extracellular spaces to be taken up by adjacent cells, where they can cause further misfolding and aggregation of protein.22 Previous work in animal models suggested a prion proteinlike spread of α-synuclein.23 This finding can have long-term therapeutic implications, as preventing extracellular release of abnormal form of α-synuclein will prevent the spread of pathologic protein. This can form the basis of neuroprotection in patients with PD.24
It has been proposed that α-synuclein accumulation and extracellular release initiates an immune response that leads to activation of microglia. This has been shown in PD animal models, overexpressing α-synuclein. In 2008 Park and colleagues demonstrated that microglial activation is enhanced by monomeric α-synuclein, not by the aggregated variant.25 Other studies have reported activated microglia around dopaminergic cells in substantia nigra.26 Sulzer and colleagues showed that peptides from α-synuclein can act as antigens and trigger an autoimmune reaction via T cells.27 PD may be associated with certain HLA-haplotypes.28 In other words, α-synuclein can induce neurodegeneration via different mechanisms, including alteration in synaptic vesicle transmission, mitochondrial dysfunction, neuroinflammation, and induction of humoral immunity.
Immunization
Due to these observations, there had been huge interest in developing antibody-based therapies for PD. A similar approach had been tested in Alzheimer disease (AD). Intracellular tangles of tau protein and extracellular aggregates of amyloid are the pathologic substrates in AD. Clinical trials utilizing antibodies targeting amyloid showed reduction in abnormal protein accumulation but no significant improvement in cognition.29 In addition, adverse events (AEs), such as vasogenic edema and intracerebral hemorrhage, were reported.30 Careful analysis of the data suggested that inadequate patient selection or targeting only amyloid, may have contributed to unfavorable results.31 Since then, more recent clinical trials have focused on careful patient selection, use of second generation anti-amyloid antibodies and immunotherapies targeting tau.32
Several studies have tested immunotherapies in PD animal models with the aim of targeting α-synuclein. Immunotherapies can be instituted in 2 ways: active immunization in which the immune system is stimulated to produce antibodies against α-synuclein or passive immunization in which antibodies against α-synuclein are administered directly. Once α-synuclein antibodies have crossed the blood-brain barrier, they are hypothesized to clear the existing α-synuclein. Animal studies have demonstrated the presence of these antibodies within the neurons. The mechanism of entry is unknown. Once inside the cells, the antibodies activate the lysosomal clearance, affecting intracellular accumulation of α-synuclein. Extracellularly, they can bind to receptors on scavenger cells, mainly microglia, activating them to facilitate uptake of extracellular α-synuclein. Binding of the antibodies to α-synuclein directly prevents the uptake of toxic protein by the cells, blocking the transfer and spread of PD pathology.33
Active Immunization
Active immunization against α-synuclein was demonstrated by Masliah and colleagues almost a decade ago. They administered recombinant human α-synuclein in transgenic mice expressing α-synuclein under the control of platelet-derived growth factor β. Reduction of accumulated α-synuclein in neurons with mild microglia activation was noted. It was proposed that the antibodies produced were able to bind to abnormal α-synuclein, were recognized by the lysosomal pathways, and degraded.34 Ghochikyan and colleagues developed vaccines by using α-synuclein-derived peptides. This induced formation of antibodies against α-synuclein in Lewy-bodies and neurites.35 Over time, other animal studies have been able to expand on these results.36
AFFiRiS, an Austrian biotechnology company, has developed 2 peptide vaccines PD01A and PD03A. Both peptides when administered to PD animal models caused antibody-based immune response against aggregated α-synuclein. Humoral autoimmune response was not observed in these studies; no neuroinflammation or neurotoxicity was noted. These peptides did not affect levels of physiologic α-synuclein, targeting only the aggregated form.37 These animal models showed improved motor and cognitive function. Similar results were noted in multiple system atrophy (MSA) animal models.38,39
The first human phase 1, randomized, parallel-group, single-center study recruited 32 subjects with early PD. Twelve subjects each were included in low- or high-dose treatment group, and 8 were included in the control group. Test subjects randomly received 4 vaccinations of low- or high-dose PD01A. Both doses were well tolerated, and no drug-related serious AEs were reported. The study confirmed the tolerability and safety of subcutaneous PD01A vaccine administration. These subjects were included in a 12-month, phase 1b follow-up extension study, AFF008E. In 2018, it was reported that administration of 6 doses of PD01A, 4 primary and 2 booster immunization, was safe. The vaccine showed a clear immune response against the peptide and cross-reactivity against α-synuclein targeted epitope. Booster doses stabilized the antibody titers. Significant increase in antibody titers against PD01A was seen over time, which was translated into a humoral immune response against α-synuclein. In addition, PD01A antibodies also were reported in cerebrospinal fluid.40
AFFiRiS presented results of a phase 1 randomized, placebo-controlled trial in 2017, confirming the safety of PD03A in patients with PD. The study showed a clear dose-dependent immune response against the peptide and cross-reactivity against α-synuclein targeted epitope.41 AFFiRiS recently presented results of another phase 1 clinical study assessing the safety and tolerability of vaccines PD01A and PD03A in patients with early MSA. Both vaccines were well tolerated, and PD01A induced an immune response against the peptide and α-synuclein epitope.42 These results have provided hope for further endeavors to develop active immunization strategies for PD.
Passive Immunization
Passive immunization against α-synuclein was first reported by Masliah and colleagues in 2011. A monoclonal antibody against the C-terminus of α-synuclein, 9E4, was injected into a transgenic mouse model of PD. There was reduction in α-synuclein aggregates in the brain along with improvement in motor and cognitive impairment.43 The C-terminus of α-synuclein plays a key role in the pathogenesis of PD. Changes in the C-terminus of α-synuclein induces formation of α-synuclein oligomers and subsequent neuronal spread. Antibody binds to the C-terminus and prevents structural changes that can lead to oligomerization of α-synuclein. Since the first study by Masliah, few other immunization studies utilized different antibodies against the C-terminus of α-synuclein. It was shown in a mouse model that binding of such antibodies promoted clearance of the α-synuclein by microglia.44
Based on these animal studies, Prothena Biosciences (South San Francisco, CA) designed a phase 1, double-blind, randomized, placebo-controlled clinical trial of prasinezumab (investigational monoclonal antibody against C-terminus of α-synuclein), in subjects without PD. The results showed that it was well tolerated, and there was dose-dependent reduction in the levels of free
BIIB054 is another monoclonal antibody that targets the N-terminal of α-synuclein. In animal models, antibodies targeting the N-terminus reduced α-synuclein triggered cell death and reduced the number of activated microglia.48 BIIB054, from Biogen (Cambridge, MA), was studied in 40 healthy subjects and was well tolerated with a favorable safety profile and could cross the blood-brain barrier. Like the prasinezumab study, this also was an ascending-dose study to assess safety and tolerability. In 2018, a randomized, double-blind, placebo-controlled, single-ascending dose study in patients with PD reported that BIIB054 was well tolerated, and the presence of BIIB054-synuclein complexes in the plasma were confirmed.49 A phase 2, multicenter, randomized, double-blind, placebo-controlled study (SPARK) with an active-treatment dose-blinded period, designed to evaluate the safety, pharmacokinetics, and the pharmacodynamics of BIIB054 is currently recruiting patients with PD.
Finally, BioArctic (Stockholm, Sweden) developed antibodies that are selective for oligomeric forms of α-synuclein, which it licensed to AbbVie (North Chicago, Il).50 These antibodies do not target the N- or C-terminus of α-synuclein. Since α-synuclein oligomers play an important role in the pathogenesis of PD, targeting them with antibodies at an early stage may prove to be an effective strategy for removal of pathogenic α-synuclein. Clinical trials are forthcoming.
Conclusions
Immunotherapy against α-synuclein has provided a new therapeutic avenue in the field of neuroprotection. Results from the first human clinical trial are promising, but despite these results, more work is needed to clarify the role of α-synuclein in the pathogenesis of PD in humans. Most of the work concerning α-synuclein aggregation and propagation has been reported in animal models. Whether similar process exists in humans is a debatable question. Similarly, more knowledge is needed about how and where in the human brain antibodies act to give neuroprotective effects. Timing of administration of immunotherapies in real time will be a crucial question.
PD is clinically evident once 80% of dopaminergic neurons in substantia nigra are lost due to neurodegeneration. Should immunotherapy be administered to symptomatic patients with PD, or if it will be beneficial only for presymptomatic, high-risk patients needs to be determined. Like AD trials, not only careful selection of patients, but determination of optimal timing for treatment will be essential. As the understanding of PD pathogenesis and therapeutics evolves, it will become clear whether immunization targeting α-synuclein will modify disease progression.
1. Marras C, Beck JC, Bower JH, et al; Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4(1):21. doi:10.1038/s41531-018-0058-0
2. Mantri S, Duda JE, Morley JF. Early and accurate identification of Parkinson disease among US veterans. Fed Pract. 2019;36(suppl 4):S18-S23. doi:10.12788/fp.37-0034
3. Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis. 2017;7(suppl 1):S71-S85. doi:10.3233/JPD-179001
4. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388(6645):839-840. doi:10.1038/42166
5. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211. doi:10.1016/s0197-4580(02)00065-9
6. Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79(6):1044-1066. doi:10.1016/j.neuron.2013.09.004
7. Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. α-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663-1667. doi:10.1126/science.1195227
8. Binolfi A, Fernández CO, Sica MP, Delfino JM, Santos J. Recognition between a short unstructured peptide and a partially folded fragment leads to the thioredoxin fold sharing native-like dynamics. Proteins. 2012;80(5):1448-1464. doi:10.1002/prot.24043
9. Fauvet B, Mbefo MK, Fares MB, et al. α-synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287(19):15345-15364. doi:10.1074/jbc.M111.318949.
10. Wang W, Perovic I, Chittuluru J, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108(43):17797-17802. doi:10.1073/pnas.1113260108
11. Bellucci A, Zaltieri M, Navarria L, Grigoletto J, Missale C, Spano P. From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson’s disease. Brain Res. 2012;1476:183-202. doi:10.1016/j.brainres.2012.04.014
12. Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Südhof TC. Properties of native α-synuclein. Nature. 2013;498(7453):E4-E7.
13. Burré J, Sharma M, Südhof TC. α-synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA. 2014;111(40):E4274-E4283. doi:10.1073/pnas.1416598111
14. Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23(2):1-13. doi:10.1038/nm.4269
15. Burré J, Sharma M, Südhof TC. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J Neurosci. 2015;35(13):5221-5232. doi:10.1523/JNEUROSCI.4650-14.2015
16. Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24(8):1888-1896. doi:10.1523/JNEUROSCI.3809-03.2004
17. Rideout HJ, Dietrich P, Wang Q, Dauer WT, Stefanis L . α-synuclein is required for the fibrillar nature of ubiquitinated inclusions induced by proteasomal inhibition in primary neurons. J Biol Chem. 2004;279(45):46915-46920. doi:10.1074/jbc.M405146200
18. Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40(4):200-210. doi:10.1016/j.tibs.2015.02.003
19. Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochem Biophys Acta. 2010;1802(1):29-44. doi:10.1016/j.bbadis.2009.08.013
20. Lee HJ, Bae EJ, Lee SJ. Extracellular α-synuclein: a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10(2):92-98. doi:10.1038/nrneurol.2013.275
21. Colom-Cadena M, Pegueroles J, Herrmann AG, et al. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain. 2017;140(12):3204-3214. doi:10.1093/brain/awx275
22. Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57-71. doi:10.1016/j.neuron.2011.08.033
23. Masuda-Suzukake M, Nonaka T, Hosokawa M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136(pt 4):1128-1138. doi:10.1093/brain/awt037
24. Hasegawa M, Nonaka T, Masuda-Suzukake M. Prion-like mechanisms and potential therapeutic targets in neurodegenerative disorders. Pharmacol Ther. 2017;172:22-33. doi:10.1016/j.pharmthera.2016.11.010
25. Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric α-synuclein, not aggregated alpha-synuclein: implications for Parkinson’s disease. Glia. 2008;56(11):1215-1223. doi:10.1002/glia.20691
26. Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 2013;8(1):189-201. doi:10.1007/s11481-013-9435-y
27. Sulzer D, Alcalay RN, Garretti F, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546(7660):656-661. doi:10.1038/nature22815
28. Hamza TH, Zabetian CP, Tenesa A, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genetics. 2010;42(9):781-785. doi:10.1038/ng.642
29. Holmes C, Boche D, Wilkinson D, et al. Long term effects of Aβ42 immunisation in Alzheimer’s disease: follow up of a randomized, placebo-controlled phase I trial. Lancet. 2008;372(9634):216-223. doi:10.1016/S0140-6736(08)61075-2
30. Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241-249. doi:10.1016/S1474-4422(12)70015-7
31. Wisniewski T, Goñi F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):499-507. doi:10.1016/j.bcp.2013.12.020
32. Herline K, Drummond E, Wisniewski T. Recent advancements toward therapeutic vaccines against Alzheimer’s disease. Expert Rev Vaccines. 2018;17(8):707-721. doi:10.1080/14760584.2018.1500905
33. Bergstrom AL, Kallunki P, Fog K. Development of passive immunotherapies for synucleopathies. Mov Disord. 2015;31(2):203-213. doi:10.1002/mds.26481
34. Masliah E, Rockenstein E, Adame A, et al. Effects of α-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46(6):857-868. doi:10.1016/j.neuron.2005.05.010
35. Ghochikyan A, Petrushina I, Davtyan H, et al. Immunogenicity of epitope vaccines targeting different B cell antigenic determinants of human α-synuclein: feasibility study. Neurosci Lett. 2014;560:86-91. doi:10.1016/j.neulet.2013.12.028
36. Sanchez-Guajardo V, Annibali A, Jensen PH, Romero-Ramos M. α-synuclein vaccination prevents the accumulation of Parkinson’s disease-like pathologic inclusions in striatum in association with regulatory T cell recruitment in a rat model. J Neuropathol Exp Neurol. 2013;72(7):624-645. doi:10.1097/NEN.0b013e31829768d2
37. Mandler M, Valera E, Rockenstein E, et al. Next generation active immunization approach for synucleinopathies: Implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014;127(6):861-879. doi:10.1007/s00401-014-1256-4
38. Mandler M, Valera E, Rockenstein E, et al. Active immunization against α-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multisystem atrophy. Mol Neurodegen. 2015;10:721. doi:10.1186/s13024-015-0008-9
39. Schneeberger A, Tierney L, Mandler M. Active immunization therapies. Mov Disord. 2015;31(2):214-224. doi:10.1002/mds.26377
40. Zella SMA, Metzdorf J, Ciftci E, et al. Emerging immunotherapies for Parkinson disease. Neurol Ther. 2019;8(1):29-44. doi:10.1007/s40120-018-0122-z
41. AFFiRiS AG. AFFiRiS announces top line results of first-in-human clinical study using AFFITOPE PD03A, confirming immunogenicity and safety profile in Parkinson’s disease patients. https://affiris.com/wp-content/uploads/2018/10/praff011prefinal0607wo-embargo-1.pdf. Published June 7, 2017. Accessed July 29, 2020.
42. AFFiRiS AG. AFFiRiS announces results of a phase I clinical study using AFFITOPEs PD01A and PD03A, confirming safety and tolerability for both compounds as well as immunogenicity for PD01A in early MSA patients. http://sympath-project.eu/wp-content/uploads/PR_AFF009_V1.pdf Published March 1, 2018. Accessed July 29, 2020.
43. Masliah E, Rockenstein E, Mante M, et al. Passive immunization reduces behavioral and neuropathological deficits in an alphasynuclein transgenic model of Lewy body disease. PLoS One. 2011;6(4):e19338. doi:10.1371/journal.pone.0019338
44. Bae EJ, Lee HJ, Rockenstein E, et al. Antibody aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32(39):1345-13469. doi:10.1523/JNEUROSCI.1292-12.2012
45. Schenk DB, Koller M, Ness DK, et al. First‐in‐human assessment of PRX002, an anti–α‐synuclein monoclonal antibody, in healthy volunteers. Mov Disord. 2017;32(2):211-218. doi:10.1002/mds.26878.
46. Jankovic J, Goodman I, Safirstein B, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α -synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2018;75(10):1206-1214. doi:10.1001/jamaneurol.2018.1487
47. Jankovic J. Pathogenesis-targeted therapeutic strategies in Parkinson’s disease. Mov Disord. 2019;34(1):41-44. doi:10.1002/mds.27534
48. Shahaduzzaman M, Nash K, Hudson C, et al. Anti-human α-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-α-synuclein rat model of Parkinson’s disease. PLoS One. 2015;10(2):E0116841. doi:10.1371/journal.pone.0116841
49. Brys M, Hung S, Fanning L, et al. Randomized, double-blind, placebo-controlled, single ascending dose study of anti-α-synuclein antibody BIIB054 in patients with Parkinson disease. Neurology. 2018;90(suppl 15):S26.001. doi:10.1002/mds.27738
50. Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target α-synuclein pathology. Exp Neurol. 2017;298(pt B):225-235. doi:10.1016/j.expneurol.2017.10.003
Parkinson disease (PD) is a progressive neurodegenerative disorder, characterized by diverse clinical symptoms. PD can present with rest tremor, bradykinesia, rigidity, falls, postural instability, and multiple nonmotor symptoms. Marras and colleagues estimated in a comprehensive meta-analysis that there were 680,000 individuals with PD in the US in 2010; this number is expected to double by 2030 based on the US Census Bureau population projections.1 An estimated 110,000 veterans may be affected by PD; hence, understanding of PD pathology, clinical progression, and effective treatment strategies is of paramount importance to the Veterans Health Administration (VHA).2
The exact pathogenesis underlying clinical features is still being studied. Pathologic diagnosis of PD relies on loss of dopamine neurons in the substantia nigra and accumulation of the abnormal protein, α-synuclein, in the form of Lewy bodies and Lewy neurites. Lewy bodies and neurites accumulate predominantly in the substantia nigra in addition to other brain stem nuclei and cerebral cortex. Lewy bodies are intraneuronal inclusions with a hyaline core and a pale peripheral halo. Central core stains positive for α-synuclein.3,4 Lewy neurites are widespread and are believed to play a larger role in the pathogenesis of PD compared with those of Lewy bodies.5
α-Synuclein
α-synuclein is a small 140 amino-acid protein with a N-terminal region that can interact with cell membranes and a highly acidic unstructured C-terminal region.6 α-synuclein is physiologically present in the presynaptic terminals of neurons and involved in synaptic plasticity and vesicle trafficking.7 There are different hypotheses about the native structure of α-synuclein. The first suggests that it exists in tetrameric form and may be broken down to monomer, which is the pathogenic form of α-synuclein. The second hypothesis suggests that it exists primarily in monomeric form, whereas other studies have shown that both forms exist and with pathologic changes, monomer accumulates in abundance and is neurotoxic.8-11 Work by Burré and colleagues shows that native α-synuclein exists in 2 forms: a soluble, cytosolic α-synuclein, which is monomeric, and a membrane-bound multimeric form.12,13
Alteration in aggregation properties of this protein is believed to play a central role in the pathogenesis of PD.14,15 Pathologic α-synuclein exists in insoluble forms that can aggregate into oligomers and fibrillar structures.16 Lysosomal dysfunction may promote accumulation of insoluble α-synuclein. Prior work has shown that several degradation pathways in lysosomes, including the ubiquitin-proteasome system and autophagy-lysosomal pathway, are down regulated, thus contributing to the accumulation of abnormal α-synuclein.17,18 Accumulation of pathologic α-synuclein leads to mitochondrial dysfunction in PD animal models, contributing further to neurotoxicity.19,20 Aggregates of phosphorylated α-synuclein have been demonstrated in dementia with Lewy body.21
In addition, α-synuclein aggregates may be released into extracellular spaces to be taken up by adjacent cells, where they can cause further misfolding and aggregation of protein.22 Previous work in animal models suggested a prion proteinlike spread of α-synuclein.23 This finding can have long-term therapeutic implications, as preventing extracellular release of abnormal form of α-synuclein will prevent the spread of pathologic protein. This can form the basis of neuroprotection in patients with PD.24
It has been proposed that α-synuclein accumulation and extracellular release initiates an immune response that leads to activation of microglia. This has been shown in PD animal models, overexpressing α-synuclein. In 2008 Park and colleagues demonstrated that microglial activation is enhanced by monomeric α-synuclein, not by the aggregated variant.25 Other studies have reported activated microglia around dopaminergic cells in substantia nigra.26 Sulzer and colleagues showed that peptides from α-synuclein can act as antigens and trigger an autoimmune reaction via T cells.27 PD may be associated with certain HLA-haplotypes.28 In other words, α-synuclein can induce neurodegeneration via different mechanisms, including alteration in synaptic vesicle transmission, mitochondrial dysfunction, neuroinflammation, and induction of humoral immunity.
Immunization
Due to these observations, there had been huge interest in developing antibody-based therapies for PD. A similar approach had been tested in Alzheimer disease (AD). Intracellular tangles of tau protein and extracellular aggregates of amyloid are the pathologic substrates in AD. Clinical trials utilizing antibodies targeting amyloid showed reduction in abnormal protein accumulation but no significant improvement in cognition.29 In addition, adverse events (AEs), such as vasogenic edema and intracerebral hemorrhage, were reported.30 Careful analysis of the data suggested that inadequate patient selection or targeting only amyloid, may have contributed to unfavorable results.31 Since then, more recent clinical trials have focused on careful patient selection, use of second generation anti-amyloid antibodies and immunotherapies targeting tau.32
Several studies have tested immunotherapies in PD animal models with the aim of targeting α-synuclein. Immunotherapies can be instituted in 2 ways: active immunization in which the immune system is stimulated to produce antibodies against α-synuclein or passive immunization in which antibodies against α-synuclein are administered directly. Once α-synuclein antibodies have crossed the blood-brain barrier, they are hypothesized to clear the existing α-synuclein. Animal studies have demonstrated the presence of these antibodies within the neurons. The mechanism of entry is unknown. Once inside the cells, the antibodies activate the lysosomal clearance, affecting intracellular accumulation of α-synuclein. Extracellularly, they can bind to receptors on scavenger cells, mainly microglia, activating them to facilitate uptake of extracellular α-synuclein. Binding of the antibodies to α-synuclein directly prevents the uptake of toxic protein by the cells, blocking the transfer and spread of PD pathology.33
Active Immunization
Active immunization against α-synuclein was demonstrated by Masliah and colleagues almost a decade ago. They administered recombinant human α-synuclein in transgenic mice expressing α-synuclein under the control of platelet-derived growth factor β. Reduction of accumulated α-synuclein in neurons with mild microglia activation was noted. It was proposed that the antibodies produced were able to bind to abnormal α-synuclein, were recognized by the lysosomal pathways, and degraded.34 Ghochikyan and colleagues developed vaccines by using α-synuclein-derived peptides. This induced formation of antibodies against α-synuclein in Lewy-bodies and neurites.35 Over time, other animal studies have been able to expand on these results.36
AFFiRiS, an Austrian biotechnology company, has developed 2 peptide vaccines PD01A and PD03A. Both peptides when administered to PD animal models caused antibody-based immune response against aggregated α-synuclein. Humoral autoimmune response was not observed in these studies; no neuroinflammation or neurotoxicity was noted. These peptides did not affect levels of physiologic α-synuclein, targeting only the aggregated form.37 These animal models showed improved motor and cognitive function. Similar results were noted in multiple system atrophy (MSA) animal models.38,39
The first human phase 1, randomized, parallel-group, single-center study recruited 32 subjects with early PD. Twelve subjects each were included in low- or high-dose treatment group, and 8 were included in the control group. Test subjects randomly received 4 vaccinations of low- or high-dose PD01A. Both doses were well tolerated, and no drug-related serious AEs were reported. The study confirmed the tolerability and safety of subcutaneous PD01A vaccine administration. These subjects were included in a 12-month, phase 1b follow-up extension study, AFF008E. In 2018, it was reported that administration of 6 doses of PD01A, 4 primary and 2 booster immunization, was safe. The vaccine showed a clear immune response against the peptide and cross-reactivity against α-synuclein targeted epitope. Booster doses stabilized the antibody titers. Significant increase in antibody titers against PD01A was seen over time, which was translated into a humoral immune response against α-synuclein. In addition, PD01A antibodies also were reported in cerebrospinal fluid.40
AFFiRiS presented results of a phase 1 randomized, placebo-controlled trial in 2017, confirming the safety of PD03A in patients with PD. The study showed a clear dose-dependent immune response against the peptide and cross-reactivity against α-synuclein targeted epitope.41 AFFiRiS recently presented results of another phase 1 clinical study assessing the safety and tolerability of vaccines PD01A and PD03A in patients with early MSA. Both vaccines were well tolerated, and PD01A induced an immune response against the peptide and α-synuclein epitope.42 These results have provided hope for further endeavors to develop active immunization strategies for PD.
Passive Immunization
Passive immunization against α-synuclein was first reported by Masliah and colleagues in 2011. A monoclonal antibody against the C-terminus of α-synuclein, 9E4, was injected into a transgenic mouse model of PD. There was reduction in α-synuclein aggregates in the brain along with improvement in motor and cognitive impairment.43 The C-terminus of α-synuclein plays a key role in the pathogenesis of PD. Changes in the C-terminus of α-synuclein induces formation of α-synuclein oligomers and subsequent neuronal spread. Antibody binds to the C-terminus and prevents structural changes that can lead to oligomerization of α-synuclein. Since the first study by Masliah, few other immunization studies utilized different antibodies against the C-terminus of α-synuclein. It was shown in a mouse model that binding of such antibodies promoted clearance of the α-synuclein by microglia.44
Based on these animal studies, Prothena Biosciences (South San Francisco, CA) designed a phase 1, double-blind, randomized, placebo-controlled clinical trial of prasinezumab (investigational monoclonal antibody against C-terminus of α-synuclein), in subjects without PD. The results showed that it was well tolerated, and there was dose-dependent reduction in the levels of free
BIIB054 is another monoclonal antibody that targets the N-terminal of α-synuclein. In animal models, antibodies targeting the N-terminus reduced α-synuclein triggered cell death and reduced the number of activated microglia.48 BIIB054, from Biogen (Cambridge, MA), was studied in 40 healthy subjects and was well tolerated with a favorable safety profile and could cross the blood-brain barrier. Like the prasinezumab study, this also was an ascending-dose study to assess safety and tolerability. In 2018, a randomized, double-blind, placebo-controlled, single-ascending dose study in patients with PD reported that BIIB054 was well tolerated, and the presence of BIIB054-synuclein complexes in the plasma were confirmed.49 A phase 2, multicenter, randomized, double-blind, placebo-controlled study (SPARK) with an active-treatment dose-blinded period, designed to evaluate the safety, pharmacokinetics, and the pharmacodynamics of BIIB054 is currently recruiting patients with PD.
Finally, BioArctic (Stockholm, Sweden) developed antibodies that are selective for oligomeric forms of α-synuclein, which it licensed to AbbVie (North Chicago, Il).50 These antibodies do not target the N- or C-terminus of α-synuclein. Since α-synuclein oligomers play an important role in the pathogenesis of PD, targeting them with antibodies at an early stage may prove to be an effective strategy for removal of pathogenic α-synuclein. Clinical trials are forthcoming.
Conclusions
Immunotherapy against α-synuclein has provided a new therapeutic avenue in the field of neuroprotection. Results from the first human clinical trial are promising, but despite these results, more work is needed to clarify the role of α-synuclein in the pathogenesis of PD in humans. Most of the work concerning α-synuclein aggregation and propagation has been reported in animal models. Whether similar process exists in humans is a debatable question. Similarly, more knowledge is needed about how and where in the human brain antibodies act to give neuroprotective effects. Timing of administration of immunotherapies in real time will be a crucial question.
PD is clinically evident once 80% of dopaminergic neurons in substantia nigra are lost due to neurodegeneration. Should immunotherapy be administered to symptomatic patients with PD, or if it will be beneficial only for presymptomatic, high-risk patients needs to be determined. Like AD trials, not only careful selection of patients, but determination of optimal timing for treatment will be essential. As the understanding of PD pathogenesis and therapeutics evolves, it will become clear whether immunization targeting α-synuclein will modify disease progression.
Parkinson disease (PD) is a progressive neurodegenerative disorder, characterized by diverse clinical symptoms. PD can present with rest tremor, bradykinesia, rigidity, falls, postural instability, and multiple nonmotor symptoms. Marras and colleagues estimated in a comprehensive meta-analysis that there were 680,000 individuals with PD in the US in 2010; this number is expected to double by 2030 based on the US Census Bureau population projections.1 An estimated 110,000 veterans may be affected by PD; hence, understanding of PD pathology, clinical progression, and effective treatment strategies is of paramount importance to the Veterans Health Administration (VHA).2
The exact pathogenesis underlying clinical features is still being studied. Pathologic diagnosis of PD relies on loss of dopamine neurons in the substantia nigra and accumulation of the abnormal protein, α-synuclein, in the form of Lewy bodies and Lewy neurites. Lewy bodies and neurites accumulate predominantly in the substantia nigra in addition to other brain stem nuclei and cerebral cortex. Lewy bodies are intraneuronal inclusions with a hyaline core and a pale peripheral halo. Central core stains positive for α-synuclein.3,4 Lewy neurites are widespread and are believed to play a larger role in the pathogenesis of PD compared with those of Lewy bodies.5
α-Synuclein
α-synuclein is a small 140 amino-acid protein with a N-terminal region that can interact with cell membranes and a highly acidic unstructured C-terminal region.6 α-synuclein is physiologically present in the presynaptic terminals of neurons and involved in synaptic plasticity and vesicle trafficking.7 There are different hypotheses about the native structure of α-synuclein. The first suggests that it exists in tetrameric form and may be broken down to monomer, which is the pathogenic form of α-synuclein. The second hypothesis suggests that it exists primarily in monomeric form, whereas other studies have shown that both forms exist and with pathologic changes, monomer accumulates in abundance and is neurotoxic.8-11 Work by Burré and colleagues shows that native α-synuclein exists in 2 forms: a soluble, cytosolic α-synuclein, which is monomeric, and a membrane-bound multimeric form.12,13
Alteration in aggregation properties of this protein is believed to play a central role in the pathogenesis of PD.14,15 Pathologic α-synuclein exists in insoluble forms that can aggregate into oligomers and fibrillar structures.16 Lysosomal dysfunction may promote accumulation of insoluble α-synuclein. Prior work has shown that several degradation pathways in lysosomes, including the ubiquitin-proteasome system and autophagy-lysosomal pathway, are down regulated, thus contributing to the accumulation of abnormal α-synuclein.17,18 Accumulation of pathologic α-synuclein leads to mitochondrial dysfunction in PD animal models, contributing further to neurotoxicity.19,20 Aggregates of phosphorylated α-synuclein have been demonstrated in dementia with Lewy body.21
In addition, α-synuclein aggregates may be released into extracellular spaces to be taken up by adjacent cells, where they can cause further misfolding and aggregation of protein.22 Previous work in animal models suggested a prion proteinlike spread of α-synuclein.23 This finding can have long-term therapeutic implications, as preventing extracellular release of abnormal form of α-synuclein will prevent the spread of pathologic protein. This can form the basis of neuroprotection in patients with PD.24
It has been proposed that α-synuclein accumulation and extracellular release initiates an immune response that leads to activation of microglia. This has been shown in PD animal models, overexpressing α-synuclein. In 2008 Park and colleagues demonstrated that microglial activation is enhanced by monomeric α-synuclein, not by the aggregated variant.25 Other studies have reported activated microglia around dopaminergic cells in substantia nigra.26 Sulzer and colleagues showed that peptides from α-synuclein can act as antigens and trigger an autoimmune reaction via T cells.27 PD may be associated with certain HLA-haplotypes.28 In other words, α-synuclein can induce neurodegeneration via different mechanisms, including alteration in synaptic vesicle transmission, mitochondrial dysfunction, neuroinflammation, and induction of humoral immunity.
Immunization
Due to these observations, there had been huge interest in developing antibody-based therapies for PD. A similar approach had been tested in Alzheimer disease (AD). Intracellular tangles of tau protein and extracellular aggregates of amyloid are the pathologic substrates in AD. Clinical trials utilizing antibodies targeting amyloid showed reduction in abnormal protein accumulation but no significant improvement in cognition.29 In addition, adverse events (AEs), such as vasogenic edema and intracerebral hemorrhage, were reported.30 Careful analysis of the data suggested that inadequate patient selection or targeting only amyloid, may have contributed to unfavorable results.31 Since then, more recent clinical trials have focused on careful patient selection, use of second generation anti-amyloid antibodies and immunotherapies targeting tau.32
Several studies have tested immunotherapies in PD animal models with the aim of targeting α-synuclein. Immunotherapies can be instituted in 2 ways: active immunization in which the immune system is stimulated to produce antibodies against α-synuclein or passive immunization in which antibodies against α-synuclein are administered directly. Once α-synuclein antibodies have crossed the blood-brain barrier, they are hypothesized to clear the existing α-synuclein. Animal studies have demonstrated the presence of these antibodies within the neurons. The mechanism of entry is unknown. Once inside the cells, the antibodies activate the lysosomal clearance, affecting intracellular accumulation of α-synuclein. Extracellularly, they can bind to receptors on scavenger cells, mainly microglia, activating them to facilitate uptake of extracellular α-synuclein. Binding of the antibodies to α-synuclein directly prevents the uptake of toxic protein by the cells, blocking the transfer and spread of PD pathology.33
Active Immunization
Active immunization against α-synuclein was demonstrated by Masliah and colleagues almost a decade ago. They administered recombinant human α-synuclein in transgenic mice expressing α-synuclein under the control of platelet-derived growth factor β. Reduction of accumulated α-synuclein in neurons with mild microglia activation was noted. It was proposed that the antibodies produced were able to bind to abnormal α-synuclein, were recognized by the lysosomal pathways, and degraded.34 Ghochikyan and colleagues developed vaccines by using α-synuclein-derived peptides. This induced formation of antibodies against α-synuclein in Lewy-bodies and neurites.35 Over time, other animal studies have been able to expand on these results.36
AFFiRiS, an Austrian biotechnology company, has developed 2 peptide vaccines PD01A and PD03A. Both peptides when administered to PD animal models caused antibody-based immune response against aggregated α-synuclein. Humoral autoimmune response was not observed in these studies; no neuroinflammation or neurotoxicity was noted. These peptides did not affect levels of physiologic α-synuclein, targeting only the aggregated form.37 These animal models showed improved motor and cognitive function. Similar results were noted in multiple system atrophy (MSA) animal models.38,39
The first human phase 1, randomized, parallel-group, single-center study recruited 32 subjects with early PD. Twelve subjects each were included in low- or high-dose treatment group, and 8 were included in the control group. Test subjects randomly received 4 vaccinations of low- or high-dose PD01A. Both doses were well tolerated, and no drug-related serious AEs were reported. The study confirmed the tolerability and safety of subcutaneous PD01A vaccine administration. These subjects were included in a 12-month, phase 1b follow-up extension study, AFF008E. In 2018, it was reported that administration of 6 doses of PD01A, 4 primary and 2 booster immunization, was safe. The vaccine showed a clear immune response against the peptide and cross-reactivity against α-synuclein targeted epitope. Booster doses stabilized the antibody titers. Significant increase in antibody titers against PD01A was seen over time, which was translated into a humoral immune response against α-synuclein. In addition, PD01A antibodies also were reported in cerebrospinal fluid.40
AFFiRiS presented results of a phase 1 randomized, placebo-controlled trial in 2017, confirming the safety of PD03A in patients with PD. The study showed a clear dose-dependent immune response against the peptide and cross-reactivity against α-synuclein targeted epitope.41 AFFiRiS recently presented results of another phase 1 clinical study assessing the safety and tolerability of vaccines PD01A and PD03A in patients with early MSA. Both vaccines were well tolerated, and PD01A induced an immune response against the peptide and α-synuclein epitope.42 These results have provided hope for further endeavors to develop active immunization strategies for PD.
Passive Immunization
Passive immunization against α-synuclein was first reported by Masliah and colleagues in 2011. A monoclonal antibody against the C-terminus of α-synuclein, 9E4, was injected into a transgenic mouse model of PD. There was reduction in α-synuclein aggregates in the brain along with improvement in motor and cognitive impairment.43 The C-terminus of α-synuclein plays a key role in the pathogenesis of PD. Changes in the C-terminus of α-synuclein induces formation of α-synuclein oligomers and subsequent neuronal spread. Antibody binds to the C-terminus and prevents structural changes that can lead to oligomerization of α-synuclein. Since the first study by Masliah, few other immunization studies utilized different antibodies against the C-terminus of α-synuclein. It was shown in a mouse model that binding of such antibodies promoted clearance of the α-synuclein by microglia.44
Based on these animal studies, Prothena Biosciences (South San Francisco, CA) designed a phase 1, double-blind, randomized, placebo-controlled clinical trial of prasinezumab (investigational monoclonal antibody against C-terminus of α-synuclein), in subjects without PD. The results showed that it was well tolerated, and there was dose-dependent reduction in the levels of free
BIIB054 is another monoclonal antibody that targets the N-terminal of α-synuclein. In animal models, antibodies targeting the N-terminus reduced α-synuclein triggered cell death and reduced the number of activated microglia.48 BIIB054, from Biogen (Cambridge, MA), was studied in 40 healthy subjects and was well tolerated with a favorable safety profile and could cross the blood-brain barrier. Like the prasinezumab study, this also was an ascending-dose study to assess safety and tolerability. In 2018, a randomized, double-blind, placebo-controlled, single-ascending dose study in patients with PD reported that BIIB054 was well tolerated, and the presence of BIIB054-synuclein complexes in the plasma were confirmed.49 A phase 2, multicenter, randomized, double-blind, placebo-controlled study (SPARK) with an active-treatment dose-blinded period, designed to evaluate the safety, pharmacokinetics, and the pharmacodynamics of BIIB054 is currently recruiting patients with PD.
Finally, BioArctic (Stockholm, Sweden) developed antibodies that are selective for oligomeric forms of α-synuclein, which it licensed to AbbVie (North Chicago, Il).50 These antibodies do not target the N- or C-terminus of α-synuclein. Since α-synuclein oligomers play an important role in the pathogenesis of PD, targeting them with antibodies at an early stage may prove to be an effective strategy for removal of pathogenic α-synuclein. Clinical trials are forthcoming.
Conclusions
Immunotherapy against α-synuclein has provided a new therapeutic avenue in the field of neuroprotection. Results from the first human clinical trial are promising, but despite these results, more work is needed to clarify the role of α-synuclein in the pathogenesis of PD in humans. Most of the work concerning α-synuclein aggregation and propagation has been reported in animal models. Whether similar process exists in humans is a debatable question. Similarly, more knowledge is needed about how and where in the human brain antibodies act to give neuroprotective effects. Timing of administration of immunotherapies in real time will be a crucial question.
PD is clinically evident once 80% of dopaminergic neurons in substantia nigra are lost due to neurodegeneration. Should immunotherapy be administered to symptomatic patients with PD, or if it will be beneficial only for presymptomatic, high-risk patients needs to be determined. Like AD trials, not only careful selection of patients, but determination of optimal timing for treatment will be essential. As the understanding of PD pathogenesis and therapeutics evolves, it will become clear whether immunization targeting α-synuclein will modify disease progression.
1. Marras C, Beck JC, Bower JH, et al; Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4(1):21. doi:10.1038/s41531-018-0058-0
2. Mantri S, Duda JE, Morley JF. Early and accurate identification of Parkinson disease among US veterans. Fed Pract. 2019;36(suppl 4):S18-S23. doi:10.12788/fp.37-0034
3. Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis. 2017;7(suppl 1):S71-S85. doi:10.3233/JPD-179001
4. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388(6645):839-840. doi:10.1038/42166
5. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211. doi:10.1016/s0197-4580(02)00065-9
6. Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79(6):1044-1066. doi:10.1016/j.neuron.2013.09.004
7. Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. α-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663-1667. doi:10.1126/science.1195227
8. Binolfi A, Fernández CO, Sica MP, Delfino JM, Santos J. Recognition between a short unstructured peptide and a partially folded fragment leads to the thioredoxin fold sharing native-like dynamics. Proteins. 2012;80(5):1448-1464. doi:10.1002/prot.24043
9. Fauvet B, Mbefo MK, Fares MB, et al. α-synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287(19):15345-15364. doi:10.1074/jbc.M111.318949.
10. Wang W, Perovic I, Chittuluru J, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108(43):17797-17802. doi:10.1073/pnas.1113260108
11. Bellucci A, Zaltieri M, Navarria L, Grigoletto J, Missale C, Spano P. From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson’s disease. Brain Res. 2012;1476:183-202. doi:10.1016/j.brainres.2012.04.014
12. Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Südhof TC. Properties of native α-synuclein. Nature. 2013;498(7453):E4-E7.
13. Burré J, Sharma M, Südhof TC. α-synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA. 2014;111(40):E4274-E4283. doi:10.1073/pnas.1416598111
14. Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23(2):1-13. doi:10.1038/nm.4269
15. Burré J, Sharma M, Südhof TC. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J Neurosci. 2015;35(13):5221-5232. doi:10.1523/JNEUROSCI.4650-14.2015
16. Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24(8):1888-1896. doi:10.1523/JNEUROSCI.3809-03.2004
17. Rideout HJ, Dietrich P, Wang Q, Dauer WT, Stefanis L . α-synuclein is required for the fibrillar nature of ubiquitinated inclusions induced by proteasomal inhibition in primary neurons. J Biol Chem. 2004;279(45):46915-46920. doi:10.1074/jbc.M405146200
18. Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40(4):200-210. doi:10.1016/j.tibs.2015.02.003
19. Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochem Biophys Acta. 2010;1802(1):29-44. doi:10.1016/j.bbadis.2009.08.013
20. Lee HJ, Bae EJ, Lee SJ. Extracellular α-synuclein: a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10(2):92-98. doi:10.1038/nrneurol.2013.275
21. Colom-Cadena M, Pegueroles J, Herrmann AG, et al. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain. 2017;140(12):3204-3214. doi:10.1093/brain/awx275
22. Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57-71. doi:10.1016/j.neuron.2011.08.033
23. Masuda-Suzukake M, Nonaka T, Hosokawa M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136(pt 4):1128-1138. doi:10.1093/brain/awt037
24. Hasegawa M, Nonaka T, Masuda-Suzukake M. Prion-like mechanisms and potential therapeutic targets in neurodegenerative disorders. Pharmacol Ther. 2017;172:22-33. doi:10.1016/j.pharmthera.2016.11.010
25. Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric α-synuclein, not aggregated alpha-synuclein: implications for Parkinson’s disease. Glia. 2008;56(11):1215-1223. doi:10.1002/glia.20691
26. Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 2013;8(1):189-201. doi:10.1007/s11481-013-9435-y
27. Sulzer D, Alcalay RN, Garretti F, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546(7660):656-661. doi:10.1038/nature22815
28. Hamza TH, Zabetian CP, Tenesa A, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genetics. 2010;42(9):781-785. doi:10.1038/ng.642
29. Holmes C, Boche D, Wilkinson D, et al. Long term effects of Aβ42 immunisation in Alzheimer’s disease: follow up of a randomized, placebo-controlled phase I trial. Lancet. 2008;372(9634):216-223. doi:10.1016/S0140-6736(08)61075-2
30. Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241-249. doi:10.1016/S1474-4422(12)70015-7
31. Wisniewski T, Goñi F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):499-507. doi:10.1016/j.bcp.2013.12.020
32. Herline K, Drummond E, Wisniewski T. Recent advancements toward therapeutic vaccines against Alzheimer’s disease. Expert Rev Vaccines. 2018;17(8):707-721. doi:10.1080/14760584.2018.1500905
33. Bergstrom AL, Kallunki P, Fog K. Development of passive immunotherapies for synucleopathies. Mov Disord. 2015;31(2):203-213. doi:10.1002/mds.26481
34. Masliah E, Rockenstein E, Adame A, et al. Effects of α-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46(6):857-868. doi:10.1016/j.neuron.2005.05.010
35. Ghochikyan A, Petrushina I, Davtyan H, et al. Immunogenicity of epitope vaccines targeting different B cell antigenic determinants of human α-synuclein: feasibility study. Neurosci Lett. 2014;560:86-91. doi:10.1016/j.neulet.2013.12.028
36. Sanchez-Guajardo V, Annibali A, Jensen PH, Romero-Ramos M. α-synuclein vaccination prevents the accumulation of Parkinson’s disease-like pathologic inclusions in striatum in association with regulatory T cell recruitment in a rat model. J Neuropathol Exp Neurol. 2013;72(7):624-645. doi:10.1097/NEN.0b013e31829768d2
37. Mandler M, Valera E, Rockenstein E, et al. Next generation active immunization approach for synucleinopathies: Implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014;127(6):861-879. doi:10.1007/s00401-014-1256-4
38. Mandler M, Valera E, Rockenstein E, et al. Active immunization against α-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multisystem atrophy. Mol Neurodegen. 2015;10:721. doi:10.1186/s13024-015-0008-9
39. Schneeberger A, Tierney L, Mandler M. Active immunization therapies. Mov Disord. 2015;31(2):214-224. doi:10.1002/mds.26377
40. Zella SMA, Metzdorf J, Ciftci E, et al. Emerging immunotherapies for Parkinson disease. Neurol Ther. 2019;8(1):29-44. doi:10.1007/s40120-018-0122-z
41. AFFiRiS AG. AFFiRiS announces top line results of first-in-human clinical study using AFFITOPE PD03A, confirming immunogenicity and safety profile in Parkinson’s disease patients. https://affiris.com/wp-content/uploads/2018/10/praff011prefinal0607wo-embargo-1.pdf. Published June 7, 2017. Accessed July 29, 2020.
42. AFFiRiS AG. AFFiRiS announces results of a phase I clinical study using AFFITOPEs PD01A and PD03A, confirming safety and tolerability for both compounds as well as immunogenicity for PD01A in early MSA patients. http://sympath-project.eu/wp-content/uploads/PR_AFF009_V1.pdf Published March 1, 2018. Accessed July 29, 2020.
43. Masliah E, Rockenstein E, Mante M, et al. Passive immunization reduces behavioral and neuropathological deficits in an alphasynuclein transgenic model of Lewy body disease. PLoS One. 2011;6(4):e19338. doi:10.1371/journal.pone.0019338
44. Bae EJ, Lee HJ, Rockenstein E, et al. Antibody aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32(39):1345-13469. doi:10.1523/JNEUROSCI.1292-12.2012
45. Schenk DB, Koller M, Ness DK, et al. First‐in‐human assessment of PRX002, an anti–α‐synuclein monoclonal antibody, in healthy volunteers. Mov Disord. 2017;32(2):211-218. doi:10.1002/mds.26878.
46. Jankovic J, Goodman I, Safirstein B, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α -synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2018;75(10):1206-1214. doi:10.1001/jamaneurol.2018.1487
47. Jankovic J. Pathogenesis-targeted therapeutic strategies in Parkinson’s disease. Mov Disord. 2019;34(1):41-44. doi:10.1002/mds.27534
48. Shahaduzzaman M, Nash K, Hudson C, et al. Anti-human α-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-α-synuclein rat model of Parkinson’s disease. PLoS One. 2015;10(2):E0116841. doi:10.1371/journal.pone.0116841
49. Brys M, Hung S, Fanning L, et al. Randomized, double-blind, placebo-controlled, single ascending dose study of anti-α-synuclein antibody BIIB054 in patients with Parkinson disease. Neurology. 2018;90(suppl 15):S26.001. doi:10.1002/mds.27738
50. Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target α-synuclein pathology. Exp Neurol. 2017;298(pt B):225-235. doi:10.1016/j.expneurol.2017.10.003
1. Marras C, Beck JC, Bower JH, et al; Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4(1):21. doi:10.1038/s41531-018-0058-0
2. Mantri S, Duda JE, Morley JF. Early and accurate identification of Parkinson disease among US veterans. Fed Pract. 2019;36(suppl 4):S18-S23. doi:10.12788/fp.37-0034
3. Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis. 2017;7(suppl 1):S71-S85. doi:10.3233/JPD-179001
4. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388(6645):839-840. doi:10.1038/42166
5. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211. doi:10.1016/s0197-4580(02)00065-9
6. Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79(6):1044-1066. doi:10.1016/j.neuron.2013.09.004
7. Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. α-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663-1667. doi:10.1126/science.1195227
8. Binolfi A, Fernández CO, Sica MP, Delfino JM, Santos J. Recognition between a short unstructured peptide and a partially folded fragment leads to the thioredoxin fold sharing native-like dynamics. Proteins. 2012;80(5):1448-1464. doi:10.1002/prot.24043
9. Fauvet B, Mbefo MK, Fares MB, et al. α-synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287(19):15345-15364. doi:10.1074/jbc.M111.318949.
10. Wang W, Perovic I, Chittuluru J, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108(43):17797-17802. doi:10.1073/pnas.1113260108
11. Bellucci A, Zaltieri M, Navarria L, Grigoletto J, Missale C, Spano P. From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson’s disease. Brain Res. 2012;1476:183-202. doi:10.1016/j.brainres.2012.04.014
12. Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Südhof TC. Properties of native α-synuclein. Nature. 2013;498(7453):E4-E7.
13. Burré J, Sharma M, Südhof TC. α-synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA. 2014;111(40):E4274-E4283. doi:10.1073/pnas.1416598111
14. Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23(2):1-13. doi:10.1038/nm.4269
15. Burré J, Sharma M, Südhof TC. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J Neurosci. 2015;35(13):5221-5232. doi:10.1523/JNEUROSCI.4650-14.2015
16. Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24(8):1888-1896. doi:10.1523/JNEUROSCI.3809-03.2004
17. Rideout HJ, Dietrich P, Wang Q, Dauer WT, Stefanis L . α-synuclein is required for the fibrillar nature of ubiquitinated inclusions induced by proteasomal inhibition in primary neurons. J Biol Chem. 2004;279(45):46915-46920. doi:10.1074/jbc.M405146200
18. Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40(4):200-210. doi:10.1016/j.tibs.2015.02.003
19. Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochem Biophys Acta. 2010;1802(1):29-44. doi:10.1016/j.bbadis.2009.08.013
20. Lee HJ, Bae EJ, Lee SJ. Extracellular α-synuclein: a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10(2):92-98. doi:10.1038/nrneurol.2013.275
21. Colom-Cadena M, Pegueroles J, Herrmann AG, et al. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain. 2017;140(12):3204-3214. doi:10.1093/brain/awx275
22. Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57-71. doi:10.1016/j.neuron.2011.08.033
23. Masuda-Suzukake M, Nonaka T, Hosokawa M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136(pt 4):1128-1138. doi:10.1093/brain/awt037
24. Hasegawa M, Nonaka T, Masuda-Suzukake M. Prion-like mechanisms and potential therapeutic targets in neurodegenerative disorders. Pharmacol Ther. 2017;172:22-33. doi:10.1016/j.pharmthera.2016.11.010
25. Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric α-synuclein, not aggregated alpha-synuclein: implications for Parkinson’s disease. Glia. 2008;56(11):1215-1223. doi:10.1002/glia.20691
26. Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 2013;8(1):189-201. doi:10.1007/s11481-013-9435-y
27. Sulzer D, Alcalay RN, Garretti F, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546(7660):656-661. doi:10.1038/nature22815
28. Hamza TH, Zabetian CP, Tenesa A, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genetics. 2010;42(9):781-785. doi:10.1038/ng.642
29. Holmes C, Boche D, Wilkinson D, et al. Long term effects of Aβ42 immunisation in Alzheimer’s disease: follow up of a randomized, placebo-controlled phase I trial. Lancet. 2008;372(9634):216-223. doi:10.1016/S0140-6736(08)61075-2
30. Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241-249. doi:10.1016/S1474-4422(12)70015-7
31. Wisniewski T, Goñi F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):499-507. doi:10.1016/j.bcp.2013.12.020
32. Herline K, Drummond E, Wisniewski T. Recent advancements toward therapeutic vaccines against Alzheimer’s disease. Expert Rev Vaccines. 2018;17(8):707-721. doi:10.1080/14760584.2018.1500905
33. Bergstrom AL, Kallunki P, Fog K. Development of passive immunotherapies for synucleopathies. Mov Disord. 2015;31(2):203-213. doi:10.1002/mds.26481
34. Masliah E, Rockenstein E, Adame A, et al. Effects of α-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46(6):857-868. doi:10.1016/j.neuron.2005.05.010
35. Ghochikyan A, Petrushina I, Davtyan H, et al. Immunogenicity of epitope vaccines targeting different B cell antigenic determinants of human α-synuclein: feasibility study. Neurosci Lett. 2014;560:86-91. doi:10.1016/j.neulet.2013.12.028
36. Sanchez-Guajardo V, Annibali A, Jensen PH, Romero-Ramos M. α-synuclein vaccination prevents the accumulation of Parkinson’s disease-like pathologic inclusions in striatum in association with regulatory T cell recruitment in a rat model. J Neuropathol Exp Neurol. 2013;72(7):624-645. doi:10.1097/NEN.0b013e31829768d2
37. Mandler M, Valera E, Rockenstein E, et al. Next generation active immunization approach for synucleinopathies: Implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014;127(6):861-879. doi:10.1007/s00401-014-1256-4
38. Mandler M, Valera E, Rockenstein E, et al. Active immunization against α-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multisystem atrophy. Mol Neurodegen. 2015;10:721. doi:10.1186/s13024-015-0008-9
39. Schneeberger A, Tierney L, Mandler M. Active immunization therapies. Mov Disord. 2015;31(2):214-224. doi:10.1002/mds.26377
40. Zella SMA, Metzdorf J, Ciftci E, et al. Emerging immunotherapies for Parkinson disease. Neurol Ther. 2019;8(1):29-44. doi:10.1007/s40120-018-0122-z
41. AFFiRiS AG. AFFiRiS announces top line results of first-in-human clinical study using AFFITOPE PD03A, confirming immunogenicity and safety profile in Parkinson’s disease patients. https://affiris.com/wp-content/uploads/2018/10/praff011prefinal0607wo-embargo-1.pdf. Published June 7, 2017. Accessed July 29, 2020.
42. AFFiRiS AG. AFFiRiS announces results of a phase I clinical study using AFFITOPEs PD01A and PD03A, confirming safety and tolerability for both compounds as well as immunogenicity for PD01A in early MSA patients. http://sympath-project.eu/wp-content/uploads/PR_AFF009_V1.pdf Published March 1, 2018. Accessed July 29, 2020.
43. Masliah E, Rockenstein E, Mante M, et al. Passive immunization reduces behavioral and neuropathological deficits in an alphasynuclein transgenic model of Lewy body disease. PLoS One. 2011;6(4):e19338. doi:10.1371/journal.pone.0019338
44. Bae EJ, Lee HJ, Rockenstein E, et al. Antibody aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32(39):1345-13469. doi:10.1523/JNEUROSCI.1292-12.2012
45. Schenk DB, Koller M, Ness DK, et al. First‐in‐human assessment of PRX002, an anti–α‐synuclein monoclonal antibody, in healthy volunteers. Mov Disord. 2017;32(2):211-218. doi:10.1002/mds.26878.
46. Jankovic J, Goodman I, Safirstein B, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α -synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2018;75(10):1206-1214. doi:10.1001/jamaneurol.2018.1487
47. Jankovic J. Pathogenesis-targeted therapeutic strategies in Parkinson’s disease. Mov Disord. 2019;34(1):41-44. doi:10.1002/mds.27534
48. Shahaduzzaman M, Nash K, Hudson C, et al. Anti-human α-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-α-synuclein rat model of Parkinson’s disease. PLoS One. 2015;10(2):E0116841. doi:10.1371/journal.pone.0116841
49. Brys M, Hung S, Fanning L, et al. Randomized, double-blind, placebo-controlled, single ascending dose study of anti-α-synuclein antibody BIIB054 in patients with Parkinson disease. Neurology. 2018;90(suppl 15):S26.001. doi:10.1002/mds.27738
50. Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target α-synuclein pathology. Exp Neurol. 2017;298(pt B):225-235. doi:10.1016/j.expneurol.2017.10.003
